Supplementary Online Content
|
|
|
- Edwin King
- 6 years ago
- Views:
Transcription
1 Supplementary Online Content Montagut C, Argilés G, Ciardiello F, et al. Efficacy of Sym4 in patients with metastatic colorectal cancer with acquired resistance to anti-egfr therapy and molecularly selected by circulating tumor DNA analyses: a phase 2 randomized clinical trial. Published online February 8, 218. JAMA Oncology. doi:1.11/jamaoncol emethods. efigure 1. Overview of the 7 genes included in the Guardant36 version 2.9 panel efigure 2. Kaplan-Meier survival estimates for Overall Survival in ITT population efigure 3. Baseline Genotyping for Genomic Biomarker Analysis for Patients Included in the Sym4-5 Study efigure 4. Number of genomic alterations (single nucleotide variants, copy number variants, indels, and fusions) identified in circulating tumor DNA from patients (N=193), listed by gene. efigure 5. Patient distribution and frequency of EGFR single nucleotide variant missense mutations efigure 6. Structural modeling of EGFR and mapping of EGFR ECD mutations identified in the patient cohort efigure 7. Dose-response curves showing the effect of the indicated antibodies on cell viability in NIH-3T3 cells stably overexpressing WT or mutant EGF efigure 8. Ability of Sym4 to block ligand induced phosphorylation of EGFR in NIH-3T3 cells transfected with either WT or mutant EGFR efigure 9. Total EGFR levels after 48 hours of treatment with the indicated antibodies, as determined by Simple Western analysis efigure 1. Venn diagrams depicting the number (fraction of all profiled patients in parentheses) of patients harboring concurrent mutations in the EGFR ECD (G465E, G465R, S464L, S492R, V441D, and V441G) and KRAS/NRAS exons 2, 3, and 4 (RAS), as well as BRAF V6E, at various mutant allele frequencies (MAFs) efigure 11. Examples of tumor growth curves in PDX models efigure 12. Bar graphs depicting overall survival (OS) for each genetically profiled patient efigure 13, 14 and 15. Oncoprints depicting the full ctdna profiles of patients treated with Sym4 12 mg/kg (efigure 13), Sym4 9/6 mg/kg (efigure 14), or investigator s choice (efigure 15) efigure 16. Number of genetic alterations in all ctdna profiled patients compared to patients harboring EGFR ECD mutations etable 1. Response in ITT population (evaluable patients) etable 2. Overall survival subsets analysis etable 3. Incidence of treatment emergent adverse effects (TEAE) etable 4. Baseline characteristics of DNmCRC and TNmCRC populations This supplementary material has been provided by the authors to give readers additional information about their work. 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
2 emethods NIH-3T3 cells (ATCC) stably overexpressing WT EGFR or EGFR ECD mutations were generated using retroviral transduction. Full length human EGFR constructs were synthesized (by Genscript) and subcloned into the retroviral vector pqcxip (Clontech). The presence of specific EGFR ECD mutations was confirmed by sequencing. A VSV-G pseudotyped retrovirus (Cell Biolabs) was produced using the Phoenix-AMPHO packaging cell line (ATCC), and filtered supernatants containing 8 µg/ml polybrene (Sigma) were used to infect NIH-3T3 cells. Cells were selected in 2 µg/ml puromycin (Thermo Fisher). 2-fold serial dilution of antibodies starting from 25 µg/ml was used to generate dose-response curves. Cells were cultured in the presence of antibodies in medium containing 2% FBS. After 96 hours of culture cell viability was determined using the WST-1 assay (Roche Diagnostics). To assess antibody binding, A 4-fold serial dilution of unlabeled antibodies was used to generate doseresponse curves. Transfected cells were washed and incubated with goat anti-human IgG (H+L)-Alexa Fluor 647 (R&D Systems) at a 1:25 dilution for 3 minutes, before a fluorescence readout was measured using the ique Screener platform (IntelliCyt). For quantification of Total EGFR and pegfr Levels by Simple Western, lysates were generated using Pierce RIPA buffer containing protease inhibitors (Thermo Scientific) and phosphatase inhibitors (Calbiochem). Samples for Simple Western analysis were diluted to.2 µg/µl in a master mix containing internal fluorescent standards and reducing agent, and were processed per standard protocol using a Sally Sue instrument (ProteinSimple). Antibodies against total EGFR (C74B9), pegfr (Y168), and Pan- Actin (all from Cell Signaling Technology) were diluted 1:5. CRC PDX tumor xenografts were derived from surgical specimens from cancer patients and were established and characterized at EPO-GmbH, Germany or Oncotest, Germany. After transplantation of 2 2 mm tumor fragments to NMRI-Foxn1nu mice, tumors were measured at least twice weekly. When tumors reached 5-25 mm3, preferably 8-2 mm3, animals were distributed into experimental groups with the aim of having comparable median and mean group tumor volumes of approximately 1-2 mm3, and treatment was initiated. The experiment was performed with 1 animals/group and three groups/model: vehicle control, Sym4, and cetuximab. Sym4 and cetuximab were administered at a dose of 3 mg/kg intraperitoneally (i.p.) twice weekly for 5 weeks (9-1 doses in total). Analysis of the Patient Subgroup Excluded due to Medical Practice Inconsistent with the Standard Therapy of Patients with mcrc For patients enrolled in the Sym4 Phase 2 study there was a notable disparity in OS between patients treated in Russia and those treated in other countries. Median OS for all treatments combined was 8.9 months for all patients excluding those in Russia (i.e., the EU and US only) vs months in Russia. The median duration of treatment (all arms) was nearly 4 times longer for patients from Russia (36 months) than for the EU and US patients (9.1 months). Also, 25% of the EU and US patients had EGFR ECD mutations vs. none of the patients from Russia. Because of these disparities, ad hoc analyses excluding patients enrolled by the Russian sites were done to remove this confounding country effect. The data obtained support the suggestion that the patients in Russia were less refractory to standard EGFR moab / more sensitive to therapy in general and to treatment on the three arms of this protocol specifically. PDX Models CRC PDX tumor xenografts were derived from surgical specimens from cancer patients and were established and characterized at EPO-GmbH, Germany or Oncotest, Germany. After transplantation of 2 2 mm tumor fragments to NMRI-Foxn1nu mice, tumors were measured at least twice weekly. When tumors reached 5-25 mm3, preferably 8-2 mm3, animals were distributed into experimental groups with the aim of having comparable median and mean group tumor volumes of approximately 1-2 mm3, and treatment was initiated. The experiment was performed with 1 animals/group and three groups/model: vehicle control, Sym4, and cetuximab. Sym4 and cetuximab were administered at a dose of 3 mg/kg intraperitoneally (i.p.) twice weekly for 5 weeks (9-1 doses in total). 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
