01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
|
|
|
- Brice Paul
- 5 years ago
- Views:
Transcription
1 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of UTMB hospitals, clinics, outpatient surgical center, contract workers, volunteers, and students. Exposures to blood borne pathogens will be managed as described in sections to follow for any occupational exposure of healthcare workers or students. UTMB will assume responsibility for testing of a source patient in the care of a UTMB facility. Occupational exposures to blood and body fluids are defined as: Percutaneous injury (e.g. needle stick, laceration with a sharp object) Contact of mucous membranes or ocular membranes (mucosal exposure) Contact of non-intact skin (e.g. skin that is chapped, abraded) with Blood or other potentially infectious fluid (semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluids; bloody body fluids and unfixed tissue). Contacts Employee Health Clinic: Fax: emphlthc@utmb.edu Student Health: Fax: (409) Infection Control & Healthcare Epidemiology: During office hours: After hours pager: Online forms and instructions: Page 1 of 11
2 Procedures A. Initial care of exposed area: exposed employee will cleanse site of percutaneous exposure or rinse exposed mucous membranes for permucosal exposure before reporting the exposure. B. Reporting an exposure: 1. Healthcare worker or student on main campus: report exposure to supervisor or supervising instructor/faculty. 2. First responder exposed to a UTMB patient during pre-hospital care: notify emergency department (ED) charge nurse and agency s infection control officer. 3. Employee: complete Blood Borne Pathogen Exposure and Notification Form, access at and take completed form at exposure evaluation (see Section D.1) 4. Student: complete student injury form. 5. Correctional employee exposed to a UTMB patient: Report exposure TDCU triage nurse C. Obtaining a blood sample from source patient: 1. The priority for testing is to secure a specimen on the source patient, when known, and test the source patient for HIV, hepatitis B and hepatitis C (see Section D.2). 2. Request charge nurse for patient s location (hospital/urgent care center/clinic) to obtain verbal consent and blood sample. If patient is no longer in the care of UTMB, call the lab to determine if there is an adequate serum sample drawn for routine clinical care. Obtain verbal consent for HIV testing when feasible. Use manual lab slips (available from Employee Health and also at to order labs. Obtain 1 serum separator tube and label with source patient s name and medical record number. D. Exposure evaluation, counseling, and management of employee or student 1. Location: a. Employee exposure: After arranging source patient testing, employee should report to the nearest UTMB ED. Employees also have the option to be evaluated by the Employee Health Clinic during office hours (9 AM-4:00 PM). Employees at remote locations may use a facility designated by Clinic management for remote locations. The completed Blood Borne Pathogen Exposure and Notification Form can be faxed or scanned to the Employee Health Clinic for follow-up on the next business day. b. UTMB students: 1) Exposed on a UTMB campus: report to UTMB Student Health during office hours. After hours, report to the designated UTMB emergency department. Page 2 of 11
3 2) Student at off-site rotation at facility not operated by UTMB: report exposure per facility policy to assure source patient testing, then report exposure to UTMB student health. 3) School of Medicine students who are designated as Austinbased or Houston-based should follow the prearranged process with the appropriate facilities per the School of Medicine agreements. c. Non-UTMB students: 1) Student-school who has contract with UTMB (Galveston College): Follow UTMB student process by reporting to the Student Health Clinic as above. 2) Student-school who does not have contract with UTMB Student Health: follow process outlined by school. Inform student that they should see their primary care provider for any additional follow-up and treatment indicated. d. Correctional care officer: 1) Register as a patient in the ED on the main campus for initial evaluation and prophylaxis as indicated. e. First responders: 1) May register as a patient in ED for initial evaluation and/or to start HIV prophylaxis 2. Laboratory tests for source patients The source patient will be tested for the following: HIV antigen/antibody combo test, HBsAg, anti-hepatitis B core antibody, and HCV antibody with reflex HCV PCR if antibody positive. a. Source patient testing at EDs: perform onsite rapid HIV screening test. Result is typically available within 30 minutes. HIV prophylaxis treatment decision will be based on this test result (see Section F). Send remainder of sample to Sample on the main campus, CSW (core laboratory) via courier for confirmatory HIV test (if onsite rapid HIV test is positive) and for hepatitis B and C. b. Additional tests to be performed at the Main campus laboratory: Sample should be sent to Sample, CSW for all testing. 