Context-Specific Grasp Movement Representation in Macaque Ventral Premotor Cortex
|
|
|
- Barry Barnard Norman
- 5 years ago
- Views:
Transcription
1 The Journal of Neuroscience, November 10, (45): ehavioral/systems/cognitive Context-Specific Grasp Movement Representation in Macaque Ventral Premotor Cortex Marie-Christine Fluet, 1 * Markus. aumann, 1 * and Hansjörg Scherberger 1,2,3 1 Institute of Neuroinformatics, University of Zürich and ETH Zürich, CH-8057 Zürich, Switzerland, and 2 Deutsches Primatenzentrum GmbH and 3 Department of iology, University of Göttingen, D Göttingen, Germany Hand grasping requires the transformation of sensory signals to hand movements. Neurons in area F5 (ventral premotor cortex) represent specific grasp movements (e.g., precision grip) as well as object features like orientation, and are involved in movement preparation and execution. Here, we examined how F5 neurons represent context-dependent grasping actions in macaques. We used a delayed grasping task in which animals grasped a handle either with a power or a precision grip depending on context information. dditionally, object orientation was varied to investigate how visual object features are integrated with context information. In 420 neurons from two animals, object orientation and grip type were equally encoded during the instruction epoch (27% and 26% of all cells, respectively). While orientation representation dropped during movement execution, grip type representation increased (20% vs 43%). ccording to tuning onset and offset, we classified neurons as sensory, sensorimotor, or motor. Grip type tuning was predominantly sensorimotor (28%) or motor(25%), whereas orientation-tuned cells were mainly sensory(11%) or sensorimotor(15%) and often also represented grip type (86%). Conversely, only 44% of grip-type tuned cells were also orientation-tuned. Furthermore, we found marked differences in the incidence of preferred conditions (power vs precision grips and middle vs extreme orientations) and in the anatomical distribution of the various cell classes. These results reveal important differences in how grip type and object orientation is processed in F5 and suggest that anatomically and functionally separable cell classes collaborate to generate hand grasping commands. Introduction Hand grasping is a goal-directed behavior that is largely driven by object characteristics, like shape, orientation, and size. It therefore requires the transformation of sensory information into appropriate hand motor commands. The ventral premotor cortex (PMv) in the frontal cortex, specifically area F5, participates in such visuomotor transformations for grasping (Gentilucci et al., 1983; Murata et al., 1997; Fogassi et al., 2001; Cerri et al., 2003; Umilta et al., 2007). natomically, area F5 is strongly and reciprocally connected to the parietal cortex, in particular to the anterior intraparietal area (IP) (Matelli et al., 1986; Luppino et al., 1999; Tanné-Gariépy et al., 2002; orra et al., 2008), which also contains hand grasping signals (Taira et al., 1990; Sakata et al., 1995; Murata et al., 2000; aumann et al., 2009). t the motor output side, F5 has direct projections to the hand area of primary motor cortex (Matsumura and Kubota, 1979; Muakkassa and Strick, 1979; Matelli et al., 1986; Dum and Strick, 2005) and to the spinal cord (Dum and Strick, 1991; He et al., 1993; orra et al., Received June 23, 2010; revised Sept. 9, 2010; accepted Sept. 14, This work was supported by the Swiss National Science Foundation (Grants /1 and /1), the Swiss NationalCenterforCompetenceinResearch NeuralPlasticityandRepair, theeuropeancommission(international Reintegration Grant NeuroGrasp ), the University of Zurich (Grant FK2004), the Zurich Center for Integrative Human Physiology (fellowship to M.C.F.), and the Swiss cademy of Medical Sciences (fellowship to M...). We thank. Disler and G. Stichel for animal care and the Institute of Neuroinformatics for administrative assistance. *M.C.F. and M... contributed equally to this work. Correspondence should be addressed to Hansjörg Scherberger, Deutsches Primatenzentrum GmbH, Kellnerweg 4, D Göttingen, Germany. hscherberger@dpz.eu. DOI: /JNEUROSCI Copyright 2010 the authors /10/ $15.00/0 2010). Furthermore, it is causally related to grasping execution (Fogassi et al., 2001). Functionally, neurons in F5 are selectively active for specific grip types, like precision, whole hand, or power grips (Gentilucci et al., 1988; Murata et al., 1997; Raos et al., 2006; Stark et al., 2007). One type of cells, motor neurons, respond only during grasping execution, whereas others, so-called visuomotor neurons, also respond during visual presentation of specific objects (Murata et al., 1997). In addition, F5 activity is modulated by the object orientation (Kakei et al., 2001; Raos et al., 2006). In these previous investigations, the shape of the object always determined the grip type to be applied. However, objects can often be grasped with various grips depending on behavioral context. Context-specific, or rule-based, information has been demonstrated in frontal cortex (White and Wise, 1999; Hoshi et al., 2000; Wallis et al., 2001; memori and Sawaguchi, 2006) and in parietal cortex (Gottlieb and Goldberg, 1999; Kalaska et al., 2003; Gail and ndersen, 2006; Scherberger and ndersen, 2007). Recently, we demonstrated such context-specific grasp movement signals in IP (aumann et al., 2009). Since IP and F5 are intimately connected, we hypothesize that F5 also contains contextspecific information that might contribute, in addition to sensory information, to grasp movement preparation. To test this hypothesis, we recorded single-unit activity in F5 while macaque monkeys were instructed, by an external context cue, to grasp a handle with one of two grip types (power or precision grip). In addition, object orientation was varied to investigate how visual object features are integrated with context information. We found context-dependent grasp planning activ-
2 15176 J. Neurosci., November 10, (45): Fluet et al. Grasp Movement Representation in F5 ity in F5 that appeared after cue presentation and motor grasp activity that became active at movement execution. We classified F5 neurons as sensory, sensorimotor, and motor for grip type and object orientation according to the extent of their respective tuning in the task, and found functional and anatomical differences between these groups. Materials and Methods asic procedures. Single-unit activity was recorded from area F5 in two awake female rhesus macaque monkeys (animals L and J). ll procedures and animal care were in accordance with the guidelines set by the Veterinary Office of the Canton of Zurich and the guidelines for the care and use of mammals in neuroscience and behavioral research (National Research Council, 2003). Methods have been extensively described previously (aumann et al., 2009). In short, monkeys were seated in a primate chair and trained to perform a grasping task with their left hand. They faced a handle placed at the level of the chest and at a distance of 30 cm. The handle could be grasped either with a power grip (opposition of fingers and palm) or a precision grip (opposition of index and thumb pads). Inside the handle, two touch sensors were placed in small, clearly visible recessions to detect the contact of the animal s Figure1. Taskparadigmandrecordingsites., Pictureofthehandle. Twosensorsingrooves(redcircle, onlyonesensorvisible) detectedtheexecutionoftheprecisiongrip, andalightbarrier(redline) detectedtheinsertionofthemonkeyhandintothehandle duringpowergripexecution., Representationofthetimecourseofthedelayedgraspingtask. Thetaskisdividedintofourepochs: fixation, cue, planning, and movement. The entire task is performed in the dark except for the cue period in which the handle is visibleandacoloredledindicatesthegriptype(powerorprecision). Thelightsareprojectedontothemiddleofthehandlethrough ahalfmirror. C, MRIsectionshowingacoronalviewofmonkeyJ. Redlineindicatestheorientationoftherecordingchamber, white crosses indicate image artifacts from two titanium bone screws located within (not below) the skull. D, MRI section of the right hemisphere of monkey L in the plane of the recording chamber. Superimposed on the image are the recording sites in area F5 (yellow dots). E, Same as D for monkey J. thumb and index finger during precision grips (Fig. 1, red dot; only one sensor visible). Power grips were detected by an infrared light barrier inside the opening of the handle (Fig. 1, red line). Grip type was instructed by two colored LEDs that were projected on the center of the handle by a half mirror positioned between the animal s eyes and the target. The handle was rotatable and was presented in five different orientations (upright and 25 and 50 to the left and right) and two spotlights could illuminate it from the left and right side in an otherwise dark experimental room. Two capacitive touch sensors (model EC3016NPPL; Carlo Gavazzi) were placed at the level of the animals waist as handrest buttons. nimals had to fixate a red LED that was projected on the center of the handle. Eye movements were measured using an optical eye tracker (ISCN) and custom-written software implemented in LabView Realtime (National Instruments) was used to control the behavioral task. ehavioral paradigm. Monkeys were first trained in a delayed grasping task in which they were required to grasp a handle in five possible orientations (upright and 25 and 50 to the right or left) with either a power grip or a precision grip. This led to 10 grasp conditions that were presented in a pseudorandom order. To initiate a trial, monkeys sat in darkness and placed each hand on a handrest button. The handle was then positioned in one of the five orientations and subsequently a red fixation LED switched on. From then on, the animal was required to fixate while keeping both hands at the handrest buttons (fixation period: duration, ms; mean, 900 ms). In the following cue period (duration, 600 ms), the object was illuminated to reveal its orientation. The color of an additional LED presented next to the fixation LED indicated which grip type to perform, either a power grip (green light) or a precision grip (white light). The spotlights and the instructing LED were then switched off while the fixation light remained on for a variable period (duration, ms; mean, 900 ms) during which the monkey had to remember the grasping instructions (planning period). The red fixation LED was then dimmed, which instructed the animal to reach and grasp the object in the dark. The movement period started with the go-signal and ended when the sensors on the handle were activated. Each correct trial was rewarded with a fixed amount of juice, which was delivered immediately after the grasp target was correctly acquired. The trial sequence is presented in Figure 1. Surgical procedures and imaging. When the animal was fully trained, an MRI scan was performed to locate anatomical landmarks for the implantation of a recording chamber. For this, the animal was sedated (ketamine and xylazine, 10 and 0.5 mg/kg, i.m., respectively), placed in the scanner (GE Healthcare 1.5T) in a prone position, and T1-weighted volumetric images were obtained of the brain and skull (aumann et al., 2009). The stereotaxic location of the arcuate sulcus was then obtained to guide the placement of the recording chamber. Next, a surgery was performed to implant a head post (titanium cylinder, 18 mm diameter) and an oval-shaped recording chamber (material PEEK; outer dimensions, mm) stereotaxically over the right hemisphere on top of F5; both were secured to the skull with titanium bone screws. Initially, no skull trephination was performed. fter recovery from surgery, a second MRI scan was obtained with the chamber filled with contrast medium (Gadolinium solution diluted in saline, 1:2000). This allowed mapping of the brain inside of the recording chamber. Finally, a second surgery was performed for the craniotomy. Recordings were performed in the posterior bank of the inferior limb of the arcuate sulcus (area F5). Figure 1, C E, show a coronal view of monkey J and recording maps in the plane of the recording chamber for monkey L and J, respectively. ll surgical procedures were performed under sterile conditions and general anesthesia (induction with ketamine 10 mg/kg, i.m., atropine 0.05 mg/kg, s.c., followed by intubation, isofluorane 1 2%, and analgesia with 0.01 mg/kg buprenorphene, s.c.). Heart and respiration rate, electrocardiogram, O 2 saturation, and body temperature were continuously monitored, and systemic antibiotics and analgesics were administered for several days after each surgery. nimals were allowed to recover for at least 1 week before behavioral training or recording experiments commenced. Neural recordings. Single-unit (spiking) activity was recorded using platinum/tungsten electrodes with quartz-glass coating (impedance, 1 2 M at 1 khz) that were positioned by a five-channel micromanipulator (MiniMatrix; Thomas Recording) that was attached to the recording
