The Role and Mechanism of Fibroblast Growth Factor Receptor 2 in Cellular Transformation
|
|
|
- Laurence Murphy
- 5 years ago
- Views:
Transcription
1 The Role and Mechanism of Fibroblast Growth Factor Receptor 2 in Cellular Transformation By Jiyoung Cha A dissertation submitted to the faculty of University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Pharmacology. Chapel Hill 2007 Approved by: Dr. Channing J. Der (advisor) Dr. Keith Burridge Dr. Adrienne D. Cox Dr. Lee M. Graves Dr. Klaus Hahn
2 ABSTRACT Jiyoung Cha: The Role and Mechanism of Fibroblast Growth Factor Receptor 2 in Cellular Transformation (Under the direction of Channing J. Der) Our laboratory recently identified fibroblast growth factor receptor 2 (FGFR2) as a transforming gene using a cdna expression library screen for novel oncogenes expressed in human breast carcinoma cells. An important feature and mode of FGFR2 function is the expression of structural variants of FGFR2 that are encoded by transcripts that arise alternative gene splicing. The tissue-restricted nature of some splice variant transcripts, together with the functional and biological differences of FGFR2 splice variant protein products, provides mechanisms of regulation of receptor function distinct from regulation of expression of receptor ligands. The first major splicing event occurs in the sequences encoding the aminoterminal extracellular domain, involving the third immunoglobulin (Ig-III)-like domain, to generate either the epithelial cell-specific IIIb or mesenchymal cell-specific IIIc isoforms. The second major splicing occurs in sequences that encode the intracellular carboxyl-terminus, resulting in three splice variants that differ in their carboxyl-terminal sequences have been identified (designated C1, C2 and C3). My studies have focused on defining the role of these two major alternative splicing ii
3 events in FGFR2 function and oncogenesis. First, I found preferential expression of the mesenchymal-specific FGFR2 IIIc splice variant in invasive breast carcinomas. FGFR2 IIIc expression was associated with loss of expression of epithelial cellspecific and the gain of expression of mesenchymal cell-specific markers. These observations suggest that IIIb to IIIc exon switching may be involved in tumor cell invasion and metastasis. Additionally, I evaluated the signaling and transforming activity of the epithelial cell-specific isoform in mammary epithelial cells. Second, I compared the transforming potency of the C1, C2 and C3 splicing variants and found that a hierarchy of transforming activity that correlated with progressive loss of carboxyl terminal sequences. I determined that one mechanism for the enhanced transforming activity of FGFR2 IIIb C3 involves loss of the 770 YLDL sorting motif and impaired internalization and a gain of ligand-independent FRS2 activation. Together, my studies provide further evidence for alternative gene splicing as a mechanism for causing aberrant FGFR2 function in promoting human oncogenesis. iii
4 ACKNOWLEDGEMENTS I would like to thank many people for helping me during my doctoral work. First, I would like to thank my advisor Dr. Channing J. Der for his excellent scientific guidance, tremendous support and patience throughout this work. Without him, this dissertation would not have been possible. I also wish to thank my committee members Drs. Adrienne Cox, Keith Burridge, Lee Graves and Klaus Hahn for their valuable suggestions for my work. I would like to thank for past and present members of the Der lab for their generosity for taking time to help me. Finally, I am especially gratefor for my parents and brothers for all their love and support throughout my work. iv
5 TABLE OF CONTENTS LIST OF FIGURES... viii LIST OF ABBREVIATIONS... x Chapter 1: Introduction... 1 I. Receptor Tyrosine Kinases (RTKs)... 1 A. Introduction to RTKs... 1 B. Intracellular signaling pathways regulated by RTKs... 4 C. Negative regulation of RTKs D. RTKs as therapeutic targets for target-based anti-cancer drug development 11 II. FGFR family of RTK A. Identification of FGFs B. Structural diversity of FGFRs C. Alternative gene splicing of FGFR2: generation of functionally-distinct proteins from a single gene D. FGFR signaling E. FGFR mutations in human skeletal disorders F. FGFRs and cancer III. FGFR2 and oncogenesis v
6 A. Opposing roles for FGFR2 in oncogenesis B. Cell type-specific differences in transformation C. Potential role of FGFR2 in epithelial to mesenchymal transition (EMT) and cancer development Chapter 2: Cell Context-dependent Mechanisms of FGFR2 IIIb Transformation of Fibroblast and Epithelial Cells I. Abstract II. Introduction III. Materials and methods IV. Results A. Identification of the FGFR2 IIIb C2 Splice Variant as a Transforming Protein from T-47D Breast Carcinoma Cells B. Aberrant Expression of the Mesenchymal FGFR2 IIIc Isoform in Invasive Breast Cancer Cell Lines C. Distinct Transforming Activities of FGFR2 IIIb C2 in NIH 3T3 Fibroblasts and RIE-1 Epithelial Cells D. FGFR2 IIIb C2 Causes Activation of Ras, ERK and AKT and Upregulation of Cyclin D1 Protein Levels in NIH 3T3 But Not RIE-1 Cells E. MEK and PI3K Activities Are Required for FGFR2 IIIb C2-induced Anchorage-Independent Growth of RIE-1 Cells F. KGF Stimulates Morphologic Transformation and Increases Cyclin D1 Protein Levels in FGFR2 IIIb C2-transformed RIE-1 Cells G. FGFR2 IIIb C2-transformed RIE-1 Cells Show Enhanced ERK Activity, Increased Cyclin D1 Protein Levels, and Upregulation Of EGFR Autocrine Loop in Confluent Cultures, But Not in Sub-Confluent Cultures V. Discussion Chapter 3: Aberrant Receptor Internalization and Enhanced FRS2-Dependent Signaling Contributes to the Ligand-Independent Transforming Activity of the Fibroblast Growth Factor Receptor 2 IIIb C3 isoform vi
7 I. Abstract II. Introduction III. Materials and methods IV. Results A. Loss of carboxyl-terminal sequences enhances FGFR2 IIIb transforming potency B. Mutation of Y770F alone activates FGFR2 IIIb C1 transforming activity C. The 770YXXL motif is required for ligand-stimulated FGFR2 IIIb C1 internalization D. Loss of Y and L of 770YXXL motif cooperates to enhance transformation.. 83 E. Loss of Y770 impairs ligand stimulated FGFR2 IIIb C1 activation of PLCγ.. 85 F.Loss of Y770 of FGFR2 C1 induces sustained activation of FRS2, but not ERK G. FRS2 activity is required for increased FGFR2 IIIb C1 transforming activity by Y770F mutation but not L773A mutation V. Discussion Chapter 4: Conclusion and Future studies REFERENCES vii
8 LIST OF FIGURES Figure 1-1. The basic modular anatomy of RTKs Figure 1-2. Receptor tyrosine kinase (RTK) families... 5 Figure 1-3. Docking proteins function as platforms for the recruitment of signaling proteins to the activated RTKs... 7 Figure 1-4. Intracellular signaling pathways regulated by RTKs Figure 1-5. Alternative splicing of FGFR2 and differential ligand stimulation Figure 1-6. FGFR2 IIIb and KGF create a paracrine signaling loop between epithelial and mesenchymal cells Figure 1-7. Alternative splicing of FGFR2 IIIb and carboxyl-terminal sequence variation Figure 1-8. FGFR carboxyl terminal sequences Figure 1-9. FGFR cytoplasmic signaling Figure Possible roles for Y770 in FGFR2 function Figure FGFR2 mutations in craniosynostosis syndromes and cancer Figure FGFR1 chromosomal translocation in myeloproliferative syndrome (EMS) Figure Regulators of EMT Figure 2-1. Human FGFR2 isoforms Figure 2-2. Expression of transcriptions encoding amino- and carboxyl-terminal isoforms of FGFR Figure 2-3. Distinct transforming activities of FGFR2 IIIb C2 in NIH 3T3 fibroblasts and RIE-1 epithelial cells Figure 2-4. FGFR2 IIIb C 2 activates Ras and Ras-mediated signaling in NIH 3T3 but not RIE-1 cells Figure 2-5. MEK and PI3K activities are required for FGFR2 IIIb C2-induced anchorage-independent growth of RIE-1 cells viii
9 Figure 2-6. KGF stimulation induces morphologic transformation and increases cyclin D1 protein levels in FGFR2 IIIb C2-transformed, but not control, RIE-1 cells Figure 2-7. FGFR2 IIIb C2-transformed RIE-1 cells show enhanced ERK activity, increased cyclin D1 protein levels, and upregulation of an EGFR-dependent autocrine loop in confluent, but not sub-confluent, cultures Figure 3-1. Loss of carboxyl-terminal sequences enhances FGFR2 IIIb transforming potency Figure 3-2. Mutation of Y770F alone activates FGFR2 IIIb C1 transforming activity Figure 3-3. The 770 YXXL motif is required for ligand-stimulated FGFR2 IIIb C1 internalization Figure 3-4. Mutation of the Y and L residues of the 770 YXXL motif cooperates to enhance FGFR2 IIIb C1 induction of anchorage-independent growth transformation Figure 3-5. Mutation of Y770 impairs ligand-stimulated FGFR2 IIIb C1-dependent activation of PLCγ Figure 3-6. Mutation of Y770 of FGFR2 IIIb C1 causes sustained activation of FRS Figure 3-7. FRS2 activity is required for increased FGFR2 IIIb C1 transforming activity caused by the Y770F but not L773A mutation Figure 3-8. Role of altered signaling caused by deletion of the 770 YXXL motif in FGFR2 IIIb C3 transforming activity Figure 4-1. Gene splicing regulation of FGFR2 function Figure 4-2. Two distinct FGFR2 alternative splicing events, which alter the primary sequence of the extracellular IgIII domain and cytoplasmic carboxyl-terminus, might be critical contributors of cancer progression ix
10 LIST OF ABBREVIATIONS DAG EGF EMT ERK FGF diacylglycerol epidermal growth factor epithelial to mesenchymal transition extracelluar signal-regulated kinase fibroblast growth factor FRS2 fibroblast growth factor receptor substrate 2 KGF GAP GDP GEF GSK3 GST GTP HGF Ig IR MAPK MEK NF-κB NSCLC PCR keratinocyte growth fctor GTPase-activating protein guanine nucleotide di-phosphate guanine nucleotide exchange factor glycogen synthase kinase-3 glutathione S-trasnferase guanine nucleotide tri-phosphate hepatocyte growth factor immunoglobulin-like insulin receptor Mitogen-activated protein kinase Mitogen-activated/extracellular signal-regulated protein kinase nuclear factor κb non-small-cell- lung cnacer polymerase chain reaction x
11 PDGF PDK PH PI3K PIP 2 PIP 3 PLC PTEN Rb RBD RTK platelet-derived growth factor phosphoinositide-dependent protein kinase plekstrin homology domain phosphatidylinositol 3-kinase phosphatidylinositol-bis-phosphate phosphatidylinositol-tri-phosphate phospholipase C Phosphatase and Tensin homolog deleted on chromosome Ten Retinoblastoma Ras biding domain receptor tyrosine kinase SH2 src homology 2 SH3 src homology 3 STAT TGF-β VEGF signal transduction and activator of transcription transforming growth factor β vascular endothelial growth factor xi
12 Chapter 1: Introduction The direction of my dissertation research was based on the unpublished identification of the fibroblast growth factor receptor 2 (FGFR2) receptor tyrosine kinase (RTK), isoform IIIb C2, in an expression library screen for novel oncogenes expressed in human breast cancers. Since the aberrant expression and function of RTKs is a widespread and common mechanism of human oncogenesis, my studies have focused on elucidating the biological activities and signaling mechanisms by which FGFR2 may promote the uncontrolled proliferation of human cancer cells. In this introduction, I first provide an overview of RTKs and their involvement in human cancers and I summarize some of the key signaling mechanisms by which RTKs regulate normal and neoplastic cell growth. I then focus on the FGFR family of RTKs and their roles in human developmental disorders and cancer. I. Receptor Tyrosine Kinases (RTKs) A. Introduction to RTKs. RTKs play a critical role in the regulation of multiple cellular processes including cell proliferation, differentiation, survival, migration and angiogenesis (1-4). RTKs contain an extracellular ligand binding domain that is usually glycosylated. The extracellular domain is connected to the cytoplasmic kinase domain by a single transmembrane helix. The cytoplasmic domain contains a protein tyrosine kinase (PTK) domain and carboxyl-terminal regulatory sequences that are subjected to autophosphorylation (Fig.1-1). With the exception of the insulin receptor (IR) family 1
13 of RTKs, all known RTKs are monomers in the cell membrane. Members of the IR family are disulfide linked dimers of two polypeptide chains forming a heterodimer (Fig.1-2). In general, signalling by RTKs requires ligand-induced receptor dimerization, which results in tyrosine autophosphorylation of their cytoplasmic domains. Tyrosine autophosphorylation sites serve as binding sites for Src homology 2 (SH2) and protein tyrosine-binding (PTB) domains that are found on a diverse variety of cytoplasmic signaling proteins. Signaling proteins containing SH2 and PTB domains are modular in nature. Many of these proteins possess intrinsic enzymatic activities and addition protein-protein modules that can interact with other signaling molecules. Examples of protein modules involved in protein protein interaction commonly found on signaling proteins are SH3, WW, LIM, PX, EH, EVH1, and PDZ domains. Protein modules involved in membrane signaling can also recognize molecules other than proteins. For example, pleckstrin homology (PH) domains recognize specific plasma membrane-associated phospholipids and thus allow the target protein to translocate to the membrane, where they can be activated by other signaling molecules (Fig.1-1). A large family of SH2/PTB domain containing proteins possesses intrinsic enzymatic activities. For instance, Src kinases and phospholipase Cγ (PLCγ) possess PTK and phospholipase activity, respectively (Fig.1-1). On the other hand, another family of SH2 domain-containing proteins, so-called adaptor proteins, does not possess intrinsic enzymatic activities. These adaptor proteins (e.g., Grb2, Nck, Crk, Shc) utilize their SH2 and/or SH3 domains to interact with effector molecules that are involved in the regulation of cytoplasmic signal transduction networks. 2
14 N-glycosylation sites EC Membrane Juxtamembrane region Tyrosine kinase Carboxyl-terminal regulatory domain TK Y- Y- P P PTB SH2 Membrane Membrane PH SH3 PDZ FIVE Enzymatic activity Cellular targets Figure 1-1. The basic modular anatomy of RTKs. RTKs contain an extracellular ligand binding domain that is usually glycosylated. The extracellular domain is connected to the cytoplasmic kinase domain by a single transmembrane helix. The cytoplasmic domain contains a protein tyrosine kinase (PTK) domain and carboxyl-terminal regulatory sequences. Tyrosine autophosphorylation sites serve as binding sites for SH2 and PTB domains found on a diverse variety of cytoplasmic signaling proteins. 3
15 For example, the adaptor protein Grb2 binds to tyrosine autophosphorylation sites of activated RTKs via its SH2 domain and recruits the Ras guanine nucleotide exchange factor (GEF) Sos via its SH3 domains and recognition of proline-rich sequences on Sos (4, 5). Another important family of proteins that involved in relaying signals from the activated receptors to downstream pathways is docking proteins (Fig. 1-3). Docking proteins contain a membrane targeting signal in their amino-termini and multiple phosphorylatable tyrosine sites in their carboxyl-termini. Whereas some docking proteins are attached to the cell membrane by a myristoyl fatty acid anchor (e.g., fibroblast receptor substrate 2 (FRS2)), most docking proteins are associated with the cell membrane via PH domains found in their amino-termini (e.g., insulin receptor substrate (IRS), Gab, Dok). In addition to the membrane targeting signal, most docking proteins contain specific domains such as PTB domains that allow the docking proteins to bind to the tyrosine phosphorylated receptors. Thus, docking proteins can function as platforms for the recruitment of signaling proteins to the activated RTKs. For instance, IRS binds to the activated insulin receptor (IR) via its PTB domain and recruits SH2-domain containing signaling molecules to the phosphorylated tyrosine residues in its carboxyl-terminus (4, 6). B. Intracellular signaling pathways regulated by RTKs The Ras-Raf-MEK-ERK mitogen-activated protein kinase (MAPK) pathway is generally considered to be one of the most central and important membrane-tonucleus signaling pathways in eukaryotes (7). Upon ligand stimulation, the Grb2 adaptor protein binds to phosphorylated tyrosine residues of RTKs via its SH2 4
16 EGF Receptor Insulin Receptor NGF Receptor PDGF Receptor FGF Receptor VEGF Receptor EPH Receptor Ig-like domain Ig-like domain Acid box Cys-rich domain SS SS SS Fibronectin like domain Tyrosine kinase Figure 1-2. Receptor tyrosine kinase (RTK) families. The extracellular ligand-binding domain of FGFR is composed of three immunoglobulin (Ig) like domains and a cluster of acidic and serine/threonine amino acids known as the acid box between Ig-I and Ig-II domains. 5
17 domain and forms complex with Sos via it SH3 domain (Figure 1-4). Recruitment of the Grb2/Sos complex to activated RTKs allows the normally cytosolic Sos to translocate to the plasma membrane where Sos can stimulate the exchange of GTP for GDP on the plasma membrane-bound small G protein Ras. Alternatively, the Grb2/Sos complex can be recruited to the plasma membrane by binding to the docking proteins such as IRS or FRS2 that become tyrosine phosphorylated in response to activation of RTKs such as FGFR and IR family of RTKs. Once activated, Ras-GTP interacts with a multitude of effector proteins, with the Raf serine/threonine kinases, the phosphatidylinositol 3-kinases (PI3Ks), and the RalGEFs the best characterized and understood. Activated Raf activates the MEK1 and MEK2 dual specificity protein kinases by phosphorylation on tandem serine residues in the activation loop of the kinase domain. Then, activated MEK stimulates ERK1 and ERK2 activation by phosphorylation on Thr and Tyr residues in the activation loop of their kinase domains. Activated ERK MAPKs then translocate into the nucleus where they phosphorylate and activate Ets family and other transcription factors, to cause changes in gene expression that regulate cell proliferation and survival (4, 8). The PI3K lipid kinases can be also activated by RTKs (9). The class I PI3Ks are comprised of heterodimers that consist of a regulatory subunit p85 and catalytic subunit p110. The regulatory subunit p85 contains an SH2 domain that can bind to the phosphorylated tyrosine residues of activated RTKs and recruit PI3K to the plasma membrane (Fig. 1-4). Alternatively, activated Ras can bind directly to the p110 subunit and recruit the enzyme to the plasma membrane. 6
18 FRS2 Myristyl PTB Tyrosine phosphorylation sites P P P P P IRS PH PTB P P P P P P P Gab PH P P P P P P Dok PH P P P P P Figure 1-3. Docking proteins function as platforms for the recruitment of signaling proteins to the activated RTKs Docking proteins contain a membrane targeting signal in amino-termini and multiple phosphorylatable tyrosine sites in carboxyl-termini. Whereas some docking proteins are attached to the cell membrane by a myristyl anchor (FRS2), most docking proteins are associated with the cell membrane via a PH domain at their aminoterminus (IRS, Gab. Dok). Some docking proteins (FRS2, IRS) contain PTB domains that allow the docking proteins to bind to the tyrosine phosphorylated receptors. 7
19 Activated PI3K catalyses the phosphorylation of phosphatidylinositols (PtdIns), including PtdIns(4)P and PtdIns(4,5)P2, and converted to PtdIns(3,4)P2 and PtdIns(3,4,5)P3, respectively. Subsequently, PtdIns(3,4,5)P3 recruits downstream signaling proteins that contain PH domains. The most well characterized signaling proteins activated by PI3K-generated PtdIns(3,4,5)P3 are the serine/threonine kinases Akt (also called protein kinase B; PKB) and phosphoinositide-dependent kinase 1 (PDK1). Once PI3K is activated, the plasma membrane-associated PtdIns(3,4,5)P3 interacts with the PH domains of Akt and PDK1 and recruit these two proteins together at the plasma membrane where PDK1 and other kinases phosphorylate Akt. These phosphorylations activate Akt and activated Akt then phosphorylates other proteins that are involved in cell growth, cell migration, and cell survival. For example, it has been shown that Akt activation leads to phosphorylation and inactivation of apoptotic protein Bad by creating a binding site for proteins and preventing Bad from binding to Bcl-2 family members Bcl-2 and Bcl- XL. Similarly, activated Akt phosphorylates the Forkhead-related transcription factor 1 (FKHR-L1) and creates a binding site for the family of proteins. Since the complex of FKHR-L1 and is retained in the cytosol, FKHR-L1 is inactive and cannot stimulate transcription of target genes. Another well characterized Akt target protein is glycogen synthase kinase-3 (GSK3) that is constitutively active in unstimulated cells. Phosphorylation of GSK3 by Akt inactivates GSK3, resulting in the activation of pathways that are normally inhibited by GSK3. The termination of PI3K signaling by degradation of PtdIns(3,4,5)P3 can be mediated by PTEN and SHIP, two phosphoinositide-specific phosphatases that dephosphorylate the 3 and 8
20 PIP3 PIP3 Ras Raf Sos Grb2 P TK P TK P85 P110 PI3K PH AKT P PH PDK1 MEK P P P MAPK FKHR-L1 BAD GSK3 Figure 1-4. Intracellular signaling pathways regulated by RTKs. The Ras-Raf-MEK-ERK MAPK and the PI3K-Akt pathways are the two most important signaling pathways regulated by RTKs that promote cell proliferation and cell survival. 9
21 5 positions of the inositol ring of phosphoinositides, respectively. Loss of the PTEN tumor suppressor protein, causing PI3K activation, has been implicated in cancer development (9, 10). The importance of the Raf-MEK-ERK and PI3K-Akt signaling pathways in RTK-mediated growth transformation is also supported by the discovery of mutationally-activated B-Raf and p110 alpha in human cancers (11, 12). C. Negative regulation of RTKs To maintain normal cell homeostasis, the activity of RTKs must be tightly regulated and appropriately balanced. The failure of negative regulation of RTKs can lead to aberrant activation of RTK signaling and may contribute to cancer development. Several mechanisms exist for the negative regulation of RTK activity after ligand stimulation, including receptor downregulation and inhibition by tyrosine phosphatases. Receptor downregulation is a major mechanism of negative regulation of RTKs (13, 14). Ligand binding to RTKs induces clathrin-dependent receptor endocytosis, following migration to multivesicular bodies and degradation in the lysosomal compartment. One of the most important signaling proteins involved in this process is the Cbl ubiquitin ligase. Subsequent to the activation of several RTKs such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGF), and hepatocyte growth factor receptor (HGFR), Cbl is recruited to the phosphorylated tyrosine residue of activated RTK via its SH2 domain. Then, Cbl becomes phosphorylated on a tyrosine residue adjacent to the RING finger domain that has E3 ubiquitin ligase activity. This leads to activation of the ubiquitin E3 ligase 10
