Young neurons born in the developing or adult brain have to
|
|
|
- Gloria Green
- 5 years ago
- Views:
Transcription
1 Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex Hynek Wichterle*, Manuel Alvarez-Dolado, Lynda Erskine, and Arturo Alvarez-Buylla *The Rockefeller University, New York, NY 10021; Brain Tumor Research Center, University of California, San Francisco, CA ; and Department of Pathology, Columbia University, New York, NY Communicated by Fernando Nottebohm, Field Research Center for Ecology and Ethology, The Rockefeller University, Millbrook, NY, November 27, 2002 (received for review February 28, 2002) Young neurons born in the medial ganglionic eminence (MGE) migrate a long distance dorsally, giving rise to several types of interneurons in neocortex. The mechanisms that facilitate selective dorsal dispersion of MGE cells while restricting their movement ventrally into neighboring regions are not known. Using microtransplantation into fetal brain slices and onto dissociated substrate cells on floating filters (spot assay), we demonstrate that ventral forebrain regions neighboring the MGE are nonpermissive for MGE cell migration, whereas the dorsal regions leading to the neocortex are increasingly permissive. Spot assay experiments using filters with different pore sizes indicate that the permissive factors are not diffusible. We also show that MGE cells respond to chemoattractive and inhibitory factors diffusing from the neocortex and ventromedial forebrain, respectively. We propose that the final extent and regional specificity of MGE cell dispersion is largely dictated by contact guidance through a selectively permissive environment, flanked by nonpermissive tissues. In addition, we propose that chemotactic guidance cues superimposed over the permissive corridor facilitate efficient dorsal migration of MGE cells. Young neurons born in the developing or adult brain have to migrate along precise pathways to find the correct sites for their final differentiation. This process is essential for proper brain development and function. Some young neurons travel long distances tangentially and settle in brain regions far from their birthplaces. The medial and lateral ganglionic eminences (MGE and LGE) are the best established sources of tangentially migrating neurons in the developing forebrain. Early in development, LGE cells migrate ventrally and anteriorly, giving rise to medium spiny neurons of the dorsal and ventral striatum (including the nucleus accumbens and olfactory tubercle; refs. 1 5) and to interneurons in the olfactory bulb (2, 3, 6). Proliferating neural stem cells derived from the LGE remain in the subventricular zone (SVZ) of the postnatal lateral ventricle (2), where they establish a germinal region, which persists into adulthood (7, 8). Young neurons born in the postnatal SVZ migrate rostrally to the olfactory bulb where they differentiate into granule and periglomerular interneurons (9, 10). It has been suggested that migration of LGE and SVZ cells is guided by repulsive factors of the Slit family secreted from the ventricular zone of the LGE in the embryo (11) and from the caudal septum (12) and choroid plexus (13) in the postnatal brain. Alternatively, it has been proposed that Slit factors normally inhibit SVZ cell migration and that this inhibitory effect is neutralized by factor(s) secreted from astrocytes along the migratory route (14). In contrast to LGE cells, which migrate preferentially ventrally or anteriorly, MGE cells migrate dorsally and spread across most of the dorsal forebrain. Their main target is the developing neocortex (2, 15 18), but they also populate the dorsal striatum (19), amygdala, globus pallidus (2), and hippocampus (20). Interestingly, although MGE cells disperse across a relatively large region within the dorsal brain, they do not migrate into neighboring ventral regions (hypothalamus, preoptic area, septum, or olfactory bulb; ref. 2). The mechanisms restricting and guiding MGE cell migration into neocortex are not well understood. Recently, it has been proposed that MGE cells expressing low levels of semaphorin receptor neuropilin settle preferentially in the striatum (21). In addition, it has been reported that TAG-1 adhesion molecule expressed on corticofugal axons serves as a guidance cue for migrating MGE cells (22). However, many MGE cells migrate within the neocortical lower intermediate (or subventricular) zone, which is poor on corticofugal axons (2), suggesting that additional mechanisms might be involved in the guidance of MGE cells. Here, we demonstrate that ventral forebrain regions neighboring the MGE are nonpermissive for MGE cell migration, whereas the dorsal regions leading to the neocortex are increasingly permissive. We propose that the extent of MGE cell dispersion is dictated by the pattern of permissive and nonpermissive areas in the developing forebrain. Moreover, we show that MGE cell migration is influenced by inhibitory factors secreted from the ventromedial forebrain and chemoattractive factors produced in the developing neocortex. We suggest that this chemotactic gradient might provide the directional information for the dorsal migration within the permissive corridor. Materials and Methods Transplantation in Slice Cultures. Brains were dissected from day-old (E14.5) mouse embryos in L-15 medium (GIBCO). Forebrains were cut transversally into 300- to 400- m-thick sections. Slices containing hypothalamus, MGE, LGE, and cortex were placed on polycarbonate filters (0.4- m pore size; Nuclepore) floating on NB medium [neurobasal medium supplemented with 2 mm L-glutamine, penicillin streptomycin, and B27 supplement (all from GIBCO)]. Germinal zones of MGE were dissected, dissociated, and labeled with PKH26 fluorescent dye (Sigma) as described (2). Labeled MGE cells were collected by centrifugation, and pellets were cut into smaller explants ( 200 m in diameter). MGE explants were inserted into different regions of embryonic brain slices on floating filters. Slices were cultured for 48 h and fixed in 3% paraformaldehyde, and the distribution of migrating MGE cells was analyzed. Spot Assay. Selected brain regions were dissected from E14.5 embryos, collected in Pipes buffer (20 mm Pipes, ph mm NaCl 5 mmkcl 25 mm glucose), and triturated by re- Abbreviations: MGE, medial ganglionic eminence; LGE, lateral ganglionic eminence; SVZ, subventricular zone; E14.5, embryo day Present address: Center for Neurobiology and Behavior, Columbia University, New York, NY Present address: Departments of Visual Science and Molecular Genetics, Institute of Ophthalmology, University College London, London ECIV9EL, United Kingdom. To whom correspondence should be addressed at: Brain Tumor Research Center, Box 0520, University of California, 533 Parnassus Avenue, San Francisco, CA abuylla@itsa.ucsf.edu. NEUROSCIENCE cgi doi pnas PNAS January 21, 2003 vol. 100 no
