Re: Integrated Oncology Management Program with evicore healthcare: Update on codes requiring precertification
|
|
|
- Rhoda Logan
- 6 years ago
- Views:
Transcription
1 Ajani Nimmagadda, MD Senior Medical Director <Date> <Provider Firstname> <Provider Lastname>, <Designation> <Street Address> <City>, <ST> <Zip code> Re: Integrated Oncology Management Program with evicore healthcare: Update on codes requiring precertification Dear <Recipient>, Our Integrated Oncology Management Program, administrated by evicore healthcare, provides utilization management services, including precertification, to help ensure our customers receive coverage for medically necessary, evidence-based care. Through this program, you are able to obtain precertification for covered medical and pharmacy medications with a single request, supporting a streamlined, integrated approach to cancer treatment that aligns with National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology. As such, we want to make you aware that effective January 1, 2018, we will add 53 Healthcare Common Procedure Coding System (HCPCS) codes to the list of medical oncology and oral chemotherapy medications that will require precertification under our Integrated Oncology Management Program. Please note that for customers currently receiving these impacted medications, we will continue to cover them without additional review until a patient s current regime is complete, or for six months (whichever comes first). After that time, you will need to submit a new precertification request. Any changes to a current regime will require medical necessity review through the precertification process. What this means for you With the addition of the 53 HCPCS codes, you will continue to submit precertification requests through evicore for: All chemotherapy medications, which include primary chemotherapy and supportive drugs (e.g., medical injectables and infusions) as well as oral chemotherapy medications. Chemotherapy medications that have not yet received an assigned code and will be billed under a miscellaneous HCPCS code. New chemotherapy regimens and changes to existing chemotherapy regimens. For the list of affected medications, please log in to the Cigna for Health Care Professionals website (CignaforHCP.com > Resources > Reimbursement and Payment Policies > Precertification Policies > Oncology Drugs Requiring Precertification). How to request precertification The preferred and most convenient method to submit precertification requests is through evicore s dedicated website at evicore.com/pages/providerlogin.aspx. evicore s proprietary software streamlines the process, enabling you to open a case, enter customer data, answer clinical questions, and receive a coverage determination on a complete episode of care within two to five minutes. Note: This program applies to commercial business only. (over)
2 You can also upload additional clinical information to support any precertification request that is currently in a pending status. The Clinical Upload Feature is available by logging in to the dedicated website and selecting Upload Additional Clinical in the Authorization Lookup tab. While submitting requests through the website is highly encouraged, you can also request precertification through our dedicated telephone number, (7:00 a.m. 10:00 p.m. EST, Monday - Friday). evicore s medical oncologist reviewers are available to support you as needed from 7:00 a.m. 10:00 p.m. EST, Monday Friday, for standard requests and urgent pediatric precertification requests. Additional information For additional information about our Integrated Oncology Management Program, please visit our dedicated website at evicore.com/cigna/pages/medicaloncology.aspx, or call Cigna Customer Service at Cigna ( ). Thank you for the care you provide our customers. Sincerely, Dr. Ajani Nimmagadda Senior Medical Director All Cigna products and services are provided exclusively by or through operating subsidiaries of Cigna Corporation, including Cigna Health and Life Insurance Company, Connecticut General Life Insurance Company, Cigna Behavioral Health, Inc., and HMO or service company subsidiaries of Cigna Health Corporation. The Cigna name, logo, and other Cigna marks are owned by Cigna Intellectual THN
