Mechanism of linezolid-induced NLRP3 inflammasome activation
|
|
|
- Lilian Cooper
- 6 years ago
- Views:
Transcription
1 University of Iowa Iowa Research Online Theses and Dissertations 2012 Mechanism of linezolid-induced NLRP3 inflammasome activation Qiong He University of Iowa Copyright 2012 Qiong He This thesis is available at Iowa Research Online: Recommended Citation He, Qiong. "Mechanism of linezolid-induced NLRP3 inflammasome activation." MS (Master of Science) thesis, University of Iowa, Follow this and additional works at: Part of the Other Cell and Developmental Biology Commons
2 MECHANISM OF LINEZOLID-INDUCED NLRP3 INFLAMMASOME ACTIVATION by Qiong He A thesis submitted in partial fulfillment of the requirements for Interdisciplinary Studies-Master of Science degree in Immunology in the Graduate College of The University of Iowa July 2012 Thesis Supervisor: Assistant Professor Fayyaz Sutterwala
3 Graduate College The University of Iowa Iowa City, Iowa CERTIFICATE OF APPROVAL MASTER'S THESIS This is to certify that the Master's thesis of Qiong He has been approved by the Examining Committee for the thesis requirement for the Interdisciplinary Studies-Master of Science degree in Immunology at the July 2012 graduation. Thesis Committee: Fayyaz Sutterwala, Thesis Supervisor Mary Wilson John Harty
4 ACKNOWLEDGMENTS I would like to thank my advisor, Dr. Fayyaz Sutterwala for his support and patience. I would also like to thank Dr. Suzanne Cassel and my thesis committee for their input. Additionally, I would like to thank everyone in the Inflammation Program. Finally, I would like to thank my family for their endless support and all the friends for their generous help. ii
5 ABSTRACT Activation of the NLRP3 inflammasome has been shown in response to numerous activators; here we show that the treatment with the oxazolidinone antibiotic linezolid results in both the NLRP3-dependent in vitro release of the proinflammatory cytokine IL- 1 and in vivo neutrophilic influx following its intraperitoneal administration. Clinical use of linezolid is commonly limited by hematologic side effects; herein we also show NLRP3-deficiency protected animals against linezolid-induced effects on the bone marrow. Importantly, all previously described activators of the NLRP3 inflammasome have required the generation of reactive oxygen species (ROS). Linezolid is however unique amongst NLRP3 agonists in that its ability to activate the NLRP3 inflammasome occurs in a ROS-independent manner. The pathways for ROS-dependent and ROSindependent NLRP3 activation converge upon mitochondrial dysfunction and specifically the mitochondrial lipid cardiolipin. We demonstrated that interference with cardiolipin synthesis specifically inhibits NLRP3 inflammasome activation. These findings firstly suggests that ROS generation is not the canonical activator of NLRP3 but rather an intermediary step leading to the mitochondrial perturbation that is tied to NLRP3 inflammasome activation and also implicate the involvement of mitochondrial lipid cardiolipin in this process; secondarily, linezolid-induced NLRP3 activation may account for the in vivo toxicity associated with prolonged usage of this antibiotic. iii
6 TABLE OF CONTENTS LIST OF TABLES... v LIST OF FIGURES... vi LIST OF ABBREVIATIONS... viii CHAPTER I INTRODUCTION TO THE INFLAMMASOME, LINEZOLID AND STERILE INFLAMMATION... 1 The NLR inflammasomes... 3 Linezolid... 7 Sterile inflammation... 8 Mechanisms for NLRP3 inflammasome activation... 9 Cardiolipin CHAPTER II MATERIALS AND METHODS Antibiotics In vitro stimulation ELISAs Mice In vivo peritonitis In vivo myelosuppression model Flow cytometry RT Real-time PCR sirna transfection Immunoblotting CHAPTER III LINEZOLID-INDUCED ACTIVATION OF NLRP3 INFLAMMASOME Secretion of IL-1β in response to linezolid required a priming step Linezolid induces the secretion of IL-1β but not TNFα or IL-6 in vitro Linezolid induces the secretion of IL-1β is not secondary to cell death Linezolid induces IL-1β secretion in an NLRP3-dependent manner Linezolid induces NLRP3-dependent inflammatory responses in vivo Linezolid mediated activation of the NLRP3 inflammasome in vitro requires a potassium efflux Linezolid-induced NLRP3 inflammasome activation is independent of ROS Mitochondrial dysfunction is required for NLRP3 inflammasome activation Cardiolipin is required for NLRP3 inflammasome activation CHAPTER IV DISCUSSION CHAPTER V FUTURE DIRECTIONS REFERENCES iv
7 LIST OF TABLES Table 1 Primer sequences for real-time PCR of human cardiolipin Synthase (hcls) and GAPDH Table 2 Various activators of the NLRP3 inflammsome v
8 LIST OF FIGURES Figure 1 Schematic representation of cardiolipin biosynthetic pathway Figure 2 IL-1β secretion by LPS-primed or unprimed J774.A1 macrophage in response to linezolid Figure 3 Inflammatory cytokines secretion by J774.A1 macrophage in response to linezolid Figure 4 Linezolid induces caspase-1-dependent IL-1β secretion Figure 5 Linezolid elicits rapid IL-1β secretion from primed J774A.1 macrophages Figure 6 Linezolid mediated IL-1βsecretion is independent of cell death Figure 7 Antibiotics screening Figure 8 Linezolid induces NLRP3-dependent inflammation in vitro Figure 9 Linezolid induces NLRP3-dependent neutrophilic influx in vivo Figure 10 Linezolid induces NLRP3-dependent effects on bone marrow in vivo Figure 11 Linezolid mediated IL-1β secretion is independent of phagocytosis pathway Figure 12 Linezolid-induced IL-1β secretion does not require purinergic receptor P2X7R Figure 13 Linezolid-induced IL-1β secretion requires a potassium efflux Figure 14 Linezolid-induced NLRP3 inflammasome activation is independent of ROS generation Figure 15 Mitochondrial ROS is dispensable for linezolid-induced NLRP3 inflammasome activation Figure 16 Linezolid-induced NLRP3 inflammasome activation requires a loss of m Figure 17 Cyclosporine A (CsA) rescues perturbation of mitochondrial membrane potential in response to NLRP3 inflammasome stimuli Figure 18 DPI stabilizes mitochondrial membrane potential in response to ROSdependent NLRP3 inflammasome stimuli Figure 19 RNAi knockdown of human Cardiolipin Synthase (hcls) Figure 20 Cardiolipin is required for NLRP3 inflammasome but not NLRC4 inflammasome activation vi
9 Figure 21 Knockdown of cardiolipin results in decreased caspase-1 activation by macrophages Figure 22 Mitochondrial control of NLRP3 inflammasome vii
10 LIST OF ABBREVIATIONS AIM2 Absent in melanoma 2 Alum ASC ATCC ATP BMDMs CARD CDP-DAG CFU CHAB Aluminum hydroxide Apoptosis-associated speck-like protein containing a CARD American Type Culture Collection Adenosine-5'-triphosphate Bone marrow derived macrophages Caspase recruitment domain Cytidine diphosphate-diacylglycerol Colony forming unit Difco cysteine heart agar supplemented with 9% sheep red blood cells CLR CsA DAMPs DNA dsdna ELISA FBS GAPDH HIN-200 HMDS h LDH LeTx C-type lectin receptors Cyclosporine A Danger-associated molecular patterns Deoxyribonucleic acid Double-stranded deoxyribonucleic acid Enzyme-linked immunosorbent assay Fetal bovine serum Glyceraldehyde-3-phosphate dehydrogenase 200 amino acid hemopoietic IFN- inducible nuclear proteins Exa-methyl-disilazane Lactate dehydrogenase Lethal toxin IFI16 Interferon alpha-inducible protein 16 IFN Interferon viii
11 IFIX IL-1α IL-1β IL-1R Interferon inducible protein X Interleukin 1α Interleukin 1β Interleukin 1 receptor IL-18 Interleukin 18 LPS LRR LVS MDP MMP MOI MPT MRSA MSU NACHT NADPH NAIP NLR Lipopolysaccharide Leucine-rich repeat domain Live Vaccine Strain Muramyl dipeptide Mitochondrial membrane potential Multiplicity of infection Mitochondrial membrane permeability transition Methicillin-resistant Staphylococcus aureus Monosodium Urate Nucleotide-binding and oligomerization domain Nicotinamide adenine dinucleotide phosphate-oxidase NLR family, apoptosis inhibitory protein Nucleotide-binding domain leucine-rich repeat containing receptors NLRP1 Nucleotide-binding domain leucine-rich repeat containing receptors with a pyrin domain 1 NLRP3 Nucleotide-binding domain leucine-rich repeat containing receptors with a pyrin domain 3 NLRP6 Nucleotide-binding domain leucine-rich repeat containing receptors with a pyrin domain 6 NLRP10 Nucleotide-binding domain leucine-rich repeat containing receptors with a pyrin domain 10 ix
12 NLRP12 Nucleotide-binding domain leucine-rich repeat containing receptors with a pyrin domain 12 NLRC4 Nucleotide-binding domain leucine-rich repeat containing receptors with a CARD domain 4 NOD PAMPs PBS PG PGP PI Nucleotide-binding oligomerization containing domain Pathogen-associated molecular patterns Phosphate buffered saline Phosphatidylglycerol Phosphatidylglycerophosphate Phosphatidylinositol PLCS3 Phospholipid scramblase 3 PVDF PYD PYHIN RA RLH RNA rrna RNAi ROS PRR sirna SNP T3SS T4SS TLR TNF-α Polyvinylidene fluoride Pyrin domain Pyrin and HIN200 domain containing protein Rheumatoid arthritis RIG-I-like RNA helicases Ribonucleic acid Ribsomal RNA RNA interference Reactive Oxygen Species Pattern recognition receptor Small interfering RNA Single nucleotide polymorphism Type 3-secretion system Type 4-secretion system Toll-like receptor Tumor necrosis factor α x
13 VRE WT Δψm Vancomycin-resistant enterococcus Wild type Mitochondrial membrane potential xi
14 1 CHAPTER I INTRODUCTION TO THE INFLAMMASOME, LINEZOLID AND STERILE INFLAMMATION Germline encoded pattern recognition receptors (PRR) are important cellular sensors that are capable of orchestrating initial responses to a wide range of pathogens and sterile inflammatory insults. Currently, four different classes of PRRs have been identified, including Toll-like receptors (TLR), RIG-I-like RNA helicases (RLH), C-type lectin receptors (CLR) and nucleotide-binding domain leucine-rich repeat containing receptors (NLR) (Medzhitov, 2009). These germline-encoded PRRs are capable of recognizing highly conserved, pathogen-associated molecular patterns (PAMPs), such as lippopolysaccharide (LPS). The release of danger signals, e.g.adenosine-5'-triphosphate (ATP), or danger-associated molecular patterns (DAMPs) induced by either pathogens or sterile inflammatory insults can also be sensed by PRR. The TLR family is one of the best-characterized PRR families sharing similar structural domains, N-terminal leucinerich repeats (LRRs) and a transmembrane region followed by a cytoplasmic Toll/IL-1R homology (TIR) domain. 10 TLRs have been identified in humans and 12 in mice. Different TLRs recognize the different molecular patterns of microorganisms and selfcomponents. The NLR family is a recently described group of innate immune proteins as intracellular surveillance molecules for sensing and responding to exogenous pathogens and endogenous danger signals. The NLR family of cytoplasmic proteins contains 23 members in humans and 34 members in the mouse (Martinon et al., 2009). Evolutionarily conserved NLR receptors share a distinguished domain architecture consisting of a central nucleotide-binding domain called the NACHT domain that is located between an N-terminal effector domain and a C-terminal leucine-rich repeat domain (Inohara et al., 2005; Martinon et al., 2009). The leucine-rich repeat domain serves an autoregulatory role for NLR activation and has been implicated in ligand sensing; however, the
15 2 mechanism and ligands involved in this interaction remain unknown. The N-terminal of most NLRs harbor protein-binding motifs, typically a caspase recruitment domain (CARD), a pyrin domain (PYD) or a baculovirus IAP repeat domain (BIR domain). Caspase-1-dependent inflammation has been shown to be effected by different NLRs including NOD1, NOD2, NLRP1, NLRP2, NLRP3, NLRP6, NLRP7 and NLRP12 (Eisenbarth et al., 2012; Mariathasan, 2007). Among the NLRs, to date NLRP1(also known as NALP1 or DEFCAP), NLRP3 (also known as Cryopyrin, CIAS1 or NALP3), NLRC4 (also known as CARD12 or IPAF) and NLRP7 have been shown to assemble inflammasomes with defined physiological roles in vivo (Agostini et al., 2004; Khare et al., 2012; Martinon et al., 2002; Sutterwala et al., 2007). The inflammasome is a multi-protein complex consisting of a cytosolic PRR, either an NLR or the PYHIN family member AIM2, the adaptor molecule ASC (apoptosis-associated speck-like protein containing a CARD) and the cysteine protease caspase-1 (Mariathasan and Monack, 2007; Martinon et al., 2009). The formation of inflammasome facilitates the activation of caspase-1 and promotes subsequent processing and secretion of proinflammatory cytokines interleukin 1β (IL-1β) and interleukin -18 (IL-18). Additionally, a cell death pathway termed pyrotosis has been shown dependent on caspase-1 (Cervantes et al., 2008; Fink and Cookson, 2005). Unlike apoptosis, pyrotosis does not rely on classical pro-apoptotic initiator and effector caspases (caspase- 3, caspase-8 and capsase-9) and is characterized by plasma-membrane breakdown. The cytosolic receptor AIM2 (absent in melanoma 2), is the first non-nlr family member identified that can form a caspase-1 activating inflammasome (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). AIM2 is unique in a family of interferon-inducible HIN-200 domain and pyrin domain (PYD)-possessing proteins, known as PYHIN molecules for its cytosol localization (Rathinam et al., 2010). The PYHIN family also contains three other members, IFIX, IFI16, and MNDA. Given their nuclear localization, it is unlikely that any of the three other members of the PYHIN
