Urgent Safety Notice. Recall. concerning the. sterile Trauma Plate Systems
|
|
|
- Shona Singleton
- 5 years ago
- Views:
Transcription
1 aap Implantate AG Lorenzweg 5 D Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.: Sender: Recipient: CAPA aap Implantate AG, Lorenzweg 5, Berlin, Germany User, Head of Orthopedic Surgery, Head of Orthopedics; Clinical Director, CEO, Sales Partner ldentification of medical devices affected: Medical device: Product description: Osteosynthesis, trauma implant Findannex A Product number: Lot code: Findannex A alllots Page 1 of7 aap Implantate AG I Lorenzweg SI ::l Bedin Germany Vorstand Bruke Seyoum Alemu Marek Hahn I Vorsitzender des Aufsichtsrats B ense Visser Tel Fax: AG Chorlattenburg HRB VAl DE Steuernummer 29/532/00534 Deutsche Bank AG Konto: 160 3:.>0 500 I Bll: I BAN: DE I BIC/SWIFT: DEUlDEBB Commerzbonk AG Konto: I Bll: IBAN: DE I BIC/SWIFT: CDBADEFF
2 Dear customer, we would like to inform you about particular circumstances relating to sterile trauma plates. Description of the problern including the identified cause: Background for the corrective action includinq the description of the product problern aap Implantate AG induces a recall of unused sterile packed trauma plate systems with product numbers as mentioned in annex A. All batches are involved. The concerned sterile trauma plates and hinges have been marketed with a sterile barrier system and an outer packaging. The sterile barrier system is realized by a combination of an inner and outer sealed peel pouch. Within the framewerk of the revalidation of transport and single device packaging aap lmplatate AG has discovered that with regard to the sterile barrier system of the concerning products a darnage or deterioration of the sterile packaging in unfavorable cases cannot be excluded. Consequently, the sterility of product can not Ionger be guaranteed. Implantation of unsterile products can Iead to infection. lnfections could unwantedly impair the healing progress and patient well-being, which is why the aap Implantate AG has decided to recall all sterile trauma plate systems. for patients, users and third parties in case of further usaqe of the product, includinq evaluation of risks high Sterility of outer and inner peel pouch is not impaired, because the marketed product is not exposed to the extreme constellation of the transport and packaging validation. Noshort-term health consequences (injury or illness), that result form the No long-term health consequences (injury or illness), that result form the The manufacture has received no complaints or objections of the market, that indicate a link to the described problem. Therefore the of occurrence of sterile barrier systemdarnage is classified as low. Page 2 of 7
3 Low Sterility of the outer peel pouch is impaired, but sterility within the inner peel pouch is still intact. Sterility of the product persists while the product is handled and introduced into the sterile area. No short-term health consequences (injury or illness), that result form the aag No long-term health consequences (injury or illness), that resu lt form the The damaged outer peel pouch can Iead to unsterility of the inner peel pouch. A contamination of the sterile area can occur du ring transfer from unsterile area into the operating area. On basis of an intact inner sterile barrier the implant remains sterile. Thus, t he risk of a patient's infection is assessed as low. A damaged outer sterile barrier can cause an impairment of the sterile area though, which in turn increases the infection risk of the patient. Very low Sterility of the outer and inner peel pouch is impaired. The sterility of the product can be compromised by the defective packaging. Due to impairment of the sterile barriers and the handling of the product du ring introduction into t he sterile area t he sterility of the product is impaired. Short-term health consequences can be wound infection, that require a treatment beyond the standards of care. Long-term health consequences can be infections, that Iead to a revision surgery, unless the infection can be fought alternatively. The of unsterility is classified as very low, because this kind of packaging has been used on the market for many years and as yet no relating incidents have occurred. Furthermore, it should be noted that surgeons administer antibiotics intra operative as weil as post operative in order to reduce the risk of infection. Page 3 of 7
