Cigna Medical Coverage Policy
|
|
|
- Garry Taylor
- 6 years ago
- Views:
Transcription
1 Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background Coding/Billing Information References Effective Date... 2/15/2017 Next Review Date.11/15/2017 Coverage Policy Number Related Coverage Resources Autologous Platelet Derived Growth Factors (Platelet-Rich Plasma [PRP]) Bone Graft Substitutes for Use in Bone Repair Breast Reconstruction Following Mastectomy or Lumpectomy Electrical Stimulation Therapy and Devices Hyperbaric Oxygen Therapy, Systemic & Topical Injectable Fillers Lumbar Fusion for Spinal Instability and Degenerative Disc Conditions, Including Sacroiliac Fusion Negative Pressure Wound Therapy/Vacuum- Assisted Closure (VAC) for Non-Healing Wounds Plantar Fasciitis Treatments Pulsed Electromagnetic Therapy Scar Revision INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. Coverage Policies relate exclusively to the administration of health benefit plans. Coverage Policies are not recommendations for treatment and should never be used as treatment guidelines. In certain markets, delegated vendor guidelines may be used to support medical necessity and other coverage determinations. Proprietary information of Cigna. Copyright 2017 Cigna Coverage Policy Cigna covers each of the following as medically necessary for wound closure: autologous skin graft (CPT Codes ) unprocessed allogeneic human, cadaver-derived skin graft (CPT Codes ) unprocessed allogeneic pig skin derived skin graft (CPT Codes ) Cigna covers each of the following products as indicated: Skin Substitute Indication Criteria Application CPT / HCPCS Codes AlloDerm Breast reconstruction Covered as medically necessary when used in association with a covered, Product HCPCS Codes Q4116 Page 1 of 90
2 Skin Substitute Indication Criteria Application CPT / HCPCS Codes medically necessary breast AlloMax Apligraf Breast reconstruction Diabetic foot ulcer reconstruction procedure Covered as medically necessary when used in association with a covered, medically necessary breast reconstruction procedure Covered as medically necessary when ALL of the following criteria are met: full-thickness diabetic foot ulcer of greater than three weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of 0.70 When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications at a minimum of one week intervals, for up to a maximum of four in 12 weeks are considered medically necessary when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) Product HCPCS Codes C Q4101 Apligraf Venous stasis ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Covered as medically necessary when BOTH of the following criteria are met: partial- or full-thickness venous stasis ulcer of greater than four weeks duration for which standard wound therapy has failed treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of Q4101 When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial Page 2 of 90
3 Skin Substitute Indication Criteria Application CPT / HCPCS Codes application additional applications at a minimum of one week intervals, for up to a maximum of four in 12 weeks when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) Product HCPCS Codes Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Biobrane Burn wound Covered as medically necessary when used for temporary covering of a partial-thickness freshly debrided or excised burn wound Biobrane-L Burn wound Covered as medically necessary when BOTH of the following criteria are met: temporary covering of a partialthickness freshly debrided or excised burn wound DermACELL DermACELL Breast reconstruction Diabetic foot ulcer adjunct to meshed autograft Covered as medically necessary when used in association with a covered, medically necessary breast reconstruction procedure Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness diabetic foot ulcer of greater than four weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of 0.70 Not Applicable Not Applicable Q Q4122 When the above medical necessity criteria are met, treatment is limited to a total of two applications. Dermagraft Diabetic foot ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Covered as medically necessary when ALL of the following criteria are met: full-thickness diabetic foot ulcer of greater than six weeks duration for which standard therapy has failed type I or type II diabetes mellitus Q4106 Page 3 of 90
4 Skin Substitute Indication Criteria Application CPT / HCPCS Codes with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of 0.70 Product HCPCS Codes When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications for up to a maximum of eight in 12 weeks when there is evidence of wound healing (e.g., signs of epithelialization and reduction in ulcer size) Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Epicel Burn wound Covered as medically necessary when used according to the U.S. Food and Drug Administration (FDA)-approved Humanitarian Device Exemption (HDE) for an individual with deep dermal or full-thickness burns comprising a total body surface area of greater than or equal to 30% EpiFix Amniotic Membrane Diabetic foot ulcer Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness, diabetic foot ulcer of greater than four weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of C5271-C Q4131 When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications may be applied at a minimum of one week intervals, for up to a maximum of four in 12 weeks are considered Page 4 of 90
5 Skin Substitute Indication Criteria Application CPT / HCPCS Codes medically necessary when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) Product HCPCS Codes EpiFix Amniotic Membrane Venous stasis ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Covered as medically necessary when BOTH of the following criteria are met: partial- or full-thickness venous stasis ulcer of greater than four weeks duration for which standard wound therapy has failed treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of Q4131 When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications at a minimum of one week intervals, for up to a maximum of four in 12 weeks when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) FlexHD Acellular Hydrated Dermis Grafix Breast reconstruction Diabetic foot ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Covered as medically necessary when used in association with a covered, medically necessary breast reconstruction procedure. Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness diabetic foot ulcer of greater than four weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of Q Q4132 Q4133 Page 5 of 90
