In Vitro Assessment of Osteoblast Behavior in. Craniosynostosis
|
|
|
- Ferdinand Reynolds
- 5 years ago
- Views:
Transcription
1 In Vitro Assessment of Osteoblast Behavior in Craniosynostosis by Tatiana Karine Simon Cypel A thesis submitted in conformity with the requirements for the degree of Master of Science The Institute of Medical Science University of Toronto Copyright by Tatiana Karine Simon Cypel 2011
2 IN VITRO ASSESSMENT OF OSTEOBLAST BEHAVIOR IN CRANIOSYNOSTOSIS Tatiana Karine Simon Cypel Masters of Science The Institute of Medical Science University of Toronto 2011 ASTRACT Introduction: The objective of this study is to investigate the role of osteoblasts in the pathophysiology of premature suture fusion in infants. Hypothesis: Regional variations in osteoblast function and cell signalling exist in calvaria of infants with craniosynostosis. Methods: Bone and periosteal tissue from fused and patent cranial sutures and adjacent bone were harvested from infants undergoing surgery for craniosynostosis and used to develop primary osteoblast cell cultures. Dural tissue was obtained from neurosurgical procedures in order to generate an osteoblast-dural co-culture. Osteoblast proliferation, differentiation, mineralization, protein expression (Noggin, BMP3 and Runx2) and response to exogenous FGF2 stimulation were assessed. Results: Cell cultures demonstrated significant (p<0.05) regional variations in osteoblast proliferation, alkaline phosphatase and in vitro bone nodule formation. The ii
3 expression of anti-osteogenic molecules (Noggin and BMP3) was decreased in osteoblasts from fused suture regions. Expression of Runx2 was increased in fused suture osteoblasts in dural co-culture. Conclusion: The creation of a pro-osteogenic environment through the decreased expression of anti-osteogenic signalling molecules and increased expression of osteogenic factors may be responsible for premature suture fusion in infants. iii
4 AKNOWLEDGEMENTS I would like to thank Dr. Christopher Forrest for giving me the opportunity to work in the exciting and very important field of craniofacial care and research. Dr. Forrest has always taken time to ensure that I had all the support required to complete my work and he was always keen to provide me opportunities in order to increase my level of knowledge. Furthermore, his qualified guidance and enthusiastic supervision in my research project, along with his commitment to patient care, have provided me with a role model of a contemporary surgeon. I would like to also thank Dr. Cho Pang for these two years. Dr. Pang taught me how to think and work as a scientist. Drs Iona Leong, Cho Pang and Peter Dirks, as members of my Program Advisory Committee, have been invaluable in providing their expert guidance and ideas to further enrich my research and make it productive. The research outlined here would not have been possible without the technical and intellectual support of my colleagues in the Craniofacial Surgery Department ( Dr. John Phillips and the craniofacial clinical fellows) and Neurosurgery Department (Dr. Rutka and iv
5 Dr. Drake). Homa Ashrafpour and Ning Huang, as our lab manager and technician respectively, have done excellent work in keeping the lab efficient and supporting my experiments. Thanks to Balram Sukhu, his expertise in bone cell culture and osteoblast behavior made this work possible. I also would like to thank Dr. Rinaldo De Angeli Pinto, chair of the Division of Plastic Surgery (Federal University of Rio Grande do Sul) where I performed my plastic surgery training in Brazil. Dr. De Angeli was for me, an example of a superb, ethical, and highly competent surgeon. He created the surgical personality I currently have and hopefully I will carry that for my entire career. I would like to acknowledge the contribution of Nicole Gojska in assisting with technical work in the conditioned culture medium project. No research would be possible without sufficient resources. I would like to thank the Craniofacial Care and Research Funding, The SickKids Start up Funding, The Komedyplast and Amercian Society of Craniofacial Surgery, The Physician s Service Incorporate Foundation and the American Society of Maxillofacial Surgeons for supporting and making this work possible. v
6 DEDICATION This thesis is dedicated to my family: my parents and two sisters who have always supported me and stood behind me and my husband Marcelo, who has shared my challenges and successes with the greatest of understanding, patience and support. vi
7 TABLE OF CONTENTS Page I. List of Tables x II. List of Figures x III. List of Appendices xiii IV. List of Abbreviations xiv V. Introduction 1 (a) Overview of Craniosynostosis 2 - Classification 3 - Functional Problems Associated with Craniosynostosis 5 - Surgical Treatment 7 (b) Pathogenesis of Craniosynostosis 9 - Embryology of Cranial Suture 9 - Normal Skull and Suture Growth 10 - Normal Suture Fusion 10 - Historical Theories of Craniosynostosis 11 - Current Theories 12 vii
8 - The Role of Anti-osteogenic Signalling 13 - The Role of Dura Mater 17 - The Role of Runx The Role of FGFs 20 - Experimental Models for Craniosynostosis Research 22 - Effect of Culture Medium Composition on Osteoblast Function 23 VI. Hypothesis 27 VII. Material and Methods 30 - In Vitro Human Osteoblast Cell Culture Model 31 - Statistical Analyses 38 VII. Results - Demographics 40 - Histology 41 - Collagen I Expression 42 - Validation of Cell Culture Model 43 - Medium Composition 45 - Osteoblast Proliferation 47 - Runx2 Expression 52 - Alkaline Phosphatase Activity 54 - Mineralization 58 - Transmission Electron Microscopy 63 - Expression of Anti-osteogenic Signalling Molecules 64 - Dura Mater Expression of FGF2 and TGF-β1 70 viii
9 - Recombinant Human FGF2 Stimulation 71 VIII. Discussion 77 IX. Conclusion 93 X. Future Directions 96 XI. References 99 XII. Appendices Detailed Protocols 111 ix
10 LIST OF TABLES Page Table 1: Classification of Craniosynostosis 3 Table 2: Demographics of Patients with Craniosynostosis Enrolled in the Study 40 LIST OF FIGURES Figure 1: Single-suture Synostosis Phenotypic Expression 4 Page Figure 2: Mechanism that BMP3 and Noggin Cause Inhibition of Bone Formation 16 Figure 3: The Role of Runx2 in Osteogenic Differentiation 19 Figure 4: Potential Mechanisms of Craniosynostosis 26 Figure 5: Hematoxylin-and eosin Staining for Bone Tissue Sampling 41 Figure 6: Immunohistochemistry Staining for Collagen I 42 Figure 7: Evidences of Osteoblasts in our Cell Culture Model 43 Figure 8: MTT Assays of Human Cranial Suture-derived Osteoblasts 46 Figure 9: Cellular Growth Prior and Post-subculture 47 Figure 10: Osteoblast Proliferation Rates (MTT) 48 Figure 11: Proliferation Rates for Syndromic Patients 49 x
11 Figure 12: Osteoblast Proliferation Rates in Co-culture with Dura Mater 50 Figure 13: Expression of Runx2 Demonstrated by Immunohistochemistry 52 Figure 14: Expression of Runx2 by Western Blot 53 Figure 15: Alkaline Phosphatase Assay Assessing Osteoblast Differentiation Rates 54 Figure 16: Alkaline Phosphatase Activity 55 Figure 17: Analysis by qrt-pcr of Alkaline Phosphatase and Osteocalcin 56 Figure 18: AP for Osteoblast Co-cultured with Dura Mater Cells 57 Figure 19: Bone Nodule Formation Alizarin Red Assay 59 Figure 20: Bone Nodule Formation at Days 14 and Figure 21: Mineralization at Days 21 and Figure 22: Mineralization for Osteoblasts Co-cultured with Dura Mater 62 Figure 23: Transmission Electron Microscopy of Bone Nodules 63 Figure 24: Immunohistochemistry Analysis of Noggin in Tissue Samples 64 Figure 25: Graphic Analysis for Noggin in the Tissue Samples 65 Figure 26: Immunohistochemistry Analysis of BMP3 in Tissue Samples 66 Figure 27: Graphic Analysis for BMP3 in the Tissue Samples 67 Figure 28: Western Blot Analysis of BMP3 and Noggin 68 xi
12 Figure 29: Expression of Pro-osteogenic Molecules in the Conditioned Medium from Dura Mater Cells 70 Figure 30: Proliferation Rates of Osteoblasts Stimulated with FGF2 72 Figure 31: Proliferation Rates after Stimulation with FGF2 (MTT) 73 Figure 32: AP Staining after Stimulation with FGF2 75 Figure 33: APA after Stimulation with FGF2 76 xii
13 LIST OF APPENDICES Page I. Calvarial Osteogenic Cell Culture 111 II. Medium Composition Study 114 III. MTT Assay 116 IV. Alkaline Phosphatase Activity 117 V. Alkaline Phosphatase Staining 118 VI. Alizarin Red Staining 119 VII. Transmission Electron Microscopy 119 VIII. Histology 120 IX. Immunohistochemistry and Immunofluoresence 120 X. Western Blot 121 XI. Real Time PCR 124 XII. Materials 126 xiii
14 LIST OF ABREVIATIONS AMEM (αamem) ANOVA AP APA Alpha Modified Eagle Medium Analysis of Variance Alkaline Phosphatase Alkaline Phosphatase Activity BMP2 Bone Morphogenic Protein 2 BMP3 Bone Morphogenic Protein 3 BMP4 Bone Morphogenic Protein 4 BMP7 Bone Morphogenic Protein 7 CT DMEM Computed Tomography Dulbeco Modified Eagle Medium Cbfa1 Core binding factor alpha 1 DNA FBS FGFs FGFR Deoxyribonucleic acid Fetal Bovine Serum Fibroblast Growth Factors Fibroblast Growth Factor Receptor FGF2 Fibroblast Growth Factor 2 FS IHC I-SMADS MTT PS Fused Suture Immunohistochemistry Inhibitory Smads Methylthiazoletetrazolium Patent Suture xiv
15 qrt-pcr REB RNA SMAD TEM TGF-β WB Real Time Quantitative Reverse Transcripition PCR Research Ethical Board Ribonucleic Acid Sma and Mad Related Proteins Transmission Electron Microscopy Transforming Growth Factor-β Western Blot xv
16 Introduction 1
17 OVERVIEW OF CRANIOSYNOSTOSIS Craniosynostosis, the premature fusion of one or more cranial sutures, is a relatively common congenital disorder, affecting as many as 1 in 2000 to 2500 live births worldwide 1. Hippocrates provided the first description of this anomaly in 100 B.C. He noted the variable appearance of the calvarial deformity and correlated it with the pattern of cranial suture involvement. In 1851, Virchow was the first one to recognize that cranial sutures were responsible for growth of the calvarium at right angles to the suture and that premature fusion resulted in growth arrest at right angles to the suture with compensatory growth at patent sutures 2. Premature ossification of cranial sutures may lead to a number of serious morphologic and functional consequences, such as increased intracranial pressure, developmental delay, visual and hearing impairment and can require major staged reconstructive procedures to correct the condition. Despite its prevalence, the cause of craniosynostosis still remains largely unknown. Craniosynostosis may be classified by the number of involved sutures, etiology or whether the condition is associated with a syndrome or not (Table 1). 2
18 Table 1: Classification of Craniosynostosis Anatomical Single Suture Sagittal Metopic Multiple Sutures Any combination possible usually bi-coronal Unicoronal Lambdoid Minor suture (fronto-sphenoid, zygomatico-temporal) Etiology Primary Non-Syndromic Scaphycephaly (sagittal) Trigonocephaly (metopic) Anterior plagiocephaly (unicoronal) Posterior plagiocephaly (lambdoid) Turribrachycephaly (bi-coronal) Oxycephaly (delayed bicoronal) Syndromic Apert s syndrome Crouzon s syndrome Pfeiffer syndrome Jackson-Weiss syndrome Carpenter syndrome Muenke syndrome Saethre-Chotzen syndrome Secondary Biomechanical (shunt) Bone metabolic disorders Nutritional The major cranial sutures involved (in order of decreasing frequency) are the sagittal, metopic, coronal and lambdoid sutures and each give a characteristic shape to the cranial vault (Figure 1). 3
19 Figure 1: Single-suture Synostosis Phenotypic Expression: Single suture nonsyndromic craniosynostosis phenotypic and radiologic (CT) features. Single suture craniosynostosis is usually an isolated phenomenon and has a low incidence of familial occurrence, generally considered to be less than 5%. The highest incidence of familial occurrence is with sagittal synostosis, which has been reported to be as high as 8% 3. While the majority of craniosynostosis occur due to unknown causes, craniosynostosis can occur as a result of metabolic disorders (e.g., hyperthyroidism), malformations (e.g., holoprosencephaly, microcephaly, shunted hydrocephalus, 4
20 encephalocele), drug exposure (e.g., valproic acid, phenytoin) or mucopolysaccharidosis (e.g., Hurler s syndrome, Morquio s syndrome). FUNCTIONAL PROBLEMS ASSOCIATED WITH CRANIOSYNSTOSIS Premature fusion of the cranial sutures may be associated with a variety of clinical problems ranging from cranial facial dysmorphism to increased intracranial pressure due to cranio-cerebral disproportion. The severity and extent of involvement is dependent upon the number of affected sutures and the presence or absence of an associated syndrome. This section details the potential functional problems associated with craniosynostosis. Increased Intracranial Pressure: Elevated intracranial pressure may be associated with craniosynostosis 4. Renier et al. published the first major study to measure the preoperative and postoperative differences in intracranial pressure in infants with craniosynostosis. Nonsyndromic patients overall had a 14% incidence of elevated intracranial pressure before surgery. The number of involved sutures affects the probability that intracranial pressure will be elevated. Preoperatively, 8.9% of the patients with one suture affected had elevated intracranial pressure but those with multiple sutures involved had a 45% incidence of intracranial hypertension. The effect of craniosynostosis, elevated intracranial pressure, and syndromic disease on intelligence is less clear. In general, for most nonsyndromic patients, intelligence is normal with single-suture involvement (>90% IQ 90) but decreases with multiple suture involvement (78% IQ 90) 5. For syndromic patients, the degree of developmental delay is highly variable, depending on the syndrome and severity of disease. 5
21 Orbits: Exorbitism is a significant component for syndromic patients with craniosynostosis. Hypoplastic orbits and a retruded midface can cause globe and corneal exposure, which can result in corneal injury and exposure keratitis. The majority of orbital problems occur relatively early, in the first 5 years of life which represents the main period of orbital growth. Airway: Syndromic craniosynostosis may be associated with airway compromise that requires early recognition and treatment to prevent cardiopulmonary and neurologic sequelae 6. This may be due to midface retrusion and choanal atresia resulting in airway obstruction. The reported incidence of airway obstruction in syndromic craniosynostosis ranges from 40% to 100% 6. Many of these children will additionally have lower airway anomalies that include tracheomalacia, complete cartilaginous tracheas, and granulations. Airway obstruction and hypoxia during sleep may present as snoring, noisy respirations, apneic episodes, paradoxical chest movements, persistent restlessness, feeding difficulties, failure to thrive, hypertension, daytime fatigue, and cardiopulmonary or neurologic impairment 7. Complications of persistent airway obstruction include respiratory infections, cor pulmonale, neurologic dysfunction and brain damage. 7 Neurodevelopment Particularly problematic is the issue of intelligence and neurocognitive development. Virtanen et al 8 contended that in a series of patients undergoing early operative correction for sagittal craniosynostosis, certain indices of neurocognitive performance were below those of age-matched control subjects and remained delayed throughout the period of examination after surgery. Other authors contended that although young children with craniosynostosis are often normal from a mental standpoint, there is an increase in frequency of psychomotor problems as they develop 5. 6
