Cycles and separations in a model of the renal medulla
|
|
|
- Debra Short
- 5 years ago
- Views:
Transcription
1 Cycles and separations in a model of the renal medulla S. RANDALL THOMAS Institut Nationale de la Santé et de la Recherche Médicale, Unité 467 Necker Faculty of Medicine, Paris Cedex 15, France Thomas, S. Randall. Cycles and separations in a model of the renal medulla. Am. J. Physiol. 275 (Renal Physiol. 44): F671 F690, This study gives the first quantitative analysis of net steady-state transmural fluxes of water, urea, and NaCl in a numerical model of the rat renal medulla in antidiuresis, revealing the model s predictions of water, urea, and NaCl cycling patterns. These predictions are compared both to in vivo micropuncture data from the literature and to earlier qualitative proposals (e.g., K. V. Lemley and W. Kriz. Kidney Int. 31: , 1987) of cycling and exchange patterns based on medullary anatomy and available permeability and transport parameter measurements. The analysis is based on our most recent three-dimensional model [X. Wang, S. R. Thomas, and A. S. Wexler. Am. J. Physiol. 274 (Renal Physiol. 43): F413 F424, 1998]. In general agreement with earlier proposed patterns, this analysis predicts the following: 1) important water short-circuiting from descending structures to ascending vasa recta in most medullary regions, 2) massive urea recycling in the upper inner medulla, 3) a progressive increase of the ratio of urea to total osmoles along the corticopapillary axis, 4) urea dumped from the collecting ducts (CD) into the deep papilla is returned to the cortex essentially via outer medullary short vasa recta, bearing witness to a shift from the long descending limbs and vasa recta of the inner medulla (IM) to short structures in the outer medulla (OM). The analysis also shows that the known radial heterogeneity of the inner stripe (IS) implies unequal osmolalities in long descending limbs, vasa recta, and CDs entering the IM across the OM/IM border and explains the model s unorthodox osmolality profile along the CD. In conflict with micropuncture evidence of a doubling of urea flow in superficial Henle s loops (SHL) between the end proximal and early distal tubule (T. Armsen and H. W. Reinhardt. Pflügers Arch. 326: , 1971), the model predicts net urea loss from SHL due to the model s inclusion of nonneglible measured urea permeability of medullary thick ascending limbs [M. A. Knepper, Am. J. Physiol. 245 (Renal Fluid Electrolyte Physiol. 14): F634 F639, 1983]. We present a suite of adjusted model permeabilities that improves agreement with the micropuncture data on this point. In conclusion, this modeling analysis of solute and water recycling serves as a quantitative check on qualitative propositions in the literature and allows closer critical comparison of model behavior with published experimental results than was heretofore possible. mathematical model; urine concentrating mechanism; salt and urea recycling THE URINARY CONCENTRATING MECHANISM remains a mystery despite a long history of both experiment and modeling efforts. The basic enigma centers on our inability to explain the origin of the corticopapillary gradient of osmolality in the passive inner medulla, i.e., in the absence of evidence for active transepithelial salt transport. The earliest explanation, the passive hypothesis (PH), was set down in 1972 by Stephenson (35) and by Kokko and Rector (17). Modeling studies based on this hypothesis predicted the permeabilities in various nephron segments in order for the PH to explain the inner medullary gradient. When it became possible to measure these permeabilities in in vitro microdissected tubules, they were found not to agree with the model s requirements. In particular, salt and urea permeabilities in the inner medullary (IM) portion of the long descending limbs (LDL) were found to be higher in species that concentrate well than in those, such as the rabbit, that cannot elaborate a concentrated urine, whereas the PH requires exactly the opposite. In the context of the PH, solute entry (especially urea entry) into the LDL compromises the inner medullary osmotic gradient (21, 36, 43). Although it is tempting to question the validity of the permeability measurements, it is also clear from micropuncture studies at the papillary tip (9) that there is considerable urea entry into long Henle s limbs somewhere between the end of the proximal tubule and the tip of the medulla, i.e., either in the outer or inner medulla or both. Therefore, we are brought to the conclusion that the PH misses some essential feature of the concentrating process. Up to now, virtually all modeling studies testing new hypotheses or the effects of new permeability measurements or anatomical juxtapositions have judged the degree of success of new model features based on a single criterion, namely, the predicted osmolality of fluid leaving the collecting duct (CD). There have been no studies that employ model predictions of the cycling of water and solutes among the various nephron and vascular pathways and compare them with the several thoughtful discussions in the literature based on anatomy and permeability data. In particular, Lemley and Kriz (22), Bankir and de Rouffignac (3), Jamison and Robertson (13), de Rouffignac (30), and others have made detailed suggestions about probable recycling paths for water, urea, and salt founded in the general framework of the PH but enhanced by additional knowledge of the system. A recent model (44) (hereafter called the WKM model, for Wexler-Kalaba-Marsh) incorporated as much detail as possible, and it seems to be an improvement over earlier models, since it concentrates better (however, WKM is not without its critics, see Ref. 37). However, it remains the case that increasing salt and/or urea permeabilities along the inner medullary descending limb in these models leads to worse, not better, inner medullary osmotic concentration; so there remains a fundamental problem. In hopes of suggesting some new directions for research, the present study explores the detailed recycling patterns predicted by our most recent incarnation of the WKM model (41), compares these patterns with those predicted by the authors mentioned above, and also compares model predictions of fractional deliveries /98 $5.00 Copyright 1998 the American Physiological Society F671
2 F672 MEDULLARY SOLUTE AND WATER CYCLING to structures in the papillary tip and in accessible surface loops with available experimental data. Since this model is currently our best attempt to represent present hypotheses, this study is a gauge of the extent to which the suggested recycling patterns are in fact consistent with known anatomy and permeability measurements. We find that on most points the suggested patterns match those observed in the simulation, but the exceptions are interesting and may be important for improving our understanding of this system. Glossary OM Outer medulla IM Inner medulla OS Outer stripe IS Inner stripe UIM Upper inner medulla TZ Transition zone LIM Lower inner medulla LDL Long descending limb LAL Long ascending limb LHL Long Henle s loop SDL Short descending limb SAL Short ascending limb SHL Short Henle s loop LDV Long descending vasa recta LAVn Long ascending vasa recta LVR Long vasa recta SVR Short vasa recta SDV Short descending vasa recta SAVn Short ascending vasa recta CD Collecting duct OMCD Outer medullary collecting duct IMCD Inner medullary collecting duct GFR glomerular filtration rate SNGFR Single-nephron glomerular filtration rate FL i Filtered load of substance i SNFL i F i,f ij (x) %FD i %SNFD i FE i (TF/P) i (U/P) i U osm METHODS Model Description Single-nephron filtered load of substance i Tubular flow of i, tubular flow of i in tubule j at point x, where x 0 is corticomedullary border, and x L 0.6 cm is papillary tip Fractional delivery of i at a given point x as a percent of total filtered load, 100 F i (x)/ FL i Fractional delivery of i per tubule at a given point x as a percent of singlenephron filtered load, 100 (F i (x)/n(x))/ SNFL i, also equal to (TF/P) i /(TF/P) inulin Fractional excretion of i, F CD i (L)/FL i Ratio of tubular fluid to plasma concentrations of i Ratio of urinary to plasma concentrations of i Urine osmolality (mosmol/l) The present study treats only the case of the antidiuretic rat kidney. The mathematical description of tubular flow and transport and the numerical solver are described in previous work (38, 42, 44). These steady-state models were based on existing qualitative and quantitative descriptions of rat renal medullary anatomy [see especially Lemley and Kriz (22)]. The present analysis uses our most recent model (41), which, compared with the original WKM model, more faithfully represents the anatomy of the IM and OM and measured permeabilities in all segments. The architectural organization of the renal medulla is shown in Fig. 1. Figure 1, left, shows transverse cuts at each medullary level, indicating the relative positions of each tubular structure and giving the connection strengths used to calculate solute and volume exchanges between structures. In each medullary zone, transmural and convective connections between structures are represented by straight lines and curved arrows, respectively. Each circle represents a collection of a given type of tubules or vessels, the numbers of which used in the model are listed in Table 1. The distances between structures in the diagram are not to scale; relative anatomic distances between the respective tubes in the model are accounted for by the values of the connections strengths. Figure 1, right, gives a view of these relationships along the corticomedullary axis to illustrate the continuity from one medullary zone to the next, but since it is flattened to two dimensions, it does not faithfully represent the spatial distribution of the structures. In accordance with the assumed tubular distribution (Table 1), the ratio of SDL population to LDL population is two to one in outer medulla (3, 22); the ratio of the Henle s loop population to the collecting duct population is six to one in outer medulla ( 5 to 1 in Ref. 16). At the outer-inner medullary border, the ratio of the number of long loops to collecting ducts is two to one, and the ratio becomes one to one as they approach the tip of the papilla (16). The model structure thus explicitly represents the six nephrons associated with a single OMCD, but it is scaled according to total numbers of nephrons at each medullary level in rat kidney. Thus