«Xin Yan Susan Fan» Copyright by «Xin Yan Susan Fan» «2012»
|
|
|
- Madlyn Sparks
- 5 years ago
- Views:
Transcription
1 «Effect of Ephrin-B3 on the Survival of Adult Rat Spinal Cord Derived Neural Stem/Progenitor Cells In Vitro and After Transplantation into the Injured Rat Spinal Cord» by «Xin Yan Susan Fan» A thesis submitted in conformity with the requirements for the degree of «Master of Science» «Institute of Medical Science» University of Toronto Copyright by «Xin Yan Susan Fan» «2012»
2 «Effect of Ephrin-B3 on the Survival of Adult Rat Spinal Cord Derived Neural Stem/Progenitor Cells In Vitro and After Transplantation into the Injured Rat Spinal Cord» «Xin Yan Susan Fan» «Master of Science» «Institute of Medical Science» University of Toronto «2012» Abstract Survival of transplanted neural stem/progenitor cells (NSPC) is limited after spinal cord injury (SCI). This project tested whether ephrin-b3 could enhance the survival of spinal cord derived NSPC based on the report that ephrin-b3 enhanced the survival of endogenous NSPC in the mouse brain. Preclustered ephrin-b3-fc was tested, and preclustered Fc fragments and phosphate-buffered saline (PBS) were used as controls. This study showed that spinal cord derived NSPC and normal and injured rat spinal cord expressed EphA4 receptors. In culture, ephrin-b3-fc increased the survival of NSPC at 1µg/mL (p<0.05), but Fc fragments reduced NSPC survival dose-dependently. In the injured spinal cord, infusion of ephrin-b3-fc increased the proliferation of endogenous ependymal cells compared with infusion of PBS (p<0.05). However, in the injured cord, infusion of either ephrin-b3-fc or Fc fragments caused a 20-fold reduction in the survival of transplanted NSPC (p<0.001). Thus, after SCI, ephrin-b3-fc and Fc fragments are toxic to transplanted NSPC. ii
3 Acknowledgements Completing this project has helped me mature both in research and as an individual, and I am grateful to everyone who guided me through the past years. I am deeply grateful to my supervisor, Dr. Charles Tator, for providing me the opportunity to pursue my research interest. His blend of passion, insight, knowledge, patience and humour has guided and encouraged me throughout the past years, and his saying that research is not a straight line will continue to push me forward in my future career. I am also grateful to my committee members, Dr. Cindi Morshead and Dr. Michael Fehlings, for their advice, guidance and encouragement. I would like to thank members of Dr. Tator s laboratory: Dr. Andrea Mothe for teaching me countless research techniques and offering her knowledge throughout this project, and Linda Lee and Rita van Bendegem for their technical knowledge and assistance. Thank you also to Dr. Howard Kim for assistance with the MTS assay, Sydney Dennis- Birnbaum for assistance with some of the data collection, and Dr. David G. Wilkinson and Dr. Jeffrey Henderson for their generous gifts. Finally, I would like to thank my parents for their endless support. With sincerest regards, Susan Fan iii
4 Table of Contents Abstract ii Acknowledgements iii Table of Contents iv List of Tables vi List of Figures vii List of Abbreviations viii 1 Introduction Anatomy of the Spinal Cord Traumatic Injury to the Spinal Cord Primary and Secondary Injury of the Spinal Cord Animal Models in Spinal Cord Injury Cell-based Therapies for Spinal Cord Injury Adult spinal cord derived neural stem/progenitor cells Transplantation of adult spinal cord derived neural stem/progenitor cells Ephrin ligands and Eph receptors in the Central Nervous System Ephrin-B3 ligands and EphA4 receptors in the Central Nervous System Ephrin-B3 ligands and EphA4 receptors in the brain Ephrin-B3 ligands and EphA4 receptors in the spinal cord Hypothesis and Objectives for Present Work 28 2 Materials and Methods Animals Isolation and Culturing of Neural Stem/Progenitor Cells Preclustering of compounds Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vitro EphA4 expression in spinal cord NSPC in vitro MTS cell survival assay Cell proliferation assay Surgeries conducted to examine the effect of ephrin-b3-fc in vivo Spinal Cord Injury Transplantation of adult spinal cord derived NPSC Intrathecal infusion Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vivo Histology and Immunohistochemistry EphA4 expression in vivo Effect of ephrin-b3-fc on the proliferation of endogenous periventricular ependymal cells in the adult spinal cord Effect of ephrin-b3-fc on the survival of transplanted spinal cord NSPC in the injured adult spinal cord Statistical Analysis 49 3 Results EphA4 expression in vitro and in vivo 49 iv
5 3.2 Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vitro MTS cell survival assay Cell proliferation assay Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vivo Proliferation of ependymal cells in the normal and injured adult spinal cord Survival of transplanted spinal cord NSPC 64 4 Discussion Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vitro Effect of ephrin-b3-fc on the proliferation of ependymal cells in the normal and injured adult spinal cord Effect of ephrin-b3-fc on the survival of transplanted NSPC in the injured spinal cord 75 5 Conclusions and Future Directions Summary Limitations of the present study Examination of ligand-receptor interaction Removal of growth factors in the in vitro experiments Limited outcome measures Limited forms of ligand controls Co-delivery of ligand with NSPC transplant versus pre-treating NSPC with ligand prior to transplantation Impact of results Directions for future research Publications, presentations/posters, and awards arising from this work 92 6 References 93 v
6 List of Tables TABLE 1: A brief summary of secondary mechanisms in spinal cord injury 5 TABLE 2: A brief summary of cell death mechanisms 7 TABLE 3: Examples of traumatic SCI models in rats 10 TABLE 4: Examples of cells that have been transplanted in vivo after SCI 14 TABLE 5: Survival of transplanted NSPC can be influenced by the conditions of NSPC and the time and location of transplant. 19 TABLE 6: Treatment groups and the number of animals used (with and without intrathecal [IT] compound delivery) 31 TABLE 7: In vitro effects of ephrin-b3-fc and Fc on NSPC assessed by MTS and Ki67 assays 36 vi
7 List of Figures FIGURE 1: Schematic diagram showing the anatomy of the spinal cord in cross section and the stem/progenitor cells in the ependymal zone. 3 FIGURE 2: Schematic diagram showing the interaction between ephrin-b3 and EphA4 receptor 26 FIGURE 3: Images of the clip, clip compression injury, and mini-osmotic pump 40 FIGURE 4: Schematic diagrams describing surgeries and timeilne of experiments with intrathecal delivery 44 FIGURE 5: Immunostaining of the EphA4 receptor in cultured brain and spinal cord derived NSPC in spinal spinal cord derived NSPC post-transplant 51 FIGURE 6: Immunostaining of EphA4 receptor in the normal and injured spinal cord 54 FIGURE 7: Survival and proliferation of dissociated spinal cord derived NSPC in culture 57 FIGURE 8: Effect of intrathecal delivery of preclustered ephrin-b3-fc or Fc fragments on the endogenous spinal cord ependymal cells 60 FIGURE 9: Sample images showing cells labeled for Ki67 at the ependymal region of the spinal cord. 63 FIGURE 10: Effect of intrathecal delivery of preclustered ephrin-b3-fc or Fc fragments on transplanted NSPC in the injured spinal cord. 65 vii
8 List of Abbreviations BDNF camp CNS Co-IP CsA CSF DAPI EGF EphA4R Fc FcR FGF2 GDNF GFAP GFP GFP+ brain-derived neurotrophic factor cyclic adenosine monophosphate central nervous system protein complex immunoprecipitation cyclosporine A cerebrospinal fluid 4,6-diamidino-2-phenyl-indole epidermal growth factor EphA4 receptor crystallizable fragment Fc receptor (receptor of crystallizable fragment) basic fibroblast growth factor glial cell line-derived neurotrophic factor glial fibrillary acidic protein green fluorescent protein green fluorescent protein positive (expressing green fluorescent protein) GPI GTPase hegf hfgf2 IgG glycophosphatidylinositol guanosine triphosphate hydrolases human epidermal growth factor human basic fibroblast growth factor immunoglobulin G viii
9 IT LGF m±se MTS intrathecal (infusion) liver growth factor mean ± standard error [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4- sulfophenyl)-2h-tetrazolium)] NGF NSPC PBS PDGF PDZ PLGA rpm SCI SVZ TNF UV nerve growth factor neural stem/progenitor cells phosphate buffered saline platelet-derived growth factor post-synaptic density protein zona occludens (binding domain) poly(lactic acid-co-glycolic acid) revolutions per minute spinal cord injury subventricular zone tumour necrosis factor ultraviolet ray ix
10 1 Introduction In mammals, the spinal cord is the main pathway for the transmission of sensory and motor information between the brain and other parts of the body [1]. Traumatic injury of the spinal cord is especially debilitating and significantly reduces the quality of life of patients due to paralysis, sensory loss, autonomic damage and often intractable pain resulting from the injury [1-4]. At present, there is no clinical cure for individuals suffering from spinal cord injury (SCI). Recent research on SCI has mainly focused on neural regeneration with the ultimate goal of reconnecting the damaged pathways to restore sensory and motor functions. To address neural regeneration, spinal cord derived neural stem/progenitor cells (NSPC) have been isolated, cultured and transplanted SCI environments [5-13]. From these studies, spinal cord NSPC have been observed to self-renew and to be multipotent (generate all cells of the neural lineage); moreover, spinal cord NSPC have been observed to regenerate functional myelin and encourage functional recovery in rodents [14-16]. Nonetheless, in the SCI environment, the survival of transplanted spinal cord NSPC is drastically reduced (even with immunosuppression and optimized time and location of transplant) [11,16,17]. Growth factors have been co-delivered to the spinal cord in attempts to either optimize the environment for transplanted NSPC or to directly enhance the survival of the transplanted cells [10,18]. Although the survival of the transplanted cells was improved, the drawbacks of growth factor administration include uncontrolled proliferation of transplant and proliferative mass lesions from adjacent tissues, which could further damage the injured spinal cord [19-21]. In an attempt to find alternative agents for enhancing the survival of transplanted NSPC, this project examined the effect of ephrin-b3 on adult rat spinal cord derived NSPC because ephrin-b3 has been shown to increase 1
11 the survival of endogenous NSPC in the adult mouse brain without eliciting uncontrolled proliferation [22,23]. 1.1 Anatomy of the Spinal Cord The spinal cord is a part of the central nervous system (CNS) together with the brain [1]. Situated within the vertebral column, the spinal cord is a long tubular bundle of nervous tissue that originates below the brainstem and extends until the lumbar vertebra. Based on the regions of innervation, the spinal cord is commonly divided into 5 major segments (in accordance with the labeling of the vertebral column) starting from below the brainstem: cervical, thoracic, lumbar, sacral and coccygeal. On the outside, the spinal cord is protected by the dura mater, the arachnoid mater (with thin processes similar to spider webs) and the subarachnoid space (containing cerebrospinal fliud [CSF] that circulates around the brain and in the ventricles). Figure 1 shows the gross anatomy of the spinal cord in cross section. The spinal cord is sub-divided into three regions. The white matter forming the outer regions of the spinal cord is mainly constituted of myelinated axons that transmit motor and sensory information. The white appearance reflects the colour of myelin, which is formed by oligodendrocytes. The white matter also contains other glial cells, such as astrocytes and microglia, which maintain a supportive environment. The butterfly-shaped inner regions form the grey matter that is mainly composed of neuronal cell bodies. The grey matter also contains glial cells (for supportive functions), dendrites and unmyelinated axons. The most central region of the spinal cord contains the central canal surrounded by the ependymal cells. 2
12 The central canal is connected to the fourth ventricle of the brain. The central canal contains CSF that runs through the entire length of the spinal cord. Figure 1: Schematic diagram showing the anatomy of the spinal cord in cross section (left; distances are not to scale) and the stem/progenitor cells in the ependymal zone (right). The pink coloured cells represent neural stem cells going through symmetrical (generating two pink coloured cells) and asymmetrical (generating one pink and one blue coloured cell) divisions. The blue and green coloured cells represent progenitor cells. The layers of cells surrounding the central canal have gained increasing attention in recent decades due to the discovery of NSPC in this region [5,6,24]. Figure 1 shows an illustration of the cells surrounding the central canal. The ependymal zone is the thin layer of cells lining central canal. Neural stem cells are present in the ependymal zone and are able to self-renew and differentiate into cells of neural origins. These neural stem cells can undergo either symmetrical or asymmetrical division, which renew the number of neural stem cells and generate progenitor cells. In asymmetrical division, the progenitor cells migrate towards the subependymal zone (layers of cells surrounding the ependymal zone), where they will receive further cues for differentiation. The ependymal and subependymal regions can be isolated from the spinal cord 3
13 and cultured in vitro to obtain neurospheres, which contain many neural stem and progenitor cells. However, because the neural stem cells readily produce progenitor cells, the two populations are not separated and are addressed together as neural stem/progenitor cells (NSPC) [25]. Different from pluripotent fetal or embryonic stem cells, the multipotent adult NSPC can only produce cells of neural origins, including glial cells (astrocytes and oligodendrocytes) and neurons [5,8]. Nevertheless, the advantage of using adult NSPC over fetal or embryonic stem cells for the purpose of transplantation into the injured spinal cord is that these cells are present throughout adulthood, can be isolated from adult spinal cords, and avert the ethical limitations and tumorigenic potential surrounding the use of fetal or embryonic stem cells. 1.2 Traumatic Injury to the Spinal Cord Traumatic injury to the spinal cord, such as contusion, compression or laceration, disrupts motor, sensory and/or autonomic functions at the site of injury and at all the levels below the injury site [1]. Depending on the degree of damage (location, size and severity of injury), traumatic SCI can result in permanent disabilities, such as paralysis, loss of sensation and intractable pain. Importantly, the injury site often spreads beyond the initial site of traumatic impact since injury activates mechanisms that lead to degeneration or apoptosis of the penumbra regions surrounding the site of injury [26,27]. Hence, the effect of injury can be divided into two phases, which include the damage from the primary trauma and the damage from secondary mechanisms of degeneration and apoptosis. 4
