Policy Specific Section: October 7, 2011 January 30, 2015
|
|
|
- Beverley Richard
- 6 years ago
- Views:
Transcription
1 Medical Policy Bio-Engineered Skin and Soft Tissue Substitutes Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: October 7, 2011 January 30, 2015 Definitions of Decision Determinations Medically Necessary: A treatment, procedure or drug is medically necessary only when it has been established as safe and effective for the particular symptoms or diagnosis, is not investigational or experimental, is not being provided primarily for the convenience of the patient or the provider, and is provided at the most appropriate level to treat the condition. Investigational/Experimental: A treatment, procedure or drug is investigational when it has not been recognized as safe and effective for use in treating the particular condition in accordance with generally accepted professional medical standards. This includes services where approval by the federal or state governmental is required prior to use, but has not yet been granted. Split Evaluation: Blue Shield of California / Blue Shield of California Life & Health Insurance Company (Blue Shield) policy review can result in a Split Evaluation, where a treatment, procedure or drug will be considered to be investigational for certain indications or conditions, but will be deemed safe and effective for other indications or conditions, and therefore potentially medically necessary in those instances. Description Bio-engineered skin and soft tissue substitutes may be derived from human tissue (autologous or allogeneic), non-human tissue (xenographic), synthetic materials, or a composite of these materials. Bio-engineered skin and soft tissue substitutes are being evaluated for a variety of conditions, including breast reconstruction and to aid healing of lower extremity ulcers and
2 severe burns. Acellular dermal matrix products are also being evaluated in the repair of a variety of soft tissues. Policy The following bio-engineered skin or soft tissue substitutes may be considered medically necessary when one of the following exists: Breast reconstructive surgery, post-mastectomy using allogeneic acellular dermal matrix (ADM) products* (i.e., AlloDerm, AlloMax, DermaMatrix, FlexHD, GraftJacket ) Chronic, non-infected, full-thickness lower extremity ulcers due to diabetes: ο Apligraf ** ο Dermagraft ** Chronic, non-infected, partial- or full-thickness lower extremity skin ulcers due to venous insufficiency which has not adequately responded following a one month period of conventional ulcer therapy: ο Apligraf ** ο Oasis Wound Matrix*** Dystrophic epidermolysis bullosa: ο OrCel (for the treatment of mitten-hand deformity when standard wound therapy has failed and when provided in accordance with the Humanitarian Device Exemption [HDE] specifications of the United States Food and Drug Administration [FDA])**** Second- and third-degree burns: ο Epicel (for the treatment of deep dermal or full-thickness burns comprising a total body surface area of greater than or equal to 30% when provided in accordance with the HDE specifications of the FDA)**** ο TransCyte ** ο Integra Dermal Regeneration Template ** * Banked human tissue. ** FDA PMA approved. *** FDA 510(k) cleared. **** FDA-approved under a humanitarian device exemption (HDE). All other applications of the above listed bio-engineered skin and soft tissue substitutes are considered investigational. All other bio-engineered skin and soft tissue substitutes not listed above are considered investigational including, but not limited to, the following: ACell UBM Hydated Wound Dressing ACell UBM Lyophilized Wound Dressing 2 of 40
3 AlloPatch HD AlloSkin AlloSkin RT Amniofix Aongen Collagen Matrix ArthroFlex (FlexGraft) Atlas Wound Matrix Avagen Wound Dressing Avaulta Plus Biobrane BioDfence/BioDfactor CellerateRX Collagen Sponge (Innocoll) Collagen Wound Dressing (Oasis Research) Collaguard CollaSorb CollaWound Collexa Collieva Conexa Coreleader Colla-Pad CorMatrix CRXa Cymetra Dermadapt Wound Dressing DressSkin Durepair Regeneration Matrix Endoform Dermal Template ENDURAgen Epifix Excellagen E-Z Derm FortaDerm Wound Dressing GammaGraft Grafix CORE Grafix PRIME GraftJacket Regenerative Tissue Matrix GraftJacket Xpress, injectable HA Absorbent Wound Dressing Helicoll Hyalomatrix (Laserskin ) Hyalomatrix PA 3 of 40
4 hmatrix Integra Flowable Wound Matrix Integra Bilayer Wound Matrix Integra Matrix Wound Dressing Jaloskin MatriDerm MatriStem Burn Matrix MatriStem Micromatrix MatriStem Wound Matrix Matrix Collagen Wound Dressing Matrix HD MediHoney Mediskin MemoDerm Oasis Burn Matrix Oasis Ultra Tri-Layer Matrix Permacol PriMatrix Primatrix Dermal Repair Scaffold Puros Dermis Repliform Repriza SIS Wound Dressing II SS Matrix Stimulen Collagen StrataGraft Strattice (xenograft) Suprathel SurgiMend Talymed TenoGlide TheraForm Standard/Sheet TheraSkin Unite Unite Biomatrix Veritas Collagen Matrix Policy Guideline CPT codes to are reported for the application of skin replacements and skin substitutes. CPT codes to are specific to autografts and tissue-cultured autografts. 4 of 40
5 CPT codes to are specific to skin substitute grafts. The add-on CPT code (implantation of biologic implant (e.g., acellular dermal matrix) for soft tissue reinforcement (i.e., breast, trunk) is specific for the use of these materials as an implant (list separately in addition to code for the primary procedure). The HCPCS codes for these products that are used in outpatient and office settings are listed in the Coding Section of the policy. There are also HCPCS modifiers to indicate whether the skin substitute is or is not used as a graft (i.e., surface use versus use as an implant): JC: Skin substitute used as a graft JD: Skin substitute not used as a graft Internal Information There is an MD Determination Form for this Medical Policy. It can be found on the following Web page: Documentation Required for Clinical Review History and physical including: ο Previous treatment plan and response ο Specific diagnosis requiring skin or soft tissue substitute Progress notes for the past six months Exact brand name of skin or soft tissue substitute to be used Post Service Procedure report(s) Skin or soft tissue substitute invoice (if applicable) The materials provided to you are guidelines used by this plan to authorize, modify, or deny care for persons with similar illness or conditions. Specific care and treatment may vary depending on individual need and the benefits covered under your contract. These Policies are subject to change as new information becomes available. 5 of 40
6 APPENDIX to Bio-Engineered Skin and Soft Tissue Substitutes Policy Prior Authorization Requirements This service (or procedure) is considered medically necessary in certain instances and investigational in others (refer to policy for details). For instances when the indication is medically necessary, clinical evidence is required to determine medical necessity. For instances when the indication is investigational, you may submit additional information to the Prior Authorization Department. Within five days before the actual date of service, the Provider MUST confirm with Blue Shield of California / Blue Shield of California Life & Health Insurance Company (Blue Shield) that the member's health plan coverage is still in effect. Blue Shield reserves the right to revoke an authorization prior to services being rendered based on cancellation of the member's eligibility. Final determination of benefits will be made after review of the claim for limitations or exclusions. Questions regarding the applicability of this policy should also be directed to the Prior Authorization Department. Please call or visit the Provider Portal Evidence Basis for the Policy Rationale Background Bio-engineered skin and soft tissue substitutes may be either acellular or cellular. Acellular products (i.e., cadaveric human dermis with cellular material removed) contain a matrix or scaffold composed of materials such as collagen, hyaluronic acid, and fibronectin. Cellular products contain living cells such as fibroblasts and keratinocytes within a matrix. The cells contained within the matrix may be autologous, allogeneic, or derived from other species (e.g., bovine, porcine). Skin substitutes may also be composed of dermal cells, epidermal cells, or a combination of dermal and epidermal cells, and may provide growth factors to stimulate healing. Tissue-engineered skin substitutes can be used as either temporary or permanent wound coverings. There are a large number of potential applications for artificial skin and soft tissue products. One large category is non-healing wounds, which potentially encompasses diabetic neuropathic ulcers, vascular insufficiency ulcers, and pressure ulcers. A substantial minority of such wounds do not heal adequately with standard wound care, leading to prolonged morbidity and increased 6 of 40
