Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells
|
|
|
- Ellen Craig
- 6 years ago
- Views:
Transcription
1 1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute of Molecular Genetics, IGM-CNR, Unit of Bologna c/o IOR, Via di Barbiano 1/10, I Bologna, Italy Abstract Prelamin A is the precursor protein of lamin A, a major constituent of the nuclear lamina in higher eukaryotes. Increasing attention to prelamin A processing and function has been given after the discovery, from 2002 to 2004, of diseases caused by prelamin A accumulation. These diseases, belonging to the group of laminopathies and mostly featuring LMNA mutations, are characterized, at the clinical level, by different degrees of accelerated aging, and adipose tissue, skin and bone abnormalities. The outcome of studies conducted in the last few years consists of three major findings. First, prelamin A is processed at different rates under physiological conditions depending on the differentiation state of the cell. This means that, for instance, in muscle cells, prelamin A itself plays a biological role, besides production of mature lamin A. Secondly, prelamin A post-translational modifications give rise to different processing intermediates, which elicit different effects in the nucleus, mostly by modification of the chromatin arrangement. Thirdly, there is a threshold of toxicity, especially of the farnesylated form of prelamin A, whose accumulation is obviously linked to cell and organism senescence. The present review is focused on prelamin A-mediated nuclear envelope modifications that are upstream of chromatin dynamics and gene expression mechanisms regulated by the lamin A precursor. Introduction The LMNA gene (OMIM ), encoding the A-type lamins, was characterized in 1993 [1] and subsequently mapped to chromosome 1q21.2 q21.3 [2]. The discovery of mutations in emerin [3] and lamin A/C [4] in 1994 and 1999 respectively, as the cause of EDMD (Emery Dreifuss muscular dystrophy), brought to the attention of researchers the nuclear envelope as a structure implicated in fundamental mechanisms of tissue homoeostasis. A few years later, FPLD2 (Dunnigan-type familial partial lipodystrophy) [5] and a form of Charcot Marie Tooth neuropathy [6] were associated with lamin A/C mutations, widening the spectrum of LMNA-linked diseases, currently referred to as laminopathies. A key discovery occurred in 2002, when the homozygous R527H-coding mutation in LMNA was identified as the cause of MADA (mandibuloacral dysplasia with type A lipodystrophy), a disease featuring accelerated aging [7]. This finding suggested that lamin A/C may regulate cellular and organism senescence. In the Key words: emerin, Emery Dreifuss muscular dystrophy (EDMD), prelamin A, progeroid laminopathy, SUN1, SUN2. Abbreviations used: AFCMe, N-acetyl-S-farnesyl-l-cysteine methyl ester; BAF, barrier to autointegration factor; EDMD, Emery Dreifuss muscular dystrophy; EDMD1, X-linked EDMD; FPLD2, Dunnigan-type familial partial lipodystrophy; FTI, farnesyltransferase inhibitor; HGPS, Hutchinson Gilford progeria syndrome; HP1α, heterochromatin protein 1α; ICMT, isoprenylcysteine carboxymethyltransferase; LAP2α, lamina-associated polypeptide 2α; LINC, linker of nucleoskeleton and cytoskeleton; MADA, mandibuloacral dysplasia with type A lipodystrophy; MADB, mandibuloacral dysplasia with type B lipodystrophy; NARF, nuclear prelamin A recognition factor; PPARγ, peroxisome-proliferator-activated receptor γ ; RCE1, Rasconverting enzyme 1; RD, restrictive dermopathy; SREBP1, sterol-regulatory-element-binding protein 1; ZMPSTE24, zinc-dependent metalloproteinase Ste24 homologue. 1 giovanna.lattanzi@cnr.it following 2 years, MADB (mandibuloacral dysplasia with type B lipodystrophy) [8] and RD (restrictive dermopathy) [9] were linked to mutations in the prelamin A endoprotease ZMPSTE24 (zinc-dependent metalloproteinase Ste24 homologue), and HGPS (Hutchinson Gilford progeria syndrome) was associated with an aberrant splicing of the LMNA gene [10,11], giving rise to a truncated form of farnesylated prelamin A. Prelamin A accumulation was found in all of the progeroid laminopathies, and it is now established that diseases associated with prelamin A accumulation feature various degrees of adipose tissue, skin and bone defects, and accelerated aging. How prelamin A might act in these disorders, by modifying nuclear envelope dynamics, is discussed in the present review. The LMNA gene The LMNA gene encoding the A-type lamins is located on chromosome 1q21.2 q21.3 and spans 12 exons [1,2]. The mrna is alternatively spliced into four transcripts, which encode lamin A, lamin C, lamin A 10 and lamin C2 [12]. Most differentiated cells express lamin A and lamin C, whereas lamin C2 is mostly found in germinal cells and lamin A 10 expression has so far been elusive. Lamin A Lamin A is the most represented A-type lamin, obtained after post-translational processing of its precursor protein, called prelamin A [13]. Recent studies have shown that lamin A Biochem. Soc. Trans. (2011) 39, ; doi: /bst
2 Nuclear Envelope Disease and Chromatin Organization 1699 is not expressed in ipscs (induced pluripotent stem cells) and it is also absent from neural progenitors [14], a finding of utmost importance for the understanding of lamin-linked diseases. Lamin C Lamin C is expressed in most tissues expressing lamin A and co-polymerizes with lamin A at the nuclear lamina [15]. However, differences in the relative expression of lamin A compared with lamin C are observed by Western blot analysis, which could reflect either different stability of the alternatively spliced products, depending on the cell type or different solubility. Our recent data show that lamin C is much less prone to lysosomal degradation with respect to lamin A [16]. Both lamin A and lamin C can be also found in the nucleoplasm, probably in a different polymerization state. The function of lamin C is considered overlapping and redundant with lamin A [17], although a deep understanding of the role of lamin C is still lacking. Prelamin A Whereas lamin C is produced as mature protein, lamin A is translated as a 74 kda precursor, which undergoes four steps of post-translational modifications, including farnesylation, double endoprotease cleavage and carboxymethylation [18]. These modifications occur at the C-terminal CaaX motif (where a is an aliphatic residue), a sequence shared by farnesylated proteins, in which C is cysteine, the target of protein farnesyl transferase which catalyses prelamin A farnesylation. In human prelamin A, the aax sequence consists of a serine, an isoleucine and a methionine residue (SIM motif), and the methionine residue directs the addition of the C 15 farnesyl residue to cysteine. Following farnesylation, the aax tripeptide is cleaved by ZMPSTE24 or RCE1 (Ras-converting enzyme 1) and the C-terminal cysteine residue is carboxymethylated by ICMT (isoprenylcysteine carboxymethyltransferase). The second ZMPSTE24-mediated cleavage of 15 amino acids at the C- terminus of prelamin A leads to removal of the farnesyl residue and yields mature lamin A. The first cleavage step of prelamin A is zinc-dependent, whereas the second appears to be independent of zinc concentrations. This feature allows us to use different inhibitors to block prelamin A processing and obtain different processing intermediates. For instance, o-phenanthroline, a zinc chelator, can be used to accumulate full-length farnesylated prelamin A in cells, whereas AFCMe (N-acetyl-S-farnesyl-L-cysteine methyl ester) [19], a non-competitor peptide, can be used to accumulate carboxymethylated farnesylated prelamin A. FTIs (farnesyltransferase inhibitors) and statins, including mevinolin, can be used to accumulate unprocessed (nonfarnesylated) prelamin A [19]. Prelamin A processing is altered in laminopathies featuring premature aging and/or lipodystrophy, including HGPS, atypical Werner syndrome, RD, FPLD2 and MADA, as well as in MADB, associated with mutations of the ZMPSTE24 endoprotease gene [18]. Prelamin A partners Prelamin A partners can be split into two groups: enzymes that catalyse and regulate prelamin A maturation, and molecules that interact with prelamin A and modify nuclear envelope/chromatin dynamics. In the first group are the protein farnesyltransferases, which farnesylate the newly transcribed full-length