14 1.2.1 Primary and Secondary Injury of the Spinal Cord The damage incurred in the primary phase of traumatic injury to the spinal cord occurs rapidly [26,27]. Immediately upon the initial physical tissue injury, cell necrosis starts at the site of injury accompanied by a vascular response of hemorrhage and then edema. In addition to the loss of tissue, the initial impact triggers disturbances in the balance of ions and neurotransmitters between the intracellular space (of surviving cells), the cell membrane and the extracellular fluid. Moreover, necrotic cells rupture and release apoptotic factors, such as lysosomes, that negatively affect the surrounding cells. Furthermore, the presence of cellular debris, edema and damage to the vasculature trigger a heightened inflammatory response, which consists of the infiltration of immune cells (such as neutrophils, monocytes, macrophages and T-lymphocytes) and the release of further pro-inflammatory molecules (such as interleukins and tumor necrosis factor-α [TNFα]). The combination of the loss of ion and neurotransmitter homeostasis, release of apoptotic factors and the aggravation of inflammation activates secondary mechanisms, which enlarge the injury to the spinal cord. Secondary mechanisms can be initiated within minutes after the primary injury and can last from weeks to months [26,27]. The list of mechanisms involved in the secondary injury has become increasingly more extensive as more studies are focusing on this time window for developing therapeutic strategies. Table 1 provides a brief list of some of the well-studied secondary mechanisms. Table 1: A brief summary of secondary mechanisms in spinal cord injury Secondary Mechanism Initiating Event Downstream Effect Calcium influx [28] Loss of ion homeostasis Further compromise of cell membrane permeability Damage to mitochondria Excitotoxicity Activation of caspases and 5
15 Glutamate influx [29,30] Synthesis of free radicals [28] Inflammation [31] Demyelination [30] Change in local vasculature [32,33] Cavitation and glial scar formation [18] Apoptosis [34] Loss of neurotransmitter homeostasis Calcium influx and mitochondrial damage Cell necrosis and apoptosis Hemorrhage and edema Inflammation Glutamate excitotoxicity Free radical damage Initial trauma Hemorrhage and edema Cell necrosis and apoptosis Inflammation and infiltration of immune cells Activation of reactive astrocytes (from trauma, ischemia and inflammation) Secretion of apoptotic factors from necrotic cells Initial trauma, hemorrhage and edema Ion and neurotransmitter influx Free radical damage Ischemia Secretion of growth-inhibiting molecules calpains (lead to cell apoptosis and degeneration) Calcium influx Excitotoxicity Lipid peroxidation (leads to cell membrane lysis and degradation of cytoskeleton or organelles resulting in cell necrosis or apoptosis) Infiltration of immune cells Release of cytokines and free radicals Induction of cavitation and scar formation Loss of conduction Degeneration of axons (Wallerian and retrograde degeneration) Loss of or damage to neurons Ischemia Production of free radicals Physical obstacle to growth Secretion of growth-inhibiting molecules Prevention of regeneration Cell loss and enlargement of injury Loss of conduction and function Based on Table 1, it is evident that the various processes involved in the secondary mechanisms are interconnected and could perpetuate one another resulting in further degeneration and loss of cells beyond the primary injury site. In addition to the enlarged injury site, the loss of cells and 6
16 function extends beyond the impact site of the primary injury due to various mechanisms of cell death, such as progressive loss of cells through apoptosis [34], and due to anterograde (Wallerian) and retrograde (dieback) degeneration in the white matter tracts [35]. Table 2 provides a summarized list of some of the mechanisms of cell death that are relevant to an SCI environment (other forms of cell death, such as cornification in the eye and cell-cell entosis, are not included). These mechanisms of cell death affect both endogenous and transplanted cells thereby limiting the regenerative potential of both the endogenous repair mechanisms in the spinal cord and applied therapeutic strategies. Table 2 provides a summarized list of some of the mechanisms of cell death that are relevant to an SCI environment (other forms of cell death, such as cornification in the eye and cell-cell entosis, are not included). These mechanisms of cell death affect both endogenous and transplanted cells thereby limiting the regenerative potential of both the endogenous repair mechanisms in the spinal cord and applied therapeutic strategies. Table 2: A brief summary of cell death mechanisms Mechanism Properties Apoptosis Programmed cell death that is characterized by [36] fragmentation of nuclear material, shrinking of the cells and blebbing of the cell membrane Apoptosis is regulated by caspases (in general, initiator caspases include caspase-2, 8, 9 and 10, which cleave and activate effector caspases, such as 3, 6 and 7, which activate apoptosis), Bcl-2 family, and/or cytochrome c release from mitochondria, which can be triggered by activation of various pathways, such as the Fas receptor, Necrosis [36,37] Autophagy [38,39] tumor necrotic factor, and cytokines. Cell death triggered by extensive physiochemical stress, such as heat, osmotic shock, traumatic injury and reactive oxygen species Necrotic cells are characterized by rapid swelling of the cytoplasm and culminating in rupture of the cell membrane thereby release degraded organelle and other toxic intracellular molecules Cell death generated through degradation of a cell s organelles and enlargement of vacuoles through the cell s own lysosomes 7
17 Paraptosis [40] Necroptosis [37] Anoikis [41] Autophagy can be triggered through infection, damage or stress to cell, and nutrient starvation The process of autophagy is independent of apoptotic regulators but can be coupled to apoptosis through caspase-dependent pathways (such as caspase 3, 7 and 8) Macroautophagy: Triggered by phosphoinositide-3-kinases and the autophagy-related gene 6 Organelles are sequestered in a vacuole and then fused to lysosomes for degradation by lysosomal hydrolases Microautophagy: Lysosome directly wrap around organelle for degradation Chaperone-mediated autophagy: Triggered by hsc-70 containing chaperone complex, which then translocates organelles to lysosomes A form of programmed necrotic cell death Cellular morphology of cells undergoing paraptosis is similar to a combination of necrotic and autophagic cell death: absence of nuclear fragmentation, loss of membrane integrity, breakdown of organelles, cytoplasmic vacuolization Paraptosis is triggered by the mitogen-activated protein kinases pathways (which also regulates apoptosis), but downstream effectors are independent of caspases and Bcl-2 family (which are key regulators in apoptotic cell death) A form of programmed necrotic cell death Cellular morphology of cells undergoing necroptosis is similar to necrotic cell death: absence of nuclear fragmentation, loss of membrane integrity, and breakdown of organelles Necroptosis is triggered by death receptors that regulate apoptotic cell death, such as Fas receptor, but downstream effectors are independent of caspases, Bcl-2 family or cytochrome c release from mitochondria (which are key regulators in apoptotic cell death) Anchorage-dependent programmed apoptotic cell death Detachment of cell from extracellular matrix cause arrest in G1 phase and activation of cell death pathways that are linked to the caspase- and cytochrome c-dependent apoptotic pathways Activation of Rho-associated knases (which are involved in assembly of cytoskeleton) triggers caspase-3 initiated apoptosis Activation of cell cycle regulators cyclin-dependent kinases 4 and 6 triggers caspase-9 dependent apoptosis 8
18 Ischemic Cell Death (oncosis and pyroptosis) [42] There is also coupled activation of death receptors and caspase-8 through Fas-associated protein with death domain that triggers apoptosis (such as through caspase-8 activation of caspase-3 and caspase-9) Cell death triggered by response to infection thereby initiating inflammation Cytoplasmic vacuolization (similar to that observed in autophagy) and swelling of organelles Autophagy and several programmed cell death pathways can be activated including apoptosis, oncosis and pyroptosis Oncosis: caspase-independent initiation of vacuolization and swelling Pyroptosis: capase-1 dependent initiation of vacuolization and swelling Animal Models in Spinal Cord Injury Animal models have been extremely important for examining the mechanisms of SCI and for assessing therapeutic strategies since many evaluations are not readily applicable in human patients. Examples of animals used for studying SCI include mice and rats, cats, dogs, pigs and non-human primates [9,43-47]. In recent decades, rats have been an increasingly common model for SCI research due to their ease of housing and handling, accessibility, and close pathological profile to clinical SCI [47,48]; hence, rats were chosen as the model animal for the experiments presented in this thesis. Various techniques have been developed to generate experimental traumatic injury in order to study different aspects of SCI; Table 3 provides a list of some common examples of traumatic SCI models in rats (this table summarizes SCI generated through traumatic physical impact and does not include other techniques, such as chemically-induced SCI). 9
19 Table 3: Examples of traumatic SCI models in rats Model Brief Description Advantages Limitations Weight drop [49] Clip compression [50,51] Electromechanical impactor (Ohio State University [OSU] device) [52] Infinite Horizon [IH] impactor [53] Various size weights are dropped perpendicular to the dorsal surface of the exposed spinal cord from graded heights Modified aneurysm clips are calibrated into graded compression forces by weight. The blades of the clip are placed ventral and dorsal to the exposed spinal cord, and the cord is compressed between the two blades of the clip. An impactor tip presses onto the dorsal surface of the exposed spinal cord. The kinematics of the impactor tip is monitored through a solenoid-controlled air cylinder Computer-monitored impactor tip that senses the surface of the exposed spinal cord and displaces the dorsal surface of the cord until a pre-set force is achieved Generate dynamic compression to exposed spinal cord tissue Generate varying degrees of injury based on calibration of compression force and duration of compression Simulates clinical condition of both ventral and dorsal compression Low cost Generate varying degrees of injury Allow monitoring of kinematics (e.g. force, velocity, or tissue displacement) Generate varying degrees of injury Allow monitoring of kinematics (e.g. force, velocity, or tissue displacement) Difficult to produce varying degree of injury (most injuries generated are severe) Variations in extent of injury Possibility of injuring the spinal cord during placement of the blades of the clip Lack of simulation of ventral compression Not commercially available Cannot vary the duration of compression for clinically relevant times Lack of simulation for ventral compression Cannot vary the duration of compression for clinically relevant times Minimal Injury [54] A bent 30 gauge needle is manually inserted into the lateral columns of the spinal cord. Insertions are performed bilaterally with the needle tip moving approximately 10 Preserve the integrity of the central canal Stimulate the proliferation and migration of endogenous ependymal cells Low severity of injury that does not simulate clinical conditions
20 Hemisection [35] Complete transection [55] Focal myelotomy [35] 2mm into the cord. A portion (usually dorsal or lateral) of the spinal cord is surgically severed or removed with a blade Spinal cord is completely severed surgically with a blade The central region of the spinal cord is severed surgically with a blade while the lateral portions of the cord remain intact Allow tracing of the endogenous ependymal cells Allow observation of the mechanisms inhibiting or promoting axon degeneration and regeneration after severance Spinal cord is still connected at the uninjured side Allow observation of the mechanisms inhibiting or promoting axon degeneration and regeneration after severance Generate consistent injury across trials Allow observation of the mechanisms inhibiting or promoting axon degeneration and regeneration after severance Spinal cord is still connected laterally Not typically observed clinically More significant loss of function than compression or contusion Not typically observed clinically Significant loss of function Not typically observed clinically More significant loss of function than compression or contusion The clip compression technique is a well-established model that closely simulates the clinical condition of compression to the spinal cord by encompassing the effects of both ventral and dorsal compression on the cord. First developed Rivlin and Tator [50], the clip used in the clip compression model contains a spring that can be calibrated to generate graded degrees of injury. The force generated by the spring controlled clip is calibrated by weight; for example, a 56g clip generates a severe injury (significant observable swelling and hemorrhage at the injury site immediately after removal of the clip, and large amount of tissue loss and significant functional 11
21 loss thereafter) whereas a 12g clip generates a minor injury (less swelling, hemorrhage and tissue and functional loss). Depending on the type of clip used, graded degrees of injury can be generated for assessing different types of treatment strategies. In the present project, a 26g clip was chosen to generate a moderate injury model in order to study the survival of endogenous and transplanted NSPC. This moderate injury model provide sufficient trauma to initiate inflammatory, apoptotic and scarring responses (as a result of both primary and secondary injury) that will considerably influence the survival of endogenous and transplanted NSPC. At the same time, a moderately injured spinal cord contains relatively intact tissue in the areas surrounding the injury site that allows the assessment of the endogenous NSPC. Conversely, the ependymal region at the injury site is largely destroyed. Moreover, the relatively intact tissue in the areas surrounding the injury site provides an environment that permits the integration of transplanted NSPC, whereas a severe injury would not permit the survival of any transplanted NSPC thereby preventing any assessment of possible therapeutic strategies using transplanted NSPC. Treatment strategies with the SCI models are commonly applied in three different time periods [11]. The acute period (for both rats and human patients) describes the effects of SCI starting at the time of impact and lasts up to 7 days, which encompasses the effect from both the primary and the beginning of secondary injury. The subacute period starts approximately 7 to 9 days after the initial time of impact, and secondary mechanisms predominate in this period, including the progression of the apoptosis and degeneration of the injury site and the penumbra regions while the edema and the heightened inflammatory response from the primary injury begin to subside. The glial scar has not been established during the subacute period. In the chronic period, primary and secondary injury mechanisms have largely been stabilized and the glial scar is firmly 12