total GFR (as given, e.g., in Table 3) is not here numerically equal to whole kidney GFR but rather equals a value six times greater than the SNGFR. Boundary conditions and parameters. The model treats the renal medulla from the corticomedullary border down to the papillary tip. The boundary conditions (flow rates and concentrations) are specified for all the descending limbs and vasas recta at the corticomedullary border, for mass conservation in the descending and ascending structures at the loop bends, and for solute and fluid reabsorption at the end of the late distal tubule (entry to the CD). Proximal tubules are assumed to reabsorb two-thirds of filtered NaCl and volume flow and one-third of filtered urea, so at the corticomedullary border, single-tubule flow rates in LDL and SDL are 10 nl/min and NaCl and urea concentrations are 140 and 18 mm. The cortical portions of the distal tubules and cortical collecting ducts are not treated explicitly. Instead, fluid and solute entry into the medullary collecting ducts are calculated from values at the top of the ascending limbs, using three assumptions: 1) fluid entering the medullary CD is isosmotic to blood; 2) NaCl concentration is 35 mm; and 3) urea delivery to the CD is 85% of its delivery to the beginning of the distal tubules, i.e., the distal tubules reabsorb 15% of the urea delivered to them by the ascending limbs. The detailed formulation is given in previous work (38, 42, 44). Model parameters (see Table 3 of Ref. 41) assume values of tubular and vascular transport properties based on available experimental measurements in rat and hamster in antidiuresis. We assumed that the length of the medulla is 6 mm divided as: outer stripe, 0.7 mm; inner stripe, 1.3 mm; upper portion of the inner medulla, 1.3 mm; transition zone, 0.2 mm; and lower portion of the inner medulla, 2.5 mm. Numerical method. The numerical package based on collocation, COLNEW (available in the netlib archives on the internet at was used to solve the models. Detailed descriptions of the system
3 MEDULLARY SOLUTE AND WATER CYCLING F673 Fig. 1. Model illustration. Left: placement of each structure in cross section through each medullary region. Right: tubes in a flattened 2-dimensional projection, approximately in their correct positions. Values in left (e.g., 0.33, 0.25) indicate connection strengths from Henle s loops and from descending vasa recta to ascending vasa recta, reflecting their relative proximities in each region. Distances are not to scale. Arrows indicate convective connections. equations and numerical scheme have been given previously (38, 42, 44). The computations were performed in double precision on an IBM RS/ or RS/ H running AIX. The predictions converged after calculating over two meshes and one or two iterations for each mesh, using the initial set of estimations saved from previous runs to reduce numerical instability. On an IBM RS/ , one iteration takes about 1 to 2 CPU seconds, and a run from a previous solution to convergence is completed in less than 10 s. Solute and Water Cycles: Flux Calculations In a model where a single loop-structure represents not one but a collection of tubes that turns back at various depths, also called n-nephron shunt models or lumped n-nephron models in the literature, one cannot calculate the amount of, say, urea secreted into or absorbed from the inner medullary interstitium by all LDL simply by subtracting the amount delivered to LDL at the tip from the amount entering the inner medulla at the OM/IM border, since most of the transport is due not to transmural flux but to shunts reducing the number of LDL, i.e., in our model, the ratio of the number of long Henle s loops traversing the OM to the number arriving at the papillary tip is 128, reflecting the fact that, in the rat, for 10,000 LDL only about 75 arrive at the tip (10, 16, 18). Because of these shunts, it is necessary in descending structures to subtract (and in ascending structures to add) the shunt flows to obtain the transmural flux into the interstitium. Although these shunt transfers from descending to ascending limbs of Henle and vasa recta are simple convective flows, they are not readily separable from the transmural fluxes, since solute concentrations at each level depend on cumulative transfers up to the given point. A program was written to calculate net transmural flux from each tube within an arbitrary medullary slice, i.e., between any two medullary depths, from the output data. To illustrate the description of the new cycles calculations, Fig. 2 schematically depicts a portion of the shunted tubular structure that represents the long Henle s limbs, illustrating the early returning loops in the inner medulla. The diagram Table 1. Numbers of individual tubes in each region OS IS IM Short Henle s loops 4 4 SDV 8 8 n SDV 8(1 IS ) x x 1 x 2 x 1 Long Henle s loops 2 2 2e 12.13(x x2) CD 1 1 e 10.40(x x2) LDV 4 4 4e 12.13(x x2) See Glossary for descriptions of tubule segment abbreviations; x, distance in cm, varies from 0 at the corticomedullary border to 0.6 cm at papillary tip; x cm is the OS/IS border, x cm is the OM/IM border; IS, small fraction given to avoid division by zero; n SDV, number of SDV at level x. Fig. 2. Schematic illustration of loop structure with shunt return, for flux calculations.
4 F674 MEDULLARY SOLUTE AND WATER CYCLING applies equally to flows of volume, urea, or NaCl. The model (for more detail, see Ref. 44) uses the following differential equations for conservation of mass within a section of lumped tubule and df d (x) J dx d (x) F shunt (x) (1) df a (x) J dx a (x) F shunt (x) (2) where F d and F a are tubular (i.e., luminal) flow rates within the descending and ascending lumped structures, respectively (flows toward the papilla are positive, and those away from papilla are negative), J d and J a are the fluxes across the descending and ascending tubular walls at depth x (i.e., loss from the tubular lumen; secretion into lumen if the value is negative), and F shunt is the shunt flow at depth x, which is given by F shunt (x) F d(x) dn(x) (3) n(x) dx where n(x) is the number of individual tubes represented by the lumped structure at depth x, sof d (x)/n(x) is the singlenephron flow at x. The number of long limbs and vasa recta decreases exponentially within the inner medulla, falling at the papillary tip (x 0.6 cm) to 1 128th of their value at the OM/IM border (x cm), according to which has the derivative n(x) n(x 0 )exp[ 12.13(x x 0 )] (4) dn(x) dx 12.13n(x) (5) We know the values of the tubule flows F d and F a at every point (from the simulation output) and are interested in calculating the integral fluxes from descending tubules and from ascending tubules J d x1 x 2 J d dx (6) J a x1 x 2 J a dx (7) within any given slice, i.e., within a region from arbitrary depth x 1 to x 2. Illustrating the calculation first for J d,we substitute in Eq. 6 from Eq. 1 J d x1 x 2 1 F shunt(x) df d(x) dx 2 dx (F d (x 1 ) F d (x 2 )) x1 x 2 F shunt (x) dx Substitution for F shunt (x) from Eq. 3 gives J d (F d (x 1 ) F d (x 2 )) x1 x 2 (8) 1 F d(x) dn(x) n(x) dx 2 dx (9) Since n(x) and dn(x)/dx are available analytically (Eqs. 4 and 5), and F d (x) is available at every point from the simulation output, we can approximate the integral on the right-handside as the sum x1 x 2 1 F d(x) dn(x) n(x) dx 2 dx N i 1 1 F d(x 1 i ) dn(x 1 i ) n(x 1 i ) dx F d(x 2 i ) dn(x 2 i ) n(x 2 i ) dx 2 x 2 (10) where N (x 2 x 1 )/ x, x i 1 x 1 (i 1) x, and x i2 x 1 i x. Similarly, for flux from the ascending tubule, we have J a (F a (x 1 ) F a (x 2 )) x1 x 2 F shunt (x) dx (11) with the same approximation for the integral of F shunt. As a check on the accuracy of these calculations, we took advantage of the fact that water permeability along the LAL is zero in the model, so LAL volume flux calculated using this method must give values arbitrarily near zero. By this criterion, it was necessary to chop each medullary region of interest into at least 30 slices. The same method was used for LVR, but since each LDV gives rise in the model to two LAV, we have, instead of Eq. 2 df lav (x) dx LDV F shunt (x) J a (x) 2 (12) Likewise, for the SVR in the inner stripe, each SDV gives rise to four SAV, so we have RESULTS df sav (x) dx SDV F shunt (x) J a (x) 4 (13) Since the following discussion is rather detailed, we first present the basic model results in three different ways, to make things more accessible. Basic results Standard output: Volume flows and concentrations. Table 2 presents the basic model output, namely, volume flows and solute concentrations and osmolality, plus TF/P inulin values, in each tube structure at key medullary levels (the actual simulation output table gives the results over a much finer mesh and to greater precision). Slight differences from the numbers in table 4 of Wang et al. (41) are due to stricter error tolerance here (attested by the smaller % imbalances). These numbers are used for construction of Tables 3 and 4. Figure 3A shows the profiles of urea and salt concentration and of osmolality in each structure along the corticopapillary axis. We reiterate that in a lumped model such as this one, these values represent the lumped averages over the population of each tube type at a given level. In the actual kidney, it must be the case that fluid in LALs that turn back in the upper inner medulla does not have the same composition as fluid at the same depth in LALs of longer loops. It is thus legitimate to ask whether such models give accurate results. Wang et al.
5 Table 2. Basic results x, mm LDV LAV1 SDV LAV2 LDL SAV1 LAL SAV2 SDL SAV3 SAL SAV4 CD Node Volume flows, nl/min (imbalance % ) o i x u m l Urea concentrations, mm (imbalance % ) o i ,134 x , ,221 u , , m , , l NaCl concentrations, mm (imbalance % ) o i x u m l Osmotic pressures, mosm o , i ,419 1,415 x ,458 1, ,487 u ,483 1, ,130 1,157 1,155 1,150 1,241 1,482 1,488 m 3.4 1,204 1,238 1,233 1,227 1,283 1,489 1, ,315 1,354 1,390 1,373 1,325 1,484 1,605 l ,468 1,468 1,468 1,469 1,386 1, ,519 1,519 1,519 1,525 1,525 1,435 TF/P inulin o i x u m l Mass imbalance figures, ideally zero, are a measure of the degree of convergence of the solution method; o and i, midpoints of outer and inner stripe of outer medulla, respectively; u, m, and l, midpoints of the upper portion, transition zone, and lower portion of inner medulla, respectively; x is bottom of inner stripe, immediately before the OM/IM border. Position is along corticomedullary axis, where indicates border between adjacent stripes or zones.
