Massimo Cristofanilli*, Chusilp Charnsangavej and Gabriel N. Hortobagyi*
|
|
|
- Irma Barton
- 5 years ago
- Views:
Transcription
1 ANGIOGENESIS MODULATION IN CANCER RESEARCH: NOVEL CLINICAL APPROACHES Massimo Cristofanilli*, Chusilp Charnsangavej and Gabriel N. Hortobagyi* Angiogenesis the formation of new blood vessels is essential for tumour progression and metastasis. Consequently, the modulation of tumour angiogenesis using novel agents has become a highly active area of investigation in cancer research, from the bench to the clinic. However, the great therapeutic potential of these agents has yet to be realized, which could, in part, be because the traditional strategies that are used in clinical trials for anticancer therapies are not appropriate for assessing the efficacy of agents that modulate angiogenesis. Here, we discuss methods for monitoring the biological activity of angiogenic modulators, and innovative approaches to trial design that might facilitate the integration of these agents into anticancer therapy. *Department of Breast Medical Oncology and Department of Diagnostic Radiology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, Texas 77030, USA. Correspondence to M.C. mcristof@mdanderson.org doi: /nrd819 Angiogenesis the development of new blood vessels from pre-existing vasculature takes place primarily during embryonic development, but also occurs in adults in a highly regulated manner during physiological processes such as wound healing, ovulation and menstruation 1. The prominent role of angiogenesis in cancer biology was first described by Folkman 2, who postulated and described the phenomenon of tumour dormancy in the absence of neovascularization. It is now recognized that angiogenesis is not only essential for tumour growth, but is also implicated in the initial progression from a pre-malignant tumour to an invasive cancer, and in the growth of dormant micrometastases into clinically detectable metastatic lesions 3. In the past decade, much angiogenesis research has focused on the clinical applications of this knowledge, from methodologies aimed at assessing and quantifying angiogenesis 4 through to the development of novel agents to modulate angiogenesis. More than 30 agents have entered clinical trials in cancer patients, but so far no therapy based on angiogenic modulation has shown sufficient clinical benefit to be approved for such an indication. So, have we developed the necessary expertise for the design of appropriate clinical trials? Are we aware of the limitations in our capacity to understand and define treatment efficacy and clinical benefits? This review focuses on approaches that are aimed at exploiting the potential of angiogenic modulation in the treatment of cancer. Overview of tumour angiogenesis In order for a tumour to grow beyond a certain size (~2 mm 3 ), it must develop a network of blood vessels to supply nutrients and oxygen and to remove waste products (for recent reviews, see REFS 1,5,6). Angiogenesis is complex in both physiological and pathophysiological processes, and is regulated through the production of several pro-angiogenic and anti-angiogenic factors. Normally, inhibitory factors, such as endostatin, predominate, but various signals can tip the finely tuned balance in favour of angiogenesis the angiogenic switch 7,8. Angiogenic factors in particular, basic fibroblast growth factor (bfgf) and vascular endothelial growth factor 9 (VEGF) activate endothelial cells, which leads to the secretion and activation of matrix metalloproteinases 10 (MMPs) and plasminogen activators (FIG. 1). This results in the degradation of the basement membrane, which allows the endothelial cells to invade the surrounding matrix. As these cells migrate, NATURE REVIEWS DRUG DISCOVERY VOLUME 1 JUNE
2 Figure 1 Simplified overview of some key steps in tumour angiogenesis. Tumour cells release pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), which diffuse into nearby tissues and bind to receptors on the endothelial cells of pre-existing blood vessels, leading to their activation. Such interactions between endothelial cells and tumour cells lead to the secretion and activation of various proteolytic enzymes, such as matrix metalloproteinases (MMPs), which degrade the basement membrane and the extracellular matrix. Degradation allows activated endothelial cells which are stimulated to proliferate by growth factors to migrate towards the tumour. Integrin molecules, such as α v -integrin, help to pull the sprouting new blood vessel forward. The endothelial cells deposit a new basement membrane and secrete growth factors, such as platelet-derived growth factor (PDGF), which attract supporting cells to stabilize the new vessel. PDGFR, PDGF receptor; VEGFR, VEGF receptor. ANGIOPOIETINS Angiopoietins are a novel family of proteins that specifically recognize and bind to the endothelial-cell-specific Tie2- receptor tyrosine kinase, and have been shown to be crucially involved in establishing the mature vascular network. they proliferate stimulated by growth factors such as bfgf and VEGF and eventually differentiate to form a new, lumen-containing vessel. Finally, the endothelial cells deposit a new basement membrane and secrete growth factors, such as platelet-derived growth factor (PDGF), which attract supporting cells to stabilize the new vessel 1. Several other factors, such as ANGIOPOIETINS, are also involved in this part of the process, which is less defined than the earlier steps and is often incomplete. Expanded knowledge of tumour angiogenesis has spurred the development of a significant number of novel agents with defined molecular targets These agents regulate angiogenesis at various steps of the cascade, and this translates to modulation of the process and inhibition of tumour growth. On the basis of their proposed mechanisms of action, angiogenic modulators can be subdivided into several categories: modulators of proteolytic enzymes 10 12,27 ; inhibitors of endothelial-cell proliferation and/or survival ; upstream modulators ; and undefined (TABLE 1). Many of these agents are now in clinical trials (TABLE 2). Problems with traditional trial design The usual clinical development of a cytotoxic chemotherapeutic agent involves Phase I, II and III trials, and is based on the following concepts: first, that the agent is associated with dose-dependent toxicity; second, that there is an upper limit for dose escalation, which is defined as the dose-limiting toxicity (DLT); third, that the maximum-tolerated dose (MTD) has a higher probability of shrinking tumours (defined as objective remission) and improving palliation of symptoms; and finally, that the agent or combination regimens that are associated with tumour shrinkage might prolong survival. 416 JUNE 2002 VOLUME 1
3 Table 1 Summary of categories of angiogenic modulator Target Class of agent Selected drugs Blocking matrix breakdown MMPs MMP inhibitors BMS , COL-3, Neovastat UPA system Urokinase/plasmin inhibitors WX-UK1, WK293 Inhibiting endothelial-cell proliferation and/or endothelial-cell survival VEGF signalling rhumab-vegf Avastin Ribozyme targeting the mrna for VEGF Angiozyme Small-molecule RTK inhibitors ZD6474, SU6668, NM3 PDGF signalling Small-molecule RTK inhibitor Gleevec, SU6668 Plasminogen, collagen XVIII, Endogenous inhibitors Endostatin, angiostatin, 2ME2 others Aminopeptidase N and others Antivascular agents ZD6126, combrestatin, NGR peptides Integrins rhumab-α v Vitaxin Upstream modulators HER2/NEU signalling Monoclonal antibody to HER2 Trastuzumab EGF signalling Monoclonal antibody to EGFR Cetuximab, ABX-EGF Small-molecule RTK inhibitors ZD1839, OSI774 RAS FTIs SCH66336, R15777 Non-specific or unknown mechanism of action Calcium influx Calcium-channel inhibitor Carboxyamidotriazole COX-2 COX-2 inhibitor Celecoxib p53 TP53-based gene therapy INGN-201, ONYX-15 Hypoxic cells Hypoxia agents Tirapazamine, AQ4N NF-κB Proteasome inhibitors PS-341 Unknown Thalidomide COX-2, cycloxygenase-2; EGF, epidermal growth factor; EGFR, EGF receptor; FTI, farnesyl-transferase inhibitor; HER2/NEU, avian erythroblastic leukaemia viral oncogene homologue 2; 2ME2; 2-methoxyestradiol; MMP, matrix metalloproteinase; mrna, messenger RNA; NF-κB, nuclear factor-κb; PDGF, platelet-derived growth factor; rhumab-α v, humanized monoclonal antibodies that target α v -integrin; rhumab-vegf, humanized monoclonal antibodies that target VEGF; RTK, receptor tyrosine kinase; UPA, urokinase plasminogen activator; VEGF, vascular endothelial growth factor. By contrast, in early Phase I/II trials, angiogenic modulators have shown modest toxic effects and are mainly cytostatic, slowing or stopping the tumour growth and the development of metastases without producing an objective remission (FIG. 2).So,traditional end points for Phase I and II studies, represented by DLT and MTD as well as measurement of objective response, might not be adequate end points for angiogenic modulators. In particular, there is increasing concern that by using approaches that are based on traditional end points, potentially interesting angiogenic modulators might be rejected prematurely: inducing a stable tumour is a clinically useful outcome, especially if the patient is asymptomatic and the stability is prolonged and associated with minimal drug-related toxicity. Consequently, attention has been directed recently towards correlative studies for the establishment of surrogate biomarkers that might serve as tools for the in vivo assessment of the biological activity of angiogenic modulators. Furthermore, the use of imaging technologies has been extensively incorporated into the design of clinical trials for the early evaluation of these novel agents. So far, several angiogenic modulators have been tested in Phase III trials, most of which have been MMP inhibitors (MMPIs). However, these studies, which were carried out mainly in advanced stages of nonsmall-cell lung cancer (NSCLC), small-cell lung cancer (SCLC) and prostate and ovarian cancers,have been extremely disappointing. Possible causes of the prominent failures of several MMPIs are discussed in BOX 1. It is clear that the early phases of clinical development of angiogenic modulators are vital for realizing the therapeutic potential of this class of agent. Methods are needed to enable early identification of the most promising agents from the many that have anti-angiogenic activity in preclinical studies. Novel approaches are needed to ensure that potentially useful agents are not rejected owing to apparent lack of efficacy, and to facilitate the optimal design of large-scale Phase III trials. In the remainder of this review, we first describe the role of imaging technologies in assessing the biological activity of angiogenic modulators, and then discuss Phase I and Phase II trials for such agents, drawing on experiences in the clinic so far. Imaging studies and tumour angiogenesis The possibility of non-invasive monitoring of tumour response has led to growing interest in the use of imaging techniques in trials of angiogenic modulators. Imaging techniques have been used to monitor the NATURE REVIEWS DRUG DISCOVERY VOLUME 1 JUNE
