number Done by Corrected by Doctor Nafeth Abu tarboush
|
|
|
- Evelyn Dorsey
- 5 years ago
- Views:
Transcription
1 number 2 Done by MamoonMohamadAlqtaminAlq Corrected by Moayyad Al-shafei Doctor Nafeth Abu tarboush 1 P a g e
2 In the previous lecture, we have started talking about plasma proteins (concept, classification ). In this lecture we will continue talking about them, and we will talk briefly about albumin. Plasma proteins half-life The time that is needed for the protein to reach its half concentration. Can be determined through isotope labeling studies (I131) We label the protein with (I131), then introduce it into the circulation and keep observing it until it reaches half the concentration, and in this way the half-life is determined. Plasma proteins vary in their half-lives; Albumin (20 days) and Haptoglobin (5 days) Diseases can affect the protein s half-life, for example; 1. In the case of Crohn s disease, albumin s half-life may be reduced to 1 day. 2. Protein-losing gastroenteropathyis characterized by the loss of plasma proteins into the GI tract. Functions of plasma proteins General Functions Specific functions 1- General functions;it means all plasma proteins do them due to sharing some common features such as; *All proteins are built from amino acids * They are found in the blood especially in the plasma **Note: any function for any plasma protein that is a result from its unique structure (side chains of its amino acids, arrangement of its amino acids..) Is not considered a general function for all plasma proteins. a) A nutritive role: all plasma proteins can be broken down to give energy (metabolism of plasma proteins) in the absence of fats and carbohydrates. 2 P a g e
3 b) Maintenance of blood ph (amphoteric property): by working as buffering agents; because all proteins have a free carboxylgroup and a free amino group which can accept or donate protons. c) Contribution to blood viscosity: all plasma proteins are glycoproteins (attached to carbohydrates) except for albumin. The addition of carbohydrates to plasma proteins will increase the negative charges, so water molecules will attach strongly to plasma proteins in order to increase viscosity. Albumin is a simple protein because it already has a higher negative charge, so glycosylation is not necessary for it. d) Maintenance of blood osmotic pressure: it is a result of dissolved solids in the blood; mainly plasma proteins, and always in the opposite direction of the hydrostatic pressure. 2- Specific functions: for certain plasma proteins; a) Enzymes (e.g. rennin, coagulation factors, lipases) b) Humeral immunity (immunoglobulins) c) Blood coagulation factors d) Hormonal (Erythropoietin) e) Transport proteins (Transferrin, Thyroxin binding globulin, Apolipoprotein). Starling forces Normal blood pressure (120/80) ** extra info ** Blood is a liquid, so it forms pressure on the walls of blood vessels (hydrostatic pressure). This pressure is trying to get water outside the capillaries into the interstitial fluid in a process called Filtration. Another type of pressure is found due to presence of plasma proteins called osmotic pressure. This pressure is trying to get water inside the capillaries(reabsorption). In the arterial side of capillaries: the hydrostatic pressure is higher than the oncotic pressure (filtration will occur). In the venous side: the oncotic pressure is higher than the hydrostatic pressure (reabsorption will occur). 3 P a g e
4 *In normal conditions, the net movement of water is zero because filtration in the arterial side is equal to reabsorption in the venous side). **Assume that you have a pathogenic reason that leads to the reduction of plasma proteins concentrations. Oncotic pressure reabsorption Accumulation of water in the interstitial fluid will result in edema. In the case of liver failure: the concentration of plasma proteins will decrease, so the oncotic pressure will decrease. Suppose that the oncotic pressure is 20 mmhg instead of 25, we will get this result: In the arterial end =20 (20 filtration) In the Venous end 10-20= -10 (10 reabsorption) More filtration than reabsorption:this causes Edema. Acute phase proteins ( الدكتور ركز ع هاد الموضوع سؤاله اكيد في االمتحان ) In the case of acute infection or chronic infection, cancerinflammatory processes start to release inflammatory mediators (markers, signals) such as Interleukin -1that is exported by certain cells and binds to liver cells. **In the cytosol of liver cells, there is a transcription factor called nuclear factor kappa-b (NFkB) in the inactive form. 4 P a g e