6 Skin Substitute Indication Criteria Application CPT / HCPCS Codes Product HCPCS Codes When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications at a minimum of one week intervals, for up to a maximum of four in 12 weeks are considered medically necessary when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) GraftJacket Regenerative Tissue Matrix Diabetic foot ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness, diabetic foot ulcer of greater than four weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of Q4107 Integra Dermal Regeneration Template Integra Bilayer Matrix Wound Dressing Integra Matrix Wound Dressing Burn wound When the above medical necessity criteria are met, one application is considered medically necessary. Covered as medically necessary when BOTH of the following criteria are met: postexcisional treatment of a fullthickness or deep partial-thickness burn sufficient autograft is not available at time of excision or is contraindicated Q4105 Q4104 Q4108 Integra Meshed Bilayer Wound Matrix Integra Dermal Regeneration Template/ Omnigraft Dermal Regeneration Matrix Diabetic Foot Ulcer Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness diabetic foot ulcer of greater than six weeks duration for which standard wound C Q4105 Page 6 of 90
7 Skin Substitute Indication Criteria Application CPT / HCPCS Codes therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of 0.70 Product HCPCS Codes When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications at a minimum of one week intervals, for up to a maximum of four in 12 weeks are considered medically necessary when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) NeoForm Dermis Oasis Wound Matrix Oasis Ultra Tri- Layer Matrix Breast reconstruction Diabetic foot ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status Covered as medically necessary when used in association with a covered, medically necessary breast reconstruction procedure Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness, diabetic foot ulcer of greater than four weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of C C5275-C5278 Q4102 Q4124 Oasis Wound Matrix Oasis Ultra Tri- Layer Matrix Venous stasis ulcer Covered as medically necessary when BOTH of the following criteria are met: partial or full-thickness, lower extremity venous stasis ulcer of four weeks duration for which standard wound therapy has failed treated foot has adequate blood supply as evidenced by either the C5271-C5278 Q4102 Q4124 Page 7 of 90
8 Skin Substitute Indication Criteria Application CPT / HCPCS Codes presence of a palpable pedal pulse or an ankle-brachial index (ABI) of 0.70 Product HCPCS Codes TheraSkin Diabetic foot ulcer Covered as medically necessary when ALL of the following criteria are met: partial or full-thickness, diabetic foot ulcer of greater than four weeks duration for which standard wound therapy has failed type 1 or type 2 diabetes mellitus with a hemoglobin A1c (HbA1C) less than 12% treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of Q4121 When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application additional applications may be applied at a minimum of one week intervals, for up to a maximum of four in 12 weeks are considered medically necessary when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) TheraSkin Venous stasis ulcer Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Covered as medically necessary when BOTH of the following criteria are met: partial- or full-thickness venous stasis ulcer of greater than four weeks duration for which standard wound therapy has failed treated foot has adequate blood supply as evidenced by either the presence of a palpable pedal pulse or an ankle-brachial index (ABI) of Q4121 When the above medical necessity criteria are met, the following conditions of coverage apply: treatment is limited to one initial application Page 8 of 90
9 Skin Substitute Indication Criteria Application CPT / HCPCS Codes additional applications at a minimum of one week intervals, for up to a maximum of four in 12 weeks when evidence of wound healing is present (e.g., signs of epithelialization and reduction in ulcer size) Product HCPCS Codes Additional applications beyond 12 weeks are considered not medically necessary regardless of wound status. Transcyte Burn wound Covered as medically necessary when used for temporary covering of a surgically excised deep partial- or fullthickness burn wound as a covering prior to autografting C5271-C5278 Cigna does not cover any of the products listed above for ANY unlisted indication because each is considered experimental, investigational, or unproven. Cigna does not cover ANY of the following products because each is considered experimental, investigational, or unproven for ANY indication (this list may not be all-inclusive): Skin Substitute Reason(s) for Request (this list may not be all inclusive) Application CPT / HCPCS Codes Product HCPCS Codes C1768 ActiveBarrier Wound care C5271-C5278 ActiveMatrix flowable Connective tissue repair No specific Acuseal Cardiovascular Patch Cardiovascular reconstruction No specific Adherus Dural Sealant Dural repair No specific Affinity Wound care Q4159 Allopatch HD Tendon augmentation No specific Q4128 AlloSkin Wound care C5271-C5278 Q4115 Q4141 AlloSkin RT Wound care Q4123 Allowrap Wound care Q4150 AmnioBand/Guardian Wound care Q4151 AmnioBand Particulate Wound Care C5271-C5278 AmnioCare Tendon/nerve repair No specific Q4168 Q4168 AmnioClear Wound care Surgical barrier AmnioClear LTC flowable Knee pain and inflammation No specific Page 9 of 90
10 Skin Substitute Reason(s) for Request (this list may not be all inclusive) Application CPT / HCPCS Codes Product HCPCS Codes AmnioExCel/BioDExCel Wound care Q4137 Soft tissue repair Amniofix Amniotic Membrane Tendon/nerve repair No specific AmnioHeal Plus Wound care C5271-C5278 AmnioMatrix Wound care Soft tissue repair C5271-C5278 Q4139 V2790 AmnioMTM Injectable Wound care Soft tissue repair No specific AmnioPro Membrane Wound care Q4163 AmnioPro Flow Wound care No specific Q4162 Amniovo Soft tissue repair Tendon repair No specific Architect Biomatrix Wound care Q4147 Artacent Surgical barrier No specific Q4169 ArthroFlex (FlexGraft ) Shoulder reconstruction No specific Q4125 Achilles tendon repair Avance Nerve Graft Peripheral nerve repair AxoGuard Nerve Connector Peripheral nerve repair Bio-ConneKt Wound care Q4161 C5271-C5278 BioDfactor Wound care Soft tissue repair C5271-C5278 BioDfence Surgical wrap/barrier No specific Q4140 Tendon repair BioDfence DryFlex Surgical wrap/barrier No specific Q4138 Tendon repair BioDRestore flowable Soft tissue repair No specific Biodesign Dural Graft Dural Repair No specific Q4161 Biodesign (Surgisis ) AFP Anal Fistula Plug Anal and rectal fistula repair C1781 Biodesign (Surgisis ) Hiatal Hernia Matrix Hernia repair C1781 Biodesign (Surgisis ) Inguinal Hernia Matrix Hernia repair C1781 Biodesign (Surgisis ) RVP Recto-Vaginal Fistula Plug Recto-vaginal fístula repair No specific C1781 BioFix Wound care C5271-C5278 BioVance Wound care Q4154 CardioCel Pericardial closure No specific Cardiac or vascular defect repair Clarix Regenerative Matrix Surgical covering/wrap/barrier No specific Page 10 of 90
