Functional assessment of white and brown adipocyte development and energy metabolism in cell culture
|
|
|
- Stuart Harrington
- 6 years ago
- Views:
Transcription
1 Journal of Cell Science 18, (1995) Printed in Great Britain The Company of Biologists Limited 1995 JCS Functional assessment of white and brown adipocyte development and energy metabolism in cell culture Dissociation of terminal differentiation and thermogenesis in brown adipocytes Susanne Klaus*, Marina Ely, Dag Encke and Gerhard Heldmaier Fachbereich Biologie/Zoologie, Philipps Universität Marburg, Karl v. Frisch Str., 3543 Marburg, Germany *Author for correspondence SUMMARY We investigated the effect of insulin, triiodothyronine (T 3 ) and dexamethasone (a synthetic glucocorticoid) on differentiation, lipid metabolism and thermogenesis of preadipocytes isolated from white fat (WAT) and brown fat (BAT) from the Siberian dwarf hamster (Phodopus sungorus). Cell cultures from WAT and BAT were chronically treated with the above hormones alone or in any combination. After differentiation (day 8 or 9 of culture) we measured the following parameters: adipogenic index (number size of adipocytes), protein content, lipolysis, cell respiration, and expression of the uncoupling protein UCP, which is unique to mitochondria of brown adipocytes. nsulin was the most important adipogenic factor for brown and white adipocytes and necessary for terminal differentiation, whereas dexamethasone alone completely inhibited differentiation. T 3 had no effect on adipogenesis in WAT cultures, but further increased insulin stimulated adipogenesis in BAT cultures. Basal lipolysis was higher in WAT than in BAT cultures except when dexamethasone was present, which stimulated lipolysis in both culture types to the same extent. T 3 had a pronounced dose dependent lipolytic effect on WAT cultures but very little effect on BAT cultures. Respiration rates were generally higher in differentiated adipocytes than in fibroblast like cells. T 3 had no effect on thermogenesis in WAT cultures but increased thermogenesis in BAT cultures, and this was further elevated by insulin. UCP expression in BAT cultures could be detected by western blot in insulin treated, T 3 treated and insulin + T 3 treated cultures with highest expression in the latter. These results imply a possible dissociation of terminal differentiation and thermogenic function of brown adipocytes. n WAT cultures there was also a low level of UCP detectable in the insulin + T 3 treated cultures. mmuno-fluorescence microscopy analysis revealed the presence of UCP in 1-15% of adipocytes from WAT cultures (in BAT cultures: 9%), indicating the presence of some brown preadipocytes in typical WAT deposits. Key words: cell respiration, lipolysis, uncoupling protein, insulin, triiodothyronine, dexamethasone NTRODUCTON White adipose tissue (WAT) and brown adipose tissue (BAT) both play an important role in mammalian energy equilibrium because they are important for energy storage (WAT) as well as energy dissipation (BAT). The thermogenic i.e. energy dissipating function of brown fat is important for maintenance of body temperature in small and newborn mammals, and for arousal from the hypothermic state in hibernators. t is also the source of the so-called diet induced thermogenesis, which can be observed after overeating in several animal models (Rothwell and Stock, 1986). Brown fat is, in contrast to white fat, highly innervated and vascularised. Morphologically, brown adipocytes can be distinguished from white adipocytes by multilocular lipid inclusions and numerous, well developed mitochondria, whereas white adipocytes are characterized by one large lipid inclusion and very few mitochondria (reviewed by Néchad, 1986). Brown fat mitochondria are equipped with a unique protein, called uncoupling protein (UCP) which is a proton translocator in the inner mitochondrial membrane and functions as an uncoupler of the mitochondrial respiratory chain (reviewed by Nicholls et al., 1986; Ricquier et al., 1991; Klaus et al., 1991a). A UCP containing adipocyte is considered a brown adipocyte and it has repeatedly been reported that typical white fat deposits can contain UCP expressing fat cells (Lonçar, 1991; Cousin et al., 1992). Although white and brown adipocytes have quite different physiological functions, it is not yet clear if they represent truly different cell types or if a conversion from one type into the other is possible. n larger mammals an apparent conversion from BAT into WAT takes place (Casteilla et al., 1989), but so far there exists no strong evidence for a possible conversion from WAT into BAT. Adipocyte cell cultures are widely employed as model systems for terminal cell differentiation and numerous white preadipocyte cell lines have been studied for many years (reviewed by Cornelius et al., 1994). The first brown
2 3172 S. Klaus and others preadipocyte cell lines have been characterized only very recently (Klaus et al., 1994; Kozak and Kozak, 1994). Although cell lines can be useful tools, they suffer the disadvantage that as they are immortalized they might differ considerably from tissue preadipocytes in regard to development and energy metabolism. Development of white as well as brown preadipocytes has therefore also been studied in stromal-vascular preadipocytes isolated from WAT and BAT of different species (Cornelius et al., 1994 and references within; Rehnmark et al., 1989; Casteilla et al., 1991; Klaus et al., 1991b; Champigny et al., 1992). Due to species and culture condition differences, results from the different studies even concerning only one given adipocyte type are occasionally quite controversial. To draw conclusions from comparisons of different studies concerning brown and white preadipocyte differentiation seems thus rather arguable. However, to our knowledge no extensive comparative studies have been performed on development and metabolism from preadipocytes isolated from WAT and BAT from the same animal and cultured in parallel under identical conditions. There are numerous hormones and other factors which play a role in proliferation and differentiation of adipocytes (reviewed by Ailhaud, 199; Ailhaud et al., 1992; Butterwirth, 1994). nsulin and T 3 were shown to be important for terminal differentiation in some adipocyte cell models (Grimaldi et al., 1982; Ailhaud, 199; Hauner, 199; Darimont et al., 1993). Glucocorticoids are also known to be involved in fat metabolism and adipocyte terminal differentiation, although their effects seem to be different, depending on the time of addition relative to the cell cycle (Xu and Björntorp, 199). As the hormones mentioned have also been reported to be involved in brown fat thermogenic function (Park et al., 1989; Geloen and Trayhurn, 199; Reiter et al., 199) we decided to study the effect of chronic treatment with insulin, T 3 and dexamethasone (a synthetic glucocorticoid) on differentiation and development of energy metabolism of brown and white preadipocytes in cell culture. We chose a comparative approach using stromal-vascular preadipocytes isolated from WAT and BAT of the Siberian hamster (Phodopus sungorus). This species has already proved an excellent system for the study of brown adipocyte differentiation (Klaus et al., 1991b). The Siberian hamster also possesses abundant white fat deposits and is prone to considerable seasonal changes in body fat, which makes it a good candidate for the study of white adipocyte development. Furthermore, we investigated the metabolic activity of adipocytes cultured in vitro by measuring cell respiration in order to relate these data to the physiological function of the two adipose types in vivo. MATERALS AND METHODS Cell cultures nguinal white fat and several deposits of brown fat (axillar, suprasternal, interscapular, dorsal-cervical) from Siberian hamsters (Phodopus sungorus) aged 4 to 6 weeks and kept at thermoneutrality (23 C) were used for tissue cultures. Fat deposits from 4 to 6 animals of both sexes were pooled for every tissue culture. The stromal-vascular fraction was obtained after collagenase treatment as described by Klaus et al. (1991b). Cells were inoculated in Petri dishes (1 cm or 6 cm diameter) at approximately 1,5-2, cells/cm 2. Cells were grown at 37 C in air with 5% CO 2 content and 1% relative humidity in cell culture medium (5% modified Eagle s medium (Gibco/BRL) and 5% F12 Ham s F12 medium (Gibco/BRL) supplemented with NaHCO 3 (1.2 g/l), biotin (4 mg/l), Ca-panthotenate (2 mg/l), glutamine (5 mm), glucose (4.5 g/l) and Hepes (15 mm, ph 7.4), penicillin G (6.25 mg/l), and streptomycin (5 mg/l)). Until the third day of culture, the medium was supplemented with 1% fetal calf serum (FCS) then it was changed to 7% FCS. The medium was changed at days 1 and 3. Hormonal supplements were added at day 3 unless otherwise indicated. The medium referred to as standard culture medium was supplemented with 17 nm insulin and 1 nm T 3. At the indicated day of harvest, cell culture medium was aspirated and dishes with cells were directly frozen and kept at 2 C until further analysis. Morphological cell analysis Adipocytes were counted directly in the dish with a phase-contrast microscope at a 1 magnification with an ocular grid corresponding to a total surface of 1 mm 2. At least four randomly chosen fields were counted for each dish. Adipocytes could be distinguished from preadipocytes by the presence of visible lipid droplets. For better visualization, lipids were stained with O Red Oil and only cells positive for this stain were considered as adipocytes. Adipocyte size was measured at a 5 magnification using an ocular scale. For each cell the width and the length were measured and multiplied in order to obtain a surface index (expressed in µm 2 ). Total cell number was assessed directly in aliquots of the suspended cells used for respiration studies, using a Thoma counting chamber. Lipolysis The amount of glycerol released into the medium was determined as an index for lipolytic activity of cells. Glycerol determination was performed using a kit from Boehringer Mannheim (No ) following the manufacturer s instructions, except that the test volume was scaled down to 6 µl. Samples of cell culture medium (5-1 µl) were drawn on the indicated days and frozen until measurement. The glycerol content of medium containing 7% FCS (approximately 1.5 mg/ml) was subtracted from each value. Cell respiration Respiration was measured with a Clark type electrode at 31 C. This temperature was chosen because at 37 C the electrodes were not very stable and showed considerable drift. Cell medium was aspirated and cells from one 1 cm dish were suspended in 1 to 2 ml of cell culture medium (without FCS) containing 5 mm EDTA, which detaches cells from the bottom. The culture medium used for respiration was saturated with air, containing 5% CO 2. The cell suspension (1 ml, which was gently pipetted several times to ensure detachment of cells) was immediately filled into the reaction chamber of the Clark electrode, and oxygen consumption was continuously monitored on a chart recorder. After a few minutes, isoproterenol (.1 µm) was added for measurement of β-adrenergic stimulation of cell respiration. Finally, maximum i.e. uncoupled respiration rates were obtained by addition of an uncoupler (FCCP,.1 µm). For each cell preparation we thus obtained values for unstimulated, stimulated and uncoupled respiration. For calculation of oxygen consumption, the medium was considered to contain 433 nmol O/ml. An aliquot of the cell suspension was used for cell counting (see above). Protein content was determined by the method of Bradford. Cells were recovered after the experiment and frozen for further analysis (e.g. western blot). Western blot analysis For western blot analysis total cell homogenates were prepared by sonification of cells in PBS. A 2 µg sample of total protein was used for SDS-PAGE and immuno-blotting using a polyclonal antibody against Siberian hamster UCP as described (Klaus et al., 1991b),