4 MICROVASCULATURE A network of vessels that have diameters of less than 100 µm, which are beyond the resolution of conventional angiography. effects of cancer therapy in clinical practice for many years, and changes in tumour size have correlated well with clinical outcome. However, angiogenic modulators might not produce the cytoreductive effects that are achieved with conventional chemotherapy or radiation therapy. Therefore, monitoring changes in tumour size might not be appropriate, and new imaging techniques might be needed to monitor the effects of anti-angiogenic therapy. In spite of the regulated nature of the angiogenic process, tumour microcirculation differs profoundly from that of normal organs in three ways: first, the flow characteristics, and sometimes the blood volume, of the MICROVASCULATURE; second, the microvascular permeability; and third, the increased fractional volume of the extravascular extracellular space 28. The network of blood vessels in many solid tumours has been shown to differ markedly from normal hierarchical branching patterns and to contain gaps in which tumour cells lack close contact with perfusing vessels, which ultimately leads to altered permeability. It seems that VEGF is a major regulator of vascular permeability, and this process is mediated through activation of endothelialcell signalling pathways, such as phosphatidylinositol 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) pathways 29. This phenomenon has been Table 2 Selected angiogenic modulators in clinical trials* Drug Sponsor Trials Mechanism Drugs that block matrix breakdown COL-3 Collagenex Phase I/II brain cancer, Kaposi s sarcoma MMP inihibitor Neovastat Aeterna Phase II multiple myeloma; Phase III renal-cell (kidney) cancer, MMP inhibitor non-small-cell lung cancer BMS Bristol-Myers Phase I/II Kaposi s sarcoma; Phase II/III advanced or metastatic MMP inhibitor Squibb non-small-cell lung cancer Drugs that target endothelial-cell proliferation and/or endothelial-cell survival SU6668 SUGEN Phase I advanced tumours Blocks VEGF, FGF and PDGF receptor signalling Interferon-α Commercially Phase II/III (search NCI trials database for listings using Inhibition of bfgf and VEGF available cytokine therapy rather than anti-angiogenesis therapy ) production Anti-VEGF NCI Phase II metastatic renal-cell cancer, non-hodgkin s lymphoma, Monoclonal antibody to VEGF antibody Genentech metastatic prostate cancer, inflammatory breast cancer, advanced or recurrent cervical cancer, non-small-cell lung cancer; Phase II with chemotherapy in untreated advanced colorectal cancer, metastatic breast cancer; Phase II/III advanced non-small-cell lung cancer; Phase III metastatic breast cancer; Phase III with chemotherapy in untreated metastatic breast cancer Angiozyme Ribozyme Phase II solid tumours Disruption of VEGF signalling Pharmaceuticals Endostatin EntreMed Phase I/II malignant metastatic melanoma; Phase II neuroendocrine Endogenous inhibitor of angiogenesis tumours Squalamine Genera Phase I advanced cancers; Phase II non-small-cell lung cancer, Inhibits the sodium hydrogen Pharmaceuticals ovarian cancer, brain cancer exchanger NHE3 2ME2 EntreMed Phase I solid-tumour studies Inhibition of endothelial cells Medi-522 MedImmune Phase I/II refractory advanced colorectal cancer Antibody that blocks integrins on the (Vitaxin II) surface of endothelial cells EMD Merck & Phase I AIDS-related Kaposi s sarcoma, Phase I/II progressive Small-molecule blocker of integrins Company or recurrent anaplastic glioma on the surface of endothelial cells Drugs with non-specific or undefined mechanism of action CAI NCI Phase I in combination against solid tumours; Phase II ovarian cancer, Inhibitor of calcium influx metastatic renal-cell cancer Celecoxib Pharmacia Phase I prostate cancer; Phase I/II cervical cancer; Phase II basal- COX-2 inhibitor Corporation cell cancer, metastatic breast cancer Interleukin-12 Genetics Phase I/II Kaposi s sarcoma Upregulation of interferon-γ and IP-10 IM862 Cytran Phase II untreated metastatic cancers of the colon and rectum, Unknown mechanism ovarian cancer Thalidomide Celgene Phase I malignant glioma; Phase I/II advanced melanoma; Phase II Unknown ovarian cancer, metastatic prostate cancer, liver cancer, metastatic melanoma, CLL, multiple myeloma; Phase II with chemotherapy against solid tumours, refractory ovarian cancer; Phase III non-smallcell lung cancer, non-metastatic prostate cancer, refractory multiple myeloma, renal cancer *Adapted from information on the National Cancer Institute (NCI) web site. Naturally occuring. Commercially available; approved for leprosy. Extract from dogfish shark liver. bfgf, basic FGF; CAI, carboxyamidotriazole; CLL, chronic lymphocytic leukaemia; COX-2, cyclooxygenase-2; FGF, fibroblast growth factor; IP-10, interferon-γ-inducible protein; 2ME2, 2-methoxyoestradiol; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor. 418 JUNE 2002 VOLUME 1
5 Phase Cytotoxic agent Angiogenic modulator I DLT, MTD Optimal biological dose End point II Objective remission Biological activity III Survival benefit Figure 2 Representation of the clinical drug development process. The suggested differences in end points between studies that are targeted at cytotoxic agents compared with studies to test angiogenic modulators are depicted. DLT, dose-limiting toxicity; MTD, maximum-tolerated dose. MAGNETIC RESONANCE IMAGING The use of radio waves in the presence of a magnetic field to extract information from certain atomic nuclei (most commonly hydrogen; for example, in water). Tissues can be differentiated by differences in their water densities. Tumours can be traced, as tumour tissue has a different water density from surrounding healthy tissue. CONTRAST AGENTS Contrast agents enhance the differences between normal and abnormal tissue in imaging studies. shown in various solid tumours to lead to blood flow that is spatially and temporally more heterogeneous than the efficient, uniform perfusion of normal organs and tissues, leading to a compromised metabolic microenvironment. It has been shown that both ph and O 2 partial pressure (po 2 ) decrease with increasing distance from the tumour vessels, which results in acidic and hypoxic conditions 30. In fact, one of the characteristic changes in the microenvironment that occurs in solid tumours is a reduction in oxygenation. Low tumour oxygen levels have been associated with increased tumour growth and metastatic potential 31. Tumour vascularity can be characterized by several physiological parameters, including tumour blood flow, tumour blood volume, tumour po 2 (REF. 32), tumour interstitial pressure 33, vascular permeability and mean transit time 34. These parameters, which are based on mathematical models that are used to calculate the distribution of contrast materials or tracers over time, could be suitable for monitoring the effects of anti-angiogenic therapy. In this section, we discuss imaging techniques that have been investigated in experimental studies of angiogenic activity, focusing on the techniques that are now available for use in clinical trials. Magnetic resonance imaging. MAGNETIC RESONANCE IMAGING (MRI) has been studied extensively and found to correlate more directly with tumour angiogenesis than do other imaging techniques 35,36. MRI techniques for quantification of angiogenesis can be classified into intrinsic and contrast-enhanced methods. The intrinsic contrast technique uses pulse sequences that are sensitive to water-molecule motion or to the distortion of magneticfield homogeneity by deoxyhaemoglobin. The latter technique, which is known as blood-oxygen-leveldependent (BOLD) contrast 37,38, has been tested in various animal models, but has limited use in clinical trials because of its low contrast-to-noise ratio. Microcirculation is defined as having three compartments: the microvasculature; the interstitium or extravascular extracellular space; and the intracellular space 39,40. Much of the success of MRI so far can be attributed to its ability to provide high-resolution images of tumours that depict perfusion and permeability of the smallest vessels, especially in the capillary network. Because the MRI CONTRAST AGENTS that have been used so far in dynamic contrast-enhanced magnetic resonance are not taken into cells, only the microvasculature and the extravascular extracellular space are usually studied by this methodology. Two types of intravenous MRI contrast agent have been investigated: small-molecule gadolinium chelates and macromolecular contrast agents 35,41.The small-molecule contrast media are distributed into the vascular space and rapidly enter the extravascular and extracellular spaces. Several pulse sequences and mathematical models are available to calculate various physiological parameters, including tissue perfusion, vascular volume fraction and permeability fraction. These parameters have been tested extensively and compared with other standard approaches for the assessment of tumour angiogenesis, such as vessel count. Studies of breast cancer, cervical cancer and brain tumours have shown that enhancement on MRI correlates well with the degree of angiogenesis that is detected using histopathology 42,43, but the results have not been uniform 44,45, and so could not be used to validate this technique. Macromolecular contrast agents, such as albumin Gd DTPA, have been used to show that vascular permeability and vascular volume fraction correlate with vessel count in animal models 38. A study of a mammary tumour model in athymic rats showed a 98% decrease in vascular permeability within 24 hours after a single dose of anti-vegf antibody, and a 71% decrease after a 3-dose, 7-day course of treatment compared with results in the control group 46,36. However, macromolecular contrast agents are not widely available in clinical practice. A few clinical studies have proposed contrastenhanced MRI as a possible imaging method for monitoring therapeutic interventions with potential anti-angiogenic mechanisms. For example, in a Phase II clinical trial of androgen-deprivation treatment in prostate cancer 45, a significant decrease in the permeability surface-area product of the tumours after treatment was shown, and the change correlated well with a decrease in the serum prostate-specific-antigen level. NATURE REVIEWS DRUG DISCOVERY VOLUME 1 JUNE
6 Box 1 Experiences with matrix-metalloproteinase inhibitors Matrix-metalloproteinase inhibitors (MMPIs) have been tested in Phase III trials in several solid malignancies. Most of these studies did not show any survival benefit, and MMPIs were generally associated with increased toxicity. Several reasons have been put forward to explain this failure 82,83, the most important being the early initiation of advanced testing (Phase III) without the appropriate safety and efficacy indications from Phase I/II trials. The significant side-effects (mainly musculoskeletal pain) that have been associated with several of these agents might have led to patient non-compliance in trials. Further explanations include the choice of an inappropriate model (advanced-stage refractory diseases) in spite of preclinical testing in animal models that had indicated an advantage at an early stage of disease 84. ULTRASONOGRAPHY The use of sound waves above the audible frequency to detect and characterize tumours. Echoes that are reflected off normal and abnormal tissues are captured by a computer to create two-dimensional images. SINGLE-PHOTON-EMISSION COMPUTED TOMOGRAPHY The detection and quantification of γ-emitting radionuclides, such as 99m Tc, 111 In, 123 I or 125 I. POSITRON-EMISSION TOMOGRAPHY An imaging technique that is used to detect decaying nuclides, such as 15 O, 13 N, 11 C, 18 F, 124 I and 94m Tc. COMPUTED TOMOGRAPHY A technique that exploits the differences in absorption of X- rays by different tissues to give high-contrast images of anatomical structures. Computed tomography has relatively poor soft-tissue contrast, so iodinated contrast agents, which perfuse different tissue types at different rates, are commonly used to delineate tumours. Ultrasonography. Recent technical advances in ULTRA- SONOGRAPHY using a high-frequency probe, harmonic imaging and intravenous contrast agents make this technique attractive for assessing angiogenic activity and monitoring anti-angiogenic therapy 47,48. Most ultrasonography contrast agents are formulations of encapsulated fluorocarbon gas that forms microbubbles that are ~2 3 µm in diameter. After they are intravenously injected, these microbubbles are distributed in the arteries, pass through the capillaries and remain in the blood pool. They produce strong ultrasonography signals mainly in the arterial system, particularly in the colour Doppler mode. Using appropriate techniques, such as pulse-inversion imaging or intermittent imaging after transient disruption of microbubbles, tumour blood flow and blood volume can be assessed. Most of the clinical applications of contrastenhanced colour Doppler ultrasonography are found in breast and prostate-gland studies, and they have been used to characterize benign and malignant tumours 49,50. So far, ultrasonography has not been used to monitor anti-angiogenic therapy in clinical trials, probably because the technique has limited imaging resolution and is operator dependent. However, new techniques such as harmonic imaging and high-frequency ultrasonography might be useful for examining experimental animal tumour models 51,52 and, in future, in the clinic. Recently, Donnelly et al. 51 showed that ultrasonography could reveal decreased tumour blood flow after radiation, or after radiation and administration of tumour-necrosis factor-α (TNF-α) adenovirus vector, in a small-animal tumour model. Nuclear medicine studies. Many radionuclide tracers have been used to study tissue blood flow and capillary permeability on SINGLE-PHOTON-EMISSION COMPUTED TOMOGRAPHY (SPECT) or POSITRON-EMISSION TOMOGRAPHY (PET) images. These include 15 O-labelled water, Technetium-99m sestamibi ( 94m Tc), Tallium-201 for measuring tumour blood flow, 15 O-labelled carbon monoxide and 94m Tc-labelled red blood cells for monitoring blood volume 53. New tracers are being developed to target specific receptors that are associated with angiogenesis 54. In addition, 18 F-fluorodeoxyglucose