22 Aesthetic/Psychosocial Aesthetic considerations are more difficult to quantify than the objective values of protecting vision, reducing intracranial pressure, or improving occlusion. Infants with craniosynostosis have visibly altered craniofacial appearance, which varies in relation to the location and extent of suture involvement. The possibility of permanent abnormality in facial or cranial appearance seems to greatly affect parents decisions to have their infant undergo craniofacial surgery. Many surgeons believe that the primary indication for cranioplasty in isolated synostosis is cosmetic rather than functional 9. There is clear evidence in other groups of children that even mild deviations from typical facial appearance can have significant impact on psychological adjustment. Congenital defects involving an infant s face and skull seem to evoke particularly strong emotional responses from parents, who must contend with a host of potentially stressful events and circumstances, including the infant s unusual appearance, potentially life-threatening surgeries and other medical procedures, and the possibility of future neuropsychological and educational problems. All of these factors can potentially affect parents responsiveness and adaptation to the infant with craniofacial abnormality 10. SURGICAL TREATMENT The field of craniofacial surgery has existed for only a short time in the overall history of medicine. Of course, the patients have always been there, with a birth prevalence of craniosynostosis of approximately 1 in 2000 live births 1 ; but it is only since Paul Tessier began his pioneering work in the late 1960s that the field of craniomaxillofacial reconstruction developed. Since the 1970s the specialty of craniofacial surgery has grown to include multidisciplinary teams treating a wide variety of patients. 7
23 Indications for Treatment In nonsyndromic patients, individual cranial sutures may be fused, resulting in an abnormality of shape requiring cranio-orbital reshaping, but the midface is generally unaffected. In syndromic craniosynostosis, in addition to cranial suture abnormalities, other facial skeletal anomalies exist including a shortened cranial base, orbital hypoplasia, midface and zygomatic retrusion to name a few. The reasons for surgical intervention in the nonsyndromic group may be primarily aesthetic; in the syndromic group, they are multifactorial. Aesthetic considerations are more difficult to quantify. Nevertheless, the benefits of improving appearance on psychosocial adjustment have been evaluated across many different populations 11. Timing of Surgery Advances in pediatric anesthesia and intensive care have allowed extensive cranial reconstructions in infancy that previously were available only to adolescents and adults. Concerns for the negative effect of early intervention on skeletal growth were reduced by studies demonstrating that in syndromic patients, the midface was deficient whether patients had early surgery or not 12, 13. McCarthy and Cutting 14 proposed that the first procedure, cranial vault remodeling and fronto-orbital advancement, be performed between 6 and 9 months of age. At an earlier age, the bone is more fragile; at a later age (>18 months), residual calvarial defects will fail to reossify. Early surgery is also important because of rapid growth of the brain, which more than doubles in volume during the first year of life 15. 8
24 Pathogenesis of Craniosynostosis During cranial development, adjustment to the expanding brain takes place by bone deposition at sutural margins and on the ectocranial surface of the calvarium, and by bone resorption on the endocranial surface 16. The cranial sutures, as primary areas of growth 16, 17 during the expansion of the cranium play a pivotal role. Although a descriptive knowledge of suture morphogenesis and function is well reported 16, 18, the spatial and temporal regulation of bone deposition, resorption, and remodeling are not well understood 19. Embryology of Cranial Suture Sutural morphogenesis occurs in midgestation when enlarging bone plates in the primordial cranium come into apposition 16, 17. Mesenchymal cell populations along the expanding osteogenic fronts differentiate into osteoblasts and contribute to the formation of osteoid and the mineralization of new bone 17, 18, bringing opposing bones into close approximation. An intervening zone of immature mesenchymal tissue is thereby created between them, forming the blastema of the suture 18, 20 Suture mesenchymal cells continue to proliferate and organize within the fibrous suture matrix, which acquires the characteristic appearance of the mature, multilamellar suture 17, 18. Simultaneously, populations of mesenchymal cells bordering the suture along the osteogenic fronts continue to differentiate into osteoblasts, contributing to the formation of new bone 17, 18. From the time of their formation, sutures are extremely active centers of cellular proliferation, cellular differentiation, tissue synthesis, and remodeling. The intricate regulation of these processes allows for the completion of cranial morphogenesis while preventing the formation of bone 9
25 across the sutural space, an event which occurs only in the pathologic condition of craniosynostosis or part of normal suture fusion later in adult life 19, 21, 22. Normal Skull and Suture Growth Intramembranous ossification of the skull begins at the end of the second month of gestation. A center of osteogenesis develops directly in vascularized mesenchyme. Expansion of the ossification center proceeds rapidly via appositional growth. Initially cancellous bone forms, but as trabeculae thicken and the bone becomes less porous, it become compact bone. Eventually each intramembranous cranial bone has enlarged to the point at which it articulates with an adjacent bone via a syndesmosis or sutures. Growth then proceeds at the sutures 19. Growth at the suture area is a secondary, compensatory, and mechanically obligatory event following the primary growth of the enclosed brain and ocular globes. The bones of the calvaria are displaced outward by the enlarging brain. Each bone of the domed skull roof responds to the expansion of the brain by depositing new bone at the contact edges of the sutures. Normal Suture Fusion Functioning sutures are the sites of continuous bone deposition and resorption. Initially, sutures are straight edges of bone separated by connective tissue. Gradually, interdigitations develop and become more prominent with time 19. For interdigitations to form, develop, and interlock, the distribution of osteoblasts along sutural bone must be uneven with clumps of osteoblasts at the tip of each interdigitation 23. Sutural interdigitations 10
26 may permit adjustive movements and/or stress reduction. Their architecture may depend on the types and distribution of forces. Suture closure has been attributed to vascular, hormonal, genetic, mechanical, and local factors. Biomechanical factors have been a perennial favorite mechanism 24. The cause of suture closure is still unclear. There may be one or possibly more than one mechanism. The relationship between suture closure, cessation of growth, and functional demands across sutures poses questions about various biological relationships. Does cessation of growth lead to suture fusion? The growth of the human brain ceases prior to the onset of osseous fusion of the cranial sutures. With this is a delay from completion of brain growth to sutural fusion in the 20s and 30s. Historical Theories of Craniosynostosis Although certain cranial deformities arise from mechanical or functional causes (e.g., plagiocephaly and hydrocephaly), the molecular basis of the majority of craniofacial abnormalities is becoming increasingly evident through advancements in molecular biology. Early explanations of cranial suture fusion included anectodal associations with intrauterine constraint, uterine malformations, decreased amniotic fluid, or breech presentation. Ozaki et al 25 performed ultrastructural analysis of sagittal sutures in the process of fusion. Their analysis revealed several new facts: 1. Premature fusion of sutures was found to begin centrally in the suture. 2. It began endocranially as opposed to both endocranially and ectocranially in normal sutures. 11
27 3. It exhibited a disorganized ultrastructure of lower density than in normal sutures. Molecular biology has now taken us beyond the speculative explanations of mechanical causes to the roots of abnormal suture development. This progression is particularly evident in autosomal dominant syndromic craniosynostosis subtypes. Current Theories Recent work has demonstrated that fusion of the calvarial sutures is mediated by locally elaborated soluble growth factors, leading some to speculate that external biomechanical forces play little role in suture development. Historically, the theory that fetal head constraint may play a critical role in the pathogenesis of many cases of nonsyndromic craniosynostosis has been supplanted by humoral theories although it is possible that cranial biomechanical stresses experienced in fetal and early life might be the trigger that leads to dural cytokine signalling involved with suture fusion and/or patency 26. On the other hand, research focused on the molecular mechanisms underlying normal cranial suture fusion has demonstrated the importance of dura mater mediated cell signalling in the complex process of fusion of normal cranial sutures. It is hypothesized that the dura mater acts as a regionally specific endogenous tissue engineer, releasing growth factors in a specific orchestrated fashion that cause the overlying cranial suture to close in a predictable fashion. Possible candidates for these growth factors include fibroblast growth factor (FGF), and transforming growth factor-β (TGF-β) isoforms. In addition to the effects of these locally released growth factors, cranial suture development has been shown to be influenced by anti-osteogenic signalling molecules such 12
28 as Noggin and Bone Morphogenic Protein 3 (BMP3), which are upregulated in patent sutures during the normal process of suture fusion thereby maintaining the cranial suture patency 27, 28. Furthermore, the role of Runx2, a transcription factor that is a marker of osteoblast differentiation, has been implicated in the process of normal cranial suture fusion. Runx2 is found in osteogenic fronts and sutural mesenchyme and has been demonstrated to be upregulated in fusing sutures during the process of normal suture fusion. Runx2 up regulation enhances differentiation and bone production leading to earlier suture fusion. This factor has been shown to regulate the expression of a number of proteins, including osteocalcin, produced by the mature osteoblast and responsible for its bone formation 29. The Role of Anti-osteogenic Signalling Noggin Noggin, a secreted BMP2/4 antagonist produced by osteoblasts and released in the extracellular matrix, is important in the process of normal suture fusion 27. Noggin is a polypeptide that inhibits Transforming Growth Factor β (TGF-β) signal transduction by binding to TGF-β family ligands and preventing them from binding to their correspondent receptors (Figure 2). Down-regulation of pro-osteogenic BMP signalling (part of the TGF-β superfamily) is then observed which maintains suture patency 27. On the other hand, downregulation of Noggin expression results in disinhibition of pro-osteogenic BMP signalling (BMP2,4,7) increasing bone formation which leads to suture fusion and may be one of the molecular mechanisms involved in the pathophysiology of craniosynostosis. Research findings in a murine model of normal suture fusion demonstrate that downregulation of Noggin expression in the normally fusing posterior frontal suture increased 13
29 bone formation with resulting suture fusion. In a normal non-fusing suture (sagittal), increased Noggin expression results in suture patency 27. It has also been shown that the suture-specific dura mater is an independent source of Noggin. Cultured dura mater cells from patent sutures expressed high levels of Noggin protein, whereas the dura mater from the fusing posterior frontal suture expressed almost undetectable levels of Noggin 30. Experiments in a rabbit model of congenital coronal craniosynostosis also demonstrated the interaction between Noggin and premature suture fusion. Fusing sutures showed low Noggin expression 29. Underexpression of Noggin was also found in the dura and coronal mesenchyme prior to suture fusion. In contrast, in the same model, the patent coronal and sagittal sutures expressed normal levels of Noggin leading to suture patency 29. Despite the current findings strongly suggesting an important role for Noggin in maintaining suture patency in animal models of normal suture fusion, there is a lack of understanding about Noggin expression and its interactions in infants with craniosynostosis. Bone Morphogenic Proteins The BMPs are growth factors secreted by osteoblasts and released in the extracellular matrix. They are part of the TGF-β superfamily, which are well known for their ability to induce the formation of bone and cartilage. The actions of these growth factors are highly concentration dependent and influence a number of cellular processes. For instance, BMP2, 4 and 7 have been shown to promote cellular chemotaxis and proliferation at low extracellular concentrations and to induce cellular differentiation and bone formation at high extracellular concentrations
30 BMP3 is an antagonist of BMP2 and BMP4 32. Rather than impeding BMP signalling of bone formation by binding to a ligand and preventing specific ligand-receptor interactions (as does Noggin), BMP3 activates a TGF-β/activin specific response pathway 33 (Figure 2). Activin is a member of the TGF-β superfamily, and antagonizes the BMP pathway by competing for SMAD proteins; SMAD proteins are transcription factors that regulate the expression of genes involved in the modulation of the activity of TGF-β ligands involved in osteoblast differentiation and bone formation 34. BMP3 has been implicated in the process of normal suture fusion in mice 28. Altough the source of BMP3 during normal suture fusion is not clear, its expression pattern is consistent with that of an antagonist playing a role in suture fusion and patency. BMP3 levels decreased in the posterior frontal suture during suture fusion and were maintained or increased in the patent sagittal suture 35. It is speculated that BMP3 may be negatively regulated by osteogenic factors such as FGF2 and TGF-β1 which are differentially expressed in the fusing posterior frontal and sagittal suture complexes 36, 37. These factors are noted to increase in the dura mater underlying the fusing posterior frontal suture during fusion when compared with the patent sagittal suture
31 Role of Noggin and BMP3 Signalling Noggin Binds to BMP2 blocking the stimulus for bone formation BMP2 Promotes bone formation BMP3 BMP-3 activates a TGF-β/activin-specific response antagonizing osteogenic signaling Activin Response Pathway Antagonize bone formation by competing for SMADS protein No signalling SMAD Activation and Nuclear Translocation Resulting in Bone Formation Figure 2: Mechanism by which BMP3 and Noggin cause inhibition of bone formation. Schematic of Noggin and BMP3-mediated antagonism of bone morphogenetic protein (BMP) signalling. Antagonists such as Noggin bind to BMP ligand and prevent ligand-receptor interactions. BMP3 binds to TGF-β/activin receptors and blocks BMP signalling downstream of activated BMP receptor complexes. Despite the insights in Noggin and BMP3 expression during the normal suture fusion process, it is not clear if down-regulation of these anti-osteogenic molecules during premature suture fusion is a cause or the effect of pro-osteogenic activation leading to premature ossification in craniosynostosis. Additional investigation of the expression of osteogenic antagonists and their regulation will further advance our knowledge of the 16