2 peated pipetting (25 35 times) using P200 pipette tips. Undissociated pieces were left to settle in the bottom of the tube, and dissociated cells were transferred into a new Eppendorf tube and collected by centrifugation (5 min, 800 g). Cells were washed in NB medium supplemented with 5% horse serum and 10 g ml DNase I (Sigma), centrifuged and resuspended at a final concentration of cells per l in L-15 medium with DNase I. Cell suspension was spotted onto polycarbonate filters ( 1 l per spot) floating on NB medium. PKH26-labeled MGE aggregates were placed in the center of each spot, and filters were cultured for 48 h, fixed, and photographed, and the extent of MGE cell migration was analyzed by using NIH IMAGE v1.62 software. For flipped filter assays, cortical and hypothalamic cell suspensions were spotted onto 0.1- m and 0.8- m pore size filters (Nuclepore) and cultured for 7 days on the surface of NB medium supplemented with 5% horse serum. Filters were lifted and gently placed face down in a new 35-mm petri dish, 300 l of fresh NB medium was added to the dishes, and PKH26- labeled MGE reaggregates were deposited on filters opposite to cultured tissue. As a control, some filters were maintained face up and MGE aggregates were placed directly on spots of cultured cells. Coculture Assays. Tested brain regions were dissected from E14.5 mouse embryos and cut into small explants ( m in diameter). MGE germinal zones were dissociated, reaggregated, and cut into explants as described above. MGE explants were embedded in a drop of collagen type I gel (Vitrogen; Cohesion Technologies, Palo Alto, CA) in the proximity ( m) of tested explants. Explants were cocultured for 24 30hinNB medium, fixed with 3% paraformaldehyde, and photographed, and cell migration was analyzed by using the NIH IMAGE software and its macro programming language. (Briefly, the software determined the center of MGE explant and drew through the center two perpendicular lines in such a way that they divided the MGE explant and migrating cells into four quadrants: proximal quadrant facing the tested tissue, distal quadrant facing away, and left and right quadrants. Finally, the software determined the number of MGE cells in each quadrant; calculated the percentage difference between the proximal and distal quadrants and the total number of migrating cells; and exported results to Microsoft EXCEL for further statistical analysis). For some experiments, human embryonic kidney cells (HEK293) were transiently transfected with xslit2 or Slit1 expression vectors (12). Transfected cells were pelleted by centrifugation, and pellets were allowed to reaggregate in culture overnight. Aggregates of HEK293 cells were cut into small explants and used in cocultures with MGE explants as described above. In Situ Hybridization. In situ hybridization, using digoxigeninlabeled riboprobes, was performed on 100- m vibratome sections of E14.5 mouse embryos as described (23). Rat cdnas encoding slit1 and -2 (24) were used as templates for riboprobe synthesis. Fig. 1. Different regions of the developing brain are differentially permissive for MGE cell migration. (A) PKH26-labeled MGE cells (black) were transplanted into neocortex in brain slice cultures (arrow). Forty-eight hours later, many MGE cells had dispersed throughout the neocortex. Note that only a few cells crossed the boundary back to the LGE (broken line). (B) MGE cells grafted into the hypothalamus remained at the graft site (arrow), unable to penetrate into the host brain tissue. (C) Diagram of the spot assay. Dissociated cells (gray) were spotted on polycarbonate membrane floating on the surface of culture medium in a petri dish, and a reaggregate of labeled MGE cells (black) was placed in the center of the spot. Cells were cultured for 48 h. (D) Labeled MGE cells (black) readily disperse through neocortical cells. (E) MGE cells do not migrate into a spot of hypothalamic cells. (F) Quantification of MGE cell migration through spots of different brain regions (number of cells per spot SD). (G) Migration of MGE cells through spots containing mixed neocortical and hypothalamic cells. The percentage indicates the amount of neocortical cells in a particular spot (number of cells per spot SD). NCx, neocortex; HT, hypothalamus; mmge, mantle zone of the MGE; mlge, mantle zone of the LGE. Scale bars 400 m. Results Neocortex Is Selectively Permissive for MGE Cell Migration. Using embryonic brain slices (17) as well as in utero fate mapping (2), we have previously reported that MGE cells migrate to the developing neocortex but avoid the ventromedial forebrain (for the purpose of this study we use the term ventromedial forebrain to describe regions into which MGE cells do not migrate; these regions include hypothalamus, preoptic area, septum, and olfactory bulb). Several mechanisms could explain these observations: (i) the ventral forebrain may be nonpermissive for MGE cell migration, (ii) there may be a nonpermissive boundary between the medial ganglionic eminence and the ventral forebrain, or (iii) ventromedial forebrain is permissive, but directional guidance cues instruct MGE cells to migrate dorsally. To distinguish between these three possibilities, we transplanted labeled E14 MGE cells ectopically into the neocortex and ventromedial forebrain in embryonic brain slices in vitro. After 2 days in vitro, large numbers of labeled cells ( 230 cells per slice) were observed dispersing from MGE grafts placed into the host neocortex, but only rarely did these cells migrate ventrally into the LGE (Fig. 1A). Graft-derived cells were bipolar, with a long, often bifurcated, leading process tipped with a prominent growth cone, a structure typical of tangentially migrating neuronal precursors (25, 26). In contrast, when MGE cells were transplanted into the host hypothalamus, thalamus or septum, none or only few cells migrated into the host tissue (fewer than five cells per slice; Fig. 1B). Occasionally, we observed short processes extending from MGE grafts, suggesting that MGE cells survived within the graft but were unable to migrate into ventromedial forebrain tissue. These results suggested that the cgi doi pnas Wichterle et al.
3 developing diencephalon is largely nonpermissive for MGE cell migration. Increasingly Permissive Pathway Connects MGE with Neocortex. Microtransplantation experiments suggested that different embryonic brain regions have different capacities to support MGE cell dispersion. However, it was difficult to analyze MGE cell migration quantitatively after microtransplantation, because the different tested regions had different shapes and uneven cellular compositions. To assess the capacity of various embryonic brain regions to support migration of MGE cells, we developed a simple and easily quantifiable spot migration assay (Fig. 1 C E). Briefly, the substrate neural tissue was dissected and dissociated, and high-density cell suspension was spotted onto floating filters in culture dishes. Labeled MGE cells were placed in the center of each spot, and the number of MGE cells migrating into the tested tissue was quantified after 48 h in culture. All results obtained from spot assays were confirmed by direct transplantation into brain slices, implying that spot assays can be used as quantitative indicators of permissiveness for MGE cell migration. Of all tested areas, the embryonic neocortex stands out as the best substrate for MGE cell migration ( 500 migrating MGE cells per spot; Fig. 1 D and F). Intermediate migratory support ( cells per spot) was provided by the hippocampus, cerebellum (rhombic lip), LGE, and the mantle region of the LGE (mlge, which consists mainly of the paleocortex; Fig. 1F, data not shown). The mantle region of the MGE (mmge, which consists mainly of the ventral striatum), the entire hypothalamus, thalamus, septum, and midbrain did not support migration of MGE cells ( 50 cells per spot; Fig. 1 E and F, data not shown). These results are consistent with the hypothesis that forebrain regions neighboring the ventral MGE are nonpermissive for MGE cell migration. In contrast, the region between the MGE and the cortex contains cells that provide an increasingly permissive migratory support for MGE cells. Because we observed a graded capacity