3 efigure 1. Overview of the 7 genes included in the Guardant36 version 2.9 panel. Genes were sequenced in critical exon regions except for those highlighted in bold, where the full exon was sequenced. efigure 2. Kaplan-Meier survival estimates for Overall Survival in ITT population 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
4 efigure 3 Mutations, % Rec.. EGFR G465E/R/V 3 S464L 2 G12A/C/D/F/R/V Q61H/K/L V441D/F/G/I S492N/R 1 A59E/G/T Furin-like Rec.. GF_.. Pkinase_Tyr Ras Mutations, % KRAS Mutations, % BRAF D594G/N N581H/I/Y V6E RBD C1_1 Pkinase_Tyr Mutations, % G12A/C/D/R/S/V Q61H/K/L/R Ras NRAS efigure 3. Baseline Genotyping for Genomic Biomarker Analysis for Patients Included in the Sym4-5 Study Lollipop plots of missense mutations identified in the EGFR, BRAF, KRAS, and NRAS genes in the present study (top half of each plot) compared with data obtained from The Cancer Genome Atlas (bottom half of each plot). Amino acid alterations detected at mutational hotspots are depicted for each gene. 218 American Medical Association. All rights reserved. (Reprinted) Downloaded From: on 6/27/218
5 efigure 4. Number of genomic alterations (single nucleotide variants, copy number variants, indels, and fusions) identified in circulating tumor DNA from patients (N=193), listed by gene. efigure 5. Patient distribution and frequency of EGFR single nucleotide variant missense mutations. N=Number of patients with each mutation; %=Percentage of the total number of nonsilent single nucleotide variant mutations in EGFR detected in the patients. 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
6 efigure 6. Structural modeling of EGFR and mapping of EGFR ECD mutations identified in the patient cohort. The four amino acid positions that were most frequently mutated (G465, S464, V441, and S492) are highlighted. efigure 7. Dose-response curves showing the effect of the indicated antibodies on cell viability in NIH- 3T3 cells stably overexpressing WT or mutant EGFR. Each data point represents the mean of three replicates ±SD. 15 WT 15 V441D 15 S464L Cell viability (%) (relative to untreated) Sym4 Sym4 Sym Antibody concentration [ g/ml] Antibody concentration [ g/ml] Antibody concentration [ g/ml] 1 15 G465R 15 S492R Sym4 Sym Antibody concentration [ g/ml] Antibody concentration [ g/ml] 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
7 efigure 8. Ability of Sym4 to block ligand induced phosphorylation of EGFR in NIH-3T3 cells transfected with either WT or mutant EGFR. Cells were cultured in the presence of the indicated drugs for 4 hours and stimulated with 1 nm EGF for 1 minutes. pegfr (Tyr168) levels were determined by Simple Western analysis. The pegfr signal intensity was normalized to pan-actin (loading control) and is presented as a percentage of the signal in unstimulated control cells. Each bar represents the mean of three replicates. Error bars represent SD. WT nm EGF V441D nm EGF S464L nm EGF G465R nm EGF 5 S492R + 1 nm EGF Relative pegfr levels 2 1 Relative pegfr levels Relative pegfr levels Relative pegfr levels Relative pegfr levels Unstimulated Untreated Sym4 Unstimulated Untreated Sym4 Unstimulated Untreated Sym4 Unstimulated Untreated Sym4 Unstimulated Untreated Sym4 efigure 9. Total EGFR levels after 48 hours of treatment with the indicated antibodies, as determined by Simple Western analysis. EGFR signal intensity was normalized to pan-actin (loading control) and is presented as a percentage of the signal in untreated control cells. Each bar represents the mean of three replicates. Error bars represent SD. WT V441D S464L 125 T= 48 hours 125 T= 48 hours 125 T= 48 hours Total EGFR levels Total EGFR levels Total EGFR levels T= hours Sym4 T= hours Sym4 T= hours Sym4 G465R S492R 125 T= 48 hours 125 T= 48 hours Total EGFR levels Total EGFR levels T= hours Sym4 T= hours Sym4 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
8 efigure 1. Venn diagrams depicting the number (fraction of all profiled patients in parentheses) of patients harboring concurrent mutations in the EGFR ECD (G465E, G465R, S464L, S492R, V441D, and V441G) and KRAS/NRAS exons 2, 3, and 4 (RAS), as well as BRAF V6E, at various mutant allele frequencies (MAFs): RAS ALL MAF, RAS>1%MAF, RAS>2% MAF, RAS>3% MAF, RAS>4% MAF, RAS>5% MAF, RAS>1% MAF, RAS>2% MAF, RAS>25% MAF, and RAS>5% MAF. efigure 11. (A), (B), and (C): Examples of tumor growth curves in PDX models. Animals were treated with vehicle (black), cetuximab (maroon), or Sym4 (green) (3 mg/kg i.p. twice weekly). The gray area marks the treatment period. (D) Waterfall plot showing tumor growth response at day 28, or the closest day to day 28, in 36 CRC PDX models treated with cetuximab (maroon) or Sym4 (green). PD: Progressive disease; SD: Stable disease; PR/CR: Partial response/complete response. (E) Mutations found in the PDX models: KRAS (green), NRAS (maroon), and BRAF (blue) 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
9 efigure 12. Bar graphs depicting overall survival (OS) for each genetically profiled patient. Patients are grouped by treatment and sorted by increasing OS. The oncoprints denote patients with EGFR ECD mutations (G465R, G465E, S464L, S492R, V441D, and V441G), KRAS mutations in exon 2, 3, or 4 at all MAFs (KRAS) and at MAF>2% (KRAS MAF>2%), NRAS mutations in exon 2, 3, or 4 at all MAFs (NRAS) and at MAF>2% (NRAS MAF>2%), MET and ERBB2 gene amplifications (copy number >5), and BRAF V6E. 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
10 efigure 13, 14 and 15. Oncoprints depicting the full ctdna profiles of patients treated with Sym4 12 mg/kg (efigure 13), Sym4 9/6 mg/kg (efigure 14), or investigator s choice (efigure 15). For all figures, the patients are sorted by overall survival, with poorest performing patients to the left. % denotes the fraction of patients in the treatment group with alterations in the specific gene. Amplifications are defined as more than five copies; gain is defined as copy number of more than 2.2 and less than five copies. 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