3. Laboratory tests for exposed individual a. On all employees/students: HIV antigen/antibody combo test, HCV antibody and total anti-hbc (see Section E). b. In addition, HBV surface antibody should be ordered for employees/students who have a history of HBV immunization prior to coming to UTMB. c. Additional laboratory tests required if Employee/Student is starting prophylaxis for HIV exposure/possible exposure CBC (use additional lavender top tube for CBC), complete metabolic panel (CMP). Females in reproductive age must have a urine or serum pregnancy test. Page 3 of 11
4 4. Laboratory test results will be reported to student health for students and to employee health for everyone else. If the exposed person does not belong to one of the following categories, results will be forwarded to Infection Control & Healthcare Epidemiology: UTMB employee, UTMB student, student from Galveston College (contract with UTMB student health), correctional officer. 5. Follow-up of initial test results and counseling and prophylaxis a. UTMB employee: Employee Health clinicians will review the source patient and employee tests. They will provide counseling, prophylaxis (if not initiated in ED), or vaccination as indicated, and instructions for any additional follow-up testing or assessment by a specialist (e.g. hepatologist, infectious disease physician, or obstetrician). b. Non-UTMB employee: 1) For counseling and additional evaluation, report to the employee s company/agency designated occupational health provider. 2) Source patient s results are reviewed by Infection Control & Healthcare Epidemiology and communicated after removing patient 3) identifiers to company/agency designated infection control officer or occupational health provider 4) Any follow up care will be provided either by the agency s designated occupational health provider or the exposed person s personal physician. c. UTMB student or student from school with UTMB student health contract: 1) Source patient results are reviewed by Student Health clinicians to determine need for additional prophylaxis, testing, vaccination, or treatment. 2) Any follow up care will be provided either by Student Health clinicians with appropriate referrals to specialists as needed. d. Non-UTMB student 1) Any follow-up care will be through a healthcare provider designated by the school or the student s personal healthcare provider. Report to individual designated by school and follow school policy regarding evaluation and post-exposure testing/treatment. 2) Source patient s results without patient identifiers are communicated to designated school official by Infection Control & Healthcare Epidemiology. e. Correctional care officer: 2) Any follow-up care will be arranged through the TDCJ liaison. f. First responder: Page 4 of 11
5 1) Any follow-up care will be provided by the agency s designated occupational health provider. 2) Source patient s results without patient identifiers are communicated to designated occupational health provider by Infection Control & Healthcare Epidemiology. E. General guidelines for follow-up testing of exposed employee/student: Follow-up is determined by the results of the initial lab work drawn on the source of the occupational exposure. It proceeds as follows: Known HIV(+) source: Obtain HIV antibody at exposure and at 6 weeks, 3 months and 6 months Known HBV (+) source: Obtain Total anti-hbc at exposure and obtain HBsAg and Total anti-hbc at 6 months Known HCV(+) source: Obtain HCV antibody at exposure and obtain HCV Qual RNA (PCR) at 6 weeks, 3 months and 6 months Unknown source: Obtain HIV & HCV antibody at exposure and at 3 months and 6 months; and also obtain Total anti-hbc at exposure and HBsAg and Total anti-hbc at 6 months Special Precautions after a known HIV(+) or high risk exposure Do not share a toothbrush. Gums can bleed easily, getting blood on the toothbrush. Do not share razors as blood may go undetected on the blade. Avoid pregnancy until HIV infection is ruled out (i.e. generally 6 months following exposure, but up to one year). Use safe sex practices-male/female latex condoms for barrier protection or abstain from sex during the follow-up period until HIV infection has been ruled out. Do not donate blood, plasma, organs, tissue or semen during the follow-up period. Seek medical evaluation for any acute illness that occurs during the follow-up period. F. HIV exposure prophylaxis specifics 1. Post-exposure prophylaxis and testing for HIV exposure a. Risk of HIV Infection The average risk of HIV infection due to all types of reported percutaneous exposures to HIV-infected blood is 0.3%. A percutaneous exposure is defined as a needle stick or laceration/puncture with a sharp object. The risk appears to be greater than 0.3% for exposure to HIV (+) patients involving deep injury, visible blood on the device causing the injury or a device previously placed in the source patient s vein or artery. Page 5 of 11