of different brain tissues to support MGE cell migration we wanted to assess whether different concentrations of permissive and nonpermissive factors could account for this result. We mixed cortical and hypothalamic cells in different ratios and measured the extent of MGE cell migration through the mixed spots. We found that, depending on the ratio of cortical to hypothalamic cells in the mixture, we could gradually change the extent of MGE cell migration, suggesting that neither one of the two environments dominated over the other (Fig. 1G). Thus, differential permissiveness of forebrain tissues could be achieved by changes in the concentration of permissive and or nonpermissive factors in different brain regions. Neocortical Permissive Factors Are Not Diffusible. We wondered whether neocortical factors that permitted the migration of MGE cells are diffusible or associated with neocortical cells. We modified the spot migration assay by culturing neocortical and hypothalamic cells on polycarbonate membranes with either 0.8- m pores (which allow direct cell cell contact across the filter) or 0.1- m pores (which allow only diffusion of secreted molecules). Cells were cultured for 7 days in vitro to allow deposition of extracellular molecules or extension of cellular processes through the pores in filters to the side facing culture medium. After this period, we flipped some of the filters and placed labeled MGE cells on the membrane opposite to the spots (Fig. 2A). As a control, we kept some filters right side up and placed MGE cells directly on the spots of neocortical or hypothalamic cells. Cultured neocortex and hypothalamus retained their specificity with respect to MGE cell migration. MGE cells dispersed through the cultured neocortical cells ( cells per spot) whereas almost no cells migrated through the hypothalamic cells (fewer than five cells per spot). On the flipped Fig. 2. The permissive nature of the neocortex is not mediated by diffusible molecules. (A) Diagram of the flipped spot assay. Dissociated cells were spotted on floating polycarbonate membranes and cultured for 7 days. Membranes were flipped upside down, and reaggregate of MGE cells was placed on the opposite side to the cultured spot of tissue. Cells were cultured for an additional 48 h. (B) PKH26-labeled MGE cells (black) do not migrate when placed opposite to hypothalamic tissue cultured on membranes with 0.8- m pores. (C) Labeled MGE cells (black) disperse when placed opposite to neocortical tissue on membranes with 0.8- m pores, which allow cell cell contact. (D) Higher magnification of cells migrating opposite neocortical spot on 0.8- m membrane. (E) MGE cells do not migrate when placed opposite to neocortical tissue on 0.1- m membranes, which do not allow cell cell contact. NCx, neocortex; HT, hypothalamus. filters, there were no MGE cells migrating either on polycarbonate filters alone or on sides opposite to the hypothalamus regardless of the pore size (Fig. 2B). When MGE cells were placed on flipped 0.8- m filters with cultured neocortical cells, MGE cells migrated throughout the area opposite to the neocortical spots (67 34 cells per spot; Fig. 2 C and D). In contrast, no MGE cell migration was observed on the opposite sides of neocortical tissue cultured on the 0.1- m filter (Fig. 2E). We concluded that MGE cells migrating through the neocortical tissue use migratory substrates associated with the surface of neocortical cells. Chemotactic Guidance of MGE Cells. To test whether long range diffusible factors were involved in the guidance of MGE cells through the permissive corridor, we cocultured MGE explants with pieces of developing neocortex (the target tissue where MGE cells migrate to) and hypothalamus (the neighboring tissue into which MGE cells do not migrate) embedded in collagen gels. To avoid the inherent asymmetry of MGE explants (which is caused by the asymmetrically located ventricular zone cells within explants), we dissociated and reaggregated MGE cells. Aggregates were cut into small explants and embedded next to the explants of tested tissue. The dorsal hypothalamus and lateral neocortex were microdissected into explants that contained mainly the germinal zones, and explants that contained mainly the mantle regions. When MGE aggregates were cultured in the proximity ( 200 m) of the germinal zones from the dorsal hypothalamus, significantly more cells were found in the distal quadrant (53.6%) than in the proximal (46.4%; n 17, t test P 0.03; Fig. 3 A and C). To determine whether factors diffusing from the hypothalamus are NEUROSCIENCE Wichterle et al. PNAS January 21, 2003 vol. 100 no
4 (50 13) than the control distal quadrant (62 12), while the proximal quadrant contained more cells (67 21) as compared with the control proximal quadrant (61 13), we concluded that the neocortex releases a chemoattractive factor. Fig. 3. Diffusible molecules influence MGE cell migration. (A) Coculture of MGE reaggregate with hypothalamic explant. More MGE cells migrate distally than proximally to the explant. (B) Coculture of MGE reaggregate with neocortical explant. More MGE cells migrate proximally than distally. (C) Quantification of the difference in the number of cells found in the distal and proximal quadrant (percentage difference SD). (D) Quantification of the total number of MGE cells migrating out of the explant (number of migrating cells SD). (E) In situ hybridization reveals strong expression of Slit1 in the hypothalamic region (arrow) next to the MGE. Slit1 is also weakly expressed in cortical plate (arrowhead), the target area of MGE cell migration. (F) In situ hybridization reveals strong expression of Slit2 in the hypothalamic region (arrow). (G) Mixing of neocortical cells with HEK293 cells expressing Slit1 or Slit2 does not result in suppression of MGE cell migration in the spot assay (the inhibitory activity of Slit-expressing cells was confirmed in coculture assays) (number of cells per spot SD). NCx, neocortex; HT, hypothalamus. chemorepulsive or inhibitory, we compared the absolute numbers of migrating MGE cells. The total number of neurons emerging from MGE aggregates cultured in the proximity of hypothalamic germinal zones was significantly lower than the number of MGE cells migrating alone in collagen gel or cocultured next to HEK293 cells (human embryonic kidney cell line used as a heterologous cell type, which did not affect MGE cell migration in the coculture assay; P 0.01, t test; Fig. 3D), suggesting that hypothalamic explants reduce the motility of MGE cells. Moreover, the number of MGE cells emerging at the distal quadrant was significantly lower (44 14) than the number of distal MGE cells cocultured with HEK293 cells (62 12; P 0.01, t test). These findings allow us to conclude that factors secreted from the hypothalamus act as inhibitors of MGE cell motility rather than as chemorepulsive factors (in which case more MGE cells would be observed in the distal quadrant as compared with control explants and the total number of migrating cells would be unchanged). When MGE was cultured adjacent to germinal zones of the neocortex, significantly more cells were detected in the proximal (56.6%), compared with the distal, quadrant (43.4%; n 15, P 0.02, t test; Fig. 3 B and C). The total number of cells emerging from MGE aggregates cultured close to the neocortical germinal zones (239 62) was not significantly different from the number of cells emerging from control aggregates (259 45, P 0.7, t test; Fig. 3D). Because the distal quadrant contained fewer cells Slit Factors Are Potential