SURGERY FOR MOVEMENT DISORDERS
|
|
|
- Ernest Ward
- 5 years ago
- Views:
Transcription
1 MOVEMENT DISORDERS SURGERY FOR MOVEMENT DISORDERS Ali R. Rezai, M.D. Center for Neurological Restoration, and Department of Neurosurgery, Cleveland Clinic, Cleveland, Ohio Andre G. Machado, M.D., Ph.D. Center for Neurological Restoration, and Department of Neurosurgery, Cleveland Clinic, Cleveland, Ohio Milind Deogaonkar, M.D. Center for Neurological Restoration, and Department of Neurosurgery, Cleveland Clinic, Cleveland, Ohio MOVEMENT DISORDERS, SUCH as Parkinson s disease, tremor, and dystonia, are among the most common neurological conditions and affect millions of patients. Although medications are the mainstay of therapy for movement disorders, neurosurgery has played an important role in their management for the past 50 years. Surgery is now a viable and safe option for patients with medically intractable Parkinson s disease, essential tremor, and dystonia. In this article, we provide a review of the history, neurocircuitry, indication, technical aspects, outcomes, complications, and emerging neurosurgical approaches for the treatment of movement disorders. KEY WORDS: Deep brain stimulation, Dystonia, Essential tremor, Globus pallidus pars interna, Movement disorders, Parkinson s disease, Stereotaxis, Subthalamic nucleus, Ventralis intermedius nucleus Neurosurgery 62[SHC Suppl 2]:SHC809 SHC839, 2008 DOI: /01.NEU B9 Hooman Azmi, M.D. Center for Neurological Restoration, and Department of Neurosurgery, Cleveland Clinic, Cleveland, Ohio Cynthia Kubu, Ph.D. Center for Neurological Restoration, and Department of Psychiatry, Cleveland Clinic, Cleveland, Ohio Nicholas M. Boulis, M.D. Center for Neurological Restoration, and Department of Neurosurgery, Cleveland Clinic, Cleveland, Ohio Reprint requests: Ali R. Rezai, M.D., Center for Neurological Restoration, 9500 Euclid Avenue, Desk S31, Cleveland OH rezaia@ccf.org Received, May 30, Accepted, November 1, ONLINE DIGITAL VIDEO Historical Perspective Various surgical approaches, such as resection, lesioning, stimulation, and others, have been used to treat patients with movement disorders. Craniotomies were performed for the resection of the motor cortex (68), cerebral peduncles (381, 382), and a variety of subcortical lesioning procedures (326). Irving Cooper (72a) first reported the effects of ligation of the choroidal artery for Parkinson s disease (PD) in Six patients were treated with eight ligations, which resulted in significant alleviation of rest tremor, rigidity, and contralateral cogwheeling. It was not until the introduction of stereotaxis by Spiegel et al. (328) in 1947, and later by Leksell (206) in 1949, that a more accurate, less invasive, and more consistent placement of lesions in various subcortical locations became feasible. The development of stereotaxy led to a variety of lesioning procedures of the basal ganglia and the thalamus for the treatment of rigidity and tremor in the 1950s and 1960s. Various surgical techniques, lesion locations, lesion sizes, and outcomes were reported (77, 256, 327, 392). The motor thalamus and the pallidal targets lying in the ventral and posterior portions of the globus pallidus internus (GPi) as well as the pallidal projections were considered to be the most effective targets. However, it was the advent of L-dopa in the mid-1960s and its significant clinical benefits that led to a dramatic decrease in surgery for PD. For the next 20 years, surgery for movement disorders was predominantly limited to thalamotomy (8, , 149, 167, 254, 275, 343) for the treatment of tremor and pallidotomy and thalamotomy for dystonia (224, 341, 371, 383). PD surgery was rarely performed during this time. It was not until the late 1980s that there was a reemergence of interest in the neurosurgical treatment for PD due to the increasing realization of the limitations of PD medications and the side effects of L-dopa. This led to a resurgence of lesioning surgeries such as pallidotomies for PD. The initial Leksell (336) target of pallidal lesions for treatment of PD was modified and repopularized by Laitinen et al. (196, 197). Original analytical descriptions of thalamic nuclei and circuitry by Hassler (142), Hassler et al. (143), and Macchi and Jones (230) and basal ganglia circuitry by Delong et al. (81, 82) also served as a foundational substrate for newer targets for therapeutic interventions using stereotactic techniques. The ability of electrical impulses to modify functional outcome in certain brain regions was identified almost 200 years ago, in 1809, by Rolando (98). Aldini had previously attempted to stimulate the brains of executed criminals immediately after death by applying current from voltaic piles (98). The use of electrical stimulation to understand and map the function of the human brain and its circuitry became commonplace in the 20th century (4, 64, 65, 291). Early explorations by Hassler et al. revealed that acute low-frequency stimulation during stereotactic exploration for ablation of VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC809
2 REZAI ET AL. the pallidum could augment tremor, whereas high-frequency stimulation at 25 to 100 Hz had the opposite effect (143). These observations paved the way for the future development of chronic electrical stimulation therapies for the management of movement disorders. The first systematic use of chronic deep brain stimulation (DBS) for the treatment of movement disorders is attributed to Bechtereva et al. (22) in Russia. Beginning in 1967, they reported benefits with chronic DBS of the thalamus, striatum, and pallidum. But it was not until the 1980s that Brice and McLellan (54), Blond and Siegfried (52), Siegfried and Shulman (319), and Benabid et al. (34, 36) published reports of the use of chronic electrical stimulation or DBS for the treatment of movement disorders, thus ushering in a new era of functional neurosurgery for movement disorders. DBS has similar efficacy as that reported with various lesioning procedures (e.g., pallidotomy, thalamotomy). However, the superior safety profile of DBS relative to lesioning procedures, particularly bilateral thalamotomy and pallidotomy, has made it the procedure of choice in countries where access to this technology is available DBS, with its inherent features of reversibility and adjustability, has gained popularity and emerged as the neurosurgical standard of care for movement disorders such as PD, dystonia, and essential tremor over the past 20 years (6, 9, 25, 32, 33, 37, 70, 101, 187, 193, 201, 202, 227, 252, 268, 274, 280, 293, 294, 343, 351, 357, 394). Since its inception, more than 40,000 DBS implants have been performed in more than 500 centers worldwide (28). In addition to the widespread use of DBS for movement disorders, a number of clinical investigations using DBS are under way to explore its safety and efficacy for conditions such as Tourette s syndrome (14, 88, 111, 155, 243, 266, 322, 366), chronic pain (48, 69, 119, 188, 210, 295), and psychiatric disorders such as depression (60, 109, 238, 310, 314) and obsessive-compulsive disorder (OCD) (73, 121, 122, 190, 242, 386). Because DBS is the most commonly used neurosurgical procedure for the treatment of movement disorders, it is the major focus of this article. Neural Circuitry of Movement Disorders Traditionally, surgical procedures have targeted the known anatomic subcortical gray or white matter regions implicated in the circuitry and the pathophysiology of movement disorders. As discussed above, a long history of lesioning procedures for the treatment of movement disorders has provided a wealth of empirical evidence supporting the presumed underlying neurocircuity associated with the aberrant motor symptoms. During the past two decades, technological advances in structural and functional brain imaging and physiological brain mapping, coupled with animal research, have further advanced our understanding of the underlying neurocircuitry of specific movement disorders, and led to additional refinement of the surgical targets. The use of animal models has contributed significantly to a better understanding of the pathophysiology and underlying neurocircuitry of PD and other movement disorders (2, 29, 39, 41, 53, 56, 62, 83, 99, 120, 133, 135, 144, 152, 204, 205, 209, 223, 234, 239, 241, 246, 248, 249, 262, 273, 283, 292, 320, 325, 332, 340, 343, 385, 391). These data provided support for the concept of the cortico-striatal-pallidal-thalamic-cortical (CSPTC) circuits. Alexander et al. (7) hypothesized that a network of five parallel, segregated circuits exists that underlies a variety of functions. These circuits originate in various regions of the frontal lobes and then traverse through different nodes in the striatum, pallidum, and thalamus before returning to their cortical points of origin. One of these circuits underlies complex motor function and is implicated in the pathophysiology of PD. The concept of a CSPTC motor circuit or loop implies that a number of the nodes involved in the circuit are potential targets for neuromodulation including neurosurgical procedures such as lesioning and DBS, somatic or stem cells, or gene therapy. In the CSPTC circuitry model, the striatal structures, such as the caudate and putamen, serve as the input structures, whereas the GPi and substantia nigra pars reticulata (SNr) are the primary output structures. The motor circuit originates in the precentral motor regions (especially Brodmann areas 4 and 6). Information passing through the basal ganglia is organized anatomically though direct and indirect pathways within the CSPTC circuit (Fig. 1). Information in the direct pathway passes monosynaptically from the putamen to the output structures of the basal ganglia, the GPi, and the SNr. Information from the indirect pathway passes multisynaptically through the globus pallidus externa (GPe) and the subthalamic nucleus (STN) before terminating on the GPi/SNr. The information from both the direct and indirect pathways then projects to various thalamic relay nuclei, including the ventral oralis anterior (Voa) and ventral oralis posterior (Vop) nuclei (in Hassler s nomenclature) (230). This information is then projected back to the frontal region of origin, thereby closing the circuit. The direct and indirect pathways appear to balance one another. The direct pathway is presumed to be responsible for the initiation of action and the indirect pathway for the braking of action or the ability to switch from one action to another. Inhibitory γ-aminobutyric acid (GABA)ergic projections predominate in these pathways. Other than the excitatory glutamatergic projections from the cortex to the basal ganglia and the returning excitatory thalamocortical projections, the only projections within the deep subcortical brain structures that are excitatory are the glutamatergic projections coursing from the STN to the GPi/SNr. The most common surgical targeted regions in this circuit are the STN, the GPi, and their associated CSPTC motor circuit white matter inputs and outputs. There are other emerging targets in the CSPTC motor loop that are being further investigated, but, given the scope of this review, they will not be discussed. Dopamine is one of the most powerful neurotransmitters influencing the motor CSPTC circuit. Dopamine can have either an excitatory or inhibitory role on striatal neurons, depending on the dopamine receptor subtype: D1 receptors are associated with an excitatory effect, whereas D2 receptors are inhibitory. In general, dopaminergic inputs to the striatum serve to reduce SHC810 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
3 SURGERY FOR MOVEMENT DISORDERS A FIGURE 1. A, cortico-striato-pallido-thalamo-cortical (CSPTC) neural circuitry in normal state. B, CSPTC in Parkinson s disease (PD). SNc, substantia nigra pars compacta; GPe, globus pallidus externus; STN, subthalamic nucleus; GPi, globus pallidus internus; SNr, substantia nigra reticulata. basal ganglia output and subsequently disinhibit thalamocortical activity. Dopaminergic activity may also ultimately facilitate activity through the direct pathway over the indirect pathway, but this hypothesis is still under debate (110). PD is a disorder characterized primarily by dopamine loss in the substantia nigra pars compacta, which results in the classic motor symptoms of PD, including tremor, rigidity, bradykinesia, gait difficulties, and postural changes. The reduced dopaminergic input associated with PD causes an increase in activity through the indirect rather than the direct pathway. This results in hyperactivity of the GPi/SNr and subsequent inhibition of thalamocortical activation further downstream, which leads to reduced frontal cortical activity and the classic motor symptoms of PD (261, 263). The STN is one of the major driving forces behind the increased activity of the GPi and SNr output nuclei. Given their unique role in the CSPTC motor circuit, the most common surgical targets are the STN, GPi, and their associated CSPTC motor circuit white matter inputs and outputs. There are other emerging targets in the CSPTC motor loop which are being further investigated, but, given the scope of this review, they will not be discussed. Recent advances in the pathophysiology of PD reveal that in addition to the abnormal frequency (hyper- or hypoactivity) between the structures of the CSPTC circuit, there is disordered neurophysiological rhythmic activities and patterns in these regions as well. Hashimoto et al. (141) assessed the effects of high-frequency (HF) STN DBS in the primate 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine model of parkinsonism. HF DBS resulted in modifications in the pathological pattern of pallidal activity. Before stimulation, spontaneous pallidal activity was irregular, with varying intervals. Stimulation resulted in a higher frequency and regular pattern of spike activity, which correlated with improvements in parkinsonian signs. Further investigation on activity patterns has also been pursued by other groups. Synchronous low-frequency activity (in the β band) has been identified in the basal ganglia by Brown (55), Kühn et al. (189), Pogosyan et al. (287), and Trottenberg et al. B (353) and correlated to bradykinesia. These results corroborate the recent view that the pathology of PD extends beyond abnormal frequencies and involves more complex interactions within the CSPTC circuit. Although the pathophysiology of dystonia may be related to a disruption of the activity within the CSPTC circuit, the exact mechanisms are still uncertain. No gross morphological changes involving specific components of the CSPTC motor circuit or links to specific neurotransmitters have been consistently identified for dystonia. Electrophysiological studies indicate that the abnormality is not as simple as increased or decreased communication rates between the motor CSPTC loop components, but rather, a disruption of the communication pattern between two structures (370). Under this perspective, the effects of DBS could be mediated by a lesion-like effect that disrupts abnormal connectivity, similar to that which might occur after DBS for the treatment of PD. Thus far, the motor GPi and ventral lateral (Voa/Vop) thalamus have been the primary surgical targets for dystonia. The pathophysiology of essential tremor most likely involves components of the cerebellum, motor thalamus, and relevant frontal cortices, and, unlike PD and dystonia, it does not necessarily involve the CSPTC circuit. Essential tremor is probably related to frontocerebellar circuits in which axons from the cerebellum synapse on thalamic neurons that project to the cortex. Studies of patients with essential tremor who are undergoing positron emission tomography and functional magnetic resonance imaging (MRI) indicate hyperactivity of the cerebellum and thalamus (58, 387). These findings provide support to the well-established clinical knowledge that ablation of the cerebellar thalamus is highly effective in alleviating tremor. DBS of the ventralis intermediate nucleus (VIM) is demonstrated to be equally effective (275, 343) in alleviating tremor, and a recent study demonstrated that VIM DBS affects the excitability of the cerebellothalamocortical pathway (247). The concept of CSPTC circuits and other neurocircuits is critically important in functional neurosurgery with respect to providing guidance regarding targets and sites of intervention. These circuits are a model of a network of interconnected regions that is implicated in normal motor functioning and abnormal functioning associated with various disease processes. The surgically accessible components of this network are potential targets of intervention, which can include lesioning procedures, DBS, or other neurorestorative approaches involving somatic or stem cells and gene therapy. Mechanism of DBS Action The placement of stereotactic lesions and DBS reflect two different methods of neuromodulation. Whereas lesioning destroys a given volume of tissue, DBS exerts a reversible electrical field on the surrounding nervous tissue elements. The underlying mechanism of action of DBS remains a point of debate and active research. There appears to be a combination of inhibition of neurons, modulation of abnormal patterns of activity, and activation of axons. Initial observations suggested that HF stimulation caused inhibition of the cellu- VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC811
4 REZAI ET AL. lar activity in the nucleus, thereby mimicking a transient lesion-like effect. Studies show that DBS can induce inhibition of cellular activity or of neural network functions by either the jamming of neural transmission through stimulated nuclei, by direct inhibition of membrane action potentials, by retrograde activation of upstream neuronal structures, or by the shutting down of neurotransmitter release in the STN (38). Recent experiments demonstrate that HF stimulation of the STN is accompanied by increased release of glutamate and dopamine in the STN and striatum, respectively (203, 338). Similarly, increased release of GABA in axons from afferent connections was observed in the GPi of parkinsonian patients during DBS surgery (40). It has also been demonstrated that activation of a DBS lead placed in the ventral thalamus can rapidly disrupt local synaptic function and neuronal firing, and thereby lead to a functional deafferentation and/or functional inactivation (13). A recent study of PD patients after placement of DBS electrodes in the STN suggests that HF stimulation produces early inhibition with subsequent rebound excitation and another period of inhibition. These findings suggest that the observed inhibitory activity reflects neuronal hyperpolarization (96). These data further suggest that STN neuronal inhibition may be accompanied by direct excitation of the cell and/or its axon. In summary, the growing literature demonstrates the complexity of the motor system and the potential mechanisms of DBS action. The prevailing hypotheses postulate inhibition at the neuronal level, activation at the axonal level, as well as interruption of excessive and abnormally patterned neuronal activity in the STN, GPi, and the interconnected components of the CSPTC network (20, 39, 133, 135, 264, 309, 385). Surgery: The Team Surgery for movement disorders is most optimally managed in the context of a multidisciplinary team. In addition to the neurosurgeon, this team should include a movement disorders neurologist, a neuropsychologist, a neurophysiologist, and physician extenders such as nurse practitioners and physician s assistants. The movement disorders neurologist can help confirm an accurate diagnosis and rule out atypical parkinsonism or psychogenic movement disorders. The benefit of surgery for a particular movement disorder is largely dependent on accurate diagnosis, as it is the underlying pathophysiology and neurocircuitry of the specific movement disorder that is influenced by surgery. The movement disorders neurologist can also optimize medications for individuals who have not had adequate medication trials. Occasionally, medication adjustments by an expert can significantly improve the functioning of a patient such that he or she no longer requires surgery. The DBS programming can be performed by the neurosurgeon, neurologist, or physician extenders. As DBS programming results in changes in motor symptoms, there must be close attention to concomitant medication adjustments coupled with rehabilitation (e.g., physical and occupational therapies) to optimize an individual patient s motor outcome. Surgical Patient Selection Criteria In general, patients must be able to tolerate the various components of surgery and have the cognitive skills and social support structure to comply with the demands of surgery and the postoperative care. For those undergoing DBS surgery, both the patient and the family members need to have a detailed understanding of reasonable outcomes, potential complications, and the multiple steps involved in the preoperative assessments, surgery, and perioperative and follow-up care. The patient needs to cooperate with follow-up programming and medication adjustments in the outpatient setting. Additionally, the patient and family should have realistic expectations about surgical outcome, and they should understand that the surgery will not cure the disease or stop its natural progression. Neurosurgery for movement disorders can provide improvements in disabling motor symptoms and motor function. It is important to provide accurate information to the patient and family members regarding those symptoms that are likely to respond to surgery versus those that are not. Patients should be in stable overall health with respect to cardiac, pulmonary, and systemic conditions such as hypertension, diabetes, and cancer. Patients who require anticoagulants, such as warfarin or antiplatelet medication, must be able to tolerate complete withdrawal from these medications before surgery. Consultation with other medical specialists may be required before proceeding with surgery. In recent years, there has been increasing recognition of the neurobehavioral changes associated with PD and other movement disorders, including cognitive, mood, and personality changes. Neuropsychological assessment is recommended as part of the preoperative assessment to determine candidacy for neurosurgical intervention for the treatment of movement disorders. The neuropsychological assessment should include assessment of cognition, neuropsychiatric symptoms, social support, and goals for surgery. Patients with severe cognitive dysfunction or dementia on neuropsychological examination should be excluded from surgical intervention. Patients with mild cognitive impairment or frontal dysexecutive syndrome may still undergo surgery, but these individuals should have a strong social support structure and receive extra counseling, along with family members, regarding the potential for increased risks for cognitive impairment and confusion postsurgery. Psychiatric conditions such as anxiety, depression, and mania must be identified and medically optimized by a specialist preoperatively. Neurosurgical intervention in patients with delusional psychosis or severe personality disorder, such as borderline personality disorder, is generally not recommended. PD: Selection Criteria PD is a progressive neurodegenerative disorder resulting in prominent motor abnormalities such as bradykinesia (slowness of movement), rigidity (muscle stiffness), tremor, and gait and postural instabilities. In PD, there is progressive degeneration of dopaminergic neurons. Administration of L-dopa and SHC812 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