6 F676 MEDULLARY SOLUTE AND WATER CYCLING
7 MEDULLARY SOLUTE AND WATER CYCLING F677 (40) demonstrated that lumped, shunted n-nephron models are an excellent approximation to discrete n-tube models, if all tubes of a given type are assumed to have similar permeability and transport properties at each level. Two points are especially revealing about the behavior of this model and will be discussed further below: 1) fluid composition and osmolality are predicted by the model to be widely different in LDV, LDL, and CD as they descend into the IM across the OM/IM border, a reflection of the inclusion in this model of the lateral inhomogeneity of the inner stripe; and 2) the predicted profiles along the CD are unlike those for the other tubes or for the IM as a whole: osmolality, for example, reaches its maximum before the OM/IM border instead of steadily increasing through the IM as is usually thought to occur (more on this below). Total tubular flows as a percent of total filtered loads. Table 3 and Fig. 3B present the same results as Table 2 and Fig. 3A but in a different form, namely, as the tubular flow rates not only of water but also of urea (F v c u ), salt (F v c s ), and total osmoles [F v (c u 1.82 c s )] all scaled as a percent of their respective total filtered loads. These flows represent the sums of flows in, say, all LDL, represented in the model by the lumped LDL structure. These values make clear the reduction of flows toward the papillary tip due to the reduced numbers of nephrons and vasa recta, and permit evaluation of the contribution of each structure to global balance. For instance, if we look at just the flows entering and leaving the IM via the whole population of long loops of Henle (LDL and LAL), we see that 1) the LDL enter the IM carrying 3.14% of GFR and leave in LAL carrying 3.65%, showing a small net gain of water, which occurs in the LDL, since LAL are impermeable to water; 2) the LDL carry 21.9% of FL u, the filtered load of urea, into the IM and LAL carry 45.8% back out to the OM, more than doubling the urea flow within the long loops within the IM; 3) the LDL enter the IM carrying 11.3% of FL s, the filtered load of salt, but LAL carry only 9.63% back up to the OM, for a net loss of 1.67% of the filtered salt load; 4) the net osmole contribution of Henle s loops to the IM is 1% of the filtered load of osmoles ( %). One point worth noticing, given the extent of urea exchange and recycling between Henle s loops and the vascular vessels (discussed below), is that total urea delivery to the medulla is 116% of the total urea load filtered into the nephrons, since Henle s loops deliver 66.6% of FL u to the medulla (22.2% in LDL and 44.4% in SDL) and the vasa recta deliver the equivalent of another 50% of FL u (16.6% in LDV and 33.3% in SDV). Tubular fractional deliveries. From the values in Table 3 alone it is not possible to know whether individual long nephrons gain or lose solutes or water along their course in the IM. Table 4 and Fig. 3C give average flows per tube as a percent of single-nephron filtered loads, which is often called the fractional delivery (given here as percentages) and is the same as reporting 100 (TF/P) i /(TF/P) inulin from micropuncture data. These values are best for evaluation of net gain or loss of solutes or water along individual nephrons of a given type and can be directly compared with micropuncture data (see below). From Table 4 and Fig. 3C, one can see that luminal water and salt flows change very little in the IM along the longest LDLs, whereas luminal urea flow increases 10-fold, attaining 658% of the single-nephron filtered load of urea at the hairpin turn, though we see in Table 3 that total urea flow at the tip in the whole (small) population of longest LDLs amounts to only 1.71% of the total filtered load of urea. Table 5 presents some results from micropuncture experiments in the literature for comparison with the simulation results. The experimental data given here are meant only to indicate physiological ranges for the nondiuretic rat kidney, not to give a consistent set of data for a given kidney, since no study has measured all the relevant parameters under identical conditions. Exchange and Recycling of Urea, Salt, and Water Tables 6 and 7 give the results of the flux calculations explained previously. Table 6 gives the amounts of volume, urea, salt, and total osmols transported into (positive values) or out of (negative values) each medullary region from each tubule population by transmural flux, expressed as a percentage of total GFR or total filtered loads of solutes. Table 7 presents the same results but scaled as a fraction of the respective flows at the tip of LDL. Figure 4, A C, illustrates the main cycling patterns for water, urea, and salt deduced from these numbers, which we explain here and discuss below in relation to the literature. Let us define some terminology: we will use the word recycling to describe transfers from ascending to neighboring descending tubes, which tend to keep a substance from escaping into higher regions of the medulla; the word short-circuit will be applied especially to water flows effectively shunted from descending to neighboring ascending tubes, thereby reducing total volume flow in the next deeper slice; instead of referring to (re)absorption and secretion, we will use the terms dumping (for transport out of a tubule) and uptake (for transport into a tubule). Water cycles (as percent of total GFR to all model nephrons). Starting our description in the deepest region, LIM, we see (Fig. 4A; Tables 6 and 7) that the CD and to a lesser extent the LDV dump volume, all of which is recovered by the ascending vasa recta. In the Fig. 3. Corticopapillary profiles along each tube predicted by the model simulation. Top graphs in each of A Cshow profiles for short nephrons and short vasa recta (and for OMCD in some). Bottom graphs in each of A C show profiles for long nephrons, long vasa recta, and CD. A: profiles of urea and NaCl concentrations and osmolality (from Table 2). B: profiles of fractional deliveries (FD) as percents of total (tot) filtered loads, i.e., 100 F i (x)/fl i (x), where i is urea, salt, or osmoles (from Table 3). C: profiles of single-nephron fractional deliveries (SNFD) as percents of single-nephron filtered loads, i.e., 100 (F i (x)/n i (x))/snfl i (x), where i is urea, salt, or osmoles (from Table 4).
8 F678 MEDULLARY SOLUTE AND WATER CYCLING Table 3. Total fractional deliveries: percent of GFR and total filtered loads x, mm LDV LAV1 SDV LAV2 LDL SAV1 LAL SAV2 SDL SAV3 SAL SAV4 CD Volume flow, % of GFR o i x u m l tot Urea flow, % of FL u o i x u m l tot Flow of NaCl, % of FL s o i x u m l tot Flow of osmoles, % of FL osm o i x u m l GFR nl/min (SNGFR 30 nl/min), plasma urea 9.0 mm, plasma NaCl mm, FL tot u 1,620 pmol/min, FL tot s 25,200 tot pmol/min, FL osm 47,484 posmol/min. Subscripts indicate urea (u), salt (s), or osmoles (osm); superscript tot indicates total. narrow transition zone, not only LDV and CD but also LDL dump a bit of volume on their way down, all of which is carried back up by the LAVs. We thus see that total volume flow at the tip is reduced due not only to the smaller number of tubes but also to a water short-circuit which transfers water from the downward-flowing tubes to the upward-flowing vasa recta. This behavior matches the usual notions of water cycling in the deep inner medulla, except perhaps for the lack of water loss from LDL in the deepest part of the papilla. In the upper inner medulla, this simulation s water recycling picture is less classic. We see that in the descending structures that there is only a very slight water loss from the CD, and there is massive loss from the LDV, but that not all of this water is carried back up
9 MEDULLARY SOLUTE AND WATER CYCLING F679 Table 4. Single-nephron fractional deliveries: percent of SNGFR and single-nephron filtered loads x, mm LDV LAV1 SDV LAV2 LDL SAV1 LAL SAV2 SDL SAV3 SAL SAV4 CD Single-nephron volume flow, % of SNGFR o i x u m l Single-nephron urea flow, % of SNFL urea o i x u m l Single-nephron NaCl flow, % of SNFL NaCl o i x u m l in the LAVs; a considerable amount is taken into the neighboring descending limbs of Henle! This is because the LDL fluid is relatively hypertonic as it enters the IM from the inner stripe, a point to which we come back, below. In the OM, water movements in this simulation conform to the usual expectations; namely, there is massive osmotic water loss from descending structures, especially in the outer stripe, and this lost water is carried up to the cortex by the ascending vasa recta. We nonetheless note that the model predicts no water loss from SDL in the inner stripe (it predicts in fact a slight gain of water), despite the high water permeability of SDL. Instead, the rise in osmolality along this part of the SDL is entirely due to urea entry. This is interesting in light of recent reports that only the early part of inner stripe SDL expresses the aquaporin channel AQP-1 (24), whereas only the terminal portion of SDL expresses the urea transporter UT2 (short transcript) (25). Table 5. Typical micropuncture data (TF/P) inulin SNFD v, % [Na], mm SNFD s, % [urea], mm SNFD u,% End PCT 1.8 to 3 (3) 33 to 56 (33) 140 (140) 50 (33.3) 8.6 (18) 50 (66.6) LDL tip 5 11 (10.8) 9 20 (9.2) 280 (484) (31.8) 175 (644) 108 (659) Early DCT 6.8 (7.9) 14.7 (12.6) 36 (30.2) 4.4 (2.7) 41 (41.8) 100 (58.8) Late DCT (39) 5 8 (2.55) 29 (35) 1.3 (0.64) 66 (199) 75 (56.6) End CD (urine) (730) (0.14) 27 (312) 0.13 (0.3) (866) (13.1) SNFD i, single-nephron fractional delivery of i, as percent of single-nephron filtered load (s, NaCl; u, urea; v, volume). Values are from Refs. 1, 6, 9, 12, and 20, all on antidiuretic rats or golden hamsters, but no single study measured all parameters. Values in parentheses are from the present simulation results.