32 complex cascades regulating suture fusion and patency in infants with syndromic and nonsyndromic craniosynostosis. The Role of Dura Mater Central to many studies of cranial sutures has been the role of the dura mater. Historically, dura mater was thought to be a conduit for tensile forces transmitted from the expanding neurocranium 38, 39. The formation of sutures was seen as a byproduct of this mechanical phenomenon, forming along dural reflections in both normal and disease states 39. While several animal models have established the importance of dura mater in the regeneration of normal cranial bone 40 and sutures in developing animals, evidence suggests that cell signalling and humoral mechanisms are more important than biomechanical forces with respect to bone regeneration 41. Recent findings suggest that dura mater modulates calvarial ossification in many ways, including providing a source of osteoblastic precursors and/or supplying osteogenic cytokines 42. Evidences suggest that the underlying dura mater also influences the behavior of the overlying suture complex by means of paracrine signalling 43. The dura mater underlying the cranial suture complex is one of several sources of FGF2 and TGF-β1cytokines in vivo; however it remains unclear whether the levels of these growth factors produced by dura mater are capable of down-regulating anti-osteogenic molecules expression in osteoblasts to favor a pro-osteogenic enviroment and promote premature suture fusion 44. Li et al 44 demonstrated direct evidence for a paracrine effect of juvenile dura mater cells on osteoblasts by showing that dura mater derived FGF2 mediates mitogenic activity in calvarial osteoblasts which is inhibited by neutralizing FGF2 45. Osteoblasts demonstrated significantly increased proliferation when combined with juvenile dura mater cells in co- 17
33 culture or when dura mater cell-conditioned medium was applied to them. Moreover high levels of FGF2 protein were detected in juvenile dura mater cells and their conditioned medium. In contrast low levels of FGF2 protein were detected in adult dura mater cells and not detectable levels in their conditioned medium. This study reinforced the idea that FGF2 might be an important paracrine signalling factor in vivo supplied by the underlying dura mater to stimulate the overlying calvarial osteoblasts 44. The idea that dura mater derived from immature animals is osteoinductive and/or osteogenic in nature was further supported by studies in which heterotopic transplantation of the dura mater into epithelial mesenchymal pockets in adult rats caused ectopic bone formation 42. Furthermore, when dura mater from adult guinea pigs (18 months of age) was grafted into the base of calvarial defects created in syngeneic infant guinea pigs (3 to 4 weeks old), incomplete reossification was observed 40. In contrast, dura mater taken from an infant rat and placed into the calvarial defect of an adult rat markedly enhanced reossification 46. Therefore immature dura mater seems to have a strong influence on the development of bone formation in vivo and as such we may expect the dura mater to have similar importance in suture regulation. Despite the advancements in the understanding of the pivotal role of dura mater in premature suture fusion there still is a lack of information regarding the interactions of dura mater pro-osteogenic signalling and anti-osteogenic molecules leading to premature calvarial ossification in infants with craniosynostosis. The Role of Runx2 Polyomavirus enhancer binding protein 2/core binding factor Alpha 1 (Cbfa1) or currently denominated Runx2 is a master transcription factor that has been shown to regulate 18
34 osteoblast differentiation stimulating osteogenic gene transcription through a cascade, starting with BMP-2 binding to its receptor (BMPR-II). This binding activates a SMAD (signal transducers for the members of the transforming growth factor-beta superfamily) signalling cascade, ultimately activating Runx2 and stimulating osteogenesis (Figure 3). It also regulates the expression of a wide variety of genes responsible for the osteoblast phenotype and function including osteocalcin and TGF-β 47. The latter provides a direct link between transcriptional regulation and growth factor activity. Figure 3: Role of Runx2 in osteogenic differentiation. BMP2 binds to its receptor (BMPR-II). This binding activates a SMAD signalling cascade, ultimately activating Runx2 and stimulating osteogenic gene transcription. Reflecting its major role in bone formation, Runx2 levels have been shown to be elevated in areas of normal suture formation in mice 48. Mutations in which Runx2 is absent demonstrate defects in osteogenesis 49. These studies provide a sound basis for an effort to 19
35 examine the activity of specific transcription factors such as Cbfa1/Runx2 in osteoblasts derived from fused sutures and to determine whether they play a causative role in sutural closure associated with craniosynostosis. The Role of Fibroblast Growth Factors Fibroblast Growth Factor 2 is a member of the fibroblast growth factor family involved in angiogenesis, wound healing, and embryonic development 50. The FGFs are heparin-binding proteins and interactions with cell-surface associated heparin sulfate proteoglycans have been shown to be essential for FGF signal transduction. FGFs are key players in the processes of proliferation and differentiation of wide variety of cells and tissues. Several observations indicate that FGFs may play an important role in the control of osteogenesis during skeletal development. FGF2 is a potent mesodermal inducer during embryogenesis and FGF receptors (FGFRs) are strongly expressed in developing bones 50. Studies in bovine calvaria cells showed that FGF2 is produced by osteoblasts and accumulates in the bone matrix 51. In bovine and rodent calvaria-derived cells, the effects of FGF on bone cell proliferation and differentiation appear to be opposite. FGF1 and FGF2 stimulate cell proliferation but inhibit alkaline phosphatase (AP) activity and reduce collagen type I (ColI) and osteocalcin (OC) expression, indicating that FGF2 has independent effects on calvarial cell proliferation. The effects of FGFs on osteoblastic cell differentiation and bone matrix formation in long-term culture are however conflicting, since positive 52, 53 and negative effects 54 have been reported, depending on the cell culture system. Considering that dura mater cells may be a source of FGF2 and anti-osteogenic molecules such as Noggin may be regulated by FGF2, it is important to know if this 20
36 molecule is the key element in the pathophysiology of craniosynostosis, which may corroborate with the hypotheses that dura mater influences osteoblast behavior at the fused suture site through release of FGF2 leading to decreased Noggin and BMP3 expression. It is also unclear if osteoblasts from different sites are defective and not able to respond equally to FGF2 stimulation, or if there is an up-regulation of this molecule at the fused suture site. While blocking FGF signalling 30 or FGF2 activity 55 prevents cranial suture fusion or osteogenesis, respectively, the findings suggest that exogenous FGF signalling is capable of suppressing Noggin expression during cranial suture fusion. Many researchers have concentrated efforts on investigating the genetic basis of syndromic craniosynostosis and the functional consequences of mutations involving the fibroblast growth factor receptor (FGFR) gene. Recent findings shown that point mutations in FGFR-1 and FGFR-2 induce premature cranial ossification suggesting that FGF is an important regulator of bone-forming cells during human calvaria (HC) osteogenesis 56. Mutations of three FGFRs account for most causes of syndromic craniosynostosis, including Crouzon s, Crouzon s with acanthosis nigricans, Pfeiffer s, Apert s, Muenke s, Beare- Stevenson, and Jackson-Weiss syndromes 57. On activation, the FGFR immunoglobulin-like bonding regions form dimers, activating the intracellular tyrosine kinase. Subsequent downstream effects on the nucleus influence cellular proliferation, differentiation, and migration. Characterization of specific mutations in genes that cause craniosynostosis is a step forward understanding the mechanism of normal and abnormal development of calvarial bone. However, various approaches of research in this area are still needed to help unravel the complex interaction of gene products that participate in signalling pathways
37 Taken all findings together, FGF2 may guide suture fate (patency versus fusion). It is also important to clarify the role of FGF2 in proliferation and differentiation, and to investigate if different concentrations at the suture site are responsible for the regional differences in osteoblast behavior. Furthermore, it is important to elucidate whether dura mater is the source of FGF2 and evaluate the ability of osteoblast from different sites to respond to FGF2 stimulation, clarifying one step of cascade that leads to craniosynostosis in infants. Experimental Models for Craniosynostosis Research Over the past several years, investigation of the biology underlying programmed posterior frontal suture fusion in rats and mice has been taken as a means of understanding the pathology seen clinically in human craniosynostosis 44. The murine model has been thought to be an excellent system with which to study suture development and molecular specification between mice and humans 45. In the mouse, the posterofrontal suture lies between two frontal bones, and the sagittal suture lies between the two parietal bones. The posterofrontal suture undergoes fusion in a predictable manner on postnatal days 8 to 10, whereas other sutures remain patent 28. This is analogous to humans, in which the metopic suture fuses in infancy and the other sutures remain patent well into adulthood. Thus, the murine posterofrontal and the sagittal sutures taken in juxtaposition, as exemplars of normal suture fusion and patency, respectively, allow for insight into both the normal coordination of suture fusion and possible mechanisms of craniosynostosis 19. Currently, the most representative animal model of craniosynsostosis is the rabbit craniosynostosis strain from the University of Pittsburgh 58. In this model, pathologic suture 22
38 fusion begins in utero, causing cranial vault deformities such as plagiocephaly in unilateral coronal suture synostosis and brachycephaly in bilateral synostosis 59. This model has made it possible to investigate the biomolecular mechanisms involved in craniosynostosis, including the role of anti-osteogenic molecules such as Noggin and pro-osteogenic factors such as Runx2. Although there are few studies utilizing a limited number of discarded samples from patients with craniosynostosis 60, to date there are no reliable models of cell cultures derived from human calvarial bone using a large number of patients and well representing the wide spectrum of craniosynostosis in infants. Effect of Culture Medium Composition on Osteoblast Function The use of normal and fused suture osteoblasts derived from cranial bones of patients with syndromic and non-syndromic craniosynostosis provides a valuable model for investigating molecular and cellular defects associated with this significant disorder in infants 60. However, there is little uniformity in the conditions used in human osteoblast cell cultures, particularly the concentration of reagents present in the media. Initial studies by Coelho et al 61 analyzed the effects of two widely used culture media, Dulbecco s modified Eagle s medium (DMEM) and minimum essential medium Eagle Alpha modification (α- MEM) on human osteoblastic characteristics, including cell viability and alkaline phosphatase (AP) activity 61. These studies demonstrated that DMEM, a less nutrient-rich medium when compared to α-mem, appears to demonstrate higher values of cell proliferation and growth 61, 62. Subsequently, many studies have found that the optimal concentration of fetal bovine serum (FBS) to supplement the culture medium is FBS 10%, 23
39 for it produces the highest proliferation rates 62, 63. The success of cranial suture biology in explaining the pathophysiological mechanism of craniosynostosis is predicated in replicable and efficient cell culture systems that are representative of in vivo cellular dynamics. Therefore, the standardization of culture conditions, especially the medium and the presence of essential compounds, is critical for developing future applications of cranial suture research in reconstructive medicine. Summary of Research Current research has proposed the molecular basis of craniosynostosis based on normal suture fusion animal models. It has been shown that the patency of the suture line depends on the balance between pro-osteogenic and anti-osteogenic signalling molecules, and the imbalance between those may be responsible for premature suture fusion. However, is not clear which changes in the cranial suture enviroment are responsible for this phenomenon. To elucidate the mechanisms locally involved in premature suture fusion we propose to create a human model of osteoblast cell culture by obtaining calvarial bone samples from patent sutures, fused sutures and adjacent bone from infants affected by this condition. First, we propose to evaluate the samples histologically to confirm regional variations between suture sites and adjacent bone. We then aim to search in the surrounding enviroment for factors that may contribute to the imbalance between pro- and anti-osteogenic signalling. It has been shown that dura mater may be the source of growth factors, such as FGF2 and TGF-β that orchestrate this complex process of suture fusion and patency. However, the interaction between osteoblast and the underlying dura mater has not been well characterized, especially in humans. By combining dura mater cells and osteoblasts from humans (using a co-culture model) we hope to be able to better evaluate osteoblast behavior. 24
40 We plan to evaluate important mediators, some pro-osteogenic (FGF2) and other antiosteogenic (Noggin and BMP3) that may be regulated by dura mater paracrine signalling or may be imbalanced at the suture site affecting bone formation. Noggin and BMP3 have been shown to downregulate ossification at the suture site in order to maintain suture patency during the normal suture fusion process. Alternatively, it has been hypothesized that the excessive bone formation at the fused suture site may be due a defect in differentiation, function or both in fused suture osteoblasts, independent of humoral signalling from the surrounding microenvironment. A potential candidate to explain these defects is the transcriptional factor Runx2. Runx2 controls osteoblast differentiation and expression of proteins such as osteocalcin responsible for bone forming function. As such we plan to evaluate protein levels of Runx2 in fused and patent sutures. The strength of this research is based on the establishment of a human model of osteoblast cell culture harvested from patients affected by craniosynostosis. We also plan to develop a model that combines human osteoblasts with human dura mater cells, establishing a more physiological environment to evaluate osteoblast behavior. Using this model, we have the possibility to surpass the normal suture fusion animal model and search in more detail the mechanisms of craniosynostosis counting on a large number of human samples representing the wide spectrum of this condition. 25
41 Figure 4. Potential mechanisms of craniosynostosis Figure 4: The patency of the suture line depends on the balance between proosteogenic and anti-osteogenic signalling molecules. It is possible that an imbalance between the pro-osteogenic and anti-osteogenic signalling molecules is responsible for the development of craniosynostosis as seen in the panel on the right. 26
42 Hypothesis Regional variations in osteoblast function and cell signalling exist in calvaria of infants with craniosynostosis. SPECIFIC AIMS: I) To develop a reliable osteoblast cell culture from calvaria of infants undergoing surgery for craniosynostosis repair. Rationale: The use of osteoblast cells derived from cranial bones of patients with craniosynostosis may provide a valuable model for investigating the molecular and cellular abnormalities associated with this disorder. In order to develop a valid technique for osteoblast cell culture, the effects of differing media will be used to determine the optimal growth conditions. To validate our bone cell culture model and demonstrate the presence of osteoblasts, we will assess cellular proliferation, Runx2 expression, alkaline phosphatase, osteocalcin, collagen I expression and mineralization. To further confirm our findings of in vitro bone formation, ultrastructural analysis of the samples will be performed by Transmission Electron Microscopy. II) To assess regional variations in osteoblast behavior with and without dura mater cells in co-culture. Rationale: Although variations in osteoblast activity have been shown in murine models of normal suture fusion, no studies have been performed using human osteoblasts and dura mater cells in vitro from infants with craniosynostosis. Osteoblast behavior will be studied in 27
