The detection and characterization of circulating tumor
|
|
|
- Aubrey Griffin
- 6 years ago
- Views:
Transcription
1 ORIGINAL ARTICLE Analysis of Circulating Tumor Cells in Patients with Non-small Cell Lung Cancer Using Epithelial Marker-Dependent and -Independent Approaches Matthew G. Krebs, MBChB, PhD,* Jian-Mei Hou, PhD,* Robert Sloane, BSc,* Lee Lancashire, PhD,* Lynsey Priest, BSc,* Daisuke Nonaka, MD, Tim H. Ward, PhD,* Alison Backen, PhD, Glen Clack, MD, Andrew Hughes, PhD, Malcolm Ranson, MD, PhD,* Fiona H. Blackhall, MD, PhD,* and Caroline Dive, PhD* Introduction: Epithelial circulating tumor cells (CTCs) are detectable in patients with non-small cell lung cancer (NSCLC). However, epithelial to mesenchymal transition, a widely reported prerequisite for metastasis, may lead to an underestimation of CTC number. We compared directly an epithelial marker-dependent (CellSearch) and a marker-independent (isolation by size of epithelial tumor cells [ISET]) technology platform for the ability to identify CTCs. Molecular characteristics of CTCs were also explored. Methods: Paired peripheral blood samples were collected from 40 chemonäive, stages IIIA to IV NSCLC patients. CTCs were enumerated by Epithelial Cell Adhesion Molecule-based immunomagnetic capture (CellSearch, Veridex) and by filtration (ISET, RareCell Diagnostics). CTCs isolated by filtration were assessed by immunohistochemistry for epithelial marker expression (cytokeratins, Epithelial Cell Adhesion Molecule, epidermal growth factor receptor) and for proliferation status (Ki67). Results: CTCs were detected using ISET in 32 of 40 (80%) patients compared with 9 of 40 (23%) patients using CellSearch. A subpopulation of CTCs isolated by ISET did not express epithelial markers. Circulating tumor microemboli (CTM, clusters of 3 CTCs) were observed in 43% patients using ISET but were undetectable by CellSearch. Up to 62% of single CTCs were positive for the *Clinical and Experimental Pharmacology Group, Paterson Institute for Cancer Research; School of Cancer and Enabling Sciences, University of Manchester; Manchester Cancer Research Centre and Manchester Academic Health Sciences Centre; Departments of Medical Oncology and Histopathology, The Christie NHS Foundation Trust, Manchester, United Kingdom; and AstraZeneca Pharmaceuticals, Alderley Park, United Kingdom. Disclosure: Tim H. Ward, PhD, was employed by the University of Manchester. Alison Backen, PhD, is an employee of Paterson Institute. Glen Clack, MD, and Andrew Hughes, PhD, are employees of and hold stock in AstraZeneca. Fiona H. Blackhall, MD, PhD, has received an EU framework FP6 grant. Caroline Dive, PhD, is an employee of Paterson Institute, has received grants for research, and has been invited to speak at meetings on translational research. Address for correspondence: Caroline Dive or Fiona Blackhall, Paterson Institute for Cancer Research, Wilmslow Road, Manchester M20 4BX, United Kingdom. cdive@picr.man.ac.uk or Fiona.blackhall@ christie.nhs.uk Copyright 2011 by the International Association for the Study of Lung Cancer ISSN: /11/ proliferation marker Ki67, whereas cells within CTM were nonproliferative. Conclusions: Both technology platforms detected NSCLC CTCs. ISET detected higher numbers of CTCs including epithelial marker negative tumor cells. ISET also isolated CTM and permitted molecular characterization. Combined with our previous CellSearch data confirming CTC number as an independent prognostic biomarker for NSCLC, we propose that this complementary dual technology approach to CTC analysis allows more complete exploration of CTCs in patients with NSCLC. Key Words: Circulating tumor cells, Non-small cell lung cancer, CellSearch, ISET. (J Thorac Oncol. 2011;XX: ) The detection and characterization of circulating tumor cells (CTCs) in cancer patients offer as yet untapped potential to further understand the biology of human cancer metastasis and to identify novel treatment strategies. Recent advances in technology have paved the way to reproducible CTC detection and enumeration and begun to reveal their potential as a real-time, minimally invasive, virtual biopsy (reviewed in Hou et al. 1 ). The robust, semiautomated Cell- Search platform (Veridex, LLC, Raritan, NJ) has been used to demonstrate prognostic significance of CTC numbers in patients with metastatic breast, prostate, and colorectal cancers 2 7 (reviewed by Krebs et al. 8 ) and subsequently has been approved by the US Food and Drug Administration as a prognostic biomarker and as an aid to monitoring treatment response in these disease types. We recently reported that CellSearch detection of 5 CTCs (per 7.5 ml of blood) in patients with advanced non-small cell lung cancer (NSCLC) is a poor prognostic factor and that a change in CTC number after a single cycle of standard-of-care chemotherapy predicts survival outcome. 9 However, two-thirds of patients with stage IV NSCLC had no detectable CTCs, and CTCs were detectable in less than 5% stage III patients using this technology platform. Detection of CTCs using CellSearch is dependent on tumor cell expression of Epithelial Cell Adhesion Molecule Journal of Thoracic Oncology Volume XX, Number XX, XXX
2 Krebs et al. Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 (EpCam), an epithelial cell marker. However, the paradigm of epithelial to mesenchymal transition (EMT) as a predominant mechanism for tumor cell invasion and metastasis raises the possibility that not all cells in the circulation will express epithelial markers Thus, we hypothesized that the low prevalence of CTCs detected with the CellSearch technology in patients with advanced NSCLC, although strongly prognostic, may be due to the loss of EpCam expression. Therefore, we explored an antigen/epcam-independent approach for the ability to identify CTCs and for the potential to perform molecular characterization. ISET(RareCell Diagnostics, Paris, France) is a filterbased, size exclusion technology capable of isolating CTCs independently of their expression of any particular marker. Using this technique, CTCs have been observed previously in patients with hepatocellular carcinoma, breast carcinoma, melanoma, and more recently in patients with early-stage, resectable NSCLC In a previous pilot study using ISET, we noted that CTCs were detectable in patients with advanced-stage NSCLC, with heterogeneity in both epithelial (E-cadherin and cytokeratin [CK]) and mesenchymal (Ncadherin and vimentin) marker expression. 20 In addition, circulating tumor microemboli (CTM; defined as clusters of cells with three or more nuclei) were identified, whereas CTM were not detected in our previous evaluation of tumor cells in 101 patients with NSCLC using CellSearch. 9 Our goal was to compare the CellSearch (epithelial dependent) and ISET (antigen/epcam independent) platforms directly for the ability to isolate CTCs in a cohort of NSCLC patients. Secondary objectives were to explore the prevalence of CTM in NSCLC patients and to examine molecular characteristics of cells isolated by filtration, specifically in relation to their epithelial marker expression and proliferative status. PATIENTS AND METHODS Patient Eligibility and Clinical Characteristics This was a prospective study conducted at the Christie Hospital, Manchester, United Kingdom. Patients with chemonäive, histologically proven NSCLC were eligible. Inclusion criteria included radiologically confirmed stage IIIA or IIIB/IV disease; World Health Organization performance status 0 2; age 18 years; and standard-of-care chemotherapy was to be administered. Patients with a history of prior malignancy, within the previous 5 years, were excluded. All patients provided written informed consent according to ethics board approved study protocols. Data were collected for age, ethnicity, histological subtype, stage of disease, smoking status, sites of metastases, and treatment received. Blood Sampling Peripheral blood (a maximum of 20 ml) samples were collected up to 1 hour before patients commencing their first cycle of chemotherapy. Blood (10 ml) was collected in a CellSave preservative tube (Veridex LLC, NJ), stored at room temperature, and processed according to standard Veridex protocols within 96 hours of collection. A further 8 to 10 ml was collected in an ethylenediaminetetraacetic acid tube (Becton Dickinson, NJ), stored at 4 C, and processed by ISET within 4 hours of collection. Samples were processed in accordance with the UK Clinical Trials regulations for compliance to Good Clinical Practice for laboratories. 21 CTC Detection by CellSearch Samples were analyzed as previously described. 22,23 A CTC was defined according to the criteria of round to oval morphology, cell size more than 4 m, 4,6-diamidino-2- phenylindole (DAPI) positive nucleus, CK positive staining, and absence of CD45 expression. CTC number is reported per 7.5 ml of blood. The sensitivity, accuracy, linearity, and reproducibility of the CellSearch system have been previously described. 22,24 Samples were considered positive for CTCs if 2 per 7.5 ml of blood were detected as one CTC has previously been reported as a normal finding. 22 CTC Detection by ISET Blood samples were divided into 1 ml aliquots and diluted 1:10 with red cell lysis buffer, containing 0.8% formaldehyde (RareCell Diagnostics), as per manufacturer s instructions. Each aliquot was placed into an individual well of a 10-well ISET filter module (consisting of a polycarbonate track-etched-type membrane punctured by 8- m cylindrical pores, supported beneath a 10-well plastic reservoir) Filtration was achieved by attaching the module to the ISET instrument and by applying regulated, gentle suction. Unfiltered cells were deposited on a 0.6-cm diameter, circular spot on the membrane beneath each well. After filtration, each spot was washed once with phosphate-buffered saline and then the membrane disassembled from the module and allowed to air dry at room temperature. Filters were subsequently stored at 20 C. The sensitivity of the ISET system has been previously reported as 1 cell per ml, 15,17,18 and similar results were found in our study (see Supplemental Table 1, Previous studies have reported that CTCs are not detectable in blood samples from healthy donors using the ISET technique. 18,19 Identification of CTCs Isolated on ISET Filters Tumor cells were identified according to absence of the leukocyte common antigen, CD45, by immunohistochemistry (IHC) and by their large, hyperchromatic, irregular-shaped nuclei. Individual spots (each representing 1 ml of filtered blood) were excised from the filter and placed in ph 6 antigen retrieval buffer (S1699, Dako) in a 99 C waterbath for 40 minutes. Spots were washed in tris-buffered saline, placed in 0.2% triton for 10 minutes to permeabilize cell membranes, and exposed for 30 minutes to 3% hydrogen peroxide methanol solution to block endogenous peroxidases. Spots were subsequently washed in water and incubated overnight at 4 C with monoclonal mouse antihuman CD45 primary antibody (clone T29/33, Dako) diluted 1:30 in antibody diluent (S0809, Dako). CD45 staining was achieved with standard Envision Kits and the Liquid DAB Substrate Chromagen System according to manufacturer s instructions (K5007, Dako) and counterstained with Gill s hematoxylin. Spots were mounted on glass slides and scanned at 400 magnification using an Olympus BX52 microscope linked 2 Copyright 2011 by the International Association for the Study of Lung Cancer
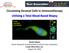