3 WAT BAT White versus brown adipocytes 3173 PT no addition T 3 PT insulin insulin + T 3 PT dex dex + T 3 PT V insulin + dex insulin + dex + T 3 Fig. 1. Microphotograph of cultured stromal-vascular cells isolated from white adipose tissue (WAT) and brown adipose tissue (BAT). Preadipocytes were cultured from day 3 in medium containing 7% FCS and the indicated supplements. At day 1 of culture cells were stained with O Red Oil which specifically stains neutral lipids. Four different phenotypes (PT) were obtained depending on hormonal supplements. Bar, 4 µm. except that proteins were blotted on Hybond-C membrane (Amersham) and an enhanced chemoluminescence (ECL) western blotting detection system (Amersham, Germany) was used with a horseradish peroxidase-conjugated second antibody according to the manufacturer s protocol. Signals were quantified by densitometrical scanning of the films. ndirect immuno-fluorescence microscopy Suspensions of the stromal-vascular fraction of brown or white fat tissues were dropped into wells of a multiwell-coverslip. After 2 hours samples were covered with cell culture medium and cells were cultured as indicated above. At day 8 or 9 coverslips were washed with ice-cold PBS and fixed in ice-cold 95% ethanol/5% acetic acid for at least 1 minutes. Coverslips were washed 3 times in cold PBS. Cells were then permeabilized consecutively at 2 C in 5% acetone, 1% acetone, and 5% acetone for 1 minute each. The following steps were performed at room temperature unless otherwise indicated. After washing in PBS cells were incubated with 2% FCS in PBS for 4 minutes. After the PBS wash samples were incubated with the first primary antibody (chicken serum against LPL, 1:1,, diluted with F dilution buffer (.5% bovine serum albumin, 1% gelatin,.1% Tween- 2) for 1 hour in a moist chamber. Coverslips were washed 3 times in PBS. Cells were incubated with second antibody (FTC-conjugated goat anti-chicken gg, 1:1 (DAKO GmbH, Hamburg, Germany) diluted with F buffer) for 1 hour in a moist chamber. After 3 washes in PBS antibodies were fixed with 95% ethanol/5% acetic acid at 2 C for 2 minutes. Then this procedure was repeated for a second primary antibody (rabbit serum against UCP, 1:1, diluted in F buffer) and second antibody (rhodamine-conjugated swine anti-rabbit gg, 1:4 diluted in F dilution buffer). After 3 washes in PBS samples were stained with the DNA-specific dye DAP (1 µg/ml, Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) for 2 minutes. The coverslips were mounted in 5% glycerol, 2.5% DAPCO (1,4-diazabicyclo octan, Merck, Darmstadt, Germany) for stabilization of fluorescence. Samples were examined using a Zeiss Axiophot microscope equipped with fluorescence optics (4/.9 neofluar objective). RESULTS Adipogenesis n order to evaluate the effect of different hormones on white and brown adipocytes in culture, we supplemented the cell culture medium (containing 7% FCS) from day 3 onward, with insulin (17 nm), T 3 (1 nm), and/or dexamethasone (1 µm). This experimental design resulted in 8 different groups: (1) no addition ( ); (2) insulin (); (3) T 3 (T3); (4) dexamethasone (Dex); (5) insulin + T 3 (+T3); (6) insulin + dexamethasone (+Dex); (7) T 3 + dexamethasone (T3+Dex); (8) insulin + T 3 + dexamethasone (+T3+Dex). n preliminary experiments, medium supplemented with charcoal treated FCS or non treated FCS was compared in order to evaluate a possible T 3 contamination of FCS. However, no difference in either cell morphology or respiration could be detected between the two FCS types (not shown). The different hormonal treatments resulted in 4 different phenotypes, both in BAT and WAT cultures (Fig. 1). n phenotype clusters of rounded cells with very little lipid accumulation were located amongst fibroblast like cells. This corresponds to the groups with no addition or only T 3 addition (groups 1 and 3). We shall refer to these cells as poorly differentiated adipocytes. Phenotype was characterized by clusters of fully differentiated, mature adipocytes with numerous, big lipid droplets. This corresponded to the groups with insulin and
4 3174 S. Klaus and others insulin + T 3 addition (groups 2 and 5). n phenotype no apparent terminal differentiation took place, cells resembled fibroblast like precursor cells with no lipid inclusion (termed non differentiated cells). This was the case in cells treated with dexamethasone and dexamethasone + T 3 (groups 4 and 7). Finally, phenotype V was characterized by numerous single adipocytes which were not arranged in the typical cluster pattern, but rather singled out. This corresponded to the groups treated with insulin + dexamethasone and insulin + dexamethasone + T 3 (groups 6 and 8). Taken together these results mean that T 3 had no influence on the phenotype of cultured WAT and BAT cells; insulin, however, was important for lipid accumulation, i.e. acquirement of a terminally differentiated phenotype. Dexamethasone completely inhibited adipocyte differentiation when added alone (Table 1) and together with insulin it still prevented the formation of the typical cluster pattern of differentiated adipocytes. We investigated more closely the different adipogenic actions of the different hormones (Table 1). The total number of lipid containing cells (including both poorly and fully differentiated adipocytes) was significantly higher when cultures were treated with insulin (1 and 91 adipocytes/mm 2 in WAT and BAT, respectively) than in the cultures treated only with T 3 or without hormonal supplements. n this respect no difference was found between cultures of BAT and WAT. Nevertheless, in phenotype there was a difference in size between white and brown adipocytes. As can be seen in Table 1, adipocytes from cultured WAT were significantly bigger than adipocytes from BAT cultures when treated with insulin or insulin + T 3. nterestingly, when insulin was present, T 3 had a positive effect on adipocyte size in BAT cultures but not in WAT cultures. T 3 alone had no effect on either adipocyte size or number. As adipocyte size correlates with lipid accumulation, we defined an adipogenic index by multiplying the number of lipid containing cells with the adipocyte size. The adipogenic index is thus an indicator for the adipose conversion of a given cell culture. Fig. 2 shows the adipogenic index of cell cultures from WAT and BAT treated with different hormones. n both cell culture types the adipogenic index was very low in the absence of insulin. n the presence of insulin the adipogenic index of WAT cultures was twice as high as that of BAT cultures. T 3 had no influence on WAT cultures, but increased the adipogenic index of BAT cultures by about 45% in the presence of insulin. When dexamethasone was present in addition to insulin, the adipogenic index of both adipocyte culture types was the same. Protein content increased with differentiation state and in both cultures the protein content was significantly elevated only in phenotype cultures, i.e. after treatment with insulin or insulin + T 3 (Table 1). This result corroborates the importance of insulin for terminal differentiation. Lipolysis Basal lipolytic activity was distinctly different in BAT and WAT cultures. WAT cultures supplemented with insulin and T 3 released over 5 times as much glycerol into the medium as corresponding BAT cultures (Table 1). When adipocyte differentiation was inhibited by the addition of dexamethasone, there was no glycerol release at all, which means that lipolysis was restricted to differentiated adipocytes. (Negative values indicate that there was even a slight uptake of glycerol from the culture medium in these cases.) As the number of adipocytes was different depending on culture conditions, we adipogenic index (au) T3 Dex +T3 +Dex T3+Dex Fig. 2. Adipogenic index (number size of adipocytes) from cultured stromal-vascular cells isolated from white adipose tissue (open bars) and brown adipose tissue (filled bars). Preadipocytes were cultured from day 3 in medium containing 7% FCS and indicated hormonal supplements (see text for abbreviations). Size and number of adipocytes were measured at day 8 of culture and adipogenic index was calculated from the mean values listed in Table 1. Table 1. Effect of culture medium supplements on adipogenesis and lipolysis Total cell Number of Adipocyte Total protein Glycerol released number adipocytes surface index content into medium (per mm 2 ) (per mm 2 ) (µm 2 ) (mg/1 6 cells) (µg/plate) WAT BAT WAT BAT WAT BAT WAT BAT WAT BAT None 246±23 241± ± ± ±18 258±32.67±.6.63±.8 78±18 21±1 275±21 294± ±5.3* 91.2±2.9* 957±68* 46±22*,.97±.6*.93±.7* 25±3* 92±32*, T3 268±21 239± ± ± ±3 274±2.66±.3.74±.5 111±29 36±11*, Dex 199±15 215± ±.6.54±.11 6±6* 27±8* +T3 295±23 281± ±5.2* 85.5±5.2*, 913±39* 76±38*, 1.2±.6* 1.5±.12* 28±26* 53±12*, +Dex 34±46 275± ±4.1* 74.8±5.3* 591±3* 611±4*.59±.6.54± ±46* 583±37* T3+Dex 189±3 213±37 n.d. n.d..62±.9.63±.18 27±19* 15±19 +T3+Dex 229±23 212±2 84.6±7.4* 53.6±1. n.d. n.d..66±.4.64±.7 125±99* 748±13* Cell number and size index was obtained as indicated in the text. Protein content was determined by a Bradford assay. All values are means + s.e.m. from multiple experiments (at least 5 different cell cultures) from day 8 or 9 of culture. *Value significantly different (P<.5) from non-treated control. Value of BAT culture significantly different (P<.5) from corresponding WAT culture. n.d. not determined.