5 When the interleukin-1 binds to the liver cells,(nfkb ) will be activated andtransported to the nucleus, where it will bind to certain promoters on the DNA that activate producing mrna which is then translated into several types of proteins (acute phase proteins): 1) C-reactive protein 2) α1 antitrypsin 3) Haptoglobin 4) Fibrinogen. **Others plasma proteins won t change or may reduce in concentration(negative acute phase proteins). 1) Albumin 2) Prealbumin 3) Transferrin. Albumin; The main transporter in the blood ( مترو االنفاق ) The Major Protein in Human Plasma, 69 kda, half-life (20 days) The main contributor to the colloid osmotic ( oncotic )pressure (75-80%) (the highest concentration) Synthesized as a preproprotein in the liver, then some modifications occur to reach the final shape. Hexapeptide (6 amino acids) Prepro Albumin Pro albumin Albumin Signal peptide The liver produces 12 g/day of albumin (25% of total protein synthesis), that s why we can use the concentration of albumin in liver function tests. It is made of one polypeptide chain, 585 amino acids, and 17 disulfide bonds(so its final shape is fixed). 5 P a g e
6 Proteases subdivide albumin into 3 domainseach domain has a certain function. One of these domains is called drug binding domain or albumin binding domain. Albumin is ellipsoidal in shape (viscosity). It is anionic at ph 7.4 with 20 negative charges. Albumin binds various ligands: 1) Free fatty acids (FFA) 2) Certain steroid hormones 3) Plasma tryptophan Remember tryptophan is non polar (so it needs transport proteins in the blood). 4) Metals: Calcium, copper and heavy metals. 5) Bilirubin: all structures in our bodies must undergo metabolism such as :heme group ( in the liver ), then it is converted into bilirubin in a certain way (the pathway is not included). *Bilirubin accumulates in the liver then it is transferred to the Gallbladder. *In some liver diseases such as Hepatitis A, bilirubin goes to the blood, then to the tissues. 6) Drugs: sulfonamides, penicillin G, dicumarol, aspirin on the binding domain of albumin. Drug-drug interaction Note: Albumin is the site that binds many drugs, so we may find more than one drug that binds to the same site on albumin. *The drug with the higher concentration and higher affinity will bind. *The free site of the drug affects the body (not the one that binds to albumin) 1. Bilirubin-Aspirin interaction Children and newborns must not take aspirin; because it binds on albumin instead of bilirubin. Therefore accumulation of bilirubin will occur in brain and that leads to mental retardation(kernicterus) Bilirubin toxicity. 6 P a g e
7 2. Phenytoin-dicoumarol interaction Phenytoin is a treatment for Epilepsy. Dicoumarol is an anticoagulant substance. Both are competitive ligands, and must not be taken at the same time. Analbuminemia(no albumin) There are human cases of analbuminemia that are rare. Transferred through autosomal recessive inheritance. One of the underlying causes is a mutation that affects splicing. Patients show moderate edema. Note: because albumin has the highest concentration in the plasma,hypoalbuminemia means hypoproteinemia. In the case of analbuminemia: we predict that severeedema will occur, but in real situations, moderate edemaoccurs because the liver compensates by producing another plasma proteins to retain the general function of plasma proteins (Maintenance of oncotic pressure). Normal Analbuminemia Hypoalbuminemia Edema seen in conditions where albumin level in the blood is less than 2 g/dl. It is caused by; 1) Malnutrition (generalized edema) 2) Nephrotic syndrome 3) Cirrhosis (mainly ascites) 4) Gastrointestinal loss (Protein-losing gastroenteropathy) reducing the halflife of albumin. 7 P a g e
8 Hyperalbuminemia Dehydration causes a relative increase (not an actual increase) in the plasma proteins concentration, because their concentration is mainly constant. However, when water levels decrease, the concentration of plasma proteins will relatively increase. 8 P a g e
Plasma proteins Quantitatively, proteins are the most important part of the soluble components of the blood plasma.