4 {or patients, that were treated with concerninq products, includinq evaluation of risks high Sterility of outer and inner peel pouch is not impaired, because the marketed product is not exposed to the extreme constellation of the transport and packaging validation. Noshort-term health consequences (injury or illness), that result form the No long-term health consequences (injury or illness), that result form the The manufacture has received no complaints or objections of the market, that indicate a link to the described problem. Therefore the of occurrence of sterile barrier system darnage is classified as low. Low Sterility of the out er peel pouch is impa ired, but sterility within the inner peel pouch is still intact. Sterility of the product persists while the product is handled and introduced into the sterile area. Noshort-term health consequences (injury or illness), that result form the No long-term health consequences (injury or illness), that result form the The damaged outer peel pouch ca n Iead to unsterility of the inner peel pouch. A contamination of the sterile area can occur du ring transfer from unsterile area into the operating area. On basis of a sterile product provided by an intact inner sterile barrier, a low risk remains that the patient will be infected, nevertheless, by the contaminated sterile area. lnfections due to product or operation area emerge with high possibility within 3 month after implantation of the product. Very low Sterility of the outer and inner peel pouch is impaired. The sterility of the product can be compromised by the defective packaging. Due to impairment of the sterile barriers and the handling of the product du ring introduction into the sterile area the sterility of the product is impaired. Short-term health consequences ca n be wound infection, that require a treatment beyond the standards of care. Long-term health consequences can be infections, that Iead to a revision surgery, unless the infection can be fought alternatively. The of unsterility is classified as very low, because this kind of packaging has been used on the market for many years and as yet no relating incidents have occurred. lnfections due to product or operation area emerge with high possibility within 3 month after implantation of the product. Page 4 of 7
5 aag What actions does the recipient now need to implement? Pieasetake the following actions without delay: 1. Piease immediately remove all products (see Annex A) from your stock to ensure that they can not be used. 2. With this Ietter you will receive a confirmation form, please complete it completely, sign it and send it back to us after receiving this information. lf you do not have any affected products, please fill out the confirmation form and fa x it to 0049 (0) or mail it to incident@aap.de. 3. Piease return all affected products immediately to us. Recommendation for patients or treatment/aftercare of patients, which were treated with potentially concerned products The general risk of non-sterility of the concerned products is considered as very low. Reason s for this are given on the one hand by using a double sterile packaging, by which sterility is still ensured even at darnage of one foil, on the other hand based on the fact that no dient complaint has ever occurred despite years of usage of this packaging. ln the extremely unlikely event of unsterile im plant application this might Iead to an infection of patient which, consequently, makes an appropriate treatment necessa ry. Patients that were treated with the concerning products of the recall should therefore be checked in close-knit interval including the monitaring of relevant inflammation parameters, such as erythrocyte Sedimentation rate (ESR), C-reaktive protein (CRP) or leucocytes. lnfection that could be caused by unsterile implants would be at short-term visible in shape of an inflammation that ought to be immediately responded to. However, if no correlating sign emerges after 8-10 weeks clinic, the risk to patient with regards to an implant issue can be classified as very low. Forwarding the safety notice: 1. Piease ensure that all users of the specified products in your organization and all other applicable persons receive notification of this "Urgent Safety Notice". lfthe products have been tran sferred to third parties, please torward a copy of this safety notice or inform the contact person specified below. 2. Piease retain this information at least until all affected products have been returned to us. The national regulators have been informed of this action. The Federallnstitute for Drugs and Medical Devices has received a copy of this "Urgent Safty Notice". Contact: Should you have any queries, please do not hesitate to contact: aap Implantate AG Lorenzweg Berlin, Germany Denis Kühn Medical Device Safety Officer incident@aap.de Tel. +49 (0) Fax +49 (0) Yours truly, Director Quality Assurance & Regulatory Affairs Page 5 of 7
6 II Confirmation of recall of Sterile Trauma Plate Systems Pieasereturn this form by fax or mail to us immediately, even if you no Ionger have any stock of the listed product. D We confirm the receipt of this information. There is no stock of the product concerned. ln the column "Return quantity in pieces" this was noted with the quantity 0. D We confirm the receipt of this information. There is still stock of the product concerned, which will be collected from us. Piease enclose this form of confirmation of recall of the return. Product description Lot-number Quantity of aap supplied Return quantity in pieces all I confirm the complete examination of our stocks Clinic: Print Name: Telephone number: Signature/Date/Stamp Piease return this form to one of the following addresses: Fax number:_ Postal address: 030/ incident@aap.de aap Implantate AG attn: Return Department Lorenzweg Berlin Page 6 of 7
