Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange
|
|
|
- Ginger Davis
- 5 years ago
- Views:
Transcription
1 Am J Physiol Renal Physiol 299: F265 F272, First published April 14, 2010; doi: /ajprenal Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange Justin Yuan and Thomas L. Pannabecker Department of Physiology, University of Arizona Health Sciences Center, Tucson, Arizona Submitted 4 February 2010; accepted in final form 12 April 2010 Yuan J, Pannabecker TL. Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange. Am J Physiol Renal Physiol 299: F265 F272, First published April 14, 2010; doi: /ajprenal Pathways and densities of descending vasa recta (DVR) and ascending vasa recta (AVR) in the outer zone of the inner medulla (IM) were evaluated to better understand medullary countercurrent exchange. Nearly all urea transporter B (UT-B)-positive DVR, those vessels exhibiting a continuous endothelium, descend with little or no branching exclusively through the intercluster region. All DVR have a terminal fenestrated (PV-1-positive) segment that partially overlaps with the UT-B-positive segment. This fenestrated segment descends a distance equal to 15% of the length of the connecting UT-B-positive segment before formation of the first branch. The onset of branching is indicative of vessel entry into the intracluster region. The number density of UT-B-positive DVR at 3,000 m below the OM-IM boundary is 60% lower than the density at 400 m below the OM-IM boundary, a result of DVR joining to fenestrated interconnecting vessels and an overall decline in UT-B expression. AVR that lie in the intercluster region (designated AVR 2 ) lie distant from CDs and ascend to the OM-IM boundary with little or no branching. AVR 2a represent a subcategory of AVR 2 that abut DVR. The mean DVR length (combined UT-B- and PV-1-positive segments) nearly equals the mean AVR 2a length, implying a degree of overall equivalence in fluid and solute countercurrent exchange may exist. The AVR 2 /DVR ratio is 2:1, and the AVR 2a /DVR ratio is 1:1; however, the AVR/DVR ratio determined for the full complement of fenestrated vessels is 4:1. The excess fenestrated vessels include vessels of the intracluster region (designated AVR 1). Countercurrent exchange between vasa recta occurs predominantly in the intercluster region. This architecture supports previous functional estimates of capillary fluid uptake in the renal IM. concentrating mechanism; countercurrent system; computer-assisted reconstruction; NaCl transport; urea transport DURING THE PAST TWO DECADES, the three-dimensional architecture of the renal medulla has become increasingly pertinent to the development of advanced computational models of medullary function (4, 10, 11, 13, 30, 31). Recent advances have been made in understanding the geometrical relationships of collecting ducts (CDs) and nephrons of the outer medulla (OM) and inner medulla (IM) (25, 32 34). However, much remains to be learned about compartmentation arising from dynamic flow of fluid and solutes between these structures, compartmentation that is essential for understanding the role of the renal medulla in processes of urine concentration, sodium and water balance, and the long-term control of arterial pressure (5, 19). For each of these processes, blood flow through the medullary vasculature is highly integrated with epithelial Address for reprint requests and other correspondence: T. L. Pannabecker, Univ. of Arizona Health Sciences Center, Dept. of Physiology, AHSC 4130, 1501 N. Campbell Ave., Tucson, AZ ( pannabec@u.arizona.edu). fluid and solute fluxes, and a more complete understanding of vessel architecture and connectivity between descending vasa recta (DVR), interconnecting capillary networks, and ascending vasa recta (AVR) will more clearly define this compartmentation. IM vasculature consists of two types: vessels with a continuous endothelium (DVR), expressing the urea transporter B (UT-B), and vessels with a discontinuous (fenestrated) endothelium (AVR), expressing PV-1, a component of the fenestral diaphragm. A type of AVR commonly referred to as interconnecting or communicating capillaries, exhibits no known structural or functional distinctions but is considered to be spatially resolved from remaining vessels (19). DVR and AVR exhibit little or no branching, and interconnecting capillaries generally exhibit repeated branching and form sparse networks (17, 19, 27). With the tip of the papilla as a reference point, the DVR are all afferent (that is, they carry blood in a descending direction) and AVR and interconnecting capillaries are generally considered efferent. However, functional studies of Zimmerhackl and colleagues (36) suggest that 10 15% of DVR may have terminal fenestrated segments that carry blood in a descending direction. DVR, grouped in vascular bundles, enter the IM and at various levels give rise to interconnecting capillaries, which form networks that route blood flow along the corticopapillary axis. Some DVR, albeit relatively few, descend to the tip of the papilla before breaking up into capillary networks (17, 27). At various levels, interconnecting capillaries join AVR that are grouped with DVR in vascular bundles. These AVR then ascend along the corticopapillary axis, exiting the IM into the OM. DVR and some AVR participate in countercurrent solute exchange, a process considered important for preservation of the IM interstitial solute gradient. AVR and interconnecting capillaries theoretically could also engage in countercurrent exchange with descending thin limbs of Henle (DTLs), prebend segments, and CDs. Exchanges in a cocurrent fashion potentially occur between AVR and ascending thin limbs of Henle (ATLs) (23 25). In all cases, the parallel expression of fluid and solute channels and transporters in adjacent vessels and tubules would define, in part, the exchange processes that take place. One important goal in understanding renal medullary function is to clearly define the limits to which vascular networks, perhaps variably, lead to homogeneous or heterogeneous interstitial compartmental composition. The analysis reported herein provides a framework for quantitative studies that explore how fluid and solute are distributed and exchanged amongst medullary compartments by way of vascular pathways /10 Copyright 2010 the American Physiological Society F265
