Tight junction biology and kidney dysfunction
|
|
|
- Rodger Wilkins
- 6 years ago
- Views:
Transcription
1 Am J Physiol Renal Physiol 290: F20 F34, 2006; doi: /ajprenal Tight junction biology and kidney dysfunction David B. N. Lee, 1,2 Edmund Huang, 2,3 and Harry J. Ward 2,4 Divisions of Nephrology, 1 Veterans Affairs Greater Los Angeles Healthcare System (VISN 22), 3 UCLA Medical Center and 4 King/Drew Medical Center and 2 David Geffen School of Medicine, University of California, Los Angeles, California Lee, David B. N., Edmund Huang, and Harry J. Ward. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol 290: F20 F34, 2006; doi: /ajprenal The epithelial tight junction (TJ) has three major functions. As a gate, it serves as a regulatory barrier separating and maintaining biological fluid compartments of different composition. As a fence, it generates and maintains the apicobasal polarity of cells that form the confluent epithelium. Finally, the TJ proteins form a trafficking and signaling platform that regulates cell growth, proliferation, differentiation, and dedifferentiation. Six examples are selected that illustrate the emerging link between TJ dysfunction and kidney disease. First, the glomerular slit diaphragm (GSD) is evolved, in part, from the TJ and, on maturation, exhibits all three functions of the TJ. GSD dysfunction leads to proteinuria and, in some instances, podocyte dedifferentiation and proliferation. Second, accumulating evidence supports epithelial-mesenchymal transformation (EMT) as a major player in renal fibrosis, the final common pathway that leads to end-stage renal failure. EMT is characterized by a loss of cell-cell contact and apicobasal polarity, which are hallmarks of TJ dysfunction. Third, in autosomal dominant polycystic kidney disease, mutations of the polycystins may disrupt their known interactions with the apical junction complex, of which the TJ is a major component. This can lead to disturbances in epithelial polarity regulation with consequent abnormal tubulogenesis and cyst formation. Fourth, evidence for epithelial barrier and polarity dysregulation in the pathogenesis of ischemic acute renal failure will be summarized. Fifth, the association between mutations of paracellin-1, the first TJ channel identified, and clinical disorders of magnesium and calcium wasting and bovine renal fibrosis will be used to highlight an integral TJ protein that can serve multiple TJ functions. Finally, the role of WNK4 protein kinase in shunting chloride across the TJ of the distal nephron will be addressed. kidney disease; zonula occludens THE TIGHT JUNCTION (TJ) FORMS a circumferential belt around an epithelial or endothelial cell, separating the plasma membrane into an apical and a basolateral domain. The belt from one cell adjoins belts from adjacent cells, thereby forming a sheet of cellular barrier between the external environment, e.g., the luminal content of the intestine or the renal tubule, from the regulated internal environment, i.e., the interstitial fluid (155). In the blood-brain (53) or the blood-testis (27) barrier, the TJ also separates and maintains the integrity of specialized fluid compartments within the internal environment. As a boundary between the apical and the basolateral plasma membrane, the TJ demarcates the asymmetric distribution of protein and lipid molecules between these two domains, thereby generating polarity in the two-dimensional plane of the plasma membrane. More recent studies indicate that the TJ congregates polarity-regulating protein complexes that participate in polarized membrane trafficking and serves as a spatial landmark for vesicle docking (94, 182), suggesting that it also regulates polarity in the three-dimensional space of the cell cytoplasm. The net effect is the establishment of a apicobasal polarity that is a defining characteristic of all epithelium (40). Address for reprint requests and other correspondence: D. B. N. Lee, Dept. of Medicine (111), VA Medical Ctr, Plummer St., Sepulveda, CA ( dbnlee@ucla.edu). The TJ proteins that regulate epithelial polarity also control cell proliferation and differentiation (12) and participate in epithelial-mesenchymal transformation (46, 129) and carcinogenesis (84). In addition to its role as a platform for trafficking and signaling macromolecular complexes, the TJ proteins also serve as viral receptors mediating viral budding and infection (8, 29, 161, 163) and as targets for bacterial pathogens and their virulence factors (143, 161). Situated immediately basal to the TJ is the adherens junction (AJ), also a continuous, circumferential cell-cell junction belt (35). Both junction belts exhibit a similar molecular architecture consisting of transmembrane-bridging proteins networking with a juxtaposed cytoplasmic platform of protein complexes, which are, in turn, linked to the actin cytoskeleton (Fig. 1). In the ensuing discussion on the role of TJ in kidney dysfunction, it is important to appreciate that the TJ is evolved from and stabilized by the AJ (4, 47, 118, 159) and is structurally and functionally interrelated to the AJ. For example, the generation and maintenance of epithelial cell polarity, traditionally considered functions of the TJ (31), are more likely functions involving both the TJ and the AJ (25, 72). Although cell polarity is a phenomenon observed in both unicellular and multicellular organisms (25), in our discussion polarity refers to the apicobasal polarity that is a characteristic of epithelium and endothelium and is strictly dependent on the formation and F20
2 Invited Review F21 Fig. 1. Schematic and simplified molecular architecture of the tight junction, the adherens junction, and the glomerular slit diaphragm. Each junction complex consists of 2 protein assemblies, the transmembrane-bridging proteins and the cytoplasmic protein platform, and the actin cytoskeleton. Some components of the protein assemblies in each junction complex are listed in Table 1. integrity of cell-cell contacts (72). The interrelationship between cell polarity and cell growth, differentiation, and dedifferentiation has received increasing interest (12, 54, 172) and is one focus of this discussion. In this review, we will address selected conditions in which the biology of TJ and clinical disorders of the kidney have sufficiently interwoven to provide newer insights and allow provocative speculations. GLOMERULAR SLIT DIAPHRAGM, PROTEINURIA, AND PODOCYTE DEDIFFERENTIATION AND PROLIFERATION Glomerular Slit Diaphragm: A Hybrid Between TJ and AJ The glomerular slit diaphragm (GSD) represents the junction structure that links the interdigitating foot processes from neighboring glomerular visceral epithelial cells (the podocytes) (132). Developmentally, the podocytes are evolved from primordial columnar epithelial cells linked together by an apical junction complex containing both the TJ and the AJ. As the epithelial cells develop into podocytes, each with its elaborate network of foot processes, the early apical TJ and AJ migrate basally and ultimately morph into a single GSD that links the interdigitating foot processes from adjacent podocytes (76, 128, 141). It should be appreciated that while the TJ or the AJ links one cell to another cell forming an epithelium, the GSD links one cellular process (a foot process) from one podocyte to the interdigitating cellular process of another podocyte forming a podothelium (Fig. 2). The mature GSD retains features of both the TJ and the AJ. Immunostaining for the TJ-associated protein zonula occludens-1 (ZO-1) in adult rat kidney reveals the greatest intensity at the insertion sites of the GSD into the foot processes (140). Also, like the TJ, the GSD functions as a barrier (the gate function of the TJ) (31) that imposes selective permeability between the blood and the urinary space (117, 153), acts as a fence (31) that segregates the apical and the basolateral plasma membrane domains of the podocyte (104, 141), and serves as a signaling platform (11). However, unlike the TJ, which tightly approximates the plasma membranes from adjacent cells, thereby obliterating the intervening intercellular space (35), the GSD spans an intercellular space of 25 nm (128) to nm (132). This separation of the plasma membrane from adjacent foot processes resembles that in the AJ, where juxtaposed cell membranes are separated by a distance of 25 nm (35). Although ZO-1 is a TJ marker in confluent epithelial sheets, in nonepithelial cells (61) and in epithelial cells before the formation of the TJ (4, 159), this protein is localized at the AJ. Indeed, several studies have observed the presence of core AJ molecules, i.e., the cadherins, the catenins, or both in early glomerular visceral epithelium (44, 150, 158). In mature GSD, however, some investigators have observed cadherins (130, 150) or catenins (119), whereas others have failed to find either Fig. 2. Apical junctions of the epithelial cells and the glomerular slit diaphragm of the podocytes. A: tight junction (TJ) and the adherens junction (AJ) link adjacent cells. B: glomerular slit diaphragm (GSD) links adjacent cell processes (the foot processes). Pcb, podocyte cell body; Pcp, podocyte cell process; Pfp, podocyte foot process; GSD, glomerular slit diaphragm. Only primary cell processes of the podocytes are represented. The figure is not drawn to proportion or scale.