5 White versus brown adipocytes 3175 calculated the glycerol released per adipocyte which is shown in Fig. 3. White adipocytes released consistently more glycerol than brown adipocytes, unless dexamethasone + insulin were supplemented. Dexamethasone had in both fat culture types a pronounced lipolytic effect (in the presence of insulin) throughout the whole culture period. nterestingly, dexamethasone induced lipolysis was further elevated by the presence of T 3 in BAT as well as in WAT cultures. n order to examine more closely the effects of dexamethasone and T 3 on lipolysis of brown and white adipocytes, we measured the glycerol release induced by 48 hours treatment with different concentrations of dexamethasone and T 3 (Fig. 4). At 1 nm, dexamethasone already had a pronounced lipolytic effect on both WAT and BAT cultures. T 3 on the other hand, strongly increased lipolysis in WAT cultures in a dose dependent matter, but had little effect on BAT cultures even at high concentrations. Cell respiration Using a Clark type electrode we could measure respiration rates glycerol released (pg / adipocyte) T3 +T3 +Dex +T3+Dex Fig. 3. Lipolysis of adipocytes from WAT (open bars) and BAT cultures (filled bars). Preadipocytes were cultured from day 3 in medium containing 7% FCS and indicated hormonal supplements (see text for abbreviations). Glycerol content of the culture medium was measured at day 8 of culture and the glycerol released per adipocyte was calculated from the mean values listed in Table 1. i.e. metabolic activity of cultured BAT and WAT cells. n Table 2 the mean respiration rates of BAT and WAT cultures grown with different hormonal supplements are summarized. n both culture types respiration rates were lowest when adipocyte differentiation was inhibited by the presence of dexamethasone, i.e. in phenotype. n the presence of insulin, when differentiated adipocytes were present, unstimulated as well as maximum (uncoupled) respiration was always higher. As we had estimated the number of differentiated adipocytes in the different A B µg glycerol / plate µg glycerol / plate WAT BAT dexamethasone (M) WAT BAT T3 (M) Fig. 4. Dose response curve of lipolysis of WAT and BAT cultures treated with dexamethasone or T 3 for 48 hours. Preadipocytes were cultured from day 3 in 3 cm Petri dishes in medium containing 7% FCS and 17 nm insulin. At day 6 dexamethasone (A) or T 3 (B) were added and glycerol content in the medium was measured 48 hours later (day 8). Glycerol content before the addition was subtracted from the values. Table 2. Effect of culture medium supplements on cell respiration Respiration Respiration Respiration non stimulated +isoproterenol +FCCP (nmol O/min per 1 6 cells) (nmol O/min per 1 6 cells) (nmol O/min per 1 6 cells) WAT BAT WAT BAT WAT BAT None 9.4±.9 9.1± ± ± ± ± ± ± ± ±6.9* 28.3± ±5.9* T3 1.7± ±4.2*, 13.7± ±5.2*, 17.± ±5.5* Dex 2.7±1.5* 3.5±1.1* 3.7± ± ±2.7 7.±2.8 +T3 28.6±6.5* 52.7±6.6*, 35.4±7.2* 72.5±1.7*, 42.3±6.9* 85.4±11.2*, +Dex 15.4± ± ± ± ± ±4.5 T3+Dex 5.3± ± ± ±1.3* 11.3± ±2.5 +T3+Dex 19.1± ± ± ± ± ±6. Respiration rates of dispersed cells from WAT and BAT cultures with different hormonal supplements. All values are means ± s.e.m. from at least 4 different cell cultures at day 9 of culture. *Value significantly different (P<.5) from non-treated control. Value of BAT culture significantly different (P<.5) from corresponding WAT culture.
6 3176 S. Klaus and others nmol O / min / 1 6 adipocytes A: non stimulated - - T3 T3 +T3 +T3 +Dex +Dex +T3+Dex B: isoproterenol induced increase +T3+Dex Fig. 5. Respiration rates of adipocytes from WAT (open bars) and BAT cultures (filled bars). Preadipocytes were cultured from day 3 in medium containing 7% FCS and indicated hormonal supplements (see text for abbreviations). Respiration was measured at day 9 and adipocyte specific respiration rates were calculated from the values listed in Tables 1 and 2. UCP / 16 cells (%) T3 Dex +T3+Dex T3+Dex +Dex +T3 Fig. 6. Western blot analysis of UCP expression in total cell homogenates from WAT (open bars) and BAT (filled bars) cultures at day 9. Preadipocytes were cultured from day 3 in medium containing 7% FCS and indicated hormonal supplements (see text for abbreviations). A 2 µg sample of total cell protein was separated by SDS-PAGE, blotted onto Hybond C membrane and probed with anti- UCP immune serum using an ECL detection system. Signals were quantified by densitometrical scanning. Maximum UCP expression was set at 1% and relative UCP expression in 1 6 cells was calculated. Means of three independently performed cell cultures are shown. culture conditions as well as the total cell number (Table 1), we could thus calculate the respiration per adipocyte, by subtraction of respiration of non differentiated cells (mean value of 3.9 nmol O/min per 1 6 cells). Fig. 5 shows the non stimulated respiration and the isoproterenol induced increase in respiration of adipocytes from BAT and WAT cultures. t should be noted that even in WAT cultures the respiration of mature adipocytes was over 1 times higher than that of non differentiated cells (46 nmol O/min per 1 6 adipocytes versus 3.9 nmol O/min per 1 6 cells) and there is an excellent correlation between respiration and adipogenic index. An increased respiratory capacity can thus be considered as an additional marker of terminal differentiation for white adipocytes. n both culture types non stimulated respiration as well as β-adrenergic induced increase in respiration was highest when cells were grown with insulin + T 3. However, in this case adipocytes of BAT cultures had much higher respiration rates than those of WAT cultures. This is also reflected in cytochrome c oxidase activity, which was 84 nmol O/min per 1 6 total cells in WAT cultures compared to 147 nmol O/min per 1 6 total cells in BAT cultures (both treated with insulin + T 3 ). A further difference between WAT and BAT cultures was the increased respiration of poorly differentiated BAT adipocytes due to T 3 alone, without addition of insulin. The β- adrenergic stimulation of adipocyte respiration, i.e. thermogenesis was two and three times higher in BAT cultures than in WAT cultures supplemented with T 3 and insulin + T 3, respectively. Fully differentiated brown adipocytes cultured in standard medium (insulin + T 3 ) increased their respiratory capacity by 4 times as compared to non differentiated cells. The maximum, uncoupled respiration of 26 nmol O/min per 1 6 adipocytes was reached by brown adipocytes treated with insulin + T 3. This is very similar to respiration rates found by Nedergaard et al. (1977), who evaluated respiration of freshly isolated adipocytes from Syrian hamster brown fat. They report noradrenaline stimulated respiration rates of about 23 nmol O/min per 1 6 freshly isolated adipocytes. UCP expression As the uncoupling protein UCP represents a qualitative marker for brown adipocytes, we analyzed the UCP expression in the cell cultures by western blot analysis and immuno-fluorescent assay. Fig. 6 shows the level of UCP expression in total cell homogenates (2 µg protein of WAT and BAT cultures). n BAT cultures UCP expression was only significant in cells treated with insulin, T 3, and insulin + T 3 with highest levels always in insulin + T 3 treated cultures. This pattern of UCP expression is very much like the one of respiration rates (Fig. 5). Taken together with the adipogenic index this means that in brown adipocytes it is possible to dissociate to a certain extent terminal differentiation from thermogenic function. As UCP expression is restricted to brown adipocytes, we did not expect a UCP signal in WAT cultures. However, a weak signal was apparent in the insulin + T 3 treated cells which could indicate the presence of brown adipocytes in this cultures. As western blot analysis does not permit us to discriminate between changes in UCP expression per cell and the number of UCP expressing cells, we performed an immuno-fluorescence microscopy study to address this problem. Fig. 7 shows typical examples of immuno-fluorescent detection of lipopro-