16 3 family are capable of forming inflammasomes (Rathinam et al., 2010). In response to cytosolic double stranded DNA (dsdna) that recognized by C-terminal HIN-200 domain, AIM2 inflammasome is activated with ensuing caspase-1 activation, resulting in the processing and secretion of IL-1β and IL-18 (Burckstummer et al., 2009; Fernandes- Alnemri et al., 2009; Hornung et al., 2009). Activation of AIM2 following recognition of dsdna facilitates the interaction of the AIM2 PYD with ASC through homotypic PYD- PYD interactions, allowing ASC to recruit caspase-1 via its CARD domain. The NLR inflammasomes Like AIM2, the NLR family members NLRP1, NLRP3, NLRC4 and NLRP7 have been suggested to assemble into an inflammasome in response to a variety of different stimuli (Khare et al., 2012). In all cases, assembly of an inflammasome results in the maturation of caspase-1 with the subsequent release of IL-1β. The NLRP3 inflammasome is the most extensively characterized amongst inflammasomes. The NLRP3 inflammasome is a multiprotein complex consisting of the nucleotide-binding domain leucine-rich repeat containing (NLR) family member NLRP3, the adaptor protein ASC and the cysteine protease caspase-1 (Agostini et al., 2004). The assembly of NLRP3 inflammasome triggers caspase-1 activation in response to cellular danger resulting in the IL-1 and IL-18 secretion (Carlsson et al., 2010; Craven et al., 2009; Kanneganti et al., 2006b; Mariathasan et al., 2006; Martinon et al., 2006; Sutterwala et al., 2006). The NLRP3 inflammasome is activated by a diverse array of stimuli including both PAMPs and endogenous host-derived DAMPs indicative of cellular damage (Table 2). NLRP3 inflammasome has been shown to be particularly important in response to several bacterial pathogens.(abdul-sater et al., 2009; Carlsson et al., 2010; Craven et al., 2009; Duncan et al., 2009; Gurcel et al., 2006; Harder et al., 2009; He et al., 2010; Mariathasan et al., 2006; Toma et al., 2010). Both Gram-positive and Gram-negative bacteria have been shown to be capable of activating the NLRP3
17 4 inflammasome through production of pore forming toxins. For example, the potassium ionophore nigericin derived from Streptomyces hygroscopicus, maitotoxin produced by the dinoflagellate Gambierdiscus toxicus (Mariathasan et al., 2006). Staphylococcus aureus activated NLRP3 inflammasome dependent on hemolysins and bacterial lipoproteins secreted in culture supernatants ((Munoz-Planillo et al., 2009). Similarly, Neisseria gonorrhoeae activates NLRP3 inflammasome that is dependent upon the secreted virulence factor lipo-oligosaccharide (Duncan et al., 2009). In addition, the bacterial pathogens Porphyromonas gingivalis (Huang et al., 2009), Chlamydia pneumoniae (He et al., 2010) and C. trachomatis (Abdul-Sater et al., 2009) are also capable of activating the NLRP3 inflammasome, however, the contributing factor or factors for activation has not been identified. Pathogens other than bacteria, such as viruses, parasites, and fungi, activate the NLRP3 inflammasome (Dostert et al., 2009; Gross et al., 2009; Hise et al., 2009; Joly et al., 2009; Kanneganti et al., 2006a; Muruve et al., 2008; Said-Sadier et al., 2010). NLRP3 can also be activated by an array of non-bacterial stimuli. Sterile particles such as aluminum hydroxide (alum), silica, monosodium urate (MSU) and asbestos can also activate the NLRP3 inflammasome, presumably through a signal following the disruption of crystal-containing phagosomes (Hornung et al., 2008). Endogenous danger signal, ATP, triggers NLRP3 inflammasome activation through purinergic P2X7 receptor stimulation (Mariathasan et al., 2006). Similar to cytosolic nucleic acid sensors RIG-I and AIM2, NLRP3 has also been shown to be activated in response to cytoplasmic DNA (Muruve et al., 2008), which may play a role in NLRP3 inflammasome activation in response to sterile inflammatory insults. Mutation of the NLRP3 gene has been identified in people to be responsible for autoinflammatory syndromes, Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and neonatal-onset multisystem inflammatory disease. All diseases that linked to mutation of NLRP3 resulting in cryopyrin hyperaction are known as cryopyrin-
18 5 associated periodic syndrome (CAPS) (Dode et al., 2002; Feldmann et al., 2002; Hoffman et al., 2001). The NLRC4 inflammasome is also capable of inducing activation of caspase-1 with the ensuing release of IL-1β, IL-18 and pyroptosis. The microbial component flagellin and type III or type IV secretion system carrying Gram-negative bacteria, such as Salmonella, Shigella and Pseudomonas, induce the activation of the NLRC4 inflammasome. NLRC4 contains an N-terminal CARD, a central NACHT domain and C- terminal LRRs (Martinon et al., 2009). Despite the potential that NLRC4 and caspase-1 may interact directly through a CARD-CARD binding, their interaction appears to be more complex, since impairment of caspase-1 activation and IL-1β secretion in response to pathogens such as Salmonella, Shigella and Pseudomonas have been observed in ASCdeficient macrophages implicative important role of ASC in NLRC4-mediated caspase-1 activation (Mariathasan et al., 2004; Sutterwala et al., 2007; Suzuki et al., 2007). ASCdeficient macrophages infected with these pathogens only shows partial affected pyroptosis comparing with WT macrophages (Broz et al., 2010). Unlike the NLRP3 inflammasome, which appears to recognize danger signal that caused by a pathogen on the host cell, NLRC4 is directly activated in response to microbial components. S. typhimurium and L. pneumophila strains deficient in flagellin show marked defect in their ability to activate macrophage caspase-1 (Franchi et al., 2006; Miao et al., 2006; Molofsky et al., 2006; Ren et al., 2006). The direct delivery of purified flagellin by transfection confirmed the requirement of flagellin in NLRC4- dependent caspase-1 activation (Miao et al., 2008). Pathogens possessing functional bacterial type III (T3SS) or type IV (T4SS) secretion system, such as Salmonella, Legionella, Shigella and Pseudomonas, require delivery of flagellin to the cytosol, to activate the NLRC4 inflammasome (Mariathasan et al., 2004; Sutterwala et al., 2007; Suzuki et al., 2007). The NLRC4 inflammasome can also be activated independently of flagellin. The non-flagellated bacterium S. flexneri as well as flagellin-deficient strains of
19 6 P. aeruginosa can efficiently activate caspase-1 in an NLRC4- dependent manner (Sutterwala et al., 2007; Suzuki et al., 2007). This apparent discrepancy in the requirement for flagellin in NLRC4 inflammasome activation was recently explained in an elegant study by Miao and colleagues (Miao et al., 2010). NLRC4 inflammasome is activated in response to the basal body rod component of the T3SS apparatus from S. typhimurium (PrgJ), Burkholderia pseudomallei (BsaK), Escherichia coli (EprJ and EscI), S. Flexneri (MxiI), and P. aeruginosa (PscI) (Miao et al., 2010). Given structural similarity of a sequence motif between rod proteins and flagellin, both the rod proteins and flagellin activate NLRC4. NAIP5 (also known as BIRC1E), a BIR-domaincontaining NLR protein, has been shown a requirement of L. pneumophila infection, flagellin-induced caspase-1 activation (Lightfield et al., 2008). Zhao and colleagues have suggested NAIP2, a functional analogue of NAIP5, serving as a specific inflammasome receptor for T3SS rod proteins such as Salmonella PrgJ and Burkholderia Bsa (Zhao et al., 2011). These finding sheds lights on the mechanism of NLRC4 inflammasome activation in recognition of microbial moieties. NLRP1 protein differs from all the other NLRP proteins in the C-terminal region, containing a C-terminal Function to Find Domain (FIIND) and CARD domain allowing CARD-CARD-mediated recruitment and maturation of caspase-1 and subsequently cleavage of pro- IL-1β (Martinon et al., 2009). The mouse has three orthologs of NLRP1 and, unlike human NLRP1, the murine orthologs lack a functional PYD and hence fail to interact with ASC. As predicted, the adaptor molecule ASC was not required for NLRP1 inflammasome activation; however, its presence did enhance caspase-1 activation (Faustin et al., 2007). The murine NLRP1b isoform NLRP1b gene has been identified to be responsible for macrophage susceptibility to Bacillus anthracis lethal toxin (LeTx), whereas human ortholog is involved in keratinocytes destruction in response to UV-irradiation (Boyden and Dietrich, 2006; Faustin and Reed, 2008; Feldmeyer et al., 2007). Hsu et al. also
20 7 described an important role for NLRP1 in LeTx-induced IL-1β secretion in response to the intact pathogen B.anthracis (Hsu et al., 2008). NOD2 is also required for B. anthracis-induced IL-1β secretion, suggesting that NOD2 may be part of a NLRP1 inflammasome complex (Hsu et al., 2008). The NLRP1 inflammasome can also be directly activated by muramyl dipeptide (MDP), a component of peptidoglycans produced by Gram-positive and Gram-negative bacteria. (Bruey et al., 2007; Faustin et al., 2007). Genetic variants in the NLRP1 encoding gene have been reported to associate with Alzheimer disease (AD) (Pontillo et al., 2011a) and NLRP1 single nucleotide polymorphisms (SNP) have been implicated in the onset and progression of autoimmune diseases systemic sclerosis related fibrosing alveolitis, celiac disease and rheumatoid arthritis (RA) (Dieude et al., 2011; Pontillo et al., 2011b; Sui et al., 2011). Linezolid Linezolid is the first synthetic antibacterial agent belonging to the oxazolidinone class of antibiotics (Moellering, 2003; Paladino, 2002) and has been embraced as an effective tool in the treatment of infections caused by antibiotic resistant bacteria, including vancomycin-resistant enterococcus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) (Ament et al., 2002). It has also demonstrated usefulness as part of multi-drug regimens in the treatment of atypical mycobacterial infections (Anger et al., 2010; Sood et al., 2006). Linezolid is currently the only antibacterial agent with good activity against MRSA that can be administered orally as well as intravenously. Linezolid inhibits bacterial protein synthesis by binding to 23S ribsomal RNA (rrna) in the catalytic site of the 50S ribosome. Unlike other known protein synthesis antibiotics, no adverse effect on the peptide elongation or translation termination steps of protein synthesis was observed for linezolid treatment (Leach et al., 2011; Moellering, 2003b; Paladino, 2002). A number of pathologies associated with these antibiotic resistant pathogens, such as endocarditis and osteomyelitis, require prolonged antibiotic therapy
21 8 lasting up to 8 weeks. This duration of therapy can be potentially problematic as linezolid has been associated with myelosuppression at rates as high as 32% in patients receiving this antibiotic (Attassi et al., 2002; Dawson et al., 2005; Plachouras et al., 2006; Takahashi et al., 2010). Linezolid presumably has a suppressive effect on the bone marrow but the mechanism is not well defined (French, 2003). Mitochondrial toxicity has been implicated in linezolid-induced myelosuppression although the precise mechanism behind this remains unclear (De Vriese et al., 2006; McKee et al., 2006; Soriano et al., 2005). Sterile inflammation Sterile inflammation induced by dead cells triggers a robust inflammatory response including netrophilic influx and monocyte recruitment (Iyer et al., 2009; Kono and Rock, 2008). Damaged cells are thought to release DAMPs that can alert the innate immune system to the impending tissue damage. Sensing the cytosolic DAMPs is mediated by germ line encoded PRRs. The best described class of these receptors are Toll-like receptors (TLRs) (Trinchieri and Sher, 2007). There is evidence that DAMPs, such as high-mobility group box 1 protein (HMGB1), heat-shock proteins, amyloid-β peptide and DNA released from damaged cells can stimulate TLRs and mediate the inflammatory cascade (Imaeda et al., 2009; Ohashi et al., 2000; Stewart et al., 2010; Yu et al., 2006). Besides TLRs, another class of PRRs, NOD-like receptors (NLRs) are suggested in the recognition of DAMPs, e.g. ATP release from necrotic cells (Chen et al., 2007; Ghiringhelli et al., 2009; Iyer et al., 2009; Li et al., 2001; Stewart et al., 2010). Another DAMP, nucleic acid DNA from damaged cells, was also found to stimulate the production of pro- IL-1β and pro-il-18 (Imaeda et al., 2009). This raises the possibility that RIG-I and AIM2 may also play a role in sterile inflammatory responses to necrotic cell death. Iyer et al. showed that macrophages challenged with cells that had undergone specific forms of necrotic cell death but not all necrosis were capable of activating
22 9 caspase-1(iyer et al., 2009), highlighting the heterogeneity of different mechanisms of necrotic cell death. Mechanisms for NLRP3 inflammasome activation The NLRP3 inflammasome activation is believed to require a two-signal mechanism. Stimulation with TLR agnonists (priming) results in NF-κB activation and subsequent synthesis of NLRP3 and precursor forms of the cytokines IL-1β (Bauernfeind et al., 2009). Further stimulation (activation) to these cells by providing the second signaling, leads to NLRP3 inflammasome activation. Numerous in vitro studies have provided priming via microbial products acting on TLR, e.g. LPS, however, the initial priming step in vivo is not required for the induction of pro IL-1β (Chen et al., 2006; Guarda et al., 2011). The activation of the NLRP3 inflammasome has been an area of intense investigation; the divergent qualities of the activators of the NLRP3 inflammasome have led to speculation that the activators converge on a common pathway with a final endogenous ligand activating NLRP3. Given the structural dissimilarity of these stimuli, it is unlikely that NLRP3 is directly interacting with all of agonists. It is however plausible that a single ligand interacts with NLRP3 and the identified stimuli in fact serve to facilitate the release, modification or recognition of that ligand. Determining the direct ligand of NLRP3 requires understanding the common signaling pathways and/or common intersecting points shared between various pathways. One event that is required for NLRP3 inflammasome activation by all known activators is potassium efflux (Lamkanfi et al., 2009; Lightfield et al., 2008). Inhibition of potassium movement, by increasing extracellular potassium concentrations, results in the abrogation of NLRP3 inflammasome (Cassel et al., 2008b; Dostert et al., 2008; Eisenbarth et al., 2008; Franchi et al., 2007). Inhibition of NLRP3 inflammasome assembly was observed in solution with K+ concentration over 70mM in vitro (Petrilli et al., 2007). The exact role of the