 Plasma proteins 42 Plasma proteins Quantitatively, proteins are the most important part of the soluble components of the blood plasma. concentrations of between 60 and 80 g L 1, they constitute approximately
Plasma proteins 42 Plasma proteins Quantitatively, proteins are the most important part of the soluble components of the blood plasma. concentrations of between 60 and 80 g L 1, they constitute approximately
Omar Alnairat. Tamer Barakat. Bahaa Abdelrahim. Dr.Nafez
 1 Omar Alnairat Tamer Barakat Bahaa Abdelrahim Dr.Nafez It s the chemistry inside living cells. What is biochemistry? Biochemistry consists of the structure and function of macromolecules (in the previous
1 Omar Alnairat Tamer Barakat Bahaa Abdelrahim Dr.Nafez It s the chemistry inside living cells. What is biochemistry? Biochemistry consists of the structure and function of macromolecules (in the previous
Separation of Main Proteins in Plasma and Serum
 BCH 471 Experiment (2) Separation of Main Proteins in Plasma and Serum PLASMA PROTEINS Mw The main plasma proteins are: þ Albumin (36-50 g/l), Mw 66.241kDa. þ Globulins (18-32 g/l), Mw of globulins Cover
BCH 471 Experiment (2) Separation of Main Proteins in Plasma and Serum PLASMA PROTEINS Mw The main plasma proteins are: þ Albumin (36-50 g/l), Mw 66.241kDa. þ Globulins (18-32 g/l), Mw of globulins Cover
Rq : Serum = plasma w/ fibrinogen and other other proteins involved in clotting removed.
 Functions of the blood Transport Nutritive Respiratory Excretory Hormone transport Temperature regulation Acid base balance ph (7.30 7.45) Protective (immunology) Rq : It comprises both ECF (plasma) &
Functions of the blood Transport Nutritive Respiratory Excretory Hormone transport Temperature regulation Acid base balance ph (7.30 7.45) Protective (immunology) Rq : It comprises both ECF (plasma) &
Diabetes. Albumin. Analyte Information
 Diabetes Albumin Analyte Information -1-2014-05-02 Albumin Introduction Albumin consists of a single polypeptide chain of 585 amino acids with molecular weight of 66.5 kda. The chain is characterized by
Diabetes Albumin Analyte Information -1-2014-05-02 Albumin Introduction Albumin consists of a single polypeptide chain of 585 amino acids with molecular weight of 66.5 kda. The chain is characterized by
Body fluids. Lecture 13:
 Lecture 13: Body fluids Body fluids are distributed in compartments: A. Intracellular compartment: inside the cells of the body (two thirds) B. Extracellular compartment: (one third) it is divided into
Lecture 13: Body fluids Body fluids are distributed in compartments: A. Intracellular compartment: inside the cells of the body (two thirds) B. Extracellular compartment: (one third) it is divided into
Laboratory diagnosis of plasma proteins and plasma enzymes
 Laboratory diagnosis of plasma proteins and plasma enzymes Functions of plasma proteins Function: transport humoral immunity enzymes protease inhibitors maintenance of oncotic pressure buffering Example:
Laboratory diagnosis of plasma proteins and plasma enzymes Functions of plasma proteins Function: transport humoral immunity enzymes protease inhibitors maintenance of oncotic pressure buffering Example:
Anemia Cases For Discussion
 Anemia Cases For Discussion Objective Goal: Determine the nature of the clinical problems. Identify the hematologic problems using the clinical and laboratory data. Answer the questions at the end of the
Anemia Cases For Discussion Objective Goal: Determine the nature of the clinical problems. Identify the hematologic problems using the clinical and laboratory data. Answer the questions at the end of the
PLASMA PROTIENS. 3)What are the plasma proteins,their functions generally and specifically and processes associated with these proteins?