3 Fluet et al. Grasp Movement Representation in F5 J. Neurosci., November 10, (45): C D Figure 2. Firing rate histograms and raster plots of four example neurons. D, Left, Precision grip trials. Right, Power grip trials. The five orientations are represented by different colors. Graphs are doubly aligned to the end of the cue and the release of the handrest button (black arrowheads). Realignment occurs at 0.6 s and is marked by a gap in the curves and the spike rasters. lack vertical lines mark cue onset, cue offset, and the go-signal. The dotted line within the movement epoch indicates the handrest release and the gray line the (mean) time of handle contact., Neuron modulated by grip type and orientation from the cue until completion of the task., Neuron modulated by grip type only, starting from the cue until task completion. C, Neuron showing no task modulation during cue and planning, but clear grip-type tuning during movement execution. D, Neuron modulated by grip type and orientation exclusively in the cue period. chamber. Neural signals were amplified ( 400), digitized with 16-bit resolution at 30 ks/s using Cerebus neural signal processor (lackrock Microsystems), and stored to disc together with the behavioral data. To coarsely monitor the tuning properties of the recorded neurons during data acquisition, spike detection was performed in real time (Cerebus hardware) and analyzed for various task conditions using Matlab (Math- Works). However, all quantitative analysis was performed offline, as described below. Data analysis. Raw signals were high-pass filtered offline (cutoff, 500 Hz) and single units were isolated using principal component analysis techniques (offline sorter; Plexon). We included in our database all single units that were stably recorded during at least seven trials per condition (minimum of 70 trials). Peristimulus time histograms were generated using a gamma distribution as a causal kernel (parameters: shape 1.5; rate 30) (aumann et al., 2009). Population histograms were obtained by averaging individual peristimulus time histograms for the entire population. ll statistical tests, however, were based on exact spike counts without any smoothing. For the generation of the population-averaged histogram, the preferred and nonpreferred grip type and orientation were determined for each cell from the mean firing rates in the time interval from cue onset to the end of the movement, which were averaged across all trials of the same grip type or object orientation. The preferred grip type was then defined as the grip with the highest mean rate and the nonpreferred grip type was the other one. The preferred orientation was defined as the orientation with the highest mean firing rate and the nonpreferred orientation was taken as the orientation at 75 angular distance from the preferred one. This definition was chosen so that the nonpreferred direction was not taken exclusively from the extreme orientations ( 50 ). For the vertical position we selected randomly between the 50 and 50 position. Figure 3. Population firing rate of the 420 cells during the preferred and nonpreferred conditions (grip type and orientation). lignment and definition of time epochs as in Figure 2. To determine the distribution of preferred conditions in the population, the same definition of preferred grip type and orientation was used, but based on the firing rate in the epoch in question or in the time interval from tuning onset to offset. To test the statistical significance of tuning for grip type and object orientation in a particular task epoch, we first determined in each trial the mean firing rate (spike count/length of epoch) and then performed a two-way NOV (factors grip type and orientation, p 0.01). Only positive main effects were taken into account. In addition, we required that the neuron fired at least with five spikes/s in the preferred condition. t the population level, we used a two-proportion z test (two-tailed version) to determine whether the number of cells with significant tuning (grip type or orientation) significantly changed between two task epochs (Zar, 1999).
4 15178 J. Neurosci., November 10, (45): Fluet et al. Grasp Movement Representation in F5 Table 1. Grip-type and orientation tuning across epochs Orientation tuning Grip-type tuning Cue Planning Movement Number of cells Cue Planning Movement Number of cells 33 (8%) 42 (10%) 28 (7%) 22 (5%) 8 (2%) 16 (4%) 41 (10%) 35 (8%) 12 (3%) 29 (7%) 29 (7%) 19 (5%) 29 (7%) 93 (22%) 240 (57%) 164 (39%) Number (and percentage) of cells for possible combinations of orientation tuning and grip-type tuning during the cue, planning, and movement epochs. Significant tuning is indicated by, no tuning by. For this, the SE of the sampling distribution difference between two proportions was calculated as SE p 1 p 1 n 1 1 n 2, with p n 1 p 1 n 2 p 2 n 1 n 2 the pooled sample proportion, n 1 and n 2 the size of samples 1 and 2 (n 1 n cells), and p 1 and p 2 the first and second sample proportion. From this, the z score was calculated as z p 1 p 2 and the corresponding p value obtained from the (cumulative) normal distribution. SE We further investigated the significance of grip type and orientation tuning in a sliding window analysis. We performed a two-way NOV ( p 0.01) in a 200-ms-width window that was shifted by 50 ms. In plots, the number of significant cells in each bin was reported at the center position of each window. Due to the variable length of the planning period, trials were doubly aligned for this analysis; up until 0.6 s after cue off, they were aligned to the cue offset, after that, they were aligned to the release of the handrest button. This approach proved to be very uncritical, as no cell showed any specific activity change around the time of realignment. nalyses based on the stepping window NOV (like the tuning onset/offset, see below) therefore inherited this realignment. The time of half-maximum activity during the cue period was calculated as the time when the number of tuned cells reached the middle between the initial value at cue onset and the maximum during the cue period. For this calculation, the number of significantly tuned cells, measured at 50 ms steps, was linearly interpolated to reach a time resolution of 2 ms (Matlab command interp). Then the time delay between the start of orientation and the start of grip type activity was taken as the difference between the two half-maximum times. To assess the significance of the observed difference, we used a Monte Carlo method that calculated the above mentioned time delay at each time step 1000 times with the labels grip type tuning and orientation tuning randomly shuffled. The 95th percentile of this null-distribution was then taken as the threshold for significance. Tuning onset for grip type or orientation was determined from the two-way NOV analysis and was defined as the first of at least five consecutive windows with significant tuning. Similarly, tuning offset was defined as the last of at least five such consecutive windows. Cells were classified according to their tuning onset (t_on) and offset (t_off) as sensory (t_on t_off 0.7 s), sensorimotor (t_on 0.7 s t_off), or motor (t_off t_on 0.7 s). ll times were relative to the end of the cue epoch. The combined encoding of grip type and orientation was investigated using joint distributions. Figure 4. Grip type and orientation tuning in the population (n 420)., Percentage of cells that are tuned for grip type (black) and object orientation (gray) during each task epoch (fixation, cue, planning, and movement) (NOV, p 0.01)., Percentage of tuned cells for grip type (black) and object orientation (gray) in 200 ms time windows (NOV, p 0.01; curves report data at the center of each window). lignment and definition of time epochs as in Figure 2. Results Tuning for grip type and orientation We recorded a total of 420 single units from area F5 in the right hemisphere of two monkeys (monkey L, 113 units; monkey J, 307 units) during a delayed grasping task, in which two grip types (power and precision grip) were performed with five different object orientations. Four typical neurons are presented in Figure 2. The first neuron was jointly modulated by orientation and grip type, starting shortly after cue onset and extending until movement execution was finished. Clearly, this neuron preferred precision grips with the handle tilted to leftward orientations ( 25 and 50 ). The second neuron (Fig. 2) showed an increased firing rate specifically for precision grip trials, independent of handle orientation. It represented only the grip type, but not the object orientation, from cue presentation until the end of the movement. The third neuron encoded the grip type with a preference for power grip, however, only during movement execution. Finally, the fourth neuron was modulated by grip type and orientation exclusively during the cue epoch. These example neurons reflect main classes of F5 neurons with sensory, sensorimotor, and motor activity. Condition-specific modulation of the firing rate was also seen in the neural population. Figure 3 shows a population histogram of all 420 recorded cells, in which the preferred and nonpreferred
5 Fluet et al. Grasp Movement Representation in F5 J. Neurosci., November 10, (45): Figure5. Preferredgriptypeandorientationofsignificantlytunedcellsineachtaskepoch., Numberofgriptype-specificcells preferring precision (white) and power grip (black) during the cue, planning, and movement epoch., Number of orientationspecific cells preferring individual object orientations ( 50, 25, 0, 25, 50 ) during cue, planning, and movement. Figure 6. Tuning consistency between different task epochs., Grip type tuning consistency. Number of cells that kept (black), altered (gray), or lost (white) their tuning from cue to planning and from the planning to the movement epoch., Orientation tuning consistency. Fraction of cells as a function of shift from their preferred orientation (in degrees) from cue to planning and from planning to movement. n.t., No longer tuned. grip types and orientations were pooled separately. It demonstrates that the handle orientation and the context-cued grip type were represented in the F5 population starting from the cue epoch, long before motor execution. Results stayed the same when the activity of individual cells was normalized before pooling, confirming that the result did not rely on a few cells with particularly strong responses. Epoch analysis To quantify grip type and orientation tuning of individual F5 neurons in each task epoch (cue, planning, and movement), we performed a two-way NOV (factors grip type and object orientation, p 0.01) on the firing rate in each task epoch. Table 1 reports the frequency of the different tuning patterns in the three task epochs. Overall, 71% of the neurons were tuned for at least one of these two parameters in at least one epoch. Specifically, 61% of the cells were tuned for grip type and 43% were tuned for object orientation. Grip type and orientation tuning did not only show a large difference of representation (61% vs 43%), but also different progression during the task (Fig. 4 ). While in the cue period, the percentage of grip-type tuned neurons was the same as for orientation-tuned neurons (27% vs 26%), grip-type-specific neurons became more frequent during the course of the task (planning epoch, 27%; movement, 43%), whereas the number of orientation-specific neurons decreased (planning epoch, 24%; movement, 20%). two-proportion z test revealed that the number of grip-type-specific cells did not change between the cue and planning epoch ( p 0.8), but it increased significantly between planning and movement ( p 0.05). In contrast, the number of orientation-specific cells tended to decrease during the task (cue to movement, p 0.02). Cells without significant tuning for grip type or orientation often showed a task modulation with respect to baseline activity, which was unspecific for the tested conditions (cue epoch, 49% of all nontuned cells; planning, 64%; movement, 78%; t test, p 0.01). These neurons could possibly be tuned for grasp conditions other than the ones tested here. However, it is interesting to note that unspecific responses during the cue and planning epoch often consisted of a decrease below baseline activity (cue, 88 cells lower, 38 cells larger; planning, 109 lower, 61 larger), while during movement execution the majority of untuned cells increased their activity compared with baseline (68 lower, 97 larger). Some of these neurons might therefore constitute a timing signal and represent temporal information independent of a specific grip type. We then investigated the representation of grip type and orientation in F5 with a finer time resolution using a two-way NOV in a small time window that was shifted along the time axis (window size, 200 ms; step size, 50 ms; p 0.01). We found that both the number of orientation and grip-type tuned cells showed a steep increase during the cue period; however, orientation tuning appeared earlier than the grip-type representation (Fig. 4). Using the time difference between the half maxima of the two ascending curves, we quantified that orientation tuning was leading grip-type tuning by 94 ms in the population (p 0.01, Monte Carlo procedure; see Materials and Methods). Orientation information therefore appeared more rapidly in F5 than the grip-type instruction, which had to be inferred abstractly from an LED color. This can be seen also in the population histogram (Fig. 3), where the curves indicating the preferred and nonpreferred object orientation split earlier than the curves of the preferred and nonpreferred grip types. t the end of the cue period, however, the number of cells representing grip type was higher than for orientation (Fig. 4 ). oth groups tended to decrease during the planning epoch. Yet, during movement execution, the number of cells representing grip type approximately doubled, whereas orientation tuning recovered only slightly. It therefore seems that grip type representation is the overall dom-
