Available online at
|
|
|
- Cory Charlotte Waters
- 5 years ago
- Views:
Transcription
1 Available online at R Experimental Cell Research 291 (2003) Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy fibroblasts: altered intermolecular interaction with emerin and implications for gene transcription Cristina Capanni, b Vittoria Cenni, b Elisabetta Mattioli, b Patrizia Sabatelli, a Andrea Ognibene, b Marta Columbaro, c Veena K. Parnaik, d Manfred Wehnert, e Nadir M. Maraldi, a,b Stefano Squarzoni, a and Giovanna Lattanzi a, * a ITOI, CNR, Unit of Bologna, c/o IOR, Bologna, Italy b Laboratory of Cell Biology, Istituti Ortopedici Rizzoli, Bologna, Italy c Neuromuscular Unit, Istituti Ortopedici Rizzoli, Bologna, Italy d Centre for Cellular and Molecular Biology, Hyderabad , India e Institute of Human Genetics, University of Greifswald, Germany Received 10 December 2002, revised version received 9 July 2003 Abstract Familial partial lipodystrophy is an autosomal dominant disease caused by mutations of the LMNA gene encoding alternatively spliced lamins A and C. Abnormal distribution of body fat and insulin resistance characterize the clinical phenotype. In this study, we analyzed primary fibroblast cultures from a patient carrying an R482L lamin A/C mutation by a morphological and biochemical approach. Abnormalities were observed consisting of nuclear lamin A/C aggregates mostly localized close to the nuclear lamina. These aggregates were not bound to either DNA-containing structures or RNA splicing intranuclear compartments. In addition, emerin did not colocalize with nuclear lamin A/C aggregates. Interestingly, emerin failed to interact with lamin A in R482L mutated fibroblasts in vivo, while the interaction with lamin C was preserved in vitro, as determined by coimmunoprecipitation experiments. The presence of lamin A/C nuclear aggregates was restricted to actively transcribing cells, and it was increased in insulin-treated fibroblasts. In fibroblasts carrying lamin A/C nuclear aggregates, a reduced incorporation of bromouridine was observed, demonstrating that mutated lamin A/C in FPLD cells interferes with RNA transcription Elsevier Inc. All rights reserved. Keywords: Lamin A/C; Familial partial lipodystrophy; Lamin-emerin interaction; Chromatin disorganization; Intranuclear lamin A/C speckles; Gene transcription Introduction * Corresponding author. ITOI, CNR, Unit of Bologna, c/o IOR, Via di Barbiano 1/10, I Bologna, Italy. address: lattanzi@jolly.bo.cnr.it (G. Lattanzi). Familial partial lipodystrophy (FPLD, MIM ) is an inherited disease caused by mutations of the LMNA gene coding for alternatively spliced lamins A and C [1]. The clinical phenotype of FPLD is characterized by loss of fat in the trunk and limbs and accumulation of fat tissue in neck and face occurring during the peripubertal phase [2]. Additionally, variable degrees of resistance to insulin action, together with hypertriglyceridemia, may occur [2]. In FPLD, LMNA missense mutations substitute a highly conserved arginine at position 482, or lysine at position 486 in the C-terminal globular domain of lamin A/C [1]. In the last few years, several diseases have been associated to lamin A/C mutations, either dominant such as FPLD, autosomal Emery-Dreifuss muscular dystrophy (EDMD2) [3], dilated cardiomyopathy and conduction system disease (CMD-CD) [4], and limb girdle muscular dystrophy 1B (LGMD 1B) [5], or recessive such as autosomal recessive Emery-Dreifuss muscular dystrophy [6], mandibuloacral /$ see front matter 2003 Elsevier Inc. All rights reserved. doi: /s (03)
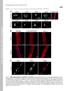
2 C. Capanni et al. / Experimental Cell Research 291 (2003) dysplasia [7], and Charcot-Marie Tooth neuropathy type 2 [8]. Recently, Hutchison-Gilford progeria syndrome has been ascribed to laminopathies, as lamin A/C mutations and nuclear defects have been found in patients [9 11]. The increasing number of LMNA-related diseases strongly suggests that lamin A/C is involved in a number of seemingly unrelated tissue-specific functions. This hypothesis is further supported by the well-known up-regulation of lamin A/C expression in differentiated cells [12 16]. In the knockout mouse model for lamin A/C, a phenotype resembling Emery-Dreifuss muscular dystrophy with Charcot-Marie Tooth neuropathy-like changes has been described, while adipose tissue and insulin sensitivity are nearly normal [17,18]. Lamin A/C interacts with the nuclear envelope protein emerin [19 23], the protein absent or mutated in X-linked Emery-Dreifuss muscular dystrophy (EDMD1). Moreover, anchorage of emerin at the nuclear rim is dependent upon lamin A/C interaction [17]. Emerin expression and localization are not affected in FPLD [24], while emerin binding to lamin A/C in FPLD cells was not studied as far as direct molecular interaction is concerned. In this study, we analyzed lamin A/C emerin interaction in cultured FPLD fibroblasts from a patient carrying an R482L mutation. Coimmunoprecipitation experiments showed that emerin does not interact with lamin A in FPLD. We also considered the possibility that lack of interaction between emerin and lamin might cause redistribution of one or both proteins in the nucleus. Altered lamin distribution with dysmorphic budding nuclei in FPLD fibroblasts carrying R482Q and R482W mutations has been reported, while the authors did not find altered distribution of emerin in FPLD cells [24]. In accordance with these reported data, we never found mislocalization of emerin in R482L FPLD fibroblasts. Nevertheless, lamin A/C was redistributed into nuclear aggregates in a high percentage of cells, forming a pattern not described before in FPLD. Lamins have been implicated in many nuclear activities including nuclear growth, DNA replication, and apoptosis [25] and, more recently, in RNA transcription [26,27]. Abnormal localization of lamin A/C was previously described in cells transfected with lamin A mutants [26,28 30] and could be associated with alteration of the transcriptional machinery. We checked the transcription rate of FPLD fibroblasts and found reduced RNA polymerase II activity in cells carrying abnormal lamin A/C aggregates. Fig. 1. Lamin A does not coprecipitate with emerin in R482L FPLD fibroblasts. Emerin was immunoprecipitated from whole cell lysates of control (C), EDMD2 (LMNA mutation R401C), and FPLD (LMNA mutation R482L) fibroblasts. The immuno-complexes were separated by SDS- PAGE followed by immunoblotting with anti-emerin and anti-lamin A/C antibodies. Immunoprecipitation with anti-emerin antibody is shown in the left panel; immunoblotting of whole lysastes with anti-lamin A/C and anti-emerin antibody is shown in the middle panel; immuno precipitation of a FPLD lysate with anti-lamin A/C antibody is shown in the right panel. Molecular weight markers are indicated in kda. Materials and methods Cell culture and synchronization Skin biopsies were obtained from FPLD patient G-9956 carrying an R482L lamin A/C mutation and from unaffected control or EDMD2 (patient G-10877; LMNA mutation R401C) patients following a written consent. Fibroblast cultures were established from skin biopsy explants and grown in D-MEM containing 10% fetal calf serum and antibiotics. Cells at passages 10 to 15 were employed. Accumulation of cells in G1 phase was obtained by serum starvation of semiconfluent fibroblast cultures [31], while synchronization of fibroblasts in the G2-M phase of the cell cycle was obtained by demecolcine treatment (25 ng/ml for 24 h) and release [32]. G0 fibroblasts (resting cells) were obtained by serum deprivation of confluent fibroblast cultures for 7 days. All these cells along with not-synchronous fibroblasts (NS) were subjected to methanol fixation and double immunofluorescence staining with anti-pcna and anti-lamin A/C. PCNA staining (not shown) allowed us to Fig. 2. Structural abnormalities of the nuclei in R482L FPLD cells. (A) Control nuclei showed lamin A/C and lamin B staining at the nuclear rim. (B) Lamin A/C nuclear aggregates are detected in 15 20% of FPLD fibroblast nuclei. (C) In FPLD nuclei, lamin B is absent from the blebbed areas (*) in which lamin A/C organization is also altered. A nucleus carrying both nuclear lamin A/C aggregates and anomalous distribution of lamin B is shown. Bar, 10 m. Fig. 3. Emerin and lamin A/C do not colocalize to nuclear lamin A/C aggregates in R482L FPLD cells. Control fibroblasts are shown in A, B, and C. EDMD2 fibroblasts are shown in D, E, and F: nuclear lamin A/C aggregates are not observed in EDMD2 nuclei, while nuclear lamina abnormalities can be detected in these cells as well as in FPLD fibroblasts (Fig. 2). For FPLD fibroblasts (G I ) different focal planes are shown: pictures obtained at the nuclear lamina plane are shown in G, H, and I; pictures obtained at the equatorial plane of the nucleus are shown in G, H, and I. As shown in G and I (arrows), lamin A/C nuclear aggregates are mostly observed at the nuclear lamina level, while just a few aggregates are detected at the middle plane of the nucleus, i.e., at internal nuclear sites (G, I ). No aggregates appear in anti-emerin staining (H, H ) Bar, 10 m.
