PHYSIOLOGICAL SCALING FACTORS AND MECHANISTIC MODELS FOR PREDICTION OF RENAL CLEARANCE FROM IN VITRO DATA
|
|
|
- Bridget Bell
- 5 years ago
- Views:
Transcription
1 PHYSIOLOGICAL SCALING FACTORS AND MECHANISTIC MODELS FOR PREDICTION OF RENAL CLEARANCE FROM IN VITRO DATA A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Medical and Human Sciences 2016 DANIEL SCOTCHER Manchester School of Pharmacy
2 Contents Contents... 2 List of Figures... 6 List of Tables... 9 Abstract Declaration Copyright statement Abbreviations Acknowledgements Chapter 1. Key to opening kidney for in vitro-in vivo extrapolation entrance: The existing holes that prevent prediction of human renal drug disposition in health and disease! Introduction Defining the need for more quantitative models Getting a handle on renal drug elimination: Use of in vitro systems In vitro systems for studying renal drug metabolism Measurement of renal passive tubular permeability in vitro In vitro systems to study active transport in kidney Mechanistic modelling of in vitro transporter kinetic data Understanding the physiology of kidney: The key system data in PBPK models Kidney weight, volume and blood flow Tubular flow rates and ph regulation Nephron number Tubule dimensions and surface area Proximal tubule cell number Microsomal and cytosolic protein content of kidney Amount of specific drug metabolising enzymes in kidney Amount of specific drug transporters in kidney Use of models for studying pharmacokinetics in kidney: Current status and IVIVE opportunities IVIVE and models of renal drug metabolism IVIVE and modelling of tubular excretion of drugs Prediction of renal drug-drug interactions within PBPK paradigm Assessing dosage adjustment in chronic kidney disease Perspective on current efforts Conclusion Aims of project Chapter 2. Measurement of microsomal and cytosolic protein kidney and in vitro-in vivo extrapolation of renal metabolic clearance Introduction Aims Methods Isolation of microsomal protein from dog kidney Isolation of microsomal and cytosolic protein from human and rat kidney Estimation of microsomal and cytosolic protein contents of tissues Prediction of mycophenolic acid glucuronidation clearance in vivo Data Analysis Results
3 Characterisation and optimisation of protein marker assays Estimation of microsomal protein content in dog kidney cortex and liver and comparison with intestine Estimation of MPPGK and CPPGK in human kidney cortex In vitro glucuronidation of mycophenolic acid by human kidney microsomes and IVIVE scaling Discussion Suitability of microsomal and cytosol protein markers for correction of protein losses Species and tissue differences in subcellular protein content estimates Impact on updated MPPGK scaling factors on renal metabolic clearance predictions Conclusion Chapter 3. Development of novel methodology for measurement of proximal tubule cell number in human kidney Background Aims Methods Reagents Source of human and pig kidney Sampling and embedding of pig kidney Microtome sectioning and mounting Deparaffinising and rehydration Periodic acid Schiff Orange G stain Immunohistochemistry Dehydration, mounting and scanning of slides Results Stereological processing of kidney Staining and immunohistochemistry Discussion Identification of proximal tubule cells in histology sections Conclusion Chapter 4. Novel minimal physiologically-based model for the prediction of passive tubular reabsorption and renal excretion clearance Introduction Aims Methods Clinical data collation Calculation of observed clearance ratio and F reab In vitro permeability data, physico-chemical properties and drug affinity for renal transporter proteins Overall structure of the minimal model of tubular reabsorption Minimal model of tubular reabsorption: Physiological system parameters Empirical relationship between P app and observed F reab Calibration of Caco-2 permeability data Data analysis Results Collation of a comprehensive renal clearance database Prediction of CL R from glomerular filtration only Prediction of F reab using the minimal physiologically-based tubular reabsorption model Empirical relationship between F reab and P app and calibration approach Discussion
4 4.4.1 Physiological considerations for predicting tubular reabsorption Validity of Caco-2 cell monolayers as in vitro model for renal tubular reabsorption Application of the mechanistic tubular reabsorption model and existing gaps Conclusion Chapter 5. In vitro-in vivo extrapolation for prediction of tubular reabsorption using a physiologically based pharmacokinetic kidney model Introduction Aims Methods Clinical data sources Workflow for PBPK model construction and verification for caffeine, theophylline, linezolid Modification of the tubular reabsorption in MechKiM: Physiological parameters and scaling approach Simulation of urine flow dependent CL R Data Analysis Results PBPK models without mechanistic models of kidney Prediction of CL R using MechKiM Simulation of urine flow dependant CL R Discussion IVIVE linked PBPK model for prediction of tubular reabsorption in healthy subjects IVIVE linked PBPK model for simulation of tubular reabsorption in patients: Potential utility and future implications Conclusion Chapter 6. SimCYP model of tubular secretion for digoxin and simulation of change in CL R due to aging and renal impairment Introduction Aims Methods Clinical data sources Verification of basal PBPK model of digoxin IVIVE and optimisation of tubular secretion in PBPK model Simulation of digoxin CL R in elderly and renal impairment populations Data Analysis Results Optimisation of digoxin kidney transporter kinetic parameters Simulation of digoxin pharmacokinetics in special populations: effects of age and renal impairment Discussion Development of mechanistic kidney model for digoxin Simulation of digoxin renal drug disposition in renal impairment: Implications for drug toxicity Conclusion Chapter Acknowledgements Final Discussion Measurement of microsomal and cytosolic protein recoveries in kidney: Theory and practice Balancing purity, yield and activity for microsomal preparation Markers of microsomal and cytosolic protein recovery in kidney
5 Assessing the relevance of renal drug metabolism Predicting the relevance of metabolites in DDIs and toxicity in kidney Opportunities to improve prediction of human renal drug disposition Accounting for multiple interacting processes Simulation of intra-cellular or tissue concentrations Challenges associated with development and validation of CL R IVIVE and mechanistic kidney model Availability of appropriate clinical data for refining and validating specific parameters Technical and practical issues with obtaining useful physiological data for kidney Relationship between systems parameters of models and measurements of physiological features Conclusions References Chapter Appendices Appendix to Chapter Calculating proximal tubule cell number Appendix to Chapter Appendix to Chapter Calculation of collecting duct surface area Microvilli expansion factors Derivation of P app calibration Results Appendix to Chapter Appendix to Chapter Word Count:
6 List of Figures Figure 1.1 Proposed IVIVE scaling approach for renal transporter data implemented in the SimCYP MechKiM module Figure 1.2 Schematic view of a nephron and collecting duct depicting the structural characteristics of epithelial cells forming various regions Figure 1.3 Chronological presentation of literature reporting experimental measurement of the microsomal protein content in kidney (open boxes) and liver (shaded boxes) for human (blue boxes) and rat (red boxes) (lower section), as well as studies using some of these data to inform the MPPGK scaling factor in order to perform IVIVE of human kidney microsomal metabolism data (upper section) Figure 1.4 Comparison of predictions of AUC ratio for renal uptake transporter mediated DDIs with observed data (226, 235) Figure 2.1 Representative UV/ Vis absorbance spectra from dithionite difference assay in dog kidney homogenate and microsomes Figure 2.2 Inter-assay variability of CYP content measurements was similar to the inter-batch variability in paired homogenate and microsomes prepared from kidney tissue of a single dog Figure 2.3 Inter-assay variability of G6Pase activity was greater than the inter-batch variability in paired homogenate and microsomes prepared from a single human kidney donor Figure 2.4 GST activity in human kidney homogenate, microsomes and cytosol from donor B Figure 2.5 Comparison of MPPGK and MPPGL in dog (n = 17 dogs) using either CYP content ( ) or G6Pase activity ( ) as the microsomal protein marker Figure 2.6 Bland-Altman plots: Difference in MPPG measured using CYP content vs. G6Pase activity as microsomal protein marker Figure 2.7 G6Pase activity measured in 31 human kidney homogenate and microsomes Figure 2.8 GST activity measured in 31 human kidney homogenate, microsomes and cytosols Figure 2.9 Microsomal (MPPGK) and cytosolic (CPPGK) protein content of kidney, and homogenate protein yields, in 31 human kidney samples Figure 2.10 Comparison of G6Pase activity and mycophenolic acid CL int,u,ugt,hkm for human kidney microsomes from 13 donors Figure 2.11 Kidney: liver ratios of mycophenolic acid CL int,u,ugt calculated using CL int,u,ugt,hkm data obtained in individual (solid bars) or XenoTech pooled (striped bars) human kidney microsomes which were scaled either by MPPGK as previously published (green; (182)) or measured in the current study (blue); scaled CL int,u,ugt,hlm was 9.32 ml/ min/ g liver, which was estimated in comparable assay conditions (i.e. in presence of BSA) (38) Figure 2.12 Impact of different assumptions on the prediction accuracy of mycophenolic acid CL UGT Figure 2.13 Kidney: liver ratios of mycophenolic acid CL met,ugt calculated based on assumptions of either the Whole kidney contributing to glucuronidation (green and blue) or Cortex only contributing (purple) using CL int,u,ugt,hkm data obtained in individual (solid bars) or XenoTech pooled (striped bars) human kidney microsomes Figure 3.1 Different stages of sampling of formalin fixed pig kidney using systematic uniformly random sampling Figure 3.2 Representative images of the same virtual slide of pig kidney with increasing magnification from left to right Figure 3.3 Representative images of cortex of 5 or 3 µm thick human kidney sections stained with Periodic acid Schiff - Orange G counterstained with Gill s No. 2 haematoxylin Figure 3.4 Representative images of cortex 5 or 3 µm thick pig kidney sections stained with Periodic acid Schiff - Orange G counterstained with Gill s haematoxylin Figure 3.5 Representative images of medulla of 5 or 3 µm thick human and pig kidney sections stained with Periodic acid Schiff - Orange G counterstained with Gill s haematoxylin Figure 3.6 Representative images of human kidney sections stained using anti-villin antibody (brown) to identify proximal tubules in cortex, using two dilutions of the primary antibody
7 Figure 3.7 Representative images of pig kidney sections stained using anti-villin antibody (brown) to identify proximal tubules in cortex, using two dilutions of the primary antibody Figure 3.8 Staining for villin in kidney cortex are abolished in the negative (no primary antibody) control Figure 3.9 Human and pig kidney medulla contained tubules which were stained using anti-villin antibody (brown) and counterstained with Papanicolaou s haematoxylin (blue) Figure 4.1 Schematic diagram of the minimal physiologically-based model for tubular reabsorption of drugs in the kidney Figure 4.2 Average LogD oct (ph 7.4) of drugs grouped by low (< 0.25), medium ( ) and high (0.75-1) F reab. The values for the classification system were arbitrarily chosen Figure 4.3 Number of drugs classified as having high (0.75-1; blue), moderate ( ; red) and low (<0.25; green) observed F reab values Figure 4.4 Comparison of predicted and observed CL R using CL R,filt alone to predict CL R Figure 4.5 Comparison of observed and predicted F reab using the mechanistic tubular reabsorption model and P app data obtained in Caco-2 cells Figure 4.6 Comparison between observed and predicted CL R by the mechanistic tubular reabsorption model Figure 4.7 Best-fit curve and 90% confidence interval of the Hill equation to the P app and F reab data for 45 drugs (solid and dashed lines respectively) Figure 4.8 Prediction of CL R using the minimal model following calibration of P app data using reference drugs (n=45 drugs) Figure 4.9 Comparison of the predicted CL R / observed CL R ratio obtained using the reabsorption model with (orange circles) and without (blue open triangles) correction for the presence/ absence of microvilli Figure 4.10 Sensitivity analysis of urine flow rate on predicted CL R for hypothetical drugs compared against observed data for theophylline Figure 5.1 Structure of the mechanistic kidney model (MechKiM) nested within the whole body physiologically based pharmacokinetic model in the SimCYP simulator (78) Figure 5.2 Workflow for development and verification of the full PBPK models for caffeine, theophylline and linezolid Figure 5.3 Representative simulated plasma concentration-time profiles using initial PBPK models, without mechanistic prediction of CL R Figure 5.4 Simulated CL R using different assumptions for calculating physiological input parameters and scaling factors using MechKiM in SimCYP Figure 5.5 Sensitivity analysis of simulated CL R against tubular surface area, with or without correction for microvilli (MV) Figure 5.6 Impact of changes to urine flow rates on simulated CL R in a population representative Figure 6.1 Representative simulated plasma concentration-time profile of digoxin using default full- PBPK model in SimCYP, without activation of MechKiM Figure 6.2 Mean simulated digoxin plasma concentration time profiles (i.v. administration of 1 mg digoxin) for intermediate PBPK models used during development of the mechanistic kidney model Figure 6.3 Simulated digoxin CL R (A) and C max, PT-1 (B) at different input values for the kidney transporter kinetic parameters Figure 6.4 Simulated digoxin CL R (A), AUC 0- (B) and C max, PT-1 (C) at different input values for the f u,kidney,cell parameter Figure 6.5 Estimation of OATP4C1 CL int,t parameter using a sensitivity analysis approach, by simulating digoxin CL R in population representatives with different serum creatinine values Figure 6.6 Correlation of simulated CL R with GFR and OATP4C1 abundance in virtual populations Figure 6.7 Impact of reduced renal secretion on simulated digoxin AUC ratio (top panel) and CL R (middle panel) or C max,pt-1 ratio (bottom panel) in renal impairment populations
8 Figure 6.8 Simulation of digoxin CL R in population representative mode with changes in different systems parameters performed to represent changes in the case of renal impairment Figure 7.1 Impact of renal impairment-associated changes in transporter abundance on pharmacokinetics of drugs at local and systemic levels Figure 8.1 A summary of different methods to isolate kidney microsomes from tissue collated from the literature Figure 8.2 Assessment of linearity of G6Pase activity in dog kidney with respect to assay protein concentration (A) and impact on relationship between assay protein concentration and the estimated microsomal protein recovery (B) Figure 8.3 Assessment of linearity of ADH activity in rat kidney with respect to assay protein concentration (A) and impact on relationship between assay protein concentration and the estimated cytosolic protein recovery (B) Figure 8.4 Assessment of GST activity linearity in rat kidney respect to assay protein concentration (A) and impact on relationship between assay protein concentration and the estimated cytosolic protein enrichment factor (B) Figure 8.5 Comparison of MPPGK and CPPGK for human kidney microsomes from 31 donors. 279 Figure 8.6 Fraction of mycophenolic acid (MPA) remaining over time in individual donor or pooled human kidney microsomes (0.25 mg/ ml) during glucuronidation substrate depletion assay Figure 8.7 Exponential function used to calculate TSA IMCD Figure 8.8 Comparison of CL R predicted using the minimal model of tubular reabsorption in combination with Caco-2 P app from the ph7.4:7.4 configured assay, with observed CL R (n=32 drugs) Figure 8.9 Comparison of plasma concentration-time profiles for different PBPK models for caffeine, theophylline and linezolid Figure 8.10 Signal intensity for peptides analysed by LC-MS/MS obtained from a single donor human kidney microsomal preparation
9 List of Tables Table 1.1 Summary of key drug metabolising enzyme isoforms in human kidney Table 1.2 Summary of key drug transporters in human and rodent kidney a Table 1.3 Summary and comparison of the features on in vitro models for active secretion Table 1.4 Example literature reports of differences or changes in kidney size in relation to renal function in different aetiologies of kidney disease a Table 1.5 Literature survey of tubular water reabsorption along nephron tubule Table 1.6 Literature survey of tubular section diameters. Mean values, with ranges in square parenthesis Table 1.7 Literature survey of tubular section lengths. Mean values, with ranges in square parenthesis Table 1.8 Summary of literature reported values for Human MPPGK and corresponding MPPGL38 Table 1.9 Calculation of AUC ratio for renal transporter mediated DDIs using static model, and comparison with published predictions using PBPK model and observed values. See Figure 1.4 for summary Table 2.1 Parameters used in calculation of MPPGK and CPPGK from human and dog kidney samples Table 2.2 CYP content, G6Pase activity and MPPG measured in homogenate and microsomal samples prepared from fresh dog kidney, frozen dog kidney and frozen dog liver. Average values are presented, with CVs in parentheses. G6Pase activity was not measured in samples prepared from fresh dog kidney. Data for individual dogs are presented in the appendix, Table Table 2.3 Comparison of scaled mycophenolic acid CL int,u,ugt,hkm and predicted CL UGT under various assumptions. Mean values from 13 individual human kidney microsomes are shown, with CVs in parentheses, and data from XenoTech pooled human kidney microsomes in square brackets. Data for individual donors are listed in the appendix, Table Table 4.1 Physiological parameter values used for tubular compartments in the minimal physiologically-based reabsorption model Table 4.2 Regional filtrate flow rates along nephron tubule, from which midpoint TFR i values were derived for model. The contribution of overall filtrate reabsorption from each region of nephron is also indicated (see Table 1.5 for full literature analysis) Table 4.3 In vivo, physico-chemical properties and in vitro data for 45 drugs used to assess the minimal model of tubular reabsorption Table 4.4 Assessment of the physiologically-based tubular reabsorption model for prediction of CL R. Performance of the mechanistic model was assessed initially for all drugs with a measured Caco-2 P app value with the exception of those that showed evidence of net secretion (clearance ratio >1.5). Subsequently, the tubular reabsorption model was reassessed after excluding drugs currently identified as substrates for drug transporters expressed within kidney Table 4.5 Overview of various CL R predictions for 45 drugs compared with observed CL R Table 4.6 Assessment of the predictive performance of various CL R prediction methods using gmfe and % predicted within 3-fold of observed CL R Table 4.7 Reference drugs used for calibration of Caco-2 P app data Table 5.1 SimCYP input parameters for caffeine, theophylline and linezolid. The majority of parameters were already implemented in existing SimCYP compound files, or taken from literature (382, ). See text for details of optimised parameters. Coefficient of variation (%) is given in parentheses where applicable Table 5.2 Clinical trials used for verification and refinement of the compound files in v14.1 of the SimCYP simulator prior to simulations using the MechKiM. Basic dosage and demographic information are shown. All subjects were healthy participants Table 5.3 Tubular surface area values used as IVIVE scaling factors for calculation of CL PD input parameters. Values were calculated from collated literature data shown in Chapter 1, Tables 1.6 and 1.7. Simulations were performed using the midpoint tubular flow rates, as used in the static model described in Chapter
10 Table 5.4 Tubular flow rate values used in MechKiM to simulate change in urine flow rate in healthy virtual subjects. See text for full details Table 6.1 SimCYP input parameters for default digoxin full-pbpk model (version 14.1), as reported by Neuhoff et al (382) Table 6.2 Clinical trials used for verification of the digoxin compound file in v14.1 of the SimCYP simulator, prior to simulations using the MechKiM. Basic dosage and demographic information are shown. All subjects were healthy participants Table 6.3 In vitro transport kinetics data for digoxin and the P-gp and OATP4C1 transporters, collated from the literature Table 6.4 Reported transporter expression of P-gp in Caco-2 and human organs. The kidney: Caco-2 REF used in the mechanistic kidney model was 1.51 based on mrna data (98) Table 6.5 Observed digoxin CL R values published in literature in healthy subjects Table 6.6 Parameters used to simulate digoxin CL R. Reduction in filtration and secretion was performed to represent changes in renal impairment. The simulated population representative of the Healthy volunteers population had an age, weight and BSA of 20 years, 81 kg and 1.98 m 2 respectively. The serum creatinine (input parameter of model) was calculated for each scenario using the Cockcroft-Gault equation (459), based on the target GFR and the age, weight and BSA of the population representative Table 6.7 Values of the OATP4C1 CL int,t parameter estimated by various methods, and subsequent simulated digoxin CL R in healthy volunteers, using the i.v. trial design from (416) Table 8.1 Expression of SLC Family Drug Transporters in the Human and Rodent Kidney. Summary presented in Table 1.2 in the main text Table 8.2 Expression of ABC Family Drug Transporters in the Human and Rodent Kidney. Summary presented in Table 1.2 in the main text Table 8.3 Expression of OATP Family Drug Transporters in the Human and Rodent Kidney. Summary presented in Table 1.2 in the main text Table 8.4 HPLC elution gradient for mycophenolic acid and warfarin (IS) Table 8.5 Microsomal protein marker data and MPPG estimates in kidney, liver and intestine for individual dogs. Data for intestine were provided by Dr Oliver Hatley Table 8.6 Demographics, protein recovery marker activities, subcellular protein content estimates, and mycophenolic acid in vitro glucuronidation data and IVIVE in individual human kidney samples Table 8.7 Database of clinical CL R values collated from the scientific literature. CL R values were calculated using plasma and urine drug concentration data measured in the same healthy subjects, and were normalised for subject weight and body surface area where necessary Table 8.8 Human kidney drug transporters reported to interact with drugs in database of 157 drugs (at substrate level) Table 8.9 Specific studies or trials excluded from the database of CL R values, and reasons for exclusion
11 Abstract The kidneys have a significant role in drug elimination through both metabolic and excretory routes. Despite a recent paradigm shift towards systems pharmacology approaches, prediction of renal drug disposition using bottom-up and mechanistic modelling approaches remains underdeveloped. Lack of gold-standard in vitro assays and corresponding in vitro-in vivo extrapolation (IVIVE) approaches for prediction of renal metabolic (CL R,met ) and excretory (CL R ) clearances contribute to this. A comprehensive literature analysis of quantitative physiological data to inform renal IVIVE scaling factors and systems parameters relevant for physiologically based pharmacokinetic (PBPK) kidney models was initially performed to identify existing knowledge gaps. Following this, microsomal protein content in dog kidney cortex (MPPGK) and liver (MPPGL) were measured in 17 samples from the same animal. Mean dog MPPGK (44.0 mg/ g kidney) and MPPGL (63.6 mg/ g liver) obtained using glucose-6-phosphatase activity as the microsomal protein marker where systematically higher than when CYP content was used as the marker (33.9 mg/ g kidney and 41.1 mg/ g liver respectively). Dog MPPGK was lower than MPPGL, with no direct correlation between the organs. In addition to dog, MPPGK and cytosolic protein per gram kidney (CPPGK) were obtained from 31 human samples, which represent the largest dataset currently available. Mean human MPPGK (25.7 mg/ g kidney) and CPPGK (52.7 mg/ g kidney), were measured using glucose-6-phosphatase and glutathione-s-transferase activities as recovery markers, respectively. Activity of prepared kidney microsomes was assessed using mycophenolic acid glucuronidation as a marker. Novel scaling factor of 25.7 mg/ g kidney was applied for IVIVE of mycophenolic acid microsomal glucuronidation data, resulting in a 2-fold increase in scaled intrinsic clearance compared with data scaled by the commonly used literature MPPGK value (12.8 mg/ g kidney). In addition to the microsomal scaling factor, several elements of a modified stereology method were developed for quantifying human proximal tubule cellularity. The methods included implementation of a systematic uniform random sampling protocol and investigation of tinctorial and immunohistochemistry based staining approaches that could be used identify and count proximal tubule cells in histology sections. A range of mechanistic models for prediction of CL R via either tubular reabsorption or active secretion were developed. A novel 5-compartment model for prediction of tubular reabsorption and CL R from Caco-2 apparent permeability data was developed. This model accounted for relevant physiological complexities of the kidney, such as regional differences in tubular filtrate flow rates and tubular surface area, including consideration of the impact of microvilli. The model predicted the CL R of 45 drugs with overall good accuracy (geometric mean fold error of 1.96), although a systematic under-prediction was noted for basic drugs. The novel 5-compartment model represents an important addition to the IVIVE toolbox for physiologically-based prediction of renal tubular reabsorption and CL R and can be implemented in the more complex mechanistic kidney models, as shown in the case of prediction of urine flow dependent CL R of theophylline and caffeine. Final part of the Thesis focused on the refinement of digoxin PBPK kidney model and its ability to predict effect of aging and renal impairment on digoxin CL R. The analysis has identified that reducing either the proximal tubule cellularity or OATP4C1 abundance parameters in the mechanistic model recovers well observed reduced tubular secretion and CL R of digoxin in renal impairment populations whereas no effect of modification of P-gp abundance was observed. Conversely, reducing the proximal tubule cellularity, OATP4C1 abundance or P-gp abundance parameters in the model resulted in negligible change, decreased or increased accumulation of digoxin in proximal tubule cells, respectively. In conclusion, the current study provides to date the most comprehensive kidney microsomal and cytosolic metabolic scaling factors, together with revised database on renal physiological data necessary for quantitative prediction of renal drug disposition. Mechanistic modelling work shown here has highlighted a need for physiological data from different population groups to inform kidney model parameters, in order to improve the scope and utility of such models within the systems pharmacology paradigm. 11
12 Declaration Some of the work referred to in the thesis has been submitted in support of applications for other degrees or qualification of this or any other university or other institute of learning. In particular: The data provided on the dog intestinal samples in Chapter 2 was provided by Dr Oliver Hatley, who generated the data as part of his thesis submitted to the University of Manchester for the degree of Doctor of Philosophy Some of the data collation and simulations presented in Chapter 6 were performed with the assistance of Amir Khalifa and Sahar Fallaha, who presented some of these results in their respective 4 th year project reports, submitted to the University of Manchester in partial fulfilment of the MPharm degree Copyright statement i. The author of this thesis (including any appendices and/or schedules to this thesis) owns certain copyright or related rights in it (the Copyright ) and s/he has given The University of Manchester certain rights to use such Copyright, including for administrative purposes. ii. Copies of this thesis, either in full or in extracts and whether in hard or electronic copy, may be made only in accordance with the Copyright, Designs and Patents Act 1988 (as amended) and regulations issued under it or, where appropriate, in accordance with licensing agreements which the University has from time to time. This page must form part of any such copies made. iii. The ownership of certain Copyright, patents, designs, trademarks and other intellectual property (the Intellectual Property ) and any reproductions of copyright works in the thesis, for example graphs and tables ( Reproductions ), which may be described in this thesis, may not be owned by the author and may be owned by third parties. Such Intellectual Property and Reproductions cannot and must not be made available for use without the prior written permission of the owner(s) of the relevant Intellectual Property and/or Reproductions. iv. Further information on the conditions under which disclosure, publication and commercialisation of this thesis, the Copyright and any Intellectual Property University IP Policy (see in any relevant Thesis restriction declarations deposited in the University Library, The University Library s regulations (see and in The University s policy on Presentation of Theses 12
13 Abbreviations ADH = alcohol dehydrogenase AKI = acute kidney disease AKR = aldo-keto reductase ALDH = alcohol dehydrogenase AP = apical AUC = area under the curve AUCR = AUC ratio BCA = bicinchoninic acid BDDCS = biopharmaceutical drug disposition classification system BLAST = basic local alignment search tool BSA = bovine serum albumin BL = basolateral CES = carboxylesterase CD = collecting duct CDNB = 1-Chloro-2,4-dinitrobenzene CHO = Chinese hamster ovary CKD = chronic kidney disease CL CR = creatinine clearance CL h,met,ugt = hepatic glucuronidation clearance CL int = intrinsic clearance CL R,int,reab,i = intrinsic renal tubular reabsorption clearance of the i th tubular region CL int,t = intrinsic transport mediated clearance CL int,ugt = intrinsic clearance by glucuronidation CL int,ugt,hkm = intrinsic clearance by glucuronidation in human kidney microsomes CL int,u,ugt = unbound intrinsic clearance by glucuronidation CL int,u,ugt,hkm = unbound intrinsic clearance by glucuronidation in human kidney microsomes CL int,ugt,hlm = intrinsic clearance by glucuronidation in human liver microsomes CL IV = intravenous clearance CL met = metabolic clearance CL max = maximal intrinsic clearance CL PD,x = passive permeability clearance across membrane x CL R = renal excretion of unchanged drug CL R,filt = renal filtration clearance CL R,met = renal metabolic clearance CL R,met,UGT = renal glucuronidation clearance CL R,sec = renal secretory clearance CL UGT = systemic clearance via glucuronidation C max = maximum concentration C max,u = maximum unbound concentration CMFT = Central Manchester University Hospitals NHS Foundation Trust CPPGK = cytosolic protein per gram kidney CPPGL = cytosolic protein per gram kidney CRF = chronic renal failure CTA = computed tomography angiography CV = coefficient of variation CYP = cytochrome P450 DDI = drug-drug interaction DT = distal tubule EBCT = electron-beam computerised tomography EDTA = ethylenediaminetetraacetic acid egfr = estimated glomerular filtration rate FDA = Food and Drug Administration 13
14 FMO = flavin-containing monooxygenase f m,ugt = fraction of metabolism due to glucuronidation F reab = fraction reabsorbed F reab = fraction of the equilibrium reached between urine and plasma f u,b = fraction unbound in blood f u,inc = fraction unbound in incubation f u,kidney,cell = fraction unbound in kidney cell f u,p = fraction drug unbound in plasma G6P = glucose-6-phosphate G6Pase = glucose-6-phosphatase GFR = glomerular filtration rate gmfe = geometric fold error GST = glutathione-s-transferase HEK = human embryonic kidney HKC = human kidney cytosol HKH = human kidney homogenate HKM = human kidney microsomes HLM = human liver microsomes HNF = hepatocyte nuclear factor IC 50 = half maximal inhibitory concentration IC 50,app = apparent half maximal inhibitory concentration ISEF = intersystem extrapolation factor ITC = international transporter consortium i.v. = intravenous IVIVE = in vitro-in vivo extrapolation k= elimination rate constant K i = inhibition constant K m = Michaelis-Menten constant K m,app = apparent Michaelis-Menten constant K p = tissue: plasma partition coefficient LC-MS/MS = liquid chromatography with tandem mass spectrometry LogD x = octanol-buffer (ph x) distribution coefficient LogP = octanol-water partition coefficient LoH = loop of Henle MATE = multidrug and toxic compound extrusion MALDI = matrix-assisted laser desorption/ionization MDCK = Madin-Darby canine kidney MDR = multidrug resistance MechKiM = mechanistic kidney model nested in SimCYP PBPK model MgCl 2 = magnesium chloride MPA = mycophenolic acid MPPGI = microsomal protein per gram intestine MPPGK = microsomal protein per gram kidney MPPGL = microsomal protein per gram liver MRI = magnetic resonance imaging mrna = messenger ribonucleic acid MRP = multidrug resistance-associated protein MV = microvilli NADH = reduced nicotinamide-adenine dinucleotide NADPH = reduced nicotinamide-adenine dinucleotide phosphate NCR = NADPH cytochrome c reductase NDI nephrogenic diabetes insipidus NRES = national research ethics service NSAID = non-steroidal anti-inflammatory drug OAT = organic anion transporter 14
15 OATP = organic anion transporter protein OCT = organic cation transporter OCTN = carnitine/ organic cation transporter P app = apparent permeability P app,calibrated = calibrated apparent permeability PBPK = physiologically-based pharmacokinetic PBS = phosphate buffered saline PCT = proximal convoluted tubule PEPT = peptide transporter PET = positron emission tomography P-gp = P-glycoprotein pk a = acid ionization constant P mem = membrane permeability PST = proximal straight tubule PT = proximal tubule PTC = proximal tubule cell PTCPGK = proximal tubule cells per gram kidney QconCAT = quantification concatemer Q h = liver blood flow qpcr = real-time quantitative polymerase chain reaction Q R = renal blood flow QSAR = quantitative structure-activity relationship QSAR = quantitative structure-pharmacokinetic relationship R 2 = coefficient of determination R B = blood to plasma concentration ratio RAF = relative activity factor REC = research ethics committee REF = relative expression factor RI = renal impairment RMSE = root mean squared error rugt = recombinant UGT S1/ S2/ S3 = segment 1/ 2/ 3 of proximal tubule S9 = 9000g supernatant S9PPGK = 9000g supernatant protein per gram kidney SD = standard deviation SLGT = sodium/glucose cotransporter SNP = single nucleotide polymorphism TF/P x = tubular fluid to plasma concentration ratio of substance x TFR = tubular flow rate TSA = tubular surface area TSCR = Taussky-Shorr colour reagent UDP = uridine diphosphate UDPGA = uridine diphosphate glucuronic acid UF = urine flow rate UGT = uridine 5 -diphosphate glucuronosyltransferase V max = maximum rate of reaction V ss = volume of distribution at steady state 15
16 Acknowledgements I would like to express my special appreciation to my supervisors Professor Amin Rostami- Hodjegan, Dr Aleksandra Galetin and Dr Christopher Jones for their expertise, support, and enthusiasm, and for giving me the opportunity to complete a PhD at the University of Manchester. Thank you for allowing me to pursue my own ideas, and providing invaluable insight and guidance whenever it was needed. I would also like to acknowledge BBSRC and AstraZeneca for funding this PhD project. Within the Centre for Applied Pharmacokinetic Research (CAPKR) I have worked alongside a brilliant group of people and good friends. Thanks to Oliver Hatley for his help at the start of the project, as well as sharing some of his data for analysis in this thesis. I am also grateful to David Hallifax for helping with the LC-MS/MS analysis of mycophenolic acid, to Andres Olivares-Morales for his insights on microvilli, to Katherine Gill for advice on microsomal glucuronidation assays and to Frauke Assmus for help with phys-chem properties. I would also like to thank Brahim Achour for useful discussions and his efforts with LC-MS/MS proteomics analysis. Jay Brown and colleagues (CMFT Biobank), Colin Brown and Sarah Billington (Newcastle University) provided the human kidney tissue samples that were so important for the project, for which I am extremely grateful. Within the University of Manchester, I was fortunate to meet and learn from a stereology enthusiast in Kate Widdows at the Institute of Human Development, as well as histology expert Peter Walker at the Histology core facility, and I am grateful for their assistance. Thank you to Peter March and colleagues at the Bio-Imaging facility for scanning the histology slides. Also thanks to Sahar Fallaha, Amir Khalifa and Saira Iqbal for their assistance with literature analyses and running simulations during their undergraduate final year projects. I would like to also thank Mark Wenlock and Neil Shearer (AstraZeneca) for generating the pk a and LogD data, as well as Sybille Neuhoff (Certara), Maria Posada and Steve Hall (Eli Lilly) for helpful comments and insight on different elements of the work. Thanks also to Lesley Wright (University of Manchester) for assistance with many administrative issues. I take this opportunity to recognise some important people who inspired and mentored me in educational and professional capacities before this PhD began: Richard Windsor, Mark Lees, Steve Brasier, Cerian Ayres, Anna Pedret-Dunn and Wayne Thomas. To Adam, Rhys, Gilroy, Olli, Lucy and Dave, thank you for being such great friends. I value the support of Diana and Malcolm, especially for helping me to settle in Manchester. To Mum and Dad, thank you for always being a reliable source of advice and encouragement. Finally, thank you to my girlfriend Laura, for her unfailing support and motivation during the course of the PhD. 16
17 Chapter 1. Key to opening kidney for in vitro-in vivo extrapolation entrance: The existing holes that prevent prediction of human renal drug disposition in health and disease! Parts of this chapter were adapted from a publication and a manuscript submitted for peer review by the author as follows: Scotcher D, Jones C, Posada MM, Galetin A and Rostami-Hodjegan A. Key to opening kidney for in vitro-in vivo extrapolation entrance: The existing holes that prevent prediction of human renal drug disposition in health and disease! Manuscript submitted for publication. Scotcher D, Jones C, Rostami-Hodjegan A and Galetin A. Novel minimal physiologicallybased model for the prediction of passive tubular reabsorption and renal excretion clearance. European Journal of Pharmaceutical Sciences doi: /j.ejps Introduction The recent paradigm shift to systems pharmacology approaches in drug development set out an increased usage of quantitative concepts for linking in vitro observations on drug characteristics to their biological behaviour, in whole biological systems and in vivo conditions. Models of systems and data in biomedicine are constantly evolving. Many complex aspects of drug disposition related to the hepatic handling of the drugs have been addressed over the last decade using systems pharmacology models, with implications for population variability in pharmacokinetics (1) as well as adverse events in liver such as drug induced liver injury (2). The same cannot be claimed for predicting renal disposition under various conditions or the covariates determining nephrotoxicity. The kidneys have a significant role in the clearance of many drugs. Within the top 200 drugs prescribed in the United States of America in 2010, 32% of them had 25% of the absorbed dose excreted unchanged in urine (3). The kidney is also involved in drug metabolism due to expression of a number of drug metabolising enzymes (summary in Table 1.1). In this Chapter the progresses required to achieve quantitative predictions of renal drug disposition using in vitro in vivo extrapolation (IVIVE) approaches is examined. The need for high quality in vitro drug data generated using appropriate experimental systems is emphasized. Current knowledge of the kidney anatomy and physiology at a quantitative level, which informs IVIVE scaling factors and model parameters, is critically assessed to reveal existing gaps that exist. Finally, the availability and suitability of mechanistic models of renal drug excretion and/ or metabolism, and dynamics of drug disposition in kidney cells, in the context of IVIVE for kinetics and safety issues, are discussed. 17
18 Table 1.1 Summary of key drug metabolising enzyme isoforms in human kidney Protein Other species Other organ expression Kidney distribution Substrates Genetic polymorphism studied? References CYP3A5 Cynomolgus monkey High in liver Cortex and medulla Ifosfamide, cyclosporine A CYP3A5*3 variant causes truncation and reduced expression in proximal tubule (4-9) CYP2D6 FMO1 Rat (isoforms CYP2D1-5) Rat, mouse, cynomolgus Higher in liver Liver of several species, not in human Paediatric; adult expression possibly low; Cortex > Medulla, Highest in PT and Loop of Henle PT Dextromethorphan, bufuralol S-methyl N,Ndiethyldithiocarbomate, sulphides Extensive/ poor metabolisers characterised Correlated with variable catalytic activity (10-12) (13-18) ADH/ ALDH Ubiquitous Higher in liver ADH: PT ALDH: throughout kidney ADH- alcohols, ALDH - aldehyde metabolites - (19-21) CES2 Several isoforms in rat and mouse Liver and intestine PT, Bowman's capsule Irinotecan, prodrugs Two functionally-deficient variants reported (22-28) AKR1A1 Mouse/ rat isoforms differ to human Ubiquitous, highest in kidney PT PT, Bowman's capsule Carbonyl containing substrates, daunorubicin 2 allelic variants have reduced daunorubicin metabolism (29-32) UGT1A9 UGT1A family members in most lab species Kidney > liver PT, DT, LoH, CD Propofol, mycophenolic acid, lorcaserin, edaverone UGT1A9 SNP has effect on SN-38, but not flavopiridol, glucuronidation (33-40) UGT2B7 UGT2 family members in most lab species Liver, intestine PT, DT, LoH, CD Efavirenz, zidovudine UGT2B7 SNPs have effect on efavirenz metabolism (34, 35, 38-44) 18
19 1.2 Defining the need for more quantitative models Quantitative knowledge of human physiology can be used to help address a variety of systems pharmacology questions. Specifically, physiologically-based pharmacokinetic (PBPK) models and in vitro systems, now widely used in pharmacokinetics research, can be integrated through the IVIVE paradigm (1). While other methods for prediction of pharmacokinetic parameters, such as quantitative structure-activity relationships (QSAR) and scaling from pre-clinical species, may give adequate prediction accuracy, IVIVE approaches based on physiological assumptions are favoured because of the mechanistic basis and advantages this approach brings (1). Further, species differences in the expression or function of drug metabolising enzymes and transporters in kidney is a potential limitation of the interspecies scaling approach for certain drugs (Table 1.1 and 1.2). Validated mechanistic models have the advantage in their ability to predict the impact of pathophysiological changes such as those encountered in chronic kidney disease in population sub-groups which have not been investigated in clinical studies. As a high proportion of drugs (>40%) approved in 2013 and 2014 did not have dose recommendations for severe renal impairment (45), mechanistic models may guide the design of clinical studies and dose recommendations (46). The collation of appropriate clinical data is necessary for assessing predictions of pharmacokinetic parameters and profiles. CL R can be readily calculated from clinical data by comparing the excreted drug in urine and drug concentrations in plasma, though the common practice does not involve repeated assessment of the drug excretion in short intervals in the urine and more commonly the cumulated excretion is measured instead. However, even when there are regular urine samples alongside plasma samples, drug accumulation and entrapment in the kidney can cause delays between the apparent clearance of drug from the plasma and appearance of drug in urine, even though renal excretion may be the dominant process. An example is that of aminoglycosides that bind to the megalin protein causing the endocytosis and subsequent renal accumulation of these drugs (47). This accumulation may be a contributing factor of aminoglycoside nephrotoxicity, highlighting the importance of understanding drug concentrations within the kidney. 19
20 Table 1.2 Summary of key drug transporters in human and rodent kidney a Transporter Human? Rat/ Mouse? Comments OCT2/ Oct2 BL BL OAT1/ Oat1 BL BL OAT2/ Oat2 BL / AP Relevance of OAT2 to transport in human kidney debated; Immunohistochemistry indicates Oat2 localised to loop of Henle in rat OAT3/ Oat3 BL / BL OAT4 AP - Oat5 - AP OCTN1/ Octn1 /? AP Both positive and negative findings for expression in human kidney OCTN2/ Octn2 AP AP Octn3 - AP PEPT1/ Pept1? AP PEPT2/ Pept2 AP AP MATE1/ Mate1 AP AP MATE2-K AP - Human MATE2-K belongs to class II subgroup of MATE transporters, no rodent ortholog exists; Rodent Mate2, expressed predominantly in testes, belongs to class 20
21 Transporter Human? Rat/ Mouse? Comments III subgroup, and has been proposed to be renamed Mate3 but this is not in common use (48) MDR1/ Mdr1a & Mdr1b AP /? Both positive and negative findings for expression in rodent kidney MRP2/ Mrp2 AP AP MRP4/ Mrp4 AP? Oatp1a1 - AP Oatp1a3 - AP Oatp1a6 - AP OATP4C1/ Oatp4c1?? Conflicting literature reports for rat Oatp4c1 localisation; apical localisation supported by functional activity data (33, 49, 50); Human OATP4C1 localisation to basolateral membrane hypothesised based on functional activity data alone (50). a Further details and references in the appendixes, Tables 8.1, 8.2 and 8.3; Strong evidence of expression in kidney; Transporter not present in particular species or not expressed in kidney; / Evidence for expression equivocal; AP apical membrane; BL basolateral membrane; - Not relevant;? Not determined or conflicting data. 21
22 The simultaneous involvement of renal metabolism and excretion may lead to erroneous interpretation of urine drug concentration data (i.e., CL R ) and can subsequently impact analyses of renal excretion mechanisms (51, 52). This leads to problems for assessment of predictions of CL R and CL R,met using IVIVE and other methods. The isolated perfused kidney model can be used to determine the occurrence of intrarenal metabolism, and is therefore a useful tool for establishing, refining and validating generic IVIVE approaches in pre-clinical species (53, 54). To infer the presence of CL R,met in human, the rate of appearance of metabolite in urine can be compared with the concentration of metabolite in plasma. If the apparent CL R of the metabolite is greater than the renal blood flow or CL R of metabolite following direct administration of metabolite alone, this indicates metabolite formation in kidneys (51, 55). In the case of mycophenolic acid acylglucuronidation, evidence for renal metabolism was inferred after comparison of the AUC and renal excretion of the metabolite following intravenous (i.v.) and oral administrations (56). In addition, for a few drugs such as propofol, CL R,met can be investigated by analysing plasma drug and/ or metabolite concentrations during the anhepatic stage of liver transplant (57). 1.3 Getting a handle on renal drug elimination: Use of in vitro systems In vitro systems for studying renal drug metabolism The major sources of kidney drug metabolising enzymes for in vitro assays are human kidney subcellular fractions and recombinant enzyme expression systems (38, 58-60). Recombinantlyexpressed enzymes are useful for determining the major isoforms responsible for the metabolism of a drug. However high variability in abundance, not only between enzymes, but also between batches for the same enzyme, has been reported for recombinantly-expressed UGTs (e.g., 30.6% CV for rugt1a4) (59). In addition, UGTs expressed using insect cells may have a substantial amount of inactive protein present (61). Therefore, the use of metabolic rate data from recombinant UGTs in a quantitative setting should include correction for enzyme abundance as well as presence of inactive enzyme (60). Human kidney microsomes (and other subcellular fractions such as 9000g supernatant (S9) or cytosol (28)) are a useful in vitro system for assessing the overall capacity of the kidney to metabolise a drug. Identifying the responsible isoforms through reaction phenotyping requires isoform selective chemical inhibitors to be further characterised. Various studies have proposed selective inhibitors for UGT1A9 (e.g., niflumic acid and diflunisal), and UGT2B7 (e.g., fluconazole), although some of these have been reported to also inhibit other UGTs, albeit at higher concentrations (62, 63). It is important to consider the source of human kidney subcellular fractions used in drug metabolism studies, as although kidney cortex (or unspecified region) derived samples are often used, expression and activity of UGTs have also been demonstrated in the medulla (43, 58, 60). Studies with human liver microsomes have highlighted the necessity for inclusion of alamethicin in microsomal assay to overcome the latency issue associated with UGTs (63, 64). Furthermore, the addition of albumin to account for the inhibitory effect of fatty acids released during microsomal 22
23 incubations has been implemented in the in vitro assays to investigate renal drug metabolism (38, 65). Analogous to hepatic cytochrome P450 (CYP) and UGTs, evidence of atypical enzyme kinetics (i.e., not following Michaelis-Menten behaviour) has been reported in human kidney microsomes (43). Such atypical kinetics requires appropriate modelling of in vitro data and subsequent IVIVE. For example in the case of auto-activation, determination of maximal intrinsic clearance (CL max ) is proposed as a substitute for standard CL int in the scaling process (66) Measurement of renal passive tubular permeability in vitro Passive renal tubular reabsorption of drugs has been correlated with lipophilicity and other physicochemical properties (67-69). Recently a quantitative structure-pharmacokinetic relationship (QSPKR) model was developed that allowed prediction of reabsorption clearance, although prior information on the dominant process (reabsorption or secretion) and/ or BDDCS class was required (70). The permeability data following apical-basolateral transport across LLC-PK1 cells were proposed for the assessment of tubular reabsorption in the human proximal tubule (71). For the remaining nephron tubule (loop of Henle through collecting duct), methods for culture of renal tubule cells have been published, but are not routinely used for permeability and drug transport studies (72, 73). In contrast, the collecting duct derived MDCK cell line is routinely used for drug transport studies (including transfection with human drug transporters), typically in the context of oral absorption and brain penetration (74). Similarly, Caco-2 cells are widely used as an in vitro model of intestinal permeability, and could potentially be used as a surrogate in vitro model for renal tubular permeability In vitro systems to study active transport in kidney Active tubular secretion and reabsorption in kidney are mediated by drug transporters expressed in the cells of the proximal tubule epithelium (Table 1.2).Various in vitro models to study active transport of drug in kidney have been reported, although a gold-standard assay format is currently lacking, as summarised in Table 1.3. The selection of the most appropriate in vitro system will depend on the question/ hypothesis being investigated and constraints such as cost or availability of fresh human kidney tissue. There is some overlap with in vitro models of drug transport in the liver, as recently reviewed (75). Importantly, the choice of in vitro system will determine the necessary scaling factors needed for IVIVE (Figure 1.1). 23
24 Table 1.3 Summary and comparison of the features on in vitro models for active secretion Feature Membrane vesicles Transfected cells Immortalised kidney cell lines Primary cultured renal tubule cells Kidney slices Next generation ( kidney-on-a-chip ) Transporter expression Dependent on source (mammalian/ insect cell expression system or kidney tissue) Consistent Can be controlled in some systems Differences to kidney Consistent Generally lower than kidney Full complement possible Inter-individual variability Dependent on culturing conditions/ tissue quality Inter-individual variability Dependent on tissue quality Not yet investigated Availability Dependent on source (mammalian/ insect cell expression system or kidney tissue) Commercial Cell culture relatively easy/ routine Mixed availability, some commercial Fresh human kidney required Expertise required to isolate and culture cells Fresh human kidney required Tissue slicer required Very early stages of development Limited to a few specialist laboratories Physiological representation Very limited Very limited May lack some transporters and morphological features Morphologically representative Lacks 3D structure Lacks other cells (endothelial and interstitial) Most physiology is retained Kidney region (cortex/ medulla) should be known Proposed to be better than traditional in vitro systems Not yet demonstrated Main application Efflux transport Screening assays Transporter kinetic parameters (K m, V max, K i) Toxicity Holistic uptake/ transport studies and assessment of DDI potential Uptake studies Development ongoing Scaling factors REF/ RAF REF/ RAF/ ISEF Surface area (P app) PTCPGK (Apparent K m & V max or CL int) a REF/ RAF may also be required Surface area (P app) PTCPGK (Apparent K m & V max or CL int) a REF/ RAF may also be required Kidney weight Allometric and functional scaling (76) PTCPGK, REF/ RAF (77) a Additionally REF/ RAF scalars may be required to account for changes in expression/ abundance/ activity of transporters, which may occur during isolation, transfection (immortalised cells only) and/ or cell culture. Abbreviations: DDI Drug-drug interaction; ISEF Intersystem extrapolation factor; PTCPGK Proximal tubule cells per gram kidney; REF Relative expression factor; RAF Relative activity factor; 24
25 Figure 1.1 Proposed IVIVE scaling approach for renal transporter data implemented in the SimCYP MechKiM module J max maximum flux rate; K m Michaelis-Menten constant; CL u,int,t unbound intrinsic transporter clearance; REF relative expression factor; PTCPGK proximal tubule cells per gram kidney. With kind permission from Springer Science+Business Media: Neuhoff S, Gaohua L, Burt H, Jamei M, Li L, Tucker GT, et al. Accounting for transporters in renal clearance: Towards a mechanistic kidney model (Mech KiM). In: Sugiyama Y, Bente S, editors. Transporters in drug development. New York: Springer; 2013, Figure 7.4, page 165. (78) Transfected cells Cell lines such as Madin-Darby Canine Kidney-II (MDCK-II), Chinese Hamster Ovary (CHO), Human Embryonic Kidney (HEK)-293, and HeLa can be stably or transiently transfected to express renal drug transporters (79-81). Transfected cell lines are widely used and commercially available, and allow measurement of kinetic parameters for specific transporter(s) and drug combinations, including determination of IC 50 and inhibitory potency (K i ) values. Uptake and efflux can be studied simultaneously using multiple-transfection of transporters in a single cell line (80, 82). While tight control of transporter expression is possible (i.e., low between occasion variability), the relative expression/ activity of multiple transporters in transfected cells may not represent that found in vivo Primary cultured renal tubule cells Primary renal tubule cells can be cultured in vitro for several cell generations whilst maintaining multiple characteristics of the cells of origin (83-85). In addition, expression and function of several key drug transporters and enzymes are well maintained following a few days in culture, although reduced expression may be expected after longer culture times (84-86). Such models of proximal tubule drug transport enable a holistic understanding to be obtained; for example, reduction in functional activity due to inhibition or knockdown of one or more transporters can be investigated. Inter-individual variability can also be investigated, which should be distinguished from other factors such as differences in tissue quality and experimental variability. Extended research using primary cells depends on the availability of a consistent supply of quality human tissue, while 25
26 obtaining reliable estimates of kinetic parameters, especially for specific transporters, can be challenging Kidney slices Kidney slices can be used to investigate drug uptake at the basolateral membrane, but not tubular reabsorption (87). An advantage of kidney slices is that the interactions of multiple substrates, inhibitors and endogenous transporters can be researched within the same system (87, 88). Imaging technology (e.g., confocal microscopy, imaging mass spectrometry) can be used to indicate the localisation of uptake and inhibition of renal drug transporter substrates, including those with therapeutic or toxicological effects in kidney (89, 90). Observed variability in kidney slice uptake of drugs can be affected by inter-batch and/ or inter-individual variability (91), which must be considered for any IVIVE strategy, e.g., normalising data against the relative activity/ content of a marker in each kidney slice batch (87). The main limitation of human tissue slice experiments is obtaining a consistent supply of quality tissue Other cell lines, membrane vesicles and kidney-on-a-chip Several cell lines derived from kidney such as LLC-PK1, Caki-1, HK-2, ci-ptec, RPTEC/TERT1 and HKC have been characterised, with some commercially available (e.g., and generally useful for investigating renal tubule function and toxicity in vitro (92-95). For example ci-ptec cells were used to investigate the impact of CYP3A5 and P-gp (MDR1) genetic variation on tacrolimus metabolism, which could allow mechanistic insights into nephrotoxicity associated with this calcineurin inhibitor (96). A crucial limitation for some of these cell lines is that functional expression of drug transporters or other renal characteristics can be lost (97, 98). Cell membrane vesicles can be isolated from tissue samples, cell lines and recombinantly expressed transporter systems (99, 100), allowing multiple or individual transporters to be studied. The inside-out vesicular transport assay is particularly useful because transport mediated by efflux transporters can be studied, which can be challenging when using standard cell based assays. The use of membrane vesicles is limited to drugs with low lipophilicity/ passive permeability, as drugs with high permeability will not be trapped within the vesicles following uptake (99). Recent advances in molecular and cellular biology, combined with micro-engineering, have led to a number of proposed next generation in vitro models of the kidney and other organs. These models may incorporate features such as co-culture of multiple cell types (epithelium, endothelial and pericytes), fluid flow (e.g., microfluidic devices such as organ-on-a-chip ), and 3D cell culture, which are suggested to provide more physiologically representative systems (76, 77, ). Other emerging technologies, including stem cell science and 3D bio-printing, offer further potential for the development of the next generation in vitro models (104, 105). The application of these technologies to address questions on renal drug disposition remains a challenge for the future. A key question is whether next generation in vitro models offer sufficient advantages over traditional methods, which justify the additional effort and expertise required to generate suitable data. 26
27 1.3.4 Mechanistic modelling of in vitro transporter kinetic data Estimation of transporter kinetic parameters from in vitro assay data is a key step for successful IVIVE of transporter mediated drug disposition, with recommendations recently published by the International Transporter Consortium (106). Key considerations pertinent to IVIVE of renal clearance are highlighted below. While uptake CL int,t data can be used in IVIVE approaches, consideration of full kinetic profile in vitro (i.e., estimation of transporter K m and V max ) is preferable (107), to account for potential saturation issues. Uptake transporter kinetic parameters can be estimated from cell based in vitro uptake assays using the conventional two-step method (79), as reported for hepatocytes (108, 109). An alternative method would be to use mechanistic compartmental modelling based on simultaneous fitting of uptake rates, bi-directional passive diffusion and intracellular binding (108); so far, there is no evidence of development of such complex cellular models for the mechanistic description of process occurring in the renal tubular cells. Passive permeability data are frequently reported as P app or net flux values using proximal tubule cell monolayers or efflux transporter transfected MDCK cells (71, 80, 82, 86); these data are subsequently scaled by tubular surface area (71). Estimation of efflux transporter kinetic (K m,app, J max ) and inhibition (IC 50,app ) parameters is generally based on the extracellular medium concentrations of either substrate or inhibitors. Parameter estimates obtained this way are often dependent on efflux transporter expression levels (110, 111) and this type of analysis is currently considered inadequate (106). Compartmental modelling approaches are recommended for estimation of mechanistic efflux transporter kinetic parameters in monolayer assays (106, 110, 111). These models differ in their complexity and may also consider membrane partitioning and organelle (lysosomes) sequestration, in addition to ionised drug permeation and consideration of the unstirred water layer (106, ). The key advantage of these models is consideration of the interaction of an efflux transporter with the unbound intracellular drug concentration, as opposed to nominal incubation concentration. The application of such mechanistic models is vital for generation of mechanistic in vitro parameters describing kinetics of renal transporters to be used subsequently in PBPK models. This is of particular importance considering some of the complexities associated with renal transporters. For example, under appropriate in vitro conditions (e.g., expression system, ph gradient/ membrane potential), some renal drug transporters (including MATE1, MATE2-K and OAT4) that are expressed on the apical membrane of proximal tubule cells in vivo, can act as both uptake and efflux transporters (79, 80), highlighting the importance of careful interpretation of such in vitro data when translating to in vivo. 27
28 1.4 Understanding the physiology of kidney: The key system data in PBPK models Mechanistic models of kidney represent a simplification of kidney anatomy and physiology (Figure 1.2), but due to the complexity of the organ may still incorporate a large number of parameters. Indeed, in silico models of kidney physiology are typically implemented for rat ( ), for which detailed system data are generally more widely available than for human, and is associated with a larger amount of in vivo and in situ measurements on relevant input-output relationships. This section will critically assess the availability of human renal physiology data which can inform mechanistic kidney model system parameters, in particular PBPK models. Several system parameters can be used, in conjunction with the corresponding parameter for the in vitro system, to derive scaling factors for IVIVE of in vitro pharmacokinetics data (Figure 1.1), as highlighted below for the relevant physiological features. Therefore a strong understanding of renal physiology, and acknowledgement of the existing gaps or uncertainties, is necessary for using mechanistic models and IVIVE to investigate drug disposition in kidney Kidney weight, volume and blood flow Kidney weight or volume and renal blood flow are found as parameters in whole-body PBPK models, whether or not a mechanistic kidney model is implemented, and are relevant to both renal excretion and renal metabolism of drugs. Kidney weight may also be used as an IVIVE scaling factor for in vitro data generated in kidney slice assays. Data on the weight and volume of human kidney, including potential covariation with factors such as age, gender, ethnicity, body weight and/ or body height, have been reported and collated previously (38, ). Inter-study variability may be low when using the same method to measure kidney size, whereas there appears to be systematic differences between the different methodological approaches (120, 121). The decrease in kidney volume with age in adults (approx. 23 ml per decade after 50 years old) appears to be driven primarily by a decrease in cortical volume (approx. 18 ml per decade after 50 years old) (122). Data in paediatric populations have been collated elsewhere (123). Kidney weight/ volume have been reported to both increase and decrease in kidney disease, depending on the stage and underlying cause of disease (124, 125) (Table 1.4). In fact kidney size and cortical volume have been proposed as markers to aid diagnosis of kidney diseases (122, 126). Literature analysis of human kidney blood flow (approx ml/ min/ kg, 20% of cardiac output) and associated inter-individual variability has been published (38). The structural organisation of the intra-renal vasculature, which is intrinsic to the physiological functioning of the kidney, has been well characterised (127, 128). 28
29 Figure 1.2 Schematic view of a nephron and collecting duct depicting the structural characteristics of epithelial cells forming various regions MARIEB, ELAINE N.; HOEHN, KATJA N., HUMAN ANATOMY & PHYSIOLOGY, 10th Edition, Printed and electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, NJ (129) 29
30 Table 1.4 Example literature reports of differences or changes in kidney size in relation to renal function in different aetiologies of kidney disease a Disease Reported changes in kidney size with decrease in renal function b Comments Methods References Autosomal dominant polycystic kidney disease Increase (volume) Rate of increase in kidney volume was equivalent to rate of increase in cyst volume EBCT, MRI (130, 131) Acquired cystic kidney disease Small decrease (length) No differences or decrease in renal function and increase in kidney size associated with presence/ absence of cysts in subjects without kidney failure Sonography ( ) Hypertensive nephrosclerosis Decrease (length) Sonography (136) Chronic ischaemic renal disease Decrease (length) Spiral CTA, sonography (136, 137) Chronic glomerulonephritis Decrease (volume, length) Sonography (125, 136, 138) Diabetic nephropathy Increase in early stages, decrease in later stages (length, volume) Prior to/ during early kidney disease progression, moderately increased albuminuria is associated with glomerular hyperfiltration and increased renal volume in type 1 (insulin-dependent) diabetes, and in some cases of type 2 (non insulin-dependent) diabetes Sonography (125, 136, ) HIV-associated nephropathy No changes found Sonography (143) CKD aetiology unspecified/ mixed Decrease (size) Some results not significant Sonography ( ) a No distinctions/ inferences are made here regarding cause vs effect relationships of renal size and renal function in the different diseases, as pathophysiological mechanisms of kidney disease can form a cycle of disease progression (148); b Renal function assessed by various methods, including CL cr, egfr and CL inulin ; CTA computed tomography angiography, CRF chronic renal failure, EBCT electron-beam computerised tomography, MRI Magnetic resonance imaging 30
31 1.4.2 Tubular flow rates and ph regulation Glomerular filtration rate is a fundamental physiological parameter of mechanistic kidney models. Glomerular filtration rates in various population groups are widely reported in the literature (123). For example, glomerular filtration rate increases rapidly after birth from around 20-30% of the adult value, reaching the adult level soon after 12 months of age (149). After the age of 30 years, glomerular filtration rate declines with aging, although some uncertainties exist around the actual rate due to normal aging, which has been reported at a loss of ml/ min/ 1.73 m 2 per decade (150, 151). Water reabsorption across the nephron tubule is mediated by various aquaporin water channel proteins. Aquaporin channels are expressed at specific sections of the nephron tubule, namely the proximal tubule, the descending limb of the loop of Henle, and in response to vasopressin (a.k.a. antidiuretic hormone) at the connecting tubule, which for simplicity here is considered as part of the late distal tubule, and collecting duct. As such, water reabsorption varies along the nephron tubule resulting in regional differences in tubular flow rates (Table 1.5). Regional tubular flow rates may be important to consider in mechanistic models of active of passive tubular reabsorption. Measurements of tubular filtrate reabsorption using the micro-puncture technique can be used to infer regional tubular flow rates based on the tubular fluid to plasma concentration ratio of inulin (TF/P inulin ). This technique, which cannot be used in humans for ethical reasons, can only be used for measurements of tubular regions that are present in the cortex, immediately below the renal capsule (i.e., proximal convoluted tubule (S1 and S2), and distal convoluted tubule). Although data are readily available for rat, rodent urine is typically more concentrated than that in human, and so rat data are not included in the literature analysis in Table 1.5. However, a single micropuncture study was found using rhesus monkey (152), which was included in the analysis. Human tubular flow rates can also be inferred from differential changes in urine composition/ flow rate in response to vasopressin levels between healthy and hereditary nephrogenic diabetes insipidus patients (153, 154). While it is generally accepted that around two-thirds of filtrate is reabsorbed in the proximal convoluted tubule, it has been suggested that after accounting for the straight portion, the entire proximal tubule reabsorbs up to 90% of the tubular filtrate following glomerular filtration (155). However, this is incompatible with reports that up to 15% of filtered water can appear in the urine during excessive water diuresis, when the distal tubule and collecting duct regions of the nephron become impermeable to water (154). Further values of human tubular filtrate reabsorption have been reported in uncited secondary sources, anecdotal assumed values used for calculations without citation of source, as well as values used as inputs for mathematical models of fluid transport in kidney and values generated by such models (Table 1.5). The micro-puncture technique can be used to study regional differences in tubular filtrate ph; studies in rat indicate that the urine (ph 6.1) is more acidic than the proximal tubule filtrate (ph 6.7) in control conditions, but each of these can vary under different pathophysiological states such as acidosis (156). 31
32 Table 1.5 Literature survey of tubular water reabsorption along nephron tubule Fraction water reabsorption Comments Type of source/ Methods Reference PT LoH DT DT+CD a CD Calculated from TF/P inulin Micropuncture; Rhesus monkey (152) Observations during maximal water diuresis/ NDI (157) PT/LoH: assumed; CD: difference between presence and absence of vasopressin (154) Cited Smith 1947 Book (158) DT and CD ranges: water diuresis and antidiuresis Simulation using model of human renal medulla (159) Mathematical model (160) Cited Uttamsingh et al 1985 Mathematical model (153) (0.66 PCT; 0.25 PST) Review article (155) Book chapter (161) MechKiM; Cited Pitts 1974 and Hall 2010 Book chapter; PBPK model (78) Ranges a Some studies published a single value or range for the DT and CD in combination. The value for the minimal model was calculated by addition of values for DT and CD. NDI nephrogenic diabetes insipidus, PCT proximal convoluted tubule, PST proximal straight tubule 32
33 1.4.3 Nephron number Nephron number becomes a particularly important parameter of kidney models when drawing inferences or scaling data to the level of whole kidney based on that of single nephrons. For example the tubular surface area of a single average nephron can be used in conjunction with nephron number to estimate the total tubular surface area in the whole kidney. Nephron number has been extensively studied through the measurement of glomerular number (118, 119, 162). While there are about 900,000 nephrons per human kidney on average, large inter-individual variability exist in human nephron number (ranging from 210,000 to 2,702,000; (162)). Factors such as age, kidney weight and birth weight have been suggested as covariates, although such findings are generally inconsistent between studies (119, 162, 163). The collecting duct may be considered, from the perspective of embryonic origin, distinct from the nephron proper (164). The collecting ducts form a branched tubular structure in the inner medulla, and each cortical collecting duct accepts the filtrate from several distal tubules (165). Therefore there is a drastic reduction in the number of distinct tubules between the beginning of the cortical collecting ducts (approx. 90,000 per kidney) and the ducts of Bellini (approx. 250 per kidney) Tubule dimensions and surface area Tubular surface area, including regional differences, is an important consideration for mechanistic models of tubular reabsorption, and is used to calculate scaling factors for IVIVE of apparent permeability data from in vitro monolayer assays (Table 1.3) (71). The nephron tubules consist of structurally different sections which are specifically adapted for various functional roles, reflected by a variety of different cell types (Figure 1.2). As such, factors which determine the surface area of any given section, such as tubule diameter and tubule length, will vary between tubule sections and between the same sections of different nephrons. In addition to the length, diameter and number of nephrons, the presence of plasma membrane structures such as microvilli, microplicae and basolateral infolding will impact the effective surface area for a given region of the nephron. Measurements on the dimensions of the nephron tubule are typically made either using histological analysis of the kidney, or following the isolation of tubule sections which may be used for in vitro experiments. Literature analysis of the length and diameter of the different regions of the nephron tubule shows that data are generally sparse, and reported values typically vary within and between studies (Tables 1.6 and 1.7). As an example, reported values of the length and diameter of the human proximal tubule range from mm and µm, respectively. These parameters may be dependent on inter-individual factors; proximal tubule length appears to be age dependent, increasing during childhood and early adulthood, and declining after around years old (117). 33
34 Table 1.6 Literature survey of tubular section diameters. Mean values, with ranges in square parenthesis. Tubule diameter (µm) Comments Type of source/ Methods Reference PT LoH DT CD [50 65] [14 22] [20 50] 200 Outer diameters; CD is at terminal end (ducts of Bellini) [ ] [28 53] [51 60] - PT: Convoluted Book (158) Maceration in HCl; 5 tubules from 1 kidney (166) Luminal diameter Morphometric analysis (167) [ ] [ ] [ ] [ ] Outer diameter; CD is for cortical collecting duct and outer medulla collecting duct. Maceration in HCl (168) MechKiM; Cited Pitts 1974 and Hall 2010 Book chapter; PBPK model (78) [50 300] Diameter of CD changes with distance from apex of renal pyramid, in agreement with two cited microscopy studies Urography (169) References not cited Model of human renal medulla (159) - 22 [14 50] [25 35] Model of human renal medulla (170) LoH is for thick ascending limb only; CD is range of cortical (25 µm) and medullary (35 µm) portions Ranges Model of human nephron (171) a Diameter of collecting ducts changes between cortex and outer medulla (50 µm), and the inner medulla. Within the inner medulla, as the collecting ducts progress toward the apex, number of collecting ducts reduces, while the diameter of the collecting ducts increase (from 40 to 200 µm). 34
35 Table 1.7 Literature survey of tubular section lengths. Mean values, with ranges in square parenthesis. Tubule length (mm) PT LoH DT CD [12 24] [6 32] [2 9] 22 Comments Type of source/ Methods Reference LoH: Thin limbs 0 14 mm; Thick ascending limbs Total length exc. CD is mm Book (158) 19.7 [ ] Maceration in HCl; 104 tubules from 3 kidneys (172) [ ] [ ] [ ] - Adult data and complete tubule sections only. LoH: Thin limbs mm; Thick ascending limbs mm. Maceration in HCl; 5 tubules from 1 kidney (166) 14.0 [ ] [ ] - LoH: Thin limbs mm; Thick ascending limbs 9.0 mm. Total length exc. CD is mm Peter, K. (1909); data reported as comparator Average for age range years old Maceration in HCl (117) LoH is for thick ascending limb only Microdissection by collagenase digestion (168) MechKiM; Cited Pitts 1974 and Hall 2010 Book chapter; PBPK model (78) (166) References not cited; Medulla only CD: Cortical CD 10 mm; Medullary CD 10 mm; Papillary CD 2 mm. Width of outer medulla 10 mm; width of inner medulla 10 mm 20 [5 30] [19 21] LoH is for thick ascending limb only; CD is combination of cortical (10 mm) and medullary (15 mm) portions Cortex (6 8 mm), outer medulla (2 mm) and inner medulla (13 mm) Model of human renal medulla (159) Model of human nephron (173) Model of human renal medulla Model of renal cortex and medulla (170) (174) Model of human nephron (171) Estimated thickness of kidney section thicknesses (175) Ranges a Collecting ducts separated into three sub-sections, with lengths of 8, 2 and 11 mm for the cortex, outer medulla and inner medulla respectively (175). 35
36 1.4.5 Proximal tubule cell number The proximal tubule cell number is used as a systems parameter in PBPK kidney models, and could be used as a scaling factor for IVIVE of in vitro data from cell based assays (Table 1.3 and Figure 1.1). An estimate of the proximal tubule cell number in human kidney is currently not reported in the literature. Data exist in preclinical species, where a single study reported a mean number of rat proximal tubule cells of 92 million cells, with a corresponding mean kidney weight of 0.99 g (176). Stereology, which has been used for counting glomeruli number in kidney, is proposed as a suitable method for measuring absolute numbers of proximal tubule cells (177). In the absence of directly measured values, human proximal tubule cell number can be inferred indirectly using relevant data from disparate literature sources (see appendix, section for full details). Calculated values range from 30.2 to million proximal tubule cells per gram kidney, in agreement with a report that 70 million cells, primarily of proximal tubule origin, can be isolated from 1 g of human renal cortical tissue (178). These calculated values are based on numerous assumptions, and should therefore be treated as approximations Microsomal and cytosolic protein content of kidney The amount of microsomal and cytosolic protein in an organ are used as scaling factors for IVIVE of in vitro metabolism data generated in the relevant sub-cellular fractions (179). In the kidney, these scaling factors are the microsomal protein per gram kidney (MPPGK) and cytosolic protein per gram kidney (CPPGK). Summary of the four studies reporting microsomal recovery in kidney microsomes are summarised in Table 1.8. There is over 5-fold difference between the highest and lowest study averages (180, 181), although data from the two most recent studies are in closer agreement (60, 182). The highest MPPGK value is reported for cortex (180), and this may be attributable to high amount of endoplasmic reticulum in cortex compared to medulla (60). Due to the low number of subjects (total of 23) and methodological differences (cortex vs mixed kidney or unspecified), it is challenging to assess the impact of potential covariates (e.g., age and gender) on the kidney microsomal protein content, as previously reported for liver microsomal protein (183). No data currently exist for human CPPGK (28). A key challenge for measuring the microsomal and cytosolic protein contents of kidney is the selection of appropriate markers to estimate protein recoveries. CYP content and NADPH cytochrome c reductase (NCR) activity are the predominantly used microsomal protein markers for liver (184). However, CYP content and NCR activity in kidney microsomes are approximately one tenth and one third of that in liver microsomes respectively, which is generally consistent across species (185). As such CYP content is not typically used as a microsomal protein marker in kidney, with NCR and glucose-6-phosphatase (G6Pase) activity being preferred (Table 1.8). Although predominantly associated with the endoplasmic reticulum, which is the predominant component of the microsomal fraction, some studies suggest that CYP, NCR and G6Pase are each also localised to some extent to the nuclear envelope, mitochondria and/ or Golgi apparatus ( ) (NB. Golgi membranes are components of microsomal fractions under typical preparation conditions (189)). However, the non-microsomal components constitute a minor proportion of the total cellular content for these markers. Therefore although the markers are known not to be purely microsomal, this is expected to have very minor impact on estimates of 36
37 microsomal protein recoveries. Few studies have aimed to critically assess cytosolic protein markers and their suitability for estimating the cytosolic protein content of liver (190), whereas no studies have reported assessment of CPPGK. Cytosolic protein markers investigated for liver are alcohol dehydrogenase (ADH) and glutathione-s-transferase (190). 37
38 Table 1.8 Summary of literature reported values for Human MPPGK and corresponding MPPGL Study Mean MPPGK ± SD Donor number (Age ± SD) [Number male] Kidney tissue source Kidney region Fresh/ frozen tissue Marker for correction factor Microsomal Preparation Centrifugation Stages MPPGL ± SD (Donor age ± SD [n]) Knights et al. (2016) (60) 9.3 ± (68.6 ± 10.7) [3] Tumour Mixed (cortex & medulla) Not specified NADPH cytochrome c reductase 10000g, g (191) NA Al-Jahdari et al. (2006) (182) 12.8 ± (67.2 ± 12.3) [4] Renal tumour Region not specified Frozen NADPH cytochrome c reductase 9000g (192) NA Pacifici et al. (1988) (181) 5.3 ± (53 ± 16.0) [2] Not Specified Region not specified Frozen None specified, protein content measured 9000g, g 32.3 ± 2.3 (36 ± 7 [6]) * Jakobsson and Cinti (1973) (180) 32.0 ± (52.8 ± 18.2) [5] Post Mortem Cortex Fresh Glucose-6- phosphatase 12000g, g NA Weighted mean ± SD 13.6 ± 11.0 (59.4 ± 16.6) *Predicted MPGGL for a 36 year-old is 39.4 mg protein/ g liver, using model proposed by Barter et al. (2008). 38