 1 PLASMA PROTIENS Refer to slide #1 * Goals from these 2 lectures: 1)What is the plasma? How can we get it? 2)What are the different components of the plasma? 3)What are the plasma proteins,their functions
1 PLASMA PROTIENS Refer to slide #1 * Goals from these 2 lectures: 1)What is the plasma? How can we get it? 2)What are the different components of the plasma? 3)What are the plasma proteins,their functions
Please check the slides
 Quick review of main concepts: The major plasma proteins are : albumin, globulin and fibrenogen globulin consists of 3 types: α, β and γ α globulin is divided into 2 types : α1 (includes α1 antitrypsin
Quick review of main concepts: The major plasma proteins are : albumin, globulin and fibrenogen globulin consists of 3 types: α, β and γ α globulin is divided into 2 types : α1 (includes α1 antitrypsin
number Done by Corrected by Doctor
 number 9 Done by Nazek Hyasat Corrected by Bahaa Najjar & mohammed AL-shrouf Doctor Alia Shatnawi HOW DO DRUGS WORK??? You know that receptors are targeted by drugs, the question now is how do these drugs
number 9 Done by Nazek Hyasat Corrected by Bahaa Najjar & mohammed AL-shrouf Doctor Alia Shatnawi HOW DO DRUGS WORK??? You know that receptors are targeted by drugs, the question now is how do these drugs
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
Chapter 6 Reading Guide
 Chapter 6 Reading Guide 1. Describe the structure of an amino acid. 2. What s the difference between an amino acid and a protein? Where do dipeptides, tripeptides and polypeptides fit in? 3. How many amino
Chapter 6 Reading Guide 1. Describe the structure of an amino acid. 2. What s the difference between an amino acid and a protein? Where do dipeptides, tripeptides and polypeptides fit in? 3. How many amino
Receptors Functions and Signal Transduction- L4- L5
 Receptors Functions and Signal Transduction- L4- L5 Faisal I. Mohammed, MD, PhD University of Jordan 1 PKC Phosphorylates many substrates, can activate kinase pathway, gene regulation PLC- signaling pathway
Receptors Functions and Signal Transduction- L4- L5 Faisal I. Mohammed, MD, PhD University of Jordan 1 PKC Phosphorylates many substrates, can activate kinase pathway, gene regulation PLC- signaling pathway
Cardiovascular Module
 Cardiovascular Module Cardiovascular Physiology Lect. Six Microcirculation & Lymphatics (Edema formation) Prof. Dr. Najeeb Hassan Mohammed The microcirculation and the lymphatic system The microcirculation
Cardiovascular Module Cardiovascular Physiology Lect. Six Microcirculation & Lymphatics (Edema formation) Prof. Dr. Najeeb Hassan Mohammed The microcirculation and the lymphatic system The microcirculation
Hormones, Receptors and Receptor-Hormone Interactions
 Classification of Hormones Hormones, Receptors and Receptor-Hormone Interactions Synthesis of Protein Hormones and Amine Hormones Hormone Activity Locations of Receptors Mechanisms of Hormone Action Types
Classification of Hormones Hormones, Receptors and Receptor-Hormone Interactions Synthesis of Protein Hormones and Amine Hormones Hormone Activity Locations of Receptors Mechanisms of Hormone Action Types
Good Afternoon! 11/30/18
 Good Afternoon! 11/30/18 1. The term polar refers to a molecule that. A. Is cold B. Has two of the same charges C. Has two opposing charges D. Contains a hydrogen bond 2. Electrons on a water molecule
Good Afternoon! 11/30/18 1. The term polar refers to a molecule that. A. Is cold B. Has two of the same charges C. Has two opposing charges D. Contains a hydrogen bond 2. Electrons on a water molecule
Blood Vessels. Chapter 20
 Blood Vessels Chapter 20 Summary of the Characteristics of Arteries and Veins Characteristic Artery Vein Wall thickness thick thin Shape in cross section round flattened Thickest tunic media externa Collagen
Blood Vessels Chapter 20 Summary of the Characteristics of Arteries and Veins Characteristic Artery Vein Wall thickness thick thin Shape in cross section round flattened Thickest tunic media externa Collagen
the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids
 the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids and their sub-units; the role of lipids in the plasma
the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids and their sub-units; the role of lipids in the plasma
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Receptors Functions and Signal Transduction L1- L2
 Receptors Functions and Signal Transduction L1- L2 Faisal I. Mohammed, MD, PhD University of Jordan 1 Introduction to Physiology (0501110) Summer 2012 Subject Lecture No. Lecturer Pages in the 11 th edition.
Receptors Functions and Signal Transduction L1- L2 Faisal I. Mohammed, MD, PhD University of Jordan 1 Introduction to Physiology (0501110) Summer 2012 Subject Lecture No. Lecturer Pages in the 11 th edition.
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
18. PANCREATIC FUNCTION AND METABOLISM. Pancreatic secretions ISLETS OF LANGERHANS. Insulin
 18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
Any of these questions could be asked as open question or lab question, thus study them well
 Any of these questions could be asked as open question or lab question, thus study them well describe the factors which regulate cardiac output describe the sympathetic and parasympathetic control of heart
Any of these questions could be asked as open question or lab question, thus study them well describe the factors which regulate cardiac output describe the sympathetic and parasympathetic control of heart
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
Water compartments inside and outside cells maintain a balanced distribution of total body water.
