Kidney function in early diabetes: the tubular hypothesis of glomerular filtration
|
|
|
- Angelica Wilcox
- 6 years ago
- Views:
Transcription
1 Invited Review Am J Physiol Renal Physiol 286: F8 F15, 2004; /ajprenal Kidney function in early diabetes: the tubular hypothesis of glomerular filtration Scott C. Thomson, 1 Volker Vallon, 2 and Roland C. Blantz 1 1 Division of Nephrology/Hypertension, Department of Medicine, University of California, and Veterans Affairs Medical Center, San Diego, California ; and 2 Department of Pharmacology, University of Tubingen, Tubingen, Germany Thomson, Scott C., Volker Vallon, and Roland C. Blantz. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8 F15, 2004; /ajprenal At the onset of diabetes mellitus, the glomerular filtration rate becomes supranormal. Discovery science has identified many abnormalities in the early diabetic kidney that apparently contribute to this phenotype. A serviceable understanding of the early diabetic kidney requires this information to fit together. It is the purpose of this article to present an archetype that explains multiple nuances of kidney function in early diabetes. We refer to this archetype as the tubular hypothesis of glomerular filtration. Its basic tenet is that strange effects of diabetes on glomerular filtration stem from primary effects on the proximal tubule or loop of Henle that impact glomerular filtration by feedback through the macula densa. This theory can explain diabetic hyperfiltration, a paradoxical effect of dietary salt on glomerular filtration rate in diabetes, and the renal response to dietary protein and amino acid infusion in diabetes. The discussion centers on the kidney as an integrated system of parts rather than on the specific cellular mechanisms that comprise those parts. tubuloglomerular feedback; glomerulotubular balance; juxtaglomerular apparatus DIABETES MELLITUS IS THE COMMONEST and fastest growing cause of end-stage renal disease in the developed world (32). The pecuniary impact of this disease and the cost it exacts in human suffering justify the ongoing concerted effort to understand it. INTRODUCTION TO DIABETES AND THE KIDNEY We know that diabetes affects the kidney in stages. At the very onset of diabetes, the kidney grows large and the glomerular filtration rate (GFR) becomes supranormal (14). Most recent basic and clinical research have slanted toward sclerosis and kidney failure that occur many years later. However, the original idea that the early hemodynamic phenotype provokes the subsequent demise of a diabetic kidney has not been abandoned (15). Scientific discovery has identified many abnormalities in the early diabetic kidney. Each of these may be viewed as pieces of a puzzle. A serviceable understanding of the early diabetic kidney requires those pieces to fit together. It is the purpose of this article to assemble some of those pieces into an archetype that is serviceable inasmuch as it explains multiple nuances of kidney function in early diabetes. We refer to this archetype as the tubular hypothesis of glomerular filtration. It is fashioned around a basic tenet: that strange effects of diabetes on glomerular filtration stem from primary effects on the proximal tubule or loop of Henle that impact glomerular filtration by feedback through the macula densa. This theory can explain diabetic hyperfiltration, a paradoxical effect of dietary salt on GFR in diabetes, and the renal response Address for reprint requests and other correspondence: S. Thomson, Div. of Nephrology/Hypertension, UCSD, and San Diego VA Health Care System, 3350 La Jolla Village Dr., San Diego, CA ( sthomson@ucsd.edu). to dietary protein and amino acid infusion in diabetes. This discussion will focus on control theory and phenomenology and not discuss specific cellular mechanisms except when necessary to illustrate principles. This is not intended to slight the research of those whose discoveries would normally be cited in a review article about kidney function in diabetes. A partial list of those discoveries appears in Table 1. Also, therapeutic concepts related to the tubular hypothesis are presented elsewhere (34) and not discussed here. PARADIGM FOR CONTROL OF GFR The Starling forces and capillary surface that determine GFR are directly influenced by various effectors that include nerves, hormones, size and condition of the glomerulus, and tubuloglomerular feedback (TGF) from the macula densa. Physiological effectors of GFR generally function as elements in negative feedback systems that link GFR to some other physiological parameter (Fig. 1). For example, glomerular filtration is linked to extracellular fluid volume (ECF) through multiple parallel feedback loops that respectively incorporate the renal nerves, renin-angiotensin system (RAS), and natriuretic peptides (ANP). Glomerular filtration is linked to the concentration of salt in the tubular fluid that reaches the macula densa (MD[NaCl]) by TGF. GFR and size of the kidney are also linked in a negative feedback arrangement, although how a feedback signal derives from GFR that influences kidney growth remains a mystery. If an external perturbation is applied to one element of a linear feedback loop, part of the perturbation will be compensated for by feedback around the loop, and the remainder will manifest as a residual error in that element. The efficiency of a negative feedback system is determined by its open-loop gain. F8
2 Table 1. Some primary vascular mediators of diabetic hyperfiltration Renal prostaglandins (18) Natriuretic peptide (18) Nitric oxide (18) Hyperglycemia (18) Ketones (18) Low insulin (18) Glucagon (18) Growth hormone (18) Kallikrein-kinin (5) Sorbitol (5) Dysfunctional Ca 2 channels in afferent arteriole (5) Excess K channels in afferent arteriole (10) Impaired electromechanical coupling in the afferent arteriole (4) Most primary vascular mediators are discussed in reference tests or review articles, shown in parentheses. The open-loop gain is the ratio of the compensated portion of the original disturbance to the residual error. A system that is perfectly efficient has an infinite open-loop gain. A system that buffers half a disturbance has an open-loop gain of unity. When a parameter is protected by multiple feedback loops arranged in parallel, the overall gain of the system for stabilizing that parameter is the sum of the open-loop gains of the individual loops. For the example contained in Fig. 1, the renal nerves, RAS, and ANP will additively compensate for any outside disturbances in ECF. Feedback loops that are arranged in parallel also compete for control of the system, and the leverage exerted by an individual loop is determined by its own open-loop gain relative to the competition. For example, the feedback loops that link GFR to ECF compete for control of GFR with the loop that links GFR to macula densa salt (Fig. 1). The open-loop gain referable to a disturbance in ECF is directly proportional to the impact of ECF on GFR. However, any change in GFR will alter MD[NaCl], eliciting a TGF response that affects GFR in the opposite direction. Hence, the homeostatic efficiency of the renal nerves, RAS, and ANP system for regulating ECF will decline as the TGF system becomes more efficient. Moreover, disturbing the ECF will impact the MD[NaCl] in this system, even though the two