23 10 potassium efflux has yet to be elucidated; however the formation of the NLRP3 inflammasome may require a low potassium environment. Another cellular event, release of ROS is recently postulated an absolute requirement for NLRP3 inflammasome activation via the use of pharmacological inhibitors, the source of ROS is identified as mitochondrial origin (Babelova et al., 2009; Cassel et al., 2008b; Gross et al., 2009; Nakahira et al., 2011a; Pulskens et al., 2008; Rouschop et al., 2005; Zhou et al., 2011). For particulate activators of the NLRP3 inflammasome, the requirement of phagocytosis has been demonstrated in nearly all reported particulate stimuli (Dostert et al., 2009; Dostert et al., 2008; Duewell et al., 2010; Hornung et al., 2008; Martinon et al., 2006). Lysosomal membrane disruption following particulate uptake has also been postulated to play a role in NLRP3 inflammasome activation (Dostert et al., 2008; Hornung et al., 2008). It is plausible that particulate stimuli, like silica, induce K+ efflux through the impaired membrane to activate NLRP3 inflammasome and recent study demonstrated that the potassium channel is crucial for membrane depolarization and uptake of different kinds of particles (Link et al., 2010). A number of studies have shown that release of the lysosomal protease cathepsin B into the cytosol is necessary for IL-1β release in response to a number of the phagocytosis dependent mediators (Babelova et al., 2009; Dostert et al., 2008; Hornung et al., 2008). However, cathepsin B-deficient macrophages showed no defect in IL-1β secretion compared with wild-type macrophages in response to monosodium urate and alum (Dostert et al., 2009). This discrepancy in role of cathepsin B in NLRP3 inflammasome activation needs further studies. Unlike particulate activators, ATP, another potent activator of NLRP3 inflammasome, binds the purinergic receptor P2X7 triggering the formation of a ion hemichannel, allowing K+ efflux through membrane pores and lowering cytoplasmic K+
24 11 levels (Mariathasan et al., 2006; Pelegrin and Surprenant, 2006; Perregaux and Gabel, 1994; Sutterwala et al., 2006). Microbial moieties activate the NLRP3 inflammasome by different mechanisms. Synthesis of the pore forming toxin, allowing the delivery of bacterial components to the cytosol, triggers NLRP3 activation (Nakagawa et al., 2004). Similarly, Staphylococcus aureus hemolysins (α and β) induces caspase-1 maturation in conjunction with released lipoproteins independently of P2X7R suggesting a role for bacterial toxins and hemolysins in fulfilling the second signal necessary for inflammasome activation (Munoz-Planillo et al., 2009). In fungal infection, the Dectin-CARD9 signaling pathway through syk kinase has been suggested to regulate transcriptional up-regulation of proinflammatory cytokines (Gross et al., 2009; Poeck and Ruland, 2010). Inhibition of syk kinase reduces the NLRP3 inflammasome activation and hence IL-1β secretion (Gross et al., 2009; Said-Sadier et al., 2010). These observations thus implicate syk kinase signaling may contribute to the NLRP3 inflammasome activation by providing the necessary signals required either for its up-regulation at the transcriptional level and/or for its assembly by a yet unidentified mechanism. Cardiolipin In eukaryotes, cardiolipin predominantly localizes in the inner mitochondrial membrane where it comprises approximately 16% of total phospholipids. Cardiolipin is an anionic phospholipid with a unique structure, consisting of three glycerol backbones and four acyl chains (Houtkooper and Vaz, 2008; Lecocq and Ballou, 1964; Schlame et al., 2000). Cardiolipin biosynthesis processes via three sequential enzymatic reactions and all required enzymes that are highly conserved in eukaryotes are present in mitochondria (Schlame and Haldar, 1993) (Figure 1). First, cytidine diphosphatediacylglycerol (CDP-DAG) is converted to phosphatidylglycerophosphate (PGP) catalyzed by PGP synthase. Following this, PGP phosphatase dephosphorylates PGP to
25 12 phosphatidylglycerol (PG). In the final step, cardiolipin synthase (CLS), a highly conserved enzyme, catalyzes the formation of cardiolipin from PG and CDP-DAG (Schlame and Hostetler, 1997). Remodeling of acyl chains is needed after cardiolipin has been synthesized. Deacylation/reacylation reaction is the first potential mechanism for cardiolipin remodeling (Ma et al., 1999; Xu et al., 2003). Besides acylation, the enzyme which has been shown involved in the remodeling of cardiolipin acyl chains and associated with overall abundance of cardiolipin in vivo is tafazzin (Neuwald, 1997; Vreken et al., 2000; Xu et al., 2006). It has been known that cardiolipin, as other phospholipids, maintains the membrane integrity. Besides structural function, cardiolipin has been shown to be required for the optimal activity of several enzymes in the mitochondria (Bisaccia and Palmieri, 1984; Muller et al., 1985; Schlattner and Wallimann, 2000). Cardiolipin has been implicated in various metabolic pathways and is associated with different complexes of the respiratory chain, including NADH dehydrogenase (complex I) (Fry and Green, 1981), ubiquinol:cytochrome c reductase (complex III) (Gomez and Robinson, 1999; Yu and Yu, 1980), cytochrome c oxidase (complex IV) (Sedlak and Robinson, 1999), and ATP synthase (complex V) (Eble et al., 1990). Depletion of cardiolipin results in perturbation of mitochondrial function, reduction of mitochondrial membrane potential and instability of electron transport chain supercomplexes (Choi et al., 2007; Jiang et al., 2000; Pfeiffer et al., 2003; Zhang et al., 2003). Paradoxically, knockdown of cardiolipin synthase (Δcrd1 mutants) in yeast does not cause marked defects in mitochondrial membrane potential, oxidative phosphorylation machinery (Huang et al., 2008). This discrepancy could be explained by the divergent ability between mammalian cells and yeast to supplant function of cardiolipin by elevated phosphatidylglycerol (Jiang et al., 2000).
26 13 In addition to the role of cardiolipin in maintenance of mitochondrial structure and function, cardiolipin is intimately involved in the apoptosis by interacting with proapoptotic proteins such as cytochrome c and tbid (Choi et al., 2007; Gonzalvez and Gottlieb, 2007; Lutter et al., 2000; Nomura et al., 2000). Release of cytochrome c from mitochondria requires the dissociation of its interactions with cardiolipin and cardiolipin peroxidation may facilitate cytochrome c detachment from the inner membrane (Nomura et al., 2000; Shidoji et al., 1999). Previous studies have been implicated that cardiolipinrich membranes provides a docking site for truncated Bcl-2 family protein, tbid, and facilitate cytochrome c release (Esposti et al., 2003; Kim et al., 2004; Liu et al., 2005; Liu et al., 2004). Insight with respect to the importance of cardiolipin in mitochondrial metabolism and apoptosis increases; it will be informative to investigate relation between innate immune response and metabolism.
27 Figure 1 Schematic representation of cardiolipin biosynthetic pathway. (modified from Yang Y et al. J. Biol. Chem. 2004). Sequential conversion of phosphatidic acid to cytidine 5'-diphosphate (CDP)-diacylglycerol (CDP- DAG), phosphatidylglycerol phosphate (PGP), phosphatidylglycerol (PG), and finally cardiolipin (CL). CDP-DAG can be converted to phosphatidylinositol (PI) by incorporation of insitol. 14
28 15 CHAPTER II MATERIALS AND METHODS Antibiotics All antibiotics used in this study were purchased from Sigma with the exception of vancomycin (Santa Cruz Biotechnology, Inc.) and tigecycline (Tygacil ; Pfizer). All in vitro assays were performed using linezolid from Sigma. In vivo studies were performed using an intravenous formulation of linezolid (Zyvox ; Pfizer). Both formulations of linezolid induced similar amounts of IL-1 secretion from J774A.1 macrophages challenged in vitro. In vitro stimulation Bone marrow macrophages were generated as described in Sutterwala et al. (Sutterwala et al., 1997). Briefly mice were euthanized and femurs and tibias were removed; bone marrow was flushed with complete media with antibiotics (DMEM supplemented with 10% FCS and 2 mm L-glutamine with 17.9 U/ml penicillin and 17.9 μg/ml streptomycin). Bone marrow was pelleted by centrifugation at 320 x g for 10 minutes. The media was aspirated and the pellet resuspended in complete media with antibiotics. Cells were cultured for at least 7 days in complete media with antibiotics supplemented with 20% L929 cell conditioned media in 100x15 mm polystyrene petri dishes at 37ºC and 5% CO2 in a humidified incubator. To remove the cells for plating the media was aspirated and cold Versene (Gibco) added; the plates were returned to the incubator for 10 minutes. Cells were collected and mixed 1:1 with complete media (DMEM supplemented with 10% FCS and 2 mm L-glutamine). The cells were pelleted at 320 x g at 4ºC and resuspended in complete media. The cells were enumerated by counting a dilution of cells on a hemocytometer and the cells were brought to a final concentration of 1 x 10 6 cells/ml in complete media. 5 x 10 5 cells/well were plated in 24 well tissue culture plates and incubated overnight at 37ºC and 5% CO 2 in a humidified incubator. The J774A.1 mouse macrophage cell line and the THP1 human monocytic cell
29 16 line were obtained from the American Type Culture Collection (ATCC). Human PBMC were isolated from blood of healthy volunteers by centrifugation through a Ficoll- Isopaque density gradient (GE healthcare). Informed consent was obtained from each individual following a protocol approved by the Institutional Review Board for human subjects at the University of Iowa. Unless indicated, macrophages and PBMC were primed with 50 ng/ml LPS from E. coli serotype 0111:B4 (Invivogen) for 4 hours; THP-1 cells were differentiated by 50 nm phorbol myristate acetate (PMA, Sigma) for 3 hours prior to stimulation with Macrophages and PBMC were stimulated with either 100 μg /ml of linezolid or the indicated concentration of antibiotic, 50 μg/cm 2 of silica (Min-U-Sil-5; Pennsylvania Glass Sand Corporation), F.tularensis LVS (multiplicity of infection of 50:1), P. aeruginosa (multiplicity of infection of 10:1), 20 μm nigericin (Sigma), 5 mm ATP (Sigma) or 0.5 μg /ml Imject alum (Pierce).For ATP and nigericin treated cells, media was replaced with fresh media 20 min following stimulation. At the indicated time post stimulation supernatants were collected and assayed for IL-1, TNF- and IL-6. Macrophage cell death was determined at the indicated time points by measuring LDH release using a cytotoxicity (LDH) detection kit (Promega). The inhibitors z-yvad-fmk (20 M; Calbiochem), cytochalasin D (20 M; Calbiochem), Mito-TEMPO (500 M; Enzo) and cyclosporine A (20 M; Alexis) were added at the indicated time prior to stimulation. LPS-primed J774A.1 macrophages were left untreated or pretreated for 30 min with ROS inhibitors DPI (20 M; Calbiochem), APDC (20 M; Sigma), and NAC (1 mm; Sigma) prior to stimulation. Inhibition of potassium efflux was performed as previously described (Cassel et al., 2008). ELISAs Antibodies for the mouse ILantibody clone 30311, biotin conjugated secondary antibody catalog number BAF401). Antibody pairs for the mouse TNF- mouse IL-6, human IL-1β and human TNF-α
30 17 ELISAs were from ebiosciences (primary antibody for TNF-α Clone 1F3F304, biotin conjugated secondary antibody for TNF-α clones MP6-xT22 and MP6-xT3, primary antibody for IL-6 Clone MP5-20F3, biotin conjugated secondary antibody for IL-6 MP5-32C11). Nunc Maxisorp plates were coated with the primary antibody overnight at 4 o C (at a concentration of 4 μg/ml (mouse IL-1β), 2 μg/ml (mouse IL-6 and TNF-α), or 2 μg/ml (human IL-1β and TNF-α in PBS). Following coating, plates were washed with PBS with 0.05% Tween 20 and blocked for 1 hour at room temperature with 200 μl/well of 10% FBS in PBS. Plates were washed and samples and standards were transferred into the plates (100 μl/well for IL-1β and 100 μl/well for TNF-α and IL-6). Plates were covered and incubated overnight at 4ºC. Plates were washed and the biotin conjugated secondary antibody (100 μl/well) was added (200 ng/ml (IL-1β), 2 μg/ml (IL-6 and TNFα)) in 10% FBS in PBS and incubated for 1 hour at room temperature. Plates were washed and 100 μl/well of a 1:2000 dilution of HRP conjugated to strept/avidin D (Vector Laboratories A-2004) in 10% FBS in PBS was added. Plates were washed and 100 μl/well of TMB substrate (KPL) was added to each well and when the standard curve obtained a gradated blue color 50 μl/well of stop solution was added to the entire plate (KPL). Absorbance at 450 nm was then assessed spectrophotometrically and the concentration of the cytokine of interest was calculated by fitting the absorbance of the sample well to a 4-parameter curve generated by plotting the absorbance of the standard curve. Mice The generation of ASC-, caspase-1-, NLRP3-, NLRC4-, P2X7R- deficient mice have been described previously (Fernandes-Alnemri et al., Kuida et al., 1995, Lara- Tejero et al., 2006, and Sutterwala et al., 2006). Caspase-1-, ASC-, NLRC4-and NLRP3- deficient mice were backcrossed onto the C57BL/6 genetic background for at least 9 generations. Age and sex-matched C57BL/6 mice purchased from NCI were used as WT
31 18 controls. The University of Iowa Institutional Animal Care and Use Committee approved all protocols used in this study. In vivo peritonitis Mice (6-8 wk old) were injected intraperitoneally with the indicated dose of linezolid (200 mg/kg) or dextrose 5% in water (D5W). 16 hours later animals were euthanized and peritoneal lavage performed. Mouse was euthanized by cervical dislocation and sterilize mouse with 70% ethanol. Cold PBS was injected and intraperitoneal cavity was thoroughly washed with injected PBS by swishing around mice. Cell suspensions were transferred to a 15ml Conical collection tubes and total cells were enumerated by Flow cytometry. PE-neutrophil (Cedar lane labs, CL8993PE, at a concentration of 2 μg/ml) and FITC-Ly-6G (Ebioscience, R86-8C5, 2 μg/ml) antibody were used for the markers for neutrophils. The number of neutrophils (Ly-6G+ 7/4+) in the lavage was assessed by flow cytometry as described (Chen et al., 2007). Mice found to be in a moribund state for more than 4 hours were considered terminal and euthanized. In vivo myelosuppression model Mice were injected intraperitoneally daily with linezolid at a dose of 200 mg/kg for 12 days. Control mice received an equal volume of D5W. On day 12 femurs were harvested, fixed, decalcified, embedded in paraffin, sectioned at 4mm and stained with hematoxylin and eosin (H&E). Femur sections were examined for overall cellularity using a high-resolution microscope (BX51, Olympus). Digital images were collected at 600X (DP72, Olympus) and the mature stages of myeloid and erythroid cells were counted in each section with two sections counted per femur. The erythroid elements were identified as small round cells with a deeply basophilic nucleus. The granulocytes were identified by the bilobed nucleus.
