Thalamic Mechanisms Mediating the Sedating Effects of General Anesthesia
|
|
|
- Cecilia O’Brien’
- 5 years ago
- Views:
Transcription
1 Thalamic Mechanisms Mediating the Sedating Effects of General Anesthesia by Bahar (Lia) Mesbah-Oskui A thesis submitted in conformity with the requirements for the degree of Doctorate of Philosophy Institute of Medical Sciences University of Toronto Copyright by Lia Mesbah-Oskui 2016
2 Thalamic Mechanisms Mediating the Sedating Effects of General Anesthesia Abstract Bahar (Lia) Mesbah-Oskui Doctorate of Philosophy Institute of Medical Sciences University of Toronto 2016 Alterations in thalamic activity are associated with electrocortical patterns that characterize nonrapid eye movement (non-rem) sleep and general anesthetic-induced sedation and loss-ofconsciousness. Both non-rem sleep and general anesthesia are states that are characterized by increased inhibitory γ aminobutyric acid (GABA)-ergic signaling in the thalamus. There are three forms of GABA type A (GABA A ) receptor-mediated inhibition that have been identified in thalamocortical neurons: (1) phasic inhibition mediated by the transient activation of synaptic GABA A receptors, (2) tonic inhibition mediated by the prolonged activation of extrasynaptic GABA A receptors, and (3) spillover inhibition mediated by the transient activation of extrasynaptic GABA A receptors. The separate impact of each of these forms of thalamic GABAergic inhibition on electrocortical activity is unclear and such an understanding would assist in elucidating the thalamic mechanisms mediating the changes in electrocortical activity that signal sedation and loss-of-consciousness. The experiments presented in this thesis identify the electrocortical effects of enhanced thalamic GABA A receptor-mediated tonic and spillover inhibition in vivo. The electrocortical changes elicited by a prototypic GABA A receptor-targeting general anesthetic agent when it is administered directly into the thalamus are characterized and compared to the effects identified with separate manipulations of GABA A receptor-mediated ii
3 inhibition. These studies show that enhanced thalamic tonic inhibition in mice promotes electrocortical patterns associated with deep non-rem sleep. Specifically, enhanced GABA A receptor-mediated tonic inhibition in the thalamus elicits a state-independent increase in 1-4 Hz electrocortical activity and a decrease in sleep spindle-like oscillations. These effects were altered by blockade of thalamic T-type Ca 2+ channels. Importantly, microperfusion of the general anesthetic etomidate into the thalamus elicits changes in electrocortical activity that are distinct form those associated with enhanced thalamic tonic inhibition. Moreover, the action of etomidate at the thalamus is sufficient to elicit an electrocortical signature associated with anestheticinduced loss-of-consciousness, but only during non-rem sleep. Finally, enhanced GABA A receptor-mediated spillover inhibition in the thalamus fully recapitulates the electrocortical effects identified with etomidate at the thalamus. Together, the findings of this thesis indicate that alterations in thalamic spillover inhibition could underlie the changes in electrocortical activity that signal anesthetic-induced loss-of-consciousness. iii
4 Acknowledgments I would like to thank my supervisor, Dr. Richard L. Horner, for his thoughtful guidance throughout my doctorate studies. I am particularly appreciative of the time that he invested teaching me how to write an effective and clear scientific manuscript. I would also like to thank my committee members, Dr. Beverley A. Orser and Dr. Paul Frankland, for their constructive feedback and valuable suggestions. I would like to offer further thanks to Dr. Orser for the additional advice and resources that she generously shared with me during my doctorate studies. My colleagues in the laboratory also deserve big thanks for their help, advice, and friendship throughout my studies. Finally, I would like to thank my friends and my family for their constant support. I am also grateful to the funding agencies that provided support for the research presented in this thesis. Specifically, this work was supported by the Canadian Institutes of Health Research (RLH), Sleep and Biological Rhythms (LM-O) a CIHR-funded research and training program (RLH) and the Ontario Graduate Scholarship Program (LM-O). I am also appreciative of the institutional awards that I received through the School of Graduate Studies at the University of Toronto that were allotted toward travel expenses for attendance at international scientific meetings where I had the opportunity to present my research. iv
5 Contributions Chapter 1: Introduction This chapter was written solely by Lia Mesbah-Oskui and contains segments from a published article review that was also written solely by Lia Mesbah-Oskui. Citation: Mesbah-Oskui, L (2015) A thalamic origin to the electrocortical patterns associated with transitions into anesthetic-induced loss-of-consciousness. J Neurosci 35: Chapter 2: Aims & Hypotheses This chapter was written solely by Lia Mesbah-Oskui for this dissertation. Chapter 3: Extrasynaptic thalamic δ-subunit containing GABA A receptors promote electrocortical signatures of deep non-rem sleep but do not mediate the effects of etomidate at the thalamus in vivo This chapter presents research that was published by Lia Mesbah-Oskui, Beverley A. Orser, and Richard L. Horner. Lia Mesbah-Oskui and Richard L. Horner designed the study. Lia Mesbah- Oskui performed all research. Lia Mesbah-Oskui and Beverley A. Orser contributed unpublished reagents/analytical tools. Lia Mesbah-Oskui analyzed data. Lia Mesbah-Oskui and Richard L. Horner wrote the paper. Citation: Mesbah-Oskui L, Orser BA, Horner RL (2014) Thalamic δ-subunit containing GABA A receptors promote electrocortical signatures of deep non-rem sleep but do not mediate the effects of etomidate at the thalamus in vivo. J Neurosci 37: Chapter 4: Enhanced thalamic spillover inhibition during non-rem sleep triggers an electrocortical signature of anesthetic hypnosis This chapter presents research by Lia Mesbah-Oskui and Richard L. Horner that is currently in press. Lia Mesbah-Oskui and Richard L. Horner designed the study. Lia Mesbah-Oskui performed all research, contributed unpublished analytical tools, and analyzed all data. Lia Mesbah-Oskui and Richard L. Horner wrote the paper. v
6 Citation: Mesbah-Oskui L, Horner RL (2016) Enhanced thalamic spillover inhibition during non rapideye-movement sleep triggers an electrocortical signature of anesthetic hypnosis. Anesthesiology. In press. Chapter 5: General Discussion This chapter was written solely by Lia Mesbah-Oskui for this dissertation. Chapter 6: Future Directions This chapter was written solely by Lia Mesbah-Oskui for this dissertation. vi
7 Table of Contents ACKNOWLEDGMENTS... IV CONTRIBUTIONS...V TABLE OF CONTENTS...VII LIST OF TABLES... XI LIST OF FIGURES...XII LIST OF ABBREVIATIONS... XV CHAPTER 1 INTRODUCTION ANATOMY AND CONNECTIVITY OF THE THALAMUS THE THALAMUS, SLEEP, AND ATTENTION SLEEP REGULATION AND T-TYPE CA 2+ CHANNELS SLEEP SPINDLES DELTA (1-4 HZ) WAVES ATTENTION ANESTHESIA AND THE THALAMUS LOSS-OF-CONSCIOUSNESS AND ANESTHESIA COMMON MOLECULAR TARGETS OF GENERAL ANESTHETICS CORTICAL ACTIVITY DURING GENERAL ANESTHESIA THALAMIC ACTIVITY DURING GENERAL ANESTHESIA: BRAIN IMAGING STUDIES THALAMIC ACTIVITY DURING GENERAL ANESTHESIA: MULTI-UNIT AND SINGLE-UNIT RECORDINGS THALAMIC GABA A RECEPTORS AND GENERAL ANESTHETICS OTHER MOLECULAR TARGETS OF GENERAL ANESTHETICS IN THE THALAMUS SYNTHESIS...63 CHAPTER 2 AIMS & HYPOTHESES...67 CHAPTER 3 EXTRASYNAPTIC THALAMIC δ-subunit CONTAINING GABA A RECEPTORS PROMOTE ELECTROCORTICAL SIGNATURES OF DEEP NON-REM vii
8 SLEEP BUT DO NOT MEDIATE THE EFFECTS OF ETOMIDATE AT THE THALAMUS IN VIVO INTRODUCTION MATERIALS & METHODS ANIMAL CARE SURGERY HABITUATION EXPERIMENTAL PROTOCOL SIGNAL ACQUISITION AND ANALYSIS OF SLEEP-WAKE STATES IDENTIFICATION AND CHARACTERIZATION OF SPINDLE-LIKE OSCILLATIONS TEMPORAL ANALYSIS OF STATE TRANSITIONS STATISTICAL ANALYSIS RESULTS THALAMIC δ-subunit CONTAINING GABA A RECEPTOR MODULATION AND EEG SPECTRAL POWER THALAMIC δ-subunit CONTAINING GABA A RECEPTOR MODULATION AND SPINDLE-LIKE OSCILLATIONS THALAMIC δ-subunit CONTAINING GABA A RECEPTOR MODULATION AND STATE-SPACE DYNAMICS MICROPERFUSION OF ETOMIDATE INTO THE VENTROBASAL COMPLEX EFFECTS EEG POWER ETOMIDATE AT THE VENTROBASAL COMPLEX AND SPINDLE-LIKE OSCILLATIONS ETOMIDATE AT THE VENTROBASAL COMPLEX AND STATE-SPACE DYNAMICS ETOMIDATE AT THE VENTROBASAL COMPLEX INCREASES REM SLEEP EXPRESSION THE EFFECTS OF ETOMIDATE AT THE VENTROBASAL COMPLEX LARGELY PERSIST DURING BLOCKADE OF GABA RE-UPTAKE DISCUSSION THALAMIC δgaba A RECEPTOR ACTIVITY, SEDATION, AND SLEEP ETOMIDATE AT THE THALAMUS AND ELECTROCORTICAL SIGNATURES OF ANESTHETIC INDUCTION THE THALAMUS, SLEEP, AND ANESTHESIA viii
9 CHAPTER 4 ENHANCED THALAMIC SPILLOVER-INHIBITION DURING NON- REM SLEEP TRIGGERS AN ELECTROCORTICAL SIGNATURE OF ANESTHETIC HYPNOSIS INTRODUCTION MATERIALS & METHODS ANIMAL CARE EXPERIMENTAL PROTOCOL SIGNAL ACQUISITION AND ANALYSIS OF SLEEP-WAKE STATES ANALYSIS OF TRANSITIONS INTO NON-REM SLEEP STATISTICAL ANALYSIS RESULTS ETOMIDATE AT THE THALAMUS INCREASES ALPHA-BETA ELECTROCORTICAL ACTIVITY AND SPINDLE-LIKE OSCILLATIONS DURING NON-REM SLEEP PHARMACOLOGICALLY ENHANCED THALAMIC GABAERGIC SPILLOVER INHIBITION FULLY RECAPITULATES THE EFFECTS OF ETOMIDATE AT THE THALAMUS PHARMACOLOGICALLY ENHANCED THALAMIC TONIC INHIBITION ELICITS ALTERATIONS IN ELECTROCORTICAL ACTIVITY THAT ARE DISTINCT FROM THOSE IDENTIFIED WITH ENHANCED THALAMIC SPILLOVER INHIBITION ETOMIDATE AND DS2 AT THE THALAMUS AMPLIFY AN ELECTROCORTICAL SIGNATURE THAT CHARACTERIZES ENTRY INTO NON-REM SLEEP DISCUSSION CHAPTER 5 GENERAL DISCUSSION GABA A RECEPTOR MEDIATED TONIC INHIBITION IN THE THALAMUS GABA A RECEPTOR MEDIATED SPILLOVER INHIBITION IN THE THALAMUS FACTORS THAT COULD FAVOUR SPILLOVER INHIBITION OVER TONIC INHIBITION FUNCTIONAL IMPLICATIONS OF ENHANCED THALAMIC SPILLOVER INHIBITION GABA A RECEPTOR TARGETING GENERAL ANESTHETICS AT THE THALAMUS GENERAL ANESTHESIA AND NON-REM SLEEP ix
10 5.3.2 THE THALAMUS AND GENERAL ANESTHESIA CHAPTER 6 FUTURE DIRECTIONS REFERENCES COPYRIGHT ACKNOWLEDGEMENTS x
11 List of Tables Table 1. List of general anesthetic agents that are commonly used clinically in human patients...36 xi
12 List of Figures Figure 1. Schematic of thalamus illustrating localization of major nuclei...6 Figure 2. Basic network circuitry of the thalamocortico-corticothalamic system...9 Figure 3. Schematic depicting the major projections of the ascending arousal system...11 Figure 4. Electroencephalographic recording of surface activity during a transition from non- REM (NREM) sleep to wakefulness in mice...14 Figure 5. Schematic illustrating the major projections from the ventrolateral preoptic (VLPO) nucleus that promote cortical de-activation and non-rem sleep through inhibition of ascending arousal inputs...16 Figure 6. Schematic of thalamocortico-corticothalamic signaling in the presence and absence of excitatory input from arousal nuclei...19 Figure 7. Traces illustrating the two modes of action potential firing identified in thalamic neurons...20 Figure 8. Schematic illustrating three major network types...34 Figure 9. Electrocortical activity during propofol-induced general anesthesia in human patients...45 Figure 10. Comparison of electrocortical activity during anesthetic-induced loss-ofconsciousness with three commonly used general anesthetic agents...46 Figure 11. Schematic of the three different forms of GABA A receptor-mediated inhibition identified in thalamocortical neurons...52 Figure 12. Experimental protocol and localization of microdialysis probes in the thalamus...79 Figure 13. Promoting thalamic δgaba A receptor activity with THIP at the ventrobasal complex increases 1-4 Hz electrocortical activity during NREM sleep and waking...87 xii
13 Figure 14. Promoting thalamic δgaba A receptor activity with THIP at the ventrobasal complex decreases both sigma power and the incidence of spindle-like oscillations...92 Figure 15. Thalamic δgaba A receptor activity facilitates rapid transitions into the spectral domain characteristic of deep non-rem sleep...93 Figure 16. Etomidate at the ventrobasal complex decreases 1-4 Hz electrocortical activity in both wild-type and Gabrd -/- mice (i.e., opposite to the effects of THIP)...97 Figure 17. Etomidate at the ventrobasal complex promotes sigma power and increases the incidence and duration of spindle-like oscillations via a δgaba A receptor-independent mechanism (i.e., unlike the effects of THIP)...99 Figure 18. Etomidate had no effect on the temporal dynamics of state transitions in spectral space Figure 19. Etomidate at the ventrobasal complex increased REM sleep expression independent of δgaba A receptor expression (i.e., effects were observed in wild-type and Gabrd -/- mice)..102 Figure 20. The effects of etomidate on electrocortical activity persist with increased GABA concentrations in the ventrobasal complex Figure 21. Summary of experimental methods Figure 22. Microperfusion of etomidate into the ventrobasal complex of wild-type mice increases alpha-beta electrocortical activity, sleep spindles, and NREM sleep through T-type Ca 2+ channel independent alterations in thalamocortical activity in vivo Figure 23. Microperfusion of TTA-P2 into the thalamus of freely behaving wild-type mice elicits alterations in electrocortical activity consistent with reduced T-type Ca 2+ channel activity in the thalamus Figure 24. Microperfusion of DS2 into the thalamus of freely behaving Gabrd -/- mice does not alter electrocortical activity or sleep-wake state durations xiii
14 Figure 25. Promoting thalamic spillover inhibition with microperfusion of DS2 into the ventrobasal complex of wild-type mice fully recapitulates the alterations in electrocortical activity and sleep-wake state behaviour identified with etomidate at the thalamus Figure 26. THIP-induced increases in tonic extrasynaptic GABA A receptor-mediated inhibition in the thalamus elicits distinct alterations in electrocortical activity that differ from DS2 and require T-type Ca 2+ channel activity in vivo Figure 27. Microperfusion of etomidate or DS2 into the thalamus of wild-type mice amplifies an electrocortical signature characterizing transitions into NREM sleep Figure 28. Proposed effect of GABA A receptor-targeting general anesthetics on the thalamus xiv
15 List of Abbreviations 1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(S)-3- piperidinecarboxylic acid 1,2,5,6-Tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3- pyridinecarboxylic acid hydrochloride 3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4- fluoro-piperidin-4-ylmethyl]-benzamide 4-Chloro-N-[2-(2-thienyl)imidazol[1,2-a]pyridin-3-yl]benzamide 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol 5-hydroxytryptamine 2 receptors 5-hydroxytryptamine/serotonin α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor δ γ aminobutyric acid type A receptor subunit knockout mice δ-subunit containing γ aminobutyric acid type A receptor γ aminobutyric acid γ aminobutyric acid type A receptor acetylcholine analysis of variance artificial cerebrospinal fluid basal forebrain Bispectral monitor Ca 2+ -dependent small-conductance type 2 K + channels cyclic adenosine monophosphate dorsal raphé dopamine electroencephalogram electromyogram SNAP-5114 NO-711 TTA-P2 DS2 THIP 5-HT 2 receptors 5-HT AMPA receptor Gabrd -/- mice δgaba A receptor GABA GABA A receptor ACh ANOVA acsf BF BIS monitor SK2 channels camp DR DA EEG EMG xv
16 evoked inhibitory postsynaptic current fast Fourier transform galanin histamine histamine type-1 receptor hyperpolarization-activated cation nonselective channel inhibitory postsynaptic current inhibitory postsynaptic potential lateral hypothalamus laterodorsal tegmental nucleus locus coeruleus melanin-concentrating hormone muscarinic type-1 receptor N-methyl-D-aspartate receptors noradrenaline non-rapid eye movement sleep oral pontine nucleus orexin pedunculopontine tegmental nucleus perifornical area rapid eye movement sleep repeated measures reticular thalamic nucleus standard error of the mean State and Response Entropy monitor second transmembrane domain spindle-like oscillation eipsc FFT Gal His H1 receptor HCN channel IPSC IPSP LHA LDT LC MCH M1 receptor NMDA receptor NA non-rem sleep PnO ORX PPT Pef REM RM RTN SEM M-Entropy monitor TM2 SLO xvi
17 transcranial magnetic stimulation tuberomammillary nucleus ventral periaqueductal gray ventroposterolateral thalamic nucleus ventroposteromedial thalamic nucleus TMS TMN vpag VPL thalamic nucleus VPM thalamic nucleus xvii
18 1 Chapter 1 Introduction Some of the contents in this section have been published previously: Mesbah-Oskui, L (2015) A thalamic origin to the electrocortical patterns associated with transitions into anesthetic-induced loss-of-consciousness. J Neurosci 35: PMID: The thalamus is a major hub of the subcortical connectome and plays a pivotal role in sensory processing, arousal state regulation, and attention (Steriade, 2001; van den Heuvel and Sporns, 2011). Its function as a relay station is well established, with all sensory input, save for olfactory, passing first through the thalamus where it is then relayed to primary, secondary, and associative cortical areas (Steriade, 1997). This thalamic relay of sensory information to the cortex is disrupted during states that are characterized by reduced consciousness and awareness, such as non-rapid eye movement (non-rem) sleep and absence seizures (Steriade et al., 1993b; Huguenard and McCormick, 2007). Moreover, midline thalamic damage can elicit a vegetative state in humans, while lesions that are restricted to the posterior thalamus are associated with contralateral hemianesthesia (i.e., loss of conscious proprioception) (Rafal and Posner, 1987; Kinney et al., 1994; Zeman, 1997). It is therefore not surprising that alterations in thalamic activity are thought to underlie general anesthetic-induced sedation and hypnosis (i.e., loss-of-consciousness) (Alkire et al., 2008). The thalamus is one of the first brain regions to exhibit marked alterations in metabolism and blood flow during general anesthesia (Alkire et al., 2008; Franks, 2008). The exact impact of
19 2 these alterations, however, is unclear. Specifically, how general anesthetic-induced alterations in thalamocortical activity relate to changes in cortical activity and consciousness are unknown and an active area of research. Understanding the role of the thalamus in mediating anestheticinduced hypnosis will help elucidate the neural correlates of consciousness. This understanding could further inform the development of better indices to assess peri- and intra-operative awareness and could facilitate the targeted design of new general anesthetic agents that lack undesirable side effects such as post-operative confusion and cognitive dysfunction. 1.1 Anatomy and connectivity of the thalamus Anatomically the thalamus is well positioned to function as a gateway for the transmission of sensory information to primary, secondary, and associative cortical areas. Moreover, the dense reciprocal connectivity between the thalamus and cortex allows the thalamus to influence cortical signaling (Guillery, 1995; Reinhold et al., 2015). Thus, the thalamus is positioned to not only influence the flow of information to the cortex, but can also impact the integration of this information across cortical areas, two processes that are considered fundamental to consciousness according to the Information and Integration Theory of Consciousness (discussed in more detail in section Consciousness and anesthesia) (Tononi, 2004). The thalamus constitutes the largest part of the diencephalon, which also contains the hypothalamus, and is densely innervated by afferents from the brainstem, retina, spinothalamic tract, and cortex. Thalamic efferents primarily target the cortex, but projections are also sent to subcortical regions such as the basal ganglia (Groenewegen and Berendse, 1994; Steriade, 1997). The strong reciprocal, largely excitatory, connectivity between the thalamus and cortex is permissive to the generation of synchronized neuronal activity across large areas of the brain.
20 3 Indeed, enhanced reciprocal activity in this circuit is implicated in both mediating and generating oscillatory patterns of cortical activity that occur during non-rem sleep and exhibit high levels of coherence across the cortex (discussed in further detail in section 1.2 The thalamus, sleep, and attention) (Steriade et al., 1993b). Structurally, the thalamus can be subdivided into a number of nuclear masses that can be further distinguished by the types of sensory inputs that they receive, integrate, and transmit (Fig. 1). Functionally, the activity of these thalamic nuclei is implicated in conscious state (i.e., subjective experience), attention, memory, motor activity, and perception (Steriade, 1997). Of the approximately 40 nuclei that have been identified to comprise the thalamus, there are a number of them that have been extensively studied in both humans and animals. These include the lateral and medial geniculate nuclei, ventroposterolateral and ventroposteromedial nuclei (i.e., ventrobasal complex), intralaminar and central medial nuclei, and the reticular thalamic nucleus (RTN) (Fig. 1A and B). The lateral and medial geniculate nuclei serve as the visual and auditory relay nuclei of the thalamus, respectively, and are located below the posterior part of the thalamus (Steriade, 1997) (Fig. 1A). The lateral geniculate nucleus receives inputs from the retina, primary and secondary visual cortices, superior colliculus, the reticular formation of the pons, and the medulla. Its efferents primarily target the visual cortex (Sherman and Guillery, 2002). The medial geniculate nucleus receives both direct and indirect input from the lateral lemniscus. It also receives input from the auditory area of the cerebral cortex. Predictably, this region of the thalamus projects to the primary auditory cortex and other surrounding auditory areas (Suga and Ma, 2003). Thus, it is reasonable to posit that the action of general anesthetics at the lateral and medial geniculate nuclei of the thalamus facilitates a breakdown in the transmission of visual and
21 4 auditory information to the cortex where this sensory information is integrated with top-down information (e.g., expectations) to form a conscious percept (as postulated by models of consciousness) (Bastos et al., 2012). Indeed, neurons from these thalamic nuclei show significant anesthetic-induced alterations in activity in vitro (discussed in more detail in sections ). The ventroposterolateral and ventroposteromedial nuclei of the thalamus are located in the ventral tier of thalamic nuclei and act as thalamic somatosensory relay nuclei (Steriade, 1997) (Fig. 1A and B). Afferents to these nuclei come from the medial lemniscal and spinothalamic tracts. Efferents from the ventroposterolateral and ventroposteromedial nuclei target the primary and secondary somatosensory areas of the cortex (Andersen et al., 1964). Functionally, this region is implicated in mediating motor activity and somatosensory perception (Steriade, 1997). Because of their proximity and similar functional roles, these two nuclei are often grouped together and referred to as the ventrobasal complex. Lesions to this region of the thalamus are associated with hemianesthesia in humans, where patients lack or have significantly impaired perception of movement and spatial orientation (Rafal and Posner, 1987). This area of the thalamus also exhibits relatively high expression (compared to other thalamic nuclei) of receptor subtypes that are major targets of commonly used general anesthetics (for more detail please see section Common molecular targets of general anesthetics) and as such was the region of the thalamus that we targeted for pharmacological manipulation for this thesis (Talley et al., 1999; Pirker et al., 2000). The intralaminar and central medial nuclei of the thalamus are consistently implicated in mediating cortical activation and sensorimotor integration (Van der Werf et al., 2002). This region of the thalamus receives inputs from the spinothalamic tract, reticular formation,
22 5 cerebellum, substantia nigra, and globus pallidus (Steriade, 1997; Van der Werf et al., 2002). Efferents of the intralaminar nuclei project diffusely throughout the cortex. (Van der Werf et al., 2002). Similarly, the central medial nucleus, which is located directly underneath (i.e., ventral to) the intralaminar nuclei (Fig. 1B), sends projections to many regions of the brain (Van der Werf et al., 2002). As such, these two groups of thalamic nuclei are often termed nonspecific. Recordings of neuronal population activity in this region of the thalamus indicate that the nonspecific nuclei of the thalamus are particularly sensitive to general anesthetic agents (Baker et al., 2014). Moreover, pharmacological manipulation of these nuclei during general anesthesia can alter the effects of general anesthetic agents administered systemically in rats in vivo (discussed in more detail in section Other molecular targets of general anesthetics in the thalamus) (Alkire et al., 2007; Alkire et al., 2009). Thus, the nonspecific nuclei of the thalamus appear to play a significant role in mediating anesthetic-induced hypnosis. The cells that comprise the relay, association, and nonspecific nuclei of the thalamus are largely excitatory. Indeed, in certain species, such as mice and rats, these nuclei appear to be comprised exclusively of excitatory neurons, although there is some evidence indicating the presence of inhibitory neurons in the lateral geniculate nucleus of rats (Steriade, 1997). However, in humans, non-human primates, and cats approximately 20 % of the neurons comprising thalamic relay, association, and nonspecific nuclei are inhibitory (Steriade, 1997). In contrast to the excitatory thalamic neurons, which possess long-range projections that target cortical layers, inhibitory thalamic neurons possess short-range projections that are confined to the thalamic nucleus in which they are located (Steriade, 1997). These thalamic inhibitory neurons are commonly referred to as local-circuit interneurons. The functional significance of these localcircuit interneurons in mediating thalamic activity is unclear, although they have been implicated in mediating the relay of visual information from the thalamus to the cortex (Hirsch et al., 2015).
23 6 To my knowledge there are no studies that have characterized the effect of general anesthetic agents on thalamic local-circuit interneuron activity. Figure 1. Schematic of thalamus illustrating localization of major nuclei. A, Exterior organization of thalamic nuclei. The reticular nucleus is made transparent in order to visualize the nuclei located directly underneath it. B, Coronal section of one lobe of the thalamus illustrating the internal localization of the ventroposteromedial, central medial, and intralaminar nuclei. The midline nuclei are not depicted in this schematic, but are located between the two thalamic lobes. The RTN is distinct from the rest of the thalamus in that it does not send any projections to the cortex and is comprised of neurons that are exclusively inhibitory. The RTN, instead, exhibits extensive intra-rtn connectivity and reciprocal connectivity with the rest of the thalamus (Pinault, 2004) (Fig. 2). The RTN also receives collateral inputs from cortical neurons that project to the thalamus (Pinault, 2004) (Fig. 2). Thus, the RTN is in a unique position to regulate thalamocortico-corticothalamic signaling because of its connectivity and inhibitory projections. The RTN also receives cholinergic input from the ascending arousal system, which
24 7 is involved in the regulation of sleep-wake behaviour (discussed in more detail in section Sleep regulation and T-type Ca 2+ channels) (Woolf and Butcher, 1986). Structurally, the RTN is comprised of a thin sheet of cells (approximately 1 mm in diameter in humans) that cover the lateral aspect of the thalamus (Pinault, 2004) (Fig. 1). The RTN is physically separated from the main body of the thalamus by the external medullary lamina, which is a thin sheet of white matter. This region of the thalamus can be subdivided into partially overlapping sensory sectors that receive topographically organized sensory information from visual, auditory, somatosensory, gustatory, visceral, motor, and limbic systems (Pinault, 2004; Zikopoulos and Barbas, 2007). Activity of neurons in the RTN refines the receptive fields and modulates the response times of corresponding thalamocortical neurons (Pinault, 2004). Specifically, RTN cells play a critical role in limiting the duration of thalamocortical responses (Hartings et al., 2000; Pinault, 2004). Given the neuroanatomy of the RTN and its strong influence on thalamocortical activity it is implicated in mediating attentiveness (discussed in more detail in section Attention) and as such alterations in RTN activity may play a critical role in mediating anesthetic-induced sedation and loss-of-consciousness. The thalamus is also comprised of anterior, medial, midline, and lateral nuclear groups, which are functionally implicated in mediating alertness, attention, emotion, memory, and perception (Steriade, 2001) (Fig. 1). The anterior nuclear groups receive inputs from the mammilary body and project to the limbic system, cingulate gyrus, and parahippocampal gyrus (Irle and Markowitsch; Guillery, 1995). This region of the thalamus also exhibits extensive reciprocal connections with the prefrontal cortex and the anterior cingulate and medial frontal gyri (Irle and Markowitsch). The anterior thalamic nuclear group also receives olfactory inputs from the cortex (Price and Slotnick, 1983). The medial nuclei of the thalamus exhibit extensive
25 8 interconnectivity with all other thalamic nuclei and are implicated in memory consolidation (Mitchell and Chakraborty, 2013; Saalmann, 2014). The midline nuclei are similarly implicated in memory consolidation, but are also associated with arousal (Vertes, 2006). Afferents from the hypothalamus, periaqueductal gray, spinothalamic tract, and medullary and pontine reticular formation target the midline nuclei (Van der Werf et al., 2002). This region of the thalamus also receives arousal related input from the locus coeruleus, raphé nuclei, and midbrain. The lateral nuclear group contains some of the most well characterized association nuclei, such as the pulvinar (Graybiel and Berson, 1980). Efferents from these regions project to association areas in the parieto-temporal cortex and visual and visual association areas in the occipital and posterior part of the temporal cortex (Graybiel and Berson, 1980). Anesthetic-induced alterations in neuronal activity in these thalamic groups have not been as extensively characterized as other thalamic regions, such as the ventrobasal complex, RTN, and nonspecific nuclei. However, given their functional roles in alertness, attention, cognition, and perception, alterations in the activity of thalamocortical neurons in these regions could feasibly influence anesthetic-induced loss-ofconsciousness.
26 9 Figure 2. Basic network circuitry of the thalamocortico-corticothalamic system. Note that the reticular thalamic nucleus (red cells) receives axon collaterals from both corticothalamic (gray cells) and thalamocortical projections (blue cells). Also note the absence of projections from the reticular thalamic nucleus to the cortex. Solid lines denote excitatory projections and dashed lines denote inhibitory projections. 1.2 The thalamus, sleep, and attention A series of seminal studies led by Mircea Steriade served to establish the pivotal role of the thalamus in mediating brain activity patterns associated with sleep. These studies showed that the thalamus plays a major role in modulating sleep spindles and slow 1-4 Hz (delta) waves, electrocortical patterns that are considered hallmarks of non-rem sleep and decreased arousability. Many studies have since built upon these initial findings and further elucidate the cellular and molecular components that position the thalamus to orchestrate these electrocortical patterns.
27 Sleep regulation and T-type Ca 2+ channels Excitatory projections to the thalamus from the brainstem comprise one of the two major pathways of the ascending arousal system, (Saper et al., 2001; Saper et al., 2005; Franks, 2008) (Fig. 3). The ascending arousal system is located in the upper brainstem and can be subdivided into a dorsal and ventral pathway, each of which is comprised of discrete cell populations and neurotransmitters. The dorsal pathway of the ascending arousal system innervates midline, intralaminar, relay, and reticular thalamic nuclei and is comprised of midbrain, pontine, and medullary reticular formation glutamatergic neurons and cholinergic neurons from the pedunculopontine and laterodorsal tegmental nuclei located in the upper pons (Fig. 3). In contrast, the ventral pathway of the ascending arousal system innervates the hypothalamus, midbrain, and basal forebrain and is comprised of glutamatergic projections from the parabrachial nucleus, noradrenergic projections from the locus coeruleus, serotonergic projections from the dorsal raphé, and dopaminergic projections from the periaqueductal gray (Fig. 3). The neuronal fibers that comprise the ventral pathway of the ascending arousal system synapse onto glutamatergic, histaminergic, and orexinergic/hypocretin neurons in the posterior/lateral hypothalamus.
28 11 Figure 3. Schematic depicting the major projections of the ascending arousal system. Cholinergic (ACh) input (yellow traces) from the pedunculopontine (PPT) and laterodorsal tegmental (LDT) nuclei in the upper pons constitute one of the major sources of excitatory input to the relay nuclei and RTN of the thalamus. The red traces denote a second pathway that effectively activates the cortex and promotes the relay of sensory information from the thalamus to cortical neurons. This pathway is comprised of monoaminergic cells, from the tuberomammillary nucleus (TMN) containing histamine (His), the ventral periaqueductal gray (vpag) containing dopamine (DA), the dorsal and median raphé nuclei containing serotonin (5-HT), and locus coeruleus (LC) containing noradrenaline (NA). Peptidergic neurons in the lateral hypothalamus (LHA) containing orexin (ORX) or melanin-concentrating hormone (MCH), and cholinergic (ACh) and GABAergic neurons from the basal forebrain (BF) also contribute to this pathway. Image was taken with permission from Saper et al. (Saper et al., 2005). The ascending arousal system plays a critical role in promoting wakefulness. Lesions that disconnect these wake-promoting projections from the brainstem to the diencephalon are associated with prolonged sleepiness (Economo, 1931). Indeed, the critical role of this pathway in mediating sleep-wake behaviour was first identified in the early 1900s by the Viennese
29 12 neurologist Constantin von Economo when he was investigating the pathophysiology underlying encephalitis lethargica. This illness was characterized by excessive sleepiness, with patients typically sleeping more than 20 hours each day (Economo, 1931). These patients possessed lesions at the junction of the midbrain and diencephalon, effectively severing ascending arousal input from the brainstem to the thalamus, hypothalamus, and basal forebrain. Cholinergic inputs from the pedunculopontine and laterodorsal tegmental nuclei of the brainstem provide the major source of excitatory drive to the thalamus from the ascending arousal system (Woolf and Butcher, 1986; Steriade et al., 1988) (Fig. 3). These cholinergic projections target both thalamic relay nuclei, where acetylcholine directly excites thalamocortical neurons through nicotinic and muscarinic type-1 (M1) receptors, and the RTN, where acetylcholine inhibits neurons through M2 receptors (Steriade et al., 1988; McCormick, 1989). The pedunculopontine and laterodorsal tegmental nuclei also target and excite neurons in the oral pontine reticular formation and mesencephalic reticular nucleus, regions that directly excite thalamocortical neurons through glutamatergic projections (Datta, 1995). Thus, acetylcholine release from the pedunculopontine and laterodorsal tegmental nuclei excites thalamocortical neurons both directly and indirectly. The activity of the pedunculopontine and laterodorsal tegmental nuclei is greatly reduced during non-rem sleep, but otherwise high during both wakefulness and REM sleep where electrocortical activity is similarly characterized as low amplitude and high frequency (Hallanger et al., 1987; Strecker et al., 2000; Saper et al., 2001; Lydic and Baghdoyan, 2005). Noradrenergic input from the locus coeruleus, serotonergic input from the raphé nucleus, and histaminergic input from the tuberomammilary nucleus also have an excitatory effect on thalamocortical neurons and promote wakefulness (McCormick, 1989; Pape and McCormick,
30 ; McCormick and Bal, 1997). In the thalamus, noradrenaline elicits depolarization of thalamocortical neurons, and subsequently promotes the tonic firing of action potentials in these neurons (the firing modes exhibited by thalamocortical neurons are discussed in more detail at the end of this section), by eliciting an α 1 -adrenergic receptor-mediated suppression of a resting potassium leak current in combination with a β-adrenoceptor mediated increase in the hyperpolarization activated cation current (McCormick, 1989; Pape and McCormick, 1989; McCormick et al., 1991). Serotonin similarly promotes depolarization of thalamocortical neurons by enhancing the hyperpolarization activated cation current through its actions on 5- hydroxytryptamine 2 (5-HT 2 ) receptors (Pape and McCormick, 1989). The effect of histamine on thalamocortical neurons is also excitatory and is mediated through histamine type-1 (H1), H2, and possibly H3 receptors (Bouthenet et al., 1988; Ruat et al., 1990; McCormick and Williamson, 1991; Lee et al., 2004b). Application of histamine to thalamocortical neurons from the dorsal lateral geniculate nucleus elicits a slow depolarization through blockade of a potassium leak current (McCormick and Williamson, 1991). Moreover, histamine also enhances the hyperpolarization activated cation current in thalamocortical neurons, similar to both noradrenaline and serotonin (McCormick and Williamson, 1991). Both noradrenaline and serotonin also have an excitatory effect on the inhibitory neurons of the RTN (Kayama et al., 1982; McCormick and Wang, 1991). Specifically, both neurotransmitters promote tonic action potential firing and inhibit high frequency bursts (~200 Hz) of action potentials in RTN neurons. Such steady trains of inhibitory input from the RTN to thalamocortical neurons have been shown to mediate directed attention in visual and cross-modal (visual and auditory) attention tasks (discussed in more detail in section Attention) (Halassa et al., 2014; Wimmer et al., 2015). Histaminergic receptors are also expressed in RTN neurons
31 14 where histamine would presumably have an excitatory effect on inhibitory neurons, although no such relationship has been published to my knowledge (Jin et al., 2002). Increased neuronal activity in nuclei that comprise the ascending arousal system (i.e., locus coeruleus, dorsal raphé, tuberomammillary nucleus) is associated with transitions in cortical activity from patterns that characterize non-rem sleep (i.e., coherent high amplitude, low frequency activity in large cortical neuronal populations) to patterns that characterize wakefulness (i.e., low amplitude, high frequency activity) (Saper et al., 2001; Saper et al., 2005) (Fig. 4). Functionally, transitions into non-rem sleep occur when arousal-associated nuclei are actively inhibited by projections from the ventrolateral pre-optic area of the hypothalamus (Sherin et al., 1998) (Fig. 5). Neurons of the ventrolateral pre-optic area exhibit a significant increase in firing rate at the onset of non-rem sleep (Sherin et al., 1996; Gaus et al., 2002). Moreover, the firing rate of these neurons increases with increasing sleep depth (as indexed by increasing 1-4 Hz, delta frequency electrocortical activity) (Szymusiak et al., 1998). Lesions to the ventrolateral pre-optic area are associated with significant reductions in the amount of non- REM sleep and REM sleep in animals (Lu et al., 2000). Figure 4. Electroencephalographic recording of surface activity during a transition from non-rem (NREM) sleep to wakefulness in mice. Note the difference in cortical activity during NREM sleep, where it is high amplitude and low frequency, and wakefulness, where it is low amplitude and high frequency.