are not elements of the same feedback loop. In other words, a disturbance in one feedback loop will spill over into all the other loops. A disturbance in ECF will reset MD[NaCl] to a new value. As TGF becomes more efficient, the influence of ECF over MD[NaCl] will shrink. If GFR becomes more sensitive to ECF via the renal nerves, RAS, or ANP, then the impact of ECF on MD[NaCl] will grow larger. The foregoing example is made without reference to the dynamics of these systems or the known cross talk that occurs between the various components of this system (i.e., direct interactions between renal nerves and RAS, renin suppression by the macula densa, etc.). It also ignores important neurohumoral mechanisms that link ECF to tubular reabsorption. However, it illustrates the point that any parameter linked to GFR by negative feedback will influence all other parameters linked to GFR, even when the parameters are not linked to each other in any other way. Therefore, it is not surprising that almost anything associated with glomerular filtration can appear abnormal in diabetes. Looking for the effector that appears most abnormal may not be a good way to establish the primary cause of a change in GFR, because a large effect of diabetes on the value of a parameter may be the result of a primary disturbance or change in the open-loop gain of a parallel feedback loop that does not even contain that parameter. DEFINING VASCULAR AND TUBULAR EVENTS Invited Review Our theory states that the macula densa is the dominant influence over glomerular filtration in early diabetes. One way to test the theory with respect to diabetic hyperfiltration would be to quantify the impact of each putative mediator of glomerular filtration, including MD[NaCl], and rank them. A partial list is shown in Table 1. The list is too long for this approach to be tractable. Instead, we begin by sorting all possible effectors of glomerular filtration into one of two categories. One category contains only the tubular reabsorption- MD[NaCl]-TGF mechanism. The other category contains all other possible effectors, including effectors that remain to be discovered. Any event that alters GFR by way of affecting MD[NaCl] is referred to as a primary tubular event. All other events that alter GFR are referred to as primary vascular events. Some examples of primary vascular events include changes in any element contained in the feedback loops that link GFR to ECF in Fig. 1. These might include abnormal sensing of the ECF, abnormal transduction of information about the ECF into a neurohormonal signal, or an altered response to the signal by the renal microvessels. A primary tubular effect is anything, except GFR, that affects reabsorption upstream from the macula densa. GFR affects tubular reabsorption via glomerulotubular balance (GTB). For present purposes, GTB refers to the instantaneous effect of GFR on tubular reabsorption due to kinetic interaction of the tubular fluid with an existing transport machinery. In contrast to GTB, a primary change in tubular reabsorption results from a change in the amount of transport machinery or its functional state. A primary change in tubular reabsorption causes GFR to change through the physiological actions of TGF. A primary vascular effect on GFR causes a secondary change in tubular reabsorption through the physiological actions of GTB (see Fig. 2). Several neurohumoral systems, most notably the renal nerves and RAS, exert both primary tubular and vascular effects. Fig. 1. Effectors that influence tubuloglomerular feedback (TGF) of negative feedback loops that link glomerular filtration rate (GFR) to other physiological parameters. ECF, extracellular fluid volume; MD[NaCl], salt concentration of tubular fluid at the macula densa; Nerves, efferent sympathetic renal nerves; RAS, renin-angiotensin system; ANP, atrial natriuretic peptide hormones from the heart; solid lines, proportional effects; dashed lines, inverse effects. A negative feedback loop must contain an odd number of inverse effects. F9
3 Invited Review F10 Fig. 2. GFR is determined by primary vascular events, TGF, and TGF resetting. MD[NaCl] is determined by glomerulotubular balance (GTB) and primary tubular events. There are 2 negative feedback loops linking MD[NaCl] and GFR. One loop passes through TGF, and the other through TGF resetting. Both TGF resetting and primary vascular events will impact the TGF relationship. TGF resetting and primary vascular events differ because the former is mediated within the juxtaglomerular apparatus. DIABETIC HYPERFILTRATION AS A PRIMARY TUBULAR EVENT Because parallel feedback loops compete for control of any parameter(s) they share, GFR must be determined by a balance of forces between primary vascular and primary tubular events. This model accounts for all possible causes of a change in GFR. GFR cannot increase unless there is a dominant primary vasodilation and/or a dominant primary increase in tubular reabsorption to cause the increase. Because GFR and MD[NaCl] are connected in a feedback loop, no single disturbance can affect GFR without secondarily affecting MD[NaCl] and vice versa. However, primary vascular and tubular events that have the same effect on GFR will have contrary effects on MD[NaCl]. If GFR increases due to a primary vascular effect, then MD[NaCl] must also increase. If GFR increases due to a primary tubular effect, then MD[NaCl] must decrease. Therefore, if one knows how MD[NaCl] is affected during hyperfiltration, then one can ascribe a vascular or tubular cause to hyperfiltration. Indeed, increased proximal reabsorption has been noted in hyperfiltering patients with early type 1 (16) or type 2 (12) diabetes based on lithium clearances. These and other authors have ignored the macula densa mechanism and posited excessive proximal reabsorption as a cause of systemic volume expansion leading to hemodynamic consequences in diabetes (1). ECF expansion is an essential intermediary if diabetic hyperfiltration is to be explained by this mechanism. However, ECF expansion is not a uniform finding in diabetes, and its link to hyperfiltration is refuted by data showing immediate and progressive hyperfiltration throughout the first 2 wk of streptozotocin diabetes, despite a persistently reduced ECF (31). Surprisingly, little attention was paid to the diabetic proximal tubule during the heyday of micropuncture, although we have now accumulated a fair quantity of such data. The present concern is to distinguish primary changes in tubular reabsorption from secondary changes in reabsorption that begin as primary vascular events, then impact tubular reabsorption via GTB. When hyperfiltration is accompanied by increased reabsorption, this distinction may not be trivial. For example, fluid collected from the late proximal tubule may reveal no effect of diabetes on fractional reabsorption, giving the impression that, in a hyperfiltering diabetic nephron, net proximal reabsorption increases purely due to GTB. However, this impression hinges on the supposition that GTB confers constant fractional reabsorption. In fact, when GTB operates normally, fractional reabsorption declines as GFR increases. Therefore, when one is testing for primary differences in tubular reabsorption, adjusting for differences in GFR may not be as simple as comparing fractional reabsorption. To