39 1.4.7 Amount of specific drug metabolising enzymes in kidney Drug metabolising enzyme abundance and expression data can be used as systems parameters in PBPK models, to consider inter-individual variabilities in drug metabolism. Expression and activity data suggest that UGT1A9 and UGT2B7 are the major UGT enzymes in human kidney, with UGT1A9 expressed at levels close to or higher than those measured in liver (193, 194). These findings are in agreement with the majority of quantitative abundance data from commercially available pooled human kidney microsomes, acquired using targeted liquid chromatography with tandem mass spectrometry (LC-MS/MS) proteomics methods (34, 40, 59). Large variability in both mrna expression (72-85% CV, n=11) and protein abundance (76-159% CV, n=10) has been noted for UGT1A6, 1A9 and 2B7 in kidney homogenates prepared from unspecified regions of healthy kidney (39). Lower expression and abundances were observed in tumoral kidney homogenates, although variabilities were similar to those in healthy kidney (39). Lower variabilities in UGT1A6, 1A9 and 2B7 abundance were observed in human kidney cortex (48-61% CV, n=5), mixed kidney (30-46% CV, n=5) and kidney medulla microsomes (32-44% CV, n=5) (60). The differences in the two studies could be due to the low number of individual samples, which may not be sufficient to accurately determine inter-individual variability, or contribution of technical variabilities which were not assessed in either study. Absolute abundance data generated using targeted LC-MS/MS can vary between studies and laboratories (195); therefore, future work is required to assess inter-laboratory variability and to facilitate standardisation of proteomics methods Amount of specific drug transporters in kidney PBPK kidney models can include systems parameters for transporter expression and abundances, including inter-individual variability, for simulation of renal secretion and active reabsorption. Transporter expression and abundance data in kidney may be used to determine REF scalars for IVIVE of in vitro data generated using transporter-transfected cell lines (Table 1.3). Several studies have measured the mrna expression of the kidney drug transporters e.g., (3, 91, 98). The overall trend suggests substantial inter-individual variabilities in the expression of drug transporters, consistent with limited available functional activity data (91). Quantitative proteomics transporter abundance data are available for human organs such as intestine liver and brain ( ), as well as rat kidney (200), but data for human kidney are lacking. Although data are available concerning renal developmental patterns of transporters in rodent species, minimal data are available for human (201). LC-MS/MS methods are currently favoured for measuring transporter abundance measurements in tissue homogenates and sub-cellular fractions due to the high precision and ability to assess inter-individual and inter-study variability in transporter expression (197). Complimentary technologies such as matrix-assisted laser desorption/ionization (MALDI)-imaging mass spectrometry, secondary ion mass spectrometry and flow cytometry ( ) may allow quantitative analysis of transporter localisation at the tissue and/ or sub-cellular scales (e.g., total protein vs. functional protein) to be possible in the future. However, the mass spectrometry-based methods require much further development before analysis of large proteins such as drug transporters is possible (202). 39
40 1.5 Use of models for studying pharmacokinetics in kidney: Current status and IVIVE opportunities IVIVE and models of renal drug metabolism As discussed above, the lack of suitable clinical data is a particular limitation for assessment of IVIVE based predictions of renal drug metabolism. However, several examples of IVIVE of human kidney microsomal metabolism data have been reported, mostly within the last decade (Figure 1.3). The scaling of in vitro microsomal metabolism data using the microsomal protein content as IVIVE scaling factor is widely used for scaling of hepatic metabolism data (179). This approach has been adopted for the IVIVE of renal drug metabolism. Chronological data shown in Figure 1.3 illustrate that until 2006, microsomal protein content measured in human liver, rat liver and rat kidney were used for IVIVE of human kidney microsomal metabolism data. The most commonly used MPPGK value of 12.8 mg/ g kidney is substantially lower than the values reported for human liver (182, 183). Due to a lack of CPPGK data, the combination of the human MPPGK and the liver cytosolic scaling factor has been applied for the IVIVE of renal hydrolysis clearance data generated in S9 fractions (28). Enzyme abundances were found to be between 7- to 11-fold and 19- to 43-fold higher in recombinant expression systems than in human kidney microsomes for UGT1A9 and UGT2B7 respectively (40, 59, 60). These data suggest that for calculation of the appropriate relative expression factor (REF) values for IVIVE, enzyme abundance data should be collected for every batch of recombinantly-expressed UGT. In addition, the presence of inactive forms of recombinant UGTs in insect cell expression systems may invalidate the assumption of proportional abundanceactivity relationship, which REF-based scaling relies upon (60, 61). These considerations, along with currently high costs and technical challenges associated with quantitative proteomics, suggest that a more pragmatic approach is to determine relative activity factors (RAF) using selective probe substrates, which have been suggested for UGT1A9 (e.g., propofol) and UGT2B7 (e.g., 3 -azidothymidine) (62, 63, 205). Assumption of the perfusion rate-limited kidney models, analogous to those traditionally used for hepatic metabolism, have been applied for IVIVE of renal metabolic clearance (38, 60, 182, 205). There is a trend for under-prediction of overall glucuronidation clearance using IVIVE, even when both hepatic and renal glucuronidation are accounted for (38, 60). Use of the well-stirred model may bias IVIVE predictions for renal metabolism (38). Although no suitable kidney-specific perfusion rate-limiting model has yet been proposed as an alternative, kidney specific permeability-rate-limiting mechanistic models of drug metabolism, suitable for investigating excretion-metabolism interplay of drugs and metabolites, have been reported (54, 206). In such models, the impact of co-localised expression (in the cortex or medulla, or particular tubular region) of relevant enzymes and transporters could be an important consideration. Therefore although mixed kidney microsomes (containing both medulla and cortex) are proposed as suitable when using the well-stirred model (60), kidney cortex microsomes, as well as cortex specific MPPGK, could be more appropriate for certain IVIVE and modelling strategies. 40
41 Figure 1.3 Chronological presentation of literature reporting experimental measurement of the microsomal protein content in kidney (open boxes) and liver (shaded boxes) for human (blue boxes) and rat (red boxes) (lower section), as well as studies using some of these data to inform the MPPGK scaling factor in order to perform IVIVE of human kidney microsomal metabolism data (upper section). Arrows indicate the cited source of microsomal protein content data used to inform the MPPGK scaling factor for each study. References: (17, 28, 38, 60, , 205, ). 41
42 PBPK models allow for simulation of plasma concentration-time profiles, although few examples are currently available for renal drug metabolism. Direct IVIVE scaling of renal, intestinal and hepatic metabolism of propofol, in combination with perfusion-limited organ models, resulted in under-prediction of metabolic clearance, and required empirical scaling factors to describe the observed concentration-time profiles (216) IVIVE and modelling of tubular excretion of drugs Filtration clearance can be predicted from the glomerular filtration rate and the fraction unbound of drug in plasma (f u, p ) whereas predicting secretion and/ or reabsorption is more challenging. Static models typically consider filtration, secretion and reabsorption in isolation and as separate mechanisms contributing to overall CL R (51, 106, 218). Mechanistic models of renal drug excretion, static or dynamic, need to account for the overall anatomical organisation of the nephron. Such representation is found in the structures of models of passive renal tubular reabsorption, some of which can describe urine flow dependent CL R (67, ). A mechanistic model has recently been reported that incorporates IVIVE of passive tubular reabsorption from in vitro P app data (71). In this model, tubular surface area of the proximal tubule is used as an IVIVE scaling factor, although other regions of the nephron such as the loop of Henle and collecting duct are not considered. Various models of active secretion and reabsorption have been proposed ( ). Both active secretion and reabsorption can be described using well-stirred or parallel tube models, relating the intrinsic secretion clearance to the renal blood flow (secretion), or the tubular filtrate flow (reabsorption) (71, 218). More recently a PBPK model of active tubular reabsorption in rat was proposed that incorporated K m values obtained in vitro data, although V max parameters were estimated by fitting to in vivo data (225). Empirical optimisation of transporter activities or REF/ RAF parameters using clinical data are typically required to adequately describe clinically observed CL R (79, 226). However, one study found that REF scaling was not necessary for IVIVE (227). In this study, it was assumed that the uptake activity in 1 mg protein in transfected HEK cells was equivalent to the activity found in 1 million proximal tubule cells in vivo, although justification for this assumption was not given. Where studies have required empirical optimisation of transporter activities of REF/ RAF, uptake is often found to be the rate-limiting step in the renal tubular secretion of a drug. In these cases, which typically involve the OAT1 and OAT3 transporters, obtaining reliable estimates of the rate of efflux from clinical plasma concentration time profiles and/ or CL R data is challenging, and can render the parameter numerically non-identifiable (79, 226, 227). In order to obtain reliable parameter estimates for efflux transporters, drug concentrations in the tubular cells and/ or urinary excretion curves over short time intervals are required for optimisation (79). For uptake into kidney tissue slices, empirical correction was required for IVIVE, which was proposed to account for blood flow mediated perfusion found in vivo, but is not represented in the in vitro assay (228). With respect to cell based assays, opportunities for completely bottom-up extrapolation from in vitro data to predict renal drug disposition are currently limited by a lack of physiological data to inform relevant scaling factors, such as transporter/ enzyme abundances, 42
43 and tubule cellularity (Figure 1.1). A similar problem is encountered with detailed PBPK models of kidney, which may require numerous assumptions on systems parameters. Therefore, reduced, semi-physiological and/ or static physiological models may provide a pragmatic method for predicting renal drug-drug interactions (DDI), nonlinear pharmacokinetics and drug-disease interactions for well-defined populations Prediction of renal drug-drug interactions within PBPK paradigm Decision trees have been proposed for prediction of renal DDIs ( ) with the main aim to prioritise in vitro and clinical DDI studies, ensuring appropriate labelling requirements are met. Recent studies have reported static models (232, 233) and a PBPK kidney model (78) for prediction of transporter mediated renal DDIs. By using the same transporter kinetics parameters as those reported for the PBPK models (79, 226, 227), AUC ratios for renal transporter DDIs with 5 victim drugs were predicted well using the static model (232). AUC ratio predictions were generally in agreement with predictions based on the PBPK approach for three inhibitors investigated, with neither approach providing a clear advantage over the other (Figure 1.4 and Table 1.9). The analysis highlights that if kidney is not relevant with respect to pharmacology or drug toxicity, static models may be sufficient for assessing clinical relevance of renal DDIs (232, 233). The advantages of PBPK kidney models are more apparent when simulating complex DDIs involving multiple organs and mechanisms, including metabolism-transport interplay. Examples include the possibility of simultaneous inhibition (or induction) of both uptake and efflux transport (79, 226, 227), and potential contribution of renally-formed metabolites as perpetrators, as proposed for the DDI between NSAIDs and methotrexate (234). The relative importance and potential impact of assumptions made on the model structure should be considered, in addition to the confidence in the experimental data which inform model parameters. Sensitivity analyses may be used to assess the impact of individual parameters on the CL R, AUC and intracellular and tubular concentrations of the victim drug. Further, where a perpetrator drug shows permeability-limited pharmacokinetic properties, relevant perpetrator concentrations should be used, for example intracellular concentrations for inhibition of efflux transporters, and filtrate concentrations for inhibition of apical uptake transporters. 43
44 Figure 1.4 Comparison of predictions of AUC ratio for renal uptake transporter mediated DDIs with observed data (226, 235). AUC ratios were predicted using the static model (232), or taken from published predictions using PBPK model (79, 226, 227). Full details are listed in Table
45 Table 1.9 Calculation of AUC ratio for renal transporter mediated DDIs using static model, and comparison with published predictions using PBPK model and observed values. See Figure 1.4 for summary. PBPK Study Posada et al, 2015 (79) Hsu et al, 2014 (226) Hsu et al, 2014 (226) Hsu et al, 2014 (226) Li et al, 2014 (227) Victim Drug Pemetrexed Oseltamavir carboxylate Cidofivir Cefuroxime Veliparib CL (L/h) 5.6 (235) 19 (226) 12.8 (226) 11 (226) 34.0 * f e a 0.8 (235) 1 * 0.89 * 1 * 0.7 (236) CL R (L/ h) a 4.48 * 19 (226) 11.4 (226) 11 (226) 23.8 (236) f u,p,victim 0.19 (235) 0.97 (226) 0.9 (226) 0.67 (226) 0.49 (236) CL R,filt (L/ h) b CL Rsec (L/ h) b Perpetrator drug (transporter) Ibuprofen (OAT3) Probenecid (OAT3) Probenecid (OAT3) Probenecid (OAT3) Cimetidine (OCT2) Inhibitor C max (µm) (237) (238) e (238) 9.4 (239) f u,p, inhibitor (226) 0.1 (226) 0.1 (226) 0.81 (240) Inhibitor C max,u (µm) 1.6 (235) 28.1 f 52.1 f 24.4 f 7.6 f K i (µm) 2.1 (235) (226) g (226) g (226) g (227, 241) h DDI index c AUC Ratio Static (232) PBPK d Observed 1.2 (235) 2.5 (226) 1.3 (226) 1.4 (226) ND a Data for plasma or IV clearance (CL), renal clearance (CL R ) or fraction of drug recovered unchanged in urine (f e ) were collated where available. Where data not available, values were calculated assuming f e = CL R / CL, indicated by *; b Filtration clearance (CL R,filt ) calculated assuming glomerular filtration rate of 7.2 L/ h. Secretion clearance (CL R,sec ) calculated assuming negligible reabsorption (CL R,sec = CL R CL R,filt ); c DDI index is calculated as C max,u / K i ; d data extracted from figures using GetData graph digitiser where necessary ( e C max of probenecid after 2 g SD; f Inhibitor C max,u calculated using reported C max and f u,p ; g Range of K i values represents sensitivity analysis performed in PBPK study (226). In vitro K i values range from µm (226, 241); h OCT2 K i of cimetidine SimCYP default compound file is 0.25 µm, which was obtained by fitting to recover clinically observed transporter mediated DDIs. Reported cimetidine IC 50 values for OCT2 range from µm (241); ND - Clinical DDI study not reported 45
46 1.5.4 Assessing dosage adjustment in chronic kidney disease Population pharmacokinetic models can be used to investigate the covariate relationship between kidney function (e.g., creatinine clearance) and pharmacokinetic parameters for a drug, and devising appropriate dose recommendations (242). Such dosage adjustments are typically based on either estimated glomerular filtration rate (egfr), calculated using the modification of diet in renal disease (MDRD) equation, or creatinine clearance (CL cr ), which can be measured but is typically calculated using the Cockcroft-Gault (C-G) equation (243). The limitations and differences between methods for assessing renal function, and implications for pharmacokinetics, are subject to wider research activities and ongoing debates (244, 245). When determining recommended dose adjustments in renally impaired patients for labelling, latest guidance suggests considering whether egfr or CL cr are assessed as categorical or continuous variables, and whether recommendations are based on predefined categories of renal function, or on pharmacokinetics and exposure-response outcomes (243, 246). Lack of relevant physiological data undermines the use of current kidney PBPK models for investigating pharmacokinetic changes in kidney disease. Collating these data is challenging, especially because patients may exhibit co-morbidities that could be involved in kidney disease initiation/ progression, or independently change certain physiological parameters (e.g., kidney size, as shown in Table 1.4) (242, 247, 248). Recent attempts to standardise definitions and classifications of chronic kidney disease and acute kidney injury may confound the collation and categorisation of physiological data taken from historical scientific literature (249, 250). Further, chronic kidney disease has various susceptibility and initiation factors which may be relevant for pharmacokinetics (251). For example kidney damage can occur without changes in GFR, due to compensation by hyper-filtration in the remaining functional nephrons, which may be inferred from albuminuria (252). Studies that have been reported to-date are restricted to investigating impact of CL R following changes in GFR and plasma protein binding in renally-impaired patients (226, 227, 253). However, CL R may reduce linearly with GFR for many drugs, even when secretion or reabsorption contribute, in accordance with the intact nephron hypothesis (254, 255). For mechanistic kidney models, accounting for reduced tubular secretion in a physiological manner is necessary (226). Other PBPK efforts have focused on understanding effects of renal impairment on non-renal elimination, with system parameters typically estimated by back-calculation of clinical data (256). 1.6 Perspective on current efforts Opportunities to make use of IVIVE and mechanistic models of renal drug disposition are found in various situations when assessing the impact of patient factors such as DDIs, genetic polymorphisms, renal impairment and understanding of drug-induced kidney injury. Systems data represent an essential component for allowing such models to be used quantitatively. Ongoing work aimed at measurement of protein abundances for renal drug metabolising enzymes and drug transporters remains a high priority. Abundance data from large cohorts of individuals will allow for co-variates and protein-protein correlations to be established, while assessing the impact of 46
47 particular demographic features such as age and renal impairment which will be challenged by sample availability in particular patient groups. Using suitable techniques to quantify differences in protein abundance in different regions of the nephron, such as the convoluted vs. straight portion of proximal tubule would allow for more detailed models to be used (78, 225). For some systems data that can be measured in human and preclinical species, such as blood flow, microsomal protein recoveries, proximal tubule cellularity and protein abundances, species differences can be established. For other physiological features such as regional tubular filtrate flow rates and ph, where direct access to the intact, functional kidney is required for measurements, preclinical data may be used as surrogate for systems parameters in models of human kidney. The uncertainties around parameters such as filtrate flow rate and ph may eventually limit the level of complexity that can be built into mechanistic models of human kidney (or the level of certainty in absolute values of systems parameters in complex models). This particularly applies when comparing to published models of rat kidney, which have incorporated features such as exponential decline in proximal tubule filtrate flow and compliant tubules (114). Despite uncertainties around relevant human kidney systems parameters, several modelling efforts have attempted to account for some of the various complexities of kidney biology (78, 219, 225). A recent study demonstrated that accounting for electrochemical gradients on OCT2-mediated secretion could be important for assessment of DDIs involving OCT and MATE transporters (257). The importance of accounting for the impact of urine ph on proton gradient dependent drug transport by MATE1 and MATE2-K, as described in vitro (258), should also be assessed. The development and use of appropriate functions to describe drug transport process kinetics, which refine or replace the widely used but potentially inappropriate Michaelis-Menten model, represents opportunities to improve mechanistic models of kidney drug disposition. Ongoing work will likely result in new and improved IVIVE approaches for quantitative predictions of CL R and renal metabolism. In cases where prediction of pharmacokinetic parameters alone may be sufficient (e.g., drug discovery and pre-clinical development), static models will be favoured for IVIVE due to computational simplicity and low data requirements. Conversely, PBPK models enable prediction of pharmacokinetic profiles, with the additional advantage of allowing simulation of tissue and intracellular drug concentrations in a dynamic manner. Mechanistic understanding of the dynamics of renal drug disposition, especially intracellular concentrations, can help to improve the translation of animal nephrotoxicity studies, by allowing for species differences in renal blood flow, drug transporters (e.g., MATE2-K; Table 1.2) and drug metabolising enzymes, including differences in protein abundances, to be accounted for. To complement the modelling and simulation, imaging approaches enable measurement of tissue and intracellular drug concentrations in kidney. Available tools for studying intracellular concentrations have previously been reviewed for liver and are in principle applicable to kidney (259, 260). In particular, techniques such as fluorescence confocal microscopy and imaging mass spectrometry enable assessment of intracellular drug concentrations in tissue following animal in vivo studies (89, 90). For clinical development, positron emission tomography (PET) imaging may be a useful tool, although spatial resolution is at the mm level and metabolites are indistinguishable from parent drug using this technique, which could confound interpretation of such data (261). 47
48 PBPK models also allow simulation of complex DDIs, such as those involving metabolismtransporter interplay and/ or multiple organs. For example some metabolites, such as acylglucuronides, may be unstable in plasma. In these cases local formation of metabolite in kidney, aside from possibility of pharmacology/ toxicity effects, could lead to DDIs which may not be expected from plasma concentrations alone. PBPK models also offer opportunities to simulate different drug dosing regimens, guiding the design of future clinical studies and informing product labelling, or simulate effects in population groups for which clinical studies are challenging or impossible because of practical and/ or ethical issues (46, 227, 229). For the reasons above, there is currently a lot of interest in developing pediatric PBPK models (262), as well as PBPK models of renal impairment (227). As discussed above, during the progression of certain kidney diseases, renal physiological changes (e.g., kidney size, number of nephrons or proximal tubule cells) are not always reflected by apparent changes in function when assessed using certain biomarkers (e.g., GFR and creatinine clearance) (252). This may be important for understanding potential changes in drug concentrations in kidney due to different causes/ stages of disease, thereby indicating potential subjects at risk of drug-induced kidney injury. Further research is needed to assess whether consideration of these extra levels of complexity are necessary and/ or advantageous. 1.7 Conclusion In addition to the requisite clinical data, IVIVE approaches depend on both high quality in vitro data and sufficient knowledge of human physiology. Various in vitro systems of kidney drug elimination are available, but generally lack the level of development and characterisation obtained for hepatic in vitro systems and corresponding models. Pre-existing mechanistic models of kidney are currently under-used for IVIVE approaches, despite covering a wide range of application areas and often being simple to implement. PBPK kidney models are gaining increasing attention within academia and industry, particularly because of their potential for prediction of complex and/ or unstudied clinical situations (DDIs, renal impairment, transportmetabolism interplay). However, there is a conspicuous need for additional physiological data to inform IVIVE scaling factors and relevant system parameters of complex PBPK models. Validation of the models may also necessitate inclusion of elements within clinical studies which are not routinely considered during drug development. For instance, understanding the dynamics of drug disposition in kidney will be facilitated by more frequent monitoring of the drug concentrations in the urine as opposed to a single integral recovery of the drug in the urine over long period of time. 48
49 1.8 Aims of project The assessment of the literature described above highlighted several areas in which quantitative data on kidney anatomy and physiology are currently either sparse or lacking. This was addressed by the first aim of the thesis, as described in Chapter 2, which was to measure the microsomal and cytosolic protein content the microsomal and cytosolic protein content of kidney samples from human and dog. The microsomal protein content of dog kidney cortex was experimentally assessed using CYP content and G6Pase activity as microsomal protein recovery markers, allowing for critical comparison of data generated using different markers. In addition, the impact of using fresh and frozen kidney tissue on MPPGK estimates was investigated, using CYP content to measure the microsomal protein recovery factor. In addition, microsomal content of dog liver was measured in matched samples (i.e., from the same animals), allowing for direct comparison of MPPGK and microsomal protein per gram liver (MPPGL). Comparison of the MPPGK and MPPGL with microsomal protein content data in the matched dog intestinal samples was also performed (data from Oliver Hatley). Following the dog studies, microsomal protein content of human kidney cortex from 31 donors was measured using G6Pase activity as the recovery marker. The CPPGK was also measured for these kidney samples, using GST activity as cytosolic protein recovery marker, which included accounting for the contribution of microsomal GST enzyme to the activity measured in kidney homogenates. In addition to estimation of MPPGK and CPPGK, the functional activity of the prepared human kidney microsomes was investigated in 13 donors. Mycophenolic acid, a probe substrate for UGT1A9, was selected as a marker, and CL int,ugt,hkm were obtained using the substrate depletion method. The in vitro microsomal CL int,ugt,hkm data were used to assess the impact of different scaling factors on the scaled intrinsic clearance (per g kidney) and the predicted in vivo glucuronidation clearance. The different IVIVE scenarios were (a) scaling the in vitro data using either the estimates of MPPGK obtained in the current study or the literature value commonly used for IVIVE, and (b) assuming that either the whole kidney or only the kidney cortex contributes to renal mycophenolic acid glucuronidation. The next aim, as described in Chapter 3, was to investigate the suitability and feasibility of methods for measuring proximal tubule cellularity in human kidney. A novel approach for estimation of proximal tubule cellularity of whole kidney, based on stereological estimation of cellularity in a selectively sampled piece of kidney, was explored. Sampling and histology protocols were developed, to allow validation of the stereology-based approach using porcine kidney. Two different staining approaches that might enable unambiguous identification of proximal tubule cells in histology sections of human and porcine kidney were investigated. These staining approaches were a tinctorial stain (Periodic acid Schiff - Orange G) and immunohistochemical staining for villin. The aim of Chapter 4 was to develop a model for prediction of tubular reabsorption, and subsequently CL R, using IVIVE. The literature analysis above revealed models of passive tubular reabsorption of drugs have been reported that are (a) mechanistic, (b) account for relevant physiological complexities of the kidney such as regional differences in the nephron tubule, (c) incorporate IVIVE, and/ or (d) relevant to human. However, a model that incorporates all of these features is currently lacking. A static mechanistic model for predicting extent of passive tubular 49
50 reabsorption from in vitro Caco-2 permeability data and tubular physiological parameters was developed. The model used regional tubular surface areas as IVIVE scaling factors. Data acquired from the literature analysis above was used to inform the tubular surface area (Tables 1.6 and 1.7) and tubular flow rate (Table 1.5) parameters of the model. From a database of in vivo CL R values for 157 drugs collated from the literature, a subset of 45 selected drugs, for which filtration or reabsorption were dominant, was used to assess the model. In vitro permeability data generated in Caco-2 cell monolayer under ph gradient conditions was used to predict the CL R for these 45 drugs using the mechanistic model. An empirical calibration approach, which accounts for the impact of inter-assay and inter-laboratory variability of in vitro permeability measurements on the prediction of CL R using the model, was proposed. This empirical calibration approach was assessed using 11 reference drugs (internal dataset). The ability of the static mechanistic model of tubular reabsorption to predict urine flow dependent CL R was also assessed. The final aim, as described in Chapter 5 and Chapter 6, was to employ the dynamic mechanistic kidney model currently implemented in the SimCYP simulator (MechKiM). The focus was on (a) modification of the model to implement mechanistic IVIVE for tubular reabsorption, (b) simulating the impact of urine flow on CL R, and (c) prediction of CL R in renal impairment using digoxin as an example, due to the abundance of clinical data. The system parameters and scaling factors of the static reabsorption model were adapted and incorporated into a PBPK kidney model and used to predict the CL R of three drugs undergoing different levels of tubular reabsorption (Chapter 5). Urine flow dependent CL R of these drugs was then simulated and compared to observed data where available. A PBPK kidney model for digoxin was developed that accounted for filtration, secretion and reabsorption processes (Chapter 6). Sensitivity analysis was used to determine the parameters which were most important to optimise in order to simulate digoxin CL R in healthy subjects. Following optimisation of the relevant parameters, the pharmacokinetics of digoxin was simulated in geriatric, moderate renal impairment and severe renal impairment populations, initially assuming that no changes in renal secretion occur in these populations. The impact of reducing different system parameters (proximal tubule cellularity or kidney transporter abundances) of the PBPK kidney model on simulated digoxin CL R and proximal tubule cell concentrations was then compared. Recommendations were made on future research, which should aim to continue quantifying physiological features of kidney relevant to renal drug disposition. 50
51 Chapter 2. Measurement of microsomal and cytosolic protein kidney and in vitro-in vivo extrapolation of renal metabolic clearance 2.1 Introduction In vitro drug metabolism data obtained in enriched preparations of sub-cellular fractions such as microsomes or cytosol are commonly scaled using IVIVE to predict clearance in vivo (28, 38, 263). These scaling approaches rely upon robust estimates of physiologically relevant scaling factors, including the protein content of the sub-cellular fraction in the tissue or organ of interest. Although such scaling factors have been well characterised in liver (e.g., MPPGL and cytosolic protein per gram liver (CPPGL)) for human and several preclinical species (179, 184, 190, 264), less data have been reported for extrahepatic tissues such as kidney. Notably, data are completely lacking for MPPGK and CPPGK in preclinical species. Although several studies have reported microsomal protein yields for rat kidney (Figure 1.3), with some studies also reporting the corresponding data for liver, none of these clearly stated that the protein recovery was estimated and accounted for. MPPGK values (study means) for human range from 5.3 to 32.0 mg/ g kidney, based on 4 literature reports and a total of 23 donors (Table 1.8), although the value of 12.8 mg/ g kidney, based on 5 donors, is most commonly used in the literature for IVIVE of renal drug metabolism data (Figure 1.3). There were several differences between the design of these studies which may have contributed to the differences between the reported MPPGK values, including selection of microsomal protein marker and region of kidney used (e.g., cortex, mixed or unspecified). As such, assessment of true biological variability in MPPGK is not currently possible based on the available literature data. In addition, no studies were found reporting CPPGK in human, and so an estimate of cytosolic protein content of liver is currently used as a surrogate when a value is needed for IVIVE (28, 38). Both inter-individual variability (e.g., impact of age, gender, ethnicity and disease states) (183, 265) and intra-individual variability (e.g. circadian rhythms and diet) (266) may contribute to overall biological variability in drug metabolising enzyme expression. Genetic polymorphisms are a key source of inter-individual variability in the function and expression levels of drug metabolising enzymes and transporters ( ). The expression levels of drug metabolising enzymes and transporters are regulated by various transcription factors (e.g., pregnane X receptor, hepatocyte nuclear factors (HNF)) ( ). Transcription factors may determine expression of these proteins, contributing to the inter-correlations observed within protein families (197, 274, 275). While baseline transcription factor expression will vary between organs/ tissues, expression can also be modulated across organs by circulating substances (e.g., inflammatory cytokines, bile acids and uremic toxins) (276, 277). Factors such as inflammation, infection, cancer, and kidney and liver disease may alter the levels of these circulating substances and therefore modulate enzyme and transporter expression (276, 278, 279). As such, expression levels of a specific protein in multiple individuals may correlate between different organs; however, assessing such correlation requires access to the relevant matched tissue samples. 51
52 Assessment of microsomal or cytosolic protein contents depends on the use of markers of the sub-cellular fraction of interest. Such markers are used quantitatively to correct for protein losses during centrifugation. CYP content is frequently used as a marker for liver microsomal protein (183) but may not be suitable for kidney, due to the lower CYP content of kidney (207, 280); therefore alternative markers such as G6Pase and NCR activity are preferred for kidney microsomes (Table 1.8). The activities of glutathione-s-transferase (GST) and alcohol dehydrogenase (ADH) reported in the literature were used to estimate the cytosolic protein content of human liver (190). However, a more thorough assessment of the suitability of these enzymes as cytosolic protein markers for kidney is currently lacking Aims The aim of this study was to characterise the microsomal and cytosolic protein content, as well as the functional activity of kidney samples from human and dog. Microsomal protein content of dog kidney cortex and liver was characterised, and the impact of using CYP content and G6Pase activity as markers to measure microsomal protein recovery was assessed for both tissues. In addition, the impact of using fresh and frozen tissue to prepare dog kidney homogenates and microsomes on subsequent CYP content measurements and MPPGK estimates were assessed. Microsomal protein recovery in liver, kidney and intestine was compared using matched samples (MPPGI data were provided by Dr Oliver Hatley). MPPGK was then characterised for 31 human kidney cortex samples using G6Pase activity as the recovery marker (demographics information such as age and gender available for 13 donors allowing investigation of potential covariates of MPPGK). The functional activity of human kidney microsomes from 13 donors and a single commercially acquired pool were characterised using a mycophenolic acid glucuronidation substrate depletion assay. The mycophenolic acid unbound intrinsic clearance by glucuronidation in human kidney microsomes (CL int,u,ugt,hkm ) data were scaled by both historical MPPGK and the newly acquired MPPGK data to assess the impact on predicted renal metabolic clearance. To preserve human samples, suitability of ADH activity and GST activity as potential cytosolic protein markers for estimating cytosolic protein recoveries were investigated using rat kidney. Subsequently GST activity was used as the cytosolic protein marker, following correction for microsomal GST, to characterise human CPPGK for the first time in 31 kidney cortex samples. 52
53 2.2 Methods Isolation of microsomal protein from dog kidney Reagents Chemicals were purchased from Sigma-Aldrich (Gillingham, Dorset, UK), unless otherwise specified. Homogenisation buffer was PBS with 0.5 mm EDTA, 5 mm histidine and 0.25 M sucrose, ph 7.4. Storage buffer was 100 mm Trizma with 0.5 mm EDTA in deionised water, ph 7.4. CYP assay buffer was 25 mm potassium phosphate buffer ph 7.4 with 1.5 % w/v potassium chloride and 30 % v/v glycerol (Fisher Scientific, Loughborough, UK). Glucose-6-phosphatase (G6Pase) assay buffer was 100 mm BIS-TRIS, ph 6.5. Taussky-Shorr colour reagent (TSCR) (281) was 0.18 M ferrous sulphate heptahydrate, 1 % w/v ammonium molybdate in 0.5 M sulphuric acid Sample collection and perfusion Kidneys and livers from 19 beagle dogs were obtained from necropsy at AstraZeneca (Alderley Park, Macclesfield, UK), according to institutional guidelines in compliance with national and regional legislation. Livers were transferred to the lab in PBS buffer on ice; kidneys were transferred in PBS buffer containing 9 U/ ml heparin on ice. Kidneys were perfused with PBS containing 9 U/ ml heparin at 37 C at 8 ml/ min for 15 min through the renal artery. All subsequent processes were performed on ice unless specified. Kidneys were cut in half, decapsulated, and each kidney half was blotted to remove excess liquid and weighed. Kidney halves from one kidney were frozen at -80 C, with the other kidney used to prepare homogenate. Pieces of liver (approx g) were washed in PBS buffer, weighed, and frozen at -80 C Dog homogenate and microsome preparation Homogenisation and centrifugation methods used for preparation of kidney microsomes vary, but generally follow the same core strategy that involves an initial centrifugation of homogenate at around g to remove cellular debris and larger organelles, followed by ultracentrifugation of the resulting supernatant at g to obtain the microsomal protein pellet (appendix, Figure 8.1). The method applied in the current study, which was consistent with this core strategy, was based on the in-house method developed by Dr Oliver Hatley for the intestine, with modifications to optimise homogenisation of kidney. For samples stored at -80 C, frozen dog tissue samples were rapidly thawed at 37 C, washed in PBS, blotted and weighed. Kidney cortex ( g) and liver ( g) were minced with scissors and homogenised with 4-5 ml/ g mince of homogenisation buffer. Homogenisation was initially with a rotor-stator homogeniser (Omni International, Kennesaw, GA) with a 10 mm x 95 mm probe. Bursts of 20s with 30s rest on ice were used, until no intact pieces of kidney mince were apparent upon visual assessment. The number of bursts for each sample depended on the starting weight of the minced tissue, but required no more than 8 bursts for kidney and 4 bursts for liver. Samples were further homogenised using a VibraCell ultrasonic processor (Sonics & Materials, Inc., Newtown, CT) for two bursts of 10s, separated with a 30 s resting period on ice to prevent excessive heat building up. Homogenate was filtered through 170 µm nylon mesh 53
54 (Plastok Associates, Birkenhead, Merseyside, UK). Homogenate volumes were measured, and aliquots stored on ice for analysis. Liver and kidney homogenates were centrifuged at 9000 g at 4 C for 15 min using an Optima LE-80K ultracentrifuge with a Type 50.2Ti rotor (Beckman Coulter (UK) Ltd., High Wycombe, Buckinghamshire, UK). Supernatants were further centrifuged at g at 4 C for 70 min. Aliquots of the cytosol were retained. The microsomal pellet was resuspended in storage buffer using a handheld Potter-Elvehjem homogeniser. Samples were stored at -80 C Microsomal protein markers in dog samples Frozen samples were thawed rapidly at room temperature, and kept on ice until used (282). Protein in homogenate, microsomes and cytosol was determined using a Micro bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology #23227), following manufacturer s instructions. Absorbance (562 nm) was measured with a Tecan Safire microplate reader with XFluor4 software (Reading, Berkshire, UK). Results were processed with Microsoft Excel. Cytochrome P450 (CYP) content of homogenate and microsomal samples were measured following the dithionite difference spectroscopy method of Matsubara et al. (283). Samples were diluted to 2 mg/ ml in CYP assay buffer and bubbled (approx. 1 bubble per second) for 1 min with carbon monoxide. 1 ml of diluted samples were then dispensed into each of two semi-micro cuvettes (VWR, Pennsylvania, USA), and baseline absorbance spectrum measured ( nm) using a UV-2401-PC dual beam spectrophotometer with UVPC software (Shimadzu, Milton Keynes, Buckinghamshire, UK). 10 µl of freshly prepared sodium dithionite (200 mg/ ml in CYP assay buffer) was added to the sample cuvette. The sample cuvette was inverted 4 times, left to stand for 4 min, and then the absorbance spectrum measured. Cytochrome P450 content (nmol/ mg protein) was calculated using a molar extinction coefficient (A ) of (283). Various endogenous contaminants such as methaemoglobin, cytochrome b 5, and cytochrome oxidase can potentially interfere with CYP content measurements in microsomal samples ( ). Preliminary experiments were therefore performed to investigate the effect of chemically reducing these contaminants on CYP content measurements and subsequent calculation of MPPG. CYP content measurements using kidney homogenate and microsomes were taken with succinate (5 mm), NADH (0.05 mm) or ascorbic acid and phenazine ethosulfate (0.25 mm and 2.5 µm respectively) added to the CYP assay buffer, and compared with measurements in the standard buffer. Glucose-6-phosphatase activity was measured in duplicate using a spectrophotometric method (287). Homogenate and microsomal protein and glucose-6-phosphate (G6P) were pre-incubated separately in G6Pase assay buffer at 37 C for 10 min. Homogenate and microsomes (0.25 mg/ ml) were added to the G6P (1 mm) to initiate the reaction, and an aliquot immediately quenched (3:1) in 20% trichloroacetic acid (TCA) on ice (t=0 min). Further aliquots were quenched at 5, 15, 30 and 60 min. Following centrifugation at 4000 rpm for 10 min, samples and phosphorous standards were added in 1:1 ratio to TSCR. Absorbance (660 nm) was measured with a Tecan Safire platereader with XFluor4 software. Results were processed with Microsoft Excel. G6Pase activity was expressed as nmol inorganic phosphate (P i ) formed/ min/ mg protein based on the initial linear rate of P i formation. 54
55 2.2.2 Isolation of microsomal and cytosolic protein from human and rat kidney Reagents XenoTech mixed gender pooled (13 donors) human kidney microsomes (lot ; 4- methylumbelliferone glucuronidation activity of 105 nmol/ min/ mg protein) were obtained from Tebu-bio (Peterborough, Cambs, UK). Chemicals were purchased from Sigma-Aldrich (Gillingham, Dorset, UK), unless otherwise specified. Homogenisation buffer was 25 mm Trizma, 0.5 mm EDTA, 5 mm histidine, 0.25 M sucrose, ph 7.4. Trizma was used as alternative to PBS to reduce background signal in G6Pase assay. Storage buffer, G6Pase assay buffer and Taussky- Shorr colour reagent were prepared as described above for the dog. Mycophenolic acid glucuronidation assay buffer was 0.1 M phosphate buffer containing 3.45 mm MgCl 2, 1.15 mm EDTA and 115 µm saccharic acid lactone Sample collection and storage Normal human kidney cortex pieces from nephrectomy patients (n=13), excised from the pole of the kidney contralateral to the tumour site, were obtained from the Biobank, Central Manchester University Hospitals NHS Foundation Trust (CMFT), UK. Kidney cortex pieces were snap frozen within 1 h of excision (Jay Brown, personal communication), and stored at -80 C. Ethical approval for this research was obtained from NRES Committee London - Camberwell St Giles (REC ref. 13/LO/1896), with samples stored under Human Tissue Authority licence. Frozen human kidney cortex homogenates (n=18) were generously provided by Dr Colin Brown, Newcastle University (Newcastle, UK). These homogenates were prepared from renal cortex from healthy kidneys unsuitable for transplant. Homogenates from Newcastle University were stored at -80 C until used. No information on the time delay between organ isolation and storage was available. Rat kidneys were obtained from 250 to 300 g Sprague-Dawley rats (Charles River, Margate, Kent, UK), and after a brief rinse in PBS buffer, were stored at -80 C until used Homogenate and microsomal preparation A single batch of homogenate and microsomes were prepared for each donor, with the exception of donor B1246, for which an initial batch was prepared for use in preliminary experiments; data generated during preliminary experiments were not included in analyses of the main dataset. Frozen human kidney samples were rapidly thawed at 37 C, washed in PBS, blotted dry and weighed. Finely minced human kidney cortex samples ( g) or whole rat kidneys ( g) were homogenised with 4-5 ml/ g mince of homogenisation buffer. Homogenisation was initially with a rotor-stator homogeniser (Dremel UK, Middlesex, UK). Bursts of 20s with 30s rest on ice were used, until no intact pieces of kidney mince were apparent upon visual assessment. This typically required 3 6 bursts, depending on the starting weight of kidney mince. Samples were further homogenised using an Omni Ruptor 400 Ultrasonic homogeniser (Omni International, Kennesaw, GA) for two bursts of 10 s, separated with a 30 s resting period on ice. Homogenate was filtered through 170 µm nylon mesh (Plastok Associates). Homogenates from Newcastle 55
56 University were thawed rapidly at 37 C and then kept on ice until use. Total kidney homogenate volumes were measured, and aliquots stored on ice for analysis. Human kidney homogenates were centrifuged at 9000 g at 4 C for 15 min using an Optima TLX- 120 Ultracentrifuge with an MLA-80 rotor (Beckman Coulter (UK) Ltd). After removing aliquots for analysis (1-2 ml, stored on ice), supernatants (S9) were further centrifuged at g at 4 C for 70 min. Aliquots of the cytosol were stored on ice for analysis. The microsomal pellet was resuspended in storage buffer using a vortex mixer and pipette. Aliquots were taken for protein content analysis; remaining microsomal samples were stored at -80 C Microsomal and cytosolic protein markers in human samples On the day of microsomal preparation, protein content in homogenate, S9, microsomes and cytosol was determined in triplicate using a Micro BCA protein assay kit (Pierce Biotechnology #23227), following manufacturer s instructions. Absorbance (562 nm) was measured with a SpectraMax 190 platereader (Molecular Devices, Sunnyvale, CA), with bovine serum albumin used as calibration standard. All activity assays were performed on samples which had undergone 4 or less freeze thaw cycles. Glucose-6-phosphatase activity was measured in duplicate using the spectrophotometric method described above for the dog samples; absorbance (660 nm) was measured with a SpectraMax 190 platereader. Glutathione-S-transferase (GST) activity was measured in human and rat kidney homogenate, microsome and cytosol samples using an assay kit (Sigma #CS0410) following manufacturer s instructions with the following modification; samples were initially prepared in 0.1 M sodium phosphate buffer ph 6.5 with 1% Triton X-100 (288), due to inadequate volume of sample buffer provided with the assay kit. GST activity was measured using protein concentrations of 10 µg/ ml (determined following preliminary optimisation experiments using rat kidney samples), with substrate concentrations of 100 and 200 µm for 1-Chloro-2,4-dinitrobenzene (CDNB) and L- glutathione (GSH) respectively. Absorbance (340 nm) was measured at appropriate timepoints up to 10 min using SpectraMax 190 platereader. Results were processed with Microsoft Excel. GST activity was expressed as nmol/ min/ mg protein based on the initial linear rate of A340, using an extinction coefficient ( A 340 ) of 9.6 mm -1 cm -1 for CDNB conjugate. Alcohol dehydrogenase (ADH) activity was measured in rat kidney homogenate, microsomes and cytosol samples using an assay kit (Sigma #MAK053) following manufacturer s instructions. NADH formation was assessed using ethanol (0.2 M) as substrate at protein concentrations ranging mg/ ml. NADH concentrations were measured at using a SpectraMax 190 platereader (450 nm) at timepoints up to 60 min. ADH activity was expressed as nmol/ min/ mg protein based on the initial linear NADH formation rate Mycophenolic acid glucuronidation depletion assay in human kidney microsomes Mycophenolic acid was selected as a clinically relevant marker to assess the metabolic activity of the human kidney microsomes, and investigate the variability of UGT activity within the kidney samples. Mycophenolic acid has previously been shown to undergo renal glucuronidation in vitro in human liver and kidney microsomes (38, 289), with UGT1A9 the major isoform involved in the renal metabolism, and UGT2B7 having a lesser role (289). Microsomal glucuronidation substrate 56
57 depletion intrinsic clearance assays were performed as previously reported (38), with 1 ml incubations performed in triplicate with a single no-cofactor control. Due to low availability of microsomal protein, only one replicate for each donor was performed. The assay was also performed in XenoTech pooled human kidney microsomes. Human kidney microsomes (0.25 mg/ ml) were activated by pre-incubation with 50 µg/ mg protein alamethicin in assay buffer for 15 min on ice. Mycophenolic acid (assay concentration 1 µm) was pre-incubated with alamethicinactivated microsomes and bovine serum albumin (BSA; assay concentration 1%) for 5 min in assay buffer at 37 C shaking at 900 rpm (Eppendorf thermomixer (Hamburg, Germany)). Reaction was initiated by addition of uridine-diphosphate-glucuronic acid (UDPGA; assay concentration 5 mm). Following incubation at 37 C with shaking at 900 rpm, aliquots of the incubation mixture were quenched in two volumes of ice cold acetonitrile containing 1 µm warfarin (internal standard) at appropriate time-points between 0 and 60 min inclusive. There was minimal depletion of mycophenolic acid after 60 min at 0.25 mg/ ml for one sample (B1140), and therefore a modified assay, with protein concentration of 0.5 mg/ ml and time-points extended to 90 min, was used for this donor. Quenched samples were stored at -20 C for at least 1 h, then centrifuged at 9000 rpm for 20 min. Aliquots of supernatant were analysed by liquid chromatography-mass spectrometry (LC-MS/MS) for mycophenolic acid concentration using matrix-matched calibration standards (0-5 µm). To preserve individual donor human kidney microsome samples, XenoTech pooled human kidney microsomes were used for preparing calibration standards. LC-MS/MS analysis was performed using an Agilent 1100 HPLC system (Stockport, Cheshire, UK) coupled to a Micromass Quattro Ultima triple quadruple mass spectrometer (Waters, Elstree, Hertfordshire, UK). Liquid chromatography was performed using a Luna C18 (3 µ, 50 x 4.6 mm) column (Phenomenex, Torrance, USA) and a flow rate of 1 ml/ min; the elution gradient is listed in the appendix, Table 8.4. The retention times of mycophenolic acid and warfarin were 4.21 and 4.49 min respectively. For mass spectrometry, source temperature, desolvation temperature, desolvation gas flow rate, cone gas flow rate and capillary voltage were 125 C, 350 C, 600 L/ h, 50 L/ h and 3.5 kv respectively. Selective reaction monitoring (SRM) of mycophenolic acid and warfarin with negative electrospray ionisation was performed; SRM transitions of precursor to product ions (m/z) were for mycophenolic acid and for warfarin. Cone voltage and collision voltage were 90 V and 25 ev for mycophenolic acid, and 130 V and 19 ev for warfarin, respectively Estimation of microsomal and cytosolic protein contents of tissues Various parameters (Table 2.1) including yields of total protein and microsomal marker in subcellular fractions from a microsomal preparation, as well as the recovery factor of the microsomal protein were calculated (Eq ). This recovery factor was used to calculate MPPGK (Eq. 2.6). In addition, a microsomal or cytosolic protein enrichment factor was calculated (Eq. 2.7) 57
58 Table 2.1 Parameters used in calculation of MPPGK and CPPGK from human and dog kidney samples Parameter Description Units Abs_Prot x [Prot] x V x, total V x, aliquot Absolute protein yield in homogenate or subfraction (x) Protein concentration of homogenate or subfraction (x) Volume of homogenate or sub-fraction (x), before aliquots are taken for analysis where applicable Volume of homogenate or sub-fraction aliquot taken for analysis mg mg/ ml ml ml Marker Kid Marker x Activity or content of subcellular protein marker in kidney tissue Activity or content of subcellular protein marker in homogenate, microsomes or cytosol (x) W Kid Weight of starting kidney tissue mince g nmol/ g kidney (CYP) nmol/ min/ g kidney (G6Pase) nmol/ min/ g kidney (GST) nmol/ mg protein (CYP) nmol/ min/ mg protein (G6Pase) nmol/ min/ mg protein (GST) Yield Marker, theor Yield Marker, actual Theoretical Yield of subcellular protein marker from homogenate, accounting for aliquot removal Actual Yield of subcellular protein marker from homogenate nmol (CYP) nmol/ min (G6Pase) nmol/ min (GST) nmol (CYP) nmol/ min (G6Pase) nmol/ min (GST) Recovery X Percent recovery % Enrichment x Mic_ Prot Hom Mic_GST Hom Yield GST,theor,corrected Enrichment factor of subcellular protein (x) Amount of microsomal protein in the homogenate, based on starting tissue weight and the MPPGK. Activity of GST in the homogenate which was attributable to microsomal isoform(s) Theoretical cytosolic GST activity yield. The GST activity yield in the homogenate that was attributed to the cytosolic fraction (i.e. corrected for the microsomal GST activity) mg nmol/ min nmol/ min S9_contribution Hom Theoretical percent contribution of the microsomal protein and cytosolic protein (i.e. S9 fraction) to overall protein in homogenate % Where x represents either homogenate (Hom), 9000g supernatant (S9) or microsomes (Mic). 58
59 Abs_Prot x = [Prot] x V x, total 2.1 Marker Kid = Marker Hom Abs_Prot Hom W Kid 2.2 Yield Marker, Theor = Marker Hom [Prot] Hom V Hom, total - V Hom, aliquot V Hom, total V S9, total - V S9, aliquot V S9, total 2.3 Yield Marker, Actual = Marker x Abs_Prot x V Mic, total 2.4 Recovery x = Yield Marker, actual Yield Marker, theor 2.5 MPPGK = Abs_Prot Mic Recovery Mic W Kid 2.6 Enrichment x = Marker x Marker hom 2.7 Analogous equations can be used to calculate the cytosolic protein recovery and cytosolic protein per gram kidney (CPPGK) through use of cytosolic protein markers. In the case of GST, although the predominant isoforms are found in the cytosolic fraction, some GSTs are also found in the endoplasmic reticulum component of the microsomal fraction (280, 290). In the current study, GST activity was noted in the microsomal fraction from rat and human kidney (see results section 2.3.1). Therefore, MPPGK for each human kidney donor, estimated using G6Pase activity as microsomal protein marker, was used to account for the GST activity attributable to the microsomal GST in each human kidney homogenate (Eq ). This corrected GST activity yield in homogenate was then compared with the GST activity yield in the cytosolic fraction to account for cytosolic protein losses during the fractionation procedure, and subsequently CPPGK (Eq and 2.12). To ensure that the estimates of MPPGK and CPPGK were physiologically feasible, their combined value was compared with the amount of homogenate protein obtained per gram of kidney for each donor. The combined value should reflect the S9 protein content per gram kidney. Therefore, the value calculated was expressed as the percent contribution of the S9 fraction to overall protein in the homogenate (Eq. 2.13). Mic_Prot Hom = MPPGK W Kid 2.8 Mic_GST Hom = Mic_Prot Hom GST Mic 2.9 Yield GST, Theor,corrected = Yield GST, Theor - Mic_GST Hom 2.10 Recovery Cyt = Yield GST, actual Yield GST, theor 2.11 CPPGK = Abs_Prot Cyt Recovery Cyt W Kid 2.12 S9_contribution Hom = MPPGK + CPPGK 100 (%) Abs_Prot Hom WKid
60 2.2.4 Prediction of mycophenolic acid glucuronidation clearance in vivo Human kidney microsomal intrinsic clearance (CL int,ugt,hkm ; µl/ min/ mg microsomal protein) for mycophenolic acid was calculated from the elimination rate constant (k; min -1 ) and the microsomal protein concentration of the incubation (mg/ ml) using Eq. 2.14; k was calculated from the slope of the linear correlation of the natural log-fraction remaining (average of triplicate incubations at each time-point) versus time. In vitro CL int,ugt,hkm data for each donor were corrected for the fraction unbound in the incubation (f u,inc ; 0.18 at all microsomal protein concentrations, obtained in the presence of BSA, as previously reported (38)) to calculate the unbound intrinsic clearance (CL int,u,ugt,hkm ). CL int,u,ugt,hkm data were scaled (to units ml/ min/ g kidney) using MPPGK with two approaches: (a) scaling to total kidney; (b) scaling to kidney cortex. Scaled CL int,u,ugt,hkm were then scaled by either kidney weight (assumed to be 4.5 g/ kg body weight; (38)) or by kidney cortex weight (approx. 68% of kidney weight, 3.1 g/ kg body weight (291)) and in vivo mycophenolic acid renal glucuronidation clearance (CL R,met,UGT ) was predicted using the wellstirred model, assuming renal- or cortex- blood flow (Q R ) of 16.4 or 13.2 ml/ min / kg respectively, fraction unbound in plasma (f u,p ) of 0.01, and blood to plasma concentration ratio (R B ) of 0.6 (38), using Eq Predicted mycophenolic acid overall glucuronidation clearance was calculated as the sum of the renal and hepatic (CL h,met,ugt ) glucuronidation clearances (Eq. 2.16). CL h,met,ugt was calculated as for CL R,met,UGT using Eq. 2.15, using scaled CL int,u,ugt,hlm of 9.32 ml/ min/ g liver, also obtained in the presence of BSA, as reported in Gill et al (38). MPPGL was 40 mg/ g liver, liver weight was 21.4 g/ kg body weight, and hepatic blood flow (Q h ) was 20.7 ml/ min/ kg, as previously reported (38). Observed mycophenolic acid CL UGT was 3.97 ml/ min/ kg. This value was calculated by Gill et al (38), based on a plasma i.v. clearance of 2.49 ml/ min/ kg which was corrected for the renal excretion (0.01 ml/ min/ kg) and fraction metabolised by UGT (f m,ugt ; 0.95). CL int,ugt,hkm = k V amount of microsomal protein in incubation 2.14 CL R,met,UGT = Q R f u,p R B CL int,u,ugt,hkm Q R + f u,p R B CL int,u,ugt,hkm 2.15 CL UGT = CL h,met, UGT + CL R,met,UGT Data Analysis CYP content and MPPGI data for 14 dog intestinal samples were provided by Dr Oliver Hatley. These data were obtained from different regions of the intestine, with each region being defined as one sixth of the entire intestine by length. The initial three regions were defined as proximal 1, 2 and 3, while the final region was defined as distal. Average (mean) values were calculated, with variability estimated using the coefficient of variation (CV; %). Inter-assay variability (%) was estimated as the average between-assay CV for each set of samples. Data were analysed using MS Excel. Student s t-test (paired, 2-tailed) was used to statistically compare means; P<0.05 was considered significant; the unpaired t-test was used for comparison of CYP content in homogenates prepared from fresh and frozen kidney, due to differences in the number of samples in each group. 60
61 Absorbance units (AU) 2.3 Results Characterisation and optimisation of protein marker assays CYP content assay The sodium dithionite difference spectra approach was used to measure CYP content in dog kidney, as reported in the literature (283). Compared with the liver, A450 signal was generally weak in kidney, but sufficient for quantification. In addition to the absorbance peak at 450 nm, broad peaks were observed at approximately 426 nm in homogenate, and 430 nm in microsomes, which may have interfered with the A450 measurement, and therefore affect CYP content measurement and MPPGK estimates. Literature analysis revealed that dithionite reduction of cytochrome b 5, cytochrome oxidase and methaemoglobin could cause absorbance peaks within the nm range. Inclusion of sodium ascorbate and phenazine ethosulfate, reported to reduce methaemoglobin (284), did not cause a change in the dithionite difference spectra for dog kidney microsomes, although a small shift in the 426 nm peak to 430 nm was noted (data not shown). However this appeared to have minimal impact on the quantification of CYP content. Inclusion of NADH and sodium succinate, which are reported to reduce cytochrome b 5 and cytochrome oxidase (284, 285), in the CYP content assay buffer caused a change in the spectra of homogenate (approx nm) and microsomes (approx nm; Figure 2.1). As no major change in baseline or peak at 450 nm was observed, neither the CYP measurements in homogenate and microsome samples, nor the estimates of MPPGK, were affected. Therefore, the sodium dithionite difference spectra assay as reported in the literature, i.e., without modification of buffer constituents, was considered sufficient for estimation of MPPGK in dog Wavelength (nm) Figure 2.1 Representative UV/ Vis absorbance spectra from dithionite difference assay in dog kidney homogenate and microsomes. Buffer was modified to reduce cytochrome b 5, cytochrome oxidase by inclusion of NADH and sodium succinate. Lines represent homogenate with normal ( ) and modified buffer ( ), and microsome with normal ( ) and modified buffer ( ). Data are the mean of duplicate measurements from a single experiment. 61
62 CYP content (nmol/ mg protein) An indication of inter-assay variability of CYP content measurements was obtained following repeat (n=2 or n=3) measurements in four batches of homogenates and microsomes from three dogs (i.e., two batches prepared from same dog). On average, the inter-assay variability of CYP content was 10% and 5% for homogenate and microsomes respectively, and 14% for the calculated microsomal protein enrichment factor. Based on data from one dog for which two separate batches of microsomes were prepared, the inter-assay variability in CYP content measurement was similar to the apparent inter-batch variability (Figure 2.2). This trend was also noted for the calculated CYP content enrichment factor (approx. 12% variability for inter-batch and inter-assay) Homogenate Batch 1 Homogenate Batch 2 Microsomes Batch 1 Microsomes Batch Assay 1 Assay 2 Assay 3 Assay 4 Figure 2.2 Inter-assay variability of CYP content measurements was similar to the interbatch variability in paired homogenate and microsomes prepared from kidney tissue of a single dog. Each bar represents the mean of two measurements from a single CYP content experiment in a single batch of homogenate or microsomes. For assay 4, measured CYP content varied by 13% and 1% between the two batches of homogenate and microsomes respectively G6Pase activity assay Dog kidney G6Pase activity appeared to be linear with respect to protein concentration in both homogenate and microsomes, but not directly proportional (i.e. intercept 0; appendix, Figure 8.2). Activity could not be reliably quantified at the lower protein concentrations (0.1 and 0.05 mg/ ml) for homogenate. The resultant microsomal protein recovery factors calculated for each assay protein concentration did not appear to show protein dependency. Therefore G6Pase activity was considered a suitable marker to estimate microsomal protein losses. Inter-assay variability of G6Pase activity was assessed initially in dog kidney homogenate and microsomes. G6Pase activity was measured in a single set of samples prepared from kidneys of three different dogs in three separate assays. The average inter-assay variability (CV) of G6Pase activity was 20.6% and 19.8% for homogenate and microsomes respectively. G6Pase activity enrichment factor interassay variability was estimated to be 14% by re-calculation for each assay and each dog. 62
63 G6Pase activity (nmol/ min/ mg protein) For a single human kidney sample (donor B1246), homogenate and microsomes were prepared in two separate batches on different days. For each batch, G6Pase activity was measured in two separate assays, with one of these assays common for both batches. Based on these preliminary data, the inter-assay variability of G6Pase assay appeared to be greater than the inter-batch variability (Figure 2.3). Considering all four batches of human kidney homogenate and microsomes, from 3 kidney samples, for which G6Pase activity was measured twice, the average inter-assay variability in G6Pase activity was 15% and 19% for homogenate and microsomes respectively. This resulted in an average inter-assay variability of 18% for the calculated G6Pase activity enrichment factor (range 3% - 39%) Homogenate Batch 1 Homogenate Batch 2 Microsomes Batch 1 Microsomes Batch 2 Assay 1 Assay 2 Assay 3 Figure 2.3 Inter-assay variability of G6Pase activity was greater than the inter-batch variability in paired homogenate and microsomes prepared from a single human kidney donor. Each bar represents the mean of three incubations from a single G6Pase activity experiment in a single batch of homogenate or microsomes GST and ADH activity assay Preliminary experiments with rat kidney homogenate and cytosol were performed to assess suitability of ADH activity as a marker of cytosolic protein losses during fractionation. The data quality from the assay (attempted twice) was generally sub-optimal. ADH activity appeared to be non-linear with respect to protein, resulting in estimates of cytosolic protein recovery which varied depending on the protein concentration used in the ADH activity assay (appendix, Figure 8.3). Due to these two factors (sub-optimal data and non-linearity) ADH activity was not selected as a suitable cytosolic protein marker. Rat kidney GST activity appeared to be non-linear with respect to protein concentration in both homogenate and cytosol (data presented in appendix, Figure 8.4). GST activity could be reliably quantified at the lower protein concentrations (2.5 and 5 µg/ ml), albeit with lower reproducibility in homogenate. As cytosolic protein recovery could not be assessed with these data (see below for correction of activity attributable to microsomal GST isoform), estimated enrichment factor of 63
64 GST activity (nmol/ min) cytosolic protein (ratio of GST activity in cytosol: GST activity in homogenate) at each protein concentration was assessed. Assay protein concentration did not appear to affect the apparent enrichment factor (appendix, Figure 8.4). As microsomal GST isoforms have been reported (290), a single exploratory assay was used to investigate whether the microsomal GST activity could potentially affect the estimation of human kidney cytosolic protein recovery using GST activity as a marker. Substantial GST activity was noted in human kidney microsomes, suggesting that GST activity in human kidney homogenate was attributable to both cytosolic and microsomal isoforms (Figure 2.4). Therefore, when estimating cytosolic protein recovery using GST activity, the enzyme activity in homogenate attributable to microsomal isoforms was accounted for by making use of the independently (different protein marker) estimated MPPGK values (Eq ) HKH HKM HKC Figure 2.4 GST activity in human kidney homogenate, microsomes and cytosol from donor B1246 Protein concentrations were 50 µg/ ml, which were higher than typically used (10 µg/ ml) to ensure detection of potential GST activity in microsomal fraction. Mean and standard deviation (error bars) of data from three incubations in a single experiment are shown. HKH Human kidney homogenate; HKM Human kidney microsomes; HKC Human kidney cytosol Estimation of microsomal protein content in dog kidney cortex and liver and comparison with intestine Liver and kidney samples were obtained from a total of 17 dogs, with an age range of years, and weights of kg. Liver weights were g, while kidney weights were g. Average CYP content in dog kidney homogenate prepared from frozen kidney tissue was nmol/ mg protein (n=17), which was significantly lower (P<0.05) than that in homogenate prepared from fresh kidney tissue (0.056 nmol/ mg protein; n=14) (Table 2.2). Both CYP content and G6Pase activity were statistically significantly lower (P<0.05) in dog kidney compared with corresponding livers (considering samples prepared from frozen tissue only). Mean 64
65 CYP content for dog kidney microsomes was over 3-fold higher than for intestinal microsomes (considering samples prepared from fresh tissue only). No trends were apparent in the CYP content or G6Pase activity between liver and kidney, following visual assessment of the data. Mean MPPGK in dog kidney was 43.1 mg/ g kidney when CYP content was used as microsomal protein marker and samples were prepared from fresh kidney (Table 2.2), with individual values ranging mg/ g kidney. This was on average 27% higher than the corresponding value when samples were prepared from frozen kidney. Individual values of MPPGK, MPPGL and MPPGI are listed in the appendix, Table 8.5. MPPGK was on average 18% or 31% lower than MPPGL when CYP content or G6Pase activity were used as microsomal protein marker (Table 2.2), although this difference varied between dogs; i.e., there was no apparent correlation in MPPGK and MPPGL (Figure 2.5). Both MPPGL and MPPGK were consistently greater than MPPGI for all regions of intestine studied, with no trends apparent, either when considering data for each region separately, or the data for all intestinal regions collectively. No clear trends between either MPPGL or MPPGK and factors such as age or dog weight were apparent (data not shown). Dog microsomal protein content was lower when using CYP content than when using G6Pase activity as microsomal marker, by 23% for MPPGK and 35% for MPPGL (Table 2.2). Bland-Altman plots show the 95% confidence interval for the mean difference between the markers do not overlap with the line of unity (difference = 0), suggesting systematic bias (Figure 2.6). 65
66 Table 2.2 CYP content, G6Pase activity and MPPG measured in homogenate and microsomal samples prepared from fresh dog kidney, frozen dog kidney and frozen dog liver. Average values are presented, with CVs in parentheses. G6Pase activity was not measured in samples prepared from fresh dog kidney. Data for individual dogs are presented in the appendix, Table 8.5. CYP content (nmol/ mg protein) G6Pase activity (nmol/ min/ mg protein) MPPG (mg/ g tissue) Homogenate Microsomes Homogenate Microsomes CYP content G6Pase activity Fresh tissue (n=14) Dog kidney (24%) (23%) Not measured Not measured 43.1 (22%) Not measured Dog Intestine a Data not available (27%) Not measured Not measured 6.5 (61%) Not measured Frozen tissue (n=17) Dog kidney (16%) (15%) 19.9 (16%) 62.1 (16%) 33.9 (18%) 44.0 (16%) Dog liver (19%) (20%) 23.8 (15%) 91.2 (18%) 41.1 (12%) 63.6 (18%) a Data for dog intestine were kindly provided by Dr Oliver Hatley, and represent data pooled from several intestinal regions. 66
67 MPPGL (mg/ g liver) MPPGK (mg/ g kidney) Figure 2.5 Comparison of MPPGK and MPPGL in dog (n = 17 dogs) using either CYP content ( ) or G6Pase activity ( ) as the microsomal protein marker. Each point represents microsomal scalar measured using a single batch of homogenates and microsomes from a single dog. 67
68 Difference: MPPGL (CYP) - MPPGL (G6Pase) Difference: MPPGK (CYP) - MPPGK (G6Pase) A Average of CYP and G6Pase MPPGK measurements (mg/ g) 10 0 B Average of CYP and G6Pase MPPGL measurements (mg/ g) Figure 2.6 Bland-Altman plots: Difference in MPPG measured using CYP content vs. G6Pase activity as microsomal protein marker. Points on graphs represent measurements made in kidney (A) or liver (B) microsome and homogenate samples. Blue lines represent mean (solid) and 95% confidence interval of mean (dashed) difference between MPPGs. Red dotted lines represent 95% limits of agreement. Thin black lines represent line of unity. 68
69 B1140 B1150 B1194 B1209 B1223 B1246 B1258 B1274 B1285 B1300 B1327 B1375 B1387 NC1 NC2 NC3 NC4 NC5 NC6 NC7 NC8 NC9 NC10 NC11 NC12 NC13 NC14 NC15 NC16 NC17 NC18 G6Pase activity (nmol/min/ mg) Estimation of MPPGK and CPPGK in human kidney cortex Average G6Pase activities of human kidney homogenate and microsomes were 8.26 and nmol/ min/ mg protein (n=31 kidney samples), with CVs of 64% and 57% respectively (Figure 2.7). The G6Pase activities were higher in samples obtained from Newcastle University (9.23 and nmol/ min/ mg protein in homogenate and microsomes respectively; n=18) compared with those obtained from CMFT Biobank (6.93 and nmol/ min/ mg protein in homogenate and microsomes respectively; n=13). Average GST activities of human kidney homogenate, microsomes and cytosol were 208, 104 and 303 nmol/ min/ mg protein respectively (n=31); CVs were between 43% and 48% (Figure 2.8). GST activities were higher in samples obtained from Newcastle University (234, 112 and 357 nmol/ min/ mg protein in homogenate, microsomes and cytosol; n=18) compared with those obtained from CMFT Biobank (172, 94 and 229 nmol/ min/ mg protein in homogenate, microsomes and cytosol respectively; n=13) Homogenate Microsomes Donor ID Figure 2.7 G6Pase activity measured in 31 human kidney homogenate and microsomes. B# and NC# indicate samples acquired from the CMFT Biobank or Newcastle University respectively. Each bar typically represent n=1 measurements per donor, although for some samples bars represent the average of n=2 measurements. Individual values are listed in the appendix, Table
70 B1140 B1150 B1194 B1209 B1223 B1246 B1258 B1274 B1258 B1300 B1327 B1375 B1387 NC1 NC2 NC3 NC4 NC5 NC6 NC7 NC8 NC9 NC10 NC11 NC12 NC13 NC14 NC15 NC16 NC17 NC18 GST activity (nmol/ min/ mg protein) Homogenate Microsome Cytosol Donor ID Figure 2.8 GST activity measured in 31 human kidney homogenate, microsomes and cytosols. B# and NC# indicate samples acquired from the CMFT Biobank or Newcastle University respectively. Each bar represents n=1 measurements per donor. Individual values are listed in the appendix, Table 8.6. Average MPPGK in human obtained from all 31 samples was 25.7 mg/ g kidney, with CV of 26% (Figure 2.9). MPPGK of samples obtained from CMFT Biobank was greater than that in samples obtained from Newcastle University (28.4 (n=13) vs 23.7 (n=18) mg/ g kidney). Observed variability of MPPGK in samples obtained from CMFT Biobank was half that of samples obtained from Newcastle University (16% (n=13) vs 32% (n=18)). Microsomal GST activity, scaled using MPPGK to units of nmol/ min/ g kidney, represented on average 15% of the GST activity yield in human kidney homogenate. Following correction for activity attributable to microsomal GST isoform(s) in the homogenate, average human CPPGK was 52.7 mg/ g kidney, with 34% CV (Figure 2.9). CPPGK of samples obtained from CMFT Biobank was greater than that in samples obtained from Newcastle University (62.6 (n=13) vs 45.5 (n=18) mg/ g kidney). There was no apparent trend between MPPGK and CPPGK (appendix, Figure 8.5). The average S9 protein per gram kidney (i.e., MPPGK + CPPGK) was 78.3, 91.0 and 69.2 mg/ g kidney for all samples (n=31), CMFT Biobank samples only (n=13) and Newcastle University samples only respectively (n=18). Theoretical contribution of the S9 protein to the protein content of homogenate was 89% on average, although the value exceeded 100% for 5 out of 31 samples (Figure 2.9). Based on 13 donors for whom demographics data were available, no trends between human MPPGK or CPPGK and factors such as age, gender and weight were found following visual analysis of the data (not shown). 70
71 B1140 B1150 B1194 B1209 B1223 B1246 B1258 B1274 B1258 B1300 B1327 B1375 B1387 NC1 NC2 NC3 NC4 NC5 NC6 NC7 NC8 NC9 NC10 NC11 NC12 NC13 NC14 NC15 NC16 NC17 NC18 mg protein/ g kidney Homogenate protein yield MPPGK CPPGK Donor ID Figure 2.9 Microsomal (MPPGK) and cytosolic (CPPGK) protein content of kidney, and homogenate protein yields, in 31 human kidney samples. Combined value of MPPGK and CPPGK in each donor represents the estimated S9 protein per gram of kidney; if physiologically plausible, this value should not exceed the homogenate protein yield. B# and NC# indicate samples acquired from the CMFT Biobank or Newcastle University respectively. Each bar represents n=1 batch of homogenate/ microsomes/ cytosol per donor. Individual values are listed in the appendix, Table In vitro glucuronidation of mycophenolic acid by human kidney microsomes and IVIVE scaling Mycophenolic acid CL int,u,ugt,hkm was measured in 13 individual human kidney microsomes and XenoTech pooled kidney microsomes. For donor B1140 an extended assay (up to 90 min) and an increased protein concentration (0.5 vs 0.25 mg/ ml in standard assay) were used (see section ). Individual depletion plots for each donor are presented in the appendix, Figure 8.6. Average CL int,u,ugt,hkm in the 13 donors was 905 µl/ min/ mg microsomal protein, with 52% CV and range µl/ min/ mg microsomal protein. This average value was 2-fold lower than that observed in commercially sourced pooled microsomes in the current study (1843 µl/ min/ mg protein) and that previously reported (1370 µl/ min/ mg protein (38)). No depletion of mycophenolic acid was observed in the no-cofactor control for any donors. There appeared to be a positive correlation between mycophenolic acid CL int,u,ugt,hkm and G6Pase activity (Figure 2.10). 71
72 G6Pase activity (nmol/ min/ mg protein) y = 0.014x R² = Mycophenolic acid CL int,u,ugt,hkm (µl/ min/ mg protein) Figure 2.10 Comparison of G6Pase activity and mycophenolic acid CL int,u,ugt,hkm for human kidney microsomes from 13 donors. Linear regression line and corresponding equation and R 2 are shown. Individual values are listed in the appendix, Table 8.6. Scaled mycophenolic acid CL int,u,ugt,hkm (per g organ weight), using either the donor-specific MPPGK or the overall average MPPGK (25.7 mg/ g kidney; n=31) measured in the current study, were on average 2.16-fold higher or 2-fold higher than when the commonly used MPPGK value (12.8 mg/ g kidney) was used for scaling (Table 2.3). These differences were reflected in the kidney: liver ratios for CL int,u,ugt, which were calculated using published data for liver, obtained using comparable in vitro assay conditions to the current study (e.g. presence of BSA; (38)) (Figure 2.11). After accounting for predicted hepatic glucuronidation (CL h,met,ugt ) and predicting CL R,met,UGT based on whole kidney weight and blood flow, predicted CL UGT values were on average 23% higher and 20% higher using individual MPPGK or overall average MPPGK values from the current study compared with when the commonly used MPPGK was applied. This had limited effect on apparent success of the IVIVE prediction for mycophenolic acid CL UGT, with predicted values all falling within 1.5-fold of the observed value (Figure 2.12). The kidney: liver ratio for predicted CL met,ugt, which was estimated using a CL h,met,ugt of 2.86 ml/ min/ kg (38), was greater when using either the donor-specific MPPGK values (kidney: liver ratio = 0.58) or the overall average MPPGK value (kidney: liver ratio = 0.54) for scaling, compared with when the previously published MPPGK of 12.8 mg/ g was used (kidney: liver ratio = 0.28) (Figure 2.13). Use of these scaling factors is associated with the assumption that the microsomes prepared in the current study were representative of the whole kidney, which is uniform with respect to activity of drug metabolising enzymes. As the microsomes were prepared from kidney cortex, scaling of CL int,u,ugt,hkm and prediction of CL R,met,UGT was repeated using adjusted kidney weight and blood flow parameters to align with an assumption that mycophenolic acid glucuronidation occurs only in the kidney cortex. Use of the cortex specific parameters resulted in a reduction of 31% for both the predicted CL R,met,UGT and the kidney: liver ratio for CL met,ugt, compared with the assumption of uniform activity throughout whole kidney (Table 2.3 and Figure 2.13). Scaled CL int,u,ugt,hkm and corresponding kidney: liver ratios for the XenoTech pooled human kidney microsomes were both 72
73 2-fold higher than the average value for the individual human kidney microsomes (Table 2.3 and Figure 2.11); this difference was propagated into the predicted CL R,met,UGT and corresponding kidney: liver ratios (Table 2.3 and Figure 2.13). For example, when scaling the in vitro CL int,u,ugt,hkm using the overall average MPPGK from all 31 donors of the current study, and predicting CL R,met,UGT using whole kidney weight and renal blood flow, kidney: liver ratio for CL met,ugt was 1.02 for the XenoTech pooled human kidney microsomes, compared to an average of 0.54 (48% CV) for the individual human kidney microsomes. 73
74 Table 2.3 Comparison of scaled mycophenolic acid CL int,u,ugt,hkm and predicted CL UGT under various assumptions. Mean values from 13 individual human kidney microsomes are shown, with CVs in parentheses, and data from XenoTech pooled human kidney microsomes in square brackets. Data for individual donors are listed in the appendix, Table 8.6. Published MPPGK + Whole kidney a, b New MPPGK (individual) New MPPGK (average) + + Whole kidney b, c Whole kidney b, d New MPPGK (individual) New MPPGK (average) + + Cortex only c, e Cortex only c, e CL int,u,ugt,hkm (µl/ min/ mg protein) (n=13) (52%) [1843] MPPGK (mg/ g kidney) (16%) (16%) 25.7 Scaled CL int,u,ugt,hkm (ml/ min/ g kidney) Kidney: Liver ratio for scaled CL int,u,ugt f Kidney weight (g/ kg body weight) f u,p g R B g 11.6 (52%) [23.6] 1.24 (52%) [1.88] 25.1 (50%) 2.70 (50%) 23.2 (52%) [47.3] 2.49 (52%) [5.08] 25.1 (50%) 2.70 (50%) 23.2 (52%) [47.3] 2.49 (52%) [5.08] 4.5 g 4.5 g 4.5 g 3.1 h 3.1 h Q R (ml/ min/ kg) 16.4 g 16.4 g 16.4 g 13.2 i 13.2 i Predicted CL R,met,UGT (ml/ min/ kg) 0.82 (49%) [1.60] Kidney: Liver ratio for predicted CL met,ugt 0.28 (49%) [0.56] Predicted CL UGT (ml/ min/ kg) j 3.68 (11%) [4.46] 1.65 (46%) 0.58 (46%) 4.52 (17%) 1.54 (48%) [2.92] 0.54 (48%) [1.02] 4.41 (17%) [5.78] 1.15 (47%) 0.40 (47%) 4.01 (13%) 1.07 (48%) [2.04] 0.37 (48%) [0.71] 3.93 (13%) [4.91] Mean Predicted/ Observed 0.93 (11%) 1.11 (17%) 0.99 (13%) k 1.14 (17%) 1.01 (13%) CL UGT [1.13] [1.46] [1.24] a Published MPPGK mg/ g (182) kidney used to scale CL int,u,ugt,hkm for all donors; b Whole kidney - kidney weight and renal blood flow used for scaling and in wellstirred model for CL R,met,UGT prediction; c New MPPGK (individual) - donor-specific MPPGK values measured in the current study (Figure 2.9) used to scale CL int,u,ugt,hkm ; d New MPPGK (average) - average MPPGK value for all 31 donors measured in the current study (Figure 2.9) used to scale CL int,u,ugt,hkm ; e Cortex only Kidney weight and renal blood flow parameters were replaced with kidney cortex weight and cortex blood flow respectively; f CL int,u,ugt,hlm was 9.32 ml/ min/ g liver, which is based on in vitro measurements in the presence of BSA (38); g (38); h Calculated by assuming that cortex comprises 68% of kidney weight (291), and kidney weight is 4.5 g/ kg (38); i Calculated by assuming that cortex blood flow is 80% of renal blood flow (291, 292), and renal blood flow is 16.4 ml/ min/ kg (38); j CL h,met,ugt (2.86 ml/ min/ kg) calculated as per (38); k Observed CL UGT was 3.96 ml/ min/ kg (38).
