Supplemental information
|
|
|
- Paul Osborne
- 5 years ago
- Views:
Transcription
1 Supplemental information Frequent reconstitution of IDH2 mutant clonal multilineage hematopoiesis following chemotherapy for acute myeloid leukemia Daniel H. Wiseman, Emma L. Williams, Deepti P. Wilks, Hui Sun Leong, Tim D. D. Somerville, Michael W. Dennis, Eduard A. Struys, Abdellatif Bakkali, Gajja S. Salomons and Tim C. P. Somervaille Figure S1. Sequential IDH1 mutant allele frequencies in AML patients achieving morphologic CR. IDH1 MAFs at presentation, at complete morphologic remission and following completion of intensive chemotherapy in six IDH1 mut AML patients treated with chemotherapy alone. Remission status is indicated (CR1, first complete remission; Rel, relapsed). MAFs were determined by digital PCR. Biobank identifiers are shown. 1
2 IDH1 IDH2 DNMT3A ASXL1 BCOR NPM1 R132 S R132 H R132 H R132 H R132 H R132 G R132 C R132 C R132 C FLT3 ITD TKD ITD ITD ITD NRAS KRAS PTPN11 CSF3R JAK2 CBL SRSF2 SF3B1 U2AF1 ZRSR2 RUNX1 GATA2 CEBPA STAG2 RAD21 TP53 PTEN PHF6 FBXW7 R132 C R172 K R172 K Figure S2. Mutation heat map. Concomitantly mutated genes in presentation samples of AML (n=21) or RAEB (n=2) patients exhibiting an IDH1 or IDH2 mutation who achieved complete morphologic remission following chemotherapy. 2
3 IDH MAF (Digital PCR) R 2 = P < IDH MAF (TruSight Myeloid NGS) Figure S3. Concordance of digital PCR and targeted next generation sequencing (TruSight Myeloid Panel) for measurement of IDH mutant allele frequency. Eighty BM or blood samples were tested by both methods. R 2 = Pearson correlation coefficient. Digital PCR assays were previously demonstrated as quantitative to 0.1%. 8 Loss of concordance at lower MAFs reflects the impact of systematic high throughput sequencing noise. S1 3
4 Figure S4. Somatic mutations identified by targeted next generation sequencing (TruSight Myeloid Panel) at presentation and onset of complete morphological remission in IDH1-mutated patients. Red circles show MAFs at presentation. Blue squares show MAFs at CR1 following one or two cycles of chemotherapy. [X] indicates location of gene on the X chromosome. 4
5 Figure S5. Somatic mutations identified by targeted next generation sequencing (TruSight Myeloid Panel) at presentation and onset of complete morphological remission in IDH2-mutated patients. Red circles show MAFs at presentation. Blue squares show MAFs at CR1 following one or two cycles of chemotherapy. [X] indicates location of gene on the X chromosome. 5
6 Figure S6. Sequential mutant allele frequencies in selected IDH2 mutated patients in CR1. Graphs show MAFs for somatic mutations identified by targeted next generation sequencing (TruSight Myeloid Panel) at presentation, onset of complete morphological remission and at the indicated follow up time points during sustained remissions in IDH2-mutated patients. 6
7 Figure S7. Gating strategies and outcomes for flow sorting experiments. Flow cytometry plots show gating strategies for sorting experiments with (a) blood mononuclear cells, (b) bone marrow CD34 - cells and (c) bone marrow immunophenotypic hematopoietic stem cells (HSC) and multipotent progenitor cells (MPP). Purities of sorted populations from (a) and (b) were %, as determined by morphological analysis of cytospin preparations and post-sort immunophenotypic analyses. In view of the small number of sorted cells in (c) post-sort analyses were not possible. 7
8 Figure S8. Multilineage contribution of IDH1 R132G mutant clonal hematopoiesis in remission. Graphs show IDH1 R132G versus NPM1 MAFs at the indicated time points and in the indicated cell populations in patient BB355. The remission sample was collected 22 months following presentation. 8
9 Table S1. Characteristics and outcome data for an IDH-mutant AML/RAEB cohort achieving complete morphologic remission (n=23). ID # Age/ Gender Disease Blast (%) IDH MUTATION Gene CDS AA MAF (%) Karyotype 85 66/M RAEB 13% M IDH2 c.419g>a p M Normal 93 58/M AML 35% M IDH2 c.515g>a p.r172k 25.7 M Normal /F AML 97% M IDH1 c.394c>a p.r132s 47.2 M Normal /M AML 28% M IDH2 c.419g>a p B Normal /M RAEB 14% M IDH1 c.394c>t p.r132c 7.3 B del(20q) /M AML 80% M IDH2 c.419g>a p B Normal Treatment ADE x2 CR1 Rel1: Azacitidine ADE x1 (Ref) FLAGIda x1 CR1; HSCT in CR1 DA x2 CR1 Rel1: FLAGIda x1 + HDAC x1 CR2; (unfit for HSCT) Rel2: Azacitidine ADE+GO (Ref) Clofarabine x1 CR1; HSCT in CR1 DA+GO x1, FLAGIda x1 CR1; HSCT in CR1 Rel1: Azacitidine ADE+GO x1, ADE x1 CR1; HSCT in CR1 Follow Up (Months) /F AML 80% M IDH2 c.419g>a p M Normal ADE+GO x1, ADE x1, HDAC x2 CR /M AML 62% M IDH1 c.394c>t p.r132c 26.0 B +8 DA x2 CR1; HSCT in CR /M AML 20% M IDH1 c.395g>a p.r132h 19.9 B Normal /M AML 44% M IDH1 c.394c>t p.r132c 3.0 B Normal DA x2, HDAC x2 CR1 Rel1: FLAGIda x1 CR2; HSCT in CR2 DA x1 CR1 (unfit for further intensive chemotherapy) Rel1: Azacitidine /F AML 90% M IDH2 c.419g>a p M Normal DA x2, HDAC x2 CR /F AML 93% M IDH1 c.395g>a p.r132h 49.1 M Normal DA x2, HDAC x2 CR /M AML 56% M IDH2 c.515g>a p.r172k 37.3 M Normal /F AML 65% M IDH2 c.419g>a p M Normal DA (refractory) FLAGIda x2 CR1; (no HSCT donor option) Rel1: Supportive care DA x1, HDAC x2 CR1 Rel1: Azacitidine + Vorinostat (ongoing) Outcome Died (Relapse) Died (Relapse) Died (Relapse) Died (in CR1): Early HSCT complications Died (Relapse) Died (in CR1): Late HSCT complications (CR2) Died (Relapse) (Relapse) (Relapse) /M AML 82% M IDH1 c.394c>g p.r132g 38.6 B Normal DA x1 CR
10 (unfit for further intensive chemotherapy) /M AML 50% M IDH2 c.419g>a p B Normal DA x2, HDAC x1 CR /M AML 36% M IDH2 c.419g>a p B /M AML 23% M IDH1 c.394c>t p.r132c 10.6 B Normal DA (refractory) FLAGIda x2 CR1; (no HSCT donor option) DA x2, HDAC x1 CR1; HSCT in CR /M AML 35% M IDH2 c.419g>a p M Normal DA x2, HDAC x2 CR /M AML 95% B IDH2 c.419g>a p B Normal DA x2, HDAC x2 CR1; Rel1: FLAGIda x1 CR2; (HSCT in CR2 planned but early relapse) Rel2: Sorafenib /M AML 39% M IDH2 c.419g>a p M +Y LDAC x7 CR1 after 2 nd cycle (ongoing) /M AML 35% M IDH1 c.395g>a p.r132h 6.4 B Normal DA+Ganetespib x3 CR /M AML 95% M IDH1 c.395g>a p.r132h 43.6 M Normal DA x2 CR1 (ongoing); (Awaiting HSCT in CR1) (Relapse) For blast percentages and IDH mutant allele frequencies (MAF) the results from the presentation bone marrow ( M ) or blood sample ( B ) are shown. Blood samples were used for analyses only where a bone marrow test was not performed at presentation, or where there was insufficient surplus bone marrow sample for biobanking. Blast percentages are from morphologic analysis of bone marrow or blood smears. IDH MAF was determined by digital PCR. Treatment regimens: ADE: cytarabine, daunorubicin, etoposide; DA: daunorubicin, cytarabine; HDAC: high dose cytarabine; LDAC: low dose cytarabine; FLAGIda: fludarabine, cytarabine, idarubicin, granulocyte-colony stimulating factor; GO: gemtuzumab ozogamicin; HSCT: hematopoietic stem cell transplant. Disease status is recorded on 1 st February All deaths were due to relapsed/refractory disease unless indicated. Other abbreviations: AML = acute myeloid leukemia; RAEB = refractory anemia with excess blasts; CR = complete remission; Rel = relapsed disease. 10
11 Table S2. Blood counts of individuals with IDH-mutant clonal hematopoiesis during sustained remission, at the latest time point indicated in Figure 1f. All values are within age and sex specific reference ranges except those in pink colored boxes, where normal ranges are shown. Biobank ID IDH2 Hemoglobin (g/l) Total white cell count (x10 9 /l) Neutrophils (x10 9 /l) Monocytes (x10 9 /l) Platelets (x10 9 /l) BB ( ) ( ) 331 BB BB BB ( ) BB BB IDH1 R132H BB IDH1 R132G BB
