Cancer develops as a result of the accumulation of somatic
|
|
|
- Shona Fleming
- 5 years ago
- Views:
Transcription
1 Cancer-mutation network and the number and specificity of driver mutations Jaime Iranzo a,1, Iñigo Martincorena b, and Eugene V. Koonin a,1 a National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethesda, MD 20894; and b Wellcome Trust Sanger Institute, CB10 1SA Hinxton, Cambridgeshire, United Kingdom Contributed by Eugene V. Koonin, May 9, 2018 (sent for review February 21, 2018; reviewed by Katerina Gurova and Sergei Maslov) Cancer genomics has produced extensive information on cancerassociated genes, but the number and specificity of cancer-driver mutations remains a matter of debate. We constructed a bipartite network in which 7,665 tumors from 30 cancer types are connected via shared mutations in 198 previously identified cancer genes. We show that about 27% of the tumors can be assigned to statistically supported modules, most of which encompass one or two cancer types. The rest of the tumors belong to a diffuse network component suggesting lower gene specificity of driver mutations. Linear regression of the mutational loads in cancer genes was used to estimate the number of drivers required for the onset of different cancers. The mean number of drivers in known cancer genes is approximately two, with a range of one to five. Cancers that are associated with modules had more drivers than those from the diffuse network component, suggesting that unidentified and/or interchangeable drivers exist in the latter. cancer types driver mutations passenger mutations bipartite networks community detection Cancer develops as a result of the accumulation of somatic mutations and other genetic alterations that impair celldivision checkpoints, resulting in abnormal cell proliferation and eventually tumorigenesis (1, 2). Such mutations are known as drivers, and their characterization is central to understanding the early steps of tumor progression (3, 4). During the last decade, comparative analyses of large collections of cancer genomes have led to the identification of overlapping sets of genes that are typically associated to cancer, i.e., that harbor a significant excess of mutations in tumors and show signatures of positive selection (5 11). The continued identification of cancer genes provides insights into the processes and pathways involved in tumorigenesis as well as possible therapy targets (12 17). Like any other gene in the genome, cancer genes are expected to accumulate passenger mutations that do not contribute to or even hinder cancer progression (18 20). Therefore, although cancer genes often harbor driver mutations, only a fraction of the mutations found in these genes are actual drivers (9 11). Distinguishing driver mutations from passenger ones poses a formidable challenge for cancer genomics. The number of driver mutations required for the onset of cancer is a fundamental question that remains a matter of debate (3, 9, 21 23). Classical approaches to this problem use age incidence curves to infer the number of rate-limiting steps in tumorigenesis, each of which is assumed to be associated with a unique driver mutation; these estimates, however, are sensitive to changes in mutation and replication rates during tumor progression (22 25). A modified method has been proposed that compares the incidence of cancer across risk groups with different mutation rates, but this approach applies only to cancers with relatively well-defined risk groups, such as lung and colorectal cancer (21). Recent measurements of selection in cancer genomes have provided quantitative estimates of the number of positively selected mutations, i.e., drivers, per tumor, ranging from less than one in thyroid and testicular cancers to more than 10 in endometrial and colorectal cancers (9). Given the novelty of these findings, a comparison with independent inference approaches appears highly desirable. A second major question about cancer-driver mutations refers to their specificity in different cancer types. Some tumors show recurrent mutation patterns, such as the oncogenic fusion BCR- ABL in chronic myeloid leukemia (26) or the inactivation of specific tumor suppressors, e.g., RB1 in retinoblastoma (27). Other tumors appear to result from interchangeable mutations in a pool of genes involved in key signaling pathways, such as the receptor tyrosine kinase/ras/raf pathway in lung adenocarcinoma (28). Between these two extremes, intermediate degrees of specificity are observed in many cancer types (8, 29, 30). Furthermore, although numerous recent studies on cancer mutational landscapes have yielded extensive lists of genes that are mutated in various cancers (31 33), a quantitative understanding of the extent to which the current tumor classification captures the existence of specific sets of driver mutations is lacking. Here, we combine tools for network analysis and multivariate statistics to assess the number and specificity of cancer-driver mutations in 30 cancer types. We show that an unsupervised community-detection approach applied to the bipartite network of somatic mutations in cancer recovers modules consisting of mutually specific tumors and genes that (i) are consistent with the tumor histology and (ii) are enriched in putative driver mutations. We used multivariate statistical analysis to estimate the characteristic number of driver mutations in known cancer genes required for the onset of each cancer type. Notably, the average age of onset for different cancer types correlates with the predicted number of drivers. Furthermore, cancers that are not associated with the specific Significance Cancer genomics yields a wealth of information on cancerassociated mutations in various cancer types, but current understanding of the number and tissue specificity of the driver mutations remains limited. We applied mathematical methods for network analysis to identify distinct modules linking tumors to driver mutations. About 27% of the tumors belong to such modules, whereas the rest form a diffuse component of the gene tumor network. The cancers from the diffuse component show an onset later in life than those in the modules and have fewer associated known drivers, implying the existence of multiple unidentified and/or interchangeable drivers in the former. Author contributions: J.I. and E.V.K. designed research; J.I. performed research; J.I., I.M., and E.V.K. analyzed data; and J.I. and E.V.K. wrote the paper. Reviewers: K.G., Roswell Park Cancer Institute; and S.M., University of Illinois at Urbana Champaign. The authors declare no conflict of interest. Published under the PNAS license. 1 To whom correspondence may be addressed. jaime.iranzosanz@nih.gov or koonin@ ncbi.nlm.nih.gov. This article contains supporting information online at /pnas /-/DCSupplemental. Published online June 12, E6010 E6019 PNAS vol. 115 no. 26
2 modules in the gene tumor network appear later in life than expected based on the general trend. Results Cancer Mutational Landscape as a Partially Modular Network. Somatic mutations in a set of tumors can be collectively represented as a bipartite network, that is, a network with two classes of nodes. In such a network, nodes of one class correspond to tumor samples, and nodes of the other class correspond to cancer genes. Mutations are represented as edges that connect each tumor sample with the genes mutated in it; conversely, each gene is linked to the tumors in which it carries a mutation(s). Using this approach, we built the network of somatic mutations from The Cancer Genome Atlas (TCGA), a collection that consists of 7,665 tumor samples from 30 cancer types (Fig. 1 A and B), focusing on coding mutations in 198 recurrently mutated cancer genes (Methods). The structure of the network is characterized by a relatively long-tailed degree distribution (i.e., the distribution of the number of connections between a given node and other nodes in the network) (Fig. 1C), which is indicative of the pronounced heterogeneity in the frequencies of mutations across genes and tumors. Also, the network has small clustering coefficients (i.e., the fraction of neighbors of a node that share connections with a second node) (Fig. 1D), due to the sparsity of mutations in the dataset. Within the bipartite network framework, the association between mutually specific sets of genes and cancer types becomes manifest by the existence of groups of nodes (tumors and genes) that are densely connected with members of the same group but poorly connected with the rest of the network. Such groups are called modules, and a network with such structure is said to be modular (34). We tested the modular nature of the cancer mutation network by computing its Barber s modularity index (35) and comparing it with 200 random networks with the same degree distribution (Fig. 1E). The result of this comparison supported the existence of a significant degree of mutual specificity between cancer types and cancer genes (P < 10 20,Welch s t test), which demonstrates the ability of the network approach to detect a wellknown feature of cancer mutational landscapes (3, 8, 29). To further investigate the specificity of the mutation landscapes, we identified the modules of the network and assessed their statistical significance. To that end, we first applied a battery of module-detection algorithms and then pruned all genes and tumors for which the specificity patterns were compatible with a random null model (Methods). The analysis revealed the existence of 12 modules with a significance threshold P < Each of these modules contains tumors and genes that are, to a certain extent, mutually specific. Accordingly, tumors in a module typically harbor mutations in genes from the same module, whereas the constituent genes are more frequently mutated in tumors that belong to the same module. Overall, the statistically significant modules comprise 27% of the samples and 66 (33%) cancer-associated genes (Table 1). Before proceeding with a more detailed dissection of the genes and cancer types represented in each module, we evaluated their biological relevance by characterizing the mutations that correspond to intra- and intermodule connections. To that end, we split the cancer-associated genes into oncogenes and tumor-suppressor genes (TSGs) and for each of these groups calculated the percentage of coding mutations that truncate the gene product and the percentage of copy number alterations that involve gene loss. We obtained these percentages separately for mutations affecting samples and genes that belong to the same module (intramodule mutations) and for those involving samples and genes from different modules (intermodule mutations). Because driver mutations in TSGs entail loss of function (11, 36), the percentage of truncations and large deletions in TSGs is indicative of the enrichment of a set of mutations in putative drivers. In the case of oncogenes, driver mutations are typically missense substitutions in mutational hotspots and/or gene amplifications (11, 36). Accordingly, the enrichment of a set of oncogene mutations in putative drivers results in a smaller percentage of truncations and deletions. As shown in Fig. 1F, intramodule mutations in TSGs include a significantly higher percentage of truncations than intermodule mutations, whereas the opposite holds for oncogenes. A similar trend is observed in copy number variation data: Compared with intermodule alterations, intramodule alterations encompass a significantly higher percentage of losses when affecting TSG and, a significantly higher percentage of amplifications when affecting oncogenes. Overall, we conclude that intramodule mutations are significantly enriched in putative drivers compared with intermodule mutations. In other words, mutations that affect mutually specific genes and tumors (i.e., genes and samples from the same module) are more likely to be cancer drivers than those affecting genes and samples that belong to different modules. To test whether intermodule mutations in oncogenes and TSGs include any drivers or only a background of passenger mutations, we obtained baseline values for the percentage of truncations and gene losses in genes that are not associated with cancer. Mutations in those genes are expected to be passengers and thus serve as a reference of the percentage of truncations and deletions expected for passenger mutations. The deviations with respect to the baseline shown in Fig. 1F imply that some intermodule mutations involving oncogenes and TSGs and gene losses involving TSGs are also relevant for tumor progression. Such deviations remain after removing the most widespread cancer genes across tissues (TP53, PIK3CA, and ARID1A), indicating that potential intermodule drivers are not limited to such genes (SI Appendix, Fig.S1). A closer inspection of intermodule mutations highlights the oncogenes BRAF (primarily assigned to the melanoma/thyroid cancer module 12; see below) and IDH1 (primarily assigned to the lowergrade glioma module 6) as major sources of intermodule drivers. Thus, mutations in BRAF are likely drivers in melanomas from module 11, and mutations in IDH1 are likely drivers in acute myeloid leukemias (module 9), with missense mutations representing 96% of the coding mutations in both genes compared with the 84% baseline in genes not associated to cancer. Similarly, the TSGs STAG2, KDM6A, PIK3R1, MAP3K1, and CDH1, with 50 60% of truncating mutations (baseline is 16%), constitute probable intermodule drivers, with MAP3K1 and CDH1 being more relevant in breast cancer. Specificity Modules for Cancer Types and Cancer Genes. As shown in Table 1, the composition of the gene-tumor specificity modules strongly correlates with the histological classification of tumors. Most modules include tumors from one or two cancer types, together with genes for which mutation frequencies are significantly higher in cancers of those types (Fig. 2). The mutual specificity analysis recovers some well-established features of cancer mutational landscapes, such as the associations between thyroid cancer and BRAF and between colorectal cancer and APC, KRAS, TP53, and SMAD4. The colorectal cancer module includes two additional genes that are mutated in a smaller fraction of samples, namely, ubiquitin ligase FBXW7 and transcription factor TCF7L2. Most samples from pancreatic adenocarcinoma cluster in the same module as colorectal cancer, in agreement with a significant excess of mutations in KRAS, TP53, and SMAD4 in both tumor types. Acute myeloid leukemia constitutes a single module, with the genes DNMT3A, FLT3, and NPM1 mutated in >30% of samples and CEBPA, IDH2, RUNX1, andwt1 mutated at lower frequencies. Testicular germ cell cancer, head and neck squamous cell carcinoma, and urothelial bladder carcinoma also form separate modules, which, however, comprise a smaller fraction of the samples from each cancer type. The sets of associated genes include KIT in testicular cancer; NOTCH1, CASP8, HLA-A, and HRAS in head and neck cancer; and FGFR3, KDM6A, and STAG2 in bladder cancer. Clear cell kidney carcinoma clusters MEDICAL SCIENCES PNAS PLUS Iranzo et al. PNAS vol. 115 no. 26 E6011
3 Fig. 1. Structure of the cancer mutation network. (A) Bipartite network of somatic mutations in tumors from the TCGA. Samples are arranged by cancer type along the x axis (black/gray/white bars); cancer genes are sorted by module along the y axis (colors indicate module assignations). Samples from the same cancer type and genes from the same module are sorted by number of connections. The upper and left semiaxes contain genes and samples that belong to statistically significant modules. The rest of the nodes were assigned to the best-match extended module with which they share the highest similarity (see text); they are representedinthe lower (genes) and right (samples) semiaxes. Links connect samples and genes affected by at least one nonsynonymous somatic mutation. Links between two nodes from the same module (intramodule links) are drawn in distinctive colors; intermodule links appear in gray. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower-grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma. (B) Alternative representation of the cancer mutation network with a force-directed drawing method. Node colors indicate module assignations. (C) Node degree distribution per sample (black) and per gene (blue). (D) Clustering coefficient of samples (black) and genes (blue) as a function of the node degree (bipartite clustering coefficient calculated as in ref. 67). (E) Modularity of the cancer mutation network, quantified by its Barber s modularity index (Q b ) and compared with 200 random networks with the same degree distribution. The modularity distribution for the original network results from 200 realizations of the communitydetection algorithm, each yielding slightly different sets of modules. The lack of overlap reveals a highly significant modular structure (P < 10 20,Welch s t test). (F) Differences in the functional spectrum of mutations between intramodule and intermodule links (significant modules only). In TSGs, higher percentages of truncating mutations (with respect to all coding mutations) and severe losses [with respect to all copy number variants (CNV)] indicate enrichment in putative drivers. For oncogenes (OG), driver enrichment is associated with lower percentages of truncating mutations and severe losses. *P < 0.05, ***P < E Iranzo et al.