75 Published MPPGK New MPPGK (individual) New MPPGK (average) Kidney: liver ratio of scaled mycophenolic acid CL int,u,ugt Figure 2.11 Kidney: liver ratios of mycophenolic acid CL int,u,ugt calculated using CL int,u,ugt,hkm data obtained in individual (solid bars) or XenoTech pooled (striped bars) human kidney microsomes which were scaled either by MPPGK as previously published (green; (182)) or measured in the current study (blue); scaled CL int,u,ugt,hlm was 9.32 ml/ min/ g liver, which was estimated in comparable assay conditions (i.e. in presence of BSA) (38). Data from individual human kidney microsomes were scaled by either the corresponding MPPGK measured for each donor ( New MPPGK (individual) ), or by the overall average MPPGK of all 31 donors in the current study ( New MPPGK (average) ). Bars represent mean values; error bars represent 1 standard deviation. Predicted/ Observed CL UGT Old MPPGK + Whole kidney New MPPGK + Whole kidney New MPPGK + Cortex only Figure 2.12 Impact of different assumptions on the prediction accuracy of mycophenolic acid CL UGT. Predictions used either CL int,u,ugt,hkm from individual donor ( ) or XenoTech pooled ( ) human kidney microsomes. Scaling in the New MPPGK scenarios used the MPPGK values for each individual donor, except in the case of the XenoTech pooled human kidney microsomes, for which the overall average MPPGK from all 31 donors was used; Old MPPGK scenario involved scaling all data by an MPPGK value of 12.8 mg/ g kidney (38). Cortex only incorporated correction of kidney weight and renal blood flow to match an assumption that only the cortex contributes to renal glucuronidation, whereas Whole kidney had no such correction. See Table 2.3 for further details. Solid horizontal line represents line of unity. 75
76 Published MPPGK + Whole kidney New MPPGK (individual) + Whole kidney New MPPGK (average) + Whole kidney New MPPGK (individual) + Cortex only New MPPGK (average) + Cortex only Kidney: liver ratio of mycophenolic acid CL met,ugt Figure 2.13 Kidney: liver ratios of mycophenolic acid CL met,ugt calculated based on assumptions of either the Whole kidney contributing to glucuronidation (green and blue) or Cortex only contributing (purple) using CL int,u,ugt,hkm data obtained in individual (solid bars) or XenoTech pooled (striped bars) human kidney microsomes. Data from individual human kidney microsomes were scaled by either a published MPPGK value ( Published MPPGK ; green (182)), the MPPGK values measured in the current study ( New MPPGK ; blue and purple). Scaling with MPPGK values from the current study was either by the individual value for each donor or by the overall average MPPGK of all 31 donors (see Table 2.3 for details). Predicted CL h,met,ugt was 2.86 ml/ min/ kg, as previously reported (38). Bars represent mean values; error bars represent 1 standard deviation. 2.4 Discussion Microsomal and cytosolic protein contents in tissues of human and pre-clinical species are used as scaling factors for IVIVE of microsomal metabolism data to predict in vivo clearance of drugs. Information on microsomal scalars is widely available for human liver, including inter-individual variability, as well as for liver of several pre-clinical species. Conversely with regard to human kidney, literature data concerning the microsomal protein content are limited, with data for only 23 donors, spread across four different studies, whereas data on the cytosolic protein are completely lacking. Studies measuring the microsomal and cytosolic protein of kidney in pre-clinical species that explicitly account for protein recoveries of the fractionation procedure are completely lacking. In the current study, the microsomal protein content of dog kidney was measured using two different microsomal protein recovery markers, and compared with the corresponding values in matched liver and intestine samples. Further, the microsomal and cytosolic protein content was measured in 31 human kidney samples. For 13 of these samples, the functional activity was assessed using a mycophenolic glucuronidation substrate depletion assay. These data were used to assess the impact of different MPPGK values, as well as different assumptions concerning the contribution of whole kidney versus only the cortex to renal drug glucuronidation, on predictions of in vivo mycophenolic acid glucuronidation clearance. 76
77 2.4.1 Suitability of microsomal and cytosol protein markers for correction of protein losses CYP content in dog liver homogenate and microsomes was readily quantified using the dithionite difference spectra approach, with values comparable with previously published values (264). There are challenges surrounding the preparation of kidney microsomes (i.e. difficult to effectively homogenise; microsomes often contaminated with mitochondrial protein) and measurement of CYP content in kidney samples (i.e. low content in kidney; spectral interference from other haemoproteins) (180, 293). Evidence from preliminary studies suggested that potential bias introduced to CYP content measurements, and subsequent MPPGK estimates, due to spectral interference from contaminating haemoproteins, could not be distinguished from inter-assay variability without extensive analyses. Such extensive analyses were outside of the scope of the current study, and would have been challenging due to the limited samples available. Furthermore, when using a consistent spectral method for all samples, the dog kidney microsomal CYP content measured in the current study (0.205 and nmol/ mg protein in homogenate from fresh and frozen kidney cortex respectively; Table 2.2) were comparable with a value reported using a customised spectral method (0.223 nmol/ mg protein; (293)). Therefore, the standard dithionite difference spectra approach (283) was used in the current study for measuring CYP content in dog kidney. Due to the limitations of CYP content as a microsomal protein marker for kidney, G6Pase activity was selected as a possible alternative microsomal protein marker for correction of protein losses during centrifugation. The estimated microsomal protein recoveries in dog (frozen tissue) were lower when using G6Pase activity as microsomal protein marker than when using CYP content, in both liver (38% for G6Pase and 58% for CYP content) and kidney (40% for G6Pase and 53% for CYP content). Subsequently the microsomal protein content estimates were higher when using G6Pase activity in both kidney and liver. The presence of G6Pase in the nuclear envelope, which has been reported in both liver and kidney (294, 295), is a potential contributing factor to this difference. However, this is unlikely to fully explain the marker related differences in microsomal protein content, as the overall G6Pase activity of the nuclear envelope is small compared to that in the endoplasmic reticulum ( ). Using the methodology applied in the current study, distinguishing between the presence of true nuclear envelope-g6pase in the 9000g pellet and loss of microsomal-g6pase into the 9000g pellet (e.g., due to incomplete homogenisation), would not be possible. Despite the potential for over-estimation of MPPG values, G6Pase activity was still considered more reliable than CYP content as the microsomal protein marker for dog kidney, and was therefore used for human kidney samples. The principal reason for this decision was the low sensitivity of the CYP content assay, which could become a limitation particularly in human kidney because of expected high biological variability (relative to dogs which are raised in controlled conditions). Indeed the observed variability of G6Pase activity in human kidney samples was over twice that in dog kidney. A correlation between G6Pase activity and mycophenolic acid CL int,u,ugt,hkm was observed (Figure 2.10). Plausible explanations include a sub-optimal or inconsistent homogenisation protocol with respect to preserving enzyme activity, or a common mechanism regulating the kidney expression 77
78 of G6Pase and UGT isoform(s) responsible for mycophenolic acid metabolism. However, a preliminary experiment was performed which allowed comparison of G6Pase activity in two batches of human kidney microsomes from the same donor. There was good reproducibility in G6Pase activity between the two batches (Figure 2.3), from which good reproducibility in homogenisation may be inferred, although further studies involving multiple microsome batches prepared from donors with both high and low enzyme activities could provide further assurances. Furthermore, HNFs are homeobox transcription factors (298, 299); members of the HNF1 and HNF4 families may be involved in the regulation of G6Pase ( ), UGT1A9 (273, 303, 304), and UGT2B7 (273, 304) expression. From an evolutionary perspective, close regulation of G6Pase and UGT expression is rationalised due to the close positions of D-glucose and glucose- 6-phosphate (substrate and product of G6Pase mediated reaction) and UDPGA (cofactor for UGT mediated glucuronidation) in the cellular metabolic pathway ( [accessed 5/2/2016]). Taken together, co-regulation of G6Pase and UGT enzymes is a more likely explanation for the observed correlation between G6Pase activity and mycophenolic acid CL int,u,ugt,hkm. Quantitative enzyme abundance data for G6Pase and relevant UGTs could be used to investigate this assertion. Alternative microsomal protein markers such as NADPH cytochrome c reductase activity have been previously used for estimating kidney microsomal protein recoveries (60, 182). However, the high mitochondrial content of kidney cortex gives rise to high levels of cytochrome c oxidase in the homogenate, and potentially the microsomal fraction as a contaminant. Cytochrome c oxidase catalyses the re-oxidation of cytochrome c (i.e., reverse reaction), but may be inhibited by presence of potassium cyanide in the NADPH cytochrome c reductase assay buffer (305). Both ADH and GST activity have been suggested as potential cytosolic protein markers to obtain estimates of cytosolic protein recoveries (190). In the current study implementation of the ADH activity assay was challenging and ineffective, which is a likely cause of the observed non-linearity with respect to protein concentration that has not been reported previously. Therefore, GST activity was used as the human cytosolic protein marker, despite a major limitation, namely the presence of microsomal GST isoforms (280). GST activity in human kidney cytosol was higher than that in microsomes, in agreement with similar findings for human liver (306). Average GST activities in human kidney microsomes were higher than a literature value by approximately one order of magnitude (307); conversely GST activities in human kidney cytosols were on average lower than previous reported values for normal human kidney (308, 309). Analogous to the situation with G6Pase, discerning the potential presence of cross-contamination of the true microsomal and cytosolic GST isoforms across the isolated subcellular fractions requires further studies involving cytochemistry studies (e.g. (295)), using purified or washed fractions, as previously reported for liver (310). Ignoring the proportion of GST activity in homogenate attributed to microsomal isoforms (15%) when calculating the cytosolic protein recovery estimate resulted in an increase in the average estimated CPPGK by 16%. In the extreme case, this 16% difference represents a potential systematic under- or under-prediction (depending on which assumption taken vs real situation) of in vivo metabolic clearance when using CPPGK as an IVIVE scaling factor for in vitro cytosolic metabolism data. 78
79 2.4.2 Species and tissue differences in subcellular protein content estimates The direct comparison of microsomal content of liver and kidney in matched samples (from the same animals) revealed that although MPPGL was higher than MPPGK on average, these values did not appear to be correlated. The mean MPPGK value in dog was higher than the corresponding value in human, in agreement with literature data suggesting a similar relationship for MPPGL (184, 266, 311). The variability observed in MPPGK in dog was lower compared to human, despite similar inter-assay variability in G6Pase activities, indicating greater biological variability in human MPPGK. Although kidneys were obtained from dogs of a wide range of ages, which may be expected to increase the biological variability in MPPG (183), the lower variability observed for dog may reflect more controlled living environments and breeding. The low number of dogs from which tissues were available, in combination with restricted population variability and large contribution of inter-assay variability, reduces the power of the current study for identifying true underlying covariates of MPPGK in dog. The number of kidney samples used to estimate human MPPGK in the current study (n=31) was greater than the entire combined samples reported so far (n=23 across 4 studies; Table 1.8), and therefore provides a more reliable indicator of true biological variability in this microsomal scalar. The mean MPPGK of the current study is in agreement with that previously reported for kidney cortex microsomes (180), but higher than values from unspecified regions or mixed kidney (60, 182). Demographic information such as age, gender and medical history of donors were available for only the 13 kidney samples from CMFT Biobank. This was an insufficient number of samples for assessment of any potential demographic covariates of MPPGK such as those investigated for MPPGL (183). However, the donors of the CMFT Biobank kidney samples were aged years at the time of nephrectomy, which represents a sub-section of the overall adult population, a trend consistent with previous studies (Table 1.8). Further data are therefore required for younger subjects in order to investigate potential MPPGK-age relationships. The average human CPPGK was approximately two thirds of the value reported for CPPGL (190). However to the author s knowledge the potential contribution of microsomal GST isoforms within the liver homogenate was not accounted for when this was used as the cytosolic protein marker in the liver. Previous IVIVE studies have highlighted the lack of cytosolic and S9 scaling factors for human kidney, using values obtained in liver as surrogate (28). The estimated human S9 protein per gram kidney, based on the combined values of MPPGK and CPPGK, was 78.3 mg/ g kidney (26% CV). This is lower than the corresponding value for liver (121 mg/ g liver), as well as an estimated value of 93.5 mg/ g kidney, which was calculated from an MPPGK value of 12.8 mg/ g kidney and CPPGL value of 80.7 mg/ g liver (28). 79
80 2.4.3 Impact on updated MPPGK scaling factors on renal metabolic clearance predictions The MPPGK values measured in the current study were on average approximately double the most commonly used value for IVIVE of renal drug metabolism data (Figure 1.3) (182). The IVIVE scaling of mycophenolic acid renal glucuronidation suggested that use of the published MPPGK value results in substantially lower predicted CL R,met,UGT than when MPPGK values obtained in the current study are used. This analysis suggests potential under-estimation of the contribution of kidney to overall metabolic clearance in several previous studies (Figure 2.13). In the scenario where the CL int,u,ugt,hkm measured in XenoTech pooled human kidney microsomes was scaled by the overall average MPPGK (25.7 mg/ g kidney) for the 31 donors, and it was assumed a contribution from the whole kidney to predict CL R,met,UGT, the kidney: liver ratio for CL met,ugt was 1.02, indicating an equal contribution of liver and kidney to mycophenolic acid glucuronidation clearance in vivo. Mycophenolic acid is an immunosuppressant with a narrow therapeutic window and large intra- and inter-individual variability in pharmacokinetics (312). Therapeutic drug monitoring has been proposed for this drug to maintain target exposure levels in plasma (313). The variable CL int,u,ugt,hkm observed in the current study (Figure 2.10) is consistent with the interindividual variability in mycophenolic acid clinical pharmacokinetics, and may indicate that renal glucuronidation could contribute to the overall variability in vivo. Several patient factors have been identified as covariates of mycophenolic acid pharmacokinetic parameters, including single nucleotide polymorphisms (SNP) in UGT1A9 and 2B7 (269), which mediate mycophenolic acid glucuronidation in vitro (289). Such polymorphisms could contribute to the inter-individual variability in the in vitro CL int,u,ugt,hkm observed in the current study, although genotyping data for these samples are not currently available for these samples. A recent study by Knights et al highlighted the differences in glucuronidation activity between the cortex and medulla regions of the kidney; for example, the average cortex: medulla ratios of UGT activity (pmol glucuronide formed/ min/ mg microsomal protein) in human kidney microsomes were 1.4, 5.2 and 10.5 for deferiprone, propofol and zidovudine, which were used as probe substrates of UGT1A6, 1A9 and 2B7 respectively (60). The authors proposed that mixed kidney microsomes (i.e., containing both cortex and medulla) were appropriate for IVIVE predictions of renal drug metabolism. This assertion was supported by the finding that glucuronidation rates in microsomes prepared from only cortex or medulla were comparable for deferiprone and propofol when activity was normalised for UGT enzyme abundance of UGT1A6 and 1A9 respectively (60). However the authors also indicated that not only is the UGT abundance (pmol/ mg microsomal protein) lower in the medulla, but also the medulla has a lower content of endoplasmic reticulum, the primary constituent of the typical microsomal fraction, in relation to cortex. Together, this would suggest a very minor role of medulla in overall renal glucuronidation, although measurement of MPPGK in the medulla would be required to confirm this, and this value is not currently available. Moreover, the assertion that mixed kidney microsomes are appropriate for IVIVE of renal drug metabolism relies upon additional assumptions. Firstly, it assumes the mixed kidney microsomes adequately represent the ratio of cortex and medulla found in the whole kidney (approx. two thirds cortex); secondly, it assumes that blood flow is equal throughout the kidney (by using the well-stirred model). While ensuring the first assumption is valid could be 80
81 challenging given the scarcity of human kidney tissue samples, it remains plausible. However, the cortex receives approximately 4 times as much of the renal blood flow as the medulla, limiting the validity of the second assumption. In the current study the renal cortex glucuronidation clearance of mycophenolic acid was estimated by modifying the kidney weight and renal blood flow parameters in the well-stirred model (Table 2.3). As expected, the predicted CL R,met,UGT for cortex was lower than when scaling using whole kidney weight and blood flow, although higher than when the common MPPGK value was used for scaling. These differences highlight the importance of knowing the source (cortex/ medulla/ mixed) of microsomal protein being used, as well using MPPGK scalars for IVIVE of renal drug metabolism data which are appropriate for the source of kidney microsomes used for in vitro assays Conclusion Subcellular fraction protein marker assays, used for estimating recovery factors following differential centrifugation, can be associated with certain limitations. In the current study, various limitations relevant to kidney microsomes and cytosol were encountered and investigated. MPPGK in dog was characterised for the first time (n=17), and, in the first study to compare matched kidney, liver and intestinal samples from the same animals, MPPGK estimated from frozen dog samples were lower than MPPGL, but higher than MPPGI, with no direct correlations between values. Mean human MPPGK in kidney cortex, measured for 31 donors (25.7 mg/ g kidney), was found to be on average 2-fold higher than the literature value commonly used for IVIVE scaling of renal metabolism data; the scalar was lower than the corresponding value in liver. Large inter-individual differences in microsomal glucuronidation of mycophenolic acid were observed in human kidney for a subset of 13 donors (20-fold difference between highest and lowest), in agreement with the large inter-individual variability in the pharmacokinetics of this drug. IVIVE scaling showed that for mycophenolic acid, use of the novel MPPGK values generated in the current study had a larger impact, compared with using the literature MPPGK value, on scaled intrinsic glucuronidation clearance (per gram kidney), than on predicted overall glucuronidation clearance (which also accounted for the hepatic glucuronidation clearance). The estimate of human CPPGK (n=31 donors), which was measured for the first time incorporating a novel approach for correction of microsomal GST activity during measurement of the cytosolic protein recovery, was lower than literature estimates of CPPGL. Further work is needed to better understand and verify the assumptions taken when estimating MPPGK and CPPGK, and to improve upon the size of current dataset to allow for covariate analyses. 81
82 Chapter 3. Development of novel methodology for measurement of proximal tubule cell number in human kidney 3.1 Background Physiological data obtained using many different experimental techniques are required to inform IVIVE scaling factors and the system parameters in PBPK models (1). Recent attempts to develop novel IVIVE approaches for predicting renal clearance, as well as mechanistic kidney models, have highlighted gaps in current knowledge of the anatomy and physiology of kidney (Chapter 1, sections 1.4 and 1.5). The critical analysis of the literature reported in Chapter 1 (section 1.4.5) revealed that no measurement of proximal tubule cell numbers in human kidney has been published, while a mean value of 92.0 ± 9.5 million proximal tubule cells was reported per rat kidney (176). In Chapter 1, section (details in appendix, section 8.1.1), human proximal tubule cell number was indirectly calculated from other reported measurements such as proximal tubule length and number of cells per length of proximal tubule, although calculated values varied from 30 to 209 million proximal tubule cells, depending on assumptions taken. The value of 60 million that is used as the PTCPGK parameter in the SimCYP MechKiM is derived from approximate cell yields following collagenase digestion and isolation from human kidney cortex (78, 314). However, this isolation procedure did not account for cell losses during the isolation procedure, and no enrichment or purification steps were used, such as flow cytometry, magnetic separation or Percoll density-gradient centrifugation, as reported in other studies (86, ). Therefore the cell yields reported could be for a mixed cortical cell population, and not only for proximal tubule cells. Stereology methods are statistically based approaches for obtaining unbiased estimates of structural parameters (177). Such approaches have been developed to estimate the number and size of biological structures, which have a variety of 3-dimensional shapes/ geometries, from 2- dimensional images such as microscopy slides. The physical or optical dissectors, combined with the fractionator, are stereological approaches that have been used to measure the number of glomeruli and proximal tubule cells in kidney (176). The fractionator-physical dissector method involves sampling the tissue/ organ of interest to obtain a known volume fraction that is sampled from. For a large organ like the kidney, this fraction which is used for counting the biological objects of interest represents a very small proportion of the entire organ and involves sampling the organ at multiple stages: a. Systematic slicing of tissue into multiple pieces and selection of a fraction of these pieces to embed as paraffin blocks (block sampling fraction; x-, y- and z-axes) using systematic uniformly random sampling (177). b. Selecting paraffin section pairs representing a known volume fraction of each block for mounting and histological preparation (section sampling fraction; z-axis) c. Systematic sampling of 2-dimensional area of each selected section is dictated by the number and size of the counting frames used (area sampling fraction; x- and y-axes) 82
83 The number of objects in each counting frame is measured using the physical dissector, in which objects unique to the reference tissue section, and not present in the paired look-up section, are counted (318). The optical dissector provides a more efficient approach for estimating particle numbers than the physical dissector. However the optical dissector uses thick sections which are less suited for some histochemistry techniques, particularly immunohistochemistry, compared with the thin sections used for the physical dissector (177). A prerequisite of stereological approaches is that all objects under study must be unambiguously identifiable. Consequentially the whole organ/ tissue/ region of interest must be available for study. This presents a challenge when studying large organs, particularly human organs. The use of biopsies for stereological estimation of glomeruli number was proposed, using pig kidney as a model due to anatomical similarity with human kidney (319). Although difficulties associated with precisely determining biopsy volume was highlighted as a concern, systematic bias was not apparent. Furthermore, the requirement for unambiguous identification of objects of interest necessitates a histochemistry method which allows proximal tubule cells to be visually distinguished from other renal cell types, including cells of other tubular regions of the nephron (e.g., distal tubule). Various histochemical staining approaches are available. For example, tinctorial stains are simple to perform, requiring minimal optimisation for the specific tissue and species of interest, and are low in cost, but may result in variable staining intensity, particularly between tissue sections (320). Conversely, immunohistochemistry techniques are typically more expensive than tinctorial stains, and may require extensive optimisation and validation, but usually permit clearer distinction between positive and negative stained areas than tinctorial stains (320) Aims The aim of this study was to develop methodology for measurement of proximal tubule cellularity in porcine kidney, and apply the developed strategy to human. It was hypothesised that single, larger pieces of kidney, which were available from nephrectomy patients, may be suitable for stereology based estimation of proximal tubule cell number. However, the use of kidney pieces would inherently invalidate a central tenet of stereology approaches, namely an equal opportunity of all objects of interest (i.e., proximal tubule cells) to be sampled. Therefore, in order to test the hypothesis stated above, pig kidney was selected as a suitable model to compare proximal tubule cell counts in kidney pieces and whole kidney performed using stereology, to assess the extent of any potential bias. In order to unambiguously identify porcine proximal tubule cells, two approaches were investigated. Initially a trichrome tinctorial staining approach (Periodic acid Schiff Orange G) stain was used; the second approach was immunohistochemistry (anti-villin antibody). 83
84 3.2 Methods Reagents EnVision+ Dual Link System-HRP (DAB+) (K406511) was obtained from Dako UK Ltd (Cambridgeshire, UK). Anti-human, anti-villin mouse monoclonal antibody [3E5G11] N-terminal (ab201989) was obtained from Abcam (Cambridgeshire, UK). Xylene, ethanol, Schiff reagent, orange g, phosphotungstic acid, Gill s No. 2 haematoxylin and periodic acid were obtained from Fisher Scientific UK Ltd (Leicestershire, UK). Papanicolaou's haematoxylin was obtained from VWR (Leicestershire, UK) Source of human and pig kidney Formalin-fixed pig kidney was a kind gift from Dr Tahera Ansari (Northwick Park Institute for Medical Research, Middlesex, UK). Formalin fixed-paraffin embedded human kidney was obtained from Biobank, Central Manchester University Hospitals NHS Foundation Trust ( [last accessed 28/02/2016]). The storage of the human kidney tissue and experimental procedures were performed under ethical approval (NRES Committee London - Camberwell St Giles, REC Ref: 13/LO/1896) Sampling and embedding of pig kidney Sampling of pig kidney was performed using systematic uniformly random sampling. After removal of the surrounding adipose tissue, formalin fixed pig kidney was cut into 16 slices. Every second slice was sampled with the first slice selected randomly (1 or 2) using a coin toss. Each of these 8 slices was cut into 8 strips, and every second strip was sampled as before. These strips were lined up end to end, and cut into 1 cm pieces. Every fifth piece was selected, starting with a random piece between 1 and 5. The selected pieces were processed by Dr Kate Widdows (Institute of Human Development, The University of Manchester), whereby tissue was dehydrated and infiltrated with paraffin wax, and then embedded into paraffin blocks Microtome sectioning and mounting Paraffin embedded human and pig kidney blocks, were chilled on ice, and then sectioned at a nominal thickness of 3 and 5 µm using a Leica RM2255 rotary microtome (Leica Microsystems (UK) Ltd, Milton Keynes, UK). Sections were flattened using a waterbath at 45 C, mounted onto SuperFrost Plus glass slides (Fisher Scientific UK Ltd), and dried overnight at 37 C. Slides were then stored at room temperature Deparaffinising and rehydration Slides were placed in a series of solvents at room temperature to remove paraffin wax and rehydrate sections in the following order: two changes of xylene (5 min), two changes of 100% ethanol (3 min), one change of 90% ethanol (3 min), one change of 70% ethanol (3 min) and two changes of deionised water (3 min). Slides were kept in deionised water at room temperature until being used for staining or immunohistochemistry. 84
85 3.2.6 Periodic acid Schiff Orange G stain Periodic acid Schiff - Orange G staining of kidney was performed as a potential method for identifying proximal tubule cells (321). Periodic acid Schiff stains the brush border membrane of proximal tubule cells, although inter-species differences in staining intensity have been reported, as well as differences between sub-sections of the proximal tubule (321). The in-house method described herein was provided by Dr Pete Walker (University of Manchester, UK). After deparaffinising and rehydration (section 3.2.5), kidney sections were covered with 1% periodic acid for 10 min, and then rinsed in deionised water. Sections were then covered with Schiff s reagent for 10 min, rinsed with tap water until the sections appeared pink, then rinsed with deionised water. Sections were covered with filtered Gill s No.2 Haematoxylin for 1.5 min, blued by rinsing with tepid tap water for, covered with 2% orange G dissolved in phosphotungstic acid for 1 min, and then rinsed with deionised water. Sections were briefly examined under a microscope to ensure appropriate staining intensity, and then dehydrated and mounted (section 3.2.8) Immunohistochemistry Antibodies have been previously reported for proximal tubule specific proteins in human and preclinical species (315, 322). However some of these proteins are specific to sub-sections of proximal tubule (i.e. S1, S2 or S3) (315) or are also expressed in other sections of the nephron tubule (322). Further, some antibodies were not commercially available, or those available were not predicted to be reactive across species. Villin is a member of an actin-binding protein family capable of both polymerising and depolymerising actin filaments, and therefore has a role in microvilli formation (323). Villin has been localised to the brush border of proximal tubule cells by immunohistochemistry (324, 325). Anti-human, anti-villin antibody [3E5G11] N-terminal (ab201989) had predicted reactivity with pig and other species ( [accessed 12/10/2015]). The NCBI Basic Local Alignment Search Tool (BLAST) blastp algorithm ( [accessed 12/10/2015]) was used to search for alignment between the anti-villin antibody immunogen protein sequence and the non-redundant protein sequence for pig (taxid:9823). After deparaffinising and rehydration (section 3.2.5), heat induced epitope retrieval was performed by incubating slides in 100 mm citrate buffer ph 6.0 at 97.5 C, followed by a cooling period of 10 min at room temperature. Sections were rinsed with deionised water. Immunohistochemistry steps were performed using a Thermo Scientific Shandon Sequenza immunostaining centre (Fisher Scientific, Pennsylvania, USA), which uses the Coverplate system. Incubation and wash steps were performed at room temperature unless specified. Sections were washed three times with PBS. Sections were incubated with 2 drops of Dual Endogenous Enzyme Block for 8 min, and then washed twice with PBS. Dual Endogenous Enzyme Block contains 0.3% hydrogen peroxide which blocks endogenous peroxidases, thereby preventing potential non-specific staining of tissue sections following addition of the substrate-chromogen solution (see below). Sections were incubated with 150 µl of either PBS (negative control) or anti-villin antibody (primary antibody) diluted 1:200 or 1:400 in PBS overnight at 4 C. Sections were returned to room 85
86 temperature and washed with PBS. Sections were incubated with 2 drops of labelled polymer (horseradish peroxidase labelled polymer conjugated to goat anti-mouse and goat anti-rabbit immunoglobulins) for 30 min, and then washed three times with PBS. Substrate-chromogen solution was prepared following manufacturer s instructions (1 drop of 3,3 -diaminobenzidine chromogen per 1 ml PBS). Sections were then incubated with substrate-chromogen solution for 5 min, followed by three washes with deionised water. Sections were then counterstained by covering with filtered Papanicolaou s haematoxylin, diluted 1:20, for 1 min, and then rinsed with tap water Dehydration, mounting and scanning of slides Slides were placed in a series of solvents at room temperature to dehydrate sections in the following order: twice in deionised water (3 min), once each in 70% and 90% ethanol (3 min), twice in 100% ethanol (3 min), and twice in xylene (5 min). Slides were mounted with a coverslip using distyrene plasticizer xylene. Slides were scanned using a Pannoramic 250 Flash II slide-scanner (3DHISTECH Ltd., Budapest, Hungary), by Dr Peter March and colleagues (Bioimaging facility, University of Manchester) to obtain the virtual slides. Visualisation of virtual slides was performed using Pannoramic Viewer (version ; 3DHISTECH Ltd.), from which representative images were taken. 86
87 3.3 Results Stereological processing of kidney The formalin fixed pig kidneys were initially sectioned into 16 equal slices (Figure 3.1a), from which 8 were selected by systematic uniformly random sampling (Figure 3.1b), and each cut into 8 strips (Figure 3.1c). Half of these strips were selected by systematic uniformly random sampling, put in a line (Figure 3.1d), cut at every 1 cm, and then 1/5 of the 1 cm pieces were processed into paraffin blocks. From these blocks, several sections of 5 µm and 3 µm were taken and mounted onto slides. A B C D Figure 3.1 Different stages of sampling of formalin fixed pig kidney using systematic uniformly random sampling. See main text for details 87
88 3.3.2 Staining and immunohistochemistry Virtual slides allowed for whole images of kidney sections, including both cortex and medulla, to be visually assessed at different magnifications (Figure 3.2). Figure 3.2 Representative images of the same virtual slide of pig kidney with increasing magnification from left to right. Images are taken from 3 µm thick pig kidney sections stained with Periodic acid Schiff - Orange G and counterstained with Gill s haematoxylin. Initially, human and pig kidney were stained using PAS Orange G, which coloured the brush border and the basement membranes purple, the erythrocytes and acidophilic cells orange (stronger staining of erythrocytes), and nuclei dark brown (Figure 3.3 and Figure 3.4). The brush border of proximal tubules was stained in both species and so proximal tubules could usually be identified in kidney cortex. In addition, pig proximal tubule cells (acidophilic) were typically stained orange in the cytoplasm, allowing further differentiation from other tubular cells (Figure 3.4). However this was not the case for human, in which the cytoplasmic staining of tubular cells was more uniform. Furthermore, in some cases the overall staining was very strong (e.g. Figure 3.4, 5 µm) or brush border staining was weak (e.g. Figure 3.3, 3 µm), such that unambiguous determination of proximal tubules would be challenging in these sections. Staining patterns consistent with proximal tubule cells were not observed in medulla of kidney sections from either pig or human (Figure 3.5). 88
89 Low Magnification High Magnification 5 µm 3 µm Figure 3.3 Representative images of cortex of 5 or 3 µm thick human kidney sections stained with Periodic acid Schiff - Orange G counterstained with Gill s No. 2 haematoxylin. Representative images were taken from virtual slides at two magnifications. Nuclei were stained dark brown, erythrocytes and acidophilic cells stained orange, and basement membrane and brush border stained purple. The staining of brush borders was weaker in the 3 µm thick sections compared to 5 µm. 89
90 Low Magnification High Magnification 5 µm 3 µm Figure 3.4 Representative images of cortex 5 or 3 µm thick pig kidney sections stained with Periodic acid Schiff - Orange G counterstained with Gill s haematoxylin. Representative images were taken from virtual slides at two magnifications. Nuclei were stained dark brown, erythrocytes and acidophilic cells stained orange, and basement membrane and brush border stained purple. The staining of was stronger in the 5 µm thick sections compared to 3 µm, and so it more difficult to distinguish specific structures in the thicker sections. 90
91 Human Pig 5 µm 3 µm Figure 3.5 Representative images of medulla of 5 or 3 µm thick human and pig kidney sections stained with Periodic acid Schiff - Orange G counterstained with Gill s haematoxylin. Representative images were taken from virtual slides. Nuclei were stained dark brown, erythrocytes and acidophilic cells stained orange and basement membrane and brush border stained purple. There was 93% protein sequence identity of the immunogen of anti-human anti-villin antibody and the analogous fragment of pig villin (accession Q ), with an expect value of 3e-139, using NCBI blastp algorithm. This suggested high sequence similarity between the two species. The anti-villin antibody strongly stained proximal tubules in both human (Figure 3.6) and pig (Figure 3.7) kidney cortex in both 5 and 3 µm thick sections. Staining for villin in human using both 1:200 and 1:400 dilutions appeared to stain other tubules in addition to proximal tubule, whereas staining appeared to be more specific in pig kidney cortex, especially in 3 µm sections. There appeared to be tiny cracks or lines in the human kidney cortex sections, suggestive of problems during tissue processing and/ or microtomy. Glomeruli were not stained (Figure 3.6 and Figure 3.7), and no staining for proximal tubules occurred in the negative control (no primary antibody) (Figure 3.8). Staining of some tubules with anti-villin occurred in the medulla of both human and pig kidney sections (Figure 3.9); increased dilution of the anti-villin antibody reduced the strength of this staining. 91
92 1:200 dilution 1:400 dilution 5 µm 3 µm Figure 3.6 Representative images of human kidney sections stained using anti-villin antibody (brown) to identify proximal tubules in cortex, using two dilutions of the primary antibody. Representative images were taken from virtual slides. Sections were 5 or 3 µm thick and counterstained with Papanicolaou s haematoxylin (blue). 92
93 1:200 dilution 1:400 dilution 5 µm 3 µm Figure 3.7 Representative images of pig kidney sections stained using anti-villin antibody (brown) to identify proximal tubules in cortex, using two dilutions of the primary antibody. Representative images were taken from virtual slides. Sections were 5 or 3 µm thick and counterstained with Papanicolaou s haematoxylin (blue). Human Pig 5 µm Figure 3.8 Staining for villin in kidney cortex are abolished in the negative (no primary antibody) control. Representative images were taken from virtual slides. 5 µm thick human and pig kidney sections were processed as described as for figures 3.3 and 3.4, with the exception that sections were incubated in the presence of PBS instead of anti-villin antibody. Nuclei were stained with Papanicolaou s haematoxylin (blue). 93
94 1:200 dilution 1:400 dilution Human Pig Figure 3.9 Human and pig kidney medulla contained tubules which were stained using antivillin antibody (brown) and counterstained with Papanicolaou s haematoxylin (blue). Representative images were taken from virtual slides of 5 µm sections. 3.4 Discussion Quantitative information on the proximal tubule cell number in human kidney is currently lacking. Although values can be estimated indirectly, no study to-date has directly measured this using appropriate methodology. The current study investigated development of novel stereology and histology based methods to determine proximal tubule cell number in human kidney tissue Identification of proximal tubule cells in histology sections A pre-requisite of stereology techniques is the opportunity to sample all objects of interest, which may include biological structures (e.g., glomeruli, tubules or vasculature), specific cell types or subcellular features (e.g., microvilli or mitochondria). In the context of the current study, this implies that every proximal tubule cell in the kidney has an equal opportunity to be counted at the start of the study. This requires that the sampling strategy used must be appropriate and statistically non-biased, and it is possible to unambiguously identify proximal tubule cells from other cell types and extracellular matrix in kidney. In order to develop methodology for stereologybased counting of proximal tubule cells, two different approaches were investigated for distinguishing proximal tubule cells from other cells in the kidney. Although the final aim was to count proximal tubule cells in human kidney, due to scarcity of human whole kidney samples available for research, an intermediate aim was to develop a modified-stereology approach which uses available kidney pieces rather than entire organs, and assess the precision and potential 94
95 bias of such an approach. Pig kidney was selected as the model to develop and validate this modified-stereology approach. Periodic acid Schiff - Orange G stained the proximal tubule cells well in both human and rat, although in some cases unambiguous visual distinction of proximal tubule cells from other tubular cells was challenging. In addition, staining intensity was sometimes inconsistent between sections of the same thickness and species, even when staining was performed on the same occasion (not shown). Immunohistochemistry was proposed as an alternative which could obviate the limitation of tinctorial staining with respect to distinction of positive and negative cells. In absence of commercially available antibodies raised against proximal tubule specific antigens in pig, species cross-reactivity of a potentially suitable anti-human antibody was investigated. Cross-reactivity of the anti-human anti-villin antibody with the pig antigen was anticipated because of the high similarity of the immunogen sequences found between the species using the BLAST alignment. Experimentally, the anti-human anti-villin antibody was found to be reactive with pig, with strong staining of tubules in kidney cortex observed (Figure 3.7). In addition, the positive and negative staining of cells was visually distinct for the immunohistochemistry approach, as noted for the lack of staining of glomerular cells and the no-primary negative control, overcoming the limitation identified for the tinctorial stain (Figure 3.8). In the 3 µm kidney cortex sections, villin staining was stronger at the apical membrane of proximal tubule cells (Figure 3.6 and Figure 3.7), which is in agreement with previous findings (326). However, for 5 µm cortex sections, and also in medulla sections, strong staining for villin appeared to occur throughout tubular cells (i.e. no apparent ultrastructural localisation). For human kidney cortex sections, especially 5 µm sections, a potential reason for this apparent non-localised staining was sub-optimal quality of sections (Figure 3.6), possibly due to damage to the paraffin block. Further, thicker sections contain larger amounts of antigen, which results in stronger staining intensity compared with thinner sections for the same antibody dilutions. Therefore an alternative reason for apparent non-localised staining could be that the primary anti-body was not diluted enough (manufacturer s recommended working range 1/200 1/100 [Accessed 9/12/2015]). Staining observed in specific tubules of the medulla represents a substantial limitation to the use of the anti-villin antibody for identifying proximal tubule cells for stereological counting. Although the proximal straight tubules extend into the outer stripe of the outer medulla, the tubules stained by anti-villin in the medulla section, which are characterised by wide open lumens, do not appear to be proximal tubules. Instead the tubules stained for villin in medulla sections could be intercalated cells of the collecting tubule. Although the collecting tubule does not have a brush border, microplicae and microvilli are present on the apical membranes of type A and type B intercalated cells respectively (327). Furthermore, villin expression has been observed in the apical region of cultured type B intercalated cells (328). Alternatively, the staining for villin of tubules in the medulla could be caused by reactivity of the antibody to actin-binding proteins other than villin. Like villin, actin-binding proteins adseverin and gelsolin are members of the gelsolin superfamily of proteins and are expressed in kidney (329). In particular, adseverin expression was localised throughout the cytoplasm of intercalated cells and, 95
96 to a lesser extent, principle cells, of collecting ducts in the cortex and papilla (330), in agreement with the pattern of staining observed for anti-villin in the current study. Gelsolin was expressed in collecting duct cells and localised predominantly towards the apical membrane and also in the cytoplasm to a lesser extend (330, 331). To further explore the possibility of potential crossreactivity of the anti-villin antibody with other actin-binding proteins, the anti-villin immunogen protein sequence was aligned against the sequences of other gelsolin superfamily members using the NCBI blastp algorithm. Following alignment, sequence identities were 59%, 52% and 59% for gelsolin isoform b (Accession NP_ ), adseverin isoform 1 (NP_ ) and Gelsolin sub-domain 1-like domain (containing the putative actin binding site; cd11290) respectively, with Expect (E) values less than 5e-46. When these alignment results are considered alongside the similar staining patterns from anti-villin observed in the current study and for adseverin in previous studies, the hypothesis that the anti-villin antibody has reactivity with adseverin (and/ or gelsolin) seems plausible. Alternative proximal tubule cell markers which may be suitable for immunohistochemistry have been suggested. For example leucine aminopeptidase is expressed in both the straight and convoluted proximal tubule (315). However finding suitable commercially available antibodies with known (or predicted) reactivity with pig is a current limitation for the study of several markers of proximal tubules. Further, Phaseolus vulgaris etythroagglutinin (PHA-E) is a lectin which has been used to stain human and pig proximal tubule cells (332, 333). Lectins such as PHA-E suitable for histochemistry (e.g. biotinylated or fluorescein labelled) are commercially available. Overall, alternative approaches to unambiguously identify proximal tubule cells in human and pig kidney sections are available, and should be considered for future work Conclusion Several aspects of a stereology method for measuring proximal tubule cellularity were developed. These included implementation of a systematic uniform random sampling protocol, and assessment of different approaches for identifying proximal tubule cells. Although the tinctorial stain could be used to identify proximal tubule cells in most cases, unambiguous identification was challenging in some virtual slides. Therefore further work should investigate if other potential antibodies and histology staining techniques offer any improvements. 96
97 Chapter 4. Novel minimal physiologically-based model for the prediction of passive tubular reabsorption and renal excretion clearance This chapter was adapted from a publication by the author: Scotcher D, Jones C, Rostami-Hodjegan A and Galetin A. Novel minimal physiologicallybased model for the prediction of passive tubular reabsorption and renal excretion clearance. European Journal of Pharmaceutical Sciences doi: /j.ejps Introduction Renal excretion is considered a major route of elimination for many drugs (e.g., metformin, acyclovir and digoxin) (3, 51, 69). Prediction of human renal excretion clearance (CL R ) prior to commencing first-in-man clinical studies currently relies on the use of in silico methods (based on physico-chemical properties) (68-70, 334) and/ or allometric scaling (335, 336). Despite wide use of these methods, they do not provide mechanistic insight into the underlying processes contributing to renal excretion and have limited ability to account for any changes in the renal physiology. Mechanistic understanding of various pharmacokinetic (PK) processes has become a necessary part of model-informed decision making for special populations (e.g., obese or patients with renal impairment), as well as devising dosage regimens for use in such populations (45). The mechanistic approach becomes even more important when certain sub-groups ( complex patients) exhibit various co-morbidities which make clinical studies very difficult, if not impossible (337). Thus, understanding various elements of renal excretion may offer advantages through prediction of potential differences in various patients under the framework of physiologically-based pharmacokinetic (PBPK) modelling (338). In addition, many currently developed drugs undergo extensive active tubular secretion (3) for which prediction of CL R by mechanistic PBPK models (78, 79, 107) is considered more promising in comparison with in silico and allometric scaling. While efforts have been made at predicting renal metabolic clearance from in vitro data (38, 216), successful prediction of CL R using in vitro-in vivo extrapolation (IVIVE) remains a challenge. In order to quantitatively and mechanistically predict CL R using IVIVE, each of the contributing processes (filtration, active secretion and tubular reabsorption, Eq. 4.1) must be considered independently. CL R = (CL R,filt + CL R,sec ) (1- F reab ) 4.1 Filtration clearance (CL R,filt ) is readily predicted from glomerular filtration rate (GFR) and fraction unbound in plasma (f u,p ). In cases where both secretion and reabsorption contribute to elimination, confidence in prediction of the fraction reabsorbed (F reab ) is equally important as the accurate prediction of renal secretion clearance (CL R,sec ). Whereas reabsorption is predominantly a passive process, secretion is actively mediated by a range of drug transporters expressed in the kidney such as OAT1, OAT3, OCT2 and MATE2-K (3). A number of mathematical models concerning physiological functions of the kidney (e.g., urine concentrating mechanism, solute transport regulation) (114, 339) exist, but may not be readily 97
98 adaptable for use in renal PBPK models. Further, these models were developed based on physiological and experimental data in rat kidney, for which analogous data in human (e.g., from micro-puncture studies) are lacking. Recently, a static model for the prediction of CL R using in vitro permeability data from LLC-PK 1 cell monolayers was proposed and its performance was assessed against a relatively small and restricted dataset (71). The model considered both active secretion and tubular reabsorption, and used the proximal tubule surface area as the IVIVE scaling factor for the apparent permeability (P app ) data. However, the remaining tubular regions (e.g., collecting duct), which may contribute to passive tubular reabsorption, were not considered (71). A dynamic kidney model that facilitates IVIVE of renal transporter kinetics and passive permeability has recently been reported (78). Although very promising, paucity of data on relevant physiological scaling factors and some of the system data (e.g., transporter abundance) restrain model application and validation. In addition, adequate consideration of the heterogeneity of the renal tubule, important for prediction of passive permeability clearance in each tubular segment, is lacking. Current reports on the use of physiologically-based kidney models for bottom-up prediction of renal drug disposition often rely on clinical plasma and/or urine drug concentration data for derivation/ optimisation of transporter kinetic parameters and their scaling factors (87, 107, 225, 226), analogous to the trends seen with prediction of hepatic clearance (106). For example, IVIVE of human CL R,sec using in vitro precision cut kidney slice uptake assays required an empirical scaling factor of 10 in order to obtain agreement between predicted and observed values (87). In addition, parameter estimation of the OAT3 maximal uptake rate (V max ) scaling factor, using plasma concentration-time profiles, was required to account for differences in transporter expression and activity between the in vitro transfected cell system and the kidney in vivo in order to predict pemetrexed CL R using a PBPK kidney model (79) Aims The aim of this study was to develop a mechanistic model to predict extent of passive tubular reabsorption from in vitro permeability data and tubular physiological parameters. The second aim was to assess the ability of the model developed to predict CL R for a range of drugs for which filtration or reabsorption appeared to be the dominant mechanisms contributing to CL R. The physiological aspects of the model were informed from data collated following an extensive literature analysis. A database of in vivo CL R and corresponding F reab were collated for 157 drugs. For a subset of 45 selected drugs, in vitro permeability data were generated in Caco-2 cell monolayer under ph gradient conditions. Subsequently, the tubular reabsorption model developed was applied to predict regional and overall passive tubular reabsorption for the selected drug subset (n=45). An empirical calibration approach was proposed to account for the effect of inter-assay/ laboratory variation in P app on the IVIVE of F reab using a set of reference drugs as calibrators (n=11). The novel mechanistic 5-compartment model developed enables prediction of the contribution of passive tubular reabsorption to CL R in a physiologically-based manner and is seen as an integral part of complex kidney models 98
99 4.2 Methods Clinical data collation CL R data were collated from literature sources and, wherever possible, data were acquired from primary studies. Further data were gathered from review papers where sufficient details on the trial design had been reported. In addition, data from unpublished clinical studies available at were also included in the analysis. Where CL R values were not reported in the study, Eq. 4.2 and 4.3 were used to calculate CL R from published urinary excretion and plasma concentration data. Reports of a drug not being detected unchanged in urine, or having negligible CL R, were not considered for collation. Data available in graphical format were digitized using GetData Graph Digitizer v2.25 ( CL R = Amount excreted in urine 0-t AUC 0-t 4.2 CL R = Urinary excretion rate C p, midpoint 4.3 where AUC 0-t represents the area under the plasma concentration-time profile, and C p,midpoint represents the plasma concentration at the midpoint of the urinary collection interval from which the urinary excretion rate was measured. Only CL R data acquired following administration of a drug to healthy adult subjects were included in the database. Data from diseased, obese, elderly or alcoholic subjects were excluded, but exclusion criteria based on sex or ethnicity were not applied. Data acquired after co-administration of multiple drugs (e.g., from drug-drug interaction studies) were generally excluded. An exception was made for trimethoprim and sulfamethoxazole because these drugs are generally coadministered and there is a paucity of data following single drug administration. These studies were considered acceptable as there have been no reports in the literature of interactions, at the level of renal excretion, between sulfamethoxazole-trimethoprim. Aminoglycosides (amikacin, gentamicin, isepamicin, netilmicin, sisomicin and tobramycin) were excluded. These drugs are reported to accumulate in proximal tubule cells, possibly due to endocytotic luminal uptake mediated by the megalin receptor, causing nephrotoxicity (47, 340, 341). Drugs with enantiomer specific renal excretion were excluded, an example being cetirizine (342). In contrast to previous databases (69), CL R data in this database are reported as absolute values, i.e. without normalisation for body weight or body surface area. Normalisation was not considered as the majority of literature studies (>75%) reported absolute CL R values and substantial portion of studies did not report either body weight or body surface area of subjects. In addition, recent publications favour the use of absolute values of markers like GFR and creatinine clearance for the drug dosing recommendation, in contrast to body surface area normalised values ( ). In cases where CL R data were reported following normalisation to body weight (e.g. ml/ min/ kg or ml/min/70 kg), CL R data were corrected according to the mean, or midpoint of the range, weight reported in the study. The CL R data were corrected using an assumed body weight of 70 kg for those studies in which body weights had not been reported, which could have introduced some bias. An analogous approach was applied for CL R data reported following normalisation to body 99
100 surface area, using a standard value of 1.73 m 2 for studies where subject body surface area data was not reported. When clearance values for the same drug were available from multiple sources, anomalies in studies/ trials reported for individual drugs were initially identified using the I 2 statistic for data heterogeneity (Eq. 4.4 and 4.5) (347, 348). High heterogeneity was observed for 20 drugs (I 2 greater than 0.5), of which 8 had very high heterogeneity (I 2 greater than 0.75). The presence and subsequent exclusion of anomalous studies/ trials was identified for drugs with I 2 greater than 0.5 through visual analyses of study/ trial mean and standard deviation data. I 2 = 100% Q - df Q where Q is Cochran s heterogeneity statistic, and df is the degrees of freedom 4.4 Q = (y i - y i σ 2 i 1 ) σ 2 i σ 2 i 2 where y i is the mean CL R reported by study i, and σ 2 i is the variance in CL R reported by study i. 4.5 In addition to CL R, any measured or estimated creatinine clearances and glomerular filtration rates (GFR) that had been reported in the same clinical studies were collated where available. These data were corrected for any normalisations that had been applied, as performed for CL R. Data reported on f u,p were also collated from the same study. Further f u,p data were obtained using other literature sources. Where non-linear plasma protein binding was reported for a drug, the f u,p, at concentrations consistent with plasma concentrations reported for CL R clinical studies were used for the analysis. Overall weighted mean CL R and standard deviation were calculated using Eq. 4.6 and 4.7, respectively. The f u,p data were collated from a variety of in vivo and in vitro sources using a number of different experimental techniques (ultrafiltration or membrane dialysis). Therefore, the average f u,p value for each drug was obtained without applying a weighting, whilst ensuring good agreement was achieved between the data used to obtain the average. J WX = j=1 n j x j J j=1 n j where WXˉ is the weighted mean, n j is the number of subjects in the j th study, and xˉ j is the mean of the j th study. Here a study is defined as the data associated with a group of subjects being administered a specific dose regime, on a particular occasion, with n number of subjects. 4.6 σ= [ J [(σ j 2 +x j 2 J j=1 )n j ]]- [( j=1 n j ) WX 2] J j=1 n j 4.7 where σ is the overall weighted standard deviation and σ j is the standard deviation of the j th study 100
101 4.2.2 Calculation of observed clearance ratio and F reab The clearance ratio was calculated from clinical data using Eq. 4.8 and 4.9, in agreement with reported studies (334, 349). Drugs with a clearance ratio greater than 1.5 were considered to undergo net secretion, and were therefore excluded from subsequent analyses and assessment of the model developed for prediction of tubular reabsorption. For the remaining drugs the fraction reabsorbed F reab was calculated using Eq CL R, filt = GFR f u,p 4.8 Clearance Ratio= CL R CL R, filt 4.9 F reab = 1 - CL R CL R, filt 4.10 N.B. In certain instances, F reab was calculated to be negative using Eq For these drugs (6/45 drugs), data were presented in figures as F reab = 0 (actual negative values were used for numerical analyses). The most extreme example was atenolol, which had f u,p of 0.97; the observed weighted overall CL R for atenolol was 145 ± 48 ml/min which exceeded GFR assumed in the current study. GFR values for healthy subjects may vary as a result of biological as well as inter-individual variation and also the method of measurement. As the more robust methods for measuring GFR, such as inulin or iohexol renal clearance, are impractical and time consuming (350), GFR measurements during clinical studies are typically based on the renal clearance of endogenous creatinine. This method is associated with a degree of inaccuracy, due to the proposed contribution of active secretion by the OCT2, MATE1 and MATE2-K transporters to creatinine clearance ( ). More frequently GFR is not measured but estimated using creatinine plasma concentrations using either the Cockcroft-Gault or Modification of Diet in Renal Disease equations (354). Individual GFR values for subjects in clinical trials databases were generally not available. Therefore, for the purposes of this study, a GFR value of 120 ml/min was assumed for all drugs, consistent with precedent set in the literature and value used in the recently developed physiologically-based kidney model (78, 150) In vitro permeability data, physico-chemical properties and drug affinity for renal transporter proteins In the absence of a robust and validated in vitro model for assessing passive permeability in the renal nephron tubule, it was hypothesised that permeability data obtained in Caco-2 cell monolayers may offer a potential substitute. Apparent permeability (P app ) of drugs in the apical to basolateral direction across Caco-2 cell monolayers was measured using an AstraZeneca inhouse assay (Hilgendorf, C. and Fredlund, L., Intrinsic permeability in-vitro - a transporter independent measure of Caco-2 permeability in drug design and development; Poster presented at World Conference on Drug Absorption, Transport and Delivery (WCDATD): Responding to Challenging Situations; Uppsala, Sweden June 24-26, 2013). The assay was performed using the apical to basolateral ph gradient format of ph 6.5 to ph 7.4. Permeability assays were performed in the presence of an efflux transporter inhibitor cocktail (50 µm quinidine, 20 µm sulfasalazine, 100 µm benzbromarone) over a 2 h incubation period. The ph gradient was applied in order to 101
102 mimic typical conditions observed in the renal tubule, where the urine ph can vary between 4.5 and 8, but is typically more acidic relative to plasma (ph ) (355). The efflux transporter inhibitor cocktail was used to minimise the effect of efflux transporters expressed in Caco-2 cells (e.g. P-glycoprotein, BCRP and MRP2) on the estimate of apical to basolateral P app of substrates for such transporters. P app data were obtained for a subset of drugs that exhibited a variety of physico-chemical properties, range of CL R, F reab and expected P app values (based on historical AstraZeneca in-house data and literature analysis of data generated under isotonic ph 7.4 conditions). The octanol-buffer (ph 7.4) distribution coefficient (LogD 7.4 ) and pk a data were generated using slight modification of the shake-flask (356) and potentiometric in-house assays respectively (assays and data analysis performed by Mark Wenlock and Neil Shearer (AstraZeneca, UK)). Where measured LogD 7.4 or pk a data could not be obtained, values reported in the BioByte Masterfile database (BioByte Corporation, Claremont, CA, USA, or calculated using ACD/LogD program v (Advanced Chemistry Development, Inc., Toronto, On, Canada, ) were used. The pk a values associated with ionisable centres identified within a given drug were used to calculate the fraction of drug ionised at ph 6.5. Drugs were then classified into groups as follows: Drugs with >50% unionised at ph 6.5 were classified as Neutral ; drugs with >50% ionised as mono-/ di- / tri-protic acids or bases were classified as Acid or Base, respectively; Zwitterions were classified as drugs with >50% ionised, with the major ionised species having no net charge; all other drugs were classified as amphoteric. The LogD 6.5 of acids and bases were estimated from LogD 7.4 and pk a data assuming that only the neutral species partitions into the octanol phase (357). LogD 6.5 for neutral, amphoteric and zwitterion drugs were assumed to be equal to LogD 7.4. Although melagatran, oxytetracycline and tetracycline have an isoelectric point > 7.4 or < 6.5, the difference between LogD 7.4 and LogD 6.5 predicted by ACD was less than 0.5, confirming the validity of the assumption above. The potential impact of renal transporter mediated secretion of drugs on the assessment of the prediction of CL R using the minimal model of reabsorption was investigated. A thorough literature search was performed in PubMed ( to identify drugs reported to be substrates of OAT1, OAT3, OAT4, OATP4C1, OCT2, OCTN1, OCTN2, MATE1, MATE2K, P-gp, MRP2, MRP4 or BCRP (3). To expand this dataset, the UCSF-FDA Transportal and TPsearch databases were also searched (241, 358). Only reports of drugs interacting as substrates of human drug transporters were included. In addition, clinical data indicating occurrence of renal transporter mediated drug-drug interactions (i.e., decrease in CL R following co-administration of a second drug compared to control) were used as indirect evidence of drugs being substrates of renal drug transporters. 102
103 4.2.4 Overall structure of the minimal model of tubular reabsorption In the proposed model, the nephron is represented as five compartments, namely the glomerulus and four tubular regions depicted in Figure 4.1. The tubular compartments in the model, listed in anatomical order starting from the glomerulus are: the proximal tubule (PT), the loop of Henle (LoH), the distal tubule (DT) and the collecting duct (CD). In the absence of active processes, tubular reabsorption proceeds until the urinary concentration is in equilibrium with the unbound drug plasma concentration. Thus a F reab value of 1, and CL R of 0, is not strictly possible in this case. The minimal model of reabsorption was developed by introducing an intermediary parameter, F reab, the value of which could range from 0 1, representing the fraction of the equilibrium reached between urine and plasma. The relationship between F reab and F reab is shown in Eq The predicted overall F reab was calculated from the predicted F reab in each (i) tubular region (F reab,i ), as shown in Eq Regional F reab,i (Eq. 4.13) were predicted from the intrinsic permeability clearance of the drug of interest for each tubule region (CL R, int,reab,i ) and the tubular flow rate (TFR) of the filtrate/ urine in the corresponding region (TFR i), adapted from previous studies (71). F reab ' = F reab CL R, filt CL R, filt - (UF f u,p ) 4.11 where UF is the urine flow, which was assumed to be 1 ml/min F reab ' = 1- (1-F reab, i ') 4.12 F reab, i ' = CL R, int, reab, i TFR i + CL R, int, reab, i 4.13 CL R, int, reab,i for each drug and tubular region combination was calculated using an IVIVE approach, as per Eq (71). CL R, int, reab, i = P app TSA i 4.14 where TSA i is the tubule surface area for each individual tubular region. 103
104 CL R, filt Q GFR 1 - F reab F reab TFR PT TSA PT CL R, int, reab, PT F reab,pt Q PT-LOH TFR LOH TSA LoH CL R, int, reab, LoH F reab,loh Q LOH-DT TFR DT TSA DT CL R, int, reab, DT F reab,dt Q DT-CD TFR CD TSA CD CL R, int, reab, CD F reab,cd Q UF CL R Figure 4.1 Schematic diagram of the minimal physiologically-based model for tubular reabsorption of drugs in the kidney. The nephron is represented by a glomerulus compartment in addition to four compartments representing different regions of the nephron tubule (proximal tubule (PT), loop of Henle (LoH), distal tubule (DT) and collecting duct (CD) in descending anatomical order). Physiological parameters, tubular flow rate (TFR i ) and tubular surface area (TSA i ), for each individual tubular region are indicated. Tubular filtrate flow (Q i ) is represented by grey arrows connecting tubular compartments and used to calculate TFR i (average midpoint flow rates). The intrinsic reabsorption clearance of each individual region (CL R,int,reab,i ) is calculated using the corresponding TSA i. Total renal excretion clearance (CL R ) is obtained from the filtration clearance (CL R,filt ) and the overall fraction reabsorbed (F reab ), by rearrangement of Eq
105 4.2.5 Minimal model of tubular reabsorption: Physiological system parameters Final values of physiological input parameters for the four tubular compartments are shown in Table 4.1, with the full detail on the individual parameters (e.g. tubular region diameters and length) described in Chapter 1, section 1.4. In general, TFR i values were the midpoint flow rates for each tubular compartment, calculated as the average of the flow rates at the beginning and end of each tubular region (Table 4.2). Table 4.1 Physiological parameter values used for tubular compartments in the minimal physiologically-based reabsorption model TSA i (m 2 ) TFR i (ml/ min) a PT ( ) LoH 0.16 b 33.6 ( ) DT 0.21 b 17.8 ( ) CD b,c 6.3 ( ) PT Proximal tubule; LoH Loop of Henle; DT Distal tubule; CD Collecting duct; a Values represent midpoint flow rates, ranges in parentheses represent flows at beginning and end of tubule regions. b TSA LoH, TSA DT and TSA CD calculated accounting for microvilli. c TSA CD includes an exponential function for calculating surface area of inner medulla CD. Table 4.2 Regional filtrate flow rates along nephron tubule, from which midpoint TFR i values were derived for model. The contribution of overall filtrate reabsorption from each region of nephron is also indicated (see Table 1.5 for full literature analysis). Flow rate at beginning of tubule section Flow rate at end of tubule section TFR i (ml/ min) Fraction reabsorbed for section PT LoH DT CD TSA i values were initially calculated following the assumption of the surface area of a cylinder, using length and diameter of tubular region (Tables 1.6 and 1.7), and the number of nephrons was assumed to be 900,000 nephrons/ kidney. Special consideration was made for the CD compartment, due to the merging of nephrons to form the cortical CD, and merging of CDs in the inner medulla. To account for this, the number of nephrons was reduced to 90,000 nephrons/ kidney for the cortical and outer medulla CD, with surface area calculated following assumption of a cylinder. The surface area of the inner medulla CD was calculated using an exponential function shown in Eq (details provided in the appendix, section 8.3.3) which accounts for the 105
106 concomitant decrease in number and increase in diameter of CD, as they traverse towards the renal pelvis. C x = (d 0 NCD 0 π)e ( ( x F n ) ln 2 1 d 0 F ( d n )) Where d 0 and d n are the diameter of inner medulla collecting ducts at the papilla apex and at the outer medulla-inner medulla boundary, NCD 0 is the number of inner medulla CD at the papilla apex, and F is the number of fusion events. C x represents the total circumference of inner medulla CD throughout this region at x mm from the papilla apex. The inner medulla CD surface area is the area under the curve of this function between 0 and n, where n is the length of the inner medulla CD (length = inner medulla width = 11 mm). Microvilli are present extensively in epithelial cells of the proximal tubule where they form a brush border; in contrast, microvilli are sparse in tubular cells of other regions (359, 360). Additionally, there is evidence of the expression of microvilli in the Caco-2 cells. Therefore, a microvilli correction factor was applied to TSA i values in the DT, LoH and CD compartments to account for the differential presence of microvilli along the renal tubule relative to the PT region and Caco-2 cells (see appendix, section for further details. This approach resulted in a 7.5-fold decrease in the estimated surface area compared to that calculated using the assumption of open cylinder for the LoH, DT and CD compartments. The method used here is analogous to a recently published approach to estimate regional specific effective permeability for intestinal PBPK models used to predict drug absorption (361) Empirical relationship between P app and observed F reab The empirical relationship between P app and F reab was best described by the Hill model (Eq. 4.16), consistent with the relationship already defined between P app across Caco-2 cell monolayers and fraction absorbed following oral administration (362) F reab ' = P app a b a + P app a 4.16 Where a represents the slope factor and b is the value of P app at which F reab equals 0.5. The Hill model was fitted to the data using nonlinear regression to estimate best-fit values and 95% confidence interval (CI) of parameters Calibration of Caco-2 permeability data Permeability data from Caco-2 cell monolayer assays generally vary between assay formats and laboratories (362). To account for this and to allow the Caco-2 data in the present study to be transferable to other assay formats/ laboratories, a P app -F reab calibration method was explored by splitting the available P app and F reab data into reference and internal validation subsets. It is important to clarify that this calibration is not intended to predict F reab using a data driven empirical model, analogous to quantitative structure-pharmacokinetic relationship models, which require formal internal and external validation techniques e.g. (70). The calibration approach is proposed as a pragmatic method to account for inter-assay/ inter-laboratory differences, although herein it is applied to the current dataset only, and not external data as intended for future studies. 106
107 The reference drugs (n=11) were selected to cover a range of P app and F reab values and are representative of the overall relationship between P app and F reab. The relationship between P app and the predicted F reab based on the minimal reabsorption model was best described by the Hill model, analogous to the relationship between P app and observed F reab discussed above. Empirical calibration was performed using the reference dataset of 11 drugs to account for any discrepancy between predicted and observed F reab. This approach allowed subsequent calculation of calibrated P app values for the drugs in the internal validation dataset (n=34), using Eq (see appendix, section for derivation). P app,calibrated = b ( a 2) 1 P a app 1 b 2 ( a 2 a 1 ) 4.17 Where a 1 and b 1 are the slope factor and P app value at which F reab is equal to 0.5 for the P app vs F reab Hill equation fitted against data predicted by the minimal model for the reference dataset; a 2 and b 2 are the Hill equation parameters obtained from fitting P app values for the reference drugs (P app,ref ) and corresponding observed F reab Data analysis Nonlinear regression was carried out using MATLAB R2012a (The MathWorks Inc., Natick, MA, USA, All other data analyses were performed using MS Excel. The model performance was assessed based on the R 2 of the predicted vs. observed linear regression, and by considering the number and percent of drugs predicted within 3-fold of the observed CL R. The performance of models with different physiological complexity was assessed for drugs with low (F reab <0.25), medium (F reab = ) and high passive tubular reabsorption (F reab >0.75). These cut-off values were arbitrarily selected to assess potential differences in trends between these groups of drugs. Bias and precision in predicting CL R and F reab were calculated as geometric fold error (gmfe) in Eq and root mean squared error (rmse) in Eq (363). The gmfe indicates an absolute deviation from the observed data, as this metric does not allow over- and under-predictions to cancel each other out. 1 gmfe = 10n log 10 (Predicted Observed ) 4.18 RMSE = 1 n (log(observed) - log(predicted))