3 Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 Circulating Tumor Cells in Non-small Cell Lung Cancer FIGURE 1. Biological controls for immunohistochemistry of circulating tumor cells (CTCs) isolated by ISET. (A) Positive-staining controls and (B) negative-staining controls for each of the markers used in this study to characterize CTCs/CTM. Control staining was performed on cell lines or peripheral blood mononuclear cells (PBMCs), cytospun onto glass slides. All images were acquired at 400 magnification using a Bioview imaging system. ISET, isolation by size of epithelial tumor cells; CTM, circulating tumor microemboli. A B Mean Number Tumor Cells Recovered (per ml) Number of Filter Spots Counted C E D FIGURE 2. Exclusion of nontumor cell contaminants on ISET filters and circulating tumor cell (CTC) enumeration strategy. A, CD45 immunostaining of H460 tumor cells spiked into healthy donor blood and processed through the ISET filtration system to exclude white blood cells. Filter pores appear dark and circular or cylindrical (black arrows), white blood cells stain brown due to DAB-substrate reaction (red arrow), and tumor cells appear blue due to the hematoxylin counterstain (blue arrow). B, Bar chart demonstrating the mean and SE for CTC number depending on the number of spots analyzed. Four spots or more exhibited the most representative mean CTC value. C, VE-cadherin (CD144) staining on clinical samples processed by ISET to exclude mature circulating endothelial cells (CECs). CECs exhibited small, round, or oval, pale nuclei with low nuclear-to-cytoplasmic ratio and were positive for CD144 (blue arrows). Nuclei appear blue due to hematoxylin counterstain. A cluster of CECs is shown in the central panel. Filter pores appear dark and circular (black arrows). D, CD45 staining on clinical samples processed by ISET. Cells with CEC morphology are seen with hematoxylin-stained nuclei (blue arrows). These cells were considered to be mature CECs on the basis on their morphological similarity with CD144-positive cells (C) and absence of CD45 expression. E, Typical CD45 negative squamous skin cells exhibiting small, round, pyknotic nuclei with abundant cytoplasm (blue arrows). Filter pores appear dark and circular (exemplified by black arrow). ISET, isolation by size of epithelial tumor cells. with image analysis software (Allegro Plus, Bioview, Rehovot, Israel). Staining for CD45 was optimized using cytospins of peripheral blood mononuclear cells as the positive control and human NSCLC H460 cells (American Type Culture Collection) as the negative control (Figure 1). H460 cells were spiked into healthy donor blood, and staining was validated on filtered spots (Figure 2A). Images were reviewed manually and cells assigned as tumor if they had (i) negative CD45 expression, (ii) presence of hyperchromatic nuclei, (iii) irregularly shaped nuclei, (iv) nuclear size 12 m, (v) nuclear to cytoplasmic ratio more than 50%, and/or (vi) presence of clusters of CD45 negative cells containing three or more nuclei with morphology as described in points (ii) to (v), defined, in this study, as CTM. Images were reviewed by a Consultant Histopathologist (Dr. Daisuke Nonaka). For each clinical sample, an average CTC number was determined from four spots and extrapolated to 7.5 ml for direct comparison with CellSearch (see Statistical Considerations section for determination of optimum number of spots to stain for reliable enumeration). Copyright 2011 by the International Association for the Study of Lung Cancer 3
4 Krebs et al. Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 TABLE 1. Number of Membrane Spots (Generated after ISET Blood Filtration) Interrogated with a Range of Antibodies to Explore Molecular Characteristics of Isolated Tumor Cells Marker Number of Patients Evaluated (1 Spot Each) Antibody and Concentration Used Cells Used as Positive Control for IHC a Cells Used as Negative Control for IHC a EpCam 9 Anti-human EpCam; clone VU-1D9, Thermo Scientific; 1:100 SW620 PBMCs Cytokeratin 9 Anti-human cytokeratin; clone C-11, Thermo Scientific; 1:100 WiDr PBMCs (4, 5, 6, 8, 10, 13, 18) EGFR 4 Anti-human epidermal growth factor receptor clone E30, Dako; 1:25 WiDr SW620 VE-cadherin 4 Anti-human endothelial cell marker CD144; Clone 16B1, Huvecs PBMCs ebioscience; 1:50 Ki67 14 Anti-human Ki-67; clone MIB-1, Dako; 1:100 H460 PBMCs IgG control 4 IgG1 control; clone G01, Dako; 1:25 H1299 Positive- and negative-staining controls are listed. a SW620 and WiDr are colorectal cancer cell lines; H460 and H1299 are non-small cell lung cancer cell lines, and Huvecs are an endothelial cell line. ISET, isolation by size of epithelial tumor cells; IHC, immunohistochemistry; PBMCs, peripheral blood mononuclear cells; EGFR, epidermal growth factor receptor; EpCam, Epithelial Cell Adhesion Molecule. Additional Characterization of CTCs on ISET Filters To further characterize the captured cells, a series of filter spots from patient samples were stained for three epithelial markers (EpCam, CKs, and epidermal growth factor receptor [EGFR]). In addition, the proliferative status of CTCs and CTM was examined using Ki67. An endothelial marker (VE-cadherin) was used to exclude the presence of circulating endothelial cells (CECs). After enumeration, which used four filter spots per patient, there were up to six spots remaining per patient for CTC molecular characterization (some patients had less depending on the total amount of blood acquired). To evaluate the full range of markers, a single filter spot per patient was used for each marker. To maximize the probability of finding CTCs on a single spot, only patients with 15 or more CTCs per 7.5 ml of blood by ISET, equating to an average of 2 CTCs per spot, were chosen for this aspect of the study. With a limited number of spots exhibiting this criterion, the numbers of patients evaluated for each marker are detailed in Table 1. Slides from archival tumor biopsies, procured at diagnosis, were obtained from four patients with the highest numbers of CTCs/CTM for comparison of morphology. Hematoxylin and eosin or Papanicalou staining was performed by pathologists at the referring hospitals (as part of the diagnostic process). Slides were scanned at 400 magnification using an Olympus BX52 microscope linked with image analysis software (Allegro Plus, Bioview, Rehovot, Israel) for direct comparison with ISET-isolated CTCs/CTM. Cell Culture Cytospins were prepared from cultured cell lines or peripheral blood mononuclear cells for use as positive or negative staining controls for IHC (see Table 1 and Figure 1). Details of cell culture methodology are included in Supplementary data ( Statistical Considerations Concordance between CTC numbers detected by Cell- Search and ISET were analyzed by Bland-Altman plots. Pitman s variance ratio test was used to assess agreement. Enumeration of CTCs/CTM by ISET is a nonautomated, labor-intensive, and lengthy process. To determine the minimum number of spots to stain with CD45 for a robust and representative CTC count per milliliter, 10 ml of healthy donor blood was collected in ethylenediaminetetraacetic acid tubes and spiked with 200 NCI-H1299 human lung cancer cells. Samples were processed according to ISET methodology and recovered tumor cells counted on each of the 10 spots, each containing cells trapped from 1 ml of blood. CTC counts were then calculated from all possible combinations of spots (i.e., any 1, any 2, any 3, etc.) and mean CTC number ( SE) with 95% confidence intervals calculated for each (Figure 2B). The total number of cells recovered was 110 (mean 11). Enumerating CTCs from only 1, 2, or 3 spots exhibited a wide error in mean CTC number estimation. Analyzing four spots or more provided an accurate estimation of the true mean (mean SE , 95% confidence interval: ). Replicate experiments confirmed four spots to provide the most reliable estimation, and therefore each patient had four spots stained with CD45 for CTC enumeration, and CTC numbers were extrapolated to 7.5 ml for comparison with CellSearch. RESULTS Patient Demographics Forty-five patients with NSCLC were recruited to the study between April 2008 and May Five patients were not evaluable for CTC analysis because of insufficient blood volume for processing by both CellSearch and ISET. Thus, 40 patients with paired blood samples were evaluable for the study. Patient characteristics are reported in Table 2. 4 Copyright 2011 by the International Association for the Study of Lung Cancer
5 Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 Circulating Tumor Cells in Non-small Cell Lung Cancer TABLE 2. Patient Demographics Patients Characteristic (N 40) Age at baseline, yr Median 68 Range Sex, n (%) Female 18 (45) Male 22 (55) Race, n (%) White British 39 (97.5) South Asian 1 (2.5) Stage at diagnosis, n (%) IIIA 5 (13) IIIB 12 (30) IV 23 (57) Histological subtype, n (%) Squamous cell carcinoma (SCC) 15 (37) Adenocarcinoma 10 (25) Poorly differentiated 2 (5) Bronchioloalveolar 2 (5) NSCLC (not otherwise specified) 11 (28) Baseline WHO PS, n (%) 0 3 (8) 1 29 (72) 2 8 (20) Smoking status, n (%) Current smoker 12 (30) Ex-smoker 16 (40) Never-smoker 2 (5) Not documented 10 (25) NSCLC, non-small cell lung cancer; WHO, World Health Organization; PS, performance status. CTC Enumeration CellSearch versus ISET CTCs were detected using ISET in 32 (80%) patients out of the 40 evaluated compared with only 9 (23%) patients using CellSearch. Seven patients had CTCs detected by both ISET and CellSearch; 2 patients had CTCs detected by CellSearch only, and 25 patients had CTCs detected by ISET only. Considering both techniques together, 34 (85%) patients had detectable CTCs. Numbers of CTCs isolated, according to disease stage, are detailed in Table 3. The numbers of CTCs detected by ISET (mean, 71; range, ) were higher than those detected by CellSearch (mean, 4; range, 0 78). There was no statistical concordance between the numbers of CTCs detected by the two techniques using Bland Altman analysis (Pitman s test of difference in variance, n 40, p 0.001, r 0.988). CTC Morphology by CellSearch and ISET Examples of images of cells isolated by CellSearch and ISET are shown in Figures 3A, B. The size range of cells detected by CellSearch was 4 to 18 m and by ISET was 12 to 30 m. Cellular morphology was seen with greater clarity using ISET (in conjunction with hematoxylin staining and IHC) compared with CellSearch. Circulating Tumor Microemboli CTM, which we define here as contiguous clusters of cells containing three or more nuclei, were observed by ISET in 15 (38%) of 40 patients including 10 of 23 patients with stage IV disease, 5 of 12 patients with stage IIIB disease, and 0 of 5 patients with stage IIIA disease (Table 3). There was no association between histological subtype and the presence of CTM (Fisher s exact test p 0.44). The number of intact nuclei within a single CTM ranged from 3 to 45 and the size of CTM ranged from 20 to 130 m in diameter. Examples of typical CTM morphology, confirmed as malignant phenotype by pathology review, are shown in Figure 3C. Their appearance showed morphological similarity to clusters of cells within the corresponding primary tumor biopsy/cytology specimens (Figure 3D). In contrast, no CTM were detected in any of the 40 patients in parallel blood samples analyzed by CellSearch. CECs and Contaminant Skin Cells Previous studies, using CellSearch technology, have shown a normal finding of 1 to 20 CECs per milliliter of blood in healthy individuals and up to 387 CECs per milliliter of blood in patients with lung cancer. 25 Therefore, it is critical that CECs (reported size 10 m. 26 ) are not falsely designated as CTCs on ISET filters. This issue was explored by staining filters for the endothelial marker VE-cadherin (CD144) that is not expressed on tumor or on white blood cells. CECs were identified by positive VE-cadherin staining and exhibited morphology clearly distinct from CTCs/CTM (Figure 2C). CECs displayed regular-shaped, round, or oval nuclei, low nuclear-to-cytoplasmic ratio, and paler hematoxylin-stained nuclei compared with CTCs/CTM which exhibited irregular-shaped, large, hyperchromatic nuclei with high nuclear-to-cytoplasmic ratio. On the basis of these morphological characteristics, mature CECs could be identified and discounted when using CD45 negative selection alone (Figure 2D). However, a caveat is that progenitor CECs (a minority population) can express CD45 weakly and may not be excluded by negative selection using CD45. Contaminant skin cells were infrequently detected using ISET and were readily identified as polygonal-shaped cells with abundant cytoplasm and small round pyknotic nuclei with absence of CD45 expression (Figure 2E). EpCam Staining in CTCs Isolated by ISET To explore the hypothesis that a proportion of CTCs in patients with NSCLC may be EpCam negative, a series of nine patient spots were subjected to EpCam staining by IHC (positive and negative controls presented in Figure 1). All CTCs/CTM detected from the nine patients tested were negative for EpCam expression (Figure 4A). Nonspecific nonmembranous staining was seen in a minority of CTM, but this staining was considered to be negative (representative image in Figure 4B). Of the nine patients, six (67%) had no CTCs detected by CellSearch, whereas ISET detected between 12 and 188 CTCs/7.5 ml of blood. The other three patients had between 3 and 18 CTCs/7.5 ml of blood detected by Cell- Search but higher numbers of cells isolated by ISET (17 84 CTCs/7.5 ml of blood). Copyright 2011 by the International Association for the Study of Lung Cancer 5
