Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients
|
|
|
- Alexina Reeves
- 5 years ago
- Views:
Transcription
1 Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki 3, Toshiyuki Araki 3 and Ichizo Nishino 1 1 Department of Neuromuscular Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Ogawa-higashi, Kodaira, Tokyo , Japan 2 Neuroscience Research Institute, AIST, Central 6, Tsukuba, Ibaraki , Japan 3 Department of Peripheral Nervous System Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Kodaira, Tokyo , Japan *Author for correspondence (hayasi_y@ncnp.go.jp Accepted 9 August , Published by The Company of Biologists Ltd doi: /jcs Summary Mutations in LMNA, which encodes A-type nuclear lamins, cause various human diseases, including myopathy, cardiomyopathy, lipodystrophy and progeria syndrome. To date, little is known about how mutations in a single gene cause a wide variety of diseases. Here, by characterizing an antibody that specifically recognizes the phosphorylation of Ser458 of A-type lamins, we uncover findings that might contribute to our understanding of laminopathies. This antibody only reacts with nuclei in muscle biopsies from myopathy patients with mutations in the Ig-fold motif of A-type lamins. Ser458 phosphorylation is not seen in muscles from control patients or patients with any other neuromuscular diseases. In vitro analysis confirmed that only lamin A mutants associated with myopathy induce phosphorylation of Ser458, whereas lipodystrophy- or progeria-associated mutants do not. We also found that Akt1 directly phosphorylates Ser458 of lamin A with myopathy-related mutations in vitro. These results suggest that Ser458 phosphorylation of A- type lamins correlates with striated muscle laminopathies; this might be useful for the early diagnosis of LMNA-associated myopathies. We propose that disease-specific phosphorylation of A-type lamins by Akt1 contributes to myopathy caused by LMNA mutations. Key words: Laminopathy, A-type lamins, EDMD, LGMD1B, Akt Introduction Mutations in LMNA cause at least 13 human hereditary diseases, collectively termed laminopathies. These include striated muscle diseases, such as autosomal dominant and recessive forms of Emery- Dreifuss muscular dystrophy (AD/AR-EDMD) (Bonne et al., 1999; Raffaele Di Barletta et al., 2000), limb-girdle muscular dystrophy type 1B (LGMD1B) (Muchir et al., 2000), LMNA-related congenital muscular dystrophy (L-CMD) (Quijano-Roy et al., 2008) and dilated cardiomyopathy (Fatkin et al., 1999), and other conditions including Hutchinson-Gilford progeria syndrome (HGPS) (De Sandre- Giovannoli et al., 2003; Eriksson et al., 2003), atypical Werner syndrome (Chen et al., 2003), mandibuloacral dysplasia (MAD) (Novelli et al., 2002) and Dunnigan-type familial partial lipodystrophy (FPLD) (Shackleton et al., 2000). It is still not clear how mutations in LMNA cause such a wide variety of tissue-specific degenerative diseases, although A-type lamins are ubiquitously expressed. A-type lamins, of which lamin A and lamin C are the predominant somatic cell isoforms, are type V intermediate filament proteins that form the nuclear lamina, a meshwork on the nucleoplasmic side of the inner nuclear membrane (Burke and Stewart, 2002; Capell and Collins, 2006). Like all intermediate filament proteins, lamins have a tripartite structure consisting of an N-terminal head, a central coiled-coil rod domain and a large globular C-terminal tail. The C-terminal tail domain has an immunoglobulin-like fold (Ig-fold) motif (residues ); these motifs are known to be involved in protein protein interactions (Dechat et al., 2000; Lee et al., 2001; Sakaki et al., 2001; Zastrow et al., 2006; Zastrow et al., 2004). In addition to their primary role in providing mechanical support for nuclear membranes to maintain nuclear shape and size (Goldman et al., 2004), lamin filaments are believed to play important roles in mitosis (Tsai et al., 2006), chromatin organization (Glass et al., 1993; Park et al., 2009; Taniura et al., 1995), transcription (Spann et al., 2002) and DNA replication (Moir et al., 2000; Spann et al., 1997). According to the PhosphoSite database ( phosphosite.org/), more than 30 phosphorylation sites in human A-type lamins have been reported. Phosphorylation of Thr19, Ser22 and Ser392 leads to depolymerization of lamin filaments during nuclear envelope breakdown in mitosis and meiosis (Haas and Jost, 1993; Heald and McKeon, 1990; Peter et al., 1990; Ward and Kirschner, 1990), but the physiological importance of the phosphorylation of other sites in A-type lamins is largely unknown. Previously, Cenni et al. reported that N-terminal phosphorylation of lamin A was specifically decreased in the muscles of four AD-EDMD or LGMD1B patients (Cenni et al., 2005), implicating unidentified lamin A phosphorylation sites in the pathomechanism of AD-EDMD and LGMD1B. Here, we produced site- and phosphorylation-state-specific antibodies against human A-type lamins, and found that the antibody against phosphorylated Ser458 specifically detects myopathy patients who have mutations within the Ig-fold motif of the C-terminal tail domain of A-type lamins. In vitro expression analysis revealed that Ser458 phosphorylation was specific to myopathy-causing LMNA mutations, because it was not detected in cells with mutations related to FPLD or progeria. These results imply that Ser458 phosphorylation might have particular roles in the pathomechanism of LMNA-associated myopathy.