3 ONCOLOGY DRUGS REQUIRING PRECERTIFICATION THROUGH EVICORE HEALTHCARE For Providers November 2017 The following oncology drugs require precertification through evicore healthcare. For additional information about the Integrated Oncology Management Program, please visit the dedicated program website at evicore.com/cigna/pages/medicaloncology.aspx. * Indicates a drug with dual indications. Requests for non-oncology indications should be submitted to Cigna. ** Indicates a drug requiring authorization under Pharmacy benefit only. ***Beginning 01/01/2018 these oncology drugs will require precertification through evicore Healthcare. HCPCS BRAND NAME GENERIC NAME ROUTE OF ADMINISTRATION C9485 LARTRUVO OLARATUMAB INJECTION J0641 ***FUSILEV ***LEVOLEUCOVORIN CALCIUM J0881 *ARANESP *DARBEPOETIN ALFA IN POLYSORBAT INJECTION J0885 *EPOGEN *EPOETIN ALFA INJECTION J0885 *PROCRIT *EPOETIN ALFA INJECTION J0894 ***DACOGEN ***DECITABINE J0897 *PROLIA *DENOSUMAB SUB-Q J0897 XGEVA DENOSUMAB SUB-Q J1442 *NEUPOGEN *FILGRASTIM INJECTION J1453 ***EMEND ***FOSAPREPITANT DIMEGLUMINE J1930 *SOMATULINE DEPOT *LANREOTIDE ACETATE SUBCUTANEOUS J2353 *SANDOSTATIN LAR *OCTREOTIDE ACETATE,MI-SPHERES INTRAMUSCULAR DEPOT J2354 *OCTREOTIDE *OCTREOTIDE ACETATE INJECTION ACETATE J2469 ***ALOXI ***PALONOSETRON HCL J2505 NEULASTA PEGFILGRASTIM SUBCUTANEOUS J2860 SYLVANT SILTUXIMAB INTRAVEN J3262 *ACTEMRA *TOCILIZUMAB INTRAVEN J3490 SUSTOL GRANISETRON SUB-Q J3490 TECENTRIQ ATEZOLIZUMAB J3490 UNITUXIN DINUTUXIMAB THN Cigna. Some content provided under license. Page 1 of 5
4 J8597 *SYNDROS *DRONABINOL ORAL J8700 TEMODAR TEMOZOLOMIDE ORAL J9000 ***DOXORUBICIN HCL ***DOXORUBICIN HCL J9015 ***PROLEUKIN ***ALDESLEUKIN J9017 ***TRISENOX ***ARSENIC TRIOXIDE J9019 ERWINAZE ASPARAGINASE (ERWINIA CHRYSAN) INJECTION J9025 */***AZACITIDINE */***AZACITIDINE INJECTION J9027 ***CLOLAR ***CLOFARABINE J9032 BELEODAQ BELINOSTAT J9033 TREANDA BENDAMUSTINE HCL J9034 BENDEKA BENDAMUSTINE HCL J9035 *AVASTIN *BEVACIZUMAB J9039 BLINCYTO BLINATUMOMAB J9039 BLINATUMOMAB BLINATUMOMAB J9040 ***BLEOMYCIN ***BLEOMYCIN SULFATE INJECTION SULFATE J9041 ***VELCADE ***BORTEZOMIB INJECTION J9042 ADCETRIS BRENTUXIMAB VEDOTIN J9043 JEVTANA CABAZITAXEL J9045 ***CARBOPLATIN ***CARBOPLATIN J9047 KYPROLIS CARFILZOMIB J9055 ERBITUX CETUXIMAB J9060 ***CISPLATIN ***CISPLATIN J9065 */***CLADRIBINE */***CLADRIBINE J9098 ***DEPOCYT ***CYTARABINE LIPOSOME/PF INTRATHECAL J9100 ***CYTARABINE ***CYTARABINE INJECTION J9120 ***COSMEGEN ***DACTINOMYCIN J9130 ***DACARBAZINE ***DACARBAZINE J9145 DARZALEX DARATUMUMAB J9150 ***DAUNORUBICIN ***DAUNORUBICIN HCL HCL J9155 **FIRMAGON **DEGARELIX ACETATE SUBCUTANEOUS J9171 ***DOCEFREZ ***DOCETAXEL J9176 EMPLICITI ELOTUZUMAB J9178 ***ELLENCE ***EPIRUBICIN HCL J9179 HALAVEN ERIBULIN MESYLATE J9181 ***ETOPOPHOS ***ETOPOSIDE PHOSPHATE J9185 ***FLUDARABINE ***FLUDARABINE PHOSPHATE PHOSPHATE J9190 ***FLUOROURACIL ***FLUOROURACIL J9200 ***FLOXURIDINE ***FLOXURIDINE INJECTION J9201 ***GEMCITABINE HCL ***GEMCITABINE HCL THN Cigna. Some content provided under license. Page 2 of 5