5 SURGERY FOR MOVEMENT DISORDERS synthetic dopamine agonists is the mainstay of medical treatment of PD. However, over time, patients experience a less favorable response to medications and may begin a cycle that includes increasing medication doses and multiple medications with disabling side effects. Dose escalations can be associated with motor fluctuations and troublesome dyskinesias (83, 251, 260). Despite major advances in the understanding of the pathophysiology of PD and improvements in pharmacological management, there are a substantial number of patients who are considered refractory to medical management. Such medically refractory patients with significant motor complications and disability can benefit from DBS of the STN or GPi. Neurosurgery has been shown to consistently benefit only patients with idiopathic PD. Atypical parkinsonism (supranuclear palsy, nigrostriatal degeneration, etc.) or other disorders with parkinsonian features have not been shown to respond favorably to surgery. In general, surgery is most likely to benefit symptoms affecting the extremities rather than axial symptoms that involve posture, balance, gait, and speech. Surgical candidates typically have more than one of the following symptoms: severe tremors; off-medication-related rigidity, freezing, dystonia, and bradykinesia; on-medication-related dyskinesias; and significantly disabling on-off-medication motor fluctuations. One of the most important predictors of neurosurgical treatment response is the patient s response to L-dopa. Patients who demonstrate a significant improvement in motor symptoms during L-dopa off-medication versus on-medication states are most likely to benefit from surgery. The only exception to this general rule involves tremor. Tremor is the only identified motor symptom that can improve with DBS regardless of response to off-on-medication testing. Consequently, a formal off-on test of L-dopa responsiveness can be very helpful in the selection of the best surgical candidates. Tremor: Selection Criteria Essential tremor is a benign condition (32, 173, 198, 222) that can be managed for many years with medications. In those patients who have disabling extremity tremor despite optimal medication management, surgery using the VIM target becomes an option. In general, patients with resting and distal postural tremor fare the best with surgery, followed by those with proximal postural tremor. Patients with intention/action tremor tend to benefit to a lesser degree. The more proximal and the action/intention features of tremor are the most difficult and challenging tremor characteristics to treat surgically (44, 85, 169). Head, neck, and lower-extremity tremors are also more difficult to treat than upper-extremity tremors. Tremors involving the head, neck, and axial regions usually require bilateral surgery. Dystonia: Selection Criteria DBS offers a therapeutically viable option for patients with severe, primary dystonia and also for a small subset of patients with secondary dystonia. The key to favorable responses after DBS in patients with dystonia is proper patient selection. Patients who are refractory to all conservative measures, including medication trials (anticholinergics, baclofen, benzodiazepines, or other muscle relaxants) and botulinum toxin injections are potential candidates. Dystonia is a heterogenous condition with variable expression. It can be classified into primary or secondary dystonia according to etiology. Primary idiopathic dystonia refers to dystonia with no discernible etiological factor responsible for its onset. Patients with primary idiopathic dystonia have normal imaging findings, cerebrospinal fluid composition, and laboratory test examinations. A subset of patients with primary dystonia have a DYT-1 mutation on chromosome 9q (12). Secondary dystonia refers to dystonia that is associated with a clearly preexisting, identifiable brain insult such as perinatal hypoxia, stroke, trauma, toxin exposure, or infectious sequelae. Tardive dystonia is another subset of dystonia that results from super-sensitivity of the postsynaptic dopamine striatal receptors due to long-term administration of dopamine receptorblocking agents such as neuroleptics (105, 219, 395). Dystonia can also be classified according to the affected body part. In focal dystonia, a single region of the body is affected, such as in blepharospasm (eyes), cervical dystonia/torticollis (neck), and spasmodic dysphonia or laryngeal dystonia (182). In segmental dystonia, two or more adjacent body parts are affected, such as cranial-cervical dystonia, crural dystonia, or brachial dystonia. Generalized dystonia refers to dystonia involving most body parts. Primary, generalized dystonia of DYT-1-positive (184, 195, 201) or non-dyt-1 types, as well as patients with idiopathic cervical dystonia can obtain the best motor benefits with bilateral GPi DBS (363). Patients with juvenile-onset idiopathic dystonia whose age of onset is older than 5 years and who do not have multiple orthopedic deformities also have a good response to surgery (280). Appendicular symptoms (e.g., those affecting the limbs) appear to respond better than axial symptoms (201). With regard to focal dystonia, ideal surgical candidates are those with cervical dystonia (201, 331). The results of DBS for secondary dystonia are inconsistent. In general, DBS for secondary dystonia is less effective than for primary generalized dystonia, particularly in those patients with an identifiable structural brain abnormality. The only exception is tardive dystonia, which has been reported to respond well to surgery in a small number of patients (92, 331, 396). Surgical Targets The three most common targets for movement disorder surgery are the STN, GPi, and VIM thalamus. GPi and STN DBS improve PD symptoms (e.g., tremor, rigidity, and bradykinesia) and also reduce drug-induced dyskinesias. STN DBS also reduces the medication burden, thereby reducing medicationassociated side effects (80, 59, 368). Both the STN and the GPi have corresponding associative (cognitive), limbic, and motor territories that require accurate surgical targeting of the motor component. Presently, the most commonly used target for DBS therapy to treat PD is the STN, followed by the GPi. The GPi is also the most commonly used target for dystonia (66, 100, 159, VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC813
6 REZAI ET AL. 185, 201, 280, 312, 331, 350, 351, 355, 396). The VIM target is the main target used for non-parkinsonian tremor. The VIM is very effective in alleviating PD-associated tremors, but is not effective in the treatment of other cardinal PD symptoms. (342, 343). Thus, VIM DBS surgery is rarely performed for PD treatment. The STN Target The STN was previously not considered a target because of the fear of causing hemiballismus. However, in 1990, Bergman et al. (42) showed that a lesion in the STN of a nonhuman primate model could reverse the symptoms of PD. This early work, coupled with the evolving concept of the flexibility (e.g., reversibility and adjustability) inherent in DBS for the treatment of movement disorders, resulted in Benabid et al. (34) and Pollak et al. (288) applying STN DBS for the treatment of PD initially in 1993, with report of a subsequent case series in 1995 (215). Since that time, STN DBS has become the most common target for DBS surgery for PD. Targeting the STN has been demonstrated to effectively treat the entire spectrum of advanced PD symptoms of tremor, rigidity, bradykinesia, motor fluctuations, and drug-induced dyskinesias, while also consistently reducing the need for dopaminergic medication postoperatively. Anatomically, the STN (also called the corpus luysi) is an almond-shaped nucleus located on the inner surface of the peduncular portion of the internal capsule. The STN is surrounded by several key structures that need to be considered carefully (Fig. 2). This includes the anterior and laterally situated internal capsule, through which corticospinal and corticobulbar fibers pass. Anteromedially lie the fibers of Cranial Nerve III, the posteromedial hypothalamus, and portions of the fields of Forel. The red nucleus, fibers with cerebellothalamic projections, and the prelemniscal radiations are situated posteromedially. Dorsal to the STN is the zona incerta and Forel s field H2 that separate it from the ventral border of the motor thalamus. The cerebral peduncle and the substantia nigra are situated ventral to the STN (Fig. 3, A C). The GPi Target The GPi target is used for PD treatment less commonly than the STN. However, the GPi is currently the most common target for treating dystonia (364) despite reports of using thalamic (Voa, ventrolateral) (92) and subthalamic DBS targets (162, 373) for dystonia. The GPi DBS target is the posteroventrolateral GPi, which is the predominant motor territory of the nucleus (Fig. 4). The globus pallidus is divided into two anatomic segments: internal (GPi) and external (GPe). Although these segments are separated by the medial medullary lamina, the pallidal neurons from each segment are similar and, for the most part, morphologically indistinguishable. The GPi is bound laterally and dorsally by the GPe. Medially, the GPi is bound by the internal capsule. Ventrally, it is close to the optic tracts (Fig. 5, A and B). The therapeutic sensorimotor territory of the GPi is ventral and posterior, and the somatotopy places the face and arm posterior and ventral, and the leg central and more dorsal (350). The striatal afferents terminate in the GPe, as do the FIGURE 2. A three-dimensional depiction of the STN and structures around it in the midbrain-diencephalic region. Note the close proximity of the STN to the red nucleus (RN), cerebral peduncles, and substantia nigra (SN). afferents coming from the intralaminar nuclei of the thalamus and STN. Pallidal efferents pass through the major routes of pallidal outflow (the ansa lenticularis and lenticular fasciculus) primarily to the Vop nucleus of the thalamus, but they also interdigitate with the afferents to the VIM. The VIM Target The VIM is the common lesioning and DBS target used for the treatment of tremor (Fig. 3, B and C) (1, 6, 25, 33, 35 37, 63, 108, 113, 126, 164, 173, 174, 191, 221, 222, 226, 231, 267, 297, 377). In the somatotopic organization of the VIM nucleus face, responsive cells lie medially, followed by the upper extremity more lateral, and the lower extremity is the most lateral, situated closely to the internal capsule (Fig. 6). The VIM nucleus of the thalamus has neurons that fire in synchronous bursts with the tremor frequency and are called tremor cells (TCs). TCs are believed by some to act as tremorigenic pacemakers (178, 222). There is a significant confusion and controversy surrounding the nomenclature of the thalamic nuclei (178, 179). The DBS target for tremor control is the electrophysiologically defined VIM (178). This electrophysiologically defined motor thalamus (VIM) has TCs and kinesthetic cells, and it lies immediately anterior to the cutaneous receptive cells, which lie in the sensory thalamus (178). The somatosensory relay nucleus ventralis caudalis (VC) of the thalamus lies immediately posterior to VIM. The VC has specific neurons that respond to tactile stimulation in small, receptive fields. The Vop nucleus lies immediately anterior to the VIM. The internal capsule lies lateral to the VIM. The Vop receives affer- SHC814 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
7 SURGERY FOR MOVEMENT DISORDERS A B FIGURE 3. Location of the STN and its relation to the surrounding structures is depicted on axial (A), coronal (B), and sagittal (C) sections of the Schaltenbrand and Wahren atlas. Note the close proximity of the STN to the internal capsule (IC), red nucleus (RN), and substantia nigra (SNR). The coronal (B) and sagittal (C) sections also show the ventral and dorsal tier of thalamic nuclei, including the ventral caudal nucleus (VC), ventral intermediate nucleus (VIM), and ventral oralis anterior and posterior nuclei (Voa and Vop, respectively). ATh, anterior thalamic nucleus complex; MTh, medial thalamic nucleus complex. (From, Schaltenbrand G, Wahren W: Atlas for Stereotaxy of the Human Brain. Stuttgart, Thieme Medical Publishers, Reproduced with permission [313].) A C B FIGURE 5. High-resolution axial magnetic resonance imaging (MRI) scans showing the globus pallidus and optic tract. A, inversion recovery sequence showing the caudate nucleus, putamen, globus pallidus (GP), IC, and thalamus. B, axial T2-weighted MRI scan showing the trajectory of the intended placement of the deep brain stimulation (DBS) lead with its tip just dorsal to the optic tract. ents from pallidal neurons (230) and the VIM receives afferents from the cerebellar neurons (cerebellothalamic fibers). There is some degree of overlap and interdigitation between these two nuclei (230). DBS Surgical Technique In this section, we review the general principles and techniques of DBS surgery, which is the most common surgical treatment for movement disorders today. Although there is FIGURE 4. Location of the GPi and its relation to the surrounding structures as depicted on the axial section of the Schaltenbrand and Wahren atlas. Note the close proximity of the GPi medially to the IC and laterally to the GPe. The dark boundary between the GPi and GPe is the external lamina. (From, Schaltenbrand G, Wahren W: Atlas for Stereotaxy of the Human Brain. Stuttgart, Thieme Medical Publishers, Reproduced with permission [313].) FIGURE 6. Somatotopic arrangement of the ventral relay nuclei shown on an axial section including VC, VIM, Voa, and Vop. The general scheme in the thalamic somatotopy includes having the leg placed more laterally and the face placed more medial with an upper limb in between the two. It also shows the somatotopy in the internal capsule. In this classic figure, the GPi homunculus does not strictly represent the GPi somatotopy, as has been suggested with more recent data. (From, Schaltenbrand G, Wahren W: Atlas for Stereotaxy of the Human Brain. Stuttgart, Thieme Medical Publishers, Reproduced with permission [313].) VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC815
8 REZAI ET AL. general agreement about the efficacy of DBS for movement disorders, there is some variance in the protocols for placement of DBS leads (220, 272, 323, 358). The surgical technique has its foundation in stereotactic principles. It has evolved from strong reliance on stereotactic atlases and incorporates advances in imaging and neurophysiological mapping techniques. At present, most neurosurgeons performing DBS use a variety of these approaches to localize the target of interest. These variations are a result of training patterns, surgeon preferences, and surgical practice logistics. There is no single correct approach, as long as outcomes are good and complications are kept to a minimum. The lack of randomized prospective studies comparing one approach to another is also a major barrier to advancement and standardization in this field. The basic components of DBS implantation surgery involve stereotactic anatomic targeting, physiological target verification, DBS lead implantation, and implantable pulse generator (IPG) or power-source placement. The components of the surgery can all be done in one setting or in stages, depending on the group s preference. We review these components, highlighting common practice patterns and acknowledging the variance of practices across centers. Headframes and Acquisition of Stereotactic Coordinates The least debated aspect of the surgery is the method chosen for acquisition of stereotactic coordinates. Currently, both frame-based and frameless techniques are commercially available for localization within the stereotactic space. Frame-based Systems The frame-based approach is the gold standard that has been used for many years with proven precision and reliability. A variety of headframes can be used, such as the Leksell (Elekta, Stockholm, Sweden), Cosman-Roberts-Wells (Radionics, Burlington, MA), Riechert-Mundinger (Fischer-Leibinger, Freiburg, Germany), and other commercially available systems. Based on a survey of North American centers that perform DBS surgeries, it appears that the Cosman-Roberts-Wells frame is the most commonly used, followed by the Leksell frame (Fig. 7) (271). The stereotactic accuracy of each frame has been well established (233), and these frame-based approaches represent the standard of care after decades of clinical use, consistency, and dependability. Placement of the headframe is achieved under local anesthesia. The frame should be placed parallel to a line extending from the lateral canthus to the tragus, to approximately parallel the anterior commissure (AC)-posterior commissure (PC) line (Fig. 8). Frameless Systems The introduction of the frameless technique and device for DBS lead placement (90, 150) has provided an alternative approach that has been embraced by some groups. The framebased fiducials have been replaced by small screws that are visible on computed tomography, which are secured to the patient s cranium before the surgery (Fig. 9). The images FIGURE 7. Leksell stereotactic frame (Elekta, Stockholm, Sweden). The system is based on the center-of-arc principle, and the basic components are the Cartesian coordinate frame, which is attached to the patient s head using four pins, and the semicircular arc. obtained preoperatively are then loaded into a surgical navigation computer, and the fiducials are registered. The frameless assembly is then used to plan a trajectory to the target of interest. The reported advantages of the frameless systems are related to arguments of increased efficiency of surgical planning and imaging acquisition before the day of surgery and enhancement of the patient s comfort with less immobilization of the head and neck (Fig. 10). Currently, there is no major advantage to using one system versus another; the surgeon s preference guides the selection process. Relatively few centers perform frameless DBS surgeries compared with the number that perform frame-based DBS. A randomized prospective study is necessary to determine the levels of patient comfort, precision, outcome, and efficiency inherent in one system versus another. Imaging FIGURE 8. Method for placement of the Leksell headframe. The frame placement is performed under local anesthesia with the patient sitting up. The frame should be placed parallel to a line extending from the lateral canthus to the tragus (orbitomeatal line depicted in red) to approximate the plane of the anterior commissure-posterior commissure (AC-PC) line. Ventriculography In the late 1960s, Guiot et al. (128, 129) defined the position of various deep nuclei based on the distance between the AC and PC and the height of the thalamus, as obtained from ventriculography (Fig. 11). This method was the cornerstone of functional neurosurgery for decades and is still used in several centers worldwide (30, 34, 177). However, the advent of modern imaging has, for the most part, replaced ventriculography with computed tomographic (CT) and MRI scans. CT Scans A thin-cut stereotactic CT scan (approximately 2-mm slices, with no gap and no gantry tilt) can be easily obtained to localize the AC and PC and subsequently be computationally fused with an MRI scan on a stereotactic planning station. CT scans are free from the image distortions inherent to MRI and allow the stereotactic space to be defined with a high degree of accuracy. SHC816 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
9 SURGERY FOR MOVEMENT DISORDERS FIGURE 9. Three-dimensional image showing fiducials placed for frameless registration using a Nexframe (Medtronic, Minneapolis, MN) frameless stereotactic system. The fiducials are small screws that are visible on computed tomography, which are secured to the patient s cranium before the surgery. The preoperatively obtained images are loaded into a surgical navigation computer and the fiducials are registered. The frameless assembly is then used to plan a trajectory to the target of interest. MRI Scans FIGURE 10. Intraoperative image of Nexframe and Nexdrive (Medtronic) systems in use for frameless stereotactic placement of a DBS lead. Nexframe is a disposable device that fits over the Navigus stimloc base (Medtronic) and allows DBS procedures to be performed directly in the operating room or MRI environments without the use of a conventional stereotactic headframe. Once Nexframe is aligned with the intended trajectory using imageguidance software, it functions as a stable cranium-mounted guide for the introduction of the DBS lead. On the top is a disposable microdrive that also acts as a simplified interface for microelectrode recording (MER) and is used for MER and final implantation of neurostimulating electrodes. MRI is the imaging modality of choice in stereotactic targeting and planning. Various sequences can be used. The most common are a T1-weighted, volumetric acquisition of the whole brain with gadolinium enhancement, a T2-weighted axial and coronal acquisition, and inversion recovery (IR) sequences. The T2-weighted and IR sequences delineate the STN and GPi well. The thalamic nuclei, however, are not visualized well on MRI scans of 3 T or less. Anatomic Targeting Anatomic targeting is the initial method for localizing the structures of interest. The goal is to achieve the most precise localization using multiple data sources. Different centers use various combinations of anatomic targeting strategies. In general, one can target via an indirect method using reformatted anatomic atlases and formulas of known distances, or via direct targeting approaches. The STN and GPi can be directly visualized on T2-weighted and IR MRI scans. Currently, imaging resolution is not sufficient to visualize the VIM. Emphasis here is placed on anatomic targeting of the STN, because it is the most common target used for PD, but brief descriptions of GPi and VIM targeting are also included. FIGURE 11. Schematic diagram showing the procedure of ventriculography and location of the AC and PC. Usually radiopaque dye such as iohexol is injected into the lateral ventricle through a frontal parasagittal burr hole, anterior to the coronal suture. Ventriculogram images are obtained in lateral and anteroposterior views with standard magnification by using orthogonal x-ray imaging with a fixed distance. The stereotactic coordinates of the AC, the PC, and the theoretical target points relative to the AC-PC line are then calculated. A FIGURE 12. T1-weighted MRI scans showing stereotactic location of the AC and the PC on axial (A), coronal (B), and sagittal (C) sections. Indirect Targeting Formulas and Brain Atlas Approaches Indirect targeting techniques use the stereotactic coordinates of the AC and the PC as determined by imaging (Fig. 12). The locations of the STN, GPi, and VIM can be subsequently determined based on their average anatomic distances with respect to the AC, PC, and midcommissural point (MCP). Typical anatomic coordinates for the sensorimotor components of these nuclei can be calculated. This includes the STN (11 13 mm lateral to the midline, 4 5 mm ventral to the AC-PC plane, and 3 4 mm posterior to the MCP), the GPi (19 21 mm lateral to the midline, 2 3 mm anterior to the MCP, and 4 5 mm ventral to AC-PC plane), and the VIM upper extremity target (11 12 mm lateral to the wall of the third ventricle, at the level of the AC- B C VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC817
10 REZAI ET AL. PC plane, and anteroposterior location between two- and threetwelfths of the AC-PC distance anterior to the PC). A standardized brain atlas can be used to locate the x, y. and z coordinates of the STN, GPi, and VIM in relation to the MCP (Figs. 3 and 4). The stereotactic atlas can be stretched and morphed using surgical navigation software to better fit each patient s anatomy. However, despite these technological advances, it is important to realize the limitations of the stereotactic atlases. The data in most atlases are based on a small number of brains. The Schaltenbrand and Wahren atlas (313) uses one brain for the frontal series and one brain for the axial and sagittal series. The Talairach and Tournoux atlas is based on one brain (339). The morphology and position of the STN is different in each atlas (257, 301), and the actual size and the position of the STN are highly variable among patients (136, 301) and within the stereotactic atlases. Direct Targeting With the advances in neuroimaging technology, direct visualization of the various nuclei has become possible. Although computed tomography offers excellent stereotactic precision, it can be difficult to visualize various targets and periventricular landmarks (375) when using it. MRI offers the advantage of excellent anatomic resolution in multiple planes. This allows for localization of the AC and the PC on T1-weighted images (Fig. 12), the visualization of the pallidum on IR and T2- weighted sequences (Fig. 5), and identification of the STN on axial, sagittal, and coronal T2-weighted images (Fig. 13) (32). The advantage of directly visualizing deep targets is implicit; one works with the patient s individual anatomic variation rather than relying on a fixed brain that was sectioned several decades ago. Some centers rely entirely on MRI scans to calculate anatomic targets (272, 330, 334). There are questions, however, regarding the accuracy of the exact location of these targets within the stereotactic space because of distortion on MRI scans (311). To reduce the possibility of MRI-related inaccuracies, several centers use a protocol of merging the anatomically superior MRI scans to stereotactically acquired CT scans (19, 220, 323, 375). Several authors have described strategies to further refine image-based targeting. Arguing that the relationship of the AC- PC line and the STN may be variable and inconsistent, they propose the use of a landmark that is physically closer to the target of interest (16, 24, 78, 330). In 2000, Bejjani et al. (24) described using the anterior border of the red nucleus as a landmark for the AP coordinate of the STN. This approach has also been used by others (17, 78). Axial and coronal T2- weighted images are particularly important for adequate visualization of the STN, as a sharp contrast can often be observed between the nucleus and the surrounding white matter. The red nucleus and the STN can be clearly visualized. The STN lies anterior and lateral to the red nucleus and, in this regard, the anterior border of the red nucleus can be used as a landmark for the STN target (Figs. 3 and 13). Starr (330) later described the relationship between the center of the red nucleus and the middle of the electrode array as another internal landmark for A targeting the STN. In the authors experience, the interpeduncular distance can also serve as a good surrogate for the laterality of the STN target (unpublished data). The possibility of directly visualizing and targeting the STN and GPi has brought forth innovative imaging application possibilities for DBS surgery. The use of intraoperative MRI to perform DBS surgery is being investigated by Martin et al. (235). Their preliminary results show that successful DBS implantation can be performed in patients under general anesthesia with only anatomic targeting. This approach has multiple inherent advantages that will facilitate its acceptance and widespread use once additional studies demonstrate that the safety and efficacy are equivalent to the traditional techniques. Trajectory Planning Imaging is necessary for accurate targeting as well as for planning of the surgical trajectory to the target. The strategy is to avoid surface and subcortical vessels and to have an angle of approach that passes through a large segment of the structure of interest. The precise entry point may be refined on the planning console, such that the trajectory passes through the crown of a gyrus rather than into a sulcus, as well as away from the vessels associated with the wall of the ventricle, thereby helping to avoid hemorrhagic complications (Fig. 14). Neurophysiological Assessment and Verification B FIGURE 13. High resolution axial (A) and coronal (B) T2-weighted MRI scans showing the STN and structures in close proximity to it. STN, subthalamic nucleus; SNR, substantia nigra reticulate; RN, red nucleus. All surgeries for movement disorders are initially based on anatomic targeting techniques. However, physiological verification of these targets is a necessary step before final implantation of the DBS electrode can occur. This is crucial because anatomic inaccuracies due to image distortion, brain shift, cerebrospinal fluid loss, and pneumocephalus can lead to final target deviation (124). A DBS lead that is misplaced by as little as 2 mm can result in inaccuracy when locating the final target. In a more practical sense, neurophysiological techniques are necessary to refine lead positioning within a target and to optimize clinical outcome and minimize stimulation-related side effects. In the lesioning era preceding DBS, physiological verification was a major requirement before the creation of SHC818 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