 Chapter 9 Water Balance Chapter 9 Lesson 9.1 Key Concepts Water compartments inside and outside cells maintain a balanced distribution of total body water. The concentration of various solute particles
Chapter 9 Water Balance Chapter 9 Lesson 9.1 Key Concepts Water compartments inside and outside cells maintain a balanced distribution of total body water. The concentration of various solute particles
About This Chapter. Hormones The classification of hormones Control of hormone release Hormone interactions Endocrine pathologies Hormone evolution
 About This Chapter Hormones The classification of hormones Control of hormone release Hormone interactions Endocrine pathologies Hormone evolution Hormones: Function Control Rates of enzymatic reactions
About This Chapter Hormones The classification of hormones Control of hormone release Hormone interactions Endocrine pathologies Hormone evolution Hormones: Function Control Rates of enzymatic reactions
Body Fluids and Fluid Compartments
 Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Biology 12 June 2001 Provincial Examination
 Biology 12 June 2001 rovincial Examination ANWER KEY / CORING GUIDE CURRICULUM: Organizers 1. Cell Biology 2. Cell rocesses and Applications 3. Human Biology ub-organizers A, B, C, D E, F, G, H I, J, K,
Biology 12 June 2001 rovincial Examination ANWER KEY / CORING GUIDE CURRICULUM: Organizers 1. Cell Biology 2. Cell rocesses and Applications 3. Human Biology ub-organizers A, B, C, D E, F, G, H I, J, K,
Sheet #10 Dr. Eman Al-khateeb 21/4/2014. Body fluid I. -what type of IV(Intravenous) fluid should be given to this patient
 Body fluid I Introduction : before we start talking about body fluid I want to mention a few point why we give you these lectures, as mentioned in the first lectures with doctor faisal we have a balanced
Body fluid I Introduction : before we start talking about body fluid I want to mention a few point why we give you these lectures, as mentioned in the first lectures with doctor faisal we have a balanced
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Name: Date: Block: Biology 12
 Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
PHSI2006/2906: Integrated Physiology B
 PHSI2006/2906: Integrated Physiology B TOPIC 1: RESPIRATION 1. The Mechanics of Breathing...2 2. Work of Breathing....5 3. Pulmonary Gas Exchange.. 10 4. Transport of Oxygen...16 5. Control of Respiration...20
PHSI2006/2906: Integrated Physiology B TOPIC 1: RESPIRATION 1. The Mechanics of Breathing...2 2. Work of Breathing....5 3. Pulmonary Gas Exchange.. 10 4. Transport of Oxygen...16 5. Control of Respiration...20
Microcirculation and Edema. Faisal I. Mohammed MD, PhD.
 Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
DNA and Protein Synthesis Practice
 Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs
Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs
Tala Saleh. Riham Abu Arrah, Abdallah AlQawasmeh. Yanal Shafagoj
 27 Tala Saleh Riham Abu Arrah, Abdallah AlQawasmeh Yanal Shafagoj Cardiovascular system Think of the following situation: 5 Cancerous cells (for example: Lymphoma cells) are placed in a proper medium with
27 Tala Saleh Riham Abu Arrah, Abdallah AlQawasmeh Yanal Shafagoj Cardiovascular system Think of the following situation: 5 Cancerous cells (for example: Lymphoma cells) are placed in a proper medium with
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
The Digestive System. What is the advantage of a one-way gut? If you swallow something, is it really inside you?