3 F22 cadherins (119, 158, 178) or catenins (44, 150, 158). Instead, Inoue and associates (60) reported the expression of FAT, another member of the cadherin superfamily, in the adult rat kidney, particularly in the podocytes, where it stains at the podocyte attachment of the GSD with its cytoplasmic domain colocalizing with ZO-1. FAT has a large extracellular domain containing 34 tandem cadherin-like repeats, five epidermal growth factor (EGF)-like repeats, and a laminin A-G domain. The authors suggested that this bulky adhesive molecule would well serve the function of bridging the relatively wide intercellular space between the foot processes. GSD Proteins are Responsible for its Barrier Function The molecular architecture of the GSD junction complex also resembles that of the TJ and AJ and consists of a collection of transmembrane-bridging proteins networking with a juxtaposed cytoplasmic platform of protein complexes, which, in turn, is linked to the actin cytoskeleton (Fig. 1). The major transmembrane and cytoplasmic plaque proteins of the GSD are listed in Table 1. Recent recognition that mutations of genes that express these proteins cause nephrotic syndrome lends strong support for GSD as a selective barrier that prevents protein loss into the urinary space (71, 104, 146). The three components of the GSD junction complex function as a unit, and the dysfunction of a single member affects the structure and function of both the GSD and the actin cytoskeleton, producing the characteristic effaced podocyte phenotype of the proteinuric states (104). Morphologically, the effaced podocytes revert back into primordial epithelial cells, linked by apical TJ-like structures into a continuous sheet that blankets the basement membrane (80). Conceptually, it is difficult to understand why the rather flimsy-looking GSD linking the relatively widely separated foot processes of the podocytes is more effective than this tightly packed epithelial sheet at preventing protein loss into the urinary space. One explanation is that protein flux into the urinary space is mediated through a transcellular endocytotic pathway rather than through the intercellular conduit of the flattened epithelium (36, 59). Another theory proposes that the Table 1. Selected examples of proteins in the molecular architecture of the tight junction, adherens junction, and glomerular slit diaphragm Transmembrane-Bridging Proteins Cytoplasmic Protein Plaque Tight junction (42) Occludin ZO-1 The claudins ZO-2 JAM ZO-3 Adherens junction (106) Cadherins -Catenin -Catenin Vinculin -Actinin Glomerular slit diaphragm Nephrin (NPHS1) ZO-1 ( -) (see text). Podocin (NPHS2)* CD2AP NEPH1 (NEPH1) -Actinin-4 (ACTN4) FAT1 - and -Catenins P-cadherin Reference nos. are in parentheses. ZO, zonula occludens; JAM, junctional adhesion molecule. *Both NH 2 and COOH terminals are cytosolic linked by hairpin domain in plasma membrane. Reported by some but not others (see text). Gene mutations associated with phenotypic proteinuric states. transformed glomerular visceral epithelium detaches from the basement membrane, leaving areas denuded of cell covering and consequent unimpeded protein flux into the urinary space (165, 180). Neither of these theories could be confirmed in other studies, leading to a third theory that the GSD-transformed TJ represents an abnormally leaky junction complex (80, 121). It is important to realize that the reverted epithelium with TJ complexes, albeit leaky to proteins (predominantly albumin), continues its life-sustaining function as a barrier between the blood and the urinary compartments. Even in the case of protein loss, a daily albumin excretion of 40 g in a patient with a glomerular filtration rate (GFR) of 100 liters/day represents only 1% of the albumin that could have been lost (assuming a normal blood albumin concentration of 40 g/l) or 2% if blood albumin level is only half that of normal. The observation that podocyte effacement with loss of GSD can also be seen in minimal-change disease during remission (nonproteinuric) (80) suggests the transformed TJ, like the GSD, has the potential to function as a barrier that excludes protein leakage. GSD May Regulate Podocyte Polarity and Proliferation Molecular linkage between the apical junction complex and epithelial cell growth pattern is well established. The transfection of an oncogenic Raf-1 into a rat parotid epithelial cell line leads to a complete loss of TJ function and the transformation of the monolayer into a stratified phenotype exhibiting no contact inhibition in cell growth (84). Introduction of exogenous occludin into these transformed cells results in the reappearance of TJ and restoration of the normal monolayer phenotype with a contact inhibition pattern of cell growth. The AJ has also been shown to play an important role in contact inhibition of cell growth in both the epithelium (17) and the endothelium (10). Contact inhibition (also known as densitydependent inhibition) refers to the physiological phenomenon in which growth ceases as cells reach confluent density and come into contact with each other. This process has been associated with the development of the apical junction complex (64). Accumulating evidence supports a close linkage between the regulation of cell polarity and the control of cell proliferation (12, 172). As mentioned earlier, the GSD, like the TJ, also maintains podocyte apical-basal polarity (104, 141) and serves as a signaling platform (11). In experimental crescentic glomerulonephritis, foot process effacement and the disappearance of GSD are associated with the loss of podocyte polarity, as reflected by the apical migration of the basolateral membrane protein 3 -integrin. The luminal membrane also begins secreting extracellular matrix material, normally the function of the basal membrane domain (83). Podocyte effacement associated with massive proteinuria is reversible in some cases but not in others. Minimal-change disease and membranous nephropathy are examples of the reversible cases in which the disappearance of the foot processes and GSD is associated with actin cytoskeleton reorganization, the retention of all mature podocyte markers, and the absence of cell proliferation. In contrast, in collapsing focal segmental glomerulosclerosis (FSGS), the foot-process effacement is associated with the loss of the actin-based cytoskeleton, reduction or disappearance of markers of differentiated
4 podocytes (dedifferentiation), and cell proliferation (6). The loss of GSD integrity and podocyte polarity associated with podocyte dedifferentiation and proliferation bears close resemblance to events described in epithelium associated with the loss of TJ integrity. In human immunodeficiency virus-associated nephropathy, a recognized cause of collapsing FSGS, podocyte proliferation is attributed to a loss of contact inhibition (142). Summary Developmentally, the GSD appears to be a product of both the TJ and AJ. Like the TJ, it functions as a selective barrier and participates in the regulation of apicobasal polarity and cell growth, differentiation, and dedifferentiation. Structural and functional disruption of the GSD gives rise to pathophysiological changes in the podothelium similar to those in the epithelium associated with TJ dysfunction. EPITHELIAL-MESENCHYMAL TRANSITION AND RENAL FIBROSIS Tubulointerstitial fibrosis, characterized by glomerulosclerosis, interstitial fibrosis, and tubular atrophy, is the final common pathway to end-stage renal disease (62, 89, 184). Epithelial-Mesenchymal Transition and its Role in Renal Fibrosis Resident fibroblasts and the infiltrated mononuclear cells have been considered the major players in renal fibrosis. However, accumulating evidence suggests that new fibroblasts are also derived from tubular epithelial cells through a process termed epithelial-mesenchymal transformation (EMT) and that the infiltrating monocytes/macrophages may, in fact, play a beneficial role through their phagocytic activity on extracellular (ECM) matrix and apoptotic cells (62, 89, 184). EMT in epithelium is characterized by the disruption of epithelial junction complexes and the loss of cell polarity, transforming stationary epithelial cells into migratory mesenchymal fibroblast-like cells. Normal epithelial cell interacts with ECM through anchorage between its basal plasma membrane domain and the basement membrane. The transformed cell, on the other hand, loses this domain-specific anchorage and acquires the ability to invade the ECM (137). In embryonic development, EMT