protein, ZMPSTE24, which is required for the two cleavage steps of prelamin A processing, and ICMT, which catalyses prelamin A methylation, whose relevance for protein processing and intermolecular interactions is still not well understood [20]. A still open question in prelamin A studies, which deserves investigation, regards the mechanism(s) regulating expression and activity of prelamin A processing enzymes. Besides the zincdependent activity of ZMPSTE24, little is known about signalling pathways and biochemical factors capable of modulating prelamin A binding and catalytic activity by of those enzymes. Another open question is whether prelamin A copolymerizes with other lamins during its maturation process. Delbarre et al. [21] showed that accumulation of progerin, the truncated form of farnesylated prelamin A found in HGPS, affects the nuclear lamina composition, leading to formation of co-polymers of lamin B, lamin A and progerin itself. On the other hand, since the RCE1 endoprotease, which catalyses prelamin B cleavage, can also intervene in the first prelamin A-processing step, it is conceivable that altered prelamin A processing could interfere with prelamin B maturation. However, the polymerization status of prelamin A under physiological conditions is not yet obvious. The first prelamin A-binding partner was identified in 1999 by Barton and Worman [22]. It is a nuclear envelope protein called NARF (nuclear prelamin A recognition factor), which selectively interacts with farnesylated prelamin A [22]. This feature makes it particularly interesting to investigate the fate of NARF in laminopathic cells accumulating farnesylated prelamin A with deleterious effects. Another prelamin A-binding partner was identified in 2002 by the Shackleton group [23]: it is the transcription factor SREBP1 (sterol-regulatory-element-binding protein 1), which regulates adipocyte-specific gene transcription, including transactivation of PPARγ (peroxisome-proliferatoractivated receptor γ ) [23]. In 2005, we discovered that the cleaved active form of SREBP1 binds with high affinity to both farnesylated and non-farnesylated prelamin A and prelamin A influences its nuclear translocation [24]. Importantly, PPARγ levels are reduced when prelamin A sequesters SREBP1 at the nuclear envelope [24,25]. The regulation of PPARγ levels in adipocytes is also dependent on LAP2α (lamina-associated polypeptide 2α), another prelamin A-binding partner, which is localized in the nucleoplasm [26]. It is conceivable that a complex protein platform, including both prelamin A and LAP2α,
3 1700 Biochemical Society Transactions (2011) Volume 39, part 6 might regulate PPARγ activation. An interplay between prelamin A and LAP2α has been also demonstrated in murine myoblasts, where the interaction occurs at the early stages of myogenic differentiation, within a platform also including the heterochromatin constituent HP1α (heterochromatin protein 1α) [27]. Thus not only regulation of transcription factors, but also mechanisms implicated in chromatin remodelling, appear to be associated with prelamin A modulation. In this respect, it is noteworthy that prelamin A is able to bind BAF (barrier to autointegration factor), a DNA-binding protein located both in the cytoplasm and in the nucleus that is recruited to the nuclear membrane by prelamin A interaction [28]. These observations and others that will follow indicate a physiological role of prelamin A in cells that is not limited to localization or modulated production of mature lamin A. Prelamin A-dependent mechanisms The first function ascribed to prelamin A was to direct localization of lamin A to the nuclear periphery, beneath the nuclear envelope [29]. The farnesylated residue at the C-terminus of prelamin A should favour interactions with nuclear membrane constituents, thus allowing the mature lamin A to properly localize. ZMPSTE24 activity has been demonstrated in the nucleus [30], which should support the hypothesis that lamin A maturation occurs at the site where the protein is expected to localize. However, non-farnesylated prelamin A, either obtained by drug treatment of cells or by transfection of unprocessable prelamin A constructs, is able to properly reach the lamina [13], strongly suggesting that the role of the farnesylated prelamin A residue is mostly to mediate functional intermolecular interactions. The first mechanism dependent on prelamin A levels is the above-mentioned regulation of SREBP1 translocation into the nucleus [24]. Such a role in the modulation of transcription factor availability has been also described for lamin A, for instance in the regulation of c-fos levels within the nucleoplasm [31]. In this context, modulation of the amount of prelamin A, at either the transcriptional or the post-transcriptional level, or both, should in turn regulate gene expression. The relevance of prelamin A in the regulation of the transcription factor PPARγ is supported further by data showing that acquired partial lipodystrophy in patients undergoing highly active anti-retroviral therapy is caused by accumulation of farnesylated prelamin A, which in turn down-regulates PPARγ expression [25,32]. Balanced PPARγ levels are relevant not only for adipocyte differentiation, but also for the fate of stem cells that will differentiate into bone precursors or muscle progenitors [33]. Thus the study of PPARγ regulation in laminopathic cells deserves further study and will probably open new therapeutic perspectives, not just for adipose tissue and metabolic laminopathies. In muscle cells, prelamin A regulates expression of caveolin 3, a membrane protein induced in differentiated myoblasts and located at the sarcolemma of muscle fibres [27]. Other proteins, including troponin T, are also modulated in mouse muscle in response to prelamin A increase, thus suggesting that prelamin A could act indirectly through regulation of upstream genes or large-scale chromatin remodelling. In human myoblasts, prelamin A regulates the expression level of SUN1, a nuclear envelope protein, which is part of the so-called LINC (linker of nucleoskeleton and cytoskeleton) complex [34]. LINC proteins, also including lamins and nesprins, play a key role in nucleocytoplasmic interplay, mostly by mediating the nucleoskeleton connection to the cytoskeleton. SUN1 is required for nuclear positioning and, in Dictyostelium, has been shown to connect centrosomes to centromere clusters [35]. The role of prelamin A in the modulation of SUN1 is particularly relevant. The prelamin A form implicated in this function is the farnesylated and carboxymethylated protein [36]. Non-farnesylated prelamin A, in contrast, down-regulates SUN1 mrna and protein levels [34]. This mechanism is regulated not only at the transcriptional level, but also through stabilization of the protein at the nuclear envelope. The key role of the prelamin A SUN1 interaction in muscle cells resides in nuclear positioning within myotubes and muscle fibres. Lack of farnesylated prelamin A in muscle causes myonuclear clustering and misshapen nuclei, two events which could be related to each other, since both of them are likely to involve centrosome regulation [34]. Another key nuclear membrane protein whose localization and function appears to be dependent on prelamin A is emerin [37]. Emerin binds both non-farnesylated and farnesylated prelamin A, but is only required for the localization of the unprocessed form. Lack of interplay with emerin causes disorganization of the nuclear lamina, and prelamin A shows a honeycomb appearance when labelled by fluorescent antibodies [37]. Our results show that honeycomb structures correspond to sites where the lamina is deeply affected, whereas the whole arrangement of the nuclear membrane is substantially preserved. We suggest that prelamin A emerin interplay is required for the proper assemblage of the nuclear lamina in G 1 -phase cells. This is supported by the observation that the highest frequency of honeycomb structures in cells bearing LMNA or emerin mutations is found in G 1 -phase cells. Provided that emerin may form at least six distinct multiprotein complexes at the nuclear envelope [38], it remains to be established in which of them prelamin A takes part. The interplay between prelamin A and BAF [28], which clearly influences BAF localization, is particularly intriguing. Since BAF is a chromatin-binding protein, the main significance of its interplay with the lamin A precursor resides in the regulation of chromatin arrangement, which is probably the best recognized function of prelamin A. It is conceivable that different prelamin A levels could determine the amount of BAF recruited to the nuclear envelope, thus limiting its chromatin interaction [28]. We have shown that all of the prelamin A-processing intermediates may influence chromatin dynamics in a very specific and reproducible way [13]. Non-farnesylated prelamin A localizes at the nuclear periphery as well as in intranuclear aggregates, where