3 124 C. Capanni et al. / Experimental Cell Research 291 (2003)
4 C. Capanni et al. / Experimental Cell Research 291 (2003) Fig. 4. Electron microscopy analysis of R482L FPLD fibroblast nuclei. Alterations of the chromatin pattern were observed in FPLD nuclei (A, C, D), compared to controls (B): they consisted mainly in lack of heterochromatin at the nuclear periphery, close to the nuclear lamina (A, C, D) (arrows). Detachment of heterochromatin from the nuclear lamina was also observed (A, arrowhead). Nuclear pores were generally absent in the areas corresponding to the peripheral heterochromatin loss (D, arrows). Bars: (A, D), 0.5 m, (B, C), 1 m.
5 126 C. Capanni et al. / Experimental Cell Research 291 (2003) distinguish between S-phase cells and G1 or G2 cells. The G2/M population of synchronized fibroblasts is referred to as G2 in the text and figure legends, as only G2 cells were considered in our analysis of lamin A/C aggregate distribution. Insulin treatment (10 M, Sigma) of serum-starved fibroblasts was performed for 4 h. FACS analysis To check the distribution of cells in the cell cycle, FPLD fibroblasts and controls at 50% of confluence were retrieved by trypsin detachment, fixed in 70% ethanol for 5 h, resuspended in PBS containing 5 g/ml RNase for 15 min at 37 C, washed in PBS and counter-stained with propidium iodide. Samples were analyzed by a FACStar Plus flow cytometer (Becton Dickinson, Palo Alto, CA) equipped with an argon ion laser tuned at 488-nm wavelength, 50 mw light output. Antibodies Anti-lamin A/C goat polyclonal antibody (Santa Cruz) or anti-lamin A/C monoclonal antibody (Novocastra) were used for immunofluorescence staining and for Western blot analysis. The anti-lamin A/C monoclonal antibody was used for the immunoprecipitation experiments. Anti-lamin A 2H10 [33] was employed for immunofluorescence staining. Monoclonal anti-emerin and anti-lamin B2 were obtained from Novocastra. Anti-SC35 monoclonal antibody was purchased from Sigma, and anti-pcna monoclonal antibody from Santa Cruz. Monoclonal anti-brdu antibody was from BD-PharMingen. Secondary antibodies were CY3-conjugated anti-mouse IgG (Sigma), FITC-conjugated anti-goat IgG (DAKO), FITC-conjugated anti-mouse IgM (Sigma), HRP conjugated anti-mouse Ig, and anti-goat Ig (Amersham-Pharmacia-Biotech). Immunoprecipitation and Western blotting Subconfluent cells were extracted by addition of RIPA buffer (20 mm Tris-HCl, ph 7.0, 1% NP-40, 150 mm NaCl, 10% glycerol, 10 mm EDTA, 20 mm NaF, 5 mm sodium pyrophosphate, 1 mm Na 3 VO 4, 1 mm PMSF, 10 g/ml leupeptin, 10 g/ml pepstatin) at 4 C. Precleared cell lysates were incubated for 3 h with anti-emerin, or anti-lamin A/C antibody (3 g per sample), or mouse IgG to provide a negative control, plus 30 l of 50% (v/v) of protein A/G agarose slurry (Santa Cruz) at 4 C with gentle rocking. Pellets were washed twice with PBS plus 1% NP-40, twice in TNE (10 mm Tris-Cl, ph 7.5, 100 mm NaCl, 1 mm EDTA), and boiled in Laemmli sample buffer. Immunoprecipitated proteins and whole cell lysates obtained in RIPA buffer were subjected to Western blotting. Samples were loaded onto 12% SDS-PAGE gel, blotted onto nitrocellulose, and probed with specific anti-lamin A/C or emerin antibodies or HRP-conjugated secondary antibodies and developed with enhanced chemiluminescence (ECL) reaction (Amersham-Pharmacia-Biotech). In situ nuclear matrix preparation Unfixed fibroblast cultures were washed in PBS, treated with a buffer containing 10 mm Tris-HCl (ph 7.4), 150 mm NaCl, 5 mm MgCl 2, 1% NP-40, 1 mm PMSF, 10 g/ml leupeptin, and aprotinin for 15 min at room temperature. Detergent-treated cells were washed twice in the absence of NP-40, subjected to DNase treatment (20 U/ml for 1 h at room temperature) and high salt extraction in 2 M NaCl containing buffer for 5 min at room temperature. This treatment led to complete removal of chromatin (as demonstrated by the absence of DAPI staining of nuclei, not shown). Samples were fixed with 2% paraformaldehyde and processed for immunofluorescence labeling. In situ transcription Living fibroblasts were treated according to published protocols [26]. Briefly, cells were permeabilized in a buffer containing 20 mm Tris-HCl (ph 7.4), 5 mm MgCl 2, 0.5 mm EGTA, 25% glycerol, 10 g/ml digitonin, 1 mm PMSF, RNasin (20 U/ml). Transcription assay was performed for 5 min in a buffer containing 50 mm Tris-HCl, ph 7.4, 100 mm KCl, 10 mm MgCl 2, 0.5 mm EGTA, 25% glycerol, 2 mm ATP, 0.5 mm CTP, 0.5 mm GTP, 0.5 mm BrUTP, 1 mm PMSF and RNasin (20 U/ml) (Promega). -Amanitin (10 g/ml) (Sigma) was added to the transcription buffer to obtain negative controls (not shown). Cells were washed in PBS and fixed in 2% paraformaldehyde at room temperature. Incorporated nucleotides were revealed by immunofluorescence labeling with anti-brdu antibody. Chromatin was counterstained with 4,6-diamidino-2-phenylindole (DAPI, 0.1 g/ml) (Sigma). Immunofluorescence Fibroblasts grown on coverslips were fixed with methanol at 20 C or with 2% paraformaldehyde at 4 C. Paraformaldehyde-fixed fibroblasts were permeabilized with 0.15% Triton X-100 in PBS. All preparations were incubated with PBS containing 4% BSA to saturate nonspecific binding. Incubation with primary antibodies was performed overnight at 4 C, while secondary antibodies were applied for 1 h at room temperature. Slides were mounted with an antifade reagent in glycerol and observed with a Nikon E 600 fluorescence microscope equipped with a digital camera. Electron microscopy FPLD fibroblasts and controls were fixed with 2.5% glutaraldehyde 0.1 M phosphate buffer, postfixed with 1%