32 15 The ventrolateral pre-optic area receives reciprocal inhibitory input from arousal nuclei, specifically the locus coeruleus and raphé nucleus (Chou et al., 2002). This bi-stable feature of the neuronal circuitry mediating sleep-wake behaviour forms the foundation of the Flip-Flop Explanation of sleep and wakefulness, where transitions between two states are discrete and possess no intermediate levels (Saper et al., 2001). Such a circuit fits with the natural characteristics of sleep, where transitions are often abrupt, with little time spent in transitional states (typically less than 1-2 % of time) (Saper et al., 2005). The abrupt nature of sleep-wake transitions is also reminiscent of transitions into anesthetic-induced loss-of-consciousness, which suggests that the neuronal network underlying sleep-wake regulation may also functionally contribute to general anesthetic-induced sedation and loss-of-consciousness. Indeed, there is a body of literature that supports this reasoning. Specifically, The Shared Circuits hypothesis of sleep and anesthesia, proposed approximately twenty years ago, posits that general anesthetics preferentially recruit sleep-regulating brain circuitry to generate and/or support sedation, hypnosis, amnesia, and analgesia (Lyidc and Biebuyck, 1994; Lydic, 1996; Lydic and Baghdoyan, 2005). In the following two decades numerous studies, employing a variety of methodological approaches, have provided results that are consistent with this hypothesis and these studies are discussed in further detail in section General anesthesia and non-rem sleep. The inhibition of arousal nuclei during non-rem sleep removes a major source of excitatory input from the thalamus and this effect promotes hyperpolarization of thalamocortical and RTN neurons (Steriade et al., 1993b). Specifically, thalamocortical neurons and neurons of the RTN exhibit a decrease in resting membrane potential from approximately -58 mv to less than -65 mv (Steriade et al., 1993b; McCormick and Bal, 1997). This hyperpolarization facilitates a T-type Ca 2+ channel-dependent switch in the firing mode of thalamic action
33 16 potentials, which can generate and promote some of the cortical activity patterns associated with non-rem sleep (particularly sleep spindles and delta waves; discussed in more detail in sections Sleep spindles and Delta (1-4 Hz) waves) (Steriade et al., 1993b) (Figs. 6 and 7). Figure 5. Schematic illustrating the major projections from the ventrolateral preoptic (VLPO) nucleus that promote cortical de-activation and non-rem sleep through inhibition of ascending arousal inputs. The VLPO sends inhibitory projections to the wake-promoting monoaminergic cell groups (red) in the tuberomammillary nucleus (TMN), ventral periaqueductal gray (vpag) region, raphé nucleus, and locus coeruleus (LC). The VLPO also innervates neurons in the lateral hypothalamus (LHA; green), including the perifornical (PeF) orexin (ORX) neurons, and interneurons in the cholinergic (ACh) (yellow) pedunculopontine (PPT) and laterodorsal tegmental nuclei (LDT). 5-HT: serotonin; GABA: γ aminobutyric acid; DA: dopamine; Gal: galanin; His: histamine; NA: noradrenaline. Image was taken with permission from Saper et al. (Saper et al., 2005). T-type Ca 2+ channels are widely expressed in neurons of the RTN and dorsal thalamus and play a critical role in mediating thalamic activity (McCormick and Bal, 1997) (Fig. 7). T- type Ca 2+ channels are expressed most heavily on the dendrites of thalamic neurons at distances
34 17 exceeding 100 µm from the soma, and exhibit a gradual decrease in expression at distances closer to the soma (Crandall et al., 2010). There are 3 isoforms of T-type Ca 2+ channels expressed within the thalamus. The Ca v 3.3 (α1i) isoform is heavily expressed in the RTN, while the Ca v 3.2 (α1h) isoform is also present but in much smaller amounts (Talley et al., 1999). Thalamocortical neurons similarly express relatively low amounts of the Ca v 3.2 isoform but they also exhibit heavy expression of the Ca v 3.1 (α1g) isoform, which is not expressed in the RTN (Talley et al., 1999). T-type Ca 2+ channels are de-inactivated at hyperpolarized resting membrane potentials (Perez-Reyes, 2003) (Fig. 7A and B). As such, T-type Ca 2+ channels in the thalamus are widely de-inactivated during non-rem sleep when depolarizing monoaminergic, cholinergic, and histaminergic inputs from the wake-promoting nuclei of the ascending arousal system are suppressed. De-inactivation of these channels triggers a switch from a tonic mode of activity, characterized by a typical train of single action potentials, to a burst mode (Suzuki and Rogawski, 1989) (Fig. 7). This burst mode is characterized by high frequency, approximately several hundred Hz, bursts of action potentials (Fig. 7B). Extensive, lidocaine-induced, lesions to the thalamus in rats are not associated with any overt alterations in sleep-wake behaviour, although sleep spindles appear to be eliminated and delta-theta frequency electrocortical activity is reduced during non-rem sleep (Fuller et al., 2011). It is important to note that electrocortical activity and sleep-wake behaviour were recorded one week after thalamic lesions were induced and the RTN and lateral and medial geniculate nuclei were intact or partially preserved in most of the rats studied (Fuller et al., 2011). In contrast, acute alterations in thalamocortical activity have been directly associated with changes in sleep-wake behaviour. Specifically, an optogenetic-induced (i.e., optical activation of
35 18 a light-sensitive receptor whose expression is coupled genetically to markers that are selectively expressed by a desired cell group) increase in inhibition of the RTN, mediated through inhibitory projections from the lateral hypothalamus, elicited a disinhibition of thalamocortical activity and increase in the amount of wakefulness in mice (Herrera et al., 2015). Moreover, similar optogenetic manipulations of RTN activity during general anesthesia in mice significantly alter cortical activity patterns, promoting patterns that are typically associated with a decreased depth of anesthesia (discussed in more detail in section Thalamic GABA A receptors and general anesthetics) (Herrera et al., 2015). These findings provide further support to the hypothesis that general anesthetics elicit sedation and loss-of-consciousness, at least in part, by preferentially recruiting brain regions that regulate sleep-wake state.
36 19 Figure 6. Schematic of thalamocortico-corticothalamic signaling in the presence and absence of excitatory input from arousal nuclei. A, During wakefulness thalamocortical neurons receive excitatory input from the ascending arousal system that elicits depolarization of thalamocortical neurons and promotes tonic action potential firing in these neurons. This mode of activity in the thalamus is permissive to the flow of sensory information through the thalamus to the cortex. B, In the absence of excitatory input from the ascending arousal system, as occurs during non-rem sleep, thalamic neurons become hyperpolarized, and this hyperpolarization promotes the burst mode of action potential firing in the thalamus. This mode of activity obstructs the relay of sensory information from afferent inputs through the thalamus to the cortex.
37 20 Figure 7. Traces illustrating the two modes of action potential firing identified in thalamic neurons. A, at hyperpolarized resting membrane potentials (i.e., < -65 mv) thalamic neurons exhibit a burst, or oscillatory, mode of action potential firing that is characterized by transient high-frequency bursts of action potentials. Conversely, at more depolarized resting membrane potentials (approximately -58 mv) thalamic neurons typically exhibit a tonic mode of action potential firing that is characterized by prototypic single trains of action potentials. B, the oscillatory, or burst mode, of firing in thalamic neurons is triggered by the de-inactivation of T-type Ca 2+ channels at hyperpolarized resting membrane potentials. Activation of these channels triggers a low-threshold Ca 2+ current (I T ) that then depolarizes the thalamic neuron to levels that trigger typical Na + /K + current-mediated action potentials. The pacing of these bursts of action potentials is determined by the hyperpolarization-activated cation current (I h ), also known as the pacemaker current. C, an expanded trace that illustrates tonic action potential firing by a thalamocortical neuron. Image was taken with permission from McCormick and Bal (McCormick and Bal, 1997).
38 Sleep spindles The RTN is essential for triggering sleep spindles, which are characterized as transient 7-14 Hz oscillations in electrocortical activity that occur during light non-rem sleep in humans, nonhuman primates, dogs, cats, and rodents (Hsieh et al., 2008; Luthi, 2013). Sleep spindles have a prototypic waxing and waning structure and typically last 1-3 seconds and recur every 3-10 seconds (Luthi, 2013). Isolation of the RTN through thalamic transections (for acute experiments) and RTN lesions (for chronic experiments) eliminates the occurrence of sleep spindles in both the dorsal thalamus and cortex (Steriade et al., 1985; Steriade et al., 1987). Sleep spindles persist, however, in the de-afferented RTN and are paced by an assortment of intrinsic ionic currents that allow RTN neurons to individually oscillate in the frequency range of spindles (Steriade et al., 1987). Specifically, T-type Ca 2+ channels and Ca 2+ -dependent small-conductance type 2 (SK2) K + channels endow RTN neurons with an intrinsic spindle pace-setting ability (Cueni et al., 2008; Astori et al., 2011; Wimmer et al., 2012). Functionally, the occurrence of sleep spindles is associated with a significant diminution in sensory perception, as indexed by pronounced reductions in arousability from sleep in humans and animals (i.e., an increased arousal threshold) (Pivik et al., 1999; Wimmer et al., 2012). Sleep spindles are also implicated in mediating learning and memory, as their density increases significantly following learning tasks in both humans and rodents and further correlates with subsequent performance on memory tasks (Fogel and Smith, 2011). It remains to be confirmed if this relationship between spindle density and memory performance is causal or indirectly related to other processes, such as hippocampal sharp wave ripples, that temporally coincide with the occurrence of sleep spindles and reduced arousability from non-rem sleep (Siapas and Wilson, 1998).
39 22 Co-localization of Ca v 3.3 channels and SK2 channels on RTN dendrites suggest that these channels play a critical role in mediating sleep spindles (Luthi, 2013). Experimental evidence supports this hypothesis. Specifically, mice that over-express the SK2 channel exhibit enhanced Ca v 3.3-mediated burst firing in their RTN neurons, which is further associated with increased spindle activity at the termination of non-rem sleep (when spindle activity typically peaks) (Wimmer et al., 2012; Luthi, 2013). Moreover, these mice exhibit more consolidated non- REM sleep (i.e., less sleep fragmentation) and are less likely to be aroused from sleep when exposed to white noise (Wimmer et al., 2012). RTN neurons from mice that lack the Ca v 3.3 isoform exhibit no burst firing (Astori et al., 2011). Sleep spindles are also absent in the Ca v 3.3 knockout mice during non-rem sleep, which indicates that expression of this T-type Ca 2+ channel isoform is necessary in the RTN for sleep spindle generation (Astori et al., 2011). Conversely, mice that lack the Ca v 3.1 isoform have normal spindle-like activity during non-rem sleep, but exhibit no burst firing in thalamocortical neurons (Lee et al., 2013). Together, these findings identify a critical role for RTN neuron burst firing in the generation and maintenance of sleep spindles during non-rem sleep. This interpretation is consistent with recent optogenetic studies that have demonstrated optogenetically-induced burst firing in the RTN is sufficient to trigger cortical spindle-like oscillations during non-rem sleep in mice (Halassa et al., 2011). Exactly how sleep spindles are triggered and terminated endogenously is unclear. It is speculated that corticothalamic-rtn synapses underlie the initiation of sleep spindles. Specifically, excitatory glutamatergic input from corticothalamic axon collaterals to RTN neurons can trigger α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptordependent burst firing in the RTN, which can then elicit a sleep spindle (Fuentealba and Steriade, 2005). The propagation of spindles to the cortex, on the other hand, is mediated through the RTN-thalamocortico-corticothalamic pathway. Specifically, widespread burst firing in the RTN
40 23 can effectively tune large populations of thalamocortical neurons to the intrinsic spindle rhythm of RTN neuron activity. Thalamocortical neurons can then recruit large populations of corticothalamic neurons. Indeed, when many thalamocortical neurons fire simultaneously, strong excitatory volleys are observed in both sensory and prefrontal cortices (Peyrache et al., 2011). The termination of sleep spindles is thought to involve lateral inhibition within the RTN (Steriade et al., 1993b) (Fig. 2). There are also intrinsic ionic mechanisms in RTN and thalamocortical neurons that act to reduce burst propensity. For example, during repetitive burst discharge in thalamocortical neurons there is a gradual activation of Ca 2+ -sensitive adenylate cyclases that ultimately activate hyperpolarization-activated cation nonselective (HCN) channels, which reduce the excitability of thalamocortical neurons and disfacilitate the thalamocortical- RTN signaling loop that is critical to the propagation of sleep spindles (Luthi and McCormick, 1999). The RTN-thalamocortico-corticothalamic circuitry underlying the generation, propagation, and termination of sleep spindles is also implicated in mediating epileptiform activity in idiopathic generalized epilepsies, of which the most common types are childhood and juvenile absence epilepsy, juvenile myoclonic epilepsy (patients exhibit absence seizures and transient muscle twitches), and generalized tonic/clonic epilepsy (Wolf and Goosses, 1986; Loiseau et al., 1991). Idiopathic generalized epilepsies are characterized by epileptiform activity that consists of synchronized, approximately 3 Hz, spike-wave cortical discharges (as recorded through the electroencephalogram). These spike-wave discharges have been posited to arise from hyper-synchronous activity in the spindle-generating RTN-thalamocortico-corticothalamic system that would, in essence, make spike-wave discharges perversions of typical sleep spindles (McCormick and Bal, 1997; Beenhakker and Huguenard, 2009).
41 24 This reasoning that epileptiform spike-wave discharges are generated through alterations in neuronal activity in the sleep spindle network is supported through qualitative and quantitative measures. Specifically, electroencephalogram (EEG) recordings show that cortical spike-wave discharges exhibit similar durations and are recorded in many different cortical regions like sleep spindles (Beenhakker and Huguenard, 2009). Additionally, thalamic activity is phase-locked to the timing of spike-wave discharges recorded in the EEG during absence seizures in humans (Williams, 1953). Current source density profiles (which identify the spatial positioning of current sinks across cortical layers) recorded at the cortex also identify similarities during spindle oscillations and spike-wave discharges (Kandel and Buzsaki, 1997). Moreover, alterations in thalamic inhibitory signaling can influence the probability of absence seizures in rodent animal models (discussed in more detail in section Thalamic GABA A receptors and general anesthetics) (Cope et al., 2009). It is also worth noting that both spindles and spike-wave discharges can be recorded in vitro in the same thalamic slice preparations. Specifically, spindle oscillations can give way to spike-wave discharges when thalamic slices are treated with pharmacological agents that block inhibitory signaling (von Krosigk et al., 1993; Huguenard and Prince, 1994; Kim et al., 1997; Sanchez-Vives and McCormick, 1997). The association between sleep spindles and absence seizures suggests that alterations in the activity of the spindle-generating circuitry in the thalamus can effectively trigger states that are associated with reduced awareness and perception. The action of general anesthetic agents on this network of neurons may play a critical role in mediating anesthetic-induced sedation and loss-of-consciousness. Indeed, modeling studies would suggest that alterations in this network underlie the changes in electrocortical activity that occur during general anesthetic-induced hypnosis (discussed in more detail in sections Cortical activity during general anesthesia) (Ching et al., 2010; Vijayan et al., 2013b, a). Thus, it is reasonable to posit that general
42 25 anesthetics could effectively lock thalamic signaling into a spindle-like mode of activity by promoting the initiation and/or impeding the termination of spindle-like oscillations. The studies that comprise this thesis are designed, in part, to identify the effect of general anesthetic agents on sleep spindle activity in vivo Delta (1-4 Hz) waves In contrast to sleep spindles, the thalamus is not essential for the generation of cortical 1-4 Hz, delta waves. Specifically, thalamic lesions in anesthetized cats do not alter cortical delta activity (Steriade et al., 1991). Moreover, cortical neurons have the intrinsic ability to generate slow, less than 1 Hz, oscillations and 1-4 Hz delta oscillations in the absence of thalamic input (Connors et al., 1982; Steriade et al., 1993a; Amzica and Steriade, 1998). Importantly, however, the thalamus does appear to play a significant role in tuning and synchronizing this brain activity pattern, with acute and chronic alterations in thalamic activity and channel expression directly affecting the frequency and power of delta waves during non-rem sleep in rodents (Steriade et al., 1991; Lee et al., 2004a; David et al., 2013; Lewis et al., 2015). Thalamocortical neurons exhibit intrinsic delta oscillations during non-rem sleep, where they become markedly more hyperpolarized due to the removal of excitatory input from the ascending arousal system (discussed in more detail in section Sleep regulation and T-type Ca 2+ channels) (McCormick and Pape, 1990; Leresche et al., 1991; Steriade et al., 1991; Dossi et al., 1992). In vivo and in vitro studies have identified that this delta oscillation activity pattern in thalamocortical neurons is mediated through T-type Ca 2+ channels (Crunelli et al., 2014). Specifically, during natural non-rem sleep the membrane potential of thalamocortical neurons commonly decreases to -65 mv or lower, which de-inactivates T-type Ca 2+ channels. The
43 26 subsequent interplay of T-type Ca 2+ channel and hyperpolarization-activated cation currents in thalamocortical neurons then triggers a burst mode of action potential firing that occurs in the delta frequency (i.e., 1-4 Hz) range (Steriade et al., 1993b) (Fig. 7). Mice that lack T-type Ca 2+ channels in thalamocortical neurons (i.e., Ca v 3.1 knockouts) exhibit a significant reduction in delta waves during non-rem sleep (Lee et al., 2004a). Depolarizing cholinergic, monoaminergic, and histaminergic neurotransmitters, that are characteristically released by afferents from the ascending arousal system into the thalamus during wakefulness and, in the case of cholinergic input, rapid eye movement (REM) sleep, effectively abolish burst firing and low-frequency rhythms in the thalamocortical system (McCormick and Bal, 1997) (Fig. 3). RTN-thalamocortical signaling influences 1-4 Hz electrocortical activity during both non-rem sleep and wakefulness (Herrera et al., 2015; Lewis et al., 2015). Local optogenetically-induced tonic activation of neurons in the RTN, which effectively decreases thalamocortical activity, is sufficient to induce delta wave activity in spatially restricted regions of the cortex in mice (Lewis et al., 2015). This tonic activation of the RTN is further associated with behavioural changes that are consistent with decreased arousal, such as decreased muscle tone and locomotor activity (Lewis et al., 2015). Moreover, optogenetically-induced tonic activation of the RTN in sleeping mice increases delta wave activity and decreases spindle amplitude (Lewis et al., 2015). Consistent with these findings, optogenetically-induced inhibition of the RTN is associated with elevated thalamocortical activity and decreased 1-4 Hz electrocortical activity during non-rem sleep (Herrera et al., 2015). It is well established that delta oscillations occur in the thalamocortical system when thalamocortical neurons are more hyperpolarized than they are during sleep spindle oscillations (Nunez et al., 1992). This mutual exclusivity between spindle and delta oscillations in
44 27 intracellular recordings of thalamocortical neurons is consistent with EEG recordings in humans and animals where light non-rem sleep is characterized by the presence of spindle rhythms, whereas deep non-rem sleep is dominated by delta waves (Steriade et al., 1993b). Nonetheless, delta oscillations in the thalamocortico-corticothalamic system are functionally similar to sleep spindles in that they are associated with the impaired relay of sensory information from the thalamus to the cortex. Cortical delta frequency activity is significantly elevated during anesthetic-induced hypnosis with a number of commonly used general anesthetic agents (Brown et al., 2010). The role of the thalamus, however, in mediating this anesthetic-induced increase in cortical delta signaling is unclear and is another topic that is investigated in this thesis Attention It has been over thirty years since Francis Crick proposed a functional role for the RTN as an attentional searchlight (Crick, 1984). Attention is a process that enhances some information in the environment while muting other surrounding information. Functionally, attention sub-serves focused processing of particular, potentially salient, aspects of the environment. The attentional searchlight hypothesis posits that the RTN functions as this internal searchlight and alters neuronal activity to focus on the aspect of environmental information that is to be actively processed (how the aspect of the environment to be processed is selected is likely a result of topdown processing) (Crick, 1984). Crick s hypothesis can be regarded as an extension of the work of Anne Treisman who first proposed the existence of an internal spotlight guiding attention and facilitating the serial processing of environmental subsections (Treisman, 1977; Treisman and Gelade, 1980).
45 28 The attentional searchlight hypothesis was largely informed by the neuroanatomy of the RTN, thalamus, and cortex and the impact that the RTN could have on the flow of sensory information from the thalamus to the cortex. All thalamocortical and corticothalamic projections pass through the RTN, since the dorsal thalamus is effectively encased by the RTN (Pinault, 2004) (Figs. 1 and 2). Many of these thalamocortical and corticothalamic projections send collateral excitatory axons to the RTN where they are topographically organized. Given the inhibitory nature of the RTN, excitatory input from axon collaterals of thalamic and cortical projections effectively positions the RTN to tune the strength of signals relayed between the thalamus and cortex. It has only been in the past few years, however, that the methodology to systematically assess Crick s proposed role for the RTN has become available. These studies provide compelling evidence that indicates a critical role for the thalamus in guiding attention. Two functionally distinct populations of RTN neurons have been identified in mice and can be distinguished through their anatomical basis (Halassa et al., 2014). Increased activity of RTN neurons that project to sensory thalamic nuclei is correlated (R = 0.42) with sleep spindles (Halassa et al., 2011; Halassa et al., 2014). Conversely, increased activity of RTN neurons that project to limbic thalamic nuclei is associated with arousal as activity of these neurons is negatively correlated with EEG delta power (i.e., amplitude) and is elevated during wakefulness (Halassa et al., 2014). Spindle-correlated RTN neurons that project to the visual relay nuclei of the thalamus exhibit significant reductions in activity during an attentional phase of a visual detection task in mice, which is consistent with previous findings from macaque monkeys where RTN neurons that putatively projected to the visual lateral geniculate nucleus exhibited reduced activity during visual attention (McAlonan et al., 2008; Halassa et al., 2014).
46 29 This modality-specific reduction in sensory-projecting RTN neuron activity is also associated with performance on a cross-modal attention task. Specifically, the activity of neurons in the visual RTN is differentially modulated during a task where mice have to select between conflicting auditory and visual stimuli, as prompted through a preceding binaurally emitted noise, that indicates food reward location (Wimmer et al., 2015). When mice were prompted to attend to vision the firing rates in the visual thalamus-projecting RTN decreased (presumably dis-inhibiting the thalamocortical visual relay neurons that these RTN neurons project to), whereas the firing rates increased when mice had to attend to audition (presumably inhibiting the thalamocortical visual relay neurons) (Wimmer et al., 2015). Collectively these findings indicate that a breakdown in the selectivity of RTN firing to the thalamus may underlie anesthetic-induced sedation and hypnosis by eliciting a decrease in attention and perception. A breakdown in selectivity of RTN signaling to thalamic nuclei could be achieved through homogenization of neuronal firing across RTN neurons. General anesthetics could achieve such an effect by eliciting and potentiating non-rem like input to the RTN, which would then promote widespread T-type Ca 2+ channel-dependent burst firing across the RTN. 1.3 Anesthesia and the thalamus An early de-activation of the thalamus has been identified during anesthetic-induced loss-ofconsciousness in several brain imaging studies (Alkire et al., 2008; Franks, 2008). Brain imaging techniques, however, are not ideally suited to identify alterations in subcortical activity and/or offer indirect indices of neuronal activity with poor temporal resolution (Menon and Kim, 1999; Mishra et al., 2011). Moreover, there is accumulating evidence that indicates a more complex role for the thalamus, beyond a basic reduction in signaling, during anesthetic-induced hypnosis.
47 Loss-of-consciousness and anesthesia Anesthetic-induced loss-of-consciousness is often signaled clinically by the absence of behavioural response to auditory commands or tactile stimulation. Consciousness is generally defined by the presence of subjective experience (Pandit et al., 2015). Such subjective experience can persist in the absence of behavioural responsiveness. For example, dreaming or dreamlike mentation is a state that is characterized by a lack of behavioural responsiveness to environmental stimuli, however subjective experience persists. Indeed, more than a quarter of patients anesthetized with propofol or desflurane report dreaming (Leslie et al., 2009). Moreover, the actual number of patients experiencing dreamlike mentation during anesthesia is likely higher since the amnestic effect of general anesthetics would likely impair patient recall. This disconnected form of consciousness during anesthesia, where perception is not connected to the physical environment, is not necessarily an undesirable state as the main goals of general anesthesia continue to be fulfilled. That is, patients who experience dreaming during anesthesia still lack awareness and perception of surgery, experience nociceptive blockade, and are immobile during surgery. Consciousness during general anesthesia is a problem when perception of the physical environment persists. There are some that argue that even this form of consciousness is permissible so long as the amnestic effects of the general anesthetic agent are strong enough to abolish patient recall. While this utilitarian approach to general anesthesia may not be entirely wrong it is ethically dubious. Ideally, tools that assess depth of anesthesia would be able to rapidly distinguish between environmentally connected and disconnected consciousness as well as unconsciousness (Sanders et al., 2012).
48 31 The isolated forearm technique is a method that is employed to assess consciousness during general anesthesia (Bruhn et al., 2006). For this technique a tourniquet is applied to the arm of the patient after the induction of general anesthesia but before the application of paralytic agents, allowing mobility of the arm, while the rest of the body is paralyzed. Patients are then asked questions or given commands that they can then respond to through hand signals (e.g., squeezing the hand of a doctor). Typically, patients that respond during the isolated forearm technique exhibit goal-directed responses and do not exhibit spontaneous hand movement (Sanders et al., 2012). Often patients that continue communicating through hand gestures during anesthesia fail to recall this communication post-operatively (Kerssens et al., 2003). There are also patients that provide full post-operative recall of surgery but explain feeling uninterested in responding to verbal commands during surgery (Alkire et al., 2008). This lack of willful responsiveness, posited to result from anesthetic-induced suppression of neuronal activity in brain regions associated with executive function, further complicates the identification of awareness during general anesthesia using the isolated forearm technique (Alkire et al., 2008). There are also EEG-based techniques for assessing the depth of anesthesia (Bruhn et al., 2006). There are three commercially available EEG-based monitors that are used clinically to assess depth of anesthesia: the Bispectral (BIS) monitor, the Narcotrend monitor, and the State and Response Entropy monitor (M-Entropy). All three of these monitors display the raw EEG and provide dimensionless measures of the depth of anesthesia that range from 0 (i.e., brain dead) to 100 (i.e., awake). Given the proprietary nature of these monitors there is limited information regarding how they calculate depth of anesthesia. All three of these monitors examine the EEG collected from electrodes located on the patient s forehead (Bruhn et al., 2006).
49 32 The BIS index is calculated through analysis of the power spectrum and phase spectrum of the EEG using fast Fourier transforms (FFT) and is described as being quantified through analysis of the coupling of phase angles from different frequencies. The BIS index is a proprietary combination of the SynchFastSlow sub-variable, which is defined as the log of the ratio of the sum of all bispectral peaks in the area from Hz over the sum of the bispectrum from Hz, and a sub-parameter from the frequency domain ( β ratio ) and a sub-variable from the time domain ( burst suppression ) (Rampil, 1998; Bruhn et al., 2000; Bruhn et al., 2006). In contrast, the Narcotrend index is related to visual sleep scoring analysis and also considers a number of frequency and time variables that can discriminate between different, visually-determined substages of the EEG (Kreuer et al., 2004; Bruhn et al., 2006). Finally, M-Entropy examines the regularity and frequency distribution of the EEG using nonlinear dynamics to predict the probability of future amplitude values. Two spectral entropy indicators are considered in these calculations: state entropy, which is calculated from Hz, and response entropy, which is calculated from Hz (Vanluchene et al., 2004). A major limitation of all of these monitors of depth-of-anesthesia is that none of them make a distinction between the different kinds of general anesthetic agents used, nor do they consider the age and/or morbidities of patients, factors which significantly, and distinctly, influence the spectral composition of the EEG during general anesthesia (Purdon et al., 2015). Comparisons of the isolated forearm technique with the EEG-based techniques outlined above indicate discrepancies between these two methods for assessing depth of anesthesia. Some reports identify that both Narcotrend and BIS indices associated with loss-of-consciousness can be achieved while patients still exhibit consciousness as assessed through the isolated forearm technique or patient response to verbal command (Schneider et al., 2004; Russell, 2006, 2013). Unlike the EEG based indices, the isolated forearm technique does not provide a clear gradient
50 33 of measures that is, it provides an all or none measure of consciousness that does not allow for the preemptive administration of more general anesthesia to maintain an unconscious or disconnected conscious state. It is worth noting that there is some recent discussion on whether the isolated forearm technique is truly all or none, with some arguing that this test can provide readouts for 4 levels of consciousness (Pandit et al., 2015). A breakdown in cortical connectivity is hypothesized to ultimately underlie loss-ofconsciousness (Alkire et al., 2008). Transcranial magnetic stimulation (TMS) of the cortex during wakefulness results in prolonged activation (i.e., > 300 ms) of multiple cortical areas as identified through functional magnetic resonance imaging (Massimini et al., 2007). In contrast, during general anesthesia and non-rem sleep, TMS results in cortical activation that is far more spatially restricted and short lasting (Massimini et al., 2007; Lewis et al., 2012). These findings identify a breakdown in the integration of sensory information across cortical areas during states that are associated with unconsciousness. This breakdown in the spatiotemporal complexity of cortical stimulation with TMS is also associated with dreamless propofol- and xenon-induced general anesthesia (i.e., loss-of-consciousness) (Sarasso et al., 2015). Importantly, in vivo brain imaging and multi-unit recordings implicate the thalamus in mediating this breakdown in cortical connectivity during general anesthesia (Ni Mhuircheartaigh et al., 2013; Reinhold et al., 2015). The thalamocortical system exhibits small-world network organization (Achard et al., 2006). Such networks are characterized by high levels of local connectivity, with short path lengths, and possess comparatively fewer long-range connections (Fig. 8). Thus, disruption of only a few long-range connections is required to effectively dismantle transmission in such a network. General anesthetics are posited to elicit hypnosis by disrupting long-range connectivity in the thalamocortical system (Alkire et al., 2008; Gili et al., 2013). Indeed, isolation of the
51 34 thalamocortical system has been associated with both the onset and maintenance of anestheticinduced loss-of-consciousness (Ni Mhuircheartaigh et al., 2013). Figure 8. Schematic illustrating three major network types. Regular networks (left) exhibit no disorganization, very high levels of local connectivity (i.e., clustering), and relatively large path lengths between nodes, as there are no long-range projections. Small-world networks (centre), in contrast, possess a few long-range connections that significantly reduce the overall path length between nodes and create hubs (i.e., nodes that are connected to many other nodes). Small-world networks also exhibit high levels of clustering. Random networks (right) possess very short path lengths between nodes; however, these networks are also characterized by lack of clustering. Image was taken with permission from Watts and Strogatz (Watts and Strogatz, 1998) Common molecular targets of general anesthetics There is a great deal of information regarding the major molecular targets of all commonly used general anesthetic agents (Rudolph and Antkowiak, 2004; Franks, 2008) (Table 1). Chief among these targets is the γ aminobutyric acid (GABA) type A (GABA A ) receptor (Rudolph and Antkowiak, 2004; Bonin and Orser, 2008; Franks, 2008). There are also a number of other molecular targets that are affected by commonly used general anesthetic agents. These targets
52 35 include two-pore domain K + channels, N-methyl-D-aspartate (NMDA) receptors, glycine receptors, and HCN channels (Rudolph and Antkowiak, 2004; Franks, 2008). The action of general anesthetics at these molecular targets ultimately achieves a state that is characterized by hypnosis, immobility, amnesia, and analgesia. GABA is the main inhibitory neurotransmitter in the brain, and augmentation of GABAergic neuronal activity promotes natural sleep (Saper et al., 2001). Activation of GABA A receptors elicits neuronal hyperpolarization by increasing the influx of Cl - ions. These receptors are widely expressed throughout the brain, although the subunits that comprise GABA A receptors do exhibit some regional specificity in their expression patterns (Olsen and Sieghart, 2009). GABA A receptors are heteropentameric and constructed from an array of subunits (α1-6, β1-3, γ1-3, δ, π, θ, ε, and ρ1-3) (Olsen and Sieghart, 2009). These receptors are typically found assembled with one of two preferred stoichiometries: 2α:2β:γ or 2α:2β:δ. Almost all commonly used general anesthetics have been found to potentiate GABA A receptor activity and/or directly activate these receptors (Rudolph and Antkowiak, 2004; Franks, 2008) (Table 1). This effect includes most volatile general anesthetic and intravenous general anesthetic agents.