43 osteogenic monocultures and co-cultures with dura mater cells by MTT (proliferation rates), immunocytochemistry and Western Blot for Runx2 expression, alkaline phosphatase assay (differentiation), mineralization assay, and Transmission Electron Microscopy (TEM). Quantitative Real Time Polymerase Chain Reaction (qrt-pcr) will be performed to evaluate the DNA expression of alkaline phosphatase and osteocalcin to confirm the cellular level of differentiation. It is anticipated that these experiments will demonstrate regional variations in osteoblast proliferation and differentiation which will provide the basis for aim III. III) To investigate the role of anti-osteogenic signalling on human osteoblasts in vitro with and without dura mater cells in co-culture. Rationale: It has been demonstrated that Noggin and BMP3 are important signalling molecules in models of normal suture fusion but their role in craniosynostosis is unknown. Noggin and BMP3 both down-regulate bone formation around the suture promoting suture patency. These experiments (Western blot and immunohistochemistry) will investigate the expression of these molecules in patent and fused sutures in infants with craniosynostosis in order to determine if regional variations in expression exist. This findings may have significance with respect to understanding the pathophysiology of craniosynostosis in humans. IV) To investigate the role of dura mater paracrine signalling in the pathophysiology of craniosynostosis in humans. Rationale: Dura mater underlying the cranial suture complex is one of several sources of FGF2 and TGF-β1cytokines in vivo. However, it remains unclear whether growth factors 28
44 produced by dura mater are capable of down-regulating BMP3 and Noggin expression in osteoblasts and up-regulate Runx2 expression thereby influencing osteoblast behavior. The aim of these experiments is to determine if dura mater cells in co-culture influence osteoblasts through paracrine signalling through secretion of FGF2 and TGF-β cytokines. V) To assess the effects of exogenous administration of FGF2 on osteoblast function in vitro. Rationale: It has been demonstrated that FGF2 signalling is of central importance for premature cranial suture fusion and might be an important paracrine signalling factor from underlying dura mater to overlying calvarial osteoblasts. These experiments will investigate the effects of exogenous administration of FGF2 to our cell culture and compare with the effects produced by dura mater cells in a co-culture model. 29
45 Material and Methods 30
46 Tissue Sampling: Patient Population: Patients sequentially selected with syndromic and non-syndromic craniosynostosis (3 months - 3 years old) scheduled to undergo elective cranial vault reshaping for craniosynostosis at The Hospital for Sick Children between July 2008 and September 2010 were enrolled in this study. Informed consent was obtained. This research received REB approval. Patients with previous cranial vault surgery were excluded from the study. Bone samples: During surgery, bone samples (5x5mm) and periosteum (1x1cm) were obtained from fused and patent cranial sutures and non-suture bone, during the normal course of the procedure. The sex, age, side, suture site and type of craniosynostosis was to be registered. Tissue samples from fused suture, patent suture and adjacent non sutural bone were processed for routine histology to confirm the origin of each sample, confirming the patency or fusion of the suture line, and collagen I staining (See Appendix pg.120) 60. Dura Mater: Samples of dura mater (patients between 3 to 17 years old) were obtained from patients undergoing surgery for epilepsy and used to develop cell co-culture models. Aim I) To develop a reliable osteoblast cell culture from infants undergoing surgery for craniosynostosis repair. Bone and periosteal tissue samples were taken from the operating room, transported in ice in 50ml tubes containing αmem and 5x penicillin-streptomycin and processed immediately for cell culture. Samples were sequentially digested in a collagenase (Sigma/Aldrich C0130) mixture at 37º C for 20 min and centrifuged at 700 rpm for 8 minutes. The pellet was resuspended in αmem (Wisent Bio-Cat# ) containing 10-7 M 31
47 dexamethasone (Sigma/Aldrich Cat# D8893) and 15% FBS and plated in 75cm 2 tissue culture flasks (SARSTEDT Cat# ) (See Appendix pg.111). At subconfluence, cultures were trypsinized (0.05% Trypsin-EDTA-Wisent Bio Cat# ) and seeded into the tissue culture plates 24 wells/5000 cells per well and 96 wells/1000 cells per well (SARSTEDT cat# ) for analysis (See Appendix pg.112). Medium changes were done every 2-3 days. At 1 week after subculture, the medium was additionally supplemented with 1mM ß-glycophosphate (Sigma/Aldrich cat# G6251) and 50μg/ml Ascorbic acid (Sigma/Aldrich Cat# A2218) 64. Identification of cultured cells was performed by phase contrast microscopy. In order to validate our bone cell culture model and demonstrate the presence of osteoblasts, we assessed cellular proliferation, Runx2 expression, alkaline phosphatase staining, osteocalcin and collagen I expression and mineralization. To further confirm our findings of in vitro bone formation, ultrastructural analysis of the samples was performed by TEM. i) Proliferation Rates: To assess proliferation in cells derived from infants with craniosynotosis, the standard MTT assay (Sigma Ref. 5655) - (See Appendix pg.116) was employed at days 1,3,5 and 7 following subculture. This assay assesses mitochondrial dehydrogenase activity and can serve as an indirect measure of cellular proliferation 65. ii) Differentiation Rates: At the same time points, alkaline phosphatase activity was analyzed using the standard ρ-nitrophenil phosphate assay (pnp, Sigma Ref ) - (See Appendix pg.117). The final alkaline phosphatase activity was adjusted per protein content (µg) and time of assay incubation (h) 64. After 32
48 confluence in cell culture, imunnocytochemistry for Runx2 (abcan 54868) was performed (See Appendix pg.120) 58. iii) qrt-pcr: Osteoblast differentiation was also evaluated by gene expression of Osteocalcin, which represents the latest marker of osteoblast differentiation (Primers - Forward: GGCAGCGAGGTAGTGAAGAG and Reverse: CTGGAGAGGAGCAGAACTGG) and Alkaline Phosphatase (Primers Forward: CGTGGCTAAGAATGTCATCATTGTT and Reverse: TGGTGGAGCTGACCCTTGA) examined by real time PCR. HPRT was used as the housekeeping gene (See Appendix pg.124) 66. iv) IHC: Collagen I (Mouse monoclonal to Colagen I abcan 6308) expression was evaluated by Immunocytochemistry (See Appendix pg.120). v) Mineralization assay: Subsequently, cells were grown for 21 and 28 days following subculture and were analyzed for mineralized bone nodule formation via Alizarin Red S assay (Sigma Ref. A5533) - (See Appendix pg.119) 64. vi) Transmission Electron Microscopy: Mineralized nodules grown on coverslips were fixed in 2% glutaraldehyde and processed for Transmission Electron Microscopy to confirm bone formation and structure (See Appendix pg 119) 64. The effects of differing media composition on cell culture was assessed in order to optimize the culture settings for osteoblast growth and differentiation. Human osteogenic cells from patients (n=7) with craniosynostosis were cultured in αmem (Wisent Bio-Cat# ) containing 10-7 M dexamethasone (Sigma/Aldrich Cat# D8893), supplemented with 33
49 i) 1% FBS, ii) 10% FBS, iii) 15% FBS or iv) ascorbic acid and β-glycophosphate. Experimental culture conditions were compared on the basis of active cell growth (MTT reduction assay) and differentiation (AP assay). Aim II) To assess regional variations in osteoblast behavior with and without dura mater cells in co-culture. Osteoblasts obtained from regions of fused suture, patent suture and adjacent non sutural bone and periosteum were cultured in αmem containing 10-7 M dexamethasone, supplemented with 15% FBS for 7 days and then supplemented with ascorbic acid 50µg/1ml of medium and 1% β-glycophosphate. Proliferation rates, differentiation, including alkaline phosphatase, collagen I and osteocalcin and mineralization were assessed in all 3 regions. In order to determine if dura mater cells exerted any influence on osteoblast behavior, cocultures were established with dura mater samples from neurosurgical patients and proliferation rates, differentiation and mineralization were assessed. Results of proliferation were also stratified in syndromic and non-syndromic patients. Dura mater sample was taken from the operating room, transported in ice in 14ml tube containing αmem and 5x penicillin-streptomycin and processed immediately for cell culture. Samples were sequentially digested in a collagenase (Sigma/Aldrich C0130) mixture at 37º C for 20 min and centrifuged at 700 rpm for 8 minutes. The pellet was resuspended in αmem (Wisent Bio-Cat# ) containing 10-7 M dexamethasone (Sigma/Aldrich Cat# D8893) and 1% FBS and plated in 25cm 2 tissue culture flasks (SARSTEDT Cat# ) (See Appendix pg.111) 44. At subconfluence, cultures were trypsinized 34
50 (0.05% Trypsin-EDTA-Wisent Bio Cat# ) and seeded into the tissue culture plates 6 wells/50000 for analysis (See Appendix pg.112). Medium changes were done every 2-3 days. i) Co-Culture Model: We plated 5.0x10 4 first-passage osteoblasts per well in six-well tissue culture plates (SARSTEDT Cat# ) and 2.0x10 4 dura mater cells onto correspondenting co-culture filter inserts (VWR Cat# ). The inserts have a pore size of 0.4µm. Cells are cultured separately in standard medium until both cell populations were confluent. Dura mater cellseeded filter inserts are then combined with the six-well plates of osteoblasts and cultured in αmem containing 10-7 M dexamethasone, supplemented with 1% FBS for 10 days. The medium is changed every other day up to 10 days when cells and medium were collected for Western blot analysis. Osteoblasts cultured with empty co-culture inserts serve as a control 67. Aim III) To investigate the role of anti-osteogenic signalling on human osteoblasts in vitro with and without dura mater cells in co-culture. Tissue samples from fused suture, patent suture and adjacent non sutural bone were sent for immunohistochemical analysis of Noggin and BMP3 expression. These molecules were investigated in tissue samples to obtain true representation of tissue expression of Noggin/BMP3 in vivo without the influence of cell culture. Osteoblasts obtained from regions of fused suture, patent suture and adjacent non sutural bone, and periosteum were cultured as described in Aim II. In order to determine the influence of dura mater cells on Noggin and BMP3 expression, a co-culture was established and medium from osteoblasts 35
51 alone or in co-culture was collected at day 10. Protein expression was measured by Western Blot. i) Investigation of Noggin and BMP3 expression by immunohistochemistry Samples were demineralized with EDTA, fixed in 10% formalin, embedded in paraffin, microtomized (5µm) and stained with rabbit polyclonal antibody to Noggin (abcam) used in dilution 1:20 or rabbit polyclonal antibody to BMP3 (R&D System) used in dilution 1:5 to analyze their spatial expression patterns in fused and patent sutures and non-suture bones. Detection was performed with Goat Polymer (Biocaremedical) for BMP3 and ABC Ellite System (Vector) for Noggin and Collagen I. 3,3 Diaminobenzidine (DAB) was used as chromogen. Semi-quantitative analysis of the staining was carried out. The staining intensity in the extracellular matrix (Noggin and BMP3) was evaluated using semi-quantitative scoring system: no staining (0), low staining (1), intermediate staining (2), and strong staining (3). The results were evaluated by 2 independent investigators and averaged. ii) Detection of Noggin and BMP3 by Western Blot : Western blot analysis for Noggin (abcam 16054) and BMP3 (abcam71500) was carried out in osteoblasts alone and in co-culture with dura mater cells (See Appendix pg.121). β-actin was used as a positive control. Results were analyzed by densitometry and expressed as protein density
52 Aim IV) To investigate the role of dura mater paracrine signalling in the pathophysiology of craniosynostosis in humans. In order to search for potential growth factors responsible for the dura mater paracrine signalling, dura mater cells were grown for 10 days and conditioned medium was collected at different time points to determine the expression of FGF2 and TGF-β, both described in the literature as the primary growth factors secreted by dura mater that may influence osteoblast behavior. i) Detection of FGF2 and TGF-β by Western blot : Conditioned Medium from dura mater cells culture was collected at days 3,5 7 and 10 after subculture. Western blot analysis was carried out for FGF2 (ab57059) and TGF-β (ab27969) expression (See Appendix pg.121). β-actin was used as a positive control. Results were analyzed by densitometry and expressed as protein density 29. Aim V) To assess the effects of exogenous administration of FGF2 on osteoblast function in vitro. In Aim IV we examined the expression of endogenous FGF2 in dura-mater cells. The aim in these experiments was to evaluate osteoblast behavior under stimulation by exogenous human recombinant FGF2. Osteoblasts obtained from regions of fused suture, patent suture and adjacent non-sutural bone were cultured alone or with increasing doses of human recombinant FGF2. In order to evaluate osteoblast capacity to respond to stimulation, proliferation and differentation rates were assessed. i) Human Recombinant FGF2 Stimulation: Cells were plated onto 6 and 24-well plates at a density of and 5000 cells/well respectively. After overnight attachment, cells were treated with osteogenic differentiation media (αmem 37
53 containing 10-7 M dexamethasone, supplemented with 15% FBS, ascorbic acid 50µg/1ml of medium and 1% β-glycophosphate) supplemented with human recombinant FGF2 (5, 10, 50 and 100ng/ml) 58 or only osteogenic media as a control. Medium was changed every 2-3 days. FGF2 was added at each medium change. MTT and Alkaline Phosphatase were performed at days 1, 3, 5 and 7. Alkaline Phosphatase staining was performed at 1 week to assess early osteogenic differentiation. Statistical Analyses Statistical analyses were performed using Graph Pad Prism and data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was applied for comparison of sample groups in the MTT, Alkaline Phosphatase and Mineralization Assays, Western Blot density, Immunohistochemistry density and qrt-pcr. Differences in values between groups were evaluated using Tukey s test. Two-way analysis of variance (ANOVA) with repeated measurements was applied when more variables were evaluated for all three groups. Significance was established at p < Research Ethics Board: This research has been approved by the Research Ethical Board at The Hospital for Sick Children ( ). Consent was obtained from parents in all cases. 38
54 Results 39
55 Demographics Forty-five patients were enrolled in the study from July of 2008 to September of Twenty-eight were male and seventeen female. Mean age of the non-syndromic patients was 7.5 ± 2.5 months (range from 3 months old to 13 months old). Forty patients had single suture non-syndromic craniosynostosis. Samples were obtained from five syndromic patients (3 Apert s Syndrome, 1 Crouzon Syndrome and 1 patient with chromosome 7p deletion). The sagittal suture was the most frequently involved followed by the metopic suture (Table 2). All surgeries were performed uneventfully and there were no mortalities or significant morbidities. Cases n=45 Age (months) Male Female Sagittal Coronal Metopic Lambdoid Syndromic 12.6± n=5 Nonsyndromic n=40 7.5± Table 2: Demographics of patients with craniosynostosis enrolled in the study. Histology Histology was performed in tissue samples using hematoxylin-and-eosin staining to confirm the tissue architecture of the cranial site from where the samples were harvested. Histological evaluation of the patent, fused and adjacent bone confirmed the clinical observations of presence or absence of a suture. Hematoxylin- and eosin-stained sections of the control bone showed mature lamellar bone and hematopoietic marrow. Sections of patent sutures showed fibrous connective tissue flanked on both sides by calvarial bony plates. 40