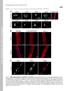
2 (22) Results Ser458 phosphorylation of A-type lamins in the muscles of LMNA-associated myopathy patients To examine the phosphorylation state of A-type lamins, we raised rabbit polyclonal antibodies against three phosphorylated A-type lamin peptides Ser5-P, Thr416-P and Ser458-P (Fig. 1A); these serine and threonine residues are well conserved between species. We performed immunohistochemistry of muscle specimens from 17 genetically confirmed LMNA-associated myopathy patients, including AD-EDMD, LGMD1B and L-CMD patients. Using the purified anti-phospho-ser458 antibody (anti-ser458- P Ab), we found clear nuclear staining in muscles from patients with LMNA-associated myopathies, but not in control muscles (Fig. 1Ba d). Notably, strong immunoreaction to anti-ser458-p Ab was observed in all eight patients carrying a mutation within the Ig-fold motif, whereas the remaining nine patients with mutations outside of the Ig-fold domain showed barely detectable nuclear staining (Table 1). Ser458 is an evolutionarily highly conserved residue within the Ig-fold motif (amino acids ) of A-type lamins (supplementary material Fig. S1A). Anti-Ser458- P Ab staining was independent of patient age and sex, and independent of the nature or severity of myopathy, because positive staining for Ser458-P was seen in AD-EDMD, LGMD1B, L-CMD and infantile inflammatory myopathy (IIM) cases. Positive staining with the anti-ser458-p antibody was detected in myonuclei [both peripheral and centrally located (Fig. 1Bb,d,e,g)], vascular endothelial and smooth muscle cell nuclei (Fig. 1Bi), and the nuclei of unidentified cells outside the basal lamina (Fig. 1Bf,h). Double staining with anti-ser458-p and anti- Pax7 antibodies also revealed positive staining in nuclei of satellite cells in patient muscles (Fig. 1Bj l). Double staining with antibodies for Ser458-P and pan-a-type lamins showed that 69.0±7.3% of nuclei were immunostained in patient muscles with mutations in the Ig-fold domain. These results suggest that the Ser458 phosphorylation might be common in LMNA-associated myopathy patients with mutations in the Ig-fold domain of A-type lamins. Consistent with the immunohistochemistry results, the anti- Ser458-P Ab detected two main bands at 70 kda and 65 kda, corresponding to lamins A and C, respectively, in muscle from laminopathy patients, but not in unaffected control muscle (Fig. 2). No Ser458 phosphorylation of A-type lamins in patients with other neuromuscular disorders To determine whether Ser458 phosphorylation is specific to LMNAassociated myopathy, we immunostained muscle specimens from patients with other neuromuscular diseases, including X-linked EDMD (X-EDMD (Fig. 3c,d), Duchenne muscular dystrophy (DMD) (Fig. 3e,f), Becker muscular dystrophy (BMD) (Fig. 3g,h), LGMD2A (Fig. 3i,j), LGMD2B (Fig. 3k,l), sporadic inclusion body myositis (sibm) (Fig. 3,m,n), idiopathic polymyositis (PM) (Fig. 3o,p) and myotonic dystrophy type 1 (MyD1) (Fig. 3q,r). As shown in Fig. 3, Ser458 phosphorylation was only observed in Fig. 1. Ser458 of A-type lamins is specifically phosphorylated in muscles from LMNA-associated myopathy patients. (A) Schematic model of three antibodies against phosphorylated A-type lamins (Ser5-P Ab, Thr416-P Ab and Ser458-P Ab). Amino acid residues form an Ig-fold structure in the C-terminal globular tail domain. (B) Immunohistochemistry of human muscles with anti-ser458-p antibody. Nuclei in patient muscle (P11) show positive staining for anti-ser458-p antibody (b,d), whereas no positive nuclear staining is seen in control muscle (a,c). Anti-Ser458-P Ab is green and anti-merosin antibody is red. (c,d) Merged images. Blue is DAPI staining. (e,f) Magnified images of boxes in b. (g,h) Magnified images of boxes in d. Note that centrally placed nuclei and nuclei in non-muscle cells outside of the basal lamina (arrowheads) are immunostained with anti-ser458-p Ab. (i) Nuclei in blood vessels in the patient muscle (P14) are also immunostained with anti-ser458-p Ab. (j l) Double staining of LMNA-associated myopathy patient muscle (P12) with anti-ser458-p Ab (j) and anti-pax7 (k) reveals colocalization (l), indicating the occurrence of Ser458 phosphorylation in the patient satellite cells. Scale bars: 20 m.
3 Myopathy-specific lamin phosphorylation 3895 Table 1. Summary of anti-ser458-p antibody staining of A-type lamins from 17 LMNA-associated myopathy patients Patient Age Sex Clinical diagnosis LMNA mutation Domain Anti-Ser458-P Ab P1 3y 11m F LGMD1B p.r28q Head P2 2y 1m F IIM p.k32 deletion Head P3 1y M IIM p.r41s Rod P4 1y 6m F L-CMD p.r41c Rod P5 2y 8m F LGMD1B p.r249q Rod P6 5y 3m F LGMD1B p.s303p Rod P7 47y M LGMD1B p.k311r Rod P8 2y 5m M IIM p.e358k Rod P9 1y 9m F L-CMD p.e444_d446 duplication Tail (Ig fold) + P10 11y 1m F EDMD p.r453w Tail (Ig fold) + P11 4y 5m F LGMD1B p.r453w Tail (Ig fold) + P12 4y F LGMD1B p.r453w Tail (Ig fold) + P13 5y F IIM p.n456h Tail (Ig fold) + P14 7y 2m M LGMD1B p.w514r Tail (Ig fold) + P15 25y F LGMD1B p.r527p Tail (Ig fold) + P16 2y 11m M LGMD1B p.t528k Tail (Ig fold) + P17 52y F LGMD1B p.v547x Tail patients with LMNA-associated myopathy (Fig. 3a,b), but not with other muscle diseases such as X-EDMD (Fig. 3c,d), which is clinically indistinguishable from AD-EDMD. This result suggests that Ser458 phosphorylation is likely to be specific to LMNA mutations. In contrast to the anti-ser458-p antibody, the anti-ser5-p antibody strongly recognized nuclei in all human muscles examined, with no disease or mutation specificity. The anti-thr416- P antibody showed very weak staining in nuclei, with no difference between control and patients (supplementary material Fig. S1B,C). Ser458 phosphorylation of A-type lamins in AD-EDMD patient fibroblasts Because Ser458 phosphorylation of A-type lamins was also observed in the nuclei of non-muscle tissues (Fig. 1B), we performed immunocytochemistry using cultured skin fibroblasts from one unaffected person and two AD-EDMD patients with either a Leu102Pro mutation in the rod domain or a Arg453Trp mutation in the Ig-fold domain. Anti-Ser458-P Ab staining was observed only in the fibroblasts with the Arg453Trp mutation, but not in control and Leu102Pro mutant fibroblasts (Fig. 4). All nuclei in the Arg453Trp fibroblasts were immunopositive, implying that Ser458 phosphorylation might be independent of the cell cycle. These results suggested that Ser458 phosphorylation requires mutations in the Ig-fold motif of A-type lamins and can occur in non-muscle cells. Ser458 phosphorylation of A-type lamins in transfected cells Next, we analyzed the specificity of anti-ser458-p Ab to phosphorylation by examining ectopically expressed FLAG-tagged lamin A and FLAG-tagged Arg453Trp mutant lamin A, which is the most common mutation in LMNA-associated myopathy. Anti- Ser458-P Ab strongly detected the ectopic FLAG Arg453Trp mutant in a western blot analysis and slightly detected even wildtype lamin A when overexpressed in COS-7 cells (Fig. 5A). Importantly, alkaline phosphatase treatment reduced the immunoreactivity of anti-ser458-p Ab to background levels (Fig. 5A, IP), strongly suggesting that anti-ser458-p Ab specifically recognizes the phosphorylation of Ser458 of mutant A-type lamins. To further verify the specificity of anti-ser458-p Ab, we transfected C2 myoblasts to express FLAG-tagged wild-type lamin A or FLAG-tagged lamin A bearing either the Ser458Ala or Arg453Trp mutation, or both mutations (FLAG Arg453Trp/ Ser458Ala) (Fig. 5B). Anti-Ser458-P Ab immunostained only the nuclei that expressed FLAG Arg453Trp, whereas the nuclei that expressed FLAG Arg453Trp/Ser458Ala were barely detectable with anti-ser458-p Ab. These results confirmed the specificity of anti-ser458-p Ab to Ser458 phosphorylation. Ser458 phosphorylation is not detected in other laminopathies As several mutations associated with FPLD, MAD and HGPS are known to be located within the Ig-fold motif of A-type lamins, we wanted to find out whether Ser458 phosphorylation also occurs in such mutants. We therefore transfected lamin A mutants associated with AD-EDMD (Leu140Pro, Arg453Trp, Arg527Pro, Leu530Pro) (Bonne et al., 1999; Boriani et al., 2003), LGMD1B (Tyr481His) (Kitaguchi et al., 2001), FPLD (Gly465Asp, Arg482Trp, Lys486Asn) (Shackleton et al., 2000; Speckman et al., 2000), MAD (Arg471Cys and Arg527His) (Cao and Hegele, 2003; Novelli et al., 2002) and HGPS (Met540Thr) (Verstraeten et al., 2006) into C2 myoblasts, and checked immunoreactivity with anti-ser458-p Ab. As expected, Ig-fold mutants Arg453Trp, Arg527Pro, Leu530Pro and Tyr481His were detected by anti-ser458-p antibody (Fig. 6). By contrast, rod domain mutant Leu140Pro formed intranuclear foci that were not detected by anti-ser458-p Ab. Interestingly, lamin A mutations associated with FPLD (Gly465Asp, Arg482Trp, Lys486Asn), MAD (Arg471Cys, Arg527His) and Fig. 2. Western blot analysis of patient muscle with anti-ser458-p Ab. Anti-Ser458-P Ab specifically recognizes proteins of ~70 kda and 65 kda, corresponding to lamin A and lamin C, respectively. Representative data from patient 13 (P13) are shown. C: control, P: patient.