22 function of Cbl and following receptor ubiquitination by Cbl. Receptor ubiquitination facilitates endocytosis of the RTK via clathrin-coated pits and targets the RTK to the degradation pathway, thus contributing to signal attenuation. In Chapter Three, I evaluate the possible role of Y770, which is part of a putative YXXL motif that may regulate receptor internalization and degradation, in aberrant FGFR2 signaling in cancer (Fig. 1-10). Protein tyrosine phosphatases (PTP) play an important role in regulating RTKs activity and their downstream signaling pathways (15, 16). Dephosphorylation of activation loop sites in RTKs leads to inactivation of the kinase domain and inhibition of RTK activity. While receptor downregulation causes definitive inhibition of signaling, dephosphorylation of RTK leads to transient and reversible inhibition that interferes with the strength and duration of the signal in a defined time period. Although most of PTPs are involved in the negative regulation of RTK signaling, some PTPs also function as positive regulators of RTK signal transduction. For example, the SH2 domain-containing PTP, SHP2, is an essential mediator of signaling for several RTKs. D. RTKs as therapeutic targets for target-based anti-cancer drug development When aberrantly activated, by mutation or overexpession in cancer, many RTKs can become potent oncoproteins. The most well chracterized RTKs implicated in the cancer development are the EGFR family of RTKs (17, 18). The EGFR family consists of four RTKs, EGFR/ErbB1, HER2(neu)/ ErbB2, HER3/ErbB3, and 11
23 HER4/ErbB4 (19). While several ligands (e.g., EGF, TGFα, HB-EGF) for EGFR have been found, a ligand for ErbB2 has not been identified. The ligands for ErbB3 and ErbB4 are the neuregulins (NRG). Stimulation with EGF or NRG induces either homo- or hetero-dimerization of different pairs of members of the EGFR family. ErbB2 may function as a heterodimeric partner with the other members of the family, and hence, be activated by ligands that recognize the dimerization partner. Activation of the EGFR family RTKs leads to activation of multiple downstream signaling pathways, including the Ras-Raf-MEK-ERK MAPK pathway, the PI3K-Akt pathway, and the signal transduction and activator of transcription (STAT) pathway. EGFRs are deregulated in cancer cells in several ways. First, EGFRs can be deregulated by overexpression of EGFRs or their ligands. EGFRs overexpression by gene amplication has been found in many different human cancers, including breast and lung carcinomas (20). Second, EGFRs can be deregulated by activating mutations. Recently, somatic mutations that increase the sensitivity of the receptor to its ligand and alter receptor signaling were identified in the EGFR tyrosine kinase domain in subgroup of patients with non small-cell lung cancer (NSCLC) (21, 22). EGFRs can be inhibited pharmacologically by multiple mechanisms (23). First, catalytic activity of the EGFRs can be inhibited by small molecules blocking the ATP binding site of the receptor. Gefitinib (Iressa) and erlotinib (Tarceva) are EGFRspecific small molecule tyrosine kinase inhibitors (TKIs) and approved by the Food and Drug Administration (FDA) for the treatment of advanced and metastatic NSCLCs. Second, EGFRs can be inactivated by neutralizing antibodies against EGFR or their ligands. Examples include trastuzumab (Herceptin), a recombinant 12
24 humanized monoclonal antibody against ErbB2, and cetuximab (Erbitux), a chimeric antibody against EGFR (23). In addition to the EGFR family of RTKs, there are several other RTKs implicated in cancer (5). The MET RTK has been found to be overexpressed in melanoma and musculoskeletal tumors, and activated by point mutations in NSCLC and renal carcinoma. Activating point mutations and fusions of the Ret/glial-derived neurotrophic factor receptor (GDNFR) occur in multiple endocrine neoplasia and thyroid carcinoma. Vascular endothelial growth factor (VEGF) and its RTKs (VEGFR-1 and VEGFR-2) have been found to be overexpressed in many cancers, including NSCLCs, breast, prostate, and colorectal cancers. Bevacizumab (Avastin), a recombinant humanized anti-vegf monoclonal antibody, has been shown to significantly improve survival rate when used in combination with standard first-line chemotherapy in patients with advanced NSCLC. Several small molecule VEGFR inhibitors have also shown promise in clinical trials in NSCLC (24). II. FGFR family of RTK A. Identification of FGFs Fibroblast growth factors (FGFs) are a large family of growth factors that mediate a variety of cellular responses including cell proliferation, differentiation, migration, and angiogenesis (1, 2). The activities of FGFs are mediated by binding to FGFRs, a family of receptor tyrosine kinases (RTKs), with heparan sulfate proteoglycan as a cofactor. To date, 22 Fgf genes have been found in humans. The first FGF was isolated from bovine pituitary gland as a mitogen that could stimulate the growth of fibroblast cell lines. It was termed basic FGF (bfgf/fgf2) based on 13
25 its high isoelectric point (25). A second FGF was subsequently isolated from brain extracts and termed acidic FGF (afgf/fgf1) based on its lower isoelectric point when compared to bfgf (26). Since then, at least 22 members of the mammalian FGF have been identified. FGF3 (INT-2) (27), FGF4 (28) and FGF5 (29) were originally identified as oncogenes, while FGF6 was identified based on sequence homology to FGF4 (30). FGF7 (keratinocyte growth factor; KGF) was identified as an epithelial-specific mitogen from conditioned medium of a human embryonic lung fibroblasts (31, 32). Unlike FGF1 and FGF2, FGF7/KGF is mitogenic for keratinocytes but not for fibroblasts or endothelial cells. FGF8 was purified as an androgen-induced growth factor from the conditioned medium of mouse mammary carcinoma cells (33) and FGF9 was identified as glial activating factor that stimulate growth of glial cells (34). FGF10 was initially identified from rat embryos by homology based PCR (35). FGF10 protein sequence is similar to FGF7. Like FGF7, FGF10 is expressed in stromal cells and mitogenic to keratinocytes. The remaining FGFs were identified based on sequence information rather than the isolation of growth-promoting factor from tissue or cell llines (3). B. Structural diversity of FGFRs To date, four FGFR genes have been found in humans. Like other RTKs, all four FGFRs (FGFR1 4) are comprised of an extracellular ligand-binding domain, a single transmembrane domain and a cytoplasmic domain with tyrosine kinase activity and additional regulatory sequences in carboxyl-terminus (Fig. 1-2). The extracellular ligand-binding domain of FGFR consists of a signal peptide, three 14
26 immunoglobulin (Ig) like domains (designated as IgI, IgII and IgIII domains; also called D1-D3) as well as a cluster of acidic and serine/threonine amino acids known as the acid box between the IgI and IgII domains (1, 3) (Figs.1-2 and 1-5). In addition to multiple ligands and receptors, the complexity of FGF signaling is further diversified by the fact that the FGFR genes encode multiple structural variants that are generated by alternative gene splicing that occurs both in the extracellular and intracellular regions of the FGFRs (1, 3). Thus, a single FGFR gene can encode multiple, functionally distinct receptors that differ in ligand binding specificity and cytoplasmic signaling activity. In FGFR1-3, a major splicing event involves the exon encoding the Ig-I domain and determines whether the IgI domain is included in the mature receptors. FGFR splicing variants lacking the IgI domain are still able to bind to ligands and biologically functional, indicating that IgI domain is not critical for FGF binding and FGFR function. A second alternative splicing event occurs in the IgIII domain of FGFR1-3 by using two alternative exons (IIIb and IIIc) encoding the carboxyl terminal half of the IgIII domain and the common exon IIIa (encoding the amino terminal half of the Ig-III domain) in a mutually exclusive fashion (Fig. 1-5). These alternative splicing events occur in a tissue-specific manner, resulting in either mesenchymal cell-specific IIIc or epithelial cell-specific IIIb isoforms. In addition, alternative splicing of the sequences that encode the IgIII domain determines the ligand binding specificity of FGFRs. Table 1 summarizes the binding specificity of FGFR isoforms to different FGF ligands. The FGFR4 gene is unique in that there is no variant that differ in Ig-III domain of FGFR4. Of the four FGFRs, the best characterized is FGFR1. Since the focus of my dissertation 15
27 research has been FGFR2, for the remaining introduction I have concentrated on FGFR2. C. Alternative gene splicing of FGFR2: generation of functionally-distinct proteins from a single gene The typical example of splicing variation in IgIII domain is the IIIb and IIIc isoforms of FGFR2. The mesenchymal specific FGFR2 IIIc (also called bacterial expressed kinase; BEK) was isolated originally by the screening of a mouse liver expression cdna library in λgt11 with antiphosphotyrosine antibodies (36, 37). Subsequently, the epithelial cell-specific FGFR2 IIIb was isolated in an expression library biological screen using FGF7/KGF-expressing NIH 3T3 mouse fibroblasts. FGFR2 IIIb was isolated originally from the transformed foci of NIH 3T3 fibroblasts that were transfected with a keratinocyte expression cdna library (38). The two alternative IgIII splicing variants of FGFR2 show different ligand binding characteristics. While FGFR2 IIIb binds FGF7/KGF and FGF10, but not FGF2, FGFR2 IIIc binds FGF2, but not FGF7 and FGF10 (Fig. 1-5). Whereas most FGFs bind more than one FGFR isoforms (Table 1), FGF7/KGF is unique among FGFs in that it binds exclusively to FGFR2 IIIb. Importantly, the tissue expression patterns of the ligands are generally opposite to the expression distribution of their receptors, resulting in the creation of paracrine signaling loops facilitated by epithelial and mesenchymal cell interactions during normal development (Fig 1-6). Another important FGFR2 splicing event occurs in sequences that encode the carboxyl-terminal cytoplasmic domain of FGFR2. To date, at least three splice variants of FGFR2 IIIb that differ in their carboxyl-terminal sequences have been 16
28 identified (designated C1, C2 and C3) (39). The C1 and C2 variants are generated from the same exon with two different splice acceptor sites, while the C3 variants are generated from a separate exon (Fig.1-7). The C2-type carboxyl-terminus is 34 amino acids shorter than the C1-type carboxyl-terminus and C3-type carboxylterminus is 19 amino acids shorter than C2-type carboxyl-terminus. These sequence differences result in differential retention of five tyrosine residues that may serve as sites of receptor autophosphorylation and docking sites for cytoplasmic signaling proteins (Fig. 1-8). One previous study found that expression of the C3 isoform was increased in gastric cancer cell lines, suggesting that aberrant expression of the C3 splicing variant may contribute to cancer development (39). Furthermore, the C3 variant that lacks carboxyl-terminal sequences was shown to be more transforming than C1 variant in NIH 3T3 fibroblasts (39) and human mammary epithelial cells (40). Using a retrovirus-based cdna expression library, derived from mrna isolated from the T-47D human breast carcinoma cell line, our laboratory isolated the wild type sequences for the FGFR2 IIIb C2 isoform. Since little has been done to characterize the signaling and biological activity of this isoform, I chose to study this unique FGFR2 isoform to evaluate and establish its role in breast cancer growth. To date, the majority of studies evaluating FGFR2 IIIb function have been done in fibroblast cells. Since FGFR2 IIIb proteins are normally epithelial cell-restricted in expression, and upregulated in cancers that arise from epithelial cells, studies of FGFR2 IIIb function in epithelial cells will provide a more physiologically-relevant approach. Furthermore, the studies in fibroblasts are complicated by the fact that fibroblasts express the ligands for FGFR2 IIIb. In chapter two, I summarize my studies 17
29 FGFR2 IIIb (KGFR/K-sam-II) Epithelial Cell Restricted IgI IgII IgIII FGFR2 IIIc (Bek/K-sam-I) Mesenchymal Cell Restricted FGF1 FGF3 FGF7/KGF FGF10 FGF22 6 TK TK 822 aa 821 aa IIIa IIIb IIIc 10 FGF1 FGF2 FGF4 FGF6 FGF9 FGF17 FGF18 Figure 1-5. Alternative splicing of FGFR2 and differential ligand stimulation. Alternative splicing occurs in IgIII domain of FGFR2 by using two alternative exons (IIIb and IIIc) encoding the carboxyl terminal half of the Ig III domain and the common exon IIIa (encoding the amino terminal half of the Ig-III domain) in a mutually exclusive fashion. 18
30 FGFR2 IIIc Paracrine signaling loop KGF KGF FGFR2 IIIb KGF KGF Fibroblasts Epithelial Cells Figure 1-6. FGFR2 IIIb and KGF create a paracrine signaling loop between epithelial and mesenchymal cells. The epithelial cell specific FGFR2 IIIb is stimulated by KGF that is secreted by mesenchymal cells, resulting in the formation of paracrine signaling loop. KGF does not bind to the mesenchymal specific FGFR2 IIIc. 19
31 evaluating the transforming and signaling activities of FGFR2 IIIb C2 in epithelial cells. D. FGFR signaling Like other RTKs, upon ligand binding, FGFRs dimerize and result in autophosphorylation on several tyrosine residues in their carboxyl terminal sequences. The docking proteins that recognize the carboxyl terminal sequences of FGFRs, and the signaling pathways that they regulate, have been bestcharacterized with FGFR1. Then, phosphorylated tyrosine residues recruit other signaling molecules to the activated FGFRs. One key signaling molecule recruited to activated FGFRs is phospholipase C gamma (PLCγ). Another important signaling molecule, FRS2 (FGF receptor substrate 2), is bound persistently to inactive FGFR. However, upon ligand binding, FRS2 is phosphorylated and activated by the activated FGFR (1, 6) (Fig.1-9) FRS2 is a docking protein and two members of the FRS2 family have been identified. FRS2α and FRS2β are structurally similar, and both of them contain myristyl. anchors and PTB domains in their amino-termini and multiple tyrosine phosphorylation sites in their carboxyl-termini (41). FRS2α contains binding sites for the adaptor protein Grb2 (41) as well as protein tyrosine phosphatase Shp2 (42). Activation of FGFRs lead to tyrosine phosphorylation of FRS2α, and the tyrosine phosphorylated FRS2α provides binding sites for the SH2 domains of Grb2 and Shp2. Once associated with FRS2α, Shp2 is tyrosine phosphorylated and recruits Grb2 indirectly to the FRS2α. Then, Grb2 forms a complex with Sos via its SH3 domain and this complex formation allows Sos to translocate to the plasma membrane where it can stimulate Ras activation, leading 20
32 TK TK TK C1 and C2 are encoded by splice variants of the same exon; C3 is encoded by a separate exon TK2 C3 C2 C1 IIIb C1 IIIb C2 IIIb C3 Figure 1-7. Alternative splicing of FGFR2 IIIb and carboxyl-terminal sequence variation. The C1 and C2 variants are generated from the same exon with two different splice acceptor sites, while the C3 variants are generated from a separate exon. The C2- type carboxyl-terminus is 34 amino-acid shorter than the C1-type carboxyl-terminus and C3-type carboxyl-terminus is 19 amino-acid shorter than C2-type carboxylterminus. 21
33 A. FGFR2 TK IIIb C1 IIIb C2 IIIb C EYLDLSQPLEQYSPSYPDTRSSCSSGDDSVFSPDPMPYEPCLPQYPHINGSVKT EYLDLSQPLEPYSPCYPDPR I B. FGFR1: Y766 FGFR2: Y770 FGFR1 FGFR2 FGFR3 FGFR4 Figure 1-8. FGFR carboxyl terminal sequences. (A) Carboxyl terminal variants of FGFR2 encoded by alternative splice variants of FGFR2. (B) Conservation of tyrosine residues in FGFR carboxyl terminal sequences. 22
34 to activation of the ERK MAPK and other Ras effector signaling pathways. In addition, Grb2 can recruit the docking protein Gab1, which is then tyrosine phosphorylated by FGFR activation (43). The tyrosine phosphorylated Gab1 recruits SH2 domain-containing signaling molecules, in particular PI3K, resulting in activation of the PI3K-AKT cell survival pathway (Fig 1-8). In Chapter two, I described my studies evaluating the importance of FRS2 adaptor function in FGFR2-mediated growth transformation. FGFR signaling also activates phosphatidylinositol (PI) hydrolysis. In FGFR1, autophosphorylation on Y766 in the carboxyl-terminus serves as a binding site for the SH2 domain of PLCγ, resulting in tyrosine phosphorylation of PLCγ (44, 45) (Fig. 1-10). Phosphorylation and activation of PLCγ leads to stimulation of PI hydrolysis and the generation of the two second messengers, diacylglycerol and Ins(1,4,5)P3. These two second messengers cause the activation of protein kinase C and the Ca2+ release from intracellular stores, respectively (46) (Fig.1-9). The Y766 residue in FGFR1 is well conserved in all four FGFR family members (Fig. 1-8). Recently, it was shown that mutant FGFR2 IIIb in which Y770 is replaced with phenylalanine cannot bind and phosphorylate PLCγ, whereas it was not determined whether this mutant fails to activate PI hydrolysis (47). My studies in Chapter Three evaluated the role of FGFR2 activation of PLCγ in FGFR2-mediated growth transformation. E. FGFR mutations in human skeletal disorders Activating mutations in FGFR1-3 gene have been associated with several skeletal disorders, including chondrodysplasia syndromes (dwarfism) and craniosynostosis syndromes (premature fusion of the cranial sutures) (48, 49). 23
35 Craniosynostosis syndromes are mostly caused by gain-of-function mutations in FGFR2 and include the Pfeifer, Crouzon and Apert syndromes (Fig.1-11). One class of mutations causing Pfeifer and Crouzon syndromes involves the loss a cysteine (for example, C278F and C342R) or gain of a cysteine (for example, W290C and S351C). The consequence of this class of mutations is to generate odd numbers of cysteine residues in the IgIII domain that facilitates the formation of intermolecular disulfide bonds causing ligand-independent dimerization. Another class of mutations causing Apert syndrome involves a missense mutation in one of two missense mutations (S252W or P253R) in the linker region between the IgII and IgIII domains of FGFR2 that is involved in ligand binding. It was demonstrated that these mutations in FGFR2 allow the mesenchymally-expressed mutant FGFR2 IIIc to be activated by mesenchymal cell expressed ligands such as FGF7 and FGF10 and the epithelial cell expressed mutant FGFR2b to be activated by epithelial cell expressed ligands such as FGF2, 6, and 9, resulting in the creation of aberrant autocrine growth signaling loop (50, 51). F. FGFRs and cancer FGFRs have been implicated in cancer by several mechanisms including activating mutations and chromosomal translocations. First, the activating FGFRs mutations involved in developmental defects have been also found in cancer patients. For example, somatic activating mutations of FGFR3 in bladder and cervical carcinomas were found at identical positions (R248C, S249C, G372C and K652E), where germline mutations of FGFR3 causing dwarfism also occur (52). In addition, activating FGFR2 somatic mutations (for example, S267P and S252W) that 24
36 Ras Raf Sos Grb2 P FRS2 P P Gab1 Shp2 P TK TK Y P PLCγ PIP 2 MEK PI3K P DAG + IP3 ERK AKT PKC Ca 2+ Figure 1-9. FGFR cytoplasmic signaling. Activation of FGFRs leads to tyrosine phosphorylation of FRS2, and the phosphorylated FRS2α provides binding sites for the SH2 domains of Grb2 and Shp2. Tyrosine phosphorylated Shp2 can recruit Grb2 indirectly to associate with FRS2. Then, Grb2 forms a complex with Sos via its SH3 domain. In addition, Grb2 can recruit the docking protein Gab1, which is subsequently tyrosine phosphorylated and causes activation of the PI3K-Akt pathway. In FGFR2, autophosphorylation of Y770 in carboxyl-terminus serves as a binding site for the SH2 domain of PLCγ, resulting in tyrosine phosphorylation and activation of PLCγ. 25
37 A. PLCγ P B. PIP 2 DAG + IP3 Y770 L D L PKC Ca 2+ Endosome Lysosome Second messenger signaling Receptor endocytosis, internalization, degradation Figure Possible roles for Y770 in FGFR2 function. (A) Second messenger signaling. (B) Receptor internalization and degradation. 26
38 Apert syndrome FGFR2 S252W FGFR2 P253R Crouzon syndrome FGFR2 C278F FGFR2 C342R FGFR2 W290C FGFR2 S351C Figure FGFR2 mutations in craniosynostosis syndromes and cancer. Craniosynostosis syndromes are characterized by premature fusion of the cranial sutures and include the Pfeifer, Crouzon and Apert syndromes. Interestingly, activating somatic mutations of FGFR2 identical to germ line mutations in craniosynostosis syndromes (for example, S252W and W290C, indicated as red) were also identified in gastric, endometrial and lung carcinomas. 27
39 FGFR1 ZNF198 Constitutively active ZNF 198-FGFR1 fusion kinase Dimerization domain Chromosomal + translocation TK Transcription activator TK TK Figure FGFR1 chromosomal translocation in myeloproliferative syndrome (EMS). The most common chromosomal translocation in EMS is t(8;13)(p11;q12) and generates a ZNF198-FGFR1 fusion protein that contains the zinc finger motif and proline-rich domain of ZNF198 and the intracellular tyrosine kinase domain of FGFR1. The zinc finger motif and proline-rich domains in amino-terminus of ZNF198-FGFR1 facilitate the dimerization of the fusion protein, resulting in the creation of a constitutively activated tyrosine kinase. 28