56 Sections of fused sutures showed an absence of the fibrous connective tissue zone, which was replaced by lamellar bone. Some sites showed remnants of fibrous tissue in areas of partial osseous obliteration and areas of bone remodeling (Figure 5). Control Patent Suture Fused Suture LB HM FT B H&E x50 magnification DAPI LB FT B HM H&E x100 magnification Figure 5: Histological sections of control bone, patent and fused suture bone with H&E stain. The control bone showed lamellar bone (LB) and hematopoietic marrow (HM). The patent suture showed fibrous connective tissue (FT) flanked on both sides by calvarial bony plates (not seen) representing the normal patent suture. The fused suture showed the absence of the fibrous connective tissue zone, replaced by bone (B). 41
57 Collagen I Expression Collagen I is secreted by mature bone in the extracellular matrix. Collagen I expression in tissue samples from non-sutural adjacent bone, patent and fused sutures was assessed by IHC. Staining for Collagen I was positive for all three sites. The control bone showed areas of mature bone. The patent suture showed an intense staining at the fibrous connective tissue representing the normal patent suture. Stained cross-section of fused suture showed intense collagen I staining, with areas of mature bone and remodeling (Figure 6). Negative Control Control Bone 100x Patent Suture Fused Suture Figure 6: Immunohistochemistry staining for Collagen I. Stained sections show positive expression of collagen I in fused bone, patent bone and adjacent non-sutural bone, demonstrating formation of extracellular bone matrix (Magnification 100x). 42
58 Aim I) To develop a reliable osteoblast cell culture from calvarial of infants undergoing surgery for craniosynostosis repair. After culture of bone samples and periosteum, osteoblast growth was evaluated daily by phase contrast microscopy up to confluence (usually 5 to 7 days after culture). Bone samples and periosteum demonstrated the same potential for osteoblast retrival (4.5 x 10 6 versus 4.2 x 10 6 ) after 5 days. As such, osteoblasts from bone samples were preferred for all experiments due to the in vivo relation between dura mater and calvarial bones. Developing a valid bone cell culture model from calvaria of infants with craniosynostosis In order to demonstrate the presence of osteoblasts in our cell culture model, we assessed cellular proliferation, Runx2, alkaline phosphatase, collagen I and osteocalcin expression and mineralization. To further confirm our findings of in vitro bone formation, ultrastructural analysis of the samples was performed, demonstrating features of in vitro osteogenesis, including mineral depositions and collagen fibrils in the extracellular matrix. A ring pattern for electron diffraction studies was characteristic of normal hydroxyapatite (Figure 7). 43
59 44
60 Figure 7: Evidences of osteoblasts in our cell culture model and in vitro bone formation: Patent suture cells were able to proliferate in vitro (A) and differentiate in osteoblasts as shown by the expression of differentiation markers such as alkaline phosphatase, Runx2, collagen I and osteocalcin (B). Mineralization and Bone Formation was achieved after 28 days in osteogenic conditions. Ultrastructural analysis of the samples was performed, demonstrating features of in vitro osteogenesis, including mineral depositions and collagen fibrils in the extracellular matrix. A ring pattern for electron diffraction studies was characteristic of normal hydroxyapatite (C). Effect of medium composition on cellular proliferation Fused suture osteoblasts, independent of the experimental conditions or time-point, demonstrated higher growth rates than the patent and control sutures. The addition of ascorbic acid and β-glycerophosphate to the osteogenic medium resulted in significantly higher growth rates for the fused suture osteoblasts when compared αmem (1% FBS) and αmem (15% FBS) on day 3 (p<0.05) (Figure 8A). There was no significant differences in proliferation rates at days 5,7 and 10 independent of medium composition. 45
61 Figure 8: MTT assays of human cranial suture-derived osteoblasts. All cultures in 1%, 10%, 15% FBS or 15% FBS + ascorbic acid (50µg)/1ml of medium. Values are mean±sd; n=7, *p<0.05. Differences in FBS concentration did not significantly affect the growth of cranial suture-derived osteoblasts from fused suture, patent suture and adjacent bone. Moreover, there was no significant difference in alkaline phosphatase activity independent of the medium composition and therefore we chose for the model system the medium consisting of αmem containing 10-7 M dexamethasone, supplemented with 15% FBS for 7 days and then supplemented with ascorbic acid (50µg)/1ml of medium and 1% β-glycerophosphate for the experiments. 46
62 Aim II) To assess regional variations in osteoblast behavior with and without dura mater cells in co-culture. Osteoblast Proliferation Cells were grown until confluence and then were subcultured (Figure 9). Proliferation rates of control bone, patent suture and fused suture osteoblasts were evaluated in triplicate by MTT assay. Figure 9: Cellular growth prior and post-subculture. A) Cranial suture-derived culture prior to subculture three days after harvesting. Osteoblasts from fused sutures achieved confluence earlier than those from patent sutures or non-sutural adjacent bone. B) Cranial suture-derived subculture at day 3. 47
63 OD/1000 cells The osteoblasts from the fused sutures exhibited a significant (p < 0.01) increased rate of growth, compared with those derived from the control and patent suture at days 5 and 7 (Figure 10). All Cases * * * Control Bone Patent Suture Fused Suture 5 * p<0.02 n=33 per group 0 Day 1 Day 3 Day 5 Day 7 Figure 10: Osteoblast proliferation rates (MTT): Fused suture cells showed a significantly higher rate of proliferation at time-point 3 (p<0.05) when compared to control bone and time-points 5 (p<0.001) and 7 (p<0.001) when compared with control and patent suture osteoblasts at the same time-points. Proliferation rates were significantly lower for syndromic cases in all three groups when compared with non-syndromic patients (Figure 11). For this reason we decided to exclude samples from syndromic patients for the subsequent experiments in order to not confound the findings. 48
64 OD/1000 cells OD/1000 cells A) Non-Syndromic Cases * * * Control Bone Patent Suture Fused Suture 5 * p<0.02 n=28 per group 0 Day 1 Day 3 Day 5 Day 7 B) Syndromic Cases Control Bone Patent Suture Fused Suture 5 p=0.8 n=5 per group 0 Day 1 Day 3 Day 5 Day 7 Figure 11: Proliferation Rates for Syndromic Patients: Proliferation rates were significantly higher for non-syndromic patients (A) when compared with syndromic patients (B). Also the difference in proliferation rates between sites was not significant (p=0.8) in the syndromic patients. 49
65 OD/1000 cells OD/1000 cells Co-Culture of Osteoblasts and Dura Mater Cells: Proliferation Rates Osteoblasts from control bone, fused suture and patent suture co-cultured with dura mater were compared with their counterparts without dura mater cells (Figure 12). Adding dural cells to the osteoblast culture does not change significantly the proliferation rates for fused suture and control bone. However, patent suture osteoblasts in co-culture with dura demonstrated a significantly greater (p=0.001) proliferation rate at day 7 when compared with patent suture osteoblasts without dura. A) Control Bone Subculture without Dura Subculture with Dura p=0.27 (two-way ANOVA) n= 28 each group without dura n= 6 each group with dura 0 Day 1 Day 3 Day 5 Day 7 B) 15 Patent Suture * Subculture without Dura Subculture with Dura 10 5 p=0.001 (two-way ANOVA) n= 28 each group without dura n= 6 each group with dura 0 Day 1 Day 3 Day 5 Day 7 50
66 OD/1000 cells C) Fused Suture Subculture without Dura Subculture with Dura 5 p=0.36 (two-way ANOVA) n= 28 each group without dura n= 6 each group with dura 0 Day 1 Day 3 Day 5 Day 7 Figure 12: Osteoblast proliferation rates in co-culture with dura mater: Co-culture with dura mater cells does not change significantly (p>0.05) the proliferation rates for fused suture and control bone. Patent suture osteoblasts in co-culture with dura mater demonstrated a significantly greater (p=0.001) proliferation rate at day 7 when compared with patent suture osteoblasts without dura mater. Runx2 Expression Runx2 is a transcriptional factor that controls osteoblast differentiation through the regulation of osteogenic proteins, including osteocalcin and osteopontin. Immunocytochemical analysis of Runx2 showed expression of this molecule in all three groups, demonstrating that cells from adjacent bone, patent suture and fused suture are able to differentiate in osteoblasts (Figure 13). 51
67 Negative Control Control Bone Patent Suture Fused Suture Dapi 400x Runx-2 400x Figure 13: Expression of Runx2 demonstrated by Immunocytochemistry (nuclear staining). Control bone, patent suture and fused suture cells were similarly positive for Runx2, indicating that cells from all three groups were able to differentiate. However, Western blot analysis of Runx2 showed an up regulation of this molecule in the control group and fused suture group with and without dura mater when compared with patent suture. This difference was significantly (p<0.05) greater when osteoblasts 52
68 % Relative Control derived from fused suture were compared without and with co-culture with dura mater cells (Figure 14). Runx-2 57kDa ß-actin 43kDa SaOs2 C C+D P P+D F F+D Runx2 Expression * SaOs2: Human osteosarcoma cell line C: Control Bone C+D: Control Bone + Dural cells P: Patent Suture P+D: Patent Suture + Dural cells F: Fused Suture F+D: Fused Suture + Dural cells 50 0 Control Bone Control Bone + Dura Mater Patent Suture Patent Suture + Dura Mater Fused Suture Fused Suture + Dura Mater p=0.024 n=6 per group Figure 14: Expression of Runx2 by Western Blot. Runx2 showed up regulation in the control and fused suture group when compared with patent suture. Expression of Runx2 was significantly (p=0.024) increased when osteoblasts from the fused suture were combined in co-culture with dura mater cells compared with monoculture. 53
69 mmol of pnp/h/µg of protein Alkaline Phosphatase Activity Alkaline Phosphatase activity increased steadily for the first 3 days. Rapid progression was noticed at day 5 and 7 for all three groups. This finding, taken together with other markers, demonstrated that cells grown in culture differentiate into osteoblasts. The increased levels of AP are consistent with the increase in cell numbers. Osteoblasts from the fused sutures showed higher levels of AP expression compared to control and patent suture (Figure 15) Alkaline Phosphatase *** ** * Control Bone Patent Suture Fused Suture p<0.05 n=28 per group 0.0 Day 1 Day 3 Day 5 Day 7 Figure 15: Alkaline Phosphatase Assay: Results of AP measurement, taken together with other markers of differentiation, suggest that osteoblasts are present in culture. The 54
70 increased levels of AP are consistent with the increased number of cells at days 5 and 7. ( * p<0.05 control versus patent suture; ** p<0.05 fused suture versus patent suture and *** p<0.05 fused suture versus control and patent bone). Final AP concentration is expressed as mmol of pnp per hour per µg of protein. Figure 16: Alkaline Phosphatase Activity (Fast Blue BB salt - dark blue staining) in cell cultures: Osteoblasts derived from the fused suture demonstrated multiple layers and an increased number of cells at day 7. 55
71 Expression of mrna qrt-pcr 6 4 Control Bone Patent Suture Fused Suture 2 p=0.66 (AP) p=0.11 (Osteocalcin) n=6 per group 0 AP Osteocalcin Figure 17: Analysis by qrt-pcr of alkaline phosphatase and osteocalcin gene expression: Expression of alkaline phosphatase and osteocalcin mrna are not significantly different between osteoblasts from various regions of the infant calvarium. mrna levels were measured by qrt-pcr and normalized to HPRT mrna. 56
72 mmol of pnp/h/µg of protein mmol of pnp/h/µg of protein Co-Culture of Osteoblasts and Dura Mater Cells: Alkaline Phosphatase AP was expressed by control bone, patent suture and fused suture osteoblasts when combined with dura mater cells and expression increased with time (Figure 18). Adding dural cells to the control bone and patent suture osteoblasts enhanced their differentiation when compared with their culture without dura mater. A) Control Bone * * * Culture with Dura Culture without Dura p= (two-way ANOVA) n= 28 each group without dura n= 6 each group with dura 0.0 Day 1 Day 3 Day 5 Day 7 B) Patent Suture * Culture with Dura Culture without Dura * p=0.002 (two-way ANOVA) n= 28 each group without dura n= 6 each group with dura 0.0 Day 1 Day 3 Day 5 Day 7 57
73 mmol of pnp/h/µg of protein C) Fused Suture Culture with Dura Culture without Dura p<0.04 (two-way ANOVA) n= 28 each group without dura n= 6 each group with dura 0.0 Day 1 Day 3 Day 5 Day 7 Figure 18: AP in osteoblasts co-culture with dura mater cells: Osteoblasts cocultured with dura mater cells demonstrate increased AP expression over time. Co-culture with dura mater cells significantly enhanced control bone (A) differentiation at days 3,5 and 7 and patent suture osteoblasts (B) differentiation at days 3 and 7 compared with osteoblasts without dura mater and reduced differentiation of fused suture osteoblasts (C). Final AP concentration is expressed as mmol of pnp per hour per µg of protein. Mineralization In cultures containing β-glycerophosphate (10mM) and ascorbic acid (50µg/ml), the secretion of extracellular matrix leads to progressive mineralization and eventual bone nodule formation by day 14 (Figure 20). Osteoblasts from all three sites were capable of forming bone nodules in vitro, as evidenced by extensive Alizarin Red Staining. However, at 28 days osteoblasts from fused sutures demonstrated significantly (p<0.05) greater mineralization compared to osteoblasts from patent sutures (Figure 19). Bone nodules were detected earlier in osteoblasts from fused sutures (11±3 days) compared with control (13±4 days) and patent sutures (16±4 days) osteoblasts (Figure 20). Formation of bone 58
74 OD/1000 cells nodules was significantly (p<0.0001) more robust for osteoblasts from fused suture (65±9 mm 2 mean area ± SD) when compared with control (34±4 mm 2 ) and patent suture osteoblast (22±2 mm 2 ). Mineralization * ** Control Bone Patent Suture Fused Suture 2 1 *p<0.05 n=21 0 Day 21 Day 28 Figure 19: Bone Nodule Formation (Alizarin Red Assay). Osteoblasts from control bone demonstrated significantly (*p<0.01) greater mineralization when compared with patent suture at day 28. Osteoblasts from the fused suture demonstrated significantly (**p<0.05) greater mineralization when compared with control bone and patent suture osteoblasts at day 28 post subculture. 59
75 Figure 20: Bone Nodule Formation at days 14 and 18 (Alizarin Red S Staining): Formation of bone nodules in cultures derived from fused sutures was detected earlier (11±3 days) compared with that of control (13±4 days) and patent sutures (16±4 days). Arrow shows bone nodule formation. 60
76 100x 100x 100x Control 21 days Patent Suture 21days Fused Suture 21days 100x 100x 100x Control 28 days Patent Suture 28 days Fused Suture 28 days Figure 21: Mineralization at days 21 and 28 (Alizarin Red Staining). Formation of bone nodules in cultures derived from fused sutures was significantly (p<0.001) more robust, compared with that of normal sutures and control areas at day 28. Arrow shows bone nodule formation. 61
77 Co-Culture of Osteoblasts and Dura Mater Cells: Mineralization Mineralization rates in osteoblasts co-cultured with dura mater cells were not different between groups. The addition of dural cells to culture resulted in qualitative differences in all three groups with reduced nodule formation and mineralization (Figure 22). Figure 22: Bone nodule formation in osteogenic cultures with and without dural coculture at day 28 (Alizarin Red S Staining). Mineralization in osteoblasts co-cultured with dura mater cells was not different between groups. 62