11 Skin Substitute Reason(s) for Request (this list may not be all inclusive) Application CPT / HCPCS Codes Clarix Flo Integumental tissue repair No specific Conexa Tendon repair No specific Cormatrix CanGaroo Protect Implantable electronic device pocket No Specific ECM Envelope Code CorMatrix ECM for Cardiac Intracardiac patch No specific Tissue Repair CorMatrix ECM for Carotid Carotid artery repair No specific Repair CorMatrix ECM for Pericardial Pericardial repair No specific Closure CryoSkin Wound care C5271-C5278 Cygnus Wound care Nerve wrap C5271-C5278 Cytal Wound care C5271-C5278 Product HCPCS Codes Q4155 C1781 Q4170 Q4119 Q4120 Q4166 Q4112 Cymetra Integumental tissue repair No specific DermaMatrix Acellular Dermis Facial soft tissue defects Breast reconstruction C1781 DermaPure Wound care Q4152 DermaSpan Wound covering Q4126 Tendon repair Dermavest Wound care Q4153 Duraform Dural repair No specific DuraGen Dural repair No specific DuraGuard Dural repair No specific DuraMatrix Dural repair No specific DuraSeal Dural Sealant System Dural repair No specific DuraSeal Spine Sealant System Dural repair No specific Durepair Regeneration Matrix Dural repair No specific Endoform Dermal Template Wound care C5271-C5278 Epifix Injectable Wound care No specific Q4145 EpiCord Wound care Q4131 Excellagen Wound care C5271-C5278 Q4149 Page 11 of 90
12 Skin Substitute Reason(s) for Request (this list may not be all inclusive) Application CPT / HCPCS Codes Product HCPCS Codes Q4136 EZ Derm Wound care C5271-C5278 FloGraft flowable Tendonitis No specific Soft tissue trauma Fortaderm /Puraply Wound care C9349 C5271-C5278 Q4172 GammaGraft Wound care Q4111 C5271-C5278 GORE BIO-A Fistula Plug Anorectal fistulas C1781 Gore Bio A Tissue Soft Tissue Reinforcement Reinforcement C1781 GraftJacket Xpress Wound care No specific Q4113 Helicoll Wound care Q4164 hmatrix Integumental tissue repair No specific Q4134 Hyalomatrix PA Wound care Q4117 C5271-C5278 HydroFix Vaso Shield Vessel guard No specific Integra Flowable Wound Matrix Wound care No specific Q4114 Interfyl Integumental tissue repair No specific Q4171 Keramatrix Wound care Q4165 C5271-C5278 MariGen/Kerecis Omega3 Wound Wound care Q4158 MatriStem Wound care C5271-C5278 Q4118 Q4119 Q4120 Matrix HD Wound care Q4128 Tendon repair Mediskin Wound care Q4135 C5271-C5278 MemoDerm Wound care Q4126 Tendon repair Miroderm Wound care Q4175 C5271-C5278 Neox Wound Matrix Wound care Q4148 Q4156 Neox 100 Wound care Q4156 Neox 1K Wound care Q4148 Neox Flo Wound care No specific Q4155 NeuraGen Nerve Guide Peripheral nerve repair C NeuraWrap Nerve Protector Peripheral nerve repair C NuCel Tendon repair No specific Page 12 of 90
13 Skin Substitute Reason(s) for Request (this list may not be all inclusive) Application CPT / HCPCS Codes No specific Product HCPCS Codes Q4160 Nucel Bioactive Amniotic Tissue Repair Suspension NuShield Orthopaedics Tendon repair No specific NuShield Spine Dura repair No specific Q4160 Oasis Burn Matrix Burn wounds Q4103 Orcel Burn wounds C5271-C5278 OrthADAPT Bioimplant Soft tissue reinforcement C1781 OsseoGuard Oral defects C5275-C5276 Ovation Wound healing C5271-C5278 PalinGen Flow Soft tissue repair No specific Q4174 PalinGen Xplus Soft tissue repair No specific Q4173 Peri-Guard Repair Patch Soft tissue repair Pericardial and intracardiac repair No specific C1781 Peri-Strips Dry Staple line reinforcement No specific Permacol Soft tissue reinforcement/repair C Preclude Dura Substitute Dural repair No specific Preclude Pericardial Membrane Pericardial repair No specific Preclude Vessel Guard Vessel covering No specific Preserve Paraderm Dermal Tissue Matrix Integumental tissue repair No specific PriMatrix Wound care Q4110 ProMatriX Wound care No specific PX50 /PX50 Plus Damaged or inadequate tissue repair No specific Repriza Reconstructive surgery Breast reconstruction Abdominal wall repair Q4174 Q4143 Restore Orthobiologic Soft Tissue Soft tissue reinforcement Implant C1781 Revitalon Wound care Q4157 Seamguard Staple Line Reinforcement Staple line reinforcement No specific SERI Surgical Scaffold Soft tissue reinforcement/repair SJM Pericardial Patch with Pericardial repair No specific EnCap AC Technology C1781 Page 13 of 90
14 Skin Substitute Reason(s) for Request (this list may not be all inclusive) Application CPT / HCPCS Codes SportMesh Soft tissue reinforcement SteriShield Soft tissue reinforcement/repair Strattice Reconstructive Tissue Soft tissue reinforcement/repair Matrix Product HCPCS Codes C1781 Q4130 C1781 Stravix Integumental tissue repair No specific SurgiMend Breast reconstruction C9358; C9360 Talymed Wound care Q4127 TenoGlide Tendon Protector Tendon repair No specific C9356 Sheet TenSIX Wound care Q4146 Tendon repair TissueMend Soft tissue repair Tendon repair No specific C1781 Tornier BioFiber Absorbable Biological Scaffold Soft tissue reinforcement/repair C1781 Tornier Collagen Coated BioFiber Scaffold Soft tissue reinforcement/repair C1781 TruSkin Wound care Q4167 C5271-C5278 Unite Biomatrix Wound care Q4129 C5271-C5278 Vascu-Guard Peripheral vascular reconstruction No specific VersaShield Wound care Soft tissue covering C5271-C5278 Veritas Collagen Matrix Soft tissue reinforcement/repair C9354 Veritas Collagen Matrix Peri-Strips Dry Staple line reinforcement No specific Viaflow /Viaflow C Connective tissue repair No specific C1781 WoundEx Membrane Wound Care Q4163 WoundEx Flow Integumental tissue repair No specific XCM Biologic Vascular Patch Soft tissue reinforcement/repair XCeed Wound care No specific Xenform Soft tissue reinforcement/repair XenMatrix Surgical Graft Soft tissue reinforcement/repair XenoSure Biologic Vascular Patch Cardiac reconstruction/repair No specific Vascular reconstruction/repair Q4162 Q4142 C1781 C1781 Page 14 of 90
15 General Background Autologous Skin Grafts and Cadaver-Derived Skin Grafts Autologous skin grafts and the use of fresh, unprocessed allogeneic cadaver-derived skin grafts are established procedures for wound care. Autologous skin grafts, or autografts, refer to tissue transplanted from one location to another in the same individual. Autografts are referred to as partial-thickness or split-thickness graft. Autografts are ideal because there is no risk of rejection. In some cases, the area of healthy skin available for harvesting may be inadequate to cover the wound area. In these cases, the best choice is human skin taken from human cadavers, consisting of both epidermal and dermal skin layers. These unprocessed, allogeneic cadaver-derived skin grafts (allografts or homograft) are used for temporary coverage of excised wounds. Cadaver skin grafts may be kept fresh for up to 14 days or may be cryopreserved or glycerol-preserved (GPA). Unprocessed cadaveric skin is a widely used skin substitute. Fresh pig s skin that has been specially treated and contains only the dermis layer has been used for coverage of partial thickness burns and excised wounds prior to grafting. There are various ways to sterilize and preserve pigskin. In general, the pigskin is treated with a solution (e.g., providine-iodine), placed in normal saline with an antibiotic, soaked in a solution to sterilize it, rinsed and refrigerated or frozen. Fresh skin stored in normal saline is viable for up to 72 hours. When autografts, unprocessed human cadaver skin or unprocessed pig s skin graft are not available tissue-engineered skin substitutes which include processed human cadaver skin and pig skin may be an option (Ruszczak and Elston, 2013; Ahmad et al., 2010; Ge et al., 2010; Paul, 2008). Tissue Engineered Skin Substitutes Tissue-engineered skin substitutes (i.e., human skin equivalents [HSE]), also referred to as artificial skin, are bioengineered skin products and may be either acellular or cellular. Acellular (i.e., cadaveric human dermis with cellular material removed) products contain a matrix or scaffold composed of materials such as collagen, hyaluronic acid, and