4 Nuclear Envelope Disease and Chromatin Organization 1701 it recruits heterochromatin, as demonstrated by staining HP1α and trimethylated histone H3 (Lys 9 ). Farnesylated carboxymethylated prelamin A does not bind HP1α, but causes loss of heterochromatin domains at the nuclear periphery. LAP2α is part of a complex that contains nonfarnesylated prelamin A and HP1α [13], which is consistent with the possibility that a protein platform including prelamin A and its nucleoplasmic binding partner might regulate chromatin dynamics in stem cells [39], with the aim to direct cell fate. In fact, modulation of chromatin remodelling by prelamin A could be a fine tool to modify expression of large groups of genes, as occurs during development, cell differentiation or aging. This hypothesis is strongly supported by data obtained in the study of syndromic laminopathies and lipodystrophies, which are characterized by prelamin A accumulation [18,40 42]. Prelamin A and disease Laminopathies featuring prelamin A accumulation Prelamin A accumulation is the hallmark of progeroid laminopathies. MADA and MADB and atypical Werner syndrome cells accumulate prelamin A bearing point mutations or wild-type prelamin A, as in the case of MADB [17]. In most cases of HGPS, a truncated prelamin A form, called progerin, is accumulated [43]. Moreover, wild-type prelamin A is recovered, instead of lamin A, in RD, a lethal developmental disorder, where null mutations of ZMPSTE24 impair lamin A maturation [44]. Prelamin A is also accumulated in lipodystrophies, either caused by LMNA missense mutations [24,32,40,45] or by drugs containing protease inhibitors, which alter ZMPSTE24 functionality [24,45]. Finally, statins, which are used worldwide to treat hypercholesterolaemia, bisphosphonates, widely used in osteoporosis treatment, and FTIs, which are used in chemotherapy of tumours, induce accumulation of unprocessed prelamin A in patients [18]. The toxicity of unprocessed prelamin A is obviously low, as suggested by the safety and efficacy of statins in the general population. Nevertheless, studies performed in animal models and clinical data show that accumulation of mutated non-farnesylated prelamin A may cause cardiomyopathy or lipodystrophy [20,46]. At the molecular level, things are a bit more complicated. Non-farnesylated (unprocessed) prelamin A accumulates in intranuclear aggregates and recruits heterochromatin [13]. However, our studies indicate that this prelamin A form is maintained at non-toxic levels in cells through scavenging mechanisms [47]. The toxicity of farnesylated prelamin A is well established. This means that, whereas proper levels of this protein isoform are required for normal activity of nuclei, as demonstrated in adipocytes and myoblasts [24,34], an increase in farnesylated prelamin A has deleterious effects. The reasons for such a toxicity are not completely understood. However, several aspects have been defined in recent years. The present review focuses on modifications of the nuclear envelope that occur in laminopathies featuring prelamin A accumulation. The first and best recognized defect caused by farnesylated prelamin A accumulation is nuclear envelope enlargement and misshaping, which can be detected using any antilamin antibodies or using antibodies directed against integral membrane proteins [13,19]. At the ultrastructural level, nuclear envelope invaginations can be observed both in cells treated with drugs that induce farnesylated prelamin A accumulation (AFCMe) [19] and in HGPS, MADA or RD fibroblasts [43,44,48]. Since enlargement of the nuclear membrane has been also reported in nuclei overexpressing B- type lamins [49], which are farnesylated, it is obvious that the farnesyl residue mediates such an effect. However, prelamin A partners involved in nuclear envelope modification have not been identified. The second effect of farnesylated prelamin A accumulation in laminopathic cells is nuclear lamina thickening. This aspect is particularly represented in MADA [48] and HGPS nuclei [43], whereas it is rarely observed in RD cells. We hypothesize that thickening of the lamina also requires mature lamin A, which is not produced in RD. Honeycomb appearance of the nuclear envelope is observed in MADA using antiemerin [48] (Figure 1) or anti-sun2 (D. Camozzi, M.R. D Apice, E. Schena, V. Cenni, M. Columbaro, C. Capanni, N.M. Maraldi, S. Squarzoni, M. Ortolani, G. Novelli and G. Lattanzi, unpublished work) antibodies. In nuclei showing emerin defects, prelamin A shows a distinct staining pattern [48], indicating an indirect effect of mutated prelamin A. Failure of lamin A and/or prelamin A to bind emerin could cause altered nuclear envelope/lamina organization, as shown in FPLD2 cells [50]. At the ultrastructural examination, the altered organization of the nuclear membrane proteins does not correspond to nuclear envelope holes [48]. It is conceivable that farnesylated prelamin A interferes with binding of emerin or SUN2 to nuclear lamina constituents, including mature lamin A, which are necessary for proper targeting of those nuclear membrane constituents. Emerin localization is HGPS cells overlaps with progerin, but honeycomb structures are not observed, although nuclear blebs are formed [37,43]. These aspects deserve further investigation. Importantly, SUN1, another nuclear envelope constituent, is increased in HGPS nuclei featuring accumulation of both progerin and wild-type prelamin A [38]. In fact, whereas accumulation of progerin lowers SUN1 levels, as seen in Western blot analysis, accumulation of wild-type farnesylated prelamin A, as occurs in a high percentage of late-passage HGPS fibroblast nuclei, up-regulates SUN1 [38], suggesting that the 50 amino acids lacking in the progerin sequence are necessary for prelamin A-dependent up-regulation and/or anchorage of SUN1. Emery Dreifuss muscular dystrophy On the other hand, prelamin A interplay with emerin is also important for laminopathies affecting skeletal muscle. In
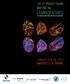
5 1702 Biochemical Society Transactions (2011) Volume 39, part 6 Figure 1 Emerin organization is altered in MADA nuclei Prelamin A [detected by anti-(prelamin A) Sc-6214 antibody and FITC-conjugated secondary antibody, green] forms intranuclear aggregates. Emerin [detected by anti-emerin antibody from Monosan and TRITC (tetramethylrhodamine β-isothiocyanate)-conjugated secondary antibody, red] shows a honeycomb distribution in the nuclear envelope of the enlarged MADA nucleus (arrowhead). Nuclear blebs are observed in MADA cells. Control, control fibroblast nuclei, MADA, MADA fibroblast nuclei. DAPI (4,6-diamidino-2-phenylindole) was used to stain DNA. Scale bar, 10 μm. homoeostasis and aging. Understanding how prelamin A levels are regulated in normal cells, either through modulation of the processing rate or through degradation mechanisms, will represent an important advance in lamin A research and will open new perspectives for the therapy of laminopathies. Acknowledgements The scientific work of Cristina Capanni, Marta Columbaro, Elisabetta Mattioli, Vittoria Cenni and Stefano Squarzoni is gratefully acknowledged. Manfred Wehnert, Giuseppe Novelli, Nicolas Levy, Luciano Merlini, patients and their families are greatfully acknowledged for providing cell samples used in these studies. Funding This work was supported by grants from the Associazione Italiana Progeria Sammy Basso (A.I.Pro.Sa.B.), Italy, the Italian Ministero dell Istruzione, dell Università e della Ricerca Scientifica (MIUR) Progetti di Ricerca di Interesse Nazionale (PRIN) 2008 (to G.L.), Fondo Investimenti Ricerca di Base (FIRB) MIUR 2010 and Fondazione Carisbo, Italy. fact, the absence of emerin, as occurs in cells from