6 15180 J. Neurosci., November 10, (45): Fluet et al. Grasp Movement Representation in F5 inant feature in F5, even though orientation information appears first. Grip Type Orientation Specific coding in task epochs To investigate the preference for specific grip types and orientations, we examined their distribution across different task epochs (cue, planning, movement). We found an overrepresentation of precision grip- versus power grip-preferring cells (Fig. 5) with a tendency to increase from the cue (57% precision) to the movement epoch (64% precision). For orientation tuning, we found a predominance of extreme orientations ( 50 ) over middle orientations ( 25, 0, and 25 ) (Fig. 5). lthough the number of neurons coding for extreme orientations remained approximately constant during the task, neurons representing middle orientations were clearly reduced during movement execution, which resulted in a relative increase of cells preferring extreme orientations from cue (60%) to movement execution (72%). These changes in representation suggest functional dynamics in the coding of F5 that we examine below. We first asked whether these shifts in the distributions of preferred grip type and orientation in F5 were due to shifts in the preference of individual neurons across the task or to different subpopulations being active in different task epochs. For this, we determined the percentage of tuned neurons that maintained or changed their specific tuning from one task epoch to the next (cue planning, planning movement). For grip-type tuning, very few cells changed their tuning preference; most cells either maintained their grip-type preference or lost their tuning (Fig. 6 ). Similarly, the preferred orientation of individual cells was nearly constant across task epochs (Fig. 6 ). Tuning shifts occurred mainly to neighboring orientations, for which the firing rate was often not significantly different. However, a considerable number of cells lost their orientation tuning from cue to planning and planning to movement. Therefore, since most cells encoded grip type and object orientation consistently across task epochs, the observed changes in the statistics of the preferred conditions (Fig. 5) must be caused by different subpopulations of cells that are active at different times in the task. Cell classification ased on the sliding window analysis described above (Fig. 4), we defined the tuning onset of an individual neuron as the first occurrence of five consecutive tuned windows. Figure 7, and, shows the time windows with significant tuning for all cells in the population. For grip type (Fig. 7) and object orientation (Fig. 7), cells are ordered according to their tuning onset. Corresponding histograms of tuning onset (Fig. 7C,D) revealed three C E Figure 7. Time of significant tuning of grip type and orientation., Sliding window analysis indicating the times with significant grip-type tuning (black lines) for each cell (NOV, p 0.01; window size, 200 ms; step, 50 ms; centered in the middle of the window). Cells are ordered by tuning onset; red dots indicate tuning offset., Same as, for orientation tuning. C D, Histograms of tuning onset of grip type and orientation. E F, Histograms of tuning offset for grip type and orientation. D F important characteristics of F5 activity: (1) one group of cells showed an early tuning onset for grip type, (2) another group showed a late tuning onset for grip type, and (3) one group showed an early tuning onset for orientation. These groups are illustrated by the example neurons in Figure 2. Furthermore, tuning offset was defined as the last occurrence of five consecutive tuned windows and is indicated by a red dot in Figure 7, and. The corresponding tuning offset histograms (Fig. 7E,F) indicate that grip-type tuned cells remained tuned predominantly until movement execution, whereas orientation tuned cells faded off during the course of the task. To relate tuning onset and offset of individual cells, we generated scatter plots that describe their joint distribution (Fig. 8). For grip-type tuning (Fig. 8 ), the joint distribution suggested a classification of three neuronal classes: (1) sensory (S) cells with early onset and offset of tuning (t_on t_off 0.7 s), (2) sensorimotor (SM) cells with early tuning onset and tuning persistence until movement execution (t_on 0.7 s t_off), and (3) motor (M)
7 Fluet et al. Grasp Movement Representation in F5 J. Neurosci., November 10, (45): Figure 8. Scatter plots of tuning onset and offset.,, Cell classification based on the tuning onset and tuning offset for grip type () and orientation (). The separation between the S, SM, and M classes is set at 0.7 s (gray lines). Table 2. Joint distribution of cell classes for grip type and object orientation Type Orientation S SM M Never Total S (11%) SM (15%) M (8%) Never (67%) Total 43 (10%) 119 (28%) 107 (25%) 151 (36%) 420 (100%) Joint distribution of sensory, sensorimotor, motor, and untuned (Never) cells for grip type (columns) and object orientation (rows) in the neural population. Percentages are relative to the entire population (420 cells). cells with late tuning onset (t_off t_on 0.7 s). The exact threshold level (we chose 0.7 s after cue offset) was thereby not critical. ny value between 0.4 and 1 s returned very similar results, as the tuning onset and offset histograms suggest (Fig. 7C F). Using this classification scheme, we found 43 S cells (10%), 119 SM cells (28%), 107 M cells (25%) for grip-type tuning, and 151 untuned cells (36%). Likewise, the same classification scheme was applied for orientation tuning (Fig. 8), which gave us 45 (11%) S cells, 61 (15%) SM cells, 32 (8%) M cells, and 282 (67%) untuned cells for object orientation. This classification expands previous schemes that distinguish only between motor and visuomotor cells, but do not recognize purely sensory cells due to the different nature of their task (Murata et al., 1997; Raos et al., 2006). The joint distribution of grip-type and orientation classes is shown in Table 2. It demonstrates a highly significant interaction between the type and orientation classes (Pearson s 2, p 0.01). Most orientation-tuned cells were of S and SM class (106 of 138 cells), and a large majority of them were also grip-type tuned (94 of 106 cells), mainly in the S or SM class (79 of 106 cells). In contrast, many of the cells with early grip-type tuning (S and SM) were never orientation tuned (71 of 162), and if they were, they were predominantly of S or SM class for orientation (79 of 91 cells). These results are remarkably different from IP, where a considerable number of visual neurons represent the object orientation alone, independent from grip type (aumann et al., 2009). Moreover, in F5, an overwhelming majority of M cells for grip type was never orientation tuned (79 of 107 cells). This suggests that object orientation information is relevant in F5 primarily during grasp planning (S and SM cells), but much less during movement execution (M cells). Specific coding of cell classes The specific coding of the different cell classes showed marked differences for S, SM, and M cells (Fig. 9). For grip-type tuning (Fig. 9), S cells were tuned for precision and power grips equally often, whereas SM and M cells showed a clear overrepresentation of precision grips. n overrepresentation of precision grips has been described in motor areas and could be due to the fact that complex grip types may need more neural resources for planning and execution than simpler grips (Muir and Lemon, 1983; Lemon et al., 2004; Umilta et al., 2007). In contrast, the balanced ratio of S cells suggests that these cells might employ a more abstract representation with equal resources for both grips. similar difference appeared for orientation tuning (Fig. 9). S cells showed a rather balanced representation of extreme and middle orientations (53% vs 47%), whereas SM and M cells coded predominantly for extreme orientations (75% and 85%, respectively). This overrepresentation of extreme orientations could reflect a motor coding along the pronation supination dimension, whereas the more balanced representation would be compatible with a visual representation of orientation. Together, these results suggest that SM and M cells represent motor-related signals, whereas S cells may convey a more sensory or cognitive representation of grip type and object orientation. Interestingly, such a balanced representation of grip type and object orientation was also found in IP for cells with an early tuning onset in this task (aumann et al., 2009). This suggests that IP cells with early tuning onset are particularly coupled with S cells in F5. natomical cell distribution Finally, we tested whether the cell classes have different anatomical distributions within F5. For this, we ordered all cells in the population along their dorsoventral recording position along the arcuate sulcus and distributed them into eight bins such that each bin contained the same number of cells. In each of these bins, we determined the fraction of S, SM, and M cells for grip type and orientation and found that early and late onset tuned cells had complementary distributions along the arcuate sulcus (Fig. 10). Cells with early grip type tuning (S and SM cells) were concentrated mainly in two areas: the most ventral recording site (Fig. 10, top left, bins 7 8) and a dorsal site (Fig. 10, top left, bins 2 3). S and SM cells with early orientation tuning showed a similar distribution with peaks in bins 2 and 8 (Fig. 10, top right). In contrast, cells with late tuning onset (M cells) were concentrated at recording sites in between the early (S and SM) cells. This can be best seen for cells with late grip-type tuning (Fig. 10, bottom left, peaks in bins 4 6 and bin 1) and somewhat less clearly for M cells of orientation tuning due to their smaller group size (Fig. 10, bottom right). similar analysis in the direction normal to the sulcus orientation and in the depth direction showed no concentration effects (data not shown). These results suggest that sensory input is not uniformly present across F5 but rather concentrated in some subregions. These subregions seem to coincide with subareas of F5 with strong connectivity to IP. Indeed, Figure 3 in orra et al. (2008) suggests that the anatomical projections from IP to F5 are not
8 15182 J. Neurosci., November 10, (45): Fluet et al. Grasp Movement Representation in F5 uniformly distributed along the inferior ramus of the arcuate sulcus; although the authors did not quantify this effect, in all of their cases two separate spots with high labeling are present in F5, a ventral one and a more dorsal one. In contrast, M cells that are predominantly located in the intermediate subregion (bins 3 6) might be more strongly connected to the primary motor area (M1). Discussion We investigated the representation of grip type and object orientation in F5 neurons while macaque monkeys performed a delayed grasping task, in which grip type was instructed by context information (Fig. 1). F5 neurons represented the grip type and object orientation from instruction to movement execution, suggesting that F5 plays an important role for the transformation of object and context information for grasping (Figs. 2, 3; Table 1). Object orientation was represented strongest during instruction, whereas grip-type representation was overall larger and strongest during movement execution (Fig. 4). t the population level, precision grips were overrepresented compared with power grips, and cells preferring middle orientations strongly declined toward movement execution (Fig. 5). Since individual cells kept the same preference for grip type and object orientation across active task epochs (Fig. 6), different subpopulations of cells must be active in different task epochs (Fig. 7). cell classification based on the tuning onset and offset led to three classes of cells (sensory, sensorimotor, and motor), both for grip type and object orientation (Fig. 8). When comparing both classifications, most orientation-selective cells were also grip-type tuned, whereas motor cells coded predominantly grip type (Table 2). Interestingly, the preference ratio for power versus precision grips and for middle versus extreme orientations was markedly different for sensory and latetuned (SM and M) cells (Fig. 9), and motor cells followed a distinct anatomical distribution within F5 (Fig. 10). These results reveal important differences in how grip type and object orientation are processed in F5 and how different cell groups might collaborate to transform sensory signals into grasp movement commands. Functional connectivity The representation of grip type and object orientation in F5 increased rapidly after cue instruction with object orientation leading by 90 ms. This might indicate different processing pathways for object orientation and grip type instruction. Figure 9. Specific tuning for grip type and orientation (orient) in cell classes., Percentage of grip type-specific cells preferring precision (white) versus power grip (black) for sensory, sensorimotor, and motor cells., Histogram, Number of cells preferring individual object orientations ( 50, 25, 0, 25, 50 ) for sensory, sensorimotor, and motor cells. Pie chart, Percentage of orientation-specific cells preferring extreme orientations (black) or middle orientations (gray). Figure10. natomicaldistributionofcellclasses. Percentageofsensory(whitebars), sensorimotor(graybars), andmotorcells (black bars) for grip type (left) and object orientation (right) as a function of their position along the inferior arcuate sulcus. Object information is most likely propagated from visual and parietal areas to F5. In particular, area IP contains a strong representation of objects (aumann et al., 2009) and is strongly and reciprocally connected to F5 (Matelli et al., 1986; Tanné- Gariépy et al., 2002; orra et al., 2008). The medial posterior parietal area (V6) also represents object orientation before