11 SURGERY FOR MOVEMENT DISORDERS FIGURE 14. Navigation view on Framelink (Medtronic) software using a contrast enhanced, T1-weighted MRI scan showing the enhancing vessels in the periventricular region. The trajectory (blue arrows and lines) is carefully planned to avoid these deep vessels and more superficial cortical vessels. The right upper panel demonstrates the images in a plane perpendicular to the planned trajectory (red point). lesions. Presently used physiological techniques include microelectrode recording (MER), semi-mer, macrostimulation, and DBS lead stimulation. The degree of dependence on these techniques varies widely. The exact detail of mapping for each target is beyond the scope of this article; however, generalities are provided and details can be obtained in the references provided. MER The MER technique uses microelectrodes with high impedances (typically 0.4 mω) with a tip diameter in the range of 2 to 4 μm (124, 158, 207). These microelectrodes are capable of recording single units as well as delivering stimulation in the microamp range (typically 100 ma). A hydraulic or electrical microdrive is used to advance a microelectrode in submillimetric steps. United States Food and Drug Administrationapproved microelectrodes are commercially available and are made of tungsten or platinum/iridium. Some centers use a single MER penetration as confirmation of the anatomic targets, whereas others rely on one or more tracks that reveal a set of acceptable criteria, such as an approximately 5-mm long area of STN (231). There are also centers that use a multiple-track penetration approach for very detailed physiological mapping of the borders of the nuclei. The hope is to improve treatment efficacy and limit postoperative side effects that are related to undesired stimulation of bordering structures (123, 373). In a survey of 36 DBS centers in North America, Ondo and Bronte-Stewart (269) found that 97% of centers use MER for assistance in lead placement. The average number of tracks was 2.3 per electrode, with a range of 1 to 18 tracks. They also reported that most centers use macrostimulation to assess the final clinical response. Although most centers advance one microelectrode at a time, several centers advance multiple microelectrodes simultaneously and assess a larger area of the target (24, 31). Delivering stimulation through the microelectrode, when feasible, is performed at some centers to assess side effects resulting from proximity of the track to other structures such as the internal capsule (124, 372). MER allows for the delineation of the physiological signature of various nuclei and white matter tracts. Single neurons, multiunit activities, and local field potentials can be discerned with characteristic sound and visual expressions. The frequency and pattern of activity are observed, thus helping to confirm location based on characteristic physiological signatures. The boundaries between white matter and nuclei are important to distinguish, as are the length of the desired nucleus and an assessment of the surrounding structures. The MER physiology of the STN, GPi, and VIM is discussed briefly below. The STN The information obtained in the track, such as the presence or absence of thalamus or SNr, could also aid in determining the trajectory in relation to the nucleus, i.e., medial, lateral, anterior, or posterior. Figure 15 shows a sample MER trajectory aimed at the STN. The thalamus is typically the initial structure encountered by the MER. The specific thalamic nuclei recorded depend on the AP angle of approach, but typically include the nucleus reticularis (Rt), the Voa, and the Vop. There are two typical cell activities: bursting units (interburst frequency, Hz) and irregular tonic firing ( 28 Hz) cells. The background activity is substantially less dense than the background activity of the STN. After exiting the thalamus, a decrease of background activity coupled with the resolution of, generally, fewer firing units indicate the zona incerta (ZI) and fields of Forel. Activity in these areas has a similar bimodal distribution of bursting and tonic firing units, usually with low firing rates. A substantial increase in background neuronal activity signals the entry into the STN. This increase in background activity, perhaps the most distinguishing characteristic of the STN compared with the other structures encountered in this procedure, can precede the resolution of single-unit activity indicative of the STN by 1 to 2 mm. Mean firing rates have been reported in the 34- to 47-Hz range, with standard deviations in the 25-Hz range. Bursting units are common. The pattern of activity is typically irregular. Cells that respond to passive movement of the limbs are encountered in the dorsolateral part of the STN. Within this motor area, lower extremity-related units tend to be VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC819
12 REZAI ET AL. FIGURE 15. Representative samples of neuronal recordings in a typical tract while targeting the STN. All of the samples are 1 second in length and demonstrate the typical firing pattern at each of those nuclei. (Reproduced with permission from Thieme). more medial than upper extremity-related units. An abrupt decrease in background noise is indicative of exiting the STN and entering the SNr. The gap between the STN and the SNr can vary from a few hundred microns to 3 mm. In general, the features that distinguish the SNr from the STN include higher firing rates (50 70 Hz), a paucity of kinesthetic-responsive units, and a more regular firing pattern. The GPi During MER, the general objectives of the mapping strategies are to define a long segment of sensorimotor GPi, determine the border regions between the GPi and the GPe, identify the optic tract at the bottom of the trajectory by means of visual evoked potentials, and distinguish the internal capsule, which is medially and posteriorly located (124). Several authors advocate use of more than one track to gather this information (10, 23, 125, 130, 181, 372). Although some groups use only one or two tracks (15, 277), there are insufficient data to state which method of neurophysiology is superior for mapping the GPi and which mapping strategy has a better outcome. In their review, Gross et al. (124) stated that, based on the existing neurophysiological data, it is unclear how much the final target within the GPi is modified. The VIM Historically, the VIM target has been localized between two- and three-twelfths of the AC-PC distance anterior to the PC (128, 129). Most centers target the sensory nucleus because of its consistent somatotopic arrangement, which can be easily identified during MER by eliciting evoked responses from tactile-stimulation-responding neurons (231). The general strategy is then to identify the border between the sensory nucleus and the VIM where the cerebellar afferents are received. Once the border is identified and the somatotopic laterality is established, the DBS electrode can be placed approximately 3 to 4 mm anterior to the border of thalamic sensory relay nucleus to avoid current spread into the sensory nucleus and development of persistent paresthesias as a side effect of stimulation. Despite the widespread use of MER, whether the utility of MER approaches is superior to other methods is an area of debate and controversy. There are centers that still use only macrostimulation, and they report comparable results (137). There is a need for a prospective randomized study comparing MER to non-mer approaches to reconcile this issue. In addition to MER, there are centers that use semi-microelectrodes to map the corresponding nuclei and white matter. Semimicroelectrodes have lower impedances and cannot discriminate single neurons, but do provide good physiological data regarding the structures being traversed (108, 393). Macroelectrode Mapping and Stimulation Macrostimulation involves stimulation in the range of milliamps to determine benefits and side effects. There are several different ways of delivering macrostimulation. Macrostimulation can be performed with a lesioning probe or, most commonly in the United States, the DBS electrode itself can be used as the macroelectrode (269). This is advantageous as the results obtained during surgery are likely to be reproduced with chronic stimulation from the DBS. Still, macroelectrode/dbs stimulation is one of the important steps for DBS surgery as it provides insight into therapeutic efficacy and stimulation-induced side effects. DBS Electrode Implantation In planning the implantation, it is important to understand that the active site of chronic stimulation may not be the bottom of the target at the bottom of the trajectory. In the STN implant, for example, the bottom contact of the quadripolar STN electrode is seldom used because the optimal site for stimulation is believed to be at the dorsolateral segment of the nucleus or immediately dorsal to it (146, 308). The two commercially available electrodes have four contacts of 1.5 mm in height and 1.27 mm in diameter and differ only in the spacing between contacts: 1.5 mm in the 3387 model and 0.5 mm in the 3389 model (Medtronic, Minneapolis, MN) (Fig. 16). Fluoroscopy is used at many centers to monitor the DBS lead implantation and ascertain that it is assuming a straight trajectory that does not deviate from the intended target. Once implanted, the electrode may cause a microlesional effect that is manifested by transient improvements in tremors and, in the case of PD, rigidity and bradykinesia. Such an effect is seldom observed during GPi surgery for dystonia. With DBS intraoperative test stimulation, the patients are assessed for clinical benefits and side effects. The typical parameters mirror the settings used for chronic stimulation SHC820 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
13 SURGERY FOR MOVEMENT DISORDERS A B C FIGURE 16. A, the two commercially available electrodes each have four contacts of 1.5 mm in height and 1.27 mm in diameter and differ only in the spacing between contacts, as illustrated in B. C, the placement of the DBS lead, connectors, and implantable pulse generator (IPG) in a human. (A and C obtained from B, printed with permission from Wiley-Liss). and include 1 to 5 V, 90 μs pulse width, and 130 Hz frequency. The larger the difference between clinical improvement thresholds and side-effects thresholds, the better the therapeutic window of stimulation for the patient. During macrostimulation, the patient is monitored for symptomatic improvement such as tremor, rigidity, and bradykinesia. Dyskinesias may appear during stimulation and are generally a positive predictor of the efficacy of chronic stimulation (157, 290). The importance of side-effect determination should be underscored, especially for patients in whom the therapeutic efficacy is unclear or situations in which the patient s cooperation is hampered (30, 31, 330). Once the DBS electrode is implanted at the final location, it must be secured to the burr hole at the cranium. Continuous fluoroscopy is helpful to monitor the potential for electrode displacement. Anchoring and securing the lead can be achieved by various techniques depending on the surgeon s preference and expertise. These include securing the lead to the cranium with ligature embedded in dental cement or using mini-plates and screws, DBS manufacturer-provided plastic burr hole ring and cap, or the Medtronic Stim-Loc anchoring device (Medtronic). Once secured, the distal end of the DBS lead is attached to an extension wire or to a connector that will protect the contacts. The distal tip is tunneled subcutaneously to the parietal/occipital region. The excess lead can be coiled around the burr hole device or placed along the path of tunneling to serve as strain relief. Implantation of the Pulse Generator The second stage of the DBS procedure is implantation of the implantable pulse generator (IPG), also referred to as the neurostimulator, and placement of the extension lead that connects the DBS lead to the IPG. Currently, there are two types of available IPGs: single channel (Medtronic Soletra) for one DBS lead, and dual channel (Medtronic Kinetra) for two leads. This is the last step of surgery, and it is performed under general anesthesia. This step can be performed the same day or in a delayed or staged fashion. The patient is placed in a supine position, with the head turned to the opposite side of the intended site of IPG implantation. In brief, an infraclavicular subcutaneous pocket is created for the IPG, and the proximal end of the DBS electrode is exposed in the parietal region. A subcutaneously implanted extension wire is tunneled from the parietal region to the infraclavicular pocket, thus connecting the DBS electrode to the IPG pocket in the chest. The most common location for the IPG placement is infraclavicular, and it is typically marked 1 to 2 cm below the clavicle and 4 cm away from the midline or 2 cm from the lateral manubrial border. However, certain patients may require placement in other locations due to body VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC821
14 REZAI ET AL. habitus (very thin patients), age (pediatric patients), activities and hobbies, a history of previous surgery in the region, or cosmetic reasons. Outcomes of DBS for Movement Disorders The literature relevant to movement disorder surgery is extensive: There are more than 1000 published articles pertaining to DBS for movement disorders. In addition to the retrospective and case report format of much of the literature, the reversibility feature (turning DBS on and off) on-demand allows for controlled, blinded assessments, making it one of the better-studied neurosurgical interventions. In addition, validated rating scales for movement disorders have been established and are used in most surgical trials. These standardized, disease-specific rating scales allow for outcomes to be expressed in a more objective fashion that is specific to the disease of interest. STN and GPi DBS for PD (see video at web site) DBS has become the surgical procedure of choice for movement disorders, replacing stereotactic ablative procedures, for the most part, in countries where access to this technology is available. Outcomes from DBS are expressed more frequently as absolute or as percent score reductions in the Unified Parkinson s Disease Rating Scale (UPDRS) Part III (motor) during the medication-off state. Thus, UPDRS Part III is a standard outcome scale indicating motor benefits from a therapy. Data on the impact of DBS upon activities of daily living (ADLs), percent reduction in dyskinesias, or incremental on time periods without dyskinesias are inconsistently reported. Reductions in dyskinesias can be considered as a direct effect of DBS or may be secondary to a reduction in medication requirements (183, 369). Prospective studies have reported on the outcomes of GPi and STN DBS for the cardinal symptoms of PD. Both targets are shown to be beneficial (177, 183), although a trend exists among these studies to indicate that STN DBS is more effective. In addition, STN DBS tends to allow for a greater reduction in the postoperative medication burden with consequent reduction in dyskinesias (80, 86, 181, 183, 282, 303). Direct comparisons of GPi versus STN stimulation have been performed in small samples of patients. The outcome data from these studies were not conclusive enough to exclude the GPi as an accepted DBS target for PD (368) and generally corroborated the advantage of using the STN in improving UPDRS Part III scores and L-dopa intake (15, 59). In addition, a recent report of long-term bilateral pallidal stimulation in 11 PD patients confirmed the therapy s sustained efficacy in alleviating dyskinesias. However, motor scores that had been alleviated in the first year deteriorated during the 5-year follow-up to an extent greater than would be expected from disease progression alone. The lost motor benefits were not regained with additional programming, but were successfully recaptured in four patients by repositioning the electrodes from the GPi to the STN (378). As discussed below (in Complications of DBS Surgery), it is possible that STN stimulation is more prone to cognitive and behavioral complications (see Cognitive and Neurobehavioral Outcome and Complications with DBS). However, outcomes from upcoming randomized, prospective large studies are expected to provide more insights into the relative efficacy and risks associated with STN versus GPi DBS for the treatment of PD. The encouraging results of STN DBS originally reported by Benabid and the pioneering Grenoble group (26, 31, 180, 181, , 289) motivated a large number of studies in the past decade that have further validated the safety and efficacy of this procedure (80, 67, 79, 93, 94, 102, 107, 134, 145, 160, 165, 208, 212, 225, 258, 271, 276, 281, 304, 307, 315, 318, 344, 359, 360, 362, 394). A meta-analysis of the literature published in 2006 reviewed the literature from 1993 to The mean reduction in UPDRS Part III scores among the 34 articles included in the study was 52% (comparing the DBS-on, medication-off state to the medication-off, DBS-off state). There was a large variation in reported outcomes, ranging from 82 to 17%. The mean reduction in UPDRS Part II scores was 49.9%, ranging from 72 to 29.5%. As noted above, the preoperative response to L-dopa is considered a good outcome predictor of response to surgery and is, therefore, a heavily considered determination regarding a patient s candidacy for surgery. The correlation between L-dopa response and positive outcomes after STN DBS was confirmed by this meta-analysis as well as other studies (384). A few prospective, controlled studies have provided fundamental contributions to the DBS literature and merit more detailed discussion. In 2001, the DBS for PD Study Group reported on the outcomes of 96 patients undergoing STN and 38 patients undergoing GPi DBS. The improvements in UPDRS Part III motor subscores at the time of the 3-month follow-up (assessed with double-blind evaluations after patients were randomly assigned to stimulation-on or -off states) were 49 and 37% for these groups, respectively. A continuation of this study was reported in 2005 with 3-year or longer follow-up period for 69 patients from the initial study. The 3-year or longer follow-up data demonstrated that the effects of DBS for PD are long-lasting. The long-term benefits of DBS were later substantiated by Krack et al. (177). Forty-nine consecutive patients treated with bilateral STN DBS were assessed at 1, 3, and 5 years after implantation. The mean reductions in UPDRS Part III scores at these time points were 66, 59, and 54%, respectively. ADLs were also improved. A significant decrease in efficacy was observed when the first and fifth years after surgery were compared. Nevertheless, most of these patients were dependent upon others before surgery and continued to enjoy independence throughout the entire follow-up period. Similar 5-year follow-up results were reported by Schüpbach et al. (315), with 54% reductions in UPDRS Part III scores and a 40% maintained reduction in UPDRS Part II scores. Additional longterm outcome studies with follow-up periods ranging from 2 to 4 years reported on mean UPDRS Part III reductions of 48% in 25 patients (170), 43% in 20 patients (367), 55% in 22 patients SHC822 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
15 SURGERY FOR MOVEMENT DISORDERS (271), 57% in 29 patients (148), and 45% in 20 patients (365). The latter study also reported on complete withdrawal of medication (replaced by stimulation) in 10 out of 20 patients. Such a dramatic and early reduction of medication intake may have accounted for some of the complications observed by the authors, such as dysarthria and cognitive problems (200). Thalamic (VIM) DBS for Tremor (see video at web site) The standardized assessment of tremor can be achieved via the Tremor Rating Scale (TRS) (151, 329). Stereotactic thalamotomies targeted at the VIM nucleus are well-established procedures for the management of tremors from PD or essential tremor (8, 103, , 166, 167, , 343). In managing patients with PD, thalamotomies alleviate tremors without significantly affecting the other cardinal symptoms of PD. Unilateral thalamotomies are considered relatively safe, but bilateral procedures carry an elevated risk of neurological deficits such as dysarthria and cognitive deterioration (237). Chronic stimulation was considered a potential alternative to thalamotomy, at least partly because the known tremor-alleviating effects of acute stimulation were used for physiological confirmation during ablative stereotactic interventions (116, 255). In addition, thalamic chronic stimulation had already been demonstrated to be feasible and safe for patients undergoing stereotactic interventions for chronic pain conditions (153, 154, 192, 211, , 354). Benabid et al. (35, 36) initially applied thalamic stimulation contralateral to thalamotomy in patients with PD. Their preliminary experiences revealed a greater efficacy of thalamotomy over stimulation. In 1991, the results of chronic VIM stimulation for tremor were reported in a series of 32 patients with essential tremor or PD who had undergone 43 thalamic stimulation implants (11 patients underwent bilateral stimulation). At a mean follow-up period of 13 months, 88% of the implanted DBS electrodes resulted in major or complete relief from tremors. DBS, initially considered an alternative to stereotactic thalamotomy, gradually became the surgical procedure of choice for the treatment of essential tremor, as it demonstrated similar efficacy rates and lower risks (174, 275, 316, 343). A direct comparison between thalamotomy and thalamic stimulation was reported by Schuurman et al. (316). Seventy patients with PD, essential tremor, or tremor from multiple sclerosis were randomized to stimulation or ablative surgery groups. Patients with unilateral symptoms underwent a single intervention contralateral to the symptoms. Patients with bilateral tremors underwent either bilateral thalamic stimulation or a unilateral thalamotomy with contralateral stimulation. Patients with PD and essential tremor who had undergone thalamic stimulation performed significantly better in ADLs than those undergoing ablation. Sixteen adverse effects occurred among the patients randomized to the thalamotomy group. In comparison, only six patients with thalamic stimulation experienced adverse effects, which were successfully resolved with stimulation cessation. Pahwa et al. (275) reported similar results when comparing the outcomes of 17 patients undergoing thalamic stimulation to the outcomes of 17 patients who had previously undergone thalamotomy. Although the effects from tremor suppression were very similar in both groups, complications, particularly intracerebral hemorrhages, were more common among patients with thalamotomies (35 versus 0%). Likewise, cognitive deterioration and hemiparesis occurred, respectively, in 29 and 12% of patients who had undergone thalamotomies but in none of those with thalamic stimulation. Although thalamic stimulation is chronically effective for most patients (37, 57, 186, 213, 222, 228, 293, 316), reductions in efficacy during longer-term follow-up periods have been reported (32, 173). The vast majority of thalamic DBS procedures have been targeted at the upper extremity function. However, lower extremity, head/neck, and axial tremor are also common problems that negatively impact quality of life for patients with essential tremor. Putzke et al. (293) reported on the outcomes of 22 patients with head, voice, or trunk tremor undergoing bilateral, staged, DBS thalamic implants. Bilateral stimulation was more effective than unilateral stimulation in alleviating axial tremors; however, as for bilateral thalamotomies, the rate of neurological complications was higher in patients who underwent bilateral stimulation. Dysarthria was observed in 27% of patients with bilateral stimulation, whereas none of those undergoing unilateral stimulation experienced the same problem. Likewise, disequilibrium was more common during bilateral stimulation. Although unilateral stimulation was comparatively less effective, it still demonstrated a significant reduction in axial tremors when compared with the preoperative baseline and stimulation-off periods. These findings are supported by the work of Koller et al. (172) in a prospective assessment of 38 patients with head tremor undergoing unilateral thalamic stimulation. In this study, 71% of patients presented with tremor alleviation at 3 months. The effects remained generally stable over the long-term (1 yr) follow-up period. Stimulation settings varied minimally during this period, further corroborating the stability of the effects. Although staged bilateral procedures are often preferred for axial symptoms, they may not be safer than simultaneous implantation procedures (337). GPi DBS for Dystonia Stereotactic ablative surgery of the GPi (pallidotomy) has been attempted in the past in patients with generalized dystonia. Encouraging results have been reported (91, 92, 150, 371) but, unlike reports of thalamotomies for treating tremor, the best results are not observed immediately, but rather, after several weeks or months (218, 224). Unilateral and, in particular, bilateral pallidotomies may carry a higher risk of neurological morbidity, including lethargy and hemiparesis (270, 345), even in the absence of hemorrhagic complications. Excellent results from bilateral stimulation of the GPi were initially reported for an 8-year-old child with severe dystonia (75). In this age group, bilateral pallidotomies were considered by the authors to be particularly risky (74), and DBS was attempted as a compassionate, last-resort alternative. The results encouraged formal assessment of this technique with prospective cohorts. In most VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC823
