Positive Modulation of Pepsinogen Secretion by Gastric Acidity After Vagal Cholinergic Stimulation 1
|
|
|
- Gwenda Norman
- 5 years ago
- Views:
Transcription
1 /97/ $03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 283, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. JPET 283: , 1997 Positive Modulation of Pepsinogen Secretion by Gastric Acidity After Vagal Cholinergic Stimulation 1 CORRADO BLANDIZZI, ROCCHINA COLUCCI, DIEGO CARIGNANI, GLORIA LAZZERI and MARIO DEL TACCA Division of Pharmacology and Chemotherapy, Department of Oncology, University of Pisa, Pisa, Italy Accepted for publication August 26, 1997 ABSTRACT Parallel increments of gastric acid and pepsinogen secretion generally occur after the application of cholinergic stimuli. However, it still remains to be established whether the changes in acid output associated with cholinergic stimulation play a role in regulation of the concomitant peptic secretory activity. In the present study, an anesthetized rat model was used for the evaluation of pepsinogen secretion in order to pursue a dual purpose: 1) to assess the relative functional relevance of direct and acid-dependent control exerted by cholinergic pathways on pepsinogen output; 2) to characterize the mechanisms through which changes in acidity within the stomach lumen may affect the peptic secretory activity of gastric mucosa. Bethanechol, 2-deoxy-D-glucose or electrical vagal stimulation caused parallel and atropine-sensitive increments of peptic and acid secretions. Omeprazole, a selective inhibitor of gastric H :K -adenosintriphosphatase, blocked the increase in acid but not pepsinogen secretion induced by bethanechol. However, 2-deoxy-D-glucose or electrical vagal stimulation failed to increase either pepsinogen or acid secretion in omeprazolepretreated rats. When tested in animals pretreated with both omeprazole and physostigmine (a drug able to prevent the enzymatic breakdown of vagally released ACh through the Vagal cholinergic stimuli and muscarinic receptor agonists evoke parallel increments of gastric acid and pepsinogen secretions in a variety of experimental models as well as in humans (Hersey, 1987; Smith and Torres, 1990; Hirschowitz and Groarke, 1993). However, although a great deal is known about the control of acid secretion mediated by cholinergic pathways (Hirschowitz and Groarke, 1993; Lloyd and Debas, 1994; Welsh et al., 1994), the mechanisms that account for the in vivo regulation of peptic secretion are still unclear (Basson et al., 1988; Raufman, 1992). In particular, it is matter of debate whether the changes in acid output associated with cholinergic stimulation might play any role in regulation of the concomitant peptic secretory activity (Basson et al., 1988; Smith and Torres, 1990). Received for publication March 31, The present work was supported in part by the Italian Ministry of University and Scientific Research (40% 60% funds). blockade of acetylcholinesterase), 2-deoxy-D-glucose or electrical vagal stimulation significantly increased pepsinogen secretion without affecting acid secretion. In omeprazole-pretreated rats, perfusion of the gastric lumen with acid solutions caused a ph-dependent and atropine-sensitive increase in peptic output only when applied in combination with electrical vagal stimulation. Functional ablation of capsaicin-sensitive sensory neurons did not modify the gastric secretory responses induced by bethanechol or electrical vagal stimulation. However, after topical application of lidocaine to the gastric mucosal surface, bethanechol stimulated both peptic and acid outputs, whereas electrical vagal stimulation only evoked acid secretion without affecting basal peptic output. The present results indicate that the activation of muscarinic receptors by vagally released ACh is not sufficient by itself to stimulate pepsinogen secretion and that a facilitatory action mediated by acid secretion is necessary to allow an increment of peptic output in response to vagal cholinergic stimuli. It is suggested that such facilitatory input is driven to chief cells by local intramural reflexes that involve capsaicin-insensitive intrinsic nerves. Several studies, based on binding, molecular biology and functional techniques, have shown that both gastric chief cells and parietal cells are provided with muscarinic receptors, the activation of which increases intracellular calcium concentration and leads to subsequent stimulation of pepsinogen and acid secretions, respectively (Raufman, 1992; Hersey, 1994; Soll and Berglindh, 1994). In addition, pharmacological experiments performed on isolated rat stomach demonstrated that gastric glands receive direct innervation from cholinergic fibers (Welsh et al., 1994). These findings would suggest that endogenous ACh, released from vagal cholinergic fibers, causes a parallel stimulation of pepsinogen and acid secretions through a simultaneous activation of muscarinic receptors located on chief cells and parietal cells, respectively (Hersey, 1994; Soll and Berglindh, 1994). However, both in vivo and in vitro studies indicated that pepsinogen secretion in response to stimulation of chief cells appears ABBREVIATION: 2DG, 2-deoxy-D-glucose. 1043
2 1044 Blandizzi et al. Vol. 283 to require a flow of water and acid from adjacent parietal cells (Hersey, 1987; Sandvik et al., 1987) and that acidity in the stomach lumen is able to stimulate the pepsinogen output from gastric mucosa (Johnson, 1972; Smith and Torres, 1990). This suggests that changes in local hydrogen ion concentration evoked by cholinergic stimulants might be, at least in part, responsible for the parallel variations of the peptic secretory output. Previous attempts to discriminate between direct and aciddependent cholinergic control of pepsinogen secretion were based mainly on the use of in vitro experimental models (Hersey et al., 1983; Sandvik et al., 1987; Basson et al., 1988). However, under such conditions an assessment of the secretory actions mediated by endogenously released ACh is not usually allowed, and the effects of hydrogen ions on chief cells cannot be clearly established because of methodological problems related to the presence of incubation media (Basson et al., 1988). In addition, data concerning the putative changes of peptic output induced by gastric lumen acidification after suppression of endogenous acid production are still lacking. Therefore, in the present study, an in vivo gastric preparation was used for the evaluation of pepsinogen secretion, under different experimental conditions, to pursue a dual purpose: 1) to assess the relative functional relevance of direct and acid-dependent control exerted by cholinergic pathways on pepsinogen output after the activation of muscarinic receptors by endogenous ACh or an exogenously administered muscarinic agonist; 2) to characterize the mechanisms through which changes in acidity within the stomach lumen may affect the peptic secretory activity of gastric mucosa. Materials and Methods Animals The experiments were carried out on male Wistar rats weighing 200 to 220 g. The animals were fed standard laboratory chow and tap water ad libitum and were not used for at least 1 week after their delivery to the laboratory. The animals were housed, six in a cage, in temperature-controlled rooms on a 12-h light cycle at C and 50 60% humidity. Their care and handling were in accordance with the provisions of the European Economic Community (EEC) Council Directive , recognized and adopted by the Italian government. Twenty-four hours before the experiments, the animals were maintained in single cages provided with wire net bottoms and were deprived of food. Free access to water was allowed until 1 h before the experiment. Perfusion of the Gastric Lumen in Anesthetized Rats Continuous perfusion of the rat stomach in situ was carried out, following the procedure previously reported (Blandizzi et al., 1995). The animals were anesthetized with urethane (1.2 g/kg) administered i.p., and the trachea was surgically exposed and cannulated by a polyethylene catheter to ensure a patent airway. A polyethylene catheter was introduced into the esophagus and advanced as far as 5 mm beyond the gastroesophageal junction. After a midline laparotomy, the proximal duodenum was exposed and its wall incised. Then a second polyethylene catheter was introduced into the duodenum and pushed forward until its tip was about 5 mm beyond the pylorus. The stomach lumen was perfused continuously at a rate of 1 ml/min with saline solution (154 mm NaCl) at 37 C (ph ) unless otherwise stated, and 15-min effluent fractions were collected. The effluent samples were used for the quantitative evaluation of both pepsinogen and acid secretions. Evaluation of Pepsinogen and Acid Secretion Pepsin levels in the gastric effluent were determined as previously reported (Blandizzi et al., 1995). Briefly, 2 ml of 2.5% bovine hemoglobin plus 0.5 ml of 0.3 N HCl and 0.5 ml of gastric effluent were maintained in separate tubes at 37 C for 10 min and then mixed. Mixtures were incubated for 10 min at 37 C, and the reaction was stopped by the addition of 5 ml 0.3 N trichloroacetic acid. After agitation and filtration, optical density was measured at 280 nm by an Uvikon 930 Spectrophotometer (Kontron Instruments, Milan, Italy). The results were compared to a standard curve, which was generated in an identical manner using known amounts of porcine pepsin (1 g 3 peptic units), and were expressed as micrograms of pepsin. The acidity in the gastric perfusate was measured with an autotitrator ph meter (PHM 85, Radiometer, Copenhagen, Denmark) by automatic potentiometric titration to ph 7.0 with 0.01 N NaOH and was expressed as EqH. After the surgical preparation of animals, basal gastric secretion was allowed to stabilize for 30 min. At the end of this period, we collected two consecutive 15-min effluent fractions to assess basal secretory values. Both pepsinogen and acid secretions were then monitored at 15-min intervals for an additional 120 min. The peptic and acid outputs obtained during the 120-min period after the collection of basal effluent samples were calculated and expressed as g of pepsin/120 min and EqH /120 min, respectively. Experimental Procedures Effects of bethanechol, of 2DG and of electrical vagal stimulation under basal secretory conditions. The first set of experiments was carried out on rats with intact vagus nerves. The gastric secretory activity of these animals was evoked by bethanechol (0.3, 1 and 3 mg/kg), a well-known muscarinic receptor agonist, or 2DG (200 mg/kg), a centrally acting stimulant of vagal efferent cholinergic pathways (Eisenberg et al., 1966). Both these drugs were administered i.v. as a bolus immediately after the collection of basal effluent samples. The second series of experiments was performed on rats whose vagus nerves were carefully separated from the carotid arteries and cut at the cervical level 30 min before the collection of basal effluent samples began. The effects of bethanechol or 2DG on gastric secretions were reassessed in animals that underwent this vagotomy procedure. In addition, in a group of vagotomized animals, the distal end of the left vagus nerve was placed on a bipolar platinum electrode and immersed in paraffin oil. In this case, after the collection of basal effluent samples, the gastric peptic and acid secretions were evoked by continuous electrical stimulation of the left vagus nerve (120 min). The stimulus parameters were square-wave pulses 0.5 ms in duration, delivered at 5 Hz with supramaximal intensity (10 V) by means of a Grass S5 stimulator (Grass Instruments, Quincy, MA) (Blandizzi et al., 1992). Effects of bethanechol, of 2DG and of electrical vagal stimulation in animals pretreated with omeprazole. A group of experiments was designed to assess the effects of bethanechol, of 2DG and of electrical vagal stimulation on pepsinogen secretion in the presence of a complete blockade of the acid secretory function of gastric parietal cells. For this purpose, 90 min before the collection of basal effluent samples began, animals were pretreated with omeprazole (30 mg/kg i.v.), a benzimidazole derivative that inhibits gastric acid secretion through a selective blockade of H :K -adenosintriphosphatase (Fellenius et al., 1981) without interfering with receptor or signal transduction pathways of gastric secretory cells (Clissold and Campoli-Richards, 1986). The dose of omeprazole was selected because of its ability to suppress acutely both basal and stimulated gastric acid secretion in anesthetized rats (Blandizzi et al., 1995). Effects of perfusion of the gastric lumen with acid solutions. The influence exerted on pepsinogen secretion by topical application of acid solutions at various ph on the surface of gastric