7 risk of mortality. Non-healing lower extremity wounds represent an ongoing risk for infection, sepsis, limb amputation and death. Bio-engineered skin and soft tissue substitutes have the potential to improve rates of healing and reduce secondary complications. Other situations in which bio-engineered skin products might substitute for living skin grafts include certain postsurgical states such as breast reconstruction, in which skin coverage is inadequate for the procedure performed, or for surgical wounds in patients with compromised ability to heal. Second- and third-degree burns are another situation where artificial skin products may substitute for autografts or allografts. Certain primary dermatologic conditions that involve large areas of skin breakdown, such as bullous diseases, may also be conditions in which artificial skin products can be considered as substitutes for skin grafts. Acellular dermal matrix (ADM) products are also being evaluated in the repair of other soft tissues including rotator cuff repair, following oral and facial surgery, hernias, and a variety of other conditions. Regulatory Status There are a large number of artificial skin products that are commercially available or in development. The following summary of commercially available skin substitutes describes those products that have substantial relevant evidence on efficacy. Information on other artificial skin and soft tissue substitutes that are available in the U.S. may be found in a 2012 Technology Assessment from the Agency for Healthcare Research and Quality (Synder et al., 2012). Acellular Dermal Matrix Allograft ADM products derived from donated human skin tissue are supplied by U.S. AATBcompliant tissue banks using the standards of the American Association of Tissue Banks (AATB) and U.S. Food and Drug Administration's (FDA) guidelines. The processing removes the cellular components (i.e., epidermis and all viable dermal cells) that can lead to rejection and infection. Acellular dermal matrix products from human skin tissue are regarded as minimally processed and not significantly changed in structure from the natural material; the FDA classifies it as banked human tissue and therefore does not require FDA approval. AlloDerm (LifeCell Corporation, Branchburg, NJ) is an ADM (allograft) tissuereplacement product that is created from native human skin and processed so that the basement membrane and cellular matrix remain intact. An injectable micronized form of AlloDerm (Cymetra) is also available. AlloMax Surgical Graft (Bard Davol Inc., Murray Hill, NJ) is an acellular non-crosslinked human dermis allograft. It is classified as banked human tissue and does not require FDA approval (AlloMax was previously marketed as NeoForm ). DermaMatrix (Synthes) is an ADM (allograft) derived from donated human skin tissue. DermaMatrix Acellular Dermis is processed by the Musculoskeletal Transplant Foundation (MTF ). GraftJacket Regenerative Tissue Matrix (Kinetic Concepts Inc., (KCI), San Antonio, TX) is an acellular regenerative tissue matrix processed from screened donated human skin supplied from U.S. tissue banks. The allograft is processed minimally to remove the epidermal and dermal cells while preserving dermal structure. 7 of 40
8 PriMatrix (TEI Biosciences Inc., Boston, MA) is a xenogeneic ADM processed from fetal bovine dermis. It is indicated through the U.S. Food and Drug Administration's (FDA) 510(k) process for partial and full-thickness wounds; diabetic, pressure, and venous stasis ulcers; surgical wounds; and tunneling, draining, and traumatic wounds. Amniotic Membrane Amniotic membrane is harvested immediately after birth, cleaned, sterilized, and either fresh frozen or dehydrated. Human amniotic membrane is considered to be minimally processed and not significantly changed in structure from the natural material; FDA classifies it as banked human tissue and therefore, it does not require FDA approval. EpiFix and Amniofix (both from MiMedix) are commercially available sources of dehydrated human amniotic membrane. EpiFix is provided in sheets and Amniofix is an injectable form of micronized amniotic membrane. Other amniotic membrane products are AmnioClear (Musculoskeletal Transplant Foundation ), AmnioGraft (Bio-Tissue), and BioDfense and BioDDryFlex (both from BioD). Collagen Scaffold OASIS Wound Matrix (Cook Biotech Inc., West Lafayette, IN) is a xenographic collagen scaffold derived from porcine small intestinal mucosa. It was cleared by the FDA's 510(k) process in 2000 for the management of partial and full-thickness wounds including pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled undermined wounds, surgical wounds, trauma wounds, and draining wounds. Living Cell Therapy Apligraf (Organogenesis Inc., Canton, MA) is a bilayered cell therapy composed of an epidermal layer of living human keratinocytes and a dermal layer of living human fibroblasts. Apligraf is supplied as needed, in 1 size, with a shelf-life of 10 days. It was FDA approved in 1998 for use in conjunction with compression therapy for the treatment of non-infected, partial and full-thickness skin ulcers due to venous insufficiency and in 2001 for full-thickness neuropathic diabetic lower extremity ulcers non-responsive to standard wound therapy. Dermagraft (formerly Advanced Biohealing Inc., Westport, CT, no Organogenesis) is composed of cryopreserved human-derived fibroblasts and collagen derived from newborn human foreskin and cultured on a bioabsorbable mesh. Dermagraft has been approved by the FDA for repair of diabetic foot ulcers. Epicel (Genzyme Biosurgery, Cambridge, MA) is a cultured epithelial autograft and is FDA approved under a humanitarian device exemption (HDE) for the treatment of deep dermal or full-thickness burns comprising a total body surface area of greater than or equal to 30%. It may be used in conjunction with split-thickness autografts or alone in patients for whom split-thickness autografts may not be an option due to the severity and extent of their burns. OrCel (Forticell Bioscience Inc., New York, NY) (formerly called Composite Cultured Skin) is an absorbable allogeneic bilayered cellular matrix, made of bovine collagen, in which human dermal cells have been cultured. It was approved by FDA premarket 8 of 40
9 Biosynthetic approval (PMA) for healing donor site wounds in burn victims and under a humanitarian device exemption (HDE) for use in patients with recessive dystrophic epidermolysis bullosa undergoing hand reconstruction surgery to close and heal wounds created by the surgery, including those at donor sites. Biobrane /Biobrane-L (Smith & Nephew Inc., Memphis TN) is a biosynthetic wound dressing constructed of a silicon film with a nylon fabric partially imbedded into the film. The fabric creates a complex three-dimensional structure of trifilament thread which chemically binds collagen. A blood/sera clot in the nylon matrix, adheres the dressing to the wound until epithelialization occurs. Integra Dermal Regeneration Template (Integra LifeSciences, Plainsboro, NJ) is a bovine, collagen/glycosaminoglycan dermal replacement covered by a silicone temporary epidermal substitute. It is FDA approved for use in postexcisional treatment of lifethreatening full-thickness or deep partial-thickness thermal injury where sufficient autograft is not available at the time of excision or not desirable because of the physiological condition of the patient. Integra Matrix Wound Dressing and Integra meshed Bilayer Wound Matrix are substantially equivalent skin substitutes that are FDA 510(k) approved for other indications. TransCyte (Advanced BioHealing Inc., Westport, CT) consists of human dermal fibroblasts grown on nylon mesh, combined with a synthetic epidermal layer and was approved by the FDA in TransCyte is intended to be used as a temporary covering over burns until autografting is possible. It can also be used as a temporary covering for some burn wounds that heal without autografting. Literature Review The following is a summary of the key literature to date. Breast Reconstruction AlloDerm Systematic Reviews: Two systematic reviews from 2012 found an increased rate of complications with ADM-assisted breast reconstruction (Ho et al., 2012; Kim et al., 2012). One meta-analysis of 16 retrospective studies found a higher likelihood of seroma (pooled odds ratio [OR]: 3.9; 95% CI: 2.4 to 6.2), infection (pooled OR: 2.7; 95% CI: 1.1 to 6.4) and reconstructive failure (pooled OR: 3.0; 95% CI: 1.3 to 6.8) when compared to breast reconstruction using traditional musculofascial flaps (Ho et al., 2012). Another meta-analysis that compared 19 studies using ADM (n = 2,037) with 35 studies using submuscular reconstruction (n = 12,847) found an increased risk of total complications (relative risk [RR]: 2.05; 95% CI: 1.55 to 2.70), seroma (RR: 2.73; 95% CI: 1.67 to 4.46), infection (RR: 2.47; 95% CI: 1.71 to 3.57), and reconstructive failure (RR: 2.80; 95% CI: ) with ADM (Kim et al., 2012). These metaanalyses are limited by the poor quality of included studies and significant heterogeneity. Randomized Controlled Trials (RCTs): In 2012, McCarthy et al. reported a multicenter blinded randomized controlled trial of AlloDerm in two-stage expander/implant reconstruction. Seventy patients were randomized to Alloderm ADM-assisted tissue expander/implant reconstruction or 9 of 40