16 REZAI ET AL. of the literature, treatment outcomes for generalized dystonia are measured using the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS), whereas treatment outcomes for torticollis are assessed using the Toronto West Spasmodic Torticollis Rating Scale. The former has a total of 120 points (higher is worse) and takes into account the severity at each segment as well as the provoking factors. The latter scale, which is specific for torticollis, takes into account severity, disability, and pain. In 2004, Coubes et al. (74) reported on the long-term results of 31 patients with primary generalized dystonia, 14 of whom were positive for the DYT-1 gene mutation. There was an overall mean improvement of 50% in the BFMDRS at 3 months, with additional gains observed at the 2-year follow-up evaluation, reaching a mean improvement of 65%. DYT-1-positive patients had improved more (74%) than patients with DYT-1- negative disease (58%). Subsequent series also found better outcomes among primary dystonia patients (92), with most dramatic improvements observed in patients with disease of early onset (184). Gradual improvement (or maintenance) of results after 3 years has been demonstrated (364), further validating DBS for treatment of generalized dystonia. Significant and sustained long-term improvements have been reported in patients with spasmodic torticollis (49). Pallidal stimulation for dystonia has been formally assessed in prospective, controlled, multicenter studies. Results from a series of 22 patients with primary generalized dystonia (seven of whom were DYT-1 positive) were described by Vidailhet and the French Stimulation du Pallidum Interne dans la Dystonie Study Group (363). At the 3-month follow-up, investigators who were blind to the status of stimulation assessed dystonia severity through video recordings. At 12 months, the dystonia-movement scores had decreased to a mean of 21, compared with a baseline preoperative mean score of Similar blinding methodologies were used by Kupsch and the DBS for Dystonia Study Group (194) to assess patients with primary generalized or segmental dystonia. Greater reductions in the dystonia-movement scores were evident in the stimulation group (15.8 points, 39.3%) than in the sham stimulation group (1.4 points, 4.9%). Surprisingly, there were no significant differences in the degree of amelioration of patients who were positive for the DYT-1 mutation versus those who were negative for the mutation. Significant differences were also not apparent when the outcomes of patients with generalized dystonia were compared with those with segmental dystonia. In summary, GPi or STN DBS has been demonstrated to be effective in alleviating the symptoms of medically refractory PD in multiple reports in the literature. These results were confirmed by prospective series with double-blinded assessments and were largely sustained at 5-year follow-up evaluations. A trend exists in favor of STN versus GPi DBS that must be verified once the outcome of additional studies comparing GPi and STN is available. Chronic stimulation of the VIM thalamus has become the procedure of choice for the treatment of tremors because of its associated high efficacy rates and low risks. VIM DBS is also highly effective for PD tremor, but it is rarely performed today for PD because both STN and GPi DBS significantly improve tremor as well as other manifestations of PD. Patients with axial tremors tend to benefit from bilateral stimulation, which carries a higher risk of adverse neurological effects. Bilateral pallidal (GPi) DBS is safe and effective for alleviating primary generalized and segmental dystonia, but the results may not be evident until after several weeks or months of stimulation. Patients who are positive for the DYT-1 mutation and those with disease of early onset may experience greater benefits. In reviewing the outcomes of movement-disorder surgery, it is important to emphasize that there should be more randomized, prospective, and controlled studies in the future. In addition, systematic and standardized assessment, reporting, and publication of outcome and complications are necessary. This includes the use of published and accepted standardized rating scales for tremor, PD, and dystonia, with evaluations performed by a blinded observer who is unaware of the stimulation status (on versus off). The implementation and publication of standard and rigorous study designs and outcome reporting will facilitate the acceptance and development of DBS surgery as a standard therapeutic modality for movement disorders. Cognitive and Neurobehavioral Outcome and Complications with DBS The vast majority of data documenting neurobehavioral outcomes after DBS involve patients who underwent surgery in the STN. The most common neuropsychiatric side effect in the immediate postoperative period after STN DBS is transient confusion, with an incidence that ranges between 1 and 36% (5, 18, 61, 72, 95, 107, 131, 138, 177, 244, 250, 281, 285, 286, 303, 352, 380, 389, 390). Evidence of greater neuropsychological deficits before surgery is significantly associated with increased confusion after surgery (284). In general, the data suggest that the most frequently observed long-term neuropsychological change after STN DBS is a decline in word fluency. A recent meta-analysis of the available data confirmed this finding (281). The meta-analysis revealed much smaller, yet significant, declines on measures assessing executive function, verbal learning, and memory. These findings are consistent with those reviewed elsewhere in the literature (380, 390). However, it is critical to recall that the majority of available neuropsychological outcome studies have not included control groups, did not statistically control for potential practice effects, and generally consisted of small groups, resulting in reduced power; this makes it very difficult to ascertain significant cognitive effects unless the related effect sizes were very large. Virtually no studies have clearly identified potential risk factors for increased cognitive decline after STN DBS. Aybek et al. (18) recently reported that the incidence of conversion to dementia in PD patients who underwent STN DBS over a 3-year follow-up period was similar to the incidence reported for medically treated patients. Furthermore, risk factors for the development of dementia in surgical patients were similar to those identified in non-surgical PD patients and included increased age, presence of hallucinations, and reduced scores SHC824 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
17 SURGERY FOR MOVEMENT DISORDERS on measures of executive cognitive function. The preliminary data suggest that DBS in the STN may result in greater cognitive and neurobehavioral changes than GPi DBS in patients with PD (15, 303, 378). This observation might reflect the relatively smaller size of the STN with the increased proximity of associative (cognitive), limbic, and motor circuits, and the subsequent increased likelihood of misplaced electrodes and/or current spread to non-motor circuits. More detailed, prospective studies are necessary to ascertain the relative neurobehavioral risks associated with STN versus GPi DBS for the treatment of PD. Despite the methodological concerns and relatively limited data, it appears that STN DBS is a relatively safe procedure from a neuropsychological perspective in wellselected patients. Neuropsychiatric symptoms have also been reported after STN DBS, and several studies have reported hypomania, depression, apathy, and suicidality (380). Postoperative hypomania was reported in 4 to 15% of STN patients in four studies (79, 147, 177, 306), and postoperative depression has been reported to occur in up to 1.5 to 25% of patients (147, 156, 177, 236, 245, 272, 302, 306, 348, 358, 379). The extent to which medication changes after surgery contributed to the reported changes in mood state is not well known. Overall group depression scores have been reported to improve at 3 and 12 months after surgery in multiple studies (79, 89, 285, 379); however, most studies relied on reporting group mean depression scores, which may be misleading. A more accurate indication of the number of patients who met criteria for depression before and after surgery is a more clinically relevant variable. Some uncontrolled series have documented suicide attempts and/or suicides after placement of DBS electrodes in the STN (45, 177), but, once again, there are no data to indicate a clear relationship to stimulation. Within the first 3 postoperative months, apathy, which can respond to administration of dopaminergic medication, can occur, although the incidence is unknown. In contrast, more permanent apathy was identified in 12% of patients, in whom 80% had associated decreases on executive cognitive measures (177). It is important to recognize that all of the neuropsychiatric symptoms previously described are evident in medically managed nonsurgical patients with PD. Consequently, it is very difficult to ascertain, on the basis of the current literature, the extent to which STN DBS is truly associated with increased neuropsychiatric symptoms versus the role of underlying ongoing disease progression. The neurobehavioral outcome literature after DBS in the GPi and VIM is much more limited than that pertaining to STN DBS. Many studies examining cognitive and neuropsychiatric outcomes after GPi DBS documented no significant declines. Isolated studies identified mild declines on measures of word fluency and visuoconstructional skills (380). There are very few studies examining neurobehavioral outcomes after VIM stimulation, and interpretation of the available data is confounded by small samples of patients with mixed diagnoses. In general, most of the data revealed no significant cognitive or neuropsychiatric declines. Improvements in verbal memory were documented in two studies, whereas declines in word fluency were documented after left-sided VIM stimulation in one study (380). In summary, the neurobehavioral outcome literature suggest that the cognitive effects associated with DBS in the STN, GPi, and VIM are relatively minor in well-selected patients. The neuropsychiatric outcome data are more limited, and surgeons should be alert to the possibility of neuropsychiatric symptoms after surgery, particularly STN DBS, but it is still unknown whether these symptoms reflect neurosurgical/neurostimulation effects or ongoing disease progression. Complications of DBS Surgery Understanding the complications of any surgical procedure helps in anticipating, preventing, recognizing, and promptly intervening on such occasions. The complications of DBS surgery can be mainly classified into four categories. These include intracranial hemorrhages, infections, hardware-related issues, and stimulation-related complications. The incidence of reported complications is variable among centers and has been changing over the past few years as a result of the increased experience of the surgical team, advancement in techniques, and improvement in devices. Another factor contributing to the observed variability in complication reporting is the lack of a systemic and consistent process for complication definition, recording, and assessment. For example, not all centers reporting outcomes obtain postoperative imaging to evaluate for a hemorrhage that is nonsymptomatic. Instead, imaging is only performed if there is clinical change in the patients. Similarly, the reporting of infections is inconsistent because the definition of infection varies (i.e., an infection that requires hardware explantation versus a superficial wound infection). Intracranial Hemorrhage Intracranial hemorrhage is one of the most important complications of movement-disorder surgery. Intraoperative hemorrhages are reported to occur in 0.2 to 12.5% of all STN DBS cases (43, 46, 47, 50, 84, 121, 139, 161, 171, 173, 175, 177, 211, 212, 229, 347, 356, 361). The correlation of hemorrhage with the type of procedure is an area of controversy. According to Blomstedt and Hariz (50), there is no significant difference between the hemorrhage risk in lesioning (1.6%) versus DBS surgeries. Terao et al. (347), however, reported a lesion surgery bleeding rate of 15.8% (thalamotomy, 21.7%; pallidotomy, 11.8%) versus a hemorrhage rate in DBS operations of 3.4% (347). Hemorrhages can be extradural, subdural, and intraparenchymal. Intraparenchymal hemorrhages are the most common and typically occur in the tract of the electrode or in the periventricular region in close proximity to vessels associated with the ventricles (Fig. 17). The size of the hemorrhages is generally small. There is little agreement on the predictors of intraoperative hemorrhages in patients who undergo DBS. The common factors identified include: 1) High blood pressure: the practices of carefully controlling blood pressure and painstakingly planning the trajectory, avoiding vasculature seen on the contrast-enhanced preop- VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC825
18 REZAI ET AL. A FIGURE 17. Axial non-contrast computed tomographic (CT) scans showing intraparenchymal hemorrhage during the placement of a DBS electrode. A, hemorrhage at the thalamus (target site). B, hemorrhage at the periventricular region with a small intraventricular extension. erative images, help reduce the incidence of hemorrhages. There is a statistically significant association of hemorrhagic complications with hypertension (118). Bleeding occurs in 10.71% of hypertensive patients and 0.91% of those who were normotensive (P ). The same study also documents that the combination of MER and hypertension increases the risk of hemorrhage to 16.67% (118). 2) MER: an increased incidence of bleeding in hypertensive patients who underwent MER (P 0.034) was observed by Gorgulho et al. (118). There are reports that strongly implicate MER as a risk factor for hemorrhage (137), and there are studies that state otherwise (46, 47, 372). It is difficult to draw any conclusion from the available literature pertaining to increased risk of hemorrhage with MER. 3) Target: some studies have documented that the GPi is more prone to hemorrhagic complication compared with the STN or the thalamus. Binder et al. (47) have shown a 7% risk of GPi hemorrhage compared with 2.2% risk of STN hemorrhage. In 2001, the DBS study group reported similar results (GPi, 9.8%; STN, 2.9%) (80). It has been suggested that anatomic peculiarity of the vasculature in the GPi region may be responsible for the increased incidence of hemorrhage (47). The GPi is supplied by the lenticulostriate arteries that come from the anterior circulation. These arteries are more prone to the effects of hypertension and may also be developmentally different (47). 4) Trajectory planning: the use of image fusion of CT and MRI scans helps in performing accurate targeting. The images that help most in avoiding hemorrhagic complications are the postcontrast T1-weighted MRI scans. These images reveal small paraventricular, sulcal, intraventricular, and ependymal vessels (Fig. 14) and assist with the planning of a vessel-free trajectory. Infections Reported infection rates for DBS surgery vary widely, from less than 1% to as high as 15% (11, 43, 46, 47, 51, 50, 76, 80, 84, B FIGURE 18. Intraoperative image obtained during removal of an infected IPG showing the infected IPG with subsequent wound breakdown. 87, 112, 118, 124, 168, 171, 175, 194, 212, 229, 259, 265, 346, 349, 356, 361, 374, 376). This is probably because different clinical definitions are used for identifying infection. The criteria for diagnosing infections are not well defined in the reported literature. DBSrelated infections have a variable presentation in terms of time and location. Typically, the infection presents within 3 months after surgery, and the most common site was at the IPG (Fig. 18) (51, 80, 168, 194, 229, 349, 356, 374, 376). Infections of the IPG tend to present soon after surgery, as do infections at the burr hole. Infections at the connector may be related to erosions. This scenario was more common in the past, when the extension connectors were larger. The introduction of the lower-profile extension connector has significantly reduced the incidence of erosions. Clinically, the infections presented as cellulitis, erythema, drainage, dehiscence, or stitch abscess. The common bacterial pathogens isolated are Staphylococcus aureus, S. epidermidis, Serratia sp., Klebsiella sp., and sometimes, Escherichia coli and mixed flora. Most of the published data fail to address any specific predictors for the infections. The risk of brain abscess from DBS infection is extremely low, with only one case reported (240). The important management decision is whether to remove or keep the hardware. Very superficial infections can be treated with oral or intravenous antibiotics. Deep-tracking infections require surgical intervention. However, if there is no purulent or necrotic material in direct contact with the hardware, debridement, irrigation, and a hardware-sparing approach can work. If the pus is in direct contact with the hardware or if the hardware is exposed, it should be promptly removed. In most cases with localized infection, partial hardware-removal strategies can be successfully used, such as removal of the infected IPG and retaining of the DBS lead that is not infected. After several weeks of antibiotic therapy, the removed hardware can be safely replaced. Hardware-related Complications Hardware-related complications are the most common, with a varying incidence that ranges from 2.7 to 50% (27, 71, 138, 140, 175, 229, 232, 279). These include DBS electrode fracture, extension wire failure, lead migration, skin erosion, IPG malfunction, and pain over the pulse generator (11, 43, 46, 47, 50, 76, 84, 87, 112, 118, 124, 168, 171, 175, 212, 265, 347, 356, 361). They can be subdivided into complications associated with the lead, those associated with the extension wires, and the IPG (51, 71, 132, 175, 229, 265, 278, 321, 376). Because the brain lead is the most delicate part of the hardware, it can malfunction or get damaged due to a variety of SHC826 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
19 SURGERY FOR MOVEMENT DISORDERS FIGURE 19. Lateral cranial x- ray showing two DBS leads, one of which is broken with a curved end (arrow). This patient presented with loss of efficacy and local pain due to the hook-like part of the electrode pressing on the scalp. causes. These include lead fracture, lead erosion, and lead malfunction. The fractures are most often the result of a fall or trauma (Fig. 19). However, fractures can occur secondary to migration of the connector to the neck (296) or even with unusual conditions, such as compulsive twisting (232). Lead erosion is not commonly observed and is usually caused by superficial placement of the lead. Lead malfunctions are generally related to connector migration in the cervical region that causes stress on the lead and results in multiple open or short circuits. Complications associated with extension wires are more uncommon than of the DBS lead because the extension material is more robust. Extension wire complications include erosions (if placed superficially), fractures, and pain and/or tightness that result from superficial placement (51, 71, 132, 175, 229, 265, 278, 321, 376). Complications associated with the IPG include erosion, caudal migration of IPGs, and shocking sensations at the IPG site (51, 71, 87, 132, 175, 265, 278, 321, 376). Subfascial implantation of the IPG is an alternative to subcutaneous implantation and may be advantageous in very thin patients. Stimulation-related Complications These are complications associated with programming of the DBS system after surgery. For the most part, these complications are reversible and require vigilance of the programmer. However, if a DBS lead is placed suboptimally, even the most expert programming cannot help, and the patient may require revision surgery. The most common stimulation-induced complications are dyskinesias, worsening of axial symptoms, speech dysfunction, capsular stimulation, and ocular symptoms. Stimulationinduced dyskinesias can be a good sign of accurate placement and are generally self-limiting (179, 217). They are observed after STN DBS and may require adjustment in stimulation parameters or change of dosage of dopaminergic medications (87, 217). Worsening of axial symptoms such as freezing and balance and gait disturbance have been reported in some studies (59, 87, 104, 127, 170, 271, 394). It has been suggested that changing the active contact and adjusting the stimulation parameters are generally helpful (87, 127). Dysarthria or hypophonia as a side effect of STN stimulation is observed in 4 to 17% of patients (59, 87, 104, 127, 170, 271, 394). The etiology is considered to be the spread of current to the capsule. Moving the active contact away from the capsule or reducing the amplitude may help reduce this side effect (87). Capsular stimulation can also cause muscle contraction that can be focal or generalized on the side contralateral to the stimulation. The treatment for this is steering the current away from the capsule by adjusting the stimulation-field parameters (87). Stimulation can also induce side effects in ocular movements causing either monocular deviation (spread to oculomotor nucleus), conjugate gaze deviation (spread to capsule) or eyelid apraxia (87, 179). Overall, the stimulation-induced complications must be monitored with the neurologist and the programming team. In summary, as the number of DBS surgeries increases, there will be more complications. The hypervigilance of the team is necessary to avoid, recognize, and manage complications associated with DBS implants. Neurorestorative Surgical Approaches for PD DBS is currently the best surgical approach for movement disorders with respect to safety, efficacy, and proven track record. However, DBS is an implantable device, with its associated complications, and it treats the symptoms of the patient and not the underlying disease. In this context, restorative strategies aimed at treating the underlying disease pathophysiology are important. A variety of surgical neurorestorative approaches have been developed in an attempt to prevent the loss of nigral neurons and stimulate the regeneration of nigrostriatal projections. These include the delivery of protein trophic factors, the delivery of therapeutic genes, and the transplantation of a variety of potentially restorative cell types. Delivery of Therapeutic Growth Factors Translational research on growth factors for PD has focused on the glial-derived neurotrophic factor (GDNF), family ligands, and neurturin. Much of the literature supports the ability of these proteins to protect dopaminergic neurons from a variety of toxic insults in cell culture and rodent and primate models (3). These observations led to two separate open-label studies of GDNF infusions into the human parkinsonian putamen (114, 324). Both open-label studies demonstrated significant improvements in UPDRS scores in the recipients. The second study demonstrated a gradual return to baseline in the first year after cessation of GDNF delivery. Enthusiasm generated by these open-label trials led to an international, multicenter, randomized placebo-controlled trial sponsored by Amgen (Thousand Oaks, CA) and Medtronic. In this study, patients were randomized to receive either GDNF or vehicle through bilateral putamenal catheters. The approach was abandoned by the sponsors after the safety and efficacy data were reviewed, including the development of anti-gdnf antibodies in some of the patients, and the observation that some monkeys had developed cerebellar lesions attributed to putamenal GDNF therapy. Results of the study at the time of termination revealed no significant difference between the placebo and treatment groups, although positron-emission tomographic imaging revealed increased dopamine production in the treatment group in the region immediately adjacent to the cannula tip (199). Proponents of intraparenchymal GDNF infusion therapy cite potential problems with catheter design and drug dis- VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC827
20 REZAI ET AL. tribution as possible reasons for the trial s failure (317). Ongoing research with a naturally-occurring structural and functional analog of GDNF, neurturin, suggest that this growth factor may also offer a potential means of dopamine neuron protection via intraparenchymal delivery. Delivery of Therapeutic Genes Two separate restorative gene-therapy strategies have entered clinical trials: trophic factor gene delivery and dopamine replacement. Ceregene (San Diego, CA) has taken the lead on stereotactic growth factor gene therapy with its product CERE- 120, an adeno-associated viral vector for the delivery of the gene for neurturin. The basis for the Ceregene trial can be found in the primate studies of striatal GDNF gene delivery with viral vectors, which demonstrated protection of striatal dopamine delivery with tyrosine hydroxylase staining, positron-emission tomography, and behavior studies in two different monkey models. Preclinical studies by Ceregene demonstrated that CERE-120 could similarly protect dopamine neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkey model (176), as well as enhance dopamine markers and activity in aged primates. The Phase I CERE-120 trial tested the safety and tolerability of putamenal CERE-120 injection in 12 patients. Surgery consisted of four injections targeting the putamen on each side, with two deposits at each injection site. Open-label follow-up of this initial cohort for 6 months has revealed no serious adverse events or decline in neurological condition. UPDRS off scores were reported to improve by roughly 40% in these Phase I patients at 12 months postdosing compared with each patient s own baseline (P 0.001) (333). On the basis of these results, a Phase II sham-surgery, controlled, multicenter trial was initated in December The next strategy, alteration of neurotransmitter production, has been pursued by three groups. The first trial (Genzyme, Westborough, MA) uses an adeno-associated viral vector to deliver the gene for aromatic amino acid L-dopa decarboxylase (AADC). Initial experiments demonstrated that AADC expression in the putamen could restore striatal dopamine when L- dopa was administered in animal models of PD (21). This strategy provides a potential means to control the amount of dopamine produced through the amount of L-dopa administered. The investigators hypothesized that this approach would prevent the development of runaway dyskinesias such as those that complicated fetal mesencephalic transplants. This team (21) conducted extensive preclinical studies, including an examination of the role of convection-enhanced delivery in viral injection as well as testing of a silicon tubing injection cannula. The issue of ideal cannula design has emerged as important to prevent vector-cannula binding with a resulting decrease in the titer delivered. The ongoing Phase I trial has included patients at the University of California, San Francisco with bilateral intrastriatal adeno-associated viral-aadc injections. To date, there have been no serious adverse events. Positron-emission tomography studies in initial patients demonstrated a 15% increase in striatal AADC activity 6 months after injection. A second trial has been pursued by Oxford Biomedica (Oxford, England) for stereotactic injection of a lentiviral vector carrying three separate transgenes: tyrosine hydroxylase, AADC, and guanosine triphosphate cyclohydrolase I. Together, these gene products are capable of driving autonomous production of L-dopa with subsequent dopamine production. Thus, unlike the Genzyme strategy, the Biomedica vector, named Prosavin, will drive dopamine production independent of L-dopa administration (388). The company plans a Prosavin trial in either England or France. A third gene-therapy approach has been advanced by Neurologix (Fort Lee, NJ). This approach induces subthalamic and pallidal inhibition through the expression of glutamate decarboxylase in the STN. Glutamate decarboxylase is the enzyme that changes glutamate to GABA. Thus, inhibition is affected by the production of GABA in the STN, and a reduction in efferent glutamate. Twelve patients received unilateral STN injection at three escalating doses (n 4 patients per group) to minimize risk. Investigators reported sustained improvements in UPDRS motor scores at 12 months (24% off state and 27% on state), with no serious adverse events related to gene therapy. Positron-emission tomographic studies confirmed reduction in thalamic metabolism (163). As the Neurologix project was the first PD gene therapy trial to pass through regulation, particular care was required in addressing safety over efficacy (as reflected in the decision to inject unilaterally). The authors note that effects were proportionally larger on the side contralateral to infusion, which lends weight to the hypothesis that genebased STN inhibition can provide a benefit beyond placebo. Cell Transplantation Delivery The approach of cell transplantation has the longest history, and perhaps, the most complex landscape. A wide variety of cells have been studied as potential means to provide neuroprotection and potentially replace lost dopamine neurons. Initial efforts used autografts of adrenal medullary cells as a potential source of dopamine but failed to show consistent improvements (97). Subsequent efforts involved the transplantation of fetal mesencephalic tissue containing nigral dopamine cells transplanted into the putamen. Results in this study proved inconsistent as well, with some patients showing improvements. However, a subset of patients developed an exacerbation of dyskinesias thought to be a result of heterogeneous and uncontrolled production of dopamine throughout the putamen (106, 305). Contemporary efforts at neural transplantation have involved putamenal implantation of retinal pigment epithelial cells grown in a bead matrix called Spheramine (Titan, San Francisco, CA). Retinal pigment epithelial cells can produce L-dopa, but also appear to have trophic/protective effects on adjoining neurons (305, 335). An ongoing Phase II, controlled, multicenter trial is under way to assess the safety and efficacy of Spheramine implants. Advances in stem cell technology have made it possible to manufacture dopaminergic neurons. A wide variety of protocols have been developed for the production of dopamine cells from embryonic stem cells, fetal-derived uncommitted and SHC828 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