fibronectin. The construction of the matrix allows easy access by host cells during the healing process. Cellular products contain living cells such as fibroblasts and keratinocytes within a matrix. The cells contained within a matrix may be allogeneic (i.e., obtained from another individual) or autologous (i.e., obtained from the same individual). Some products are derived from other species (e.g., bovine, porcine) and are referred to as a xenograft. Skin substitutes are generally comprised of epidermal cells, dermal cells or may be composites (i.e., a combination of dermal and epidermal). The substitutes can be used as either temporary or permanent wound coverings (Ho, et al., 2005; Sibbald, et al., 2005). Grafting techniques utilized to apply skin substitutes include autografting (i.e., tissue transplanted from one part of the body to another), allografting (i.e., transplant from one individual to another of the same species), and xenografting (i.e., a graft from one species to another unlike species). Skin substitutes have been proposed for the treatment of multiple conditions including breast reconstruction and chronic wounds nonresponsive to standard therapy. During breast reconstruction, acellular dermal skin substitutes (i.e., AlloDerm, AlloMax) are primarily used in the setting of tissue expander and breast implant reconstruction. Patients should be in overall good health and have no underlying condition that would restrict blood flow or interfere with the normal healing process (e.g., uncontrolled diabetes, hypertension, previous surgery). These matrixes may be indicated when there is insufficient tissue expander or implant coverage by the pectoralis major muscle and additional coverage is required, as may be the case in a very thin patient; if there is viable but compromised or thin post-mastectomy skin flaps that are at risk of dehiscence or necrosis; or if there is a need to re-establish the inframammary fold and lateral mammary fold landmarks. When used in appropriate candidates, these skin substitutes are proposed to improve control over placement of the inframammary fold and final breast contour, enhance use of available mastectomy skin, reduce the number of expander fills necessary, reduce time to complete expansion and eventual implant exchange, potential improved management of a threatened implant, reduce the need for explantation and the potential for reduction in the incidence of capsular contracture. However, there are ongoing concerns regarding the increased risk of seroma and infection, a higher risk of an implant having to be removed, and tissue flap death (American Cancer Society, 2013; Nguyen, et al., 2011; Sbitany and Serletti, 2011). A chronic wound is defined as a wound that does not heal in the time expected based upon the patient s age, comorbidities, and wound etiology. A wound that has not healed within 30 days to three months is considered chronic. Different types of chronic wounds include lower extremity diabetic neuropathic ulcers, venous ulcers and burn wounds. Treatment depends on the type of wound, wound location, and wound size. The wound should be free of infection, coagulum, sinus tracts, tunnels, cellulitis, eschar and necrotic tissue. There should be no exposure of joints, tendons, ligaments or bone. Adequate blood supply to the affected area should be evidenced by a palpable pedal pulse or an ankle-brachial index (ABI) of Page 15 of 90
16 Standard wound therapy for a foot ulcer in a type 1 or type 2 diabetic includes avoidance of mechanical stressors on the ulcerated extremity (i.e., off-loading), wound cleansing and debridement, management of infection with antibiotic therapy and application of saline-soaked gauze. It is essential that routine medical management of diabetes and the presence of a hemoglobin A1C (HbA1C) of less than 12% be achieved to maximize complete healing of the wound. The mainstay of conventional wound therapy for lower extremity venous stasis ulcers is compression therapy (e.g., compression stockings, Unna boots, elastic wraps). Surgical debridement of the wound, zinc paste gauze and non-weight bearing regimens may also be used. Skin substitutes may be indicated for the treatment of a wound that is not healing in response to conventional therapy. The underlying medical condition, such as hypertension, should be adequately managed to foster complete healing. To date evidence is lacking supporting superiority of one product over another for the treatment of lower extremity wound therapy (Santema, et al., 2016; Hayes, Aug 2015). U.S. Food and Drug Administration (FDA) Depending on the purpose of the product and how it functions, skin substitutes are regulated by the FDA premarket approval (PMA) process, 510(k) premarket notification process, or the FDA regulations for banked human tissue. Products that are classified by the FDA as an interactive wound and burn dressing are approved under the PMA process as a class III, high-risk device and require clinical data to support their claims for use. These devices may be used as a long-term skin substitute or a temporary synthetic skin substitute. They actively promote healing by interacting directly or indirectly with the body tissues. Examples of these devices include Apligraf (Organogenesis Inc., Canton, MA) and Dermagraft (Advanced BioHealing, Inc., LaJolla, CA). Other wound care devices are approved by the 510(k) process, and their primary purpose is to protect the wound and provide a scaffold for healing. They may or may not be integrated into the body tissue. Some devices are rejected by the body after approximately ten days to several weeks and removed prior to definitive wound therapy or skin grafting. Integra Bilayer Matrix Wound Dressing (BMWD) (Integra LifeSciences Corp., Plainsboro, NJ) and Oasis Wound Matrix (Cook Biotech, Inc., West Lafayette, IN) are examples of these devices. Donated skin that requires minimal processing and is not significantly changed in structure from its natural form is classified by the FDA as banked human tissue, is not considered a medical device, and does not require PMA or 510(k) approval. Donated skin is regulated by the American Association of Tissue Banks (AATB) and the FDA guidelines for banked human tissue. AATB oversees a voluntary accreditation program and the FDA focuses on preventing the transmission of communicable diseases by requiring donor screening and testing. Tissue establishments must register with the FDA and list each cell or tissue produced. An example of a banked human tissue product is AlloDerm, an acellular dermal matrix (FDA, 2009; FDA, 2004). Skin Substitutes The safety and efficacy of the skin substitutes listed below are supported by the evidence in the published peerreviewed scientific literature and/or are established treatment options for the discussed indications. AlloDerm - Breast Reconstruction AlloDerm (LifeCell Corporation, Branchburg, NJ) is a human acellular dermal matrix allograft classified as banked human tissue by the FDA because it is minimally processed and not significantly changed in structure from the natural material. AlloDerm is an established treatment option and is supported by the evidence in the published peer-reviewed scientific literature for tissue repair during postmastectomy breast reconstruction (Kim, et al., 2012; Jansen and Macadam, 2011; Nguyen, et al., 2011; Chun, et al., 2010; Spear, et al., 2008; Bindingnavele, et al., 2007; Breuing and Colwell, 2007; Zienowicz, et al., 2007; Salzberg, 2006; Breuing, et al., 2005; Nahabedian, 2005; Gamboa-Bobadilla, 2006). AlloDerm is available in two forms. One product is a Tissue Regeneration Matrix and the other is a Ready to Use matrix. The Ready to Use with a contour shape was specifically designed for breast reconstruction. AlloDerm Other Indications Page 16 of 90