22 established. The chronic period begins approximately 4 weeks post-injury in rats [18] and approximately 2 to 3 months post-injury for patients. 1.3 Cell-based Therapies for Spinal Cord Injury The adult mammalian spinal cord contains endogenous NSPC that are self-renewing and multipotent [5,6,24]. In response to injury, these endogenous NSPC will proliferate, differentiate and migrate towards the site of injury in attempts to repair the damaged spinal cord [54,56-61]. Unlike other species (such as amphibians), in the mammalian central nervous system, the regenerative potential of the endogenous NSPC is limited due to the small number of NSPC generated and the hostile environment in the injury and penumbra regions [58,62]. Kojima and Tator [62] have delivered a combination of human epidermal growth factor (hegf) and human basic fibroblast growth factor (hfgf2) in attempts to encourage regeneration from the endogenous NSPC. The combination of hegf and hfgf2 considerably encouraged the proliferation and migration of the endogenous NSPC and resulted in modest functional recovery [62]. However, regeneration, remyelination of demyelinated axons, and re-innervation of target cells and functional recovery were difficult to achieve by stimulation of endogenous NSPC. To augment endogenous repair mechanisms after SCI, various strategies have been tested in vivo, including transplantation of tissue, transplantation of cells and delivery of neuroprotective or growth-promoting molecules. For example, peripheral nerve grafts have been transplanted into the injury site to provide a bridge for regenerating cells and axons across the injury site [63,64]. This strategy presented evidence that certain CNS axons could regenerate in a modified environment and that transplanted materials could enhance regeneration in the CNS, although 13
23 functional recovery was limited. Similar to transplantation of peripheral nerve grafts, embryonic CNS tissue, such as fetal spinal cord, have also been transplanted after SCI although the tissue sources are limited by ethical concerns [65,66]. Nonetheless, grafts in general act as a relay station for disrupted connections instead of regenerating lost cells. To replace damaged or lost cells, cells of numerous origins have been transplanted in vivo. Table 4 presents a brief list of the cells that have been transplanted into the injured spinal cord. Table 4: Examples of cells that have been transplanted in vivo after SCI Cell Type Purpose Limitations Schwann cells [67,68] Remyelinate demyelinated axons thereby improving Regenerate one cell type Immune/inflammatory cells [31] Olfactory ensheathing glia [68,69] Embryonic cell derived stem/progenitor cells [70] Adult mesenchymal stem cells [16,71] Adult neural stem/progenitor cells conduction and function Modify the injury environment by transplanting activated macrophages or monocytes that are preincubated with PNS tissue (these transplanted immune cells are different from the detrimental ones activated in the CNS); this modified environment then encourages regeneration Encourage regeneration and remyelination Regenerate damaged or lost cells (embryonic stem/progenitor cells are highly potent and exhibit strong survival after transplant) Modify the injury environment by providing a scaffold and by secreting neuroprotective or growthpromoting molecules Regenerate damaged or lost cells Regenerate damaged or lost cells 14 Initial reports of improved functional in experimental models Some studies have reported increased cavitation at the injury site and worsening of hindlimb recovery Mechanisms of positive effects remain to be examined Increased chance of tumorigenesis due to high potency Ethical limitations Limited differentiation of cells into neural origin Limited functional improvement Survival is limited posttransplant
24 [16,17] Engineered stem/progenitor cells (such as induced pluripotent cells and transfected cells to overexpress certain genes) [72,73] Regenerate damaged or lost cells with improved potency, survival, adhesion, and migratory and differentiating capabilities Safety remains a major concern since genetic modification is usually involved (might increase the possibility of tumorigenesis) Aside from replacing damaged or dead cells, cell-based therapies have also been associated with improved recovery due to the secretion of neuroprotective or growth-promoting molecules by the transplanted cells. In attempts to modify the SCI environment, various neuroprotective and/or growth-promoting molecules have been delivered to the spinal cord, including growth factors (such as EGF, FGF2, brain-derived neurotrophic factor, nerve growth factor and platelet-derived growth factor) [10,18,20], cyclic adenosine monophosphate (camp) [55], small guanosine triphosphate hydrolases (GTPase) (such as Rho inhibitors and inhibitors of Rho kinases) [74], and chondroitinase ABC [10,18]. These neuroprotective and growth-promoting molecules are often delivered in combination with cell-based therapies to improve the survival and regenerative potential of the transplanted cells Adult spinal cord derived neural stem/progenitor cells NSPC are present in the ependymal zone lining the central canal of the adult spinal cord. When cultured in a supportive environment (such as the presence of growth factors and the maintenance of physiological temperature and humidity), the cells isolated from the ependymal zones will self-renew, proliferate and form free floating neurospheres [8,25]. In an undifferentiated condition, the neurospheres express a large variety of markers, including nestin (which labels neural stem cells), RC1 (which labels radial glia and neural progenitors), Olig2 (which labels oligodendrocyte precursors), RIP (which labels immature and mature 15
25 oligodendrocytes), and small amounts of glial fibrillary acidic protein (GFAP, which labels differentiated astrocytes) and BIIIT (which labels cellular bodies and processes of young neurons). In a differentiating condition, the neurospheres continue to express the aforementioned markers although the amount of nestin expression is significantly reduced whereas the expression of GFAP, RIP and BIIIT is significantly increased. This change in expression is expected since differentiation reduces the number of stem/progenitors and increases the number of differentiated progeny, such as astrocytes, oligodendrocytes and neurons. Different from the brain (such as fetal brain and adult forebrain) derived and fetal spinal cord derived NSPC, which predominantly differentiate into astrocytes [75-77], the adult spinal cord derived NSPC show predominant expression of oligodendrocytes and radial glia. Kulbatski et al.[8] reported 58% of cells in adult spinal cord derived NSPC neurospheres expressed RIP whereas only 18% expressed GFAP after differentiation. The authors also reported 73% of cells expressed RC1 after differentiation, with no significant difference in the percentage of RC1 expression between the undifferentiated and differentiated states. In addition, Kulbatski et al.[8] reported myelin production (in the absence of and wrapped around axon fibers) and the ability to phagocytose cellular debris (in order to maintain the local microenvironment) by adult spinal cord derived NSPC. These in vitro properties of adult spinal cord derived NSPC raise the possibility of culturing and transplanting adult spinal cord derived NSPC as a cell replacement therapy after SCI. In addition to multipotentiality, adult spinal cord derived NSPC could be especially important in regenerating oligodendrocytes (and thereby myelin) because oligodendrocytes are subject to critical damage and loss as a result of SCI. Moreover, the production of radial glia, which are important for directing and supporting migrating neurons during development, could provide support for both endogenous and transplanted NSPC after 16
26 SCI. Furthermore, the ability to phagocytose cellular debris could indicate a potential use of transplanted adult spinal cord derived NSPC as neurospheres for reducing the amount of cellular debris produced after SCI, thereby promoting a less hostile environment for regeneration and repair Transplantation of adult spinal cord derived neural stem/progenitor cells Based on their properties of self-renewal and multipotentiality in vitro [8,25], adult spinal cord derived NSPC have been transplanted into the rat spinal cord to examine their regenerative properties in vivo. In the normal spinal cord, transplanted NSPC maintain their ability to proliferate although proliferation is limited to only hours to a few days post-transplantation in the absence of the co-delivery of additional factors (such as growth factors) [11,16,17]. During the first few hours to days post-transplantation, transplanted NSPC express stem/progenitor markers (such as nestin and NG2). Under the influence of the host environment, migration and differentiation of the transplanted NSPC are observed. Interestingly, Mothe et al.[17] reported that in the absence of the co-delivery of additional differentiating factors, transplanted adult spinal cord derived NSPC predominantly differentiated into oligodendrocytes at one week posttransplant. The authors reported more than 80% of transplanted NSPC expressed the oligodendrocyte marker, CC1, whereas only approximately 2% expressed the GFAP astrocytic maker, and there was no detection of any neuronal markers, such as MAP2 or NeuN [17]. Therefore, adult spinal cord derived NSPC maintain their in vitro properties of proliferation, multipotentiality and preferential differentiation into oligodendrocytes post-transplant. Nevertheless, there was a significant reduction in the survival of transplanted NSPC [11,16,17], especially in comparison to the survival of NSPC in culture. Different from the optimal 17
27 conditions provided in culture, the host environment post-transplant lacks scaffolds or matrix and contains diverse cues that could be growth-inhibiting, apoptotic or necrotic. Death of transplanted NSPC is most prevalent during the first week post-transplant as reported by Mothe et al.[17]. The authors found a 33% reduction in cell survival after transplantation of NSPC into the normal spinal cord at one week post-transplant in comparison to one day post-transplant, although this reduction in cell survival tapered after the first week and live NSPC were be detected 6 weeks post-transplant signifying that transplanted NSPC had the potential to be integrated into the host system [17]. In the injured spinal cord, transplanted NSPC maintain some of their in vitro properties similar to those demonstrated in the normal spinal cord [11,16]. For example, transplanted NSPC proliferate transiently, show multipotentiality and differentiate into oligodendrocyte in large numbers. However, different from transplantation into the normal cord, differentiated progenies contain higher numbers of astrocytes. For example, Parr et al. [11] reported that, following transplantation into the spinal cord with a 27g clip compression injury, approximately 60% of transplanted NSPC expressed the CC1 oligodendrocyte marker, 20% expressed the GFAP astrocytic maker, and 1% expressed the MAP2 neuronal marker. Notably, the survival of transplanted NSPC is drastically reduced in the injured spinal cord even with immunosuppression to prevent allogenic rejection [11,16]. To improve the survival of transplanted NSPC, the conditions of NSPC and the time and location of transplant were modified and optimized (Table 5). NSPC transplanted as neurospheres result in better survival than transplantation as dissociated cells since dissociation causes cell death prior to transplantation; moreover, neurospheres provide a more supportive micoenvironment (such as the ability to phagocytose cellular debris) thereby enhancing cell survival post- 18
28 transplant [8]. Transplantation at a subacute post-injury time period result in better cell survival since the injury environment is more stabilized in the subacute period, when edema, hemorrhage and inflammation are lessened while the glial scar is not yet established [11]. NSPC transplanted rostral and caudal to the injury site survive better than cells transplanted directly into the site of injury; this is because the injury site lacks scaffolds and contains many apoptotic and growthinhibiting factors, which are toxic to cell survival [11]. Table 5: Survival of transplanted NSPC can be influenced by the conditions of NSPC and the time and location of transplant. Variable Differences in Survival of Transplanted NSPC Passage number and days in culture (prior to transplant) Transplantation of NSPC as neurospheres Neurospheres at passages 2 to 4 at divisions 5 to 7 have smaller amount of dead cells (number of dead cells within neurospheres increase from 1-4% at passage 2 to 12-16% at passage 4) [8] NSPC transplanted as neurospheres show 3.5 times better survival than transplanted as dissociated cells at 2 weeks post-transplant [17] Location of transplant At 1 week post-transplant [11] Rostral and caudal transplantation to injury site: 1.1% survival Transplantation to injury site: 0.1% survival At 2 weeks post-transplant [11] Rostral and caudal transplantation to injury site: 0.4% survival Transplantation to injury site: 0.1% survival Time of transplant At 1 week post-transplant [11] Subacute transplantation (9 days after injury): 6.5% survival Acute transplantation (immediately after injury): 1% survival Chronic transplantation (28 days after injury): 2% survival At 2 weeks post-transplant [11] Subacute transplantation: 2% survival Acute transplantation: 0.2% survival Chronic transplantation: 1% survival 19
29 Nonetheless, even with immunosuppression and optimized time and location of transplant, survival of transplanted NSPC is still very limited in the injured spinal cord. Typically, only approximately 3-10% of transplanted NSPC are alive at one week post-transplant and less than 2% are present at 12 weeks post-transplant [11,16]. This low survival of transplanted NSPC impedes their promotion of regeneration. To augment the survival of transplanted NSPC, infusion of growth factors has led to promising results. For instance, Karimi-Abdolrezaee et al.[10] increased the survival of transplanted NSPC to 37% at 8 weeks post-transplant through the intrathecal administration of the combination of EGF, FGF2 and platelet-derived growth factor (PDGF) for seven days. However, the major drawback of growth factor administration is uncontrolled proliferation of transplanted cells and proliferative mass lesions from adjacent tissues [19-21]. In some cases, the uncontrolled proliferation resulted in neoplasms that further compress and damage the injured spinal cord. In an attempt to find alternative agents for enhancing the survival of transplanted NSPC, the present study examined the effect of ephrin-b3 on adult rat spinal cord derived NSPC because ephrin-b3 has been shown to increase the survival of endogenous NSPC in the mouse brain [22,23,78,79]. 1.4 Ephrin ligands and Eph receptors in the Central Nervous System Ephrin ligands and Eph receptors (ephrin-eph) represent the largest sub-family of tyrosine receptor kinases in the central nervous system [80,81]. To date, 9 ephrin and 16 Eph have been identified for their diverse roles, originally recognized in the developing nervous system, and more recently in the adult nervous system, immune activation, angiogenesis and tumorigenesis [80,82-85]. The ephrin-eph are divided into A and B subclasses based on sequence homology 20