108 4.3 Results Collation of a comprehensive renal clearance database CL R, f u,p and transporter interaction data were collated for 157 drugs; details are listed in the appendix, Tables 8.7 and 8.8. On average 4 5 clinical studies and 40 CL R measurements were obtained per individual drug, although this varied depending on availability of data. No attempt was made to separate intra- and inter-subject variability. Following analysis using the I 2 statistic, three studies/ trials were classified as anomalous results and excluded; details are listed in the appendix, Table 8.9. Overall weighted mean CL R ranged from (isoxicam) to 526 ml/min (metformin), while f u,p ranged from 0.01 (olmesartan) to 1 (metformin). Measurements and estimates of GFR (n=1686) were reported from 200 clinical studies (28% of studies collated). Only two of the studies collated used inulin to measure GFR; the remainder used either creatinine clearance, estimated GFR from plasma creatinine concentrations, or did not specify the method. Overall weighted mean GFR was ± 25.2 ml/min, with study mean values ranging from 69.2 to ml/min. Of the 157 drugs, 72 were classified as net secreted (clearance ratio > 1.5). As such, they were regarded unsuitable for assessing a model of tubular reabsorption and therefore excluded from further analysis. Glomerular filtration or reabsorption was the dominant mechanism for the remaining 85 drugs (clearance ratio < 1.5). Of these, a representative subset of 45 drugs was selected for the assessment of the predictive performance of the mechanistic tubular reabsorption model. These drugs covered a range of CL R values, from 0.02 (isoxicam) to ml/min (atenolol); the extent of tubular reabsorption ranged from none (six drugs including atenolol and verapamil) to reaching complete equilibrium (F reab = 1, isoxicam). Drugs were also selected to ensure a range of physico-chemical properties were represented, although neutral and basic drugs represented the majority, with 15 and 16 drugs, out of the set of 45, falling into these categories, respectively (Table 4.3). Approximately one third of the drugs have been reported to be substrates of human drug transporters known to be expressed in the kidney. Corresponding P app values obtained in the Caco-2 cells under the ph gradient conditions covered approximately 3 orders of magnitude, as shown in Table 4.3. Lipophilic drugs, and those unionised at physiological ph, were associated with low CL R, (Figures 4.2 and 4.3), in agreement with previous studies (68, 69). No clear trends could be established between LogD 7.4 or LogD 6.5 and observed F reab of 45 drugs investigated (data not shown). High LogD 7.4 did not appear to be predictive of high tubular reabsorption (F reab >0.75) as a wide range of LogD 7.4 values were associated with drugs in moderate to low F reab category (Figure 4.2). The majority of neutral drugs (62%) had high tubular reabsorption (F reab > 0.75), whereas the majority of ionised drugs had low F reab (<0.25). The percent of acidic, basic, zwitterion and amphoteric drugs with F reab < 0.25 was 43%, 56%, 61% and 100%, respectively (Figure 4.3). 108
109 Figure 4.2 Average LogD oct (ph 7.4) of drugs grouped by low (< 0.25), medium ( ) and high (0.75-1) F reab. The values for the classification system were arbitrarily chosen. Error bars indicate 1 standard deviation. Both measured and calculated LogD oct (ph 7.4) data were included. 109
110 Figure 4.3 Number of drugs classified as having high (0.75-1; blue), moderate ( ; red) and low (<0.25; green) observed F reab values. The values for the classification system were arbitrarily chosen. (A) All drugs for which filtration of reabsorption was the dominant mechanism of CL R (n=85). Drugs predominantly neutral (n=24) (B) or ionised as acids (n=14) (C), bases (n=25) (D), zwitterions (n=18) (E) or amphoteric (n=2) (F) at ph
111 Table 4.3 In vivo, physico-chemical properties and in vitro data for 45 drugs used to assess the minimal model of tubular reabsorption. References for CL R and indications of transporter affinity are provided in the appendix, Tables 8.7 and 8.8 Drug CL R (ml/ min) F reab a P app ( 10-6 cm/s) f u,p pk a (acid) pk a (base) Ionisation at ph 6.5 b LogD c Indication of 7.4 LogD 6.5 transporter affinity Antipyrine Neutral N/A Aprindine , 5.85 d Base N/A Atenolol Base c OCT2 Betamethasone Neutral P-gp Betaxolol Base N/A Caffeine Neutral 0.11 c 0.11 N/A Chlorpheniramine , 5.15 Base N/A Chlorpropamide Acid N/A Citalopram d Base N/A Dapsone Neutral N/A Difloxacin Zwitterion N/A Doxepin d Base N/A Fluconazole Neutral P-gp Gabapentin d d Zwitterion OCTN1 Grepafloxacin d 8.74 d Zwitterion P-gp Imipramine Base N/A Irbesartan Acid N/A Isoxicam Acid N/A 111
112 Drug CL R (ml/ min) F reab a P app ( 10-6 cm/s) f u,p pk a (acid) pk a (base) Ionisation at ph 6.5 b LogD c Indication of 7.4 LogD 6.5 transporter affinity Levetiracetam Neutral c N/A Linezolid Neutral N/A Melagatran , 8.16 d Amphoteric P-gp Metoprolol Base OCT2 Metronidazole Neutral N/A Mexiletine Base N/A Moclobemide d Base N/A Moxifloxacin Zwitterion P-gp, MRP2 Oxprenolol Base N/A Oxytetracycline , 7.32, 9.11 d d Zwitterion -4.7 c P-gp Pefloxacin Zwitterion P-gp Prednisolone Neutral 1.62 c 1.62 P-gp Prednisone Neutral Weak P-gp interaction Probenecid Acid N/A Propafenone d Base N/A Propylthiouracil Neutral N/A Ribavirin Neutral c N/A Ropivacaine Base N/A Sparfloxacin Zwitterion P-gp
113 Drug CL R (ml/ min) F reab a P app ( 10-6 cm/s) f u,p pk a (acid) pk a (base) Ionisation at ph 6.5 b LogD c Indication of 7.4 LogD 6.5 transporter affinity Sulfamethoxazole Acid N/A Tetracycline , 7.63, 8.77 d d Zwitterion Weak OAT3 interaction Theophylline Neutral N/A Tocainide d Base N/A Topiramate d - Neutral MATE2K Venlafaxine Base N/A Verapamil Base P-gp, OCTN1, OCTN2 Voriconazole Neutral N/A a Apparent negative F reab are result of inclusion criteria (clearance ratio < 1.5) and were changed to 0 for graphical presentation; b Drugs were classified using the predominant species (>50%) at ph 6.5, using either measured or calculated pk a data; c LogD 6.5 calculated from pk a and LogD 7.4, as described in the Methods; d LogD 7.4 and pk a values predicted using ACD (v.14.02) or obtained from BioByte Masterfile (otherwise measured experimentally as described in the Methods); N/A No information available whether a drug was a substrate for renal drug transporters. 113
114 Predicted CL R (ml/ min) Prediction of CL R from glomerular filtration only Predictive performance of the tubular reabsorption model was assessed using a subset of 45 drugs. Initial analysis was performed by assessing CL R prediction assuming that the glomerular filtration was the only contributing mechanism (Eq. 4.8). This approach resulted in general overprediction of CL R, as shown in Figure 4.4 and Table 4.4; the predicted CL R for each individual drug are listed in Table 4.5. The extent of over-prediction (> 3-fold) was particularly apparent for neutral (10 out of 15 drugs) and acidic drugs (4 out of 5 drugs). Antipyrine (neutral), caffeine (neutral), and isoxicam (acid) were the most pronounced outliers and the extent of over-prediction of CL R ranged from 75- to 202- fold for these drugs. Conversely, the majority of basic drugs were predicted well, especially betaxolol, citalopram, metoprolol and venlafaxine. For these four basic drugs, the assumption of glomerular filtration in isolation resulted in predicted CL R within 10% of the observed values Observed CL R (ml/ min) Figure 4.4 Comparison of predicted and observed CL R using CL R,filt alone to predict CL R. Neutral ( ), basic ( ), acidic ( ), zwitterion ( ) and amphoteric ( ) drugs are indicated. Solid and dashed lines represent line of unity and 3-fold error, respectively 114
115 Table 4.4 Assessment of the physiologically-based tubular reabsorption model for prediction of CL R. Performance of the mechanistic model was assessed initially for all drugs with a measured Caco-2 P app value with the exception of those that showed evidence of net secretion (clearance ratio >1.5). Subsequently, the tubular reabsorption model was reassessed after excluding drugs currently identified as substrates for drug transporters expressed within kidney. R 2 # (%) of drugs within 3-fold of observed CL R CL R,filt only gmfe RMSE All drugs (45) (58%) Neutral (15) (33%) Acid (5) (20%) Basic (16) (88%) Zwitterion (8) (63%) Amphoteric (1) N/A 1 (100%) Minimal model All drugs (45) (87%) Neutral (15) (87%) Acid (5) (80%) Basic (16) (88%) Zwitterion (8) (88%) Amphoteric (1) N/A 1 (100%) Non-substrates of renal transporters (29) (86%) Neutral (10) (80%) Acid (5) (80%) Basic (13) (92%) Zwitterion (1) N/A 1 (100%) Amphoteric (0) N/A N/A N/A N/A 115
116 Table 4.5 Overview of various CL R predictions for 45 drugs compared with observed CL R Drug Name Apparent F reab Apparent F reab Reference drug for P app -F reab calibration? Observed a Filtration only b Reabsorption model c CL R (ml/ min) P app -F reab calibration d Proximal tubule only e No correction for microvilli f Antipyrine Yes Aprindine No Atenolol No Betamethasone No Betaxolol No Caffeine Yes Chlorpheniramine Yes Chlorpropamide No Citalopram No Dapsone No Difloxacin No Doxepin No Fluconazole No Gabapentin No Grepafloxacin No Imipramine No Irbesartan No Isoxicam No
117 Drug Name Apparent F reab Apparent F reab Reference drug for P app -F reab calibration? Observed a Filtration only b Reabsorption model c CL R (ml/ min) P app -F reab calibration d Proximal tubule only e No correction for microvilli f Levetiracetam No Linezolid Yes Melagatran No Metoprolol No Metronidazole No Mexiletine No Moclobemide No Moxifloxacin No Oxprenolol Yes Oxytetracycline Yes Pefloxacin No Prednisolone No Prednisone No Probenecid No Propafenone No Propylthiouracil No Ribavirin Yes Ropivacaine No
118 Drug Name Apparent F reab Apparent F reab Reference drug for P app -F reab calibration? Observed a Filtration only b Reabsorption model c CL R (ml/ min) P app -F reab calibration d Proximal tubule only e No correction for microvilli f Sparfloxacin Yes Sulfamethoxazole No Tetracycline No Theophylline Yes Tocainide Yes Topiramate No Venlafaxine No Verapamil No Voriconazole Yes a Observed CL R is overall weighted mean of values obtained from a literature survey; b CL R,filt calculated using Eq. 4.7; c CL R predicted using tubular reabsorption model, as per Eq ; d CL R predicted using the tubular reabsorption model after calibration of P app data using Eq. 4.14; e CL R predicted using a model with only one tubular compartment representing proximal tubule (main contributor to reabsorption predicted by the model); f No correction was made for surface area attributable to presence/ absence of microvilli when calculating CL R,int,reab,i. 118
119 4.3.3 Prediction of F reab using the minimal physiologically-based tubular reabsorption model The IVIVE approach was used to predict the F reab and CL R from Caco-2 P app data using the minimal mechanistic tubular reabsorption model, as outlined in the Methods (Eq ). The observed F reab were calculated from reported CL R and f u,p data, assuming the GFR value of 120 ml/min (Figure 4.5). In some instances, apparently negative F reab and F reab values were obtained (e.g. atenolol and verapamil); this is an artefact of the inclusion criteria applied, given that the renal clearance ratio was used as cut off for net secretion (> 1.5). Drugs with apparent negative F reab values were plotted as zero in the figures presented herein. Overall there was a good agreement between predicted and observed F reab, albeit with over-prediction of F reab for some drugs with moderate P app values (~20-40 x 10-6 cm/s). The predicted CL R were calculated for 45 drugs using the predicted F reab, as well as GFR and urine flow (Table 4.5). There was a good agreement between predicted and observed CL R data with <2-fold bias for the whole dataset (Figure 4.6 and Table 4.4). In particular, this trend was evident for neutral drugs (gmfe = 1.86), where 87% of CL R were predicted within 3-fold error of the observed value with antipyrine and caffeine being the only exceptions (over-prediction of 4.1 and 6.8-fold, respectively). In the case of basic drugs, a general CL R under-prediction trend was noted, as well as poor precision (RMSE = 31.0), in agreement with the over-prediction of F reab seen for this class of drugs (Figure 4.5). Consideration of both glomerular filtration and reabsorption reduced the prediction accuracy in the case of betaxolol, citalopram, metoprolol and venlafaxine. However, the predicted CL R was still within 50-65% of the observed data. Despite this overall CL R under-prediction trend, it is important to note that the values for the majority of basic drugs (14/16) were predicted within 3-fold of the observed. Mexiletine and verapamil were the most pronounced outliers as the predicted CL R represented only 16 and 29% of the observed value, respectively. Consideration of ph gradient and ionization was of particular relevance for this class of drugs, as the use of P app data obtained under isotonic ph 7.4 conditions resulted in pronounced under-prediction of CL R for half of basic drugs in the dataset, with predicted CL R <35% of the observed value (appendix, Figure 8.8). Excluding drugs which were known/ reported substrates of human kidney transporters had negligible impact on the success of CL R prediction (Table 4.4). In contrast, failing to account for presence/ absence of microvilli expressed by Caco-2 cell monolayers and nephron tubular cells resulted in reduced prediction success with only 27% of drugs predicted within 3-fold of the observed CL R. Comparison of predictive performances of different models stratified according to low, medium and highly reabsorption drug status is shown in Table
120 F reab P app (x 10-6 cm/ s) 100 Predicted/ Observed F reab P app (x 10-6 cm/ s) Figure 4.5 Comparison of observed and predicted F reab using the mechanistic tubular reabsorption model and P app data obtained in Caco-2 cells. Panel a: Predicted relationship between F reab and P app is shown by the solid black line, whereas observed data are shown by symbols; Panel b: Predicted/ Observed F reab are plotted as symbols, whereas solid and dashed black lines indicate Predicted/ Observed = 1 and 3-fold error, respectively. Symbols indicate neutral ( ), basic ( ), acidic ( ), zwitterion ( ) and amphoteric ( ) drugs. Drugs with negative values for observed F reab are plotted as F reab = 0 in Panel A, or Predicted/ Observed F reab = 100 in Panel B, as described in the Methods. 120
121 Predicted CL R (ml/ min) Observed CL R (ml/ min) Figure 4.6 Comparison between observed and predicted CL R by the mechanistic tubular reabsorption model Symbols indicate neutral ( ), basic ( ), acidic ( ), zwitterion ( ) and amphoteric ( ) drugs respectively. Solid and dashed lines represent line of unity and 3-fold error, respectively. The inset shows the data for lower CL R values for clarity 121
122 Table 4.6 Assessment of the predictive performance of various CL R prediction methods using gmfe and % predicted within 3-fold of observed CL R gmfe (% predicted within 3-fold of observed) Filtration only a No correction for microvilli b Proximal tubule only c Reabsorption model d P app -F reab calibration e All drugs (n = 45) 3.73 (58%) 5.35 (27%) 2.17 (76%) 1.96 (87%) 1.65 (91%) Low F reab (n = 17) 1.17 (100%) 5.02 (35%) 1.59 (94%) 1.97 (88%) 1.34 (94%) Medium F reab (n=12) 2.56 (75%) 8.52 (17%) 1.44 (92%) 1.90 (92%) 1.73 (92%) High F reab (n = 16) (0%) 4.03 (25%) 4.11 (44%) 2.01 (81%) 1.98 (88%) a CL R,filt calculated using Eq. 4.7; b No correction was made for surface area attributable to presence/ absence of microvilli when calculating CL R,int,reab,i ; c CL R predicted using a model with only one tubular compartment representing proximal tubule (main contributor to reabsorption predicted by the model); d CL R predicted using tubular reabsorption model, as per Eq ; e CL R predicted using the tubular reabsorption model after calibration of P app data using Eq. 4.14, data are for all drugs including reference subset. 122
123 4.3.4 Empirical relationship between F reab and P app and calibration approach The Hill model was fitted to the observed F reab and P app data for the 45 drugs selected (Figure 4.7). The best-fit value and 95% confidence interval for Caco-2 P app corresponding to F reab = 0.5 was estimated to be 34.4 ( ) 10-6 cm/s. In addition, the Hill model was fitted to the P app values and F reab predicted by the mechanistic tubular reabsorption model for 45 drugs. The resultant estimate of P app corresponding to F reab = 0.5 was 14.8 ( ) 10-6 cm/s. In order to evaluate the application of P app -F reab calibration (Eq. 4.17), 11 drugs were selected as reference calibrator drugs (P app,ref ) covering a representative range of F reab and P app values (Table 4.7). The remaining 34 drugs were treated as an internal validation set. Following the fitting of the Hill equation to observed F reab and P app,ref data for these 11 reference drugs, the bestfit estimates for a 2 and b 2 were 2.74 and cm/s. These were used to calculate values of P app,calibrated for the validation dataset, and were subsequently applied for prediction of F reab, F reab and CL R using the minimal physiologically-based reabsorption model, as done initially. Use of this calibrated approach led to considerable improvement in the predictive performance with 31/ 34 drugs in the internal validation set predicted within 3-fold of the observed CL R (gmfe = 1.73, Figure 4.8). Comparable success was seen for the full dataset, with 41/ 45 drugs predicted within 3-fold of the observed CL R, and reduced bias (gmfe = 1.65) compared to the model before applying the P app calibration. Particular improvement in the prediction of CL R following the P app -F reab calibration was apparent for basic drugs, as 15/ 16 drugs were successfully predicted. An increase in prediction accuracy was also observed for neutral drugs (e.g., prednisone) and zwitterions (e.g., moxifloxacin) with moderate P app values ( cm/s). The calibration approach resulted in marginal overall improvement in the prediction of CL R for drugs with F reab >0.75. However, substantial improvement was noted for 9/ 11 highly reabsorbed drugs (F reab > 0.9), including isoxicam, probenecid, caffeine and antipyrine. 123
124 F reab ' P app (x 10-6 cm/ s) Figure 4.7 Best-fit curve and 90% confidence interval of the Hill equation to the P app and F reab data for 45 drugs (solid and dashed lines respectively). Symbols indicate neutral ( ), basic ( ), acidic ( ), zwitterion ( ) and amphoteric ( ) drugs. Drugs with negative values for observed F reab are plotted as F reab = 0, as described in the text Table 4.7 Reference drugs used for calibration of Caco-2 P app data a CL Drug R P F app,ref P app,ref,calibrated (ml/min) reab (x 10-6 cm/s) (x 10-6 cm/s) Ionisation at ph 6.5 Antipyrine Neutral Caffeine Neutral Chlorpropamide Acid Linezolid Neutral Oxprenolol Base Oxytetracycline Zwitterion Ribavirin Neutral Sparfloxacin Zwitterion Theophylline Neutral Tocainide Base Voriconazole Neutral a P app,ref,calibrated calculated using Eq after fitting of the Hill equation to the Caco-2 P app data and either observed or predicted (using minimal model of reabsorption) F reab. 124
125 PredictedCL R (ml/ min) Observed CL R (ml/ min) Figure 4.8 Prediction of CL R using the minimal model following calibration of P app data using reference drugs (n=45 drugs). Symbols indicate neutral ( ), basic ( ), acidic ( ), zwitterion ( ) and amphoteric ( ) drugs in the internal validation dataset and drugs in the reference dataset ( ; n=11). Solid and dashed lines represent line of unity and 3-fold error, respectively. The inset shows the data for lower CL R values for clarity 4.4 Discussion Physiological considerations for predicting tubular reabsorption The 5-compartment minimal physiologically-based model was developed for the prediction of tubular reabsorption. Although static, the model accounted for physiological differences between regions of the nephron and captured complex underlying physiology of the kidney in a mechanistic manner. In addition to the model development, a comprehensive database of physiological parameters with relevance to pharmacokinetics of drugs in human kidney was collated. Quantitative human physiological data were sparse in general and available from a few primary research articles, but were supported by data in preclinical species where possible (see Chapter 1, Tables 1.5, 1.6 and 1.7). For some physiological parameters, such as glomerular filtration rate, considerable inter-individual variability, as well as bias in some of the commonly used methods has been reported (150, 350, 351). The lack of data and information on variability in reported values for physiological parameters will inevitably result in a level of uncertainty associated with 125
126 the relevant input model parameters. For example, calculation of apparent F reab was dependent on the value of GFR; the sensitivity of F reab to changes in GFR was evident for drugs exhibiting low tubular reabsorption (F reab <0.25). However, as the reported data on the inter-individual variability in GFR were limited in the clinical studies in the database, its potential impact on the estimation of apparent F reab was not considered in the current analysis. Predictive performance of the mechanistic tubular reabsorption model was assessed against a representative set of 45 drugs, focusing in particular on the impact of the tubular surface area in the model development (Table 4.1 and Table 4.6). The mechanistic tubular reabsorption model was applied to predict both regional and overall F reab for the selected dataset. The analysis has shown that reabsorption in the proximal tubule compartment was the major contributor to the overall predicted F reab for most of the drugs. Consideration of tubular reabsorption solely in this region within the model had marginal impact on the prediction of CL R for drugs with low F reab (e.g., atenolol and melagatran). In contrast, this approach resulted in reduced CL R prediction accuracy compared with the ultimate 5-compartment model for extensively reabsorbed drugs such as antipyrine and isoxicam (Table 4.6). The reabsorption in other tubular regions was not considered in the previously reported static model (71). However, it is important to note that in the study by Kunze et al. only one drug in the dataset exhibited notable net reabsorption (desipramine, apparent F reab of 0.31) and drugs showing extensive reabsorption (F reab ~ 1, CL R approaching 1-2 ml/min, (51)) were not included in the analysis. The 5-compartment tubular reabsorption model was also able to account for regional differences in the expression of microvilli and subsequent effect on the surface area to be used for scaling of permeability data. A pronounced under-prediction of CL R was observed for most of the drugs in the dataset if differences in microvilli related surface area between Caco-2 monolayers and the loop of Henle, distal tubule and collecting duct regions were ignored (Figure 4.9); the exceptions were drugs with low P app (e.g., melagatran). All of the above emphasizes the necessity for appropriate interpretation and implementation of complex physiological features of human kidney in the model to allow mechanistic prediction of CL R. 126
127 Predicted CL R / Observed CL R P app (cm/ s x 10-6 ) Figure 4.9 Comparison of the predicted CL R / observed CL R ratio obtained using the reabsorption model with (orange circles) and without (blue open triangles) correction for the presence/ absence of microvilli. Predicted CL R / observed CL R ratios of 1 (solid), 0.33 and 3 (dashed) are indicated by horizontal lines (i.e. unity and 3-fold error respectively). The model includes a correction factor (7.5-fold reduction), applied to the tubular surface area parameter (TSA i ) of the loop of Henle, distal tubule and collecting duct compartments of the model. This correction factor accounts for the scarcity/ absence of microvilli in these tubular sections, in contrast to Caco-2 cells (and proximal tubule cells), which have a distinctive brush border Validity of Caco-2 cell monolayers as in vitro model for renal tubular reabsorption The ability to cross biological membranes is an important determinant of rate of absorption and rate and route of elimination (364, 365). Existing in vitro proximal tubule models express a range of functional drug transporters, confounding measurement of passive permeability in the nephron (71, 86, 366). Although primary cultured collecting duct cells can be used for this purpose, these methods require a consistent supply of high quality kidney tissue (367). Caco-2 cell monolayer assay performed in the presence of a transporter inhibitor cocktail is widely used to measure passive permeability and was therefore considered in the current work. Alternatively, permeability data obtained in MDCK cells (74, 368) can also be considered for prediction of F reab. Further studies are needed to validate their application in the current model, in particular the adequate implementation of the microvilli/ surface area considerations highlighted above. Differences in endogenous transporter expression and activity between cell lines are a potential limitation for using MDCK cells to generate P app data to use in the model. However, inclusion of a transporter inhibitor cocktail in the assay media, as per the current study, could be used to reduce any transporter related divergences in P app data from different cell lines. 127
128 Beyond inter-system differences in permeability (e.g. Caco-2 vs. MDCK vs. nephron tubule cells), inter-laboratory variability has been widely reported for experimental data generated in either Caco-2 or MDCK cells (362, 369). Reference compounds are often used to standardise in vitro assay data and minimise the impact of this variability on the subsequent IVIVE (88, 362, 370). The P app -F reab calibration approach proposed here can be used in that context. While this method resulted in the improved prediction of CL R (Figure 4.8 and Table 4.6), the choice of drugs used as reference dataset was fundamental. As such, the P app -F reab calibration proposed here should be applied with caution; further work is needed to refine and validate this approach using an external dataset of Caco-2 (or MDCK) P app data. The Caco-2 permeability data were measured under a ph-gradient in order to mimic the slightly acidic nature of urine typically found in vivo. The ph consideration was particularly important for basic drugs because the use of permeability data under iso-ph conditions resulted in systematic under-prediction of CL R for this class (appendix, Figure 8.8). However, despite the use of the phgradient assay conditions, an apparent over-prediction of F reab was still evident for some basic drugs, mostly those with moderate P app (approx cm/s). Two ph-related mechanisms could potentially contribute to this under-prediction. Firstly, a reduced fraction of unionised drug in acidic conditions may affect the permeation rate of the total (ionised and unionised) drug. Secondly, for basic drugs the concentration gradient of the unionised drug in vivo could be reduced in acidic conditions (due to water reabsorption), or in extreme cases reversed which would not be represented by the typical in vitro permeability assay. Additionally, it is important to consider that if left uncontrolled, urine ph can vary substantially in clinical studies (371), which can confound subsequent pharmacokinetic analyses and estimation of CL R and F reab used for model validation. Finally, potential contribution of tubular reabsorption via active transport in vivo via as yet unknown transporter-substrate interactions should not be disregarded Application of the mechanistic tubular reabsorption model and existing gaps Use of the mechanistic tubular reabsorption model in conjunction with in vitro permeability data in a pure bottom-up manner resulted in CL R prediction accuracy comparable with quantitative structure-pharmacokinetic relationships and allometric approaches. In contrast to other methods, the added advantage of this IVIVE approach is the mechanistic insight into renal drug elimination because of the physiological nature of the model. This model provides solid foundation to inform future PBPK efforts towards understanding mechanisms behind changes in CL R following pathophysiological changes in kidney, by accounting for the effects of factors such as age and renal impairment on the values of physiological parameters. For example, the lengths of each region of the tubule used in the model are representative of values reported for the adult population (Chapter 1, Table 1.7); accounting for reported changes in proximal tubule length due to age (117, 372), as well as any changes in other physiological parameters, could be used to investigate potential differences in tubular reabsorption in young adult, paediatric and geriatric populations. Accuracy of CL R prediction using the tubular reabsorption model was consistent across all ionisation groups, with a slightly higher bias seen for acidic drugs (Table 4.4). CL R was over- 128
129 predicted by more than 4-fold for three drugs (antipyrine, caffeine and isoxicam), which all had apparent F reab values For these drugs, predicted CL R were very sensitive to even minor relative changes in F reab. However, the differences between predicted and observed CL R for drugs with very high F reab represented 15% of the plasma clearance of these drugs in healthy subjects, as they are also extensively metabolised by the liver ( ). Therefore, potential errors in prediction of CL R for extensively reabsorbed drugs were less likely to have a substantial impact on prediction of the overall in vivo clearance for such drugs. The dataset used for model assessment here included several drugs (e.g., caffeine, antipyrine) which are extensively reabsorbed and are known to exhibit urine flow and ph dependent CL R ( ). It is beyond capability (and purpose) of the model to describe/ predict urine flow dependent CL R quantitatively, as reported by some of the previous modelling efforts (78, 219, 220). However, modulation of the TFR CD and urine flow rate parameters in the model can be used to indicate whether a drug is expected to exhibit urine flow dependent CL R, as shown by the sensitivity analysis presented in Figure It is anticipated that incorporation of the IVIVE for tubular reabsorption into existing PBPK kidney models, e.g., (78), might allow for more accurate predictions of changes in tubular reabsorption and CL R due to changes in urine flow rates. Whereas IVIVE for renal drug metabolism has been investigated in several studies (38, 60), prediction of CL R,sec using IVIVE is currently challenging due to lack of a gold standard in vitro system and physiologically relevant scaling factors (71, 79, 87, 107, 226). Static models of CL R,sec (87) could be used alongside the current reabsorption model for prediction of CL R in a simple approach using Eq. 4.1; however, such an approach is unlikely to be adequate when metabolism is simultaneously involved. While consideration of metabolism and secretion was outside of the scope of the current model, these elements have been parameterised in other models with various levels of complexity e.g., (78). Such models could be adapted to additionally incorporate mechanistic description of tubular reabsorption using the principles of the current model. In addition, refinement of systems parameters of existing PBPK kidney models could be possible using the data on kidney physiology collated in the current study. Although some models allow for metabolism-transport interplay to be investigated (78), obtaining suitable clinical data to validate such models remains challenging (51). Finally, considering a conservation of the tissue level organisation between mammalian species (e.g., regional differentiation of nephron), the mechanistic tubular reabsorption model could be adapted for prediction of F reab and CL R in preclinical species, by accounting for specific differences in surface area and flow rate parameters. 129
130 Figure 4.10 Sensitivity analysis of urine flow rate on predicted CL R for hypothetical drugs compared against observed data for theophylline. Hypothetical drugs had Caco-2 P app values ranging from cm/ s, resulting in predicted F reab values ranging from assuming a urine flow rate of 1 ml/ min (using Eq ). Predicted F reab, and subsequently CL R, were re-calculated by changing the urine flow rate parameter to values ranging from ml/ min (Eq. 4.9), and TFR CD (Eq. 4.11) after recalculation of the mid-point tubular flow rate (Table 4.2). The % change in CL R is calculated using CL R predicted when urine flow rate = 1 ml/ min as baseline. Observed data for urine flow and matching theophylline CL R were extracted from (379), and % change was calculated, using as baseline the overall weighted mean theophylline CL R obtained from the literature survey in the present study (appendix, Table 8.7). The logarithm line of best fit for the observed data was plotted using MS Excel Conclusion A novel 5-compartment mechanistic tubular reabsorption model was developed for prediction of F reab and CL R from Caco-2 P app data. A database of clinical CL R values for 157 drugs was collated, as well as a comprehensive database of physiological parameters with relevance to IVIVE of renal excretion clearance. The mechanistic model successfully predicted CL R for 45 chemically diverse drugs for which filtration or reabsorption appeared to be the dominant mechanism. In addition, empirical P app -F reab calibration method was proposed to account for inter-assay variability in permeability data. The physiological assumptions of the model represent an excellent basis for future studies, e.g., simultaneous consideration of secretion and reabsorption for mechanistic predictions of CL R and prediction of F reab in different pathophysiological conditions (e.g., renal impairment). Overall, the mechanistic model represents an important addition to the currently existing IVIVE toolbox, and a step towards enabling physiologically-based predictions of renal tubular reabsorption, and its contribution to CL R. 130
131 Chapter 5. In vitro-in vivo extrapolation for prediction of tubular reabsorption using a physiologically based pharmacokinetic kidney model 5.1 Introduction IVIVE represents an integral part of mechanistic organ models (1, 380). In the previous chapter, a static mechanistic model of tubular reabsorption was developed to predict the tubular reabsorption for a number of drugs using IVIVE. This model could be incorporated within a wider static mechanistic model of renal excretion, which could provide an opportunity for both active secretion and passive reabsorption to be accounted for when predicting CL R. However, several limitations of the static model were identified. One such limitation was that urine-flow dependent CL R could not be adequately described (Figure 4.10). Furthermore, accounting for active secretion and passive reabsorption in proximal tubule separately (as opposed to simultaneously) does not account for any potential interplay of these processes. In contrast, a PBPK model incorporating a mechanistic model for kidney may not be susceptible to these limitations. PBPK models allow not only the prediction of pharmacokinetic parameters, but also simulation of drug exposure over time in plasma and individual tissues/ organs. A key advantage of PBPK models includes the flexibility of perturbing the system (e.g., impact of DDIs and effects of disease) to allow extrapolation, by changing appropriate system-specific parameters of the model (1). The SimCYP simulator is a widely used PBPK modelling and simulation platform (265, 381), which includes the Mechanistic Kidney Model (MechKiM) module (382). MechKiM represents the nephron as a number of compartments, as shown in Figure 5.1 The glomerulus (blood) and Bowman s capsule (tubular filtrate) make up the renal corpuscle and are each represented by a compartment. The nephron tubule is divided into seven regions, representing proximal tubule subsections S1, S2 and S3, the loop of Henle, the distal tubule and the cortical and medullary collecting duct, with compartments representing the blood, tubular fluid and tubular cells of each region. Despite simplification of many aspects of human kidney, MechKiM still remains a complex mechanistic model, requiring information concerning many physiological parameters (382). Critical analysis of the experimental data informing parameter values in MechKiM reveals that for several parameters there is large uncertainty, and/ or there are few or no studies reporting relevant data (e.g., proximal tubule cellularity, Chapter 1, section 1.4.5). 131
132 Figure 5.1 Structure of the mechanistic kidney model (MechKiM) nested within the whole body physiologically based pharmacokinetic model in the SimCYP simulator (78). The model comprises different compartments which represent the proximal tubule (S1, S2 and S3; yellow), loop of Henle (pale yellow), distal tubule (orange) and collecting duct (cortical and medullary; light blue) regions of the nephron tubule, as well as corresponding tubular cells (green) and vasculature (pink). With kind permission from Springer Science+Business Media: Neuhoff S, Gaohua L, Burt H, Jamei M, Li L, Tucker GT, et al. Accounting for transporters in renal clearance: Towards a mechanistic kidney model (Mech KiM). In: Sugiyama Y, Bente S, editors. Transporters in drug development. New York: Springer; 2013, Figure 7.4, page 165. (78) When systems or drug specific parameter data are lacking, parameter estimation, using clinical pharmacokinetic data, may be necessary to obtain appropriate input values. For models with many parameters, such as MechKiM (Figure 5.1), identifiability may be a problem associated with parameter estimation (383). Furthermore, such an approach may not allow separation of drugand system- specific information, restricting the opportunity to extrapolate into new populations with different pathophysiological features. For example, the passive permeability across the tubular epithelium is currently parameterised by a permeability clearance (CL PD ) in MechKiM, rather than a drug-specific apparent permeability (P app ) and a system-specific tubular surface area (TSA). This contrasts with the IVIVE approach for prediction of tubular reabsorption used in Chapter 4, which has separate P app and TSA parameters Aims The aim of this study was to implement the IVIVE approach developed for prediction of renal tubular reabsorption (Chapter 4) within the SimCYP MechKiM, and simulate urine flow dependent CL R. Caffeine, theophylline and linezolid were selected because they represented a range of F reab values (0.99, 0.91 and 0.58 respectively (Table 4.5)), and for caffeine and theophylline, clinical data were available on the effect of urine flow on their CL R. PBPK models for these drugs were initially developed and/ or verified in the SimCYP simulator without activation of MechKiM module. Following activation of the MechKiM module, tubular surface area (scaling factors) and tubular flow rate (model systems parameters) values were adapted to allow for the differences in the 132
133 compartmental structures of the mechanistic static model from Chapter 4 and the MechKiM. The CL R of caffeine, theophylline and linezolid was then predicted using in vitro permeability data in conjunction with relevant system parameters, and compared with the predictions of the static model. The impact of different assumptions concerning the tubular surface area and tubular flow rates on predictions of CL R were investigated. Further, the ability of the PBPK kidney model to predict the impact of changes in urine flow on CL R for the three drugs was assessed, including comparison against observed data where available. 5.2 Methods Clinical data sources Mean plasma concentration-time profiles and pharmacokinetic parameters were collated from the scientific literature. Pharmacokinetic parameters of interest were the area under the curve (AUC) for the plasma concentration-time profile, the intravenous (i.v.) and oral clearance (CL/ F), volume of distribution at steady state (V ss ) and the renal excretion clearance (CL R ). Where necessary data were digitised using GetData Digitizer (version 2, Collation of CL R data was done as described in Chapter 4, sections and Workflow for PBPK model construction and verification for caffeine, theophylline, linezolid All simulations presented herein were performed using the SimCYP population-based PBPK simulator software, version 14, release 1 (Certara, Sheffield, UK) (265, 381). All simulations involving caffeine, theophylline and linezolid were performed using the default Healthy volunteers population template file provided with the SimCYP simulator. Many key input parameters for each drug (without implementation of the mechanistic kidney model (MechKiM)) were already published (linezolid) in previous versions of the SimCYP simulator and implemented as compound files provided with the new version (caffeine and theophylline) ( ). The workflow used for refinement and verification of compound files in the full PBPK model, as required for use of MechKiM, is shown in Figure 5.2. In full-pbpk model mode, distribution parameters including V ss and tissue-to-plasma partition coefficients were predicted using prediction method 2 (387, 388). Linezolid predicted K p s were optimised by an empirical scalar (same factor for all tissues) which was adjusted in order to recover the observed V SS of 0.7 L/ kg (385). No refinement of predicted K p was necessary for caffeine and theophylline (K p scalar = 1). Metabolic clearance and CL R 1 input parameters for caffeine and theophylline PBPK models were not changed from the default values. Linezolid CL R 1 parameter was informed from the literature analysis in Chapter 4 (Table 4.3). The metabolic clearance parameters for linezolid were obtained using back-calculation of CL int from available i.v. CL data (389), using the well-stirred model and correcting for CL R and fraction metabolised (f m ) by CYP3A4 (0.1), with the remaining elimination assigned to Additional Clearance in the liver. 1 MechKiM was not activated at the initial stage of model refinement/ verification, so CL R was defined using a single input parameter 133
134 Following verification of the clearance and distribution parameters, f a, k a and Caco-2 P app parameters in the first-order absorption model were optimised (for linezolid only) and verified (caffeine, theophylline and linezolid). The f a (1) and k a (2.8 h -1 ) parameters for linezolid were taken as previously published (385), which were based on data from unpublished clinical studies for which formulation and trial design information such as fasted or fed state were not reported. The Caco-2 P app ( P Caco-2 parameter) in the absorption model for linezolid was consistent with that used for IVIVE of tubular reabsorption ( cm/ s in ph 6.5: 7.4 assay format; Chapter 4, Table 4.3), which was calibrated with P app data for atenolol, verapamil and metoprolol as references in the Permeability Calibrator toolbox. Data used for verification were taken from separate clinical studies to those used for parameter refinement and developing the model. During verification and refinement of PBPK models, simulations followed trial designs (dosing route, amount, and frequency; number of individuals and age of subjects) as reported in the respective publications. 20 trials were used for each simulation for verification/ refinement of caffeine, theophylline and linezolid. The clinical studies used for verification and refinement of each drug are listed in Table
135 Figure 5.2 Workflow for development and verification of the full PBPK models for caffeine, theophylline and linezolid. See text for details. Studies used as Verification and Refinement clinical data sets are shown in Table 5.2 With the exception of selecting the full PBPK distribution model as required for use of MechKiM, parameter refinement steps were not required for caffeine and theophylline. Therefore validation against observed data following i.v. administration was not required for these drugs. 135
136 Table 5.1 SimCYP input parameters for caffeine, theophylline and linezolid. The majority of parameters were already implemented in existing SimCYP compound files, or taken from literature (382, ). See text for details of optimised parameters. Coefficient of variation (%) is given in parentheses where applicable. Phys-chem Parameter Caffeine Theophylline Linezolid Mol Weight (g/mol) log P Compound Type Monoprotic base Ampholyte Monoprotic Base pk a pk a 2 N/A 0.99 N/A B/P f u Absorption Absorption model 1 st order 1 st order 1 st order f a 1 (30%) 1 (30%) 1 (30%) k a (1/h) 2.18 (30%) 6 (30%) 2.8 (30%) Q gut Input Predicted Predicted Predicted Permeability assay P Caco-2 P Caco-2 P Caco-2 P app,caco-2 (10E-06 cm/ s) P app,caco-2 Scalar Distribution Distribution Model Full PBPK Full PBPK Full PBPK V ss mode Predicted Predicted Predicted Prediction method Method 2 Method 2 Method 2 K p Scalar Elimination Clearance Type Enzyme Kinetics Enzyme Kinetics Enzyme Kinetics In vitro metabolic system Recombinant Recombinant Recombinant Pathway N1-demethylation N1-demethylation Pathway 1 Enzyme CYP1A2 CYP1A2 CYP3A4 V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A CL int (µl/ min/ pmol of isoform) N/A N/A f u,mic Pathway N1-demethylation N3-demethylation N/A Enzyme CYP2E1 CYP1A2 N/A V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A f u,mic 1 1 N/A Pathway N3-demethylation N3-demethylation N/A Enzyme CYP1A2 CYP2D6 N/A V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A f u,mic 1 1 N/A Pathway N7-demethylation 8-OH N/A Enzyme CYP1A2 CYP1A2 N/A V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A f u,mic 1 1 N/A Pathway N7-demethylation 8-OH N/A Enzyme CYP2E1 CYP2D6 N/A 136
137 Parameter Caffeine Theophylline Linezolid V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A f u,mic 1 1 N/A Pathway OH 8-OH N/A Enzyme CYP1A2 CYP2E1 N/A V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A f u,mic 1 1 N/A Pathway OH 8-OH N/A Enzyme CYP2E1 CYP3A4 N/A V max (µmol/ min/ pmol of isoform) N/A K m (µm) N/A f u,mic 1 1 N/A Pathway OH N/A N/A Enzyme CYP3A4 N/A N/A V max (µmol/ min/ pmol of isoform) 1.8 N/A N/A K m (µm) N/A N/A f u,mic 1 N/A N/A Additional HLM CL int (µl/ min/ mg protein) N/A N/A (30%) Additional HLM f u,mic N/A N/A 0.97 Active Uptake into Hepatocyte CL R (L/ h) a a CL R values for initial PBPK models before activation of MechKiM, derived from clinical data. B/P, blood to plasma partition ratio; CL int, intrinsic clearance; CL R, renal clearance; f a, fraction absorbed; f u fraction unbound in plasma; f u,mic, fraction unbound in microsomes; k a, absorption rate constant; K m, Michaelis-Menten constant; K p, tissue to plasma partition coefficient; log P, logarithm of the octanol-water partition coefficient; P caco-2, permeability across Caco-2 cell monolayers; pk a, acid dissociation constant; Q gut hybrid parameter of blood flow and drug permeability; V max, maximum rate of metabolism; V ss, volume of distribution at steady state. 137
138 Table 5.2 Clinical trials used for verification and refinement of the compound files in v14.1 of the SimCYP simulator prior to simulations using the MechKiM. Basic dosage and demographic information are shown. All subjects were healthy participants. Drug Reference Optimisation/ Verification Dose Information Subjects Information Caffeine (390) Refinement 270 mg SD Oral 6 male, years (391) Verification 50 mg Oral SD 5 male, 21 to 36 years (391) Verification 300 mg Oral SD 5 male, 1 female, 21 to 36 years (391) Verification 500 mg Oral SD 5 male, 1 female, 21 to 36 years (391) Verification 750 mg Oral SD 5 male, 1 female, 21 to 36 years Theophylline (390) Refinement 250 mg SD Oral 6 male, years (392) Verification 125 mg SD Oral 4 male, 4 female, years (392) Verification 250 mg SD Oral 4 male, 4 female, years (392) Verification 375 mg SD Oral 4 male, 4 female, years (392) Verification 500 mg SD Oral 4 male, 4 female, years Linezolid (389) Refinement 500 mg q12h IV 5 male, 1 female, years (389) Refinement 625 mg q12h IV 6 male, years (389) Refinement 375 mg q12h Oral 4 male, 2 female, years (389) Refinement 500 mg q12h Oral 4 male, 2 female, years (389) Refinement 625 mg q12h Oral 4 male, 2 female, years (393) Verification 375 mg SD IV 7 male, 5 female, years (393) Verification 375 mg SD Oral 7 male, 5 female, years (394) Verification 500 mg SD Oral 4 male, years (394) Verification 500 mg SD Oral 3 female years 138
139 5.2.3 Modification of the tubular reabsorption in MechKiM: Physiological parameters and scaling approach The MechKiM parameterises passive permeability of drugs across the tubular epithelium as permeability clearances through the apical (CL PD,apical ) and basolateral (CL PD,basolateral ) membranes. An IVIVE approach was adapted from the static model used for prediction of passive tubular reabsorption in Chapter 4. Apparent permeability (P app ) across Caco-2 cell monolayers, obtained using the ph gradient format of ph 6.5 to ph 7.4 and in the presence of an efflux transporter inhibitor cocktail (50 µm quinidine, 20 µm sulfasalazine, 100 µm benzbromarone) was used as in vitro input, as described in section Apparent membrane permeability (P mem ) was calculated on the basis that resistance is the inverse of permeability, and by assuming that the membrane resistance associated with the Caco-2 cell monolayer is attributable to the sum of resistances associated with the apical and basolateral membranes (Eq. 5.1; (395, 396)). This assumes that the permeability (or resistance) of drugs across the apical membrane is equal to that of the basolateral membrane, that there is no significant accumulation or binding of drug within the cell, that the assay is performed under sink conditions, and ignores potential effects of the filter support, aqueous boundary layer, paracellular pathway. 1 P app = 2 P mem 5.1 P mem was scaled to CL PD,apical and CL PD,basolateral using tubular surface area as IVIVE scaling factor for each i th tubular section represented by the model (Eq. 5.2). CL PD,i = P mem TSA i 5.2 Tubular surface areas used were as described in Chapter 4, section Tubular surface area of the proximal tubule, loop of Henle and distal tubule were recalculated using the highest and lowest values collated for the length and diameter of these tubular sections, assuming 900,000 nephrons per kidney. The surface area for collecting duct was consistent with the assumptions detailed in section 4.2.5, which accounts for the merging of nephrons to join the cortical collecting duct, and the branching structure of the collecting duct in the inner medulla. Surface area values were also recalculated with and without correction for microvilli related surface area (7.5-fold) for the loop of Henle, distal tubule and collecting duct sections. To allow compatibility with the MechKiM model, the surface areas for each tubular section were expressed per million proximal tubule cells by correcting surface area by tubule cellularity (60 million tubule cells per gram kidney) and kidney weight (341 g per individual). The final surface area values for each tubular section under the various assumptions tested are listed in Table 5.3. The tubular flow rate input parameter values were the same as those used for the static model (Chapter 4), with one exception. As MechKiM automatically changes the GFR to mirror user changes to the tubular flow rate in the proximal tubule compartment, the flow rate in the proximal tubule was set to 120 ml/ min. The tubular flow rates in the loop of Henle, distal tubule, collecting 139
140 duct and urine were 33.6, 17.8, 6.0 and 1.0 ml/ min, respectively 2, representing midpoint flow rates in these tubular sections. The corresponding default values in MechKiM were 43.2, 24.0, 11.6 and 1.0 ml/ min respectively, which represent flow rates and the beginning of each tubular region. Prediction of CL R of caffeine, theophylline and linezolid using MechKiM was assessed. Simulations were performed in 100 virtual healthy subjects following a single oral administration of drug. A sensitivity analysis was performed by repeating simulation of CL R using the recalculated tubular surface area and CL PD values described above. Simulated CL R values were assessed by comparison with observed data; criteria for successful prediction was within 2-fold of observed values. 2 Technical note: Upon saving either the population or workspace file in SimCYP simulator, tubular flow rates will return to default values. Therefore simulations must be run immediately after changing the tubular flow rate values in the kidney section of the user interface without saving. Further, changing the tubular flow rates for either the proximal tubule or bladder compartments will result in automatic recalculating of the flow rates for the remaining compartments. 140
141 Table 5.3 Tubular surface area values used as IVIVE scaling factors for calculation of CL PD input parameters. Values were calculated from collated literature data shown in Chapter 1, Tables 1.6 and 1.7. Simulations were performed using the midpoint tubular flow rates, as used in the static model described in Chapter 4. Tubular Section Proximal Tubule a Loop of Henle Distal Tubule Cortical Collecting Duct Medullary Collecting Duct Reabsorption model Surface area (cm 2 / million tubule cells) With MV correction b Without MV correction Lowest Surface area (cm 2 / million tubule cells) Highest Surface area (cm 2 / million tubule cells) With MV correction With MV correction a Proximal tubule represented as three equivalent compartments in mode (other sections represented by one compartment each); b The surface areas are equivalent to those used for the static model of tubular reabsorption (Chapter 4) 141
142 5.2.4 Simulation of urine flow dependent CL R Effects of variations of urine flow on CL R were simulated for each drug. Urine flow rates were varied from 0.2 ml/ min to 11.6 ml/ min, with the assumption that changes in urine flow rate were caused by changes in water reabsorption that occurred only in the collecting duct. This is in accordance with current understanding of the physiological regulation of water balance via a feedback mechanism involving osmoreceptor, arginine vasopressin, and aquaporin (397). The value of 0.2 ml/ min ensured that the lowest observed flow rate in the available clinical data was included in the sensitivity analysis. The highest flow rate investigated (11.6 ml/ min) was determined by the assumed flow rate at the beginning of the collecting duct. Tubular flow rate input parameter values used for the sensitivity analysis are listed in Table 5.4. Higher urine flow rate values (up to approx.15 ml/ min) have been reported in human under extreme water diuresis (154, 157). In addition the permeability of the connecting tubule to water is mediated in part by aquaporin-2 and regulated by vasopressin (397). The connecting tubule is considered an intermediate segment between the distal tubule and collecting duct, which differ in their embryonic origin. The connecting duct is not explicitly defined within the static reabsorption model or MechKiM. Based on the assumptions used for calculating the collecting duct surface area, the connecting tubule should be considered as part of the distal tubule in the context of the current study. Therefore additional simulations were performed by altering the tubular flow rate parameters of the distal tubule, collecting duct and urine flow rate compartments, with urine flow rates between 0.2 and 20 ml/ min Data Analysis Microsoft Excel 2010 was used for data analysis. 142
143 Table 5.4 Tubular flow rate values used in MechKiM to simulate change in urine flow rate in healthy virtual subjects. See text for full details. Urine flow rate (ml/ min) Tubular flow rate parameter value (ml/ min) Proximal tubule Loop of Henle Distal Tubule Collecting Duct Bladder a The tubular flow rates associated with urine flow rate 1 ml/ min are equivalent to those used in the static tubular reabsorption model (with exception of proximal tubule) 143
144 5.3 Results PBPK models without mechanistic models of kidney Compound specific input parameters for the PBPK models of drugs investigated, prior to activation of MechKiM, are listed in Table 5.1. The compound files for caffeine and theophylline did not require refinement for any parameters. The optimised K p scalar parameter for linezolid was 1.8. The metabolic intrinsic clearance parameters for linezolid were estimated from the clinical data as described in the methods. The simulated concentration-time profiles, as well as key pharmacokinetic parameters were generally in good agreement with observed data for all drugs, although the simulated T max appeared to be earlier than that observed for linezolid (Figure 5.3). As accurate description of oral absorption was not considered an essential feature of the model for the purpose of the current study, further refinement of oral absorption was not performed. 144
145 Systemic Concentration (mg/l) Systemic Concentration (mg/l) Systemic Concentration (mg/l) 10 Caffeine Time (h) Theophylline Time (h) Linezolid Time (h) Figure 5.3 Representative simulated plasma concentration-time profiles using initial PBPK models, without mechanistic prediction of CL R Initial PBPK models used a single input parameter derived from clinical data for CL R (i.e. MechKiM was not activated). Pharmacokinetics was simulated following single dose oral administrations (300 mg, 250 mg and 500 mg for caffeine, theophylline and linezolid respectively ( )). Mean (solid lines) 5th and 95th percentiles (dashed lines) of all simulated individuals are compared with mean observed data (points). 145
146 5.3.2 Prediction of CL R using MechKiM CL R was predicted for 3 drugs investigated using MechKiM and following different model assumptions. As expected for the drugs investigated (caffeine, theophylline and linezolid), simulation of CL R without consideration of passive tubular reabsorption (i.e., considering glomerular filtration only) resulted in substantial over-prediction of CL R (Figure 5.4). The IVIVE approach for passive tubular reabsorption was implemented using the MechKiM together with the full-pbpk model of the SimCYP simulator. When physiological features relevant to tubular surface area (i.e., impact of microvilli and collecting duct merging) and tubular flow rates (i.e., use of midpoint values) were considered, simulated CL R was in close agreement with observed values for theophylline (1.2-fold of observed), with over- and under-prediction of CL R noted for caffeine (5.9-fold of observed) and linezolid (0.34-fold of observed) respectively (Figure 5.4). These differences had negligible impact on the simulated plasma concentration for caffeine, whereas a small impact was noted for linezolid (appendix, Figure 8.9). Simulated CL R using MechKiM was lower than CL R predicted using the static model (Chapter 4) for the three drugs investigated, when tubular flow rates and consideration of microvilli related surface area were consistently considered (Figure 5.4). Overall the inconsistencies between simulated and observed CL R were similar to those noted for the static model. Use of the refined mid-point flow rates improved predictive performance of the model for caffeine and theophylline, in comparison to using the default flow rates; the opposite trend was observed for linezolid (Figure 5.4). Using the highest or lowest published tubular section dimensions for calculating the tubular surface area scaling factors resulted in approx fold change in simulated CL R (Figure 5.5). Greater sensitivity in simulated CL R was noted when including/ omitting correction for microvilli in the surface area scalars. 146
147 100 Caffeine Theophylline Linezolid Predicted/ Observed CL R 10 1 Predicted/ Observed CL R 10 1 Predicted/ Observed CL R Default flow rates Refined (midpoint) flow rates 0.1 Default flow rates Refined (midpoint) flow rates 0.1 Default flow rates Refined (midpoint) flow rates Figure 5.4 Simulated CL R using different assumptions for calculating physiological input parameters and scaling factors using MechKiM in SimCYP. Predicted CL R for each of the three drugs was the mean of 100 simulations in virtual subjects. Simulations performed either accounting for glomerular filtration only (i.e., assuming no tubular reabsorption or accounting for both filtration and reabsorption, by incorporation of the IVIVE scaling for passive permeability, with correction for the absence of microvilli in loop of Henle, distal tubule and collecting duct sections of the nephron in the surface area scaling. Simulations for each drug were repeated using either the default tubular flow rates specified in MechKiM (representing flow rates at beginning of tubular region, Table 4.2), or with the refined flow rates tubular flow rates (representing mid-point flow rates in each tubular region Table 4.2). Error bars represent the 5 th and 95 th centiles for 100 simulated virtual subjects. Predictions of CL R using static model (Chapter 4, Table 4.6) for each drug are overlaid. 147
148 Caffeine with MV correction no MV correction Theophylline with MV correction no MV correction Linezolid with MV correction no MV correction Fold change in simulated CL R compared with reabsorption model Figure 5.5 Sensitivity analysis of simulated CL R against tubular surface area, with or without correction for microvilli (MV). All simulations were performed using the refined (midpoint) flow rates. The surface areas used for the static model and implemented in MechKiM (i.e., Reabsorption model in Table 5.3, including correction for MV) were used as the baseline scenario (as per Figure 5.4) (i.e., fold change = 1, horizontal line). Error bars represent fold change in CL R when surface areas were recalculated using the lowest and highest dimensions of nephron regions obtained from literature survey (Table 5.2) Simulation of urine flow dependant CL R The impact of changes in urine flow rate on the simulated CL R of reabsorbed drugs was assessed (Figure 5.6), by changing the relevant tubular flow rate parameters in MechKiM (Table 5.4). The relative change in CL R, compared to CL R at the default urine flow rate (1 ml/ min), followed a similar trend for all three drugs. The biggest impact of urine flow rate changes within the drugs studied was for caffeine. There was reasonable agreement with observed trends at low urine flow rates for caffeine and theophylline, but a tendency to under-predict the extent of change in CL R at high urine flow rates for theophylline (Figure 5.6). Prediction of changes in CL R at different urine flow rates by MechKiM showed better consistency with observed data than predictions using the static model. A greater impact of urine flow on CL R was noted for a theoretical drug (based on caffeine) with P app value approx. 6 times higher than caffeine (P app = cm/ s), which would represent a drug with very high permeability (N.B. the highest P app value in the current study was cm/ s for antipyrine Table 4.3). Further simulations were performed to assess any potential impact of making the assumption that urine flow rates changes occur because of proportional variation in water reabsorption in the distal tubule and collecting duct, rather than just the collecting duct. The assumption that both distal tubule and collecting duct play a role allowed simulation of CL R at higher urine flow rates (up to 20 ml/ min), although otherwise there were only minor differences compared to the initial assumption of only collecting duct contributing (data not shown). 148
149 10 Caffeine 10 Theophylline Fold Change in CL R Fold Change in CL R 1 MechKiM Static model (Ch. 4) 0.1 Observed Urine flow (ml/ min) 10 1 Linezolid MechKiM Static (Ch. 4) Urine flow (ml/ min) Fold Change in CL R Fold change in CL R 1 MechKiM Static model (Ch. 4) 0.1 Observed Urine flow (ml/ min) 10 1 Comparison (MechKiM only) Caffeine Linezolid Theophylline 0.1 Theoretical highly reabsorbed drug Urine flow (ml/ min) Figure 5.6 Impact of changes to urine flow rates on simulated CL R in a population representative Fold change in simulated CL R (lines) of caffeine, theophylline, linezolid and a theoretical drug with very high F reab was calculated using simulated CL R at urine flow = 1 ml/ min as baseline for each drug. Observed data for caffeine and theophylline were taken from published literature (377, 398). 149
150 5.4 Discussion Several mechanistic kidney models describing the passive tubular reabsorption of drugs have been reported allowing simulation of urine flow dependent CL R of drugs with different permeability values (67, 220, 221). However, none of these kidney models have been implemented within a PBPK model, which would allow investigation of the effect of urine flow on simulated plasma concentration time profiles. More recently a mechanistic kidney model was implemented within the PBPK model of the SimCYP simulator (382). However, no studies to date have demonstrated the utility of MechKiM for actual drugs with moderate or extensive tubular reabsorption, and therefore little is currently known about the suitability or performance of these elements of the model. In the current study, the features of the SimCYP mechanistic kidney model which concern passive tubular reabsorption were used for predictions of urine-flow dependent CL R for relevant drugs. This was the first time that passive permeability parameters of a PBPK kidney model had been informed by IVIVE, which was achieved by adapting the scaling approach described in Chapter 4. Overall the SimCYP mechanistic kidney performed reasonably well in predicting absolute tubular reabsorption and CL R for three drugs. Predicted CL R using MechKiM were 1.2-fold, 5.9-fold and 0.34-fold of observed CL R for caffeine, theophylline and linezolid, respectively, consistent with predictions using the static model (1.4-fold, 6.5-fold, and 0.58-fold of observed for caffeine, theophylline and linezolid, respectively. Furthermore, use of MechKiM allowed improved prediction of the relationship between urine flow and relative change in CL R for caffeine and theophylline compared with using the static model IVIVE linked PBPK model for prediction of tubular reabsorption in healthy subjects Prediction of CL R for 3 selected drugs which exhibit tubular reabsorption in the kidney was based on the use of regional tubular surface areas as IVIVE scaling factors for P app data to calculate the CL PD input parameter. Comparison of predicted and observed CL R suggests that the IVIVE linked PBPK model of kidney can be used to predict CL R for reabsorbed drugs, although some differences may be expected for some drugs (Figure 5.4). CL R predictions were lower than those using the static model (Chapter 4), especially in the case of linezolid where there was a 40% difference between the models (Figure 5.4). Additional simulations with more drugs may be necessary to understand the reason for these differences. However, possible reasons for the difference between predictions using the MechKiM and the static model include the model structure, as there are seven tubular compartments in MechKiM compared with four in the static model, with unequal assignment of tubular surface area in the cortex and medulla collecting duct in MechKiM. Insufficient assumptions when calculating P mem from Caco-2 P app data, or that the PBPK model considers compartmental drug concentrations when simulating CL R (whereas static approach does not account for tubular, cell or blood concentrations) are other possible explanations for differences in CL R predictions by the two models. Based on the 3 drugs investigated, the IVIVE linked PBPK model appears to perform as well as the static model for the prediction of tubular reabsorption and CL R in healthy subjects. 150
151 As previously noted for the static model, there was strong dependency of simulated CL R on the values of system-specific input parameters/ physiological scaling factors, especially surface area (Figure 5.5). For example, application of the highest and lowest length and diameter of tubular sections to re-calculate surface areas shifted the predicted caffeine CL R to 0.6-fold and 2.1-fold of that when dimensions applied for the IVIVE predictions were used. Further, not correcting for the impact of microvilli on the tubular surface area shifted the predicted caffeine CL R to 0.2-fold of that when microvilli were accounted for. System parameter values may vary depending on key assumptions and sources of physiological data, and may be influenced by methodological and/ or pathophysiological factors. A key concept in PBPK models is the separation of system and drugspecific parameters. Future PBPK models of kidney may benefit from separating the tubular surface area (system parameter) from drug permeability, rather than relying on the composite CL PD parameter currently implemented in MechKiM. This principle was applied within complex models of physiological solute transport in rat kidney, which were applied to simulate glucose transport by the SLGT transporters, including inhibition (114, 399). Complex kidney models developed for pre-clinical species are more easily validated due to the availability of experimental data obtained using invasive methods (e.g., micro-puncture studies (400)), which cannot be obtained in humans for ethical reasons. As such, the validation of complex models of human kidney relies more upon clinical data obtained using less invasive methods, such as measurement of urinary and plasma concentrations of solutes under various conditions. For example, measurements of relative changes in CL R following changes in urine flow rates can be performed under controlled (e.g., administration of diuretics, increased water intake) or uncontrolled conditions (e.g., intra- and inter-individual variability). The lack of experimental data obtained from micro-puncture studies is a key limitation for further validation of the assumptions of the model, such as the relative permeabilities of the various regions of the tubule. In particular, the current study assumed that changes in urine flow rate were attributable to changes in water reabsorption in the collecting duct. This is supported by clinical data in water diuresis and by molecular physiology implicating the role of vasopressin in regulating aquaporin-2 mediated water reabsorption in collecting duct (397). Modulation of water reabsorption may also occur in the connecting tubules because aquaporin-2 is expressed in this intermediate section of the nephron tubule (397). This was represented in additional simulations by changing the distal tubule flow rate parameter in proportion to changes in collecting duct flow rates, but this had little impact on simulated CL R (data not shown). Although this did allow for simulations at higher flow rates than were possible by changing the collecting duct flow rate parameter alone (up to 20 ml/ min possible), this is unlikely to be a good representation of the physiological reality. This is because the connecting tubule is small compared to the distal tubule, and data from a rat micropuncture study indicates larger effect of vasopressin on the TF/P inulin of the final urine compared to the late distal tubule (401). In contrast, data from a single clinical study suggest that the caffeine induced increase in urine flow rate may be (at least in part) due to reduced fluid reabsorption in the proximal and distal tubules (402). Although these data were from urinary lithium measurements, and not direct measurements of the proximal tubule filtrate, other studies investigating the molecular 151
152 pharmacology of methylxanthine induced diuresis report data that are consistent (but not confirmatory) with the suggestion that caffeine affects proximal tubule water reabsorption (403). A dominant role of proximal tubule in the variation of urine flow rate contrasts with the assumptions made when simulating urine flow rate changes in the PBPK kidney model of the current study, as well as previous modelling studies (220). Micro-puncture studies comparing TF/P inulin in early and late proximal tubule, distal tubule and urine in rats with or without administration of caffeine would allow for targeted measurements to assess the impact of caffeine on different tubular regions. Such studies could allow PBPK kidney models for animals to be assessed and refined, prior to inter-species extrapolation to inform the systems parameters in the human model IVIVE linked PBPK model for simulation of tubular reabsorption in patients: Potential utility and future implications The PBPK model for tubular reabsorption was able to capture the overall trends in urine flow dependent CL R of caffeine and theophylline observed in the reported clinical data (Figure 5.6). Furthermore, the model predicted that the CL R for drugs with highest permeability, and therefore highest F reab, would be the most sensitive to changes in urine flow, in agreement with previous studies (67, 220, 221). Other kidney models were often able to capture the relationship between urine flow and CL R, for example for theophylline (220, 379), by fitting models to the data. The current study is the first to use a bottom-up, IVIVE approach to predict CL R at different urine flow rates. These results provide supporting evidence that MechKiM may have utility for simulation of drug concentrations in tubular filtrate in different regions of the nephron. This has potential implications for assessing nephrotoxicity, in particular drug-induced crystal nephropathy (404). Risk of drug concentrations in the distal tubule and collecting duct reaching levels where precipitation could occur might be studied by coupling the simulated urine ph and drug concentrations in relation to drug solubility. However, drugs which undergo tubular secretion, as well as patients with renal impairment, often feature in reports of crystal nephropathy. Mechanistic PBPK kidney models that can reliably account for physiology related changes in renal impairment, including impact on drug transporters are lacking. Other physiological factors linked to crystal nephropathy such as volume depletion should also be considered (405). Therefore, much work is needed in order to further develop mechanistic models of kidney before benefits in drug development or clinical situations will be gained Conclusion This was the first study to implement an IVIVE approach for predicting CL R of reabsorbed drugs in MechKiM. The use of the PBPK kidney model allowed for simulation of urine flow dependent CL R, which was a limitation arising from the use of the static mechanistic model. This simulation study represents an initial step towards development of mechanistic kidney models for studying pharmacokinetic variability arising from different clinical scenarios and patient characteristics. Following further development, it may be possible to extend such kidney models to predict not only pharmacokinetics, but also the risk/ probabilities of clinical outcomes under various scenarios. 152
153 Chapter 6. SimCYP model of tubular secretion for digoxin and simulation of change in CL R due to aging and renal impairment 6.1 Introduction Physiologically-based pharmacokinetic (PBPK) models are being increasingly used for mechanistic prediction of the pharmacokinetics of drugs, and for addressing regulatory issues (338, 406, 407). PBPK models are structured to account for drug specific and system specific parameters, as well as the study trial design (e.g., dose regimen). This structure is proposed to facilitate extrapolation outside of the studied population, potentially identifying particular high risk patient groups or sub-groups (1, 407). These predictive scenarios can include drug-drug interactions (DDIs), special populations (e.g., renal impairment, geriatrics), including so-called complex patients and complex DDIs, in which performing prospective clinical studies with adequate numbers of patients may be unrealistic (337). Although there is a relatively strong confidence in the use of PBPK models when CYP mediated metabolism is the dominant route of elimination, less certainty is given when transporters contribute (106, 408). For example, a study investigating renal impairment related changes in CYP metabolism using a PBPK model was reported earlier than analogous studies on the impact of renal impairment on renal secretion (226, 227, 409). A major challenge for physiologically-based models of kidney is accommodating the complexity and heterogeneity of this organ, at the tissue and cellular levels, while simultaneously ensuring system specific parameters can be reliably and unambiguously informed. For example, the mechanistic kidney model (MechKiM) implemented in the SimCYP simulator can facilitate in vitroin vivo extrapolation (IVIVE) of renal transporter kinetics data (78). However, physiological scaling factors required for IVIVE, such as transporter abundance and proximal tubule cellularity, are lacking (Chapter 1, section 1.4). MechKiM may also facilitate extrapolation of renal drug disposition from healthy subjects to subjects with renal impairment. Different renal impairment conditions may reduce drug clearance by both glomerular filtration and tubular secretion (226, 410). However, the mechanism(s) behind reduced renal secretion associated with renal impairment or other underlying diseases are still not well understood. Physiological data supporting any particular mechanism are lacking and typically restricted to rodent models (226, 411). Digoxin is a cardiac glycoside used in the treatment of heart failure and atrial fibrillation (412). A narrow therapeutic index, as well as severe consequences of toxicity, represent limitations for digoxin treatment (413). Recently a PBPK model for digoxin was developed which incorporated permeability limited modules for the liver and gut (382, 414). The model accounted for the role of P-gp on digoxin pharmacokinetics in both these organs, and was applied to investigate the DDI potential of verapamil and norverapamil (inhibition) and rifampicin (induction) on digoxin pharmacokinetics. Although P-gp is also expressed in the kidney, the mechanistic kidney model for digoxin was not developed. However the authors did highlight the possibility to extend the PBPK model of digoxin to include this, and hypothesised that inhibition of renal P-gp could affect simulated renal concentrations of digoxin, but was unlikely to affect plasma concentrations (382, 153