6 C. Capanni et al. / Experimental Cell Research 291 (2003) OsO 4 in veronal buffer, dehydrated in ethanol, scraped, and embedded in epoxy resin. Ultrathin sections were cut from two different embedded blocks of mutated and control cells, respectively, counterstained with uranyl acetate-lead citrate, and observed with a Philips EM 400 T electron microscope, operated at 100 KV. For each sample, about 250 nuclei were evaluated. Results Lamin A and emerin do not interact in cell lysates from FPLD R482L fibroblasts Lamin A/C has been demonstrated to bind directly emerin [20]. This interaction has been shown to involve mainly lamin A as for the strength of binding [21]. It depends mostly on lamin A for retaining emerin at the nuclear envelope [23]. The sequence that binds emerin includes amino acids 384 to 566 [21] in the tail domain shared by lamins A and C. Since the mutated FPLD sequence is within this domain, we immunoprecipitated FPLD fibroblast lysates with anti-emerin antibody to check if emerinlamin binding was affected by the R482L mutation. An immunoblot of the immunoprecipitated complex showed that lamin A failed to coprecipitate with emerin in these cells (Fig. 1), while the two proteins coprecipitated in control fibroblasts as well as in EDMD2 fibroblasts cultured under the same conditions (Fig. 1). On the contrary, lamin C was still bound to emerin in FPLD fibroblasts (Fig. 1). The amount of both lamin A and C was not affected in the whole cell lysates from FPLD fibroblasts suggesting that the proteins are expressed at normal levels (Fig. 1). Moreover, the emerin-immunoblotted band was not reduced in FPLD whole cell lysates with respect to controls (Fig. 1). As expected, immunoprecipitation of lamin A/C from FPLD fibroblasts with an anti-lamin A/C monoclonal antibody allowed us to detect coprecipitation of emerin (Fig. 1), thus demonstrating that the interaction between emerin and lamin A/C is preserved through lamin C binding. Lamin A/C distribution is dramatically affected in FPLD R482L fibroblasts To check lamin A/C distribution in cultured FPLD fibroblasts we immunolabeled methanol or paraformaldehyde fixed cells with anti-lamin A/C (Fig. 2). Nuclear lamina staining was observed in 80% of labeled fibroblasts, while a marked reduction of labeling was observed at the nuclear rim in 15 20% of FPLD cells (338 out of 1890 nuclei), with lamin A/C being mainly distributed into nuclear aggregates (Fig. 2A). The same pattern of distribution was observed with an anti-lamin A antibody (not shown). The number of nuclear lamin A/C aggregates varied from 10 to 30 per nucleus and the aggregates appeared to be uniformly distributed throughout the nuclei (Fig. 2). Lamin A/C aggregates were not observed in control fibroblasts (Fig. 2). We immunolabeled the same samples with anti-lamin B2 antibody and found that lamin A and lamin B2 colocalized in some, but not all the intranuclear aggregates (Fig. 2). This finding suggests that partial redistribution of lamin B in these FPLD fibroblasts does occur. In addition, alterations of the lamin A/C lattice (Fig. 2B) were observed in about 15% of fibroblasts as compared with only 4% in controls and 10% in EDMD2 fibroblasts (Fig. 3). In FPLD cells, lamin A/C was irregularly organized at one pole of the nucleus, with a sponge-like appearance, and lamin B2 was absent from the same area (Fig. 2B). This finding is in agreement with data reported by Vigouroux et al. [24]. Interestingly, in 3.5% of fibroblasts we could observe both these lamina abnormalities and the presence of lamin A/C aggregates (Fig. 2B). It is conceivable that both defects result from impaired interaction of lamin A/C with other nuclear binding partners. Emerin is not detected at nuclear lamin A/C aggregates Lamin A/C aggregates were not observed in control fibroblasts from either unaffected controls or EDMD2 patient carrying R401C LMNA mutation (Fig. 3A). We wondered if emerin was still retained at the nuclear envelope in R482L FPLD fibroblasts or if it was relocalized due to the lack of binding to lamin A as observed by immunoprecipitation (Fig. 1A) or as a consequence of the apparent mislocalization of lamin A/C. Double immunofluorescence labeling using antibodies against lamin A/C and emerin (Fig. 3) showed that emerin localization was not affected in FPLD fibroblasts compared to control fibroblasts (Fig. 3), while the intensity of emerin staining appeared slightly reduced in cells bearing the lamin A/C aggregates (Fig. 3). In EDMD2 fibroblasts lamin A/C aggregates were not found, while nuclear lamina holes and thickening of the nuclear rim were observed (Fig. 3). In FPLD cells, emerin was never detected at the lamin A/C aggregates (Fig. 3). Double labeling of emerin and lamin A/C also allowed us to define the exact localization of lamin A/C aggregates observed in FPLD fibroblasts. As also shown in Fig. 3, the aggregates are not visible in the equatorial plane of the nucleus, i.e., they are mostly localized close to the nuclear lamina. Peripheral heterochromatin distribution is altered in FPLD nuclei Electron microscopy of FPLD fibroblast nuclei showed an altered distribution of peripheral chromatin in a high percentage of nuclei (about 20%, 53 nuclei out of 250 observed) compared to controls (no alterations detected)
7 128 C. Capanni et al. / Experimental Cell Research 291 (2003) Fig. 5. Lamin A/C aggregates partially colocalize with intranuclear lamin A/C sites and splicing factor components. (A) Anti-lamin A (2H10) antibody was used to label intranuclear lamin A foci in control and FPLD fibroblasts. 2H10-labeled sites, mostly, did not colocalize with lamin A/C aggregates of R482L FPLD cells. However, in a minor percentage the aggregates colocalized with internal lamin A foci (arrowhead). (B) Antisplicing factor SC35 antibody staining allowed us to demonstrate that redistribution of splicing sites by okadaic acid treatment does not affect lamin A/C aggregates. SC35 and lamin A/C were double-labeled in control fibroblasts and in R482L FPLD fibroblasts before and after okadaic acid treatment. Bar, 2.4 m for (A) and 10 m for (B). (Fig. 4). Lack of heterochromatin was observed at the nuclear periphery, close to the nuclear lamina (arrows); in these areas the nucleoplasm appeared less electron dense than normal (Figs. 4A and C); nuclear pores were generally absent in the areas corresponding to the peripheral heterochromatin loss (Fig. 4D). Detachment of chromatin from the nuclear lamina was also observed (arrowhead Fig. 4A). Neither thickening of the nuclear lamina nor nuclear envelope breaches were detected. These observations indicate that altered organization of the nuclear lamina can affect the chromatin arrangement. This is consistent with previously published data demonstrating altered chromatin organization in EDMD cells and muscle tissue bearing lamin A/C [34] or emerin [35] mutations.