3 1997 Gastric Acidity and Pepsinogen Secretion 1045 mucosa was studied both under basal conditions and in the presence of electrical vagal stimulation. In this case, the anesthetized rats first were pretreated with omeprazole, to suppress endogenous acid production, and then were subjected to bilateral cervical vagotomy. Perfusion of the gastric lumen was performed with saline up to the collection of basal effluent samples and then was continued until the end of the experimental period with one of the following acid solutions: 0.1 mm HCl plus mm NaCl (ph 4.0); 1 mm HCl plus 153 mm NaCl (ph 3.0); 10 mm HCl plus 144 NaCl (ph 2.0); 100 mm HCl plus 54 mm NaCl (ph 1.0). Effects of bethanechol and of electrical vagal stimulation on animals subjected to systemic capsaicinization or intragastric application of lidocaine. A group of experiments was designed to assess whether bethanechol and whether electrical vagal stimulation was able to affect gastric pepsinogen and acid secretions from vagotomized rats in the presence of a systemic ablation of capsaicin-sensitive sensory nerve fibers. For this purpose, some animals were given a dose of 125 mg/kg capsaicin s.c., as previously reported by Pabst et al. (1993). Ten days after this treatment, the animals were used for the assessment of gastric pepsinogen and acid secretions. One day before the experiment, the efficacy of capsaicin treatment was checked by instilling a drop of a capsaicin solution (0.1 mg/ml in saline solution) into one eye of each rat. Capsaicintreated rats were expected not to react by wiping their eyes, but whenever an animal responded with wiping, the afflicted eye was immediately and extensively rinsed with water. An additional series of experiments was performed to investigate the effects of bethanechol and of electrical vagal stimulation on both pepsinogen and acid secretions from vagotomized rats after topical application of the local anesthetic lidocaine to the gastric mucosal surface. In this case, pre-exposure of the gastric mucosa to lidocaine was carried out according to the procedure reported by Mercer et al. (1994) with minor modifications. After the collection of two basal effluent samples, the perfusion was interrupted, and the gastric lumen was gently emptied and then filled with 3 ml of 4% lidocaine. Fifteen minutes later the stomach was emptied, rinsed by gently flushing with 6 ml of saline and filled again with 3 ml of saline. Perfusion of the gastric lumen with saline was then resumed and continued up to the end of the experimental period. An interval of 5 min was allowed to elapse between the restart of gastric perfusion and either bethanechol injection or electrical vagal stimulation. Effects of physostigmine and atropine. In some experiments the enzymatic breakdown of vagally released ACh was prevented in order to promote its accumulation over physiological concentrations. For this purpose, 5 min before they received 2DG injection or electrical vagal stimulation, some animals were treated with the acetylcholinesterase inhibitor physostigmine (1 mg/kg i.v.). In those experiments where the involvement of muscarinic receptors in the gastric secretory responses was assessed, atropine 1 mg/kg i.v. was always administered 5 min before collection of the second basal effluent sample ended. Drugs The following drugs and reagents were used: urethane ethyl carbamate, crystalline porcine pepsin, lyophilized bovine hemoglobin, bethanechol chloride, 2DG, physostigmine sulfate, capsaicin, aminophylline, terbutaline, lidocaine (Sigma, St. Louis, MO), omeprazole (kindly provided by Malesci, Florence, Italy) and atropine sulfate (BDH Chemicals, Poole, England). Other reagents were of analytical grade. Bethanechol, 2DG, atropine, physostigmine and lidocaine were dissolved in saline immediately before use. Omeprazole was initially dissolved in polyethyleneglycole (PEG, molecular weight 400) and then diluted with 7 mm NaHCO 3 to a final concentration of 50% PEG (v/v). All drugs administered i.v. were injected in a volume of 0.25 ml/rat. Capsaicin was dissolved (12.5 mg/ml) in a vehicle composed by 10% ethanol, 10% Tween 80 and 80% saline solution (v/v/v). The total dose of capsaicin (125 mg/kg s.c.) was administered under ether anesthesia in four injections over two consecutive days (first day: 25 mg/kg in the morning and 25 mg/kg in the late afternoon; second day: 25 mg/kg in the morning and 50 mg/kg in the late afternoon). To counteract the respiratory impairment associated with the administration of capsaicin, rats received atropine (0.2 mg/kg i.p.), terbutaline (0.2 mg/kg i.p.) and aminophylline (20 mg/kg i.p.) 10 min before the first and third capsaicin injections. Statistics Results are given as mean S.E. The significance of differences was evaluated by Student t test or one-way analysis of variance (ANOVA) followed by post-hoc analysis by Student-Newman-Keuls test, and P values less than.05 were considered significant; n indicates the number of experiments. Results Evaluation of basal pepsinogen and acid secretions. In control animals with intact vagus nerves (n 6), basal gastric pepsinogen and acid secretions, assessed after a 30- min stabilization, accounted for g of pepsin/15 min and EqH /15 min, respectively, and these values remained nearly constant until the end of the experiments (120 min). In addition, when control animals underwent bilateral cervical vagotomy (n 6), basal pepsinogen and acid secretions accounted for g of pepsin/15 min and EqH /15 min, respectively. These values did not differ significantly from those obtained in control rats with intact vagus nerves, and they remained at a steady level throughout the experiment. In a series of preliminary experiments, both basal pepsinogen and acid outputs were monitored in rats, with or without intact vagus nerves, after pretreatment with omeprazole or atropine (n 6 for each drug). An almost complete inhibition of basal acid secretion, but not of peptic output, was detected only in rats undergoing pretreatment with omeprazole; atropine did not significantly affect basal peptic or acid secretory activities (fig. 1). Effects of bethanechol on pepsinogen and acid secretions. In animals with intact vagus nerves, bethanechol (0.3, 1 and 3 mg/kg i.v.) caused a dose-dependent and parallel increase in both pepsinogen and acid secretions, the maximal effects occurring at the dose of 1 mg/kg (fig. 2,A and B). The Fig. 1. Anesthetized rats with perfused gastric lumen. Effects of omeprazole 30 mg/kg i.v. (OME), of atropine 1 mg/kg i.v. (ATR), of bilateral cervical vagotomy (VAG), of omeprazole 30 mg/kg i.v. plus vagotomy (OME VAG) and of atropine 1 mg/kg i.v. plus vagotomy (ATR VAG) on basal pepsinogen (panel A) and acid secretion (panel B). Each column represents the mean value obtained from six animals SE (vertical bars). *P.05: significant difference from control values (CON).
4 1046 Blandizzi et al. Vol. 283 Fig. 2. Anesthetized rats with perfused gastric lumen. Effects of bethanechol 0.3 (BET-0.3), 1 (BET-1) and 3 (BET-3) mg/kg i.v. (panels A, B), and of bethanechol 1 mg/kg i.v., administered either alone (BET-1) or in the presence of bilateral cervical vagotomy (BET-1 VAG), atropine 1 mg/kg i.v. (BET-1 ATR), omeprazole 30 mg/kg i.v. (BET- 1 OME) and omeprazole 30 mg/kg i.v. plus atropine 1 mg/kg i.v. (BET OME ATR) (panels C, D), on basal pepsinogen (panels A, C) and acid secretion (panels B, D). Each column represents the mean value obtained from 8 to 10 animals S.E. (vertical bars). *P.05: significant difference from control values (CON); a P.05: significant difference from bethanechol 1 mg/kg alone (BET-1). excitatory responses elicited by bethanechol were not significantly affected by bilateral cervical vagotomy, while they were completely prevented by pretreatment with atropine (fig. 2,C and D). When administered to rats pretreated with omeprazole, bethanechol failed to stimulate acid secretion, but it was still able to evoke an atropine-sensitive increase in pepsinogen output that did not differ from the increase observed in omeprazole-untreated rats (fig. 2,C and D). Effects of 2DG and of electrical vagal stimulation on pepsinogen and acid secretions. In animals with intact vagus nerves, the administration of 2DG (200 mg/kg i.v.) induced significant and simultaneous increases in pepsinogen and acid outputs (fig. 3,A and B). Both of these effects were prevented by bilateral cervical vagotomy as well as by atropine (fig. 3,A and B). However, in contrast with the results obtained in the presence of bethanechol, 2DG failed to stimulate either pepsinogen or acid secretion in omeprazolepretreated animals (fig. 3,A and B). Both pepsinogen and acid outputs increased simultaneously after the application of electrical stimulation to the left vagus nerve, and these effects were completely prevented by atropine. However, analogously to 2DG, electrical vagal stimulation was also not able to modify basal pepsinogen or acid secretions after pretreatment of animals with omeprazole (fig. 3,C and D). Fig. 3. Anesthetized rats with perfused gastric lumen. Effects of 2DG 200 mg/kg i.v. (2DG) (panels A, B) or electrical vagal stimulation of the left vagus nerve (EVS: 0.5 ms, 5 Hz, 10 V for 120 min) (panels C, D) on basal pepsinogen (panels A, C) and acid secretion (panels B, D). 2DG was administered either alone or in the presence of bilateral cervical vagotomy (2DG VAG), atropine 1 mg/kg i.v. (2DG ATR), or omeprazole 30 mg/kg i.v. (2DG OME) (panels A, B). Electrical vagal stimulation was applied either alone or in the presence of atropine 1 mg/kg i.v. (EVS ATR) or omeprazole 30 mg/kg i.v. (EVS OME) (panels C, D). Each column represents the mean value obtained from 8 to 10 animals S.E. (vertical bars). *P.05: significant difference from control values (CON); a P.05: significant difference from either 2DG alone (panels A, B) or EVS alone (panels C, D). In animals where the enzymatic breakdown of ACh was prevented by acute pretreatment with physostigmine, 2DG injection or electrical vagal stimulation evoked atropine-sensitive increases in both peptic and acid outputs (fig. 4). However, whereas acid secretion in response to 2DG or vagal stimulation did not differ from that obtained in physostigmine-untreated rats, the increments in peptic values were higher in the presence of physostigmine than in its absence (P.05), and they were similar to those induced by bethanechol 1 mg/kg in physostigmine-untreated animals. In addition, when physostigmine was administered to animals pretreated with omeprazole, both 2DG and vagal stimulation were still able to induce marked and atropine-sensitive increments in pepsinogen secretion (fig. 4,A and C), although they did not exert any stimulant action on acid output (fig. 4,B and D). These peptic responses did not differ from that obtained after bethanechol injection to omeprazole-pretreated rats. Effects of perfusion of the gastric lumen with acid solutions. The influence on pepsinogen secretion exerted by topical intragastric application of solutions with different ph values was studied in animals subjected to bilateral cervical vagotomy and pretreatment with omeprazole. Under these