6 The risk of HIV infection is considered to be much lower if the source HIV patient is on antiretroviral treatment and has undetectable HIV viral load. b. Prescriptions: 1) Initial dose: Pharmacy has stocked several locations, including emergency departments, urgent care centers and some clinics with the initial 24-hour dose if source patient is known HIV positive, or if the source patient is unavailable for testing. In these circumstances, the first dose of prophylaxis must begin within 4 hours of exposure or as soon as possible. In other situations, where the source sample is being tested for HIV, the initial prophylaxis dose can be delayed for up to 72 hours until HIV test result is available. 2) When continued prophylaxis is required, the prescription will be faxed to the Pharmacy. The Pharmacy will arrange to deliver medication to employees who do not work on the main campus via courier. c. Prophylaxis Regimens for Exposure to HIV (Revised September, 2013) Prophylaxis is being offered to employees and students who have a percutaneous injury or contamination of mucous membranes or nonintact skin exposure to HIV during the performance of their duties. Prophylaxis in these circumstances is voluntary. The CDC says, Because most occupational exposures to HIV do not result in infection transmission, potential toxicity must be carefully considered when prescribing post-exposure prophylaxis (PEP) Page 6 of 11
7 Medication Schedule RECOMMENDATIONS FOR NON-PREGNANT PERSONS: Dolutegravir 50 mg tablet (Tivicay) - One tablet by mouth with or without food once daily for 4 weeks. AND Emtricitabine/Tenofovir 200mg/300mg tablet (Truvada) - One tablet by mouth with or without food once daily for 4 weeks. RECOMMENDATIONS FOR PERSONS KNOWN TO BE PREGNANT: Emtricitabine/Tenofovir 200mg/300mg tablet (Truvada) - One tablet by mouth with or without food once daily for 4 weeks AND Raltegravir 400 mg tab/dose Twice daily for 4 weeks Side effects associated with Dolutegravir (Tivicay) and Raltegravir (Isentress) include nausea, diarrhea, headache, dizziness, abnormal dreams and difficulty sleeping. Rarely, these medications may cause jaundice with dark urine and yellowing of the skin or eyes. Side effects associated with emtricitabine/tenofovir (Truvada) include nausea, vomiting, diarrhea, abdominal pain, dizziness, gas, loss of appetite, headache, rash, skin discoloration, joint pain and muscle pain. Rarely, this medication may cause jaundice with dark urine and yellowing of the skin or eyes. Situations for which Infectious Diseases/HIV Expert Consultation for Human Immunodeficiency Virus (HIV) postexposure prophylaxis (PEP) is recommended. Provision of PEP should not be delayed while awaiting expert consultation. Delayed (ie, later than 72 hours) exposure report. Interval after which benefits from PEP are undefined. Breastfeeding in the exposed person If source person s virus is known or suspected to be resistant to one or more of the drugs considered for PEP, selection of drugs to which the source person s virus is unlikely to be resistant is recommended. Page 7 of 11
8 Toxicity of the initial PEP regimen Symptoms (eg, gastrointestinal symptoms and others) are often manageable without changing the PEP regimen by prescribing antimotility or antiemetic agents. Serious medical illness in the exposed person who is already taking multiple medications may increase the risk of drug toxicity and drugdrug interactions. G. Post-exposure Prophylaxis for Hepatitis B and Hepatitis C 1. Risk of Hepatitis B or Hepatitis C Infection The average risk of Hepatitis B virus (HBV) infection in susceptible persons after percutaneous exposure to HBVinfected blood is 6 30%. The risk of Hepatitis C virus (HCV) infection after percutaneous exposure to HCV-infected blood is 7.4% (95% CI 3.9%-12.5%). 