7 II Annex A to FSN sterile trauma plate systems Model Number PA PA PA PF PF PF PF PF PF PF PF PF PF PH PH PH PH PK PK PK PK PK PK PK PO PO PO K EN -Title LOQTEQ Dist. Anterolat. Tibia Plate 3.5, 14 holes, L 209, R Titanium, sterile LOQTEQ Dist. Anterolat. Tibia Plate 3.5, 14 holes, L 209, L Titanium, sterile LOQTEQ VA Hinge for periprosthetics 3.5, 2 pcs. Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 9 holes, L 243, R Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 11 holes, L 279, R Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 13 holes, L 314, R Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 15 holes, L 350, R Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 17 holes, L 386, R Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 9 holes, L 243, L Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 11 holes, L 279, L Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 13 holes, L 314, L Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 15 holes, L 350, L Titanium, sterile LOQTEQ Dist. Lateral Femur Plate PP, 17 holes, L 386, L Titanium, sterile LOQTEQ Prox. Humerus Plate 3.5, 14 holes, L 221 Titanium, sterile LOQTEQ Prox. Humerus Plate 3.5, 16 holes, L 247 Titanium, sterile LOQTEQ Distal Dorsolat. Humerus Plate, 11 holes, L 206, R Titanium, sterile LOQTEQ Distal Dorsolat. Humerus Plate, 11 holes, L 206, L Titanium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 6 holes, L 76, R Titanium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 8 holes, L 101, R Tit anium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 10 holes, L 121, R Titanium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 6 holes, L 76, L Titanium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 7 holes, L 88, L Titanium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 8 holes, L 101, L Titanium, sterile LOQTEQ Clavicle Shaft Plate 3.5, 10 holes, L 121, L Titanium, sterile LOQTEQ High Tibia Osteotomy Plate 4.5 Titanium, sterile LOQTEQ Distal Femur Osteotomy Plate 4.5, R Titanium, sterile LOQTEQ Distal Femur Osteotomy Plate 4.5, L Titanium, sterile LOQTEQ Cerclage button, large fragment, 2 pcs. Titanium, sterile Page 7 of 7
Elbow System Anatomy:
 Elbow System Elbow System Anatomy: Olecranon Fossa Medial Lateral Ulna Olecranon Radius 2 Elbow System AO-classification of distal humerus fractures 3 3 3 Elbow System Case example Transkondylar elbow
Elbow System Elbow System Anatomy: Olecranon Fossa Medial Lateral Ulna Olecranon Radius 2 Elbow System AO-classification of distal humerus fractures 3 3 3 Elbow System Case example Transkondylar elbow
Distal Lateral Femur Plate PP. Surgical Technique
 1 Disclaimer This surgical technique is exclusively intended for medical professionals, especially physicians, and therefore may not be regarded as a source of information for non-medical persons. The
1 Disclaimer This surgical technique is exclusively intended for medical professionals, especially physicians, and therefore may not be regarded as a source of information for non-medical persons. The
URGENT FIELD SAFETY NOTICE
 AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
International Trauma Course Berlin
 International Trauma Course Berlin Lectures & Cad Lab Upper and Lower Extremities August 16-18, 2018 Locking Compression Technology by aap Dear Course Participants, It is our great pleasure to welcome
International Trauma Course Berlin Lectures & Cad Lab Upper and Lower Extremities August 16-18, 2018 Locking Compression Technology by aap Dear Course Participants, It is our great pleasure to welcome
NovoPen Echo URGENT MEDICAL DEVICE RECALL
 NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
Locking Compression Technology by aap
 Minimally Invasive Locking Compression Technology by aap Minimally Invasive 1 Disclaimer This surgical technique is exclusively intended for medical professionals, especially physicians, and therefore
Minimally Invasive Locking Compression Technology by aap Minimally Invasive 1 Disclaimer This surgical technique is exclusively intended for medical professionals, especially physicians, and therefore
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Urgent Notice September 16, 2014
 Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
SECTION VIII: ANNEX A- FEMALE CONDOM INSPECTION, SAMPLING AND TESTING SPECIFICATIONS PREQUALIFICATION TESTING
 SECTION VIII: ANNEX A- FEMALE CONDOM INSPECTION, SAMPLING AND TESTING SPECIFICATIONS PREQUALIFICATION TESTING **Note: The WHO/UNFPA Specification is being reviewed for updates and this Annex is subject
SECTION VIII: ANNEX A- FEMALE CONDOM INSPECTION, SAMPLING AND TESTING SPECIFICATIONS PREQUALIFICATION TESTING **Note: The WHO/UNFPA Specification is being reviewed for updates and this Annex is subject
URGENT MEDICAL DEVICE CORRECTION. Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps
 URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system.