21 SURGERY FOR MOVEMENT DISORDERS committed progenitors, and even adult progenitors harvested from the subventricular zone. Parallel strategies have been developed to purify the dopaminergic cells from nondopaminergic cells in these preparations with the hope of creating a sustainable, practical source for pure, differentiated, human dopaminergic cells. Finally, a fusion of gene-delivery technology with transplantation (ex vivo gene transfer) provides a potential means to control these cells, hence reducing the potential for the runaway dyskinesias to occur that were observed in the fetal transplant. Surgery for Movement Disorders: Future Directions The field of functional neurosurgery has witnessed a renaissance over the past 20 years. This development has been fueled by progress in the neurobiology of movement disorders, surgical technical advancements, therapeutic device developments, and innovative approaches. The growth in our understanding of the neural circuitry of the disease has determined and refined our surgical targets. This increased understanding of the neurobiology of movement disorders will guide the discovery of additional targets for surgical exploration and translational clinical research. The evolution of stereotactic surgical tools and techniques is facilitating safe and minimally invasive approaches that enable neurosurgeons to target various brain structures with reliable accuracy. This, coupled with rapid advances in imaging technology and capabilities, will play an important role in improving our capability to visualize brain structures and function with unparalleled resolution. At present, DBS is the standard therapy of choice for movement-disorder patients who are medication intractable and who meet the surgical selection criteria. DBS has a proven safety and efficacy profile and long-term outcomes have been demonstrated by prospective, randomized studies. Technological advances in DBS systems have already resulted in improvements and will continue to do so. The next-generation DBS systems will be smaller and rechargeable, with current steering features and built-in sensing capabilities. A number of clinical trials are under way to explore the utility of gene therapy and cell transplantation and stem cell approaches with promising preliminary outcomes. In the near future, these technologies will provide additional options for neurosurgical management of movement disorders. Functional neurosurgery is one of the most exciting and rapidly growing areas in neurosurgery. Despite significant advances, this arena of neurosurgery is still in its infancy. The movement-disorder surgery evolution can be argued to be a model approach for development of neurosurgical therapies. The lessons learned from movement-disorder surgical experience are already being applied to surgical treatment of psychiatric and other chronic neurological disorders. REFERENCES 1. Abosch A, Lozano A: Stereotactic neurosurgery for movement disorders. Can J Neurol Sci 30 [Suppl 1]:S72 S82, Agid Y: Parkinson s disease: Pathophysiology. Lancet 337: , Airaksinen MS, Saarma M: The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: , Akert K, Koella WP, Hess R Jr: Sleep produced by electrical stimulation of the thalamus. Am J Physiol 168: , Alegret M, Junque C, Valldeoriola F, Vendrell P, Pilleri M, Rumia J, Tolosa E: Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch Neurol 58: , Alesch F, Pinter MM, Helscher RJ, Fertl L, Benabid AL, Koos WT: Stimulation of the ventral intermediate thalamic nucleus in tremor dominated Parkinson s disease and essential tremor. Acta Neurochir (Wien) 136:75 81, Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: , Alterman RL, Kall BA, Cohen H, Kelly PJ: Stereotactic ventrolateral thalamotomy: Is ventriculography necessary? Neurosurgery 37: , Alterman RL, Shils JL, Miravite J, Tagliati M: Lower stimulation frequency can enhance tolerability and efficacy of pallidal deep brain stimulation for dystonia. Mov Disord 22: , Alterman RL, Sterio D, Beric A, Kelly PJ: Microelectrode recording during posteroventral pallidotomy: Impact on target selection and complications. Neurosurgery 44: , Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN: Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery 58 [Suppl]:ONS96 ONS102, Anca MH, Zaccai TF, Badarna S, Lozano AM, Lang AE, Giladi N: Natural history of Oppenheim s dystonia (DYT1) in Israel. J Child Neurol 18: , Anderson T, Hu B, Pittman Q, Kiss ZH: Mechanisms of deep brain stimulation: An intracellular study in rat thalamus. J Physiol 559: , Anderson TR, Hu B, Iremonger K, Kiss ZH: Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulationinduced tremor arrest. J Neurosci 26: , Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP: Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 62: , Andrade-Souza YM, Schwalb JM, Hamani C, Eltahawy H, Hoque T, Saint- Cyr J, Lozano AM: Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in Parkinson s disease. Neurosurgery 56: , Andrade-Souza YM, Schwalb JM, Hamani C, Hoque T, Saint-Cyr J, Lozano AM: Comparison of 2-dimensional magnetic resonance imaging and 3-planar reconstruction methods for targeting the subthalamic nucleus in Parkinson disease. Surg Neurol 63: , Aybek S, Gronchi-Perrin A, Verney A, Chiuvé SC, Villemure JG, Bukhard PR, Vingerhoets FJ: Long-term cognitive profile and incidence of dementia after STN-DBS in Parkinson s disease. Mov Disord 22: , Aziz TZ, Nandi D, Parkin S, Liu X, Giladi N, Bain P, Gregory RG, Joint C, Scott RB, Stein JF: Targeting the subthalamic nucleus. Stereotact Funct Neurosurg 77:87 90, Baev KV, Greene KA, Marciano FF, Samanta JE, Shetter AG, Smith KA, Stacy MA, Spetzler RF: Physiology and pathophysiology of cortico-basal gangliathalamocortical loops: Theoretical and practical aspects. Prog Neuropsychopharmacol Biol Psychiatry 26: , Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J: Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther 14: , Bechtereva NP, Bondartchuk AN, Smirnov VM, Meliutcheva LA, Shandurina AN: Method of electrostimulation of the deep brain structures in treatment of some chronic diseases. Confin Neurol 37: , Bejjani B, Damier P, Arnulf I, Bonnet AM, Vidailhet M, Dormont D, Pidoux B, Cornu P, Marsault C, Agid Y: Pallidal stimulation for Parkinson s disease. Two targets? Neurology 49: , Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, Bonnet AM, Marsault C, Agid Y, Philippon J, Cornu P: Bilateral subthalamic stimulation for Parkinson s disease by using three-dimensional stereotactic magnetic VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC829
22 REZAI ET AL. resonance imaging and electrophysiological guidance. J Neurosurg 92: , Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollak P: Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord 13 [Suppl 3]: , Benabid AL, Benazzouz A, Limousin P, Koudsie A, Krack P, Piallat B, Pollak P: Dyskinesias and the subthalamic nucleus. Ann Neurol 47 [Suppl 1]:S189 S192, Benabid AL, Chabardes S, Seigneuret E: Deep-brain stimulation in Parkinson s disease: Long-term efficacy and safety What happened this year? Curr Opin Neurol 18: , Benabid AL, Deuschl G, Lang AE, Lyons KE, Rezai AR: Deep brain stimulation for Parkinson s disease. Mov Disord 21 [Suppl 14]:S168 S170, Benabid AL, Koudsie A, Benazzouz A, Fraix V, Ashraf A, Le Bas JF, Chabardes S, Pollak P: Subthalamic stimulation for Parkinson s disease. Arch Med Res 31: , Benabid AL, Koudsie A, Benazzouz A, Le Bas JF, Pollak P: Imaging of subthalamic nucleus and ventralis intermedius of the thalamus. Mov Disord 17 [Suppl 3]:S123 S129, Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P: Deep brain stimulation of the subthalamic nucleus for Parkinson s disease: Methodologic aspects and clinical criteria. Neurology 55 [Suppl 6]:S40 S44, Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A: Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 84: , Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J: Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: , Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J: Acute and long-term effects of subthalamic nucleus stimulation in Parkinson s disease. Stereotact Funct Neurosurg 62:76 84, Benabid AL, Pollak P, Hommel M, Gaio JM, de Rougemont J, Perret J: Treatment of Parkinson tremor by chronic stimulation of the ventral intermediate nucleus of the thalamus [in French]. Rev Neurol (Paris) 145: , Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J: Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 50: , Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J: Chronic VIM thalamic stimulation in Parkinson s disease, essential tremor and extrapyramidal dyskinesias. Acta Neurochir Suppl (Wien) 58:39 44, Benabid AL, Wallace B, Mitrofanis J, Xia R, Piallat B, Chabardes S, Berger F: A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg 105: , Benazzouz A, Hallett M: Mechanism of action of deep brain stimulation. Neurology 55 [Suppl 6]:S13 S16, Benazzouz A, Piallat B, Ni ZG, Koudsie A, Pollak P, Benabid AL: Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson s disease. Cell Transplant 9: , Bergman H, Deuschl G: Pathophysiology of Parkinson s disease: From clinical neurology to basic neuroscience and back. Mov Disord 17 [Suppl 3]:S28 S40, Bergman H, Wichmann T, DeLong MR: Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249: , Beric A, Kelly PJ, Rezai A, Sterio D, Mogilner A, Zonenshayn M, Kopell B: Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg 77:73 78, Berk C, Carr J, Sinden M, Martzke J, Honey CR: Thalamic deep brain stimulation for the treatment of tremor due to multiple sclerosis: A prospective study of tremor and quality of life. J Neurosurg 97: , Berney A, Vingerhoets F, Perrin A, Guex P, Villemure JG, Burkhard PR, Benkelfat C, Ghika J: Effect on mood of subthalamic DBS for Parkinson s disease: A consecutive series of 24 patients. Neurology 59: , Binder DK, Rau G, Starr PA: Hemorrhagic complications of microelectrodeguided deep brain stimulation. Stereotact Funct Neurosurg 80:28 31, Binder DK, Rau GM, Starr PA: Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery 56: , Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ: Deep brain stimulation for pain relief: A meta-analysis. J Clin Neurosci 12: , Bittar RG, Yianni J, Wang S, Liu X, Nandi D, Joint C, Scott R, Bain PG, Gregory R, Stein J, Aziz TZ: Stereotactic and Functional Neurosurgery Resident Award: Deep brain stimulation for generalized dystonia and spasmodic torticollis: Rate and extent of postoperative improvement. Clin Neurosurg 52: , Blomstedt P, Hariz MI: Are complications less common in deep brain stimulation than in ablative procedures for movement disorders? Stereotact Funct Neurosurg 84:72 81, Blomstedt P, Hariz MI: Hardware-related complications of deep brain stimulation: A ten year experience. Acta Neurochir (Wien) 147: , Blond S, Siegfried J: Thalamic stimulation for the treatment of tremor and other movement disorders. Acta Neurochir Suppl (Wien) 52: , Bonnet AM, Houeto JL: Pathophysiology of Parkinson s disease. Biomed Pharmacother 53: , Brice J, McLellan L: Suppression of intention tremor by contingent deepbrain stimulation. Lancet 1: , Brown P: Bad oscillations in Parkinson s disease. J Neural Transm Suppl 70:27 30, Brown P: Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson s disease. Mov Disord 18: , Bryant JA, De Salles A, Cabatan C, Frysinger R, Behnke E, Bronstein J: The impact of thalamic stimulation on activities of daily living for essential tremor. Surg Neurol 59: , Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH: Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol 41:32 40, Burchiel KJ, Anderson VC, Favre J, Hammerstad JP: Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson s disease: Results of a randomized, blinded pilot study. Neurosurgery 45: , Carpenter LL: Neurostimulation in resistant depression. J Psychopharmacol 20 [Suppl]:35 40, Castelli L, Perozzo P, Zibetti M, Crivelli B, Morabito U, Lanotte M, Cossa F, Bergamasco B, Lopiano L: Chronic deep brain stimulation of the subthalamic nucleus for Parkinson s disease: Effects on cognition, mood, anxiety and personality traits. Eur Neurol 55: , Ceballos-Baumann AO: Functional imaging in Parkinson s disease: activation studies with PET, fmri and SPECT. J Neurol 250 [Suppl 1]:I15 I23, Ceballos-Baumann AO, Boecker H, Fogel W, Alesch F, Bartenstein P, Conrad B, Diederich N, von Falkenhayn I, Moringlane JR, Schwaiger M, Tronnier VM: Thalamic stimulation for essential tremor activates motor and deactivates vestibular cortex. Neurology 56: , Chapman WP, Livingston KE, Poppen JL: Effect upon blood pressure of electrical stimulation of tips of temporal lobes in man. J Neurophysiol 13:65 71, Chapman WP, Livingston RB, Livingston KE: Frontal lobotomy and electrical stimulation of orbital surface of frontal lobes; effect on respiration and on blood pressure in man. Arch Neurol Psychiatry 62: , Chen CC, Kuhn AA, Trottenberg T, Kupsch A, Schneider GH, Brown P: Neuronal activity in globus pallidus interna can be synchronized to local field potential activity over 3 12 Hz in patients with dystonia. Exp Neurol 202: , Cintas P, Simonetta-Moreau M, Ory F, Brefel-Courbon C, Fabre N, Chaynes P, Sabatier J, Sol JC, Rascol O, Berry I, Lazorthes Y: Deep brain stimulation for parkinson s disease: Correlation between intraoperative subthalamic nucleus neurophysiology and most effective contacts. Stereotact Funct Neurosurg 80: , Cobb S, Pool JL, Scarff J, Schwab RS, Walker AE, White JC: Section of U fibers of motor cortex in cases of paralysis agitans (Parkinson s disease); report of 9 cases. Arch Neurol Psychiatry 64:57 59, SHC830 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
23 SURGERY FOR MOVEMENT DISORDERS 69. Coffey RJ, Lozano AM: Neurostimulation for chronic noncancer pain: An evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg 105: , Cohen OS, Hassin-Baer S, Spiegelmann R: Deep brain stimulation of the internal globus pallidus for refractory tardive dystonia. Parkinsonism Relat Disord 13: , Constantoyannis C, Berk C, Honey CR, Mendez I, Brownstone RM: Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci 32: , Contarino MF, Daniele A, Sibilia AH, Romito LM, Bentivoglio AR, Gainotti G, Albanese A: Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson s disease. J Neurol Neurosurg Psychiatry 78: , a. Cooper IS: Ligation of the anterior choroidal artery for involuntary movements; parkinsonism. Psychiatr Q 27: , Cosyns P, Gabriels L, Nuttin B: Deep brain stimulation in treatment refractory obsessive compulsive disorder. Verh K Acad Geneeskd Belg 65: , Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, Picot MC, Tuffery S, Claustres M, Echenne B, Frerebeau P: Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: Long-term results. J Neurosurg 101: , Coubes P, Echenne B, Roubertie A, Vayssiere N, Tuffery S, Humbertclaude V, Cambonie G, Claustres M, Frerebeau P: Treatment of early-onset generalized dystonia by chronic bilateral stimulation of the internal globus pallidus. Apropos of a case [in French]. Neurochirurgie 45: , Coubes P, Vayssiere N, El Fertit H, Hemm S, Cif L, Kienlen J, Bonafe A, Frerebeau P: Deep brain stimulation for dystonia. Surgical technique. Stereotact Funct Neurosurg 78: , Crevier PH: Various considerations on pallidotomy for relief of abnormal movements [in French]. Union Med Can 86: , Cuny E, Guehl D, Burbaud P, Gross C, Dousset V, Rougier A: Lack of agreement between direct magnetic resonance imaging and statistical determination of a subthalamic target: The role of electrophysiological guidance. J Neurosurg 97: , Daniele A, Albanese A, Contarino MF, Zinzi P, Barbier A, Gasparini F, Romito LM, Bentivoglio AR, Scerrati M: Cognitive and behavioural effects of chronic stimulation of the subthalamic nucleus in patients with Parkinson s disease. J Neurol Neurosurg Psychiatry 74: , Deep-Brain Stimulation for Parkinson s Disease Study Group: Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson s disease. N Engl J Med 345: , DeLong MR, Crutcher MD, Georgopoulos AP: Primate globus pallidus and subthalamic nucleus: Functional organization. J Neurophysiol 53: , Delong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE: Functional organization of the basal ganglia: Contributions of single-cell recording studies. Ciba Found Symp 107:64 82, Deogaonkar M, Subramanian T: Pathophysiological basis of drug-induced dyskinesias in Parkinson s disease. Brain Res Brain Res Rev 50: , De Salles AA, Frighetto L, Behnke E, Sinha S, Tseng L, Torres R, Lee M, Cabatan-Awang C, Frysinger R: Functional neurosurgery in the MRI environment. Minim Invasive Neurosurg 47: , Deuschl G, Bain P: Deep brain stimulation for tremor [correction of trauma]: Patient selection and evaluation. Mov Disord 17 [Suppl 3]:S102 S111, Deuschl G, Fogel W, Hahne M, Kupsch A, Müller D, Oechsner M, Sommer U, Ulm G, Vogt T, Volkmann J: Deep-brain stimulation for Parkinson s disease. J Neurol 249 [Suppl 3]:36 39, Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE, Rodriguez-Oroz MC, Tamma F, Tröster AI, Vitek JL, Volkmann J, Voon V: Deep brain stimulation: Postoperative issues. Mov Disord 21 [Suppl 14]:S219 S237, Diallo R, Welter ML, Mallet L: Therapeutic management of tics in Tourette s syndrome [in French]. Rev Neurol (Paris) 163: , Dujardin K, Defebvre L, Krystkowiak P, Blond S, Destee A: Influence of chronic bilateral stimulation of the subthalamic nucleus on cognitive function in Parkinson s disease. J Neurol 248: , Eljamel MS, Tulley M, Spillane K: A simple stereotactic method for frameless deep brain stimulation. Stereotact Funct Neurosurg 85:6 10, Eltahawy HA, Feinstein A, Khan F, Saint-Cyr J, Lang AE, Lozano AM: Bilateral globus pallidus internus deep brain stimulation in tardive dyskinesia: A case report. Mov Disord 19: , Eltahawy HA, Saint-Cyr J, Giladi N, Lang AE, Lozano AM: Primary dystonia is more responsive than secondary dystonia to pallidal interventions: Outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery 54: , Eriksen SK, Tuite PJ, Maxwell RE, Sullivan M, Low WC, Ebner TJ: Bilateral subthalamic nucleus stimulation for the treatment of Parkinson s disease: Results of six patients. J Neurosci Nurs 35: , Erola T, Heikkinen ER, Haapaniemi T, Tuominen J, Juolasmaa A, Myllylä VV: Efficacy of bilateral subthalamic nucleus (STN) stimulation in Parkinson s disease. Acta Neurochir (Wien) 148: , Fields JA, Troster AI: Cognitive outcomes after deep brain stimulation for Parkinson s disease: A review of initial studies and recommendations for future research. Brain Cogn 42: , Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO: Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res 156: , Fine A: Transplantation of adrenal tissue into the central nervous system. Brain Res Brain Res Rev 15: , Finger S: Origins of Neuroscience. New York, Oxford University Press, 1994, p Foffani G, Ardolino G, Meda B, Egidi M, Rampini P, Caputo E, Baselli G, Priori A: Altered subthalamo-pallidal synchronisation in parkinsonian dyskinesias. J Neurol Neurosurg Psychiatry 76: , Foncke EM, Bour LJ, Speelman JD, Koelman JH, Tijssen MA: Local field potentials and oscillatory activity of the internal globus pallidus in myoclonus-dystonia. Mov Disord 22: , Foote KD, Okun MS: Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: Two leads may be better than one: Technical note. Neurosurgery 56 [Suppl]: E445, Ford B, Winfield L, Pullman SL, Frucht SJ, Du Y, Greene P, Cheringal JH, Yu Q, Cote LJ, Fahn S, McKhann GM 2nd, Goodman RR: Subthalamic nucleus stimulation in advanced Parkinson s disease: Blinded assessments at one year follow up. J Neurol Neurosurg Psychiatry 75: , Fox MW, Ahlskog JE, Kelly PJ: Stereotactic ventrolateralis thalamotomy for medically refractory tremor in post-levodopa era Parkinson s disease patients. J Neurosurg 75: , Fraix V, Pollak P, Moro E, Chabardes S, Xie J, Ardouin C, Benabid AL: Subthalamic nucleus stimulation in tremor dominant parkinsonian patients with previous thalamic surgery. J Neurol Neurosurg Psychiatry 76: , Franzini A, Marras C, Ferroli P, Zorzi G, Bugiani O, Romito L, Broggi G: Longterm high-frequency bilateral pallidal stimulation for neuroleptic-induced tardive dystonia. Report of two cases. J Neurosurg 102: , Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S: Transplantation of embryonic dopamine neurons for severe Parkinson s disease. N Engl J Med 344: , Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, Chabardes S, Foote K, Benabid AL, Pollak P: Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson s disease. J Neurol Neurosurg Psychiatry 75: , Garonzik IM, Hua SE, Ohara S, Lenz FA: Intraoperative microelectrode and semi-microelectrode recording during the physiological localization of the thalamic nucleus ventral intermediate. Mov Disord 17 [Suppl 3]:S135 S144, George MS, Nahas Z, Borckardt JJ, Anderson B, Foust MJ, Burns C, Kose S, Short EB: Brain stimulation for the treatment of psychiatric disorders. Curr Opin Psychiatry 20: , Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR: D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: , Gilbert DL, Lipps TD: Tourette s Syndrome. Curr Treat Options Neurol 7: , VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC831