17 AlloDerm has been proposed as a treatment option for various other conditions including: reconstruction after excision of skin and soft tissue malignancies, abdominal wall reconstruction and/or hernia repair, tympanoplasty, lower eyelid surgery, Frey s syndrome (a complication of parotid excision), cleft palate repair; various oral surgery procedures including gingival recession, empty nose syndrome, burns and postburn scar contractures and nasal contour deformities. In addition, AlloDerm has been investigated for placement over implantable cardioverter-defibrillators and cardiac pacemakers to prevent skin erosion, scalp reconstruction and hand resurfacing. Studies are primarily in the form of case series or retrospective reviews with small patient populations (n=6-58) and short-term follow-ups (e.g., 3 68 months). Comparative studies to established therapies with randomization are lacking. There is insufficient evidence in the published peer-reviewed scientific literature to support the efficacy of AlloDerm for these indications. Abdominal Wall Reconstruction: Case series (n=10) (DeMoya, et al., 2008) and retrospective reviews (Lee, et al., 2009; Bellows, et al., 2007; Patton, et al., 2007; Schuster, et al., 2006) (n=18-67) with 2 16 months follow-up have evaluated the use of AlloDerm during contaminated abdominal wall reconstructive surgery. Diagnosis included infected fascia with dehiscence, complex ventral hernia, and dehiscence and/or evisceration. Typically the wounds were contaminated or dirty. Hernia recurrence rates up to 64% were reported. Complication rates were as high as 43% and included wound infections, fistulas, wound dehiscence, graft infection, postoperative intra-abdominal bleeding, and evisceration. Some cases required repeat surgery and/or removal of the AlloDerm. The authors reported that 100% of the patients experienced either significant abdominal laxity or a hernia following the application of AlloDerm (De Moya, et al., 2008); due to the high overall rate of hernia recurrence when the wound was left open, they could not support the use of AlloDerm unless the wound could be closed postoperatively (Shuster, et al., 2006); ongoing studies are required to address further refinements of surgical technique and to analyze long-term outcomes related to the durability (Patton, et al., 2007); and lastly, long-term outcomes are unknown and are critical to fully establish the durability and functional properties of remodeling of AlloDerm grafts when used as tissue prosthesis during abdominal wall repair (Bellows, et al., 2007). Cleft Palate Repair: A systematic review of the literature included nine nonrandomized studies (n=166) that evaluated AlloDerm for cleft palate repair during primary palatoplasty (n=92) and palatal fistula repair (n=74). There was insufficient evidence to support AlloDerm for this indication (Aldekhayel, et al., 2012). Frey s syndrome: Parotidectomy exposes the postganglionic parasympathetic fibers, which can cause severance and inappropriate regeneration. It is proposed that the syndrome is prevented by putting a barrier between skin and the auriculotemporal nerve to prevent the switched parasympathetic fibers from innervating the sweat glands or skin, thus preventing Frey syndrome. It has been proposed that AlloDerm can alleviate the gustatory sweating associated with Frey s syndrome following parotid excision (Zeng, et al., 2012; Sinha et al., 2003; Govindaraj, et al., 2001). Zeng et al. (2012) conducted a systematic review and meta-analysis of randomized and quais-randomized controlled trials to evaluate the effectiveness of AlloDerm for preventing Frey syndrome after parotidectomy. Five studies (n=409) met inclusion criteria. The primary outcome measure was the incidence of Frey syndrome (objective or subjective). Secondary outcomes included facial contour, wound infection, rejection, seroma or salivary fistula and facial nerve paralysis. Meta-analyses of 2 4 trials showed a significant reduction in objective incidence (p< ) and subjective incidence (p< ) of Frey syndrome and salivary fistula (p=0.02). There was no statistically significant reduction in the incidence of facial nerve paralysis (p=0.51), incidence of seroma/sialocele (p=0.40) or improvement in facial contour. There were no significant differences in wound infection between the two groups and no cases of implant extrusion with AlloDerm. The authors noted that limitations of this study included: the number of studies contributing substantial data to the meta-analysis was small and the authors could not fully assess the effects of important clinical factors that may have influenced outcomes, possible problems with concealment, lack of blinding, loss of patients to follow-up and possible publication bias. Additional well-designed randomized controlled trials with large patient populations are needed to confirm the efficacy of AlloDerm for this subpopulation. Hernia Repair: Case series (n=11 70) (Bluebond-Langner, et al., 2008; Misra, et al., 2008; Aycock, et al., 2007) and retrospective reviews (n=37 165) (Diaz, et al., 2009; Lee, et al., 2008; Jin, et al., 2007) evaluated the application of AlloDerm during hernia repairs (e.g., parastomal hernia, hiatal hernia, incisional hernia, ventral hernia). Follow-ups ranged from 8-37 months. Complication rates were as high as 44%. Diaz, et al. (2000) reported a 17.1% overall hernia recurrence rate, 40% surgical site infections, and 11.6% postoperative fistulas. Other studies reported postoperative ileus (24.2%), wound seroma (12.9%), and intrabdominal abscess (9.6%). Page 17 of 90
18 In one study, seven of nine patients required reoperation due to postoperative abdominal wall laxity which was associated with infection and larger defects. Outcomes varied based on the type of surgical procedure performed, the type and number of AlloDerm sheets used, presence or absence of fecal contamination, and patient comorbidities (e.g., diabetes mellitus). The evidence in the published peer-reviewed scientific literature does not support the efficacy of AlloDerm for hernia repair. Lower Eyelid Surgery: AlloDerm is proposed as an alternative to hard palate grafting used in the surgical repair of lower eyelid retraction following blepharoplasty. However, studies are primarily in the form of retrospective reviews with small patient populations and the authors reported less that beneficial clinical outcomes were not seen with the addition of AlloDerm (Li, et al., 2005; Taban, et al., 2005). Oral Surgery: AlloDerm has been proposed for closure of oral harvest sites, oral cavity reconstruction, and the treatment of gingival recession. Studies are primarily in the form of case reports or case series with small patient populations. Published randomized controlled