11 Downloaded From: on 6/27/ American Medical Association. All rights reserved.
12 Downloaded From: on 6/27/ American Medical Association. All rights reserved.
13 Downloaded From: on 6/27/ American Medical Association. All rights reserved.
14 efigure 16. Number of genetic alterations in all ctdna profiled patients compared to patients harboring EGFR ECD mutations. 5 Genetic alteration count in all patients vs. EGFR-ECD mutation positive patients 4 Number of genetic alterations ALL (N=193) All ECD (N=49) 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
15 etable 1. Response in ITT population (evaluable patients) Arm A Sym4 12 mg/kg (N=83) Arm B Sym4 9/6 mg/kg (N=86) Arm C Investigators Choice (N=85) Best Overall Response All Countries, n (%) CR (1.4) PR 11 (14.1) 8 (9.6) 1 (1.4) SD 4 (51.3) 47 (56.6) 37 (52.9) PD 27 (34.6) 28 (33.7) 31 (44.3) Not evaluable Disease Control Rate All Countries, % (n/n evaluable) CR+PR+SD 65.4 (51/78) 66.2 (55/83) 55.7 (39/7) etable 2. Overall survival subsets analysis 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
16 etable 3. Incidence of treatment emergent adverse effects (TEAE) Arm A Sym4 12 mg/kg (N a T =83) Arm B Sym4 9/6 mg/kg (N T =84) Arm C Investigators Choice (N T =78) Any TEAE 83 (1) 84 (1) 67 (85.9) Any Related TEAE 81 (97.6) 8 (95.2) 46 (59.) Serious TEAE 27 (32.5) 23 (27.4) 12 (15.4) Serious Related TEAE 9 (1.8) 6 (7.1) 2 (2.6) TEAE leading to dose reduction 29 (34.9) 17 (2.2) 8 (1.3) TEAE leading to study treatment 12 (14.5) 5 (6.) 6 (7.7) discontinuation Related TEAE leading to study 9 (1.8) 2 (2.4) 3 (3.8) treatment discontinuation TEAE of Grade 3 67 (8.7) 53 (63.1) 25 (32.1) Related TEAE of Grade 3 58 (69.9) 41 (48.8) 9 (11.5) TEAE resulting in death 4 (4.8) 4 (4.8) 3 (3.8) Related TEAE resulting in death Dermatologic toxicity b 78 (94.) 98 (92.9) 8 (1.3) Dermatologic toxicity 3 45 (54.2) 31 (36.9) 1 (1.3) Hypomagnesemia 57 (68.7) 47 (56.) 6 (7.7) Hypomagnesemia 3 27 (32.5) 14 (16.7) Gastrointestinal disorders c 43 (51.8) 41 (48.8) 37 (47.4) Gastrointestinal disorders 3 13 (15.7) 6 (7.1) 6 (7.7) Infections and infestations 41 (49.4) 39 (46.4) 11 (14.1) Infections and infestations 3 8 (9.6) 8 (9.5) 2 (2.6) Infusion reaction 2 (24.1) 15 (17.9) Hypokalemia 1 (12.) 4 (4.8) 3 (3.8) a Number of patients who received study treatment. b Dermatologic toxicity includes any AE terms described in the Dermatologic Toxicity sections in the 215 package inserts for cetuximab, panitumumab, or necitumumab, as well as AE terms under infectious sequelae in the cetuximab package insert unless all AEs with a specific Preferred Term are unrelated. c Gastrointestinal disorders include all AEs under the MedDRA System Organ Class. 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
17 etable 4. Baseline characteristics of DNmCRC and TNmCRC populations DNmCRC a Age mean ± s.d. b, years Sex, N (%) Race, N (%) Male Female White Other or N/A ECOG PS, N (%) Number of prior mcrc treatments d, N (%) Prior anti-egfr mab therapies, N (%) only & only Arm A Sym4 12 mg/kg (N=6) Arm B Sym4 9/6 mg/kg (N=57) Arm C Investigators Choice (N=51) 63 ± ± ± (69.4) 37 (64.9) 33 (64.7) 19 (3.6) 2 (35.1) 18 (35.3) 52 (83.9) 48 (84.2) 42 (82.4) 1 (16.1) 9 (15.8) 9 (17.6) 28 (45.2) 28 (49.1) 26 (51.) 34 (54.8) 28 (49.1) 25 (49.) - 1 (1.8) c - 14 (22.6) 6 (1.5) 1 (19.6) 23 (37.1) 19 (33.3) 18 (35.3) 25 (4.3) 32 (56.1) 23 (45.1) 42 (67.7) 33 (57.9) 28 (54.9) 9 (14.5) 1 (17.5) 1 (19.6) 11 (17.7) 14 (24.6) 13 (25.5) 81 ± ± ± 44.2 Time since last anti-egfr mab therapy, days mean ± s.d. TNmCRC e Sym4 12 mg/kg (N=47) Sym4 9/6 mg/kg (N=46) Age mean ± s.d., years 63 ± ± ± 12. Sex, N (%) Male 35 (74.5) 29 (63.) 21 (55.3) Female 12 (25.5) 17 (37.) 17 (44.7) Race, N (%) White 4 (85.1) 4 (87.) 32 (84.2) Other or N/A 7 (14.9) 6 (13.) 6 (15.8) ECOG PS, N (%) 27 (57.4) 24 (52.2) 17 (44.7) 1 2 (42.6) 21 (45.7) 21 (55.3) 2-1 (2.2) c - Number of prior mcrc treatments d, N (%) Prior anti-egfr mab therapies, N (%) Time since last anti-egfr mab therapy, days mean ± s.d. Investigators Choice (N=38) 2 1 (21.3) 5 (1.9) 8 (21.1) 3 2 (42.6) 17 (37.) 14 (36.8) 4 17 (36.2) 24 (52.2) 16 (42.1) only 33 (7.2) 29 (63.) 24 (63.2) & 7 (14.9) 6 (13.) 6 (15.8) 7 (14.9) 11 (23.9) 8 (21.1) only 78 ± ± ± American Medical Association. All rights reserved. Downloaded From: on 6/27/218
18 EFFICACY DATA, Overall Survival, Months Population per Protocol with DNmCRC, N=17 (88%) Sym4 12 mg/kg 8.9 (6.2, 12.4) N= (6.8, 13.1) N=47 Sym4 9/6 mg/kg 11.9 (9.7, 13.8) N= (9.7, 14.7) N=46 Investigators Choice 8.4 (6.4, 1.) N= (6.3, 8.8) N=38 Population per Protocol with TNmCRC, N=131 (68%) a DNmCRC: Double Negative metastatic Colorectal Cancer. b Standard deviation. c Patient had an ECOG performance status of 1 at screening and therefore met eligibility criteria. d All patients received at least one anti-egfr antibody containing prior cancer therapy. e TNmCRC: Triple Negative metastatic Colorectal Cancer. 218 American Medical Association. All rights reserved. Downloaded From: on 6/27/218
Supplementary Online Content
 Supplementary Online Content Venook AP, Niedzwiecki D, Lenz H-J, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced
Supplementary Online Content Venook AP, Niedzwiecki D, Lenz H-J, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced
Daniele Santini University Campus Bio-Medico Rome, Italy
 Daniele Santini University Campus Bio-Medico Rome, Italy Anti EGFR therapy and colorectal cancer Cetuximab or Panitumumab Adapted from Ciardiello F. and Tortora G. NEJM 2008;358:1160-74 Who will benefit
Daniele Santini University Campus Bio-Medico Rome, Italy Anti EGFR therapy and colorectal cancer Cetuximab or Panitumumab Adapted from Ciardiello F. and Tortora G. NEJM 2008;358:1160-74 Who will benefit
Frequency(%) KRAS G12 KRAS G13 KRAS A146 KRAS Q61 KRAS K117N PIK3CA H1047 PIK3CA E545 PIK3CA E542K PIK3CA Q546. EGFR exon19 NFS-indel EGFR L858R
 Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Frequency(%) 1 a b ALK FS-indel ALK R1Q HRAS Q61R HRAS G13R IDH R17K IDH R14Q MET exon14 SS-indel KIT D8Y KIT L76P KIT exon11 NFS-indel SMAD4 R361 IDH1 R13 CTNNB1 S37 CTNNB1 S4 AKT1 E17K ERBB D769H ERBB
Urinary ctdna Platform for Diagnosis and Cancer Treatment Monitoring. Summit August 19,2015
 Urinary ctdna Platform for Diagnosis and Cancer Treatment Monitoring Mark G. Erlander, Ph.D., CSO CHI Next Generation Summit August 19,2015 Circulating Tumor DNA (ctdna) Tumor cells Main Advantages of
Urinary ctdna Platform for Diagnosis and Cancer Treatment Monitoring Mark G. Erlander, Ph.D., CSO CHI Next Generation Summit August 19,2015 Circulating Tumor DNA (ctdna) Tumor cells Main Advantages of
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
METASTATIC COLORECTAL CANCER: TUMOR MUTATIONAL ANALYSIS AND ITS IMPACT ON CHEMOTHERAPY SUMA SATTI, MD
 METASTATIC COLORECTAL CANCER: TUMOR MUTATIONAL ANALYSIS AND ITS IMPACT ON CHEMOTHERAPY SUMA SATTI, MD INTRODUCTION Second leading cause of cancer related death in the United States. 136,830 cases in 2014