12 Table S3: Putative somatic mutations identified in 23 RAEB/AML genomes by targeted resequencing using Illumina TruSight Myeloid Panel. Shown for each genomic variant are genomic location, the consequence of the base change on the protein coding sequence, the COSMIC ID (if found in the Catalogue of Somatic Mutations in Cancer Database) S2 and the calculated mutant allele frequency (MAF%) at presentation and remission (after one or two cycles of induction chemotherapy). ID # Chr Position Gene Variant Type CDS AA Change COSMIC PRESENTATION REMISSION Reads Variant MAF% Reads Variant MAF% IDH2 C>T Missense c.419g>a p. COSM DNMT3A G>A Missense c.2644c>t p.r882c COSM X BCOR -AC/ +AT CBL -AT Complex frameshift Frameshift del (truncation) c.3266_3267gt >ATCA c.1131_1132del AT p.r1089hfs*25 COSM p.f378pfs* FBXW7 A>T Missense c.1336t>a p.w446r X PHF6 G>A Missense c.421g>a p.d141n COSM RUNX1 C>T Missense c.611g>a p.r204 COSM SRSF2 G>A Missense c.284c>t p.p95l COSM X STAG2 A>G Missense c.185a>g p.k62r IDH2 C>T Missense c.515g>a p.r172k COSM SF3B1 T>C Missense c.2098a>g p.k700e COSM U2AF1 G>A Missense c.101c>t p.s34f COSM DNMT3A T>C Missense c.1403a>g p.k468r COSM IDH1 G>T Missense c.394c>a p.r132s COSM NPM1 +TC DNMT3A C>T FLT3 +36bp +56bp +64bp CEBPA -GTCGAGAC Splice donor variant p.w288cfs*12 COSM c g>a n/a ITD (x3) n/a n/a (ITD) - n/a n/a 7.21 n/a n/a 0.00 Frameshift del (truncation) c.180_189delgt CCATCGAC p.i62afs*95 COSM PTEN T>C Missense c.712t>c p.f238l COSM RAD21 A>T Missense c.463t>a p.l155i X STAG2 G>A Missense c.3134g>a p.r1045 COSM
13 IDH2 C>T Missense c.419g>a p. COSM DNMT3A C>G Missense c.2095g>c p.g699r NPM1 +TC p.w288cfs*12 COSM IDH1 G>A Missense c.394c>t p.r132c COSM DNMT3A A>C Missense c.2251t>g p.f751v DNMT3A A>G Missense c.2264t>c p.f755s U2AF1 G>A Missense c.101c>t p.s34f X BCOR +T ASXL1 +G c.2264dupa p.y755* c.1926_1927ins G p.g646wfs*12 COSM IDH2 C>T Missense c.419g>a p. COSM DNMT3A G>A Missense c.2644c>t p.r882c COSM SRSF2 G>A Missense c.284c>t p.p95l COSM X ZRSR2 G>A Missense c.61g>a p.a21t IDH2 C>T Missense c.419g>a p. COSM NPM1 +TC p.w288cfs*12 COSM SRSF2 G>T Missense c.284c>g p.p95r COSM CEBPA G>A Missense c.41c>t p.p14l GATA2 -C Frameshift del (truncation) c.599delg p.g200vfs*18 COSM IDH1 G>A Missense c.394c>t p.r132c COSM DNMT3A C>T Missense c.2645g>a p.r882h COSM X BCOR G>A Missense c.2729c>t p.p910l RUNX1 +AGGA c.1237_1240dup TCCT p.y414ffs* SRSF2 G>T Missense c.284c>g p.p95r COSM IDH1 C>T Missense c.395g>a p.r132h COSM DNMT3A C>T Missense c.2645g>a p.r882h COSM GATA2 C>G Missense c.469g>c p.a157p NPM1 +TC p.w288cfs*12 COSM RUNX1 A>C Missense c.469g>c p.v419g NRAS C>T Missense c.38g>a p.g13d COSM IDH1 G>A Missense c.394c>t p.r132c COSM
14 DNMT3A C>T Missense c.1990g>a p.e664k COSM X STAG2 +ACT c.3435_3436ins ACA p.h1148lfs* TP53 A>T Stop-gain c.1043t>a TP53 L348* COSM IDH2 C>T Missense c.419g>a p. COSM NPM1 +TC p.w288cfs*12 COSM FLT3 +31bp ITD n/a n/a (ITD) - n/a n/a n/a n/a IDH1 C>T Missense c.395g>a p.r132h NPM1 +TC p.w288cfs*12 COSM DNMT3A G>A Stop-gain c.1792c>t p.r598* COSM CSF3R A>G Missense c.2420t>c p.l807p IDH2 C>T Missense c.515g>a p.r172k COSM DNMT3A C>T Missense c.1904g>a p.r IDH2 C>T Missense c.419g>a p. COSM DNMT3A C>T Missense c.2645g>a p.r882h COSM RUNX1 +CC c.366_367dupg G p.d123gfs11* COSM NRAS C>A Missense c.34g>t p.g12c COSM CEBPA -G Frameshift del (truncation) c.590delc p.p197rfs* CSF3R G>A Missense c.1853c>t p.t618i ASXL1 +G c.1926_1927ins G p.g646wfs*12 COSM IDH1 G>C Missense c.394c>g p.r132g COSM NPM1 +TC p.w288cfs*12 COSM FLT3 +61bp ITD n/a n/a (ITD) - n/a n/a n/a n/a IDH2 C>T Missense c.419g>a p. COSM NPM1 +TC p.w288cfs*12 COSM SRSF2 G>A Missense c.284c>t p.p95l COSM IDH2 C>T Missense c.419g>a p. COSM RUNX1 +A c.1047dupt p.p350fs* SRSF2 G>T Missense c.284c>g p.p95r COSM X BCOR G>A Stop-gain c.4540c>t p.r1514* COSM IDH1 G>A Missense c.394c>t p.r132c COSM
15 CSF3R C>T Missense c.2429g>a p.s810n DNMT3A C>T Missense c.2645g>a p.r882h COSM RUNX1 C>T Missense c.602g>a p.r201 COSM SRSF2 G>T Missense c.284c>g p.p95r COSM IDH2 C>T Missense c.419g>a p. COSM DNMT3A A>C Missense c.1640t>g p.l547r NPM1 +TC p.w288cfs*12 COSM PTPN11 C>T Missense c.1505c>t p.s502l PTPN11 G>A Missense c.1508g>a p.g503e COSM X BCOR C>A Missense c.757g>t p.v253f X BCOR A>G Missense c.794t>c p.m265t IDH2 C>T Missense c.419g>a p. COSM DNMT3A C>T Missense c.2645g>a p.r882h COSM NPM1 +TC p.w288cfs*12 COSM FLT3 +41bp ITD n/a n/a (ITD) - n/a n/a n/a n/a RAD21 G>A Missense c.1631c>t p.a544v IDH2 C>T Missense c.419g>a p. COSM JAK2 G>T Missense c.1849g>t p.v617f COSM NPM1 +TC p.w288cfs*12 COSM IDH1 C>T Missense c.395g>a p.r132h COSM DNMT3A G>C Missense c.2141c>g p.s714c COSM FLT3 T>C Missense c.2516a>g p.d839g COSM KRAS T>A Missense c.182a>t p.61l COSM NPM1 +TC p.w288cfs*12 COSM PTPN11 G>C Missense c.181g>c p.d61h COSM IDH1 C>T Missense c.395g>a p.r132h COSM NPM GAGGAAG/ +GAAGTTTCTC GCC Complex frameshift c.868_876ga GGAAG>GAAG TTTCTCGCC p.w290efs* PTPN11 G>T Missense c.181g>t p.d61y COSM
16 Table S4. Flow sorting antibody combinations Blood Anti-CD66B Anti-CD16 Anti-CD19 Anti-CD3 Fluorochrome Clone Supplier FITC -Cy7 APC-eFluor780 G10F5 3G8 SJ25C1 UCHT1 BD Biosciences BD Biosciences Bone Marrow (CD34-depleted) Anti-CD34 FITC Anti-CD33 -Cy7 Anti-CD3 APC-eFluor780 Anti-CD19 efluor450 Anti-CD235a Bone Marrow (CD34-enriched) (Primary incubation) lineage cocktail Anti-CD2 Anti-CD3 Anti-CD4 Anti-CD7 Anti-CD8 Anti-CD11b Anti-CD14 Anti-CD19 Anti-CD20 Anti-CD56 Anti-CD235a (Secondary incubation) Anti-CD34 FITC Anti-CD38 -Cy7 Anti-CD10 BV-421 Anti-CD90 BV-510 Anti-CD45RA AlexaFluor700 Anti-CD110 APC Anti-CD123 BV-786 AC136 WM-53 UCHT1 SJ25C1 HIR2 RPA-2.10 UCHT1 L3T4 ebio124-1d1 OKT8 ICRF44 61D3 SJ25C1 2H7 B159 HIR2 AC136 HIT2 HI10a 5E10 HI G3 Miltenyi Biotec BD Biosciences BD Biosciences Miltenyi Biotec BD Biosciences BD Biosciences BD Biosciences BD Pharmingen BD Biosciences 16
17 Table S5. TruSight Myeloid Sequencing Panel genes Gene Targeted Region / Targeted Region / Gene Exons Exons ABL1 4-6 JAK3 13 ASXL1 12 KDM6A Full ATRX 8-10; KIT 2; 8-11; 13; 17 BCOR Full KRAS 2-3 BCORL1 Full MLL (KMT2A) 5-8 BRAF 15 MPL 10 CALR 9 MYD CBL 8-9 NOTCH ; 34 CBLB 9-10 NPM1 12 CBLC 9-10 NRAS 2-3 CDKN2A Full PDGFRA 12; 14; 18 CEBPA Full PHF6 Full CSF3R PTEN 5; 7 CUX1 Full PTPN11 3; 13 DNMT3A Full RAD21 Full ETV6 Full RUNX1 Full EZH2 Full SETBP1 4 (partial) FBXW SF3B FLT ; 20 SMC1A 2; 11; GATA1 2 SMC3 10; 13; 19; 23; 25; 28 GATA2 2-6 SRSF2 1 GNAS 8-9 STAG2 Full HRAS 2-3 TET IDH1 4 TP IDH2 4 U2AF1 2; 6 IKZF1 Full WT1 7; 9 JAK2 12; 14 ZRSR2 Full 17
18 Materials and methods Study design, genotyping, digital PCR and plasma 2-HG measurements The 23 IDH mutant cases were from a cohort of 241 adult patients with MDS or AML presenting to The Christie NHS Foundation Trust, Manchester, UK, of whom 46 were genotyped by Sanger sequencing as IDH1- or IDH2-mutated. IDH genotyping was performed as described. 8 Tissue and plasma samples were from the Manchester Cancer Research Centre Biobank, instituted with the approval of the South Manchester Research Ethics Committee. Analyses and experiments were ethically approved by the Biobank s scientific sub-committee. Bone marrow (BM) and blood mononuclear cells (MNCs) and platelet-poor plasma samples were cryopreserved at presentation and multiple time points during follow up, as described. S3,S4 All IDHmutated patients for whom at least presentation and remission samples were available, and who received chemotherapy to complete remission (CR) (n=23), were included in the study. IDH (and NPM1, where present) mutant allele frequency (MAF) was determined for each serial MNC sample in the study cohort by quantitative digital PCR (dpcr), as previously described. 8 Plasma 2-hydroxyglutarate was assayed as previously described. 