by Mannitol during Hypoperfusion of the Kidney
|
|
|
- Allan Armstrong
- 5 years ago
- Views:
Transcription
1 Restoration and Maintenance of Glomerular Filtration by Mannitol during Hypoperfusion of the Kidney C. RicHARD MORRIS, EDWARD A. ALEXANDER, FRANK J. BRUNS, and NORMAN G. LEVINSKY From the Department of Medicine, Boston University School of Medicine, Boston University Medical Center and Boston University Medical Service, Boston City Hospital, Boston, Massachusetts A B S T R A C T Glomerular filtration (GF) during progressive reduction of renal perfusion pressure by aortic clamping was studied in hydropenic rats and in rats infused with isotonic saline, hypertonic saline, or mannitol. As judged by visual observation of Lissamine green movements in superficial nephrons, GF was absent in hydropenic or saline-loaded rats at 40 mm Hg aortic pressure, but continued in some nephrons of all rats infused with mannitol and of some rats infused with hypertonic saline. Urine flow persisted only in rats infused with mannitol. By use of the qualitative Hanssen technique, it was found that all glomeruli in superficial and deep portions of the cortex were perfused at 40 mm Hg in all groups of rats. By the same method, GF continued in 1% of nephrons in hydropenic rats, 12% of nephrons in isotonic saline-loaded rats, and 78% of nephrons in rats infused with mannitol. By means of a quantitative Hanssen technique, GF was 5.8 nl/min per nephron in mannitol-infused rats and not measurable (<0.5 nl) in hydropenic rats. Superficial and deep nephrons were similar in both qualitative and quantitative studies. Although urine flow did not persist in rats infused with hypertonic saline, GF was detected in four of seven studies by the Hanssen method (mean, 9.1 nl/min per nephron). In additional experiments, mannitol infused after perfusion pressure had already been- lowered to 40 mm Hg in hydropenic rats reestablished urine flow and GF (mean, 9.8 nl/min). Furosemide, isotonic and hypertonic saline did not restart urine flow; however, GF (Lissamine green) was restarted by hypertonic saline. We conclude that mannitol can maintain or reestablish by an extratubular mechanism GF which otherwise would not occur during renal hypoperfusion. Hypertonic saline has a similar effect on GF in some cases, but urine Received for publication 20 September 1971 and in revised form 13 January flow is not maintained, implying of filtrate. complete reabsorption INTRODUCTION In previous studies (1) in the dog, we found that prior infusion of small amounts of mannitol would maintain glomerular filtration (GF)' even when renal perfusion pressure was reduced to less than 40 mm Hg by aortic clamping. In hydropenic dogs, there was no evidence of GF at such reduced pressures. Infusion of large volumes of isotonic saline did not maintain GF, indicating that the effect of mannitol was not due to dilution of plasma proteins. These observations suggested that mannitol might maintain GF in the hypoperfused kidney by some novel mechanism. GF was detected by visual evaluation of the movement of Lissamine green dye in superficial nephrons in these studies. The limitations of this method precluded firm conclusions regarding several important aspects of these experiments. Recently, modifications (2, 3) of the Hanssen method (4) have become available which permit more definitive and quantitative evaluation of GF in individual nephrons. We have applied these methods to extend our evaluation of the effects of mannitol on GF during hypoperfusion of the kidney. In studies in rats reported in this paper, we found that prior infusion of mannitol maintains GF which otherwise would stop when renal arterial pressure is reduced to 40 mm Hg. Moreover, when given after hypoperfusion has been induced, mannitol will restart GF which has already stopped. Therefore, mannitol apparently can maintain or reestablish GF by a mechanism which does not depend on its presence within tubular lumina. 'Abbreviations used in this paper: GF, glomerular filtration; SNGFR, single nephron glomerular filtration rate. The Journal of Clinical Investigation Volume
2 METHODS M\ale Sprague-Dawley rats, weighing g, were deprived of food and water for hr, and anesthetized with intraperitoneal Inactin (Promonta, Hamburg, West Germany) (100 mg/kg body weight). They were placed on a heated micropuncture table and maintained at normal body temperature. A tracheostomy was performed and catheters were placed in the left jugular vein for fluid infusion, in the right jugular vein for injections of 10%o Lissamine green or radiolabeled sodium ferrocyanide, and in the left femoral artery for arterial blood collection and determination of mean arterial pressure. Both ureters were cannulated near the renal pelvis. A No. 10 polyethylene catheter, passed retrograde from the right femoral artery to an intra-aortic position just above the left renal artery, was used for rapid injections of 10% Lissamine green or 20% sodium ferrocyanide. A modified Blalock clamp was positioned around the aorta proximal to both renal arteries and a ligature was placed loosely around the renal hilus. The left kidney was suspended in a Lucite holder, illuminated with a fiberoptic light source, and bathed with mineral oil maintained at 370 C. Fluid losses incurred during surgical preparation were not replaced. Experiments were performed only if the proximal transit time was < 12 sec, dye rapidly left the distal tubules, and arterial blood pressure was > 95 mm Hg. Blood pressure below the arterial clamp was measured continuously with a mercury manometer. Urine flow was determined utilizing timed collections in calibrated No. 50 or No. 10 polyethylene catheters permitting measurement of volumes greater than 0.05 Ml. Clearance of inulin was determined utilizing inulin-3h (New England Nuclear Corp., Boston, Mass.). Proximal tubular transit times and visual estimations of the per cent of functioning surface tubules were determined by intravenous injection of Al of 10% Lissamine green. Proximal transit time was defined as the time between appearance of the dye on the surface of the kidney in blood vessels ("vascular flush") and the disappearance from the latest proximal tubular segments in the "rosette." Especially at reduced pressures, an occasional tubular segment in a microscopic field remained filled with dye for an indefinite period; these segments were disregarded in calculating transit times. The fraction of tubular segments in which Lissamine green appeared was estimated by observation of the kidney surface visible in one X 25 microscopic field. necessarily only semiquantitative and are Such estimates are reported as approximate fractional values. Single nephron glomerular filtration rate (SNGFR) at reduced perfusion pressure was assessed qualitatively using a modification of the histochemical microdissection technique described by Hanssen (2-4). A single, continuous, intravenous infusion of 0.8 ml of 20% unlabeled sodium ferrocyanide