4 Table 1. Module no. Composition of the 12 statistically significant modules in the cancer mutation network Cancer types with genes VHL, PBRM1, SETD2,andBAP1, among others. Some samples from mesothelioma and papillary kidney carcinoma are also assigned to that module, mostly because of mutations in SETD2 and BAP1. Notably, some cancer types are distributed among more than one module. Thus, glioblastoma is represented in two modules, one characterized by mutations in EGFR and PTEN and the other by mutations in IDH1, ATRX, and TP53. Such subdivision is consistent with previous reports, which relate the second group with the glioblastoma-cpg island methylator phenotype (37 39). The same module also includes most samples from lower-grade glioma and some representatives from sarcoma (although the latter typically lack mutations in IDH1). Similarly, melanoma is divided into tumors with mutated BRAF on a low mutational background, which cluster with thyroid cancer, and samples with mutations in a larger set of genes (including NRAS, KDR, ILR7, and PTPRB), which constitute a separate module. Finally, breast cancer splits between a breast cancer-only module characterized by mutations in GATA3 and TBX3 and a larger module that includes uterine (endometrial and carcinosarcoma) and prostate cancers, with PIK3CA as the signature gene. In terms of histological types, the PIK3CA module is significantly enriched in lobular breast tumors (46% compared with 13% in the GATA3/ TBX3 module and 17% in the entire dataset; P < 10 6, χ 2 test). Genes mutated in >30% tumors from the module Genes mutated in <30% tumors from the module 1 Bladder (BLCA, 10%) FGFR3, KDM6A, STAG2 ERCC2, EP300 2 Breast (BRCA, 12%) GATA3, TBX3 3 Endometrium (UCEC, 57%], uterus-cs (UCS, 24%), breast (BRCA, 17%), prostate (PRAD, 6%), stomach (STAD, 6%) ARID1A, PIK3CA, PTEN BCOR, CBFB, CCND1, CDH1, CTNNB1, FGFR2, FOXA1, MAP2K4, MAP3K1, MAX, MED12, PIK3R1, PPP2RIA, RUNX1, SPOP 4 Colon (COAD, 41%), rectum (READ, 62%], APC*, KRAS, TP53 FBXW7, SMAD4, TCF7L2 pancreas (PAAD, 36%), uterus-cs (UCS, 11%) 5 Glioblastoma (GBM, 13%) EGFR PTEN 6 Liver (LGG, 38%), sarcoma (SARC, 9%), ATRX, IDH1, TP53 glioblastoma (GBM, 6%) 7 Head and neck (HNSC, 6%) CASP8, HLA-A, HRAS, NOTCH1 8 Kidney-RCC (KIRC, 40%), mesothelioma PBRM1, SETD2, VHL ATM, BAP1, KDM5C, NF2, PTPN11 (MESO, 16%), Kidney-RP(KIRP, 6%) 9 AML (LAML, 38%) DNMT3A, FLT3, NPM1 CEBPA, IDH2, RUNX1, WT1 10 Testis (TGCT, 25%) KIT 11 Melanoma (SKCM, 14%) IL7R, KDR, NRAS, PDGFRA, CARD11, CBLB, MET, PPP6C, RAC1 PTPRB 12 Thyroid (THCA, 72%), melanoma (SKCM, 15%) BRAF AKT1 Tumor samples are grouped by cancer type, with the TCGA abbreviated name in parentheses. The percentage next to the TCGA abbreviation indicates the fraction of tumors from that class that are present in the module (only classes represented by >5% of their samples are shown). Genes are presented in two columns according to the prevalence of mutations within the module. AML, acute myeloid leukemia; kidney-rcc, kidney renal clear cell carcinoma; kidney-rp, kidney renal papillary cell carcinoma; READ, rectum adenocarcinoma; UCEC, uterine corpus endometrial carcinoma; uterus-cs, uterine carcinosarcoma. Other cancer abbreviations are given in the legend of Fig. 1 and in SI Appendix, Table S1. *Mutations in APC are typically absent from pancreatic cancer. Glioblastoma samples in this module belong to the glioblastoma-cpg island methylator phenotype subtype. Mutations in IDH1 are typically absent from sarcoma. Table 2. In terms of the molecular subtypes, both modules include breast tumors that mostly belong to the luminal subtype (estrogen receptor-positive), whereas most of the basal-like breast tumors are not assigned to any significant module (P < 10 10, χ 2 test). Among all the modules, the one that combines breast, uterine, and prostate cancers stands out for its size and diversity. This module contains the largest number of genes, with many of those mutated in less than 30% of the samples. Moreover, two of its constituent histologies (breast cancer and uterine carcinosarcoma) are split between this and other modules. To further dissect the specificity of the mutations affecting these cancer types, we reanalyzed the subnetwork composed by all cancer genes and samples from breast, prostate, and uterine (endometrial and carcinosarcoma) cancers. This analysis yielded four significant modules that are dominated by each of the four cancer types (Table 2). The list of module-specific genes is consistent with the findings of the global analysis. Notably, the reanalysis places most breast cancer samples inasinglemodulewithgenescdh1, PIK3CA, GATA3,andTBX3, whereas uterine carcinosarcoma clusters with genes FBXW7, PPP2RIA, and TP53 (the samples without mutations in PPP2RIA were formerly assigned to the colorectal cancer module). Specific modules for prostate and endometrial cancer are also clearly delineated, the former with SPOP and FOXA1 and the latter with ARID1A, CTNNB1, PI3KR1, andpten, among other genes. Modules in the subnetwork of breast, prostate, endometrial, and uterine cancers Genes Breast, % Prostate, % Endometrium, % Uterus-CS, % MEDICAL SCIENCES PNAS PLUS CBFB, CDH1, GATA3, MAP2K4, MAP3K1, PIK3CA, RUNX1, TBL1XR1, TBX3 ARID1A, BCOR, CCND1, CIC, CTNNB1, CUX1, ESR1, FGFR2, KRAS, MAX, PIK3CA, PIK3R1, PTEN FOXA1, SPOP FBXW7, PPP2RIA, TP Numbers indicate the percentage of samples from a given cancer type associated with the module. Iranzo et al. PNAS vol. 115 no. 26 E6013
5 Fig. 2. Cancer genes mutated at significantly distinct rates in different modules and cancer types. Tumors that do and do not belong to specificity modules are shown in A and B, respectively. Owing to their mixed nature, bladder, prostate, head and neck, and testicular cancers appear twice, with samples assigned to significant modules in A and the rest in B. Only genes that belong to specificity modules are shown. Significance was evaluated with a two-tailed Fisher s exact test; red and blue indicate that mutations in a gene are over- and underrepresented, respectively, in a group of tumors. Two Major Modes of Driver Accumulation. The statistically significant modules of mutual tumor gene specificity include 27% of the tumors in the TCGA. There are at least two alternative explanations for why 73% of samples remain unassigned. The first possibility is that, despite having mutational patterns compatible with one of the modules, the unassigned samples do not reach the required threshold of statistical significance. That would be the case if mutations affecting module-specific genes occurred in noncoding regions, involved copy number variants, or occurred in functionally equivalent genes not included in our list of cancer genes. The second possibility is that unassigned samples account for the exchangeability of cancer genes. Under such a scenario, some cancer types might not be specifically associated to any set of genes. To evaluate the first possibility, we built a set of best-match extended modules by attaching unassigned samples and genes to the module with which they shared the most connections (SI Appendix, Table S2). We would expect that, if the specific association between tumors and genes held for most samples within a cancer type, the extended modules would recover unassigned samples from the same cancer types as those already assigned to the original modules. Indeed, the best-match extended modules cluster >75% of the samples from rectum, pancreas, kidney (clear cell), acute myeloid leukemia, thyroid, and melanoma, and 50 75% of the samples from lower-grade glioma, mesothelioma, colon, and endometrial cancer in a tissue-specific way (Fig. 3A). In contrast, cancer types that were absent from the original modules, such as stomach and lung, appear distributed among multiple extended modules, with typically <25% of the samples assigned to the same module (Fig. 3B). The only exception is ovarian cancer, which does not appear in any of the significant modules although 70% of the samples are recovered as members of the same extended module as lower-grade glioma. The apparent cause is the tight association between ovarian cancer and TP53, which is mutated in almost 90% of samples, against a low background of somatic mutations (40). We confirmed the existence of a diffuse (nonspecific) mode of driver accumulation by running the module-detection pipeline without removal of nonsignificant members. The resulting set of statistically relaxed modules consists of 12 modules with a counterpart among the original (statistically significant) modules, 16 minor modules with a single gene each and small sample sizes, and a giant, nonsignificant module that includes 20% of the samples and 90 (45%) genes (SI Appendix, Table S3). The appearance of nonsignificant (pseudo)modules is a well-known artifact that results from applying module detection algorithms to large networks with partial or no modular structure (41, 42). In the context of cancers and the associated genes, the giant pseudomodule accounts for the nonmodular (diffuse) component of the cancer mutation network, which comprises exchangeable cancer genes that are not specifically associated with particular groups of tumors. Cancer types differ with respect to their contribution to this component. Thus, only 10 20% of samples from cancer types that are represented in the original modules are assigned to the diffuse component, whereas the fraction rises to more than 25% in other cancers (P < 0.01, Wilcoxon test) (Fig. 3 A and B). Taken together, these findings indicate that cancer types can be conceptually split into two major groups: (i) those that accumulate driver mutations in specific sets of cancer genes and are accordingly clustered into distinct modules (Table 1) and (ii) those that accumulate exchangeable driver mutations in a non tissuespecific manner, such as stomach and lung cancers. Although most cancer types can be clearly placed in one of those two extreme categories, there are some mixed cases (Fig. 3C). For example, bladder cancer generates its own module that includes genes KDM6A, FGFR3, and STAG2. However, only 10% of bladder tumors are assigned to that module (30% in the best-match extension), whereas about 40% belong to the diffuse component and accumulate mutations in a less specific manner (Fig. 2B). Similar, albeit less extreme, trends are observed for head and neck and testicular cancers. Such a mixed mutational landscape, modular for some subsets of the samples but nonspecific for others, seems to mirror the heterogeneity of these cancer types. E Iranzo et al.