5 J9202 **ZOLADEX **GOSERELIN ACETATE SUBCUTANEOUS J9205 ONIVYDE IRINOTECAN LIPOSOMAL J9206 ***CAMPTOSAR ***IRINOTECAN HCL J9207 ***IXEMPRA ***IXABEPILONE J9208 ***IFEX ***IFOSFAMIDE J9211 ***IDAMYCIN PFS ***IDARUBICIN HCL J9214 *INTRON A *INTERFERON ALFA-2B,RECOMB. INJECTION J9216 *ACTIMMUNE *INTERFERON GAMMA-1B,RECOMB. SUBCUTANEOUS J9217 **LUPRON DEPOT **LEUPROLIDE ACETATE INTRAMUSCULAR J9218 **LEUPROLIDE **LEUPROLIDE ACETATE SUBCUTANEOUS ACETATE J9225 VANTAS HISTRELIN AC IMPLANTATION J9228 YERVOY IPILIMUMAB J9230 ***MUSTARGEN ***MECHLORETHAMINE HCL INJECTION J9245 ***ALKERAN ***MELPHALAN HCL J9261 ***ARRANON ***NELARABINE J9262 SYNRIBO OMACETAXINE MEPESUCCINATE SUBCUTANEOUS J9263 ***OXALIPLATIN ***OXALIPLATIN J9264 ABRAXANE PACLITAXEL PROTEIN-BOUND J9266 ***ONCASPAR ***PEGASPARGASE INJECTION J9267 ***PACLITAXEL ***PACLITAXEL J9268 ***NIPENT ***PENTOSTATIN J9271 KEYTRUDA PEMBROLIZUMAB J9280 ***MITOMYCIN ***MITOMYCIN J9293 ***MITOXANTRONE ***MITOXANTRONE HCL HCL J9295 PORTRAZZA NECITUMUMAB J9299 OPDIVO NIVOLUMAB J9301 GAZYVA OBINUTUZUMAB J9302 ***ARZERRA ***OFATUMUMAB J9303 VECTIBIX PANITUMUMAB J9305 ALIMTA PEMETREXED DISODIUM J9306 PERJETA PERTUZUMAB J9307 ***FOLOTYN ***PRALATREXATE J9308 CYRAMZA RAMUCIRUMAB J9310 *RITUXAN *RITUXIMAB J9315 ***ISTODAX ***ROMIDEPSIN J9320 ***ZANOSAR ***STREPTOZOCIN J9325 IMLYGIC TALIMOGENE LAHERPAREPVEC INJECTION J9328 ***TEMODAR ***TEMOZOLOMIDE J9330 ***TORISEL ***TEMSIROLIMUS J9340 ***THIOTEPA ***THIOTEPA INJECTION THN Cigna. Some content provided under license. Page 3 of 5
6 J9351 ***HYCAMTIN ***TOPOTECAN HCL J9352 YONDELIS TRABECTEDIN J9354 KADCYLA ADO-TRASTUZUMAB EMTANSINE J9355 HERCEPTIN TRASTUZUMAB J9360 ***VINBLASTINE ***VINBLASTINE SULFATE SULFATE J9370 ***VINCASAR PFS ***VINCRISTINE SULFATE J9371 MARQIBO VINCRISTINE SULFATE LIPOSOMAL J9390 ***NAVELBINE ***VINORELBINE TARTRATE J9395 */***FASLODEX */***FULVESTRANT INTRAMUSCULAR J9400 ZALTRAP ZIV-AFLIBERCEPT J9999 BAVENCIO AVELUMAB J9999 IMFINZI DURVALUMAB J9999 VYXEOS LIPOSOME DAUNORUBICIN/CYTARABINE LIPOS J9999 RITUXAN HYCELA RITUXIMAB/HYALURONIDASE,HUMAN SUBCUTANEOUS J9999 BESPONSA INOTUZUMAB OZOGAMICIN J9999 ALIQOPA COPANLISIB DI-HCL J9999 MYLOTARG GEMTUZUMAB OZOGAMICIN Q2017 ***TENIPOSIDE ***TENIPOSIDE Q2043 PROVENGE SIPULEUCEL-T/LACTATED RINGERS Q2050 ***DOXIL ***DOXORUBICIN HCL PEG-LIPOSOMAL S0145 *PEGASYS *PEGINTERFERON ALFA-2A SUBCUTANEOUS S0148 *PEGINTRON *PEGINTERFERON ALFA-2B SUBCUTANEOUS S0148 *SYLATRON *PEGINTERFERON ALFA-2B SUBCUTANEOUS #N/A *KINERET *ANAKINRA SUBCUTANEOUS #N/A *AFINITOR *EVEROLIMUS ORAL #N/A *AFINITOR DISPERZ *EVEROLIMUS ORAL #N/A ALECENSA ALECTINIB HCL ORAL #N/A BOSULIF BOSUTINIB ORAL #N/A CABOMETYX CABOZANTINIB S-MALATE ORAL #N/A CAPRELSA VANDETANIB ORAL #N/A COMETRIQ CABOZANTINIB S-MALATE ORAL #N/A COTELLIC COBIMETINIB FUMARATE ORAL #N/A ERIVEDGE VISMODEGIB ORAL #N/A FARYDAK PANOBINOSTAT LACTATE ORAL #N/A GILOTRIF AFATINIB DIMALEATE ORAL #N/A GLEEVEC IMATINIB MESYLATE ORAL #N/A IBRANCE PALBOCICLIB ORAL #N/A ICLUSIG PONATINIB HCL ORAL #N/A IMBRUVICA IBRUTINIB ORAL #N/A INLYTA AXITINIB ORAL #N/A IRESSA GEFITINIB ORAL THN Cigna. Some content provided under license. Page 4 of 5
7 #N/A *JAKAFI *RUXOLITINIB PHOSPHATE ORAL #N/A LENVIMA LENVATINIB MESYLATE ORAL #N/A LONSURF TRIFLURIDINE/TIPIRACIL HCL ORAL #N/A LYNPARZA OLAPARIB ORAL #N/A MEKINIST TRAMETINIB DIMETHYL SULFOXIDE ORAL #N/A NEXAVAR SORAFENIB TOSYLATE ORAL #N/A NINLARO IXAZOMIB CITRATE ORAL #N/A ODOMZO SONIDEGIB PHOSPHATE ORAL #N/A POMALYST POMALIDOMIDE ORAL #N/A *REVLIMID *LENALIDOMIDE ORAL #N/A RUBRACA RUCAPARIB CAMSYLATE ORAL #N/A SPRYCEL DASATINIB ORAL #N/A STIVARGA REGORAFENIB ORAL #N/A SUTENT SUNITINIB MALATE ORAL #N/A TAFINLAR DABRAFENIB MESYLATE ORAL #N/A TAGRISSO OSIMERTINIB MESYLATE ORAL #N/A TARCEVA ERLOTINIB HCL ORAL #N/A TASIGNA NILOTINIB HCL ORAL #N/A *THALOMID *THALIDOMIDE ORAL #N/A TYKERB LAPATINIB DITOSYLATE ORAL #N/A VENCLEXTA VENETOCLAX ORAL #N/A VOTRIENT PAZOPANIB HCL ORAL #N/A XALKORI CRIZOTINIB ORAL #N/A XTANDI ENZALUTAMIDE ORAL #N/A ZELBORAF VEMURAFENIB ORAL #N/A ZOLINZA VORINOSTAT ORAL #N/A ZYDELIG IDELALISIB ORAL #N/A ZYKADIA CERITINIB ORAL #N/A ZYTIGA ABIRATERONE ACETATE ORAL #N/A KISQALI RIBOCICLIB SUCCINATE ORAL #N/A XERMELO TELOTRISTAT ETIPRATE ORAL #N/A ZEJULA NIRAPARIB ORAL #N/A ALUNBRIG BRIGATINIB ORAL #N/A RYDAPT MIDOSTAURIN ORAL #N/A IDHIFA ENASIDENIB MESYLATE ORAL #N/A NERLYNX NERATINIB MALEATE ORAL #N/A VERZENIO ABEMACICLIB ORAL #N/A CALQUENCE ACALABRUTINIB ORAL THN Cigna. Some content provided under license. Page 5 of 5