2. Testing and vaccination regimens for Hepatitis B: a. For vaccinated HCW (who have written documentation of a complete, 3-dose of Hepatitis B vaccine series) with subsequent 10 miu/ml, testing the source patient for HBsAg is unnecessary. b. Exposed HCW/Student has never received Hepatitis B vaccine 1) Offer HBV vaccine if source is known to be positive for hepatitis B or is high risk for hepatitis B or source is unknown, (e.g., needle puncture through a trash bag) and employee/student has not been vaccinated against hepatitis B. 2) Offer Hepatitis B Immune Globulin 0.06 ml/kg IM if source is known to be positive for hepatitis B, or is high risk for hepatitis B or source is unknown, (e.g. needle puncture through a trash bag) and employee/student has not been vaccinated against hepatitis B.Exposed HCW/Student has received 3 doses of Hepatitis B vaccine twice and anti-hbs < 10 miu/ml 3) For vaccinated HCW/Student (who have written documentation of Hepatitis B vaccination) with anti-hbs < 10 miu/ml after two complete 3-dose Hepatitis B vaccine series, the source patient should be tested for HbsAg as soon as possible after the exposure. If the source patient is HbsAg-positive or has unknown HbsAg status, HCW/Student should receive 2 doses of HBIG. The first dose should be administered as soon as possible after exposure and the second dose should be administered 1 month later. If the source patient is HbsAg-negative neither HBIG nor Hepatitis B vaccine is necessary. 4) Exposed HCW/Student has received 3 doses of Hepatitis B vaccine but has not been tested for anti- Page 8 of 11
9 HBs: For vaccinated HCW/Student (who have written documentation of a complete, 3-dose Hepatitis B vaccine series) without previous anti-hbs testing, the HCW/Student should be tested for anti-hbs and the source patient (if known) should be tested for HbsAg as soon as possible after the exposure. Testing the source patient and the HCW/Student should occur simultaneously; testing the source patient should not be delayed while waiting for the HCW/Student anti-hbs test results, and likewise, testing the HCW/Student should not be delayed while waiting for the source patient HbsAg results. 5) Exposed HCW/Student has < 10 miu/ml anti-hbs and source patient positive or has unknown HbsAg status. If the HCW/Student has anti-hbs < 10 miu/ml and the source patient is HbsAg- positive or has unknown HbsAg status, the HCW/Student should receive 1 dose of HBIG and be revaccinated as soon as possible after the exposure. The HCW/Student should then receive the second 2 doses to complete the second Hepatitis B vaccine series (6 doses total when accounting for the original 3-dose series) according to the vaccination schedule. To document the HCW s/student s vaccine response status for future exposures, anti-hbs testing should be performed 1-2 months after the last dose of vaccine. 6) Exposed HCW/Student has anti-hbs < 10 miu/ml and the source patient is HbsAg negative. If the HCW/Student has anti-hbs < 10 miu/ml and the source patient is HbsAg-negative, the HCW/Student should receive an additional Hepatitis B vaccine dose, followed by repeat anti-hbs testing 1-2 months later. HCWs/Students whose anti-hbs remains < 10 miu/ml should undergo revaccination with 2 more doses (6 doses total when accounting for the original 3-dose series). To document the HCW s/student s vaccine response status for future exposures, anti-hbs testing should be performed 1-2 months after the last dose of vaccine. Page 9 of 11
10 7) Exposed HCW/Student has anti-hbs 10 miu/ml at time of exposure. If the HCW/Student has anti-hbs 10 miu/ml at the time of exposure, no postexposure HBV management is necessary, regardless of the source patient s HbsAg status. 8) Exposed HCW/Student unvaccinated or incompletely vaccinated (including those who refused vaccination). For unvaccinated or incompletely vaccinated HCW/Student (including those who refused vaccination), the source patient should be tested for HbsAg as soon as possible after the exposure. Testing unvaccinated or incompletely vaccinated HCW/Student for anti-hbs is not necessary and is potentially misleading, because anti-hbs 10 miu/ml as a correlate of vaccine-induced protection has only been determined for persons who have completed an approved vaccination series. If the source patient is HbsAg-positive or has unknown HbsAg status, the HCW/Student should receive one dose of HBIG and one dose of Hepatitis B vaccine administered as soon as possible after the exposure. The HCW/Student should complete the Hepatitis B vaccine series according to the vaccination schedule. To document the HCW s/student s vaccine response status for future exposures, anti-hbs testing should be performed approximately 1-2 months after the last dose of vaccine. Because anti-hbs testing of HCW/Student who received HBIG should be performed after anti-hbs from HBIG is no longer detectable (6 months after administration), it will likely be necessary to defer anti-hbs testing for a period longer than 1-2 months after the last vaccine dose. 3. Testing and follow-up recommendations for Hepatitis C. Employees/Students testing positive for Hepatitis C Qualitative RNA (PCR) at 6 weeks, 3 months or 6 months, will be referred immediately to a hepatologist for treatment. Page 10 of 11