6 Copy Number Alterations. We further explored the specificity of driver events by jointly considering somatic mutations and copy number alterations that affect cancer genes. To reduce the number of noninformative connections, only amplifications of oncogenes and losses of TSGs were added to the network of somatic mutations. The addition of copy number alterations does not significantly change the results described so far (SI Appendix, Tables S4 and S5). The same significant modules were recovered, although their composition in terms of cancer types is slightly less clear. Besides the modules described above, the addition of the copy number alterations resulted in five new modules, none of which showed an obvious correspondence with a particular cancer type. Four of these modules are associated with arm-level alterations affecting chromosome arms 7q (genes BRAF, EZH2, MET, and SMO), 9q (genes ABL1, GNAQ, and KLF4), and 17p (genes MAP2K4 and NCOR1) and the X chromosome region Xp11 (genes BCOR, GATA1, KDM5C, andkdm6a). Thefifthofthesenew modules accounts for the frequent loss of 56 cancer genes (mostly TSGs) distributed across the genome. Moreover, some genes in which copy number alterations are more recurrent than somatic mutations, such as ERBB1 in breast cancer, become assigned to significant modules. Overall, the inclusion of oncogene amplifications and TSG losses does not reveal the existence of specific modules that were not already detected by the analysis of somatic mutations, although this outcome could be biased by the fact that most cancer genes in our study were identified through mutations. The minor changes in the network structure caused by the inclusion of copy number alterations seem related to the chromosomal location of cancer genes and the opposite trends toward amplification and deletion in oncogenes and TSG, respectively. Average Number of Driver Mutations. Identification of driver mutations is confounded by the numerous passenger mutations that are typically found in cancer genomes. Passenger mutations in the coding regions appear to dominate even in cancer genes (9, 43), resulting in a strong correlation between the number of mutations in cancer genes and noncancer (passenger) genes (R = 0.87, P < )(SI Appendix, Fig.S2). To obtain an estimate of the number of driver mutations in different tumors, we built a general linear model with the number of coding mutations (substitutions and small indels) in cancer genes as the dependent variable, the number of coding mutations in putative passenger genes as the explanatory variable, and the cancer type as grouping factor. Due to the pervasive abundance of passenger mutations, a major feature of the model is the strong correlation between mutations in cancer genes Fig. 3. Classification of cancer types according to the gene specificity of their driver mutations. (A and B) Fraction of samples assigned to statistically significant (solid bars) and best-match extended (semitransparent bars) modules obtained by reassigning nonsignificant samples and genes to the significant modules with which they share the largest number of connections. Black diamonds indicate the fraction of samples assigned to the largest nonsignificant pseudomodule. Cancer types without major contributions to any significant module are shown in B. Bar colors refer to the best-match extended module that contains most samples from each type. (C) Principal component analysis of cancer types based on the fraction of samples assigned to statistically significant modules, best-match extended modules, and the largest nonsignificant pseudomodule. The percentages of the total variance explained by the first and second components are 88.5% and 8.6%, respectively. Special cases discussed in the text are labeled: BLCA, bladder cancer; HNSC, head and neck cancer; OV, ovarian cancer; PRAD, prostate cancer; TGCT, testicular cancer. and noncancer genes. In this context, the intercepts (which depend on the cancer type) can be interpreted as the excess of mutations in cancer genes that is not attributable to the same causes that lead to the accumulation of mutations in passenger genes. Thus, these intercepts constitute a proxy for the number of driver mutations in known cancer genes. We found that 75% of the variance in the number of mutations in cancer genes is explained by the number of mutations in passenger genes [P < 10 20, analysis of covariance (ANCOVA)], which is indicative of a common trend of mutation accumulation in both gene classes (Fig. 4A). Considering all tumors together and controlling for the nonuniform abundances of different cancer types, the mean number of driver mutations per tumor in known cancer genes was estimated as 1.72 ± 0.09 (95% CI). Differences in the intercepts across tumor classes are statistically significant but explain only 4% of the total variability in the data (Fig. 4 A and B and SI Appendix, Table S6). Such low explanatory power could be due to the heterogeneity of the samples within the same cancer type and the possible occurrence of driver mutations in noncoding regions or in genes that do not belong to our list of 198 cancer genes; indeed, 12% of the samples lack coding mutations in these genes. Both limitations can be mitigated by analyzing only samples from significant modules, which represent more homogeneous subsets of tumors enriched in putative driver mutations. Thus, to assess how the number of driver mutations varies across tumors, we repeated the regression analysis with the samples that were assigned to significant modules, using both the assignment to the modules and the cancer type as the grouping variables (Fig. 4A and SI Appendix, Table S7). In samples from significant modules, differences in the number of drivers across cancer types explain 20% of the observed variance in the number of mutations in cancer genes. The predicted number of driver mutations in these samples ranges from values near 1 in testicular cancer, thyroid carcinoma, and the subset of melanoma with low mutational load to values between 4 and 5 in bladder and endometrial cancers (Fig. 4C). The average number of drivers per tumor in colorectal cancer is 3.23 ± 0.26, consistent with previous estimates based on epidemiological models (21, 44) and measures of positive selection in cancer genes (9). Notably, the estimates of the average number of driver mutations in the two groups of melanomas differ by almost 3 (3.52 ± 0.60 vs ± 0.45, respectively), which could be related to the higher mutation burden observed in the first group. Overall, both the number of drivers and the fraction of mutations that are inferred to be drivers tend to be larger in samples MEDICAL SCIENCES PNAS PLUS Iranzo et al. PNAS vol. 115 no. 26 E6015
7 Fig. 4. Estimation of the average number of driver mutations per tumor in 198 cancer genes. (A) Regression between the number of coding mutations in cancer genes (y axis) and noncancer genes (x axis). Colored circles correspond to samples from significant modules. The solid lines show the fit to an ANCOVA model with class- or module-specific slopes and intercepts, y = ðα + α i Þ + ðβ + β i Þx + «, when considering all samples (gray) or samples from significant modules (colored). The global R 2 of the model are 0.83 and 0.75, respectively (P < in both cases). Vertical and horizontal axes were jointly scaled in all panels to allow comparison of slopes. (B and C) The intercepts that correspond to the estimated number of driver mutations are represented in B (all cancer types) and C (members of significant modules); error bars represent 95% CIs. (D and E) The number of drivers correlates with the number of intramodule mutations (Spearman srho= 0.815, P < 0.001) (D) and with the age at diagnosis (Spearman srho= 0.527, P = 0.003) (E).The solid line in D is a visual reference that shows the 1:1 correspondence between the number of drivers and the number of intramodule mutations. Solid lines in E are fits to the curve y = atx=ð1 + axþ derived from the model of Armitage and Doll (22), where T = 75 is the average lifespan in the absence of cancer and a is the proportionality constant between the number of drivers and rate-limiting steps (light gray, a = 2.5, all tumors; dark gray, a = 1.5, tumors from significant modules). (F) Comparison of the number of driver mutations in the main set of 198 cancer genes and in an extended set of 369 known cancer genes. from the significant specificity modules. Moreover, the estimated number of driver mutations in these samples closely matches the average number of module-specific (i.e., intramodule) mutations per tumor (P < 0.001, Spearman s ρ = 0.815) (Fig. 4D). These two findings indicate that module-specific genes are the principal target of actual driver mutations in cancer types that belong to significant modules. The only notable exception occurs for bladder cancer (see the deviation from the 1:1 trend in Fig. 4D). In this cancer type, the average number of module-specific mutations falls well below the estimated number of drivers, suggesting the existence of driver mutations in cancer genes that do not belong to the module. There is a strong positive correlation between the estimated number of drivers in a cancer type and the average age at which the respective cancers are diagnosed (Fig. 4E). This result holds regardless of whether the number of drivers is estimated for the complete set of samples of the given cancer type (P = 0.003, Spearman s ρ = 0.527) or for the samples assigned to significant modules only (P = 0.005, ρ = 0.693). After controlling for the differences in the number of drivers, cancer types that are and are not associated with significant modules (i.e., with and without specific sets of cancer genes) differ significantly in the average age at diagnosis, the former appearing earlier in life E Iranzo et al.