7 White versus brown adipocytes 3177 T 3 nsulin + T 3 T 3 nsulin + T 3 WAT Fig. 7. Microphotograph of cultured stromal-vascular cells isolated from white adipose tissue (WAT) and brown adipose tissue (BAT). Preadipocytes were cultured from day 3 in medium containing 7% FCS, supplemented with T 3 (1 nm) alone or insulin (17 nm) + T 3 (1 nm). : phase contrast image with DAP stain of nucleus (blue), : immuno-fluorescent imaging of LPL (FTC, green) : immuno-fluorescent imaging of UCP (rhodamine, red).,, and are from the same microscopic field. Bar, 4 µm. BAT tein lipase (LPL) and UCP in WAT and BAT cultures treated only with T 3 or insulin + T 3. LPL was used as a marker for adipocyte differentiation and we found an excellent correlation between cells which we considered as adipocytes by visual criteria (i.e. lipid inclusions) and LPL positive cells. UCPexpression was always restricted to LPL-positive cells. t was found in about 9% of adipocytes from BAT cultures and in 1-13% of adipocytes from WAT cultures (Fig. 8) under standard culture conditions (insulin + T 3 ). This means that the low expression of UCP found by western blot analysis in WAT cultures was due to the existence of a certain percentage of brown adipocytes within WAT cultures and not to a low leaky expression of UCP in white adipocytes. The immunofluorescent image (Fig. 7) also confirms the expression of UCP in some cells of BAT cultures treated only with T 3 although these cells do not resemble fully differentiated adipocytes. Using immuno-fluorescence microscopy it is now possible to discriminate clearly between white and brown adipocytes. DSCUSSON Due to the quite opposite physiological functions of brown fat and white fat in mammalian energy metabolism, the comparative study of white and brown adipocyte development and metabolism is important for the elucidation of cellular components of energy metabolism. For small mammals like the Siberian hamster, brown fat is a powerful tool for survival in the cold due to its thermogenic function. White fat is very important as an energy store and its mass and metabolic activity is known to be highly regulated in this species. n our investigation we therefore focused on several function-related parameters that are known to be important in vivo conditions and for the differentiation state of cultured cells. Terminal differentiation Dexamethasone completely inhibited white and brown adipocyte differentiation without having an effect on total cell number, i.e. proliferation (Table 1). This is somewhat surprising, as it is known to be implicated in terminal adipocyte differentiation (reviewed by Ailhaud et al., 1992; Cornelius et al., 1994). But as our culture medium contained 7% FCS, possible interactions with serum factors cannot be excluded. Furthermore the effect of dexamethasone was shown to be different depending on the time of its addition (Xu and Björntorp, 199) and in our cultures it was added before confluence. Anyway, the complete inhibition of adipocyte differentiation by dexamethasone provides us with excellent control groups. Our culture conditions thus permit us to distinguish between 3 distinct differentiation types (non differentiated cells, poorly differen-
8 3178 S. Klaus and others % of adipocytes expressing UCP day 8 day 9 1 Fig. 8. UCP expressing adipocytes in cultures from WAT (open bars) and BAT (filled bars) at day 8 and 9. Preadipocytes were cultured from day 3 in medium containing 7% FCS, supplemented with insulin (17 nm) and T 3 (1 nm). UCP expression was limited to LPL expressing cells which were considered adipocytes. Shown are the percentages of adipocytes (+ s.e.m.) positive for UCP from multiple wells of one cell culture. tiated adipocytes and fully differentiated adipocytes), both by morphological and by metabolic criteria. Non differentiated cells showed no phenotypic characteristics of adipocytes (rounded shape, lipid accumulation) had low respiration rates (approximately 4 nmol O/min per 1 6 cells) and no lipolytic activity. These cells were indistinguishable in WAT and BAT cultures. Poorly differentiated adipocytes were rounded, displayed few lipid inclusions, showed basal lipolysis, and had severalfold increased respiration rates (e.g. 27 nmol O/min per 1 6 adipocytes in non treated WAT cultures). Finally, fully differentiated adipocytes were characterized by large triglyceride accumulations (i.e. larger size), basal lipolytic activities and further increased respiration rates (e.g. 45 nmol O/min per 1 6 adipocytes in non treated WAT cultures). Differentiation of many cell types is accompanied by increased protein synthesis and triglyceride accumulation is generally considered a marker for terminal adipocyte differentiation (Ailhaud et al., 1992; Cornelius et al., 1994). Applying these two criteria, it is obvious that insulin was the prerequisite for terminal differentiation of both white and brown adipocytes, because it significantly increased protein content and adipocyte size (as an index for lipid accumulation) in both WAT and BAT cultures (Table 1). Furthermore it increased the number of cells differentiating into adipocytes (Table 1) resulting in a 7-fold increase in the adipogenic index of WAT cultures (Fig. 2). For WAT cultures insulin was even the only hormonal factor necessary for terminal differentiation. t cannot be excluded that this effect of insulin was mediated by GF-1 receptors rather than insulin receptors as the concentration used (17 nm) is rather supra physiological and therefore likely to act via GF-1 receptors (reviewed by Cornelius et al., 1994). The hormonal regulation of brown adipocyte terminal differentiation proved to be more complex. Adipocyte size (i.e. lipid accumulation) was significantly increased by about 65% when T 3 was present in addition to insulin (Table 1). A similar picture evolves if we consider cell respiration as a marker for terminal differentiation, because T 3 further increased basal brown adipocyte respiration to 165 nmol O/min per 1 6 adipocytes as compared to 72 nmol O/min per 1 6 adipocytes with insulin alone (Fig. 5). Lipid metabolism The main function of adipose tissue lies in its capacity for lipid storage and lipid mobilization and both white and brown adipocytes are equipped with the necessary machinery for lipogenesis and lipolysis. The hormonal regulation, however of lipid storage and lipid mobilization can be quite different in white and brown fat. Lipoprotein lipase activity for example is decreased in white fat upon adrenergic stimulation, but is increased in brown fat (Carneheim et al., 1988). The hormonal regulation of lipogenic and lipolytic capacity in developing white and brown adipocytes is thus of special interest. Here we used adipocyte size as an index of triglyceride accumulation and glycerol release into the medium as an index of the lipolytic activity. Lipid accumulation has already extensively been discussed above, but it is obvious that in our culture system white adipocytes had a higher lipogenic capacity as well as basal lipolytic activity than brown adipocytes (Table 1, Fig. 3). The lipolytic capacity, however, was the same in both adipocyte types because it was stimulated to exactly the same extent by dexamethasone (Fig. 3) and in the same dose dependent manner (Fig. 4). The lipolytic action of T 3 on the other hand was very different on white and brown adipocytes. Treated with 1 µm T 3, WAT cultures released 4 times more glycerol within 48 hours than did BAT cultures (Fig. 4). Taken together, these results imply higher triglyceride turnover rates in white adipocytes and a more stringent regulation of lipolysis in brown adipocytes. Thermogenesis Thermogenesis in brown adipocytes is due to the uncoupling protein UCP whose expression is unique to the mitochondria of brown adipocytes (Cannon et al., 1982; Ricquier et al., 1992). UCP is therefore classically considered the qualitative marker protein for brown adipocytes as compared to white adipocytes and often used as an index for thermogenic capacity. However, from a physiological point of view, the important difference between the two adipose types is the high respiratory i.e. heat producing capacity of brown adipocytes compared to the very low respiratory capacity of white adipocytes. Brown fat thermogenesis is under the control of the sympathetic nervous system, acting through β-adrenergic receptors (Seydoux and Girardier, 1978; Bukowiecki et al., 1981). n order to investigate more directly the thermogenesis of cultured adipocytes, we measured UCP expression as well as unstimulated respiration and the stimulation of respiration by a β-adrenergic agonist (isoproterenol). This method has been used to evaluate the thermogenesis of brown fat cells isolated from the brown fat of animals adapted to different temperatures (Bukowiecki et al., 198; Locke et al., 1982). To our knowledge, respiration measurements have only once been applied to cultured preadipocytes from WAT and BAT (Néchad et al., 1983); however, those respiration rates were very low compared to our data and no stimulation by β-adrenergic agonist was found.
