POLICIES AND PROCEDURE MANUAL
|
|
|
- Cordelia Arnold
- 6 years ago
- Views:
Transcription
1 POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a policy of coverage regarding III. Responsibility: A. Medical Directors B. Medical Management IV. Required Definitions 1. Attachment a supporting document that is developed and maintained by the policy writer or department requiring/authoring the policy. 2. Exhibit a supporting document developed and maintained in a department other than the department requiring/authoring the policy. 3. Devised the date the policy was implemented. 4. Revised the date of every revision to the policy, including typographical and grammatical changes. 5. Reviewed the date documenting the annual review if the policy has no revisions necessary. V. Additional Definitions Medical Necessity or Medically Necessary means Covered Services rendered by a Health Care Provider that the Plan determines are: a. appropriate for the symptoms and diagnosis or treatment of the Member's condition, illness, disease or injury; b. provided for the diagnosis, and the direct care and treatment of the Member's condition, illness disease or injury; c. in accordance with current standards of good medical treatment practiced by the general medical community. d. not primarily for the convenience of the Member, or the Member's Health Care Provider; and e. the most appropriate source or level of service that can safely be provided to the Member. When applied to hospitalization, this further means that the Member requires acute care as an inpatient due to the nature of the services rendered or the Member's condition, and the Member cannot receive safe or adequate care as an outpatient. Medicaid Business Segment Medical Necessity shall mean a service or benefit that is compensable under the Medical Assistance Program and if it meets any one of the following standards: (i) (ii) (iii) The service or benefit will, or is reasonably expected to, prevent the onset of an illness, condition or disability. The service or benefit will, or is reasonably expected to, reduce or ameliorate the physical, mental or development effects of an illness, condition, injury or disability. The service or benefit will assist the Member to achieve or maintain maximum functional
2 capacity in performing daily activities, taking into account both the functional capacity of the Member and those functional capacities that are appropriate for members of the same age. DESCRIPTION: Tissue engineered skin substitutes have been developed to replace autografts, allografts and xenograft in the treatment of extensive burn injuries and hard-to-heal, chronic wounds associated with non-burn etiologies such as venous ulcers and diabetic foot ulcers. The goal of tissue engineered skin substitute therapy is to provide a temporary biologic dressing that encourages skin tissue regeneration and wound healing through its own natural contingent of growth factors and proteins. It delivers new cells to the wound, which are able to adjust to the microenvironment of the wound and stimulate healing. INDICATIONS: Tissue engineered skin substitutes may be considered medically necessary when used for the appropriate FDA approved indications. Specific criteria may also apply as listed below: Apligraf is a bilayered, living skin equivalent which is indicated for treatment of: 1. Venous insufficiency ulcers when all of the following criteria are met: The ulcer is a non-infected partial or full thickness ulcer at least 1.0cm 2 in size due to clinically documented venous insufficiency; The ulcer has been present for a minimum of 4 weeks, has been treated with conventional non-surgical therapy (including, but not limited to: debridement, off-loading, compression dressings, wet and/or dry dressings, cleansing and nutritional support) for at least 4 weeks and has failed to respond (e.g., no significant decrease in wound size, failure to show signs of granulation or progression toward closure). 2. Neuropathic diabetic foot ulcers when all of the following criteria are met: Physician documentation of medical management for clinically documented type 1 or type 2 diabetes The wound is a non-infected full thickness neuropathic diabetic foot ulcer and has been present for a minimum of 3 weeks and at least 1.0cm 2 in size due to clinically documented diabetic neuropathy The ulcer is located on the plantar, medial or lateral surface of the foot and is free of infection, tunnels, tracts, cellulitis, eschar or obvious necrotic material The ulcer extends through the dermis but does not involve the tendon, muscle, capsule or exposure to bone Conservative treatment measures including a non-weight bearing regimen, debridement and acceptable methods of wound care have been tried for a minimum of 3 weeks and there has been failure to respond to conservative measures The extremity is free of Charcot s arthropathy There is adequate arterial blood supply to support tissue growth Dermagraft is a cryopreserved dermal substitute derived from human fibroblasts and is indicated for the treatment of diabetic foot ulcers when all of the following criteria are met: Physician documentation of medical management for clinically documented type 1 or type 2 diabetes The wound is a non-infected full thickness diabetic foot ulcer and has been present for a minimum of 3 weeks and at least 1.0cm 2 in size The ulcer is located on the plantar, medial or lateral surface of the foot and is free of infection, tunnels, tracts, cellulitis, eschar or obvious necrotic material The ulcer extends through the dermis but does not involve the tendon, muscle, capsule or exposure to bone Conservative treatment measures including a non-weight bearing regimen, debridement and acceptable methods of wound care have been tried for a minimum of 3 weeks and there has been failure to respond to conservative measures. The extremity is free of Charcot s arthropathy There is adequate arterial blood supply to support tissue growth Biobrane (biosynthetic dressing) is a knitted nylon fabric bonded to an ultra-thin silicone rubber membrane coated with a protein (gelatin) and is indicated for the treatment of thermal injuries, superficial scald burn or flame injury of the hand when: The burn is superficial, partial-thickness with limited involvement of the dermis (less than or equal to 25% total body surface area); and The burn is clean, non-infected, and free of nonviable tissue and coagulation eschar.