8 (P = 0.036, difference = 6.7 rank units, ANCOVA on ranktransformed data). Although the 198 genes included in the cancer mutation network likely harbor a large fraction of driver mutations, recent studies have extended the list of well-supported cancer genes beyond those included in the network (45). To assess the contribution of other cancer genes to tumor initiation, we extended the regression analysis to include a larger set of 369 high-confidence cancer genes (Fig. 4F). The average number of driver mutations per tumor in the extended dataset is 1.96 ± 0.12, which constitutes a 10% increase compared with the estimate obtained with the original set of 198 cancer genes (P < 0.001, Student s t test). Larger differences were observed for bladder, head and neck, endometrial, and kidney (chromophobe) cancers, suggesting that some genes from the extended list have a key role in those types of cancer. For the other cancer types, however, we conclude that the 198 genes included in the cancer mutation network account for most of the driver substitutions and small indels that occur in known cancer genes. Discussion It is well established that tumors accumulate recurrent mutations more often in some genes than in others. This phenomenon underlies the discovery of oncogenes and TSGs and has led to the identification of central pathways for tumorigenesis and tumor progression (7, 8, 11, 13, 46). Here we went a step further and tested the extent to which the association between genes and cancer types is mutually specific and suffices to define coherent, biologically meaningful groups of tumors. Although related to previous research on the detection of significantly mutated genes and tumor classification (29, 47, 48), our approach differed in three major aspects. First, tumor samples were not classified a priori based on their histology, which enabled us to test whether different cancer types are distinguishable by comparison of their mutational landscapes alone. Second, genes and samples were jointly clustered in a single step, so that the resulting network modules reflect mutual specificity. Third, because our network-based clustering is conducive to rigorous statistical testing, we could discriminate between cancer types that do and do not show a significant degree of specificity in their sets of mutated genes. Previous studies involving 12 major cancer types have shown that tissue-specific clusters can be automatically identified from genomic and transcriptomic features, suggesting the existence of a consistent molecular basis for a tissue-based classification of tumors (29). Our analysis provides a generalization of that result to a more diverse dataset that included 30 cancer types and 198 cancer genes, revealing major differences among cancer types. Thus, colorectal, pancreatic, endometrial, kidney (clear cell), breast, thyroid, and brain cancers, and acute myeloid leukemia, sarcoma, mesothelioma, melanoma, and uterine carcinosarcoma are significantly associated with mutations in tissue-specific sets of genes. In contrast, stomach, esophagus, and lung cancers, among others, follow a more diffuse, less specific mode of driver accumulation. Some cancers, such as bladder, prostate, testicular (germ cell), and head-and-neck squamous cancer, show a mixed picture, with a significant specificity of mutations observed in only a fraction of samples. The observed specificity patterns of cancer genes could originate from at least two, not mutually exclusive, causes. Biases in mutation and/or repair could make some tissues more prone to accumulating mutations in certain genes (e.g., due to differences in transcription levels, chromatin configuration, and exposure to mutational processes) (49), although such biases are unlikely to account for the large differences observed across cancer types (50). A more important factor is the tissue specificity of the pathways that control cell proliferation, which have to be overcome for tumor progression through mutations in different genes (51). This view is supported by experimental research on the functional mechanisms by which APC and KRAS mutations lead to colorectal cancer (52, 53) and combined VHL-BAP1 mutations lead to clear cell renal cell carcinoma (54). Along similar lines, recent analysis of synonymous and nonsynonymous substitutions in cancer genomes has shown that positive selection promotes the fixation of somatic mutations in a gene- and tissue-specific manner, implying that selection pressures during tumorigenesis are tissue specific (9, 10). The absence of detectable specificity patterns in some cancers might be affected by inherent limitations of community-detection algorithms on large, partially modular networks such as the one we analyzed here. In particular, small sample size could compromise the identification of modules for thymoma, adrenocortical, cervical, and kidney (chromophobe) carcinomas. Additionally, it could be hard to find specificity patterns in cancers with high mutational load, such as lung and stomach cancer, due to their low signal-tonoise (drivers-to-passengers) ratio. Nonetheless, the detection of significant modules for bladder cancer and melanoma, which both have high mutational loads (3, 55), implies that a high mutation burden does not critically affect the performance of the module detection algorithm. Finally, relevant specificity modules based on copy number variation or gene rearrangements could remain undetected if these large-scale mutations involve genes that are not considered here. It should be noted that the absence of specific sets of driver genes for some cancer types does not imply that such cancers cannot be clustered on the basis of other molecular features, as has been shown for lung adenocarcinoma and lung squamous carcinoma based on transcriptomics (29). The second major theme of this study is the estimation of the number of driver mutations affecting cancer genes in different cancer types. By comparing the number of mutations in cancer genes and noncancer genes, we inferred an average of approximately two driver mutations (substitutions and small indels) in known cancer genes per tumor, with significant variation (from less than one to more than five) across cancer types. Our results generally agree with previously reported numbers based on mutations under positive selection, providing independent support for such values (9). Remarkably, there is a connection between driver mutations and tissue-specific cancer genes. Specifically, the number of driver mutations in different cancer types shows a 1:1 correspondence (some minor variations notwithstanding) with the number of intramodule mutations, that is, with the number of mutations in tissue-specific genes. We further show that the number of driver mutations strongly and positively correlates with the mean age of cancer onset, as one would expect if the number of driver mutations was proportional (yet not necessarily equal) to the number of rate-limiting steps in tumorigenesis (22, 24). Supporting this view, many of the tissuespecific cancer genes detected in this study are targets of mutations that appear as early clonal events in the trunk of singletumor evolutionary trees and likely reflect crucial steps in tumor progression (56). That is, for example, the case for VHL and PBRM1 in clear cell renal cell carcinoma (often accompanied by parallel subclonal mutations in SETD2) (57), for DNMT3A and NPM1 in acute myeloid leukemia (often with parallel subclonal mutations in FLT3) (58), for KRAS, TP53, and SMAD4 in pancreatic cancer (59), and for APC, KRAS, and TP53 in colorectal cancer (60). Based on our observations, we propose that the cancer phenotype can be reached in two ways: via driver mutations in tissuespecific cancer genes (the modular mode) or via mutations in a broader set of genes, some of which have not yet been identified as cancer genes (the diffuse mode). Tumors from the same tissue typically follow one of these routes preferentially, which makes it possible to classify cancers as modular or diffuse. We found an intriguing link between the onset of a cancer and the specificity of cancer genes: Modular cancer types (those that carry mutations in specific genes) tend to appear earlier in life than expected given MEDICAL SCIENCES PNAS PLUS Iranzo et al. PNAS vol. 115 no. 26 E6017
9 their estimated number of driver mutations. We suspect that this difference could be explained by the requirement for additional driver mutations in genes that are currently not classified as cancer-associated in the case of the diffuse cancer types and/or by stronger effects of driver mutations in the modular cancers. The results of this work show that rigorous statistical methods for community detection in bipartite networks can shed light on the relationships between different types of tumors and cancer genes. The modularity of the gene tumor network analyzed here is relatively low, with only about one in four tumor samples included in significant modules. This fraction is likely to increase as more tumors are sequenced and additional cancer genes are identified. Nevertheless, the good agreement between the numbers of driver mutations estimated here and those obtained by other methods, as well as the significant difference in the age of cancer onset between the modular and the diffuse components of the network, suggest that the difference between the two modes of carcinogenesis revealed by this analysis withstands the test of time. This distinction between tumors caused by driver mutations in limited sets of tissuespecific genes and those caused by mutations in interchangeable genes that are only weakly linked to specific tumor types could have important implications for understanding cancer evolution as well as for diagnostic and therapeutic approaches. Methods Data: Tumors and Mutations. TCGA public mutation calls were downloaded from TCGA, on January Mutations were reannotated with the Ensembl Variant Effect Predictor (VEP) software (61); information on the affected gene, type of mutation, and coarse-grained impact was collected. The classification of genes into oncogenes and TSGs was extracted from ref. 62. Network Construction. An unweighted, undirected, bipartite network of somatic mutations in cancers was built by connecting tumor sample nodes to gene nodes whenever a small mutation in the coding region was identified in a given gene in a given sample. For this purpose, the following were considered as coding mutations: missense and nonsense substitutions, loss of a stop codon, mutations affecting splice donor or acceptor sites, and in-frame and out-of-frame indels. To keep the network size tractable and maximize the signal-to-noise ratio, only genes with a known association with cancer were included in the network. Specifically, we used the list of 198 cancer genes reported in ref. 3, which includes the curated list of 174 mutated genes from the COSMIC database (version 73) (31) and any other gene from the Cancer Gene Census database found recurrently mutated in ref. 7. Samples without any mutation in cancer genes and samples with >3,000 coding mutations (hypermutators) were excluded from the network. The resulting network consisted of a single connected component with 7,665 samples and 198 genes, with a density of connections of Module Detection. Following recent methodological advances in network analysis, a consensus clustering approach was used to identify the modules of the network (63). In the first step, maximization of Barber s modularity index was performed in 200 replicas of the network with the MODULAR software (simulated annealing algorithm, default parameters) (64), which yielded 200 alternative partitions. To test, from a global perspective, if the cancer mutation network has a significant modular structure, we used the Chung Lu model (65) to generate 200 random bipartite networks with the same expected gene- and sample-degree distributions, ran MODULAR on them, and compared the modularities of the resulting partitions with those of the cancer mutation network, using a Welch s t test. In the second step, the 200 alternative partitions of the cancer mutation network were used to build a consensus matrix by assigning to each pair of nodes a value equal to the fraction of replicas in which both nodes were assigned to the same module. A distance matrix was then defined as one minus the consensus matrix, and the consensus partition was finally obtained by performing hierarchical clustering on the distance matrix [the unweighted pair group method with arithmetic mean (UPGMA) method], implemented by the linkage function in MATLAB version R2015a. The number of clusters was chosen to maximize the Barber s modularity index of the consensus partition with respect to the original network. We refer to the clusters in this consensus partition (MODULAR + UPGMA) as the statistically relaxed modules. The composition of these clusters is described in Two Major Modes of Driver Accumulation and in SI Appendix, Table S3. To evaluate the significance of each module separately and to filter out the genes and samples that do not follow a modular pattern, we ran the OSLOM (Order Statistics Local Optimization Method) software (42) with the options -singlet -r 0 -hr 0 -t hint, using the modules from the previous step as the reference partition. The significance threshold was set to With these settings, OSLOM evaluates the probability that nodes from a random (nonmodular) network display the connection patterns observed in the reference partition and removes those nodes that do not reach the required significance threshold. Additionally, OSLOM accounts for the existence of overlapping modules by reassigning nodes to more than one module when statistically supported. The result is a filtered partition that includes only nodes (genes and samples) with a statistically significant modular structure, sometimes assigned to more than one module (see for example the double assignation of TP53 in Table 1). The main results of our study are based on the analysis of these 12 statistically significant modules obtained by the combination of MODULAR + UPGMA + OSLOM (Table 1). The same pipeline was used to study the structure of the joint network of somatic mutations and copy number alterations (Copy Number Alterations and SI Appendix, Tables S4 and S5). Together with the statistically relaxed and statistically significant modules, we built a set of best-match extended modules by attaching every node that is missing from the statistically significant partition to the module that best matches its pattern of connections. By construction, the best-match extended partition has the same number of modules as the statistically significant partition, but it includes all the genes and samples. The best-match extended partition was obtained as a secondary output of OSLOM (output file tp_without_singletons when the program is run with option -singlet), which reassigns the nodes that do not reach the significance threshold to the module that minimizes their P value. The best-match extended modules are described in Two Major Modes of Driver Accumulation,Fig.3,andSI Appendix, Table S2. To test whether the results were robust to the choice of the network partitioning method, we repeated the network analysis using Infomap as the module detection software (66), both in the first (MODULAR) and second (UPGMA) steps of the consensus clustering pipeline. In the second case, all entries with values <0.4 in the consensus matrix were set to 0, and Infomap was run on the network defined by the consensus matrix constructed with this threshold. Both the unipartite and bipartite versions of Infomap (options set to find hard partitions with two levels of hierarchy) were run, followed by the assessment of module significance with OSLOM. In all cases, the results were similar to those presented here (SI Appendix, Table S8). Estimation of the Number of Driver Mutations. To estimate the number of driver mutations in different cancer types, we considered all small coding mutations affecting cancer genes and noncancer (passenger) genes. The function aoctool in MATLAB R2015a was used to fit the number of mutations in cancer genes to a separate lines linear model with cancer type and number of mutations in passenger genes as independent variables. The statistical significance of the model parameters was evaluated with an ANCOVA with cancer types as factors and the number of mutations in passenger genes as covariable. The entire analysis was carried out twice. First, all the samples in the dataset, classified into cancer types, were analyzed. Then, the analysis was limited to the samples that were represented in any of the significant modules. Under the second approach, cancer types represented by fewer than 20 samples were excluded, and cancer types assigned to more than one significant module were split to obtain module-specific estimates. Although the interaction term between cancer types and number of mutations in passenger genes explains only <3% of the observed variance, we chosetokeepitinthemodel(and consequently use different slopes to fit each cancer type) based on the high statistical significance of this term (P < ) and the difference in the Akaike Information Criterion (AIC) between models with global and factorwise slopes (ΔAIC > 100). The association between the number of drivers and the age of diagnosis was initially evaluated through a Spearman s correlation analysis. To further assess whether cancer types associated or not with significant modules differ in the age at diagnosis while controlling for the number of driver mutations, an ANCOVA was performed on the rank-converted data with the number of drivers as a covariable and the inclusion into a significant module as a binary factor. To extend our estimates of the number of driver mutations to known cancer genes not included in the cancer mutation network, we used the list of 369 significantly mutated genes compiled in ref. 9. E Iranzo et al.