78 Transmission Electron Microscopy Electron microscopy of cell cultures of control bone, patent suture and fused sutures revealed ultrastructural features of in vitro osteogenesis, including mineral depositions and collagen fibrils in the extracellular matrix (Figure 23). Figure 23: Transmission Electron Microscopy of Bone Nodule Formation: Sections showing ultrastructural features of in vitro osteogenesis, including collagen fibrils, mineral and osteoblasts. Cell cultures from control bone, patent suture and fused suture were able to form bone. Aim III) To investigate the role of anti-osteogenic signalling on human osteoblasts in vitro with and without dura mater cells in co-culture. 63
79 Expression of anti-osteogenic signalling molecules The anti-osteogenic molecules Noggin and BMP3 were analyzed in tissue samples from control bone, patent suture and fused suture by IHC. The expression of Noggin and BMP3 in the osteoblasts from patent suture was greater than expression levels in control bone and fusing suture (Figure 24). Figure 24: Immunohistochemistry analysis of Noggin in tissue samples. Noggin expression (expressed as a brown pigment in the extracellular matrix) was found to be greater in the osteoblasts from patent suture when compared to control bone or fused suture. 64
80 Semi-quantitative score 4 3 Noggin Expression * * p< n= Control Bone Patent Suture Fused Suture Figure 25: Quantification of Immunohistochemical analysis for Noggin in the tissue samples. Expression of Noggin was significantly (p<0.0001) greater in osteoblasts from patent sutures when compared with the control bone and fused suture. The staining intensity was evaluated using semi-quantitative scoring system: no staining (0), low staining (1), intermediate staining (2), and strong staining (3). The results were evaluated by 2 independent investigators and averaged.. 65
81 Negative Control Patent Suture Control Bone 200x Fused Suture 200x 200x 200x Figure 26: Immunohistochemistry analysis of BMP3 in tissue samples. BMP3 expression was greater in the extracellular matrix of patent sutures when compared to fused sutures. 66
82 Semi-quantitative score 4 BMP3 Expression * * 3 p< n=6 per group Control Bone Patent Suture Fused Suture Figure 27: Quantification of Immunohistochemical analysis for BMP3 in the tissue samples. BMP3 expression was significantly (p<0.0001) higher in patent sutures when compared with control and fused suture. The staining intensity was evaluated using semiquantitative scoring system. In order to further confirm these findings and to evaluate the influence of dura mater cells in co-culture on Noggin and BMP3 expression, Western Blot analysis was performed in control bone, patent suture and fused suture samples after cell culture. BMP3 expression (protein expression) was significantly up regulated in the patent suture when co-cultured with dura mater cells but not in control and fused suture osteoblasts (Figure 28A). Noggin expression was enhanced in all three groups when in co-culture with dura mater cells (Figure 67
83 % Relative Control 28B). The significant differences previously observed in the immunohistochemical analysis of calvarial tissue (figures 25 and 27) were not observed in the WB analysis of the three sites without dura mater. A) BMP-3 53kDa SaOs2 C C+D P P+D F F+D ß-actin 43kDa BMP-3 Expression * SaOs2: Human osteosarcoma cell line C: Control Bone C+D: Control Bone + Dural cells P: Patent Suture P+D: Patent Suture + Dural cells F: Fused Suture F+D: Fused Suture + Dural cells 50 0 Control Bone Control Bone + Dura Mater Patent Suture Patent Suture + Dura Mater Fused Suture Fused Suture + Dura Mater p< n=6 Mean SD
84 % Relative Control B) Noggin SaOs2 C C+D P P+D F F+D 26kDa ß-actin 43kDa Noggin Expression * * * SaOs2: Human osteosarcoma cell line C: Control Bone C+D: Control Bone + Dural cells P: Patent Suture P+D: Patent Suture + Dural cells F: Fused Suture F+D: Fused Suture + Dural cells 50 0 Control Bone Control Bone + Dura Mater Patent Suture Patent Suture + Dura Mater Fused Suture Fused Suture + Dura Mater p< n=6 Mean SD Figure 28: Western Blot analysis of BMP3 and Noggin after 10 days of co-culture in osteogenic medium. WB analysis of the osteogenic medium showed an increased in Noggin and BMP3 protein expression when control bone, patent suture and fused suture were compared. A) BMP3 expression was significantly (p<0.0001) up regulated only in patent suture osteoblasts co-cultured with dura mater cells. B) Noggin expression showed significantly (p<0.0001) increased expression in all three groups when osteoblasts were cocultured with dura mater cells compared with monocultures. Aim IV) To investigate the role of dura mater paracrine signalling in the pathophysiology of craniosynostosis in humans. 69
85 TGF- B1 Density FGF-2 Density Dura Mater Expression of FGF2 and TGF-β1 In order to understand the influence of dura mater cells on osteoblast behavior, we then searched for candidate molecules that may be responsible for dura mater paracrine signalling modifying osteoblast behavior. We chose FGF2 and TGF-β1 to examine. Western Blot of the conditioned medium obtained at days 3,5,7 and 10 from dural cultures demonstrate the presence of FGF2 and TGF-β1. TGF-β1 levels remained consistent throughout the study period whereas FGF2 levels declined after day 3 (Figure 29). Dura from 3 year old patient Dura from 11 years old patient TGF-ß1 44kDa FGF2 47.5kDa D3 D5 D7 D10 D3 D5 D7 D10 Actin 43KDa TGF- B1 Mean SD Day 3: Day 5: Day 7: Day 10: FGF2 Mean SD Day 3: Day 5: Day 7: Day 10: Day 3 Day 5 Day 7 Day 10 0 Day 3 Day 5 Day 7 Day 10 Figure 29: Expression of pro-osteogenic molecules in the conditioned medium from dura mater cells. TGF-ß1 did not show significant variation between days. FGF2 had a two-fold higher expression at day 3 when compared with the subsequent days. Western blot analyses by densitometry. 70
86 Aim V) To assess the effects of exogenous administration of FGF2 on osteoblast function in vitro. Recombinant Human FGF2 Stimulation FGF2 was expressed in higher concentration in the medium from dura mater cells when compared with TGF-β1. We then decided to add exogenous FGF2 to control bone, patent suture and fused suture osteoblast cell cultures to evaluate the ability of osteoblasts to respond to this stimulation. The addition of FGF2 to culture medium enhanced cellular proliferation at all 4 concentrations for the three groups in a dose dependent fashion. Osteoblasts from fused suture were more sensitive to lower doses of stimulation (5ng/ml p<0.05) at day 7 than the other two groups. 71
87 Proliferation No stimulation 5ng/ml 10ng/ml 50ng/ml 100ng/ml 200x Control Bone 200x Patent Bone 200x Fused Bone Figure 30: Proliferation of osteoblasts stimulated with FGF2. At 3 days of incubation, an increase in proliferation was observed for all three groups independent of the FGF2 concentration compared with non-stimulated osteoblasts shown in the photos of cell culture plates. 72
88 OD/1000 cells OD/1000 cells OD/1000 cells A) MTT-Control Bone * * * * * Day 1 Day 3 Day 5 Day 7 Control Bone 5 ng/ml 10ng/ml 50ng/ml 100ng/ml Co-culture with Dura Mater p<0.0001(two-way ANOVA) n=4 B) MTT-Patent Suture * * Patent Suture 5ng/ml 10ng/ml 50ng/ml 100ng/ml Co-culture with Dura Mater 0 Day 1 Day 3 Day 5 Day 7 p<0.0001(two-way ANOVA) n=4 C) MTT-Fused Suture * * * * * * * * Day 1 Day 3 Day 5 Day 7 Fused Suture 5ng/ml 10ng/ml 50ng/ml 100ng/ml Co-culture with Dura Mater p<0.0001(two-way ANOVA) n=4 Figure 31: Proliferation Rates after Stimulation with FGF2 (MTT): Stimulation with recombinant human FGF2 significantly (p<0.0001) increases the rate of proliferation 73
89 for all three groups (in a dose dependent manner) compared with osteoblasts without stimulation. A) In the control bone group proliferation is significantly greater with 50ng/ml (p<0.05) at days 5 and 7; 100ng/ml (p<0.01) at days 5 and in co-culture with dura mater cells at days 5 (p<0.001) and 7 (p<0.01). B) In the patent suture group proliferation is significantly greater in co-culture with dura mater cells at day 5 (p<0.05) and 7 (p<0.001) C) In the fused suture group proliferation is significantly greater with 5ng/ml at day 7(p<0.05); 50 ng/ml at days 3(p<0.05),5 (p<0.01)and 7(p<0.05) and with 100ng/ml at days 5 and 7(p<0.0) and in co-culture with dura mater cells at days 5 and 7 (p<0.001). 74
90 Differentiation Osteoblasts cultured with exogenous FGF2 demonstrated an increase in alkaline phosphatase expression in all three groups at days 3,5 and 7. However the differences between groups were not significant. No stimulation 5ng/ml 10ng/ml 50ng/ml 100ng/ml 200x Control Bone 200x Patent Bone Fused Bone 200x Figure 32: AP expression after stimulation with FGF2. At 3 days of incubation, increased alkaline phosphatase staining is seen in all three groups treated with FGF2 independent of the FGF2 concentration. 75
Interactions of the endocrine system, bone and oral health
 Interactions of the endocrine system, bone and oral health All bones are not equal! Dense high proportion of cortical bone High proportion of trabecular bone Mandible Functions: mastication, respiration,
Interactions of the endocrine system, bone and oral health All bones are not equal! Dense high proportion of cortical bone High proportion of trabecular bone Mandible Functions: mastication, respiration,
What is Craniosynostosis?
 What is Craniosynostosis? Craniosynostosis is defined as the premature closure of the cranial sutures (what some people refer to as soft spots). This results in restricted and abnormal growth of the head.
What is Craniosynostosis? Craniosynostosis is defined as the premature closure of the cranial sutures (what some people refer to as soft spots). This results in restricted and abnormal growth of the head.
Lecture 3: Skeletogenesis and diseases
 Jilin University School of Stomatology Skeletogenesis Lecture 3: Skeletogenesis and diseases Aug. 21, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Bone Development Mouse embryo, E14.5 Mouse embryo, E18.0
Jilin University School of Stomatology Skeletogenesis Lecture 3: Skeletogenesis and diseases Aug. 21, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Bone Development Mouse embryo, E14.5 Mouse embryo, E18.0
Department of Neurosurgery. Differentiating Craniosynostosis from Positional Plagiocephaly
 Department of Neurosurgery Differentiating Craniosynostosis from Positional Plagiocephaly The number of infants with head shape deformities has risen over the past several years, likely due to increased
Department of Neurosurgery Differentiating Craniosynostosis from Positional Plagiocephaly The number of infants with head shape deformities has risen over the past several years, likely due to increased
T HERE is an unusual and interesting variety of craniosynostosis in
 SURGICAL TREATMENT OF CONGENITAL ANOMALIES OF THE CORONAL AND METOPIC SUTURES TECHNICAL NOTE DONALD D. MATSON, M.D. Neurosurgical Service, The Children's Medical Center, and Deparlment of Surgery, Itarvard
SURGICAL TREATMENT OF CONGENITAL ANOMALIES OF THE CORONAL AND METOPIC SUTURES TECHNICAL NOTE DONALD D. MATSON, M.D. Neurosurgical Service, The Children's Medical Center, and Deparlment of Surgery, Itarvard
Craniosynostosis. Diagnosis and Treatment
 Craniosynostosis Diagnosis and Treatment 2015 For more information about the Weill Cornell Craniofacial Program ABOUT The Weill Cornell Craniofacial Program takes a multidisciplinary approach to treating
Craniosynostosis Diagnosis and Treatment 2015 For more information about the Weill Cornell Craniofacial Program ABOUT The Weill Cornell Craniofacial Program takes a multidisciplinary approach to treating
Coding For Craniosynostosis. Peggy Feeley RHIA, CCS, CCS-P, COC AHIMA Approved ICD-10-CM/PCS Trainer
 Coding For Craniosynostosis Peggy Feeley RHIA, CCS, CCS-P, COC AHIMA Approved ICD-10-CM/PCS Trainer Cranial sagittal Synostosis Cranium job is to protect the brain The top portion of the skull, which protects
Coding For Craniosynostosis Peggy Feeley RHIA, CCS, CCS-P, COC AHIMA Approved ICD-10-CM/PCS Trainer Cranial sagittal Synostosis Cranium job is to protect the brain The top portion of the skull, which protects
Craniosynostosis and Plagiocephaly
 Craniosynostosis and Plagiocephaly Andrew Jea MD MHA FAAP Professor and Chief Section of Pediatric Neurosurgery Riley Hospital for Children Department of Neurosurgery Indiana University School of Medicine
Craniosynostosis and Plagiocephaly Andrew Jea MD MHA FAAP Professor and Chief Section of Pediatric Neurosurgery Riley Hospital for Children Department of Neurosurgery Indiana University School of Medicine
International Journal of Current Research and Academic Review ISSN: Volume 3 Number 1 (January-2015) pp
 International Journal of Current Research and Academic Review ISSN: 47 Volume Number (January) pp. 66 www.ijcrar.com Clinical Profile of Patients with Craniosynostosis: A Descriptive Study Nagaraj V. Gadwal*
International Journal of Current Research and Academic Review ISSN: 47 Volume Number (January) pp. 66 www.ijcrar.com Clinical Profile of Patients with Craniosynostosis: A Descriptive Study Nagaraj V. Gadwal*
NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM
 LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway
 Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway Jieyuan Zhang, Xiaolin Liu, Haiyan Li, Chunyuan Chen, Bin Hu, Xin Niu, Qing
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway Jieyuan Zhang, Xiaolin Liu, Haiyan Li, Chunyuan Chen, Bin Hu, Xin Niu, Qing
Skeletal System. Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section
 Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
pressure 6-7 and cranial growth restriction.
 Josephine Jung graduated from Medical School in Berlin in 2014. After completing her M.D. Thesis in Berlin she moved to London and is working as a Clinical Research Fellow in Neurosurgery at King s College
Josephine Jung graduated from Medical School in Berlin in 2014. After completing her M.D. Thesis in Berlin she moved to London and is working as a Clinical Research Fellow in Neurosurgery at King s College
CASE REPORT Pan-Suture Synostosis After Posterior Vault Distraction
 CASE REPORT Pan-Suture Synostosis After Posterior Vault Distraction Katrina F. Chu, BA, a Stephen R. Sullivan, MD, MPH, a,b and Helena O. Taylor, MD, PhD a,b a Warren Alpert Medical School of Brown University;
CASE REPORT Pan-Suture Synostosis After Posterior Vault Distraction Katrina F. Chu, BA, a Stephen R. Sullivan, MD, MPH, a,b and Helena O. Taylor, MD, PhD a,b a Warren Alpert Medical School of Brown University;
Prevention Diagnosis
 Prevention and Management of Positional Skull Deformities in Infants John Persing, MD, Hector James, MD, Jack Swanson, MD, John Kattwinkel, MD, Committee on Practice and Ambulatory Medicine, Section on
Prevention and Management of Positional Skull Deformities in Infants John Persing, MD, Hector James, MD, Jack Swanson, MD, John Kattwinkel, MD, Committee on Practice and Ambulatory Medicine, Section on
Mac Med Review 2016; 70(2): Case report
 Mac Med Review 2016; 70(2): 99-103 99 DOI:10.1515/mmr-2016-0019 Case report CARPENTER SYNDROME CASE REPORT AND TREATMENT Vladimir Mirchevski 1, Elizabeta Zogovska 2, Aleksandar Chaparoski 1, Venko Filipce
Mac Med Review 2016; 70(2): 99-103 99 DOI:10.1515/mmr-2016-0019 Case report CARPENTER SYNDROME CASE REPORT AND TREATMENT Vladimir Mirchevski 1, Elizabeta Zogovska 2, Aleksandar Chaparoski 1, Venko Filipce
FORMATION OF BONE. Intramembranous Ossification. Bone-Lec-10-Prof.Dr.Adnan Albideri
 FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
4.3 Surgical Management of anterior skull synostosis
 ISPN course 23 rd Nov, 2015 Cranial & Craniofacial disorders 4.3 Surgical Management of anterior skull synostosis Kazuaki Shimoji, Masakazu Miyajima and Hajime Arai Department of Neurosurgery, Juntendo
ISPN course 23 rd Nov, 2015 Cranial & Craniofacial disorders 4.3 Surgical Management of anterior skull synostosis Kazuaki Shimoji, Masakazu Miyajima and Hajime Arai Department of Neurosurgery, Juntendo
Sample page. Neurosurgery/Neurology. A comprehensive illustrated guide to coding and reimbursement CODING COMPANION
 CODING COMPNION 2018 Neurosurgery/Neurology comprehensive illustrated guide to coding and reimbursement POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com.