3 Orcel is an absorbable bilayered cellular matrix, made of bovine collagen, in which human dermal cells have been cultured and is considered medically necessary for the treatment of healing donor site wounds in burn victims, and for use in persons with dystrophic epidermolysis bullosa undergoing hand reconstruction surgery to close and heal wounds created by the surgery, including those at donor sites when used in accordance with the FDA s Humanitarian Device Exemption. TransCyte consists of human dermal fibroblasts grown on nylon mesh, combined with a synthetic epidermal layer and is considered medically necessary for the temporary wound covering for surgically excised full-thickness and deep partial-thickness thermal burn wounds in persons who require such a covering before autograft placement. Integra Dermal Regeneration Template, Matrix Wound Dressing, Bilayer Matrix Wound Dressing, and Meshed Bilayer Wound Matrix an acellular, biodegradable copolymer matrix coated is considered medically necessary for postexcision treatment of full thickness of deep partial thickness burns when autografting is not feasible due to the lack of suitable healthy tissue or the member s weak physiological state. Alloderm (acellular dermal matrix allograft) is considered medically necessary when used In association with a covered, medically necessary breast reconstruction procedure. Surgical repair of complex abdominal wall wounds (e.g., due to infection, fascial defect, etc.) ENT/Head & Neck reconstructive procedures Neoform (solvent-dehydrated, gamma-irradiated preserved human allograft) is considered medically necessary when used in association with a covered, medically necessary breast reconstruction procedure. AlloMax is considered medically necessary when used in association with a covered, medically necessary breast reconstruction procedure. Oasis Wound Matrix is considered medically necessary when used to treat the following: 1. Partial or full thickness venous insufficiency or diabetic ulcers of the lower extremity when the following criteria are met: The ulcer has been present for a minimum of 4 weeks, has been treated with conventional non-surgical therapy (including, but not limited to: debridement, off-loading, compression dressings, wet and/or dry dressings, cleansing and nutritional support) for at least 4 weeks and has failed to respond (e.g., no significant decrease in wound size, failure to show signs of granulation or progression toward closure). 2. Pressure ulcers 3. Surgical or traumatic wounds 4. Draining wounds Epicel is considered medically necessary when used for the treatment of deep dermal or full thickness burns of 30% or more of the total body surface area in accordance with the FDA s Humanitarian Device Exemption Graftjacket Regenerative Tissue Matrix is considered medically necessary when used for the treatment of diabetic foot ulcers when the following criteria are met: Physician documentation of medical management for clinically documented type 1 or type 2 diabetes The wound is a non-infected full thickness neuropathic diabetic foot ulcer and has been present for a minimum of 3 weeks and at least 1.0cm 2 in size due to clinically documented diabetic neuropathy The ulcer is located on the plantar, medial or lateral surface of the foot and is free of infection, tunnels, tracts, cellulitis, eschar or obvious necrotic material The ulcer extends through the dermis but does not involve the tendon, muscle, capsule or exposure to bone Conservative treatment measures including a non-weight bearing regimen, debridement and acceptable methods of wound care have been tried for a minimum of 3 weeks and there has been failure to respond to conservative measures The extremity is free of Charcot s arthropathy There is adequate arterial blood supply to support tissue growth
4 Any tissue engineered skin substitute not specifically addressed in this document should be considered as requiring prior authorization. CONTRAINDICATIONS: Clinically infected wounds Ulcers with sinus tracts Known allergies to bovine collagen Apligraf and Dermagraft are not approved for use in the treatment of acute surgical wounds, pressure sores or burns. LIMITATIONS: For the purpose of this policy, re-application is referring to an additional application of skin substitutes to the same ulcer within the same treatment period. Re-treatment is referencing a new treatment period where the same ulcer is being treated again because the initial treatment has most likely failed. For Apligraf the following limitations apply and will be considered NON-COVERED as being not medically necessary: The efficacy of treatment with Apligraf beyond five applications at no less than weekly intervals has not been established. Reapplication when initial application has resulted in no measurable response Retreatment within 1 year following the last successful application Retreatment following unsuccessful treatment defined for the purposes of this policy as 5 failed Apligraf applications For Dermagraft: The following limitation applies and will be considered NON-COVERED as being not medically necessary: Retreatment within 1 year following the last successful application Reapplication when initial application has resulted in no measurable response Retreatment of an ulcer following the unsuccessful treatment where it consisted of 8 failed Dermagraft applications Data regarding Permacol Biologic porcine mesh implant is limited to small case series, retrospective case reviews, and non-randomized comparison studies. There is minimal evidence in the peer-reviewed, medical literature of its effectiveness as an alternative to synthetic meshes, that it provides any advantage over other available surgical mesh, and information on the potential complications associated with its use is lacking. Requests for this product will be considered on a per case basis. EXCLUSIONS: The use of tissue engineered skin equivalents in any application outside of the current FDA approvals is considered experimental, investigational or unproven and is NOT COVERED. Due to limited studies, small study populations, variable outcomes and/ or poor study designs, there is insufficient evidence in the current published peer-reviewed medical literature to fully evaluate the clinical utility of any of the following products. Their use is limited to the FDA approved indication. AlloDerm RTM Ready to Conexa Integra Matrix Wound Repriza Use AlloMax CorMatrix InteguPly Restore Orthobiologic AlloMend CRXa Jaloskin Seamguard Allopatch HD CryoSkin LiquidGen SportMesh Alloskin Cuffpatch MariGen Omega3 SS Matrix Alloskin RT Cymetra Matriderm Stimulen Collagen AlloWrap DeNovo NT Graft Matristem StrataGraft AmnioCare Dermacell Matrix HD Strattice AmnioExcel Dermadapt Wound MediHoney Suprathel Dressing AmnioFix DermaMatrix Medeor SurgiMend