9 White versus brown adipocytes 3179 Our results show clearly that development of increased thermogenic capacities of brown adipocytes depends largely on the hormonal environment during differentiation. T 3 proved to be very important as assessed by respiration measurements as well as UCP detection (Figs 5, 6, and 7). T 3 was able to increase thermogenesis as well as UCP expression alone and synergistically with insulin. This corroborates and extends previous studies which have shown the importance of T 3 for UCP expression in vivo (Silva and Bianco, 199; Reiter et al., 199) and in vitro (Rehnmark et al., 199; Klaus et al., 1991b; Guerra et al., 1994). n cultured WAT preadipocytes a small effect of T 3 on thermogenesis was observed (Fig. 5B). But if we take into account that 1 to 15% of adipocytes in WAT cultures are really brown adipocytes (Fig. 8) and subtract the respective respiration rates, the β-adrenergic induced increase in respiration of white adipocytes was very similar in all experimental groups (between 6.5 and 8.5 nmol O/min per 1 6 adipocytes) and thus 7 to 8 times lower than the value for brown adipocytes treated with isulin + T 3 (61 nmol O/min per 1 6 adipocytes). This increase in respiration is surprisingly similar to the norepinephrine induced stimulation of respiration of brown adipocytes isolated from warm adapted guinea pigs (Locke et al., 1982; Rafael et al., 1986). Adipocytes isolated from cold adapted guinea pigs and thermoneutrally kept rats were reported to show up to 1-fold increased respiration rates upon stimulation by β-adrenergic agonists (Locke et al., 1982, Bukowiecki et al., 198, Rafael et al., 1986). t will be interesting to use our culture system in order to investigate if the presence of β-adenergic agonists will improve the thermogenic capacity of brown adipocytes differentiated in vitro. Overall, a very good correlation of adipocyte thermogenesis (Fig. 5B) with UCP expression (Fig. 6) indicates that UCP is functional in brown adipocytes differentiated in vitro. Relatively high unstimulated respiration rates in our study (up to 164 nmol O/min per 1 6 adipocytes) might indicate that respiration of these cells is, in culture, already partially uncoupled in the absence of adrenergic stimulation. Although in the inguinal fat pad that we used for isolation of preadipocytes normally no UCP (protein or mrna) could be detected (data not shown), we could detect UCP in total cell homogenates of WAT cultures treated with insulin + T 3 (Fig. 6). Our immuno-fluorescent results prove that this was not due to an expression leakage but that UCP expression was confined to discrete sub-populations of adipocytes. This corroborates results obtained in vivo, indicating the presence of UCP expressing fat cells i.e. brown adipocytes, in typical white fat deposits (Lonçar, 1991; Cousin et al., 1992). t will be interesting to investigate if β-adrenergic stimulation during adipocyte development might increase the number of UCP expressing cells, i.e. recruit new brown adipocytes in WAT cultures. This is now possible using immuno-fluorescent detection of UCP expression. Considering the very low metabolic activity of white fat in vivo, the respiration rates of cultured white adipocytes seem to be rather high. This is partly due to the presence of brown adipocytes as shown in Fig. 8. However, it is also possible that mitochondria are more abundant during early development of white adipocytes and reduced later on. Therefore it will be interesting to investigate the thermogenic capacity of WAT and BAT cultures during long term cultures. Conclusions Using a comparative approach we could clearly demonstrate a different determination of brown preadipocytes as compared to white preadipocytes. The major difference between the two preadipocyte types lies in their reaction to T 3, which evokes different metabolic responses in the two preadipocyte types. The presence of T 3 is necessary for the development of maximal thermogenic capacity of brown adipocytes but does not increase the thermogenic capacity of white adipocytes. t remains to be established if this is due to different characteristics of T 3 binding to nuclear receptors or post receptor binding events. Our results also demonstrate for the first time in brown adipocytes a dissociation of the thermogenic function from the lipid storage function. The hormonal regulation of the latter appears to be very similar in white and brown adipocytes, whereas the ability to develop thermogenic functions seems to be intrinsic to brown preadipocytes. Thanks are due to Norbert Radomski from the University in Gießen (Germany) who helped us to set up the indirect immuno-fluorescent microscopy. This study was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to S. Klaus (Kl 613/2-1 and Kl 613/2-2) and G. Heldmaier (SFB 35). REFERENCES Ailhaud, G. (199). Extracellular factors, signalling pathways and differentiation of adipose precursor cells. Curr. Opin. Cell Biol. 2, Ailhaud, G., Grimaldi, P. and Négrel, R. (1992). Cellular and molecular aspects of adipose tissue development. Annu. Rev. Nutr. 12, Bianco, A. C., Sheng, X. and Silva, J. E (1988). Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J. Biol. Chem. 263, Bronnikov, G., Houstek, J. and Nedergaard, J. (1992). β-adrenergic, campmediated stimulation of proliferation of brown fat cells in primary culture. J. Biol. Chem. 267, Bukowiecki, L., Folléa, N., Paradis, A. and Collet, A. J. (198). Stereospecific stimulation of brown adipose tissue respiration by catecholamines via β1-adrenergic receptors. Am. J. Physiol. 238, E552- E563. Bukowiecki, L., Folléa, N., Lupien, J. and Paradis, A., (1981). Metabolic relationships between lipolysis and respiration in rat brown adipocytes. J. Biol. Chem. 256, Butterwirth, S. C. (1994). Molecular events in adipocyte development. Pharmac. Ther. 61, Carneheim, C., Nedergaard, J. and Cannon, B. (1988). Cold-induced β- adrenergic recruitment of lipoprotein lipase in brown fat is due to increased transcription. Am. J. Physiol. 254, E155-E161. Casteilla, L., Champigny, O., Bouillaud, F., Robelin, J. and Ricquier, D. (1989). Sequential changes in the expression of mitochondrial protein mrna during the development of brown adipose tissue in bovine and ovine species. Biochem. J. 257, Casteilla, L., Nougués, J., Reyne, Y. and Ricquier, D. (1991). Differentiation of ovine brown adipocyte precursor cells in a chemically defined serum-free medium. mportance of glucocorticoids and age of animals. Eur. J. Biochem. 198, Champigny, O., Holloway, B. R. and Ricquier, D. (1992). Regulation of UCP gene expression in brown adipocytes differentiated in primary culture: effects of a new β-adrenoceptor agonist. Mol. Cell. Endocrinol. 86, Cannon, B., Hedin, A. and Nedergaard, J. (1982). Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett. 15, Cornelius, P., MacDougald, O. A. and Lane, M. D. (1994). Regulation of adipocyte development. Annu. Rev. Nutr. 14, Cousin, B., Cinti, S., Morroni, M., Raimbault, S., Ricquier, D., Pénicaud, L. and Casteilla, L. (1992). Occurrence of brown adipocytes in rat white
10 318 S. Klaus and others adipose tissue: molecular and morphological characterization. J. Cell Science 13, Darimont, C., Gaillard, D., Ailhaud, G. and Négrel, R. (1993). Terminal differentiation of mouse preadipocyte cells: adipogenic and anti mitogenic role of triiodothyronine. Mol. Cell. Endocrinol. 98, Geloen, A. and Trayhurn, P. (199). Regulation of uncoupling protein in brown adipose tissue by insulin. Am. J. Physiol. 258, R418-R424. Grimaldi, P., Dijan, P., Négrel, R. and Ailhaud, G. (1982). Differentiation of Ob17 preadipocytes to adipocytes: requirements of adipose conversion factor(s) for fat cell cluster formation. EMBO J. 1, Guerra, C., Porra, A., Roncero, C., Benito, M. and Fernandez, M. (1994). Triiodothyronine induces the expression of the uncoupling protein in long term fetal rat brown adipocyte primary cultures: role of nuclear thyroid hormone receptor expression. Endocrinology 134, Hauner, H. (199). Complete adipose differentiation of 3T3-L1 cells in a chemically defined medium: comparison to serum-containing culture conditions. Endocrinology 127, Klaus, S., Casteilla, L., Bouillaud, F. and Ricquier, D. (1991a). The uncoupling protein UCP: A membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. nt. J. Biochem. 23, Klaus, S., Cassard-Doulcier, A.-M. and Ricquier, D. (1991b). Development of Phodopus sungorus brown preadipocytes in primary cell culture: effect of an atypical beta-adrenergic agonist, insulin, and triiodothyronin on differentiation, mitochondrial development, and expression of the uncoupling protein UCP. J. Cell Biol. 115, Klaus, S., Choy, L., Champigny, O., Cassard-Doulcier, A.-M., Ross, S., Spiegelman, B. and Ricquier, D. (1994). Characterization of the novel brown adipocyte cell line HB 1B. Adrenergic pathways involved in regulation of uncoupling protein gene expression. J. Cell Sci. 17, Kopecky, J., Baudysova, M., Zanotti, F., Janikova, D., Pavelka, S. and Houstek, J. (199). Synthesis of mitochondrial uncoupling protein in brown adipocytes differentiated in cell culture. J. Biol. Chem. 265, Kozak, U. C. and Kozak, L. P. (1994). Norepinephrine-dependent selection of brown adipocyte cell lines. Endocrinology 134, Locke, R. M., Rial, E. and Nicholls, D. G. (1982). The acute regulation of mitochondrial proton-conductance in cells and mitochondria from the brown fat of cold-adapted and warm-adapted guinea pigs. Eur. J. Biochem. 129, Lonçar, D. (1991). Convertible adipose tissue in mice. Cell. Tiss. Res. 266, Nedergaard, J., Cannon, B. and Lindberg, O. (1977). Microcalorimetry of isolated mammalian cells. Nature 267, Néchad, M., Kuusela, P., Carneheim, C., Björntorp, P., Nedergaard, J. and Cannon, B. (1983). Development of brown fat cells in monolayer culture.. Morphological and biochemical distinction from white fat cells in culture. Exp. Cell Res. 149, Néchad, M. (1986). Structure and development of brown adipose tissue. n Brown Adipose Tissue (ed. P. Trayhurn and D. G. Nicholls), pp London: E. Arnold Ltd. Nicholls, D. G., Cunningham, S. and Rial, E. (1986). The bioenergetic mechanism of brown adipose tissue mitochondria. n Brown Adipose Tissue. (ed. P. Trayhurn, and D. G. Nicholls), pp London: E. Arnold Ltd. Park, L. R. A., Mount, D. B. and Himms-Hagen, J. (1989). Role of T 3 in thermogenic and trophic responses of brown adipose tissue to cold. Am. J. Physiol. 257, E81-E87. Rafael, J., Fesser, W. and Nicholls, D. G. (1986). Cold adaptation in guinea pig at level of isolated brown adipocyte. Am. J. Physiol. 25, C1-C9. Reiter, J. R., Klaus, S., Ebbinghaus, C., Heldmaier, G., Redlin, U., Ricquier, D., Vaughan, M. K. and Steinlechner, S. (199). nhibition of 5 deiodination of thryroxine suppresses the cold-induced increase in brown adipose tissue mrna for mitochondrial uncoupling protein without influencing lipoprotein lipase activity. Endocrinology 126, Rehnmark, S., Kopecky, J., Jacobsson, A., Néchad, M., Herron, D., Nelson, B. D., Obregon, M.-J., Nedergaard, J. and Cannon, B. (1989). Brown adipocytes differentiated in vitro can express the gene for the uncoupling protein thermogenin: effects of hypothyroidism and norepinephrine. Exp. Cell Res. 182, Rehnmark, S., Néchad, M., Herron, D., Cannon, B. and Nedergaard, J. (199). Alpha- and beta-adrenergic induction of the expression of the uncoupling protein thermogenin in brown adipocytes differentiated in culture. J. Biol. Chem. 265, Ricquier, D., Casteilla, L. and Bouillaud, F. (1991). Molecular studies of the uncoupling protein. FASEB J. 5, Ricquier, D., Raimbault, S., Champigny, O., Miroux, B. and Bouillaud, F. (1992). The uncoupling protein is not expressed in rat liver. FEBS Lett. 33, Rothwell, N. and Stock, M. (1986). Brown adipose tissue and diet-induced thermogenesis. n Brown Adipose Tissue (ed. P. Trayhurn and D. G. Nicholls), pp London: E. Arnold Ltd. Seydoux, J. and Girardier, L. (1978). Control of brown fat thermogenesis by the sympathetic nervous system. Experientia Suppl. 32, Silva, J. and Bianco, C. (199). Role of local thyroxine 5 deiodinase on the response of brown adipose tissue (BAT) to β-adrenergic stimulation. n Obesity: Towards a Molecular Approach, UCLA Symposium on Molecular and Cellular Biology, New Series (ed. G. Bray et al.), pp Wiley- Liss, nc., New York. Xu, X. and Björntorp, P. (199). Effects of dexamethasone on multiplication and differentiation of rat adipose precursor cells. Exp. Cell Res. 189, (Received 19 June Accepted 25 July 1995)