The Cancer Genome Atlas Pan-cancer analysis Katherine A. Hoadley
 The Cancer Genome Atlas Pan-cancer analysis Katherine A. Hoadley Department of Genetics Lineberger Comprehensive Cancer Center The University of North Carolina at Chapel Hill What is TCGA? The Cancer Genome
The Cancer Genome Atlas Pan-cancer analysis Katherine A. Hoadley Department of Genetics Lineberger Comprehensive Cancer Center The University of North Carolina at Chapel Hill What is TCGA? The Cancer Genome
Exploring TCGA Pan-Cancer Data at the UCSC Cancer Genomics Browser
 Exploring TCGA Pan-Cancer Data at the UCSC Cancer Genomics Browser Melissa S. Cline 1*, Brian Craft 1, Teresa Swatloski 1, Mary Goldman 1, Singer Ma 1, David Haussler 1, Jingchun Zhu 1 1 Center for Biomolecular
Exploring TCGA Pan-Cancer Data at the UCSC Cancer Genomics Browser Melissa S. Cline 1*, Brian Craft 1, Teresa Swatloski 1, Mary Goldman 1, Singer Ma 1, David Haussler 1, Jingchun Zhu 1 1 Center for Biomolecular
Machine-Learning on Prediction of Inherited Genomic Susceptibility for 20 Major Cancers
 Machine-Learning on Prediction of Inherited Genomic Susceptibility for 20 Major Cancers Sung-Hou Kim University of California Berkeley, CA Global Bio Conference 2017 MFDS, Seoul, Korea June 28, 2017 Cancer
Machine-Learning on Prediction of Inherited Genomic Susceptibility for 20 Major Cancers Sung-Hou Kim University of California Berkeley, CA Global Bio Conference 2017 MFDS, Seoul, Korea June 28, 2017 Cancer
Session 4 Rebecca Poulos
 The Cancer Genome Atlas (TCGA) & International Cancer Genome Consortium (ICGC) Session 4 Rebecca Poulos Prince of Wales Clinical School Introductory bioinformatics for human genomics workshop, UNSW 20
The Cancer Genome Atlas (TCGA) & International Cancer Genome Consortium (ICGC) Session 4 Rebecca Poulos Prince of Wales Clinical School Introductory bioinformatics for human genomics workshop, UNSW 20
Session 4 Rebecca Poulos
 The Cancer Genome Atlas (TCGA) & International Cancer Genome Consortium (ICGC) Session 4 Rebecca Poulos Prince of Wales Clinical School Introductory bioinformatics for human genomics workshop, UNSW 28
The Cancer Genome Atlas (TCGA) & International Cancer Genome Consortium (ICGC) Session 4 Rebecca Poulos Prince of Wales Clinical School Introductory bioinformatics for human genomics workshop, UNSW 28
Supplementary Figure 1: LUMP Leukocytes unmethylabon to infer tumor purity
 Supplementary Figure 1: LUMP Leukocytes unmethylabon to infer tumor purity A Consistently unmethylated sites (30%) in 21 cancer types 174,696
Supplementary Figure 1: LUMP Leukocytes unmethylabon to infer tumor purity A Consistently unmethylated sites (30%) in 21 cancer types 174,696
Supplementary Figure 1. Copy Number Alterations TP53 Mutation Type. C-class TP53 WT. TP53 mut. Nature Genetics: doi: /ng.
 Supplementary Figure a Copy Number Alterations in M-class b TP53 Mutation Type Recurrent Copy Number Alterations 8 6 4 2 TP53 WT TP53 mut TP53-mutated samples (%) 7 6 5 4 3 2 Missense Truncating M-class
Supplementary Figure a Copy Number Alterations in M-class b TP53 Mutation Type Recurrent Copy Number Alterations 8 6 4 2 TP53 WT TP53 mut TP53-mutated samples (%) 7 6 5 4 3 2 Missense Truncating M-class
Identification of Tissue Independent Cancer Driver Genes
 Identification of Tissue Independent Cancer Driver Genes Alexandros Manolakos, Idoia Ochoa, Kartik Venkat Supervisor: Olivier Gevaert Abstract Identification of genomic patterns in tumors is an important
Identification of Tissue Independent Cancer Driver Genes Alexandros Manolakos, Idoia Ochoa, Kartik Venkat Supervisor: Olivier Gevaert Abstract Identification of genomic patterns in tumors is an important
Supplemental Information. Integrated Genomic Analysis of the Ubiquitin. Pathway across Cancer Types
 Cell Reports, Volume 23 Supplemental Information Integrated Genomic Analysis of the Ubiquitin Pathway across Zhongqi Ge, Jake S. Leighton, Yumeng Wang, Xinxin Peng, Zhongyuan Chen, Hu Chen, Yutong Sun,
Cell Reports, Volume 23 Supplemental Information Integrated Genomic Analysis of the Ubiquitin Pathway across Zhongqi Ge, Jake S. Leighton, Yumeng Wang, Xinxin Peng, Zhongyuan Chen, Hu Chen, Yutong Sun,
Nature Getetics: doi: /ng.3471
 Supplementary Figure 1 Summary of exome sequencing data. ( a ) Exome tumor normal sample sizes for bladder cancer (BLCA), breast cancer (BRCA), carcinoid (CARC), chronic lymphocytic leukemia (CLLX), colorectal
Supplementary Figure 1 Summary of exome sequencing data. ( a ) Exome tumor normal sample sizes for bladder cancer (BLCA), breast cancer (BRCA), carcinoid (CARC), chronic lymphocytic leukemia (CLLX), colorectal
Plasma-Seq conducted with blood from male individuals without cancer.
 Supplementary Figures Supplementary Figure 1 Plasma-Seq conducted with blood from male individuals without cancer. Copy number patterns established from plasma samples of male individuals without cancer
Supplementary Figures Supplementary Figure 1 Plasma-Seq conducted with blood from male individuals without cancer. Copy number patterns established from plasma samples of male individuals without cancer
Nature Genetics: doi: /ng Supplementary Figure 1. SEER data for male and female cancer incidence from
 Supplementary Figure 1 SEER data for male and female cancer incidence from 1975 2013. (a,b) Incidence rates of oral cavity and pharynx cancer (a) and leukemia (b) are plotted, grouped by males (blue),
Supplementary Figure 1 SEER data for male and female cancer incidence from 1975 2013. (a,b) Incidence rates of oral cavity and pharynx cancer (a) and leukemia (b) are plotted, grouped by males (blue),
Clinical Grade Genomic Profiling: The Time Has Come
 Clinical Grade Genomic Profiling: The Time Has Come Gary Palmer, MD, JD, MBA, MPH Senior Vice President, Medical Affairs Foundation Medicine, Inc. Oct. 22, 2013 1 Why We Are Here A Shared Vision At Foundation
Clinical Grade Genomic Profiling: The Time Has Come Gary Palmer, MD, JD, MBA, MPH Senior Vice President, Medical Affairs Foundation Medicine, Inc. Oct. 22, 2013 1 Why We Are Here A Shared Vision At Foundation
The Cancer Genome Atlas & International Cancer Genome Consortium
 The Cancer Genome Atlas & International Cancer Genome Consortium Session 3 Dr Jason Wong Prince of Wales Clinical School Introductory bioinformatics for human genomics workshop, UNSW 31 st July 2014 1
The Cancer Genome Atlas & International Cancer Genome Consortium Session 3 Dr Jason Wong Prince of Wales Clinical School Introductory bioinformatics for human genomics workshop, UNSW 31 st July 2014 1
Distinct cellular functional profiles in pan-cancer expression analysis of cancers with alterations in oncogenes c-myc and n-myc
 Honors Theses Biology Spring 2018 Distinct cellular functional profiles in pan-cancer expression analysis of cancers with alterations in oncogenes c-myc and n-myc Anne B. Richardson Whitman College Penrose
Honors Theses Biology Spring 2018 Distinct cellular functional profiles in pan-cancer expression analysis of cancers with alterations in oncogenes c-myc and n-myc Anne B. Richardson Whitman College Penrose
Fluxion Biosciences and Swift Biosciences Somatic variant detection from liquid biopsy samples using targeted NGS
 APPLICATION NOTE Fluxion Biosciences and Swift Biosciences OVERVIEW This application note describes a robust method for detecting somatic mutations from liquid biopsy samples by combining circulating tumor
APPLICATION NOTE Fluxion Biosciences and Swift Biosciences OVERVIEW This application note describes a robust method for detecting somatic mutations from liquid biopsy samples by combining circulating tumor
Ahrim Youn 1,2, Kyung In Kim 2, Raul Rabadan 3,4, Benjamin Tycko 5, Yufeng Shen 3,4,6 and Shuang Wang 1*
 Youn et al. BMC Medical Genomics (2018) 11:98 https://doi.org/10.1186/s12920-018-0425-z RESEARCH ARTICLE Open Access A pan-cancer analysis of driver gene mutations, DNA methylation and gene expressions
Youn et al. BMC Medical Genomics (2018) 11:98 https://doi.org/10.1186/s12920-018-0425-z RESEARCH ARTICLE Open Access A pan-cancer analysis of driver gene mutations, DNA methylation and gene expressions
The Center for PERSONALIZED DIAGNOSTICS
 The Center for PERSONALIZED DIAGNOSTICS Precision Diagnostics for Personalized Medicine A joint initiative between The Department of Pathology and Laboratory Medicine & The Abramson Cancer Center The (CPD)
The Center for PERSONALIZED DIAGNOSTICS Precision Diagnostics for Personalized Medicine A joint initiative between The Department of Pathology and Laboratory Medicine & The Abramson Cancer Center The (CPD)
Expanded View Figures
 Molecular Systems iology Tumor CNs reflect metabolic selection Nicholas Graham et al Expanded View Figures Human primary tumors CN CN characterization by unsupervised PC Human Signature Human Signature
Molecular Systems iology Tumor CNs reflect metabolic selection Nicholas Graham et al Expanded View Figures Human primary tumors CN CN characterization by unsupervised PC Human Signature Human Signature
Targeted Agent and Profiling Utilization Registry (TAPUR ) Study. February 2018
 Targeted Agent and Profiling Utilization Registry (TAPUR ) Study February 2018 Precision Medicine Therapies designed to target the molecular alteration that aids cancer development 30 TARGET gene alterations
Targeted Agent and Profiling Utilization Registry (TAPUR ) Study February 2018 Precision Medicine Therapies designed to target the molecular alteration that aids cancer development 30 TARGET gene alterations
Genomic Medicine: What every pathologist needs to know
 Genomic Medicine: What every pathologist needs to know Stephen P. Ethier, Ph.D. Professor, Department of Pathology and Laboratory Medicine, MUSC Director, MUSC Center for Genomic Medicine Genomics and
Genomic Medicine: What every pathologist needs to know Stephen P. Ethier, Ph.D. Professor, Department of Pathology and Laboratory Medicine, MUSC Director, MUSC Center for Genomic Medicine Genomics and
Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins
 Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins February 3-5, 2016 Lansdowne Resort, Leesburg, VA Molecular
Identification and clinical detection of genetic alterations of pre-neoplastic lesions Time for the PML ome? David Sidransky MD Johns Hopkins February 3-5, 2016 Lansdowne Resort, Leesburg, VA Molecular
Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ
 Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ G E NETIC T E CHNOLOGIST MEDICAL G E NETICS, CARDIFF To Cover Background to the project Choice of panel Validation process Genes on panel, Protocol
Illumina Trusight Myeloid Panel validation A R FHAN R A FIQ G E NETIC T E CHNOLOGIST MEDICAL G E NETICS, CARDIFF To Cover Background to the project Choice of panel Validation process Genes on panel, Protocol
User s Manual Version 1.0
 User s Manual Version 1.0 #639 Longmian Avenue, Jiangning District, Nanjing,211198,P.R.China. http://tcoa.cpu.edu.cn/ Contact us at xiaosheng.wang@cpu.edu.cn for technical issue and questions Catalogue
User s Manual Version 1.0 #639 Longmian Avenue, Jiangning District, Nanjing,211198,P.R.China. http://tcoa.cpu.edu.cn/ Contact us at xiaosheng.wang@cpu.edu.cn for technical issue and questions Catalogue
Frequency(%) KRAS G12 KRAS G13 KRAS A146 KRAS Q61 KRAS K117N PIK3CA H1047 PIK3CA E545 PIK3CA E542K PIK3CA Q546. EGFR exon19 NFS-indel EGFR L858R
 Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Nature Genetics: doi: /ng Supplementary Figure 1. Mutational signatures in BCC compared to melanoma.