9 Fluet et al. Grasp Movement Representation in F5 J. Neurosci., November 10, (45): grasping (Fattori et al., 2009) and might potentially also contribute to grasp planning (attaglini et al., 2002; Fattori et al., 2010). Grip type, on the other hand, was instructed by a context cue (colored light), which required retrieving a learned association for correct interpretation. rain areas involved in such arbitrary visuomotor mapping, in addition to the premotor cortex, are the prefrontal cortex, the hippocampus, and the basal ganglia (Murray et al., 2000). F5 receives projections from prefrontal cortex (Matelli et al., 1986; arbas and Pandya, 1987; Petrides and Pandya, 2002), and this multisynaptic pathway could therefore explain the longer processing time for context information. Our observation that many F5 cells represent the grip type at the time of movement execution corresponds well with the strong direct connections of F5 to primary motor cortex (Matsumura and Kubota, 1979; Muakkassa and Strick, 1979; Matelli et al., 1986; Dum and Strick, 2005) and the spinal cord (Dum and Strick, 1991; He et al., 1993; orra et al., 2010). This suggests a fairly direct role of F5 for generating hand-grasping movements. The weak representation of object orientation during movement could indicate that wrist orientation, necessary to align the hand with the object, is perhaps more strongly represented elsewhere, e.g., in dorsal premotor cortex (Raos et al., 2004; Stark et al., 2007). However, object orientation is clearly present in F5, suggesting that it might be necessary for the selection of grip types or for motor coordination. Our evidence that cells in F5 are functionally clustered is in contrast to Raos et al. (2006), who studied a fourfold smaller dataset (108 cells). Subregions with predominantly sensory activity (S and SM cells) colocated in two spots most ventrally and dorsally in F5. They seem to coincide with regions of strong connectivity to IP (orra et al., 2008, their Fig. 3), whereas the intermediate region of our recordings was predominantly active during movement execution (M cells). This intermediate region is likely located well within the posterior subdivision of F5 (F5p) (Nelissen et al., 2005; elmalih et al., 2009), which is strongly connected to the hand area of primary motor cortex (Matsumura and Kubota, 1979; Muakkassa and Strick, 1979; Matelli et al., 1986; Dum and Strick, 2005). These functional connections place F5 right at the center of cortical hand movement control. Comparison to previous work Previous studies of F5 have classified neurons in F5 as motor or visuomotor depending on the neural activity during grasping in the light and dark (Murata et al., 1997; Raos et al., 2006). Motor neurons were active only during movement execution, whereas visuomotor neurons responded during object presentation. This is compatible with our definition of M and SM cells. In our task, however, visual information was present only during cue and not in the following planning and movement execution epochs. Nonetheless, F5 encoded the instructed grip type during cue and planning in the absence of vision, which highlights its role for sensorimotor transformation. The decline of orientation tuning during the course of the task was not observed by Raos et al. (2006), which is likely explained by the fact that their objects remained visible throughout the task. This also prevented the detection of sensory cells. Comparison with IP Our recordings were made in the same animals and often during the same sessions as recordings in IP with the same paradigm (aumann et al., 2009). This allowed a direct comparison with results in IP. s expected from their strong direct and reciprocal connections (Matelli et al., 1986; Luppino et al., 1999; orra et al., 2008), grip type and object orientation were represented similarly in F5 and IP. For example, IP cells also showed a bimodal distribution of the onset of grip-type tuning with peaks during the cue and movement epoch, and a large percentage of IP cells represented object orientation throughout the task (aumann et al., 2009). These findings strongly suggest that sensorimotor transformation for hand grasping occurs in a distributed network including premotor and parietal cortices, similar to what is known for eye and arm movements control (Rizzolatti and Luppino, 2001; ndersen and uneo, 2002; Gold and Shadlen, 2007; Pesaran et al., 2008; Gail et al., 2009). The main difference to F5 is that IP represents object orientation more strongly and persistently. This is evident from the fraction of orientation-tuned cells in the movement epoch (IP, 55%; F5, 20%) and throughout the task (IP, 77%; F5, 43%) (aumann et al., 2009). Furthermore, the representation of object orientation in F5 is much weaker and more coupled to griptype representation. Nevertheless, object information is present in F5, in particular in S and SM cells, which could simply reflect the strong connectivity to IP, or might be necessary for appropriate grip-type selection or the coordination of grasping and reaching actions (Jeannerod et al., 1995). In this respect, it remains to be seen whether F5 neurons code the object orientation or the intended wrist orientation of the upcoming movement. Possible coding schemes The preference for a particular grip type or object orientation remained largely constant in individual cells (Fig. 6). Our neural classification based on tuning onset and offset was therefore justified. ut which roles do these cell classes play for the sensorimotor processing of hand movements? S cells preferred power and precision grips in equal numbers and likewise represented object orientations almost equally. This could point to a high-level (visual, cognitive, or abstract) coding scheme that places equal weight on different alternatives. In contrast, SM and M cells showed an overrepresentation of precision grips and extreme orientations. This could indicate a motor code, since precision grips are arguably more difficult to perform and might require more cortical resources than power grips. Likewise, the motor code of wrist orientation might be implemented in a push pull mechanism for pronation and supination, which could require a strong representation of extreme orientations. Therefore, the coding scheme of SM and M cells could be regarded as more motor-related than abstract, whereas S cells might encode a high-level representation. In contrast, sensory and sensorimotor cells in IP have been classified as sensory or abstract (aumann et al., 2009). In other words, the coding scheme in IP is determined by the tuning onset, whereas in F5 it is the tuning offset. This places F5 neurons closer to the motor cortex, whereas IP codes grasp movements predominantly in sensory or abstract terms during movement preparation. In summary, our results suggest that F5 is involved in contextspecific grasp movement planning, for which different subpopulations are functionally connected to parietal, motor, and frontal areas. This emphasizes an integrative role of F5 for the planning of hand movements on the basis of sensory, contextual, and, most likely, volitional information. References memori K, Sawaguchi T (2006) Rule-dependent shifting of sensorimotor representation in the primate prefrontal cortex. Eur J Neurosci 23: ndersen R, uneo C (2002) Intentional maps in posterior parietal cortex. nnu Rev Neurosci 25:
10 15184 J. Neurosci., November 10, (45): Fluet et al. Grasp Movement Representation in F5 arbas H, Pandya DN (1987) rchitecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: attaglini PP, Muzur, Galletti C, Skrap M, rovelli, Fattori P (2002) Effects of lesions to area V6 in monkeys. Exp rain Res 144: aumann M, Fluet MC, Scherberger H (2009) Context-specific grasp movement representation in the macaque anterior intraparietal area. J Neurosci 29: elmalih, orra E, Contini M, Gerbella M, Rozzi S, Luppino G (2009) Multimodal architectonic subdivision of the rostral part (area F5) of the macaque ventral premotor cortex. J Comp Neurol 512: orra E, elmalih, Calzavara R, Gerbella M, Murata, Rozzi S, Luppino G (2008) Cortical connections of the macaque anterior intraparietal (IP) area. Cereb Cortex 18: orra E, elmalih, Gerbella M, Rozzi S, Luppino G (2010) Projections of the hand field of the macaque ventral premotor area F5 to the brainstem and spinal cord. J Comp Neurol 518: Cerri G, Shimazu H, Maier M, Lemon RN (2003) Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol 90: Dum RP, Strick PL (1991) The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: Dum RP, Strick PL (2005) Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci 25: Fattori P, reveglieri R, Marzocchi N, Filippini D, osco, Galletti C (2009) Hand orientation during reach-to-grasp movements modulates neuronal activity in the medial posterior parietal area V6. J Neurosci 29: Fattori P, Raos V, reveglieri R, osco, Marzocchi N, Galletti C (2010) The dorsomedial pathway is not just for reaching: grasping neurons in the medial parieto-occipital cortex of the macaque monkey. J Neurosci 30: Fogassi L, Gallese V, uccino G, Craighero L, Fadiga L, Rizzolatti G (2001) Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. rain 124: Gail, ndersen R (2006) Neural dynamics in monkey parietal reach region reflect context-specific sensorimotor transformations. J Neurosci 26: Gail, Klaes C, Westendorff S (2009) Implementation of spatial transformation rules for goal-directed reaching via gain modulation in monkey parietal and premotor cortex. J Neurosci 29: Gentilucci M, Scandolara C, Pigarev IN, Rizzolatti G (1983) Visual responses in the postarcuate cortex (area 6) of the monkey that are independent of eye position. Exp rain Res 50: Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G (1988) Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp rain Res 71: Gold JI, Shadlen MN (2007) The neural basis of decision making. nnu Rev Neurosci 30: Gottlieb J, Goldberg ME (1999) ctivity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci 2: He SQ, Dum RP, Strick PL (1993) Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13: Hoshi E, Shima K, Tanji J (2000) Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol 83: Jeannerod M, rbib M, Rizzolatti G, Sakata H (1995) Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18: Kakei S, Hoffman DS, Strick PL (2001) Direction of action is represented in the ventral premotor cortex. Nat Neurosci 4: Kalaska JF, Cisek P, Gosselin-Kessiby N (2003) Mechanisms of selection and guidance of reaching movements in the parietal lobe. dv Neurol 93: Lemon RN, Kirkwood P, Maier M, Nakajima K, Nathan P (2004) Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog rain Res 143: Luppino G, Murata, Govoni P, Matelli M (1999) Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas IP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp rain Res 128: Matelli M, Camarda R, Glickstein M, Rizzolatti G (1986) fferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol 251: Matsumura M, Kubota K (1979) Cortical projection to hand-arm motor area from post-arcuate area in macaque monkeys: a histological study of retrograde transport of horseradish peroxidase. Neurosci Lett 11: Muakkassa KF, Strick PL (1979) Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized premotor areas. rain Res 177: Muir R, Lemon RN (1983) Corticospinal neurons with a special role in precision grip. rain Res 261: Murata, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G (1997) Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol 78: Murata, Gallese V, Luppino G, Kaseda M, Sakata H (2000) Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area IP. J Neurophysiol 83: Murray E, ussey TJ, Wise SP (2000) Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Exp rain Res 133: National Research Council (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, D.C.: National cademies. Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban G (2005) Observing others: multiple action representation in the frontal lobe. Science 310: Pesaran, Nelson MJ, ndersen R (2008) Free choice activates a decision circuit between frontal and parietal cortex. Nature 453: Petrides M, Pandya DN (2002) Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16: Raos V, Umiltá M, Gallese V, Fogassi L (2004) Functional properties of grasping-related neurons in the dorsal premotor area f2 of the macaque monkey. J Neurophysiol 92: Raos V, Umiltá M, Murata, Fogassi L, Gallese V (2006) Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J Neurophysiol 95: Rizzolatti G, Luppino G (2001) The cortical motor system. Neuron 31: Sakata H, Taira M, Murata, Mine S (1995) Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex 5: Scherberger H, ndersen R (2007) Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci 27: Stark E, sher I, beles M (2007) Encoding of reach and grasp by single neurons in premotor cortex is independent of recording site. J Neurophysiol 97: Taira M, Mine S, Georgopoulos P, Murata, Sakata H (1990) Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp rain Res 83: Tanné-Gariépy J, Rouiller EM, oussaoud D (2002) Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp rain Res 145: Umilta M, rochier T, Spinks RL, Lemon RN (2007) Simultaneous recording of macaque premotor and primary motor cortex neuronal populations reveals different functional contributions to visuomotor grasp. J Neurophysiol 98: Wallis JD, nderson KC, Miller EK (2001) Single neurons in prefrontal cortex encode abstract rules. Nature 411: White IM, Wise SP (1999) Rule-dependent neuronal activity in the prefrontal cortex. Exp rain Res 126: Zar JH (1999) iostatistical analysis. Upper Saddle River, New Jersey: Prentice-Hall.