40 are identical to the germ line mutations associated with craniosynostosis syndromes (Fig.1-11) were identified in gastric and endometrial carcinomas (53, 54). Moreover, systemic analyses of somatic mutations in human cancer genomes identified somatic mutations of FGFR1 and FGFR2 in lung cancers at identical positions (P252T in FGFR1 and W290C in FGFR2) where germ line mutations that cause Pfeifer syndrome occurs (55, 56). Chromosomal translocations often result in the creation of tumor-specific fusion proteins. These fusion proteins are considered to play an important role in the development of hematological malignancies. Translocation and fusion between FGFR1 and at least four alternative partner genes have been identified in 8P11 myeloproliferative syndrome (EMS). The most common chromosomal translocation in EMS is t(8;13)(p11;q12) and generates ZNF198- FGFR1 fusion protein that contains the zinc finger motif and proline-rich domain of ZNF198 and the intracellular tyrosine kinase domain of FGFR1 (57). The zinc finger motif and proline-rich domains in amino-terminus of ZNF198-FGFR1 facilitate the dimerization of fusion protein, resulting in the creation of a constitutively activatedtyrosine kinase (Fig.1-12). Subsequently, other FGFR1 fusion partner genes, including FOP (58), CEP110 (59) and BCR (60), have been identified. Like ZNF198-FGFR1, all these fusion proteins contain non-fgfr1 dimerization domains that are fused to the tyrosine kinase domain of FGFR1, resulting in constitutive activation of FGFR1 kinase activity. A constitutively active form of FGFR2 fusion protein was identified in rat osteosarcoma (ROS) cells (termed as FGFR2-ROS) (61). The FGFR2-ROS contains an altered carboxyl terminus generated from chromosomal rearrangement with a novel gene, designated FGFR activating gene 1 29
41 (FRAG1). The FGFR2-ROS fusion protein appears to form constitutive dimmers, resulting in constitutively activated tyrosine kinase. Fusion of the ETV6/TEL gene to FGFR3 was found in a patient with peripheral T-cell lymphoma (PTCL) with a t(4;12)(p16;p13) translocation (62). The TEL-FGFR3 fusion protein consists of the HLH domain of ETV6/TEL in amino-terminus and the tyrosine kinase domain of FGFR3 in carboxyl-terminus. TEL-FGFR3 fusion protein displays constitutive kinase activity that is mediated by the oligomerization of the HLH domain (63). A third mechanism by with FGFR function can be deregulated in human cancers involves changes in gene splicing and the preferential expression of functionally-distinct forms of FGFR2 in cancers. In my studies in Chapter Two, I describe my studies suggesting that differential gene splicing and expression of the C2 and C3 isoforms of FGFR2 IIIb results in preferential expression of transforming variants of this receptor. Additionally, I describe a shift in gene splicing in invasive breast cancers that favors expression of the normally mesenchymal-restricted FGFR2 IIIc isoform. This isoform may then promote EMT and breast cancer progression. III. FGFR2 and oncogenesis A. Opposing roles for FGFR2 in oncogenesis Although the role of FGFR2 IIIb in organ development has been well studied, much less is known regarding the role of FGFR2 IIIb in oncogenesis. In particular, there are conflicting observations for the role of FGFR2 IIIb in oncogenesis. While some reports demonstrated that FGFR2 IIIb is a transforming oncoprotein, other 30
42 reports showed that FGFR2 IIIb functions as a tumor suppressor. For example, ectopic expression of FGFR2 IIIb in human prostate tumor PC-3 cell lines (64) or salivary adenocarcinoma cells (65) resulted in reduced cell growth. Furthermore, expression of FGFR2 IIIb was shown to be downregulated in bladder carcinomas (66) and restoration of FGFR2 IIIb in human bladder carcinoma cells that lack FGFR2 IIIb caused reduced cell growth (67). In contrast, there are evidences suggesting that FGFR2 IIIb is an oncoprotein. FGFR2 IIIb was shown to be overexpressed in poorly differentiated types of stomach cancers and expression of FGFR2 IIIb caused transformation of NIH 3T3 fibroblasts (39). Moreover, it was shown that the Fgfr2 IIIb gene was amplified in the SUM-52PE breast cancer cell line and ectopic expression of FGFR2 IIIb in the immortalized human mammary epithelial cell line H16N2 induced growth transformation (40, 68). The basis for the seemingly opposing roles of FGFR2 IIIb in cellular transformation and oncogenesis is not known, and may reflect cellular genetic context differences in FGFR2 function. That such opposing functions can be ascribed to a single signaling protein is not surprising and has been seen for other oncoproteins, such as Ras, and are discussed in the next section. B. Cell type-specific differences in transformation. The accumulated evidences indicate that cell type-specific differences exist in cellular transformation and oncogenesis. For example, transforming growth factor β (TGF-β) inhibits the growth of epithelial cells, whereas TGF-β can induce oncogenic transformation of fibroblasts (69, 70). Furthermore, we and others have previously 31
43 found that although activating Ras or Raf transforms rodent fibroblasts, only Ras but not Raf transforms epithelial cells (71, 72), suggesting distinct mechanisms exist for Ras transformation in epithelial cells and fibroblasts. Although previous studies showed FGFR2 IIIb transformed both fibroblasts and epithelial cells (38-40), whether FGFR2 IIIb causes transformation of fibroblasts and epithelial cells by distinct or common, mechanisms has not been determined. C. Potential role of FGFR2 in epithelial to mesenchymal transition (EMT) and cancer development EMT is a fundamental process for morphogenesis during embryonic development and is also implicated in the carcinoma progression (73-75). EMT is characterized by the loss of cell polarity and cell-cell contacts, gain of fibroblastic morphology, and loss of epithelial (E-cadherin, β-catenin) and gain of mesenchymal (N-cadherin, vimentin and fibronectin) marker expression (Fig. 1-13). Several developmentally important genes that induce EMT during embryonic morphogenesis have been also implicated in tumor progression. For example, Snail and the closely related gene Slug, which are essential for gastrulation during embryonic development, have shown to be transcriptional repressors of E-cadherin and mediators of EMT in human invasive carcinoma cells (76-78). More recently, Twist, a key regulator of embryonic morphogenesis, was found to play an essential role in breast cancer metastasis by loss of E-cadherin-mediated cell-cell adhesion, activation of mesenchymal markers, and induction of cell motility (79). Several growth factors, including TGFβ, HGF/SF (hepatocyte growth factor/scatter factor), 32
44 FGF, and EGF have been shown to induce EMT by activating Ras and ERK MAPK pathway or the PI3K-Akt pathway in certain epithelial cells (78, 80, 81) (Fig.1-11). There is evidence that FGFR2 IIIb signaling may induce EMT. Thiery and colleagues showed that FGF1 or FGF7, but not FGF2 can induce EMT in NBT-II rat bladder carcinoma cells that express FGFR2 IIIb, but not FGFR1 or FGFR2 IIIc (81). They also showed that treatment of FGF1 induced an FGFR2 IIIb to FGFR2 IIIc exon switch in NBT-II cells. Interestingly, this exon IIIb to IIIc splicing switch is accompanied by a gain of a fibroblastic morphology and enhanced cell motility. A loss of FGFR2 IIIb expression and gain of FGFR2 IIIc expression was also observed in rat prostate tumor progression model and prostate cancer cells (82, 83). Taken together, these observations suggest that aberrant expression of mesenchymal specific FGFR2 IIIc in epithelial cells might contribute to cancer progression by inducing EMT. However, the possible role of FGFR2 IIIc in cancer progression has not been explored. In my studies in Chapter two, I explored the possible role of alternative gene splicing and expression of the normally mesenchymal cell-restricted FGFR2 IIIc isoform in invasive breast cancers as a mediator and/or marker of EMT. In summary, despite the strong evidence that aberrant FGFR function may promote oncogenesis, the role of gene splice and the specific involvement of FGFRmediated signaling in cancer development and growth are areas that remain poorly understood. In Chapter Two, I explore cell context differences in FGFR2 transforming activity and the role of alternative splice in altering FGFR2 biological function in breast cancer growth and invasion, as well as EMT. In Chapter Three, I evaluate the mechanistic consequences of alternative gene splicing and loss of 33
45 carboxyl terminal sequences in activating FGFR2 transforming activity. In particular, these studies critically evaluate the key cytoplasmic signaling events that may promote FGFR2 growth transformation and implicate aberrant receptor endocytosis as an important mechanism for FGFR2 activation in cancer. My studies establish gene splicing as an important mechanism of FGFR2 activation in cancer, and they further validate FGFR2 as an oncogene that promotes epithelial cell transformation. 34
46 FGF, EGF, HGF TGFβ Ras Smad Twist Snail/Slug Loss of epithelial markers : E-cadherin, Cytokeratine Gain of mesenchymal markers: N-cadherin, Vimentin, Smooth-muscle actin EMT Figure Regulators of EMT. E-cadherin repressors such as Snail and Twist induce EMT, which is characterized by a loss of cell-cell contacts, a gain of fibroblastic morphology, and a loss of expression of epithelial and gain of expression of mesenchymal markers. Growth factor receptor mediated Ras activation or TGFβ/smad pathways induce EMT by increasing Snail expression. Snail and Twist induce EMT by downregulating E- cadherin expression. 35
47 Chapter 2: Cell Context-dependent Mechanisms of FGFR2 IIIb Transformation of Fibroblast and Epithelial Cells I. Abstract We identified the IIIb C2 epithelial cell-specific splice variant of fibroblast growth factor receptor 2 (FGFR2 IIIb C2) receptor tyrosine kinase in a screen for activated oncogenes expressed in T-47D human breast carcinoma cells. We found FGFR2 IIIb C2 expression in breast carcinoma cell lines and additionally, expression of the mesenchymal-specific FGFR2 IIIc splice variant in invasive breast carcinomas. Furthermore, we found FGFR2 IIIc expression was associated with loss of epithelial and gain of mesenchymal markers. Although FGFR2 IIIb is expressed in epithelial cells, previous studies on FGFR2 IIIb transformation have focused on fibroblasts. Since cell-type specific differences exist in cellular transformation and oncogenesis, we compared the transforming activities of FGFR2 IIIb C2 in NIH 3T3 fibroblasts and RIE-1 intestinal epithelial cells. FGFR2 IIIb C2 caused growth transformation of both cell types but morphologic transformation of only NIH 3T3 cells. FGFR2 IIIb C2- transformed NIH 3T3 but not RIE-1 cells showed persistent activation of Ras and increased cyclin D1 protein expression. NIH 3T3, but not RIE-1, cells express keratinocyte growth factor (KGF), a ligand for FGFR2 IIIb C2. Ectopic treatment with KGF caused FGFR2 IIIb C2-dependent morphologic transformation of RIE-1 cells, as well as cyclin D1 upregulation, indicating that both ligand-independent and 36
48 stromal cell derived ligand-dependent mechanisms contribute to epithelial cell transformation. FGFR2 IIIb C2 transformation of RIE-1 cells was also dependent on an epidermal growth factor receptor autocrine mechanism. Our results support distinct mechanisms of FGFR2 IIIb C2 transformation of fibroblast and epithelial cells. II. Introduction Fibroblast growth factors (FGFs) comprise a large family of structurally related growth factors (22 human members) that mediate a variety of cellular responses that include cell proliferation, differentiation, migration, and angiogenesis (1, 2, 84). The activities of FGFs are mediated by their binding to a family of four receptor tyrosine kinases (RTKs), designated FGFR1-4. FGFRs are comprised of an extracellular domain that consists of two or three immunoglobulin (Ig)-like domains, a single transmembrane domain and an intracellular catalytic tyrosine kinase domain and flanking regulatory sequences (Fig. 1A). In addition to multiple ligands and receptors, the complexity of FGF signaling is further diversified by the fact that the FGFR genes encode multiple structural variants that are generated by alternative gene splicing (1, 2, 84). Most importantly, in the case of FGFR1-3, alternative RNA splicing and exon utilization of sequences encoding the carboxyl-terminal half of the third Ig-like domain III results in the expression of either the IIIb or IIIc isoform of the FGFRs (Fig. 1A). This alternative splicing occurs in a tissue-specific manner and determines the ligand binding specificity of FGFRs. For example, FGFR2 IIIb (also called KGFR and K-sam-II) binds FGF7 (also called keratinocyte growth factor; KGF) and FGF10, but not FGF2, whereas the FGFR2 IIIc (also called Bek/K-sam-I) binds FGF2 and FGF18, but not 37
49 FGF7 and FGF10. Additionally, ligand and receptor expression can be tissue restricted and expressed in a non-overlapping tissue distribution. FGFR2 IIIb expression is restricted exclusively to normal epithelial cells, while FGFR2 IIIc isoform is generally expressed in normal mesenchymal cells. In contrast, expression of the ligands for FGFR2 IIIb (KGF/FGF7 and FGF10) is restricted to mesenchymal cells, and ligands for the mesenchymal-restricted receptor are generally expressed in epithelial cells, resulting in the creation of paracrine signaling loops facilitated by epithelial and mesenchymal cell interactions during normal development. However, exon switching and expression of FGFR2 IIIc has been described in epithelial cell tumor progression. A loss of FGFR2 IIIb expression and gain of FGFR2 IIIc expression was observed in rat prostate tumor progression from androgendependence to androgen-independence (83). A similar exon switch has been described for NBT-II rat bladder carcinoma cells, where the onset of FGFR2 IIIc expression was correalted with epithelial-mesenchymal transition (EMT), a process associated with tumor progression and invasion (75). Alternative splicing also results in FGFR2 variation in carboxyl-terminal sequences. To date, at least three carboxyl-terminal splice variants of FGFR2 IIIb have been identified, designated C1, C2 and C3 (39) (Fig. 1B). The C2-type carboxyl-terminus is 34 amino-acid shorter than the C1-type carboxyl terminus, whereas the C3-type carboxyl terminus is 53 amino-acid shorter than C1-type carboxyl terminus. These sequence differences result in differential retention of tyrosine residues that may serve as sites of receptor autophosphorylation and docking sites for cytoplasmic signaling proteins. Enhanced expression of the C3 38
50 isoform was seen in gastric cancer cell lines (39). Ethier and colleagues found that normal human mammary epithelial cells express FGFR2 IIIb C1 and C2, but C3 isoform expression found only in the SUM-52 breast tumor cell line (68). Previous studies showed that FGFR2 IIIb C1 and IIIb C3 could promote growth transformation of NIH 3T3 mouse fibroblast (39) and H16N2 immortalized human mammary epithelial cells (40), with C3 exhibiting greater transforming activity. However, the transforming activity of FGFR2 IIIb C2 has not been evaluated. FGFRs are activated by ligand-induced dimerization, causing stimulation of their intrinsic tyrosine kinase activity, tyrosine autophophorylation, and recruitment of signaling proteins to specific phosphorylated tyrosine residues in their cytoplasmic carboxyl-termini (1). FGFRs activate the FGFR substrate 2 (FRS2) docking proteins. FGFR phosphorylation of FRS2 creates phosphorylated tyrosine docking sites for the Grb2 adaptor protein. Recruitment of Grb2 in complex with the SOS Rasspecific guanine nucleotide exchange factor causes activation of Ras and the ERK mitogen-activated protein kinase (MAPK) cascade. Ras can also activate the phosphatidylinositol 3-kinase (PI3K)-AKT serine/threonine kinase pathway in some cell types. FRS2-dependent FGFRs signaling can also activate the PI3K-AKT pathway through Grb2 and the Grb2-associated docking protein Gab1. Since the majority of studies evaluating FGFR signaling are based on transient stimulation with FGFs that can activate multiple FGFRs (for example, FGF1 binds and stimulates all four FGFR1-4 isoforms), it is not clear whether the identified FGFR signaling activities described above are common for all four FGFRs. Furthermore, the 39
51 majority of studies have focused on FGFR1 and there has been little effort made in studying the signaling activities that mediate FGFR2 IIIb oncogenesis. The accumulated evidences indicate that cell type-specific differences exist in cellular transformation. For example, transforming growth factor β (TGF-β) inhibits the growth of epithelial cells, whereas TGF-β induces oncogenic transformation of fibroblasts (69, 70, 85). Furthermore, we and others have previously found that although activating Ras or Raf transforms fibroblasts, only Ras but not Raf transforms epithelial cells, suggesting distinct mechanisms exist for Ras transformation in epithelial cells and fibroblasts (71, 72). The majority of studies have evaluated the transforming potential of FGFR2 IIIb in NIH 3T3 mouse fibroblasts (38, 39, 86). Since fibroblasts and not epithelial cells express FGFR2 IIIb ligands, the biological analyses of FGFR2 IIIb in fibroblasts is complicated by an autocrine growth loop not present in epithelial cells. Since FGFR2 IIIb is an epithelial cell-specific splice variant, an evaluation of FGFR2 IIIb signaling and biological activity in an epithelial cell model system may provide a more physiologically-relevant assessment of the role and mechanism of aberrant FGFR2 IIIb function in human epithelial cell-derived carcinomas. Therefore, in the present study, we evaluated the transforming activities and signaling mechanisms of FGFR2 IIIb in epithelial cells. Since we identified the C2 isoform of FGFR2 IIIb in a screen for activated oncogenes, and determined that C2 expression was characteristic of the majority of breast carcinoma cells, we focused our analyses on this isoform. We found that while FGFR2 IIIb C2 caused growth transformation of NIH 3T3 fibroblasts and RIE-1 epithelial cells, FGFR2 IIIb C2 transformation of RIE-1 cells was mediated 40
52 primarily by a ligand-independent mechanism that did not involve activation of Ras and Ras-mediated signaling. Furthermore, FGFR2 IIIb C2 transformation of RIE-1 cells was mediated, in part, by induction of an epidermal growth factor receptordependent autocrine mechanism. Finally, we found that expression of the mesenchyme-specific FGFR2 IIIc isoform in invasive breast carcinomas. Taken together, our results suggest that FGFR2 IIIb causes growth transformation by distinct signaling mechanisms in fibroblast and epithelial cells, and that isoform switching might be a mechanism for FGFR2 deregulation in breast cancer progression. III. Materials and methods Cell Culture and Plasmid Expression Vectors NIH 3T3, RIE-1, MCF-10A, NMuMG, and human breast carcinoma cell lines were maintained as we have described previously (72, 87). Telomerase-immortalized human mammary epithelial cells (HMEC; provided by R. Weinberg, MIT) were maintained in MEGM growth medium (Clonetics). The cdna sequence encoding FGFR2 IIIb C2 was subcloned into the 5' blunt end and 3' SalI site of the pbabe-puro retroviral vector. The pctv3 and pbabe-puro H-Ras(61L) expression vectors were described previously (88). Expression Library Screening Messenger RNA was purified from 2.8 x 10 7 T-47D cells and double stranded cdna was synthesized and used to generate the cdna library in the pctv1b retroviral vector as described (89, 90). The T-47D cdna plasmid library was then converted 41
53 into infectious retrovirus by transfection of 293T cells. Rat-1 fibroblasts (5 x 10 5 ) and RIE-1 cells (7 x 10 5 ) were plated in 10 cm dishes (ten each) the day prior to infection with retrovirus expressing the T-47D cdna expression library. Genomic DNA was isolated from transformed foci and cdnas were recovered by PCR as described (90). FGFR2 Isoform Expression Analyses To evaluate the expression of FGFR2 C1, C2 and C3 splicing variants in human breast cells, total cellular RNA was isolated from each cell line and reverse transcribed. The resulting cdnas were amplified by PCR for 35 cycles. The primer sequences used for amplification of FGFR2 C1, C2 and C3 cdna fragments were described previously (39). Our analysis of exon IIIb and IIIc expression was done as described previously (82), with PCR amplification of the cdna sequences for 50 cycles. Transformation Assays For primary focus formation assays, NIH 3T3 fibroblasts were plated at 2 x 10 5 cells per 60 mm dish. The following day, cells were transiently transfected with the pbabepuro empty vector, or encoding H-Ras(61L) or FGFR2 IIIb C2. For secondary focus formation assays, RIE-1 cells and NIH 3T3 fibroblasts were stably infected with the pbabe-puro empty vector or encoding H-Ras(61L) or FGFR2 IIIb C2. After infection, cells were selected in growth medium supplemented with puromycin (2 µg/ml). Multiple drug-resistant colonies were pooled and replated into 60 mm dishes and maintained in growth medium for three weeks before the appearance of foci of transformed cells was photographed. To determine the growth rate on plastic and saturation density, mass populations of NIH 3T3 and RIE-1 cells stably expressing 42
54 FGFR2 IIIb C2 were plated at 5 x 10 4 cells per 60 mm dishes. Cells were then trypsinized and duplicate dishes were counted every three days for days. Parallel cultures of NIH 3T3 and RIE-1 cells stably-infected with the empty pbabepuro vector, or encoding activated H-Ras(61L), were included for negative and positive controls, respectively. To determine the anchorage-independent growth, NIH 3T3 and RIE-1 cells stably expressing either empty vector or FGFR2 IIIb C2 were suspended in 0.4% bacto-agar in growth medium at 5 x 10 4 cells per 60 mm dish. The single cell suspensions were layered on top of 0.6% bacto-agar in growth media. After days, colonies were stained with 2 mg/ml MTT (tetrazolium salt 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and the average number of colonies on duplicate dishes was calculated. Signaling Analyses To determine the level of Ras activation, we utilized a GST fusion protein containing the Ras-binding domain (RBD) of Raf-1 as we have described previously (91, 92). To determine ERK and AKT activation, cells were lysed and then analyzed for active ERK and AKT by immunoblot analyses with antibodies specifically detect phosporylated ERK and AKT (Cell Signaling Technology), respectively. The membranes were stripped and reprobed with antibodies for total ERK (Santa Cruz Biotechnology, sc93) and AKT (Cell Signaling Technology), respectively. To determine cyclin D1 protein expression levels, immunoblot analyses were performed with anti-cyclin D1 antibody (Santa Cruz Biotechnology). To determine Rb phosphorylation and inactivation, immunoblot analyses were performed with 43
