Occupational Postexposure Prophylaxis
|
|
|
- Marcus Derrick Sanders
- 5 years ago
- Views:
Transcription
1 National HIV Curriculum PDF created July 1, 2018, 11:59 pm Occupational Postexposure Prophylaxis This is a PDF version of the following document: Section 5: Prevention of HIV Topic 3: Occupational Postexposure Prophylaxis You can always find the most up to date version of this document at Introduction Background Although exposure prevention remains the primary strategy for reducing occupationally acquired HIV infection, appropriate postexposure management is an important element of workplace safety. In 1990, the Centers for Disease Control and Prevention (CDC) issued a statement that management of occupational exposure to HIV should consider use of zidovudine for postexposure prophylaxis (PEP).[1] The first iteration of the U.S. Public Health Service recommendations advocating the use of PEP dates back to 1996.[2] As more data emerged and more antiretroviral medications became available, the occupational PEP guidelines were updated four times (Figure 1), with the most recent of these published as the 2013 U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV.[3,4,5,6] Occupational exposures, particularly those known to involve risk for HIV transmission, are urgent medical matters and clinicians should be familiar with updated PEP guidelines. In addition, all health care facilities and clinics should have policies and procedures in place to ensure that appropriate mechanisms are available for timely management. Issues related to the management of nonoccupational exposures to HIV are addressed in the Topic Review Nonoccupational Postexposure Prophylaxis. Management of exposure to other blood borne pathogens (hepatitis B virus or hepatitis C virus) is not addressed in this Topic Review. Definition of Health Care Worker/Health Care Personnel For the purposes of initiating PEP, the Occupational PEP Guidelines use the term health care personnel to refer to all paid and unpaid persons working in health care settings who have the potential for exposure to infectious materials, including body substances (blood, tissue, and specific body fluids), contaminated medical supplies and equipment, and contaminated environmental surfaces.[3] Health care personnel (also called health care workers) might include, but are not limited to, emergency medical service personnel, dental personnel, laboratory personnel, autopsy personnel, nurses, nursing assistants, physicians, technicians, therapists, pharmacists, students and trainees, contractual staff not employed by the health care facility, and persons not directly involved in patient care but potentially exposed to blood and body fluids (e.g. clerical, dietary, housekeeping, security, maintenance, and volunteer personnel). In addition, the 2013 Occupational PEP Guidelines note the same principles of exposure management could be used for similar types of exposures that occur in other occupational settings, such as might occur with public safety officers. Estimated Risk for Occupational Acquisition of HIV In the situation where a health care worker suffers a percutaneous exposure to HIV-infected blood and does not take PEP, the estimated risk for HIV acquisition is approximately 0.2 to 0.3%.[7,8,9] After a mucous membrane exposure, such as eye or mouth contact with blood, the risk is approximately 0.09%.[3] In the United States, from 1985 to 2013, a total of 58 confirmed and 150 Page 1/29
2 possible cases of occupational transmission of HIV had been reported to the CDC; only one of the confirmed cases occurred after 1999 and that case involved a laboratory worker who had a needle puncture wound while working with a live HIV culture (Figure 2).[10] Of the 58 documented cases of occupationally-acquired HIV, 49 resulted from a percutaneous cut or puncture (Figure 3).[10] Transmission of HIV with blood contact of non-intact skin has been documented in case reports, but most experts consider this risk significantly lower than with a mucous membrane exposure. Epidemiological studies have identified several factors associated with increased risk of HIV transmission following an occupational exposure: a larger quantity of blood from the source patient (device visibly contaminated with blood, needle recently used in an artery or vein, larger bore needle, deeper injury) and a source patient with a diagnosis of terminal AIDS (Table 1).[11] Note that in earlier years of the HIV epidemic, terminal AIDS was considered a surrogate marker for a high plasma HIV RNA level typically seen in late-stage AIDS. Page 2/29
3 Rationale for Occupational PEP When an individual has a percutaneous or mucous membrane exposure to HIV-contaminated blood or fluids, replication of HIV first occurs in the dendritic cells of the skin and mucosa before spreading through lymphatic vessels and developing into systemic infection; the delay in spread of HIV is thought to leave a window of opportunity for PEP to prevent establishment of chronic HIV infection. A landmark study published in 1997 provided the first convincing evidence that PEP significantly decreased the risk of occupational HIV acquisition following a needlestick injury.[11] In this report, investigators performed a case-control study of needlestick injuries involving health care workers and demonstrated that zidovudine PEP, which was typically taken for at least 4 weeks, reduced the risk of seroconversion by 81% if implemented within 4 hours of the exposure.[11] This study, along with CDC recommendations, led to the widespread use of antiretroviral therapy for occupational PEP in the late 1990s. From the year 2000 onward, occupationally-acquired HIV infection in the United States has become exceedingly rare,[10] a finding that indirectly supports the efficacy of PEP. In addition, there are data supporting the use of PEP based on small observational studies among humans and animal transmission models.[12,13] There have been no published randomized control trials that evaluated PEP for occupational exposures to HIV, and due to ethical and logistical reasons, it is unlikely that such studies will be performed in the future. On the basis of available data, there is a strong rationale for using PEP for health care personnel exposed to HIV.[3] Page 3/29
4 Risk Assessment of the Occupational Exposure Event General Approach It is important first to determine whether the exposure involving the health care worker actually warrants PEP. In general, this assessment should initially obtain four key items of information: (1) HIV status of the source patient, (2) type of body fluid involved in the exposure, (3) nature of the exposure (percutaneous, mucous membrane, or contact with non-intact skin), and (4) timing of when the exposure occurred.[6] If the exposure is deemed an occupational exposure to a source patient known to be infected with HIV, additional information should be obtained, such as the source patient s most recent HIV RNA level (also known as viral load), their current antiretroviral treatment (if any), and any history of HIV drug resistance. Further, as part of the overall exposure evaluation, the risk assessment for transmission of hepatitis B virus or hepatitis C virus should be undertaken. Determining HIV Status of Source Patient The use of PEP applies to situations in which a health care worker has experienced an exposure to blood or body fluids from a source patient with documented or suspected HIV infection. The HIV status of the source patient, if unknown, should be determined to guide appropriate use of PEP.