circumvent this problem, we artificially activated TGF as a tool in manipulating single-nephron GFR (SNGFR). Data are thus obtained for expressing proximal reabsorption as a function of SNGFR in individual nephrons. This approach unmasks a major primary increase in proximal reabsorption in rats with early streptozotocin diabetes (29) (see Fig. 3). For this primary increase in proximal reabsorption to be the dominant cause of glomerular hyperfiltration, the diabetic nephron must operate with MD[NaCl] below normal. In contrast, when a primary vascular event is the dominant cause of hyperfiltration, MD[NaCl] must be above normal. In fact, all published data on the ionic content of the early distal nephron in diabetes list values substantially below normal (20, 23, 33, 35, 37). It is clear that the diabetic kidney meets the requirement for low MD[NaCl] necessary for a primary increase in tubular reabsorption to be the dominant cause of hyperfiltration. Another requirement of the tubular hypothesis is for the TGF system to respond to the reduced MD[NaCl] by increasing SNGFR. Indeed, the range of the TGF response is preserved in diabetes, although the slope of the response is reduced (29, 33). Also, if hyperfiltration is mediated by a reduced TGF signal, then there should be evidence that TGF is less engaged by the ambient MD[NaCl] in diabetes. The usual way to test for TGF activation is via the proximal-distal (P-D) difference in SNGFR. A greater P-D difference corresponds to greater TGF activation. Technical pitfalls result in a high coefficient of variation in measuring the P-D difference. Nonetheless, we have shown the P-D difference to be small in diabetes (23). In other words, there is upward deviation in the ambient SNGFR along the TGF curve in diabetes, suggesting that the diabetic Fig. 3. Proximal reabsorption represented by transtubular concentration differences (TF/P) for inulin sampled from the late proximal tubule. Singlenephron GFR (SNGFR) was made an independent variable, using microperfusion of Henle s loop to activate TGF. Higher SNGFR values were obtained during 0 loop perfusion. Lower SNGFR values were obtained while perfusion to maximum TGF activation. Controlling for SNGFR, TF/P is greater in diabetes, confirming a primary increase in proximal reabsorption that was not evident during 0 loop perfusion. In the absence of TGF, SNGFR is greater in diabetes, indicating an upward resetting of the TGF curve. Blocking ornithine decarboxylase with difluoromethyl ornithine (DFMO) eliminated the primary increase in proximal reabsorption and TGF resetting by diabetes. DM, diabetes mellitis; Con, control. Adapted from Ref. 29. *P 0.05.
4 nephron has converted low MD[NaCl] into hyperfiltration. A hypothetical illustration is provided in Fig. 4. However, the entire increase in SNGFR cannot be explained by a smaller P-D difference, because SNGFR collected from the proximal tubule also invariably increases in diabetes. As TGF is inoperative during proximal tubular fluid collections, this implies that the TGF curve itself must also shift upward in diabetes. It is a challenge for the tubular hypothesis to account for this upward resetting of the TGF curve. As conventionally defined, TGF confers a specific dependence of SNGFR on MD[NaCl] from one minute to the next. Changes in SNGFR that are due to TGF are represented by moving from one point to another along a given TGF curve. However, the curve itself may be influenced by events outside of the juxtaglomerular apparatus (JGA). In fact, any primary vascular effect on GFR can be represented by a change in the TGF curve (see Fig. 4). Therefore, the upward shift in the TGF curve in diabetes may represent a primary vascular event mediated by any of the factors listed in Table 1. When TGF is reset upward by external events such as plasma volume expansion, the operating point migrates downward along to the TGF curve and TGF becomes more activated (27). The same would be expected for any vascular cause of upward resetting. This is contrary to what occurs in diabetes, where the state of TGF activation lessens despite an upward shift in the TGF curve. Therefore, one might conclude that the tubule is the dominant controller of SNGFR with a primary vascular effect as close runner-up and that, under slightly different starting conditions, the roles could be reversed. The tubular hypothesis of glomerular filtration would be much more compelling if TGF resetting were also shown to arise from the tubule. TGF RESETTING: TUBULAR OR VASCULAR EVENT? We have defined primary tubular events as being mediated by TGF and have pointed out that any primary vascular effect on GFR can be represented as a resetting of TGF. However, Fig. 4. A: primary increase in MD[NaCl] followed by rightward resetting of TGF. Resetting is mediated through the macula densa and occurs during 1 h when a sustained stimulus is applied to TGF. Adapted from Refs. 28 and 30. B: primary decrease in MD[NaCl] followed by upward resetting of TGF. MD[NaCl] is stimulated to increase by withdrawing a proximal tubular diuretic, and resetting occurs during the next several hours. Adapted from Ref. 26. We hypothesize that this is also the mechanism for TGF resetting in early diabetes. Solid curves, prior to resetting; dashed curves, after resetting. this does not imply that all events that cause TGF to reset are mediated by nerves or circulating hormones, or begin with changes in the renal vessels. An event that occurs in the JGA and causes GFR to change, but is not TGF, fulfills the criteria for a primary vascular event (see Fig. 2). In fact, the JGA does mediate TGF resetting in response to sustained increments in MD[NaCl]. Such resetting normally maintains the operating point of a nephron along the steep portion of the TGF curve (28, 30). A TGF response occurs within seconds whenever there is a change in MD[NaCl]. In contrast, resetting of TGF by MD[NaCl] begins after min. It is likely that those events involved with the initial resetting of TGF are different from those that maintain it hours or days later. Most experimentation in this area has been done using microperfusion or a proximal tubular diuretic to impose a sustained increase in MD[NaCl] for up to 2 h. Increasing MD[NaCl] by these maneuvers causes rightward resetting of TGF over min (28, 30). In diabetes, however, the stimulus for resetting must come from a decrease in MD[NaCl] applied for hours or days and must yield an upward resetting of TGF. Data are published in which TGF resetting was tested in response to several hours of increased proximal reabsorption. This was done by first giving a carbonic anhydrase inhibitor to suppress proximal reabsorption for 24 h, then withdrawing the drug to induce the increase in proximal reabsorption relative to the baseline that was established during carbonic anhydrase inhibition. Micropuncture data obtained 8 10 h after withdrawal of the carbonic anhydrase inhibitor revealed hyperfiltration and a clear upward resetting of the TGF curve, notwithstanding that the animals had been in negative fluid balance for the prior 24 h (26). Therefore, resetting of TGF by the JGA can overcome an opposing influence of some magnitude from the ECF and is capable of causing the type of upward TGF resetting seen in early diabetes (see Fig. 4). The foregoing example demonstrates that a