Inhibitory Cues Secreted from the Ventromedial Forebrain. Slit molecules were previously identified as potent chemorepulsive factors guiding axonal outgrowth (23, 27 29) and migration of postnatal SVZ and embryonic LGE cells (11 13). Moreover, it has been shown that Slit factors inhibit postnatal SVZ cell motility (14). We therefore decided to analyze the expression pattern of Slit factors in the developing hypothalamus. It has been reported that both Slit1 and Slit2 are strongly expressed in the embryonic septum (29), preoptic area (23), thalamus, and epithalamus (30, 31), where they act as guidance cues for the olfactory bulb and retinal ganglion cell efferent axons. To complement these studies, we analyzed the expression of Slit molecules in hypothalamic regions directly adjacent to the MGE. In situ hybridization revealed a strong expression of both Slit1 and Slit2 in the periventricular regions in the hypothalamus neighboring the MGE (Fig. 3 E and F). Therefore, we decided to directly test whether MGE cell migration is directional in a gradient of Slit factors. Mouse Slit1 (Slit1) and Xenopus Slit (xslit2) [which is homologous to mouse Slit2 (12)] were transiently expressed in HEK293 cells. When MGE cells were placed next to reaggregates of mock-transfected HEK293 cells, MGE cell migration was symmetrical (Fig. 3C). In contrast, MGE cells cocultured next to cells transfected with either Slit1 or xslit2 migrated asymmetrically (Fig. 3C). This effect was more pronounced with xslit2 (n 17, t test P 0.001). The total number of cells migrating from MGE reaggregates was significantly decreased in cocultures with cells expressing xslit2 as compared with mock-transfected HEK293 cells (P 0.01; Fig. 3D). Similarly, like in cocultures with hypothalamic germinal zones, there were fewer cells in the distal quadrant as compared with controls. These findings demonstrate that MGE cells can respond to a gradient of Slit factors similarly as they respond to hypothalamic germinal zone explants in vitro, indicating that Slit factors secreted from the hypothalamus, preoptic area or septum might provide guidance for MGE cells in vivo. xslit2 Is Not Sufficient To Create a Nonpermissive Environment. The ability of Slit molecules to reduce MGE cell motility prompted us to examine whether Slit factors might be responsible for the nonpermissive nature of the ventromedial forebrain. We analyzed the ability of xslit2 to block MGE migration within cortical cells by using the spot migration assays. When neocortical cells were mixed with mslit1-transfected, xslit2-transfected, or mock transfected HEK293 cells (3:1) and spotted on floating filters, there was no significant difference in the extent of MGE cell migration (Fig. 3G). In contrast, migration of MGE cells in neocortical tissue, which is mixed 3:1 with hypothalamic cells, is reduced by 49% (Fig. 1G). These findings indicate that Slit factors alone are not sufficient to generate the nonpermissive environment encountered in the ventromedial forebrain. Discussion The minimum requirement for cell migration is the presence of a permissive environment that allows random dispersion of migratory cells. The overall extent and shape of cell dispersal can be influenced by boundaries between permissive and nonpermissive environments. Directional migration can be achieved by two general principles: contact guidance and diffusible gradients (32). Contact guidance relies on an increasingly permissive environment connecting the source of migrating cells with the target area. Directional migration within regions of uniform cgi doi pnas Wichterle et al.
5 Fig. 4. Model of the guidance of MGE cells to the neocortex. MGE is ventromedially surrounded with a nonpermissive tissue (dark gray). In contrast, neocortex is the most permissive tissue in the central nervous system for MGE cell migration (light gray). Inhibitory factors are secreted from the ventromedial forebrain (including Slit1 and Slit2), which suppress MGE cell migration in that direction. Neocortex secretes a factor(s) that seems to act as a chemoattractant for migrating MGE cells. NCx, neocortex; HT, hypothalamus. permissiveness can be achieved by superposition of gradients of diffusible guidance cues over the permissive region. In contrast to the relatively extensive body of work focusing on diffusible molecules involved in the guidance of cell migration, the distribution and molecular nature of permissive and nonpermissive substrates in the developing central nervous system remain poorly understood. Here, we have developed a simple assay that facilitated mapping of permissive substrates for MGE cell migration in the developing forebrain. We demonstrated that all major forebrain areas into which MGE cells normally do not migrate are nonpermissive for MGE cells. Moreover, an increasingly permissive corridor leads from MGE to the neocortex. Therefore, permissive and nonpermissive environments might play a primary role in determining the regional specificity and overall direction of MGE cell migration (Fig. 4). The molecular nature of the permissive and nonpermissive substrates has not been determined. However, we show that the permissive substrate used by migrating MGE cells is associated with the surface of neocortical cells. Previous reports suggest that MGE cells use TAG-1 adhesion molecules expressed on the surface of corticofugal axons as a substrate for their migration (22). Recently, we reported that the majority of MGE cells enter the neocortex via a region adjacent to the cortical ventricular zone, but only relatively few cells migrate through the axon-rich upper intermediate zone (2). When labeled MGE cells were cocultured with neocortical explants extending multiple neurites, MGE cells selectively migrated into cell rich neocortical tissue avoiding axonal bundles (H.W. and A.A.-B., unpublished observations). In vitro experiments demonstrated that MGE cells are capable of long distance migration in a complex extracellular substrate (matrigel) as well as within simple collagen type I substrate (17). Therefore, it is likely that, besides TAG-1 adhesion molecule, there are other permissive substrates, possibly produced by cortical subventricular or ventricular zone cells, that are used by tangentially migrating MGE cells. In addition to a selectively permissive environment, directional migration of MGE cells might be facilitated by chemotactic gradients. We present evidence that the neocortex secretes chemoattractive factor(s) and that ventromedial forebrain secretes factor(s) inhibiting cell motility (Fig. 4). We do not know the molecular nature of the chemoattractive factor secreted from the neocortex. It has been shown that neocortex produces hepatocyte growth factor (HGF), which acts as a motogen (stimulates migration) for tangentially migrating cells (33). It remains to be determined whether HGF could also act as a chemoattractant for MGE cells. Slit factors were previously shown to inhibit migration of postnatal SVZ neuronal precursors (29). Similarly, we demonstrate that Slit factors are abundantly expressed in ventromedial forebrain and that these factors mimic the inhibitory activity detected in the dorsal hypothalamus. Besides being strongly expressed in ventromedial forebrain, Slit1 is also weakly expressed in the cortical plate, the target area of MGE cell migration (ref. 11; Fig. 3E). It is likely that either MGE cells lose their responsiveness to Slit factors (34 36), the permissive environment in the neocortex dominates over Slit inhibition, or neocortical tissue blocks Slit signaling (14). All of these possibilities are consistent with the observation that MGE cell migration is not suppressed when neocortical cells are mixed with Slit-expressing HEK293 cells. Interestingly, we observed that the addition of a high concentration of heparin (10 units ml) to culture medium significantly increases (1.9-fold) the number of MGE cells migrating through neocortical tissue in spot assays (H.W. and A.A.