8 C. Capanni et al. / Experimental Cell Research 291 (2003) A/C aggregates were not affected by the treatment. Thus, we can conclude that the nuclear aggregates do not correspond to lamin A/C-containing structures present inside the nucleus in normal cells [33], but represent abnormal structures that form close to the nuclear lamina as a consequence of R482L lamin A/C mutation. The lamin A/C aggregates observed in FPLD nuclei were not extracted by detergent treatment and were still detectable after DNase digestion and high salt extraction (Fig. 6), thus suggesting that they were bound to the nuclear matrix. The presence of lamin A/C aggregates is increased in G1 and G2 cells Fig. 6. Double staining of emerin and lamin A/C was performed in in situ nuclear matrix preparations. FPLD fibroblasts carrying lamin A/C aggregates show a slightly reduced emerin staining at the nuclear rim. In FPLD nuclei, lamin A/C aggregates are bound to the nuclear matrix after DNase treatment and high salt extraction. Bar, 10 m. Lamin A/C aggregates do not correspond to intranuclear lamin A/C sites Intranuclear lamin A foci have been previously described in G1 cells [31] and also in interphase cells by labeling with a specific antibody [33]. We wondered if lamin A/C aggregates observed in FPLD fibroblast nuclei could correspond to intranuclear structures observed in normal cells and could be detected in FPLD cells better than in control fibroblasts due to epitope unmasking. Immunolabeling of FPLD fibroblasts was performed with the 2H10 antibody, which selectively binds to lamin A at intranuclear sites [33]. In most nuclei, the lamin A/C aggregates detected by anti-lamin A/C antibody did not colocalize with 2H10 labeled sites (Fig. 5). It should be noted that intranuclear lamin A foci are found throughout the nucleus (except the nucleolus) whereas the abnormal aggregates are mostly localized toward the nuclear periphery. However, in a lower percentage of cells, the two fluorescence signals partially coincided (Fig. 5). This finding suggests that the abnormal distribution of lamin A/C in FPLD fibroblasts could represent an alteration of a dynamic situation, at least in part involving intranuclear lamin A foci. To check if lamin A/C aggregates were structurally or functionally related to intranuclear lamin A/C foci known to be associated with mrna splicing compartments, we treated FPLD fibroblasts with okadaic acid. Okadaic acid is a phosphatase inhibitor known to cause redistribution of splicing factor SC35 and of several components of the splicing factor complexes [36]. As shown in Fig. 5, SC35 was redistributed after okadaic acid incubation, but lamin To rule out the possibility that mutated lamin A/C could interfere with cell cycle progression, fluorescence-activated cell-sorting analysis (FACS analysis) of cultured FPLD fibroblasts was performed. A distribution of the FPLD fibroblast population in the cell cycle phases comparable to that observed in control fibroblasts was detected (not shown). Immunofluorescence labeling with anti-lamin A/C antibody was then performed on synchronized FPLD fibroblasts, to evaluate the rate of lamin A/C aggregate formation in the different cell cycle phases. Fibroblasts synchronized in the G1 phase by serum starvation and release or in the G2 phase by demecolcine treatment showed increased lamin A/C aggregates with respect to nonsynchronous FPLD cells (Fig. 7). In addition, starved cells obtained after 7 days of serum deprivation, completely lost lamin A/C aggregates. We did not observe any lamin A/C aggregates in S phase cells (as determined by proliferating cell nuclear antigen (PCNA) and lamin A/C double staining of asynchronous Fig. 7. Increased nuclear lamin A/C aggregates in G 1 cells. The nuclear aggregates are not detected in G0 fibroblasts and in S-phase cells. Control (C) and mutant R482L (FPLD) fibroblasts were synchronized as described in Materials and methods and double-stained with lamin A/C and PCNA to detect cells in different phases of the cell cycle. In G1 cells the percentage of nuclei with lamin aggregates was 35% (out of 1500 examined). Starved cells (starved) were obtained after 7 days of serum depletion and treated with 10 M insulin for 4 h (insulin). In insulin-treated fibroblasts 32% of nuclei (out of 1320 examined) showed lamin aggregates. The mean of the results of three different counts performed in different samples / standard deviation are reported per each point. (NS, not synchronous; G1, G1 phase; S, S phase; G2, G2 phase; G0, G0 phase.)
9 130 C. Capanni et al. / Experimental Cell Research 291 (2003) cells, not shown). Thus, abnormal lamin A/C structures did not occur in G0 cells or, alternatively, in cells with low transcriptional activity. This finding suggests that formation of lamin A/C aggregates is a failure of correct lamin A/C assembly into a functionally active structure. This hypothesis is supported by the observation that insulin treatment of serum-depleted FPLD cells raised to 32% the percentage of nuclei bearing lamin A/C aggregates (Fig. 7). Transcriptional activity is altered in FPLD R482L fibroblasts To check whether abnormal lamin A/C organization could interfere with RNA polymerase II activity, we performed an in situ transcription assay that allowed us to determine the relative amount of incorporated bromouridine (BrU) into transcribed RNA. A bright punctate staining was observed in the nuclei of control fibroblasts (Fig. 8A) and in FPLD nuclei with normal lamin A/C organization at the nuclear lamina. In all FPLD fibroblasts bearing abnormal lamin A/C aggregates (15 20% of the total population) we found strongly reduced incorporation of BrU as determined by immunofluorescence (Fig. 8B). These findings demonstrate that the R482L mutation of LMNA, which causes FPLD, interferes with RNA polymerase II-mediated transcription. In fact, in cells showing complete absence of BrU fluorescence in the nucleoplasm, nucleolar staining was often observed (not shown), suggesting that RNA polymerase I-mediated transcription is not affected. It should be noted that DAPI staining of nuclei (Fig. 8A) allowed us to rule out the possibility that BrU-negative nuclei were apoptotic: in fact, neither hypercondensed chromatin nor micronuclei were found in fibroblasts showing reduced transcriptional activity. Discussion The overall evaluation of our results shows that:(1) mutation of lamin A/C at R482 affects interaction of the nuclear lamina protein with both nuclear envelope (emerin) and nuclear lamina (lamin B2) components; (2) in R482L FPLD fibroblasts, lamin A/C is redistributed in a significant percentage of nuclei and an altered nuclear lamina assembly is observed mostly in G1 and G2 cells, but not in G0 fibroblasts; (3) in FPLD fibroblast nuclei, altered heterochromatin distribution close to the nuclear lamina occurs and heterochromatic areas are frequently lacking; (4) the lamin A/C aggregates observed in FPLD fibroblasts are not bound to DNA or splicing factor compartments, but a low percentage of aggregates colocalize with intranuclear lamin A sites; (5) a reduced RNA transcription rate is observed in FPLD nuclei carrying lamin A/C aggregates, as determined by in situ transcription assay. R482L lamin A/C mutation causes disruption of lamin organization In FPLD fibroblast nuclei carrying an R482L mutation of lamin A/C, we observed the formation of lamin A/C aggregates localized close to the nuclear lamina. This abnormal distribution of lamin A/C has never been observed before in FPLD nuclei and can be strictly related to the amino acid substitution present in these cells. An altered organization of lamin A/C, similar to the one observed in this study, has been described by Lloyd et al.