10 F680 MEDULLARY SOLUTE AND WATER CYCLING Table 6. Net fluxes across tubule walls within each medullary region, expressed as percent of total filtered load LDV LAV1 LAV2 LVR tot LDL LAL LHL tot CD SDL SAL SHL tot SDV SAV1 SAV2 SAV3 SAV4 SVR tot Volume fluxes, percent of GFR OS IS OMtot UIM E TZ E LIM E IMtot E Medtot E Urea fluxes, percent of FL u tot OS E IS OMtot E UIM TZ LIM IMtot Medtot NaCl fluxes, percent of FL s tot OS IS OMtot UIM TZ LIM E-05 IMtot Medtot tot Osmole fluxes, percent of FL osm OS IS OMtot UIM TZ LIM IMtot Medtot
11 MEDULLARY SOLUTE AND WATER CYCLING F681 Table 7. Net fluxes across tubule walls within each medullary region, expressed as fraction of LDL flows at the papillary tip LDV LAV1 LAV2 LVR tot LDL LAL LHL tot CD SDL SAL SHL tot SDV SAV1 SAV2 SAV3 SAV4 SVR tot Volume flux, as fraction of FvLDL (tip) OS IS OMtot UIM TZ E LIM IMtot Medtot Urea flux, as fraction of FuLDL (tip) OS E IS OMtot E UIM TZ LIM IMtot Medtot Salt flux, as fraction of FsLDL (tip) OS IS OMtot UIM TZ LIM IMtot Medtot Osmolar flux, as fraction of FosmLDL (tip) OS IS OMtot UIM TZ LIM IMtot Medtot
12 Fig. 4. Fluxes in each medullary region. Arrow bars represent fluxes out of/into each structure, and the relative thickness of the arrows roughly indicates the relative flux values in a given region (actual values given in Table 6). Note that the algebraic sum of fluxes at any given level is zero, by mass conservation. A: water fluxes. Note that OS water fluxes are about 100-fold greater than those in the papilla (LIM), so arrow thicknesses cannot be exactly to scale. B: urea fluxes. C: salt fluxes.
13 MEDULLARY SOLUTE AND WATER CYCLING F683 With respect to the crucial question of the concentration of urine on its final path, that is, along the CD, the CDs lose only 1.55% and 0.6% of GFR in the OS and IS, respectively, and then 0.2% in the LIM, but due to the low volume flow entering the CD (only 2.55% of GFR, see Table 3), this volume reabsorption suffices to raise (TF/P) inulin (Table 2) from 39 at the top of the OM to 730 at the exit from the papilla. Note, however, that the maximum osmolality in CDs is reached here within the inner stripe, which is contrary not only to the usual notions of the system s behavior but also apparently to the scant direct experimental data on the question (23, 24), a point to which we will come back, below. We also point out (Table 2) that the (TF/P) inulin only doubles along LDV and SDV in the OM and reaches a value of only 3.8 at the papillary tip. In SDL, (TF/P) inulin reaches 8.4 at the bottom of the OS and then actually falls slightly to 7.9 at the bottom of IS. Along the LDL, (TF/P) inulin rises from 3 to 10.5 within the OM and is only 10.8 at the papillary tip, so net water flux is negligible along the LDL in the IM. Nonetheless, the value of (TF/P) inulin attained in LDL at the papillary tip closely matches the reported value of 11 found in the literature (9, 20). Urea cycles. UREA CYCLES IN THE IM. As with the above account of water cycles, we begin in the deep papilla and work upward (Fig. 4B). The discussion of inner medullary urea exchanges centers naturally around the destiny of the urea dumped there from the CDs. The CDs in this simulation dump into the LIM an amount of urea equivalent to nearly one-third of the total filtered load of urea (Table 6) (also equivalent to 7.7 times the total osmoles delivered to the papillary tip in LDLs, Table 7). This urea load is picked up by all the other structures in the region, descending as well as ascending (except for LAV1). In the TZ, the CDs dump 8.3% of FL u. A third of this urea is picked up by the LDV, and the rest enters Henle s loops, both LDL and LAL. In this region, the ascending vasa rectae already start losing urea back to their surroundings, i.e., it is recycled back toward the papilla via the LDV. In the UIM, the small urea loss from the CD (3.4% of FL u ) is picked up entirely and carried deeper into the papilla by the LDL. Much more important in this region is the massive urea recycling from ascending to descending tubes: i.e., 20.5% of FL u from LAL to LDL and 104.6% of FL u from LAV1 and LAV2 to LDV and LDL. This recycling can be revealed in the model only by the present new flux calculations and cannot be measured in vivo. To summarize the net steady-state urea transfers within the IM among the three tube systems (see Table 6, columns CD, LHL tot, and LVR tot ) in this simulation (quantities as %FL u ): in LIM, of the 31.7% of FL u lost from IMCD, somewhat more is picked up by the vasa recta (17.8%) than by Henle s loops (13.8%); in zone TZ, of the 8.3% of FL u dumped by IMCD, more goes to Henle s loops (5.3%) than to the vasa recta (3%); in the UIM zone, the amounts of urea transferred from IMCD (3.4%) and vasa recta (1.4%) to Henle s loops are dwarfed by the amounts of urea recycled from ascending to descending limbs of vasa recta and Henle s loops. UREA CYCLES IN THE OM. The lateral inhomogeneity of especially the inner stripe, typified by the grouping of all descending vasa recta and of the long (but not the short) ascending vasa recta into vascular bundles (VB), are crucial to understanding urea movements. It is also worth recalling that the urea permeability of OMCD is very low even in antidiuresis, so urea transfers occur only among the non-cd sturctures. Within the vascular bundles, fluid in the LAV1 and LAV2 arrives carrying the equivialent of 80% of FL u from their trip into the urea-rich IM; they dump a total of 49% of FL u as they pass through the IS and another 9.9% in the OS and carry the remaining equivalent of 21% of FL u back to the cortex. Within the IS, about half of the urea dumped in the VB by LAVs is recycled to the inner medulla via the LDV; of the other half, most is picked up by the SDV, which are situated in the perimeter of the VB (before splitting off into the interbundle region to form capillary beds and thence SAVs), and the rest (5.8% of FL u ) is picked up by the SDL, as is usually supposed, since they run close to the VB in the IS in rats and have high urea permeability. Within the interbundle region of the IS in this simulation, the LAL dump about one-fourth of their urea (10.8% of FL u ), all of which is returned to the blood via SAVs. Urea fluxes are virtually absent along LDL and SAL of the inner stripe. In the OS, the LAL and SAL each dump 7.7% of FL u, and the SDL also dumps 3.3% of FL u. The equivalent of 7.6% of FL u is recycled deeper into the medulla via the LDV, and the rest is picked up by the SDV. There is also considerable urea recycling in the OS by transfer from ascending to descending short vasa recta. In summary, for urea balance in the OM (see the tot columns of Tables 6 and 7), the long vasa recta dump 15% of FL u and Henle s long loops (essentially the LAL) dump 18.8% of FL u. The short limbs of Henle are also net urea contributors (5.2% of FL u ), since the SAL dump more in the OS than is picked up by the SDL during their inner stripe excursion near the vascular bundles. This whole urea load is of course picked up by the short vasa recta system, since net inflow equals net outflow in the steady state. Salt cycles. Along the CD, although salt reabsorption is quantitatively very small, it is crucial for the organism s salt balance; absolute NaCl reabsorption along the IMCD in antidiuresis has been reported as high as 3% of FLs (34), about 10 times the rate of fractional salt excretion. Moreover, sodium concentration along the CD may rise in the OM before falling again in the IM (5). However, models of the medullary countercurrent system, the present one included, do not yet do justice to the role of the CD in salt regulation; they lump all salt into one pool labeled NaCl, whereas renal handling of sodium and potassium (to say nothing of ammonium, phosphate, etc.) are in fact quite different, especially in the distal nephron and CD. For instance, by free-flow micropuncture at both the base and tip of IMCD, Diezi et al. (5) showed clearly that Na concen-
14 F684 MEDULLARY SOLUTE AND WATER CYCLING tration ([Na ]) falls and [K ] rises along the IMCD. The minimal salt reabsorption included in the present model ( 0.1% of FL s ) avoids some problems of earlier models, but proper treatment of this question must await more complete models. In the inner medulla (Fig. 4C; Tables 6 and 7), salt flux along the LDL is outward but is very slight due to their relatively low permeability, but the LAL dump salt along their whole length, as posited by the familiar passive hypothesis (17, 35). However, this salt is not recycled back down into the papilla. Somewhat surprisingly, the LDV also dump salt along their whole length within the IM (even more than by the LAL), because water is drawn out faster than salt, maintaining a favorable gradient for salt exit even as the LDV fluid descends into a region of increasing salt concentration. The salt dumped from these structures and from the CD (by the active pumps) is carried up to the OM by the LAVs; thus vasa recta salt balance is negative at every level in the IM, i.e., the vasa recta carry salt upward out of every successive IM slice. In the outer medulla, SAL and LAL actively dump 20.6% and 8.9% of FL s, respectively, with most of it being pumped out in the IS (see Tables 6 and 7). Though a very small part of this salt is recycled to the IM in the LDL, the great majority is carried back to the general circulation in the short vasa recta. In accord with established facts, given the water impermeability of the LAL and SAL, this salt dumping also dilutes the LAL and SAL and renders the OM hyperosmotic with respect to the fluid in entering descending tubes. In addition, as the WKM models in general and the present model in particular reflect, the inner stripe has not only an axial (i.e., corticopapillary) but also a radial (i.e., from vascular bundles to