-B., unpublished results). One possible explanation of this result is that heparin, which has been shown to disrupt interactions between Slit factors and heparan sulfate proteoglycan glypican-1 (37 39), interferes with Slit-mediated suppression of MGE cell migration normally occurring in the developing neocortex. MGE cells move at high speed over long distances within the developing brain. This behavior allows them to contribute to the histogenesis of distant brain regions like neocortex. This remarkable migratory potential is restricted during development by precise boundaries between the MGE and neighboring brain regions where these cells are not allowed to invade. Boundary formation and parcelation is an important mechanism to control the navigation of both axons and neural cells (32). It will be interesting to identify permissive factors that allow MGE cell migration into the neocortex and to develop animal models in which this migration is disrupted. It has been recognized that defects in neuronal migration can result in severe brain abnormalities and intractable epilepsy (40, 41). It is tempting to speculate that disrupted tangential migration of inhibitory interneurons originating in the MGE contributes to these pathologies (42). The unique migratory potential of MGE cells may also have applications in cell replacement therapies in certain neurodegenerative diseases as MGE cells are the only primary neuronal precursors known to be able to disperse when grafted into the adult brain (17). The present results could help develop methods to steer MGE cells to specific locations after transplantation in the adult brain. We thank Dr. Carol Mason for constructive discussion and critical reading of the manuscript. We thank Cynthia Yaschine for help with the preparation of the manuscript. We thank Dr. Yi Rao for providing us with Slit1 and xslit2 constructs and Dr. Marc Tessier-Lavigne for providing us with probes for Slit1 and Slit2. H.W. was the recipient of a DeWitt Wallace Reader s Digest fellowship. L.E. was the recipient of European Molecular Biology Organization and Human Frontier Science Program fellowships. This work was supported by National Institute of Child Health and Human Development Grant HD32116 (to A.A.-B.). NEUROSCIENCE 1. Olsson, M., Campbell, K., Wictorin, K. & Bjorklund, A. (1995) Neuroscience 69, Wichterle, H., Turnbull, D. H., Nery, S., Fishell, G. & Alvarez-Buylla, A. (2001) Development (Cambridge, U.K.) 128, Corbin, J. G., Gaiano, N., Machold, R. P., Langston, A. & Fishell, G. (2000) Development (Cambridge, U.K.) 127, Yun, K., Potter, S. & Rubenstein, J. L. (2001) Development (Cambridge, U.K.) 128, Wichterle et al. PNAS January 21, 2003 vol. 100 no
6 5. Toresson, H., Potter, S. S. & Campbell, K. (2000) Development (Cambridge, U.K.) 127, Anderson, S. A., Eisenstat, D. D., Shi, L. & Rubenstein, J. L. (1997) Science 278, Altman, J. (1969) J. Comp. Neurol. 137, Lois, C. & Alvarez-Buylla, A. (1993) Proc. Natl. Acad. Sci. USA 90, Lois, C. & Alvarez-Buylla, A. (1994) Science 264, Luskin, M. B. (1993) Neuron 11, Zhu, Y., Li, H., Zhou, L., Wu, J. Y. & Rao, Y. (1999) Neuron 23, Wu, W., Wong, K., Chen, J., Jiang, Z., Dupuis, S., Wu, J. Y. & Rao, Y. (1999) Nature 400, Hu, H. (1999) Neuron 23, Mason, H. A., Ito, S. & Corfas, G. (2001) J. Neurosci. 21, Sussel, L., Marin, O., Kimura, S. & Rubenstein, J. L. (1999) Development (Cambridge, U.K.) 126, Lavdas, A. A., Grigoriou, M., Pachnis, V. & Parnavelas, J. G. (1999) J. Neurosci. 19, Wichterle, H., Garcia-Verdugo, J. M., Herrera, D. G. & Alvarez-Buylla, A. (1999) Nat. Neurosci. 2, Anderson, S. A., Marin, O., Horn, C., Jennings, K. & Rubenstein, J. L. (2001) Development (Cambridge, U.K.) 128, Marin, O., Anderson, S. A. & Rubenstein, J. L. (2000) J. Neurosci. 20, Pleasure, S. J., Anderson, S., Hevner, R., Bagri, A., Marin, O., Lowenstein, D. H. & Rubenstein, J. L. (2000) Neuron 28, Marin, O., Yaron, A., Bagri, A., Tessier-Lavigne, M. & Rubenstein, J. L. (2001) Science 293, Denaxa, M., Chan, C. H., Schachner, M., Parnavelas, J. G. & Karagogeos, D. (2001) Development (Cambridge, U.K.) 128, Erskine, L., Williams, S. E., Brose, K., Kidd, T., Rachel, R. A., Goodman, C. S., Tessier-Lavigne, M. & Mason, C. A. (2000) J. Neurosci. 20, Brose, K., Bland, K. S., Wang, K. H., Arnott, D., Henzel, W., Goodman, C. S., Tessier-Lavigne, M. & Kidd, T. (1999) Cell 96, Kishi, K. (1987) J. Comp. Neurol. 258, Wichterle, H., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1997) Neuron 18, Brose, K. & Tessier-Lavigne, M. (2000) Curr. Opin. Neurobiol. 10, Kidd, T., Bland, K. S. & Goodman, C. S. (1999) Cell 96, Nguyen Ba-Charvet, K. T., Brose, K., Marillat, V., Kidd, T., Goodman, C. S., Tessier-Lavigne, M., Sotelo, C. & Chedotal, A. (1999) Neuron 22, Niclou, S. P., Jia, L. & Raper, J. A. (2000) J. Neurosci. 20, Ringstedt, T., Braisted, J. E., Brose, K., Kidd, T., Goodman, C., Tessier- Lavigne, M. & O Leary, D. D. (2000) J. Neurosci. 20, Tessier-Lavigne, M. & Goodman, C. S. (1996) Science 274, Powell, E. M., Mars, W. M. & Levitt, P. (2001) Neuron 30, Kramer, S. G., Kidd, T., Simpson, J. H. & Goodman, C. S. (2001) Science 292, Rajagopalan, S., Nicolas, E., Vivancos, V., Berger, J. & Dickson, B. J. (2000) Neuron 28, Simpson, J. H., Kidd, T., Bland, K. S. & Goodman, C. S. (2000) Neuron 28, Hu, H. (2001) Nat. Neurosci. 4, Liang, Y., Annan, R. S., Carr, S. A., Popp, S., Mevissen, M., Margolis, R. K. & Margolis, R. U. (1999) J. Biol. Chem. 274, Ronca, F., Andersen, J. S., Paech, V. & Margolis, R. U. (2001) J. Biol. Chem. 276, Allen, K. M. & Walsh, C. A. (1999) Epilepsy Res. 36, Rakic, P. (2000) Adv. Neurol. 84, Anderson, S., Mione, M., Yun, K. & Rubenstein, J. L. (1999) Cereb. Cortex 9, cgi doi pnas Wichterle et al.
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches
 During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Progenitors are Contained within Unique Domains and Tangentially Fixed. EMBRYO ADULT Migratory Behavior
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Progenitors are Contained within Unique Domains and Tangentially Fixed. EMBRYO ADULT Migratory Behavior
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches
 During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Two mayor forms of neuronal migration: Radial and Tangential Leber & Sanes, 95 How do young neurons actually
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Two mayor forms of neuronal migration: Radial and Tangential Leber & Sanes, 95 How do young neurons actually
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
ErbB4 migrazione I parte. 3- ErbB4- NRG1
 ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain
 review Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain Joshua G. Corbin, Susana Nery and Gord Fishell Developmental Genetics Program and the Department of Cell Biology,
review Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain Joshua G. Corbin, Susana Nery and Gord Fishell Developmental Genetics Program and the Department of Cell Biology,
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012
 Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
NIH Public Access Author Manuscript Science. Author manuscript; available in PMC 2011 September 1.