has been well characterized in monitoring glucose metabolism in tumours, and is one of the most sensitive methods for tracking treatment effects. However, there is a lack of clinical data to recommend the routine use of this technique for monitoring anti-angiogenic therapy. Computed tomography. The distribution of contrast material after intravenous injection during COMPUTED TOMOGRAPHY (CT) examination has been extensively investigated since the early 1980s. The model for measuring blood flow to the organs has been in clinical use, but has not gained wide acceptance because of the lack of proper clinical application. Recent interest in tumour angiogenesis, and in the development of new software that makes quantification simpler, has encouraged renewed interest in this application 55,56. Only a few mathematical models are used at present, including the Fick principle and the deconvolution method 57. The technique requires consecutive data acquisition over 30 to 50 seconds with a temporal resolution of 1 second or shorter, or every 3 5 seconds after contrast injection, depending on the model. The image data are then calculated for tissue blood flow, blood volume, mean transit time and permeability surface-area product using arteries as the arterial input. Reproducibility of cerebral-blood-flow and of cerebral-bloodvolume measurements obtained with CT in rabbit brains have been reported to be 13% and 7%, respectively, but the value of the permeability-surface-area product has not been well validated 57. At present, a functional CT technique has been incorporated into a few clinical trials at our institution. A decrease in blood flow and blood volume was observed in patients who were treated in a Phase II clinical trial of thalidomide for metastatic renal-cell carcinoma (FIG. 3). Phase I: focus on biological end points Phase I or dose-finding trials have traditionally been developed to define the highest safe dose of the agent to be used in further drug testing (typically Phase II and III trials). Novel biological agents, including angiogenic modulators, seem to be non-toxic at doses that achieve biologically effective concentrations. The pharmacodynamic as well as biological activity and eventually the clinical benefit might depend on the schedule of administration. Consequently, a doseescalation trial that incorporates a specific biological end point for the agent, in addition to and not replacing that of toxicity, might be appropriate. Even so, such a study to define the optimal biological dose would probably require more patients than are typically enrolled in a Phase I study. Several biological end points might be considered in the evaluation of the activity of angiogenic modulators. These include, but are not limited to, the various 420 JUNE 2002 VOLUME 1
7 a b Figure 3 Imaging studies to monitor tumour angiogenesis. Blood-flow maps a before treatment and b six months after treatment of a patient with metastatic renal-cell carcinoma with thalidomide. The blood flow decreased from 127 ml min g 1 to 14 ml min g 1 of tissue. The arrows in the figure identify the area of the tumour. The colour coding indicates the range of blood flow: red yellow, high blood flow; blue, low blood flow. steps that are involved in the inhibition of endothelial-cell proliferation. Endothelial cells are interesting models to evaluate because they represent the main cell target of angiogenic modulators and because variations in endothelial-cell apoptosis (ECA) 58, alterations in endothelial-cell signalling 59 and variations in the number of circulating endothelial progenitor cells (cepcs; mainly mononucleated cells) 60 might represent the most appropriate surrogate biomarkers of activity of angiogenesis modulators. However, these approaches, although scientifically attractive, are associated with several problems, indicating that the evaluation of endothelial-cell biology is far from the ideal biomarker for clinical testing. In addition to issues related to reproducibility and validation, it is obvious that the need to repeatedly approach the tumour with an invasive and potentially dangerous procedure might be limited by accessibility of the target lesion and ethical issues. One of the proposed, less invasive approaches to the development of surrogate biomarkers is the measurement of circulating levels of angiogenic factors, such as serum VEGF, bfgf and PDGF 61. Elevated serum levels of VEGF have been associated with prognostic significance in most solid malignancies, and have been used, but not validated, as predictors of recurrence and markers of response in several studies 61. Existing data with respect to other soluble factors, such as bfgf, are limited and less conclusive. Several Phase I studies that tested different classes of angiogenic modulator have been completed, and a brief summary of some of the most interesting trials is reported in some detail to better delineate the problems that are associated with the testing of these agents. Semaxanib. Semaxanib (SU5416) is a small-molecule tyrosine-kinase inhibitor that targets the VEGF receptor FLK1 on endothelial cells, inhibiting VEGF-mediated FLK1 signalling and the proliferation of endothelial cells in vitro 62. In vivo studies of SU5416, in which various tumour cell lines were implanted subcutaneously into immunocompromised mice, showed that SU5416 significantly suppresses tumour growth against a broad spectrum of tumour types in which growth is driven by various growth factors, such as PDGF, the epidermal-growth-factor receptor (EGFR) and HER2 (REF. 63). SU5416 has been studied in seven Phase I or Phase I/II clinical trials: six involving the therapy of patients with advanced malignancies and one for AIDS-related Kaposi s sarcoma. In the first clinical protocol, SU5416 was administered twice weekly for four weeks followed by an optional two-week rest 64. The three patients who were treated at the highest administered dose of 190 mg m 2 of body surface area experienced DLT within 3 6 hours after the first dose. DLTs consisted of headache, nausea and vomiting, which were reversible within hours of onset without sequelae. On the basis of this trial, 145 mg m 2 given twice weekly was established to be the MTD and recommended Phase II/III dose. The significant incidence of toxicity and the lack of targeted improvement in clinical benefit over standard cytotoxic treatment in a Phase III trial in advanced colon cancer led to a recent disappointing withdrawal of this agent from clinical development. Avastin. Another angiogenic modulator that targets VEGF recombinant human anti-vegf (Avastin) seems to show more promising results. Twenty-five patients with refractory solid malignancies were initially treated in a single-agent Phase I trial with escalating doses of recombinant human anti-vegf (rhumab-vegf) 65. The doses ranged from 0.1 to 10 mg kg 1 of body weight, and were given over a 90-minute infusion that was NATURE REVIEWS DRUG DISCOVERY VOLUME 1 JUNE
8 Box 2 Optimal Phase II testing The issue of a single-arm Phase II trial for evaluating the efficacy of new therapies on the basis of a historical comparison as opposed to randomized design was introduced in 1974 by Gehan and Freireich 85. Their arguments in favour of a historical-control comparison include, but are not limited to, the possibility of offering to patients a promising novel treatment without the ethical limitations that are imposed by the randomization process, and with an obvious substantial saving of resources. Furthermore, the reproducibility of the results could be guaranteed by carefully selecting the patients who are enrolled for clinical characteristics and tumour type. This selection process would allow a more uniform group to be tested and should allow reasonable comparability with a historical group of patients. In 1989, Simon 86 attempted to better define the issue of optimal Phase II testing by introducing the concept of two-stage design to minimize the sample size in case of lack of clinical activity (first-stage stopping). Drugs that show early evidence of objective activity will proceed to complete the second stage of the trial and eventually move into Phase III testing. GRADE III OR IV TOXICITIES For each adverse event (AE) that is associated with a specific treatment, grades are assigned and defined using a scale from 0 to V. Grade III, severe and undesirable AE; grade IV, life threatening or disabling AE. repeated on days 28, 35 and 42. There were no GRADE III OR IV TOXICITIES, although two patients had major haemorrhagic events. No objective response was observed and twelve patients experienced stable disease. Serum rhumab-vegf and free VEGF were measured, which showed that the basal serum VEGF concentrations ranged from less than 20 to 281 pg ml 1 (lower limit 20 pg ml 1 ). The free serum VEGF concentrations were reduced, and at doses of 0.3mg kg 1 were below the detectable limit of the assay, which indicates that measurement of serum VEGF could serve as a surrogate biomarker for this particular class of agent. rhumab- VEGF has been further tested in Phase I studies in combination with several cytotoxic regimens, and the results have indicated the safety of this approach for more advanced Phase II/III trials 66. BAY A further example of minimal toxicity associated with angiogenic modulators is provided by the orally available MMPI BAY , which was administered in a Phase I trial in 21 patients with refractory solid malignancies 67. Patients received daily oral doses that ranged from 100 to 1,600 mg for a 28-day course. As with Avastin, no DLT was reached, and the dose escalation was discontinued because pharmacokinetic studies showed a plateau in concentration (not a linear increase) that indicated saturable drug absorption. Serum levels of MMP2 and MMP9 were not significantly changed during the treatment, indicating that they could not serve as markers for monitoring the activity of MMPIs. Vitaxin. Vitaxin, an anti-α ν -integrin antibody, interferes with blood-vessel formation by inducing apoptosis in newly generated endothelial cells. Seventeen patients were treated weekly with increasing doses of Vitaxin for six weeks (0.1 4 mg kg 1 ) 68. The dose range was selected on the basis of in vitro concentration data, which showed the concentration of drug that was required to saturate the α ν receptor and to block endothelial-cell migration.. Escalation beyond 4 mg per kg per week was limited by drug availability. Once again, the treatment was well tolerated, with little or no toxicity overall. A total of fourteen patients were evaluable for response: one patient had a partial response (at the lower dose level) and seven patients achieved disease stability. These results are encouraging, and support the α ν receptor as a potentially relevant target for anti-angiogenic therapy. Endostatin. Endostatin, which is the carboxy-terminal, 20,000-Da fragment of collagen XVIII, can inhibit angiogenesis and tumour growth in an endothelial-cellspecific manner 17. It is a potent inducer of endothelialcell apoptosis, and can also inhibit cell proliferation and migration, but the mechanism behind these effects is poorly understood so far. Animal studies showed that recombinant endostatin strongly inhibits the growth of various mouse and xenotransplanted human tumours 69. Three initial Phase I studies have investigated daily intravenous infusion schedules of 20 minutes (2 studies) and 1 hour (1 study) in adult humans with refractory solid tumours A standard dose-escalation scheme and pharmacokinetic studies were carried out in all investigations. The pharmacokinetic results were linear at all doses. One of these studies 73 was done at The University of Texas M. D. Anderson Cancer Center. Twenty-five patients were treated with a daily 20-min infusion of recombinant human endostatin at doses between 15 mg m 2 and 600 mg m 2. No grade III IV toxicity was observed, so no DLT or MTD could be defined. The MTD was defined by preclinical studies as described 69 and by limitations in drug supply. Patients were allowed to continue their treatment for as long as they did not develop cancer-related symptoms and/or until reaching 100% documented tumour growth. Investigators in this study also attempted to evaluate ECA and tumour-cell death in patients who presented with disease that was amenable to tissue biopsy 73. Seventeen patients from whom tumour biopsy samples were obtained both before and after treatment (56 days) were included in the analysis 74. Endostatin treatment resulted in a significant increase in apoptotic endothelial cells (5.3-fold, P = ). A 2.2-fold increase in apoptotic tumour cells was observed post treatment, although it was not statistically significant. The increases in apoptotic endothelial cells and tumour-cell death were significantly correlated (P = 0.002). Interestingly, these changes were not significantly correlated with the dose. 422 JUNE 2002 VOLUME 1