53 36 Table 1. List of general anesthetic agents that are commonly used clinically in human patients. General Anesthetic Molecular Targets Mode of Delivery Use Desflurane GABA A receptor (Nishikawa et al., 2002; Nishikawa and Harrison, 2003), nicotinic acetylcholine receptor (Paul et al., 2002) Inhalational Induction Enflurane GABA A receptor (Mihic et al., 1997), glycine receptor (Mihic et al., 1997), NMDA receptor (Petrenko et al., 2014), nicotinic acetylcholine receptor (McKenzie et al., 1995) Inhalational Induction & maintenance Etomidate GABA A receptor (Forman, 2011) Intravenous Induction & maintenance Halothane GABA A receptor (Yamauchi et al., 2002; Jurd et al., 2003), two-pore domain K+ channel (Patel et al., 1999), glycine receptor (Yamauchi et al., 2002), nicotinic acetylcholine receptor (Eckenhoff, 1996), NMDA receptor (Kitamura et al.) Inhalational Induction & maintenance Isoflurane GABA A receptor (Grasshoff and Antkowiak, 2006; Jia et al., 2008b), glycine receptor (Grasshoff and Antkowiak, 2006), NMDA receptor (Petrenko et al., 2014), HCN channel (Chen et al., 2009b), two-pore domain K+ channel (Patel et al., 1999), nicotinic acetylcholine receptor (McKenzie et al., 1995; Scheller et al., 1997) Inhalational Maintenance Ketamine NMDA receptor (Petrenko et al., 2014), nicotinic acetylcholine receptor (Scheller et al., 1996), HCN channel (Chen et al., 2009a) Intravenous Induction Propofol GABA A receptor (Ying and Goldstein, 2005a), glycine receptor (Hales and Lambert, 1991; Dong and Xu, 2002), HCN channel (Ying et al., 2006) Intravenous Induction & maintenance Sevoflurane GABA A receptor (Nishikawa et al., 2002; Nishikawa and Harrison, 2003), glycine receptor (Grasshoff and Antkowiak, 2004), NMDA receptor (Petrenko et al., 2014), nicotinic acetylcholine receptor (Scheller et al., 1997) Inhalational Induction Thiopental GABA A receptor (Lukatch and MacIver, 1996), glycine receptor (Daniels and Roberts, 1998), nicotinic acetylcholine receptor (Downie et al., 2000) Intravenous Induction Mutational studies have characterized the influence of specific GABA A receptor subunits on general anesthetic endpoints. Specifically, α subunit mutations greatly influence the
54 37 analgesic, amnestic, sedative, and hypnotic effects of volatile general anesthetics, but these mutations generally have little effect on the anesthetic endpoints of intravenous general anesthetics (Mihic et al., 1997; Krasowski et al., 1998; Koltchine et al., 1999; Krasowski and Harrison, 2000; Jenkins et al., 2001; Nishikawa et al., 2002; Schofield and Harrison, 2005). There are, however, exceptions to this generalization. For example, α5-subunit containing GABA A receptors are implicated in mediating memory impairment following sedation with intravenous general anesthetics, such as etomidate (Cheng et al., 2006; Zurek et al., 2014). Mutations of the β GABA A receptor subunit also exhibit a robust association with the potency of both volatile and intravenous general anesthetic agents. Specifically, point mutations in the β2 and β3 GABA A receptor subunits can reduce the duration of loss-of-righting reflex (a well established index for loss-of-consciousness in animals) with the intravenous general anesthetic agents etomidate and propofol as well as with the volatile general anesthetic agent enflurane in mice (Belelli et al., 1997; Krasowski et al., 1998; Carlson et al., 2000; Krasowski and Harrison, 2000; Krasowski et al., 2001; Siegwart et al., 2002; Chang et al., 2003; Richardson et al., 2007). The immobilizing actions of etomidate, enflurane, and halothane (another volatile general anesthetic agent) were also impaired in β3 GABA A receptor subunit point mutant and/or knockout mice (Quinlan et al., 1998; Jurd et al., 2003; Reynolds et al., 2003). Two-pore domain K + channels, NMDA receptors, glycine receptors, and HCN channels also exhibit significant alterations in their activity with certain commonly used general anesthetic agents (Franks, 2008). Regardless of the molecular target, however, the effect of general anesthetics on neuronal activity is ultimately inhibitory. Specifically, general anesthetic agents can promote inhibition through direct increases in inhibitory channel/receptor activity and/or through blockade of excitatory channel/receptor activity. Thus, it is clear that whatever
55 38 alterations in neuronal activity are responsible for triggering general anesthetic-induced sedation and loss-of-consciousness they are ultimately initiated through elevated/potentiated inhibitory signaling. Two-pore domain K + channels mediate a potassium leak current that influences the resting membrane potential of neurons (Lesage and Lazdunski, 2000). Activation of these channels promotes hyperpolarization through the efflux of K + ions, while blockade of these channels promotes depolarization. Indeed, neurotransmitters associated with cortical activation such as acetylcholine, serotonin, and noradrenaline inhibit two-pore domain K + channels and promote neuronal excitability through a G protein-coupled receptor-dependent pathway (Mathie et al., 2010). Similar to GABA A receptors, two-pore domain K + channels are expressed widely throughout the brain (Talley et al., 1999). Two-pore domain K + channels exhibit significant sensitivity to halogenated volatile general anesthetic agents, such as isoflurane and halothane (Patel et al., 1999). These agents promote two-pore domain K + channel activity and thus hyperpolarization of neurons. Indeed mice that lack TASK-3 channels, which are an acidsensitive member of the two-pore domain K + channel family, exhibit reduced sensitivity to halothane (Linden et al., 2007). In contrast, propofol-induced loss-of-righting reflex was unaltered in TASK-3 knockout mice, which is unsurprising considering the molecular targets of propofol (i.e., primarily GABA A receptors) (Linden et al., 2007) (Table 1). NMDA receptors are distinct from both GABA A receptors and two-pore domain K + channels in that their activation promotes depolarization, and subsequent excitation of neurons. These receptors require both glutamate, which is the major excitatory neurotransmitter in the central nervous system, and the co-agonist glycine or D-serine to bind to them for activation. NMDA receptor activation promotes neuronal excitation through Ca 2+ influx. A number of
56 39 commonly used general anesthetic agents block NMDA receptor activity by binding to active NMDA receptors and occluding the flow of Ca 2+ across the receptor (Petrenko et al., 2014). Blockade of these receptors by general anesthetics is associated with a number of behavioural abnormalities that occur before loss-of-consciousness (van Berckel et al.; Becker et al., 2003). These abnormalities include hallucinations, dissociative states, euphoria, and dysphoria. Such alterations in behaviour are not associated with general anesthetic agents that target other receptors and/or channels and are posited to arise from the preferential actions of NMDA receptor-blocking general anesthetics at inhibitory interneurons, which are typically more active than pyramidal neurons under baseline conditions (Olney and Farber, 1995; Seamans, 2008; Purdon et al., 2015). Such an action would lead to an initial disinhibition of the cortex, and lossof-consciousness would then occur once NMDA receptors on excitatory glutamatergic neurons are blocked. Ketamine is perhaps the most well-known and commonly used NMDA receptortargeting general anesthetic agent, although a number of volatile general anesthetics (that also possess other molecular targets; see Table 1 for examples) elicit blockade of NMDA receptor activity as well. Mice that lack expression of the GluN2A NMDA receptor subunit exhibit resistance to the hypnotic effects of ketamine (Petrenko et al., 2004). Similarly mutation of the transmembrane segment of the GluN2A NMDA receptor subunit is associated with reduced NMDA receptor sensitivity to halothane, isoflurane, cyclopropane, and xenon in a heterologous system where human wild-type and mutant NMDA receptors were expressed in Xenopus oocytes (Ogata et al., 2006). Glycine receptors are similar to, and often co-localize with, GABA A receptors (Betz, 1991). Activation of these receptors is inhibitory, eliciting Cl - ion influx and hyperpolarization. Volatile anesthetics, such as enflurane and isoflurane, potentiate glycine receptor activity in recombinant systems, where chimaeric glycine receptors (composed of the glycine receptor α1
57 40 sequence from the amino terminus to a junction site where the GABA-receptor ρ1 sequence was expressed until the carboxy terminus) were expressed in Xenopus oocytes (Mihic et al., 1997). The effect of intravenous general anesthetics on glycine receptors is less clear. Propofol appears to have no effect on glycine receptors in hippocampal neurons, however it does potentiate glycine-induced currents at clinically relevant concentrations in acutely dissociated rat spinal dorsal horn neurons (Hales and Lambert, 1991; Hara et al., 1993; Patten et al., 2001; Dong and Xu, 2002). Thus, glycine receptors may play a role in mediating the analgesic effects of intravenous general anesthetic agents, but do not appear to play a clear role in their amnestic, sedative, or hypnotic effects. Activation of HCN channels occurs at hyperpolarized membrane potentials (i.e., < -50 mv) and elicits a mixed cation current that is commonly referred to as a pacemaker current (also known as I h ). These channels are gated by cyclic adenosine monophosphate (camp) and are permeable to both Na + and K + ions. Activation of HCN channels can contribute to the maintenance and recovery of resting membrane potentials and action potential firing. Volatile anesthetics like halothane and enflurane effectively dampen HCN channel activity by shifting their activation to even lower membrane potentials and decreasing their current conductance (Tokimasa et al., 1990; Sirois et al., 2002). Both propofol and ketamine similarly impair HCN channel activity through blockade in heterologous systems (Bojak et al., 2013). Consistent with these findings, the hypnotic potency of both ketamine and propofol is significantly reduced in HCN1 (one of four subunits that make up HCN channels) knockout mice (Chen et al., 2009a). Blockade of these channels in the thalamus by general anesthetics may serve to lock RTNthalamocortico-corticothalamic signaling into an aberrant spindle generating mode, as HCN channel activation is implicated in mediating the termination of sleep spindles (for more detail see section Sleep spindles) (Luthi and McCormick, 1999).
58 41 General anesthetic action at all of these molecular targets would ultimately promote a reduction in the resting membrane potential of a neuron. At hyperpolarized resting membrane potentials, RTN neurons and thalamocortical neurons can enter into a burst mode of action potential firing. Given the connectivity between these thalamic regions and the cortex it is possible that this neuronal system is responsible for orchestrating the breakdown in sensory processing and subsequent changes in cortical activity that signal anesthetic-induced loss-ofconsciousness Cortical activity during general anesthesia It has been approximately 80 years since general anesthetic-induced alterations in cortical activity were first described (Gibbs et al., 1937). Since then numerous studies have characterized distinct patterns of cortical activity that are associated with varying doses and stages of general anesthesia. The alterations in cortical activity, as measured through EEG recordings, elicited by general anesthetics vary according to their molecular targets (Purdon et al., 2015). Specifically, general anesthetics that target GABA A receptors typically elicit an increase in delta (1-4 Hz) and alpha-beta (8-22 Hz) frequency activities while general anesthetics that target NMDA receptors elicit an increase in gamma (25-40 Hz) frequency activity (discussed in more detail in the following paragraphs). Despite this documented heterogeneity, however, the current electrocortical indices used to assess depth of anesthesia are the same across all general anesthetic agents (discussed in section Consciousness and anesthesia) (Bruhn et al., 2006; Purdon et al., 2015). Appreciating these differences and the neurophysiology that underlies their generation will be a key step in the development of more precise indices of depth of anesthesia and will further aid in elucidating the neural correlates of consciousness.
59 42 Propofol is a prototypic intravenous GABA A receptor-targeting general anesthetic agent that elicits a significant increase in 1-4 Hz (i.e., delta) and 8-22 Hz (i.e., alpha-beta) electrocortical activity during sedation and hypnosis (as judged by loss of behavioural responsiveness to an auditory cue) (Purdon et al., 2013) (Fig. 9A). Specifically, during the induction of anesthesia with propofol there is a significant increase in the amplitude of delta waves in the EEG. This increase in delta activity can be attributed to the effect of propofol on brainstem arousal nuclei, where it potentiates inhibitory GABAergic input from the sleep promoting pre-optic area of the hypothalamus. This effect of propofol at the brainstem effectively dampens excitatory input to the cortex, which promotes intrinsic delta frequency activity (Devor and Zalkind, 2001; Lewis et al., 2012; Purdon et al., 2013). Loss-of-consciousness, as indexed by loss of behavioural responsiveness to verbal command, with propofol is also associated with increased delta frequency cortical activity, but there is another key electrocortical marker that distinguishes this maintenance stage of anesthesia from the initial induction. This signature is characterized by an increase and anteriorization of alpha-beta frequency electrocortical activity (Tinker et al., 1977; Cimenser et al., 2011; Purdon et al., 2013; Akeju et al., 2014) (Fig. 9B). This frontal alpha-beta rhythm shows a high degree of coherence, which is not seen with the delta frequency activity signature, suggesting the involvement of other brain regions in its development. A number of modeling studies have identified that anesthetic-induced alterations in thalamocortico-corticothalamic signaling in the same network that generates sleep spindles would be sufficient to elicit this key signature of anesthetic hypnosis with certain general anesthetic agents (Ching et al., 2010; Vijayan et al., 2013a; Ching and Brown, 2014). The sufficiency of thalamocortico-corticothalamic signaling in generating this alpha-beta signature of anesthetic-induced loss-of-consciousness has not,
60 43 however, been demonstrated in vivo and this is one of the aims of the studies that comprise this thesis. Emergence from propofol-induced anesthesia is characterized by a return of low amplitude, high frequency activity in the EEG (Purdon et al., 2013) (Fig. 9A and B). A reversal in the anteriorization of alpha-beta electrocortical activity and breakdown in delta power also occur at this point (Purdon et al., 2013). Administration of other GABA A receptor-targeting general anesthetics, like etomidate, sevoflurane, and thiopental, are also associated with increased delta and alpha-beta frequency EEG activity during loss-of-consciousness, although these changes have not been characterized as extensively as the electrocortical alterations associated with propofol (Kuizenga et al., 2001; Andrada et al., 2012) (Fig. 10). Halothane, a volatile two-pore domain K + channel-targeting general anesthetic agent (that also has other molecular targets; see Table 1 for detail), elicits alterations in EEG activity that are similar to those associated with GABA A receptor-targeting general anesthetic agents. Specifically, halothane-induced hypnosis is associated with an increase in delta and alpha-beta frequency EEG activity (Findeiss et al., 1969; Jäntti and Sloan, 2008). Moreover, increasing concentrations of halothane in human patients is correlated with increased power in the Hz and Hz bands (Yli-Hankala et al., 1989). This increase in alpha-beta frequency activity was also most pronounced in frontal EEG regions (Yli-Hankala et al., 1989). Modeling studies indicate that halothane can effectively elicit an anteriorization of electrocortical alpha activity through thalamic-dependent mechanisms that are similar to those associated with the GABA A receptor-targeting general anesthetic agent propofol (Vijayan et al., 2013b). In contrast to the EEG patterns associated with GABA A receptor and two-pore domain K + channel-targeting general anesthetic agents, ketamine, an NMDA receptor-targeting general
61 44 anesthetic, elicits a low amplitude, high frequency pattern of EEG activity (Fig. 9A and B). Specifically, ketamine promotes fast beta-gamma frequency oscillations (described as Hz in some publications and Hz in others) and irregular slow frequency oscillations in the EEG during anesthetic-induced hypnosis (Jäntti and Sloan, 2008; Bojak et al., 2013; Purdon et al., 2015) (Fig. 9A and B). The increase in beta-gamma frequency activity is also noted to dissipate several minutes before recovery of behavioural responsiveness to verbal command (Purdon et al., 2015). The actions of ketamine at projections from the parabrachial nucleus and median pontine reticular formation to the basal forebrain are suggested to mediate the betagamma frequency signature seen at the loss-of-consciousness (Brown et al., 2011).
62 45 Figure 9. Electrocortical activity during propofol-induced general anesthesia in human patients. A, Power spectrum depicting electrocortical activity during administration of propofol. Sedation with propofol is associated with an increase in slow, 1-4 Hz activity. Loss-of-consciousness is marked by an increase in alpha-beta frequency (8-22 Hz) activity. Warmer colours denote increased power. The period associated with propofol-induced sedation and loss-of-consciousness (as judged by loss of responsiveness to verbal command) is demarcated by the white trace. The red bar at the top of the spectrogram, labeled maintenance, denotes the period the patient was unconscious. B, Loss-of-consciousness with propofol is also associated with an anteriorization of alpha-beta activity. LOC: loss-of-consciousness; ROC: recovery of consciousness. This image was taken with permission from Purdon et al. (Purdon et al., 2015).
63 46 Figure 10. Comparison of electrocortical activity during anesthetic-induced loss-of-consciousness with three commonly used general anesthetic agents. A, Raw electroencephalogram recording of cortical activity during general anesthesia with propofol, sevoflurane, and ketamine. Note the comparatively high frequency, low amplitude electrocortical signature associated with ketamine-induced loss-ofconsciousness compared to the high amplitude, lower frequency activity associated with propofol and sevoflurane. B, Spectral composition of the raw electroencephalogram traces. Warm colours depict higher activity in the corresponding frequency range (y axis). Note the coherent increase in alpha-beta frequency activity associated with propofol- and sevoflurane-induced loss-of-consciousness. This image was taken with permission from Purdon et al. (Purdon et al., 2015) Thalamic activity during general anesthesia: brain imaging studies Brain imaging studies have consistently shown that most general anesthetic agents, with the exception of ketamine (discussed in some detail in section Cortical activity and anesthesia), elicit global reductions in cerebral blood flow and/or metabolism when unconsciousness is achieved (Fiset et al., 1999; Bonhomme et al., 2001; Kaisti et al., 2003;
64 47 Laitio et al., 2007). There are a number of specific brain regions that consistently exhibit marked alterations at the point of anesthetic induced loss-of-consciousness. Of these common alterations, thalamic deactivation is a feature that is often identified during anesthetic hypnosis with intravenous and inhaled general anesthetic agents. For example, propofol, sevoflurane (a GABA A receptor-targeting inhalational general anesthetic that also has secondary effects on twopore domain K + channels), and xenon (an inhalational anesthetic that is believed to elicit its effects largely through its action on NMDA receptors although it also activates two-pore domain K + channels) elicit a deactivation of the thalamus at the point of loss-of-consciousness (i.e., the point at which patients no longer respond to a verbal command or tactile stimulation) (Fiset et al., 1999; Alkire et al., 2000; Bonhomme et al., 2001; Kaisti et al., 2003; White and Alkire, 2003). Associative cortices, such as the precuneus and cuneus and the parieto-occipital cortex, also commonly exhibit deactivation during anesthetic-induced hypnosis with a number of widely used general anesthetic agents, such as propofol (Alkire et al., 2008). These particular associative cortices, like the thalamus, are considered hubs of activity (i.e., they are relatively heavily innervated by long range projections from other brain regions) and a breakdown in their activity would have significant consequences on the processing and integration of information that subserves conscious perception (Tomasi and Volkow, 2011). Brain imaging studies identify a significant temporal link between anesthetic-induced hypnosis and altered thalamic activity. Alterations in cerebral blood flow during vibrotactile stimulation are well documented in the thalamus. Importantly, propofol eliminates these alterations only once loss-of-consciousness occurs (Bonhomme et al., 2001). Conversely, changes in cerebral blood flow at somatosensory cortices during vibrotactile stimulation are suppressed at lighter, sedative doses of propofol where patients still respond to verbal command but report feeling drowsy and/or exhibit slurred speech (Bonhomme et al., 2001). Brain imaging
65 48 studies also show that thalamocortico-corticothalamic interactions breakdown significantly during unconsciousness with halothane and isoflurane, further indicating that the alterations in thalamic activity during anesthetic-induced hypnosis bear functional relevance (i.e., these alterations significantly contribute to anesthetic-induced loss-of-consciousness) (White and Alkire, 2003). Functional magnetic resonance imaging with concomitant EEG recording also identify a breakdown in the relay of sensory information from the thalamus to the cortex in humans during propofol-induced hypnosis (Ni Mhuircheartaigh et al., 2013). In humans there is an increase in slow wave activity that eventually plateaus during loss-of-consciousness with gradually increasing levels of administered propofol. Once cortical slow wave activity has reached this plateau there is an isolation of the thalamocortical system, as indicated by an absence of response to sensory stimuli (Ni Mhuircheartaigh et al., 2013). Lower frequency (10-12 Hz) sleep spindlelike oscillations recorded from the prefrontal cortex also are significantly increased at the point of thalamocortical isolation with propofol (Ni Mhuircheartaigh et al., 2013). These findings support modeling studies that suggest the spindle-generating thalamocortico-corticothalamic circuit is engaged and contributes to the electrocortical patterns associated with anestheticinduced hypnosis (Ching and Brown, 2014). The studies presented in this thesis are designed, in part, to validate this hypothesis in vivo Thalamic activity during general anesthesia: multi-unit and single-unit recordings Local field potential recordings provide direct measurements of neuronal population activity in spatially discrete regions of the brain. Such recordings can help elucidate the temporal relation
66 49 between alterations in neuronal activity in the thalamus and cortex during the onset and maintenance of general anesthetic-induced loss-of-consciousness. Indeed, local field potential recordings have identified a number of key alterations in neuronal activity that occur first in the thalamus before spreading to the cortex during propofol-induced loss-of-consciousness in rats (Baker et al., 2014). Specifically, a brief increase in Hz power that occurs during transitions into propofol-induced loss-of-righting reflex occurs first in the central medial thalamus before spreading to the anterior cingulate cortex and barrel cortex (Baker et al., 2014). The central medial thalamus is characterized as a nonspecific thalamic nucleus (i.e., one that is not involved in the discrete relay of sensory information) that projects diffusely to the cortex. This transient Hz signature that occurred first at the thalamus was also found to occur during transitions into natural non-rem sleep, indicating that general anesthetic agents elicit non-rem like changes in thalamic input (Baker et al., 2014). In both cases, the increase in Hz power was also accompanied by a shift to a lower dominant frequency in this Hz band. Transitions into propofol-induced loss-of-righting reflex were also associated with a sharp increase in 8 Hz electrocortical signaling, which was not seen during non-rem sleep and is consistent with EEG recordings in humans and animals during loss-of-consciousness with propofol or etomidate (Kuizenga et al., 2001; Andrada et al., 2012; Purdon et al., 2013; Baker et al., 2014). These local field potential recordings indicate that while a non-rem-like signature occurs at the onset of propofol-induced loss-of-consciousness, there are subsequent alterations in cortical activity that are distinct from those associated with non-rem sleep. The experiments that comprise this thesis provide further insight on the thalamic mechanisms that mediate the electrocortical patterns that distinguish non-rem sleep from general anesthesia. Activity exceeding approximately 30 Hz is significantly depressed in thalamocortical neurons of the dorsal lateral geniculate nucleus during isoflurane-induced general anesthesia in
67 50 mice (Reinhold et al., 2015). In this experiment isoflurane was delivered alone or in combination with an intraperitoneal injection of urethane (an intravenous general anesthetic agent that is commonly used in veterinary medicine and acts on GABA A receptors, glycine receptors, and NMDA receptors) and/or chlorprothixene (an antipsychotic that also acts as a local anesthetic, targeting a number of receptors including the H1 histamine receptor). This anesthetic-induced reduction in thalamocortical activity corresponded to a decrease in spontaneous thalamocortical action potential firing and was directly associated with disrupted cortical processing of visual information (Reinhold et al., 2015). Such a break down in sensory information processing is regarded as a pre-requisite for anesthetic-induced hypnosis (Tononi, 2004). These findings support the hypothesis that a breakdown in the relay of information from the thalamus to the cortex contributes significantly to loss-of-consciousness. Reductions in the spontaneous firing rate of thalamocortical neurons during anestheticinduced hypnosis are consistently identified in both rodents and cats. Both propofol and etomidate-induced general anesthesia elicit an approximate 40 % decrease in the spontaneous firing rate of thalamocortical neurons in cats (Andrada et al., 2012). These alterations in thalamocortical activity are further associated with a shift in the EEG from low amplitude, high frequency activity to high amplitude, low frequency activity, where peak amplitude (i.e., power) was identified at Hz with both propofol and etomidate (Andrada et al., 2012). Etomidate also elicited an increase in 7-8 Hz power (Andrada et al., 2012). Intraperitoneal injections of urethane similarly decreased the spontaneous firing rate of thalamocortical neurons in mice (Huh and Cho, 2013). This reduction in spontaneous activity may be permissive to the recruitment of large populations of thalamocortical neurons to the intrinsic sleep-spindle like rhythm set by GABAergic inputs from the RTN. Given the reciprocal connectivity between the thalamus and cortex, such signaling could rapidly spread to the cortex. It remains unclear, however, exactly
68 51 how general anesthetics elicit this effect on thalamocortical neurons at the cellular and molecular level, a topic that is investigated in this thesis Thalamic GABA A receptors and general anesthetics The effects of GABA A receptor-targeting general anesthetics on thalamocortical neurons have been studied greatly in vitro. There are three different forms of GABA A receptor-mediated inhibition that have been identified in thalamocortical neurons and can be altered by GABA A receptor-targeting general anesthetics (Fig. 11A-C). Specifically, thalamocortical neurons exhibit phasic inhibition that is characterized by the transient activation of synaptic GABA A receptors, tonic inhibition that is characterized by the prolonged activation of extrasynaptic GABA A receptors by ambient GABA, and, the most recently identified, spillover inhibition that is characterized by the transient activation of extrasynaptic GABA A receptors by spillover GABA from the synapse (Belelli et al., 2005; Cope et al., 2005; Farrant and Nusser, 2005; Jia et al., 2005; Herd et al., 2013; Rovo et al., 2014) (Fig. 11A-C). Inhibitory post-synaptic potentials (IPSPs) elicited through spillover inhibition can be distinguished from those elicited through phasic inhibition by their decay constant (i.e., the time that it takes for an IPSP to decay or for the membrane potential to be restored) (Fig. 11A-C). Specifically, spillover inhibition is characterized by a GABA A receptor-mediated slow current with a decay constant typically greater than 30 ms (Farrant and Nusser, 2005; Capogna and Pearce, 2011). Phasic inhibition, conversely, is characterized by a GABA A receptor-mediated fast current with a decay constant that is around 5 ms (Farrant and Nusser, 2005; Capogna and Pearce, 2011).
69 52 Figure 11. Schematic of the three different forms of GABA A receptor-mediated inhibition identified in thalamocortical neurons. A, Phasic inhibition is elicited by the brief, transient activation of synaptic GABA A receptors by high levels of GABA that are released at the synapse. B, Tonic inhibition is elicited by the activation of extrasynaptic GABA A receptors by ambient GABA. C, Spillover inhibition is elicited by the transient activation of extrasynaptic GABA A receptors by GABA that is released at the synapse. Current traces were taken with permission from Herd et al. (Herd et al., 2013). GABA A receptors of the thalamus are comprised of the α1, α3, α4, β1, β2, β3, γ2, and δ GABA A receptor subunits, as identified through immunohistochemistry (Pirker et al., 2000). Through the use of pharmacological manipulation and genetic mutations two distinct populations of thalamocortical GABA A receptors have been identified and associated with phasic and tonic
70 53 inhibition, respectively. Specifically, α4β2δ subunit-containing GABA A receptors are consistently linked to tonic GABA A receptor-mediated conductance in thalamocortical neurons, while α1β2γ2 subunit-containing GABA A receptors are consistently linked to phasic inhibition in thalamocortical neurons (Sohal et al., 2003; Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005; Herd et al., 2014; Rovo et al., 2014). Knockout of the α4 or δ subunit results in a significant decrease of tonic GABA A receptor conductance in thalamocortical neurons of the ventrobasal complex (Cope et al., 2005; Herd et al., 2013). Moreover, administration of 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), a selective agonist of δ-subunit containing GABA A receptors, significantly increases tonic inhibition in thalamocortical neurons in vitro and in vivo (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005; Cope et al., 2009; Wimmer et al., 2015). Expression of these GABA A receptor subunits (in addition to the α5 and α6 subunits that are heavily expressed in other regions of the brain such as the hippocampus, neocortex, and cerebellum) is associated with a number of biophysical properties that promote a persistent, long-lived (i.e., tonic) form of inhibition (Farrant and Nusser, 2005). These extrasynaptic receptors are the most sensitive to GABA, such that they can be activated by concentrations of GABA that are approximately two to three orders of magnitude less than the concentrations needed to activate synaptic GABA A receptors (i.e., a few micromolar compared to the millimolar range) (Farrant and Nusser, 2005; Belelli et al., 2009). As such, these receptors can be activated in a paracrine fashion through ambient GABA that does not necessarily originate from the synapse. Such non-vesicular GABA release can be mediated through reverse transport of GABA by GABA carriers or release from glial cells (Attwell et al.; Lee et al., 2010). RTN neurons do not exhibit a tonic GABA A receptormediated conductance and are not believed to express extrasynaptic GABA A receptors (Pirker et
71 54 al., 2000; Belelli et al., 2005). Indeed, immunohistochemistry experiments indicate that the δ and the α4 GABA A receptor subunits do not appear to be expressed in the RTN (Pirker et al., 2000). Extrasynaptic GABA A receptors in thalamocortical neurons also exhibit significant sensitivity to ethanol and have further been associated with mediating absence seizures (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Glykys et al., 2007; Jia et al., 2008a; Cope et al., 2009). Concentrations of ethanol that are associated with social drinking and typically elicit light to moderate intoxication (i.e., mm) enhance tonic inhibition in thalamocortical neurons from the ventrobasal complex (Jia et al., 2008a). This effect of ethanol was not apparent in mice that lacked extrasynaptic α4-subunit containing GABA A receptors (Jia et al., 2008a). Similarly, tonic inhibition is increased in thalamocortical neurons just preceding and during epileptic sharp wave discharges that are characteristic of absence seizures (Cope et al., 2009). These seizures last several seconds and present behaviourally as episodes of inattentiveness that are often accompanied by staring in patients with absence epilepsy. Suppression of extrasynaptic δ-subunit containing GABA A receptor activity in the thalamus of rats that are prone to absence epilepsy decreased the incidence of seizures and was further associated with a reduction in tonic inhibition. Specifically, intra-thalamic microinfusion of the antisense oligodeoxynucleotide to the δ GABA A receptor subunit in genetic absence epilepsy rats from Strasbourg (GAERS) reduced the incidence of spontaneous seizures in these animals (Cope et al., 2009). The association between enhanced extrasynaptic GABA A receptor activity in the thalamus with intoxication and pathological lapses in perception indicate a potential role for thalamic extrasynaptic GABA A receptor-mediated activity in arousal and conscious perception. Phasic/synaptic GABA A receptor-conductance in thalamocortical neurons is consistently linked with α1β2γ2 subunit-containing GABA A receptors (Fig. 11A). These receptors
72 55 desensitize far more rapidly than extrasynaptic GABA A receptors (approximately 5 ms vs. seconds to minutes) and also possess comparatively greater current amplitudes (approximately 100 pa vs. 40 pa) (Farrant and Nusser, 2005) (Fig. 11A and B). Viral mediated knockout of thalamic γ2 GABA A receptor subunits, which are necessary for the synaptic localization of GABA A receptors, does not alter the spectral composition of the EEG during non-rem sleep in mice (Essrich et al., 1998; Rovo et al., 2014). Moreover, these mice exhibit normal sleep spindlelike oscillations as indexed by the number of spindle cycles, and incidence and duration of spindle-like oscillations (Rovo et al., 2014). These findings indicate that alterations in synaptic GABA A receptor-mediated inhibition in the thalamus are not necessary to mediate the changes in thalamocortical activity that precipitate sleep spindles and delta waves during non-rem sleep. Importantly, knockout of the α1 or γ2 GABA A receptor subunit in thalamocortical neurons does not eliminate the ability to evoke RTN-triggered inhibitory postsynaptic currents (eipscs) in thalamocortical neurons (Herd et al., 2013; Rovo et al., 2014). These findings indicate that transient IPSCs can be triggered in the absence of synaptic GABA A receptors in thalamocortical neurons and provide compelling evidence for extrasynaptic GABA A receptormediated spillover inhibition in the thalamus. The eipscs elicited in the absence of synaptic GABA A receptors were of a longer duration (i.e., had a greater decay constant), smaller amplitude, and exhibited less charge transfer than eipscs measured in wild-type thalamocortical neurons, as would be expected if they were mediated solely through extrasynaptic GABA A receptors (Herd et al., 2013; Rovo et al., 2014). Pharmacologically enhancing the sensitivity of extrasynaptic δ-subunit containing GABA A receptors to GABA has been shown to alter eipscs in thalamocortical neurons, again indicating that extrasynaptic GABA A receptors in the thalamus can be activated in a phasic
73 56 manner (Herd et al., 2013; Rovo et al., 2014). Specifically, administration of 4-Chloro-N-[2-(2- thienyl)imidazol[1,2-a]pyridin-3-yl]benzamide (DS2), which is a positive allosteric modulator of extrasynaptic δ-subunit containing GABA A receptors (i.e., it promotes GABA-mediated activation of δ-subunit containing GABA A receptors), elicits prolonged eipscs with greater charge transfer (Jensen et al., 2013). Genetic knockout of δ-subunit containing GABA A receptors eliminates this effect of DS2 on thalamocortical neurons, indicating that this effect of DS2 on eipscs is indeed mediated through these extrasynaptic GABA A receptors (Herd et al., 2013). Among the general anesthetics that potentiate/activate GABA A receptors, the effects of the intravenous agents propofol and etomidate, along with the inhalational agent isoflurane, have been extensively studied in thalamocortical neurons (Belelli et al., 1997; Belelli et al., 2005; Ying and Goldstein, 2005b, a; Jia et al., 2008b; Herd et al., 2014). Both etomidate and propofol exhibit selectivity for the second transmembrane domain (TM2) of the β2 and β3 GABA A receptor subunits (Bali and Akabas, 2004; Li et al., 2006). Treatment of thalamocortical neurons from the ventrobasal complex with etomidate results in prolonged miniature IPSCs, with no effect on the rise time or amplitude of these IPSCs (Belelli et al., 1997; Herd et al., 2014). This effect of etomidate is absent in mice that possess a point mutation in the β2 GABA A receptor subunit (asparagine to serine at the 265 th residue; located in TM2) (Belelli et al., 1997). Thalamocortical neurons of these mice exhibit no overt alterations in phasic inhibition (Belelli et al., 1997). However, these mice do exhibit markedly lower sensitivity to etomidate. Specifically, the hypnotic and sedative potency of etomidate is greatly reduced in the β2 N265S mice, indicating that β2-subunit expressing GABA A receptors play a critical role in mediating etomidate-induced loss of consciousness (Belelli et al., 1997). The β2 subunit is expressed in both synaptic and extrasynaptic GABA A receptors in thalamocortical neurons; however the effect of β2 mutations