2 F266 METHODS Animals. Young male Munich-Wistar rats (average wt, 90 g) were purchased from Harlan (Indianapolis, IN). The animals were euthanized with pentobarbital sodium (0.2 ml/100 g body wt) or with CO 2. All experiments were conducted in accordance with The Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 1996) and approved by the Institutional Animal Care and Use Committee. Tissue preparation and immunohistochemistry. Kidneys were prepared for immunohistochemistry as described previously (23, 24). Serial, transverse sections 1 m thick and no greater than 5 m apart were prepared for each kidney. DVR were labeled with a polyclonal antibody raised in rabbits against rat UT-B (diluted 1:200, provided by Drs. Jeff Sands and Janet Klein, Emory University). Fenestrated vessels were labeled with a polyclonal antibody raised in chickens against rat PV-1, a plasmalemmal vesicle protein formerly known as gp68 (diluted 1:500, provided by Radu Stan, Dartmouth College). PV-1 is a component of fenestral diaphragms. The structural and functional role of PV-1 is poorly understood. In the rat IM, the AVR and interconnecting capillaries are fenestrated and believed to have diaphragms. CDs were labeled for aquaporin-2 (AQP2; diluted 1:200, raised in goats, Santa Cruz Biotechnology). Secondary antibodies conjugated to fluorescent probes (Invitrogen/Molecular Probes or Jackson ImmunoResearch) were applied as described previously (23, 24). Sections were mounted with Dako fluorescent mounting medium (Carpinteria, CA) and were viewed with epifluorescence microscopy. Image analysis. Separate stacks of digitized, serial images were generated by capturing AQP2, UT-B, or PV-1 immunofluorescence from each tissue section. Partial three-dimensional reconstructions were created for some, but not all tissue. Quantitative analyses were carried out on two-dimensional images using PhotoShop (Adobe) and the Image Processing Toolkit (Reindeer Graphics). Determining CD cluster boundaries with a Euclidean distance map. Each pixel in the background is assigned a luminosity value equal to its distance from the nearest edge of any CD; in this case, pixels at a greater distance are assigned a darker value (28). This value encodes the straight-line distance to the nearest point on any CD edge. The Euclidean distance map (EDM) is then skeletonized to produce a boundary around each CD. The process of skeletonization sets pixels in white if they have neighbors that are darker. The boundaries are then linked so as to encompass individual CD clusters. Boundaries are represented in images as contour lines that correspond to the linear array of pixels that are most distant from any CD. The volume shrinkage factor for ethanol dehydration of rat medullary tissue has been reported to be 20% (1), and the linear shrinkage factor has been reported to be 20 25% (6). Thus the linear distances that we report would underestimate the distances measured for fresh tissue by a maximum of 20 25%. Statistical analyses. Data combined from three or more samples are reported as means SE (n number of replicates). The statistical significance of differences between the means for each category of multiple data sets was determined with ANOVA and Duncan s post hoc test (P 0.05). RESULTS Clusters of CDs that coalesce as they descend the corticopapillary axis form the organizing motif in the outer zone of the IM (the first mm of the IM). At the OM-IM boundary, each primary CD cluster consists of 6 12 CDs that coalesce into a single CD deep in the outer zone of the IM. The terminal CDs from these primary CD clusters also coalesce to form a single CD mm below the OM-IM boundary (in the inner zone of the IM). Therefore, about five to six of the primary clusters seen at the OM-IM boundary make up a larger secondary CD cluster that ends at or near the ducts of Bellini. This organization of CDs produces two clearly defined interstitial regions in the transverse dimension, the intercluster and intracluster regions (26). The intercluster region is composed of the interstitial space that separates CDs of adjacent primary clusters. The boundary for each primary CD cluster is defined by the EDM border (see METHODS). This border lies in the intercluster region. The intracluster region is the region encompassed by CDs of each cluster (8). DTLs, ATLs, and blood vessels are arranged within and outside clusters in an organized radial fashion. This distinctive radial tubulovascular organization may be important in establishing functionally distinct radial compartments; integration of radial and axial compartmentation may be essential to the urine concentrating mechanism (12, 14, 25). Vascular bundles in intercluster region of the outer zone of the IM. Vascular bundles carrying DVR (UT-B-positive vessels) and associated AVR 2 (PV-1-positive vessels) lie within the intercluster region (intercluster AVR are designated AVR 2 ). For the first several hundred micrometers below the OM-IM boundary, these vessels remain relatively tightly packed (Fig. 1). However, even in this region of the IM, these vascular bundles are more loosely organized than those of the OM, with wider interstitial space between vessels (19). As the UT-B-positive DVR descend through the outer IM, the bundles break up and DVR tend to fan out from each other along the EDM borders, remaining compartmentalized within the intercluster region throughout their entire descent of the IM outer zone. This architecture can be seen for DVR and AVR 2 in transverse sections at 400 and 1,500 m below the OM-IM boundary (Fig. 2). DVR and AVR 2 in the nine vascular bundles of Fig. 2A are the same as those shown in the bundles of Fig. 2B. Fenestrated descending vessels of the outer zone of the IM. All UT-B-positive DVR lying within the intercluster regions of two secondary CD clusters were reconstructed beginning at a Fig. 1. Five vascular bundles from a transverse section at 100 m below the outer medullary (OM)-inner medullary (IM) boundary [collecting ducts (CDs), aquaporin-2 (AQP2)/blue; descending vasa recta (DVR), urea transporter B (UT-B)/green; vessels of the intracluster region (AVR 1) and in the intercluster region (AVR 2), PV-1/red]. Vascular bundles are located predominantly in the intercluster region, distant from CDs. In the intracluster region, fenestrated vessels abut CDs. Scale bar 100 m.
