Proteins that bind A-type lamins: integrating isolated clues
|
|
|
- Peter Walters
- 5 years ago
- Views:
Transcription
1 Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine, 725 N. Wolfe Street, Baltimore, MD 21205, USA *Author for correspondence ( Journal of Cell Science 117, Published by The Company of Biologists 2004 doi: /jcs Summary What do such diverse molecules as DNA, actin, retinoblastoma protein and protein kinase Cα all have in common? They and additional partners bind A-type lamins, which form stable filaments in animal cell nuclei. Mutations in A-type lamins cause a bewildering range of tissue-specific diseases, termed laminopathies, including Emery-Dreifuss muscular dystrophy and the devastating Hutchinson-Gilford progeria syndrome, which mimics premature aging. Considered individually and collectively, partners for A-type lamins form four loose groups: architectural partners, chromatin partners, generegulatory partners and signaling partners. We describe 16 partners in detail, summarize their binding sites in A-type lamins, and sketch portraits of ternary complexes and functional pathways that might depend on lamins in vivo. On the basis of our limited current knowledge, we propose lamin-associated complexes with multiple components relevant to nuclear structure (e.g. emerin, nesprin 1α, actin) or signaling and gene regulation (e.g. LAP2α, retinoblastoma, E2F-DP heterodimers, genes) as food for thought. Testing these ideas will deepen our understanding of nuclear function and human disease. Key words: Laminopathy, Emerin, Emery-Dreifuss muscular dystrophy, Hutchinson-Gilford progeria syndrome, Nuclear envelope Introduction Lamins are intermediate filament proteins found only in the nuclei of multicellular eukaryotes. They form stable filaments at the nuclear inner membrane and stable structures in the nucleoplasm that might or might not be filamentous. Lamins are important for nuclear architecture; they provide mechanical strength, determine nuclear shape, and anchor and space the nuclear pore complexes. Perhaps more surprisingly, lamins are also essential for DNA replication and mrna transcription (Goldman et al., 2002). Mammalian cells encode both A-type and B-type lamins, which are highly related but can be distinguished biochemically and functionally. The most crucial distinction is that B-type lamins, encoded by two human genes (LMNB1 and LMNB2), are essential for viability of individual cells, whereas A-type lamins (encoded by LMNA) are dispensable. Loss of either lamin B1 or lamin B2 is lethal to dividing cells (Harborth et al., 2001), which is consistent with fundamental roles in replication and transcription. However, the basis for the dependence of metazoans on B-type lamins is still unknown. A-type lamins, although dispensable during development in mice, have key roles in differentiated tissues. Humans synthesize four A-type lamins (A, C, C2 and A 10 ), which are generated by alternative mrna splicing of the LMNA gene (Fisher et al., 1986; Furukawa et al., 1994; Machiels et al., 1996; McKeon et al., 1986). A-type lamins begin to be expressed in a tissue-specific manner during development, and cell-type-appropriate isoforms of A-type lamins continue to be expressed in most differentiated adult cells (Machiels et al., 1996; Rober et al., 1989). A-type lamins appeared late in metazoan evolution; they are not found in the nematode Caenorhabditis elegans, which has differentiated cells and tissues, but are found in more complicated eukaryotes, including Drosophila, birds and vertebrates (Cohen et al., 2001; Zimek et al., 2003). Despite their clearly different roles, the conserved similarities between A- and B- type lamins suggest similar mechanisms of function in the nucleus. Like other intermediate filament proteins, lamins consist of an N-terminal head domain, a central coiled-coil (rod) domain responsible for dimerization, and a large globular C-terminal tail. The tail includes an immunoglobulin (Ig)-fold domain, the backbone organization of which is unique to lamins, formed by residues (Dhe-Paganon et al., 2002; Krimm et al., 2002). All lamins except lamin C and lamin C2 also contain a CAAX (Cys-aliphatic-aliphatic-any residue) motif at the C- terminus, which is farnesylated on Cys in vivo prior to proteolytic removal of the AAX sequence. Prelamin A is then carboxymethylated and re-cleaved to remove the modified Cys plus 14 additional residues ( ), yielding mature lamin A (Fig. 1) (Moir et al., 1995). A site-specific protease named Zmpste24 is proposed to perform both cleavage events (Pendas et al., 2002). Lamin A is (so far) the only known substrate for Zmpste24 in mammals. Consistent with lamin A being a major substrate is the observation that loss of Zmpste24 causes muscular dystrophy and bone degeneration phenotypes in mice (Bergo et al., 2002; Pendas et al., 2002; see below) and mandibuloacral dysplasia in humans (Agarwal et al., 2003), disorders associated with compromised A-type lamin function. The assembly mechanisms and structures of lamin filaments are reviewed elsewhere (Goldman et al., 2002; Stuurman et al., 1998). However, several properties are noteworthy. First, the abundance and organization of lamin filaments vary between
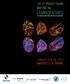
2 980 Journal of Cell Science 117 (7) Tail (lamin C) Fig. 1. Regions in lamins A and C to which partners bind. The structural domains (head, rod, tail) of prelamin A are shown. Exons 1-12 encoding residues 1- Head Rod domain Tail (mature lamin A) are numbered; the last 496 residue encoded by each exon Lamin C (except exon 11) is given above The Ig-fold domain, which includes exons 8 and 9, is shaded Lamin A gray. Residues of lamin A 12 and lamin C are identical.? Lamin B? Unique lamin C tail residues are produced by Actin Actin alternative mrna splicing; thus LAP2α the extreme C-terminal regions of lamins A and C may have?? nesprin Nesprin 1 α1α?? distinct binding properties. The? LAP1? zigzag represents the farnesyl moiety on prelamin A; the DNA farnesylated C-terminal peptide Hist is normally removed by? BAF? proteolytic cleavage after residue Emerin 646 (bold; dotted line) to generate mature lamin A. SREBP1 a/c Colored bars indicate the region(s) in lamins A and C required for each named partner to bind, as detailed in Table 1 (e.g. actin can bind two different MOK2 Rb E1B 19K regions in the tail). For partners with question marks, the binding region in lamin A/C is unmapped. Based on current 12(S)-lipoxygenase PKCα NARF incomplete knowledge, interactions were loosely color-coded (top to bottom) as blue (architectural), orange (chromatin), yellow (gene regulation), pink (signaling) and green (unknown). Some partners (e.g. BAF) will continue to defy categorization until more is known about their functions. species and cell types. In Xenopus oocyte nuclei, lamins form square networks of 10 nm filaments, all of which are tightly associated with the inner membrane (Stuurman et al., 1998). By contrast, in mammalian somatic nuclei, a significant number of lamins are found in the nuclear interior (Moir et al., 2000). The ultrastructure of interior lamins is unknown, except that they are unlikely to form 10 nm filaments. Second, although A-B heterodimers can form in vitro (Georgatos et al., 1988; Schirmer et al., 2001; Ye and Worman, 1995), there is growing evidence that A-type and B-type lamins form independent networks in vivo (Izumi et al., 2000; Moir et al., 2000; Haraguchi et al., 2001). Finally, and most interestingly from our current viewpoint, lamin polymers comprise a threedimensional network that displays at least a million copies of each binding site in the exposed rod and tail domains of each lamin, per nucleus. (HeLa cell nuclei contain ~10 6 copies each of lamins A, B1 and C) (L. Gerace, personal communication). Most biochemical studies have measured partner binding to lamin dimers, not polymers; thus the corresponding interaction in vivo could have increased avidity owing to the large number of available sites on lamin polymers. It is also possible that lamins form a variety of distinct oligomers or polymers that have distinct docking properties. The variety of human diseases linked to A-type lamins suggests that these sites, and the partners that bind to them, are important for nuclear function. Laminopathies At least seven diseases are caused by mutations in LMNA (Burke and Stewart, 2002; Östlund and Worman, 2003). Each disease affects specific combinations of tissues such as the heart, skeletal muscle and tendons (Emery-Dreifuss muscular dystrophy; EDMD), skeletal muscle and heart (limb-girdle muscular dystrophy 1B), the heart only (dilated cardiomyopathy type 1A), neurons and muscle (Charcot- Marie-Tooth disorder type 2B1), adipocytes (Dunnigan-type familial partial lipodystrophy), or adipocytes and bone (mandibuloacral dysplasia). The most devastating laminopathy is Hutchinson-Gilford progeria syndrome (HGPS), which affects multiple tissues and resembles premature aging (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003; Chen et al., 2003). Patients who have this disease usually die in their teens. Whereas most laminopathies are caused by missense mutations spread throughout the molecule, the most frequent known cause of HGPS is a point mutation that creates a new splice donor site, resulting in the deletion of 50 residues ( ), including the proteolytic cleavage site at residue 646.