 The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
Blood. Biol 105 Lecture 14 Chapter 11
 Blood Biol 105 Lecture 14 Chapter 11 Outline I. Overview of blood II. Functions of blood III. Composition of blood IV. Composition of plasma V. Composition of formed elements VI. Platelets VII. White blood
Blood Biol 105 Lecture 14 Chapter 11 Outline I. Overview of blood II. Functions of blood III. Composition of blood IV. Composition of plasma V. Composition of formed elements VI. Platelets VII. White blood
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Like other Biological Macromolecules such as polysaccharides and nucleic acids, Proteins are essential parts of all living organisms and participate
 Like other Biological Macromolecules such as polysaccharides and nucleic acids, Proteins are essential parts of all living organisms and participate in every process within cells. The RDA of protein intake
Like other Biological Macromolecules such as polysaccharides and nucleic acids, Proteins are essential parts of all living organisms and participate in every process within cells. The RDA of protein intake
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Chapter 19. Openstax: Chapter 18. Blood
 Chapter 19 Blood Openstax: Chapter 18 Chapter 19 Learning Outcomes After completing Chapter 19, you will be able to: 1. Describe the components and major functions of blood and list the physical characteristics
Chapter 19 Blood Openstax: Chapter 18 Chapter 19 Learning Outcomes After completing Chapter 19, you will be able to: 1. Describe the components and major functions of blood and list the physical characteristics
Principles of Fluid Balance
 Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Hyperemia, Congestion, and Edema
 Hyperemia, Congestion, and Edema Hyperemia Acute, actively increased blood flow Tissues look red (erythema) Congestion Chronic, passively reduced outflow Tissues look pale or blue (cyanosis) Edema Water
Hyperemia, Congestion, and Edema Hyperemia Acute, actively increased blood flow Tissues look red (erythema) Congestion Chronic, passively reduced outflow Tissues look pale or blue (cyanosis) Edema Water
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Proteins. Dr. Basima Sadiq Jaff. /3 rd class of pharmacy. PhD. Clinical Biochemistry
 Proteins /3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry a Greek word that means of first importance. It is a very important class of food molecules that provide organisms not
Proteins /3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry a Greek word that means of first importance. It is a very important class of food molecules that provide organisms not
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Microcirculation and Edema- L1 L2
 Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Hematology. The Study of blood
 Hematology The Study of blood Average adult = 8-10 pints of blood Composition: PLASMA liquid portion of blood without cellular components Serum plasma after a blood clot is formed Cellular elements are
Hematology The Study of blood Average adult = 8-10 pints of blood Composition: PLASMA liquid portion of blood without cellular components Serum plasma after a blood clot is formed Cellular elements are
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Unit 1: Biochemistry
 Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
A. Lipids: Water-Insoluble Molecules
 Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Biological Substances found in Living Tissues Lecture Series 3 Macromolecules: Their Structure and Function A. Lipids: Water-Insoluble Lipids can form large biological molecules, but these aggregations
Laith Abu Shekha. Omar Sami. Ebaa Alzayadneh
 24 Laith Abu Shekha Omar Sami Ebaa Alzayadneh Signal Transduction Please note that it s very important to refer to the slides. Introduction: Through these five lectures, we should know the basics of signal
24 Laith Abu Shekha Omar Sami Ebaa Alzayadneh Signal Transduction Please note that it s very important to refer to the slides. Introduction: Through these five lectures, we should know the basics of signal
Microcirculation. Lecture Block 11 (contributions from Brett Burton)
 Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
By the name of Allah
 By the name of Allah Receptors function and signal transduction ( Hormones and receptors Types) We were talking about receptors of the neurotransmitters; we have 2 types of receptors: 1- Ionotropic receptors
By the name of Allah Receptors function and signal transduction ( Hormones and receptors Types) We were talking about receptors of the neurotransmitters; we have 2 types of receptors: 1- Ionotropic receptors
Ch 11: Endocrine System
 Ch 11: Endocrine System SLOs Describe the chemical nature of hormones and define the terms proand prepro-hormone. Explain mechanism of action of steroid and thyroid hormones Create chart to distinguish