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Population coding of grasp and laterality-related information in the macaque fronto-parietal network
 www.nature.com/scientificreports Received: 5 September 2017 Accepted: 11 January 2018 Published: xx xx xxxx OPEN Population coding of grasp and laterality-related information in the macaque fronto-parietal
www.nature.com/scientificreports Received: 5 September 2017 Accepted: 11 January 2018 Published: xx xx xxxx OPEN Population coding of grasp and laterality-related information in the macaque fronto-parietal
Movement-related activity during goal-directed hand actions in the monkey ventrolateral prefrontal cortex
 European Journal of Neuroscience, Vol. 42, pp. 2882 2894, 2015 doi:10.1111/ejn.13040 COGNITIVE NEUROSCIENCE Movement-related activity during goal-directed hand actions in the monkey ventrolateral prefrontal
European Journal of Neuroscience, Vol. 42, pp. 2882 2894, 2015 doi:10.1111/ejn.13040 COGNITIVE NEUROSCIENCE Movement-related activity during goal-directed hand actions in the monkey ventrolateral prefrontal
 Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Functional Properties of Grasping-Related Neurons in the Ventral Premotor Area F5 of the Macaque Monkey
 J Neurophysiol 95: 79 729, 6. First published October 26, 5; doi:1.1152/jn.463.5. Functional Properties of Grasping-Related Neurons in the Ventral Premotor Area F5 of the Macaque Monkey Vassilis Raos,
J Neurophysiol 95: 79 729, 6. First published October 26, 5; doi:1.1152/jn.463.5. Functional Properties of Grasping-Related Neurons in the Ventral Premotor Area F5 of the Macaque Monkey Vassilis Raos,
Parietofrontal Circuits for Action and Space Perception in the Macaque Monkey
 NeuroImage 14, S27 S32 (2001) doi:10.1006/nimg.2001.0835, available online at http://www.idealibrary.com on Parietofrontal Circuits for Action and Space Perception in the Macaque Monkey Massimo Matelli
NeuroImage 14, S27 S32 (2001) doi:10.1006/nimg.2001.0835, available online at http://www.idealibrary.com on Parietofrontal Circuits for Action and Space Perception in the Macaque Monkey Massimo Matelli
Report. View-Based Encoding of Actions in Mirror Neurons of Area F5 in Macaque Premotor Cortex
 Current Biology 21, 144 148, January 25, 2011 ª2011 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2010.12.022 View-Based Encoding of Actions in Mirror Neurons of Area F5 in Macaque Premotor Cortex
Current Biology 21, 144 148, January 25, 2011 ª2011 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2010.12.022 View-Based Encoding of Actions in Mirror Neurons of Area F5 in Macaque Premotor Cortex
Supplementary materials for: Executive control processes underlying multi- item working memory
 Supplementary materials for: Executive control processes underlying multi- item working memory Antonio H. Lara & Jonathan D. Wallis Supplementary Figure 1 Supplementary Figure 1. Behavioral measures of
Supplementary materials for: Executive control processes underlying multi- item working memory Antonio H. Lara & Jonathan D. Wallis Supplementary Figure 1 Supplementary Figure 1. Behavioral measures of
Supplemental material
 Supplemental material Research Article Title: The dorsomedial pathway is not just for reaching: Grasping neurons in the medial parieto-occipital cortex of the macaque monkey Abbreviated title: Authors:
Supplemental material Research Article Title: The dorsomedial pathway is not just for reaching: Grasping neurons in the medial parieto-occipital cortex of the macaque monkey Abbreviated title: Authors:
Direction of action is represented in the ventral premotor cortex
 articles 21 Nature Publishing Group http://neurosci.nature.com Direction of action is represented in the ventral premotor cortex 21 Nature Publishing Group http://neurosci.nature.com Shinji Kakei 1,2,4,
articles 21 Nature Publishing Group http://neurosci.nature.com Direction of action is represented in the ventral premotor cortex 21 Nature Publishing Group http://neurosci.nature.com Shinji Kakei 1,2,4,
Sum of Neurally Distinct Stimulus- and Task-Related Components.
 SUPPLEMENTARY MATERIAL for Cardoso et al. 22 The Neuroimaging Signal is a Linear Sum of Neurally Distinct Stimulus- and Task-Related Components. : Appendix: Homogeneous Linear ( Null ) and Modified Linear
SUPPLEMENTARY MATERIAL for Cardoso et al. 22 The Neuroimaging Signal is a Linear Sum of Neurally Distinct Stimulus- and Task-Related Components. : Appendix: Homogeneous Linear ( Null ) and Modified Linear
Transformation of a Virtual Action Plan into a Motor Plan in the Premotor Cortex
 The Journal of Neuroscience, October 8, 2008 28(41):10287 10297 10287 Behavioral/Systems/Cognitive Transformation of a Virtual Action Plan into a Motor Plan in the Premotor Cortex Yoshihisa Nakayama, Tomoko
The Journal of Neuroscience, October 8, 2008 28(41):10287 10297 10287 Behavioral/Systems/Cognitive Transformation of a Virtual Action Plan into a Motor Plan in the Premotor Cortex Yoshihisa Nakayama, Tomoko
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature14066 Supplementary discussion Gradual accumulation of evidence for or against different choices has been implicated in many types of decision-making, including value-based decisions
doi:10.1038/nature14066 Supplementary discussion Gradual accumulation of evidence for or against different choices has been implicated in many types of decision-making, including value-based decisions
Limb-Specific Representation for Reaching in the Posterior Parietal Cortex
 6128 The Journal of Neuroscience, June 11, 2008 28(24):6128 6140 Behavioral/Systems/Cognitive Limb-Specific Representation for Reaching in the Posterior Parietal Cortex Steve W. C. Chang, Anthony R. Dickinson,
6128 The Journal of Neuroscience, June 11, 2008 28(24):6128 6140 Behavioral/Systems/Cognitive Limb-Specific Representation for Reaching in the Posterior Parietal Cortex Steve W. C. Chang, Anthony R. Dickinson,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11239 Introduction The first Supplementary Figure shows additional regions of fmri activation evoked by the task. The second, sixth, and eighth shows an alternative way of analyzing reaction
doi:10.1038/nature11239 Introduction The first Supplementary Figure shows additional regions of fmri activation evoked by the task. The second, sixth, and eighth shows an alternative way of analyzing reaction
Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more
 1 Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more strongly when an image was negative than when the same
1 Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more strongly when an image was negative than when the same
Neurophysiology of systems
 Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
The Cortical Motor System
 Neuron, Vol. 31, 889 901, September 27, 2001, Copyright 2001 by Cell Press The Cortical Motor System Review Giacomo Rizzolatti 1 and Giuseppe Luppino Istituto di Fisiologia Umana Università di Parma Via
Neuron, Vol. 31, 889 901, September 27, 2001, Copyright 2001 by Cell Press The Cortical Motor System Review Giacomo Rizzolatti 1 and Giuseppe Luppino Istituto di Fisiologia Umana Università di Parma Via
When vision guides movement: a functional imaging study of the monkey brain
 NeuroImage 19 (2003) 959 967 www.elsevier.com/locate/ynimg When vision guides movement: a functional imaging study of the monkey brain Georgia G. Gregoriou a,b and Helen E. Savaki a,b, * a Department of
NeuroImage 19 (2003) 959 967 www.elsevier.com/locate/ynimg When vision guides movement: a functional imaging study of the monkey brain Georgia G. Gregoriou a,b and Helen E. Savaki a,b, * a Department of
Supplementary Information. Gauge size. midline. arcuate 10 < n < 15 5 < n < 10 1 < n < < n < 15 5 < n < 10 1 < n < 5. principal principal
 Supplementary Information set set = Reward = Reward Gauge size Gauge size 3 Numer of correct trials 3 Numer of correct trials Supplementary Fig.. Principle of the Gauge increase. The gauge size (y axis)
Supplementary Information set set = Reward = Reward Gauge size Gauge size 3 Numer of correct trials 3 Numer of correct trials Supplementary Fig.. Principle of the Gauge increase. The gauge size (y axis)
From Rule to Response: Neuronal Processes in the Premotor and Prefrontal Cortex
 J Neurophysiol 90: 1790 1806, 2003. First published May 7, 2003; 10.1152/jn.00086.2003. From Rule to Response: Neuronal Processes in the Premotor and Prefrontal Cortex Jonathan D. Wallis and Earl K. Miller
J Neurophysiol 90: 1790 1806, 2003. First published May 7, 2003; 10.1152/jn.00086.2003. From Rule to Response: Neuronal Processes in the Premotor and Prefrontal Cortex Jonathan D. Wallis and Earl K. Miller
Supporting Information
 Supporting Information Lingnau et al. 10.1073/pnas.0902262106 Fig. S1. Material presented during motor act observation (A) and execution (B). Each row shows one of the 8 different motor acts. Columns in
Supporting Information Lingnau et al. 10.1073/pnas.0902262106 Fig. S1. Material presented during motor act observation (A) and execution (B). Each row shows one of the 8 different motor acts. Columns in
Grasping Execution and Grasping Observation Activity of Single Neurons in the Macaque Anterior Intraparietal Area
 Grasping Execution and Grasping Observation Activity of Single Neurons in the Macaque Anterior Intraparietal Area Pierpaolo Pani, Tom Theys, Maria C. Romero, and Peter Janssen Abstract Primates use vision
Grasping Execution and Grasping Observation Activity of Single Neurons in the Macaque Anterior Intraparietal Area Pierpaolo Pani, Tom Theys, Maria C. Romero, and Peter Janssen Abstract Primates use vision
Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load
 Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load Intro Examine attention response functions Compare an attention-demanding
Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load Intro Examine attention response functions Compare an attention-demanding
Mirror neurons. Romana Umrianova
 Mirror neurons Romana Umrianova The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations Giacomo Rizzolatti and Corrado Sinigaglia Mechanism that unifies action
Mirror neurons Romana Umrianova The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations Giacomo Rizzolatti and Corrado Sinigaglia Mechanism that unifies action
Selectivity for Three-Dimensional Shape and Grasping-Related Activity in the Macaque Ventral Premotor Cortex
 12038 The Journal of Neuroscience, August 29, 2012 32(35):12038 12050 Behavioral/Systems/Cognitive Selectivity for Three-Dimensional Shape and Grasping-Related Activity in the Macaque Ventral Premotor
12038 The Journal of Neuroscience, August 29, 2012 32(35):12038 12050 Behavioral/Systems/Cognitive Selectivity for Three-Dimensional Shape and Grasping-Related Activity in the Macaque Ventral Premotor
Coordination among the body segments during reach-to-grasp action involving the trunk
 Exp Brain Res (1998) 123:346±350 Springer-Verlag 1998 RESEARCH NOTE Jinsung Wang George E. Stelmach Coordination among the body segments during reach-to-grasp action involving the trunk Received: 1 April
Exp Brain Res (1998) 123:346±350 Springer-Verlag 1998 RESEARCH NOTE Jinsung Wang George E. Stelmach Coordination among the body segments during reach-to-grasp action involving the trunk Received: 1 April
Ventrolateral Prefrontal Neuronal Activity Related to Active Controlled Memory Retrieval in Nonhuman Primates
 Cerebral Cortex 2007;17:i27-i40 doi:10.1093/cercor/bhm086 Ventrolateral Prefrontal Neuronal Activity Related to Active Controlled Memory Retrieval in Nonhuman Primates Genevie` ve Cadoret and Michael Petrides
Cerebral Cortex 2007;17:i27-i40 doi:10.1093/cercor/bhm086 Ventrolateral Prefrontal Neuronal Activity Related to Active Controlled Memory Retrieval in Nonhuman Primates Genevie` ve Cadoret and Michael Petrides
Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex
 The Journal of Neuroscience, March 11, 29 29(1):3259 327 3259 Behavioral/Systems/Cognitive Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex Steven W. Kennerley and Jonathan D.