trials have included small, heterogeneous patient populations (e.g., n=10 23) and short-term follow-ups. Overall, studies have not reported a significant difference with the use of AlloDerm for these indications. Jamal et al. (2010) conducted a randomized controlled trial to compare AlloDerm (n=10) closure to primary closure (n=10) of oral harvest sites for buccal mucosa grafts for urethroplasty. A single graft was harvested from one cheek. Based on questionnaire scores, there were no significant differences in postoperative oral pain, neurosensory deficits, or mouth tightness between the two groups. Although the difference was not statistically significant, there was a trend in the AlloDerm group toward more difficulty with mastication at three weeks, and three-, six-, and 12-month follow-ups. A significant difference was reported in cheek swelling at three weeks with 80% of the AlloDerm group compared to 30% of the primary closure group (p=0.01). The authors noted that AlloDerm offered no significant advantages when compared with primary closure and its use appeared to be an unnecessary step. In a prospective nonrandomized study, Girod et al. (2009) compared the efficacy of AlloDerm (n=22) to split thickness skin graft (STSG) (n=12) in patients who underwent surgical resection of oral cavity tumors followed by reconstruction. The surgeries were performed by two different surgeons. The time from date of surgery to enrollment in the study was 22 months for the AlloDerm group and 12 months for the STSG group. There was a higher pre- and post-operative prevalence of radiotherapy exposure in the AlloDerm (45%) compared to the STSG group (17%). A higher graft failure rate was seen in the AlloDerm group (14% vs. 0%), but was not statistically significant. There was a significant difference in the distribution of graft sites with more tongue patients in the AlloDerm group and more floor-of-mouth patients in the STSG group. AlloDerm grafts resulted in a more normal appearing mucosal surface. Although the AlloDerm patients scored higher on the Global Health Status, Functional, and Symptom scores on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items/head and Neck 35 (EORTC QLQ-C30/H&N35) tool, the differences were not significant. Histopathology comparisons (n=12) showed less fibrous tissue and keratinization of the epithelium in the AlloDerm patients. Mahajan et al. (2007), in a randomized controlled trial, evaluated the effectiveness of AlloDerm in the treatment of gingival recession. Fourteen patients were randomly assigned to the AlloDerm group (AlloDerm and coronally positioned flap [CPF]; n=7) or the CPF group (CPF alone; n=7). The defect coverage in the AlloDerm group was 97.14% compared to 77.42% in the CPF group, which was statistically significant (p<0.05). CPF produced statistically significant better results (p<0.03) in patient comfort. There were no significant differences between the two groups in the remaining clinical outcomes and overall patient satisfaction. A randomized study by Rahmani and Lades (2006) compared AlloDerm to conventional grafting. Fourteen patients with 20 gingival recessions of Miller s grade I and II were included in the study. Outcomes were measured at baseline and at six months after surgery and included: recession height, recession width, probing depth, attached gingiva, keratinized gingiva, and clinical attachment level. Differences in the mean change between the two groups were not significant in any of the parameters. Gapski et al. (2005) conducted a systematic review and meta-analysis to compare the efficacy of acellular dermal matrix (ADM) (AlloDerm) based root coverage increase in keratinized tissues to commonly used mucogingival surgeries for the treatment of gingival recession and to increase the width of attached gingiva. Eight randomized controlled trials met inclusion criteria. Four studies were eligible for comparisons between ADM-based root coverage and free autogenous connective tissue graft (CTG): two for comparisons between ADM based root coverage and coronally advanced flap (CAF) and two for comparisons between ADM-based Page 18 of 90
19 augmentation of keratinized gingiva (KG) and free gingival graft (FGG). There were no statistically significant differences between groups for any of the outcomes measured (recession coverage, keratinized tissue formation, probing depths, and clinical attachment levels). Due to the heterogeneity in study design and analysis and lack of data, meta-analysis could not be performed. AlloMax AlloMax Surgical Graft (Bard Davol, Inc. Warwick, RI) is an acellular non-cross-linked human dermis allograft. Because AlloMax is a natural human product it is classified as banked human tissue and does not require FDA approval. It is regulated by the American Association of Tissue Banks and the FDA guidelines for banked human tissue. The AlloMax Surgical Graft for Breast Reconstruction (previously marketed as NeoForm ) is proposed for post-mastectomy breast reconstruction and is an established skin substitute for this indication (Bard, 2016). The AlloMax Surgical Graft for Hernia and Abdominal Wall Repair is proposed for hernia or other complex abdominal wall repairs when a synthetic prosthesis is contraindicated or inappropriate. There is insufficient evidence in the published peer-reviewed scientific literature to support the safety and efficacy of AlloMax for hernia and abdominal wall repair. Studies have primarily been in the form of case reports for hernia repair (e.g., hiatal hernia, incisional hernia) and abdominal wall reconstruction (Bard, 2015). Apligraf Apligraf (Organogenesis Inc., Canton, MA) (also known as Graftskin), a bilayered living skin equivalent with bovine reagents, is FDA PMA approved for use in conjunction with compression therapy for the treatment of noninfected, partial and full-thickness skin ulcers due to venous insufficiency and for full-thickness neuropathic diabetic foot ulcers nonresponsive to standard wound therapy. Based on the results of clinical trials, Apligraf may be appropriate when used for the treatment of type I and type 2 diabetics when the patient is under routine medical management and has a hemoglobin A1C (HbA1C) less than 12%. The ulcer should be free of sinus tracts, tunnels, cellulitis, eschar and necrotic tissue. Adequate blood supply to the treated foot (i.e., palpable pedal pulse or an ankle-brachial index [ABI] of 0.70) is necessary for healing to occur. One application of Apligraf is initially indicated. If Apligraf coverage is less than 100% and the wound is not progressing, up to a total of four applications in a twelve week period of time may be used. The safety and efficacy of more than five applications has not been reported in the published peer-reviewed literature (Organogenesis, 2010; FDA, 2000). Apligraf is an accepted treatment modality for chronic noninfected, full-thickness lower extremity venous stasis ulcers of at least one month duration that are nonresponsive to medical management. The ulcer should be free of cellulitis, eschar, sinus tracts, tunnels, necrotic tissue and osteomyelitis and have adequate arterial blood supply to support healing as determined by a palpable pedal pulse or an ankle-brachial index (ABI) of Apligraf is used in conjunction with