10 to submuscular tissue expander/implant placement. There were no significant differences between the groups in the primary outcomes of immediate postoperative pain (54.6 AlloDerm and 42.8 control on a 100-point visual analog score) or pain during the expansion phase (17.0 Alloderm and 4.6 control), or in the secondary outcome of rate of tissue expansion (91 days AlloDerm and 108 days control) and patient-reported physical well-being. There was no significant difference in adverse events, although the total number of adverse events was small. Phase 2 of the study will evaluate long-term outcomes. Controlled Studies: Preminger and colleagues (2008) evaluated the impact of Alloderm on expansion rates in immediate tissue expander/implant reconstruction in a retrospective matched cohort study. Forty-five patients underwent reconstruction with Alloderm while 45 received standard reconstruction. Subjects were matched for expander size (+/-100 milliliter (ml), history of irradiation, and indication for mastectomy. There were no significant differences in initial filling volume, mean number of postoperative expansions, mean rate of postoperative tissue expansion, or in the incidence of postoperative complications. Aesthetic outcomes were not addressed. In 2008, Colwell and Breuing reported on 10 patients who had mastopexy with dermal slings, five patients were given cadaveric (Alloderm ) and five were given autologous tissue. Patients maintained projection and breast base width after 6 months to 3 years. Uncontrolled Studies: A number of case series have also demonstrated that this approach can provide tissue coverage of implants and tissue expanders (Salzberg, 2006; Breuing & Colwell, 2007). AlloDerm has been reported in nipple reconstructive surgery in a case series on 30 nipple reconstructive procedures performed at 1 institution (Garramone & Lam, 2007). Use of AlloDerm has also been reported in a small series (n=3) to correct breast implant-related problems (malposition, symmastia, and rippling) (Baxter, 2003). Other: Liu et al. (2011) reported postoperative complications in breast reconstruction with (n = 266) or without (n = 204) AlloDerm. Radiation therapy, body mass index (BMI), intraoperative use of tumescent solution, and medical comorbidities were similar between the two groups, but there were twice as many smokers and the implants were larger in the AlloDerm group. There was a trend for a higher rate of major infections that required prosthesis removal in the AlloDerm group (4.9% vs. 2.5%, p = 0.172) and a statistically significant increase in overall wound infection rate (6.8% versus 2.5%). The overall surgical complication rate was significantly higher in the AlloDerm group (19.5% vs. 12.3%). Multivariate analysis indicated that the use of ADM, smoking, higher BMI, higher initial volume, and bigger implant size were associated with a higher overall surgical complication rate. This study was limited by the retrospective analysis and differences between groups at baseline. Bindingnavele et al., (2007) reviewed charts of 41 patients (65 breasts) who had staged breast reconstruction with acellular cadaveric dermis to report postoperative complication rates. Rates for wound infection, expander removal, hematoma, and seroma were 3.1%, 1.5%, 1.5%, and 4.6%, respectively. The authors concluded that based on low rates of complications and good cosmetic outcomes, the technology should be in the repertoire of plastic surgeons, and follow-up was required to evaluate long-term outcomes. 10 of 40
11 AlloDerm versus DermaMatrix or FlexHD A 2013 retrospective review by Liu et al. compared complication rates following breast reconstruction with AlloDerm or FlexHD in 382 consecutive women (547 breasts). Eighty-one percent of the sample were immediate reconstructions; 165 used AlloDerm, and 97 used FlexHD. Mean follow-up was 6.4 months. Compared with breast reconstruction without use of AlloDerm or FlexHD, ADM had a higher rate of delayed healing (20.2% vs 10.3%), although this finding might be related to differences in fill volumes. In univariate analysis, there were no significant differences in complications (return to the operating room, surgical site infection, seroma, hematoma, delayed healing, or implant loss) between AlloDerm and FlexHD. In multivariate analysis, there were no significant differences between AlloDerm or FlexHD for the return to the operating room, surgical site infection, seroma, or delayed healing. Independent risk factors for implant loss included the use of FlexHD, single-stage reconstruction, and smoking. Another retrospective review from 2013 compared complication rates following use of AlloDerm (n=136) or FlexHD (n=233) in a consecutive series of 255 patients (369 breasts) (Seth et al., 2013). Total complication rates for the two products were similar (19.1% for AlloDerm and 19.3% for FlexHD). Analysis by type of complication showed no significant difference between the two, and regression analysis controlling for differences in baseline measures found that the type of ADM was not a risk factor for any complication. Brooke et al. (2012) conducted a retrospective review of complication rates when AllDerm (n=49), DermaMatrix (n=110), or FlexHD (n=62) was used for tissue expander breast reconstruction. (15) Clinically significant complications were defined as cellulitis, abscess, seroma, expander leak or puncture, skin necrosis, wound dehiscence, or hematoma. The total clinically significant complication rate was 22% with AlloDerm, 15% with DermaMatrix, and 16% with FlexHD (not significantly different). Infectious complication rates for the 3 products were the same at 10%. When compared with breast reconstruction without an ADM (n=64), there was no significant difference in the total complication rate (17% vs 11%), but there was a trend toward a higher incidence of infectious complications (10% vs 2%, p=0.09) This small amount of evidence from retrospective comparative studies does not show any difference in outcomes among different types of ADM products. Interpositional Graft after Parotidectomy AlloDerm In 2003, Sinha et al. reported the use of AlloDerm acellular human dermal matrix as an interpositional physical barrier to prevent the development of Frey syndrome (gustatory sweating) after parotidectomy. Thirty patients were divided into three groups; it was not described if the assignments were randomized. One group underwent superficial parotidectomy with reconstruction of the defect with AlloDerm, a second group had superficial parotidectomy without placement of an interpositional barrier, and the third group underwent deep-plane rhytidectomy without disruption of the parotid fascia. At minimum one-year follow-up, there was a subjective incidence of Frey syndrome in one patient treated with AlloDerm and five patients in the second group. The objective incidence of Frey syndrome, measured with the Minor starch-iodine test, was two patients treated with AlloDerm and eight patients in the second group. None of the patients in the third group who underwent deep-plane rhytidectomy without 11 of 40