32 19 Flow cytometry For mitochondrial dysfunction study, mitochondrial membrane potential ( m) was measured using the fluorescent MitoProbe JC-1 (Invitrogen). Cells were washed with pre-warmed HBSS and resuspend in 1 ml HBSS. 10μl of JC-1 stock solution was added to each tube and final concentration of JC-1 dye was 20μM. Cells were incubated in the 37ºC incubator for minutes. Positive control, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), was added with final concentration of 2 μm and incubated for 5 minutes in 37ºC incubator. Data were acquired with C6 Flow Cytometer and Accuri analysis software (Accuri Cytometers) RT Real-time PCR Total RNA was extracted by using RNeasy Mini Kit (Qiagen). Concentration of RNA was assessed by Nanodrop 1000 (Thermo). 0.2 mg of total RNA was subjected to cdna synthesis using superscript III first-strand synthesis kit (Invitrogen). Primer sequences are shown in Table 2.1. SYBR green real-time PCR amplification was performed on a 7000 real-time PCR system (Applied Biosystems). sirna transfection Three duplexes of human cardiolipin synthase (hcls) sirnas and scrambledcontrol sirna were purchased from Origene. Lipofectamine 2000 (Invitrogen) was used to transfect these sirnas into THP-1 cells with 1:1 ratio.thp-1 cells were plated in 6-well plate at 0.5X10 5 /well/ml on day 1 with RPMI-1640 medium excluding serum and antibiotics. On day 2, cells were transfected with sirnas or scrambled-control sirna and cultured in Opti-MEM (Invitrogen). The final concentration of sirnas was 100nM. 8 hours post transfection complexes containing sirna were washed out, normal RPMI medium was added, and the cells were allowed to incubate for an additional 40 hours. On day 4, the THP-1 cells were differentiated by 50nM PMA for 3 hours and stimulated with linezolid (100 g/ml), of silica (50 g/cm2), F. tularensis LVS
33 20 (multiplicity of infection of 50:1), P. aeruginosa PKA (multiplicity of infection of 10:1), nigericin (20 μm) and ATP (5 mm). Supernatants were harvested for the measurement of IL-1β and TNF-α by using ELISA. Complementary DNA was generated 48 hrs post transfection and real-time PCR performed using a SYBR Green PCR master mix (Applied Biosystems) and the sequences of oligonucleotides were used are shown in Table 1. Immunoblotting Immunoblotting was performed as previously described (Cassel et al., 2008). Samples were prepared according to instructions provided by NuPage (Invitrogen). Samples were run on a 4-12% Bis-Tris NuPage gel and transferred to PVDF. Blots were blocked for 1 hour at room temperature in 5% non-fat dry milk dissolved in PBS % Tween 20. To detect caspase-1, a rabbit polyclonal anti-mouse caspase-1 p10 antibody (clone SC-514) (Santa Cruz Biotechnology) and a rabbit monoclonal anti- human caspase-1 p20 antibody (D7F10) (Cell Signaling) were used. Anti-pro-caspase1 antibody was purchased from Santa Cruz (clone SC-622).Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected using an antibody from Calbiochem (clone 6C5).
34 21 Table 1 Primer sequences for real-time PCR of human cardiolipin Synthase (hcls) and GAPDH Gene Sequence hcls Sense Anti-sense 5'-CCCAGTTCTGGGCTATTTG-3' 5'-TCAAGAGCACTTCCCAAAGC-3' GAPDH Sense Anti-sense 5'-GAAGGTGAAGGTCGGAGTC-3' 5'-GAAGATGGTGATGGGATTTC-3'
35 22 CHAPTER III LINEZOLID-INDUCED ACTIVATION OF NLRP3 INFLAMMASOME The NLRP3 inflammasome is a multiprotein complex consisting of the nucleotide-binding domain leucine-rich repeat containing (NLR) family member NLRP3, the adaptor protein ASC and the cysteine protease caspase-1 (Agostini et al., 2004). The NLRP3 inflammasome can activate caspase-1 in response to cellular danger resulting in the processing and secretion of the proinflammatory cytokines IL-1 and IL-18 (Kanneganti et al., 2006b; Mariathasan et al., 2006; Martinon et al., 2006; Sutterwala et al., 2006). A diverse array of stimuli can activate the NLRP3 inflammasome including both pathogen associated molecular patterns and endogenous host-derived molecules indicative of cellular damage (Table 2). The divergent qualities of the NLRP3 inflammasome agonists have led to the supposition that the activators converge on a common pathway with a final endogenous ligand activating NLRP3. An aspect that many activators of the NLRP3 inflammasome share is their ability to generate ROS (Martinon, 2010). Blockade of ROS with pharmacological inhibitors blocks activation of the NLRP3 inflammasome, suggesting that the generation of ROS is a required upstream event for NLRP3 activation (Cassel et al., 2008a; Cruz et al., 2007; Dostert et al., 2008; Petrilli et al., 2007b; Zhou et al., 2011). Recent studies have focused on elucidating the cellular source of this ROS and have identified it to be of mitochondrial origin and independent of the NADPH oxidase pathway (Meissner et al., 2008; Nakahira et al., 2011b; van Bruggen et al., 2010; Zhou et al., 2011). We demonstrate that oxazolidinone class of antibiotics (Moellering, 2003a), linezolid, is capable of activating the NLRP3 inflammasome. Activation of the NLRP3 inflammasome by linezolid results in an in vivo inflammatory response with an associated suppression of bone marrow erythroid precursors, consistent with the hematologic
36 23 anomalies seen in patients that have been ascribed to direct effects of linezolid on mitochondria. In contrast to other activators of the NLRP3 inflammasome, linezolidinduced activation occurs in an ROS-independent manner. However, activation of the NLRP3 inflammasome by linezolid was associated with a loss of mitochondrial membrane potential, and this was consistent for all other NLRP3 agonists tested. We further confirm the central role of the mitochondria in NLRP3 activation by demonstrating that disruption of the cardiolipin synthesis pathway results in impaired NLRP3 inflammasome activation. The novel finding of an ROS independent pathway to NLRP3 inflammasome activation and the requirement for cardiolipin in this process sheds light on the mechanism by which the NLRP3 inflammasome is activated and suggests that mitochondrial disruption is ultimately responsible for NLRP3 inflammasome activation. Secretion of IL-1β in response to linezolid required a priming step Activation of the inflammasome and subsequent secretion of IL-1β requires two separate signals, a priming step and an activation step. We showed that linezolid alone did not induce IL-1β secretion (Figure 2), consistent with the actions of other stimuli of the inflammasome such as silica and ATP that require prior macrophage priming in order to activate caspase-1 (Cassel et al., 2008b; Mariathasan et al., 2006; Sutterwala et al., 2006). Linezolid induces the secretion of IL-1β but not TNFα or IL-6 in vitro The mechanism by which linezolid induces myelosuppression has not been elucidated. As inflammatory mediators have been shown to have both positive and negative effects on myeloid and erythroid precursors we asked if linezolid was capable of inducing an inflammatory response. In contrast to LPS, we showed that linezolid was a poor inducer of TNF-α and IL-6 production by the murine macrophage J774A.1 cell line (Figure 3A). However, treatment of LPS-primed macrophages with linezolid resulted in
37 24 the secretion of IL-1β in a dose-dependent manner (Figure 3B). Linezolid was also capable of inducing IL-1β secretion from human peripheral blood mononuclear cells (PBMC) and the human monocytic THP1 cell line via a mechanism that was inhibited by the caspase-1 inhibitor z-yvad-fmk (Figure 4). Collectively, these results demonstrate that linezolid was capable of inducing the caspase-1-dependent secretion of IL-1β from both human PBMC and mouse macrophages. In addition, we showed that linezolidinduced secretion of IL-1β occurred rapidly with maximal secretion by 6 hrs post challenge (Figure 5). Linezolid induces the secretion of IL-1β is not secondary to cell death Recent studies have demonstrated that necrotic cells are capable of inducing macrophage inflammasome activation (Ghiringhelli et al., 2009; Iyer et al., 2009; Li et al., 2009). To ensure that the IL-1β secretion observed was not secondary to linezolidinduced cytotoxicity we assessed the degree of cell death in macrophages following linezolid treatment by measuring lactate dehydrogenase (LDH) release as a marker of cellular damage. As shown in Figure 6, LDH release was minimal at 6 hrs after linezolid treatment, by which time maximal IL-1β release had already been achieved. These data suggest that linezolid-induced inflammasome activation and IL-1β secretion were not a consequence of danger-associated molecular pattern release from damaged cells. Linezolid inhibits bacterial protein synthesis by binding to the bacterial 23S ribosomal RNA of the 50S subunit and preventing a functional 70S initiation complex from forming, which is an essential for the bacterial translation process. To determine if other antibiotics were capable of inducing IL-1β secretion we challenged J774.A1 macrophages with selected antibiotics from multiple different classes of antimicrobials, including those that target protein synthesis as their mechanism of action. Only gramicidin induced substantial IL-1β secretion from macrophages (Figure 7), consistent in part with a recent study by Allam and colleagues (Allam et al., 2011). Gramicidin is a
38 25 heterogeneous mixture of Gramicidin A, B, and C, which are linear polypeptides capable of forming ion channels in the bacterial cell membrane. Like valinomycin, a known activator of the NLRP3 inflammasome, the gramicidin ion channel is selective for monovalent cations and in particular potassium (Wallace, 1990). Based on these data, the capacity of linezolid to trigger IL-1β secretion was not shared by antibiotics with a similar mechanism of antibacterial action, but rather a property relatively unique to linezolid. Linezolid induces IL-1β secretion in an NLRP3-dependent manner To determine if the NLRP3 inflammasome was involved in linezolid-induced IL- 1β secretion we challenged LPS-primed bone marrow-derived macrophages (BMDMφ) from wild-type (WT), NLRP3-, ASC-, caspase-1-, and Nlrc4-deficient mice with linezolid in vitro. NLRP3-, ASC-, and caspase-1 BMDMφ displayed a marked defect in their ability to process and secrete IL-1β in response to linezolid compared to WT BMDMφ (Figure 8A). In contrast, BMDMφ deficient in Nlrc4, which is required for caspase-1 activation in response to flagellin and infection with type III and type IV secretion system carrying Gram-negative bacteria (Sutterwala and Flavell, 2009), secreted IL-1β in response to linezolid (Figure 8A). Caspase-1 activation involves autocatalytic processing of the 45 kda procaspase- 1 to generate two subunits, p20 and p10. Caspase-1 activation in LPS-primed WT BMDMφ stimulated with linezolid was detected in immunoblots by the appearance of the p10 cleavage product (Figure 8B). We did not observe caspase-1 activation in response to linezolid in either NLRP3- or ASC-deficient LPS-primed BMDMφ (Figure 8B). Taken together these findings suggest that linezolid was capable of inducing caspase-1-mediated IL-1β secretion in a manner dependent on the NLRP3 inflammasome.
