A Comprehensive Guide for the Recognition and Classification of Distinct Stages of Hair Follicle Morphogenesis
|
|
|
- Emery Jordan
- 5 years ago
- Views:
Transcription
1 REVIEW ARTICLE A Comprehensive Guide for the Recognition and Classification of Distinct Stages of Hair Follicle Morphogenesis Ralf Paus, Sven Müller-Röver,* Carina van der Veen, Marcus Maurer, Stefan Eichmüller, Gao Ling, Udo Hofmann, Kerstin Foitzik, Lars Mecklenburg, ** and Bori Handjiski Department of Dermatology, Charité, Humboldt University, Berlin, and University Hospital Eppendorf, University of Hamburg, Hamburg, Germany; *Center for Cutaneous Research, Queen Mary and Westfield College, London, U.K.; Clinical Cooperation Unit for Dermato-Oncology (DKFZ), Department of Dermatology, Klinikum Mannheim, University of Heidelberg, Mannheim, Germany; Department of Pathology, Uppsala University Hospital, Uppsala, Sweden; Department of Pathology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, U.S.A.; **Department of Pathology, School of Veterinary Medicine, Hannover, Germany Numerous spontaneous and experimentally induced mouse mutations develop a hair phenotype, which is often associated with more or less discrete abnormalities in hair follicle development. In order to recognize these, it is critically important to be able to determine and to classify accurately the major stages of normal murine hair follicle morphogenesis. As an aid, we propose a pragmatic and comprehensive guide, modified after previous suggestions by Hardy, and provide a list of easily recognizable classification criteria, illustrated by representative micrographs. Basic and more advanced criteria are distinguished, the former being applicable to all mouse strains and requiring only simple histologic stains (hematoxylin and eosin, Giemsa, periodic acid Schiff, alkaline phosphatase activity), the latter serving as auxiliary criteria, which require a pigmented mouse strain (like C57BL/ 6J) or immunohistochemistry (interleukin-1 receptor type I, transforming growth factor-β receptor type II). In addition, we present simplified, computergenerated schematic drawings for the standardized recording and reporting of gene and antigen expression patterns during hair follicle development. This classification aid serves as a basic introduction into the field of hair follicle morphogenesis, aims at standardizing the presentation of related hair research data, and should become a useful tool when screening new mouse mutants for discrete abnormalities of hair follicle morphogenesis (compared with the respective wild type) in a highly reproducible, easily applicable, and quantifiable manner. Key words: alkaline phosphatase/ C57BL/6J mouse/dermal papilla/interleukin-1 receptor/ transforming growth factor-β receptor. J Invest Dermatol 113: , 1999 After decades of relative quiescence, basic and applied hair research have witnessed an impressive renaissance over the past 10 y. This has resulted to a major extent from the application of modern tools of molecular biology, and from the use of murine hair research models to the ancient challenge of defining the elusive mechanisms of hair growth control (Stenn et al, 1991; Hardy, 1992; Paus, 1996; Stenn et al, 1996, 1998; Philpott and Paus, 1998; Paus and Cotsarchis, 1999). In particular, an ever-increasing number of spontaneous or experimentally generated mouse mutations has provided invaluable insights into the functional significance of selected gene products in Manuscript received January 5, 1999; revised June 30, 1999; accepted for publication July 5, Reprint requests to: Department of Dermatology, University Hospital Eppendorf, University of Hamburg, Martinistr. 52, D Hamburg, Germany. paus@uke.uni-hamburg.de Abbreviations: AP, alkaline phosphatase; DP, dermal papilla; HF, hair follicle; IL-1-RI, interleukin 1 receptor type 1; IR, immunoreactivity; IRS, inner root sheath; NCAM, neural cell-adhesion molecule; ORS outer root sheath; PAS, periodic acid Schiff reaction; p.p. post partum ( days of postnatal life); SG, sebaceous gland; TGF-β-RII, transforming growth factor-β receptor type II. the control of hair follicle (HF) morphogenesis and cycling (Sundberg, 1994; Stenn et al, 1996; Paus and Cotsarchis, 1999; Philpott and Paus, 1998). Often enough, however, the published analyses of the hair phenotype of a new mouse mutation, from a hair researcher s point of view, are highly unsatisfactory because they tend to be very superficial, inaccurate, and/or incomplete. One basic, but essential requirement for any researcher with an interest in HF biology, is to acquire the ability to recognize and classify accurately the various stages of murine HF development (morphogenesis) (Hardy, 1992; Philpott and Paus, 1998). Unfortunately, despite a large body of literature on selected aspects of murine HF development, dating back almost a century (Oyama, 1904; Dry, 1926; Gibbs, 1941; Hardy, 1949; Butcher, 1951; Chase, 1951; Davidson and Hardy, 1952), there is not a single pragmatic guide that comprehensively and quickly introduces newcomers to the intricacies of HF classification without the need to first digest dozens of studies, each of which covers only selected aspects of HF morphogenesis. This study strives to provide such a guide, which does not require any prior knowledge of HF biology (useful introductions into the latter may be found, for example, in Hardy, 1992; Paus, 1996; Stenn et al, 1996, 1998; Paus and Cotsarchis, 1999). This comprehensive guide to HF morphogenesis aims at standardizing the presentation of hair research data, and should become a useful tool when screening X/99/$14.00 Copyright 1999 by The Society for Investigative Dermatology, Inc. 523
2 524 PAUS ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY Table I. Glossary of anatomical terms frequently used in hair research a Term Bulb Bulbous peg (syn.: Bulbuszapfen) Bulge (syn.: Wulst) Dermal papilla (DP) Hair canal Hair cone Hair germ (syn.: placode, Haarkeim) Hair peg (syn.: Haarzapfen) Hair shaft Infundibulum Inner root sheath (IRS) Isthmus Outer root sheath (ORS) Pre-germ (syn.: primitive hair germ) Definition Prominent, onion-shaped thickening on the proximal end of the HF, consisting of relatively undifferentiated matrix cells, HF melanocytes, as well as of proximal ORS cells Elongated and more differentiated column of epithelial cells with massive bulb-like aggregation of matrix keratinocytes on the proximal end, recognizable parts of IRS, egg-shaped DP and two solid swellings on the posterior side as a hair bulge and an early sebaceous gland Convex extension on the distal part of the ORS, near the epidermis, seat of epithelial follicle stem cells and point of insertion of the muscle arrector pili A mesodermal part of the HF, which consists of closely packed mesenchymal cells, framed by the enlarged bulb matrix on the distal end Tube-like connection between epidermal surface and most distal part of the IRS, demarcated by surrounding ORS. First recognizable part of IRS (pale epithelial layer or Henle s layer) which starts to develop as a cone-shaped structure above the DP Bud-like thickening of the pre-germ plaque in the epidermis consisting of elongated keratinocytes whose proximal end is capped by numerous aggregated mesenchymal cells in the dermis Solid column of epithelial keratinocytes growing into the dermis with a concave proximal end which surrounds partially a compact ball of mesenchymal cells of the future DP The hair per se, composed of trichocytes ( terminally differentiated HF keratinocytes), organized in the form of hair cuticle, cortex and medulla, and surrounded by the IRS (or ORS at the level of the hair canal) Most distal part of the HF including hair canal and distal ORS, limited proximally by the duct of SG and laterally by cells of the ORS Multilayered structure composed of terminally differentiated HF keratinocytes surrounded by the ORS, consisting of the pale epithelial layer or Henle s layer as well as Huxley s layers and cuticle, which covers the hair shaft up to the hair canal. Small part of the HF between insertion of the sebaceous gland and the bulge Outermost sheath of HF keratinocytes, which merges distally into the basal layer of epidermis and proximally into the hair bulb; its distal part is often subdivided into a supra- and infrainfundibular portion (see: infundibulum) Plaque of epidermal cells, recognizable as a crowding of nuclei in the basal layer of the epidermis a For introductory references, see: Stöhr, 1903; Oyama, 1904; Pinkus, 1910; Dry, 1926; Hentschel, 1930; Hardy, 1949, 1951; Fleischhauer, 1953; Montagna and van Scott, 1958; Pinkus, 1958; Straile et al, 1961; Parakkal, 1969a; Cotsarelis et al, 1990; Holbrook and Minami, 1991; Abell, 1994; Paus et al, 1994b; Paus and Cotsarchis, 1999 new mouse mutants for discrete abnormalities of HF morphogenesis (compared with the respective wild type) in a highly reproducible, easily applicable, and quantifiable manner. It uses only simple histochemical and widely available immunohistologic staining techniques [hematoxylin eosin, periodic acid Schiff (PAS) reaction, Oil Red O, alkaline phosphatase activity (AP); interleukin-1 receptor type 1 (IL-1-RI), TGF-β receptor type II (TGF-β-RII)]. In addition, we suggest some slight modifications of previously proposed classification schemes (e.g., Stöhr, 1903; Hardy, 1949, 1951; Pinkus, 1958; Hardy, 1992). Among the many different HF subtypes (e.g., vibrissae, tylotrich, and non-tylotrich pelage HF) (Sundberg, 1994), this guide is focused exclusively on murine non-tylotrich pelage follicles, which comprise the vast majority of the truncal fur coat of mice, and most of which develop in the perinatal period (Vielkind et al, 1995; Vielkind and Hardy, 1996; Paus et al, 1997). Nevertheless, the current guide is also broadly applicable to all other HF types seen in any of the haired mammalian species, as they all exhibit the same basic morphologic principles of development. Rather than dealing