154 414). In addition to P-gp, uptake of digoxin into the tubular cells from the plasma, mediated by the OATP4C1 transporter, contributes to digoxin renal secretion (49, 415) Aims This study aimed to develop a PBPK kidney model for digoxin, and use this to mechanistically investigate potential effects of renal impairment on renal drug disposition. A mechanistic kidney model for digoxin was developed using the SimCYP simulator (v14, release 1), with IVIVE used to inform model parameters where possible. The IVIVE approach was unsuitable for the OATP4C1 transporter intrinsic clearance (CL int,t ) parameter. Therefore estimation of this parameter using clinical data was performed using a sensitivity analysis approach that accounted for the uncertainty in the contribution of glomerular filtration to the observed digoxin CL R. The developed model was subsequently used to simulate changes in digoxin CL R due to aging and moderate and severe renal impairment. In the case of renal impairment, different assumptions concerning the mechanism by which digoxin secretion is potentially reduced, namely the impact of OATP4C1 abundance and proximal tubule cellularity, were investigated to assess the impact of such assumptions on the simulated digoxin renal disposition. 6.2 Methods Clinical data sources Mean plasma concentration-time profiles and pharmacokinetic parameters were collated from the scientific literature. Pharmacokinetic parameters of interest were the area under the curve (AUC) for the plasma concentration-time profile, the intravenous (i.v.) clearance (CL) and oral clearance (CL/F), volume of distribution at steady state (V ss ) and the renal excretion clearance (CL R ). Where necessary data were digitised using GetData Digitizer (version 2, Verification of basal PBPK model of digoxin All simulations presented herein were performed using the SimCYP population-based PBPK simulator software, version 14, release 1 (SimCYP, Sheffield, UK) (265, 381). Initial simulations were performed using the default Healthy volunteers population file provided with the SimCYP simulator. Optimised parameters for the full PBPK model of digoxin, including mechanistic models of liver and intestine, were recently published in version 12.2 of the simulator (382). The default compound file provided with version 14.1 of the SimCYP simulator (Table 6.1) was verified against clinical data to ensure consistency between different versions of the software. Simulations were performed following the design of the clinical studies listed in Table 6.2, with 10 trials for each set of simulations. 154
155 Table 6.1 SimCYP input parameters for default digoxin full-pbpk model (version 14.1), as reported by Neuhoff et al (382). Parameter Value Phys-chem Mol Weight (g/mol) log P Compound Type Neutral B/P 1.07 f u 0.71 Absorption Absorption model ADAM Permeability assay P app,caco-2 (10E-06 cm/s) 12.7 P Caco-2 P app,caco-2 Scalar 1 P eff,man Duodenum (10E-4 cm/s) 0.5 P eff,man Jejunum I (10E-4 cm/s) 4.67 P eff,man Jejunum II (10E-4 cm/s) 4.67 P eff,man Ileum I (10E-4 cm/s) 4.67 P eff,man Ileum II (10E-4 cm/s) 3.67 P eff,man Ileum III (10E-4 cm/s) 2.67 P eff,man Ileum IV (10E-4 cm/s) 1.67 P eff,man Colon (10E-4 cm/s) 0.1 Input Form Solution Distribution Distribution Model Full PBPK V ss mode Predicted Prediction method Method 2 K p muscle 7.35 K p adipose 10.8 K p Scalar 1 Elimination Clearance Type In vitro metabolic system Enzyme Kinetics Recombinant Additional Hep CL int (µl/ min/ million hepatocytes) 0.37 (30%) Additional Hep f u,mic 1 Active Uptake into Hepatocyte 1 CL R (L/ h) 9.66 Transport Assume Colon SS Organ/Tissue Transporter Location Function No Gut ABCB1 (P-gp) Apical Efflux J max (pmol/min/million cells) 434 K m (µm) 177 A (cm²) 1 System User RAF/REF 2 Organ/Tissue Liver 155
156 Parameter Value CL PD (ml/min/million cells) 0.1 f u,iw Type f u,ew Type Transporter Location Function Predicted Predicted ABCB1 (P-gp) Canalicular Efflux J max (pmol/min/million cells) 434 K m (µm) 177 System User RAF/REF 1.5 A, area; B/P, blood to plasma partition ratio; CL int, intrinsic clearance; CL PD, passive diffusion clearance; CL R, renal clearance; f a, fraction absorbed; f u fraction unbound in plasma; f u,ew, fraction unbound in extracellular water; f u,iw, fraction unbound in intracellular water; f u,mic, fraction unbound in microsomes; Hep, Hepatocyte HLM, human liver microsomes; J max, Maximum rate of transport; k a, absorption rate constant; K m, Michaelis-Menten constant; K p, tissue to plasma partition coefficient; log P, logarithm of the octanol-water partition coefficient; P app,caco-2, permeability across Caco-2 cell monolayers; P eff,man, Human jejunum permeability; pk a, acid dissociation constant; Q gut hybrid parameter of blood flow and drug permeability; RAF/REF, Relative activity factor/ relative expression factor; V max, maximum rate of metabolism; V ss, volume of distribution at steady state. Table 6.2 Clinical trials used for verification of the digoxin compound file in v14.1 of the SimCYP simulator, prior to simulations using the MechKiM. Basic dosage and demographic information are shown. All subjects were healthy participants. Dose Information Subjects Information Reference 1 mg SD i.v. 8 male, years (416) 1 mg SD Oral 8 male, years (416) 0.01 mg/ kg i.v. SD 12 male, years (417) 0.75 mg Oral SD 12 male, years (417) 0.5 mg Oral SD 12 male, years (418) SD Single dose; i.v. intravenous IVIVE and optimisation of tubular secretion in PBPK model Secretion is considered a substantial contributing mechanism of digoxin renal elimination. Digoxin has low passive permeability across cell monolayers, even in the presence of P-gp inhibitors (literature Caco-2 P app cm/ s; various assay formats used ( )). There are mixed reports from clinical studies suggesting possible urine flow dependent CL R of digoxin ( ). Therefore, passive tubular reabsorption may have a minor role in vivo, and was accounted for in MechKiM using the passive diffusion clearance (CL PD ) parameter. Using the static model of tubular reabsorption (Chapter 4), the Caco-2 P app range indicated above would result in predicted fraction reabsorbed (F reab ) of The static model of tubular reabsorption has not been validated for external Caco-2 datasets. Furthermore, in depth mechanistic investigation of passive tubular reabsorption was not the focus of the digoxin simulations. Therefore SimCYP default values for tubular flow rate parameters were used, and a value of 0.01 µl/ min/ million tubule cells was assigned to the CL PD parameters for all tubular compartments. This resulted in a simulated F reab of 0.12, in agreement with the range predicted using the static model of tubular reabsorption using the literature data. Fraction unbound in kidney cells (f u, kidney,cell ) was predicted using the MechKiM module to be 0.51, using the Rodgers and Rowland method (388). 156
157 Digoxin secretion in kidney is considered to be mediated predominantly by the OATP4C1 and P- gp transporters, expressed on the basolateral and apical proximal tubule membranes, respectively (49, 50, 415, 426). A wide range of in vitro K m and V max values were available for P-gp in the literature, with two studies reporting in vitro data for OATP4C1 (Table 6.3). In addition, quantitative expression data in kidney and relevant in vitro systems were available for P-gp (Table 6.4), but not for OATP4C1. For consistency, the P-gp K m and V max parameter values in the mechanistic kidney model were the same as those implemented in the pre-existing mechanistic models for liver and intestine (382, 427). Due to the lack of in vitro V max values reported in the literature, OATP4C1 CL int,t was estimated from reported uptake rate (dx/ dt) and initial substrate concentration (C) data (49, 428), using Eq. 6.1 which assumes that C << K m. CL int,t = dx dt C 6.1 As the transporter expression/ abundance data needed to inform the REF scaling factor were lacking, in vitro OATP4C1 CL int,t was normalised to proximal tubule cellularity (Table 6.3) as previously attempted for OCT2 uptake activity (227). Normalisation was performed by assuming equal expression/ activity of OATP4C1 in transfected cells and proximal tubule cells, and a cellularity of 13 million MDCK cells per mg protein (429). Although large uncertainty is associated with the number of proximal tubule cells per gram of kidney (PTCPGK) in human (section 1.4.5), a value of 60 million cells/ gram kidney was used in accordance with previous simulation studies (78, 226, 227). As the IVIVE approach outlined above did not successfully predict clinically observed digoxin CL R, OATP4C1 CL int,t was optimised using two different approaches. Firstly parameter estimation was performed by fitting the model to the observed plasma concentration profiles of two representative clinical studies with i.v. administration, using the automated parameter estimation module of the SimCYP simulator. Secondly, the observed CL R collated from multiple clinical studies (Table 6.5) was used to optimise OATP4C1 CL int,t using a detailed sensitivity analysis. Digoxin pharmacokinetics was then simulated, and compared against the observed data (417, 430). 157
158 Table 6.3 In vitro transport kinetics data for digoxin and the P-gp and OATP4C1 transporters, collated from the literature Transporter Parameter Value System Reference 177 Caco-2 (431) 73 Caco-2 (432) K m (µm) 181 Sf9-MDR1 liposomes (433) 130 Caco-2 (434) 1000 MDCK-MDR1 (434) P-gp 58 Caco-2 (435) 434 Caco-2 (431) 57 Caco-2 (432) V max (pmol/ min/ cm 2 ) 126 Caco-2 (434) 594 MDCK-MDR1 (434) 2167 Caco-2 (435) V max (pmol/ min/ mg) Sf9-MDR1 liposomes (433) K m (µm) 7.8 MDCK-OATP4C1 (49) Reported uptake 0.37 µm (pmol/ 30 min/ mg) a 0.2 MDCK-OATP4C1 (49) OATP4C1 Reported active 0.1 µm (pmol/ 5 min/ 200,000 cells) a 27 CHO-OATP4C1 (428) Normalised uptake clearance (µl/ MDCK-OATP4C1 (49) min/ million cells) b 270 CHO-OATP4C1 (428) a Active uptake = uptake in transporter-transfected cells uptake in mock-transfected cells; b Uptake clearance was estimated from uptake rate and assay concentration using Eq. 6.1, assuming 13 million MDCK cells per mg protein (429). 158
159 Table 6.4 Reported transporter expression of P-gp in Caco-2 and human organs. The kidney: Caco-2 REF used in the mechanistic kidney model was 1.51 based on mrna data (98). Expression ratio Method Published values b Relative Expression Reference Jejunum: Caco-2 Western Blot 2064 : (431) Jejunum: Caco-2 qpcr : (98) Liver: Small intestine qpcr : (98) Liver: Small intestine qpcr : (436) Liver: Small intestine qpcr : (437) Kidney: liver qpcr : (98) Kidney: Liver qpcr : (436) Kidney: Liver qpcr : (437) Kidney: small intestine qpcr : (98) Kidney: Small intestine qpcr : (436) Kidney: Small intestine qpcr : (437) a There were no data available for relative expression of OATP4C1 in kidney compared with transfected cell lines; b Values for qpcr are relative gene expression, using either geometric mean of cyclophilin A and MVP (98), GADPH (436) or peptidylprolyl isomerase A (437), whereas values for western blot are integrated optical densitometry intensity (431). 159
160 Table 6.5 Observed digoxin CL R values published in literature in healthy subjects Study # CL R (ml/ min) Number of subjects Reference (438) (Oral) (IV) 8 8 (416) (439) (417) (440) (441) (442) (443) (444) (445) (446) (447) (448) (449) (450) (451) (452) (453) (454) Weighted average = ml/ min; Range = ml/ min Simulation of digoxin CL R in elderly and renal impairment populations Digoxin pharmacokinetics was simulated in 100 virtual subjects following i.v. infusion (30 min) of 0.75 mg in different virtual populations, with other trial design parameters such as age range and proportion of females of the virtual subjects determined by the general values of the relevant population files. The virtual populations used for simulations included the Geriatric NEC, RenalGFR_30-60 and RenalGFR_less_30 populations, which are supplied with the SimCYP simulator. Geriatric NEC population accounts for changes in the age-sex distribution, weights and heights and kidney size of subjects, whereas the RenalGFR populations account for changes in GFR, protein binding by albumin, haematocrit, kidney weight and renal blood flow. In addition the RenalGFR_30-60 and RenalGFR_less_30 populations were modified by reducing the value of the parameter representing number of proximal tubule cells per gram of kidney. Reducing proximal tubule cells per gram kidney was intended to mechanistically represent changes in secretion resulting from tubular cell damage, which may occur during renal impairment and injury (and possibly aging) ( ). Another potential mechanism of changes to renal secretion of drugs due to renal impairment is the change in expression of drug transporters by proximal tubule, 160
161 although data to support this are relatively sparse (458). Reduced expression of kidney drug transporters were represented in MechKiM by assigning relative abundances for the OATP4C1 and P-gp transporters in kidney in the Poor Transporter (PT) phenotype as a proportion the SimCYP default Extensive Transporter (ET) phenotype value of 1, and setting the frequency of PT in the modified population to 1. Relative abundance of P-gp in liver and gut remained the same. AUC ratio (AUCR) was calculated using Eq. 6.2 AUCR = AUC RI AUC Control 6.2 where AUC RI and AUC Control are the mean digoxin AUC in renal impairment subjects (GFR < 60 ml/ min/ ) and mean digoxin AUC in healthy volunteers or patients without renal impairment (GFR > 60 ml/ min/ ). CL R ratio and maximum concentration of digoxin in proximal tubule segment 1 cells (C max,pt-1 ) ratio were calculated in an analogous manner. Separately, simulations were performed in the population representative mode following changes in systems parameters in the kidney model, using the Healthy volunteers population file as a template. In these simulations, digoxin CL R was simulated following changes in GFR either alone or in combination with proportional changes in the OATP4C1 abundance or PTCPGK parameter (see Table 6.6 for details). Table 6.6 Parameters used to simulate digoxin CL R. Reduction in filtration and secretion was performed to represent changes in renal impairment. The simulated population representative of the Healthy volunteers population had an age, weight and BSA of 20 years, 81 kg and 1.98 m 2 respectively. The serum creatinine (input parameter of model) was calculated for each scenario using the Cockcroft-Gault equation (459), based on the target GFR and the age, weight and BSA of the population representative. GFR (ml/ min/ 1.73 m 2 ) Serum creatinine concentration (µmol/ L) OATP4C1 abundance PTCPGK (million PTC/ g kidney) * * Relative change in GFR for each scenario was calculated using the value of ml/ min/ m 2 as baseline, and this relative change was applied to the OATP4C1 abundance or PTCPGK parameters Data Analysis Microsoft Excel 2010 was used for data analysis. 161
162 6.3 Results Optimisation of digoxin kidney transporter kinetic parameters Initial analysis was performed using the full PBPK model without mechanistic kidney model activated. Verification was performed using several clinical studies (Table 6.2) using the default full-pbpk model (382). The simulated concentration-time profiles, as well as key pharmacokinetic parameters were generally in good agreement with observed data (Figure 6.1). Assuming CL R was negligible increased the simulated AUC 0- by 144% compared with the default model, due to underestimation of the clearance (Figure 6.2). Following activation of the MechKiM module and accounting for either only glomerular filtration, or both glomerular filtration and passive tubular reabsorption, resulted in simulated digoxin CL R of 98.8 ml/ min, or 87.3 ml/ min, respectively, which were both lower compared to the overall weighted mean observed value of ml/ min (Table 6.5). Digoxin plasma concentration time profiles for these scenarios are presented in Figure 6.2; simulated AUC 0- was 29% and 39% higher than that using the default model for the filtration only and filtration and reabsorption scenarios respectively. Based on these scenarios, F reab was calculated to be Figure 6.1 Representative simulated plasma concentration-time profile of digoxin using default full-pbpk model in SimCYP, without activation of MechKiM. Mean (solid lines), 5th and 95th percentiles (dashed lines) simulated plasma concentrations of all virtual subjects are overlaid with mean observed data (circles) (417). 162
163 Figure 6.2 Mean simulated digoxin plasma concentration time profiles (i.v. administration of 1 mg digoxin) for intermediate PBPK models used during development of the mechanistic kidney model. Lines represent digoxin PBPK model with CL R = 0 ml/ min ( ), CL R defined by a single input value (136.1 ml/ min) based on the literature analysis ( ) and CL R simulated using the mechanistic kidney model, accounting for only glomerular filtration ( ), glomerular filtration and reabsorption ( ) or glomerular filtration, reabsorption and active secretion ( ). The sensitivity of simulated digoxin pharmacokinetic parameters on model parameters was assessed in order to determine their relative importance. Simulated digoxin CL R was highly sensitive to changes in OATP4C1 CL int,t, in contrast to the marginal effect of P-gp REF/ RAF (Figure 6.3, A); C max, PT-1 was sensitive to both OATP4C1 CL int,t and P-gp REF/ RAF input parameters (Figure 6.3, B). The simulated digoxin CL R and AUC 0- were insensitive to changes in f u,kidney,cell, with only small changes noted at lower values of f u,kidney,cell (Figure 6.4, A and B). C max, PT- 1 was sensitive to changes in f u,kidney,cell at values below approx. 0.4 (Figure 6.4, C). 163
164 A B Figure 6.3 Simulated digoxin CL R (A) and C max, PT-1 (B) at different input values for the kidney transporter kinetic parameters. Insets show the graphs presented on logarithmic scales. Values of OATP4C1 CL int,t and P-gp REF/ RAF were varied using the automated sensitivity analysis tool in the SimCYP simulator in a population representative following the clinical trial design reported previously (416). 164
165 A B C Figure 6.4 Simulated digoxin CL R (A), AUC 0- (B) and C max, PT-1 (C) at different input values for the f u,kidney,cell parameter. f u,kidney,cell was varied using the automated sensitivity analysis tool in the SimCYP simulator in a population representative, following the clinical trial design reported previously (416). f u,kidney,cell predicted using the Rodgers and Rowland method used for simulation of digoxin pharmacokinetics was 0.51 (387). 165
166 Three studies reported P-gp relative expression between kidney and intestine (Table 6.4). Multiplying these values by the intestine: Caco-2 REF of 2.04 used in the SimCYP gut module gave kidney: Caco-2 REFs ranging from 0.78 to The P-gp REF for Caco-2 cells: kidney used in the digoxin model (1.51) was calculated using mrna expression data reported in both systems by the same study (98). In vitro OATP4C1 mediated uptake clearance of digoxin was calculated from two uptake rate values reported in the literature (Table 6.7). In vitro uptake clearance values varied by approx. 3 orders of magnitude, and the use of these values to inform the OATP4C1 CL int,t parameter (i.e., IVIVE) resulted in over 8-fold difference in simulated CL R (Table 6.7), with neither value in good agreement with observed CL R (Table 6.5). Alternatively, OATP4C1 CL int,t was obtained by parameter estimation by fitting the model to the observed plasma concentration-time profiles following i.v. administration. Fitting of the model to the plasma concentration-time profiles from two representative clinical studies using the automated parameter estimation module resulted in OATP4C1 values that were over 7-fold apart (Table 6.7). Subsequently, mean simulated CL R values differed by 1.7-fold and were within the range of reported CL R from clinical studies (Table 6.5). Table 6.7 Values of the OATP4C1 CL int,t parameter estimated by various methods, and subsequent simulated digoxin CL R in healthy volunteers, using the i.v. trial design from (416). Method for estimating CL int,t OATP4C1 CL int,t value (µl/ min/ million PTC) Simulated digoxin CL R (ml/ min) IVIVE - in vitro data from (49) IVIVE - in vitro data from (428) Parameter estimation using fitting to plasma concentration-time profile from (430) Parameter estimation using fitting to plasma concentration-time profile from (417) Parameter estimation using sensitivity analysis vs. overall weighted mean CL R - trial design from (430) Parameter estimation using sensitivity analysis vs. overall weighted mean CL R - trial design from (417) A sensitivity analysis based approach was next used for estimation of the OATP4C1 CL int,t parameter. The overall weighted mean observed CL R of digoxin obtained from extensive literature search (136.1 ml/ min; n = 214 healthy subjects) was used as the optimal value (Table 6.5). Using a population representative, digoxin pharmacokinetics were simulated using different OATP4C1 CL int,t and serum creatinine input parameter values. The values for OATP4C1 CL int,t 166
167 resulting in optimal simulated CL R were dependent on serum creatinine (Figure 6.5). The estimated OATP4C1 CL int,t value, based on the sensitivity analysis approach using a serum creatinine value of 80 µmol/ L, was 4.14 µl/ min/ million proximal tubule cells (Figure 6.5). A serum creatinine value of 80 µmol/ L was an assumed average value, because several clinical studies used in the literature analysis did not report serum creatinine, creatinine clearance or other measurements/ estimates of GFR of subjects. The comparison of simulated (coloured mesh) and observed (grey plane) digoxin CL R in Figure 6.5 indicates a range of plausible values for optimised OATP4C1 CL int,t (i.e., various intersections between simulated and observed CL R ), depending on the assumed serum creatinine for the virtual subjects. Following optimisation of the OATP4C1 CL int,t, simulation of digoxin pharmacokinetics using a separate clinical trial design (416) resulted in CL R in agreement with observed data. In addition, simulated digoxin plasma concentrations using the PBPK model incorporating the optimised OATP4C1 CL int,t were in close agreement with plasma concentrations simulated using a single input parameter (i.e. prior to activation of MechKiM) to define the CL R of digoxin (Figure 6.2). Figure 6.5 Estimation of OATP4C1 CL int,t parameter using a sensitivity analysis approach, by simulating digoxin CL R in population representatives with different serum creatinine values. The coloured meshes and grey horizontal plane indicate the simulated CL R and the overall weighted mean CL R obtained from the literature analysis (136.1 ml/ min; n = 214 healthy subjects) respectively. Values of OATP4C1 CL int,t and serum creatinine parameters were varied using the automated sensitivity analysis tool in the SimCYP simulator. The optimal OATP4C1 value was taken at the intersection (yellow star) of the simulated digoxin CL R with the observed CL R at a serum creatinine value of 80 µmol/ L (which corresponds to simulated GFR ~120 ml/ min), as indicated by the blue arrows. The sensitivity analysis was performed twice using clinical trial designs reported previously (417, 430). 167
168 6.3.2 Simulation of digoxin pharmacokinetics in special populations: effects of age and renal impairment Mean digoxin CL R simulated in elderly virtual subjects ( Sim-Geriatric NEC population file) was 90.7 ml/ min, which was 31% lower than that in simulated in the simulated healthy volunteers. This change was comparable to the relative change observed in clinical study (digoxin CL R was 36% lower in elderly subjects than young subjects (460)), but lower than the relative change in simulated GFR (44% lower in elderly, compared with 54% observed (460)). Mean simulated digoxin CL R in moderate (GFR_30-60) and severe (GFR_less_30) renal impairment virtual populations were 50% and 66% lower than in the simulated healthy volunteers. The mean GFR of the virtual subjects with moderate and severe renal impairment were 64% and 82% lower than in the simulated healthy volunteers. Comparison of observed clinical data with simulated CL R and GFR in the healthy, moderate renal impairment and severe renal impairment virtual populations are shown in Figure 6.6 (top panel). Although some agreement between predicted and observed data was noted, some virtual subjects within the renal impairment populations had higher CL R values than observed CL R in patients with comparable GFR. These overestimated simulated CL R values were associated with high OATP4C1 relative expression in the virtual subjects. Average simulated AUCR in moderate renal impairment (1.4) was in agreement with that calculated from clinical data (1.3), whereas in severe renal impairment the simulated AUCR (1.5) was lower than the AUCR based on clinical data (3.3) (461). GFR and OATP4C1 relative abundance had similar coefficient of determination (R 2 ) of the line of best fit with simulated CL R in healthy virtual subjects (Figure 6.6, bottom panel). For the virtual subjects with renal impairment, there was a much weaker correlation (i.e., lower R 2 ) between GFR and simulated CL R, but a stronger correlation between OATP4C1 relative abundance and CL R. Stronger correlation of simulated CL R with number of proximal tubule cells per gram kidney in renal impairment compared with healthy virtual subjects was also noted (data not shown). Changes to OATP4C1 relative abundance ( ) in the renal impairment virtual populations had similar impact on CL R and AUCR as when comparable changes in proximal tubule cell number ( million proximal tubule cells/ g kidney) were made (Figure 6.7). Reduced proximal tubule cell number had minimal impact on simulated digoxin concentrations in proximal tubule cells, whereas reduced OATP4C1 or P-gp abundance decreased or increased digoxin intra-cellular concentrations respectively. Further simulations were performed in population representative mode, using the Healthy Volunteers population as a template, whereby GFR was altered either alone or alongside proportionally altered OATP4C1 abundance or PTCPGK (Figure 6.8). As noted for Figure 6.6 when systems parameters contributing to renal secretion (OATP4C1 abundance and PTCPGK) were not considered (i.e., only serum creatinine parameter changed to reduce GFR), the impact of renal impairment on digoxin CL R was under-estimated by simulations when compared to the observed data. Accounting for changes in tubular secretion in renal impairment, assuming that either OATP4C1 abundance or PTCPGK are affected proportionally to changes in GFR resulted in improved agreement between simulated and observed digoxin CL R (Figure 6.8). 168
169 Figure 6.6 Correlation of simulated CL R with GFR and OATP4C1 abundance in virtual populations. Top Panel: Simulated CL R and GFR (creatinine clearance (CL CR ) for observed data) in healthy ( ) and moderate ( ) and severe ( ) renal impairment virtual subjects, in comparison with reported clinical data ( ) (461, 462); Bottom panel: Simulated CL R and GFR in healthy ( ) and moderate ( ) and severe ( ) renal impairment virtual subjects. Solid coloured lines represent linear lines of best fit for data from each simulation or clinical study, with relevant equations and R 2 shown in boxes at top of each panel. 169
170 Figure 6.7 Impact of reduced renal secretion on simulated digoxin AUC ratio (top panel) and CL R (middle panel) or C max,pt-1 ratio (bottom panel) in renal impairment populations. Renal secretion was reduced either by changing the kidney OATP4C1 or P-gp relative abundance parameters, or by reducing the PTCPGK parameter by a proportional amount. Lines represent changes in PTCPGK in moderate renal impairment ( ) and severe renal impairment ( ), OATP4C1 abundance in moderate renal impairment ( ) and severe renal impairment ( ), and P-gp abundance in moderate renal impairment ( ) and severe renal impairment ( ). Each scenario was simulated in 100 virtual subjects. Solid horizontal black line (ratio = 1) represents the healthy volunteer population; estimated CL R ratios for the average moderate (GFR = 46.5 ml/ min/ 1.73 m 2 ; ) and severe (GFR = 23.5 ml/ min/ 1.73 m 2 ; ) renal impairment were calculated based on the correlation of GFR and CL R for the observed data (Table 6.5, Top panel). Relative change of PTCPGK or transporter abundance of 1 indicates that the default moderate or severe renal impairment population in the SimCYP simulator was used 170
171 Figure 6.8 Simulation of digoxin CL R in population representative mode with changes in different systems parameters performed to represent changes in the case of renal impairment. Glomerular filtration rate (range ml/ min/ 1.73 m 2 ) was changed by altering the serum creatinine parameter ( µmol/ L); OATP4C1 abundance and PTCPGK parameters were altered by a factor proportional to the relative change in GFR from the population representative of the default Healthy volunteers population (GFR = ml/ min/ 1.73 m 2 ; serum creatinine = 76.5 µmol/ L). Lines represent simulations performed with changes in GFR alone ( ), both GFR and OATP4C1 abundance ( ), or both GFR and PTCPGK ( ). Reported clinical data ( ) are overlaid (461, 462). 6.4 Discussion Development of mechanistic kidney model for digoxin A PBPK model for digoxin was previously published that incorporated permeability-limited organ models for the gut and liver in the SimCYP simulator. This model was developed particularly to investigate the role of both hepatic and intestinal P-gp in DDIs with digoxin (382, 414). However, the roles of P-gp and other relevant transporters in the renal disposition of digoxin, and the potential role of these transporters in digoxin DDIs, were not accounted for in these studies (382, 414). In the current study, the permeability-limited kidney model (MechKiM) for digoxin was developed, which accounted for OATP4C1 and P-gp mediated uptake and efflux into the proximal tubule cells, respectively. Based on the available clinical data for digoxin, the P-gp transporter kinetic parameter in MechKiM was practically non-identifiable, and so the accuracy of the IVIVE approach for the P-gp REF parameter could not be assessed, consistent with previous modelling studies involving other drugs and renal efflux transporters (79, 226). Therefore the model developed was not suitable for simulation of P-gp mediated digoxin DDIs. However, the proposition of Neuhoff et al. that P-gp inhibition is unlikely to have a substantial effect on digoxin plasma concentrations (382) is supported by the finding in the current study that uptake into proximal tubule cells was the rate limiting step of the simulated renal secretion of digoxin (Figure 6.3). 171
172 Prediction of CL R of digoxin using an IVIVE approach was not successful, in contrast to a previous study using a very similar approach using MechKiM (227). This was due to the inability of the IVIVE approach to obtain a robust estimate of the OATP4C1 CL int,t, based on the available in vitro data and scaling factors. In addition, use of in vitro uptake transporter kinetics data from different literature sources resulted in large differences in predicted CL R (Table 6.7). It is expected that the availability of transporter abundance data in in vitro systems and human kidney will improve not only the understanding of inter-system differences in in vitro transporter kinetics, but also IVIVE predictions. An alternative approach to estimate transporter kinetic parameters involves using the available clinical data (383). The parameter estimation approach using plasma concentration data from two clinical studies resulted in two different OATP4C1 CL int,t values, although these were in closer agreement with each other than the values obtained using the IVIVE approach. The sensitivity analysis approach for optimisation of OATP4C1 CL int,t also depended upon literature data for digoxin CL R. In an attempt to minimise subjectivity related to the choice of literature data used to define the observed CL R used for optimisation, a value obtained from a meta-analysis of 19 studies with 214 subjects was used (Table 6.5). However, given the large number of clinical studies and variability in digoxin pharmacokinetics, differences in literature analyses of digoxin CL R are apparent. For example, the literature analysis in the current study (weighted mean CL R = ml/ min) included a larger number of subjects than the analysis of Chapter 4 (weighted mean CL R = ml/ min) and also that of Neuhoff et al (weighted mean CL R = ml/ min; (382)). The differences in the weighted mean digoxin CL R between the literature analyses, as well as variability in digoxin plasma concentration data, can each affect optimisation of model parameters. Overall this indicates that optimisation of PBPK model parameters using literature data is associated with some uncertainty because of subjectivity in the selection of the literature studies. In the current study, the average of the CL int,t values obtained using parameter estimation (4.65 µl/ min/ million proximal tubule cells) was approximately 12% higher than that obtained from the sensitivity analysis approach (4.14 µl/ min/ million proximal tubule cells). More detailed and objective analyses with respect to population variability, uncertainty and confidence intervals of prediction, as per previous recommendations (383, 463), were outside the scope of the present study Simulation of digoxin renal drug disposition in renal impairment: Implications for drug toxicity Dose adjustment for drugs eliminated predominantly by renal filtration (and secretion) in elderly subjects is informed by the ratio of the patient s egfr or CL CR to that of normal renal function ( ). Such dosage adjustment is important for a drug like digoxin, which has a narrow therapeutic index and potentially severe toxic effects, even with minor increases in plasma concentration (466). In the current study, the impact of age (i.e., geriatric vs. healthy volunteer virtual populations) on digoxin CL R was simulated and compared to clinical data. The GFR of virtual subjects, calculated using the Cockcroft-Gault equation in the SimCYP simulator (64), was 48% lower in the geriatric population compared to the healthy population. In agreement with that observed in a clinical study (460), the magnitude of difference in simulated digoxin CL R between 172
173 elderly and young virtual subjects was smaller than the difference in GFR (simulated) and CL CR (observed). This finding supports the proposal that despite physiological changes in kidney during aging (117), proximal tubule secretion is largely retained in elderly subjects without kidney disease (467). Simulated digoxin CL R was lower in the renal impairment virtual populations than in healthy volunteers, in agreement with a previous study (226). There were some disparities when compared with clinical data, especially when secretion was assumed not to change in renal impairment (Figure 6.6 and Figure 6.8). In contrast to clinical data suggesting good correlation between digoxin CL R and CL CR in both healthy and renal impairment subjects (461, 462), in the current study, the correlation between simulated digoxin CL R and GFR became weaker with increasing severity of renal impairment. Such disparity may reflect the non-proportional change of inulin clearance (i.e. true GFR ) and CL CR following renal impairment ( ). Additional clinical studies in healthy and renal impairment subjects, comparing patient-matched inulin clearance, CL CR and digoxin CL R (or other renally secreted drugs) could help clarify this issue. However, the assumption that renal secretion of digoxin does not change in renal impairment contradicts the postulates of the intact nephron hypothesis (252, 254). In addition, there are data, albeit from a limited number of studies, indicating there are changes in renal transporter expression and activity in rodent models of renal impairment (411). Therefore, a more likely cause of the disparity between the simulated and clinical data in Figure 6.6 is that renal impairment associated changes in tubular secretion were not initially accounted for. This is supported by a previous simulation study which found that a >10-fold reduction in PTCPGK was required to predict the effects of renal impairment on the CL R of cefuroxime and oseltamivir carboxylate, which undergo tubular secretion (226). Various underlying physiological factors have been proposed with respect to reduced tubular secretion in renal impairment, including transporter inhibition by uremic serum, loss of proximal tubule cells and decreases in transporter expression levels (226, 279, 411, 471). Therefore, simulation of changes to OATP4C1 abundance, P-gp abundance and proximal tubule cellularity were all considered in the current study in order to mimic renal impairment. Changes to the OATP4C1 abundance and PTCPGK parameters resulted in similar changes to simulated AUCR and CL R, although changes to P-gp abundance had negligible effect on these pharmacokinetic parameters (Figure 6.7). In particular, reducing OATP4C1 abundance or PTCPGK proportionally to changes in GFR resulted in changes in simulated digoxin CL R in agreement with clinical observations (Figure 6.8). Conversely, simulated digoxin C max,pt-1 was insensitive to changes in PTCPGK, but was affected by changes in the transporter abundance parameters (Figure 6.7). The interplay of these parameters could therefore be important for understanding potential risk of proximal tubule drug toxicity (or loss of efficacy if a drug has a therapeutic target in proximal tubule cells) in patients with underlying renal impairment (405). Furthermore, incorporation of cellularity/ transporter covariation may be important for simulation of complex DDIs, such as those involving concomitant induction and/ or inhibition of multiple enzymes and/ or transporters by parent drugs and/ or their metabolites in patient population sub-groups. Further information on impact of renal impairment on human renal blood flow, transporter abundances, cellularity and 173
174 other physiological parameters affecting tubular secretion is needed to support future modelling and simulation efforts. The current study investigated the use of a PBPK kidney model for simulating pharmacokinetic changes in renal impairment from the bottom-up perspective. It is this bottom-up approach which relies upon experimentally obtained physiological data to inform changes in the system parameters of renal impairment populations in the model. The top-down approach is an alternative approach which uses clinical data to determine generic CL R scaling factors and system parameter values for simulating renal impairment associated changes in pharmacokinetics with PBPK models (255, 256). Given the lack of transporter abundance data in human kidney for healthy and renally impaired individuals, the top-down approach offers a promising alternative to inform these PBPK model parameters Conclusion A mechanistic kidney model for digoxin was developed, which accounted for the roles of OATP4C1 and P-gp in its tubular secretion. The lack of requisite physiological data, in particular relative transporter abundance between the in vitro systems and the human kidney, prevented successful use of IVIVE to inform the OATP4C1 CL int,t parameter for CL R prediction. In contrast, estimation of the OATP4C1 CL int,t parameter using sensitivity analysis allowed for simulation of CL R which was in agreement with clinical data. The sensitivity analysis used for OATP4C1 optimisation highlighted the need for appropriate consideration of covariates such as GFR, which may be important for obtaining accurate estimates of transporter kinetic parameters. When changes in filtration are only considered, the simulation of digoxin pharmacokinetics in renal impairment under-estimated the observed magnitude of changes in digoxin CL R compared to healthy subjects. Sensitivity analysis demonstrated that additional consideration of reduced renal secretion, attributed to changes in either proximal tubule cellularity or OATP4C1 abundance, could account for the differences between observed and simulated digoxin CL R ratio for renal impairment. However, systems data on the actual changes in human kidney physiology relevant to renal secretion are currently limited. Based on the simulation results of the current study, further work is needed to develop physiologically-based models of renal impairment which adequately represent the underlying biological changes due to disease, at the organ, cell and molecular level. Such models could lead to improvements in accuracy of pharmacokinetics predictions, in particular intra-cellular drug concentrations, with potential implications for understanding of drug efficacy and toxicity. 6.5 Acknowledgements Amir Khalifa and Sahar Fallaha contributed to this work by assisting with literature analyses and preliminary modelling and simulation efforts as part of their undergraduate studies. 174
175 Chapter 7. Final Discussion The kidney is an important route of elimination for many drugs and their metabolites. This elimination is either via renal excretion, renal metabolism, or a combination of both, and can therefore significantly contribute to the pharmacokinetic profiles of drugs. In vitro assays and IVIVE approaches have been increasingly used for prediction of pharmacokinetic parameters, particularly hepatic metabolic clearance (1, 179, 472). Improved use of these tools over the last two decades has coincided with reduced drug development attrition rates due to poor pharmacokinetics, as well as the increasing development of more metabolically stable drugs (473, 474). With the development of more metabolically stable drugs, there has been an increasing need for adequate IVIVE strategies for prediction of renal elimination. Despite recent studies, IVIVE approaches for prediction of renal elimination currently lag behind that of hepatic elimination (71, 87). This could be because of previous trends towards the development of drugs that are primarily hepatically eliminated, or a general perception that human CL R can be predicted reasonably well from pre-clinical species using allometric scaling, reducing the need for mechanistic predictive tools. Although literature reports exist that give examples of successful allometric scaling (335), this approach does not provide mechanistic insight, and known species differences in renal drug transporters (Table 1.2) will confound prediction of CL R for some drugs. More recently there has been an increasing interest in the development and applications of mechanistic models of renal drug elimination, as well as a desire for robust IVIVE approaches for kidney, within industry and regulatory bodies (78, 107, 225, 226). Therefore, identifying the key physiological elements of kidney necessary to inform IVIVE scaling factors and experimentally obtaining some of these data, together with defining knowledge gaps in model parameters, represent the valuable scientific contribution of this work. In the current study, an extensive literature analysis was performed to identify the key in vitro systems that are currently used (or in development) for investigating renal drug elimination and disposition (Chapter 1). This analysis additionally included the important collation of kidney physiological parameters required to scale the data generated in these in vitro systems to in vivo and to inform system parameters in mechanistic models of kidney. Due to sparse availability of data in the literature, the microsomal and cytosolic protein contents were measured in 31 human kidney cortex samples (Chapter 2). This study represents the only available data for cytosolic protein in the kidney, and more than doubles the currently available data for microsomal protein. The measurement of mycophenolic acid CL int,ugt,hkm in kidney microsomes prepared from 13 donors revealed large inter-individual variability in the UGT1A9 activity in the human kidney microsomes (20-fold difference). In addition to scaling factors, the Thesis has developed a range of mechanistic models of relevance for the prediction of CL R from in vitro data. Minimal mechanistic model for prediction of passive tubular reabsorption developed was validated against a dataset of 45 drugs (Chapter 4) and subsequently implemented within an existing PBPK kidney model (Chapter 5) to predict the effect of urine flow on CL R. Initial analysis has highlighted that the same PBPK kidney model (MechKiM), was not successful for IVIVE of active tubular secretion of digoxin using the data available in the literature (Chapter 6). Therefore, estimation of drug specific parameters was 175
176 performed using clinical data from a large cohort of studies to refine further the digoxin mechanistic kidney model. Simulation results indicated that robust estimates of specific physiological parameters relevant to drug secretion, such as transporter abundance and proximal tubule cellularity, were important in different scenarios, depending on the purpose of the modelling exercise. Progress was made towards the measurement of proximal tubule cells in human kidney using a modified stereology-based approach by developing relevant histology protocols. For the first time, cross-reactivity of a commercially available anti-human anti-villin antibody with pig was demonstrated (Chapter 3). In addition, and unexpectedly, staining of non-proximal tubules in the medulla of human and pig kidney by anti-villin antibody was noted. The sections below highlighted areas where the current research should be continued, and will discuss the wider implications of current findings. The importance of utilising the wide range of scientific techniques and technologies which are now available to further develop, refine and validate the IVIVE approaches and mechanistic kidney models for renal clearance predictions will be emphasised. 7.1 Measurement of microsomal and cytosolic protein recoveries in kidney: Theory and practice The preparation of highly purified subcellular fractions and the use of highly specific protein markers localised to only the fraction of interest are theoretically the prerequisites for accurately estimating subcellular fraction protein recoveries. Deviation from this scenario could represent an invalid assumption when estimating microsomal protein recovery and lead to inaccuracies in MPPG (or CPPG) data. However, when considering the practicalities of preparing microsomal fractions, the availability of suitable protein markers and the biological nature of the system being investigated, it is evident that deviations from the theoretical scenario are likely, if not inevitable. This section will discuss some of the deviations and uncertainties relevant to the current study, with respect to both the preparation of human kidney sub-cellular fractions, and the use of protein markers, and implications for the measurement of MPPGK and CPPGK Balancing purity, yield and activity for microsomal preparation Although exact procedures for isolation of the microsomal fraction vary between studies (see appendix, Figure 8.1), most follow a generic two-stage differential centrifugation approach as used in the current study. This generic differential centrifugation preparation yields a microsomal fraction which contains several organelles that may each be isolated through further subfractionated of the microsomes (475). While the endoplasmic reticulum is the main constituent in microsomes, the plasma membrane and Golgi are also expected (475, 476). Microsomes may also contain contaminants such as mitochondrial fragments and cytosolic constituents. Contamination with mitochondria is a particular issue for the kidney, which requires more vigour during the homogenisation stage than softer organs such as liver (180). Such contamination may be reduced by various approaches, including washing pellets and performing repetitive centrifugation steps (214, 310, ). Attempts to reduce contamination are also likely to reduce the yield of microsomal protein, and are not typically used when preparing microsomes for assessment of drug metabolism activities or quantification of enzyme abundances (appendix, 176
177 Figure 8.1) (191, 266). Repetitive centrifugation steps prolong the time that fractions are being handled and manipulated, which may be detrimental to enzyme activity. It was important that the microsomes in the current study were prepared and treated in a typical manner, with a focus on preservation of enzyme activities to allow for comparison with current literature on renal drug metabolism. For this over-riding reason, aside from ensuring suitable care when separating supernatant and pellet fractions, extensive modifications of the protocol to reduce non-microsomal contamination were not performed in the current study Markers of microsomal and cytosolic protein recovery in kidney Estimates of microsomal protein recoveries following microsome preparation for dog kidney and liver differed depending on whether G6Pase activity or CYP content were used as protein marker (Table 2.2). There are several limitations to the use of certain marker assays for assessing microsomal protein recoveries (section 2.4.1), and these limitations could contribute to the discrepancy between the two markers investigated in dog. A third marker may be required to investigate whether data from either CYP content or G6Pase provide the more reliable estimates of microsomal protein recoveries, and subsequently MPPGK and MPPGL. Recent studies have used NADPH cytochrome c reductase as a microsomal protein marker for human kidney (60, 182). Further studies that directly compare kidney microsomal protein recoveries estimated using G6Pase activity, CYP content and NADPH cytochrome c reductase are therefore recommended. In the current study, it was decided that G6Pase activity would be a more suitable microsomal protein marker in human kidney than CYP content, primarily because CYP content could not be detected in some human kidney samples in previous studies (180, 182). In addition, human kidney CYP content was expected to show high variability, in part due to the polymorphic expression of key renal CYP enzymes such as CYP3A5 (9). High inter-individual variability was observed in G6Pase activity in human kidney samples, although activity was readily measured in all donors including those with the lowest activity (Chapter 2, Figure 2.10). A further reason for preference of G6Pase activity over CYP content was the difference in the average MPPGK estimated using CYP content when using either dog kidney that had been freshly collected or undergone a freezethaw cycle (Chapter 2, Table 2.2). Literature evidence to suggest freezing tissue can affect CYP content measurements, and subsequently estimates of microsomal protein recovery, are equivocal (184, 264, 480). Information on the impact of freezing of G6Pase activity in fresh and frozen tissue samples could not be found in the literature, and could be the subject of future studies in order to establish whether this could have been a confounding factor in the current study. When assessing the suitability of any protein marker, the extent of its localisation in organelles that are not in the fraction of interest should be considered. This was identified as a potential issue for GST activity in Chapter 2, because GST has both cytosolic and microsomal isoforms. In this case, quantifying the GST activity in the microsomes was possible and was accounted for when estimating CPPGK (Chapter 2, Figure 2.8). A large body of evidence suggests that CYP content, G6Pase activity and cytochrome c reductase activity are localised to non-microsomal organelles, such as the nuclear envelope, mitochondria and cytosol, in addition to those of the microsomes (479, ). It has been suggested that the non-microsomal CYP content and cytochrome c 177
178 reductase activity (catalysed by novel reductase 1) are minor compared to the microsomes, and was assumed to have negligible impact on MPPGL estimates (186, 482). In addition, the G6Pase activity in the nuclear envelope, mitochondria and cytosol combined is reported to only represent up to 7 % of the total activity in homogenate (295, 297). Very few studies have considered the suitability of particular cytosolic protein markers for the specific purpose of estimating recoveries following differential centrifugation (190). As noted for the microsomal recovery estimates using CYP content and G6Pase activity as markers, protein recovery measurements could be marker dependent. Therefore, it would be prudent to corroborate the cytosolic protein recovery estimates obtained using GST activity with a second (and possibly third) marker. Literature evidence supports the use of ADH activity as a cytosolic protein marker, although issues with the ADH activity assay were encountered in the current study (Chapter 2, section ). Although issues were encountered with GST activity as a marker, it is important to note that human CPPGK data obtained in the current study are unique and novel data. These scaling factors can now be applied for different IVIVE scenarios, such as prediction of renal metabolic clearance via cytosolic enzymes (e.g., renal carboxylesterases). As matching dog liver and kidney cytosol samples (i.e., from same animals) are available, it would be possible to perform a direct comparison of the cytosolic protein contents of these organs, analogous to the data generated for MPPGL and MPPGK (Chapter 2, Figure 2.5), which has not previously been investigated. 7.2 Assessing the relevance of renal drug metabolism Under-prediction of in vivo glucuronidation clearance from human liver microsomal metabolism data has been reported by several studies (64, 209). This trend for under-prediction persists despite optimisation of in vitro assay conditions, such as activation of microsomes using alamethicin, or sequestration of fatty acids using BSA (38, 64, 489). For some drugs, particularly those for which metabolism by UGT1A9 is substantial, consideration of renal glucuronidation improves prediction of overall in vivo clearance using IVIVE (38). The establishment of tissue and species-specific scaling factors is an important requirement for successful IVIVE of microsomal metabolism data. Therefore, MPPGK in human and dog was measured for a number of donors. The mean value of this scalar obtained for dog (44.0 mg/ g kidney) was higher than that in human (25.7 mg/ g kidney) using the G6Pase activity as microsomal protein marker in both cases. The mean value for human MPPGK obtained in the current study was approximately 2-fold higher than the literature value commonly used for IVIVE of renal metabolic data (Chapter 1, Figure 1.3). From this finding it could be inferred that previous studies have under-estimated renal glucuronidation. However, the source of tissue used to prepare microsomes is an important factor to consider. Whereas kidney cortex was used in the current study (25.7 mg/ g kidney), the source was not specified in the study by Al-Jahdari et al. (12.8 mg/ g kidney) (182). This explanation is supported by the other reports of MPPGK measurements, as another value reported using kidney cortex was generally higher than a value reported for mixed kidney microsomes (summary in Table 1.8). In addition, the recent study by Knights et al. reported higher abundance of UGTs in 178
179 the cortex compared with medulla (60). There is also evidence of differences in metabolic capacity and blood flow across regions of the kidney (216). Therefore, in the current study, it was important to assess the impact of assuming that either the whole kidney or only the cortex contributes to renal glucuronidation clearance, and the subsequent effect on the predicted kidney: liver ratio for CL met,ugt. The scaling of mycophenolic acid CL int,u,ugt,hkm data using the MPPGK value obtained in the current study, without correction for blood flow and kidney weight, resulted in 93% higher kidney: liver ratio for CL met,ugt compared to using the value reported by Al-Jahdari et al. (182) (Figure 2.13), as well as 20% higher predicted CL UGT (Table 2.3). However, correction for the blood flow and kidney weight, thereby assuming only cortex is contributing to renal glucuronidation of mycophenolic acid, reduced these differences by two thirds. This suggests that underestimation of overall CL R,met,UGT associated with using the MPPGK scaling factor reported by Al- Jahdari et al. is unlikely or minor. Overall it is recommended that if kidney microsomal protein used for in vitro metabolism assays is known to be derived from cortex tissue, then cortex specific MPPGK scalar for prediction of CL R,met,UGT should be also used, in conjunction with correction for the blood flow and kidney weight parameters. A permeability-limited model of renal metabolism has been used to describe renal elimination of preformed and generated metabolites in the isolated perfused rat kidney (206). This model has compartments representing the renal plasma, renal tissue and the tubular filtrate, with separate uptake and efflux parameters for the parent drug and metabolite on both the apical and basolateral membranes of the renal tissue, as well as intrinsic metabolic clearance parameter in the renal tissue compartment. This model could be expanded to account for regional differences in the cortex and medulla, with parameters informed using IVIVE approaches for both the renal drug metabolism (Chapter 2) and tubular reabsorption (Chapter 4) elements. However, accounting for the dynamic filtrate flow rate and subsequent impact on filtrate drug concentrations, as well as the structural organisation of the renal vasculature, could be more challenging. In contrast these elements are implemented in the SimCYP MechKiM, which accounts for cortex/ medulla differences in metabolic capacity and blood flows (78). The IVIVE for renal metabolism is implemented in MechKiM module, and metabolism is assumed to occur only in the cortex, specifically the proximal tubule cell compartments. Therefore, the cortex specific MPPGK value obtained in Chapter 2 could be used to update the corresponding parameter of the MechKiM, in addition to IVIVE of tubular reabsorption implemented in Chapter 5. Excretion of mycophenolic acid glucuronide in the urine is the main route for final elimination of mycophenolic acid from the body (490). SNPs in the promotor and coding regions of UGT1A8, 1A9 and 2B7 genes are reported to influence mycophenolic acid glucuronidation rates and exposure. This suggests an important role for glucuronidation, by liver, kidney and intestine, in the elimination of mycophenolic acid and in the inter-individual variability of the pharmacokinetics of this drug (269, 491, 492). In the current study, predicted mycophenolic acid CL UGT, accounting for the role of both liver and kidney, was in agreement with the observed clearance (Figure 2.12), consistent with previous findings (38). The observed CL UGT was estimated using i.v. clearance of mycophenolic acid published in a clinical study, in which a second peak occurred in the plasma concentration-time profile, consistent with enterohepatic recirculation (56). However, predictions of 179
180 CL UGT using the well-stirred model, as performed in the current study and Gill et al. (38), do not account for enterohepatic recirculation. The mean predicted kidney: liver ratio of CL met,ugt ranged from depending on the assumptions used for scaling (Table 2.3). However the actual contribution of renal glucuronidation to overall final elimination and inter-individual variability in pharmacokinetics of mycophenolic acid could be higher than this suggests. This is because the proportion of glucuronide formed in liver that undergoes recirculation will not contribute to the final elimination. In addition, mycophenolic acid CL int,u,ugt, scaled per g of tissue, was higher in kidney compared to liver, and this difference was larger when MPPGK from the current study was used compared with the commonly used literature value (Table 2.3). To investigate this further, a PBPK model could be developed which features renal and hepatic glucuronidation of mycophenolic acid, transporter mediated uptake and efflux of mycophenolic acid parent drug and glucuronide metabolites by kidney (OAT3, MRP2 and MRP4 (269, 493, 494)) and liver (OATP1B1, OATP1B3, MRP2, MRP3 and MRP4 (269, )), and the enterohepatic recirculation. Although pharmacokinetic models of mycophenolic acid enterohepatic recirculation have been reported, these do not consider the separate contributions to glucuronidation of liver and kidney (498, 499). Such models could be particularly valuable to investigate the impact of hepatic impairment and DDIs, which can affect the pharmacokinetics of mycophenolic acid (500). In vitro studies have so far used human kidney microsomes to investigate renal glucuronidation of mycophenolic acid, with separate assays involving transfected cell lines to study the transport of mycophenolic acid and the glucuronide metabolite (Chapter 2, (38, 269, 493, 494)). However, use of primary cultured proximal tubule cells or immortalised kidney cell lines are more physiologically representative (Table 1.3) and could allow for more holistic studies on the metabolism-transporter interplay, as has been performed for this drug in sandwich-cultured human hepatocytes (497) Predicting the relevance of metabolites in DDIs and toxicity in kidney Mechanistic models of kidney could be used to improve simulation of renal DDIs by accounting for the inhibitor concentrations at the site of action, rather than using plasma concentrations as surrogate, analogous to previous studies on the hepatic and intestinal DDIs (106, 501, 502). The parameters of these models can be informed through IVIVE of passive permeability and drug metabolism, as explored in Chapters 2, 4 and 5, and, following improvements in appropriate scaling factors, IVIVE of tubular secretion. In this way, the simultaneous roles of multiple transporters/ enzymes and inhibition of these by multiple drugs and metabolites can be simulated. An example of a complex DDI is that between NSAIDs and methotrexate. Clinical data suggest that co-administration of NSAIDs can reduce the renal secretion of methotrexate (503). These data are supported by in vitro studies demonstrating that NSAIDs inhibit renal drug transporters that mediate methotrexate renal secretion, such as OAT1, OAT3, OAT4, MRP2 and MRP4 ( ). Some NSAIDs such as ibuprofen, diclofenac and naproxen undergo glucuronidation via UGT1A9 and 2B7, as reported in human kidney microsomes in vitro (38, 209, 508, 509). Recently, NSAID glucuronides were reported to inhibit MRP2 and MRP4 mediated efflux of methotrexate in vitro, with potencies that in some cases exceeded those of the parent NSAID drugs (234). It was therefore hypothesised that NSAID glucuronides could contribute to the clinical DDI between NSAIDs and methotrexate (234). These kinds of complex DDI scenarios, involving interplay of multiple processes and effects of both parent and metabolite, can be accommodated by 180
181 physiologically-based mechanistic models to determine the clinical relevance of DDI risk associated with NSAID glucuronides. The parent and glucuronide concentrations in both the plasma and tubular cells could be simulated, although data needed to inform the PBPK model parameters (e.g., K p and elimination rates/ routes) for the glucuronide are likely to be limited. It may be important to account for the contributions of both the pre-formed (circulating) and the intrarenally formed glucuronides, as well as the impact of renal metabolism on the local concentrations of the parent drug. This may require consideration of cortex and medulla differences in the MPPGK scaling factor and blood flows, highlighting a potential advantage of using cortical microsomes, as per the current study (Table 2.3). IVIVE of renal drug metabolism could therefore be useful to inform some of the requisite parameters for simulation of complex renal DDIs using mechanistic kidney models. However, in the example of the NSAID-methotrexate DDI, the development of a model for methotrexate that accounts for the active and passive processes involved would be needed, which is associated with several challenges, as highlighted in Chapter 6 and below. Renal drug metabolism can play an important role in the detoxification of nephrotoxic drugs (510). Conversely, renally formed drug metabolites have been implicated in causing drug-induced kidney injury. For example, although glutathione conjugation of drugs is typically a detoxification pathway for drugs and environmental contaminants, in some cases reactive metabolites can be subsequently formed from glutathione adducts in the kidney, e.g., efavirenz and trichloroethylene (178, ). The formation of these reactive metabolites is a possible mechanism behind observed species differences in proximal tubule necrosis and nephrotoxicity associated with such xenobiotics (511, 513, 514). Furthermore, metabolites formed by CYP3A are likely to contribute to ifosfamide and cyclosporine A related nephrotoxity (515, 516). In vitro data and clinical studies indicate that polymorphic expression of CYP3A5 results in inter-individual variability in the intrarenal formation of ifosfamide and cyclosporine A metabolites (6, 517, 518). In these examples, in vitro data provided qualitative information to improve or substantiate the understanding of the role of drug metabolism in drug induced kidney injury. Development of multiscale and mechanistic models to describe the role of drugs and their metabolites in nephrotoxicity (e.g., adverse outcome pathways), integrated with PBPK kidney models (as explored in Chapter 5 and 6), could be used to quantitatively predict the impact of different factors such as species, genotype and dosing regimens (e.g., DDIs) on drug induced nephrotoxicity (519, 520). Accurate IVIVE scaling factors, such as MPPGK and CPPGK which were measured in the current study (Figure 2.9), and physiological data, such as enzyme abundances, are crucial parameters required, in addition to in vitro metabolism data for the drug(s) of interest. Quantitative information on differences in drug metabolising enzymes between different species could therefore be used to improve the use of pre-clinical species in toxicology assessment and translation to human. This is also an example in which modelling approaches could facilitate the clinical application of genomics and proteomics data for stratified or personalised medicine (1). 181
182 7.3 Opportunities to improve prediction of human renal drug disposition Although mechanistic models of CL R theoretically support IVIVE to inform model parameters, IVIVE for CL R is currently poorly developed (78, 106, 107). Improving this situation is likely to yield several opportunities to maximise the use of data from in vitro and preclinical in vivo studies for prediction of human renal drug disposition in clinical situations. Some examples of particular relevance to renal drug metabolism were given in the previous section; here the discussion focuses on the role of renal drug transport and excretion, including suggestions on potential ways in which mechanistic models may be used in the future Accounting for multiple interacting processes An advantage of mechanistic kidney models is the possibility to integrate multiple processes contributing to the renal disposition of drugs. In the current study IVIVE approaches for several of these processes were explored and developed, including renal metabolism (Chapter 2), tubular reabsorption (Chapters 4 and 5) and active secretion (Chapter 6). It is important to note that the contribution of multiple processes can confound the interpretation of clinical data which are used for model validation. Returning to methotrexate as an exemplar, this drug is reported to undergo both active secretion, which is saturable at high doses, and active reabsorption which is saturable at low doses (521). Methotrexate also exhibits urine flow and urine ph dependent CL R (522), from which the involvement of passive tubular reabsorption may be inferred. Urine alkalinisation and hyper-hydration is required during high dose methotrexate treatment to prevent methotrexate induced kidney injury through crystalluria (522, 523). Based on clinical data alone, separation of active secretion and active reabsorption elements to define appropriate parameters in a PBPK model was not possible, despite the availability of in vitro data to inform the binding affinities (K m ) of methotrexate for various kidney transporters (224, 494, 506). IVIVE would therefore be useful to inform the maximal transport rate (V max ) parameters of a more mechanistic kidney model for methotrexate. The passive permeability parameters of such a mechanistic kidney model could be informed using tubular reabsorption model with urine flow consideration as demonstrated in Chapter 5 (Figure 5.6). Development of a mechanistic kidney model for methotrexate and drugs with similar properties could be used to investigate several clinical scenarios, including the impact of different patient factors on urinary concentrations of methotrexate at different flow rates and ph of urine, as well as simulation of renal DDIs, for example with NSAIDs Simulation of intra-cellular or tissue concentrations Preclinical in vivo studies can be used to investigate the potential for drug accumulation in the kidney, as well as the impact of renal impairment on renal drug disposition. For example, digoxin kidney: plasma concentration ratio increased 4-fold in chronic renal failure rats compared with control animals (411). Obtaining equivalent data in human is challenging and requires use of noninvasive imaging such as PET, which involves specialist equipment and expertise, and does not allow distinction of parent drugs from metabolites (261). The lack of such clinical data restricted the initial development of the digoxin PBPK kidney model, particularly for validating or refining the P-gp REF parameter (Chapter 6, section 6.3.1). In addition, the limited availability of transporter 182
183 abundance data and other relevant physiological data in kidney restrict the opportunities to apply the model for extrapolation beyond healthy subjects. As such, the analyses presented in Chapter 6 investigated hypothetical scenarios based on plausible changes in the system parameters of the kidney model (e.g., Figures 6.7 and 6.8). Therefore, translation of knowledge and understanding of renal drug disposition in preclinical species to human would be advantageous. However, translation from preclinical to human may be confounded by species differences in renal drug transporters (Chpter 1, Table 1.2) (106). For example, the OATP4C1 transporter, which mediates digoxin uptake into proximal tubule cells, is thought to be expressed on the basolateral membrane in human, but the apical membrane in rat (33, 50). Additional evidence supporting the proposed species differences in OATP4C1/ Oatp4c1 localisation comes from unpublished data from rat and human primary cultured proximal tubule cell monolayers, indicating that the direction of digoxin net flux is absorptive in rat but secretory in human (Chung G, Billington S, Brown C. Marked Species Difference in the Handling of Digoxin by Primary Human and Rat Proximal Tubule Cells. Poster T2286, presented at the AAPS Annual Meeting and Exposition, San Diego, CA, November 2014). Quantitative knowledge of these species differences in renal transporters (abundance and localisation) and other physiological features of kidney (e.g., proximal tubule cellularity), when available, could be used to inform species specific systems parameters of PBPK kidney models such as MechKiM that was used for Chapter 6. For example a mechanistic model of rat kidney for healthy and chronic renal failure animals could include the relative differences in the protein expression of several renal transporters, including Oatp4c1 and P-gp that were reported (411). Likewise species specific drug parameters such as K m and V max can be obtained, using in vitro systems derived from, or representative of, kidney of particular species, along with relevant IVIVE scaling factors (e.g., REF). Therefore, mechanistic models and IVIVE approaches may allow for more accurate translation of preclinical findings to human. The mechanistic rat kidney model for digoxin could be developed initially using available in vitro transporter kinetics data (e.g., (49)) and refined using observed rat digoxin CL R and kidney: plasma concentration ratio data from healthy rats (411). Subsequently the ability of the model to simulate the changes in digoxin kidney concentrations and CL R in chronic renal failure rats based on transporter expression changes could be investigated. If successful, this could provide confidence in using the PBPK kidney model of digoxin developed in Chapter 6 for predicting changes in the renal drug disposition of digoxin in human, including intra-cellular concentrations. Theoretically, following validation using digoxin, the separation of drug specific and system specific parameters would allow the renal impairment model to be applied to other drugs. Transporter mediated uptake and subsequent accumulation of certain drugs, including cisplatin and antiretroviral drugs, is associated with nephrotoxicity (103, ). Investigation of population variability using PBPK kidney models could enable the identification of particular patient characteristics (e.g., age, gender and ethnicity) and physiological parameters (e.g., glomerular filtration rate, transporter abundances and kidney size) that are associated with increased or decreased accumulation of drugs in the proximal tubule. Further, simulated proximal tubule cell concentrations could be used as part of a quantitative IVIVE strategy for in vitro toxicity assessment (520). As demonstrated in Chapter 6 using digoxin, accurate simulation of drug concentrations in proximal tubule cells requires robust estimates of transporter kinetic parameters, 183
184 as well as key systems parameters such as proximal tubule cellularity. Obtaining the requisite data to inform IVIVE scaling factors and systems parameters in PBPK kidney models could therefore provide new opportunities to quantitatively predict the accumulation of drugs in proximal tubule cells in clinical scenarios, which could be used to better understand risks of nephrotoxicity. 7.4 Challenges associated with development and validation of CL R IVIVE and mechanistic kidney model As highlighted above, having robust parameter values for system-specific and drug-specific features of mechanistic kidney models is associated with several advantages. However, there are a number of challenges which prevent these parameter values being measured, estimated and/ or validated Availability of appropriate clinical data for refining and validating specific parameters While IVIVE is a promising approach to inform parameters in mechanistic kidney models, clinical data are often needed to refine the values of such parameters (79, 225, 226), and to validate the model before extrapolation to new clinical scenarios is possible (227). In Chapter 6, digoxin CL R data were used to inform the OATP4C1 CL int,t parameter that represented the uptake into the proximal tubule cells (Figure 6.5). Uptake is the rate-limiting step in the renal secretion of the drug and any delay between uptake into proximal tubule cells and efflux into the tubular filtrate is not apparent from the clinical urinary excretion data. For digoxin, neither the plasma concentrationtime profile nor the CL R were sensitive to changes in the efflux transport rate parameter (Figure 6.3). Therefore, the available clinical data were not informative enough for either refinement or validation of the efflux transport parameter of the digoxin model consistent with previous studies (79, 226). Conversely, the concentration of digoxin in proximal tubule cells was sensitive to changes in the efflux transport parameter (Figure 6.3). While PET imaging can be used to obtain clinical information on drug concentrations in particular organs, the spatial resolution of this technique is in the mm range (261, 527, 528). This level of spatial resolution does not allow distinction between the vasculature, cellular and tubular spaces of the nephron, although it can allow differentiation between cortex and medulla. Quantitative PET imaging data might provide enough information to allow refinement/ validation of parameters in the PBPK kidney model necessary to simulate kidney drug concentrations (i.e., volume-weighted average concentration of relevant sub-compartments). An analogous approach has been applied to derive GFR data from magnetic resonance renography data using a compartmental model (529). Validation of the use of PET imaging data to unambiguously inform otherwise nonidentifiable parameters of PBPK kidney models could be performed initially using pre-clinical species. Such validation could involve the parallel use of invasive imaging techniques such as fluorescence confocal microscopy or imaging mass spectrometry which provide greater spatial resolution (89, 90). 184