11 References: 1. Kuhar DT, Henderson DK, Struble KA, Heneine W, et al. Updated US Public Health Service Guidelines for the of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis. Infect Control Hosp Epidemiol 2013;34: Centers for Disease Control and Prevention. CDC Guidance for Evaluating Health-Care Personnel for Hepatitis B Virus Protection and for Administering Postexposure, 2013;62(No. RR-10): Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C. Clinical Infectious Diseases, Volume 67, Issue 10, 30 October 2018, Pages Available at: 4. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: Accessed December 19, Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at: Accessed December 19, Page 11 of 11
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Prophylaxis
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Blood-borne Pathogens Exposure Policy
 Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Bloodborne Pathogen Exposure STUDENT. Blood Borne Pathogen Exposure. Student. Rev:
 Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
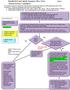 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Blood Borne Pathogen Exposure Employee
 Blood Borne Pathogen Exposure Employee INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Employee INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
You will now begin the Bloodborne Pathogen Refresher Training.
 You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS
 TITLE: Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS Page 1 of 2 POLICY: The College of Nursing shall maintain a bloodborne pathogen
TITLE: Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS Page 1 of 2 POLICY: The College of Nursing shall maintain a bloodborne pathogen
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
An International Antiviral Society-USA
 Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Bloodborne Pathogens. Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
PEDIATRIC EMERGENCY DEPARTMENT CLINICAL GUIDELINE: NON- OCCUPATIONAL EXPOSURE TO BLOOD-BORNE PATHOGENS (HIV, Hepatitis B, AND Hepatitis C)
 General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Bloodborne Pathogens Training
 Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
Revised April WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER
 Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV
 Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
Goldenrod Hills Community Action. Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR
 Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Central Franciscan Health Rensselaer Blood and Body Fluid Exposures Procedure COPY
 Current Status: Active PolicyStat ID: 2625043 Original: 7/18/2011 Last Reviewed: 8/19/2016 Last Revised: 8/19/2016 Next Review: 8/19/2019 Responsible Party: Kristin Deno: Manager Policy Area: References:
Current Status: Active PolicyStat ID: 2625043 Original: 7/18/2011 Last Reviewed: 8/19/2016 Last Revised: 8/19/2016 Next Review: 8/19/2019 Responsible Party: Kristin Deno: Manager Policy Area: References:
Emergency Department Blood/Body Fluid Exposure Instructions
 Emergency Department Blood/Body Fluid Exposure Instructions For employees sent to the Emergency Department after hours to be evaluated for needle stick / body fluid exposure 1. Floor or unit Charge RN
Emergency Department Blood/Body Fluid Exposure Instructions For employees sent to the Emergency Department after hours to be evaluated for needle stick / body fluid exposure 1. Floor or unit Charge RN
Blood Borne Pathogens (BBP)
 Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
Chapter 2 Hepatitis B Overview
 Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
BAY-ARENAC BEHAVIORAL HEALTH AUTHORITY POLICIES AND PROCEDURES MANUAL
 Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
B. Tasks and Procedures where employees, students or contractors can be exposed to bloodborne pathogens:
 Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Environmental Health and Safety Offices BLOODBORNE PATHOGENS
 Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM
 Page 1 of 13 BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM INTRODUCTION The attached materials will assist in teaching the information about bloodborne pathogens for health care workers as required
Page 1 of 13 BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM INTRODUCTION The attached materials will assist in teaching the information about bloodborne pathogens for health care workers as required
Blood-Borne Pathogens and Post-Exposure Prophylaxis
 Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood borne Pathogen
 Blood borne Pathogen Training For Certified Nursing Assistants Meets the Blood borne Pathogens & Infection Control Update (Formerly HIV/AIDS) 1 0 In-service Hour Meets the Blood borne Pathogens & Infection
Blood borne Pathogen Training For Certified Nursing Assistants Meets the Blood borne Pathogens & Infection Control Update (Formerly HIV/AIDS) 1 0 In-service Hour Meets the Blood borne Pathogens & Infection
Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis).
 LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1
 PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
Healthcare Professionals
 EMPLOYEE HEALTH Employee Health: Screening and immunization programs Counseling, follow up work restrictions Analysis and trending of occupational exposure incidents Assess risk for occupational exposure
EMPLOYEE HEALTH Employee Health: Screening and immunization programs Counseling, follow up work restrictions Analysis and trending of occupational exposure incidents Assess risk for occupational exposure
Needles and Sharps Exposure: How do We Proceed?
 Needles and Sharps Exposure: How do We Proceed? UMAYYA MUSHARRAFIEH,MD AMERICAN UNIVERSITY OF BEIRUT MEDICAL CENTER JUNE 14, 2013 Health care workers who use or may be exposed to needles are at increased
Needles and Sharps Exposure: How do We Proceed? UMAYYA MUSHARRAFIEH,MD AMERICAN UNIVERSITY OF BEIRUT MEDICAL CENTER JUNE 14, 2013 Health care workers who use or may be exposed to needles are at increased
Post Blood & Body Fluid Exposure Checklist
 Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
EMPLOYEE HEALTH TABLE OF CONTENTS
 EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
Hepatitis C Virus (HCV)
 Clinical Practice Guidelines Hepatitis C Virus (HCV) OBJECTIVE The purpose is to guide the appropriate diagnosis and management of Hepatitis C Virus (HCV). GUIDELINE These are only guidelines, and are
Clinical Practice Guidelines Hepatitis C Virus (HCV) OBJECTIVE The purpose is to guide the appropriate diagnosis and management of Hepatitis C Virus (HCV). GUIDELINE These are only guidelines, and are
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Hepatitis B at a Glance
 Return completed form, preferably within 30 days of U.S. date of arrival, to address on reverse side of this form. Review overseas medical exam if available and document immunization dates. Indicate if
Return completed form, preferably within 30 days of U.S. date of arrival, to address on reverse side of this form. Review overseas medical exam if available and document immunization dates. Indicate if
SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification
 SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States.
SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States.
C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官
 C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官 HCV:Structure and Classification Unclassified virus, Member of the flavivirus family (other members yellow fever and dengue) Enveloped single stranded RNA virus Humans
C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官 HCV:Structure and Classification Unclassified virus, Member of the flavivirus family (other members yellow fever and dengue) Enveloped single stranded RNA virus Humans
Training for Employees of Taylor Special Care Services, Inc.
 Training for Employees of Taylor Special Care Services, Inc. TSCS Taylor Special Care Services housing staffing counseling on-going support Simon Pop, MBA Chief Operating Officer 2015 2016 Guidelines:
Training for Employees of Taylor Special Care Services, Inc. TSCS Taylor Special Care Services housing staffing counseling on-going support Simon Pop, MBA Chief Operating Officer 2015 2016 Guidelines:
Pre and Post Exposure Prophylaxis. Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018
 Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Hepatitis B Control. Table of Contents
 Control Table of Contents 1.0 INTRODUCTION... 1 2.0 GOALS... 1 3.0 DEFINITIONS... 1 4.0 CASE MANAGEMENT... 3 5.0 HEPATITIS B POST- EXPOSURE MANAGEMENT... 4 TABLE 1: HEPATITIS B POST EXPOSURE PROPHYLAXIS...
Control Table of Contents 1.0 INTRODUCTION... 1 2.0 GOALS... 1 3.0 DEFINITIONS... 1 4.0 CASE MANAGEMENT... 3 5.0 HEPATITIS B POST- EXPOSURE MANAGEMENT... 4 TABLE 1: HEPATITIS B POST EXPOSURE PROPHYLAXIS...
Bloodborne Pathogen Module. Chelmsford Public Schools September, 2016
 Bloodborne Pathogen Module Chelmsford Public Schools September, 2016 Why is this important? v OSHA BB Pathogen Standard anyone whose job requires exposure to BB pathogens is required to complete training
Bloodborne Pathogen Module Chelmsford Public Schools September, 2016 Why is this important? v OSHA BB Pathogen Standard anyone whose job requires exposure to BB pathogens is required to complete training
NEOMED ACADEMIC POLICY
 (A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
(A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
Bloodborne Pathogens Training (OHS_BIO500) Course Material
 Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
Bloodborne Pathogens Training. IEA, Inc.