8,S5 Flow sorting of hematopoietic stem/progenitor and downstream lineage populations Fresh BM and blood were obtained from two cases with residual clonal IDH2 hematopoiesis (BB161, BB287) at remission time points 40 and 18 months following presentation, respectively. Analyses were also performed on cryopreserved remission material from BB months following presentation. Fresh blood was also obtained from one case with residual clonal IDH1 R132G hematopoiesis (BB355) 22 months after presentation. For BM experiments, MNCs were isolated by density gradient centrifugation (Lymphoprep, Stem Cell Technologies, Cambridge, UK). The CD34 + compartment was isolated by magnetic bead separation using an automacs Pro (Miltenyi Biotec, Bisley, UK), according to manufacturer protocols. Blood leukocytes were isolated by buffy coat separation to preserve neutrophil content. The CD34 enriched BM, CD34-depleted BM and peripheral blood cell fractions were each separately sorted by fluorescence activated cell sorting (FACS) on a BD FACSAria TM III cell sorter (BD Biosciences, Oxford, UK). Live-dead discrimination was by inspection of forward/side scatter and 7-AAD incorporation. Antibody panels and gating strategies are indicated in Table S4 and Figure S7 respectively. Population purities were confirmed, where cell numbers permitted, by post-sort flow analyses and inspection of morphology on cytospin preparations. Colony forming assays, single colony expansion and colony genotyping Fresh CD34 + BM cells from BB161 and BB287 were harvested and plated in Methocult H4230 (Stem Cell Technologies) (2000 cells/ml, in triplicate) supplemented with the following cytokines: IL-3 20ng/mL, IL-6 20ng/mL, IL-11 10ng/mL, FLT3L 50ng/mL, SCF 50ng/mL, TPO 50ng/mL (all from Peprotech, London, UK), G-CSF 50ng/mL (Chugai, London, UK) and EPO 4U/mL (Janssen Cilag, High Wycombe, UK). For single cell sorting of immunophenotypic HSCs, CD Lin - cells were flow sorted directly into U-bottom 96 well plates containing 100uL of Methocult per well supplemented with cytokines. Plates were incubated at 37 C 18
19 and 5% CO 2 for 14 days. Colonies were typed by inverted microscopy, plucked and genomic DNA extracted using the Taqman Sample-To-SNP Kit (Life Technologies, Paisley, UK) according to manufacturer s instructions. DNA was pre-amplified using Taqman PreAmp MasterMix (Life Technologies), according to the manufacturer s protocol. Amplified DNA was genotyped by allelic discrimination dpcr. 5μL reaction mixes containing 1μL DNA with 2.5μL 2X Taqman GTXpress Master Mix, 0.25μL 20X Taqman IDH2 SNP genotyping assay and 1.25μL water were subjected to the following thermal cycling conditions on an ABI Prism 7900HT (Applied Biosystems): 95 C for 20s, followed by 40 cycles of 95 C for 3s and 60 C for 20s. ROX was used as passive reference. Targeted next generation sequencing Illumina TruSight Myeloid Panel Genomic DNA from BM or blood MNCs collected at presentation and remission time points was subjected to targeted next generation sequencing (NGS) using a NextSeq 500 sequencer (Illumina, San Diego, CA, USA). Illumina s amplicon-based TruSight Myeloid Sequencing Panel interrogates 56 genes recurrently mutated in myeloid neoplasms (Table S5), using a proprietary multiplexed oligonucleotide pool covering each region of interest. Each primer includes universal adaptor sequences, subsequently incorporated in the amplification reaction. Amplicons are of standardized length (~250bp) for an overall library size of 141 kb. Libraries were generated according to the manufacturer s protocol. Briefly, genomic DNA was quantified using a ubit DNA BR assay kit (Life Technologies, Carlsbad, CA, USA) and diluted to 50ng in 96 well plates. Oligonucleotides were hybridized to regions of interest, followed by an extension-ligation reaction and PCR amplification, incorporating unique combinations of i5/i7 index sequences to permit multiplexing up to 96 samples per sequencing run. Successful amplification was confirmed using a DNA 1000 kit and the 2100 Bioanalyzer system (Agilent Technologies, CA, USA). Libraries were purified using AMPure magnetic beads (Agencourt, Brea, CA, USA) and bead-normalized according to the TruSight protocol. Libraries were pooled (5 L per library, 96 per pool) and quantified by PCR to determine molarity for loading onto the NextSeq flow cell to achieve optimal cluster density ( k/m 2 ). The pooled library was denatured, diluted and loaded onto a reagent cartridge according to Illumina s protocol. Paired end (150bp) sequencing was performed on the NextSeq 500 sequencer (Illumina) with 96 samples multiplexed on a single NextSeq 500 High Output run (300 cycles). Alignments, variant calling and annotation Data analysis was performed within Illumina s online BaseSpace genomics analysis platform. FAST files were aligned to human genome reference GRCh37/hg19 by the TruSeq Amplicon App (v2.0; Illumina; using a banded Smith-Waterman algorithm. Variant calling was performed by Somatic Variant Caller (v ; Illumina) using default parameters. The resulting gvcf files were uploaded to Variant Studio (v2.2.3; Illumina; for downstream filtering and annotation (from RefSeq database) of high confidence variants. Integrative Genomics Viewer (v2.1.2; Broad 19
20 Institute/Illumina; S6 was used to confirm regional coverage, visualize read alignments and confirm variant calls. Variant allele frequency was calculated as the fraction of mutated reads versus total number of reads covering that base. For mutation discovery on presentation samples, sequencing coverage of 250X (bi-directional) and minimum variant frequency of 5% were used as lower thresholds for variant calling, as employed previously. S7 Filters were applied to exclude known germline SNPs with population frequency >1%. However, since unequivocally pathogenic/somatic SNVs also have dbsnp database entries (including all common AML-associated IDH1/2 mutations), presence on dbsnp was not itself an exclusion criterion. All variants passing initial filtering were manually interrogated and those suspicious for germline polymorphisms were excluded. To screen for lower frequency variants, all those detected at 1-5% frequency were manually interrogated and included if they (a) corresponded to a COSMIC database entry S1 with multiple reports in hematopoietic tissue of a confirmed somatic variant; (b) were supported by >40 individual variant reads; and (c) were convincingly absent (or present at <20% of the presentation sample level) in the corresponding remission sample. Among the six additional variants included by this approach were two known sub-clonal IDH1 mutations (BB253: IDH1 R132C 1.9%; BB485: IDH1 R132H 2.9%), both previously detected and quantitated to a similar level by highly sensitive digital PCR and both of which had been initially excluded by the TruSight analysis pipeline. A single presentation sample (BB85) displayed significantly lower coverage (total 0.89 million reads vs average 3.69 million reads per sample), and yielded an unusually high number of variants passing the standard filters (n=16, versus median of four high confidence mutations for the remainder of the cohort). For this case more stringent, manual filtering was applied to minimize false positive calls leading to exclusion of seven possible variants. FLT3 internal tandem duplications (ITDs) are not detected by the standard alignment and variant calling pipelines for the TruSight Myeloid Panel. Paired reads were re-aligned using BWA-MEM (v0.7.12; S8 and the revised BAM files were uploaded to two alternative variant callers: Pindel (v0.2.4) S9 and ITDseek, S10 both of which have been reported to call FLT3-ITD successfully from TruSight/TruSeq targeted sequencing data. S7,S10 ITDseek outperformed Pindel, calling all FLT3-ITDs that were concurrently identified through standard PCR and gel electrophoresis on corresponding presentation gdna samples, as previously described. S11 FLT3-ITD burden was quantified by running PCR products on a Bioanalyzer 2100 High Sensitivity DNA chip (Agilent Technologies, CA, USA), as previously described. S12 20