185 7.4.2 Technical and practical issues with obtaining useful physiological data for kidney The literature analysis in Chapter 1, section 1.4 highlighted a number of areas where quantitative information on renal physiology is either missing or is scarce. This may be partly because obtaining a reliable source of high quality human kidney tissue is challenging. In this respect, the 31 human kidney samples obtained in the current study for measurement of MPPGK and CPPGK represents a highly valuable resource. Access to tissue may be particularly relevant in explaining the lack of direct measurements of proximal tubule cellularity in human kidney, because stereological techniques require access to the entire organ of interest to sample from in order to maintain statistical validity (177). A possible solution for this, namely the use of a piece of kidney that is expected to be representative of the whole organ, was explored in Chapter 3 of this Thesis. The proximal tubule cellularity is aimed to be used as a scaling factor (i.e., PTCPGK, Figure 1.1) for IVIVE of in vitro data generated in immortalised proximal tubule cell lines and primary cultured proximal tubule cells (Table 1.3). In addition to human tissue requirements, there are important technical challenges involved in estimating this physiological parameter using stereology, notably the requirement for unambiguous identification of proximal tubule cells, which requires the use of selective histochemical staining techniques (see section 3.3.2). In the current study, the commercially sourced anti-villin antibody stained not only the proximal tubule cells in human kidney histology sections (Figure 3.6), but also tubules that were non-proximal (Figure 3.9). This finding suggests that either (a) villin is expressed in non-proximal regions of the nephron tubule, and therefore is not a suitable marker for proximal tubule, or (b) that the antibody used in the current study was not specific to villin. Technical challenges also apply to quantitative proteomics using LC-MS/MS for measurement of enzyme and transporter abundances. Lack of OATP4C1 abundance data in human kidney and transfected cell lines (necessary for calculation of REF scaling factors), was identified in Chapter 6 as a reason that IVIVE of digoxin CL int,t could not be done. In fact, no data have yet been published on the abundance of drug transporters in human kidney. Conversely, two studies have so far reported abundances of UGT1A6, 1A9 and 2B7 in individual kidney samples, using targeted LC-MS/MS proteomics (39, 60), although methodological differences makes direct comparison of the studies challenging. As each study had a limited number of samples available, information on the inter-individual variability is currently limited. QconCAT technology allows for the simultaneous quantification of multiple proteotypic peptides from different proteins using a single quantification standard, facilitating efficient and cost effective analysis (530). Although this approach has been used in-house to quantify abundances of drug metabolising enzymes and transporters in human liver and intestine (196, 530, 531), no studies have yet reported application for human kidney. It is not currently known whether factors specific to kidney or the microsomal preparation procedures used in the current study (Chapter 2) would necessitate further optimisation of protocols (e.g., sample preparation, digestion or instrument settings) for LC-MS/MS quantification using QconCAT. Preliminary experiments were performed using a single donor, in which LC-MS/MS analysis demonstrated that peptides representing three plasma membrane proteins, namely ATP1A1, P-gp and MRP2, could be detected using the QconCAT in-house proteomic methods (appendix, Figure 8.10; LC-MS/MS experiments performed by Dr Brahim Achour). Further 185
186 challenges, relevant to the wider field of quantitative proteomics, have been identified (195). The evaluation of accuracy of different approaches and distinguishing true biological variability from technical and inter- and intra-laboratory sources of variability, are considered particularly important. The contribution of these other sources of variability should be considered during analysis of co-variate relationships between different proteins, as it has been suggested that technical co-variability may contribute to apparent correlations in protein expression data (532). It is important to highlight that some of the systems parameters of human mechanistic kidney models are informed by physiological data obtained in pre-clinical species. In Chapters 4 and 5, tubular flow rates were important systems parameters for the prediction of F reab and CL R (Figure 5.4). Data on the tubular flow rate and ph in different regions of the nephron, measured during micro-puncture experiments, were most widely available in rat (Table 1.5) because of the invasive nature of this technique, which cannot be performed in human. There were clinical evidence (e.g., impact of vasopressin, water diuresis and/ or nephrogenic diabetes insipidus on urine flow rates (152, 154)) that support the use of these particular data from animals in the human models. Conversely, clinical data that support the use of animal tubular filtrate ph data to inform the parameters of models of human kidney are lacking. Therefore, any future work aiming to incorporate the effects of filtrate ph on the ionisation, permeability and ph partitioning of drugs in the mechanistic reabsorption model should consider this uncertainty in parameter values Relationship between systems parameters of models and measurements of physiological features GFR is a fundamental parameter of PBPK kidney models. The measurement of true GFR (mgfr) using inulin or iothalamate renal excretion clearance is more time-consuming, resource intensive and invasive than obtaining an estimated value (egfr) from serum creatinine or CL CR (244, 351, 463). Therefore, during clinical trials in which renal functions of patients are reported, as well as in clinical practice, egfr or estimated CL CR are usually reported. In contrast, mechanistic models rely upon a GFR parameter to calculate CL R,filt of the drug in the simulated population. In Chapter 6, the sensitivity analysis approach employed for estimation of OATP4C1 CL int,t also considered the potential impact of GFR on digoxin CL R (Figure 6.5). This was achieved by including the serum creatinine parameter in the sensitivity analysis, which was varied from 50 to 115 µmol/ L) to give GFR values ranging from 103 to 238 ml/ min for the simulated population representative. In the SimCYP PBPK kidney model, serum creatinine and demographics (e.g., age, gender) values for virtual subjects are randomly generated from predefined distributions, with GFRs subsequently calculated using conventional equations designed for clinical use (459, 533). However, this is a miss-specification of the major causal relationship between GFR and serum creatinine concentrations (assuming any feedback mechanisms which exist have relatively minor effect), because in reality the serum creatinine concentrations are an outcome of synthesis and elimination rates of creatinine (534, 535). With respect to demographics, potential relationships between mgfr and age, gender and ethnicity in healthy subjects have been explored by numerous studies, as reviewed previously (150), with further data since published (464). In addition, demographic factors can influence the generation rate of creatinine independently of any changes in GFR (534). Therefore, PBPK models of kidney, which are concerned primarily with the 186
187 mechanistic determinants of pharmacokinetics should define the actual GFR parameter as a function of the demographics (which excludes serum creatinine) for each virtual subject. Clarifying the underlying mechanisms behind changes in tubular drug secretion in renal impairment may not be a priority when the purpose of the modelling exercise does not include other factors (e.g., DDIs), and is restricted to understanding potential changes in systemic drug concentrations. However, understanding such mechanisms is important when the focus shifts to complex DDIs and/ or potential changes in intracellular concentrations. As shown in Figure 7.1, reduced transporter abundance per kidney could be attributed to decreases in (a) transporter abundance per proximal tubule cell, (b) proximal tubule cellularity per gram kidney, or (c) kidney weight. Obtaining the requisite data to differentiate (a) and (b) represents a substantial challenge, especially when one recognises that renal impairment may have a number of aetiologies. Changes to physiological characteristics of kidney may vary between renal impairment aetiologies, as was noted for kidney weight in Chapter 1 (Table 1.4). For example, although renal impairment is typically associated with atrophy, tubule hypertrophy has been observed in the remnant kidney model in rats (252, 536), while tubule hypertrophy and proximal tubule cell hyperplasia have been noted in diffuse kidney diseases in human (124). Such findings highlight the need for more physiological data to inform mechanistic models of kidney in health and disease states. In addition to the challenges associated with renal impairment aetiology, there are practical challenges associated with obtaining the requisite systems data for simulating changes in intracellular drug concentrations in kidney disease. In particular, current LC-MS/MS proteomics methodology measure protein abundances in tissue homogenates or sub-cellular fractions, and therefore generates data normalised to the protein content or weight of the original tissue (200, 266, 530, 531). Such data do not directly correspond to systems parameters in the PBPK kidney model used in Chapter 6, where transporter abundances are normalised to proximal tubule cell number (Figure 7.1). Experimentally obtaining transporter abundance normalised to proximal tubule cell number would be very difficult. Theoretically, this could be achieved by proteomic quantification of protein abundance in lysates (or sub-cellular fractions) of proximal tubule cells that have been freshly isolated and purified from human kidney tissue. Isolation and purification of proximal tubule cells currently requires use of flow cytometry and/ or magnetic cell separation techniques (204, 315, 316, 537). Fresh kidney tissue often becomes available at short notice, making it difficult to coordinate and organise access to the equipment and technical expertise required for proximal tubule cell purification. Cryopreservation of isolated human kidney cells could provide a solution to this problem, and formed part of a study in which flow cytometry was used directly to quantify the abundances of hepatic transporters (204). An alternative approach, whereby proximal tubule cellularity (stereology) and transporter abundances (LC-MS/MS using tissue homogenates or subcellular fractions) are measured separately in the same tissue sample, might be more reliable in terms of practicality, although interpretation of such data would be less straightforward. In addition, MALDI-imaging mass spectrometry and secondary ion mass spectrometry could be used in the future to quantify protein abundances at the cellular level; 187
188 however, further improvements in instrument capabilities would be required, particularly the ability to quantify large molecules (202). Figure 7.1 Impact of renal impairment-associated changes in transporter abundance on pharmacokinetics of drugs at local and systemic levels. For simulation renal impairment associated changes in CL R,sec and urine/ plasma exposure, models accounting for altered transporter abundance (and activity) at the organ level (yellow arrow) is sufficient. For understanding liabilities associated with drug concentrations in proximal tubule cells (PTC) (i.e., renal toxicity or efficacy), models accounting for changes in transporter abundance at the cellular level are required. Understanding the covariate- and casualrelationships of the physiological parameters associated with proximal tubule cellularity and volume, as well as transporter abundance would enable more physiologically relevant models to be developed. 7.5 Conclusions The current study has made important and substantial contributions to improve the use of scaling factors and mechanistic models for prediction of renal drug disposition. The microsomal protein recovery experiments in dog kidney and liver highlighted that microsomal protein markers are important to consider and can affect the estimates of microsomal protein recovery. No correlation was found between MPPGK and MPPGL using matched dog tissues. The average MPPGK for human kidney cortex in the current study (25.7 mg/ g kidney; n=31 donors) was 2-fold the literature value commonly used for IVIVE of human kidney microsomal metabolism data. When scaling kidney microsomal metabolism data to predict CL R,met, it is important to consider the 188
189 source of the microsomes (cortex, medulla or mixed), and use the corresponding regional or overall MPPGK, blood flow and kidney weight. Average human CPPGK (52.7 mg/ g kidney; n=31) obtained in the current study was lower than literature data for the corresponding value in liver (CPPGL), a trend that was consistent with human and dog MPPGK and MPPGL. Therefore, tissue specific scaling factors are recommended for predicting metabolic clearance using the IVIVE approach. Development of the novel 5-compartment model for prediction of tubular reabsorption from Caco-2 data using IVIVE required careful consideration of physiological features of the nephron, particularly regional variations in tubular surface area and filtrate flow rate. The model showed good overall CL R prediction accuracy for the dataset of 45 chemically diverse drugs, but systematic under-prediction was evident for basic drugs. Further work is needed to investigate the impact of accounting for the effect of tubular filtrate ph on the ionisation and subsequently the permeability, particularly for basic drugs. In addition, the relevance of ph gradient between the tubular filtrate and plasma to the concentration gradient of the unionised form of drugs within the proximal tubule should be explored, to investigate the possibility of passive secretion occurring in this region of the nephron. Validation of the tubular reabsorption model with an external in vitro permeability dataset would be beneficial. The current study refined drug metabolism scaling factors and model structure and relevant physiological parameters for mechanistic prediction of tubular reabsorption using the bottom-up approach. However, analogous IVIVE methods were less successful for active transport processes in kidney. In the current study, IVIVE for the uptake transporter kinetic parameter was limited by the lack of data. Alternative approaches (use of clinical data) were considered for the estimation of OATP4C1 uptake parameter. In contrast, plasma data were not sufficient to validate or optimise the efflux transporter IVIVE scaling factor. The ability to simulate tubular and intracellular drug concentrations of parent drugs and metabolites are key advantages of using mechanistic kidney models. These properties could be particularly important for prediction of complex DDIs and potential for drug induced kidney injury. Future work is recommended to measure proximal tubule cellularity and enzyme and transporter abundances in human kidney to inform systems parameters of these models. Parallel measurement of these features in relevant in vitro systems to derive new scaling factors should refine further IVIVE methods to predict renal drug elimination from in vitro data. In addition, further work is needed to (a) acquire physiological data on kidney for a range of different patient characteristics (e.g., age, renal impairment, ethnicity) to inform model parameters for different populations, (b) develop novel methods to quantify transporter abundance per proximal tubule cell, and (c) investigate the use of imaging techniques to measure drug concentrations at the tissue and, preferably, cellular levels, that are suitable for refining and validating transporter kinetic parameters in mechanistic kidney models. 189
190 References 1. Rostami Hodjegan A. Physiologically based pharmacokinetics joined with in vitro in vivo extrapolation of ADME: A marriage under the arch of systems pharmacology. Clin Pharmacol Ther. 2012;92(1): Shoda LK, Woodhead JL, Siler SQ, Watkins PB, Howell BA. Linking physiology to toxicity using dilisym, a mechanistic mathematical model of drug induced liver injury. Biopharm Drug Dispos. 2014;35(1): Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53(1): Koch I, Weil R, Wolbold R, Brockmoller J, Hustert E, Burk O, et al. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mrna. Drug Metab Dispos. 2002;30(10): Haehner BD, Gorski JC, Vandenbranden M, Wrighton SA, Janardan SK, Watkins PB, et al. Bimodal distribution of renal cytochrome P450 3A activity in humans. Mol Pharmacol. 1996;50(1): McCune JS, Risler LJ, Phillips BR, Thummel KE, Blough D, Shen DD. Contribution of CYP3A5 to hepatic and renal ifosfamide n-dechloroethylation. Drug Metab Dispos. 2005;33(7): Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4): Schuetz EG, Schuetz JD, Grogan WM, Narayfejestoth A, Fejestoth G, Raucy J, et al. Expression of cytochrome-p450 3a in amphibian, rat, and human kidney. Arch Biochem Biophys. 1992;294(1): Bolbrinker J, Seeberg S, Schostak M, Kempkensteffen C, Baelde H, de Heer E, et al. CYP3A5 genotype-phenotype analysis in the human kidney reveals a strong sitespecific expression of CYP3A5 in the proximal tubule in carriers of the CYP3A5* 1 allele. Drug Metab Dispos. 2012;40(4): Pavek P, Dvorak Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr Drug Metab. 2008;9(2): Baker JR, Satarug S, Reilly PEB, Edwards RJ, Ariyoshi N, Kamataki T, et al. Relationships between non-occupational cadmium exposure and expression of nine cytochrome P450 forms in human liver and kidney cortex samples. Biochem Pharmacol. 2001;62(6): Ekins S, Wrighton SA. The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev. 1999;31(3): Yeung CK, Lang DH, Thummel KE, Rettie AE. Immunoquantitation of FMO1 in human liver, kidney, and intestine. Drug Metab Dispos. 2000;28(9): Dolphin CT, Cullingford TE, Shephard EA, Smith RL, Phillips IR. Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FMO3 and FMO4. Eur J Biochem. 1996;235(3): Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 2006;46: Furnes B, Schlenk D. Evaluation of xenobiotic n- and s-oxidation by variant flavincontaining monooxygenase 1 (FMO1) enzymes. Toxicol Sci. 2004;78(2): Pike MG, Mays DC, Macomber DW, Lipsky JJ. Metabolism of a disulfiram metabolite, s-methyl n,n-diethyldithiocarbamate by flavin monooxygenase in human renal microsomes. Drug Metab Dispos. 2001;29(2): Uno Y, Shimizu M, Yamazaki H. Molecular and functional characterization of flavin-containing monooxygenases in cynomolgus macaque. Biochem Pharmacol. 2013;85(12): Buhler R, Pestalozzi D, Hess M, Vonwartburg JP. Immunohistochemical localization of alcohol-dehydrogenase in human-kidney, endocrine organs and brain. Pharmacol Biochem Behav. 1983;18(suppl 1):
191 20. Ambroziak W, Izaguirre G, Pietruszko R. Metabolism of retinaldehyde and other aldehydes in soluble extracts of human liver and kidney. J Biol Chem. 1999;274(47): Vasiliou V, Pappa A, Petersen DR. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact. 2000;129(1-2): Barthel BL, Torres RC, Hyatt JL, Edwards CC, Hatfield MJ, Potter PM, et al. Identification of human intestinal carboxylesterase as the primary enzyme for activation of a doxazolidine carbamate prodrug. J Med Chem. 2008;51(2): Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13(2): Holmes RS, Wright MW, Laulederkind SJF, Cox LA, Hosokawa M, Imai T, et al. Recommended nomenclature for five mammalian carboxylesterase gene families: Human, mouse, and rat genes and proteins. Mamm Genome. 2010;21(9-10): Hatfield MJ, Tsurkan L, Garrett M, Shaver TM, Hyatt JL, Edwards CC, et al. Organ-specific carboxylesterase profiling identifies the small intestine and kidney as major contributors of activation of the anticancer prodrug CPT-11. Biochem Pharmacol. 2011;81(1): Kubo T, Kim SR, Sai K, Saito Y, Nakajima T, Matsumoto K, et al. Functional characterization of three naturally occurring single nucleotide polymorphisms in the CES2 gene encoding carboxylesterase 2 (hce-2). Drug Metab Dispos. 2005;33(10): Xu G, Zhang WH, Ma MK, McLeod HL. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clin Cancer Res. 2002;8(8): Nishimuta H, Houston JB, Galetin A. Hepatic, intestinal, renal, and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog, and rat: Implications for in vitro in vivo extrapolation of clearance of prodrugs. Drug Metab Dispos. 2014;42(9): Knight LP, Primiano T, Groopman JD, Kensler TW, Sutter TR. cdna cloning, expression and activity of a second human aflatoxin b1-metabolizing member of the aldoketo reductase superfamily, AKR7A3. Carcinogenesis. 1999;20(7): Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40(4): Bains OS, Takahashi RH, Pfeifer TA, Grigliatti TA, Reid RE, Riggs KW. Two allelic variants of aldo-keto reductase 1A1 exhibit reduced in vitro metabolism of daunorubicin. Drug Metab Dispos. 2008;36(5): Barski OA, Papusha VZ, Ivanova MM, Rudman DM, Finegold MJ. Developmental expression and function of aldehyde reductase in proximal tubules of the kidney. Am J Physiol Renal Physiol. 2005;289(1):F200-F Kuo K-L, Zhu H, McNamara PJ, Leggas M. Localization and functional characterization of the rat oatp4c1 transporter in an in vitro cell system and rat tissues. PLoS ONE. 2012;7(6):e Harbourt DE, Fallon JK, Ito S, Baba T, Ritter JK, Glish GL, et al. Quantification of human uridine-diphosphate glucuronosyl transferase 1A isoforms in liver, intestine, and kidney using nanobore liquid chromatography tandem mass spectrometry. Anal Chem. 2012;84(1): Sadeque AJM, Usmani KA, Palamar S, Cerny MA, Chen WCG. Identification of human UDP-glucuronosyltransferases involved in n-carbamoyl glucuronidation of lorcaserin. Drug Metab Dispos. 2012;40(4): Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15(10): Villeneuve L, Girard H, Fortier LC, Gagne JF, Guillemette C. Novel functional polymorphisms in the UGT1A7 and UGT1A9 glucuronidating enzymes in caucasian and african-american subjects and their impact on the metabolism of 7-ethyl-10- hydroxycamptothecin and flavopiridol anticancer drugs. J Pharmacol Exp Ther. 2003;307(1):
192 38. Gill KL, Houston JB, Galetin A. Characterization of in vitro glucuronidation clearance of a range of drugs in human kidney microsomes: Comparison with liver and intestinal glucuronidation and impact of albumin. Drug Metab Dispos. 2012;40(4): Margaillan G, Rouleau M, Fallon JK, Caron P, Villeneuve L, Turcotte V, et al. Quantitative profiling of human renal UDP-glucuronosyltransferases and glucuronidation activity: A comparison of normal and tumoral kidney tissues. Drug Metab Dispos. 2015;43(4): Sato Y, Nagata M, Tetsuka K, Tamura K, Miyashita A, Kawamura A, et al. Optimized methods for targeted peptide-based quantification of human uridine 5 - diphosphate-glucuronosyltransferases in biological specimens using liquid chromatography tandem mass spectrometry. Drug Metab Dispos. 2014;42(5): Gu YC, Tingle MD, Wilson WR. Glucuronidation of anticancer prodrug PR-104A: Species differences, identification of human UDP-glucuronosyltransferases, and implications for therapy. J Pharmacol Exp Ther. 2011;337(3): Knights KM, Miners JO. Renal UDP-glucuronosyltransferases and the glucuronidation of xenobiotics and endogenous mediators. Drug Metab Rev. 2010;42(1): Gaganis P, Miners JO, Brennan JS, Thomas A, Knights KM. Human renal cortical and medullary UDP-glucuronosyltransferases (UGTs): Immunohistochemical localization of UGT2B7 and UGT1A enzymes and kinetic characterization of s-naproxen glucuronidation. J Pharmacol Exp Ther. 2007;323(2): Kaji H, Kume T. Regioselective glucuronidation of denopamine: Marked species differences and identification of human UDP-glucuronosyltransferase isoform. Drug Metab Dispos. 2005;33(3): Jadhav PR, Cook J, Sinha V, Zhao P, Rostami-Hodjegan A, Sahasrabudhe V, et al. A proposal for scientific framework enabling specific population drug dosing recommendations. J Clin Pharmacol. 2015;55(10): Wagner C, Zhao P, Pan Y, Hsu V, Grillo J, Huang S, et al. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: Report of an FDA public workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4(4): Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004;19(3): Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol Rev. 2010;62(1): Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A. 2004;101(10): He J, Yu Y, Prasad B, Chen X, Unadkat JD. Mechanism of an unusual, but clinically significant, digoxin bupropion drug interaction. Biopharm Drug Dispos. 2014;35(5): Tucker G. Measurement of the renal clearance of drugs. Br J Clin Pharmacol. 1981;12(6): Smith DE, Kugler AR. Influence of intrarenal metabolism on the analysis of renal drug transport mechanisms. J Pharm Sci. 1994;83(10): Wang J, Evans AM, Knights KM, Miners JO. Differential disposition of intra renal generated and preformed glucuronides: Studies with 4 methylumbelliferone and 4 methylumbelliferyl glucuronide in the filtering and nonfiltering isolated perfused rat kidney. J Pharm Pharmacol. 2011;63(4): Geng W, Pang KS. Differences in excretion of hippurate, as a metabolite of benzoate and as an administered species, in the single-pass isolated perfused rat kidney explained. J Pharmacol Exp Ther. 1999;288(2): Vree TB, van Ewijk-Beneken Kolmer EWJ, Wuis EW, Hekster YA, Broekman MMM. Interindividual variation in the capacity-limited renal glucuronidation of probenecid by humans. Pharm World Sci. 1993;15(5):
193 56. Bullingham R, Monroe S, Nicholls A, Hale M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single dose oral and intravenous administration. J Clin Pharmacol. 1996;36(4): Veroli P, O'kelly B, Bertrand F, Trouvin J, Farinotti R, Ecoffey C. Extrahepatic metabolism of propofol in man during the anhepatic phase of orthotopic liver transplantation. Br J Anaesth. 1992;68(2): Kerdpin O, Knights KM, Elliot DJ, Miners JO. In vitro characterisation of human renal and hepatic frusemide glucuronidation and identification of the UDPglucuronosyltransferase enzymes involved in this pathway. Biochem Pharmacol. 2008;76(2): Fallon JK, Neubert H, Goosen TC, Smith PC. Targeted precise quantification of 12 human recombinant uridine-diphosphate glucuronosyl transferase 1A and 2B isoforms using nano-ultra-high-performance liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Drug Metab Dispos. 2013;41(12): Knights KM, Spencer SM, Fallon JK, Chau N, Smith PC, Miners JO. Scaling factors for the in vitro in vivo extrapolation (IV ive) of renal drug and xenobiotic glucuronidation clearance. Br J Clin Pharmacol. 2016;DOI: /bcp Zhang H, Patana A-S, Mackenzie PI, Ikushiro S, Goldman A, Finel M. Human UDP-glucuronosyltransferase expression in insect cells: Ratio of active to inactive recombinant proteins and the effects of a c-terminal his-tag on glucuronidation kinetics. Drug Metab Dispos. 2012;40(10): Miners JO, Mackenzie PI, Knights KM. The prediction of drug-glucuronidation parameters in humans: UDP-glucuronosyltransferase enzyme-selective substrate and inhibitor probes for reaction phenotyping and in vitro-in vivo extrapolation of drug clearance and drug-drug interaction potential. Drug Metab Rev. 2010;42(1): Walsky RL, Bauman JN, Bourcier K, Giddens G, Lapham K, Negahban A, et al. Optimized assays for human UDP-glucuronosyltransferase (UGT) activities: Altered alamethicin concentration and utility to screen for UGT inhibitors. Drug Metab Dispos. 2012;40(5): Kilford PJ, Stringer R, Sohal B, Houston JB, Galetin A. Prediction of drug clearance by glucuronidation from in vitro data: Use of combined cytochrome P450 and UDP-glucuronosyltransferase cofactors in alamethicin-activated human liver microsomes. Drug Metab Dispos. 2009;37(1): Rowland A, Knights KM, Mackenzie PI, Miners JO. Characterization of the binding of drugs to human intestinal fatty acid binding protein (ifabp): Potential role of ifabp as an alternative to albumin for in vitro-in vivo extrapolation of drug kinetic parameters. Drug Metab Dispos. 2009;37(7): Houston JB, Galetin A. Modelling atypical cyp3a4 kinetics: Principles and pragmatism. Arch Biochem Biophys. 2005;433(2): Mayer JM, Hall SD, Rowland M. Relationship between lipophilicity and tubular reabsorption for a series of 5 alkyl 5 ethylbarbituric acids in the isolated perfused rat kidney preparation. J Pharm Sci. 1988;77(4): Paine SW, Barton P, Bird J, Denton R, Menochet K, Smith A, et al. A rapid computational filter for predicting the rate of human renal clearance. J Mol Graph Model. 2010;29(4): Varma MV, Feng B, Obach RS, Troutman MD, Chupka J, Miller HR, et al. Physicochemical determinants of human renal clearance. J Med Chem. 2009;52(15): Dave RA, Morris ME. Quantitative structure-pharmacokinetic relationships for the prediction of renal clearance in humans. Drug Metab Dispos. 2015;43(1): Kunze A, Huwyler J, Poller B, Gutmann H, Camenisch G. In vitro-in vivo extrapolation method to predict human renal clearance of drugs. J Pharm Sci. 2014;103(3): Scott DM. Differentiation in vitro of primary cultures and transfected cell lines of epithelial cells derived from the thick ascending limb of henle's loop. Differentiation. 1987;36(1):
194 73. Mooren FC, Kinne RK. Intracellular calcium in primary cultures of rat renal inner medullary collecting duct cells during variations of extracellular osmolality. Pflugers Arch. 1994;427(5-6): Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick H, et al. MDCK (madin darby canine kidney) cells: A tool for membrane permeability screening. J Pharm Sci. 1999;88(1): Brouwer KL, Keppler D, Hoffmaster KA, Bow DA, Cheng Y, Lai Y, et al. In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther. 2013;94(1): Wikswo JP, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip. 2013;13(18): Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R. Kidney-on-achip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2015;34(2): Neuhoff S, Gaohua L, Burt H, Jamei M, Li L, Tucker GT, et al. Accounting for transporters in renal clearance: Towards a mechanistic kidney model (Mech KiM). Transporters in drug development: Springer; p Posada MM, Bacon JA, Schneck KB, Tirona RG, Kim RB, Higgins JW, et al. Prediction of renal transporter mediated drug-drug interactions for pemetrexed using physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2015;43(3): Konig J, Zolk O, Singer K, Hoffmann C, Fromm MF. Double-transfected MDCK cells expressing human OCT1/MATE1 or OCT2/MATE1: Determinants of uptake and transcellular translocation of organic cations. Br J Pharmacol. 2011;163(3): Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11(3): Müller F, König J, Hoier E, Mandery K, Fromm MF. Role of organic cation transporter OCT2 and multidrug and toxin extrusion proteins MATE1 and MATE2-k for transport and drug interactions of the antiviral lamivudine. Biochem Pharmacol. 2013;86(6): Baer PC, Bereiter-Hahn J, Schubert R, Geiger H. Differentiation status of human renal proximal and distal tubular epithelial cells in vitro: Differential expression of characteristic markers. Cells Tissues Organs. 2006;184(1): Lash LH, Putt DA, Cai H. Drug metabolism enzyme expression and activity in primary cultures of human proximal tubular cells. Toxicology. 2008;244(1): Lash LH, Putt DA, Cai HL. Membrane transport function in primary cultures of human proximal tubular cells. Toxicology. 2006;228(2-3): Brown CD, Sayer R, Windass AS, Haslam IS, De Broe ME, D'Haese PC, et al. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol. 2008;233(3): Watanabe T, Kusuhara H, Watanabe T, Debori Y, Maeda K, Kondo T, et al. Prediction of the overall renal tubular secretion and hepatic clearance of anionic drugs and a renal drug-drug interaction involving organic anion transporter 3 in humans by in vitro uptake experiments. Drug Metab Dispos. 2011;39(6): Hasegawa M, Kusuhara H, Endou H, Sugiyama Y. Contribution of organic anion transporters to the renal uptake of anionic compounds and nucleoside derivatives in rat. J Pharmacol Exp Ther. 2003;305(3): Nagle MA, Truong DM, Dnyanmote AV, Ahn S-Y, Eraly SA, Wu W, et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem. 2011;286(1): Takai N, Tanaka Y, Saji H. Quantification of small molecule drugs in biological tissue sections by imaging mass spectrometry using surrogate tissue-based calibration standards. Mass Spectrom (Tokyo). 2014;3(1):A Nozaki Y, Kusuhara H, Kondo T, Hasegawa M, Shiroyanagi Y, Nakazawa H, et al. Characterization of the uptake of organic anion transporter (OAT) 1 and OAT3 substrates by human kidney slices. J Pharmacol Exp Ther. 2007;321(1):
195 92. Bens M, Vandewalle A. Cell models for studying renal physiology. Pflugers Arch. 2008;457(1): Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van der Velden TJ, Russel FG, et al. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010;339(2): Glube N, Giessl A, Wolfrum U, Langguth P. Caki-1 cells represent an in vitro model system for studying the human proximal tubule epithelium. Nephron Experimental Nephrology. 2007;107(2):e Aschauer L, Carta G, Vogelsang N, Schlatter E, Jennings P. Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol In Vitro. 2015;30(1): Knops N, van den Heuvel LP, Masereeuw R, Bongaers I, de Loor H, Levtchenko E, et al. The functional implications of common genetic variation in CYP3A5 and ABCB1 in human proximal tubule cells. Mol Pharm. 2015;12(3): Ahlin G, Hilgendorf C, Karlsson J, Szigyarto CA, Uhlen M, Artursson P. Endogenous gene and protein expression of drug-transporting proteins in cell lines routinely used in drug discovery programs. Drug Metab Dispos. 2009;37(12): Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35(8): Glavinas H, Mehn D, Jani M, Oosterhuis B, Heredi-Szabo K, Krajcsi P. Utilization of membrane vesicle preparations to study drug-abc transporter interactions. Expert Opin Drug Metab Toxicol. 2008;4(6): Karlsson JE, Heddle C, Rozkov A, Rotticci-Mulder J, Tuvesson O, Hilgendorf C, et al. High-activity p-glycoprotein, multidrug resistance protein 2, and breast cancer resistance protein membrane vesicles prepared from transiently transfected human embryonic kidney 293-epstein-barr virus nuclear antigen cells. Drug Metab Dispos. 2010;38(4): Kelly EJ, Wang Z, Voellinger JL, Yeung CK, Shen DD, Thummel KE, et al. Innovations in preclinical biology: Ex vivo engineering of a human kidney tissue microperfusion system. Stem Cell Res Ther. 2013;4(suppl 1):S Davies J. Engineered renal tissue as a potential platform for pharmacokinetic and nephrotoxicity testing. Drug Discov Today. 2014;19(6): Jang K-J, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh K-Y, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integrative Biology. 2013;5(9): Sciancalepore AG, Sallustio F, Girardo S, Passione LG, Camposeo A, Mele E, et al. A bioartificial renal tubule device embedding human renal stem/progenitor cells. PLoS ONE. 2014;9(1):e Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8): Zamek Gliszczynski MJ, Lee CA, Poirier A, Bentz J, Chu X, Ellens H, et al. ITC recommendations for transporter kinetic parameter estimation and translational modeling of transport mediated PK and DDIs in humans. Clin Pharmacol Ther. 2013;94(1): Felmlee MA, Dave RA, Morris ME. Mechanistic models describing active renal reabsorption and secretion: A simulation-based study. AAPS J. 2013;15(1): Ménochet K, Kenworthy KE, Houston JB, Galetin A. Simultaneous assessment of uptake and metabolism in rat hepatocytes: A comprehensive mechanistic model. J Pharmacol Exp Ther. 2012;341(1): Poirier A, Lavé T, Portmann R, Brun M-E, Senner F, Kansy M, et al. Design, data analysis, and simulation of in vitro drug transport kinetic experiments using a mechanistic in vitro model. Drug Metab Dispos. 2008;36(12): Kalvass JC, Pollack GM. Kinetic considerations for the quantitative assessment of efflux activity and inhibition: Implications for understanding and predicting the effects of efflux inhibition. Pharm Res. 2007;24(2):
196 111. Korzekwa K, Nagar S. Compartmental models for apical efflux by p-glycoprotein: Part 2 a theoretical study on transporter kinetic parameters. Pharm Res. 2014;31(2): Nagar S, Tucker J, Weiskircher EA, Bhoopathy S, Hidalgo IJ, Korzekwa K. Compartmental models for apical efflux by p-glycoprotein part 1: Evaluation of model complexity. Pharm Res. 2014;31(2): Ghosh A, Scott DO, Maurer TS. Towards a unified model of passive drug permeation i: Origins of the unstirred water layer with applications to ionic permeation. Eur J Pharm Sci. 2014;52: Weinstein AM. A mathematical model of rat proximal tubule and loop of henle. Am J Physiol Renal Physiol. 2015;308(10):F1076-F Edwards A. Modeling transport in the kidney: Investigating function and dysfunction. Am J Physiol Renal Physiol. 2010;298(3):F475-F Randall Thomas S. Kidney modeling and systems physiology. Wiley Interdiscip Rev Syst Biol Med. 2009;1(2): Darmady E, Offer J, Woodhouse M. The parameters of the ageing kidney. J Pathol. 1973;109(3): Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. 2003;63(suppl 83):S31-S Nyengaard J, Bendtsen T. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232(2): Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, et al. Renal volume measurements: Accuracy and repeatability of US compared with that of MR imaging 1. Radiology. 1999;211(3): Di Leo G, Di Terlizzi F, Flor N, Morganti A, Sardanelli F. Measurement of renal volume using respiratory-gated MRI in subjects without known kidney disease: Intraobserver, interobserver, and interstudy reproducibility. Eur J Radiol. 2011;80(3):e212-e Wang X, Vrtiska TJ, Avula RT, Walters LR, Chakkera HA, Kremers WK, et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85(3): DeWoskin RS, Thompson CM. Renal clearance parameters for PBPK model analysis of early lifestage differences in the disposition of environmental toxicants. Regul Toxicol Pharm. 2008;51(1): Garcia-Caceres U, Ortega J. Studies of tubular alterations in diffuse renal disease. II. Quantitative evaluation of cellularity and length of proximal convoluted tubules in the kidney of lupus nephritis. Am J Pathol. 1967;50(6): Buturović-Ponikvar J, Višnar-Perovič A. Ultrasonography in chronic renal failure. Eur J Radiol. 2003;46(2): Ozmen C, Akin D, Bilek S, Bayrak A, Senturk S, Nazaroglu H. Ultrasound as a diagnostic tool to differentiate acute from chronic renal failure. Clin Nephrol. 2010;74(1): Kriz W, Bankir L, Bulger RE, Burg MB, Goncharevskaya OA, Imai M, et al. A standard nomenclature for structures of the kidney. Pflugers Arch. 1988;411(1): Nordsletten DA, Blackett S, Bentley MD, Ritman EL, Smith NP. Structural morphology of renal vasculature. Am J Physiol Heart Circ Physiol. 2006;291(1):H296- H Marieb EN, Hoehn K. Human anatomy & physiology. 10th ed. New York: Pearson Education; King BF, Reed JE, Bergstralh EJ, Sheedy PF, Torres VE. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11(8): Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7(3):
197 132. Al-Said J, O'Neill WC. Reduced kidney size in patients with simple renal cysts. Kidney Int. 2003;64(3): Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O'Neill WC. Reduced renal function in patients with simple renal cysts. Kidney Int. 2004;65(6): Lee HS, Kim EJ, Kim SJ, Lee YK, Yoon JW, Noh JW. The changes of renal function in apparent healthy individuals with a simple renal cyst. Kidney Res Clin Pract. 2007;26(5): Ponte B, Pruijm M, Ackermann D, Vuistiner P, Guessous I, Ehret G, et al. Copeptin is associated with kidney length, renal function, and prevalence of simple cysts in a population-based study. J Am Soc Nephrol. 2014;26(6): Kariyanna SS, Light RP, Agarwal R. A longitudinal study of kidney structure and function in adults. Nephrol Dial Transplant. 2010;25(4): Mounier-Vehier C, Lions C, Devos P, Jaboureck O, Willoteaux S, Carre A, et al. Cortical thickness: An early morphological marker of atherosclerotic renal disease. Kidney Int. 2002;61(2): Sanusi AA, Arogundade FA, Famurewa O, Akintomide AO, Soyinka FO, Ojo OE, et al. Relationship of ultrasonographically determined kidney volume with measured GFR, calculated creatinine clearance and other parameters in chronic kidney disease (CKD). Nephrol Dial Transplant. 2009;24(5): Matsumoto N, Ishimura E, Taniwaki H, Emoto M, Shoji T, Kawagishi T, et al. Diabetes mellitus worsens intrarenal hemodynamic abnormalities in nondialyzed patients with chronic renal failure. Nephron. 2000;86(1): Gragnoli G, Signorini AM, Tanganelli I, Fondelli C, Borgogni P, Borgogni L, et al. Prevalence of glomerular hyperfiltration and nephromegaly in normo-and microalbuminuric type 2 diabetic patients. Nephron. 1993;65(2): Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non insulin-dependent diabetes mellitus. Kidney Int. 1999;55(1): Inomata S. Renal hypertrophy as a prognostic index for the progression of diabetic renal disease in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1993;7(1): Atta MG, Longenecker JC, Fine DM, Nagajothi N, Grover DS, Wu J, et al. Sonography as a predictor of human immunodeficiency virus associated nephropathy. J Ultrasound Med. 2004;23(5): Beland MD, Walle NL, Machan JT, Cronan JJ. Renal cortical thickness measured at ultrasound: Is it better than renal length as an indicator of renal function in chronic kidney disease? Am J Roentgenol. 2010;195(2):W146-W Mustafiz M, Rahman M, Islam M, Mohiuddin A. Correlation of ultrasonographically determined renal cortical thickness and renal length with estimated glomerular filtration rate in chronic kidney disease patients. Bangladesh Med Res Counc Bull. 2014;39(2): Kim HC, Yang DM, Jin W, Lee SH. Relation between total renal volume and renal function: Usefulness of 3D sonographic measurements with a matrix array transducer. Am J Roentgenol. 2010;194(2):W186-W Jovanovic D, Gasic B, Pavlovic S, Naumovic R. Correlation of kidney size with kidney function and anthropometric parameters in healthy subjects and patients with chronic kidney diseases. Ren Fail. 2013;35(6): Taal M, Brenner B. Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int. 2006;70(10): Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants part i. Clin Pharmacokinet. 2002;41(12): Delanaye P, Schaeffner E, Ebert N, Cavalier E, Mariat C, Krzesinski J, et al. Normal reference values for glomerular filtration rate: What do we really know? Nephrol Dial Transplant. 2012;27(7): Malmgren L, McGuigan FE, Berglundh S, Westman K, Christensson A, Åkesson K. Declining estimated glomerular filtration rate and its association with mortality and comorbidity over 10 years in elderly women. Nephron. 2015;130(4):
198 152. Bennett C, Brenner B, Berliner R. Micropuncture study of nephron function in the rhesus monkey. J Clin Invest. 1968;47(1): Goldstein LJ, Rypins EB. A computer model of the kidney. Comput Methods Programs Biomed. 1992;37(3): Weitzman RE, Kleeman CR. The clinical physiology of water metabolism: Part II: Renal mechanisms for urinary concentration; diabetes insipidus. West J Med. 1979;131(6): Knauf H, Mutschler E. Pharmacodynamic and kinetic considerations on diuretics as a basis for differential therapy. Klin Wochenschr. 1991;69(6): Malnic G, Aires MDM, Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972;222(1): Smith HW. The excretion of water. Bull N Y Acad Med. 1947;23(4): Pitts RF. Physiology of the kidney and body fluids: An introductory text: Chicago: Year Book Medical Publishers; Cage P, Carson E, Britton K. A model of the human renal medulla. Comput Biomed Res. 1977;10(6): Uttamsingh R, Leaning M, Bushman J, Carson E, Finkelstein L. Mathematical model of the human renal system. Med Biol Eng Comput. 1985;23(6): Hall JE. Textbook of medical physiology / john e. Hall, arthur c. Guyton. 12th ed. ed. Guyton AC, editor. Philadelphia, Pa.: Saunders Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011;20(1): Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int. 2003;63(6): Benz-Bohm G. Urinary tract embryology, anatomy and anatomical variants. Pediatric uroradiology: Springer; p Kriz W. Structural organization of the renal medulla: Comparative and functional aspects. Am J Physiol Regul Integr Comp Physiol. 1981;241(1):R3-R Pai H-C. Dissection of nephrons from the human kidney. J Anat. 1935;69(Pt 3): Møller J, Skriver E. Quantitative ultrastructure of human proximal tubules and cortical interstitium in chronic renal disease (hydronephrosis). Virchows Arch A Pathol Anat Histopathol. 1985;406(4): Chabardes D, Gagnan-Brunette M, Imbert-Teboul M, Gontcharevskaia O, Montegut M, Clique A, et al. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest. 1980;65(2): Ohlson L. Normal collecting ducts: Visualization at urography. Radiology. 1989;170(1 Pt 1): Jacquez JA, Foster D, Daniels E. Solute concentration in the kidney i. A model of the renal medulla and its limit cases. Math Biosci. 1976;32(3): Roman RJ, Sias FR. Network computer analysis of the human kidney. Math Modelling. 1986;7(5): Oliver J, MacDowell M. The structural and functional aspects of the handling of glucose by the nephrons and the kidney and their correlation by means of structuralfunctional equivalents. J Clin Invest. 1961;40(7): Koushanpour E, Tarica R, Stevens W. Mathematical simulation of normal nephron function in rat and man. J Theor Biol. 1971;31(2): Jacquez JA, Carnahan B, Abbrecht P. A model of the renal cortex and medulla. Math Biosci. 1967;1(2): Wallace D, Christensen M, Reif G, Belibi F, Thrasher B, Herrell D, et al. Electrolyte and fluid secretion by cultured human inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2002;283(6):F1337-F Nyengaard J, Flyvbjerg A, Rasch R. The impact of renal growth, regression and regrowth in experimental diabetes mellitus on number and size of proximal and distal tubular cells in the rat kidney. Diabetologia. 1993;36(11):
199 177. Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999;10(5): Cummings BS, Lash LH. Metabolism and toxicity of trichloroethylene and s-(1, 2- dichlorovinyl)-l-cysteine in freshly isolated human proximal tubular cells. Toxicol Sci. 2000;53(2): Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol. 1994;47(9): Jakobsson SV, Cinti DL. Studies on cytochrome P-450-containing monooxygenase system in human kidney-cortex microsomes. J Pharmacol Exp Ther. 1973;185(2): Pacifici GM, Franchi M, Bencini C, Repetti F, Dilascio N, Muraro GB. Tissue distribution of drug-metabolizing-enzymes in humans. Xenobiotica. 1988;18(7): Al-Jahdari WS, Yamamoto K, Hiraoka H, Nakamura K, Goto F, Horiuchi R. Prediction of total propofol clearance based on enzyme activities in microsomes from human kidney and liver. Eur J Clin Pharmacol. 2006;62(7): Barter ZE, Chowdry JE, Harlow JR, Snawder JE, Lipscomb JC, Rostami-Hodjegan A. Covariation of human microsomal protein per gram of liver with age: Absence of influence of operator and sample storage may justify interlaboratory data pooling. Drug Metab Dispos. 2008;36(12): Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: Reaching a consensus on values of human micro-somal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8(1): Litterst C, Mimnaugh E, Reagan R, Gram T. Comparison of in vitro drug metabolism by lung, liver, and kidney of several common laboratory species. Drug Metab Dispos. 1974;3(4): Amar-Costesec A, Beaufay H, Wibo M, Thinès-Sempoux D, Feytmans E, Robbi M, et al. Analytical study of microsomes and isolated subcellular membranes from rat liver. II. Preparation and composition of the microsomal fraction. J Cell Biol. 1974;61(1): Taira Y, Redick J, Baron J. An immunohistochemical study on the localization and distribution of NADPH-cytochrome c (P-450) reductase in rat liver. Mol Pharmacol. 1980;17(3): Matsuura S, Masuda R, Omori K, Negishi M, Tashiro Y. Distribution and induction of cytochrome P-450 in rat liver nuclear envelope. J Cell Biol. 1981;91(1): Bergeron J, Ehrenreich J, Siekevitz P, Palade G. Golgi fractions prepared from rat liver homogenates II. Biochemical characterization. J Cell Biol. 1973;59(1): Cubitt HE, Houston JB, Galetin A. Prediction of human drug clearance by multiple metabolic pathways: Integration of hepatic and intestinal microsomal and cytosolic data. Drug Metab Dispos. 2011;39(5): Tsoutsikos P, Miners JO, Stapleton A, Thomas A, Sallustio BC, Knights KM. Evidence that unsaturated fatty acids are potent inhibitors of renal UDPglucuronosyltransferases (UGT): Kinetic studies using human kidney cortical microsomes and recombinant UGT1A9 and UGT2B7. Biochem Pharmacol. 2004;67(1): Kitagawa H, Kamataki T. Studies on drug metabolism. XII. Activity of liver microsomal drug-metabolizing enzymes in human liver. Chem Pharm Bull (Tokyo). 1971;19(4): Court MH, Zhang XL, Ding XX, Yee KK, Hesse LM, Finel M. Quantitative distribution of mrnas encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica. 2012;42(3): Ohno S, Nakajin S. Determination of mrna expression of human UDPglucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2009;37(1): Al Feteisi H, Achour B, Rostami-Hodjegan A, Barber J. Translational value of liquid chromatography coupled with tandem mass spectrometry-based quantitative proteomics 199
200 for in vitro in vivo extrapolation of drug metabolism and transport and considerations in selecting appropriate techniques. Expert Opin Drug Metab Toxicol. 2015;11(9): Harwood M, Achour B, Russell M, Carlson G, Warhurst G, Rostami-Hodjegan A. Application of an LC-MS/MS method for the simultaneous quantification of human intestinal transporter proteins absolute abundance using a QconCAT technique. J Pharm Biomed Anal. 2015;110: Badée J, Achour B, Rostami-Hodjegan A, Galetin A. Meta-analysis of expression of hepatic organic anion transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab Dispos. 2015;43(4): Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CE, et al. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos. 2015;43(3): Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood brain barrier transporters and receptors. J Neurochem. 2011;117(2): Uchida Y, Toyohara T, Ohtsuki S, Moriyama Y, Abe T, Terasaki T. Quantitative targeted absolute proteomics for 28 transporters in brush border and basolateral membrane fractions of rat kidney. J Pharm Sci. 2016;105(2): Brouwer K, Aleksunes L, Brandys B, Giacoia G, Knipp G, Lukacova V, et al. Human ontogeny of drug transporters: Review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther. 2015;98(3): Fletcher JS, Vickerman JC. Secondary ion mass spectrometry: Characterizing complex samples in two and three dimensions. Anal Chem. 2012;85(2): Balluff B, Schöne C, Höfler H, Walch A. MALDI imaging mass spectrometry for direct tissue analysis: Technological advancements and recent applications. Histochem Cell Biol. 2011;136(3): Hogg K, Thomas J, Ashford D, Cartwright J, Coldwell R, Weston DJ, et al. Quantification of proteins by flow cytometry: Quantification of human hepatic transporter P-gp and OATP1B1 using flow cytometry and mass spectrometry. Methods. 2015;82: Gibson CR, Lu P, Maciolek C, Wudarski C, Barter Z, Rowland-Yeo K, et al. Using human recombinant UDP-glucuronosyltransferase isoforms and a relative activity factor approach to model total body clearance of laropiprant (MK-0524) in humans. Xenobiotica. 2013;43(12): de Lannoy IA, Hirayama H, Pang KS. A physiological model for renal drug metabolism: Enalapril esterolysis to enalaprilat in the isolated perfused rat kidney. J Pharmacokinet Biopharm. 1990;18(6): Litterst CL, Mimnaugh EG, Reagan RL, Gram TE. Comparison of in vitro drugmetabolism by lung, liver, and kidney of several common laboratory species. Drug Metab Dispos. 1975;3(4): Knights KM, Winner LK, Elliot DJ, Bowalgaha K, Miners JO. Aldosterone glucuronidation by human liver and kidney microsomes and recombinant UDPglucuronosyltransferases: Inhibition by NSAIDs. Br J Clin Pharmacol. 2009;68(3): Soars MG, Burchell B, Riley RJ. In vitro analysis of human drug glucuronidation and prediction of in vivo metabolic clearance. J Pharmacol Exp Ther. 2002;301(1): Orellana M, Araya J, Guajardo V, Rodrigo R. Modulation of cytochrome P450 activity in the kidney of rats following long-term red wine exposure. Comp Biochem Physiol, C: Toxicol Pharmacol. 2002;132(3): Aitio A, Vainio H. Udpglucuronosyltransferase and mixed function oxidase activity in microsomes prepared by differential centrifugation and calcium aggregation. Acta Pharmacol Toxicol (Copenh). 1976;39(5): Jakobsson SV. Subfractionation and properties of rat kidney cortex microsomal fraction. Exp Cell Res. 1974;84(1): Bowalgaha K, Miners JO. The glucuronidation of mycophenolic acid by human liver, kidney and jejunum microsomes. Br J Clin Pharmacol. 2001;52(5):
201 214. Sausen PJ, Elfarra AA. Cysteine conjugate s-oxidase - characterization of a novel enzymatic-activity in rat hepatic and renal microsomes. J Biol Chem. 1990;265(11): Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1): Gill KL, Gertz M, Houston JB, Galetin A. Application of a physiologically based pharmacokinetic model to assess propofol hepatic and renal glucuronidation in isolation: Utility of in vitro and in vivo data. Drug Metab Dispos. 2013;41(4): Du J, You T, Chen X, Zhong D. Stereoselective glucuronidation of ornidazole in humans: Predominant contribution of UDP-glucuronosyltransferases 1A9 and 2B7. Drug Metab Dispos. 2013;41(7): Janků I. Physiological modelling of renal drug clearance. Eur J Clin Pharmacol. 1993;44(6): Hall S, Rowland M. Relationship between renal clearance, protein binding and urine flow for digitoxin, a compound of low clearance in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1984;228(1): Tang Liu D, Tozer TN, Riegelman S. Dependence of renal clearance on urine flow: A mathematical model and its application. J Pharm Sci. 1983;72(2): Komiya I. Urine flow dependence of renal clearance and interrelation of renal reabsorption and physicochemical properties of drugs. Drug Metab Dispos. 1986;14(2): Felmlee MA, Wang Q, Cui D, Roiko SA, Morris ME. Mechanistic toxicokinetic model for γ-hydroxybutyric acid: Inhibition of active renal reabsorption as a potential therapeutic strategy. AAPS J. 2010;12(3): Russel FG, Wouterse AC, van Ginneken CA. Physiologically based pharmacokinetic model for the renal clearance of phenolsulfonphthalein and the interaction with probenecid and salicyluric acid in the dog. J Pharmacokinet Biopharm. 1987;15(4): Ogungbenro K, Aarons L. Physiologically based pharmacokinetic modelling of methotrexate and 6-mercaptopurine in adults and children. Part 1: Methotrexate. J Pharmacokinet Pharmacodyn. 2014;41(2): Dave RA, Morris ME. Semi-mechanistic kidney model incorporating physiologically-relevant fluid reabsorption and transporter-mediated renal reabsorption: Pharmacokinetics of γ-hydroxybutyric acid and l-lactate in rats. J Pharmacokinet Pharmacodyn. 2015;42(5): Hsu V, de LT Vieira M, Zhao P, Zhang L, Zheng JH, Nordmark A, et al. Towards quantitation of the effects of renal impairment and probenecid inhibition on kidney uptake and efflux transporters, using physiologically based pharmacokinetic modelling and simulations. Clin Pharmacokinet. 2014;53(3): Li J, Kim S, Sha X, Wiegand R, Wu J, LoRusso P. Complex disease, gene, and drug drug interactions: Impacts of renal function, CYP2D6 phenotype, and OCT2 activity on veliparib pharmacokinetics. Clin Cancer Res. 2014;20(15): Watanabe T, Maeda K, Kondo T, Nakayama H, Horita S, Kusuhara H, et al. Prediction of the hepatic and renal clearance of transporter substrates in rats using in vitro uptake experiments. Drug Metab Dispos. 2009;37(7): US Food Drug Admin. Guidance for industry: Drug interaction studies study design, data analysis, implications for dosing, and labeling recommendations. (2012) US Food and Drug Administration, Silver Spring, MD Tweedie D, Polli JW, Berglund EG, Huang SM, Zhang L, Poirier A, et al. Transporter studies in drug development: Experience to date and follow up on decision trees from the international transporter consortium. Clin Pharmacol Ther. 2013;94(1): European Medicines Agency. Guideline on the investigation of drug interactions. (2012) Committee for Human Medicinal Products (CHMP), London 201
202 232. Feng B, Hurst S, Lu Y, Varma MV, Rotter CJ, El-Kattan A, et al. Quantitative prediction of renal transporter-mediated clinical drug drug interactions. Mol Pharm. 2013;10(11): Maeda K, Tian Y, Fujita T, Ikeda Y, Kumagai Y, Kondo T, et al. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur J Pharm Sci. 2014;59: Kawase A, Yamamoto T, Egashira S, Iwaki M. Stereoselective inhibition of methotrexate excretion by glucuronides of nonsteroidal anti-inflammatory drugs via multidrug resistance proteins 2 and 4. J Pharmacol Exp Ther. 2016;356(2): Sweeney CJ, Takimoto CH, Latz JE, Baker SD, Murry DJ, Krull JH, et al. Two drug interaction studies evaluating the pharmacokinetics and toxicity of pemetrexed when coadministered with aspirin or ibuprofen in patients with advanced cancer. Clin Cancer Res. 2006;12(2): Kikuchi R, Lao Y, Bow DA, Chiou WJ, Andracki ME, Carr RA, et al. Prediction of clinical drug drug interactions of veliparib (abt 888) with human renal transporters (OAT1, OAT3, OCT2, MATE1, and MATE2K). J Pharm Sci. 2013;102(12): Holodniy M, Penzak SR, Straight TM, Davey RT, Lee KK, Goetz MB, et al. Pharmacokinetics and tolerability of oseltamivir combined with probenecid. Antimicrob Agents Chemother. 2008;52(9): Selen A, Amidon G, Welling P. Pharmacokinetics of probenecid following oral doses to human volunteers. J Pharm Sci. 1982;71(11): Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of metformin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol. 1987;23(5): Kalvass JC, Maurer TS, Pollack GM. Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: Comparison of unbound concentration ratios to in vivo p-glycoprotein efflux ratios. Drug Metab Dispos. 2007;35(4): Morrissey K, Wen C, Johns S, Zhang L, Huang S, Giacomini K. The UCSF-FDA transportal: A public drug transporter database. Clin Pharmacol Ther. 2012;92(5): Tortorici MA, Cutler DL, Hazra A, Nolin TD, Rowland Yeo K, Venkatakrishnan K. Emerging areas of research in the assessment of pharmacokinetics in patients with chronic kidney disease. J Clin Pharmacol. 2015;55(3): US Food Drug Admin. Guidance for industry: Pharmacokinetics in patients with impaired renal function study design, data analysis, and impact on dosing and labeling (revision 1). (2010) US Food and Drug Administration, Silver Spring, MD Delanaye P, Mariat C. The applicability of egfr equations to different populations. Nat Rev Nephrol. 2013;9(9): Chung SM, Lee DJ, Hand A, Young P, Vaidyanathan J, Sahajwalla C. Kidney function changes with aging in adults: Comparison between cross sectional and longitudinal data analyses in renal function assessment. Biopharm Drug Dispos. 2015;36(9): Matzke GR, Aronoff GR, Atkinson AJ, Bennett WM, Decker BS, Eckardt K-U, et al. Drug dosing consideration in patients with acute and chronic kidney disease a clinical update from kidney disease: Improving global outcomes (KDIGO). Kidney Int. 2011;80(11): Jameson K, Jick S, Hagberg K, Ambegaonkar B, Giles A, O'Donoghue D. Prevalence and management of chronic kidney disease in primary care patients in the uk. Int J Clin Pract. 2014;68(9): Nitsch D. Chronic kidney disease: Epidemiology and causes. Practical nephrology: Springer; p Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int. 2011;80(1): Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury risk and outcomes. Nat Rev Nephrol. 2013;9(2):
203 251. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139(2): Schnaper HW. Remnant nephron physiology and the progression of chronic kidney disease. Pediatr Nephrol. 2014;29(2): Rodieux F, Wilbaux M, van den Anker JN, Pfister M. Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin Pharmacokinet. 2015;54(12): Bricker NS, Morrin PA, Kime SW. The pathologic physiology of chronic bright's disease: An exposition of the intact nephron hypothesis. Am J Med. 1960;28(1): Sayama H, Takubo H, Komura H, Kogayu M, Iwaki M. Application of a physiologically based pharmacokinetic model informed by a top-down approach for the prediction of pharmacokinetics in chronic kidney disease patients. AAPS J. 2014;16(5): Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol. 2011;4(2): Burt H, Neuhoff S, Almond L, Gaohua L, Harwood M, Jamei M, et al. Metformin and cimetidine: Physiologically based pharmacokinetic modelling to investigate transporter mediated drug drug interactions. Eur J Pharm Sci. 2016; doi: /j.ejps Komatsu T, Hiasa M, Miyaji T, Kanamoto T, Matsumoto T, Otsuka M, et al. Characterization of the human MATE2 proton-coupled polyspecific organic cation exporter. Int J Biochem Cell Biol. 2011;43(6): Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, et al. Intracellular drug concentrations and transporters: Measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94(1): Dollery C. Intracellular drug concentrations. Clin Pharmacol Ther. 2013;93(3): Matthews PM, Rabiner EA, Passchier J, Gunn RN. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol. 2012;73(2): Allegaert K, Smits A, van den Anker JN. Physiologically based pharmacokinetic modeling in pediatric drug development: A clinician s request for a more integrated approach. J Biomed Biotechnol. 2012: Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27(11): Smith R, Jones R, Ballard P, Griffiths H. Determination of microsome and hepatocyte scaling factors for in vitro/in vivo extrapolation in the rat and dog. Xenobiotica. 2008;38(11): Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5(2): Heikkinen AT, Friedlein A, Matondo M, Hatley OJ, Petsalo A, Juvonen R, et al. Quantitative ADME proteomics CYP and UGT enzymes in the beagle dog liver and intestine. Pharm Res. 2015;32(1): Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther. 2009;86(3): Yee S, Nguyen A, Brown C, Savic R, Zhang Y, Castro R, et al. Reduced renal clearance of cefotaxime in asians with a low-frequency polymorphism of OAT3 (SLC22A8). J Pharm Sci. 2013;102(9): Fukuda T, Goebel J, Cox S, Maseck D, Zhang K, Sherbotie JR, et al. UGT1A9, UGT2B7 and MRP2 genotypes can predict mycophenolic acid pharmacokinetic variability in pediatric kidney transplant recipients. Ther Drug Monit. 2012;34(6): Ihunnah CA, Jiang M, Xie W. Nuclear receptor pxr, transcriptional circuits and metabolic relevance. BBA-Mol Basis Dis. 2011;1812(8):
204 271. Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem Pharmacol. 2006;71(10): Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drugmetabolizing enzymes and drug transporters: Implications for interindividual variability in response to drugs. J Clin Pharmacol. 2007;47(5): Ramírez J, Mirkov S, Zhang W, Chen P, Das S, Liu W, et al. Hepatocyte nuclear factor-1 alpha is associated with ugt1a1, UGT1A9 and UGT2B7 mrna expression in human liver. The pharmacogenomics journal. 2008;8(2): Achour B, Rostami Hodjegan A, Barber J. Protein expression of various hepatic uridine 5 diphosphate glucuronosyltransferase (UGT) enzymes and their intercorrelations: A meta analysis. Biopharm Drug Dispos. 2014;35(6): Achour B, Barber J, Rostami-Hodjegan A. Expression of hepatic drug-metabolizing cytochrome p450 enzymes and their intercorrelations: A meta-analysis. Drug Metab Dispos. 2014;42(8): Zollner G, Marschall H-U, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: Pathogenetic and therapeutic considerations. Mol Pharm. 2006;3(3): Ding X, Staudinger JL. Repression of pxr-mediated induction of hepatic CYP3A gene expression by protein kinase c. Biochem Pharmacol. 2005;69(5): Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2): Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther. 2006;109(1): Song W, Yu L, Peng Z. Targeted label-free approach for quantification of epoxide hydrolase and glutathione transferases in microsomes. Anal Biochem. 2015;478: Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953;202(2): Pearce RE, McIntyre CJ, Madan A, Sanzgiri U, Draper AJ, Bullock PL, et al. Effects of freezing, thawing, and storing human liver microsomes on cytochrome P450 activity. Arch Biochem Biophys. 1996;331(2): Matsubara T, Koike M, Touchi A, Tochino Y, Sugeno K. Quantitative determination of cytochrome P-450 in rat liver homogenate. Anal Biochem. 1976;75(2): Burke M, Orrenius S. Isolation and comparison of endoplasmic reticulum membranes and their mixed function oxidase activities from mammalian extrahepatic tissues. Pharmacol Ther. 1979;7(3): Estabrook R, Werringloer J. The measurement of difference spectra: Application to the cytochromes of microsomes. Methods Enzymol. 1978;52: Johannesen KA, DePierre JW. Measurement of cytochrome P-450 in the presence of large amounts of contaminating hemoglobin and methemoglobin. Anal Biochem. 1978;86(2): Nordlie RC, Arion WJ. [111] glucose-6-phosphatase. Methods Enzymol. 1966;9: Ji Y, Toader V, Bennett BM. Regulation of microsomal and cytosolic glutathione s- transferase activities by s-nitrosylation. Biochem Pharmacol. 2002;63(8): Picard N, Ratanasavanh D, Prémaud A, Le Meur Y, Marquet P. Identification of the UDP-glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos. 2005;33(1): Hayes JD, Pulford DJ. The glutathione s-transferase supergene family: Regulation of gst and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance part II. Crit Rev Biochem Mol Biol. 1995;30(6): Lerman L, Flickinger A, Sheedy 2nd P, Turner S. Reproducibility of human kidney perfusion and volume determinations with electron beam computed tomography. Invest Radiol. 1996;31(4): Vallée J-P, Lazeyras F, Khan H, Terrier F. Absolute renal blood flow quantification by dynamic MRI and Gd-DTPA. Eur Radiol. 2000;10(8):
205 293. Ohno Y, Kawanishi T, Takahashi A, Kasuya Y, Omori Y. A new device for the determination of microsomal cytochrome P-450 in renal tissue preparations from various species contaminated with mitochondria and hemoglobin. Jap J Pharmacol. 1982;32(4): Kanamura S. Ultrastructural localization of glucose-6-phosphatase activity in proximal convoluted tubule cells of rat kidney. Histochemie. 1971;28(4): Kartenbeck J, Jarasch E, Franke W. Nuclear membranes from mammalian liver: Vi. Glucose-6-phosphatase in rat liver, a cytochemical and biochemical study. Exp Cell Res. 1973;81(1): Nordlie RC. Multifunctional glucose-6-phosphatase: Cellular biology. Life Sci. 1979;24(26): Franke WW, Deumling B, Ermen B, Jarasch E-D, Kleinig H. Nuclear membranes from mammalian liver i. Isolation procedure and general characterization. J Cell Biol. 1970;46(2): Mendel DB, Hansen LP, Graves MK, Conley PB, Crabtree GR. Hnf-1 alpha and hnf-1 beta (vhnf-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991;5(6): Yu D-D, Guo S-W, Jing Y-Y, Dong Y-L, Wei L-X. A review on hepatocyte nuclear factor-1beta and tumor. Cell Biosci. 2015;5(1): Rajas F, Gautier A, Bady I, Montano S, Mithieux G. Polyunsaturated fatty acyl coenzyme a suppress the glucose-6-phosphatase promoter activity by modulating the DNA binding of hepatocyte nuclear factor 4α. J Biol Chem. 2002;277(18): Schmoll D, Allan BB, Burchell A. Cloning and sequencing of the 5 region of the human glucose 6 phosphatase gene: Transcriptional regulation by camp, insulin and glucocorticoids in h4iie hepatoma cells. FEBS Lett. 1996;383(1-2): Lin B, Morris DW, Chou JY. The role of hnf1α, hnf3γ, and cyclic amp in glucose-6- phosphatase gene activation. Biochemistry (Mosc). 1997;36(46): Mackenzie PI, Hu DG, Gardner-Stephen DA. The regulation of UDPglucuronosyltransferase genes by tissue-specific and ligand-activated transcription factors. Drug Metab Rev. 2010;42(1): Hu DG, Meech R, McKinnon RA, Mackenzie PI. Transcriptional regulation of human UDP-glucuronosyltransferase genes. Drug Metab Rev. 2014;46(4): Hogeboom GH, Schneider WC. Cytochemical studies of mammalian tissues III. Isocitric dehydrogenase and triphosphopyridine nucleotide-cytochrome c reductase of mouse liver. J Biol Chem. 1950;186(2): Prabhu KS, Reddy PV, Jones EC, Liken AD, Reddy CC. Characterization of a class alpha glutathione-s-transferase with glutathione peroxidase activity in human liver microsomes. Arch Biochem Biophys. 2004;424(1): Morgenstern R, Lundqvist G, Andersson G, Balk L, Depierre JW. The distribution of microsomal glutathione transferase among different organelles, different organs, and different organisms. Biochem Pharmacol. 1984;33(22): Simic T, Mimic-Oka J, Ille K, Savic-Radojevic A, Reljic Z. Isoenzyme profile of glutathione s-transferases in human kidney. Urol Res. 2001;29(1): Simić T, Mimić-Oka J, Ille K, Dragičević D, Savić-Radojević A. Glutathione s- transferase isoenzyme profile in non-tumor and tumor human kidney tissue. World J Urol. 2003;20(6): Morgenstern R, Meijer J, Depierre J, Ernster L. Characterization of rat-liver microsomal glutathione s-transferase activity. Eur J Biochem. 1980;104(1): Heikkinen AT, Friedlein A, Lamerz J, Jakob P, Cutler P, Fowler S, et al. Mass spectrometry-based quantification of CYP enzymes to establish in vitro/in vivo scaling factors for intestinal and hepatic metabolism in beagle dog. Pharm Res. 2012;29(7): Dong M, Fukuda T, Vinks AA. Optimization of mycophenolic acid therapy using clinical pharmacometrics. Drug Metab Pharmacokinet. 2014;29(1): Tett SE, Saint-Marcoux F, Staatz CE, Brunet M, Vinks AA, Miura M, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev. 2011;25(2):
206 314. Cummings BS, Lasker JM, Lash LH. Expression of glutathione-dependent enzymes and cytochrome p450s in freshly isolated and primary cultures of proximal tubular cells from human kidney. J Pharmacol Exp Ther. 2000;293(2): Helbert MJ, Dauwe SE, Van der Biest I, Nouwen EJ, De Broe ME. Immunodissection of the human proximal nephron: Flow sorting of s1s2s3, s1s2 and s3 proximal tubular cells. Kidney Int. 1997;52(2): Legouis D, Bataille A, Hertig A, Vandermeersch S, Simon N, Rondeau E, et al. Ex vivo analysis of renal proximal tubular cells. BMC Cell Biol. 2015;16: Lash LH, Tokarz JJ. Isolation of two distinct populations of cells from fat kidney cortex and their use in the study of chemical-induced toxicity. Anal Biochem. 1989;182(2): Bertram JF. Counting in the kidney. Kidney Int. 2001;59(2): Lødrup AB, Karstoft K, Dissing TH, Pedersen M, Nyengaard JR. Kidney biopsies can be used for estimations of glomerular number and volume: A pig study. Virchows Arch. 2008;452(4): Taylor C, Levenson R. Quantification of immunohistochemistry issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4): Longley JB, Fisher ER. Alkaline phosphatase and the periodic acid schiff reaction in the proximal tubule of the vertebrate kidney. A study in segmental differentiation. Anat Rec. 1954;120(1): Bauchet A-L, Masson R, Guffroy M, Slaoui M. Immunohistochemical identification of kidney nephron segments in the dog, rat, mouse, and cynomolgus monkey. Toxicol Pathol. 2011;39(7): Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: Villin s perspective. FEBS Lett. 2008;582(14): Gröne H, Weber K, Helmchen U, Osborn M. Villin--a marker of brush border differentiation and cellular origin in human renal cell carcinoma. Am J Pathol. 1986;124(2): Brown D, Lee R, Bonventre JV. Redistribution of villin to proximal tubule basolateral membranes after ischemia and reperfusion. Am J Physiol Renal Physiol. 1997;273(6):F1003-F Kuehn EW, Park KM, Somlo S, Bonventre JV. Kidney injury molecule-1 expression in murine polycystic kidney disease. Am J Physiol Renal Physiol. 2002;283(6):F1326- F Roy A, Al-bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol. 2015;10(2): Vijayakumar S, Takito J, Hikita C, Al-Awqati Q. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: Processes that resemble terminal differentiation. J Cell Biol. 1999;144(5): Ghoshdastider U, Popp D, Burtnick LD, Robinson RC. The expanding superfamily of gelsolin homology domain proteins. Cytoskeleton. 2013;70(11): Lueck A, Brown D, Kwiatkowski DJ. The actin-binding proteins adseverin and gelsolin are both highly expressed but differentially localized in kidney and intestine. J Cell Sci. 1998;111(24): Hartwig J, Brown D, Ausiello D, Stossel T, Orci L. Polarization of gelsolin and actin binding protein in kidney epithelial cells. Journal of Histochemistry & Cytochemistry. 1990;38(8): Engel U, Breborowicz D, Bøg-Hansen T, Francis D. Lectin staining of renal tubules in normal kidney. APMIS. 1997;105(1): Zhu XY, Urbieta Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31(1): Ito S, Ando H, Ose A, Kitamura Y, Ando T, Kusuhara H, et al. Relationship between the urinary excretion mechanisms of drugs and their physicochemical properties. J Pharm Sci. 2013;102(9):
207 335. Paine SW, Ménochet K, Denton R, McGinnity DF, Riley RJ. Prediction of human renal clearance from preclinical species for a diverse set of drugs that exhibit both active secretion and net reabsorption. Drug Metab Dispos. 2011;39(6): Huh Y, Smith DE, Rose Feng M. Interspecies scaling and prediction of human clearance: Comparison of small-and macro-molecule drugs. Xenobiotica. 2011;41(11): Rostami Hodjegan A. Complex patients complex DDI: Is there a straight way forward? Biopharm Drug Dispos. 2015;36(2): Zhao P, Zhang L, Grillo J, Liu Q, Bullock J, Moon Y, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89(2): Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol. 2011;300(2):F356-F Schmitz C, Hilpert J, Jacobsen C, Boensch C, Christensen E, Luft F, et al. Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem. 2002;277(1): Moestrup SK, Cui S, Vorum H, Bregengård C, Bjørn S, Norris K, et al. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest. 1995;96(3): Strolin BM, Whomsley R, Mathy F, Jacques P, Espie P, Canning M. Stereoselective renal tubular secretion of levocetirizine and dextrocetirizine, the two enantiomers of the h1-antihistamine cetirizine. Fundam Clin Pharmacol. 2008;22(1): Dooley MJ, Poole SG. Poor correlation between body surface area and glomerular filtration rate. Cancer Chemother Pharmacol. 2000;46(6): Pai MP. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv Chronic Kidney Dis. 2010;17(5):e53-e Chew Harris JS, Chin PK, Florkowski CM, George P, Endre Z. Removal of body surface area normalisation improves raw measured GFR estimation by the CKD epi equation and drug dosing in the obese. Intern Med J. 2015;45(7): Jones GR. Estimating renal function for drug dosing decisions. Clin Biochem Rev. 2011;32(2): Higgins J, Thompson SG. Quantifying heterogeneity in a meta analysis. Stat Med. 2002;21(11): Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414): Giacomini KM, Huang S-M, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3): Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almén T. Determining 'true' glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2008;42(3): Soveri I, Berg U, Björk J, Elinder C, Grubb A, Mejare I, et al. Measuring GFR: A systematic review. Am J Kidney Dis. 2014;64(3): Urakami Y, Kimura N, Okuda M, Inui K. Creatinine transport by basolateral organic cation transporter hoct2 in the human kidney. Pharm Res. 2004;21(6): Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-k, human multidrug and toxin extrusions/h (+)-organic cation antiporters. Biochem Pharmacol. 2007;74(2): Nyman H, Dowling T, Hudson J, Peter W, Joy M, Nolin T. Comparative evaluation of the cockcroft-gault equation and the modification of diet in renal disease (mdrd) study equation for drug dosing: An opinion of the nephrology practice and research network of the american college of clinical pharmacy. Pharmacotherapy. 2011;31(11): Simerville J, Maxted W, Pahira J. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):
208 356. Wenlock MC, Potter T, Barton P, Austin RP. A method for measuring the lipophilicity of compounds in mixtures of 10. J Biomol Screen. 2011;16(3): Kah M, Brown CD. Logd: Lipophilicity for ionisable compounds. Chemosphere. 2008;72(10): Ozawa N, Shimizu T, Morita R, Yokono Y, Ochiai T, Munesada K, et al. Transporter database, tp-search: A web-accessible comprehensive database for research in pharmacokinetics of drugs. Pharm Res. 2004;21(11): Welling L, Welling D. Relationship between structure and function in renal proximal tubule. J Electron Microsc Tech. 1988;9(2): Welling L, Evan A, Welling D. Shape of cells and extracellular channels in rabbit cortical collecting ducts. Kidney Int. 1981;20(2): Olivares-Morales A, Lennernäs H, Aarons L, Rostami-Hodjegan A. Translating human effective jejunal intestinal permeability to surface-dependent intrinsic permeability: A pragmatic method for a more mechanistic prediction of regional oral drug absorption. AAPS J. 2015;17(5): Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Del Rev. 2001;46: Gertz M, Harrison A, Houston JB, Galetin A. Prediction of human intestinal firstpass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data. Drug Metab Dispos. 2010;38(7): Varma MV, Steyn SJ, Allerton C, El-Kattan AF. Predicting clearance mechanism in drug discovery: Extended clearance classification system (eccs). Pharm Res. 2015;32(12): Smith D, Artursson P, Avdeef A, Di L, Ecker GF, Faller B, et al. Passive lipoidal diffusion and carrier-mediated cell uptake are both important mechanisms of membrane permeation in drug disposition. Mol Pharm. 2014;11(6): Fouda A, Fauth C, Roch-Ramel F. Transport of organic cations by kidney epithelial cell line LLC-PK1. J Pharmacol Exp Ther. 1990;252(1): Trifillis A, Kahng M. Characterization of an in vitro system of human renal papillary collecting duct cells. In Vitro Cell Dev Biol. 1990;26(5): Avdeef A, Tam KY. How well can the Caco-2/madin darby canine kidney models predict effective human jejunal permeability? J Med Chem. 2010;53(9): Bentz J, O Connor MP, Bednarczyk D, Coleman J, Lee C, Palm J, et al. Variability in p-glycoprotein inhibitory potency (ic50) using various in vitro experimental systems: Implications for universal digoxin drug-drug interaction risk assessment decision criteria. Drug Metab Dispos. 2013;41(7): Sohlenius-Sternbeck A-K, Jones C, Ferguson D, Middleton BJ, Projean D, Floby E, et al. Practical use of the regression offset approach for the prediction of in vivo intrinsic clearance from hepatocytes. Xenobiotica. 2012;42(9): Özdemir M, Crewe KH, Tucker GT, Rostami Hodjegan A. Assessment of in vivo CYP2D6 activity: Differential sensitivity of commonly used probes to urine ph. J Clin Pharmacol. 2004;44(12): Fetterman G, Shuplock N, Philipp F, Gregg H. The growth and maturation of human glomeruli and proximal convolutions from term to adulthood: Studies by microdissection. Pediatrics. 1965;35(4): Shaw P, Houston J, Rowland M, Hopkins K, Thiercelin J, Morselli P. Antipyrine metabolite kinetics in healthy human volunteers during multiple dosing of phenytoin and carbamazepine. Br J Clin Pharmacol. 1985;20(6): Tang-Liu D, Williams R, Riegelman S. Nonlinear theophylline elimination. Clin Pharmacol Ther. 1982;31(3): Huffman D, Shoeman D, Azarnoff D. Correlation of the plasma elimination of antipyrine and the appearance of 4-hydroxy antipyrine in the urine of man. Biochem Pharmacol. 1974;23(2): Mawer G, Lee H. Value of forced diuresis in acute barbiturate poisoning. BMJ. 1968;2(5608):