39 26 Table 2 Various activators of the NLRP3 inflammsome Author Reference Bacteria Products Muramyl dipeptide Bacterial Listeria monocytogenes Staphylococcus aureus Mycobacterium Marinum Porphyromonas Gingivalis Neisseria gonorrhoeae Mycobacterium tuberculosis Chlamydia pneumonia Salmonella typhimurium Pan, Q et al Mariathasan, S et al and Warren, SE et al Mariathasan, S et al Koo, IC et al Huang, MT et al Duncan, JA et al Mishra, BB et al He, X et al Broz et al J Leukoc Bio. 2007;82(1): Nature 2006; 440(7081): and J Immunol. 2008;180(11): Nature 2006;440(7081): Cell Microbiol. 2008;10(9): J Immunol. 2009;182(4): J Immunol. 2009;182(10): Cell Microbiol. 2010; 12(8): J Immunol. 2010; 184(10): J Exp Med. 2010; 207(8): Streptococcus pyogenes Vibrio cholera Harder et al J Immunol. 2009;183(9): Toma et al J Immunol. 2010;184(9): Viruses and Viral Products Kanneganti, TD et al and Ichinohe, T et al Influenza virus and Thomas, PG et al Adenovirus Muruve, DA et at Sendai virus Modified vaccinia virus Ankara (MVA) dsrna Kanneganti, TD et al Delaloye et al Kanneganti, TD et al J Biol Chem ; 281(48): and J Exp Med ; 206(1):79-87 and Immunity ;30(4): Nature ;452(7183):103-7 J Biol Chem (48): PLoS Pathog. 2009;5(6):e J Biol Chem ;281(48):3656-8
40 27 Table 2 continued poly (I:C) viral RNA Fungal Pathogens Candida albicans Aspergillus fumigatus Saccharomyces cerevisiae Kanneganti, TD et al Kanneganti, TD et al Gross, O et al and Hise, AG et al and Joly, S et al Said-Sadier, N et al Kumar et al and Lamkanfi et al J Biol Chem ;281(48): J Biol Chem ;281(48): Nature. 2009; 459(7245):433-6 and Cell Host Microbe. 2009; 5(5): and J Immunol. 2009;183(6): PLoS One. 2010;5(4):e10008 J Immunol. 2009;183(12): and J Biol Chem ;284(31): Sterile insults Silica Dostert, C. et al and Cassel, S. L. et al and Hornung, V. et al. Science. 2008; 320(5876) and Proc. Natl. Acad. Sci : and Nat. Immunol : Asbestos Dostert, C. et al Science. 2008; 320(5876) Aluminum hydroxide Eisenbarth, S. C. et al and Li, H. et al and Kool, M. et al Nature. 2008; 453(7198) and J Immunol. 2008;181(1) and J. Immunol : and Eur. J. Immunol : Monosodium urate Martinon, F. et al. Nature : (MSU) Calcium Pyrophosphate Martinon, F. et al. Nature : Dihydrate amyloid-β Halle, A. et al. Nat. Immunol : ATP Mariathasan S et al. Nature 440(7081): Cholesterol crystals Duewell, P. et al. and Rajamäki, K. et al. Nature.2010; 464, and PLoS ONE 2010; 5, e11765
41 Figure 2 IL-1β secretion by LPS-primed or unprimed J774.A1 macrophage in response to linezolid. J774A.1 macrophages were either primed with 50 ng/ml of LPS for 4 hrs or left untreated. Cells were then stimulated with either 100μg/ml linezolid or silica; culture supernatants were collected 18 hrs later and IL-1β release measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of two independent experiments. 28
42 Figure 3 Inflammatory cytokines secretion by J774.A1 macrophage in response to linezolid. (A) J774A.1 mouse macrophages were stimulated with either linezolid (100μg/ml) or LPS; culture supernatants were collected 18 hrs later and TNF- and ILrelease measured by ELISA. (B) LPS primed J774A.1 macrophages were stimulated with either silica or linezolid; culture supernatants were collected 18 hrs later and IL-1β release measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of two independent experiments. 29
43 30 Figure 4 Linezolid induces caspase-1-dependent IL-1β secretion. (A) LPS-primed human PBMC were incubated with 20 μm z-yvad-fmk for 1 hr prior to the addition of silica or linezolid (100μg/ml). 18 hrs after stimulation culture supernatants were collected and IL-1β release measured by ELISA. (B) PMA-differentiated THP1 cells were incubated with 20 μm z-yvad-fmk for 1 hr prior to the addition of silica or linezolid. 18 hrs after stimulation culture supernatants were collected and IL- ssessed by ELISA Determinations were performed in triplicate and expressed as the mean ± SD. Data are representative of two independent experiments.
44 Figure 5 Linezolid elicits rapid IL-1β secretion from primed J774A.1 macrophages. LPS-primed J774A.1 macrophages were stimulated with either linezolid (100μg/ml) or silica for the indicated time. The amount of IL-1β secreted into culture supernatants was determined by ELISA. The values are the mean ± SD. The data are representative of two independent experiments. 31
45 Figure 6 Linezolid mediated IL-1βsecretion is independent of cell death. LPS-primed J774A.1 macrophages were stimulated with linezolid; culture supernatants were collected at the indicated times and cytotoxicity measured by LDH release and expressed as a percentage of LDH release by Triton X-100 detergent. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of three independent experiments. 32
46 Figure 7 Antibiotics screening. LPS-primed J774A.1 macrophages were challenged with silica or 1, 10 and 100 μg/ml of the indicated antibiotic for 18 hrs; ILsupernatant was measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of two independent experiments. 33
47 34 Linezolid induces NLRP3-dependent inflammatory responses in vivo To examine if the NLRP3 inflammasome was involved in the inflammatory response to linezolid in vivo we injected linezolid intraperitoneally into WT mice or mice deficient in NLRP3, ASC or caspase-1 (Figure 9). Sixteen hours after challenge with linezolid, WT mice displayed a marked influx of neutrophils into the peritoneal cavity compared to mice that received an equal volume of the control buffer, dextrose 5% in water (D5W). This neutrophil influx was significantly diminished in mice deficient in components of the NLRP3 inflammasome, suggesting that the in vivo inflammatory response to linezolid was also dependent upon the NLRP3 inflammasome (Figure 9). As linezolid therapy has been associated with the adverse clinical side effect of myelosuppression (Attassi et al., 2002; Dawson et al., 2005; Gerson et al., 2002; Taketani et al., 2009), and in particular with the finding of increased myeloid/erythroid ratio on bone marrow examination (Dawson et al., 2005; Green et al., 2001; Taketani et al., 2009), we examined the effect of linezolid therapy on myeloid and erythroid cells in the bone marrow of mice treated with 200 mg/kg/day of linezolid for 12 days. Bone marrow from linezolid-treated WT mice had significantly decreased mature erythroid cells and increased myeloid cells compared to that of control animals (Figure 10A and B). In contrast, there was no difference observed in either erythroid or myeloid cells in linezolid-treated NLRP3-deficient mice compared to control animals (Figure 10A and B). In contrast, there was no difference observed in either erythroid or myeloid cells in linezolid-treated NLRP3-deficient mice compared to control animals (Figure 10A and B). There were no differences in total cell number per high powered field in both wild type and NLRP3-/- mice (Figure 10A and B). Taken together these data demonstrate that linezolid induced inflammatory responses in vivo and its effect on the bone marrow is dependent upon the NLRP3 inflammasome.
48 Figure 8 Linezolid induces NLRP3-dependent inflammation in vitro. (A) LPS-primed BMMφ from WT, NLRP3-, ASC-, caspase-1-, or Nlrc4-deficient mice were stimulated with either linezolid or silica. Culture supernatants were collected at 18 hrs after stimulation and IL-1β release measured by ELISA. (B) Lysates from LPS-primed WT, NLRP3-, ASC-, or Nlrc4-deficient BMMφ stimulated with linezolid for 18 hrs were immunoblotted with antibodies against the p10 subunit of caspase-1. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of four (A) and three (B) separate experiments. 35
49 Figure 9 Linezolid induces NLRP3-dependent neutrophilic influx in vivo. Linezolid induces NLRP3-dependent neutrophilic influx in vivo. WT, NLRP3 ASC and caspase-1-deficient mice (n=5) were challenged with 200 mg/kg of linezolid intraperitoneally; 16 hrs later neutrophil influx into the peritoneum was determined by flow cytometry. Control WT mice (n=3) were challenged intraperitoneally with D5W. Results are representative of at least three independent experiments. *p<0.01 by two-tailed Mann-Whitney test. 36
50 Figure 10 Linezolid induces NLRP3-dependent effects on bone marrow in vivo. (A)WT and NLRP3-deficient mice received daily intraperitoneal injections of linezolid (200 mg/kg) (WT, n=9; NLRP3-/-, n=9) or an equal volume of control D5W (WT, n=10; NLRP3-/-, n=6). 12 days later femurs were sectioned and stained with H&E. (A) Representative 600X images are shown. Open arrows indicate myeloid cells and black arrows represent erythroid cells. (B)The mature stages of myeloid and erythroid cells were counted in each section with two sections counted per femur. Results are pooled from two independent experiments and expressed as the mean ± SEM. * p < 0.01 by two tailed Mann-Whitney test. 37
51 38 Hence the activation of the NLRP3 inflammasome by linezolid may contribute to the in vivo toxicity associated with linezolid therapy. Although the contribution of the NLRP3 inflammasome to immune defense against selected pathogens precludes deliberate blockade of the NLRP3 inflammasome in order to abrogate the myelosuppression associated with linezolid, future generations of oxazolidinone antibiotics should however be screened for their ability to activate the inflammasome in order to limit possible toxicities. Linezolid mediated activation of the NLRP3 inflammasome in vitro requires a potassium efflux To gain mechanistic insights into how linezolid activates the NLRP3 inflammasome, we examined pathways required for NLRP3 inflammasome activation in response to other agonists. Particulate agonists such as monosodium urate (MSU), silica and alum require internalization by the macrophage in order to activate the NLRP3 inflammasome (Cassel et al., 2008; Eisenbarth et al., 2008; Martinon and Glimcher, 2006). Given its lipophilic nature that allows it to cross the cell membrane, we predicted linezolid s activation of the NLRP3 inflammasome would be independent of the phagocytic pathway. Treatment of LPS-primed J774A.1 macrophages with cytochalasin D, which inhibits actin polymerization, inhibited silica-induced, but not ATP-mediated, IL-1β secretion as expected (Cassel et al., 2008). Cytochalasin D did not inhibit linezolid from inducing macrophage IL-1β secretion (Figure 11). These data demonstrate that, as predicted by its molecular structure, active endocytosis of linezolid is not required for NLRP3 inflammasome activation. The cell surface receptor P2X7R has been implicated in NLRP3 inflammasome activation in response to extracellular ATP (Mehta et al., 2001; Solle et al., 2001). Linezolid-mediated IL-1β secretion remained intact in the absence of the P2X7R (Figure 12) suggesting that the P2X7R was not required for linezolid-induced inflammasome
52 39 activation. Additionally, this also suggests that linezolid-induced NLRP3 inflammasome activation was not secondary to ATP release from damaged cells as has been observed for the chemotherapeutic agent doxorubicin (Ghiringhelli et al., 2009). A common step required by all known NLRP3 agonists to induce caspase-1 activation is the efflux of cellular potassium (Petrilli et al., 2007a). Preventing the potassium efflux by increasing extracellular potassium concentrations inhibits caspase-1 activation in response to stimuli that activate NLRP3. Consistent with this, increased extracellular potassium significantly inhibited linezolid-induced IL-1β secretion from LPS-primed J774A.1 macrophages (Figure 13). As expected, increased extracellular potassium also inhibited NLRP3 dependent silica-induced, but not AIM2-dependent Francisella tularensis LVS-induced IL-1β production (Figure 13). Hence linezolid, like all other known NLRP3 stimuli, required a cellular potassium efflux to induce macrophage IL-1β secretion. Linezolid-induced NLRP3 inflammasome activation is independent of ROS ROS generation has also been suggested to be an absolute requirement for activation of the NLRP3 inflammasome, as no activators have yet been described that are capable of activating the NLRP3 inflammasome independently of ROS ((Dostert et al., 2008; Martinon, 2010; Tschopp and Schroder, 2010). Two recent studies have further suggested that the source of this ROS is mitochondrial in origin (Nakahira et al., 2011a; Zhou et al., 2011). To examine the role of ROS in linezolid-induced NLRP3 inflammasome activation we utilized pharmacologic inhibitors of ROS. Macrophages were pretreated with the free radical scavenger N-acetylcysteine (NAC), the flavoprotein inhibitor diphenylene iodonium (DPI), or the metabotropic glutamate receptor 3 agonist (2R, 4R)-4-aminopyrrolidine-2, 4-dicarboxylate (APDC) prior to challenge with either linezolid, silica, nigericin, ATP, or alum. As previously observed (Cassel et al., 2008;
53 40 Dostert et al., 2008; Petrilli et al., 2007; Zhou et al., 2011), the ROS inhibitors NAC, DPI and APDC blocked silica, nigericin, ATP, and alum-induced IL-1β secretion and caspase- 1 activation (Figure 14A and B). Unexpectedly, NAC, DPI and APDC all failed to inhibit linezolid-induced IL-1β secretion (Figure 14A). Caspase-1 activation induced by linezolid was also not inhibited by DPI treatment (Figure 14B). In order to more closely examine the effect of inhibition of mitochondria-derived ROS, macrophages were treated with the mitochondria- targeted antioxidant Mito-TEMPO. As previously shown by Nakahira et al., Mito-TEMPO inhibited ATP-mediated IL-1β secretion and caspase-1 activation (Figure 15A and B) (Nakahira et al., 2011a). In contrast, but consistent with our other data, Mito-TEMPO failed to suppress linezolid induced ILcaspase-1 activation (Figure 15A and B). Taken together, these data implicate an ROSindependent mechanism for linezolid-induced NLRP3 inflammasome activation. Mitochondrial dysfunction is required for NLRP3 inflammasome activation Linezolid inhibits bacterial protein synthesis by preventing the fusion of 30S and 50S ribosomal subunits to form the 70S initiation complex (Lopez et al., 2004). Due to similarities between the ribosomes of bacteria and mitochondria linezolid has been implicated in the inhibition of mitochondrial protein synthesis. This has been demonstrated by showing that both amounts and activity of mitochondrial respiratory chain complexes are decreased in animals and patients receiving linezolid (De Vriese et al., 2006; McKee et al., 2006; Palenzuela et al., 2005; Soriano et al., 2005). To determine if linezolid has more immediate effects on mitochondrial function we examined the effect of linezolid on the mitochondrial membrane potential (Δψm). The ROS-generating activators silica, nigericin and the ROS-independent activator linezolid all had a similar effect on the Δψm (Figure 16; the respiratory chain uncoupler CCCP is shown as a positive control), as monitored using the cationic dye MitoProbe JC-1, which reflects
54 Figure 11 Linezolid mediated IL-1β secretion is independent of phagocytosis pathway. LPS primed J774A.1 macrophages were incubated with 20 μm cytochalasin D for 30 min prior to the addition of linezolid, silica or ATP. 18 hrs later culture supernatants were collected and IL-1β release measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of two independent experiments. 41
55 42 Figure 12 Linezolid-induced IL-1β secretion does not require purinergic receptor P2X7R. LPS-primed WT and P2X7R-/- lated with linezolid, silica or ATP. 18 hrs after stimulation culture supernatants were collected and IL- 1β ns were performed in triplicate and expressed as the mean ± SD. Results are representative of two independent experiments.