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
OPENING KEYNOTE: PRECISION MEDICINE AT THE INFLECTION POINT. Session PM1, March 5, 2018 Damon Hostin, CEO, Precision Medicine Alliance, LLC
 OPENING KEYNOTE: PRECISION MEDICINE AT THE INFLECTION POINT Session PM1, March 5, 2018 Damon Hostin, CEO, Precision Medicine Alliance, LLC 1 Conflicts of Interest Damon Hostin Has no real or apparent conflicts
OPENING KEYNOTE: PRECISION MEDICINE AT THE INFLECTION POINT Session PM1, March 5, 2018 Damon Hostin, CEO, Precision Medicine Alliance, LLC 1 Conflicts of Interest Damon Hostin Has no real or apparent conflicts
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Self Administered Oncology Agents Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
Self Administered Oncology Agents Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY]
![INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY] INJECTION, INOTUZUMAB OZOGAMICIN, 0.1 MG [BESPONSA ] [C CODES FOR FACILITY USE ONLY]](/thumbs/85/92112268.jpg) Commercial Medical Oncology Program Review Code List 3rd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of
Commercial Medical Oncology Program Review Code List 3rd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of
Genetics in Cancer Therapy. Raju Kucherlapati, Ph.D. Harvard Medical School
 Genetics in Cancer Therapy Raju Kucherlapati, Ph.D. Harvard Medical School RK Financial interests I do not intend to discuss an off-label use of a product during this activity AVEO Pharmaceuticals KEW
Genetics in Cancer Therapy Raju Kucherlapati, Ph.D. Harvard Medical School RK Financial interests I do not intend to discuss an off-label use of a product during this activity AVEO Pharmaceuticals KEW
CPT Service Description Effective Date
 Medical Oncology Program Review Code List 2 nd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of January, April,
Medical Oncology Program Review Code List 2 nd Quarter 2018 This list is subject to change once per quarter. Changes will be posted to the BCBSNC website at www.bcbsnc.com by the 10th day of January, April,
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Prior Authorization Proposal: Oncology Agents
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Chemotherapy 101 for Radiation Oncology Workers
 Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Formulary Chemotherapy Agents: (Current as of 6/2018) Therapeutic Class
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Self Administered Oncology Agents Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
Self Administered Oncology Agents Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
Pharmacy Medical Necessity Guidelines: Oral Cancer Medications
 Pharmacy Medical Necessity Guidelines: Effective: February 18, 2019 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED)
Pharmacy Medical Necessity Guidelines: Effective: February 18, 2019 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED)
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
Drug Name. J0129 Injection, abatacept (Orencia ), 10 mg Effective 01/01/2014. J0178 Injection, aflibercept (Eylea ), 1 mg Effective 04/01/2015
 J0129 Injection, abatacept (Orencia ), 10 J0178 Injection, aflibercept (Eylea ), 1 J0256 J0257 J0585 J0586 J0587 J0588 J0597 J0641 J0717 J0800 Injection, alpha 1-proteinase inhibitor, human (Aralast NP,
J0129 Injection, abatacept (Orencia ), 10 J0178 Injection, aflibercept (Eylea ), 1 J0256 J0257 J0585 J0586 J0587 J0588 J0597 J0641 J0717 J0800 Injection, alpha 1-proteinase inhibitor, human (Aralast NP,