24 REZAI ET AL Gill CE, Konrad PE, Davis TL, Charles D: Deep brain stimulation for Parkinson s disease: The Vanderbilt University Medical Center experience, Tenn Med 100:45 47, Giller CA, Dewey RB Jr: Ventralis intermedius thalamotomy can succeed when ventralis intermedius thalamic stimulation fails: Report of 2 cases for tremor. Stereotact Funct Neurosurg 79:51 56, Gill SS, Patel NK, Hotton GR, O Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P: Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9: , Goldman MS, Ahlskog JE, Kelly PJ: The symptomatic and functional outcome of stereotactic thalamotomy for medically intractable essential tremor. J Neurosurg 76: , Goldman MS, Kelly PJ: Stereotactic thalamotomy for medically intractable essential tremor. Stereotact Funct Neurosurg 58:22 25, Goldman MS, Kelly PJ: Symptomatic and functional outcome of stereotactic ventralis lateralis thalamotomy for intention tremor. J Neurosurg 77: , Gorgulho A, De Salles AA, Frighetto L, Behnke E: Incidence of hemorrhage associated with electrophysiological studies performed using macroelectrodes and microelectrodes in functional neurosurgery. J Neurosurg 102: , Green AL, Nandi D, Armstrong G, Carter H, Aziz T: Post-herpetic trigeminal neuralgia treated with deep brain stimulation. J Clin Neurosci 10: , Greenamyre JT: Ottorino Rossi Award New targets for therapy in Parkinson s disease: Pathogenesis and pathophysiology. Funct Neurol 15:67 80, Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA: Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 31: , Greenberg BD, Rezai AR: Mechanisms and the current state of deep brain stimulation in neuropsychiatry. CNS Spectr 8: , Gross CE, Boraud T, Guehl D, Bioulac B, Bezard E: From experimentation to the surgical treatment of Parkinson s disease: Prelude or suite in basal ganglia research? Prog Neurobiol 59: , Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL: Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson s disease and tremor. Mov Disord 21 [Suppl 14]:S259 S283, Gross RE, Lombardi WJ, Hutchison WD, Narula S, Saint-Cyr JA, Dostrovsky JO, Tasker RR, Lang AE, Lozano AM: Variability in lesion location after microelectrode-guided pallidotomy for Parkinson s disease: Anatomical, physiological, and technical factors that determine lesion distribution. J Neurosurg 90: , Gross RE, Lozano AM: Advances in neurostimulation for movement disorders. Neurol Res 22: , Guehl D, Cuny E, Benazzouz A, Rougier A, Tison F, Machado S, Grabot D, Gross C, Bioulac B, Burbaud P: Side-effects of subthalamic stimulation in Parkinson s disease: Clinical evolution and predictive factors. Eur J Neurol 13: , Guiot G, Arfel G, Derome P, Kahn A: Neurophysiologic control procedures for sterotaxic thalamotomy [in French]. Neurochirurgie 14: , Guiot G, Derome P, Trigo JC: Intention tremor: The best indication for stereotaxic surgery [in French]. Presse Med 75: , Guridi J, Rodriguez-Oroz MC, Lozano AM, Moro E, Albanese A, Nuttin B, Gybels J, Ramos E, Obeso JA: Targeting the basal ganglia for deep brain stimulation in Parkinson s disease. Neurology 55 [Suppl 6]:S21 S28, Halbig TD, Gruber D, Kopp UA, Schneider GH, Trottenberg T, Kupsch A: Pallidal stimulation in dystonia: Effects on cognition, mood, and quality of life. J Neurol Neurosurg Psychiatry 76: , Hamani C, Lozano AM: Hardware-related complications of deep brain stimulation: A review of the published literature. Stereotact Funct Neurosurg 84: , Hamani C, Lozano AM: Physiology and pathophysiology of Parkinson s disease. Ann N Y Acad Sci 991:15 21, Hamani C, Richter E, Schwalb JM, Lozano AM: Bilateral subthalamic nucleus stimulation for Parkinson s disease: A systematic review of the clinical literature. Neurosurgery 56: , Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM: The subthalamic nucleus in the context of movement disorders. Brain 127:4 20, Hamid NA, Mitchell RD, Mocroft P, Westby GW, Milner J, Pall H: Targeting the subthalamic nucleus for deep brain stimulation: Technical approach and fusion of pre- and postoperative MR images to define accuracy of lead placement. J Neurol Neurosurg Psychiatry 76: , Hariz M, Fodstad H: Do microelectrode techniques increase accuracy or decrease risks in pallidotomy and deep brain stimulation? A critical review of the literature. Stereotact Funct Neurosurg 72: , Hariz MI: Complications of deep brain stimulation surgery. Mov Disord 17 [Suppl 3]:S162 S166, Hariz MI, Fodstad H: Do microelectrode techniques increase accuracy or decrease risks in pallidotomy and deep brain stimulation? A critical review of the literature. Stereotact Funct Neurosurg 72: , Hariz MI, Johansson F: Hardware failure in parkinsonian patients with chronic subthalamic nucleus stimulation is a medical emergency. Mov Disord 16: , Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL: Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: , Hassler R: Anatomy of the thalamus [in German]. Arch Psychiatr Nervenkr 184: , Hassler R, Riechert T, Mundinger F, Umbach W, Ganglberger JA: Physiological observations in stereotaxic operations in extrapyramidal motor disturbances. Brain 83: , Heimer G, Rivlin M, Israel Z, Bergman H: Synchronizing activity of basal ganglia and pathophysiology of Parkinson s disease. J Neural Transm Suppl:17 20, Hertel F, Züchner M, Weimar I, Gemmar P, Noll B, Bettag M, Decker C: Implantation of electrodes for deep brain stimulation of the subthalamic nucleus in advanced Parkinson s disease with the aid of intraoperative microrecording under general anesthesia. Neurosurgery 59:E1138, Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B, Weinert D, Pfister G, Müller D, Mehdorn HM, Deuschl G, Volkmann J: Most effective stimulation site in subthalamic deep brain stimulation for Parkinson s disease. Mov Disord 19: , Herzog J, Reiff J, Krack P, Witt K, Schrader B, Müller D, Deuschl G: Manic episode with psychotic symptoms induced by subthalamic nucleus stimulation in a patient with Parkinson s disease. Mov Disord 18: , Herzog J, Volkmann J, Krack P, Kopper F, Pötter M, Lorenz D, Steinbach M, Klebe S, Hamel W, Schrader B, Weinert D, Müller D, Mehdorn HM, Deuschl G: Two-year follow-up of subthalamic deep brain stimulation in Parkinson s disease. Mov Disord 18: , Hitchcock E, Flint GA, Gutowski NJ: Thalamotomy for movement disorders: A critical appraisal. Acta Neurochir Suppl (Wien) 39:61 65, Holloway KL, Gaede SE, Starr PA, Rosenow JM, Ramakrishnan V, Henderson JM: Frameless stereotaxy using bone fiducial markers for deep brain stimulation. J Neurosurg 103: , Hooper J, Taylor R, Pentland B, Whittle IR: Rater reliability of Fahn s tremor rating scale in patients with multiple sclerosis. Arch Phys Med Rehabil 79: , Hornykiewicz O, Kish SJ: Biochemical pathophysiology of Parkinson s disease. Adv Neurol 45:19 34, Hosobuchi Y: Chronic brain stimulation for the treatment of intractable pain. Res Clin Stud Headache 5: , Hosobuchi Y: Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases ( ). J Neurosurg 64: , Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P, Pidoux B, Dormont D, Cornu P, Agid Y: Tourette s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry 76: , Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, du Moncel ST, Bonnet AM, Pidoux B, Dormont D, Cornu P, Agid Y: Behavioural disorders, Parkinson s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry 72: , 2002 (comment) Houeto JL, Mesnage V, Welter ML, Mallet L, Agid Y, Bejjani BP: Subthalamic DBS replaces levodopa in Parkinson s disease: Two-year follow-up. Neurology 60: , SHC832 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
25 SURGERY FOR MOVEMENT DISORDERS 158. Hubel D: Tungsten microelectrode from recording from single units. Science 125: , Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM, Lang AE, Dostrovsky JO, Hutchison WD, Moro E: Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology 68: , Iansek R, Rosenfeld JV, Huxham FE: Deep brain stimulation of the subthalamic nucleus in Parkinson s disease. Med J Aust 177: , Jeon SW, Shure MA, Baker KB, Huang D, Rollins AM, Chahlavi A, Rezai AR: A feasibility study of optical coherence tomography for guiding deep brain probes. J Neurosci Methods 154:96 101, Kahilogullari G, Ugur HC, Savas A, Dirik EB, Akbostanci MC, Elibol B, Kanpolat Y: Management of a hemidystonic patient with thalamotomy, campotomy and cervical dorsal root entry zone operation. Stereotact Funct Neurosurg 83: , Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ: Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson s disease: An open label, phase I trial. Lancet 369: , Katayama Y, Kano T, Kobayashi K, Oshima H, Fukaya C, Yamamoto T: Difference in surgical strategies between thalamotomy and thalamic deep brain stimulation for tremor control. J Neurol 252 [Suppl 4]:IV17-IV22, Katayama Y, Oshima H, Kano T, Kobayashi K, Fukaya C, Yamamoto T: Direct effect of subthalamic nucleus stimulation on levodopa-induced peakdose dyskinesia in patients with Parkinson s disease. Stereotact Funct Neurosurg 84: , Kelly PJ: Microelectrode recording for the somatotopic placement of stereotactic thalamic lesions in the treatment of parkinsonian and cerebellar intention tremor. Appl Neurophysiol 43: , Kelly PJ, Ahlskog JE, Goerss SJ, Daube JR, Duffy JR, Kall BA: Computerassisted stereotactic ventralis lateralis thalamotomy with microelectrode recording control in patients with Parkinson s disease. Mayo Clin Proc 62: , Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, Jankovic J: Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg 106: , Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, Tashiro K: Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology 55: , Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE: Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg 99: , Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, Lang AE, Deuschl G: Subthalamic nucleus deep brain stimulation: Summary and meta-analysis of outcomes. Mov Disord 21 [Suppl 14]:S290 S304, Koller WC, Lyons KE, Wilkinson SB, Pahwa R: Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 14: , Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R: Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 16: , Koller WC, Pahwa PR, Lyons KE, Wilkinson SB: Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology 55 [Suppl 6]:S29 S33, Kondziolka D, Whiting D, Germanwala A, Oh M: Hardware-related complications after placement of thalamic deep brain stimulator systems. Stereotact Funct Neurosurg 79: , Kordower JH: In vivo gene delivery of glial cell line-derived neurotrophic factor for Parkinson s disease. Ann Neurol 53 [Suppl 3]:S120 S134, Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P: Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson s disease. N Engl J Med 349: , Krack P, Dostrovsky J, Ilinsky I, Kultas-Ilinsky K, Lenz F, Lozano AM, Vitek J: Surgery of the motor thalamus: Problems with the present nomenclatures. Mov Disord 17 [Suppl 3]:S2 S8, Krack P, Fraix V, Mendes A, Benabid AL, Pollak P: Postoperative management of subthalamic nucleus stimulation for Parkinson s disease. Mov Disord 17 [Suppl 3 ]:S188 S197, Krack P, Limousin P, Benabid AL, Pollak P: Chronic stimulation of subthalamic nucleus improves levodopa-induced dyskinesias in Parkinson s disease. Lancet 350:1676, Krack P, Pollak P, Limousin P, Hoffmann D, Xie J, Benazzouz A, Benabid AL: Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson s disease. Brain 121: , Kraft SP, Lang AE: Cranial dystonia, blepharospasm and hemifacial spasm: Clinical features and treatment, including the use of botulinum toxin. CMAJ 139: , Krause M, Fogel W, Heck A, Hacke W, Bonsanto M, Trenkwalder C, Tronnier V: Deep brain stimulation for the treatment of Parkinson s disease: Subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry 70: , Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V: Pallidal stimulation for dystonia. Neurosurgery 55: , Krause M, Fogel W, Tronnier V, Pohle S, Hörtnagel K, Thyen U, Volkmann J: Long-term benefit to pallidal deep brain stimulation in a case of dystonia secondary to pantothenate kinase-associated neurodegeneration. Mov Disord 21: , Krauss JK, Simpson RK Jr, Ondo WG, Pohle T, Burgunder JM, Jankovic J: Concepts and methods in chronic thalamic stimulation for treatment of tremor: Technique and application. Neurosurgery 48: , Krauss JK, Yianni J, Loher TJ, Aziz TZ: Deep brain stimulation for dystonia. J Clin Neurophysiol 21:18 30, Kringelbach ML, Jenkinson N, Green AL, Owen SL, Hansen PC, Cornelissen PL, Holliday IE, Stein J, Aziz TZ: Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport 18: , Kühn AA, Kupsch A, Schneider GH, Brown P: Reduction in subthalamic 8 35 Hz oscillatory activity correlates with clinical improvement in Parkinson s disease. Eur J Neurosci 23: , Deleted in proof Kumar K, Kelly M, Toth C: Deep brain stimulation of the ventral intermediate nucleus of the thalamus for control of tremors in Parkinson s disease and essential tremor. Stereotact Funct Neurosurg 72:47 61, Kumar K, Wyant GM: Deep brain stimulation for alleviating chronic intractable pain. Can J Surg 28:20 22, Kumar R, Lozano AM, Sime E, Lang AE: Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology 61: , Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Müller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J; Deep Brain Stimulation for Dystonia Study Group: Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355: , Kupsch A, Klaffke S, Kühn AA, Meissner W, Arnold G, Schneider GH, Maier-Hauff K, Trottenberg T: The effects of frequency in pallidal deep brain stimulation for primary dystonia. J Neurol 250: , Laitinen LV, Bergenheim AT, Hariz MI: Leksell s posteroventral pallidotomy in the treatment of Parkinson s disease. J Neurosurg 76:53 61, Laitinen LV, Bergenheim AT, Hariz MI: Ventroposterolateral pallidotomy can abolish all parkinsonian symptoms. Stereotact Funct Neurosurg 58:14 21, Lambert D, Waters CH: Essential Tremor. Curr Treat Options Neurol 1:6 13, Lang AE, Gill S, Patel NK, Lozano AM, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M: Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59: , Lang AE, Kleiner-Fisman G, Saint-Cyr JA, Miyasaki J, Lozano A: Subthalamic DBS replaces levodopa in Parkinson s disease: Two-year followup. Neurology 60: , VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC833
26 REZAI ET AL Lee JY, Deogaonkar M, Rezai A: Deep brain stimulation of globus pallidus internus for dystonia. Parkinsonism Relat Disord 13: , Lee JY, Kondziolka D: Thalamic deep brain stimulation for management of essential tremor. J Neurosurg 103: , Lee KH, Chang SY, Roberts DW, Kim U: Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus. J Neurosurg 101: , Lee M, Epstein FJ, Rezai AR, Zagzag D: Nonneoplastic intramedullary spinal cord lesions mimicking tumors. Neurosurgery 43: , Lee RG: Physiology of the basal ganglia and pathophysiology of Parkinson s disease. Can J Neurol Sci 14 [Suppl 3]: , Leksell L: A stereotactic apparatus for intracerebral surgery. Acta Chir Scand 99: , Lenz FA, Dostrovsky JO, Kwan HC, Tasker RR, Yamashiro K, Murphy JT: Methods for microstimulation and recording of single neurons and evoked potentials in the human central nervous system. J Neurosurg 68: , Lévesque MF, Taylor S, Rogers R, Le MT, Swope D: Subthalamic stimulation in Parkinson s disease. Preliminary results. Stereotact Funct Neurosurg 72: , Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO: Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson s disease. Brain 125: , Levy RM: Deep brain stimulation for the treatment of intractable pain. Neurosurg Clin N Am 14: , Levy RM, Lamb S, Adams JE: Treatment of chronic pain by deep brain stimulation: Long term follow-up and review of the literature. Neurosurgery 21: , Liang GS, Chou KL, Baltuch GH, Jaggi JL, Loveland-Jones C, Leng L, Maccarone H, Hurtig HI, Colcher A, Stern MB, Kleiner-Fisman G, Simuni T, Siderowf AD: Long-term outcomes of bilateral subthalamic nucleus stimulation in patients with advanced Parkinson s disease. Stereotact Funct Neurosurg 84: , Limousin-Dowsey P: Thalamic stimulation in essential tremor. Lancet Neurol 3:80, Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL: Electrical stimulation of the subthalamic nucleus in advanced Parkinson s disease. N Engl J Med 339: , Limousin P, Pollak P, Benazzouz A, Hoffmann D, Broussolle E, Perret JE, Benabid AL: Bilateral subthalamic nucleus stimulation for severe Parkinson s disease. Mov Disord 10: , Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL: Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345:91 95, Limousin P, Pollak P, Hoffmann D, Benazzouz A, Perret JE, Benabid AL: Abnormal involuntary movements induced by subthalamic nucleus stimulation in parkinsonian patients. Mov Disord 11: , Lin JJ, Lin SZ, Lin GY, Chang DC, Lee CC: Treatment of intractable generalized dystonia by bilateral posteroventral pallidotomy one-year results. Zhonghua Yi Xue Za Zhi (Taipei) 64: , Lipp A, Tank J, Trottenberg T, Kupsch A, Arnold G, Jordan J: Sympathetic activation due to deep brain stimulation in the region of the STN. Neurology 65: , Liu X, Rowe J, Nandi D, Hayward G, Parkin S, Stein J, Aziz T: Localisation of the subthalamic nucleus using Radionics Image Fusion and Stereoplan combined with field potential recording. A technical note. Stereotact Funct Neurosurg 76:63 73, Loher TJ, Gutbrod K, Fravi NL, Pohle T, Burgunder JM, Krauss JK: Thalamic stimulation for tremor. Subtle changes in episodic memory are related to stimulation per se and not to a microthalamotomy effect. J Neurol 250: , Lozano AM: Vim thalamic stimulation for tremor. Arch Med Res 31: , Lozano AM, Carella F: Physiologic studies in the human brain in movement disorders. Parkinsonism Relat Disord 8: , Lozano AM, Kumar R, Gross RE, Giladi N, Hutchison WD, Dostrovsky JO, Lang AE: Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord 12: , Lyons KE, Davis JT, Pahwa R: Subthalamic nucleus stimulation in Parkinson s disease patients intolerant to levodopa. Stereotact Funct Neurosurg 85: , Lyons KE, Pahwa R: Deep brain stimulation and essential tremor. J Clin Neurophysiol 21:2 5, Lyons KE, Pahwa R: Deep brain stimulation in Parkinson s disease. Curr Neurol Neurosci Rep 4: , Lyons KE, Pahwa R: Long-term benefits in quality of life provided by bilateral subthalamic stimulation in patients with Parkinson disease. J Neurosurg 103: , Lyons KE, Wilkinson SB, Overman J, Pahwa R: Surgical and hardware complications of subthalamic stimulation: A series of 160 procedures. Neurology 63: , Macchi G, Jones EG: Toward an agreement on terminology of nuclear and subnuclear divisions of the motor thalamus. J Neurosurg 86: , Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL: Deep brain stimulation for Parkinson s disease: Surgical technique and perioperative management. Mov Disord 21 [Suppl 14]:S247 S258, Machado AG, Hiremath GK, Salazar F, Rezai AR: Fracture of subthalamic nucleus deep brain stimulation hardware as a result of compulsive manipulation: Case report. Neurosurgery 57:E1318, Maciunas RJ, Galloway RL Jr, Latimer JW: The application accuracy of stereotactic frames. Neurosurgery 35: , Marceglia S, Foffani G, Bianchi AM, Baselli G, Tamma F, Egidi M, Priori A: Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson s disease. J Physiol 571: , Martin AJ, Larson PS, Ostrem JL, Keith Sootsman W, Talke P, Weber OM, Levesque N, Myers J, Starr PA: Placement of deep brain stimulator electrodes using real-time high-field interventional magnetic resonance imaging. Magn Reson Med 54: , Martínez-Martín P, Valldeoriola F, Tolosa E, Pilleri M, Molinuevo JL, Rumià J, Ferrer E: Bilateral subthalamic nucleus stimulation and quality of life in advanced Parkinson s disease. Mov Disord 17: , Matsumoto K, Asano T, Baba T, Miyamoto T, Ohmoto T: Long-term followup results of bilateral thalamotomy for parkinsonism. Appl Neurophysiol 39: , Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH: Deep brain stimulation for treatment-resistant depression. Neuron 45: , McLeod JG: Pathophysiology of Parkinson s disease. Aust N Z J Med 1 [Suppl 1]:19 23, Merello M, Cammarota A, Leiguarda R, Pikielny R: Delayed intracerebral electrode infection after bilateral STN implantation for Parkinson s disease. Case report. Mov Disord 16: , Meyer JS, Magee KR: Current views concerning the etiology, pathophysiology, and treatment of Parkinson s disease. J Neuropsychiatr 5: , Millet B, Jaafari N: Treatment of obsessive-compulsive disorder [in French]. Rev Prat 57:53 57, Mink JW, Walkup J, Frey KA, Como P, Cath D, Delong MR, Erenberg G, Jankovic J, Juncos J, Leckman JF, Swerdlow N, Visser-Vandewalle V, Vitek JL; Tourette Syndrome Association, Inc.: Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord 21: , Miyawaki E, Perlmutter JS, Tröster AI, Videen TO, Koller WC: The behavioral complications of pallidal stimulation: A case report. Brain Cogn 42: , Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, Ferrer E: Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol 57: , Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO: Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson s disease, essential tremor, and pain. J Neurophysiol 93: , Molnar GF, Sailer A, Gunraj CA, Lang AE, Lozano AM, Chen R: Thalamic deep brain stimulation activates the cerebellothalamocortical pathway. Neurology 63: , Moore DJ, West AB, Dawson VL, Dawson TM: Molecular pathophysiology of Parkinson s disease. Annu Rev Neurosci 28:57 87, SHC834 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
27 SURGERY FOR MOVEMENT DISORDERS 249. Moore RY: Organization of midbrain dopamine systems and the pathophysiology of Parkinson s disease. Parkinsonism Relat Disord 9 [Suppl 2]:S65 S71, Morrison CE, Borod JC, Perrine K, Beric A, Brin MF, Rezai A, Kelly P, Sterio D, Germano I, Weisz D, Olanow CW: Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson s disease. Arch Clin Neuropsychol 19: , Mouradian MM, Heuser IJ, Baronti F, Fabbrini G, Juncos JL, Chase TN: Pathogenesis of dyskinesias in Parkinson s disease. Ann Neurol 25: , Murata J, Kitagawa M, Uesugi H, Saito H, Iwasaki Y, Kikuchi S, Tashiro K, Sawamura Y: Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg 99: , Narabayashi H: Neurophysiological ideas on pallidotomy and ventrolateral thalamotomy for hyperkinesis. Confin Neurol 22: , Narabayashi H, Maeda T, Yokochi F: Long-term follow-up study of nucleus ventralis intermedius and ventrolateralis thalamotomy using a microelectrode technique in parkinsonism. Appl Neurophysiol 50: , Narabayashi H, Ohye C: Nucleus ventralis intermedius of human thalamus. Trans Am Neurol Assoc 99: , Narabayashi H, Shimazu H, Fujita Y, Shikiba S, Nagao T, Nagahata M: Procaine-oil-wax pallidotomy for double athetosis and spastic states in infantile cerebral palsy: Report of 80 cases. Neurology 10:61 69, Niemann K, Naujokat C, Pohl G, Wollner C, von Keyserlingk D: Verification of the Schaltenbrand and Wahren stereotactic atlas. Acta Neurochir (Wien) 129:72 81, Nilsson MH, Törnqvist AL, Rehncrona S: Deep-brain stimulation in the subthalamic nuclei improves balance performance in patients with Parkinson s disease, when tested without anti-parkinsonian medication. Acta Neurol Scand 111: , Novak KE, Nenonene EK, Bernstein LP, Vergenz S, Medalle G, Prager JM, Eller TW, Cozzens JW, Rezak M: Two cases of ischemia associated with subthalamic nucleus stimulator implantation for advanced Parkinson s disease. Mov Disord 21: , Obeso JA, Grandas F, Vaamonde J, Luquin MR, Artieda J, Lera G, Rodriguez ME, Martinez-Lage JM: Motor complications associated with chronic levodopa therapy in Parkinson s disease. Neurology 39 [Suppl 2]:11 19, Obeso JA, Rodriguez-Oroz MC, Rodriguez M, DeLong MR, Olanow CW: Pathophysiology of levodopa-induced dyskinesias in Parkinson s disease: Problems with the current model. Ann Neurol 47 [Suppl 1]:S22 S34, Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW: Pathophysiology of the basal ganglia in Parkinson s disease. Trends Neurosci 23 [Suppl 10]:S8 S19, Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR: Pathophysiologic basis of surgery for Parkinson s disease. Neurology 55 [Suppl 6]:S7 S12, Obeso JA, Rodríguez MC, Gorospe A, Guridi J, Alvarez L, Macias R: Surgical treatment of Parkinson s disease. Baillieres Clin Neurol 6: , Oh MY, Abosch A, Kim SH, Lang AE, Lozano AM: Long-term hardwarerelated complications of deep brain stimulation. Neurosurgery 50: , Okun MS, Rodriguez RL, Mikos A, Miller K, Kellison I, Kirsch-Darrow L, Wint DP, Springer U, Fernandez HH, Foote KD, Crucian G, Bowers D: Deep brain stimulation and the role of the neuropsychologist. Clin Neuropsychol 21: , Ondo W, Dat Vuong K, Almaguer M, Jankovic J, Simpson RK: Thalamic deep brain stimulation: Effects on the nontarget limbs. Mov Disord 16: , Ondo WG: Essential tremor: Treatment options. Curr Treat Options Neurol 8: , Ondo WG, Bronte-Stewart H: The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg 83: , Ondo WG, Desaloms JM, Jankovic J, Grossman RG: Pallidotomy for generalized dystonia. Mov Disord 13: , Østergaard K, Aa Sunde N: Evolution of Parkinson s disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord 21: , Østergaard K, Sunde N, Dupont E: Effects of bilateral stimulation of the subthalamic nucleus in patients with severe Parkinson s disease and motor fluctuations. Mov Disord 17: , Pahapill PA, Lozano AM: The pedunculopontine nucleus and Parkinson s disease. Brain 123: , Pahwa R, Lyons KE, Wilkinson SB, Simpson RK Jr, Ondo WG, Tarsy D, Norregaard T, Hubble JP, Smith DA, Hauser RA, Jankovic J: Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg 104: , Pahwa R, Lyons KE, Wilkinson SB, Tröster AI, Overman J, Kieltyka J, Koller WC: Comparison of thalamotomy to deep brain stimulation of the thalamus in essential tremor. Mov Disord 16: , Pahwa R, Wilkinson SB, Overman J, Lyons KE: Preoperative clinical predictors of response to bilateral subthalamic stimulation in patients with Parkinson s disease. Stereotact Funct Neurosurg 83:80 83, Pahwa R, Wilkinson S, Smith D, Lyons K, Miyawaki E, Koller WC: High-frequency stimulation of the globus pallidus for the treatment of Parkinson s disease. Neurology 49: , Paluzzi A, Bain PG, Liu X, Yianni J, Kumarendran K, Aziz TZ: Pregnancy in dystonic women with in situ deep brain stimulators. Mov Disord 21: , Paluzzi A, Belli A, Bain P, Liu X, Aziz TM: Operative and hardware complications of deep brain stimulation for movement disorders. Br J Neurosurg 20: , Parr JR, Green AL, Joint C, Andrew M, Gregory RP, Scott RB, McShane MA, Aziz TZ: Deep brain stimulation in childhood: An effective treatment for early onset generalised idiopathic dystonia. Arch Dis Child 92: , Parsons TD, Rogers SA, Braaten AJ, Woods SP, Tröster AI: Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson s disease: A meta-analysis. Lancet Neurol 5: , Peppe A, Pierantozzi M, Bassi A, Altibrandi MG, Brusa L, Stefani A, Stanzione P, Mazzone P: Stimulation of the subthalamic nucleus compared with the globus pallidus internus in patients with Parkinson disease. J Neurosurg 101: , Perlmutter JS: New insights into the pathophysiology of Parkinson s disease: The challenge of positron emission tomography. Trends Neurosci 11: , Pilitsis JG, Rezai AR, Boulis NM, Henderson JM, Busch RM, Kubu CS: A preliminary study of transient confusional states following bilateral subthalamic stimulation for Parkinson s disease. Stereotact Funct Neurosurg 83:67 70, Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, Bonnet AM, Pollak P, Benabid AL, Agid Y: Neuropsychological changes between off and on STN or GPi stimulation in Parkinson s disease. Neurology 55: , Pillon B, Ardouin C, Dujardin K, Vittini P, Pelissolo A, Cottencin O, Vercueil L, Houeto JL, Krystkowiak P, Agid Y, Destée A, Pollak P, Vidailhet M: Preservation of cognitive function in dystonia treated by pallidal stimulation. Neurology 66: , Pogosyan A, Kühn AA, Trottenberg T, Schneider GH, Kupsch A, Brown P: Elevations in local gamma activity are accompanied by changes in the firing rate and information coding capacity of neurons in the region of the subthalamic nucleus in Parkinson s disease. Exp Neurol 202: , Pollak P, Benabid AL, Gervason CL, Hoffmann D, Seigneuret E, Perret J: Long-term effects of chronic stimulation of the ventral intermediate thalamic nucleus in different types of tremor. Adv Neurol 60: , Pollak P, Benabid AL, Gross C, Gao DM, Laurent A, Benazzouz A, Hoffmann D, Gentil M, Perret J: Effects of the stimulation of the subthalamic nucleus in Parkinson disease [in French]. Rev Neurol (Paris) 149: , Pollak P, Krack P, Fraix V, Mendes A, Moro E, Chabardes S, Benabid AL: Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson s disease. Mov Disord 17 [Suppl 3]:S155 S161, Pool JL, Ransohoff J: Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol 12: , Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM: Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson s disease. Exp Neurol 189: , VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC835