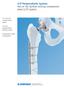 LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system. 4.5 mm LCP curved broad plates 5.0 mm periprosthetic locking screws Orthopaedic cable components LCP Periprosthetic
LCP Periprosthetic System. Part of the Synthes locking compression plate (LCP) system. 4.5 mm LCP curved broad plates 5.0 mm periprosthetic locking screws Orthopaedic cable components LCP Periprosthetic
Guidelines for Product Recall or Withdrawal
 REPUBLIC OF KENYA PHARMACY AND POISONS BOARD Guidelines for Product Recall or Withdrawal Edition 1 Date of release for publication June 2006 Date of implementation June 2006 This document has been prepared
REPUBLIC OF KENYA PHARMACY AND POISONS BOARD Guidelines for Product Recall or Withdrawal Edition 1 Date of release for publication June 2006 Date of implementation June 2006 This document has been prepared
Manometer Tray, Jamshidi Needle Biopsy, Illinois (TJ) Needle Aspiration, Jamshidi (TJ) Needle Bone Marrow, Thoracentesis/Paracentesis Kit
 URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
Elbow Plating System Surgical Technique
 Locking Compression Technology by aap 1 Disclaimer This surgical technique is exclusively intended for medical professionals, especially physicians, and therefore may not be regarded as a source of information
Locking Compression Technology by aap 1 Disclaimer This surgical technique is exclusively intended for medical professionals, especially physicians, and therefore may not be regarded as a source of information
PROXIMAL TIBIAL PLATE
 SURGICAL NÁSTROJE TECHNIQUE PRO ARTROSKOPII PROXIMAL INSTRUMENTS TIBIAL FOR PLATE ARTHROSCOPY Proximal Tibial Plate Description of medical device The Proximal Tibial Plate is used in epyphyseal and metaphyseal
SURGICAL NÁSTROJE TECHNIQUE PRO ARTROSKOPII PROXIMAL INSTRUMENTS TIBIAL FOR PLATE ARTHROSCOPY Proximal Tibial Plate Description of medical device The Proximal Tibial Plate is used in epyphyseal and metaphyseal
Hoffmann II Compact External Fixation System
 Trauma Hoffmann II Compact External Fixation System Modular System for Upper Extremity Foot Introduction In 1938, Raoul Hoffmann, a surgeon from Geneva, Switzerland, designed a revolutionary External Fixation
Trauma Hoffmann II Compact External Fixation System Modular System for Upper Extremity Foot Introduction In 1938, Raoul Hoffmann, a surgeon from Geneva, Switzerland, designed a revolutionary External Fixation
AST Standards of Practice for Monitoring Sterility
 AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been
AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been
INTRAMEDULLARY NAILING TIBIA AND FEMUR 1.3 TIBIAL AND FEMORAL NAILING FEMORAL NAILING WITH RETROGRADE INSERTION IMPLANTS AND OPERATING MANUAL
 1.3 TIBIA AND FEMORA NAIING FEMORA NAIING WITH RETROGRADE INSERTION IMPANTS AND OPERATING MANUA INTRAMEDUARY NAIING TIBIA AND FEMUR Medical Products Manufacturing and Trading td. General informations
1.3 TIBIA AND FEMORA NAIING FEMORA NAIING WITH RETROGRADE INSERTION IMPANTS AND OPERATING MANUA INTRAMEDUARY NAIING TIBIA AND FEMUR Medical Products Manufacturing and Trading td. General informations
Complex process High costs
 With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking 4 Control
With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking 4 Control
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
URGENT FIELD SAFETY NOTICE
 August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
Ordering information. LCP Locking Compression Plate. Combine without compromise.
 Ordering information LCP Locking Compression Plate. Combine without compromise. Table of Contents Small Fragment System Large Fragment System LCP Instruments Emergency Instruments Implants Humerus Plates,
Ordering information LCP Locking Compression Plate. Combine without compromise. Table of Contents Small Fragment System Large Fragment System LCP Instruments Emergency Instruments Implants Humerus Plates,
New York State Department of Health. Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine
 New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
LCP Distal Femur Plates. Shape based on Distal Femur LISS Plate design.