with fetal HF morphogenesis, this guide depicts neonatal HF development in pigmented C57BL/6J mice, because most researchers will find it easiest to study neonatal rather than fetal HF morphogenesis. Furthermore, we explain how the delicate task of obtaining morphologically satisfactory longitudinal cryosections through the HF can best be mastered, which is essential for a wide range of immunohistochemical studies on murine HF (Fig 1). Finally, a glossary of anatomical terms frequently used in hair research is also provided so as to avoid terminologic confusion (Table I). HOW TO USE THE GUIDE Here, we provide a pragmatic method for obtaining morphologically satisfactory longitudinal cryosections through the HF by using the harvesting and embedding technique developed by U. Hofmann (Paus et al, 1994c) (Fig 1 and legend for details). Furthermore, we provide a comprehensive guide (Fig 2) that is structured as follows: the left-hand column shows a standardized, computer-generated schematic drawing of nine distinct stages of HF morphogenesis (stages 0 8), modified after previous suggestions by Hardy et al (Hardy, 1949, 1951, 1992) (for greater simplicity, we have omitted the subdivision of developmental stage 3 into three separate substages suggested by Hardy). Major anatomical regions of the developing HF are outlined and the corresponding terms are explained in a glossary (Table I). The central column provides a list of easily recognizable classification criteria, which are then illustrated by three representative micrographs of non-tylotrich pelage HF from C57BL/6J mouse neonatal dorsal skin, depicted on the right-hand side of Fig 2. Basic and auxiliary criteria are distinguished in the central column (separated from each other by a dotted line; top: basic criteria, bottom: auxiliary criteria). The basic criteria listed are applicable to all mouse strains, as they require only simple histologic stains (Giemsa, PAS, hematoxylin eosin) and are not dependent on the presence of pigment granules (melanin) in the HF. The auxiliary criteria shown here, which further aid in classification, require a pigmented mouse strain (such as C57BL/6J), histochemistry (AP technique) or immunohistochemistry (IL-1-RI, TGF-β-receptor type II). The figure legend of Fig 2 explains the corresponding (immuno-) histochemical stains, and the day after birth in neonatal C57BL/6J mice when the depicted dorsal skin samples were harvested. BASIC AND AUXILIARY CRITERIA FOR THE RECOGNITION AND CLASSIFICATION OF DISTINCT STAGES OF HF MORPHOGENESIS HF length During the progression of HF development, the length of developing HF is precisely coupled to the stage of
3 VOL. 113, NO. 4 OCTOBER 1999 HAIR FOLLICLE MORPHOGENESIS GUIDE 525 Figure 1. Embedding procedure for obtaining longitudinal cryosections of mouse pelage HF. As developed by U. Hofmann (Paus et al, 1994c). Tissue banks used for this guide were prepared from neonatal dorsal skin of C57BL/6J mice obtained from Charles River (Sulzfeld, Germany) as described (Paus et al, 1997; Botchkarev et al, 1998a). Skin samples (1 3 cm) were obtained from a well-defined dorsal skin region precisely located above the spinal cord at the thoracolumbar region with each end at the same distance (µ1.5 cm) from the smallest part of the neck and the insertion of the tail, respectively. Dorsal skin has been dissected at the level of the subcutis just below the panniculus carnosus ( subcutaneous muscle layer). For routine histology, biopsies from the upper half of the dissected dorsal skin were cut parallel to the spine to obtain longitudinal HF sections (steps 1 3). After step 6 [freezing the tissue into liquid nitrogen (N 2 )] it is of crucial importance to avoid any thawing of the skin sample by dipping it again in liquid nitrogen after every consecutive step (steps 7, 9, 11). Thawing inevitably leads to freezing thawing artifacts such as fast protein degradation, antigen masking, and increased background during immunohistochemistry.
4 526 PAUS ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY HF morphogenesis (Fig 3). Assessing the length of the HF allows one to obtain a rough idea on whether the developing HF is in an early, middle, or late stage of its morphogenesis. An additional parameter is the location of the most proximal part of the HF in the dermis (stages 1 5) or in the subcutis (stages 6 8).
5 VOL. 113, NO. 4 OCTOBER 1999 HAIR FOLLICLE MORPHOGENESIS GUIDE 527 Time-scale of HF development in C57BL/6J mice Approximately 10% of murine pelage HF have entered into stage 8 at day 3, about 35% at day 5, and about 100% at day 9 (Paus et al, 1998). The first morphologic signs of synchronized catagen development can be detected about days after birth (Gibbs, 1941; Hardy, 1949; Botchkarev et al, 1998a, b; Müller-Röver et al, 1998; Paus et al, 1998).
6 528 PAUS ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY Stage-specific criteria for each stage of HF development Stage 0 The first stage of HF development (Fig 2, stage 0), also described as pregerm stage (Pinkus, 1958), is characterized as an accumulation of nuclei (Pinkus, 1910, 1958; Fleischhauer, 1953), which is virtually impossible to recognize with routine histologic techniques (Fig 2A). We have previously identified TGF-β-RII immunoreactivity, however, as a useful marker for the pregerm stage of HF development in an otherwise morphologically homogeneous epidermis (Fig 2A): the pregerm stage of HF development can easily be detected as sharply demarcated, strongly TGF-β-RII plaques of epidermal keratinocytes in the basal and suprabasal cell layers of the epidermis (Fig 2B) (Paus et al, 1997). Stage 1 Confusingly, stage 1 of HF morphogenesis has been described under various names, such as hair germ (Oyama, 1904; Dry, 1926; Chase, 1951; Holbrook and Minami, 1991), germ plate (Chase, 1951), early hair germ (Pinkus, 1958), follicle plug (Hardy, 1949; Hardy and Lyne, 1956), or placode (Hardy and Vielkind, 1996; Vielkind and Hardy, 1996) (Table I). During stage 1 of HF development (Fig 2, stage 1), the pregerm develops into a circumscribed, morphologically recognizable epidermal thickening which is composed of enlarged, palisade-like oriented keratinocytes in the basal layer of the epidermis (Fig 2C) (called, in the context of this guide, hair germ ). In contrast to regular epidermal keratinocytes, stage 1 HF keratinocytes display a vertically polarized orientation (the proximal pole points towards the panniculus carnosus, whereas the distal pole points towards the stratum corneum). Stage 1 of HF development is furthermore characterized by a localized increase in the number of dermal fibroblasts in close proximity to the epidermal thickening. These fibroblasts begin to display a slightly altered orientation (Fig 2C), as a preparation towards their subsequent aggregation. These future dermal papilla (DP) fibroblasts can be visualized by the AP technique as they become AP (in contrast to surrounding interfollicular dermal fibroblasts). In contrast to mature HF (Handjiski et al, 1994), at this early time point in HF morphogenesis, however, AP activity is still very weak, and is easily missed (Fig 2D). The increasing Figure 3. Schematic representation of the increasing length of the developing HF and the localization of the most proximal part of the HF in the dermis (stages 1 5) or in the subcutis (stages 6 8) during the progression of HF morphogenesis. Note the developmentally controlled changes in skin thickness and HF angle. Figure 2. A comprehensive guide for the recognition and classification of distinct stages of murine HF morphogenesis. The left-hand column shows a computer-generated schematic drawing of nine distinct stages of HF morphogenesis, modified after Hardy (Hardy, 1949; Hardy, 1951; Hardy, 1992) and Paus et al (1997). The second column summarizes simple basic parameters for HF staging (above dotted line), and auxiliary criteria for more precise staging (below dotted line). Depicted is the development of pigmented non-tylotrich pelage HF in the dorsal skin of neonatal C57BL/6J mice (days 1 8 p.p.; note: the day p.p. listed indicates, when during postnatal skin and HF development the corresponding dorsal skin sample was harvested), which is largely representative of the key developmental steps in the morphogenesis of all HF of any mammalian species. Note: for graphical reasons the angles of the HF to the epidermis have been increased. The following staining techniques were employed: A, C, F, I, L, O, R, U, X: Giemsa staining technique (Romeis, 1991). D, G, J, M, P, S, V, Y: AP technique (Handjiski et al, 1994) as marker of the configuration and localization of the DP fibroblasts. B, E, H, K, Q, Z: TGF-β RII immunoreactivity (Paus et al, 1997). N: IL-1-RI immunoreactivity (Eichmüller et al, 1998). Both markers are used to differentiate the length of the developing TGF-β RII-negative and IL-1-RI -negative IRS framed by the TGF-β-RII and IL-1-RI keratinocytes of the follicular cord and the ORS. T, W: Oil Red O staining (Romeis, 1991) as marker of the developing SG and the sebum-filled hair canal. Stage 0, day 1 p.p. (A) morphologically homogeneous epidermis (a), no visible follicles (note: at this day p.p., one also finds numerous pelage HF in various stages of their morphogenesis that had already developed in the pre- and perinatal period, as HF development does not appear to be synchronized).