9 Box 3 Response criteria The World Health Organization (WHO) definitions for objective tumour response, which were published in the 1979 WHO Handbook, have been the most commonly used criteria around the world. In 1992, toxicity criteria, end-point definitions and response criteria 87 were more rigorously revised by the Southwest Oncology Group and other cooperative groups in cooperation with the National Cancer Institute, and these have been applied to clinical investigations over the past decade. In 1999, new guidelines for response assessment known as the RECIST criteria (Response Evaluation Criteria in Solid Tumors) were developed, essentially to solve some problems associated with the standard WHO criteria: first, to establish uniformity in the methods for recording and assessing changes in the size of measurable disease; second, to address the necessity to define a minimum lesion size as well as to introduce a definition of target lesions in comparison to non-target lesions when dealing with a more diffuse metastatic process; third, to amend the definition of progressive disease to take into consideration the target lesions and the overall tumour load; and last, to establish the role of novel imaging techniques, such as magnetic resonance imaging, in measuring the initial disease status and for assessment of response. It has been suggested that cepcs might have an important role in vascularization through vasculogenesis, as established by in vivo tumour models. Folkman s group analysed cepcs in cancer patients receiving endostatin therapy (60 mg per m 2 per day). Blood samples from healthy volunteers (n = 3) and from patients with cancer (n = 4) were collected at different time points during the treatment 75. Peripheral mononuclear cells were purified and immunostained with antivascular VEGFR2 (anti-vegfr2) antibodies. The number of vasculogenic cells identified with this technique in blood samples that were collected before the treatment were approximately 100-fold higher than those in samples from healthy volunteers ( as opposed to 3 4). Furthermore, treatment with endostatin lowered the number of VEGFR2-positive cells in three out of four patients. These results are extremely preliminary and must be validated in larger cohorts of patients, but indicate that cepcs might have a significant role in the process of metastases formation. Phase II: appropriate measures of efficacy? To design Phase II clinical trials for biological agents is a challenging task. In general, Phase II trials are singlearm studies with no internal controls; in this setting, historical experience is used to define the true response rates that are necessary to generate interest in pursuing further development of the agent (BOX 2). These estimated response rates facilitate the determination of the sample size to be investigated. Assessment of the efficacy of the agents is usually done through an accurate measurement of the shrinkage of the tumour mass defined as objective remission which represents essentially a surrogate marker of efficacy. Recently, the criteria for measurement of response have been modified, with the bidimensional criteria defined by the World Health Organization (BOX 3) which have been used for many years being replaced by the unidimensional criteria defined by the European Organisation for Research and Treatment of Cancer (EORTC) Response Evaluation Criteria in Solid Tumours Group (RECIST) 76. Single-arm Phase II trial designs are not perfectly suited for studying angiogenic modulators, possibly because there is a low probability of an objective measurable response. To overcome these methodological problems, various proposals have been made. One example is that a clinical end point that is expected to be affected by the agent that is, progression-free survival be substituted for objective response. In such a standard design, a predetermined patient population is treated, and progression-free survival is targeted as the end point. The main flaw in this design is that if the agent is totally inactive, it will take a long time (possibly 2 3 years) and unnecessary treatments for a large number of patients to show it. Another approach would be to investigate innovative statistical designs to maximize the number of patients who are treated with the angiogenic modulator that is, enrichment design in a relatively short period of time 77,78. These novel designs would attempt to study a homogeneous group of patients who are more likely to show a benefit from the treatment and concurrently to reduce the costs and delays that are associated with a traditional Phase II or Phase III approach. An example is the randomized discontinuation design, an innovative design that has been applied to other areas of investigation in medicine and that has recently been proposed for the investigation of cytostatic agents in oncology 79,80. The aim of this trial design is to select a subset of enrolled patients who are more homogeneous with respect to important prognostic factors than the group of patients that would otherwise be randomized in the trial. Such a design has been applied to the development of carboxyamidotriazole (CAI) in patients with metastatic kidney cancer. CAI is a novel oral agent with a mechanism of action that is postulated to be inhibition of calcium-mediated signal transduction 81. It is an inhibitor of receptor-gated calcium channels, and results in the inhibition of phospholipase Cγ and phospholipase A 2 phosphorylation. In vitro, this has been shown to inhibit tumour-cell motility, invasion and angiogenesis by inhibiting bfgf stimulation of endothelial-cell proliferation. The Cancer and Leukemia Group B (CALGB) is evaluating CAI at present using a randomized discontinuation design (study CLB-69901). In this trial, with an expected maximum of 335 patients accrued, CAI is administered to all enrolled patients for the first 16 weeks, and then disease response is assessed radiologically (first stage). Afterwards, patients whose disease showed no response cease to receive the treatment, NATURE REVIEWS DRUG DISCOVERY VOLUME 1 JUNE
10 whereas those with objective disease response will continue to receive treatment. The group of patients with stable disease will be randomized to receive CAI or a placebo (second stage) for a further 16 weeks. The patients who receive the placebo can resume treatment with CAI once tumour progression is shown. This design is an attempt to address the heterogeneity of kidney cancer while trying to define the biological activity of the agent. The initial analysis will determine the possibility of stopping the trial early if the risk of progression is greater than expected and declaring the agent inactive (that is, the estimated risk of progression by 16 weeks is 85% or greater, with 90% certainty). If, on the other hand, the estimated risk of progressive disease is less than expected (that is, the risk of progressive disease is 40% or less, with 90% certainty), the study would be stopped and the agent considered active. Subsequent analysis will probably focus on the most interesting group of patients and, clearly, the biology of the tumour as well as the activity of CAI will become evident by looking into the events that are detected in the placebo group. In fact, a faster-growing tumour and an active drug will amplify differences between the two groups, thereby revealing the activity of the agent. The trial represents one of the most innovative examples of statistical design that has been applied to the study of angiogenic modulators. These statistical approaches have been criticized for several reasons, including the issue of early discontinuation that is not considered advisable for these agents, but there is hope that they will provide a more flexible model for future investigations to test angiogenic modulators. It should be emphasized that these novel strategies, although conceptually interesting and well founded, are far from perfect and do not encompass the whole complexity of tumour biology. For example, the heterogeneity in clinical tumour growth could be related to various biological factors, including, but not limited to, the complex hetereogeneity in angiogenesis among tumours in different disease locations and even in the same lesion. In spite of this, it seems that this trial design, compared with a traditional statistical approach, will better serve the dual objectives of showing a possible clinical benefit as well as reducing the number of patients and resources dedicated to ineffective treatments. This objective will be even better achieved in the selected case of randomized discontinuation design being applied to the comparison of an investigational combination regimen (that is, the use of an angiogenic modulator with chemotherapy) with standard cytotoxic chemotherapy. Combination therapies offer the theoretical advantage of using the cytotoxic agents as a debulking machine, with the angiogenic modulator affecting the endothelial component of the newly proliferating microvasculature, which could theoretically greatly reduce the growth potential of the tumour. So, an early use of such combinations might potentially increase the efficacy of traditional treatments, which could translate to higher objective remissions and better long-term control. In this context, we can see the value of maintaining traditional end points of efficacy. Conclusions Crucial aspects of the complex phenomenon of tumour angiogenesis have been clarified by many investigators, which has led to the development of a significant number of novel agents with defined molecular targets. These novel treatments are usually associated with only minimal side effects, and they produce mostly cytostatic effects on disease, which suggests that patients are more likely to comply with study treatment and are able to receive prolonged treatment. These factors have also challenged the way in which we organize clinical trials, and it might be that if we do not modify our approach to clinical investigation, drug development could be affected, and there could be serious consequences for patient care and for the research community. New trial designs add a layer of complexity to an already complex task, and require close cooperation between investigators from different specialities, such as pathology, radiology and biostatistics). Essentially, a multidisciplinary approach is needed for efficacious translational investigations. It seems clear that the end points for dose-defining trials (Phase I) and efficacy trials (Phase II) should be reconsidered. We strongly recommend the extensive use of correlative studies in the early phases of drug development to establish surrogate biomarkers for use in efficacy trials. In this regard, less invasive and more easily reproducible approaches such as measurement of serum levels of circulating angiogenic factors, even if fairly promising as surrogate biomarkers still require a clear definition of methodologies and well-designed validation studies. Imaging studies could have a key role in assessing the efficacy of treatments. As described above, various imaging modalities can be selected for this purpose, and the choice of imaging study should be based on an accurate evaluation of the novel agents in preclinical models. In fact, different angiogenic modulators could have different effects on imaging parameters at different time points. For example, the anti-vegfr2 antibody has been shown to decrease vascular permeability within 24 hours of treatment 36,41. However, measurement of po 2 of the tumours showed an initial decrease in po 2 followed by an increase in po 2 a few weeks later 34. Percentage changes in functional-imaging parameters, such as tumour blood flow, blood volume and capillary permeability, might be used to monitor treatment and compare it with the immediate (that is, objective remission) or, more realistically, late effects (that is, clinical benefit, improvement in time to progression and overall survival time) of the treatment. It is hoped that the introduction of target-specific contrast agents will improve functional imaging. Finally, it needs to be pointed out that a careful selection of the clinical setting for the investigation (for example, tumour type and stage of disease) must be carried out before expensive, definitive Phase III clinical trials are initiated. This selection will increase the probability of finding the appropriate clinical indication for angiogenic modulators. 424 JUNE 2002 VOLUME 1
Backgrounder. 1. What are targeted therapies? 2. How do targeted therapies work?