55 antibody specific for the phosphorylated (Ser 780), inactivated form of Rb (Cell signaling technology). Conditional Media Assays RIE-1 cells stably expressing either empty vector or FGFR2 IIIb C2 were plated and cultured in growth medium. After 3 or 12 days after plating, conditioned media from either vector infected cells or FGFR2 IIIb C2 expressing cells were collected by passage through 0.45 µm filter and added onto subconfluent parental RIE-1 cells. Where indicated, the cells were pretreated with either the U0126 MEK inhibitor (Promega) or the gefitinib EGFR inhibitor (a gift from Dr. Shelley Earp, UNC-Chapel Hill). IV. Results A. Identification of the FGFR2 IIIb C2 Splice Variant as a Transforming Protein from T-47D Breast Carcinoma Cells. To identify novel oncogenes involved in breast epithelial cell growth transformation, we generated a retrovirus-based cdna expression library from mrna expressed in T-47D human breast carcinoma cells. The cdna expression library was introduced into Rat-1 fibroblasts and RIE-1 epithelial cells. One 2.3 kb cdna sequence was identified independently as a sequence that induced focus formation in both our Rat-1 and RIE-1 screenings. This sequence encodes for FGFR2 IIIb C2, the epithelial cell-specific splice variant of FGFR2 (Fig. 2-1). 44
56 Figure 2-1. Human FGFR2 isoforms. A. Structure of FGFR2 amino-terminal isoforms. Cell type-specific alternative exon utilization and gene splicing results in formation of two amino-terminal variants, the epithelial cell-specific IIIb isoform and the mesenchymal-specific IIIc isoform. Abbreviations: Ig, immunoglobulin-like; TM, transmembrane; TK, tyrosine kinase domain. B. Carboxyl-terminal sequence comparison of FGFR2 IIIb splice variants. Carboxyl-terminal amino sequence comparison of FGFR2 IIIb C1, C2 and C3 splice variants. The known or putative tyrosine phosphorylation sites are bolded and indicated by their residue number. FGFR2 IIIb C1 (822 aa), C2 (788 aa) and C3 (769 aa) differ in the retention of these tyrosine residues as well as divergence in the underlined residues. 45
57 B. Aberrant Expression of the Mesenchymal FGFR2 IIIc Isoform in Invasive Breast Cancer Cell Lines. The SUM-52 breast cancer cell line expresses nine distinct FGFR2 IIIb isoforms that include the C1, C2 and C3 variants (68). However, aside from this cell line, a systematic analysis of established breast carcinoma cell lines for FGFR2 IIIb isoform expression has not been done. Therefore, we performed RT-PCR analyses to determine transcription of FGFR2 IIIb carboxyl-terminal isoforms in the immortalized, nontransformed MCF-10A human breast epithelial cell line and a panel of breast carcinoma cell lines. We found that MCF-10A cells expressed predominantly C1, and very weakly, the C2 and C3 isoforms (Fig. 2-2A). In contrast, the majority of tumor cell lines (6 of 9) showed significant expression of all three isoforms. To date, there has been limited analysis of the expression of the mesenchymal-specific FGFR2 IIIc in breast cancer cells. Whereas exon switching and preferential expression of FGFR2 IIIc has been associated with tumor progression in two rat carcinoma models (75, 83), analyses of breast cancer tissue found exon switching to favor IIIc expression was associated with patients with advanced clinical staging (93). Therefore, we utilized exon III-specific primers together with restriction enzyme analyses (82), to detect the expression of FGFR2 IIIb and IIIc in breast carcinoma cell lines (Fig. 2-2B). Since exon IIIb contains a unique AvaI site, whereas exon IIIc contains two HincII sites, digestion with these two enzymes allows detection of these two isoforms. We found that FGFR2 IIIb expression was characteristic of untransformed MCF-10A and noninvasive (BT-474, 46
58 ZR-75-1, MCF-7, T-47D, MDA-MB-468, and SK-BR-3) breast cell lines, whereas FGFR2 IIIc was detected in all three invasive breast carcinoma cell lines (Hs578T, BT-549 and MDA-MB-231). Previously, we verified that the Hs578T, BT-549 and MDA-MB-231, but not the untransformed MCF-10A or other carcinoma cell lines, showed invasive activity in vitro by using the Matrigel invasion and other assays (87). We also found that expression of FGFR2 IIIc was correlated with an epithelial-tomesenchymal transition (EMT), as indicated by the loss of epithelial (E-cadherin) and gain of mesenchymal (N-cadherin and vimentin) protein expression (Fig. 2-2B). Thus, breast cancer progression may involve two distinct alternative splicing events, with expression of FGFR2 IIIb C2/C3 isoforms associated with the progress from normal breast epithelia to non-invasive breast cancer, and the conversion to the mesenchymal FGFR2 IIIc isoform in invasive breast cancer cells. Previous studies have determined that FGFR2 IIIb C3 exhibits greater transforming activity than FGFR2 IIIb C1 (39, 40). The transforming activity of the IIIb C2 isoform has not been evaluated. Therefore, for the remainder of this study, we focused our analyses on the biological activity of the FGFR2 IIIb C2 isoform isolated in our transformation screen. Our identification of FGFR2 IIIb C2 as a transforming protein in T-47D breast carcinoma cells suggests that FGFR2 IIIb C2 will function as an oncogene and promote breast epithelial cell growth transformation. To address this possibility, we ectopically-expressed FGFR2 IIIb C2 in untransformed NMuMG mouse mammary epithelial cells, the spontaneously-immortalized, untransformed MCF-10A and telomerase-immortalized HMEC human breast epithelial cell lines. All three cell 47
59 lines have been shown previously to be sensitive to activated Ras-mediated growth transformation (94-97). Whereas empty-vector infected cells did not grow in soft agar, we found that FGFR2 IIIb C2-expressing NMuMG, MCF-10A and HMEC cells showed the ability to form colonies when suspended in soft agar (Fig. 2-2C and data not shown), supporting an oncogene function for FGFR2 in T-47D breast carcinoma growth. C. Distinct Transforming Activities of FGFR2 IIIb C2 in NIH 3T3 Fibroblasts and RIE-1 Epithelial Cells. Since previous studies evaluating FGFR2 IIIb function in NIH 3T3 mouse fibroblasts are complicated by fibroblast production of FGFR2 IIIb ligand, we focused our analyses using the RIE-1 cell line, a well-characterized rodent epithelial cell model system for the analyses of oncogene transformation. Since previous studies used NIH 3T3 cells, we performed parallel analyses in NIH 3T3 cells to identify possible cell type differences in FGFR2 IIIb C2 transforming activity. As expected from the identification of FGFR2 IIIb C2 in both our screens with Rat-1 and RIE-1 cells, we found that FGFR2 IIIb C2 caused potent formation of foci of transformed cells in NIH 3T3, RIE-1 and Rat-1 cells (Fig. 2-3A; data not shown). Formation of foci of transformed cells is indicative of a loss of density-dependent growth inhibition and/or morphologic transformation. Therefore, we determined if FGFR2 IIIb C2 can cause these cellular parameters of oncogenesis. First, we examined whether expression of FGFR2 IIIb C2 can cause loss of density dependent growth inhibition in NIH 3T3 fibroblasts and RIE-1 cells. Since FGFR2 IIIb 48
60 signaling can involve activation of Ras, we utilized activated H-Ras(61L) as a positive control for these analyses. First, we found that expression of FGFR2 IIIb C2 induced primary focus formation in NIH 3T3 fibroblasts. Next, we established RIE-1 cells stably infected with the pbabe-puro empty vector, or encoding FGFR2 IIIb C2 or activated H- Ras(61L) (designated RIE(vector), RIE(FGFR2), RIE(Ras), respectively) and performed secondary focus formation assays. Whereas RIE(vector) cells showed no focus-forming activity, RIE(FGFR2) cells showed potent focus-forming activity (Fig. 2-3A). Second, we determined whether FGFR2 IIIb C2 caused morphologic transformation of NIH 3T3 fibroblasts and RIE-1 cells. For these analyses, NIH 3T3 fibroblasts were stably infected with the pbabe-puro empty vector, or encoding FGFR2 IIIb C2 or activated H-Ras(61L) (designated NIH(vector), NIH(FGFR2), NIH(Ras), respectively). To our surprise, although FGFR2 IIIb C2 caused focus formation in both NIH 3T3 fibroblasts and RIE-1 cells (Fig. 2-3A), only NIH 3T3 fibroblasts but not RIE-1 cells were morphologically transformed by FGFR2 IIIb C2 (Fig. 2-3B). NIH(FGFR2) cells showed a highly retractile, elongated and spindleshaped cell morphology that was essentially identical to that of NIH(Ras) transformed cells. In contrast, the morphology of RIE(FGFR2) cells was indistinguishable from the RIE(vector) cells (Fig. 2-3B). We next evaluated the growth rate and saturation density of NIH 3T3 fibroblasts and RIE-1 cells stably expressing FGFR2 IIIb C2. When the cultures were at subconfluent densities (between day 0 and 6 post plating; Fig. 2-3C), NIH(FGFR2), but not RIE(FGFR2), 49
61 A MCF-10A BT474 ZR-75 MCF-7 T-47D MDA-MB-468 SK-BR-3 Hs578T BT549 MDA-MB-231 C1 C2 B IIIb AvaI NT MCF-10A BT474 ZR-75 Non-invasive MCF-7 T-47D MDA-MB-468 SK-BR-3 Hs578T Invasive BT549 MDA-MB-231 HAUHAUHAUHAUHAUHAUHAUHAUHAUHAU IIIc HincII C3 E-cadherin GAPDH Matrigel Vimentin N-Cadherin C Colonies Per Dish MCF-10A Vector FGFR2 Colonies Per Dish NMuMG Vector FGFR2 Figure 2-2. Expression of transcriptions encoding amino- and carboxylterminal isoforms of FGFR2. A. Expression of FGFR2 C1, C2 and C3 variants in MCF-10A and human breast carcinoma cells. RT-PCR products were fractionated on an agarose gel and stained with ethidium bromide. Matrigel (+) indicates that invasive or (-) non-invasive breast carcinoma cell lines as we have determined previously (87). B. Expression of the mesenchyme-specific IIIc isoform in invasive breast carcinoma cell lines. RT-PCR products were obtained using FGFR2 exon III specific primers and digested with either AvaI or HincII (U=uncut, A=AvaI digest and H=HincII digest). Full length, uncut PCR products generate 367 bp and 364 bp bands for IIIb and IIIc isoforms, respectively. The presence of 249 bp and 118 bp bands following digestion with AvaI is indicative of FGFR2 IIIb. The presence of 125 bp, 120 bp and 119 bp bands following digestion with HincII is indicative of FGFR2 IIIc. Immunoblot analyses were done using antibody against E-cadherin, vimentin and N-cadherin for total cell lysates of the indicated cell lines. C. FGFR2 IIIb induces anchorage-independent growth of NMuMG and MCF-10A cells. NMuMG and MCF-10A cells stablyexpressing either the empty vector or encoding FGFR2 IIIb C2 (designated FGFR2) were suspended in 0.4% soft agar and the number of proliferating colonies was quantitated after 3-4 weeks. Data shown are the average of two dishes and representative of two or three independent assays. 50
62 cells displayed an increased growth rate when compared with their empty vector counterparts. However, at confluent densities (after day 6), both NIH(FGFR2) and RIE(FGFR2) cells showed increased growth rates when compared with NIH(vector) and RIE(vector) cells, respectively (Fig. 2-3C). In contrast, both NIH(Ras) and RIE(Ras) cells showed increased growth rates in both subconfluent and confluent cultures, indicating that FGFR2 IIIb C2, but not Ras, causes distinct growth promoting activities for fibroblasts and epithelial cells. Finally, we assessed whether FGFR2 IIIb C2 can induce anchorage-independent growth of NIH 3T3 fibroblasts and RIE-1 cells and we found that both NIH(FGFR2) and RIE(FGFR2) cells, but not their empty vector counterparts, formed colonies in soft agar (Fig. 2-3D). D. FGFR2 IIIb C2 Causes Activation of Ras, ERK and AKT and Upregulation of Cyclin D1 Protein Levels in NIH 3T3 But Not RIE-1 Cells. FGF stimulation activates multiple downstream signaling networks, including the Ras and ERK mitogen-activated protein kinase cascade (MAPK) (1). Surprisingly, little has been reported regarding whether FGFR2 IIIb is an activator of Ras and Ras-mediated signaling pathways. Furthermore, most of studies done for FGFR2 IIIb signaling have focused on transient activation of FGFR2 IIIb (47, ) rather than sustained activation of FGFR2 IIIb in transformed cells. Therefore, we focused on characterizing the consequences of sustained activation of FGFR2 IIIb in stablytransformed cells. First, we determined whether FGFR2 IIIb C2 caused sustained activation of Ras. To measure Ras activity, we performed a pull down assay utilizing 51
63 A B FGFR2 RIE-1 Vector NIH 3T3 NIH 3T3 RIE-1 Vector FGFR2 Ras C D 5 Number of Cells (x10 ) Vector FGFR2 NIH 3T3 H-Ras(61L) Days 5 Number of Cells (10 ) Vector FGFR2 RIE-1 H-Ras(61L) Days Vector FGFR2 NIH 3T3 RIE-1 Figure 2-3. Distinct transforming activities of FGFR2 IIIb C2 in NIH 3T3 fibroblasts and RIE-1 epithelial cells. A. FGFR2 IIIb C2 induces focus formation in NIH 3T3 and RIE-1 cells. NIH 3T3 cells were transfected with either the empty pbabe-puro vector (Vector) or encoding FGFR2 IIIb C2 (FGFR2) and the appearance of foci of transformed cells were photographed after 14 days in culture (left panel). RIE-1 cells were infected with the empty pctv3 vector or encoding FGFR2 IIIb C2. Drug-resistant colonies were pooled and replated for secondary focus formation analyses. Cells were grown to confluence for 21 days before the appearance of foci of transformed cells was photographed (middle panel). The cells were then fixed and stained with crystal violet (total dish; right panel). B. FGFR2 IIIb C2 induces morphologic transformation of NIH 3T3 but not RIE-1 cells. C. FGFR2 IIIb C2 shows distinct growth promoting activity in NIH 3T3 and RIE-1 cells dependent on cell density. D. FGFR2 IIIb C2 induces anchorage-independent growth of NIH 3T3 and RIE-1 cells. 52
64 a GST fusion protein containing the GTP-dependent Ras-binding domain from the Ras effector, the Raf-1 serine/threonine kinase (GST-Ras-RBD). As expected, the H-Ras-transformed NIH 3T3 and RIE-1 cells showed greatly elevated levels of Ras- GTP. Surprisingly, we found that NIH(FGFR2) but not RIE(FGFR2) cells showed an elevated level of activated GTP-bound Ras (Fig. 2-4A). Next, we determined whether FGFR2 IIIb C2 caused sustained activation of downstream effector pathways of Ras. The two best-characterized effector pathways of Ras important for growth transformation are the Raf-MEK-ERK MAPK and the PI3K-AKT pathways. For these analyses, we utilized immunoblot analyses with phospho-specific antibody that recognizes the phosphorylated and activated forms of ERK1 and ERK2, or AKT. As we have showed previously (102), activated Ras causes sustained ERK activation in NIH 3T3 and RIE-1 cells, and sustained AKT activation in NIH 3T3 but not RIE-1 cells (Fig. 2-4B). Expression of FGFR2 IIIb C2 also led to sustained activation of ERK1/2 and AKT in NIH 3T3 fibroblasts, although the degree of activation was less than that seen in NIH(Ras) cells. In contrast, we found no increase in activated ERK or AKT levels in RIE(FGFR2) cells (Fig. 2-4B). Finally, we evaluated the consequences of FGFR2 IIIb C2 transformation on altered cell cycle regulation. Both the Raf-MEK-ERK and PI3K-AKT pathways have been shown to contribute to Ras-mediated upregulation of cyclin D1, a key positive regulator of cell cycle progression through G1 (103). Therefore, we examined whether FGFR2 IIIb C2 increased the steady-state levels of cyclin D1 protein. Consistent with the ERK and AKT activity levels, we found that cyclin D1 protein levels are upregulated in NIH(FGFR2) but not RIE(FGFR2) cells (Fig. 2-4B). These 53
65 Figure 2-4. FGFR2 IIIb C 2 activates Ras and Ras-mediated signaling in NIH 3T3 but not RIE-1 cells. A. NIH 3T3 and RIE-1 cells stably expressing the indicated proteins were assayed for the amount of activated GTP-bound Ras by GST-Raf-RBD pull down analyses. GTP-bound and total Ras expression was determined by immunoblotting with antipan-ras antibodies. B. Ras signaling activation. Cells were lysed and assayed for ERK and AKT activation by immunoblotting with antibodies that recognize phosphorylated ERK and AKT (designated P-ERK and P-AKT). Same blots were reprobed with anti-erk or anti-akt antibodies to determine total ERK and AKT protein expression. Cyclin D1 protein expression was determined by immunoblotting with cyclin D1 antibody. Data shown are representative of three independent experiments. 54
66 results suggest that FGFR2 IIIb C2 causes sustained activation of Ras and Rasmediated effector signaling in NIH 3T3 but not RIE-1 cells. E. MEK and PI3K Activities Are Required for FGFR2 IIIb C2-induced Anchorage-Independent Growth of RIE-1 Cells. Although we found that FGFR2 IIIb C2 expression did not cause persistent activation of Ras-mediated signaling pathways in RIE-1 cells, it is possible that the basal activity of these signaling pathways might be necessary for FGFR2 IIIb C2 - mediated growth transformation of RIE-1 cells. We found that treatment with either the U0126 MEK inhibitor or the LY PI3K inhibitor blocked the anchorageindependent growth of RIE(FGFR2) cells (Fig. 2-5A and B), indicating that both basal MEK and PI3K activities are required for FGFR2 IIIb C2-mediated transformation of RIE-1 cells. F. KGF Stimulates Morphologic Transformation and Increases Cyclin D1 Protein Levels in FGFR2 IIIb C2-transformed RIE-1 Cells. Since fibroblasts, but not epithelial cells, express the ligand (KGF/FGF7) for FGFR2 IIIb, it is expected that FGFR2 IIIb C2 will be stimulated by an autocrine mechanism in fibroblast but not epithelial cells. This may account for the absence of Ras activation that we observed in RIE(FGFR2) cells (Fig. 2-4). Thus, we postulated that exogenous ligand stimulation of RIE(FGFR2) cells should mimic the biological and signaling consequences of FGFR2 IIIb C2 seen in NIH(FGFR2) cells. We found that sustained KGF stimulation (for two days) caused morphologic transformation of RIE(FGFR2) but not control RIE(vector) cells (Fig. 2-6A), indicating that the 55
67 morphologic transformation seen with NIH(FGFR2) cells (Fig. 2-3B) is dependent on ligand stimulation of FGFR2 IIIb C2. Figure 2-5. MEK and PI3K activities are required for FGFR2 IIIb C2-induced anchorage-independent growth of RIE-1 cells. A. RIE-1 cells stably expressing empty vector or FGFR2 IIIb C2 were suspended in 0.4% soft agar. The cells were incubated for three weeks in the absence or presence of 10 μm LY (PI3K inhibitor) or 30 µm U0126 (MEK inhibitor). Cells were photographed about three weeks after plating. B. Colonies per dish were counted two weeks after plating. Data shown are the average of duplicate dishes, with the bars indicating standard deviation, and are representative of two independent experiments. 56
68 The transformed morphology of KGF-stimulated RIE(FGFR2) cells (Fig. 2-6A) was similar to that seen with RIE(Ras) cells (Fig. 2-3B), with cells exhibiting a refractile, elongated and fibroblastic morphology. This suggested that KGF stimulation may cause sustained Ras activation in RIE(FGFR2) cells. First, we assessed whether sustained KGF stimulation led to constitutive activation of ERK and AKT in RIE(FGFR2) cells. Surprisingly, KGF stimulation did not increase the steady-state level of ERK phosphorylation levels in RIE(FGFR2) cells (Fig. 2-6B). As expected, since activated H-Ras(61L) did not increase AKT phosphorylation in RIE-1 cells, while H-Ras(61L) did increase AKT phosphorylation in NIH 3T3 fibroblasts (Fig. 2-4B), no increase in AKT activity was seen in RIE(FGFR2) cells. However, we did find that sustained KGF increased cyclin D1 protein expression levels in RIE(FGFR2) cells. Cyclin D1 forms a complex with cyclin-dependent kinases CDK4/6 and phosphorylates and inactivates the retinoblastoma tumor suppressor protein (Rb). Thus, we further assessed if the increased cyclin D1 protein levels in RIE(FGFR2) cells stimulated with KGF corresponded to hyperphosphorylation and inactivation of Rb. Immunoblot analyses with phospho-specific Rb antibody that recognizes the phosphorylated and inactive form of Rb revealed that KGF stimulation caused Rb hyperphosphorylation in RIE(FGFR2) cells (Fig. 2-6B). 57
69 A B Vehicle KGF Vector FGFR2 Ras Vector FGFR2 Ras Vector P-Erk Total Erk P-Akt FGFR2 Total Akt Cyclin D1 P-Rb Vehicle KGF Figure 2-6. KGF stimulation induces morphologic transformation and increases cyclin D1 protein levels in FGFR2 IIIb C2-transformed, but not control, RIE-1 cells. A. RIE-1 cells stably infected with either the empty vector or encoding FGFR2 IIIb C2 were maintained in growth medium supplemented either 0.1% bovine serum albumin (Vehicle) or 50 ng/ml KGF and changes in cell morphology were evaluated after two days. B. RIE-1 cells stably expressing the indicated proteins were treated with either vehicle or 50 ng/ml KGF. After 48 h, cell lysates were prepared and analyzed by immunoblot analyses for activated ERK and AKT. Cyclin D1 protein expression was determined by immunoblotting with cyclin D1 antibody and Rb inactivation was determined by immunoblotting with a phospho specific Rb (Ser 780) antibody. 58