 Supplementary Figure 1 Mutational signatures in BCC compared to melanoma. (a) The effect of transcription-coupled repair as a function of gene expression in BCC. Tumor type specific gene expression levels
Supplementary Figure 1 Mutational signatures in BCC compared to melanoma. (a) The effect of transcription-coupled repair as a function of gene expression in BCC. Tumor type specific gene expression levels
Clinically Useful Next Generation Sequencing and Molecular Testing in Gliomas MacLean P. Nasrallah, MD PhD
 Clinically Useful Next Generation Sequencing and Molecular Testing in Gliomas MacLean P. Nasrallah, MD PhD Neuropathology Fellow Division of Neuropathology Center for Personalized Diagnosis (CPD) Glial
Clinically Useful Next Generation Sequencing and Molecular Testing in Gliomas MacLean P. Nasrallah, MD PhD Neuropathology Fellow Division of Neuropathology Center for Personalized Diagnosis (CPD) Glial
Clustered mutations of oncogenes and tumor suppressors.
 Supplementary Figure 1 Clustered mutations of oncogenes and tumor suppressors. For each oncogene (red dots) and tumor suppressor (blue dots), the number of mutations found in an intramolecular cluster
Supplementary Figure 1 Clustered mutations of oncogenes and tumor suppressors. For each oncogene (red dots) and tumor suppressor (blue dots), the number of mutations found in an intramolecular cluster
Supplementary Tables. Supplementary Figures
 Supplementary Files for Zehir, Benayed et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients Supplementary Tables Supplementary Table 1: Sample
Supplementary Files for Zehir, Benayed et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients Supplementary Tables Supplementary Table 1: Sample
Out-Patient Billing CPT Codes
 Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
Out-Patient Billing CPT Codes Updated Date: August 3, 08 Client Billed Molecular Tests HPV DNA Tissue Testing 8764 No Medicare Billed - Molecular Tests NeoARRAY NeoARRAY SNP/Cytogenetic No 89 NeoLAB NeoLAB
SureSelect Cancer All-In-One Custom and Catalog NGS Assays
 SureSelect Cancer All-In-One Custom and Catalog NGS Assays Detect all cancer-relevant variants in a single SureSelect assay SNV Indel TL SNV Indel TL Single DNA input Single AIO assay Single data analysis
SureSelect Cancer All-In-One Custom and Catalog NGS Assays Detect all cancer-relevant variants in a single SureSelect assay SNV Indel TL SNV Indel TL Single DNA input Single AIO assay Single data analysis
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making
 Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
OncoPPi Portal A Cancer Protein Interaction Network to Inform Therapeutic Strategies
 OncoPPi Portal A Cancer Protein Interaction Network to Inform Therapeutic Strategies 2017 Contents Datasets... 2 Protein-protein interaction dataset... 2 Set of known PPIs... 3 Domain-domain interactions...
OncoPPi Portal A Cancer Protein Interaction Network to Inform Therapeutic Strategies 2017 Contents Datasets... 2 Protein-protein interaction dataset... 2 Set of known PPIs... 3 Domain-domain interactions...
File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables. File Name: Peer Review File Description:
 File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables File Name: Peer Review File Description: Primer Name Sequence (5'-3') AT ( C) RT-PCR USP21 F 5'-TTCCCATGGCTCCTTCCACATGAT-3'
File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables File Name: Peer Review File Description: Primer Name Sequence (5'-3') AT ( C) RT-PCR USP21 F 5'-TTCCCATGGCTCCTTCCACATGAT-3'
Nature Genetics: doi: /ng Supplementary Figure 1. Workflow of CDR3 sequence assembly from RNA-seq data.
 Supplementary Figure 1 Workflow of CDR3 sequence assembly from RNA-seq data. Paired-end short-read RNA-seq data were mapped to human reference genome hg19, and unmapped reads in the TCR regions were extracted
Supplementary Figure 1 Workflow of CDR3 sequence assembly from RNA-seq data. Paired-end short-read RNA-seq data were mapped to human reference genome hg19, and unmapped reads in the TCR regions were extracted
IntelliGENSM. Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community.
 IntelliGENSM Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community. NGS TRANSFORMS GENOMIC TESTING Background Cancers may emerge as a result of somatically
IntelliGENSM Integrated Oncology is making next generation sequencing faster and more accessible to the oncology community. NGS TRANSFORMS GENOMIC TESTING Background Cancers may emerge as a result of somatically
Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope
 Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope National Medical Center Disclosures I have no disclosures
Patricia Aoun MD, MPH Professor and Vice-Chair for Clinical Affairs Medical Director, Clinical Laboratories Department of Pathology City of Hope National Medical Center Disclosures I have no disclosures
Protein Domain-Centric Approach to Study Cancer Somatic Mutations from High-throughput Sequencing Studies
 Protein Domain-Centric Approach to Study Cancer Somatic Mutations from High-throughput Sequencing Studies Dr. Maricel G. Kann Assistant Professor Dept of Biological Sciences UMBC 2 The term protein domain
Protein Domain-Centric Approach to Study Cancer Somatic Mutations from High-throughput Sequencing Studies Dr. Maricel G. Kann Assistant Professor Dept of Biological Sciences UMBC 2 The term protein domain
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
NeoTYPE Cancer Profiles 30+ Multimethod Assays for Hematologic Diseases and Solid Tumors Molecular FISH Anatomic Pathology The next generation of diagnostic, prognostic, and therapeutic assessment What
The 16th KJC Bioinformatics Symposium Integrative analysis identifies potential DNA methylation biomarkers for pan-cancer diagnosis and prognosis
 The 16th KJC Bioinformatics Symposium Integrative analysis identifies potential DNA methylation biomarkers for pan-cancer diagnosis and prognosis Tieliu Shi tlshi@bio.ecnu.edu.cn The Center for bioinformatics
The 16th KJC Bioinformatics Symposium Integrative analysis identifies potential DNA methylation biomarkers for pan-cancer diagnosis and prognosis Tieliu Shi tlshi@bio.ecnu.edu.cn The Center for bioinformatics
Secuenciación masiva: papel en la toma de decisiones
 Secuenciación masiva: papel en la toma de decisiones Cancer is a Genetic Disease Development of cancer is driven by the acquisition of somatic genetic alterations: Nonsynonymous point mutations: missense.
Secuenciación masiva: papel en la toma de decisiones Cancer is a Genetic Disease Development of cancer is driven by the acquisition of somatic genetic alterations: Nonsynonymous point mutations: missense.