4 123 (22) 3896 Fig. 3. Immunohistochemical analysis of other neuromuscular diseases. Muscles from myopathic patients were immunostained with anti-ser458-p Ab (green) and anti-merosin (red). (a,b) AD-EDMD with Arg453Trp mutation in LMNA (P11), (c,d) X-EDMD, (e,f) DMD, (g,h) BMD, (i,j) LGMD2A, (k,l) LGMD2B, (m,n) sibm, (o,p) PM, (q,r) MyD1. (b,d,f,h,j,l,n,p,r) Merged images. Nuclei were stained with DAPI (blue). Scale bars: 20 m. HGPS (Met540Thr), which are all located in the Ig-fold motif, were negative for anti-ser458-p Ab. These results suggested that Ser458 in A-type lamins might be specifically phosphorylated in laminopathies related to myopathy, but not in other laminopathies even with mutations in the Ig-fold domain. Akt1 directly phosphorylates Ser458 of lamin A The sequence around Ser458 contains the Akt consensus sequence RxRxxS. To test whether Akt phosphorylates Ser458 of lamin A, COS-7 cells were co-transfected with wild-type Akt (wtakt HA) or myristoylated Akt (myrakt HA), which is regarded as a constitutively active form (Andjelkovic et al., 1997; Manning and Cantley, 2007), and FLAG-tagged lamin A constructs. The cell lysates were immunoprecipitated with FLAG M2 agarose and detected with anti-ser458-p Ab. Coexpression of wtakt HA enhanced Ser458 phosphorylation of the Arg453Trp mutant; this signal became even stronger in cells co-transfected with myrakt HA (Fig. 7A). By contrast, only background levels of Ser458 phosphorylation were detected for wild-type lamin A and the double (Arg453Trp/Ser458Ala) mutant (Fig. 7A). The same results were obtained by co-transfection in C2 myotubes (supplementary material Fig. S2). To determine whether Ser458 phosphorylation is sensitive to the specific amino acid present at position Arg527, we co-transfected COS-7 cells with wtakt HA plus either the myopathy-causing Arg527Pro mutation or the MAD-causing Arg527His mutation of FLAG-tagged lamin A (Fig. 7B). Only background levels of Ser458 phosphorylation were detected with wild-type and His-substituted lamin A, suggesting specific recognition of myopathic Arg527Pro-mutated lamin A by Akt1. To determine whether Akt1 directly phosphorylates Ser458 of lamin A, we performed an in vitro kinase assay using the purified C-terminal tail domain of lamin A (LA-T, amino acids ) and a recombinant, constitutively active form of Akt1 (rakt).
5 Myopathy-specific lamin phosphorylation 3897 Fig. 4. Immunocytochemistry of human fibroblasts. Only fibroblasts from an AD-EDMD patient with an Arg453Trp mutation in the Ig-fold domain are detected with anti-ser458-p Ab (h), whereas nuclear staining of cells from a control and an AD-EDMD patient with a Leu102Pro mutation is barely detectable (b,e). (a,d,g) Staining with anti-lamin A antibody, (b,e,h) staining with anti-ser458-p Ab, (c,f,i) merged images of anti-ser458-p Ab and DAPI. Scale bars: 20 m. Consistently, LA-T with the Arg453Trp mutation was phosphorylated by rakt in vitro (Fig. 7C). rakt failed to phosphorylate LA-T with Arg453Trp/Ser458Ala double mutations, indicating that Ser458 is a genuine Akt phosphorylation site. We conclude that Ser458 in lamin A is specifically phosphorylated by Akt1, even in non-muscle cells, when the Ig-fold domain bears mutations that cause myopathy; Ser458 is not phosphorylated when lamin A bears mutations, even in the Ig-fold, that cause disease in other tissues. Discussion For the first time, we have identified a disease-related phosphorylation site of A-type lamins using the antibody that specifically recognizes the phosphorylation of Ser458 of A-type lamins. Cenni et al. previously reported a monoclonal antibody, named SW2-30, that recognizes the phosphorylation of the N- terminal region of A-type lamins in normal muscles, but immunoreactions were barely detectable in myonuclei from AD- EDMD and LGMD1B patients (Cenni et al., 2005). The precise phosphorylation site was not determined, but the change in phosphorylation state between control and patients was the reverse of that seen with Ser458. In contrast to previously reported cellcycle-specific phosphorylation (Heald and McKeon, 1990; Peter et al., 1990; Ward and Kirschner, 1990), Ser458 phosphorylation is independent of cell cycle, because the all nuclei in replicating fibroblasts from patients with the Arg453Trp mutation were immunopositive. The early diagnosis of LMNA-associated myopathy is particularly important because the patients eventually develop severe cardiac problems with conduction defects, with high mortality (Bonne et al., 2003; Taylor et al., 2003). Because patients show a wide variety of clinicopathological features and recent research has revealed the spectrum of LMNA-associated myopathy to be broader than Fig. 5. Anti-Ser458-P Ab specifically recognizes the phosphorylation of Ser458 of lamin A. (A) Western blot analysis of transfected cells. FLAGtagged lamin A constructs were overexpressed in COS-7 cells. The cell lysates were subjected to immunoprecipitation with anti-flag M2 antibody. The immunoprecipitates were incubated with (+) or without ( ) calf intestine alkaline phosphatase, and then resolved by SDS-PAGE. WT: wild type, RW: Arg453Trp mutant. (B) Substitution of Ser458 to Ala diminishes the immunoreactivity of anti-ser458-p against Arg453Trp mutant lamin A. Wildtype or mutant lamin A were overexpressed in C2 myoblasts, and then transfected cells were double immunostained with anti-flag Ab (red) and anti-ser458-p Ab (green). Nuclei were stained with DAPI (blue). previously understood (Quijano-Roy et al., 2008), simple screening using disease-specific phospho-a-type lamin antibodies should be quite useful. Interestingly, the position of the mutation in LMNA is important for positive staining by anti-ser458-p Ab (Table 1). About 30% of AD-EDMD and LGMD1B patients previously reported have the mutation within the Ig-fold motif (Leiden Muscular Dystrophy Pages; The Arg453Trp mutation, which is the most common mutation in AD-EDMD, is also located within this motif. Anti-Ser458-P antibody also detected the nuclei in skin fibroblasts and the vascular endothelial cells of patients, suggesting that skin biopsy, which is less invasive for the patient, might be used for the diagnosis. We have revealed that Akt1 phosphorylates Ser458 of myopathy-related mutant lamin A. A recent study also reported that Ser404 of lamin A is phosphorylated by Akt (Cenni et al., 2008). Interestingly, Ser458 of wild-type lamin A was not phosphorylated by Akt1 even in vitro (Fig. 7C), suggesting that