74 57 on anesthetic-induced alterations in thalamocortical IPSC duration suggests a role for thalamic β2-subunit containing extrasynaptic GABA A receptor-mediated spillover inhibition in mediating anesthetic-induced hypnosis with etomidate. Similar to etomidate, propofol also significantly increases the decay time and charge transfer of GABA A receptor-mediated IPSCs in thalamocortical neurons, again indicating a possible phasic activation of extrasynaptic GABA A receptors (Ying and Goldstein, 2005a). However, in contrast to etomidate, propofol also increases the amplitude of IPSCs in these neurons (Ying and Goldstein, 2005a). A similar point mutation as the one described above for the β2 GABA A receptor subunit, in the β3 GABA A receptor subunit also alters the potency of anesthesia with propofol and etomidate. Specifically, replacing the asparagine at the 265 th residue with methionine in the β3 subunit reduces the duration of loss-of-righting reflex elicited by etomidate and propofol (Jurd et al., 2003; Reynolds et al., 2003; Zecharia et al., 2009). The analgesic effects of these intravenous general anesthetics are also abolished in mice carrying the β3 N265M mutation (Zecharia et al., 2009). In vitro recordings identify a diminished effect of both etomidate and propofol on GABA A receptor-mediated currents in cortical neurons collected from these mice (Zecharia et al., 2009). To date, no such recordings in thalamocortical neurons from mice carrying mutations in the β GABA A receptor subunit with propofol have been published. Given the similarity between the effects of propofol and etomidate, it is expected that the effects of propofol on GABA A receptor-mediated IPSCs would be altered in the β mutant mice in a manner that is similar to the alterations identified with etomidate (Pirker et al., 2000). Both etomidate and propofol significantly enhance extrasynaptic GABA A receptormediated tonic inhibition in thalamocortical neurons and recombinant systems (Belelli et al., 1997; Bieda and MacIver, 2004; Meera et al., 2009). Moreover, the hypnotic potency of
75 58 etomidate and propofol was enhanced in female mice that were heterozygous for a point mutation in the γ2 GABA A receptor subunit and exhibited elevated levels of extrasynaptic α4- subunit containing GABA A receptors in the dentate gyrus and thalamus (Kretschmannova et al., 2013). These female mice did not appear to exhibit regular estrus cycling, which suggests that the typical alterations in extrasynaptic δ-subunit containing GABA A receptors that occur during the female estrus cycle did not occur in these mice (Maguire et al., 2005; Kretschmannova et al., 2013). The inhalational general anesthetic isoflurane also promotes extrasynaptic GABA A receptor-mediated inhibition in thalamocortical neurons from the ventrobasal complex (Jia et al., 2008b). Heterologous expression systems further identified that isoflurane directly activates α4β2δ subunit-containing GABA A receptors expressed in human embryonic kidney (HEK) 293 cells (Jia et al., 2008b). Isoflurane also directly activates α1β2γ2 GABA A receptors and potentiates postsynaptic GABA A receptor-mediated currents, indicating that it effects phasic GABA A receptor-mediated inhibition as well (Jia et al., 2008b). Most recently the effects of etomidate and DS2, an agent whose pharmacology positions it to be an effective promoter of GABA A receptor-mediated spillover inhibition, at thalamocortical neurons that received GABAergic input from the RTN were compared in vitro (Herd et al., 2014). Both etomidate and DS2 increased the duration of eipscs and enhanced charge conductance (Herd et al., 2014). Propofol and isoflurane are similarly associated with prolonged IPSCs, suggesting that this may be a common effect elicited by general anesthetic agents (Hales and Lambert, 1991; Jones and Harrison, 1993). Enhanced GABA A receptormediated spillover inhibition in the thalamus may thus mediate some of the effects of general anesthesia, particularly sedation and hypnosis. Importantly, while RTN neurons do not exhibit any tonic GABA A receptor mediated current, they do exhibit a GABA A receptor-mediated slow
76 59 current that is associated with expression of the β3 GABA A receptor subunit (Zhang et al., 1997; Belelli et al., 2005; Capogna and Pearce, 2011). As such, anesthetic-induced increases in spillover inhibition in thalamocortical neurons could effectively tune thalamocortical signaling to the frequency of RTN signaling, which could promote thalamocortical-rtn signaling in a spindle frequency-like range. Consistent with the putative role of the thalamus in mediating anesthetic-induced sedation and hypnosis, microinjection of GABA agonists into the nonspecific intralaminar thalamic nuclei promotes sleep and slow EEG activity in rats (Miller and Ferrendelli, 1990). Additionally, optogenetic stimulation of inhibitory inputs to the RTN from the lateral hypothalamus, which would indirectly decrease inhibitory input to the rest of the thalamus and promote spontaneous thalamic activity, elicited a switch from isoelectric electrocortical activity to sustained bursting (an electrocortical pattern that indicates a relatively lower depth of anesthesia) in the cortex of mice during isoflurane anesthesia (Brown et al., 2010; Herrera et al., 2015). Moreover, this optogenetic stimulation protocol effectively restored loss-of-righting reflex in some of the mice studied (Herrera et al., 2015). It is worth mentioning that other regions, such as the hippocampus and spinal cord, and in a number of instances other molecular targets, such as glycine receptors, are implicated in mediating the amnestic and immobilizing effects of commonly used general anesthetic agents (Rudolph and Antkowiak, 2004; Bonin and Orser, 2008; Franks, 2008). Typically the dose of anesthesia required to impair memory is much lower than the dose needed to elicit immobility, indicating that these two effects of anesthesia may be mediated through actions on distinct molecular and/or neural substrates. Extrasynaptic α5 and α4-subunit containing GABA A receptors in the hippocampus are consistently implicated in mediating the amnestic effects of
77 60 inhalational and intravenous general anesthetic agents (Bonin and Orser, 2008; Zurek et al., 2014). In contrast, the action of general anesthetics on GABA A receptors, glycine receptors, and other targets in spinal neurons is implicated in eliciting anesthetic-induced immobility (Rudolph and Antkowiak, 2004) Other molecular targets of general anesthetics in the thalamus In addition to GABA A receptors, there are a number of other channels in the thalamus that exhibit sensitivity to commonly used general anesthetic agents. Among these other molecular targets of general anesthetics are the two-pore domain K + channels, voltage-gated Ca 2+ channels, voltage-gated K + channels, and HCN channels. Alterations in the activity of these channels are ultimately associated with reduced spontaneous thalamocortical activity. The volatile general anesthetic halothane elicits significant effects on two-pore domain K + channels (Patel et al., 1999). Exposure of thalamocortical neurons to halothane results in hyperpolarization of the membrane potential, which can trigger a switch from tonic to low threshold spike (i.e., burst) activity (Meuth et al., 2003) (Fig. 7). The effect of halothane on thalamocortical neurons could not be entirely attributed to its action on two-pore domain K + channels, as the reversal potential of the halothane sensitive current was positive to that associated with the K + reversal potential (Meuth et al., 2003). This finding indicates that there are other molecular targets on thalamocortical neurons that are also affected by halothane, such as Na + or Ca 2+ channels, and contribute to the changes in thalamocortical firing. Shaker-related K + channels have also been implicated in mediating some of the effects of volatile anesthetics on thalamocortical neurons (Alkire et al., 2009; Lioudyno et al., 2013). In
78 61 heterologous expression systems low concentrations of sevoflurane, isoflurane, and desflurane potentiate K v 1 channel activity (Lioudyno et al., 2013). Thalamocortical neurons from the murine central medial thalamic nucleus exhibit a reduction in the firing frequency of action potentials when they are treated with sevoflurane in vitro and inhibition of K v 1.3 channels eliminated this effect of sevoflurane (Lioudyno et al., 2013). Moreover, microinjection of an antibody to the K v 1.2 channel into the central medial thalamus effectively restores loss-ofrighting reflex in rodents anesthetized with desflurane (Alkire et al., 2009). These findings suggest that K v 1 channels may contribute to the alterations in thalamocortical activity that underlie loss-of-consciousness with some volatile general anesthetic agents. R-type Ca 2+ channels, which mediate high threshold spiking, are implicated in mediating some of the effects of isoflurane-induced general anesthesia. Specifically, mice that lack expression of the R-type Ca 2+ channel isoform Ca v 2.3, which is expressed by RTN neurons, exhibit alterations in burst suppression activity with isoflurane as measured through EEG recordings (Joksovic et al., 2009). Moreover, treatment of RTN neurons with Ni 2+ or SNX-482 (a peptidyl toxin) to selectively block R-type Ca 2+ channel activity attenuated the reduction of eipscs with isoflurane in RTN neurons (Joksovic et al., 2009). This group also identified an inhibitory effect of general anesthetics on T-type Ca 2+ channels, which are critical mediators of the firing mode of thalamocortical neurons (discussed in more detail in section Sleep spindles and Delta (1-4 Hz) waves) (Suzuki and Rogawski, 1989; Joksovic et al., 2005). Specifically, Ca v 3.1 channels were blocked by propofol, etomidate, isoflurane, and ketamine in stably transfected HEK 293 cells (Todorovic et al., 2000; Joksovic et al., 2005). It should be noted that the concentrations of propofol and etomidate that elicited blockade of Ca v 3.1 channel activity were well above those that are considered clinically relevant (IC 50 propofol approximately 20.5 µm compared to clinically relevant concentrations of approximately 1-3 µm;
79 62 IC 50 etomidate approximately 161 µm compared to clinically relevant concentration of approximately 1-3 µm) (Todorovic et al., 2000). Consistent with these findings, mice that lack Ca v 3.1 channels exhibit unaltered sensitivity to propofol (Petrenko et al., 2007). These mice did, however, exhibit a greater latency to loss-of-righting reflex induced with the volatile general anesthetic agents isoflurane, halothane, and sevoflurane, indicating that Ca v 3.1 T-type Ca 2+ channel de-inactivation may play a significant role in mediating anesthetic-induced loss-ofconsciousness with volatile general anesthetic agents (Petrenko et al., 2007). It is important to note that Ca v 3.1 channels are also highly expressed in brain regions other than the thalamus, such as the cortex, hippocampus, striatum, and corpus callosum and as such it is possible that Ca v 3.1 channels in non-thalamic brain regions mediate loss-of-righting reflex with inhalational anesthetics (Yunker et al., 2003; Petrenko et al., 2007). The hyperpolarization activated cation current (I h ) in thalamocortical neurons from the murine ventrobasal complex exhibits significant alterations with propofol (Ying et al., 2006). Whole cell current clamp recordings in brain slices identified a reduction in I h conductance and slowed activation with propofol (Ying et al., 2006). Moreover, this suppression of I h with propofol was associated with a reduction in the regularity and frequency of delta oscillations in thalamocortical neurons in vitro (Ying et al., 2006). This effect of propofol persisted, with the alterations in I h activity being identified after removal of anesthesia. This long-lived effect of propofol was associated with a reduction in HCN2 and HCN4 expression, as identified through immunolabeling in brain slices collected from mice that had received an intraperitoneal injection of propofol or vehicle (mice were sacrificed 3 and 24 hours later) (Ying et al., 2006). Given that this effect of propofol extends beyond the period of unconsciousness, it is possible that the general anesthetic-induced alterations in I h conductance in the thalamus contribute to
80 63 postoperative cognitive dysfunction, an undesirable side effect that is experienced by approximately a quarter of elderly patients and is associated with alterations in GABA A receptormediated inhibition in the hippocampus (Moller et al.; Zurek et al., 2014). Microinjection of nicotine into the central medial thalamus can effectively elicit a return to consciousness in rodents anesthetized with the volatile general anesthetic sevoflurane, implicating nicotinic acetylcholine receptors as another putative molecular target for general anesthetic agents in the thalamus (Alkire et al., 2007). Both propofol and isoflurane have been shown to inhibit activity of these receptors in heterologous expression systems (where nicotinic acetylcholine receptor subunits were prepared from chicken complementary deoxyribonucleic acid and expressed in Xenopus oocytes) at sub-anesthetic doses (Flood et al., 1997). Blockade of nicotinic acetylcholine receptors in the central medial thalamus with microinjection of mecamylamine did not effect the induction of anesthetic hypnosis with sevoflurane in rats, although it did prevent nicotine-induced arousal (Alkire et al., 2007). Thus, general anestheticinduced alterations in thalamic nicotinic acetylcholine receptor activity may play a role in emergence from anesthetic hypnosis, but does not appear to be critically involved in the induction or maintenance of anesthetic-induced loss-of-consciousness. 1.4 Synthesis This introduction has provided evidence identifying a temporal link between alterations in thalamic activity and anesthetic-induced sedation and loss-of-consciousness. Furthermore, the functional significance of these alterations has been demonstrated directly through a number of in vivo manipulations in animal models, and is also implicated indirectly through imaging studies in humans (Fiset et al., 1999; Alkire et al., 2000; Bonhomme et al., 2001; Kaisti et al., 2003;
81 64 White and Alkire, 2003; Alkire et al., 2007; Alkire et al., 2009; Gili et al., 2013; Lioudyno et al., 2013; Ni Mhuircheartaigh et al., 2013; Baker et al., 2014; Herrera et al., 2015; Reinhold et al., 2015). This link between thalamic activity and consciousness is readily reconcilable with the endogenous role of the thalamus in mediating attention and electrocortical rhythms of non-rem sleep that are associated with reduced sensory perception (Rafal and Posner, 1987; Steriade et al., 1987; Van der Werf et al., 2002; Lee et al., 2004a; Cueni et al., 2008; McAlonan et al., 2008; Fogel and Smith, 2011; Halassa et al., 2011; Wimmer et al., 2012; David et al., 2013; Halassa et al., 2014; Herrera et al., 2015; Lewis et al., 2015; Wimmer et al., 2015). Importantly, however, the molecular and cellular underpinnings that mediate the changes in thalamocortical activity that precipitate alterations in cortical activity that occur with reduced arousal and perception are unclear. Identification of these thalamic mechanisms is a focus of this thesis. Studies conducted in vitro identify pronounced alterations in GABAergic activity in thalamocortical neurons with a number of commonly used general anesthetic agents (Belelli et al., 1997; Belelli et al., 2005; Cope et al., 2005; Ying and Goldstein, 2005b, a; Jia et al., 2008b; Belelli et al., 2009; Herd et al., 2014). These in vitro studies identify alterations in tonic, phasic, and spillover GABAergic inhibition. How these alterations in thalamic GABAergic activity relate to the changes in cortical activity that signal anesthetic-induced loss-of-consciousness has not been investigated directly. Moreover, it remains to be established whether the alterations in thalamic phasic, tonic, and spillover GABAergic inhibition contribute equally or differentially to the changes in electrocortical activity that are identified during anesthetic-induced sedation and hypnosis. It is important to establish if and how cortical activity is altered when thalamocortical neurons are exposed to a commonly used general anesthetic agent in vivo. Such a study would
82 65 identify whether the action of general anesthetics at the thalamus is sufficient to elicit the changes in electrocortical activity that signal general anesthesia, as posited by modeling studies (Ching et al., 2010; Vijayan et al., 2013a, b). It is also critical to characterize the individual impact of alterations in thalamic tonic, phasic, and spillover GABAergic inhibition on cortical activity. Comparison of these findings to those identified with a general anesthetic at the thalamus would elucidate which form(s) of GABAergic inhibition play a functional role in orchestrating the changes in cortical activity elicited during the induction and maintenance of anesthetic-induced loss-of-consciousness. Understanding the role of thalamic T-type Ca 2+ channel activity in mediating the general anesthetic induced changes in thalamocortical neuron activity would also provide meaningful new knowledge. De-inactivation of T-type Ca 2+ channels plays a critical role in mediating the firing mode of thalamic neurons and significantly influences endogenous 1-4 Hz electrocortical activity (Suzuki and Rogawski, 1989; Lee et al., 2004a). These channels are de-inactivated at hyperpolarized resting membrane potentials, which can be triggered through enhanced GABA A receptor mediated tonic inhibition (Cope et al., 2005). Numerous commonly used GABA A receptor targeting general anesthetic agents enhance tonic inhibition in thalamocortical neurons in vitro (Belelli et al., 2005; Jia et al., 2008b). Thus, anesthetic-induced de-inactivation of thalamic T-type Ca 2+ channels may contribute significantly to the alterations in cortical activity that are associated with anesthetic hypnosis. Conversely, thalamic T-type Ca 2+ channels may play no role in mediating anesthetic-induced changes in cortical activity. Indeed, there is evidence that strongly indicates that expression and/or activity of T-type Ca 2+ channels is not necessary to mediate the effects of a number of commonly used general anesthetic agents (Todorovic et al., 2000; Joksovic et al., 2005; Petrenko et al., 2007).
83 66 Elucidating the impact and nature of thalamocortical alterations elicited by commonly used general anesthetic agents will generate new knowledge of the functional mechanisms mediating anesthetic-induced sedation and loss-of-consciousness. Such knowledge can be used to inform the development of improved general anesthetic agents that lack undesirable side effects, such as post-operative cognitive dysfunction. Moreover, the findings from such studies will generate new knowledge regarding the neural correlates of consciousness, which has implications that extend beyond general anesthesia, and could inform the development of effective therapies for minimally conscious patients of which currently none exist.
84 67 Chapter 2 Aims & Hypotheses The studies that comprise this thesis where designed to assess the role of thalamic GABAergic activity in mediating the changes in electrocortical activity that signal general anesthetic-induced loss-of-consciousness. Rationale and Hypothesis: Given previous in vitro work that shows enhanced tonic inhibition in thalamocortical neurons can trigger a switch in thalamocortical activity to a T-type Ca 2+ channel-dependent mode of activity that promotes slow, 1-4 Hz electrocortical signaling (Steriade et al., 1993b; Cope et al., 2005), we hypothesize that enhanced thalamic GABA A receptor-mediated tonic inhibition will increase 1-4 Hz electrocortical activity in vivo and this effect will require normal thalamic T-type Ca 2+ channel activity. Aim 1: Show that enhanced thalamic tonic inhibition elicits a significant increase in 1-4 Hz power in the EEG in vivo (addressed in the studies presented in Chapter 3). Aim 2: Show that this effect of enhanced thalamic tonic inhibition on electrocortical activity is significantly altered when thalamic T-type Ca 2+ channels are blocked (addressed in the studies presented in Chapter 4). Rationale and Hypothesis: Given modeling studies that identify the sleep-spindle generating circuitry in the thalamus as a key site mediating the alpha-beta frequency electrocortical signature elicited by GABA A receptor-targeting general anesthetic agents at and during loss-ofconsciousness (Ching et al., 2010; Vijayan et al., 2013a; Ching and Brown, 2014), we
85 68 hypothesize that delivery of a prototypic intravenous GABA A receptor-targeting general anesthetic agent into the thalamus is sufficient to elicit this electrocortical signature of anesthetic-induced hypnosis and that this effect of the general anesthetic at the thalamus on electrocortical activity does not require T-type Ca 2+ channel-dependent changes in thalamocortical activity (since such changes are associated with low-frequency electrocortical activity (Steriade et al., 1993b)). Aim 3: Show that microperfusion of the GABA A receptor-targeting general anesthetic etomidate into the thalamus elicits an increase in alpha-beta frequency power in the EEG (addressed in the studies presented in Chapters 3 and 4). Aim 4: Show that this electrocortical effect of etomidate at the thalamus is not altered by blockade of thalamic T-type Ca 2+ channels (addressed in the studies presented in Chapter 4). Rationale and Hypothesis: Given that the prototypic GABA A receptor-targeting general anesthetic agent etomidate acts on both synaptic and extrasynaptic GABA A receptors to promote GABAergic spillover inhibition in thalamocortical neurons in vitro (Herd et al., 2014) and the implicated role of the thalamus in mediating the alpha-beta frequency electrocortical pattern that signals anesthetic-induced loss-of-consciousness (Ching et al., 2010; Vijayan et al., 2013a; Baker et al., 2014) we hypothesize that enhanced thalamic GABAergic spillover inhibition will promote alpha-beta frequency electrocortical activity and fully recapitulate all of the other electrocortical effects associated with etomidate at the thalamus (examined in Aims 3 and 4). We also anticipate that this effect of thalamic spillover inhibition on electrocortical activity is not
86 69 mediated through T-type Ca 2+ channel dependent changes in thalamocortical activity (like what we anticipate with etomidate). Aim 5: Show that enhanced thalamic spillover inhibition elicits an increase in alpha-beta frequency power in the EEG and fully recapitulates all of the other changes in electrocortical activity that were identified with a GABA A receptor-targeting general anesthetic at the thalamus (addressed in the studies presented in Chapters 3 and 4). Aim 6: Show that the electrocortical changes associated with enhanced thalamic spillover inhibition are not altered by blockade of thalamic T-type Ca 2+ channels (addressed in the studies presented in Chapter 4).
87 70 Chapter 3 Extrasynaptic thalamic δ-subunit containing GABA A receptors promote electrocortical signatures of deep non-rem sleep but do not mediate the effects of etomidate at the thalamus in vivo This chapter is modified from the following: Mesbah-Oskui L, Orser BA, Horner, RL (2014) Thalamic δ-subunit containing GABA A receptors promote electrocortical signatures of deep non-rem sleep but do not mediate the effects of etomidate at the thalamus in vivo. J Neurosci 37: PMID: Introduction γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain, and augmentation of GABAergic neuronal activity promotes natural sleep (Saper et al., 2001; Franks, 2008). Many commonly used anesthetic and sedating agents enhance neuronal inhibition via interactions with binding sites on GABA A receptors, including a subset containing the δ-subunit (δgaba A receptors) that are localized extrasynaptically (Belelli et al., 2005; Cope et al., 2005; Farrant and Nusser, 2005; Drasbek and Jensen, 2006). The thalamus is a key structure controlling the state of brain arousal and δgaba A receptors are highly expressed in the thalamus, particularly in the ventrobasal complex (Lopes da Silva, 1991; McCormick and Bal, 1997; Pirker et al., 2000). Activation of δgaba A receptors in the ventrobasal complex elicits a tonic hyperpolarization in vitro, which promotes a change in cell firing pattern from a tonic to a bursting mode (Cope et al., 2005). This bursting mode of
88 71 thalamocortical cell firing observed in vitro is consistent with the patterns observed in thalamocortical neurons during both non-rem sleep as well as anesthetic-induced loss-ofconsciousness with certain general anesthetics (Hirsch et al., 1983; Steriade et al., 1991; Steriade, 1993). However, the role of thalamic δgaba A receptors in mediating the electrocortical effects of sedative and anesthetic agents that target GABA A receptors has not yet been investigated in vivo. Generating this knowledge is important because activation of thalamic δgaba A receptors is suspected of mediating the sedating and hypnotic effects of intravenous anesthetics, such as the widely used general anesthetic etomidate (Belelli et al., 2005; Meera et al., 2009; Kretschmannova et al., 2013; Herd et al., 2014). Using targeted manipulation of the ventrobasal complex of freely behaving wild-type mice, and transgenic mice lacking δgaba A receptors (Gabrd -/- ), we tested two hypotheses in vivo: (1) Activating thalamic δgaba A receptors by local perfusion of the δgaba A receptorpreferring agonist THIP (Storustovu and Ebert, 2003; Winsky-Sommerer et al., 2007; Meera et al., 2011) will elicit electrocortical signatures that are consistent with widespread thalamocortical burst firing such as increased delta oscillations (1-4 Hz) and reciprocal changes in spindle-like (7-14 Hz) oscillations; and (2) These electrocortical signatures will be recapitulated by etomidate if the electrocortical effects of etomidate at the thalamus are also modulated through δgaba A receptors. Here we show that local activation of thalamic δgaba A receptors with THIP elicits changes in electrocortical activity in vivo that are consistent with the first hypothesis. Importantly, however, these receptors were not necessary in mediating the electrocortical effects of etomidate at the thalamus, and further suggest that the effects of etomidate are more likely mediated through non-δ likely synaptic GABA A receptors (Uchida et al., 1995; Hill-Venning
89 72 et al., 1997). Moreover, etomidate at the thalamus elicited changes in global electrocortical activity that are characteristic of anesthetic induction with the most commonly used intravenous general anesthetics etomidate and propofol (Kuizenga et al., 2001). Collectively, these findings suggest different effects of enhanced tonic versus phasic inhibition in the thalamus on electrocortical activity, which further implicate potential mechanisms that distinguish natural sleep from anesthesia. 3.2 Materials & Methods Animal Care Experiments were performed on 3-6 month old male GABA A receptor δ-subunit knockout (Gabrd -/- ) mice and their wild-type controls (C57BL/6 SvJ129). We investigated only male mice because δ-subunit containing GABA A receptor expression and function are significantly modulated by the estrous cycle (Maguire et al., 2005). Gabrd -/- mice were generated as previously described (Mihalek et al., 1999). Briefly, δ-subunit specific primers for exon 2 (5ʹ - CAGGGCAATGAATGACATTG-3ʹ ) and 3 (5ʹ -CAAGCGCCACATTCACAG-3ʹ ) were used to isolate a 9.5-kb PstI restriction fragment from a strain 129/SvJ P1 phage library (Genome Systems, St. Louis). The replacement-type DNA targeting vector was made by inserting the MC1Neo gene into a HindIII site on exon 4. This vector was linearized and electroporated into R1 embryonic stem cells. Correctly targeted embryonic stem cells were then microinjected into C57BL/6J blastocysts. Highly chimaeric males were then mated with C57BL/6J females
90 73 (Jackson Laboratory). Heterozygous (Gabrd +/- ) Agouti offspring were interbred to produce wildtype (Gabrd +/+ ) and homozygous null (Gabrd -/- ) mice. Mice were housed with their littermates in filtered cages with controlled lighting (lights on: hr). Mice also had free access to sterile food and water. A total of 54 mice were studied. All procedures were performed in compliance with the requirements of the Canadian Council on Animal Care, and were approved by the University of Toronto Animal Care Committee Surgery All surgical procedures were performed under aseptic conditions with mice under general anesthesia. Anesthesia was induced by inhaled isoflurane (3 %) with the animal in an induction chamber. Following induction of anesthesia, isoflurane was administered to mice through a gas anesthesia mask placed over the snout (1.5 2 %). Addition of oxygen to the inspired air (50 % oxygen to 50 % air) was used to ensure healthy oxygenation of the mouse throughout surgery. The mice were also given buprenorphine ( mg/kg subcutaneous) as an analgesic and atropine (1 mg/kg, intraperitoneal) to control airway secretions. Following the onset of surgical anesthesia, as judged by abolition of hind limb withdrawal and corneal blink reflex, the head and neck regions of the mouse were shaved, prepped with betadine and draped. The mice were then placed on a heating pad and body temperature was maintained between C. Lubricant eye ointment was placed on the corneas to prevent drying. All surgical instruments were autoclaved prior to use, while some other instruments that were unsuitable for autoclaving were soaked in the chemosterilant Accel.
91 74 For recording muscle activity (i.e., electromyogram (EMG) recording), two insulated stainless steel wires were implanted subcutaneously into the dorsal neck muscles. The neck muscles were first exposed by blunt dissection following small midline incisions over the dorsum of the neck. The mouse was then placed in a stereotaxic apparatus and a midline incision was made from the frontal bone to the nuchal crest and joined to the neck incision. The neck incision was closed using 4-0 absorbable sutures. Mice were also implanted with stainless steel EEG electrodes for recording of electrocortical activity and determination of sleep-wake states. Electrodes were positioned in the left frontal cortex, right anterior parietal cortex, and left cerebellum (serving as a ground). All electrodes were connected to pins in a miniature connector (ED90267-ND, Digi-Key Co., Thief River Falls, MN). Two microdialysis guide cannulae (CXG- 4, Eicom, San Diego, CA) were also stereotaxically positioned 3.0 mm above the right and left ventrobasal complex of the thalamus (1.6 mm posterior to lambda, 2 mm lateral to midline, and 0.25 mm ventral to lambda), i.e. probes were positioned bilaterally. The connector and guide cannulae were secured to the skull with dental acrylic. At the end of surgery mice received a bolus dose of sterile lactated Ringer s solution via intraperitoneal injection for fluid loading, and were monitored until they had come out of anesthesia and appeared to be recovering as evidenced by normal locomotor activity. Animals were given at least 9 days to recover, and to return back to their pre-surgical weights, prior to habituation and experiments. Following surgery, mice were housed individually in cages and received soft food (mash and Napa nectar) for the first two post-operative days.
92 Habituation Recordings were performed in noise-attenuated and electrically shielded chambers (EPC-010, BRS/LVE Inc., Laurel, MD). Recording chambers housed a large, open-topped Plexiglas bowl (Rodent Bowl, MD, 1514, BAS Inc., West Lafayette, IN). Inside the bowl, mice had ad libitum access to food and water. A video camera, mounted to the chamber ceiling, permitted visual monitoring of mouse behaviour. Mice were habituated through daily 20-minute exposures for 4 consecutive days. During each exposure, mice were connected to a lightweight counterbalanced recording cable. On the fifth day, mice were left overnight and experimental recordings and microdialysis perfusion commenced the following morning. The chamber and Plexiglas bowls were cleaned with a 70 % ethanol solution between each recording session and fresh bedding and food was laid down each time Experimental protocol Mice were gently restrained on the morning of experiments as the dummy cannulae were removed from their guides and the microdialysis probes were inserted (CX-6-01, Eicom, San Diego, CA). Probes projected 3 mm from the tip of the guide, targeting the ventrobasal complex. The membrane tips of the probes were 1 mm long and 0.22 mm wide with a molecular weight cutoff of 50,000 Daltons. The probes were continuously flushed with artificial cerebrospinal fluid (acsf) at a flow rate of 2.1 µl/min. ACSF was bubbled with CO 2 to a ph of 7.38 ± 0.01 and warmed to 37 ºC. The composition (in mm) of acsf was: 125 NaCl, 3 KCl, 1 KH 2 PO 4, 2 CaCl 2, 1 MgSO 4, 25 NaHCO 3, and 30 D-glucose. All experiments were performed during the day, when the mice normally sleep. In all of the mice studied, the data collected during the first hour following probe insertion were excluded
93 76 from analysis. The entire experimental protocol occurred over a 5-hour period ( hr). Data obtained in the first half hour following switching of the microdialysis perfusate were also excluded from analyses. THIP, a δgaba A receptor-preferring agonist, and etomidate, a general anesthetic agent that promotes extrasynaptic GABA A receptor activity (Forman, 2011; Meera et al., 2011), were purchased from Tocris (Bristol, UK). Two concentrations of THIP (10 and 50 µm) and etomidate (10 and 30 µm) were prepared in acsf. Importantly, the amount of media that diffuses across the microdialysis probe membrane into tissue is reported to be % of the original perfusate concentration (Portas et al., 1996; Grace et al., 2014). Thus, the effective concentrations of THIP at the ventrobasal complex are expected to have been approximately and 5-9 µm, while the expected effective concentrations of etomidate at the thalamus were approximately and µm at the probe tip. The concentrations of THIP used in this study were selected based on clinically relevant doses in humans, with serum and plasma concentrations in the same range as our effective concentrations enhancing sleep quality and sedation (Madsen et al., 1983; Faulhaber et al., 1997). Additionally, the effects of THIP at synaptic GABA A receptors are minimal at these concentrations, whereas there is modulation of extrasynaptic GABA A receptors (Brown et al., 2002; Belelli et al., 2005; Jia et al., 2005; Drasbek and Jensen, 2006). The concentrations for etomidate were selected after consideration of the plasma concentrations that yield hypnosis in humans and loss-of-righting reflex in mice, with concentrations in this same range (1-5 µm) eliciting both of these effects (De Paepe et al., 1999; Benkwitz et al., 2007). In addition, previous in vitro studies report that at concentrations in this range, etomidate elicits increased extrasynaptic GABA A receptor-mediated tonic inhibition in thalamocortical neurons of the ventrobasal complex (Belelli et al., 2005; Forman, 2011; Herd et al., 2014).
94 77 Importantly, at the effective concentrations used in this study, the ability of etomidate to promote GABA A receptor activity depends on the presence of GABA, since etomidate likely acts as a positive allosteric modulator rather than an agonist of GABA A receptors at these concentrations (Forman, 2011). This requirement for GABA differs for THIP, which acts as a δgaba A receptor-preferring agonist even at low concentrations (Meera et al., 2011). To ensure that ambient GABA concentrations were sufficient for etomidate-mediated actions on both synaptic and extrasynaptic GABA A receptors, additional experiments were conducted where etomidate was microperfused into the ventrobasal complex together with blockers of GABA transporters (GAT) -1 and -3. Specifically, 10 and 30 µm solutions of etomidate were prepared in acsf containing 100 µm 1,2,5,6-Tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3- pyridinecarboxylic acid hydrochloride (NO-711) (Sigma-Aldrich, St. Louis, MO), a GAT-1 inhibitor, and 300 µm 1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(S)-3-piperidinecarboxylic acid (SNAP-5114) (Tocris, Bristol, UK), a GAT-3 inhibitor. GAT-1 and -3 serve as the primary mediators of synaptic GABA clearance in the thalamus (De Biasi et al., 1998; Beenhakker and Huguenard, 2010). The concentrations of NO-711 and SNAP-5114 used in this study were chosen because they increase extracellular GABA concentrations in vivo using the same methodology of reverse microdialysis as our study and also do not generate spike-wave discharges (Smith et al., 2007; Cope et al., 2009; Kersante et al., 2013). Additionally, in vitro inhibition of these transporters, using similar effective concentrations of NO-711 and SNAP- 5114, greatly enhances extrasynaptic GABA A receptor-mediated effects on IPSC duration (i.e. spill-over inhibition) in thalamocortical neurons (Herd et al., 2013). Baseline recordings were performed in each mouse while the ventrobasal complex was microperfused with acsf for 2 hours. The perfusion media was then switched to THIP (n = 9 wild-type mice and n = 9 Gabrd -/- mice), etomidate (n = 9 wild-type mice and n = 9 Gabrd -/-
95 78 mice), or etomidate with NO-711 and SNAP-5114 (n = 6 wild-type mice). In the first study, mice received 10 µm THIP for 1.5 hours, followed by 50 µm THIP for another 1.5 hours. In the second (separate) study, the ventrobasal complex was perfused with 10 µm etomidate for 1.5 hours and then 30 µm etomidate for the additional 1.5 hours. A third study was conducted that was identical to the second study, except for the inclusion of 100 µm NO-711 and 300 µm SNAP-5114 in the acsf that contained etomidate. Finally, a fourth time control study was conducted where the perfusion medium was maintained solely as acsf, (n = 6 wild-type mice and n = 6 Gabrd -/- mice; Fig. 12). These time controls ensured that none of the identified sleepstate effects of THIP and/or etomidate could be attributed to time-of-day influences. At the end of the in vivo recordings, the mice were deeply anesthetized with 5 % isoflurane and transcardially perfused with 0.9 % saline, followed by 10 % formalin solution while the microdialysis probes were left in the brain. This procedure allowed for stable fixation of the brains for the subsequent localization of probe sites by histology. Brains were then dissected and immersion-fixed in 10 % formalin for 2 days at room temperature. Following fixation, the brains were transferred to a 30 % sucrose solution for 2 days at 4 C. Brains were subsequently cut coronally into 50 µm slices using a cryostat (Leica, CM 1850, Wetzlar, Germany). Sections were then mounted on slides and dried overnight. Neutral red staining was performed the following day. Probe site location was verified through imaging of the stained sections using the Infinity Capture software package (Lumenera, Ottawa, ON).