Ch 11: Endocrine System SLOs Describe the chemical nature of hormones and define the terms proand prepro-hormone. Explain mechanism of action of steroid and thyroid hormones Create chart to distinguish
Essential Components of Food
 Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting
 Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting Question No. 1 of 10 Question 1. Which of the following statements about the nucleus is correct? Question #01 A. The
Molecular Cell Biology Problem Drill 16: Intracellular Compartment and Protein Sorting Question No. 1 of 10 Question 1. Which of the following statements about the nucleus is correct? Question #01 A. The
Albumin (serum, plasma)
 Albumin (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Albumin (plasma or serum) 1.2 Alternative names None (note that albumen is a protein found in avian eggs) 1.3 NLMC code 1.4
Albumin (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Albumin (plasma or serum) 1.2 Alternative names None (note that albumen is a protein found in avian eggs) 1.3 NLMC code 1.4
numbe r Done by Corrected Docto Alia Shatnawi
 numbe r 9 Done by Nazek Hyasat Corrected Bahaa Najjar & mohammed AL-shrouf Docto Alia Shatnawi HOW DO DRUGS WORK??? You know that receptor targets by the drugs, the question now how these drugs work on
numbe r 9 Done by Nazek Hyasat Corrected Bahaa Najjar & mohammed AL-shrouf Docto Alia Shatnawi HOW DO DRUGS WORK??? You know that receptor targets by the drugs, the question now how these drugs work on
Module C CHEMISTRY & CELL BIOLOGY REVIEW
 Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
Week 3 The Pancreas: Pancreatic ph buffering:
 Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
BIOLOGY 111. CHAPTER 2: The Chemistry of Life Biological Molecules
 BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
Biochemistry Macromolecules and Enzymes. Unit 02
 Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
Filtration and Reabsorption Amount Filter/d
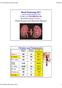 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Close to site of release (at synapse); binds to receptors in
 Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
March 19 th Batool Aqel
 March 19 th - 2013 6 Batool Aqel Hormones That Bind to Nuclear Receptor Proteins Hormones bind to their receptors.whether the receptor is found in the nucleus or the cytoplasm, at the end they are translocated
March 19 th - 2013 6 Batool Aqel Hormones That Bind to Nuclear Receptor Proteins Hormones bind to their receptors.whether the receptor is found in the nucleus or the cytoplasm, at the end they are translocated
The Structure and Function of Large Biological Molecules
 NAME DATE Chapter 5 - The Structure and Function of Large Biological Molecules Guided Reading Concept 5.1: Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall
NAME DATE Chapter 5 - The Structure and Function of Large Biological Molecules Guided Reading Concept 5.1: Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall
Q1: Circle the best correct answer: (15 marks)
 Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
UNIT 2 DIABETES REVIEW
 UNIT 2 DIABETES REVIEW Pancreas is unable to make insulin. Therefore, glucose cannot get into the cells for energy. Insulin is made, but cell receptors do not work at getting recognizing that insulin.
UNIT 2 DIABETES REVIEW Pancreas is unable to make insulin. Therefore, glucose cannot get into the cells for energy. Insulin is made, but cell receptors do not work at getting recognizing that insulin.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
EDEMA. Basic Course of Diagnosis
 EDEMA Basic Course of Diagnosis Definition A clinical apparent increase in the interstitial fluid volume. Distribution: local general Special form: ascites hydrothorax Pathogenesis Total body water(tbw):
EDEMA Basic Course of Diagnosis Definition A clinical apparent increase in the interstitial fluid volume. Distribution: local general Special form: ascites hydrothorax Pathogenesis Total body water(tbw):
Blood. Plasma. The liquid part of blood is called plasma. 1. Pale yellow fluid; forms more than half the blood volume.
 11 Blood FOCUS: Blood consists of plasma and formed elements. The plasma is 91% water with dissolved or suspended molecules, including albumin, globulins, and fibrinogen. The formed elements include erythrocytes,
11 Blood FOCUS: Blood consists of plasma and formed elements. The plasma is 91% water with dissolved or suspended molecules, including albumin, globulins, and fibrinogen. The formed elements include erythrocytes,
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
General Pathophysiology PROTEIN METABOLISM
 General Pathophysiology PROTEIN METABOLISM 1. The degradation of proteins is selectively disturbed when there is deficit of (pick the most complete answer): 1. Saliva, amylase, lipase, carboxypeptidases.
General Pathophysiology PROTEIN METABOLISM 1. The degradation of proteins is selectively disturbed when there is deficit of (pick the most complete answer): 1. Saliva, amylase, lipase, carboxypeptidases.