 LCP Distal Femur Plates. Shape based on Distal Femur LISS Plate design. Preshaped plate Round threaded holes in plate head Combi holes in plate shaft LCP Distal Femur Plates Plates The LCP Distal Femur
LCP Distal Femur Plates. Shape based on Distal Femur LISS Plate design. Preshaped plate Round threaded holes in plate head Combi holes in plate shaft LCP Distal Femur Plates Plates The LCP Distal Femur
Guidelines for the Recall or Withdrawal of a Medical Product
 National Drug Authority Rumee Towers Plot 19, Lumumba Avenue P. O. Box 23096 Kampala, Uganda. Tel: +256-0414 - 255665/347391/2 E-mail: ndaug@nda.or.ug Website: http://www.nda.or.ug TABLE OF CONTENTS 0.2
National Drug Authority Rumee Towers Plot 19, Lumumba Avenue P. O. Box 23096 Kampala, Uganda. Tel: +256-0414 - 255665/347391/2 E-mail: ndaug@nda.or.ug Website: http://www.nda.or.ug TABLE OF CONTENTS 0.2
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Part 1: General requirements
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
2.4 mm LCP Volar Column Distal Radius Plates. Part of the 2.4 mm LCP Distal Radius System.
 2.4 mm LCP Volar Column Distal Radius Plates. Part of the 2.4 mm LCP Distal Radius System. 12 anatomically shaped volar plates Multiple screw options for fixedangle support to articular surface Combi holes
2.4 mm LCP Volar Column Distal Radius Plates. Part of the 2.4 mm LCP Distal Radius System. 12 anatomically shaped volar plates Multiple screw options for fixedangle support to articular surface Combi holes
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Please see the attached drug alert for onward transmission as below.
 Chief Medical Officer Directorate Pharmacy and Medicines Division T: 0131-244 2528 E: irene.fazakerley@gov.scot IMMEDIATE MESSAGE TO: 1. Directors of Pharmacy 2. Medical Directors NHS Boards 18 April 2018
Chief Medical Officer Directorate Pharmacy and Medicines Division T: 0131-244 2528 E: irene.fazakerley@gov.scot IMMEDIATE MESSAGE TO: 1. Directors of Pharmacy 2. Medical Directors NHS Boards 18 April 2018
Complex process High costs
 MTP With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking
MTP With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER
 URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan
 URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Suspected Defective Product Report
 Defective Product Report Form European Medicines Agency Inspections Suspected Defective Product Report London, 9 February 2007 Doc. Ref. EMEA/INS/GMP/75464/2007 1. Origin of Report Shaded areas to be completed
Defective Product Report Form European Medicines Agency Inspections Suspected Defective Product Report London, 9 February 2007 Doc. Ref. EMEA/INS/GMP/75464/2007 1. Origin of Report Shaded areas to be completed
1 Delivery. 4 Control. 3 Unpacking. 2 Storage. 6 Cleaning. 8 Control. 9 Packaging of the kit. 11 Surgery. 16 Traceability. 18 Packaging of the kit
 Clavicle With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline High costs Complex process Stocks Control Cleaning Decontamination
Clavicle With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline High costs Complex process Stocks Control Cleaning Decontamination
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL
 URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
ISO 13485:2016 Medical Devices Training Handbook
 ISO 13485:2016 Medical Devices Training Handbook Presented By Maria Mylonas Page 1 of 16 pages How to use this handbook The handbook is organized to focus on particular skills and revisions. These lessons
ISO 13485:2016 Medical Devices Training Handbook Presented By Maria Mylonas Page 1 of 16 pages How to use this handbook The handbook is organized to focus on particular skills and revisions. These lessons
Urgent Medical Device Recall
 PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
Urgent Field Safety Notice
 Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