6 Krebs et al. Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 TABLE 3. Comparison of the Number of CTCs and CTM Identified by CellSearch and ISET Expressed per 7.5 ml Blood Patient ID Stage Histology CTC No. by CellSearch per 7.5 ml Blood CTC No. by ISET per 7.5 ml Blood CTM No. by ISET per 7.5 ml Blood 93 IIIA SCC IIIA SCC IIIA NOS IIIA SCC IIIA SCC Mean SE IIIB BCA IIIB SCC IIIB Adeno IIIB Poor diff IIIB SCC IIIB Adeno IIIB SCC IIIB NOS IIIB SCC IIIB NOS IIIB Adeno IIIB SCC Mean SE IV NOS IV SCC IV Adeno IV SCC IV Adeno IV Adeno IV Poor diff IV NOS IV Adeno IV SCC IV Adeno IV NOS IV NOS IV SCC IV SCC IV BCA IV NOS IV NOS IV Adeno IV Adeno IV NOS IV SCC IV NOS Mean SE Adeno, Adenocarcinoma; BCA, bronchioalveolar; CTCs, circulating tumor cells; CTM, circulating tumor microemboli; NOS, not otherwise specified; Poor diff, poorly differentiated; SCC, squamous cell carcinoma. Molecular Characterization of CTCs/CTM by ISET To further characterize CTCs/CTM identified by ISET, cells were stained by IHC for the epithelial markers CKs and EGFR (positive and negative controls shown in Figure 1). Of the nine patients tested for CK staining, eight had identifiable CTCs/CTM on the single spot tested. All CTM, where present, were positive for CK, whereas the proportion of positive single cells was more heterogeneous, ranging from 0 to 90% between patients (representative images in Figure 4C). Of the four patients tested for EGFR staining, all had identifiable CTCs/CTM on the single spot tested. All CTM were again positive for EGFR, whereas the proportion of positive single cells was heterogeneous, ranging from 0 to 85% between patients (representative images in Figure 4D). 6 Copyright 2011 by the International Association for the Study of Lung Cancer
7 Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 Circulating Tumor Cells in Non-small Cell Lung Cancer A Composite CK-PE DAPI CD45 - APC C D B FIGURE 3. Circulating tumor cells (CTCs) and circulating tumor microemboli (CTM) from patients with non-small cell lung cancer (NSCLC) using CellSearch and ISET in comparison to primary tumor biopsies. A, Representative images of cells isolated by CellSearch. CTCs are defined as positive for cytokeratin and DAPI, CD45 negative, and cell size 4 m. B, Representative images of cells isolated by ISET. CTCs exhibited large, hyperchromatic, irregular-shaped nuclei (identified by hematoxylin counterstain), cell size 12 m, high nuclear-to-cytoplasmic ratio, and were CD45 negative. C, Representative images of CTM from NSCLC patients. CTM were CD45 negative, containing groups of irregularly shaped/pleomophic nuclei and enlarged nucleoli (stained by hematoxylin counterstain). D, Matched primary tumor biopsies/cytology specimens (top and middle panels stained with hematoxylin and eosin; bottom panel stained with papanicolau) show striking morphological similarity between groups of cells within tumor and CTM in a paired blood sample (adjacent images in panel C show CTM from matched patient blood samples). APC, allophycocyanin; CK, cytokeratin; PE, phycoerythrin; ISET, isolation by size of epithelial tumor cells. CTC and CTM Proliferative Status The proliferative status of CTCs and cells within CTM was explored using Ki67 in 14 patients (11, stage IV; 2, stage IIIB; and 1, stage IIIA disease). CTCs and/or CTM were identified in all cases. The proportion of CTCs staining positive for Ki67 ranged from 0 to 62% between patients with no trend according to the disease stage. In contrast, all CTM were negative for Ki67 staining (Figure 4E). DISCUSSION The introduction of the CellSearch platform has enabled robust and reliable CTC enumeration in several epithelial tumor types, 2 7,22 including NSCLC, 9 but the technique is dependent on the presence of EpCAM expression on CTCs. With EMT increasingly being reported as an important mechanism for cancer cell invasion and metastasis (with the loss of epithelial markers), this study explored the filtration-based, antigen-independent ISET system and found that higher numbers of CTCs were detected in advanced NSCLC patients compared with CellSearch. Eighty percent of patients were positive for CTCs by ISET compared with 23% by Cell- Search, in support of our hypothesis. A minority of patients were positive for CTCs by CellSearch but were negative by ISET, thus changes in protein expression cannot solely account for the differences in cell capture between the two systems. Individual CTCs isolated by ISET (18 30 m) tended to be larger than those isolated by CellSearch (4 18 m), and these physical characteristics may also contribute to the differences in numbers of CTCs isolated by each system. One of the most intriguing findings of this study was the isolation and prevalence of CTM by ISET. Preclinical in vivo studies have demonstrated that CTM may be an important mechanism of metastasis with survival advantages over single cells and greater propensity to seed distant metastates Although reported in preclinical models, studies investigating CTM in humans have been less forthcoming, mainly due to the Copyright 2011 by the International Association for the Study of Lung Cancer 7
8 Krebs et al. Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 FIGURE 4. Molecular characterization of circulating tumor cells (CTCs)/circulating tumor microemboli (CTM) isolated by ISET. Filtered membrane spots were interrogated with a range of antibodies by immunohistochemistry. A, EpCam staining in two CTCs and CTM which were all negative for this marker. B, Equivocal EpCam staining within a CTM. C, Cytokeratin staining was heterogeneous with the images of negative CTCs shown on the left and a positive CTC on the right. D, Variably positive epidermal growth factor receptor (EGFR) staining in two CTM (left and bottom right images) and a CTC (top right). E, Ki67 (proliferative status) was positive with nuclear staining in the majority of CTCs (four images shown on the left) and was negative in all CTM (representative image shown on the right). All images were taken at 400 magnification and representative images are shown. ISET, isolation by size of epithelial tumor cells. difficulty in isolating clusters of cells from the blood. However, anecdotal observations of CTM in clinical samples have been reported in several cancer types using filtration techniques,15 19 and here CTM were identified in 43% of patients with stage IIIB/IV NSCLC by ISET. Curiously, CTM were never isolated by matched samples processed by CellSearch, and this was also the case in our previous evaluation of 101 patients with advanced NSCLC by CellSearch.9 A possible explanation may relate to magnetic beads (employed by CellSearch) having insufficient capacity to enrich CTM of large physical size (upto 130 m) and weight. It is important to consider, however, the possibility that CTM may be a methodological artifact of filtration. The evidence to suggest that this is not the case is that using ISET, CTM are not seen universally in patients with NSCLC; that cell groups are not forced together when single cells are spiked into human blood unless intentionally spiked as groups of cells; and that in SCLC, CTM are seen in a subset of patients using both ISET and CellSearch, perhaps because the groups of cells are smaller and 8 lighter or because they express more EpCam or for both reasons.20,33 Furthermore, CTM in this study showed clear morphological similarities with primary tumor biopsy/cytology specimens (Figures 3C, D). A number of other studies have detected CTM using nonfiltration approaches in patients with renal, prostate, and colorectal cancers Stott et al.37 demonstrated the presence of CTM in patients with advanced NSCLC or prostate cancer using the novel herringbone-chip which is a microfluidic mixing device with EpCam-coated chevrons. The design of this device facilitates the mixing of blood as it passes through the chip allowing contact and attachment of cells with the antibody-coated surface. Cells are then directly visualized by light microscopy, and CTM were identified in a small number of patients. This lends support to the existence of CTM in patients with NSCLC as a real entity and not a methodological artifact. Here we demonstrate that the ISET system is versatile for the molecular characterization of CTCs and CTM by IHC. This is an invaluable tool as CTC research moves beyond CTC enumeration. For example, to identify predictive and/or Copyright 2011 by the International Association for the Study of Lung Cancer