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Self Administered Oncology Agents Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
Self Administered Oncology Agents Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
ORAL ONCOLOGY CRITERIA
 ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
ORAL ONCOLOGY CRITERIA
 ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
Provider Administered Drug Program (PADP) and Physician Administered Drug VPSS List
 Provider Administered Drug Program (PADP) and Physician Administered Drug VPSS List Code Drug Name Effective and/or Term Date J0129 Injection, abatacept (Orencia ), 10 mg J0178 Injection, aflibercept (Eylea
Provider Administered Drug Program (PADP) and Physician Administered Drug VPSS List Code Drug Name Effective and/or Term Date J0129 Injection, abatacept (Orencia ), 10 mg J0178 Injection, aflibercept (Eylea
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data
 Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
ANTIEMETICS UTILIZATION MANAGEMENT CRITERIA
 ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
ANTIEMETICS [Akynzeo, Anzemet, Cesamet, Emend, Sancuso, Varubi, Zuplenz ] DRUG CLASS: UTILIZATION MANAGEMENT CRITERIA 5-HT 3 Receptor Antagonists 5-HT 3 Receptor Antagonist and Substance P/Neurokinin (NK
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year
 ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
ORAL ONCOLOGY CRITERIA LENGTH OF AUTHORIZATION: Varies; Maximum of one year REVIEW CRITERIA: Drug Name Indication & Dosage Age Limit Quantity per day AFINITOR (everolimus) AFINITOR DISPERZ (everolimus)
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Overview of Cancer. Laura Bingell RN Transition Center Nurse for MFP (607)
 Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Pharmacy Policy Bulletin
 Pharmacy Policy Bulletin Title: Policy #: Oncology Agents Rx.01.67 Application of pharmacy policy is determined by benefits and contracts. Benefits may vary based on product line, group, or contract. Some
Pharmacy Policy Bulletin Title: Policy #: Oncology Agents Rx.01.67 Application of pharmacy policy is determined by benefits and contracts. Benefits may vary based on product line, group, or contract. Some
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi )
 09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
09-J2000-60 Original Effective Date: 06/15/16 Reviewed: 04/11/18 Revised: 01/01/19 Subject: NK-1 receptor antagonist injectable therapy (Emend, Cinvanti, Varubi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Specialty Drug List - Sorted by Therapeutic Class Developed for the Mississippi Division of Medicaid by Mercer
 ANTI-INFECTIVE ABELCET 100 MG/20 ML VIAL 4/1/2017 ANTI-INFECTIVE AMBISOME 50 MG VIAL 4/1/2017 ANTI-INFECTIVE ANCOBON 250 MG CAPSULE 4/1/2017 ANTI-INFECTIVE ANCOBON 500 MG CAPSULE 4/1/2017 ANTI-INFECTIVE
ANTI-INFECTIVE ABELCET 100 MG/20 ML VIAL 4/1/2017 ANTI-INFECTIVE AMBISOME 50 MG VIAL 4/1/2017 ANTI-INFECTIVE ANCOBON 250 MG CAPSULE 4/1/2017 ANTI-INFECTIVE ANCOBON 500 MG CAPSULE 4/1/2017 ANTI-INFECTIVE
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Bladder Cancer Pathways (Urothelial)
 Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Cancer Therapy Update in 2017
 Cancer Therapy Update in 2017 Editor: Le Wang, M.D., Ph.D Medical Oncology and Hematology (Board-Certified) Ibrutinib (Imbruvica) Pharmacyclics 1 /19/2017 Lymphoma Marginal Zone Lymphoma (MZL), Relapsed/Refractory
Cancer Therapy Update in 2017 Editor: Le Wang, M.D., Ph.D Medical Oncology and Hematology (Board-Certified) Ibrutinib (Imbruvica) Pharmacyclics 1 /19/2017 Lymphoma Marginal Zone Lymphoma (MZL), Relapsed/Refractory
Bladder Cancer (Urothelial) Pathways
 Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Leukemia. Treatment of. compendia. Associated ICD-9-CM Codes: Drug & Administration. managedcareoncology.com