5 1997 Gastric Acidity and Pepsinogen Secretion 1047 Fig. 4. Anesthetized rats with perfused gastric lumen and pretreatment with physostigmine. Effects of 2DG 200 mg/kg i.v. (2DG) (panels A, B) or electrical stimulation of the left vagus nerve (EVS: 0.5 ms, 5 Hz, 10 V for 120 min) (panels C, D) on basal pepsinogen (panels A, C) and acid secretion (panels B, D). 2DG was administered either alone or in the presence of atropine 1 mg/kg i.v. (2DG ATR), omeprazole 30 mg/kg i.v. (2DG OME) or omeprazole 30 mg/kg i.v. plus atropine 1 mg/kg i.v. (2DG OME ATR) (panels A, B). Electrical vagal stimulation was applied either alone or in the presence of atropine 1 mg/kg i.v. (EVS ATR), omeprazole 30 mg/kg i.v. (EVS OME) or omeprazole 30 mg/kg i.v. plus atropine 1 mg/kg i.v. (EVS OME ATR) (panels C, D). Each column represents the mean value obtained from 8 to 10 animals S.E. (vertical bars). *P.05: significant difference from control values (CON); a P.05: significant difference from either 2DG alone (panels A, B) or EVS alone (panels C, D). Fig. 5. Anesthetized rats with perfused gastric lumen, bilateral cervical vagotomy and pretreatment with omeprazole. A) Pepsinogen secretion in the presence of intraluminal perfusion with solutions at ph 7, 4, 3, 2 or 1. B) Pepsinogen secretion in the presence of electrical stimulation of the left vagus nerve (0.5 ms, 5 Hz, 10 V for 120 min) plus intraluminal perfusion with solutions at ph 7, 4, 3, 2 or 1 or with solution at ph 2 after pretreatment with atropine 1 mg/kg i.v. (ph 2 ATR). Each column represents the mean value obtained from 6 to 8 animals S.E. (vertical bars). *P.05: significant difference from values obtained at ph 7; a P.05: significant difference from values obtained at ph 2. Fig. 6. Anesthetized rats with perfused gastric lumen and bilateral cervical vagotomy. Effects of systemic capsaicinization (o) or intragastric application of lidocaine ( ) on pepsinogen (A) or acid secretion (B) under basal conditions, treatment with bethanechol 1 mg/kg i.v. (BET-1) or electrical stimulation of the left vagus nerve (EVS: 0.5 ms, 5 Hz, 10 V for 120 min). Each column represents the mean value obtained from 8 animals S.E. (vertical bars). *P.05: significant difference from basal values; a P.05: significant difference from control values ( ). conditions, perfusion of the gastric lumen with solutions at ph 4.0, 3.0, 2.0 or 1.0 did not modify basal pepsinogen secretion (fig. 5A). However, when gastric perfusion with one of these solutions was carried out in combination with electrical stimulation of the left vagus nerve, the pepsinogen output increased in a ph-dependent manner, the maximal increment occurring at ph 2.0 (fig. 5B). Pretreatment with atropine prevented the peptic response evoked by gastric perfusion with acid solution at ph 2.0 combined with electrical vagal stimulation (fig. 5B). Effects of bethanechol and of electrical vagal stimulation on pepsinogen and acid secretions after pretreatment with capsaicin or lidocaine. The functional ablation of capsaicin-sensitive sensory neurons by systemic pretreatment with capsaicin did not significantly modify basal gastric secretions and failed also to affect the stimulant actions of bethanechol or electrical vagal stimulation on pepsinogen and acid outputs (fig. 6,A and B). In animals whose gastric mucosa was exposed to lidocaine, both basal peptic and acid outputs did not differ significantly from those measured in lidocaine-untreated animals. In addition, after topical application of lidocaine to the gastric mucosal surface, bethanechol increased both pepsinogen and acid secretions to an extent similar to that induced by the same drug in lidocaine-untreated rats (fig. 6,A and B). However, under the same conditions, electrical stimulation of the left vagus nerve caused a significant increase in acid secretion without affecting basal pepsinogen output (fig. 6,A and B). Discussion Current interest in studying the mechanisms underlying the regulation of gastric pepsin secretion arises from recognition of the relevant role played by this enzyme in the pathophysiology of various digestive disorders, including peptic ulcer (Samloff, 1989; Raufman, 1992) and gastritis associated with Helicobacter pylori infection (Cave and Cave, 1991; Lamers, 1992). As far as the cholinergic control of
6 1048 Blandizzi et al. Vol. 283 pepsinogen secretion is concerned, it is known that a parallel increase in peptic and acid outputs generally occurs after the application of cholinergic stimuli (Smith and Torres, 1990; Hirschowitz and Groarke, 1993), but it remains to be established whether peptic hypersecretion depends mainly on a direct activation of chief cells or on a stimulant action exerted on these cells by the concomitant increase in acid secretory output (Basson et al., 1988; Raufman, 1992). In the present study, it was possible to dissociate direct from acid-dependent cholinergic regulation of pepsinogen secretion, and we obtained evidence that the secretory response of chief cells to endogenous ACh is driven by the parallel increment of acid output. The present results showing that, despite a total inhibition of acid output, the basal pepsinogen secretion continued unchanged after treatment of animals with omeprazole, are in agreement with findings obtained from fundic mucosal sheets (Basson et al., 1988) and suggest that in the present model, the basal pepsinogen output is independent of acid secretion. It must be noted also that in this preparation, the anesthesia with urethane depresses the vagal cholinergic outflow to the stomach (Maggi and Meli, 1986). Accordingly, atropine or bilateral cervical vagotomy did not modify basal peptic and acid outputs, which indicates that the anesthetized animal is not subjected to an endogenous tonic cholinergic control, and therefore it represents an useful model to test the influence of applied cholinergic stimuli on gastric secretory functions. It has also been reported that urethane stimulates both synthesis and release of gastric somatostatin (Yang et al., 1990). However, with regard to putative interferences elicited by this peptide in the present study, it should be considered that muscarinic receptors mediate inhibitory effects on somatostatin secretion (Del Tacca et al., 1987), so the application of cholinergic stimuli was expected to counterbalance the somatostatin-releasing action of urethane. In addition, because an increase in gastrin release may occur as a consequence of omeprazole administration (Wilde and McTavish, 1994), the presence of a somatostatin background might have counteracted the possible interference of gastrin in the excitatory effects evoked by cholinergic stimuli. Putative differences in the peptic response of chief cells to the application of an exogenous cholinergic agonist or to the release of endogenous ACh were investigated in experiments where gastric secretion was elicited by bethanechol, 2DG or electrical vagal stimulation, either in the absence or in the presence of omeprazole. In the absence of omeprazole, these stimulants induced parallel and atropine-sensitive increments of both peptic and acid outputs, which indicates the involvement of muscarinic receptors. However, quite surprising results were obtained when the same stimuli were applied after blockade of acid secretion by omeprazole. Under these conditions, bethanechol was still able to evoke an atropine-sensitive increase in pepsinogen secretion, whereas neither 2DG nor electrical vagal stimulation affected basal peptic output. These findings are compatible with the hypothesis that below a threshold level of muscarinic receptor activation, acid secretion may exert an excitatory influence on the peptic secretory response of chief cells to cholinergic stimuli. In particular, the results obtained in the presence of omeprazole suggest that a background of acid secretion plays a pivotal role in facilitating peptic hypersecretion promoted by endogenous ACh concentrations, which are likely to lead to submaximal and/or short-lasting occupation of muscarinic receptors. In contrast, according to our data as well as those yielded by previous in vitro studies (Hersey et al., 1983; Basson et al., 1988), acid secretion does not appear to be necessary to increase the pepsinogen secretion when muscarinic receptors are activated by pharmacological concentrations of exogenous agonists, such as bethanechol or carbachol, and it rather appears that parietal cells and chief cells can be stimulated simultaneously by these agents only by virtue of sharing similar receptors (Basson et al., 1988). In support of this view, in animals where endogenous ACh concentrations were raised to pharmacological levels by pretreatment with physostigmine, both the injection of 2DG and the stimulation of vagus nerve induced marked and atropinesensitive increments in pepsinogen secretion regardless of whether omeprazole-induced acid inhibition was present. In the present study, a series of experiments was designed in the attempt to gain more insight into the mechanisms underlying the in vivo interaction between hydrogen ions and chief cells. For this purpose, we took care to suppress endogenous acid production by pretreatment with omeprazole, and the gastric mucosal surface was continuously irrigated with iso-osmotic solutions at ph ranging from 4 to 1. Because under these peculiar conditions a ph-dependent and atropine-sensitive increment of pepsinogen output could be achieved only after electrical stimulation of vagus nerve, it is conceivable that hydrogen ions are not able to promote by themselves a direct stimulation of pepsinogen secretion but that, rather, they facilitate the activation of chief cells in response to the release of endogenous ACh. This conclusion is in line with our data showing that basal pepsinogen secretion remained unchanged after suppression of acid secretion by omeprazole, and it is consistent with the findings of previous studies indicating a lack of direct stimulant influence by gastric acidity on the secretory function of chief cells (Puurunen, 1979; Kleveland et al., 1987). Indeed, no significant changes in basal pepsinogen secretion from urethane-anesthetized rats were observed after perfusion of the gastric lumen with iso-osmotic NaCl solutions containing HCl from 0.01 to 100 mm (Puurunen, 1979). Analogously, intragastric perfusion with 1 mm HCl failed to affect basal pepsinogen secretion from isolated and vascularly perfused rat stomach (Kleveland et al., 1987). It must be noted also that although observations arguing in favor of a direct pepsigogic action exerted by luminal hydrogen ions have been previously reported (Johnson, 1972; Smith and Torres, 1990), they probably reflected the occurrence of methodological bias. For instance, significant increments of pepsinogen secretion were detected after topical application of acid solutions to the gastric mucosa in humans (Bynum and Johnson, 1975; Smith and Torres, 1990), dogs (Johnson, 1972), cats and rabbits (Descroix-Vagne et al., 1993). In all these cases, however, the peptic response did not appear clearly to be related to ph changes, but rather to concomitant gastric distension or osmotic stimulation by test solutions (Descroix-Vagne et al., 1993). Accordingly, distension of the stomach wall and osmolarity of the gastric contents are well recognized as stimulants of pepsin secretion in different mammalian species, acting by gastrin release or local cholinergic reflexes (Descroix-Vagne et al., 1993; Sengupta and Gebhart, 1994). In addition, when incubated in the
7 1997 Gastric Acidity and Pepsinogen Secretion 1049 presence of acidified media, isolated gastric gland preparations increased their pepsinogen release, but under those conditions, chief cells were subjected to alterations in intracellular ph that occur only after marked back-diffusion of acid through a damaged gastric mucosal barrier (Norris and Hersey, 1983). There is evidence in the literature to support the view that changes in hydrogen ion concentration might modulate pepsinogen secretion indirectly by interacting with ph-sensitive mucosal chemoreceptors, which in turn would sensitize chief cells to the stimulant action of endogenous cholinergic pathways. Indeed, electrophysiological studies demonstrated the existence of gastric mucosal receptors that respond to low ph in various species (Norris and Hersey, 1983; Yamamoto et al., 1994a). In addition, the rat stomach is provided with two distinct populations of sensory fibers, belonging to either capsaicin-sensitive afferent nerves or capsaicin-insensitive intrinsic neurons, which can affect some gastric functions in response to changes in intraluminal ph (Geppetti et al., 1991). Because capsaicin-sensitive nerves of the GI mucosa have been implicated in the control of various digestive functions (Sharkey et al., 1991; Pabst et al., 1993), we attempted first to ascertain whether capsaicin-sensitive fibers might be involved in the sensitizing action exerted by hydrogen ions on chief cells. The present data, which show that bethanechol or vagal stimulation was still able to induce simultaneous increments of acid and peptic outputs after pretreatment with capsaicin, indicate that systemic capsaicinization did not influence the functional status of chief cells and suggest that capsaicin-sensitive sensory nerves are not implicated in the mechanisms linking pepsinogen secretion to changes in hydrogen ion concentration. In accordance with these findings, Sharkey et al. (1991) showed that in urethane-anesthetized rats subjected to vagal stimulation at 4 Hz, the gastric secretory response is totally independent of the activation of capsaicin-sensitive afferent fibers. In the present study, different results from those obtained after systemic capsaicinization were obtained when gastric mucosa was acutely exposed