allows the migration of epithelial cells to distant sites and forms tissues such as the mesoderm and the neural crest (33, 49). In mature tissue, it is responsible for the progression of noninvasive tumor cells into malignant, metastatic cancers (160). Evidence for epithelial-derived fibroblasts in renal fibrosis has been demonstrated in transgenic mice genetically engineered to express the LacZ marker in renal tubular epithelia (63). Unilateral ureteral obstruction in R26R mice produces progressive interstitial fibrosis and complete destruction of the kidney in 3 4 wk. The normal contralateral kidney prevents the development of azotemia. Following an obstructive injury, LacZ-expressing (tubular) cells lose their epithelial morphology and organization and migrate into the interstitium in large numbers. These epithelial-derived cells expand by cell division and express fibroblast-specific protein-1 (FSP1). These transformed cells synthesize collagen, as reflected by the expression of heat shock protein 47, which is known to increase to Invited Review F23 chaperon type I collagen in cells activated to synthesize collagen (107). Role of TJ and AJ in EMT The association between EMT and structural and functional alterations in TJ and AJ is well established. Transforming growth factor- 1 (TGF- 1 )-induced EMT is associated with reduced expression of ZO-1 (114, 174, 184) and E-cadherin (177). In a mouse hepatic cell line, oncogenic Raf-1-induced EMT is associated with a marked downregulation of occludin and claudin-2 expression at both the transcriptional and translational levels (82). Snail, a zinc-finger transcription factor, is another important player implicated in the activation of EMT (109). It binds to three E-boxes in the promoter of the human E-cadherin gene, blocking its transcription (9). Madin-Darby canine kidney (MDCK) epithelial cells transfected with Snail downregulate E-cadherin expression, increase expression of mesenchymal markers (vimentin and fibronectin), transform to a fibroblastoid phenotype, and exhibit tumorigenic invasive properties (23). Snail protein is also present endogenously and is associated with a loss of E-cadherin expression in invasive mouse and human tumors and in epithelial tumor cell lines (9, 23). However, Snail-mediated phenotypic changes cannot be accounted for completely by a loss of E-cadherin. Blockage of E-cadherin expression by stable antisense transfection leads to the acquisition of invasive and metastatic properties but is insufficient to induce full-scale EMT (23). A more recent study demonstrated that EMT induced in cultured mouse epithelial cells is associated with concomitant repression in the transcription and translation of claudins-3, -4, -7, and occludin (58). Snail binds to the E-boxes of the promoters of the genes of these TJ proteins, with consequent complete repression of their promoter activity. Snail inhibition of occludin transcription is confirmed in MDCK cells. However, the transcription of claudin-1 (unlike the mouse claudins-3, -4, -7 mentioned above) and ZO-1 are unaffected, and their downregulation is attributed to posttranscriptional events (113). Thus Snail-induced EMT is associated with downregulation of TJ and AJ proteins at both the transcriptional and posttranscriptional levels. Spatiotemporal studies indicate that loss of cell-cell adhesion and cell polarity precedes full-scale EMT (62, 89, 184). In rat lung carcinogenesis, losses of ZO-1 and E-cadherin expression are early events that precede EMT, raising the possibility of a cause-and-effect relationship (14). In primary culture of porcine thyroid epithelium treated with both TGF- and epidermal growth factor (EGF), reduced expression of claudin-1 and occludin occurred in the first 24 h, accompanied by a loss of transepithelial resistance (TER) and a marked increase in the paracellular flux of [ 3 H]inulin. Both the expression and localization of E-cadherin are not altered at 24 h, although the clear loss of its expression did occur by 48 h and was associated with fibroblastic transformation of the epithelial cells (46). These data suggest that alterations in TJ precede those in AJ and the evolution of EMT. A coordinated role of TJ and AJ in EMT is demonstrated using MDCK-I monolayers and COOH-terminal deletion mutants of ZO-1. Wild-type ZO-1 contains five major domains, PDZ-PDZ-PDZ-SH3-GUK. A deletion mutant (ZO-1-PDZ) constructed by the removal of the GUK and the SH3 domains
5 F24 loses its localization to the plasma membrane at the TJ and migrates into a cytoplasmic pool. This is associated with a dramatic EMT. Transfected cells no longer form confluent, polarized monolayers that generate TER in two-dimensional cultures or form cysts lined by polarized epithelium in threedimensional cultures. Other features of EMT include a reduction in epithelial TJ and AJ marker gene expression and an augmentation of mesenchymal marker gene expression. The EMT is also associated with the activation of a -catenin signaling pathway. Ectopic expression of the adenomatous polyposis coli tumor suppressor gene, which is known to downregulate -catenin signaling, reverts the transformed phenotype of mutant ZO-1-PDZ cells (129). These data support the notion that the translocation of the TJ protein ZO-1 from plasma membrane to the cytoplasm leads to structural and functional dysfunction of the TJ and AJ, probably through the activation of the -catenin signaling pathway. More recent studies further established a pivotal role of TJ in EMT (7, 116, 152). TGF- signals through TGF- type I and type II transmembrane receptors (TGF RI and TGF RII, respectively). Occludin binds and promotes the recruitment of TGF RI to the TJ. TGF- induction of EMT is followed by the additional recruitment of TGF RII to the same junction complex (7). In addition to occludin, TGF RI also directly binds partitioning-defective protein 6 (Par6) (116). Phosphorylation of Par6, a known regulator of epithelial cell polarity and TJ assembly, by TGF RII is necessary for TGF -dependent EMT in mammary gland epithelial cells (116). Phosphorylated Par6 binds and redistributes Smurf1 to the TJ. Smurf1 is an E3 ubiquitin ligase and mediates localized ubiquitination and degradation of RhoA, which is an important modulator of TJ assembly and stability. These observations suggest that TJ disassembly is an early event in EMT and is mediated through a series of events including the phosphorylation of Par6, the recruitment of Smurf1 to the TJ, and the modulation of localized degradation of RhoA (116). Summary EMT plays an important role in renal fibrosis. Structural and functional alterations in both the TJ and AJ are important components in the genesis of EMT. POLYCYSTINS, CELL POLARITY, TUBULOGENESIS, AND AUTOSOMAL DOMINANT POLYCYSTIC KIDNEY DISEASE Autosomal dominant polycystic kidney disease (ADPKD) is manifested by defects in the structure and function of the polarized epithelial kidney cells, with consequent abnormal cell growth and formation of numerous fluid-filled cysts (30, 169). The ability to form cysts would suggest the TJ continues to function as a barrier in ADPKD. Indeed, morphologically and functionally normal TJ has been reported in cultured monolayers of cells isolated from proximal tubule-derived cysts from ADPKD kidneys (26). On the other hand, a body of information suggests that ADPKD is associated with alterations in the apical junction complex and dysregulation of polarity and cell growth. This information is briefly reviewed here because TJ is a component of the apical junction complex and is functionally important in the regulation of epithelial polarity and cell growth and regulation. Polycystins Almost all cases of ADPKD are the consequence of mutations in either the PKD1 (type I ADPKD) or the PKD2 (type II ADPKD) gene that encodes the proteins polycystin-1 (PDK1) and polycystin-2 (PKD2), respectively. The two proteins function as a receptor-ion channel complex, with PKD1 transducing mechanical cues that gate a Ca 2 -permeant PKD2 channel, thereby initiating a cascade of Ca 2 signals (108). Thus lossof-function mutations in either protein predictably give rise to qualitatively similar phenotypic manifestations (30, 81, 169). The PKD protein complex has been localized to all three membrane domains of renal tubular cells: the apical cilium, the lateral cell-cell junction complex, and the basal cell membranematrix anchorage (169). Thus it links all components of the extracellular environment surrounding the cell membrane to the intracellular actin-tubelin