 NIH Public Access Author Manuscript Published in final edited form as: Science. 2010 February 26; 327(5969): 1145 1148. doi:10.1126/science.1183962. Cortical Plasticity Induced by Inhibitory Neuron Transplantation
NIH Public Access Author Manuscript Published in final edited form as: Science. 2010 February 26; 327(5969): 1145 1148. doi:10.1126/science.1183962. Cortical Plasticity Induced by Inhibitory Neuron Transplantation
Prss56, a novel marker of adult neurogenesis in the mouse brain. - Supplemental Figures 1 to 5- Brain Structure and Function
 Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70%
 Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
4/18/2011. Physiology 67 Lecture on Neural Development
 Physiology 67 Lecture on Neural Development 1 2 3 4 5 6 Neural cell categories After the ectodermal tissue has folded into the neural tube, another series of signaling interactions determine the type of
Physiology 67 Lecture on Neural Development 1 2 3 4 5 6 Neural cell categories After the ectodermal tissue has folded into the neural tube, another series of signaling interactions determine the type of
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Cellular Therapies December 9, 2013
 Cellular Therapies December 9, 2013 Scott C. Baraban, Ph.D. University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Neurona Therapeutics Co-founder BioCrea - Consultant
Cellular Therapies December 9, 2013 Scott C. Baraban, Ph.D. University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Neurona Therapeutics Co-founder BioCrea - Consultant
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex?
 Prague Medical Report / Vol. 107 (2006) No. 1, p. 71 80 71) Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex? Šimonová Z., Dutt J. Department of Neuroscience of the Institute
Prague Medical Report / Vol. 107 (2006) No. 1, p. 71 80 71) Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex? Šimonová Z., Dutt J. Department of Neuroscience of the Institute
Central nervous system (CNS): brain and spinal cord Collections of cell body and dendrites (grey matter) are called nuclei/nucleus Nucleus can also
 Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Neocortex. Cortical Structures in the Brain. Neocortex Facts. Laminar Organization. Bark-like (cortical) structures: Shepherd (2004) Chapter 12
 Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
The neuron. Biocytin labeled pyramidal neuron recorded in piriform cortex
 The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
Carl P. Wonders* and Stewart A. Anderson
 The origin and specification of cortical interneurons Carl P. Wonders* and Stewart A. Anderson Abstract GABA-containing interneurons are crucial to both the development and function of the cerebral cortex.
The origin and specification of cortical interneurons Carl P. Wonders* and Stewart A. Anderson Abstract GABA-containing interneurons are crucial to both the development and function of the cerebral cortex.
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Nature Protocols: doi: /nprot Supplementary Figure 1. Effect of different enzymes on CD13 and CD31 populations.
 Supplementary Figure 1 Effect of different enzymes on CD13 and CD31 populations. Comparison of cortex dissociated for 30 minutes with collagenase/dispase digestion (a) or papain (b). Papain degrades the
Supplementary Figure 1 Effect of different enzymes on CD13 and CD31 populations. Comparison of cortex dissociated for 30 minutes with collagenase/dispase digestion (a) or papain (b). Papain degrades the
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex
 The Journal of Neuroscience, May 15, 2002, 22(10):4002 4014 Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex Christopher B. Reid 1,2 and Christopher A. Walsh
The Journal of Neuroscience, May 15, 2002, 22(10):4002 4014 Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex Christopher B. Reid 1,2 and Christopher A. Walsh
Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors
 Article Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors Pierre Flandin, 1 Yangu Zhao, 2 Daniel Vogt, 1 Juhee Jeong, 1 Jason Long, 1,3
Article Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh that Controls the Generation of Interneuron Progenitors Pierre Flandin, 1 Yangu Zhao, 2 Daniel Vogt, 1 Juhee Jeong, 1 Jason Long, 1,3
Sheep Brain Dissection
 Sheep Brain Dissection Mammalian brains have many features in common. Human brains may not be available, so sheep brains often are dissected as an aid to understanding the mammalian brain since he general
Sheep Brain Dissection Mammalian brains have many features in common. Human brains may not be available, so sheep brains often are dissected as an aid to understanding the mammalian brain since he general
THE ORGANIZATION OF AMYGDALOPETAL PROJECTIONS FROM THE LATERAL HYPOTHALAMUS AND PREOPTIC AREA IN THE RAT
 ACTA NEUROBIOL. EXP. 1977, 37: 247-252 THE ORGANIZATION OF AMYGDALOPETAL PROJECTIONS FROM THE LATERAL HYPOTHALAMUS AND PREOPTIC AREA IN THE RAT Liliana NITECKA, Olgierd NARKIEWICZ and Czeslaw JAKIEL Department
ACTA NEUROBIOL. EXP. 1977, 37: 247-252 THE ORGANIZATION OF AMYGDALOPETAL PROJECTIONS FROM THE LATERAL HYPOTHALAMUS AND PREOPTIC AREA IN THE RAT Liliana NITECKA, Olgierd NARKIEWICZ and Czeslaw JAKIEL Department
Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain
 Development 128, 527-540 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV1621 527 Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain Susana
Development 128, 527-540 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV1621 527 Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain Susana
The neuron. Biocytin labeled pyramidal neuron recorded in piriform cortex
 The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution
 Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain
 Supplementary Information Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain Yuko Okamura-Oho a, b, *, Kazuro Shimokawa c, Masaomi Nishimura b,
Supplementary Information Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain Yuko Okamura-Oho a, b, *, Kazuro Shimokawa c, Masaomi Nishimura b,
Recipes for Making Human Interneurons from Stem Cells Require Multiple Factors, Careful Timing, and Long Maturation Periods
 Current Literature In Basic Science Recipes for Making Human Interneurons from Stem Cells Require Multiple Factors, Careful Timing, and Long Maturation Periods Directed Differentiation and Functional Maturation
Current Literature In Basic Science Recipes for Making Human Interneurons from Stem Cells Require Multiple Factors, Careful Timing, and Long Maturation Periods Directed Differentiation and Functional Maturation
Neuronal migration in the adult brain: are we there yet?
 Neuronal migration in the adult brain: are we there yet? H. Troy Ghashghaei*, Cary Lai and E. S. Anton* Abstract The generation and targeting of appropriate numbers and types of neurons to where they are
Neuronal migration in the adult brain: are we there yet? H. Troy Ghashghaei*, Cary Lai and E. S. Anton* Abstract The generation and targeting of appropriate numbers and types of neurons to where they are
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
9.14 Classes #21-23: Visual systems
 9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion
 ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Development of the Central Nervous System
 Development of the Central Nervous System an ongoing process, through adolescence and maybe even adult hood? the nervous system is plastic Experience plays a key role Dire consequences when something goes
Development of the Central Nervous System an ongoing process, through adolescence and maybe even adult hood? the nervous system is plastic Experience plays a key role Dire consequences when something goes
Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling
 Bjorke et al. Neural Development (2016) 11:18 DOI 10.1186/s13064-016-0073-y RESEARCH ARTICLE Open Access Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling Brielle Bjorke
Bjorke et al. Neural Development (2016) 11:18 DOI 10.1186/s13064-016-0073-y RESEARCH ARTICLE Open Access Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling Brielle Bjorke
Neuroanatomy. Assistant Professor of Anatomy Faculty of Medicine The University of Jordan Dr Maha ELBeltagy
 Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
New Neurons Clear the Path of Astrocytic Processes for Their Rapid Migration in the Adult Brain
 Please cite this article in press as: Kaneko et al., New s Clear the Path of Astrocytic Processes for Their Rapid Migration in the Adult Brain, (2010), doi:10.1016/j.neuron.2010.06.018 Ar ticle New s Clear
Please cite this article in press as: Kaneko et al., New s Clear the Path of Astrocytic Processes for Their Rapid Migration in the Adult Brain, (2010), doi:10.1016/j.neuron.2010.06.018 Ar ticle New s Clear
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture
 Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
1. The basic anatomy of the Central Nervous System (CNS)
 Psyc 311A, fall 2008 Conference week 1 Sept 9 th to 11 th TA: Jürgen Germann; e-mail: jurgen.germann@mcgill.ca Overview: 1. The basic anatomy of the Central Nervous System (CNS) 2. Cells of the CNS 3.