The Journal of Neuroscience, March 11, 29 29(1):3259 327 3259 Behavioral/Systems/Cognitive Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex Steven W. Kennerley and Jonathan D.
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
What We Know Currently about Mirror Neurons
 Current Biology 23, R1057 R1062, December 2, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.10.051 What We Know Currently about Mirror Neurons Minireview J.M. Kilner and
Current Biology 23, R1057 R1062, December 2, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.10.051 What We Know Currently about Mirror Neurons Minireview J.M. Kilner and
Reward prediction based on stimulus categorization in. primate lateral prefrontal cortex
 Reward prediction based on stimulus categorization in primate lateral prefrontal cortex Xiaochuan Pan, Kosuke Sawa, Ichiro Tsuda, Minoro Tsukada, Masamichi Sakagami Supplementary Information This PDF file
Reward prediction based on stimulus categorization in primate lateral prefrontal cortex Xiaochuan Pan, Kosuke Sawa, Ichiro Tsuda, Minoro Tsukada, Masamichi Sakagami Supplementary Information This PDF file
Nature Neuroscience doi: /nn Supplementary Figure 1. Characterization of viral injections.
 Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
The organization of the cortical motor system: new concepts
 Electroencephalography and clinical Neurophysiology 106 (1998) 283 296 Invited review The organization of the cortical motor system: new concepts G. Rizzolatti*, G. Luppino, M. Matelli Istituto di Fisiologia
Electroencephalography and clinical Neurophysiology 106 (1998) 283 296 Invited review The organization of the cortical motor system: new concepts G. Rizzolatti*, G. Luppino, M. Matelli Istituto di Fisiologia
Human Paleoneurology and the Evolution of the Parietal Cortex
 PARIETAL LOBE The Parietal Lobes develop at about the age of 5 years. They function to give the individual perspective and to help them understand space, touch, and volume. The location of the parietal
PARIETAL LOBE The Parietal Lobes develop at about the age of 5 years. They function to give the individual perspective and to help them understand space, touch, and volume. The location of the parietal
Decoding a Perceptual Decision Process across Cortex
 Article Decoding a Perceptual Decision Process across Cortex Adrián Hernández, 1 Verónica Nácher, 1 Rogelio Luna, 1 Antonio Zainos, 1 Luis Lemus, 1 Manuel Alvarez, 1 Yuriria Vázquez, 1 Liliana Camarillo,
Article Decoding a Perceptual Decision Process across Cortex Adrián Hernández, 1 Verónica Nácher, 1 Rogelio Luna, 1 Antonio Zainos, 1 Luis Lemus, 1 Manuel Alvarez, 1 Yuriria Vázquez, 1 Liliana Camarillo,
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
U.S. copyright law (title 17 of U.S. code) governs the reproduction and redistribution of copyrighted material.
 U.S. copyright law (title 17 of U.S. code) governs the reproduction and redistribution of copyrighted material. The Journal of Neuroscience, June 15, 2003 23(12):5235 5246 5235 A Comparison of Primate
U.S. copyright law (title 17 of U.S. code) governs the reproduction and redistribution of copyrighted material. The Journal of Neuroscience, June 15, 2003 23(12):5235 5246 5235 A Comparison of Primate
The Dorsomedial Pathway Is Not Just for Reaching: Grasping Neurons in the Medial Parieto-Occipital Cortex of the Macaque Monkey
 342 The Journal of Neuroscience, January 6, 2010 30(1):342 349 Behavioral/Systems/Cognitive The Dorsomedial Pathway Is Not Just for Reaching: Grasping Neurons in the Medial Parieto-Occipital Cortex of
342 The Journal of Neuroscience, January 6, 2010 30(1):342 349 Behavioral/Systems/Cognitive The Dorsomedial Pathway Is Not Just for Reaching: Grasping Neurons in the Medial Parieto-Occipital Cortex of
Modest Gaze-Related Discharge Modulation in Monkey Dorsal Premotor Cortex During a Reaching Task Performed With Free Fixation
 J Neurophysiol 88: 1064 1072, 2002; 10.1152/jn.09951.2001. RAPID COMMUNICATION Modest Gaze-Related Discharge Modulation in Monkey Dorsal Premotor Cortex During a Reaching Task Performed With Free Fixation
J Neurophysiol 88: 1064 1072, 2002; 10.1152/jn.09951.2001. RAPID COMMUNICATION Modest Gaze-Related Discharge Modulation in Monkey Dorsal Premotor Cortex During a Reaching Task Performed With Free Fixation
Synchrony and the attentional state
 Synchrony and the attentional state Ernst Niebur, Dept. of Neuroscience & Krieger Mind/Brain Institute Johns Hopkins University niebur@jhu.edu Collaborators Arup Roy monkey monkey Peter monkey Steven monkey
Synchrony and the attentional state Ernst Niebur, Dept. of Neuroscience & Krieger Mind/Brain Institute Johns Hopkins University niebur@jhu.edu Collaborators Arup Roy monkey monkey Peter monkey Steven monkey
Hand kinematics during reaching and grasping in the macaque monkey
 Behavioural Brain Research 117 (2000) 75 82 www.elsevier.com/locate/bbr Research report Hand kinematics during reaching and grasping in the macaque monkey Alice C. Roy, Yves Paulignan, Alessandro Farnè
Behavioural Brain Research 117 (2000) 75 82 www.elsevier.com/locate/bbr Research report Hand kinematics during reaching and grasping in the macaque monkey Alice C. Roy, Yves Paulignan, Alessandro Farnè
Presupplementary Motor Area Activation during Sequence Learning Reflects Visuo-Motor Association
 The Journal of Neuroscience, 1999, Vol. 19 RC1 1of6 Presupplementary Motor Area Activation during Sequence Learning Reflects Visuo-Motor Association Katsuyuki Sakai, 1,2 Okihide Hikosaka, 1 Satoru Miyauchi,
The Journal of Neuroscience, 1999, Vol. 19 RC1 1of6 Presupplementary Motor Area Activation during Sequence Learning Reflects Visuo-Motor Association Katsuyuki Sakai, 1,2 Okihide Hikosaka, 1 Satoru Miyauchi,
Parietal Reach Region Encodes Reach Depth Using Retinal Disparity and Vergence Angle Signals
 J Neurophysiol 12: 85 816, 29. First published May 13, 29; doi:1.12/jn.9359.28. Parietal Reach Region Encodes Reach Depth Using Retinal Disparity and Vergence Angle Signals Rajan Bhattacharyya, 1 Sam Musallam,
J Neurophysiol 12: 85 816, 29. First published May 13, 29; doi:1.12/jn.9359.28. Parietal Reach Region Encodes Reach Depth Using Retinal Disparity and Vergence Angle Signals Rajan Bhattacharyya, 1 Sam Musallam,
CISC 3250 Systems Neuroscience
 CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
NIH Public Access Author Manuscript Nature. Author manuscript; available in PMC 2009 August 17.
 NIH Public Access Author Manuscript Published in final edited form as: Nature. 2008 May 15; 453(7193): 406 409. doi:10.1038/nature06849. Free choice activates a decision circuit between frontal and parietal
NIH Public Access Author Manuscript Published in final edited form as: Nature. 2008 May 15; 453(7193): 406 409. doi:10.1038/nature06849. Free choice activates a decision circuit between frontal and parietal
Geography of the Forehead
 5. Brain Areas Geography of the Forehead Everyone thinks the brain is so complicated, but let s look at the facts. The frontal lobe, for example, is located in the front! And the temporal lobe is where
5. Brain Areas Geography of the Forehead Everyone thinks the brain is so complicated, but let s look at the facts. The frontal lobe, for example, is located in the front! And the temporal lobe is where
Representation of negative motivational value in the primate
 Representation of negative motivational value in the primate lateral habenula Masayuki Matsumoto & Okihide Hikosaka Supplementary Figure 1 Anticipatory licking and blinking. (a, b) The average normalized
Representation of negative motivational value in the primate lateral habenula Masayuki Matsumoto & Okihide Hikosaka Supplementary Figure 1 Anticipatory licking and blinking. (a, b) The average normalized
P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center. Wednesday, 16 March 2009, 1:00p.m. 2:00p.m.
 Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Prefrontal Activity During Serial Probe Reproduction Task: Encoding, Mnemonic, and Retrieval Processes
 Prefrontal Activity During Serial Probe Reproduction Task: Encoding, Mnemonic, and Retrieval Processes Masato Inoue and Akichika Mikami JN 95:1008-1041, 2006. First published Oct 5, 2005; doi:10.1152/jn.00552.2005
Prefrontal Activity During Serial Probe Reproduction Task: Encoding, Mnemonic, and Retrieval Processes Masato Inoue and Akichika Mikami JN 95:1008-1041, 2006. First published Oct 5, 2005; doi:10.1152/jn.00552.2005
NIH Public Access Author Manuscript J Neurophysiol. Author manuscript; available in PMC 2010 May 12.