3 Proteins that bind A-type lamins 981 *ND, not determined. Table 1. Current knowledge of binding regions in A-type lamins and each partner Binding region Partner in partner Binding region in lamin A/C Actin ND* Lamins A and C residues (Zastrow et al., unpublished) Lamin A residues (Sasseville and Langelier, 1998) BAF ND ND DNA Minor groove Lamins A and C residues (Stierle et al., 2003) E1B 19K Residues Lamins A and C residues (Rao et al., 1997) Emerin Residues Lamins A and C residues (Lee et al., 2001; Sakaki et al., 2001) Histones ND Lamin C residues (Taniura et al., 1995) Lamin B ND ND LAP1 ND ND LAP2α Residues Lamins A and C residues (Dechat et al., 2000) 12(S)-LOX ND Lamin A residues (Tang et al., 2000) MOK2 Residues Lamins A and C residues (Dreuillet et al., 2002) Narf ND Farnesylated prelamin A residues (Barton and Worman, 1999) Nesprin 1α Residues ND (Mislow et al., 2002a) PKCα Residues Lamin A residues (Martelli et al., 2002) Residues Rb Residues ND for lamin C (Markiewicz et al., 2002) Residues Lamins A and C residues (Ozaki et al., 1994) SREBP Residues Lamin A residues (Lloyd et al., 2002) Thus, HGPS patients are predicted to produce an internally deleted form of the farnesylated and carboxymethylated lamin A precursor. Zmpste24-null mice lack the processing protease and also have bone- and muscle-disorder phenotypes (Bergo et al., 2002). This suggests that severe laminopathy, if not progeria, might correlate with the presence of unprocessed lamin A precursors. Interestingly, a protein named nuclear prelamin A recognition factor (Narf), which is expressed at its highest levels in heart, skeletal muscle and brain, localizes to the nuclear envelope and intranuclear foci and binds specifically to farnesylated lamin A precursors (Barton and Worman, 1999). Thus, these forms of lamin A have the potential to saturate Narf, the function of which is unknown. When expressed exogenously, disease-associated lamins can disturb the organization of endogenous lamins (e.g. Raharjo et al., 2001; Capanni et al., 2003). In the few cases studied, laminopathy mutations can disrupt binding of lamins to specific partners (see below). The first genetic model for laminopathy was the LMNAknockout mouse; these mice appear normal at birth but develop severe muscular dystrophy and die within eight weeks (Sullivan et al., 1999). In the first reported human LMNA-null case, a baby possessing a homozygous nonsense mutation in LMNA, with no detectable A-type lamin proteins, died after premature birth, exhibiting severe joint contractures, muscular dystrophy, fibrosis and absence of muscle fibers in the diaphragm (Muchir et al., 2003). This premature birth and death cannot be attributed exclusively to loss of A-type lamins, because of parental consanguinity and the consequent potential for homozygous defects in other genes. Nonetheless, the resemblance to laminopathy suggests that A-type lamins, although not essential for cell viability, are essential for human life. Mice have been constructed to express only the L530Pmutated forms of lamins A and C, which causes EDMD in humans (Mounkes et al., 2003). A side-effect of the targeting strategy was that the knocked-in L530P allele also changed the mrna splicing pattern to yield two predicted protein products: mature A-type lamins that lack exon 9, and a C- terminally-truncated lamin A that has 19 extra (intronencoded) internal residues (see supplementary data in Mounkes et al., 2003). Neither mutation is expected to disrupt C-terminal processing of lamins, but this possibility has not been ruled out. These mice have progeria-like pathologies of bone and skin, and die prematurely (Mounkes et al., 2003). Loss of human Zmpste24 gene activity causes severe mandibuloacral dysplasia with progeroid appearance and general lipodystrophy (Agarwal et al., 2003), which is altogether less severe than HGPS. Thus, large deletions in the lamin A/C tail (i.e. loss of 50 residues in HGPS, or loss of exon 9 residues in L530P-knock-in mice) might correlate with progeria, and must certainly disrupt lamin organization and interactions. The effects of progeric mutations on the assembly and partner-binding activities of A-type lamins will be important to test. Many plausible disease mechanisms have been proposed. Some models emphasize possible disruption of the mechanical properties of lamin filaments, which control the shape, stiffness and structural integrity of nuclei. Other models view lamins as sites ( scaffolds ) for the assembly of other proteins that regulate transcription, cell fate or apoptosis (Burke and Stewart, 2002; Goldman et al., 2002). Both models are probably correct. We have taken a fresh look at the published binding partners for A-type lamins (Table 1, Fig. 1). Some bind A-type lamins in two-hybrid assays and in vitro, but their in vivo significance remains untested. In other cases, the interactions have also been studied in vivo. Many partners were familiar to us, but others were not. They form four loose groups: architectural partners, chromatin partners, generegulatory partners and signaling partners. Clearly, some partners fit multiple categories. What emerges, however, is that a rich variety of protein complexes and biochemical pathways could depend on lamins in vivo. Architectural partners We loosely defined architectural partners as those that (1) form
4 982 Journal of Cell Science 117 (7) stable complexes during interphase (on the basis of their resistance to chemical extraction or their immobility in FRAP experiments), (2) bind tightly to A-type lamins in vitro, or (3) have known structural roles in other contexts (e.g. actin). Such partners might connect lamins to the nuclear envelope, chromatin or other subnuclear structures (Burke and Stewart, 2002; Cohen et al., 2001). All integral membrane proteins that bind to lamins fit this definition, including lamina-associated polypeptide 1 (LAP1) and emerin (Foisner and Gerace, 1993; Östlund et al., 1999). LAP1 has an unusually large lumenal domain (Foisner and Gerace, 1993; Martin et al., 1995) and is expressed as multiple isoforms but has not been further characterized. More information is available for emerin, which is relatively immobile in the nuclear inner membrane (Östlund et al., 1999) and binds lamin A with an affinity of 40 nm in vitro (Holaska et al., 2003) (reviewed by Bengtsson and Wilson, 2004). Interestingly, emerin also binds directly to barrier-to-autointegration factor (BAF), a DNA-binding chromatin protein, and might thereby anchor lamins and chromatin to the nuclear envelope during interphase (Lee et al., 2001). Other nuclear membrane proteins (e.g. LAP2β) bind to B-type lamins, BAF, DNA and chromatin, extending this architectural theme (Cai et al., 2001; Foisner and Gerace, 1993; Furukawa, 1999; Shumaker et al., 2001). Emerin and LAP2β both belong to the LEM-domain family of nuclear proteins (Lin et al., 2000). The defining feature of LEM-domain proteins is their LEM domain: a folded motif of at least 40 residues that binds directly to BAF (Zheng et al., 2000; Cai et al., 2001). The LEM-domain family in vertebrates also includes at least six splice forms of LAP2 (Berger et al., 1996), MAN1 (which binds Smad transcription factors and regulates development) (Osada et al., 2003; Raju et al., 2003) and a smaller MAN1-related protein provisionally named Lem2 (Lee and Wilson, 2004), which is conserved in metazoans and essential for viability in C. elegans embryos (Lee et al., 2000; Liu et al., 2003). Lem2 (also known as NET-25) was identified in a proteomic study that also revealed more than 60 additional potential nuclear membrane proteins (Schirmer et al., 2003). We assume that many of these novel proteins bind