EDMD1 (X-linked EDMD) causes mislocalization of unprocessable non-farnesylated prelamin A, a defect rescued by emerin expression [37]. Thus emerin is, in turn, necessary for fulllength prelamin A localization. How altered localization of full-length prelamin A, which is normally processed in EDMD1, affects the disease, remains to be established. We showed recently that SUN1 levels are up-regulated by farnesylated prelamin A, which accumulates in muscle cells, where prelamin A further anchors SUN1 to the nuclear envelope [34]. Recruitment of SUN1 in myotubes is necessary for proper positioning of myonuclei [51]. Thus reduced levels of farnesylated prelamin A, as observed in EDMD2 (autosomal dominant EDMD), fail to elicit SUN1 up-regulation and causes clustering of nuclei in differentiated muscle [34]. Clustering of muscle nuclei [52] could represent a pathogenetic factor in EDMD. Conclusions Expanding our knowledge of prelamin A function under normal or pathological conditions has brought to scientists attention a major role of prelamin A nuclear membrane protein interplay. Membrane proteins so far characterized for their interaction with prelamin A are NARF, emerin, SUN1 and BAF. Importantly, BAF mutations have been recently associated with a form of progeria showing severely impaired bone development [53]. The latter finding supports the hypothesis that platforms containing prelamin A, nuclear membrane proteins and chromatin-binding molecules regulate major mechanisms of human development, tissue References 1 Lin, F. and Worman, H.J. (1993) Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, Wydner, K.L., McNeil, J.A., Lin, F., Worman, H.J. and Lawrence, J.B. (1996) Chromosomal assignment of human nuclear envelope protein genes LMNA, LMNB1, and LBR by fluorescence in situ hybridization. Genomics 32, Bione, S., Maestrini, E., Rivella, S., Mancini, M., Regis, S., Romeo, G. and Toniolo, D. (1994) Identification of a novel X-linked gene responsible for Emery Dreifuss muscular dystrophy. Nat. Genet. 8, Bonne, G., Di Barletta, M.R., Varnous, S., Becane, H.M., Hammouda, E.H., Merlini, L., Muntoni, F., Greenberg, C.R., Gary, F., Urtizberea, J.A. et al. (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery Dreifuss muscular dystrophy. Nat. Genet. 21, Shackleton, S., Lloyd, D.J., Jackson, S.N., Evans, R., Niermeijer, M.F., Singh, B.M., Schmidt, H., Brabant, G., Kumar, S., Durrington, P.N. et al. (2000) LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat. Genet. 24, De Sandre-Giovannoli, A., Chaouch, M., Kozlov, S., Vallat, J.M., Tazir, M., Kassouri, N., Szepetowski, P., Hammadouche, T., Vandenberghe, A., Stewart, C.L. et al. (2002) Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot Marie Tooth disorder type 2) and mouse. Am. J. Hum. Genet. 70, Novelli, G., Muchir, A., Sangiuolo, F., Helbling-Leclerc, A., D Apice, M.R., Massart, C., Capon, F., Sbraccia, P., Federici, M., Lauro, R. et al. (2002) Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am. J. Hum. Genet. 71, Agarwal, A.K., Fryns, J.P., Auchus, R.J. and Garg, A. (2003) Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12, Navarro, C.L., De Sandre-Giovannoli, A., Bernard, R., Boccaccio, I., Boyer, A., Genevieve, D., Hadj-Rabia, S., Gaudy-Marqueste, C., Smitt, H.S., Vabres, P. et al. (2004) Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum. Mol. Genet. 13,
6 Nuclear Envelope Disease and Chromatin Organization Eriksson, M., Brown, W.T., Gordon, L.B., Glynn, M.W., Singer, J., Scott, L., Erdos, M.R., Robbins, C.M., Moses, T.Y., Berglund, P. et al. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson Gilford progeria syndrome. Nature 423, De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C.L., Munnich, A., Le Merrer, M. and Levy, N. (2003) Lamin A truncation in Hutchinson Gilford progeria. Science 300, Maraldi, N.M. and Lattanzi, G. (2005) Linkage of lamins to fidelity of gene transcription. Crit. Rev. Eukaryotic Gene Expression 15, Lattanzi, G., Columbaro, M., Mattioli, E., Cenni, V., Camozzi, D., Wehnert, M., Santi, S., Riccio, M., Del Coco, R., Maraldi, N.M. et al. (2007) Pre-lamin A processing is linked to heterochromatin organization. J. Cell. Biochem. 102, Zhang, J., Lian, Q., Zhu, G., Zhou, F., Sui, L., Tan, C., Mutalif, R.A., Navasankari, R., Zhang, Y., Tse, H.F. et al. (2011) A human ipsc model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, Moir, R.D., Quinlan, R.A. and Stewart, M. (1990) Expression and characterization of human lamin C. FEBS Lett. 268, Cenni, V., Capanni, C., Columbaro, M., Ortolani, M., D Apice, M.R., Novelli, G., Fini, M., Marmiroli, E., Scarano, N.M., Maraldi, S. et al. (2011) Autophagic degradation of famesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur. J. Histochem., doi: /ejh.2011.e36 17 Fong, L.G., Ng, J.K., Lammerding, J., Vickers, T.A., Meta, M., Cote, N., Gavino, B., Qiao, X., Chang, S.Y., Young, S.R. et al. (2006) Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Invest. 116, Maraldi, N.M. and Lattanzi, G. (2007) Involvement of prelamin A in laminopathies. Crit. Rev. Eukaryotic Gene Expression 17, Mattioli, E., Columbaro, M., Capanni, C., Santi, S., Maraldi, N.M., D Apice, M.R., Novelli, G., Riccio, M., Squarzoni, S., Foisner, R. and Lattanzi, G. (2008) Drugs affecting prelamin A processing: effects on heterochromatin organization. Exp. Cell Res. 314, Davies, B.S., Coffinier, C., Yang, S.H., Barnes, R.H., 2nd, Jung, H.J., Young, S.G. and Fong, L.G. (2011) Investigating the purpose of prelamin A processing. Nucleus 2, Delbarre, E., Tramier, M., Coppey-Moisan, M., Gaillard, C., Courvalin, J.C. and Buendia, B. (2006) The truncated prelamin A in Hutchinson Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum. Mol. Genet. 15, Barton, R.M. and Worman, H.J. (1999) Prenylated prelamin A interacts with Narf, a novel nuclear protein. J. Biol. Chem. 274, Lloyd, D.J., Trembath, R.C. and Shackleton, S. (2002) A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum. Mol. Genet. 11, Capanni, C., Mattioli, E., Columbaro, M., Lucarelli, E., Parnaik, V.K., Novelli, G., Wehnert, M., Cenni, V., Maraldi, N.M., Squarzoni, S. and Lattanzi, G. (2005) Altered pre-lamin A processing is a common mechanism leading to lipodystrophy. Hum. Mol. Genet. 14, Caron, M., Auclair, M., Sterlingot, H., Kornprobst, M. and Capeau, J. (2003) Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS 17, Dorner, D., Vlcek, S., Foeger, N., Gajewski, A., Makolm, C., Gotzmann, J., Hutchison, C.J. and Foisner, R. (2006) Lamina-associated polypeptide 2α regulates cell cycle progression and differentiation via the retinoblastoma E2F pathway. J. Cell Biol. 173, Capanni, C., Del Coco, R., Squarzoni, S., Columbaro, M., Mattioli, E., Camozzi, D., Rocchi, A., Scotlandi, K., Maraldi, N., Foisner, R. and Lattanzi, G. (2008) Prelamin A is involved in early steps of muscle differentiation. Exp. Cell Res. 314, Capanni, C., Cenni, V., Haraguchi, T., Squarzoni, S., Schüchner, S., Ogris, E., Novelli, G., Maraldi, N.M. and Lattanzi, G. (2010) Lamin A precursor induces barrier-to-autointegration factor nuclear localization. Cell Cycle 9, Lutz, R.J., Trujillo, M.A., Denham, K.S., Wenger, L. and Sinensky, M. (1992) Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc. Natl. Acad. Sci. U.S.A. 89, Barrowman, J., Hamblet, C., George, C.M. and Michaelis, S. (2008) Analysis of prelamin A biogenesis reveals the nucleus to be a CaaX processing compartment. Mol. Biol. Cell 19, Gonzalez, J.M., Navarro-Puche, A., Casar, B., Crespo, P. and Andres, V. (2008) Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-fos at the nuclear envelope. J. Cell Biol. 183, Caron, M., Auclair, M., Donadille, B., Bereziat, V., Guerci, B., Laville, M., Narbonne, H., Bodemer, C., Lascols, O., Capeau, J. and Vigouroux, C. (2007) Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ. 14, Kawai, M. and Rosen, C.J. (2011) PPARγ : a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 6, Mattioli, E., Columbaro, M., Capanni, C., Maraldi, N.M., Cenni, V., Scotlandi, K., Marino, M.T., Merlini, L., Squarzoni, S. and Lattanzi, G. (2011) Prelamin A-mediated recruitment of SUN1 to the nuclear envelope directs nuclear positioning in human muscle. Cell Death Differ. 