cytoskeleton and the protein signaling networks, rendering it an ideal candidate for generating and maintaining cell polarity and cell orientation. Direct association between polycystin-2 and the actin microfilament (85) and centrosome protein pericentrin (65) has been demonstrated. Cell Polarity, Polycystins, Tubulogenesis, and Cystogenesis A large body of evidence suggests tubulogenesis is coupled closely to an orchestrated modification and regulation of cellcell contact and polarity (90, 166, 183). In this context, the polycystins have been shown to induce tubule formation (15). MDCK cells form cysts in three-dimensional collagen gel matrix and differentiate into tubules in the presence of hepatocyte growth factor (HGF) (101). Interestingly, overexpression of human PKD1 in these cells is sufficient to induce tubulogenesis in the absence of HGF. Clones that lose the expression of recombinant PKD1 lose the ability to form tubules and revert back to forming cysts (16). With use of the same model, it has been shown that in HGF-induced tubulogenesis the development of tubular cell polarity and the formation of tubular lumen coincide with the expression of endogenous (canine) PKD-1 at intercellular contacts (19). Compared with the tubules, PKD1 mrna is markedly downregulated in cysts and the PKD1 protein is mislocated from the plasma membrane to cytoplasmic pools (19). Finally, growth factors, including HGF, induce cell polarity generation and branching tubulogenesis in normal human renal epithelial cells but not in epithelial cells from cysts of ADPKD (which express mutated proteins) (24), again suggesting the importance of PKD proteins in normal tubulogenesis. Polycystins and the Apical Junction Complex There is evidence supporting the interplay between each of the three components of the apical junction complex [TJ, AJ, and the desmosomes or the desmosomal junction (DJ)] with the polycystins and tubulogenesis. The exocyst, an eight-protein complex, is involved in the biogenesis of cell polarity from yeast to mammals (179). In the early stages of cell-cell contact formation, the exocyst is associated with the AJ, but as the apical junction complex matures, the bulk of the exocyst is sorted to the TJ (179). In the three-dimensional MDCK II cell culture model, the exocyst has been shown to play a central role in tubulogenesis. Its expression in sprouting tubules syn-
6 chronizes with the orchestrated changes in cell polarity and lumen formation (88). The observation that in ADPKD cells the exocyst is depleted from the plasma membrane (26) is consistent with the thesis that this polarity-regulating complex at the TJ is important in normal tubulogenesis. More recently, the presence of exocyst has also been demonstrated in the primary cilium of MDCK II cells (133). There is also a molecular and functional association between the AJ and the polycystins. Endogenous PKD1 is found in a complex with E-cadherin and the catenins, the major components of AJ (52). In ADPKD cells, cellular E-cadherin expression is lower than that in normal kidney cells and is translocated from the plasma membrane into the cytoplasm (26). These observations raise the possibility that PKD1 mutations disrupt both the normal AJ cytoarchitecture and the integrity of the apical junction complex, resulting in a loss of cell polarity regulation that is necessary for normal tubulogenesis. Finally, the polycystins have also been localized to the third component of the apical junction complex, the DJ (19, 139). Like the TJ and AJ, the DJ is an intercellular adhesive junction (35). However, the two major transmembrane desmosomal glycoproteins, desmogleins and desmocollins, exhibit little or no adhesive activities (28, 75). PKD1, on the other hand, displays strong homophilic interaction between its multiple extracellular Ig-like domains (56) and has been proposed to mediate DJ adhesion (19). In HGF-induced tubulogenesis, AJ and DJ play prominent roles, particularly during the phase of modified polarity (166). The presence in the apical membrane of some proteins normally localized to the basolateral membrane, e.g, Na -K - ATPase and EGF receptors, provides further evidence supporting the presence of a polarity defect in ADPKD (169). This can be attributed to a defect in the fence function of the TJ resulting in protein mistargeting. Another possibility is a dysfunctional TJ, and its partner components of the apical junction complex, can lead to epithelial cell dedifferentiation. The abnormal polarization may represent a reversion of tissue to a developmentally more primitive state. Na -K -ATPase in the normal fetus is made up of 1 2 -subunits and is localized apically, whereas in the adult kidney it is complexed as 1 1 -subunits and is localized basolaterally. In ADPKD kidneys, Na -K -ATPase is expressed as 1 2 in the apical plasma membrane (170), suggesting dedifferentiation into fetal epithelium. In a similar fashion, fetal epidermal growth factor receptor (EGFR) is expressed apically as a heterodimer (one copy each of EGFR and erb-b2), whereas the adult EGFR is basal in location and expressed as a homodimer (two copies of EGFR). In ADPKD, EGFR expression and localization duplicate the fetal pattern (169). This abnormal apical localization of EGFR, coupled with the finding that ADPKD patients secrete mitogenic concentrations of EGF into the lumen of the cysts (131), creates a sustained cycle of autocrine-paracrine stimulation of proliferation in the cysts (169). Summary Evidence for interactions among components of the apical junction complex, the polycystins, and polarity and growth regulation in ADPKD is beginning to emerge. The role of TJ, a component of the apical junction complex and a major player Invited Review in epithelial polarity regulation, in ADPKD is relatively unexplored and deserves further study. BARRIER AND POLARITY DYSREGULATION IN ISCHEMIC ACUTE RENAL FAILURE F25 About 50% of acute renal failure (ARF) encountered in hospital practice is attributable to hypoxic or ischemic injuries (151). The pathogenetic mechanism of ischemic ARF is complex and remains poorly defined. Sublethal, Reversible Ischemic Experimental Model The experimental model of sublethal and reversible ischemic injury provides an important conceptual advance over the original notion that ARF is predominantly the result of tubular necrosis ( acute tubular necrosis ) (87). This sublethal model is characterized by the reversible loss of epithelial barrier function and polarity (20, 144) and is the central theme of this review. The examination of ischemia-induced epithelial structural and functional changes is greatly facilitated by the use of cultured epithelium subjected to ATP depletion, a model that mimics molecular and cellular effects of renal ischemia, which is also characterized by a rapid depletion of cellular ATP (20, 87). ARF, TJ, and AJ In general, studies in ARF suggest the involvement of both TJ and AJ, reiterating the importance of both junction structures in the maintenance and regulation of epithelial permeability and polarity. Both the TJ and the AJ are intimately associated with the perijunctional actin-myosin ring (3, 157). The apical actin cytoskeleton, particularly that of the proximal tubular cells, is particularly sensitive to ischemia, exhibiting changes and loss of the majority of F-actin within 5 min (70). Among the early consequences of this perturbation are the alteration in junction complexes and cell polarity (97). Rho GTPases regulate actin cytoskeleton and cell adhesion (37, 48) and play a central role in cell polarization (38). Activation and inhibition of Rho ATPase signaling, respectively increases and decreases protection of MDCK II monolayer TJ from damage during ATP depletion (43). The association of ischemia or ATP-depletion with TJ alterations is documented in clinical (78), animal (99), and in vitro cell culture studies (22, 91). Altered cellular distribution of the TJ proteins occludin (43), ZO-1 (5, 43), and ZO-1, ZO-2, and cingulin in combination (154) has been observed with ATP depletion. ATP depletion also causes rapid internalization of E-cadherin, suggesting disruption of the AJ (92). Degradation of E-cadherin and disruption of its interaction with the catenins have been demonstrated in ischemic whole kidney and in ATP-depleted cultured MDCK monolayers (21). Reduced expression and redistribution of ZO-1 and AJ proteins ( -, -, and -catenins) have also been documented in renal allografts with postischemic injury (78). One mechanism for the reduction in GFR in ARF is the backleak of the filtrate across the damaged tubular epithelium and intratubular obstruction (Fig. 3). Evidence for backleak of glomerular filtrate is derived from both animal (32) and human studies (78, 102, 105). Recent demonstration of TJ and AJ abnormalities discussed above provides the molecular and