A-type lamins. Further work will undoubtedly increase our appreciation of the complexity of the nuclear lamina network and possibly also extend its range of known functions. The best-studied architectural partner for A-type lamins in the nuclear interior is LAP2α, which has separate binding sites for BAF, A-type lamins and chromatin (Dechat et al., 1998; Dechat et al., 2000; Vlcek et al., 1999). LAP2α binds tightly to A-type lamins during interphase (Dechat et al., 2000), but has dynamic architectural roles during nuclear envelope assembly (Vlcek et al., 2002). It is proposed to tether A-type lamins to intranuclear sites and to cooperate with lamins in organizing chromatin (Foisner, 2003). LAP2α (and other LEM-domain proteins) also binds transcriptional regulators (see below), establishing a theme in which architectural partners for lamins also influence gene regulation, directly or indirectly. We do not yet know whether B-type lamins are direct architectural partners for A-type lamins in vivo. A- and B-type lamins have distinct assembly pathways in vivo (Moir et al., 2000), which could mean they form distinct networks. However, B-type lamins might set the stage for the assembly of A-type lamins. A-type lamins are known to be architectural partners for each other, since lamin C depends on lamin A for its localization and assembly in the nucleus (Vaughan et al., 2001). Actin, actin-related proteins and numerous actin-binding proteins (including a nuclear-specific isoform of myosin I) are present in the nucleus, where their functions are now slowly emerging (Pederson and Aebi, 2002). There is currently no evidence for long actin filaments (F-actin) in the nucleus. However, actin can also form a multitude of special dimers, short protofilaments and tubular, flat or branched oligomers that would suit the chromatin-dominated nuclear space (Pederson and Aebi, 2002). Interestingly, nuclear actin polymers adopt a unique conformation that is recognized by specific antibodies (Milankov and De Boni, 1993). Actin binds directly to two regions in the lamin A/C tail: residues (in the Ig-fold domain) (M.S.Z. and K.L.W., unpublished) and residues (Sasseville and Langelier, 1998). A-type lamin filaments might thus bind to actin polymers in the nucleus. The possibility that actin polymers are architectural partners for lamin filaments deserves serious attention. Nesprin 1α, a 131 kda nuclear membrane protein, binds directly to A-type lamins and emerin (Mislow et al., 2002a; Mislow et al., 2002b). All nesprin-family proteins (also known as Syne, Myne and NUANCE) have multiple spectrin repeat (SR) domains, and many also have an actin-binding domain (Zhang et al., 2002; Zhang et al., 2001; Starr and Han, 2003). Human nesprins are encoded by two genes, which yield multiple protein isoforms through alternative mrna splicing and use of internal transcription initiation sites. These isoforms range in mass from huge (~1 MDa for nesprin 1) to modest (e.g. 131 kda for nesprin 1α) (Mislow et al., 2002a; Zhang et al., 2002; Zhang et al., 2001). Nesprins are anchored to specific membranes, such as the Golgi complex or the outer or inner membrane of the nuclear envelope (Gough et al., 2003; Mislow et al., 2002b; Zhang et al., 2001). Nesprin 1α was proposed to anchor lamin A and emerin at the inner nuclear membrane. However, this model was overthrown by the finding that nesprin 1α and emerin both mislocalize to the endoplasmic reticulum (ER) in human fibroblasts that lack A-type lamins (Muchir et al., 2003). Transient expression of either lamin A or lamin C is sufficient to relocalize both membrane proteins to the nuclear envelope. These results suggest an architectural hierarchy based on the integrity of A-type lamins. Chromatin partners Lamins bind DNA directly but nonspecifically in vitro, by contacting the minor groove (Luderus et al., 1994). The DNAbinding region is identical in lamin A and lamin C, and includes both the Ig-fold domain and the nuclear localization signal in the tail (Stierle et al., 2003). The affinity of lamin A for DNA is 420 nm (Stierle et al., 2003). The R482W missense mutation, which causes lipodystrophy, reduces this affinity for DNA by seven-fold (to 2.95 µm), and also weakens binding of lamin A to the adipocyte differentiation factor sterol response element-binding protein (SREBP; see below). DNA is wound around core histone octamers to form nucleosomes, the fundamental unit of chromatin structure. The human lamin A/C tail binds to mixtures of core histones with an apparent affinity of 300 nm (Taniura et al., 1995), which is similar to its affinity for naked DNA. Since histones and DNA
5 Proteins that bind A-type lamins 983 are abundant in the nucleus, given such an affinity A-type lamins are probably always close to chromatin. This would allow lamins to serve as scaffolds for multiprotein complexes associated with chromatin. BAF is essential for cell viability in C. elegans (Zheng et al., 2000) and Drosophila (Furukawa et al., 2003). It binds to lamin A with 1 µm affinity in vitro (Holaska et al., 2003) and also binds to LEM-domain proteins (Furukawa, 1999; Lee et al., 2001; Shumaker et al., 2001; Liu et al., 2003). However, BAF also qualifies as a chromatin partner for lamins because it binds nonspecifically to double-stranded DNA (Zheng et al., 2000; Shumaker et al., 2001) and co-localizes with chromatin in certain cell types (e.g. Drosophila embryos) (Furukawa et al., 2003). Live-cell studies using GFP-fused BAF show direct binding to emerin at the nuclear envelope but also show that GFP-BAF is highly mobile during interphase (Shimi et al., 2004). In Drosophila embryos, loss of BAF causes chromatin and nuclear envelope defects (Furukawa et al., 2003). Additional mitotic defects seen in these embryos (e.g. lack of cyclin expression) might reflect a role for BAF in gene expression (see below). Nonetheless, excess BAF present during nuclear assembly can profoundly alter chromatin structure in Xenopus egg extracts (Segura-Totten et al., 2002), confirming its importance as an architectural partner but not revealing the mechanism. We can only speculate that BAF serves as a chromatin partner for lamin A by rapidly interlinking chromatin, LEM-domain proteins and lamins. Gene-regulatory partners Lamin A/C filaments are thought to provide scaffolds for protein complexes that regulate gene expression (Cohen et al., 2001). Hypophosphorylated retinoblastoma protein (Rb) binds specifically to A-type lamins in vitro (Mancini et al., 1994; Markiewicz et al., 2002; Ozaki et al., 1994). Rb is a transcriptional regulator that has central roles in cell-cycle control. Hypo-phosphorylated Rb binds to E2F-DP heterodimers and blocks E2F-dependent gene expression through a variety of mechanisms, including the recruitment of histone deacetylase complexes (HDACs) (Chau and Wang, 2003). Rb also regulates expression of apoptosis proteaseactivating factor 1 (Apaf1) and thus apoptosis, which is proposed to contribute to muscle loss in EDMD patients (Bonne et al., 2003). During G1 phase, hypophosphorylated (repressive) Rb is anchored to the nucleoskeleton by its pocket-c domain, which binds directly to A-type lamins and LAP2α (Mancini et al., 1994; Markiewicz et al., 2002). Overexpression of LAP2α interferes with cell-cycle progression and leads to apoptosis (Vlcek et al., 2002), which supports scaffolding models in which Rb activity depends on its attachment to lamins and LAP2α (Fig. 2). Such complexes might be weakened by disease-causing mutations, because Rb and LAP2α interact with the rod and rod/tail domains, respectively, of lamins A and C (Fig. 1). Biochemical studies using a different repressor of E2F activity, germ-cell-less (GCL), independently support scaffolding models, showing that GCL, emerin and lamin A form stable tertiary complexes in vitro (Holaska et al., 2003). Indeed, stable, lamin-based scaffolds might be useful to tether the enormous (1-2 MDa) chromatin-remodeling machines recruited by Rb and other regulators (Neely and Workman, 2002) (Fig. 2). SREBP1a and SREBP1c are additional lamin-binding proteins. Encoded by alternatively spliced mrnas, they are both basic helix-loop-helix leucine zipper transcription factors that, when activated, are released from the ER by proteolytic cleavage and move into the nucleus. Once in the nucleus, they activate genes required for cholesterol biosynthesis and lipogenesis (reviewed by Horton, 2002) and promote adipocyte differentiation (Kim and Spiegelman, 1996). SREBP proteins bind to the Ig-fold domain of the lamin A/C tail, as do many other partners (Fig. 1). Interestingly, lipodystrophy-causing mutations in A-type lamins reduce SREBP binding by 25-40% (Lloyd et al., 2002). It will be important to determine which other partners (in addition to SREBP and DNA) are affected by lipodystrophy-causing mutations. MOK2 is a DNA-binding transcriptional repressor that interacts with lamins A and C. It represses genes activated by cone-rod homeobox protein (Crx) transcription factors by competing with them for binding sites (Dreuillet et al., 2002). MOK2 also binds to RNA in vitro, and might thus influence RNA processing (Arranz et al., 1997). Like Rb, MOK2 binds to the coil 2 region of A-type lamins (Dreuillet et al., 2002) (Fig. 1). It therefore seems that A-type lamins have binding site(s) for transcriptional repressors not only in their tail domain, but also in the rod domain. Optimal access to rod domain sites might require a specific oligomeric (dimer, tetramer or octamer) or assembly (filamentous) state. Another gene-regulatory partner is BAF (see above). BAF binds directly to several different homeodomain transcription activators, including Crx, and represses Crx-dependent gene expression in retinal cells (Wang et al., 2002) (reviewed by Segura-Totten and Wilson, 2004). Thus, BAF and MOK2 both repress CRX-activated genes, but by different mechanisms (Fig. 2). It is curious that two out of four identified laminbinding repressors influence Crx-regulated genes, but this might simply reflect the low number of proteins studied so far. The BAF-binding partner emerin is proposed to be a fifth gene-regulatory partner for A-type lamins. Other LEM-domain proteins (e.g. LAP2β and MAN1) that bind lamin B inhibit gene expression in vivo (Liu et al., 2003; Nili et al., 2001; Osada et al., 2003). Emerin has not yet been tested for such activity in vivo. However, it does form a complex with lamin A and the transcriptional repressor GCL in vitro (Holaska et al., 2003). Emerin is very special among known binding partners for lamin A/C, because loss of emerin causes the same disease EDMD as many dominant mutations in A-type lamins (Bione et al., 1994). Perhaps in future other laminopathies will be analogously paired to loss of a specific lamin-binding protein(s). Meanwhile the wide expression of emerin in human tissues, and functional overlap between emerin and other LEM-domain proteins make it difficult to pinpoint which function(s) of emerin are critical to the EDMD disease mechanism (Bengtsson and Wilson, 2004). Further molecular and in vivo dissection of emerin functions is needed to clarify its role in lamin A/C complexes, which might combine roles in nuclear architecture (Fig. 2) and gene regulation. Various gene regulators thus bind A-type lamins and/or emerin, and this supports the idea that dysregulation of gene expression is an important contributing factor in laminopathies.
6 984 Journal of Cell Science 117 (7) Fig. 2. Speculative cartoon to integrate isolated clues, and stimulate thought. A few interactions described for A- type lamins in the text are illustrated here, speculatively, in the context of lamin filaments near the nuclear inner membrane (IM) or associated with chromatin. Integral membrane proteins nesprin 1α (Nesprin) and emerin (EM) are embedded at the IM. Many other laminbinding IM proteins are not depicted. NPC, nuclear pore complex. NS, nucleosome. OM, nuclear outer membrane. (Top left) Emerin, representing all LEM-domain proteins, binds BAF. BAF and MOK bind lamins in vitro and inhibit Crx-dependent gene activation in vivo, implicating both proteins in lamindependent gene-regulation events. Not depicted are potential gene-regulatory complexes involving nuclear membrane proteins LAP2β (Nili et al., 2001; Foisner, 2003) or lamin B receptor (Östlund and Worman, 2003). OM IM Nesprin CRX BAF EM MOK ns Lamins CRX-activated genes ns HDAC Cytoplasm Lamins 12(S)-LOX Emerin has many direct binding partners including nesprin-1α, lamins, actin (not depicted), BAF and several transcription regulators (not shown) (Bengtsson and Wilson, 2004). The number of distinct oligomeric complexes that include emerin is not known. (Top right) Laminbinding enzyme 12(S)-lipoxygenase [12(S)-LOX] converts arachidonic acid (AA) to 12(S)-hydroxyeicosatetraenoic acid [12(S)-HETE]. We speculate that this reaction occurs in a signaling complex near the inner membrane, where 12(S)-HETE then activates lamin-bound protein kinase Cα (PKCα), leading to phosphorylation of unknown target proteins. (Bottom center) Pairwise interactions, summarized in the text, suggest that one or multiple oligomeric gene-regulation complexes might be formed by LAP2α, A-type lamins, retinoblastoma (Rb), E2F/DP heterodimers (gene-specific activators), chromatin-silencing histone deacetylase complex (HDAC), BAF and additional unidentified chromatin partners for LAP2α (Vlcek et al., 2002).? ns AA Rb 12(S)-HETE? OH LAP2α PKCα BAF E2F DPP? Chromatin NPC Furthermore, studies with LEM-domain proteins (emerin, LAP2α and LAP2β) and transcriptional regulators (GCL, BAF and Rb) also provide the best current evidence that lamins scaffold multiprotein complexes (Holaska et al., 2003; Markiewicz et al., 2002; Nili et al., 2001). Interestingly, the gene regulators known to bind A-type lamins are all repressors. Gene silencing is certainly key to cell differentiation, but it is premature to assume that all gene regulators act repressively when complexed with A-type lamins. Signaling partners Signal transduction pathways regulate many activities in the nucleus. Three signaling proteins are known to bind A-type lamins in vivo; lamin filaments might therefore provide a structural basis for signaling. Protein kinase C α (PKCα) a serine-threonine kinase activated by many signals, including diacylglycerol (DAG) and 12(S)-hydroxyeicosatetraenoic acid [12(S)-HETE] binds to the C-terminal half of the lamin A/C tail (Martelli et al., 2002) (Fig. 1). The lamin-binding and substrate-binding regions of PKCα are distinct. In response to elevated DAG levels, PKCα translocates to the nucleus and interacts with A-type lamins, which suggests that signal transduction by PKCα might involve its attachment to a lamin scaffold (Martelli et al., 2000; Ron and Kazanietz, 1999) (Fig. 2). 12(S)-lipoxygenase [12(S)-LOX] also binds to the lamin A/C tail, perhaps overlapping the binding site for PKCα (Tang et al., 2000) (Fig. 1). Interestingly, 12(S)-LOX is part of a lipidsignaling pathway that converts arachidonic acid to 12(S)- HETE, which activates PKCα in prostate tumor cells (Liu et al., 1994). The 12(S)-LOX protein is detected in both the cytoplasm and nucleus in western blots of cell fractions (Tang et al., 2000). We speculate that PKCα might translocate to the nucleus and co-dock with 12(S)-LOX on lamin A/C filaments (Fig. 2). Alternatively, nuclear-localized PKCα might be activated in a 12(S)-LOX-dependent manner. The knowledge that PKCα and 12(S)-LOX are both direct partners for lamin A can be used to test the hypothesis that lamin scaffolds are important for PKCα-mediated signal transduction. The third signaling partner, E1B 19K, is an adenovirus early protein (Cuconati and White, 2002). E1B 19K binds directly to lamin A in two-hybrid assays, and cofractionates with