Next generation histopathological diagnosis for precision medicine in solid cancers
 Next generation histopathological diagnosis for precision medicine in solid cancers from genomics to clinical application Aldo Scarpa ARC-NET Applied Research on Cancer Department of Pathology and Diagnostics
Next generation histopathological diagnosis for precision medicine in solid cancers from genomics to clinical application Aldo Scarpa ARC-NET Applied Research on Cancer Department of Pathology and Diagnostics
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Pan-cancer analysis of global and local DNA methylation variation a) Variations in global DNA methylation are shown as measured by averaging the genome-wide
Supplementary Figures Supplementary Figure 1. Pan-cancer analysis of global and local DNA methylation variation a) Variations in global DNA methylation are shown as measured by averaging the genome-wide
COSMIC - Catalogue of Somatic Mutations in Cancer
 COSMIC - Catalogue of Somatic Mutations in Cancer http://cancer.sanger.ac.uk/cosmic https://academic.oup.com/nar/articl e-lookup/doi/10.1093/nar/gkw1121 Data In Large-scale systematic screens Detailed
COSMIC - Catalogue of Somatic Mutations in Cancer http://cancer.sanger.ac.uk/cosmic https://academic.oup.com/nar/articl e-lookup/doi/10.1093/nar/gkw1121 Data In Large-scale systematic screens Detailed
Whole Genome and Transcriptome Analysis of Anaplastic Meningioma. Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute
 Whole Genome and Transcriptome Analysis of Anaplastic Meningioma Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute Outline Anaplastic meningioma compared to other cancers Whole genomes
Whole Genome and Transcriptome Analysis of Anaplastic Meningioma Patrick Tarpey Cancer Genome Project Wellcome Trust Sanger Institute Outline Anaplastic meningioma compared to other cancers Whole genomes
Personalised cancer care Information for Medical Specialists. A new way to unlock treatment options for your patients
 Personalised cancer care Information for Medical Specialists A new way to unlock treatment options for your patients Contents Optimised for clinical benefit 4 Development history 4 Full FIND IT panel vs
Personalised cancer care Information for Medical Specialists A new way to unlock treatment options for your patients Contents Optimised for clinical benefit 4 Development history 4 Full FIND IT panel vs
Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities
 Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities Sujana Movva 1, Wenhsiang Wen 2, Wangjuh Chen 2, Sherri Z. Millis 2, Margaret von Mehren 1, Zoran
Predictive biomarker profiling of > 1,900 sarcomas: Identification of potential novel treatment modalities Sujana Movva 1, Wenhsiang Wen 2, Wangjuh Chen 2, Sherri Z. Millis 2, Margaret von Mehren 1, Zoran
Nicholas Borcherding, Nicholas L. Bormann, Andrew Voigt, Weizhou Zhang 1-4
 SOFTWARE TOOL ARTICLE TRGAted: A web tool for survival analysis using protein data in the Cancer Genome Atlas. [version 1; referees: 1 approved] Nicholas Borcherding, Nicholas L. Bormann, Andrew Voigt,
SOFTWARE TOOL ARTICLE TRGAted: A web tool for survival analysis using protein data in the Cancer Genome Atlas. [version 1; referees: 1 approved] Nicholas Borcherding, Nicholas L. Bormann, Andrew Voigt,
Dr David Guttery Senior PDRA Dept. of Cancer Studies and CRUK Leicester Centre University of Leicester
 Dr David Guttery Senior PDRA Dept. of Cancer Studies and CRUK Leicester Centre University of Leicester dsg6@le.ac.uk CFDNA/CTDNA Circulating-free AS A LIQUID DNA BIOPSY (cfdna) Tumour Biopsy Liquid Biopsy
Dr David Guttery Senior PDRA Dept. of Cancer Studies and CRUK Leicester Centre University of Leicester dsg6@le.ac.uk CFDNA/CTDNA Circulating-free AS A LIQUID DNA BIOPSY (cfdna) Tumour Biopsy Liquid Biopsy
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Systematic investigation of cancer-associated somatic point mutations in SNP databases HyunChul Jung 1,2, Thomas Bleazard 3, Jongkeun Lee 1 and Dongwan Hong 1 1. Cancer Genomics
SUPPLEMENTARY INFORMATION Systematic investigation of cancer-associated somatic point mutations in SNP databases HyunChul Jung 1,2, Thomas Bleazard 3, Jongkeun Lee 1 and Dongwan Hong 1 1. Cancer Genomics
Inferring Biological Meaning from Cap Analysis Gene Expression Data
 Inferring Biological Meaning from Cap Analysis Gene Expression Data HRYSOULA PAPADAKIS 1. Introduction This project is inspired by the recent development of the Cap analysis gene expression (CAGE) method,
Inferring Biological Meaning from Cap Analysis Gene Expression Data HRYSOULA PAPADAKIS 1. Introduction This project is inspired by the recent development of the Cap analysis gene expression (CAGE) method,
ARTICLE. Mutational landscape and significance across12majorcancertypes. OPEN doi: /nature12634
 ARTICLE OPEN doi:1.138/nature12634 Mutational landscape and significance across12majorcancertypes Cyriac Kandoth 1 *, Michael D. McLellan 1 *, Fabio Vandin 2, Kai Ye 1,3, Beifang Niu 1, Charles Lu 1, Mingchao
ARTICLE OPEN doi:1.138/nature12634 Mutational landscape and significance across12majorcancertypes Cyriac Kandoth 1 *, Michael D. McLellan 1 *, Fabio Vandin 2, Kai Ye 1,3, Beifang Niu 1, Charles Lu 1, Mingchao
RNA SEQUENCING AND DATA ANALYSIS
 RNA SEQUENCING AND DATA ANALYSIS Download slides and package http://odin.mdacc.tmc.edu/~rverhaak/package.zip http://odin.mdacc.tmc.edu/~rverhaak/rna-seqlecture.zip Overview Introduction into the topic
RNA SEQUENCING AND DATA ANALYSIS Download slides and package http://odin.mdacc.tmc.edu/~rverhaak/package.zip http://odin.mdacc.tmc.edu/~rverhaak/rna-seqlecture.zip Overview Introduction into the topic
ACTIVITY 2: EXAMINING CANCER PATIENT DATA
 OVERVIEW Refer to the Overview of Cancer Discovery Activities for Key Concepts and Learning Objectives, Curriculum Connections, and Prior Knowledge, as well as background information, references, and additional
OVERVIEW Refer to the Overview of Cancer Discovery Activities for Key Concepts and Learning Objectives, Curriculum Connections, and Prior Knowledge, as well as background information, references, and additional
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature13898 Supplementary Information Table 1 Kras mutation status of carcinogen-induced mouse lung adenomas Tumour Treatment Strain Grade Genotype Kras status (WES)* Kras status (Sanger) 32T1
doi:10.1038/nature13898 Supplementary Information Table 1 Kras mutation status of carcinogen-induced mouse lung adenomas Tumour Treatment Strain Grade Genotype Kras status (WES)* Kras status (Sanger) 32T1
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/7/283/283ra54/dc1 Supplementary Materials for Clonal status of actionable driver events and the timing of mutational processes in cancer evolution
www.sciencetranslationalmedicine.org/cgi/content/full/7/283/283ra54/dc1 Supplementary Materials for Clonal status of actionable driver events and the timing of mutational processes in cancer evolution
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature12912 Figure S1. Distribution of mutation rates and spectra across 4,742 tumor-normal (TN) pairs from 21 tumor types, as in Figure 1 from Lawrence et al. Nature
SUPPLEMENTARY INFORMATION doi:1.138/nature12912 Figure S1. Distribution of mutation rates and spectra across 4,742 tumor-normal (TN) pairs from 21 tumor types, as in Figure 1 from Lawrence et al. Nature
Accel-Amplicon Panels
 Accel-Amplicon Panels Amplicon sequencing has emerged as a reliable, cost-effective method for ultra-deep targeted sequencing. This highly adaptable approach is especially applicable for in-depth interrogation
Accel-Amplicon Panels Amplicon sequencing has emerged as a reliable, cost-effective method for ultra-deep targeted sequencing. This highly adaptable approach is especially applicable for in-depth interrogation
Cancer-Drug Associations: A Complex System
 Cancer-Drug Associations: A Complex System Ertugrul Dalkic 1,2,3, Xuewei Wang 1,4, Neil Wright 1,5, Christina Chan 1,2,3,4,6,7 * 1 Center for Systems Biology, Michigan State University, East Lansing, Michigan,
Cancer-Drug Associations: A Complex System Ertugrul Dalkic 1,2,3, Xuewei Wang 1,4, Neil Wright 1,5, Christina Chan 1,2,3,4,6,7 * 1 Center for Systems Biology, Michigan State University, East Lansing, Michigan,
Supplementary Figure 1. Estimation of tumour content
 Supplementary Figure 1. Estimation of tumour content a, Approach used to estimate the tumour content in S13T1/T2, S6T1/T2, S3T1/T2 and S12T1/T2. Tissue and tumour areas were evaluated by two independent
Supplementary Figure 1. Estimation of tumour content a, Approach used to estimate the tumour content in S13T1/T2, S6T1/T2, S3T1/T2 and S12T1/T2. Tissue and tumour areas were evaluated by two independent
Characterisation of structural variation in breast. cancer genomes using paired-end sequencing on. the Illumina Genome Analyser
 Characterisation of structural variation in breast cancer genomes using paired-end sequencing on the Illumina Genome Analyser Phil Stephens Cancer Genome Project Why is it important to study cancer? Why
Characterisation of structural variation in breast cancer genomes using paired-end sequencing on the Illumina Genome Analyser Phil Stephens Cancer Genome Project Why is it important to study cancer? Why
NGS in tissue and liquid biopsy
 NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes
 Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes Nature Genetics 47, 106-114 (2015) doi:101038/ng3168 Max Leiserson RECOMB 2015 April
Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes Nature Genetics 47, 106-114 (2015) doi:101038/ng3168 Max Leiserson RECOMB 2015 April
The mutations that drive cancer. Paul Edwards. Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge
 The mutations that drive cancer Paul Edwards Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge Previously on Cancer... hereditary predisposition Normal Cell Slightly
The mutations that drive cancer Paul Edwards Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge Previously on Cancer... hereditary predisposition Normal Cell Slightly
August 17, Dear Valued Client:
 August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
August 7, 08 Re: CMS Announces 6-Month Period of Enforcement Discretion for Laboratory Date of Service Exception Policy Under the Medicare Clinical Laboratory Fee Schedule (the 4 Day Rule ) Dear Valued
Cancer Informatics Lecture
 Cancer Informatics Lecture Mayo-UIUC Computational Genomics Course June 22, 2018 Krishna Rani Kalari Ph.D. Associate Professor 2017 MFMER 3702274-1 Outline The Cancer Genome Atlas (TCGA) Genomic Data Commons
Cancer Informatics Lecture Mayo-UIUC Computational Genomics Course June 22, 2018 Krishna Rani Kalari Ph.D. Associate Professor 2017 MFMER 3702274-1 Outline The Cancer Genome Atlas (TCGA) Genomic Data Commons
Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each
 Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each mutated gene and the panel of 125 cancer-driving genes
Supplementary Figure 1. Cytoscape bioinformatics toolset was used to create the network of protein-protein interactions between the product of each mutated gene and the panel of 125 cancer-driving genes
Results and Discussion of Receptor Tyrosine Kinase. Activation
 Results and Discussion of Receptor Tyrosine Kinase Activation To demonstrate the contribution which RCytoscape s molecular maps can make to biological understanding via exploratory data analysis, we here
Results and Discussion of Receptor Tyrosine Kinase Activation To demonstrate the contribution which RCytoscape s molecular maps can make to biological understanding via exploratory data analysis, we here
Jennifer Hauenstein Oncology Cytogenetics Emory University Hospital Atlanta, GA
 Comparison of Genomic Coverage using Affymetrix OncoScan Array and Illumina TruSight Tumor 170 NGS Panel for Detection of Copy Number Abnormalities in Clinical GBM Specimens Jennifer Hauenstein Oncology
Comparison of Genomic Coverage using Affymetrix OncoScan Array and Illumina TruSight Tumor 170 NGS Panel for Detection of Copy Number Abnormalities in Clinical GBM Specimens Jennifer Hauenstein Oncology
Next Generation Sequencing in Haematological Malignancy: A European Perspective. Wolfgang Kern, Munich Leukemia Laboratory
 Next Generation Sequencing in Haematological Malignancy: A European Perspective Wolfgang Kern, Munich Leukemia Laboratory Diagnostic Methods Cytomorphology Cytogenetics Immunophenotype Histology FISH Molecular
Next Generation Sequencing in Haematological Malignancy: A European Perspective Wolfgang Kern, Munich Leukemia Laboratory Diagnostic Methods Cytomorphology Cytogenetics Immunophenotype Histology FISH Molecular
Information for You and Your Family
 Information for You and Your Family What is Prevention? Cancer prevention is action taken to lower the chance of getting cancer. In 2017, more than 1.6 million people will be diagnosed with cancer in the
Information for You and Your Family What is Prevention? Cancer prevention is action taken to lower the chance of getting cancer. In 2017, more than 1.6 million people will be diagnosed with cancer in the
About OMICS Group Conferences
 About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
Relationship between genomic features and distributions of RS1 and RS3 rearrangements in breast cancer genomes.
 Supplementary Figure 1 Relationship between genomic features and distributions of RS1 and RS3 rearrangements in breast cancer genomes. (a,b) Values of coefficients associated with genomic features, separately
Supplementary Figure 1 Relationship between genomic features and distributions of RS1 and RS3 rearrangements in breast cancer genomes. (a,b) Values of coefficients associated with genomic features, separately
Transform genomic data into real-life results
 CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
MEDICAL POLICY Genetic Testing for Breast and Ovarian Cancers
 POLICY: PG0067 ORIGINAL EFFECTIVE: 07/30/02 LAST REVIEW: 01/25/18 MEDICAL POLICY Genetic Testing for Breast and Ovarian Cancers GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0067 ORIGINAL EFFECTIVE: 07/30/02 LAST REVIEW: 01/25/18 MEDICAL POLICY Genetic Testing for Breast and Ovarian Cancers GUIDELINES This policy does not certify benefits or authorization of benefits,
Click to edit Master /tle style
 Click to edit Master /tle style Tel: (314) 747-7337 Toll Free: (866) 450-7697 Fax: (314) 747-7336 Email: gps@wustl.edu Website: gps.wustl.edu GENETIC TESTING IN CANCER Ka/nka Vigh-Conrad, PhD Genomics
Click to edit Master /tle style Tel: (314) 747-7337 Toll Free: (866) 450-7697 Fax: (314) 747-7336 Email: gps@wustl.edu Website: gps.wustl.edu GENETIC TESTING IN CANCER Ka/nka Vigh-Conrad, PhD Genomics
Introduction to Cancer Bioinformatics and cancer biology. Anthony Gitter Cancer Bioinformatics (BMI 826/CS 838) January 20, 2015
 Introduction to Cancer Bioinformatics and cancer biology Anthony Gitter Cancer Bioinformatics (BMI 826/CS 838) January 20, 2015 Why cancer bioinformatics? Devastating disease, no cure on the horizon Major
Introduction to Cancer Bioinformatics and cancer biology Anthony Gitter Cancer Bioinformatics (BMI 826/CS 838) January 20, 2015 Why cancer bioinformatics? Devastating disease, no cure on the horizon Major
TCGA. The Cancer Genome Atlas
 TCGA The Cancer Genome Atlas TCGA: History and Goal History: Started in 2005 by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) with $110 Million to catalogue
TCGA The Cancer Genome Atlas TCGA: History and Goal History: Started in 2005 by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) with $110 Million to catalogue
Supplemental Figure legends
 Supplemental Figure legends Supplemental Figure S1 Frequently mutated genes. Frequently mutated genes (mutated in at least four patients) with information about mutation frequency, RNA-expression and copy-number.