 Backgrounder TARGETED THERAPIES FOR CANCER 1. What are targeted therapies? 2. How do targeted therapies work? 3. What are some of the different types of targeted therapy? 4. What are the potential benefits
Backgrounder TARGETED THERAPIES FOR CANCER 1. What are targeted therapies? 2. How do targeted therapies work? 3. What are some of the different types of targeted therapy? 4. What are the potential benefits
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL. PhD THESIS
 UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL PhD THESIS THE IMPORTANCE OF TUMOR ANGIOGENESIS IN CEREBRAL TUMOR DIAGNOSIS AND THERAPY ABSTRACT PhD COORDINATOR: Prof. univ. dr. DRICU Anica PhD
UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA PhD SCHOOL PhD THESIS THE IMPORTANCE OF TUMOR ANGIOGENESIS IN CEREBRAL TUMOR DIAGNOSIS AND THERAPY ABSTRACT PhD COORDINATOR: Prof. univ. dr. DRICU Anica PhD
DAWNING OF THE AGE OF ANGIOGENESIS
 DAWNING OF THE AGE OF ANGIOGENESIS Bob Leibowitz, M.D. DIPLOMATE AMERICAN BOARDS OF INTERNAL MEDICINE AND SUBSPECIALTIES OF MEDICAL ONCOLOGY AND HEMATOLOGY December 1997 April 2004 (Revised) Angiogenesis
DAWNING OF THE AGE OF ANGIOGENESIS Bob Leibowitz, M.D. DIPLOMATE AMERICAN BOARDS OF INTERNAL MEDICINE AND SUBSPECIALTIES OF MEDICAL ONCOLOGY AND HEMATOLOGY December 1997 April 2004 (Revised) Angiogenesis
Perfusion Physics. ICMRI2018 March 29-31, 2018 Grand Hilton Hotel, Seoul, Korea. Asian Forum Ⅱ: Perfusion MRI SY24-1.
 SY24-1 Perfusion Physics Hiroyuki Kabasawa MR Collaborations and Development, GE Healthcare, Tokyo, Japan Perfusion is referred as the blood supply to micro capillary in tissue. Perfusion parameter such
SY24-1 Perfusion Physics Hiroyuki Kabasawa MR Collaborations and Development, GE Healthcare, Tokyo, Japan Perfusion is referred as the blood supply to micro capillary in tissue. Perfusion parameter such
Functional aspects of anatomical imaging techniques
 Functional aspects of anatomical imaging techniques Nilendu Purandare Associate Professor & Consultant Radiologist Tata Memorial Centre Functional/metabolic/molecular imaging (radioisotope scanning) PET
Functional aspects of anatomical imaging techniques Nilendu Purandare Associate Professor & Consultant Radiologist Tata Memorial Centre Functional/metabolic/molecular imaging (radioisotope scanning) PET
1. The metastatic cascade. 3. Pathologic features of metastasis. 4. Therapeutic ramifications. Which malignant cells will metastasize?
 1. The metastatic cascade 3. Pathologic features of metastasis 4. Therapeutic ramifications Sir James Paget (1814-1899) British Surgeon/ Pathologist Paget s disease of Paget s disease of the nipple (intraductal
1. The metastatic cascade 3. Pathologic features of metastasis 4. Therapeutic ramifications Sir James Paget (1814-1899) British Surgeon/ Pathologist Paget s disease of Paget s disease of the nipple (intraductal
PATHOBIOLOGY OF NEOPLASIA
 PATHOBIOLOGY OF NEOPLASIA Department of Pathology Gadjah Mada University School of Medicine dr. Harijadi Blok Biomedis, 6 Maret 2009 [12] 3/17/2009 1 The pathobiology of neoplasia Normal cells Malignant
PATHOBIOLOGY OF NEOPLASIA Department of Pathology Gadjah Mada University School of Medicine dr. Harijadi Blok Biomedis, 6 Maret 2009 [12] 3/17/2009 1 The pathobiology of neoplasia Normal cells Malignant
The Angiopoietin Axis in Cancer
 Ang2 Ang1 The Angiopoietin Axis in Cancer Tie2 An Overview: The Angiopoietin Axis Plays an Essential Role in the Regulation of Tumor Angiogenesis Growth of a tumor beyond a limiting size is dependent upon
Ang2 Ang1 The Angiopoietin Axis in Cancer Tie2 An Overview: The Angiopoietin Axis Plays an Essential Role in the Regulation of Tumor Angiogenesis Growth of a tumor beyond a limiting size is dependent upon
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
Introduction to Targeted Therapy
 Introduction to Targeted Therapy Cancer remains the second leading cause of death in the United States, despite the significant advances in cancer therapy made over the past several decades. Many factors
Introduction to Targeted Therapy Cancer remains the second leading cause of death in the United States, despite the significant advances in cancer therapy made over the past several decades. Many factors
Brain Tumor Treatment
 Scan for mobile link. Brain Tumor Treatment Brain Tumors Overview A brain tumor is a group of abnormal cells that grows in or around the brain. Tumors can directly destroy healthy brain cells. They can
Scan for mobile link. Brain Tumor Treatment Brain Tumors Overview A brain tumor is a group of abnormal cells that grows in or around the brain. Tumors can directly destroy healthy brain cells. They can
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 21-23, 2013 Acknowledgment Elizabeth Eisenhauer for some slides! Learning Objectives At the end of the session the participant should
Phase II Cancer Trials: When and How Course for New Investigators August 21-23, 2013 Acknowledgment Elizabeth Eisenhauer for some slides! Learning Objectives At the end of the session the participant should
Oncolytic Viruses: Reovirus
 T S X : O N C N A S D A Q : O N C Y International Society for Biological Therapy of Cancer 2008 Oncology Biologics Development Primer Oncolytic Viruses: Reovirus REOLYSIN - mode of action REOLYSIN contains
T S X : O N C N A S D A Q : O N C Y International Society for Biological Therapy of Cancer 2008 Oncology Biologics Development Primer Oncolytic Viruses: Reovirus REOLYSIN - mode of action REOLYSIN contains
Molecular Imaging and Cancer
 Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
EGFR Antibody. Necitumumab, LY , IMC-11F8. Drug Discovery Platform: Cancer Cell Signaling
 EGFR Antibody Necitumumab, LY3012211, IMC-11F8 Derived from Yarden Y and Shilo BZ 1 ; Schneider MR and Wolf E. 2 Drug Discovery Platform: Cancer Cell Signaling A Single-Arm, Multicenter, Open-Label, Phase
EGFR Antibody Necitumumab, LY3012211, IMC-11F8 Derived from Yarden Y and Shilo BZ 1 ; Schneider MR and Wolf E. 2 Drug Discovery Platform: Cancer Cell Signaling A Single-Arm, Multicenter, Open-Label, Phase
Breast Cancer Diagnosis, Treatment and Follow-up
 Breast Cancer Diagnosis, Treatment and Follow-up What is breast cancer? Each of the body s organs, including the breast, is made up of many types of cells. Normally, healthy cells grow and divide to produce
Breast Cancer Diagnosis, Treatment and Follow-up What is breast cancer? Each of the body s organs, including the breast, is made up of many types of cells. Normally, healthy cells grow and divide to produce
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
Cancer as a disease of development; Developmental therapies: Anti- Angiogenesis; Stem cells and tissue regeneration.
 Cancer as a disease of development; Developmental therapies: Anti- Angiogenesis; Stem cells and tissue regeneration Mitesh Shrestha What is Cancer? Unrestricted cell growth: tumor cell population 1x10^9
Cancer as a disease of development; Developmental therapies: Anti- Angiogenesis; Stem cells and tissue regeneration Mitesh Shrestha What is Cancer? Unrestricted cell growth: tumor cell population 1x10^9
It s s Always Something!