12 disruption of the parotid fascia had subjective or objective Frey syndrome. There were no adverse effects. A 2008 publication from Asia compared use of allogeneic ADM (RENOV) in 168 patients who had superficial or partial parotidectomy (Ye et al., 2008). Sixty-four patients received an ADM and 104 patients had superficial or partial parotidectomy alone. The size of the graft depended on the amount of tissue required to restore the normal facial contour. The method of assignment to the two groups was not described. At a median follow-up of 16 months (range, 11 to 27 months), the subjective incidence of Frey syndrome was 2% in the ADM group compared with 61% in controls. Objective assessment, performed in 30 patients randomly selected from each group, found an incidence of Frey syndrome in two patients (7%) treated with ADM and 24 patients (80%) in the control group. One patient in the ADM group and 18 patients in the control group developed a parotid fistula. DermaMatrix DermaMatrix is an ADM that differs from AlloDerm in several ways; it can be stored at room temperature (versus refrigerated), it has a shelf-life of three years (versus two years), and it can be rehydrated in three minutes (versus 30 minutes). Athavale et al. (2011) evaluated complications of AlloDerm and DermaMatrix in a retrospective review of 100 patients treated between 2001 and 2009 at a single U.S. institution. Exclusion criteria for the study included presence of malignancy on final surgical pathology report, incomplete medical records, previous history of radiation therapy to the head and neck region, and additional procedures to the region of the parotid gland. Initially, only AlloDerm was used; this changed to a 20/80 ratio of AlloDerm/DermaMatrix due to more readily available stock of DermaMatrix. Complications were defined as any outcome that required procedural intervention for resolution (seroma/sialocele formation, infected fluid collection, and/or serosanguinous fluid collection). The authors identified eight complications in 31 DermaMatrix implants (26%) compared with five complications in 69 AlloDerm implants (7%). The complication rate did not differ for total parotidectomies but was higher for DermaMatrix compared to AlloDerm for subtotal parotidectomies (37% versus 8%). Nearly half of all complications were seroma/sialocele formation. Double-blind RCTs with longer follow-up are needed to evaluate this procedure. Tendon Repair GraftJacket In 2012, Barber et al. reported an industry-sponsored multi-center randomized controlled trial of augmentation with GraftJacket acellular human dermal matrix for arthroscopic repair of large (>3 centimeter (cm)) rotator cuff tears involving two tendons. Twenty-two patients were randomized to GraftJacket augmentation and 20 patients were randomized to no augmentation. At a mean follow-up of 24 months (range, 12 to 38 months) the American Shoulder and Elbow Surgeons (ASES) score improved from 48.5 to 98.9 in the GraftJacket group and from 46.0 to 94.8 in the control group (p = 0.035). The Constant score improved from 41 to 91.9 in the GraftJacket group and from 45.8 to 85.3 in the control group (p = 0.008). The University of California, Los Angeles score was not significantly different between the groups. Gadolinium- 12 of 40
13 enhanced magnetic resonance imaging (MRI) scans showed intact cuffs in 85% of repairs in the GraftJacket group and 40% of repairs in the control group. However, no correlation was found between MRI findings and clinical outcomes. Rotator cuff retears occurred in three patients (14%) in the GraftJacket group and nine patients (45%) in the control group. Although these results were promising, additional study with a larger number of patients is needed. Fistula Repair Acellular Dermal Matrix A study from Asia compared a xenogeneic ADM (J-I type, J.Y. Life Tissue Engineering Co., China) with endorectal advancement flap (ERAF) for the treatment of complex anorectal fistula in a randomized study with 90 consecutive patients (ba-bai-ke-re et al., 2010). Follow-up was performed at two days, 2, 4, 6, 12 weeks, and five months after surgery. Success was defined as closure of all external opening, absence of drainage without further intervention, and absence of abscess formation. Success was observed in 82.2% of the ADM group. Fistula recurred in two (4.45%) patients in the ADM group compared with 13 (28.89%) patients in the ERAF group. Healing time was reduced (7.5 versus 24.5 days) and quality of life was rated higher in the ADM group (85.9 versus 65.3). No significant difference was observed in the incontinence and anal deformity rate between the two groups. This product is not cleared for marketing in the United States, although the manufacturing process was reported to be similar to the Surgisis AFP (anal fistula plug) (Cook Biotech Inc., West Lafayette, IN). Surgical Repair of Hernias A 2011 systematic review included 30 level III and level IV articles on ADM for abdominal wall reconstruction (Zhong et al., 2011). No RCTs or high-quality comparative studies (level I or II) were identified. Examples of the level III studies are described next. Alloderm Gupta et al. (2006) compared the efficacy and complications associated with the use of AlloDerm and Surgisis bioactive mesh (Cook Biotech Incorporated, West Lafayette, IN) in 74 patients who underwent ventral hernia repair. The first 41 procedures were performed using Surgisis Gold 8-ply mesh formed from porcine small intestine sub-mucosa, and the remaining 33 patients had ventral hernia repair with AlloDerm. Patients were seen seven to 10 days after discharge from the hospital and at six weeks. Any signs of wound infection, diastasis, hernia recurrence, changes in bowel habits, and seroma formation were evaluated. The use of the AlloDerm mesh resulted in eight hernia recurrences (24%). Fifteen of the AlloDerm patients (45%) developed a diastasis or bulging at the repair site. Seroma formation was only a problem in two patients. In 2007, Espinosa-de-los-Monteros and colleagues retrospectively reviewed 39 abdominal wall reconstructions with AlloDerm performed in 37 patients and compared them with 39 randomly selected cases. They reported a significant decrease in recurrence rates when human cadaveric acellular dermis was added as an overlay to primary closure plus rectus muscle advancement and imbrication in patients with medium-sized hernias. However, no differences were observed when adding human cadaveric acellular dermis as an overlay to patients with large-size hernias treated with underlay mesh. 13 of 40
14 A 2013 study by Bochicchio and colleagues compared AlloDerm with FlexHD for complicated hernia surgery. From 2005 to 2007, AlloDerm was used to repair large (>200 cm²) symptomatic complicated ventral hernia that resulted from trauma or emergency surgery (n=55). From 2008 to 2010, FlexHD was used to repair large complicated ventral hernia in patients meeting the same criteria (n=40). The two groups were comparable at baseline. At 1-year follow-up, all of the AlloDerm patients were diagnosed with hernia recurrence (abdominal laxity, functional recurrence, or true recurrence) requiring a second repair. Eleven patients (31%) in the FlexHD group required a second repair. This comparative study is limited by the use of nonconcurrent comparisons, which is prone to selection bias and does not control for temporal trends in outcomes. The limited evidence available at this time does not support the use of AlloDerm in hernia repair. Oral Surgery AlloDerm In 2008, Novaes and de Barros described three randomized trials from their research group that examined use of ADM in root coverage therapy and alveolar ridge augmentation. Two trials used ADM in both the study and control groups and are not described here. A third trial compared ADM with subepithelial connective tissue graft in 30 gingival recessions (nine patients). At six months post surgery, the ADM showed recession reduction of 1.83 millimeters (mm) while subepithelial connective tissue graft showed recession reduction of 2.10 mm; these were not significantly different. A non-randomized cohort study compared Alloderm with the gold standard of split thickness skin grafts in 34 patients who underwent oral cavity reconstruction following surgical removal of tumors (Girod et al., 2009). Patients were enrolled after surgical treatment for evaluation at a tertiary care center and divided into two cohorts according to the reconstruction method used, which was based on surgeon preference. Twenty-two patients had been treated with Alloderm, and 12 had been treated with split thickness skin grafts. The location of the grafts (Alloderm versus autograft) were on the tongue (54% versus 25%), floor of mouth (9% versus 50%), tongue and floor of mouth (23% versus 8%), buccal (9% versus 0%), or other (5% versus 17%). More patients in the Alloderm group were treated with radiation therapy (45% versus 17%), and the graft failure rate was higher (14% versus 0%). Radiation therapy had a significantly negative impact for both groups. Histology on a subset of the patients showed increased inflammation, fibrosis, and elastic fibers with split thickness skin grafts. Functional status and quality of life were generally similar in the two groups. Interpretation of these results is limited by the differences between the groups at baseline. Laryngoplasty There are several reports with short-term follow-up of micronized AlloDerm (Cymetra) injection for laryngoplasty. In 2005, Milstein et al. reported mean 11.2 month follow-up (range, 1 to 35 months) of Cymetra injection in 20 patients with unilateral vocal-fold paralysis. Pre- and postoperative digital voice samples and video stroboscopy were rated on a four-point scale by a panel of three voice experts who were blinded to the pre- or post-operative status. Compared with preoperative measures, Cymetra improved voice quality (from 3.23 to 1.65), glottal closure 14 of 40