 Drug & compendia Treatment of Leukemia With each publication, Managed Care Oncology s Drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
Drug & compendia Treatment of Leukemia With each publication, Managed Care Oncology s Drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
NICE-approved drugs for specialities which fall outside of the services currently provided by the Trust
 NICE-approved drugs for specialities which fall outside of the services currently provided by the Trust Drug NICE-approved use NICE TA link Abiraterone Abiraterone Adalimumab Adalimumab Adalimumab Adefovir
NICE-approved drugs for specialities which fall outside of the services currently provided by the Trust Drug NICE-approved use NICE TA link Abiraterone Abiraterone Adalimumab Adalimumab Adalimumab Adefovir
ALKYLATING AGENTS (22)
 Serving the United States, Canada and North Central America MEMBER OF TEXAS HEALTH CARE ENTITIES A PRIVATE MEMBERSHIP MEDICAL ASSOCIATION 3105 MAIN STREET, ROWLETT, TX 75088 TEL: 214-299-9449 office@rgccusa.com
Serving the United States, Canada and North Central America MEMBER OF TEXAS HEALTH CARE ENTITIES A PRIVATE MEMBERSHIP MEDICAL ASSOCIATION 3105 MAIN STREET, ROWLETT, TX 75088 TEL: 214-299-9449 office@rgccusa.com
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care
 Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care Presented as a Live Webinar Wednesday, April 13, 2016 12:00 p.m. 1:00 p.m. ET Tuesday,
Chemotherapy-induced Nausea and Vomiting: The Pharmacist s Role in Integrating Clinical Guidelines into Patient Care Presented as a Live Webinar Wednesday, April 13, 2016 12:00 p.m. 1:00 p.m. ET Tuesday,
Targeted Cancer Therapies
 Targeted Cancer Therapies Primary Care Training Programme 14 th February 2018 Sin Chong Lau Consultant in Medical Oncology Financial Disclosure Honoraria: Amgen, Pfizer, Roche, Sanofi, Servier Meetings:
Targeted Cancer Therapies Primary Care Training Programme 14 th February 2018 Sin Chong Lau Consultant in Medical Oncology Financial Disclosure Honoraria: Amgen, Pfizer, Roche, Sanofi, Servier Meetings:
Corporate Medical Policy
 Antiemetic Injection Therapy Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: antiemetic_injection_therapy 5/2015 3/2017 3/2018 3/2017 Description of Procedure
Antiemetic Injection Therapy Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: antiemetic_injection_therapy 5/2015 3/2017 3/2018 3/2017 Description of Procedure
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
Opening Address: A Quick Survey Thinking 25 Years Backwards & Forwards
 Opening Address: A Quick Survey Thinking 25 Years Backwards & Forwards Jeffrey M. Bockman, PhD Vice President Defined Health 24 th Annual Cancer Progress Conference March 4 th 5 th, 2014 March 4 th 5 th,
Opening Address: A Quick Survey Thinking 25 Years Backwards & Forwards Jeffrey M. Bockman, PhD Vice President Defined Health 24 th Annual Cancer Progress Conference March 4 th 5 th, 2014 March 4 th 5 th,
intolerance to, contraindication to, or therapeutic failure on a minimum 3 month trial of Inflectra*
 What s New Medical Pharmaceutical Policy March 2018 Updates MBP 5.0 Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)- Updated policy Remicade (infliximab), Inflectra (infliximab-dyyb)
What s New Medical Pharmaceutical Policy March 2018 Updates MBP 5.0 Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)- Updated policy Remicade (infliximab), Inflectra (infliximab-dyyb)
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
MABCAMPATH mg 3 INJ GENZYME BIOPHARMACEUTICALS. MABCAMPBATH mg 3 INJ GENZYME BIOPHARMACEUTICALS
 ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
Clinical Therapeutic Intelligence Report: 2015 Year in Review
 Clinical Therapeutic Intelligence Report: 2015 Year in Review This issue highlights the many ground-breaking therapies approved by the U.S. Food and Drug Administration over the course of 2015. Highlights