7 Proteins that bind A-type lamins 985 lamins in vivo. In adenovirus-infected cells, E1B 19K, which shares a low level of sequence similarity with Bcl-2, localizes to the ER and nuclear membranes, and blocks apoptosis in a lamin-dependent manner (Rao et al., 1997). E1B 19K also interacts with (and potentially inactivates) a death-promoting repressor named Btf (Kasof et al., 1999), which binds to emerin in vitro (Haraguchi et al., 2004). Does E1B 19K bind lamin A in order to co-assemble with Btf on emerin-lamin complexes, or might it displace Btf? By considering each strand in this web of interactions, we may be able to understand how each partner uses lamin A. Perspectives There are at least a million copies of each binding site on A- type lamins per nucleus. This repetition should ensure that all binding partners have access to lamin structures. To understand the functions of A-type lamins, we must now search for new binding partners, particularly those expressed in human tissues affected by laminopathy. However, it would be a mistake to neglect known partners, which provide tools to take this field to the next level. Old-fashioned (but still powerful) biochemical analysis, specific functional assays both in vitro and in vivo, and real-time analysis in living cells will all be needed if we are to understand how and why these complexes interact with lamins and what goes wrong in disease. Known partners for A-type lamins can also be mined for structural information to facilitate discovery of new partners. For example, partners whose binding sites on A-type lamins overlap (e.g. MOK2, Rb and E1B 19K in Fig. 1) might share a lamin-binding motif. Such motifs might allow investigators to identify potentially disease-relevant lamin-binding proteins rapidly in databases. We are hopeful that further work on lamin A/C partners, both known and novel, will lead to a better understanding of nuclear lamins and clinical treatments for diseases in which they are implicated. References Agarwal, A. K., Fryns, J.-P., Auchus, R. J. and Garg, A. (2003). Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12, Arranz, V., Harper, F., Florentin, Y., Puvion, E., Kress, M. and Ernoult- Lange, M. (1997). Human and mouse MOK2 proteins are associated with nuclear ribonucleoprotein components and bind specifically to RNA and DNA through their zinc finger domains. Mol. Cell. Biol. 17, Barton, R. M. and Worman, H. J. (1999). Prenylated prelamin A interacts with Narf, a novel nuclear protein. J. Biol. Chem. 274, Bengtsson, L. and Wilson, K. L. (2004). Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr. Opin. Cell Biol. 16, Berger, R., Theodor, L., Shoham, J., Gokkel, E., Brok-Simoni, F., Avraham, K. B., Copeland, N. G., Jenkins, N. A., Rechavi, G. and Simon, A. J. (1996). The characterization and localization of the mouse thymopoietin/lamina-associated polypeptide 2 gene and its alternatively spliced products. Genome Res. 6, Bergo, M. O., Gavino, B., Ross, J., Schmidt, W. K., Hong, C., Kendall, L. V., Mohr, A., Meta, M., Genant, H., Jiang, Y. et al. (2002). Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. USA 99, Bione, S., Maestrini, E., Rivella, S., Mancini, M., Regis, S., Romeo, G. and Toniolo, D. (1994). Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 8, Bonne, G., Yaou, R. B., Beroud, C., Boriani, G., Brown, S., de Visser, M., Duboc, D., Ellis, J., Hausmanowa-Petrusewicz, I., Lattanzi, G. et al. (2003). 108th ENMC International Workshop, 3rd Workshop of the MYO- CLUSTER project: EUROMEN, 7th International Emery-Dreifuss Muscular Dystrophy (EDMD) Workshop, September 2002, Naarden, The Netherlands. Neuromuscul. Disord. 13, Burke, B. and Stewart, C. (2002). Life at the edge: the nuclear envelope and human disease. Nat. Rev. Mol. Cell. Biol. 3, Cai, M., Huang, Y., Ghirlando, R., Wilson, K. L., Craigie, R. and Clore, G. M. (2001). Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. EMBO J. 20, Capanni, C., Cenni, V., Mattioli, E., Sabatelli, P., Ognibene, A., Columbaro, M., Parnaik, V. K., Wehnert, M., Maraldi, N. M., Squarzoni, S. and Lattanzi, G. (2003). Failure of lamin A/C to functionally assemble in R482L mutated partial lipodystrophy fibroblasts: altered intermolecular interaction with emerin and implications for gene transcription. Exp. Cell Res. 291, Chau, B. N. and Wang, J. Y. J. (2003). Coordinated regulation of life and death by RB. Nat. Rev. Cancer 3, Chen, L., Lee, L., Kudlow, B. A., Dos Santos, H. G., Sletvold, O., Shafeghati, Y., Botha, E. G., Garg, A, Hanson, N. B., Martin, G. M. et al. (2003). LMNA mutations in atypical Werner s syndrome. Lancet 362, Cohen, M., Lee, K. K., Wilson, K. L. and Gruenbaum, Y. (2001). Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem. Sci. 26, Cuconati, A. and White, E. (2002). Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 16, De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C. L., Munnich, A., Le Merrer, M. and Levy, N. (2003). Lamin A truncation in Hutchinson-Gilford progeria. Science 300, Dechat, T., Gotzmann, J., Stockinger, A., Harris, C. A., Talle, M. A., Siekierka, J. J. and Foisner, R. (1998). Detergent-salt resistance of LAP2α in interphase nuclei and phosphorylation-dependent association with chromosomes early in nuclear assembly implies functions in nuclear structure dynamics. EMBO J. 17, Dechat, T., Korbei, B., Vaughan, O. A., Vlcek, S., Hutchison, C. J. and Foisner, R. (2000). Lamina-associated polypeptide 2α binds intranuclear A- type lamins. J. Cell Sci. 113, Dhe-Paganon, S., Werner, E. D., Chi, Y. I. and Shoelson, S. E. (2002). Structure of the globular tail of nuclear lamin. J. Biol. Chem. 277, Dreuillet, C., Tillit, J., Kress, M. and Ernoult-Lange, M. (2002). In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res. 30, Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P. et al. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, Fisher, D. Z., Chaudhary, N. and Blobel, G. (1986). cdna sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. USA 83, Foisner, R. and Gerace, L. (1993). Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73, Foisner, R. (2003). Cell cycle dynamics of the nuclear envelope. Scientific World Journal 3, Furukawa, K., Inagaki, H. and Hotta, Y. (1994). Identification and cloning of an mrna coding for a germ cell-specific A-type lamin in mice. Exp. Cell Res. 212, Furukawa, K. (1999). LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2-chromatin interaction. J. Cell Sci. 112, Furukawa, K., Sugiyama, S., Osouda, S., Goto, H., Inagaki, M., Horigome, T., Omata, S., McConnell, M., Fisher, P. A. and Nishida, Y. (2003). Barrier-to-autointegration factor plays crucial roles in cell cycle progression and nuclear organization in Drosophila. J. Cell Sci. 116, Georgatos, S. D., Stournaras, C. and Blobel, G. (1988). Heterotypic and homotypic associations between the nuclear lamins: site-specificity and control by phosphorylation. Proc. Natl. Acad. Sci. USA 85, Goldman, R. D., Gruenbaum, Y., Moir, R. D., Shumaker, D. K. and Spann, T. P. (2002). Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16, Gough, L. L., Fan, J., Chu, S., Winnick, S. and Beck, K. A. (2003). Golgi localization of Syne-1. Mol. Biol. Cell 14, Haraguchi, T., Koujin, T., Segura-Totten, M., Lee, K. K., Matsuoka, Y., Yoneda, Y., Wilson, K. L. and Hiraoka, Y. (2001). BAF is required for