28 REZAI ET AL Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE: Bilateral thalamic deep brain stimulation: Midline tremor control. J Neurol Neurosurg Psychiatry 76: , Putzke JD, Wharen RE Jr, Obwegeser AA, Wszolek ZK, Lucas JA, Turk MF, Uitti RJ: Thalamic deep brain stimulation for essential tremor: Recommendations for long-term outcome analysis. Can J Neurol Sci 31: , Rasche D, Rinaldi PC, Young RF, Tronnier VM: Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus 21:E8, Rezai AR, Kopell BH, Gross RE, Vitek JL, Sharan AD, Limousin P, Benabid AL: Deep brain stimulation for Parkinson s disease: Surgical issues. Mov Disord 21 [Suppl 14]:S197 S218, Rezai AR, Lozano AM, Crawley AP, Joy ML, Davis KD, Kwan CL, Dostrovsky JO, Tasker RR, Mikulis DJ: Thalamic stimulation and functional magnetic resonance imaging: Localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg 90: , Richardson DE: Brain stimulation for pain control. IEEE Trans Biomed Eng 23: , Richardson DE: Thalamic stimulation in the control of pain. South Med J 73: , Richardson DE, Akil H: Pain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sites. J Neurosurg 47: , Richter EO, Hoque T, Halliday W, Lozano AM, Saint-Cyr JA: Determining the position and size of the subthalamic nucleus based on magnetic resonance imaging results in patients with advanced Parkinson disease. J Neurosurg 100: , Rodriguez-Oroz MC, Gorospe A, Guridi J, Ramos E, Linazasoro G, Rodriguez-Palmero M, Obeso JA: Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson s disease. Neurology 55 [Suppl 6]:S45 S51, Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N: Bilateral deep brain stimulation in Parkinson s disease: A multicentre study with 4 years follow-up. Brain 128: , Rodriguez-Oroz MC, Zamarbide I, Guridi J, Palmero MR, Obeso JA: Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson s disease 4 years after surgery: Double blind and open label evaluation. J Neurol Neurosurg Psychiatry 75: , Roitberg B, Urbaniak K, Emborg M: Cell transplantation for Parkinson s disease. Neurol Res 26: , Romito LM, Raja M, Daniele A, Contarino MF, Bentivoglio AR, Barbier A, Scerrati M, Albanese A: Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson s disease. Mov Disord 17: , Romito LM, Scerrati M, Contarino MF, Iacoangeli M, Bentivoglio AR, Albanese A: Bilateral high frequency subthalamic stimulation in Parkinson s disease: Long-term neurological follow-up. J Neurosurg Sci 47: , Saint-Cyr JA, Hoque T, Pereira LC, Dostrovsky JO, Hutchison WD, Mikulis DJ, Abosch A, Sime E, Lang AE, Lozano AM: Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg 97: , Santens P, Boon P, Van Roost D, Caemaert J: The pathophysiology of motor symptoms in Parkinson s disease. Acta Neurol Belg 103: , Sartorius A, Henn FA: Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses 69: , Schad LR, Boesecke R, Schlegel W, Hartmann GH, Sturm V, Strauss LG, Lorenz WJ: Three dimensional image correlation of CT, MR, and PET studies in radiotherapy treatment planning of brain tumors. J Comput Assist Tomogr 11: , Schadt CR, Charles PD, Davis TL, Konrad PE: Thalamotomy, DBS-Vim, and DBS-GPi for generalized dystonia: A case report. Tenn Med 100:38 39, Schaltenbrand G, Wahren W: Atlas for Stereotaxy of the Human Brain. Stuttgart, Thieme Medical Publishers, 1977, pp Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V: Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology, Schüpbach WM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, Czernecki V, Maltête D, Hartmann A, Mallet L, Pidoux B, Dormont D, Navarro S, Cornu P, Mallet A, Agid Y: Stimulation of the subthalamic nucleus in Parkinson s disease: A 5 year follow up. J Neurol Neurosurg Psychiatry 76: , Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP, Speelman JD: A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 342: , Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW: Crossroads in GDNF therapy for Parkinson s disease. Mov Disord 21: , Siderowf A, Jaggi JL, Xie SX, Loveland-Jones C, Leng L, Hurtig H, Colcher A, Stern M, Chou KL, Liang G, Maccarone H, Simuni T, Baltuch G: Long-term effects of bilateral subthalamic nucleus stimulation on health-related quality of life in advanced Parkinson s disease. Mov Disord 21: , Siegfried J, Shulman J: Deep brain stimulation. Pacing Clin Electrophysiol 10: , Silberstein P, Pogosyan A, Kühn AA, Hotton G, Tisch S, Kupsch A, Dowsey- Limousin P, Hariz MI, Brown P: Cortico-cortical coupling in Parkinson s disease and its modulation by therapy. Brain 128: , Sixel-Döring F, Trenkwalder C, Kappus C, Hellwig D: Abscess at the implant site following apical parodontitis. Hardware-related complications of deep brain stimulation [in German]. Nervenarzt 77: , Skidmore FM, Rodriguez RL, Fernandez HH, Goodman WK, Foote KD, Okun MS: Lessons learned in deep brain stimulation for movement and neuropsychiatric disorders. CNS Spectr 11: , Slavin KV, Thulborn KR, Wess C, Nersesyan H: Direct visualization of the human subthalamic nucleus with 3T MR imaging. AJNR Am J Neuroradiol 27:80 84, Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, Walton A, Wagner R, Young AB: Unilateral intraputaminal glial cell line-derived neurotrophic factor in patients with Parkinson disease: Response to 1 year each of treatment and withdrawal. Neurosurg Focus 20:E1, Snyder SH, D Amato RJ: MPTP: A neurotoxin relevant to the pathophysiology of Parkinson s disease. The 1985 George C. Cotzias lecture. Neurology 36: , Spiegel EA, Wycis HT: Effect of thalamic and pallidal lesions upon involuntary movements in choreoathetosis. Trans Am Neurol Assoc 51: , Spiegel EA, Wycis HT, Baird HW 3rd: Pallidotomy and pallido-amygdalotomy in certain types of convulsive disorders. Trans Am Neurol Assoc 82:51 54, Spiegel EA, Wycis HT, Marks M, Lee AJ: Stereotaxic apparatus for operations on the human brain. Science 106: , Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J: Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord 22: , Starr PA: Placement of deep brain stimulators into the subthalamic nucleus or Globus pallidus internus: Technical approach. Stereotact Funct Neurosurg 79: , Starr PA, Turner RS, Rau G, Lindsey N, Heath S, Volz M, Ostrem JL, Marks WJ Jr: Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: Techniques, electrode locations, and outcomes. J Neurosurg 104: , Starr PA, Vitek JL, Bakay RA: Ablative surgery and deep brain stimulation for Parkinson s disease. Neurosurgery 43: , Starr P, Verhagen L, Larson P, Bakay E, Taylor R, Cahn-Weiner D, Bartus R, Ostrem J, Marks W: Intrastriatal gene transfer with AAV-neurturin for Parkinson s disease: Results of a phase I trial. Presented at the 75th Annual Meeting of the American Association of Neurological Surgeons, Washington, D.C., April 16, Sterio D, Zonenshayn M, Mogilner AY, Rezai AR, Kiprovski K, Kelly PJ, Beric A: Neurophysiological refinement of subthalamic nucleus targeting. Neurosurgery 50:58 69, SHC836 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
29 SURGERY FOR MOVEMENT DISORDERS 335. Stover NP, Bakay RAE, Subramanian T, Raiser CD, Cornfeldt ML, Schweikert AW, Allen RC, Watts RL: Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Arch Neurol 62: , Svennilson E, Torvik A, Lowe R, Leksell L: Treatment of parkinsonism by stereotatic thermolesions in the pallidal region. A clinical evaluation of 81 cases. Acta Psychiatr Scand 35: , Taha JM, Janszen MA, Favre J: Thalamic deep brain stimulation for the treatment of head, voice, and bilateral limb tremor. J Neurosurg 91:68 72, Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A: Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J 17: , Talairach J, Tournoux P: Co-planar Stereotactic Atlas of the Human Brain. Stuttgart, Thieme Verlag, 1988, pp Tapper VJ: Pathophysiology, assessment, and treatment of Parkinson s disease. Nurse Pract 22:76 78, Tasker RR: Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol 49: , Tasker RR: Tremor of parkinsonism and stereotactic thalamotomy. Mayo Clin Proc 62: , Tasker RR, Munz M, Junn FS, Kiss ZH, Davis K, Dostrovsky JO, Lozano AM: Deep brain stimulation and thalamotomy for tremor compared. Acta Neurochir Suppl 68:49 53, Tavella A, Bergamasco B, Bosticco E, Lanotte M, Perozzo P, Rizzone M, Torre E, Lopiano L: Deep brain stimulation of the subthalamic nucleus in Parkinson s disease: Long-term follow-up. Neurol Sci 23 [Suppl 2]:S111 S112, Teive HA, Sá DS, Grande CV, Antoniuk A, Werneck LC: Bilateral pallidotomy for generalized dystonia. Arq Neuropsiquiatr 59: , Terao T, Okiyama R, Takahashi H, Yokochi F, Taniguchi M, Hamada I, Hasegawa N: Comparison and examination of stereotactic surgical complications in movement disorders [in Japanese]. No Shinkei Geka 31: , Terao T, Takahashi H, Yokochi F, Taniguchi M, Okiyama R, Hamada I: Hemorrhagic complication of stereotactic surgery in patients with movement disorders. J Neurosurg 98: , Thobois S, Mertens P, Guenot M, Hermier M, Mollion H, Bouvard M, Chazot G, Broussolle E, Sindou M: Subthalamic nucleus stimulation in Parkinson s disease: Clinical evaluation of 18 patients. J Neurol 249: , Tir M, Devos D, Blond S, Touzet G, Reyns N, Duhamel A, Cottencin O, Dujardin K, Cassim F, Destée A, Defebvre L, Krystkowiak P: Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of parkinsonian patients. Neurosurgery 61: , Tisch S, Rothwell JC, Bhatia KP, Quinn N, Zrinzo L, Jahanshahi M, Ashkan K, Hariz M, Limousin P: Pallidal stimulation modifies after-effects of paired associative stimulation on motor cortex excitability in primary generalised dystonia. Exp Neurol 206:80 85, Tisch S, Zrinzo L, Limousin P, Bhatia KP, Quinn N, Ashkan K, Hariz M: The effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia. J Neurol Neurosurg Psychiatry, Trépanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA: Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson s disease. Brain Cogn 42: , Trottenberg T, Fogelson N, Kühn AA, Kivi A, Kupsch A, Schneider GH, Brown P: Subthalamic gamma activity in patients with Parkinson s disease. Exp Neurol 200:56 65, Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T: Deafferentation pain and stimulation of the thalamic sensory relay nucleus: Clinical and experimental study. Appl Neurophysiol 48: , Uc EY, Follett KA: Deep brain stimulation in movement disorders. Semin Neurol 27: , Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, Baltuch GH: Deep brain stimulation for movement disorders: Morbidity and mortality in 109 patients. J Neurosurg 98: , Ushe M, Mink JW, Revilla FJ, Wernle A, Schneider Gibson P, McGee-Minnich L, Hong M, Rich KM, Lyons KE, Pahwa R, Perlmutter JS: Effect of stimulation frequency on tremor suppression in essential tremor. Mov Disord 19: , Valldeoriola F, Pilleri M, Tolosa E, Molinuevo JL, Rumià J, Ferrer E: Bilateral subthalamic stimulation monotherapy in advanced Parkinson s disease: Long-term follow-up of patients. Mov Disord 17: , Varma TR, Fox SH, Eldridge PR, Littlechild P, Byrne P, Forster A, Marshall A, Cameron H, McIver K, Fletcher N, Steiger M: Deep brain stimulation of the subthalamic nucleus: Effectiveness in advanced Parkinson s disease patients previously reliant on apomorphine. J Neurol Neurosurg Psychiatry 74: , Vergani F, Landi A, Antonini A, Sganzerla EP: Bilateral subthalamic deep brain stimulation in a patient with Parkinson s disease who had previously undergone thalamotomy and autologous adrenal grafting in the caudate nucleus: Case report. Neurosurgery 59:E1140, Vesper J, Haak S, Ostertag C, Nikkhah G: Subthalamic nucleus deep brain stimulation in elderly patients Analysis of outcome and complications. BMC Neurol 7:7, Vesper J, Klostermann F, Stockhammer F, Funk T, Brock M: Results of chronic subthalamic nucleus stimulation for Parkinson s disease: A 1-year follow-up study. Surg Neurol 57: , Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tézenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destée A, Pollak P: Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352: , Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, Bardinet E, Benabid AL, Navarro S, Dormont D, Grand S, Blond S, Ardouin C, Pillon B, Dujardin K, Hahn-Barma V, Agid Y, Destée A, Pollak P; French SPIDY Study Group: Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: A prospective 3 year follow-up study. Lancet Neurol 6: , Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J: Subthalamic DBS replaces levodopa in Parkinson s disease: Two-year followup. Neurology 58: , Visser-Vandewalle V, Ackermans L, van der Linden C, Temel Y, Tijssen MA, Schruers KR, Nederveen P, Kleijer M, Boon P, Weber W, Cath D: Deep brain stimulation in Gilles de la Tourette s syndrome. Neurosurgery 58:E590, Visser-Vandewalle V, van der Linden C, Temel Y, Celik H, Ackermans L, Spinc le G, Caemaert J: Long-term effects of bilateral subthalamic nucleus stimulation in advanced Parkinson disease: A four year follow-up study. Parkinsonism Relat Disord 11: , Vitek JL: Deep brain stimulation for Parkinson s disease. A critical re-evaluation of STN versus GPi DBS. Stereotact Funct Neurosurg 78: , Vitek JL: Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord 17 [Suppl 3]:S69 S72, Vitek JL: Pathophysiology of dystonia: A neuronal model. Mov Disord 17 [Suppl 3]:S49 S62, Vitek JL, Bakay RA: The role of pallidotomy in Parkinson s disease and dystonia. Curr Opin Neurol 10: , Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR: Microelectrode-guided pallidotomy: Technical approach and its application in medically intractable Parkinson s disease. J Neurosurg 88: , Vitek JL, Zhang J, Evatt M, Mewes K, DeLong MR, Hashimoto T, Triche S, Bakay RA: GPi pallidotomy for dystonia: Clinical outcome and neuronal activity. Adv Neurol 78: , Voges J, Hilker R, Bötzel K, Kiening KL, Kloss M, Kupsch A, Schnitzler A, Schneider GH, Steude U, Deuschl G, Pinsker MO: Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov Disord, 22: , Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund HJ, Sturm V: Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: Correlation of therapeutic effect with anatomical electrode position. J Neurosurg 96: , Voges J, Waerzeggers Y, Maarouf M, Lehrke R, Koulousakis A, Lenartz D, Sturm V: Deep-brain stimulation: Long-term analysis of complications caused by hardware and surgery experiences from a single centre. J Neurol Neurosurg Psychiatry 77: , VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC837
30 REZAI ET AL Volkmann J: Deep brain stimulation for the treatment of Parkinson s disease. J Clin Neurophysiol 21:6 17, Volkmann J, Allert N, Voges J, Sturm V, Schnitzler A, Freund HJ: Long-term results of bilateral pallidal stimulation in Parkinson s disease. Ann Neurol 55: , Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V: Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology 56: , Voon V, Kubu C, Krack P, Houeto JL, Troster AI: Deep brain stimulation: Neuropsychological and neuropsychiatric issues. Mov Disord 21 [Suppl 14]:S305 S327, Walker AE: Cerebral pedunculotomy for the relief of involuntary movements; hemiballismus. Acta Psychiatr Neurol 24: , Walker AE: Cerebral pedunculotomy for the relief of involuntary movements. II. Parkinsonian tremor. J Nerv Ment Dis 116: , Weetman J, Anderson IM, Gregory RP, Gill SS: Bilateral posteroventral pallidotomy for severe antipsychotic induced tardive dyskinesia and dystonia. J Neurol Neurosurg Psychiatry 63: , Welter ML, Houeto JL, Tezenas du Montcel S, Mesnage V, Bonnet AM, Pillon B, Arnulf I, Pidoux B, Dormont D, Cornu P, Agid Y: Clinical predictive factors of subthalamic stimulation in Parkinson s disease. Brain 125: , Wichmann T, DeLong MR: Pathophysiology of Parkinson s disease: The MPTP primate model of the human disorder. Ann N Y Acad Sci 991: , Wichmann T, Delong MR: Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52: , Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ: A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol 52: , Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND: Lentivirus-mediated gene transfer to the central nervous system: Therapeutic and research applications. Hum Gene Ther 17:1 9, Woods SP, Fields JA, Lyons KE, Koller WC, Wilkinson SB, Pahwa R, Troster AI: Neuropsychological and quality of life changes following unilateral thalamic deep brain stimulation in Parkinson s disease: A one-year follow-up. Acta Neurochir (Wien) 143: , Woods SP, Fields JA, Troster AI: Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson s disease: A critical review. Neuropsychol Rev 12: , Wooten GF: Progress in understanding the pathophysiology of treatmentrelated fluctuations in Parkinson s disease. Ann Neurol 24: , Wycis HT, Baird HW, Spiegel EA: Pallidotomy and pallido-amygdalotomy in certain types of convulsive disorders. Confin Neurol 17:67 68, Yokoyama T, Sugiyama K, Nishizawa S, Yokota N, Ohta S, Uemura K: Subthalamic nucleus stimulation for gait disturbance in Parkinson s disease. Neurosurgery 45:41 49, Zhang JG, Zhang K, Ma Y, Hu WH, Yang AC, Chu JS, Wu ST, Ge M, Zhang Y, Wang ZC: Follow-up of bilateral subthalamic deep brain stimulation for Parkinson s disease. Acta Neurochir Suppl 99:43 47, Zhang JG, Zhang K, Wang ZC: Deep brain stimulation in the treatment of tardive dystonia. Chin Med J (Engl) 119: , Zhang JG, Zhang K, Wang ZC, Ge M, Ma Y: Deep brain stimulation in the treatment of secondary dystonia. Chin Med J (Engl) 119: , Acknowledgments We thank Charles Steiner, M.E., Martha Tobin, M.A., and Christine Moore, A.A., for their expert technical assistance in the preparation of this manuscript. COMMENTS This ambitious article shows what a privilege it is to participate in the extraordinary world of movement disorder surgery. This narrow subspecialty is paradigmatic of the golden age of neurosurgery, combining so many of the elements that fascinate and inspire us: Its rise has been fueled by seminal discoveries in basal ganglia physiology, the technical approaches are rapidly evolving, and it represents a coming era where elective neurosurgery can greatly enhance quality of life for a prolonged period in serious neurological diseases. Philip A. Starr San Francisco, California Deep brain stimulation for movement disorders joined the mainstream of neurosurgical practice only in the last 10 years, yet as noted by Rezai et al. (who have played no small role in increasing access to this procedure for patients with movement disorders as well as other more novel indications), more than 1000 articles have been published on this topic. A detailed, comprehensive review of this complex field would require a whole supplement (1), but the authors provide a review that nevertheless addresses all of the major issues and is in a very readable form. Certain aspects of surgical technique tend to inspire extensive debate among functional neurosurgeons, such as the use of framed vs. frameless devices, or the use of microelectrode recording techniques. Rezai et al. provide balanced views of these areas, correctly pointing out that these and other issues can only be resolved with prospective, randomized, blinded trials. However, it is not feasible to subject every nuance of functional neurosurgical procedures to such a metric. Ten years later, we still do not know from prospective randomized trials (Class 1 evidence) whether the subthalamic nucleus (STN) truly is a superior target to the globus pallidus internus, and what the relative risks and benefits are (although two such trials will be arriving soon, one from Emory and the Cleveland Clinic, and the other from the multicenter National Institutes of Health/Veteran s Administration trial). Ten years later, we must still suffer the endless debate about the utility of microelectrode recording, now obfuscating the pioneering work on new potential targets for Parkinson s disease (2, 3). As new indications, e.g., depression (7), and new targets, e.g., pedunculopontine nucleus (PPN), arrive on the landscape, new and old issues will arise, providing more fodder for prospective, randomized, clinical trials: Which is better, STN, PPN, STN + PPN, or STN then PPN (4, 5)? Which is better, gene therapy or cell therapy or deep brain stimulation? If gene therapy is better, then which gene therapy? Anterior internal capsule or Area 25 for depression? Medtronic (Minneapolis, MN) or Advanced Neuromodulation Systems (Plano, TX)? Which method is better: yours, or mine, or both, or neither? And has anyone yet subjected prospective, randomized, double-blind, sham/placebo-controlled trials to a prospective, randomized, clinical trial? Two such trials seemingly eliminated fetal cell transplantation for Parkinson s disease, but were the trials fair? Perhaps we will even see this subject revisited in the years to come (6). If Rezai et al. reflects the state of the art for movement disorders alone 10 years later and approximately a century after surgery for movement disorders began, I suspect functional neurosurgeons will be well occupied for the next century too. Robert E. Gross Atlanta, Georgia 1. Benabid AL, Deuschl G, Lang AE, Rezai AR: Deep brain stimulation for Parkinson s disease. Mov Disord 21 [Suppl 14]:S168 S170, Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH: Deep brain stimulation for treatment-resistant depression. Neuron 45: , Mazzone P, Stanzione P, Lozano A, Scarnati E, Peppe A, Galati S, Stefani A: Reply: The peripeduncular and pedunculopontine nuclei: A putative dispute not discouraging the effort to define a clinically relevant target. Brain 130:E74, 2007 (comment). SHC838 VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT
31 SURGERY FOR MOVEMENT DISORDERS 4. Plaha P, Gill SS: Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson s disease. Neuroreport 16: , Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P: Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson s disease. Brain 130: , Tuszynski M: Rebuilding the brain: Resurgence of fetal grafting. Nat Neurosci 10: , Zrinzo L, Zrinzo LV, Hariz M: The pedunculopontine and peripeduncular nuclei: A tale of two structures. Brain 130:E73, In this article, Rezai et al. provide an excellent overview of the current status of movement disorder neurosurgery. The information is provided in a well-organized manner and the literature review is extensive. The conclusions presented are based on the available literature, gray areas in our knowledge are appropriately highlighted, and professional biases are minimized. This is a comprehensive first read for anyone interested in participating in this exciting field of neurosurgery. Ron L. Alterman Bronx, New York Rezai et al. provide an outstanding review of the history, present status, and possible future of movement disorder surgery that has progressed from gross ablations to precise lesioning to neuroaugmentation and neuromodulation. Initially based on empiricism and a crude understanding of the neural circuitry of the motor system, functional neurosurgery has been ever more refined by better understanding of extrapyramidal neurophysiology and cellular neurochemistry. As a result of modern advances in basic science, future procedures may go beyond simple manipulation of large cellular populations for the control of symptoms (as we do now with deep brain stimulation neuroaugmentation) to neurochemically target-specific cellular groups within these populations. In addition, genetically programmed stem cells may be used to repopulate central nervous system regions whose cells have been decimated by the underlying disease process. However, the ultimate therapy may focus on identifying and reversing the neurotoxic triggers that result in selective loss of neuromelanin-containing dopaminergic neurons in the substantia nigra. Patrick J. Kelly New York, New York Dissection of the brain, (engraving, after A. Vesalius, Fabrica, 1543), T. Geminus, Compendiosa totius anatomie delineatio. London, J. Herford, (From: Roberts KB, Tomlinson JDW: The Fabric of the Body: European Traditions of Anatomical Illustration. Oxford, Clarendon Press, 1992). VOLUME 62 NUMBER 2 FEBRUARY 2008 SUPPLEMENT SHC839
Anatomy of the basal ganglia. Dana Cohen Gonda Brain Research Center, room 410
 Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
VL VA BASAL GANGLIA. FUNCTIONAl COMPONENTS. Function Component Deficits Start/initiation Basal Ganglia Spontan movements
 BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
Deep Brain Stimulation: Surgical Process
 Deep Brain Stimulation: Surgical Process Kia Shahlaie, MD, PhD Assistant Professor Bronte Endowed Chair in Epilepsy Research Director of Functional Neurosurgery Minimally Invasive Neurosurgery Department
Deep Brain Stimulation: Surgical Process Kia Shahlaie, MD, PhD Assistant Professor Bronte Endowed Chair in Epilepsy Research Director of Functional Neurosurgery Minimally Invasive Neurosurgery Department
COGNITIVE SCIENCE 107A. Motor Systems: Basal Ganglia. Jaime A. Pineda, Ph.D.
 COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
Making Things Happen 2: Motor Disorders
 Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Basal Ganglia George R. Leichnetz, Ph.D.
 Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Damage on one side.. (Notes) Just remember: Unilateral damage to basal ganglia causes contralateral symptoms.
 Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Connections of basal ganglia
 Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD
 PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD WHAT IS DEEP BRAIN STIMULATION? WHY SHOULD YOU CONSIDER DBS SURGERY FOR YOUR PATIENTS? HOW DOES DBS WORK? DBS electrical stimulation overrides abnormal
PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD WHAT IS DEEP BRAIN STIMULATION? WHY SHOULD YOU CONSIDER DBS SURGERY FOR YOUR PATIENTS? HOW DOES DBS WORK? DBS electrical stimulation overrides abnormal
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Functional Neuroanatomy and Physiology for Movement Disorders
 Functional Neuroanatomy and Physiology for Movement Disorders Chang, Won Seok Division of Stereotactic and Functional Neurosurgery, Department of Neurosurgery, Brain Research Institute, Yonsei University
Functional Neuroanatomy and Physiology for Movement Disorders Chang, Won Seok Division of Stereotactic and Functional Neurosurgery, Department of Neurosurgery, Brain Research Institute, Yonsei University
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: SUR Policy No: 395 1/1/19 Medical Policy Committee Approved Date: 8/17; 2/18; 12/18 Medical Officer Date APPLIES TO: Medicare Only See Policy CPT/HCPCS CODE section below
Effective Date: 1/1/2019 Section: SUR Policy No: 395 1/1/19 Medical Policy Committee Approved Date: 8/17; 2/18; 12/18 Medical Officer Date APPLIES TO: Medicare Only See Policy CPT/HCPCS CODE section below
The motor regulator. 1) Basal ganglia/nucleus
 The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
Deep Brain Stimulation: Indications and Ethical Applications
 Deep Brain Stimulation Overview Kara D. Beasley, DO, MBe, FACOS Boulder Neurosurgical and Spine Associates (303) 562-1372 Deep Brain Stimulation: Indications and Ethical Applications Instrument of Change
Deep Brain Stimulation Overview Kara D. Beasley, DO, MBe, FACOS Boulder Neurosurgical and Spine Associates (303) 562-1372 Deep Brain Stimulation: Indications and Ethical Applications Instrument of Change
Surgical Treatment for Movement Disorders
 Surgical Treatment for Movement Disorders Seth F Oliveria, MD PhD The Oregon Clinic Neurosurgery Director of Functional Neurosurgery: Providence Brain and Spine Institute Portland, OR Providence St Vincent
Surgical Treatment for Movement Disorders Seth F Oliveria, MD PhD The Oregon Clinic Neurosurgery Director of Functional Neurosurgery: Providence Brain and Spine Institute Portland, OR Providence St Vincent
Punit Agrawal, DO Clinical Assistant Professor of Neurology Division of Movement Disorders OSU Department of Neurology
 Deep Brain Stimulation for Movement Disorders Punit Agrawal, DO Clinical Assistant Professor of Neurology Division of Movement Disorders OSU Department of Neurology History of DBS 1 History of DBS 1987
Deep Brain Stimulation for Movement Disorders Punit Agrawal, DO Clinical Assistant Professor of Neurology Division of Movement Disorders OSU Department of Neurology History of DBS 1 History of DBS 1987
Lecture XIII. Brain Diseases I - Parkinsonism! Brain Diseases I!
 Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Basal ganglia Sujata Sofat, class of 2009
 Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Biological Bases of Behavior. 8: Control of Movement
 Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Deep Brain Stimulation: Patient selection
 Deep Brain Stimulation: Patient selection Halim Fadil, MD Movement Disorders Neurologist Kane Hall Barry Neurology Bedford/Keller, TX 1991: Thalamic (Vim) DBS for tremor Benabid AL, et al. Lancet. 1991;337(8738):403-406.