to lidocaine in order to induce a local surface anesthesia. Lidocaine-pretreatment did not impair the ability of parietal or chief cells to respond to secretory inputs, as demonstrated by the significant and parallel increase in both acid and pepsinogen secretions after bethanechol administration. However, under the same conditions, vagal stimulation evoked acid secretion but did not affect basal peptic output, which suggests that mucosal afferent fibers, probably belonging to capsaicin-insensitive intrinsic neurons, drive an acid-mediated facilitatory input to chief cells, allowing a peptic secretory output in response to the activation of muscarinic receptors by endogenous ACh. In support of this view, previous studies indicated that capsaicin-insensitive intrinsic neurons of rat stomach can be activated by low ph levels to promote a reflex increase in mucosal blood flow (Geppetti et al., 1991). Although the existence of a local intramural reflex linking pepsinogen output to changes in acid secretion may represent a possible explanation for our findings, two points remain to be clarified: 1) how gastric intrinsic neurons can detect ph variations and 2) how these neurons can transmit their information to chief cells. With regard to the first point, it is hard to believe that under normal conditions, hydrogen ions can penetrate the gastric epithelium to reach the free endings of intrinsic sensory fibers, and it is generally considered that such nerve terminals may be connected to special sensory structures sensitive to acidic ph (Yamamoto et al., 1994a). Accordingly, enteroendocrine or enterochromaffin cells of the mucosal epithelium are currently regarded as possible transducer elements for ph variations (Sengupta and Gebhart, 1994; Yamamoto et al., 1994a), and it has been postulated that, once activated by gastric acidification, these cells release a specific factor that, in turn, stimulates local sensory nerve terminals (Yamamoto et al., 1994b). As far as the second issue is concerned, the activation of a local aciddependent reflex might theoretically result either in an enhancement of ACh release from cholinergic terminals or in the release (or co-release) of a distinct transmitter acting on chief cells to facilitate the pepsigogic action of ACh. However, further investigations are needed to clarify this issue. In conclusion, the present results suggest that the activation of muscarinic receptors on chief cells by vagally released ACh is not sufficient to increase peptic output and that an acid-mediated facilitatory action is necessary to allow pepsinogen hypersecretion in response to stimulation by endogenous cholinergic pathways. According to our findings, it may be proposed that such acid-dependent facilitatory input is driven to chief cells by a local gastric intramural reflex that involves capsaicin-insensitive intrinsic neurons. Overall, the hypothesis emerging from these findings would reconcile the conflicting results of previous reports that have indicated, on the one hand, a close parallel between acid and peptic hypersecretory responses to cholinergic stimulation in vivo and, on the other, the ability of chief cells to be activated by muscarinic agonists in vitro independently of acid secretion. Acknowledgments The experiments were carried out with the technical assistance of Mr. Bruno Stacchini. References BASSON, M. D., ADRIAN, T. E. AND MODLIN, I. M.: Dissociation of pepsinogen and acid secretion in the guinea-pig. Gastroenterology 95: , BLANDIZZI, C., BERNARDINI, M. C., NATALE G., MARTINOTTI, E. AND DEL TACCA, M.: Peripheral 2-hydroxy-saclofen-sensitive GABA-B receptors mediate both vagal-dependent and vagal-independent acid secretory responses in rats. J. Auton. Pharmacol. 12: , BLANDIZZI, C., COLUCCI, R., CARIGNANI, D., NATALE, G., LAZZERI, G., CREMA, F.AND DEL TACCA, M.: Role of peripheral GABA B receptors in the regulation of pepsinogen secretion in anaesthetized rats. Eur. J. Pharmacol. 294: , BYNUM, T. E. AND JOHNSON, L. R.: Stimulation of human pepsin output by topical hydrochloric acid. Am. J. Dig. Dis. 20: , CAVE, T.R. AND CAVE, D.R.:Helicobacter pylori stimulates pepsin secretion from isolated rabbit gastric glands. Scand. J. Gastroenterol. 26: suppl. 181; 9 14, CLISSOLD, S. P. AND CAMPOLI-RICHARDS, D. M.: Omeprazole: A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peptic ulcer. Drugs 32: 15 47, DEL TACCA, M., SOLDANI, G., POLLONI, A., BERNARDINI, C., COSTA, F. AND BELLINI, M.: The effects of the antimuscarinic drugs pirenzepine and atropine on plasma portal levels of somatostatin and gastrin in the dog. J. Endocrinol. Invest. 10: , DESCROIX-VAGNE, M., PERRET, J. P., DAOUD-EL BABA, M., GROS, I., RAKOTOMALALA, H., DESVIGNE, A., JOURDAN, G. AND NICOL, P.: Interaction between pepsin and acid secretion during fundic perfusion in cat and rabbit. Comp. Biochem Physiol 104A: , EISENBERG, M. M., EMAS, G. S. AND GROSSMAN, M. I.: Comparison of the effect of 2-deoxy-D-glucose and insulin on gastric acid secretion in dogs. Surgery 60: , FELLENIUS, E., BERGLINDH, T., SACHS, G., OLBE, L., ELANDER, B., SJOSTRAND, S. E. AND WALLMARK, B.: Substituted benzimidazoles inhibit gastric acid secretion by blocking (H -K )ATPase. Nature (Lond.) 290: , GEPPETTI, P., TRAMONTANA, M., EVANGELISTA, S., RENZI, S., MAGGI, C. A., FUSCO,
8 1050 Blandizzi et al. Vol. 283 B. M. AND DEL BIANCO, E.: Differential effect on neuropeptide release of different concentrations of hydrogen ions on afferent and intrinsic neurons of the rat stomach. Gastroenterology 101: , HERSEY, S. J.: Pepsinogen secretion. In Physiology of the Gastrointestinal Tract, 2nd ed., ed. by L. R. Johnson, pp , Raven Press, New York, HERSEY, S. J.: Gastric secretion of pepsins. In Physiology of the Gastrointestinal Tract, 3rd ed., ed. by L. R. Johnson, pp , Raven Press, New York, HERSEY, S. J., MILLER, M., MAY, D. AND NORRIS, S. H.: Lack of interaction between acid and pepsinogen secretion in isolated gastric glands. Am. J. Physiol. 245: G775 G779, HIRSCHOWITZ, B. I. AND GROARKE, J.: Vagal effects on acid and pepsin secretion and serum gastrin in duodenal ulcer and control. Dig. Dis. Sci. 38: , JOHNSON, L. R.: Regulation of pepsin secretion by topical acid in the stomach. Am. J. Physiol. 223: , KLEVELAND, P. M., WALDUM, H. L., PETERSEN, H. AND SANDBERG, P.: Pepsin secretion by the totally isolated, vascularly perfused rat stomach. Scand. J. Gastroenterol. 22: , LAMERS, C. B. H. W.: Gastric secretory abnormalities in duodenal ulcer: Primary or secondary to Helicobacter pylori infection? Scand. J. Gastroenterol. 27: (suppl. 194), , LLOYD, K. C. L. AND DEBAS, H. T.: Peripheral regulation of gastric acid secretion. In Physiology of the Gastrointestinal Tract, 3rd ed., ed. by L. R. Johnson, pp , Raven Press, New York, MAGGI, C. A. AND MELI, A.: Suitability of urethane anaesthesia for physiopharmacological investigation in various systems. Part 3: Other systems and conclusions. Experientia 42: , MERCER, D. W., RITCHIE, W. P. AND DEMPSEY, D. T.: Sensory neuron-mediated gastric mucosal protection is blocked by cyclooxygenase inhibition. Surgery 115: , NORRIS, S. H. AND HERSEY, S. J.: ph dependence of pepsinogen and acid secretion in isolated gastric glands. Am. J. Physiol. 245: G730 G738, PABST, M. A., SCHONINKLE, E. AND HOLZER, P.: Ablation of capsaicin-sensitive afferent nerves impairs defence but not rapid repair of rat gastric mucosa. Gut 34: , PUURUNEN, J.: Effect of intragastric acid on pepsinogen secretion in the rat. Eur. J. Pharmacol. 55: , RAUFMAN, J. P.: Gastric chief cells: Receptors and signal transduction mechanisms. Gastroenterology 102: , SAMLOFF, I. M.: Peptic ulcer: The many proteases of aggression. Gastroenterology 96: , SANDVIK, A. K., KLEVELAND, P. M. AND WALDUM, H. L.: Stimulated pepsin secretion after omeprazole-induced acid suppression in the totally isolated, vascularly perfused rat stomach. Scand. J. Gastroenterol. 22: , SENGUPTA, J. N. AND GEBHART, G. F.: Gastrointestinal afferent fibres and sensation. In Physiology of the Gastrointestinal Tract, 3rd ed., ed. by L. R. Johnson, pp , Raven Press, New York, SHARKEY, K. A., OLAND, C. D., KIRK, D. R. AND DAVISON, J. S.: Capsaicin-sensitive vagal stimulation-induced gastric acid secretion in the rat: Evidence for cholinergic vagal afferents. Br. J. Pharmacol. 103: , SMITH, J. L. AND TORRES, E. L.: Effect of topical acid on pepsinogen secretion in man. Scand. J. Gastroenterol. 25: , SOLL, A. H. AND BERGLINDH, T.: Receptors that regulate gastric acid secretory function. In Physiology of the Gastrointestinal Tract, 3rd ed., ed. by L. R. Johnson, pp , Raven Press, New York, WELSH, N. J., SHANKLEY, N. P. AND BLACK, J. M.: Comparative analysis of the vagal stimulation of gastric acid secretion in rodent isolated stomach preparations. Br. J. Pharmacol. 112: 93 96, WILDE, M. I. AND MCTAVISH, D.: Omeprazole. An update of its pharmacology and therapeutic use in acid-related disorders. Drugs 48: , YAMAMOTO, O., MATSUNAGA, Y., HAGA, N. AND ITOH, Z.: Vagovagal inhibition of motilin-induced phase III contractions by antral acidification in dog stomach. Am. J. Physiol. 267: G129 G134, 1994a. YAMAMOTO, O., MATSUNAGA, Y., HAGA, N., MIZUMOTO, A. AND ITOH, Z.: Inhibition of phase III activity by acidifying stomach in vagally denervated and innervated dogs with gastric pouches. Gastroenterology 106: , 1994b. YANG, H., WONG, H., WU, V., WALSH, J. H. AND TACHÉ, Y.: Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anaesthetized rats. Gastroenterology 99: , Send reprint requests to: Prof. Mario Del Tacca, M.D., Division of Pharmacology and Chemotherapy, Department of Oncology, University of Pisa, Via Roma, 55, I Pisa, Italy.
PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID
 GASTROENTEROLOGY Copyriht 1972 by The Williams & Wilkins Co. Vol. 62. No. 3 Printed in U.S. A. PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID LEONARD R. JOHNSON, PH.D. Department of Physiology
GASTROENTEROLOGY Copyriht 1972 by The Williams & Wilkins Co. Vol. 62. No. 3 Printed in U.S. A. PEPSIN SECRETION DURING DAMAGE BY ETHANOL AND SALICYLIC ACID LEONARD R. JOHNSON, PH.D. Department of Physiology
Cytochemical Quantification of Physiologic Regulation of Oxyntic Cell Carbonic Anhydrase a
 Cytochemical Quantification of Physiologic Regulation of Oxyntic Cell Carbonic Anhydrase a A. I. VINIKb and A. A. HELDSINGER Department of Internal Medicine University of Michigan Medical School Ann Arbor,
Cytochemical Quantification of Physiologic Regulation of Oxyntic Cell Carbonic Anhydrase a A. I. VINIKb and A. A. HELDSINGER Department of Internal Medicine University of Michigan Medical School Ann Arbor,
GI Secretion 1: Salivary and Gastric Secretion Jack Grider, Ph.D.
 GI Secretion 1: Salivary and Gastric Secretion Jack Grider, Ph.D. OBJECTIVES: 1. List the volumes of secretion by various regions. 2. Predict the components of salivary secretion at different flow rates.
GI Secretion 1: Salivary and Gastric Secretion Jack Grider, Ph.D. OBJECTIVES: 1. List the volumes of secretion by various regions. 2. Predict the components of salivary secretion at different flow rates.
to food and histamine
 Gut, 97,, 53-57 Maximal acid response of Pavlov pouches to food and histamine A. MARVIN BROOKS AND MORTON I. GROSSMAN From the Veterans Administration Center and UCLA School of Medicine, Departments of
Gut, 97,, 53-57 Maximal acid response of Pavlov pouches to food and histamine A. MARVIN BROOKS AND MORTON I. GROSSMAN From the Veterans Administration Center and UCLA School of Medicine, Departments of
Overview of digestion or, gut reactions - to food
 Key concepts in Digestion. Indigestion module Overview of digestion or, gut reactions - to food Prof. Barry Campbell Gastroenterology Cellular & Molecular Physiology e-mail: bjcampbl@liv.ac.uk http://pcwww.liv.ac.uk/~bjcampbl
Key concepts in Digestion. Indigestion module Overview of digestion or, gut reactions - to food Prof. Barry Campbell Gastroenterology Cellular & Molecular Physiology e-mail: bjcampbl@liv.ac.uk http://pcwww.liv.ac.uk/~bjcampbl
PEPSIN STIMULATED BY TOPICAL HYDROCHLORIC AND ACETIC ACIDS
 GASTROENTEROLOGY Copyright 1972 by The Williams & Wilkins Co. Vol. 62, No.1 Printed in U.S.A. PEPSN STMULATED BY TOPCAL HYDROCHLORC AND ACETC ACDS LEONARD R. JOHNSON, PH.D. Department of Physiology and
GASTROENTEROLOGY Copyright 1972 by The Williams & Wilkins Co. Vol. 62, No.1 Printed in U.S.A. PEPSN STMULATED BY TOPCAL HYDROCHLORC AND ACETC ACDS LEONARD R. JOHNSON, PH.D. Department of Physiology and
Soft palate elevates, closing off the nasopharynx. Hard palate Tongue Bolus Epiglottis. Glottis Larynx moves up and forward.