5 Amniomatrix DermaSpan Mediskin Surgisis (including Surgisis AFP Anal Fistula Plug, Surgisis Gold Hernia Repair Grafts, and Surgisis ) AmnioMTM DressSkin Memoderm Talymed AmnioShield Duraform Menaflex TenoGlide Aongen Collagen Matrix Duragen XS Meso BioMatrix TenSIX Architect Extracellular Duragen Plus Neoform Dermis TheraForm Matrix Standard/Sheet ArthroFlex DuraMatrix NEOX 100 Quick-Peel TissueMend Atlas Wound Matrix Durepair Regeneration NEOX 1k Wound Matrix TranzGraft Matrix Avance Nerve Graft Endobon Xenograft NEOX FLO NuCel Unite Granules Avaulta Plus Endoform Neuragen Veritas Collagen Matrix AxoGuard nerve ENDURAgen NeuraWrap X-Repair connector AxoGuard nerve protector Epicel Neuroflex XCM Biologic Biobrane EpiDex NeuroMatrix Xelma BioDDryFlex EpiFix NeuroMend XenMatrix Biodesign Excellagen NuCel Xwrap (Hydro, DRY, and ECM) BioDExCel EZ Derm NuShield BioDfactor FlexHD Oasis BioDfence FloGraft OrthADAPT BioDOptix FortaDerm Wound OsseoGuard Dressing BioFiber Gammagraft Ovation C-QUR GORE Bio A Pelvicol Celaderm Grafix CORE Pelvisoft CellerateRX Grafix PRIME Peri-Guard Repair Patch CelluTome GraftJacket Peri-Strips Dry CLARIX 100 Quick-Peel Graftjacket Xpress Permacol injectable CLARIX 1k HA Absorbent Wound PriMatrix Dressing CLARIX FLO Helicoll Promogran CollaFix hmatrix PTFE felt Collamend Hyalomatrix Puracol CollaSorb Inforce Puros Dermis CollaWound Integra Dermal Regeneration Repliform Other Associated Key Words: Human Skin Equivalents Note: A complete description of the process by which a given technology or service is evaluated and determined to be experimental, investigational or unproven is outlined in MP 15 - Experimental Investigational or Unproven Services or Treatment. CODING ASSOCIATED WITH:
6 The coding listed in this document may not represent the comprehensive range of codes that may be associated with this service. HCPCS CODES: J7340 Dermal and epidermal, tissue of human origin, with or without bioengineered or processed elements, with metabolically active elements, per square centimeter (Apligraf, OrCel, TransCyte) J7342 Dermal tissue, of human origin, with or without other bioengineered or processed elements, with metabolically active elements, per square centimeter (Dermagraft) Tissue cultured skin autograft, trunk, arms, legs; first 25 sq cm or less Tissue cultured skin autograft, trunk, arms, legs; additional 1 sq cm to 75 sq cm Tissue cultured skin autograft, trunk, arms, legs; each additional 100 sq cm, or each additional 1% of body area of infants and children, or part thereof Tissue cultured skin autograft, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits; first 25 sq cm or less Tissue cultured skin autograft, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits; additional 1 sq cm to 75 sq cm Tissue cultured skin autograft, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits; each additional 100 sq cm, or each additional 1% of body area of infants and children, or part thereof application of skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm; first 25 cm or less wound surface area each additional 25 sq cm wound surface area, or part thereof application of skin substitute graft to trunk, arms, legs, total wound surface area greater than or equal to 100 sq cm; first 100 sq cm wound surface area, or 1% of body area of infants and children each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 sq cm; first 25 sq cm or less wound surface area each additional 25 sq cm wound surface area, or part thereof application of skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 sq cm; first 100 sq cm wound surface area, or 1% of body area infants and children each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof Acellular xenograft implant, first 100 sq. cm or less, or one percent of body area of infants and children Acellular xenograft implant, each additional 100 sq. cm, or one percent of body area of infants and children or part thereof Implantation of biologic implant (eg, acellular dermal matrix) for soft tissue reinforcement (eg, breast, trunk) C9352 Microporous collagen implantable tube (NeuraGen Nerve Guide), per centimeter length C9353 Microporous collagen implantable slit tube (NeuraWrap Nerve Protector), per centimeter length C9354 Acellular pericardial tissue matrix of non-human origin (Veritas), per square centimeter C9355 Collagen nerve cuff (NeuroMatrix), per 0.5 centimeter length C9356 Tendon, porous matrix of cross-linked collagen and glycosaminoglycan matrix (TenoGlide Tendon Protector Sheet), per square centimeter C9358 Dermal substitute, native, non-denatured collagen, fetal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters C9360 Dermal substitute, native, non-denatured collagen, neonatal bovine origin (SurgiMend Collagen Matrix), per 0.5 square centimeters C9361 Collagen matrix nerve wrap (NeuroMend Collagen Nerve Wrap), per 0.5 centimeter length C9363 Skin substitute, Integra Meshed Bilayer Wound Matrix, per square centimeter C9364 Porcine implant, Permacol, per square centimeter G0428 Collagen meniscus implant procedure for filling meniscal defects (e.g., CMI, collagen scaffold, Menaflex) Q4100 Skin substitute, not otherwise classified [when specified as TransCyte] Q4101 Apligraf, per square cm Q4102 Skin substitute, Oasis wound matrix, per sq cm Q4103 Oasis Burn Matrix, per square centimeter Q4104 Integra Bilayer Matrix Wound Dressing (BMWD), per square centimeter Q4105 Integra Dermal Regeneration Template (DRT), per square centimeter Q4106 Dermagraft, per square centimeter Q4107 Graftjacket, per square centimeter Q4108 Integra Matrix, per square centimeter