9 Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 Circulating Tumor Cells in Non-small Cell Lung Cancer pharmacodynamic biomarkers to facilitate drug development, drug target levels and downstream effects of target inhibition of a novel agent may be analyzed through serial sampling of CTCs rather than undertaking serial biopsies, often challenging to obtain in lung cancer patients. CTC characterization may also provide novel insight into the biology and mechanisms of metastasis such as EMT. In this study, as proof of principle that characterization is achievable, IHC was performed on ISET filters on an exploratory basis and cells interrogated for epithelial markers and proliferative status. Analysis of EpCam expression on ISET-isolated CTCs/ CTM showed that all cells were negative for this marker, consistent with lack of CTC detection by CellSearch in the majority (six of nine) of these patients. In three of nine patients, CTCs were detected unexpectedly by CellSearch but in much lower numbers than those isolated by ISET. The reasons for this may relate to the concept of partial EMT 10 (where cells express both epithelial and mesenchymal characteristics) and differential sensitivity of the assays or may be due to the choice of EpCam antibody used. Analysis of the epithelial markers EGFR and CK showed that single cells were heterogeneous for these markers, again supporting the concept that at least a proportion of circulating cells lack epithelial characteristics. In addition, a recent study by Hofman et al. 38 compared CellSearch and ISET for the detection of CTCs in patients with early-stage, resectable NSCLC. Similar findings were reported in that higher numbers of CTCs were isolated by ISET compared with CellSearch, and a proportion of cells isolated by ISET lacked epithelial characteristics. Cells within CTM tended to be positive for EGFR and CK expression, in our study, and that they seem to maintain their cell cell interactions within CTM may suggest that they undergo EMT to a lesser degree than single CTCs. These hypotheses warrant further interrogation and provide an impetus to examine further CTC and CTM biology. As an initial step, we compared the proliferative activity (by Ki67 staining) of cells within CTM and single CTCs and revealed that cells within CTM were nonproliferative, in contrast to a majority subpopulation of solitary CTCs. This suggests that at the time of sampling, CTM do not represent groups of cells actively dividing during transit in the blood, rather the lack of proliferation is more consistent with the hypothesis that cell clusters break off from the primary tumor, invade the stroma and local vasculature en mass, and appear in the blood as the product of collective migration. 29 In contrast, up to 62% of single CTCs stained positively for Ki67. In a previous study of breast cancer patients, single CTCs were negative for Ki The difference in the proliferative status between breast cancer CTCs and lung cancer CTCs is consistent with disseminated breast cancer cells existing for many years in the bone marrow with a more protracted clinical course The natural history of lung cancer is more rapid and aggressive than the majority of breast cancers, and the finding of Ki67-positive CTCs may thus mirror the differential kinetics of disease progression. The biological and clinical significance of the observed heterogeneity of CTCs/ CTM within and between NSCLC patients remains unclear, but the opportunity now exists to explore this in further detail. TABLE 4. Advantages and Disadvantages of the CellSearch and ISET Techniques for the Isolation of CTCs from Patients with Non-small Cell Lung Cancer CellSearch EpCam-Based Pros FDA approved for prognostic use (and as an aid to monitoring treatment) in patients with breast, prostate, and colorectal cancer Robust, reliable, reproducible, semiautomated processing Stains with CK/CD45/DAPI and option to stain with one further antibody, i.e., multiparameter staining Nonsize dependent capable of detecting smaller CTCs Standardized kits Cons EpCam dependent may miss cells if they have undergone EMT process (i.e., lack epithelial markers) Expensive Limited ability to further characterize cells unless present in high numbers Numbers of CTCs low in NSCLC and no CTM detectable ISET Filtration-Based Nonantigen dependent able to detect wider range and higher number of cells than CellSearch Able to isolate CTM Better image quality of cells by IHC Cheaper than CellSearch (but still expensive) Cells amenable to interrogation by IHC, IF, FISH, and potentially DNA/RNA extraction for genetic analyses potential to explore CTC biology Filters can be stored at 20 C for later analysis Exploratory needs further validation before routine clinical use Manual processing limits robustness and reproducibility (but potential to semi-automate) Size dependent may miss cells less than 8 m in size This study used single-staining IHC but multiparameter staining readily achievable by IHC or IF FDA, Food and Drug Administration; CK, cytokeratin; FISH, fluorescent in situ hybridization; IF, immunofluorescence; IHC, immunohistochemistry; CTC, circulating tumor cell; NSCLC, non-small cell lung cancer; CTM, circulating tumor microemboli; EMT, epithelial to mesenchymal transition; EpCam, Epithelial Cell Adhesion Molecule. There are relative advantages and disadvantages to both CTC isolation techniques used in this study, but when used together they have a complementary role. ISET isolated higher numbers of CTCs and CTM than CellSearch, was versatile for molecular characterization of cells, and has the useful attribute that it allows storage of filters at 20 C for analysis of a completed patient cohort of samples at a later time. However, ISET currently lacks the acknowledged sufficient level of robust and reliable validation from regulatory agencies as afforded to CellSearch 22 with respect to accuracy and reproducibility for CTC enumeration, and this currently restricts ISET use for formally accredited CTC enumeration and prognostication. For this reason, ISET numbers were not correlated with clinical characteristics or clinical outcomes in this study. Our previous study showed the strong prognostic utility of CellSearch detected CTC number and the association of CTCs with stage IV disease and Copyright 2011 by the International Association for the Study of Lung Cancer 9
10 Krebs et al. Journal of Thoracic Oncology Volume XX, Number XX, XXX 2011 presence of liver or bone metastases compared with other sites of metastatic disease. Advantages and disadvantages of the CellSearch and ISET techniques are detailed in Table 4. This study demonstrates the complementary use of two different technologies to explore and characterize CTCs/CTM in patients with NSCLC. Our findings highlight a rationale for using combinations of approaches to delineate how CTCs/CTM can be evaluated optimally to further understanding of metastasis biology with the goal of improved therapeutics and clinical management of patients with NSCLC. ACKNOWLEDGMENTS Supported by a Clinical Pharmacology Fellowship from Cancer Research UK and AstraZeneca Ltd. (to M.K.) and through Cancer Research UK grant C147/A12328 (to C.D.) and European Union CHEMORES FP6 contract LSHC-CT (to F.B.). REFERENCES 1. Hou J-M, Krebs M, Ward T, et al. Circulating tumor cells, enumeration and beyond. Cancers 2010;2: Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351: Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23: Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12: Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26: Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20: de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14: Krebs MG, Hou J-M, Ward T, et al. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol 2010;2: Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29: Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006;66: Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2: Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006;7: Raimondi C, Gradilone A, Naso G, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat 2011;130: Mikolajczyk SD, Millar LS, Tsinberg P, et al. Detection of EpCAMnegative and cytokeratin-negative circulating tumor cells in peripheral blood. J Oncol 2011;2011: Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 2000;156: Vona G, Estepa L, Beroud C, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 2004;39: Pinzani P, Salvadori B, Simi L, et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol 2006;37: De Giorgi V, Pinzani P, Salvianti F, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol 2010;130: Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17: Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178: The Medicines for Human Use (Clinical Trials) Regulations (UK Gov) Available at: Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10: Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol 2010;2010: Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13: Rowand JL, Martin G, Doyle GV, et al. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A 2007;71: Woywodt A, Goldberg C, Scheer J, et al. An improved assay for enumeration of circulating endothelial cells. Ann Hematol 2004;83: Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 1973;9: Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 1976;36: Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009;10: Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci 2009;122: Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res 1974;34: Alpaugh ML, Tomlinson JS, Kasraeian S, et al. Cooperative role of E-cadherin and sialyl-lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene 2002;21: Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175: Kats-Ugurlu G, Roodink I, de Weijert M, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol 2009;219: Brandt B, Junker R, Griwatz C, et al. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res 1996; 56: Molnar B, Ladanyi A, Tanko L, et al. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res 2001;7: Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA 2010;107: Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-smallcell lung carcinoma: comparison of the efficacy of the CellSearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129: Muller V, Stahmann N, Riethdorf S, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res 2005;11: Hedley BD, Chambers AF. Tumor dormancy and metastasis. Adv Cancer Res 2009;102: Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 2004;10: Copyright 2011 by the International Association for the Study of Lung Cancer
In most industrialized countries, primary lung cancer is. Circulating Tumor Cells in Pulmonary Venous Blood of Primary Lung Cancer Patients
 Circulating Tumor Cells in Pulmonary Venous Blood of Primary Lung Cancer Patients Yoshitomo Okumura, MD, Fumihiro Tanaka, MD, PhD, Kazue Yoneda, Masaki Hashimoto, MD, Teruhisa Takuwa, MD, Nobuyuki Kondo,
Circulating Tumor Cells in Pulmonary Venous Blood of Primary Lung Cancer Patients Yoshitomo Okumura, MD, Fumihiro Tanaka, MD, PhD, Kazue Yoneda, Masaki Hashimoto, MD, Teruhisa Takuwa, MD, Nobuyuki Kondo,
A Novel CTC-Detecting Technique Using TelomeScan and Its Clinical Applications
 A Novel CTC-Detecting Technique Using TelomeScan and Its Clinical Applications Yasuo Urata CEO and President Oncolys BioPharma Inc. February 16, 2013 Telomere Length is a Limiting Factor for Cell Replication
A Novel CTC-Detecting Technique Using TelomeScan and Its Clinical Applications Yasuo Urata CEO and President Oncolys BioPharma Inc. February 16, 2013 Telomere Length is a Limiting Factor for Cell Replication
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Finite-element analysis of cell cluster dynamics in different cluster trap architectures. (a) Cluster-Chip (b) Filter (c) A structure identical to the Cluster-Chip except that one
Supplementary Figure 1 Finite-element analysis of cell cluster dynamics in different cluster trap architectures. (a) Cluster-Chip (b) Filter (c) A structure identical to the Cluster-Chip except that one
High Sensitivity Immunomagnetic CTC Isolation as Compared to Alternative Isolation Methods
 High Sensitivity Immunomagnetic CTC Isolation as Compared to Alternative Isolation Methods 1. Introduction: An overview of CTC isolation methods 2. Challenges for direct comparisons of CTC recovery 3.
High Sensitivity Immunomagnetic CTC Isolation as Compared to Alternative Isolation Methods 1. Introduction: An overview of CTC isolation methods 2. Challenges for direct comparisons of CTC recovery 3.
Biomarker Utility of Circulating Tumor Cells in Metastatic Cutaneous Melanoma
 ORIGINAL ARTICLE See related commentary on pg 146 Biomarker Utility of Circulating Tumor Cells in Metastatic Cutaneous Melanoma Leila Khoja 1, Paul Lorigan 2,3,CongZhou 1, Matthew Lancashire 1, Jessica
ORIGINAL ARTICLE See related commentary on pg 146 Biomarker Utility of Circulating Tumor Cells in Metastatic Cutaneous Melanoma Leila Khoja 1, Paul Lorigan 2,3,CongZhou 1, Matthew Lancashire 1, Jessica
Challenges for use of CTCs as a Diagnostic. Farideh Z. Bischoff, Ph.D. Interim CSO Sr. Director, Translational Clinical Development Biocept, Inc.