ab Adipogenesis Assay Kit (Cell-Based)
 ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
Differences in the response of UCP1 mrna to hormonal stimulation between rat and mouse
 1 Differences in the response of UCP1 mrna to hormonal stimulation between rat and mouse primary cultures of brown adipocytes Arturo Hernandez 1 *, Raquel Martinez de Mena*, Eva Martin, and Maria-Jesus
1 Differences in the response of UCP1 mrna to hormonal stimulation between rat and mouse primary cultures of brown adipocytes Arturo Hernandez 1 *, Raquel Martinez de Mena*, Eva Martin, and Maria-Jesus
Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization
 Journal of Cell Science 103, 931-942 (1992) Printed in Great Britain The Company of Biologists Limited 1992 931 Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization
Journal of Cell Science 103, 931-942 (1992) Printed in Great Britain The Company of Biologists Limited 1992 931 Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization
Growth factor regulation of uncoupling protein-1 mrna expression in brown adipocytes
 Am J Physiol Cell Physiol 282: C105 C112, 2002. First published October 3, 2001; 10.1152/ajpcell.01396.2000. Growth factor regulation of uncoupling protein-1 mrna expression in brown adipocytes BIBIAN
Am J Physiol Cell Physiol 282: C105 C112, 2002. First published October 3, 2001; 10.1152/ajpcell.01396.2000. Growth factor regulation of uncoupling protein-1 mrna expression in brown adipocytes BIBIAN
BEIGE AND BROWN FAT: BASIC BIOLOGY AND NOVEL THERAPEUTICS Dr. Carl Ascoli
 BEIGE AND BROWN FAT: BASIC BIOLOGY AND NOVEL THERAPEUTICS Dr. Carl Ascoli Symposium Co-Chairs: Bruce M. Spiegelman (Harvard/Dana Farber) and Sven Enerbäck (U.Gothenburg) April 17-23, 2015 Snowbird Resort,
BEIGE AND BROWN FAT: BASIC BIOLOGY AND NOVEL THERAPEUTICS Dr. Carl Ascoli Symposium Co-Chairs: Bruce M. Spiegelman (Harvard/Dana Farber) and Sven Enerbäck (U.Gothenburg) April 17-23, 2015 Snowbird Resort,
For the rapid, sensitive and accurate measurement of Glycerol in cell cultures.
 ab185433 Lipolysis Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Glycerol in cell cultures. This product is for research use only and is not intended
ab185433 Lipolysis Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Glycerol in cell cultures. This product is for research use only and is not intended
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well
 Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
In The Name Of God. In The Name Of. EMRI Modeling Group
 In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
In The Name Of God In The Name Of God EMRI Modeling Group Cells work together in functionally related groups called tissues Types of tissues: Epithelial lining and covering Connective support Muscle movement
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
GPR120 *** * * Liver BAT iwat ewat mwat Ileum Colon. UCP1 mrna ***
 a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
a GPR120 GPR120 mrna/ppia mrna Arbitrary Units 150 100 50 Liver BAT iwat ewat mwat Ileum Colon b UCP1 mrna Fold induction 20 15 10 5 - camp camp SB202190 - - - H89 - - - - - GW7647 Supplementary Figure
Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set
 Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Influenza A H1N1 (Swine Flu 2009) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit
 2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
2009 H1N1 Influenza ( Swine Flu ) Hemagglutinin ELISA kit Catalog Number : SEK001 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as
Application Note. Introduction
 Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
exposed operative area for presence of small islands (±1-2 cm) of adipose tissue from
 Supplemental Material Details for the biopsy procedure During surgery, both the surgeon (N.B.) and the researcher (G.V.) visually inspected the exposed operative area for presence of small islands (±1-2
Supplemental Material Details for the biopsy procedure During surgery, both the surgeon (N.B.) and the researcher (G.V.) visually inspected the exposed operative area for presence of small islands (±1-2
Metabolically functional brown adipose tissue can be pharmacologically stimulated
 J. Physiol. (1981), 314, pp. 85-89 85 With I text figure Printed in Great Britain THERMOGENESIS IN NORMAL RABBITS AND RATS: NO ROLE FOR BROWN ADIPOSE TISSUE? BY J. M. BROCKWAY AND G. E. LOBLEY From the
J. Physiol. (1981), 314, pp. 85-89 85 With I text figure Printed in Great Britain THERMOGENESIS IN NORMAL RABBITS AND RATS: NO ROLE FOR BROWN ADIPOSE TISSUE? BY J. M. BROCKWAY AND G. E. LOBLEY From the
Lipid (Oil Red O) staining Kit
 Lipid (Oil Red O) staining Kit Catalog Number KA4541 1 Kit Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Lipid (Oil Red O) staining Kit Catalog Number KA4541 1 Kit Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit
 INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
Lecithin Cholesterol Acyltransferase (LCAT) ELISA Kit
 Product Manual Lecithin Cholesterol Acyltransferase (LCAT) ELISA Kit Catalog Number STA-616 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is a lipid sterol
Product Manual Lecithin Cholesterol Acyltransferase (LCAT) ELISA Kit Catalog Number STA-616 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is a lipid sterol
Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at
 Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at doses of 0.1, 0.5 and 1 mg/kg on cumulative food intake
Fig. S1. Dose-response effects of acute administration of the β3 adrenoceptor agonists CL316243, BRL37344, ICI215,001, ZD7114, ZD2079 and CGP12177 at doses of 0.1, 0.5 and 1 mg/kg on cumulative food intake
Human Apolipoprotein A1 EIA Kit
 A helping hand for your research Product Manual Human Apolipoprotein A1 EIA Kit Catalog Number: 83901 96 assays 1 Table of Content Product Description 3 Assay Principle 3 Kit Components 3 Storage 4 Reagent
A helping hand for your research Product Manual Human Apolipoprotein A1 EIA Kit Catalog Number: 83901 96 assays 1 Table of Content Product Description 3 Assay Principle 3 Kit Components 3 Storage 4 Reagent
Burning Fat for Body Shaping and Cellulite Treatment english. F. Wandrey, R. Sacher, D. Schmid, F. Suter, F. Zülli
 09/16 Volume 142 Thannhausen, September 9, 2016 02 09 2016 english Burning Fat for Body Shaping and Cellulite Treatment F. Wandrey, R. Sacher, D. Schmid, F. Suter, F. Zülli personal care anti-cellulite
09/16 Volume 142 Thannhausen, September 9, 2016 02 09 2016 english Burning Fat for Body Shaping and Cellulite Treatment F. Wandrey, R. Sacher, D. Schmid, F. Suter, F. Zülli personal care anti-cellulite
Differentiation and characterization in primary culture of white adipose tissue brown adipocyte-like cells
 (29) 33, 68 686 & 29 Macmillan Publishers Limited All rights reserved 37-565/9 $32. ORIGINAL ARTICLE www.nature.com/ijo Differentiation and characterization in primary culture of white adipose tissue brown
(29) 33, 68 686 & 29 Macmillan Publishers Limited All rights reserved 37-565/9 $32. ORIGINAL ARTICLE www.nature.com/ijo Differentiation and characterization in primary culture of white adipose tissue brown
Instructions. Fuse-It-mRNA easy. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Influenza A H7N9 (A/Anhui/1/2013) Hemagglutinin / HA ELISA Pair Set
 Influenza A H7N9 (A/Anhui/1/2013) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK40103 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Influenza A H7N9 (A/Anhui/1/2013) Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK40103 To achieve the best assay results, this manual must be read carefully before using this product and the assay
Human Leptin ELISA Kit
 Product Manual Human Leptin ELISA Kit Catalog Numbers MET-5057 MET-5057-5 96 assays 5 x 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Leptin is a polypeptide hormone
Product Manual Human Leptin ELISA Kit Catalog Numbers MET-5057 MET-5057-5 96 assays 5 x 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Leptin is a polypeptide hormone
Free Fatty Acid Uptake Assay Kit (Fluorometric)
 ab176768 Free Fatty Acid Uptake Assay Kit (Fluorometric) Instructions for Use For measurement of fatty acid uptake in cells containing fatty acid transporters. This product is for research use only and
ab176768 Free Fatty Acid Uptake Assay Kit (Fluorometric) Instructions for Use For measurement of fatty acid uptake in cells containing fatty acid transporters. This product is for research use only and
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Free Fatty Acid Assay Kit (Fluorometric)
 Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
Product Manual Free Fatty Acid Assay Kit (Fluorometric) Catalog Number STA-619 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Triglycerides (TAG) are a type of lipid
BROWN ADIPOSE TISSUE, β3-adrenergic RECEPTORS, AND OBESITY
 Annu. Rev. Med. 1997. 48:307 16 Copyright 1997 by Annual Reviews Inc. All rights reserved BROWN ADIPOSE TISSUE, β3-adrenergic RECEPTORS, AND OBESITY B. B. Lowell, MD, PhD, and J. S. Flier, MD Division
Annu. Rev. Med. 1997. 48:307 16 Copyright 1997 by Annual Reviews Inc. All rights reserved BROWN ADIPOSE TISSUE, β3-adrenergic RECEPTORS, AND OBESITY B. B. Lowell, MD, PhD, and J. S. Flier, MD Division
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
Metabolic Solutions Development Company, Kalamazoo, USA.