209 377. Birkett D, Miners J. Caffeine renal clearance and urine caffeine concentrations during steady state dosing. Implications for monitoring caffeine intake during sports events. Br J Clin Pharmacol. 1991;31(4): Taylor G, Blaschke T. Measurement of antipyrine half life from urinary drug concentrations [letter]. Br J Clin Pharmacol. 1984;18(4): Tang-Liu DD-S, Tozer TN, Riegelman S. Urine flow-dependence of theophylline renal clearance in man. J Pharmacokinet Biopharm. 1982;10(4): Galetin A. Rationalizing underprediction of drug clearance from enzyme and transporter kinetic data: From in vitro tools to mechanistic modeling. In: Nagar S, Argikar AU, Tweedie JD, editors. Enzyme kinetics in drug metabolism: Fundamentals and applications. Totowa, NJ: Humana Press; p Jamei M, Marciniak S, Edwards D, Wragg K, Feng K, Barnett A, et al. The simcyp population based simulator: Architecture, implementation, and quality assurance. In Silico Pharmacol. 2013;1(1): Neuhoff S, Yeo KR, Barter Z, Jamei M, Turner DB, Rostami Hodjegan A. Application of permeability limited physiologically based pharmacokinetic models: Part i digoxin pharmacokinetics incorporating p glycoprotein mediated efflux. J Pharm Sci. 2013;102(9): Tsamandouras N, Rostami Hodjegan A, Aarons L. Combining the bottom up and top down approaches in pharmacokinetic modelling: Fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2015;79(1): Plowchalk DR, Yeo KR. Prediction of drug clearance in a smoking population: Modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur J Clin Pharmacol. 2012;68(6): Gandelman K, Zhu T, Fahmi OA, Glue P, Lian K, Obach RS, et al. Unexpected effect of rifampin on the pharmacokinetics of linezolid: In silico and in vitro approaches to explain its mechanism. J Clin Pharmacol. 2011;51(2): Howgate E, Rowland Yeo K, Proctor N, Tucker G, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: Impact of inter-individual variability. Xenobiotica. 2006;36(6): Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6): Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6): Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH. Pharmacokinetics and tolerance of single-and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother. 2003;51(5): Lelo A, Birkett D, Robson R, Miners J. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Br J Clin Pharmacol. 1986;22(2): Newton R, Broughton L, Lind M, Morrison P, Rogers H, Bradbrook I. Plasma and salivary pharmacokinetics of caffeine in man. Eur J Clin Pharmacol. 1981;21(1): Rovei V, Chanoine F, Benedetti SM. Pharmacokinetics of theophylline: A doserange study. Br J Clin Pharmacol. 1982;14(6): Welshman IR, Sisson TA, Jungbluth GL, Stalker DJ, Hopkins NK. Linezolid absolute bioavailability and the effect of food on oral bioavailability. Biopharm Drug Dispos. 2001;22(3): Slatter J, Stalker D, Feenstra K, Welshman I, Bruss J, Sams J, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14) c] linezolid to healthy human subjects. Drug Metab Dispos. 2001;29(8): Avdeef A. Absorption and drug development: Solubility, permeability, and charge state. Hoboken, New Jersey, US: John Wiley & Sons; Krämer SD. Quantitative aspects of drug permeation across in vitro and in vivo barriers. Eur J Pharm Sci. 2015;DOI: /j.ejps
210 397. Knepper MA, Kwon T-H, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372(14): Blanchard J, Sawers S. Relationship between urine flow rate and renal clearance of caffeine in man. J Clin Pharmacol. 1983;23(4): Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol. 2007;292(4):F1164-F Senekjian HO, Knight TF, Weinman EJ. Micropuncture study of the handling of gentamicin by the rat kidney. Kidney Int. 1981;19(3): Field MJ, Stanton BA, Giebisch GH. Influence of adh on renal potassium handling: A micropuncture and microperfusion study. Kidney Int. 1984;25(3): Shirley D, Walter S, Noormohamed F. Natriuretic effect of caffeine: Assessment of segmental sodium reabsorption in humans. Clin Sci. 2002;103(5): Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine a1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313(1): Yarlagadda SG, Perazella MA. Drug-induced crystal nephropathy: An update. Expert Opin Drug Saf. 2008;7(2): Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4(7): Zhao P, Rowland M, Huang SM. Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther. 2012;92(1): Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51: Jones H, Chen Y, Gibson C, Heimbach T, Parrott N, Peters S, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3): Zhao P, Vieira MdL, Grillo JA, Song P, Wu TC, Zheng JH, et al. Evaluation of exposure change of nonrenally eliminated drugs in patients with chronic kidney disease using physiologically based pharmacokinetic modeling and simulation. J Clin Pharmacol. 2012;52(S1):91S-108S Sakurai Y, Motohashi H, Ueo H, Masuda S, Saito H, Okuda M, et al. Expression levels of renal organic anion transporters (oats) and their correlation with anionic drug excretion in patients with renal diseases. Pharm Res. 2004;21(1): Naud J, Michaud J, Beauchemin S, Hébert M-J, Roger M, Lefrancois S, et al. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos. 2011;39(8): Allen LA, Fonarow GC, Simon DN, Thomas LE, Marzec LN, Pokorney SD, et al. Digoxin use and subsequent outcomes among patients in a contemporary atrial fibrillation cohort. J Am Coll Cardiol. 2015;65(25): See I, Shehab N, Kegler SR, Laskar SR, Budnitz DS. Emergency department visits and hospitalizations for digoxin toxicity united states, 2005 to Circulation: Heart Failure. 2014;7(1): Neuhoff S, Yeo KR, Barter Z, Jamei M, Turner DB, Rostami Hodjegan A. Application of permeability limited physiologically based pharmacokinetic models: Part IIprediction of p glycoprotein mediated drug drug interactions with digoxin. J Pharm Sci. 2013;102(9): Lee C, Kalvass J, Galetin A, Zamek Gliszczynski M. ITC commentary on the prediction of digoxin clinical drug drug interactions from in vitro transporter assays. Clin Pharmacol Ther. 2014;96(3): Greiner B, Eichelbaum M, Fritz P, Kreichgauer H-P, von Richter O, Zundler J, et al. The role of intestinal p-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104(2): Rengelshausen J, Göggelmann C, Burhenne J, Riedel KD, Ludwig J, Weiss J, et al. Contribution of increased oral bioavailability and reduced nonglomerular renal 210
211 clearance of digoxin to the digoxin clarithromycin interaction. Br J Clin Pharmacol. 2003;56(1): Tayrouz Y, Ding R, Burhenne J, Riedel KD, Weiss J, Hoppe Tichy T, et al. Pharmacokinetic and pharmaceutic interaction between digoxin and cremophor rh40. Clin Pharmacol Ther. 2003;73(5): Zhang S, Morris ME. Effect of the flavonoids biochanin a and silymarin on the p- glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm Res. 2003;20(8): Djuv A, Nilsen OG. Caco 2 cell methodology and inhibition of the p glycoprotein transport of digoxin by aloe vera juice. Phytother Res. 2008;22(12): Fossati L, Dechaume R, Hardillier E, Chevillon D, Prevost C, Bolze S, et al. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int J Pharm. 2008;360(1): Neuhoff S, Ungell A-L, Zamora I, Artursson P. ph-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: Implications for drug drug interactions. Pharm Res. 2003;20(8): Halkin H, Sheiner L, Peck C, Melmon K. Determinants of the renal clearance of digoxin. Clin Pharmacol Ther. 1975;17(4): Steiness E. Renal tubular secretion of digoxin. Circulation. 1974;50(1): Steiness E, Waldorff S, Hansen P. Renal digoxin clearance: Dependence on plasma digoxin and diuresis. Eur J Clin Pharmacol. 1982;23(2): Tanigawara Y, Okamura N, Hirai M, Yasuhara M, Ueda K, Kioka N, et al. Transport of digoxin by human p-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1). J Pharmacol Exp Ther. 1992;263(2): Troutman MD, Thakker DR. Efflux ratio cannot assess p-glycoprotein-mediated attenuation of absorptive transport: Asymmetric effect of p-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003;20(8): Chu XY, Bleasby K, Yabut J, Cai XX, Chan GH, Hafey MJ, et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4c1, and multidrug resistance p-glycoprotein. J Pharmacol Exp Ther. 2007;321(2): Richardson JC, Scalera V, Simmons NL. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta. 1981;673(1): Kramer WG, Kolibash AJ, Lewis RP, Bathala MS, Visconti JA, Reaming RH. Pharmacokinetics of digoxin: Relationship between response intensity and predicted compartmental drug levels in man. J Pharmacokinet Biopharm. 1979;7(1): Troutman MD, Thakker DR. Novel experimental parameters to quantify the modulation of absorptive and secretory transport of compounds by p-glycoprotein in cell culture models of intestinal epithelium. Pharm Res. 2003;20(8): Collett A, Tanianis-Hughes J, Hallifax D, Warhurst G. Predicting p-glycoprotein effects on oral absorption: Correlation of transport in Caco-2 with drug pharmacokinetics in wild-type and mdr1a(-/-) mice in vivo. Pharm Res. 2004;21(5): Kimura Y, Kioka N, Kato H, Matsuo M, Ueda K. Modulation of drug-stimulated atpase activity of human MDR1/p-glycoprotein by cholesterol. Biochem J. 2007;401(2): Korjamo T, Kemilainen H, Heikkinen AT, Monkkonen J. Decrease in intracellular concentration causes the shift in k-m value of efflux pump substrates. Drug Metab Dispos. 2007;35(9): Stephens RH, O'Neill CA, Warhurst A, Carlson GL, Rowland M, Warhurst G. Kinetic profiling of p-glycoprotein-mediated drug efflux in rat and human intestinal epithelia. J Pharmacol Exp Ther. 2001;296(2): Miki Y, Suzuki T, Tazawa C, Blumberg B, Sasano H. Steroid and xenobiotic receptor (sxr), cytochrome P450 3a4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol. 2005;231(1-2):
212 437. Nishimura M, Naito S. Tissue-specific mrna expression profiles of human ATPbinding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20(6): Verstuyft C, Strabach S, El Morabet H, Kerb R, Brinkmann U, Dubert L, et al. Dipyridamole enhances digoxin bioavailability via p glycoprotein inhibition. Clin Pharmacol Ther. 2003;73(1): Jalava K-M, Partanen J, Neuvonen PJ. Itraconazole decreases renal clearance of digoxin. Ther Drug Monit. 1997;19(6): Pedersen K, Dorph-Pedersen A, Hvidt S, Klitgaard N, Pedersen K. The long-term effect of verapamil on plasma digoxin concentration and renal digoxin clearance in healthy subjects. Eur J Clin Pharmacol. 1982;22(2): Koup JR, Greenblatt DJ, Jusko WJ, Smith TW, Koch-Weser J. Pharmacokinetics of digoxin in normal subjects after intravenous bolus and infusion doses. J Pharmacokinet Biopharm. 1975;3(3): Erik Pedersen K, Dorph Pedersen A, Hvidt S, Anders Klitgaard N, Nielsen Kudsk F. Digoxin verapamil interaction. Clin Pharmacol Ther. 1981;30(3): Schwartz JB, Migliore PJ. Effect of nifedipine on serum digoxin concentration and renal digoxin clearance. Clin Pharmacol Ther. 1984;36(1): Leahey EB, Bigger JT, Butler VP, Reiffel JA, O'Connell GC, Scaffidi LE, et al. Quinidine-digoxin interaction: Time course and pharmacokinetics. Am J Cardiol. 1981;48(6): Hedman A, Angelin B, Arvidsson A, Dahlqvist R, Nilsson B. Interactions in the renal and biliary elimination of digoxin: Stereoselective difference between quinine and quinidine. Clin Pharmacol Ther. 1990;47(1): Fenster PE, White NW, Hanson CD. Pharmacokinetic evaluation of the digoxinamiodarone interaction. J Am Coll Cardiol. 1985;5(1): Becquemont L, Verstuyft C, Kerb R, Brinkmann U, Lebot M, Jaillon P, et al. Effect of grapefruit juice on digoxin pharmacokinetics in humans. Clin Pharmacol Ther. 2001;70(4): Westphal K, Weinbrenner A, Giessmann T, Stuhr M, Franke G, Zschiesche M, et al. Oral bioavailability of digoxin is enhanced by talinolol: Evidence for involvement of intestinal p glycoprotein. Clin Pharmacol Ther. 2000;68(1): Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, et al. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76(1): Rameis H, Magometschnigg D, Ganzinger U. The diltiazem digoxin interaction. Clin Pharmacol Ther. 1984;36(2): Sumner D, Russell A. Digoxin pharmacokinetics: Multicompartmental analysis and its clinical implications. Br J Clin Pharmacol. 1976;3(2): Hedman A, Angelin B, Arvidsson A, Dahlqvist R. Digoxin-interactions in man: Spironolactone reduces renal but not biliary digoxin clearance. Eur J Clin Pharmacol. 1992;42(5): Penzak SR, Shen JM, Alfaro RM, Remaley AT, Natarajan V, Falloon J. Ritonavir decreases the nonrenal clearance of digoxin in healthy volunteers with known MDR1 genotypes. Ther Drug Monit. 2004;26(3): Kovarik JM, Rigaudy L, Guerret M, Gerbeau C, Rost KL. Longitudinal assessment of a p glycoprotein mediated drug interaction of valspodar on digoxin. Clin Pharmacol Ther. 1999;66(4): Andreucci M, Faga T, Pisani A, Sabbatini M, Russo D, Michael A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. Scientific World J. 2014;2014: Nangaku M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1): Wang X, Bonventre JV, Parrish AR. The aging kidney: Increased susceptibility to nephrotoxicity. Int J Mol Sci. 2014;15(9): Wang L, Sweet DH. Renal organic anion transporters (SLC22 family): Expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013;15(1):
213 459. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1): Ewy G, Kapadia G, Yao L, Lullin M, Marcus F. Digoxin metabolism in the elderly. Circulation. 1969;39(4): Okada RD, Hager WD, Graves PE, Mayersohn M, Perrier DG, Marcus FI. Relationship between plasma concentration and dose of digoxin in patients with and without renal impairment. Circulation. 1978;58(6): Bloom PM, Nelp WB, Tuell SH. Relationship of the excretion of tritiated digoxin to renal function. Am J Med Sci. 1966;251(2): Elinder C-G, Bárány P, Heimbürger O. The use of estimated glomerular filtration rate for dose adjustment of medications in the elderly. Drug Aging. 2014;31(7): Blake GM, Sibley-Allen C, Hilton R, Burnapp L, Moghul MR, Goldsmith D. Glomerular filtration rate in prospective living kidney donors. Int Urol Nephrol. 2013;45(5): Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75(10): Currie GM, Wheat JM, Kiat H. Pharmacokinetic considerations for digoxin in older people. Open Cardiovasc Med J. 2011;5: Musso CG, Oreopoulos DG. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119(Suppl. 1): Bauer JH, Brooks CS, Burch RN. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis. 1982;2(3): Lin Y-c, Bansal N, Vittinghoff E, Go AS, Hsu C-y. Determinants of the creatinine clearance to glomerular filtration rate ratio in patients with chronic kidney disease: A cross-sectional study. BMC Nephrol. 2013;14(1): Bennett WM, Porter GA. Endogenous creatinine clearance as a clinical measure of glomerular filtration rate. BMJ. 1971;4(5779): Komazawa H, Yamaguchi H, Hidaka K, Ogura J, Kobayashi M, Iseki K. Renal uptake of substrates for organic anion transporters oat1 and oat3 and organic cation transporters oct1 and oct2 is altered in rats with adenine induced chronic renal failure. J Pharm Sci. 2013;102(3): Masimirembwa CM, Bredberg U, Andersson TB. Metabolic stability for drug discovery and development. Clin Pharmacokinet. 2003;42(6): Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8): Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14(7): Beaufay H, Amar-Costesec A, Thinès-Sempoux D, Wibo M, Robbi M, Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. 3. Subfractionation of the microsomal fraction by isopycnic and differential centrifugation in density gradients. J Cell Biol. 1974;61(1): Bergeron JJ, Posner BI, Josefsberg Z, Sikstrom R. Intracellular polypeptide hormone receptors. J Biol Chem. 1978;253(11): Prough RA, Patrizi VW, Okita RT, Masters BSS, Jakobsson SW. Characteristics of benzo (a) pyrene metabolism by kidney, liver, and lung microsomal fractions from rodents and humans. Cancer Res. 1979;39(4): Palade GE, Siekevitz P. Liver microsomes an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956;2(2): Brunner G, Bygrave F. Microsomal marker enzymes and their limitations in distinguishing the outer membrane of rat liver mitochondria from the microsomes. Eur J Biochem. 1969;8(4): Yamazaki H, Inoue K, Turvy CG, Guengerich FP, Shimada T. Effects of freezing, thawing, and storage of human liver samples on the microsomal contents and activities of cytochrome P450 enzymes. Drug Metab Dispos. 1997;25(2):
214 481. Guengerich FP. Similarity of nuclear and microsomal cytochromes P-450 in the in vitro activation of aflatoxin b 1. Biochem Pharmacol. 1979;28(19): Wilson Z, Rostami Hodjegan A, Burn J, Tooley A, Boyle J, Ellis S, et al. Interindividual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br J Clin Pharmacol. 2003;56(4): Paine MJ, Garner AP, Powell D, Sibbald J, Sales M, Pratt N, et al. Cloning and characterization of a novel human dual flavin reductase. J Biol Chem. 2000;275(2): Schnaitman C, Greenawalt JW. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968;38(1): Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria a biochemical and morphological study. J Cell Biol. 1967;32(2): Fahl W, Jefcoate C, Kasper C. Characteristics of benzo (a) pyrene metabolism and cytochrome P-450 heterogeneity in rat liver nuclear envelope and comparison to microsomal membrane. J Biol Chem. 1978;253(9): Tice LW, Barrnett RJ. The fine structural localization of glucose-6-phosphatase in rat liver. J Histochem Cytochem. 1962;10(6): Kashnig DM, Kasper CB. Isolation, morphology, and composition of the nuclear membrane from rat liver. J Biol Chem. 1969;244(14): Miners JO, Knights KM, Houston JB, Mackenzie PI. In vitro in vivo correlation for drugs and other compounds eliminated by glucuronidation in humans: Pitfalls and promises. Biochem Pharmacol. 2006;71(11): Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34(6): Bernard O, Guillemette C. The main role of UGT1A9 in the hepatic metabolism of mycophenolic acid and the effects of naturally occurring variants. Drug Metab Dispos. 2004;32(8): Levesque E, Delage R, Benoit Biancamano MO, Caron P, Bernard O, Couture F, et al. The impact of ugt1a8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81(3): Uwai Y, Motohashi H, Tsuji Y, Ueo H, Katsura T, Inui K-i. Interaction and transport characteristics of mycophenolic acid and its glucuronide via human organic anion transporters hoat1 and hoat3. Biochem Pharmacol. 2007;74(1): El-Sheikh AA, Koenderink JB, Wouterse AC, van den Broek PH, Verweij VG, Masereeuw R, et al. Renal glucuronidation and multidrug resistance protein 2-/multidrug resistance protein 4-mediated efflux of mycophenolic acid: Interaction with cyclosporine and tacrolimus. Transl Res. 2014;164(1): Picard N, Yee SW, Woillard JB, Lebranchu Y, Le Meur Y, Giacomini KM, et al. The role of organic anion transporting polypeptides and their common genetic variants in mycophenolic acid pharmacokinetics. Clin Pharmacol Ther. 2010;87(1): Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE. Pharmgkb summary: Mycophenolic acid pathway. Pharmacogenet Genomics. 2014;24(1): Matsunaga N, Wada S, Nakanishi T, Ikenaga M, Ogawa M, Tamai I. Mathematical modeling of the in vitro hepatic disposition of mycophenolic acid and its glucuronide in sandwich-cultured human hepatocytes. Mol Pharm. 2013;11(2): Yau WP, Vathsala A, Lou HX, Zhou S, Chan E. Mechanism based enterohepatic circulation model of mycophenolic acid and its glucuronide metabolite: Assessment of impact of cyclosporine dose in asian renal transplant patients. J Clin Pharmacol. 2009;49(6): Sherwin CM, Sagcal Gironella ACP, Fukuda T, Brunner HI, Vinks AA. Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with childhood onset systemic lupus erythematosus. Br J Clin Pharmacol. 2012;73(5):
215 500. Shipkova M, Armstrong VW, Oellerich M, Wieland E. Mycophenolate mofetil in organ transplantation: Focus on metabolism, safety and tolerability. Expert Opin Drug Metab Toxicol. 2005;1(3): Gertz M, Cartwright CM, Hobbs MJ, Kenworthy KE, Rowland M, Houston JB, et al. Cyclosporine inhibition of hepatic and intestinal cyp3a4, uptake and efflux transporters: Application of PBPK modeling in the assessment of drug-drug interaction potential. Pharm Res. 2013;30(3): Tsamandouras N, Dickinson G, Guo Y, Hall S, Rostami-Hodjegan A, Galetin A, et al. Development and application of a mechanistic pharmacokinetic model for simvastatin and its active metabolite simvastatin acid using an integrated population PBPK approach. Pharm Res. 2015;32(6): Kremer J, Hamilton R. The effects of nonsteroidal antiinflammatory drugs on methotrexate (mtx) pharmacokinetics: Impairment of renal clearance of mtx at weekly maintenance doses but not at 7.5 mg. J Rheumatol. 1995;22(11): El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007;320(1): Khamdang S, Takeda M, Noshiro R, Narikawa S, Enomoto A, Anzai N, et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 2002;303(2): Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002;302(2): Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, Miyamoto E, et al. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur J Pharmacol. 2008;596(1): Kuehl GE, Lampe JW, Potter JD, Bigler J. Glucuronidation of nonsteroidal antiinflammatory drugs: Identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos. 2005;33(7): Gaganis P, Miners JO, Knights KM. Glucuronidation of fenamates: Kinetic studies using human kidney cortical microsomes and recombinant UDP-glucuronosyltransferase (UGT) 1A9 and 2B7. Biochem Pharmacol. 2007;73(10): Lohr JW, Willsky GR, Acara MA. Renal drug metabolism. Pharmacol Rev. 1998;50(1): Mutlib AE, Gerson RJ, Meunier PC, Haley PJ, Chen H, Gan LS, et al. The speciesdependent metabolism of efavirenz produces a nephrotoxic glutathione conjugate in rats. Toxicol Appl Pharmacol. 2000;169(1): Cummings BS, Parker JC, Lash LH. Cytochrome P450-dependent metabolism of trichloroethylene in rat kidney. Toxicol Sci. 2001;60(1): Lash LH, Putt DA, Huang P, Hueni SE, Parker JC. Modulation of hepatic and renal metabolism and toxicity of trichloroethylene and perchloroethylene by alterations in status of cytochrome P450 and glutathione. Toxicology. 2007;235(1): Dekant W. Chemical-induced nephrotoxicity mediated by glutathione s-conjugate formation. Toxicol Lett. 2001;124(1): Copeland KR, Yatscoff RW. Comparison of the effects of cyclosporine and its metabolites on the release of prostacyclin and endothelin from mesangial cells. Transplantation. 1992;53(3): Nissim I, Horyn O, Daikhin Y, Nissim I, Luhovyy B, Phillips PC, et al. Ifosfamideinduced nephrotoxicity: Mechanism and prevention. Cancer Res. 2006;66(15): Zheng S, Tasnif Y, Hebert MF, Davis CL, Shitara Y, Calamia JC, et al. CYP3A5 gene variation influences cyclosporine a metabolite formation and renal cyclosporine disposition. Transplantation. 2013;95(6): Dai Y, Iwanaga K, Lin YS, Hebert MF, Davis CL, Huang WL, et al. In vitro metabolism of cyclosporine a by human kidney CYP3A5. Biochem Pharmacol. 2004;68(9):
216 519. Adeleye Y, Andersen M, Clewell R, Davies M, Dent M, Edwards S, et al. Implementing toxicity testing in the 21st century (tt21c): Making safety decisions using toxicity pathways, and progress in a prototype risk assessment. Toxicology. 2015;332: Yoon M, Campbell JL, Andersen ME, Clewell HJ. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol. 2012;42(8): Hendel J, Nyfors A. Nonlinear renal elimination kinetics of methotrexate due to saturation of renal tubular reabsorption. Eur J Clin Pharmacol. 1984;26(1): Sand T, Jacobsen S. Effect of urine ph and flow on renal clearance of methotrexate. Eur J Clin Pharmacol. 1981;19(6): Rouch JA, Burton B, Dabb A, Brown V, Seung AH, Kinsman K, et al. Comparison of enteral and parenteral methods of urine alkalinization in patients receiving high-dose methotrexate. J Oncol Pharm Pract. 2015;DOI: / Uwai Y, Ida H, Tsuji Y, Katsura T, Inui K. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hoat1, hoat3, and hoct2). Pharm Res. 2007;24(4): Kalyesubula R, Perazella MA. Nephrotoxicity of haart. AIDS research and treatment. 2011; Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. The American journal of the medical sciences. 2007;334(2): Mukai H, Ozaki D, Cui Y, Kuboyama T, Yamato-Nagata H, Onoe K, et al. Quantitative evaluation of the improvement in the pharmacokinetics of a nucleic acid drug delivery system by dynamic pet imaging with 18 f-incorporated oligodeoxynucleotides. J Controlled Release. 2014;180: Moses WW. Fundamental limits of spatial resolution in pet. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2011;648:S236-S Lee VS, Rusinek H, Bokacheva L, Huang AJ, Oesingmann N, Chen Q, et al. Renal function measurements from MR renography and a simplified multicompartmental model. American Journal of Physiology-Renal Physiology. 2007;292(5):F1548-F Achour B, Al-Majdoub ZM, Al Feteisi H, Elmorsi Y, Rostami-Hodjegan A, Barber J. Ten years of QconCATs: Application of multiplexed quantification to small medically relevant proteomes. Int J Mass spectrom. 2015;391: Russell MR, Achour B, Mckenzie EA, Lopez R, Harwood MD, Rostami-Hodjegan A, et al. Alternative fusion protein strategies to express recalcitrant QconCAT proteins for quantitative proteomics of human drug metabolizing enzymes and transporters. J Proteome Res. 2013;12(12): Heikkinen AT, Lignet F, Cutler P, Parrott N. The role of quantitative ADME proteomics to support construction of physiologically based pharmacokinetic models for use in small molecule drug development. Proteomics Clin Appl. 2015;9(7-8): Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130(6): Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20(11): Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23): Miskell CA, Simpson DP. Hyperplasia precedes increased glomerular filtration rate in rat remnant kidney. Kidney Int. 1990;37(2): Baer PC, Nockher WA, Haase W, Scherberich JE. Isolation of proximal and distal tubule cells from human kidney by immunomagnetic separation: Technical note. Kidney Int. 1997;52(5): Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: A resource for investigations into drug disposition. Xenobiotica. 2006;36(10-11):
217 539. Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303(2): Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13(4): Augustine LM, Markelewicz RJ, Jr., Boekelheide K, Cherrington NJ. Xenobiotic and endobiotic transporter mrna expression in the blood-testis barrier. Drug Metab Dispos. 2005;33(1): Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 2003;31(2): Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mrnas in the choroid plexus of rats. Drug Metab Dispos. 2003;31(11): Karbach U, Kricke J, Meyer-Wentrup F, Gorboulev V, Volk C, Loffing-Cueni D, et al. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol. 2000;279(4):F Sugawara-Yokoo M, Urakami Y, Koyama H, Fujikura K, Masuda S, Saito H, et al. Differential localization of organic cation transporters roct1 and roct2 in the basolateral membrane of rat kidney proximal tubules. Histochem Cell Biol. 2000;114(3): Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34(3): Cheng Y, Vapurcuyan A, Shahidullah M, Aleksunes LM, Pelis RM. Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab Dispos. 2012;40(3): Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7): Wu XA, Huang W, Ganapathy ME, Wang HP, Kekuda R, Conway SJ, et al. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am J Physiol Renal Physiol. 2000;279(3):F449-F Kamiie J, Ohtsuki S, Iwase R, Unine K, Katsukura Y, Yanai K, et al. Quantitative atlas of membrane transporter proteins: Development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008;25(6): Wu X, George RL, Huang W, Wang HP, Conway SJ, Leibach FH, et al. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. BBA-Biomembranes. 2000;1466(1-2): Tamai I, Nakanishi T, Kobayashi D, China K, Kosugi Y, Nezu J, et al. Involvement of OCTN1 (SLC22A4) in ph-dependent transport of organic cations. Mol Pharm. 2004;1(1): Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17(8): Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, et al. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther. 1999;290(3): Tamai I, China K, Sai Y, Kobayashi D, Nezu J, Kawahara E, et al. Na+-coupled transport of l-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. BBA-Biomembranes. 2001;1512(2): Cano MM, Calonge ML, Ilundain AA. Expression of OCTN2 and OCTN3 in the apical membrane of rat renal cortex and medulla. J Cell Physiol. 2010;223(2): Tamai I, Ohashi R, Nezu J, Sai Y, Kobayashi D, Oku A, et al. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J Biol Chem. 2000;275(51):
218 558. Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol Renal Physiol. 1999;276(1):F122-F Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, et al. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J Am Soc Nephrol. 1999;10(3): Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, et al. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol. 2004;287(1):F124-F Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem. 2006;281(8): Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, et al. Murine renal organic anion transporters moat1 and moat3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol. 2005;289(5):C1075-C Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, et al. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther. 2002;301(3): Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol. 2002;13(4): Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and pgp expression, function and therapeutic drug response: A critical review and recommendations for future research. Pharmacogenom J. 2007;7(3): Cha SH, Sekine T, Fukushima J, Kanai Y, Kobayashi Y, Goya T, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59(5): Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, et al. Functional characterization of rat organic anion transporter 5 (slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther. 2005;315(2): Babu E, Takeda M, Narikawa S, Kobayashi Y, Enomoto A, Tojo A, et al. Role of human organic anion transporter 4 in the transport of ochratoxin a. BBA-Mol Cell Res. 2002;1590(1): Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, et al. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94(3): Ikarashi R, Shibasaki K, Yamaguchi A. Immunohistochemical studies of organic anion transporters and urate transporter 1 expression in human salivary gland. Acta Odontol Scand. 2012;71(2): Cheng XG, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos. 2009;37(11): Lu H, Klaassen C. Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mrna in rodents. Peptides. 2006;27(4): Smith DE, Pavlova A, Berger UV, Hediger MA, Yang TX, Huang YNG, et al. Tubular localization and tissue distribution of peptide transporters in rat kidney. Pharm Res. 1998;15(8): Shen H, Smith DE, Yang TX, Huang YNG, Schnermann JB, Brosius FC. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mrna and protein in rat kidney. Am J Physiol Renal Physiol. 1999;276(5):F658-F Noshiro R, Anzai N, Sakata T, Miyazaki H, Terada T, Shin H, et al. The PDZ domain protein PDZK1 interacts with human peptide transporter PEPT2 and enhances its transport activity. Kidney Int. 2006;70(2): Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A. 2005;102(50):
219 577. Nishihara K, Masuda S, Ji L, Katsura T, Inui KI. Pharmacokinetic significance of luminal multidrug and toxin extrusion 1 in chronic renal failure rats. Biochem Pharmacol. 2007;73(9): Lickteig AJ, Cheng XG, Augustine LM, Klaassen CD, Cherrington NJ. Tissue distribution, ontogeny and induction of the transporters multidrug and toxin extrusion (MATE) 1 and MATE2 mrna expression levels in mice. Life Sci. 2008;83(1-2): Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci. 2006;95(1): Huls M, Brown C, Windass A, Sayer R, van den Heuvel J, Heemskerk S, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73(2): Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular-localization of the multidrug-resistance gene-product p-glycoprotein in normal human-tissues. Proc Natl Acad Sci U S A. 1987;84(21): Haenisch S, Zimmermann U, Dazert E, Wruck CJ, Dazert P, Siegmund S, et al. Influence of polymorphisms of ABCB1 and ABCC2 on mrna and protein expression in normal and cancerous kidney cortex. Pharmacogenom J. 2007;7(1): Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, et al. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47(6): Maher JM, Slitt AL, Cherrington NJ, Cheng XG, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (MRP) family in mice. Drug Metab Dispos. 2005;33(7): Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, et al. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med. 1998;188(5): Schaub TP, Kartenbeck J, Konig J, Spring H, Dorsam J, Staehler G, et al. Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. J Am Soc Nephrol. 1999;10(6): Scheffer GL, Hu HF, Pijnenborg ACLM, Wijnholds J, Bergen AAB, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82(4): van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: Putative efflux pump for urinary camp and cgmp. J Am Soc Nephrol. 2002;13(3): Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, et al. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8(8): Scheffer GL, Kool M, Heijn M, de Haas M, Pijnenborg AC, Wijnholds J, et al. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and MDR3 p-glycoprotein with a panel of monoclonal antibodies. Cancer Res. 2000;60(18): Kuroda M, Kobayashi Y, Tanaka Y, Itani T, Mifuji R, Araki J, et al. Increased hepatic and renal expressions of multidrug resistance-associated protein 3 in eisai hyperbilirubinuria rats. J Gastroenterol Hepatol. 2004;19(2): Bergwerk AJ, Shi XY, Ford AC, Kanai N, Jacquemin E, Burk RD, et al. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am J Physiol Gastrointest Liver Physiol. 1996;271(2):G231-G Cheng XG, Maher J, Lu H, Klaassen CD. Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol. 2006;70(4): Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1a2 (oatp1a2) - implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280(10):
220 595. Nomura T, Chang HY, Lu R, Hankin J, Murphy RC, Schuster VL. Prostaglandin signaling in the renal collecting duct - release, reuptake, and oxidation in the same cell. J Biol Chem. 2005;280(31): Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, et al. Molecular characterization of human and rat organic anion transporter OATP-d. Am J Physiol Renal Physiol. 2003;285(6):F1188-F Welling L, Welling D, Holsapple J, Evan A. Morphometric analysis of distinct microanatomy near the base of proximal tubule cells. Am J Physiol Renal Physiol. 1987;253(1):F126-F Welling LW, Welling DJ. Shape of epithelial cells and intercellular channels in the rabbit proximal nephron. Kidney Int. 1976;9(5): Garg L, Knepper MA, Burg MB. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol Renal Physiol. 1981;240(6):F536-F Tiedemann K, Welling LW, Basto P. Structural and functional comparison of mesonephric and metanephric proximal tubules. Pediatr Nephrol. 1987;1(3): Gonçalves V, Sobrinho-Simões M. Comparative morphometric study of the cells of the third proximal segment of the rat kidney under different conditions of fixation. Experientia. 1977;33(6): Amet Y, Berthou F, Fournier G, Dréano Y, Bardou L, Clèdes J, et al. Cytochrome P450 4a and 2e1 expression in human kidney microsomes. Biochem Pharmacol. 1997;53(6): Soars MG, Riley RJ, Findlay KA, Coffey MJ, Burchell B. Evidence for significant differences in microsomal drug glucuronidation by canine and human liver and kidney. Drug Metab Dispos. 2001;29(2): McGurk KA, Brierley CH, Burchell B. Drug glucuronidation by human renal UDPglucuronosyltransferases. Biochem Pharmacol. 1998;55(7): Dohn DR, Anders MW. Assay of cysteine conjugate beta-lyase activity with s-(2- benzothiazolyl)cysteine as the substrate. Anal Biochem. 1982;120(2): Ozaki H, Sugihara K, Tamura Y, Fujino C, Watanabe Y, Uramaru N, et al. Hydrolytic metabolism of phenyl and benzyl salicylates, fragrances and flavoring agents in foods, by microsomes of rat and human tissues. Food Chem Toxicol. 2015;86: Lash LH, Qian W, Putt DA, Desai K, Elfarra AA, Sicuri AR, et al. Glutathione conjugation of perchloroethylene in rats and mice in vitro: Sex-, species-, and tissuedependent differences. Toxicol Appl Pharmacol. 1998;150(1): Wise RW, Zenser TV, Kadlubar FF, Davis BB. Metabolic activation of carcinogenic aromatic amines by dog bladder and kidney prostaglandin h synthase. Cancer Res. 1984;44(5): Nässberger L, Bergstrand A, DePierre JW. Biochemical effects of gentamicin on rat kidney cortex: I. Analytical subfractionation of control tissue. Exp Mol Pathol. 1987;46(2): Zordoky BN, Anwar-Mohamed A, Aboutabl ME, El-Kadi AO. Acute doxorubicin toxicity differentially alters cytochrome P450 expression and arachidonic acid metabolism in rat kidney and liver. Drug Metab Dispos. 2011;39(8): Sharer JE, Duescher RJ, Elfarra AA. Species and tissue differences in the microsomal oxidation of 1,3-butadiene and the glutathione conjugation of butadiene monoxide in mice and rats - possible role in 1,3-butadiene-induced toxicity. Drug Metab Dispos. 1992;20(5): Jakobsson SW, Okita RT, Mock NI, Masters BSS, Buja LM, Prough RA. Monooxygenase activities of human liver, lung, and kidney microsomes a study of 42 post mortem cases. Acta Pharmacol Toxicol (Copenh). 1982;50(5): Okita RT, Jakobsson SW, Prough RA, Masters BSS. Lauric acid hydroxylation in human liver and kidney cortex microsomes. Biochem Pharmacol. 1979;28(23): Wistrand PJ, Knuuttila KG. Renal membrane-bound carbonic anhydrase. Purification and properties. Kidney Int. 1989;35(3): Taub ME, Ludwig-Schwellinger E, Ishiguro N, Kishimoto W, Yu H, Wagner K, et al. Sex-, species-, and tissue-specific metabolism of empagliflozin in male mouse kidney forms an unstable hemiacetal metabolite (m466/2) that degrades to 4-220
221 hydroxycrotonaldehyde, a reactive and cytotoxic species. Chem Res Toxicol. 2015;28(1): Lory P, Gilg A, Horster M. Renal countercurrent system: Role of collecting duct convergence and pelvic urea predicted from a mathematical model. J Math Biol. 1983;16(3): Loffing J, Kaissling B. Sodium and calcium transport pathways along the mammalian distal nephron: From rabbit to human. Am J Physiol Renal Physiol. 2003;284(4):F628-F Nielsen S, Kwon T, Fenton R, Praetorius J. Anatomy of the kidney. In: Taal M, Chertow G, Marsden P, Skorecki K, Yu A, Brenner B, editors. Brenner and rector's the kidney. 9th ed. Philadelphia, PA: Elsevier; p Kainer R. A geometric model of the rat kidney. Anat Embryol (Berl). 1975;147(1): Takahashi-Iwanaga H, Iwata Y, Adachi K, Fujita T. The histotopography and ultrastructure of the thin limb of the henle's loop: A scanning electron microscopic study of the rat kidney. Arch Histol Cytol. 1989;52(4): Orloff J, Berliner RW. Handbook of physiology. Section 8: Renal physiology. Washington: American Physiological Society; Palay SL, Karlin LJ. An electron microscopic study of the intestinal villus: II. The pathway of fat absorption. J Biophys Biochem Cytol. 1959;5(3): Welling L, Welling D. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int. 1975;8(6): Helander H, Fändriks L. Surface area of the digestive tract-revisited. Scand J Gastroenterol. 2014;49(6): Roux A, Aubert P, Guedon J, Flouvat B. Pharmacokinetics of acebutolol in patients with all grades of renal failure. Eur J Clin Pharmacol. 1980;17(5): Smith R, Warren D, Renwick A, George C. Acebutolol pharmacokinetics in renal failure. Br J Clin Pharmacol. 1983;16(3): Lilja J, Raaska K, Neuvonen P. Effects of grapefruit juice on the pharmacokinetics of acebutolol. Br J Clin Pharmacol. 2005;60(6): Roux A, Le Liboux A, Delhotal B, Gaillot J, Flouvat B. Pharmacokinetics in man of acebutolol and hydrochlorothiazide as single agents and in combination. Eur J Clin Pharmacol. 1983;24(6): Coombs T, Coulson C, Smith V. Blood plasma binding of acebutolol and diacetolol in man. Br J Clin Pharmacol. 1980;9(4): Kukes V, Gneushev E, Mamedov T, Gneusheva I. Acebutolol and diacetolol: Their binding to plasma proteins and erythrocytes and secretion with the saliva. Farmakol Toksikol. 1991;54(1): Coyle J, Boudoulas H, Lima J. Acecainide pharmacokinetics in normal subjects of known acetylator phenotype. Biopharm Drug Dispos. 1991;12(8): Connolly S, Kates R. Clinical pharmacokinetics of n-acetylprocainamide. Clin Pharmacokinet. 1982;7(3): Critchley J, Critchley L, Anderson P, Tomlinson B. Differences in the single-oraldose pharmacokinetics and urinary excretion of paracetamol and its conjugates between hong kong chinese and caucasian subjects. J Clin Phar Ther. 2005;30(2): Herrera A, Scott D, Lunte C. Microdialysis sampling for determination of plasma protein binding of drugs. Pharm Res. 1990;7(10): Brigden D, Bye A, Fowle A, Rogers H. Human pharmacokinetics of acyclovir (an antiviral agent) following rapid intravenous injection. J Antimicrob Chemoth. 1981;7(4): Soul-Lawton J, Weatherley B, Posner J, Layton G, Peck R. Lack of interaction between valaciclovir, the l-valyl ester of aciclovir, and digoxin. Br J Clin Pharmacol. 1998;45(1): De Bony F, Tod M, Bidault R, On N, Posner J, Rolan P. Multiple interactions of cimetidine and probenecid with valaciclovir and its metabolite acyclovir. Antimicrob Agents Chemother. 2002;46(2):
222 638. Blum M, Liao S, de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med. 1982;73(1A): Kearney B, Ramanathan S, Cheng A, Ebrahimi R, Shah J. Systemic and renal pharmacokinetics of adefovir and tenofovir upon coadministration. J Clin Pharmacol. 2005;45(8): Cundy K, Barditch-Crovo P, Walker R, Collier A, Ebeling D, Toole J, et al. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1995;39(11): Breithaupt B, Tittel M. Kinetics of allopurinol after single intravenous and oral doses. Noninteraction with benzbromarone and hydrochlorothiazide. Eur J Clin Pharmacol. 1982;22(1): Elion G, Kovensky A, Hitchings G. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966;15(7): Jansat J, Costa J, Salvà P, Fernandez F, Martinez-Tobed A. Absolute bioavailability, pharmacokinetics, and urinary excretion of the novel antimigraine agent almotriptan in healthy male volunteers. J Clin Pharmacol. 2002;42(12): Fleishaker J, Sisson T, Carel B, Azie N. Pharmacokinetic interaction between verapamil and almotriptan in healthy volunteers. Clin Pharmacol Ther. 2000;67(5): Gaudry S, Sitar D, Smyth D, McKenzie J, Aoki F. Gender and age as factors in the inhibition of renal clearance of amantadine by quinine and quinidine. Clin Pharmacol Ther. 1993;54(1): Cook J, Silverman M, Schelling D, Nix D, Schentag J, Brown R, et al. Multipledose pharmacokinetics and safety of oral amifloxacin in healthy volunteers. Antimicrob Agents Chemother. 1990;34(6): Moffat AC, Osselton MD, Widdop B. Clarke's analysis of drugs and poisons. London: Pharmaceutical press; Horber F, Frey F, Descoeudres C, Murray A, Reubi F. Differential effect of impaired renal function on the kinetics of clavulanic acid and amoxicillin. Antimicrob Agents Chemother. 1986;29(4): Sjövall J, Westerlund D, Alván G. Renal excretion of intravenously infused amoxycillin and ampicillin. Br J Clin Pharmacol. 1985;19(2): Brogden R, Heel R, Speight T, Avery G. Amoxycillin injectable: A review of its antibacterial spectrum, pharmacokinetics and therapeutic use. Drugs. 1979;18(3): Blum R, Kohli R, Harrison N, Schentag J. Pharmacokinetics of ampicillin (2.0 grams) and sulbactam (1.0 gram) coadministered to subjects with normal and abnormal renal function and with end-stage renal disease on hemodialysis. Antimicrob Agents Chemother. 1989;33(9): Barza M, Weinstein L. Pharmacokinetics of the penicillins in man. Clin Pharmacokinet. 1976;1(4): Staiger C, Schlicht F, Walter E, Gundert-Remy U, Hildebrandt R, de Vries J, et al. Effect of single and multiple doses of sulphinpyrazone on antipyrine metabolism and urinary excretion of 6-beta-hydroxycortisol. Eur J Clin Pharmacol. 1983;25(6): Bax N, Lennard M, Tucker G. Inhibition of antipyrine metabolism by betaadrenoceptor antagonists. Br J Clin Pharmacol. 1981;12(6): Kochansky C, McMasters D, Lu P, Koeplinger K, Kerr H, Shou M, et al. Impact of ph on plasma protein binding in equilibrium dialysis. Mol Pharm. 2008;5(3): Lode H, Elvers A, Koeppe P, Borner K. Comparative pharmacokinetics of apalcillin and piperacillin. Antimicrob Agents Chemother. 1984;25(1): Kobari T, Itoh T, Hirakawa T, Namekawa H, Suzuki T, Satoh T, et al. Dosedependent pharmacokinetics of aprindine in healthy volunteers. Eur J Clin Pharmacol. 1984;26(1): Andreasen F, Husted S, Jakobsen P, Jensen E. The binding of aprindine to serum proteins with statistical considerations concerning the analysis of binding data. Acta Pharmacol Toxicol (Copenh). 1980;46(2):
223 659. Teirlynck O, Belpaire F, Andreasen F. Binding of aprindine and moxaprindine to human serum, alpha 1-acid glycoprotein and serum of healthy and diseased humans. Eur J Clin Pharmacol. 1982;21(5): Barber H, Hawksworth G, Kitteringham N, Petersen J, Petrie J, Swann J. Protein binding of atenolol and propranolol to human serum albumin and in human plasma [proceedings]. Br J Clin Pharmacol. 1978;6(5):446P Mason W, Winer N, Kochak G, Cohen I, Bell R. Kinetics and absolute bioavailability of atenolol. Clin Pharmacol Ther. 1979;25(4): McAinsh J, Holmes B, Smith S, Hood D, Warren D. Atenolol kinetics in renal failure. Clin Pharmacol Ther. 1980;28(3): Belpaire F, Bogaert M, Rosseneu M. Binding of beta-adrenoceptor blocking drugs to human serum albumin, to alpha 1-acid glycoprotein and to human serum. Eur J Clin Pharmacol. 1982;22(3): Fitzgerald J, Ruffin R, Smedstad K, Roberts R, McAinsh J. Studies on the pharmacokinetics and pharmacodynamics of atenolol in man. Eur J Clin Pharmacol. 1978;13(2): Kirch W, Köhler H, Mutschler E, Schäfer M. Pharmacokinetics of atenolol in relation to renal function. Eur J Clin Pharmacol. 1981;19(1): Mason W, Kochak G, Winer N, Cohen I. Effect of exercise on renal clearance of atenolol. J Pharm Sci. 1980;69(3): Lander R, Henderson R, Pyszczynski D. Pharmacokinetic comparison of 5 g of azlocillin every 8 h and 4 g every 6 h in healthy volunteers. Antimicrob Agents Chemother. 1989;33(5): Leroy A, Humbert G, Godin M, Fillastre J. Pharmacokinetics of azlocillin in subjects with normal and impaired renal function. Antimicrob Agents Chemother. 1980;17(3): Nathwani D, Wood M. Penicillins. A current review of their clinical pharmacology and therapeutic use. Drugs. 1993;45(6): Bergan T. Review of the pharmacokinetics and dose dependency of azlocillin in normal subjects and patients with renal insufficiency. J Antimicrob Chemoth. 1983;11(suppl B): Vinks AA, van Rossem RN, Mathôt RA, Heijerman HG, Mouton JW. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using monte carlo simulation. Antimicrob Agents Chemother. 2007;51(9): Swabb E, Sugerman A, McKinstry D. Multiple-dose pharmacokinetics of the monobactam azthreonam (sq 26,776) in healthy subjects. Antimicrob Agents Chemother. 1983;23(1): Rumble R, Roberts M, Scott A. The effect of posture on the pharmacokinetics of intravenous benzylpenicillin. Eur J Clin Pharmacol. 1986;30(6): Petersen M, Nation R, McBride W, Ashley J, Moore R. Pharmacokinetics of betamethasone in healthy adults after intravenous administration. Eur J Clin Pharmacol. 1983;25(5): Ludden T, Boyle D, Gieseker D, Kennedy G, Crawford M, Ludden L, et al. Absolute bioavailability and dose proportionality of betaxolol in normal healthy subjects. J Pharm Sci. 1988;77(9): Bianchetti G, Thiercelin J, Thenot J. Pharmacokinetics of betaxolol in middle aged patients. Eur J Clin Pharmacol. 1986;31(2): McDevitt D. Comparison of pharmacokinetic properties of beta-adrenoceptor blocking drugs. Eur Heart J. 1987;8(suppl M): Bühring K, Sailer H, Faro H, Leopold G, Pabst J, Garbe A. Pharmacokinetics and metabolism of bisoprolol-14c in three animal species and in humans. J Cardiovasc Pharmacol. 1986;8(suppl 11):S21-S Le Coz F, Sauleman P, Poirier J, Cuche J, Midavaine M, Rames A, et al. Oral pharmacokinetics of bisoprolol in resting and exercising healthy volunteers. J Cardiovasc Pharmacol. 1991;18(1):
224 680. Leopold G, Pabst J, Ungethüm W, Bühring K. Basic pharmacokinetics of bisoprolol, a new highly beta 1-selective adrenoceptor antagonist. J Clin Pharmacol. 1986;26(8): Horikiri Y, Suzuki T, Mizobe M. Pharmacokinetics and metabolism of bisoprolol enantiomers in humans. J Pharm Sci. 1998;87(3): Blanchard J. Protein binding of caffeine in young and elderly males. J Pharm Sci. 1982;71(12): Sinhvi S, Duchin K, Willard D, McKinstry D, Migdalof B. Renal handling of captopril: Effect of probenecid. Clin Pharmacol Ther. 1982;32(2): Lin S, Wei Y, Li M, Wang S. Effect of ethanol or/and captopril on the secondary structure of human serum albumin before and after protein binding. Eur J Pharm Biopharm. 2004;57(3): Itoh T, Ishida M, Onuki Y, Tsuda Y, Shimada H, Yamada H. Stereoselective renal tubular secretion of carbenicillin. Antimicrob Agents Chemother. 1993;37(11): Wise E, Armstrong G, Brown R, Andrews J. The pharmacokinetics and tissue penetration of ceftazidime and cefamandole in healthy volunteers. J Antimicrob Chemoth. 1981;8(suppl B): Berkhout J, Visser L, van den Broek P, van de Klundert J, Mattie H. Clinical pharmacokinetics of cefamandole and ceftazidime administered by continuous intravenous infusion. Antimicrob Agents Chemother. 2003;47(6): Lee FH, Pfeffer M, Van Harken DR, Smyth RD, Hottendorf GH. Comparative pharmacokinetics of ceforanide (bl-s786r) and cefazolin in laboratory animals and humans. Antimicrob Agents Chemother. 1980;17(2): Nakagawa K, Koyama M, Tachibana A, Komiya M, Kikuchi Y, Yano K. Pharmacokinetics of cefotetan (ym09330) in humans. Antimicrob Agents Chemother. 1982;22(6): Smyth RD, Pfeffer M, Donald AG, Van Harken R, Hottendorf GH. Clinical pharmacokinetics and safety of high doses of ceforanide (bl-s786r) and cefazolin. Antimicrob Agents Chemother. 1979;16(5): Lanao J, Vicente M, Dominguez-Gil A. Pharmacokinetics of cefazolin administered as a new drug delivery system in healthy volunteers. Biopharm Drug Dispos. 1988;9(4): Vella-Brincat JW, Begg EJ, Kirkpatrick CM, Zhang M, Chambers ST, Gallagher K. Protein binding of cefazolin is saturable in vivo both between and within patients. Br J Clin Pharmacol. 2007;63(6): Lavillaureix J, Brogard J, Pinget M, Ledoux F. Dosage adjustments of cefazolin according to the pharmacokinetics of this new cephalosporin. Infection. 1975;3(2): Ohashi K, Tsunoo M, Tsuneoka K. Pharmacokinetics and protein binding of cefazolin and cephalothin in patients with cirrhosis. J Antimicrob Chemoth. 1986;17(3): Barbhaiya R, Forgue S, Gleason C, Knupp C, Pittman K, Weidler D, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992;36(3): Barbhaiya R, Forgue S, Gleason C, Knupp C, Pittman K, Weidler D, et al. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990;34(6): Barbhaiya R, Forgue S, Shyu W, Papp E, Pittman K. High-pressure liquid chromatographic analysis of bmy in plasma and urine. Antimicrob Agents Chemother. 1987;31(1): Kessler R, Bies M, Buck R, Chisholm D, Pursiano T, Tsai Y, et al. Comparison of a new cephalosporin, bmy 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985;27(2): Faulkner R, Yacobi A, Barone J, Kaplan S, Silber B. Pharmacokinetic profile of cefixime in man. Pediatr Infect Dis J. 1987;6(10):
225 700. Guay D, Meatherall R, Harding G, Brown G. Pharmacokinetics of cefixime (cl 284,635; fk 027) in healthy subjects and patients with renal insufficiency. Antimicrob Agents Chemother. 1986;30(3): Ohkawa M, Orito M, Sugata T, Shimamura M, Sawaki M, Nakashita E, et al. Pharmacokinetics of cefmetazole in normal subjects and in patients with impaired renal function. Antimicrob Agents Chemother. 1980;18(3): Tan J, Salstrom S, Signs S, Hoffman H, File T. Pharmacokinetics of intravenous cefmetazole with emphasis on comparison between predicted theoretical levels in tissue and actual skin window fluid levels. Antimicrob Agents Chemother. 1989;33(6): Lenfant B, Namour F, Logeais C, Coussediere D, Rivault O, Bryskier A, et al. Pharmacokinetics of cefodizime following single doses of 0.5, 1.0, 2.0, and 3.0 grams administered intravenously to healthy volunteers. Antimicrob Agents Chemother. 1995;39(9): Loffreda A, Lampa E, Lucarelli C, Amorena M, Contaldi C, Calderaro V, et al. Pharmacokinetics of cefodizime in patients with various degrees of renal failure. Chemotherapy. 1999;45(1): Scaglione F, Demartini G, Arcidiacono MM, Dugnani S, Fraschini F. Serum protein binding and extravascular diffusion of cefodizime and ceftriaxone. Clin Drug Invest. 1997;14(3): Brockmeier D, Dagrosa E. Pharmacokinetic profile of cefodizime. Infection. 1992;20(suppl 1):S14-S Barré J. Pharmacokinetics of cefodizime: A review of the data on file. J Antimicrob Chemoth. 1990;26(suppl C): Bryskier A, Procyk T, Tremblay D, Lenfant B, Fourtillan J. Pharmacokinetics of cefodizime administered intravenously as a single-dose (1.0 and 2.0 g) to healthy adult volunteers. J Antimicrob Chemoth. 1990;26(suppl C): Bryskier A, Procyk T, Tremblay D, Lenfant B, Fourtillan J. The pharmacokinetics of cefodizime following intravenous and intramuscular administration of a single dose of 1.0 g. J Antimicrob Chemoth. 1990;26(suppl C): Conte Jr J. Pharmacokinetics of cefodizime in volunteers with normal or impaired renal function. J Clin Pharmacol. 1994;34(11): Barriere S, Hatheway G, Gambertoglio J, Lin E, Conte Jr J. Pharmacokinetics of cefonicid, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1982;21(6): Benson J, Boudinot F, Pennell A, Cunningham F, DiPiro J. In vitro protein binding of cefonicid and cefuroxime in adult and neonatal sera. Antimicrob Agents Chemother. 1993;37(6): Fillastre J, Fourtillan J, Leroy A, Ramis N, Lefevre M, Reumont G, et al. Pharmacokinetics of cefonicid in uraemic patients. J Antimicrob Chemoth. 1986;18(2): Gonik B, Feldman S, Pickering L, Doughtie C. Pharmacokinetics of cefoperazone in the parturient. Antimicrob Agents Chemother. 1986;30(6): Guglielmo B, Flaherty J, Woods T, LaFollette G, Gambertoglio J. Pharmacokinetics of cefoperazone and tobramycin alone and in combination. Antimicrob Agents Chemother. 1987;31(2): Lam Y, Duroux M, Gambertoglio J, Barriere S, Guglielmo B. Effect of protein binding on serum bactericidal activities of ceftazidime and cefoperazone in healthy volunteers. Antimicrob Agents Chemother. 1988;32(3): Pfeffer M, Gaver R, Van Harken D. Human pharmacokinetics of a new braodspectrum parenteral cephalosporin antibiotic, ceforanide. J Pharm Sci. 1980;69(4): Gerding D, Van Etta L, Peterson L. Role of serum protein binding and multiple antibiotic doses in the extravascular distribution of ceftizoxime and cefotaxime. Antimicrob Agents Chemother. 1982;22(5): Esmieu F, Guibert J, Rosenkilde H, Ho I, Le Go A. Pharmacokinetics of cefotaxime in normal human volunteers. J Antimicrob Chemoth. 1980;6(suppl A):
226 720. Harding S, Monro A, Thornton J, Ayrton J, Hogg M. The comparative pharmacokinetics of ceftazidime and cefotaxime in healthy volunteers. J Antimicrob Chemoth. 1981;8(suppl B): Scaglione F, Raichi M, Fraschini F. Serum protein binding and extravascular diffusion of methoxyimino cephalosporins. Time courses of free and total concentrations of cefotaxime and ceftriaxone in serum and pleural exudate. J Antimicrob Chemoth. 1990;26(suppl A): Smith B, LeFrock J, Thyrum P, Doret B, Yeh C, Onesti G, et al. Cefotetan pharmacokinetics in volunteers with various degrees of renal function. Antimicrob Agents Chemother. 1986;29(5): Martin C, Thomachot L, Albanese J. Clinical pharmacokinetics of cefotetan. Clin Pharmacokinet. 1994;26(4): Carver P, Nightingale C, Quintiliani R. Pharmacokinetics and pharmacodynamics of total and unbound cefoxitin and cefotetan in healthy volunteers. J Antimicrob Chemoth. 1989;23(1): Yates R, Adam H, Donnelly R, Houghton H, Charlesworth E, Laws E. Pharmacokinetics and tolerance of single intravenous doses of cefotetan disodium in male caucasian volunteers. J Antimicrob Chemoth. 1983;11(suppl A): Zimmerman J, Cohen A, Thyrum P. Absolute bioavailability and noncompartmental analysis of intravenous and intramuscular cefotan (cefotetan) in normal volunteers. J Clin Pharmacol. 1989;29(2): Brisson A, Bryskier A, Millerioux L, Fourtillan J. Pharmacokinetics of cefotiam administered intravenously and intramuscularly to healthy adults. Antimicrob Agents Chemother. 1984;26(4): Querol-Ferrer V, Zini R, Tillement J. The blood binding of cefotiam and cyclohexanol, metabolites of the prodrug cefotiam hexetil, in-vitro. J Pharm Pharmacol. 1991;43(12): Bulitta J, Kinzig M, Landersdorfer C, Holzgrabe U, Stephan U, Sörgel F. Comparable population pharmacokinetics and pharmacodynamic breakpoints of cefpirome in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother. 2011;55(6): Maass L, Malerczyk V, Verho M. Pharmacokinetics of cefpirome (hr 810), a new cephalosporin derivative administered intramuscularly and intravenously to healthy volunteers. Infection. 1987;15(3): Steiner I, Langenberger H, Marsik C, Mayer B, Fischer M, Georgopoulos A, et al. Effect of norepinephrine on cefpirome tissue concentrations in healthy subjects. J Antimicrob Chemoth. 2004;53(3): Müller M, Rohde B, Kovar A, Georgopoulos A, Eichler H, Derendorf H. Relationship between serum and free interstitial concentrations of cefodizime and cefpirome in muscle and subcutaneous adipose tissue of healthy volunteers measured by microdialysis. J Clin Pharmacol. 1997;37(12): Ljungberg B, Nilsson-Ehle I. Comparative pharmacokinetics of ceftazidime in young, healthy and elderly, acutely ill males. Eur J Clin Pharmacol. 1988;34(2): Kowalsky S, Echols R, Venezia A, Andrews E. Pharmacokinetics of ceftizoxime in subjects with various degrees of renal function. Antimicrob Agents Chemother. 1983;24(2): LeBel M, Paone R, Lewis G. Effect of probenecid on the pharmacokinetics of ceftizoxime. J Antimicrob Chemoth. 1983;12(2): Aderounmu A, Salako L, Lindström B, Walker O, Ekman L. Comparison of the pharmacokinetics of chloroquine after single intravenous and intramuscular administration in healthy africans. Br J Clin Pharmacol. 1986;22(5): Ofori-Adjei D, Ericsson O, Lindström B, Sjöqvist F. Protein binding of chloroquine enantiomers and desethylchloroquine. Br J Clin Pharmacol. 1986;22(3): Walker O, Birkett D, Alván G, Gustafsson L, Sjöqvist F. Characterization of chloroquine plasma protein binding in man. Br J Clin Pharmacol. 1983;15(3): Onyeji C, Toriola T, Ogunbona F. Lack of pharmacokinetic interaction between chloroquine and imipramine. Ther Drug Monit. 1993;15(1):
227 740. Koch K, O'Connor-Semmes R, Davis I, Yin Y. Stereoselective pharmacokinetics of chlorpheniramine and the effect of ranitidine. J Pharm Sci. 1998;87(9): Peets E, Jackson M, Symchowicz S. Metabolism of chlorpheniramine maleate in man. J Pharmacol Exp Ther. 1972;180(2): Neuvonen P, Kärkkäinen S, Lehtovaara R. Pharmacokinetics of chlorpropamide in epileptic patients: Effects of enzyme induction and urine ph on chlorpropamide elimination. Eur J Clin Pharmacol. 1987;32(3): Dieterle W, Wagner J, Faigle J. Binding of chlorthalidone (hygroton ) to blood components in man. Eur J Clin Pharmacol. 1976;10(1): Fleuren H, Thien T, Verwey-van Wissen C, van Rossum J. Absolute bioavailability of chlorthalidone in man: A cross-over study after intravenous and oral administration. Eur J Clin Pharmacol. 1979;15(1): Mulley B, Parr G, Rye R. Pharmacokinetics of chlorthalidone. Dependence of biological half life on blood carbonic anhydrase levels. Eur J Clin Pharmacol. 1980;17(3): Riess W, Dubach U, Burckhardt D, Theobald W, Vuillard P, Zimmerli M. Pharmacokinetic studies with chlorthalidone (hygroton) in man. Eur J Clin Pharmacol. 1977;12(5): Gisclon L, Boyd R, Williams R, Giacomini K. The effect of probenecid on the renal elimination of cimetidine. Clin Pharmacol Ther. 1989;45(4): Taylor D, Cresswell P, Bartlett D. The metabolism and elimination of cimetidine, a histamine h2-receptor antagonist, in the rat, dog, and man. Drug Metab Dispos. 1978;6(1): Joos B, Ledergerber B, Flepp M, Bettex J, Lüthy R, Siegenthaler W. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother. 1985;27(3): Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother. 2000;44(10): Allard S, Kinzig M, Boivin G, Sörgel F, LeBel M. Intravenous ciprofloxacin disposition in obesity. Clin Pharmacol Ther. 1993;54(4): Borner K, Höffken G, Lode H, Koeppe P, Prinzing C, Glatzel P, et al. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986;5(2): Zlotos G, Oehlmann M, Nickel P, Holzgrabe U. Determination of protein binding of gyrase inhibitors by means of continuous ultrafiltration. J Pharm Biomed Anal. 1998;18(4): Waters N, Jones R, Williams G, Sohal B. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J Pharm Sci. 2008;97(10): Holford N. Clinical pharmacokinetics. Drug data handbook. 3rd ed. Auckland, N.Z: Adis International; Herrlin K, Yasui-Furukori N, Tybring G, Widén J, Gustafsson LL, Bertilsson L. Metabolism of citalopram enantiomers in CYP2C19/CYP2D6 phenotyped panels of healthy swedes. Br J Clin Pharmacol. 2003;56(4): Sidhu J, Priskorn M, Poulsen M, Segonzac A, Grollier G, Larsen F. Steady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humans. Chirality. 1997;9(7): Joffe P, Larsen F, Pedersen V, Ring-Larsen H, Aaes-Jørgensen T, Sidhu J. Single-dose pharmacokinetics of citalopram in patients with moderate renal insufficiency or hepatic cirrhosis compared with healthy subjects. Eur J Clin Pharmacol. 1998;54(3): Spigset O, Hägg S, Stegmayr B, Dahlqvist R. Citalopram pharmacokinetics in patients with chronic renal failure and the effect of haemodialysis. Eur J Clin Pharmacol. 2000;56(9-10):
228 760. Fredricson OK. Kinetics of citalopram in man; plasma levels in patients. Prog Neuro-Psychopharmacol Biol Psychiatry. 1982;6(3): Randinitis EJ, Koup JR, Rausch G, Abel R, Bron NJ, Hounslow NJ, et al. Clinafloxacin pharmacokinetics in subjects with various degrees of renal function. Antimicrob Agents Chemother. 2001;45(9): Wise R, Ashby J, Andrews J. In vitro activity of pd 127,391, an enhanced-spectrum quinolone. Antimicrob Agents Chemother. 1988;32(8): Bron N, Dorr M, Mant T, Webb C, Vassos A. The tolerance and pharmacokinetics of clinafloxacin (ci-960) in healthy subjects. J Antimicrob Chemoth. 1996;38(6): May D, Porter J, Uetrecht J, Wilkinson G, Branch R. The contribution of n- hydroxylation and acetylation to dapsone pharmacokinetics in normal subjects. Clin Pharmacol Ther. 1990;48(6): Rudorfer M, Lane E, Chang W, Zhang M, Potter W. Desipramine pharmacokinetics in chinese and caucasian volunteers. Br J Clin Pharmacol. 1984;17(4): Ciraulo D, Barnhill J, Jaffe J. Clinical pharmacokinetics of imipramine and desipramine in alcoholics and normal volunteers. Clin Pharmacol Ther. 1988;43(5): Spina E, Avenoso A, Campo G, Caputi A, Perucca E. The effect of carbamazepine on the 2-hydroxylation of desipramine. Psychopharmacology. 1995;117(4): Spina E, Avenoso A, Campo G, Caputi A, Perucca E. Phenobarbital induces the 2- hydroxylation of desipramine. Ther Drug Monit. 1996;18(1): Earhart R, Tutsch K, Koeller J, Rodriguez R, Robins H, Vogel C, et al. Pharmacokinetics of (+)-1, 2-di (3, 5-dioxopiperazin-1-yl) propane intravenous infusions in adult cancer patients. Cancer Res. 1982;42(12): Brier M, Gaylor S, McGovren J, Glue P, Fang A, Aronoff G. Pharmacokinetics of dexrazoxane in subjects with impaired kidney function. J Clin Pharmacol. 2011;51(5): Granneman G, Snyder K, Shu V. Difloxacin metabolism and pharmacokinetics in humans after single oral doses. Antimicrob Agents Chemother. 1986;30(5): Zlotos G, Bücker A, Kinzig-Schippers M, Sorgel F, Holzgrabe U. Plasma protein binding of gyrase inhibitors. J Pharm Sci. 1998;87(2): Hedman A, Angelin B, Arvidsson A, Dahlqvist R. No effect of probenecid on the renal and biliary clearances of digoxin in man. Br J Clin Pharmacol. 1991;32(1): Belz G, Doering W, Munkes R, Matthews J. Interaction between digoxin and calcium antagonists and antiarrhythmic drugs. Clin Pharmacol Ther. 1983;33(4): Koytchev R, Alken R, Mayer O. Effect of diprafenone on the pharmacokinetics of digoxin. Eur J Clin Pharmacol. 1996;50(1-2): Lukas D, De Martino A. Binding of digitoxin and some related cardenolides to human plasma proteins. J Clin Invest. 1969;48(6): Larsen F, Priskorn M, Overø K. Lack of citalopram effect on oral digoxin pharmacokinetics. J Clin Pharmacol. 2001;41(3): Shoaf S, Ohzone Y, Ninomiya S, Furukawa M, Bricmont P, Kashiyama E, et al. In vitro p-glycoprotein interactions and steady-state pharmacokinetic interactions between tolvaptan and digoxin in healthy subjects. J Clin Pharmacol. 2011;51(5): Tsutsumi K, Kotegawa T, Kuranari M, Otani Y, Morimoto T, Matsuki S, et al. The effect of erythromycin and clarithromycin on the pharmacokinetics of intravenous digoxin in healthy volunteers. J Clin Pharmacol. 2002;42(10): Kubitza D, Becka M, Roth A, Mueck W. Absence of clinically relevant interactions between rivaroxaban--an oral, direct factor xa inhibitor--and digoxin or atorvastatin in healthy subjects. J Int Med Res,. 2012;40(5): Hinderling P, Hartmann D. Pharmacokinetics of digoxin and main metabolites/derivatives in healthy humans. Ther Drug Monit. 1991;13(5): Boyd R, Chin S, Don-Pedro O, Verotta D, Sheiner L, Williams R, et al. The pharmacokinetics and pharmacodynamics of diltiazem and its metabolites in healthy adults after a single oral dose. Clin Pharmacol Ther. 1989;46(4): Hung J, Hackett P, Gordon S, Ilett K. Pharmacokinetics of diltiazem in patients with unstable angina pectoris. Clin Pharmacol Ther. 1988;43(4):
229 784. Kwong T, Sparks J, Sparks C. Lipoprotein and protein binding of the calcium channel blocker diltiazem. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine [N.Y.]; p Abel S, Nichols D, Brearley C, Eve M. Effect of cimetidine and ranitidine on pharmacokinetics and pharmacodynamics of a single dose of dofetilide. Br J Clin Pharmacol. 2000;49(1): Mounsey J, DiMarco J. Cardiovascular drugs. Dofetilide. Circulation. 2000;102(21): Mahmood I. Interspecies scaling: Role of protein binding in the prediction of clearance from animals to humans. J Clin Pharmacol. 2000;40(12): Virtanen R, Iisalo E, Irjala K. Protein binding of doxepin and desmethyldoxepin. Acta Pharmacol Toxicol (Copenh). 1982;51(2): Virtanen R, Scheinin M, Iisalo E. Single dose pharmacokinetics of doxepin in healthy volunteers. Acta Pharmacol Toxicol (Copenh). 1980;47(5): Yan J, Hubbard J, McKay G, Korchinski E, Midha K. Absolute bioavailability and stereoselective pharmacokinetics of doxepin. Xenobiotica. 2002;32(7): Bury R, Becker G, Kincaid-Smith P, Moulds R, Whitworth J. Elimination of enoxacin in renal disease. Clin Pharmacol Ther. 1987;41(4): Chang T, Black A, Dunky A, Wolf R, Sedman A, Latts J, et al. Pharmacokinetics of intravenous and oral enoxacin in healthy volunteers. J Antimicrob Chemoth. 1988;21(suppl B): Somogyi A, Bochner F. The absorption and disposition of enoxacin in healthy subjects. J Clin Pharmacol. 1988;28(8): Zhai S, Wei X, Parker B, Kunze K, Vestal R. Relation between plasma and saliva concentrations of enoxacin, ciprofloxacin, and theophylline. Ther Drug Monit. 1996;18(6): Okerholm R, Chan K, Lang J, Thompson G, Ruberg S. Biotransformation and pharmacokinetic overview of enoximone and its sulfoxide metabolite. Am J Cardiol. 1987;60(5): Alken R, Belz G, Haegele K, Meinicke T, Schechter P. Kinetics of fenoximone, a new cardiotonic, in healthy subjects. Clin Pharmacol Ther. 1984;36(2): Hook R, Boxenbaum H, Thompson G, Okerholm R. Human serum and plasma protein binding of enoximone and its sulfoxide metabolite. J Pharm Sci. 1988;77(12): Morita S, Sawai Y, Heeg J, Koike Y. Pharmacokinetics of enoximone after various intravenous administrations to healthy volunteers. J Pharm Sci. 1995;84(2): Borgå O, Andersson K, Edholm L, Fagerström P, Lunell E, Persson C. Enprofylline kinetics in healthy subjects after single doses. Clin Pharmacol Ther. 1983;34(6): Borgå O, Larsson R, Lunell E. Effects of probenecid on enprofylline kinetics in man. Eur J Clin Pharmacol. 1986;30(2): Lunell E, Borgå O, Larsson R. Pharmacokinetics of enprofylline in patients with impaired renal function after a single intravenous dose. Eur J Clin Pharmacol. 1984;26(1): Tegnér K, Borgå O, Svensson I. Protein binding of enprofylline. Eur J Clin Pharmacol. 1983;25(5): Russell T, Stoltz M, Weir S. Pharmacokinetics, pharmacodynamics, and tolerance of single-and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharmacol Ther. 1998;64(6): Yasui-Furukori N, Uno T, Sugawara K, Tateishi T. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin Pharmacol Ther. 2005;77(1): Shiba K, Saito A, Shimada J, Hori S, Kaji M, Miyahara T, et al. Renal handling of fleroxacin in rabbits, dogs, and humans. Antimicrob Agents Chemother. 1990;34(1): Stuck A, Frey F, Heizmann P, Brandt R, Weidekamm E. Pharmacokinetics and metabolism of intravenous and oral fleroxacin in subjects with normal and impaired renal 229
230 function and in patients on continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1989;33(3): Landersdorfer CB, Kirkpatrick CM, Kinzig M, Bulitta JB, Holzgrabe U, Sörgel F. Inhibition of flucloxacillin tubular renal secretion by piperacillin. Br J Clin Pharmacol. 2008;66(5): Sutherland R, Croydon E, Rolinson G. Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin. Br Med J. 1970;4(5733): Humphrey M, Jevons S, Tarbit M. Pharmacokinetic evaluation of uk-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother. 1985;28(5): Gross A, McLachlan A, Minns I, Beal J, Tett S. Simultaneous administration of a cocktail of markers to measure renal drug elimination pathways: Absence of a pharmacokinetic interaction between fluconazole and sinistrin, p-aminohippuric acid and pindolol. Br J Clin Pharmacol. 2001;51(6): Sobue S, Tan K, Layton G, Leclerc V, Weil A. The effects of renal impairment on the pharmacokinetics and safety of fosfluconazole and fluconazole following a single intravenous bolus injection of fosfluconazole. Br J Clin Pharmacol. 2004;57(6): Buchan P, Keywood C, Wade A, Ward C. Clinical pharmacokinetics of frovatriptan. Headache. 2002;42(suppl 2):S54-S Vree T, van den Biggelaar-Martea M, Verwey-van Wissen C. Probenecid inhibits the renal clearance of frusemide and its acyl glucuronide. Br J Clin Pharmacol. 1995;39(6): Sudoh T, Fujimura A, Shiga T, Sasaki M, Harada K, Tateishi T, et al. Renal clearance of lomefloxacin is decreased by furosemide. Eur J Clin Pharmacol. 1994;46(3): Chennavasin P, Seiwell R, Brater D, Liang W. Pharmacodynamic analysis of the furosemide-probenecid interaction in man. Kidney Int. 1979;16(2): Ekblom M, Hammarlund-Udenaes M, Lundqvist T, Sjöberg P. Potential use of microdialysis in pharmacokinetics: A protein binding study. Pharm Res. 1992;9(1): Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg. 2000;91(1): Hooper W, Kavanagh M, Herkes G, Eadie M. Lack of a pharmacokinetic interaction between phenobarbitone and gabapentin. Br J Clin Pharmacol. 1991;31(2): Blum R, Comstock T, Sica D, Schultz R, Keller E, Reetze P, et al. Pharmacokinetics of gabapentin in subjects with various degrees of renal function. Clin Pharmacol Ther. 1994;56(2): Urban T, Brown C, Castro R, Shah N, Mercer R, Huang Y, et al. Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther. 2008;83(3): Radulovic L, Türck D, von Hodenberg A, Vollmer K, McNally W, DeHart P, et al. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1995;23(4): Boyd R, Türck D, Abel R, Sedman A, Bockbrader H. Effects of age and gender on single-dose pharmacokinetics of gabapentin. Epilepsia. 1999;40(4): Bickel U, Thomsen T, Weber W, Fischer J, Bachus R, Nitz M, et al. Pharmacokinetics of galanthamine in humans and corresponding cholinesterase inhibition. Clin Pharmacol Ther. 1991;50(4): Tariot P. Current status and new developments with galantamine in the treatment of alzheimer's disease. Expert Opin Pharmacother. 2001;2(12): Zhao Q, Brett M, Van Osselaer N, Huang F, Raoult A, Van Peer A, et al. Galantamine pharmacokinetics, safety, and tolerability profiles are similar in healthy caucasian and japanese subjects. J Clin Pharmacol. 2002;42(9): Zhao Q, Iyer G, Verhaeghe T, Truyen L. Pharmacokinetics and safety of galantamine in subjects with hepatic impairment and healthy volunteers. J Clin Pharmacol. 2002;42(4):
231 827. Gajjar D, Bello A, Ge Z, Christopher L, Grasela D. Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects. Antimicrob Agents Chemother. 2003;47(7): Van Wart S, Phillips L, Ludwig EA, Russo R, Gajjar DA, Bello A, et al. Population pharmacokinetics and pharmacodynamics of garenoxacin in patients with communityacquired respiratory tract infections. Antimicrob Agents Chemother. 2004;48(12): Nakashima M, Uematsu T, Kosuge K, Kusajima H, Ooie T, Masuda Y, et al. Single-and multiple-dose pharmacokinetics of am-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob Agents Chemother. 1995;39(12): Singh S, Mehta J. Measurement of drug-protein binding by immobilized human serum albumin-hplc and comparison with ultrafiltration. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;834(1-2): Zhang X, Overholser B, Kays M, Sowinski K. Gatifloxacin pharmacokinetics in healthy men and women. J Clin Pharmacol. 2006;46(10): Swaisland H, Laight A, Stafford L, Jones H, Morris C, Dane A, et al. Pharmacokinetics and tolerability of the orally active selective epidermal growth factor receptor tyrosine kinase inhibitor zd1839 in healthy volunteers. Clin Pharmacokinet. 2001;40(4): Li J, Brahmer J, Messersmith W, Hidalgo M, Baker S. Binding of gefitinib, an inhibitor of epidermal growth factor receptor-tyrosine kinase, to plasma proteins and blood cells: In vitro and in cancer patients. Invest New Drugs. 2006;24(4): McKillop D, Hutchison M, Partridge E, Bushby N, Cooper C, Clarkson-Jones J, et al. Metabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and man. Xenobiotica. 2004;34(10): Allen A, Bygate E, Oliver S, Johnson M, Ward C, Cheon A, et al. Pharmacokinetics and tolerability of gemifloxacin (sb ) after administration of single oral doses to healthy volunteers. Antimicrob Agents Chemother. 2000;44(6): Allen A, Bygate E, Vousden M, Oliver S, Johnson M, Ward C, et al. Multiple-dose pharmacokinetics and tolerability of gemifloxacin administered orally to healthy volunteers. Antimicrob Agents Chemother. 2001;45(2): Islinger F, Bouw R, Stahl M, Lackner E, Zeleny P, Brunner M, et al. Concentrations of gemifloxacin at the target site in healthy volunteers after a single oral dose. Antimicrob Agents Chemother. 2004;48(11): Landersdorfer CB, Kirkpatrick CM, Kinzig M, Bulitta JB, Holzgrabe U, Drusano GL, et al. Competitive inhibition of renal tubular secretion of gemifloxacin by probenecid. Antimicrob Agents Chemother. 2009;53(9): Gee T, Andrews J, Ashby J, Marshall G, Wise R. Pharmacokinetics and tissue penetration of gemifloxacin following a single oral dose. J Antimicrob Chemoth. 2001;47(4): Efthymiopoulos C, Bramer S, Maroli A. Pharmacokinetics of grepafloxacin after oral administration of single and repeat doses in healthy young males. Clin Pharmacokinet. 1997;33(1): Borgå O, Azarnoff D, Forshell G, Sjöqvist F. Plasma protein binding of tricyclic anti-depressants in man. Biochem Pharmacol. 1969;18(9): Brinkschulte M, Breyer-Pfaff U. Binding of tricyclic antidepressants and perazine to human plasma. Methodology and findings in normals. Naunyn-Schmiedeberg's Arch Pharmacol. 1979;308(1): Nyberg G, Mårtensson E. Determination of free fractions of tricyclic antidepressants. Naunyn-Schmiedeberg's Arch Pharmacol. 1984;327(3): Sutfin T, DeVane C, Jusko W. The analysis and disposition of imipramine and its active metabolites in man. Psychopharmacology. 1984;82(4): Morsing P, Adler G, Brandt-Eliasson U, Karp L, Ohlson K, Renberg L, et al. Mechanistic differences of various at1-receptor blockers in isolated vessels of different origin. Hypertension. 1999;33(6): Marino M, Vachharajani N. Pharmacokinetics of irbesartan are not altered in special populations. J Cardiovasc Pharmacol. 2002;40(1):