8 986 Journal of Cell Science 117 (7) emerin assembly into the reforming nuclear envelope. J. Cell Sci. 114, Haraguchi, T., Holaska, J. M., Yamane, M., Koujin, T., Hashiguchi, N., Mori, C., Wilson, K. L. and Hiraoka, Y. (2004). Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur. J. Biochem. (in press). Harborth, J., Elbashir, S. M., Bechert, K., Tuschl, T. and Weber, K. (2001). Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 114, Holaska, J. M., Lee, K. K., Kowalski, A. K. and Wilson, K. L. (2003). Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J. Biol. Chem. 278, Horton, J. D. (2002). Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem. Soc. Trans. 30, Izumi, M., Vaughan, O. A., Hutchison, C. J. and Gilbert, D. M. (2000). Head and/or CaaX domain deletions of lamin proteins disrupt preformed lamin A and C but not lamin B structure in mammalian cells. Mol. Biol. Cell 11, Kasof, G. M., Goyal, L. and White, E. (1999). Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol. Cell. Biol. 19, Kim, J. B. and Spiegelman, B. M. (1996). ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes. Dev. 10, Krimm, I., Ostlund, C., Gilquin, B., Couprie, J., Hossenlopp, P., Mornon, J. P., Bonne, G., Courvalin, J. C., Worman, H. J. and Zinn-Justin, S. (2002). The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure (Camb.) 10, Lee, K. K., Gruenbaum, Y., Spann, P., Liu, J. and Wilson, K. L. (2000). C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol. Biol. Cell 11, Lee, K. K., Haraguchi, T., Lee, R. S., Koujin, T., Hiraoka, Y. and Wilson, K. L. (2001). Distinct functional domains in emerin bind lamin A and DNAbridging protein BAF. J. Cell Sci. 114, Lee, K. K. and Wilson, K. L. (2004). All in the family: evidence for three new LEM-domain proteins Lem3, Lem2 (NET-25) and Lem1 in the human genome. In Communication and Gene Regulation at the Nuclear Envelope (ed. D. Evans, J. Bryant and C. J. Hutchison). Oxford: BIOS Scientific Publishers (in press). Lin, F., Blake, D. L., Callebaut, I., Skerjanc, I. S., Holmer, L., McBurney, M. W., Paulin-Levasseur, M. and Worman, H. J. (2000). MAN1, an inner nuclear membrane protein that shares the LEM domain with laminaassociated polypeptide 2 and emerin. J. Biol. Chem. 275, Liu, B., Maher, R. J., Hannun, Y. A., Porter, A. T. and Honn, K. V. (1994). 12(S)-HETE enhancement of prostate tumor cell invasion: selective role of PKC alpha. J. Natl. Cancer Inst. 86, Liu, J., Lee, K. K., Segura-Totten, M., Neufeld, E., Wilson, K. L. and Gruenbaum, Y. (2003). MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 100, Lloyd, D. J., Trembath, R. C. and Shackleton, S. (2002). A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum. Mol. Genet. 11, Luderus, M. E., den Blaauwen, J. L., de Smit, O. J., Compton, D. A. and van Driel, R. (1994). Binding of matrix attachment regions to lamin polymers involves single-stranded regions and the minor groove. Mol. Cell. Biol. 14, Machiels, B. M., Zorenc, A. H., Endert, J. M., Kuijpers, H. J., van Eys, G. J., Ramaekers, F. C. and Broers, J. L. (1996). An alternative splicing product of the lamin A/C gene lacks exon 10. J. Biol. Chem. 271, Mancini, M. A., Shan, B., Nickerson, J. A., Penman, S. and Lee, W. H. (1994). The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc. Natl. Acad. Sci. USA 91, Markiewicz, E., Dechat, T., Foisner, R., Quinlan, R. A. and Hutchison, C. J. (2002). Lamin A/C binding protein LAP2α is required for nuclear anchorage of retinoblastoma protein. Mol. Biol. Cell 13, Martelli, A. M., Tabellini, G., Bortul, R., Manzoli, L., Bareggi, R., Baldini, G., Grill, V., Zweyer, M., Narducci, P. and Cocco, L. (2000). Enhanced nuclear diacylglycerol kinase activity in response to a mitogenic stimulation of quiescent Swiss 3T3 cells with insulin-like growth factor I. Cancer Res. 60, Martelli, A. M., Bortul, R., Tabellini, G., Faenza, I., Cappellini, A., Bareggi, R., Manzoli, L. and Cocco, L. (2002). Molecular characterization of protein kinase C-alpha binding to lamin A. J. Cell Biochem. 86, Martin, L., Crimaudo, C. and Gerace, L. (1995). cdna cloning and characterization of lamina-associated polypeptide 1C (LAP1C), an integral protein of the inner nuclear membrane. J. Biol. Chem. 270, McKeon, F. D., Kirschner, M. W. and Caput, D. (1986). Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319, Milankov, K. and De Boni, U. (1993). Cytochemical localization of actin and myosin aggregates in interphase nuclei in situ. Exp. Cell Res. 209, Mislow, J. M. K., Holaska, J. M., Kim, M. S., Lee, K. K., Segura-Totten, M., Wilson, K. L. and McNally, E. M. (2002a). Nesprin-1α self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 525, Mislow, J. M. K., Kim, M. S., Davis, D. B. and McNally, E. M. (2002b). Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C. J. Cell Sci. 115, Moir, R. D., Spann, T. P. and Goldman, R. D. (1995). The dynamic properties and possible functions of nuclear lamins. Int. Rev. Cytol. 162B, Moir, R. D., Yoon, M., Khuon, S. and Goldman, R. D. (2000). Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151, Mounkes, L. C., Kozlov, S., Hernandez, L., Sullivan, T. and Stewart, C. L. (2003). A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423, Muchir, A., van Engelen, B. G., Lammens, M., Mislow, J. M., McNally, E., Schwartz, K. and Bonne, G. (2003). Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp. Cell Res. 291, Neely, K. E. and Workman, J. L. (2002). Histone acetylation and chromatin remodeling: which comes first? Mol. Genet. Metab. 76, 1-5. Nili, E., Cojocaru, G. S., Kalma, Y., Ginsberg, D., Copeland, N. G., Gilbert, D. J., Jenkins, N. A., Berger, R., Shaklai, S., Amariglio, N. et al. (2001). Nuclear membrane protein LAP2β mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less). J. Cell Sci. 114, Osada, S., Ohmori, S. Y. and Taira, M. (2003). XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development 130, Östlund, C., Ellenberg, J., Hallberg, E., Lippincott-Schwartz, J. and Worman, H. J. (1999). Intracellular trafficking of emerin, the Emery- Dreifuss muscular dystrophy protein. J. Cell Sci. 112, Östlund, C. and Worman, H. J. (2003). Nuclear envelope proteins and neuromuscular diseases. Muscle Nerve 27, Ozaki, T., Saijo, M., Murakami, K., Enomoto, H., Taya, Y. and Sakiyama, S. (1994). Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene 9, Pederson, T. and Aebi, U. (2002). Actin in the nucleus: what form and what for? J. Struct. Biol. 140, 3-9. Pendas, A. M., Zhou, Z., Cadinanos, J., Freije, J. M., Wang, J., Hultenby, K., Astudillo, A., Wernerson, A., Rodriguez, F., Tryggvason, K. and Lopez-Otin, C. (2002). Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31, Raharjo, W. H., Enarson, P., Sullivan, T., Stewart, C. L. and Burke, B. (2001). Nuclear envelope defects associated with LMNA mutations causing dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci. 114, Raju, G. P., Dimova, N., Klein, P. S. and Huang, H. C. (2003). SANE, a novel LEM domain protein, regulates bone morphogenetic protein signaling through interaction with Smad1. J. Biol. Chem. 278, Rao, L., Modha, D. and White, E. (1997). The E1B 19K protein associates with lamins in vivo and its proper localization is required for inhibition of apoptosis. Oncogene 15, Rober, R. A., Weber, K. and Osborn, M. (1989). Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development 105,
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans
 MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans Jun Liu*, Kenneth K. Lee, Miriam Segura-Totten, Ester Neufeld, Katherine L.
MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans Jun Liu*, Kenneth K. Lee, Miriam Segura-Totten, Ester Neufeld, Katherine L.