7 Q4110 PriMatrix, per square centimeter Q4111 Gammagraft, per square centimeter Q4112 Cymetra, injectable, 1 cc Q4113 Graftjacket Xpress, injectable, 1 cc Q4114 Integra Flowable Wound Matrix, injectable, 1 cc Q4115 Alloskin, per square centimeter Q4116 Skin substitute, alloderm, per square centimeter Q4117 Hyalomatrix, per square centimeter Q4118 Matristem micromatrix, 1 mg Q4121 Theraskin, per square centimeter Q4122 Dermacell, per square centimeter Q4123 AlloSkin RT, per square centimeter Q4124 Oasis Ultra Tri-Layer Wound Matrix, per square centimeter Q4125 ArthroFlex, per square centimeter Q4126 Memoderm, dermaspan, tranzgraft or integuply, per square centimeter Q4127 Talymed, per square centimeter Q4128 FlexHD, AlloPatch HD, or Matrix HD, per square centimeter Q4130 Strattice, per square centimeter Q4131 EpiFix, per square centimeter Q4132 Grafix CORE, per square centimeter Q4133 Grafix PRIME, per square centimeter Q4134 hmatrix, per square centimeter Q4135 Mediskin, per square centimeter Q4136 EZ-derm, per square centimeter Q4137 Amnioexcel or biodexcel, per square centimeter Q4138 Biodfence dryflex, per square centimeter Q4139 Amniomatrix or biodmatrix, injectable, 1 cc Q4140 Biodfence, per square centimeter Q4141 Alloskin ac, per square centimeter Q4142 Xcm biologic tissue matrix, per square centimeter Q4143 Repriza, per square centimeter Q4145 Epifix, injectable, 1 mg Q4146 Tensix, per square centimeter Q4147 Architect extracellular matrix, per square centimeter Q4148 Neox 1k, per square centimeter Q4149 Excellagen, 0.1 cc Q4150 Allowrap DS or Dry, per square centimeter Q4151 AmnioBand or Guardian, per square centimeter Q4152 DermaPure, per square centimeter Q4153 Dermavest, per square centimeter Q4154 Biovance, per square centimeter Q4155 NeoxFlo or ClarixFlo, 1 mg Q4156 Neox 100, per square centimeter Q4157 Revitalon, per square centimeter Q4158 MariGen, per square centimeter Q4159 Affinity, per square centimeter Q4160 NuShield, per square centimeter Q4161 Bio-connekt wound matrix, per square centimeter Q4162 Amniopro flow, bioskin flow, biorenew flow, woundex flow, amniogen-a, amniogen-c, 0.5cc Q4163 Amniopro, bioskin, biorenew, woundex, amniogen-45, amniogen-200, per square centimeter Q4164 Helicoll, per square centimeter Q4165 Keramatrix, per square centimeter Q4166 Cytal, per square centimeter Q4167 Truskin, per square centimeter Q4168 Amnioband, 1 mg Q4169 Artacent wound, per square centimeter Q4170 Cygnus, per square centimeter Q4171 Interfyl, 1 mg Q4172 Puraply or puraply am, per square centimeter Q4173 Palingen or palingen xplus, per square centimeter Q4174 Palingen or promatrx, 0.36 mg per 0.25 cc Q4175 Miroderm, per square centimeter
8 LINE OF BUSINESS: Eligibility and contract specific benefits, limitations and/or exclusions will apply. Coverage statements found in the line of business specific benefit document will supersede this policy. For Medicare, applicable LCD s and NCD s will supercede this policy. For PA Medicaid Business segment, this policy applies as written. REFERENCES: Aristidis V, Falanga V, Armstrong DG, Sabolinski ML, Graftskin, a Human Skin Equivalent, Is Effective in the management of Noninfected Neuropathic Diabetic Foot Ulcers A Prospective Randomized Multicenter Clinical Trial, Diabetes Care 24: , 2001 Falanga V, Tissue Engineering in Wound Repair, Advances in Skin and Wound Care, 13(2 Suppl):15-19, May-June 2000 Kirsner RS, Fastenau J, Falabella A, Valencia I, Long R, Eaglestein WH, Clinical and Economic Outcomes with Graftskin for Hard-to-Heal Venous Leg Ulcers: A Single Center Experience, Dermatologic Surgery 28:81-82, 2002 Sibbald RG, Torrance GW, Walker V, Attard C, MacNeil P, Cost-effectiveness of Apligraf in the Treatment of Venous Leg Ulcers, Ostomy Wound Management 47(8):36-46, Aug 2001 Schonfeld WH, Villa KF, Fastenau JM, Mazonson PD, Falanga V, An Economic Assessment of Apligraf (Graftskin) for the Treatment of Hard to Heal Venous Leg Ulcers, Wound Repair and Regeneration 8(4): , July-Aug 2000 Kaiser Permanente Technology Assessment Program, TEC Review, Graftskin for the Treatment of Skin Ulcers, Vol 16(12):1-24, Nov 2001 Medical Device Approvals, Dermagraft, Medical Device Approvals, Apligraf Graftskin, Managed Care-Current Best Practices-Treatment Pathways, Recent Advances in Treating Chronic Diabetic Foot Ulcers, Winifred S. Hayes, Hayes Inc. Online. Skin Substitutes for Wound Healing. Feb. 1, Updated February 10, Updated Feb. 9, ECRI, HTAIS Windows on Medical Technology, Bioengineered Skin and Dermal Replacement for Chronic Diabetic Ulcers. Feb Mostow EN, Haraway GD, Dalsing M, Hodde JP, Kling D, and the Oasis Venous Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg Ulcer: A randomized clinical trial. J Vas Surg 2005;41: ECRI, HTAIS Custom Hotline Response, Skin Substitute (OASIS) for Wound Management. December 12,2005 Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for Diabetic Ulcers. Adv. Skin Wound Care Jun; 18(5 Pt 1): Still J, Glat P, Silverstein P, Griswols J. Mozingo D. The use of collagen sponge/living cell composite material to treat donor sites in burn patients. Burns.2003 Dec;29(8) Phillips LG, Robson MC, Smith DJ, Phillips WA, Gracia WD, McHugh TP, Sullivan WG, Mathoney K, Swartz K, Meltzer T. Uses and abuses of a biosynthetic dressing for partial skin thickness burns. Burns 1989 Aug;15(4): Lang EM, Eiberg CA, Brandis M, Stark BG. Biobrane in the treatment of burn and scald injuries in children. Annals of Plastic Surgery November;55(5): Lal S, Barrow RE, Wolf SE, Chinkes DL, Hart DW, Heggers JP, Herndon DN. Biobrane improves wound healing in burned children without increased risk of infection. Shock 2000 Sep; 14(3):314-8.