[3] In many health care settings, clinicians have access to FDA-approved rapid HIV tests that can provide a preliminary determination of a source patient s HIV serostatus within 30 minutes. The FDA-approved rapid HIV antibody tests perform well in detecting persons with chronic HIV infection, but have poor sensitivity in detecting acute or very recent HIV infection. The new rapid fourth-generation Alere Determine HIV-1/2 Ag/Ab test has also shown relatively poor performance in detecting persons with recent infection;[14,15] this contrasts with the relatively high sensitivity of the non-rapid fourthgeneration tests Architect HIV-1/2 Ag/Ab Combo test and Bio-Rad GS HIV Comb Ag/Ab EIA, which have approximately 85% sensitivity in detecting recent (acute) HIV infection.[16,17] Any preliminary positive rapid test result requires confirmation with additional HIV testing. If a rapid HIV test is not available, PEP should not be delayed while waiting for the test results. In the situation where a source patient s HIV status is initially unknown and the health care worker starts on antiretroviral PEP, discontinuation of the antiretroviral PEP is warranted if HIV testing subsequently shows the source patient is not infected with HIV. Source Patient in Seroconversion Window Period Persons with very recent acquisition of HIV can have detectable HIV RNA levels with a negative HIV antibody test result (Figure 4), and thus potentially transmit HIV in the setting of this seroconversion window period (Figure 5).[18] Nevertheless, there have been no documented occupational transmissions of HIV related to an exposure involving a source patient with very recent acquisition of HIV who was in the seroconversion window period. In addition, when using newer recommended fourth-generation combination antigen-antibody immunoassays, the window period is shortened by approximately 1 week (Figure 6).[18] Accordingly, administering PEP despite a negative HIV antibody test is not recommended, unless the source patient is known to have signs or symptoms that strongly suggest acute HIV infection. In addition, routine use of HIV RNA testing of the source patient is not recommended to determine HIV serostatus for decision-making regarding PEP. Relative Risks of Infectious Body Fluids As part of the evaluation for an occupational exposure to HIV, it is important to determine what type and quantity of body fluid from the source patient is involved in the exposure (Figure 7).[3] Blood or visibly bloody fluids are considered the most potentially infectious body fluids. Other body fluids also considered potentially infectious, but lower risk than blood or visibly bloody fluids, include semen, vaginal fluids, and fluids found in normally sterile areas of the body (cerebrospinal, synovial, pleural, pericardial, peritoneal, and amniotic). The risk of transmission from vomitus, feces, urine, nasal Page 4/29
5 secretions, sputum, sweat and tears is not considered potentially infectious unless they are visibly bloody.[3] Type of Exposure In the initial evaluation of the health care worker, it is essential to determine if the exposure involved (1) percutaneous injury, (2) mucous membrane exposure, or (3) contact with non-intact skin. The contact of blood or body fluid with intact skin does not confer risk of HIV transmission and thus does not warrant further evaluation or postexposure management. For needlestick injuries, additional information should include whether or not the skin of the health care worker was punctured, the type and gauge of needle involved, whether the injury sustained was deep or shallow, and if visible blood was present on the needle. Timing of Exposure As part of the initial evaluation, determination should be made regarding when the exposure took place. Health care workers with an occupational exposure to HIV should immediately seek care, since available data suggest that PEP should be started as soon as possible. Most health care workers promptly seek evaluation after an exposure, but certain circumstances can arise that result in a significant delay. It is particularly important to determine if the delay is longer than 72 hours from the incident, and expert consultation is recommended if that situation arises. Defining an At-Risk Exposure For the purposes of initiating PEP, the U.S. guidelines define an at-risk exposure as contact of blood, tissue, or other potentially infectious body fluids from a person with known or suspected HIV via (1) percutaneous injury (e.g. a needlestick or cut with a sharp object), (2) mucous membrane exposure, or (3) contact with non-intact skin (e.g. exposed skin that is chapped, abraded, or afflicted with dermatitis).[3] Page 5/29
6 Recommendations for Occupational PEP Initial Approach Following Exposure Event It is extremely important that health care workers have an immediate evaluation and consultation following an exposure event that may have involved contact with HIV-contaminated blood or body fluids. Prior to any discussions regarding PEP, it is essential to make sure the health care worker has adequately decontaminated the wound or mucous membranes. In addition, one of the goals of the initial evaluation is to make sure the health care worker promptly receives the first doses of antiretroviral medications for PEP, if indicated. All health care facilities should have a plan in place for administering appropriate evaluation and management for a health care worker who has an occupational exposure to a blood-borne pathogen. Wound Decontamination All exposure events should prompt an immediate effort to decontaminate the area of the body exposed to potentially infectious fluids. If the wound involved a needlestick injury, wash the area thoroughly with soap and water (if the health care worker was wearing a glove, the glove should be removed prior to washing the wound). The health care workers should not squeeze or milk the injury site. For an exposure to a mucous membrane, the health care worker should thoroughly wash and irrigate the area with a large volume of saline. If the exposure involves skin (intact or nonintact), the skin should be thoroughly washed with soap and water. Timing for Initiation of Antiretroviral Therapy Occupational exposures should be considered an urgent medical issue and addressed immediately. Animal studies have shown that PEP is most effective when started as early as possible after exposure (Figure 8).[12] Thus, health care workers should receive their first dose of antiretroviral medications promptly after the exposure, ideally within 30 to 90 minutes after the exposure event. In some situations, significant delays occur, usually because the health care worker failed to initially consider the exposure event significant enough to seek evaluation. When a delay occurs, but is less than 72 hours, PEP should still be given (if warranted by the exposure). If the delay extends past 72 hours, PEP is likely to be less effective and expert consultation should be obtained; in this situation, some experts will consider giving PEP, particularly in cases considered very high risk for HIV acquisition. Three-Drug Regimens for Occupational PEP The 2013 Occupational PEP Guidelines recommend the use of three or more antiretroviral medications when PEP is indicated.[3] Prior guidelines utilized a more complicated decision process, whereby either a two-drug (basic) or three-drug (expanded) regimen was chosen based on factors related to the risk of the exposure and the HIV status of the source patient. The new recommendation to use three or more medications for all exposures when PEP is indicated has provided a uniform approach that has simplified the management of occupational PEP. The 2013 Occupational PEP Guidelines note that in certain circumstances, such as difficulty with antiretroviral medication availability, anticipated toxicity, or adherence concerns, a two-drug regimen could be considered if administered with expert consultation. Nevertheless, with the widespread availability of convenient, safe, and well-tolerated antiretroviral medications, most experts would strongly recommend using a three-drug regimen for occupational PEP. Preferred Regimens for Occupational PEP The preferred regimen recommended in the 2013 Occupational PEP Guidelines consists of tenofovir DF-emtricitabine plus raltegravir (Table 2).[3] Large-scale studies involving persons with HIV Page 6/29