 Challenges for use of CTCs as a Diagnostic Farideh Z. ischoff, Ph.D. Interim CSO Sr. Director, Translational Clinical Development iocept, Inc. Current Technology for CTC Testing Existing CTC testing platform
Challenges for use of CTCs as a Diagnostic Farideh Z. ischoff, Ph.D. Interim CSO Sr. Director, Translational Clinical Development iocept, Inc. Current Technology for CTC Testing Existing CTC testing platform
The concept of circulating tumor cells (CTCs) was not a
 Original Article Folate Receptor Positive Circulating Tumor Cell Detected by LT-PCR Based Method as a Diagnostic Biomarker for Non Small-Cell Lung Cancer Xiaoxia Chen, MD,* Fei Zhou,* Xuefei Li, PhD, Guohua
Original Article Folate Receptor Positive Circulating Tumor Cell Detected by LT-PCR Based Method as a Diagnostic Biomarker for Non Small-Cell Lung Cancer Xiaoxia Chen, MD,* Fei Zhou,* Xuefei Li, PhD, Guohua
Detection of Circulating Tumor Cells Harboring a Unique ALK-Rearrangement in ALK- Positive Non-Small-Cell Lung Cancer.
 Detection of Circulating Tumor Cells Harboring a Unique ALK-Rearrangement in ALK- Positive Non-Small-Cell Lung Cancer Pailler, et al Data Supplement Table S1. Numbers and Percentages of ALK-Rearranged
Detection of Circulating Tumor Cells Harboring a Unique ALK-Rearrangement in ALK- Positive Non-Small-Cell Lung Cancer Pailler, et al Data Supplement Table S1. Numbers and Percentages of ALK-Rearranged
Detection of Circulating Tumor Cells Harboring a Unique ALK Rearrangement in ALK-Positive Non Small-Cell Lung Cancer
 Published Ahead of Print on May 13, 2013 as 10.1200/JCO.2012.44.5932 The latest version is at http://jco.ascopubs.org/cgi/doi/10.1200/jco.2012.44.5932 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P
Published Ahead of Print on May 13, 2013 as 10.1200/JCO.2012.44.5932 The latest version is at http://jco.ascopubs.org/cgi/doi/10.1200/jco.2012.44.5932 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P
Circulating tumor cells as biomarker for hormonal treatment in breast and prostate cancer. Michal Mego
 National Cancer Institute, Slovakia Translational Research Unit Circulating tumor cells as biomarker for hormonal treatment in breast and prostate cancer Michal Mego 2 nd Department of Oncology, Faculty
National Cancer Institute, Slovakia Translational Research Unit Circulating tumor cells as biomarker for hormonal treatment in breast and prostate cancer Michal Mego 2 nd Department of Oncology, Faculty
Incorporating pharmacodynamic, response and patient selection biomarkers. Paul Elvin PhD Chief Translational Science Officer Aptus Clinical
 Incorporating pharmacodynamic, response and patient selection biomarkers Paul Elvin PhD Chief Translational Science Officer Aptus Clinical 22 Oncology drug development Biomarkers key for: Strong hypothesis
Incorporating pharmacodynamic, response and patient selection biomarkers Paul Elvin PhD Chief Translational Science Officer Aptus Clinical 22 Oncology drug development Biomarkers key for: Strong hypothesis
Page: 1 of 10. Detection of Circulating Tumor Cells in the Management of Patients with Cancer
 Last Review Status/Date: September 2014 Page: 1 of 10 Management of Patients with Cancer Description The prognosis of cancer patients is often determined by the occurrence of metastatic disease. Studies
Last Review Status/Date: September 2014 Page: 1 of 10 Management of Patients with Cancer Description The prognosis of cancer patients is often determined by the occurrence of metastatic disease. Studies
METACELL. PERSONALIZED CANCER THERAPY USING CIRCULATING TUMOR CELLS (CTCs) METACELL LIQUID BIOPSY
 METACELL PERSONALIZED CANCER THERAPY USING CIRCULATING TUMOR CELLS (s) AN EASY WAY TO LIQUID BIOPSY MORE THAN A METASTATIC CELL IN BLOOD A STEP TOWARDS PERSONALIZED CANCER TREATMENT LIQUID BIOPSY REAL-TIME
METACELL PERSONALIZED CANCER THERAPY USING CIRCULATING TUMOR CELLS (s) AN EASY WAY TO LIQUID BIOPSY MORE THAN A METASTATIC CELL IN BLOOD A STEP TOWARDS PERSONALIZED CANCER TREATMENT LIQUID BIOPSY REAL-TIME
Cancers of unknown primary : Knowing the unknown. Prof. Ahmed Hossain Professor of Medicine SSMC
 Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Review Article Significance of Circulating Tumor Cells Detected by thecellsearchsysteminpatientswithmetastaticbreast Colorectal and Prostate Cancer
 Oncology Volume, Article ID 67, 8 pages doi:.55//67 Review Article Significance of Circulating Tumor Cells Detected by thecellsearchsysteminpatientswithmetastaticbreast Colorectal and Prostate Cancer M.
Oncology Volume, Article ID 67, 8 pages doi:.55//67 Review Article Significance of Circulating Tumor Cells Detected by thecellsearchsysteminpatientswithmetastaticbreast Colorectal and Prostate Cancer M.
Liquid Biopsy: Implications for Cancer Staging & Therapy
 Prof. Klaus Pantel, MD, PhD Institut für Tumorbiologie Liquid Biopsy: Implications for Cancer Staging & Therapy Tumor cell dissemination and cancer dormancy Primary tumor Local relapse Cancer cells disseminate
Prof. Klaus Pantel, MD, PhD Institut für Tumorbiologie Liquid Biopsy: Implications for Cancer Staging & Therapy Tumor cell dissemination and cancer dormancy Primary tumor Local relapse Cancer cells disseminate
Cover Letter. Reviewer 1:
 Cover Letter Michael Yang, M.D., Ph.D. Managing Editor of Cancer Research Frontiers 1188 Willis Ave, #109, Albertson, NY 11507, USA Phone: +1-917-426-1571 http://cancer-research-frontiers.org/ Dear Dr.
Cover Letter Michael Yang, M.D., Ph.D. Managing Editor of Cancer Research Frontiers 1188 Willis Ave, #109, Albertson, NY 11507, USA Phone: +1-917-426-1571 http://cancer-research-frontiers.org/ Dear Dr.
Circulating Tumor Cells in Solid Tumor in Metastatic and Localized Stages
 Circulating Tumor Cells in Solid Tumor in and Stages LUISA M. MAESTRO 1, JAVIER SASTRE 2, SARA B. RAFAEL 1, SILVIA B. VEGANZONES 1, MARTA VIDAURRETA 1, MIGUEL MARTÍN 2, CARLOS OLIVIER 3, VIRGINIA B. DE
Circulating Tumor Cells in Solid Tumor in and Stages LUISA M. MAESTRO 1, JAVIER SASTRE 2, SARA B. RAFAEL 1, SILVIA B. VEGANZONES 1, MARTA VIDAURRETA 1, MIGUEL MARTÍN 2, CARLOS OLIVIER 3, VIRGINIA B. DE
Circulating Tumor Cells (CTC) Technologies
 Table of Contents MIR036 I. SCOPE AND METHODOLOGY Scope of the Study Analytics and data presented in this report pertain to several parameters such as - Research Methodology This report is uniquely researched
Table of Contents MIR036 I. SCOPE AND METHODOLOGY Scope of the Study Analytics and data presented in this report pertain to several parameters such as - Research Methodology This report is uniquely researched
Layered-IHC (L-IHC): A novel and robust approach to multiplexed immunohistochemistry So many markers and so little tissue
 Page 1 The need for multiplex detection of tissue biomarkers. There is a constant and growing demand for increased biomarker analysis in human tissue specimens. Analysis of tissue biomarkers is key to
Page 1 The need for multiplex detection of tissue biomarkers. There is a constant and growing demand for increased biomarker analysis in human tissue specimens. Analysis of tissue biomarkers is key to
Circulating Stromal Cells in Immunotherapy Utilizing a Total Blood Based Biopsy
 Circulating Stromal Cells in Immunotherapy Utilizing a Total Blood Based Biopsy Cancer associated macrophage CTCs Daniel Adams Senior Research Scientist/Head of Clinical Core Laboratory Creatv MicroTech,
Circulating Stromal Cells in Immunotherapy Utilizing a Total Blood Based Biopsy Cancer associated macrophage CTCs Daniel Adams Senior Research Scientist/Head of Clinical Core Laboratory Creatv MicroTech,
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer
 Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Page: 1 of 10. Detection of Circulating Tumor Cells in the Management of Patients with Cancer
 Last Review Status/Date: September 2015 Page: 1 of 10 Management of Patients with Cancer Description The prognosis of cancer patients is often determined by the occurrence of metastatic disease. Studies
Last Review Status/Date: September 2015 Page: 1 of 10 Management of Patients with Cancer Description The prognosis of cancer patients is often determined by the occurrence of metastatic disease. Studies
Prospective Clinical Study of Circulating Tumor Cells For Colorectal Cancer Screening
 Prospective Clinical Study of Circulating Tumor Cells For Colorectal Cancer Screening Results From a Multi-Year 620-Sample Study CRC: SLOW GROWING, PREVENTABLE POLYP TO CANCER CAN TAKE 5 TO 15 YEARS CANCER
Prospective Clinical Study of Circulating Tumor Cells For Colorectal Cancer Screening Results From a Multi-Year 620-Sample Study CRC: SLOW GROWING, PREVENTABLE POLYP TO CANCER CAN TAKE 5 TO 15 YEARS CANCER
HER2 CISH pharmdx TM Kit Interpretation Guide Breast Cancer
 P A T H O L O G Y HER2 CISH pharmdx TM Kit Interpretation Guide Breast Cancer FROM CERTAINTY COMES TRUST For in vitro diagnostic use HER2 CISH pharmdx Kit HER2 CISH pharmdx Kit is intended for dual-color
P A T H O L O G Y HER2 CISH pharmdx TM Kit Interpretation Guide Breast Cancer FROM CERTAINTY COMES TRUST For in vitro diagnostic use HER2 CISH pharmdx Kit HER2 CISH pharmdx Kit is intended for dual-color
NPQR Quality Payment Program (QPP) Measures 21_18247_LS.
 NPQR Quality Payment Program (QPP) Measures 21_18247_LS MEASURE ID: QPP 99 MEASURE TITLE: Breast Cancer Resection Pathology Reporting pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes)
NPQR Quality Payment Program (QPP) Measures 21_18247_LS MEASURE ID: QPP 99 MEASURE TITLE: Breast Cancer Resection Pathology Reporting pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes)
Survey Results Q1. How would you best describe your organization?