9 Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in seconddegree pediatric burns. Plast Reconstr Surg Jan;105(1):62-5. Ou LF, Lee SY, Chen YC, Yang RS, Tang YW. Use of Biobrane in pediatric scald burns experience in 106 children. Burns Feb;24(1): Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial-thickness burns; a prospective, randomized trial using Transcyte. ANZ J Surg Aug;74(8): Noordenbos J, Dore C, Hansbrough JF. Safety and efficacy of Transcyte for the treatment of partial-thickness burns. J Burn Care Rehabilitation 1999 July-Aug.;20(4): Purdue GF, Hunt JL, Still JM Jr., Law EJ, Herndon DN, Goldfarb JW, Schiller WR, Hansbrough JF, Hickerson WL, Himel HN, Kealey GP, Twomey J, Misavage AE, Solem LD, Davis M, Totoritis M, Gentzkow GD. A multi-center clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryoperserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil Jan-Feb; 18(1 pt 1):52-7. Hansbrough JF, Mozingo DW, Kealey GP, Davis M, Gidner A, Gentzkow GD. Clinical Trial of a biosynthetic temporary skin replacement, Dermagraft-Transitional Covering, compared with cryoperserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil Jan-Feb;18(1 Pt 1):43-51 Centers for Medicare and Medicaid Services. National Coverage Determination.Porcine Skin and Gradient Pressure Dressings. Manual Section Number O Donnell TF. Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcers. J Vasc Surg 2006;44: Schuster R, Singh J, Safadi BY, Wren SM. The use of acellular dermal matrix for contaminated abdominal wall defects; wound status predicts success. Am J Surg 2006;192: Ho C, Tran K, Hux M, et al. Artificial skin grafts in chronic wound care: A meta-analysis of clinical efficacy and a review of cost-effectiveness. Technology Report No 52. Ottawa, ON: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial-thickness burns: a prospective, randomized trial using Transcyte TM. ANZ Journal of Surgery. August 2004;74(8): Amani H, Dougherty WR, Blome-Eberwine S. Use of Transcyte and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns 2006 Nov;32(7): Still J, Glat B, Silverstein P, et al. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns Dec. 2003;29(8): Enoch S, Grey JE, Harding KG. ABC of wound healing. Recent advances and emerging treatments. BMJ April 2006;322: Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24(1): Heitland A, Piatkowski A, Noah EM, Pallua N. Update on the use of collagen/glycosaminoglycate skin substitute-six years of experiences with artificial skin in 15 German burn centers. Burns. 2004;30(5): Fette A. Integra artificial skin in use for full-thickness burn surgery: Benefits or harms on patient outcome. Technol Health Care. 2005;13(6): ECRI Institute, HTAIS Hotline report. Permacol Porcine Dermal Implant for Hernia Repair. 9/17/2007. Armellino, MF, De, SG, Scardi, F, Forner, AL, Ambrosino, F, Bellotti, R, Robustelli, U, De, SG. [Use of Permacol in complicated incisional hernia]. Chir Ital. 2006;58(5):
10 Parker, DM, Armstrong, PJ, Frizzi, JD, North, JH, Jr. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg. 2006;63(4): Shaikh, FM, Giri, SK, Durrani, S, Waldron, D,Grace, PA. Experience with Porcine Acellular Dermal Collagen Implant in One-stage Tension-free Reconstruction of Acute and Chronic Abdominal Wall Defects. World J Surg Hsu PW, Salgado CJ, Kent K, et al. Evaluation of porcine dermal collagen (Permacol) used in abdominal wall reconstruction. J Plast Reconstr Aesthet Surg Mitchell IC, Garcia NM, Barber R, et al. Permacol: A potential biologic patch alternative in congenital diaphragmatic hernia repair. J Pediatr Surg. 2008;43(12): Saray A. Porcine dermal collagen (Permacol) for facial contour augmentation: preliminary report. Aesthetic Plast Surg. 2003;27(5): Hammond TM, Chin-Aleong J, Navsaria H, Williams NS. Human in vivo cellular response to a cross-linked acellular collagen implant. Br J Surg Apr;95(4): Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, Tallis A, Turner WB, Vayser D, Winters C, Armstrong DG. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J Apr Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg Jul;57(1):1-5. ECRI Institute. AlloDerm versus Autograft for Tissue Regeneration. Plymouth Meeting (PA): ECRI Institute; 2008 Dec p. [ECRI hotine response]. ECRI Institute. Porcine-derived Extracellular Matrix (OASIS Wound Matrix) for Wound Management. Plymouth Meeting (PA): ECRI Institute; 2009 April 24. 9p. [ECRI hotine response]. ECRI Institute. Bioengineered Skin and Dermal Cell Replacement for Chronic Diabetic and Venous Ulcers. Plymouth Meeting (PA): ECRI Institute; 2010 March 26. 9p. [ECRI hotine response]. Hunt, D. Diabetes: foot ulcers and amputations. Clin Evid (Online) National Institutes for Health and Clinical Excellence (NICE). Closure of anal fistula using a suturable bioprosthetic plug. June Accessible at Rosales MA, Bruntz M, et al. Gamma-irradiated human skin allograft: a potential treatment modality for lower extremity ulcers. Int. Wound J. 2004;1(3): Cancio LC, Horvath EE, et al. Burn Support for Operation Iraqi Freedom and Related Operations, 2003 to J Burn Care & Rehab 2005;26: Purde GF et al. A multiple center clinical trial of biosynthetic skin replacement, dermagraft, compared with cryopreserved human cadaveric skin for temperature coverage of excised burn wounds. J Burn Care Rehabil 1997;18(1): Winifred S. Hayes, Hayes Inc. Biosynthetic Tissue-Engineered Skin Substitutes for Wound Healing. January 14, Updated January 21, 2011 Hubik, DJ, Wasiak, J, Paul, E, Cleland, H. Biobrane: A retrospective analysis of outcomes at a specialist adult burns centre. Burns 2011 June: 37(4): Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation Plast Reconstr Surg Dec;124(6): Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care Jan;23(1):34-8.