3 F267 Fig. 2. Transverse sections that include 2 secondary CD clusters (see RESULTS for definition of secondary CD cluster) (CDs, AQP2/blue; DVR, UT-B/green; AVR1 and AVR2, PV-1/red). A: 400 m below the OM-IM boundary. B: 1,500 m below the OM-IM boundary. Within bundles, DVR descend from, and AVR2 ascend to, the upper boundary of reconstruction at 400 m below the OM-IM boundary. Each secondary CD cluster consists of 5 primary clusters; secondary cluster 1, bottom 5 white borders; secondary cluster 2, top 5 white borders (compare with Figs. 1 3 in Ref. 26). Four vascular bundles (VB) are associated with cluster 1, and 5 vascular bundles are associated with cluster 2 (outlined with green). Inset: magnification of vascular bundle 2 from cluster 1 (arrows) (CDs, AQP2/blue; DVR, UT-B/green; AVR2, PV-1/overdrawn with white; AVR1, PV-1/red). Scale bars 100 m (figure) and 25 m (inset). point 400 m below the OM-IM boundary and continuing at least as far as their first branch point. Beyond this point, the degree of branching becomes more frequent, signaling initiation of a capillary network, and presumably ascending flow. Few DVR within these two clusters directly join AVR in a hairpin-type loop configuration, at least through the first 3,200 m below the OM-IM boundary; instead, they connect to fenestrated vessels that form networks within the intracluster region (8). Each UTB-positive descending vas rectum overlaps with a PV-1-positive segment at its terminus for virtually all reconstructed DVR. This confirms earlier observations (23). The vascular arrangement is depicted diagrammatically in Fig. 3. Along this overlap, in the axial descent, there is a gradual reduction in UT-B expression and a gradual increase in PV-1 expression. The lengths of the overlapping UT-B- and PV-1positive segments for DVR in reconstructed secondary CD clusters 1 and 2 averaged (n 42) and m (n 46; means SE), respectively. The terminal fenestrated segment (PV1-positive, UT-B-negative) continues to descend a variable distance before forming its first branch, for 75% of all DVR. Fenestrated descending segments (distinguishable only for reconstructed vessels) are designated DVR. For the remaining 25% of DVR, a secondary fenestrated vessel immediately branches off from the DVR at the beginning of the terminal (primary) fenestrated segment. The primary fenestrated segment that continues descending beyond this secondary branch potentially carries blood in either descending or ascending direction. For vessels of clusters 1 and 2, these segments measured (n 32) and m (n 31; means SE), respectively, equal to 14 4 and 18 4% of the length of the connecting UT-B-positive DVR. The length of the terminal fenestrated segment is not significantly correlated with the length of the connecting UT- B-positive descending vas rectum segment (R and for clusters 1 and 2). AVR/DVR number density ratios of the outer zone of the IM. The number densities of all PV-1-positive vessels and all UT-B-positive vessels within random IM transverse sections, such as the section shown in Fig. 1, are summarized in Fig. 4A. Through the first 2,000 m of the IM, the PV-1/UT-B number density ratio ranges between 4.2 and 5.9. At levels deeper than 2,000 m, the fractional decrease in number density of UT-B-positive vessels is greater than the fractional decrease in number density of PV-1-positive vessels, resulting in a very high PV-1/UT-B ratio. UTB-positive vessels are nearly undetectable at deeper than 3,000 m below the OM-IM boundary (24). As noted, some fraction of PV-1-positive (fenestrated) vessels are actually descending vessels. To assess this fraction, we analyzed vessels in two clusters that have been reconstructed through the first 3,200 m below the OM-IM boundary. In addition to distinguishing fenestrated descending segments that might otherwise be considered as ascending, these reconstructions make clear distinctions between AVR2 that ascend through the intercluster region, and AVR1, those AVR that ascend through the intracluster region (8). The AVR/DVR ratio of these vessels through the first 1,500 m below the OM-IM boundary is 4:1, rising at deeper levels (Fig. 4B). For the outer zone of the IM, the highest percentage of fenestrated DVR occurs at 3,000 m below the OM-IM boundary (Table 1). AVR/DVR number density ratios of countercurrent exchanging vessels in intercluster region. AVR2 of the outer zone of the IM do not abut CDs and can be spatially discriminated from PV-1-positive vessels that lie in the intracluster region and do abut CDs (AVR1) (Figs. 1 and 2). AVR2 likely play a dominant role in the process of countercurrent exchange with DVR due
4 F268 (including fenestrated descending segments) and apposing nonbranching AVR 2a within nine vascular bundles from two secondary CD clusters were compared to evaluate this degree of coupling between adjacent vessels. These vessel lengths are depicted in Supplemental Figs. 1 and 2 (all supplementary material for this article is available on the AJP-Renal Physiology website). As noted, apposing AVR 2a include those AVR that do not abut CDs and do abut DVR in transverse sections, as shown for vascular bundles in Fig. 2A. All vessels that lie within the EDM border plus those lying within 1 vessel diameter outside the EDM border were included for each bundle. These criteria are sufficient to identify all DVR and most AVR 2a that form individual vascular bundles. The bundles consist of variable numbers of DVR and AVR 2a. Vessel lengths within bundles are highly variable (Fig. 5). The lengths of AVR 2a in bundles of two secondary CD clusters are longer on average than the lengths of UT-B-positive DVR (reaching statistical significance in one of two secondary CD clusters) and are not significantly different from the lengths of UT-Bpositive DVR including their fenestrated descending segments Fig. 3. Diagram of vasa recta architecture in the outer zone of the IM. UT-B-positive (shaded light, nonfenestrated) DVR descends along the corticopapillary axis. UT-B-positive segment overlaps (small bracket) with PV-1 (shaded dark, fenestrated) and is continuous with a descending PV-1-positive, UT-B-negative DVR (large bracket). This PV-1-positive segment is continuous with AVR 1, which lie within the CD cluster (intracluster region) (8). AVR 1 connect to AVR 2 in vascular bundles of the intercluster region. Arrows denote blood flow direction. AVR 1, PV-1-positive intracluster AVR and interconnecting capillaries that do abut CDs; AVR 2, ascending PV-1-positive intercluster vessels that do not abut CDs, and do or do not abut DVR. to their proximity to DVR. For random transverse sections from various depths below the OM-IM boundary, we determined the number densities of all fenestrated vessels that do not abut CDs and 1) that do or do not abut UT-B-positive DVR (AVR 2 )or2) that do abut UT-B-positive DVR (AVR 2a ). Note that AVR 2a are a subset of AVR 2. The distribution of AVR 1 is assessed in a separate manuscript (8). Through