7 infection have shown tenofovir DF-emtricitabine plus raltegravir is safe, highly effective, well tolerated, and has minimal drug interactions.[19] The recommended dosing for this regimen is tenofovir DF-emtricitabine once a day and raltegravir twice a day. From a practical standpoint, the first doses of antiretroviral PEP should include both tenofovir DF-emtricitabine and raltegravir (the health care workers should NOT take raltegravir alone for the first dose). Since the release of these guidelines, a once daily 1200 mg raltegravir dose (two 600 mg tablets) has been approved by the United States Food and Drug Administration (FDA) for treatment of persons with HIV infection; there are, however, no data with the use of once daily raltegravir for PEP. Use of Dolutegravir for Occupational PEP Since the release of the 2013 Occupational PEP Guidelines, the integrase inhibitor dolutegravir received FDA approval and has emerged as a first-line antiretroviral medication for initial therapy in persons with HIV infection. Based on the excellent experience with dolutegravir in persons with HIV infection, some experts have substituted dolutegravir for raltegravir for use in occupational PEP. Dolutegravir is dosed once daily, has a higher genetic barrier to resistance than raltegravir, and is active against some HIV isolates that have resistance to raltegravir.[20] More extensive safety data exists with raltegravir than dolutegravir in pregnancy and in breastfeeding. In addition, on May 18, 2018, an FDA Safety Alert was posted that warned of potential serious neural tube birth defects in infants born to mothers who received dolutegravir at the time of becoming pregnant or early in the first trimester. On May 30, 2018, the HHS Antiretroviral Guideline Panels issued Recommendations Regarding Dolutegravir that address the use of dolutegravir in adults and adolescents with HIV who are pregnant or of child-bearing potential. Note that dolutegravir is also a component of two fixeddose combinations: dolutegravir-abacavir-lamivudine and dolutegravir-rilpivirine. Alternative Regimens for PEP Alternative regimens are provided in the Occupational PEP Guidelines if concern exists that the source patient has drug resistance to any agent of the preferred regimen, as well as for circumstances, such as in a rural area, when a site may only have access to alternative medications until preferred medications can be obtained through ordering.[3] Certain medications should be avoided in selecting alternative regimens for PEP. For example, the medication nevirapine should never be used in the provision of PEP due to the potential risk of fatal hepatotoxicity and serious skin reactions.[21] Similarly, abacavir should not be used for PEP without expert consultation because of the risk of developing a potentially fatal abacavir hypersensitivity reaction; although HLA- B*5701 testing can be performed and predict those at risk to develop the hypersensitivity reaction, it may take several days for results to return and thus abacavir is not practical for inclusion in a PEP regimen. Duration of Therapy The 2013 U.S. Public Health Service Occupational Postexposure Prophylaxis guidelines recommend that health care workers who initiate antiretroviral therapy for PEP should complete a 28-day course.[3] Studies involving macaques have shown that PEP given for 28 days is more effective than 10 days, which is more effective than 3 days.[12] The pervading theory is that, in most instances, PEP aborts a very early and limited HIV infection, rather than truly preventing any cell in the body from becoming infected with HIV. Thus, prompt initiation of PEP is important to minimize the tissue involvement, and continuing therapy for 28 days is important to control HIV replication while allowing sufficient time for localized immune responses to clear out a very limited HIV infection. Use of PEP if the Source Patient has Undetectable HIV RNA Level If the source patient has a recent undetectable HIV RNA level, the risk of transmission in an occupational exposure is likely significantly reduced. Nevertheless, undetectable plasma HIV RNA in Page 7/29
8 the source patient does not eliminate the possibility that transmission of HIV could occur, especially with a needlestick injury. Plasma viral load measurements reflect only the level of cell-free virus in the peripheral blood, not the persistence of HIV in latently infected cells present in whole blood. Thus, even when the source patient with HIV infection has an undetectable plasma HIV RNA level, the guidelines recommend that PEP should still be offered.[3] Choice of PEP Regimen with Known or Suspected HIV Drug Resistance Rare cases of documented PEP failure have occurred due to transmission of drug-resistant HIV from the source patient.[22,23] In the case of known or suspected resistance in the source virus to antiretroviral medications, expert consultation is strongly advised. If access to an expert is not immediately available, initiation of standard PEP should take place without delay; the regimen can be adjusted after initiation as additional information or advice from an expert becomes available. Recommended Situations to Obtain Expert Consultation The 2013 Occupational PEP Guidelines recommend expert consultation for HIV-related occupational exposures in the following situations:[3] Delay of greater than 72 hours from exposure to evaluation Unknown source (e.g. needle in sharps disposal container or laundry, source patient declines HIV testing) Known or suspected antiretroviral drug resistance of the source virus Known or suspected pregnancy in the exposed health care worker Breastfeeding in the exposed health care worker Serious medical illness in the exposed health care worker Development of toxicity of the initial PEP regimen. Expert Consultation: National Clinician Consultation Center PEPline Expert consultation can obtained by calling the National Clinician Consultation Center s PostExposure Prophylaxis PEPline at (888) The hours for PEPline phone consultation are 9 a.m.-9 p.m. EST seven days a week. Page 8/29