6 (22) Fig. 6. Myopathy-specific phosphorylation of Ser458 of lamin A. Lamin A mutants that cause AD-EDMD, LGMD1B, FPLD, HGPS or MAD were overexpressed in C2 myoblasts. Transfected cells were double immunostained with anti-flag Ab (upper panel, red) and anti-ser458-p Ab (lower panel, green). Ser458 is buried in wild-type lamin A. Ser458 was found to be partly buried and structural studies have showed that the Arg453Trp mutation destabilizes the three-dimensional structure of the Ig-fold motif, whereas the mutations observed in FPLD (Arg482Trp and Arg482Gln) do not (Krimm et al., 2002). We propose that LMNA mutations that make Ser458 easily accessible to Akt1 phosphorylation predispose patients to muscle pathology. It is nonetheless perplexing that EDMD and LGMD1B patients with mutations outside the Ig-fold domain have no detectable Ser458 phosphorylation. We speculate that mutations outside the Ig-fold motif might cause myopathy by different mechanisms, for example, by disrupting protein protein interactions or lamin filament formation, because rod domain mutations such as Leu140Pro can disrupt polymerization and mislocalize lamins. Although further studies are needed to understand these mechanisms, antibodies that recognize Ser458-phosphorylated lamin A will be immediately useful for the differential diagnosis of a large fraction of LMNA-associated myopathies. Materials and Methods Clinical materials All clinical materials used in this study were obtained for diagnostic purposes with informed consent. The studies were approved by the Ethical Committee of the National Center of Neurology and Psychiatry. Mutation analysis Genomic DNA was isolated from peripheral lymphocytes or muscle specimens using standard techniques. All LMNA exons and their flanking intronic regions were Fig. 7. Akt1 directly phosphorylates Ser458 of lamin A with myopathyrelated mutations. (A) COS-7 cells were transfected with Akt HA constructs (wtakt HA or myrakt HA) and FLAG lamin A constructs (WT: wild type, RW: Arg453Trp mutant, RW/SA: Arg453Trp/Ser458Ala mutant) for 30 hours. FLAG lamin A was immunoprecipitated (IP), then probed with anti-ser458-p and anti-flag M2 antibodies. Whole-cell lysates were also probed with antiphospho-akt Ser473 (anti-pakt), and anti-ha and anti-actin antibodies to confirm equivalent levels of expression. (B) COS-7 cells were transfected with FLAG lamin A constructs alone or co-transfected with wtakt HA for 30 hours. FLAG lamin A was immunoprecipitated, then immunoblotted as described above. (C) Purified His-tagged C-terminal tail domain of lamin A (LA-T) was phosphorylated in vitro in the presence (+) or absence ( ) of recombinant active Akt (rakt). The membranes were probed with anti-ser458- P and anti-his tag antibodies. sequenced directly for all 17 patients using an ABI PRISM 3100 automated sequencer (PE Applied Biosystems, Foster City, CA). Primer sequences are shown in supplementary material Table S1. Antibody production and purification The following peptides for immunization were synthesized by MBL (Ina, Nagano, Japan). For phospho-a-type lamins: Ser5-P (Met-Glu-Thr-Pro-phosphoSer-Gln-Arg- Arg-Ala-Thr-Cys); Thr416-P (AcetylVal-phosphoThr-Lys-Lys-Arg-Lys-Leu-Glu- Cys); Ser458-P (Cys-Leu-Arg-Asn-Lys-phosphoSer-Asn-Glu-Asp-Gln-Ser). For non-phospho-a-type lamins: Ser5 (Met-Glu-Thr-Pro-Ser-Gln-Arg-Arg-Ala-Thr-Cys); Thr416 (AcetylVal-Thr-Lys-Lys-Arg-Lys-Leu-Glu-Cys); Ser458 (Cys-Leu-Arg-Asn- Lys-Ser-Asn-Glu-Asp-Gln-Ser). To obtain the specific antibody for phospho-a-type lamins, rabbits were immunized with the phospho-peptides conjugated to keyhole limpet hemocyanin (KLH). Antisera were purified by affinity chromatography at 4 C. First, antisera were passed through HiTrap NHS-activated HP columns (GE Healthcare UK, Buckinghamshire, UK) coupled with non-phospho-a-type lamin peptides. Then, the flow-through fractions were collected. Next, the flow-through fractions were passed through the columns coupled with phospho-a-type lamin peptides. Antibodies were eluted with 0.15 M glycine HCl (ph 2.7), concentrated by Amicon ultra-4 (Millipore, Bedford, MA, USA) and dialyzed with PBS at 4 C overnight. Approximately 0.2 g/ l of antibody was obtained. Immunohistochemistry Biopsied muscle specimens were frozen in isopentane cooled in liquid nitrogen. Serial frozen sections of 6 m thickness were fixed in cold acetone for 5 minutes at
7 Myopathy-specific lamin phosphorylation 3899 room temperature. After blocking with PBS containing 2% BSA and 5% heatinactivated normal goat serum, the sections were incubated with primary antibodies for 2 hours at 37 C. Primary antibodies used in this study are: rabbit anti-phospho- A-type lamin polyclonal antibodies at 1:50; mouse anti-human merosin (M-chain) monoclonal antibody (5H2) (Chemicon International, Temecula, CA) at 1:400; rabbit anti-lamin A polyclonal antibody (Sakaki et al., 2001) at 1:400; mouse anti-pax7 monoclonal antibody (Developmental Studies Hybridoma Bank, Iowa City, IA) at 1:300. Sections were incubated with anti-rabbit IgG antibody conjugated with Alexa- Fluor-488 and anti-mouse IgG antibody conjugated with Alexa-Fluor-568 (Invitrogen, Carlsbad, CA) at 1:500 for 45 minutes at room temperature. To enhance the immunoreactions, we used Can Get Signal immunostain solution A (Toyobo, Osaka, Japan) to dilute primary and secondary antibodies. Sections were observed under a Zeiss Axiophot 2 microscope (Carl Zeiss, Oberkochen, Germany). Immunoblot analysis For biopsied muscle specimens, 20 slices of 10 m cryosections were immediately sonicated in SDS sample buffer [50 mm Tris-HCl (ph 6.8), 2% SDS, 1% glycerol (v/v), 0.1% bromophenol blue, 6% 2-mercaptoethanol] and boiled for 5 minutes at 95 C. The lysates were centrifuged at 100,000 g at 4 C for 5 minutes. The supernatants were subjected to SDS-PAGE and the proteins were transferred to Immobilon-P