standard wound care therapy. One initial application is used and the wound is observed to see if the graft adheres to the skin. If less than 50% adherence is observed, additional applications may be indicated for up to a maximum of four applications in 12 weeks. Any underlying medical condition that may deter healing should be adequately managed (Organogenesis, 2010; FDA, 1998). Systematic reviews and randomized controlled trials (DiDomenica, et al., 2011; Steinberg, et al., 2010; Edmonds, et al., 2009; Curran and Plosker, 2002; Veves, et al., 2001; Falange, et al., 1999; Falange et al., 1998) support the safety and efficacy of Apligraf for these indications. Biobrane /Biobrane -L Biobrane/Biobrane-L (Smith and Nephew, Inc., Largo, FL) are synthetic, bilaminate, collagen-based composites. Under the FDA PMA approval, Biobrane is indicated for use as a temporary covering of partial-thickness, freshly debrided or excised burn wounds in the absence of coagulum, eschare and necrotic tissue. Biobrane-L is also a temporary covering used as an adjunct until autografting is clinically appropriate. Biobrane L is a less complex nylon fabric for use when less aggressive adhesion is needed (UDL Laboratories, 2013). Randomized controlled trials and retrospective reviews support the safety and effectiveness of Biobrane for the treatment of partialthickness burns (Lang, et al., 2005; Lal, et al., 2000). Biobrane has also been proposed for the treatment of toxic epidermal necrolysis, paraneoplastic pemphigus, dermabrasion, skin graft harvesting, laser resurfacing, and other types of chronic wounds that cannot be immediately closed (e.g., open sternotomy, venous ulcers), but there is insufficient evidence to support Biobrane for these indications (Whitaker, et al., 2008). Page 19 of 90
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
MEDICAL POLICY No R8 SKIN SUBSTITUTES & SOFT TISSUE GRAFTS
 Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures
 POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds
 Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
What s New Medical Policy Updates April 2017
 What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
IHCP banner page. IHCP to cover CPT code IHCP to cover CPT code INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Tissue-Engineered Skin Substitutes
 Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Bioengineered Skin and Soft Tissue Substitutes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Policy Specific Section: October 7, 2011 January 30, 2015
 Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Medical Policy. MP Bioengineered Skin and Soft Tissue Substitutes
 Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Number: (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end
 1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Skin Substitutes for Wound Care AHM
 Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12
 Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
THE WOUND SOLUTION. (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix)
 (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
(Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy
 Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid
 02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Integra. Tissue Technologies. Limit uncertainty with a leader in collagen technology
 Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft
 Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
2012 Head and Neck Reconstruction/ENT Repair Coding Observations
 Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Over the years, mucogingival surgery
 The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation and Root Coverage: A Case Series Saroff Stephen Andrew Saroff, DDS, MSD 1 Abstract Over the years, mucogingival surgery has developed
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation and Root Coverage: A Case Series Saroff Stephen Andrew Saroff, DDS, MSD 1 Abstract Over the years, mucogingival surgery has developed
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls. Daniel Kuebler, PhD Franciscan University of Steubenville
 Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls Daniel Kuebler, PhD Franciscan University of Steubenville Benjamin D. Smith & Daniel A. Grande. The current state of scaffolds for
Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls Daniel Kuebler, PhD Franciscan University of Steubenville Benjamin D. Smith & Daniel A. Grande. The current state of scaffolds for
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
Surgical Therapy. Tuesday, April 2, 13. Alessan"o Geminiani, DDS, MS
 Surgical Therapy Alessan"o Geminiani, DDS, MS Periodontal Flap: a surgical procedure in which incisions are made in the gingiva or mucosa to allow for separation of the epithelium and connective tissues
Surgical Therapy Alessan"o Geminiani, DDS, MS Periodontal Flap: a surgical procedure in which incisions are made in the gingiva or mucosa to allow for separation of the epithelium and connective tissues
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
A wide variety of membrane options
 A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
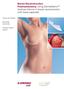 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction
 A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm
 Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
CGS Medicare Policy Primer
 CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
BASICS OF BURN MANAGEMENT
 BASICS OF BURN MANAGEMENT Dr S M Keswani Cosmetic Surgeon National Burns Centre, Airoli,Navi-Mumbai Breach Candy Hospital Wockhardt Hospital National Burns Centre, Airoli, Navi-Mumbai. CLASSIFICATION 1.
BASICS OF BURN MANAGEMENT Dr S M Keswani Cosmetic Surgeon National Burns Centre, Airoli,Navi-Mumbai Breach Candy Hospital Wockhardt Hospital National Burns Centre, Airoli, Navi-Mumbai. CLASSIFICATION 1.
ALLIANCE OF WOUND CARE STAKEHOLDERS. Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015
 ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
Review Services Update September 2015
 Review Services Update September 2015 Unless otherwise indicated, prior authorization guidelines are applicable to members with active Group Health coverage. Prior Authorization Updates Procedure Notifications
Review Services Update September 2015 Unless otherwise indicated, prior authorization guidelines are applicable to members with active Group Health coverage. Prior Authorization Updates Procedure Notifications
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
medically necessary as the evidence is insufficient to determine the effects of the technology on health outcomes.
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
clevelandclinic.org/transplant
 Hannah Hicks bone and soft tissue TRANSPL ANT RECIPIENT Dr. Joyce said I could wear high heels in the future. I m just happy about that. Hannah Hicks, 15, Solon, Ohio. Hannah was diagnosed with a solid
Hannah Hicks bone and soft tissue TRANSPL ANT RECIPIENT Dr. Joyce said I could wear high heels in the future. I m just happy about that. Hannah Hicks, 15, Solon, Ohio. Hannah was diagnosed with a solid
Skin Deep. Agenda. Burns Wounds Debridement Evaluation and Management Services. Presented by: Mike Strong, SFM The Work Comp Experts.