macula densa mechanism for upward TGF resetting is a plausible explanation for what occurs in diabetes, but doesn t prove it. It would be more convincing to demonstrate that one could prevent TGF resetting by specifically preventing hyperreabsorption, and it would be best to prevent hyperreabsorption by blocking the mechanism that leads to hyperreabsorption in the first place. In theory, proximal reabsorption might be increased in diabetes due to nerves or hormones that impinge directly on the proximal tubule, as a result of increased sodium-glucose cotransport, or because the tubule grows larger overall. It is difficult to use drugs that target the neurohumoral effectors of tubular reabsorption to alter the macula densa control of GFR because these same drugs will have confounding direct effects on the glomerulus. Also, there is no convincing evidence that diabetic hyperreabsorption is directly mediated by overactivity of one hormone or neurotransmitter in the tubule. Acutely blocking sodium-glucose cotransport with phlorizin does reduce proximal reabsorption, increase MD[NaCl], and eliminate hyperfiltration (37). However, this is akin to activating TGF with a proximal tubular diuretic after TGF is already reset and doesn t address the role of the tubule in long-term resetting of the TGF curve. GROWTH IS A TUBULAR EVENT Invited Review F11 All else being equal, the diabetic proximal tubule should reabsorb more because it is larger. The proximal tubule accounts for most of the increased kidney mass in early diabetes,
5 Invited Review F12 and growth of the early diabetic kidney can be attenuated by blocking ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis (19). ODC is not expressed in the diabetic glomerulus. Therefore, we blocked ODC as a means of examining the primary role of the tubule in hyperfiltration and TGF resetting. Difluoromethyl ornithine (DFMO), the ODC antagonist given during the first 7 days of streptozotocin diabetes, attenuated kidney growth and hyperfiltration in exact proportion. In fact, across groups that included nondiabetic and diabetic animals with and without DFMO, kidney weight predicted 95% of the difference in GFR. Furthermore, DFMO completely prevented both the primary increase in proximal reabsorption and the upward resetting of TGF in diabetes, as assessed by micropuncture. DFMO had no effect on any of these parameters in control animals (29). These findings imply the following sequence of events: diabetes causes the proximal tubule to grow. As the tubule grows, it reabsorbs more, thus causing MD[NaCl] to decline incrementally. As MD[NaCl] declines, GFR increases through the TGF mechanism, while TGF resets upward through a slower macula densa mechanism. The present discussion is devoted to the consequences of kidney growth rather than its cause. For this purpose, DFMO was a useful tool. We have since identified the distal nephron as the main site of increased ODC expression in early diabetes and confirmed that blocking this pool of ODC prevents hyperplasia of the proximal tubule. These preliminary findings warrant speculation that the distal nephron could exert a paracrine influence on growth of the proximal tubule (6). DOES INVOKING THE MACULA DENSA EXCLUDE A PRIMARY VASCULAR EFFECT? Several defects in the afferent arteriole have been identified in diabetes that are expected to cause vasodilation. Some of these are listed in Table 1. Why doesn t a residual primary vasodilatory effect of diabetes become apparent when the primary tubular effect is eliminated? It is plausible that the reduced efficiency of electromechanical coupling in the afferent arteriole as reported in diabetes (4) could be part of the mechanism for upward TGF resetting. This hypothesis has not been tested. Another potential explanation is that primary defects in vasomotion are neutralized by strong vasoconstrictor influences that arise via feedback from a deranged systemic milieu in experimental diabetes. If those systemic influences were removed, then the diabetic kidney might hyperfilter independently of the tubular mechanism. One logical way to remove confounding systemic vasoconstrictor influences might be to expand the ECF by feeding a high-salt diet. However, we find that dietary salt has just the opposite effect on hemodynamics in the diabetic kidney. circumstances. For example, hypertensive African-Americans manifest this behavior (17). In contrast, it would be counterintuitive for GFR to vary inversely with dietary salt. Nonetheless, this salt paradox occurs in diabetes. The salt paradox was first noted in the form of renal vasodilation and augmented hyperfiltration among rats placed on a low-salt diet for 7 days after several weeks of streptozotocin diabetes (38). The paradox was subsequently confirmed using a high-salt diet in rats with early or established diabetes (36). Renal blood flow and GFR have also been shown to vary inversely with dietary salt in hyperfiltering diabetic patients (13). For good measure, the effect of dietary salt on the renin-angiotensin-aldosterone axis was essentially intact in these subjects. The salt paradox cannot be explained by any primary vascular mechanism but can be explained in light of the tubular hypothesis, as outlined in Fig. 5. Changes in salt intake are sensed as a disturbance in ECF. In turn, ECF impacts various nerves and hormones that exert primary effects on the glomerulus and tubule. The efficiency of these nerves and hormones can be altered by disease, but their intrinsic character cannot. For example, diabetes may reduce the amount of renal nerve traffic or renin secretion that results from a given decrease in ECF, but diabetes cannot convert norepinephrine or angiotensin II into renal vasodilators. It is also important to note that the primary vascular and tubular limbs of the response to ECF are arranged in parallel. Therefore, it is possible for a disease or condition to simultaneously strengthen the primary tubular response and weaken the primary vascular response that arises from a given disturbance in ECF. An upward perturbation in ECF will act through the baroreceptors to elicit a primary vasodilation and a primary decrease in tubular reabsorption. If the entire primary decrease in tubular reabsorption occurs downstream from the macula densa, then there will be an upward deviation in MD[NaCl] that results purely from the primary vascular effect, filtered by GTB. In turn, TGF will partially buffer the primary vascular THE SALT PARADOX Any long-term change in salt intake must be matched precisely by a change in salt excretion. For salt excretion to change, there must be a change in GFR and/or tubular reabsorption. Because the normal kidney can adjust salt excretion to accommodate a wide range of dietary intake while GFR remains relatively constant, the tubule must mediate salt balance in most cases. However, it is not surprising that the kidney can respond to a high-salt diet by increasing GFR under some Fig. 5. Control system model for linking GFR to dietary salt by parallel pathways that include primary vascular or primary tubular effects. If the primary tubular effects dominate, GFR will vary inversely with dietary salt. TB salt, total body salt; solid lines, proportional effects; dashed lines, inverse effects. A negative feedback loop must contain an odd number of inverse effects.