the first 3,000 m of the outer zone of the IM the AVR 2a /DVR ratio ranges between 0.71 and 1.17 (Table 2). In fact, each descending vas rectum may abut as many as two to three AVR as a result of compact bundling (Fig. 2A, inset). Through the first 3,000 m of the outer zone of the IM, the AVR 2 /DVR ratio ranges between 1.69 and 2.51 (Table 2). Notably, the AVR 2a /DVR ratios are significantly lower than the AVR 2 /DVR ratios along nearly the entire corticopapillary axis of the outer IM (ANOVA; P 0.05); the AVR 2a /DVR ratio is 1.0, and the AVR 2 /DVR ratio is 2.0. The AVR 2 /DVR ratios and AVR 2a /DVR ratios that were determined in random transverse sections from multiple kidneys are comparable to values determined for reconstructed vessels in two secondary CD clusters from a single kidney (Table 1). Relative lengths of DVR and AVR 2a within vascular bundles of the outer zone of the IM. The lengths of descending and ascending vessels that lie adjacent to each other along the corticopapillary axis should provide an index of the relative degree of fluid and solute countercurrent exchange that takes place along the corticopapillary axis. The lengths of DVR Fig. 4. Number densities and ratios of IM blood vessels. A: number densities and ratios for UT-B-positive and PV-1-positive vessels that lie within random cross-sectional areas of the IM from 3 kidneys. Values are means SE. Ratio means marked with an asterisk are not significantly different from each other (ANOVA; P 0.05). B: number densities and ratios for reconstructed DVR and AVR that lie within the borders of CD clusters 1 and 2. Tracing UT-B-positive DVR and their fenestrated segments becomes increasingly less accurate beyond 2 mm for technical reasons. Incomplete recognition of fenestrated DVR combined with biological variation probably account for higher AVR/DVR ratios at 2,000 m below the OM-IM boundary. Compare with Fig. 4 in Ref. 8.
5 Table 1. Number densities and ratios of reconstructed ascending vasa recta (ascending fenestrated vessels) and descending vasa recta (UT-B-positive or PV-1-positive descending vessels) that lie within cross-sectional areas defined by borders of 2 secondary CD clusters Distance Below OM-IM Boundary, m Number of Vessels/mm ,500 2,000 3,000 Cluster 1 AVR 2 1,098 1, AVR 2a DVR %DVR fen AVR 2/DVR ratio AVR 2a/DVR ratio Cluster 2 AVR AVR 2a DVR %DVR fen AVR 2/DVR ratio AVR 2a/DVR ratio UT-B, urea transporter B; OM, outer medulla; IM, inner medulla; AVR, ascending vasa recta; DVR, descending vasa recta. AVR that do not abut collecting ducts (CDs) and either do or do not abut DVR (AVR 2) lie primarily within the intercluster region (Fig. 2A). AVR that do not abut CDs and do abut DVR (AVR 2a) lie solely within the intercluster region (Fig. 2A). %DVR fen, percentage of DVR that are fenestrated. (Fig. 6). The fenestrated descending segments bring the mean DVR lengths nearer to the mean AVR 2a lengths. Although vascular pairs repeatedly dissociate and reassociate with different partners along the corticopapillary axis, countercurrent exchange overall appears to occur between a relatively fixed ratio and nearly equal lengths of AVR 2a and DVR within the outer zone of the IM. The lengths of ascending vessels within bundles give an indication of the approximate depths at which AVR 1 networks (8) feed into the countercurrent exchange process. For two secondary CD clusters that were reconstructed between the interval of 400 and 3,200 m below the OM-IM boundary, nearly every vascular bundle includes at least several AVR 2a that are shorter than and several that are longer than the length of the longest DVR (Supplemental Figs. 1 and 2). Thus most DVR are sufficiently long so as to connect to AVR 2a that lie within the same bundle. Also, for most bundles there are relatively few DVR that extend to levels deeper than 3,200 m below the OM-IM boundary. DISCUSSION F269 Three-dimensional functional reconstructions of IM vasa recta reveal a number of spatial relationships that may be significant for the process of vascular countercurrent exchange and the urinary concentrating mechanism. Previous studies have shown that CD clusters form the organizing motif in the IM with nephrons and vessels arranged within and around them in an organized fashion (22, 23). This architecture leads to two distinct interstitial compartments in the transverse dimension, the intercluster and intracluster interstitial regions. The vascular elements associated with these two compartments are summarized in Fig. 3. In addition, there appears to be a repeating array of discrete compartments that we refer to as interstitial nodal spaces (INSs) that are formed by two fenestrated capillaries adjacent to a CD, with one or two ATLs or prebend segments lying opposite the CD (23). We and our colleagues have hypothesized that INSs may play a significant role in a solute-separation, solute-mixing mechanism for concentrating urine in the IM (14, 21, 25). We have shown here that unbranched fenestrated vessels that are positioned within the intercluster region and near UTB-positive DVR within the vascular bundles (AVR 2, Tables 1 and 2) constitute a significant fraction of the intercluster vessels at each transverse level examined. Their spatial orientation, such as their position within the intercluster region, their distance from CDs, their proximity to DVR, and their unbranched segmentation uniquely characterize these vessels as AVR 2. These characteristics of AVR 2 distinguish them from fenestrated vessels that typically lie within m ofacd (23) and which undergo branching and fusing to form capillary networks (AVR 1 ). Importantly, AVR 1, which lie in the intracluster region, connect to AVR 2, which lie in the intercluster region. We have described elsewhere the intracluster architecture of AVR 1 in detail for reconstructed IM vascular networks (8). For purposes of categorizing populations of fenestrated vessels that are structurally and possibly functionally distinct, we consider any ascending vas rectum that abuts a CD (AVR 1 ) to lie within the CD cluster. However, some of these vessels may have one surface abutting a CD and an opposite surface facing DVR in the intercluster region; these vessels could be Table 2. Number densities and ratios of UT-B-positive vessels (DVR) and PV-1-positive vessels (AVR) that lie within random cross-sectional areas of IM from 3 kidneys Distance Below OM-IM Boundary, m Number of Vessels/mm ,500 2,000 3,000 AVR AVR 2a DVR AVR 2/DVR ratio ANOVA 3,9 4,10 1,5,7,11 2,6,8,12 AVR 2a/DVR ratio ANOVA 1,2 3,4,5,6 7,8 9,10,11,12 Values are means SE. AVR that do not abut CDs and either do or do not abut DVR (AVR 2) lie primarily within the intercluster region (Fig. 2A). AVR that do not abut CDs and do abut DVR (AVR 2a) lie solely within the intercluster region (Fig. 2A). Fenestrated descending vessels cannot be discerned in these images. Ratio means that share a common numeral (ANOVA, P 0.05) are significantly different from each other.