 NIH Public Access Author Manuscript Published in final edited form as: J Neurophysiol. 2007 February ; 97(2): 1656 1670. doi:10.1152/jn.01031.2006. Neurophysiology of Prehension. II. Response Diversity
NIH Public Access Author Manuscript Published in final edited form as: J Neurophysiol. 2007 February ; 97(2): 1656 1670. doi:10.1152/jn.01031.2006. Neurophysiology of Prehension. II. Response Diversity
Common Neural Substrate for Processing Depth and Direction Signals for Reaching in the Monkey Medial Posterior Parietal Cortex
 Cerebral Cortex doi:10.1093/cercor/bht021 Cerebral Cortex Advance Access published February 4, 2013 Common Neural Substrate for Processing Depth and Direction Signals for Reaching in the Monkey Medial
Cerebral Cortex doi:10.1093/cercor/bht021 Cerebral Cortex Advance Access published February 4, 2013 Common Neural Substrate for Processing Depth and Direction Signals for Reaching in the Monkey Medial
Motor Systems I Cortex. Reading: BCP Chapter 14
 Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Supplemental Material
 1 Supplemental Material Golomb, J.D, and Kanwisher, N. (2012). Higher-level visual cortex represents retinotopic, not spatiotopic, object location. Cerebral Cortex. Contents: - Supplemental Figures S1-S3
1 Supplemental Material Golomb, J.D, and Kanwisher, N. (2012). Higher-level visual cortex represents retinotopic, not spatiotopic, object location. Cerebral Cortex. Contents: - Supplemental Figures S1-S3
Cerebrum-Cerebral Hemispheres. Cuneyt Mirzanli Istanbul Gelisim University
 Cerebrum-Cerebral Hemispheres Cuneyt Mirzanli Istanbul Gelisim University The largest part of the brain. Ovoid shape. Two incompletely separated cerebral hemispheres. The outer surface of the cerebral
Cerebrum-Cerebral Hemispheres Cuneyt Mirzanli Istanbul Gelisim University The largest part of the brain. Ovoid shape. Two incompletely separated cerebral hemispheres. The outer surface of the cerebral
Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements
 Y. Isomura et al. 1 Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements Yoshikazu Isomura, Rie Harukuni, Takashi Takekawa, Hidenori Aizawa & Tomoki Fukai
Y. Isomura et al. 1 Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements Yoshikazu Isomura, Rie Harukuni, Takashi Takekawa, Hidenori Aizawa & Tomoki Fukai
Selective Attention. Modes of Control. Domains of Selection
 The New Yorker (2/7/5) Selective Attention Perception and awareness are necessarily selective (cell phone while driving): attention gates access to awareness Selective attention is deployed via two modes
The New Yorker (2/7/5) Selective Attention Perception and awareness are necessarily selective (cell phone while driving): attention gates access to awareness Selective attention is deployed via two modes
Observational Learning Based on Models of Overlapping Pathways
 Observational Learning Based on Models of Overlapping Pathways Emmanouil Hourdakis and Panos Trahanias Institute of Computer Science, Foundation for Research and Technology Hellas (FORTH) Science and Technology
Observational Learning Based on Models of Overlapping Pathways Emmanouil Hourdakis and Panos Trahanias Institute of Computer Science, Foundation for Research and Technology Hellas (FORTH) Science and Technology
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,100 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,100 116,000 120M Open access books available International authors and editors Downloads Our
How do individuals with congenital blindness form a conscious representation of a world they have never seen? brain. deprived of sight?
 How do individuals with congenital blindness form a conscious representation of a world they have never seen? What happens to visual-devoted brain structure in individuals who are born deprived of sight?
How do individuals with congenital blindness form a conscious representation of a world they have never seen? What happens to visual-devoted brain structure in individuals who are born deprived of sight?
Deliverable Item 4.5 Final Results of the Biological Experiments: Monkey data, TMS and Behavioural Development
 MIRROR IST 2000-28159 Mirror Neurons based Object Recognition Deliverable Item 4.5 Final Results of the Biological Experiments: Monkey data, TMS and Behavioural Development Delivery Date: November 21 st,
MIRROR IST 2000-28159 Mirror Neurons based Object Recognition Deliverable Item 4.5 Final Results of the Biological Experiments: Monkey data, TMS and Behavioural Development Delivery Date: November 21 st,
Prof. Greg Francis 7/31/15
 s PSY 200 Greg Francis Lecture 06 How do you recognize your grandmother? Action potential With enough excitatory input, a cell produces an action potential that sends a signal down its axon to other cells
s PSY 200 Greg Francis Lecture 06 How do you recognize your grandmother? Action potential With enough excitatory input, a cell produces an action potential that sends a signal down its axon to other cells
Double dissociation of value computations in orbitofrontal and anterior cingulate neurons
 Supplementary Information for: Double dissociation of value computations in orbitofrontal and anterior cingulate neurons Steven W. Kennerley, Timothy E. J. Behrens & Jonathan D. Wallis Content list: Supplementary
Supplementary Information for: Double dissociation of value computations in orbitofrontal and anterior cingulate neurons Steven W. Kennerley, Timothy E. J. Behrens & Jonathan D. Wallis Content list: Supplementary
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Finding the Central Sulcus MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Medical Neuroscience Tutorial Notes Finding the Central Sulcus MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Giacomo Rizzolatti - selected references
 Giacomo Rizzolatti - selected references 1 Rizzolatti, G., Semi, A. A., & Fabbri-Destro, M. (2014). Linking psychoanalysis with neuroscience: the concept of ego. Neuropsychologia, 55, 143-148. Notes: Through
Giacomo Rizzolatti - selected references 1 Rizzolatti, G., Semi, A. A., & Fabbri-Destro, M. (2014). Linking psychoanalysis with neuroscience: the concept of ego. Neuropsychologia, 55, 143-148. Notes: Through
Supplementary Information Methods Subjects The study was comprised of 84 chronic pain patients with either chronic back pain (CBP) or osteoarthritis
 Supplementary Information Methods Subjects The study was comprised of 84 chronic pain patients with either chronic back pain (CBP) or osteoarthritis (OA). All subjects provided informed consent to procedures
Supplementary Information Methods Subjects The study was comprised of 84 chronic pain patients with either chronic back pain (CBP) or osteoarthritis (OA). All subjects provided informed consent to procedures
Theta sequences are essential for internally generated hippocampal firing fields.
 Theta sequences are essential for internally generated hippocampal firing fields. Yingxue Wang, Sandro Romani, Brian Lustig, Anthony Leonardo, Eva Pastalkova Supplementary Materials Supplementary Modeling
Theta sequences are essential for internally generated hippocampal firing fields. Yingxue Wang, Sandro Romani, Brian Lustig, Anthony Leonardo, Eva Pastalkova Supplementary Materials Supplementary Modeling
Cognitive Modelling Themes in Neural Computation. Tom Hartley
 Cognitive Modelling Themes in Neural Computation Tom Hartley t.hartley@psychology.york.ac.uk Typical Model Neuron x i w ij x j =f(σw ij x j ) w jk x k McCulloch & Pitts (1943), Rosenblatt (1957) Net input:
Cognitive Modelling Themes in Neural Computation Tom Hartley t.hartley@psychology.york.ac.uk Typical Model Neuron x i w ij x j =f(σw ij x j ) w jk x k McCulloch & Pitts (1943), Rosenblatt (1957) Net input:
Supplementary Data for "Single-trial learning of novel stimuli by individual. neurons of the human hippocampus-amygdala complex"
 Supplementary Data for "Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex" Figure S1. Behavioral performance of all subjects. Recognition performance
Supplementary Data for "Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex" Figure S1. Behavioral performance of all subjects. Recognition performance
The Frontal Lobes. Anatomy of the Frontal Lobes. Anatomy of the Frontal Lobes 3/2/2011. Portrait: Losing Frontal-Lobe Functions. Readings: KW Ch.
 The Frontal Lobes Readings: KW Ch. 16 Portrait: Losing Frontal-Lobe Functions E.L. Highly organized college professor Became disorganized, showed little emotion, and began to miss deadlines Scores on intelligence
The Frontal Lobes Readings: KW Ch. 16 Portrait: Losing Frontal-Lobe Functions E.L. Highly organized college professor Became disorganized, showed little emotion, and began to miss deadlines Scores on intelligence
Strick Lecture 3 March 22, 2017 Page 1
 Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Behavioral training.
 Supplementary Figure 1 Behavioral training. a, Mazes used for behavioral training. Asterisks indicate reward location. Only some example mazes are shown (for example, right choice and not left choice maze
Supplementary Figure 1 Behavioral training. a, Mazes used for behavioral training. Asterisks indicate reward location. Only some example mazes are shown (for example, right choice and not left choice maze
Supplemental Material
 Supplemental Material Recording technique Multi-unit activity (MUA) was recorded from electrodes that were chronically implanted (Teflon-coated platinum-iridium wires) in the primary visual cortex representing
Supplemental Material Recording technique Multi-unit activity (MUA) was recorded from electrodes that were chronically implanted (Teflon-coated platinum-iridium wires) in the primary visual cortex representing
Ch.20 Dynamic Cue Combination in Distributional Population Code Networks. Ka Yeon Kim Biopsychology
 Ch.20 Dynamic Cue Combination in Distributional Population Code Networks Ka Yeon Kim Biopsychology Applying the coding scheme to dynamic cue combination (Experiment, Kording&Wolpert,2004) Dynamic sensorymotor
Ch.20 Dynamic Cue Combination in Distributional Population Code Networks Ka Yeon Kim Biopsychology Applying the coding scheme to dynamic cue combination (Experiment, Kording&Wolpert,2004) Dynamic sensorymotor
Comparison of Associative Learning- Related Signals in the Macaque Perirhinal Cortex and Hippocampus
 Cerebral Cortex May 2009;19:1064--1078 doi:10.1093/cercor/bhn156 Advance Access publication October 20, 2008 Comparison of Associative Learning- Related Signals in the Macaque Perirhinal Cortex and Hippocampus
Cerebral Cortex May 2009;19:1064--1078 doi:10.1093/cercor/bhn156 Advance Access publication October 20, 2008 Comparison of Associative Learning- Related Signals in the Macaque Perirhinal Cortex and Hippocampus
Effects of Attention on MT and MST Neuronal Activity During Pursuit Initiation
 Effects of Attention on MT and MST Neuronal Activity During Pursuit Initiation GREGG H. RECANZONE 1 AND ROBERT H. WURTZ 2 1 Center for Neuroscience and Section of Neurobiology, Physiology and Behavior,
Effects of Attention on MT and MST Neuronal Activity During Pursuit Initiation GREGG H. RECANZONE 1 AND ROBERT H. WURTZ 2 1 Center for Neuroscience and Section of Neurobiology, Physiology and Behavior,
Voluntary Movements. Lu Chen, Ph.D. MCB, UC Berkeley. Outline. Organization of the motor cortex (somatotopic) Corticospinal projection
 Voluntary Movements Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Organization of the motor cortex (somatotopic) Corticospinal projection Physiology of motor neurons Direction representation, population coding
Voluntary Movements Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Organization of the motor cortex (somatotopic) Corticospinal projection Physiology of motor neurons Direction representation, population coding
A system in the human brain for predicting the actions of others
 A system in the human brain for predicting the actions of others Narender Ramnani 1 & R Christopher Miall 2 The ability to attribute mental states to others, and therefore to predict others behavior, is
A system in the human brain for predicting the actions of others Narender Ramnani 1 & R Christopher Miall 2 The ability to attribute mental states to others, and therefore to predict others behavior, is
in Motion analysis TRAMA Project September th 2007
 First Course Basics in Motion analysis TRAMA Project September 10-12 th 2007 Prof. Guy CHERON Laboratory of Neurophysiology and Movement Biomechanics Université Libre de Bruxelles, Belgium Objectives Neurophysiology
First Course Basics in Motion analysis TRAMA Project September 10-12 th 2007 Prof. Guy CHERON Laboratory of Neurophysiology and Movement Biomechanics Université Libre de Bruxelles, Belgium Objectives Neurophysiology
Characterization of serial order encoding in the monkey anterior cingulate sulcus
 HAL Archives Ouvertes!France Author Manuscript Accepted for publication in a peer reviewed journal. Published in final edited form as: Eur J Neurosci. 2001 September ; 14(6): 1041 1046. Characterization
HAL Archives Ouvertes!France Author Manuscript Accepted for publication in a peer reviewed journal. Published in final edited form as: Eur J Neurosci. 2001 September ; 14(6): 1041 1046. Characterization
PUBBLICAZIONI Pubblicazioni peer reviewed in extenso
 PUBBLICAZIONI Pubblicazioni peer reviewed in extenso 1. Gerbella M, Rozzi S, Rizzolatti G. The extended object-grasping network. Exp Brain Res. 2017 Jul 26 2. Ferrari PF, Gerbella M, Coudé G, Rozzi S.