70 G. FGFR2 IIIb C2-transformed RIE-1 Cells Show Enhanced ERK Activity, Increased Cyclin D1 Protein Levels, and Upregulation Of EGFR Autocrine Loop in Confluent Cultures, But Not in Sub-Confluent Cultures. We observed that FGFR2 IIIb C2 but not Ras caused distinct growth promoting activities for fibroblasts and epithelial cells at different cell densities (Fig. 2-3C). Thus, we compared the signaling activity of RIE(FGFR2) and control RIE(vector) cells at subconfluent (~70% confluency) and post-confluent densities. For these analyses, the cultures were evaluated either three days after subculturing (for sub-confluent cultures) or after 12 days (for confluent cultures) and harvested for immunoblot analyses. We observed sustained ERK, but not AKT, activation and increased cyclin D1 protein levels in confluent but not in sub-confluent RIE(FGFR2) cells (Fig. 2-7A). Thus, sustained and elevated ERK activation may be required to facilitate the density-independent growth of RIE(FGFR2) cells. One of the important traits that distinguish cancer cells from normal cells is the acquisition of self-sufficiency in growth signals by creating an autocrine signaling loop (104). Therefore, we next examined the possibility that autocrine growth factors might be induced in confluent but not in sub-confluent RIE(FGFR2) cells. For these analyses, either RIE(vector) cells or RIE(FGFR2) cells maintained in culture until they reached 70% confluence (three days post plating) or for 12 days when cells had maintained at confluent density for eight days. The conditioned media were then harvested from these cultures and added to parental RIE-1 cells. While conditioned medium from confluent RIE(FGFR2) cultures (designated D12 FGFR2-CM) caused morphologic transformation and cell scattering when added to parental RIE-1 cells, 59
71 conditioned medium from sub-confluent RIE(FGFR2) cultures (designated D3 FGFR2-CM) showed no activity (Fig. 2-7B). In contrast, conditioned medium from either confluent or sub-confluent RIE(vector) cells did not induce transformation of parental RIE-1 cells. Previously, our lab and others observed Ras caused upregulated expression and secretion of the transforming growth factor alpha (TGFα) epidermal growth factor receptor (EGFR) ligand that activated an EGFR-dependent autocrine growth and morphologic transformation in RIE-1 cells (72, 105) Thus, we examined the possibility that an EGFR autocrine signaling loop might be upregulated in confluent RIE(FGFR2) cells. We observed that treatment with the gefitinib EGFR-specific kinase inhibitor as well as with U0126 prevented the morphologic transformation of parental RIE-1 cells caused by conditioned medium from confluent RIE(FGFR2) cells (Fig. 2-7C). Thus, FGFR2 IIIb caused the upregulation of an EGFR-dependent autocrine signaling loop in confluent, but not subconfluent, RIE-1 cells. 60
72 A Sub-confluent Post-confluent Vector FGFR2 Ras Vector FGFR2 Ras B D3 Vector-CM D12 Vector-CM P-ERK ERK P-AKT Total ERK Cyclin D1 D3 FGFR2-CM D12 FGFR2-CM C DMSO U0126 Gefitinib D12 FGFR2-CM Figure 2-7. FGFR2 IIIb C2-transformed RIE-1 cells show enhanced ERK activity, increased cyclin D1 protein levels, and upregulation of an EGFR-dependent autocrine loop in confluent, but not sub-confluent, cultures. A. RIE-1 cells stably expressing the indicated proteins were plated and cultured in growth media for either 3 or 12 days. Sub-confluent (cultured for 3 days) or confluent (cultured for 12 days) RIE-1 cultures were harvested and analyzed for activated ERK and AKT, and cyclin D1 protein levels by immunoblot analyses as described above B. RIE-1 cells stably infected with the empty vector or encoding FGFR2 IIIb were plated in growth medium. After 3 (D3) or 12 (D12) days after plating, conditioned medium from either vector control (Vector CM) or FGFR2 IIIb C2-transformed RIE-1 cells (FGFR2-CM) were collected and added to parental RIE-1 cells. Cells were photographed after 36 h. C. The gefitinib EGFR-specific kinase inhibitor and U0126 prevented the morphologic transformation of parental RIE-1 cells caused by D12 FGFR2-CM. 61
73 V. Discussion We identified FGFR2 IIIb C2 as a transforming gene expressed in human breast carcinoma cells. Although FGFR2 IIIb is expressed exclusively in normal epithelial cells, the majority of studies evaluating FGFR2 IIIb transformation have been done in fibroblasts (38, 39, 86). Since fibroblasts express FGFR2 IIIb ligands, the analyses of FGFR2 IIIb transformation in fibroblasts are complicated by an autocrine mechanism. Since cell type-specific differences exist in mechanisms of cellular transformation and oncogenesis, we have compared FGFR2 IIIb C2 transforming and signaling activities in NIH 3T3 fibroblasts and RIE-1 epithelial cells. Our observations suggest that FGFR2 IIIb-mediated transformation of epithelial cells may involve ligand-independent signaling that is independent of Ras activation, but additionally does require upregulation of an EGFR-dependent autocrine growth loop. Previous studies found low expression of the C1 isoform of FGFR2 IIIb in normal mammary epithelial cells, with greatly elevated C1, as well as C2 and C3, expression in SUM-52 human breast cancer cells (68). We extended these observations and found enhanced FGFR2 IIIb C1, C2 and C3 expression in 7 of 9 breast carcinoma cell lines evaluated, when compared to the levels seen in immortalized MCF-10A human breast epithelial cell line. Interestingly, the two cell lines that lacked FGFR2 IIIb expression harbor mutationally-activated Ras. FGFR2 IIIb C3 exhibited higher transforming activity when expressed in NIH 3T3 fibroblasts (39) or in H16N2 immortalized human mammary epithelial cells (40). We have determined that FGFR2 IIIb C2 also displays greater transforming potency than FGFR2 IIIb C1 (data not shown). Hence, increased expression of C2 and C3 may 62
74 contribute to the aberrant growth of breast carcinoma cells. Furthermore, we identified expression of the mesenchymal-specific FGFR2 IIIc isoform in invasive breast carcinomas. A similar switch from FGFR2 IIIb to IIIc has been observed in a rat prostate tumor progression model, where IIIc expression promoted progression to malignant tumors which were independent of the stroma (83). Our cell line observations are consistent with a previous study that found a shift to IIIc expression in more advanced tumor stages (93). We also found that expression of FGFR2 IIIc correlated with an epithelial-mesenchymal transition (EMT), as indicated by the loss of epithelial (E-cadherin) and gain of mesenchymal (N-cadherin and vimentin) protein expression. Since FGFR expression has been shown to promote EMT (75), perhaps the enhanced expression of FGFR2 IIIc in invasive breast carcinomas may promote EMT and increased tumor cell invasion. Expression of FGFR2 IIIc may also render breast cancer cells independent of stromal cell-derived ligands. Although FGFR2 IIIb C2 expression induced focus formation and soft agar growth of both NIH 3T3 fibroblasts and RIE-1 epithelial cells, only NIH 3T3 fibroblasts were morphologically transformed by FGFR2 IIIb C2. However, when we treated RIE(FGFR2) cells with exogenous KGF, RIE(FGFR2) cells became morphologically transformed. These data indicate that FGFR2 IIIb-induced morphologic transformation is dependent on stromal cell-derived KGF. In addition, we found that FGFR2 IIIb C2 caused upregulation of cyclin D1 protein levels in NIH 3T3 fibroblasts but not RIE-1 cells. However, KGF stimulation did increase cyclin D1 protein levels, leading to inactivation of the Rb tumor suppressor. These observations indicate that FGFR2 IIIb can induce growth transformation of epithelial 63
75 cells in a ligand-independent manner, but that paracrine stimulation by stromal cellderived KGF will also contribute to FGFR2 IIIb C2-mediated transformation of epithelial cells. It has become clear that fibroblasts can regulate adjacent epithelia by secretion of growth factors and direct epithelial-mesenchymal interactions. In particular, several lines of evidences indicate that fibroblasts within the tumor stroma, so called carcinoma-associated fibroblasts (CAFs), possess distinct biological properties from normal fibroblasts and play a role as a key modifier of cancer initiation and progression ( ). Several oncogenic signals, including TGF-β, hepatocyte growth factor (HGF) and stromal cell-derived factor 1 (SDF-1) were shown to be upregulated in CAFs compared with normal fibroblasts (110). It is possible that KGF might be also upregulated in CAFs and aberrantly activate FGFR2 IIIb in adjacent epithelial cells and contribute to cancer development. Since KGF-stimulated FGFR2 IIIb C2-transformed cells showed characteristics that resembled Ras-transformed RIE-1 cells, the enhanced transformation caused by KGF is likely to involve activation of Ras. However, unlike mutant Ras-transformed RIE-1 cells, KGF stimulation did not cause activation of ERK in FGFR2 IIIb C2-transformed RIE-1 cells. This difference may reflect a qualitative or quantitative difference in effector utilization by mutationally activated Ras when compared to upstream activation of endogenous wild type Ras. The signaling mechanisms for ligand-independent growth transformation are not clear, but the absence of Ras activation in FGFR2 IIIb C2-transformed RIE-1 cells suggests that it does not involve Ras-mediated signaling. Other FGFR2 IIIb C2 64
76 signaling activities that may facilitate growth transformation include activation of PLC gamma and stimulation of second messenger production. We observed that FGFR2 IIIb C2 caused distinct growth-promoting properties in RIE-1 cells dependent on cell density (Fig. 2-3C). At subconfluent cell densities, we did not observe activation of ERK and upregulation of cyclin D1 in RIE(FGFR2) cells. In contrast, at confluent cell densities, FGFR2 IIIb C2 increased ERK activity and cyclin D1 protein levels in RIE-1 cells. We determined that confluent RIE(FGFR2) cells secreted factors that caused EGFR-dependent morphologic transformation of RIE-1 cells. We showed previously that activated Ras caused upregulation of EGF family ligands that were necessary for growth and morphologic transformation of RIE-1 cells (72, 105). Conventionally, signaling activities are analyzed when cells are subconfluent and exponentially growing. However, our data indicate that cell density can be an important factor for determining signaling activity. Thus, our observations emphasize the importance of evaluating signaling activity in a same context where biological activity is obtained. Differences in oncogenemediated signaling when evaluated in exponentially growing cells in cell culture versus a tumor mass where cells are at confluent cell densities, and in contact with stromal tissue, may account for why the potent inhibitory activities seen with signal transduction inhibitors have not reliably mirrored activities against a tumor mass. In summary, our studies provide further validation of a positive role for the tumor-associated expression of the IIIb C2 splice variant of FGFR 2 in breast cancer growth. Additionally, our studies support both ligand-independent and stromal cell derived ligand-dependent mechanisms by which FGFR2 IIIb may promote 65
77 oncogenesis in epithelial cells. Our future studies will focus on elucidation of the ligand-independent, Ras-independent mechanisms of FGFR2 IIIb transformation. 66
78 Chapter 3: Aberrant Receptor Internalization and Enhanced FRS2-Dependent Signaling Contributes to the Ligand-Independent Transforming Activity of the Fibroblast Growth Factor Receptor 2 IIIb C3 isoform I. Abstract Alternative gene splicing generates variants of fibroblast growth factor receptor 2 (FGFR2) IIIb that differ in the length of carboxyl-terminal sequences (designated C1, C2 and C3) and preferential expression of C2 and C3 isoforms is associated with oncogenesis. C2 and C3 lack the 34 or 53 carboxyl-terminal residues of C1, respectively, and we determined that progressive loss of carboxylterminus sequences enhanced the transforming potency of FGFR2 IIIb. The highly transforming C3 variant lacks five tyrosine residues present in C1 and we found that the loss of Y770 alone enhanced FGFR2 IIIb C1 transforming activity. Furthermore, concurrent mutation of Y770 and L773 in the 770 YXXL motif cooperated to disrupt FGFR2 IIIb C1 internalization and enhance transforming activity, similar to that seen with the C3 isoform. We also determined that the Y770F mutation decreased PLCγ activity, but enhanced ligand-independent fibroblast growth factor receptor substrate 2 (FRS2) activation, which was required for transformation. Our data support a dual mechanism where loss of Y770 impairs receptor internalization and promotes ligandindependent activation of FRS2, contributing to the enhanced transforming activity of FGFR2 IIIb C3. 67
79 II. Introduction Fibroblast growth factors (FGFs) comprise a large family of structurally related growth factors (22 human members) that mediate a variety of cellular responses that include cell proliferation, differentiation, migration, and angiogenesis (1-3, 84). The activities of FGFs are mediated by their binding to a family of four receptor tyrosine kinases (RTKs), designated FGFR1-4. FGFRs are comprised of an extracellular domain that consists of two or three immunoglobulin (Ig)-like domains, a single transmembrane domain and an intracellular catalytic tyrosine kinase domain and flanking regulatory sequences (Fig. 1A). An important feature and mode of regulation of FGFR2 function is that structural variants of FGFR2 are generated by numerous alternative gene splicing events that generate transcripts that encode proteins altered in both the extracellular and intracellular regions of the FGFR2. To date, more than 20 alternative splicing variants of FGFR2 have been identified. The first major splicing event occurs in the second half of the third Ig-like domain (designated Ig-III domain). Tissue-specific inclusion of either exon IIIb or exon IIIc that encode for the second half of the Ig-III domain generates either the epithelial cell-specific IIIb or mesenchymal cell-specific IIIc isoforms. This alternative splicing determines the ligand binding specificity of FGFR2. While FGFR2 IIIb (also called keratinocyte growth factor receptor; KGFR) binds FGF7 (also called KGF) and FGF10, but not FGF2, FGFR2 IIIc (also called BEK) binds FGF2, but not FGF7 and FGF10. The second major splicing occurs in sequences that encode the carboxylterminus of FGFR2. To date, at least three splice variants of FGFR2 IIIb that differ in 68
80 their carboxyl-terminal sequences have been identified (designated C1, C2 and C3) (39). The C2-type carboxyl-terminus is 34 amino-acid shorter than the C1-type carboxyl-terminus and C3-type carboxyl-terminus is 19 amino-acid shorter than C2- type carboxyl-terminus. (Fig. 1A). These sequence differences result in differential retention of tyrosine residues that may serve as sites of receptor autophosphorylation and docking sites for cytoplasmic signaling proteins. Previous study found that expression of the C3 isoform was increased in gastric cancer cell lines (39). We also observed enhanced expression of C2 and C3 isoforms in a majority of human breast carcinoma cell lines when compared to nontransformed MCF-10A human mammary epithelial cells (J.Y. Cha, G.W. Reuther, Q.T. Lambert and C.J. Der, submitted for publication), suggesting that aberrant expression of the C2 or C3 splicing variants may contribute to cancer development. Furthermore, the C3 variant that lacks carboxyl-terminal sequences was shown to be more transforming than the C1 variant when expressed ectopically in NIH 3T3 fibroblasts and human mammary epithelial cells (39, 40). However, whether C2 variant is more (or less) transforming than the C1 (or C3) variant has not been determined. Furthermore, the mechanism(s) for the enhanced transforming activity of the C3 variant that lacks carboxyl-terminal sequences remains to be elucidated. Like other RTKs, FGFRs are activated by ligand-induced dimerization, causing stimulation of their intrinsic tyrosine kinase activity, tyrosine autophophorylation, and recruitment of signaling proteins to specific phosphorylated tyrosine residues in their cytoplasmic carboxyl-termini. The two best characterized downstream signaling components of FGFRs are phospholipase C-γ (PLCγ) and 69
81 FGF receptor substrate 2 (FRS2). In FGFR1, Y766 in the carboxyl-terminus is the major autophosphorylation site on FGFR1 and serves as a binding site for the Src homology 2 (SH2) domain of PLCγ, resulting in tyrosine phosphorylation of PLCγ (45, 111). Phosphorylation and activation of PLCγ leads to stimulation of phosphatidylinositol (PI) hydrolysis and the generation of the two second messengers, diacylglycerol and Ins(1,4,5)P3. These two second messengers cause the activation of protein kinase C and the Ca 2+ release from intracellular stores, respectively (46). The Y766 residue in FGFR1 is well conserved in all four FGFR family members and corresponds to Y770 in FGFR2 IIIb (Fig. 1B). Recently, it was shown that a Y770F missense mutant of FGFR2 IIIb C1 cannot bind and phosphorylate PLCγ (47). However, whether this mutant fails to activate PI hydrolysis, and whether altered PLCγ binding and activation contributes to FGFR transforming activity, remain unresolved. Unlike PLCγ, FRS2 association with FGFR is constitutive and independent of ligand stimulation and receptor phosphorylation (112). FRS2 is a docking protein that binds to a conserved sequence within the juxtamembrane domain of FGFRs. Ligand-stimulated activation of FGFRs leads to phosphorylation of multiple tyrosine residues in the carboxyl-terminus of FRS2. One well-characterized FRS2 effector is the Grb2 adaptor protein which binds to tyrosine phosphorylated FRS2 via its SH2 domain and forms a stable complex with the Sos Ras guanine nucleotide exchange factor. This complex formation allows Sos to translocate to the plasma membrane where it can stimulate the exchange of GTP for GDP on the Ras small GTPase. 70
82 Activated Ras then activates the Raf-MEK-ERK mitogen-activated protein kinase (MAPK) cascade (1, 6). A balance between positive and negative signaling is critical for maintaining normal cell physiology. One mechanism of negative regulation of RTK signaling involves ligand-mediated receptor internalization and lysosomal degradation (13, 14). Therefore, disruption of receptor internalization could lead to aberrant receptor activation and contribute to the development of human disease including cancer. Interestingly, FGFR2 IIIb C1 contains two putative YXXΦ tyrosine-based sorting motifs (where X is any amino acid and Φ is a bulky hydrophobic amino acid) in carboxyl-terminal sequences (113). The C2-type carboxyl terminus lacks one YXXΦ motif ( 813 YPHI), while the C3-type carboxyl terminus lacks two YXXΦ motifs ( 770 YLDL and 813 YPHI) (Fig. 1A), suggesting that the loss of the YXXΦ motif(s) might impair receptor internalization and contribute to the enhanced transforming activity of the FGFR2 IIIb C3 variant. To date, a detailed comparative analyses of the transforming potential of FGFR2 IIIb C1, C2 and C3 using the same cell system has not been done. In the present study, we compared the transforming potency of FGFR2 IIIb C1, C2 and C3 splicing variants and found that the C3 variant is considerably more transforming than the C2 variant, and the C2 variant is modestly enhanced in transforming activity when compared to the weakly transforming C1 variant. We determined that one mechanism for the enhanced transforming activity of FGFR2 IIIb C3 involves loss of the 770 YLDL sorting motif and impaired internalization. In addition, we determined that mutation of Y770 led to a loss of PLCγ activation, but a gain of ligand- 71
83 independent FRS2 activation. Finally, we found that FRS2 activity was required for the enhanced transforming activity of FGFR2 IIIb C1 mutant receptors that lack Y770. Taken together, our data support a model where the potent transforming activity of the FGFR2 IIIb C3 splice variant is mediated, in part, by a mechanism involving loss of the 770 YLDL motif, resulting in impaired receptor internalization and enhanced FRS2 signaling. III. Materials and methods Plasmid expression vectors Human FGFR2 IIIb C1, C2 and C3 cdna sequences were generated by PCR amplification from a T-47D breast cancer cell cdna library and subsequently cloned into the pbabe-puro retrovirus mammalian expression vector (5'-SalI and 3'-BamHI sites). The FGFR2 IIIb C2 (QST) cdna sequence (deletion of FGFR2 IIIb C1 residues ) was created by PCR amplification from FGFR2 IIIb C1 cdna sequence and cloned into pbabe-puro (5'-SalI and 3'-BamHI sites). Additional cdna sequences encoding misssense mutations were created by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene) and verified by DNA sequencing. Oligonucleotides designed to create the appropriate mutations to encode the indicated amino acid substitutions are as follows: 5'- CACAACCAATGAGGAATTCTTGGACCTCAGCCAACC-3' (Y770F); 5'- GCCAACCTCTCGAACAGTTTTCACCTAGTTACCCTG-3' (Y780F); 5'- CAGTATTCACCTAGTTTCCCTGACACAAGAAG-3' (Y784F); 5'-CCA GACCCCATGCCTTTCGAACCATGCCTTCCT-3' (Y806F); 5'- CATGCCTTCCTCAGTTTCCACACATAAACGGC-3' (Y813F); 5'- 72
84 GAGGAATTCTTGGACGCCAGCCAACCTCTCGAACAG-3' (Y770F/L773A); 5'- CCAATGAGGAAGCCTTGGACGCCAGCCAACCTCTCG-3' (Y770A/L773A); 5'- GAGGAATACTTGGACGCCAGCCAACCTCTCG-3' (L773A); 5'- GCACAAGCTGACCGCACGTATCGCCGCGCGGAGACAGG-3' (K421A/P424A/L425A). Cell culture and transformation assays Rat-1 and RIE-1 cells were maintained in Dulbecco s modified minimum essential medium (DMEM-H) supplemented with 10% fetal calf serum. For secondary focus formation assays, Rat-1 and RIE-1 cells were stably-infected with pbabe-puro constructs encoding wild type FGFR2 IIIb isoforms and missense mutants of FGFR2 IIIb C1. Expression of ectopically-introduced FGFR2 IIIb C1 proteins was determined by immunoblot analyses with anti-fgfr2 antibody (sc-122; Santa Cruz Biotechnology). After infection, cells were selected in growth medium supplemented with puromycin (2 µg/ml). Drug resistant colonies were pooled and replated into 60 mm dishes and maintained in growth medium for 2-3 weeks. Cells were then fixed and stained with crystal violet. To determine anchorage-independent growth potential, Rat-1 cells stably expressing either the pbabe-puro empty vector or encoding various FGFR2 IIIb proteins were suspended in 0.4% bacto-agar in growth medium at 5 x 10 4 cells per 60 mm dish. The single cell suspensions were layered on top of 0.6% bacto-agar in growth media. After 2-3 weeks, colonies were stained with 2 mg/ml MTT (tetrazolium salt 3,[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide) and the average number of colonies on duplicate dishes was calculated. 73