(b) the hair germ can only be visualized as well-demarcated plaques of TGF-β-RII intraepidermal keratinocytes (b) that are morphologically indistinguishable from their TGF-β-RII-negative neighbors (a). Stage 1, day 1 p.p. (C) early hair germ (a) with aggregation of mesenchymal cells below the epidermal thickening (b). (D) only weakly AP mesenchymal cells (d) closely attached to the hair germ (epidermal thickening) (a). (E) the hair germ can only be precisely visualized as TGF-β-RII keratinocytes (c) in the basal layer of the epidermis. Stage 2, day 2 p.p. (F) enlarged hair germ (a) with a convex proximal end and closely attached mesenchymal cells (b). (G) AP dermal fibroblasts (d) below the elongated hair plug (a). (H) TGF-β-RII IR demarcates the convex end of the hair germ (c). Stage 3, days 2 3 p.p. (I) enlarged hair peg (a) with concave proximal end; the central group of keratinocytes (b) displays a columnar arrangement radially to the follicular axis; the dermal fibroblasts form a rounded, more oval-shaped DP (c). (J) AP staining reveals an oval-shaped DP (e) partially embedded in the concave end of the hair plug (a). (K) IL1-RI IR shows the length of the hair peg (d) and indicates its concave end (a). Stage 4, day 3 p.p. (L) developing bulb (a) and cone-shaped formation of the IRS (b). (M) developing bulb (a) enclosing the AP DP (e). (N) IL-1R IR of the developing ORS (d) demarcates the IL-1R -negative DP (c) and the developing IL-1R-negative IRS (b). Stage 5, days 3 4 p.p. (O) elongating IRS (a), developing bulge (b), first sebocytes (c), almost completely enclosed DP (d) and the first melanine granules (h). (p) AP IR reveals that the DP (f) is completely enclosed by hair bulb keratinocytes (d); the first melanine granules (h) are recognizable above its distal pole. (Q) the TGF-β-RII ORS keratinocytes (e) demarcate the elongating TGF-β-RII-negative IRS (a) and the TGF-β-RII-negative DP (d). Stage 6, day 4 p.p. (R) developing sebocytes (a), IRS (c) contains hair shaft (g) with melanin granules; DP (d) is almost completely enclosed and located deeply in the subcutis. (S) hair shaft with melanin granules (g) in the IRS (c), which still had not reached the hair canal (b), AP DP (e) deeply located in the subcutis. (T) Oil Red O sebum in the hair canal (b), Oil Red O SG (f), melanin in the hair shaft (g) still enclosed by the IRS (c). Stage 7, days 4 5 p.p. (U) tip of the hair shaft leaves the epidermis (a) in the close vicinity of the infundibulum of the SG (b). (V) the tip of the hair shaft (a) leaves the TGF-β-RII-negative IRS which is precisely demarcated by the TGF-β-RII ORS keratinocytes (c). (W) the tip of the hair shaft (a) in the Oil Red O hair canal close to the Oil Red O SG (b). Stage 8, days 5 8 p.p. (X) HF at maximal length (a) with a prominent hair shaft (b) emerging through the epidermis. (Y) AP DP (d) is located in the deep subcutis. (Z) HF at maximal length (a) revealed by TGF-β-RII ORS keratinocytes (c). Scale bars: 50 µm. Please note: upper case letters in the left-hand corner label the image whereas lower case letters identify tissues corresponding to the lower case letters of the criteria listed in the central column.
7 VOL. 113, NO. 4 OCTOBER 1999 HAIR FOLLICLE MORPHOGENESIS GUIDE 529 condensation of dermal cells below the epidermal thickening is a crucial event during HF morphogenesis and indicates intensive epithelial mesenchymal interactions, which initiate the actual HF formation (Hardy, 1992; Oro and Scott, 1998; Philpott and Paus, 1998). Stage 1 hair germs can be detected as circumscribed, strongly TGF-β-RII areas in the basal layer of the epidermis (Paus et al, 1997) (Fig 2E). Morphologically, stage 1 hair germs frequently display a bilateral symmetry, and show as yet no anterior posterior orientation in either murine (Hardy, 1949; Chase, 1951; Hardy, 1951; Chase, 1954) or human skin (Stöhr, 1903; Pinkus, 1958; Holbrook and Minami, 1991). Stage 2 In stage 2 of HF morphogenesis (Fig 2, stage 2), the hair germ develops into a more prominent and enlarged, broad column of epidermal keratinocytes (Fig 2F) (Stöhr, 1903; Pinkus, 1958). Like human hair germs (Pinkus, 1910, 1958), murine stage 2 hair germs start to show an anterior posterior orientation (oblique angle to the epidermis, with the proximal end of the hair germ tilted towards the head, and the distal end towards the tail region) (Fig 2F, G). Our findings during neonatal HF morphogenesis in C57BL/6J mice are in contrast to Chase (1954), who reported changes in the growth direction of the hair germ at the earliest on day 3 after birth, whereas we found an anterior posterior orientation of stage 2 hair germs already at day 1 p.p. Stage 2 hair germs in mice display multiple mitotic figures (not shown), and are almost all strongly positive for the proliferation marker Ki67 (Magerl et al, 1998). This supports Pinkus view, who had postulated that the hair germ does not elongate as a result of epidermal invagination (i.e., downward movement of epithelial cells), but primarily as a consequence of massive epithelial cell proliferation in a wellcircumscribed patch of basal layer epidermal keratinocytes (Pinkus, 1958). Stage 2 hair germs are also characterized by a convex proximal end, covered by a cap of several mesenchymal cells easily distinguished by hematoxylin eosin (not shown) or Giemsa (Fig 2F). This cap-like condensation of mesenchymal cells generates a strong AP color reaction, indicating a substantial increase in stringently localized AP enzyme activity in the precursor cells of DP and connective tissue sheath fibroblasts of the future HF (Fig 2G), whereas the enlarged epithelial hair germ shows strong IL-1-RI (not shown) and TGF-β-RII immunoreactivity (Fig 2H). This stage is morphologically identical to the prepapilla stage 2 (Hardy, 1949; Hardy and Lyne, 1956), previously described as hair plug (Hardy, 1949; Hardy and Vielkind, 1996). Stage 3 In stage 3 (Fig 2, stage 3), the hair peg (Stöhr, 1903; Oyama, 1904; Dry, 1926; Pinkus, 1958) has become a solid, elongated column of epithelial cells, which consists of multiple layers of keratinocytes that become concentrically arranged around the follicular axis (Fig 2I) (Robins and Breathnach, 1969). The DP is now recognizable as a ball-shaped, strongly AP condensation of fibroblasts in the developing cavity of the future DP at the proximal end of the epithelial column (Fig 2J). An excellent marker to visualize the length of the HF and the developing papillary cavity is the strong IL-1-RI immunoreactivity (not shown) as well as the strong TGF-β-RII immunoreactivity (Fig 2K) of all follicular keratinocytes (both antigens are not markedly expressed on dermal fibroblasts; Paus et al, 1997; Eichmüller et al, 1998). Hardy has described this stage as the papilla stage (Hardy, 1949; Hardy and Lyne, 1956), and has split it into several, rather similar substages, according to changes in the form of the DP (Hardy, 1992; Hardy and Vielkind, 1996). We have omitted this overly complex distinction in order to simplify HF classification. Stage 4 During stage 4 (Fig 2, stage 4), the hair peg has become more elongated and displays the first characteristics of adolescent HF: a bulb-like thickening of its proximal portion, and a prominent, wide DP cavity (Fig 2L). At this time, the pale epithelial layer or Henle s layer of the inner root sheath (IRS) starts to develop as a cone-shaped structure above the DP (Fig 2L; Oyama, 1904; Hardy, 1949, 1951) that can be distinguished by standard hematoxylin eosin or Giemsa staining. The DP is now longer than wide, is embedded by more than 50% in the DP cavity formed by the surrounding hair matrix, and shows strong AP positivity (Fig 2M). The entire hair peg except for the IRS is also strongly TGF-β- RII (not shown) as well as strongly IL-1-RI (Fig 2N). This stage corresponds to the so-called fiber cone or hair cone stage of Hardy (Hardy, 1949; Hardy and Lyne, 1956). Stage 5 The subsequent bulbus peg stage (Fig 2, stage 5) (Stöhr, 1903; Pinkus, 1958) displays the following light microscopic characteristics: an elongation of the IRS halfway up through the follicle (called hair cone by Hardy, 1949); an enlarged future bulge; above the developing bulge region of the future outer root sheath (ORS), the first sebocytes are seen to develop in the midst of the distal HF epithelium, recognized as very large Oil Red O cells (not shown, cf. Fig 2T) that are easily recognizable by their size and their more lucid cytoplasm (Fig 2O). The oval-shaped DP of loose consistency is now almost completely enclosed by the hair matrix (Fig 2O). In mice with pigmented skin, such as C57BL/6J, light microscopically visible melanin formation starts during stage 5, and the first melanin granules become detectable in the precortex region (Fig 2O) above the distal pole of the strongly AP DP (Fig 2P). Except for the IRS, all follicular keratinocytes are strongly TGF-β- RII (Fig 2Q) as well as strongly IL-1-RI (not shown). This stage has been defined as advanced hair cone stage (Hardy and Lyne, 1956) or hair canal stage (Hardy, 1949), as the hair canal also starts to develop at this time (this is not easy to identify by light microscopy). Stage 6 In stage 6 (Fig 2, stage 6), the HF has reached the deep subcutis (Fig 2R) at an angle of approximately 40 to the epidermis (note: in Fig 2 the angles have been increased for graphic reasons). The hair canal is now visible by light microscopy, the multilayered IRS extends to the level of the hair canal, and contains a hair shaft (Fig 2R). The strongly AP DP becomes thinner and more elongated, and is completely enclosed by the developing bulb (Fig 2) (Robins and Breathnach, 1970). The Oil Red O sebocytes (Fig 2T) now begin to form the sebaceous gland (SG). Also, in stage 6, melanin granules are visible in the proximal hair shaft (Fig 2R). This stage corresponds to the hair formation stage of Hardy (Hardy, 1949; Hardy and Lyne, 1956). Stage 7 Stage 7 of HF development (Fig 2, stage 7) is characterized by the following criteria: the tip of the hair shaft leaves the IRS and enters the hair canal at the level of the infundibulum of the enlarged SG, the latter is now located on the posterior wall of the HF (Fig 2U). Compared with stage 6 (Fig 3R), the strongly AP DP is almost completely enclosed by the bulb and more narrow (Fig 3U). This stage corresponds to the hair in hair canal stage (Hardy, 1949) or hair in the epidermis stage (Hardy and Lyne, 1956). Stage 8 In stage 8 of HF morphogenesis (Fig 2, stage 8), the HF acquires its maximal length and reaches the subcutaneous muscle layer (panniculus carnosus), and a prominent hair shaft emerges through the epidermis (Fig 2X). Morphologically, the HF now resembles a mature anagen VI HF (Parakkal, 1969b). SUMMARY OF THE IMMUNOHISTOLOGIC MARKERS USED FOR HF STAGING These stages of neonatal HF morphogenesis in mice can be identified by applying the basic criteria described above to skin sections stained by standard hematoxylin eosin or Giemsa techniques. The listed auxiliary criteria (melanin, AP activity, TGF-β- RII and IL-1-RI immunoreactivity) are helpful to dissect additional differences in order to distinguish individual stages during HF morphogenesis. The AP staining technique is the simplest method and is invaluable for visualizing the exact dimensions and configuration of the DP, which is otherwise often misinterpreted as part of