was given over a period equal to one-half the proximal tubular transit time. Immediately thereafter the entire renal hilus was ligated, and the kidney was excised and transferred to a solution of 2-methyl butane previously cooled to a temperature of - 750C to - 780C by immersion in an acetone-solid C02 slush. After complete freezing (45 sec), the kidney was removed and fragmented into pieces approximately 2-5 mm thick. These fragments were then transferred to a 50%o alcoholic FeCl, solution previously cooled to - 32'C to - 34C and placed in a freezer at - 25'C. After hr freeze-dry exchange in FeCl,, the renal fragments were washed with distilled water and placed in 20%o hydrochloric acid for maceration. After 24 hr incubation at 270C, the fragments were again washed and placed 1556 C. R. Morris, E. A. Alexander, F. J. Bruns, and N. G. Levinsky in acetic acid-ferric chloride solution (100 mg/100 ml ferric chloride and 1% acetic acid) and reincubated hr after maceration, 50 "superficial" nephrons (those in which at least one convolution touches the cortical surface) and 50 "deep" nephrons (those located in the innermost third of the cortex) were microdissected from four randomly selected fragments, and the presence of precipitated Prussian blue granules within glomeruli and tubules was noted. Superficial or deep glomeruli and their attached tubules were dissected as encountered without knowledge of intratubular Prussian blue content. Quantitative SNGFR was determined using Hanssen's technique as modified by de Rouffignac, Deiss, and Bonvalet (3). An infusion of sodium ferrocyanide containing sufficient unlabeled and 14C-labeled decahydrate sodium ferrocyanide (New England Nuclear Corp., Boston, Mass.) to produce and maintain a plasma concentration of 1.5 mm and ACi/ml was given intravenously. During equilibration, an intra-aortic injection of 50 Al of 10% Lissamine green was used to determine proximal tubular transit time and the time between injection and appearance of Lissamine green in surface capillaries ("appearance time"). After 10 min of equilibration, duplicate arterial plasma samples were drawn for sodium ferrocyanide-"c concentration. Immediately thereafter, 70 pl of 20% unlabeled sodium ferrocyanide was injected intra-aortically as a single, rapid, "marker bolus," and the time was recorded. After a period sec less than the proximal tubular transit time, the entire renal hilus was ligated, and the kidney was excised and frozen. After freeze substitution and maceration as described above, at least three superficial and three deep nephrons were dissected from each of four randomly selected renal fragments. The entire proximal nephron (glomerulus and proximal tubule) was dissected free, divided at the front of the visible "marker bolus" of ferric ferrocyanide (Prussian blue), transferred to a scintillation vial containing 10 ml of Aquasol (New England Nuclear Corp., Boston, Mass.), and counted in a Beckman LS-200 Beta scintillation counter (Beckman Instruments, Inc., Fullerton, Calif.) 5-jd plasma samples were counted similarly. SNGFR was calculated using the standard clearance equation in which UV represented intratubular radioactivity divided by the period of time between injection of the marker bolus and renal excision, minus appearance time. The minimum detectable SNGFR was 0.5 nl/min. This was determined by maintaining the level of radioactivity in the plasma high enough so that during the time the kidney was perfused with isotope, and assuming SNGFR was 0.5 nl/min or higher, the amount of filtrate formed would contain sufficient radioactivity to count at least twice background. In the case of the hydropenic group of rats (see below), qualitative studies had indicated that there was no visible intratubular marker bolus. Hence, the entire length of the proximal tubule to the end of the pars recta was dissected and counted for radioactivity. The entire period between the start of the ferrocyanide-14c infusion and excision of the kidney (about 10 min) was considered to be the time for calculation of nephron clearances. Nephrons were counted for at least 100 min. The radioactivity recorded from nephrons prepared as described reflects both filtered sodium ferrocyanide (intratubular) and sodium ferrocyanide present within the glomerular capillaries and along the outer surface of the tubule. Radioactivity not attributable to filtration was determined in hydropenic animals subjected to the same reduction in renal perfusion pressure (40 mm Hg) used in the experi-
3 mental animal. In addition, ureteral pressure was increased to 60 mm Hg in an attempt to produce a condition in which filtration would be unlikely. After 10 min equilibration with sodium ferrocyanide at the same plasma concentration as that used experimentally, renal excision, freeze-dry exchange with FeCl3 and maceration were performed. Three superficial and three deep nephrons were dissected from each of four randomly selected renal fragments. The entire proximal nephron was dissected free, divided at the end of the pars recta, and counted. A mean value of 9 cpm for this extratubular radioactivity was thereby obtained and this value was subtrated from the radioactivity present in all experimental nephron samples. In actual experiments, nephrons from hydropenic rats without elevated ureteral pressure counted at the same rate, i.e., 9 cpm/nephron, as nephrons in these rats used to determined extratubular contamination. Since there was a 10 min "clearance" period and plasma radioactivity was about 5 cpm/nl, calculated SNGFR would still be <0.2 nl/min if all 9 counts were considered to represent filtration. However, in the mannitol and 1.7% saline groups, in which filtration occurred, cpm/nephron were only above background. Hence, the calculated SNGFR given in the text would increase substantially if the 9 cpm/nephron found in the obstructed, hypoperfused kidneys had not been subtracted. Animals were divided into two major groups, those receiving continuous infusions before and during serial reductions in renal perfusion pressure (prehypoperfusion group) and those receiving a similar volume given acutely after establishment of renal perfusion pressure at 40 mm Hg for at least 30 min (posthypoperfusion group). Animals in the prehypoperfusion group received one of the following infusions: 0.85% sodium chloride at 0.02 ml/min (hydropenic), 0.85% sodium chloride, 1.7% sodium chloride, or 5% mannitol in 0.85% sodium chloride, all administered at 0.11 ml/ min. Total volume infused was approximately 3-5% body weight at the time of observations at 40 mm Hg. After 1 hr of infusion, proximal tubular transit time, distribution of Lissamine green in surface nephrons, and urine flow were determined during three consecutive 10-min periods. The same observations were made after serial reduction in renal perfusion pressures to 60 and 40 mm Hg. After more than 40 min of established oliguria at a perfusion pressure of 40 mm Hg, the kidney was