6 F270 Fig. 5. Comparisons between mean lengths of DVR (including PV-1-positive segment) and AVR 2a for vessels of 4 vascular bundles from secondary CD cluster 1 (black circles) and for 5 vascular bundles from secondary CD cluster 2 (white squares). Values are means SE. The 2 clusters and 9 bundles are outlined in Fig. 2A. DVR and AVR 2a lie within the reconstructed interval of 400 and 3,200 m below the OM-IM boundary. Line of identity is shown. uniquely positioned to engage in countercurrent exchange and also interact with INSs. IM blood flow is a dynamic process involving multiple regulatory mechanisms constrained within a fixed architecture (8, 18, 19). Blood flow rates and flow patterns likely influence the degree to which fluid and solute derived from different endothelial and epithelial compartments become intermixed (35) and the extent of fluid and solute countercurrent exchange that occurs within each vascular bundle. The marked variation in length between DVR and AVR 2a for each vascular bundle (Fig. 5), combined with near equivalent average length overall for DVR and AVR 2a within each secondary CD cluster (Fig. 6) imply that a degree of countercurrent exchange uniformity may exist for each bundle. This would be particularly true if DVR-to-AVR 2a connections exist as a uniform, cascading arrangement along the corticopapillary axis. However, since for some bundles, a number of AVR 2a in the IM outer zone are longer than UT-B-positive DVR (Supplemental Figs. 1 and 2), some fraction of countercurrent exchange appears to occur between AVR 2a that arise from the deep IM and DVR that recycle solutes to shallower levels. This would seem at odds with a system whose primary goal is to recycle urea to the deepest IM. Blood flow through AVR 1 will further influence countercurrent systems. In this regard, the arrays of INSs or microdomains that lie alongside CDs in the intracluster region may be sites of high osmolality due to reabsorption of NaCl, urea, and water from ATLs and CDs (14, 25). Abutting capillaries may provide a low-resistance sink for water and solutes. Solutes are either retained within AVR 1 of the intracluster region or pass into the intercluster region as AVR 1 ascend the corticopapillary axis. The extent to which vessels distribute solutes among these two compartments may further promote or constrain the multiple countercurrent systems that have been hypothesized to exist in the intracluster and intercluster regions (25). The AVR/DVR number density ratio is important in accounting for mass balance of fluid and solute flows into and out of the IM. All vessels leaving the IM are generally considered to pass into the OM by way of vascular bundles (19), although there is evidence that some take an alternate pathway (8). The AVR 2 /DVR ratio of 2:1, determined for vessels positioned in the intercluster region along the entire corticopapillary axis to a point 3,000 m below the OM-IM boundary (outer zone of the IM) (Tables 1 and 2) provides a structural basis supporting previous functional studies (2, 16, 36) that have derived a comparable ratio. Notably, Zimmerhackl et al. (36) visualized blood flow patterns with videomicroscopy and combined these data with protein concentration determinations in the Munich- Wistar rat, and estimated the functional AVR/DVR ratio to lie between 2.1:1 and 2.4:1 at 2,000 m above the papilla tip. Böttcher and Steinhausen (2) estimated an AVR/DVR ratio of 2.2:1 for the Munich-Wistar rat based on blood flow patterns in situ for exposed papilla at 500 m above the papilla tip on the papillary surface. Also, for the hamster, Marsh and Segel (16) reported a mean AVR/DVR ratio of 1.71:1, estimated from blood flow patterns in situ for exposed papilla at 3,000 m below the OM-IM boundary on the papillary surface. On the other hand, an AVR/DVR ratio of between about 4:1 and 6:1, based on number densities of all fenestrated and UTB-positive vessels along the outer zone of the IM (Fig. 4A), approximates the ratio of 3.96:1 determined at 2,000 m Fig. 6. Lengths of vessel segments in vascular bundles of the outer zone of the IM. A: secondary CD cluster 1 (n 4). B: secondary CD cluster 2 (n 5). Values are means SE. Sample means that share a common symbol are not significantly different from each other (ANOVA; P 0.05).