Psyc 311A, fall 2008 Conference week 1 Sept 9 th to 11 th TA: Jürgen Germann; e-mail: jurgen.germann@mcgill.ca Overview: 1. The basic anatomy of the Central Nervous System (CNS) 2. Cells of the CNS 3.
Behavioral and Motivational mechanisms of Brain. Limbic system and the Hypothalamus
 Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
The Nervous System 7PART B. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7PART B What is a reflex? What is a reflex? What is meant by the statement that
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7PART B What is a reflex? What is a reflex? What is meant by the statement that
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A
 MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
Introduction to the Central Nervous System: Internal Structure
 Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
EGFRs mediate chemotactic migration in the developing telencephalon
 Development 128, 4203-4216 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV9789 4203 EGFRs mediate chemotactic migration in the developing telencephalon Damira Caric, Heather
Development 128, 4203-4216 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV9789 4203 EGFRs mediate chemotactic migration in the developing telencephalon Damira Caric, Heather
Yasuhiko Matsumori, Jialing Liu, Philip R. Weinstein, Takamasa Kayama ABSTRACT
 Yamagata Med J 2003 21 2) 171-175 Yasuhiko Matsumori, Jialing Liu, Philip R. Weinstein, Takamasa Kayama Department of Neurosurgery, Yamagata University School of Medicine, Yamagata, Japan Department of
Yamagata Med J 2003 21 2) 171-175 Yasuhiko Matsumori, Jialing Liu, Philip R. Weinstein, Takamasa Kayama Department of Neurosurgery, Yamagata University School of Medicine, Yamagata, Japan Department of
nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
 Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo
 Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo Stewart A. Anderson 2, Christine E. Kaznowski 1, Carrie Horn, John L.R. Rubenstein and Susan K. McConnell 1 Department of Psychiatry,
Distinct Origins of Neocortical Projection Neurons and Interneurons In Vivo Stewart A. Anderson 2, Christine E. Kaznowski 1, Carrie Horn, John L.R. Rubenstein and Susan K. McConnell 1 Department of Psychiatry,
CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain
 Research article 565 CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain Christopher Gregg and Samuel Weiss* Genes and Research
Research article 565 CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain Christopher Gregg and Samuel Weiss* Genes and Research
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Cortical Organization. Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:.
 Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
The human brain. of cognition need to make sense gives the structure of the brain (duh). ! What is the basic physiology of this organ?
 The human brain The human brain! What is the basic physiology of this organ?! Understanding the parts of this organ provides a hypothesis space for its function perhaps different parts perform different
The human brain The human brain! What is the basic physiology of this organ?! Understanding the parts of this organ provides a hypothesis space for its function perhaps different parts perform different
Gene co-expression networks in the mouse, monkey, and human brain July 16, Jeremy Miller Scientist I
 Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Development of Brain Stem, Cerebellum and Cerebrum
 Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
4) Modification of Development by Sensory Experience (Nurture)
 Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Development and specification of GABAergic cortical interneurons
 Kelsom and Lu Cell & Bioscience 2013, 3:19 Cell & Bioscience REVIEW Development and specification of GABAergic cortical interneurons Corey Kelsom and Wange Lu * Open Access Abstract GABAergic interneurons
Kelsom and Lu Cell & Bioscience 2013, 3:19 Cell & Bioscience REVIEW Development and specification of GABAergic cortical interneurons Corey Kelsom and Wange Lu * Open Access Abstract GABAergic interneurons
Axon Guidance. Matthew Blewitt
 Axon Guidance Matthew Blewitt Overview Axonal development PNS regeneration CNS regeneration Future directions Brain Repair Axonal Development Neurite growth cone www.anatomy.unimelb.edu.au http://kalil.anatomy.wisc.edu/articles/kalil_dent_curropneurobiol_2005.pdf
Axon Guidance Matthew Blewitt Overview Axonal development PNS regeneration CNS regeneration Future directions Brain Repair Axonal Development Neurite growth cone www.anatomy.unimelb.edu.au http://kalil.anatomy.wisc.edu/articles/kalil_dent_curropneurobiol_2005.pdf
The nervous system regulates most body systems using direct connections called nerves. It enables you to sense and respond to stimuli
 The nervous system regulates most body systems using direct connections called nerves. It enables you to sense and respond to stimuli The basic function of nervous system are: Receive sensory input internal
The nervous system regulates most body systems using direct connections called nerves. It enables you to sense and respond to stimuli The basic function of nervous system are: Receive sensory input internal
Detailed protocol Only dissected human brain samples are stored. The microdissection is performed on frozen brains and the samples are kept on -70 C.
 2008 Detailed protocol Only dissected human brain samples are stored. The microdissection is performed on frozen brains and the samples are kept on -70 C. BrainNet Europe II Project Co-ordinator: Prof.
2008 Detailed protocol Only dissected human brain samples are stored. The microdissection is performed on frozen brains and the samples are kept on -70 C. BrainNet Europe II Project Co-ordinator: Prof.