PUBBLICAZIONI Pubblicazioni peer reviewed in extenso 1. Gerbella M, Rozzi S, Rizzolatti G. The extended object-grasping network. Exp Brain Res. 2017 Jul 26 2. Ferrari PF, Gerbella M, Coudé G, Rozzi S.
The mirror-neuron system: a Bayesian perspective
 CE: madhu ED: Susan Koshy Op: srini WNR: LWW_WNR_4050 COMMISSIONED REVIEW NEUROREPORT The mirror-neuron system: a Bayesian perspective James M. Kilner, Karl J. Friston and Chris D. Frith WellcomeTrust
CE: madhu ED: Susan Koshy Op: srini WNR: LWW_WNR_4050 COMMISSIONED REVIEW NEUROREPORT The mirror-neuron system: a Bayesian perspective James M. Kilner, Karl J. Friston and Chris D. Frith WellcomeTrust
Computational Explorations in Cognitive Neuroscience Chapter 7: Large-Scale Brain Area Functional Organization
 Computational Explorations in Cognitive Neuroscience Chapter 7: Large-Scale Brain Area Functional Organization 1 7.1 Overview This chapter aims to provide a framework for modeling cognitive phenomena based
Computational Explorations in Cognitive Neuroscience Chapter 7: Large-Scale Brain Area Functional Organization 1 7.1 Overview This chapter aims to provide a framework for modeling cognitive phenomena based
TMS Disruption of Time Encoding in Human Primary Visual Cortex Molly Bryan Beauchamp Lab
 TMS Disruption of Time Encoding in Human Primary Visual Cortex Molly Bryan Beauchamp Lab This report details my summer research project for the REU Theoretical and Computational Neuroscience program as
TMS Disruption of Time Encoding in Human Primary Visual Cortex Molly Bryan Beauchamp Lab This report details my summer research project for the REU Theoretical and Computational Neuroscience program as
Parietal Reach Region Encodes Reach Depth Using Retinal Disparity and Vergence Angle Signals
 Articles in PresS. J Neurophysiol (May 13, 2009). doi:10.1152/jn.90359.2008 1 Parietal Reach Region Encodes Reach Depth Using Retinal Disparity and Vergence Angle Signals Authors: Rajan Bhattacharyya 1,
Articles in PresS. J Neurophysiol (May 13, 2009). doi:10.1152/jn.90359.2008 1 Parietal Reach Region Encodes Reach Depth Using Retinal Disparity and Vergence Angle Signals Authors: Rajan Bhattacharyya 1,
Electrical recording with micro- and macroelectrodes from the cerebellum of man
 Electrical recording with micro- and macroelectrodes from the cerebellum of man D. GRAHAM SLAUGHTER, M.D., BLAINE S. NASHOLD, Jn., M.D., AND GEORGE G. SOMJEN, M.D. The Division of Neurosurgery, and the
Electrical recording with micro- and macroelectrodes from the cerebellum of man D. GRAHAM SLAUGHTER, M.D., BLAINE S. NASHOLD, Jn., M.D., AND GEORGE G. SOMJEN, M.D. The Division of Neurosurgery, and the
Supplemental Information. Gamma and the Coordination of Spiking. Activity in Early Visual Cortex. Supplemental Information Inventory:
 Neuron, Volume 77 Supplemental Information Gamma and the Coordination of Spiking Activity in Early Visual Cortex Xiaoxuan Jia, Seiji Tanabe, and Adam Kohn Supplemental Information Inventory: Supplementary
Neuron, Volume 77 Supplemental Information Gamma and the Coordination of Spiking Activity in Early Visual Cortex Xiaoxuan Jia, Seiji Tanabe, and Adam Kohn Supplemental Information Inventory: Supplementary
HST.583 Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Fall 2006
 MIT OpenCourseWare http://ocw.mit.edu HST.583 Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Fall 2006 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
MIT OpenCourseWare http://ocw.mit.edu HST.583 Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Fall 2006 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
THE PREFRONTAL CORTEX. Connections. Dorsolateral FrontalCortex (DFPC) Inputs
 THE PREFRONTAL CORTEX Connections Dorsolateral FrontalCortex (DFPC) Inputs The DPFC receives inputs predominantly from somatosensory, visual and auditory cortical association areas in the parietal, occipital
THE PREFRONTAL CORTEX Connections Dorsolateral FrontalCortex (DFPC) Inputs The DPFC receives inputs predominantly from somatosensory, visual and auditory cortical association areas in the parietal, occipital
Cerebellum: little brain. Cerebellum. gross divisions
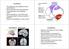 Cerebellum The anatomy of the cerebellum and its gross divisions Its principal input and output pathways The organization of the cerebellar cortex Role of climbing vs. mossy fibre input The parallel-fibre/
Cerebellum The anatomy of the cerebellum and its gross divisions Its principal input and output pathways The organization of the cerebellar cortex Role of climbing vs. mossy fibre input The parallel-fibre/
Dissociation of Response Variability from Firing Rate Effects in Frontal Eye Field Neurons during Visual Stimulation, Working Memory, and Attention
 2204 The Journal of Neuroscience, February 8, 2012 32(6):2204 2216 Behavioral/Systems/Cognitive Dissociation of Response Variability from Firing Rate Effects in Frontal Eye Field Neurons during Visual
2204 The Journal of Neuroscience, February 8, 2012 32(6):2204 2216 Behavioral/Systems/Cognitive Dissociation of Response Variability from Firing Rate Effects in Frontal Eye Field Neurons during Visual
Supplementary Information
 Supplementary Information The neural correlates of subjective value during intertemporal choice Joseph W. Kable and Paul W. Glimcher a 10 0 b 10 0 10 1 10 1 Discount rate k 10 2 Discount rate k 10 2 10
Supplementary Information The neural correlates of subjective value during intertemporal choice Joseph W. Kable and Paul W. Glimcher a 10 0 b 10 0 10 1 10 1 Discount rate k 10 2 Discount rate k 10 2 10
5th Mini-Symposium on Cognition, Decision-making and Social Function: In Memory of Kang Cheng
 5th Mini-Symposium on Cognition, Decision-making and Social Function: In Memory of Kang Cheng 13:30-13:35 Opening 13:30 17:30 13:35-14:00 Metacognition in Value-based Decision-making Dr. Xiaohong Wan (Beijing
5th Mini-Symposium on Cognition, Decision-making and Social Function: In Memory of Kang Cheng 13:30-13:35 Opening 13:30 17:30 13:35-14:00 Metacognition in Value-based Decision-making Dr. Xiaohong Wan (Beijing
Auditory Peripersonal Space in Humans: a Case of Auditory Tactile Extinction
 Neurocase (2001) Vol. 7, pp. 97 103 Oxford University Press 2001 Auditory Peripersonal Space in Humans: a Case of Auditory Tactile Extinction Elisabetta Làdavas 1, Francesco Pavani 1,2 and Alessandro Farnè
Neurocase (2001) Vol. 7, pp. 97 103 Oxford University Press 2001 Auditory Peripersonal Space in Humans: a Case of Auditory Tactile Extinction Elisabetta Làdavas 1, Francesco Pavani 1,2 and Alessandro Farnè
Visual Selection and Attention
 Visual Selection and Attention Retrieve Information Select what to observe No time to focus on every object Overt Selections Performed by eye movements Covert Selections Performed by visual attention 2
Visual Selection and Attention Retrieve Information Select what to observe No time to focus on every object Overt Selections Performed by eye movements Covert Selections Performed by visual attention 2
Morton-Style Factorial Coding of Color in Primary Visual Cortex
 Morton-Style Factorial Coding of Color in Primary Visual Cortex Javier R. Movellan Institute for Neural Computation University of California San Diego La Jolla, CA 92093-0515 movellan@inc.ucsd.edu Thomas
Morton-Style Factorial Coding of Color in Primary Visual Cortex Javier R. Movellan Institute for Neural Computation University of California San Diego La Jolla, CA 92093-0515 movellan@inc.ucsd.edu Thomas
Movement Sequence-Related Activity Reflecting Numerical Order of Components in Supplementary and Presupplementary Motor Areas
 RAPID COMMUNICATION Movement Sequence-Related Activity Reflecting Numerical Order of Components in Supplementary and Presupplementary Motor Areas WILLIAM T. CLOWER AND GARRETT E. ALEXANDER Department of
RAPID COMMUNICATION Movement Sequence-Related Activity Reflecting Numerical Order of Components in Supplementary and Presupplementary Motor Areas WILLIAM T. CLOWER AND GARRETT E. ALEXANDER Department of
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Rules of apparent motion: The shortest-path constraint: objects will take the shortest path between flashed positions.
 Rules of apparent motion: The shortest-path constraint: objects will take the shortest path between flashed positions. The box interrupts the apparent motion. The box interrupts the apparent motion.
Rules of apparent motion: The shortest-path constraint: objects will take the shortest path between flashed positions. The box interrupts the apparent motion. The box interrupts the apparent motion.
Exploring the pulvinar path to visual cortex
 C. Kennard & R.J. Leigh (Eds.) Progress in Brain Research, Vol. 171 ISSN 0079-6123 Copyright r 2008 Elsevier B.V. All rights reserved CHAPTER 5.14 Exploring the pulvinar path to visual cortex Rebecca A.
C. Kennard & R.J. Leigh (Eds.) Progress in Brain Research, Vol. 171 ISSN 0079-6123 Copyright r 2008 Elsevier B.V. All rights reserved CHAPTER 5.14 Exploring the pulvinar path to visual cortex Rebecca A.