7 F26 Fig. 3. Acute renal failure (ARF): the back-leak and intratubular obstruction hypothesis. A: normal tubular cells linked by TJ into an epithelial sheet that forms a selective barrier (the gate function) between the tubular fluid compartment and the interstitium. The fence function of the TJ regulates the apicobasal polarity, segregating molecules such as the integrins to the basal plasma membrane domain forming the adhesive anchorage of the cell-basement membrane junction. B: ischemia-induced TJ dysfunction leads to a loss of barrier function, allowing the leakage of glomerular filtrate (tubular fluid) back to the interstitium and a loss of polarity function, allowing the apical migration of basal molecules such as the integrins. C: loss of basal anchorage molecules allows cell or cell fragments to exfoliate from the basement membrane and adhere and aggregate with other exfoliated cells or fragments within the tubular lumen or with other anchored cells with apical adhesive molecules. The obstruction-induced increase in pressure proximal to the obstruction can both oppose glomerular filtration as well as accelerate backleak of tubular fluid across the denuded basement membrane. cellular basis for these observations. The transtubular backleak of glomerular filtrate reflects a loss of TJ barrier function. Ischemic insults also cause dysregulation in the fence function of TJ (Fig. 3). Apical fluorescent phosphatidyl choline (PC) has been documented to diffuse to the basolateral membrane domain in ATP-depleted monolayers, suggesting that the TJ molecular fence is compromised (5). In rat proximal tubule cells, Brown and associates (18) demonstrated a redistribution of apical microvillar actin along with the actin binding protein villin from the apical to the basolateral plasma membrane within 1hofreperfusion after ischemia. Restoration of normal polarization follows continued reperfusion. This loss of polarity is accompanied by structural changes, including amorphous transformation of distinct finger-like microvilli and the formation of extracellular vesicles with cytoplasmic contents ( blebs ), which are either endocytosed or released into the tubular lumen. The loss of microvilli reduces apical membrane surface area and consequent decrease in tubular reabsorptive capacity, whereas the aggregation of membrane blebs and cytoplasmic contents released into the tubular lumen can cause intratubular obstruction (97). This will be further discussed below. The disruption of polarity is also manifested by the apical localization of basolateral membrane proteins, such as the integrins (Fig. 3), which normally anchor tubular cells to the basement membrane. ATP depletion results in the redistribution of 1 -integrin subunits to the apical membrane, and the loss of anchorage allows the exfoliation of viable cells into the tubular lumen (86, 100). Cell detachment would be expected to expose a greater area for glomerular filtrate backleak. The integrins, which are major cell adhesion receptors, promote cell-cell aggregation of exfoliated cells with one another or with normally anchored cells expressing apical integrins (Fig. 3). This constitutes a second cause for obstruction (see discussion of membrane bleb aggregation in the previous paragraph) of tubular fluid flow, with a consequent increase in tubular pressure proximal to the obstruction, thereby magnifying the backleak of glomerular filtrate. Tubular cell detachment (45, 115, 123) and obstruction (32, 149) have been reported in both clinical and experimental studies. Ischemic damage also causes ectopic localization of Na - K -ATPase to either the cytoplasmic compartment (77, 162) or the apical membrane (98). In addition, a reduction in its expression has also been reported (79). It has been proposed that Na -K -ATPase plays a direct role in TJ assembly, and an ischemic-induced alteration in its expression and localization may disturb TJ assembly with consequent loss of permeability and polarity regulation ( ). Basally located Na -K -ATPase plays a major role in the tubular reabsorption of sodium. The ectopic localization of Na -K -ATPase away from the basolateral membrane, particularly in tubular segments proximal to the macula densa, is expected to enhance distal sodium delivery in renal ischemic injuries (77, 78). Reduction in proximal sodium reabsorption is compounded by the decrease in apical surface area for reabsorption, as discussed earlier. It is proposed that this increase in sodium delivery to the macula densa activates tubuloglomerular feedback and results in a reduction in GFR. This constitutes an important alternative mechanism linking polarity dysregulation to ischemia-induced reduction in GFR because evidence for tubular obstruction is not a consistent finding in animal (93) and human (78) studies. Indeed, a prior study implicated an afferent vasoconstriction (consequence of tubuloglomerular feedback) rather than tubular obstruction as the cause for the depression in transcapillary hydraulic pressure difference and loss of GFR in postischemic renal allografts (2).
8 Endothelial Junction Complex in ARF Alterations in the structure and function of the endothelial cell junction complex may also contribute to ischemic damage by causing microvascular dysfunction (97). In a rodent model of acute ischemic renal failure, early disruption of the actin cytoskeleton is followed by the loss of staining of endothelial cell-cell junctions and an increase in endothelial permeability (147). Summary Experimental and clinical studies support important associations between epithelial barrier and polarity dysregulation and ARF. Emerging evidence suggests similar association between the endothelial apical junction complex and ARF. PARACELLIN-1, Mg 2, AND Ca 2 WASTING AND RENAL FIBROSIS Fig. 4. Paracellular Mg 2 and Ca 2 absorption in thick ascending limb of the loop of Henle (TAL). Na -K -2Cl cotransporter translocates these ions from lumen into cell in an electroneutral fashion. K recycles back to the lumen through the K channel (ROMK), maintaining a lumen-positive gradient of 8 mv. Na exits the cell through the basally located Na -K -ATPase and Cl through the Cl channels Ka and Kb (ClC-Ka and ClC-Kb). The positive lumen potential drives the Mg 2 and Ca 2 across the TJ and through the paracellular conduit. Invited Review F27 Paracellin-1/Claudin-16 and Mg 2 Wasting Renal Mg 2 reabsorption occurs mostly through the paracellular pathway in the thick ascending limb of Henle (TAL) (122). In the TAL, electrically neutral lumen-to-cell cotransport of Na -K -2Cl is followed by the recycling of K through renal outer medullary K (ROMK) channel back into the lumen, thereby generating a lumen-positive potential (Fig. 4). This transepithelial voltage drives the positively charged Mg 2 and Ca 2 through the paracellular conduit in an absorptive direction. The high Mg 2 and Ca 2 conductance through this intercellular pathway contrasts with its water impermeability (74), and the molecular mechanism for this divalent ion conductance has been elucidated recently. Positional cloning has identified a human gene, paracellin-1 (PCLN-1), that encodes the protein, paracellin-1 (PCLN-1) (145). This protein is colocalized with occludin at the TJ of the TAL of the loop of Henle and, when mutated, causes renal Mg 2 wasting in the syndrome of familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC). Of the 10 mutations identified in 1 series, 8 were homozygous, and 2 compound heterozygous (145). In a another series of 33 patients, 19 homozygous and 13 compound heterozygous mutations were documented, with 1 patient demonstrating no mutation within the coding sequence (164). PCLN-1 exhibits structural and sequence homology to the claudin family of tetraspan transmembrane TJ proteins with two extracellular loops (156, 181) and is now classified as claudin-16. Its relationship to paracellular Mg 2 handling has led to the concept that the TJ is populated with channels that mediate selective and regulated transport of solutes across the paracellular conduit (173, 181). Among the many other phenotypic manifestations of this disorder is the frequent association with deterioration in renal function, terminating, in many cases, in