18, Xiong, H., Rivero, F., Euteneuer, U., Mondal, S., Mana-Capelli, S., Larochelle, D., Vogel, A., Gassen, B. and Noegel, A.A. (2008) Dictyostelium Sun-1 connects the centrosome to chromatin and ensures genome stability. Traffic 9, Haque, F., Mazzeo, D., Patel, J.T., Smallwood, D.T., Ellis, J.A., Shanahan, C.M. and Shackleton, S. (2010) Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J. Biol. Chem. 285, Capanni, C., Del Coco, R., Mattioli, E., Camozzi, D., Columbaro, M., Schena, E., Merlini, L., Squarzoni, S., Maraldi, N.M. and Lattanzi, G. (2009) Emerin prelamin A interplay in human fibroblasts. Biol. Cell 101, Holaska, J.M. and Wilson, K.L. (2007) An emerin proteome : purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mrna splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry 46, Naetar, N., Korbei, B., Kozlov, S., Kerenyi, M.A., Dorner, D., Kral, R., Gotic, I., Fuchs, P., Cohen, T.V., Bittner, R. et al. (2008) Loss of nucleoplasmic LAP2α lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat. Cell Biol. 10, Araujo-Vilar, D., Lattanzi, G., Gonzalez-Mendez, B., Costa-Freitas, A.T., Prieto, D., Columbaro, M., Mattioli, E., Victoria, B., Martinez-Sanchez, N., Ramazanova, A. et al. (2009) Site-dependent differences in both prelamin A and adipogenic genes in subcutaneous adipose tissue of patients with type 2 familial partial lipodystrophy. J. Med. Genet. 46, Columbaro, M., Capanni, C., Mattioli, E., Novelli, G., Parnaik, V.K., Squarzoni, S., Maraldi, N.M. and Lattanzi, G. (2005) Rescue of heterochromatin organization in Hutchinson Gilford progeria by drug treatment. Cell. Mol. Life Sci. 62, Bruston, F., Delbarre, E., Ostlund, C., Worman, H.J., Buendia, B. and Duband-Goulet, I. (2010) Loss of a DNA binding site within the tail of prelamin A contributes to altered heterochromatin anchorage by progerin. FEBS Lett. 584, Columbaro, M., Capanni, C., Mattioli, E., Novelli, G., Parnaik, V.K., Squarzoni, S., Maraldi, N.M. and Lattanzi, G. (2005) Rescue of heterochromatin organization in Hutchinson Gilford progeria by drug treatment. Cell. Mol. Life Sci. 62, Columbaro, M., Mattioli, E., Schena, E., Capanni, C., Cenni, V., Levy, N., Navarro, C.L., Del Coco, R., Squarzoni, S., Camozzi, D. et al. (2010) Prelamin A processing and functional effects in restrictive dermopathy. Cell Cycle 9, Caron-Debarle, M., Lagathu, C., Boccara, F., Vigouroux, C. and Capeau, J. (2010) HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol. Med. 16, Le Dour, C., Schneebeli, S., Bakiri, F., Darcel, F., Jacquemont, M.L., Maubert, M.A., Auclair, M., Jeziorowska, D., Reznik, Y., Bereziat, V. et al. (2011) A homozygous mutation of prelamin-a preventing its farnesylation and maturation leads to a severe lipodystrophic phenotype: new insights into the pathogenicity of nonfarnesylated prelamin-a. J. Clin. Endocrinol. Metab. 96, E856 E Marmiroli, S., Bertacchini, J., Beretti, F., Cenni, V., Guida, M., De Pol, A., Maraldi, N.M. and Lattanzi, G. (2009) A-type lamins and signaling: the PI 3-kinase/Akt pathway moves forward. J. Cell. Physiol. 220, Filesi, I., Gullotta, F., Lattanzi, G., D Apice, M.R., Capanni, C., Nardone, A.M., Columbaro, M., Scarano, G., Mattioli, E., Sabatelli, P. et al. (2005) Alterations of nuclear envelope and chromatin organization in mandibuloacral dysplasia, a rare form of laminopathy. Physiol. Genomics 23,
7 1704 Biochemical Society Transactions (2011) Volume 39, part 6 49 Prufert, K., Vogel, A. and Krohne, G. (2004) The lamin CxxM motif promotes nuclear membrane growth. J. Cell Sci. 117, Capanni, C., Cenni, V., Mattioli, E., Sabatelli, P., Ognibene, A., Columbaro, M., Parnaik, V.K., Wehnert, M., Maraldi, N.M., Squarzoni, S. and Lattanzi, G. (2003) Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy fibroblasts: altered intermolecular interaction with emerin and implications for gene transcription. Exp. Cell Res. 291, Lei, K., Zhang, X., Ding, X., Guo, X., Chen, M., Zhu, B., Xu, T., Zhuang, Y., Xu, R. and Han, M. (2009) SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl. Acad. Sci. U.S.A. 106, Mejat, A., Decostre, V., Li, J., Renou, L., Kesari, A., Hantai, D., Stewart, C.L., Xiao, X., Hoffman, E., Bonne, G. and Misteli, T. (2009) Lamin A/C-mediated neuromuscular junction defects in Emery Dreifuss muscular dystrophy. J. Cell Biol. 184, Puente, X.S., Quesada, V., Osorio, F.G., Cabanillas, R., Cadinanos, J., Fraile, J.M., Ordonez, G.R., Puente, D.A., Gutierrez-Fernandez, A., Fanjul-Fernandez, M. et al. (2011) Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am. J. Hum. Genet. 88, Received 14 July 2011 doi: /bst
Non-commercial use only
 European Journal of Histochemistry 2011; volume 55:e36 Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria V. Cenni, 1 C. Capanni, 1 M. Columbaro, 2 M.
European Journal of Histochemistry 2011; volume 55:e36 Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria V. Cenni, 1 C. Capanni, 1 M. Columbaro, 2 M.
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
Keywords: BAF, BANF1, prelamin A, lamin A/C, laminopathies, emerin, EDMD1. Introduction
 Report Cell Cycle 11:19, 3568-3577; October 1, 2012; 2012 Landes Bioscience Familial partial lipodystrophy, mandibuloacral dysplasia and restrictive dermopathy feature barrier-to-autointegration factor
Report Cell Cycle 11:19, 3568-3577; October 1, 2012; 2012 Landes Bioscience Familial partial lipodystrophy, mandibuloacral dysplasia and restrictive dermopathy feature barrier-to-autointegration factor
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy
 The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
THURSDAY, JANUARY
 THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies
 Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Laminopathies and the long strange trip from basic cell biology to therapy
 Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24
 Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins
 Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
When Lamins Go Bad: Nuclear Structure and Disease
 Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1
 Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Available online at
 Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Importance of molecular cell biology investigations in human medicine in the story of the Hutchinson-Gilford progeria syndrome
 Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome
 Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome Brian C. Capell*, Michael R. Erdos*, James P. Madigan, James J. Fiordalisi, Renee
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome Brian C. Capell*, Michael R. Erdos*, James P. Madigan, James J. Fiordalisi, Renee
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins
 /, Vol. 6, No. 10 Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins Camilla Evangelisti 1, Pia Bernasconi 2, Paola
/, Vol. 6, No. 10 Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins Camilla Evangelisti 1, Pia Bernasconi 2, Paola
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,100 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,100 116,000 120M Open access books available International authors and editors Downloads Our
All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype
 /, Vol. 6, No. 30 All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype Camilla Pellegrini 1,2, Marta Columbaro 2,3, Cristina Capanni 1,2, Maria Rosaria D Apice
/, Vol. 6, No. 30 All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype Camilla Pellegrini 1,2, Marta Columbaro 2,3, Cristina Capanni 1,2, Maria Rosaria D Apice
Nuclear envelopathies: a complex LINC between nuclear envelope and pathology
 Janin et al. Orphanet Journal of Rare Diseases (2017) 12:147 DOI 10.1186/s13023-017-0698-x REVIEW Nuclear envelopathies: a complex LINC between nuclear envelope and pathology Alexandre Janin 1,2,3,4, Delphine
Janin et al. Orphanet Journal of Rare Diseases (2017) 12:147 DOI 10.1186/s13023-017-0698-x REVIEW Nuclear envelopathies: a complex LINC between nuclear envelope and pathology Alexandre Janin 1,2,3,4, Delphine
Journal of Cell Science Accepted manuscript
 2014. Published by The Company of Biologists Ltd. Dysregulated Affinity of Lamin A to SUN1 Induces Nuclear and Endoplasmic Reticulum Aberrancies in Progeric Laminopathies Zi-Jie Chen 1, Wan-Ping Wang 1,
2014. Published by The Company of Biologists Ltd. Dysregulated Affinity of Lamin A to SUN1 Induces Nuclear and Endoplasmic Reticulum Aberrancies in Progeric Laminopathies Zi-Jie Chen 1, Wan-Ping Wang 1,
Laminopathies: A consequence of mechanical stress or gene expression disturbances?