96 79 Figure 12. Experimental protocol and localization of microdialysis probes in the thalamus. A, Schematic illustrating the four major experiments and treatment groups, and the duration for each agent microperfused into the ventrobasal complex. Nine wild-type and 9 Gabrd -/- mice were used for the study of THIP at the ventrobasal complex (top). Nine wild-type and 9 Gabrd -/- mice were used for the study of etomidate (middle top). Six wild-type mice were used for the study of GABA re-uptake inhibition with etomidate (middle bottom). Six wild-type and 6 Gabrd -/- mice were used for the time control study (bottom). B, Schematic indicating the targeting of the microdialysis probe locations to the ventrobasal complex (top) and exemplar histological images indicating probe locations (bottom). The arrows indicate the locations of the tip of the microdialysis membranes. C, Schematics illustrating the locations of the microdialysis membranes for all of the mice studied. Numbers indicate the distance posterior to bregma (Franklin and Paxinos, 2007). Each probe track is made translucent to allow for visualization of probe site distribution across all the mice studied. The ventrobasal complex is comprised of the ventral
97 80 posteromedial (VPM) and ventroposterolateral (VPL) thalamic nuclei. D, Exemplar EEG and EMG recordings collected during the experiments Signal acquisition and analysis of sleep-wake states Signals were acquired using Spike 2 software (1401 interface, CED Ltd., Cambridge, UK). The EEG and EMG signals were amplified 10,000 and 5,000 times, filtered between Hz and 100-1,000 Hz, and digitized at 2,000 Hz and 500 Hz, respectively (Super-Z headstage amplifiers and BMA-400 amplifiers/filters, CWE Inc., Ardmore, PA). Sleep-wake states were scored by analyzing EEG and EMG signals in consecutive 5- second epochs. For each epoch, the corresponding sleep-wake state was defined using an automatic scoring protocol (Costa-Miserachs et al., 2003). The accuracy of the scoring procedure was confirmed visually for each epoch. The amount of time spent in wakefulness, non-rem and REM sleep was calculated as a percentage of the total recording time for each treatment in each animal. A period of sustained (stable) wakefulness or sleep was defined as a series of epochs in one state lasting > 30 seconds. Transitions between states were calculated as a percentage of the total number of transitions, per treatment, in each animal. Electrocortical activity was further analyzed using a FFT algorithm (SUDSA22 script, Spike2 software, CED Inc.). Power spectra were generated for every 5-second epoch of the EEG that was scored for sleep-wake state, through the analysis of overlapping 1,024-sample segments that were windowed with a raised cosine (Hanning) and subjected to a FFT. Each 5-second epoch was analyzed for absolute power in five bandwidths: delta (1-4 Hz), theta (4-8 Hz), alpha (8-12 Hz), beta (12-30 Hz), and sigma (10-15 Hz). The absolute power in each bandwidth was
98 81 divided by the total power (1-30 Hz) in each epoch and the normalized powers were sorted according to their corresponding sleep-wake state. The mean power in each frequency band for each sleep-wake state was then calculated for each treatment in each subject Identification and characterization of spindle-like oscillations The incidence and features of spindle-like oscillations were identified using custom-written routines in MATLAB (MATLAB R12, Mathworks, Natick, MA). The EEG during non-rem sleep was analyzed with overlapping segments of 4,096 samples, windowed using a raised cosine (Hanning window) and subjected to a FFT to yield the power spectrum with a resolution of 0.25 Hz. To identify and characterize spindle-like oscillations the powers from Hz and 5-15 Hz were removed to optimize fitting of data from Hz and Hz according to a power law: Log 10 (F(x)) = mlog 10 (x) + b; F(x) = (10 b )(x m ) where x represents frequency, m represents slope, and b is the y-intercept. The expected power in the sleep spindle range (7-14 Hz) was then predicted from this power law. This value was then used as a threshold for the identification of spindle-like oscillations (for more details on the fit of this power law and the suitability of the threshold in detecting spindle-like oscillations see section Thalamic δ-subunit containing GABA A receptor modulation and spindle-like oscillations and Fig. 14). The EEG was then bandpass filtered from 7-14 Hz and down-sampled to 200 Hz (i.e., smoothed). The root-mean square of the down-sampled 7-14 Hz EEG was calculated with a window length of 200 samples (i.e., 1 s of data sampled at 200 Hz) and overlap
99 82 of 150 samples (i.e., 0.75 s). Whenever the resulting root-mean squared EEG surpassed the predicted 7-14 Hz threshold, and did so for a period of time consistent with spindle-like oscillation duration (0.5-3 s) (Astori et al., 2013), these events were identified as spindle-like oscillations. The incidence of these spindle-like oscillations was also calculated by dividing their total number by the total time spent in non-rem sleep for a given recording period Temporal analysis of state transitions We also investigated the temporal dynamics of state transitions by analyzing trajectories in cortical activity using two-dimensional state space plots (Gervasoni et al., 2004; Diniz Behn et al., 2010). The absolute power in three bandwidths (1-9, 7-9, and 1-19 Hz) was analyzed for every 5-second epoch of the EEG (FFT size 1024, Hanning window, i.e. as described above). The ratio of 7-9/1-9 Hz was then calculated for each epoch, providing a measure for the relative contribution to the signal of faster frequencies in the1-9 Hz range. An increase in the 7-9/1-9 Hz ratio indicates a relative shift to faster frequencies, as occurs during transitions from non-rem sleep to wakefulness or REM sleep. Values were then smoothed using a moving average function (smooth, MATLAB, window-length of five 5-s epochs). To permit inter-subject comparisons, the smoothed data was then normalized according to the transformation: x = (xmin) / (max-min), where x is the normalized value, x is the original value, min is the minimum value for the dataset, and max is the maximum value. The resulting normalized power ratio plotted against the 1-19 Hz frequency band generates distinct clusters of EEG frequency-derived data points that correspond to sleep-wake states (Gervasoni et al., 2004; Diniz Behn et al., 2010). Defining these data points to clusters was done mathematically and without a priori visual classification into sleep-wake states (function kmeans, MATLAB, 10 replicates of the function
100 83 before classifying data points to a cluster). The total number of clusters assigned per recording (2 to 3) was determined by assessing the fit of data points to their defined cluster, through comparisons of their distance to other cluster centers (function silhouette, MATLAB); 3 clusters were initially assigned for each recording, but if the data points of a cluster exhibited poor grouping (i.e., a mean silhouette value < 0.5, MATLAB) clustering was performed with 2 defined clusters. The temporal dynamics of stable state transitions were then investigated by calculating the spectral distance traveled between clusters Statistical analysis The effects of THIP and etomidate on sleep-wake structure, electrocortical activity, spindle-like oscillations, and state dynamics were assessed using a 2-way repeated measures (RM) analysis of variance (ANOVA), with the factors being: 1) drug treatment (i.e., acsf, followed by the separate interventions with THIP or etomidate) and 2) genotype (i.e., wild-type and Gabrd -/- mice). The influence of ambient GABA concentrations on any of the identified effects of etomidate were further evaluated within wild-type mice using a 2-way RM ANOVA with the factors being drug treatment and group (i.e., with or without GABA re-uptake inhibitors). Comparisons between test and time-control mice were made during corresponding times of the experiment, i.e. during the same times of recording when the test mice received acsf and the different concentrations of THIP or etomidate, again using a 2-way ANOVA with the factors being group (i.e., drug vs. time-control) and genotype. Bonferroni-corrected p values were used to test statistical significance when post hoc comparisons were performed. Differences were considered significant if p < Analyses were performed using SigmaStat software (SPSS, San Jose, CA). Data are expressed as mean ± standard error of the mean (SEM).
101 Results The experimental protocol performed in the three groups of animals is outlined in Figure 12, and a sample of the original EEG and EMG signals are also shown. Figure 12 also shows an example of histological sections from a single animal, which illustrates the location of the two microdialysis probes on either side of the midline in the ventrobasal complexes of the thalamus. The locations of the microdialysis probes in all of the mice used in the study are also illustrated on standard cross-sections. The probes were successfully implanted into the ventrobasal complex in all animals. THIP First we identified the effects of modulation of thalamic δgaba A receptors on electrocortical activity in freely behaving wild-type mice and Gabrd -/- mice. Here we show that microperfusion of the δgaba A receptor-preferring agonist THIP into the ventrobasal complex of the thalamus produced significant effects on electrocortical activity in wild-type mice but not in Gabrd -/- mice, i.e., the effects of THIP were dependent on δgaba A receptors. THIP: (i) increased 1-4Hz power in wakefulness and non-rem sleep, consistent with its sedating effects; (ii) reduced spindle-like oscillations in non-rem sleep; and (iii) increased the speed of stable transitions into non-rem sleep, indicating effects on state-space dynamics. These results are detailed below.
102 Thalamic δ-subunit containing GABA A receptor modulation and EEG spectral power Non-REM sleep: In agreement with the hypothesis, microperfusion of the δgaba A receptorpreferring agonist THIP into the ventrobasal complex, significantly increased power in the 1-4 Hz EEG frequency band in non-rem sleep in wild-type mice, but not in the Gabrd -/- mice (Fig. 13A-C). Analysis of the group data in Figure 13C indicated that there was a significant effect of THIP at the ventrobasal complex on 1-4 Hz EEG power in non-rem sleep, with this response being dependent on genotype (F 2, 31 = 11.85, p < 0.001, 2-way RM-ANOVA). Further analysis identified that in wild-type mice, 50 µm THIP increased 1-4 Hz EEG power in non-rem sleep compared to both acsf and 10 µm THIP (each p < 0.002, post hoc paired t-test). Importantly, however, and in agreement with the main hypothesis, no such effects of THIP on 1-4 Hz EEG activity were observed in the Gabrd -/- mice (each p > 0.06; Fig. 13C). As well as the increased 1-4 Hz EEG power elicited by THIP at the ventrobasal complex of the thalamus in the wild-type mice, there was decreased theta (4-8 Hz) and alpha (8-12 Hz) activity in non-rem sleep (each p < 0.01, post hoc paired t-test), i.e., there was a general shift to increased lower frequency EEG power produced by the δgaba A receptor-preferring agonist. Importantly, again, no such effects were observed in the Gabrd -/- mice (each p > 0.61). Nevertheless, there was an increase in beta (12-30 Hz) EEG power in non-rem sleep during microperfusion of 50 µm THIP into the ventrobasal complex in the Gabrd -/- mice compared to baseline recordings with acsf (p = 0.002), with this being the only significant effect of THIP observed in the Gabrd -/- mice. Figure 13C also shows that in non-rem sleep there was a baseline difference in 1-4 Hz EEG power between genotypes in the absence of THIP (i.e., during microperfusion of acsf into
103 86 the ventrobasal complex), with 1-4 Hz power being greater in the Gabrd -/- mice compared to the wild-type mice (p = 0.006, post hoc t-test). However, such a baseline difference was not observed in wakefulness or REM sleep (Fig. 13C). Wakefulness: As with the effects observed in non-rem sleep that supported the main hypothesis, 50 µm THIP at the ventrobasal complex, to increase tonic neuronal inhibition, also increased 1-4 Hz EEG power in wakefulness in the wild-type mice compared to both acsf and 10 µm THIP (each p < 0.04, post hoc paired t-test). Again, no such effects were observed in the Gabrd -/- mice (each p > 0.50). The increased 1-4 Hz EEG power elicited by THIP in the wildtype mice was also associated with a corresponding decrease in alpha and beta EEG power in waking (each p < 0.01), again reflecting a general shift to increased lower frequency electrocortical activity caused by thalamic δgaba A receptor modulation. Also in agreement with the main hypothesis, no such effects were observed in the Gabrd -/- mice (each p > 0.61). REM: There was no effect of THIP at the ventrobasal complex on any measure of EEG activity, in any frequency band, in REM sleep (all p > 0.05; Fig. 13B-D).
104 87 Figure 13. Promoting thalamic δgaba A receptor activity with THIP at the ventrobasal complex increases 1-4 Hz electrocortical activity during NREM sleep and waking. A, Example power spectrums and hypnograms from a wild-type and Gabrd -/- mouse during microperfusion of the ventrobasal complex with acsf and 50 µm THIP. Each plot illustrates the last 30 minutes of the respective treatment session. Note the increase in lower frequency power in the wild-type mouse with THIP and the absence of such an effect in the Gabrd -/- mouse. B, Continuous frequency plots illustrating the percent change in power with 50 µm THIP relative to acsf during NREM sleep, waking, and REM sleep. Each trace illustrates the
105 88 mean ± the standard error of the mean (SEM). C, Changes in 1-4 Hz power with 10 and 50 µm THIP. Note the increase in 1-4 Hz power with microperfusion of 50 µm THIP into the ventrobasal complex during NREM sleep and waking in wild-type mice, and the absence of these effects in Gabrd -/- mice. D, Additional changes in electrocortical activity with THIP. Plots illustrate the percent increase or decrease in theta (4-8 Hz), alpha (8-12 Hz), and beta (12-30 Hz) power with 50 µm THIP relative to acsf. 2-way RM-ANOVA, * p < 0.05, ** p < 0.01, *** p < See section Thalamic δ-subunit containing GABA A receptor modulation and EEG spectral power for further details. NREM: non-rem Thalamic δ-subunit containing GABA A receptor modulation and spindle-like oscillations We next examined the effects of thalamic δgaba A receptor activation on the incidence and duration of spindle-like oscillations. This is because sleep spindles are generated by the thalamus, and spindles are most prevalent in periods of non-rem sleep with reduced 1-4 Hz EEG power, i.e., lighter periods of non-rem sleep (Nunez et al., 1992; Steriade et al., 1993b). Sigma power: Hz EEG power during non-rem sleep is often taken to indicate spindlelike activity (Astori et al., 2013). Figure 14A shows that microperfusion of 50 µm THIP into the ventrobasal complex of the thalamus significantly decreased Hz EEG power in non-rem sleep in wild-type mice compared to both acsf and 10 µm THIP (each p < 0.028, post hoc paired t-test). Together, the effect of pharmacological manipulation of thalamic tonic neuronal inhibition in decreasing Hz power (Fig. 14A) and increasing 1-4 Hz power (Fig. 14C) in the wild-type mice is consistent with a reciprocal relationship between these frequency bands during non-rem sleep (Nunez et al., 1992; Steriade et al., 1993b).
106 89 Importantly, this effect of δgaba A receptor modulation at the ventrobasal complex on Hz activity was also dependent on genotype (F 2, 31 = 7.83, p = 0.002, 2-way RM- ANOVA); no significant effects of THIP were observed in the Gabrd -/- mice (each p > 0.06, post hoc paired t-test; Fig. 14A). Figure 14A also shows that in the absence of THIP (i.e., during baseline recordings with acsf) Hz EEG power was lower in the Gabrd -/- mice compared to the wild-type mice (p = 0.020, post hoc t-test). This difference is also consistent with the elevated baseline 1-4 Hz power seen during non-rem sleep in the Gabrd -/- mice (Fig. 13C). Incidence and duration of spindle-like oscillations (7-14 Hz): Figure 14B-C shows the automated detection of individual transient spindle-like oscillations from electrocortical activity, i.e., as opposed to sigma power that occurs throughout non-rem sleep (described in previous section). First, we fit a power law to the EEG data obtained during non-rem sleep in each mouse with each treatment. An example of this fit is shown in Figure 14B. This fit was then used to identify the baseline power in the 7-14 Hz frequency band during non-rem sleep, in order to set a threshold value for identifying individual transient spindle-like oscillations that add to the power in this frequency range (for more detail see section Identification and characterization of spindle-like oscillations for details). When 7-14 Hz power was transiently increased above this threshold for between 0.5 and 3 seconds then spindle-like oscillations were identified (Astori et al., 2013) (Fig. 14C). The group data in Figure 14D show that the effect of THIP on the incidence of spindlelike oscillations depended on genotype (F 2, 32 = 7.32, p = 0.002, 2-way RM-ANOVA). Further analysis identified that spindle-like oscillations were reduced with 50 µm THIP in the wild-type mice only (p = 0.022, post hoc paired t-test). This effect of promoting tonic neuronal inhibition at
107 90 the ventrobasal complex on reducing spindle-like oscillations in non-rem sleep in the wild-type mice is consistent with the reduced sigma power observed in Figure 14A. There was no effect of either THIP, or genotype, on the duration of spindle-like oscillations (Fig. 14E), suggesting that, once triggered, spindle-like oscillations were normally propagated and terminated irrespective of δgaba A receptor modulation Thalamic δ-subunit containing GABA A receptor modulation and state-space dynamics Next we identified if pharmacologically induced thalamic δgaba A receptor modulation altered the trajectory between behavioural states in these freely behaving mice. This was identified through analysis of the speed of transitions between EEG-defined spectral boundaries that defined the states of wakefulness and sleep, i.e., speed as derived from measures in the spectral domain (Fig. 15A and B). Figure 15A shows the clustering of data points associated with pre-defined frequency bands in time (for more detail see section Temporal analysis of state transitions). These points cluster in spectral space in regions that correspond to the states of wakefulness, non-rem and REM sleep, together termed state space (Gervasoni et al., 2004; Diniz Behn et al., 2010). Figure 15B shows a close-up of three transitions into stable (i.e., > 30 second duration) periods of non-rem sleep, from preceding periods of wakefulness, in one wild-type and one Gabrd -/- mouse. Example transitions are shown for microperfusion of acsf into the ventrobasal complex, and for modulation of thalamic δgaba A receptors with 10 and 50 µm THIP.
108 91 Group data show that promoting thalamic δgaba A receptor-mediated inhibition increased the speed of transitions into non-rem sleep as identified from EEG-defined spectral state space (Fig. 15C). Further analysis identified that the effect of THIP at the ventrobasal complex on the trajectory between behavioural states was specific for non-rem sleep and depended on genotype (F 2, 31 = 3.49, p = 0.043, 2-way RM-ANOVA). The speed of transitions into non-rem sleep in the spectral domain was increased with 50 µm THIP compared to acsf and 10 µm THIP in wild-type mice (each p < 0.014, post hoc paired t-test). This effect of increased tonic thalamic neuronal inhibition in facilitating EEG transitions into regions of the spectral domain consistent with deep non-rem sleep was only observed in the wild-type mice and not in the Gabrd -/- mice (each p > 0.43, post hoc paired t-test). Amounts of sleep-wake states: Although there were effects of THIP at the ventrobasal complex on EEG spectral power within sleep-wake states (Fig. 13 and 14) and state-space dynamics (Fig. 15), there were no effects of THIP on the amounts of wakefulness, non-rem and REM sleep (F 2, 30 < 2.38, p > 0.110, 2-way RM-ANOVA). Nor were there any effects of THIP on sleep-wake distribution between genotypes (F 2, 30 < 1.18, p > 0.322, 2-way RM-ANOVA). To further confirm these data, additional time-control experiments were performed with continued microperfusion of acsf into the ventrobasal complex of wild-type and Gabrd -/- mice. There was no difference in the amounts of wakefulness, non-rem and REM sleep in the time control experiments compared to the experiments with THIP (each F 1, 26 < 2.48, p > 0.127, 2-way ANOVA) and this absence of effect did not depend on genotype (each F 1, 26 < 1.73, p > 0.200, 2- way ANOVA).
109 92 Figure 14. Promoting thalamic δgaba A receptor activity with THIP at the ventrobasal complex decreases both sigma power and the incidence of spindle-like oscillations. A, 50 µm THIP at the ventrobasal complex decreases Hz sigma power in wild-type mice. Also note the baseline difference in sigma power between wild-type and Gabrd -/- mice. B, Example of the threshold defining protocol for the automatic detection of spindle-like oscillations. A power law is used to predict the power in the spindle range (7-14 Hz). See section Identification and characterization of spindle-like oscillations for further description and application. C, Example of spindle-like oscillation detection. When the filtered 7-14 Hz EEG surpasses the predefined spindle-threshold these events qualify as spindle-like oscillations if they meet a duration criteria of seconds. denotes events that failed to meet duration criteria, while * denote events that met this criterion and were identified as spindle-like
110 93 oscillations. D, The incidence of spindle-like oscillations is reduced in wild-type mice with 50 µm THIP at the ventrobasal complex. Note the lack of effect in Gabrd -/- mice. E, THIP had no effect on the average duration of spindle like oscillations. 2-way RM-ANOVA, * p < 0.05, ** p < Figure 15. Thalamic δgaba A receptor activity facilitates rapid transitions into the spectral domain characteristic of deep non-rem sleep. A, Example state space plots from a wild-type and Gabrd -/- mouse during microperfusion of the thalamus with acsf and 50 µm THIP. B, Exemplar state space plots depicting stable transitions from waking into non-rem sleep during microperfusion of the thalamus with acsf, 10 and 50 µm THIP. Note the increased spectral distance travelled between states with 50 µm THIP (black trace) in wild-type mice. C, Movement through spectral space into the region occupied by non-rem sleep is significantly facilitated by 50 µm THIP at the ventrobasal complex of wild-type mice but this effect is not apparent in Gabrd -/- mice. THIP at the ventrobasal complex had no effect on the speed of spectral transitions into wakefulness or REM sleep. 2-way RM-ANOVA, * p < 0.05, ** p < 0.01.
111 94 Etomidate Next we identified if the effects on electrocortical activity of microperfusion of etomidate into the ventrobasal complex were mediated by δgaba A receptors. Here we show that compared to THIP, microperfusion of etomidate into the ventrobasal complex produced different effects on electrocortical activity that were independent of δgaba A receptors. In summary, etomidate: (i) decreased 1-4 Hz power, increased 8-12 Hz and/or Hz power in all sleep-wake states; (ii) increased spindle-like oscillations; (iii) had no effect on the trajectory between behavioural states as analyzed by state space dynamics; (iv) increased REM sleep expression; and (v) that these effects largely persisted under conditions that favored potentiation of extrasynaptic GABA A receptors by etomidate with co-application of GAT-1 and -3 inhibitors to the thalamic perfusion media Microperfusion of etomidate into the ventrobasal complex effects EEG power Non-REM sleep: In contrast to the effects of THIP at the ventrobasal complex, which promoted electrocortical signatures of deep non-rem sleep an effect that depended on the presence of δgaba A receptors, as it occurred only in the wild-type mice (Fig. 13 to 15), microperfusion of etomidate into the same region of the thalamus significantly decreased power in the 1-4 Hz EEG frequency band in non-rem sleep in both the wild-type and the Gabrd -/- mice (Fig. 16A-C). Analysis of the group data in Figure 16C indicated that there was a significant effect of etomidate at the ventrobasal complex on 1-4 Hz EEG power in non-rem sleep (F 2, 32 = 35.79, p < 0.001, 2-way RM-ANOVA), with this response to treatment being independent of genotype
112 95 (F 2, 32 = 1.56, p = 0.225), i.e., the pattern of response was independent of δgaba A receptors. Unlike THIP, however, which increased 1-4 Hz EEG power in non-rem sleep, 10 and 30 µm etomidate decreased 1-4 Hz EEG power compared to acsf (each p < 0.001, post hoc paired t- test). There was an independent effect of genotype on 1-4 Hz EEG power (F 1, 16 = 4.55, p = 0.049, 2-way RM-ANOVA), with wild-type mice exhibiting significantly lower 1-4 Hz power at baseline, consistent with the baseline differences that were identified in the study with THIP (post hoc t-test, p = 0.028). As well as the decreased 1-4 Hz EEG power elicited by etomidate at the ventrobasal complex of the thalamus, there was increased alpha (8-12 Hz) and beta (12-30 Hz) EEG activity in non-rem sleep (both F 2, 32 > 16.60, p < 0.001, 2-way RM-ANOVAs; Fig. 16D). These responses were independent of genotype as they occurred in both the wild-type and Gabrd -/- mice (both F 2, 32 < 0.55, p > 0.546, 2-way RM-ANOVA; Fig. 16D). These data indicate that there was a general shift to decreased lower frequency EEG power and increased higher frequency power produced by etomidate at the ventrobasal complex, with this response being independent of δgaba A receptors. Wakefulness and REM sleep: Consistent with the effects of etomidate at the ventrobasal complex on 1-4 Hz EEG power in non-rem sleep, there were also significant effects of etomidate on the spectral composition of the EEG in wakefulness and REM sleep. These effects are illustrated in Figure 16B-D. As for non-rem sleep, etomidate also decreased 1-4 Hz EEG power in wakefulness and REM sleep, despite the decrease in ambient GABA that is associated with both of these states compared to non-rem sleep (Saper et al., 2001). This reduction in 1-4 Hz EEG power with etomidate occurred regardless of genotype (each p > 0.07, 2-way RM- ANOVA) and was significant for 10 and 30 µm etomidate in waking (each p < 0.041, post hoc
113 96 paired t-test) and in REM sleep (each p < 0.023). Figure 16D shows that this general shift to decreased slower-frequency EEG activity elicited by etomidate was also accompanied by significantly increased Hz EEG power in the wild-type and Gabrd -/- mice in wakefulness (F 2, 32 = 13.46, p < 0.001, 2-way RM-ANOVA) and 8-12 Hz EEG power in REM sleep (F 2, 28 = 8.53, p = 0.001) Etomidate at the ventrobasal complex and spindle-like oscillations Here we show that unlike the effects of THIP at the ventrobasal complex, which produced a δgaba A receptor-specific reduction in spindle-like oscillations in non-rem sleep, etomidate increased both sigma power and the incidence and duration of spindle-like oscillations in non- REM sleep, with these effects being independent of genotype. Sigma power: Figure 17A shows that there was a significant effect of etomidate at the ventrobasal complex of the thalamus on Hz EEG power in non-rem sleep (F 2, 32 = 47.20, p < 0.001, 2-way RM-ANOVA) that was dose-dependent (each p < versus acsf, post hoc paired t-test). This effect of etomidate on sigma power was not influenced by genotype (F 2, 32 = 0.48, p = 0.625, 2-way RM-ANOVA). There was, however, an independent effect of genotype on sigma power (F 1, 16 = 7.17, p = 0.017), with wild-type mice exhibiting significantly higher Hz power at all doses of etomidate (each p < 0.025, post hoc t-test).
114 97 Figure 16. Etomidate at the ventrobasal complex decreases 1-4 Hz electrocortical activity in both wildtype and Gabrd -/- mice (i.e., opposite to the effects of THIP). A, Exemplar power spectrums and hypnograms from a wild-type and Gabrd -/- mouse during microperfusion of the ventrobasal complex with acsf and 30 µm etomidate. Each plot illustrates the last 30 minutes of the respective treatment session. Note the decrease in lower frequency power and increase in higher frequency power in wild-type and
115 98 Gabrd -/- mice with etomidate. B, Continuous frequency plots illustrating the percent change in power with 30 µm etomidate relative to acsf during NREM sleep, waking, and REM sleep. Each trace illustrates the mean ± SEM. C, Changes in 1-4 Hz power with 10 and 30 µm etomidate. Note the decrease in 1-4 Hz power with 10 and 30 µm etomidate during NREM sleep, waking, and REM sleep in wild-type and Gabrd -/- mice. D, Additional changes in electrocortical activity with etomidate. Plots illustrate the percent increase or decrease in theta (4-8 Hz), alpha (8-12 Hz), and beta (12-30 Hz) power with 30 µm etomidate relative to acsf. Note the increase in alpha and/or beta power in wild-type and Gabrd -/- mice during all sleep-wake states. 2-way RM-ANOVA, * p < 0.05, ** p < 0.01, *** p < NREM: non-rem. Incidence and duration of spindle-like oscillations (7-14Hz): The group data in Figure 17B-C show that etomidate at the ventrobasal complex of the thalamus increased the incidence and duration of spindle-like oscillations (both F 2, 32 > 14.07, p < 0.001, 2-way RM-ANOVA) with this effect being evident in both wild-type mice and Gabrd -/- mice (F 2, 32 < 0.73, p > 0.490). Consistent with the genotypic differences in sigma power identified above and in the study with THIP, an effect of genotype on spindle-like oscillations was also identified, with reduced incidence and duration in the Gabrd -/- mice (both F 1, 16 > 5.83, p < 0.029, 2-way RM-ANOVA; Fig. 17B-C).
116 99 Figure 17. Etomidate at the ventrobasal complex promotes sigma power and increases the incidence and duration of spindle-like oscillations via a δgaba A receptor-independent mechanism (i.e., unlike the effects of THIP). A, 10 and 30 µm etomidate significantly increased sigma (10-15 Hz) power during NREM sleep. B, 30 µm etomidate at the ventrobasal complex increased the incidence and C, duration of spindle-like oscillations. Note the differences in sigma power, and spindle-like oscillation incidence and duration between genotypes at baseline and with etomidate. 2-way RM-ANOVA, * p < 0.05, ** p < 0.01, *** p < SLO: Spindle Like Oscillation. NREM: non-rem Etomidate at the ventrobasal complex and state-space dynamics We next identified if etomidate altered the trajectory between behavioural states. As with the experiments with THIP, this was identified from analysis of the speed of transitions between EEG-defined spectral boundaries that defined the states of wakefulness and sleep. Unlike with THIP at the ventrobasal complex, however, which increased the speed of stable transitions into non-rem sleep (Fig. 15), there was no effect of etomidate on such EEG transitions between behavioural states (each F 2, 31 < 2.12, p > 0.136, 2-way RM-ANOVA; Fig. 18).
117 100 Figure 18. Etomidate had no effect on the temporal dynamics of state transitions in spectral space. A, Exemplar state space plots from a wild-type and Gabrd -/- mouse during microperfusion of the thalamus with acsf and 30 µm etomidate. B, Etomidate had no effect on the speed of transitions between states in the spectral domain in both wild-type and Gabrd -/- mice Etomidate at the ventrobasal complex increases REM sleep expression Figure 19 shows that microperfusion of etomidate into the ventrobasal complex of the thalamus increased REM sleep expression (F 2, 32 = 6.07, p = 0.006, 2-way RM-ANOVA), with this effect being independent of genotype (F 2, 32 = 0.11, p = 0.901). The significant increase in REM sleep amount occurred with 30 µm etomidate (p = 0.004, post hoc paired t-test; Fig. 19A-B). There were no effects of etomidate at the ventrobasal complex on the amounts of wakefulness or non- REM sleep (both F 2, 32 < 1.82, p > 0.178, 2-way RM-ANOVA; Fig. 19B). There were also more transitions into, and out of, REM sleep with etomidate at the ventrobasal complex (both F 2, 32 >
118 , p < 0.020, 2-way RM-ANOVA; Fig. 19A,C). This increase in REM sleep amount with etomidate was associated with a decrease in the proportion of direct transitions from non-rem sleep to wakefulness (F 2, 32 = 5.20, p = 0.011; Fig. 19C). There were no effects of etomidate on transitions into non-rem sleep (F 2, 32 = 1.18, p = 0.320; Fig. 19C). A genotypic effect on the proportion of direct transitions from non-rem sleep to wakefulness was also identified (F 1, 16 = 6.42, p < 0.022, 1-way RM-ANOVA), with Gabrd -/- mice exhibiting a higher proportion of direct transitions from non-rem sleep to wakefulness at baseline and with 30 µm etomidate (both p < 0.023, post hoc t-test). This finding is consistent with the trend toward a lower proportion of overall transitions from REM sleep to wakefulness (Fig. 19C). Importantly, and as mentioned above, this effect on state dynamics was not associated with any overall differences in the time spent in each state. The increase in REM sleep amounts with etomidate at the ventrobasal complex was due to the effects of drug per se, and not simply due to the fact that the etomidate interventions were performed after the interventions with acsf. This is because additional experiments showed that the amounts of REM sleep with 30 µm etomidate were significantly increased compared to the same time of day as the time control experiments (F 1, 26 = 4.63, p = 0.041, 2-way ANOVA; Fig. 19D). The time control experiments used sham interventions with acsf instead of switches between etomidate concentrations.
119 102 Figure 19. Etomidate at the ventrobasal complex increased REM sleep expression independent of δgaba A receptor expression (i.e., effects were observed in wild-type and Gabrd -/- mice). A, Exemplar hypnograms highlighting the increase in REM sleep expression with 30 µm etomidate. B, 30 µm etomidate at the thalamus had no effect on the relative amount of wakefulness or NREM sleep, but significantly increased the amount of REM sleep. 2-way RM-ANOVA. C, 30 µm etomidate significantly increased the number of transitions into- and out of- REM sleep. This was associated with a significant
120 103 decrease in the number of direct transitions from NREM sleep to wakefulness. 2-way RM-ANOVA. D, Comparison of the amounts of sleep and wakefulness between test mice and time control mice that received sham treatment (i.e., continuous microperfusion of acsf) at the ventrobasal complex at the corresponding time-points where test mice received etomidate. Mice receiving 30 µm etomidate exhibited a greater amount of REM sleep relative to time controls. Note the additional effects of 10 µm etomidate on the amount of wakefulness and NREM sleep. 2-way ANOVA. * p < 0.05, ** p < 0.01, *** p < NREM: non-rem The effects of etomidate at the ventrobasal complex largely persist during blockade of GABA re-uptake Since etomidate acts as a positive allosteric modulator of GABA A receptors, its ability to potentiate the activity of these receptors requires GABA (Forman, 2011). Given the possible decrease in endogenous extracellular GABA levels that could occur with reverse microdialysis, the actions of etomidate on extrasynaptic GABA A receptors could be reduced by the intervention. Thus, we conducted additional experiments in wild-type mice where etomidate was co-applied with the GAT-1 and -3 inhibitors NO-711 (100 µm) and SNAP-5114 (300 µm). As shown in Figure 20, the effects of etomidate on electrocortical activity largely persisted in mice that received NO-711 and SNAP-5114 in addition to etomidate (Fig. 20A-D). Consistent with previous findings (Fig. 16), we identified an effect of etomidate on 1-4 Hz power during each of waking, non-rem sleep (both F 2, 26 > 4.01, p < 0.031, 2-way RM ANOVA) and REM sleep (F 2, 23 = 9.42, p = 0.001; Fig. 20A). This effect of etomidate on 1-4 Hz EEG power was not altered by the inclusion of NO-711 and SNAP-5114 to the perfusion media (each F < 2.69, p > 0.089). Post hoc analyses confirmed that microperfusion of 30 µm etomidate into the ventrobasal complex decreased 1-4 Hz power during all sleep-wake states (each p <
121 , versus acsf, post hoc paired t-test). Likewise, 10 µm etomidate decreased 1-4 Hz EEG in non-rem and REM sleep (both p < 0.005, versus acsf). The effects of etomidate on 8-12 Hz power during non-rem and REM sleep and on Hz power during waking were similarly not altered by blockade of GABA re-uptake (each p > 0.498; Fig. 20B). Of all of the comparisons the only difference observed between groups receiving etomidate with and without the co-application of the GABA transporter inhibitors was a reduction in the etomidate-induced increase in Hz power in non-rem sleep (F 2, 26 = 4.70, p = 0.018; Fig. 20B). There were no significant effects of NO-711 and SNAP-5114 on the etomidate-induced increase in sigma power, and spindle-like oscillation incidence and duration (all F 2, 26 < 3.00, p > 0.068; Fig. 20C). Finally, while the effects of etomidate on sleep-wake structure were independent of NO-711 and SNAP-5114 (all F 2, 26 < 1.16, p > 0.290), the significant increase in REM sleep observed with etomidate alone was no longer identified (Fig. 19D and 20D). There was no independent effect of NO-711 and SNAP-5114 identified in any of the comparisons of sleep-wake structure (all F 1, 13 < 3.15, p > 0.100).
122 105 Figure 20. The effects of etomidate on electrocortical activity persist with increased GABA concentrations in the ventrobasal complex. A, Microperfusion of 30 µm etomidate into the ventrobasal complex decreased 1-4 Hz EEG power during all sleep-wake states in mice receiving etomidate alone or co-application of the GABA-re-uptake inhibitors NO-711 and SNAP B, Additional changes in electrocortical activity in different frequency bands with etomidate in the absence and presence of the GABA re-uptake inhibitors. Plots illustrate the percent change in theta (4-8 Hz), alpha (8-12 Hz), and beta
123 106 (12-30 Hz) power with 30 µm etomidate relative to acsf. With the exception of beta power during NREM sleep, note that the effects of etomidate on electrocortical activity persisted in mice that also received GABA re-uptake inhibitors into the ventrobasal complex. C, Etomidate at the ventrobasal complex increased the incidence and duration of spindle-like oscillations. Again, note the absence of differences between mice receiving etomidate with or without the GABA re-uptake inhibitors. D, The amount of time spent in each sleep-wake state was also unaffected by etomidate and/or inhibition of GABA re-uptake. 2-way RM ANOVA. * p < 0.05, ** p < 0.01, *** p < NREM: non-rem. 3.4 Discussion Here we show that thalamic δgaba A receptor activation in vivo using localized microperfusion of THIP into the ventrobasal complex of freely behaving mice promotes electrocortical signatures commonly associated with the deep stages of non-rem sleep. These include an increase in 1-4 Hz EEG activity and a decrease in spindle-like oscillations, with these effects being observed in wild-type but not Gabrd -/- mice. These findings are consistent with the sedating property of THIP and its effects on electrocortical activity when administered systemically in humans and rodents (Faulhaber et al., 1997; Vyazovskiy et al., 2005; Cremers and Ebert, 2007). Importantly, we also show that etomidate at the thalamus does not recapitulate the electrocortical effects of THIP, with etomidate eliciting changes in electrocortical activity that were largely independent of δgaba A receptor expression. Moreover, the changes in electrocortical activity that were identified with etomidate are consistent with the global changes in electrocortical activity that are reported during induction of anesthesia with etomidate in humans (Kuizenga et al., 2001). Collectively, these findings implicate thalamic δgaba A receptors in mediating the electrocortical markers of deep non-rem sleep and suggest a role
124 107 for non-δgaba A receptors in the thalamus in mediating the changes in electrocortical activity during induction of anesthesia with etomidate Thalamic δgaba A receptor activity, sedation, and sleep The effects of THIP at the thalamus on cortical 1-4 Hz signaling require δgaba A receptor expression, suggesting they are mediated by enhanced δgaba A receptor-mediated tonic inhibition (Cope et al., 2005). Increasing tonic inhibition would effectively hyperpolarize the resting membrane potential of thalamocortical neurons to levels that enable burst-firing and, ultimately, promote 1-4 Hz cortical activity (McCormick and Bal, 1997). This interpretation is further supported by the decrease in sigma power and spindle-like oscillations that were also identified with THIP at the thalamus of wild-type mice. Together, our findings support the general scheme whereby enhanced thalamic δgaba A receptor-mediated tonic inhibition hyperpolarizes the resting membrane potential of thalamocortical neurons to levels that support 1-4 Hz oscillations at the expense of sleep spindles (Nunez et al., 1992). Enhanced thalamic δgaba A receptor activity was also associated with increased speed of stable transitions into the spectral space occupied by non-rem sleep. While transitions into non-rem sleep occurred more rapidly in the spectral domain, there were no identifiable effects of THIP on the amount non-rem sleep per se, as can occur in some species (Schultz et al., 1981; Madsen et al., 1983). Although systemic administration of THIP in mice has no effect on the amount of non-rem sleep (Vyazovskiy et al., 2005; Winsky-Sommerer et al., 2007), it does produce a generalized increase in 1-4 Hz electrocortical power, as observed in our study, consistent with its sedating action. The results of our study identify the thalamus as a potential key site of action for the sedating property of THIP.