11 Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31(4): Stiefel D, Schiestl C, Meuli M. Integra Artificial Skin for burn scar revision in adolescents and children. Burns.2010;36(1): Oh SJ, Kim Y. Combined AlloDerm and thin skin grafting for the treatment of postburn dyspigmented scar contracture of the upper extremity. J Plast Reconstr Aesthet Surg. 2011;64(2): Iorio ML, Goldstein J, Adams M, et al. Functional limb salvage in the diabetic patient: the use of a collagen bilayer matrix and risk factors for amptutation. Plast Reconstr Surg. 2011;127(1): Centers for Medicare & Medicaid Services (CMS). HCPCS public meeting summary report for: drugs/biologics/radiopharmaceuticals/radiologic imaging agents. Novitas Solutions, Inc. Local Carrier Determination (LCD) for Wound Care and Cellular and/or Tissue-Based Products for Wounds (CTPs) (L27547). Accessed 2/14/14. This policy will be revised as necessary and reviewed no less than annually. Devised: 6/02 Revised: 6/03 (Coding), 6/04; 6/05 (Coding); 03/06 (additional criteria, coding); 04/07; 2/08 (criteria); 2/09 (criteria); 4/10 (criteria); 7/11 (contradiction, exclusions, coding); 4/12 (indications, coding, references), 2/14 (indication, reference, removed exclusion); 2/15 Add Product names remove PA requirement); 1/17 (add products, remove exclusions) Reviewed: 4/13, 3/16
What s New Medical Policy Updates April 2017
 What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds
 Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
MEDICAL POLICY No R8 SKIN SUBSTITUTES & SOFT TISSUE GRAFTS
 Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures
 POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
IHCP banner page. IHCP to cover CPT code IHCP to cover CPT code INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy
 Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid
 02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bioengineered Skin and Soft Tissue Substitutes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Tissue-Engineered Skin Substitutes
 Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Medical Policy. MP Bioengineered Skin and Soft Tissue Substitutes
 Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
Number: (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end
 1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Skin Substitutes for Wound Care AHM
 Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12
 Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
Policy Specific Section: October 7, 2011 January 30, 2015
 Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
CGS Medicare Policy Primer
 CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
Palmetto Medicare Policy Primer
 Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Surgical Preparation Codes for Skin Replacement Surgery** Hospital Outpatient/Ambulatory Surgical Center Setting
 2018 National Medicare Reimbursement Rate Summary* for Integra Dermal Regeneration Template, & Office Settings Integra LifeSciences Corporation compiles this summary of Medicare payment rates to provide
2018 National Medicare Reimbursement Rate Summary* for Integra Dermal Regeneration Template, & Office Settings Integra LifeSciences Corporation compiles this summary of Medicare payment rates to provide
medically necessary as the evidence is insufficient to determine the effects of the technology on health outcomes.
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
MOVE BEYOND. to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair
 MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Cahaba Medicare Policy Primer 1,2 for Apligraf
 Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Summary of Package Insert 1 for PuraPly Wound Matrix
 Summary of Package Insert 1 for PuraPly Wound Matrix For NGS Indications Indicated for the management of wounds including: Partial and full-thickness wounds Venous ulcers Diabetic ulcers Drainage wounds
Summary of Package Insert 1 for PuraPly Wound Matrix For NGS Indications Indicated for the management of wounds including: Partial and full-thickness wounds Venous ulcers Diabetic ulcers Drainage wounds
Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix
 Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix For States with Non-Published Policies-Novitas Indications Indicated for the management of wounds as an effective barrier to resist microbial
Summary of Package Insert 1 for PuraPly Antimicrobial Wound Matrix For States with Non-Published Policies-Novitas Indications Indicated for the management of wounds as an effective barrier to resist microbial
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Skin Deep. Agenda. Burns Wounds Debridement Evaluation and Management Services. Presented by: Mike Strong, SFM The Work Comp Experts.