CDs) osmolality gradient. Total osmoles. One of the basic tenets of the classic view of the concentrating mechanism is that at the OM/IM border the descending structures, LDV, LDL, and CD, enter the IM with nearly equivalent osmolalities and that the rising LAL fluid is dilute relative to the other structures at the same level as it leaves the IM (not all workers insist that LAL fluid must be dilute as it leaves the IM; see Refs. 4, 21, 30). The present model contradicts both of these tenets, namely, we see (Table 2) that at the OM/IM border c osmldv c osmldl c osmcd, reflecting the respective positions of LDV, LDL, and CD along the radial osmolality gradient in the IS. Furthermore, despite the considerable loss of water-free osmoles along the LAL within the IM, LAL is here an osmotically equilibrating rather than a diluting segment in the IM. Although these two points both seem quite straightforward consequences of the anatomic and permeability characteristics represented by the model, the matter of the profiles along the CD remains troublesome. This will be discussed below. As we saw above, the LDL lose salt and gain urea as they descend within the IM. Since LDL osmolality increases from 984 to 1,525 mosmol/kgh 2 O within the IM in this simulation, the LDL clearly gain more urea osmoles than the salt osmoles they lose, recalling that (TF/P) inulin (Table 2) in the LDL that reach the tip is virtually identical to mean (TF/P) inulin of LDL at the OM/IM border, so their increased osmolality is due here not to water loss but virtually exclusively to solute (i.e., urea) entry. DISCUSSION For the discussion that follows, we remind the reader that our purpose is not to justify the present model (41) but rather to explore its predictions in detail, since to date it is the most accurate representation of the available experimental measurements and anatomic data. As such, this model analysis serves two purposes: 1) it serves as a quantitative check on qualitative extrapolations that have been based on these same data; and 2) by our detailed analysis, we hope to clarify directions for future improvements and new measurements. To facilitate the discussion in comparison with the experimental literature, Fig. 4, A C, illustrates the results given in Tables 6 and 7, showing the basic patterns of intertubular exchanges and recycling of water, urea, and salt, respectively, for the present model. Summary of Water Cycles This model s water exchanges basically conform to the accepted idea of water short-circuiting in each medullary region; that is, with few exceptions, the descending structures lose water and ascending vasa recta take up water. In combination with the greatly reduced number of loops and capillaries toward the papilla, this results in a progressive reduction of total volume toward the papilla, thus reducing the amount of osmotic work needed to render the deep papilla hyperosmotic. The model predicts exceptions to this water shortcircuiting pattern in descending Henle s loops where water uptake accompanies high urea influx, namely, in the LDL of the deep papilla (uptake of urea dumped form the terminal IMCD), in LDL of the UIM (massive recycling of urea dumped from parallel ascending LAL and LAV), and in SDL running close to vascular bundles in the inner stripe (uptake of urea dumped from LAV in the vascular bundles and also form nearby LAL). A close look at Tables 6 and 7 reveals (see columns LVR tot and LHL tot, row IM tot ) that in the IM, the net volume receivers are in fact LDL due to their considerable (for the region) water uptake in the UIM, not the LVR, which despite their considerable water short-circuiting from LDV to LAV actually have a small net water loss within the IM of this model. Summary of Salt Cycles Salt is actively transported out of thick ascending limbs (LAL and SAL) in the outer medulla, diluting the tubule lumen due to the negligible water permeability of these segments. This is recognized as the basic motor for the concentrating mechanism. It is also known that there is salt transport (not only of sodium but also of
15 MEDULLARY SOLUTE AND WATER CYCLING F685 potassium, to say nothing here of ammonium and bicarbonate) along the CD throughout the medulla, but it has generally been assumed in modeling studies that these transport systems transfer no net osmoles, being based on one-for-one ion exchangers, and should thus have minimal effect on the concentrating mechanism, a view which may merit revision. The present model follows tradition and neglects this CD transport except within the UIM, where we now include slight net active salt transport (41, 42), which is too small to affect overall medullary salt balance significantly but is crucial for the organism s salt balance. Improving the CD solute transport is desirable but must be done by accomodating other solutes, including at least KCl. Within the IM, according to the passive hypothesis, passive salt loss from LAL, made possible by high interstitial urea thanks to urea dumped from the CD (17, 35), is taken to be the single effect for buildup of the IM osmotic gradient. In the present model, the LAL does in fact dump salt along its whole IM ascent; this salt is not here recycled into the parallel descending Henle s limbs but is instead carried up and out of the IM by the ascending vasa recta, which corresponds to Stephenson s analysis of the passive mechanism (36). It is sometimes suggested (2, 3, 22, 30) that some or most of the salt transported out of the ascending limbs in the OM is recycled down into the IM in the LDL, which have a moderately high measured salt permeability, but in this model osmotic equilibration along the LDL in the OM by water loss occurs fast enough to keep the salt gradient small across the LDL wall; consequently, the amount of salt diffusing into the LDL within the OM (0.3% of FL s, see Table 6) is only a negligible fraction of that dumped into the OM by the SAL and LAL (20.6% and 8.9% of Fl s, respectively). We thus see that in this simulation virtually all of the salt actively transported out of ascending limbs in the OM is returned directly to the general circulation via the vasa recta. The role of this active salt transport in the concentrating mechanism is thus to dilute the ascending limb fluid and concentrate the descending fluid not by adding salt but by drawing water out of the LDL and CD (but not from SDL in the inner stripe, which is concentrated by urea uptake as mentioned above). Summary of Urea Cycles Although urea is apparently transported passively everywhere in the kidney under normal conditions, it has long been recognized that it plays a central role in the concentrating mechanism, though the exact nature of that role remains one of the central questions about how the concentrating mechanism works. We are thus particularly interested to see just what the present model predicts about urea handling, given its close conformity to measured permeabilities and anatomical arrangements. Before summarizing the details of the urea exchanges within each region, it is useful to look at the big picture, starting with the primary event of interest, namely, the dumping of 43.4% of the filtered load of urea into the IM by the IMCD. What is the ultimate fate of this dumped urea in this simulation? From Table 3 we can calculate the net dumping or uptake of urea in each tube type over the whole medulla by subtracting urea outflow in the ascending tubes at the corticomedullary border from urea inflow in the descending tubes. Thus for LVR F LVR,u (0) FD LDV,u (FD LAV1,u FD LAV2,u ) (14) remembering that upward flows are negative. Similar equations apply for SVR, LHL, and SHL. Results of these calculations are given in Table 8. From this table, we see that in this simulation 1) nearly all of the urea dumped from IMCD in the inner medulla actually ends up returning to the cortex via the SVR, and 2) Henle s loops make no net contribution of urea to the medulla, since the net amount picked up by the long loops is identical to the net amount dumped by the short loops. Since the SVR do not extend into the IM, there is clearly massive urea exchange within the outer medulla from the LAV and LAL, which carry the urea up from the IM, to other tubes. Figure 4B shows the urea paths in this simulation. Within the IM, the two main features are urea dumping by the IMCD and massive urea recycling between descending and ascending branches of both Henle s loops and the vasa recta. To better appreciate the extent of inner medullary urea recycling, we can point out (Table 6) that the LDL take up an amount of urea equal to 36% of FL u on their way down within the IM and the LAL take up another 8% of FL u in the LIM and TZ before they start dumping urea further up in the IM, for a total of 44% taken up by Henle s loops. LDV take up an amount of urea equal to 122% of FL u and LAV2 take up another 14% in the deepest medulla, for a total of 136% of FL u taken up by vasa recta. This amounts to a grand total of 180% of FL u taken up by vasa recta and Henle s loops, whereas only 43.4% of FL u is dumped by the IMCD. The difference, or the equivalent of 137% of FL u, is recycled within the IM, where total volume flow is only a small fraction of GFR. Within the OM, in accord with classic predictions, urea from LAV is recycled to LDV and SDV within the vascular bundles of the IS and also within the OS. More surprising, however, is the role of the short Henle loops in this model; they pick up considerable urea as they pass near the VB in the IS, as is generally supposed, but then they dump an even greater quantity of urea in the OS before returning to the cortex (urea dumping Table 8. Global medullary urea balance for Henle s loops and vasa recta (%FL u ) Tube Type Input Output Net Urea Dumped LVR SVR Vasa recta total LHL SHL Henle s loop total All values are in % FL u.