85 Internalization assays Rat-1 cells that stably express either wild type or mutant FGFR2 IIIb proteins were serum-starved for 20 h and then incubated with either vehicle (BSA) or 50 ng/ml KGF (R&D systems) for 40 min at 37ºC to allow internalization. Cells were then fixed (4% paraformaldehyde in PBS), washed, and permeabilized (0.1% Triton X-100). Next, cells were immunostained with rabbit polyclonal FGFR2 antibody (sc-122; Santa Cruz Biotechnology) to determine FGFR2 IIIb subcellular location by confocal microscope. To quantify receptor internalization, the number of positive internalization cells was counted under the confocal microscope. Positive internalization was scored only in cases where all surface staining was lost and almost all receptors were internalized to punctuate vesicular structure. The average number of positive internalization cells on three independent assays was calculated and 100 to 200 cells were examined for each independent assay. Signaling analyses To determine PLCγ activation, cells were lysed and then analyzed for tyrosinephosphorylated PLCγ by immunoprecipitation with anti-plcγ antibody (sc-7290, clone E-12; Santa Cruz Biotechnology) followed by immunoblotting with antiphosphotyrosine antibody (#05-321, clone 4G10; Upstate Biotechnology). Then, the same membrane was stripped and reprobed with PLCγ antibody for total PLCγ expression. PI hydrolysis assays were performed to measure PLCγ activity as we have described previously (114). Briefly, Rat-1 cells stably expressing either wild type FGFR2 IIIb C1 or 770F mutant were labeled with myo-[ 3 H] inositol in inositolfree medium. Then, cells were stimulated with either KGF, vehicle (BSA, negative 74
86 control), thrombin (positive control) or lysophosphatidic acid (LPA; positive control) for 20 min in medium supplemented with 10 mm LiCl. Accumulation of [ 3 H]inositol phosphates was quantitated as described previously (114). To determine FRS2 activation, cells were lysed and then analyzed for tyrosine-phosphorylated FRS2 by immunoprecipitation with anti-frs2 antibody (sc-8318; Santa Cruz Biotechnology) followed by immunoblotting with anti-phosphotyrosine antibody. To determine ERK activation, cells were lysed and then analyzed for active ERK by immunoblot analyses with antibody specifically detect phosporylated ERK (#9106; Cell Signaling Technology). The same membrane was stripped and reprobed with anti-erk antibody (#9102; Cell Signaling Technology) for total ERK expression. IV. Results A. Loss of carboxyl-terminal sequences enhances FGFR2 IIIb transforming potency. Three splice variants of FGFR2 IIIb that differ in their carboxyl-terminal sequences have been identified (C1, C2 and C3) (39). The C2-type carboxyl - terminus is 34 amino- acid shorter than the C1-type carboxyl-terminus, and C3-type carboxyl-terminus is 19 amino-acid shorter than C2-type carboxyl-terminus, resulting in the progressive loss of tyrosine residues (Fig. 3-1A). Previous reports demonstrated that FGFR2 IIIb C3 is more transforming than FGFR2 IIIb C1 in NIH 3T3 fibroblasts and human mammary epithelial cells (39, 40). However, whether the FGFR2 IIIb C2 variant is more (or less) transforming than the FGFR2 IIIb C3 (or C1) variant has not been determined. Since we recently identified the C2 variant in a biological screen for novel oncogenes expressed in breast cancer (J.Y. Cha, G.W. 75
87 Reuther, Q.T. Lambert and C.J. Der, submitted for publication), we initiated studies to compare the transforming potencies of C1, C2 and C3 in the same cell systems. To determine the role of carboxyl-terminal sequences in FGFR2 IIIb transformation, we compared the transforming potency of FGFR2 IIIb C1, C2 and C3 variants by examining two aspects of growth transformation, loss of densitydependent growth inhibition and acquisition of anchorage-independent growth potential. For these analyses, we established Rat-1 rat fibroblasts and RIE-1 rat intestinal epithelial cells stably expressing the FGFR2 IIIb C1, C2 or C3 variants and performed secondary focus formation and soft agar growth transformation assays. First, we compared the ability of the three FGFR2 IIIb variants to induce loss of density-dependent growth inhibition by quantitating the appearance of foci of multilayered cells in confluent cultures. Cells stably-transfected with the empty vector or expressing the C1 variant exhibited no focus-forming activity. In contrast, we found that cells expressing the C2 variant exhibited limited focus-forming activity, whereas the C3 variant caused significantly greater focus-forming activity than the C2 variant in RIE-1 (Fig. 3-1C) as well as Rat-1 (data not shown) cells. Second, we compared the ability of three FGFR2 IIIb variants to promote anchorageindependent growth by soft agar assay. Consistent with their relative focus-forming potencies, the C3 variant induced 3.7-fold more soft agar colonies than the C2 variant, and the C2 variant induced 2.1-fold great formation of soft agar colonies than C1 variant in Rat-1 cells (Fig. 3-1D). The same hierarchy of transformation potency was also seen in soft agar colony formation analyses of RIE-1 and MCF- 10A human breast epithelial cells (data not shown). These data indicate that multiple 76
88 A IgI IgII IgIII TM TK B Y766 for FGFR1 C1 s-s s-s s-s TK YYYYY 822 Y770 for FGFR2 FGFR1 NQEYLDLSMPLDQYSPSFPDTRSSTCSSGEDSVFSHEPLPEEPCLPRHPAQLANGGLKRR C2 s-s s-s s-s TK 788 YYY FGFR2 FGFR3 FGFR4 NEEYLDLSQPLEQYSPSYPDTRSS-CSSGDDSVFSPDPMPYEPCLPQYP--HINGSVKT- TDEYLDLSAPFEQYSPGGQDTPSS-SSSGDDSVFAHDLLP-----PAPP---SSGGSRT- SEEYLDLRLTFGPYSPSGGDASST-CSSS-DSVFSHDPLPLGS--SSFP---FGSGVQT- C3 s-s s-s s-s TK C1 EYLDLSQPLEQYSPSYPDTRSSCSSGDDSVFSPDPMPYEPCLPQYPHINGSVKT C2 EYLDLSQPLEPYSPCYPDPR C3 I C D Vector FGFR2 C2 FGFR2 C1 FGFR2 C3 Colonies Per DIsh Vector C1 C2 C3 FGFR2 Figure 3-1. Loss of carboxyl-terminal sequences enhances FGFR2 IIIb transforming potency. A. Carboxyl-terminal sequence comparison of three FGFR2 IIIb splice variants (C1, C2, C3). Known or putative phosphorylated tyrosine residues are indicated. B. Conservation of carboxyl-terminal tyrosines in FGFR family members. Of the five tyrosine residues present in the C1 but not C3 variant of FGFR2, only Y770 (Y766 in FGFR1) and Y780 (Y776 in FGFR1) are conserved in all four receptors. Receptor autophosphorylation of Y770 of FGFR2 IIIb (analogous to Y766 of FGFR1 and Y760 of FGFR3) creates a recognition site for the SH2 domain of PLCγ. C. Loss of carboxyl-terminal sequences enhances FGFR2-induced focus formation in RIE-1 cells. Mass populations of RIE-1 cells that stably express the indicated FGFR2 IIIb carboxyl-terminal splice variants were assayed for focus-forming activity using a secondary focus formation assay. Cells were plated and allowed to grow for 21 days, and then the appearance of foci of transformed cells was monitored. The cultures were fixed and stained with crystal violet and focus-forming activity was quantitated. D. Loss of carboxyl-terminal sequences enhances FGFR2-induced soft agar growth of Rat-1 cells. Mass populations of Rat-1 cells that stably-expressed the indicated FGFR2 variants were assayed for their ability to grow in soft agar. The number of colonies was quantitated after 21 days. 77
89 carboxyl-terminal sequences function as negative regulators of FGFR2 IIIb transforming activity, with the loss of residues within the region spanning 769 to 788 causing the most significant activation of transforming activity. B. Mutation of Y770F alone activates FGFR2 IIIb C1 transforming activity Next, we determined the mechanism(s) for the enhanced transforming activity of FGFR2 IIIb variants lacking carboxyl-terminal sequences. The carboxyl-terminal domain tyrosine residues of RTKs are sites of autophosphorylation and critical for their cytoplasmic signaling and growth regulatory activities. The C3 variant lacks five tyrosine residues (Y770, Y780, Y784, Y806, Y813), while the C2 variant lacks two tyrosine residues (Y806, Y813) present in the C1 isoform (Fig. 3-1A). We speculated that the loss of specific tyrosine residue(s) might account for the enhanced transforming activity of the C2 and (or) C3 variants. To address this possibility, we introduced phenylalanine substitutions at each of the five tyrosine residues (Y770F, Y780F, Y784F, Y806F, Y813F) of the carboxyl-terminus of the weakly transforming C1 variant. Since loss of multiple tyrosine residues might be required for enhanced transforming activity, we also generated double (F2; Y813/806F), triple (F3; Y813/806/784F), quadruple (F4; Y813/806/784/770F) and quintuple (F5; Y813/806/784/780/Y770F) mutants. We then established mass populations of Rat-1 cells that stably-expressed wild type and mutant FGFR2 IIIb C1 proteins and compared their transforming potency by evaluating anchorageindependent growth potential in soft agar assays (Figs. 3-2A and 3-2B). We found that the Y770F mutation alone promoted colony formation in soft agar (~600 78
90 colonies per dish), while receptors with the Y780F, Y784F, Y806F or Y813F mutations showed the same weak colony forming activity (~200 colonies per dish) as cells expressing the wild type (WT) FGFR2 IIIb C1 receptor (Figs. 3-2A and 3-2B). In addition, we found that the F2 double or F3 triple mutant receptors showed the same transforming potency as WT FGFR2 IIIb C1 (Fig. 3-2B), while the F4 quadruple and F5 quintuple mutants that included the Y770F mutation promoted colony formation in soft agar to the same extent as the Y770F single mutant (Fig. 3-2B). To exclude the possibility that the enhanced transforming potency caused by the Y770F mutation might be due to greater expression, we analyzed the steady-state level of protein expression of all FGFR2 IIIb C1 proteins tested above by immunoblot analyses with an anti-fgfr2 antibody generated against a carboxyl-terminal sequence found only in the C1 isoform, and therefore, cannot recognize expression of the C2 and C3 variants. We found that all FGFR2 IIIb mutants were expressed at a similar level as WT FGFR2 IIIb C1 (Fig. 3-2C). Together, these results indicate that loss of the Y770 residue, but not other tyrosine residues (Y780, Y784, Y806 and Y813), contributes to the increased transforming potency of the C3 variant that lacks Y770 residue. However, since the C2 variant does retain the Y770 residue (Fig. 3-1A), the loss of the Y770 residue is not the basis for the increased transforming activity of the C2 variant. It is notable that the C2 variant lacks 34 carboxyl-terminal residues found in the C1 variant, but additionally, is also divergent from the C1 variant for three amino acids at positions 779, 783 and 787 (Fig. 3-1A). While the C1 variant contains 779Q/783S/787T, the C2 variant contains 779P/783C/787P. 79
91 Figure 3-2. Mutation of Y770F alone activates FGFR2 IIIb C1 transforming activity. A. Rat-1 cells that stably expressed the indicated FGFR2 IIIb proteins were assayed for their ability to grow in soft agar. Cells were suspended in 0.4% soft agar and photographed 21 days after plating. B. The number of colonies was quantitated after 21 days. Data shown are the average of duplicate dishes, with the bars indicating standard deviation, and are representative of two independent experiments. C. Rat-1 cells that stably express the indicated FGFR2 IIIb proteins were assayed for their FGFR2 IIIb protein expression levels by immunoblot analyses with FGFR2 antibody against a peptide sequence in the FGFR2 IIIb C1 carboxyl-terminal sequence. This sequence has been deleted in the FGFR2 IIIb C2 and FGFR2 IIIb C3 variants; therefore, we cannot determine the level of expression of these two isoforms with this antibody. Blot analysis with anti-beta actin was done to verify equivalent total protein loading. 80
92 Therefore, we speculated that differences in these three amino acids might contribute to the increased transforming potency of the C2 variant. To address this possibility, we generated a truncation mutant of C1 variant that has same length as C2 variant but contains the 779Q/783S/787T sequence (designated C2 (QST)) rather than the 779P/783C/787P sequence. We found that this C2 (QST) mutant exhibited a similar transforming potency as the C2 variant (Fig. 3-2B), indicating that the loss of the carboxyl-terminal 34 amino acids (from 789 residue to 822 residue), and not differences in 779, 783 and 787 residues is responsible for increased transforming potency of C2 variant. C. The 770YXXL motif is required for ligand-stimulated FGFR2 IIIb C1 internalization. Next, we sought to determine how mutation of the Y770 residue enhanced FGFR2 IIIb C3 transforming activity. Interestingly, the Y770 residue and flanking sequences ( 770 YLDL) correspond to the YXXΦ motif (Φ = bulky hydrophobic residue) that is known to be the major determinate for endocytosis of many transmembrane proteins (113). Consistent with this possibility, mutation of the analogous tyrosine residue in FGFR1 (Y766F) resulted in decreased receptor internalization, and decreased ligand-induced receptor downregulation and degradation (115). However, another study found that a Y770F mutation did not cause impaired FGFR2 IIIb internalization (47). Thus, a clear role for the 770 YXXL motif in FGFR2 IIIb receptor internalization is presently unresolved. One possible resolution to the apparently opposing conclusions with FGFR1 and FGFR2 may be that, since there is evidence that the tyrosine residue of the YXXΦ sorting motif can be substituted with aromatic 81
93 amino acids such as phenylalanine or tryptophan ( ), we postulated that if Y770 is substituted with an alanine instead of phenylalanine, the YXXΦ sorting signal might be disrupted. Alternatively, it is also possible that both the Y770 and L773 residues of 770 YXXL motif might be required for FGFR2 internalization, and that concurrent mutation of both residues will be required to disrupt its function. To determine whether the Y770 and/or L773 residues are required for FGFR2 IIIb C1 internalization, we generated mutant cdna sequences encoding four FGFR2 IIIb C1 missense mutants (Y770F, Y770F/L773A, Y770A/L773A and L773A) and compared the ability of WT and mutant receptors to undergo ligand-stimulated internalization. For these analyses, Rat-1 cells that stably-expressed WT or mutant FGFR2 IIIb C1 proteins were stimulated with either vehicle (BSA) or 50 ng/ml KGF and immunostained with anti-fgfr2 antibody to determine the subcellular localization of receptors. In unstimulated cells, the WT and all four mutant receptors were found on the cell surface as well as in the intracellular compartment (Fig. 3-3B). After 40 min of KGF stimulation, the majority of the WT receptor (~89% internalized) was internalized to punctuate vesicular structure (Figs. 3-3A and 3-3B). We found that the Y770F mutation slightly impaired the ability of FGFR2 IIIb C1 to internalize (~71% internalized), while the L773A substitution caused a more strongly impaired ability of FGFR2 IIIb C1 to internalize (~42% internalized) (Figs. 3-3A and 3-3B). These data indicate that although both Y770 and L773 residues contribute to FGFR2 IIIb C1 internalization, the L773 residue is more critical than the Y770 residue for FGFR2 IIIb C1 internalization. We also found that the Y770F/L773A (~23% internalized) double mutant more strongly impaired the ability of FGFR2 IIIb C1 to 82
94 internalize when compared to the Y770F or L773A single mutations (Figs. 3-3A and 3-3B), indicating that both Y770 and L773 are required for efficient ligand-stimulated FGFR2 IIIb C1 internalization. In addition, the Y770F/L773A (~23% internalized) and Y770A/L773A (~26% internalized) double mutants showed similar abilities to internalize, indicating that Y770 cannot be replaced with an aromatic acid such as phenylalanine (Fig. 3-3). D. Loss of Y and L of 770YXXL motif cooperates to enhance transformation. We next determined whether disruption of 770 YXXL motif to cause impaired internalization correlated directly with an enhanced transforming activity of FGFR2 IIIb C1. For these analyses, we established Rat-1 cells that stably-expressed WT and mutant FGFR2 IIIb C1 proteins (Y770F, Y770F/L773A, Y770A/L773A and L773A) and compared transforming potency by colony formation in soft agar assays. Although the L773A mutant (~42% internalized) showed greater impairment in internalization when compared to Y770F mutant (~71% internalized) (Fig. 3-3A), the L773A mutant (~4-fold more transforming than WT) showed slightly weaker transforming activity than Y770F mutant (~6-fold more transforming than WT) (Fig. 3-4A). Nevertheless, we did observe a general correlation with the degree of impaired internalization, with both the Y770F/L773A and Y770A/L773A double mutants (~10-fold more transforming than WT) exhibiting higher transforming activity when compared to either single mutant (Fig. 3-4A), suggesting that concurrent loss of the tyrosine and leucine residues of the 770 YXXL motif cooperate to enhance the transforming activity of the carboxyl-terminus truncated FGFR2 IIIb. 83
95 A WT Y770F Y770F/L773A Y770A/L773A L773A KGF Control B % of cells internalized WT Y770F Y770F/L773A Y770A/L773A L773A Figure 3-3. The 770 YXXL motif is required for ligand-stimulated FGFR2 IIIb C1 internalization. A. Rat-1 cells that stably express the indicated FGFR2 IIIb proteins were serumstarved for 20 h and then incubated with either vehicle (BSA) or 50 ng/ml KGF for 40 min at 37ºC. The cells were fixed, permeabilized, and immunostained with anti- FGFR2 antibody to determine FGFR2 IIIb subcellular location by confocal microscopy. B. Quantification of the internalization of wild type and mutant FGFR2 IIIb C1 proteins. Rat-1 cells that stably express the indicated FGFR2 IIIb proteins were serum-starved for 20 h and then incubated with either vehicle (BSA) or 50 ng/ml KGF for 40 min at 37ºC. Data are expressed as percentage of cells that internalized receptors with the bars indicating standard deviation and are representative of three independent experiments. 84
96 To exclude the possibility that different expression level of proteins could have an effect on transforming potency, we determined all FGFR2 IIIb proteins tested above were expressed at comparable levels (Fig. 3-4B). E. Loss of Y770 impairs ligand stimulated FGFR2 IIIb C1 activation of PLCγ. While the L773A mutant exhibited greater impairment in internalization than the Y770F mutant (Fig. 3-3A), the Y770F mutant showed greater transforming activity than the L773A mutant (Fig. 3-4A). These observations suggest that impaired receptor internalization as well as some other mechanism(s) might contribute to enhanced transforming activity of Y770F mutant. Interestingly, the Y770 residue that corresponds to the 770 YXXL protein sorting motif was also shown to be a binding site for PLCγ (45, 111). Recently, it was shown that a Y770F missense mutant of FGFR2 IIIb C1 cannot bind and phosphorylate PLCγ, and impaired, rather than enhanced, ligand-stimulated mitogenic activity (47). While they showed that this mutation impaired KGF-stimulated ERK activation, whether this mutant fails to activate PI hydrolysis was not determined. To further evaluate the role of Y770F in FGFR2 IIIb regulation of PLCγ signaling and transforming activity, and the consequences of mutation of other 770 YXXL motif residues, we first determined whether these mutations decreased ligand-stimulated PLCγ phosphorylation. Once FGFR is stimulated and autophosphorylated, the SH2 domains of PLCγ bind to tyrosine phosphorylated FGFR. Binding of PLCγ to the activated FGFR facilitates its own tyrosine phosphorylation by the FGFR (4). To 85
97 determine if loss of Y770 causes decreased tyrosine phosphorylation of PLCγ, Rat-1 cells stably-expressing a series of FGFR2 IIIb C1 mutants (Y770F, Y770F/L773A, Y770A/L773A and L773A), as well as the WT C1, C2 and C3 variants, were stimulated with either KGF (50 ng/ml) or vehicle (BSA in PBS) for 20 min. The cells were then lysed and immunoprecipitated with PLCγ antibody followed by immunoblot analyses with phosphotyrosine antibody. In unstimulated cells, none of the FGFR2 IIIb proteins evaluated showed elevated tyrosine phosphorylation of endogenous PLCγ. However, after stimulation with KGF, as expected the WT FGFR2 IIIb C1 and C2, and not the C3 variants showed increased tyrosine phosphorylated PLCγ. Interestingly, we observed that the WT C2 variant showed more strongly tyrosine phosphorylated PLCγ when compared to WT C1. Moreover, we found that all three FGFR2 IIIb C1 mutants that lack the Y770 residue (Y770F, Y770F/L773A, and Y770A/L773A) failed to exhibit elevated tyrosine-phosphorylated PLCγ, while the L773A mutant retained the ability to stimulate tyrosine phosphorylation of PLCγ (Fig. 3-5A). Since tyrosine phosphorylation of PLCγ is essential for its activation it was expected that the Y770F mutant receptor would not activate PLCγ. To test this idea directly, [ 3 H]inositol phosphate accumulation was quantified in cells expressing either WT FGFR2 IIIb C1 or the Y770F mutant receptor. Both thrombin and LPA promoted [ 3 H]inostiol phosphate accumulation in Rat-1 cells expressing empty vector, but no endogenous response to KGF was observed (Fig. 3-5B). 86
98 A 1400 B FGFR2 C1 Colonies Per Dish Vector WT Y770F Y770F/L773A Y770A/L773A L773A Vector WT Y770F Y770F/L773A Y770A/L773A L773A FGFR2 β-actin Figure 3-4. Mutation of the Y and L residues of the 770 YXXL motif cooperates to enhance FGFR2 IIIb C1 induction of anchorage-independent growth transformation. A. FGFR2 IIIb C1 770 YXXL motif mutants exhibit enhanced colony formation in soft agar. Rat-1 cells that stably express the indicated FGFR2 IIIb proteins were suspended in 0.4% soft agar and allowed to grow for 14 days before the number of colonies was quantitated. Data shown are the average of duplicate dishes, with the bars indicating standard deviation, and are representative of three independent experiments. B. Wild type and 770 YXXL motif mutants of FGFR2 IIIb C1 are stablyexpressed at comparable levels. Rat-1 cells that stably-expressed the indicated FGFR2 IIIb proteins were assayed for their FGFR2 IIIb protein expression levels by immunoblot analyses with anti-fgfr2 antibody. Total cell lysates were also blotted with anti-β-actin to verify equivalent total protein. 87