A locking plate system that expands a surgeon s options in trauma surgery. Zimmer NCB Plating System
 A locking plate system that expands a surgeon s options in trauma surgery Zimmer NCB Plating System The Power of Choice The power of having true intraoperative options is at your fingertips. Using standard
A locking plate system that expands a surgeon s options in trauma surgery Zimmer NCB Plating System The Power of Choice The power of having true intraoperative options is at your fingertips. Using standard
Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA Telephone: 510/
 Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Recall Guidelines. for Chinese Medicine Products
 Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
VBOSS TM System Overview. Vertebral Body Support System
 VBOSS TM System Overview Vertebral Body Support System Introduction The Vertebral Body Support System (VBOSS TM ) provides structural anterior column support using precisely fit titanium implants. Each
VBOSS TM System Overview Vertebral Body Support System Introduction The Vertebral Body Support System (VBOSS TM ) provides structural anterior column support using precisely fit titanium implants. Each
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Surgical Technique. Cannulated Angled Blade Plate 3.5 and 4.5, 90
 Surgical Technique Cannulated Angled Blade Plate 3.5 and 4.5, 90 Cannulated Angled Blade Plate 3.5 and 4.5, 90 Table of contents Indications/Contraindications 2 Implants 3 Surgical technique 5 Implant
Surgical Technique Cannulated Angled Blade Plate 3.5 and 4.5, 90 Cannulated Angled Blade Plate 3.5 and 4.5, 90 Table of contents Indications/Contraindications 2 Implants 3 Surgical technique 5 Implant
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
STIMULAN POWER TO TRANSFORM OUTCOMES
 STIMULAN POWER TO TRANSFORM OUTCOMES Perfect partner for your infection management strategy In contrast to today s growing economic and performance challenges, STIMULAN has been shown to transform outcomes
STIMULAN POWER TO TRANSFORM OUTCOMES Perfect partner for your infection management strategy In contrast to today s growing economic and performance challenges, STIMULAN has been shown to transform outcomes
AST Guidelines for Best Practices in Monitoring Sterility
 1 AST Guidelines for Best Practices in Monitoring Sterility Approved October 2008 Revised February 2015 Revised April 14, 2017 Introduction The following Guidelines for Best Practices were researched and
1 AST Guidelines for Best Practices in Monitoring Sterility Approved October 2008 Revised February 2015 Revised April 14, 2017 Introduction The following Guidelines for Best Practices were researched and
CABLE GRIP SYSTEM COMPREHENSIVE CABLE GRIP SYSTEM. Family Ties
 CABLE GRIP SYSTEM COMPREHENSIVE CABLE GRIP SYSTEM Family Ties Contoured for Optimal Fit with Proximal Femur The GTR cradles the bone anatomically while the strategically placed proximal and distal fins
CABLE GRIP SYSTEM COMPREHENSIVE CABLE GRIP SYSTEM Family Ties Contoured for Optimal Fit with Proximal Femur The GTR cradles the bone anatomically while the strategically placed proximal and distal fins
3.5 mm LCP Tibial Plateau Sets. Configuration options.
 3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
4766 Research Dr. San Antonio, TX insightdentalsystems.com
 OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
O.S.2-C & O.S.2-V. Static Staple - Stainless steel
 O.S.2-C & O.S.2-V Compression Staple PEEK Static Staple - Stainless steel 2 O.S.2 - TABLE OF CONTENTS O.S.2-C 3 Product description 4 Indications 8 Surgical technique 9 O.S.2-V 13 Product description 14
O.S.2-C & O.S.2-V Compression Staple PEEK Static Staple - Stainless steel 2 O.S.2 - TABLE OF CONTENTS O.S.2-C 3 Product description 4 Indications 8 Surgical technique 9 O.S.2-V 13 Product description 14
SP MEDIKAL NEWS. of the Helix device as batch monitoring device but also the big advantages when compared to detection possibilities of CI and BI s.