56 43 Figure 13 Linezolid-induced IL-1β secretion requires a potassium efflux. LPS-primed J774A.1 macrophages were incubated in either high NaCl or high KCl containing media and then stimulated with either linezolid, silica or F. tularensis LVS (MOI 50:1). 18 hrs later culture supernatants were collected and IL-1β eterminations were performed in triplicate and expressed as the mean ± SD. Results are representative of two independent experiments.
57 44 mitochondrial depolarization through a reduction in its red fluorescence (upper right panel). To confirm that mitochondrial membrane depolarization was required for activation of the NLRP3 inflammasome, J774A.1 macrophages were pretreated with cyclosporine A, a potent inhibitor of mitochondrial membrane permeability transition (MPT). Blockade of MPT with cyclosporine A significantly stabilized the disruption of Δψm caused both by the ROS-generating activators (ATP and nigericin) as well as by the ROS-independent activator linezolid (Figure 17). Cyclosporine A also markedly inhibited the secretion of IL-1β, but not TNF α, from LPS-primed J774A.1 macrophages challenged with linezolid, silica and nigericin (Figure 16). Together these observations suggest that NLRP3 inflammasome activation was dependent on the MPT with the generation in order to induce NLRP3 inflammasome activation. In contrast, the ROS inhibitor DPI was only capable of stabilizing m disruption due to the ROS-dependent activators silica, ATP and nigericin, but not in response to linezolid (Figure 18).. Together these observations suggest that NLRP3 inflammasome activation was dependent on MPT with the subsequent disruption of m. Additionally, the function of ROS for NLRP3 inflammasome activators such as silica, ATP and nigericin may be solely to trigger MPT, which linezolid can achieve independently of ROS. Mitochondria play a crucial role in orchestrating the activation of the NLRP3 inflammasome as evidenced by two recent studies suggesting that generation of ROS by the mitochondrial respiratory chain as well as MPT are required for NLRP3 inflammasome activation (Tschopp and Schroder, 2010; Zhou et al., 2011). Our work demonstrates that the oxazolidinone antibiotic linezolid was a potent activator of the NLRP3 inflammasome. More importantly, we show linezolid-induced NLRP3 inflammasome activation occurred independently of mitochondrial ROS generation. The identification of an NLRP3 agonist that does not rely on ROS as an intermediary for
58 45 inflammasome activation suggests that the pathway for NLRP3 inflammasome activation is mediated through the direct sensing of mitochondrial dysfunction. Cardiolipin is required for NLRP3 inflammasome activation Cardiolipin, a phospholipid the predominantly found in the mitochondria has been suggested playing a significant role in the mitochondrial integrity and function. A critical step in the production of cardiolipin is the conversion of phosphatidylglycerol to cardiolipin by the mitochondrial enzyme cardiolipin synthase (CLS). It has been shown in our lab that cardiolipin binds to NLRP3 of both mouse and human origin. In order to investigate the role of cardiolipin in the NLRP3 inflammsome activation, we employed the sirna to knockdown the CLS in human monocytic cell line THP-1. We observed knockdown of CLS in THP-1 cells with two different sirna constructs resulted in a 51% and 48% decrease in CLS mrna respectively as measured by quantitative RT-PCR (Figure 19). Importantly, sirna knockdown of cardiolipin synthase significantly impaired the release of IL-1 in THP-1 cells following stimulation with the ROSdependent NLRP3 activators silica and ATP as well as the ROS-independent activator linezolid (Figure 20). Inhibition of cardiolipin synthase did not globally inhibit inflammatory responses as release of TNF was unimpaired by CLS sirna treatment (Figure 20). In addition, activation of the AIM2 and Nlrc4 inflammasomes remained intact as shown by unimpaired IL-1 release following treatment with F. tularensis LVS and Pseudomonas auerginosa, respectively (Figure 20). Consistent with diminished IL- 1 secretion in response to silica, ATP and linezolid following CLS sirna treatment we also observed a marked defect in caspase-1 activation in CLS sirna treated cells specifically in response to NLRP3 agonists (Figure 21). Together, these data suggest the mitochondrial lipid cardiolipin is required for NLRP3 activation in response to both ROS-dependent and ROS-independent activators.
59 Figure 14 Linezolid-induced NLRP3 inflammasome activation is independent of ROS generation. (A) LPS-primed J774A.1 macrophages were pretreated with the ROS inhibitors DPI (20 μm), APDC (20 μm) or NAC (1 mm) for 30 min prior to stimulation with linezolid, silica, nigericin, ATP or alum. 18 hrs later supernatants were collected and IL-1β secretion measured by ELISA. (B) Lysates from LPS-primed J774A.1 macrophages challenged with linezolid, silica or ATP in the absence or presence of DPI were immunoblotted with santibodies against the p10 subunit of caspase-1. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of three (A) and two (B) separate experiments. 46
60 Figure 15 Mitochondrial ROS is dispensable for linezolid-induced NLRP3 inflammasome activation. (A) LPS-primed J774A.1 macrophages were pretreated with the antioxidant Mito-TEMPO (500 μm) for 1 hr prior to stimulation Lysates from LPS-primed J774A.1 macrophages challenged with linezolid, silica or ATP. 18 hrs later supernatants were collected and IL-1β secretion measured by ELISA.* p = by two-tailed unpaired Student s t-test. (B) Lysates from LPS-primed J774A.1 macrophages challenged with linezolid, silica or ATP in the absence or presence of Mito-TEMPO were immunoblotted with antibodies against the p10 subunit of caspase-1. Determinations in Fig A were performed in triplicate and expressed as the mean ± SD. Results are representative of two separate experiments. 47
61 Figure 16 Linezolid-induced NLRP3 inflammasome activation requires a loss of m. LPS-primed J774A.1 cells were pretreated with 20 M CsA for 1 hr and then challenged with linezolid, silica or nigericin for 6 hrs. Supernatants were collected and cytokine secretions analyzed by ELISA. Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of three separate experiments. * p = 0.003, ** p = by two tailed unpaired Student s t-test. 48
62 Figure 17 Cyclosporine A (CsA) rescues perturbation of mitochondrial membrane potential in response to NLRP3 inflammasome stimuli. (A)LPS-primed J774A.1 macrophage were incubated with 20 μm CsA for 20 min and then stimulated with linezolid, ATP or nigericin for a further 2 hrs. Cells were treated with the respiratory chain uncoupler CCCP as a positive control. Cells were harvested and stained with MitoProbe JC-1 an mitochondrial membrane potential (Δψm) was determined by FACS. Results are representative of three independent experiments. (B) Quantification of the ratio of FL2 (red fluorescence) and FL1 (green fluorescence) in response to linezolid, silica and ATP with or without CsA treatment. 49
63 Figure 18 DPI stabilizes mitochondrial membrane potential in response to ROSdependent NLRP3 inflammasome stimuli. (A)LPS-primed J774A.1 macrophage were incubated with 20 μm DPI for 20 min and then stimulated with linezolid, ATP or nigericin for a further 30 min and silica for a further 2 hrs. Cells were treated with the respiratory chain uncoupler CCCP as a positive control. Cells were harvested and stained with MitoProbe JC-1 and mitochondrial membrane potential (Δψm) was determined by FACS. Results are representative of three independent experiments. (B) Quantification of the ratio of FL2 (red fluorescence) and FL1 (green fluorescence) in response to linezolid, silica and ATP with or without DPI treatment. 50
64 Figure 19 RNAi knockdown of human Cardiolipin Synthase (hcls). Real-time quantitative RT-PCR analysis of hcls mrna expression levels in control and hcls-rnai cells. THP-1 cells transfected with controlled small interference (si) RNAs (scramble RNA) or sirna directed against hcls (si CLS; two different sirna sequences were used, 1 and 2) were harvested. Total RNA was isolated, reverse-transcribed, and analyzed by real-time quantitative PCR with GAPDH or hcls primers. The hcls mrna expression levels were normalized to GAPDH. Data represent mean± SEM of three independent experiments. 51
65 Figure 20 Cardiolipin is required for NLRP3 inflammasome but not NLRC4 inflammasome activation (A) PMA-differentiated THP-1 cells plated in 24-well plates were treated with linezolid (100µg/ml), silica (50 µg /cm2), ATP (5mM) and F.tularensis LVS (multiplicity of infection of 50:1). 16 hrs later supernatants were collected and IL-1β, TNF α (A) secretion measured by ELISA. (B) PMA differentiated THP-1 cells plated in 24-well plates were treated with P. aeruginosa PAK strain (MOI 10:1) for 6 hours. Culture supernatants were collected and IL-1 and TNF release measured by ELISA Determinations were performed in triplicate and expressed as the mean ± SD. Results are representative of two separate experiments. Results are representative of three (A), two(b) independent experiments. *p< 0.05, ** p<0.01, *** p<
66 Figure 21 Knockdown of cardiolipin results in decreased caspase-1 activation by macrophages. PMA-differentiated THP-1 cells plated in 24-well plates were treated with linezolid (100µg/ml), silica (50 µg /cm2), ATP (5mM) and F.tularensis LVS (multiplicity of infection of 50:1). Immunoblot analysis of caspase-1 in lysates (Lys) and supernatants (Sup) of THP-1 cells. 53
67 54 CHAPTER IV DISCUSSION Linezolid is an oxazolidinone antibiotic that inhibits bacterial ribosomal protein synthesis. Given its potent anticidal activity, it has been extensively used for clinical treatment of Gram-positive bacteria infection, including MRSA. Prolonged therapy can be problematic because of its toxicity on hematological compartment. The mechanism of suppressive effect on bone marrow is elusive. In this study, we show that linezolid induces a NLRP3-dependent inflammation in vivo. Linezolid treatment for over 12 days causes significant reduction of erythroid precursors in WT mice, whereas, the decrease is markedly alleviated in NLRP3-/- mice. The finding that linezolid activates the NLRP3 inflammasome and in doing so may be a factor in the adverse side effect of linezolidinduced myelosuppression has important clinical implications. Although the contribution of the NLRP3 inflammasome to immune defense against selected pathogens precludes deliberate blockade of the NLRP3 inflammasome in order to abrogate this myelosuppression, future generations of oxazolidinone antibiotics should be screened for their ability to activate the inflammasome in order to limit possible toxicities. Among various agonists, we confirm that linezolid is a unique activator of NLRP3 inflammasome independent of ROS production, but mitochondrial perturbation is required for inflammasome activation. The diverse molecules capable of activating the NLRP3 inflammasome are structurally and biologically unrelated making it unlikely that they directly interact with NLRP3. Instead it is more likely that these dissimilar agonists converge on a shared pathway leading to NLRP3 inflammasome activation. Common steps in NLRP3 inflammasome activation that are found for all known activators until now have included the efflux of potassium from the cell (Petrilli et al., 2007) and the generation of mitochondrial ROS (Nakahira et al., 2011; Zhou et al., 2011). However, in this study we find that ROS is dispensable for NLRP3 inflammasome activation in response to the oxazolidinone antibiotic linezolid, suggesting that ROS generation is not
68 55 an absolute requirement for NLRP3 inflammasome activation as has been previously postulated. Mitochondria play a crucial role in orchestrating the activation of the NLRP3 inflammasome as evidenced by two recent studies showing the generation of ROS by the mitochondrial respiratory chain are required for NLRP3 inflammasome activation (Nakahira et al., 2011; Zhou et al., 2011). Compromised mitochondrial membrane potential has been observed in response to different activators of NLRP3 inflammasome, indicative a common pathway for inflammasome activation. Disruption of cellular cardiolipin synthesis by treatment of macrophages with sirna knockdown of CLS resulted is specific defects in NLRP3, but not NLRC4 or AIM2, inflammasome activation suggesting that cardiolipin plays a critical role in NLRP3 inflammasome activation. In this study we found that mitochondrial dysfunction is required for the NLRP3 inflammasome activation. Based on the current model that has been postulated to describe the mitochondria in NLRP3 inflammasome activation, cardiolipin is released from damaged or stressful mitochondria and sensed by NLRP3 which leads to the inflammatory cytokine secretion. Given the fact ROS is dispensable for linezolid induced NLRP3 inflammasome activation, generation of ROS is less possible to be recognized directly by NLRP3 rather than acts as a feedback to mitochondria which causes more damage to the mitochondrial, ultimately results in more release of cardiolipin to the cytosol (). It has been long known that mitochondria modulate metabolic pathways involving fatty acid, amino acid and steroid metabolism. In addition to these critical life-supporting roles, mitochondria play a central part in the execution of apoptotic cell death (Danial and Korsmeyer, 2004; Newmeyer and Ferguson-Miller, 2003). The similarities between NLRP3 inflammasome activation and that of apoptosome-induced apoptosis are remarkable. Jürg Tschopp recently drew parallels between these two pathways when he noted that low intracellular potassium is required for activation of the apoptosome as well
69 56 as for activation of the NLRP3 inflammasome (Cain et al., 2001; Tschopp, 2011). Additionally, interference with the important mitochondrial membrane channel VDAC not only inhibits NLRP3 inflammasome mediated IL-1 release (Zhou et al., 2011), but is also known to play a role in apoptosis induction by the pro-apoptotic members of the Bcl-2 family. Peroxidation of cardiolipin results in the release of bound cytochrome c into the cytosol where it binds to Apaf-1 (Garrido et al., 2006; Kagan et al., 2005). Apaf- 1 oligomerization occurs to form the apoptosome, which serves as a platform for caspase- 9 activation (Wang, 2001). Cardiolipin also plays an important role in the extrinsic apoptotic pathway where cardiolipin in the outer mitochondrial membrane binds to caspase-8 and acts as an activating platform (Gonzalvez et al., 2008). Overall, cardiolipin serves as a central switch in the mitochondrial apoptotic program, makes cardiolipin a potentially interesting target for therapeutic intervention in diseases in which cell death is dysregulated, such as cancer.(liu et al., 2005). Hence it appears that multiple cell death pathways converge on mitochondrial cardiolipin, including both the intrinsic and extrinsic apoptotic pathways and now also possibly NLRP3 inflammasome mediated pyroptosis. It remains unclear what factors are required to induce NLRP3 inflammasome activation without additionally activating apoptosis. A growing body of experimental evidence implicates the mitochondrial DNA (mtdna), endogenous DAMP, is sensed by PRR and triggers assembly, activation of NLRP3 inflammasome (Nakahira et al., 2011; Shimada et al., 2012). It may be that mitochondrial DNA in concert with cardiolipin serve as a regulator in determining whether mitochondrial dysfunction will result in NLRP3 inflammasome activation, necrosis or apoptosis.