21 Supplemental references S1. Fuller CW, Middendorf LR, Benner SA, Church GM, Harris T, Huang X et al. The challenges of sequencing by synthesis. Nat Biotechnol 2009; 27: S2. Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res 2015; 43: D S3. Greystoke BF, Huang X, Wilks DP, Atkinson S, Somervaille TCP. Very high frequencies of leukaemiainitiating cells in precursor T-acute lymphoblastic leukaemia may be obscured by cryopreservation. Br J Haematol 2013; 163: S4. Wiseman DH, Small HF, Wilks DP, Waddell ID, Dennis MW, Ogilvie DJ, Somervaille TCP. Elevated plasma 2-hydroxyglutarate in acute myeloid leukaemia: association with the IDH1 SNP rs and severe renal impairment. Br J Haematol 2014; 166: S5. Struys EA, Jansen EEW, Verhoeven NM, Jakobs C. Measurement of urinary D- and L-2- hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-l-tartaric anhydride. Clin Chem 2004; 50: S6. Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G et al. Integrative genomics viewer. Nat Biotechnol 2011; 29: S7. Luthra R, Patel KP, Reddy NG, Haghshenas V, Routbort MJ, Harmon MA et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica 2014; 99: S8. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2010; 26: S9. Ye K, Schulz MH, Long, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 2009; 25: S10. Au CH, Wa A, Ho DN, Chan TL, Ma ESK. Clinical evaluation of panel testing by next-generation sequencing (NGS) for gene mutations in myeloid neoplasms. Diagn Pathol 2016; 11: 11. S11. Shih L-Y, Huang C-F, Wu J-H, Lin T-L, Dunn P, Wang P-N et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood 2002; 100: S12. Mills KI, Gilkes AF, Walsh V, Sweeney M, Gale R. Rapid and sensitive detection of internal tandem duplication and activating loop mutations of FLT3. Br J Haematol 2005; 130:
Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ
 Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ G E NETIC T E CHNOLOGIST MEDICAL G E NETICS, CARDIFF To Cover Background to the project Choice of panel Validation process Genes on panel, Protocol
Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ G E NETIC T E CHNOLOGIST MEDICAL G E NETICS, CARDIFF To Cover Background to the project Choice of panel Validation process Genes on panel, Protocol
Next Generation Sequencing in Haematological Malignancy: A European Perspective. Wolfgang Kern, Munich Leukemia Laboratory
 Next Generation Sequencing in Haematological Malignancy: A European Perspective Wolfgang Kern, Munich Leukemia Laboratory Diagnostic Methods Cytomorphology Cytogenetics Immunophenotype Histology FISH Molecular
Next Generation Sequencing in Haematological Malignancy: A European Perspective Wolfgang Kern, Munich Leukemia Laboratory Diagnostic Methods Cytomorphology Cytogenetics Immunophenotype Histology FISH Molecular
Next generation sequencing analysis - A UK perspective. Nicholas Lea
 Next generation sequencing analysis - A UK perspective Nicholas Lea King s HMDC LMH is part of an integrated pathology service at King s Haematological Malignancy Diagnostic Centre (HMDC) HMDC serves population
Next generation sequencing analysis - A UK perspective Nicholas Lea King s HMDC LMH is part of an integrated pathology service at King s Haematological Malignancy Diagnostic Centre (HMDC) HMDC serves population
Supplementary Figure 1
 Count Count Supplementary Figure 1 Coverage per amplicon for error-corrected sequencing experiments. Errorcorrected consensus sequence (ECCS) coverage was calculated for each of the 568 amplicons in the
Count Count Supplementary Figure 1 Coverage per amplicon for error-corrected sequencing experiments. Errorcorrected consensus sequence (ECCS) coverage was calculated for each of the 568 amplicons in the
Blastic Plasmacytoid Dendritic Cell Neoplasm with DNMT3A and TET2 mutations (SH )
 Blastic Plasmacytoid Dendritic Cell Neoplasm with DNMT3A and TET2 mutations (SH2017-0314) Habibe Kurt, Joseph D. Khoury, Carlos E. Bueso-Ramos, Jeffrey L. Jorgensen, Guilin Tang, L. Jeffrey Medeiros, and
Blastic Plasmacytoid Dendritic Cell Neoplasm with DNMT3A and TET2 mutations (SH2017-0314) Habibe Kurt, Joseph D. Khoury, Carlos E. Bueso-Ramos, Jeffrey L. Jorgensen, Guilin Tang, L. Jeffrey Medeiros, and
Introduction of an NGS gene panel into the Haemato-Oncology MPN service
 Introduction of an NGS gene panel into the Haemato-Oncology MPN service Dr. Anna Skowronska, Dr Jane Bryon, Dr Samuel Clokie, Dr Yvonne Wallis and Professor Mike Griffiths West Midlands Regional Genetics
Introduction of an NGS gene panel into the Haemato-Oncology MPN service Dr. Anna Skowronska, Dr Jane Bryon, Dr Samuel Clokie, Dr Yvonne Wallis and Professor Mike Griffiths West Midlands Regional Genetics
Accel-Amplicon Panels
 Accel-Amplicon Panels Amplicon sequencing has emerged as a reliable, cost-effective method for ultra-deep targeted sequencing. This highly adaptable approach is especially applicable for in-depth interrogation
Accel-Amplicon Panels Amplicon sequencing has emerged as a reliable, cost-effective method for ultra-deep targeted sequencing. This highly adaptable approach is especially applicable for in-depth interrogation
Fluxion Biosciences and Swift Biosciences Somatic variant detection from liquid biopsy samples using targeted NGS
 APPLICATION NOTE Fluxion Biosciences and Swift Biosciences OVERVIEW This application note describes a robust method for detecting somatic mutations from liquid biopsy samples by combining circulating tumor
APPLICATION NOTE Fluxion Biosciences and Swift Biosciences OVERVIEW This application note describes a robust method for detecting somatic mutations from liquid biopsy samples by combining circulating tumor
Please Silence Your Cell Phones. Thank You
 Please Silence Your Cell Phones Thank You Utility of NGS and Comprehensive Genomic Profiling in Hematopathology Practice Maria E. Arcila M.D. Memorial Sloan Kettering Cancer Center New York, NY Disclosure
Please Silence Your Cell Phones Thank You Utility of NGS and Comprehensive Genomic Profiling in Hematopathology Practice Maria E. Arcila M.D. Memorial Sloan Kettering Cancer Center New York, NY Disclosure
Laboratory Service Report
 Client C7028846-DLP Rochester Rochester, N 55901 Specimen Type Peripheral blood CR PDF Report available at: https://test.mmlaccess.com/reports/c7028846-zwselwql7p.ashx Indication for Test DS CR Pathogenic
Client C7028846-DLP Rochester Rochester, N 55901 Specimen Type Peripheral blood CR PDF Report available at: https://test.mmlaccess.com/reports/c7028846-zwselwql7p.ashx Indication for Test DS CR Pathogenic
Concomitant WT1 mutations predicted poor prognosis in CEBPA double-mutated acute myeloid leukemia
 Concomitant WT1 mutations predicted poor prognosis in CEBPA double-mutated acute myeloid leukemia Feng-Ming Tien, Hsin-An Hou, Jih-Luh Tang, Yuan-Yeh Kuo, Chien-Yuan Chen, Cheng-Hong Tsai, Ming Yao, Chi-Cheng
Concomitant WT1 mutations predicted poor prognosis in CEBPA double-mutated acute myeloid leukemia Feng-Ming Tien, Hsin-An Hou, Jih-Luh Tang, Yuan-Yeh Kuo, Chien-Yuan Chen, Cheng-Hong Tsai, Ming Yao, Chi-Cheng
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
The Center for PERSONALIZED DIAGNOSTICS
 The Center for PERSONALIZED DIAGNOSTICS Precision Diagnostics for Personalized Medicine A joint initiative between The Department of Pathology and Laboratory Medicine & The Abramson Cancer Center The (CPD)
The Center for PERSONALIZED DIAGNOSTICS Precision Diagnostics for Personalized Medicine A joint initiative between The Department of Pathology and Laboratory Medicine & The Abramson Cancer Center The (CPD)
Overview. Methods 9/11/2017. Next Generation Sequencing and Precision Medicine in Hematological Malignancies. Genotyping in hematology
 Overview Next Generation Sequencing and Precision Medicine in Hematological Malignancies Sharathkumar Bhagavathi, MD University of Iowa Carver College of Medicine NGS as a genotyping platform in hematopathology
Overview Next Generation Sequencing and Precision Medicine in Hematological Malignancies Sharathkumar Bhagavathi, MD University of Iowa Carver College of Medicine NGS as a genotyping platform in hematopathology
ADRL Advanced Diagnostics Research Laboratory
 ADRL Advanced Diagnostics Research Laboratory John DeCoteau, MD FRCP Department of Pathology, Division of Hematopathology University of Saskatchewan Saskatchewan Cancer Agency ADRL Project Objectives New
ADRL Advanced Diagnostics Research Laboratory John DeCoteau, MD FRCP Department of Pathology, Division of Hematopathology University of Saskatchewan Saskatchewan Cancer Agency ADRL Project Objectives New
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
Laboratory Service Report
 Specimen Type Peripheral blood CR PDF Report available at: https://test.mmlaccess.com/reports/c7028846-ih2xuglwpq.ashx Indication for Test DS CR Pathogenic utations Detected CR 1. JAK2: c.1849g>t;p.val617phe