 Survey Results Q1. How would you best describe your organization? Q2. How high would you rate the priority of Circulating Tumor Cells in you organization? Q3. What do you think is the biggest challenge
Survey Results Q1. How would you best describe your organization? Q2. How high would you rate the priority of Circulating Tumor Cells in you organization? Q3. What do you think is the biggest challenge
Evolution of Pathology
 1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
Single and Multiplex Immunohistochemistry
 Single and Multiplex Immunohistochemistry Steve Westra, BS Reagent Product Specialist Leica Biosystems IHC Theory Polyclonal vs Monoclonal Polyclonal reagents Detect a multitude of epitopes Batch to batch
Single and Multiplex Immunohistochemistry Steve Westra, BS Reagent Product Specialist Leica Biosystems IHC Theory Polyclonal vs Monoclonal Polyclonal reagents Detect a multitude of epitopes Batch to batch
Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression
 Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression Mona A. Abd-Elazeem, Marwa A. Abd- Elazeem Pathology department, Faculty of Medicine, Tanta
Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression Mona A. Abd-Elazeem, Marwa A. Abd- Elazeem Pathology department, Faculty of Medicine, Tanta
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing)
 Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing) platform with laser manipulation to efficiently purify lung
Supplementary Figure 1. Identification of tumorous sphere-forming CSCs and CAF feeder cells. The LEAP (Laser-Enabled Analysis and Processing) platform with laser manipulation to efficiently purify lung
LUNG CANCER PATHOLOGY: UPDATE ON NEUROENDOCRINE LUNG TUMORS
 LUNG CANCER PATHOLOGY: UPDATE ON NEUROENDOCRINE LUNG TUMORS William D. Travis, M.D. Attending Thoracic Pathologist Memorial Sloan Kettering Cancer Center New York, NY PULMONARY NE TUMORS CLASSIFICATION
LUNG CANCER PATHOLOGY: UPDATE ON NEUROENDOCRINE LUNG TUMORS William D. Travis, M.D. Attending Thoracic Pathologist Memorial Sloan Kettering Cancer Center New York, NY PULMONARY NE TUMORS CLASSIFICATION
LUNG CANCER. pathology & molecular biology. Izidor Kern University Clinic Golnik, Slovenia
 LUNG CANCER pathology & molecular biology Izidor Kern University Clinic Golnik, Slovenia 1 Pathology and epidemiology Small biopsy & cytology SCLC 14% NSCC NOS 4% 70% 60% 50% 63% 62% 61% 62% 59% 54% 51%
LUNG CANCER pathology & molecular biology Izidor Kern University Clinic Golnik, Slovenia 1 Pathology and epidemiology Small biopsy & cytology SCLC 14% NSCC NOS 4% 70% 60% 50% 63% 62% 61% 62% 59% 54% 51%
HOW TO GET THE MOST INFORMATION FROM A TUMOR BIOPSY
 HOW TO GET THE MOST INFORMATION FROM A TUMOR BIOPSY 7 TH Annual New York Lung Cancer Symposium Saturday, November 10, 2012 William D. Travis, M.D. Attending Thoracic Pathologist Memorial Sloan Kettering
HOW TO GET THE MOST INFORMATION FROM A TUMOR BIOPSY 7 TH Annual New York Lung Cancer Symposium Saturday, November 10, 2012 William D. Travis, M.D. Attending Thoracic Pathologist Memorial Sloan Kettering
Cytological Sub-classification of Lung Cancer: Morphologic and Molecular Characteristics. Mercè Jordà, University of Miami
 Cytological Sub-classification of Lung Cancer: Morphologic and Molecular Characteristics Mercè Jordà, University of Miami Mortality Lung cancer is the most frequent cause of cancer incidence and mortality
Cytological Sub-classification of Lung Cancer: Morphologic and Molecular Characteristics Mercè Jordà, University of Miami Mortality Lung cancer is the most frequent cause of cancer incidence and mortality
The CellCollector TM technology
 The CellCollector TM technology In vivo isolation of circulating tumor cells by the CellCollector TM system Dr. Klaus Lücke (CEO) Unmet medical need in oncology: Access to tumor cells not only at the time
The CellCollector TM technology In vivo isolation of circulating tumor cells by the CellCollector TM system Dr. Klaus Lücke (CEO) Unmet medical need in oncology: Access to tumor cells not only at the time
The Presence and Persistence of Resistant and Stem Cell- Like Tumor Cells as a Major Problem in Ovarian Cancer
 Welcome! The Presence and Persistence of Resistant and Stem Cell- Like Tumor Cells as a Major Problem in Ovarian Cancer Prof. Sabine Kasimir-Bauer Department of Gynecology and Obstetrics University Hospital
Welcome! The Presence and Persistence of Resistant and Stem Cell- Like Tumor Cells as a Major Problem in Ovarian Cancer Prof. Sabine Kasimir-Bauer Department of Gynecology and Obstetrics University Hospital
Research Article Stromal Expression of CD10 in Invasive Breast Carcinoma and Its Correlation with ER, PR, HER2-neu, and Ki67
 SAGE-Hindawi Access to Research International Breast Cancer Volume 20, Article ID 47957, 4 pages doi:0.406/20/47957 Research Article Stromal Expression of CD0 in Invasive Breast Carcinoma and Its Correlation
SAGE-Hindawi Access to Research International Breast Cancer Volume 20, Article ID 47957, 4 pages doi:0.406/20/47957 Research Article Stromal Expression of CD0 in Invasive Breast Carcinoma and Its Correlation
Personalized Medicine: Lung Biopsy and Tumor
 Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Elizabeth H. Moore, MD Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Genomic testing has resulted in a paradigm shift in the
Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Elizabeth H. Moore, MD Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Genomic testing has resulted in a paradigm shift in the
Immunohistochemistry on Fluid Specimens: Technical Considerations
 Immunohistochemistry on Fluid Specimens: Technical Considerations Blake Gilks Dept of Pathology University of British Columbia, Vancouver, BC, Canada Disclosures None Learning Objectives At the end of
Immunohistochemistry on Fluid Specimens: Technical Considerations Blake Gilks Dept of Pathology University of British Columbia, Vancouver, BC, Canada Disclosures None Learning Objectives At the end of
Review Article Significance of Circulating Tumor Cells in Soft Tissue Sarcoma
 Analytical Cellular Pathology Volume 2015, Article ID 697395, 5 pages http://dx.doi.org/10.1155/2015/697395 Review Article Significance of Circulating Tumor Cells in Soft Tissue Sarcoma Chiara Nicolazzo
Analytical Cellular Pathology Volume 2015, Article ID 697395, 5 pages http://dx.doi.org/10.1155/2015/697395 Review Article Significance of Circulating Tumor Cells in Soft Tissue Sarcoma Chiara Nicolazzo
Endobronchial Ultrasound in the Diagnosis & Staging of Lung Cancer
 Endobronchial Ultrasound in the Diagnosis & Staging of Lung Cancer Dr Richard Booton PhD FRCP Lead Lung Cancer Clinician, Consultant Respiratory Physician & Speciality Director Manchester University NHS
Endobronchial Ultrasound in the Diagnosis & Staging of Lung Cancer Dr Richard Booton PhD FRCP Lead Lung Cancer Clinician, Consultant Respiratory Physician & Speciality Director Manchester University NHS
Youngnam Cho. National Cancer Center Biomarker Branch
 Youngnam Cho National Cancer Center Biomarker Branch Contents 1. Liquid Biopsy 2. Circulating Tumor Cells from Blood 3. Cell-free DNA from Blood 1. Liquid biopsy Cancer Diagnosis IMAGING TISSUE BIOPSY
Youngnam Cho National Cancer Center Biomarker Branch Contents 1. Liquid Biopsy 2. Circulating Tumor Cells from Blood 3. Cell-free DNA from Blood 1. Liquid biopsy Cancer Diagnosis IMAGING TISSUE BIOPSY
Cancer Biology Course. Invasion and Metastasis
 Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Circulating tumor cells/dna/etc for Radiation Oncologists
 Circulating tumor cells/dna/etc for Radiation Oncologists Andrew Z. Wang, M.D. Associate Professor Director of Clinical and Translational Research Department of Radiation Oncology Carolina Center for Cancer
Circulating tumor cells/dna/etc for Radiation Oncologists Andrew Z. Wang, M.D. Associate Professor Director of Clinical and Translational Research Department of Radiation Oncology Carolina Center for Cancer
CANCER. Clinical Validation of Breast Cancer Predictive Markers
 Clinical Validation of Breast Cancer Predictive Markers David Hicks, MD Loralee McMahon, MS, HTL(ASCP) CANCER The human body is composed of billions of highly regulated cells Cancer cells no longer respond
Clinical Validation of Breast Cancer Predictive Markers David Hicks, MD Loralee McMahon, MS, HTL(ASCP) CANCER The human body is composed of billions of highly regulated cells Cancer cells no longer respond
Supplementary Figure 1. Double-staining immunofluorescence analysis of invasive colon and breast cancers. Specimens from invasive ductal breast
 Supplementary Figure 1. Double-staining immunofluorescence analysis of invasive colon and breast cancers. Specimens from invasive ductal breast carcinoma (a) and colon adenocarcinoma (b) were staining
Supplementary Figure 1. Double-staining immunofluorescence analysis of invasive colon and breast cancers. Specimens from invasive ductal breast carcinoma (a) and colon adenocarcinoma (b) were staining
Corporate Medical Policy
 Corporate Medical Policy Microarray-based Gene Expression Testing for Cancers of Unknown File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microarray-based_gene_expression_testing_for_cancers_of_unknown_primary
Corporate Medical Policy Microarray-based Gene Expression Testing for Cancers of Unknown File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microarray-based_gene_expression_testing_for_cancers_of_unknown_primary
Assessment performed on Tuesday, July 29, 2014, at Lions Gate Hospital, North Vancouver
 Assessors report for ciqc Run 37: BRAF V600E (April 2014) Assessors: B Gilks, R Wolber, K Ung, P Tavassoli, J Garratt and J Won (recorder) Assessment performed on Tuesday, July 29, 2014, at Lions Gate
Assessors report for ciqc Run 37: BRAF V600E (April 2014) Assessors: B Gilks, R Wolber, K Ung, P Tavassoli, J Garratt and J Won (recorder) Assessment performed on Tuesday, July 29, 2014, at Lions Gate
Patient Selection: The Search for Immunotherapy Biomarkers
 Patient Selection: The Search for Immunotherapy Biomarkers Mark A. Socinski, MD Executive Medical Director Florida Hospital Cancer Institute Orlando, Florida Patient Selection Clinical smoking status Histologic
Patient Selection: The Search for Immunotherapy Biomarkers Mark A. Socinski, MD Executive Medical Director Florida Hospital Cancer Institute Orlando, Florida Patient Selection Clinical smoking status Histologic
Quality ID #395: Lung Cancer Reporting (Biopsy/Cytology Specimens) National Quality Strategy Domain: Communication and Care Coordination
 Quality ID #395: Lung Cancer Reporting (Biopsy/Cytology Specimens) National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE:
Quality ID #395: Lung Cancer Reporting (Biopsy/Cytology Specimens) National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE:
Inertia based microfluidic capture and characterisation of circulating tumour cells for the diagnosis of lung cancer
 Original Article Page 1 of 6 Inertia based microfluidic capture and characterisation of circulating tumour cells for the diagnosis of lung cancer Dimple Y. Chudasama 1,2,3, Daria V. Freydina 1,4, Maxim
Original Article Page 1 of 6 Inertia based microfluidic capture and characterisation of circulating tumour cells for the diagnosis of lung cancer Dimple Y. Chudasama 1,2,3, Daria V. Freydina 1,4, Maxim
Next-Generation Immunohistochemistry: Multiplex tissue imaging with mass cytometry
 Nat Met, April 2014 Nat Med, April 2014 Next-Generation Immunohistochemistry: Multiplex tissue imaging with mass cytometry Journal Club Timo Böge Overview Introduction Conventional Immunohistochemistry
Nat Met, April 2014 Nat Med, April 2014 Next-Generation Immunohistochemistry: Multiplex tissue imaging with mass cytometry Journal Club Timo Böge Overview Introduction Conventional Immunohistochemistry
The Avatar System TM Yields Biologically Relevant Results
 Application Note The Avatar System TM Yields Biologically Relevant Results Liquid biopsies stand to revolutionize the cancer field, enabling early detection and noninvasive monitoring of tumors. In the
Application Note The Avatar System TM Yields Biologically Relevant Results Liquid biopsies stand to revolutionize the cancer field, enabling early detection and noninvasive monitoring of tumors. In the
High Level of Chromosomal Instability in Circulating Tumor. Cells of ROS1-Rearranged Non-Small-Cell Lung Cancer
 Annals of Oncology Advance Access published April 6, 2015 1 High Level of Chromosomal Instability in Circulating Tumor Cells of ROS1-Rearranged Non-Small-Cell Lung Cancer E. Pailler 1,2, N. Auger 3, C.R.