end-stage renal failure (120, 164). Paracellin-1/Claudin-16 and Hypercalciuria Of interest is the report of frequent, isolated hypercalciuria with or without kidney stones (HC) in otherwise healthy family members with no additional manifestations of FHHNC (120, 164). It was proposed that these subjects represent heterozygous mutation carriers of the claudin-16 gene (164). This raises the interesting question of whether some patients with HC may in fact represent heterozygous mutations in the claudin-16 gene and manifest a dominant inheritance pattern. However, screening in a cohort of families with idiopathic hypercalciuria came up with an unexpectedly novel finding. A single homozygous substitution of a cytosine for a guanine at position 697 of the open reading frame was identified in four patients from two families (103). Unlike the FHHNC, in which all homozygous or compound heterozygous mutations affect amino acids in either the extracellular or the transmembrane segments of the claudin-16 molecule, this new homozygous mutation results in the substitution of threonine (T) for an arginine (R) (T233R) in the last amino acid triplet (threoninearginine-valine at positions ) of the COOH terminal of the claudin-16 molecule (Fig. 5). Patients present with childhood hypercalciuria, which progressively resolves, with urine Ca 2 excretion normalizing in adulthood. Another difference from the classic FHHNC is the absence of deterioration of GFR in all four patients. Urine Mg 2 excretion, measured in three patients, is slightly elevated compared with the unaffected members, but frank hypomagnesemia is detected only in one patient. This suggests that the mild renal wastage is compensated for, probably through an increase intestinal absorption. Whether renal Mg 2 handling changes with maturation is not mentioned. Using polarized MDCK epithelium, the authors demonstrated that the wild-type claudin-16 binds ZO-1, the prototypic TJ scaffold protein in polarized epithelium, and the two proteins colocalize at the TJ. They reasoned that claudin-16/zo-1 binding is most likely mediated through claudin-16 s COOH-
9 F28 Fig. 5. Claudin-16 mutations and associated phenotypes. A: homozygous or compound heterozygous mutations affecting amino acids in the extracellular and transmembrane segments of claudin-16 are associated with familial hypomagnesemia with hypercalciuria and nephrocalcinosis in humans with frequent progression to end-stage renal failure. B: homozygous mutations affecting the last 3 amino acids of the COOH terminal of claudin-16 are associated with childhood hypercalciuria that resolves with growth and maturation and no reduction in glomerular filtration rate. C: null mutation of claudin-16 is associated with bovine autosomal recessive chronic interstitial nephritis and death with renal failure. terminal threonine-arginine-valine (TRV) sequence (which resembles the type 1 PDZ-binding motif) (55, 135) with the PDZ domain of ZO-1 (41). Indeed, binding between ZO-1 and the T233R mutant is markedly impaired, and the bulk of the mutated claudin-16 is mislocalized from the plasma membrane into the lysosomes (103). The authors postulated that the mutated claudin-16 is targeted to the TJ but its residency there is markedly shortened because of its inability to tether to ZO-1, resulting in a marked reduction of channel-conducting activities at the TJ. A higher claudin-16 expression coupled with the anticipated lower Ca 2 turnover rate in adults may explain the resolution of hypercalciuria with maturation. In microperfusion of mouse cortical TAL, paracellular absorption of Ca 2 and Mg 2 is higher in tubules from adults (8 wk) than from young (4 wk) mice (171). Another study demonstrated that the threonine or the valine residue of the COOH-terminal TRV sequence of PCLN-1 is necessary for binding to ZO-1. Mutants with an alanine substitution of threonine (ARV) or valine (TRA), or with deletion of TRV (delta TRV), inhibit this PCLN-1/ZO-1 association, and PCLN-1 becomes widely distributed along the lateral membrane rather than selectively at the TJ. Mutants with an alanine substitution of arginine (TAV) behave and function as the wild-type and both exhibit apical-to-basal 45 Ca 2 transport activity at levels much higher than those in the other three mutations. Ca 2 transport is enhanced by a positive electrical potential gradient and inhibited by increasing Mg 2 concentrations (57). Paracellin-1/Claudin-16, Renal Fibrosis, and Renal Failure Null mutation of the claudin-16 gene in Japanese Black cattle (Wagyu) is associated with autosomal recessive chronic interstitial nephritis with diffuse zonal fibrosis (CINF) (51, 73, 110). Clinical manifestations include growth retardation with hoof elongation, proteinuria, and death with renal failure (111). The initial mutation (type 1) identified is characterized by a 37-kb deletion containing the first four axons of the claudin-16 gene, resulting in the absence of a claudin-16 transcript (51). This was followed by the identification of a second mutation (type 2), consisting of a 56-kb deletion containing the first four exons and 21-bp of the fifth exon (50). Compared with the normal cattle, the affected cattle exhibit a reduction in creatinine and Mg 2 clearance, an elevation in fractional excretion of Mg 2, and normal serum Mg 2 concentration (112). Earlier, we mentioned that patients with FHHNC frequently progressed to end-stage renal failure. Praga and associates (120) reported that six of eight patients required maintenance dialysis from within 1 10 yr following presentation. The two remaining patients showed mild renal insufficiency after 11 and 9 yr of follow-up, respectively (120). Twelve of 33 patients from 12 European pediatric departments developed end-stage renal failure at a median age of 14.5 yr, with a range of yr. A less aggressive progression to renal failure is reported in a series of seven Arab patients. However, the oldest of the seven patients at the last follow-up is 16 yr, and the age range in the remaining six patients is yr (69), raising the possibility that the clinical course may change with a longer follow-up. While the deterioration in renal function associated with progressive tubulointerstitial nephropathy in FHHNC has traditionally been attributed to the concomitant hypercalciuria and nephrocalcinosis, long-term correlative clinical experience has not consistently supported this association (120, 164). These authors observed that the correlation between renal failure and hypercalciuria and nephrocalcinosis is also not borne out in conditions such as distal renal tubular acidosis or antenatal Bartter syndrome. It appears that the site and extent of claudin-16 gene mutation determine the phenotypic manifestation, which can span the spectrum from a variation in channel conductance to an alteration in cell proliferation and differentiation (Fig. 5). We raise the possibility that gene mutations in some cases of FHHNC may affect claudin-16 structure and function, important not only in Mg 2 and Ca 2 reabsorption but also in cell growth regulation. The latter dysfunction can lead to renal fibrosis, which is qualitatively similar but quantitatively less aggressive than those of the null mutants in cattle. Emerging evidence supports a role for the claudins in epithelial cell growth regulation. The association between the claudins and EMT resulting in renal fibrosis has already been discussed. In
3. PODOCYTE INJURY IN GLOMERULAR DISEASES
 How to Cite this article: Podocyte Injury in Glomerular Diseases - ejifcc 20/01 2009 http://www.ifcc.org 3. PODOCYTE INJURY IN GLOMERULAR DISEASES Mirjana Sabljar Matovinović Podocytes are injured in diabetic
How to Cite this article: Podocyte Injury in Glomerular Diseases - ejifcc 20/01 2009 http://www.ifcc.org 3. PODOCYTE INJURY IN GLOMERULAR DISEASES Mirjana Sabljar Matovinović Podocytes are injured in diabetic
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Podocyte Biology and clinical applications Dr. F. Ahmadi Professor Of Nephrology TUMS
 Podocyte Biology and clinical applications Dr. F. Ahmadi Professor Of Nephrology TUMS Proteinuria is a major healthcare problem that affects several hundred million people worldwide. Proteinuria is a cardinal
Podocyte Biology and clinical applications Dr. F. Ahmadi Professor Of Nephrology TUMS Proteinuria is a major healthcare problem that affects several hundred million people worldwide. Proteinuria is a cardinal
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
CELL BIOLOGY - CLUTCH CH CELL JUNCTIONS AND TISSUES.