 Bloodborne Pathogens Training IEA, Inc. Review the potential hazard of exposure to blood and other potentially infectious materials (OPIMs). Review safe work practices to prevent occupational exposure
Bloodborne Pathogens Training IEA, Inc. Review the potential hazard of exposure to blood and other potentially infectious materials (OPIMs). Review safe work practices to prevent occupational exposure
TRANSMISSION OF BLOODBORNE PATHOGENS: HIV,HBV,HCV. Dr Daniel Kimani HIV Prevention Medical Transmission CDC, Kenya.
 TRANSMISSION OF BLOODBORNE PATHOGENS: HIV,HBV,HCV Dr Daniel Kimani HIV Prevention Medical Transmission CDC, Kenya. Nevada Hepatitis C Outbreak Tied to Las Vegas Clinic. Thousands Now At Risk for Hepatitis,
TRANSMISSION OF BLOODBORNE PATHOGENS: HIV,HBV,HCV Dr Daniel Kimani HIV Prevention Medical Transmission CDC, Kenya. Nevada Hepatitis C Outbreak Tied to Las Vegas Clinic. Thousands Now At Risk for Hepatitis,
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
Bloodborne Pathogens: Recognition and Treatment 2011
 Bloodborne Pathogens: Recognition and Treatment 2011 Occupational Health Services at The Summit Rita M. Lopez, APRN-BC, MSN 751-4189 rlopez@krmc.org OBJECTIVES DEFINE Bloodborne Pathogen Exposure WHO must
Bloodborne Pathogens: Recognition and Treatment 2011 Occupational Health Services at The Summit Rita M. Lopez, APRN-BC, MSN 751-4189 rlopez@krmc.org OBJECTIVES DEFINE Bloodborne Pathogen Exposure WHO must
Infection Control Program (ICP) ICP Components 1. Exposure Determination 2. Control Methods A. Universal Precautions
 Compliance Assistance Guideline for the February 27, 1990, OSHA Instruction CPL 2 2.44B Enforcement Procedures for Occupational Exposure to Hepatitis B Virus and Human Immunodeficiency Virus from the U.S.
Compliance Assistance Guideline for the February 27, 1990, OSHA Instruction CPL 2 2.44B Enforcement Procedures for Occupational Exposure to Hepatitis B Virus and Human Immunodeficiency Virus from the U.S.
Bloodborne Pathogens. Montclair Kimberley Academy 1
 Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
2017 BLOODBORNE PATHOGENS
 2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
Vaccine preventable health associated infections (HAI) Entesar Husain BM BCH, FRCPC
 Vaccine preventable health associated infections (HAI) Entesar Husain BM BCH, FRCPC Assistant Professor, Department of Pediatrics Faculty of Medicine Consultant Pediatric Infectious Diseases NBK Hospital
Vaccine preventable health associated infections (HAI) Entesar Husain BM BCH, FRCPC Assistant Professor, Department of Pediatrics Faculty of Medicine Consultant Pediatric Infectious Diseases NBK Hospital
Chapter 5 Serology Testing
 Chapter 5 Serology Testing 49 50 This page intentionally left blank. Diagnostic Tests for Hepatitis B Virus (HBV) Diagnosis of HBV infection (acute vs. chronic) is based on clinical, laboratory, and epidemiologic
Chapter 5 Serology Testing 49 50 This page intentionally left blank. Diagnostic Tests for Hepatitis B Virus (HBV) Diagnosis of HBV infection (acute vs. chronic) is based on clinical, laboratory, and epidemiologic
Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training
 Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training Education is the KEY Here are Gwynedd Mercy University, we recognize the importance of providing a safe working environment for
Gwynedd Mercy University Bloodborne Pathogen Safety and Awareness Training Education is the KEY Here are Gwynedd Mercy University, we recognize the importance of providing a safe working environment for
HEPATITIS B INFECTION and Pregnancy. Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011
 HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
The Bloodborne Pathogen Standard. An Overview
 The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
3/23/2016. Managing Bloodborne Pathogen Exposures. Managing Bloodborne Pathogen Exposures
 This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
Hepatitis B and C Overview, Outbreaks, and Recommendations. Viral Hepatitis Language. Types of Viral Hepatitis 7/1/2013
 Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES
 NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
NHS GREATER GLASGOW CONTROL OF INFECTION COMMITTEE GUIDELINE MANAGEMENT OF OCCUPATIONAL AND NON-OCCUPATIONAL EXPOSURES TO BLOODBORNE VIRUSES INCLUDING NEEDLESTICK INJURIES & SEXUAL EXPOSURES Effective
Management of Exposure to Needlestick Injuries & Body Fluids
 Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Management of Exposure to Needlestick Injuries & Body Fluids Clinical S.O.P. No.:28 Compiled by: Approved by: Review date: November 2016 DOCUMENT HISTORY Version Detail of purpose / change Author / edited
Hepatitis C January 26, 2018
 Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
Hepatitis C January 26, 2018 Case Investigation Guidelines Contents A. Purpose...2 B. Case Definitions...2 a. Acute Hepatitis C (2016...2 b. Chronic Hepatitis C (2016)...3 c. Perinatal Hepatitis C (2017
2014 OSHA Blood-borne Pathogens (BBP) Update JHS Annual Mandatory Education
 2014 OSHA Blood-borne Pathogens (BBP) Update 2014 JHS Annual Mandatory Education Objectives Discuss the epidemiology of Bloodborne Pathogens. List the statistics of HIV/AIDS cases Identify the correlation
2014 OSHA Blood-borne Pathogens (BBP) Update 2014 JHS Annual Mandatory Education Objectives Discuss the epidemiology of Bloodborne Pathogens. List the statistics of HIV/AIDS cases Identify the correlation
Bloodborne Pathogen Refresher Training
 Bloodborne Pathogen Refresher Training This program will review your occupational risks and the steps that you and the County must take to reduce your risks of exposure. Employees must report any occupational
Bloodborne Pathogen Refresher Training This program will review your occupational risks and the steps that you and the County must take to reduce your risks of exposure. Employees must report any occupational
determine need but regimen does not change based on risk factor ED medicine attending meets
 HIV PEP Guidelines after Sexual Assault Harborview Medical Center January 2015 Initial risk assessment and discussion with patient about risk and PEP is by clinician who does exam (SANE, Ob-Gyn resident,
HIV PEP Guidelines after Sexual Assault Harborview Medical Center January 2015 Initial risk assessment and discussion with patient about risk and PEP is by clinician who does exam (SANE, Ob-Gyn resident,
Greenwood School District 50 OSHA UPDATE BLOODBORNE PATHOGENS
 Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
ACS BLOOD BORNE PATHOGEN TRAINING
 ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
Hepatitis STARS Program. Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003
 Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Infection Prevention and Control - General Orientation
 Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
Clinical management of non-occupational and occupational exposure to blood borne pathogens. - A Pocket Reference -
 Pocket P.E.P. Clinical management of non-occupational and occupational eposure to blood borne pathogens Development Team: Deborah Yoong, PharmD 1 1, 2, 3 Kevin Gough, MD, MEd 1 Positive Care Clinic, Toronto,
Pocket P.E.P. Clinical management of non-occupational and occupational eposure to blood borne pathogens Development Team: Deborah Yoong, PharmD 1 1, 2, 3 Kevin Gough, MD, MEd 1 Positive Care Clinic, Toronto,
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: F:
 University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
Blood and Body Fluid Exposure
 Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
Blood and Body Fluid Exposure Infection Prevention and Control Contents Policy... 1 Purpose... 1 Scope/Audience... 1 Definitions... 2 Associated documents... 2 1.1 Indications for BBFE reporting... 2 1.2
Self-Instructional Packet (SIP)
 Self-Instructional Packet (SIP) Advanced Infection Prevention and Control Training Module 1 Intro to Infection Prevention Control February 11, 2013 Page 1 Learning Objectives Module One Introduction to
Self-Instructional Packet (SIP) Advanced Infection Prevention and Control Training Module 1 Intro to Infection Prevention Control February 11, 2013 Page 1 Learning Objectives Module One Introduction to