9 Baseline Evaluation and Counseling for Health Care Worker Baseline Evaluation Health care workers who have experienced occupational exposure to HIV should have prompt counseling, baseline laboratory testing, and medical evaluation (Figure 9).[3] It is important to determine if the health care worker has any pre-existing (or significant risk of) renal disease since PEP antiretroviral therapy, specifically tenofovir DF, carries potential nephrotoxicity and requires dose adjustment if renal dysfunction is present. However, the baseline evaluation should not result in a delay of giving the first dose of antiretroviral therapy for PEP. The recommended routine baseline laboratory testing for the health care worker exposed to a known or suspected HIV-positive source should include the following: Baseline HIV testing to document HIV status of the health care worker at the time of the exposure Complete blood count Renal function test Hepatic function tests Hepatitis B antibody (if indicated) Hepatitis C antibody (if indicated) Pregnancy test (if indicated) Evaluation of Management of Other Bloodborne Pathogens For health care workers exposed to a source patient with HIV infection who has coinfection with hepatitis B virus or hepatitis C virus, additional baseline testing and management considerations are needed. It is beyond the scope of this topic review to address postexposure prophylaxis for hepatitis B and/or hepatitis C. In this situation, we recommend obtaining expert consultation. Initial Counseling Health care workers who receive PEP should be informed of possible drug interactions, drug toxicities and side effects, the need for high levels of adherence with the PEP regimen, the importance of completing the entire 28-day postexposure course, and the importance of follow-up testing. Health care workers should be advised to use preventative measures (i.e., barrier contraception, avoidance of pregnancy and breastfeeding) to prevent secondary transmission of HIV, especially in the initial 6 to 12 weeks following the exposure. From a practical standpoint, this counseling should not delay the patient receiving their first doses of antiretroviral medications for PEP. Management of pregnant or breastfeeding health care workers who require PEP should involve expert consultation for counseling the risks and benefits of PEP in these settings. Page 9/29
10 Follow-Up of Health Care Worker After Exposure Event Early Reevaluation Early reevaluation of the exposed health care worker within 72 hours of exposure is strongly recommended, both to assess tolerance with the antiretroviral medications and to consider any new information about the source patient that may be available. Providing exposed health care personnel with psychological counseling should be considered an essential component of management. Follow-Up Laboratory Testing Regardless of whether a health care worker is taking PEP, reevaluation within 72 hours is recommended. If PEP is used, the health care worker should be advised about the importance of completing the prescribed regimen and should undergo close monitoring for any drug toxicities or development of acute HIV symptoms during the follow-up period. The schedule for follow-up laboratory testing requires repeat HIV testing of the health care worker at 6 weeks, 12 weeks, and 24 weeks after the occupational exposure. Alternatively, if a fourth-generation combination HIV p24 antigen-hiv antibody test is being utilized (which shortens the window period), the follow-up HIV testing schedule can be modified to 6 weeks and 16 weeks after the exposure.[3] Additional testing should include repeat complete blood counts and renal and liver function tests at 2 weeks after exposure for health care workers who initiate PEP. Further testing may be required in the event of abnormalities or drug toxicities (Figure 10).[3] Indications for HIV RNA Testing or Extended Follow-Up Routine use of HIV RNA testing of an exposed health care worker for follow-up after occupational exposure to HIV is not recommended, except in the scenario where the exposed health care worker manifests signs or symptoms suggestive of acute retroviral syndrome. In the setting of suspected acute HIV infection, it is appropriate to order a fourth-generation HIV antigen-antibody test and an HIV RNA. Based on several case reports of delayed HIV seroconversion in health care workers who also acquired hepatitis C virus from the exposure event, extended follow-up is indicated if the health care worker becomes infected with hepatitis C virus. In this situation, most experts recommend follow-up HIV testing out to 12 months, regardless of the method of HIV testing being used. Page 10/29
11 Summary Points Following an occupational exposure to HIV, PEP is an important element of workplace safety and markedly reduces the risk of HIV acquisition in an exposed health care worker. A health care worker who experiences a significant occupational HIV exposure should be evaluated for PEP as soon as possible. If PEP is initiated, the regimen should be continued for 28 days. Antiretroviral PEP medication regimens should contain 3 (or more) antiretroviral drugs for all occupational exposures to HIV; the number of medications should no longer be based on the risk of the individual exposure. The recommended regimen for PEP consists of tenofovir DF-emtricitabine plus raltegravir; this recommendation is based on this regimen s high potency, favorable side effect profile, convenient dosing, and minimal drug interactions. Some experts now use dolutegravir in place of raltegravir due to its greater potency and once-daily dosing. Initial evaluation of the exposed person should include both counseling and laboratory studies (baseline HIV testing, blood counts, and renal and hepatic function tests). Expert consultation should be sought for all cases involving drug-resistant source virus and for situations that fall outside the scope of the guidelines. Expert consultation can be obtained by calling the National Clinician Consultation Center s Post-Exposure Prophylaxis PEPline at All health care workers who experience an HIV exposure event, regardless of whether they take PEP, should be tested for the presence of HIV antibodies at baseline and following the exposure at 6, 12, and 24 weeks. If, however, a fourth-generation combination HIV p24 antigen-hiv antibody test is utilized for follow-up HIV testing of the health care worker, then follow-up testing can be performed at 6 and 16 weeks. Page 11/29
12 Citations 1. Public Health Service statement on management of occupational exposure to human immunodeficiency virus, including considerations regarding zidovudine postexposure use. MMWR Recomm Rep. 1990;39:1-14. [PubMed Abstract] 2. Centers for Disease Control and Prevention (CDC). Update: provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. MMWR Morb Mortal Wkly Rep. 1996;45: [PubMed Abstract] 3. Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol. 2013;34: [PubMed Abstract] 4. Panlilio AL, Cardo DM, Grohskopf LA, Heneine W, Ross CS. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1-17. [PubMed Abstract] 5. Public Health Service guidelines for the management of health-care worker exposures to HIV and recommendations for postexposure prophylaxis. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-33. [PubMed Abstract] 6. U.S. Public Health Service. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. MMWR Recomm Rep. 2001;50:1-52. [PubMed Abstract] 7. Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20: [PubMed Abstract] 8. Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med. 1997;102(suppl 5B):9-15. [PubMed Abstract] 9. Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28: [PubMed Abstract] 10. Joyce MP, Kuhar D, Brooks JT. Notes from the field: occupationally acquired HIV infection among health care workers - United States, MMWR Morb Mortal Wkly Rep. 2015;63: [PubMed Abstract] 11. Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337: [PubMed Abstract] - Page 12/29