 BIOLOGY AND HEALTH Journal website: www.biologyandhealth.com Narrative review Laminopathies: A consequence of mechanical stress or gene expression disturbances? Alicia Jonker Alicia Jonker I6109457 Maastricht
BIOLOGY AND HEALTH Journal website: www.biologyandhealth.com Narrative review Laminopathies: A consequence of mechanical stress or gene expression disturbances? Alicia Jonker Alicia Jonker I6109457 Maastricht
Reviews. Mechanisms Underlying Caloric Restriction, Lipid Metabolism, and Life Span Regulation Issei Komuro, Guest Editor
 Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
Progeria, the nucleolus and farnesyltransferase inhibitors
 Nuclear Envelope Disease and Chromatin Organization 2009 287 Progeria, the nucleolus and farnesyltransferase inhibitors Ishita S. Mehta*, Joanna M. Bridger* and Ian R. Kill 1 *Laboratory of Nuclear and
Nuclear Envelope Disease and Chromatin Organization 2009 287 Progeria, the nucleolus and farnesyltransferase inhibitors Ishita S. Mehta*, Joanna M. Bridger* and Ian R. Kill 1 *Laboratory of Nuclear and
Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C
 Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Lipodystrophy-Linked LMNA p.r482w Mutation Induces Clinical Early Atherosclerosis and In Vitro Endothelial Dysfunction
 Lipodystrophy-Linked LMNA p.r482w Mutation Induces Clinical Early Atherosclerosis and In Vitro Endothelial Dysfunction Guillaume Bidault, Marie Garcia, Marie-Christine Vantyghem, Pierre-Henri Ducluzeau,
Lipodystrophy-Linked LMNA p.r482w Mutation Induces Clinical Early Atherosclerosis and In Vitro Endothelial Dysfunction Guillaume Bidault, Marie Garcia, Marie-Christine Vantyghem, Pierre-Henri Ducluzeau,
The Nuclear Envelope as a Signaling Node in Development and Disease
 The Nuclear Envelope as a Signaling Node in Development and Disease William T. Dauer 1,2, * and Howard J. Worman 3,4, * 1 Department of Neurology 2 Department of Cell and Developmental Biology University
The Nuclear Envelope as a Signaling Node in Development and Disease William T. Dauer 1,2, * and Howard J. Worman 3,4, * 1 Department of Neurology 2 Department of Cell and Developmental Biology University
HMG Advance Access published August 26, 2005
 HMG Advance Access published August 26, 2005 Glynn & Glover 1 Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase
HMG Advance Access published August 26, 2005 Glynn & Glover 1 Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase
RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria syndrome
 JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
The LMNA gene encodes the A-type lamins, including lamin A
 A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two Chinese siblings: two case reports
 Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Clinical Cell Biology Organelles in Health and Disease
 Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Guilt by Association. Gavin S. Wilkie and Eric C. Schirmer. Review THE NUCLEAR ENVELOPE PROTEOME AND DISEASE*
 Review Guilt by Association THE NUCLEAR ENVELOPE PROTEOME AND DISEASE* Gavin S. Wilkie and Eric C. Schirmer The discovery that many inherited diseases are linked to interacting nuclear envelope proteins
Review Guilt by Association THE NUCLEAR ENVELOPE PROTEOME AND DISEASE* Gavin S. Wilkie and Eric C. Schirmer The discovery that many inherited diseases are linked to interacting nuclear envelope proteins
Genotype phenotype correlations in laminopathies: how does fate translate?
 Nuclear Envelope Disease and Chromatin Organization 2009 257 Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S.
Nuclear Envelope Disease and Chromatin Organization 2009 257 Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S.
H utchinson-gilford progeria syndrome (HGPS; MIM
 609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China
 Luo et al. BMC Pediatrics 2014, 14:256 CASE REPORT Open Access Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China Di-Qing Luo 1*, Xiao-Zhu
Luo et al. BMC Pediatrics 2014, 14:256 CASE REPORT Open Access Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China Di-Qing Luo 1*, Xiao-Zhu
HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells
 HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells Catherine Coffinier*, Sarah E. Hudon, Emily A. Farber*, Sandy Y. Chang*, Christine A.
HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells Catherine Coffinier*, Sarah E. Hudon, Emily A. Farber*, Sandy Y. Chang*, Christine A.
Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation
 Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation Shao H. Yang*, Martin O. Bergo, Julia I. Toth*, Xin Qiao*,
Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation Shao H. Yang*, Martin O. Bergo, Julia I. Toth*, Xin Qiao*,
MODIFIED FINAL ACTIVITY REPORT
 Contract no.: LSHM-CT-2005-018690 Project acronym: Project title: Instrument: Thematic priority: EURO-Laminopathies Nuclear Envelop-linked Rare Human Diseases: From Molecular Pathophysiology towards Clinical
Contract no.: LSHM-CT-2005-018690 Project acronym: Project title: Instrument: Thematic priority: EURO-Laminopathies Nuclear Envelop-linked Rare Human Diseases: From Molecular Pathophysiology towards Clinical
Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization
 Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
T he LMNA gene encodes two nuclear envelope proteins,
 1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A
 available at www.sciencedirect.com www.elsevier.com/locate/yexcr Research Article Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin
available at www.sciencedirect.com www.elsevier.com/locate/yexcr Research Article Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin
Proteins that bind A-type lamins: integrating isolated clues
 Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
The laminopathies: a clinical review
 Clin Genet 2006: 70: 261 274 Printed in Singapore. All rights reserved Review # 2006 The Authors Journal compilation # 2006 Blackwell Munksgaard CLINICAL GENETICS doi: 10.1111/j.1399-0004.2006.00677.x
Clin Genet 2006: 70: 261 274 Printed in Singapore. All rights reserved Review # 2006 The Authors Journal compilation # 2006 Blackwell Munksgaard CLINICAL GENETICS doi: 10.1111/j.1399-0004.2006.00677.x
Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells
 JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
IN the spring of 2007, a 2-year clinical drug trial began at
 Journal of Gerontology: BIOLOGICAL SCIENCES 2008, Vol. 63A, No. 8, 777 787 Copyright 2008 by The Gerontological Society of America Meeting Report Highlights of the 2007 Progeria Research Foundation Scientific
Journal of Gerontology: BIOLOGICAL SCIENCES 2008, Vol. 63A, No. 8, 777 787 Copyright 2008 by The Gerontological Society of America Meeting Report Highlights of the 2007 Progeria Research Foundation Scientific
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Durham E-Theses. The Application of a Statistical Model Investigating Reactive Oxygen Species in Premature Ageing Syndromes MUTER, JOANNE,RUTH
 Durham E-Theses The Application of a Statistical Model Investigating Reactive Oxygen Species in Premature Ageing Syndromes MUTER, JOANNE,RUTH How to cite: MUTER, JOANNE,RUTH (2011) The Application of a
Durham E-Theses The Application of a Statistical Model Investigating Reactive Oxygen Species in Premature Ageing Syndromes MUTER, JOANNE,RUTH How to cite: MUTER, JOANNE,RUTH (2011) The Application of a
HUTCHINSON-GILFORD progeria syndrome (HGPS)
 Journal of Gerontology: BIOLOGICAL SCIENCES 2007, Vol. 62A, No. 1, 3 8 Copyright 2007 by The Gerontological Society of America Perspectives Article Progeria of Stem Cells: Stem Cell Exhaustion in Hutchinson-Gilford