16 F686 MEDULLARY SOLUTE AND WATER CYCLING from MTAL has in fact been suggested by some authors; see, e.g., Ref. 30); that is, the model predicts that the fate of the urea they pick up is not to be carried up and around to contribute to the urea load delivered to the collecting ducts, but rather just to be transferred from the IS to the OS, where part of it is recycled via the SDV and the rest is lost to the cortex in the SAV. This OS urea loss from the SAL is of course made possible by their relatively low but certainly not negligible measured urea permeability (15). Although recently cloned urea transporters have led to localization of their expression along the nephron (25, 27, 28, 33), none has been found in the medullary thick assending limb (MTAL), which, assuming the measured permeability value is correct, suggests either that some as yet unidentified urea transporter operates in this segment or that urea may pass between the cells across the tight junctions. A more recent report confirms this tendency using purified vesicles from apical and basolateral membranes of MTAL (29), since it suggests that urea permeability of both cell membranes is very low. Whatever the eventual outcome of this question of the effective in vivo urea permeability of the outer stripe MTAL, the handling of urea by this model s short loops is clearly in disagreement with micropuncture data from surface convolutions. Armsen and Reinhardt (1) measured end proximal and early distal tubule fractional delivery of urea in Wistar rats by micropuncture under antidiuresis and varying degrees of water diuresis. They found that in accessible short loops of nondiuretic rats, FD u increased from 60% of FL u at the end of the accessible proximal convoluted tubule (PCT) to 100% at the beginning of the distal convoluted tubule (DCT), a near doubling of tubular urea flow in superficial nephrons despite a halving of volume flow [(TF/P) inulin increased from 3 to 6]. This is clear evidence of considerable net urea uptake in the superficial loops of Henle during antidiuresis, contrary to the model s prediction of a small net urea loss. This suggests, of course, that the model might work better, i.e., might produce a higher urinary osmolality and steeper IM gradient, if P u of SAL were reduced to lessen or prevent urea dumping in the OS and thus increase urea delivery to the CD. The results of testing this idea with the present model bear witness to the difficulty of second-guessing this complicated system. In a series of simulations (partial results given in Table 9), P u (SAL) was gradually reduced to 1 100th of its control value. The result was a modest decrease rather than the expected increase of U osm, from 1,434 down to 1,396 mosmol/kgh 2 O, which is explained as osmotic diuresis, i.e., the short loops did in fact deliver slightly more urea to the CD, but instead of resulting in greater urea recycling, the CD dumped the same amount of urea into the IM as before, so FE urea increased from 13.1% to 18.5% of FL u, resulting in greater urine flow [(TF/P) inulin decreased from 729 to 535]. These results suggested that the model s IMCD urea permeability is too low to permit effective recycling of the higher urea load. Subsequently increasing P u (CD) in TZ and LIM to higher measured values (7, 32) did in fact lead to increased U osm, but only if L p (CD) was also increased to higher measured values (19, 31), permitting water to follow the urea dumping at the higher rate. This set of parameter changes (indicated by an asterisk in Table 9) effectively increased the model s urea recycling (IMCD dumps 57.4% of FL u instead of 43.4%) and increased U osm to 1,630 mosmol/kgh 2 O. It is not our purpose here to develop this idea systematically, but Table 9 shows some of the interesting functional parameters in comparison with the basic simulation results from the earlier tables, and Fig. 5 shows the area-weighted slice profiles of urea concentration, salt osmoles, and total osmolality, similar to measurements in whole slices from each medullary depth according to c i j c i,j n j A j j n j A j (15) where i is urea, NaCl, or osmolality, c i,j is the concentra- Table 9. Test simulations in an effort to increase urea recycling Parameter Modifications U osm (U/P) inulin SNFD u, % of SNFL u J u Net,%ofFL u LAL(0) LDL(L) SAL(0) CD(0) CD(L) SHL CD Baseline 1, P SAL u /100 1, *P SAL u P IMCD IMCD u and L p 1, P SAL IMCD u L p 1, P SAL IMCD u P u 1, P IMCD IMCD u and L p 1,720 1, IMCD P u 1, IMCD L p 1, The following changes were made to parameter values: 1) P SAL u 10 2 (from baseline value of cm/s); 2) L p (in nl cm 2 mosm 1 min 1 ) and corresponding P f values (in µm/s in parentheses) of IMCD increased from baseline values of 16 (148), 20 (185), and 20 (185) to recent literature values of 22 (203), 22 (203), and 75 (700) in UIM, TZ, and LIM, respectively; c) P CD u increased from cm/s to recent literature value of cm/s in TZ and LIM. In the columns for SNFD u, the (0) and (L) indicate the top of the medulla and the tip of the papilla, respectively. J Net u is the net integrated flux across tubule walls along the structure within the whole medulla. The simulation marked with an asterisk (*) was used for plots in Fig. 5.
17 MEDULLARY SOLUTE AND WATER CYCLING F687 Collecting Duct Profile Fig. 5. Comparison of corticopapillary slice profiles from test simulation (thick curves) with those of the baseline simulation (thin curves). Inset: profile of the ratio of urea to total osmoles. tion of i in structure j, n j is the number of structures j at the given medullary level, and A j is the crosssectional area of the individual structures (42). The inset to Fig. 5 shows the ratio of urea to total slice osmolality along the medulla. This simulation (indicated by an asterisk in Table 9) gives results more closely in accord with experimental results on several important points, namely, increased U osm, steeper and deeper inner medullary osmolality profile, and better agreement of SNFD u values with micropuncture results, especially concerning the significant addition of urea to the short Henle s loops, which was, after all, the impetus for making these changes. Table 9 also presents results of simulations in which CD urea and water permeabilities were changed alone, with or without the reduction of SAL urea permeability. It nonetheless remains the case (results not shown) that increasing urea permeability of LDL and/or LAL in the IM in this new scenario still results in poorer performance. Osmole Cycles With respect to the literal question of the IM osmolality gradient, what matters is the net change of osmolality from one slice to the next, although of course total osmolality is composed both of salt and urea (as well as other solutes in the kidney itself). As can be seen in the inset of Fig. 5, the fraction of this model s total slice osmolality due to urea rises from 12% at the top of the OM to more than 50% in the TZ of the IM and then remains high through the remainder of the deep medulla. This increasing ratio of urea to salt osmoles is accomplished as the result of three features: urea dumping from the terminal IMCD, efficient urea recycling, and, last but not least, efficient water shortcircuiting, without which the amount of urea dumped by the CD would be insufficient. It is also interesting to note that the depth at which total osmolality levels off (instead of continuing to increase, as observed experimentally) coincides with the leveling off of the ratio of urea concentration to total osmolality. As already mentioned, the osmolality and urea concentrations along the collecting duct in this model (Fig. 3A) reach a maximum in the inner stripe instead of increasing gradually in tandem with the rest of the medulla (Fig. 5) as is usually supposed to occur. This unconventional CD profile results directly from the model s inner stripe radial heterogeneity, that is, from the fact that each tube in this region sits in a unique environment along the vascular bundle-to-cd continuum, rather than all being bathed by a common interstitial milieu (as in central core models). The immediate neighbors of the CD in this region of the model are the SAV4, i.e., a population of short ascending vasa recta that arise from vascular beds in the region of the thick ascending limbs and the CD, far from the vascular bundles. Due to the vigorous hyperosmotic salt pumping by the ascending limbs, this neighborhood develops the high osmolality that draws water out of the CDs, which in this region have high water permeability but very low salt and urea permeabilities, resulting in dramatic concentration of the contents of the CD luminal fluid. The same arguments could be invoked in favor of a mode of operation proposed by Bonventre and Lechene (4) suggesting that fluid in the LDL is hyperosmotic on exiting the IS into the upper IM and could thus supply net osmoles to the IM. So, since this is in fact a rather conventional view of the operation of the outer medullary CD, it seems reasonable that the CD profile should be different from that of Henle s loops and the vasa recta; nonetheless, the actual extent of the difference predicted by the model is surprising. It thus becomes important to confront the prediction with data. When it has been mentioned in the literature, it is generally believed that the CD profile conforms essentially to that observed in medullary slices, and three studies are most often cited in support of this belief, namely Wirz (45), Gottschalk and Mylle (8), and Gottschalk et al. (9). However, a close look at these reports reveals that they do not in fact provide direct evidence for an inner medullary osmotic gradient along the CD; the spatial resolution of the cryoscopic slice technique of Wirz (45), though ingenious, was probably too poor to give clear evidence of osmotic differences between neighboring structures at the relatively high temperatures they used ( 10 C); Gottschalk and Mylle (8) made measurements only in structures close to the papillary tip, not further up the CD near the papillary base; and Gottschalk et al. (9) did obtain evidence for a considerable osmolality gradient along the IMCD, but only in Psammomys, a species that cannot be easily compared to other rodents for both anatomic (100% long loops) and dietetic reasons (no urea problem because they eat only succulent plants). So, based only on these reports, it would be possible to suspect the truth may lie with the model s predictions. There are other data, however, some of which is in favor of and some