membranes (Millipore). The membranes were blocked with blocking buffer [2% BSA in Tris-buffered saline (TBS) containing 0.05% Tween-20] at room temperature for 1 hour, then incubated with anti-ser458-p antibody diluted in Can Get Signal solution 1 (Toyobo) at 1:1000 at 4 C overnight. The anti-ser458-p antibody was followed with Histofine simple stain MAX-PO (Nichirei Biosciences, Tsukiji, Tokyo, Japan) diluted in Can Get Signal solution 2 at 1:500 at room temperature for 45 minutes. Recognized proteins were visualized by enhanced chemiluminescence plus detection reagent (GE Healthcare). Plasmid construction and mutagenesis To generate FLAG-tagged wild-type human lamin A, the N terminus of human lamin A was amplified by PCR using the following primers: 5 -GGAATTCA- CCATGGACTACAAAGACGATGACGACAAGGAGACCCCGTCCCAGCGG-3 and 5 -CTCGCGGCTGACCACCTCTT-3. PCR product was digested with EcoRI and AccI, and subcloned into full-length human prelamin A in puc19 (a kind gift from Howard J. Worman, Columbia University, NY). FLAG-tagged lamin A cdna was cut out by digestion with EcoRI and XbaI, and subcloned into pcdna3.1 (Invitrogen). Each of the mutant lamin A constructs was made by site-directed mutagenesis (Horton et al., 1989) using the primers listed in supplementary material Table S2. Arg453Trp and Arg471Cys mutants were made by combining primers LMNA1386 and R453W-Fw with R453W-Rv and LMNA2579, and LMNA1386 and R471C-Fw with R471C-Rv and LMNA2579, respectively. Human full-length Akt1 was amplified by PCR using an Akt1 cdna construct (a gift from Yukiko Gotoh, University of Tokyo, Japan) (Masuyama et al., 2001) and subcloned into pcdna3 (Invitrogen). Then, a hemagglutinin (HA) tag was added at the C-terminal end, yielding wtakt HA. The constitutively active form of Akt (myrakt HA) was generated by adding a myristoylation site derived from murine Src tyrosine kinase to the N-terminal end of the Akt HA described above (Andjelkovic et al., 1997; Manning and Cantley, 2007). For the in vitro kinase assay, the C-terminal tail domain of lamin A (amino acids ) was amplified by PCR using the following primers: 5 -CATATGG - GTGGGGGCAGCGTCACCAAAAAG-3 and 5 -GGGATCCTTAGT CGTCCT - CAACCACAGTCACTGAGC-3. PCR product was ligated with pgem-t-easy (Promega, Madison, WI), digested with NdeI and BamHI, and subcloned into pet 15b vector (Merck, Darmstadt, Germany). The sequences of all constructs were verified by DNA sequencing. Cell culture and transfection African green-monkey kidney fibroblast cell line COS-7 cells and mouse myoblast cell line C2 cells were cultured in DMEM (Sigma, St Louis, MO) supplemented with 10% FBS (Invitrogen) at 37 C in a humidified atmosphere of 5% CO 2. Before transfection, the medium was replaced with serum-free DMEM. The cells were transiently transfected using FuGENE HD transfection reagent (Roche Diagnostics, Indianapolis, IN). After 8 12 hours transfection, DMEM supplemented with 20% FBS was added to the dishes to adjust the FBS concentration in the medium to 10%. The cells were used for each experiment 48 hours after transfection. Immunoprecipitation and alkaline phosphatase treatment COS-7 cells were transfected with 10 g wild-type lamin A or Arg453Trp lamin A construct. The cells were lysed in 1.0 ml lysis buffer containing 50 mm Tris-HCl (ph 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate and Complete Protease Inhibitor Cocktail (Roche Diagnostics). The lysates were incubated at 4 C for 30 minutes with gentle rotation and then centrifuged at 15,000 g at 4 C for 30 minutes. The supernatants were collected and their protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA). For immunoprecipitation, the protein concentration of the cleared lysates was adjusted to 1.0 g/ l and 40 l anti-flag M2 affinity gel (Sigma) was added. The mixtures were incubated at 4 C overnight. The resulting immune complexes were washed three times with TBS and two times with alkaline phosphatase buffer (AP buffer) containing 50 mm Tris-HCl (ph 9.0) and 1 mm MgCl 2. The immune complexes were incubated with calf intestine alkaline phosphatase (Takara Bio, Shiga, Japan) in AP buffer at 37 C overnight. The proteins were eluted by boiling at 95 C for 5 minutes in SDS sample buffer. Immunoblot analysis was performed as described above. To detect FLAG-tagged lamin A, we used mouse anti-flag M2 monoclonal antibody (Sigma) diluted in PBS containing 5% skim milk at 1:2500. Immunocytochemistry Human skin fibroblasts from healthy individuals (control), AD-EDMD patients with a LMNA Leu102Pro or Arg453Trp mutation, and transfected C2 myoblasts were plated onto 12 mm cover glass coated with type I collagen. The cells were fixed in methanol for 10 minutes at 20 C. The steps were as described under Immunohistochemistry. Akt kinase assay In mammalian cell experiments, COS-7 cells or C2 myoblasts were co-transfected with 5 g each of Akt HA construct and FLAG lamin A construct. The cells were lysed in 1.0 ml lysis buffer supplemented with 50 mm sodium fluoride, 1 mm sodium orthovanadate and 1 mm phenylmethylsulfonyl fluoride. FLAG lamin A was immunoprecipitated as described above. The resulting immune complexes were washed two times with lysis buffer and two times with TBS. The proteins were eluted by boiling at 95 C for 5 minutes in SDS sample buffer. Immunoblot analysis was performed as described above. For the detection of phosphorylated proteins, Blocking One-P blocking solution (Nacalai Tesque, Kyoto, Japan) was used. Mouse anti-ha monoclonal antibody (16B12 clone; Covance, Princeton, NJ) was used at 1:1000, rabbit anti-phospho-akt (Ser473) monoclonal antibody (Cell Signaling Technology, Danvers, MA) was used at 1:1000, and rabbit anti-actin polyclonal antibody (Nichirei) was used at 1:2000. For the in vitro kinase assay, the C-terminal tail domain of lamin A (LA-T) was in-vitro translated as a His-tagged protein using the S30 T7 High-Yield Protein Expression System (Promega) according to the manufacturer s instructions. The LA- T was purified with Ni-NTA agarose and eluted with elution buffer containing 50 mm NaH 2 PO 4, 300 mm NaCl, 250 mm imidazole, followed by dialysis against 20 mm MOPS-NaOH (ph 8.0), 1 mm EDTA, 