30 and membrane anchorage. The ephrin contain an approximately 160 amino acid homologous sequence in their N-terminus, and are categorized into 2 classes based on their C-terminus, which also provide membrane anchorage [80,82]. The ephrin-a (A1-6) proteins are attached to the cell membrane by a glycophosphatidylinositol (GPI) anchor, and ephrin-b (B1-3) are transmembrane proteins. The structure of Eph contains more domains and is more complex than the ephrins. All Eph (EphA1-9 and EphB1-6) are transmembrane receptors with a highly conserved globular domain on the extracellular N-terminus, which is both necessary and sufficient for ligand recognition and receptor activation. This globular domain is then attached to one unique cysteine-rich motif and two fibronectin type III motifs, which are responsible for receptor dimerization and receptor-specific response. The intracellular C-terminus region contains several domains, such as the tyrosine kinase domain, the sterile alpha motif domain and the postsynaptic density protein zona occludens (PDZ)-binding domain, that provide binding sites for downstream regulators. Because of their membrane attachment, ephrin-eph interactions require cell-cell contact. Moreover, membrane attachment allows the ephrin-eph to cluster and form ephrin-ephrin or Eph-Eph dimers [86,87]. Ephrin-Eph have been suggested to be present in lipid rafts of the cell membrane in loose clusters prior to cell contact; when contact occurs, the binding and recognition of ephrin-ephrin to Eph-Eph clusters will promote further multimerization and activate downstream regulators [88]. In spite of the A and B subclass classifications, binding within and among the ephrin-eph subclasses is promiscuous. For example, ephrin-a5 is able to bind EphA2-8 and EphB2, and ephrin-b3 can bind to EphA4 and EphB1-3 [80,89,90]. This promiscuity in binding is permitted because Eph usually have separate binding pockets or different structure of the binding pocket for ephrin-a in comparison to ephrin-b [91]. 21
31 The expression of ephrin-eph was first studied in developing nervous system of the chick embryo, where expression of ephrin-eph provided chemotaxic gradients that were important in axon path-finding and branching, cell migration, and maintenance of regional patterning [80,92]. For example, in the retinotopic mapping of retinal ganglion axons in the superior colliculus, binding between ephrin-a and EphA provides inhibitory cues that cause growth cone collapse along the anterior-posterior axis, whereas binding between ephrin-b and EphB provides attractive cues along the lateral-medial axis [92]. Similar retinotopic mapping has been later confirmed in the developing mouse nervous system [93]; moreover, later studies reported the contribution of ephrin-eph system in axon path-finding and branching, cell migration, and maintenance of regional patterning throughout the developing brain and spinal cord in the mammalian CNS [82]. Recent studies have focused on the expression and function of the ephrin-eph system in the adult CNS. Ephrin-Eph are continually expressed in the adult CNS although the expression level is significantly lower than that in the developing CNS, except in areas where constant reformations occur, such as the dendritic spines of neurons in the hippocampus [94]. In the adult CNS, ephrin- Eph are expressed by astrocytes, oligodendrocytes and neurons in both brain and spinal cord [95-100]; moreover, the ephrin-eph system promotes formation and maturation of dendritic spines (and is involved in long-term potentiation) and regulates synaptic plasticity and spinal cord pain processing [82]. Several recent studies have also described a new role of the ephrin-eph system in regulating the NSPC population in the adult mouse brain (this will be discussed in more detail in the next section) [22,23,78,79,101]. In the injured adult CNS, recent studies have revealed an upregulation in the expression of the ephrin-eph [96-100]. To date, there is limited information regarding the mechanisms that initiate 22
32 the upregulation of ephrin-eph system. In the developing CNS, the expression of the ephrin-eph system is regulated by the Vax, Tbx5, Pax, CBF1 and Hox transcription factors, Wnt signaling, and neural activity dependent serum response factor-associated transcription [102]. However, it is unknown whether these factors, relevant to developing CNS, are still responsible for the regulation of ephrin-eph in the injured adult CNS. Currently, it is suggested that proinflammatory cytokines (abundant post-injury), such as interleukin-1-beta, interferon-gamma and tumor necrosis factor alpha, could upregulate the expression of ephrin-eph through the Pax transcription factors [82,96,103]. However, the details of how inflammatory cytokines influence the activities of transcription factors remain to be determined. Upregulation of the ephrin-eph system post-injury in the adult CNS has mainly contributed to the worsening of the traumatic injury and the inhibition of regenerative mechanisms [96-100]. For example, upregulation of ephrin-b2 has contributed to the formation of the glial scar in spinal cord injury, which inhibits the regrowth of regenerating axons [99,104]. Similar effects have also been demonstrated in response to ephrin-a5 and ephrin-b3, where neurite outgrowth was inhibited [105,106]. Efforts to reduce the inhibitory effects of the upregulated ephrin-eph system after adult CNS injury have focused on the delivery of fusion proteins combining ephrin and crystallizable fragment (Fc) of the immunoglobulin G (ephrin-fc) or Eph-Fc [86,107]. The fusion of the Fc fragments to soluble ephrin or Eph allows clustering and multimerization of these proteins that mimics the natural clustering (as previously introduced), since the membrane association of the ephrin-eph is lost during the synthesis of the soluble forms of these proteins [86]. Delivery of ephrin- or Eph-Fc aims to inhibit the damaging endogenous ephrin-eph pathways. This method has conveyed success in some models; for example, Ricard et al.[22] showed that delivery of ephrin-b3-fc could inhibit the apoptotic effects of EphA4 receptors in 23
33 the subventricular zone (SVZ) NSPC in the adult mouse brain. However, the effect of delivery ephrin- or Eph-Fc remains to be examined in many more models, especially injury environments; therefore, the role and application of ephrin-eph remains a field of many on-going research. This project aims to examine one of the ephrin, ephrin-b3, in order to gain more information regarding the effect of ephrin-b3 on the survival of the spinal cord NSPC in vitro and after transplantation into the injured spinal cord. 1.5 Ephrin-B3 ligands and EphA4 receptors in the Central Nervous System The functions of ephrin-b3 and EphA4 receptor (EphA4R) have been first described in the developing central nervous system [80,81]; however, recent research has revealed that the interaction between ephrin-b3 and EphA4R is involved in regulating the survival and proliferation of adult NSPC [22,23,79,101] Ephrin-B3 ligands and EphA4 receptors in the brain In the developing central nervous system, the ephrin-eph system provides chemotaxic cues that are important in axon path-finding and branching, cell migration, and maintenance of regional patterning [80,108,109]. Regarding the developing brain, studies have typically described the interaction between ephrin-b3 and EphA4R as repulsive chemotaxic or growth-inhibiting cues [110]. For example, in the developing thalamus, ephrin-b3 and EphA4R interaction prevents the neurons in the lateral thalamus, but not those in the medial thalamus, from extending projections 24
34 to the limbic cortex [111]. However, in the adult mouse brain, recent studies have reported a positive effect of the ephrin-b3 and EphA4R interaction on the survival of NSPC in the SVZ [22,23,78,79,101]. In adult mouse brain, EphA4R is expressed in the SVZ and in neurospheres derived from the SVZ [22,23,79,101]. Furne et al.[23] reported that, for the adult mouse brain derived NSPC, EphA4R functions as a dependence receptor [112], which is pro-apoptotic in the absence of ephrin-b3 (Figure 2). 25
35 Figure 2: Schematic diagram showing the interaction between ephrin-b3 and EphA4 receptor (modified from Mehlen et al.[112]). The EphA4 is a dependence receptor that has a cleavage site at the aspartic acid (D) located at the 773/774 position (D773/774). The truncated N-terminus can then activate further caspase-dependent pathways that increase apoptosis. Ephrin-B3 binding prevents the cleavage at D773/774 and thereby encourages cell survival. 26
36 In the absence of ligand binding, Furne et al.[23] found that the intracellular region of the EphA4R is cleaved by caspase-3 at the aspartic acid (D) located at the 773/774 position (D773/774) of the amino acid sequence. This cleavage reveals a pro-apoptotic domain (addiction/dependence domain) in the N-terminus upstream of the D773/774 cleavage site, which in turn activates caspase-dependent apoptotic pathways (such as caspase-3-dependent phosphorylation of STAT1 [79]). Upon binding with ephrin-b3, the EphA4R undergoes oligomerization that prevents the cleavage of the D773/774 site thereby inhibiting the activation of downstream pro-apoptotic pathways [23]. Unlike EphA4R, ephrin-b3 is not expressed endogenous by cells in the SVZ or in neurospheres derived from the SVZ [22]. To augment the survival of NSPC, ephrin-b3 has been delivered in culture and into the lateral ventricles of the adult mouse brain [23,78,79]. With the delivery of ephrin-b3, survival of NSPC is enhanced in vitro and in vivo; meanwhile, proliferation of NSPC is reduced. These properties of ephrin-b3 suggest that it could be used to improve the survival of transplanted NSPC without inducing uncontrolled proliferation (as that observed in the use of growth factors) Ephrin-B3 ligands and EphA4 receptors in the spinal cord Similar effects in the interactions between ephrin-b3 and EphA4R have been observed in the developing brain and spinal cord [1,113]. In the developing spinal cord, interaction between ephrin-b3 and EphA4R also produces repulsive chemotaxic cues [113,114]. For example, during the development of the corticospinal tracts, interactions between ephrin-b3 and EphA4R at the midline ensure that ipsilateral projections cannot decussate and contralateral projections are averted from recrossing the midline [114]. In the adult spinal cord, EphA4R expression has been 27
37 observed in both gray and white matter, with strong staining in the substantia gelatinosa, axons and soma of glial cells [95]. In the injured adult spinal cord, the interaction between ephrin-b3 and EphA4R is not clearly defined. In injured mouse spinal cord, EphA4R expression has been observed to be upregulated in reactive astrocytes, in which EphA4R receptor co-localizes mainly with myelin [96,106]. However, in injured rat spinal cord, EphA4R expression is upregulated and observed in the axons at the injury site, especially in the axon stumps, in neuronal cell bodies and in glial cells (such as oligodendrocytes and astrocytes) [96-100]. Moreover, there are no previous reports regarding the expression of EphA4R in adult rat spinal cord derived NSPC in culture and after transplantation into the injured spinal cord. Furthermore, there are no previous reports of the effect of ephrin-b3 on the survival of endogenous or transplanted spinal cord derived NSPC. 1.6 Hypothesis and Objectives for Present Work Hypothesis This project hypothesized that ephrin-b3 could enhance the survival of adult spinal cord derived NSPC in vitro and after transplantation into a SCI environment. As introduced previously, the substantial amount of cell death in transplanted adult spinal cord derived NSPC is one major challenge that limits their regenerative potential in a SCI environment [11,16]. To encourage NSPC survival and avoid uncontrolled proliferation, ephrin- B3 was examined in this project. In the developing central nervous system, ephrin-b3 has been observed to exert similar effects as a guidance molecule in the brain and spinal cord. In studies conducted using the adult mouse brain, ephrin-b3 has been delivered in vitro and in vivo and has 28
38 been shown to reduce death of subventricular zone-derived NSPC and death of endogenous NSPC in the subventricular zone without inducing uncontrolled proliferation [22,23]. Because of the comparable effects of ephrin-b3 in the developing brain and spinal cord and the beneficial effects on NSPC survival in adult brain, this project aimed to examine whether survival of adult spinal cord derived NSPC could be enhanced through the use of ephrin-b3. The effect of ephrin- B3 has not been previously examined on adult rat spinal cord ependymal region derived NSPC in vitro or after transplantation into the injured adult rat spinal cord. Therefore, this project aimed to test the effect of ephrin-b3 as an alternative survival factor for spinal cord derived NSPC and to gain more information regarding the effect of ephrin-b3 in the adult spinal cord. Objectives To test the hypothesis of this project, the following three objectives were established: 1. To examine the expression of EphA4R in cultured adult rat spinal cord derived NSPC in vitro and post-transplant EphA4R have been shown to be expressed in adult mouse brain subventricular zone-derived NSPC [22,23], however, it is unknown whether EphA4R are expressed in adult rat spinal cord derived NSPC. Since enhanced cell survival was observed as a result of interactions between ephrin-b3 and EphA4R, whereby ephrin-b3 bind and inhibit the downstream apoptotic effect of EphA4R [22,23,112]. Therefore, it is essential for this project to first examine whether EphA4R are expressed in the adult rat spinal cord derived NSPC in order to determine if ephrin-b3 could enhance NSPC survival through interacting with the EphA4R. This project examined the expression of EphA4R both in vitro and post-transplant in order to determine if spinal cord NSPC could respond to ephrin-b3 in both conditions. 29
39 2. To assess the effect of ephrin-b3 on the survival of spinal cord NSPC in vitro Ephrin-B3 have been shown to enhance the survival of adult mouse brain subventricular zonederived NSPC [22,23], however, it is unknown if ephrin-b3 could improve the survival of spinal cord NSPC. Following the results obtained in the first objective, in the condition that spinal cord NSPC express EphA4R, it is possible that addition of ephrin-b3 into the culture media of spinal cord NSPC could encourage NSPC survival through the EphA4R pathway [22,23,112]. Moreover, this project aimed to determine the effect of ligand concentration on NSPC survival in vitro. 3. To assess the effect of ephrin-b3 on the survival of transplanted spinal cord NSPC This project aimed to examine if ephrin-b3 could be an alternative survival factor that address the problem of the significantly reduced NSPC survival after transplantation into a SCI environment. There are no previous reports on the effect of ephrin-b3 on transplanted spinal cord NSPC, therefore, this project aimed to provide information regarding the effect of ephrin- B3 in the spinal cord and on transplanted NSPC in a SCI environment. 2 Materials and Methods 2.1 Animals Adult female Wistar rats ( g) were used in all experiments (n=61 total). Transgenic rats expressing green fluorescent protein (GFP) were used for harvesting NSPC (YS Institute Inc, Ytsunomiya, Tochigi, Japan) [115], because the GFP expression allowed tracking of cells posttransplant. Wildtype adult female Wistar rats (6-8 weeks old; Charles River, St. Constant, QC) 30