METASTATIC COLORECTAL CANCER: TUMOR MUTATIONAL ANALYSIS AND ITS IMPACT ON CHEMOTHERAPY SUMA SATTI, MD INTRODUCTION Second leading cause of cancer related death in the United States. 136,830 cases in 2014
Colorectal Cancer in 2017: From Biology to the Clinics. Rodrigo Dienstmann
 Colorectal Cancer in 2017: From Biology to the Clinics Rodrigo Dienstmann MOLECULAR CLASSIFICATION Tumor cell Immune cell Tumor microenvironment Stromal cell MOLECULAR CLASSIFICATION Biomarker Tumor cell
Colorectal Cancer in 2017: From Biology to the Clinics Rodrigo Dienstmann MOLECULAR CLASSIFICATION Tumor cell Immune cell Tumor microenvironment Stromal cell MOLECULAR CLASSIFICATION Biomarker Tumor cell
Liquid biopsy: the experience of real life case studies
 Liquid biopsy: the experience of real life case studies 10 th September 2018 Beatriz Bellosillo Servicio de Anatomía Patológica Hospital del Mar, Barcelona Agenda Introduction Experience in colorectal
Liquid biopsy: the experience of real life case studies 10 th September 2018 Beatriz Bellosillo Servicio de Anatomía Patológica Hospital del Mar, Barcelona Agenda Introduction Experience in colorectal
DENOMINATOR: Adult patients with metastatic colorectal cancer who have a RAS (KRAS or NRAS) gene mutation
 Quality ID #452 (NQF 1860): Patients with Metastatic Colorectal Cancer and RAS (KRAS or NRAS) 4 with Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal Antibodies National Quality Strategy Domain:
Quality ID #452 (NQF 1860): Patients with Metastatic Colorectal Cancer and RAS (KRAS or NRAS) 4 with Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal Antibodies National Quality Strategy Domain:
Transform genomic data into real-life results
 CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
ADVANCED COLORECTAL CANCER: UNRESECTABLE OR BORDERLINE RESECTABLE (GROUP 1) CHEMOTHERAPY +/- TARGETED AGENTS. Andrés Cervantes. Professor of Medicine
 ADVANCED COLORECTAL CANCER: UNRESECTABLE OR BORDERLINE RESECTABLE (GROUP 1) CHEMOTHERAPY +/- TARGETED AGENTS Andrés Cervantes Professor of Medicine 1995 One option Advances in the treatment of mcrc 2000
ADVANCED COLORECTAL CANCER: UNRESECTABLE OR BORDERLINE RESECTABLE (GROUP 1) CHEMOTHERAPY +/- TARGETED AGENTS Andrés Cervantes Professor of Medicine 1995 One option Advances in the treatment of mcrc 2000
CURRENT STANDARD OF CARE OF COLORECTAL CANCER: THE EVOLUTION OF ESMO CLINICAL PRACTICE GUIDELINES
 CURRENT STANDARD OF CARE OF COLORECTAL CANCER: THE EVOLUTION OF ESMO CLINICAL PRACTICE GUIDELINES Fortunato Ciardiello ESMO Past-President 2018-2019 Dipartimento di Medicina di Precisione Università degli
CURRENT STANDARD OF CARE OF COLORECTAL CANCER: THE EVOLUTION OF ESMO CLINICAL PRACTICE GUIDELINES Fortunato Ciardiello ESMO Past-President 2018-2019 Dipartimento di Medicina di Precisione Università degli
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
DOSING AND INFORMATION GUIDE LEAPS AHEAD
 DOSING AND INFORMATION GUIDE In patients with WT RAS* mcrc 1 VECTIBIX (panitumumab) LEAPS AHEAD 5.6-month increase in median OS with FOLFOX vs FOLFOX alone 1 Spot the difference. CHOOSE VECTIBIX PRIME
DOSING AND INFORMATION GUIDE In patients with WT RAS* mcrc 1 VECTIBIX (panitumumab) LEAPS AHEAD 5.6-month increase in median OS with FOLFOX vs FOLFOX alone 1 Spot the difference. CHOOSE VECTIBIX PRIME
Description of Procedure or Service. Policy. Benefits Application
 Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Nature Medicine: doi: /nm.4322
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
ReDOS Trial Background
 Regorafenib Dose Optimization Study (ReDos) A Phase II Randomized Study of Lower Dose Regorafenib Compared to Standard Dose Regorafenib in Patients With Refractory Metastatic Colorectal Cancer (mcrc) Abstract
Regorafenib Dose Optimization Study (ReDos) A Phase II Randomized Study of Lower Dose Regorafenib Compared to Standard Dose Regorafenib in Patients With Refractory Metastatic Colorectal Cancer (mcrc) Abstract
Nature Medicine: doi: /nm.4078
 Supplementary Figure 1. Cetuximab induces ER stress response in DiFi cells. (a) Scheme of SILAC proteome. (b) MS-base read out of SILAC experiment. The histogram of log 2 -transformed normalized H/L ratios
Supplementary Figure 1. Cetuximab induces ER stress response in DiFi cells. (a) Scheme of SILAC proteome. (b) MS-base read out of SILAC experiment. The histogram of log 2 -transformed normalized H/L ratios
If multiple KRAS mutation tests have been performed, refer to the most recent test results.
 Measure #452 (NQF 1860): Patients with Metastatic Colorectal Cancer and KRAS Gene Mutation Spared Treatment with Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal Antibodies National Quality Strategy
Measure #452 (NQF 1860): Patients with Metastatic Colorectal Cancer and KRAS Gene Mutation Spared Treatment with Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal Antibodies National Quality Strategy
Supplementary Figure 1
 Supplementary Figure 1 6 HE-50 HE-116 E-1 HE-108 Supplementary Figure 1. Targeted drug response curves of endometrial cancer cells. Endometrial cancer cell lines were incubated with serial dilutions of
Supplementary Figure 1 6 HE-50 HE-116 E-1 HE-108 Supplementary Figure 1. Targeted drug response curves of endometrial cancer cells. Endometrial cancer cell lines were incubated with serial dilutions of
Cetuximab plus 5-FU/FA/oxaliplatin (FOLFOX-4) in the first-line treatment of metastatic colorectal cancer: a large-scale Phase II study (OPUS)
 Cetuximab plus 5-FU/FA/oxaliplatin (FOLFOX-4) in the first-line treatment of metastatic colorectal cancer: a large-scale Phase II study (OPUS) C Bokemeyer, E Staroslawska, A Makhson, I Bondarenko, JT Hartmann,
Cetuximab plus 5-FU/FA/oxaliplatin (FOLFOX-4) in the first-line treatment of metastatic colorectal cancer: a large-scale Phase II study (OPUS) C Bokemeyer, E Staroslawska, A Makhson, I Bondarenko, JT Hartmann,
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1: Generation of cetuximab-resistant cells and analysis of singlecell clones. Cetuximab-sensitive cells (LIM1215 and OXCO-2) were chronically treated with cetuximab
Supplementary Figure 1 Supplementary Figure 1: Generation of cetuximab-resistant cells and analysis of singlecell clones. Cetuximab-sensitive cells (LIM1215 and OXCO-2) were chronically treated with cetuximab
Supplementary Online Content
 Supplementary Online Content Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an
Supplementary Online Content Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc
 MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
Liquid biopsies to track clonal evolution and resistance to EGFR inhibition in mcrc
 Liquid biopsies to track clonal evolution and resistance to EGFR inhibition in mcrc Alberto Bardelli Candiolo Cancer Center IRCCs University of Torino - Medical School Disclosures Horizon discovery Biocartis
Liquid biopsies to track clonal evolution and resistance to EGFR inhibition in mcrc Alberto Bardelli Candiolo Cancer Center IRCCs University of Torino - Medical School Disclosures Horizon discovery Biocartis
Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth.
 Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth. a. Immunoblot for Usp9x protein in NRAS mutant melanoma cells
Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth. a. Immunoblot for Usp9x protein in NRAS mutant melanoma cells
Therapeutic Options for Patients with BRAF-mutant Metastatic Colorectal Cancer
 Therapeutic Options for Patients with BRAF-mutant Metastatic Colorectal Cancer Axel Grothey, M.D., Professor of Oncology, Clinical and Translational Science Division of Medical Oncology Mayo Clinic, Rochester,
Therapeutic Options for Patients with BRAF-mutant Metastatic Colorectal Cancer Axel Grothey, M.D., Professor of Oncology, Clinical and Translational Science Division of Medical Oncology Mayo Clinic, Rochester,
RAS and BRAF in metastatic colorectal cancer management
 Review Article RAS and BRAF in metastatic colorectal cancer management Jun Gong 1, May Cho 1, Marwan Fakih 2 1 Department of Medical Oncology, City of Hope National Medical Center, Duarte, CA, USA; 2 Medical
Review Article RAS and BRAF in metastatic colorectal cancer management Jun Gong 1, May Cho 1, Marwan Fakih 2 1 Department of Medical Oncology, City of Hope National Medical Center, Duarte, CA, USA; 2 Medical
Supplementary Figure 1. BMS enhances human T cell activation in vitro in a
 Supplementary Figure 1. BMS98662 enhances human T cell activation in vitro in a concentration-dependent manner. Jurkat T cells were activated with anti-cd3 and anti-cd28 antibody in the presence of titrated
Supplementary Figure 1. BMS98662 enhances human T cell activation in vitro in a concentration-dependent manner. Jurkat T cells were activated with anti-cd3 and anti-cd28 antibody in the presence of titrated
DCC-2618, a novel pan-kit and PDGFR
 DCC-2618, a novel pan-kit and PDGFRα Kinase switch control inhibitor demonstrates encouraging activity in patients (pts) with Gastrointestinal Stromal Tumors (GIST) N. Somaiah, A. Razak, M. Gordon, F.
DCC-2618, a novel pan-kit and PDGFRα Kinase switch control inhibitor demonstrates encouraging activity in patients (pts) with Gastrointestinal Stromal Tumors (GIST) N. Somaiah, A. Razak, M. Gordon, F.
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 KRAS, NRAS, and BRAF Variant Analysis in Metastatic Colorectal Cancer Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: KRAS, NRAS, and BRAF Variant Analysis
KRAS, NRAS, and BRAF Variant Analysis in Metastatic Colorectal Cancer Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: KRAS, NRAS, and BRAF Variant Analysis
NGS in tissue and liquid biopsy
 NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
AVENIO ctdna Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB
 Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/318/ra29/dc1 Supplementary Materials for Antagonism of EGFR and HER3 Enhances the Response to Inhibitors of the PI3K-Akt Pathway in Triple-Negative Breast Cancer
www.sciencesignaling.org/cgi/content/full/7/318/ra29/dc1 Supplementary Materials for Antagonism of EGFR and HER3 Enhances the Response to Inhibitors of the PI3K-Akt Pathway in Triple-Negative Breast Cancer
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits
 AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
Tumors in the Randomized German AIO study KRK-0306
 FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First- Line Treatment for Patients with Metastatic Colorectal Cancer (mcrc): Analysis of Patients with KRAS-Mutated Tumors in the Randomized German
FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First- Line Treatment for Patients with Metastatic Colorectal Cancer (mcrc): Analysis of Patients with KRAS-Mutated Tumors in the Randomized German
KRAS G13D mutation testing and anti-egfr therapy
 KRAS G13D mutation testing and anti-egfr therapy KRAS G13D mutation and anti-egfr therapy Current data do not support a need to specifically identify this mutation for assessing anti-egfr eligibility in
KRAS G13D mutation testing and anti-egfr therapy KRAS G13D mutation and anti-egfr therapy Current data do not support a need to specifically identify this mutation for assessing anti-egfr eligibility in
Basket Trials: Features, Examples, and Challenges
 : Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
: Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
ANTI-EGFR IN MCRC? Assoc. Prof. Gerald Prager, Medical University of Vienna, Austria
 IS IT TIME TO RE-CHALLENGE ANTI-EGFR IN MCRC? Assoc. Prof. Gerald Prager, Medical University of Vienna, Austria Dr. Andrea Sartore-Bianchi, Oncologia Clinica Molecolare, Niguarda Cancer Center, Milano,
IS IT TIME TO RE-CHALLENGE ANTI-EGFR IN MCRC? Assoc. Prof. Gerald Prager, Medical University of Vienna, Austria Dr. Andrea Sartore-Bianchi, Oncologia Clinica Molecolare, Niguarda Cancer Center, Milano,
DOES LOCATION MATTER IN COLORECTAL CANCER: LEFT VS RIGHT?