Annals of Oncology Advance Access published April 6, 2015 1 High Level of Chromosomal Instability in Circulating Tumor Cells of ROS1-Rearranged Non-Small-Cell Lung Cancer E. Pailler 1,2, N. Auger 3, C.R.
Human Papillomavirus Testing in Head and Neck Carcinomas
 Human Papillomavirus Testing in Head and Neck Carcinomas Guideline from the College of American Pathologists Early Online Release Publication: Archives of Pathology & Laboratory Medicine 12/18/2017 Overview
Human Papillomavirus Testing in Head and Neck Carcinomas Guideline from the College of American Pathologists Early Online Release Publication: Archives of Pathology & Laboratory Medicine 12/18/2017 Overview
A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis
 APPLICATION NOTE Cell-Free DNA Isolation Kit A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis Abstract Circulating cell-free DNA (cfdna) has been shown
APPLICATION NOTE Cell-Free DNA Isolation Kit A complete next-generation sequencing workfl ow for circulating cell-free DNA isolation and analysis Abstract Circulating cell-free DNA (cfdna) has been shown
HER2 FISH pharmdx TM Interpretation Guide - Breast Cancer
 P A T H O L O G Y HER2 FISH pharmdx TM Interpretation Guide - Breast Cancer For In Vitro Diagnostic Use FDA approved as an aid in the assessment of patients for whom Herceptin TM (trastuzumab) treatment
P A T H O L O G Y HER2 FISH pharmdx TM Interpretation Guide - Breast Cancer For In Vitro Diagnostic Use FDA approved as an aid in the assessment of patients for whom Herceptin TM (trastuzumab) treatment
Supplemental Figure S1. RANK expression on human lung cancer cells.
 Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
INTRODUCTION TO PATHOLOGICAL TECHNIQUES. 1. Types of routine biopsy procedures 2. Special exams (IHC, FISH)
 INTRODUCTION TO PATHOLOGICAL TECHNIQUES 1. Types of routine biopsy procedures 2. Special exams (IHC, FISH) Biopsy-Indications Diffuse/multifocal lesions (neoplastic, inflammatory, etc) Etiology of the
INTRODUCTION TO PATHOLOGICAL TECHNIQUES 1. Types of routine biopsy procedures 2. Special exams (IHC, FISH) Biopsy-Indications Diffuse/multifocal lesions (neoplastic, inflammatory, etc) Etiology of the
Abstract. Background. Objective
 Molecular epidemiology of clinical tissues with multi-parameter IHC Poster 237 J Ruan 1, T Hope 1, J Rheinhardt 2, D Wang 2, R Levenson 1, T Nielsen 3, H Gardner 2, C Hoyt 1 1 CRi, Woburn, Massachusetts,
Molecular epidemiology of clinical tissues with multi-parameter IHC Poster 237 J Ruan 1, T Hope 1, J Rheinhardt 2, D Wang 2, R Levenson 1, T Nielsen 3, H Gardner 2, C Hoyt 1 1 CRi, Woburn, Massachusetts,
1/23/2017. Alarice Lowe, MD Assistant Professor of Pathology Director, Circulating Tumor Cell Lab Brigham and Women s Hospital Harvard Medical School
 Application of Cytologic Techniques to Circulating Tumor Cell Specimens Alarice Lowe, MD Assistant Professor of Pathology Director, Circulating Tumor Cell Lab Brigham and Women s Hospital Harvard Medical
Application of Cytologic Techniques to Circulating Tumor Cell Specimens Alarice Lowe, MD Assistant Professor of Pathology Director, Circulating Tumor Cell Lab Brigham and Women s Hospital Harvard Medical
Disclosure of Relevant Financial Relationships NON-SMALL CELL LUNG CANCER: 70% PRESENT IN ADVANCED STAGE
 MORPHOLOGY AND MOLECULAR TESTING IN NON-SMALL CELL OF LUNG NEW FRONTIEIRS IN CYTOPATHOLOGY PRACTICE American Society for Cytopathology San Antonio, Texas Sunday March 5, 2017 Disclosure of Relevant Financial
MORPHOLOGY AND MOLECULAR TESTING IN NON-SMALL CELL OF LUNG NEW FRONTIEIRS IN CYTOPATHOLOGY PRACTICE American Society for Cytopathology San Antonio, Texas Sunday March 5, 2017 Disclosure of Relevant Financial
Quality ID #395: Lung Cancer Reporting (Biopsy/Cytology Specimens) National Quality Strategy Domain: Communication and Care Coordination
 Quality ID #395: Lung Cancer Reporting (Biopsy/Cytology Specimens) National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE:
Quality ID #395: Lung Cancer Reporting (Biopsy/Cytology Specimens) National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE:
High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer.
 Biomedical Research 2017; 28 (18): 7779-7783 ISSN 0970-938X www.biomedres.info High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer. Hu Song 1, Qi-yu Liu 2, Zhi-wei
Biomedical Research 2017; 28 (18): 7779-7783 ISSN 0970-938X www.biomedres.info High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer. Hu Song 1, Qi-yu Liu 2, Zhi-wei
Cell Migration and Invasion Assays INCUCYTE LIVE-CELL ANALYSIS SYSTEM. Real-time automated measurements of cell motility inside your incubator
 Cell Migration and Invasion Assays INCUCYTE LIVE-CELL ANALYSIS SYSTEM Real-time automated measurements of cell motility inside your incubator See the whole story Real-time cell motility visualization and
Cell Migration and Invasion Assays INCUCYTE LIVE-CELL ANALYSIS SYSTEM Real-time automated measurements of cell motility inside your incubator See the whole story Real-time cell motility visualization and
Detection of the Circulating Tumor Cells in Cancer Patients
 Detection of the Circulating Tumor Cells in Cancer Patients Athanasios Armakolas; Zacharoula Panteleakou; Adrianos Nezos; Aikaterini Tsouma; Maria Skondra; Peter Lembessis; Nikolaos Pissimissis; Michael
Detection of the Circulating Tumor Cells in Cancer Patients Athanasios Armakolas; Zacharoula Panteleakou; Adrianos Nezos; Aikaterini Tsouma; Maria Skondra; Peter Lembessis; Nikolaos Pissimissis; Michael
Award Number: W81XWH TITLE: Characterizing an EMT Signature in Breast Cancer. PRINCIPAL INVESTIGATOR: Melanie C.