CODING COMPNION 2018 Neurosurgery/Neurology comprehensive illustrated guide to coding and reimbursement POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com.
Role of oocyte-secreted factors in prevention of cumulus cell apoptosis and enhancement of oocyte developmental competence
 Role of oocyte-secreted factors in prevention of cumulus cell apoptosis and enhancement of oocyte developmental competence Tamer Hussein, MScMed Research Centre for Reproductive Health, Discipline of Obstetrics
Role of oocyte-secreted factors in prevention of cumulus cell apoptosis and enhancement of oocyte developmental competence Tamer Hussein, MScMed Research Centre for Reproductive Health, Discipline of Obstetrics
Multidisciplinary care of craniosynostosis
 Journal of Multidisciplinary Healthcare open access to scientific and medical research Open Access Full Text Article Multidisciplinary care of craniosynostosis Review edward P Buchanan 1 Yunfeng Xue 1
Journal of Multidisciplinary Healthcare open access to scientific and medical research Open Access Full Text Article Multidisciplinary care of craniosynostosis Review edward P Buchanan 1 Yunfeng Xue 1
For more information about how to cite these materials visit
 Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
intracranial anomalies
 Chapter 5: Fetal Central Nervous System 84 intracranial anomalies Hydrocephaly Dilatation of ventricular system secondary to an increase in the amount of CSF. Effects of hydrocephalus include flattening
Chapter 5: Fetal Central Nervous System 84 intracranial anomalies Hydrocephaly Dilatation of ventricular system secondary to an increase in the amount of CSF. Effects of hydrocephalus include flattening
Occipital flattening in the infant skull
 Occipital flattening in the infant skull Kant Y. Lin, M.D., Richard S. Polin, M.D., Thomas Gampper, M.D., and John A. Jane, M.D., Ph.D. Departments of Plastic Surgery and Neurological Surgery, University
Occipital flattening in the infant skull Kant Y. Lin, M.D., Richard S. Polin, M.D., Thomas Gampper, M.D., and John A. Jane, M.D., Ph.D. Departments of Plastic Surgery and Neurological Surgery, University
DOI: /01.PRS
 CME Management of Craniosynostosis Jayesh Panchal, M.D., M.B.A., and Venus Uttchin, B.A. Oklahoma City, Okla. Learning Objectives: After studying this article, the participant should be able to: 1. Review
CME Management of Craniosynostosis Jayesh Panchal, M.D., M.B.A., and Venus Uttchin, B.A. Oklahoma City, Okla. Learning Objectives: After studying this article, the participant should be able to: 1. Review
Chapter 7: Head & Neck
 Chapter 7: Head & Neck Osteology I. Overview A. Skull The cranium is composed of irregularly shaped bones that are fused together at unique joints called sutures The skull provides durable protection from
Chapter 7: Head & Neck Osteology I. Overview A. Skull The cranium is composed of irregularly shaped bones that are fused together at unique joints called sutures The skull provides durable protection from
Craniosynostosis. chapter. Jeffrey Weinzweig, MD / Stephen B. Baker, MD, DDS / Mitchel Seruya, MD AQ1
 chapter 16 AQ1 Craniosynostosis Jeffrey Weinzweig, MD / Stephen B. Baker, MD, DDS / Mitchel Seruya, MD AQ2 PATIENT EVALUATION AND SELECTION Craniosynostosis is defined as the premature closure of a cranial
chapter 16 AQ1 Craniosynostosis Jeffrey Weinzweig, MD / Stephen B. Baker, MD, DDS / Mitchel Seruya, MD AQ2 PATIENT EVALUATION AND SELECTION Craniosynostosis is defined as the premature closure of a cranial
Research Article Human Anatomy Case Report Bathrocephaly: a case report of a head shape associated with a persistent mendosal suture
 IJAE Vol. 119, n. 3: 263-267, 2014 ITALIAN JOURNAL OF ANATOMY AND EMBRYOLOGY Research Article Human Anatomy Case Report Bathrocephaly: a case report of a head shape associated with a persistent mendosal
IJAE Vol. 119, n. 3: 263-267, 2014 ITALIAN JOURNAL OF ANATOMY AND EMBRYOLOGY Research Article Human Anatomy Case Report Bathrocephaly: a case report of a head shape associated with a persistent mendosal
a guide to understanding craniosynostosis a publication of children s craniofacial association
 a guide to understanding craniosynostosis a publication of children s craniofacial association 1 a guide to understanding craniosynostosis this parent s guide to craniosynostosis is designed to answer
a guide to understanding craniosynostosis a publication of children s craniofacial association 1 a guide to understanding craniosynostosis this parent s guide to craniosynostosis is designed to answer
Ossification and Bone Remodeling
 Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Regulation of the skeletal mass through the life span
 Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
SKULL AS A WHOLE + ANTERIOR CRANIAL FOSSA
 SKULL AS A WHOLE + ANTERIOR CRANIAL FOSSA LEARNING OBJECTIVES At the end of this lecture, the student should be able to know: Parts of skeleton (axial and appendicular) Parts of skull Sutures of skull
SKULL AS A WHOLE + ANTERIOR CRANIAL FOSSA LEARNING OBJECTIVES At the end of this lecture, the student should be able to know: Parts of skeleton (axial and appendicular) Parts of skull Sutures of skull
Lecture 2: Skeletogenesis
 Jilin University School of Stomatology Skeletogenesis Lecture 2: Skeletogenesis Aug. 18, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Student will describe Development of Bone - the general anatomy of bone
Jilin University School of Stomatology Skeletogenesis Lecture 2: Skeletogenesis Aug. 18, 2015 Yuji Mishina, Ph.D. mishina@umich.edu Student will describe Development of Bone - the general anatomy of bone
Pathological condition that results from premature fusion of one or more sutures in the cranial vault;
 Pathological condition that results from premature fusion of one or more sutures in the cranial vault; Associated with a deformity of the vault and cranial base. Development Bones of the cranium The skull
Pathological condition that results from premature fusion of one or more sutures in the cranial vault; Associated with a deformity of the vault and cranial base. Development Bones of the cranium The skull
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
DISEASES WITH ABNORMAL MATRIX
 DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
Development and Growth of the Normal Cranial Vault : An Embryologic Review
 www.jkns.or.kr http://dx.doi.org/10.3340/jkns.2016.59.3.192 J Korean Neurosurg Soc 59 (3) : 192-196, 2016 Print ISSN 2005-3711 On-line ISSN 1598-7876 Copyright 2016 The Korean Neurosurgical Society Pediatric
www.jkns.or.kr http://dx.doi.org/10.3340/jkns.2016.59.3.192 J Korean Neurosurg Soc 59 (3) : 192-196, 2016 Print ISSN 2005-3711 On-line ISSN 1598-7876 Copyright 2016 The Korean Neurosurgical Society Pediatric
SKELETAL TISSUES CHAPTER 7 INTRODUCTION TO THE SKELETAL SYSTEM TYPES OF BONES
 SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
CHAPTER 6 LECTURE OUTLINE
 CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
Peggers Super Summaries Basic Sciences Bone
 Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
OPERATIVE CORRECTION BY OSTEOTOMY OF RECESSED MALAR MAXILLARY COMPOUND IN A CASE OF OXYCEPHALY
 OPERATIVE CORRECTION BY OSTEOTOMY OF RECESSED MALAR MAXILLARY COMPOUND IN A CASE OF OXYCEPHALY By Sir HAROLD GILLIES, C.B.E., F.R.C.S., and STEWART H. HARRISON, F.R.C.S., L.D.S., R.C.S. From the Plastic
OPERATIVE CORRECTION BY OSTEOTOMY OF RECESSED MALAR MAXILLARY COMPOUND IN A CASE OF OXYCEPHALY By Sir HAROLD GILLIES, C.B.E., F.R.C.S., and STEWART H. HARRISON, F.R.C.S., L.D.S., R.C.S. From the Plastic
4.1 Classification of Craniosynostosis: Therapeutical implications.
 ISPN course 23 rd Nov, 2015 Cranial & Craniofacial disorders 4.1 Classification of Craniosynostosis: Therapeutical implications. Kazuaki Shimoji, Masakazu Miyajima and Hajime Arai Department of Neurosurgery,
ISPN course 23 rd Nov, 2015 Cranial & Craniofacial disorders 4.1 Classification of Craniosynostosis: Therapeutical implications. Kazuaki Shimoji, Masakazu Miyajima and Hajime Arai Department of Neurosurgery,
JBI Database of Systematic Reviews & Implementation Reports 2013;11(11) 54-63
 Risk factors for developing recurrent raised intracranial pressure post cranial vault remodelling in nonsyndromic craniosynostosis in children: a systematic review protocol Xenia Doorenbosch MBBS 1,2 Jared
Risk factors for developing recurrent raised intracranial pressure post cranial vault remodelling in nonsyndromic craniosynostosis in children: a systematic review protocol Xenia Doorenbosch MBBS 1,2 Jared
Research Article Cytological Evaluation of Hyaluronic Acid on Wound Healing Following Extraction
 Cronicon OPEN ACCESS DENTAL SCIENCE Research Article Cytological Evaluation of Hyaluronic Acid on Wound Healing Following Extraction Gocmen Gokhan 1 *, Gonul O 1, Oktay NS 2, Pisiriciler R 2 and Goker
Cronicon OPEN ACCESS DENTAL SCIENCE Research Article Cytological Evaluation of Hyaluronic Acid on Wound Healing Following Extraction Gocmen Gokhan 1 *, Gonul O 1, Oktay NS 2, Pisiriciler R 2 and Goker
SLLF FOR TMJ CASES IN ADULT DENTITION SEVERE BRACHIFA BRACHIF FACIAL
 SLLF FOR TMJ CASES IN ADULT DENTITION SEVERE BRACHIFAFACIAL TMJ: Severe Postural Imbalance+Severe Myofascial Pain Syndrome, severe soreness Temporalis Tendon RL, Sternocleidomastoideus RL Age:39 years
SLLF FOR TMJ CASES IN ADULT DENTITION SEVERE BRACHIFAFACIAL TMJ: Severe Postural Imbalance+Severe Myofascial Pain Syndrome, severe soreness Temporalis Tendon RL, Sternocleidomastoideus RL Age:39 years
Pathology of Coronary Artery Disease
 Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
Osteology. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS
 ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS AMBRISH KAUSHAL, MALA KAMBOJ Department of Oral and Maxillofacial Pathology Career Post Graduate Institute of Dental Sciences and Hospital
ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS AMBRISH KAUSHAL, MALA KAMBOJ Department of Oral and Maxillofacial Pathology Career Post Graduate Institute of Dental Sciences and Hospital
Rama Nada. - Mousa Al-Abbadi. 1 P a g e
 - 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
- 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy
 An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy Housekeeping: I have no financial disclosures Learning objectives: Develop an understanding of bronchopulmonary dysplasia (BPD)
An Overview of Bronchopulmonary Dysplasia and Chronic Lung Disease in Infancy Housekeeping: I have no financial disclosures Learning objectives: Develop an understanding of bronchopulmonary dysplasia (BPD)
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT POLICY GUIDELINES. Page: 1 of 5. Medical Policy Title CRANIAL ORTHOTICS Policy Number 1.01.
 Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title CRANIAL ORTHOTICS Policy Number 1.01.32 Category Equipment/Supplies Effective Date 10/18/01 Revised Date 06/27/02, 07/24/03, 06/24/04,
Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title CRANIAL ORTHOTICS Policy Number 1.01.32 Category Equipment/Supplies Effective Date 10/18/01 Revised Date 06/27/02, 07/24/03, 06/24/04,
Generation of post-germinal centre myeloma plasma B cell.
 Generation of post-germinal centre myeloma. DNA DAMAGE CXCR4 Homing to Lytic lesion activation CD38 CD138 CD56 Phenotypic markers Naive Secondary lymphoid organ Multiple myeloma is a malignancy of s caused
Generation of post-germinal centre myeloma. DNA DAMAGE CXCR4 Homing to Lytic lesion activation CD38 CD138 CD56 Phenotypic markers Naive Secondary lymphoid organ Multiple myeloma is a malignancy of s caused
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Cranial Base Changes Following. Surgical Treatment of Craniosynostosis. JEFFREY L. MARSH, M.D. MicHaAeL W. VaAnniEer, M.D.