15 (from 3.21 to 1.42), and degree of vocal-fold bowing (from 2.38 to 1.36). Quality-of-life measures and patients' self-perceptions of vocal quality were also improved. In five patients (25%), the effect was temporary, and in eight patients (40%) who had follow-up of one year or longer, the improvement was maintained. Longer-term study in a larger number of patients is needed to determine the durability of this procedure and to evaluate the safety of repeat injections. Tympanoplasty Vos et al. (2005) reported a retrospective non-randomized comparison of AlloDerm versus native tissue grafts for type I tympanoplasty. Included in the study were 108 patients (25 AlloDerm, 53 fascia reconstruction, and 30 fascia plus cartilage reconstruction) treated between 2001 and One surgeon had performed 96% of the AlloDerm tympanoplasties. Operative time was reduced in the AlloDerm group (82 minutes for AlloDerm, 114 minutes for fascial cases, and 140 minutes for fascia plus cartilage). There was no significant difference in the success rate of the graft (88% for AlloDerm, 89% for fascia grafts, 96.7% for cartilage plus fascia). There was no significant difference in hearing between the groups at follow-up (time not specified). Longer-term controlled study in a larger number of patients is needed to determine the durability of this procedure. Diabetic Lower Extremity Ulcers Apilgraf In 2001, Veves and colleagues reported on a randomized prospective study on the effectiveness of Graftskin (Apligraf), a living skin equivalent, in treating non-infected non-ischemic chronic plantar diabetic foot ulcers. The study involved 24 centers in the United States, 208 patients were randomly assigned to ulcer treatment either with Graftskin (112 patients) or saline-moistened gauze (96 patients, control group). Standard state-of-the-art adjunctive therapy, including extensive surgical debridement and adequate foot off-loading, was provided in both groups. Graftskin was applied at the beginning of the study and weekly thereafter for a maximum of four weeks (maximum of five applications) or earlier if complete healing occurred. At the 12-week follow-up visit, 63 (56%) Graftskin-treated patients achieved complete wound healing compared with 36 (38%) in the control group (p = ). The Kaplan-Meier median time to complete closure was 65 days for Graftskin, significantly lower than the 90 days observed in the control group (p = ). The rate of adverse reactions was similar between the two groups with the exception of osteomyelitis and lower-limb amputations, both of which were less frequent in the Graftskin group. The authors concluded application of Graftskin for a maximum of four weeks resulted in a higher healing rate when compared with state-of-the-art treatment and was not associated with any significant side effects. This study was reviewed in a 2001 Blue Cross Blue Shield Association Technology Evaluation Center (TEC) Assessment, which concluded Apligraf (graftskin), in conjunction with good local wound care, met the TEC criteria for the treatment of diabetic ulcers that failed to respond to conservative management. In 2010, Steinberg and colleagues reported on a study of 72 subjects from Europe and Australia that assessed the safety and efficacy of Apligraf in the treatment of non-infected diabetic foot ulcers. The design and patient population of this study were similar to the 208-subject U.S. study (described above) which led to FDA approval of Apligraf for the treatment of diabetic foot 15 of 40
16 ulcers. For these studies, subjects with a non-infected neuropathic diabetic foot ulcer present for at least two weeks were enrolled in these prospective, multicenter, randomized, controlled, openlabel studies comparing Apligraf use in conjunction with standard therapy (sharp debridement, standard wound care, and off-loading) against standard therapy alone. Pooling of data was performed because of the similarity and consistency of the two studies. Efficacy and safety results were consistent across studies independent of mean ulcer duration that was significantly longer in the European study (21 months, compared to 10 months in the U.S. study). Reported adverse events by 12 weeks were comparable across treatment groups in the two studies. Efficacy measures demonstrated superiority of Apligraf treatment over control treated groups in both studies. Combining the data from both studies, 55.2% (80 of 145) of Apligraf subjects had complete would closure by 12 weeks, compared to 34.3% (46 of 134) of control subjects (p = ), and Apligraf subjects had a significantly shorter time to complete wound closure (p = ). The authors concluded both the European Union and U.S. studies exhibited superior efficacy and comparable safety for subjects treated with Apligraf compared to control subjects, and the studies provided evidence of the benefit of Apligraf in treating diabetic foot ulcers. In 2010, Kirsner and colleagues reported on analysis of 2,517 patients with diabetic neuropathic foot ulcers who were treated between 2001 and The study was a retrospective analysis using a wound-care database; the patients received advanced biological therapy (i.e., Apligraf (446 patients)), Regranex, or Procuren. In this study, advanced biological therapy was used, on average, within 28 days from the first wound clinic visit and associated with a median time to healing of 100 days. Wounds treated with engineered skin (Apligraf) as the first advanced biological therapy were 31.2% more likely to heal than wounds first treated with topical recombinant growth factor (p < 0.001), and 40.0% more likely to heal than those first treated with platelet releasate (p = 0.01). Wound size, wound grade, duration of wound, and time to initiation of advanced biological therapy affected the time to healing. Dermagraft A pivotal multicenter FDA-regulated trial randomized 314 patients with chronic diabetic ulcers to Dermagraft or control (Marston et al., 2003). Over the course of the 12-week study patients received up to eight applications of Dermagraft. All patients received pressure-reducing footwear and were encouraged to stay off their study foot as much as possible. At 12 weeks, the median percent wound closure for the Dermagraft group was 91% compared to 78% for the control group. Ulcers treated with Dermagraft closed significantly faster than ulcers treated with conventional therapy. No serious adverse events were attributed to Dermagraft. Ulcer infections developed in 10.4% of the Dermagraft patients compared to 17.9% of the control patients. Together, there was a lower rate of infection, cellulitis, and osteomyelitis in the Dermagrafttreated group (19% versus 32.5%). GraftJacket Regenerative Tissue Matrix Brigido et al. (2004) reported a small (n = 40) randomized pilot study of GraftJacket compared with conventional treatment for chronic non-healing diabetic foot ulcers. Control patients received conventional therapy with debridement, wound gel with gauze dressing, and offloading. GraftJacket patients received surgical application of the scaffold using skin staples or sutures and moistened compressive dressing. A second graft application was necessary after the 16 of 40