CLASS SET. Modeling Life s Important Compounds. AP Biology
 Modeling Life s Important Compounds AP Biology CLASS SET OBJECTIVES: Upon completion of this activity, you will be able to: Explain the connection between the sequence and the subcomponents of a biological
Modeling Life s Important Compounds AP Biology CLASS SET OBJECTIVES: Upon completion of this activity, you will be able to: Explain the connection between the sequence and the subcomponents of a biological
Biology: Life on Earth Chapter 3 Molecules of life
 Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Week 2. Macromolecules
 Week 2 In living organisms, smaller molecules are often attached to each other to make larger molecules. These smaller molecules are sometimes called monomers, and the larger molecules made from these
Week 2 In living organisms, smaller molecules are often attached to each other to make larger molecules. These smaller molecules are sometimes called monomers, and the larger molecules made from these
Chapter 13. Plasma Proteins and Enzymes. Lecturer: Dr Abeer Shnoudeh. Clinical Chemistry William Marshall
 Chapter 13 Plasma Proteins and Enzymes Lecturer: Dr Abeer Shnoudeh Clinical Chemistry William Marshall Introduction Measurement of Plasma Proteins Total Plasma Protein concentration of total protein in
Chapter 13 Plasma Proteins and Enzymes Lecturer: Dr Abeer Shnoudeh Clinical Chemistry William Marshall Introduction Measurement of Plasma Proteins Total Plasma Protein concentration of total protein in
Dergam Al-Tarawneh. - Saleem khresha. 1 P a g e
 - 11 - Dergam Al-Tarawneh - - Saleem khresha 1 P a g e This will be an exact replica of what Dr. Saleem said because weʼve already experienced that even if the science says something you should always
- 11 - Dergam Al-Tarawneh - - Saleem khresha 1 P a g e This will be an exact replica of what Dr. Saleem said because weʼve already experienced that even if the science says something you should always
Introduction to Biochemistry
 Life is Organized in Increasing Levels of Complexity Introduction to Biochemistry atom simple molecule What is the chemical makeup of living things? macromolecule organ organ system organism organelle
Life is Organized in Increasing Levels of Complexity Introduction to Biochemistry atom simple molecule What is the chemical makeup of living things? macromolecule organ organ system organism organelle
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Chapter 5: The Structure and Function of Large Biological Molecules
 Chapter 5: The Structure and Function of Large Biological Molecules 1. Name the four main classes of organic molecules found in all living things. Which of the four are classified as macromolecules. Define
Chapter 5: The Structure and Function of Large Biological Molecules 1. Name the four main classes of organic molecules found in all living things. Which of the four are classified as macromolecules. Define
2018/9/24 AMINO ACIDS. Important. 436 Notes Original slides. 438 notes Extra information
 2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
Biological Molecules Ch 2: Chemistry Comes to Life
 Outline Biological Molecules Ch 2: Chemistry Comes to Life Biol 105 Lecture 3 Reading Chapter 2 (pages 31 39) Biological Molecules Carbohydrates Lipids Amino acids and Proteins Nucleotides and Nucleic
Outline Biological Molecules Ch 2: Chemistry Comes to Life Biol 105 Lecture 3 Reading Chapter 2 (pages 31 39) Biological Molecules Carbohydrates Lipids Amino acids and Proteins Nucleotides and Nucleic
BIOL 2458 CHAPTER 19 Part 1 SI 1. List the types of extracellular fluids. 2. Intracellular fluid makes up of the body fluids. Where is it found?
 BIOL 2458 CHAPTER 19 Part 1 SI 1 1. Extracellular fluid makes up of the body fluids. List the types of extracellular fluids. 2. Intracellular fluid makes up of the body fluids. Where is it found? 3. In
BIOL 2458 CHAPTER 19 Part 1 SI 1 1. Extracellular fluid makes up of the body fluids. List the types of extracellular fluids. 2. Intracellular fluid makes up of the body fluids. Where is it found? 3. In
Biology 12 August 2007 Form A Provincial Examination Multiple-Choice Key
 Biology 12 August 2007 Form A Provincial Examination Multiple-Choice Key Cognitive Processes K = Knowledge U = Understanding H = Higher Mental Processes Weightings 22% 58% 20% Types 67 = Multiple Choice
Biology 12 August 2007 Form A Provincial Examination Multiple-Choice Key Cognitive Processes K = Knowledge U = Understanding H = Higher Mental Processes Weightings 22% 58% 20% Types 67 = Multiple Choice
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE
 Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
4/5/17. Blood. Blood. Outline. Blood: An Overview. Functions of Blood
 Outline Blood Biol 105 Chapter 11 I. Overview of blood II. Functions of blood III. Composition of blood IV. Composition of plasma V. Composition of formed elements VI. Platelets VII. White blood cells
Outline Blood Biol 105 Chapter 11 I. Overview of blood II. Functions of blood III. Composition of blood IV. Composition of plasma V. Composition of formed elements VI. Platelets VII. White blood cells
Average adult = 8-10 pints of blood. Functions:
 Average adult = 8-10 pints of blood Functions: Transports nutrients, oxygen, cellular waste products, and hormones Aids in distribution of heat Regulates acid-base balance Helps protect against infection
Average adult = 8-10 pints of blood Functions: Transports nutrients, oxygen, cellular waste products, and hormones Aids in distribution of heat Regulates acid-base balance Helps protect against infection