prepared for either qualitative or quantitative assessment of SNGFR by the appropriate method described above. Hydropenic animals in the posthypoperfusion group received 0.85% sodium chloride at 0.02 ml/min during serial reductions in renal perfusion pressure identical to those for the prehypoperfusion group. After 30 min of established oliguria at a renal perfusion pressure of 40 mm Hg, 0.85% sodium chloride, 1.7% sodium chloride, or 5% mannitol in 0.85% sodium chloride was acutely infused ( ml/min) to a final volume equaling 5% body weight. Another group of hydropenic animals received two sequential doses of furosemide: 25 mg/kg acutely followed by a 30 min infusion providing 3.5 mg/kg; then 100 mg/kg acutely, followed by an infusion providing 100 mg/kg per hr. Proximal tubular transit time, Lissamine green distribution, and urine flow were determined in each group after completion of the infusion or as soon as urine flow began. Thereafter, SNGFR and whole kidney GFR were determined by the quantitative Hanssen technique and inulin-14c clearance, respectively, in the group which received mannitol. Statistics were calculated from mean values for each experiment and the values given are means of individual experimental means +SEM. The Student t test was used to determine statistical significance. RES ULTS Prehypoperfusion infusions. Changes in urine flow in each group are shown in Fig. 1. Hydropenic rats and those infused with 0.85% or 1.7% saline were similar in their response to progressive reduction in renal perfusion pressure. In each group, severe oliguria was present during stable reduction of aortic pressure to 60 mm Hg for min; many rats in each group were anuric. At 40 mm Hg, anuria was uniformly present in each group. In contrast, in the group infused with 5% mannitol, although urine flow decreased progressively as aortic pressure was reduced, it was still 5.8±0.71!l/min at 40 mm Hg (23% of flow rate at normal presure). Appearance of Lissamine green was evaluated semiquantitatively by visual observation of all tubular segments in one low-power (X 25) microscopic field. Transit time was estimated as the time between appearance of the dye in blood vessels on the kidney surface and its disappearance from the latest proximal segments. The results are illustrated in Fig. 2. All segments filled and emptied in all groups at normal perfusion pressure. Transit time was 9.8±0.04 sec. At 60 mm Hg, occasional tubular segments continued to fill and empty in all the rats which were hydropenic or infused with 0.85% saline. Transit time was 42+4 sec in the hydropenic, sec in the isotonic saline group. When aortic pressure was reduced to 40 mm Hg, there was no evidence of tubular appearance of the dye in any rats in either the hydropenic or the isotonic saline group. Although all rats infused with 1.7% saline were anuric at 40 mm Hg, two different patterns of Lissamine green were noted. In three of the seven rats studied, there was no evidence of the dye in surface F0 Hydropenia 10 (n=13) a 0.85% (n=5) * 1.7% Saline (n=8) N Mannitol (n = 12) AORT/C PRESSURE (mm/hg) FIGURE 1 Urine flow (mean ±SEM) during progressive reduction in aortic pressure. The groups are shown in the same order at each pressure. Mannitol and GFR in Renal Ischemia 1557
4 II::: X.0D o*, 404- *x.oc*avu I -6 IAORTIC PRESSURE- CONTROL 60 mm Hg 341-1/41-0 a 0.0, K 0r xar v.0 _ 40 mmhg Or la._ 'I * 'edrw~i - v 5ZAto f PREHYPOPERFUSION /donnltol. M IT7ZMiCIJV~.LIVNf }POSTUPOERFUSIM 10rUU -.v. - FIGURE 2 Appearance of Lissamine green in superficial nephrons during progressive reduction in aortic pressure. The upper panel shows change in the proximal transit time of the dye. The lower panel shows a semiquantitative estimate of the fraction of proximal segments in one microscopic field (X 25) in which Lissamine green appeared. The L symbol represents three rats infused with 1.7%o saline, in which Lissamine green did not appear in surface tubules at 40 mm Hg; the a symbol, four rats in which the dye continued to appear in some tubules at 40 mm Hg. tubules. In each of the remaining 4 rats, however, Lissamine green appeared in about one-sixth of surface tubular segments, and transit time of the dye was sec. All rats infused with mannitol responded in a similar manner; about one-fourth of tubules filled, and transit time was 71±11 sec at 40 mm Hg. The qualitative presence or absence of glomerular perfusion was determined at 40 mm Hg in 50 superficial and 50 deep glomeruli from several random areas of kidney in each of eight hydropenic rats, five rats infused with isotonic saline, and six receiving mannitol. The presence of precipitated Prussian blue within the glomerular capillaries was interpreted as evidence of glomerular perfusion. Precipitated Prussian blue was easily appreciated, and intermediate degrees of intraglomerular precipitation were not seen (Fig. 3). 100% of glomeruli from both superficial and deep nephrons of all rats studied in each group contained Prussian blue. Thus, there was no evidence of systematic or patchy nonperfusion of glomeruli by blood at an aortic pressure of 40 mm Hg. - The presence of Prussian blue granules within the tubular lumen was interpreted as evidence of glomerular filtration (Fig. 3). The granules were readily seen and, when present, formed a scattered column over at least one-quarter the length of the proximal tubule. 50 superficial and 50 deep nephrons from several randomly selected areas of the kidney were dissected in each rat, and the number containing Prussian blue (filtering) is shown in Fig. 4. In hydropenic rats, <1% of all nephrons filtered. In rats infused with 0.85% saline, 12% filtered. In rats receiving mannitol, 76% of nephrons were filtering at 40 mm Hg perfusion pressure. The difference between the mannitol group and either the saline or the hydropenic group was highly significant (P <0.01). On the other hand, saline-expanded and hydropenic animals were not statistically different (P > 0.1). GF was also evaluated separately in superficial and deep nephrons. There were no significant differences between superficial and deep nephrons within each group. Moreover, the differences among the three groups described above were true as well of superficial and deep nephrons, treated as separate groups statistically. Single nephron glomerular filtration rates (SNGFR) estimated by the quantitative modification of the Hanssen technique are shown in Table I. In a total of 100 nephrons from five hydropenic rats, SNGFR was uniformly too low to measure (< 0.5 nl/min). There was no significant difference between SNGFR of 50 superficial and 50 deep nephrons, either in individual experiments or in total (P > 0.5). The seven rats infused with 1.7% saline fell into two groups. In the three rats in which there was no evidence of superficial nephron function as judged by appearance of Lissamine green in surface tubules, GF was uniformly absent from both superficial and deep nephrons. There were no differences either in individual experiments or in total between 37 superficial and 37 deep nephrons dissected (P > 0.2). In the other four rats infused with hypertonic saline, in which Lissamine green was seen in some nephrons, mean SNGFR was 9.5 nl/min (89 nephrons). GF was detected in more than 90% of nephrons dissected. There was no significant difference between superficial and deep nephrons in individual experiments or in the combined 45 superficial and 44 deep nephrons (P > 0.5). Six rats received an infusion of 5% mannitol. Since urine flow occurred, it was possible to measure an inulin clearance of 0.12±0.03 ml/ min. Mean SNGFR in 121 nephrons was 5.8±1.3 nl/ min. GF was measurable in more than 90% of nephrons dissected. Deep nephron GFR was significantly greater (P < 0.05) than superficial nephron GFR in two of five rats studied. When all experiments were combined, however, superficial (61 nephrons) and deep (60 neph C. R. Morris, E. A. Alexander, F. J. Bruns, and N. G. Levinsky