 AD Award Number: W81XWH-08-1-0306 TITLE: Characterizing an EMT Signature in Breast Cancer PRINCIPAL INVESTIGATOR: Melanie C. Bocanegra CONTRACTING ORGANIZATION: Leland Stanford Junior University Stanford,
AD Award Number: W81XWH-08-1-0306 TITLE: Characterizing an EMT Signature in Breast Cancer PRINCIPAL INVESTIGATOR: Melanie C. Bocanegra CONTRACTING ORGANIZATION: Leland Stanford Junior University Stanford,
Disclosures. Outline. What IS tumor budding?? Tumor Budding in Colorectal Carcinoma: What, Why, and How. I have nothing to disclose
 Tumor Budding in Colorectal Carcinoma: What, Why, and How Disclosures I have nothing to disclose Soo-Jin Cho, MD, PhD Assistant Professor UCSF Dept of Pathology Current Issues in Anatomic Pathology 2017
Tumor Budding in Colorectal Carcinoma: What, Why, and How Disclosures I have nothing to disclose Soo-Jin Cho, MD, PhD Assistant Professor UCSF Dept of Pathology Current Issues in Anatomic Pathology 2017
Thermo Scientific UltraVision Quanto for Immunohistochemistry The New Generation Micro-Polymer Detection System
 Thermo Scientific for Immunohistochemistry The New Generation Micro-Polymer Detection System highest sensitivity sharp crisp clear shorter incubation times UltraVision Quanto the new Micro-Polymer System
Thermo Scientific for Immunohistochemistry The New Generation Micro-Polymer Detection System highest sensitivity sharp crisp clear shorter incubation times UltraVision Quanto the new Micro-Polymer System
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Image analysis in IHC overview, considerations and applications
 Image analysis in IHC overview, considerations and applications Rasmus Røge, MD, Institute of Pathology, Aalborg University Hospital NordiQC workshop September 2016 Aalborg, Denmark Outline Theory Image
Image analysis in IHC overview, considerations and applications Rasmus Røge, MD, Institute of Pathology, Aalborg University Hospital NordiQC workshop September 2016 Aalborg, Denmark Outline Theory Image
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Mario Giuliano Trieste Novembre 2015
 Mario Giuliano Trieste 20-21 Novembre 2015 Metastatic Cascade Main Actors A small fraction of cells detaching from primary tumors end up forming metastatic lesions. 1 0 Tumor Circulating Tumor Cells (CTCs)
Mario Giuliano Trieste 20-21 Novembre 2015 Metastatic Cascade Main Actors A small fraction of cells detaching from primary tumors end up forming metastatic lesions. 1 0 Tumor Circulating Tumor Cells (CTCs)
CIRCULATING TUMOR CELL (CTC) DIAGNOSTICS: TECHNOLOGIES AND GLOBAL MARKETS
 CIRCULATING TUMOR CELL (CTC) DIAGNOSTICS: TECHNOLOGIES AND GLOBAL MARKETS PHM153A January 2014 Usha Nagavarapu Project Analyst ISBN: 1-56965-671-1 BCC Research 49 Walnut Park, Building 2 Wellesley, MA
CIRCULATING TUMOR CELL (CTC) DIAGNOSTICS: TECHNOLOGIES AND GLOBAL MARKETS PHM153A January 2014 Usha Nagavarapu Project Analyst ISBN: 1-56965-671-1 BCC Research 49 Walnut Park, Building 2 Wellesley, MA
Applying Genomics to Cancer 21 st September The Frequency of EGFR mutations in Lung Adenocarcinoma: The Cardiff Experience
 Applying Genomics to Cancer 21 st September 2015 The Frequency of EGFR mutations in Lung Adenocarcinoma: The Cardiff Experience Aled Daniels R Butler, R Attanoos, H Davies University Hospital of Wales
Applying Genomics to Cancer 21 st September 2015 The Frequency of EGFR mutations in Lung Adenocarcinoma: The Cardiff Experience Aled Daniels R Butler, R Attanoos, H Davies University Hospital of Wales
Lung cancer is the leading cancer of all cancer-related death
 ORIGINAL ARTICLE Preliminary Investigation of the Clinical Significance of Detecting Circulating Tumor Cells Enriched from Lung Cancer Patients Chi Wu, MD,* Huaijie Hao, MS, Longyun Li, MD,* Xiaoyun Zhou,
ORIGINAL ARTICLE Preliminary Investigation of the Clinical Significance of Detecting Circulating Tumor Cells Enriched from Lung Cancer Patients Chi Wu, MD,* Huaijie Hao, MS, Longyun Li, MD,* Xiaoyun Zhou,
NCRI Biomarkers & Imaging CSG Cell-free DNA workshop
 NCRI Biomarkers & Imaging CSG Cell-free DNA workshop Workshop Report Christie Education Centre, Manchester 30th January 2014 Sponsored by Workshop summary 86 delegates from a variety of specialities attended
NCRI Biomarkers & Imaging CSG Cell-free DNA workshop Workshop Report Christie Education Centre, Manchester 30th January 2014 Sponsored by Workshop summary 86 delegates from a variety of specialities attended
Circulating Tumor Cells in non- Metastatic Triple Negative Breast Cancer
 Circulating Tumor Cells in non- Metastatic Triple Negative Breast Cancer Carolyn Hall, Ph.D. Department of Surgical Oncology The University of Texas MD Anderson Cancer Center Triple Negative Breast Cancer
Circulating Tumor Cells in non- Metastatic Triple Negative Breast Cancer Carolyn Hall, Ph.D. Department of Surgical Oncology The University of Texas MD Anderson Cancer Center Triple Negative Breast Cancer
Results you can trust
 PRODUCT I NF OR MAT ION pharmdx Results you can trust The first and only FDA-approved PD-L1 test to assess the magnitude of treatment effect on progression-free survival in melanoma patients from OPDIVO
PRODUCT I NF OR MAT ION pharmdx Results you can trust The first and only FDA-approved PD-L1 test to assess the magnitude of treatment effect on progression-free survival in melanoma patients from OPDIVO
Presentation material is for education purposes only. All rights reserved URMC Radiology Page 1 of 98
 Presentation material is for education purposes only. All rights reserved. 2011 URMC Radiology Page 1 of 98 Radiology / Pathology Conference February 2011 Brooke Koltz, Cytopathology Resident Presentation
Presentation material is for education purposes only. All rights reserved. 2011 URMC Radiology Page 1 of 98 Radiology / Pathology Conference February 2011 Brooke Koltz, Cytopathology Resident Presentation
Post Neoadjuvant therapy: issues in interpretation
 Post Neoadjuvant therapy: issues in interpretation Disclosure: Overview D Prognostic features in assessment of post treatment specimens: Tumor size Cellularity Grade Receptors LN Neoadjuvant chemotherapy:
Post Neoadjuvant therapy: issues in interpretation Disclosure: Overview D Prognostic features in assessment of post treatment specimens: Tumor size Cellularity Grade Receptors LN Neoadjuvant chemotherapy:
Dr. dr. Primariadewi R, SpPA(K)
 Curriculum Vitae Dr. dr. Primariadewi R, SpPA(K) Education : Medical Doctor from UKRIDA Doctoral Degree from Faculty of Medicine University of Indonesia Pathologist Specialist and Consultant from Faculty
Curriculum Vitae Dr. dr. Primariadewi R, SpPA(K) Education : Medical Doctor from UKRIDA Doctoral Degree from Faculty of Medicine University of Indonesia Pathologist Specialist and Consultant from Faculty
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
* * * * Supplementary Figure 1. DS Lv CK HSA CK HSA. CK Col-3. CK Col-3. See overleaf for figure legend. Cancer cells
 Supplementary Figure 1 Cancer cells Desmoplastic stroma Hepatocytes Pre-existing sinusoidal blood vessel New blood vessel a Normal liver b Desmoplastic HGP c Pushing HGP d Replacement HGP e f g h i DS
Supplementary Figure 1 Cancer cells Desmoplastic stroma Hepatocytes Pre-existing sinusoidal blood vessel New blood vessel a Normal liver b Desmoplastic HGP c Pushing HGP d Replacement HGP e f g h i DS
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-04-1-0618 TITLE: Are Breast Tumor Stem Cells Responsible for Metastasis and Angiogenesis PRINCIPAL INVESTIGATOR: Quintin Pan, Ph.D. CONTRACTING ORGANIZATION: University of Michigan
AD Award Number: W81XWH-04-1-0618 TITLE: Are Breast Tumor Stem Cells Responsible for Metastasis and Angiogenesis PRINCIPAL INVESTIGATOR: Quintin Pan, Ph.D. CONTRACTING ORGANIZATION: University of Michigan
3/27/2017. Pulmonary Pathology Specialty Conference. Disclosure of Relevant Financial Relationships. Clinical History:
 Pulmonary Pathology Specialty Conference Saul Suster, M.D. Medical College of Wisconsin Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position
Pulmonary Pathology Specialty Conference Saul Suster, M.D. Medical College of Wisconsin Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position
Statistical Analysis of Biomarker Data
 Statistical Analysis of Biomarker Data Gary M. Clark, Ph.D. Vice President Biostatistics & Data Management Array BioPharma Inc. Boulder, CO NCIC Clinical Trials Group New Investigator Clinical Trials Course
Statistical Analysis of Biomarker Data Gary M. Clark, Ph.D. Vice President Biostatistics & Data Management Array BioPharma Inc. Boulder, CO NCIC Clinical Trials Group New Investigator Clinical Trials Course
SSM signature genes are highly expressed in residual scar tissues after preoperative radiotherapy of rectal cancer.
 Supplementary Figure 1 SSM signature genes are highly expressed in residual scar tissues after preoperative radiotherapy of rectal cancer. Scatter plots comparing expression profiles of matched pretreatment
Supplementary Figure 1 SSM signature genes are highly expressed in residual scar tissues after preoperative radiotherapy of rectal cancer. Scatter plots comparing expression profiles of matched pretreatment
Product Introduction. Product Codes: HCL029, HCL030 and HCL031. Issue
 Product Introduction Product Codes: HCL029, HCL030 and HCL031 Issue 1. 180510 Contents Introduction to Estrogen Receptor 2 ER immunohistochemistry 3 Quality control 5 Cell lines as controls 6 Estrogen
Product Introduction Product Codes: HCL029, HCL030 and HCL031 Issue 1. 180510 Contents Introduction to Estrogen Receptor 2 ER immunohistochemistry 3 Quality control 5 Cell lines as controls 6 Estrogen
CINtec PLUS Cytology. Interpretation training
 CINtec PLUS Cytology Interpretation training Objectives After reviewing this learning module, you will have a basic understanding of how to interpret CINtec PLUS Cytology, including: The mechanism of action
CINtec PLUS Cytology Interpretation training Objectives After reviewing this learning module, you will have a basic understanding of how to interpret CINtec PLUS Cytology, including: The mechanism of action
Utility of Adequate Core Biopsy Samples from Ultrasound Biopsies Needed for Today s Breast Pathology
 Utility of Adequate Core Biopsy Samples from Ultrasound Biopsies Needed for Today s Breast Pathology Ugur Ozerdem, M.D. 1 Abstract Background: There is a paradigm shift in breast biopsy philosophy. In
Utility of Adequate Core Biopsy Samples from Ultrasound Biopsies Needed for Today s Breast Pathology Ugur Ozerdem, M.D. 1 Abstract Background: There is a paradigm shift in breast biopsy philosophy. In
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury
 Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
HER2 status assessment in breast cancer. Marc van de Vijver Academic Medical Centre (AMC), Amsterdam
 HER2 status assessment in breast cancer Marc van de Vijver Academic Medical Centre (AMC), Amsterdam 13e Bossche Mamma Congres 17 th June 2015 Modern cancer therapies are based on sophisticated molecular
HER2 status assessment in breast cancer Marc van de Vijver Academic Medical Centre (AMC), Amsterdam 13e Bossche Mamma Congres 17 th June 2015 Modern cancer therapies are based on sophisticated molecular
Qué hemos aprendido hasta hoy? What have we learned so far?
 Qué hemos aprendido hasta hoy? What have we learned so far? Luís Costa Hospital de Santa Maria & Instituto de Medicina Molecular Faculdade de Medicina de Lisboa Disclosures Research Grants: Amgen; Novartis;
Qué hemos aprendido hasta hoy? What have we learned so far? Luís Costa Hospital de Santa Maria & Instituto de Medicina Molecular Faculdade de Medicina de Lisboa Disclosures Research Grants: Amgen; Novartis;
Assessment Run B HER2 IHC
 Assessment Run B24 2017 HER2 IHC Material The slide to be stained for HER2 comprised the following 5 materials: IHC: HER2 Score* (0, 1+, 2+, 3+) FISH: HER2 gene/chr 17 ratio** 1. Breast carcinoma, no.
Assessment Run B24 2017 HER2 IHC Material The slide to be stained for HER2 comprised the following 5 materials: IHC: HER2 Score* (0, 1+, 2+, 3+) FISH: HER2 gene/chr 17 ratio** 1. Breast carcinoma, no.
Evaluation of cyclin-dependent kinase inhibitor p27 and Bcl-2 protein in nonsmall cell lung cancer
 166 Turkish Journal of Cancer Volume 35, No.4, 2005 Evaluation of cyclin-dependent kinase inhibitor p27 and cl-2 protein in nonsmall cell lung cancer LEVENT DERTS Z 1, GÜLY ÖZ L M 2, LKNUR KÜKNER 2, REM
166 Turkish Journal of Cancer Volume 35, No.4, 2005 Evaluation of cyclin-dependent kinase inhibitor p27 and cl-2 protein in nonsmall cell lung cancer LEVENT DERTS Z 1, GÜLY ÖZ L M 2, LKNUR KÜKNER 2, REM