40 were used for all other experiments. All animal procedures were approved by the Animal Care Committee of the Research Institute of the University Health Network in accordance with the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care). Table 6 shows the treatment groups and the respective number of animals used. Table 6: Treatment groups and the number of animals used (with and without intrathecal [IT] compound delivery) Experiment Group Duration of IT Number of Rats Endogenous Ependymal Cells in Normal and Injured Cord with IT Delivery NSPC Transplanted into the Injured Cord with IT Delivery Endogenous Ependymal Cells without IT Delivery Delivery (days) Normal cord + PBS 7 5 Normal cord + ephrin-b3-fc 7 5 Normal cord + Fc 7 5 Injured cord + PBS 7 5 Injured cord + ephrin-b3-fc 7 5 Injured cord + Fc 7 5 Injured cord + NSPC + PBS 3 3 Injured cord + NSPC + ephrin-b3-fc 3 3 Injured cord + NSPC + Fc 3 3 Injured cord + NSPC + PBS 7 6 Injured cord + NSPC + ephrin-b3-fc 7 6 Injured cord + NSPC + Fc 7 6 Normal cord (no laminectomy or NSPC N/A 2 transplant) Injured cord (no NSPC transplant) N/A 2 Total: Isolation and Culturing of Neural Stem/Progenitor Cells GFP-positive (GFP+) NSPC were isolated from both brain and spinal cord since previous studies examined the ephrin-b3 and EphA4R in brain derived NSPC [22,23,95,101]; thus, the brain derived NSPC were used as comparisons for the spinal cord derived NSPC in detecting the presence of the EphA4R. The procedures for harvesting and passaging of NSPC have been previously described by members of Dr. Tator s laboratory (Mothe et al.[17] and Guo et al.[21]). 31
41 To briefly describe the procedures, the spinal cord and brain were excised under sterile conditions. The spinal cord was dissected, removing the overlying meninges, blood vessels and white matter and most of the gray matter, so that only the central canal region (including the ependymal, subependymal and some gray matter surrounding the central canal) remained. The brain was dissected similarly so that only the periventricular tissue remained. The excised tissues were placed in fresh Dulbecco s phosphate buffered saline (PBS) supplemented with 30% glucose and divided into small pieces (approximately 1mm 3 pieces). The pieces of tissue were then physically and chemically dissociated with 0.01% papain and 0.01% DNAse I at 37 C (Worthington Biochemical Corporation, Lakewood, NJ). The resulting cell suspension was centrifuged for 5min at 1000rpm to remove cell membrane fragments, and then resuspended in serum free media supplemented with 20ng/mL of mouse EGF, 20ng/mL of hfgf2 and 2µg/mL of heparin (all from Sigma-Aldrich, Oakville, Ontario, Canada). The serum free media contained Neurobasal-A (Gibco-Invitrogen, Burlington, Ontario, Canada), B27 (Gibco-Invitrogen), L- glutamine (Gibco-Invitrogen), penicillin/streptomycin (Gibco-Invitrogen) and hormone mix (Sigma-Aldrich). The hormone mix was composed of DMEM/F-12 (1:1), 0.6% gluclose, 2.5µg/mL insulin, 100µg/mL transferring, 5mM HEPES buffer, 3mM sodium bicarbonate, 30nM sodium selenite, 10µM putrescine and 20nM progesterone (all from Sigma-Aldrich). The cells were stored in a 37 C incubator with 100% humidity and 5% CO 2, cultured as neurospheres and passaged every 7 days. 2.3 Preclustering of compounds Ephrin-B3 ligands are naturally present in cells as transmembrane proteins, and this membrane association facilitates the oligomerization of the ligands [116]. The oligomerization of ephrin-b3 32
42 facilitates binding and activation of receptors. In the synthesis, isolation and purification of ephrin-b3 (for delivery in larger and concentrated quantities in culture or transplantation), the membrane association is lost, and the ligands are present as soluble monomers [86]. Notably, Davis et al.[86] reported that the soluble form of ephrin-b3 activates receptors very minimally due to the loss of oligomerization. To reconcile this problem, fusion proteins are synthesized containing the amino acid sequence of the human ephrin-b3 linked to the crystallizable fragment (Fc) of the human immunoglobulin G (IgG) through a linker peptide. The Fc component of the fusion protein, ephrin-b3-fc, can be targeted by anti-igg Fc specific antibodies and are clustered by the antibodies thereby allowing the oligomerization of soluble ephrin-b3 molecules (Davis et al.[86] reported that the preclustered ephrin fusion ligands are more than 100 times more potent at receptor activation than the unclustered soluble ligands). Hence, to date, the only commercially available form of ephrin-b3 is ephrin-b3-fc (purchased from R&D Systems, Minneapolis, MN). To control for possible effects exerted by the Fc fragment alone, the IgG Fc fragments (IgG Fc) (Jackson ImmunoResearch, West Grove, PA) were used as a ligand control for the ephrin-b3-fc. Prior to each experiment, the compounds (ephrin-b3-fc and Fc fragments alone) were preclustered using anti-fc antibodies (Fitzgerald Industries, Acton, MA) at room temperature for 3 hours at a ratio of 1:10 (ligand: anti-fc) as previously described [22,107]. 2.4 Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vitro 33
43 2.4.1 EphA4 expression in spinal cord NSPC in vitro The EphA4R expression was examined in both brain and spinal cord derived NSPC. Since previous studies have reported EphA4R expression in the adult rat brain and in adult mouse brain SVZ derived NSPC [22,23,95,101], this project used the GFP+ adult rat brain SVZ derived NSPC as a positive control. Brain and spinal cord derived NSPC of passage 5 and 3-5 days in vitro were fixed in 4% paraformaldehyde for 20min at room temperature. The cultures were washed with 0.1M PBS, blocked with 10% normal goat serum for 1hr at room temperature and incubated with the primary antibody anti-epha4r (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA) overnight at 4 C. Cultures were washed with 0.1M PBS and incubated with fluorescent Alexa 568 secondary antibody (Millipore, Temecula, CA) for 1hr. Thereafter, cultures were washed with 0.1M PBS and counterstained with Hoechst (Invitrogen, Burlington, Ontario) to identify the nuclei. Each staining condition was repeated in five wells, and the entire assay was repeated twice. The percentage of brain and spinal cord NSPC that expressed EphA4R were counted and averaged using the method previously described by Kulbatski et al.[8]. In brief, five fields at 20X magnification (710x530µm rectangular fields) were selected from each well, where immunofluorescent cells were examined using a Nikon Eclipse TE 300 microscope. Images were captured with a CCD camera and Bioquant Software (R&M Biometrics Inc.). EphA4R and Hoechst double-labeled cells were counted, and the EphA4R-positive cells were expressed as a percentage of the Hoechst labeled cells. 34
44 2.4.2 MTS cell survival assay The MTS tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4- sulfophenyl)-2h-tetrazolium)] assay is a colorimetric assay that measures cell survival based on the colour change of the MTS (CellTiter 96 AQueous Non-radioactive cell proliferation assay, Promega, Madison, WI). When added into the cell culture, metabolically active cells convert the MTS molecule into aqueous soluble formazan (coloured); ultraviolet absorbance of the solution is measured and reflects the quantity of formazan. This quantity is proportionate to the amount of live cells in culture. Cultured spinal cord NSPC of passages 3-5 and 3 days in vitro were assessed using the MTS assay. Spinal cord NSPC (cultured as free floating neurospheres) were centrifuged for 4min at 1500 revolutions per minute (rpm), the supernatant was removed, and the cells were resuspended and dissociated in 1mL of fresh media. A 300µL sample of the cell suspension was mixed with 100µL of fresh media and 200µL of trypan blue. 13µL of the cell suspension and trypan blue mixture was placed on each side of a hemocytometer, and the number of cells in the suspension was calculated using the hemocytometer. Based on this calculation of the total cell number, the cells were resuspended as dissociated cells at a concentration of 1000 cells/µl. These dissociated NSPC were seeded in Matrigel TM (Growth Factor Reduced BD Matrigel Matrix and diluted by a factor of 25; BD Biosciences, Mississauga, Ontario) coated wells in 24- and 96-well plates. In each well, cells were seeded in serum free media (content described in Section 2.2) supplemented with 20ng/mL of EGF, 20ng/mL of FGF and 2µg/mL of heparin for 24hr in a 37 C incubator with 100% humidity and 5% CO 2. After 24hr, the media was changed to serum free media with preclustered ephrin-b3-fc or Fc fragments (at concentrations 0.1µg/mL, 1µg/mL, 2µg/mL, 10µg/mL, and 100µg/mL) for 48hr in the incubator (Table 7). The control 35
45 wells contained serum free media with an equal volume of PBS in place of the preclustered compounds (labeled as the 0µg/mL condition). Since the MTS assay uses ultraviolet (UV) absorbance as an indicator, two additional wells were prepared in this experiment to determine the baseline UV absorbance of the culture media (the media only condition) and the baseline absorbance when all cells are dead (the media with Triton X-100 condition). Triton X-100 is a surfactant that can lyse the cell membrane and kill the cells. After 48hr of incubation with preclustered compounds or PBS in serum free media, 20µL of the MTS/PMS solution was added per 100µL of NSPC in media (resulting in a final concentration of 333µg/mL MTS and 25µM of PMS). The resulting mixture was incubated in the incubator for 1hr; thereafter, the absorbance was recorded at 490nm using an UV plate reader. Each treatment condition was repeated in four wells, and the entire assay was repeated three times. Table 7: In vitro effects of ephrin-b3-fc and Fc on NSPC assessed by MTS and Ki67 assays Condition Media Compound Compound Controls Added Concentration Initial Seeding (without addition of compound) (0-24hr) Serum free media EGF FGF2 Heparin None _ No cells (media only with no cells seeded) Addition of Compound (24-96hr) Serum free media Ephrin-B3-Fc or Fc fragments 0.1µg/mL 1µg/mL 2µg/mL 10µg/mL 100µg/mL 1.No cells (media only) 2.NSPC + Media + PBS (0µg/mL of compound) 3.NSPC + Media + PBS + 2% Triton X
46 2.4.3 Cell proliferation assay Cell proliferation was analyzed through Ki67 immunocytochemical staining. Ki67 is an antigen recognized by the anti-ki67 antibody. The Ki67 antigen is suggested to be a component of a non-histone nuclear protein that is associated with the cell cycle [117,118]. In brief, the cell cycle consists four distinct phases, 3 phases in interphase and the mitosis (M) phase. In interphase, cell growth is observed in the Gap 1 (G 1 ) phase, followed by replication of genetic material in the synthesis (S) phase, and then another period of cell growth in the Gap 2 (G 2 ) phase. Upon the completion of interphase, the cell enters mitosis, in which cell division occurs in several stages. In addition to these 4 phases of the cell cycle, a cell can exit the cell cycle and become nonproliferative or post-mitotic in the Gap 0 (G 0 ) resting phase. Cells in the G 0 phase are usually fully differentiated and are commonly described to be quiescent or senescent since they do not proliferate for long periods of time; however, these cells can re-enter the cell cycle under certain stimuli, such as trauma. Expression of Ki67 is usually localized to the nucleus during interphase and localized to the surface of the chromosomes during mitosis [119]. The level of Ki67 expression varies throughout the cell cycle with minimal expression in G 1, increased expression during the later half of S phase, and maximal expression observed during G 2 and mitosis [120]. Ki67 has a short half-life of approximately 1 hour; hence, as the cell completes mitosis, detection of Ki67 rapidly diminishes [120]. Ki67 is not detected in G 0 and early G 1. Because of this association of Ki67 with the cell cycle (and its absence in G 0 and early G 1 ), Ki67 is used in this project as an indicator for cells that are engaged in the cell cycle and have the potential to proliferate. Spinal cord NSPC were cultured, dissociated, seeded and treated with compounds in the same fashion as that described in the MTS assay (Section and Table 7). After 48hr of incubation 37
47 with preclustered compounds or PBS in serum free media, cultures were fixed in 4% paraformaldehyde for 20min at room temperature. The cultures were washed with 0.1M PBS, blocked with 10% normal goat serum for 1hr at room temperature and incubated with the primary antibody anti-ki67 (1:100) overnight at 4 C. Cultures were washed with 0.1M PBS and incubated with fluorescent Alexa 568 secondary antibody (Millipore, Temecula, CA) for 1hr. Thereafter, cultures were washed with 0.1M PBS and counterstained with Hoechst (Invitrogen, Burlington, Ontario) to identify the nuclei. Each treatment was repeated in four wells, and the entire assay was repeated three times. Quantitative analysis of the Ki67-labeling in cell cultures were conducted as previously described by Kulbatski et al.[8]. In brief, five fields at 20X magnification (710x530µm rectangular fields) were selected from each well, where immunofluorescent cells were examined using a Nikon Eclipse TE 300 microscope. Images were captured with a CCD camera and Bioquant Software (R&M Biometrics Inc.). Ki67-positive and Hoechst double-labeled cells were counted, and the Ki67-positive cells were expressed as a percentage of the Hoechst labeled cells. 2.5 Surgeries conducted to examine the effect of ephrin-b3-fc in vivo Spinal Cord Injury Adult female Wistar rats were injured through the clip compression injury model with a 26g modified aneurysm clip. The rats were anesthetized by inhalation of 4% isofluorane and a mixture of 1:2 nitrous oxide and oxygen, and then maintained at 2% isofluorane during surgery. The spinal cord was exposed through a midline incision, and a laminectomy was performed at 38
48 levels T7 and T8 (Figure 3). The spinal cord was then compressed at T7/8 by a 26g clip for one minute [50]. The wound was then sutured, and the animals recovered in a warm room (approximately 26 C). Animals used for assessing endogenous ependymal cells with intrathecal (IT) infusion in the normal spinal cord received laminectomy only (no clip compression). Animals used for assessing endogenous ependymal cells without IT infusion in the normal spinal cord did not receive any manipulations (no laminectomy or clip compression). 39
49 Figure 3: Images showing the clip (A), clip compression injury (B and C), mini-osmotic pump attached to an IT catheter (D), and the schematic diagram showing the injured spinal cord and the IT delivery setup (E). Note the sizes and distances (A-E) are all relative and not to scale. (B) White arrow points to the site of the injury and yellow arrow points to the caudal end of the IT catheter. 40