 New Insulin Sensitizers Produce Differentiation of Brown-like Adipose Cells from a Subcutaneous Fat Depot and Increase Secretion of Adiponectin in vitro William G. McDonald, Serena L. Cole, Danielle D.
New Insulin Sensitizers Produce Differentiation of Brown-like Adipose Cells from a Subcutaneous Fat Depot and Increase Secretion of Adiponectin in vitro William G. McDonald, Serena L. Cole, Danielle D.
A HISTOLOGICAL STRUCTURE AND SPECIFIC PROTEIN OF BROWN ADIPOSE TISSUE OF MONGOLIAN LAMBS
 8 A HISTOLOGICAL STRUCTURE AND SPECIFIC PROTEIN OF BROWN ADIPOSE TISSUE OF MONGOLIAN LAMBS KhorolmaaCh. 1, Demberel Sh. 2, Battsetseg B. 2, Gereltsetseg G. 1, and AndreiS. 1 1- School of veterinary medicine,
8 A HISTOLOGICAL STRUCTURE AND SPECIFIC PROTEIN OF BROWN ADIPOSE TISSUE OF MONGOLIAN LAMBS KhorolmaaCh. 1, Demberel Sh. 2, Battsetseg B. 2, Gereltsetseg G. 1, and AndreiS. 1 1- School of veterinary medicine,
Islet Assay Using the Agilent Seahorse XF24 Islet Capture Microplate
 Islet Assay Using the Agilent Seahorse XF24 Islet Capture Microplate Technical Overview Introduction Agilent Seahorse XF Analyzers are most commonly used with an adherent monolayer of cells attached to
Islet Assay Using the Agilent Seahorse XF24 Islet Capture Microplate Technical Overview Introduction Agilent Seahorse XF Analyzers are most commonly used with an adherent monolayer of cells attached to
Mouse primary keratinocytes preparation
 Mouse primary keratinocytes preparation 1. Fill a 150 X 25 mm petri dish with ice. Put newborn mice (2 3 days old) in the petri dish and insert it in an ice bucket. Leave the mice in the ice bucket for
Mouse primary keratinocytes preparation 1. Fill a 150 X 25 mm petri dish with ice. Put newborn mice (2 3 days old) in the petri dish and insert it in an ice bucket. Leave the mice in the ice bucket for
Control 7 d cold 7 d CL
 Control 7 d cold 7 d ibat iwat gwat Supplementary Figure 1. Histology of adipose tissues after cold or 3-adrenergic receptor stimulation. C57BL/6J wild-type mice were housed at 4 C or injected daily with
Control 7 d cold 7 d ibat iwat gwat Supplementary Figure 1. Histology of adipose tissues after cold or 3-adrenergic receptor stimulation. C57BL/6J wild-type mice were housed at 4 C or injected daily with
Influenza A H1N1 HA ELISA Pair Set
 Influenza A H1N1 HA ELISA Pair Set for H1N1 ( A/Puerto Rico/8/1934 ) HA Catalog Number : SEK11684 To achieve the best assay results, this manual must be read carefully before using this product and the
Influenza A H1N1 HA ELISA Pair Set for H1N1 ( A/Puerto Rico/8/1934 ) HA Catalog Number : SEK11684 To achieve the best assay results, this manual must be read carefully before using this product and the
Supplemental Information Supplementary Table 1. Tph1+/+ Tph1 / Analyte Supplementary Table 2. Tissue Vehicle LP value
 Supplemental Information Supplementary Table. Urinary and adipose tissue catecholamines in Tph +/+ and Tph / mice fed a high fat diet for weeks. Tph +/+ Tph / Analyte ewat ibat ewat ibat Urine (ng/ml)
Supplemental Information Supplementary Table. Urinary and adipose tissue catecholamines in Tph +/+ and Tph / mice fed a high fat diet for weeks. Tph +/+ Tph / Analyte ewat ibat ewat ibat Urine (ng/ml)
Islet Oxygen Consumption Assay using the XF24 Islet Capture Microplate Shirihai Lab and Seahorse Bioscience Aug
 1 Islet Oxygen Consumption Assay using the XF24 Islet Capture Microplate Shirihai Lab and Seahorse Bioscience Aug 6 2009 XF Analyzers are most commonly used with an adherent monolayer of cells attached
1 Islet Oxygen Consumption Assay using the XF24 Islet Capture Microplate Shirihai Lab and Seahorse Bioscience Aug 6 2009 XF Analyzers are most commonly used with an adherent monolayer of cells attached
Global Histone H3 Acetylation Assay Kit
 Global Histone H3 Acetylation Assay Kit Catalog Number KA0633 96 assays Version: 06 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Global Histone H3 Acetylation Assay Kit Catalog Number KA0633 96 assays Version: 06 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric)
 Version 10 Last updated 19 December 2017 ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric) For the measurement of triglycerides in various samples. This product is for research
Version 10 Last updated 19 December 2017 ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric) For the measurement of triglycerides in various samples. This product is for research
Cryopreserved HepaRG Cells and Media Supplements
 Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
ab Intracellular O 2 Probe
 ab140101 Intracellular O 2 Probe Instructions for Use For the measurement of intracellular oxygen in populations of live mammalian cells This product is for research use only and is not intended for diagnostic
ab140101 Intracellular O 2 Probe Instructions for Use For the measurement of intracellular oxygen in populations of live mammalian cells This product is for research use only and is not intended for diagnostic
Supplementary Information
 Supplementary Information GADD34-deficient mice develop obesity, nonalcoholic fatty liver disease, hepatic carcinoma and insulin resistance Naomi Nishio and Ken-ichi Isobe Department of Immunology, Nagoya
Supplementary Information GADD34-deficient mice develop obesity, nonalcoholic fatty liver disease, hepatic carcinoma and insulin resistance Naomi Nishio and Ken-ichi Isobe Department of Immunology, Nagoya
Laboratoire de Physio%gie comparée IL.A. 307, CNRSJ, Université P. et M. Curie, 4, place Jussieu, Paris Cedex 05, France
 Factors controlling brown adipose tissue development D. RICQUIER, G. MORY, F. BOUILLAUD, Michèle COMBES-GEORGE J. THIBAULT Christine BLANCHARD Laboratoire de Physio%gie comparée IL.A. 307, CNRSJ, Université
Factors controlling brown adipose tissue development D. RICQUIER, G. MORY, F. BOUILLAUD, Michèle COMBES-GEORGE J. THIBAULT Christine BLANCHARD Laboratoire de Physio%gie comparée IL.A. 307, CNRSJ, Université
MTS assay in A549 cells
 Project: VIGO MTS assay in A549 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
Project: VIGO MTS assay in A549 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
ab LDL Uptake Assay Kit (Cell-Based)
 ab133127 LDL Uptake Assay Kit (Cell-Based) Instructions for Use For the detection of LDL uptake into cultured cells. This product is for research use only and is not intended for diagnostic use. Version
ab133127 LDL Uptake Assay Kit (Cell-Based) Instructions for Use For the detection of LDL uptake into cultured cells. This product is for research use only and is not intended for diagnostic use. Version
Mamofillin New aesthetic perspective
 New aesthetic perspective info@ White adipose tissue (WAT) White adipose tissue (WAT) is the prevalent type in human adults functioning as the major storage site for the lipids absorbed from daily intake
New aesthetic perspective info@ White adipose tissue (WAT) White adipose tissue (WAT) is the prevalent type in human adults functioning as the major storage site for the lipids absorbed from daily intake
ab CytoPainter Golgi/ER Staining Kit
 ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
Product # R8132 (Explorer Kit) R8133 (Bulk Kit)
 Product Insert QBT Fatty Acid Uptake Assay Kit Product # R8132 (Explorer Kit) R8133 (Bulk Kit) Introduction About the Fatty Acid Uptake Assay Kit The homogeneous QBT Fatty Acid Uptake Assay Kit from Molecular
Product Insert QBT Fatty Acid Uptake Assay Kit Product # R8132 (Explorer Kit) R8133 (Bulk Kit) Introduction About the Fatty Acid Uptake Assay Kit The homogeneous QBT Fatty Acid Uptake Assay Kit from Molecular
Essential Medium, containing 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Huvec were cultured in
 Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
Total Phosphatidic Acid Assay Kit
 Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Kit for assay of thioredoxin
 FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by
 Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
ab65311 Cytochrome c Releasing Apoptosis Assay Kit
 ab65311 Cytochrome c Releasing Apoptosis Assay Kit Instructions for Use For the rapid, sensitive and accurate detection of Cytochrome c translocation from Mitochondria into Cytosol during Apoptosis in
ab65311 Cytochrome c Releasing Apoptosis Assay Kit Instructions for Use For the rapid, sensitive and accurate detection of Cytochrome c translocation from Mitochondria into Cytosol during Apoptosis in
Annexin V-PE Apoptosis Detection Kit
 Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Lipoprotein Lipase Activity Assay Kit (Fluorometric)
 Lipoprotein Lipase Activity Assay Kit (Fluorometric) Catalog Number KA4538 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Lipoprotein Lipase Activity Assay Kit (Fluorometric) Catalog Number KA4538 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Influenza B Hemagglutinin / HA ELISA Pair Set
 Influenza B Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK11053 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
Influenza B Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK11053 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/9/e1500781/dc1 Supplementary Materials for pnaktide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis Komal Sodhi, Kyle Maxwell,
advances.sciencemag.org/cgi/content/full/1/9/e1500781/dc1 Supplementary Materials for pnaktide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis Komal Sodhi, Kyle Maxwell,
Thyroid hormones derived from two iodinated tyrosine molecules
 Thyroid Hormones OBJECTIVES Chemical nature of the thyroid hormones How different enzymes play a role in thyroid hormone formation? And what drugs affect them? Describe Function & Metabolism of thyroid
Thyroid Hormones OBJECTIVES Chemical nature of the thyroid hormones How different enzymes play a role in thyroid hormone formation? And what drugs affect them? Describe Function & Metabolism of thyroid
Beige fat: A New Hope for Metabolic Disorder
 Young Biomedical Scientists Forum(YBSF) Beige fat: A New Hope for Metabolic Disorder Harim Kim(2) 1 Ewha University, School of Medicine, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750 Korea k3541729@ybsf21.org
Young Biomedical Scientists Forum(YBSF) Beige fat: A New Hope for Metabolic Disorder Harim Kim(2) 1 Ewha University, School of Medicine, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750 Korea k3541729@ybsf21.org
For the isolation of mitochondria from P. pastoris and other species of yeast
 ab178779 Mitochondrial Yeast Isolation Kit Instructions for Use For the isolation of mitochondria from P. pastoris and other species of yeast This product is for research use only and is not intended for
ab178779 Mitochondrial Yeast Isolation Kit Instructions for Use For the isolation of mitochondria from P. pastoris and other species of yeast This product is for research use only and is not intended for
Lipase Assay Kit. Catalog Number KA assays Version: 02. Intended for research use only.