 Presented by: Mike Strong, SFM The Work Comp Experts Agenda Wounds Debridement Evaluation and Management Services 2 1 Types of First Degree Second Degree Third Degree Rule of 9 Adults Infants Burn Coding
Presented by: Mike Strong, SFM The Work Comp Experts Agenda Wounds Debridement Evaluation and Management Services 2 1 Types of First Degree Second Degree Third Degree Rule of 9 Adults Infants Burn Coding
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Palmetto Medicare Policy Primer
 Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
BIOFIBER. Absorbable Biologic Scaffolding Solutions
 BIOFIBER Absorbable Biologic Scaffolding Solutions BIOFIBER IS ONLY THE BEGINNING BIOFIBER - CM What is BIOFIBER CM? BIOFIBER Collagen Matrix is comprised of the same P4HB material with the addition of
BIOFIBER Absorbable Biologic Scaffolding Solutions BIOFIBER IS ONLY THE BEGINNING BIOFIBER - CM What is BIOFIBER CM? BIOFIBER Collagen Matrix is comprised of the same P4HB material with the addition of
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
CorMatrix ECM Bioscaffold
 CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
Burn & Soft Tissue Service Orientation Slides
 Burn & Soft Tissue Service Orientation Slides Damien Wilson Carter, MD Director, Burn/Soft Tissue Service Sue Reeder, BSN, CWOCN Burn Resource Nurse Specialist Scope ALL Burn injuries (> Age 12) Cold injury/
Burn & Soft Tissue Service Orientation Slides Damien Wilson Carter, MD Director, Burn/Soft Tissue Service Sue Reeder, BSN, CWOCN Burn Resource Nurse Specialist Scope ALL Burn injuries (> Age 12) Cold injury/
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
We Want to Keep You Smiling. Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide
 We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
The Original remains unique.
 The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
Townie Guest Editorial. Gingival Attachment Loss: Evaluation and Surgical Options. Daniel J. Melker, DDS. fig. 1
 Gingival Attachment Loss: Evaluation and Surgical Options Daniel J. Melker, DDS Attached connective tissue (a.k.a. attached tissue) in the simplest terms is the body s only barrier between the underlying
Gingival Attachment Loss: Evaluation and Surgical Options Daniel J. Melker, DDS Attached connective tissue (a.k.a. attached tissue) in the simplest terms is the body s only barrier between the underlying
Integra TenoGlide Tendon Protector Sheet
 Integra Use of TenoGlide Tendon Protector Sheet as an Interface to Protect Extensor Tendons after Removal of Hardware from Multiple Metacarpal Fractures 1 CASE STUDY Use of Integra TenoGlide Tendon Protector
Integra Use of TenoGlide Tendon Protector Sheet as an Interface to Protect Extensor Tendons after Removal of Hardware from Multiple Metacarpal Fractures 1 CASE STUDY Use of Integra TenoGlide Tendon Protector
HeliMEND Advanced. Absorbable Collagen Membrane. Instructions for Use
 HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
Corporate Medical Policy Growth Factors in Wound Healing
 Corporate Medical Policy Growth Factors in Wound Healing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: growth_factors_in_wound_healing 4/1993 11/2017 11/2018 11/2017 Description
Corporate Medical Policy Growth Factors in Wound Healing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: growth_factors_in_wound_healing 4/1993 11/2017 11/2018 11/2017 Description
CYGNUS REIMBURSEMENT GUIDE
 CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
OSTEOCHONDRAL ALLOGRAFTS AND AUTOGRAFTS IN THE TREATMENT OF FOCAL ARTICULAR CARTILAGE LESIONS
 Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-115 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-115 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
REGENERATIONTIME. A Case Report by. Geistlich Mucograft for the treatment of multiple adjacent recession defects: A more palatable option
 A Case Report by Dr. Daniel Gober Geistlich Mucograft for the treatment of multiple adjacent recession defects: A more palatable option The Situation A 35 year old male presented in my practice with a
A Case Report by Dr. Daniel Gober Geistlich Mucograft for the treatment of multiple adjacent recession defects: A more palatable option The Situation A 35 year old male presented in my practice with a
Epicel (cultured epidermal autografts) HDE# BH Patient Information
 Epicel (cultured epidermal autografts) HDE# BH990200 Patient Information This leaflet is designed to help you understand Epicel (cultured epidermal autografts) and its use for the treatment of burn wound.
Epicel (cultured epidermal autografts) HDE# BH990200 Patient Information This leaflet is designed to help you understand Epicel (cultured epidermal autografts) and its use for the treatment of burn wound.
REGENERATIONTIME. A Case Report by. Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor
 A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
TREATMENT OF CARTILAGE LESIONS
 TREATMENT OF CARTILAGE LESIONS Angelo J. Colosimo, MD -Head Orthopaedic Surgeon University of Cincinnati Athletics -Director of Sports Medicine University of Cincinnati Medical Center -Associate Professor
TREATMENT OF CARTILAGE LESIONS Angelo J. Colosimo, MD -Head Orthopaedic Surgeon University of Cincinnati Athletics -Director of Sports Medicine University of Cincinnati Medical Center -Associate Professor
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
OSTEOCHONDRAL ALLOGRAFT RECONSTRUCTION FOR MASSIVE BONE DEFECT
 OSTEOCHONDRAL ALLOGRAFT RECONSTRUCTION FOR MASSIVE BONE DEFECT Angelo J. Colosimo, MD -Head Orthopaedic Surgeon University of Cincinnati Athletics -Director of Sports Medicine University of Cincinnati
OSTEOCHONDRAL ALLOGRAFT RECONSTRUCTION FOR MASSIVE BONE DEFECT Angelo J. Colosimo, MD -Head Orthopaedic Surgeon University of Cincinnati Athletics -Director of Sports Medicine University of Cincinnati
Allograft Based Breast Reconstruction: Opportunity for a Second Look
 Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
Allograft Based Breast Reconstruction: Opportunity for a Second Look Martin I. Newman, MD, FACS Director of Resident Education and Associate Program Director Department of Plastic and Reconstructive Surgery
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Cigna Medical Coverage Policy Subject Breast Reconstruction Following Mastectomy or Lumpectomy Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 19 References...
Wright Medical Technology, Inc Airline Road Arlington, TN phone toll-free
 References 1 Brigido SA, Boc SF, Lopez RC. Effective Management of Major Lower Extremity Wounds Using an Acellular Regenerative Tissue Matrix: A Pilot Study. Orthopedics 2004; 27(1S): pp145-149. 2 Brigido
References 1 Brigido SA, Boc SF, Lopez RC. Effective Management of Major Lower Extremity Wounds Using an Acellular Regenerative Tissue Matrix: A Pilot Study. Orthopedics 2004; 27(1S): pp145-149. 2 Brigido
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