[28] in HeLa cells transiently transfected with an E203G lamin A/C mutation, which is associated with dilated cardiomyopathy (CMD1A). These authors suggest that the E203G LMNA mutation can affect the self-assembly of the molecule, thus causing formation of intranuclear aggregates [28]. In R482L FPLD fibroblasts, it is more probable that the amino acid substitution may affect an interaction with other nuclear partner(s) [28,37] necessary for proper nuclear lamina organization. The nuclear lamin A/C aggregates present in R482L FPLD fibroblasts represent anomalous lamin A/C structures not anchored to nuclear components involved in either DNA replication or RNA splicing. Thus, it appears likely that the lamin A/C aggregates observed in FPLD R482L fibroblasts are formed as a consequence of lacking functional interaction usually occurring in normal fibroblasts. The variable impact on the lamina structure of R482L lamin A/C mutation can be explained by hypothesizing that a functional interaction occurring in a percentage of an asynchronous cell population may be impaired in FPLD fibroblasts. This hypothesis is supported by the fact that accumulation of cells in G1, as well as insulin treatment, increase the rate of altered nuclei. The latter is an intriguing result, suggesting that some posttranslational modification of lamin A/C, such as phosphorylation, may occur in the nuclei with altered lamin A/C distribution. On the other hand, insulin signaling plays a major role in adipocyte differentiation through the SREBP1-PPAR pathway [38]: thus, lamin A/C distribution in response to insulin stimulus should be investigated in an adipocyte cellular model, and intermolecular interactions between lamin A/C and its possible partner protein SREBP1 [28] or chromatin [39] should be evaluated. R482L mutated lamin A fails to interact with emerin Moreover, we demonstrate that lamin A fails to bind emerin in FPLD R482L fibroblasts, while lamin C, whose interaction with emerin is not disrupted, is sufficient to retain the nuclear envelope protein at its proper localization. The finding that lamin A and emerin do not coprecipitate in R482L FPLD fibroblasts was an unexpected finding. Other authors reported that the interaction between emerin and lamin is not affected in FPLD fibroblasts carrying different mutations [24,40] or in in vitro pull-down assays performed with lamin A/C R482W mutant [41]. We suggest that a different amino acid substitution (R482L instead of
10 C. Capanni et al. / Experimental Cell Research 291 (2003) R482Q/W) [24,37,41] elicits different effects on the intermolecular interactions involving the mutated C-terminal domain. In this respect, it is noteworthy that an Ig-like domain has been recently identified in the carboxy-terminal globular domain of lamin A, which has been suggested to be involved in intermolecular interactions [37,42]. Moreover, loss of a basic side chain at the carboxy-terminus of lamin A/C has been demonstrated in FPLD cells [37]. Thus, we hypothesize that the presence of a nonpolar amino acid such as leucine within this domain impairs lamin A emerin binding. Another explanation for the failure of emerin to bind lamin A could be the absence of an interaction with a third molecule necessary to stabilize the emerin-lamin A complex. In fact, other interactions with lamin A/C could also be altered in nuclei carrying the R482L mutation and thus elicit a pathogenic effect. As far as the site of interaction between LAP2 and lamin A/C is within the mutated C-terminal domain of lamin A/C [29], the interaction with LAP2 is possibly also interrupted in R482L FPLD. Reduced in vitro binding of lamin A/C mutants found in FPLD to the sterol response element binding protein 1 (SREBP1) has been recently demonstrated [28]. The evidence of impaired emerin lamin A binding as reported in this study represents the first evidence of altered in vivo protein protein interaction in FPLD cells. This supports the hypothesis of a major role of abnormal lamin A interference with its partner protein(s) in the pathogenesis of FPLD [37]. Chromatin disorganization is a common feature of laminopathies We previously described altered lamin distribution in EDMD1 fibroblasts [35] and altered chromatin organization in both EDMD1[35] and EDMD2 fibroblasts [34]. A markedly altered heterochromatin arrangement and areas devoid of heterochromatin have been observed also in FPLD fibroblasts (Fig. 4). This observation was not unexpected, since not only lamin A/C is mutated in FPLD, but also emerinlamin A binding is impaired in R482L FPLD cells: thus, a nuclear envelope defect is again found to be associated with altered chromatin organization. This finding suggests that a common mechanism leading to chromatin disorganization may underlie the pathology caused by nuclear envelope nuclear lamina defects [43]. This hypothesis is strongly supported by a recent report by Stierlé et al. [39] demonstrating that the lamin A/C sequence around the amino acid R482 contains a DNA-binding domain that allows a stable DNA interaction in cooperation with the NLS-containing peptide [39]. Interestingly, these authors further show that no DNA sequence specificity is required for lamin A/C binding, again suggesting that lamin A/C could be involved in a more general mechanism regulating chromatin organization. Blebbed areas lacking lamin B were recently described in FPLD fibroblasts carrying different amino acid substitutions by Vigouroux et al. [24]. Also, these authors showed that transfection of normal fibroblasts with a vector encoding an R482W mutated lamin A/C causes in a percentage of nuclei an altered lamin A/C organization with holes and lack of lamin B in the same areas [24]. This finding is consistent with our results obtained with R482L FPLD cells. Mutated R482L lamin A/C can affect RNA transcription Further evidence to the hypothesis that nuclear lamin A/C aggregates are formed due to the lack of a normal functional interaction is given by the finding that the nuclear aggregates are not found in serum-starved cells, while their frequency increases in G1 and G2 fibroblasts. Thus, the aggregates must correspond to anomalous lamin A/C structures forming in actively transcribing cells. It is noteworthy that lamin A/C aggregates were not observed in S-phase cells, so that we can rule out that they represent a failure of lamin A/C to correctly assemble into structures involved in DNA replication. On the basis of these results, we further evaluated the transcriptional activity of R482L FPLD cells. Involvement of nuclear envelope proteins in the regulation of gene expression has been demonstrated for LAP2 beta and its associated germ-cell-less protein [44]. Moreover, a report by Spann et al. [26] shows that a mutated lamin A impairs RNA polymerase II-mediated transcription in transfected cells. Our results agree with those of Spann et al. [26], as far as we demonstrated a reduced BrU incorporation in R482L-mutated nuclei bearing lamin A/C aggregates. This finding provides the first evidence that RNA transcription is affected in a laminopathy, concomitantly with failure of lamin A/C to correctly assemble at the nuclear lamina. It is noteworthy that the presence of lamin A/C aggregates in FPLD nuclei appears to be dependent on the functional status of the cell (cells accumulated in G1 showed aggregates to a higher percentage). Thus, virtually all FPLD fibroblasts could undergo an alteration of lamin A/C arrangement, and as a consequence an altered RNA transcription rate, under a determined functional condition. Lamin A/C has been shown to interact with SREBP1 [28] as well as with MOK2 transcription factor and to affect the nuclear localization of the latter [45]. Concerning the diseases associated with nuclear envelope defects, a recent expression profiling in EDMD1 cells using cdna microarray analysis showed altered expression of several genes, including LMNA [46]. Thus, altered gene expression in laminopathies, as previously speculated by several authors [16,43,47], is now gaining experimental evidence. Nevertheless, more than one mechanism is likely to be involved in the pathogenesis of laminopathies [48], and proved and potential nuclear binding partners of lamins may be linked to the pathogenetic mechanism. In this respect, it is worthy to note that a transactivation-deficient mutant of PPARG encoding peroxisome proliferator activated receptor (PPAR)- has been recently identified in a FPLD not linked to LMNA mutation [49]. The analysis of the in vivo interaction be-