 It s s Always Something! New Approaches in Brain Tumor Treatment Virginia Stark-Vance, M.D. When Something Is a Brain Tumor Brain tumors aren t rare: there are over 100,000/yr Most originate as other cancers
It s s Always Something! New Approaches in Brain Tumor Treatment Virginia Stark-Vance, M.D. When Something Is a Brain Tumor Brain tumors aren t rare: there are over 100,000/yr Most originate as other cancers
1.The metastatic cascade. 2.Pathologic features of metastasis. 3.Therapeutic ramifications
 Metastasis 1.The metastatic cascade 2.Pathologic features of metastasis 3.Therapeutic ramifications Sir James Paget (1814-1899) British Surgeon/ Pathologist Paget s disease of bone Paget s disease of the
Metastasis 1.The metastatic cascade 2.Pathologic features of metastasis 3.Therapeutic ramifications Sir James Paget (1814-1899) British Surgeon/ Pathologist Paget s disease of bone Paget s disease of the
Brain Tumors. What is a brain tumor?
 Scan for mobile link. Brain Tumors A brain tumor is a collection of abnormal cells that grows in or around the brain. It poses a risk to the healthy brain by either invading or destroying normal brain
Scan for mobile link. Brain Tumors A brain tumor is a collection of abnormal cells that grows in or around the brain. It poses a risk to the healthy brain by either invading or destroying normal brain
Phase I and Pharmacodynamic Study of High-Dose NGR-hTNF in Patients
 Phase I and Pharmacodynamic Study of High-Dose NGR-hTNF in Patients with Refractory Solid Tumors P. A. Zucali 1, A. Santoro 1, M. Simonelli 1, F. De Vincenzo 1, E. Lorenzi 1, L. Rimassa 1, L. Balzarini
Phase I and Pharmacodynamic Study of High-Dose NGR-hTNF in Patients with Refractory Solid Tumors P. A. Zucali 1, A. Santoro 1, M. Simonelli 1, F. De Vincenzo 1, E. Lorenzi 1, L. Rimassa 1, L. Balzarini
Proc. Intl. Soc. Mag. Reson. Med. 22 (2014)
 Tumor Physiology Natalie J. Serkova, PhD Department of Anesthesiology and Radiology, University of Colorado at Denver, Anschutz Medical Center, Aurora, CO This course will describe the distinct characteristics
Tumor Physiology Natalie J. Serkova, PhD Department of Anesthesiology and Radiology, University of Colorado at Denver, Anschutz Medical Center, Aurora, CO This course will describe the distinct characteristics
Challenges and Opportunities for In Vivo Imaging in Oncology
 Challenges and Opportunities for In Vivo Imaging in Oncology Daniel C. Sullivan, M.D. Unraveling of the genome and proteome, and advances in biomedical technology are changing the practice of medicine
Challenges and Opportunities for In Vivo Imaging in Oncology Daniel C. Sullivan, M.D. Unraveling of the genome and proteome, and advances in biomedical technology are changing the practice of medicine
What Radiologists do?
 Multimodality Imaging in Oncology 2018 March 5 th 9th Diagnostic Imaging in Oncology What Radiologists do? Chikako Suzuki, MD, PhD Department of Diagnostic Radiology, KS Solna Department of Molecular Medicine
Multimodality Imaging in Oncology 2018 March 5 th 9th Diagnostic Imaging in Oncology What Radiologists do? Chikako Suzuki, MD, PhD Department of Diagnostic Radiology, KS Solna Department of Molecular Medicine
Introduction to the Course and the Techniques. Jeffry R. Alger, PhD Ahmanson-Lovelace Brain Mapping Center Department of Neurology
 Introduction to the Course and the Techniques Jeffry R. Alger, PhD Ahmanson-Lovelace Brain Mapping Center Department of Neurology (jralger@ucla.edu) CTSI Neuroimaging April 2014 Rationale for the Course
Introduction to the Course and the Techniques Jeffry R. Alger, PhD Ahmanson-Lovelace Brain Mapping Center Department of Neurology (jralger@ucla.edu) CTSI Neuroimaging April 2014 Rationale for the Course
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
[A RESEARCH COORDINATOR S GUIDE]
![[A RESEARCH COORDINATOR S GUIDE] [A RESEARCH COORDINATOR S GUIDE]](/thumbs/88/117127924.jpg) 2013 COLORECTAL SURGERY GROUP Dr. Carl J. Brown Dr. Ahmer A. Karimuddin Dr. P. Terry Phang Dr. Manoj J. Raval Authored by Jennifer Lee A cartoon about colonoscopies. 1 [A RESEARCH COORDINATOR S GUIDE]
2013 COLORECTAL SURGERY GROUP Dr. Carl J. Brown Dr. Ahmer A. Karimuddin Dr. P. Terry Phang Dr. Manoj J. Raval Authored by Jennifer Lee A cartoon about colonoscopies. 1 [A RESEARCH COORDINATOR S GUIDE]
Nuclear Medicine and PET. D. J. McMahon rev cewood
 Nuclear Medicine and PET D. J. McMahon 150504 rev cewood 2018-02-15 Key Points Nuclear Medicine and PET: Imaging: Understand how Nuc Med & PET differ from Radiography & CT by the source of radiation. Be
Nuclear Medicine and PET D. J. McMahon 150504 rev cewood 2018-02-15 Key Points Nuclear Medicine and PET: Imaging: Understand how Nuc Med & PET differ from Radiography & CT by the source of radiation. Be
Anatomical and Functional MRI of the Pancreas
 Anatomical and Functional MRI of the Pancreas MA Bali, MD, T Metens, PhD Erasme Hospital Free University of Brussels Belgium mbali@ulb.ac.be Introduction The use of MRI to investigate the pancreas has
Anatomical and Functional MRI of the Pancreas MA Bali, MD, T Metens, PhD Erasme Hospital Free University of Brussels Belgium mbali@ulb.ac.be Introduction The use of MRI to investigate the pancreas has
Angiogenesis and Cancer Control: From Concept to Therapeutic Trial
 Joel Roy, France. Oil on canvas. Angiogenesis and Cancer Control: From Concept to Therapeutic Trial Steven Brem, MD Current research into angiosuppressive agents is providing cautious optimism in the field
Joel Roy, France. Oil on canvas. Angiogenesis and Cancer Control: From Concept to Therapeutic Trial Steven Brem, MD Current research into angiosuppressive agents is providing cautious optimism in the field
Nintedanib in Oncology Backgrounder
 For media outside the US, UK and Canada only Nintedanib in Oncology Backgrounder 1. What is nintedanib? 2. How does nintedanib work? 3. Data overview 4. Additional clinical data 5. Nintedanib approval
For media outside the US, UK and Canada only Nintedanib in Oncology Backgrounder 1. What is nintedanib? 2. How does nintedanib work? 3. Data overview 4. Additional clinical data 5. Nintedanib approval
Molecular Imaging and Breast Cancer
 Molecular Imaging and Breast Cancer Breast cancer forms in tissues of the breast usually in the ducts, tubes that carry milk to the nipple, and lobules, the glands that make milk. It occurs in both men
Molecular Imaging and Breast Cancer Breast cancer forms in tissues of the breast usually in the ducts, tubes that carry milk to the nipple, and lobules, the glands that make milk. It occurs in both men
BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY
 BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY Cancer is a group of more than 100 different diseases that are characterized by uncontrolled cellular growth,
BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY Cancer is a group of more than 100 different diseases that are characterized by uncontrolled cellular growth,
Inhibitors of Methionine Aminopeptidase-2 2 in the Treatment of Non-Hodgkin
 Inhibitors of Methionine Aminopeptidase-2 2 in the Treatment of Non-Hodgkin Hodgkin s s Lymphoma Targeted Cancer Therapies William Westlin, Ph.D. Vice President, Preclinical Research Discovery of Fumagillin
Inhibitors of Methionine Aminopeptidase-2 2 in the Treatment of Non-Hodgkin Hodgkin s s Lymphoma Targeted Cancer Therapies William Westlin, Ph.D. Vice President, Preclinical Research Discovery of Fumagillin
NEWS RELEASE Media Contact: Krysta Pellegrino (650) Investor Contact: Sue Morris (650) Advocacy Contact: Kristin Reed (650)
 NEWS RELEASE Media Contact: Krysta Pellegrino (650) 225-8226 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Kristin Reed (650) 467-9831 FDA APPROVES AVASTIN IN COMBINATION WITH CHEMOTHERAPY
NEWS RELEASE Media Contact: Krysta Pellegrino (650) 225-8226 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Kristin Reed (650) 467-9831 FDA APPROVES AVASTIN IN COMBINATION WITH CHEMOTHERAPY
ulcer healing role 118 Bicarbonate, prostaglandins in duodenal cytoprotection 235, 236
 Subject Index Actin cellular forms 48, 49 epidermal growth factor, cytoskeletal change induction in mucosal repair 22, 23 wound repair 64, 65 polyamine effects on cytoskeleton 49 51 S-Adenosylmethionine
Subject Index Actin cellular forms 48, 49 epidermal growth factor, cytoskeletal change induction in mucosal repair 22, 23 wound repair 64, 65 polyamine effects on cytoskeleton 49 51 S-Adenosylmethionine
CANCER IMMUNOPATHOLOGY. Eryati Darwin Faculty of Medicine Andalas University
 CANCER IMMUNOPATHOLOGY Eryati Darwin Faculty of Medicine Andalas University Padang 18 Mei 2013 INTRODUCTION Tumor: cells that continue to replicate, fail to differentiate into specialized cells, and become
CANCER IMMUNOPATHOLOGY Eryati Darwin Faculty of Medicine Andalas University Padang 18 Mei 2013 INTRODUCTION Tumor: cells that continue to replicate, fail to differentiate into specialized cells, and become
SIBLINGs, cancer's multifunctional weapons
 SIBLINGs, cancer's multifunctional weapons 6/18/08 Akeila Bellahcène and Vincent Castronovo of the Metastasis Research laboratory of the University of Liège are among the first researchers to have discovered
SIBLINGs, cancer's multifunctional weapons 6/18/08 Akeila Bellahcène and Vincent Castronovo of the Metastasis Research laboratory of the University of Liège are among the first researchers to have discovered
Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist
 Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist Moderator: S. Terence Dunn, Ph.D. Associate Professor, Pathology Director, Molecular Pathology Laboratory University
Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist Moderator: S. Terence Dunn, Ph.D. Associate Professor, Pathology Director, Molecular Pathology Laboratory University
BNC105 Results Presentation
 Creating and developing innovative therapies BNC105 Results Presentation Dr Deborah Rathjen CEO & Managing Director, Bionomics Limited Dr José Iglesias Chief Medical Officer, Bionomics Limited Dr Tom Hutson
Creating and developing innovative therapies BNC105 Results Presentation Dr Deborah Rathjen CEO & Managing Director, Bionomics Limited Dr José Iglesias Chief Medical Officer, Bionomics Limited Dr Tom Hutson
A holistic approach to targeting breast cancer part II: Micronutrient synergy. Presented by: Dr. Neha Shanker DRRI
 A holistic approach to targeting breast cancer part II: Micronutrient synergy Presented by: Dr. Neha Shanker DRRI Overview of the previous webinar In the last presentation we talked about: Increase in
A holistic approach to targeting breast cancer part II: Micronutrient synergy Presented by: Dr. Neha Shanker DRRI Overview of the previous webinar In the last presentation we talked about: Increase in
Phase 1 Trial of ALN-VSP in Cancers Involving the Liver. Annual Meeting of the Controlled Release Society August 2, 2011
 Phase 1 Trial of ALN-VSP in Cancers Involving the Liver Annual Meeting of the Controlled Release Society August 2, 2011 Agenda ALN-VSP: Background Phase 1 Trial Study Design Safety Data Tumor Response
Phase 1 Trial of ALN-VSP in Cancers Involving the Liver Annual Meeting of the Controlled Release Society August 2, 2011 Agenda ALN-VSP: Background Phase 1 Trial Study Design Safety Data Tumor Response
Chapter 10. Summary, conclusions and future perspectives
 Chapter 10 Summary, conclusions and future perspectives 10.1 SUMMARY In this thesis, a new tumor imaging tracer in nuclear medicine is studied. This 123 tracer, L-3-[ I]Iodo-alpha-methyl-tyrosine (IMT),
Chapter 10 Summary, conclusions and future perspectives 10.1 SUMMARY In this thesis, a new tumor imaging tracer in nuclear medicine is studied. This 123 tracer, L-3-[ I]Iodo-alpha-methyl-tyrosine (IMT),
Cancer Biology Course. Invasion and Metastasis
 Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Cancer Biology Course Invasion and Metastasis 2016 Lu-Hai Wang NHRI Cancer metastasis Major problem: main reason for killing cancer patients, without it cancer can be cured or controlled. Challenging questions:
Goals. Aims and design of Phase I trials. Challenges in the new era of oncology. Complex and multidisciplinary Phase I.