6 Invited Review F13 GFR by reducing MD[NaCl] is clearly less than the capacity to reduce GFR through the systemic effects of salt depletion, which, in the extreme case, will result in zero GFR. This likely explains an angiotensin-mediated doubling of renal vascular resistance reported for non-insulin-treated diabetic rats that achieved zero salt excretion on a low-salt diet despite what should have been an ongoing osmotic diuresis (2). It is not yet known what causes the diabetic proximal tubule to exhibit salt sensitivity. Thus far, we have been unable to implicate angiotensin (Munger KA, unpublished observations) or renal nerves (3) in the salt paradox. Fig. 6. Primary tubular and vascular effects of glycine (Gly) infusion or dietary protein in normal and diabetic rats. Primary vascular events are signified by resetting of the sigmoidal TGF curves. This can be mediated by systemic nerves and hormones or by the macula densa mechanism of TGF resetting. The straight solid lines represent GTB between the glomerulus and macula densa. A primary tubular event occurs when the operating point shifts to a point not on the original line. In some comparisons, the slope of the second GTB line is not known. However, it is apparent that the 2 operating points cannot lie on the same line. The arrows designate vectors from one operating point to the other. A vector that is clockwise rotated relative to the original GTB slope requires a primary decrease in reabsorption, and a vector that is counterclockwise rotated relative to the original GTB line requires a primary increase in reabsorption. LP, low protein. Adapted from Ref 23. effect but must leave a residual positive deviation in both MD[NaCl] and GFR. As long as the link between ECF and GFR is dominated by primary vascular effects, there can never be a salt paradox. However, if expanding the ECF induces a sufficient primary decrease in tubular reabsorption upstream from the macula densa, the subsequent increase in MD[NaCl] can activate TGF to overwhelm the primary vasodilation, causing GFR to decline. Thus we can explain the salt paradox if, and only if, the proximal tubule holds sway among the mechanisms that link glomerular filtration to ECF. This theory has been tested by micropuncture and confirmed in rats with established diabetes. First, the salt paradox was confirmed to apply to SNGFR. Then, dietary salt was shown to exert a strong primary negative impact on proximal reabsorption and a strong positive impact on MD[NaCl] in diabetes. Finally, high salt caused the entire TGF curve to reset downward (reduced SNGFR), but not enough to offset the entire decrease in reabsorption. Therefore, the operating point shifted down along the TGF curve as one would expect for resetting mediated in the JGA (35). We have observed the salt paradox in diabetic rats of different strains and both sexes in Germany and in the United States. The phenomenon has also been observed in diabetic patients (13). However, from the model (Fig. 5) one can envision circumstances under which the paradox would not be observed. The paradox will occur if, and only if, the primary tubular effect of ECF on GFR outweighs its primary vascular effect. Given the limited range of TGF, the capacity to increase THE TUBULAR HYPOTHESIS, DIETARY PROTEIN, AND VASODILATORY AMINO ACIDS GFR normally increases during glycine infusion. This phenomenon has been termed renal functional reserve. Renal functional reserve is absent in diverse pathophysiological conditions that are united by an apparent tendency for glycine to reduce proximal reabsorption and activate TGF (reviewed in Ref. 9). Protein feeding is another maneuver that normally causes GFR to increase. Protein feeding also increases tubular reabsorption to such an extent that MD[NaCl] declines despite increased GFR (21, 22). Therefore, primary tubular effects contribute to the increase in GFR during glycine infusion and protein feeding. As additional background to the forthcoming argument, the glycine response is absent in some rats and humans with diabetes (8, 24, 25), and a low-protein diet is proposed to normalize glomerular hemodynamics and slow the progression of kidney failure in diabetes (11, 39). The effects of dietary protein and glycine infusion vis-à-vis the tubular hypothesis of glomerular filtration were recently examined in rats after 1 mo of diabetes (14). Animals were studied before and during glycine infusion. Some were fed a low-protein diet for 1 wk beforehand. TGF activity was inferred from the difference between ambient SNGFR and the midpoint of the TGF curve. Early distal ionic content was used as a surrogate for MD[NaCl]. Data were combined with prior Fig. 7. Model containing the minimum required features to explain the data shown in Fig. 6. In normal rats, glycine exerts primary vascular and primary tubular effects that both favor increased GFR. In diabetes, the vascular influence is attenuated and the tubular effect is reversed. Because glycine stimulates proximal reabsorption in normal rats and inhibits proximal reabsorption in diabetes, it is likely that glycine affects reabsorption by more than 1 pathway with opposing influences rather than a single pathway with variable influence. Solid lines, proportional effects; dashed lines, inverse effects. A negative feedback loop must contain an odd number of inverse effects.