 The Cephalic Phase Chemical and mechanical digestion begins in the mouth Saliva is an exocrine secretion Salivary secretion is under autonomic control Softens and lubricates food Chemical digestion: salivary
The Cephalic Phase Chemical and mechanical digestion begins in the mouth Saliva is an exocrine secretion Salivary secretion is under autonomic control Softens and lubricates food Chemical digestion: salivary
Overview of digestion or, gut reactions - to food
 1 Key concepts in Digestion. Indigestion module Overview of digestion or, gut reactions to food Prof. Barry Campbell Gastroenterology Cellular & Molecular Physiology email: bjcampbl@liv.ac.uk http://pcwww.liv.ac.uk/~bjcampbl
1 Key concepts in Digestion. Indigestion module Overview of digestion or, gut reactions to food Prof. Barry Campbell Gastroenterology Cellular & Molecular Physiology email: bjcampbl@liv.ac.uk http://pcwww.liv.ac.uk/~bjcampbl
Physiological processes in the GI tract:
 Gastrointestinal physiology for medical students General principal of gastrointestinal function Motility, nervous control and blood circulation Physiological processes in the GI tract: Motility Secretion
Gastrointestinal physiology for medical students General principal of gastrointestinal function Motility, nervous control and blood circulation Physiological processes in the GI tract: Motility Secretion
Urinary system. Kidney anatomy Renal cortex Renal. Nephrons
 Urinary system Aids homeostasis by removing cellular wastes and foreign compounds, and maintains salt and water balance of plasma Kidney anatomy Renal cortex Renal pelvis Renal medulla Cortex Ureter Medulla
Urinary system Aids homeostasis by removing cellular wastes and foreign compounds, and maintains salt and water balance of plasma Kidney anatomy Renal cortex Renal pelvis Renal medulla Cortex Ureter Medulla
Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, ,
 IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
Vagal Regulation of Acid Secretion and Gastrin Release
 YALE JOURNAL OF BIOLOGY AND MEDICINE 67 (1994), pp. 145-151. Copyright 1995. All rights reserved. Vagal Regulation of Acid Secretion and Gastrin Release Haile T. Debasa and Sam H. Carvajal Department ofsurgery
YALE JOURNAL OF BIOLOGY AND MEDICINE 67 (1994), pp. 145-151. Copyright 1995. All rights reserved. Vagal Regulation of Acid Secretion and Gastrin Release Haile T. Debasa and Sam H. Carvajal Department ofsurgery
Effect of acid infusion into various levels of the intestine on gastric and pancreatic secretion in the cat
 Gut, 1969, 10, 749-753 Effect of acid infusion into various levels of the intestine on gastric and pancreatic secretion in the cat S. J. KONTUREK, J. DUBIEL, AND B. GABRY9 From the Department of Medicine,
Gut, 1969, 10, 749-753 Effect of acid infusion into various levels of the intestine on gastric and pancreatic secretion in the cat S. J. KONTUREK, J. DUBIEL, AND B. GABRY9 From the Department of Medicine,
THE EFFECT OF CIMETIDINE ON BASAL AND STIMULATED PEPSIN SECRETION IN THE ISOLATED WHOLE STOMACH OF THE RAT
 Br..1. Pharmac. ( 1 981). 73, 4146 THE EFFECT OF CIMETIDINE ON BASAL AND STIMULATED PEPSIN SECRETION IN THE ISOLATED WHOLE STOMACH OF THE RAT K.T. BUNCE', M. GREWAL & M.E. PARSONS The Research Institute,
Br..1. Pharmac. ( 1 981). 73, 4146 THE EFFECT OF CIMETIDINE ON BASAL AND STIMULATED PEPSIN SECRETION IN THE ISOLATED WHOLE STOMACH OF THE RAT K.T. BUNCE', M. GREWAL & M.E. PARSONS The Research Institute,
EFFECT OF VAGOTOMY ON PANCREATIC SECRETION STIMULATED BY ENDOGENOUS AND EXOGENOUS SECRETIN
 GASTROENTEROLOGY Copyright,. 1971 by The Williams & Wilkins Co. Vol. 60, No. 3 P>-inted in U. S. A. EFFECT OF VAGOTOMY ON PANCREATIC SECRETION STIMULATED BY ENDOGENOUS AND EXOGENOUS SECRETIN HARRIS J.
GASTROENTEROLOGY Copyright,. 1971 by The Williams & Wilkins Co. Vol. 60, No. 3 P>-inted in U. S. A. EFFECT OF VAGOTOMY ON PANCREATIC SECRETION STIMULATED BY ENDOGENOUS AND EXOGENOUS SECRETIN HARRIS J.
OPERATIVE TREATMENT OF ULCER DISEASE
 Página 1 de 8 Copyright 2001 Lippincott Williams & Wilkins Greenfield, Lazar J., Mulholland, Michael W., Oldham, Keith T., Zelenock, Gerald B., Lillemoe, Keith D. Surgery: Scientific Principles & Practice,
Página 1 de 8 Copyright 2001 Lippincott Williams & Wilkins Greenfield, Lazar J., Mulholland, Michael W., Oldham, Keith T., Zelenock, Gerald B., Lillemoe, Keith D. Surgery: Scientific Principles & Practice,
University of Buea. Faculty of Health Sciences. Programme in Medicine
 Faculty of Health Sciences University of Buea Wednesday, 28 th January 2009 Time: 8 00-10 00 Programme in Medicine MED 303 (Gastrointestinal Physiology) EXAMS (2008-2009) Identify the letter of the choice
Faculty of Health Sciences University of Buea Wednesday, 28 th January 2009 Time: 8 00-10 00 Programme in Medicine MED 303 (Gastrointestinal Physiology) EXAMS (2008-2009) Identify the letter of the choice
Our gut reactions to food or, gut reactions - to food
 Key concepts in Digestion. Our gut reactions to food or, gut reactions to food Prof. Barry Campbell Cellular & Molecular Physiology email: bjcampbl@liv.ac.uk http://pcwww.liv.ac.uk/~bjcampbl Swallowing
Key concepts in Digestion. Our gut reactions to food or, gut reactions to food Prof. Barry Campbell Cellular & Molecular Physiology email: bjcampbl@liv.ac.uk http://pcwww.liv.ac.uk/~bjcampbl Swallowing
Digestive System. - Food is ingested
 11 V. Digestive Processes in the Mouth - Food is ingested - Mechanical digestion begins (chewing) - Salivary amylase begins chemical breakdown of starch - Propulsion is initiated by Deglutition (Swallowing)
11 V. Digestive Processes in the Mouth - Food is ingested - Mechanical digestion begins (chewing) - Salivary amylase begins chemical breakdown of starch - Propulsion is initiated by Deglutition (Swallowing)
Diversion of bile and pancreatic juices from the duodenum to the jejunum has
 GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.4 Printed in U.S.A. EFFECT OF EXCLUSION, ACIDIFICATION, AND EXCISION OF THE DUODENUM ON GASTRIC ACID SECRETION AND THE PRODUCTION
GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.4 Printed in U.S.A. EFFECT OF EXCLUSION, ACIDIFICATION, AND EXCISION OF THE DUODENUM ON GASTRIC ACID SECRETION AND THE PRODUCTION
EFFECT OF CARBENOXOLONE ON THE GASTRIC MUCOSAL BARRIER IN MAN AFTER ADMINISTRATION OF TAUROCHOLIC ACID
 GASTROENTEROLOGY 64: 1101-1105, 1973 Copyright 1973 by The Williams & Wilkins Co. Vol. 64 No.6 Printed in U.S.A. EFFECT OF CARBENOXOLONE ON THE GASTRIC MUCOSAL BARRIER IN MAN AFTER ADMINISTRATION OF TAUROCHOLIC
GASTROENTEROLOGY 64: 1101-1105, 1973 Copyright 1973 by The Williams & Wilkins Co. Vol. 64 No.6 Printed in U.S.A. EFFECT OF CARBENOXOLONE ON THE GASTRIC MUCOSAL BARRIER IN MAN AFTER ADMINISTRATION OF TAUROCHOLIC
taurocholate-induced gastric mucosal damage in the rat
 Gut, 1985, 26, 77-775 Effect of cimetidine and omeprazole on aspirin- and taurocholate-induced gastric mucosal damage in the rat R J UTLEY, A S M SALIM, AND D C CARTER From the University Department of
Gut, 1985, 26, 77-775 Effect of cimetidine and omeprazole on aspirin- and taurocholate-induced gastric mucosal damage in the rat R J UTLEY, A S M SALIM, AND D C CARTER From the University Department of
MECHANISM BY WHICH FAT IN THE UPPER SMALL INTESTINE INHIBITS GASTRIC ACID
 GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.3 Printea in U.S.A. MECHANISM BY WHICH FAT IN THE UPPER SMALL INTESTINE INHIBITS GASTRIC ACID H. T. DEBAS, M.D., B. S. BEDI, M.B.,
GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.3 Printea in U.S.A. MECHANISM BY WHICH FAT IN THE UPPER SMALL INTESTINE INHIBITS GASTRIC ACID H. T. DEBAS, M.D., B. S. BEDI, M.B.,
Gastric and Intestinal secretion
 Gastric and Intestinal secretion OBJECTIVES: 1. Describe the various types of gastric cells and the secretion of each cell type. 2. Mention the components of gastric juice and the function of each component.
Gastric and Intestinal secretion OBJECTIVES: 1. Describe the various types of gastric cells and the secretion of each cell type. 2. Mention the components of gastric juice and the function of each component.
Peptic Ulcer Disease: Zollinger-Ellison Syndrome
 GASTROINTESTINAL PHYSIOLOGY 235 Case 41 Peptic Ulcer Disease: Zollinger-Ellison Syndrome Abe Rosenfeld, who is 47 years old, owns a house painting business with his brothers. The brothers pride themselves
GASTROINTESTINAL PHYSIOLOGY 235 Case 41 Peptic Ulcer Disease: Zollinger-Ellison Syndrome Abe Rosenfeld, who is 47 years old, owns a house painting business with his brothers. The brothers pride themselves
(b) Stomach s function 1. Dilution of food materials 2. Acidification of food (absorption of dietary Fe in small intestine) 3. Partial chemical digest
 (1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
(1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
Metoclopramide in gastrooesophageal reflux
 Metoclopramide in gastrooesophageal reflux C. STANCIU AND JOHN R. BENNETT From the Gastrointestinal Unit, Hull Royal Infirmary Gut, 1973, 14, 275-279 SUMMARY In 3 patients with gastrooesophageal reflux,
Metoclopramide in gastrooesophageal reflux C. STANCIU AND JOHN R. BENNETT From the Gastrointestinal Unit, Hull Royal Infirmary Gut, 1973, 14, 275-279 SUMMARY In 3 patients with gastrooesophageal reflux,
Review article: pharmacology of esomeprazole and comparisons with omeprazole
 Aliment Pharmacol Ther 2003; 17 (Suppl. 1): 5 9. Review article: pharmacology of esomeprazole and comparisons with omeprazole J. DENT Department of Gastroenterology, Hepatology and General Medicine, Royal
Aliment Pharmacol Ther 2003; 17 (Suppl. 1): 5 9. Review article: pharmacology of esomeprazole and comparisons with omeprazole J. DENT Department of Gastroenterology, Hepatology and General Medicine, Royal
(Received 16 July 1976)
 J. Phyeiol. (1977), 270, pp. 29-36 29 With 5 text-ftgure8 Printed in Great Britain THE SECRETION OF PEPSIN BY T. KONDO* AND D. F. MAGEEt From the Creighton University School of Medicine, Department of
J. Phyeiol. (1977), 270, pp. 29-36 29 With 5 text-ftgure8 Printed in Great Britain THE SECRETION OF PEPSIN BY T. KONDO* AND D. F. MAGEEt From the Creighton University School of Medicine, Department of
For more information about how to cite these materials visit
 Author: John Williams, M.D., Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author: John Williams, M.D., Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
GASTROENTEROLOGY. Official Publication of the American Gastroenterological Association. COPTBIGHT 1969 THE W,LLIAMS & W,LDN8 Co.