125 108 Some baseline differences in electrocortical activity were identified in our comparisons of wild-type and Gabrd -/- mice before drug interventions. The Gabrd -/- mice exhibit higher 1-4 Hz power and lower Hz power during non-rem sleep. This difference would be consistent with more hyperpolarized resting membrane potentials in thalamocortical neurons of Gabrd -/- mice relative to wild-types. Such genotypic differences could be attributed to compensatory changes in the expression of GABA A receptor subpopulations and/or other channels/receptors, such as the TWIK-related acid-sensitive K + channels that can also hyperpolarize the resting membrane potential of thalamocortical neurons (Brickley et al., 2001; Peng et al., 2002; Meuth et al., 2006). Microperfusion of 50 µm THIP into the ventrobasal complex increased Hz power in Gabrd -/- mice, indicating that THIP had some non-δgaba A receptor-mediated effects, potentially via effects on synaptic GABA A receptors (Brown et al., 2002; Storustovu and Ebert, 2003). Importantly, the effective delivered concentrations of THIP used in this study (for more details see section Experimental Protocol) were selected based on previous reports showing that THIP, at those concentrations, has minimal effects on synaptic GABA A receptors (Brown et al., 2002; Storustovu and Ebert, 2003; Belelli et al., 2005; Jia et al., 2005) and promotes sleep and sedation in humans (Madsen et al., 1983; Faulhaber et al., 1997). Nonetheless, it is possible that THIP also had non-δgaba A receptor-mediated effects that contributed to the changes identified in the wild-type mice. This interpretation, however, is not readily supported by our findings since all of the identified effects of THIP in the wild-type mice were absent in the Gabrd -/- mice. If actions on non-δgaba A receptors played a significant role in mediating the effects of THIP in the wild-type mice, we would expect to see similar, albeit attenuated, effects
126 109 in the Gabrd -/- mice. Given the absence of such similarities between genotypes, our findings suggest that the identified effects of THIP in the wild-type mice require δgaba A receptors. In contrast to the in vitro studies that identified the effects of acute administration of THIP/etomidate on GABA A receptor subpopulation activities (Brown et al., 2002; Storustovu and Ebert, 2003; Belelli et al., 2005; Cope et al., 2005; Drasbek and Jensen, 2006), the experiments reported here utilized a longer period of drug exposure. Such prolonged exposure to THIP and/or etomidate may have elicited changes in receptor expression and/or sensitization not identified with acute application. Nonetheless, the longer duration of exposure to THIP and etomidate used in this study is of particular relevance given the use of these agents in sedation and anesthesia Etomidate at the thalamus and electrocortical signatures of anesthetic induction Unlike with THIP, all of the effects that were identified with etomidate presented in both the wild-type and Gabrd -/- mice, indicating that these effects do not require δgaba A receptors. Some of the effects of etomidate were also in the direction opposite to that elicited by THIP, further identifying a different mechanism of action. Etomidate at the thalamus elicited reductions in 1-4 Hz power and increases in alpha and/or beta power in wild-type and Gabrd -/- mice across all sleep-wake states. The effective concentrations of etomidate used in this study were again selected based on previous in vitro studies that identified a δgaba A receptor-potentiating effect of etomidate in thalamocortical neurons at similar concentrations (Belelli et al., 2005). Importantly, an effect of etomidate on synaptic GABA A receptors was also identified at these concentrations.
127 110 The effects of etomidate at the thalamus on cortical alpha and beta power are consistent with studies modeling the effects of the intravenous general anesthetics etomidate and propofol on electrocortical activity (Talavera et al., 2009; Ching et al., 2010; Boly et al., 2012). Ching et al. further associate these cortical effects with increased thalamic GABA A receptor conductance and IPSP duration. Consistent with the modeling effects of etomidate and propofol on thalamic phasic inhibition, in vitro studies show that etomidate prolongs the decay of miniature IPSCs in neurons of the ventrobasal complex and RTN (Belelli et al., 2005). Enhanced recruitment of thalamocortical neurons through lateral connectivity between RTN neurons with etomidate could further precipitate widespread tuning of thalamocortical firing to RTN firing rates, promoting cortical alpha oscillations and facilitating the incidence and propagation of spindle-like oscillations (Suffczynski et al., 2001; Steriade, 2005). It should also be noted that while the probe tips targeted the ventrobasal complex, diffusion of etomidate to the RTN and other neighbouring thalamic nuclei is likely. Thus, the effects of etomidate reported here likely result from its actions on neurons located in the ventrobasal complex and other thalamic nuclei including the RTN. The reduction in 1-4 Hz power, increase in spindle-like oscillations, and increase in REM sleep expression with etomidate at the thalamus of both wild-type and Gabrd -/- mice, all suggest that etomidate supports a pattern of electrocortical activity that is otherwise typically recognized as light sleep but through largely δgaba A receptor-independent mechanisms. The types of sleep that etomidate at the thalamus appears to promote are often associated with dream-like and hypnogogic mentation in humans (Rowley et al., 1998; Fosse et al., 2001). Importantly, such dream-like awareness is also frequently associated with induction of anesthesia (Brown et al., 2010).
128 The thalamus, sleep, and anesthesia The extrasynaptic GABA A receptor-potentiating actions of etomidate within the thalamus have been suspected of mediating its influence on consciousness (Meera et al., 2009; Kretschmannova et al., 2013). However, etomidate has pronounced effects on both phasic and tonic inhibition given its primary molecular targets, the β 2 and β 3 GABA A receptor subunits, have synaptic and extrasynaptic localization (Uchida et al., 1995; Belelli et al., 1997; Hill-Venning et al., 1997). Unlike etomidate, THIP at concentrations similar to the effective concentrations used in this study has no effect on synaptic inhibition in the ventrobasal complex or RTN in vitro (Belelli et al., 2005). Thus, given the absence of δgaba A receptors in the RTN (Pirker et al., 2000), THIP appears to have minimal, if any, effect on RTN neurons at clinically relevant concentrations. Different influences on phasic inhibition in the thalamus and RTN could explain the differing effects of THIP and etomidate identified here. Importantly, etomidate also promotes spillover inhibition in addition to phasic and tonic inhibition (Herd et al., 2013; Herd et al., 2014). This form of inhibition is characterized by the recruitment of extra- and peri-synaptic GABA A receptors by synaptically released GABA, which ultimately prolongs evoked IPSCs. The effect of etomidate on spillover inhibition, however, requires both synaptic and extrasynaptic GABA A receptors (Herd et al., 2014). Our study indicates that thalamic δgaba A receptor-mediated tonic and spillover inhibition are not required to elicit etomidate-induced electrocortical changes, given the lack of effect of genotype. Additionally, we did not identify any major effect of increased extracellular GABA levels on the effects elicited by etomidate. This finding indicates that the δgaba A receptor-independent effects of etomidate on electrocortical activity largely persist under conditions that are favorable
129 112 to the potentiation of extrasynaptic GABA A receptors. We did, however, identify an effect of NO-711 and SNAP-5114 on the etomidate-induced increase in Hz signaling, but only during non-rem sleep. Our results suggest that THIP and etomidate at the thalamus promote different effects on the thalamo-cortico-thalamic oscillatory feedback loop. However, both increased delta power and alpha/beta power are associated with an increased coupling between the thalamus and the cortex (Lopes da Silva et al., 1980; Steriade et al., 1993b). Such an increase in cortico-thalamic connectivity would occur at the expense of cortical connectivity with other brain regions. Moreover, increasing inhibitory signaling in thalamic neurons as would be elicited by THIP and etomidate would impact the ability of these neurons to relay sensory input to the cortex. Thus, it is likely that both THIP and etomidate at the thalamus impair cortical processing of sensory information. Future studies investigating the effects of sedative and anesthetic agents at the thalamus on attention and arousal will confirm or refute this notion. The differing effects of THIP and etomidate at the thalamus have important implications in identifying the mechanisms that distinguish anesthesia from sleep. Our findings implicate, albeit indirectly, enhanced thalamic phasic inhibition in mediating the electrocortical signatures of anesthetic induction with etomidate (Kuizenga et al., 2001). Conversely, enhanced thalamic tonic inhibition promotes signatures consistent with deep non-rem sleep, the only naturally occurring brain state in which healthy human subjects have reported having minimal mentation (Hobson et al., 2000; Stickgold et al., 2001; Hobson and Pace-Schott, 2002). Further in vivo studies that assess the contribution of phasic, spillover, and tonic inhibition in the thalamus during sleep and systemic drug-induced sedation and anesthesia will serve to confirm the role of these pathways in conscious awareness.
130 113 Chapter 4 Enhanced thalamic spillover-inhibition during non-rem sleep triggers an electrocortical signature of anesthetic hypnosis This chapter is modified from the following: Mesbah-Oskui L, Horner RL (2016) Enhanced thalamic spillover-inhibition during non rapideye-movement sleep triggers an electrocortical signature of anesthetic hypnosis. Anesthesiology. In press. 4.1 Introduction The electrocortical patterns that signal anesthetic-induced loss-of-consciousness are only starting to be understood (Ching and Brown, 2014). There is an established link between increased frontal alpha-beta frequency cortical activity and the onset of the behavioural correlates that mark loss-of-consciousness with GABA A receptor-targeting general anesthetics (Ching et al., 2010; Purdon et al., 2013; Baker et al., 2014). Moreover, a critical role for the thalamus in orchestrating the emergence of this alpha-beta electrocortical signature during the induction of anesthesia has been suggested (Ching et al., 2010; Vijayan et al., 2013a; Baker et al., 2014; Ching and Brown, 2014). Specifically, in vivo local field potential recordings in rodents identify that during propofol-induced loss-of-consciousness, elevated alpha-beta activity in the thalamus precedes manifestation of the same signature in the cortex (Baker et al., 2014). Additionally, computational modeling studies indicate that increased GABAergic conductance and prolonged IPSPs in thalamocortical neurons can reinforce cortical activity in the alpha-beta range (Ching et al., 2010; Vijayan et al., 2013a). Importantly, the molecular and cellular underpinnings that could position the thalamus to orchestrate these electrocortical changes remain to be elucidated in vivo.
131 114 Modulation of thalamic GABAergic signaling can trigger state-associated changes in electrocortical activity (Steriade et al., 1993b). There are three types of GABA A receptormediated inhibition identified in thalamocortical neurons: phasic (i.e., phasic activation of synaptic GABA A receptors), tonic (i.e., tonic activation of extra- and peri-synaptic GABA A receptors) and spillover (i.e., phasic activation of extra- and peri-synaptic GABA A receptors) (Herd et al., 2013). Importantly, the alterations in thalamocortical neuron firing elicited by the general anesthetic etomidate require phasic activation of extrasynaptic GABA A receptors in vitro (Herd et al., 2014). De-inactivation and inactivation of T-type Ca 2+ channels in thalamocortical neurons plays a pivotal role in modulating their firing patterns (Steriade et al., 1993b). At typical resting membrane potentials (i.e., around -60 mv) thalamocortical neurons exhibit tonic firing patterns with inactivation of T-type Ca 2+ channels. Conversely, at more hyperpolarized resting membrane potentials thalamocortical neurons exhibit burst firing characterized by short duration and high frequency bursts of action potentials followed by periods of quiescence (Steriade et al., 1993b). Critically, this burst firing pattern is elicited by de-inactivation of T-type Ca 2+ channels and is further associated with increased 1-4 Hz electrocortical activity (Steriade et al., 1993b; Lee et al., 2004a). Here we use a combination of genetic, pharmacological, and electrophysiological approaches to identify the electrocortical effects elicited by pharmacological enhancement of phasic extrasynaptic GABA A receptor activity in the thalamus in vivo. We also identify that the same electrocortical signatures are produced by the presence of the prototypic GABA A receptortargeting general anesthetic etomidate at the thalamus in vivo. We further characterize the role of thalamic T-type Ca 2+ channels in mediating these changes in electrocortical activity, and identify
132 115 that this signature is confined to periods of non-rem sleep. Together these findings identify how a commonly used general anesthetic when acting at the thalamus can elicit the characteristic brain wave pattern associated with anesthetic hypnosis, and the necessity of an initial sleep transition to generate this signature. 4.2 Materials & Methods Animal Care Three- to 6-month old male wild-type mice (C57BL/6 SvJ129; n = 33) and Gabrd -/- mice (i.e., mice that lack δ-subunit containing GABA A Rs; n = 9) were used for all experiments (Fig. 21A). Mice were generated and housed as previously described (Mesbah-Oskui et al., 2014). All experimental procedures were approved by the University of Toronto Animal Care Committee and performed in compliance with the requirements of the Canadian Council on Animal Care Experimental protocol All surgeries were performed under sterile conditions using isoflurane anesthesia (1.5-2 %). The mice were implanted with frontal EEG and nuchal EMG electrodes for recording of electrocortical activity and sleep-wake behaviour. Two microdialysis guide cannulae (CXG-4; Eicom) were stereotaxically positioned 3.0 mm above the right and left ventrobasal complex of the thalamus, i.e., bilateral placement (1.6 mm posterior to lambda, 2 mm lateral to midline and lowered 0.25 mm ventrally; Fig. 21B-D). Following recovery from surgery, mice were habituated to the recording chamber as previously described (Mesbah-Oskui et al., 2014).
133 116 The drugs used for these studies were: (i) etomidate, a general anesthetic that acts as a positive allosteric modulator of GABA A receptors (Forman, 2011); (ii) DS2, a positive allosteric modulator of extrasynaptic δ-subunit containing GABA A receptors (δgaba A receptor) (Jensen et al., 2013); (iii) THIP, a δgaba A receptor-preferring agonist (Meera et al., 2011), and (iv) 3,5- dichloro-n-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]- benzamide (TTA-P2), a potent T-type Ca 2+ channel blocker (Dreyfus et al., 2010). Etomidate, DS2, and THIP were purchased from Tocris Bioscience, and TTA-P2 from Alomone Labs. Etomidate (30 µm), DS2 (100 µm) and THIP (50 µm) were prepared in acsf with and without TTA-P2 (300 µm). Importantly, the amount of drug that diffuses across the microdialysis probe membrane into tissue is estimated to be % of the original concentration in the perfusion medium (Portas et al., 1996; Grace et al., 2014). Thus, the effective concentration of etomidate, DS2, and THIP at the ventrobasal complex approximates µm, µm, and 5-9 µm, respectively. Similarly, the effective concentration of TTA-P2 at the thalamus is expected to approximate µm. The concentrations of THIP and etomidate were selected based on clinically relevant concentrations. Specifically, human serum and plasma levels of THIP and etomidate at the same effective concentrations as the ones used in this study elicit sedation and hypnosis (Madsen et al., 1983; Faulhaber et al., 1997; Benkwitz et al., 2007). In addition, the concentrations of THIP and etomidate used here are similar to the concentrations used in the in vitro studies identifying the effects of THIP and etomidate on tonic and phasic GABA A receptor activity in thalamocortical neurons (Belelli et al., 2005; Herd et al., 2014). Similarly, DS2 at concentrations similar to that used here promotes phasic extrasynaptic δgaba A receptor activation in thalamocortical neurons in vitro (Herd et al., 2013; Rovo et al., 2014). There are no clinically relevant doses with which
134 117 to compare with DS2 as it does not effectively cross the blood-brain barrier when administered systemically (Jensen et al., 2013). The concentration of TTA-P2 was selected based on previous in vivo and in vitro studies that characterized the specificity of TTA-P2 for low-voltage activated T-type Ca 2+ channels. Specifically, a previous in vivo study, using methods similar to ours, identified that 3 mm TTA- P2 at the ventrobasal complex of naturally sleeping rats significantly reduced thalamocortical burst firing (David et al., 2013). Importantly, 3 mm TTA-P2 at the thalamus was also associated with reduced tonic firing, suggesting that at this concentration TTA-P2 effectively dampened net thalamic activity. This decrease in total thalamic activity suggests that 3 mm TTA-P2 may also be affecting the activity of other channels in addition to T-type Ca 2+ channels. Consistent with this interpretation, in vitro studies have shown that TTA-P2 at concentrations greater than 100 µm can inhibit high-voltage activated Ca 2+ channels, which are expressed in the RTN and could affect thalamic output (Choe et al., 2011; Zaman et al., 2011). Concentrations of TTA-P2 in the low micromolar range, however, are highly selective for all T-type Ca 2+ channel isoforms (Dreyfus et al., 2010; Choe et al., 2011). Given these findings we selected a concentration of 300 µm TTA-P2, which is expected to yield an effective concentration of µm around the microdialysis probe (Portas et al., 1996; Grace et al., 2014), a concentration that is associated with selectivity for low-voltage activated T-type Ca 2+ channels. On the morning of experiments, the mice were gently restrained and microdialysis probes (CX-6-01; Eicom) that projected to the ventrobasal complex were inserted. The probes were continuously flushed with acsf at a flow rate of 2.1 µl/min. The acsf was composed of (in mm): 125 NaCl, 3 KCl, 1 KH 2 PO 4, 2 CaCl 2, 1 MgSO 4, 25 NaHCO 3, and 30 D-glucose. ACSF
135 118 was bubbled with CO 2 to a ph of 7.41 ± All experiments were performed during the day. The entire experimental protocol occurred over a 5 h period ( h). Baseline recordings were performed while acsf was microperfused bilaterally into the ventrobasal complex of each mouse for 2 h (Fig. 21A). The perfusion media was then switched to etomidate, TTA-P2, DS2, or THIP. In the first study 30 µm etomidate was delivered into the thalamus of wild-type mice (n = 9) for 1.5 h, followed by another 1.5 h period where the thalamus was microperfused with 30 µm etomidate plus 300 µm TTA-P2. A control study was also conducted where acsf containing only 300 µm TTA-P2 was microperfused into the thalamus of wild-type mice (n = 6) for 2 h following baseline recordings. The third study also served as a positive control, and was designed to confirm the specificity of DS2 for δgaba A receptors. In this study the ventrobasal complex of the thalamus of Gabrd -/- mice (n = 9) was microperfused with 100 µm DS2 for 1.5 h, followed by another 1.5 h period where they received 100 µm DS2 in acsf that also contained i) 100 µm NO-711 (Sigma-Aldrich), an inhibitor of GABA transporter 1, and (ii) 300 µm of SNAP-5114 (Tocris Bioscience), an inhibitor of GABA transporter 3. Reverse microdialysis of NO-711 and SNAP at concentrations similar to the effective concentrations used here are associated with an increase in extracellular GABA concentrations (Smith et al., 2007; Cope et al., 2009; Kersante et al., 2013). GABA re-uptake inhibitors were co-applied as part of this protocol to further increase GABA levels and promote enhanced GABA A receptor activation with DS2. In the fourth study 100 µm DS2 was microperfused into the ventrobasal of wild-type mice (n = 9) for 1.5 h, followed by another 1.5 h period where 100 µm DS2 was delivered with 300 µm TTA-P2. The fifth study was similar to the previous one, except 50 µm THIP was
136 119 microperfused into the thalamus instead of DS2. At the end of each experiment the mice were deeply anesthetized with 5 % isoflurane and the brains were removed, fixed, and sliced as previously described (Mesbah-Oskui et al., 2014). Neutral red staining was performed on dried sections to confirm probe site location (Fig. 21B). Figure 21. Summary of experimental methods. A, Schema that outlines the treatment protocols for each experimental group. B, Exemplar coronal brain sections showing the targeting of probe tips (as indicated by the arrows) to the ventrobasal complex of the thalamus. C, Exemplar raw EEG and EMG recordings from a mouse during microperfusion of the thalamus with acsf. D, Probe locations for all mice studied (n = 42). Numbers on the bottom left side of each section indicate the distance posterior from bregma (Franklin and Paxinos, 2007).
137 Signal acquisition and analysis of sleep-wake states Acquisition, filtering, and digitization of the EEG and EMG signals were performed as previously described (Mesbah-Oskui et al., 2014). Fast Fourier analysis was used to analyze general characteristics of electrocortical activity in each sleep-wake state and was performed as previously described (Mesbah-Oskui et al., 2014). Briefly, each 5 s epoch of the EEG was analyzed for absolute power in five bandwidths: 1-4 Hz, 4-8 Hz, 8-12 Hz, Hz, and Hz. The absolute power in each bandwidth was divided by the total power (1-30 Hz) in each epoch and the normalized powers were then sorted according to the corresponding sleep-wake state. The mean power in each frequency band for each sleep-wake state was then calculated for each treatment in each subject. Sleep-wake states were scored by analyzing EEG and EMG signals in consecutive 5 s epochs using an established automatic scoring protocol (Costa-Miserachs et al., 2003). The accuracy of the scoring procedure was confirmed visually and corrected manually as necessary after this visual inspection. The time spent in wakefulness, NREM sleep and REM sleep was calculated as a percentage of the total recording time for each treatment in each animal. The incidence and features of spindle-like oscillations were identified using previously described custom-written routines in MATLAB (Mesbah-Oskui et al., 2014) Analysis of transitions into non-rem sleep Only stable transitions into non-rem sleep were included in this analysis, i.e., transitions where the mouse was awake for >30 s and then transitioned into a non-rem sleep episode that also lasted >30 s. The wavelet power spectrums, calculated by taking the square of the wavelet
138 121 transform, were generated using MATLAB routines based on those provided by Torrence and Compo (Torrence and Compo, 1998). We used a Morlet Mother wavelet, defined as: ϕ(t) = π 1 t 2 4 e i2πf 0 e 2 where f 0 is the wavelet central frequency and t is a dimensionless time parameter. The value of 2πf 0 (i.e., ω 0 ) was set to 6 to satisfy the admissibility criterion, which states that for an integral function the analyzing wavelet should have a mean of zero (Farge, 1992). Alterations in wavelet power during microperfusion of the thalamus with DS2, THIP, etomidate, and/or TTA-P2 were identified by following three fixed frequency bands: 1-4 Hz, Hz, and Hz. All three of these bands exhibited significant alterations in power that exceeded a 95 % confidence level, which was calculated assuming a red-noise background that serves as an effective background spectrum (Torrence and Compo, 1998). This procedure was performed because, like electrocortical activity, red-noise exhibits increasing power for decreasing frequencies (Torrence and Compo, 1998; Baker et al., 2014). The average power in the 1-4 Hz, Hz, and Hz bands were calculated for each mouse for the 30 s period before and 30 s period after transitions into non-rem sleep Statistical analysis The effects of microperfusion of etomidate, DS2, and THIP into the thalamus, with and without TTA-P2, on electrocortical activity and sleep-wake behaviour were assessed using a 2-way RM ANOVA with the factors being (i) drug treatment and (ii) sleep-wake state. The effects of the above treatments on spindle-like oscillations were assessed using a 1-way RM ANOVA. Finally, comparisons of electrocortical activity during transitions into non-rem sleep were conducted
139 122 using a 2-way RM ANOVA with the factors being (i) drug treatment and (ii) period of transition (i.e. pre- or post-transition into NON-REM sleep). Bonferonni-corrected p values were used to test statistical significance when post hoc comparisons were performed. Differences were considered significant if p < Analyses were performed using SigmaStat software (SPSS). Data are expressed as mean ± SEM. 4.3 Results Etomidate at the thalamus increases alpha-beta electrocortical activity and spindle-like oscillations during non-rem sleep We first characterized the electrocortical signature associated with delivery of the prototypic GABA A receptor-targeting general anesthetic etomidate into the thalamus. This first experiment was performed before the subsequent manipulations of thalamic activity in order to confirm, validate and replicate the initial finding from our laboratory upon which this new study was based (Mesbah-Oskui et al., 2014). Bilateral microperfusion of 30 µm etomidate into the ventrobasal complex of freely behaving wild-type mice that were instrumented with EEG and nuchal EMG electrodes (Fig. 21A-D), elicited state-dependent changes in 1-4 Hz, 8-12 Hz, and Hz electrocortical activity (all F 4, 26 > 3.17, p < 0.031, 2-way RM ANOVA; Fig. 22A and B), which fully replicates previous findings from our laboratory (Mesbah-Oskui et al., 2014). Specifically, etomidate reduced 1-4 Hz power and increased both 8-12 Hz and Hz power, all during non-rem sleep only (post hoc paired t-test, all p < 0.021). Importantly, mice receiving etomidate at the thalamus spontaneously transitioned in and out of sleep but the EEG signature
140 123 elicited by etomidate occurred only when mice were in non-rem sleep. There was no effect of etomidate at the thalamus on 4-8 Hz electrocortical activity (F 2, 16 = 2.39, p = 0.117, 2-way RM ANOVA). These initial findings identify that the action of etomidate at the thalamus is sufficient to elicit a cortical activity pattern of anesthetic hypnosis, but only when thalamic activity has already entered a mode of firing that is set-up during non-rem sleep. Next we analyzed the effect of etomidate on spindle-like oscillations given the overlap of alpha-beta frequencies with the frequency of sleep spindles (7-14 Hz) that are generated by the thalamus (Steriade et al., 1993b). Etomidate increased Hz (sigma) power during non-rem sleep (F 2, 16 = 8.32, p = 0.003, 1-way RM ANOVA; Fig. 22C), which further suggests an increase in the density of sleep spindles. We then identified the incidence of individual spindlelike oscillations during non-rem sleep using custom-written routines in MATLAB. Consistent with the alterations in sigma power identified with etomidate at the thalamus, the incidence of spindle-like oscillations was also increased with etomidate (F 2, 16 = 15.81, p < 0.001, 1-way RM ANOVA; Fig. 22C). Etomidate did not, however, have any effect on the average duration of spindle-like oscillations (F 2, 16 = 0.78, p = 0.476, 1-way RM ANOVA), indicating that once these oscillations were triggered they proceeded normally. In addition to the alterations in electrocortical activity identified with etomidate at the thalamus there was also a decrease in the amount of wakefulness (F 4, 32 = 4.26, p = 0.007, 2-way RM ANOVA; post hoc paired t-test p = 0.035; Fig. 22D). Importantly, none of the effects identified above with etomidate at the thalamus were affected by co-application of the T-type Ca 2+ channel blocker TTA-P2 (300 µm) (post hoc paired t-test, all p > 0.555; Fig. 22A-D). This finding indicates that the changes in electrocortical activity elicited by etomidate at the thalamus were achieved by changes in thalamocortical activity that was T-type Ca 2+ channel-independent.
141 124 Figure 22. Microperfusion of etomidate into the ventrobasal complex of wild-type mice increases alphabeta electrocortical activity, sleep spindles, and NREM sleep through T-type Ca 2+ channel independent alterations in thalamocortical activity in vivo. A, Exemplar EMG and EEG recordings from a wild-type mouse during bilateral microperfusion of the ventrobasal complex with acsf (left) and etomidate (right). The vertical scale bar corresponds to the EMG trace and equals 1 mv. Hypnograms are superimposed as white traces on the EEG spectrograms. Note the decrease in slow electrocortical activity with etomidate. B, Microperfusion of etomidate into the thalamus elicits a NREM sleep-specific reduction in 1-4 Hz EEG power and increased 8-12 Hz and Hz powers. These effects persist during co-administration of TTA-P2. 2-way RM ANOVA. C, Both NREM Hz (sigma) power and the incidence of spindle-like oscillations increase with etomidate at the thalamus. Note that co-application of TTA-P2 with etomidate
142 125 does not alter these effects and that neither treatment significantly alters the duration of spindle-like oscillations. 1-way RM ANOVA. D, Etomidate at the thalamus also elicits a reduction in wakefulness that persists with co-application of TTA-P2. NREM sleep is increased with etomidate plus TTA-P2 at the thalamus. 2-way RM ANOVA. All data represent mean ± SEM. N = 9; *p < 0.05, **p < 0.01, and ***p < NREM: non-rem. We also conducted a separate study to characterize the effects of TTA-P2 at the thalamus alone. This study was important because it showed that microperfusion of TTA-P2 into the thalamus changed electrocortical activity in a manner consistent with reduced slow oscillatory activity in the thalamus, i.e., this study served as a positive control. Specifically, microperfusion of TTA-P2 into the thalamus of wild-type mice significantly altered 1-4 Hz, 4-8 Hz, 8-12 Hz, and Hz electrocortical activities in a state-dependent manner (all F 2, 9 > 4.59, p < 0.043; Fig. 23A and B). Consistent with the role of T-type Ca 2+ channels in modulating 1-4 Hz electrocortical activity (Lee et al., 2004a), we identified a significant reduction in 1-4 Hz power during non-rem sleep with microperfusion of TTA-P2 into the thalamus of wild-type mice (post hoc paired t-test, p = 0.004). While there was increased Hz power during non-rem sleep with TTA-P2 at the thalamus (F 1, 5 = 10.42, p = 0.023, 1-way RM ANOVA), there were no corresponding changes in the duration or incidence of spindle-like oscillations (both F 1, 5 < 1.42, p > 0.287, 1-way RM ANOVA; Fig. 23C). An effect of TTA-P2 was also identified during REM sleep, with TTA-P2 eliciting an increase in 4-8 Hz power and a decrease in 8-12 Hz and Hz power (all p < 0.034). Microperfusion of TTA-P2 into the thalamus did not elicit any alterations in sleep-wake state durations (F 1, 5 = 1.11, p = 0.340, 2-way RM ANOVA; Fig. 23D).
143 126 Figure 23. Microperfusion of TTA-P2 into the thalamus of freely behaving wild-type mice elicits alterations in electrocortical activity consistent with reduced T-type Ca 2+ channel activity in the thalamus. A, Exemplar EMG and EEG recordings from a wild-type mouse during microperfusion of the ventrobasal complex with acsf (left) and TTA-P2 (right). The vertical scale bar corresponds to the EMG trace and equals 1 mv. B, During NREM sleep, blockade of thalamic T-type Ca 2+ channel activity with TTA-P2 decreased 1-4 Hz EEG power and reciprocally increased 8-12 Hz and Hz powers. During REM sleep TTA-P2 at the thalamus increased 4-8 Hz EEG power and decreased 8-12 Hz and Hz powers. 2-way RM ANOVA. C, TTA-P2 at the thalamus increased Hz power during NREM sleep, but had no effect on the duration or incidence of spindle-like oscillations. 1-way RM ANOVA. D, Microperfusion of TTA-P2 into the thalamus did not alter sleep-wake state durations. 2-way RM ANOVA. All data represent mean ± SEM. N = 6; *p < 0.05, and **p < NREM: non-rem.
144 Pharmacologically enhanced thalamic GABAergic spillover inhibition fully recapitulates the effects of etomidate at the thalamus To promote spillover inhibition in vivo we microperfused the ventrobasal complex of freely behaving mice with DS2, which is a positive allosteric modulator of extrasynaptic δ-subunit containing GABA A receptors (Jensen et al., 2013). DS2 promotes δgaba A receptor-mediated spillover inhibition in thalamocortical neurons in vitro by increasing the sensitivity of extrasynaptic δgaba A receptors to GABA released from the synapse and promoting phasic activation of these receptors (Herd et al., 2013; Jensen et al., 2013; Rovo et al., 2014). After having first established that DS2 elicits no identifiable effects on in vivo electrocortical activity and sleep-wake state behaviour in mice lacking δgaba A receptors (i.e., Gabrd -/- mice) (all p > 0.08; Fig. 24A-D), we then performed a similar experiment in wild-type mice. Microperfusion of 100 µm DS2 into the thalamus of freely behaving wild-type mice fully recapitulated the state-dependent alterations in electrocortical activity identified with etomidate at the thalamus (all F 4, 26 >2.76, p < 0.044, 2-way RM ANOVA; Fig. 25A-C). Specifically, DS2 at the thalamus, like etomidate, decreased 1-4 Hz power and increased 8-12 Hz and Hz powers during non-rem sleep only (post hoc paired t-test, all p < 0.008; Fig. 25B). There was no effect of DS2 on electrocortical activity during wakefulness or REM sleep (post hoc paired t- test, all p > 0.365; Fig. 25B). Microperfusion of DS2 into the thalamus had no effect on 4-8 Hz power (F 2, 16 = 1.42, p = 0.266, 2-way RM ANOVA; Fig. 25B), also consistent with our findings with etomidate. Moreover, like the mice that received etomidate at the thalamus, mice receiving DS2 also spontaneously transitioned in and out of sleep.
145 128 Together, these initial findings indicate that using DS2 to increase the sensitivity of extrasynaptic δgaba A receptors to phasic GABA release at the thalamus (Herd et al., 2013; Jensen et al., 2013; Rovo et al., 2014) recapitulates the characteristic electrocortical signature elicited by etomidate. That this signature is confined to non-rem sleep further suggests that there is a state-associated mode of network activity that is necessary for DS2 or etomidate at the thalamus to each elicit this same distinct pattern of electrocortical activity. The increased GABAergic release at thalamic nuclei from the RTN that occurs in non-rem sleep fits with such a mode (Steriade et al., 1993b).
146 129 Figure 24. Microperfusion of DS2 into the thalamus of freely behaving Gabrd -/- mice does not alter electrocortical activity or sleep-wake state durations. A, Exemplar EMG and EEG recordings from a Gabrd -/- mouse during microperfusion of the ventrobasal complex with acsf (left) and DS2 (right). The vertical scale bar corresponds to the EMG trace and equals 1 mv. B, DS2 did not elicit any significant alterations in the spectral composition of the EEG during any sleep-wake state in the Gabrd -/- mice. Moreover, co-administration of the GABA re-uptake blockers NO-711 (GAT-1 blocker) and SNAP-5114 (GAT-3 blocker) to increase extracellular GABA levels did not elicit an effect of DS2 on electrocortical activity or sleep-wake behaviour in the Gabrd -/- mice. 2-way RM ANOVA. C, DS2 did not alter NREM sigma (10-15 Hz) power or the duration and incidence of spindle-like oscillations in the Gabrd -/- mice (1- way RM ANOVA), nor D, did it effect sleep-wake state durations (2-way RM ANOVA). All data represent mean ± SEM. N = 9. NREM: non-rem.