Journal of Gerontology: BIOLOGICAL SCIENCES 2007, Vol. 62A, No. 1, 3 8 Copyright 2007 by The Gerontological Society of America Perspectives Article Progeria of Stem Cells: Stem Cell Exhaustion in Hutchinson-Gilford
Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity
 Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity Jemima Barrowman 1, Patricia A. Wiley 2, Sarah E. Hudon-Miller 2, Christine A. Hrycyna 2 and Susan Michaelis
Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity Jemima Barrowman 1, Patricia A. Wiley 2, Sarah E. Hudon-Miller 2, Christine A. Hrycyna 2 and Susan Michaelis
Inauguraldissertation
 CHARACTERISATION OF THE MOLECULAR LINKS BETWEEN THE NUCLEAR PORE COMPLEX AND THE NUCLEAR LAMINS AND RECONSTITUTION OF THE XENOPUS OOCYTE LAMIN ASSEMBLY IN VITRO Inauguraldissertation zur Erlangung der
CHARACTERISATION OF THE MOLECULAR LINKS BETWEEN THE NUCLEAR PORE COMPLEX AND THE NUCLEAR LAMINS AND RECONSTITUTION OF THE XENOPUS OOCYTE LAMIN ASSEMBLY IN VITRO Inauguraldissertation zur Erlangung der
Lady Davis, Institute for Medical Research, Montreal, Quebec, Canada QC H3T 1E2 2
 PPAR Research Volume 2007, Article ID 81654, 7 pages doi:10.1155/2007/81654 Research Article Inhibition of Protein Farnesylation Arrests Adipogenesis and Affects PPARγ Expression and Activation in Differentiating
PPAR Research Volume 2007, Article ID 81654, 7 pages doi:10.1155/2007/81654 Research Article Inhibition of Protein Farnesylation Arrests Adipogenesis and Affects PPARγ Expression and Activation in Differentiating
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome
 Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging
 382 Biomed Environ Sci, 2013; 26(5): 382-389 Review Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging QI Ying Chun and XIE Xiao Hua # First Department of
382 Biomed Environ Sci, 2013; 26(5): 382-389 Review Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging QI Ying Chun and XIE Xiao Hua # First Department of
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Eiger BioPharmaceuticals Reports on 2018 R&D Day
 Eiger BioPharmaceuticals Reports on 2018 R&D Day Late Stage Rare and Ultra-Rare Disease Pipeline Advancing >$100M in Cash Available to Achieve Key Milestones PALO ALTO, Calif., December 11, 2018 Eiger
Eiger BioPharmaceuticals Reports on 2018 R&D Day Late Stage Rare and Ultra-Rare Disease Pipeline Advancing >$100M in Cash Available to Achieve Key Milestones PALO ALTO, Calif., December 11, 2018 Eiger
L aminopathies represent a heterogeneous group of genetic
 1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
Mutations in LMNA Modulate the Lamin A - Nesprin-2 Interaction and Cause LINC Complex Alterations
 Mutations in LMNA Modulate the Lamin A - Nesprin-2 Interaction and Cause LINC Complex Alterations Liu Yang 1, Martina Munck 1, Karthic Swaminathan 1, Larisa E. Kapinos 2, Angelika A. Noegel 1 *, Sascha
Mutations in LMNA Modulate the Lamin A - Nesprin-2 Interaction and Cause LINC Complex Alterations Liu Yang 1, Martina Munck 1, Karthic Swaminathan 1, Larisa E. Kapinos 2, Angelika A. Noegel 1 *, Sascha
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Progeria. Premature human aging: t he progerias. Progerias as models for aging. Hutchinson-Gilford syndrome. Definition:
 Premature human aging: t he progerias Reading: Genetic alterations in accelerated ageing syndromes Do they play a role in natural ageing? Monika Puzianowska- Kuznicka. Jacek Kuznicki. 2005. IJBCB, 37;
Premature human aging: t he progerias Reading: Genetic alterations in accelerated ageing syndromes Do they play a role in natural ageing? Monika Puzianowska- Kuznicka. Jacek Kuznicki. 2005. IJBCB, 37;
Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy
 RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
Nuclear Lamins in Cell Regulation and Disease
 Nuclear Lamins in Cell Regulation and Disease T. SHIMI, V. BUTIN-ISRAELI, S.A. ADAM, AND R.D. GOLDMAN Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago,
Nuclear Lamins in Cell Regulation and Disease T. SHIMI, V. BUTIN-ISRAELI, S.A. ADAM, AND R.D. GOLDMAN Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago,
ANALYSIS OF PHENOTYPE REVERSIBILITY IN HUTCHINSON-GILFORD PROGERIA SYNDROME IN MICE
 From the DEPARTMENT OF BIOSCIENCES AND NUTRITION Karolinska Institutet, Stockholm, Sweden ANALYSIS OF PHENOTYPE REVERSIBILITY IN HUTCHINSON-GILFORD PROGERIA SYNDROME IN MICE Charlotte Strandgren Stockholm
From the DEPARTMENT OF BIOSCIENCES AND NUTRITION Karolinska Institutet, Stockholm, Sweden ANALYSIS OF PHENOTYPE REVERSIBILITY IN HUTCHINSON-GILFORD PROGERIA SYNDROME IN MICE Charlotte Strandgren Stockholm
Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy
 See related Commentary on page xii Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy Casey L. Moulson, Gloriosa Go, Jennifer M. Gardner,w Allard C.
See related Commentary on page xii Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy Casey L. Moulson, Gloriosa Go, Jennifer M. Gardner,w Allard C.
LMNA mutations in atypical Werner s syndrome
 Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Alpha thalassemia mental retardation X-linked. Acquired alpha-thalassemia myelodysplastic syndrome
 Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Prelamin A and lamin A appear to be dispensable in the nuclear lamina
 Related Commentary, page 632 Research article Prelamin A and lamin A appear to be dispensable in the nuclear lamina Loren G. Fong, 1 Jennifer K. Ng, 2 Jan Lammerding, 3 Timothy A. Vickers, 4 Margarita
Related Commentary, page 632 Research article Prelamin A and lamin A appear to be dispensable in the nuclear lamina Loren G. Fong, 1 Jennifer K. Ng, 2 Jan Lammerding, 3 Timothy A. Vickers, 4 Margarita
Polycystic ovary syndrome (PCOS) is the most
 Brief Genetics Report Type A Insulin Resistance Syndrome Revealing a Novel Lamin A Mutation Jacques Young, 1 Louise Morbois-Trabut, 2 Béatrice Couzinet, 1 Olivier Lascols, 2,3 Elisabeth Dion, 4 Véronique
Brief Genetics Report Type A Insulin Resistance Syndrome Revealing a Novel Lamin A Mutation Jacques Young, 1 Louise Morbois-Trabut, 2 Béatrice Couzinet, 1 Olivier Lascols, 2,3 Elisabeth Dion, 4 Véronique
Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson- Gilford progeria fibroblasts
 /, 2017, Vol. 8, (No. 39), pp: 64809-64826 Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson- Gilford progeria fibroblasts Diana Gabriel
/, 2017, Vol. 8, (No. 39), pp: 64809-64826 Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson- Gilford progeria fibroblasts Diana Gabriel
A Comparative Study of Drosophila and Human A-Type Lamins
 Western Washington University Western CEDAR Biology College of Science and Engineering 10-26-2009 A Comparative Study of Drosophila and Human A-Type Lamins Sandra R. Schulze Western Washington University,
Western Washington University Western CEDAR Biology College of Science and Engineering 10-26-2009 A Comparative Study of Drosophila and Human A-Type Lamins Sandra R. Schulze Western Washington University,
Workshop report. 1. Introduction
 Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients
 Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism
 FEBS 25589 FEBS Letters 509 (2001) 423^429 Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism Marta Columbaro
FEBS 25589 FEBS Letters 509 (2001) 423^429 Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism Marta Columbaro
Complexity DNA. Genome RNA. Transcriptome. Protein. Proteome. Metabolites. Metabolome
 DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression. Chromatin Array of nuc
 Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Hutchinson-Gilford Progeria Syndrome. Mohamed Ibrahim
 Hutchinson-Gilford Progeria Syndrome A new treatment strategy and the role of prelamin A in oncogenesis Mohamed Ibrahim Institute of Medicine Department of Molecular and Clinical Medicine Sahlgrenska Academy
Hutchinson-Gilford Progeria Syndrome A new treatment strategy and the role of prelamin A in oncogenesis Mohamed Ibrahim Institute of Medicine Department of Molecular and Clinical Medicine Sahlgrenska Academy
Downloaded on T20:37:32Z
 Title Author(s) Altered regulation of adipogenesis with respect to disease processes Davies, Stephanie Jane Publication date 2017 Original citation Type of publication Rights Davies, S. J. 2017. Altered
Title Author(s) Altered regulation of adipogenesis with respect to disease processes Davies, Stephanie Jane Publication date 2017 Original citation Type of publication Rights Davies, S. J. 2017. Altered
MicroRNA and Male Infertility: A Potential for Diagnosis
 Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
LMNA cardiomyopathy: cell biology and genetics meet clinical medicine
 Disease Models & Mechanisms 4, 562-568 (2011) doi:10.1242/dmm.006346 : cell biology and genetics meet clinical medicine Jonathan T. Lu 1, Antoine Muchir 1,2, Peter L. Nagy 2 and Howard J. Worman 1,2, *
Disease Models & Mechanisms 4, 562-568 (2011) doi:10.1242/dmm.006346 : cell biology and genetics meet clinical medicine Jonathan T. Lu 1, Antoine Muchir 1,2, Peter L. Nagy 2 and Howard J. Worman 1,2, *
Disease Models & Mechanisms DMM Accepted manuscript
 First posted online on 24 May 2018 as 10.1242/dmm.033670 Access the most recent version at http://dmm.biologists.org/lookup/doi/10.1242/dmm.033670 ZMPSTE24 Missense Mutations that Cause Progeroid Diseases
First posted online on 24 May 2018 as 10.1242/dmm.033670 Access the most recent version at http://dmm.biologists.org/lookup/doi/10.1242/dmm.033670 ZMPSTE24 Missense Mutations that Cause Progeroid Diseases
Original Articles. Jason Cowan, MS; Duanxiang Li, MD; Jorge Gonzalez-Quintana, BS; Ana Morales, MS, CGC; Ray E. Hershberger, MD
 Original Articles Morphological Analysis of 13 LMNA Variants Identified in a Cohort of 324 Unrelated Patients With Idiopathic or Familial Dilated Cardiomyopathy Jason Cowan, MS; Duanxiang Li, MD; Jorge
Original Articles Morphological Analysis of 13 LMNA Variants Identified in a Cohort of 324 Unrelated Patients With Idiopathic or Familial Dilated Cardiomyopathy Jason Cowan, MS; Duanxiang Li, MD; Jorge
DEVELOPMENT OF A MOUSE MODEL FOR HUTCHINSON-GILFORD PROGERIA SYNDROME REVEAL DEFECTS IN ADULT STEM CELL MAINTENANCE
 From THE DEPARTMENT OF BIOSCIENCES AND NUTRITION Karolinska Institutet, Stockholm, Sweden DEVELOPMENT OF A MOUSE MODEL FOR HUTCHINSON-GILFORD PROGERIA SYNDROME REVEAL DEFECTS IN ADULT STEM CELL MAINTENANCE
From THE DEPARTMENT OF BIOSCIENCES AND NUTRITION Karolinska Institutet, Stockholm, Sweden DEVELOPMENT OF A MOUSE MODEL FOR HUTCHINSON-GILFORD PROGERIA SYNDROME REVEAL DEFECTS IN ADULT STEM CELL MAINTENANCE
Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy
 RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of β-catenin
 Research Article 401 Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of β-catenin Katarzyna Tilgner*, Kamila Wojciechowicz*, Colin
Research Article 401 Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of β-catenin Katarzyna Tilgner*, Kamila Wojciechowicz*, Colin
HHS Public Access Author manuscript Ageing Res Rev. Author manuscript; available in PMC 2018 January 01.
 Hutchinson-Gilford Progeria Syndrome: a premature aging disease caused by LMNA gene mutations Susana Gonzalo 1,*, Ray Kreienkamp 1, and Peter Askjaer 2 1 Edward A. Doisy Department of Biochemistry and
Hutchinson-Gilford Progeria Syndrome: a premature aging disease caused by LMNA gene mutations Susana Gonzalo 1,*, Ray Kreienkamp 1, and Peter Askjaer 2 1 Edward A. Doisy Department of Biochemistry and
CORRECTIONS. PNAS August 4, 2009 vol. 106 no
 MEDICAL SCIENCES Correction for A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model, by Brian C. Capell, Michelle Olive, Michael
MEDICAL SCIENCES Correction for A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model, by Brian C. Capell, Michelle Olive, Michael
Eukaryotic Gene Regulation
 Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Renata Schipp Medical Biology Department
 Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Lamin A/C mutation associated with lipodystrophy influences adipogenic differentiation of stem cells through interaction with Notch signaling
 Lamin A/C mutation associated with lipodystrophy influences adipogenic differentiation of stem cells through interaction with Notch signaling Journal: Biochemistry and Cell Biology Manuscript ID bcb-2017-0210
Lamin A/C mutation associated with lipodystrophy influences adipogenic differentiation of stem cells through interaction with Notch signaling Journal: Biochemistry and Cell Biology Manuscript ID bcb-2017-0210
Biochimica et Biophysica Acta
 Biochimica et Biophysica Acta 1812 (2011) 711 718 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis Osteoblasts from a mandibuloacral
Biochimica et Biophysica Acta 1812 (2011) 711 718 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbadis Osteoblasts from a mandibuloacral
Cerebral Small Vessel Disease and HAND in ARV-treated Subjects
 Cerebral Small Vessel Disease and HAND in ARV-treated Subjects Cristian L. Achim, MD, PhD Ronald J. Ellis, MD, PhD Virawudh Soontornniyomkij, MD ARROW, Bucharest, Romania October 5-6, 2015 Rationale and
Cerebral Small Vessel Disease and HAND in ARV-treated Subjects Cristian L. Achim, MD, PhD Ronald J. Ellis, MD, PhD Virawudh Soontornniyomkij, MD ARROW, Bucharest, Romania October 5-6, 2015 Rationale and
The nuclear envelope as a chromatin organizer
 review Nucleus 2:5, 339-349; September/October 2011; 2011 Landes Bioscience REVIEW The nuclear envelope as a chromatin organizer Nikolaj Zuleger, Michael I. Robson and Eric C. Schirmer* The Wellcome Trust
review Nucleus 2:5, 339-349; September/October 2011; 2011 Landes Bioscience REVIEW The nuclear envelope as a chromatin organizer Nikolaj Zuleger, Michael I. Robson and Eric C. Schirmer* The Wellcome Trust
2015 PhD proposal. CIFRE Grant SANOFI Centre de Recherche en Myologie Altered calcium homeostasis and LMNA-cardiomyopathy
 2015 PhD proposal Project / Research program: Project Leader: Department/Unit: CIFRE Grant SANOFI Centre de Recherche en Myologie Altered calcium homeostasis and LMNA-cardiomyopathy Antoine Muchir, PhD,
2015 PhD proposal Project / Research program: Project Leader: Department/Unit: CIFRE Grant SANOFI Centre de Recherche en Myologie Altered calcium homeostasis and LMNA-cardiomyopathy Antoine Muchir, PhD,
A new model for nuclear lamina organization
 Nuclear Envelope Diseases and Chromatin Organization 1339 A new model for nuclear lamina organization Martin W. Goldberg* 1, Jindriska Fiserova*, Irm Huttenlauch and Reimer Stick *School of Biological
Nuclear Envelope Diseases and Chromatin Organization 1339 A new model for nuclear lamina organization Martin W. Goldberg* 1, Jindriska Fiserova*, Irm Huttenlauch and Reimer Stick *School of Biological
Progerin expression disrupts critical adult stem cell functions involved in tissue repair
 www.impactaging.com AGING, December 2014, Vol 6, N 12 Research Paper Progerin expression disrupts critical adult stem cell functions involved in tissue repair Laurin Marie Pacheco 1,2, Lourdes Adriana
www.impactaging.com AGING, December 2014, Vol 6, N 12 Research Paper Progerin expression disrupts critical adult stem cell functions involved in tissue repair Laurin Marie Pacheco 1,2, Lourdes Adriana