 !! www.clutchprep.com CONCEPT: CELL-CELL ADHESION Cells must be able to bind and interact with nearby cells in order to have functional and strong tissues Cells can in two main ways - Homophilic interactions
!! www.clutchprep.com CONCEPT: CELL-CELL ADHESION Cells must be able to bind and interact with nearby cells in order to have functional and strong tissues Cells can in two main ways - Homophilic interactions
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION. Androgen deprivation therapy is the most used treatment of de novo or recurrent
 CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
Intercellular indirect communication
 Intercellular indirect communication transmission of chemical signals: sending cell signal transmitting tissue hormone medium receiving cell hormone intercellular fluid blood neurocrine neurotransmitter
Intercellular indirect communication transmission of chemical signals: sending cell signal transmitting tissue hormone medium receiving cell hormone intercellular fluid blood neurocrine neurotransmitter
Journal Club. 03/04/2012 Lama Nazzal
 Journal Club 03/04/2012 Lama Nazzal NOTCH and the kidneys Is an evolutionarily conserved cell cell communication mechanism. Is a regulator of cell specification, differentiation, and tissue patterning.
Journal Club 03/04/2012 Lama Nazzal NOTCH and the kidneys Is an evolutionarily conserved cell cell communication mechanism. Is a regulator of cell specification, differentiation, and tissue patterning.
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo
 Integration of Cells into Tissues Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo Hospital Lahore 1. How
Integration of Cells into Tissues Dr Mahmood S Choudhery, PhD, Postdoc (USA) Assistant Professor Tissue Engineering and Regenerative Medicine King Edward Medical University/Mayo Hospital Lahore 1. How
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG)
 In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
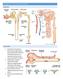 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Renal Physiology - Lectures
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Sodium and chlorine transport
 Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Tissues. Definition. A group of similar cells and their intercellular substances specialized to perform a specific function.
 Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Cell Polarity and Cancer
 Cell Polarity and Cancer Pr Jean-Paul Borg Email: jean-paul.borg@inserm.fr Features of malignant cells Steps in Malignant Progression Cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways
Cell Polarity and Cancer Pr Jean-Paul Borg Email: jean-paul.borg@inserm.fr Features of malignant cells Steps in Malignant Progression Cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Functions of the kidney:
 Diseases of renal system : Normal anatomy of renal system : Each human adult kidney weighs about 150 gm, the ureter enters the kidney at the hilum, it dilates into a funnel-shaped cavity, the pelvis, from
Diseases of renal system : Normal anatomy of renal system : Each human adult kidney weighs about 150 gm, the ureter enters the kidney at the hilum, it dilates into a funnel-shaped cavity, the pelvis, from
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Research Introduction
 Research Introduction 9.17.13 Altered metabolism in polycystic kidney disease Telomerase activity in polycystic kidney disease cells Autosomal dominant polycystic kidney disease ADPKD is the most common
Research Introduction 9.17.13 Altered metabolism in polycystic kidney disease Telomerase activity in polycystic kidney disease cells Autosomal dominant polycystic kidney disease ADPKD is the most common
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Tissues. Definition. A group of similar cells and their intercellular substances specialized to perform a specific function.
 Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Chapter 4 - Tissues Tissues Definition A group of similar cells and their intercellular substances specialized to perform a specific function. Tissues Epithelial covers exposed surfaces, lines internal
Overview of glomerular diseases
 Overview of glomerular diseases *Endothelial cells are fenestrated each fenestra: 70-100nm in diameter Contractile, capable of proliferation, makes ECM & releases mediators *Glomerular basement membrane
Overview of glomerular diseases *Endothelial cells are fenestrated each fenestra: 70-100nm in diameter Contractile, capable of proliferation, makes ECM & releases mediators *Glomerular basement membrane
Tumor microenvironment Interactions and Lung Cancer Invasiveness. Pulmonary Grand Rounds Philippe Montgrain, M.D.
 Tumor microenvironment Interactions and Lung Cancer Invasiveness Pulmonary Grand Rounds Philippe Montgrain, M.D. February 26, 2009 Objectives Review epithelial mesenchymal transition (EMT), and its implications
Tumor microenvironment Interactions and Lung Cancer Invasiveness Pulmonary Grand Rounds Philippe Montgrain, M.D. February 26, 2009 Objectives Review epithelial mesenchymal transition (EMT), and its implications
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
2. Epithelial Tissues Dr. Manal Othman
 Biology-232 GENERAL HISTOLOGY 2. Epithelial Tissues Dr. Manal Othman Anatomy Department CMMS, AGU HISTOLOGY: w Study of the structure and function of tissues and organs at the microscopic levels. w Tissues
Biology-232 GENERAL HISTOLOGY 2. Epithelial Tissues Dr. Manal Othman Anatomy Department CMMS, AGU HISTOLOGY: w Study of the structure and function of tissues and organs at the microscopic levels. w Tissues
يراهظلا( يئلاطلا جيسنلا
 Epithelium النسيج الطالئي )الظهاري( Features of Epithelium Epithelium occurs in the body as a sheet of cells that covers a body surface, lines a cavity, or forms a gland. Coverings, linings, glands. Derived
Epithelium النسيج الطالئي )الظهاري( Features of Epithelium Epithelium occurs in the body as a sheet of cells that covers a body surface, lines a cavity, or forms a gland. Coverings, linings, glands. Derived
Tissues. tissue = many cells w/ same structure and function. cell shape aids its function tissue shape aids its function
 Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Zool 3200: Cell Biology Exam 4 Part I 2/3/15
 Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name: Key Trask Zool 3200: Cell Biology Exam 4 Part I 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Distal renal tubular acidosis: genetic and clinical spectrum
 Distal renal tubular acidosis: genetic and clinical spectrum Sabrina Giglio Medical Genetics Unit, Meyer Children s University Hospital, University of Florence sabrina.giglio@meyer.it sabrinarita.giglio@unifi.it
Distal renal tubular acidosis: genetic and clinical spectrum Sabrina Giglio Medical Genetics Unit, Meyer Children s University Hospital, University of Florence sabrina.giglio@meyer.it sabrinarita.giglio@unifi.it
Epithelial Tissue. Functions include: 1. Protection 4. Absorption 2. Secretion 5. Filtration 3. Sensory reception
 Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Focal Segmental Glomerulosclerosis and the Nephro6c Syndrome Dr. A. Gangji Dr. P. Marge>s
 Focal Segmental Glomerulosclerosis and the Nephro6c Syndrome Dr. A. Gangji Dr. P. Marge>s The basic facts about proteinuria and FSGS A primer on proteinuria Endothelium and glycocalyx Podocytes Pathology
Focal Segmental Glomerulosclerosis and the Nephro6c Syndrome Dr. A. Gangji Dr. P. Marge>s The basic facts about proteinuria and FSGS A primer on proteinuria Endothelium and glycocalyx Podocytes Pathology
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19](/thumbs/77/74887026.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
The Golgi Apparatus: Shipping and Receiving Center. The Golgi apparatus. Functions of the Golgi apparatus. Lysosomes: Digestive Compartments
 The Golgi Apparatus: Shipping and Receiving Center The Golgi apparatus Receives (on the cis-side) many of the transport vesicles produced in the rough ER Consists of flattened membranous sacs called cisternae
The Golgi Apparatus: Shipping and Receiving Center The Golgi apparatus Receives (on the cis-side) many of the transport vesicles produced in the rough ER Consists of flattened membranous sacs called cisternae
Advanced Concept of Nursing- II UNIT-VI Advance Nursing Management of Genitourinary (GU) Diseases.