8 530 PAUS ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY the hair matrix and vice versa. By using the AP technique, the three-dimensional changes of the DP, which are tightly linked to defined developmental stages, can easily be visualized, thus improving the precision of staging. In mature HF, an additional, useful marker for the fibroblasts of the perifollicular connective tissue sheath and the DP is the adhesion molecule NCAM (Hardy and Vielkind, 1996; Combates et al, 1997; Müller-Röver et al, 1998; Panteleyev et al, 1998). Both AP histochemistry and NCAM immunohistochemistry also help to demarcate the proximal connective tissue sheath of the HF (Handjiski et al, 1994; Müller-Röver et al, 1998). NCAM is also strongly expressed, however, in the interfollicular dermis and on developing nerve fibers during neonatal HF morphogenesis in mice (Müller-Röver et al, 1998) so that, unfortunately, it is not particularly useful for classification during HF development. TGF-β-RII immunoreactivity is a specific marker to distinguish stage 0 pregerms and consecutive stages of HF development (Paus et al, 1997) which are not distinguishable by most other epithelial markers, including the adhesion molecules E- and P-cadherin, as these are expressed homogeneously in the epidermis during stages 0 2 (Müller-Röver and Paus, 1998; Müller-Röver et al, 1998). TGF-β-RII and IL-1-RI immunoreactivity are also valuable markers for differentiating early HF keratinocytes from dermal and DP fibroblasts. Prominent IL-1-RI staining is found essentially on all keratinocytes that are not yet terminally differentiated (Eichmüller et al, 1998), whereas TGF-β-RII immunoreactivity is more specific for HF-associated keratinocytes (Paus et al, 1997). Both epithelial markers, in our hands, proved easier to handle than the use of keratin antibodies, which can be rather tricky in the murine system, not the least due to background problems. The Oil Red O stain is helpful for visualizing the lipid products of sebocytes and the generation of sebum by the maturing SG. This allows one to detect the first sebocytes during stage 5 and the enlarged SG during stage 6 of HF development. We recommend the presentation of the hair canal during stage 7 by Oil Red O staining so as to recognize the distal end of the IRS and the tip of the hair shaft on the discrete background of the Oil Red O-stained sebum in the developing hair canal. This may be particularly welladvised in view of the postulate that SG products are involved in the degradation of the IRS at the level of the infundibulum (Williams and Stenn, 1994). ANALYSIS OF THE HF PHENOTYPE IN TRANSGENIC OR KNOCKOUT MICE Using this guide, it is possible to provide fully quantitative comparisons of abnormalities in HF morphogenesis, comparing mutant and wild-type mice, if a sufficiently large number of age-matched wild-type and mutant littermates is compared by quantitative histomorphometry, i.e., when the morphologic criteria described above are used to determine the stages of a defined number of longitudinally cut HF per mouse (e.g., 20 HF per mouse). Thus, a sufficient number of skin sections has to be prepared for each mouse to acquire enough well-cut HF sections which are obtained as summarized in Fig 1. Then, the number of HF in each stage is counted (e.g., six HF display stage III criteria, 13 HF display stage IV criteria, nine HF display stage V criteria, etc.). Quantitative histomorphometry should be applied with respect to the predominant stages of HF development at a defined time during fetal, prenatal, or neonatal HF morphogenesis [recommended minimum n 3 mutant and wild-type mice (littermates) per time point of skin harvesting; if only age-matched mutant and wild-type mice are used, a minimal n 5 each is recommended]. In order to quantitate changes, a defined number of test and control HF, has to be staged, i.e., has to be assigned to a defined stage of HF development. The total number of HF per stage of HF development (stages 1 8) can be compared quantitatively and statistically between test and control (for examples of quantitative histomorphometry, using the classification criteria listed here, see our recent studies: Botchkarev et al, 1998a, 1999; St-Jacques et al, 1998). A different approach of quantitative morphometry has been described elsewhere in more detail (Sundberg et al, 1997). This simple quantitative approach allows to identify even discrete abnormalities in the patterns and dynamics of HF morphogenesis between mutant and wild-type mice that would otherwise have escaped notice. More severe affection of the skin phenotype caused by a mutation might include numerous alterations. For a rough analysis of mutant mice the following parameters should be tested: altered balance between proliferation and apoptosis, altered number of HF, altered HF orientation and altered morphology of single follicular compartments. An easy and frequently used method to analyze alterations of proliferation and apoptosis is double-labeling with TUNEL staining (as a marker for apoptosis) and Ki67 IR (as a marker for proliferation) (Lindner et al, 1997; St Jacques et al, 1998). Unfortunately, TUNEL staining is not sensitive enough to detect the majority of apoptotic cells during early HF morphogenesis (Magerl et al, 1998) and it is strongly advised to complement TUNEL data with electronmicroscopy. Another useful method for the detection of proliferating cells is bromodeoxyuridine incorporation and staining. For determining an altered number of induced HF in mutant mice, the Hofmann technique (Fig 1) is very useful in order to get precise longitudinal sections of HF, which is essential to count and compare the number of HF per microscopic field (e.g., 50 microscopic fields per mouse) in test versus control mice. Altered HF orientation is easily detected when comparing precise longitudinal sections of mutant and wild-type skin by measuring the angle between HF and epidermis. In order to determine the infinite number of potential alterations of follicular morphology a few simple rules should be kept in mind: in order to determine the precise morphology and size of the DP in a mouse mutant [e.g., platelet-derived growth factor-a / mice which develop smaller DP compared with wild-type animals (Karlsson et al, 1999)], AP staining is a very fast and effective method. Good examples for severely disturbed HF development are Sonic hedgehog (Shh)-deficient mice (St-Jacques et al, 1998) which develop HF blocked at stage 2 of HF development. During consecutive stages these HF do not grow longer and do not display the typical anterior posterior orientation. If a mutant mouse displays similar defects, TGF-β-RII and IL-1RI IR are both very helpful to determine the morphology of the entire developing HF up to stage 3. During the subsequent stages, the IRS can be easily distinguished from the ORS as it is negative for both antigens. Particularly during the very early stages of HF development (stages 0 1) it may be difficult to determine the cause of altered morphology in later stages. TGF-β-RII IR is a useful marker to visualize the developing stage 0/1 hair germ. Other markers for developing stages 0 2 HF include lef-1 IR (van Genderen et al, 1994; Zhou et al, 1995), sonic hedgehog (Bitgood and McMahon, 1995; Iseki et al, 1996; Oro et al, 1997; Motoyama et al, 1998; St- Jacques et al 1998), BMP-2 and BMP-4 (Bitgod and McMahon, 1995; Lyons et al, 1990), and NCAM (Müller-Röver et al, 1998), as well as P-cadherin (Müller-Röver et al, 1999). All of these markers may be used to determine the size, homogeneous distribution, and keratinocyte orientation of follicular pregerms. An important disadvantage of TGF-β-RII IR and other markers is that not all pregerms in a skin section are visualized probably depending on the quality of the specimen (velocity of harvesting and embedding, fixation, etc.). Oil Red O staining is the easiest method to visualize morphologic alterations of the developing SG and the hair canal region, e.g., after the application of function blocking antibodies to plateletderived growth factor (Takakura et al, 1996) which severely disturbed murine hair canal formation. This qualitative classification method, which can be used in a quantitative histomorphometric approach (see above), is a useful tool that investigators can incorporate into their existing protocols. Many other HF and fiber types deriving from a variety of other anatomic sites (Sundberg and Hogan, 1994) are not included here,