18 F688 MEDULLARY SOLUTE AND WATER CYCLING against an osmotic gradient along the IMCD. These studies are briefly summarized as follows. Ullrich (39) measured (TF/P) inulin, [Na], [K], ph, and [NH 4 ] and total osmolality (but not urea) in fluid samples obtained by retrograde microcatheterization at various levels along the IMCD of golden hamsters in antidiuresis. That study showed clearly that along the IMCD there was considerable water and sodium reabsorption, virtually no potassium reabsorption, and a decrease of ph (dramatic increase of [NH 4 ]), but that the osmolality remained virtually constant from the OM/IM border to the papilla. This evidence thus tends to agree with the prediction of the present model, but it must be pointed out that their animals had urinary osmolality ranging from 400 to 1,200 mosmol/kgh 2 O, not frankly antidiuretic. Jamison (11) used a similar technique in rats with urinary osmolalities ranging from 800 to 1,450 mosmol/ kgh 2 O and found an average gradient of 226 mosmol/ kgh 2 O per mm along the final 1.5 mm of the IMCD. Sonnenberg (34), also using retrograde microcatheterization in rat collecting ducts, also found a considerable osmolality gradient within the IMCD under nondiuretic (U osm up to 1,400 in the experimental kidneys, and 1,800 in the control kidney of the same animals), but not under diuretic conditions, and confirmed many of the results of Ullrich (39). Marsh and Martin (23) used microcatheterization into the papillary CD of the hamster. They presented only (TF/P) inulin and fractional deliveries (or fractional excretion rates) at the end of the CD (duct of Bellini), in the ureter, and at a point 4 mm higher upstream in the CD system, which in the hamster is about two-thirds of the way up to the OM/IM border. Osmolality and urea concentration in CD fluid calculated from the data they report showed an osmolality increase from 1,550 mosmol/kgh 2 O at the upstream point to 2,300 mosmol/ kgh 2 O at the papillary tip, i.e., a gradient of 200 mosmol/kgh 2 O per mm (if linear) along the IMCD of the hamster in antidiuresis. For comparison with the present model of the rat kidney, the analogous position would be situated 2.7 mm from the tip, or just at the bottom of the UIM. In the model, CD osmolality at this point is 1,229 mosmol/kgh 2 O and is 1,496 mosmol/ kgh 2 O at the papillary tip, giving a gradient of 100 mosmol/kgh 2 O per mm, or only half as steep as that found by Marsh and Martin (23). Existing data thus mostly support a steeper IMCD osmolality gradient than that predicted by the present model. Conclusions This explicit model analysis of solute and water exchanges within the medulla confirms the general patterns that have been predicted based on anatomy and permeability data while pointing to some discrepancies, especially concerning the micropuncture data on urea absorption by short nephrons and the supposed profiles along the IMCD. A more serious problem pointing, in this author s opinion, to some fundamental lack in the present formulation, is that this model and all previous models of this system predict a lower instead of steeper inner medullary gradient if permeabilities along the inner medullary LDL are raised, whereas these permeabilities are uniformly found to be higher in species that concentrate better than in those that do not. In a recent study aimed specifically at this question, Layton et al. (21) confirmed, in chinchilla kidneys, the high urea permeability of LDL under conditions resembling those in vitro as closely as possible. This issue thus remains central to the question of the inner medullary concentrating mechanism. I suggest several possible directions for further investigation. 1) Thanks principally to the recent work of Pallone and collaborators (26), it is becoming clear that the vasa recta may play a role much more interesting than that attributed to them up to now. To mention just one possibility stemming from their work, Pallone et al. (26) found that, contrary to the notion that vasa recta allow essentially free passage to small solutes and water, the sodium permeability of OMDVR may in fact be quite low and may be principally paracellular, whereas water and urea permeabilities are high and they probably pass through the cells, In our recent presentation of the present model (41), we showed that reduction of P s in OMDVR could dramatically increase concentrating ability by favoring osmotic equilibration in the OM in these vessels by water removal instead of solute addition, thereby reducing IM blood flow. 2) Our favored hypothesis is that there may be interstitial osmolytes other than salt and urea in the inner medulla that contribute to the concentrating mechanism by drawing water out of the descending structures. This idea was formalized by Jen and Stephenson (14) and was tested by us in a model similar to the one analyzed here (38) without specification of the possible origin of the extra osmoles. Those studies made it clear that if such osmolytes are present in concentrations on the order of around 100 mosmol/ kgh 2 O or more, then they could play a very important role in the concentrating mechanism. One possible source of such osmolytes, which remains to be tested experimentally, is the metabolism of glucose to lactate, since it is known that anaerobic glycolysis is the main source of ATP in the relatively hypoxic inner medulla. Without engaging in a detailed analysis here, we postulate that it could be the case that a relatively constant rate of metabolic osmole production (no a priori reason to suspect that cells in the medulla consume more ATP during antidiuresis) might be ineffective during diuresis but that it could become important to the concentrating mechanism when volume flow to the inner medulla is reduced during the onset of antidiuresis (invoking adjustment of permeabilities in the OMDVR to reduce IM blood flow as mentioned just above), allowing a buildup by countercurrent exchange in the reduced IM volume. 3) Solutes found in relative abundance in the medulla and urine but not traditionally included in models of the medulla, such as potassium and NH 4, whose recycling paths are interesting in their own right, may
19 MEDULLARY SOLUTE AND WATER CYCLING F689 turn out to play a significant role in the concentrating mechanism. I thank Anthony Wexler for insightful critical comments and suggestions. I am grateful to Lise Bankir and Christian de Rouffignac for stimulating discussions on this problem over many years and to Lise Bankir for continually drawing my attention back to the importance of urea. Address for reprint requests: S. R. Thomas, INSERM U 467, Necker Faculty of Medicine, 156 rue de Vaugirard, Paris Cedex 15, France. Received 13 March 1998; accepted in final form 26 June REFERENCES 1. Armsen, T., and H. W. Reinhardt. Transtubular movement of urea at different degrees of water diuresis. Pflügers Arch. 326: , Bankir, L., N. Bouby, and M.-M. Trinh-Trang-Tan. The role of the kidney in the maintenance of water balance. Baillière s Clin. Endocr. Metab. 3: , Bankir, L., and C. de Rouffignac. Urinary concentrating ability: insights from comparative anatomy. Am. J. Physiol. 249 (Regulatory Integrative Comp. Physiol. 18): R643 R666, Bonventre, J. V., and C. Lechene. Renal medullary concentrating process: an integrative hypothesis. Am. J. Physiol. 239 (Renal Fluid Electrolyte Physiol. 8): F578 F588, Diezi, J., P. Michoud, J. Aceves, and G. Giebisch. Micropuncture study of electrolyte transport across papillary collecting duct of the rat. Am. J. Physiol. 224: , Elalouf, J. M., D. Chabane Sari, N. Roinel, and C. de Rouffignac. NaCl and Ca delivery at the bend of rat deep nephrons decreases during antidiuresis. Am. J. Physiol. 252 (Renal Fluid Electrolyte Physiol. 21): F1055 F1064, Gillin, A. G., and J. M. Sands. Characteristics of osmolaritystimulated urea transport in rat IMCD. Am. J. Physiol. 262 (Renal Fluid Electrolyte Physiol. 31): F1061 F1067, Gottschalk, C. W., and M. Mylle. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am. J. Physiol. 196: , Gottschalk, C. W., W. E. Lassiter, M. Mylle, K. J. Ullrich, B. Schmidt-Nielsen, R. O Dell, and G. Pehling. Micropuncture study of composition of loop of Henle fluid in desert rodents. Am. J. Physiol. 204: , Han, J. S., K. A. Thompson, C. L. Chou, and M. A. Knepper. Experimental tests of three-dimensional model of urinary concentrating mechanism. J. Am. Soc. Nephrol. 2: , Jamison, R. L. Micropuncture study of superficial and juxtamedullary nephrons in the rat. Am. J. Physiol. 218: 46 55, Jamison, R. L., J. Buerkert, and F. Lacy. A micropuncture study of Henle s thin loop in Brattleboro rats. Am. J. Physiol. 224: , Jamison, R. L., and C. R. Robertson. Recent formulations of the urinary concentrating mechanism: a status report. Kidney Int. 16: , Jen, J. F., and J. L. Stephenson. Externally driven countercurrent multiplication in a mathematical model of the urinary concentrating mechanism of the renal inner medulla. Bull. Math. Biol. 56: , Knepper, M. A. Urea transport in isolated thick ascending limbs and collecting ducts from rats. Am. J. Physiol. 245 (Renal Fluid Electrolyte Physiol. 14): F634 F639, Knepper, M. A., R. A. Danielson, G. M. Saidel, and R. S. Post. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int. 12: , Kokko, J. P., and F. C. Rector. Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 2: , Kriz, W. Structural organization of the renal medulla: comparative and functional aspects. Am. J. Physiol. 241 (Regulatory Integrative Comp. Physiol. 10): R3 R16, Lankford, S. P., C. L. Chou, Y. Terada, S. M. Wall, J. B. Wade, and M. A. Knepper. Regulation of collecting duct water permeability independent of camp-mediated AVP response. Am. J. Physiol. 261 (Renal Fluid Electrolyte Physiol. 30): F554 F566, Lassiter, W. E., C. W. Gottschalk, and M. Mylle. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am. J. Physiol. 200: , Layton, H. E., M. A. Knepper, and C. L. Chou. Permeability criteria for effective function of passive countercurrent multiplier. Am. J. Physiol. 270 (Renal Fluid Electrolyte Physiol. 39): F9 20, Lemley, K. V., and W. Kriz. Cycles and separations: the histotopography of the urinary concentrating process. Kidney Int. 31: , Marsh, D. J., and C. M. Martin. Lack of water or urea movement from pelvic urine to papilla in hydropenic hamsters. Miner. Electrolyte Metab. 3: 81 86, Nielsen, S., T. L. Pallone, B. L. Smith, E. I. Christensen, P. Agre, and A. B. Maunsbach. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am. J. Physiol. 268 (Renal Fluid Electrolyte Physiol. 37): F1023 F1037, Nielsen, S., J. Terris, C. P. Smith, M. A. Hediger, C. A. Ecelbarger, and M. A. Knepper. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc. Natl. Acad. Sci. USA 93: , Pallone, T. L., S. Nielsen, E. P. Silldorff, and S. Yang. Diffusive transport of solute in the rat medullary microcirculation. Am. J. Physiol. 269 (Renal Fluid Electrolyte Physiol. 38): F55 F63, Promeneur, D., G. Rousselet, L. Bankir, P. Bailly, J. P. Cartron, P. Ripoche, and M. M. Trinh-Trang-Tan. Evidence for distinct vascular and tubular urea transporters in the rat kidney. J. Am. Soc. Nephrol. 7: , Ripoche, P., and G. Rousselet. Urea transporters. Nephrologie 17: , Rivers, R. L., A. Blanchard, D. Eladari, F. Leviel, M. Paillard, R. Podevin, and M. L. Zeidel. Unit permeabilities of medullary thick ascending limb (mtal) apical (AP) and basolateral (BL) membranes are similar (Abstract) J. Am. Soc. Nephrol. 8: 24, de Rouffignac, C. The urinary concentrating mechanism. In: Urinary Concentrating Mechanisms, edited by R. K. H. Kinne, Basel: Karger, 1990, p Sands, J. M., M. Naruse, J. D. Jacobs, J. N. Wilcox, and J. D. Klein. Changes in aquaporin-2 protein contribute to the urine concentrating defect in rats fed a low-protein diet. J. Clin. Invest. 97: , Sands, J. M., and D. C. Schrader. An independent effect of osmolality on urea transport in rat terminal inner medullary collecting ducts. J. Clin. Invest. 88: , Shayakul, C., M. A. Knepper, C. P. Smith, S. R. Digiovanni, and M. A. Hediger. Segmental localization of urea transporter mrnas in rat kidney. Am. J. Physiol. 270 (Renal Fluid Electrolyte Physiol. 41): F654 F660, Sonnenberg, H. Medullary collecting duct function in antidiuretic and in salt- or water-diuretic rats. Am. J. Physiol. 226: , Stephenson, J. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 2: 85 94, Stephenson, J. L. Urinary concentration and dilution: models. In: Handbook of Physiology. Renal Physiology, Bethesda, MD: Am. Physiol. Soc., 1992, sect. 8, vol. II, chapt. 30, p Stephenson, J. L., J. F. Jen, H. Wang, and R. P. Twearson. Convective uphill transport of NaCl from ascending thin limb of loop of Henle. Am. J. Physiol. 268 (Renal Fluid Electrolyte Physiol. 37): F680 F692, Thomas, S. R., and A. S. Wexler. Inner medullary external osmotic driving force in a 3-D model of the renal concentrating mechanism. Am. J. Physiol. 269 (Renal Fluid Electrolyte Physiol. 38): F159 F171, Ullrich, K. J. Function of the collecting ducts. Circulation 21: , 1960.