5% glycerol, 50 mm NaCl at 4 C overnight. Approximately 0.5 g His-tagged protein was incubated with 5 ng recombinant active Akt1 (Millipore) in a reaction buffer consisting of 8 mm MOPS- NaOH (ph 7.0), 0.2 mm EDTA, 10 mm magnesium acetate, 100 mm ATP at 30 C for 30 minutes. The reaction was terminated by the addition of SDS sample buffer. Immunoblot analysis was performed as described above. For the detection of Histagged proteins, mouse anti-his-tag monoclonal antibody (Merck) was used at 1:2000. We thank May Malicdan (National Center of Neurology and Psychiatry) for reviewing the manuscript, and Kamako Goto, Mieko Ohnishi, Yuriko Kure and Megumo Ogawa (National Center of Neurology and Psychiatry) for technical assistance. This study was supported by a Research on Psychiatric and Neurological Diseases and Mental Health of Health Labour Sciences Research Grant; by a Research Grant (20B-12, 20B-13) for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare of Japan; by a grant from the Japan Foundation for Neuroscience and Mental Health; by grants from the Human Frontier Science Program; by a KAKENHI grant ( ) from the Japan Society for the Promotion of Science; by Research on Publicly Essential Drugs and Medical Devices from the Japanese Health Sciences Foundation; and by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO). Supplementary material available online at References Andjelkovic, M., Alessi, D. R., Meier, R., Fernandez, A., Lamb, N. J., Frech, M., Cron, P., Cohen, P., Lucocq, J. M. and Hemmings, B. A. (1997). Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272, Bonne, G., Di Barletta, M. R., Varnous, S., Becane, H. M., Hammouda, E. H., Merlini, L., Muntoni, F., Greenberg, C. R., Gary, F., Urtizberea, J. A. et al. (1999). Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21, Bonne, G., Yaou, R. B., Beroud, C., Boriani, G., Brown, S., de Visser, M., Duboc, D., Ellis, J., Hausmanowa-Petrusewicz, I., Lattanzi, G. et al. (2003). 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss Muscular Dystrophy (EDMD) Workshop, September 2002, Naarden, The Netherlands. Neuromuscul. Disord. 13, Boriani, G., Gallina, M., Merlini, L., Bonne, G., Toniolo, D., Amati, S., Biffi, M., Martignani, C., Frabetti, L., Bonvicini, M. et al. (2003). Clinical relevance of atrial
8 (22) fibrillation/flutter, stroke, pacemaker implant, and heart failure in Emery-Dreifuss muscular dystrophy: a long-term longitudinal study. Stroke 34, Burke, B. and Stewart, C. L. (2002). Life at the edge: the nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 3, Cao, H. and Hegele, R. A. (2003). LMNA is mutated in Hutchinson-Gilford progeria (MIM ) but not in Wiedemann-Rautenstrauch progeroid syndrome (MIM ). J. Hum. Genet. 48, Capell, B. C. and Collins, F. S. (2006). Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 7, Cenni, V., Sabatelli, P., Mattioli, E., Marmiroli, S., Capanni, C., Ognibene, A., Squarzoni, S., Maraldi, N. M., Bonne, G., Columbaro, M. et al. (2005). Lamin A N- terminal phosphorylation is associated with myoblast activation: impairment in Emery- Dreifuss muscular dystrophy. J. Med. Genet. 42, Cenni, V., Bertacchini, J., Beretti, F., Lattanzi, G., Bavelloni, A., Riccio, M., Ruzzene, M., Marin, O., Arrigoni, G., Parnaik, V. et al. (2008). Lamin A Ser404 is a nuclear target of Akt phosphorylation in C2C12 cells. J. Proteome Res. 7, Chen, L., Lee, L., Kudlow, B. A., Dos Santos, H. G., Sletvold, O., Shafeghati, Y., Botha, E. G., Garg, A., Hanson, N. B., Martin, G. M. et al. (2003). LMNA mutations in atypical Werner s syndrome. Lancet 362, De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C. L., Munnich, A., Le Merrer, M. et al. (2003). Lamin a truncation in Hutchinson-Gilford progeria. Science 300, Dechat, T., Korbei, B., Vaughan, O. A., Vlcek, S., Hutchison, C. J. and Foisner, R. (2000). Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. J. Cell Sci. 113, Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P. et al. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, Fatkin, D., MacRae, C., Sasaki, T., Wolff, M. R., Porcu, M., Frenneaux, M., Atherton, J., Vidaillet, H. J., Jr, Spudich, S., De Girolami, U. et al. (1999). Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 341, Glass, C. A., Glass, J. R., Taniura, H., Hasel, K. W., Blevitt, J. M. and Gerace, L. (1993). The alpha-helical rod domain of human lamins A and C contains a chromatin binding site. EMBO J. 12, Goldman, R. D., Shumaker, D. K., Erdos, M. R., Eriksson, M., Goldman, A. E., Gordon, L. B., Gruenbaum, Y., Khuon, S., Mendez, M., Varga, R. et al. (2004). Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 101, Haas, M. and Jost, E. (1993). Functional analysis of phosphorylation sites in human lamin A controlling lamin disassembly, nuclear transport and assembly. Eur. J. Cell Biol. 62, Heald, R. and McKeon, F. (1990). Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61, Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K. and Pease, L. R. (1989). Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, Kitaguchi, T., Matsubara, S., Sato, M., Miyamoto, K., Hirai, S., Schwartz, K. and Bonne, G. (2001). A missense mutation in the exon 8 of lamin A/C gene in a Japanese case of autosomal dominant limb-girdle muscular dystrophy and cardiac conduction block. Neuromuscul. Disord. 11, Krimm, I., Ostlund, C., Gilquin, B., Couprie, J., Hossenlopp, P., Mornon, J. P., Bonne, G., Courvalin, J. C., Worman, H. J. and Zinn-Justin, S. (2002). The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure 10, Lee, K. K., Haraguchi, T., Lee, R. S., Koujin, T., Hiraoka, Y. and Wilson, K. L. (2001). Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci. 114, Manning, B. D. and Cantley, L. C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, Masuyama, N., Oishi, K., Mori, Y., Ueno, T., Takahama, Y. and Gotoh, Y. (2001). Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J. Biol. Chem. 276, Moir, R. D., Spann, T. P., Herrmann, H. and Goldman, R. D. (2000). Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J. Cell Biol. 149, Muchir, A., Bonne, G., van der Kooi, A. J., van Meegen, M., Baas, F., Bolhuis, P. A., de Visser, M. and Schwartz, K. (2000). Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum. Mol. Genet. 9, Novelli, G., Muchir, A., Sangiuolo, F., Helbling-Leclerc, A., D Apice, M. R., Massart, C., Capon, F., Sbraccia, P., Federici, M., Lauro, R. et al. (2002). Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am. J. Hum. Genet. 71, Park, Y. E., Hayashi, Y. K., Goto, K., Komaki, H., Hayashi, Y., Inuzuka, T., Noguchi, S., Nonaka, I. and Nishino, I. (2009). Nuclear changes in skeletal muscle extend to satellite cells in autosomal dominant Emery-Dreifuss muscular dystrophy/limb-girdle muscular dystrophy 1B. Neuromuscul. Disord. 19, Peter, M., Nakagawa, J., Doree, M., Labbe, J. C. and Nigg, E. A. (1990). In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61, Quijano-Roy, S., Mbieleu, B., Bonnemann, C. G., Jeannet, P. Y., Colomer, J., Clarke, N. F., Cuisset, J. M., Roper, H., De Meirleir, L., D Amico, A. et al. (2008). De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann. Neurol. 64, Raffaele Di Barletta, M., Ricci, E., Galluzzi, G., Tonali, P., Mora, M., Morandi, L., Romorini, A., Voit, T., Orstavik, K. H., Merlini, L. et al. (2000). Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery- Dreifuss muscular dystrophy. Am. J. Hum. Genet. 66, Sakaki, M., Koike, H., Takahashi, N., Sasagawa, N., Tomioka, S., Arahata, K. and Ishiura, S. (2001). Interaction between emerin and nuclear lamins. J. Biochem. 129, Shackleton, S., Lloyd, D. J., Jackson, S. N., Evans, R., Niermeijer, M. F., Singh, B. M., Schmidt, H., Brabant, G., Kumar, S., Durrington, P. N. et al. (2000). LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat. Genet. 24, Spann, T. P., Moir, R. D., Goldman, A. E., Stick, R. and Goldman, R. D. (1997). Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 136, Spann, T. P., Goldman, A. E., Wang, C., Huang, S. and Goldman, R. D. (2002). Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J. Cell Biol. 156, Speckman, R. A., Garg, A., Du, F., Bennett, L., Veile, R., Arioglu, E., Taylor, S. I., Lovett, M. and Bowcock, A. M. (2000). Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am. J. Hum. Genet. 66, Taniura, H., Glass, C. and Gerace, L. (1995). A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J. Cell Biol. 131, Taylor, M. R., Fain, P. R., Sinagra, G., Robinson, M. L., Robertson, A. D., Carniel, E., Di Lenarda, A., Bohlmeyer, T. J., Ferguson, D. A., Brodsky, G. L. et al. (2003). Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J. Am. Coll. Cardiol. 41, Tsai, M. Y., Wang, S., Heidinger, J. M., Shumaker, D. K., Adam, S. A., Goldman, R. D. and Zheng, Y. (2006). A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 311, Verstraeten, V. L., Broers, J. L., van Steensel, M. A., Zinn-Justin, S., Ramaekers, F. C., Steijlen, P. M., Kamps, M., Kuijpers, H. J., Merckx, D., Smeets, H. J. et al. (2006). Compound heterozygosity for mutations in LMNA causes a progeria syndrome without prelamin A accumulation. Hum. Mol. Genet. 15, Ward, G. E. and Kirschner, M. W. (1990). Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell 61, Zastrow, M. S., Vlcek, S. and Wilson, K. L. (2004). Proteins that bind A-type lamins: integrating isolated clues. J. Cell Sci. 117, Zastrow, M. S., Flaherty, D. B., Benian, G. M. and Wilson, K. L. (2006). Nuclear titin interacts with A- and B-type lamins in vitro and in vivo. J. Cell Sci. 119,
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
L aminopathies represent a heterogeneous group of genetic
 1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Available online at
 Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
H utchinson-gilford progeria syndrome (HGPS; MIM
 609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
THURSDAY, JANUARY
 THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C
 Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation
 Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Genotype phenotype correlations in laminopathies: how does fate translate?
 Nuclear Envelope Disease and Chromatin Organization 2009 257 Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S.
Nuclear Envelope Disease and Chromatin Organization 2009 257 Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S.
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Table S1. Primers and fluorescent probes used for qrt-pcr analysis of relative expression levels of PPP family phosphatases. gene name forward primer, 5-3 probe, 5-3 reverse primer,
SUPPLEMENTARY MATERIAL Table S1. Primers and fluorescent probes used for qrt-pcr analysis of relative expression levels of PPP family phosphatases. gene name forward primer, 5-3 probe, 5-3 reverse primer,
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy
 MOLECULAR MEDICINE REPORTS 12: 5065-5071, 2015 Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy LI ZHANG 1, HONGRUI SHEN 2, ZHE ZHAO 2, QI BING 2 and
MOLECULAR MEDICINE REPORTS 12: 5065-5071, 2015 Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy LI ZHANG 1, HONGRUI SHEN 2, ZHE ZHAO 2, QI BING 2 and
MEK1 Assay Kit 1 Catalog # Lot # 16875
 MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
Novel LMNA Mutation in a Taiwanese Family with Autosomal Dominant Emery-Dreifuss Muscular Dystrophy
 ASE REPORT Novel LMNA Mutation in a Taiwanese Family with Autosomal Dominant Emery-Dreifuss Muscular Dystrophy Wen-hen Liang, 1 hung-yee Yuo, 2 hun-ya Liu, 3 hee-siong Lee, 4 Kanako Goto, 5 Yukiko K. Hayashi,
ASE REPORT Novel LMNA Mutation in a Taiwanese Family with Autosomal Dominant Emery-Dreifuss Muscular Dystrophy Wen-hen Liang, 1 hung-yee Yuo, 2 hun-ya Liu, 3 hee-siong Lee, 4 Kanako Goto, 5 Yukiko K. Hayashi,
LMNA mutations in atypical Werner s syndrome
 Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Anti-Lamin B1/LMNB1 Picoband Antibody
 Anti-Lamin B1/LMNB1 Picoband Antibody Catalog Number:PB9611 About LMNB1 Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene. The nuclear lamina consists of a two-dimensional matrix of proteins
Anti-Lamin B1/LMNB1 Picoband Antibody Catalog Number:PB9611 About LMNB1 Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene. The nuclear lamina consists of a two-dimensional matrix of proteins
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth.
 Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth. a. Immunoblot for Usp9x protein in NRAS mutant melanoma cells
Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth. a. Immunoblot for Usp9x protein in NRAS mutant melanoma cells
Early-Onset LMNA-Associated Muscular Dystrophy with Later Involvement of Contracture
 JCN Open Access pissn 1738-6586 / eissn 2005-5013 / J Clin Neurol 2017;13(4):405-410 / https://doi.org/10.3988/jcn.2017.13.4.405 ORIGINAL ARTICLE Early-Onset LMNA-Associated Muscular Dystrophy with Later
JCN Open Access pissn 1738-6586 / eissn 2005-5013 / J Clin Neurol 2017;13(4):405-410 / https://doi.org/10.3988/jcn.2017.13.4.405 ORIGINAL ARTICLE Early-Onset LMNA-Associated Muscular Dystrophy with Later
T he LMNA gene encodes two nuclear envelope proteins,
 1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Supplementary Materials and Methods
 Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy
 RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein
![2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein](/thumbs/86/94743397.jpg) Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Recombinant Protein Expression Retroviral system
 Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Familial DilatedCardiomyopathy Georgios K Efthimiadis, MD
 Familial DilatedCardiomyopathy Georgios K Efthimiadis, MD Dilated Cardiomyopathy Dilated LV/RV, reduced EF, in the absence of CAD valvulopathy pericardial disease Prevalence:40/100.000 persons Natural
Familial DilatedCardiomyopathy Georgios K Efthimiadis, MD Dilated Cardiomyopathy Dilated LV/RV, reduced EF, in the absence of CAD valvulopathy pericardial disease Prevalence:40/100.000 persons Natural
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
a b G75 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server.
 a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3
 Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
The rabbit femoral artery was prepared and each arterial ring was permeabilized
 Online Supplement Nakmura et al. cgmp-dependent relaxation of smooth muscle Materials and Methods Measurement of tension The rabbit femoral artery was prepared and each arterial ring was permeabilized
Online Supplement Nakmura et al. cgmp-dependent relaxation of smooth muscle Materials and Methods Measurement of tension The rabbit femoral artery was prepared and each arterial ring was permeabilized
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
Supplementary Material
 Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two Chinese siblings: two case reports
 Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo
 Research Article 239 Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo Michael S. Zastrow 1, *, Denise B. Flaherty 2, Guy M. Benian 2 and Katherine L. Wilson 1, 1 Department of Cell
Research Article 239 Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo Michael S. Zastrow 1, *, Denise B. Flaherty 2, Guy M. Benian 2 and Katherine L. Wilson 1, 1 Department of Cell
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins
 Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies
 Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1
 Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy
 The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
Immunohistochemical Study of Dystrophin Associated Glycoproteins in Limb-girdle Muscular Dystrophies
 Dystrophin Immunohistochemical Study of Dystrophin Associated Glycoproteins in Limb-girdle Muscular Dystrophies NSC 89-2314-B-002-111 88 8 1 89 7 31 ( Peroxidase -AntiPeroxidase Immnofluorescence) Abstract
Dystrophin Immunohistochemical Study of Dystrophin Associated Glycoproteins in Limb-girdle Muscular Dystrophies NSC 89-2314-B-002-111 88 8 1 89 7 31 ( Peroxidase -AntiPeroxidase Immnofluorescence) Abstract
Phospho-AKT Sampler Kit
 Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Supplementary Information
 Supplementary Information Structural basis of improved second generation 3-nitro-tyrosine trna synthetases Richard B. Cooley, Jessica L. Feldman, Camden M. Driggers, Taylor Bundy, Audrey L. Stokes, P.
Supplementary Information Structural basis of improved second generation 3-nitro-tyrosine trna synthetases Richard B. Cooley, Jessica L. Feldman, Camden M. Driggers, Taylor Bundy, Audrey L. Stokes, P.
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
The Immunoassay Guide to Successful Mass Spectrometry. Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013
 The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Electronic Supplementary Information. Table of Contents
 Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy
 RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved
 1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
1 Supplemental Figure Legends Supplemental Figure 1 ELISA scheme to measure plasma total, mature and furin-cleaved PCSK9 concentrations. 4 Plasma mature and furin-cleaved PCSK9s were measured by a sandwich
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene
 Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene YUELIN ZHANG, WEIHUA FAN, MARK KINKEMA, XIN LI, AND
Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene YUELIN ZHANG, WEIHUA FAN, MARK KINKEMA, XIN LI, AND
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
Supporting Information
 Supporting Information Fujishita et al. 10.1073/pnas.0800041105 SI Text Polyp Scoring. Intestinal polyps were counted as described (1). Briefly, the small and large intestines were excised, washed with
Supporting Information Fujishita et al. 10.1073/pnas.0800041105 SI Text Polyp Scoring. Intestinal polyps were counted as described (1). Briefly, the small and large intestines were excised, washed with
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Antibodies: LB1 buffer For 50 ml For 10ml For 30 ml Final 1 M HEPES, ph 2.5 ml 0.5 ml 1.5 ml 50mM. 5 M NaCl 1.4 ml 280 µl 0.
 Experiment: Date: Tissue: Purpose: ChIP-Seq Antibodies: 11x cross-link buffer: Regent Stock Solution Final Vol for 10 ml of 11xstock concentration 5 M NaCl 0.1M 0.2 ml 0.5 M EDTA 1 mm 20 ul 0.5 M EGTA,
Experiment: Date: Tissue: Purpose: ChIP-Seq Antibodies: 11x cross-link buffer: Regent Stock Solution Final Vol for 10 ml of 11xstock concentration 5 M NaCl 0.1M 0.2 ml 0.5 M EDTA 1 mm 20 ul 0.5 M EGTA,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2*
 Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Western Immunoblotting Preparation of Samples:
 Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Table S1. Sequence of human and mouse primers used for RT-qPCR measurements.
 Table S1. Sequence of human and mouse primers used for RT-qPCR measurements. Ca9, carbonic anhydrase IX; Ndrg1, N-myc downstream regulated gene 1; L28, ribosomal protein L28; Hif1a, hypoxia inducible factor
Table S1. Sequence of human and mouse primers used for RT-qPCR measurements. Ca9, carbonic anhydrase IX; Ndrg1, N-myc downstream regulated gene 1; L28, ribosomal protein L28; Hif1a, hypoxia inducible factor
Supplementary Figure 1: Co-localization of reconstituted L-PTC and dendritic cells
 a CD11c Na + K + ATPase Na + K + ATPase CD11c x-y CD11c Na + K + ATPase Na + K + ATPase CD11c x-z c b x-y view BoNT NAPs CD11c BoNT CD11c NAPs BoNT NAPs CD11c 90 x-z view Apical Basolateral Supplementary
a CD11c Na + K + ATPase Na + K + ATPase CD11c x-y CD11c Na + K + ATPase Na + K + ATPase CD11c x-z c b x-y view BoNT NAPs CD11c BoNT CD11c NAPs BoNT NAPs CD11c 90 x-z view Apical Basolateral Supplementary
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Proteins that bind A-type lamins: integrating isolated clues
 Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
Predictive PP1Ca binding region in BIG3 : 1,228 1,232aa (-KAVSF-) HEK293T cells *** *** *** KPL-3C cells - E E2 treatment time (h)
 Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Phenylketonuria (PKU) Structure of Phenylalanine Hydroxylase. Biol 405 Molecular Medicine
 Phenylketonuria (PKU) Structure of Phenylalanine Hydroxylase Biol 405 Molecular Medicine 1998 Crystal structure of phenylalanine hydroxylase solved. The polypeptide consists of three regions: Regulatory
Phenylketonuria (PKU) Structure of Phenylalanine Hydroxylase Biol 405 Molecular Medicine 1998 Crystal structure of phenylalanine hydroxylase solved. The polypeptide consists of three regions: Regulatory