5 ...-*} o'4 8 s....,.... i w 'hi ',.>,# s.x *.:. *e w u To A: :. i; \ i;k X i t <'Jf ''.._$;f '\A;,; And: ant, sa :\ 5'^> 7.ir,: \ as Uj.'.Le 2 mm *!1a,,mv I _... FIGURE 3 Right, the proximal tubule contains a scattered column of Prussian blue granules, which demonstrate filtration; left, a nonfiltering nephron without granules. Note that both glomeruli are darkly stained by numerous granules of Prussian blue, which indicate perfusion by blood in vivo. Glomeruli containing only rare or questionable granules were not observed. X 160. rons) SNGFR were not significantly different (P > 0.5). Assuming that two-thirds of nephrons correspond to our superficial group and that there are 30,000 nephrons per kidney, calculated kidney GFR would be ml/min, in fair agreement with the value of 0.12 ml/min actually measured. Posthypoperfusion infusions. 12 rats were subjected to progressive reduction of aortic pressure and at least 30 min of sustained anuria at 40 mm Hg. Lissamine green did not appear in tubules of any of these rats at this reduced pressure. Thereafter, each rat received a rapid infusion; special care was taken to keep aortic pressure at 40 mm Hg throughout infusion and thereafter. Two rats received 0.85% saline; neither urine flow nor Lissamine green appearance was noted in either rat. Three rats were infused with 1.7% saline. Although anuria persisted, Lissamine green appeared in about one-fifth of nephrons in each rat. Proximal transit time was 51+6 sec. Three animals received furosemide. Anuria persisted and observations of surface tubules did not demonstrate Lissamine green filling. Rats infused with mannitol responded dramatically within 5-10 min. Urine flow increased to 31.4 Al/min after infusion. About one-fifth of superficial tubular segments filled after intravenous Lissamine green and a transit time of 35±4 sec was recorded. Quantitative SNGFR was determined in four of five rats studied (Table I). SNGFR in superficial nephrons averaged 10.3±4.4 nl/min and in deep nephrons, 9.4±3.2 nl/ min; GF was detected in more than 90% of nephrons dissected. There was no significant difference between superficial and deep nephrons (P > 0.8). Calculated whole kidney GFR, using the assumptions noted above, was 0.31 nl/min, somewhat higher than the observed value of Sensitivity of methods used to detect SNGFR. In a qualitative sense, there was good agreement among the three methods used to detect SNGFR: observation of Lissamine green, qualitative Hanssen method, quantitative modification of Hanssen method. Thus, in hydropenic rats, GF was not detected by any of these methods at 40 mm Hg. In rats infused with mannitol, Mannitol and GFR in Renal Ischemia 1559
6 I0J 100 r 80 U3SuperficioI MDeep 60 F I P 0 HYDROPENIA 0.85% SALINE MlIANN ITOL (8).(5) (6) FIGURE 4 Per cent (mean ±5-EM) of nephrons which filtered when aortic pressure was reduced to 40 mm Hg. The number of rats studied is shown in parenthesis on the horizontal axis. 50 superficial and 50 deep nephrons were counted in each rat. all three methods revealed that GF was occurring. In the three rats of the prophylactic 1.7% saline group in which Lissamine green failed to appear on the kidney surface, GF was not detected by the quantitative Hanssen technique. On the other hand, quantitative agreement among the methods was not close. In the prophylactic mannitol group less than one-fourth of surface tubular segments appeared to fill with Lissamine green at 40 mm Hg (Fig. 2). By the qualitative Hanssen method, about three-fourths of superficial nephrons in this group were found to filter (Fig. 4). In studies with the quantitative method, more than 90% of superficial nephrons had detectable filtration. Since the Lissamine green method depends on observation, it is obviously semiquantitative at best and it is not surprising that dye concentration in many nephrons is too low to perceive. The different sensitivity of the qualitative and quantitative Hanssen methods may be due to the fact that the ferrocyanide marker is injected directly into the aorta at the renal arteries in the quantitative method rather than into the jugular vein, as in the qualitative technique. DISCUSSION Our previous studies -in dogs (1) were subject to three important limitations. First, the determination of GF depended on visual observation of the appearance and move-,me~nt of Lissamine green in tubular lumina. This technique is open to subjective bias and is of unknown sensitivity in detecting small amounts of GF. Second, since the first 10% of the proximal tubule is below the surface of the kidney, small amounts of GF would be missed if all the filtrate were reabsorbed in that nonvisualizable part of the tubule. Third, GF in "deep" nephrons, i.e. any T whose proximal convolutions are entirely below the surfact of the kidney, cannot be determined by this method. The Hanssen technique used in the present study circumvents all of these limitations. The sensitivity of the quantitative method as used in our experiments is sufficient to detect 0.5 nl/min of GF per nephron. GF at any point in the proximal tubule will be detected. Both deep and superficial nephrons can be studied. Despite the theoretical limitations of the Lissamine green technique, our current experiments show that there is actually good qualitative agreement between observation of Lissamine green and the Hanssen method. There is also good general correspondence between the present experiments in the rat and earlier experiments in the dog (1). In general, urine flow stopped at pressures of about mm Hg during hydropenia in each species and there was evidence that some nephrons continued to filter even in anuric rats and dogs at this pressure level. At pressures of mm Hg during hydropenia, Lissamine green observations gave no evidence of GF in superficial nephrons -in either speices. The qualitative TABLE I Summary of Nephron GER Measurements Single nephron GFR Experi- Urine ment No. Cln* flow S* D Hydropenia ml/min id/min ni/mm ni/min % NaCi, prehypoperfusion ± ± ±a ±== ± ± ± ±40.4 Mean 4-SE 0.08± ± ±1.1 Mannitol, prehypoperfusion ± ± ± ± ± ± ± ± ± ±=i0.8 Mean ASE 0.12± ± ± ±42.1 Mannitol, "posthypoperfusion" ± ± ± ± ± ± ± ±42.9 Mean ±~se ± ± ± ±-3.2 * Abbreviations: Ci., inulin clearance; 5. superficial nephrons; D, deep nephrons C. 1R. Morris, E. A. Alexander, F. J. Bruns, and N. C. Levinsky