20 F690 MEDULLARY SOLUTE AND WATER CYCLING 40. Wang, H., R. P. Tewarson, J. F. Jen, and J. L. Stephenson. A comparison of multinephron and shunt models of the renal concentrating mechanism. Appl. Math. Lett. 6: 61 65, Wang, X., S. R. Thomas, and A. S. Wexler. Outer medullary anatomy and the urine concentrating mechanism. Am. J. Physiol. 274 (Renal Physiol. 43): F413 F424, Wang, X., and A. S. Wexler. The effects of collecting duct active NaCl reabsorption and inner medullar anatomy on renal concentrating mechanism. Am. J. Physiol. 270 (Renal Fluid Electrolyte Physiol. 39): F900 F911, Wexler, A. S., R. E. Kalaba, and D. J. Marsh. Passive, one-dimensional countercurrent models do not simulate hypertonic urine formation. Am. J. Physiol. 253 (Renal Fluid Electrolyte Physiol. 22): F1020 F1030, Wexler, A. S., R. E. Kalaba, and D. J. Marsh. Threedimensional anatomy and renal concentrating mechanism. I. Modeling results. Am. J. Physiol. 260 (Renal Fluid Electrolyte Physiol. 29): F368 F383, Wirz, H. K. Lokalisation des Konzentrierungsprozesses in der Niere durch direkte Kryoskopie. Helv. Physiol. Acta 9: , 1951.
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Anatomy / Vascularisation. 2. Urine concentration. 3. Axial heterogeneity of some segments
 Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Normal Renal Function
 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Faculty version with model answers
 Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Diagram of the inner portions of the kidney
 Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi
 Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY
 URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
I. Metabolic Wastes Metabolic Waste:
 I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
Physiology (5) 2/4/2018. Mohammad jaber
 Physiology (5) 2/4/2018 Mohammad jaber Slides are embedded in the sheet in bold, some figure are not included sorry for any mistake,, lets go.. There are two types of urine : 1-diluted urine ( Excess fluid
Physiology (5) 2/4/2018 Mohammad jaber Slides are embedded in the sheet in bold, some figure are not included sorry for any mistake,, lets go.. There are two types of urine : 1-diluted urine ( Excess fluid
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
CLEARANCE AND REABSORPTION OF WATER*
 CLEARANCE AND REABSORPTION OF WATER* I. Glomerular Filtration Rate and Renal Plasma Flow A. It would be extremely useful for both practical and theoretical reasons to be able to estimate the amount of
CLEARANCE AND REABSORPTION OF WATER* I. Glomerular Filtration Rate and Renal Plasma Flow A. It would be extremely useful for both practical and theoretical reasons to be able to estimate the amount of
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System)
 NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Chapter 25: Urinary System
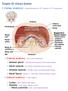 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
AP2, Lab 7 - THE URINARY SYSTEM
 AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Moayyad Al-Shafei. - Saad Hayek. - Yanal Shafaqoj. 1 P a g e
 - 5 - Moayyad Al-Shafei - Saad Hayek - Yanal Shafaqoj 1 P a g e In this lecture we are going to study the tubular reabsorption of Na+. We know that the body must maintain its homeostasis by keeping its
- 5 - Moayyad Al-Shafei - Saad Hayek - Yanal Shafaqoj 1 P a g e In this lecture we are going to study the tubular reabsorption of Na+. We know that the body must maintain its homeostasis by keeping its
Counter-Current System Regulation of Renal Functions
 Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Renal System and Excretion
 Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Class XI Chapter 19 Excretory Products and their Elimination Biology
 Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
1. remove: waste products: urea, creatinine, and uric acid foreign chemicals: drugs, water soluble vitamins, and food additives, etc.
 Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Chapter 44. Osmoregulation and Excretion
 Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
General Anatomy of Urinary System
 General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
28/04/2013 LEARNING OUTCOME C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS STUDENT ACHIEVEMENT INDICATORS URINARY SYSTEM & EXCRETION
 LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
From Blood Filtrate to Urine: A Closer Look
 Blood Vessels Associated with the Nephrons Each nephron supplied with blood by an afferent arteriole branch of renal artery divides into glomerular capillaries capillaries converge as they leave glomerulus
Blood Vessels Associated with the Nephrons Each nephron supplied with blood by an afferent arteriole branch of renal artery divides into glomerular capillaries capillaries converge as they leave glomerulus
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Long-Term Regulation of Renal Urea Transporters during Antidiuresis
 18 Electrolyte & Blood Pressure 4:18-22, 2006 3) Long-Term Regulation of Renal Transporters during Antidiuresis Dong-Un Kim, M.D. Department of Pediatrics, College of Medicine, The Catholic University
18 Electrolyte & Blood Pressure 4:18-22, 2006 3) Long-Term Regulation of Renal Transporters during Antidiuresis Dong-Un Kim, M.D. Department of Pediatrics, College of Medicine, The Catholic University
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Class XI - Biology Excretory Products and their Elimination
 Class XI - Biology Excretory Products and their Elimination MULTIPLE CHOICE QUESTIONS 1. The following substances are the excretory products in animals. Choose the least toxic form among them? a. Urea
Class XI - Biology Excretory Products and their Elimination MULTIPLE CHOICE QUESTIONS 1. The following substances are the excretory products in animals. Choose the least toxic form among them? a. Urea
Physiology (1) 27/3/2018. Hala Nsour
 Physiology (1) 27/3/2018 Hala Nsour This lecture topic is about kidney function, nephron, renal blood flow (RBF), glomerular filtration (Ma fe da3e trj3/e ll slides) The doctor started with anatomy of
Physiology (1) 27/3/2018 Hala Nsour This lecture topic is about kidney function, nephron, renal blood flow (RBF), glomerular filtration (Ma fe da3e trj3/e ll slides) The doctor started with anatomy of
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Hill et al. 2004, Fig. 27.6
 Lecture 25, 15 November 2005 Osmoregulation (Chapters 25-28) Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 1. Osmoregulation 2. Kidney Function Text: Chapters
Lecture 25, 15 November 2005 Osmoregulation (Chapters 25-28) Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 1. Osmoregulation 2. Kidney Function Text: Chapters
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Unit #4 Waste and Excretion. The Kidneys
 Unit #4 Waste and Excretion The Kidneys Renal Hilus (Hilus) the doorway of the kidney Ureter leaves this region blood and lymphatic vessels enter and exit here Renal Capsule (Capsule) smooth fibrous tissue
Unit #4 Waste and Excretion The Kidneys Renal Hilus (Hilus) the doorway of the kidney Ureter leaves this region blood and lymphatic vessels enter and exit here Renal Capsule (Capsule) smooth fibrous tissue
Outline Urinary System
 Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