70 Figure 22 Mitochondrial control of NLRP3 inflammasome. 57
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Journal club. Lama Nazzal
 Journal club Lama Nazzal Background Kidney stone disease affects about 12% of men and 5% of women during their lifetimes in the United States Intrarenal nephrocalcinosis is often asymptomatic, but can
Journal club Lama Nazzal Background Kidney stone disease affects about 12% of men and 5% of women during their lifetimes in the United States Intrarenal nephrocalcinosis is often asymptomatic, but can
Gout and Nucleic Acid Metabolism Vol.33 No
 Gout and Nucleic Acid Metabolism Vol.33 No.1 2009 1 1 2 3 in vitro 14 IgM 1 IgM IgM 1 PAMPs Pattern recognition receptors PRRs PRRs PRRs PAMPs Toll Toll-like receptor TLR PAMPs Nod Nod-like receptor NLR
Gout and Nucleic Acid Metabolism Vol.33 No.1 2009 1 1 2 3 in vitro 14 IgM 1 IgM IgM 1 PAMPs Pattern recognition receptors PRRs PRRs PRRs PAMPs Toll Toll-like receptor TLR PAMPs Nod Nod-like receptor NLR
Role of Innate Immunity in Control of Adaptive Immunity
 Role of Innate Immunity in Control of Adaptive Immunity Innate Immunity The burden of pathogen sensing is placed on the innate immune system Danger hypothesis Missing Self Based on the detection of molecular
Role of Innate Immunity in Control of Adaptive Immunity Innate Immunity The burden of pathogen sensing is placed on the innate immune system Danger hypothesis Missing Self Based on the detection of molecular
Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation
 Article Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation Shankar S. Iyer, 1,10 Qiong He, 1,2,10 John R. Janczy, 1,2,10 Eric I. Elliott, 1,3 Zhenyu Zhong, 6 Alicia K. Olivier, 4 Jeffrey
Article Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation Shankar S. Iyer, 1,10 Qiong He, 1,2,10 John R. Janczy, 1,2,10 Eric I. Elliott, 1,3 Zhenyu Zhong, 6 Alicia K. Olivier, 4 Jeffrey
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
Structure-function analysis of Nlrp1b reveals a link. between metabolism and inflammation: energy stress. triggers inflammasome activation
 Structure-function analysis of Nlrp1b reveals a link between metabolism and inflammation: energy stress triggers inflammasome activation by Kuo-Chieh Liao A thesis submitted in conformity with the requirements
Structure-function analysis of Nlrp1b reveals a link between metabolism and inflammation: energy stress triggers inflammasome activation by Kuo-Chieh Liao A thesis submitted in conformity with the requirements
Understanding the Role of the Inflammasome in Inflammation
 Understanding the Role of the Inflammasome in Inflammation Martha O Brien, Ph.D. Assay Design Group 215 Innate Immune Response in Inflammation LPS flagellin TLR-1/2/6 TLR-4 TLR-5 Adaptor proteins PAMPs,
Understanding the Role of the Inflammasome in Inflammation Martha O Brien, Ph.D. Assay Design Group 215 Innate Immune Response in Inflammation LPS flagellin TLR-1/2/6 TLR-4 TLR-5 Adaptor proteins PAMPs,
Identification of Microbes
 Identification of Microbes Recognition by PRR (pattern recognition receptors) Recognize conserved molecular patterns on microbes called pathogen associated molecular patterns (PAMPs) which are not present
Identification of Microbes Recognition by PRR (pattern recognition receptors) Recognize conserved molecular patterns on microbes called pathogen associated molecular patterns (PAMPs) which are not present
Chapter 3 The Induced Responses of Innate Immunity
 Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
INFLAMMASOME IN INTESTINAL INFLAMMATION AND CANCER. Laura Stronati ENEA - Roma
 INFLAMMASOME IN INTESTINAL INFLAMMATION AND CANCER Laura Stronati ENEA - Roma Inflammasome: definition, components and activation TLRs NODs RLRs CLRs PRRs LPS, Flagellin, DNA, RNA, etc) PAMPs DAMPs ATP,
INFLAMMASOME IN INTESTINAL INFLAMMATION AND CANCER Laura Stronati ENEA - Roma Inflammasome: definition, components and activation TLRs NODs RLRs CLRs PRRs LPS, Flagellin, DNA, RNA, etc) PAMPs DAMPs ATP,
NOD-like Receptors (NLRs) and Inflammasomes
 International Edition NOD-like Receptors (NLRs) and Inflammasomes In mammals, germ-line encoded pattern recognition receptors (PRRs) detect the presence of pathogens through recognition of pathogen-associated
International Edition NOD-like Receptors (NLRs) and Inflammasomes In mammals, germ-line encoded pattern recognition receptors (PRRs) detect the presence of pathogens through recognition of pathogen-associated
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Lecture on Innate Immunity and Inflammation
 Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation. Innate Immunity: An Evolutionary View
 Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Innate Immunity: (I) Molecules & (II) Cells
 Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Innate Immunity. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples Chapter 3. Antimicrobial peptide psoriasin
 Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Inflammation - Molecular events
 Academic lectures for students of medical schools 3rd Year updated 2004-2015 GENERAL PATHOPHYSIOLOGY Inflammation - Molecular events R. A. Benacka, MD, PhD Department of Pathophysiology Medical faculty
Academic lectures for students of medical schools 3rd Year updated 2004-2015 GENERAL PATHOPHYSIOLOGY Inflammation - Molecular events R. A. Benacka, MD, PhD Department of Pathophysiology Medical faculty
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Within the gastrointestinal (GI) tract, innate immune REVIEWS IN BASIC AND CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
 GASTROENTEROLOGY 2011;141:1986 1999 IN BASIC CLINICAL GASTROENTEROLOGY HEPATOLOGY Robert F. Schwabe and John W. Wiley, Section Editors Inflammasomes in Intestinal Inflammation and Cancer GRACE Y. CHEN*
GASTROENTEROLOGY 2011;141:1986 1999 IN BASIC CLINICAL GASTROENTEROLOGY HEPATOLOGY Robert F. Schwabe and John W. Wiley, Section Editors Inflammasomes in Intestinal Inflammation and Cancer GRACE Y. CHEN*
Immunology Part II. Innate Immunity. 18. April 2018, Ruhr-Universität Bochum Marcus Peters,
 Immunology Part II Innate Immunity 18. April 2018, Ruhr-Universität Bochum Marcus Peters, marcus.peters@rub.de Conserved structures of pathogens PAMPs are detected by Pattern Recognition Receptors PRRs
Immunology Part II Innate Immunity 18. April 2018, Ruhr-Universität Bochum Marcus Peters, marcus.peters@rub.de Conserved structures of pathogens PAMPs are detected by Pattern Recognition Receptors PRRs
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Newly Recognized Components of the Innate Immune System
 Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Sensing and reacting to microbes through the inflammasomes
 The inflammasome Sensing and reacting to microbes through the inflammasomes Luigi Franchi, Raul Muñoz-Planillo & Gabriel Núñez Inflammasomes are multiprotein complexes that activate caspase-1, which leads
The inflammasome Sensing and reacting to microbes through the inflammasomes Luigi Franchi, Raul Muñoz-Planillo & Gabriel Núñez Inflammasomes are multiprotein complexes that activate caspase-1, which leads
In vitro bactericidal assay Fig. S8 Gentamicin protection assay Phagocytosis assay
 In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
Masmudur M. Rahman and Grant McFadden* Department of Molecular Genetics and Microbiology, University of Florida, Gainesville, Florida 32610
 JOURNAL OF VIROLOGY, Dec. 2011, p. 12505 12517 Vol. 85, No. 23 0022-538X/11/$12.00 doi:10.1128/jvi.00410-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Myxoma Virus Lacking
JOURNAL OF VIROLOGY, Dec. 2011, p. 12505 12517 Vol. 85, No. 23 0022-538X/11/$12.00 doi:10.1128/jvi.00410-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Myxoma Virus Lacking
Toll-like Receptors (TLRs): Biology, Pathology and Therapeutics
 Toll-like Receptors (TLRs): Biology, Pathology and Therapeutics Dr Sarah Sasson SydPATH Registrar 23 rd June 2014 TLRs: Introduction Discovered in 1990s Recognise conserved structures in pathogens Rely
Toll-like Receptors (TLRs): Biology, Pathology and Therapeutics Dr Sarah Sasson SydPATH Registrar 23 rd June 2014 TLRs: Introduction Discovered in 1990s Recognise conserved structures in pathogens Rely
Inflammation: How to Cool the Fire Inside your Gut? REINVENTING DIAGNOSTICS
 Inflammation: How to Cool the Fire Inside your Gut? REINVENTING DIAGNOSTICS Future of Healthcare REINVENTING DIAGNOSTICS Inflammation Gut Inflammation Basis of a Healthy
Inflammation: How to Cool the Fire Inside your Gut? REINVENTING DIAGNOSTICS Future of Healthcare REINVENTING DIAGNOSTICS Inflammation Gut Inflammation Basis of a Healthy
Toll-like Receptor Signaling
 Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Innate Immunity. Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 2 August 2016
 Innate Immunity Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 2 August 2016 Objectives: Explain how innate immune system recognizes foreign substances
Innate Immunity Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 2 August 2016 Objectives: Explain how innate immune system recognizes foreign substances
Thioredoxin(TRX) oxidation mediated by mitochondrial ROS is a key event in NLRP3 Inflammasome activation
 Thioredoxin(TRX) oxidation mediated by mitochondrial ROS is a key event in NLRP3 Inflammasome activation TRX-(SH) 2 TRX-S 2 A review on the role of mitochondria and redox reactions in NLRP3 inflammasome
Thioredoxin(TRX) oxidation mediated by mitochondrial ROS is a key event in NLRP3 Inflammasome activation TRX-(SH) 2 TRX-S 2 A review on the role of mitochondria and redox reactions in NLRP3 inflammasome
THE ROLE OF AIM2 IN INFLAMMATORY GENE EXPRESSION
 MQP-JXR-1218 THE ROLE OF AIM2 IN INFLAMMATORY GENE EXPRESSION A Major Qualifying Project Report submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for
MQP-JXR-1218 THE ROLE OF AIM2 IN INFLAMMATORY GENE EXPRESSION A Major Qualifying Project Report submitted to the Faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for
A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System
 Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
Identification of ethyl pyruvate as a NLRP3 inflammasome inhibitor that preserves mitochondrial integrity
 Li et al. Molecular Medicine (2018) 24:8 https://doi.org/10.1186/s10020-018-0006-9 Molecular Medicine RESEARCH ARTICLE Open Access Identification of ethyl pyruvate as a NLRP3 inflammasome inhibitor that
Li et al. Molecular Medicine (2018) 24:8 https://doi.org/10.1186/s10020-018-0006-9 Molecular Medicine RESEARCH ARTICLE Open Access Identification of ethyl pyruvate as a NLRP3 inflammasome inhibitor that
Caspase-11 Activation in Response to Bacterial Secretion Systems That Access the Host Cytosol
 University of Pennsylvania ScholarlyCommons Department of Microbiology Perelman School of Medicine 6-6-2013 Caspase-11 Activation in Response to Bacterial Secretion Systems That Access the Host Cytosol
University of Pennsylvania ScholarlyCommons Department of Microbiology Perelman School of Medicine 6-6-2013 Caspase-11 Activation in Response to Bacterial Secretion Systems That Access the Host Cytosol
The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1b secretion but dispensable for adjuvant activity
 Eur. J. Immunol. 2008. 38: 2085 2089 DOI 10.1002/eji.200838549 Highlights 2085 The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1b secretion but dispensable for adjuvant activity
Eur. J. Immunol. 2008. 38: 2085 2089 DOI 10.1002/eji.200838549 Highlights 2085 The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1b secretion but dispensable for adjuvant activity
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Mechanisms for activation and inhibition of inflammasomes
 University of Iowa Iowa Research Online Theses and Dissertations 2014 Mechanisms for activation and inhibition of inflammasomes John Roger Janczy University of Iowa Copyright 2014 John Roger Janczy This
University of Iowa Iowa Research Online Theses and Dissertations 2014 Mechanisms for activation and inhibition of inflammasomes John Roger Janczy University of Iowa Copyright 2014 John Roger Janczy This
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Regulation of the Antimicrobial Response by NLR Proteins
 Regulation of the Antimicrobial Response by NLR Proteins Eran Elinav, 1,3 Till Strowig, 1,3 Jorge Henao-Mejia, 1,3 and Richard A. Flavell 1,2, * 1 Department of Immunobiology, Yale University School of
Regulation of the Antimicrobial Response by NLR Proteins Eran Elinav, 1,3 Till Strowig, 1,3 Jorge Henao-Mejia, 1,3 and Richard A. Flavell 1,2, * 1 Department of Immunobiology, Yale University School of
Fc receptors, phagocytosis role 128
 Subject Index Adaptive immunity dependence on innate immunity 9, 10 evolution 10 Aging anti-inflammatory agents in counteraction 202 beneficial polymorphisms 199 201 definition 18, 189 innate immunity
Subject Index Adaptive immunity dependence on innate immunity 9, 10 evolution 10 Aging anti-inflammatory agents in counteraction 202 beneficial polymorphisms 199 201 definition 18, 189 innate immunity
Cell Injury MECHANISMS OF CELL INJURY
 Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
Innate Immunity. Jan 8 th Prof. dr. sc. Ivana Novak Nakir 1