7 above the papillary tip by Holliger et al. (7) but varies from the ratio of 2:1 for the same region in the Sprague-Dawley rat (15). AVR/DVR ratios determined in the latter two studies were based on morphological criteria for the full complement of fenestrated and nonfenestrated vessels. Assuming our study and the study by Holliger et al. (7) include representative areas from the IM, our ratio of 10.8:1 for random cross-sectional areas at 3,000 m below the OM-IM boundary (Fig. 4A) accounts only for 40% of all DVR. Reconstructed vessels confirm that at 3,000 m below the OM-IM boundary the percentage of DVR that are fenestrated is 60 70% (66.7% for both clusters) (Table 1). This confirms and extends our previous report (24) and that of Kim et al. (9), citing evidence of reduced UT-B expression in the terminal papilla. Existence of a significant number of fenestrated DVR supports functional studies that have estimated total plasma outflow from the IM via AVR exceeds inflow by 30% (36), the excess outflow representing fluid uptake. A significant degree of uptake would occur in the intracluster region (8). A higher ratio of fenestrated to UT-B-positive vessels in the inner zone of the IM (the terminal mm of the IM) (3, 15, 29) suggests that UTB-mediated countercurrent urea exchange between AVR and DVR is a feature primarily of the outer zone of the IM; if a significant degree of solute recycling does occur between descending and ascending vessels in the inner zone of the IM, it likely involves primarily fenestrated vessels. A disproportionately large fraction of fenestrated descending and ascending vessels in the inner zone of the IM could account for the remarkably equivalent Na and urea permeabilities reported by Pallone et al. (20) for DVR and AVR at the papillary tip of the adult female Munich-Wistar rat. Countercurrent exchange between DVR and AVR in the outer zone of the IM may therefore be more selective for urea, and in the inner zone of the IM may more permissively include NaCl and urea, though both solutes are exchanged to some degree throughout the IM (19). This further supports the concept that the inner and outer zones play functionally distinct roles in the urine concentrating mechanism (25, 26), a concept based, in part, on the gradual disappearance of distinct intracluster and intercluster regions in the inner zone. In summary, we have introduced a set of parameters that more clearly define those fenestrated vessels that play a major role in the process of countercurrent exchange between IM DVR and AVR. This population of AVR lies outside CD clusters and accounts for, at most, 50% of all fenestrated vessels in the outer zone of the IM. Additional fenestrated vessels are spatially more closely associated with CDs (AVR 1 ). The existence of fenestrated descending segments at DVR termini tends to equalize the overall lengths of descending and ascending segments participating in countercurrent exchange. The vascular architecture described here supports former functional studies suggesting countercurrent-exchanging AVR outnumber DVR by a factor of about two (36). ACKNOWLEDGMENTS Insightful discussions about the dynamic urinary concentrating mechanism with long-time colleagues Harold Layton, Anita Layton, and Bill Dantzler are gratefully acknowledged. We thank Radu Stan of Dartmouth College, and Jeff Sands and Janet Klein of Emory University for providing antibodies. Brandi Hoopes and Collin Laufenberg contributed to image analysis as part of their undergraduate degree requirements at the University of Arizona. GRANTS This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the authors. REFERENCES F Bankir L, Fischer C, Fischer S, Jukkala K, Specht HC, Kriz W. Adaptation of the rat kidney to altered water intake and urine concentration. Pflügers Arch 412: 42 53, Bottcher W, Steinhausen M. Microcirculation of the renal papilla of rats under control conditions and after temporary ischemia. Kidney Int 10: S74 S80, Bulger RE, Trump BF. Fine structure of the rat renal papilla. Am J Anat 118: , Chen J, Layton AT, Edwards A. A mathematical model of oxygen transport in the rat outer medulla: I. Model formulation and baseline results. Am J Physiol Renal Physiol 297: F537 F548, Cowley AW Jr. Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1 R15, Han JS, Thompson KA, Chou CL, Knepper MA. Experimental tests of three-dimensional model of urinary concentrating mechanism. JAmSoc Nephrol 2(12): , Holliger C, Lemley K, Schmitt C, Thomas F, Robertson C, Jamison RL. Direct determination of vasa recta blood flow in the rat renal papilla. Circ Res 53: , Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol (doi: /ajprenal ). 9. Kim YH, Kim DU, Han KH, Jung JY, Sands JM, Knepper MA, Madsen KM, Kim J. Expression of urea transporters in the developing rat kidney. Am J Physiol Renal Physiol 282: F530 F540, Layton AT, Layton HE. A region-based model framework for the rat urine concentrating mechanism. Bull Math Biol 65: , Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346 F1366, Layton AT, Layton HE, Dantzler WH, Pannabecker TL. The mammalian urine concentrating mechanism: hypotheses and uncertainties. Physiology 24: , Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Two modes for concentrating urine in rat inner medulla. Am J Physiol Renal Physiol 287: F816 F839, Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973 F987, MacPhee PJ. Fluid uptake by the renal medullary vasa recta: An estimate based on a quantitative analysis of the distribution of fenestrae in the vasa recta of young Sprague-Dawley rats. Exp Physiol 83: 23 34, Marsh DJ, Segel LA. Analysis of countercurrent diffusion exchange in blood vessels of the renal medulla. Am J Physiol 221: , Moffat DB, Fourman J. The vascular pattern of the rat kidney. J Anat 97: , Navar LG, Inscho EW, Majid DSA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: , Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153 R1175, Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 93: , Pannabecker TL. Loop of Henle interaction with interstitial nodal spaces in the renal inner medulla. Am J Physiol Renal Physiol 295: F1744 F1751, Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767 F774, Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355 F1366, 2006.