 In The Name of God (A PROJECT OF NEW LIFE COLLEGE OF NURSING KARACHI) Advanced Concept of Nursing- II UNIT-VI Advance Nursing Management of Genitourinary (GU) Diseases. Shahzad Bashir RN, BScN, DCHN,MScN
In The Name of God (A PROJECT OF NEW LIFE COLLEGE OF NURSING KARACHI) Advanced Concept of Nursing- II UNIT-VI Advance Nursing Management of Genitourinary (GU) Diseases. Shahzad Bashir RN, BScN, DCHN,MScN
Diuretics having the quality of exciting excessive excretion of urine. OED. Inhibitors of Sodium Reabsorption Saluretics not Aquaretics
 Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
Histology / First stage The Urinary System: Introduction. Kidneys
 The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
Cellular pathophysiology of cystic kidney disease: insight into future therapies
 Int. J. Dev. Biol. 43: 457-461 (1999) Cellular pathophysiology of cystic kidney desease 457 Cellular pathophysiology of cystic kidney disease: insight into future therapies ELLIS D. AVNER*, RICHARD P.
Int. J. Dev. Biol. 43: 457-461 (1999) Cellular pathophysiology of cystic kidney desease 457 Cellular pathophysiology of cystic kidney disease: insight into future therapies ELLIS D. AVNER*, RICHARD P.
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Lecture 3: Cells and Tissues. Bio 219 Dr. Adam Ross
 Lecture 3: Cells and Tissues Bio 219 Dr. Adam Ross Cell Physiology Cell Physiology Brief review of organelles Should be mostly review Cell surrounded by plasma membrane Lipid bilayer Also surrounds organelles
Lecture 3: Cells and Tissues Bio 219 Dr. Adam Ross Cell Physiology Cell Physiology Brief review of organelles Should be mostly review Cell surrounded by plasma membrane Lipid bilayer Also surrounds organelles
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Faculty version with model answers
 Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Ischemia remains the major cause of acute renal failure (ARF) in the
 Pathophysiology of Ischemic Acute Renal Failure: Cytoskeletal Aspects Bruce A. Molitoris Robert Bacallao Ischemia remains the major cause of acute renal failure (ARF) in the adult population [1]. Clinically
Pathophysiology of Ischemic Acute Renal Failure: Cytoskeletal Aspects Bruce A. Molitoris Robert Bacallao Ischemia remains the major cause of acute renal failure (ARF) in the adult population [1]. Clinically
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Vitreous! Retinal pigment epithelium! and the visual cycle! Retinal degenerations and pigment epithelium!
 Vitreous Bruch s membrane Retinal pigment epithelium and the visual cycle Retinal degenerations and pigment epithelium Basic Science course 2017 Swiss Eye Week, Neuchâtel Ch. E. Remé, Zürich Ch.E. Remé
Vitreous Bruch s membrane Retinal pigment epithelium and the visual cycle Retinal degenerations and pigment epithelium Basic Science course 2017 Swiss Eye Week, Neuchâtel Ch. E. Remé, Zürich Ch.E. Remé
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Lecture 36: Review of membrane function
 Chem*3560 Lecture 36: Review of membrane function Membrane: Lipid bilayer with embedded or associated proteins. Bilayers: 40-70% neutral phospholipid 10-20% negative phospholipid 10-30% cholesterol 10-30%
Chem*3560 Lecture 36: Review of membrane function Membrane: Lipid bilayer with embedded or associated proteins. Bilayers: 40-70% neutral phospholipid 10-20% negative phospholipid 10-30% cholesterol 10-30%
PHSI2006/2906: Integrated Physiology B
 PHSI2006/2906: Integrated Physiology B TOPIC 1: RESPIRATION 1. The Mechanics of Breathing...2 2. Work of Breathing....5 3. Pulmonary Gas Exchange.. 10 4. Transport of Oxygen...16 5. Control of Respiration...20
PHSI2006/2906: Integrated Physiology B TOPIC 1: RESPIRATION 1. The Mechanics of Breathing...2 2. Work of Breathing....5 3. Pulmonary Gas Exchange.. 10 4. Transport of Oxygen...16 5. Control of Respiration...20
Renal Regulation of Sodium and Volume. Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM
 Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
ACID-BASE BALANCE URINE BLOOD AIR
 ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
1. Disorders of glomerular filtration
 RENAL DISEASES 1. Disorders of glomerular filtration 2. Nephrotic syndrome 3. Disorders of tubular transport 4. Oliguria and polyuria 5. Nephrolithiasis 6. Disturbances of renal blood flow 7. Acute renal
RENAL DISEASES 1. Disorders of glomerular filtration 2. Nephrotic syndrome 3. Disorders of tubular transport 4. Oliguria and polyuria 5. Nephrolithiasis 6. Disturbances of renal blood flow 7. Acute renal
Cystic Renal Disease, for USMLE Step One. Howard J. Sachs, MD
 Cystic Renal Disease, for USMLE Step One Howard J. Sachs, MD www.12daysinmarch.com The Major Players Medullary Sponge Kidney (MSK) Polycystic Kidney Disease (PKD) Autosomal Recessive: Childhood Autosomal
Cystic Renal Disease, for USMLE Step One Howard J. Sachs, MD www.12daysinmarch.com The Major Players Medullary Sponge Kidney (MSK) Polycystic Kidney Disease (PKD) Autosomal Recessive: Childhood Autosomal
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Renal physiology II. Basic renal processes. Dr Alida Koorts BMS
 Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
The Ebf1 knockout mouse and glomerular maturation
 Repository of the Max Delbrück Center for Molecular Medicine (MDC) Berlin (Germany) http://edoc.mdc-berlin.de/14009/ The Ebf1 knockout mouse and glomerular maturation Schmidt-Ott, K.M. Published in final
Repository of the Max Delbrück Center for Molecular Medicine (MDC) Berlin (Germany) http://edoc.mdc-berlin.de/14009/ The Ebf1 knockout mouse and glomerular maturation Schmidt-Ott, K.M. Published in final
Glomerular Pathology- 1 Nephrotic Syndrome. Dr. Nisreen Abu Shahin
 Glomerular Pathology- 1 Nephrotic Syndrome Dr. Nisreen Abu Shahin The Nephrotic Syndrome a clinical complex resulting from glomerular disease & includes the following: (1) massive proteinuria (3.5 gm /day
Glomerular Pathology- 1 Nephrotic Syndrome Dr. Nisreen Abu Shahin The Nephrotic Syndrome a clinical complex resulting from glomerular disease & includes the following: (1) massive proteinuria (3.5 gm /day
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Chapter 4 Cell Membrane Transport
 Chapter 4 Cell Membrane Transport Plasma Membrane Review o Functions Separate ICF / ECF Allow exchange of materials between ICF / ECF such as obtaining O2 and nutrients and getting rid of waste products
Chapter 4 Cell Membrane Transport Plasma Membrane Review o Functions Separate ICF / ECF Allow exchange of materials between ICF / ECF such as obtaining O2 and nutrients and getting rid of waste products
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