Specimen Type Peripheral blood CR PDF Report available at: https://test.mmlaccess.com/reports/c7028846-ih2xuglwpq.ashx Indication for Test DS CR Pathogenic utations Detected CR 1. JAK2: c.1849g>t;p.val617phe
Examining Genetics and Genomics of Acute Myeloid Leukemia in 2017
 Examining Genetics and Genomics of Acute Myeloid Leukemia in 2017 Elli Papaemmanuil, PhD Memorial Sloan Kettering Cancer Center New York, New York, United States Today s Talk Cancer genome introduction
Examining Genetics and Genomics of Acute Myeloid Leukemia in 2017 Elli Papaemmanuil, PhD Memorial Sloan Kettering Cancer Center New York, New York, United States Today s Talk Cancer genome introduction
SUPPLEMENTAL APPENDIX METZELER ET AL.: SPECTRUM AND PROGNOSTIC RELEVANCE OF DRIVER GENE MUTATIONS IN ACUTE MYELOID LEUKEMIA
 SUPPLEMENTAL APPENDIX TO METZELER ET AL.: SPECTRUM AND PROGNOSTIC RELEVANCE OF DRIVER GENE MUTATIONS IN ACUTE MYELOID LEUKEMIA SUPPLEMENTAL METHODS Treatment protocols In the AMLCG-1999 trial 1-3 (clinicaltrials.gov
SUPPLEMENTAL APPENDIX TO METZELER ET AL.: SPECTRUM AND PROGNOSTIC RELEVANCE OF DRIVER GENE MUTATIONS IN ACUTE MYELOID LEUKEMIA SUPPLEMENTAL METHODS Treatment protocols In the AMLCG-1999 trial 1-3 (clinicaltrials.gov
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance
Acute Myeloid Leukemia with RUNX1 and Several Co-mutations
 Case SH2017-0281 Acute Myeloid Leukemia with RUNX1 and Several Co-mutations James Bauer, MD, PhD David Yang, MD Erik Ranheim, MD, PhD Catherine Leith, MB, Bchir Clinical History Chief Complaint: 72 year
Case SH2017-0281 Acute Myeloid Leukemia with RUNX1 and Several Co-mutations James Bauer, MD, PhD David Yang, MD Erik Ranheim, MD, PhD Catherine Leith, MB, Bchir Clinical History Chief Complaint: 72 year
Out-Patient Billing CPT Codes
 Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
Supporting Information
 Supporting Information Rampal et al. 10.1073/pnas.1407792111 Fig. S1. Genetic events in leukemic transformation of chronic-phase MPNs. (A) Survival of post-mpn AML patients according to mutational status
Supporting Information Rampal et al. 10.1073/pnas.1407792111 Fig. S1. Genetic events in leukemic transformation of chronic-phase MPNs. (A) Survival of post-mpn AML patients according to mutational status
Acute leukemia and myelodysplastic syndromes
 11/01/2012 Post-ASH meeting 1 Acute leukemia and myelodysplastic syndromes Peter Vandenberghe Centrum Menselijke Erfelijkheid & Afdeling Hematologie, UZ Leuven 11/01/2012 Post-ASH meeting 2 1. Acute myeloid
11/01/2012 Post-ASH meeting 1 Acute leukemia and myelodysplastic syndromes Peter Vandenberghe Centrum Menselijke Erfelijkheid & Afdeling Hematologie, UZ Leuven 11/01/2012 Post-ASH meeting 2 1. Acute myeloid
Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research
 Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research Application Note Authors John McGuigan, Megan Manion,
Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research Application Note Authors John McGuigan, Megan Manion,
The preclinical efficacy of a novel telomerase inhibitor, imetelstat, in AML: A randomized trial in patient-derived xenografts
 The preclinical efficacy of a novel telomerase inhibitor, imetelstat, in AML: A randomized trial in patient-derived xenografts Claudia Bruedigam, Ph.D Gordon and Jessie Gilmour Leukaemia Research Laboratory
The preclinical efficacy of a novel telomerase inhibitor, imetelstat, in AML: A randomized trial in patient-derived xenografts Claudia Bruedigam, Ph.D Gordon and Jessie Gilmour Leukaemia Research Laboratory
Juan Ma 1, Jennifer Dunlap 2, Lisong Shen 1, Guang Fan 2 1
 Juan Ma 1, Jennifer Dunlap 2, Lisong Shen 1, Guang Fan 2 1 Xin Hua Hospital, Shanghai, China 2 Oregon Health & Science University, Portland, OR, United States AML is a hematopoietic neoplasms characterized
Juan Ma 1, Jennifer Dunlap 2, Lisong Shen 1, Guang Fan 2 1 Xin Hua Hospital, Shanghai, China 2 Oregon Health & Science University, Portland, OR, United States AML is a hematopoietic neoplasms characterized
Supplemental Material. The new provisional WHO entity RUNX1 mutated AML shows specific genetics without prognostic influence of dysplasia
 Supplemental Material The new provisional WHO entity RUNX1 mutated AML shows specific genetics without prognostic influence of dysplasia Torsten Haferlach, 1 Anna Stengel, 1 Sandra Eckstein, 1 Karolína
Supplemental Material The new provisional WHO entity RUNX1 mutated AML shows specific genetics without prognostic influence of dysplasia Torsten Haferlach, 1 Anna Stengel, 1 Sandra Eckstein, 1 Karolína
Frequency(%) KRAS G12 KRAS G13 KRAS A146 KRAS Q61 KRAS K117N PIK3CA H1047 PIK3CA E545 PIK3CA E542K PIK3CA Q546. EGFR exon19 NFS-indel EGFR L858R
 Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
August 17, Dear Valued Client:
 August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
The Evolving Role of Transplantation for MPN
 The Evolving Role of Transplantation for MPN (PMF, PV, ET) H.Joachim Deeg MD Fred Hutchinson Cancer Research Center, University of Washington, Seattle WA, SCCA jdeeg@fhcrc.org 10 th J. Niblack Conference
The Evolving Role of Transplantation for MPN (PMF, PV, ET) H.Joachim Deeg MD Fred Hutchinson Cancer Research Center, University of Washington, Seattle WA, SCCA jdeeg@fhcrc.org 10 th J. Niblack Conference
Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope
 Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope National Medical Center Disclosures I have no disclosures
Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope National Medical Center Disclosures I have no disclosures
Spectrum of somatically acquired mutations identified by combining WES and genome-wide DNA array analysis in the discovery cohort of 30 JMML cases.
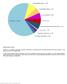 Supplementary Figure 1 Spectrum of somatically acquired mutations identified by combining WES and genome-wide DNA array analysis in the discovery cohort of 30 JMML cases. A total of 85 somatically acquired
Supplementary Figure 1 Spectrum of somatically acquired mutations identified by combining WES and genome-wide DNA array analysis in the discovery cohort of 30 JMML cases. A total of 85 somatically acquired
Molecular Markers in Acute Leukemia. Dr Muhd Zanapiah Zakaria Hospital Ampang
 Molecular Markers in Acute Leukemia Dr Muhd Zanapiah Zakaria Hospital Ampang Molecular Markers Useful at diagnosis Classify groups and prognosis Development of more specific therapies Application of risk-adjusted
Molecular Markers in Acute Leukemia Dr Muhd Zanapiah Zakaria Hospital Ampang Molecular Markers Useful at diagnosis Classify groups and prognosis Development of more specific therapies Application of risk-adjusted
Supplementary Table 2. Identified causative mutations and/or mutation candidates.
 Supplementary Table 2. Identified causative mutations and/or mutation candidates. Nonsense mutations base change aa change Average depth Result of next generation in 432 patient Hereditary form of the
Supplementary Table 2. Identified causative mutations and/or mutation candidates. Nonsense mutations base change aa change Average depth Result of next generation in 432 patient Hereditary form of the
SESSION 1 Reactive cytopenia and dysplasia
 SESSION 1 Reactive cytopenia and dysplasia Falko Fend, Tübingen & Alexandar Tzankov, Basel 1 Disclosure of speaker s interests (Potential) conflict of interest none Potentially relevant company relationships
SESSION 1 Reactive cytopenia and dysplasia Falko Fend, Tübingen & Alexandar Tzankov, Basel 1 Disclosure of speaker s interests (Potential) conflict of interest none Potentially relevant company relationships
Molecular Genetic Testing for the Diagnosis of Haematological Malignancies
 Molecular Genetic Testing for the Diagnosis of Haematological Malignancies Dr Anthony Bench Haemto-Oncology Diagnostic Service Cambrıdge Unıversıty Hospitals NHS Foundatıon Trust Cambridge UK Molecular
Molecular Genetic Testing for the Diagnosis of Haematological Malignancies Dr Anthony Bench Haemto-Oncology Diagnostic Service Cambrıdge Unıversıty Hospitals NHS Foundatıon Trust Cambridge UK Molecular
TP53 mutational profile in CLL : A retrospective study of the FILO group.