9 VOL. 113, NO. 4 OCTOBER 1999 HAIR FOLLICLE MORPHOGENESIS GUIDE 531 but can easily be incorporated into an even more comprehensive HF morphogenesis guide to be compiled in the future. In summary, this comprehensive, pragmatic, and simple guide offers an introduction into the phenomenologic essentials of HF morphogenesis. It should not only be useful for scientists with a primary interest in hair biology, but also offers researchers with only a passing interest in hair an easily handled tool for rapid, professional, and instructive analyses of the hair phenotype of any mouse mutant. The excellent technical assistance of R. Pliet and E. Hagen, and the most helpful critical suggestions of J.P. Sundberg are gratefully acknowledged. This study was supported in parts by grants from Deutsche Forschungsgemeinschaft (Pa 345/8 2) and the E.U. (Brite Euram Project No. BE ) to R.P. REFERENCES Abell E: Embryology and anatomy of the hair follicle. In: Olsen EA (ed.). Disorders of Hair Growth, New York: McGraw Hill, 1994, pp Bitgood MJ, McMahon AP: Hedgehog and Bmp genes are coexpressed at many diverse sites of cell cell interaction in the mouse embryo. Dev Biol 172: , 1995 Botchkarev V, Botchkareva N, Albers K, van der Veen C, Lewin G, Paus R: Neutrophin-3 involvement in the regulation of hair follicle morphogenesis. J Invest Dermatol 111: , 1998a Botchkarev V, Welker P, Albers K, et al: A new role for neurotrophin-3: Involvement in the regulation of hair follicle regression (catagen). Am J Pathol 153: , 1998b Botchkarev VA, Botchkareva NV, Roth W, et al: Noggin is a mesenchymally derived stimulator of the hair follicle induction. Nature Cell Biol 1: , 1999 Butcher EO: Development of the pilary system and the replacement of hair in mammals. Ann NY Acad Sci 53: , 1951 Chase HB: Critical stages of hair development and pigmentation in the mouse. Physiol Zool 24:1 9, 1951 Chase H: Growth of the hair. Physiol Rev 34: , 1954 Combates NJ, Chuong CM, Stenn KS, Prouty SM: Expression of two Ig family adhesion molecules in the murine hair cycle: DCC in the bulge epithelia and NCAM in the follicular papilla. J Invest Dermatol 109: , 1997 Cotsarelis G, Sun TT, Lavker RM: Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: , 1990 Davidson P, Hardy MH: The development of mouse vibrissae in vivo and in vitro. J Anat 86: , 1952 Dry E: The coat of the mouse (Mus musculum). J Genet , 1926 Eichmüller S, van der Veen C, Moll I, Hermes B, Hofmann U, Müller-Röver S, Paus R: Clusters of perifollicular macrophages in normal murine skin: physiological degeneration of selected hair follicles by programmed organ deletion. J Histochem Cytochem 46: , 1998 Fleischhauer K: Über die Entstehung der Haaranordnung und das Zustandekommen räumlicher Beziehungen zwischen Haaren und Schweissdrüsen. Z Zellforsch Mikroskop Anat 38: , 1953 van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R: Development of several organs that require inductive epithelial mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8: , 1994 Gibbs HF: A study of the postnatal development of the skin and hair of the mouse. Anat Rec 80:61 81, 1941 Handjiski B, Eichmüller S, Hofmann U, Czarnetzki BM, Paus R: Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol 131: , 1994 Hardy MH: The development of mouse hair in vitro with some observations on pigmentation. J Anat 83: , 1949 Hardy MH: The development of pelage hairs and vibrissae from skin in tissue culture. Ann NY Acad Sci 53: , 1951 Hardy MH: The secret life of the hair follicle. Trends Genet. 8:55 61, 1992 Hardy MH, Lyne AG: The prenatal development of wool follicles in merino sheep. Aust J Biol Sci 9: , 1956 Hardy MH, Vielkind U: Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 1. Follicle morphogenesis in wild-type mice. Acta Anat 157: , 1996 Hentschel HG: Die Bildung des Haarpigments nach Untersuchungen an Mäusen. Z Naturw 64: , 1930 Holbrook KA, Minami SI: Hair follicle embryogenesis in the human. Characterization of events in vivo and in vitro. Ann N Y Acad Sci 642: , 1991 Iseki S, Araga A, Ohuchi H, Nohno T, Yoshioka H, Hayashi F, Noji S: Sonic hedgehog is expressed in epithelial cells during development of whisker, hair, and tooth. Biochem Biophys Res Commun 218: , 1996 Karlsson L, Bondjers C, Betsholtz C: Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 126: , 1999 Lindner G, Botchkarev VA, Botchkareva NV, et al: Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol 151: , 1997 Lyons KM, Pelton RW, Hogan BL: Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2a (BMP-2A). Development 109: , 1990 Magerl M, Lindner G, Botchkareva N, Paus R: Patterns of proliferation and apoptosis during hair follicle morphogenesis. J Invest Dermatol 110:532a, 1998 Montagna W, van Scott EJ: The anatomy of the hair follicle. In: Montagna W, Ellis RA (eds). The Biology of the Hair Growth. New York: Academic Press, 1958 Motoyama J, Heng H, Crackower MA, Takabatake T, Takeshima K, Tsui LC, Hui C: Overlapping and non-overlapping Ptch2 expression with Shh during mouse embryogenesis. Mech Dev 78:81 84, 1998 Müller-Röver S, Paus R. Topobiology of the hair follicle: adhesion molecules as morphoregulatory signals during hair follicle morphogenesis. In: Chuong CM (ed.). Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes Bioscience Publishers, 1998, pp Müller-Röver S, Botchkarev VA, Peters EJM, Panteleyev A, Paus R: Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J Histochem Cytochem 46: , 1998 Müller-Röver S, Tokura Y, Welker P, Furukawa F, Wakita H, Takigawa M, Paus R: E- and P-cadherin expression during murine hair follicle development and cycling. Exp Dermatol 8: , 1999 Oro AE, Scott MP: Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell 96: , 1998 Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH Jr, Scott MP: Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276: , 1997 Oyama R: Entwicklungsgeschichte des Deckhaares der weissen Maus (Mus musculus var. alba). Arb Anat Inst Wiesbaden 23: , 1904 Panteleyev AA, van der Veen C, Rosenbach T, Müller-Röver S, Sokolov VE, Paus R: Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol 110(6): , 1998 Parakkal PF: The fine structure of anagen hair follicle of the mouse. Adv Biol Skin , 1969a Parakkal PF: Role of macrophages in collagen resorption during hair growth cycle. J Ultrastruct Res 29: , 1969b Paus R: Control of the hair cycle and hair diseases as cycling disorders. Curr Opin Dermatol 3: , 1996 Paus R, Cotsarchis G: The biology of hair follicles. N Engl J Med 341: , 1999 Paus R, Stenn KS, Link RE: Telogen skin contains an inhibitor of hair growth. Br J Dermatol 122: , 1990 Paus R, Eichmüller S, Hofmann U, Czarnetzki BM: Expression of classical and nonclassical MHC class I antigens in murine hair follicles. Br J Dermatol 131: , 1994a Paus R, Handjiski B, Czarnetzki BM, Eichmüller S: Biologie des Haarfollikels. Hautarzt 45: , 1994b Paus R, Hofmann U, Eichmüller S, Czarnetzki BM: Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br J Dermatol 130: , 1994c Paus R, Foitzik K, Welker P, Bulfone-Paus S, Eichmüller S: Transforming growth factor-β receptor type I and type II expression during murine hair follicle development and cycling. J Invest Dermatol 109: , 1997 Paus R, van der Veen C, Eichmüller S, Kopp T, Hagen E, Müller-Röver S, Hofmann U: Generation and cycling remodeling of the hair follicle immune system in mice. J Invest Dermatol 111:7 18, 1998 Philpott MP, Paus R. Principles of hair follicle morphogenesis. In: Chuong CM (ed.) Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes Bioscience Publishers, 1998, pp Pinkus F: The development of the integument. In: Kabel G, Mall S (eds). Manual of Human Embryology. Philadelphia: Lippincott, 1910, pp Pinkus H: Embryology of hair. In: Montagna W, Ellis RA (eds). The Biology of the Hair Growth. New York: Academic Press, 1958, pp 1 32 Robins EJ, Breathnach AS: Fine structure of the human foetal hair follicle at hair peg and early bulbous peg stages of development. J Anat 104: , 1969 Robins EJ, Breathnach AS: Fine structure of bulbar end of foetal hair follicle at stage of differentiation of inner root sheath. J Anat 107: , 1970 Romeis B: Mikroskopische Technik. München: Urban & Schwarzenberg, 1991 Stenn KS, Messenger AG, Baden HP: The molecular and structural biology of hair. Ann N Y Acad Sci 642:1 519, 1991 Stenn KS, Combates NJ, Eilertsen KJ, Gordon JS, Pardinas JR, Parimoo S, Prouty SM: Hair follicle growth controls. Dermatol Clinics 14: , 1996 Stenn K, Parimoo S, Prouty S: Growth of the hair follicle: A cycling and regenerating biological system. In: Chuong CM (ed.). Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes Bioscience Publishers, 1998, pp St-Jacques B, Dassule H, Karavanova I, et al: Sonic hedgehog signaling is essential for hair development. Current Biol 24: , 1998 Stöhr P: Entwicklungsgeschichte des menschlichen Wollhaares. Anat H 23:1 66, 1903 Straile W, Chase H, Arsenault C: Growth and differentiation of hair follicles between periods of activity and quiescence. J Exp Zool 148: , 1961 Sundberg JP: Handbook of Mouse Mutations with Skin and Hair Abnormalities. Boca Raton: CRC Press, 1994
10 532 PAUS ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY Sundberg JP, Hogan ME: Hair types and subtypes in the mouse. In: Handbook of Mouse Mutations with Skin and Hair Abnormalities. Boca Raton: CRC Press, 1994, pp Sundberg JP, Rourk MH, Boggess D, Hogan ME, Sundberg BA, Bertolino AP: Angora mouse mutation: altered hair cycle, follicular dystrophy, phenotypic maintenance of skin grafts, and changes in keratin expression. Vet Pathol 34: , 1997 Takakura N, Yoshida H, Konisada T, Nishikawa SI: Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J Invest Dermatol 107: , 1996 Vielkind U, Hardy MH: Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 2. Follicle morphogenesis in the hair mutants, Tabby and downy. Acta Anat 157: , 1996 Vielkind U, Sebzda MK, Gibson IR, Hardy MH: Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta Anat 152:93 109, 1995 Williams D, Stenn K: Transection level dictates the pattern of hair follicle sheath growth in vitro. Dev Biol 165: , 1994 Zhou P, Byrne C, Jacobs J, Fuchs E: Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 15: , 1995
Supporting Information
 Supporting Information Plikus et al. 10.1073/pnas.1215935110 SI Text Movies S1, S2, S3, and S4 are time-lapse recordings from individually cultured Period2 Luc vibrissa follicles show that circadian cycles
Supporting Information Plikus et al. 10.1073/pnas.1215935110 SI Text Movies S1, S2, S3, and S4 are time-lapse recordings from individually cultured Period2 Luc vibrissa follicles show that circadian cycles
Chapter 4 Opener Pearson Education, Inc.
 Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Integumentary System. Integumentary System
 1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
Skin. Kristine Krafts, M.D.
 Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Skin (Integumentary System) Wheater, Chap. 9
 Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Dr Narmeen S. Ahmad. Lab 1
 Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
INTEGUMENTARY 1-Epidermis, 2-Dermis, Structure of thick and thin skin I- Epidermis . Stratum basale
 INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
B. Incorrect! The ectoderm does not produce the dermis. C. Incorrect! The dermis is derived from the mesoderm.
 Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Anatomy and Physiology Homework: Chapters 3-4
 Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
Anatomy and Physiology Homework: Chapters 3-4 CHAPTER 3: Cells and Tissues 1. The smallest unit of living tissue is called a. All living organisms are composed of these basic units where all life processes
4 Skin and Body Membranes Study Guide
 Name: SKIN AND BODY MEMBRANES: 4 Skin and Body Membranes Study Guide Period: Body membranes, which cover body surfaces, line its cavities, and form protective sheets around organs, fall into two major
Name: SKIN AND BODY MEMBRANES: 4 Skin and Body Membranes Study Guide Period: Body membranes, which cover body surfaces, line its cavities, and form protective sheets around organs, fall into two major
(From the Arnold Biological Laboratory, Brown University, Providence, Rhode Island) Materials and Methods
 HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN XI. THF. DISTRIBUTION OF /~-GLucURONIDASE* BY WILLIAM MONTAGNA, PH.D. (From the Arnold Biological Laboratory, Brown University, Providence, Rhode Island) PLATES
HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN XI. THF. DISTRIBUTION OF /~-GLucURONIDASE* BY WILLIAM MONTAGNA, PH.D. (From the Arnold Biological Laboratory, Brown University, Providence, Rhode Island) PLATES
****************************************************************************************************** INTEGUMENTARY SYSTEM
 BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** INTEGUMENTARY SYSTEM ******************************************************************************************************
BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ****************************************************************************************************** INTEGUMENTARY SYSTEM ******************************************************************************************************
This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur.
 Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Cytological and Histological Study of Adult and Neonate Epidermis in Thick and Thin Skin of Various Anatomical Sites
 Available online on www.ijpqa.com International Journal of Pharmaceutical Quality Assurance 218; 9(2); 174-179 doi: 1.25258/ijpqa.v9i2.13642 ISSN 975 956 Research Article Cytological and Histological Study
Available online on www.ijpqa.com International Journal of Pharmaceutical Quality Assurance 218; 9(2); 174-179 doi: 1.25258/ijpqa.v9i2.13642 ISSN 975 956 Research Article Cytological and Histological Study
The Integumentary System: An Overview
 The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
THE sebaceous glands of the rabbit consist of clusters of about ten cells
 79 On the Relationship between Mammary, Sweat, and Sebaceous Glands By D. B. CARLISLE (From the Department of Zoology and Comparative Anatomy, Oxford, and the Plymouth Laboratory of the Marine Biological
79 On the Relationship between Mammary, Sweat, and Sebaceous Glands By D. B. CARLISLE (From the Department of Zoology and Comparative Anatomy, Oxford, and the Plymouth Laboratory of the Marine Biological
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Integumentary System and Body Membranes
 Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
CHAPTER 5 INTEGUMENTARY
 CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
The Integumentary System
 The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
Anatomy Ch 6: Integumentary System
 Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Hole s Essentials of Human Anatomy & Physiology
 Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Hole s Essentials of Human Anatomy & Physiology David Shier Jackie Butler Ricki Lewis Created by Dr. Melissa Eisenhauer Head Athletic Trainer/Assistant Professor Trevecca Nazarene University Chapter 6
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS
 Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Introduction. Skin and Body Membranes. Cutaneous Membranes Skin 9/14/2017. Classification of Body Membranes. Classification of Body Membranes
 Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
Chapter 05. Lecture Outline. See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes.
 Chapter 05 Lecture Outline See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 05 Lecture Outline See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Histology of Integumentary System
 Histology of Integumentary System CONTENT / Topics Integumentary System Skin, thick - Major Layers Epidermis Dermis Hair Skin, hairy and Hair Follicle Sebaceous Glands Sebaceous Gland Sweat Glands Merocrine
Histology of Integumentary System CONTENT / Topics Integumentary System Skin, thick - Major Layers Epidermis Dermis Hair Skin, hairy and Hair Follicle Sebaceous Glands Sebaceous Gland Sweat Glands Merocrine
11/8/2012. Chapter 6 Part 1 Objectives: Skin = Integument = Cutaneous Membrane. The Structure of Skin. Epidermis
 Chapter 6 Part 1 Objectives: Define organ, and associate the skin as an organ of the integumentary system. List the general functions of the skin. Describe the structure of the layers of the skin. Summarize
Chapter 6 Part 1 Objectives: Define organ, and associate the skin as an organ of the integumentary system. List the general functions of the skin. Describe the structure of the layers of the skin. Summarize
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE
 TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
Ex. 7: Integumentary
 Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Integument. Squamous Cell Carcinoma. Melanoma. Largest organ 30% of all clinical diagnoses 1/3 of all tumors
 Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs
 Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
INTEGUMENTARY SYSTEM CHAPTER 4
 INTEGUMENTARY SYSTEM CHAPTER 4 FUNCTIONS Waterproofs Protein called keratin Protection 1 st line of defense against pathogens, chemicals & abrasions Insulation Regulates heat loss by controlling blood
INTEGUMENTARY SYSTEM CHAPTER 4 FUNCTIONS Waterproofs Protein called keratin Protection 1 st line of defense against pathogens, chemicals & abrasions Insulation Regulates heat loss by controlling blood
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Ch 4. Skin and Body Membranes
 Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Unit 4 - The Skin and Body Membranes 1
 Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
SKIN HISTOLOGY the microscopic anatomy of the Integument. Mikrogeo. com
 SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
Figure 6.1 Transparency Master 37
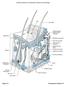 Pore Hair shaft Epidermis Dermal papillae (papillary layer of dermis) Meissner s corpuscle Dermis Free nerve ending Reticular layer of dermis Sebaceous (oil) gland Arrector pili muscle Sensory nerve fiber
Pore Hair shaft Epidermis Dermal papillae (papillary layer of dermis) Meissner s corpuscle Dermis Free nerve ending Reticular layer of dermis Sebaceous (oil) gland Arrector pili muscle Sensory nerve fiber
Ch. 4: Skin and Body Membranes
 Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
Anatomy and Physiology I Student Outline The Integumentary System. Integumentary System. Page 1
 Anatomy and Physiology I Student Outline The Integumentary System Integumentary System Page 1 Have a very clear understanding of the each particular tissue and their unique functions in each layer of the
Anatomy and Physiology I Student Outline The Integumentary System Integumentary System Page 1 Have a very clear understanding of the each particular tissue and their unique functions in each layer of the
Chapter 5. Integumentary System 5-1
 Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Lesson 3: The Human Integumentary and Fascial Systems
 Basic Human Anatomy Lesson 3: The Human Integumentary and Fascial Systems Welcome to Lesson 3 of the Basic Human Anatomy Course. Today, we ll be studying the Human Integumentary and Fascial Systems. I
Basic Human Anatomy Lesson 3: The Human Integumentary and Fascial Systems Welcome to Lesson 3 of the Basic Human Anatomy Course. Today, we ll be studying the Human Integumentary and Fascial Systems. I
LESSON ASSIGNMENT. The Human Integumentary and Fascial Systems. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 3 The Human Integumentary and Fascial Systems. TEXT ASSIGNMENT Paragraphs 3-1 through 3-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Define integumentary
LESSON ASSIGNMENT LESSON 3 The Human Integumentary and Fascial Systems. TEXT ASSIGNMENT Paragraphs 3-1 through 3-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Define integumentary
Development of the skin and its derivatives
 Development of the skin and its derivatives Resources: http://php.med.unsw.edu.au/embryology/ Larsen s Human Embryology The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam School
Development of the skin and its derivatives Resources: http://php.med.unsw.edu.au/embryology/ Larsen s Human Embryology The Developing Human: Clinically Oriented Embryology Dr Annemiek Beverdam School
BI 121 LAB. WEEK 2: Tissues (continued); Integumentary System
 BI 121 LAB 2-1 WEEK 2: Tissues (continued); Integumentary System This week you will 1) Review the four major tissue types 2) Review the characteristics of epithelial tissues. 3) Learn the major characteristics
BI 121 LAB 2-1 WEEK 2: Tissues (continued); Integumentary System This week you will 1) Review the four major tissue types 2) Review the characteristics of epithelial tissues. 3) Learn the major characteristics
Development of teeth. 5.DM - Pedo
 Development of teeth 5.DM - Pedo Tooth development process of continuous changes in predetermined order starts from dental lamina A band of ectodermal cells growing from the epithelium of the embryonic
Development of teeth 5.DM - Pedo Tooth development process of continuous changes in predetermined order starts from dental lamina A band of ectodermal cells growing from the epithelium of the embryonic
SKIN. 3. How is the skin structured around the finger joints to allow for flexible movement of the fingers?
 SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
Chapter 6 Skin and the Integumentary System. Skin Cells. Layers of Skin. Epidermis Dermis Subcutaneous layer beneath dermis not part of skin
 Chapter 6 Skin and the Integumentary System Composed of several tissues Maintains homeostasis Protective covering Retards water loss Regulates body temperature Houses sensory receptors Contains immune
Chapter 6 Skin and the Integumentary System Composed of several tissues Maintains homeostasis Protective covering Retards water loss Regulates body temperature Houses sensory receptors Contains immune
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis
2/5/2019. Organ System: Skin or Integumentary System. Hypodermis (or superficial fascia) Integumentary System - Learn and Understand
 Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1
 Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
The Integumentary System. Mosby items and derived items 2010, 2006, 2002, 1997, 1992 by Mosby, Inc., an affiliate of Elsevier Inc.
 The Integumentary System The Skin Structure two primary layers called epidermis and dermis Epidermis Outermost and thinnest primary layer of skin Composed of several layers of stratified squamous epithelium
The Integumentary System The Skin Structure two primary layers called epidermis and dermis Epidermis Outermost and thinnest primary layer of skin Composed of several layers of stratified squamous epithelium
Skin and adnexa Skin Adnexa (derivatives): hair nail sebaceous glands sweat glands mammary glands
 Integumentary system - skin and adnexa Skin and adnexa Skin Adnexa (derivatives): hair nail sebaceous glands sweat glands mammary glands Skin (cutis): structure epidermis stratified squamous keratinized
Integumentary system - skin and adnexa Skin and adnexa Skin Adnexa (derivatives): hair nail sebaceous glands sweat glands mammary glands Skin (cutis): structure epidermis stratified squamous keratinized
ABCD rule. apocrine glands. arrector pili. ceruminous glands. contact dermatitis
 ABCD rule assessing moles: asymmetric, broder irregularity, color, diameter (larger than 6mm) apocrine glands arrector pili sweat glands in the pubic and underarm areas that secrete thicker sweat, that
ABCD rule assessing moles: asymmetric, broder irregularity, color, diameter (larger than 6mm) apocrine glands arrector pili sweat glands in the pubic and underarm areas that secrete thicker sweat, that
7/10/18. Introduction. Integumentary System. Physiology. Anatomy. Structure of the Skin. Epidermis
 Introduction Integumentary System Chapter 22 Skin is largest and heaviest organ of body (7% of body weight) Houses receptors for touch, heat, cold, movement, and vibration No other body system is more
Introduction Integumentary System Chapter 22 Skin is largest and heaviest organ of body (7% of body weight) Houses receptors for touch, heat, cold, movement, and vibration No other body system is more
Supplementary Information
 Supplementary Information Figure S1: Follicular melanocytes in the wound peripheral area migrate to the epidermis in response to wounding stimuli. Dorsal skin of Trp2-LacZ mice stained with X-gal and analyzed
Supplementary Information Figure S1: Follicular melanocytes in the wound peripheral area migrate to the epidermis in response to wounding stimuli. Dorsal skin of Trp2-LacZ mice stained with X-gal and analyzed
Chapter 5: Integumentary System
 Chapter 5: Integumentary System I. Overview of the Integumentary System A. List the five major functions of the integumentary system: 1. 2. 3. 4. 5. Il. Skin A. Epidermis 1. The epidermis consists of 2.
Chapter 5: Integumentary System I. Overview of the Integumentary System A. List the five major functions of the integumentary system: 1. 2. 3. 4. 5. Il. Skin A. Epidermis 1. The epidermis consists of 2.
Integumentary System
 Chapter 5 Integumentary System 5-1 Skin: composed of dermis and epidermis Dermis. Gives structural strength. C.T. with many fibers, fibroblasts, macrophages. Some adipocytes and blood vessels. Contains
Chapter 5 Integumentary System 5-1 Skin: composed of dermis and epidermis Dermis. Gives structural strength. C.T. with many fibers, fibroblasts, macrophages. Some adipocytes and blood vessels. Contains
Cornell Notes Name: Date: Topic: CH 4
 *We are revisiting Ch 3B on body tissues (Connective) prior to our study of Ch 4 Integumentary. Start on p.90 I. Connective Tissue A. Functions of Connective 1. Protection 2. Support 3. Binding Together
*We are revisiting Ch 3B on body tissues (Connective) prior to our study of Ch 4 Integumentary. Start on p.90 I. Connective Tissue A. Functions of Connective 1. Protection 2. Support 3. Binding Together
Skin and Body Membranes
 4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
Ch 5: Integumentary System
 Ch 5: Integumentary System You gotta have skin; All you really need is skin. Skin's the thing, that if you've got it outside, It helps keep your insides in. Alan Sherman (1924-1973) Developed by John Gallagher,
Ch 5: Integumentary System You gotta have skin; All you really need is skin. Skin's the thing, that if you've got it outside, It helps keep your insides in. Alan Sherman (1924-1973) Developed by John Gallagher,
Epithelia will be discussed according to the following scheme: Type Number of layers Shape Line drawing. Squamous Cuboidal Columnar
 Epithelia Epithelia will be discussed according to the following scheme: Type Number of layers Shape Line drawing Simple Squamous Cuboidal Columnar Covering and Lining epithelium Pseudostratified Stratified
Epithelia Epithelia will be discussed according to the following scheme: Type Number of layers Shape Line drawing Simple Squamous Cuboidal Columnar Covering and Lining epithelium Pseudostratified Stratified
Trichofolliculoma of the Guinea Pig 1,2
 Trichofolliculoma of the Guinea Pig 1,2 Raymond D. Ediger, Garrett S. Dill, Jr., and Robert M. Kovatch, Aerobiology and Evaluation Laboratories and Medical Sciences Laboratories, Fort Detrick, Frederick,
Trichofolliculoma of the Guinea Pig 1,2 Raymond D. Ediger, Garrett S. Dill, Jr., and Robert M. Kovatch, Aerobiology and Evaluation Laboratories and Medical Sciences Laboratories, Fort Detrick, Frederick,
1. Introduction (Open your text to the image of a cross section of skin) i. Organ of the Integument. Connective Tissues. Epithelial Tissues
 Integumentary System 1. Introduction (Open your text to the image of a cross section of skin) A. Integumentary System i. Organ of the Integument a. Tissues Connective Tissues * Tissue / Location Relationships
Integumentary System 1. Introduction (Open your text to the image of a cross section of skin) A. Integumentary System i. Organ of the Integument a. Tissues Connective Tissues * Tissue / Location Relationships
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Study Guide for Bio 101 Lecture Exam 3
 Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Study Guide for Bio 101 Lecture Exam 3 Please note that this study guide is a listing of objectives that you are required to master for this course. However, items mentioned in class or in laboratory as
Patterns of Proliferation and Apoptosis during Murine Hair Follicle Morphogenesis
 Patterns of Proliferation and Apoptosis during Murine Hair Follicle Morphogenesis Markus Magerl,* Desmond J. Tobin,² Sven MuÈller-RoÈver,*³ Evelin Hagen,* Gerd Lindner,* Ian A. McKay,³ and Ralf Paus* *Department
Patterns of Proliferation and Apoptosis during Murine Hair Follicle Morphogenesis Markus Magerl,* Desmond J. Tobin,² Sven MuÈller-RoÈver,*³ Evelin Hagen,* Gerd Lindner,* Ian A. McKay,³ and Ralf Paus* *Department
The Integumentary System: ANATOMY Includes: - Skin (integument) MEMBRANES. PHYSIOLOGY (functions) Protection. EPITHELIAL (cont.
 Did you know. Membranes & The Integumentary System The skin is the largest organ of the human body. It has a surface area of about 25 square-feet! You shed about 1.5 pounds of skin particles each year.
Did you know. Membranes & The Integumentary System The skin is the largest organ of the human body. It has a surface area of about 25 square-feet! You shed about 1.5 pounds of skin particles each year.