7 and quantitative Hanssen experiments in rats fully confirm these visual impressions, in that GF in superficial nephrons was undetectable by either technique at 40 mm Hg. Rats and dogs preinfused with large amounts of isotonic saline responded similarly to hydropenic animals. There was no urine flow and GF was not detected by the Lissamine green technique in either species. By the qualitative Hanssen technique, 12% of nephrons appeared to filter in saline-loaded rats. In both animals, at 40 mm Hg prior infusion of mannitol maintained both urine flow and GF detectable by Lissamine green in the majority of superficial nephrons. One important difference should be noted, however. In dogs, as little as ml of isotonic mannitol was effective. This amount is equivalent to about % of body weight in a 15 kg dog. Proportionate volumes were tested in rats in preliminary experiments and proved ineffective. Only when volumes of mannitol equivalent to 3-5% of body weight had been infused were urine flow and GF (Lissamine green) maintained at 40 mm Hg in rats. Thus, the effect of mannitol to maintain GF is present in both rats and dogs, but the dog appears to be much more sensitive to this action of mannitol. We adjusted the level of plasma radioactivity so that as little as 0.5 nl of GF per nephron would have resulted in accumulation of counts equal to twice background during the 10 min interval used to measure GF in hydropenic rats. No GF was detected by this quantitative method in hydropenic rats when renal arterial pressure was reduced to 40 mm Hg. During infusion of mannitol, GF was 5.8 nl/min per nephron at the same arterial pressure. GF was 9.8 nl/min per nephron in rats infused rapidly with an equivalent volume of mannitol after GF had been interrupted by arterial clamping. These differences in GF persist for at least 30 min to 1 hr after the pressure of 40 mm has been set. GF can be restarted with mannitol within 5-10 min after the requisite amount has been infused. These data demonstrate conclusively that mannitol will maintain or restart GF which otherwise would not occur during hypoperfusion of the kidney. What mechanisms might account for this striking effect of mannitol? The rate of GF depends on the renal plasma flow and on the balance of Starling's forces across the glomerular capillaries. The three principal Starling forces are glomerular capillary oncotic pressure, glomerular capillary hydrostatic pressure, and intratubual hydrostatic pressure. Thus, four general categories of mechanisms must be considered: (a) decreased plasma oncotic pressure; (b) decreased intratubular pressure; (c) increased gloanerular capillary pressure; (d) increased renal plasma flow to glomeruli (glomerular perfusion rate). Since relatively large volumes of mannitol were infused, plasma protein concentration and hence plasma oncotic pressure, although not measured, undoubtedly fell by 15-25%. However, infusion of equal or greater volumes of isotonic saline did not maintain or reestablish GF. The calculated fall in plasma protein concentration should have been at least as great with saline as with mannitol. Indeed, about 20% of the infused volume of mannitol was excreted in the urine during the period required for stepwise reduction of arterial pressure to 40 mm, while virtually none of the saline volume was excreted. Moreover, a volume of saline equal to 5% of body weight had no effect on GF while mannitol equal to 3% of weight was effective. Thus, the effect of saline on plasma protein should have exceeded that of mannitol in some cases. Finally, in our previous studies in dogs (1), infusion of saline equal to 10% of weight failed to maintain GF, while 1/20 as great a volume of mannitol was effective. Thus, reduction of plasma oncotic pressure by simple dilution of plasma proteins does not appear to be the mechanism of the effect of mannitol on GF. For identical reasons, direct or indirect effects of expansion of extracellular fluid volume per se cannot be the explanation of this action of mannitol. We have no information on possible changes in intratubular pressure in our experiments. However, it seems reasonable that intratubular pressure would decrease during hypoperfusion of the kidney at arterial pressures below the autoregulatory range and it is known to increase during mannitol infusion (5). If similar changes occurred in our experiments, i.e. increased intratubular pressure after mannitol, this would, of course, oppose rather than enhance GF. Furthermore, the ability of mannitol to restart GF after it has already stopped would require that intratubular pressure be reduced by an extraluminal action. It is conceivable that swelling of tubular epithelial cells due to entrance of sodium and water during renal hypoperfusion could occlude tubular lumina. Mannitol might relieve swelling by osmotic removal of water from swollen cells and thereby relieve intratubular obstruction. We have no evidence pertaining to such a mechanism. Some evidence (6-8) supports the concept that redistribution of blood flow away from superficial glomeruli may occur during renal ischemia. In renal hypoperfusion induced by hemorrhage, radioxenon studies have suggested patchy ischemia of superficial cortical areas and diversions of blood flow to deep zones (6, 7). During arterial clamping, some redistribution of flow to deeper cortical areas has also been described (8). It was conceivable that redistribution of renal plasma flow away from superficial glomeruli during arterial clamping with restoration of the normal pattern of flow by mannitol might occur. Such a phenomenon could account for our present Mannitol and GFR in Renal Ischemia 1561