13 12. Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72: [PubMed Abstract] 13. Young TN, Arens FJ, Kennedy GE, Laurie JW, Rutherford Gw. Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure. Cochrane Database Syst Rev. 2007;:CD [PubMed Abstract] 14. Duong YT, Mavengere Y, Patel H, et al. Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a National Household Survey in Swaziland. J Clin Microbiol. 2014;52: [PubMed Abstract] 15. Rosenberg NE, Kamanga G, Phiri S, et al. Detection of acute HIV infection: a field evaluation of the determine HIV-1/2 Ag/Ab combo test. J Infect Dis. 2012;205: [PubMed Abstract] 16. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58 Suppl 1:e [PubMed Abstract] 17. Chavez P, Wesolowski L, Patel P, Delaney K, Owen SM. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol Dec;52 Suppl 1:S51-5. [PubMed Abstract] 18. Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Published June 27, [CDC] 19. Lennox JL, Dejesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692): [PubMed Abstract] 20. Shah BM, Schafer JJ, Desimone JA Jr. Dolutegravir: a new integrase strand transfer inhibitor for the treatment of HIV. Pharmacotherapy. 2014;34: [PubMed Abstract] 21. Centers for Disease Control and Prevention (CDC). Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, MMWR Morb Mortal Wkly Rep. 2001;49: [MMWR] 22. Beltrami EM, Luo CC, de la Torre N, Cardo DM. Transmission of drug-resistant HIV after an occupational exposure despite postexposure prophylaxis with a combination drug regimen. Infect Control Hosp Epidemiol. 2002;23: [PubMed Abstract] - Page 13/29
14 23. Lopes GI, Coelho LP, Hornke L, et al. Transmission of a multidrug-resistant HIV-1 from an occupational exposure, in São Paulo, Brazil. AIDS. 2015;29: [PubMed Abstract] - References Ford N, Irvine C, Shubber Z, et al. Adherence to HIV postexposure prophylaxis: a systematic review and meta-analysis. AIDS. 2014;28: [PubMed Abstract] Lee LM, Henderson DK. Tolerability of postexposure antiretroviral prophylaxis for occupational exposures to HIV. Drug Saf. 2001;24: [PubMed Abstract] Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2011;:CD [PubMed Abstract] U.S. Public Health Service. Updated Information Regarding Antiretroviral Agents Used as HIV Postexposure Prophylaxis for Occupational HIV Exposures. MMWR Recomm Rep. 2007;56(49): [U.S. Public Health Service] Wang SA, Panlilio AL, Doi PA, White AD, Stek M Jr, Saah A. Experience of healthcare workers taking postexposure prophylaxis after occupational HIV exposures: findings of the HIV Postexposure Prophylaxis Registry. Infect Control Hosp Epidemiol. 2000;21: [PubMed Abstract] Webster DP. Is HIV post-exposure prophylaxis required following occupational exposure to a source patient who is virologically suppressed on antiretroviral therapy? HIV Med. 2015;16:73-5. [PubMed Abstract] - Page 14/29
15 Figures Figure 1 Timeline for Occupational Postexposure Prophylaxis Recommendations in the United States Abbreviations: CDC = Centers for Disease Control and Prevention; PEP = postexposure prophylaxis; INSTI= integrase strand transfer inhibitor Illustration by David H. Spach, MD Page 15/29
16 Figure 2 Number of Confirmed Cases of Occupationally-Acquired HIV Infection in United States, This graphic shows the year-by-year distribution of the 58 cases of occupationally acquired HIV infection in the United States. Most cases occurred between 1985 and 1995 prior to the widespread use of postexposure prophylaxis. Source: Joyce MP, Kuhar D, Brooks JT. Notes from the field: occupationally acquired HIV infection among health care workers - United States, MMWR Morb Mortal Wkly Rep. 2015;63: Page 16/29
17 Figure 3 Route of Exposure for Occupationally-Acquired HIV Infection in United States, Most cases of occupational HIV infection in the United States have resulted from a percutaneous puncture or cut. Source: Joyce MP, Kuhar D, Brooks JT. Notes from the field: occupationally acquired HIV infection among health care workers - United States, MMWR Morb Mortal Wkly Rep. 2015;63: Page 17/29
18 Figure 4 Timing of HIV RNA and HIV Antibodies following HIV Acquisition Page 18/29
19 Figure 5 HIV Seroconversion Window Page 19/29
20 Figure 6 Timing of Positivity of Various Laboratory Tests Following HIV Acquisition Source: modified from Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Published June 27, Page 20/29
21 Figure 7 Relative Risk of Body Fluids Involved in Occupational Exposure to HIV Source: Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol. 2013;34: Page 21/29
22 Figure 8 (Image Series) - Tenofovir for Postexposure Prophylaxis following SIV-1 Inoculation of Macaques (Image Series) - Figure 8 (Image Series) - Tenofovir for Postexposure Prophylaxis following SIV-1 Inoculation of Macaques Image 8A: Tenofovir for Postexposure Prophylaxis following SIV-1 Inoculation of Macaques In this study, investigators inoculated 24 macaques with simian immunodeficiency virus (SIV) and then instituted various postexposure prophylaxis regimens with tenofovir, which is (R)-9-(2-phosphonylmethoxypropyl). Source: Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72: Page 22/29
23 Figure 8 (Image Series) - Tenofovir for Postexposure Prophylaxis following SIV-1 Inoculation of Macaques Image 8B: SIV Transmission Based on Timing of Initiation and Duration of PEP Among animals that received placebo, all became infected with simian immunodeficiency virus (SIV). The most effective tenofovir prevention regimen consisted of early initiation of tenofovir (at 24 hours) and long duration of postexposure prophylaxis (28 days). Source: Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72: Page 23/29
24 Figure 9 Initial Counseling and Laboratory Studies for Persons with Occupational Exposure to HIV Source: Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol. 2013;34: Page 24/29
25 Figure 10 Follow-Up Counseling and Laboratory Studies for Persons with Occupational Exposure to HIV Source: Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol. 2013;34: Page 25/29
26 Table 1. Risk Factors for HIV Seroconversion in Health Care Workers Risk Factor Deep Injury Visible Blood on Device Terminal Illness in Source Patient Needle in Source Vein/Artery Postexposure Prophylaxis with Zidovudine Source: Adjusted Odds Ratio Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337: [PubMed Abstract] Page 26/29
27 Table 2. USPS Guidelines for the Use of Antiretroviral Agents for Occupational Exposures to HIV HIV Postexposure Prophylaxis Regimens Preferred HIV Postexposure Prophylaxis Regimen Raltegravir (400 mg PO twice daily) plus Tenofovir DF-Emtricitabine (300 mg-200 mg [1 tablet] daily) Alternative HIV Postexposure Prophylaxis Regimens May combine 1 anchor drug or drug pair from the left column with 1 pair of nucleoside/nucleotide reverse-transcriptase inhibitors from the right column; prescribers unfamiliar with these agents/regimens should consult physicians familiar with the agents and their toxicitiesa Anchor Drug Nucleoside Reverse Transcriptase Inhibitors Raltegravir Tenofovir DF-emtricitabine Darunavir + ritonavir Tenofovir DF + lamivudine Etravirine Zidovudine-lamivudine Rilpivirine Zidovudine + emtricitabine Atazanavir + ritonavir Lopinavir-ritonavir The following alternative is a complete fixed-dose combination regimen, and no additional antiretrovirals are needed: Elvitegravir-cobicistat-tenofovir DF-emtricitabine Alternative Antiretroviral Agents for Use as PEP Only with Expert Consultation Abacavir Efavirenz Enfuvirtide Fosamprenavir Maraviroc Saquinavir Stavudine Antiretroviral Agents Generally Not Recommended for Use as PEP Didanosine Nelfinavir Tipranavir Antiretroviral Agents Contraindicated as PEP Nevirapine Note. For consultation or assistance with HIV PEP, contact the National Clinicians Post-Exposure Prophylaxis Hotline at telephone number or visit its website at a The alternatives regimens are listed in order of preference; however, other alternatives may be reasonable based on patient and clinician preference. Source: Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337: [PubMed Abstract] Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol. Page 27/29