A new model for nuclear lamina organization
 Nuclear Envelope Diseases and Chromatin Organization 1339 A new model for nuclear lamina organization Martin W. Goldberg* 1, Jindriska Fiserova*, Irm Huttenlauch and Reimer Stick *School of Biological
Nuclear Envelope Diseases and Chromatin Organization 1339 A new model for nuclear lamina organization Martin W. Goldberg* 1, Jindriska Fiserova*, Irm Huttenlauch and Reimer Stick *School of Biological
Lamin A and Lamin C are Differentially Dysfunctional in Autosomal Dominant Emery-Dreifuss Muscular Dystrophy
 Bayerische Julius-Maximilians- Universität Würzburg Fakultät für Biologie Lamin A and Lamin C are Differentially Dysfunctional in Autosomal Dominant Emery-Dreifuss Muscular Dystrophy Dissertation zur Erlangung
Bayerische Julius-Maximilians- Universität Würzburg Fakultät für Biologie Lamin A and Lamin C are Differentially Dysfunctional in Autosomal Dominant Emery-Dreifuss Muscular Dystrophy Dissertation zur Erlangung
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1
 Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria syndrome
 JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans
 Research Article 923 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans Yosef Gruenbaum 1, *, Kenneth K. Lee 2, *, Jun Liu 3, Merav Cohen 1 and Katherine
Research Article 923 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans Yosef Gruenbaum 1, *, Kenneth K. Lee 2, *, Jun Liu 3, Merav Cohen 1 and Katherine
The Nuclear Envelope as a Signaling Node in Development and Disease
 The Nuclear Envelope as a Signaling Node in Development and Disease William T. Dauer 1,2, * and Howard J. Worman 3,4, * 1 Department of Neurology 2 Department of Cell and Developmental Biology University
The Nuclear Envelope as a Signaling Node in Development and Disease William T. Dauer 1,2, * and Howard J. Worman 3,4, * 1 Department of Neurology 2 Department of Cell and Developmental Biology University
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy
 The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
Inauguraldissertation
 CHARACTERISATION OF THE MOLECULAR LINKS BETWEEN THE NUCLEAR PORE COMPLEX AND THE NUCLEAR LAMINS AND RECONSTITUTION OF THE XENOPUS OOCYTE LAMIN ASSEMBLY IN VITRO Inauguraldissertation zur Erlangung der
CHARACTERISATION OF THE MOLECULAR LINKS BETWEEN THE NUCLEAR PORE COMPLEX AND THE NUCLEAR LAMINS AND RECONSTITUTION OF THE XENOPUS OOCYTE LAMIN ASSEMBLY IN VITRO Inauguraldissertation zur Erlangung der
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization
 Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins
 Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Alpha thalassemia mental retardation X-linked. Acquired alpha-thalassemia myelodysplastic syndrome
 Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
BIO 5099: Molecular Biology for Computer Scientists (et al)
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
Available online at
 Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Genetics and Genomics in Medicine Chapter 6 Questions
 Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis
 APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis Dr. Ramesh Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008
APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis Dr. Ramesh Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
General Biology 1004 Chapter 11 Lecture Handout, Summer 2005 Dr. Frisby
 Slide 1 CHAPTER 11 Gene Regulation PowerPoint Lecture Slides for Essential Biology, Second Edition & Essential Biology with Physiology Presentation prepared by Chris C. Romero Neil Campbell, Jane Reece,
Slide 1 CHAPTER 11 Gene Regulation PowerPoint Lecture Slides for Essential Biology, Second Edition & Essential Biology with Physiology Presentation prepared by Chris C. Romero Neil Campbell, Jane Reece,
Chapter 11 How Genes Are Controlled
 Chapter 11 How Genes Are Controlled PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Mary
Chapter 11 How Genes Are Controlled PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture by Mary
Mitosis and the Cell Cycle
 Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
Nuclear Lamins in Cell Regulation and Disease
 Nuclear Lamins in Cell Regulation and Disease T. SHIMI, V. BUTIN-ISRAELI, S.A. ADAM, AND R.D. GOLDMAN Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago,
Nuclear Lamins in Cell Regulation and Disease T. SHIMI, V. BUTIN-ISRAELI, S.A. ADAM, AND R.D. GOLDMAN Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago,
Chapter 12. living /non-living? growth repair renew. Reproduction. Reproduction. living /non-living. fertilized egg (zygote) next chapter
 Chapter 12 How cells divide Reproduction living /non-living? growth repair renew based on cell division first mitosis - distributes identical sets of chromosomes cell cycle (life) Cell Division in Bacteria
Chapter 12 How cells divide Reproduction living /non-living? growth repair renew based on cell division first mitosis - distributes identical sets of chromosomes cell cycle (life) Cell Division in Bacteria
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being A Eukaryote. Eukaryotic Cells. Basic eukaryotes have:
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 15: Being a Eukaryote: From DNA to Protein, A Tour of the Eukaryotic Cell. Christiaan van Woudenberg Being A Eukaryote Basic eukaryotes
Renata Schipp Medical Biology Department
 Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells
 1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
Using a proteomic approach to identify proteasome interacting proteins in mammalian
 Supplementary Discussion Using a proteomic approach to identify proteasome interacting proteins in mammalian cells, we describe in the present study a novel chaperone complex that plays a key role in the
Supplementary Discussion Using a proteomic approach to identify proteasome interacting proteins in mammalian cells, we describe in the present study a novel chaperone complex that plays a key role in the
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Getting from there to here Going from egg to baby. the original
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Getting from there to here Going from egg to baby. the original
Guilt by Association. Gavin S. Wilkie and Eric C. Schirmer. Review THE NUCLEAR ENVELOPE PROTEOME AND DISEASE*
 Review Guilt by Association THE NUCLEAR ENVELOPE PROTEOME AND DISEASE* Gavin S. Wilkie and Eric C. Schirmer The discovery that many inherited diseases are linked to interacting nuclear envelope proteins
Review Guilt by Association THE NUCLEAR ENVELOPE PROTEOME AND DISEASE* Gavin S. Wilkie and Eric C. Schirmer The discovery that many inherited diseases are linked to interacting nuclear envelope proteins
Molecular Cell Biology 5068 In Class Exam 1 October 3, 2013
 Molecular Cell Biology 5068 In Class Exam 1 October 3, 2013 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Molecular Cell Biology 5068 In Class Exam 1 October 3, 2013 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
The effects of dilated cardiomyopathy and atrial fibrillation lamin A/C mutations on phosphorylated kinase C alpha cellular distribution and activity.
 The effects of dilated cardiomyopathy and atrial fibrillation lamin A/C mutations on phosphorylated kinase C alpha cellular distribution and activity. Musfira Mohamed-Uvaize, B.Sc. (Hons) Thesis submitted
The effects of dilated cardiomyopathy and atrial fibrillation lamin A/C mutations on phosphorylated kinase C alpha cellular distribution and activity. Musfira Mohamed-Uvaize, B.Sc. (Hons) Thesis submitted
Eukaryotic Gene Regulation
 Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Regulators of Cell Cycle Progression
 Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
Nuclear envelope defects in muscular dystrophy
 Biochimica et Biophysica Acta 1772 (2007) 118 127 www.elsevier.com/locate/bbadis Review Nuclear envelope defects in muscular dystrophy Kyle J. Roux, Brian Burke Department of Anatomy and Cell Biology,
Biochimica et Biophysica Acta 1772 (2007) 118 127 www.elsevier.com/locate/bbadis Review Nuclear envelope defects in muscular dystrophy Kyle J. Roux, Brian Burke Department of Anatomy and Cell Biology,
THURSDAY, JANUARY
 THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
Importance of molecular cell biology investigations in human medicine in the story of the Hutchinson-Gilford progeria syndrome
 Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Laminopathies and the long strange trip from basic cell biology to therapy
 Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
9/3/2009 DNA i DNA n euk euk yotes Organizatio Organ izatio n of o f gen ge e n tic Locati t on: In n ucleu e s material mater in e ial
 DNA in eukaryotes Organization of genetic material in eukaryotes Location: In nucleus In mitochondria DNA in eukaryotes Nuclear DNA: Long, linear molecules; Chromatin chromosomes; 10% of DNA in genes,
DNA in eukaryotes Organization of genetic material in eukaryotes Location: In nucleus In mitochondria DNA in eukaryotes Nuclear DNA: Long, linear molecules; Chromatin chromosomes; 10% of DNA in genes,
Complexity DNA. Genome RNA. Transcriptome. Protein. Proteome. Metabolites. Metabolome
 DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
Clinical Cell Biology Organelles in Health and Disease
 Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Chapter 12 The Cell Cycle: Cell Growth, Cell Division
 Chapter 12 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a period at the end of a sentence And now look at you How did you get from there to
Chapter 12 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a period at the end of a sentence And now look at you How did you get from there to
Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo
 Research Article 239 Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo Michael S. Zastrow 1, *, Denise B. Flaherty 2, Guy M. Benian 2 and Katherine L. Wilson 1, 1 Department of Cell
Research Article 239 Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo Michael S. Zastrow 1, *, Denise B. Flaherty 2, Guy M. Benian 2 and Katherine L. Wilson 1, 1 Department of Cell
Durham E-Theses. The Application of a Statistical Model Investigating Reactive Oxygen Species in Premature Ageing Syndromes MUTER, JOANNE,RUTH
 Durham E-Theses The Application of a Statistical Model Investigating Reactive Oxygen Species in Premature Ageing Syndromes MUTER, JOANNE,RUTH How to cite: MUTER, JOANNE,RUTH (2011) The Application of a
Durham E-Theses The Application of a Statistical Model Investigating Reactive Oxygen Species in Premature Ageing Syndromes MUTER, JOANNE,RUTH How to cite: MUTER, JOANNE,RUTH (2011) The Application of a
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Name: Multiple choice questions. Pick the BEST answer (2 pts ea)
 Exam 1 202 Oct. 5, 1999 Multiple choice questions. Pick the BEST answer (2 pts ea) 1. The lipids of a red blood cell membrane are all a. phospholipids b. amphipathic c. glycolipids d. unsaturated 2. The
Exam 1 202 Oct. 5, 1999 Multiple choice questions. Pick the BEST answer (2 pts ea) 1. The lipids of a red blood cell membrane are all a. phospholipids b. amphipathic c. glycolipids d. unsaturated 2. The
Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells
 JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
When Lamins Go Bad: Nuclear Structure and Disease
 Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Polyomaviridae. Spring
 Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome
 Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome Brian C. Capell*, Michael R. Erdos*, James P. Madigan, James J. Fiordalisi, Renee
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome Brian C. Capell*, Michael R. Erdos*, James P. Madigan, James J. Fiordalisi, Renee
Proteins. Length of protein varies from thousands of amino acids to only a few insulin only 51 amino acids
 Proteins Protein carbon, hydrogen, oxygen, nitrogen and often sulphur Length of protein varies from thousands of amino acids to only a few insulin only 51 amino acids During protein synthesis, amino acids
Proteins Protein carbon, hydrogen, oxygen, nitrogen and often sulphur Length of protein varies from thousands of amino acids to only a few insulin only 51 amino acids During protein synthesis, amino acids
Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle. 9.1 Multiple-Choice Questions
 Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle 9.1 Multiple-Choice Questions 1) Starting with a fertilized egg (zygote), a series of five cell divisions would produce an early embryo with how
Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle 9.1 Multiple-Choice Questions 1) Starting with a fertilized egg (zygote), a series of five cell divisions would produce an early embryo with how
The LMNA gene encodes the A-type lamins, including lamin A
 A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
H utchinson-gilford progeria syndrome (HGPS; MIM
 609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
Laminopathies: A consequence of mechanical stress or gene expression disturbances?