 TP53 mutational profile in CLL : A retrospective study of the FILO group. Fanny Baran-Marszak Hopital Avicenne Bobigny France 2nd ERIC workshop on TP53 analysis in CLL, Stresa 2017 TP53 abnormalities :
TP53 mutational profile in CLL : A retrospective study of the FILO group. Fanny Baran-Marszak Hopital Avicenne Bobigny France 2nd ERIC workshop on TP53 analysis in CLL, Stresa 2017 TP53 abnormalities :
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Information
 Supplementary Information Table of Contents Supplementary methods... 2 Figure S1 - Variable DNA yield proportional to bone marrow aspirate cellularity.... 3 Figure S2 - Mutations by clinical ontogeny group....
Supplementary Information Table of Contents Supplementary methods... 2 Figure S1 - Variable DNA yield proportional to bone marrow aspirate cellularity.... 3 Figure S2 - Mutations by clinical ontogeny group....
Management of Myelodysplastic Syndromes
 Management of Myelodysplastic Syndromes Peter L. Greenberg, MD Stanford Cancer Institute Myelodysplastic Syndromes: Clinical & Molecular Advances for Disease Classification and Prognostication MDSs: A
Management of Myelodysplastic Syndromes Peter L. Greenberg, MD Stanford Cancer Institute Myelodysplastic Syndromes: Clinical & Molecular Advances for Disease Classification and Prognostication MDSs: A
Corporate Medical Policy. Policy Effective February 23, 2018
 Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Acute Myeloid Leukemia Progress at last
 Acute Myeloid Leukemia Progress at last Bruno C. Medeiros, MD September 9, 217 Introduction Mechanisms of leukemogenesis Emerging therapies in AML Previously untreated AML Relapsed and refractory patients
Acute Myeloid Leukemia Progress at last Bruno C. Medeiros, MD September 9, 217 Introduction Mechanisms of leukemogenesis Emerging therapies in AML Previously untreated AML Relapsed and refractory patients
Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine
 Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center How the
Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center How the
West Midlands Regional Genetics Laboratory
 West Midlands Regional Genetics Laboratory Haemato-oncology service update letter October 2017 Dear colleagues, We are writing to outline the latest developments to our service, aiming to support the management
West Midlands Regional Genetics Laboratory Haemato-oncology service update letter October 2017 Dear colleagues, We are writing to outline the latest developments to our service, aiming to support the management
A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis
 APPLICATION NOTE Cell-Free DNA Isolation Kit A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis Abstract Circulating cell-free DNA (cfdna) has been shown
APPLICATION NOTE Cell-Free DNA Isolation Kit A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis Abstract Circulating cell-free DNA (cfdna) has been shown
ESTABLISHED AND EMERGING THERAPIES FOR ACUTE MYELOID LEUKAEMIA. Dr Rob Sellar UCL Cancer Institute, London, UK
 ESTABLISHED AND EMERGING THERAPIES FOR ACUTE MYELOID LEUKAEMIA Dr Rob Sellar UCL Cancer Institute, London, UK OVERVIEW Main focus on patients fit for intensive treatment Biological and Clinical Heterogeneity
ESTABLISHED AND EMERGING THERAPIES FOR ACUTE MYELOID LEUKAEMIA Dr Rob Sellar UCL Cancer Institute, London, UK OVERVIEW Main focus on patients fit for intensive treatment Biological and Clinical Heterogeneity
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits
 AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment
Kevin Kelly, MD, Phd Acute Myeloid and Lymphoid Leukemias
 Kevin Kelly, MD, Phd Acute Myeloid and Lymphoid Leukemias Relevant financial relationships in the past twelve months by presenter or spouse/partner. Speakers bureau: Novartis, Janssen, Gilead, Bayer The
Kevin Kelly, MD, Phd Acute Myeloid and Lymphoid Leukemias Relevant financial relationships in the past twelve months by presenter or spouse/partner. Speakers bureau: Novartis, Janssen, Gilead, Bayer The
Performance Characteristics BRCA MASTR Plus Dx
 Performance Characteristics BRCA MASTR Plus Dx with drmid Dx for Illumina NGS systems Manufacturer Multiplicom N.V. Galileïlaan 18 2845 Niel Belgium Table of Contents 1. Workflow... 4 2. Performance Characteristics
Performance Characteristics BRCA MASTR Plus Dx with drmid Dx for Illumina NGS systems Manufacturer Multiplicom N.V. Galileïlaan 18 2845 Niel Belgium Table of Contents 1. Workflow... 4 2. Performance Characteristics
Molecular. Oncology & Pathology. Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine. Liquid Biopsy.
 Molecular Oncology & Pathology Hereditary Cancer Somatic Cancer Liquid Biopsy Next-Gen Sequencing qpcr Sanger Sequencing Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine
Molecular Oncology & Pathology Hereditary Cancer Somatic Cancer Liquid Biopsy Next-Gen Sequencing qpcr Sanger Sequencing Diagnostic, Prognostic, Therapeutic, and Predisposition Tests in Precision Medicine
Importance of minor TP53 mutated clones in the clinic
 Importance of minor TP53 mutated clones in the clinic Davide Rossi, M.D., Ph.D. Hematology IOSI - Oncology Institute of Southern Switzerland IOR - Institute of Oncology Reserach Bellinzona - Switzerland
Importance of minor TP53 mutated clones in the clinic Davide Rossi, M.D., Ph.D. Hematology IOSI - Oncology Institute of Southern Switzerland IOR - Institute of Oncology Reserach Bellinzona - Switzerland
APPLICATIONS OF NEXT GENERATION SEQUENCING IN SOLID TUMORS - PATHOLOGIST PROSPECTIVE
 AMP COMPANION MEETING SYMPOSIUM AT USCAP 2015 NEXT-GENERATION OF PATHOLOGY: ROLE OF PATHOLOGIST IN NGS-BASED PERSONALIZED MEDICINE APPLICATIONS OF NEXT GENERATION SEQUENCING IN SOLID TUMORS - PATHOLOGIST
AMP COMPANION MEETING SYMPOSIUM AT USCAP 2015 NEXT-GENERATION OF PATHOLOGY: ROLE OF PATHOLOGIST IN NGS-BASED PERSONALIZED MEDICINE APPLICATIONS OF NEXT GENERATION SEQUENCING IN SOLID TUMORS - PATHOLOGIST
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
Whole Exome Sequenced Characteristics
 Supplementary Tables Supplementary Table 1: Patient characteristics of 45 whole exome sequenced HNSCC tumors Whole Exome Sequenced Characteristics Tumors (n=45) Age, years Median (range) 61.0 (19-90) Sex,
Supplementary Tables Supplementary Table 1: Patient characteristics of 45 whole exome sequenced HNSCC tumors Whole Exome Sequenced Characteristics Tumors (n=45) Age, years Median (range) 61.0 (19-90) Sex,
Myelodysplastic Syndromes. Post-ASH meeting 2014 Marie-Christiane Vekemans
 Myelodysplastic Syndromes Post-ASH meeting 2014 Marie-Christiane Vekemans Agenda New biological developments Risk assessment and prognostic factors New therapeutic options Agenda New biological developments
Myelodysplastic Syndromes Post-ASH meeting 2014 Marie-Christiane Vekemans Agenda New biological developments Risk assessment and prognostic factors New therapeutic options Agenda New biological developments
NGS in tissue and liquid biopsy
 NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
July 2015 Assay ID Assay Name Gene COSMIC ID Amino acid change Nucleotide change Wild type allele (VIC label) Mutant allele (FAM label)
 July 2015 Assay ID Assay Name Gene COSMIC ID Amino acid change Nucleotide change Wild type allele (VIC label) Mutant allele (FAM label) p AHLJ090 AKT1_33765 AKT1 33765 p.e17k c.49g>a C T 2 AHBKFRM BRAF_473
July 2015 Assay ID Assay Name Gene COSMIC ID Amino acid change Nucleotide change Wild type allele (VIC label) Mutant allele (FAM label) p AHLJ090 AKT1_33765 AKT1 33765 p.e17k c.49g>a C T 2 AHBKFRM BRAF_473
Impact of Biomarkers in the Management of Patients with Acute Myeloid Leukemia
 Impact of Biomarkers in the Management of Patients with Acute Myeloid Leukemia Hartmut Döhner Medical Director, Department of Internal Medicine III Director, Comprehensive Cancer Center Ulm Ulm University,
Impact of Biomarkers in the Management of Patients with Acute Myeloid Leukemia Hartmut Döhner Medical Director, Department of Internal Medicine III Director, Comprehensive Cancer Center Ulm Ulm University,
Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine
 Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center How the
Enhancing Assessment of Myeloid Leukemia in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center How the
7/12/2016 TESTING. Objectives. New Directions in Aplastic Anemia: What's on the Horizon? Better way to evaluate clonal evolution?