 Goals Aims and design of Phase I trials Challenges in the new era of oncology Complex and multidisciplinary Phase I Perspectives Drug Development...2007 Preclinical Phase I Phase II Phase III Phase IV
Goals Aims and design of Phase I trials Challenges in the new era of oncology Complex and multidisciplinary Phase I Perspectives Drug Development...2007 Preclinical Phase I Phase II Phase III Phase IV
Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment
 Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment 1 Introductions Peter Langecker, MD, PhD Executive Medical Director, Global Oncology Clinipace Worldwide Mark Shapiro Vice President
Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment 1 Introductions Peter Langecker, MD, PhD Executive Medical Director, Global Oncology Clinipace Worldwide Mark Shapiro Vice President
The Process of Angiogenesis & Inhibition of Angiogenesis and/or Lymphangiogenesis
 The Process of Angiogenesis & Inhibition of Angiogenesis and/or Lymphangiogenesis Nam Deuk Kim, Ph.D. Pusan National University Contents Part 1. The process of angiogenesis Part 2. The role of angiopoietins
The Process of Angiogenesis & Inhibition of Angiogenesis and/or Lymphangiogenesis Nam Deuk Kim, Ph.D. Pusan National University Contents Part 1. The process of angiogenesis Part 2. The role of angiopoietins
Zachary I. Hodes, M.D., Ph.D., F.A.C.C.
 Zachary I. Hodes, M.D., Ph.D., F.A.C.C. Disclamer: I personally have no financial relationship with any company mentioned today. The Care Group, LLC does have a contract with Cardium to participate in
Zachary I. Hodes, M.D., Ph.D., F.A.C.C. Disclamer: I personally have no financial relationship with any company mentioned today. The Care Group, LLC does have a contract with Cardium to participate in
Chapter Introduction
 Chapter 1 Introduction Malignancy is a life-threatening condition in which tumor cells are of a highly invasive character and penetrate normal organs and exhibit an obstinate resistance against cancer
Chapter 1 Introduction Malignancy is a life-threatening condition in which tumor cells are of a highly invasive character and penetrate normal organs and exhibit an obstinate resistance against cancer
RECRUITMENT OF PATIENTS
 Newsletter This resume of the results from the phase 1b study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
Newsletter This resume of the results from the phase 1b study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
Neoplasia 18 lecture 8. Dr Heyam Awad MD, FRCPath
 Neoplasia 18 lecture 8 Dr Heyam Awad MD, FRCPath ILOS 1. understand the angiogenic switch in tumors and factors that stimulate and inhibit angiogenesis. 2. list the steps important for tumor metastasis
Neoplasia 18 lecture 8 Dr Heyam Awad MD, FRCPath ILOS 1. understand the angiogenic switch in tumors and factors that stimulate and inhibit angiogenesis. 2. list the steps important for tumor metastasis
PTAC meeting held on 5 & 6 May (minutes for web publishing)
 PTAC meeting held on 5 & 6 May 2016 (minutes for web publishing) PTAC minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics Advisory Committee (PTAC) and
PTAC meeting held on 5 & 6 May 2016 (minutes for web publishing) PTAC minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics Advisory Committee (PTAC) and
Imaging Decisions Start Here SM
 Owing to its high resolution and wide anatomic coverage, dynamic first-pass perfusion 320-detector-row CT outperforms PET/CT for distinguishing benign from malignant lung nodules, researchers from Japan
Owing to its high resolution and wide anatomic coverage, dynamic first-pass perfusion 320-detector-row CT outperforms PET/CT for distinguishing benign from malignant lung nodules, researchers from Japan
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Subject Index. Dry desquamation, see Skin reactions, radiotherapy
 Subject Index Actinic keratosis disseminated disease 42 surgical excision 42 AIDS, see Kaposi s sarcoma Amifostine, skin reaction prophylaxis 111 Basal cell carcinoma, superficial X-ray therapy Bowen s
Subject Index Actinic keratosis disseminated disease 42 surgical excision 42 AIDS, see Kaposi s sarcoma Amifostine, skin reaction prophylaxis 111 Basal cell carcinoma, superficial X-ray therapy Bowen s
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 PET Scanning: Oncologic Applications Page 1 of 88 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: Oncologic Applications
PET Scanning: Oncologic Applications Page 1 of 88 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: Oncologic Applications
Hosts. New Treatments for Cancer
 Hosts Anees MD Associate Professor of Surgical Oncology Francine Foss MD Professor of Medical Oncology New Treatments for Cancer Guest Expert: Lieping Chen, MD, PhD United Technologies Corporation Professor
Hosts Anees MD Associate Professor of Surgical Oncology Francine Foss MD Professor of Medical Oncology New Treatments for Cancer Guest Expert: Lieping Chen, MD, PhD United Technologies Corporation Professor
!!! Oncology for Scientists (RPN 530) Metastasis and Angiogenesis Chapter 13 and 14 Oct. 28 th 2014
 Oncology for Scientists (RPN 530) Metastasis and Angiogenesis Chapter 13 and 14 Oct. 28 th 2014 Department of Cancer Genetics Masashi Muramatsu, Ph.D. About Exam First, think and understand the concepts.
Oncology for Scientists (RPN 530) Metastasis and Angiogenesis Chapter 13 and 14 Oct. 28 th 2014 Department of Cancer Genetics Masashi Muramatsu, Ph.D. About Exam First, think and understand the concepts.
Colorectal Cancer Treatment
 Scan for mobile link. Colorectal Cancer Treatment Colorectal cancer overview Colorectal cancer, also called large bowel cancer, is the term used to describe malignant tumors found in the colon and rectum.
Scan for mobile link. Colorectal Cancer Treatment Colorectal cancer overview Colorectal cancer, also called large bowel cancer, is the term used to describe malignant tumors found in the colon and rectum.
Medical imaging X-ray, CT, MRI, scintigraphy, SPECT, PET Györgyi Műzes
 Medical imaging X-ray, CT, MRI, scintigraphy, SPECT, PET Györgyi Műzes Semmelweis University, 2nd Dept. of Medicine Medical imaging: definition technical process of creating visual representations about
Medical imaging X-ray, CT, MRI, scintigraphy, SPECT, PET Györgyi Műzes Semmelweis University, 2nd Dept. of Medicine Medical imaging: definition technical process of creating visual representations about
A PHASE 1 STUDY OF TRC105 (ANTI- ADVANCED SOLID TUMORS
 ASCO 2011 Abstract Number: 3073 A PHASE 1 STUDY OF TRC105 (ANTI- CD105 ANTIBODY) IN PATIENTS WITH ADVANCED SOLID TUMORS J. W. Goldman, M. S. Gordon, H. Hurwitz, R. Pili, D. S. Mendelson, B. J. Adams, D.
ASCO 2011 Abstract Number: 3073 A PHASE 1 STUDY OF TRC105 (ANTI- CD105 ANTIBODY) IN PATIENTS WITH ADVANCED SOLID TUMORS J. W. Goldman, M. S. Gordon, H. Hurwitz, R. Pili, D. S. Mendelson, B. J. Adams, D.