99 Stable expression of the wild type form of the C1 receptor conferred a marked [ 3 H]inositol phosphate response to KGF. In contrast, KGF-promoted [ 3 H]inositol phosphate accumulation was not observed in Y770F mutant receptor-expressing cells although responses to thrombin and LPA were retained. Taken together, these data suggest that Y770 is required for activation of PLCγ by FGFR2 IIIb, and the loss of capacity to promote inositol lipid hydrolysis might contribute to enhanced transforming activity of the C3 variant that lacks Y770. F.Loss of Y770 of FGFR2 C1 induces sustained activation of FRS2, but not ERK. PLCγ-mediated signaling is generally considered to promote mitogenesis and growth ( ). Thus, the fact that Y770F enhances FGFR2 IIIb C1 transforming activity, yet results in the loss of PLCγ activation, argues that mutation of Y770 may affect other signaling activities that promote growth transformation. One possible explanation for this paradoxical observation is that PLCγ might compete with other FGFR2 IIIb effectors for binding to FGFR2 IIIb. By abolishing PLCγ binding, the Y770F mutation might relieve steric hindrance for other FGFR2 IIIb effectors and facilitate their binding to FGFR2 IIIb. Consequently, loss of PLCγ binding caused by the Y770F mutation may indirectly promote the activation of other FGFR2 IIIb effector pathways. Aside from PLCγ, the FRS2 proteins represent the next bestcharacterized effectors of FGFR signaling. Therefore, we determined whether the mutation of Y770 altered FGFR2 IIIb activation of FRS2. For these analyses, Rat-1 cells stably-expressing WT or mutants of FGFR2 IIIb C1, as well as the WT C2 and 88
100 C3 variants, were stimulated for 20 min with either KGF (50 ng/ml) or vehicle. The cells were then lysed and immunoprecipitated with either anti-frs2 antibody or antiphospho-tyrosine antibody followed by immunonblot analyses using either antiphospho-tyrosine or anti-frs2 antibody, respectively. After stimulation with KGF, all three FGFR2 IIIb variants (C1 WT, C2 and C3) showed increased levels of robustly tyrosine-phosphorylated FRS2. In addition, the Y770F and Y770A/L773A mutants tyrosine-phosphorylated FRS2 to a similar extent as was seen with the WT C1, C2 and C3 variants (Fig. 3-6A). In the absence of KGF, although the WT C1 and C2 variants that contain the Y770 residue did not exhibit increased tyrosinephosphorylated FRS2, the C3 variant that lacks the Y770 residue showed constitutively tyrosine-phosphorylated FRS2 (Figs. 3-6A and 3-6B). Furthermore, all three mutants that lack Y770 residue (Y770F, Y770F/L773A, and Y770A/L773A) showed constitutively tyrosine-phosphorylated FRS2 in the absence of KGF stimulation (Figs. 3-6A and 3-6B). These data indicate that the loss of Y770 promotes constitutive, ligand-independent activation of FRS2 to enhance FGFR2 IIIb transforming activity. Next, we determined whether the loss of Y770 further activated downstream signaling by FRS2. Once FRS2 is tyrosine- phosphorylated, it forms a complex with the Grb2 adaptor protein and the Sos guanine nucleotide exchange factor and activator of Ras. The best-characterized effector pathway of Ras is the Raf-MEK- ERK MAPK. Thus, we examined whether loss of Y770F led to activation of ERK MAPK. For these analyses, we utilized immunoblot analyses with a phospho-specific antibody that recognizes the phosphorylated and activated forms of ERK1 and ERK2. 89
101 A C1 C1 [ 3 H]IP (cmp x 10 4 ) Vector C1 WT C2 C3 Y770F Y770F/L773A Y770A/L773A L773A Vector C1 WT C2 C3 Y770F Y770F/L773A Y770A/L773A L773A P-Tyr PLCγ B Vehicle KGF Vector [ 3 H]IP (cmp x 10 4 ) C1 WT [ 3 H]IP (cmp x 10 4 ) C1 Y770F basal thrombin 0.1 mm thrombin 1mM LPA 1 mm LPA 10 mm KGF 100 ng/ml KGF 200 ng/ml basal thrombin 0.1 mm thrombin 1 mm LPA 1 mm LPA 10 mm KGF 100 ng/ml KGF 200 ng/ml basal thrombin 0.1 mm thrombin 1 mm LPA 1 mm LPA 10 mm KGF 100 ng/ml KGF 200 ng/ml Figure 3-5. Mutation of Y770 impairs ligand-stimulated FGFR2 IIIb C1- dependent activation of PLCγ. A. Mutation of Y770 but not L773 impairs KGF-stimulated phosphorylation of endogenous PLCγ. Rat-1 cells stably expressing indicated FGFR2 IIIb proteins were serum-starved for 20 h and stimulated with either vehicle or 50 ng/ml KGF for 30 min. Tyrosine phosphorylated and total PLCγ protein was determined by immunoprecipitation with anti-plcγ antibody followed by immunoblotting with antiphosphotyrosine (P-Tyr) antibody or with anti-plcγ antibody, respectively. Data shown are representative of three independent experiments. B. Mutation of Y770 impairs KGF-stimulated formation of inositol phosphates. Rat-1 cells stablyexpressing the indicated wild type (WT) or mutant FGFR2 IIIb C1 proteins were serum-starved for 20 h and stimulated with vehicle (basal), or with 100 or 200 ng/ml KGF, 1 or 10 μm thrombin or lysophophatidic acid (LPA) (positive controls) and [ 3 H]inositol phosphate (IP) accumulation was measured to assess PLCγ activity. Data shown are representative of three independent assays. 90
102 After stimulation with KGF for 20 min, all three FGFR2 IIIb variants (WT C1, C2 and C3) and mutants (Y770F, Y770F/L773A, and Y770A/L773A) phosphorylated ERK to a similar extent (data not shown). However, in the absence of KGF, none of the FGFR2 IIIb proteins tested above caused sustained activation of ERK (Fig. 3-6B). Together, these data suggest that the mutation of Y770 caused constitutive activation of FRS2, but did not promote FRS2-mediated activation of ERK. G. FRS2 activity is required for increased FGFR2 IIIb C1 transforming activity by Y770F mutation but not L773A mutation. Next, we determined whether FRS2 activity is required for increased transforming activity of FGFR2 IIIb C1 by Y770F mutation and/or L773A mutation. For these analyses, we generated mutants of FGFR2 IIIb C1 that cannot bind and activate FRS2. Previously, it was demonstrated that the phosphotyrosine-binding (PTB) domain of the FRS2 constitutively binds to juxtamembrane region of FGFR1 (112). Utilizing alanine scanning mutagenesis, the specific amino acid residues in juxtamembrane region of FGFR1 that are responsible for FRS2 binding were identified. When K419, P422, and L423 residues in juxtamembrane region were substituted with alanine, interaction between FGFR1 and the FRS2 was strongly diminished, resulting in decreased tyrosine phosphorylation of FRS2 (112). Since K419, P422, and L423 residues in juxtamembrane region of FGFR1 are well conserved in FGFR2 IIIb, we constructed a FGFR2 IIIb (K421A/P424A/L425A) mutant (designated as ΔFRS2) that is analogous to the FGFR1 (K419A/P422A/L423A) mutant. We first determined whether the ΔFRS2 mutation 91
103 A Vehicle KGF C1 C1 Vector WT Y770F Y770A/L773A C2 C3 Vector WT Y770F Y770A/L773A C2 C3 IP: FRS2 IB: P-Tyr B Vector WT Y770F C1 Y770F/L773A Y770A/L773A C2 C3 C1 WT + KGF IP: FRS2 IB: P-Tyr IP: P-Tyr IB: FRS2 IB: P-ERK IB: Total ERK Figure 3-6. Mutation of Y770 of FGFR2 IIIb C1 causes sustained activation of FRS2. A. Like FGFR2 IIIb C3, Y770 mutants of FGFR2 IIIb C1 exhibit ligand-independent phosphorylation of FRS2. Rat-1 cells stably expressing indicated FGFR2 IIIb proteins were serum starved for 20 h and stimulated with either vehicle (control) or 50 ng/ml KGF for 30 min. FRS2 activity was determined by immunoprecipitation (IP) with anti-frs2 antibody followed by immunoblotting (IB) with anti-phosphotyrosine (P-Tyr) antibody. B. Like FGFR2 IIIb C3, Y770 mutants of FGFR2 IIIb C1 cause ligand-independent sustained activation of FRS2 but not ERK. Rat-1 cells stably expressing indicated proteins were analyzed for FRS2 activity by immunoprecipitation (IP) with either anti-frs2 antibody or anti-phospho-tyrosine antibody followed by immunoblotting (IB) with either phospho-tyrosine anibody or FRS2 antibody, respectively. ERK activation was determined by immunoblot analyses using antibody that recognize activated, phosphorylated form of ERK. Data shown are representative of three independent experiments. 92
104 could abolish FRS2 activation. As expected, when the ΔFRS2 mutation was introduced into two existing mutants, Y770F (designated as (designated as Y770F/L773A/ΔFRS2), the increased FRS2 tyrosine-phosphorylation caused by the Y770F mutation was completely abolished (Fig. 3-7A). Since we determined that the ΔFRS2 mutation blocked FRS2 tyrosinephosphorylation, we further examined whether the ΔFRS2 mutation can diminish the enhanced transforming activity of the Y770F or Y770F/L773A mutants. Consistent with our observation in Fig. 4, Y770F showed ~7-fold, while Y770F/L773A showed ~11-fold higher transforming activity compared with WT C1 (Fig. 3-7B). When we introduced the ΔFRS2 mutation into the Y770F mutant, the resulting Y770F/ΔFRS2 mutant showed similar transforming activity as WT C1 (Fig. 3-7B). This observation indicates that the enhanced transforming potency by Y770F mutation is dependent on FRS2 activity. However, when we introduced ΔFRS2 mutation in Y770F/L773A mutant, although the ΔFRS2 mutation partially diminished the transforming activity of the Y770F/L773A mutant, Y770F/L773A/ΔFRS2 still exhibited ~4-fold greater transforming potency than WT C1 (Fig. 3-7B). In fact, Y770F/L773A/ΔFRS2 mutant showed similar transforming potency as L773A mutant (Fig. 3-7B), suggesting that ΔFRS2 only abolishes enhanced transforming activity caused by they770f but not L773A mutation. Taken together, these data indicate that increased FRS2 activation is required for the increased transforming activity caused by the Y770F but not L773A mutation. 93
105 Figure 3-7. FRS2 activity is required for increased FGFR2 IIIb C1 transforming activity caused by the Y770F but not L773A mutation. A. Mutation of the FRS2 binding site impairs FGFR2 IIIb YXXL mutant activation of FRS2. Rat-1 cells stably expressing indicated FGFR2 IIIb proteins were grown to confluence and were lysed and FRS2 activity was determined as described above. Total FRS2 protein levels were determined by immunoprecipitation (IP) with anti- FRS2 antibody followed by immunoblotting (IB) with anti-frs2 antibody. Total cell lyasates were analysed to determine stable FGFR2 IIIb protein expression levels by immunoblot analyses with anti-fgfr2 antibody. ΔFRS2 corresponds to the K421A/P424A/L425A mutations in the FRS2 binding site. B. Mutation of the FRS2 binding site impairs the transforming activity caused by the Y770F but not the L773A YXXL mutant of FGFR2 IIIb C1. Rat-1 cells stably expressing the indicated FGFR2 IIIb proteins were suspended in 0.4% soft agar and allowed to grow for about 14 days before the number of colonies was quantitated. Data shown are the average of duplicate dishes, with the bars indicating standard deviation, and are representative of three independent experiments. 94
106 V. Discussion Although missense mutations of FGFR2 are found in human cancers ( (54), another mechanism of FGFR2 activation in cancer involves alternative gene splicing. FGFR2 IIIb exists in at least three carboxyl-terminal variants, designated C1, C2 and C3, due to alternative gene splicing. Increased expression of the C2 and C3 splice variants has been observed in human cancers, suggesting that differential expression of specific splice variants may contribute to oncogenesis. A recent study identified frameshift mutations that caused premature truncation of the carboxyl terminal sequences lacking in FGFR2 IIIb C3, in endometrial cancers (54), supporting the importance of loss of carboxyl terminal sequences in FGFR2 activation in cancer. In this study, we compared the transforming potency of the C1, C2 and C3 splicing variants of FGFR2 IIIb and found that a hierarchy of transforming activity (C3>C2>C1) that correlated with progressive loss of carboxyl terminal sequences. C2 and C3 lack two or five carboxyl terminal tyrosine residues, respectively, that are retained in C1 and we determined that loss of the Y770 alone significantly the enhanced transforming activity of FGFR2 IIIb C1. This observation was unexpected since a previous study found that loss of Y770 impaired FGFR2 IIIb C1-mediated activation of the ERK MAPK cascade and stimulation of cell proliferation (47). Since this residue is a key component of an established PLCγ binding site and a putative YXXL tyrosine-based sorting motif, we evaluated the consequences of mutation of 770 YXXL motif on FGFR2 IIIb on PLCγ activation and receptor internalization. We found that disruption of the 770 YXXL motif in the weakly transforming C1 variant impaired receptor 95
107 internalization and enhanced transforming potency. We also found that loss of Y770 abolished PLCγ activity, but instead enhanced FRS2 activity. Together, we suggest a model (Fig. 3-8) for two distinct mechanisms where the loss of the 770 YXXL motif contributes to the enhanced transforming potency of FGFR2 IIIb C3. The loss of the 770 YXXL motif causes impaired ligand-stimulated receptor internalization to promote persistent ligand-independent signaling. In addition, loss of Y770 in the C3 variant abolishes PLCγ binding to FGFR2 IIIb, and consequently, may relieve steric hindrance and facilitate FGFR2 IIIb activation of FRS2-mediated signaling. FRS2- dependent signaling, independent of the ERK MAPK cascade, then promotes growth transformation. Our analyses, in several fibroblast and epithelial cell types (data not shown), showed that sequential loss of carboxyl-terminal sequences caused progressive enhancement in FGFR2 IIIb transforming activity, indicating that multiple carboxylterminal tyrosine residues control negative regulators of FGFR2 IIIb biological activity. However, the significantly greater transforming activity of the C3 variant, when compared to the C2 isoform, indicates the key importance of residues Y770, Y780 and/or Y784. This would be consistent with the fact that, of the five Tyr residues found in the carboxyl terminus of FGFR2 IIIb C1, only Y770 and Y780 are conserved in all four FGFR family members (Fig. 3-1B). Our mutational analyses of all five Tyr residues found that the loss of Y770 alone in C1 partially mimicked the enhanced transforming activity of the C3 isoform. When Y770 was mutated in combination with the remaining four tyrosine residues, no further enhancement in transforming activity was seen. This suggests that the loss of non-tyrosine residues 96
108 Figure 3-8. Role of altered signaling caused by deletion of the 770 YXXL motif in FGFR2 IIIb C3 transforming activity. Based on our observations with missense mutations in the 770 YXXL motif and FRS binding site of FGFR2 IIIb C1, we propose that the loss of 770 YXXL sorting motif contributes significantly to the potent transforming activity of the C3 variant. We propose that the 770 YXXL motif serves two distinct functions in the C1 isoform. First, it serves as a phosphorylation-dependent binding site for the SH2 domain of PLCγ, which promotes ligand-stimulated second messenger signaling. Second, it serves as a protein sorting signal, similar to those found in other cell surface receptors, that promotes rapid receptor internalization and endocytosis, and lysosomal degradation. We also propose that the mutation of the tyrosine residue of the 770 YXXL motif prevents PLCγ binding and activation. FRS2 association with the FGFR2 receptor is phosphorylation-independent. The loss of PLCγ binding may facilitate FGFR2 phosphorylation and activation of FRS2. Consequently, the C3 variant exhibits increased, ligand-independent FRS2 activity that leads to enhanced transformation through and unidentified FRS2 effector(s). Our results suggest that the bestcharacterized FRS2 effector, Grb2 adaptor-mediated activation of Ras and the ERK MAPK cascade, is not the basis for FRS2-dependent transformation. 97
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Chapter 15: Signal transduction
 Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Signal Transduction Pathways. Part 2
 Signal Transduction Pathways Part 2 GPCRs G-protein coupled receptors > 700 GPCRs in humans Mediate responses to senses taste, smell, sight ~ 1000 GPCRs mediate sense of smell in mouse Half of all known
Signal Transduction Pathways Part 2 GPCRs G-protein coupled receptors > 700 GPCRs in humans Mediate responses to senses taste, smell, sight ~ 1000 GPCRs mediate sense of smell in mouse Half of all known
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Cell, Volume 141. Supplemental Information Cell Signaling by Receptor Tyrosine Kinases Mark A. Lemmon and Joseph Schlessinger
 Cell, Volume 141 Supplemental Information Cell Signaling by Receptor Tyrosine Kinases Mark A. Lemmon and Joseph Schlessinger Figure S1. RTK Mutations in Diseases Locations of gain-of-function (green arrows)
Cell, Volume 141 Supplemental Information Cell Signaling by Receptor Tyrosine Kinases Mark A. Lemmon and Joseph Schlessinger Figure S1. RTK Mutations in Diseases Locations of gain-of-function (green arrows)
The elements of G protein-coupled receptor systems
 The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
Signaling Through Immune System Receptors (Ch. 7)
 Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Cellular Signaling Pathways. Signaling Overview
 Cellular Signaling Pathways Signaling Overview Signaling steps Synthesis and release of signaling molecules (ligands) by the signaling cell. Transport of the signal to the target cell Detection of the
Cellular Signaling Pathways Signaling Overview Signaling steps Synthesis and release of signaling molecules (ligands) by the signaling cell. Transport of the signal to the target cell Detection of the
MCB*4010 Midterm Exam / Winter 2008
 MCB*4010 Midterm Exam / Winter 2008 Name: ID: Instructions: Answer all 4 questions. The number of marks for each question indicates how many points you need to provide. Write your answers in point form,
MCB*4010 Midterm Exam / Winter 2008 Name: ID: Instructions: Answer all 4 questions. The number of marks for each question indicates how many points you need to provide. Write your answers in point form,
Chapter 20. Cell - Cell Signaling: Hormones and Receptors. Three general types of extracellular signaling. endocrine signaling. paracrine signaling
 Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
THE HALLMARKS OF CANCER
 THE HALLMARKS OF CANCER ONCOGENES - Most of the oncogenes were first identified in retroviruses: EGFR (ErbB), Src, Ras, Myc, PI3K and others (slightly more than 30) - Mutated cellular genes incorporated
THE HALLMARKS OF CANCER ONCOGENES - Most of the oncogenes were first identified in retroviruses: EGFR (ErbB), Src, Ras, Myc, PI3K and others (slightly more than 30) - Mutated cellular genes incorporated
Regulation of cell function by intracellular signaling
 Regulation of cell function by intracellular signaling Objectives: Regulation principle Allosteric and covalent mechanisms, Popular second messengers, Protein kinases, Kinase cascade and interaction. regulation
Regulation of cell function by intracellular signaling Objectives: Regulation principle Allosteric and covalent mechanisms, Popular second messengers, Protein kinases, Kinase cascade and interaction. regulation
Lecture: CHAPTER 13 Signal Transduction Pathways
 Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
The Tissue Engineer s Toolkit
 The Tissue Engineer s Toolkit Stimuli Detection and Response Ken Webb, Ph. D. Assistant Professor Dept. of Bioengineering Clemson University Environmental Stimulus-Cellular Response Environmental Stimuli
The Tissue Engineer s Toolkit Stimuli Detection and Response Ken Webb, Ph. D. Assistant Professor Dept. of Bioengineering Clemson University Environmental Stimulus-Cellular Response Environmental Stimuli
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Signal Transduction I
 Signal Transduction I Prof. Tianhua Zhou Department of Cell Biology Zhejiang University School of Medicine Office hours by appointment tzhou@zju.edu.cn Signal transduction: Key contents for signal transduction:
Signal Transduction I Prof. Tianhua Zhou Department of Cell Biology Zhejiang University School of Medicine Office hours by appointment tzhou@zju.edu.cn Signal transduction: Key contents for signal transduction:
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression
 Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Signal Transduction: G-Protein Coupled Receptors
 Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
1. Activated receptor tyrosine kinases (RTKs) phosphorylates themselves
 Enzyme-coupled receptors Transmembrane proteins Ligand-binding domain on the outer surface Cytoplasmic domain acts as an enzyme itself or forms a complex with enzyme 1. Activated receptor tyrosine kinases
Enzyme-coupled receptors Transmembrane proteins Ligand-binding domain on the outer surface Cytoplasmic domain acts as an enzyme itself or forms a complex with enzyme 1. Activated receptor tyrosine kinases
Signal transduction and protein kinase inhibitors. Feng Qian ( 钱峰 )
 Signal transduction and protein kinase inhibitors Feng Qian ( 钱峰 ) fengqian@sjtu.edu.cn Protein Kinases in the Human Genome 518 kinases 1.7 % of human genome Lipid kinases Nucleotide kinases Cell Signaling
Signal transduction and protein kinase inhibitors Feng Qian ( 钱峰 ) fengqian@sjtu.edu.cn Protein Kinases in the Human Genome 518 kinases 1.7 % of human genome Lipid kinases Nucleotide kinases Cell Signaling
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Concise Reference. HER2 Testing in Breast Cancer. Mary Falzon, Angelica Fasolo, Michael Gandy, Luca Gianni & Stefania Zambelli
 Concise Reference Testing in Breast Cancer Mary Falzon, Angelica Fasolo, Michael Gandy, Luca Gianni & Stefania Zambelli Extracted from Handbook of -Targeted Agents in Breast Cancer ublished by Springer
Concise Reference Testing in Breast Cancer Mary Falzon, Angelica Fasolo, Michael Gandy, Luca Gianni & Stefania Zambelli Extracted from Handbook of -Targeted Agents in Breast Cancer ublished by Springer
Effects of Second Messengers
 Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Cell cycle, signaling to cell cycle, and molecular basis of oncogenesis
 Cell cycle, signaling to cell cycle, and molecular basis of oncogenesis MUDr. Jiří Vachtenheim, CSc. CELL CYCLE - SUMMARY Basic terminology: Cyclins conserved proteins with homologous regions; their cellular
Cell cycle, signaling to cell cycle, and molecular basis of oncogenesis MUDr. Jiří Vachtenheim, CSc. CELL CYCLE - SUMMARY Basic terminology: Cyclins conserved proteins with homologous regions; their cellular
number Done by Corrected by Doctor Maha Shomaf
 number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
Propagation of the Signal
 OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
What would you observe if you fused a G1 cell with a S cell? A. Mitotic and pulverized chromosomes. B. Mitotic and compact G1 chromosomes.