 Lipase Assay Kit Catalog Number KA1654 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3 General Information...
Lipase Assay Kit Catalog Number KA1654 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3 General Information...
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Triglyceride Assay Kit-Bulk 5 Plate Kit Cat# TG-5-RB
 Triglyceride Assay Kit-Bulk 5 Plate Kit Cat# TG-5-RB INSTRUCTION MANUAL (ZBM-14) STORAGE CONDITIONS Reagents A & B, Buffers: Store at 2-8 C. Glycerol Standard -20 C For Research Use Only Not For Use In
Triglyceride Assay Kit-Bulk 5 Plate Kit Cat# TG-5-RB INSTRUCTION MANUAL (ZBM-14) STORAGE CONDITIONS Reagents A & B, Buffers: Store at 2-8 C. Glycerol Standard -20 C For Research Use Only Not For Use In
EPIGENTEK. EpiQuik Global Histone H3 Acetylation Assay Kit. Base Catalog # P-4008 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE
 EpiQuik Global Histone H3 Acetylation Assay Kit Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Global Histone H3 Acetylation Assay Kit is suitable for specifically measuring global
EpiQuik Global Histone H3 Acetylation Assay Kit Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Global Histone H3 Acetylation Assay Kit is suitable for specifically measuring global
Multi-Parameter Apoptosis Assay Kit
 Multi-Parameter Apoptosis Assay Kit Catalog Number KA1335 5 x 96 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Multi-Parameter Apoptosis Assay Kit Catalog Number KA1335 5 x 96 assays Version: 05 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Introduction Kit Components Cat. # # of vials Reagent Quantity Storage
 Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
control kda ATGL ATGLi HSL 82 GAPDH * ** *** WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi iwat gwat ibat
 body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
MBB317. Dr D MANGNALL OBESITY. Lecture 2
 MBB317 Dr D MANGNALL OBESITY Lecture 2 When the structure of the insulin receptor was first discovered it was assumed that the active beta subunit tyrosine kinase would phosphorylate some intracellular
MBB317 Dr D MANGNALL OBESITY Lecture 2 When the structure of the insulin receptor was first discovered it was assumed that the active beta subunit tyrosine kinase would phosphorylate some intracellular
Glucose Uptake Assay Kit (Red Fluorescence)
 Glucose Uptake Assay Kit (Red Fluorescence) Catalog Number KA4085 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the
Glucose Uptake Assay Kit (Red Fluorescence) Catalog Number KA4085 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the
ab Membrane fluidity kit Instructions for Use For the detection of membrane fluidity in cells
 ab189819 Membrane fluidity kit Instructions for Use For the detection of membrane fluidity in cells This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated
ab189819 Membrane fluidity kit Instructions for Use For the detection of membrane fluidity in cells This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated
Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis
 icell Skeletal Myoblasts Application Protocol Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis Introduction The skeletal muscle is one of the
icell Skeletal Myoblasts Application Protocol Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis Introduction The skeletal muscle is one of the
ab Pyruvate dehydrogenase (PDH) Enzyme Activity Dipstick Assay Kit
 ab109882 Pyruvate dehydrogenase (PDH) Enzyme Activity Dipstick Assay Kit Instructions for Use For the quantitative measurement of Pyruvate dehydrogenase (PDH) activity in samples from human, mouse, rat,
ab109882 Pyruvate dehydrogenase (PDH) Enzyme Activity Dipstick Assay Kit Instructions for Use For the quantitative measurement of Pyruvate dehydrogenase (PDH) activity in samples from human, mouse, rat,
binding capacity seen after a single injection of noradrenaline. surgical denervation.
 J. Physiol. (1984), 355, pp. 457-463 457 Printed in Great Britain EFFECTS OF DENERVATING BROWN ADIPOSE TISSUE ON THE RESPONSES TO COLD, HYPERPHAGIA AND NORADRENALINE TREATMENT IN THE RAT BY NANCY J. ROTHWELL
J. Physiol. (1984), 355, pp. 457-463 457 Printed in Great Britain EFFECTS OF DENERVATING BROWN ADIPOSE TISSUE ON THE RESPONSES TO COLD, HYPERPHAGIA AND NORADRENALINE TREATMENT IN THE RAT BY NANCY J. ROTHWELL
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets
 Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
ShapePerfection Burns fat fights cellulite
 Burns fat fights cellulite Burns fat fights cellulite Spicy Substances to Fight Cellulite and Excess Centimeters ShapePerfection is a liposoluble anti-cellulite slimming active ingredient that is based
Burns fat fights cellulite Burns fat fights cellulite Spicy Substances to Fight Cellulite and Excess Centimeters ShapePerfection is a liposoluble anti-cellulite slimming active ingredient that is based
Human LDL Receptor / LDLR ELISA Pair Set
 Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
Human LDL Receptor / LDLR ELISA Pair Set Catalog Number : SEK10231 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in
ab Hepatic Lipid Accumulation/ Steatosis Assay Kit
 ab133131 Hepatic Lipid Accumulation/ Steatosis Assay Kit Instructions for Use For evaluating steatosis risk of drug candidates using Oil Red O to stain neutral lipids in hepatocytes. This product is for
ab133131 Hepatic Lipid Accumulation/ Steatosis Assay Kit Instructions for Use For evaluating steatosis risk of drug candidates using Oil Red O to stain neutral lipids in hepatocytes. This product is for
SHORT-TERM AND LONG-TERM EFFECT OF OXYTOCIN ON THE MORPHOLOGY AND FUNCTIONAL ACTIVITY OF FEMALE RAT SUBCUTANEOUS ADIPOSE TISSUE. P.
 PROCEEDINGS OF THE BALKAN SCIENTIFIC CONFERENCE OF BIOLOGY IN PLOVDIV (BULGARIA) FROM 19 TH TILL 21 ST OF MAY 2005 (EDS B. GRUEV, M. NIKOLOVA AND A. DONEV), 2005 (P. 739 743) SHORT-TERM AND LONG-TERM EFFECT
PROCEEDINGS OF THE BALKAN SCIENTIFIC CONFERENCE OF BIOLOGY IN PLOVDIV (BULGARIA) FROM 19 TH TILL 21 ST OF MAY 2005 (EDS B. GRUEV, M. NIKOLOVA AND A. DONEV), 2005 (P. 739 743) SHORT-TERM AND LONG-TERM EFFECT
Mouse Adiponectin Immunoassay Kit
 Antibody and Immunoassay Services Li Ka Shing Faculty of Medicine, The University of Hong Kong Mouse Adiponectin Immunoassay Kit Catalogue Number: 32010 For the quantitative determination of mouse adiponectin
Antibody and Immunoassay Services Li Ka Shing Faculty of Medicine, The University of Hong Kong Mouse Adiponectin Immunoassay Kit Catalogue Number: 32010 For the quantitative determination of mouse adiponectin
Human TRPC6 Ion Channel Cell Line
 TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set
 Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or
 Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Instructions. Fuse-It-Color. Overview. Specifications
 Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Supplementary Figure 1
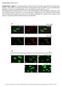 Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
H5N1 ( Avian Flu ) Hemagglutinin ELISA Pair Set
 H5N1 ( Avian Flu ) Hemagglutinin ELISA Pair Set Catalog Number : SEK002 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
H5N1 ( Avian Flu ) Hemagglutinin ELISA Pair Set Catalog Number : SEK002 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