7 Invited Review F14 information regarding TGF and GTB slopes to mathematically determine the extent to which the effects of glycine and dietary protein on GFR were due to primary vascular or primary tubular events. A low-protein diet was found to normalize MD[NaCl] in diabetes through a primary decrease in tubular reabsorption similar to that predicted for normal rats based on prior work by Seney and Wright (22). SNGFR declined in response, although not to normal. Because the kidney did not shrink to a normal size during 1 wk of a low-protein diet, it is not surprising that normalizing MD[NaCl] failed to completely normalize SNGFR. During glycine infusion in nondiabetic rats, SNGFR increased while MD[NaCl] remained constant. Lone primary vascular or tubular increases in SNGFR of this magnitude would have caused a substantial change in MD[NaCl]. Because none was observed, the glycine response in normal rats must involve both primary vasodilation and a primary increase in tubular reabsorption. Eliminating either effect would reduce the glycine response by about half. In diabetes, the primary vascular effect of glycine was minimal, and glycine caused a primary decrease in proximal reabsorption. Prior restriction of dietary protein normalized the primary vascular effect of glycine, but the primary decrease in tubular reabsorption persisted. The renal functional reserve was partially restored. Although the renal functional reserve and the response to dietary protein have been known and studied for decades, little is known of the basic mechanisms. However, it is evident that both vascular and tubular mechanisms must be invoked to describe them. A graphical representation of the above findings and a model to explain them are presented in Figs. 6 and 7. Recent work prompts speculation that NMDA-glycine receptors in the kidney could mediate the glycine response (7) and that dietary protein modulates the expression of these receptors (Munger KA, unpublished observations), although work has not been completed to fully test this hypothesis. SUMMARY The factors that influence glomerular filtration may do so by impinging directly on the vasculature or directly on the tubule upstream from the macula densa. Primary tubular effects include TGF and can lead to TGF resetting. Decreased MD[NaCl] is the sine qua non of an increase in GFR mediated through TGF. Upward TGF resetting by the macula densa can be difficult to distinguish from vascular resetting but should yield a smaller P-D difference and lesser MD[NaCl]. In the normal kidney, primary tubular events account for the response to dietary protein and half of the response to glycine infusion, but they are not involved with salt balance. This implies that salt balance is normally mediated downstream from the macula densa. In early diabetes, kidney growth causes a primary increase in proximal reabsorption that is sufficient to classify diabetic hyperfiltration as a primary tubular event. In diabetes, the proximal tubule also assumes a major role in salt balance, leading to a paradoxical effect of dietary salt on GFR. The diabetic tubule retains the ability to increase reabsorption in response to long-term dietary protein, but not in response to acute glycine infusion. The tubular hypothesis of glomerular filtration explains at least three kidney function phenotypes in early diabetes. GRANTS This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-56248, the U.S. Department of Veterans Affairs Research Service, and by the Deutsche Forschungsgemeinschaft (Va 118/3 2). REFERENCES 1. Bank N and Aynedjian HS. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Invest 86: , Bank N, Lahorra G, Aynedjian HS, and Wilkes BM. Sodium restriction corrects hyperfiltration of diabetes. Am J Physiol Renal Fluid Electrolyte Physiol 254: F668 F676, Birk C, Richter K, Huang DY, Piesch C, Luippold G, and Vallon V. The salt paradox of the early diabetic kidney is independent of renal innervation. Kidney Blood Press Res. In press. 4. Carmines PK and Fujiwara K. Altered electromechanical coupling in the renal microvasculature during the early stage of diabetes mellitus. Clin Exp Pharmacol Physiol 29: , Carmines PK, Ohishi K, and Ikenaga H. Functional impairment of renal afferent arteriolar voltage-gated calcium channels in rats with diabetes mellitus. J Clin Invest 98: , Deng A, Munger KA, Valdivielso JM, Satriano J, Lortie M, Blantz RC, and Thomson SC. Increased expression of ornithine decarboxylase in distal tubules of early diabetic rat kidneys: are polyamines paracrine hypertrophic factors? Diabetes 52: , Deng A, Valdivielso JM, Munger KA, Blantz RC, and Thomson SC. Vasodilatory-methyl-D-aspartate receptors are constitutively expressed in the rat kidney. J Am Soc Nephrol 13: , DeNicola L, Blantz RC, and Gabbai FB. Renal functional reserve in the early stage of experimental diabetes. Diabetes 41: , Gabbai FB, DeNicola L, Garcia GE, and Blantz RC. Role of angiotensin in the regulation of renal response to proteins. Semin Nephrol 15: , Ikenaga H, Bast JP, Fallet RW, and Carmines PK. Exaggerated impact of ATP-sensitive K channels on afferent arteriolar diameter in diabetes mellitus. J Am Soc Nephrol 11: , Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, and Klahr S. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. JAmSoc Nephrol 10: , Mbanya JC, Thomas TH, Taylor R, Alberti KG, and Wilkinson R. Increased proximal tubular sodium reabsorption in hypertensive patients with type 2 diabetes. Diabet Med 6: , Miller JA. Renal responses to sodium restriction in patients with early diabetes mellitus. J Am Soc Nephrol 8: , Mogensen CE. Glomerular filtration rate and renal plasma flow in shortterm and long-term juvenile diabetes mellitus. Scand J Clin Lab Invest 28: , O Bryan GT and Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 17: , O Hagan M, Howey J, and Greene SA. Increased proximal tubular reabsorption of sodium in childhood diabetes mellitus. Diabet Med 8: 44 48, Parmer RJ, Stone RA, and Cervenka JH. Renal hemodynamics in essential hypertension. Racial differences in response to changes in dietary sodium. Hypertension 24: , Parving HH, Osterby R, and Ritz E. Diabetic nephropathy. In: The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 2000, p Pedersen SB, Flyvbjerg A, and Richelsen B. Inhibition of renal ornithine decarboxylase activity prevents kidney hypertrophy in experimental diabetes. Am J Physiol Cell Physiol 264: C453 C456, Pollock CA, Lawrence JR, and Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 260: F946 F952, Seney FS, Persson EG, and Wright FD Jr. Modification of tubuloglomerular feedback signal by dietary protein. Am J Physiol Renal Fluid Electrolyte Physiol 252: F83 F90, Seney FS and Wright FD Jr. Dietary protein suppresses feedback control of glomerular filtration in rats. J Clin Invest 75: , 1985.