(From Arnold Biological Laboratory, Brown University, ProVence) P~s 3 To 5. Materials and Methods
 Published Online: 25 January, 1955 Supp Info: http://doi.org/10.1083/jcb.1.1.13 Downloaded from jcb.rupress.org on June 21, 2018 HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN IX. THE Dm~mUT~ON Ot NoN-SP~.c~Ic
Published Online: 25 January, 1955 Supp Info: http://doi.org/10.1083/jcb.1.1.13 Downloaded from jcb.rupress.org on June 21, 2018 HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN IX. THE Dm~mUT~ON Ot NoN-SP~.c~Ic
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
Tissues. Tissues - Overview. Bio 101 Laboratory 3. Epithelial Tissues and Integument
 Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Skin is a multilayered organ that covers and protects the body.
 Section 1: Skin is a multilayered organ that covers and protects the body. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the four tissue types that are found in
Section 1: Skin is a multilayered organ that covers and protects the body. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the four tissue types that are found in
Integumentary System
 Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
21 Skin and it s Accessories
 21 Skin and it s Accessories 21-001 Skin 1 2 Sweat glands Adipose tissue 3 Retinaculum cutis 21-01. Skin of the palm 1. General view. Human, H-E stain, x 3.3. Opening of sweat gland duct Stratum corneum
21 Skin and it s Accessories 21-001 Skin 1 2 Sweat glands Adipose tissue 3 Retinaculum cutis 21-01. Skin of the palm 1. General view. Human, H-E stain, x 3.3. Opening of sweat gland duct Stratum corneum
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
The Integumentary System : Embryology & Genetic Bases. Purnomo Soeharso Department of Medical Biology FMUI
 The Integumentary System : Embryology & Genetic Bases Purnomo Soeharso Department of Medical Biology FMUI Tissue organization of the skin (integumentum) : - Epidermis stratified epithelium on the outer
The Integumentary System : Embryology & Genetic Bases Purnomo Soeharso Department of Medical Biology FMUI Tissue organization of the skin (integumentum) : - Epidermis stratified epithelium on the outer
Observations on the Pathology of Lesions Associated with Stephanofilaria dinniki Round, 1964 from the Black Rhinoceros (Diceros bicornis)
 Journal of Helminthology, ~ol. XXXVIII, Nos. 1/2, 1964, pp. 171-174. Observations on the Pathology of Lesions Associated with Stephanofilaria dinniki Round, 1964 from the Black Rhinoceros (Diceros bicornis)
Journal of Helminthology, ~ol. XXXVIII, Nos. 1/2, 1964, pp. 171-174. Observations on the Pathology of Lesions Associated with Stephanofilaria dinniki Round, 1964 from the Black Rhinoceros (Diceros bicornis)
Edinburgh Research Explorer
 Edinburgh Research Explorer MGF (KIT ligand) is a chemokinetic factor for melanoblast migration into hair follicles Citation for published version: Jordan, SA & Jackson, IJ 2000, 'MGF (KIT ligand) is a
Edinburgh Research Explorer MGF (KIT ligand) is a chemokinetic factor for melanoblast migration into hair follicles Citation for published version: Jordan, SA & Jackson, IJ 2000, 'MGF (KIT ligand) is a
An Investigation of Apoptosis in Androgenetic Alopecia
 Annals of Clinical & Laboratory Science, vol. 33, no. 1, 2003 107 An Investigation of Apoptosis in Androgenetic Alopecia Michael B. Morgan 1 and Paul Rose 2 1Department of Pathology and 2 Department of
Annals of Clinical & Laboratory Science, vol. 33, no. 1, 2003 107 An Investigation of Apoptosis in Androgenetic Alopecia Michael B. Morgan 1 and Paul Rose 2 1Department of Pathology and 2 Department of
Levels of Organization
 Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
Levels of Organization Oklahoma Laws Violators can be fined, arrested or jailed for making ugly faces at a dog. Females are forbidden from doing their own hair without being licensed by the state. Dogs
IN the skin of the mouse we find that the dermal papilla of growing hair
 (240 Cyclic Changes in Polysaccharides of the Papilla of the Hair Follicle By WILLIAM MONTAGNA, HERMAN B. CHASE, JANET D. MALONE, AND HELEN P. MELARAGNO (From Brown University, Providence, Rhode Island,
(240 Cyclic Changes in Polysaccharides of the Papilla of the Hair Follicle By WILLIAM MONTAGNA, HERMAN B. CHASE, JANET D. MALONE, AND HELEN P. MELARAGNO (From Brown University, Providence, Rhode Island,
Integumentary System (Script) Slide 1: Integumentary System. Slide 2: An overview of the integumentary system
 Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Publication list Sven Hendrix (ne Müller-Röver)
 Publication list Sven Hendrix (ne Müller-Röver) Peters EMJ, Liezmann C, Spatz K, Daniltchenko M, Joachim R, Gimenez-Rivera A, Hendrix S, Botchkarev V, Brandner JM, Klapp BF: Dissecting the role of nerve
Publication list Sven Hendrix (ne Müller-Röver) Peters EMJ, Liezmann C, Spatz K, Daniltchenko M, Joachim R, Gimenez-Rivera A, Hendrix S, Botchkarev V, Brandner JM, Klapp BF: Dissecting the role of nerve
Chapter 6: Skin & the. 6.1 Skin and its Tissues 6.2 Accessory Organs of the Skin 6.3 Regulation of Body Temperature 6.4 Healing of Wounds
 Skin & the Integumentary System 6.1-6.2 September 10, 2012 Chapter 6: Skin & the Integumentary System 6.1 Skin and its Tissues 6.2 Accessory Organs of the Skin 6.3 Regulation of Body Temperature 6.4 Healing
Skin & the Integumentary System 6.1-6.2 September 10, 2012 Chapter 6: Skin & the Integumentary System 6.1 Skin and its Tissues 6.2 Accessory Organs of the Skin 6.3 Regulation of Body Temperature 6.4 Healing
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS
 INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
INTEGUMENTARY SYSTEM PART I: FUNCTIONS & EPIDERMIS Integumentary System Cutaneous membrane Epidermis (5-layers) made up of epithelial tissue only Dermis (2-layers) contains connective tissue, vessels,
Student Objectives. 7. Describe the structure of nails.
 Student Objectives When you have completed the exercises in this chapter, you will have accomplished the following objectives: The Skin 1. Name the tissue types composing the epidermis and dermis. List
Student Objectives When you have completed the exercises in this chapter, you will have accomplished the following objectives: The Skin 1. Name the tissue types composing the epidermis and dermis. List
Unit 4 The Integumentary System
 Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Skin and Body Membranes
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 4 Skin and Body Membranes Slides 4.1 4.32 Lecture Slides in PowerPoint by Jerry L. Cook Skin and Body Membranes Function
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 4 Skin and Body Membranes Slides 4.1 4.32 Lecture Slides in PowerPoint by Jerry L. Cook Skin and Body Membranes Function
Chapter 6: Skin and the Integumentary System
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 10 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Skin and the Integumentary System I. Skin and Its Tissues A. Introduction
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 10 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Skin and the Integumentary System I. Skin and Its Tissues A. Introduction
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
THE INTEGUMENTARY SYSTEM. Body Membranes & Skin
 THE INTEGUMENTARY SYSTEM Body Membranes & Skin TYPES OF MEMBRANES Epithelial Membranes includes layer of epithelial cells and connective tissue Serous Cutaneous Mucous Connective Tissue Membranes solely
THE INTEGUMENTARY SYSTEM Body Membranes & Skin TYPES OF MEMBRANES Epithelial Membranes includes layer of epithelial cells and connective tissue Serous Cutaneous Mucous Connective Tissue Membranes solely
The Integementary System. The Skin & Its Parts
 The Integementary System The Skin & Its Parts General Structure 2. Accessory structures: hair, nails, exocrine glands 1. Cutaneous membrane: various layers Major Functions 1. Protection 2. Temperature
The Integementary System The Skin & Its Parts General Structure 2. Accessory structures: hair, nails, exocrine glands 1. Cutaneous membrane: various layers Major Functions 1. Protection 2. Temperature
Chapter 6: Integumentary System
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 12 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Integumentary System I. Introduction 1. The skin is composed of of tissues.
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 12 th ed. Chapter 6: Skin and the Integumentary System Chapter 6: Integumentary System I. Introduction 1. The skin is composed of of tissues.
Cell Types in Epidermis
 Epidermis Stratified, squamous keratinized epithelium Appendages hair follicles nails sweat glands sebaceous glands mammary glands Dermis Dense, irregular connective tissue Hypodermis Superficial fascia
Epidermis Stratified, squamous keratinized epithelium Appendages hair follicles nails sweat glands sebaceous glands mammary glands Dermis Dense, irregular connective tissue Hypodermis Superficial fascia
Overview of the Integumentary System. Lab #7. Layers of the epidermis are known as strata. Organization of the Epidermis: Layers of the Epidermis
 Overview of the Integumentary System Lab #7 Integumentary System Organization of the Epidermis: Layers of the epidermis are known as strata Figure 5 2 Layers of the Epidermis Top: Free surface of skin
Overview of the Integumentary System Lab #7 Integumentary System Organization of the Epidermis: Layers of the epidermis are known as strata Figure 5 2 Layers of the Epidermis Top: Free surface of skin
Due next week in lab - Scientific America Article Select one article to read and complete article summary
 Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