50 2.5.2 Transplantation of adult spinal cord derived NPSC Transplantation of spinal cord NSPC was performed 7 days after the initial SCI (in the subacute period). On the day of transplantation, cultured adult rat spinal cord derived NSPC of passages 3-5 and 3-6 days in vitro were prepared for transplantation. Prior to transplantation, GFP+ spinal cord NSPC (cultured as free floating neurospheres) were centrifuged for 4min at 1500rpm, the supernatant was removed, and the cells were resuspended and dissociated in 1mL of fresh media. A 300µL sample of the cell suspension was mixed with 100µL of fresh media and 200µL of trypan blue. 13µL of the cell suspension and trypan blue mixture was placed on each side of a hemocytometer, and the number of cells in the suspension was calculated using the hemocytometer. Based on this calculation of the total cell number, the cells were resuspended as neurospheres (not dissociated) at a concentration of cells/µl and placed on ice until transplantation. During the transplantation surgery, animals were anesthetized by inhalation of 4% isofluorane and a mixture of 1:2 nitrous oxide and oxygen and then maintained at 2% isofluorane in order to re-expose the initial site of SCI. GFP+ neurospheres were injected approximately 1mm rostral and 1mm caudal to the injury site (Figure 4). One rostral and one caudal injection were made in the midline at a depth of 1-1.5mm into the spinal cord with a 32 gauge needle (Hamilton ; 32 gauge with 20 bevel) attached to a Hamilton syringe (Hamilton, Reno, NV). At each injection site, 2µL containing cells were injected at a rate of 2.5µL/min with a motorized pump (Model ; Stoelting, Wood Dale, IL) [17]. Starting from the day of transplantation, animals were immunosuppressed daily with 15mg/kg of cyclosporine A (CsA) (Sandimmune, Novartis, Dorval, QC, Canada) until sacrifice. Animals used for assessing endogenous ependymal cells in the normal and injured spinal cord did not receive NSPC transplantation. 41
51 These animals were immunosuppressed daily with 15mg/kg of CsA starting at the date of IT infusion until sacrifice. Animals used for assessing endogenous ependymal cells without IT infusion in the injured spinal cord did not receive NSPC transplant and were sacrificed seven days post-injury Intrathecal infusion IT infusion involves the implantation of a catheter into the intrathecal space (the subarachnoid space between the dura and the spinal cord where CSF circulates). This delivery of compounds directly into the intrathecal space averts the problem of permeability of the blood-brain or bloodspinal cord barrier, since some compounds diffuse very slowly or minimally or are incapable of diffusing through the blood-spinal cord barrier. The IT catheter is connected to a mini-osmotic pump, which pumps out compounds in a constant flow rate, thereby allowing a continuous flow and consistent dosage of compounds to be delivered for a set period of time (depending on the model of the pump used). This continuous flow and consistent dosage of IT delivery avoids the need of multiple injections (which stress the animals) and the decline of dosage concentration over time that are involved in systemic delivery methods. Hence, the present work chose to delivery compounds intrathecally when testing their effects on the survival of spinal cord NSPC. Prior to the implantation of the IT catheter and the mini-osmotic pump, 100µL of preclustered ephrin-b3-fc or Fc fragments (100µg/mL) were injected into individual mini-osmotic pumps (Alzet Model No. 1007D; Alzet Osmotic Pumps, Cupertino, CA). An equal volume of PBS was used as a control in animals not receiving preclustered ephrin-b3-fc or Fc fragments. Each pump was attached to a polyurethane catheter (Alzet Model No ) and primed in sterile saline 42
52 at 37 C overnight. The implantation of the IT catheter and the mini-osmotic pump is conducted immediately after transplantation of NSPC. A small midline durotomy was made at T9 through which the catheter was inserted into the IT space [20] (Figure 4). The tip of the catheter was directed rostrally to the T7 level, which was approximately 1mm rostral to the site of the rostral injection of NSPC. The dura was then sealed around the catheter by fibrin glue (Beriplast, Beriplast, CSL Behring, Marburg, Germany), and the catheter and pump were sutured to the subcutaneous tissues. The wound was then closed, and the animals recovered in a warm room (approximately 26 C). Preclustered ephrin-b3-fc, preclustered Fc fragments or PBS were then intrathecally delivered continuously for three or seven days at a rate of 0.5µL/hr via the catheter and pump system. The timeline of IT delivery is summarized in Figure 4. 43
53 Figure 4 44
54 Figure 4: Schematic diagrams describing the surgeries conducted in normal (A) and injured (B) spinal cord receiving IT delivery to examine the effect of compounds on the endogenous ependymal cells. The timeline for (A) and (B) are described in (C). Schematic diagrams describing the surgeries conducted to examine the effect of IT delivered compounds on transplanted spinal cord NSPC (D). The timelines for (D) are described in (E). Diagrams (A, B, D) show the relative locations of the clip compression injury, the injection sites for cell transplantation, and the placement of the IT catheter. The distances are not to scale. The suture is immobilizing the catheter preventing movement. The arrowheads in (D) show the location of the NSPC injections rostral and caudal to the injury site. (Modified from Parr et al.[20]) 45
55 2.6 Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vivo Histology and Immunohistochemistry Animals were sacrificed with a lethal intraperitoneal injection of sodium pentobarbital, and then received perfusion with 4% paraformaldehyde for fixation. The spinal cord was excised and maintained in 30% sucrose at 4 C. A 1cm segment of spinal cord was mounted and cryosectioned parasagittally into 20µm sections and collected on Superfrost slides (Fisher Scientific, Ottawa, Ontario). The following antibodies were used for immunofluorescent staining: anti-epha4r (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA) for EphA4R, anti-ki67 (1:100, Novacastra, Burlington, Ontario) for proliferating cells, anti-gfap (1:500, Chemicon, Temecula, CA) for astrocytes, and anti-cc1/apc (1:1000, Calbiochem, San Diego, CA) for oligodendrocytes. During immunostaining, sections were rehydrated with 0.1M PBS and blocked with normal goat serum in 0.1M PBS for one hour. Sections were incubated with primary antibody at 4 C overnight, washed with 0.1M PBS for 30min, and then incubated with secondary antibody conjugated to Alexa Fluor 568 (1:500) or Alexa Fluor 488 (1:500) (all from Millipore, Temecula, CA) for one hour. Negative controls were obtained by omission of the primary antibody or by the application of anti-epha4r antibody blocking peptides (at a ratio of 5:1/peptide:antibody by weight; Santa Cruz Biotechnology). Sections were counterstained with the 4,6-diamidino-2-phenyl-indole (DAPI) nuclear counterstain (Vector Laboratories, Burlington, Ontario). 46
56 2.6.2 EphA4 expression in vivo Normal and injured spinal cord (without NSPC transplant or IT delivery) were stained with anti- EphA4R antibodies to examine the endogenous expression in the rat spinal cord. These sections were also double-labeled with anti-gfap and anti-cc1 to assess if astrocytes and oligodendrocytes contribute to the endogenous EphA4R staining (as described previously in literature [95-100]). Injured spinal cord with NSPC transplant and 3 or 7 days of IT delivery were stained with anti-epha4r antibodies to examine whether transplanted NSPC expressed EphA4R in vivo. Co-localization of GFP+ and EphA4R+ cells were judged as a transplanted NSPC that express EphA4R Effect of ephrin-b3-fc on the proliferation of endogenous periventricular ependymal cells in the adult spinal cord Periventricular ependymal cells of the adult spinal cord are known to contain stem/progenitor cells [6,8,58]. In response to injury, these ependymal cells proliferate and differentiate in attempts to repair the damaged spinal cord. Since the cultured NSPC for this project was derived from the ependymal cells of the spinal cord and previous studies have shown that ephrin-b3-fc could affect the ependymal cells of the brain SVZ [6,8,58], this project examined the effect of IT delivery of ephrin-b3-fc on the endogenous ependymal cells of the normal and injured spinal cord. Upon 7 days of IT delivery (without NSPC transplant) of preclustered ephrin-b3-fc, preclustered Fc fragments or PBS, the animals were sacrificed and the spinal cord sections were stained with Ki67 to label proliferating cells. To quantitate the Ki67-labeling in the ependymal region, the ependymal cells were examined in every 8 th section (every 8 th section was 140µm apart to avoid double-counting of cells appearing on more than one section) as previously 47
57 described by Mothe et al.[17]. A total of 15 sections were examined, and images were captured on sections containing the ependymal region through a Zeiss LSM 510 confocal microscope. The Ki67 and DAPI stained cells in the ependymal region were counted for the entire length of the section, and the Ki67+ cells were expressed as a percentage of the DAPI stained cells. The total cell count was estimated using the Abercrombie method [121] for the entire cord thickness. In the analysis of the Ki67-labeling index, the injured spinal cords were analyzed in two ways. First, the Ki67-labeling of the entire length of the sections were summed together and were compared among the three IT delivery groups (preclustered ephrin-b3-fc, preclustered Fc fragments or PBS). Second, the Ki67-labeling within each IT delivery group were examined based on rostral or caudal distance from the injury site. The distances from the injury site were based on the length of the fame of image captured by the Zeiss LSM 510 confocal microscope, which in this project was 585µm (or 0.585mm) per frame. Within each group, the Ki67-labeling between the same lengths of the cord, which were of equal distance rostral or caudal from the injury site (e.g. 1.2mm rostral versus 1.2mm caudal to the injury site), were compared Effect of ephrin-b3-fc on the survival of transplanted spinal cord NSPC in the injured adult spinal cord This project focused on the effect of ephrin-b3-fc on the survival of transplanted NSPC in the injured spinal cord. The animals received either 3 days of IT delivery and sacrificed to examine the early effect of ephrin-b3-fc delivery, or received 7 days of IT delivery and sacrificed 14 days post-transplant to examine the effect of ephrin-b3-fc in a longer term. To quantitate the amount of cell survival in tissue sections, transplanted GFP+ cells were examined in every 8 th section [17]. Images of each section were captured using a Zeiss LSM 510 confocal microscope, 48
58 and GFP+ cells were counted in a total of 15 sections (every 8 th section, 140µm apart, was selected to avoid double-counting of cells appearing on more than one section). The total cell count was estimated using the Abercrombie method [121] for the entire cord thickness. 2.7 Statistical Analysis All data were presented in the form of mean ± standard error (m±se) and were analyzed using SigmaStat 3.1 (Systat, Point Richmond, CA). Statistical differences between groups were examined with one-way ANOVA and Bonferroni-adjusted pairwise multiple comparisons. In cases where only two experimental conditions were examined, statistical differences were evaluated with the Student s t-test. In all statistical tests, p<0.05 was set as the significance level unless otherwise indicated. 3 Results 3.1 EphA4 expression in vitro and in vivo In culture, immunofluorescent staining showed that cultured adult rat brain SVZ (Figure 5A-C) and spinal cord derived NSPC (Figure 5D-F) express the EphA4R. Through quantitative analysis, 80.95±2.42% of brain NSPC showed EphA4R expression and 76.84±3.85% of spinal cord NSPC showed EphA4R expression; no statistical difference is observed in the EphA4R expression between the brain and spinal cord derived NSPC. After transplanting into an injured spinal cord, many GFP+ spinal cord NSPC showed EphA4R expression at 3 and 14 days post- 49
59 transplant (Figure 5). Thus, cultured adult spinal cord NSPC express the receptor that is capable of responding to the ephrin-b3-fc ligand. 50
60 Figure 5 51
61 Figure 5: Immunostaining of the EphA4 receptor in cultured brain and spinal cord derived NSPC (A-F), and in spinal cord derived NSPC post-transplant (G-J). Dissociated brain derived NSPC in culture stained with Hoechst nuclear counterstain (A) and EphA4 (B) with colocalization of 80.95±2.42% (m±se) (merged panel C). Dissociated spinal cord derived NSPC in culture stained with Hoechst (D) and EphA4 (E) with colocalization of 76.84±3.85% (merged panel F). There was no significant difference in EphA4 staining between brain and spinal cord derived NSPC in culture. (G-J) Sample images of spinal cord derived NSPC (GFP+ in panel I) stained with DAPI nuclear counterstain (G) showing EphA4 staining (H); panel J shows the merged image. Scale bars: (A-F) 50µm and (G-J) 20µm. 52
62 In the normal and injured spinal cord (without NSPC transplant or IT delivery), anti-epha4r antibody blocking peptides were used as an additional negative control. No notable difference was observed between the negative controls obtained by the omission of the primary antibody or the use of the blocking peptide. In the normal and injured spinal cord (without NSPC transplant or IT delivery), EphA4R was expressed endogenously, and this expression co-localized with GFAP and CC1 staining. Moreover, the injured spinal cord showed a higher level of EphA4R expression than the normal spinal cord suggesting that the EphA4R is upregulated after SCI (Figure 6). 53
63 Figure 6 54
64 Figure 6: Immunostaining of EphA4 receptor in the normal and injured spinal cord. (A) Schematic diagram of a cross section of spinal cord showing the approximate region (in the boxed area) of the immunohistochemical stained images B-K. EphA4 is expressed in the normal spinal cord (A, B, and merged panel C) by astrocytes (merged panel E) and oligodendrocytes (merged panel F). EphA4 is also expressed in the injured spinal cord (G, H, and merged panel I) by astrocytes (merged panel J) and oligodendrocytes (merged panel K). Scale bars: (A-K) 50µm and insets 20µm. 55
65 3.2 Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vitro MTS cell survival assay Preclustered ephrin-b3-fc, preclustered Fc fragments or PBS was added to spinal cord NSPC in culture for 3 days (Table 7). The UV absorbance value in the MTS assay reflects the relative quantity of live cells such that the higher values of absorbance represent great amount of live cells (and vice versa). In the conditions where preclustered ephrin-b3-fc was added, most of the assessed concentrations had no effect on NSPC survival although an increase in NSPC survival was observed at a concentration of 1µg/mL (p<0.05) (Figure 7B). In contrast, the presence of Fc fragments in culture decreased NSPC survival starting at 2µg/mL with progressively greater toxicity as concentration of Fc fragments increase (p<0.05) (Figure 7A). 56
66 Figure 7: Survival and proliferation of dissociated spinal cord derived NSPC in culture with preclustered ephrin- B3-Fc or Fc fragments for 3 days. Survival of NSPC is assessed by the MTS assay (m±se) with the addition of Fc fragments (A) or ephrin-b3-fc (B). Ki67-labeling of NSPC (m±se) with the addition of Fc fragments (C) or ephrin- B3-Fc (D). Asterisk denotes a significant difference in comparison to the PBS with no preclustered compound added condition (0µg/mL) (p<0.05; Bonferroni-adjusted). Fc fragments decreased NSPC survival with increased concentrations. Ephrin-B3-Fc did not alter NSPC survival at most concentrations. Both ephrin-b3-fc and Fc fragments reduced NSPC proliferation at the highest concentration tested. 57