Differential Expression of COUP-TFI, CHL1, and Two Novel Genes in Developing Neocortex Identified by Differential Display PCR
 The Journal of Neuroscience, October 15, 2000, 20(20):7682 7690 Differential Expression of COUP-TFI, CHL1, and Two Novel Genes in Developing Neocortex Identified by Differential Display PCR Qing Liu,*
The Journal of Neuroscience, October 15, 2000, 20(20):7682 7690 Differential Expression of COUP-TFI, CHL1, and Two Novel Genes in Developing Neocortex Identified by Differential Display PCR Qing Liu,*
Seafood Contaminants causing Foodborne Diseases
 Seafood Contaminants causing Foodborne Diseases Bacteria and Viruses Vibrio species Viruses: Norwalk-like (NLV) and Hepatitis A (HAV) Industrial Compounds Mercury Polyclorinated biphenyls (PCBs) Natural
Seafood Contaminants causing Foodborne Diseases Bacteria and Viruses Vibrio species Viruses: Norwalk-like (NLV) and Hepatitis A (HAV) Industrial Compounds Mercury Polyclorinated biphenyls (PCBs) Natural
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Anterior Olfactory Nucleus
 A Anterior Olfactory Nucleus PETER C. BRUNJES, KURT R. ILLIG Department of Psychology, University of Virginia Charlottesville, VA, USA Synonyms Anterior olfactory cortex Definition The primary component
A Anterior Olfactory Nucleus PETER C. BRUNJES, KURT R. ILLIG Department of Psychology, University of Virginia Charlottesville, VA, USA Synonyms Anterior olfactory cortex Definition The primary component
Integration of GABAergic Interneurons into Cortical Cell Assemblies: Lessons from Embryos and Adults
 Integration of GABAergic Interneurons into Cortical Cell Assemblies: Lessons from Embryos and Adults Giorgia Bartolini, 1,2 Gabriele Ciceri, 1,2 and Oscar Marín 1, * 1 Instituto de Neurociencias, Consejo
Integration of GABAergic Interneurons into Cortical Cell Assemblies: Lessons from Embryos and Adults Giorgia Bartolini, 1,2 Gabriele Ciceri, 1,2 and Oscar Marín 1, * 1 Instituto de Neurociencias, Consejo
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 5 Due: Tuesday, Nov. 24, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 5 Due: Tuesday, Nov. 24, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
COGNITIVE SCIENCE 107A MIDTERM EXAM 1 - FALL Name: PID: Total Pts: /100pts
 COGNITIVE SCIENCE 107A MIDTERM EXAM 1 - FALL 2009 Name: PID: Total Pts: /100pts I. SHORT ANSWERS (5 points each for a total of 30 points) 1. Label the three meningeal layers in the following diagram. Describe
COGNITIVE SCIENCE 107A MIDTERM EXAM 1 - FALL 2009 Name: PID: Total Pts: /100pts I. SHORT ANSWERS (5 points each for a total of 30 points) 1. Label the three meningeal layers in the following diagram. Describe
Basal Ganglia. Steven McLoon Department of Neuroscience University of Minnesota
 Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
strain), were tested by growing explants of neural fold from the posterior Woronzowa,2' 3 implanting pituitaries in postlarval stages, observed
 VOL. 35, 1949 GENETICS: H. C. DALTON 277 DE VELOPMENTAL ANAL YSIS OF GENETIC DIFFERENCES IN PIGMENTATION IN THE AXOLOTL By H. CLARK DALTON DEPARTMENT OF GENETICS, CARNEGIE INSTITUTION OF WASHINGTON, COLD
VOL. 35, 1949 GENETICS: H. C. DALTON 277 DE VELOPMENTAL ANAL YSIS OF GENETIC DIFFERENCES IN PIGMENTATION IN THE AXOLOTL By H. CLARK DALTON DEPARTMENT OF GENETICS, CARNEGIE INSTITUTION OF WASHINGTON, COLD
Supplementary Figure S1: Pial and periventricular vascular networks and the dual GABA neuron streams of the early embryonic mouse telencephalon
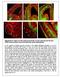 Supplementary Figure S1: Pial and periventricular vascular networks and the dual GABA neuron streams of the early embryonic mouse telencephalon a) The profile of GABA neurons arriving at the pallial-subpallial
Supplementary Figure S1: Pial and periventricular vascular networks and the dual GABA neuron streams of the early embryonic mouse telencephalon a) The profile of GABA neurons arriving at the pallial-subpallial
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Lecture 42: Final Review. Martin Wessendorf, Ph.D.
 Lecture 42: Final Review Martin Wessendorf, Ph.D. Lecture 33 cortex Heilbronner 5 lobes of the cortex Lateral view (left side) Mid-saggital view (right side) Cellular organization of cortex White matter
Lecture 42: Final Review Martin Wessendorf, Ph.D. Lecture 33 cortex Heilbronner 5 lobes of the cortex Lateral view (left side) Mid-saggital view (right side) Cellular organization of cortex White matter
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Lori Sussel 1, *, Oscar Marin 1, Shioko Kimura 2 and John L. R. Rubenstein 1, SUMMARY
 Development 126, 3359-3370 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8607 3359 Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification
Development 126, 3359-3370 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8607 3359 Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification
Replacement of Nerve-Growth Factor by Ganglionic Non-Neuronal Cells for the Survival In Vitro of Dissociated Ganglionic Neurons (culture neuroglia)
 Proc. Nat. Acad. Sci. USA VoL 69, No. 12, pp. 3556-3560, December 1972 Replacement of Nerve-Growth Factor by Ganglionic Non-Neuronal Cells for the Survival In Vitro of Dissociated Ganglionic Neurons (culture
Proc. Nat. Acad. Sci. USA VoL 69, No. 12, pp. 3556-3560, December 1972 Replacement of Nerve-Growth Factor by Ganglionic Non-Neuronal Cells for the Survival In Vitro of Dissociated Ganglionic Neurons (culture
Cellular components of CNS
 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain
 Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain E S Anton 1,8, H T Ghashghaei 1,8, Janet L Weber 2, Corey McCann 1, Tobias M Fischer 2, Isla D Cheung
Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain E S Anton 1,8, H T Ghashghaei 1,8, Janet L Weber 2, Corey McCann 1, Tobias M Fischer 2, Isla D Cheung
Nervous System. Lecture 4
 Nervous System Lecture 4 Neurons Functional unit of the nervous system Also called the nerve cell Soma or body Axon Dendrites Neuroglial cells support cells Schwann cells produce myelin in PNS Oligodendrocytes
Nervous System Lecture 4 Neurons Functional unit of the nervous system Also called the nerve cell Soma or body Axon Dendrites Neuroglial cells support cells Schwann cells produce myelin in PNS Oligodendrocytes
axion Protocol Cell Culture on Microelectrode Arrays Cell Type: QBM Cell Science - E14,15 Embryonic C57 Mouse Cortical Neurons BioSystems v. 1.
 axion BioSystems Cell Culture on Microelectrode Arrays Cell Type: QBM Cell Science - E14,15 Embryonic C57 Mouse Cortical Neurons Protocol v. 1.1 Trademarks Axion BioSystems, Inc. and the logo are trademarks
axion BioSystems Cell Culture on Microelectrode Arrays Cell Type: QBM Cell Science - E14,15 Embryonic C57 Mouse Cortical Neurons Protocol v. 1.1 Trademarks Axion BioSystems, Inc. and the logo are trademarks
Organization of the nervous system 2
 Organization of the nervous system 2 Raghav Rajan Bio 334 Neurobiology I August 22nd 2013 1 Orienting within the brain absolute axes and relative axes SUPERIOR (above) ANTERIOR (in front) Anterior/Posterior,
Organization of the nervous system 2 Raghav Rajan Bio 334 Neurobiology I August 22nd 2013 1 Orienting within the brain absolute axes and relative axes SUPERIOR (above) ANTERIOR (in front) Anterior/Posterior,
PHYSIOLOGY of LIMBIC SYSTEM
 PHYSIOLOGY of LIMBIC SYSTEM By Dr. Mudassar Ali Roomi (MBBS, M.Phil.) Assistant Professor Physiology Limbic system: (shown in dark pink) Limbic = border Definition: limbic system means the entire neuronal
PHYSIOLOGY of LIMBIC SYSTEM By Dr. Mudassar Ali Roomi (MBBS, M.Phil.) Assistant Professor Physiology Limbic system: (shown in dark pink) Limbic = border Definition: limbic system means the entire neuronal