11 132 C. Capanni et al. / Experimental Cell Research 291 (2003) Fig. 8. (A) Reduced BrU incorporation in FPLD nuclei with abnormal lamin A/C distribution. BrU incorporation in control nuclei was detected as bright fluorescence staining (CY3 labeling, red). The marked FPLD nucleus (arrows) bearing lamin A/C aggregates shows reduced BrU staining. Fluorescence staining of incorporated BrU is not affected in the FPLD nucleus with normal lamin A/C at the nuclear lamina. DAPI staining of chromatin is also shown. Bar, 5 m. (B) This figure is representative of the results obtained in three different experiments. A total of 450 nuclei were counted and the mean values for each cell population are reported as percentage of observed nuclei. To perform a quantitative analysis of reduced transcriptional activity, in situ transcription assay was performed for a shorter period (2 min), in order to obtain a BrU fluorescence signal of altered FPLD nuclei under the detection level. Under these experimental conditions, only negative nuclei were considered as showing reduced BrU incorporation. tween lamin A/C and PPAR- or SREBP1 [28] could give an insight into the altered mechanism leading to FPLD. Acknowledgments The study was supported by an EU grant MYO-Cluster QLG , by Italian Ministero della Salute, P.F. No. 83/2001 and FIRB Project No. RBNE01JJ45. M.W. was supported by a grant of the Deutsche Forschungsgemeinschaft We 1470/12-3. V.K.P. was supported by the Council for Scientific and Industrial Research, India. The authors are indebted to Dr. K. Mohnike for referring FPLD patient G-9956 to L. Cattini for the FACS analysis and to I. Mura for the technical support. References [1] S. Shackleton, D.J. Lloyd, S.N.J. Jackson, R. Evans, M.F. Niermeijer, B.M. Singh, H. Schmidt, G. Brabant, S. Kumar, P.N. Durrington, S. Gregory, S. O Rahilly, R.C. Trembath, LMNA, encoding lamin A/C, is mutated in partial lipodystrophy, Nature Genet. 24 (2000) [2] R.A. Hegele, Molecular basis of partial lipodystrophy and prospects for therapy, Trends Mol. Med. 7 (2001) [3] G. Bonne, M. Raffaele di Barletta, S. Varnous, H.M. Becane, E.H. Hammouda, L. Merlini, F. Muntoni, C.R. Greenberg, F. Gary, J.A. Urtizberea, D. Duboc, M. Fardeau, D. Toniolo, K. Schwartz, Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy, Nature Genet. 21 (1999) [4] D. Fatkin, C. MacRae, T. Sasaki, M.R. Wolff, M. Porcu, M. Frenneaux, J. Atherton, H.J. Vidaillet Jr, S. Spudich, U. De Girolami, J.G. Seidman, C. Seidman, F. Muntoni, G. Muehle, W. Johnson, B. Mc- Donough, Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease, N. Engl. J. Med. 341 (1999) [5] A. Muchir, G. Bonne, A.J. van der Kooi, M. van Meegen, F. Baas, P.A. Bolhuis, M. devisser, K. Schwartz, Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B), Hum. Mol. Genet. 9 (2000) [6] M. Raffaele di Barletta, E. Ricci, G. Galluzzi, P. Tonali, M. Mora, L. Morandi, A. Romorini, T. Voit, K. Helene Orstavik, L. Merlini, C. Trevisan, V. Biancalana, I. Housmanowa-Petrusewicz, S. Bione, R. Ricotti, K. Schwartz, G. Bonne, D. Toniolo, Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy, Am. J. Hum. Genet. 66 (2000) [7] G. Novelli, A. Muchir, F. Sangiuolo, A. Helbling-Leclerc, M.R. D Apice, C. Massart, F. Capon, P. Sbraccia, M. Federici, R. Lauro, C. Tudisco, R. Pallotta, G. Scarano, B. Dallapiccola, L. Merlini, G. Bonne, Mandibuloacral dysplasia is caused by a mutation in LMNAencoding lamin A/C, Am. J. Hum. Genet. 71 (2002)
12 C. Capanni et al. / Experimental Cell Research 291 (2003) [8] A. De Sandre-Giovannoli, M. Chaouch, S. Kozlov, J.M. Vallat, M. Tazir, N. Kassouri, P. Szepetowski, T. Hammadouche, A. Vandenberghe, C.L. Stewart, D. Grid, N. Levy, Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie- Tooth disorder type 2) and mouse, Am. J. Hum. Genet. 70 (2002) [9] Eriksson, M., Brown, W.T., Gordon, L.B., Glynn, M.W., Singer, J., Scott, L., Erdos, M.R., Robbins, C.M., Moses, T.Y., Berglund, P., Dutra, A., Pak, E., Durkin, S., Csoka, A.B., Boehnke, M., Glover, T.W., Collins, F.S., Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423 (2003) [10] De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C.L., Munnich, A., Le Merrer, M. Levy, N. Lamin A truncation in Hutchinson-Gilford progeria. Science 300 (2003) [11] Cao, H. Hegele, R.A. LMNA is mutated in Hutchinson-Gilford progeria (MIM ) but not in Wiedemann-Rautenstrauch progeroid syndrome (MIM ). J. Hum. Genet. 48 (2003) [12] D. Lourim, J.J. Lin, Expression of nuclear lamin A and musclespecific proteins in differentiating muscle cells in ovo and in vitro, J Cell Biol. 109 (1989) [13] J.L. Broers, B.M. Machiels, G.J. van Eys, H.J. Kuijpers, E.M. Manders, R. van Driel, F.C. Ramaekers, Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins, J. Cell Sci. 112 (1999) [14] R.A. Rober, K. Weber, M. Osborn, Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study, Development 105 (1989) [15] A.L. Olins, H. Herrmann, P. Lichter, M. Kratzmeier, D. Doenecke, D.E. Olins, Nuclear envelope and chromatin compositional differences comparing undifferentiated and retinoic acid- and phorbol ester-treated HL-60 cells, Exp. Cell Res. 268 (2001) [16] B. Burke, C.L. Stewart, Life at the edge: the nuclear envelope and human disease, Nature Rev. Mol. Cell Biol. 3 (2002) [17] T. Sullivan, D. Escalante-Alcalde, H. Bhatt, M. Anver, N. Bhat, K. Nagashima, C.L. Stewart, B. Burke, Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy, J. Cell Biol. 147 (1999) [18] D.A. Cutler, T. Sullivan, B. Marcus-Samuels, C.L. Stewart, M.L. Reitman, Characterization of adiposity and metabolism in Lmnadeficient mice, Biochem. Biophys. Res. Commun. 291 (2002) [19] E.A. Fairley, J. Kendrick-Jones, J.A. Ellis, The Emery-Dreifuss muscular dystrophy phenotype arises from aberrant targeting and binding of emerin at the inner nuclear membrane, J. Cell Sci. 112 (1999) [20] L. Clements, S. Manilal, D.R. Love, G.E. Morris, Direct interaction between emerin and lamin A, Biochem. Biophys. Res. Commun. 267 (2000) [21] M. Sakaki, H. Koike, N. Takahashi, N. Sasagawa, S. Tomioka, K. Arahata, S. Ishiura, Interaction between emerin and nuclear lamins, J. Biochem. 129 (2001) [22] K.K. Lee, T. Haraguchi, R.S. Lee, T. Koujin, Y. Hiraoka, K.L. Wilson, Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF, J. Cell Sci. 114 (2001) [23] A. Vaughan, M. Alvarez-Reyes, J.M. Bridger, J.L.V. Broers, F.C.S. Ramaekers, M. Wehnert, G.E. Morris, W.G.F. Whitfield, C.J. Hutchison, Both emerin and lamin C depend on lamin A for localization at the nuclear envelope, J. Cell Sci. 114 (2001) [24] C. Vigouroux, M. Auclair, E. Dubosclard, M. Pouchelet, J. Capeau, J.C Courvalin, B. Buendia, Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene, J. Cell Sci. 114 (2001) [25] Y. Gruenbaum, K.L. Wilson, A. Harel, M. Goldberg, M. Cohen, Nuclear lamins structural proteins with fundamental functions, J. Struct. Biol. 129 (2000) [26] T.S. Spann, A.E. Goldman, C. Wang, S. Huang, R.D. Goldman, Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription, J. Cell Biol. 156 (2002) [27] R.I. Kumaran, B. Muralikrishna, V.K. Parnaik, Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription, J. Cell Biol. 159 (2002) [28] D.J. Lloyd, R.C. Trembath, S. Shackleton, A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies, Hum. Mol. Genet. 11 (2002) [29] T. Dechat, B. Korbei, O.A. Vaughan, S. Vlcek, C.J. Hutchison, R. Foisner, Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins, J. Cell Sci. 113 (2000) [30] K. Bechert, M. Lagos-Quintana, J. Harborth, K. Weber, M. Osborn, Effects of expressing lamin A mutant protein causing Emery-Dreifuss muscular dystrophy and familial partial lipodystrophy in Hela cells, Exp. Cell Res. 286 (2003) [31] J.M. Bridger, I.R. Kill, M. O Farrell, C.J. Hutchison, Internal lamin structures within G1 nuclei of human dermal fibroblasts, J. Cell Sci. 104 (1993) [32] N. Adachi, M. Kobayashi, H. Koyama, Cell cycle-dependent regulation of the mouse DNA topoisomerase IIalpha gene promoter, Biochem. Biophys. Res. Commun. 230 (1997) [33] G. Jagatheesan, S. Thanumalayan, B. Muralikrishna, N. Rangaraj, A.A. Karande, V.K. Parnaik, Colocalization of intranuclear lamin foci with RNA splicing factors, J. Cell Sci. 112 (1999) [34] P. Sabatelli, G. Lattanzi, A. Ognibene, M. Columbaro, C. Capanni, L. Merlini, N.M. Maraldi, S. Squarzoni, Nuclear alterations in autosomal-dominant Emery-Dreifuss muscular dystrophy, Muscle Nerve. 24 (2001) [35] A. Ognibene, P. Sabatelli, S. Petrini, S. Squarzoni, M. Riccio, S. Santi, M. Villanova, S. Palmeri, L. Merlini, N.M. Maraldi, Nuclear changes in a case of X-linked Emery-Dreifuss muscular dystrophy, Muscle Nerve 22 (1999) [36] T. Misteli, J.F. Caceres, D.L. Spector, The dynamics of a pre-mrna splicing f factor in living cells, Nature 387 (1997) [37] S. Dhe-Paganon, E.D. Werner, Y.I. Chi, S.E. Shoelson, Structure of the globular tail of nuclear lamin, J. Biol. Chem. 277 (2002) [38] M. Caron, M. Auclair, C. Vigouroux, M. Glorian, C. Forest, J. Capeau, The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance, Diabetes 50 (2001) [39] V.V. Stierlé, J. Couprie, C. Ostlund, I. Krimm, S. Zinn-Justin, P. Hossenlopp, H.J. Worman, J.C. Courvalin, I. Duband-Goulet, The carboxyl-terminal region common to lamins A and C contains a DNA binding domain, Biochemistry 42 (2003) [40] I. Holt, L. Clements, S. Manilal, S.C. Brown, G.E. Morris, The R482Q lamin A/C mutation that causes lipodystrophy does not prevent nuclear targeting of lamin A in adipocytes or its interaction with emerin, Eur. J. Hum. Genet. 9 (2001) [41] W., Raharjo, R., Enarson, P., Sullivan, T., Stewart, C.L., Burke, B. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci [42] I. Krimm, C. Ostlund, B. Gilquin, J. Couprie, P. Hossenlopp, J.P. Mornon, G. Bonne, J.C. Courvalin, H.J. Worman, S. Zinn-Justin, The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy, Structure (Cambr.). 10 (2002)