 SP MEDIKAL NEWS Third newsletter by SP Medikal San. Ltd In this Edition: After the wintertime the spring and summertime is welcomed by many people. These seasons are giving energy and motivation. After
SP MEDIKAL NEWS Third newsletter by SP Medikal San. Ltd In this Edition: After the wintertime the spring and summertime is welcomed by many people. These seasons are giving energy and motivation. After
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Please find enclosed Dosimetry Service Licence No which replaces Dosimetry Service Licence No
 Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL
 January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
URGENT MEDICINE RECALL
 Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Compression Screw 3.2 Bioabsorbable. Product information
 Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
Compression Screw 3.2 Bioabsorbable Product information Caution The current product description is not sufficient for the immediate application of instruments and implants. Instructional training by an
with Ø Ø 1.5 mm Titanium Screws distributed by ARCS Alveolar Reconstruction System E
 Alveolar Reconstruction System with Ø 1.2 + Ø 1.5 mm Titanium Screws distributed by ARCS Alveolar Reconstruction System with Ø 1 mm Steel Screws 14.03.2017-E 14.03.2017-E 2 +49 (0) 74 64 / 98 88 0 A strong
Alveolar Reconstruction System with Ø 1.2 + Ø 1.5 mm Titanium Screws distributed by ARCS Alveolar Reconstruction System with Ø 1 mm Steel Screws 14.03.2017-E 14.03.2017-E 2 +49 (0) 74 64 / 98 88 0 A strong
CRANIAL RECONSTRUCTION SOLUTIONS
 CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV REV.8 Guideline Vigilance)
 FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV 2.12-1 REV.8 Guideline Vigilance) In Vitro Diagnostics Medical Devices : Type: Instruments for professional use. Attention :
FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV 2.12-1 REV.8 Guideline Vigilance) In Vitro Diagnostics Medical Devices : Type: Instruments for professional use. Attention :
Health Authority Abu Dhabi. Document Title: Policy for Recall of Drugs and Healthcare Products Document Ref. Number:
 Health Authority Abu Dhabi Document Title: Policy for Recall of Drugs and Healthcare Products Document Ref. Number: PPR/DMP/DR/P0001 Version 0.9 Approval Date: August 2007 Effective Date: August 2007 Last
Health Authority Abu Dhabi Document Title: Policy for Recall of Drugs and Healthcare Products Document Ref. Number: PPR/DMP/DR/P0001 Version 0.9 Approval Date: August 2007 Effective Date: August 2007 Last
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Cannulated Angled Blade Plate 3.5 and 4.5, 90.
 Cannulated Angled Blade Plate 3.5 and 4.5, 90. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Table of Contents Introduction
Cannulated Angled Blade Plate 3.5 and 4.5, 90. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Table of Contents Introduction
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
KASM Knee Articulating Spacer Mold. Surgical Summary
 KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
Electrical Markets. 3M TM Heat Shrink Tubing Promotion for Construction & Industrial. Heat Shrink Tubing USA. Made in the
 Electrical Markets 3M TM Heat Shrink Tubing Promotion for Construction & Industrial Heat Shrink Tubing USA Made in the With convenient, clearly-marked Made in USA packaging, 3M Heat Shrink Tubing will
Electrical Markets 3M TM Heat Shrink Tubing Promotion for Construction & Industrial Heat Shrink Tubing USA Made in the With convenient, clearly-marked Made in USA packaging, 3M Heat Shrink Tubing will
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation. Surgical Technique
 JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
Gentle Guided Growth to Correct Knock Knees and Bowed Legs in Children
 PATIENT INFORMATION Gentle Guided Growth to Correct Knock Knees and Bowed Legs in Children The Guided Growth System eight-plate quad-plate INTRODUCTION Children need gentle guidance and correction in many
PATIENT INFORMATION Gentle Guided Growth to Correct Knock Knees and Bowed Legs in Children The Guided Growth System eight-plate quad-plate INTRODUCTION Children need gentle guidance and correction in many
No two knees are alike
 No two knees are alike That s why we custom-fit your surgery just for you Custom-Fit Surgery: The right fit for nearly every knee It s not surprising. Research proves that differences in bone shape influence
No two knees are alike That s why we custom-fit your surgery just for you Custom-Fit Surgery: The right fit for nearly every knee It s not surprising. Research proves that differences in bone shape influence
Gamma3 TM Fragment Control Clip
 Osteosynthesis Gamma3 TM Fragment Control Clip Operative Technique Hip Fracture Introduction Indication The Fragment Control Clip is designed to stabilize rotationally unstable femoral head-neck fragments
Osteosynthesis Gamma3 TM Fragment Control Clip Operative Technique Hip Fracture Introduction Indication The Fragment Control Clip is designed to stabilize rotationally unstable femoral head-neck fragments
Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems
 Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems Designed to allow customized system configurations Variable Angle (VA) and Locking Compression Plate (LCP ) options
Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems Designed to allow customized system configurations Variable Angle (VA) and Locking Compression Plate (LCP ) options
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
Interlocking Nailing Course for Surgeons
 Credit Points Program Interlocking Nailing Course for Surgeons 6-7 February 2017 Berlin Germany Preface Dear Colleagues, Intramedullary nailing has gained widespread acceptance among orthopaedic trauma
Credit Points Program Interlocking Nailing Course for Surgeons 6-7 February 2017 Berlin Germany Preface Dear Colleagues, Intramedullary nailing has gained widespread acceptance among orthopaedic trauma
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Complex process High costs
 MTP With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking
MTP With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking
Technique Guide. 2.4 mm Variable Angle LCP Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology.