 DOES LOCATION MATTER IN COLORECTAL CANCER: LEFT VS RIGHT? By: Dr. Dominik Modest, Medical Department III, Hospital of the University of Munich, Germany Dr. Andrea Sartore-Bianchi, Niguarda Cancer Center,
DOES LOCATION MATTER IN COLORECTAL CANCER: LEFT VS RIGHT? By: Dr. Dominik Modest, Medical Department III, Hospital of the University of Munich, Germany Dr. Andrea Sartore-Bianchi, Niguarda Cancer Center,
OHTAC Recommendation. KRAS Testing for Anti-EGFR Therapy in Advanced Colorectal Cancer
 OHTAC Recommendation KRAS Testing for Anti-EGFR Therapy in Advanced Colorectal Cancer Presented to the Ontario Health Technology Advisory Committee in August, 2010 December 2010 Issue Background In February
OHTAC Recommendation KRAS Testing for Anti-EGFR Therapy in Advanced Colorectal Cancer Presented to the Ontario Health Technology Advisory Committee in August, 2010 December 2010 Issue Background In February
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making
 Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Supplementary Figure 1. Quantile-quantile (Q-Q) plots. (Panel A) Q-Q plot graphical
 Supplementary Figure 1. Quantile-quantile (Q-Q) plots. (Panel A) Q-Q plot graphical representation using all SNPs (n= 13,515,798) including the region on chromosome 1 including SORT1 which was previously
Supplementary Figure 1. Quantile-quantile (Q-Q) plots. (Panel A) Q-Q plot graphical representation using all SNPs (n= 13,515,798) including the region on chromosome 1 including SORT1 which was previously
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Online Content
 Supplementary Online Content Kris MG, Johnson BE, Berry LD, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. doi:10.1001/jama.2014.3741 etable 1. Trials
Supplementary Online Content Kris MG, Johnson BE, Berry LD, et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. doi:10.1001/jama.2014.3741 etable 1. Trials
Encouraging activity of novel pan-kit and PDGFRα inhibitor DCC-2618 in patients (pts) with gastrointestinal stromal tumor (GIST)
 2017 ESMO Proffered Paper Encouraging activity of novel pan-kit and PDGFRα inhibitor DCC-2618 in patients (pts) with gastrointestinal stromal tumor (GIST) F Janku, A Razak, M Gordon, D Flynn, M Kaufman,
2017 ESMO Proffered Paper Encouraging activity of novel pan-kit and PDGFRα inhibitor DCC-2618 in patients (pts) with gastrointestinal stromal tumor (GIST) F Janku, A Razak, M Gordon, D Flynn, M Kaufman,
Supplementary Figure 1
 Supplementary Figure 1 # nonsynonymous mutations (tngs, 1561 genes) 600 p=0.056 500 250 200 150 100 50 0 HLA-DR (-) HLA-DR (+) Supplementary Figure 1: HLA-DR(+) melanoma cell lines are associated with
Supplementary Figure 1 # nonsynonymous mutations (tngs, 1561 genes) 600 p=0.056 500 250 200 150 100 50 0 HLA-DR (-) HLA-DR (+) Supplementary Figure 1: HLA-DR(+) melanoma cell lines are associated with
Related Policies None
 Medical Policy MP 2.04.53 BCBSA Ref. Policy: 2.04.53 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Medicine Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 2.04.53 BCBSA Ref. Policy: 2.04.53 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Medicine Related Policies None DISCLAIMER Our medical policies are designed for informational
MUTATION TEST CE IVD. ctnras-braf FEATURES
 ctnras-braf CE IVD TECHNICAL SHEET IDYLLA ctnras-braf MUTATION TEST The Idylla ctnras-braf Mutation Test, performed on the Biocartis Idylla System, is an in vitro diagnostic test for the qualitative detection
ctnras-braf CE IVD TECHNICAL SHEET IDYLLA ctnras-braf MUTATION TEST The Idylla ctnras-braf Mutation Test, performed on the Biocartis Idylla System, is an in vitro diagnostic test for the qualitative detection
CTC in clinical studies: Latest reports on GI cancers
 CTC in clinical studies: Latest reports on GI cancers François-Clément Bidard, MD PhD GI cancers are characterized by Multimodal treatment strategies Treatments are adapted to tumor burden & prognosis
CTC in clinical studies: Latest reports on GI cancers François-Clément Bidard, MD PhD GI cancers are characterized by Multimodal treatment strategies Treatments are adapted to tumor burden & prognosis
PRECISION INSIGHTS. Liquid GPS. Blood-based tumor profiling and quantitative monitoring. Reveal more with cfdna + cfrna.
 PRECISION INSIGHTS Liquid GPS Blood-based tumor profiling and quantitative monitoring Reveal more with cfdna + cfrna www.nanthealth.com Why Blood-Based Tumor Profiling? Although tissue-based molecular
PRECISION INSIGHTS Liquid GPS Blood-based tumor profiling and quantitative monitoring Reveal more with cfdna + cfrna www.nanthealth.com Why Blood-Based Tumor Profiling? Although tissue-based molecular
Κίκα Πλοιαρχοπούλου. Παθολόγος Ογκολόγος Ευρωκλινική Αθηνών
 Κίκα Πλοιαρχοπούλου Παθολόγος Ογκολόγος Ευρωκλινική Αθηνών Time (months) Survival outcomes in mcrc have progressively improved over the past two decades Treatment options for many patients Multidisciplinary
Κίκα Πλοιαρχοπούλου Παθολόγος Ογκολόγος Ευρωκλινική Αθηνών Time (months) Survival outcomes in mcrc have progressively improved over the past two decades Treatment options for many patients Multidisciplinary
mtor Inhibition Specifically Sensitizes Colorectal Cancers with KRAS or BRAF Mutations to BCL-2/BCL-
 Supplementary Material for mtor Inhibition Specifically Sensitizes Colorectal Cancers with KRAS or BRAF Mutations to BCL-2/BCL- XL Inhibition by Suppressing MCL-1 Anthony C. Faber 1,2 *, Erin M. Coffee
Supplementary Material for mtor Inhibition Specifically Sensitizes Colorectal Cancers with KRAS or BRAF Mutations to BCL-2/BCL- XL Inhibition by Suppressing MCL-1 Anthony C. Faber 1,2 *, Erin M. Coffee
Incorporating biologics in the management of older patients with metastatic colorectal cancer
 Incorporating biologics in the management of older patients with metastatic colorectal cancer D Papamichael MB BS MD FRCP Cyprus Oncology Centre GSK Satellite Symposium SIOG APAC Singapore 12-13 July 2014
Incorporating biologics in the management of older patients with metastatic colorectal cancer D Papamichael MB BS MD FRCP Cyprus Oncology Centre GSK Satellite Symposium SIOG APAC Singapore 12-13 July 2014
Prognostic significance of K-Ras mutation rate in metastatic colorectal cancer patients. Bruno Vincenzi Università Campus Bio-Medico di Roma
 Prognostic significance of K-Ras mutation rate in metastatic colorectal cancer patients Bruno Vincenzi Università Campus Bio-Medico di Roma Colorectal cancer 3 rd most common cancer worldwide Approximately
Prognostic significance of K-Ras mutation rate in metastatic colorectal cancer patients Bruno Vincenzi Università Campus Bio-Medico di Roma Colorectal cancer 3 rd most common cancer worldwide Approximately
Panitumumab: The KRAS Story. Chrissie Fletcher, MSc. BSc. CStat. CSci. Director Biostatistics, Amgen Ltd
 Panitumumab: The KRAS Story Chrissie Fletcher, MSc. BSc. CStat. CSci. Director Biostatistics, Amgen Ltd Clinical Background: panitumumab in mcrc Panitumumab is a fully human IgG2 monoclonal antibody directed
Panitumumab: The KRAS Story Chrissie Fletcher, MSc. BSc. CStat. CSci. Director Biostatistics, Amgen Ltd Clinical Background: panitumumab in mcrc Panitumumab is a fully human IgG2 monoclonal antibody directed
Supplementary Online Content
 Supplementary Online Content Jänne PA, van den Heuvel MM, Barlesi F, et al. Effect of selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRASmutant
Supplementary Online Content Jänne PA, van den Heuvel MM, Barlesi F, et al. Effect of selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRASmutant
Review of the ESMO consensus conference on metastatic CRC Basis strategies ad groups (RAS, BRAF, etc) Michel Ducreux
 Review of the ESMO consensus conference on metastatic CRC Basis strategies ad groups (RAS, BRAF, etc) Michel Ducreux 2 ESMO consensus on mcrc 2016 Chairs: Co-Chairs of working groups E Van Cutsem A Sobrero
Review of the ESMO consensus conference on metastatic CRC Basis strategies ad groups (RAS, BRAF, etc) Michel Ducreux 2 ESMO consensus on mcrc 2016 Chairs: Co-Chairs of working groups E Van Cutsem A Sobrero
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader
 Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
EGFR shrna A: CCGGCGCAAGTGTAAGAAGTGCGAACTCGAGTTCGCACTTCTTACACTTGCG TTTTTG. EGFR shrna B: CCGGAGAATGTGGAATACCTAAGGCTCGAGCCTTAGGTATTCCACATTCTCTT TTTG
 Supplementary Methods Sequence of oligonucleotides used for shrna targeting EGFR EGFR shrna were obtained from the Harvard RNAi consortium. The following oligonucleotides (forward primer) were used to
Supplementary Methods Sequence of oligonucleotides used for shrna targeting EGFR EGFR shrna were obtained from the Harvard RNAi consortium. The following oligonucleotides (forward primer) were used to
Nature Genetics: doi: /ng Supplementary Figure 1. Details of sequencing analysis.