8 and previous (1) observations with Lissamine green, which are limited to superficial nephrons. However, the present data obtained with the Hanssen technique rule out total diversion of plasma flow from any groups of nephrons. We carefully chose a number of random pieces from each kidney to perform microdissections. All glomeruli in both superficial and deep zones of the cortex contained precipitated Prussian blue dye, indicating perfusion of all glomeruli, at least to some extent. It must be stressed that the appearance of dye crystals in a glomerulus is evidence only that the glomerulus was indeed perfused with blood but gives no information whatever on the rate of flow. Nevertheless, complete diversion of plasma flow from patchy superficial areas or from the superficial cortical zone as a whole is ruled out. Moreover, the excellent agreement between superficial and deep nephron GF rates in all groups of rats we studied argues strongly against redistribution of blood flow to deep nephrons as the major mechanism. In hydropenic rats, GF stopped in both deep and superficial nephrons during hypoperfusion. During mannitol infusion, both groups of nephrons filtered at about the same rate. Thus, mannitol appears to act by a mechanism which affects GF in nephrons in superficial and deep zones of the cortex more or less equally. Recently, Flores-Calle, Beck, DiBona, Marcilio, and Leaf (9) have reported that the intrarenal vasculature failed to fill normally when the renal artery was released after 2 hr of complete obstruction. Hypertonic mannitol reversed this "no-reflow" phenomenon and permitted relatively normal filling of the entire intrarenal vasculature. Based on similar work in cerebral vessels (10), these authors suggested that swelling of postglomerular capillary endothelial cells occurs during arterial occlusion, due to failure of the pump which maintains normal cell volume by extruding sodium. When arterial flow is reestablished, occlusion of postglomerular capillaries by swollen endothelial cells prevents reestablishment of renal blood flow. The authors postulated that hypertonic mannitol, relatively impermeant across cell membranes, induces water movement out of swollen cells by increasing effective serum osmolality. There are several differences between their studies and our own which lead us to doubt the applicability of their ingenious suggestion to our model. Their technique involves complete occlusion of the renal circulation for 2 hr, whereas renal blood flow, although reduced, undoubtedly continued in our experiments. This is evidenced by the appearance of Prussian blue crystals in all glomeruli in hydropenic rats. Moreover, direct measurement of renal blood flow at an arterial pressure of 45 mm in the dog reveals that it is about one-half of normal (8). It seems unlikely that endothelial cell swelling due to hypoxia would occur when these cells are exposed directly to such high absolute blood flow rates. Secondly, both in our current rat and in our previous dog studies, 5% mannitol was infused rather than the 25% solution employed by Flores-Calle and colleagues (9). According to their formulation, isotonic mannitol as used in our experiments should not be effective. Nevertheless, other of our data would fit their hypothesis. The mannitol given to rats (but not dogs) was infused in 0.85% saline; therefore, total osmolality presumably increased during the infusion. Moreover, in one-half or more of the rats, infusion of a comparable volume of 1.7% saline maintained GF at the same level as mannitol infusion, when arterial pressure was reduced to 40 mm Hg. We have no direct information on endothelial cell swelling or total renal blood flow in our rat model, however. Hence we consider it possible that in hydropenic rats in which arterial pressure is maintained at 40 mm Hg for some time, renal blood flow is reduced to very low levels by vascular endothelial obstruction which can be relieved by mannitol. Finally, it is possible that renal blood flow is reduced only moderately at 40 mm Hg in our model, as in other studies in which partial arterial clamping has been performed (8), but that increased afferent arteriolar resistance reduces effective filtration pressure to zero and thereby stops GF. Within the range of autoregulation, total renal vascular resistance falls as arterial pressure is reduced (11), and the bulk of evidence indicates that the afferent arterioles progressively dilate. However, when arterial pressure is reduced below 80 mm Hg, renal blood flow falls progressively. Some studies (12) suggest that renal vascular resistance may increase at pressures comparable to those we used in our experiments. In normal rats, about one-half of the arterial blood pressure is transmitted to the glomerular capillaries (13). If afferent arteriolar resistance fell to zero and arterial pressure were transmitted completely to glomerular capillaries, glomerular pressure in our experiments would, of course, be 40 mm Hg. If plasma oncotic pressure were 25 and intratubular pressure were reduced to about 5, net filtration pressure would approximate 10 mm Hg, i.e., about one-half the normal value of 18 (13). If total plasma flow is about one-half of normal at 40 mm Hg (8), GF would be about one-fourth the normal value. This is about the rate of nephron GF observed in rats infused with mannitol. The data would, thus, be consistent with the view that afferent arterioles are widely dilated in rats infused with mannitol at a renal artery pressure of 40. Obviously, by the same reasoning, afferent arterioles would not be fully dilated at the same arterial pressure in hydropenic rats. What might cause afferent arterioles presumably widely dilated at 80 mm Hg (11) to become less open at 40 mm Hg? One possibility is the endothelial swelling mechanism of Flores-Calle and associates (9), transferred to 1562 C. R. Morris, E. A. Alexander, F. J. Bruns, and N. G. Levinsky
9 the afferent arterioles. The arguments for and against the application of their hypothesis to our studies have been outlined already. Another possible cause of arteriolar constriction would be an effect of angiotensin, formed at the juxtaglomerular apparatus and acting locally. Although there has been controversy about the intrarenal formation of angiotensin II, some recent work (14) supports the possibility. Mannitol is known to decrease renin release from hypoperfused kidneys (15, 16). In the experiments of Fojas and Schmid (16) in dogs, mannitol reduced renin production by the kidney significantly at 50 mm Hg arterial pressure. If mannitol acts through alterations in the renin-angiotensin system in our experiments, its effect must be exerted at an extratubular locus, since it restarted GF in nephrons in which it had already stopped. Such an effect could be exerted at the juxtaglomerular apparatus or by some contraluminal effects on the macula densa cells of the distal tubule, which are thought to play a role in controlling renin secretion (17). Obviously, until there is direct evidence of increased afferent arteriolar resistance or for any of these mechanisms, the foregoing represents merely informed speculation. In conclusion, our data indicate that nephron GF can be maintained at about one-fourth of normal during hypoperfusion by mannitol or, in some cases, by hypertonic saline. However, the latter, even when it maintains GF, does not relieve anuria. Thus, we conclude that mannitol can maintain GF by an unknown action and can prevent total reabsorption of the filtrate, presumably by its intratubular action as an osmotic diuretic. Hypertonic saline shares the action on GF to some extent but lacks the osmotic diuretic effect; hence, filtrate is completely reabsorbed. The observations on maintenance of urine flow by mannitol during hypotension fit with those of Peters and Brunner (18) and the earlier work of Coelho and Bradley, using glucose or diodrast infusions (19). The latter authors