17 initial application for all patients in the GraftJacket group. Preliminary one month results showed that after a single treatment, ulcers treated with GraftJacket healed at a faster rate than conventional treatment. There were significantly greater decreases in wound length (51% versus 15%), width (50% versus 23%), area (73% versus 34%) and depth (89% versus 25%). All of the grafts incorporated into the host tissue. In 2009, Reyzelman et al. reported an industry-sponsored multicenter randomized study that compared a single application of GraftJacket versus standard of care in 86 patients with diabetic foot ulcers. Offloading was performed using a removable cast walker. Ulcer size at presentation was 3.6 square cm/squared (cm 2 ) in the GraftJacket group and 5.1 cm 2 in the control group. Eight patients, six in the study group and two in the control group, did not complete the trial. At 12 weeks, complete healing was observed in 69.6% of the GraftJacket group and 46.2% of controls. After adjusting for ulcer size at presentation, a statistically significant difference in non-healing rate was calculated, with odds of healing two times higher in the study group. Mean healing time was 5.7 weeks versus 6.8 weeks for the control group. The authors did not report if this difference was statistically significant. The median time to healing was 4.5 weeks for GraftJacket (range, 1 to 12 weeks) and seven weeks for control (range, 2 to 12 weeks). Kaplan-Meier survivorship analysis for time to complete healing at 12 weeks showed a significantly lower nonhealing rate for the study group (30.4%) compared with the control group (53.9%). The authors commented that a single application of GraftJacket, as used in this study, is often sufficient for complete healing. This study was limited by the small study population, differences in ulcer size at baseline, and the difference in the percentage of patients censored in each group. Questions also remained about whether the difference in mean time to healing was statistically or clinically significant. Additional trials with a larger number of subjects are needed to evaluate if GraftJacket Regenerative Tissue Matrix improves health outcomes in this population. EpiFix Amniotic Membrane In 2013, Zelen et al. reported an industry-sponsored, nonblinded, RCT comparing use of EpiFix (n=13) with standard of care (SOC; moist wound therapy, n=12) for diabetic foot ulcers of at least 4 weeks duration. EpiFix was applied every 2 weeks if the wound had not healed, with weekly dressing changes comprised of nonadherent dressing, moisture retentive dressing, and a compression dressing. Standard moist wound dressing was changed daily. After 4 weeks of treatment, EpiFix treated wounds had reduced in size by a mean of 97.1% compared with 32.0% for the SOC group. Healing rate, defined as complete epithelialization of the open area of the wound, was 77% for EpiFix compared with 0% for SOC. After 6 weeks of treatment, wounds were reduced by 98.4% with EpiFix treatment compared with -1.8% for standard care. The healing rate was 92% with EpiFix compared with 8% with standard treatment alone. Results of this small study are encouraging, but preliminary. Confirmation of these results in a larger trial is needed. Oasis Wound Matrix Niezgoda and colleagues (2005) compared healing rates at 12 weeks for full-thickness diabetic foot ulcers treated with Oasis Wound Matrix, an acellular wound care product, versus Regranex Gel. This was an industry-sponsored randomized controlled multicenter trial conducted at nine outpatient wound care clinics and involved 73 patients with at least one diabetic foot ulcer. 17 of 40
18 Patients were randomized to receive either Oasis Wound Matrix (n = 37) or Regranex Gel (n = 36) and a secondary dressing. Wounds were cleansed and debrided, if needed, at a weekly visit. The maximum treatment period for each patient was 12 weeks. After 12 weeks of treatment, 18 (49%) Oasis-treated patients had complete wound closure compared with 10 (28%) Regranextreated patients. Oasis treatment met the non-inferiority margin, but did not demonstrate that healing in the Oasis group was statistically superior (p = 0.055). Post-hoc subgroup analysis showed no significant difference in incidence of healing in patients with type 1 diabetes (33% versus 25%) but a significant improvement in patients with type 2 diabetes (63% versus 29%). There was also an increased healing of plantar ulcers in the Oasis group (52% versus 14%). These post-hoc findings were considered hypothesis generating. Additional studies with larger number of subjects are needed to evaluate the effect of Oasis treatment in comparison with the current standard of care. PriMatrix In 2011, Karr published a retrospective comparison of PriMatrix (a xenograft fetal bovine dermal collagen matrix) and Apligraf in 40 diabetic foot ulcers. The first 20 diabetic foot ulcers matching the inclusion and exclusion criteria for each graft were compared. Included were diabetic foot ulcers of four weeks' duration, at least 1 cm² in depth to subcutaneous tissue, healthy tissue at the ulcer, adequate arterial perfusion to heal, and able to off-load the diabetic ulcer. The products were placed on the wound with clean technique, overlapping the edges of the wound, and secured with sutures or staples. The time to complete healing for PriMatrix was 38 days with 1.5 applications compared to 87 days with two applications for Apligraf. Although promising, additional study with a larger number of subjects is needed to evaluate the effect of PriMatrix treatment in comparison with the current standard of care. Lower Extremity Ulcers Due to Venous Insufficiency Apligraf Aplicraf is a living cell therapy composed of living human keratinocytes and fibroblasts. Falanga and colleagues (1998) reported a multicenter randomized trial of Apligraf (human skin equivalent). A total of 293 patients with venous insufficiency and clinical signs of venous ulceration were randomized to compression therapy alone or compression therapy and treatment with Apligraf. Apligraf was applied up to a maximum of five (mean 3.3) times per patient during the initial three weeks. The primary endpoints were the percentage of patients with complete healing by six months after initiation of treatment and the time required for complete healing. At six months' follow-up, the percentage of patients healed was increased with Apligraf (63% versus 49%) and the median time to complete wound closure was reduced (61 days versus 181 days). Treatment with Apligraf was found to be superior to compression therapy in healing larger (> 1000 millimeter squared (mm 2 )) and deeper ulcers and ulcers of more than six months' duration. There were no symptoms or signs of rejection and the occurrence of adverse events were similar in both groups. This study was reviewed in a 2001 TEC Assessment, which concluded Apligraf (graftskin), in conjunction with good local wound care, met the TEC criteria for the treatment of venous ulcers that failed to respond to conservative management. 18 of 40
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2014 Origination: 4/2008 Next Review: 3/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 10/01/2013 Section: Surgery Place(s)
Tissue-Engineered Skin Substitutes
 Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Tissue-Engineered Skin Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO 06/01/2012 Section: Surgery Place(s) of Service: Outpatient/Office
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Page 1 of 58 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See Also: Bio-Engineered Skin and Periodontal Soft Tissue Grafting dental
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: 7.01.113 Last Review: 3/2018 Origination: 4/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Bioengineered Skin and Soft Tissue Substitutes
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Medical Policy. MP Bioengineered Skin and Soft Tissue Substitutes
 Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy MP 7.01.113 BCBSA Ref. Policy: 7.01.113 Last Review: 02/26/2018 Effective Date: 05/30/2018 Section: Surgery Related Policies 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Page 1 of 62 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Bio-Engineered Skin and Amniotic Membrane and Amniotic Fluid medical
Bio-Engineered Skin and Soft Tissue Substitutes
 Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Bio-Engineered Skin and Soft Tissue Substitutes Policy Number: Original Effective Date: MM.06.018 06/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section:
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12
 Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
Protocol Bio-Engineered Skin and Soft Tissue Substitutes (701113) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 03/12 The following Protocol contains
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
FEP Medical Policy Manual Effective Date: April 15, 2017 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors as a Treatment of Wound Healing and Other Conditions 7.01.149
MEDICAL POLICY No R8 SKIN SUBSTITUTES & SOFT TISSUE GRAFTS
 Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
Summary of Changes MEDICAL POLICY SKIN SUBSTITUTES & SOFT TISSUE GRAFTS Effective Date: August 25, 2017 Review Dates: 12/08, 8/09, 8/10, 8/11, 8/12, 4/13, 5/14, 5/15, 5/16, 5/17 Date Of Origin: December
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: 7.01.149 Amniotic Membrane and Amniotic Fluid Bioengineered Skin and Soft Tissue Substitutes Description Bioengineered skin and