147 130 Microperfusion of DS2 into the thalamus also increased Hz power during non- REM sleep, and this effect occurred with an increase in the incidence of spindle-like oscillations (both F 2, 16 > 8.67, p < 0.004, 1-way RM ANOVA; Fig. 25D and F). Microperfusion of DS2 into the thalamus did not, however, alter the duration of spindle-like oscillations (F 8, 16 = 2.19, p = 0.144, 1-way RM ANOVA; Fig. 25E). The effects of DS2 on spindle-like oscillations again fully mimic the alterations identified with etomidate. DS2 also altered sleep-wake state durations in a manner that paralleled the changes seen with etomidate, with increased non-rem sleep and decreased wakefulness (F 4, 32 = 5.41, p = 0.002, 2-way RM ANOVA; post hoc paired t-test both p < 0.008; Fig. 25G). The reduction in 1-4 Hz EEG power identified with DS2 (and etomidate) in the thalamus implicates a reduction in slow oscillatory activity in the thalamus. Since thalamic T-type Ca 2+ channels influence such electrocortical activity (Kim et al., 2001; Lee et al., 2004a), we identified the role of T-type Ca 2+ channels in mediating the effects of DS2 at the thalamus by coapplying the selective T-type Ca +2 channel blocker TTA-P2 (300 µm) into the thalamus of wildtype mice following microperfusion of DS2 alone. Co-application of TTA-P2 with DS2 to the thalamus did not alter any of the electrocortical effects identified with DS2 alone (post hoc paired t-test, all p > 0.344; Fig. 25B and C). Similarly, there were no differences in spindle-like oscillations between DS2 alone or when it was co-applied with TTA-P2 at the thalamus (post hoc paired t-test both p > 0.497; Fig. 25D-F). Co-administration of TTA-P2 with DS2 also did not alter the increased non-rem sleep occurring with DS2 alone (post hoc paired t-test, p = 0.096), although it did reverse the decrease in wakefulness (post hoc paired t-test, DS2 versus DS2 & TTA-P2, p = 0.026; acsf versus DS2 & TTA-P2, p = 0.361; Fig. 25G). Overall, these
148 131 findings indicate that thalamic T-type Ca +2 channels do not play a significant role in mediating the electrocortical effects elicited by DS2 at the thalamus, again recapitulating the findings with etomidate Figure 25. Promoting thalamic spillover inhibition with microperfusion of DS2 into the ventrobasal complex of wild-type mice fully recapitulates the alterations in electrocortical activity and sleep-wake
149 132 state behaviour identified with etomidate at the thalamus. A, Exemplar EMG and EEG recordings from a wild-type mouse as it received acsf (left) and DS2 (right) bilaterally into the ventrobasal complex. The vertical scale bar corresponds to the EMG trace and equals 1 mv. Note the reduced power at low frequencies with DS2. B, DS2 at the thalamus was associated with a NREM sleep-specific reduction in 1-4 Hz EEG power and increased 8-12 Hz and Hz powers. These effects of DS2 were not altered by co-application of the T-type Ca 2+ channel blocker TTA-P2. 2-way RM ANOVA. C, Continuous power plot showing the decrease in slow frequency electrocortical activity and increase in high frequency activity with DS2 and DS2 plus TTA-P2. The central lines denote the mean change in power, and the shaded regions correspond to the SEM. Note the similarity in the effects on electrocortical activity of DS2 and DS2 plus TTA-P2. D, Sigma power during NREM sleep was increased by DS2 and this effect did not change with co-administration of TTA-P2. E, The average duration of spindle-like oscillations was unaffected by DS2 and DS2 plus TTA-P2. F, Exemplar recordings of spindle-like oscillations during microperfusion of the thalamus with acsf and DS2 (right). The vertical scale bar equals 100 µv and the horizontal scale bar equals 1 s. The incidence of spindle-like oscillations increased with DS2 and this increase was not influenced by TTA-P2 (left). 1-way RM ANOVA. G, DS2 at the thalamus also increased the amount of NREM sleep and decreased wakefulness, an effect that was partly reversed by coadministration with TTA-P2. 2-way RM ANOVA. All data represent mean ± SEM. N = 9; *p < 0.05, **p < 0.01, and ***p < NREM: non-rem Pharmacologically enhanced thalamic tonic inhibition elicits alterations in electrocortical activity that are distinct from those identified with enhanced thalamic spillover inhibition To confirm that the effects identified with DS2 were consistent with enhanced phasic extrasynaptic δgaba A receptor activity (i.e., spillover inhibition) in the thalamus, and not due to elevated tonic δgaba A receptor activity, we conducted an additional study using THIP to
150 133 directly activate thalamic δgaba A receptors and promote tonic inhibition (Cope et al., 2005; Meera et al., 2011). We have previously shown that microperfusion of THIP into the thalamus of mice that lack δgaba A receptors has no effect on electrocortical activity or sleep-wake state durations, confirming the selectivity of THIP for extrasynaptic δgaba A receptors (Mesbah- Oskui et al., 2014). Microperfusion of THIP into the thalamus of wild-type mice elicited an increase in 1-4 Hz activity and a decrease in 8-12 Hz activity during non-rem sleep and wakefulness (both F 2, 16 = 14.06, p < 0.001, 2-way RM ANOVA; post hoc paired t-test both p < 0.046; Fig. 26A and B), which replicates previous findings from our laboratory (Mesbah-Oskui et al., 2014). These effects of THIP were significantly affected by the co-application of the T-type Ca 2+ channel blocker TTA-P2 (post hoc paired t-test, both p < 0.037). TTA-P2 led to a further increase in 1-4 Hz power and decreased 8-12 Hz power when co-applied with THIP. THIP at the thalamus also elicited state-dependent alterations in Hz EEG activity (F 2, 16 = 8.30, p = 0.003, 2-way RM ANOVA; Fig. 26A and B), with THIP causing a decrease in Hz EEG power during wakefulness (post hoc paired t-test, p = 0.007). This effect of THIP on Hz power was also significantly potentiated by co-application of TTA-P2 (post hoc paired t-test, p = 0.003). Since only 4 out of 9 mice had REM sleep during microperfusion of 50 µm THIP alone and/or in combination with 300 µm TTA-P2 into the thalamus, only the data from non-rem sleep and waking were analyzed for this study. Microperfusion of THIP into the thalamus also altered spindle-like oscillations, reducing Hz power during non-rem sleep (F 2, 16 = 24.50, p < 0.001, 1-way RM ANOVA; post hoc paired t-test, p = 0.003; Fig. 26C), an effect that was further decreased by co-administration of TTA-P2 (post hoc paired t-test, p = 0.027). Microperfusion of THIP plus TTA-P2 into the
151 134 thalamus reduced the duration and incidence of spindle-like oscillations compared to acsf (both F 2, 16 >9.51, p < 0.003, 1-way RM ANOVA; post hoc paired t-test, both p = 0.001; Fig. 26C). Co-application of THIP with TTA-P2 into the thalamus also altered sleep-wake structure (F 4, 32 = 7.97, p < 0.001, 2-way RM ANOVA; Fig. 26D), with THIP and TTA-P2 eliciting increased non- REM sleep (post hoc paired t-test, acsf vs. THIP & TTA-P2, p < 0.001; THIP vs. THIP & TTA-P2, p = 0.021) and decreased wakefulness (post hoc paired t-test, acsf vs. THIP & TTA- P2, p < 0.001; THIP vs. THIP & TTA-P2, p = 0.054). Importantly, these effects of THIP on EEG activity and sleep-wake behaviour are markedly different from those identified with DS2. This difference indicates that while both agents require δgaba A receptors to elicit their effects, these effects are likely produced by separate mechanisms that can be distinguished through their dependence on thalamic T-type Ca 2+ channel activity.
152 135 Figure 26. THIP-induced increases in tonic extrasynaptic GABA A receptor-mediated inhibition in the thalamus elicits distinct alterations in electrocortical activity that differ from DS2 and require T-type Ca 2+ channel activity in vivo. A, Exemplar EMG and EEG recordings from a mouse during microperfusion of the thalamus with acsf (left), THIP (center), and THIP with TTA-P2 (right). The vertical scale bar corresponds to the EMG traces and equals 500 µv. Note the increase in slow electrocortical activity with THIP, and the further increase in slow electrocortical activity with THIP & TTA-P2. B, Microperfusion of THIP into the thalamus of wild-type mice elicited a significant increase in 1-4 Hz EEG power and a decrease in 8-12 Hz and Hz powers during NREM sleep and wakefulness. Note that these effects were further increased by co-application of TTA-P2. The symbol * denotes significant differences between baseline (acsf) and treatment, whereas denote differences between THIP and THIP plus
153 136 TTA-P2. REM sleep was not included in these comparisons because 5 mice did not exhibit REM sleep during microperfusion of THIP and/or THIP plus TTA-P2. 2-way RM ANOVA. C, Microperfusion of THIP into the thalamus decreases NREM sigma power. Note that microperfusion of TTA-P2 with THIP elicits a further reduction in sigma power and also decreases the duration and incidence of spindle-like oscillations. 1-way RM ANOVA. D, THIP at the thalamus does not significantly effect sleep-wake state durations. Note, however, the increase in NREM sleep and the decrease in wakefulness elicited by TTA- P2 with THIP. 2-way RM ANOVA. All data represent mean ± SEM. N = 9; or *p < 0.05, or **p < 0.01, and ***p < NREM: non-rem Etomidate and DS2 at the thalamus amplify an electrocortical signature that characterizes entry into non-rem sleep Transient alterations in high-frequency cortical and subcortical activity have been identified during transitions into both non-rem sleep and propofol-induced loss-of-consciousness in rats (Baker et al., 2014). Moreover, this high frequency signature occurred in the thalamus before the cortex (Baker et al., 2014). Accordingly, we then examined transient alterations in electrocortical activity occurring during transitions into non-rem sleep using wavelet analysis. Consistent with the previous report, we identified significant alterations in high frequency EEG activity upon entry into non-rem sleep, as indicated by increases in power that exceeded a 95% confidence interval set by a red-noise background (Fig. 27A and B), the latter also used previously (Torrence and Compo, 1998; Baker et al., 2014). We then identified that microperfusion of etomidate into the thalamus increased the power of the high frequency (14-20 Hz) EEG signature characterizing entry into non-rem sleep in mice (F 2, 12 = 5.60, p = 0.019, 2-way RM ANOVA; post hoc paired t-test p = 0.048; Fig. 27A, C and E). Likewise, microperfusion of DS2 into the thalamus elicited an increase in the Hz signature following entry into non-rem sleep (F 2,
154 = 6.64, p = 0.008, 2-way RM ANOVA; post hoc paired t-test p = 0.002; Fig. 27B, C and E). The effects of DS2 and etomidate on the Hz EEG signature were unaffected by the T-type Ca 2+ channel blocker TTA-P2 (post hoc paired t-test, both p > 0.433; Fig. 27E). There were no changes in the dominant frequency of the Hz band during transitions into non-rem sleep with acsf, etomidate, or DS2 at the thalamus (all F 2, 16 < 1.95, p > 0.176, 2-way RM ANOVA; Fig. 27D and F).
155 138 Figure 27. Microperfusion of etomidate or DS2 into the thalamus of wild-type mice amplifies an electrocortical signature characterizing transitions into NREM sleep. Exemplar EEG recordings from a mouse undergoing a transition into NREM sleep during microperfusion of the thalamus with acsf (top) and A, etomidate (bottom) or B, DS2 (bottom). The vertical scale bar corresponds to the raw EEG traces and equals 100 µv. Black outlines on the spectrogram denote spectral regions that show significant alterations in power. Note the increase in power of the same high frequency EEG signature with both etomidate and DS2. C, Continuous power plot of the Hz EEG signature of NREM sleep transitions.
156 139 Note the increase in this power range with etomidate (yellow) and DS2 (blue) following entry into NREM sleep compared to the acsf control. D, Continuous plot of the dominant frequency in the Hz band during wake-nrem sleep transitions showing the general similarity in the dominant frequency across all treatments. E, Both etomidate (left) and DS2 (right) increase Hz power following transitions into NREM sleep. Co-administration of TTA-P2 with etomidate or DS2 does not significantly affect the increase in Hz power after entry into NREM sleep. 2-way RM ANOVA. F, The dominant frequency (i.e., frequency at peak power) of the Hz band is not significantly altered by administration of etomidate or DS2 into the thalamus with or without co-application of TTA-P2. 2-way RM ANOVA. All data represent mean ± SEM. N = 9; *p < 0.05, **p < 0.01, and ***p < NREM: non-rem. 4.4 Discussion Here we show that the presence of the GABA A receptor targeting general anesthetic agent etomidate at the thalamus in vivo elicits a distinct electrocortical signature that is recapitulated by pharmacological enhancement of phasic extrasynaptic GABA A receptor-mediated (spillover) inhibition at the thalamus. Both the etomidate-induced and spillover-induced EEG signatures were restricted to non-rem sleep and not mediated by T-type Ca 2+ channel de-inactivation. The absence of this effect in states outside of non-rem sleep suggests that the mode of thalamic activity set-up by an initial sleep transition then allows etomidate at the thalamus to enhance GABAergic spillover inhibition and elicit the distinct electrocortical signature. Increased GABAergic burst input from the RTN to thalamocortical neurons during non-rem sleep can satisfy this role (Steriade et al., 1993b). In the clinical context, such a non-rem-like mode of activity would necessarily be first triggered by the sedating (i.e., initial sleep-promoting) properties of GABA A receptor targeting general anesthetic agents acting at various sites in the
157 140 neuraxis (Franks, 2008). The subsequent action of the general anesthetic at the thalamus would then elicit a thalamocortical network oscillation in the alpha-beta range, a pattern that signals anesthetic hypnosis (Ching et al., 2010; Purdon et al., 2013) and is thought to impede responsiveness to external stimuli (Ching and Brown, 2014). In this model, an initial sleep-like transition is a prerequisite to generate this electrocortical signature associated with anesthetic hypnosis. Microperfusion of etomidate into the thalamus of freely behaving mice elicited an increase in non-rem alpha-beta electrocortical activity, increased spindle-like oscillations, and decreased wakefulness. These findings with etomidate replicate previous results from our laboratory that formed the foundation for this study (Mesbah-Oskui et al., 2014), and we further demonstrate that none of these effects of etomidate are sensitive to blockade of T-type Ca 2+ channels by TTA-P2. Moreover, the same results with DS2 show that the effects elicited by etomidate at the thalamus can be achieved by pharmacological enhancement of phasic activation of thalamic extrasynaptic GABA A receptors. We previously showed that microperfusion of etomidate into the thalamus of freely behaving Gabrd -/- mice elicits alterations in electrocortical activity that mimic those identified in wild-type mice (Mesbah-Oskui et al., 2014). This finding shows that extrasynaptic δgaba A receptors are not necessary in mediating the electrocortical signature produced by etomidate at the thalamus. Importantly, this finding does not eliminate a role for other non-δ subunitcontaining extra- or peri-synaptic GABA A receptors in mediating the electrocortical signature elicited by etomidate. Compensatory changes in GABA A receptor subunit expression in Gabrd -/- mice have been reported (Tretter et al., 2001; Korpi et al., 2002; Peng et al., 2002). Of particular relevance, Gabrd -/- mice exhibit greater co-assembly of α 4 and γ 2 GABA A receptor subunits as
158 141 indicated through immunoprecipitation (Korpi et al., 2002), suggesting the formation of extrasynaptic GABA A receptors with atypical subunit composition in Gabrd -/- mice. Additionally, tonic GABA A receptor-mediated inhibition persists in Gabrd -/- mice, albeit attenuated, indicating the presence of other functional extrasynaptic GABA A receptors (Cope et al., 2009). The changes in IPSP duration identified in vitro with DS2 and etomidate require expression of extrasynaptic GABA A receptors, identifying a role for spillover inhibition in mediating these changes (Herd et al., 2013; Herd et al., 2014; Rovo et al., 2014). Moreover, the effects of DS2 and etomidate on the activity of thalamocortical neurons in vitro are similar to each other (Herd et al., 2013; Herd et al., 2014), consistent with the in vivo findings with DS2 and etomidate in the present report. Specifically, whole cell recordings from the ventrobasal complex of the thalamus identify similar alterations in RTN-mediated IPSPs with DS2 and etomidate; both DS2 and etomidate are associated with increased IPSP decay times and charge transfer during presynaptic RTN spike bursts (Herd et al., 2013; Herd et al., 2014; Rovo et al., 2014). The potentiation of GABA responses and alterations in IPSP duration identified with DS2 and etomidate in vitro satisfy the conditions required by a computational model that shows such alterations in GABAergic thalamic signaling can precipitate the changes in cortical activity typically associated with loss-of-consciousness with propofol (Ching et al., 2010), another prototypic GABA A receptor-targeting intravenous general anesthetic agent. Importantly, the findings of our study indicate that the increase in alpha-beta electrocortical activity observed during the induction and maintenance of anesthesia (Ching et al., 2010; Purdon et al., 2013) can be effectively triggered by the action of the anesthetic at the thalamus, with the stipulation that
159 142 the thalamus is receiving non-rem-like inputs as the effects were restricted to non-rem sleep (Figs. 21, 25 and 27). The findings with THIP, an agent that directly activates δgaba A receptors, and the T- type Ca 2+ channel blocker TTA-P2 indicate that alterations in thalamocortical activity that are T- type Ca 2+ channel-dependent contribute to the changes in electrocortical activity identified with THIP in vivo. This suggestion is consistent with previous results obtained in vitro (Cope et al., 2005). Co-administration of TTA-P2 with THIP further increased slow electrocortical activity. This potentiating effect of TTA-P2 on slow EEG activity may reflect a diminution of thalamic activity, as THIP hyperpolarizes thalamocortical neurons to levels that can trigger a switch from tonic firing to T-type Ca 2+ channel-dependent burst firing (Cope et al., 2005). Blockade of T-type Ca 2+ channels with TTA-P2 during microperfusion of the thalamus with DS2 or etomidate did not alter any of the electrocortical effects elicited by DS2 or etomidate alone. These findings indicate that T-type Ca 2+ channel-independent alterations in thalamocortical activity underlie the changes in cortical activity identified with DS2 and etomidate. The lack of effect of TTA-P2 on the effects identified with etomidate is consistent with a previous study showing the normal onset of propofol-induced anesthesia/hypnosis in mice that lacked Ca v 3.1 (α 1G ) T-type Ca 2+ channels (Petrenko et al., 2007). These channels are strongly expressed in thalamocortical relay neurons and are necessary for burst firing in these neurons (Talley et al., 1999; Kim et al., 2001). Both DS2 and etomidate at the thalamus elicited an increase in spindle-like oscillations, a signal that characterizes light non-rem sleep. These oscillations are generated by the thalamus and are thought to require activation of thalamocortical T-type Ca 2+ channels for their initiation and propagation (Steriade et al., 1993b). Importantly, the present findings with TTA-P2 are
160 143 consistent with a recent study where normal spindle density and durations were identified in mice lacking Ca v 3.1 channels with no thalamocortical burst firing (Lee et al., 2013). Co-administration of TTA-P2 with etomidate potentiated the etomidate-induced alterations in sleep-wake behaviour. This finding is consistent with in vitro studies that identify etomidate as a blocker of T-type Ca 2+ channels (Todorovic et al., 2000; Joksovic et al., 2005). Importantly, the concentrations of etomidate associated with T-type Ca 2+ channel blockade are significantly above the clinically relevant range (IC 50 of approximately 161 µm) and so it is unlikely that the effective concentrations of etomidate used in our study resulted in any direct effect of etomidate on thalamic T-type Ca 2+ channels. It is possible, however, that the concomitant blockade of thalamic T-type Ca 2+ channels during microperfusion of etomidate into the thalamus mimicked conditions of elevated etomidate concentration and, thus, facilitated the effect of etomidate on sleep-wake behaviour. While co-administration of TTA-P2 with etomidate potentiated the etomidate-induced alterations in sleep-wake behaviour, TTA-P2 had the opposite effect when it was coadministered with DS2. Specifically, co-administration of TTA-P2 with DS2 restored the amount of wakefulness to levels that were comparable to baseline. Additionally, the increase in non- REM sleep seen with DS2 at the thalamus was attenuated with TTA-P2 (i.e., the amount of non- REM sleep associated with DS2 and TTA-P2 at the thalamus was not significantly different from the amount associated with DS2 alone or at baseline). These findings suggest that the alterations in sleep-wake behaviour triggered by DS2 at the thalamus may require T-type Ca 2+ channel deinactivation to occur. The molecular mechanisms underlying this differential effect of TTA-P2 on sleep-wake behaviour when co-administered with etomidate versus DS2 are unclear and require further study. It is possible that the effect of etomidate on sleep-wake behaviour is
161 144 mediated through its actions on GABA A receptors that are not sensitive to DS2 and do not require T-type Ca 2+ activity to influence sleep-wake behaviour. Nonetheless, given the TTA-P2-insensitive increase in alpha-beta frequency electrocortical activity and spindle-like oscillations and the association of these respective electrocortical patterns with anesthetic-induced loss-of-consciousness and decreased arousal, we anticipate that mice receiving DS2 (and etomidate) into the thalamus have an elevated threshold for arousal from non-rem sleep (Pivik et al., 1999; Wimmer et al., 2012; Purdon et al., 2015). Conversely, given the reduction in spindle-like oscillations in mice receiving THIP into the thalamus we anticipate that these mice exhibit unaltered or modestly reduced arousal thresholds. Future experiments that systematically assess arousal threshold in mice during such intrathalamic manipulations will confirm or refute these hypotheses and provide further insight on the clinical significance of the electrocortical signatures and alterations in sleep-wake behaviour that were identified in our studies. In summary, the findings in this report identify a site and mechanism of action by which a prototypic GABA A receptor targeting general anesthetic can elicit the distinctive brain wave signature that accompanies anesthetic hypnosis in humans. Future studies that characterize the functional relationship between enhanced thalamic GABAergic spillover-inhibition and cortical connectivity will further elucidate the neural correlates of consciousness and increase understanding of how general anesthetic agents trigger the reversible state of unconsciousness.
162 145 Chapter 5 General Discussion This thesis presents six major findings: 1) Enhanced GABAergic tonic inhibition in the thalamus elicits electrocortical signatures indicative of deep non-rem sleep; 2) The electrocortical patterns associated with enhanced thalamic tonic inhibition require normal thalamic T-type Ca 2+ channel activity to manifest; 3) The action of the general anesthetic etomidate at the thalamus is sufficient to elicit the changes in electrocortical activity that signal anesthetic-induced loss-ofconsciousness with GABA A receptor-targeting general anesthetic agents; 4) The changes in electrocortical activity triggered by the action of etomidate at the thalamus do not require extrasynaptic δgaba A receptors; 5) Thalamic T-type Ca 2+ channel de-inactivation is not required to mediate the changes in electrocortical activity that are elicited by etomidate in the thalamus; and 6) Enhanced GABAergic spillover inhibition in the thalamus fully recapitulates the changes in electrocortical activity that are elicited by etomidate at the thalamus. The implications of these findings with regard to electrocortical activity and the anesthetic mechanisms mediating loss-of-consciousness will be discussed in further detail in the following sections. 5.1 GABA A receptor mediated tonic inhibition in the thalamus We show that enhanced thalamic tonic inhibition mediated through extrasynaptic δgaba A receptors promotes electrocortical signatures that are associated with deep non-rem sleep in vivo. Specifically, enhanced thalamic tonic inhibition elicited a state-independent (i.e., these
163 146 changes were present during both wakefulness and non-rem sleep) increase in 1-4 Hz electrocortical activity and decrease in faster frequency activity and also decreased spindle-like oscillations during non-rem sleep. Importantly, systemic administration of THIP is associated with increased 1-4 Hz electrocortical activity in animals and humans (Lancel and Faulhaber, 1996; Faulhaber et al., 1997; Vyazovskiy et al., 2005; Winsky-Sommerer et al., 2007). Given the similarity between our findings and those that are identified with THIP administered systemically, it appears that the action of THIP at the thalamus may play a major role in mediating its effects on electrocortical activity. Treatment of thalamocortical neurons with THIP elicits an increase in tonic inhibition, which is further associated with a switch from a tonic action potential firing mode to a burst action potential firing mode in vitro (Cope et al., 2005) (see Fig. 7 for a summary on tonic versus burst mode of activity in thalamic neurons). Burst firing in thalamocortical neurons requires deinactivation of T-type Ca 2+ channels (Suzuki and Rogawski, 1989). Moreover, such burst firing in the thalamus is associated with 1-4 Hz electrocortical activity (Steriade et al., 1993b; Kim et al., 2001; Lee et al., 2004a). Thus, it is not surprising that blockade of T-type Ca 2+ channel activity significantly altered the changes in electrocortical activity associated with enhanced thalamic tonic inhibition. What does require further discussion, however, is the nature of the electrocortical changes seen with T-type Ca 2+ channel blockade during conditions of enhanced thalamic tonic inhibition with THIP. Rather than elicit a return of 1-4 Hz electrocortical activity to baseline levels, we identified a further potentiation of 1-4 Hz activity with T-type Ca 2+ channel blockade under conditions of elevated thalamic tonic inhibition. This pronounced increase in 1-4 Hz electrocortical activity (approximately % greater than baseline values, and 20 % greater
164 147 than values associated with enhanced thalamic tonic inhibition) is consistent with the changes in cortical activity that are associated with inactivation of thalamocortical neurons. Specifically, silencing of thalamocortical neuron firing is consistently associated with elevated 1-4 Hz electrocortical activity (David et al., 2013; Herrera et al., 2015; Lewis et al., 2015). Thus, it is likely that blockade of T-type Ca 2+ channel activity under conditions of enhanced thalamic tonic inhibition, where thalamocortical neurons are hyperpolarized and would necessarily enter into a T-type Ca 2+ channel-dependent burst firing mode, effectively impedes action potential firing in thalamocortical neurons. This would mean that glutamatergic input from thalamocortical neurons to corticothalamic neurons would be absent, thus removing a significant source of excitatory drive to the cortex. In the absence of thalamocortical input, recurrent signaling in the cortex is impaired and the slow rhythmic activity that cortical neurons intrinsically exhibit is favoured (Connors et al., 1982; Amzica and Steriade, 1998; Reinhold et al., 2015). THIP administered systemically elicits an increase in the amount of non-rem sleep in rats (Lancel and Faulhaber, 1996). In humans, THIP promotes slow wave sleep (i.e., deep non- REM sleep) and decreases sigma power, which suggests a reduction in the density of sleep spindles and is consistent with our findings with THIP in the ventrobasal complex of mice (Faulhaber et al., 1997). Systemic administration of THIP in mice is associated with increased slow wave (0.5-4 Hz) activity (Vyazovskiy et al., 2005). Importantly, this increase was present during both wakefulness and non-rem sleep, recapitulating our findings with THIP at the thalamus. The doses of systemically administered THIP (4 mg/kg and 6 mg/kg) that were associated with increased Hz electrocortical activity in mice also elicited low frequency (0.5-1 Hz) sharp-wave discharges. We did not identify any sharp-wave discharges in the EEG of mice that received THIP into the thalamus, suggesting that the concentrations we used were not
165 148 great enough to elicit sharp-wave discharges and/or the action of THIP at other brain regions is required to trigger sharp-wave discharges in the cortex. Systemic administration of THIP in mice is also associated with a reduction in the amount of REM sleep (Vyazovskiy et al., 2005). While we did not identify a significant reduction in REM sleep with THIP microperfused directly into the thalamus of mice, a trend was evident. Indeed a number of mice did not exhibit any REM sleep during microperfusion of the thalamus with THIP, suggesting that enhanced thalamic tonic inhibition impairs entry into and/or maintenance of REM sleep. It is possible that tonic action potential firing in the thalamus is permissive to REM sleep since this mode of thalamocortical firing is associated with cortical activity at higher frequencies that are typically associated with REM sleep and wakefulness. Thus, the burst action potential firing mode that appears to underlie the changes in electrocortical activity associated with enhanced thalamic tonic inhibition may impede the generation of the cortical activity patterns (particularly elevated theta frequency activity) indicative of REM sleep. In summary, our findings regarding the relationship between tonic inhibition in the thalamus and electrocortical activity indicate that thalamic tonic inhibition may play a functional role in mediating the depth of non-rem sleep. Deep non-rem sleep is characterized by increased 1-4 Hz electrocortical activity and is considered functionally restorative, largely because the incidence of this type of non-rem sleep increases following sleep deprivation in humans and animals (Borbély and Neuhaus; Borbély et al., 1981). Moreover, deep non-rem sleep has been suggested to play a role in the consolidation of explicit memories (Maquet, 2001). Thus, our findings suggest that pharmaceutical agents designed to target and activate extrasynaptic GABA A receptors in the thalamus and increase tonic inhibition could effectively
166 149 enhance sleep quality, which would be therapeutically beneficial to patients who experience difficulty sleeping, poor quality sleep, and/or fragmented sleep. 5.2 GABA A receptor mediated spillover inhibition in the thalamus Our findings indicate that enhanced thalamic spillover inhibition can effectively recapitulate the electrocortical signature associated with anesthetic-induced loss-of-consciousness. This suggests that alterations in thalamic GABAergic spillover inhibition could play a critical role in orchestrating the changes in cortical signaling that elicit loss of awareness and perception during general anesthesia. To promote spillover inhibition we increased the sensitivity of δgaba A receptors to GABA using DS2. Both THIP and DS2 are recognized for their effects on δgaba A receptors (Stórustovu and Ebert, 2006; Wafford et al., 2009; Jensen et al., 2013). A significant limitation of the studies presented in this thesis is that they do not show that the electrocortical effects of DS2 were elicited directly through enhanced phasic activation of δgaba A receptors. Consequently, a role for altered tonic inhibition in eliciting the electrocortical changes seen with DS2 cannot be discounted. At the effective concentrations used in our studies (approximately µm for DS2 and 5-9 µm for THIP) DS2 exhibits approximately 7 times greater efficacy than THIP at ternary α4β3δ GABA A receptors expressed in Xenopus oocytes (Lee et al., 2016). Importantly, in vitro studies measuring tonic inhibition during treatment of ventrobasal complex neurons with DS2 or THIP show that at concentrations similar to the effective concentrations used in this thesis, the tonic current elicited by DS2 is approximately 5 times less than the tonic current elicited by THIP (Cope et al., 2005; Wafford et al., 2009). Thus, the different
167 150 electrocortical effects associated with DS2 versus THIP may be due to DS2 having a more modest or pronounced effect on tonic inhibition relative to THIP. Importantly, however, given the distinct EEG signatures elicited by DS2 and THIP, along with the lack of effect of TTA-P2 on the electrocortical effects elicited by DS2 and the in vitro studies identifying a robust effect of DS2 on spillover inhibition in thalamocortical neurons, our findings are most consistent with DS2 eliciting its effects on electrocortical activity by preferentially promoting phasic activation of δgaba A receptors on thalamocortical neurons (Herd et al., 2013; Herd et al., 2014; Rovo et al., 2014) Factors that could favour spillover inhibition over tonic inhibition GABA A receptor mediated spillover inhibition is characterized by the phasic activation of periand extrasynaptic GABA A receptors, which are typically associated with a continuous, tonic form of inhibition (Belelli et al., 2009; Herd et al., 2013; Rovo et al., 2014) (for a brief summary of the types of GABA A receptor-mediated inhibition present in the thalamus see Fig. 11). The conditions that could promote phasic rather than tonic activation of these receptors remain unclear. Our findings suggest that phasic activation of extrasynaptic δgaba A receptors in the thalamus can be promoted under conditions where synaptic GABA release is elevated, as is seen during natural non-rem sleep. During non-rem sleep GABAergic neurons of the RTN typically enter into a burst mode of action potential firing, which means that thalamocortical neurons are exposed to transient, high frequency barrages of GABA (Steriade et al., 1993b; McCormick and Bal, 1997). This form of GABAergic inhibition in the thalamus could have a saturating effect, recruiting synaptic, perisynaptic, and extrasynaptic GABA A receptors.
168 151 Temporally coupling the activation of these distinct GABA A receptor families may contribute to the slowed kinetics of the IPSCs that characterize spillover GABAergic inhibition. GABA current kinetics can also be altered through the phosphorylation and dephosphorylation of the GABA A receptor (Brandon et al., 2002; Mody and Pearce, 2004). Indeed, the intracellular domain of GABA A receptors can be phosphorylated by a variety of different kinases that target serine, threonine, and tyrosine residues (Moss et al., 1992; McDonald and Moss, 1994). Studies have shown that the GABA A receptor β subunit is particularly sensitive to phosphorylation by serine/threonine kinases (Poisbeau et al., 1999; Hinkle and Macdonald, 2003). Moreover, enhanced phosphorylation of native GABA A receptors in excised patches elicits shortened IPSCs by promoting faster desensitization (Jones and Westbrook, 1997). This effect of GABA A receptor phosphorylation on IPSC duration was posited to result from a destabilization of the GABA binding site and changes in GABA A receptor gating (Jones and Westbrook, 1997). Alterations in intracellular Ca 2+ concentrations have also been implicated in mediating IPSC kinetics (Mody et al., 1991; Soltesz and Mody, 1995). Specifically, blockade of Ca 2+ release in hippocampal neurons eliminated the prolongation of IPSCs typically elicited by halothane in these neurons (Mody et al., 1991). Thus, elevations in intracellular Ca 2+ concentration may promote phasic activation of peri- and extrasynaptic GABA A receptors and result in IPSCs with a longer decay constant (i.e., prolonged IPSCs). Our results using TTA-P2 indicate that T-type Ca 2+ channel mediated increases in intracellular Ca 2+ levels are not critical in promoting spillover inhibition in thalamocortical neurons. The results with TTA-P2 do not, however, exclude a role for other channels/receptors that contribute in regulating intracellular
169 152 Ca 2+ levels in mediating phasic versus tonic activation of peri- and extrasynaptic GABA A receptors. It is also possible that GABA A receptors with distinct receptor subunit compositions underlie tonic and spillover inhibition. While our findings identified that the electrocortical effects elicited by both DS2 and THIP required δgaba A receptors, they do not provide insight on the remaining subunit composition of these receptors. GABA A receptors are pentameric and are typically comprised of 2 α subunits, 2 β subunits, and either a γ subunit (i.e., commonly synaptic GABA A receptor) or a δ subunit (i.e., extrasynaptic GABA A receptor) (Olsen and Sieghart, 2009). The effects of DS2 and THIP on GABA A receptor activity are largely influenced by expression of the δ GABA A receptor subunit, as assessed in heterologous systems using recombinant human GABA A receptors (Wafford et al., 2009; Meera et al., 2011; Jensen et al., 2013). The effects of DS2 are also influenced by the type of α GABA A receptor subunit expressed. Specifically, DS2 has a maximal effect in GABA A receptors that contain the δ GABA A receptor subunit in combination with the α4 or α6 GABA A receptor subunits (Jensen et al., 2013). Moreover, the type of β GABA A receptor subunit expressed does not significantly influence the effect of DS2 on GABA A receptor activity (more specifically the effects of DS2 were comparable across receptors that expressed either the β2 or β3 GABA A receptor subunit) (Jensen et al., 2013). In contrast, low micromolar concentrations of THIP (consistent with the effective concentrations used in our studies) appear to selectively target δ-subunit containing GABA A receptors and are not markedly influenced by the type of α GABA A receptor subunit expressed, (also assessed in heterologous expression systems using recombinant human GABA A receptors) (Stórustovu and Ebert, 2006; Meera et al., 2011; Lee et al., 2016). Immunolabelling
170 153 and immunoprecipitation, however, indicate a strong coupling of δ GABA A receptor subunits with α4 GABA A receptor subunits, suggesting that DS2 and THIP likely target GABA A receptors with the same subunit composition (or, at the very least, there is significant overlap in the receptors that DS2 and THIP target) (Pirker et al., 2000; Korpi et al., 2002). Thus, given the pharmacology of DS2 and THIP and the GABA A receptor subunit composition identified in the thalamus, it appears unlikely that different GABA A receptor subpopulations underlie the distinct electrocortical effects elicited by DS2 and THIP at the thalamus in our studies. It is quite possible that DS2 binding elicits conformational changes in δgaba A receptors that are distinct and unique from those elicited by the binding of THIP. Binding of DS2 to δgaba A receptors may result in a conformational change to the receptor that favours a GABAinduced phasic activation as opposed to a persistent, long-lived activation. It is also possible that binding of DS2 to the δgaba A receptor triggers a signaling cascade that results in chemical modifications to the receptor (like dephosphorylation or phosphorylation) that, again, favour phasic activation of these receptors. Importantly, given our findings and interpretations, we expect that such phasic activation of extrasynaptic GABA A receptors in the thalamus is not a widespread occurrence under normal, endogenous conditions Functional implications of enhanced thalamic spillover inhibition Signaling between the RTN and thalamocortical neurons plays a critical role in mediating synchronous activity in the cortex. Such interactions underlie the generation of sleep spindles, the tuning of slow, 1-4 Hz electrocortical activity, and are also implicated in triggering hypersynchronous signaling that is associated with pathological conditions such as absence epilepsy
171 154 (Steriade et al., 1993b; McCormick and Bal, 1997; Huguenard and McCormick, 2007; Beenhakker and Huguenard, 2009). Moreover, the decay kinetics of IPSCs in the RTN has been implicated in influencing the synchronicity of thalamic signaling (Sohal et al., 2000; Huntsman and Huguenard, 2006). RTN neurons exhibit an intrinsic slow GABA A receptor-mediated current, like the one that characterizes GABAergic spillover inhibition (Zhang et al., 1997). Thus, promotion of spillover inhibition in thalamocortical neurons could effectively couple thalamocortical activity to the activity of RTN neurons that exhibit slow, GABA-induced, IPSPs. The RTN expresses only the α3, β1, β3, and γ2 GABA A receptor subunits at significant levels (Pirker et al., 2000). Thus, the RTN is not associated with a great deal of heterogeneity in GABA A receptor composition (relatively speaking). Despite the limited repertoire of GABA A receptor subunits, neurons in the RTN exhibit varied IPSC kinetics (Huntsman and Huguenard, 2006). RTN neurons that exhibit slowly decaying IPSCs have been identified throughout the RTN and do not appear to exhibit any spatial specificity (Huntsman and Huguenard, 2006). Slowly decaying IPSCs are identified more frequently in RTN neurons that lack expression of the β1 GABA A receptor subunit (Huntsman and Huguenard, 2006). Moreover, it is speculated that GABA A receptors that contain the β3 GABA A receptor subunit underlie slowly decaying IPSCs in the RTN, as RTN neurons from mice that lack the β3 GABA A receptor subunit exhibit only fast (and lower amplitude) IPSCs (Huntsman et al., 1999). These knockout mice also exhibit hyper-synchronous oscillatory network activity in the thalamus, which resembles epileptiform activity that is associated with absence epilepsy (Inoue et al., 1993; Huntsman et al., 1999). Modeling studies have identified that IPSC decay kinetics in the RTN can critically influence synchronous activity in the thalamus (Sohal et al., 2000). Specifically, it has been
172 155 shown that slow IPSCs in the RTN play a preventive role in the genesis of epileptiform activity (Sohal et al., 2000). Additionally, normally occurring fast IPSCs in RTN neurons are thought to contribute to the voltage-dependent 40 Hz endogenous rhythm identified in RTN neurons in vivo, which is linked behaviourally to focused arousal (Pinault and Deschenes, 1992; Huntsman and Huguenard, 2006). Intracellular recordings from neurons in the RTN identify intrinsic firing of bursts of action potentials in the spindle frequency range (Steriade et al., 1993b; McCormick and Bal, 1997). Given the connectivity of the RTN (see section 1.1 Anatomy and connectivity of the thalamus for more detail) these bursts of action potentials effectively inhibit large populations of thalamocortical neurons in addition to RTN neurons (because of extensive reciprocal connectivity between neurons in the RTN). Traditionally, it was speculated that this rhythmic activity was then propagated through the rest of the thalamus through T-type Ca 2+ channeldependent burst firing of thalamocortical neurons (Steriade et al., 1993b; McCormick and Bal, 1997). Specifically, the bursts of inhibition from the RTN were thought to then hyperpolarize thalamocortical neurons to levels that de-inactivated T-type Ca 2+ channels and triggered burst firing. Thalamocortical action potential burst firing would then excite RTN neurons and cortical neurons and the signaling loop would propagate. However, the findings from our studies (see section Results and Fig. 23 for details) and those of others (Lee et al., 2013) provide compelling evidence that thalamocortical T-type Ca 2+ channel de-inactivation is not required to trigger sleep spindles and/or spindle frequency activity in the cortex. We propose, instead, that alterations in the kinetics of thalamocortical IPSPs, which can be achieved through enhanced GABAergic spillover inhibition, may underlie the propagation of spindle-like oscillations in the thalamocortico-corticothalamic system. Specifically, if thalamocortical neurons possess IPSPs with similar decay kinetics to RTN neurons, their subsequent repolarization and action potential
173 156 firing would temporally coincide with RTN neuronal repolarization. Given the reciprocal connectivity between thalamocortical neurons and RTN neurons, such a mode of activity in the thalamus would effectively reinforce signaling within the spindle frequency range. We posit that the action of general anesthetics on this signaling pathway elicits the increase in alpha-beta frequency (which overlaps with spindle frequencies) activity in the cortex and this signature ultimately belies a breakdown in the relay and integration of sensory information through the thalamus to the cortex. 5.3 GABA A receptor targeting general anesthetics at the thalamus Our findings show that the action of etomidate, a prototypic GABA A receptor targeting general anesthetic agent, at the thalamus is sufficient to elicit the changes in electrocortical activity that are associated with anesthetic-induced sedation and loss-of-consciousness. This electrocortical signature was most prominent during non-rem sleep. Similarly, enhanced thalamic spillover inhibition elicited the same signature and its effects were also restricted to non-rem sleep. That the effect of etomidate at the thalamus is dependent on sleep-wake state suggests that there is a state-dependent mode of activity that is required in the thalamus for etomidate to exert an effect. Specifically, our findings identify that a non-rem mode of activity is required in the thalamus for etomidate to have a major effect on electrocortical activity. This finding is consistent with a number of studies that have identified a significant association between neuronal activity in arousal-associated nuclei and anesthetic-induced loss-of-consciousness (or loss-of-righting reflex in animals).