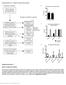 Supplementary Figure 1 Details of sequencing analysis. (a) Flow chart showing which patients fall into each category and were used for analysis. (b) Graph showing the average and median coverage for all
Supplementary Figure 1 Details of sequencing analysis. (a) Flow chart showing which patients fall into each category and were used for analysis. (b) Graph showing the average and median coverage for all
KRAS, NRAS, and BRAF Variant Analysis in Metastatic Colorectal Cancer
 KRAS, NRAS, and BRAF Variant Analysis in Metastatic Colorectal Cancer Policy Number: 2.04.53 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City
KRAS, NRAS, and BRAF Variant Analysis in Metastatic Colorectal Cancer Policy Number: 2.04.53 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The authors have presented data demonstrating activation of AKT as a common resistance mechanism in EGFR mutation positive, EGFR TKI resistant
Reviewers' comments: Reviewer #1 (Remarks to the Author): The authors have presented data demonstrating activation of AKT as a common resistance mechanism in EGFR mutation positive, EGFR TKI resistant
File name: Supplementary Information Description: Supplementary figures and supplementary tables. File name: Peer review file Description:
 File name: Supplementary Information Description: Supplementary figures and supplementary tables. File name: Peer review file Description: Supplementary Figure 1. Schematic of Ras biochemical coupled assay.
File name: Supplementary Information Description: Supplementary figures and supplementary tables. File name: Peer review file Description: Supplementary Figure 1. Schematic of Ras biochemical coupled assay.
Colorectal Cancer in the Coming Years: What Can We Expect?
 Colorectal Cancer in the Coming Years: What Can We Expect? Clara Montagut, MD, PhD Hospital Universitari del Mar, Barcelona, Spain Memorial Sloan Kettering Cancer Center, New York, United States What Are
Colorectal Cancer in the Coming Years: What Can We Expect? Clara Montagut, MD, PhD Hospital Universitari del Mar, Barcelona, Spain Memorial Sloan Kettering Cancer Center, New York, United States What Are
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
OWa 22 80) :IEZ
 Clinical Study Report: 20025409 Part 2 Date: 22 September 2008 OWa 22 80) 06 --- :IEZ Page 1 SYNOPSIS Name of the Sponsor: Name of Finished Product: Name of Active Ingredient: Immunex Corporation Panitumumab
Clinical Study Report: 20025409 Part 2 Date: 22 September 2008 OWa 22 80) 06 --- :IEZ Page 1 SYNOPSIS Name of the Sponsor: Name of Finished Product: Name of Active Ingredient: Immunex Corporation Panitumumab
MEETING SUMMARY ESMO 2018, Munich, Germany. Dr. Jenny Seligmann University of Leeds, UK HIGHLIGHTS ON COLORECTAL CANCER
 MEETING SUMMARY ESMO 2018, Munich, Germany Dr. Jenny Seligmann University of Leeds, UK HIGHLIGHTS ON COLORECTAL CANCER DISCLAIMER Please note: The views expressed within this presentation are the personal
MEETING SUMMARY ESMO 2018, Munich, Germany Dr. Jenny Seligmann University of Leeds, UK HIGHLIGHTS ON COLORECTAL CANCER DISCLAIMER Please note: The views expressed within this presentation are the personal
Supplementary Information
 Supplementary Information An orally available, small-molecule interferon inhibits viral replication Hideyuki Konishi 1, Koichi Okamoto 1, Yusuke Ohmori 1, Hitoshi Yoshino 2, Hiroshi Ohmori 1, Motooki Ashihara
Supplementary Information An orally available, small-molecule interferon inhibits viral replication Hideyuki Konishi 1, Koichi Okamoto 1, Yusuke Ohmori 1, Hitoshi Yoshino 2, Hiroshi Ohmori 1, Motooki Ashihara
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
ras Multikinase Inhibitor Multikinase Inhibitor 0.1
 a ras ** * ** * ** ** ** ** un in et m lu Se ib SL G 32 W 7 So 50 ra 74 fe ni b LY W 294 or 0 tm 02 R an ap n a in Ev my er cin ol im BE us Z2 3 En P 5 za I13 st 0 au rin D as SP ati 60 nib C 012 is 5
a ras ** * ** * ** ** ** ** un in et m lu Se ib SL G 32 W 7 So 50 ra 74 fe ni b LY W 294 or 0 tm 02 R an ap n a in Ev my er cin ol im BE us Z2 3 En P 5 za I13 st 0 au rin D as SP ati 60 nib C 012 is 5
Supplementary Tables. Supplementary Figures
 Supplementary Files for Zehir, Benayed et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients Supplementary Tables Supplementary Table 1: Sample
Supplementary Files for Zehir, Benayed et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients Supplementary Tables Supplementary Table 1: Sample
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Individualized Cancer Therapy: Chemotherapy Resistance Testing before Therapy
 Individualized Cancer Therapy: Chemotherapy Resistance Testing before Therapy 1 st st International Oncological Conference Wrocław, October 6 th, 2012 Dr. Frank Kischkel Individualized Cancer Therapy:
Individualized Cancer Therapy: Chemotherapy Resistance Testing before Therapy 1 st st International Oncological Conference Wrocław, October 6 th, 2012 Dr. Frank Kischkel Individualized Cancer Therapy:
Validation & Assay Performance Summary
 Validation & Assay Performance Summary LanthaScreen IGF-1R GripTite Cells Cat. no. K1834 Modification Detected: Phosphorylation of Multiple Tyr Residues on IGF-1R LanthaScreen Cellular Assay Validation
Validation & Assay Performance Summary LanthaScreen IGF-1R GripTite Cells Cat. no. K1834 Modification Detected: Phosphorylation of Multiple Tyr Residues on IGF-1R LanthaScreen Cellular Assay Validation
Sponsor / Company: Sanofi Drug substance(s): Docetaxel (Taxotere )
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Lentiviral Delivery of Combinatorial mirna Expression Constructs Provides Efficient Target Gene Repression.
 Supplementary Figure 1 Lentiviral Delivery of Combinatorial mirna Expression Constructs Provides Efficient Target Gene Repression. a, Design for lentiviral combinatorial mirna expression and sensor constructs.
Supplementary Figure 1 Lentiviral Delivery of Combinatorial mirna Expression Constructs Provides Efficient Target Gene Repression. a, Design for lentiviral combinatorial mirna expression and sensor constructs.