Supplemental Figure legends Supplemental Figure S1 Frequently mutated genes. Frequently mutated genes (mutated in at least four patients) with information about mutation frequency, RNA-expression and copy-number.
Insights from Sequencing the Melanoma Exome
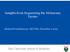 Insights from Sequencing the Melanoma Exome Michael Krauthammer, MD PhD, December 2 2015 Yale University School Yof Medicine 1 2012 Exome Screens and Results Exome Sequencing of 108 sun-exposed melanomas
Insights from Sequencing the Melanoma Exome Michael Krauthammer, MD PhD, December 2 2015 Yale University School Yof Medicine 1 2012 Exome Screens and Results Exome Sequencing of 108 sun-exposed melanomas
SUPPLEMENTARY FIGURES: Supplementary Figure 1
 SUPPLEMENTARY FIGURES: Supplementary Figure 1 Supplementary Figure 1. Glioblastoma 5hmC quantified by paired BS and oxbs treated DNA hybridized to Infinium DNA methylation arrays. Workflow depicts analytic
SUPPLEMENTARY FIGURES: Supplementary Figure 1 Supplementary Figure 1. Glioblastoma 5hmC quantified by paired BS and oxbs treated DNA hybridized to Infinium DNA methylation arrays. Workflow depicts analytic
Pan-cancer patterns of DNA methylation
 Witte et al. Genome Medicine 2014, 6:66 REVIEW Pan-cancer patterns of DNA methylation Tania Witte, Christoph Plass and Clarissa Gerhauser * Abstract The comparison of DNA methylation patterns across cancer
Witte et al. Genome Medicine 2014, 6:66 REVIEW Pan-cancer patterns of DNA methylation Tania Witte, Christoph Plass and Clarissa Gerhauser * Abstract The comparison of DNA methylation patterns across cancer
LESSON 3.2 WORKBOOK. How do normal cells become cancer cells? Workbook Lesson 3.2
 For a complete list of defined terms, see the Glossary. Transformation the process by which a cell acquires characteristics of a tumor cell. LESSON 3.2 WORKBOOK How do normal cells become cancer cells?
For a complete list of defined terms, see the Glossary. Transformation the process by which a cell acquires characteristics of a tumor cell. LESSON 3.2 WORKBOOK How do normal cells become cancer cells?
Nature Genetics: doi: /ng Supplementary Figure 1. Depths and coverages in whole-exome and targeted deep sequencing data.
 Supplementary Figure 1 Depths and coverages in whole-exome and targeted deep sequencing data. Depth (top) and coverage (bottom) of whole-exome sequencing for 38 independent JPN cases (mean depth = 130)
Supplementary Figure 1 Depths and coverages in whole-exome and targeted deep sequencing data. Depth (top) and coverage (bottom) of whole-exome sequencing for 38 independent JPN cases (mean depth = 130)
Clinical Grade Biomarkers in the Genomic Era Observations & Challenges
 Clinical Grade Biomarkers in the Genomic Era Observations & Challenges IOM Committee on Policy Issues in the Clinical Development & Use of Biomarkers for Molecularly Targeted Therapies March 31-April 1,
Clinical Grade Biomarkers in the Genomic Era Observations & Challenges IOM Committee on Policy Issues in the Clinical Development & Use of Biomarkers for Molecularly Targeted Therapies March 31-April 1,
Advances in Brain Tumor Research: Leveraging BIG data for BIG discoveries
 Advances in Brain Tumor Research: Leveraging BIG data for BIG discoveries Jill Barnholtz-Sloan, PhD Associate Professor & Associate Director for Bioinformatics and Translational Informatics jsb42@case.edu
Advances in Brain Tumor Research: Leveraging BIG data for BIG discoveries Jill Barnholtz-Sloan, PhD Associate Professor & Associate Director for Bioinformatics and Translational Informatics jsb42@case.edu
Nature Methods: doi: /nmeth.3115
 Supplementary Figure 1 Analysis of DNA methylation in a cancer cohort based on Infinium 450K data. RnBeads was used to rediscover a clinically distinct subgroup of glioblastoma patients characterized by
Supplementary Figure 1 Analysis of DNA methylation in a cancer cohort based on Infinium 450K data. RnBeads was used to rediscover a clinically distinct subgroup of glioblastoma patients characterized by
S1 Appendix: Figs A G and Table A. b Normal Generalized Fraction 0.075
 Aiello & Alter (216) PLoS One vol. 11 no. 1 e164546 S1 Appendix A-1 S1 Appendix: Figs A G and Table A a Tumor Generalized Fraction b Normal Generalized Fraction.25.5.75.25.5.75 1 53 4 59 2 58 8 57 3 48
Aiello & Alter (216) PLoS One vol. 11 no. 1 e164546 S1 Appendix A-1 S1 Appendix: Figs A G and Table A a Tumor Generalized Fraction b Normal Generalized Fraction.25.5.75.25.5.75 1 53 4 59 2 58 8 57 3 48
Liquid biopsy: the experience of real life case studies
 Liquid biopsy: the experience of real life case studies 10 th September 2018 Beatriz Bellosillo Servicio de Anatomía Patológica Hospital del Mar, Barcelona Agenda Introduction Experience in colorectal
Liquid biopsy: the experience of real life case studies 10 th September 2018 Beatriz Bellosillo Servicio de Anatomía Patológica Hospital del Mar, Barcelona Agenda Introduction Experience in colorectal
Oncogenes and Tumor Suppressors MCB 5068 November 12, 2013 Jason Weber
 Oncogenes and Tumor Suppressors MCB 5068 November 12, 2013 Jason Weber jweber@dom.wustl.edu Oncogenes & Cancer DNA Tumor Viruses Simian Virus 40 p300 prb p53 Large T Antigen Human Adenovirus p300 E1A
Oncogenes and Tumor Suppressors MCB 5068 November 12, 2013 Jason Weber jweber@dom.wustl.edu Oncogenes & Cancer DNA Tumor Viruses Simian Virus 40 p300 prb p53 Large T Antigen Human Adenovirus p300 E1A
RNA SEQUENCING AND DATA ANALYSIS
 RNA SEQUENCING AND DATA ANALYSIS Length of mrna transcripts in the human genome 5,000 5,000 4,000 3,000 2,000 4,000 1,000 0 0 200 400 600 800 3,000 2,000 1,000 0 0 2,000 4,000 6,000 8,000 10,000 Length
RNA SEQUENCING AND DATA ANALYSIS Length of mrna transcripts in the human genome 5,000 5,000 4,000 3,000 2,000 4,000 1,000 0 0 200 400 600 800 3,000 2,000 1,000 0 0 2,000 4,000 6,000 8,000 10,000 Length
LncRNA TUSC7 affects malignant tumor prognosis by regulating protein ubiquitination: a genome-wide analysis from 10,237 pancancer
 Original Article LncRNA TUSC7 affects malignant tumor prognosis by regulating protein ubiquitination: a genome-wide analysis from 10,237 pancancer patients Xiaoshun Shi 1 *, Yusong Chen 2,3 *, Allen M.
Original Article LncRNA TUSC7 affects malignant tumor prognosis by regulating protein ubiquitination: a genome-wide analysis from 10,237 pancancer patients Xiaoshun Shi 1 *, Yusong Chen 2,3 *, Allen M.
The Role of Next Generation Sequencing in Solid Tumor Mutation Testing
 The Role of Next Generation Sequencing in Solid Tumor Mutation Testing Allie H. Grossmann MD PhD Department of Pathology, University of Utah Division of Anatomic Pathology & Oncology, ARUP Laboratories
The Role of Next Generation Sequencing in Solid Tumor Mutation Testing Allie H. Grossmann MD PhD Department of Pathology, University of Utah Division of Anatomic Pathology & Oncology, ARUP Laboratories
Research Strategy: 1. Background and Significance
 Research Strategy: 1. Background and Significance 1.1. Heterogeneity is a common feature of cancer. A better understanding of this heterogeneity may present therapeutic opportunities: Intratumor heterogeneity
Research Strategy: 1. Background and Significance 1.1. Heterogeneity is a common feature of cancer. A better understanding of this heterogeneity may present therapeutic opportunities: Intratumor heterogeneity
The Cancer Genome Atlas
 The Cancer Genome Atlas July 14, 2011 Kenna M. Shaw, Ph.D. Deputy Director The Cancer Genome Atlas Program TCGA: Core Objectives Launched in 2006 as a pilot and expanded in 2009, the goals of TCGA are
The Cancer Genome Atlas July 14, 2011 Kenna M. Shaw, Ph.D. Deputy Director The Cancer Genome Atlas Program TCGA: Core Objectives Launched in 2006 as a pilot and expanded in 2009, the goals of TCGA are
Gene-microRNA network module analysis for ovarian cancer
 Gene-microRNA network module analysis for ovarian cancer Shuqin Zhang School of Mathematical Sciences Fudan University Oct. 4, 2016 Outline Introduction Materials and Methods Results Conclusions Introduction
Gene-microRNA network module analysis for ovarian cancer Shuqin Zhang School of Mathematical Sciences Fudan University Oct. 4, 2016 Outline Introduction Materials and Methods Results Conclusions Introduction
SUPPLEMENTARY APPENDIX
 SUPPLEMENTARY APPENDIX 1) Supplemental Figure 1. Histopathologic Characteristics of the Tumors in the Discovery Cohort 2) Supplemental Figure 2. Incorporation of Normal Epidermal Melanocytic Signature
SUPPLEMENTARY APPENDIX 1) Supplemental Figure 1. Histopathologic Characteristics of the Tumors in the Discovery Cohort 2) Supplemental Figure 2. Incorporation of Normal Epidermal Melanocytic Signature
CentoCancer STRIVE FOR THE MOST COMPLETE INFORMATION
 CentoCancer STRIVE FOR THE MOST COMPLETE INFORMATION CentoCancer our most comprehensive oncogenetics panel for hereditary mutations Hereditary pathogenic variants confer an increased risk of developing
CentoCancer STRIVE FOR THE MOST COMPLETE INFORMATION CentoCancer our most comprehensive oncogenetics panel for hereditary mutations Hereditary pathogenic variants confer an increased risk of developing