 New Directions in Aplastic Anemia: What's on the Horizon? Amy E. DeZern, MD; MHS Assistant Professor Hematologic Malignancies Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Baltimore, MD Objectives
New Directions in Aplastic Anemia: What's on the Horizon? Amy E. DeZern, MD; MHS Assistant Professor Hematologic Malignancies Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Baltimore, MD Objectives
Objectives. Morphology and IHC. Flow and Cyto FISH. Testing for Heme Malignancies 3/20/2013
 Molecular Markers in Hematologic Malignancy: Ways to locate the needle in the haystack. Objectives Review the types of testing for hematologic malignancies Understand rationale for molecular testing Marcie
Molecular Markers in Hematologic Malignancy: Ways to locate the needle in the haystack. Objectives Review the types of testing for hematologic malignancies Understand rationale for molecular testing Marcie
Molecular Markers. Marcie Riches, MD, MS Associate Professor University of North Carolina Scientific Director, Infection and Immune Reconstitution WC
 Molecular Markers Marcie Riches, MD, MS Associate Professor University of North Carolina Scientific Director, Infection and Immune Reconstitution WC Overview Testing methods Rationale for molecular testing
Molecular Markers Marcie Riches, MD, MS Associate Professor University of North Carolina Scientific Director, Infection and Immune Reconstitution WC Overview Testing methods Rationale for molecular testing
Variant interpretation exercise. ACGS Somatic Variant Interpretation Workshop Joanne Mason 21/09/18
 Variant interpretation exercise ACGS Somatic Variant Interpretation Workshop Joanne Mason 21/09/18 Format of exercise Compile a list of tricky variants across solid cancer and haematological malignancy.
Variant interpretation exercise ACGS Somatic Variant Interpretation Workshop Joanne Mason 21/09/18 Format of exercise Compile a list of tricky variants across solid cancer and haematological malignancy.
Reporting TP53 gene analysis results in CLL
 Reporting TP53 gene analysis results in CLL Mutations in TP53 - From discovery to clinical practice in CLL Discovery Validation Clinical practice Variant diversity *Leroy at al, Cancer Research Review
Reporting TP53 gene analysis results in CLL Mutations in TP53 - From discovery to clinical practice in CLL Discovery Validation Clinical practice Variant diversity *Leroy at al, Cancer Research Review
Mutational Impact on Diagnostic and Prognostic Evaluation of MDS
 Mutational Impact on Diagnostic and Prognostic Evaluation of MDS Elsa Bernard, PhD Papaemmanuil Lab, Computational Oncology, MSKCC MDS Foundation ASH 2018 Symposium Disclosure Research funds provided by
Mutational Impact on Diagnostic and Prognostic Evaluation of MDS Elsa Bernard, PhD Papaemmanuil Lab, Computational Oncology, MSKCC MDS Foundation ASH 2018 Symposium Disclosure Research funds provided by
Genomic Medicine: What every pathologist needs to know
 Genomic Medicine: What every pathologist needs to know Stephen P. Ethier, Ph.D. Professor, Department of Pathology and Laboratory Medicine, MUSC Director, MUSC Center for Genomic Medicine Genomics and
Genomic Medicine: What every pathologist needs to know Stephen P. Ethier, Ph.D. Professor, Department of Pathology and Laboratory Medicine, MUSC Director, MUSC Center for Genomic Medicine Genomics and
DNA-seq Bioinformatics Analysis: Copy Number Variation
 DNA-seq Bioinformatics Analysis: Copy Number Variation Elodie Girard elodie.girard@curie.fr U900 institut Curie, INSERM, Mines ParisTech, PSL Research University Paris, France NGS Applications 5C HiC DNA-seq
DNA-seq Bioinformatics Analysis: Copy Number Variation Elodie Girard elodie.girard@curie.fr U900 institut Curie, INSERM, Mines ParisTech, PSL Research University Paris, France NGS Applications 5C HiC DNA-seq
Molecular profiling in confirming the diagnosis of early myelodysplastic syndrome
 Molecular profiling of early MDS Hematopathology - March 2016 Article Molecular profiling in confirming the diagnosis of early myelodysplastic syndrome Maya Thangavelu 1,*, Ryan Olson 2, Li Li 2, Wanlong
Molecular profiling of early MDS Hematopathology - March 2016 Article Molecular profiling in confirming the diagnosis of early myelodysplastic syndrome Maya Thangavelu 1,*, Ryan Olson 2, Li Li 2, Wanlong
Nature Medicine: doi: /nm.4439
 Figure S1. Overview of the variant calling and verification process. This figure expands on Fig. 1c with details of verified variants identification in 547 additional validation samples. Somatic variants
Figure S1. Overview of the variant calling and verification process. This figure expands on Fig. 1c with details of verified variants identification in 547 additional validation samples. Somatic variants
DISCLOSURE Luca Malcovati, MD. No financial relationships to disclose
 ICUS, CCUS and CHIP Luca Malcovati, MD Department of Molecular Medicine, University of Pavia Medical School, & Department of Hematology Oncology, IRCCS Policlinico S. Matteo Foundation, Pavia, Italy DISCLOSURE
ICUS, CCUS and CHIP Luca Malcovati, MD Department of Molecular Medicine, University of Pavia Medical School, & Department of Hematology Oncology, IRCCS Policlinico S. Matteo Foundation, Pavia, Italy DISCLOSURE
Myelodysplastic syndromes and the new WHO 2016 classification
 Myelodysplastic syndromes and the new WHO 2016 classification 32nd General Annual Meeting of the Belgian Hematology Society 10-11 February 2017 Gregor Verhoef, Departement of Hematology, University Hospital
Myelodysplastic syndromes and the new WHO 2016 classification 32nd General Annual Meeting of the Belgian Hematology Society 10-11 February 2017 Gregor Verhoef, Departement of Hematology, University Hospital
Aplastic Anaemia Genomics: Current data and interpretation
 An Academic Health Sciences Centre at the heart of a world city... Aplastic Anaemia Genomics: Current data and interpretation Austin G Kulasekararaj Consultant Haematologist King s College Hospital, London
An Academic Health Sciences Centre at the heart of a world city... Aplastic Anaemia Genomics: Current data and interpretation Austin G Kulasekararaj Consultant Haematologist King s College Hospital, London
Case 1. Sa A.Wang, MD UT MD Anderson Cancer Center Houston, TX
 Case 1 Sa A.Wang, MD UT MD Anderson Cancer Center Houston, TX Disclosure of Relevant Financial Relationships The USCAP requires that anyone in a position to influence or control the content of all CME
Case 1 Sa A.Wang, MD UT MD Anderson Cancer Center Houston, TX Disclosure of Relevant Financial Relationships The USCAP requires that anyone in a position to influence or control the content of all CME
TCF3 breakpoints of TCF3-PBX1 (patients 1a 5a) and TCF3-HLF (patients 6a 9a and11a) translocations.
 Supplementary Figure 1 TCF3 breakpoints of TCF3-PBX1 (patients 1a 5a) and TCF3-HLF (patients 6a 9a and11a) translocations. The CpG motifs closest to the breakpoints are highlighted in red boxes and the
Supplementary Figure 1 TCF3 breakpoints of TCF3-PBX1 (patients 1a 5a) and TCF3-HLF (patients 6a 9a and11a) translocations. The CpG motifs closest to the breakpoints are highlighted in red boxes and the
Published Ahead of Print on April 14, 2016, as doi: /haematol Copyright 2016 Ferrata Storti Foundation.