Deep Brain Stimulation: Patient selection Halim Fadil, MD Movement Disorders Neurologist Kane Hall Barry Neurology Bedford/Keller, TX 1991: Thalamic (Vim) DBS for tremor Benabid AL, et al. Lancet. 1991;337(8738):403-406.
Chapter 8. Control of movement
 Chapter 8 Control of movement 1st Type: Skeletal Muscle Skeletal Muscle: Ones that moves us Muscles contract, limb flex Flexion: a movement of a limb that tends to bend its joints, contraction of a flexor
Chapter 8 Control of movement 1st Type: Skeletal Muscle Skeletal Muscle: Ones that moves us Muscles contract, limb flex Flexion: a movement of a limb that tends to bend its joints, contraction of a flexor
Palladotomy and Pallidal Deep Brain Stimulation
 Palladotomy and Pallidal Deep Brain Stimulation Parkinson s disease Parkinson s Disease is a common neurodegenerative disorder that affects about 1:100 individuals over the age of 60. In a small percentage
Palladotomy and Pallidal Deep Brain Stimulation Parkinson s disease Parkinson s Disease is a common neurodegenerative disorder that affects about 1:100 individuals over the age of 60. In a small percentage
Teach-SHEET Basal Ganglia
 Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
The Wonders of the Basal Ganglia
 Basal Ganglia The Wonders of the Basal Ganglia by Mackenzie Breton and Laura Strong /// https://kin450- neurophysiology.wikispaces.com/basal+ganglia Introduction The basal ganglia are a group of nuclei
Basal Ganglia The Wonders of the Basal Ganglia by Mackenzie Breton and Laura Strong /// https://kin450- neurophysiology.wikispaces.com/basal+ganglia Introduction The basal ganglia are a group of nuclei
The Surgical Management of Essential Tremor
 The Surgical Management of Essential Tremor International Essential Tremor Foundation Learning About Essential Tremor: Diagnosis and Treatment Options Albuquerque, NM September 24, 2005 Neurosurgeon Overview:
The Surgical Management of Essential Tremor International Essential Tremor Foundation Learning About Essential Tremor: Diagnosis and Treatment Options Albuquerque, NM September 24, 2005 Neurosurgeon Overview:
Deep Brain Stimulation Surgery for Parkinson s Disease
 Deep Brain Stimulation Surgery for Parkinson s Disease Demystifying Medicine 24 January 2012 Kareem A. Zaghloul, MD, PhD Staff Physician, Surgical Neurology Branch NINDS Surgery for Parkinson s Disease
Deep Brain Stimulation Surgery for Parkinson s Disease Demystifying Medicine 24 January 2012 Kareem A. Zaghloul, MD, PhD Staff Physician, Surgical Neurology Branch NINDS Surgery for Parkinson s Disease
The webinar will begin momentarily. Tractography-based Targeting for Functional Neurosurgery
 Welcome The webinar will begin momentarily. Tractography-based Targeting for Functional Neurosurgery Vibhor Krishna, MD, SM Assistant Professor, Center for Neuromoduation, Dept. of Neurosurgery and Dept.
Welcome The webinar will begin momentarily. Tractography-based Targeting for Functional Neurosurgery Vibhor Krishna, MD, SM Assistant Professor, Center for Neuromoduation, Dept. of Neurosurgery and Dept.
Basal Ganglia. Steven McLoon Department of Neuroscience University of Minnesota
 Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Subthalamic Nucleus Deep Brain Stimulation (STN-DBS)
 Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) A Neurosurgical Treatment for Parkinson s Disease Parkinson s Disease Parkinson s disease is a common neurodegenerative disorder that affects about
Subthalamic Nucleus Deep Brain Stimulation (STN-DBS) A Neurosurgical Treatment for Parkinson s Disease Parkinson s Disease Parkinson s disease is a common neurodegenerative disorder that affects about
GBME graduate course. Chapter 43. The Basal Ganglia
 GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
Surgical Treatment: Patient Edition
 Parkinson s Disease Clinic and Research Center University of California, San Francisco 505 Parnassus Ave., Rm. 795-M, Box 0114 San Francisco, CA 94143-0114 (415) 476-9276 http://pdcenter.neurology.ucsf.edu
Parkinson s Disease Clinic and Research Center University of California, San Francisco 505 Parnassus Ave., Rm. 795-M, Box 0114 San Francisco, CA 94143-0114 (415) 476-9276 http://pdcenter.neurology.ucsf.edu
Deep Brain Stimulation and Movement Disorders
 Deep Brain Stimulation and Movement Disorders Farrokh Farrokhi, MD Neurosurgery Maria Marsans, PA-C Neurosurgery Virginia Mason June 27, 2017 OBJECTIVES Understand the role of Deep Brain Stimulation (DBS)
Deep Brain Stimulation and Movement Disorders Farrokh Farrokhi, MD Neurosurgery Maria Marsans, PA-C Neurosurgery Virginia Mason June 27, 2017 OBJECTIVES Understand the role of Deep Brain Stimulation (DBS)
Deep Brain Stimulation for Parkinson s Disease & Essential Tremor
 Deep Brain Stimulation for Parkinson s Disease & Essential Tremor Albert Fenoy, MD Assistant Professor University of Texas at Houston, Health Science Center Current US Approvals Essential Tremor and Parkinsonian
Deep Brain Stimulation for Parkinson s Disease & Essential Tremor Albert Fenoy, MD Assistant Professor University of Texas at Houston, Health Science Center Current US Approvals Essential Tremor and Parkinsonian
Basal Ganglia. Introduction. Basal Ganglia at a Glance. Role of the BG
 Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Deep Brain Stimulation. Is It Right for You?
 Deep Brain Stimulation Is It Right for You? Northwestern Medicine Deep Brain Stimulation What is DBS? Northwestern Medicine Central DuPage Hospital is a regional destination for the treatment of movement
Deep Brain Stimulation Is It Right for You? Northwestern Medicine Deep Brain Stimulation What is DBS? Northwestern Medicine Central DuPage Hospital is a regional destination for the treatment of movement
Basal Ganglia. Today s lecture is about Basal Ganglia and it covers:
 Basal Ganglia Motor system is complex interaction between Lower motor neurons (spinal cord and brainstem circuits) and Upper motor neurons (pyramidal and extrapyramidal tracts) plus two main regulators
Basal Ganglia Motor system is complex interaction between Lower motor neurons (spinal cord and brainstem circuits) and Upper motor neurons (pyramidal and extrapyramidal tracts) plus two main regulators
Surgical Treatment of Movement Disorders. Surgical Treatment of Movement Disorders. New Techniques: Procedure is safer and better
 Surgical Treatment of Movement Stephen Grill, MD, PHD Johns Hopkins University and Parkinson s and Movement Center of Maryland Surgical Treatment of Movement Historical Aspects Preoperative Issues Surgical
Surgical Treatment of Movement Stephen Grill, MD, PHD Johns Hopkins University and Parkinson s and Movement Center of Maryland Surgical Treatment of Movement Historical Aspects Preoperative Issues Surgical
SUPPLEMENTAL DIGITAL CONTENT
 SUPPLEMENTAL DIGITAL CONTENT FIGURE 1. Unilateral subthalamic nucleus (STN) deep brain stimulation (DBS) electrode and internal pulse generator. Copyright 2010 Oregon Health & Science University. Used
SUPPLEMENTAL DIGITAL CONTENT FIGURE 1. Unilateral subthalamic nucleus (STN) deep brain stimulation (DBS) electrode and internal pulse generator. Copyright 2010 Oregon Health & Science University. Used
BASAL GANGLIA. Dr JAMILA EL MEDANY
 BASAL GANGLIA Dr JAMILA EL MEDANY OBJECTIVES At the end of the lecture, the student should be able to: Define basal ganglia and enumerate its components. Enumerate parts of Corpus Striatum and their important
BASAL GANGLIA Dr JAMILA EL MEDANY OBJECTIVES At the end of the lecture, the student should be able to: Define basal ganglia and enumerate its components. Enumerate parts of Corpus Striatum and their important
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Strick Lecture 4 March 29, 2006 Page 1
 Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Pallidal Deep Brain Stimulation
 Pallidal Deep Brain Stimulation Treatment for Dystonia Dystonia Dystonia is a neurological disorder characterized by involuntary muscle contractions resulting in abnormal postures. These movements may
Pallidal Deep Brain Stimulation Treatment for Dystonia Dystonia Dystonia is a neurological disorder characterized by involuntary muscle contractions resulting in abnormal postures. These movements may
Dr. Farah Nabil Abbas. MBChB, MSc, PhD
 Dr. Farah Nabil Abbas MBChB, MSc, PhD The Basal Ganglia *Functions in association with motor cortex and corticospinal pathways. *Regarded as accessory motor system besides cerebellum. *Receive most of
Dr. Farah Nabil Abbas MBChB, MSc, PhD The Basal Ganglia *Functions in association with motor cortex and corticospinal pathways. *Regarded as accessory motor system besides cerebellum. *Receive most of
EMERGING TREATMENTS FOR PARKINSON S DISEASE
 EMERGING TREATMENTS FOR PARKINSON S DISEASE Katerina Markopoulou, MD, PhD Director Neurodegenerative Diseases Program Department of Neurology NorthShore University HealthSystem Clinical Assistant Professor
EMERGING TREATMENTS FOR PARKINSON S DISEASE Katerina Markopoulou, MD, PhD Director Neurodegenerative Diseases Program Department of Neurology NorthShore University HealthSystem Clinical Assistant Professor
Neurodegenerative Disease. April 12, Cunningham. Department of Neurosciences
 Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Basal Ganglia General Info
 Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Visualization and simulated animations of pathology and symptoms of Parkinson s disease
 Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
NS219: Basal Ganglia Anatomy
 NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Motor Functions of Cerebral Cortex
 Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning
 1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
symptoms of Parkinson s disease EXCEPT.
 M. Angele Theard, M.D Asst. Professor, Washington University, St. Louis, MO Quiz team; Shobana Rajan, M.D; Suneeta Gollapudy, MD; Verghese Cherian, M.D, M. Angele Theard, MD This quiz is being published
M. Angele Theard, M.D Asst. Professor, Washington University, St. Louis, MO Quiz team; Shobana Rajan, M.D; Suneeta Gollapudy, MD; Verghese Cherian, M.D, M. Angele Theard, MD This quiz is being published
As clinical experience with deep-brain stimulation (DBS) of
 ORIGINAL RESEARCH K.V. Slavin K.R. Thulborn C. Wess H. Nersesyan Direct Visualization of the Human Subthalamic Nucleus with 3T MR Imaging BACKGROUND AND PURPOSE: Electrical stimulation of the subthalamic
ORIGINAL RESEARCH K.V. Slavin K.R. Thulborn C. Wess H. Nersesyan Direct Visualization of the Human Subthalamic Nucleus with 3T MR Imaging BACKGROUND AND PURPOSE: Electrical stimulation of the subthalamic
Deep brain stimulation: What can patients expect from it?
 MEDICAL GRAND ROUNDS ANDRE MACHADO, MD, PhD* Director, Center for Neurological Restoration, Neurological Institute, Cleveland Clinic HUBERT H. FERNANDEZ, MD Section Head, Movement Disorders, Center for
MEDICAL GRAND ROUNDS ANDRE MACHADO, MD, PhD* Director, Center for Neurological Restoration, Neurological Institute, Cleveland Clinic HUBERT H. FERNANDEZ, MD Section Head, Movement Disorders, Center for
Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease
 Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease Abstract Parkinson's disease (PD) is a neurodegenerative disease affecting the dopaminergic neurons of the
Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease Abstract Parkinson's disease (PD) is a neurodegenerative disease affecting the dopaminergic neurons of the
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
1/2/2019. Basal Ganglia & Cerebellum a quick overview. Outcomes you want to accomplish. MHD-Neuroanatomy Neuroscience Block. Basal ganglia review
 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
Extrapyramidal Motor System. Basal Ganglia or Striatum. Basal Ganglia or Striatum 3/3/2010
 Extrapyramidal Motor System Basal Ganglia or Striatum Descending extrapyramidal paths receive input from other parts of motor system: From the cerebellum From the basal ganglia or corpus striatum Caudate
Extrapyramidal Motor System Basal Ganglia or Striatum Descending extrapyramidal paths receive input from other parts of motor system: From the cerebellum From the basal ganglia or corpus striatum Caudate
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817.
 Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
High-Field in vivo Visualization of the Human Globus Pallidus Using 7T MRI
 Niederer 1 High-Field in vivo Visualization of the Human Globus Pallidus Using 7T MRI Abstract In the past several decades the introduction of high-resolution, three-dimensional modeling techniques have
Niederer 1 High-Field in vivo Visualization of the Human Globus Pallidus Using 7T MRI Abstract In the past several decades the introduction of high-resolution, three-dimensional modeling techniques have
DBS Programming. Paul S Fishman MD, PhD University of Maryland School of Medicine PFNCA 3/24/18
 DBS Programming Paul S Fishman MD, PhD University of Maryland School of Medicine PFNCA 3/24/18 Disclosure The University of Maryland has received research funding form InSightec and the Focused Ultrasound
DBS Programming Paul S Fishman MD, PhD University of Maryland School of Medicine PFNCA 3/24/18 Disclosure The University of Maryland has received research funding form InSightec and the Focused Ultrasound
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
DEEP BRAIN STIMULATION
 DEEP BRAIN STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
DEEP BRAIN STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Motor System Hierarchy
 Motor Pathways Lectures Objectives Define the terms upper and lower motor neurons with examples. Describe the corticospinal (pyramidal) tract and the direct motor pathways from the cortex to the trunk
Motor Pathways Lectures Objectives Define the terms upper and lower motor neurons with examples. Describe the corticospinal (pyramidal) tract and the direct motor pathways from the cortex to the trunk
Parkinson disease: Parkinson Disease
 Surgical Surgical treatment treatment for for Parkinson disease: Parkinson Disease the Present and the Future the Present and the Future Olga Klepitskaya, MD Associate Professor of Neurology Co-Director,
Surgical Surgical treatment treatment for for Parkinson disease: Parkinson Disease the Present and the Future the Present and the Future Olga Klepitskaya, MD Associate Professor of Neurology Co-Director,
Voluntary Movement. Ch. 14: Supplemental Images
 Voluntary Movement Ch. 14: Supplemental Images Skeletal Motor Unit: The basics Upper motor neuron: Neurons that supply input to lower motor neurons. Lower motor neuron: neuron that innervates muscles,
Voluntary Movement Ch. 14: Supplemental Images Skeletal Motor Unit: The basics Upper motor neuron: Neurons that supply input to lower motor neurons. Lower motor neuron: neuron that innervates muscles,
Functional Distinctions
 Functional Distinctions FUNCTION COMPONENT DEFICITS Start Basal Ganglia Spontaneous Movements Move UMN/LMN Cerebral Cortex Brainstem, Spinal cord Roots/peripheral nerves Plan Cerebellum Ataxia Adjust Cerebellum
Functional Distinctions FUNCTION COMPONENT DEFICITS Start Basal Ganglia Spontaneous Movements Move UMN/LMN Cerebral Cortex Brainstem, Spinal cord Roots/peripheral nerves Plan Cerebellum Ataxia Adjust Cerebellum
Thalamus and Sensory Functions of Cerebral Cortex
 Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
The Nervous System: Sensory and Motor Tracts of the Spinal Cord
 15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
10/13/2017. Disclosures. Deep Brain Stimulation in the Treatment of Movement Disorders. Deep Brain Stimulation: Objectives.
 Deep Brain Stimulation in the Treatment of Movement Disorders Disclosures None Eleanor K Orehek, M.D. Movement Disorders Specialist Noran Neurological Clinic 1 2 Objectives To provide an overview of deep
Deep Brain Stimulation in the Treatment of Movement Disorders Disclosures None Eleanor K Orehek, M.D. Movement Disorders Specialist Noran Neurological Clinic 1 2 Objectives To provide an overview of deep
Exam 2 PSYC Fall (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
 Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Shaheen Shaikh, M.D. Assistant Professor of Anesthesiology, University of Massachusetts Medical center, Worcester, MA.
 Shaheen Shaikh, M.D. Assistant Professor of Anesthesiology, University of Massachusetts Medical center, Worcester, MA. Shobana Rajan, M.D. Associate staff Anesthesiologist, Cleveland Clinic, Cleveland,
Shaheen Shaikh, M.D. Assistant Professor of Anesthesiology, University of Massachusetts Medical center, Worcester, MA. Shobana Rajan, M.D. Associate staff Anesthesiologist, Cleveland Clinic, Cleveland,
Reformatted Imaging to Define the Intercommissural Line for CT -Guided Stereotaxic Functional Neurosurgery
 Reformatted Imaging to Define the Intercommissural Line for CT -Guided Stereotaxic Functional Neurosurgery 429 Richard E. Latchaw1.2 L. Dade Lunsford 1. 2 William H. Kennedi Functional stereotaxic neurosurgery
Reformatted Imaging to Define the Intercommissural Line for CT -Guided Stereotaxic Functional Neurosurgery 429 Richard E. Latchaw1.2 L. Dade Lunsford 1. 2 William H. Kennedi Functional stereotaxic neurosurgery
Announcement. Danny to schedule a time if you are interested.
 Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Gangli della Base: un network multifunzionale
 Gangli della Base: un network multifunzionale Prof. Giovanni Abbruzzese Centro per la Malattia di Parkinson e i Disordini del Movimento DiNOGMI, Università di Genova IRCCS AOU San Martino IST Basal Ganglia
Gangli della Base: un network multifunzionale Prof. Giovanni Abbruzzese Centro per la Malattia di Parkinson e i Disordini del Movimento DiNOGMI, Università di Genova IRCCS AOU San Martino IST Basal Ganglia
神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心.
 神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心 http://www.ym.edu.tw/~cflu OUTLINE Components and Pathways of the Basal Nuclei Functions and Related Disorders of the Basal Nuclei
神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心 http://www.ym.edu.tw/~cflu OUTLINE Components and Pathways of the Basal Nuclei Functions and Related Disorders of the Basal Nuclei
Surgical treatment for Parkinson's disease. Citation Hong Kong Practitioner, 1999, v. 21 n. 3, p
 Title Surgical treatment for Parkinson's disease Author(s) Leung, GKK; Hung, KN; Fan, YW Citation Hong Kong Practitioner, 1999, v. 21 n. 3, p. 106-115 Issued Date 1999 URL http://hdl.handle.net/10722/45396
Title Surgical treatment for Parkinson's disease Author(s) Leung, GKK; Hung, KN; Fan, YW Citation Hong Kong Practitioner, 1999, v. 21 n. 3, p. 106-115 Issued Date 1999 URL http://hdl.handle.net/10722/45396
P rimary generalised dystonia (PGD) is a movement disorder
 1314 PAPER Effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia S Tisch, L Zrinzo, P Limousin, K P Bhatia, N Quinn, K Ashkan, M Hariz...
1314 PAPER Effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia S Tisch, L Zrinzo, P Limousin, K P Bhatia, N Quinn, K Ashkan, M Hariz...
Basal ganglia motor circuit
 Parkinson s Disease Basal ganglia motor circuit 1 Direct pathway (gas pedal) 2 Indirect pathway (brake) To release or augment the tonic inhibition of GPi on thalamus Direct pathway There is a tonic inhibition
Parkinson s Disease Basal ganglia motor circuit 1 Direct pathway (gas pedal) 2 Indirect pathway (brake) To release or augment the tonic inhibition of GPi on thalamus Direct pathway There is a tonic inhibition
I T IS well known that aneurysms occur at
 The Lateral Perforating Branches of the Anterior and Middle Cerebral Arteries* HARRY A. KAPLAN, M.D. Division of Neurosurgery, Seton Hall College of Medicine, and Jersey City Medical Center, Jersey City,
The Lateral Perforating Branches of the Anterior and Middle Cerebral Arteries* HARRY A. KAPLAN, M.D. Division of Neurosurgery, Seton Hall College of Medicine, and Jersey City Medical Center, Jersey City,
Basal nuclei, cerebellum and movement
 Basal nuclei, cerebellum and movement MSTN121 - Neurophysiology Session 9 Department of Myotherapy Basal Nuclei (Ganglia) Basal Nuclei (Ganglia) Role: Predict the effects of various actions, then make
Basal nuclei, cerebellum and movement MSTN121 - Neurophysiology Session 9 Department of Myotherapy Basal Nuclei (Ganglia) Basal Nuclei (Ganglia) Role: Predict the effects of various actions, then make
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document.
 Deep brain stimulation for tremor resulting from acquired brain injury Sitsapesan, H A; Holland, P; Oliphant, Z; De Pennington, N; Brittain, John-Stuart; Jenkinson, Ned ; Joint, C; Aziz, T Z; Green, A
Deep brain stimulation for tremor resulting from acquired brain injury Sitsapesan, H A; Holland, P; Oliphant, Z; De Pennington, N; Brittain, John-Stuart; Jenkinson, Ned ; Joint, C; Aziz, T Z; Green, A
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
MODULE 6: CEREBELLUM AND BASAL GANGLIA
 MODULE 6: CEREBELLUM AND BASAL GANGLIA This module will summarize the important neuroanatomical and key clinical concepts from Chapters 15 and 16 of the textbook for the course. The first part of this
MODULE 6: CEREBELLUM AND BASAL GANGLIA This module will summarize the important neuroanatomical and key clinical concepts from Chapters 15 and 16 of the textbook for the course. The first part of this
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Introduction to the Central Nervous System: Internal Structure
 Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Over the last 20 years, Deep Brain Stimulation (DBS)
 Deep Brain Stimulation in Parkinson s Disease: Past, Present, and Future Though it has been underutilized over the past two decades, deep brain stimulation is an effective intervention for many individuals
Deep Brain Stimulation in Parkinson s Disease: Past, Present, and Future Though it has been underutilized over the past two decades, deep brain stimulation is an effective intervention for many individuals
HealthTalks. Deep Brain Stimulation for Parkinson s Disease Patients
 HealthTalks Parkinson s Disease and Wellness Ali Rezai, MD Neurological Institute Director, Center for Neurological Restoration Cleveland Clinic Appointments: 216.444.4720 Deep Brain Stimulation for Parkinson
HealthTalks Parkinson s Disease and Wellness Ali Rezai, MD Neurological Institute Director, Center for Neurological Restoration Cleveland Clinic Appointments: 216.444.4720 Deep Brain Stimulation for Parkinson
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy
 Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Epilepsy & Functional Neurosurgery
 Epilepsy & Functional Neurosurgery An Introduction The LSU-Shreveport Department of Neurosurgery Presenting Authors: Neurosurgery Residents & Faculty Epilepsy Neurosurgery What is a seizure? continuous
Epilepsy & Functional Neurosurgery An Introduction The LSU-Shreveport Department of Neurosurgery Presenting Authors: Neurosurgery Residents & Faculty Epilepsy Neurosurgery What is a seizure? continuous
History of Deep Brain Stimulation in la Pitié-Salpêtrière hospital
 History of Deep Brain Stimulation in la Pitié-Salpêtrière hospital Since the beginning of Deep Brain Stimulation (DBS) in the 80 s, DBS changed very much following evolution of technologies, but also following
History of Deep Brain Stimulation in la Pitié-Salpêtrière hospital Since the beginning of Deep Brain Stimulation (DBS) in the 80 s, DBS changed very much following evolution of technologies, but also following
CN V! touch! pain! Touch! P/T!
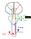 CN V! touch! pain! Touch! P/T! Visual Pathways! L! R! B! A! C! D! LT! E! F! RT! G! hypothalamospinal! and! ALS! Vestibular Pathways! 1. Posture/Balance!!falling! 2. Head Position! 3. Eye-Head Movements
CN V! touch! pain! Touch! P/T! Visual Pathways! L! R! B! A! C! D! LT! E! F! RT! G! hypothalamospinal! and! ALS! Vestibular Pathways! 1. Posture/Balance!!falling! 2. Head Position! 3. Eye-Head Movements
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Basal Nuclei (Ganglia)
 Doctor said he will not go deep within these slides because we will take them in physiology, so he will explain the anatomical structures, and he will go faster in the functions sheet in yellow Basal Nuclei
Doctor said he will not go deep within these slides because we will take them in physiology, so he will explain the anatomical structures, and he will go faster in the functions sheet in yellow Basal Nuclei