Clinical Therapeutic Intelligence Report: 2015 Year in Review This issue highlights the many ground-breaking therapies approved by the U.S. Food and Drug Administration over the course of 2015. Highlights
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Welcome. Coding uidelines Coding Guidelines Coding. Coding Guidelines Coding Guidelines. Contact Us. Edition #1 March 2018
 Coding Guidelines Coding Guidelines uidelines Coding Guidelines Coding Coding Coding Guidelines Guidelines Coding uidelines Coding Guidelines Coding Coding Chemotherapy Guidelines and Radiation Coding
Coding Guidelines Coding Guidelines uidelines Coding Guidelines Coding Coding Coding Guidelines Guidelines Coding uidelines Coding Guidelines Coding Coding Chemotherapy Guidelines and Radiation Coding
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Ovarian Cancer. compendia TREATMENT OF
 drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
SPECIALTY PHARMACY Master Clinical Drug List
 Abraxane J9264 Provider ONCOLOGY None NO Actemra J3262 Provider ARTHRITIS PA - all YES Acthar HP Gel J0800 Prov/Self Med/Pharm ENDOCRINE/METABOLIC PA - all YES Adagen J2504 Provider ENZYME DISORDERS None
Abraxane J9264 Provider ONCOLOGY None NO Actemra J3262 Provider ARTHRITIS PA - all YES Acthar HP Gel J0800 Prov/Self Med/Pharm ENDOCRINE/METABOLIC PA - all YES Adagen J2504 Provider ENZYME DISORDERS None
New Cancer Drug Update 2015/2016. Teresa Knoop, MSN, RN, AOCN Vanderbilt-Ingram Cancer Center Nashville, TN
 New Cancer Drug Update 2015/2016 Teresa Knoop, MSN, RN, AOCN Vanderbilt-Ingram Cancer Center Nashville, TN Disclosures No conflicts to disclose Objectives Identify new drugs that were FDA approved for
New Cancer Drug Update 2015/2016 Teresa Knoop, MSN, RN, AOCN Vanderbilt-Ingram Cancer Center Nashville, TN Disclosures No conflicts to disclose Objectives Identify new drugs that were FDA approved for
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Supplementary Figures
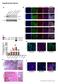 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
TFDA Tyrosine Kinase Inhibitor
 TFDA 1. 癌症診療指引420 http://www.accessdata.fda.gov/scripts/cder/daf https://www.tga.gov.au/orphan-drugs 2. http://www.nhi.gov.tw/webdata/webdata.aspx?menu=21&menu_id=713&wd_id=849&webdata_id=2919 3. http://floridacancer.com/disease-drug-info/drug-dictionary/p/p40/
TFDA 1. 癌症診療指引420 http://www.accessdata.fda.gov/scripts/cder/daf https://www.tga.gov.au/orphan-drugs 2. http://www.nhi.gov.tw/webdata/webdata.aspx?menu=21&menu_id=713&wd_id=849&webdata_id=2919 3. http://floridacancer.com/disease-drug-info/drug-dictionary/p/p40/
Chemotherapy-Induced Nausea and Vomiting: Strategies for Achieving Optimal Control
 Chemotherapy-Induced Nausea and Vomiting Strategies for Achieving Learning Objectives Describe the challenges of assessing nausea in patients undergoing chemotherapy and the impact of nausea and also vomiting
Chemotherapy-Induced Nausea and Vomiting Strategies for Achieving Learning Objectives Describe the challenges of assessing nausea in patients undergoing chemotherapy and the impact of nausea and also vomiting
Melanoma. compendia. Associated ICD-9-CM Codes: Drug & Administration
 Drug & Administration compendia Treatment of Melanoma With each publication, ManagedCare Oncology s Drug & Administration Compendia highlights a single medication or a group of medications that could be
Drug & Administration compendia Treatment of Melanoma With each publication, ManagedCare Oncology s Drug & Administration Compendia highlights a single medication or a group of medications that could be
Chapter 2 Clinical Anticancer Drugs for Cancer Treatment
 Chapter 2 Clinical Anticancer Drugs for Cancer Treatment Besides cytoreductive surgery and radiotherapy, chemotherapy is the most widely used therapeutic strategy in combating cancer. The high incidence