University of Groningen. Quantitative CT myocardial perfusion Pelgrim, Gert
 University of Groningen Quantitative CT myocardial perfusion Pelgrim, Gert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Quantitative CT myocardial perfusion Pelgrim, Gert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
Tissue repair. (3&4 of 4)
 Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
Option D: Medicinal Chemistry
 Option D: Medicinal Chemistry Basics - unstable radioactive nuclei emit radiation in the form of smaller particles alpha, beta, positron, proton, neutron, & gamma are all used in nuclear medicine unstable
Option D: Medicinal Chemistry Basics - unstable radioactive nuclei emit radiation in the form of smaller particles alpha, beta, positron, proton, neutron, & gamma are all used in nuclear medicine unstable
IVC History, Cancer Research
 Riordan Clinic IVC Academy 5 IVC History, Cancer Research O (slides 41-80) Pharmacokinetics of Oral Vitamin C using Liposomal Form* To test whether plasma vitamin C levels, following oral doses in supplemented
Riordan Clinic IVC Academy 5 IVC History, Cancer Research O (slides 41-80) Pharmacokinetics of Oral Vitamin C using Liposomal Form* To test whether plasma vitamin C levels, following oral doses in supplemented
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.PHAR.121
 Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Avastin (bevacizumab)
 Avastin (bevacizumab) Policy Number: 5.02.502 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Avastin
Avastin (bevacizumab) Policy Number: 5.02.502 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Avastin
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16
 COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
Targeted Cancer Therapies: Questions and Answers. Key Points
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Targeted Cancer Therapies:
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Targeted Cancer Therapies:
CILENT P Leblond, DIPG Meeting, Barcelone 2012
 CILENT-0902 Cilengitide (EMD121974) in combination with irradiation in children and adolescents with newly diagnosed diffuse intrinsic brainstem glioma : Phase I Study P Leblond, DIPG Meeting, Barcelone
CILENT-0902 Cilengitide (EMD121974) in combination with irradiation in children and adolescents with newly diagnosed diffuse intrinsic brainstem glioma : Phase I Study P Leblond, DIPG Meeting, Barcelone
Bone PET/MRI : Diagnostic yield in bone metastases and malignant primitive bone tumors
 Bone PET/MRI : Diagnostic yield in bone metastases and malignant primitive bone tumors Lars Stegger, Benjamin Noto Department of Nuclear Medicine University Hospital Münster, Germany Content From PET to
Bone PET/MRI : Diagnostic yield in bone metastases and malignant primitive bone tumors Lars Stegger, Benjamin Noto Department of Nuclear Medicine University Hospital Münster, Germany Content From PET to
COMENIUS-Project: SM&CLIL Radiation & Medicine
 Medical imaging refers to the techniques and processes used to create images of the human body (or parts thereof) for clinical purposes. Thanks to modern mathematics and computer technology, medical imaging
Medical imaging refers to the techniques and processes used to create images of the human body (or parts thereof) for clinical purposes. Thanks to modern mathematics and computer technology, medical imaging
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
number Done by Corrected by Doctor Maha Shomaf
 number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
Angiogenesis as a therapeutic target
 Angiogenesis as a therapeutic target Lecture Experimentelle Krebsforschung SS 07 Prof. Gerhard Christofori Institute of Biochemistry and Genetics Department of Clinical-Biological Sciences University of
Angiogenesis as a therapeutic target Lecture Experimentelle Krebsforschung SS 07 Prof. Gerhard Christofori Institute of Biochemistry and Genetics Department of Clinical-Biological Sciences University of
FGL2 A new biomarker for cancer in a simple blood test
 FGL2 A new biomarker for cancer in a simple blood test WHO IS FGL2 Human gene (chromosome 7) is 7 kb long, 2 exons, monomer protein 70 KD, tetramer in solution. Fibrinogen-like protein 2 (Fgl2), a member
FGL2 A new biomarker for cancer in a simple blood test WHO IS FGL2 Human gene (chromosome 7) is 7 kb long, 2 exons, monomer protein 70 KD, tetramer in solution. Fibrinogen-like protein 2 (Fgl2), a member
Radionuclides in Medical Imaging. Danielle Wilson
 Radionuclides in Medical Imaging Danielle Wilson Outline Definitions History and development Radionuclide applications & techniques in imaging Conclusion Definition #1 : Radionuclide An unstable nucleus
Radionuclides in Medical Imaging Danielle Wilson Outline Definitions History and development Radionuclide applications & techniques in imaging Conclusion Definition #1 : Radionuclide An unstable nucleus
Nursing s Role in the Management of New Oral Chemotherapy Agents
 Nursing s Role in the Management of New Oral Chemotherapy Agents Mechelle Barrick BSN, RN, OCN, CCRP Clinical Research Nurse Coordinator Greater Baltimore Medical Center mbarrick@gbmc.org THE NURSES ROLE
Nursing s Role in the Management of New Oral Chemotherapy Agents Mechelle Barrick BSN, RN, OCN, CCRP Clinical Research Nurse Coordinator Greater Baltimore Medical Center mbarrick@gbmc.org THE NURSES ROLE
Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice
 Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice Publication from the Dr. Rath Research Institute Experimental
Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice Publication from the Dr. Rath Research Institute Experimental
Index. mri.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Angiogenesis, and cancer of prostate, 689 690 Angiography, MR. See MR angiography. Apoptosis, MR imaging of, 637 Apparent diffusion coefficient,
Index Note: Page numbers of article titles are in boldface type. A Angiogenesis, and cancer of prostate, 689 690 Angiography, MR. See MR angiography. Apoptosis, MR imaging of, 637 Apparent diffusion coefficient,
Sulfur hexafluoride-filled microbubbles SonoVue 3-7microns diameter Blood pool agent
 Sulfur hexafluoride-filled microbubbles SonoVue 3-7microns diameter Blood pool agent Extremely good tolerance in clinical practice - No nephrotoxicity, - No thyroid interaction - No need of Blood test
Sulfur hexafluoride-filled microbubbles SonoVue 3-7microns diameter Blood pool agent Extremely good tolerance in clinical practice - No nephrotoxicity, - No thyroid interaction - No need of Blood test
Growth Factors. BIT 230 Walsh Chapter 7
 Growth Factors BIT 230 Walsh Chapter 7 3 Definitions Autocrine: a mode of hormone action in which a hormone affects the function of the cell type that produced it. Paracrine: Relating to the release of
Growth Factors BIT 230 Walsh Chapter 7 3 Definitions Autocrine: a mode of hormone action in which a hormone affects the function of the cell type that produced it. Paracrine: Relating to the release of
Breast Cancer. What is breast cancer?
 Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
New Developments in Cancer Treatment. Dulcinea Quintana, MD
 New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
Breast Cancer. What is breast cancer?
 Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
International Course on Theranostics and Molecular Radiotherapy ImmunoPET in Breast Cancer
 International Course on Theranostics and Molecular Radiotherapy ImmunoPET in Breast Cancer Geraldine Gebhart Jules Bordet Institute 5/10/2017 PLAN OF THE TALK Increasing role of antibodies in anti-cancer
International Course on Theranostics and Molecular Radiotherapy ImmunoPET in Breast Cancer Geraldine Gebhart Jules Bordet Institute 5/10/2017 PLAN OF THE TALK Increasing role of antibodies in anti-cancer
Pharmacyclics Reports Updated Clinical Results from its Phase IA Trial of its First in Human BTK- Inhibitor PCI-32765
 Contact: Ramses Erdtmann Vice President of Finance Phone: 408-215-3325 Pharmacyclics Reports Updated Clinical Results from its Phase IA Trial of its First in Human BTK- Inhibitor PCI-32765 Company to Host
Contact: Ramses Erdtmann Vice President of Finance Phone: 408-215-3325 Pharmacyclics Reports Updated Clinical Results from its Phase IA Trial of its First in Human BTK- Inhibitor PCI-32765 Company to Host
Study No Title : Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Virtual Melanoma: When, Where and How Much to Cut Yang Kuang, Arizona State University
 Virtual Melanoma: When, Where and How Much to Cut Yang Kuang, Arizona State University Based on: Eikenberry S, Thalhauser C, Kuang Y. PLoS Comput Biol. 2009, 5:e1000362. Mathematical Modeling of Melanoma
Virtual Melanoma: When, Where and How Much to Cut Yang Kuang, Arizona State University Based on: Eikenberry S, Thalhauser C, Kuang Y. PLoS Comput Biol. 2009, 5:e1000362. Mathematical Modeling of Melanoma
IVC History, Cancer Research
 Riordan Clinic IVC Academy 5 IVC History, Cancer Research O (slides 81-116) Cytokine Signaling Categories Heal the Wound! Angiogenesis - 62 Inflammation - 69 Differentiation - 53 Oncogene-Activation -
Riordan Clinic IVC Academy 5 IVC History, Cancer Research O (slides 81-116) Cytokine Signaling Categories Heal the Wound! Angiogenesis - 62 Inflammation - 69 Differentiation - 53 Oncogene-Activation -
Disorders of Cell Growth & Neoplasia
 General Pathology VPM 152 Disorders of Cell Growth & Neoplasia Lecture 3 Rate of growth, local invasion, and metastasis. Molecular basis of cancer (normal cell-cycle and cellular proliferation). Enrique
General Pathology VPM 152 Disorders of Cell Growth & Neoplasia Lecture 3 Rate of growth, local invasion, and metastasis. Molecular basis of cancer (normal cell-cycle and cellular proliferation). Enrique
T2, T2*, ute. Yeo Ju Kim. Radiology, Inha University Hospital, Incheon, Korea
 SY28-1 T2, T2*, ute Yeo Ju Kim Radiology, Inha University Hospital, Incheon, Korea T2 relaxation times relate to the rate of transverse magnetization decay, caused by the loss of phase coherence induced
SY28-1 T2, T2*, ute Yeo Ju Kim Radiology, Inha University Hospital, Incheon, Korea T2 relaxation times relate to the rate of transverse magnetization decay, caused by the loss of phase coherence induced
Functional Imaging of Cancer Using Contrast-Enhanced Ultrasound JOHN M. HUDSON
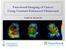 Functional Imaging of Cancer Using Contrast-Enhanced Ultrasound JOHN M. HUDSON Renal Cell Carcinoma (RCC) Anti-angiogenic Treatments for Metastatic Renal Cell Carcinoma (RCC) Sunitinib vs. Interferon Alpha
Functional Imaging of Cancer Using Contrast-Enhanced Ultrasound JOHN M. HUDSON Renal Cell Carcinoma (RCC) Anti-angiogenic Treatments for Metastatic Renal Cell Carcinoma (RCC) Sunitinib vs. Interferon Alpha
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