 Technique Guide 2.4 mm Variable Angle LCP Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Table of Contents Introduction 2.4 mm Variable Angle LCP
Technique Guide 2.4 mm Variable Angle LCP Distal Radius System. For fragment-specific fracture fixation with variable angle locking technology. Table of Contents Introduction 2.4 mm Variable Angle LCP
Subject: Temporary importation of Hartmann s (Compound Sodium Lactate) 1L from Spain to address manufacturing disruption in UK
 5 th September 2018 Subject: Temporary importation of Hartmann s (Compound Sodium Lactate) 1L from Spain to address manufacturing disruption in UK Dear Customer, In order to address shortages of critical
5 th September 2018 Subject: Temporary importation of Hartmann s (Compound Sodium Lactate) 1L from Spain to address manufacturing disruption in UK Dear Customer, In order to address shortages of critical
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 14708-7 First edition 2013-01-15 Implants for surgery Active implantable medical devices Part 7: Particular requirements for cochlear implant systems Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD ISO 14708-7 First edition 2013-01-15 Implants for surgery Active implantable medical devices Part 7: Particular requirements for cochlear implant systems Implants chirurgicaux Dispositifs
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS
 RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193. ETEST Ceftriaxone TXL32 (Ref , ) FOAM packaging
 16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
Cerasorb M DENTAL. O:\Zulassung\Cerasorb Dental Kanada 2013\Texte\Cerasorb M Dental final IFU docx
 Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Corex HOSPITAL OVERVIEW AND TECHNICAL ASSESSMENT TECHNOLOGY OVERVIEW NEW TECHNOLOGY REFERENCE GUIDE. Minimally Invasive Bone Harvester
 NEW TECHNOLOGY REFERENCE GUIDE Corex Minimally Invasive Bone Harvester HOSPITAL OVERVIEW AND TECHNICAL ASSESSMENT The Corex Bone Harvester is a supplied sterile, single patient use, manually operated trephine
NEW TECHNOLOGY REFERENCE GUIDE Corex Minimally Invasive Bone Harvester HOSPITAL OVERVIEW AND TECHNICAL ASSESSMENT The Corex Bone Harvester is a supplied sterile, single patient use, manually operated trephine
Integra. DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE
 Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
DECLARATION OF CONFORMITY To Council Directive 93/42/EEC of 14 June 1993
 DECLARATION OF CONFORMITY To Council Directive 93/42/EEC of 14 June 1993 Manufacturer: EC Authorized Representative: T.A.G. Medical Products Corporation LTD. Kibbutz Gaaton 2513000 Israel Tel: (972) 4
DECLARATION OF CONFORMITY To Council Directive 93/42/EEC of 14 June 1993 Manufacturer: EC Authorized Representative: T.A.G. Medical Products Corporation LTD. Kibbutz Gaaton 2513000 Israel Tel: (972) 4
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use.
 Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Medium External Fixator Humeral Shaft Frame. Modular frame for upper extremity use. Technique Guide Part of the Medium External Fixation System MRI Information Synthes Medium External Fixation devices
Arcos Modular Femoral Revision System
 Arcos Modular Femoral Revision System Arcos System Simplify the Complex The Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the
Arcos Modular Femoral Revision System Arcos System Simplify the Complex The Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the
The Colorado Bureau of Investigation s (CBI) Blood Alcohol Analyses Summary of Issues
 The Colorado Bureau of Investigation s (CBI) Blood Alcohol Analyses Summary of Issues On December 7, 2015, while the CBI was conducting standard quality checks, an unexplained variability of results in
The Colorado Bureau of Investigation s (CBI) Blood Alcohol Analyses Summary of Issues On December 7, 2015, while the CBI was conducting standard quality checks, an unexplained variability of results in
Dear Friends, This is your opportunity to really change someone s life for the better!
 Dear Friends, It is our pleasure to invite you to participate as a Sponsor in the Second Annual which will take place on April 19, 2018, at Moore College of Art. This unique event will showcase the teamwork
Dear Friends, It is our pleasure to invite you to participate as a Sponsor in the Second Annual which will take place on April 19, 2018, at Moore College of Art. This unique event will showcase the teamwork