 BIOLOGY AND HEALTH Journal website: www.biologyandhealth.com Narrative review Laminopathies: A consequence of mechanical stress or gene expression disturbances? Alicia Jonker Alicia Jonker I6109457 Maastricht
BIOLOGY AND HEALTH Journal website: www.biologyandhealth.com Narrative review Laminopathies: A consequence of mechanical stress or gene expression disturbances? Alicia Jonker Alicia Jonker I6109457 Maastricht
Overview of virus life cycle
 Overview of virus life cycle cell recognition and internalization release from cells progeny virus assembly membrane breaching nucleus capsid disassembly and genome release replication and translation
Overview of virus life cycle cell recognition and internalization release from cells progeny virus assembly membrane breaching nucleus capsid disassembly and genome release replication and translation
BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney
 BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney Page 2: Immune Mechanisms & Molecular Biology of Host Defence (Prof Campbell) Page 45: Infection and Implications for Cell
BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney Page 2: Immune Mechanisms & Molecular Biology of Host Defence (Prof Campbell) Page 45: Infection and Implications for Cell
How Cells Divide. Chapter 10
 How Cells Divide Chapter 10 Bacterial Cell Division Bacteria divide by binary fission. -the single, circular bacterial chromosome is replicated -replication begins at the origin of replication and proceeds
How Cells Divide Chapter 10 Bacterial Cell Division Bacteria divide by binary fission. -the single, circular bacterial chromosome is replicated -replication begins at the origin of replication and proceeds
L aminopathies represent a heterogeneous group of genetic
 1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
The nuclear lamina and heterochromatin: a complex relationship
 Nuclear Envelope Disease and Chromatin Organization 1705 The nuclear lamina and heterochromatin: a complex relationship Erin M. Bank and Yosef Gruenbaum 1 Department of Genetics, Institute of Life Sciences,
Nuclear Envelope Disease and Chromatin Organization 1705 The nuclear lamina and heterochromatin: a complex relationship Erin M. Bank and Yosef Gruenbaum 1 Department of Genetics, Institute of Life Sciences,
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Cell Cycle and Mitosis
 Cell Cycle and Mitosis Name Period A# THE CELL CYCLE The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell between its formation and the moment it replicates
Cell Cycle and Mitosis Name Period A# THE CELL CYCLE The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell between its formation and the moment it replicates
Cell Overview. Hanan Jafar BDS.MSc.PhD
 Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Control of Cell Cycle. Unit 2 Part f III
 Control of Cell Cycle Unit 2 Part f III How often do cells divide and why? The timing and rate of cell division in different parts of the plant or animals are crucial to normal growth, development and
Control of Cell Cycle Unit 2 Part f III How often do cells divide and why? The timing and rate of cell division in different parts of the plant or animals are crucial to normal growth, development and
Nucleic acids. Nucleic acids are information-rich polymers of nucleotides
 Nucleic acids Nucleic acids are information-rich polymers of nucleotides DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell RNA and DNA are made up of very similar nucleotides.
Nucleic acids Nucleic acids are information-rich polymers of nucleotides DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell RNA and DNA are made up of very similar nucleotides.
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients
 Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling
 Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling Resources: Latchman Lodish chapter 10, 20 Helmreich, chapter 11 http://www.nursa.org,
Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling Resources: Latchman Lodish chapter 10, 20 Helmreich, chapter 11 http://www.nursa.org,
Molecular biology :- Cancer genetics lecture 11
 Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
Ploidy and Human Cell Types. Cell Cycle and Mitosis. DNA and Chromosomes. Where It All Began 11/19/2014. Chapter 12 Pg
 Ploidy and Human Cell Types Cell Cycle and Mitosis Chapter 12 Pg. 228 245 Cell Types Somatic cells (body cells) have 46 chromosomes, which is the diploid chromosome number. A diploid cell is a cell with
Ploidy and Human Cell Types Cell Cycle and Mitosis Chapter 12 Pg. 228 245 Cell Types Somatic cells (body cells) have 46 chromosomes, which is the diploid chromosome number. A diploid cell is a cell with
Chapter 8 The Cell Cycle
 What molecule stores your genetic information or determines everything about you? DNA a nucleic acid How are DNA molecules arranged in the nucleus? As you can see DNA is: Chapter 8 The Cell Cycle 1. Arranged
What molecule stores your genetic information or determines everything about you? DNA a nucleic acid How are DNA molecules arranged in the nucleus? As you can see DNA is: Chapter 8 The Cell Cycle 1. Arranged
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Chapt. 10 Cell Biology and Biochemistry. The cell: Student Learning Outcomes: Describe basic features of typical human cell
 Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Chapt. 10 Cell Biology and Biochemistry Cell Chapt. 10 Cell Biology and Biochemistry The cell: Lipid bilayer membrane Student Learning Outcomes: Describe basic features of typical human cell Integral transport
Cell cycle and apoptosis
 Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
RECAP (1)! In eukaryotes, large primary transcripts are processed to smaller, mature mrnas.! What was first evidence for this precursorproduct
 RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
RECAP (1) In eukaryotes, large primary transcripts are processed to smaller, mature mrnas. What was first evidence for this precursorproduct relationship? DNA Observation: Nuclear RNA pool consists of
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression. Chromatin Array of nuc
 Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes
 Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Introduction. Biochemistry: It is the chemistry of living things (matters).
 Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
A. Major parts 1. Nucleus 2. Cytoplasm a. Contain organelles (see below) 3. Plasma membrane (To be discussed in Cellular Transport Lecture)
 Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
Cell Biology. A discipline of biology: 1. Cell structure 2. Cellular processes 3. Cell division
 The Cell Cell Biology 1 A discipline of biology: 1. Cell structure 2. Cellular processes 3. Cell division Tight connection with 1. Molecular biology 2. Biochemistry Cell theory 2 1838, 1839 Theodor Schwann
The Cell Cell Biology 1 A discipline of biology: 1. Cell structure 2. Cellular processes 3. Cell division Tight connection with 1. Molecular biology 2. Biochemistry Cell theory 2 1838, 1839 Theodor Schwann
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Structures in Cells. Cytoplasm. Lecture 5, EH1008: Biology for Public Health, Biomolecules
 Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