 Innate Immunity Jan 8 th 2018. Prof. dr. sc. Ivana Novak Nakir 1 Adaptive Innate 2 Immune system overview 1 st line of defense skin (2m 2 ) and mucosal membranes (~400m 2 ): physical barrier, lymphoid
Innate Immunity Jan 8 th 2018. Prof. dr. sc. Ivana Novak Nakir 1 Adaptive Innate 2 Immune system overview 1 st line of defense skin (2m 2 ) and mucosal membranes (~400m 2 ): physical barrier, lymphoid
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Comparison of Two Mice Strains, A/J and C57BL/6, in Caspase-1 Activity and IL-1
 Mediators of Inflammation Volume, Article ID 7873, 5 pages doi:.55//7873 Research Article Comparison of Two Mice Strains, A/J and C57BL/6, in Caspase- Activity and IL-β SecretionofMacrophage to Mycobacterium
Mediators of Inflammation Volume, Article ID 7873, 5 pages doi:.55//7873 Research Article Comparison of Two Mice Strains, A/J and C57BL/6, in Caspase- Activity and IL-β SecretionofMacrophage to Mycobacterium
Mice. Mice genetically deficient for IRAK-1, IRAK-2, IRAK-1/2, MyD88, TRIF, NLRP3,
 Supporting Information SI Text Mice. Mice genetically deficient for IRAK-1, IRAK-2, IRAK-1/2, MyD88, TRIF, NLRP3, MyD88/TRIF, TLR2/TLR4, TLR7, caspase-1, IL-1R1 and IL-18R, control C57BL/6 mice as well
Supporting Information SI Text Mice. Mice genetically deficient for IRAK-1, IRAK-2, IRAK-1/2, MyD88, TRIF, NLRP3, MyD88/TRIF, TLR2/TLR4, TLR7, caspase-1, IL-1R1 and IL-18R, control C57BL/6 mice as well
CHAPTER 4 IMMUNOLOGICAL TECHNIQUES
 CHAPTER 4 IMMUNOLOGICAL TECHNIQUES Nitroblue Tetrazolium Chloride (NBT) Reduction test NBT reduction test was evaluated by employing the method described by Hudson and Hay,1989 based upon principle that
CHAPTER 4 IMMUNOLOGICAL TECHNIQUES Nitroblue Tetrazolium Chloride (NBT) Reduction test NBT reduction test was evaluated by employing the method described by Hudson and Hay,1989 based upon principle that
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
TD-BF01: Innate immunity to microorganisms
 TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
TD-BF01: Innate immunity to microorganisms I. Toll receptors (adapted from Takeuchi, O. et al. (1999) Immunity 11:443; Kawai, T. et al. (1999) Immunity 11:115; Hemmi, H. et al. (2000) Nature 408:740; Muzio,
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Innate Immunity: (I) Molecules & (II) Cells. Part II: Cells (aka the Sentinels)
 Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
MANUAL IL-1alpha (mouse) ELISA Kit Cat. No. AG-45B-0003-KI01 [Interleukin-1 alpha (mouse) ELISA Kit]
![MANUAL IL-1alpha (mouse) ELISA Kit Cat. No. AG-45B-0003-KI01 [Interleukin-1 alpha (mouse) ELISA Kit] MANUAL IL-1alpha (mouse) ELISA Kit Cat. No. AG-45B-0003-KI01 [Interleukin-1 alpha (mouse) ELISA Kit]](/thumbs/75/71629507.jpg) MANUAL IL-1alpha (mouse) ELISA Kit [Interleukin-1 alpha (mouse) ELISA Kit] For research use only. Not for diagnostic use Version 1 (March-5-2013) Cat. No. AG-45B-0003-KI01 www.adipogen.com Table of Contents
MANUAL IL-1alpha (mouse) ELISA Kit [Interleukin-1 alpha (mouse) ELISA Kit] For research use only. Not for diagnostic use Version 1 (March-5-2013) Cat. No. AG-45B-0003-KI01 www.adipogen.com Table of Contents
Of inflammasomes and pathogens sensing of microbes by the inflammasome
 Series Host pathogen interactions OPEN ACCESS sensing of microbes by the inflammasome Franz Bauernfeind 1,2, Veit Hornung 1 * Keywords: caspase-1; inflammasome; ; NLRP3; pathogens DOI 10.1002/emmm.201201771
Series Host pathogen interactions OPEN ACCESS sensing of microbes by the inflammasome Franz Bauernfeind 1,2, Veit Hornung 1 * Keywords: caspase-1; inflammasome; ; NLRP3; pathogens DOI 10.1002/emmm.201201771
Animal Models to Understand Immunity
 Animal Models to Understand Immunity Hussein El Saghire hesaghir@sckcen.be Innate Adaptive immunity Immunity MAPK and NF-kB TLR pathways receptors Fast Slow Non-specific Specific NOD-like receptors T-cell
Animal Models to Understand Immunity Hussein El Saghire hesaghir@sckcen.be Innate Adaptive immunity Immunity MAPK and NF-kB TLR pathways receptors Fast Slow Non-specific Specific NOD-like receptors T-cell
Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TCAGCCGATTTGCTATCTCAT A
 Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
Supplementary Table 1. Q- and RT-PR primers used in this study. Primer sequences Target Sequence F Sequence R TNF-α (Tnfa) TGGTTTGTTTT GTTTGGGGTTG T hemokine (- motif) ligand 5 (cl5) GTGTTTGTTT TGGTGGTG
TLR2/MyD88/NF-kB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection
 TLR2/MyD88/NF-kB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection Jesus Segovia 1., Ahmed Sabbah 1., Victoria Mgbemena 1,
TLR2/MyD88/NF-kB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection Jesus Segovia 1., Ahmed Sabbah 1., Victoria Mgbemena 1,
Innate Immunity. Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 25 July 2017
 Innate Immunity Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 25 July 2017 Objectives: Explain how innate immune system recognizes foreign substances
Innate Immunity Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 25 July 2017 Objectives: Explain how innate immune system recognizes foreign substances
The oxidized phospholipid oxpapc protects from septic shock by targeting the non-canonical inflammasome in macrophages
 ARTICLE DOI: 1.138/s41467-18-39-3 OPEN The oxidized phospholipid protects from septic shock by targeting the non-canonical inflammasome in macrophages Lan H. Chu 1,2, Mohanalaxmi Indramohan 1, Rojo A.
ARTICLE DOI: 1.138/s41467-18-39-3 OPEN The oxidized phospholipid protects from septic shock by targeting the non-canonical inflammasome in macrophages Lan H. Chu 1,2, Mohanalaxmi Indramohan 1, Rojo A.
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Inflammasomes: too big to miss
 Science in medicine Inflammasomes: too big to miss Andrea Stutz, 1 Douglas T. Golenbock, 1 and Eicke Latz 1,2 1 Department of Infectious Diseases and Immunology, University of Massachusetts Medical School,
Science in medicine Inflammasomes: too big to miss Andrea Stutz, 1 Douglas T. Golenbock, 1 and Eicke Latz 1,2 1 Department of Infectious Diseases and Immunology, University of Massachusetts Medical School,
Supplementary information
 Supplementary information Supplementary Figure S1: Ex[Ca 2+ ]-induced IL-1ß production of monocytes primed with different TLR ligands IL-1ß release of CD14+ monocytes in response to stimulation for 16
Supplementary information Supplementary Figure S1: Ex[Ca 2+ ]-induced IL-1ß production of monocytes primed with different TLR ligands IL-1ß release of CD14+ monocytes in response to stimulation for 16
The Innate Immune Response is Conserved Throughout Evolution and is Triggered by Pattern Recognition. Lipopolysaccharide = Lipid + Polysaccharide
 The Innate Immune Response is Conserved Throughout Evolution and is Triggered by Pattern Recognition Lipopolysaccharide = Lipid + Polysaccharide E.coli Cell wall organization Lipopolysaccharide Outer membrane
The Innate Immune Response is Conserved Throughout Evolution and is Triggered by Pattern Recognition Lipopolysaccharide = Lipid + Polysaccharide E.coli Cell wall organization Lipopolysaccharide Outer membrane
Asc and Ipaf Inflammasomes Direct Distinct Pathways for Caspase-1 Activation in Response to Legionella pneumophila
 INFECTION AND IMMUNITY, May 2009, p. 1981 1991 Vol. 77, No. 5 0019-9567/09/$08.00 0 doi:10.1128/iai.01382-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Asc and Ipaf Inflammasomes
INFECTION AND IMMUNITY, May 2009, p. 1981 1991 Vol. 77, No. 5 0019-9567/09/$08.00 0 doi:10.1128/iai.01382-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Asc and Ipaf Inflammasomes
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in
 Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Innate Immunity. Natural or native immunity
 Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial Pathogens
 Phagocytosis of IgG-coated Targets by s Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial s 3 min 10 min Mast Cells Can Phagocytose Too! Extension of an F-actin-rich
Phagocytosis of IgG-coated Targets by s Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial s 3 min 10 min Mast Cells Can Phagocytose Too! Extension of an F-actin-rich
Rapid antigen-specific T cell enrichment (Rapid ARTE)
 Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Inflammasoma e malattie
 Inflammasoma e malattie Ferrara, Novembre 2017 The rediscovery of inflammation Body Defense Dummy arm Smart arm Inflammation Innate Immunity Adaptive Immunity tumor, calor, dolor, rubor Very smart Control
Inflammasoma e malattie Ferrara, Novembre 2017 The rediscovery of inflammation Body Defense Dummy arm Smart arm Inflammation Innate Immunity Adaptive Immunity tumor, calor, dolor, rubor Very smart Control
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
1. The scavenger receptor, CD36, functions as a coreceptor for which TLR? a. TLR ½ b. TLR 3 c. TLR 4 d. TLR 2/6
 Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary fig. 1. Crystals induce necroptosis does not involve caspases, TNF receptor or NLRP3. A. Mouse tubular epithelial cells were pretreated
 Supplementary fig. 1. Crystals induce necroptosis does not involve caspases, TNF receptor or NLRP3. A. Mouse tubular epithelial cells were pretreated with zvad-fmk (10µM) and exposed to calcium oxalate
Supplementary fig. 1. Crystals induce necroptosis does not involve caspases, TNF receptor or NLRP3. A. Mouse tubular epithelial cells were pretreated with zvad-fmk (10µM) and exposed to calcium oxalate
NF-κB p65 (Phospho-Thr254)
 Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 NF-κB p65 (Phospho-Thr254) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02015 Please read the provided manual
Assay Biotechnology Company www.assaybiotech.com Tel: 1-877-883-7988 Fax: 1-877-610-9758 NF-κB p65 (Phospho-Thr254) Colorimetric Cell-Based ELISA Kit Catalog #: OKAG02015 Please read the provided manual
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
LPS LPS P6 - + Supplementary Fig. 1.
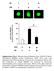 P6 LPS - - - + + + - LPS + + - - P6 + Supplementary Fig. 1. Pharmacological inhibition of the JAK/STAT blocks LPS-induced HMGB1 nuclear translocation. RAW 267.4 cells were stimulated with LPS in the absence
P6 LPS - - - + + + - LPS + + - - P6 + Supplementary Fig. 1. Pharmacological inhibition of the JAK/STAT blocks LPS-induced HMGB1 nuclear translocation. RAW 267.4 cells were stimulated with LPS in the absence
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
MTS assay in A549 cells
 Project: VIGO MTS assay in A549 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
Project: VIGO MTS assay in A549 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Overview of the immune system
 Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
3rd International Conference on Neurology & Therapeutics.
 3rd International Conference on Neurology & Therapeutics www.neuroimmunology.ca Multiple sclerosis is a devastating disease The first description of the disease was mentioned in 14th century In 1838 Dr.
3rd International Conference on Neurology & Therapeutics www.neuroimmunology.ca Multiple sclerosis is a devastating disease The first description of the disease was mentioned in 14th century In 1838 Dr.
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Pyroptosis/Caspase-1 Assay
 Pyroptosis/Caspase-1 Assay Green; Catalog #9145 & #9146 FOR RESEARCH USE ONLY. Not for use in diagnostic procedures. 1. INTRODUCTION Exposure of inflammatory effector cells like monocytes and macrophages
Pyroptosis/Caspase-1 Assay Green; Catalog #9145 & #9146 FOR RESEARCH USE ONLY. Not for use in diagnostic procedures. 1. INTRODUCTION Exposure of inflammatory effector cells like monocytes and macrophages
MTS assay in THP-1 cells
 Project: VIGO MTS assay in THP-1 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
Project: VIGO MTS assay in THP-1 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Supplementary Materials for
 immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
MCB 4211 Basic Immunology 2nd Exam; 10/26/17 Peoplesoft #:
 For this first section, circle the letter that precedes the best answer for each of the following multiple-choice questions. LOOK AT ALL ALTERNATIVES BEFORE CHOOSING YOUR ANSWER. 1. The TcR (T cell receptor)
For this first section, circle the letter that precedes the best answer for each of the following multiple-choice questions. LOOK AT ALL ALTERNATIVES BEFORE CHOOSING YOUR ANSWER. 1. The TcR (T cell receptor)
Integrated Innate Immunity Combining Activation and Effector Functions
 Section 4 Integrated Innate Immunity Combining Activation and Effector Functions 4.1 THE DETECTION OF BACTERIAL LIPOPOLYSACCHARIDE LPS is one of the most potent immune stimuli known. It is found in the
Section 4 Integrated Innate Immunity Combining Activation and Effector Functions 4.1 THE DETECTION OF BACTERIAL LIPOPOLYSACCHARIDE LPS is one of the most potent immune stimuli known. It is found in the
Chemical and Biochemical Mechanism Of Cell Injury.
 Chemical and Biochemical Mechanism Of Cell Injury. Professor Dr. M. Tariq Javed Dept. of Pathology Faculty of Vet. Science The University Of Agriculture Faisalabad Cell Injury When the cell is exposed
Chemical and Biochemical Mechanism Of Cell Injury. Professor Dr. M. Tariq Javed Dept. of Pathology Faculty of Vet. Science The University Of Agriculture Faisalabad Cell Injury When the cell is exposed