 Cranial Base Changes Following Surgical Treatment of Craniosynostosis JEFFREY L. MARSH, M.D. MicHaAeL W. VaAnniEer, M.D. Three-dimensional osseous surface images from CT scans have been used to study the
Cranial Base Changes Following Surgical Treatment of Craniosynostosis JEFFREY L. MARSH, M.D. MicHaAeL W. VaAnniEer, M.D. Three-dimensional osseous surface images from CT scans have been used to study the
Tissue repair. (3&4 of 4)
 Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Development of the skull
 Development of the skull Ágnes Nemeskéri 2017 Semmelweis University Budapest Department of Anatomy, Histology and Embryology Clinical Anatomy Research Laboratory nemeskeri.agnes@med.semmelweis-univ.hu
Development of the skull Ágnes Nemeskéri 2017 Semmelweis University Budapest Department of Anatomy, Histology and Embryology Clinical Anatomy Research Laboratory nemeskeri.agnes@med.semmelweis-univ.hu
Skeletal Development Multiple Cellular Origins. Intramembranous Bone. Endochondrial Bone. Cartilage template of the limb in the Chick wing
 Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
PhD THESIS Epigenetic mechanisms involved in stem cell differentiation
 Romanian Academy Institute of Cellular Biology and Pathology "Nicolae Simionescu" PhD THESIS Epigenetic mechanisms involved in stem cell differentiation Coordinator: Acad. Maya Simionescu PhD Student:
Romanian Academy Institute of Cellular Biology and Pathology "Nicolae Simionescu" PhD THESIS Epigenetic mechanisms involved in stem cell differentiation Coordinator: Acad. Maya Simionescu PhD Student:
Craniosynostosis - making the head fit the hat
 Craniosynostosis - making the head fit the hat Poster No.: R-0173 Congress: 2014 CSM Type: Scientific Exhibit Authors: S. Constantine, B. Clark; NORTH ADELAIDE/AU Keywords: Head and neck, Bones, Pediatric,
Craniosynostosis - making the head fit the hat Poster No.: R-0173 Congress: 2014 CSM Type: Scientific Exhibit Authors: S. Constantine, B. Clark; NORTH ADELAIDE/AU Keywords: Head and neck, Bones, Pediatric,
Chapter 6: Skeletal System: Bones and Bone Tissue
 Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Interesting Case Series. The Danger of Posterior Plagiocephaly
 Interesting Case Series The Danger of Posterior Plagiocephaly Susan Orra, BA, a,b Kashyap Komarraju Tadisina, BS, a Bahar Bassiri Gharb, MD, PhD, a Antonio Rampazzo, MD, PhD, a Gaby Doumit, MD, a and Francis
Interesting Case Series The Danger of Posterior Plagiocephaly Susan Orra, BA, a,b Kashyap Komarraju Tadisina, BS, a Bahar Bassiri Gharb, MD, PhD, a Antonio Rampazzo, MD, PhD, a Gaby Doumit, MD, a and Francis
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen. Yusuke Murasawa, Pi-chao Wang
 Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
BONE TISSUE. Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology
 BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
Bone. Development. Tim Arnett. University College London. Department of Anatomy and Developmental Biology
 Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
BARROW VOLUME 23 NUMBER CRANIOFACIAL SURGERY. Copyright 2006, Barrow
 BARROW Q U A R T E R L Y VOLUME 23 NUMBER 2 2007 BARROW NEUROLOGICAL INSTITUTE OF ST. JOSEPH S HOSPITAL AND MEDICAL CENTER PHOENIX, ARIZONA Copyright 2006, Barrow CRANIOFACIAL SURGERY Copyright 2007, Barrow
BARROW Q U A R T E R L Y VOLUME 23 NUMBER 2 2007 BARROW NEUROLOGICAL INSTITUTE OF ST. JOSEPH S HOSPITAL AND MEDICAL CENTER PHOENIX, ARIZONA Copyright 2006, Barrow CRANIOFACIAL SURGERY Copyright 2007, Barrow
Biology. Dr. Khalida Ibrahim
 Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair
 Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Emerging Surgical Technologies: Open vs. Endoscopic Craniosynostosis Repair
 Emerging Surgical Technologies: Open vs. Endoscopic Craniosynostosis Repair Petra M. Meier, MD, DEAA Senior Associate of the Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children
Emerging Surgical Technologies: Open vs. Endoscopic Craniosynostosis Repair Petra M. Meier, MD, DEAA Senior Associate of the Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children
Subject Index. AXIN2, cleft defects 24, 26
 Subject Index ADAMTS, mouse mutants and palate development 37, 38 Africa, cleft lip and palate prevalence 6, 7 Alcohol dependence, pregnancy risks for cleft 25, 61 Altitude, pregnancy risks for cleft 25,
Subject Index ADAMTS, mouse mutants and palate development 37, 38 Africa, cleft lip and palate prevalence 6, 7 Alcohol dependence, pregnancy risks for cleft 25, 61 Altitude, pregnancy risks for cleft 25,
Early neurosurgical repair in eraniofacial dysmorphism
 J Neurosurg 51:796-803, 1979 Early neurosurgical repair in eraniofacial dysmorphism HAROLD J. HOrrMAN, M.D., F.R.C.S.(C)., AND E. BRUCE HENDRICK, M.D., F.R.C.S.(C). Division of Neurosurgery, University
J Neurosurg 51:796-803, 1979 Early neurosurgical repair in eraniofacial dysmorphism HAROLD J. HOrrMAN, M.D., F.R.C.S.(C)., AND E. BRUCE HENDRICK, M.D., F.R.C.S.(C). Division of Neurosurgery, University
Functions of the Skeletal System. Chapter 6: Osseous Tissue and Bone Structure. Classification of Bones. Bone Shapes
 Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Surgery for craniosynostosis has evolved rapidly
 Surgical Treatment of Craniosynostosis: Outcome Analysis of 250 Consecutive Patients Gerald M. Sloan, MD*; Karin C. Wells, MD ; Corey Raffel, MD, PhD ; and J. Gordon McComb, MD ABSTRACT. Objective. Surgery
Surgical Treatment of Craniosynostosis: Outcome Analysis of 250 Consecutive Patients Gerald M. Sloan, MD*; Karin C. Wells, MD ; Corey Raffel, MD, PhD ; and J. Gordon McComb, MD ABSTRACT. Objective. Surgery
Dr. Heba Kalbouneh. Saba Alfayoumi. Heba Kalbouneh
 11 Dr. Heba Kalbouneh Saba Alfayoumi Heba Kalbouneh 2- Bone Bone tissue is also classified into primary bone and secondary bone. In the beginning, the first bone that is deposited by the osteoblasts is
11 Dr. Heba Kalbouneh Saba Alfayoumi Heba Kalbouneh 2- Bone Bone tissue is also classified into primary bone and secondary bone. In the beginning, the first bone that is deposited by the osteoblasts is
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
What is Hemifacial Microsomia? By Pravin K. Patel, MD and Bruce S. Bauer, MD Children s Memorial Hospital, Chicago, IL
 What is Hemifacial Microsomia? By Pravin K. Patel, MD and Bruce S. Bauer, MD Children s Memorial Hospital, Chicago, IL 773-880-4094 Early in the child s embryonic development the structures destined to
What is Hemifacial Microsomia? By Pravin K. Patel, MD and Bruce S. Bauer, MD Children s Memorial Hospital, Chicago, IL 773-880-4094 Early in the child s embryonic development the structures destined to
Središnja medicinska knjižnica.
 Središnja medicinska knjižnica Pećina, M., Vukičević, S. (2007) Biological aspects of bone, cartilage and tendon regeneration. International Orthopaedics, 31 (6). pp. 719-720. The original publication
Središnja medicinska knjižnica Pećina, M., Vukičević, S. (2007) Biological aspects of bone, cartilage and tendon regeneration. International Orthopaedics, 31 (6). pp. 719-720. The original publication
SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over 3 Weeks. A SEPARATE WORKSHEET WILL BE PROVIDED.
 BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
Cellular Physiology and Biochemistry
 Original Paper 2015 The Author(s). 2015 Published The Author(s) by S. Karger AG, Basel Published online: November 27, 2015 www.karger.com/cpb Published by S. Karger AG, Basel 2194 1421-9778/15/0376-2194$39.50/0
Original Paper 2015 The Author(s). 2015 Published The Author(s) by S. Karger AG, Basel Published online: November 27, 2015 www.karger.com/cpb Published by S. Karger AG, Basel 2194 1421-9778/15/0376-2194$39.50/0
Skeletal Tissues. Skeletal tissues. Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs.
 Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
Human, Male, Single gunshot wound
 Human, Male, Single gunshot wound Product Number: Specimen Evaluated: Skeletal Inventory: BC-152 Bone Clones replica 1 intact cranium - left inferior nasal concha absent - middle nasal conchae absent 1
Human, Male, Single gunshot wound Product Number: Specimen Evaluated: Skeletal Inventory: BC-152 Bone Clones replica 1 intact cranium - left inferior nasal concha absent - middle nasal conchae absent 1
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Bones are made of OSSEOUS TISSUE
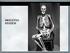 SKELETAL SYSTEM Functions of the Skeletal System Bones are made of OSSEOUS TISSUE Support and Protection Body movement Blood cell formation (bone marrow) Storage of inorganic materials (salt, calcium,
SKELETAL SYSTEM Functions of the Skeletal System Bones are made of OSSEOUS TISSUE Support and Protection Body movement Blood cell formation (bone marrow) Storage of inorganic materials (salt, calcium,
By JOHN MARQUIS CONVERSE, M.D., and DAUBERT TELSEY, D.D.S.
 THE TRIPARTITE OSTEOTOMY OF THE MID-FACE FOR ORBITAL EXPANSION AND CORRECTION OF THE DEFORMITY IN CRANIOSTENOSIS By JOHN MARQUIS CONVERSE, M.D., and DAUBERT TELSEY, D.D.S. Center for Craniofacial Anomalies
THE TRIPARTITE OSTEOTOMY OF THE MID-FACE FOR ORBITAL EXPANSION AND CORRECTION OF THE DEFORMITY IN CRANIOSTENOSIS By JOHN MARQUIS CONVERSE, M.D., and DAUBERT TELSEY, D.D.S. Center for Craniofacial Anomalies
Bone Development. V. Gilsanz and O. Ratib, Hand Bone Age, DOI / _2, Springer-Verlag Berlin Heidelberg 2012
 Bone Development 2 Skeletal maturity is a measure of development incorporating the size, shape and degree of mineralization of bone to define its proximity to full maturity. The assessment of skeletal
Bone Development 2 Skeletal maturity is a measure of development incorporating the size, shape and degree of mineralization of bone to define its proximity to full maturity. The assessment of skeletal
BONE LABORATORY DEMONSTRATIONS. These demonstrations are found on the bulletin boards outside the MCO Bookstore.
 BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
Evaluation of Staggered Osteotomy in Surgical Treatment of Trigonocephaly
 Evaluation of Staggered Osteotomy... Hassanpour et al. 28 Original 28 Evaluation of Staggered Osteotomy in Surgical Treatment of Trigonocephaly Seyed Esmail Hassanpour¹ Mohammad Reza Hadi Sichani 2 Mohammadreza
Evaluation of Staggered Osteotomy... Hassanpour et al. 28 Original 28 Evaluation of Staggered Osteotomy in Surgical Treatment of Trigonocephaly Seyed Esmail Hassanpour¹ Mohammad Reza Hadi Sichani 2 Mohammadreza
(i) Family 1. The male proband (1.III-1) from European descent was referred at
 1 Supplementary Note Clinical descriptions of families (i) Family 1. The male proband (1.III-1) from European descent was referred at age 14 because of scoliosis. He had normal development. Physical evaluation
1 Supplementary Note Clinical descriptions of families (i) Family 1. The male proband (1.III-1) from European descent was referred at age 14 because of scoliosis. He had normal development. Physical evaluation
TERMINOLOGY. portion of a bone ossified from a primary center. portion of a bone ossified from a secondary center.
 Embryology APPENDICULAR SKELETON Consists of the pectoral and the pelvic girdles and the bones of the limbs. Beginning at the 4 th \ menstrual week primordial bone patterns evolve into cartilaginous bone
Embryology APPENDICULAR SKELETON Consists of the pectoral and the pelvic girdles and the bones of the limbs. Beginning at the 4 th \ menstrual week primordial bone patterns evolve into cartilaginous bone
Advaneement-onlay: an improved technique of fronto-orbital remodeling in craniosynostosis *
 Advances & operative techniques mcns 9 Springer-Verlag 1991 Child's Nerv Syst (1991) 7:264-271 Advaneement-onlay: an improved technique of fronto-orbital remodeling in craniosynostosis * Steven R. Cohen
Advances & operative techniques mcns 9 Springer-Verlag 1991 Child's Nerv Syst (1991) 7:264-271 Advaneement-onlay: an improved technique of fronto-orbital remodeling in craniosynostosis * Steven R. Cohen
Calcification of Porcine Aortic Valvular Interstitial Cells
 Calcification of Porcine Aortic Valvular Interstitial Cells Liwen Gu 1,2* Supervisor: Craig A. Simmons 1 Department of Engineering Science, 2 Institute of Biomaterials and Biomedical Engineering, University
Calcification of Porcine Aortic Valvular Interstitial Cells Liwen Gu 1,2* Supervisor: Craig A. Simmons 1 Department of Engineering Science, 2 Institute of Biomaterials and Biomedical Engineering, University
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
CNS Embryology 5th Menstrual Week (Dorsal View)
 Imaging of the Fetal Brain; Normal & Abnormal Alfred Abuhamad, M.D. Eastern Virginia Medical School CNS Embryology 5th Menstrual Week (Dorsal View) Day 20 from fertilization Neural plate formed in ectoderm
Imaging of the Fetal Brain; Normal & Abnormal Alfred Abuhamad, M.D. Eastern Virginia Medical School CNS Embryology 5th Menstrual Week (Dorsal View) Day 20 from fertilization Neural plate formed in ectoderm
PRP Usage in Today's Implantology
 Volume 1, December 2004 www.implant.co.il PRP Usage in Today's Implantology by Dr. R. Shapira Introduction: Treating patients suffering from hematological disorders or using anticoagulant medications always
Volume 1, December 2004 www.implant.co.il PRP Usage in Today's Implantology by Dr. R. Shapira Introduction: Treating patients suffering from hematological disorders or using anticoagulant medications always
Fructose Upregulates FGF23 Expression In MC3T3 Pre-osteoblasts
 Fructose Upregulates FGF23 Expression In MC3T3 Pre-osteoblasts Edek A. Williams, B.S.E., Veronique Douard, Ph.D., Joseph M. Lomuti, B.S., Ronaldo Ferraris, Ph.D., J. C. Fritton, Ph.D.. Rutgers University,
Fructose Upregulates FGF23 Expression In MC3T3 Pre-osteoblasts Edek A. Williams, B.S.E., Veronique Douard, Ph.D., Joseph M. Lomuti, B.S., Ronaldo Ferraris, Ph.D., J. C. Fritton, Ph.D.. Rutgers University,
Functions of the Skeletal System
 SKELETAL SYSTEM Functions of the Skeletal System Support: Internal framework that supports and anchors all soft organs. Protection: Bones protect soft body organs Body movement skeletal muscle attached
SKELETAL SYSTEM Functions of the Skeletal System Support: Internal framework that supports and anchors all soft organs. Protection: Bones protect soft body organs Body movement skeletal muscle attached
Skeletal System: Skull.
 Skeletal System: Skull www.fisiokinesiterapia.biz Bones of the Skull SPLANCHNOCRANIUM Nasal (2) Maxilla (2) Lacrimal (2) Zygomatic (2) Palatine (2) Inferior concha (2) Vomer Mandible NEUROCRANIUM Frontal
Skeletal System: Skull www.fisiokinesiterapia.biz Bones of the Skull SPLANCHNOCRANIUM Nasal (2) Maxilla (2) Lacrimal (2) Zygomatic (2) Palatine (2) Inferior concha (2) Vomer Mandible NEUROCRANIUM Frontal
Cleft-Craniofacial Center
 Cleft-Craniofacial Center A Pioneering T eam 2 Welcome to the Cleft-Craniofacial Center at Children s Hospital of Pittsburgh The Cleft-Craniofacial Center at Children s Hospital of Pittsburgh has been
Cleft-Craniofacial Center A Pioneering T eam 2 Welcome to the Cleft-Craniofacial Center at Children s Hospital of Pittsburgh The Cleft-Craniofacial Center at Children s Hospital of Pittsburgh has been