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Effective Date: 1/1/2019 Section: MED Policy No: 378 Medical Officer 1/1/2019 Date Technology Assessment Committee Approved Date: 3/09; 10/14; 1/16 Medical Policy Committee Approved Date: 4/02; 4/03; 9/04;
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
Note: Page numbers of article titles are in boldface type. A Acellular dermal allografts for arthroplasty, 633 642 for plantar soft tissue augmentation, 543 555 Achilles tendon repair extracellular matrix
What s New Medical Policy Updates April 2017
 What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
What s New Medical Policy Updates April 2017 Listed below are the recent changes made to policies within the Geisinger Health Plan Medical Policy Portfolio during the month of March that will become effective
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds
 Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Use of Kerecis Omega 3 Fatty Acid Fish-skin Graft in Traumatic Wounds WINDY COLE, DPM ADJUNCT PROFESSOR AND DIRECTOR OF WOUND CARE RESEARCH KSUCPM MEDICAL DIRECTOR, WOUND CENTER, UH AHUJA HOSPITAL Goals
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Skin Replacement Therapy for Chronic Non healing Wounds in the Outpatient Setting MP-032-MD-DE Medical Management Provider
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL Policy Number: MP.083.MH Last Review Date: 11/03/2016 Effective Date: 01/01/2017
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Skin Substitutes for Wound Care AHM
 Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
Skin Substitutes for Wound Care AHM Clinical Indications Any 1 or more of the following products for wound care are considered medically necessary if the individual criteria are met Apligraf (graftskin)
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
MP.083.MH Skin Substitutes- Human Skin Equivalents
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.083.MH Skin Substitutes- Human Skin Equivalents This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP
Coding for Wound Care
 Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Coding for Wound Care ****IMPORTANT*** Disclaimer ***Information provided is to the best of our knowledge and as current as possible. ***Please verify all policy and reimbursement information with your
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
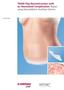 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Cigna Medical Coverage Policy Subject Tissue-Engineered Skin Substitutes Table of Contents Coverage Policy... 1 General Background... 15 Coding/Billing Information... 62 References... 66 Effective Date...
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
THE WOUND SOLUTION. (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix)
 (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
(Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft
 Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
Single-Stage Full-Thickness Scalp Reconstruction Using Acellular Dermal Matrix and Skin Graft Yoon S. Chun, MD, a and Kapil Verma, BA b a Division of Plastic and Reconstructive Surgery, Department of Surgery,
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Number: (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end
 1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
1 of 278 Number: 0244 (Replaces CPB 331) Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. I. Aetna considers the following products for wound care medically necessary according
Wound & Burn. Reimbursement & Coding Guide
 Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Wound & Burn Reimbursement & Coding Guide Wound & Burn Reimbursement and Coding Guide MicroMatrix and Cytal devices facilitate the remodeling of functional, site-appropriate tissue. Comprised of ACell
Integra. Tissue Technologies. Limit uncertainty with a leader in collagen technology
 Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Limit uncertainty with a leader in collagen technology Integra Dermal Regeneration Template PriMatrix Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy
 Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Bioengineered Skin and Soft Tissue Substitutes Corporate Medical Policy File Name: Bioengineered Skin and Soft Tissue Substitutes File Code: UM.SURG.16 Origination: 09/2016 Last Review: 10/2018 Next Review:
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
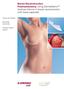 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid
 02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
02-10000-11 Original Effective Date: 01/01/01 Reviewed: 03/22/18 Revised: 01/01/19 Subject: Bio-Engineered Skin and Soft Tissue Substitutes; Amniotic Membrane and Amniotic Fluid THIS MEDICAL COVERAGE GUIDELINE
Wright Medical Technology, Inc Airline Road Arlington, TN phone toll-free
 References 1 Brigido SA, Boc SF, Lopez RC. Effective Management of Major Lower Extremity Wounds Using an Acellular Regenerative Tissue Matrix: A Pilot Study. Orthopedics 2004; 27(1S): pp145-149. 2 Brigido
References 1 Brigido SA, Boc SF, Lopez RC. Effective Management of Major Lower Extremity Wounds Using an Acellular Regenerative Tissue Matrix: A Pilot Study. Orthopedics 2004; 27(1S): pp145-149. 2 Brigido
2012 Head and Neck Reconstruction/ENT Repair Coding Observations
 Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Health Policy, Economics & Reimbursement Reimbursement Hotline Tel: 888.543.3656 Fax: 866.262.6977 reimbursement@lifecell.com www.lifecell.com 2012 Head and Neck Reconstruction/ENT Repair Coding Observations
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
MY STRATEGY FOR TREATING BURN INJURIES. Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA
 MY STRATEGY FOR TREATING BURN INJURIES Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA ASSUMPTIONS: Burns which heal to normal have best outcome. Medical risk, functional recovery,
MY STRATEGY FOR TREATING BURN INJURIES Warren Garner MD FACS Keck School of Medicine at USC Los Angeles, CA ASSUMPTIONS: Burns which heal to normal have best outcome. Medical risk, functional recovery,
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction
 A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
A Comparative Study of CG CryoDerm and AlloDerm in Direct-to-Implant Immediate Breast Reconstruction Original Article Jun Ho Lee 1, Ki Rin Park 1, Tae Gon Kim 1, Ju-Ho Ha 1, Kyu-Jin Chung 1, Yong-Ha Kim
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Commercial Medical Policy Policy Number: 2018T0592A Effective Date: October 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm
 Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
Infectious Complications Leading to Explantation in Implant-Based Breast Reconstruction With AlloDerm Minh-Doan Nguyen, MD, PhD, a Chen Chen, MS, b Salih Colakoğlu, MD, b Donald J. Morris, MD, b Adam M.
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur
 VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION
 NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
NIPPLE SPARING PRE-PECTORAL BREAST RECONSTRUCTION 42 yo female healthy athlete Right breast mass. Past medical history: none Family history: aunt with Breast cancer Candidates for nipple-sparing mastectomy
IHCP banner page. IHCP to cover CPT code IHCP to cover CPT code INDIANA HEALTH COVERAGE PROGRAMS BR JULY 3, 2018
 IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
IHCP banner page INDIANA HEALTH COVERAGE PROGRAMS BR201827 JULY 3, 2018 IHCP to cover CPT 93050 Effective August 3, 2018, the Indiana Health Coverage Programs (IHCP) will cover Current Procedural Terminology
MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures
 POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0203 ORIGINAL EFFECTIVE: 01/15/09 LAST REVIEW: 03/13/18 MEDICAL POLICY: Skin Substitutes and Wound Repair Procedures GUIDELINES This policy does not certify benefits or authorization of benefits,
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position
 Breast Surgery Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position M. Mark Mofid, MD; and Navin K. Singh, MD Background: The
Breast Surgery Pocket Conversion Made Easy: A Simple Technique Using Alloderm to Convert Subglandular Breast Implants to the Dual-Plane Position M. Mark Mofid, MD; and Navin K. Singh, MD Background: The
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Epicel (cultured epidermal autografts) HDE# BH Patient Information
 Epicel (cultured epidermal autografts) HDE# BH990200 Patient Information This leaflet is designed to help you understand Epicel (cultured epidermal autografts) and its use for the treatment of burn wound.