 GASTROENTEROLOGY Official Publication of the American Gastroenterological Association COPTBIGHT 1969 THE W,LLIAMS & W,LDN8 Co. VOLUME 56 April 1969 NUMBER 4 EFFECT OF THE VAGUS NERVE AND SALICYLATE ADMINISTRATION
GASTROENTEROLOGY Official Publication of the American Gastroenterological Association COPTBIGHT 1969 THE W,LLIAMS & W,LDN8 Co. VOLUME 56 April 1969 NUMBER 4 EFFECT OF THE VAGUS NERVE AND SALICYLATE ADMINISTRATION
KK College of Nursing Peptic Ulcer Badil D ass Dass, Lecturer 25th July, 2011
 KK College of Nursing Peptic Ulcer Badil Dass, Lecturer 25 th July, 2011 Objectives: By the end of this lecture, the students t will be able to: Define peptic pp ulcer Describe the etiology and pathology
KK College of Nursing Peptic Ulcer Badil Dass, Lecturer 25 th July, 2011 Objectives: By the end of this lecture, the students t will be able to: Define peptic pp ulcer Describe the etiology and pathology
Gastric, intestinal and colonic absorption of metoprolol in
 Br. J. clin. Pharmac. (1985), 19, 85S-89S Gastric, intestinal and colonic absorption of metoprolol in the rat J. DOMENECH', M. ALBA', J. M. MORERA', R. OBACH' & J. M. PLA DELFINA2 'Department of Pharmaceutics,
Br. J. clin. Pharmac. (1985), 19, 85S-89S Gastric, intestinal and colonic absorption of metoprolol in the rat J. DOMENECH', M. ALBA', J. M. MORERA', R. OBACH' & J. M. PLA DELFINA2 'Department of Pharmaceutics,
ACETYLCHOLINE, HISTAMINE AND GASTRIN
 Br. J. Pharmac. (1977), 61, 279-284 THE EFFECT OF ATROPINE ON ACID SECRETION STIMULATED BY ACETYLCHOLINE, HISTAMINE AND GASTRIN IN THE ISOLATED WHOLE STOMACH OF THE RAT K.T. BUNCE, GILLIAN F. MARSH & M.E.
Br. J. Pharmac. (1977), 61, 279-284 THE EFFECT OF ATROPINE ON ACID SECRETION STIMULATED BY ACETYLCHOLINE, HISTAMINE AND GASTRIN IN THE ISOLATED WHOLE STOMACH OF THE RAT K.T. BUNCE, GILLIAN F. MARSH & M.E.
ACETYLSALICYLIC ACID AND IONIC FLUXES ACROSS THE GASTRIC MUCOSA OF MAN
 GASTROENTEROLOGY Copyright 1968 by The Williams & Wilkins Co. Vol. 54, No.4, Part 1 of 2 Parts Printed in U.S.A. ACETYLSALICYLIC ACID AND IONIC FLUXES ACROSS THE GASTRIC MUCOSA OF MAN BERGEIN F. OVERHOLT,
GASTROENTEROLOGY Copyright 1968 by The Williams & Wilkins Co. Vol. 54, No.4, Part 1 of 2 Parts Printed in U.S.A. ACETYLSALICYLIC ACID AND IONIC FLUXES ACROSS THE GASTRIC MUCOSA OF MAN BERGEIN F. OVERHOLT,
INTRODUCTION TO GASTROINTESTINAL FUNCTIONS
 1 INTRODUCTION TO GASTROINTESTINAL FUNCTIONS 2 Learning outcomes List two main components that make up the digestive system Describe the 6 essential functions of the GIT List factors (neurological, hormonal
1 INTRODUCTION TO GASTROINTESTINAL FUNCTIONS 2 Learning outcomes List two main components that make up the digestive system Describe the 6 essential functions of the GIT List factors (neurological, hormonal
RELEASE OF HISTAMINE INTO GASTRIC VENOUS BLOOD FOLLOWING INJURY BY ACETIC OR SALICYLIC ACID
 GASTROENTEROLOGY Copyright 1967 by The Williams & Wilkins Co. Vol. 52, No.3 Printed in U.S.A. RELEASE OF HISTAMINE INTO GASTRIC VENOUS BLOOD FOLLOWING INJURY BY ACETIC OR SALICYLIC ACID LEONARD R. JOHNSON
GASTROENTEROLOGY Copyright 1967 by The Williams & Wilkins Co. Vol. 52, No.3 Printed in U.S.A. RELEASE OF HISTAMINE INTO GASTRIC VENOUS BLOOD FOLLOWING INJURY BY ACETIC OR SALICYLIC ACID LEONARD R. JOHNSON
Serum gastrin and gastric acid responses to meals at various ph levels in man
 Gut, 1974, 15, 526-530 Serum gastrin and gastric acid responses to meals at various ph levels in man S. J. KONTURK,1 J. BIRNAT, AND J. OLKSY From the Institute ofphysiology, Medical Academy, Krak6w, Poland,
Gut, 1974, 15, 526-530 Serum gastrin and gastric acid responses to meals at various ph levels in man S. J. KONTURK,1 J. BIRNAT, AND J. OLKSY From the Institute ofphysiology, Medical Academy, Krak6w, Poland,
susceptibility of either the axons in the dorsal and ventral roots, or the intramedullary
 213 J. Physiol. (31958) I40, 2I3-2I9 THE SITE OF ACTION OF PROCAINE ON THE ISOLATED SPINAL CORD OF THE FROG BY M. HARMEL AND J. L. MALCOLM From the Department of Physiology, State University of New York,
213 J. Physiol. (31958) I40, 2I3-2I9 THE SITE OF ACTION OF PROCAINE ON THE ISOLATED SPINAL CORD OF THE FROG BY M. HARMEL AND J. L. MALCOLM From the Department of Physiology, State University of New York,
Gastrointestinal Physiology
 Gastrointestinal Physiology Digestion and Absorption Definition Digestion: i Food dis dissolved d and broken down. Physical digestion: Propulsion and mixing of food by muscle in the alimentary tract. Chemical
Gastrointestinal Physiology Digestion and Absorption Definition Digestion: i Food dis dissolved d and broken down. Physical digestion: Propulsion and mixing of food by muscle in the alimentary tract. Chemical
THE INHIBITORY EFFECT OF STILBOESTROL ON GASTRIC SECRETION IN CATS
 Brit. J. Pharmacol. (1950), 5, 3S9. THE INHIBITORY EFFECT OF STILBOESTROL ON GASTRIC SECRETION IN CATS BY K. N. OJHA* AND D. R. WOOD From the Department of Pharmacology and Therapeutics, University of
Brit. J. Pharmacol. (1950), 5, 3S9. THE INHIBITORY EFFECT OF STILBOESTROL ON GASTRIC SECRETION IN CATS BY K. N. OJHA* AND D. R. WOOD From the Department of Pharmacology and Therapeutics, University of
Key words: acetylcholine, capsaicin, presynaptic cholinergic neurons, postsynaptic cholinergic neurong truncal vagotomy
 Key words: acetylcholine, capsaicin, presynaptic cholinergic neurons, postsynaptic cholinergic neurong truncal vagotomy Fig. 2 Effects of hexamethonium (Co) on CCK8 infusion (2.5-80ng/kglmin)-induced gallbladder
Key words: acetylcholine, capsaicin, presynaptic cholinergic neurons, postsynaptic cholinergic neurong truncal vagotomy Fig. 2 Effects of hexamethonium (Co) on CCK8 infusion (2.5-80ng/kglmin)-induced gallbladder
THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION
 Brit. J. Pharmacol. (1963), 20, 74-82. THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION BY J. H. BURN AND D. F. WEETMAN From the Biological Research Laboratories,
Brit. J. Pharmacol. (1963), 20, 74-82. THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION BY J. H. BURN AND D. F. WEETMAN From the Biological Research Laboratories,
Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate
 Pathophysiology 4 (1998) 275 280 Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate B.J. Adegunloye, O.A. Sofola
Pathophysiology 4 (1998) 275 280 Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate B.J. Adegunloye, O.A. Sofola
The actions of bombesin on gastric secretion of the dog and the rat
 Br. J. Pharmac. (1973), 49, 437-444. The actions of bombesin on gastric secretion of the dog and the rat G. BERTACCINI, V. ERSPAMER AND M. IMPICCIATORE Institutes of Pharmacology of the Universities of
Br. J. Pharmac. (1973), 49, 437-444. The actions of bombesin on gastric secretion of the dog and the rat G. BERTACCINI, V. ERSPAMER AND M. IMPICCIATORE Institutes of Pharmacology of the Universities of
THE ROLE OF GASTRINE AND ACETYLCHOLINE IN THE GASTRIC ACID SECRETION MECHANISM
 ANALELE UNIVERSITATII DIN ORADEA, Fascicula Ecotoxicologie, Zootehnie si Tehnologii de Industrie Alimentara THE ROLE OF GASTRINE AND ACETYLCHOLINE IN THE GASTRIC ACID SECRETION MECHANISM Gavril Paul, Ioana
ANALELE UNIVERSITATII DIN ORADEA, Fascicula Ecotoxicologie, Zootehnie si Tehnologii de Industrie Alimentara THE ROLE OF GASTRINE AND ACETYLCHOLINE IN THE GASTRIC ACID SECRETION MECHANISM Gavril Paul, Ioana
Gastrointestinal Motility 2: Intestinal and Colonic Motility Jack Grider, Ph.D.
 Gastrointestinal Motility 2: Intestinal and Colonic Motility Jack Grider, Ph.D. OBJECTIVES: 1. Contrast the types of motility in the small intestine. 2. Describe the neural circuits that mediate peristalsis.
Gastrointestinal Motility 2: Intestinal and Colonic Motility Jack Grider, Ph.D. OBJECTIVES: 1. Contrast the types of motility in the small intestine. 2. Describe the neural circuits that mediate peristalsis.
Why would fatty foods aggravate the patient s RUQ pain? What effect does cholecystokinin (CCK) have on gastric emptying?
 CASE 28 A 43-year-old woman presents to the emergency department with the acute onset of abdominal pain. Her pain is located to the right upper quadrant (RUQ) and radiates to the right shoulder. She reports
CASE 28 A 43-year-old woman presents to the emergency department with the acute onset of abdominal pain. Her pain is located to the right upper quadrant (RUQ) and radiates to the right shoulder. She reports
University of Essen, Essen, F.R.G.