Chapter 2 Clinical Anticancer Drugs for Cancer Treatment Besides cytoreductive surgery and radiotherapy, chemotherapy is the most widely used therapeutic strategy in combating cancer. The high incidence
Pharmacy Management Drug Policy
 SUBJECT: Oncology Clinical Review Prior Authorization (Oncology-CRPA) Rx Drugs POLICY NUMBER: Pharmacy-33 EFFECTIVE DATE: 10/13 LAST REVIEW DATE: 11/12/2018 If the member s subscriber contract excludes
SUBJECT: Oncology Clinical Review Prior Authorization (Oncology-CRPA) Rx Drugs POLICY NUMBER: Pharmacy-33 EFFECTIVE DATE: 10/13 LAST REVIEW DATE: 11/12/2018 If the member s subscriber contract excludes
Pharmacy Management Drug Policy
 SUBJECT: Oncology Clinical Review Prior Authorization (Oncology-CRPA) Rx Drugs POLICY NUMBER: Pharmacy-33 EFFECTIVE DATE: 10/13 LAST REVIEW DATE: 03/22/2018 If the member s subscriber contract excludes
SUBJECT: Oncology Clinical Review Prior Authorization (Oncology-CRPA) Rx Drugs POLICY NUMBER: Pharmacy-33 EFFECTIVE DATE: 10/13 LAST REVIEW DATE: 03/22/2018 If the member s subscriber contract excludes
StrandAdvantage Tissue-Specific Cancer Genomic Tests. Empowering Crucial First-Line Therapy Decisions for Your Patient
 StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
Vectibix (panitumumab) will be considered medically necessary when all of the following criteria are met:
 What s New Medical Pharmaceutical Policy November 2017 Updates MBP 40.0 Orencia IV (abatacept)- New Indication Orencia IV (abatacept) will be considered medically necessary when all of the following criteria
What s New Medical Pharmaceutical Policy November 2017 Updates MBP 40.0 Orencia IV (abatacept)- New Indication Orencia IV (abatacept) will be considered medically necessary when all of the following criteria
Clinical Policy: Aprepitant (Emend) Reference Number: CP.PMN.19 Effective Date: 11/06 Last Review Date: 08/17
 Clinical Policy: (Emend) Reference Number: CP.PMN.19 Effective Date: 11/06 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Emend) Reference Number: CP.PMN.19 Effective Date: 11/06 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Cancer Drug Pipeline Information for Patient Advocacy Groups. Breast Cancer. Route of Administration. Brand Name Generic Name Manufacturer Indication
 Please note: CCAN and CADTH do not represent or warrant that any of the drug products listed in the will be submitted to the pcodr program. The use of this information is solely intended for informational
Please note: CCAN and CADTH do not represent or warrant that any of the drug products listed in the will be submitted to the pcodr program. The use of this information is solely intended for informational
Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting
 Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University
Prevention and Management of cancer disease and of chemo-and radiotherapyinduced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University
Pharmacy Management Drug Policy
 SUBJECT: Oncology Clinical Review Prior Authorization (Oncology-CRPA) Medical Drugs POLICY NUMBER: Pharmacy-64 EFFECTIVE DATE: 10/13 LAST REVIEW DATE: 09/05/2018 If the member s subscriber contract excludes
SUBJECT: Oncology Clinical Review Prior Authorization (Oncology-CRPA) Medical Drugs POLICY NUMBER: Pharmacy-64 EFFECTIVE DATE: 10/13 LAST REVIEW DATE: 09/05/2018 If the member s subscriber contract excludes
Journal of Stem Cell Research
 Review Article Challenges to Cure Cancer Journal of Stem Cell Research Sudha Bansode* Associate Professor in Zoology, Shankarrao Mohite College, Akluj, Maharashtra State, India * Corresponding author Dr.
Review Article Challenges to Cure Cancer Journal of Stem Cell Research Sudha Bansode* Associate Professor in Zoology, Shankarrao Mohite College, Akluj, Maharashtra State, India * Corresponding author Dr.