28 2013;34: [PubMed Abstract] Page 28/29
29 Page 29/29 Powered by TCPDF (
Blood-Borne Pathogens and Post-Exposure Prophylaxis
 Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital
 HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV
 POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
HIV Management Update 2015
 9/30/15 HIV Management Update 2015 Larry Pineda, PharmD, PhC, BCPS Visiting Assistant Professor Pharmacy Practice and Administrative Science ljpineda@salud.unm.edu Pharmacist Learning Objectives Describe
9/30/15 HIV Management Update 2015 Larry Pineda, PharmD, PhC, BCPS Visiting Assistant Professor Pharmacy Practice and Administrative Science ljpineda@salud.unm.edu Pharmacist Learning Objectives Describe
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
An International Antiviral Society-USA
 Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV
 Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
Post-Sexual Exposure Prophylaxis (npep)
 Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Occupational HIV exposures are crisis situations demanding immediate, decisive action. Henderson, 2001
 Post-Exposure Care: A Balancing Act Alice C. Thornton, MD Southeast AIDS Training & Education Center Kentucky Local Performance Site University of Kentucky October 24, 2009 Objectives Review the goals
Post-Exposure Care: A Balancing Act Alice C. Thornton, MD Southeast AIDS Training & Education Center Kentucky Local Performance Site University of Kentucky October 24, 2009 Objectives Review the goals
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
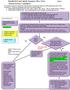 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Post Exposure Prophylaxis (PEP)
 Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
Management of sharps injuries in the healthcare setting
 Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong 1 3 1 Department of Infection, Barts Health NHS
Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong 1 3 1 Department of Infection, Barts Health NHS
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
HIV Update Objectives. Epidemiology. Epidemiology, Transmission and Natural History. Transmission Risk by Exposure. Transmission 9/29/2014
 Objectives HIV Update 2014 Jay Sizemore, MD, MPH Medical Director Chattanooga CARES Assistant Professor UTCOM Chattanooga 2October 2014 Review HIV epidemiology and screening/testing guidelines Discuss
Objectives HIV Update 2014 Jay Sizemore, MD, MPH Medical Director Chattanooga CARES Assistant Professor UTCOM Chattanooga 2October 2014 Review HIV epidemiology and screening/testing guidelines Discuss
3/23/2016. Managing Bloodborne Pathogen Exposures. Managing Bloodborne Pathogen Exposures
 This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
Postexposure prophylaxis (PEP)
 Postexposure Prophylaxis Against Human Immunodeficiency Virus MICHAEL A. TOLLE, MD, MPH, and HEIDI L. SCHWARZWALD, MD, MPH, Baylor College of Medicine, Houston, Texas Family physicians often encounter
Postexposure Prophylaxis Against Human Immunodeficiency Virus MICHAEL A. TOLLE, MD, MPH, and HEIDI L. SCHWARZWALD, MD, MPH, Baylor College of Medicine, Houston, Texas Family physicians often encounter
Bloodborne Pathogens. Montclair Kimberley Academy 1
 Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Management of Exposure to Needlestick Injuries & Body Fluids
 Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Antiretroviral Therapy During Pregnancy and Delivery: 2015 Update
 Frontier AIDS Education and Training Center Antiretroviral Therapy During Pregnancy and Delivery: 2015 Update Brian R. Wood, MD Assistant Professor of Medicine, University of Washington Medical Director,
Frontier AIDS Education and Training Center Antiretroviral Therapy During Pregnancy and Delivery: 2015 Update Brian R. Wood, MD Assistant Professor of Medicine, University of Washington Medical Director,
TORONTO GENERAL HOSPITAL HIV AMBULATORY CARE ROTATION
 TGH - ambulatory rotation page 1 of 5 TORONTO GENERAL HOSPITAL HIV AMBULATORY CARE ROTATION SITE: Immunodeficiency Clinic, Toronto General Hospital, University Health Network Location: 13 th floor, Norman
TGH - ambulatory rotation page 1 of 5 TORONTO GENERAL HOSPITAL HIV AMBULATORY CARE ROTATION SITE: Immunodeficiency Clinic, Toronto General Hospital, University Health Network Location: 13 th floor, Norman
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP)
 Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents
 Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents Visit the AIDSinfo website to access the most up-to-date guideline. Register for e-mail notification of guideline
Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents Visit the AIDSinfo website to access the most up-to-date guideline. Register for e-mail notification of guideline
Drew University Bloodborne Pathogens Exposure Control Plan and Procedures
 PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
The use of antiretroviral agents during pregnancy in Canada and compliance with North-American guidelines
 The use of antiretroviral agents during pregnancy in Canada and compliance with North-American guidelines I. Boucoiran, T. Lee, K. Tulloch, L. Sauve, L. Samson, J. Brophy, M. Boucher and D. Money For and
The use of antiretroviral agents during pregnancy in Canada and compliance with North-American guidelines I. Boucoiran, T. Lee, K. Tulloch, L. Sauve, L. Samson, J. Brophy, M. Boucher and D. Money For and
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Second-Line Therapy NORTHWEST AIDS EDUCATION AND TRAINING CENTER
 NORTHWEST AIDS EDUCATION AND TRAINING CENTER Second-Line Therapy David Spach, MD Clinical Director, Northwest AETC Professor of Medicine, Division of Infectious Diseases University of Washington Presentation
NORTHWEST AIDS EDUCATION AND TRAINING CENTER Second-Line Therapy David Spach, MD Clinical Director, Northwest AETC Professor of Medicine, Division of Infectious Diseases University of Washington Presentation
HIV Occupational Transmission and Exposure. Marsh Gelbart 2010
 HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
INDIAN JOURNAL OF DENTAL ADVANCEMENTS
 INDIAN JOURNAL OF DENTAL ADVANCEMENTS Journal homepage: www.nacd.in CASE REPORT Prophylaxis Following Occupational Exposure To HIV Manika 1, Shashikant M C 2, Shailaja S 3 Sr. Lecturer 1 & 3 Dept of Oral
INDIAN JOURNAL OF DENTAL ADVANCEMENTS Journal homepage: www.nacd.in CASE REPORT Prophylaxis Following Occupational Exposure To HIV Manika 1, Shashikant M C 2, Shailaja S 3 Sr. Lecturer 1 & 3 Dept of Oral
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers
 Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Information for Health Care Workers
 Information for Health Care Workers This Bulletin highlights precautions to be taken by health care workers who may be exposed to blood and body fluids. In general, workers should minimize direct contact
Information for Health Care Workers This Bulletin highlights precautions to be taken by health care workers who may be exposed to blood and body fluids. In general, workers should minimize direct contact
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Prophylaxis
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
HIV is primarily transmitted through un -
 CMAJ Early release, published at www.cmaj.ca on May 28, 2012. Subject to revision. Review Antiretroviral medication for preventing HIV infection in nonoccupational settings Isaac I. Bogoch MD, Eileen P.