8 Invited Review F Slomowitz LA, Deng A, Hammes J, Gabbai FB, and Thomson SC. Glomerulotubular balance, dietary protein, and the renal response to glycine in diabetic rats. Am J Physiol Regul Integr Comp Physiol 282: R1096 R1103, Slomowitz LA, Hirschberg R, and Kopple JD. Captopril augments the renal response to an amino acid infusion in diabetic adults. Am J Physiol Renal Fluid Electrolyte Physiol 255: F755 F762, Slomowitz LA, Peterson OW, and Thomson SC. Converting enzyme inhibition and the glomerular hemodynamic response to glycine in diabetic rats. J Am Soc Nephrol 10: , Thomson SC, Bachmann S, Bostanjoglo M, Ecelbarger CA, Peterson OW, Schwartz D, Bao D, and Blantz RC. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J Clin Invest 104: , Thomson SC and Blantz RC. Homeostatic efficiency of tubuloglomerular feedback in hydropenia, euvolemia, an acute volume expansion. Am J Physiol Renal Fluid Electrolyte Physiol 264: F930 F936, Thomson SC, Blantz RC, and Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F461 F468, Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, and Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: , Thomson SC, Vallon V, and Blantz RC. Acute reduction in proximal reabsorption induces resetting of tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 273: R1414 R1420, Tucker BJ, Collins R, Ziegler MG, and Blantz RC. Dissociation between glomerular hyperfiltration and extracellular volume in diabetic rats. Kidney Int 39: , US Renal Data System. USRDS 2002 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Vallon V, Blantz RC, and Thomson S. Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol Renal Fluid Electrolyte Physiol 269: F876 F883, Vallon V, Blantz RC, and Thomson SC. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol 14: , Vallon V, Huang DY, Deng A, Richter K, Blantz RC, and Thomson SC. Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol 13: , Vallon V, Kirschenmann D, Wead LM, Lortie MJ, Satriano J, Blantz RC, and Thomson SC. Effect of chronic salt loading on kidney function in early and established diabetes mellitus in rats. J Lab Clin Med 130: 76 82, Vallon V, Richter K, Blantz RC, Thomson S, and Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: , Vallon V, Wead LM, and Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol 5: , Zatz R, Meyer TW, Rennke H, and Brenner BM. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc Natl Acad Sci USA 82: , Downloaded from by on September 30, 2017
The functional unit of the kidney, the single nephron, shows
 Tubuloglomerular Feedback and the Control of Glomerular Filtration Rate Volker Vallon Institute of Pharmacology and Toxicology, University of Tübingen, D-72074 Tübingen, Germany In every nephron the glomerular
Tubuloglomerular Feedback and the Control of Glomerular Filtration Rate Volker Vallon Institute of Pharmacology and Toxicology, University of Tübingen, D-72074 Tübingen, Germany In every nephron the glomerular
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
BIOGRAPHICAL SKETCH. DEGREE (if applicable) B.S. B.S. M.D.
 BIOGRAPHICAL SKETCH Provide the following information for the key personnel in the order listed on Form Page 2. Photocopy this page or follow this format for each person. NAME Thomson, Scott C M.D. POSITION
BIOGRAPHICAL SKETCH Provide the following information for the key personnel in the order listed on Form Page 2. Photocopy this page or follow this format for each person. NAME Thomson, Scott C M.D. POSITION
Glomerular Hyperfiltration and the Salt Paradox in Early Type 1 Diabetes Mellitus: A Tubulo-Centric View
 REVIEW J Am Soc Nephrol 14: 530 537, 2003 Glomerular Hyperfiltration and the Salt Paradox in Early Type 1 Diabetes Mellitus: A Tubulo-Centric View VOLKER VALLON,* ROLAND C. BLANTZ, AND SCOTT THOMSON *Department
REVIEW J Am Soc Nephrol 14: 530 537, 2003 Glomerular Hyperfiltration and the Salt Paradox in Early Type 1 Diabetes Mellitus: A Tubulo-Centric View VOLKER VALLON,* ROLAND C. BLANTZ, AND SCOTT THOMSON *Department
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes
 Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes Scott C. Thomson, 1 Aihua Deng, 1 Dingjiu Bao, 1 Joseph Satriano, 1 Roland C. Blantz,
Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes Scott C. Thomson, 1 Aihua Deng, 1 Dingjiu Bao, 1 Joseph Satriano, 1 Roland C. Blantz,
Renal Regulation of Sodium and Volume. Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM
 Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
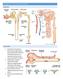 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
COMPLETE INHIBITION OF RENAL RESERVE IN CHRONIC RENAL FAILURE BY A COMBINATION OF ACEI AND ARB
 COMPLETE INHIBITION OF RENAL RESERVE IN CHRONIC RENAL FAILURE BY A COMBINATION OF ACEI AND ARB CG Musso ¹, J Reynaldi¹, N Imperiali ¹, L Algranati ¹, DG Oreopoulos ² Nephrology Department Hospital Italiano
COMPLETE INHIBITION OF RENAL RESERVE IN CHRONIC RENAL FAILURE BY A COMBINATION OF ACEI AND ARB CG Musso ¹, J Reynaldi¹, N Imperiali ¹, L Algranati ¹, DG Oreopoulos ² Nephrology Department Hospital Italiano
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
The ability of the kidneys to regulate extracellular fluid volume by altering sodium
 REGULATION OF EXTRACELLULAR FLUID VOLUME BY INTEGRATED CONTROL OF SODIUM EXCRETION Joey P. Granger Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi
REGULATION OF EXTRACELLULAR FLUID VOLUME BY INTEGRATED CONTROL OF SODIUM EXCRETION Joey P. Granger Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Regulation of fluid and electrolytes balance
 Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Physiology (4) 2/4/2018. Wael abu-anzeh
 Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Principles of Renal Physiology. 4th Edition
 Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
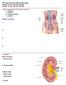 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
Urinary System BIO 250. Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat. Routes of Waste Elimination
 Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
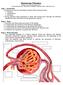 Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Renal physiology D.HAMMOUDI.MD
 Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
BLOCK REVIEW Renal Physiology. May 9, 2011 Koeppen & Stanton. EXAM May 12, Tubular Epithelium
 BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
Counter-Current System Regulation of Renal Functions
 Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Renal physiology II. Basic renal processes. Dr Alida Koorts BMS
 Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Class XI Chapter 19 Excretory Products and their Elimination Biology
 Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance
 Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance Matthew R. Weir, MD Professor and Director Division of Nephrology University of Maryland School of Medicine Overview Introduction Mechanisms
Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance Matthew R. Weir, MD Professor and Director Division of Nephrology University of Maryland School of Medicine Overview Introduction Mechanisms
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Physiology of Excretory Systems
 Physiology of Excretory Systems Fig 12-2 (a) Urea is formed by the ornithine-urea cycle in most vertebrates. Because ATP is required for the first step, nitrogen excretion in ureotelic animals is more
Physiology of Excretory Systems Fig 12-2 (a) Urea is formed by the ornithine-urea cycle in most vertebrates. Because ATP is required for the first step, nitrogen excretion in ureotelic animals is more
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
5.Which part of the nephron removes water, ions and nutrients from the blood?
 Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
Chapter 26 The Urinary System
 Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
Hyperaldosteronism: Conn's Syndrome
 RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
Hormonal Control of Osmoregulatory Functions *
 OpenStax-CNX module: m44828 1 Hormonal Control of Osmoregulatory Functions * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of
OpenStax-CNX module: m44828 1 Hormonal Control of Osmoregulatory Functions * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of
Fal Fal P h y s i o l o g y 6 1 1, S a n F r a n c i s c o S t a t e U n i v e r s i t y
 Fall 12 OSMOTIC REGULATION OF THE RENAL SYSTEM: Effects of fasting and ingestion of water, coke, or Gatorade on urine flow rate and specific gravity Dorette Franks The purpose of the physiology experiment
Fall 12 OSMOTIC REGULATION OF THE RENAL SYSTEM: Effects of fasting and ingestion of water, coke, or Gatorade on urine flow rate and specific gravity Dorette Franks The purpose of the physiology experiment
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: 100 20% of grade in class 1) During exercise, plasma levels of Renin increase moderately. Why should Renin levels be elevated during
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: 100 20% of grade in class 1) During exercise, plasma levels of Renin increase moderately. Why should Renin levels be elevated during
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
BIPN100 F12 FINAL EXAM ANSWERS Name Answer Key PID p. 1
 BIPN100 F12 FINAL EXAM ANSWERS Name Answer Key PID p. 1 READ THIS PAGE BEFORE YOU BEGIN THE EXAM. 1. Write your name on every page, in both packets of stapled-together pages. (5 points off for EACH unnamed
BIPN100 F12 FINAL EXAM ANSWERS Name Answer Key PID p. 1 READ THIS PAGE BEFORE YOU BEGIN THE EXAM. 1. Write your name on every page, in both packets of stapled-together pages. (5 points off for EACH unnamed
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D.
 Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla.
 2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Renal System and Excretion
 Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Fluid and electrolyte balance, imbalance
 Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Renal Functions: Renal Functions: Renal Function: Produce Urine
 Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
Renal Functions: Excrete metabolic waste products Reabsorb vital nutrients Regulate osmolarity: Maintain ion balance Regulate extracellular fluid volume (and thus blood pressure) Renal Functions: Regulate
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19](/thumbs/77/74887026.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Mechanisms of renal hemodynamic regulation in response to protein feeding
 Kidney nternational, Vol. 44 (1993), pp. 659 675 DTORAL RVW Mechanisms of renal hemodynamic regulation in response to protein feeding t is well established that protein ingestion causes marked changes
Kidney nternational, Vol. 44 (1993), pp. 659 675 DTORAL RVW Mechanisms of renal hemodynamic regulation in response to protein feeding t is well established that protein ingestion causes marked changes
Managing patients with renal disease
 Managing patients with renal disease Hiddo Lambers Heerspink, MD University Medical Centre Groningen, The Netherlands Asian Cardio Diabetes Forum April 23 24, 216 Kuala Lumpur, Malaysia Prevalent cases,
Managing patients with renal disease Hiddo Lambers Heerspink, MD University Medical Centre Groningen, The Netherlands Asian Cardio Diabetes Forum April 23 24, 216 Kuala Lumpur, Malaysia Prevalent cases,
Na concentration in the extracellular compartment is 140
 هللامسب Na regulation: Na concentration in the extracellular compartment is 140 meq\l. Na is important because: -It determines the volume of extracellular fluid : the more Na intake will expand extracellular
هللامسب Na regulation: Na concentration in the extracellular compartment is 140 meq\l. Na is important because: -It determines the volume of extracellular fluid : the more Na intake will expand extracellular
Outline Urinary System
 Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
Acute Kidney Injury. APSN JSN CME for Nephrology Trainees May Professor Robert Walker
 Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
General introduction of nephrology. Xiaoqiang Ding M.D., Ph.D. Department of nephrology Zhongshan Hospital, Fudan University
 General introduction of nephrology Xiaoqiang Ding M.D., Ph.D. Department of nephrology Zhongshan Hospital, Fudan University Terminology Kidney,renal Nephrology Scope of nephrology Kidney diseases and
General introduction of nephrology Xiaoqiang Ding M.D., Ph.D. Department of nephrology Zhongshan Hospital, Fudan University Terminology Kidney,renal Nephrology Scope of nephrology Kidney diseases and
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