 J. Phy8iol. (1985), 369, pp. 355-364 355 With 4 text-figure8 Printed in Great Britain GASTRIN RESPONSE TO A MEAL BEFORE AND AFTER CUTTING THE EXTRINSIC NERVES OF THE STOMACH IN THE DOG By V. E. EYSSELEIN,
J. Phy8iol. (1985), 369, pp. 355-364 355 With 4 text-figure8 Printed in Great Britain GASTRIN RESPONSE TO A MEAL BEFORE AND AFTER CUTTING THE EXTRINSIC NERVES OF THE STOMACH IN THE DOG By V. E. EYSSELEIN,
Methods. Effect oforal cimetidine on gastric acid secretion. Wistar rats (12) weighing g were housed
 Br. J. Pharmac. (1982), 76,551-555 THE EFFECT OF CIMETIDINE ON BASAL GASTRIC ACID SECRETION IN THE RAT N.S. BROUGHTON' & J.F. MORRIS Department of Human Anatomy, South Parks Road, Oxford OX1 3QX 1 The
Br. J. Pharmac. (1982), 76,551-555 THE EFFECT OF CIMETIDINE ON BASAL GASTRIC ACID SECRETION IN THE RAT N.S. BROUGHTON' & J.F. MORRIS Department of Human Anatomy, South Parks Road, Oxford OX1 3QX 1 The
Action of drugs on denervated myoepithelial cells of salivary glands
 Br. J. Pharmac. (1973), 48, 73-79. Action of drugs on denervated myoepithelial cells of salivary glands N. EMMELIN AND A. THULIN Institute of Physiology, University of Lund, Sweden Summary 1. The pressure
Br. J. Pharmac. (1973), 48, 73-79. Action of drugs on denervated myoepithelial cells of salivary glands N. EMMELIN AND A. THULIN Institute of Physiology, University of Lund, Sweden Summary 1. The pressure
(Received 5 November 1963) rabbit were 65 and 80 mm Hg, respectively. The mean arterial blood
 J. Phy8iol. (1964), 174, pp. 136-171 163 With 5 text-figure8 Printed in Great Britain AORTIC BARORCPTOR THRSHOLD AND SNSITIVITY IN RABBITS AT DIFFRNT AGS BY C. M. BLOOR* From the Nuffield Institute for
J. Phy8iol. (1964), 174, pp. 136-171 163 With 5 text-figure8 Printed in Great Britain AORTIC BARORCPTOR THRSHOLD AND SNSITIVITY IN RABBITS AT DIFFRNT AGS BY C. M. BLOOR* From the Nuffield Institute for
Biology 153/155. Equivalency Exam 60 QUESTIONS 18 PAGES
 Biology 153/155 Equivalency Exam 60 QUESTIONS 18 PAGES Student name: Student number: Signature: READ EACH QUESTION CAREFULLY. The mark value for each question is posted in the margin. Good luck! 3 1) On
Biology 153/155 Equivalency Exam 60 QUESTIONS 18 PAGES Student name: Student number: Signature: READ EACH QUESTION CAREFULLY. The mark value for each question is posted in the margin. Good luck! 3 1) On
Department of Physiology, Okayama University Medical School
 The Japanese Journal of Physiology 15, pp.243-252, 1965 Department of Physiology, Okayama University Medical School BAYLISS and STARLING 1) and others 6, 7, 9, 12, 14, 15) have reported that the stimulation
The Japanese Journal of Physiology 15, pp.243-252, 1965 Department of Physiology, Okayama University Medical School BAYLISS and STARLING 1) and others 6, 7, 9, 12, 14, 15) have reported that the stimulation
Stomach. Stomach. Nerve supply. Blood supply. Sympathe0c and parasympathe0c fibers of the autonomic nervous system
 Stomach Nerve supply Sympathe0c and parasympathe0c fibers of the autonomic nervous system Blood supply Celiac trunk, and corresponding veins (part of the hepa0c portal system) Stomach Figure 23.14a Chapter
Stomach Nerve supply Sympathe0c and parasympathe0c fibers of the autonomic nervous system Blood supply Celiac trunk, and corresponding veins (part of the hepa0c portal system) Stomach Figure 23.14a Chapter
GI Pharmacology. Dr. Alia Shatanawi 5/4/2018
 GI Pharmacology Dr. Alia Shatanawi 5/4/2018 Drugs Used in Gastrointestinal Diseases Drugs used in Peptic Ulcer Diseases. Drugs Stimulating Gastrointestinal Motility &Laxatives. Antidiarrheal Agents. Drugs
GI Pharmacology Dr. Alia Shatanawi 5/4/2018 Drugs Used in Gastrointestinal Diseases Drugs used in Peptic Ulcer Diseases. Drugs Stimulating Gastrointestinal Motility &Laxatives. Antidiarrheal Agents. Drugs
Augmentation of Cysteamine and Mepirizole-Induced Lesions in the Rat Duodenum and Stomach by Histamine or Indomethacin
 Augmentation of Cysteamine and Mepirizole-Induced Lesions in the Rat Duodenum and Stomach by Histamine or Indomethacin Hironori TANAKA, Yoshimi KUWAHARA and Susumu OKABE Department of Applied Pharmacology,
Augmentation of Cysteamine and Mepirizole-Induced Lesions in the Rat Duodenum and Stomach by Histamine or Indomethacin Hironori TANAKA, Yoshimi KUWAHARA and Susumu OKABE Department of Applied Pharmacology,
On the relationship between gastric ph and pressure
 Gut, 1979, 20, 59-63 On the relationship between gastric ph and pressure in the normal human lower oesophageal sphincter M. D. KAYE1 From the Gastroenterology Unit, Department of Medicine, University of
Gut, 1979, 20, 59-63 On the relationship between gastric ph and pressure in the normal human lower oesophageal sphincter M. D. KAYE1 From the Gastroenterology Unit, Department of Medicine, University of
Gastrointestinal Anatomy and Physiology. Bio 219 Napa Valley College Dr. Adam Ross
 Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
in gastric emptying' of the stomach was recorded by an electronic transducer (T1) attached by an air-filled tube to the second limb of
 Gut, 1963, 4, 174 Function of the pylorus and pyloric antrum in gastric emptying' A. K. ARMITAGE AND A. C. B. DEAN From the Department of Surgery, King's College Hospital Medical School, London EDITORIAL
Gut, 1963, 4, 174 Function of the pylorus and pyloric antrum in gastric emptying' A. K. ARMITAGE AND A. C. B. DEAN From the Department of Surgery, King's College Hospital Medical School, London EDITORIAL
5. Which component of the duodenal contents entering the stomach causes the most severe changes to gastric mucosa:
 Gastro-intestinal disorders 1. Which are the most common causes of chronic gastritis? 1. Toxic substances 2. Chronic stress 3. Alimentary factors 4. Endogenous noxious stimuli 5. Genetic factors 2. Chronic
Gastro-intestinal disorders 1. Which are the most common causes of chronic gastritis? 1. Toxic substances 2. Chronic stress 3. Alimentary factors 4. Endogenous noxious stimuli 5. Genetic factors 2. Chronic
January 07, ANIMALS Digestive System Stomach.notebook. The Stomach. (cardiac sphincter) bbbbbbbbbbbbbbbbbb
 (cardiac sphincter) bbbbbbbbbbbbbbbbbb 1 Location: thoracic cavity Physical description: a "J" shaped organ with muscular walls lined with folds it is the widest part of the digestive tract has 2 muscular
(cardiac sphincter) bbbbbbbbbbbbbbbbbb 1 Location: thoracic cavity Physical description: a "J" shaped organ with muscular walls lined with folds it is the widest part of the digestive tract has 2 muscular
GASTROINTESTINAL AND ANTIEMETIC DRUGS. Submitted by: Shaema M. Ali
 GASTROINTESTINAL AND ANTIEMETIC DRUGS Submitted by: Shaema M. Ali GASTROINTESTINAL AND ANTIEMETIC DRUGS by: Shaema M. Ali There are four common medical conditions involving the GI system 1) peptic ulcers
GASTROINTESTINAL AND ANTIEMETIC DRUGS Submitted by: Shaema M. Ali GASTROINTESTINAL AND ANTIEMETIC DRUGS by: Shaema M. Ali There are four common medical conditions involving the GI system 1) peptic ulcers
For more information about how to cite these materials visit
 Author: John Williams, M.D., Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author: John Williams, M.D., Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
The presence of HCl in the stomach by W. Prout in secretagogues and anti-ulcer efficacy
 74).20;51/;),20)4)+/; ##$"#%#! MMMFFH=MF 4ALEAM=HJE?A 5674-2+674-6*4951! 9674-992)91.4-48-5),04-56*)+6-41)160-56)+0 *-241-.4)+01-8--651/)564/;,741/ )56+-674;,AF=HJAJB+EE?=2DOIECO=CEAE=7ELAHIEJO+ACABA@E?EA+H=?M
74).20;51/;),20)4)+/; ##$"#%#! MMMFFH=MF 4ALEAM=HJE?A 5674-2+674-6*4951! 9674-992)91.4-48-5),04-56*)+6-41)160-56)+0 *-241-.4)+01-8--651/)564/;,741/ )56+-674;,AF=HJAJB+EE?=2DOIECO=CEAE=7ELAHIEJO+ACABA@E?EA+H=?M
SECOND MIDTERM EXAM November 15, 2011 BILD 2. Nasha 10. (10 points) Josh 2. (10 points) Josh 3. (10 points) Mary 4. (5 points) 8.
 SECOND MIDTERM EXAM November 15, 2011 BILD 2 WRITE YOUR NAME ON ALL 7 PAGES. ANSWER ALL 10 QUESTIONS (100 POINTS). CONFINE YOUR ANSWERS TO THE SPACE ALLOWED. If you would like to write on the back of the
SECOND MIDTERM EXAM November 15, 2011 BILD 2 WRITE YOUR NAME ON ALL 7 PAGES. ANSWER ALL 10 QUESTIONS (100 POINTS). CONFINE YOUR ANSWERS TO THE SPACE ALLOWED. If you would like to write on the back of the
STEIN IN-TERM EXAM -- BIOLOGY APRIL 21, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- APRIL 21, 2016 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There is
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- APRIL 21, 2016 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There is
(Received 3 June 1974)
 J. Phkyiol. (1975), 246, pp. 143-157 143 With 9 text-ftgure Printed in Great Britain HMIAL STIMULATORY MHANISM IN GASTRI SRTION By M. ISZKOWSKI, S. J. KONTURK, W. OBTULOWIZ AND J. TASLR From the Institute
J. Phkyiol. (1975), 246, pp. 143-157 143 With 9 text-ftgure Printed in Great Britain HMIAL STIMULATORY MHANISM IN GASTRI SRTION By M. ISZKOWSKI, S. J. KONTURK, W. OBTULOWIZ AND J. TASLR From the Institute
s. J. RUNE, M.D., AND F. W. HENRIKSEN, M.D.
 GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.4 Printed in U.S.A. CARBON DOXDE TENSONS N TlE PROXMAL PART OF THE CANNE GASTRONTESTNAL TRACT s. J. RUNE, M.D., AND F. W. HENRKSEN,
GASTROENTEROLOGY Copyright 1969 by The Williams & Wilkins Co. Vol. 56, No.4 Printed in U.S.A. CARBON DOXDE TENSONS N TlE PROXMAL PART OF THE CANNE GASTRONTESTNAL TRACT s. J. RUNE, M.D., AND F. W. HENRKSEN,
Chapter 1. General introduction and outline
 Chapter 1 General introduction and outline General introduction The function of the stomach comprises storage of ingested food, production of gastric secretion and mixing food with gastric secretion,
Chapter 1 General introduction and outline General introduction The function of the stomach comprises storage of ingested food, production of gastric secretion and mixing food with gastric secretion,
Understandings, Applications & Skills
 D.2 Digestion Understandings, Applications & Skills Statement D.2.U1 Nervous and hormonal mechanisms control the secretion of digestive juices. D.2.U2 Exocrine glands secrete to the surface of the body
D.2 Digestion Understandings, Applications & Skills Statement D.2.U1 Nervous and hormonal mechanisms control the secretion of digestive juices. D.2.U2 Exocrine glands secrete to the surface of the body
Capsaicin (3.3 mmol/l)
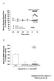 A 18 16 14 1 8 6 4 Vehicle BCTC (.8 mmol/l) Sensory deafferentation Time (min) Capsaicin (3.3 mmol/l) N=3 4 * P
A 18 16 14 1 8 6 4 Vehicle BCTC (.8 mmol/l) Sensory deafferentation Time (min) Capsaicin (3.3 mmol/l) N=3 4 * P
ISOLATED AND INNERVATED ATRIA AND VESSELS
 Brit. J. Pharmacol. (1960), 15, 117. THE ACTION OF SYMPATHETIC BLOCKING AGENTS ON ISOLATED AND INNERVATED ATRIA AND VESSELS BY S. HUKOVIC* From the Department of Pharmacology, University of Oxford (RECEIVED
Brit. J. Pharmacol. (1960), 15, 117. THE ACTION OF SYMPATHETIC BLOCKING AGENTS ON ISOLATED AND INNERVATED ATRIA AND VESSELS BY S. HUKOVIC* From the Department of Pharmacology, University of Oxford (RECEIVED
In this chapter we will study gastrointestinal (GI)
 Chapter 28 The astric Phase of the Integrated Response to a Meal 1 CHAPTER 28 The astric Phase of the Integrated Response to a Meal In this chapter we will study gastrointestinal (I) tract physiology when
Chapter 28 The astric Phase of the Integrated Response to a Meal 1 CHAPTER 28 The astric Phase of the Integrated Response to a Meal In this chapter we will study gastrointestinal (I) tract physiology when
1. ZOLLINGER - ELLISON SYNDROME
 1. ZOLLINGER - ELLISON SYNDROME Zollinger Ellison syndrome is characterized by gastric hypersecretion, ulceration (ulceration) and gastrin-producing tumor. Gastrin is a peptide hormone synthesized by the
1. ZOLLINGER - ELLISON SYNDROME Zollinger Ellison syndrome is characterized by gastric hypersecretion, ulceration (ulceration) and gastrin-producing tumor. Gastrin is a peptide hormone synthesized by the
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Gastrointestinal Physiology. Secretion
 Gastrointestinal Physiology Secretion Fig. 24.26 Functions Provided by secretory glands which serve 2 functions: - Digestive enzymes. - Lubrication and protection of the mucosa. Types of secretory structures
Gastrointestinal Physiology Secretion Fig. 24.26 Functions Provided by secretory glands which serve 2 functions: - Digestive enzymes. - Lubrication and protection of the mucosa. Types of secretory structures
Digestive system L 2. Lecturer Dr. Firdous M. Jaafar Department of Anatomy/Histology section
 Digestive system L 2 Lecturer Dr. Firdous M. Jaafar Department of Anatomy/Histology section objectives 1-Describe the general structure of digestive tract: a-mucosa. b-submucosa. c-muscularis externa d-adventitia
Digestive system L 2 Lecturer Dr. Firdous M. Jaafar Department of Anatomy/Histology section objectives 1-Describe the general structure of digestive tract: a-mucosa. b-submucosa. c-muscularis externa d-adventitia
The effect of metoclopramide on gastroduodenal
 Gut, 1971, 12, 158-163 The effect of metoclopramide on gastroduodenal and gallbladder contractions A. G. JOHNSON From the Department of Surgery, Charing Cross Hospital Medical School, London SUMMARY The
Gut, 1971, 12, 158-163 The effect of metoclopramide on gastroduodenal and gallbladder contractions A. G. JOHNSON From the Department of Surgery, Charing Cross Hospital Medical School, London SUMMARY The
This manuscript describes a dry laboratory using the virtual rat to help
 VIRTUAL RAT : A TOOL FOR UNDERSTANDING HORMONAL REGULATION OF GASTROINTESTINAL FUNCTION Christopher T. Hsu, Cynthia M. Bailey, and Stephen E. DiCarlo Department of Physiology, Wayne State University School
VIRTUAL RAT : A TOOL FOR UNDERSTANDING HORMONAL REGULATION OF GASTROINTESTINAL FUNCTION Christopher T. Hsu, Cynthia M. Bailey, and Stephen E. DiCarlo Department of Physiology, Wayne State University School
Chapter 26 The Digestive System
 Chapter 26 The Digestive System Digestive System Gastroenterology is the study of the stomach and intestine. Digestion Catabolism Absorption Anabolism The actions of the digestive system are controlled
Chapter 26 The Digestive System Digestive System Gastroenterology is the study of the stomach and intestine. Digestion Catabolism Absorption Anabolism The actions of the digestive system are controlled
Chapter 20 The Digestive System Exam Study Questions
 Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Inflammation-Induced Airway Hypersensitivity: From Ion Channels to Patients
 Inflammation-Induced Airway Hypersensitivity: From Ion Channels to Patients Lu-Yuan Lee, Ph.D. Airway Sensory Neurobiology Laboratory Department of Physiology University of Kentucky Medical Center BACKGROUND
Inflammation-Induced Airway Hypersensitivity: From Ion Channels to Patients Lu-Yuan Lee, Ph.D. Airway Sensory Neurobiology Laboratory Department of Physiology University of Kentucky Medical Center BACKGROUND
Digestive System Module 4: The Stomach *
 OpenStax-CNX module: m49286 1 Digestive System Module 4: The * Donna Browne Based on The by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0
OpenStax-CNX module: m49286 1 Digestive System Module 4: The * Donna Browne Based on The by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0
EFFECTS OF METIAMIDE AND PROPRANOLOL ON GASTRIC SECRETION IN ANESTHETIZED DOGS
 EFFECTS OF METIAMIDE AND PROPRANOLOL ON GASTRIC SECRETION IN ANESTHETIZED DOGS Susumu OKABE, Chen R. HUNG, Koji TAKEUCHI Yoshinobu TAKATA and Keijiro TAKAGI Department of Chemical Pharmacology, Faculty
EFFECTS OF METIAMIDE AND PROPRANOLOL ON GASTRIC SECRETION IN ANESTHETIZED DOGS Susumu OKABE, Chen R. HUNG, Koji TAKEUCHI Yoshinobu TAKATA and Keijiro TAKAGI Department of Chemical Pharmacology, Faculty
-Ist hour and 2nd hour.
 Vol. 12, No. 5. October. 1971. SINGAPORE MEDICAL JOURNAL 291 ASSESSMENT OF VAGOTOMY BY THE INSULIN TEST By W. P. Fung SYNOPSIS The insulin test for vagal innervation was done in 21 patients, who had vagotomy
Vol. 12, No. 5. October. 1971. SINGAPORE MEDICAL JOURNAL 291 ASSESSMENT OF VAGOTOMY BY THE INSULIN TEST By W. P. Fung SYNOPSIS The insulin test for vagal innervation was done in 21 patients, who had vagotomy
Gastrin derivatives investigated for secretory potency and for changes in gastric mucosal histamine formation
 Br. J. Pharmac. (1970), 38, 473-477. Gastrin derivatives investigated for secretory potency and for changes in gastric mucosal histamine formation ELSA ROSENGREN AND S. E. SVENSSON Institute of Physiology,
Br. J. Pharmac. (1970), 38, 473-477. Gastrin derivatives investigated for secretory potency and for changes in gastric mucosal histamine formation ELSA ROSENGREN AND S. E. SVENSSON Institute of Physiology,
The effect of sildenafil on electrostimulation-induced erection in the rat model
 (2002) 14, 251 255 ß 2002 Nature Publishing Group All rights reserved 0955-9930/02 $25.00 www.nature.com/ijir The effect of sildenafil on electrostimulation-induced erection in the rat model N Ueno 1,
(2002) 14, 251 255 ß 2002 Nature Publishing Group All rights reserved 0955-9930/02 $25.00 www.nature.com/ijir The effect of sildenafil on electrostimulation-induced erection in the rat model N Ueno 1,
al., 1972; Soli & Berglindh, 1987), and administration of H2 receptor antagonists inhibits the acid
 Br. J. Pharmacol. (1989), 96, 557-562 Histamine release in the isolated vascularly perfused stomach of the rat: regulation by autoreceptors 'Arne K. Sandvik, *Miguel J.M. Lewin & Helge L. Waldum Section
Br. J. Pharmacol. (1989), 96, 557-562 Histamine release in the isolated vascularly perfused stomach of the rat: regulation by autoreceptors 'Arne K. Sandvik, *Miguel J.M. Lewin & Helge L. Waldum Section
THE ACTION OF PHYSOSTIGMINE AND THE DISTRIBUTION OF CHOLINESTERASES IN THE CHICKEN OESOPHAGUS
 Br. J. Phannac. Chemother. (1968), 33, 531-536. THE ACTION OF PHYSOSTIGMINE AND THE DISTRIBUTION OF CHOLINESTERASES IN THE CHICKEN OESOPHAGUS BY A. L. BARTLET AND T. HASSAN From the Department of Veterinary
Br. J. Phannac. Chemother. (1968), 33, 531-536. THE ACTION OF PHYSOSTIGMINE AND THE DISTRIBUTION OF CHOLINESTERASES IN THE CHICKEN OESOPHAGUS BY A. L. BARTLET AND T. HASSAN From the Department of Veterinary
Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit
 Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
GASTROINTESTINAL PHYSIOLOGY PHYSIOLOGY DEPARTMENT KAMPALA INTERNATIONAL UNIVERSITY DAR ES SALAAM TANZANIA
 GASTROINTESTINAL PHYSIOLOGY PHYSIOLOGY DEPARTMENT KAMPALA INTERNATIONAL UNIVERSITY DAR ES SALAAM TANZANIA Anatomy of the GI Tract The GI tract is essentially a hollow tube connecting the mouth to the anus.
GASTROINTESTINAL PHYSIOLOGY PHYSIOLOGY DEPARTMENT KAMPALA INTERNATIONAL UNIVERSITY DAR ES SALAAM TANZANIA Anatomy of the GI Tract The GI tract is essentially a hollow tube connecting the mouth to the anus.
2.4 Autonomic Nervous System
 2.4 Autonomic Nervous System The ANS regulates visceral activities normally outside the realm of consciousness and voluntary control: Circulation. Digestion. Sweating. Pupillary size. The ANS consists
2.4 Autonomic Nervous System The ANS regulates visceral activities normally outside the realm of consciousness and voluntary control: Circulation. Digestion. Sweating. Pupillary size. The ANS consists
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE
 Br. J. Pharmac. Chemother. (1966), 28, 207-211. INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE BY J. AMARO, P. S. GUTH AND L. WANDERLINDER From the Department of Pharmacology,
Br. J. Pharmac. Chemother. (1966), 28, 207-211. INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE BY J. AMARO, P. S. GUTH AND L. WANDERLINDER From the Department of Pharmacology,
Studies on the Role of Cephalic-Vagal Stimulation in the Acid Secretory
 Studies on the Role of Cephalic-Vagal Stimulation in the Acid Secretory Response to Eating in Normal Human Subjects CHARLES T. RICHARDSON, JOHN H. WALSH, KATHLEEN A. COOPER, MARK FELDMAN, and JOHN S. FORDTRAN
Studies on the Role of Cephalic-Vagal Stimulation in the Acid Secretory Response to Eating in Normal Human Subjects CHARLES T. RICHARDSON, JOHN H. WALSH, KATHLEEN A. COOPER, MARK FELDMAN, and JOHN S. FORDTRAN
Glucose acts in the CNS to regulate gastric motility during hypoglycemia
 Am J Physiol Regul Integr Comp Physiol 285: R1192 R1202, 2003. First published July 17, 2003; 10.1152/ajpregu.00179.2003. acts in the CNS to regulate gastric motility during hypoglycemia Min Shi, 1 Allison
Am J Physiol Regul Integr Comp Physiol 285: R1192 R1202, 2003. First published July 17, 2003; 10.1152/ajpregu.00179.2003. acts in the CNS to regulate gastric motility during hypoglycemia Min Shi, 1 Allison