 What would you observe if you fused a G1 cell with a S cell? A. Mitotic and pulverized chromosomes. B. Mitotic and compact G1 chromosomes. C. Mostly non-compact G1 chromosomes. D. Compact G1 and G2 chromosomes.
What would you observe if you fused a G1 cell with a S cell? A. Mitotic and pulverized chromosomes. B. Mitotic and compact G1 chromosomes. C. Mostly non-compact G1 chromosomes. D. Compact G1 and G2 chromosomes.
Growth and Differentiation Phosphorylation Sampler Kit
 Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Biosignals, Chapter 8, rearranged, Part I
 Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Principles of cell signaling Lecture 4
 Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Tyrosine kinases. Cell surface receptors ligand binding. Producer cell RNA. Target cell
 Tyrosine kinases http://msbl.helsinki.fi/tkseminar roducer cell Signaling molecules Receptor binding Signal transduction Target cell Activation of Gene expression RNA Biological responses proliferation,
Tyrosine kinases http://msbl.helsinki.fi/tkseminar roducer cell Signaling molecules Receptor binding Signal transduction Target cell Activation of Gene expression RNA Biological responses proliferation,
Deregulation of signal transduction and cell cycle in Cancer
 Deregulation of signal transduction and cell cycle in Cancer Tuangporn Suthiphongchai, Ph.D. Department of Biochemistry Faculty of Science, Mahidol University Email: tuangporn.sut@mahidol.ac.th Room Pr324
Deregulation of signal transduction and cell cycle in Cancer Tuangporn Suthiphongchai, Ph.D. Department of Biochemistry Faculty of Science, Mahidol University Email: tuangporn.sut@mahidol.ac.th Room Pr324
Signal Transduction SS Gerhild van Echten-Deckert
 Signal Transduction SS 2018 Gerhild van Echten-Deckert Tel. 73 2703 E-mail: g.echten.deckert@uni-bonn.de https://www.limes-institut-bonn.de/forschung/ Focus on 2 classes of cell-surface receptors (Growth
Signal Transduction SS 2018 Gerhild van Echten-Deckert Tel. 73 2703 E-mail: g.echten.deckert@uni-bonn.de https://www.limes-institut-bonn.de/forschung/ Focus on 2 classes of cell-surface receptors (Growth
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Genetics and Cancer Ch 20
 Genetics and Cancer Ch 20 Cancer is genetic Hereditary cancers Predisposition genes Ex. some forms of colon cancer Sporadic cancers ~90% of cancers Descendants of cancerous cells all cancerous (clonal)
Genetics and Cancer Ch 20 Cancer is genetic Hereditary cancers Predisposition genes Ex. some forms of colon cancer Sporadic cancers ~90% of cancers Descendants of cancerous cells all cancerous (clonal)
Oncogenes and Tumor. supressors
 Oncogenes and Tumor supressors From history to therapeutics Serge ROCHE Neoplastic transformation TUMOR SURESSOR ONCOGENE ONCOGENES History 1911 1960 1980 2001 Transforming retrovirus RSV v-src is an oncogene
Oncogenes and Tumor supressors From history to therapeutics Serge ROCHE Neoplastic transformation TUMOR SURESSOR ONCOGENE ONCOGENES History 1911 1960 1980 2001 Transforming retrovirus RSV v-src is an oncogene
Revision. camp pathway
 االله الرحمن الرحيم بسم Revision camp pathway camp pathway Revision camp pathway Adenylate cyclase Adenylate Cyclase enzyme Adenylate cyclase catalyses the formation of camp from ATP. Stimulation or inhibition
االله الرحمن الرحيم بسم Revision camp pathway camp pathway Revision camp pathway Adenylate cyclase Adenylate Cyclase enzyme Adenylate cyclase catalyses the formation of camp from ATP. Stimulation or inhibition
VIII Curso Internacional del PIRRECV. Some molecular mechanisms of cancer
 VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
Protein kinases are enzymes that add a phosphate group to proteins according to the. ATP + protein OH > Protein OPO 3 + ADP
 Protein kinase Protein kinases are enzymes that add a phosphate group to proteins according to the following equation: 2 ATP + protein OH > Protein OPO 3 + ADP ATP represents adenosine trisphosphate, ADP
Protein kinase Protein kinases are enzymes that add a phosphate group to proteins according to the following equation: 2 ATP + protein OH > Protein OPO 3 + ADP ATP represents adenosine trisphosphate, ADP
Signal Transduction: Information Metabolism. Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire
 Signal Transduction: Information Metabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire Introduction Information Metabolism How cells receive, process and respond
Signal Transduction: Information Metabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire Introduction Information Metabolism How cells receive, process and respond
Chapter 9. Cellular Signaling
 Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Genome of Hepatitis B Virus. VIRAL ONCOGENE Dr. Yahwardiah Siregar, PhD Dr. Sry Suryani Widjaja, Mkes Biochemistry Department
 Genome of Hepatitis B Virus VIRAL ONCOGENE Dr. Yahwardiah Siregar, PhD Dr. Sry Suryani Widjaja, Mkes Biochemistry Department Proto Oncogen and Oncogen Oncogen Proteins that possess the ability to cause
Genome of Hepatitis B Virus VIRAL ONCOGENE Dr. Yahwardiah Siregar, PhD Dr. Sry Suryani Widjaja, Mkes Biochemistry Department Proto Oncogen and Oncogen Oncogen Proteins that possess the ability to cause
Computational Biology I LSM5191
 Computational Biology I LSM5191 Aylwin Ng, D.Phil Lecture 6 Notes: Control Systems in Gene Expression Pulling it all together: coordinated control of transcriptional regulatory molecules Simple Control:
Computational Biology I LSM5191 Aylwin Ng, D.Phil Lecture 6 Notes: Control Systems in Gene Expression Pulling it all together: coordinated control of transcriptional regulatory molecules Simple Control:
PHSI3009 Frontiers in Cellular Physiology 2017
 Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Computational Systems Biology: Biology X
 Bud Mishra Room 1002, 715 Broadway, Courant Institute, NYU, New York, USA L#10:(November-22-2010) Cancer and Signals 1 1 Micro-Environment Story How does the rest of our body influences the cancer cell?
Bud Mishra Room 1002, 715 Broadway, Courant Institute, NYU, New York, USA L#10:(November-22-2010) Cancer and Signals 1 1 Micro-Environment Story How does the rest of our body influences the cancer cell?
Lecture 7: Signaling Through Lymphocyte Receptors
 Lecture 7: Signaling Through Lymphocyte Receptors Questions to Consider After recognition of its cognate MHC:peptide, how does the T cell receptor activate immune response genes? What are the structural
Lecture 7: Signaling Through Lymphocyte Receptors Questions to Consider After recognition of its cognate MHC:peptide, how does the T cell receptor activate immune response genes? What are the structural
Ayman Mesleh & Leen Alnemrawi. Bayan Abusheikha. Faisal
 24 Ayman Mesleh & Leen Alnemrawi Bayan Abusheikha Faisal We were talking last time about receptors for lipid soluble hormones.the general mechanism of receptors for lipid soluble hormones: 1. Receptors
24 Ayman Mesleh & Leen Alnemrawi Bayan Abusheikha Faisal We were talking last time about receptors for lipid soluble hormones.the general mechanism of receptors for lipid soluble hormones: 1. Receptors
Phospho-AKT Sampler Kit
 Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy se
 A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
Part I => CARBS and LIPIDS. 1.7 Signal Transduction 1.7a Endocrine Hormones 1.7b Hormone Receptors
 Part I => CARBS and LIPIDS 1.7 Signal Transduction 1.7a Endocrine Hormones 1.7b Hormone Receptors Section 1.7a: Endocrine Hormones Synopsis 1.7a - Hormones are chemical messengers that play a key role
Part I => CARBS and LIPIDS 1.7 Signal Transduction 1.7a Endocrine Hormones 1.7b Hormone Receptors Section 1.7a: Endocrine Hormones Synopsis 1.7a - Hormones are chemical messengers that play a key role
Chem Lecture 10 Signal Transduction
 Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Signal-Transduction Cascades - 2. The Phosphoinositide Cascade
 Signal-Transduction Cascades - 2 The Phosphoinositide Cascade Calcium ion as a second messenger Tyrosine kinase and receptor dimerization scribd.com Faisal Khatib JU The Phosphoinositide Cascade Used by
Signal-Transduction Cascades - 2 The Phosphoinositide Cascade Calcium ion as a second messenger Tyrosine kinase and receptor dimerization scribd.com Faisal Khatib JU The Phosphoinositide Cascade Used by
BL 424 Chapter 15: Cell Signaling; Signal Transduction
 BL 424 Chapter 15: Cell Signaling; Signal Transduction All cells receive and respond to signals from their environments. The behavior of each individual cell in multicellular plants and animals must be
BL 424 Chapter 15: Cell Signaling; Signal Transduction All cells receive and respond to signals from their environments. The behavior of each individual cell in multicellular plants and animals must be
Introduction! Introduction! Introduction! Chem Lecture 10 Signal Transduction & Sensory Systems Part 2
 Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
Allosteric Inhibition of SHP2: Identification of a Potent, Selective, and Orally Efficacious Phosphatase Inhibitor!
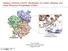 Allosteric Inhibition of SHP2: Identification of a Potent, Selective, and Orally Efficacious Phosphatase Inhibitor Allosteric pocket SHP2 Phosphatase ovel allosteric Phosphatase inhibitor Evan Carder Wipf
Allosteric Inhibition of SHP2: Identification of a Potent, Selective, and Orally Efficacious Phosphatase Inhibitor Allosteric pocket SHP2 Phosphatase ovel allosteric Phosphatase inhibitor Evan Carder Wipf
EGFR: fundamenteel en klinisch
 EGFR: fundamenteel en klinisch Guido Lammering MAASTRO Clinic Maastricht, NL What is EGFR?? The EGFR some facts 1186 amino acids 170 kda Expressed by all cells of epithelial origin Increased activation
EGFR: fundamenteel en klinisch Guido Lammering MAASTRO Clinic Maastricht, NL What is EGFR?? The EGFR some facts 1186 amino acids 170 kda Expressed by all cells of epithelial origin Increased activation
Sarah Jaar Marah Al-Darawsheh
 22 Sarah Jaar Marah Al-Darawsheh Faisal Mohammad Receptors can be membrane proteins (for water-soluble hormones/ligands) or intracellular (found in the cytosol or nucleus and bind to DNA, for lipid-soluble
22 Sarah Jaar Marah Al-Darawsheh Faisal Mohammad Receptors can be membrane proteins (for water-soluble hormones/ligands) or intracellular (found in the cytosol or nucleus and bind to DNA, for lipid-soluble
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Genetics of Cancer Lecture 32 Cancer II. Prof. Bevin Engelward, MIT Biological Engineering Department
 Genetics of Cancer Lecture 32 Cancer II rof. Bevin Engelward, MIT Biological Engineering Department Why Cancer Matters New Cancer Cases in 1997 Cancer Deaths in 1997 Genetics of Cancer: Today: What types
Genetics of Cancer Lecture 32 Cancer II rof. Bevin Engelward, MIT Biological Engineering Department Why Cancer Matters New Cancer Cases in 1997 Cancer Deaths in 1997 Genetics of Cancer: Today: What types
Molecular Oncology, oncology parameters see each test
 Molecular Oncology, oncology parameters see each test DPD deficiency Dihydropyrimidine dehydrogenase deficiency (DPD deficiency) is an autosomal recessive metabolic disorder in which there is absent or
Molecular Oncology, oncology parameters see each test DPD deficiency Dihydropyrimidine dehydrogenase deficiency (DPD deficiency) is an autosomal recessive metabolic disorder in which there is absent or
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Determination Differentiation. determinated precursor specialized cell
 Biology of Cancer -Developmental Biology: Determination and Differentiation -Cell Cycle Regulation -Tumor genes: Proto-Oncogenes, Tumor supressor genes -Tumor-Progression -Example for Tumor-Progression:
Biology of Cancer -Developmental Biology: Determination and Differentiation -Cell Cycle Regulation -Tumor genes: Proto-Oncogenes, Tumor supressor genes -Tumor-Progression -Example for Tumor-Progression:
Cell Communication. Chapter 11. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 11 Cell Communication PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
7/6/2015. Cancer Related Deaths: United States. Management of NSCLC TODAY. Emerging mutations as predictive biomarkers in lung cancer: Overview
 Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Mechanisms of Hormone Action
 Mechanisms of Hormone Action General principles: 1. Signals act over different ranges. 2. Signals have different chemical natures. 3. The same signal can induce a different response in different cells.
Mechanisms of Hormone Action General principles: 1. Signals act over different ranges. 2. Signals have different chemical natures. 3. The same signal can induce a different response in different cells.
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San
 Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
Membrane associated receptor transfers the information. Second messengers relay information
 Membrane associated receptor transfers the information Most signals are polar and large Few of the signals are nonpolar Receptors are intrinsic membrane proteins Extracellular and intracellular domains
Membrane associated receptor transfers the information Most signals are polar and large Few of the signals are nonpolar Receptors are intrinsic membrane proteins Extracellular and intracellular domains
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Vets 111/Biov 111 Cell Signalling-2. Secondary messengers the cyclic AMP intracellular signalling system
 Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
The PI3K/AKT axis. Dr. Lucio Crinò Medical Oncology Division Azienda Ospedaliera-Perugia. Introduction
 The PI3K/AKT axis Dr. Lucio Crinò Medical Oncology Division Azienda Ospedaliera-Perugia Introduction Phosphoinositide 3-kinase (PI3K) pathway are a family of lipid kinases discovered in 1980s. They have
The PI3K/AKT axis Dr. Lucio Crinò Medical Oncology Division Azienda Ospedaliera-Perugia Introduction Phosphoinositide 3-kinase (PI3K) pathway are a family of lipid kinases discovered in 1980s. They have
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Karyotype analysis reveals transloction of chromosome 22 to 9 in CML chronic myelogenous leukemia has fusion protein Bcr-Abl
 Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
Cellular Physiology (PHSI3009) Contents:
 Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
Cellular Physiology (PHSI3009) Contents: Cell membranes and communication 2 nd messenger systems G-coupled protein signalling Calcium signalling Small G-protein signalling o RAS o MAPK o PI3K RHO GTPases
MECHANISMS UNDERLYING DISTINCT EGFR VERSUS FGFR-3 AND -1 DEPENDENCY IN HUMAN BLADDER CANCER CELLS
 Texas Medical Center Library DigitalCommons@TMC UT GSBS Dissertations and Theses (Open Access) Graduate School of Biomedical Sciences 5-2014 MECHANISMS UNDERLYING DISTINCT EGFR VERSUS FGFR-3 AND -1 DEPENDENCY
Texas Medical Center Library DigitalCommons@TMC UT GSBS Dissertations and Theses (Open Access) Graduate School of Biomedical Sciences 5-2014 MECHANISMS UNDERLYING DISTINCT EGFR VERSUS FGFR-3 AND -1 DEPENDENCY
Diabetes Mellitus and Breast Cancer
 Masur K, Thévenod F, Zänker KS (eds): Diabetes and Cancer. Epidemiological Evidence and Molecular Links. Front Diabetes. Basel, Karger, 2008, vol 19, pp 97 113 Diabetes Mellitus and Breast Cancer Ido Wolf
Masur K, Thévenod F, Zänker KS (eds): Diabetes and Cancer. Epidemiological Evidence and Molecular Links. Front Diabetes. Basel, Karger, 2008, vol 19, pp 97 113 Diabetes Mellitus and Breast Cancer Ido Wolf
Regulation of Enzymatic Activity. Lesson 4
 Regulation of Enzymatic Activity Lesson 4 Regulation of Enzymatic Activity no real regulation: - regulation of enzyme expression and turnover - control of enzyme trafficking - supply of cofactors real
Regulation of Enzymatic Activity Lesson 4 Regulation of Enzymatic Activity no real regulation: - regulation of enzyme expression and turnover - control of enzyme trafficking - supply of cofactors real
Cancer genetics
 Cancer genetics General information about tumorogenesis. Cancer induced by viruses. The role of somatic mutations in cancer production. Oncogenes and Tumor Suppressor Genes (TSG). Hereditary cancer. 1
Cancer genetics General information about tumorogenesis. Cancer induced by viruses. The role of somatic mutations in cancer production. Oncogenes and Tumor Suppressor Genes (TSG). Hereditary cancer. 1
2013 W. H. Freeman and Company. 12 Signal Transduction
 2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
Cell Communication. Chapter 11. PowerPoint Lectures for Biology, Seventh Edition. Lectures by Chris Romero. Neil Campbell and Jane Reece
 Chapter 11 Cell Communication PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Overview: The Cellular Internet Cell-to-cell communication Is absolutely
Chapter 11 Cell Communication PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Overview: The Cellular Internet Cell-to-cell communication Is absolutely
G-Protein Coupled Receptors GPCRs. GPCRs
 2 type of ligands 1 G-Protein Coupled Receptors GPCRs One of the largest protein families: > 1000 type of GPCRs in mammals >3% of the human genes Major drug targets: ~ 60 % of approved drugs interact with
2 type of ligands 1 G-Protein Coupled Receptors GPCRs One of the largest protein families: > 1000 type of GPCRs in mammals >3% of the human genes Major drug targets: ~ 60 % of approved drugs interact with
G-Protein-Coupled Receptors
 Cellular Signalling Cells must be ready to respond to essential signals in their environment. These are often chemicals in the extracellular fluid (ECF) from distant locations in a multicellular organism
Cellular Signalling Cells must be ready to respond to essential signals in their environment. These are often chemicals in the extracellular fluid (ECF) from distant locations in a multicellular organism
Chapter 6: Cancer Pathways. Other Pathways. Cancer Pathways
 Chapter 6: Cancer Pathways Limited number of pathways control proliferation and differentiation Transmit signals from growth factors, hormones, cell-to-cell communications/interactions Pathways turn into
Chapter 6: Cancer Pathways Limited number of pathways control proliferation and differentiation Transmit signals from growth factors, hormones, cell-to-cell communications/interactions Pathways turn into
Mechanism and Therapeutic Potential of Statin-Mediated Inhibition of Tyrosine Kinase Receptors. Tong Tong Zhao
 Mechanism and Therapeutic Potential of Statin-Mediated Inhibition of Tyrosine Kinase Receptors Tong Tong Zhao Thesis submitted to the Faculty of Graduate and Postdoctoral Studies In partial fulfillment
Mechanism and Therapeutic Potential of Statin-Mediated Inhibition of Tyrosine Kinase Receptors Tong Tong Zhao Thesis submitted to the Faculty of Graduate and Postdoctoral Studies In partial fulfillment
Protein kinases a CRASH course. Michael Freissmuth Institute of Pharmacology
 Protein kinases a CRASH course Michael Freissmuth Institute of Pharmacology Protein kinases Outline: Kinome - multitude of kinases (why, what for?) Specificity Regulation Significance for drug development
Protein kinases a CRASH course Michael Freissmuth Institute of Pharmacology Protein kinases Outline: Kinome - multitude of kinases (why, what for?) Specificity Regulation Significance for drug development