Epicel (cultured epidermal autografts) HDE# BH990200 Patient Information This leaflet is designed to help you understand Epicel (cultured epidermal autografts) and its use for the treatment of burn wound.
Medical Policy Original Effective Date: Revised Date: Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
POLICIES AND PROCEDURE MANUAL Policy: MP075 Section: Medical Benefit Policy Subject: Tissue Engineered Skin Substitutes I. Policy: Tissue Engineered Skin Substitutes II. Purpose/Objective: To provide a
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
Care of Wound Reconstruction Patients, 2018 ed.
 Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
Care of Wound Reconstruction Patients, 2018 ed. STEVE GROSSO, MD, FACS BILLINGS PLASTIC SURGERY A Smorgasbord of Plastic Surgery Stuff Disclaimer/Conflicts of Interest It is not possible to discuss wound
Treating Massive Rotator Cuff Tears and Revisions
 Treating Massive Rotator Cuff Tears and Revisions The solutions leading surgeons are using to help achieve higher success rates and better patient outcomes. Shoulder Restoration System To learn more about
Treating Massive Rotator Cuff Tears and Revisions The solutions leading surgeons are using to help achieve higher success rates and better patient outcomes. Shoulder Restoration System To learn more about
Bioimpedance Devices for Detection and Management of Lymphedema
 2.01.82 Bioimpedance Devices for Detection and Management of Lymphedema Section 2.0 Medicine Subsection Effective Date January 30, 2015 Original Policy Date July 2, 2010 Next Review Date January 2015 Medical
2.01.82 Bioimpedance Devices for Detection and Management of Lymphedema Section 2.0 Medicine Subsection Effective Date January 30, 2015 Original Policy Date July 2, 2010 Next Review Date January 2015 Medical
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
BASICS OF BURN MANAGEMENT
 BASICS OF BURN MANAGEMENT Dr S M Keswani Cosmetic Surgeon National Burns Centre, Airoli,Navi-Mumbai Breach Candy Hospital Wockhardt Hospital National Burns Centre, Airoli, Navi-Mumbai. CLASSIFICATION 1.
BASICS OF BURN MANAGEMENT Dr S M Keswani Cosmetic Surgeon National Burns Centre, Airoli,Navi-Mumbai Breach Candy Hospital Wockhardt Hospital National Burns Centre, Airoli, Navi-Mumbai. CLASSIFICATION 1.
Clinical teaching/experi ence. Lectures/semina rs/conferences Self-directed. learning. Clinical teaching/experi ence
 Regional Medical Center (The MED) Plastic Surgery PGY-3 By the end of the Plastic Surgery at the MED, the PGY-3 residents are expected to expand and cultivate knowledge and skills developed during previous
Regional Medical Center (The MED) Plastic Surgery PGY-3 By the end of the Plastic Surgery at the MED, the PGY-3 residents are expected to expand and cultivate knowledge and skills developed during previous
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Technology Report. Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness
 Technology Report Issue 52 February 2005 Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness Publications can be requested from: CCOHTA
Technology Report Issue 52 February 2005 Artificial Skin Grafts in Chronic Wound Care: A Meta-analysis of Clinical Efficacy and a Review of Cost-effectiveness Publications can be requested from: CCOHTA
MOVE BEYOND. to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair
 MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
MOVE BEYOND to new hope for enterocutaneous fistulas. Enterocutaneous Fistula Repair to an alternative treatment option Enterocutaneous fistulas can significantly affect patient health and quality of life.
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
UvA-DARE (Digital Academic Repository) Ischemic and diabetic wounds of the lower extremity Santema, T.B. Link to publication
 UvA-DARE (Digital Academic Repository) Ischemic and diabetic wounds of the lower extremity Santema, T.B. Link to publication Citation for published version (APA): Santema, T. B. (2017). Ischemic and diabetic
UvA-DARE (Digital Academic Repository) Ischemic and diabetic wounds of the lower extremity Santema, T.B. Link to publication Citation for published version (APA): Santema, T. B. (2017). Ischemic and diabetic
Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
BIOFIBER. Absorbable Biologic Scaffolding Solutions
 BIOFIBER Absorbable Biologic Scaffolding Solutions BIOFIBER IS ONLY THE BEGINNING BIOFIBER - CM What is BIOFIBER CM? BIOFIBER Collagen Matrix is comprised of the same P4HB material with the addition of
BIOFIBER Absorbable Biologic Scaffolding Solutions BIOFIBER IS ONLY THE BEGINNING BIOFIBER - CM What is BIOFIBER CM? BIOFIBER Collagen Matrix is comprised of the same P4HB material with the addition of
Over the years, mucogingival surgery
 The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation and Root Coverage: A Case Series Saroff Stephen Andrew Saroff, DDS, MSD 1 Abstract Over the years, mucogingival surgery has developed
The Use of DynaMatrix Extracellular Membrane for Gingival Augmentation and Root Coverage: A Case Series Saroff Stephen Andrew Saroff, DDS, MSD 1 Abstract Over the years, mucogingival surgery has developed
Goals of Care. Restore shape and function after cancer
 Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
If both a standardized, validated screening tool and an evaluation of clinical factors are utilized, select Response 2.
 (M1300) Pressure Ulcer Assessment: Was this patient assessed for Risk of Developing Pressure Ulcers? 0 - No assessment conducted [Go to M1306 ] 1 - Yes, based on an evaluation of clinical factors (for
(M1300) Pressure Ulcer Assessment: Was this patient assessed for Risk of Developing Pressure Ulcers? 0 - No assessment conducted [Go to M1306 ] 1 - Yes, based on an evaluation of clinical factors (for
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
The Case FOR Oncoplastic Surgery in Small Breasts. Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA
 The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
Palmetto Medicare Policy Primer
 Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
BONE AUGMENTATION AND GRAFTING
 1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
Hemostasis Inflammatory Phase Proliferative/rebuilding Phase Maturation Phase
 The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Recombinant and Autologous Platelet-Derived Growth Factors Page 1 of 20 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Recombinant and Autologous Platelet-Derived
Recombinant and Autologous Platelet-Derived Growth Factors Page 1 of 20 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Recombinant and Autologous Platelet-Derived
Burn & Soft Tissue Service Orientation Slides
 Burn & Soft Tissue Service Orientation Slides Damien Wilson Carter, MD Director, Burn/Soft Tissue Service Sue Reeder, BSN, CWOCN Burn Resource Nurse Specialist Scope ALL Burn injuries (> Age 12) Cold injury/
Burn & Soft Tissue Service Orientation Slides Damien Wilson Carter, MD Director, Burn/Soft Tissue Service Sue Reeder, BSN, CWOCN Burn Resource Nurse Specialist Scope ALL Burn injuries (> Age 12) Cold injury/
Negative Pressure Wound Therapy
 Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