CMAJ Early release, published at www.cmaj.ca on May 28, 2012. Subject to revision. Review Antiretroviral medication for preventing HIV infection in nonoccupational settings Isaac I. Bogoch MD, Eileen P.
New federal guidelines for managing occupational exposures to HIV
 Recommendations consider new drugs and tests New federal guidelines for managing occupational exposures to HIV CHICAGO, ILL, USA (August 6, 2013) New guidelines from the United States Public Health Service
Recommendations consider new drugs and tests New federal guidelines for managing occupational exposures to HIV CHICAGO, ILL, USA (August 6, 2013) New guidelines from the United States Public Health Service
B. Tasks and Procedures where employees, students or contractors can be exposed to bloodborne pathogens:
 Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Module 1: HIV epidemiology, transmission and prevention
 Session 2 Module goals Module 1 Participants will be able to: -offer an insight into the epidemiological situation in the country and worldwide -present the HIV transmission modes and the broad approaches
Session 2 Module goals Module 1 Participants will be able to: -offer an insight into the epidemiological situation in the country and worldwide -present the HIV transmission modes and the broad approaches
Bloodborne Pathogens. Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Introduction to Blood Borne Pathogens
 Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
/AIDS HIV/ HIV Overview. Nelson L. Michael, MD, PhD Division of Retrovirology Walter Reed Army Institute of Research US Military HIV Research Program
 /AIDS HIV/ HIV Overview Nelson L. Michael, MD, PhD Division of Retrovirology Walter Reed Army Institute of Research US Military HIV Research Program www.hivresearch.org 1 WRAIR Tropical Medicine Course
/AIDS HIV/ HIV Overview Nelson L. Michael, MD, PhD Division of Retrovirology Walter Reed Army Institute of Research US Military HIV Research Program www.hivresearch.org 1 WRAIR Tropical Medicine Course
Simplifying HIV Treatment Now and in the Future
 Simplifying HIV Treatment Now and in the Future David M. Hachey, Pharm.D., AAHIVP Professor Idaho State University Department of Family Medicine Nothing Disclosure 1 Objectives List current first line
Simplifying HIV Treatment Now and in the Future David M. Hachey, Pharm.D., AAHIVP Professor Idaho State University Department of Family Medicine Nothing Disclosure 1 Objectives List current first line
Occupational Exposures to Bloodborne Pathogen (BBP) Training
 Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
Guidance for Non-HIV-Specialized Providers Caring for Persons with HIV Displaced by Disasters
 Guidance for Non-HIV-Specialized Providers Caring for Persons with HIV Displaced by Disasters Visit the AIDSinfo website to access the most up-to-date guideline. Register for e-mail notification of guideline
Guidance for Non-HIV-Specialized Providers Caring for Persons with HIV Displaced by Disasters Visit the AIDSinfo website to access the most up-to-date guideline. Register for e-mail notification of guideline
2017 BLOODBORNE PATHOGENS
 2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: F:
 University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
PREP IN PRIMARY CARE TRACY SALAMEH RN, BSN, ACRN HIV CLINICAL SPECIALIST DAKOTA AIDS EDUCATION AND TRAINING CENTER
 PREP IN PRIMARY CARE TRACY SALAMEH RN, BSN, ACRN HIV CLINICAL SPECIALIST DAKOTA AIDS EDUCATION AND TRAINING CENTER THE NEED FOR CONTINUED HIV PREVENTION Estimated new HIV infections in the US for the most
PREP IN PRIMARY CARE TRACY SALAMEH RN, BSN, ACRN HIV CLINICAL SPECIALIST DAKOTA AIDS EDUCATION AND TRAINING CENTER THE NEED FOR CONTINUED HIV PREVENTION Estimated new HIV infections in the US for the most
FAMILY HIV CENTER NJ CARES SANE
 HIV, Hepatitis B & C Postexposure Prophylaxis for Sexual Assault Victims Update for Case Managers Susan Burrows-Clark, RN, MSN, CNS, C. 10/17/11 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug
HIV, Hepatitis B & C Postexposure Prophylaxis for Sexual Assault Victims Update for Case Managers Susan Burrows-Clark, RN, MSN, CNS, C. 10/17/11 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug
Greenwood School District 50 OSHA UPDATE BLOODBORNE PATHOGENS
 Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Universal Precautions
 Universal Precautions emphasizes the need for workers and students to consider all blood and body fluids as potentially infected with HIV, HBV, and / or other blood-borne pathogens, and to adhere rigorously
Universal Precautions emphasizes the need for workers and students to consider all blood and body fluids as potentially infected with HIV, HBV, and / or other blood-borne pathogens, and to adhere rigorously
Continuing Education for Pharmacy Technicians
 Continuing Education for Pharmacy Technicians HIV/AIDS TREATMENT Michael Denaburg, Pharm.D. Birmingham, AL Objectives: 1. Identify drugs and drug classes currently used in the management of HIV infected
Continuing Education for Pharmacy Technicians HIV/AIDS TREATMENT Michael Denaburg, Pharm.D. Birmingham, AL Objectives: 1. Identify drugs and drug classes currently used in the management of HIV infected
HIV Overview. Mary Marovich, MD, DTMH Division of Retrovirology Walter Reed Army Ins?tute of Research US Military HIV Research Program
 HIV Overview Mary Marovich, MD, DTMH Division of Retrovirology Walter Reed Army Ins?tute of Research US Military HIV Research Program www.hivresearch.org 1 Outline HIV Virology, Transmission, and Pathogenesis
HIV Overview Mary Marovich, MD, DTMH Division of Retrovirology Walter Reed Army Ins?tute of Research US Military HIV Research Program www.hivresearch.org 1 Outline HIV Virology, Transmission, and Pathogenesis
Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis).
 LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents
 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents 1 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in
Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents 1 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in
Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
 PROPHYLAXISFOLLOWINGHIV, HEPB ANDC EXPOSURES: WHAT SNEW Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
PROPHYLAXISFOLLOWINGHIV, HEPB ANDC EXPOSURES: WHAT SNEW Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
HIV Treatment Guidelines
 HIV Treatment Guidelines Together, we can change the course of the HIV epidemic one woman at a time. #onewomanatatime #thewellproject What Are Treatment Guidelines? Issued by variety of global and country-based
HIV Treatment Guidelines Together, we can change the course of the HIV epidemic one woman at a time. #onewomanatatime #thewellproject What Are Treatment Guidelines? Issued by variety of global and country-based
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
NEOMED ACADEMIC POLICY
 (A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
(A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
ART and Prevention: What do we know?