assumed that GF continued even in hydropenic dogs when renal perfusion pressure was reduced below 60 mm Hg and that glucose and diodrast maintained urine flow solely by acting as osmotic diuretics to prevent complete reabsorption of filtrate. Peters and Brunner (18), on the other hand, suggested from indirect evidence that hypertonic mannitol reestablished GF which had stopped. Our data demonstrate conclusively that this suggestion is correct. A number of investigators have found that mannitol (20-23) and other hypertonic solutions (24) can increase renal blood flow and decrease renal vascular resistance in the normal dog kidney. It seems plausible that renal vascular resistance is increased in the hypoperfused rat kidney, perhaps at the afferent arterioles. Mannitol could decrease resistance to blood flow by a vascular effect, exerted either on swollen endothelial cells (9) or on smooth muscle. ACKNOWLEDGMENTS These studies were supported by National Institutes of Health Grant Nos. AM-11793, HE-07299, AM-14004, and AM REFERENCES 1. Alexander, E. A., and N. G. Levinsky Nephron function during hypotension: persistence of glomerular filtration in anuria. J. Clin. Invest. 46: 1032a. (Abstr.) 2. Baines, A. D., C. J. Baines, and C. de Rouffignac Functional heterogeneity of nephrons. I. Intraluminal flow velocities. Pfluegers Arch. Gesamte Physiol. Menschen Tiere. 308: de Rouffignac, C., S. Deiss, and J. P. Bonvalet Determination du taux individuel de filtration glomerulaire des nephrons accessibles et inaccessibles a la microponction. Pfluegers Arch. Gesamte Physiol. Menschen Tiere. 315: Hanssen, 0. E The relationship between glomerular filtration and length of the proximal convoluted tubule in mice. Acta. Pathol. Microbiol. Scand. 53: Koch, K. M., Th. Dume, H. H. Krause, and B. Ochwadt Intratubularer Druck, glomerularer Capillardruck und Glomerulumfiltrar wahrend Mannit-Diureses. Pfluegers Arch. Gesamte Physiol. Menschen Tiere. 295: Carriere, S., G. D. Thorburn, C. C. C. O'Morchoe, and A. C. Barger Intrarenal distribution of blood flow in dogs during hemorrhagic hypotension. Circ. Res. 19: Carriere, S., and B. Daigneault Effect of retransfusion after hemorrhagic hypotension on intrarenal distribution of blood flow in dogs. J. Clin. Invest. 49: McNay, J. L., and Y. Abe Pressure-dependent heterogeneity of renal cortical blood flow in dogs. Circ. Res. 27: Flores, J., D. R. DiBona, C. H. Beck, and A. Leaf The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J. Clin. Invest. 51: Ames, A., III, R. L. Wright, M. Kowada, J. M. Thurston, and G. Majno Cerebral ischemia. II. The no-reflow phenomenon. Amer. J. Pathol. 52: Thurau, K Renal hemodynamics. Amer. J. Med. 36: Bell, G., and A. M. Harper Effect of hemorrhage on blood flow through renal cortex of the dog. J. Appi. Physiol. 28: Brenner, B. M., J. L. Troy, and T. M. Daugharty The dynamics of glomerular ultrafiltration in the rat. J. Clin. Invest. 50: Bailie, M. D., F. C. Rector, Jr., and D. W. Seldin Angiotensin II in arterial and renal venous plasma and renal lymph in the dog. J. Clin. Invest. 50: Vander, A. J., and R. Miller Control of renin Mannitol and GFR in Renal Ischemia 1563
10 secretion in the anesthetized dog. Amer. J. Physiol. 207: Fojas, J. E., and H. E. Schmid Renin release, renal autoregulation, and sodium excretion in the dog. Amer. J. Physiol 219: Vander, A. J Control of renin release. Physiol. Rev. 47: Peters, G., and H. Brunner Mannitol diuresis in hemorrhagic hypotension. Amer. J. Physiol. 204: Coelho, J. B., and S. E. Bradley Function of the nephron population during hemorrhagic hypotension in the dog, with special reference to the effects of osmotic diuresis. J. Clin. Invest. 43: Berman, L. B., K. Onen, and G. Chisholm Unilateral renal hemodynamic changes with hypertonic mannitol. Proc. Soc. Exp. Biol. Med. 113: Braun, W. E., and L. S. Lilienfield Renal hemodynamic effects of hypertonic mannitol infusions. Proc. Soc. Exp. Biol. Med. 114: Goldberg, A. H., and L. S. Lilienfield Effects of hypertonic mannitol on renal vascular resistance. Proc. Soc. Exp. Biol. Med. 119: Wendling, M. G., J. W. Eckstein, and F. M. Abboud Effects of mannitol on the renal circulation. J. Lab. Clin. Med. 74: Gazitua, S., J. B. Scott, C. C. Chou, and F. J. Haddy Effect of osmolarity on canine renal vascular resistance. Amer. J. Physiol. 217: C. R. Morris, E. A. Alexander, F. J. Bruns, and N. G. Levinsky
Vittorio E. Andreucci,, Floyd C. Rector Jr., Donald W. Seldin
 Effective glomerular filtration pressure and single nephron filtration rate during hydropenia, elevated ureteral pressure, and acute volume expansion with isotonic saline Vittorio E. Andreucci,, Floyd
Effective glomerular filtration pressure and single nephron filtration rate during hydropenia, elevated ureteral pressure, and acute volume expansion with isotonic saline Vittorio E. Andreucci,, Floyd
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
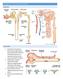
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Circulation Research. Brief Reviews. Pathogenesis of Oliguria in Acute Renal Failure. An Official Journal of the American Heart Association
 Circulation Research An Official Journal of the American Heart Association Brief Reviews JUNE 1975 VOL. 36 NO. 6 Pathogenesis of Oliguria in Acute Renal Failure By William F. Finn, William J. Arendshorst,
Circulation Research An Official Journal of the American Heart Association Brief Reviews JUNE 1975 VOL. 36 NO. 6 Pathogenesis of Oliguria in Acute Renal Failure By William F. Finn, William J. Arendshorst,
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Distal Nephron Segments on Renin Release
 The Effect of Altered Sodium Concentration in the Distal Nephron Segments on Renin Release C. ROBERT COOKE, ToRREY C. BROWN, BARRY J. ZAcHERLE, and W. GORDON WALKER From the Department of Medicine of The
The Effect of Altered Sodium Concentration in the Distal Nephron Segments on Renin Release C. ROBERT COOKE, ToRREY C. BROWN, BARRY J. ZAcHERLE, and W. GORDON WALKER From the Department of Medicine of The
during Saline Loading in the Dog
 Superficial and Juxtamedullary Nephron Function during Saline Loading in the Dog FRANK J. BRUNS, EDWARD A. ALEXANDER, ARHu NORMAN G. LEVINsY L. RILEY, and From the Departments of Medicine, Boston University
Superficial and Juxtamedullary Nephron Function during Saline Loading in the Dog FRANK J. BRUNS, EDWARD A. ALEXANDER, ARHu NORMAN G. LEVINsY L. RILEY, and From the Departments of Medicine, Boston University
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog
 Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog HENRY MANDIN, ARNOLD H ISRAELIT, FLOYD C REcroR, JR, and DONALD W SELDIN From the Department
Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog HENRY MANDIN, ARNOLD H ISRAELIT, FLOYD C REcroR, JR, and DONALD W SELDIN From the Department
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
5.Which part of the nephron removes water, ions and nutrients from the blood?
 Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
absolute rate of net transfer had occurred. These rate of sodium reabsorption by the proximal
 Journal of Clinical Investigation Vol. 44, No. 8, 1965 Micropuncture Study of the Effect of Acute Reductions in Glomerular Filtration Rate on Sodium and Water Reabsorption by the Proximal Tubules of the
Journal of Clinical Investigation Vol. 44, No. 8, 1965 Micropuncture Study of the Effect of Acute Reductions in Glomerular Filtration Rate on Sodium and Water Reabsorption by the Proximal Tubules of the
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER
 MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Control of Renin Secretion in the Dog
 of Renin Secretion in the Dog EFFECTS OF FUROSEMIDE ON THE VASCULAR AND MACULA DENSA RECEPTORS By William A. Corsini, Jerry B. Hook, and Michael D. Bailie ABSTRACT Experiments were undertaken to investigate
of Renin Secretion in the Dog EFFECTS OF FUROSEMIDE ON THE VASCULAR AND MACULA DENSA RECEPTORS By William A. Corsini, Jerry B. Hook, and Michael D. Bailie ABSTRACT Experiments were undertaken to investigate
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
1. Urinary System, General
 S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Effect of Variatidn in Dietary NaCl Intake
 Effect of Variatidn in ary NaCl Intake on the Intrarenal Distribution of Plasma Flow in the Rat ROLAND C. BLANTZ, JoH DONALD W. SELDIN D. WALLIN, FLOYD C. RECTOR, JR., and From the Department of Internal
Effect of Variatidn in ary NaCl Intake on the Intrarenal Distribution of Plasma Flow in the Rat ROLAND C. BLANTZ, JoH DONALD W. SELDIN D. WALLIN, FLOYD C. RECTOR, JR., and From the Department of Internal
Pressure Diuresis 9 Sample Student Essays
 Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Chapter 17: Urinary System
 Introduction Chapter 17: Urinary System Organs of the Urinary System REFERENCE FIGURE 17.1 2 kidneys filters the blood 2 ureters transport urine from the kidneys to the urinary bladder Urinary bladder
Introduction Chapter 17: Urinary System Organs of the Urinary System REFERENCE FIGURE 17.1 2 kidneys filters the blood 2 ureters transport urine from the kidneys to the urinary bladder Urinary bladder
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
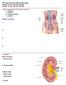 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
man of the effects of diabetes and of insulin on the maximum ability of the tubules to reabsorb glucose.
 EFFECT OF DIABETES AND INSULIN ON THE MAXIMUM CA- PACITY OF THE RENAL TUBULES TO REABSORB GLUCOSE t By SAUL J. FARBER, EUGENE Y. BERGER, AND DAVID P. EARLE (From the Department of Medicine, New York University
EFFECT OF DIABETES AND INSULIN ON THE MAXIMUM CA- PACITY OF THE RENAL TUBULES TO REABSORB GLUCOSE t By SAUL J. FARBER, EUGENE Y. BERGER, AND DAVID P. EARLE (From the Department of Medicine, New York University
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Basic Urinary Tract Anatomy and Histology
 Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
The Dynamics of Glomerular Ultrafiltration in the Rat
 The Dynamics of Glomerular Ultrafiltration in the Rat BARRY M. BRENNER, JULIA L. TROY, and TERRANCE M. DAUGHARTY From the Departments of Medicine, Veterans Administration Hospital, San Francisco, California
The Dynamics of Glomerular Ultrafiltration in the Rat BARRY M. BRENNER, JULIA L. TROY, and TERRANCE M. DAUGHARTY From the Departments of Medicine, Veterans Administration Hospital, San Francisco, California
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Preparation of Animals for Live Animal Imaging
 Preparation of Animals for Live Animal Imaging George A. Tanner, Ph.D. Department of Cellular and Integrative Physiology Indiana University School of Medicine Ideal Condition of Rats during Experiments:
Preparation of Animals for Live Animal Imaging George A. Tanner, Ph.D. Department of Cellular and Integrative Physiology Indiana University School of Medicine Ideal Condition of Rats during Experiments:
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Similarly, oliguria is commonly taken to reflect a uniform reduction of. oliguria. An analysis of such 'peaks' in clearance has been made in
 J. Physiol. (1966), 187, pp. 63-614 With 5 text-ftgures n C li 63 Printed in Great Britain CONCEALED GLO LA A TION BY E. G. BREWIN,* R. E NASHAT AND R. W.P From the Departments of Physiology and Nuclear
J. Physiol. (1966), 187, pp. 63-614 With 5 text-ftgures n C li 63 Printed in Great Britain CONCEALED GLO LA A TION BY E. G. BREWIN,* R. E NASHAT AND R. W.P From the Departments of Physiology and Nuclear
Micropuncture study of renal tubular reabsorption of calcium in normal rodentd
 Micropuncture study of renal tubular reabsorption of calcium in normal rodentd WILLIAM E. LASSITER,3 CARL W. GOTTSCHALK,4 AND MARGARET MYLLE Department of Medicine, University of North Carolina, School
Micropuncture study of renal tubular reabsorption of calcium in normal rodentd WILLIAM E. LASSITER,3 CARL W. GOTTSCHALK,4 AND MARGARET MYLLE Department of Medicine, University of North Carolina, School
The topic of normal vascular and glomerular anatomy is introduced
 Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa
 Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Lab 19 The Urinary System
 Lab 19 The Urinary System Laboratory Objectives Identify and describe the micro- and macroscopic anatomy of the kidney. Track the blood flow in and out of the kidney. Compare blood, glomerular filtrate,
Lab 19 The Urinary System Laboratory Objectives Identify and describe the micro- and macroscopic anatomy of the kidney. Track the blood flow in and out of the kidney. Compare blood, glomerular filtrate,
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
URINARY SYSTEM. Primary functions. Major organs & structures
 URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
reported. METHODS Renin was prepared from fresh rabbit kidneys by the alcohol method of Pickering (Pickering &
 106 J. Physiol. (I954) I 24, I 06-I I 2 AN ANGIOGRAPHIC STUDY OF THE EFFECT OF RENIN UPON THE RENAL CIRCULATION By P. M. DANIEL, MARJORIE M. L. PRICHARD AND J. N. WARD-McQUAID* From the NufIeld Department
106 J. Physiol. (I954) I 24, I 06-I I 2 AN ANGIOGRAPHIC STUDY OF THE EFFECT OF RENIN UPON THE RENAL CIRCULATION By P. M. DANIEL, MARJORIE M. L. PRICHARD AND J. N. WARD-McQUAID* From the NufIeld Department