 Presented by: Mike Strong, SFM The Work Comp Experts Agenda Wounds Debridement Evaluation and Management Services 2 1 Types of First Degree Second Degree Third Degree Rule of 9 Adults Infants Burn Coding
Presented by: Mike Strong, SFM The Work Comp Experts Agenda Wounds Debridement Evaluation and Management Services 2 1 Types of First Degree Second Degree Third Degree Rule of 9 Adults Infants Burn Coding
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP058 Section: Medical Benefit Policy Subject: Negative Pressure Wound Therapy I. Policy: Negative Pressure Wound Therapy II. Purpose/Objective: To provide a policy
POLICIES AND PROCEDURE MANUAL Policy: MP058 Section: Medical Benefit Policy Subject: Negative Pressure Wound Therapy I. Policy: Negative Pressure Wound Therapy II. Purpose/Objective: To provide a policy
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound
 ORIGINAL ARTICLES Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound A Pilot Study John G. Fleischli, DPM* Terese J. Laughlin, DPM* Jeffery W. Fleischli,
ORIGINAL ARTICLES Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound A Pilot Study John G. Fleischli, DPM* Terese J. Laughlin, DPM* Jeffery W. Fleischli,
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041)
 FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041) Links in PDF documents are not guaranteed to work. To follow
FUTURE Local Coverage Determination (LCD): Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds (L35041) Links in PDF documents are not guaranteed to work. To follow
CYGNUS REIMBURSEMENT GUIDE
 CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
CYGNUS REIMBURSEMENT GUIDE The CYGNUS amnion patch is an immune-privileged tissue containing the natural regenerative factors responsible for creating a wound healing environment conducive to tissue regeneration.
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
medical policy update bulletin
 January 2019 medical policy update bulletin Medical Policy, Medical Benefit Drug Policy & Coverage Determination Guideline Updates UnitedHealthcare respects the expertise of the physicians, health care
January 2019 medical policy update bulletin Medical Policy, Medical Benefit Drug Policy & Coverage Determination Guideline Updates UnitedHealthcare respects the expertise of the physicians, health care
Integra. Tissue Technologies. Limit uncertainty with a leader in collagen technology
 Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Care of Wound Reconstruction Patients, 2018 ed.
 Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
MY STRATEGY FOR TREATING BURN INJURIES. Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA
 MY STRATEGY FOR TREATING BURN INJURIES Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA ASSUMPTIONS: Burns which heal to normal have best outcome. Medical risk, functional recovery,
MY STRATEGY FOR TREATING BURN INJURIES Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA ASSUMPTIONS: Burns which heal to normal have best outcome. Medical risk, functional recovery,
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Epidemiology. Burn Rehabilitation. Epidemiology. Epidemiology. United States. United States Cause of injury. Incidence has declined
 Burn Rehabilitation Peter Esselman, MD Professor and Chair Department of Rehabilitation Medicine University of Washington Epidemiology United States 450,000 burn injuries/year in USA that receive medical
Burn Rehabilitation Peter Esselman, MD Professor and Chair Department of Rehabilitation Medicine University of Washington Epidemiology United States 450,000 burn injuries/year in USA that receive medical
2012 Head and Neck Reconstruction/ENT Repair Coding Observations
 Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Jeffrey A. Ross, DPM, MD, FACFAS Associate Professor Baylor College of Medicine Houston, Texas DFCon 2018 Houston, Texas
 Jeffrey A. Ross, DPM, MD, FACFAS Associate Professor Baylor College of Medicine Houston, Texas DFCon 2018 Houston, Texas A Bridge to Healing S.T.E.P Save The Extremity Program Brooklyn Bridge Abnormal
Jeffrey A. Ross, DPM, MD, FACFAS Associate Professor Baylor College of Medicine Houston, Texas DFCon 2018 Houston, Texas A Bridge to Healing S.T.E.P Save The Extremity Program Brooklyn Bridge Abnormal
AlloMend AlloSkin TM AlloWrap DS. AlloSkin TM AC AlloSkin TM RT AlloSkin TM REGENERATIVE WOUND CARE JOINT OPERATIONS LLC
 Allosource-Woundcare-no prices_allosource Price List 15/12/2015 17:54 Page 1 AlloMend AlloSkin TM AlloWrap DS AlloSkin TM AC AlloSkin TM RT AlloSkin TM JOINT OPERATIONS LLC Suite 20, Anchor Business Centre,
Allosource-Woundcare-no prices_allosource Price List 15/12/2015 17:54 Page 1 AlloMend AlloSkin TM AlloWrap DS AlloSkin TM AC AlloSkin TM RT AlloSkin TM JOINT OPERATIONS LLC Suite 20, Anchor Business Centre,
Burn & Soft Tissue Service Orientation Slides
 Burn & Soft Tissue Service Orientation Slides Damien Wilson Carter, MD Director, Burn/Soft Tissue Service Sue Reeder, BSN, CWOCN Burn Resource Nurse Specialist Scope ALL Burn injuries (> Age 12) Cold injury/
Burn & Soft Tissue Service Orientation Slides Damien Wilson Carter, MD Director, Burn/Soft Tissue Service Sue Reeder, BSN, CWOCN Burn Resource Nurse Specialist Scope ALL Burn injuries (> Age 12) Cold injury/
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
POLICY The proposal is to add words in red text and to delete words or statements with a strikethrough:
 Bi-Engineered Skin and Sft Tissue Substitutes DESCRIPTION Bi-engineered skin and sft tissue substitutes may be derived frm human tissue (autlgus r allgeneic), nnhuman tissue (xengraphic), synthetic materials,
Bi-Engineered Skin and Sft Tissue Substitutes DESCRIPTION Bi-engineered skin and sft tissue substitutes may be derived frm human tissue (autlgus r allgeneic), nnhuman tissue (xengraphic), synthetic materials,