67 3.2.2 Cell proliferation assay Preclustered ephrin-b3-fc, preclustered Fc fragments or PBS was added to spinal cord NSPC in culture (Table 7). Figure 7 shows that that ephrin-b3-fc or Fc fragments did not significantly alter (increase or decrease) proliferation compared to the PBS control, except at the highest concentration (100µg/mL), where proliferation was significantly decreased (p<0.001). These results suggest that ephrin-b3-fc and Fc fragments had minimal effect on NSPC proliferation except toxicity at a very large concentration in vitro. 3.3 Effect of ephrin-b3-fc on the survival and proliferation of spinal cord derived NSPC in vivo Proliferation of ependymal cells in the normal and injured adult spinal cord The effect of ephrin-b3 on periventricular cells in the normal and injured adult spinal cord was examined because these periventricular cells are known to contain stem/progenitor cells [6,8,58]. Preclustered ephrin-b3-fc (100µg/mL), preclustered Fc fragments (100µg/mL) or PBS as a negative control were infused intrathecally for 7 days (n=5/group). In the normal spinal cord (Figure 8A), no significant difference was observed among the three groups in the percentage of Ki67-positive cells. In the injured spinal cord (Figure 8B), a significant increase was observed in the ephrin-b3-fc group in comparison to the PBS group (approximately 0.4% to 1.5%; p<0.05). However, the difference between ephrin-b3-fc and Fc fragments groups did not reach 58
68 significance, and no significant difference was observed between the Fc fragments and PBS groups. Figure 8C-E show the Ki67-labeling in the injured spinal cord based on distances rostral and caudal to the injury site (re-analysis of Figure 8B). Comparisons of the Ki67-labeling were made between the same rostral and caudal lengths of spinal cord equidistant from the injury site. In the PBS and Fc fragments groups, Ki67-labeling in the range of 1.8mm to 3.5mm rostral to the injury site was significantly higher than the labeling at the respective caudal distances (p<0.001). In the ephrin-b3-fc group, Ki67-labeling in the range of 1.2mm to 4.1mm rostral to the injury site was significantly higher than the labeling at respective caudal distances (p<0.001). Moreover, for all three groups, Ki67-labeling was concentrated at approximately 1.8mm to 2.9mm with the highest labeling at approximately 2.3mm rostral to the site of injury, which corresponds with the approximate location of the tip of the IT catheter. At approximately 2.3mm rostral to the injury site, Ki67-labeling was significantly higher in the ephrin-b3-fc and Fc fragments groups than in the PBS group (p<0.001). 59
69 Figure 8 60
70 Figure 8: Effect of intrathecal delivery of preclustered ephrin-b3-fc or Fc fragments on the endogenous spinal cord ependymal cells. Preclustered ephrin-b3-fc or Fc fragments were delivered for 7 days to normal and SCI rats, and Ki67-labeling was used to measure proliferation of the endogenous spinal cord ependymal cells. Data reported as m±se, and asterisk denotes a significant difference. (A) Neither preclustered ephrin-b3-fc nor Fc fragments altered proliferation in the normal spinal cord. (B) Preclustered ephrin-b3-fc slightly increased proliferation of endogenous ependymal cells in the injured spinal cord (p<0.05; Bonferroni-adjusted). (C-E) Distribution of Ki67-labeling in the injured spinal cord. The same tissue sections from (B) are re-examined based on distance from the edge of the injury: each bin is 585µm (0.585mm), 0 represents the site of injury, positive numbers indicate distances rostral to the edge of injury, and negative numbers indicate distances caudal to the edge of injury. Distances are calculated as (0.585mm)x(distance in number of bins) and rounded to one decimal place. In all three groups in C-E, the same rostral and caudal lengths equidistant from the injury site are compared. Significantly higher Ki67-labeling is observed in the rostral side in comparison to the respective distances caudal to the injury site (brackets indicate the range of distances that contain statistically higher Ki67-labeling; p<0.001; Bonferroni-adjusted). Ki67-labeling is concentrated at approximately 1.8mm to 2.9mm with the highest labeling at approximately 2.3mm rostral to the site of injury (corresponding with the approximate location of the tip of the intrathecal catheter as show in Figure 4). At 2.3mm rostral to the injury site, Ki67-labeling was significantly higher in the ephrin-b3-fc and Fc fragments groups compared to the PBS group (p<0.001; Bonferroni-adjusted). 61
71 In comparison to animals without IT catheter implantation, a higher number of proliferating cells was observed in all the groups using IT catheter (Figure 9A-D). This experiment did not perform a cell count for the Ki67-labeled cells in the animals without IT catheter implantation. However, compared to cell counts reported previously by Namiki et al.[58] using a similar paradigm (a lighter 20g injury was used in Namiki et al.[58] experiment in comparison to the 26g injury used in the present work), in which 1-2% of ependymal cells in the normal cord were labeled by Ki67 and less than 5% of ependymal cells in the injured cord, this project found approximately 7% of Ki67-labeling in the normal spinal cord and approximately 6% of Ki67-labeling in the rostral side of the injured spinal cord. Moreover, in the injured spinal cord, this project showed significantly higher Ki67-labeling in the rostral distances relative to the injury site in comparison to the caudal distances (as mentioned above Figure 8B), whereas Namiki et al.[58] observed no difference between the rostral and caudal Ki67-labeling. 62
72 Figure 9: Sample images showing cells labeled for Ki67 confined to the ependymal region of the spinal cord. Merged confocal images showing Ki67-labeled cells counterstained with DAPI. (A) Normal cord with IT catheter, (B) injured cord with IT catheter, (C) normal cord without catheter or laminectomy, (D) injured cord without catheter or laminectomy. The yellow boxes indicate the approximate location of the insets. Note the significantly higher Ki67-labeling in animals with IT catheters (A-B) than in those without (C-D). Scale bars: (A-D) 100µm, insets in (A-D) 20µm. 63
73 3.3.2 Survival of transplanted spinal cord NSPC Immediately after the transplantation of spinal cord NSPC, preclustered ephrin-b3-fc (100µg/mL), preclustered Fc fragments (100µg/mL) or PBS as a negative control were infused intrathecally for 3 (n=3/group) or 7 (n=6/group) days. In all the 3-day IT infusion groups, many GFP+ NSPC were observed (Figure 10A-C). There was no statistical difference in the number of surviving GFP+ NSPC between the three groups although there was a trend towards slightly lower GFP+ NSPC survival in the ephrin-b3-fc and Fc fragment groups (Figure 10G; PBS group had a survival of 4.07±1.50%, Fc fragments alone group had a survival of 2.97±0.88%, and ephrin-b3-fc group had a survival of 3.25±0.93%). Interestingly, in the ephrin-b-fc and Fc fragments groups, the transplanted NSPC were more localized to the sites of injection in comparison to the PBS group, in which the cells were more widely dispersed (Figure 10A-C). In contrast, in the 7-day IT infusion groups, only a small number of surviving transplanted NSPC were observed in the ephrin-b3-fc and Fc fragments groups (Figure 10D-F); large clusters of degraded GFP+ cell debris were observed in localized collections at the sites of injection, and some GFP+ remnants were observed in macrophages (Figure 10E-F). Compared to the PBS group, a 20-fold decrease was observed in the percentage of surviving NSPC in the ephrin-b3-fc and Fc fragments groups (Figure 10H). The PBS group had a survival of 2.19±0.47% with all 6 animals showing live transplanted NSPC. In contrast, the ephrin-b3-fc group had a survival of 0.15±0.10% with only 3 out of 6 animals showing any surviving transplanted NSPC, and the Fc fragments group (n=5; one animal died during the transplantation surgery) had a survival of 0.11±0.10% with only 2 out of 5 animals showing any surviving transplanted NSPC. 64
74 65 Figure 10
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
regenerative medicine in the brain and the spinal cord spinal cord injuries
 regenerative medicine in the brain and the spinal cord spinal cord injuries primary and secondary events during SCI traumatic spinal cord injury (SCI) traumatic spinal cord injury (SCI) main goal is to
regenerative medicine in the brain and the spinal cord spinal cord injuries primary and secondary events during SCI traumatic spinal cord injury (SCI) traumatic spinal cord injury (SCI) main goal is to
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System
 Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System Question No. 1 of 10 The human body contains different types of tissue. The tissue is formed into organs and organ systems.
Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System Question No. 1 of 10 The human body contains different types of tissue. The tissue is formed into organs and organ systems.
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Introduction to pathology lecture 5/ Cell injury apoptosis. Dr H Awad 2017/18
 Introduction to pathology lecture 5/ Cell injury apoptosis Dr H Awad 2017/18 Apoptosis = programmed cell death = cell suicide= individual cell death Apoptosis cell death induced by a tightly regulated
Introduction to pathology lecture 5/ Cell injury apoptosis Dr H Awad 2017/18 Apoptosis = programmed cell death = cell suicide= individual cell death Apoptosis cell death induced by a tightly regulated
Biology 218 Human Anatomy
 Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Symptoms of spinal cord injury:
 Symptoms of spinal cord injury: involuntary muscle spasms loss of voluntary movement sensation, balance control of breathing autonomic functions (blood pressure) bladder, sexual, bowel control All due
Symptoms of spinal cord injury: involuntary muscle spasms loss of voluntary movement sensation, balance control of breathing autonomic functions (blood pressure) bladder, sexual, bowel control All due
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
The cells of the nervous system
 The cells of the nervous system LESSON N.9 - PSYCHOBIOLOGY because of the location and volume as compared to our body, the brain has always been a matter of conjecture about its fundamental role in the
The cells of the nervous system LESSON N.9 - PSYCHOBIOLOGY because of the location and volume as compared to our body, the brain has always been a matter of conjecture about its fundamental role in the
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Nerve Cell Flashcards
 1. What does the word innervates mean? Refers to a nerve supplying a muscle or organ. For example, The phrenic nerve innervates the diaphragm muscle. 2. 3 parts of the Nervous System 1. Central Nervous
1. What does the word innervates mean? Refers to a nerve supplying a muscle or organ. For example, The phrenic nerve innervates the diaphragm muscle. 2. 3 parts of the Nervous System 1. Central Nervous
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Nervous system. Dr. Rawaa Salim Hameed
 Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
BIOL241 - Lecture 12a
 Cranial Nerves, source: training.seer.cancer.gov Nervous System Overview BIOL241 - Lecture 12a 1 Topics Divisions of the NS: CNS and PNS Structure and types of neurons Synapses Structure and function of
Cranial Nerves, source: training.seer.cancer.gov Nervous System Overview BIOL241 - Lecture 12a 1 Topics Divisions of the NS: CNS and PNS Structure and types of neurons Synapses Structure and function of
Human Histology The Nervous System. Dr. Rawaa Salim Hameed
 Human Histology The Nervous System Dr. Rawaa Salim Hameed The organization of the nervous system Anatomically, the nervous system is divided into:- Neurohistology Structurally, nerve tissue consists of
Human Histology The Nervous System Dr. Rawaa Salim Hameed The organization of the nervous system Anatomically, the nervous system is divided into:- Neurohistology Structurally, nerve tissue consists of
The Nervous System PART C. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
NURSE-UP INTRODUCTION TO THE NERVOUS SYSTEM
 NURSE-UP INTRODUCTION TO THE NERVOUS SYSTEM FUNCTIONS OF THE NERVOUS SYSTEM Body s primary communication and control system. Integrates and regulates body function Collects information specialized nervous
NURSE-UP INTRODUCTION TO THE NERVOUS SYSTEM FUNCTIONS OF THE NERVOUS SYSTEM Body s primary communication and control system. Integrates and regulates body function Collects information specialized nervous
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
Nervous System. Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI
 Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
NERVOUS TISSUE. 1. Functional units of the nervous system; receive, process, store and transmit information to other neurons, muscle cells or glands.
 NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
! BIOL 2401! Week 5. Nervous System. Nervous System
 Collin County Community College! BIOL 2401! Week 5 Nervous System 1 Nervous System The process of homeostasis makes sure that the activities that occur in the body are maintained within normal physiological
Collin County Community College! BIOL 2401! Week 5 Nervous System 1 Nervous System The process of homeostasis makes sure that the activities that occur in the body are maintained within normal physiological
Cells of the Nervous System
 Cells of the Nervous System Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal Cord (in the spine) Interprets sensory input, initiates movement, and mediates complex
Cells of the Nervous System Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal Cord (in the spine) Interprets sensory input, initiates movement, and mediates complex
Chapter 8 Nervous System
 Chapter 8 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Chapter 8 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A
 MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
THE NEUROBIOLOGY OF THE NEURON AND THE NEUROGLIA
 THE NEUROBIOLOGY OF THE NEURON AND THE NEUROGLIA DEFINITION OF A NEURON Neuron is the name given to the nerve cell and all its processes. Neurons are excitable cells that are specialized for the reception
THE NEUROBIOLOGY OF THE NEURON AND THE NEUROGLIA DEFINITION OF A NEURON Neuron is the name given to the nerve cell and all its processes. Neurons are excitable cells that are specialized for the reception
Human Anatomy and Physiology I Laboratory
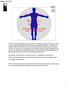 Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) Histology of Nervous Tissue, and 2) Spinal Cord, Spinal Nerves,
Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) Histology of Nervous Tissue, and 2) Spinal Cord, Spinal Nerves,
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Cell Injury MECHANISMS OF CELL INJURY
 Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
2401 : Anatomy/Physiology
 Dr. Chris Doumen Week 5 2401 : Anatomy/Physiology Introduction Neural Tissue TextBook Readings Pages 388 through 397. Make use of the figures in your textbook ; a picture is worth a thousand words! Work
Dr. Chris Doumen Week 5 2401 : Anatomy/Physiology Introduction Neural Tissue TextBook Readings Pages 388 through 397. Make use of the figures in your textbook ; a picture is worth a thousand words! Work
BI 232: Human Anatomy & Physiology
 BI 232: Human Anatomy & Physiology Roster Business Course Introduction and Syllabus Notecard Name E-mail Why you are taking the course Something interesting you did over break Lecture Tips Use the Study
BI 232: Human Anatomy & Physiology Roster Business Course Introduction and Syllabus Notecard Name E-mail Why you are taking the course Something interesting you did over break Lecture Tips Use the Study
Spinal Cord H. Ruth Clemo, Ph.D.