8 F Pannabecker TL, Dantzler WH. Three-dimensional architecture of collecting ducts, loops of Henle, and blood vessels in the renal papilla. Am J Physiol Renal Physiol 293: F696 F704, Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271 F1285, Pannabecker TL, Henderson C, Dantzler WH. Quantitative analysis of functional reconstructions reveals lateral and axial zonation in the renal inner medulla. Am J Physiol Renal Physiol 294: F1306 F1314, Rollhauser H, Kriz W, Heinke W. Das gefass-system der rattenniere. Z Zellforsch 64: , Russ JC. The Image Processing Handbook. Boca Raton, FL: CRC, Schwartz MM, Karnovsky MJ, Venkatachalam M. Ultrastructural differences between rat inner medullary descending and ascending vasa recta. Lab Invest 35: , Wexler AS, Kalaba RE, Marsh DJ. Three-dimensional anatomy and renal concentrating mechanism. I. Modeling results. Am J Physiol Renal Fluid Electrolyte Physiol 260: F368 F383, Wexler AS, Kalaba RE, Marsh DJ. Three-dimensional anatomy and renal concentrating mechanism. II. Sensitivity results. Am J Physiol Renal Fluid Electrolyte Physiol 260: F384 F394, Zhai XY, Thomsen JS, Birn H, Kristoffersen IB, Andreasen A, Christensen EI. Three-dimensional reconstruction of the mouse nephron. J Am Soc Nephrol 17: 77 88, Zhai XY, Birn H, Jensen KB, Thomsen JS, Andreasen A, Christensen EI. Digital three-dimensional reconstruction and ultrastructure of the mouse proximal tubule. J Am Soc Nephrol 14: , Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI. Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol 18: , Zimmerhackl B, Roberston CR, Jamison RL. Effect of arginine vasopressin on renal medullary blood flow. A videomicroscopic study in the rat. J Clin Invest 76: , Zimmerhackl B, Robertson CR, Jamison RL. Fluid uptake in the renal papilla by vasa recta estimated by two methods simultaneously. Am J Physiol Renal Fluid Electrolyte Physiol 248: F347 F353, 1985.
1. Anatomy / Vascularisation. 2. Urine concentration. 3. Axial heterogeneity of some segments
 Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Long-Term Regulation of Renal Urea Transporters during Antidiuresis
 18 Electrolyte & Blood Pressure 4:18-22, 2006 3) Long-Term Regulation of Renal Transporters during Antidiuresis Dong-Un Kim, M.D. Department of Pediatrics, College of Medicine, The Catholic University
18 Electrolyte & Blood Pressure 4:18-22, 2006 3) Long-Term Regulation of Renal Transporters during Antidiuresis Dong-Un Kim, M.D. Department of Pediatrics, College of Medicine, The Catholic University
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion
 Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY
 URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
Chapter 25: Urinary System
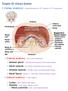 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
General Anatomy of Urinary System
 General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Cycles and separations in a model of the renal medulla
 Cycles and separations in a model of the renal medulla S. RANDALL THOMAS Institut Nationale de la Santé et de la Recherche Médicale, Unité 467 Necker Faculty of Medicine, 75730 Paris Cedex 15, France Thomas,
Cycles and separations in a model of the renal medulla S. RANDALL THOMAS Institut Nationale de la Santé et de la Recherche Médicale, Unité 467 Necker Faculty of Medicine, 75730 Paris Cedex 15, France Thomas,
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
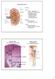 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
RELATIONSHIP ANALYSIS BETWEEN INTERSTIAL SPACE AND COLLECTING DUCT AREA WITHIN FRESH RAT KIDNEY
 RELATIONSHIP ANALYSIS BETWEEN INTERSTIAL SPACE AND COLLECTING DUCT AREA WITHIN FRESH RAT KIDNEY Item Type text; Electronic Thesis Authors NAQVI, MOHAMMAD Publisher The University of Arizona. Rights Copyright
RELATIONSHIP ANALYSIS BETWEEN INTERSTIAL SPACE AND COLLECTING DUCT AREA WITHIN FRESH RAT KIDNEY Item Type text; Electronic Thesis Authors NAQVI, MOHAMMAD Publisher The University of Arizona. Rights Copyright
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Chapter 44. Osmoregulation and Excretion
 Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
From Blood Filtrate to Urine: A Closer Look
 Blood Vessels Associated with the Nephrons Each nephron supplied with blood by an afferent arteriole branch of renal artery divides into glomerular capillaries capillaries converge as they leave glomerulus
Blood Vessels Associated with the Nephrons Each nephron supplied with blood by an afferent arteriole branch of renal artery divides into glomerular capillaries capillaries converge as they leave glomerulus
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Normal Renal Function
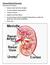 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
I. Metabolic Wastes Metabolic Waste:
 I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
Chapter 13 The Urinary System
 Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Fifth Year Biology. Excretion. Miss Rochford
 Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
Oxygen transport across vasa recta in the renal medulla
 Am J Physiol Heart Circ Physiol 283: H1042 H1055, 2002. First published May 16, 2002; 10.1152/ajpheart.00074.2002. Oxygen transport across vasa recta in the renal medulla WENSHENG ZHANG AND AUÉLIE EDWADS
Am J Physiol Heart Circ Physiol 283: H1042 H1055, 2002. First published May 16, 2002; 10.1152/ajpheart.00074.2002. Oxygen transport across vasa recta in the renal medulla WENSHENG ZHANG AND AUÉLIE EDWADS
The renal architecture is arranged elaborately to fulfill
 JASN Express. Published on November 30, 2005 as doi: 10.1681/ASN.2005080796 Three-Dimensional Reconstruction of the Mouse Nephron Xiao-Yue Zhai,* Jesper S. Thomsen, Henrik Birn,* Inger B. Kristoffersen,*
JASN Express. Published on November 30, 2005 as doi: 10.1681/ASN.2005080796 Three-Dimensional Reconstruction of the Mouse Nephron Xiao-Yue Zhai,* Jesper S. Thomsen, Henrik Birn,* Inger B. Kristoffersen,*
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Urinary System kidneys, ureters, bladder & urethra
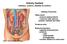 Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Renal Physiology - Lectures
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
CHAPTER 25 URINARY. Urinary system. Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1. functions
 CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
AP2, Lab 7 - THE URINARY SYSTEM
 AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Diagram of the inner portions of the kidney
 Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Nephrology - the study of the kidney. Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system
 Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Faculty version with model answers
 Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Physiology (5) 2/4/2018. Mohammad jaber
 Physiology (5) 2/4/2018 Mohammad jaber Slides are embedded in the sheet in bold, some figure are not included sorry for any mistake,, lets go.. There are two types of urine : 1-diluted urine ( Excess fluid
Physiology (5) 2/4/2018 Mohammad jaber Slides are embedded in the sheet in bold, some figure are not included sorry for any mistake,, lets go.. There are two types of urine : 1-diluted urine ( Excess fluid
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Histology / First stage The Urinary System: Introduction. Kidneys
 The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
Urinary System (Anatomy & Physiology)
 Urinary System (Anatomy & Physiology) IACLD CME, Monday, February 20, 2012 Mohammad Reza Bakhtiari, DCLS, PhD Iranian Research Organization for Science & Technology (IROST) Tehran, Iran The Urinary System
Urinary System (Anatomy & Physiology) IACLD CME, Monday, February 20, 2012 Mohammad Reza Bakhtiari, DCLS, PhD Iranian Research Organization for Science & Technology (IROST) Tehran, Iran The Urinary System
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi
 Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Urinary System. Dr. Ahmed Maher Dr. Ahmed Manhal
 Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Osmoregulation regulates solute concentrations and balances the gain and loss of water
 Ch 44 Osmoregulation & Excretion Osmoregulation regulates solute concentrations and balances the gain and loss of water Freshwater animals show adaptations that reduce water uptake and conserve solutes
Ch 44 Osmoregulation & Excretion Osmoregulation regulates solute concentrations and balances the gain and loss of water Freshwater animals show adaptations that reduce water uptake and conserve solutes
Urinary System. BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College
 Urinary System BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College Filter plasma, separate and eliminate wastes Functions Regulate blood volume and pressure Regulate osmolarity of body
Urinary System BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College Filter plasma, separate and eliminate wastes Functions Regulate blood volume and pressure Regulate osmolarity of body
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018 Intercalated cells Intercalated cells secrete either H + (Typ A) or HCO 3- (Typ B). In intercalated cells Typ A can be observed
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018 Intercalated cells Intercalated cells secrete either H + (Typ A) or HCO 3- (Typ B). In intercalated cells Typ A can be observed
Urinary System Review Questions:
 Urinary System Review Questions: 1. This system would be lined with what type of membrane? 2. What type of epithelial tissue would line the opening of the urethra (the exit of the tract)? 3. What type
Urinary System Review Questions: 1. This system would be lined with what type of membrane? 2. What type of epithelial tissue would line the opening of the urethra (the exit of the tract)? 3. What type
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Basic mechanisms of Kidney function
 Excretion Basic mechanisms of Kidney function Urine formation in Amphibians Urine formation in Mammals Urine formation in Insects Nitrogen balance Kidneys The most fundamental function of kidneys) is to
Excretion Basic mechanisms of Kidney function Urine formation in Amphibians Urine formation in Mammals Urine formation in Insects Nitrogen balance Kidneys The most fundamental function of kidneys) is to
Countercurrent system
 Kidney International, Vol. 38 (1990), pp. 695 699 Countercurrent system JEFF M. SANDS and JUHA P. KOKKO Renal Division, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA
Kidney International, Vol. 38 (1990), pp. 695 699 Countercurrent system JEFF M. SANDS and JUHA P. KOKKO Renal Division, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
The Urinary System Pearson Education, Inc.
 26 The Urinary System Introduction The urinary system does more than just get rid of liquid waste. It also: Regulates plasma ion concentrations Regulates blood volume and blood pressure Stabilizes blood
26 The Urinary System Introduction The urinary system does more than just get rid of liquid waste. It also: Regulates plasma ion concentrations Regulates blood volume and blood pressure Stabilizes blood
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
Copyright 2003 Pearson Education, Inc. publishing as Benjamin Cummings. Dr. Nabil Khouri
 Dr. Nabil Khouri Objectives: General objectives: - to identify the kidney s structures, function and location - to analyze the relationship between microscopic structure and function Specific objectives:
Dr. Nabil Khouri Objectives: General objectives: - to identify the kidney s structures, function and location - to analyze the relationship between microscopic structure and function Specific objectives:
B108 BC Urinary System *
 OpenStax-CNX module: m62388 1 B108 BC Urinary System * Melodye Gold Based on Human Biology Chapter 12.2: Urinary System Anatomy and Function by OpenStax Willy Cushwa This work is produced by OpenStax-CNX
OpenStax-CNX module: m62388 1 B108 BC Urinary System * Melodye Gold Based on Human Biology Chapter 12.2: Urinary System Anatomy and Function by OpenStax Willy Cushwa This work is produced by OpenStax-CNX
Renal Functions: Renal Functions: Renal Function: Produce Urine
 Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Excretion and Water Balance
 Excretion and Water Balance 1. Osmoregulation (water balance) a. Most marine invertebrates are osmoconformers in which the concentration of solutes in their body fluid is equal to that of their environment.
Excretion and Water Balance 1. Osmoregulation (water balance) a. Most marine invertebrates are osmoconformers in which the concentration of solutes in their body fluid is equal to that of their environment.
URINARY SYSTEM. These organs lie posterior or inferior to the. (membrane).
 URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
Chapter 24: The Urinary System
 Chapter 24: The Urinary System Overview of kidney functions n Regulation of blood ionic composition n Regulation of blood ph n Regulation of blood volume n Regulation of blood pressure n Maintenance of
Chapter 24: The Urinary System Overview of kidney functions n Regulation of blood ionic composition n Regulation of blood ph n Regulation of blood volume n Regulation of blood pressure n Maintenance of
Osmoregulation and Excretion
 Chapter 44 Osmoregulation and Excretion PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 44 Osmoregulation and Excretion PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