Novel EGFR TKI Theliatinib: An Open Label, Dose Escalation Phase I Clinical Trial
 Novel EGFR TKI Theliatinib: An Open Label, Dose Escalation Phase I Clinical Trial 2014-309-00CH1 Presenter: Jifang Gong, Beijing Cancer Hospital Lin Shen 1, Li Zhang 2, Hongyun Zhao 2, Wenfeng Fang 2,
Novel EGFR TKI Theliatinib: An Open Label, Dose Escalation Phase I Clinical Trial 2014-309-00CH1 Presenter: Jifang Gong, Beijing Cancer Hospital Lin Shen 1, Li Zhang 2, Hongyun Zhao 2, Wenfeng Fang 2,
Cell-free tumor DNA for cancer monitoring
 Learning objectives Cell-free tumor DNA for cancer monitoring Christina Lockwood, PhD, DABCC, DABMGG Department of Laboratory Medicine 1. Define circulating, cell-free tumor DNA (ctdna) 2. Understand the
Learning objectives Cell-free tumor DNA for cancer monitoring Christina Lockwood, PhD, DABCC, DABMGG Department of Laboratory Medicine 1. Define circulating, cell-free tumor DNA (ctdna) 2. Understand the
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
a b G75 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server.
 a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
Supporting Information Table of Contents
 Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
MET skipping mutation, EGFR
 New NSCLC biomarkers in clinical research: detection of MET skipping mutation, EGFR T790M, and other important biomarkers Fernando López-Ríos Laboratorio de Dianas Terapéuticas Hospital Universitario HM
New NSCLC biomarkers in clinical research: detection of MET skipping mutation, EGFR T790M, and other important biomarkers Fernando López-Ríos Laboratorio de Dianas Terapéuticas Hospital Universitario HM
p.r623c p.p976l p.d2847fs p.t2671 p.d2847fs p.r2922w p.r2370h p.c1201y p.a868v p.s952* RING_C BP PHD Cbp HAT_KAT11
 ARID2 p.r623c KMT2D p.v650fs p.p976l p.r2922w p.l1212r p.d1400h DNA binding RFX DNA binding Zinc finger KMT2C p.a51s p.d372v p.c1103* p.d2847fs p.t2671 p.d2847fs p.r4586h PHD/ RING DHHC/ PHD PHD FYR N
ARID2 p.r623c KMT2D p.v650fs p.p976l p.r2922w p.l1212r p.d1400h DNA binding RFX DNA binding Zinc finger KMT2C p.a51s p.d372v p.c1103* p.d2847fs p.t2671 p.d2847fs p.r4586h PHD/ RING DHHC/ PHD PHD FYR N
4. Aflibercept showed significant improvement in overall survival (OS), the primary
 Cost effectiveness of aflibercept (Zaltrap ) in combination with FOLFIRI in the treatment of adult patients with metastatic colorectal cancer (mcrc) that is resistant to or has progressed after an oxaliplatin
Cost effectiveness of aflibercept (Zaltrap ) in combination with FOLFIRI in the treatment of adult patients with metastatic colorectal cancer (mcrc) that is resistant to or has progressed after an oxaliplatin
Supplementary Figure 1: Equilibrium binding analyses of the interaction of efgartigimod with human FcRn
 SULEMENTARY DATA Supplementary Figure 1: Equilibrium binding analyses of the interaction of with human FcRn using SR. The coupling density of was 275 RU. Human FcRn was injected over the flow cells at
SULEMENTARY DATA Supplementary Figure 1: Equilibrium binding analyses of the interaction of with human FcRn using SR. The coupling density of was 275 RU. Human FcRn was injected over the flow cells at
underlying metastasis and recurrence in HNSCC, we analyzed two groups of patients. The
 Supplementary Figures Figure S1. Patient cohorts and study design. To define and interrogate the genetic alterations underlying metastasis and recurrence in HNSCC, we analyzed two groups of patients. The
Supplementary Figures Figure S1. Patient cohorts and study design. To define and interrogate the genetic alterations underlying metastasis and recurrence in HNSCC, we analyzed two groups of patients. The
Personalized Healthcare Update
 Dr. Kai - Oliver Wesche Market Development Manager, Personalized Healthcare QIAGEN Personalized Healthcare Update Pioneering Personalized Medicine through Partnering TOMTOVOK BKM120 Zelboraf QIAGEN partners:
Dr. Kai - Oliver Wesche Market Development Manager, Personalized Healthcare QIAGEN Personalized Healthcare Update Pioneering Personalized Medicine through Partnering TOMTOVOK BKM120 Zelboraf QIAGEN partners:
Supplementary Online Content
 Supplementary Online Content Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patientlevel data. Published online
Supplementary Online Content Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patientlevel data. Published online
Supplementary Information. Supplementary Figure 1
 Supplementary Information Supplementary Figure 1 1 Supplementary Figure 1. Functional assay of the hcas9-2a-mcherry construct (a) Gene correction of a mutant EGFP reporter cell line mediated by hcas9 or
Supplementary Information Supplementary Figure 1 1 Supplementary Figure 1. Functional assay of the hcas9-2a-mcherry construct (a) Gene correction of a mutant EGFP reporter cell line mediated by hcas9 or
Identification of BRAF mutations in melanoma lesions with the Roche z480 instrument
 Identification of BRAF mutations in melanoma lesions with the Roche z480 instrument Márta Széll IAP, Siófok May 14, 2012 Multifactorial skin diseses: much more frequent then genodermatoses exhibit familial
Identification of BRAF mutations in melanoma lesions with the Roche z480 instrument Márta Széll IAP, Siófok May 14, 2012 Multifactorial skin diseses: much more frequent then genodermatoses exhibit familial
Metastatik Kolorektal Kanser Tedavisinde Yeni Biyobelirteçler Sonrası Panitumumab. Prof. Dr. N. Faruk Aykan Antalya 22 Mart 2014
 Metastatik Kolorektal Kanser Tedavisinde Yeni Biyobelirteçler Sonrası Panitumumab Prof. Dr. N. Faruk Aykan Antalya 22 Mart 2014 1952-1953 St. Louis, ABD Kinase growth factor pathway Activated receptor
Metastatik Kolorektal Kanser Tedavisinde Yeni Biyobelirteçler Sonrası Panitumumab Prof. Dr. N. Faruk Aykan Antalya 22 Mart 2014 1952-1953 St. Louis, ABD Kinase growth factor pathway Activated receptor
Supplementary Figure 1. a. b. Relative cell viability. Nature Genetics: doi: /ng SCR shyap1-1 shyap
 Supplementary Figure 1. a. b. p-value for depletion in vehicle (DMSO) 1e-05 1e-03 1e-01 1 0 1000 2000 3000 4000 5000 Genes log2 normalized shrna counts in T0 0 2 4 6 8 sh1 shluc 0 2 4 6 8 log2 normalized
Supplementary Figure 1. a. b. p-value for depletion in vehicle (DMSO) 1e-05 1e-03 1e-01 1 0 1000 2000 3000 4000 5000 Genes log2 normalized shrna counts in T0 0 2 4 6 8 sh1 shluc 0 2 4 6 8 log2 normalized
a surface permeabilized
 a surface permeabilized RAW 64.7 P388D1 J774 b CD11b + Ly-6G - Blood Monocytes WT Supplementary Figure 1. Cell surface expression on macrophages and DCs. (a) RAW64.7, P388D1, and J774 cells were subjected
a surface permeabilized RAW 64.7 P388D1 J774 b CD11b + Ly-6G - Blood Monocytes WT Supplementary Figure 1. Cell surface expression on macrophages and DCs. (a) RAW64.7, P388D1, and J774 cells were subjected