 Published Ahead of Print on April 14, 2016, as doi:10.3324/haematol.2016.143214. Copyright 2016 Ferrata Storti Foundation. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse
Published Ahead of Print on April 14, 2016, as doi:10.3324/haematol.2016.143214. Copyright 2016 Ferrata Storti Foundation. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse
Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins
 Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins February 3-5, 2016 Lansdowne Resort, Leesburg, VA Molecular
Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins February 3-5, 2016 Lansdowne Resort, Leesburg, VA Molecular
Pediatric Oncology & Pathology Services
 Pediatric Oncology & Pathology Services Anatomic Pathology Flow Cytometry Cytogenetics Pharma Services Diagnostic, Prognostic, Predictive, and Predisposition Testing Pediatric Oncology & Pathology Services
Pediatric Oncology & Pathology Services Anatomic Pathology Flow Cytometry Cytogenetics Pharma Services Diagnostic, Prognostic, Predictive, and Predisposition Testing Pediatric Oncology & Pathology Services
Enhancing Assessment of Myeloid Disorders in the Era of Precision Medicine
 Enhancing Assessment of Myeloid Disorders in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center Objectives
Enhancing Assessment of Myeloid Disorders in the Era of Precision Medicine Michelle Afkhami, M.D. Medical Director, Clinical Molecular Diagnostic Laboratory City of Hope National Medical Center Objectives
Dr David Guttery Senior PDRA Dept. of Cancer Studies and CRUK Leicester Centre University of Leicester
 Dr David Guttery Senior PDRA Dept. of Cancer Studies and CRUK Leicester Centre University of Leicester dsg6@le.ac.uk CFDNA/CTDNA Circulating-free AS A LIQUID DNA BIOPSY (cfdna) Tumour Biopsy Liquid Biopsy
Dr David Guttery Senior PDRA Dept. of Cancer Studies and CRUK Leicester Centre University of Leicester dsg6@le.ac.uk CFDNA/CTDNA Circulating-free AS A LIQUID DNA BIOPSY (cfdna) Tumour Biopsy Liquid Biopsy
Nature Immunology: doi: /ni.3412
 Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
SUPPLEMENTARY FIG. S3. Kaplan Meier survival analysis followed with log-rank test of de novo acute myeloid leukemia patients selected by age <60, IA
 Supplementary Data Supplementary Appendix A: Treatment Protocols Treatment protocols of 123 cases patients were treated with the protocols as follows: 110 patients received standard DA (daunorubicin 45
Supplementary Data Supplementary Appendix A: Treatment Protocols Treatment protocols of 123 cases patients were treated with the protocols as follows: 110 patients received standard DA (daunorubicin 45
AVENIO ctdna Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB
 Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
Breast and ovarian cancer in Serbia: the importance of mutation detection in hereditary predisposition genes using NGS
 Breast and ovarian cancer in Serbia: the importance of mutation detection in hereditary predisposition genes using NGS dr sc. Ana Krivokuća Laboratory for molecular genetics Institute for Oncology and
Breast and ovarian cancer in Serbia: the importance of mutation detection in hereditary predisposition genes using NGS dr sc. Ana Krivokuća Laboratory for molecular genetics Institute for Oncology and
Whole Genome and Transcriptome Analysis of Anaplastic Meningioma. Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute
 Whole Genome and Transcriptome Analysis of Anaplastic Meningioma Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute Outline Anaplastic meningioma compared to other cancers Whole genomes
Whole Genome and Transcriptome Analysis of Anaplastic Meningioma Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute Outline Anaplastic meningioma compared to other cancers Whole genomes
Session II: Summary. Robert P Hasserjian, MD Professor of Pathology Massachusetts General Hospital and Harvard Medical School
 Session II: Summary Robert P Hasserjian, MD Professor of Pathology Massachusetts General Hospital and Harvard Medical School Disclosure of speaker s interests (Prefix and Last Name) (Potential) conflict
Session II: Summary Robert P Hasserjian, MD Professor of Pathology Massachusetts General Hospital and Harvard Medical School Disclosure of speaker s interests (Prefix and Last Name) (Potential) conflict
iplex genotyping IDH1 and IDH2 assays utilized the following primer sets (forward and reverse primers along with extension primers).
 Supplementary Materials Supplementary Methods iplex genotyping IDH1 and IDH2 assays utilized the following primer sets (forward and reverse primers along with extension primers). IDH1 R132H and R132L Forward:
Supplementary Materials Supplementary Methods iplex genotyping IDH1 and IDH2 assays utilized the following primer sets (forward and reverse primers along with extension primers). IDH1 R132H and R132L Forward:
Toluidin-Staining of mast cells Ear tissue was fixed with Carnoy (60% ethanol, 30% chloroform, 10% acetic acid) overnight at 4 C, afterwards
 Toluidin-Staining of mast cells Ear tissue was fixed with Carnoy (60% ethanol, 30% chloroform, 10% acetic acid) overnight at 4 C, afterwards incubated in 100 % ethanol overnight at 4 C and embedded in
Toluidin-Staining of mast cells Ear tissue was fixed with Carnoy (60% ethanol, 30% chloroform, 10% acetic acid) overnight at 4 C, afterwards incubated in 100 % ethanol overnight at 4 C and embedded in
Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each
 Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each mutated gene and the panel of 125 cancer-driving genes
Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each mutated gene and the panel of 125 cancer-driving genes
IV Simposio International Sao Paulo Nov Hematologic malignant diseases molecular information, present and future
 IV Simposio International Sao Paulo Nov 7 2012 Hematologic malignant diseases molecular information, present and future Dr. rer. nat. Alexander Kohlmann, MLL Munich Leukemia Laboratory Spectrum of Methods
IV Simposio International Sao Paulo Nov 7 2012 Hematologic malignant diseases molecular information, present and future Dr. rer. nat. Alexander Kohlmann, MLL Munich Leukemia Laboratory Spectrum of Methods
All patients with FLT3 mutant AML should receive midostaurin-based induction therapy. Not so fast!
 All patients with FLT3 mutant AML should receive midostaurin-based induction therapy Not so fast! Harry P. Erba, M.D., Ph.D. Professor, Internal Medicine Director, Hematologic Malignancy Program University
All patients with FLT3 mutant AML should receive midostaurin-based induction therapy Not so fast! Harry P. Erba, M.D., Ph.D. Professor, Internal Medicine Director, Hematologic Malignancy Program University
Objectives and Financial Disclosure
 Enhancing Assessment of Leukemia and Lymphoma with Next Generation Sequencing Michelle Afkhami, M.D. Medical Director, Clinical Molecular Laboratory Assistant Professor, Department of Pathology City of
Enhancing Assessment of Leukemia and Lymphoma with Next Generation Sequencing Michelle Afkhami, M.D. Medical Director, Clinical Molecular Laboratory Assistant Professor, Department of Pathology City of
Vertical Magnetic Separation of Circulating Tumor Cells and Somatic Genomic-Alteration Analysis in Lung Cancer Patients
 Vertical Magnetic Separation of Circulating Cells and Somatic Genomic-Alteration Analysis in Lung Cancer Patients Chang Eun Yoo 1,2#, Jong-Myeon Park 3#, Hui-Sung Moon 1,2, Je-Gun Joung 2, Dae-Soon Son
Vertical Magnetic Separation of Circulating Cells and Somatic Genomic-Alteration Analysis in Lung Cancer Patients Chang Eun Yoo 1,2#, Jong-Myeon Park 3#, Hui-Sung Moon 1,2, Je-Gun Joung 2, Dae-Soon Son
Supplementary Figure 1
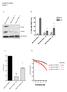 Supplementary Figure 1 A B HEC1A PTEN +/+ HEC1A PTEN -/- 16 HEC1A PTEN -/- 22 PTEN RAD51 % Cells with RAD51 foci 40 -IR +IR 30 20 10 * *! TUBULIN 0 HEC1A PTEN+/+ HEC1A PTEN-/- 16 HEC1A PTEN-/- 22 C D 1.0
Supplementary Figure 1 A B HEC1A PTEN +/+ HEC1A PTEN -/- 16 HEC1A PTEN -/- 22 PTEN RAD51 % Cells with RAD51 foci 40 -IR +IR 30 20 10 * *! TUBULIN 0 HEC1A PTEN+/+ HEC1A PTEN-/- 16 HEC1A PTEN-/- 22 C D 1.0
Chronic Myelomonocytic Leukemia with molecular abnormalities SH
 Chronic Myelomonocytic Leukemia with molecular abnormalities SH2017-0351 Madhu P. Menon MD,PhD, Juan Gomez MD, Kedar V. Inamdar MD,PhD and Kristin Karner MD Madhu P Menon, MD, PhD Henry Ford Hospital Patient
Chronic Myelomonocytic Leukemia with molecular abnormalities SH2017-0351 Madhu P. Menon MD,PhD, Juan Gomez MD, Kedar V. Inamdar MD,PhD and Kristin Karner MD Madhu P Menon, MD, PhD Henry Ford Hospital Patient
Targeted NGS in oncology and hemato-oncology using in-house designed gene panels. Joni Van der Meulen Molecular Diagnostics UZ Ghent (MDG) 24/03/2017
 Targeted NGS in oncology and hemato-oncology using in-house designed gene panels Joni Van der Meulen Molecular Diagnostics UZ Ghent (MDG) 24/03/2017 MDG = Molecular Diagnostics UZ Ghent Center for Medical
Targeted NGS in oncology and hemato-oncology using in-house designed gene panels Joni Van der Meulen Molecular Diagnostics UZ Ghent (MDG) 24/03/2017 MDG = Molecular Diagnostics UZ Ghent Center for Medical
VUmc Basispresentatie
 Clinical diagnostic cytometry Gerrit J Schuurhuis Dept of Hematology VU University Medical Center Amsterdam, Netherlands Use of immunophenotyping at diagnosis to trace residual disease after therapy 1.
Clinical diagnostic cytometry Gerrit J Schuurhuis Dept of Hematology VU University Medical Center Amsterdam, Netherlands Use of immunophenotyping at diagnosis to trace residual disease after therapy 1.
SureSelect Cancer All-In-One Custom and Catalog NGS Assays
 SureSelect Cancer All-In-One Custom and Catalog NGS Assays Detect all cancer-relevant variants in a single SureSelect assay SNV Indel TL SNV Indel TL Single DNA input Single AIO assay Single data analysis
SureSelect Cancer All-In-One Custom and Catalog NGS Assays Detect all cancer-relevant variants in a single SureSelect assay SNV Indel TL SNV Indel TL Single DNA input Single AIO assay Single data analysis
IntelliGENSM. Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community.
 IntelliGENSM Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community. NGS TRANSFORMS GENOMIC TESTING Background Cancers may emerge as a result of somatically
IntelliGENSM Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community. NGS TRANSFORMS GENOMIC TESTING Background Cancers may emerge as a result of somatically