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Case Report Treatment of severe burn with DermACELL, an acellular dermal matrix
 Int J Burn Trauma 2012;2(2):105-109 www.ijbt.org /ISSN: 2160-2126/IJBT1208002 Case Report Treatment of severe burn with DermACELL, an acellular dermal matrix Shyi-Gen Chen, Yuan-Sheng Tzeng, Chih-Hsin
Int J Burn Trauma 2012;2(2):105-109 www.ijbt.org /ISSN: 2160-2126/IJBT1208002 Case Report Treatment of severe burn with DermACELL, an acellular dermal matrix Shyi-Gen Chen, Yuan-Sheng Tzeng, Chih-Hsin
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
CLINICAL EVIDENCE Partial and Deep Partial Burns
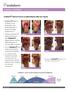 CLINICAL EVIDENCE Partial and Deep Partial Burns Endoform helps to improve re-epithelialization after burn injuries Endoform helps to facilitate tissue granulation and epithelialization in partial and
CLINICAL EVIDENCE Partial and Deep Partial Burns Endoform helps to improve re-epithelialization after burn injuries Endoform helps to facilitate tissue granulation and epithelialization in partial and
REPRESENTATIVE REPORTS USING DERMACELL AWM
 REPRESENTATIVE REPORTS USING DERMACELL AWM Documents available upon request. Clinical Reports using DermACELL AWM in Advanced Wound Management Published & Peer-Reviewed Articles, Posters, & Abstracts from
REPRESENTATIVE REPORTS USING DERMACELL AWM Documents available upon request. Clinical Reports using DermACELL AWM in Advanced Wound Management Published & Peer-Reviewed Articles, Posters, & Abstracts from
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP230 Section: Medical Benefit Policy Subject: Outpatient Pulmonary Rehabilitation I. Policy: Outpatient Pulmonary Rehabilitation II. Purpose/Objective: To provide
POLICIES AND PROCEDURE MANUAL Policy: MP230 Section: Medical Benefit Policy Subject: Outpatient Pulmonary Rehabilitation I. Policy: Outpatient Pulmonary Rehabilitation II. Purpose/Objective: To provide
OF WOUNDS SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM. AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois
 1 THE WACKY WORLD OF WOUNDS ERIN RYDELL SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois www.ahia.org Carolinas HealthCare System 2 Carolinas
1 THE WACKY WORLD OF WOUNDS ERIN RYDELL SENIOR AUDITOR CAROLINAS HEALTHCARE SYSTEM AHIA 32 nd Annual Conference August 25-28, 2013 Chicago, Illinois www.ahia.org Carolinas HealthCare System 2 Carolinas
ALLIANCE OF WOUND CARE STAKEHOLDERS. Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015
 ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Technology Report. Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness
 Technology Report Issue 52 February 2005 Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness Publications can be requested from: CCOHTA
Technology Report Issue 52 February 2005 Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness Publications can be requested from: CCOHTA
Work Relative Value Unit Repair initial incisional or ventral hernia; reducible $767.87
 Coding and Reimbursement Guide for SurgiMend for Use in Hernia Repair Procedures Hospital Department (HOPD), Setting (ASC) and Physician Fee Schedule Payment - 2018 Because rates are the only publicly
Coding and Reimbursement Guide for SurgiMend for Use in Hernia Repair Procedures Hospital Department (HOPD), Setting (ASC) and Physician Fee Schedule Payment - 2018 Because rates are the only publicly
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology
 A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
The choice for aesthetic and functional results in loss of substances 1 SKIN EXPERTISE WITH A NEW COLLAGEN TECHNOLOGY
 The choice for aesthetic and functional results in loss of substances 1 SKIN EXPERTISE WITH A NEW COLLAGEN TECHNOLOGY SYMATESE: OUR EXPERTISE IN THE FIELD OF COLLAGEN AND SKIN SYMATESE GROUP is recognized
The choice for aesthetic and functional results in loss of substances 1 SKIN EXPERTISE WITH A NEW COLLAGEN TECHNOLOGY SYMATESE: OUR EXPERTISE IN THE FIELD OF COLLAGEN AND SKIN SYMATESE GROUP is recognized
DermACELL AWM Comprehensive Reimbursement Resource Guide. Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018.
 2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
2018 Comprehensive Reimbursement Resource Guide Prepared by Musculoskeletal Clinical Regulatory Advisers, LLC. Version 01/2018. DermACELL AWM Disclaimer: This information is for educational/informational
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold
 Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is
Inpatient ICD-9-CM Mapping to ICD-10 PCS Procedures Involving the Application of PriMatrix AG Antimicrobial Dermal Repair Scaffold Effective October 1, 2015, the Centers for & Medicaid Services (CMS) is
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft
 Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
Original Article Tissue engineered skin for diabetic foot ulcers: a meta-analysis
 Int J Clin Exp Med 2015;8(10):18191-18196 www.ijcem.com /ISSN:1940-5901/IJCEM0014309 Original Article Tissue engineered skin for diabetic foot ulcers: a meta-analysis Xuedong Li 1, Geliang Xu 2, Jianqiu
Int J Clin Exp Med 2015;8(10):18191-18196 www.ijcem.com /ISSN:1940-5901/IJCEM0014309 Original Article Tissue engineered skin for diabetic foot ulcers: a meta-analysis Xuedong Li 1, Geliang Xu 2, Jianqiu
Apligraf A Reimbursement Case Study Antonio S. Montecalvo, CPA Director of Customer Support Services ISCT San Diego - May 5th, 2009
 Apligraf A Reimbursement Case Study Antonio S. Montecalvo, CPA Director of Customer Support Services ISCT San Diego - May 5th, 2009 Key Points Current Business Reimbursement Landscape FDA Approval Hospital
Apligraf A Reimbursement Case Study Antonio S. Montecalvo, CPA Director of Customer Support Services ISCT San Diego - May 5th, 2009 Key Points Current Business Reimbursement Landscape FDA Approval Hospital