 ART and Prevention: What do we know? Biomedical Issues Trip Gulick, MD, MPH Chief, Division of Infectious Diseases Professor of Medicine Weill Cornell Medical College New York City ART for Prevention:
ART and Prevention: What do we know? Biomedical Issues Trip Gulick, MD, MPH Chief, Division of Infectious Diseases Professor of Medicine Weill Cornell Medical College New York City ART for Prevention:
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
HIV in the Workplace
 OEM012_CH24.qxp 8/6/08 12:00 PM Page 441 24 HIV in the Workplace Alan L. Williams and Richard J. Thomas* Over 25 years have elapsed since the first cases of what would become known as the acquired immune
OEM012_CH24.qxp 8/6/08 12:00 PM Page 441 24 HIV in the Workplace Alan L. Williams and Richard J. Thomas* Over 25 years have elapsed since the first cases of what would become known as the acquired immune
Blood Borne Pathogens (BBP)
 Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
PHARMACOKINETICS OF ANTIRETROVIRAL AND ANTI-HCV AGENTS
 8. PHARMACOKINETICS OF ANTIRETROVIRAL AND ANTI-HCV AGENTS David Burger José Moltó Table 8.1a: INFLUENCE OF FOOD ON ABSORPTION (AREA UNDER THE CURVE) OF ANTIRETROVIRAL AGENTS NUCLEOSIDE ANALOGUES NtRTI
8. PHARMACOKINETICS OF ANTIRETROVIRAL AND ANTI-HCV AGENTS David Burger José Moltó Table 8.1a: INFLUENCE OF FOOD ON ABSORPTION (AREA UNDER THE CURVE) OF ANTIRETROVIRAL AGENTS NUCLEOSIDE ANALOGUES NtRTI
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. Training Guide for Healthcare Providers
 TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
HIV Testing. ECHO Hep C. Judith Feinberg, MD June 22, 2017
 HIV Testing ECHO Hep C Judith Feinberg, MD June 22, 2017 Overview A few basics HIV epidemiology in the US HIV testing Time course of HIV-1 infection symptoms HIV proviral DNA symptoms window period HIV
HIV Testing ECHO Hep C Judith Feinberg, MD June 22, 2017 Overview A few basics HIV epidemiology in the US HIV testing Time course of HIV-1 infection symptoms HIV proviral DNA symptoms window period HIV
Needle Stick. Mr. Fadi J. Zaben RN MSN IMET 2000, Ramallah IMET 2000
 Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
Needle Stick Mr. Fadi J. Zaben RN MSN, Ramallah 1 Objectives: Define Needle Stick. Mention the sharps. Discus the rate of incidence. Identify the person who is at risk. Discus Needle stick and infectious
Infection Control Program (ICP) ICP Components 1. Exposure Determination 2. Control Methods A. Universal Precautions
 Compliance Assistance Guideline for the February 27, 1990, OSHA Instruction CPL 2 2.44B Enforcement Procedures for Occupational Exposure to Hepatitis B Virus and Human Immunodeficiency Virus from the U.S.
Compliance Assistance Guideline for the February 27, 1990, OSHA Instruction CPL 2 2.44B Enforcement Procedures for Occupational Exposure to Hepatitis B Virus and Human Immunodeficiency Virus from the U.S.
BLOODBORNE DISEASES. Prevention of transmission for school staff. for staff not directly responsible for providing care or cleaning up blood
 BLOODBORNE DISEASES Prevention of transmission for school staff for staff not directly responsible for providing care or cleaning up blood MASSACHUSETTS DIVISION OF OCCUPATIONAL SAFETY Robert Prezioso,
BLOODBORNE DISEASES Prevention of transmission for school staff for staff not directly responsible for providing care or cleaning up blood MASSACHUSETTS DIVISION OF OCCUPATIONAL SAFETY Robert Prezioso,
Standard Precautions and HIV Postexposure Prophylaxis in the Health Care Setting
 HIV Curriculum for the Health Professional Standard Precautions and HIV Postexposure Prophylaxis in the Health Care Setting Nancy R. Calles, MSN, RN, PNP, ACRN, MPH Andreea C. Cazacu, MD Objectives 1.
HIV Curriculum for the Health Professional Standard Precautions and HIV Postexposure Prophylaxis in the Health Care Setting Nancy R. Calles, MSN, RN, PNP, ACRN, MPH Andreea C. Cazacu, MD Objectives 1.
THE HIV LIFE CYCLE. Understanding How Antiretroviral Medications Work
 THE HIV LIFE CYCLE Understanding How Antiretroviral Medications Work DEFINITIONS Host: The animal or cell that another organism lives in. In HIV human CD4 T-cells are the host for HIV. Nucleus: The core
THE HIV LIFE CYCLE Understanding How Antiretroviral Medications Work DEFINITIONS Host: The animal or cell that another organism lives in. In HIV human CD4 T-cells are the host for HIV. Nucleus: The core
You will now begin the Bloodborne Pathogen Refresher Training.
 You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
A. Background for Trainer: B. What OSHA Requires: Bloodborne Pathogens. Lesson Plan 6080a
 Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
BAY-ARENAC BEHAVIORAL HEALTH AUTHORITY POLICIES AND PROCEDURES MANUAL
 Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Environmental Health and Safety Offices BLOODBORNE PATHOGENS
 Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM
 Page 1 of 13 BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM INTRODUCTION The attached materials will assist in teaching the information about bloodborne pathogens for health care workers as required
Page 1 of 13 BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM INTRODUCTION The attached materials will assist in teaching the information about bloodborne pathogens for health care workers as required
Nursing Infectious Diseases Topics. David H. Spach, MD Professor of Medicine Division of Infectious Diseases University of Washington, Seattle
 Nursing Infectious Diseases Topics David H. Spach, MD Professor of Medicine Division of Infectious Diseases University of Washington, Seattle Clostridium difficile Free Access Via Web Source: Cohen SH,
Nursing Infectious Diseases Topics David H. Spach, MD Professor of Medicine Division of Infectious Diseases University of Washington, Seattle Clostridium difficile Free Access Via Web Source: Cohen SH,
Needles and Sharps Exposure: How do We Proceed?
 Needles and Sharps Exposure: How do We Proceed? UMAYYA MUSHARRAFIEH,MD AMERICAN UNIVERSITY OF BEIRUT MEDICAL CENTER JUNE 14, 2013 Health care workers who use or may be exposed to needles are at increased
Needles and Sharps Exposure: How do We Proceed? UMAYYA MUSHARRAFIEH,MD AMERICAN UNIVERSITY OF BEIRUT MEDICAL CENTER JUNE 14, 2013 Health care workers who use or may be exposed to needles are at increased
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. For Healthcare Providers
 Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