174 General anesthesia and non-rem sleep It has been approximately twenty years since The Shared Circuits hypothesis for sleep and general anesthesia was originally proposed (Lyidc and Biebuyck, 1994; Lydic, 1996; Lydic and Baghdoyan, 2005). There have since been numerous studies in humans, non-human primates, cats, and rodents that have indicated or identified a significant role for sleep-regulating circuitry in mediating general anesthesia, supporting The Shared Circuits hypothesis. The findings from a number of these studies are discussed in more detail below. Brain imaging studies in humans have identified similarities in brain activity patterns during non-rem sleep and anesthetic-induced loss-of-consciousness with certain kinds of general anesthetic agents (primarily targeting GABA A receptors) (Braun et al., 1997; Kajimura et al., 1999; Kaisti et al., 2003; Laureys et al., 2004). These similarities include deactivation of the thalamus, as well as a number of other brain regions that mediate sleep-wake state such as the brainstem and basal ganglia. Behavioural studies in rodents offer further evidence for a link between non-rem sleep and general anesthesia. Depriving rats of sleep, through the activation of a rotating disk once non-rem sleep is detected (through EEG recordings), is associated with a decrease in the latency to propofol- and isoflurane-induced loss-of-righting reflex (Tung et al., 2002). Furthermore, these sleep-deprived rats also exhibit an increased latency to recovery-ofrighting reflex following anesthetic hypnosis with propofol and isoflurane (Tung et al., 2002). Cats anesthetized with halothane exhibit a significant increase in spindle-like oscillations with increasing anesthetic-depth (Keifer et al., 1994). This increase in spindle-like activity during halothane-induced hypnosis was also associated with a reduction in acetylcholine release from the medial pontine reticular formation, similar to normal non-rem sleep (Keifer et al., 1994). Additionally, administration of the acetylcholinesterase inhibitor, physostigmine (which
175 158 would serve to elevate acetylcholine concentrations) during propofol-induced hypnosis in humans is sufficient to elicit recovery of consciousness (Meuret et al., 2000). Together, these studies suggest that elevated cholinergic tone, such as would be seen during wakefulness and REM sleep (Celesia and Jasper, 1966; Jasper and Tessier, 1971; Marrosu et al., 1995), could possibly antagonize general anesthetic-induced up-regulation of spindle-like activity and promote emergence from general anesthesia. Pharmacological manipulation of arousal-associated nuclei in the brainstem and posterior hypothalamus provide further evidence that indicates a role for these regions in mediating anesthetic-induced loss-of-consciousness. In vivo microinjection of relatively high concentrations of pentobarbital and other GABAergic agents into the oral pontine reticular formation and deep mesencaphlic nucleus (which send excitatory projections to the thalamus) elicits loss-of-righting reflex and EEG synchronization in rats (Devor and Zalkind, 2001; Sukhotinsky et al., 2007). It is unclear whether the association between elevated GABAergic signaling in the oral pontine reticular formation and loss-of-righting reflex identified by Devor and Zalkind bears any functional relevance to general anesthetic-induced loss-of-righting reflex. Specifically, while several other studies have identified an association between oral pontine reticular formation activity and anesthetic-induced hypnosis, measurements of GABA levels in the oral pontine reticular formation during isoflurane- and propofol- induced loss-of-righting reflex in cats and rats, respectively, indicate a reduction in GABA concentration (Vanini et al., 2008; Vanini et al., 2014). Moreover, GABA levels in the oral pontine reticular formation are highest during wakefulness and lower during REM sleep in freely-behaving cats (Vanini et al., 2011). Increasing GABA levels in the oral pontine reticular formation by reverse microdialysis of a GABA uptake inhibitor in rats is associated with increased duration to isoflurane- and propofol-induced loss-of-righting reflex, while reducing GABA levels by reverse microdialysis
176 159 of a GABA synthesis inhibitor into the oral pontine reticular formation is associated with reduced duration to isoflurane- and propofol-induced loss-righting-reflex (Vanini et al., 2008; Vanini et al., 2014). These findings indicate that alterations in GABAergic tone at the oral pontine reticular formation can functionally influence anesthetic hypnosis in a manner that is consistent with endogenous sleep-state dependent changes in GABA levels. Elevated δgaba A receptor activity in the oral pontine reticular formation is implicated in mediating the GABA-induced increase in wakefulness and resistance to isoflurane-induced loss-of-righting reflex (Vanini and Baghdoyan, 2013). The network-level mechanisms mediating this GABA-induced increase in wakefulness at the oral pontine reticular formation are unclear as glutamatergic signaling from the oral pontine reticular formation, which would presumably be dampened by GABA, promotes wakefulness. It is worth noting that microinjection of the wakepromoting neuropeptide orexin A (also known as hypocretin-1) into the oral pontine reticular formation elicits a significant increase in extracellular GABA levels consistent with what is normally seen in this brain region during wakefulness (Watson et al., 2008). Histaminergic neurons in the wake-promoting tuberomammillary nucleus also exhibit significant alterations in their activity during general anesthesia. Specifically, these neurons exhibit a significant reduction in activity during anesthetic-induced loss-of-consciousness (Mammoto et al., 1997; Nelson et al., 2002). Bilateral microinjection of muscimol, an agent that directly activates GABA A receptors, into the tuberomammillary nucleus is sufficient to elicit loss-of-righting reflex in rodents (Nelson et al., 2002; Nelson et al., 2003). Conversely, microinjection of gabazine, a GABA A receptor antagonist, into the tuberomammillary nucleus is associated with resistance to dexmedetomidine-induced (a sedative agent that targets α2- adrenoceptors) loss-of-righting reflex (Nelson et al., 2002; Nelson et al., 2003). It is also worth
177 160 noting that microinjection of propofol into the tuberomammillary nucleus failed to elicit loss-ofrighting reflex, indicating a necessary role for other brain regions in mediating anestheticinduced loss-of-consciousness (Nelson et al., 2002). Noradrenergic neurons in the wake-promoting locus coeruleus also exhibit a decrease in activity with the general anesthetic agent halothane and sedative agent dexmedetomidine (Correa-Sales et al., 1992; Sirois et al., 2000). Further evidence supporting a role for endogenous sleep-wake pathways in mediating anesthetic-induced loss-of-consciousness is provided through studies examining the role of the sleep-promoting ventrolateral preoptic area during general anesthesia. Neurons in the ventrolateral preoptic area exhibit elevated activity during anestheticinduced sedation and loss-of-consciousness with a variety of different general anesthetic agents (Nelson et al., 2002; Nelson et al., 2003; Lu et al., 2008; Moore et al., 2012). Moreover, lesions to the ventrolateral preoptic area are associated with decreased sensitivity to isoflurane, as indexed by an increase in the latency to loss-of-righting reflex with isoflurane in rats (Moore et al., 2012). Thus, systemic administration of general anesthetics can trigger a non-rem-like mode of input to the thalamus by eliciting non-rem-like changes in brain activity, such as inhibiting arousal-associated nuclei and increasing neuronal activity in the sleep-promoting ventrolateral preoptic area of the hypothalamus. There must, however, be mechanisms that are distinct to anesthetic-induced loss-of-consciousness and, therefore, not seen during natural non-rem sleep as these states are clearly not the same. Our findings provide insight regarding the mechanism that distinguishes non-rem sleep from anesthetic-induced sedation and loss-of-consciousness and this distinction arises at the thalamus.
178 The thalamus and general anesthesia Our findings suggest that once a non-rem like mode of input to the thalamus has been achieved, as would occur by the direct and indirect actions of general anesthetics on the ascending arousal system, the condition is set up for general anesthetics to exert an effect on thalamocortical activity. Our findings further indicate that general anesthetics, particularly those that target GABA A receptors, promote spillover GABAergic inhibition in the thalamus. Enhanced spillover inhibition in the thalamus during non-rem sleep is sufficient to elicit the changes in electrocortical activity that signal anesthetic-induced loss-of-consciousness (with GABA A receptor targeting general anesthetics) when general anesthetics are administered systemically (Purdon et al., 2015). We posit that this is because enhanced spillover inhibition in thalamocortical neurons elicits a slow GABA A receptor-mediated current, as has been demonstrated in vitro, and that this current effectively tunes thalamocortical activity to the rhythmic alpha-beta signaling intrinsic to neurons of the RTN during non-rem like conditions (Steriade et al., 1993b; McCormick and Bal, 1997; Herd et al., 2013; Herd et al., 2014; Rovo et al., 2014) (Fig. 28). Under these conditions the thalamocortico-corticothalamic system is effectively locked in an alpha-beta frequency loop of activity. Thus, the flow of sensory information to the cortex through the thalamus would be impeded and this would ultimately promote loss-of-consciousness by decreasing both the available information to the cortex and the integration of this information across cortical areas. Future experiments assessing the necessity of this pathway in mediating anesthetic-induced loss-of-consciousness will be essential in validating this new hypothesis.
179 162 Figure 28. Proposed effect of GABA A receptor-targeting general anesthetics on the thalamus. A, General anesthetics directly and indirectly inhibit brain regions that promote arousal and wakefulness. Specifically, many general anesthetics have been shown to inhibit neuronal activity in the locus coeruleus (LC), and tuberomammillary nucleus (TMN) (Correa-Sales et al., 1992; Mammoto et al., 1997; Sirois et al., 2000; Nelson et al., 2002). Moreover, general anesthetics have also been shown to increase neuronal activity in the ventrolateral preoptic area (VLPO), which sends inhibitory projections to the dorsal raphé (DR), LC, oral pontine nucleus (PnO), and TMN (Nelson et al., 2002; Nelson et al., 2003; Lu et al., 2008; Moore et al., 2012). Thus, the net effect of general anesthetics on neuronal activity in brain regions associated with the regulation of sleep-wake state is a significant diminution of excitatory input to the thalamus. Black traces denote inhibitory projections. White traces denote excitatory projections. B, Similar to what is seen during non-rem sleep, during general anesthesia excitatory input from the ascending arousal system to the thalamus is significantly attenuated which creates conditions
Relevance of sleep neurobiology for cognitive neuroscience and anesthesiology
 1 Relevance of sleep neurobiology for cognitive neuroscience and anesthesiology Giancarlo Vanini, MD, Helen A. Baghdoyan, PhD, and Ralph Lydic, PhD Introduction Although general anesthetics are used for
1 Relevance of sleep neurobiology for cognitive neuroscience and anesthesiology Giancarlo Vanini, MD, Helen A. Baghdoyan, PhD, and Ralph Lydic, PhD Introduction Although general anesthetics are used for
COGNITIVE SCIENCE 107A. Sensory Physiology and the Thalamus. Jaime A. Pineda, Ph.D.
 COGNITIVE SCIENCE 107A Sensory Physiology and the Thalamus Jaime A. Pineda, Ph.D. Sensory Physiology Energies (light, sound, sensation, smell, taste) Pre neural apparatus (collects, filters, amplifies)
COGNITIVE SCIENCE 107A Sensory Physiology and the Thalamus Jaime A. Pineda, Ph.D. Sensory Physiology Energies (light, sound, sensation, smell, taste) Pre neural apparatus (collects, filters, amplifies)
EEG Sleep Circadian rhythms Learning Objectives: 121, 122
 EEG Sleep Circadian rhythms Learning Objectives: 121, 122 Zoltán Lelkes Electroencenphalography Hans Berger pen time amplifier electrodes 1 The waves of the EEG gamma > 30 Hz beta: 13-30 Hz Mental activity:
EEG Sleep Circadian rhythms Learning Objectives: 121, 122 Zoltán Lelkes Electroencenphalography Hans Berger pen time amplifier electrodes 1 The waves of the EEG gamma > 30 Hz beta: 13-30 Hz Mental activity:
Embryological origin of thalamus
 diencephalon Embryological origin of thalamus The diencephalon gives rise to the: Thalamus Epithalamus (pineal gland, habenula, paraventricular n.) Hypothalamus Subthalamus (Subthalamic nuclei) The Thalamus:
diencephalon Embryological origin of thalamus The diencephalon gives rise to the: Thalamus Epithalamus (pineal gland, habenula, paraventricular n.) Hypothalamus Subthalamus (Subthalamic nuclei) The Thalamus:
Page 1 L 58. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems /2013 RETICULAR FORMATION
 Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy
 Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07)
 NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
Nervous System, Neuroanatomy, Neurotransmitters
 Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Sleep. No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness,
 Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Sleep. No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness,
 Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may have as many as 200,000
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may have as many as 200,000
Basal Ganglia. Introduction. Basal Ganglia at a Glance. Role of the BG
 Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Thalamus and Sensory Functions of Cerebral Cortex
 Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Special Lecture 6 Effects of Sleep on Seizures ; A Path to sleep neurology (Somno-neurology)
 Special Lecture 6 Effects of Sleep on Seizures ; A Path to sleep neurology (Somno-neurology) Jun Kohyama, MD Tokyo Bay Urayasi/Ichikawa Medical Center Taiwan Pediatric Epilepsy Congress 2010 Dec. 26, 2010,
Special Lecture 6 Effects of Sleep on Seizures ; A Path to sleep neurology (Somno-neurology) Jun Kohyama, MD Tokyo Bay Urayasi/Ichikawa Medical Center Taiwan Pediatric Epilepsy Congress 2010 Dec. 26, 2010,
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Thalamo-Cortical Relationships Ultrastructure of Thalamic Synaptic Glomerulus
 Central Visual Pathways V1/2 NEUR 3001 dvanced Visual Neuroscience The Lateral Geniculate Nucleus () is more than a relay station LP SC Professor Tom Salt UCL Institute of Ophthalmology Retina t.salt@ucl.ac.uk
Central Visual Pathways V1/2 NEUR 3001 dvanced Visual Neuroscience The Lateral Geniculate Nucleus () is more than a relay station LP SC Professor Tom Salt UCL Institute of Ophthalmology Retina t.salt@ucl.ac.uk
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
The Neural Control of Behavioral State
 The Neural Control of Behavioral State Learning objectives: Introduction - Behavioral State; why do we sleep? A Clinical Case - The Sleeping Beauty of Oak Park. EEG - A Neural Signature of Behavioral State.
The Neural Control of Behavioral State Learning objectives: Introduction - Behavioral State; why do we sleep? A Clinical Case - The Sleeping Beauty of Oak Park. EEG - A Neural Signature of Behavioral State.
The Central Nervous System I. Chapter 12
 The Central Nervous System I Chapter 12 The Central Nervous System The Brain and Spinal Cord Contained within the Axial Skeleton Brain Regions and Organization Medical Scheme (4 regions) 1. Cerebral Hemispheres
The Central Nervous System I Chapter 12 The Central Nervous System The Brain and Spinal Cord Contained within the Axial Skeleton Brain Regions and Organization Medical Scheme (4 regions) 1. Cerebral Hemispheres
Central Neurocircuitry Functioning during the Wake-Sleep Cycle
 Chapter 1 OOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Central Neurocircuitry Functioning during the Wake-Sleep Cycle The
Chapter 1 OOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Central Neurocircuitry Functioning during the Wake-Sleep Cycle The
Posterior White Column-Medial Lemniscal Pathway
 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Thalamocortical Dysrhythmia. Thalamocortical Fibers. Thalamocortical Loops and Information Processing
 halamocortical Loops and Information Processing 2427 halamocortical Dysrhythmia Synonyms CD A pathophysiological chain reaction at the origin of neurogenic pain. It consists of: 1) a reduction of excitatory
halamocortical Loops and Information Processing 2427 halamocortical Dysrhythmia Synonyms CD A pathophysiological chain reaction at the origin of neurogenic pain. It consists of: 1) a reduction of excitatory
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Basic Science of Representative Normal Human EEG Potentials
 Basic Science of Representative Normal Human EEG Potentials Seyed M Mirsattari, MD, PhD, FRCPC Departments of Clinical Neurological Sciences, Medical Biophysics, Diagnostic Imaging, Psychology University
Basic Science of Representative Normal Human EEG Potentials Seyed M Mirsattari, MD, PhD, FRCPC Departments of Clinical Neurological Sciences, Medical Biophysics, Diagnostic Imaging, Psychology University
Chapter 14: Integration of Nervous System Functions I. Sensation.
 Chapter 14: Integration of Nervous System Functions I. Sensation A. General Organization 1. General senses have receptors a. The somatic senses provide information about & 1. Somatic senses include: a.
Chapter 14: Integration of Nervous System Functions I. Sensation A. General Organization 1. General senses have receptors a. The somatic senses provide information about & 1. Somatic senses include: a.
Thalamic nuclei. Each thalamus has several well defined borders: Introduction. Thalamus
 Thalamic nuclei Introduction For the successful completion of any task, some sort of recognition, identification and organisation is needed. Imagine what would happen if employees in a team would just
Thalamic nuclei Introduction For the successful completion of any task, some sort of recognition, identification and organisation is needed. Imagine what would happen if employees in a team would just
Brainstem. Steven McLoon Department of Neuroscience University of Minnesota
 Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Functioning of Circuits Connecting Thalamus and Cortex
 Functioning of Circuits Connecting Thalamus and Cortex S. Murray Sherman *1 ABSTRACT Glutamatergic pathways in thalamus and cortex are divided into two distinct classes: driver, which carries the main
Functioning of Circuits Connecting Thalamus and Cortex S. Murray Sherman *1 ABSTRACT Glutamatergic pathways in thalamus and cortex are divided into two distinct classes: driver, which carries the main
NEOCORTEX. Laminar pattern 6 layers billion neurons 95 % surface of the hemisphere
 THE CEREBRAL CORTEX NEOCORTEX Laminar pattern 6 layers 10 20 billion neurons 95 % surface of the hemisphere Six Layers of Cortex LGN input Parvo Magno B-Slide 4 NEOCORTEX, types of neurons Pyramidal neurons
THE CEREBRAL CORTEX NEOCORTEX Laminar pattern 6 layers 10 20 billion neurons 95 % surface of the hemisphere Six Layers of Cortex LGN input Parvo Magno B-Slide 4 NEOCORTEX, types of neurons Pyramidal neurons
Chemical Control of Behavior and Brain 1 of 9
 Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Neuroscience of Consciousness I
 1 C83MAB: Mind and Brain Neuroscience of Consciousness I Tobias Bast, School of Psychology, University of Nottingham 2 What is consciousness? 3 Consciousness State of consciousness - Being awake/alert/attentive/responsive
1 C83MAB: Mind and Brain Neuroscience of Consciousness I Tobias Bast, School of Psychology, University of Nottingham 2 What is consciousness? 3 Consciousness State of consciousness - Being awake/alert/attentive/responsive
pdf NIH Overview Back to Course Schedule (The material below on the neuron is adapted from:
 1 of 8 6/20/2012 10:25 AM Sleep and Dreams Sleep and the Brain pdf NIH Overview 3.2-3.3 Some Basic Background: Back to Course Schedule (The material below on the neuron is adapted from: http://vv.carleton.ca/~neil/neural/neuron-a.html)
1 of 8 6/20/2012 10:25 AM Sleep and Dreams Sleep and the Brain pdf NIH Overview 3.2-3.3 Some Basic Background: Back to Course Schedule (The material below on the neuron is adapted from: http://vv.carleton.ca/~neil/neural/neuron-a.html)
Sleep-Wake Cycle I Brain Rhythms. Reading: BCP Chapter 19
 Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
SLEEP AND AROUSAL: Thalamocortical Mechanisms
 Annu. Rev. Neurosci. 1997. 20:185 215 Copyright c 1997 by Annual Reviews Inc. All rights reserved SLEEP AND AROUSAL: Thalamocortical Mechanisms David A. McCormick and Thierry Bal 1 Section of Neurobiology,
Annu. Rev. Neurosci. 1997. 20:185 215 Copyright c 1997 by Annual Reviews Inc. All rights reserved SLEEP AND AROUSAL: Thalamocortical Mechanisms David A. McCormick and Thierry Bal 1 Section of Neurobiology,
from sleep to attention lecture 4 April 9, 2012 control of sleep/wake state production II
 from sleep to attention lecture 4 April 9, 2012 control of sleep/wake state production II "From the moment of my birth, the angels of anxiety, worry, and death stood at my side, followed me out when I
from sleep to attention lecture 4 April 9, 2012 control of sleep/wake state production II "From the moment of my birth, the angels of anxiety, worry, and death stood at my side, followed me out when I
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Brown EN, Lydic R, Schiff ND, et al. General anesthesia, sleep,
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Brown EN, Lydic R, Schiff ND, et al. General anesthesia, sleep,
P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center. Wednesday, 16 March 2009, 1:00p.m. 2:00p.m.
 Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
COMA BIOLOGY. Assist. Prof. Mehmet Akif KARAMERCAN Gazi University Faculty of Medicine Department of Emergency Medicine
 COMA BIOLOGY Assist. Prof. Mehmet Akif KARAMERCAN Gazi University Faculty of Medicine Department of Emergency Medicine Outlines Definitions Classification and Major Causes Arousal Systems (Reticular Activating
COMA BIOLOGY Assist. Prof. Mehmet Akif KARAMERCAN Gazi University Faculty of Medicine Department of Emergency Medicine Outlines Definitions Classification and Major Causes Arousal Systems (Reticular Activating
nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
 Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Neural Basis of Motor Control. Chapter 4
 Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Neuroscience: Exploring the Brain, 3e. Chapter 4: The action potential
 Neuroscience: Exploring the Brain, 3e Chapter 4: The action potential Introduction Action Potential in the Nervous System Conveys information over long distances Action potential Initiated in the axon
Neuroscience: Exploring the Brain, 3e Chapter 4: The action potential Introduction Action Potential in the Nervous System Conveys information over long distances Action potential Initiated in the axon
Clinical Electroencephalography for Anesthesiologists
 Review Article David S. Warner, M.D., Editor Clinical Electroencephalography for Anesthesiologists Part I: Background and Basic Signatures Patrick L. Purdon, Ph.D., Aaron Sampson, B.S., Kara J. Pavone,
Review Article David S. Warner, M.D., Editor Clinical Electroencephalography for Anesthesiologists Part I: Background and Basic Signatures Patrick L. Purdon, Ph.D., Aaron Sampson, B.S., Kara J. Pavone,
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Full file at TEST BANK. R.H. Ettinger. Eastern Oregon University. Psychopharmacology. 1/e. R.H. Ettinger
 TEST BANK R.H. Ettinger Eastern Oregon University Psychopharmacology 1/e R.H. Ettinger Eastern Oregon University Prentice Hall Boston Columbus Indianapolis New York San Francisco Upper Saddle River Amsterdam
TEST BANK R.H. Ettinger Eastern Oregon University Psychopharmacology 1/e R.H. Ettinger Eastern Oregon University Prentice Hall Boston Columbus Indianapolis New York San Francisco Upper Saddle River Amsterdam
Anatomy of the basal ganglia. Dana Cohen Gonda Brain Research Center, room 410
 Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
 BRAIN AND ITS VITAL FUNCTIONS 1 Brain and Its Vital Functions Student s Name Institution Name Professor s Name Course Title BRAIN AND ITS VITAL FUNCTIONS 2 The brain is the integral organism and all its
BRAIN AND ITS VITAL FUNCTIONS 1 Brain and Its Vital Functions Student s Name Institution Name Professor s Name Course Title BRAIN AND ITS VITAL FUNCTIONS 2 The brain is the integral organism and all its
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Basic Mechanism for Generation of Brain Rhythms
 203 Continuing Medical Education Basic Mechanism for Generation of Brain Rhythms Wei-Hung Chen Abstract- Study of the basic mechanism of brain rhythms adds to our understanding of the underlying processes
203 Continuing Medical Education Basic Mechanism for Generation of Brain Rhythms Wei-Hung Chen Abstract- Study of the basic mechanism of brain rhythms adds to our understanding of the underlying processes
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
10/3/2016. T1 Anatomical structures are clearly identified, white matter (which has a high fat content) appears bright.
 H2O -2 atoms of Hydrogen, 1 of Oxygen Hydrogen just has one single proton and orbited by one single electron Proton has a magnetic moment similar to the earths magnetic pole Also similar to earth in that
H2O -2 atoms of Hydrogen, 1 of Oxygen Hydrogen just has one single proton and orbited by one single electron Proton has a magnetic moment similar to the earths magnetic pole Also similar to earth in that
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Cortical Organization. Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:.
 Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
Teach-SHEET Basal Ganglia
 Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Organization of the nervous system. [See Fig. 48.1]
![Organization of the nervous system. [See Fig. 48.1] Organization of the nervous system. [See Fig. 48.1]](/thumbs/90/103926552.jpg) Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
THE CENTRAL NERVOUS SYSTEM. The Brain & Spinal Cord
 THE CENTRAL NERVOUS SYSTEM The Brain & Spinal Cord Review: Nervous System Parallel Distributed Processing Composition of the CNS Nuclei: Clusters of neurons in the CNS ( neighborhoods ) Fiber Tracts/Pathways:
THE CENTRAL NERVOUS SYSTEM The Brain & Spinal Cord Review: Nervous System Parallel Distributed Processing Composition of the CNS Nuclei: Clusters of neurons in the CNS ( neighborhoods ) Fiber Tracts/Pathways:
Basal Ganglia George R. Leichnetz, Ph.D.
 Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Objectives. brain pacemaker circuits role of inhibition
 Brain Rhythms Michael O. Poulter, Ph.D. Professor, Molecular Brain Research Group Robarts Research Institute Depts of Physiology & Pharmacology, Clinical Neurological Sciences Schulich School of Medicine
Brain Rhythms Michael O. Poulter, Ph.D. Professor, Molecular Brain Research Group Robarts Research Institute Depts of Physiology & Pharmacology, Clinical Neurological Sciences Schulich School of Medicine
COGS 107B. Week 7 Section IA: Ryan Szeto OH: Wednesday CSB Kitchen
 COGS 107B Week 7 Section IA: Ryan Szeto OH: Wednesday 1PM @ CSB Kitchen MT2: Tomorrow Question 1 NE: Locus coeruleus HA: Posterior hypothalamus ACh: Two pockets- located in pons and basal forebrain DA:
COGS 107B Week 7 Section IA: Ryan Szeto OH: Wednesday 1PM @ CSB Kitchen MT2: Tomorrow Question 1 NE: Locus coeruleus HA: Posterior hypothalamus ACh: Two pockets- located in pons and basal forebrain DA:
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
Announcement. Overview. Words Describing Sectional Planes. Words Describing Spatial Orientation. Explore! Basic neuroscience terminology
 Announcement Explore! The reading list is a good place to start, especially the Perspectives section. Overview Basic neuroscience terminology A Roadmap to the course Also, check out short articles in the
Announcement Explore! The reading list is a good place to start, especially the Perspectives section. Overview Basic neuroscience terminology A Roadmap to the course Also, check out short articles in the
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Chapter 3. Biological Processes
 Biological Processes Psychology, Fifth Edition, James S. Nairne What s It For? Biological Solutions Communicating internally Initiating and coordinating behavior Regulating growth and other internal functions
Biological Processes Psychology, Fifth Edition, James S. Nairne What s It For? Biological Solutions Communicating internally Initiating and coordinating behavior Regulating growth and other internal functions
Physiology of Sleep. Dr Nervana
 Physiology of Sleep Dr Nervana Objectives: 1. Explain the difference between sleep and coma. 2. Define NREM (non-rapid eye movement, SWS) and REM (rapid eye movement) sleep. 3. Describe how NREM and REM
Physiology of Sleep Dr Nervana Objectives: 1. Explain the difference between sleep and coma. 2. Define NREM (non-rapid eye movement, SWS) and REM (rapid eye movement) sleep. 3. Describe how NREM and REM
Cogs 107b Systems Neuroscience lec9_ neuromodulators and drugs of abuse principle of the week: functional anatomy
 Cogs 107b Systems Neuroscience www.dnitz.com lec9_02042010 neuromodulators and drugs of abuse principle of the week: functional anatomy Professor Nitz circa 1986 neurotransmitters: mediating information
Cogs 107b Systems Neuroscience www.dnitz.com lec9_02042010 neuromodulators and drugs of abuse principle of the week: functional anatomy Professor Nitz circa 1986 neurotransmitters: mediating information
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Nsci 2100: Human Neuroanatomy 2017 Examination 3
 Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Primary Functions. Monitor changes. Integrate input. Initiate a response. External / internal. Process, interpret, make decisions, store information
 NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
Lecture 8. Arousal & Sleep. Cogs17 * UCSD
 Lecture 8 Arousal & Sleep Cogs17 * UCSD Arousal in the Brain Stimulated by sensory input Initiated, maintained endogenously Basal Forebrain Delivers ACh throughout cortex Arousal in the Brain Lateral Hypothalamus
Lecture 8 Arousal & Sleep Cogs17 * UCSD Arousal in the Brain Stimulated by sensory input Initiated, maintained endogenously Basal Forebrain Delivers ACh throughout cortex Arousal in the Brain Lateral Hypothalamus
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa3.0/
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
EEG Electrode Placement
 EEG Electrode Placement Classifying EEG brain waves Frequency: the number of oscillations/waves per second, measured in Hertz (Hz) reflects the firing rate of neurons alpha, beta, theta, delta Amplitude:
EEG Electrode Placement Classifying EEG brain waves Frequency: the number of oscillations/waves per second, measured in Hertz (Hz) reflects the firing rate of neurons alpha, beta, theta, delta Amplitude:
Modeling synaptic facilitation and depression in thalamocortical relay cells
 College of William and Mary W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2011 Modeling synaptic facilitation and depression in thalamocortical relay cells Olivia
College of William and Mary W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2011 Modeling synaptic facilitation and depression in thalamocortical relay cells Olivia
Course Calendar
 Clinical Neuroscience BMS 6706C Charles, Ph.D., Course Director charles.ouimet@med.fsu.edu (850) 644-2271 2004 2005 Course Calendar Click here to return to the syllabus Meeting Hours for entire semester:
Clinical Neuroscience BMS 6706C Charles, Ph.D., Course Director charles.ouimet@med.fsu.edu (850) 644-2271 2004 2005 Course Calendar Click here to return to the syllabus Meeting Hours for entire semester:
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Psychology in Your Life
 Sarah Grison Todd Heatherton Michael Gazzaniga Psychology in Your Life SECOND EDITION Chapter 2 The Role of Biology in Psychology 1 2016 W. W. Norton & Company, Inc. 2.1 How Do Our Nervous Systems Affect
Sarah Grison Todd Heatherton Michael Gazzaniga Psychology in Your Life SECOND EDITION Chapter 2 The Role of Biology in Psychology 1 2016 W. W. Norton & Company, Inc. 2.1 How Do Our Nervous Systems Affect
2/22/2012. Cerebrum CNS
 Chapter 8 outline CNS: Consists of???? Structural organization of the brain Cerebrum Diencephalon Midbrain and hindbrain Spinal cord tracts Cranial and spinal nerves Receives input from???? neurons Directs
Chapter 8 outline CNS: Consists of???? Structural organization of the brain Cerebrum Diencephalon Midbrain and hindbrain Spinal cord tracts Cranial and spinal nerves Receives input from???? neurons Directs
Motor Functions of Cerebral Cortex
 Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Reticular Formation George R. Leichnetz, Ph.D.
 Reticular Formation George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the anatomical and functional organization of the brainstem reticular formation into three general regions: median (raphe), medial
Reticular Formation George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the anatomical and functional organization of the brainstem reticular formation into three general regions: median (raphe), medial
Anatomy and Physiology (Bio 220) The Brain Chapter 14 and select portions of Chapter 16
 Anatomy and Physiology (Bio 220) The Brain Chapter 14 and select portions of Chapter 16 I. Introduction A. Appearance 1. physical 2. weight 3. relative weight B. Major parts of the brain 1. cerebrum 2.
Anatomy and Physiology (Bio 220) The Brain Chapter 14 and select portions of Chapter 16 I. Introduction A. Appearance 1. physical 2. weight 3. relative weight B. Major parts of the brain 1. cerebrum 2.
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
Acetylcholine (ACh) Action potential. Agonists. Drugs that enhance the actions of neurotransmitters.
 Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Nervous System and Brain Review. Bio 3201
 Nervous System and Brain Review Bio 3201 Dont worry about: glial cells Oligodendrocytes Satelite cells etc Nervous System - Vital to maintaining homeostasis in organisms - Comprised of : brain, spinal
Nervous System and Brain Review Bio 3201 Dont worry about: glial cells Oligodendrocytes Satelite cells etc Nervous System - Vital to maintaining homeostasis in organisms - Comprised of : brain, spinal
CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX:
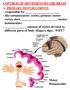 CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
b. The groove between the two crests is called 2. The neural folds move toward each other & the fuse to create a
 Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to
 CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Outline of the next three lectures
 Outline of the next three lectures Lecture 35 Anatomy of the human cerebral cortex gross and microscopic cell types connections Vascular supply of the cerebral cortex Disorders involving the cerebral cortex
Outline of the next three lectures Lecture 35 Anatomy of the human cerebral cortex gross and microscopic cell types connections Vascular supply of the cerebral cortex Disorders involving the cerebral cortex
Orientation, Development, Gross Anatomy, Blood Supply and Meninges References... 3
 Section I Orientation, Development, Gross Anatomy, Blood Supply and Meninges... 1 1 Orientation... 3 References... 3 2 Development... 7 Early Morphogenesis... 7 FormationoftheBrainRegions... 9 Histogenesis...
Section I Orientation, Development, Gross Anatomy, Blood Supply and Meninges... 1 1 Orientation... 3 References... 3 2 Development... 7 Early Morphogenesis... 7 FormationoftheBrainRegions... 9 Histogenesis...