 Spinal Cord H. Ruth Clemo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be familiar with: 1. Surface anatomy of the spinal cord. 2. Internal structure and organization
Spinal Cord H. Ruth Clemo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be familiar with: 1. Surface anatomy of the spinal cord. 2. Internal structure and organization
Nervous system part 1. Danil Hammoudi.MD
 Nervous system part 1 Danil Hammoudi.MD The central nervous system (CNS) is formed by : the brain spinal cord. These elements are enclosed within the skull and spinal vertebral canal. They are covered
Nervous system part 1 Danil Hammoudi.MD The central nervous system (CNS) is formed by : the brain spinal cord. These elements are enclosed within the skull and spinal vertebral canal. They are covered
Unit Three. I. General Functions of the Nervous System. I. General Functions of the Nervous System
 10 Refer to the following URLs. It is a good idea to print them and bring them to class. Be sure to study these along with your book. http://www.sirinet.net/~jgjohnso/nervous.html http://faculty.washington.edu/chudler/ap.html
10 Refer to the following URLs. It is a good idea to print them and bring them to class. Be sure to study these along with your book. http://www.sirinet.net/~jgjohnso/nervous.html http://faculty.washington.edu/chudler/ap.html
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
The Nervous System: Neural Tissue Pearson Education, Inc.
 13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided
 Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Nervous System (Part A-1) Module 8 -Chapter 14
 Nervous System (Part A-1) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
Nervous System (Part A-1) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
Collin County Community College BIOL Week 5. Nervous System. Nervous System
 Collin County Community College BIOL 2401 Week 5 Nervous System 1 Nervous System The process of homeostasis makes sure that the activities that occur in the body are maintained within normal physiological
Collin County Community College BIOL 2401 Week 5 Nervous System 1 Nervous System The process of homeostasis makes sure that the activities that occur in the body are maintained within normal physiological
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
Adult Nervous System
 Adult Nervous System What is the capacity of the PNS and CNS for repair? WHY? Why discuss this now? Potential for repair depends on cellular properties of nerve and glial cells. http://neuroscience.uth.tmc.edu/s1/chapter09.html
Adult Nervous System What is the capacity of the PNS and CNS for repair? WHY? Why discuss this now? Potential for repair depends on cellular properties of nerve and glial cells. http://neuroscience.uth.tmc.edu/s1/chapter09.html
A. Subdivisions of the Nervous System: 1. The two major subdivisions of the nervous system:
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
Anatomy and Physiology 1 Chapters 12 and 13 self quiz Pro, Dima Darwish,MD.
 Anatomy and Physiology 1 Chapters 12 and 13 self quiz Pro, Dima Darwish,MD. 1) Which of the following is a function of the nervous system? A) sense the internal and external environments B) integrate sensory
Anatomy and Physiology 1 Chapters 12 and 13 self quiz Pro, Dima Darwish,MD. 1) Which of the following is a function of the nervous system? A) sense the internal and external environments B) integrate sensory
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Fundamentals of the Nervous System and Nervous Tissue: Part A
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 11 Fundamentals of the Nervous System and Nervous Tissue: Part A This is Your Brain on Music Assignment 1 With your
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 11 Fundamentals of the Nervous System and Nervous Tissue: Part A This is Your Brain on Music Assignment 1 With your
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
April 29, Neurophysiology. Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University,
 April 29, 2016 Neurophysiology Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University, Cells in the brain Neurons glia 1. Astrocytes 2. Microglia
April 29, 2016 Neurophysiology Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University, Cells in the brain Neurons glia 1. Astrocytes 2. Microglia
Anatomy of the Nervous System. Brain Components
 Anatomy of the Nervous System Brain Components NERVOUS SYSTEM INTRODUCTION Is the master system of human body, controlling the functions of rest of the body systems Nervous System CLASSIFICATION A. Anatomical
Anatomy of the Nervous System Brain Components NERVOUS SYSTEM INTRODUCTION Is the master system of human body, controlling the functions of rest of the body systems Nervous System CLASSIFICATION A. Anatomical
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Chapter 13. The Spinal Cord & Spinal Nerves. Spinal Cord. Spinal Cord Protection. Meninges. Together with brain forms the CNS Functions
 Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
NERVOUS SYSTEM 1 CHAPTER 10 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
Overview of the Nervous System A. Subdivisions of the Nervous System: 1. The two major subdivisions of the nervous system:
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
Nervous tissue, charachteristics, neurons, glial cells
 Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Functional Organization of Nervous Tissue. Nervous tissue, charachteristics, neurons, glial cells. The Nervous System. The Nervous System 21/12/2010
 Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Functions of the Nervous System. Fundamentals of the Nervous System & Nervous Tissue
 Fundamentals of the Nervous System & Nervous Tissue Overview Structure cell types & structures Neurophysiology membrane potential Synapse, neurotransmitters & receptors Functions of the Nervous System
Fundamentals of the Nervous System & Nervous Tissue Overview Structure cell types & structures Neurophysiology membrane potential Synapse, neurotransmitters & receptors Functions of the Nervous System
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
Reaction to Injury & Regeneration. Steven McLoon Department of Neuroscience University of Minnesota
 Reaction to Injury & Regeneration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dec 4 (Mon) Dec 6 (Wed) adult neurogenesis injury & regeneration Dec 8 (Fri) research paper
Reaction to Injury & Regeneration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dec 4 (Mon) Dec 6 (Wed) adult neurogenesis injury & regeneration Dec 8 (Fri) research paper
Chapter 12 The Nervous System INTRODUCTION TO THE NERVOUS SYSTEM. Central Nervous System (CNS): STRUCTURE BRAIN SPINAL CORD NERVES
 Chapter 12 The Nervous System PowerPoint by John McGill Supplemental Notes by Beth Wyatt INTRODUCTION TO THE NERVOUS SYSTEM STRUCTURE BRAIN SPINAL CORD NERVES Central Nervous System (CNS): Brain Spinal
Chapter 12 The Nervous System PowerPoint by John McGill Supplemental Notes by Beth Wyatt INTRODUCTION TO THE NERVOUS SYSTEM STRUCTURE BRAIN SPINAL CORD NERVES Central Nervous System (CNS): Brain Spinal
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 7 Nerve tissue 1 Liu Jiamei
 Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Neurogenesis in Adult Central Nervous System: Death of a Dogma
 Aristotle University of Thessaloniki, Greece, Nov. 2007 Neurogenesis in Adult Central Nervous System: Death of a Dogma Anton B. Tonchev Division of Cell Biology, Varna University of Medicine, Bulgaria
Aristotle University of Thessaloniki, Greece, Nov. 2007 Neurogenesis in Adult Central Nervous System: Death of a Dogma Anton B. Tonchev Division of Cell Biology, Varna University of Medicine, Bulgaria
(3) Chemical synapse ---structure
 (3) Chemical synapse ---structure LM: in silver preparation dark brown color button-liked on the surface of cell body and dendrites called synaptic button LM: synaptic button (3) Chemical synapse ---structure
(3) Chemical synapse ---structure LM: in silver preparation dark brown color button-liked on the surface of cell body and dendrites called synaptic button LM: synaptic button (3) Chemical synapse ---structure
Chapter 11: Functional Organization of Nervous Tissue
 Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Chapter 17 Nervous System
 Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Man and his environment
 Man and his environment Dr. Elriah M. Makie 0122858517 Nervous Tissue BSc.M.Sc.MBBS Introduction The nervous system is divided into two main parts: The central nervous system (CNS) comprising the brain
Man and his environment Dr. Elriah M. Makie 0122858517 Nervous Tissue BSc.M.Sc.MBBS Introduction The nervous system is divided into two main parts: The central nervous system (CNS) comprising the brain
Chapter 7 Nervous System
 Chapter 7 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Chapter 7 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
TABLE OF CONTINENTS. PSYC1002 Notes. Neuroscience.2. Cognitive Processes Learning and Motivation. 37. Perception Mental Abilities..
 TABLE OF CONTINENTS Neuroscience.2 Cognitive Processes...21 Learning and Motivation. 37 Perception.....54 Mental Abilities.. 83 Abnormal Psychology....103 1 Topic 1: Neuroscience Outline 1. Gross anatomy
TABLE OF CONTINENTS Neuroscience.2 Cognitive Processes...21 Learning and Motivation. 37 Perception.....54 Mental Abilities.. 83 Abnormal Psychology....103 1 Topic 1: Neuroscience Outline 1. Gross anatomy
THE NERVOUS SYSTEM. Station 9 : THE SPINAL CORD
 Station 9 : THE SPINAL CORD The spinal cord is a long thin bundle of nerve cells that extends from the medulla of the brainstem all the way down the vertebral column. The spinal cord is made up of gray
Station 9 : THE SPINAL CORD The spinal cord is a long thin bundle of nerve cells that extends from the medulla of the brainstem all the way down the vertebral column. The spinal cord is made up of gray
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
25/12/50. Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury
 2 Karimi-Abdolrezaee et al. J Neuroscience 26(13):3377-89; (2006) Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury
2 Karimi-Abdolrezaee et al. J Neuroscience 26(13):3377-89; (2006) Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury
Chapter 7. The Nervous System
 Chapter 7 The Nervous System General overview of the nervous system functions Sensory input (info travels in along afferent pathways) Integration (information is processed) Sensory neurons Spinal cord
Chapter 7 The Nervous System General overview of the nervous system functions Sensory input (info travels in along afferent pathways) Integration (information is processed) Sensory neurons Spinal cord
CNS third year med students Summary of midterm material H Awad
 CNS third year med students 2018 Summary of midterm material H Awad Dear All This presentation summaries the main important topics covered in the midterm material ( lectures 1-6) There will be two questions
CNS third year med students 2018 Summary of midterm material H Awad Dear All This presentation summaries the main important topics covered in the midterm material ( lectures 1-6) There will be two questions
Meyers' A&P February 15, Unit 7. The Nervous System. I. Functions of the Nervous System. Monitors body's internal and external enviornments
 Unit 7 The Nervous System I. Functions of the Nervous System Monitors body's internal and external enviornments Integrates sensory information Coordinates voluntary & involuntary responses of many other
Unit 7 The Nervous System I. Functions of the Nervous System Monitors body's internal and external enviornments Integrates sensory information Coordinates voluntary & involuntary responses of many other
Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury
 Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D., Ph.D., Satoshi Tateda, M.D., Kenichiro Yahata, M.D.,
Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D., Ph.D., Satoshi Tateda, M.D., Kenichiro Yahata, M.D.,
The Spinal Cord & Spinal Nerves
 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward and downward travel
The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward and downward travel
Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for
 Chapter 13 Outline Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for what you need to know from Exhibits 13.1 13.4 I. INTRODUCTION A. The spinal cord and spinal nerves
Chapter 13 Outline Note: Please refer to handout Spinal Plexuses and Representative Spinal Nerves for what you need to know from Exhibits 13.1 13.4 I. INTRODUCTION A. The spinal cord and spinal nerves
The Nervous System PART A
 7 The Nervous System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Structural Classification
7 The Nervous System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Structural Classification
3/15/17. Outline. Nervous System - PNS and CNS. Two Parts of the Nervous System
 Nervous System - PNS and CNS Bio 105 Outline I. Central Nervous System vs Peripheral Nervous System II. Peripheral Nervous System A. Autonomic Nervous Systems B. Somatic Nervous Systems III. Autonomic
Nervous System - PNS and CNS Bio 105 Outline I. Central Nervous System vs Peripheral Nervous System II. Peripheral Nervous System A. Autonomic Nervous Systems B. Somatic Nervous Systems III. Autonomic
Nervous System - PNS and CNS. Bio 105
 Nervous System - PNS and CNS Bio 105 Outline I. Central Nervous System vs Peripheral Nervous System II. Peripheral Nervous System A. Autonomic Nervous Systems B. Somatic Nervous Systems III. Autonomic
Nervous System - PNS and CNS Bio 105 Outline I. Central Nervous System vs Peripheral Nervous System II. Peripheral Nervous System A. Autonomic Nervous Systems B. Somatic Nervous Systems III. Autonomic
The Nervous System. Lab Exercise 29. Objectives. Introduction
 Lab Exercise The Nervous System Objectives -You should be able to recognize a neuron and identify its components. - Be able to identify the principal components of the brain and be able to name at least
Lab Exercise The Nervous System Objectives -You should be able to recognize a neuron and identify its components. - Be able to identify the principal components of the brain and be able to name at least
Nervous Tissue. Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology
 Nervous Tissue Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology Controls and integrates all body activities within limits that maintain life Three basic functions 1. sensing changes with
Nervous Tissue Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology Controls and integrates all body activities within limits that maintain life Three basic functions 1. sensing changes with
Spinal Cord Protection. Chapter 13 The Spinal Cord & Spinal Nerves. External Anatomy of Spinal Cord. Structures Covering the Spinal Cord
 Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
The nervous system regulates most body systems using direct connections called nerves. It enables you to sense and respond to stimuli
 The nervous system regulates most body systems using direct connections called nerves. It enables you to sense and respond to stimuli The basic function of nervous system are: Receive sensory input internal
The nervous system regulates most body systems using direct connections called nerves. It enables you to sense and respond to stimuli The basic function of nervous system are: Receive sensory input internal
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury
 Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury James Guest MD, PhD, FACS Clinical Professor of Neurological Surgery, Neurosurgery and the Miami Project to
Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury James Guest MD, PhD, FACS Clinical Professor of Neurological Surgery, Neurosurgery and the Miami Project to
Structural Organization of Nervous System
 Nervous System Structural Organization of Nervous System Myelinated Neuron Myelin White, fatty material which covers nerve fibers(axons) Protects and insulates fiber Increases the rate of transmission
Nervous System Structural Organization of Nervous System Myelinated Neuron Myelin White, fatty material which covers nerve fibers(axons) Protects and insulates fiber Increases the rate of transmission
action potential afferent neuron Weblike; specifically, the weblike middle layer of the three meninges. arachnoid astrocytes autonomic nervous system
 action potential A large transient depolarization event, including polarity reversal, that is conducted along the membrane of a muscle cell or a nerve fiber. afferent neuron Nerve cell that carries impulses
action potential A large transient depolarization event, including polarity reversal, that is conducted along the membrane of a muscle cell or a nerve fiber. afferent neuron Nerve cell that carries impulses