13 134 C. Capanni et al. / Experimental Cell Research 291 (2003) [43] N.M. Maraldi, G. Lattanzi, P. Sabatelli, A. Ognibene, S.Squarzoni1, Functional domains of the nucleus: implications for Emery-Dreifuss muscular dystrophy, Neuromusc. Disorders 12 (2002) [44] E. Nili, G.S. Cojocaru, Y. Kalma, D. Ginsberg, N.G. Copeland, D.J. Gilbert, N.A. Jenkins, R. Berger, S. Shaklai, N. Amariglio, F. Brok- Simoni, A.J. Simon, G. Rechavi, Nuclear membrane protein LAP2 mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less), J. Cell Sci. 114 (2001) [45] C. Dreuillet, J. Tillit, M. Kress, M. Ernoult-Lange, In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C, Nucleic Acids Res. 30 (2002) [46] T. Tsukahara, S. Tsujino, K. Arahata, cdna microarray analysis of gene expression in fibroblasts of patients with X-linked Emery- Dreifuss muscular dystrophy, Muscle Nerve 25 (2002) [47] K.L. Wilson, The nuclear envelope, muscular dystrophy and gene expression, Trends Cell Biol. 10 (2000) [48] C.J. Hutchison, Lamins: building blocks or regulators of gene expression?, Nature Rev. Mol. Cell Biol. 3 (2002) [49] R.A. Hegele, H. Cao, C. Frankowski, S.T. Mathews, T. Leff, PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy, Diabetes 51 (2002)
Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C
 Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
T he LMNA gene encodes two nuclear envelope proteins,
 1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization
 Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
L aminopathies represent a heterogeneous group of genetic
 1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy
 RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins
 Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
H utchinson-gilford progeria syndrome (HGPS; MIM
 609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Workshop report. 1. Introduction
 Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells
 1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two Chinese siblings: two case reports
 Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy
 MOLECULAR MEDICINE REPORTS 12: 5065-5071, 2015 Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy LI ZHANG 1, HONGRUI SHEN 2, ZHE ZHAO 2, QI BING 2 and
MOLECULAR MEDICINE REPORTS 12: 5065-5071, 2015 Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy LI ZHANG 1, HONGRUI SHEN 2, ZHE ZHAO 2, QI BING 2 and
Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells
 JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies
 Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Lipodystrophies represent a heterogeneous group
 Brief Genetics Report Lamin A/C Gene Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy
Brief Genetics Report Lamin A/C Gene Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients
 Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Disruption of spermatogenesis in mice lacking A-type lamins
 Research Article 1173 Disruption of spermatogenesis in mice lacking A-type lamins Manfred Alsheimer 1, *, Bodo Liebe 2, *, Lori Sewell 3, Colin L. Stewart 3, Harry Scherthan 2, and Ricardo Benavente 1,
Research Article 1173 Disruption of spermatogenesis in mice lacking A-type lamins Manfred Alsheimer 1, *, Bodo Liebe 2, *, Lori Sewell 3, Colin L. Stewart 3, Harry Scherthan 2, and Ricardo Benavente 1,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
JCB Article. Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription
 JCB Article Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription R. Ileng Kumaran, Bhattiprolu Muralikrishna, and Veena K. Parnaik Centre
JCB Article Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription R. Ileng Kumaran, Bhattiprolu Muralikrishna, and Veena K. Parnaik Centre
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype
 Research Article 341 The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype Elizabeth A. L. Fairley 1, *, Andrew Riddell 2, Juliet A. Ellis
Research Article 341 The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype Elizabeth A. L. Fairley 1, *, Andrew Riddell 2, Juliet A. Ellis
The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans
 Research Article 923 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans Yosef Gruenbaum 1, *, Kenneth K. Lee 2, *, Jun Liu 3, Merav Cohen 1 and Katherine
Research Article 923 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans Yosef Gruenbaum 1, *, Kenneth K. Lee 2, *, Jun Liu 3, Merav Cohen 1 and Katherine
Distinct changes in intranuclear lamin A/C organization during myoblast differentiation
 RESEARCH ARTICLE 4001 Distinct changes in intranuclear lamin A/C organization during myoblast differentiation Bh. Muralikrishna, Jyotsna Dhawan, Nandini Rangaraj and Veena K. Parnaik* Centre for Cellular
RESEARCH ARTICLE 4001 Distinct changes in intranuclear lamin A/C organization during myoblast differentiation Bh. Muralikrishna, Jyotsna Dhawan, Nandini Rangaraj and Veena K. Parnaik* Centre for Cellular
Genotype phenotype correlations in laminopathies: how does fate translate?
 Nuclear Envelope Disease and Chromatin Organization 2009 257 Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S.
Nuclear Envelope Disease and Chromatin Organization 2009 257 Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S.
Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy
 RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Keywords: BAF, BANF1, prelamin A, lamin A/C, laminopathies, emerin, EDMD1. Introduction
 Report Cell Cycle 11:19, 3568-3577; October 1, 2012; 2012 Landes Bioscience Familial partial lipodystrophy, mandibuloacral dysplasia and restrictive dermopathy feature barrier-to-autointegration factor
Report Cell Cycle 11:19, 3568-3577; October 1, 2012; 2012 Landes Bioscience Familial partial lipodystrophy, mandibuloacral dysplasia and restrictive dermopathy feature barrier-to-autointegration factor
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
THURSDAY, JANUARY
 THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
LMNA mutations in atypical Werner s syndrome
 Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome
 Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Supplementary Materials and Methods
 Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Proteins that bind A-type lamins: integrating isolated clues
 Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Anti-Lamin B1/LMNB1 Picoband Antibody
 Anti-Lamin B1/LMNB1 Picoband Antibody Catalog Number:PB9611 About LMNB1 Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene. The nuclear lamina consists of a two-dimensional matrix of proteins
Anti-Lamin B1/LMNB1 Picoband Antibody Catalog Number:PB9611 About LMNB1 Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene. The nuclear lamina consists of a two-dimensional matrix of proteins
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Guilt by Association. Gavin S. Wilkie and Eric C. Schirmer. Review THE NUCLEAR ENVELOPE PROTEOME AND DISEASE*
 Review Guilt by Association THE NUCLEAR ENVELOPE PROTEOME AND DISEASE* Gavin S. Wilkie and Eric C. Schirmer The discovery that many inherited diseases are linked to interacting nuclear envelope proteins
Review Guilt by Association THE NUCLEAR ENVELOPE PROTEOME AND DISEASE* Gavin S. Wilkie and Eric C. Schirmer The discovery that many inherited diseases are linked to interacting nuclear envelope proteins
The LMNA gene encodes the A-type lamins, including lamin A
 A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Recombinant Protein Expression Retroviral system
 Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans
 MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans Jun Liu*, Kenneth K. Lee, Miriam Segura-Totten, Ester Neufeld, Katherine L.
MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans Jun Liu*, Kenneth K. Lee, Miriam Segura-Totten, Ester Neufeld, Katherine L.
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Non-commercial use only
 European Journal of Histochemistry 2011; volume 55:e36 Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria V. Cenni, 1 C. Capanni, 1 M. Columbaro, 2 M.
European Journal of Histochemistry 2011; volume 55:e36 Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria V. Cenni, 1 C. Capanni, 1 M. Columbaro, 2 M.
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy
 The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
PK15 48 (1) :58 63, (intermediate filament, IF) D ( Traub et al., 1994) IF P K15 D. ( Fuchs et al., 1994), , ( Fuchs et al., 1992) (apoptosis)
 48 (1) :58 63, 2002 A cta Zoologica S inica 3 PK15 (, 100871) D P K15 ( Porcrne Kidney215) DNA, DNA ladder ;,,,, P K15 D (intermediate filament, IF) D ( Traub et al., 1994) IF P K15 ( Porcine Kidney215),,
48 (1) :58 63, 2002 A cta Zoologica S inica 3 PK15 (, 100871) D P K15 ( Porcrne Kidney215) DNA, DNA ladder ;,,,, P K15 D (intermediate filament, IF) D ( Traub et al., 1994) IF P K15 ( Porcine Kidney215),,
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Expression of a Mutant Lamin A That Causes Emery-Dreifuss Muscular Dystrophy Inhibits In Vitro Differentiation of C2C12 Myoblasts
 REFERENCES CONTENT ALERTS Expression of a Mutant Lamin A That Causes Emery-Dreifuss Muscular Dystrophy Inhibits In Vitro Differentiation of C2C12 Myoblasts Catherine Favreau, Dominique Higuet, Jean-Claude
REFERENCES CONTENT ALERTS Expression of a Mutant Lamin A That Causes Emery-Dreifuss Muscular Dystrophy Inhibits In Vitro Differentiation of C2C12 Myoblasts Catherine Favreau, Dominique Higuet, Jean-Claude
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
The laminopathies: a clinical review
 Clin Genet 2006: 70: 261 274 Printed in Singapore. All rights reserved Review # 2006 The Authors Journal compilation # 2006 Blackwell Munksgaard CLINICAL GENETICS doi: 10.1111/j.1399-0004.2006.00677.x
Clin Genet 2006: 70: 261 274 Printed in Singapore. All rights reserved Review # 2006 The Authors Journal compilation # 2006 Blackwell Munksgaard CLINICAL GENETICS doi: 10.1111/j.1399-0004.2006.00677.x
For the rapid, sensitive and accurate measurement of apoptosis in various samples.
 ab14082 500X Annexin V-FITC Apoptosis Detection Reagent Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples. This product is for research use only and
ab14082 500X Annexin V-FITC Apoptosis Detection Reagent Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples. This product is for research use only and
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
s u p p l e m e n ta ry i n f o r m at i o n
 Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria syndrome
 JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Figure 1.
 Supplementary Figure 1. Visualization of endoplasmic reticulum-mitochondria interaction by in situ proximity ligation assay. A) Illustration of targeted proteins in mitochondria (M), endoplasmic reticulum
Supplementary Figure 1. Visualization of endoplasmic reticulum-mitochondria interaction by in situ proximity ligation assay. A) Illustration of targeted proteins in mitochondria (M), endoplasmic reticulum
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism
 FEBS 25589 FEBS Letters 509 (2001) 423^429 Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism Marta Columbaro
FEBS 25589 FEBS Letters 509 (2001) 423^429 Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism Marta Columbaro
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
When Lamins Go Bad: Nuclear Structure and Disease
 Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Embryonic Stem Cells Characterization Series
 Embryonic Stem Cells Characterization Series Lamin A/C Expression Is a Marker of Mouse and Human Embryonic Stem Cell Differentiation DAN CONSTANTINESCU, a HEATHER L. GRAY, b PAUL J. SAMMAK, b GERALD P.
Embryonic Stem Cells Characterization Series Lamin A/C Expression Is a Marker of Mouse and Human Embryonic Stem Cell Differentiation DAN CONSTANTINESCU, a HEATHER L. GRAY, b PAUL J. SAMMAK, b GERALD P.
RayBio Annexin V-FITC Apoptosis Detection Kit
 RayBio Annexin V-FITC Apoptosis Detection Kit User Manual Version 1.0 May 25, 2014 (Cat#: 68FT-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
RayBio Annexin V-FITC Apoptosis Detection Kit User Manual Version 1.0 May 25, 2014 (Cat#: 68FT-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Superior Fluorescent Labeling Dyes Spanning the Full Visible Spectrum...1. Trademarks: HiLyte Fluor (AnaSpec, Inc.)
 Table of Contents Fluor TM Labeling Dyes Superior Fluorescent Labeling Dyes Spanning the Full Visible Spectrum....1 Fluor TM 405 Dye, an Excellent Alternative to Alexa Fluor 405 & DyLight 405....2 Fluor
Table of Contents Fluor TM Labeling Dyes Superior Fluorescent Labeling Dyes Spanning the Full Visible Spectrum....1 Fluor TM 405 Dye, an Excellent Alternative to Alexa Fluor 405 & DyLight 405....2 Fluor
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Supplementary Material
 Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Lamina-associated polypeptide 2α binds intranuclear A-type lamins
 Journal of Cell Science 113, 3473-3484 (2000) Printed in Great Britain The Company of Biologists Limited 2000 JCS1581 3473 Lamina-associated polypeptide 2α binds intranuclear A-type lamins Thomas Dechat
Journal of Cell Science 113, 3473-3484 (2000) Printed in Great Britain The Company of Biologists Limited 2000 JCS1581 3473 Lamina-associated polypeptide 2α binds intranuclear A-type lamins Thomas Dechat
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Table S1. Primers and fluorescent probes used for qrt-pcr analysis of relative expression levels of PPP family phosphatases. gene name forward primer, 5-3 probe, 5-3 reverse primer,
SUPPLEMENTARY MATERIAL Table S1. Primers and fluorescent probes used for qrt-pcr analysis of relative expression levels of PPP family phosphatases. gene name forward primer, 5-3 probe, 5-3 reverse primer,
Annexin V-PE Apoptosis Detection Kit
 Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
For the rapid, sensitive and accurate quantification of Ras in various samples
 ab128504 Ras Assay Kit Instructions for Use For the rapid, sensitive and accurate quantification of Ras in various samples This product is for research use only and is not intended for diagnostic use.
ab128504 Ras Assay Kit Instructions for Use For the rapid, sensitive and accurate quantification of Ras in various samples This product is for research use only and is not intended for diagnostic use.
RayBio Annexin V-Cy5 Apoptosis Detection Kit
 RayBio Annexin V-Cy5 Apoptosis Detection Kit User Manual Version 1.0 Mar 20, 2014 (Cat#: 68C5-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
RayBio Annexin V-Cy5 Apoptosis Detection Kit User Manual Version 1.0 Mar 20, 2014 (Cat#: 68C5-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