232 847. Vachharajani N, Shyu W, Chando T, Everett D, Greene D, Barbhaiya R. Oral bioavailability and disposition characteristics of irbesartan, an angiotensin antagonist, in healthy volunteers. J Clin Pharmacol. 1998;38(8): Esquivel M, Ogilvie R, East D, Shaw Jr D, Heathcote J. Pharmacokinetic disposition of isoxicam in hepatic cirrhosis. Clin Invest Med. 1987;10(5): Bury R, Whitworth J, Saines D, Kincaid-Smith P, Moulds R. Effect of impairment of renal function on the accumulation and disposition of isoxicam. Eur J Clin Pharmacol. 1985;28(5): Edwards I, Ferry D, Campbell A. Factors affecting the kinetics of two benzothiazine non-steroidal anti-inflammatory medicines, piroxicam and isoxicam. Eur J Clin Pharmacol. 1985;28(6): Jolliet P, Simon N, Brée F, Urien S, Pagliara A, Carrupt P, et al. Blood-to-brain transfer of various oxicams: Effects of plasma binding on their brain delivery. Pharm Res. 1997;14(5): Boffito M, Back D, Blaschke T, Rowland M, Bertz R, Gerber J, et al. Protein binding in antiretroviral therapies. AIDS Res Hum Retroviruses. 2003;19(9): Johnson M, Verpooten G, Daniel M, Plumb R, Moss J, Van Caesbroeck D, et al. Single dose pharmacokinetics of lamivudine in subjects with impaired renal function and the effect of haemodialysis. Br J Clin Pharmacol. 1998;46(1): Johnson M, Moore K, Yuen G, Bye A, Pakes G. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36(1): Votano J, Parham M, Hall L, Hall L, Kier L, Oloff S, et al. QSAR modeling of human serum protein binding with several modeling techniques utilizing structureinformation representation. J Med Chem. 2006;49(24): Wootton R, Soul-Lawton J, Rolan P, Sheung C, Cooper J, Posner J. Comparison of the pharmacokinetics of lamotrigine in patients with chronic renal failure and healthy volunteers. Br J Clin Pharmacol. 1997;43(1): Yuen A, Land G, Weatherley B, Peck A. Sodium valproate acutely inhibits lamotrigine metabolism. Br J Clin Pharmacol. 1992;33(5): Rambeck B, Wolf P. Lamotrigine clinical pharmacokinetics. Clin Pharmacokinet. 1993;25(6): Cohen A, Land G, Breimer D, Yuen W, Winton C, Peck A. Lamotrigine, a new anticonvulsant: Pharmacokinetics in normal humans. Clin Pharmacol Ther. 1987;42(5): Ebert U, Thong N, Oertel R, Kirch W. Effects of rifampicin and cimetidine on pharmacokinetics and pharmacodynamics of lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2000;56(4): Scott L, Lyseng-Williamson K. Spotlight on lenalidomide in relapsed or refractory multiple myeloma. BioDrugs. 2011;25(5): Chen N, Kasserra C, Reyes J, Liu L, Lau H. Single-dose pharmacokinetics of lenalidomide in healthy volunteers: Dose proportionality, food effect, and racial sensitivity. Cancer Chemother Pharmacol. 2012;70(5): Chen N, Lau H, Kong L, Kumar G, Zeldis J, Knight R, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47(12): Radtke R. Pharmacokinetics of levetiracetam. Epilepsia. 2001;42(suppl 4): Patsalos P. Pharmacokinetic profile of levetiracetam: Toward ideal characteristics. Pharmacol Ther. 2000;85(2): Neckel U, Joukhadar C, Frossard M, Jäger W, Müller M, Mayer BX. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by highperformance liquid chromatography. Anal Chim Acta. 2002;463(2): Chien S, Rogge M, Gisclon L, Curtin C, Wong F, Natarajan J, et al. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41(10): Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O'Grady M, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47(9):
233 869. Buerger C, Plock N, Dehghanyar P, Joukhadar C, Kloft C. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob Agents Chemother. 2006;50(7): Blum R, Schultz R, Schentag J. Pharmacokinetics of lomefloxacin in renally compromised patients. Antimicrob Agents Chemother. 1990;34(12): Okezaki E, Terasaki T, Nakamura M, Nagata O, Kato H, Tsuji A. Serum protein binding of lomefloxacin, a new antimicrobial agent, and its related quinolones. J Pharm Sci. 1989;78(6): Sudoh T, Fujimura A, Harada K, Sunaga K, Ohmori M, Sakamoto K. Effect of ranitidine on renal clearance of lomefloxacin. Eur J Clin Pharmacol. 1996;51(1): Turnidge J. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs. 1999;58(3): Wise R, Andrews J, Ashby J, Matthews R. In vitro activity of lomefloxacin, a new quinolone antimicrobial agent, in comparison with those of other agents. Antimicrob Agents Chemother. 1988;32(5): Abernethy D, Greenblatt D, Divoll M, Ameer B, Shader R. Differential effect of cimetidine on drug oxidation (antipyrine and diazepam) vs. Conjugation (acetaminophen and lorazepam): Prevention of acetaminophen toxicity by cimetidine. J Pharmacol Exp Ther. 1983;224(3): Herman RJ, Van Pham JD, Szakacs CB. Disposition of lorazepam in human beings: Enterohepatic recirculation and first-pass effect. Clin Pharmacol Ther. 1989;46(1): Samara EE, Granneman RG, Witt GF, Cavanaugh JH. Effect of valproate on the pharmacokinetics and pharmacodynamics of lorazepam. J Clin Pharmacol. 1997;37(5): Abel S, Russell D, Whitlock LA, Ridgway CE, Nedderman AN, Walker DK. Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br J Clin Pharmacol. 2008;65(suppl 1): Walker D, Abel S, Comby P, Muirhead G, Nedderman A, Smith D. Species differences in the disposition of the ccr5 antagonist, uk-427,857, a new potential treatment for HIV. Drug Metab Dispos. 2005;33(4): De La Torre R, Farre M, Ortuno J, Mas M, Brenneisen R, Roset P, et al. Non linear pharmacokinetics of MDMA ( ecstasy ) in humans. Br J Clin Pharmacol. 2000;49(2): de la Torre R, Farré M, Roset P, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26(2): De Letter E, De Paepe P, Clauwaert K, Belpaire F, Lambert W, Van Bocxlaer J, et al. Is vitreous humour useful for the interpretation of 3, 4- methylenedioxymethamphetamine (MDMA) blood levels? Experimental approach with rabbits. Int J Legal Med. 2000;114(1-2): Johansson LC, Andersson M, Fager G, Gustafsson D, Eriksson UG. No influence of ethnic origin on the pharmacokinetics and pharmacodynamics of melagatran following oral administration of ximelagatran, a novel oral direct thrombin inhibitor, to healthy male volunteers. Clin Pharmacokinet. 2003;42(5): Eriksson UG, Johansson S, Attman P-O, Mulec H, Frison L, Vager G, et al. Influence of severe renal impairment on the pharmacokinetics and pharmacodynamics of oral ximelagatran and subcutaneous melagatran. Clin Pharmacokinet. 2003;42(8): Eriksson UG, Bredberg U, Hoffmann K-J, Thuresson A, Gabrielsson M, Ericsson H, et al. Absorption, distribution, metabolism, and excretion of ximelagatran, an oral direct thrombin inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2003;31(3): Eriksson UG, Bredberg U, Gislén K, Johansson LC, Frison L, Ahnoff M, et al. Pharmacokinetics and pharmacodynamics of ximelagatran, a novel oral direct thrombin inhibitor, in young healthy male subjects. Eur J Clin Pharmacol. 2003;59(1):
234 887. Freudenthaler S, Meineke I, Schreeb K, Boakye E, Gundert-Remy U, Gleiter C. Influence of urine ph and urinary flow on the renal excretion of memantine. Br J Clin Pharmacol. 1998;46(6): Jarvis B, Figgitt D. Memantine. Drug Aging. 2003;20(6): Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the n- methyl-d-aspartate (nmda) receptor antagonist memantine in man. Neurosci Lett. 1995;195(2): Periclou A, Ventura D, Rao N, Abramowitz W. Pharmacokinetic study of memantine in healthy and renally impaired subjects. Clin Pharmacol Ther. 2006;79(1): Cutler M, Urquhart B, Velenosi T, Schwabedissen H, Dresser G, Leake B, et al. In vitro and in vivo assessment of renal drug transporters in the disposition of mesna and dimesna. J Clin Pharmacol. 2012;52(4): James C, Mant T, Rogers H. Pharmacokinetics of intravenous and oral sodium 2 mercaptoethane sulphonate (mesna) in normal subjects. Br J Clin Pharmacol. 1987;23(5): Shaw I, Graham M. Mesna--a short review. Cancer Treat Rev. 1987;14(2): Pentikäinen P, Neuvonen P, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16(3): Sambol NC, Chiang J, O'Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin dependent diabetes mellitus. J Clin Pharmacol. 1996;36(11): Tucker G, Casey C, Phillips P, Connor H, Ward J, Woods H. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12(2): Abramson F. Methadone plasma protein binding: Alterations in cancer and displacement from alpha 1-acid glycoprotein. Clin Pharmacol Ther. 1982;32(5): Foster DJ, Somogyi AA, Dyer KR, White JM, Bochner F. Steady-state pharmacokinetics of (r)-and (s)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50(5): Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84(4): Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2b6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76(3): Lugo R, Satterfield K, Kern S. Pharmacokinetics of methadone. J Pain Palliat Care Pharmacother. 2005;19(4): Nilsson M-I, Widerlöv E, Meresaar U, Änggård E. Effect of urinary ph on the disposition of methadone in man. Eur J Clin Pharmacol. 1982;22(4): Kirchheiner J, Heesch C, Bauer S, Meisel C, Seringer A, Goldammer M, et al. Impact of the ultrarapid metabolizer genotype of cytochrome P450 2D6 on metoprolol pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2004;76(4): Jack DB, Kendall MJ, Dean S, Laugher SJ, Zaman R, Tenneson ME. The effect of hydralazine on the pharmacokinetics of three different beta adrenoceptor antagonists: Metoprolol, nadolol, and acebutolol. Biopharm Drug Dispos. 1982;3(1): Regårdh CG, Borg KO, Johansson R, Johnsson G, Palmer L. Pharmacokinetic studies on the selectiveβ 1-receptor antagonist metoprolol in man. J Pharmacokinet Biopharm. 1974;2(4): Loft S, Døssing M, Poulsen H, Sonne J, Olesen K-L, Simonsen K, et al. Influence of dose and route of administration on disposition of metronidazole and its major metabolites. Eur J Clin Pharmacol. 1986;30(4): Loft S, Sonne J, Poulsen H, Petersen K, Jørgensen B, Døssing M. Inhibition and induction of metronidazole and antipyrine metabolism. Eur J Clin Pharmacol. 1987;32(1):
235 908. Ralph ED, Clarke JT, Libke RD, Luthy RP, Kirby WM. Pharmacokinetics of metronidazole as determined by bioassay. Antimicrob Agents Chemother. 1974;6(6): Sanvordeker D, Chien Y, Lin T, Lambert H. Binding of metronidazole and its derivatives to plasma proteins: An assessment of drug binding phenomenon. J Pharm Sci. 1975;64(11): Grech-Belanger O, Turgeon J, Gilbert M. Stereoselective disposition of mexiletine in man. Br J Clin Pharmacol. 1986;21(5): Kwok D, Kerr C, McErlane K. Pharmacokinetics of mexiletine enantiomers in healthy human subjects. A study of the in vivo serum protein binding, salivary excretion and red blood cell distribution of the enantiomers. Xenobiotica. 1995;25(10): Mitchell B, Clements J, Pottage A, Prescott L. Mexiletine disposition: Individual variation in response to urine acidification and alkalinisation. Br J Clin Pharmacol. 1983;16(3): Pentikäinen P, Koivula I, Hiltunen H. Effect of rifampicin treatment on the kinetics of mexiletine. Eur J Clin Pharmacol. 1982;23(3): Talbot R, Nimmo J, Julian D, Clark R, Neilson J, Prescott L. Treatment of ventricular arrhythmias with mexiletine (kö 1173). Lancet. 1973;2(7826): Härtter S, Dingemanse J, Baier D, Ziegler G, Hiemke C. The role of cytochrome P450 2D6 in the metabolism of moclobemide. Eur Neuropsychopharmacol. 1996;6(3): Yu K-S, Yim D-S, Cho J-Y, Park SS, Park JY, Lee K-H, et al. Effect of omeprazole on the pharmacokinetics of moclobemide according to the genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;69(4): Fromm MF, Eckhardt K, Li S, Schänzle G, Hofmann U, Mikus G, et al. Loss of analgesic effect of morphine due to coadministration of rifampin. Pain. 1997;72(1): Glare P, Walsh T. Clinical pharmacokinetics of morphine. Ther Drug Monit. 1991;13(1): Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Clin Pharmacokinet. 1993;24(4): Milne R, Nation R, Somogyi A, Bochner F, Griggs W. The influence of renal function on the renal clearance of morphine and its glucuronide metabolites in intensivecare patients. Br J Clin Pharmacol. 1992;34(1): Bolton WK, Scheld WM, Spyker DA, Overby TL, Sande M. Pharmacokinetics of moxalactam in subjects with various degrees of renal dysfunction. Antimicrob Agents Chemother. 1980;18(6): DeSante KA, Israel KS, Brier GL, Wolny JD, Hatcher BL. Effect of probenecid on the pharmacokinetics of moxalactam. Antimicrob Agents Chemother. 1982;21(1): Kemmerich B, Lode H, Belmega G, Jendroschek T, Borner K, Koeppe P. Comparative pharmacokinetics of cefoperazone, cefotaxime, and moxalactam. Antimicrob Agents Chemother. 1983;23(3): Peterson L, Bean B, Fasching C, Korchik W, Gerding D. Pharmacokinetics, protein binding, and predicted extravascular distribution of moxalactam in normal and renal failure subjects. Antimicrob Agents Chemother. 1981;20(3): Scheld W, Spyker D, Donowitz G, Bolton W, Sande M. Moxalactam and cefazolin: Comparative pharmacokinetics in normal subjects. Antimicrob Agents Chemother. 1981;19(4): Srinivasan S, Fu K, Neu H. Pharmacokinetics of moxalactam and cefazolin compared in normal volunteers. Antimicrob Agents Chemother. 1981;19(2): Standiford H, Drusano G, Bustamante C, Rivera G, Forrest A, Tatem B, et al. Imipenem coadministered with cilastatin compared with moxalactam: Integration of serum pharmacokinetics and microbiologic activity following single-dose administration to normal volunteers. Antimicrob Agents Chemother. 1986;29(3): Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42(8):
236 929. Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(suppl 2): Stass H, Kubitza D, Halabi A, Delesen H. Pharmacokinetics of moxifloxacin, a novel 8 methoxy quinolone, in patients with renal dysfunction. Br J Clin Pharmacol. 2002;53(3): Stass H, Kubitza D, Möller JG, Delesen H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br J Clin Pharmacol. 2005;59(5): Zeitlinger M, Sauermann R, Fille M, Hausdorfer J, Leitner I, Müller M. Plasma protein binding of fluoroquinolones affects antimicrobial activity. J Antimicrob Chemoth. 2008;61(3): Waller E, Sharanevych M, Yakatan G. The effect of probenecid on nafcillin disposition. J Clin Pharmacol. 1982;22(10): Lode H, Höffken G, Olschewski P, Sievers B, Kirch A, Borner K, et al. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother. 1987;31(9): Yuk J, Nightingale C, Quintiliani R, Sweeney K. Bioavailability and pharmacokinetics of ofloxacin in healthy volunteers. Antimicrob Agents Chemother. 1991;35(2): Schwocho L, Masonson H. Pharmacokinetics of cs-866, a new angiotensin II receptor blocker, in healthy subjects. J Clin Pharmacol. 2001;41(5): Gardner S, Franks A. Olmesartan medoxomil: The seventh angiotensin receptor antagonist. Ann Pharmacother. 2003;37(1): Chrysant S, Chrysant G. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother. 2004;5(3): He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite ro Clin Pharmacokinet. 1999;37(6): Brennan B, Davies B, Cirrincione-Dall G, Morcos P, Beryozkina A, Chappey C, et al. Safety, tolerability, and pharmacokinetics of intravenous oseltamivir: Single-and multiple-dose phase i studies with healthy volunteers. Antimicrob Agents Chemother. 2012;56(9): Laethem M, Lefebvre R, Belpaire F, Vanhoe H, Bogaert M. Stereoselective pharmacokinetics of oxprenolol and its glucuronides in humans. Clin Pharmacol Ther. 1995;57(4): Green R, Brown J, Calvert R. The disposition of four tetracyclines in normal subjects. Eur J Clin Pharmacol. 1976;10(3): Kunin CM, Dornbush A, Finland M. Distribution and excretion of four tetracycline analogues in normal young men. J Clin Invest. 1959;38(11): Barre J, Houin G, Tillement J. Dose-dependent pharmacokinetic study of pefloxacin, a new antibacterial agent, in humans. J Pharm Sci. 1984;73(10): Montay G, Goueffon Y, Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984;25(4): Frydman A, Le Roux Y, Lefebvre M, Djebbar F, Fourtilllan J, Gaillot J. Pharmacokinetics of pefloxacin after repeated intravenous and oral administration (400 mg bid) in young healthy volunteers. J Antimicrob Chemother. 1986;17(suppl B): Pue M, Pratt S, Fairless A, Fowles S, Laroche J, Georgiou P, et al. Linear pharmacokinetics of penciclovir following administration of single oral doses of famciclovir 125, 250, 500 and 750 mg to healthy volunteers. J Antimicrob Chemoth. 1994;33(1): Vinh D, Aoki F. Famciclovir for the treatment of recurrent genital herpes: A clinical and pharmacological perspective. Expert Opin Pharmacother. 2006;7(16): Takabatake T, Ohta H, Yamamoto Y, Ishida Y, Hara H, Ushiogi Y, et al. Pharmacokinetics of sun 1165, a new antiarrhythmic agent, in renal dysfunction. Eur J Clin Pharmacol. 1991;40(4):
237 950. Shiga T, Hashiguchi M, Urae A, Kasanuki H, Rikihisa T. Effect of cimetidine and probenecid on pilsicainide renal clearance in humans. Clin Pharmacol Ther. 2000;67(3): Kim B, Kim J, Lim K, Kim J, Kim K, Hong J, et al. An open-label, single-dose, parallel-group, dose-increasing study comparing the pharmacokinetics and tolerability of pilsicainide hydrochloride in healthy korean and japanese male subjects. Clin Ther. 2009;31(3): Taylor E, Turner P. The distribution of propranolol, pindolol and atenolol between human erythrocytes and plasma. Br J Clin Pharmacol. 1981;12(4): Hsyu P-H, Giacomini KM. Stereoselective renal clearance of pindolol in humans. J Clin Invest. 1985;76(5): Somogyi A, Bochner F, Sallustio B. Stereoselective inhibition of pindolol renal clearance by cimetidine in humans. Clin Pharmacol Ther. 1992;51(4): Ujhelyi M, Bottorff M, Schur M, Roll K, Zhang H, Stewart J, et al. Aging effects on the organic base transporter and stereoselective renal clearance. Clin Pharmacol Ther. 1997;62(2): Aronoff G, Sloan R, Brier M, Luft F. The effect of piperacillin dose on elimination kinetics in renal impairment. Eur J Clin Pharmacol. 1983;24(4): Kyrklund C, Backman J, Neuvonen M, Neuvonen P. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther. 2003;73(6): Halstenson C, Triscari J, DeVault A, Shapiro B, Keane W, Pan H. Single-dose pharmacokinetics of pravastatin and metabolites in patients with renal impairment. J Clin Pharmacol. 1992;32(2): Singhvi S, Pan H, Morrison R, Willard D. Disposition of pravastatin sodium, a tissue-selective hmg-coa reductase inhibitor, in healthy subjects. Br J Clin Pharmacol. 1990;29(2): Powell L, Axelsen E. Corticosteroids in liver disease: Studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut. 1972;13(9): Ağabeyoğlu I, Bergstrom R, Gillespie W, Wagner J, Kay D. Plasma protein binding of prednisolone in normal volunteers and arthritic patients. Eur J Clin Pharmacol. 1979;16(6): Rose J, Yurchak A, Jusko W. Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm. 1981;9(4): Garg V, Blum R, Wilner K, Jusko W. Effect of the anti-inflammatory agent tenidap on the pharmacokinetics and pharmacodynamics of prednisolone. J Clin Pharmacol. 1992;32(3): Boudinot F, Jusko W. Plasma protein binding interaction of prednisone and prednisolone. J Steroid Biochem. 1984;21(3): Emanuelsson B, Beermann B, Paalzow L. Non-linear elimination and protein binding of probenecid. Eur J Clin Pharmacol. 1987;32(4): Vree T, Van Ewijk-Beneken KE, Wuis E, Hekster Y. Capacity-limited renal glucuronidation of probenecid by humans. A pilot vmax-finding study. Pharm Weekbl Sci. 1992;14(5): Rodvold K, Paloucek F, Jung D, Gallastegui J. Interaction of steady-state procainamide with h2-receptor antagonists cimetidine and ranitidine. Ther Drug Monit. 1987;9(4): Rocci Jr M, Kosoglou T, Ferguson R, Vlasses P. Ranitidine-induced changes in the renal and hepatic clearances of procainamide are correlated. J Pharmacol Exp Ther. 1989;248(3): Lam Y, Boyd R, Chin S, Chang D, Giacomini K. Effect of probenecid on the pharmacokinetics and pharmacodynamics of procainamide. J Clin Pharmacol. 1991;31(5): Sarre S, Van Belle K, Smolders I, Krieken G, Michotte Y. The use of microdialysis for the determination of plasma protein binding of drugs. J Pharm Biomed Anal. 1992;10(10-12):
238 971. Martin D, Shen J, Griener J, Raasch R, Patterson J, Cascio W. Effects of ofloxacin on the pharmacokinetics and pharmacodynamics of procainamide. J Clin Pharmacol. 1996;36(1): Bauer LA, Black DJ, Lill JS, Garrison J, Raisys VA, Hooton TM. Levofloxacin and ciprofloxacin decrease procainamide and n-acetylprocainamide renal clearances. Antimicrob Agents Chemother. 2005;49(4): DiGregorio G, Ruch E. Human whole blood and parotid saliva concentrations of oral and intramuscular promethazine. J Pharm Sci. 1980;69(12): Taylor G, Houston J, Shaffer J, Mawer G. Pharmacokinetics of promethazine and its sulphoxide metabolite after intravenous and oral administration to man. Br J Clin Pharmacol. 1983;15(3): Chan G, Axelson J, Price J, McErlane K, Kerr C. In vitro protein binding of propafenone in normal and uraemic human sera. Eur J Clin Pharmacol. 1989;36(5): Vozeh S, Haefeli W, Ha H, Vlcek J, Follath F. Nonlinear kinetics of propafenone metabolites in healthy man. Eur J Clin Pharmacol. 1990;38(5): Dilger K, Greiner B, Fromm M, Hofmann U, Kroemer H, Eichelbaum M. Consequences of rifampicin treatment on propafenone disposition in extensive and poor metabolizers of CYP2D6. Pharmacogenetics. 1999;9(5): Chen X, Zhong D, Blume H. Stereoselective pharmacokinetics of propafenone and its major metabolites in healthy chinese volunteers. Eur J Pharm Sci. 2000;10(1): Komura H, Iwaki M. Nonlinear pharmacokinetics of propafenone in rats and humans: Application of a substrate depletion assay using hepatocytes for assessment of nonlinearity. Drug Metab Dispos. 2005;33(6): Giles H, Roberts E, Orrego H, Sellers E. Disposition of intravenous propylthiouracil. J Clin Pharmacol. 1981;21(11-12 Pt 1): Kampmann J, Hansen JM. Serum protein binding of propylthiouracil. Br J Clin Pharmacol. 1983;16(5): Lacroix C, Guyonnaud C, Chaou M, Duwoos H, Lafont O. Interaction between allopurinol and pyrazinamide. Eur Respir J. 1988;1(9): Lacroix C, Hoang T, Nouveau J, Guyonnaud C, Laine G, Duwoos H, et al. Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol. 1989;36(4): Woo J, Cheung W, Chan R, Chan H, Cheng A, Chan K. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin Biochem. 1996;29(2): Fremstad D, Bergerud K, Haffner J, Lunde P. Increased plasma binding of quinidine after surgery: A preliminary report. Eur J Clin Pharmacol. 1976;10(6): Greenblatt D, Pfeifer H, Ochs H, Franke K, MacLaughlin D, Smith T, et al. Pharmacokinetics of quinidine in humans after intravenous, intramuscular and oral administration. J Pharmacol Exp Ther. 1977;202(2): Fremstad D, Nilsen O, Storstein L, Amlie J, Jacobsen S. Pharmacokinetics of quinidine related to plasma protein binding in man. Eur J Clin Pharmacol. 1979;15(3): Woo E, Greenblatt D. Pharmacokinetic and clinical implications of quinidine protein binding. J Pharm Sci. 1979;68(4): Rakhit A, Holford N, Guentert T, Maloney K, Riegelman S. Pharmacokinetics of quinidine and three of its metabolites in man. J Pharmacokinet Biopharm. 1984;12(1): Kaukonen K, Olkkola K, Neuvonen P. Itraconazole increases plasma concentrations of quinidine. Clin Pharmacol Ther. 1997;62(5): Damkier P, Hansen L, Brøsen K. Effect of fluvoxamine on the pharmacokinetics of quinidine. Eur J Clin Pharmacol. 1999;55(6): Damkier P, Hansen LL, Brøsen K. Effect of diclofenac, disulfiram, itraconazole, grapefruit juice and erythromycin on the pharmacokinetics of quinidine. Br J Clin Pharmacol. 1999;48(6):
239 993. Iwamoto M, Wenning L, Petry A, Laethem M, De Smet M, Kost J, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83(2): Laufer R, Paz O, Di Marco A, Bonelli F, Monteagudo E, Summa V, et al. Quantitative prediction of human clearance guiding the development of raltegravir (mk- 0518, isentress) and related HIV integrase inhibitors. Drug Metab Dispos. 2009;37(4): Widerlöv E, Termander B, Nilsson M. Effect of urinary ph on the plasma and urinary kinetics of remoxipride in man. Eur J Clin Pharmacol. 1989;37(4): Movin-Osswald G, Hammarlund-Udenaes M. Remoxipride: Pharmacokinetics and effect on plasma prolactin. Br J Clin Pharmacol. 1991;32(3): Movin-Osswald G, Boelaert J, Hammarlund-Udenaes M, Nilsson L. The pharmacokinetics of remoxipride and metabolites in patients with various degrees of renal function. Br J Clin Pharmacol. 1993;35(6): Yisak W, Farde L, von Bahr C, Nilsson L, Fredriksson G, Ogenstad S. Interaction study between remoxipride and biperiden. Psychopharmacology. 1993;111(1): N'soukpoe-Kossi C, St-Louis C, Beauregard M, Subirade M, Carpentier R, Hotchandani S, et al. Resveratrol binding to human serum albumin. J Biomol Struct Dyn. 2006;24(3): Boocock D, Faust G, Patel K, Schinas A, Brown V, Ducharme M, et al. Phase i dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16(6): Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-c/o-conjugated diglucuronides-two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52(5): Kantesaria B, Glue P. Exploring the influence of renal dysfunction on the pharmaco-kinetics of ribavirin after oral and intravenous dosing. Drug Discov Ther. 2014;8(2): Glue P, Schenker S, Gupta S, Clement RP, Zambas D, Salfi M. The single dose pharmacokinetics of ribavirin in subjects with chronic liver disease. Br J Clin Pharmacol. 2000;49(5): Preston SL, Drusano GL, Glue P, Nash J, Gupta S, McNamara P. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother. 1999;43(10): Blaschke T, Skinner M. The clinical pharmacokinetics of rifabutin. Clin Infect Dis. 1996;22(suppl 1):S15-S Polk RE, Brophy DF, Israel DS, Patron R, Sadler BM, Chittick GE, et al. Pharmacokinetic interaction between amprenavir and rifabutin or rifampin in healthy males. Antimicrob Agents Chemother. 2001;45(2): Boman G, Ringberger V. Binding of rifampicin by human plasma proteins. Eur J Clin Pharmacol. 1974;7(5): Peloquin C, Namdar R, Singleton M, Nix D. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest. 1999;115(1): Huang M, Van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen A, et al. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54(3): Mannens G, Huang M, Meuldermans W, Hendrickx J, Woestenborghs R, Heykants J. Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos. 1993;21(6): Snoeck E, Van Peer A, Sack M, Horton M, Mannens G, Woestenborghs R, et al. Influence of age, renal and liver impairment on the pharmacokinetics of risperidone in man. Psychopharmacology. 1995;122(3): Weinz C, Buetehorn U, Daehler H, Kohlsdorfer C, Pleiss U, Sandmann S, et al. Pharmacokinetics of bay an oral, direct factor xa inhibitor--in rats and dogs. Xenobiotica. 2005;35(9):
240 1013. Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor xa inhibitor. Br J Clin Pharmacol. 2010;70(5): Perzborn E, Roehrig S, Straub A, Kubitza D, Mueck W, Laux V. Rivaroxaban: A new oral factor xa inhibitor. Arterioscler Thromb Vasc Biol. 2010;30(3): Lee A, Fagan D, Lamont M, Tucker G, Halldin M, Scott D. Disposition kinetics of ropivacaine in humans. Anesth Analg. 1989;69(6): Jokinen M, Ahonen J, Neuvonen P, Olkkola K. The effect of erythromycin, fluvoxamine, and their combination on the pharmacokinetics of ropivacaine. Anesth Analg. 2000;91(5): Jokinen M, Olkkola K, Ahonen J, Neuvonen P. Effect of rifampin and tobacco smoking on the pharmacokinetics of ropivacaine. Clin Pharmacol Ther. 2001;70(4): Pere P, Salonen M, Jokinen M, Rosenberg P, Neuvonen P, Haasio J. Pharmacokinetics of ropivacaine in uremic and nonuremic patients after axillary brachial plexus block. Anesth Analg. 2003;96(2): Pere P, Ekstrand A, Salonen M, Honkanen E, Sjövall J, Henriksson J, et al. Pharmacokinetics of ropivacaine in patients with chronic renal failure. Br J Anaesth. 2011;106(4): White C. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42(9): Martin P, Warwick M, Dane A, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25(10): Imbimbo B, Broccali G, Cesana M, Crema F, Attardo-Parrinello G. Inter-and intrasubject variabilities in the pharmacokinetics of rufloxacin after single oral administration to healthy volunteers. Antimicrob Agents Chemother. 1991;35(2): Kisicki J, Griess R, Ott C, Cohen G, McCormack R, Troetel W, et al. Multiple-dose pharmacokinetics and safety of rufloxacin in normal volunteers. Antimicrob Agents Chemother. 1992;36(6): Segre G, Cerretani D, Moltoni L, Urso R. Pharmacokinetics of rufloxacin in healthy volunteers. Eur J Clin Pharmacol. 1992;42(1): Ward JK, Dow J, Dallow N, Eynott P, Milleri S, Ventresca GP. Enantiomeric disposition of inhaled, intravenous and oral racemic-salbutamol in man no evidence of enantioselective lung metabolism. Br J Clin Pharmacol. 2000;49(1): Morgan D, Paull J, Richmond B, Wilson-Evered E, Ziccone S. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986;22(5): Shi J, Ripley E, Gehr T, Sica D, Dandekar K, Hinderling P. Pharmacokinetics of sematilide in renal failure. J Clin Pharmacol. 1996;36(2): Hinderling P, Dilea C, Koziol T, Millington G. Comparative kinetics of sematilide in four species. Drug Metab Dispos. 1993;21(4): Herman G, Stevens C, Van Dyck K, Bergman A, Yi B, De Smet M, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: Results from two randomized, double-blind, placebocontrolled studies with single oral doses. Clin Pharmacol Ther. 2005;78(6): Bergman A, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-iv inhibitor: A double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28(1): Bergman A, Cote J, Yi B, Marbury T, Swan S, Smith W, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30(7): Herman G, Stein P, Thornberry N, Wagner J. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: Focus on sitagliptin. Clin Pharmacol Ther. 2007;81(5):
241 1033. Montay G, Bruno R, Vergniol J, Ebmeier M, Le Roux Y, Guimart C, et al. Pharmacokinetics of sparfloxacin in humans after single oral administration at doses of 200, 400, 600, and 800 mg. J Clin Pharmacol. 1994;34(11): Ritz M, Lode H, Fassbender M, Borner K, Koeppe P, Nord C. Multiple-dose pharmacokinetics of sparfloxacin and its influence on fecal flora. Antimicrob Agents Chemother. 1994;38(3): Kamberi M, Kotegawa T, Tsutsumi K, Nakamura K, Nakano S. Sparfloxacin pharmacokinetics in healthy volunteers: The influence of acidification and alkalinization. Eur J Clin Pharmacol. 1998;54(8): Dorr M, Johnson R, Jensen B, Magner D, Marbury T, Talbot G. Pharmacokinetics of sparfloxacin in patients with renal impairment. Clin Ther. 1999;21(7): Sharpstone P. The renal handling of trimethoprim and sulphamethoxazole in man. Postgrad Med J. 1969;45(Suppl): Vree T, Hekster Y, Baars A, Damsma J, Kleijin E. Determination of trimethoprim and sulfamethoxazole (co-trimoxazole) in body fluids of man by means of highperformance liquid chromatography. J Chromatogr. 1978;146(1): Stevens R, Laizure S, Sanders P, Stein D. Multiple-dose pharmacokinetics of 12 milligrams of trimethoprim and 60 milligrams of sulfamethoxazole per kilogram of body weight per day in healthy volunteers. Antimicrob Agents Chemother. 1993;37(3): Vree TB, van der Ven AJ, Koopmans PP, Kolmer EWvE-B, Verwey-van Wissen CP. Pharmacokinetics of sulfamethoxazole with its hydroxy metabolites and n4-acetyl-, n1-glucuronide conjugates in healthy human volunteers. Clin Drug Invest. 1995;9(1): Hu P, Jiang J, Wang H, Pietropaolo K, Chao G, Brown N, et al. Single-dose and multiple-dose pharmacokinetics and safety of telbivudine after oral administration in healthy chinese subjects. J Clin Pharmacol. 2006;46(9): Zhou X-J, Fielman BA, Lloyd DM, Chao GC, Brown NA. Pharmacokinetics of telbivudine in healthy subjects and absence of drug interaction with lamivudine or adefovir dipivoxil. Antimicrob Agents Chemother. 2006;50(7): Zhou X-J, Swan S, Smith WB, Marbury TC, Dubuc-Patrick G, Chao GC, et al. Pharmacokinetics of telbivudine in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 2007;51(12): Granneman G, Carpentier P, Morrison P, Pernet A. Pharmacokinetics of temafloxacin in humans after single oral doses. Antimicrob Agents Chemother. 1991;35(3): Granneman G, Carpentier P, Morrison P, Pernet A. Pharmacokinetics of temafloxacin in humans after multiple oral doses. Antimicrob Agents Chemother. 1992;36(2): Slocombe B, Basker M, Bentley P, Clayton J, Cole M, Comber K, et al. Brl 17421, a novel beta-lactam antibiotic, highly resistant to beta-lactamases, giving high and prolonged serum levels in humans. Antimicrob Agents Chemother. 1981;20(1): Overbosch D, van Gulpen C, Mattie H. Renal clearance of temocillin in volunteers. Drugs. 1985;29(suppl 5): Kearney B, Flaherty J, Shah J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9): Goicoechea M, Best B. Efavirenz/emtricitabine/tenofovir disoproxil fumarate fixeddose combination: First-line therapy for all? Expert Opin Pharmacother. 2007;8(3): Jaffe JM, Colaizzi JL, Poust RI, McDonald Jr RH. Effect of altered urinaryph on tetracycline and doxycycline excretion in humans. J Pharmacokinet Biopharm. 1973;1(4): Hallén B, Guilbaud O, Strömberg S, Lindeke B. Single-dose pharmacokinetics of terodiline, including a stable isotope technique for improvement of statistical evaluations. Biopharm Drug Dispos. 1988;9(3): Hallén B, Gabrielsson J, Nyambati S, Johansson A, Larsson E, Guilbaud O. Concomitant single-dose and multiple-dose pharmacokinetics of terodiline in man, with a note on its enantiomers and major metabolites. Pharmacol Toxicol. 1995;76(3):
242 1053. Webster R, Allan G, Anto-Awuakye K, Harrison A, Kidd T, Leishman D, et al. Pharmacokinetic/pharmacodynamic assessment of the effects of e4031, cisapride, terfenadine and terodiline on monophasic action potential duration in dog. Xenobiotica. 2001;31(8-9): Buss D, Leopold D, Smith A, Routledge P. Determinants of the plasma protein binding of theophylline in health. Br J Clin Pharmacol. 1983;15(4): Birkett D, Dahlqvist R, Miners J, Lelo A, Billing B. Comparison of theophylline and theobromine metabolism in man. Drug Metab Dispos. 1985;13(6): Vanholder R, Van Landschoot N, De Smet R, Schoots A, Ringoir S. Drug protein binding in chronic renal failure: Evaluation of nine drugs. Kidney Int. 1988;33(5): Liu L, Pan X, Liu H-y, Liu X-d, Yang H-w, Xie L, et al. Modulation of pharmacokinetics of theophylline by antofloxacin, a novel 8-amino-fluoroquinolone, in humans. Acta Pharmacol Sin. 2011;32(10): Fourtillan J, Courtois P, Lefebvre M, Girault J. Pharmacokinetics of oral timolol studied by mass fragmentography. Eur J Clin Pharmacol. 1981;19(3): Mäntylä R, Männistö P, Nykänen S, Koponen A, Lamminsivu U. Pharmacokinetic interactions of timolol with vasodilating drugs, food and phenobarbitone in healthy human volunteers. Eur J Clin Pharmacol. 1983;24(2): Wood S, John B, Chasseaud L, Brodie R, Baker J, Faulkner J, et al. Pharmacokinetics and metabolism of 14c-tinidazole in humans. J Antimicrob Chemoth. 1986;17(6): Wood B, Faulkner J, Monro A. The pharmacokinetics, metabolism and tissue distribution of tinidazole. J Antimicrob Chemoth. 1982;10(suppl A): Chaikin P, Alton K, Sampson C, Weintraub H. Pharmacokinetics of tinidazole in male and female subjects. J Clin Pharmacol. 1982;22(11-12): Wagstaff A, Bryson H. Tizanidine. A review of its pharmacology, clinical efficacy and tolerability in the management of spasticity associated with cerebral and spinal disorders. Drugs. 1997;53(3): Shellenberger M, Groves L, Shah J, Novack G. A controlled pharmacokinetic evaluation of tizanidine and baclofen at steady state. Drug Metab Dispos. 1999;27(2): Granfors M, Backman J, Laitila J, Neuvonen P. Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1a2. Clin Pharmacol Ther. 2005;78(4): Lalka D, Meyer M, Duce B, Elvin A. Kinetics of the oral antiarrhythmic lidocaine congener, tocainide. Clin Pharmacol Ther. 1976;19(6): Graffner C, Conradson T, Hofvendahl S, Rydén L. Tocainide kinetics after intravenous and oral administration in healthy subjects and in patients with acute myocardial infarction. Clin Pharmacol Ther. 1980;27(1): Sedman A, Bloedow D, Gal J. Serum binding of tocainide and its enantiomers in human subjects. Res Commun Chem Pathol Pharmacol. 1982;38(1): Braun J, Sörgel F, Engelmaier F, Gluth W, Gessler U. Pharmacokinetics of tocainide in patients with severe renal failure. Eur J Clin Pharmacol. 1985;28(6): McErlane K, Axelson J, Vaughan R, Kerr C, Price J, Igwemezie L, et al. Stereoselective pharmacokinetics of tocainide in human uraemic patients and in healthy subjects. Eur J Clin Pharmacol. 1990;39(4): Mallalieu NL, Lennon S, Guy T, Liu M, Luedin E, Davies BE. Lack of age and gender effects on single-dose pharmacokinetics of tomopenem (ro /cs-023), a novel carbapenem. Br J Clin Pharmacol. 2009;67(4): Mallalieu NL, Lennon S, Liu M, Kirkpatrick C, Robson R, Luedin E, et al. Effect of impaired renal function on the pharmacokinetics of tomopenem (ro /cs-023), a novel carbapenem. Antimicrob Agents Chemother. 2008;52(7): Shibayama T, Matsushita Y, Hirota T, Ikeda T, Kuwahara S. Pharmacokinetics of cs-023 (ro ), a novel parenteral carbapenem, in healthy male caucasian volunteers. Antimicrob Agents Chemother. 2006;50(12):
243 1074. Doose D, Walker S, Gisclon L, Nayak R. Single-dose pharmacokinetics and effect of food on the bioavailability of topiramate, a novel antiepileptic drug. J Clin Pharmacol. 1996;36(10): Perucca E. Pharmacokinetic profile of topiramate in comparison with other new antiepileptic drugs. Epilepsia. 1996;37(suppl 2):S8-S Johannessen S. Pharmacokinetics and interaction profile of topiramate: Review and comparison with other newer antiepileptic drugs. Epilepsia. 1997;38(suppl 1):S18- S Manitpisitkul P, Curtin CR, Shalayda K, Wang S-S, Ford L, Heald DL. Pharmacokinetics of topiramate in patients with renal impairment, end-stage renal disease undergoing hemodialysis, or hepatic impairment. Epilepsy Res. 2014;108(5): Baethke R, Golde G, Gahl G. Sulphamethoxazole/trimethoprim: Pharmacokinetic studies in patients with chronic renal failure. Eur J Clin Pharmacol. 1972;4(4): Andreasen F, Elsborg L, Husted S, Thomsen O. Pharmacokinetics of sulfadiazine and trimethoprim in man. Eur J Clin Pharmacol. 1978;14(1): Wijkström A, Westerlund D. Plasma protein binding of sulphadiazine, sulphamethoxazole and trimethoprim determined by ultrafiltration. J Pharm Biomed Anal. 1983;1(3): Teng R, Harris S, Nix D, Schentag J, Foulds G, Liston T. Pharmacokinetics and safety of trovafloxacin (cp-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemoth. 1995;36(2): Teng R, Liston T, Harris S. Multiple-dose pharmacokinetics and safety of trovafloxacin in healthy volunteers. J Antimicrob Chemoth. 1996;37(5): Vincent J, Venitz J, Teng R, Baris B, Willavize S, Polzer R, et al. Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J Antimicrob Chemoth. 1997;39(suppl 2): Gugler R, Schell A, Eichelbaum M, Fröscher W, Schulz H. Disposition of valproic acid in man. Eur J Clin Pharmacol. 1977;12(2): Cramer J, Mattson R. Valproic acid: In vitro plasma protein binding and interaction with phenytoin. Ther Drug Monit. 1979;1(1): Colussi D, Parisot C, Rossolino M, Brunner L, Lefèvre G. Protein binding in plasma of valsartan, a new angiotensin II receptor antagonist. J Clin Pharmacol. 1997;37(3): Flesch G, Müller P, Lloyd P. Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur J Clin Pharmacol. 1997;52(2): Faessel H, Gibbs M, Clark D, Rohrbacher K, Stolar M, Burstein A. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. J Clin Pharmacol. 2006;46(12): Feng B, Obach R, Burstein A, Clark D, de Morais S, Faessel H. Effect of human renal cationic transporter inhibition on the pharmacokinetics of varenicline, a new therapy for smoking cessation: An in vitro-in vivo study. Clin Pharmacol Ther. 2008;83(4): Faessel H, Obach R, Rollema H, Ravva P, Williams K, Burstein A. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49(12): Rollema H, Shrikhande A, Ward K, Tingley III F, Coe J, O'Neill B, et al. Pre-clinical properties of the α4β2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160(2): Kikkawa H, Maruyama N, Fujimoto Y, Hasunuma T. Single-and multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy japanese adult smokers. J Clin Pharmacol. 2011;51(4): DeVane C. Pharmacokinetics of the newer antidepressants: Clinical relevance. Am J Med. 1994;97(6):S13-S
244 1094. Troy S, Schultz R, Parker V, Chiang S, Blum R. The effect of renal disease on the disposition of venlafaxine. Clin Pharmacol Ther. 1994;56(1): Troy S, Parker V, Hicks D, Boudino F, Chiang S. Pharmacokinetic interaction between multiple-dose venlafaxine and single-dose lithium. J Clin Pharmacol. 1996;36(2): Lessard E, Yessine M, Hamelin B, Gauvin C, Labbé L, O'Hara G, et al. Diphenhydramine alters the disposition of venlafaxine through inhibition of CYP2D6 activity in humans. J Clin Psychopharmacol. 2001;21(2): Lombardo F, Obach R, Shalaeva M, Gao F. Prediction of human volume of distribution values for neutral and basic drugs. 2. Extended data set and leave-class-out statistics. J Med Chem. 2004;47(5): Mikus G, Eichelbaum M, Fischer C, Gumulka S, Klotz U, Kroemer H. Interaction of verapamil and cimetidine: Stereochemical aspects of drug metabolism, drug disposition and drug action. J Pharmacol Exp Ther. 1990;253(3): Lutsar I, Roffey S, Troke P. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis. 2003;37(5): Mikus G, Schöwel V, Drzewinska M, Rengelshausen J, Ding R, Riedel K, et al. Potent cytochrome P450 2C19 genotype-related interaction between voriconazole and the cytochrome P450 3a4 inhibitor ritonavir. Clin Pharmacol Ther. 2006;80(2): Scholz I, Oberwittler H, Riedel K-D, Burhenne J, Weiss J, Haefeli WE, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol. 2009;68(6): Rengelshausen J, Banfield M, Riedel K, Burhenne J, Weiss J, Thomsen T, et al. Opposite effects of short-term and long-term st john's wort intake on voriconazole pharmacokinetics. Clin Pharmacol Ther. 2005;78(1): Roffey S, Cole S, Comby P, Gibson D, Jezequel S, Nedderman A, et al. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos. 2003;31(6): Weiss J, Ten Hoevel M, Burhenne J, Walter-Sack I, Hoffmann M, Rengelshausen J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49(2): Cass L, Brown J, Pickford M, Fayinka S, Newman S, Johansson C, et al. Pharmacoscintigraphic evaluation of lung deposition of inhaled zanamivir in healthy volunteers. Clin Pharmacokinet. 1999;36(1): Weller S, Jones LS, Lou Y, Peppercorn A, Ng-Cashin J. Pharmacokinetics of zanamivir following intravenous administration to subjects with and without renal impairment. Antimicrob Agents Chemother. 2013;57(7): Cass L, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36(1): Daniel M, Barnett J, Pearson B. The low potential for drug interactions with zanamivir. Clin Pharmacokinet. 1999;36(1): Cass L, Efthymiopoulos C, Marsh J, Bye A. Effect of renal impairment on the pharmacokinetics of intravenous zanamivir. Clin Pharmacokinet. 1999;36(1): Singlas E, Pioger J, Taburet A, Colin J, Fillastre J. Zidovudine disposition in patients with severe renal impairment: Influence of hemodialysis. Clin Pharmacol Ther. 1989;46(2): Hedaya M, Elmquist W, Sawchuk R. Probenecid inhibits the metabolic and renal clearances of zidovudine (azt) in human volunteers. Pharm Res. 1990;7(4): Luzier A, Morse G. Intravascular distribution of zidovudine: Role of plasma proteins and whole blood components. Antiviral Res. 1993;21(3): Fernandez C, Gimenez F, Thuillier A, Farinotti R. Stereoselective binding of zopiclone to human plasma proteins. Chirality. 1999;11(2): Fernandez C, Maradeix V, Gimenez F, Thuillier A, Farinotti R. Pharmacokinetics of zopiclone and its enantiomers in caucasian young healthy volunteers. Drug Metab Dispos. 1993;21(6):
245 1115. Gaillot J, Heusse D, Hougton G, Marc AJ, Dreyfus J. Pharmacokinetics and metabolism of zopiclone. Pharmacology. 1983;27(suppl 2): Tornio A, Neuvonen P, Backman J. The CYP2C8 inhibitor gemfibrozil does not increase the plasma concentrations of zopiclone. Eur J Clin Pharmacol. 2006;62(8): Marc-Aurele J, Caille G, Bourgoin J. Comparison of zopiclone pharmacokinetics in patients with impaired renal function and normal subjects. Effect of hemodialysis. Sleep. 1987;10(suppl 1): Busch A, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, et al. Human neurons express the polyspecific cation transporter hoct2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol. 1998;54(2): Hill G, Cihlar T, Oo C, Ho E, Prior K, Wiltshire H, et al. The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion-correlation of in vivo and in vitro studies. Drug Metab Dispos. 2002;30(1): Li M, Anderson G, Phillips B, Kong W, Shen D, Wang J. Interactions of amoxicillin and cefaclor with human renal organic anion and peptide transporters. Drug Metab Dispos. 2006;34(4): Gerk P, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002;302(2): Jedlitschky G, Hoffmann U, Kroemer H. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin Drug Metab Toxicol. 2006;2(3): Uchida Y, Kamiie J, Ohtsuki S, Terasaki T. Multichannel liquid chromatographytandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007;24(12): Ciarimboli G, Schröter R, Neugebauer U, Vollenbröker B, Gabriëls G, Brzica H, et al. Kidney transplantation down-regulates expression of organic cation transporters, which translocate β-blockers and fluoroquinolones. Mol Pharm. 2013;10(6): Leroy A, Humbert G, Fillastre J. Pharmacokinetics of azlocillin in healthy subjects. Scand J Infect Dis Suppl. 1981;29: Bakos E, Evers R, Sinkó E, Váradi A, Borst P, Sarkadi B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol. 2000;57(4): Choi M, Kim H, Han Y, Song I, Shim C. Involvement of mrp2/mrp2 in the species different excretion route of benzylpenicillin between rat and human. Xenobiotica. 2009;39(2): Tahara H, Shono M, Kusuhara H, Kinoshita H, Fuse E, Takadate A, et al. Molecular cloning and functional analyses of OAT1 and OAT3 from cynomolgus monkey kidney. Pharm Res. 2005;22(4): Zelcer N, Huisman M, Reid G, Wielinga P, Breedveld P, Kuil A, et al. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette c2). J Biol Chem. 2003;278(26): Bachmakov I, Werner U, Endress B, Auge D, Fromm M. Characterization of betaadrenoceptor antagonists as substrates and inhibitors of the drug transporter p- glycoprotein. Fundam Clin Pharmacol. 2006;20(3): Kirch W, Rose I, Klingmann I, Pabst J, Ohnhaus E. Interaction of bisoprolol with cimetidine and rifampicin. Eur J Clin Pharmacol. 1986;31(1): Kelety B, Diekert K, Tobien J, Watzke N, Dörner W, Obrdlik P, et al. Transporter assays using solid supported membranes: A novel screening platform for drug discovery. Assay Drug Dev Technol. 2006;4(5): Ueo H, Motohashi H, Katsura T, Inui K. Human organic anion transporter hoat3 is a potent transporter of cephalosporin antibiotics, in comparison with hoat1. Biochem Pharmacol. 2005;70(7):
246 1134. Griffith R, Black H, Brier G, Wolny J. Effect of probenecid on the blood levels and urinary excretion of cefamandole. Antimicrob Agents Chemother. 1977;11(5): Pedersen J, Matsson P, Bergström C, Norinder U, Hoogstraate J, Artursson P. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2). J Med Chem. 2008;51(11): Ci L, Kusuhara H, Adachi M, Schuetz J, Takeuchi K, Sugiyama Y. Involvement of MRP4 (ABCC4) in the luminal efflux of ceftizoxime and cefazolin in the kidney. Mol Pharmacol. 2007;71(6): Russel F, Koenderink J, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29(4): Akanuma S, Uchida Y, Ohtsuki S, Kamiie J, Tachikawa M, Terasaki T, et al. Molecular-weight-dependent, anionic-substrate-preferential transport of β-lactam antibiotics via multidrug resistance-associated protein 4. Drug Metab Pharmacokinet. 2011;26(6): Kato Y, Takahara S, Kato S, Kubo Y, Sai Y, Tamai I, et al. Involvement of multidrug resistance-associated protein 2 (abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics. Drug Metab Dispos. 2008;36(6): Müller F, König J, Glaeser H, Schmidt I, Zolk O, Fromm MF, et al. Molecular mechanism of renal tubular secretion of the antimalarial drug chloroquine. Antimicrob Agents Chemother. 2011;55(7): Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker J, et al. Human breast cancer resistance protein: Interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo (4, 5-b) pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312(1): Cavet M, West M, Simmons N. Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1997;121(8): Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, et al. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007;4(1): Merino G, Alvarez A, Pulido M, Molina A, Schinkel A, Prieto J. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos. 2006;34(4): Meyer zsh, Verstuyft C, Kroemer H, Becquemont L, Kim R. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: Functional characterization, interaction with OCT2 (slc22a2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol. 2010;298(4):F997-F Park MS, Okochi H, Benet LZ. Is ciprofloxacin a substrate of p-glycoprotein? Arch Drug Inf. 2011;4(1): Crivori P, Reinach B, Pezzetta D, Poggesi I. Computational models for identifying potential p-glycoprotein substrates and inhibitors. Mol Pharm. 2006;3(1): Misiak P, Eldon M, Toothaker R, Sedman A. Effects of oral cimetidine or ranitidine on the pharmacokinetics of intravenous enoxacin. J Clin Pharmacol. 1993;33(1): Wijnands W, Vree T, Baars A, van Herwaarden C. Pharmacokinetics of enoxacin and its penetration into bronchial secretions and lung tissue. J Antimicrob Chemoth. 1988;21(suppl B): Kusuhara H, Miura M, Yasui-Furukori N, Yoshida K, Akamine Y, Yokochi M, et al. Effect of coadministration of single and multiple doses of rifampicin on the pharmacokinetics of fexofenadine enantiomers in healthy subjects. Drug Metab Dispos. 2013;41(1): Matsushima S, Maeda K, Inoue K, Ohta K, Yuasa H, Kondo T, et al. The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab Dispos. 2009;37(3):
247 1152. Tahara H, Kusuhara H, Maeda K, Koepsell H, Fuse E, Sugiyama Y. Inhibition of oat3-mediated renal uptake as a mechanism for drug-drug interaction between fexofenadine and probenecid. Drug Metab Dispos. 2006;34(5): de Lange E, Marchand S, van den Berg D, van der Sandt I, de Boer A, Delon A, et al. In vitro and in vivo investigations on fluoroquinolones; effects of the p-glycoprotein efflux transporter on brain distribution of sparfloxacin. Eur J Pharm Sci. 2000;12(2): Wang E-j, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with p glycoprotein. Antimicrob Agents Chemother. 2002;46(1): Hasannejad H, Takeda M, Taki K, Shin H, Babu E, Jutabha P, et al. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308(3): Hasegawa M, Kusuhara H, Adachi M, Schuetz J, Takeuchi K, Sugiyama Y. Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol. 2007;18(1): Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by p-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334(1): Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll R, Verweij J, et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst. 2006;98(23): Ozvegy-Laczka C, Hegedus T, Várady G, Ujhelly O, Schuetz J, Váradi A, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65(6): Jin H, Song B, Kim S, Shim W, Kim D, Chong S, et al. Transport of gemifloxacin, a 4th generation quinolone antibiotic, in the Caco-2 and engineered MDCKII cells, and potential involvement of efflux transporters in the intestinal absorption of the drug. Xenobiotica. 2013;43(4): Vadlapatla RK, Vadlapudi AD, Kwatra D, Pal D, Mitra AK. Differential effect of P-gp and MRP2 on cellular translocation of gemifloxacin. Int J Pharm. 2011;420(1): Lowes S, Simmons NL. Multiple pathways for fluoroquinolone secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 2002;135(5): Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, et al. Active intestinal secretion of new quinolone antimicrobials and the partial contribution of p-glycoprotein. J Pharm Pharmacol. 2001;53(5): Anderson P, Lamba J, Aquilante C, Schuetz E, Fletcher C. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: A pilot study. J Acquir Immune Defic Syndr. 2006;42(4): de Souza J, Benet L, Huang Y, Storpirtis S. Comparison of bidirectional lamivudine and zidovudine transport using MDCK, MDCK-MDR1, and Caco-2 cell monolayers. J Pharm Sci. 2009;98(11): Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J, et al. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008;36(8): Kim H-S, Sunwoo YE, Ryu JY, Kang H-J, Jung H-E, Song I-S, et al. The effect of ABCG2 v12m, q141k and q126x, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007;64(5): Minuesa G, Volk C, Molina-Arcas M, Gorboulev V, Erkizia I, Arndt P, et al. Transport of lamivudine [(-)-beta-l-2', 3'-dideoxy-3'-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J Pharmacol Exp Ther. 2009;329(1): Wang X, Furukawa T, Nitanda T, Okamoto M, Sugimoto Y, Akiyama S, et al. Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol. 2003;63(1): Crowe A, Teoh Y. Limited p-glycoprotein mediated efflux for anti-epileptic drugs. J Drug Target. 2006;14(5):
248 1171. Luna-Tortós C, Fedrowitz M, Löscher W. Several major antiepileptic drugs are substrates for human p-glycoprotein. Neuropharmacology. 2008;55(8): Luna-Tortós C, Fedrowitz M, Löscher W. Evaluation of transport of common antiepileptic drugs by human multidrug resistance-associated proteins (MRP1, 2 and 5) that are overexpressed in pharmacoresistant epilepsy. Neuropharmacology. 2010;58(7): Hofmeister CC, Yang X, Pichiorri F, Chen P, Rozewski DM, Johnson AJ, et al. Phase i trial of lenalidomide and cci-779 in patients with relapsed multiple myeloma: Evidence for lenalidomide cci-779 interaction via p-glycoprotein. J Clin Oncol. 2011;29(25): Kumar G, Surapeneni S, Lau H, Laskin O, Fox L. Interaction of lenalidomide with human drug transporters in vitro. Drug Metab Rev. 2008;40(suppl 3): Darnell M, Karlsson J, Owen A, Hidalgo I, Li J, Zhang W, et al. Investigation of the involvement of p-glycoprotein and multidrug resistance-associated protein 2 in the efflux of ximelagatran and its metabolites by using short hairpin RNA knockdown in Caco-2 cells. Drug Metab Dispos. 2010;38(3): Chen Y, Teranishi K, Li S, Yee SW, Hesselson S, Stryke D, et al. Genetic variants in multidrug and toxic compound extrusion 1, hmate1, alter transport function. Pharmacogenom J. 2009;9(2): Choi M, Jin Q, Jin H, Shim C, Cho D, Shin J, et al. Effects of tetraalkylammonium compounds with different affinities for organic cation transporters on the pharmacokinetics of metformin. Biopharm Drug Dispos. 2007;28(9): Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20(5): Zolk O, Solbach T, König J, Fromm M. Functional characterization of the human organic cation transporter 2 variant p. 270ala> ser. Drug Metab Dispos. 2009;37(6): Crettol S, Digon P, Golay K, Brawand M, Eap C. In vitro p-glycoprotein-mediated transport of (r)-,(s)-,(r, s)-methadone, laam and their main metabolites. Pharmacology. 2007;80(4): Kharasch ED, Hoffer C, Whittington D. The effect of quinidine, used as a probe for the involvement of p-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br J Clin Pharmacol. 2004;57(5): Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS. Role of p-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol. 2005;69(12): Störmer E, Perloff M, von Moltke L, Greenblatt D. Methadone inhibits rhodamine123 transport in Caco-2 cells. Drug Metab Dispos. 2001;29(7): Dudley AJ, Bleasby K, Brown CD. The organic cation transporter OCT2 mediates the uptake of β-adrenoceptor antagonists across the apical membrane of renal LLC-PK1 cell monolayers. Br J Pharmacol. 2000;131(1): Barot M, Gokulgandhi M, Pal D, Mitra A. In vitro moxifloxacin drug interaction with chemotherapeutics: Implications for retinoblastoma management. Exp Eye Res. 2014;118: Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier J-C, Couet W. P- glycoprotein-mediated transport of moxifloxacin in a calu-3 lung epithelial cell model. Antimicrob Agents Chemother. 2009;53(4): Chang C, Bahadduri P, Polli J, Swaan P, Ekins S. Rapid identification of p- glycoprotein substrates and inhibitors. Drug Metab Dispos. 2006;34(12): Kamiyama E, Nakai D, Mikkaichi T, Okudaira N, Okazaki O. Interaction of angiotensin II type 1 receptor blockers with P-gp substrates in Caco-2 cells and hmdr1- expressing membranes. Life Sci. 2010;86(1-2): Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, et al. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II at1-receptor. Drug Metab Dispos. 2007;35(12):
249 1190. Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, et al. Limited brain distribution of [3r, 4r, 5s]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1- carboxylate phosphate (ro ), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (oat3/slc22a8) and multidrug resistance-associated protein 4 (Mrp4/abcc4). Drug Metab Dispos. 2009;37(2): Schrickx J, Fink-Gremmels J. P-glycoprotein-mediated transport of oxytetracycline in the Caco-2 cell model. J Vet Pharmacol Ther. 2007;30(1): Brillault J, De Castro W, Couet W. Relative contributions of active mediated transport and passive diffusion of fluoroquinolones with various lipophilicities in a calu-3 lung epithelial cell model. Antimicrob Agents Chemother. 2010;54(1): Natrillo A, Vapurcuyan A, Cheng Y, Pelis R. Interaction of penciclovir with renal drug transporters (p334). 17th North American Regional ISSX Meeting October 16-20, 2011; Atlanta, GA, US Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hoat1 and hoat3. Pharmacol Res. 2012;65(2): Nakagomi-Hagihara R, Nakai D, Tokui T. Inhibition of human organic anion transporter 3 mediated pravastatin transport by gemfibrozil and the metabolites in humans. Xenobiotica. 2007;37(4): Sasaki M, Suzuki H, Ito K, Abe T, Sugiyama Y. Transcellular transport of organic anions across a double-transfected madin-darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and multidrug resistance-associated protein 2 (MRP2/ABCC2). J Biol Chem. 2002;277(8): Yates C, Chang C, Kearbey J, Yasuda K, Schuetz E, Miller D, et al. Structural determinants of p-glycoprotein-mediated transport of glucocorticoids. Pharm Res. 2003;20(11): Sato T, Masuda S, Yonezawa A, Tanihara Y, Katsura T, Inui K. Transcellular transport of organic cations in double-transfected MDCK cells expressing human organic cation transporters hoct1/hmate1 and hoct2/hmate1. Biochem Pharmacol. 2008;76(7): Somogyi A, McLean A, Heinzow B. Cimetidine-procainamide pharmacokinetic interaction in man: Evidence of competition for tubular secretion of basic drugs. Eur J Clin Pharmacol. 1983;25(3): Ohashi R, Tamai I, Yabuuchi H, Nezu J, Oku A, Sai Y, et al. Na (+)-dependent carnitine transport by organic cation transporter (OCTN2): Its pharmacological and toxicological relevance. J Pharmacol Exp Ther. 1999;291(2): Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, et al. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and ph-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289(2): Moss DM, San Kwan W, Liptrott NJ, Smith DL, Siccardi M, Khoo SH, et al. Raltegravir is a substrate for SLC22A6: A putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob Agents Chemother. 2011;55(2): Breedveld P, Pluim D, Cipriani G, Dahlhaus F, van Eijndhoven M, de Wolf C, et al. The effect of low ph on breast cancer resistance protein (ABCG2)-mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol. 2007;71(1): Zhu H, Wang J, Markowitz J, Donovan J, Gibson B, DeVane C. Risperidone and paliperidone inhibit p-glycoprotein activity in vitro. Neuropsychopharmacology. 2007;32(4): Gnoth M, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In vitro and in vivo p-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338(1): Li J, Volpe D, Wang Y, Zhang W, Bode C, Owen A, et al. Use of transporter knockdown Caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab Dispos. 2011;39(7):
250 1207. Verhulst A, Sayer R, De Broe M, D'Haese P, Brown C. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4): Windass A, Lowes S, Wang Y, Brown C. The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther. 2007;322(3): Hendrickx R, Johansson J, Lohmann C, Jenvert R, Blomgren A, Börjesson L, et al. Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013;56(18): Stein G. Drug interactions with fluoroquinolones. Am J Med. 1991;91(6):S81-S Imaoka T, Kusuhara H, Adachi M, Schuetz J, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71(2): Neumanova Z, Cerveny L, Ceckova M, Staud F. Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS. 2014;28(1): Babu E, Takeda M, Narikawa S, Kobayashi Y, Yamamoto T, Cha S, et al. Human organic anion transporters mediate the transport of tetracycline. Jap J Pharmacol. 2002;88(1): Luna-Tortós C, Rambeck B, Jürgens U, Löscher W. The antiepileptic drug topiramate is a substrate for human p-glycoprotein but not multidrug resistance proteins. Pharm Res. 2009;26(11): Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II at1-receptor, in humans. Drug Metab Dispos. 2006;34(7): Kajiwara M, Masuda S, Watanabe S, Terada T, Katsura T, Inui K. Renal tubular secretion of varenicline by multidrug and toxin extrusion (MATE) transporters. Drug Metab Pharmacokinet. 2012;27(6): Ohashi R, Tamai I, Inano A, Katsura M, Sai Y, Nezu J, et al. Studies on functional sites of organic cation/carnitine transporter OCTN2 (SLC22A5) using a ser467cys mutant protein. J Pharmacol Exp Ther. 2002;302(3): Takeda M, Khamdang S, Narikawa S, Kimura H, Kobayashi Y, Yamamoto T, et al. Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002;300(3): Tsuruoka S, Ioka T, Wakaumi M, Sakamoto K, Ookami H, Fujimura A. Severe arrhythmia as a result of the interaction of cetirizine and pilsicainide in a patient with renal insufficiency: First case presentation showing competition for excretion via renal multidrug resistance protein 1 and organic cation transporter 2. Clin Pharmacol Ther. 2006;79(4): Ho P, Ghose K, Saville D, Wanwimolruk S. Effect of grapefruit juice on pharmacokinetics and pharmacodynamics of verapamil enantiomers in healthy volunteers. Eur J Clin Pharmacol. 2000;56(9-10): Mooy J, Schols M, Muytjens A, Rahn K. Pharmacokinetics of verapamil in patients with renal failure. Eur J Clin Pharmacol. 1985;28(4):
251 Chapter 8. Appendices 8.1 Appendix to Chapter 1 251
252 Table 8.1 Expression of SLC Family Drug Transporters in the Human and Rodent Kidney. Summary presented in Table 1.2 in the main text. Human Rodent Transporter Kidney Abundance Kidney Localisation Cell Localisation Transporter Kidney Abundance Kidney Localisation Cell Localisation (Gene) (Method; Region) (Method) (Method) (Gene) (Method; Region) (Method) (Method) hoct1 (SLC22A1) + (RT-PCR; NS) (98) + (MA; NS) (538) - (RT-PCR; NS) (437) + (RT-PCR; NS) (539) PT, DT (IHC) (267) AP (IHC) (267) roct1 (Slc22a1) 5.20 fmol/ µg (MS; K) (200) c +++ (BDA; NS) (541) + (BDA; NS) (542) +++ (MA; NS) (538) S2, S3>S1 (ISH) (544) S1, S2>S3 (IHC) (544) C>OS (IHC) (545) BL (IHC, WB) (544) + (NS; NS) (267) + (BDA; NS) (543) - (RT-PCR; C) (540) + (RT-PCR) (241) moct1 (Slc22a1) +++ (BDA; NS) (546) hoct2 (SLC22A2) ++ (RT-PCR; NS) (98) +++ (MA; NS) (538) ++ (RT-PCR; NS) (437) PT (IHC) (267) PT (IHC) (540) DT (ISH, IHC) (548) a BL (IHC) (267) BL (IHC) (540) AP (IHC) (548) roct2 (Slc22a2) +++ (BDA; NS) (541) + (BDA; NS) (542) +++ (MA; NS) (538) +++ (BDA; NS) (543) OS>C (ISH) (544) S2, S3>S1 (IHC) (544) OS>C (IHC) (545) BL (IHC, WB) (544) BL (WB) (545) ++ (RT-PCR; NS) (539) ++ (RT-PCR; C) (540) + (RT-PCR; C) (547) moct2 (Slc22a2) +++ (BDA; NS; Male) (546) ++ (BDA; NS; Female) (546) hoct3 + (RT-PCR; NS) (98) C>M; PT, DT (ISH) (549) roct3 ++ (BDA; NS) (541) (SLC22A3) + (MA; NS) (538) (Slc22a3) + (BDA; NS) (542) + (RT-PCR; NS) (437) - (RT-PCR; NS) (539) +(RT-PCR; C) (540) moct3 (Slc22a3) + (MA; NS) (538) - (BDA; NS) (543) - (MS; C; M) (550) b - (BDA; NS) (546) 252
253 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) hoctn1 (SLC22A4) + (RT-PCR; NS) (98) ++ (MA; NS) (538) + (RT-PCR; NS) (437) - (RT-PCR; NS) (539) - (RT-PCR; C) (540) roctn1 (Slc22a4) moctn1 (Slc22a4) ++ (BDA; NS) (541) + (BDA; NS) (542) ++ (MA; NS) (538) - (BDA; NS) (543) OS> C, M; G, PT, DT (ISH) (551) + (BDA; NS) (546) C; PT (IHC) (552) AP (552) hoctn2 (SLC22A5) ++ (RT-PCR; NS) (98) +++ (MA; NS) (538) + (RT-PCR; NS) (437) ++ (RT-PCR; NS) (539) + (RT-PCR; C) (540) PT (IHC) (553) AP (553) roctn2 (Slc22a5) moctn2 (Slc22a5) +++ (BDA; NS) (541) + (BDA; NS) (542) +++ (MA; NS) (538) +++ (BDA; NS) (543) C; G, PT, DT (ISH) (554) PT (IHC) (555) AP (IHC) (555) ++ (BDA; NS) (546) C; PT (IHC) (552) AP (552) roctn3 (Slc22a9) S2, S3 (IHC) (556) AP (IHC) (556) moctn3 (Slc22a9) + (RT-PCR, WB; NS) (557) - (BDA; NS) (546) hoat1 (SLC22A6) +++ (RT-PCR; NS) (98) +++ (MA; NS) (538) +++ (RT-PCR; NS) (437) +++ (RT-PCR; NS) (539) PT (IHC) (558) PT (IHC) (540) BL (IHC) (558) BL (IHC) (540) BL (IHC) (547) roat1 (Slc22a6) 10.5 fmol/ µg (MS; K) (200) c +++ (BDA; NS) (541) +++ (BDA; NS) (542) ++ (BDA; NS) (543) C (WB); PT (IHC) (559) PT, Male>Female (IHC) (560) BL (IHC) (559) BL (IHC) (560) 253
254 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) ++ (RT-PCR; C) (540) ++ (RT-PCR; C) (547) moat1 (Slc22a6) 12.7 fmol/ ug (MS; C) (550) b 3.00 fmol/ µg (MS; M) (550) b C, PT (IHC) (561) S1, S2 (IHC) (562) BL (IHC) (561) BL (IHC) (562) hoat2 (SLC22A7) + (RT-PCR NS) (98) +++ (MA; NS) (538) + (RT-PCR; NS) (437) + (RT-PCR; NS) (539) + (RT-PCR; C) (540) ++ (RT-PCR; C) (547) PT (IHC) (563) BL (IHC) (563) BL (IHC) (547) roat2 (Slc22a7) moat2 (Slc22a7) + (BDA; NS) (541) ++ (BDA; NS) (542) - (BDA; NS) (543) TAL (IHC) (564) S3, F>M (IHC, WB) (565) S3, F>M (IHC, WB) (565) AP (IHC) (564) AP (IHC) (565) AP (IHC) (565) hoat3 (SLC22A8) +++ (RT-PCR; NS) (98) +++ (MA; NS) (538) ++ (RT-PCR; NS) (437) +++ (RT-PCR; NS) (539) +++ (RT-PCR; C) (540) +++ (RT-PCR; C) (547) PT (IHC) (566) PT (IHC) (540) BL (IHC) (566) BL (IHC) (540) BL (IHC) (547) roat3 (Slc22a8) moat3 (Slc22a8) 6.71 fmol/ µg (MS; K) (200) c +++ (BDA; NS) (541) +++ (BDA; NS) (542) +++ (BDA; NS) (543) 4.66 fmol/ µg (MS; C) (550) b 0.94 fmol/ µg (MS; M) (550) b PT, TAL, CD (IHC) (564) S1, S2, S3 (IHC) (567) S1, S2, S3, TAL, DT, CD (IHC) (562) BL (IHC) (564) BL (IHC) (567) BL (IHC) (562) hoat4 (SLC22A11) ++ (RT-PCR; NS) (98) + (MA; NS) (538) ++ (RT-PCR; NS) (437) + (RT-PCR; C) (540) PT (IHC) (568) PT (IHC) (569) PT (IHC) (570) AP (IHC) (568) AP (IHC) (569) AP (IHC) (570) 254
255 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) roat5 (SLC22A19) moat5 (SLC22A19) +++ (RT-PCR; NS) (567) +++ (BDA; F; NS) (571) ++ (BDA; M; NS) (571) S2, S3 (IHC) (567) AP (567) hpept1 (SLC15A1) + (RT-PCR; NS) (98) + (MA; NS) (538) + (RT-PCR; NS) (437) ++ (RT-PCR; NS) (539) rpept1 (Slc15a1) + (BDA; NS) (541) + (BDA; NS) (542) - (BDA; NS) (543) ++ (BDA; NS) (572) S1 (PCR, ISH) (573) S1 (IHC) (574) AP (IHC) (574) mpept1 (Slc15a1) - (BDA; NS) (572) hpept2 (SLC15A2) + (RT-PCR; NS) (98) ++ (MA; NS) (538) + (RT-PCR; NS) (437) PT (IHC) (575) AP (IHC) (575) rpept2 (Slc15a2) ++ (BDA; NS) (541) ++ (BDA; NS) (542) - (BDA; NS) (543) +++ (BDA; NS) (572) S3 (PCR, ISH) (573) S3 (IHC) (574) AP (IHC) (574) mpept2 (Slc15a2) +++ (BDA; NS) (572) hmate1 (SLC47A1) +++ (BDA; NS) (553) PT (IHC) (553) PT, DT (IHC) (576) AP (IHC) (553) AP (IHC) (576) rmate1 (Slc47a1) ++ (WB; NS) (577) PT (IHC) (577) AP (IHC) (577) 255
256 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) mmate1 (Slc47a1) 6.35 fmol/ µg (MS; C) (550) b 1.37 fmol/ µg (MS; M) (550) b +++ (BDA; NS) (578) PT, LoH, CD (IHC) (576) AP (IHC) (576) hmate2-k (SLC47A2) +++ (BDA; NS) (553) PT (IHC) (553) AP (IHC) (553) mmate2 (Slc47a2) - (BDA; NS) (578) Key: +++ High; ++ Medium; + Low; - Absent or not quantified; NS Not specified; RT-PCR Real time polymerase chain reaction; WB Western Blot; IHC Immunohistochemistry; BDA Branched DNA assay; MA Microarray (hybridisation); MS liquid chromatography tandem mass spectrometer (LC-MS/MS); ISH in situ hybridisation; K Whole kidney; C cortex; M medulla; OS outer stripe; G glomeruli, PT proximal tubule; S1/2/3 segment 1/2/3 of proximal tubule; TLH thin limb of loop of Henle, LoH Loop of Henle, TAL thick ascending limb of loop of Henle, DT distal tubule, CD collecting duct, BL basolateral membrane; AP brush-border (apical) membrane. a Although an early study reported hoct2 to be expressed at the apical membrane of distal tubule cells, the consensus opinion, based on subsequent studies, is that hoct2 is expressed at the basolateral membrane of the proximal tubules (579); b Proteomics data per µg of plasma membrane protein (550); c Abundance per µg of total membrane protein (200) 256
257 Table 8.2 Expression of ABC Family Drug Transporters in the Human and Rodent Kidney. Summary presented in Table 1.2 in the main text. Human Rodent Transporter Kidney Abundance Kidney Localisation Cell Localisation Transporter Kidney Abundance Kidney Localisation Cell Localisation (Gene) (Method; Region) (Method) (Method) (Gene) (Method; Region) (Method) (Method) hmdr1 (ABCB1) ++ (RT-PCR; NS) (98) ++ (MA; NS) (538) PT (IHC) (581) PT (IHC) (582) AP (IHC) (581) AP (IHC) (582) rmdr1a (Abcb1a) 0.68 fmol/ µg (MS; K) (200) b + (BDA; NS) (541) ++ (RT-PCR; NS) (437) + (RT-PCR; NS) (539) ++ (RT-PCR; NS) (580) mmdr1a - (BDA; NS) (542) - (BDA; NS) (543) ++ (RT-PCR; NS) (580) - (MS; C; M) (550) a (Abcb1a) ++ (RT-PCR; NS) (580) rmdr1b + (BDA; NS) (541) (Abcb1a) + (BDA; NS) (542) - (BDA; NS) (543) ++ (RT-PCR; NS) (580) mmdr1b - (MS; C; M) (550) a (Abcb1a) ++ (RT-PCR; NS) (580) hmrp1 + (RT-PCR; NS) (98) G, DT, CD (IHC) (583) BL (583) rmrp1 + (BDA; NS) (541) (ABCC1) ++ (MA; NS) (538) (Abcc1) + (BDA; NS) (542) + (RT-PCR; NS) - (BDA; NS) (543) 257
258 Human Rodent Transporter Kidney Abundance Kidney Localisation Cell Localisation Transporter Kidney Abundance Kidney Localisation Cell Localisation (Gene) (Method; Region) (Method) (Method) (Gene) (Method; Region) (Method) (Method) (437) - (RT-PCR; NS) (539) mmrp1 (Abcc1) - (MS; C; M) (550) a + (WB; NS) (583) + (BDA; NS) (584) G, DT, CD (IHC) (583) TLH, CD (IHC) (585) BL (IHC) (583) hmrp2 (ABCC2) ++ (RT-PCR; NS) (98) ++ (MA; NS) (538) ++ (RT-PCR; NS) (437) - (RT-PCR; NS) (539) PT (IHC) (586) PT (IHC) (587) PT (IHC) (588) AP (IHC) (586) AP (IHC) (587) AP (IHC) (588) rmrp2 (Abcc2) mmrp2 (Abcc2) ++ (BDA; NS) (541) + (BDA; NS) (542) - (BDA; NS) (543) 4.94 fmol/ µg (MS; C) (550) a - (MS; M) (550) a S1, S2, S3 (IHC) (589) AP (IHC) (589) ++ (BDA; NS) (584) hmrp3 + (RT-PCR; NS) (98) TLH, CD (IHC) (590) rmrp3 + (BDA; NS) (541) PT (IHC) (591) BL (IHC) (591) (ABCC3) ++ (MA; NS) (538) (Abcc3) + (BDA; NS) (542) + (RT-PCR; NS) (437) -(RT-PCR; NS) (539) mmrp3 (Abcc3) - (BDA; NS) (543) - (MS; C; M) (550) a + (BDA; NS) (584) + (BDA; M; NS) (271) ++ (BDA; F; NS) (271) 258
259 Human Rodent Transporter Kidney Abundance Kidney Localisation Cell Localisation Transporter Kidney Abundance Kidney Localisation Cell Localisation (Gene) (Method; Region) (Method) (Method) (Gene) (Method; Region) (Method) (Method) hmrp4 (ABCC4) ++ (RT-PCR; NS) (98) + (MA; NS) (538) PT (IHC) (588) AP (IHC) (588) rmrp4 (Abcc4) 0.54 fmol/ µg (MS; K) (200) b + (BDA; NS) (541) ++ (RT-PCR; NS) (437) - (RT-PCR; NS) (539) + (RT-PCR; NS) (580) mmrp4 (Abcc4) + (BDA; NS) (542) + (BDA; NS) (543) + (RT-PCR; NS) (580) 0.22 fmol/ µg (MS; M) (550) a 0.72 fmol/ µg (MS; C) (550) a + (BDA; M; NS) (584) ++ (BDA; F; NS) (584) + (RT-PCR; NS) (580) hmrp5 + (RT-PCR; NS) (98) rmrp5 + (BDA; NS) (541) (ABCC5) ++ (MA; NS) (538) (Abcc5) - (BDA; NS) (542) + (RT-PCR; NS) (437) - (RT-PCR; NS) (539) mmrp5 (Abcc5) - (BDA; NS) (543) - (MS; C; M) (550) a + (BDA; NS) (584) hmrp6 + (RT-PCR; NS) (98) PT (IHC) (587) BL (IHC) (587) rmrp6 + (BDA; NS) (541) (ABCC6) ++ (MA; NS) (538) (Abcc6) - (BDA; NS) (542) + (RT-PCR; NS) - (BDA; NS) (543) 259
260 Human Rodent Transporter Kidney Abundance Kidney Localisation Cell Localisation Transporter Kidney Abundance Kidney Localisation Cell Localisation (Gene) (Method; Region) (Method) (Method) (Gene) (Method; Region) (Method) (Method) (437) mmrp6 - (MS; C; M) (550) a - (RT-PCR; NS) (539) (Abcc6) - (BDA; NS) (543) + (BDA; NS) (584) hbcrp (ABCG2) + (RT-PCR; NS) (98) + (MA; NS) (538) + (RT-PCR; NS) (437) + (RT-PCR; NS) (580) PT (IHC) (580) BL (IHC) (580) rbcrp (Abcg2) mbcrp (Abcg2) 15.9 fmol/ µg (MS; K) (200) b + (RT-PCR; NS) (580) + (RT-PCR; NS) (580) Key: +++ High; ++ Medium; + Low; - Absent; NS Not specified; RT-PCR Real time polymerase chain reaction; IHC Immunohistochemistry; BDA Branched DNA assay; MA Microarray (hybridisation); MS liquid chromatography tandem mass spectrometer; ISH in situ hybridisation; K Whole kidney; C cortex; M medulla; OS outer stripe; G glomeruli, PT proximal tubule; S1/2/3 segment 1/2/3 of proximal tubule; TLH thin limb of loop of Henle, DT distal tubule, CD collecting duct, BL basolateral membrane; AP brush-border (apical) membrane. a Abundance per µg of plasma membrane protein (550); b Abundance per µg of total membrane protein (200) 260
261 Table 8.3 Expression of OATP Family Drug Transporters in the Human and Rodent Kidney. Summary presented in Table 1.2 in the main text. Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) roatp1a1 (Slco1a1) moatp1a1 (Slco1a1) +++ (BDA; NS) (541) - (BDA; NS) (542) + (BDA; NS) (543) 12.1 fmol/ µg (MS; C) (550) a 2.86 fmol/ µg (MS; M) (550) a M>F (593) S3 (IHC) (592) AP (IHC) (592) hoatp1a2 (SLCO1A2) + (RT-PCR; NS) (98) - (MA; NS) (538) - (RT-PCR; NS) (437) - (RT-PCR; NS) (539) DT (IHC) (594) AP (IHC) (594) roatp1a3 (Slco1a3) roatp1a4 (Slco1a4) moatp1a4 (Slco1a4) +++ (BDA; NS) (541) + (BDA; NS) (542) +++ (BDA; NS) (543) + (BDA; NS) (541) - (BDA; NS) (542) - (BDA; NS) (543) - (MS; C; M) (550) a 261
262 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) roatp1a5 (Slco1a5) + (BDA; NS) (541) - (BDA; NS) (542) - (BDA; NS) (543) roatp1a6 (Slco1a6) +++ (BDA; NS) (541) +++ (BDA; NS) (542) +++ (BDA; NS) (543) hoatp1b1 (SLCO1B1) - (RT-PCR; NS) (98) - (MA; NS) (538) - (RT-PCR; NS) (437) - (RT-PCR; NS) (539) roatp1b2 (Slco1b2) + (BDA; NS) (541) - (BDA; NS) (542) - (BDA; NS) (543) hoatp1b3 (SLCO1B3) + (RT-PCR; NS) (98) - (MA; NS) (538) hoatp1c1 (SLCO1C1) + (RT-PCR; NS) (98) - (MA; NS) (538) - (RT-PCR; NS) (437) roatp1c1 (Slco1c1) - (MS; C; M) (550) a hoatp2a1 (SLCO2A1) ++ (MA; NS) (538) roatp2a1 (Slco2a1) CD (IHC) (595) DT, CD (IHC) (596) AP (IHC) (595) 262
263 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) hoatp2b1 (SLCO2B1) + (RT-PCR; NS) (98) + (MA; NS) (538) + (RT-PCR; NS) (437) + (RT-PCR; NS) (539) roatp2b1 (Slco2b1) + (BDA; NS) (541) + (BDA; NS) (542) - (BDA; NS) (543) hoatp3a1 (SLCO3A1) + (RT-PCR; NS) (98) ++ (MA; NS) (538) + (RT-PCR; NS) (437) - (RT-PCR; NS) (539) roatp3a1 (Slco3a1) M>F (593) DT, CD (IHC) (596) hoatp4a1 (SLCO4A1) + (RT-PCR; NS) (98) + (MA; NS) (538) + (RT-PCR; NS) (437) roatp4a1 (Slco4a1) + (BDA; NS)(541) + (BDA; NS) (542) - (BDA; NS) (543) hoatp4c1 (SLCO4C1) ++ (RT-PCR; NS) (98) +++ (MA; NS) (538) roatp4c1 (Slco4c1) S1, S2, S3 (RT-PCR, IHC) (49) BL (IHC) (49) hoatp5a1 (SLCO5A1) - (MA; NS) (538) - (RT-PCR; NS) (437) roatp5a1 (Slco5s1) hoatp6a1 (SLCO6A1) 263
264 Human Rodent Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) Transporter (Gene) Kidney Abundance (Method; Region) Kidney Localisation (Method) Cell Localisation (Method) roatp6b1 (Slco6b1) - (BDA; NS) (541) Key: +++ High; ++ Medium; + Low; - Absent; NS Not specified; RT-PCR Real time polymerase chain reaction; IHC Immunohistochemistry; BDA Branched DNA assay; MA Microarray (hybridisation); ISH in situ hybridisation; K Whole kidney; C cortex; M medulla; OS outer stripe; S1/2/3 segment 1/2/3 of proximal tubule; DT distal tubule, CD collecting duct, BL basolateral membrane; AP brush-border (apical) membrane. a Proteomics data per µg of plasma membrane protein (550). 264
265 8.1.1 Calculating proximal tubule cell number Strategy 1. Multiplying number of proximal tubule cells per mm proximal tubule by total length of proximal tubule in kidney PTC d = 6.1 cells/ cross section proximal tubule (124) PTC l = 3.6 cells/ 100 µm proximal tubule length (124) L PT = 18 mm (Chapter 1, Table 1.7) N PT = 900,000 proximal tubules per kidney (118, 119, 162) PTC kidney = PTC d PTC l LPT N PT PTC kidney = 3.95 billion proximal tubule cells per kidney Where PTC d, PTC l and PTC kidney are the number of proximal tubule cells per cross section of proximal tubule, per 100 µm proximal tubule length and per human kidney, and L PT and N PT are the average length and number of proximal tubules per human kidney, respectively. The reported values of total proximal tubule cells per mm of proximal tubule (i.e. PTC d PTC l ) in rabbit were 278, 281 and 204 for segments S 1, S 2 and S 3, 300 and 825 cells/ mm tubule ( ), although these were not measured using stereological methodology. Analogous values for the cellularity of other regions of the nephron have also been reported for rabbit (599). The corresponding value calculated as above for human was at the lower end of this range, proximal tubule cells per mm proximal tubule. Assuming a kidney weight of 131 g (119), it follows that there are approximately 30.2 million proximal tubule cells per gram kidney. Further assuming that the cortex contributes 72% of kidney volume (and also mass) (122), 41.9 million proximal tubule cells per gram cortical kidney tissue is calculated. Strategy 2. Dividing total volume of proximal tubule epithelium wall by volume of a single proximal tubule cell. If the volume of the proximal tubule epithelium is assumed to be mm 3 / mm tubular length (167), and the proximal tubule length and number of proximal tubules per kidney assumed to be 18 mm and 900,000 tubules/ kidney as above, the total volume of proximal tubule epithelium can be calculated as mm 3 / kidney. Alternatively, if the total volume of cortex is assumed to be 106 ml/ kidney (average of male and female for all ages) (122), and that 34.9% of cortex is proximal tubule epithelium by volume (167), then the total volume of proximal tubule epithelium can be calculated as mm 3 / kidney. Using these two values as the minimum (23976 mm 3 / kidney) and maximum (36990 mm 3 / kidney) volume of proximal tubule epithelium, and 1350 and 3521 µm 3 as the minimum and maximum volumes of a single proximal tubule cell (176, 600, 601), the inferred number of proximal tubule cells ranges from 6.81 to billion cells/ kidney. This equates to 52.0 to million proximal tubule cells/ gram kidney (assuming kidney weight of 131 g (119)). 265
266 8.2 Appendix to Chapter 2 Kidney Isolation Excision only No perfusion/ wash step before freezing or homogenisation (181, 182, 191, 518, ) Perfused Saline (0.9% NaCl) (180) Saline with 0.05 M Tricine (ph 8.0) (477, 612, 613) Ringer-dextran type solution, then 10% invertose with NaHCO 3 (614) Rinse/ placed in buffer 0.25 M sucrose (211, 212) 0.25 M sucrose, 50 mm Tris HCl (ph 7.4) (207) 0.25 M sucrose, 10mM TEA, 1mM EDTA (ph 7.6) (512, 607) Saline ( % NaCl) (608, 609) 1.15% w/ v KCl (610) 0.1 M K 2PO 4, 0.15M KCl, 1.5mM EDTA (ph 7.4) (611) 50 mm Tris-HCl, 0.15 M KCl, 2 mm EDTA, ph 7.4 * Homogenisation Buffer 0.25 M sucrose (180, 207, 211, 212, 609, 610) 0.25 M sucrose, 2 mm Tris, ph 7.5 (191) 0.25 M sucrose, 5 mm HEPES, ph 7.4 (603) 0.25 M sucrose, Tris-HCl ph 7.4 (181) 0.25 M sucrose, 10 mm potassium phosphate, 1 mm EDTA, 1 tablet/ 50 ml protease inhibitor, ph 7.4 (518) 0.25 M sucrose, 1 mm NaCO 3, 1 mm EDTA, 1 mm protease inhibitor (614) 0.25 M sucrose, 10 mm TEA, 1mM EDTA, ph 7.6 (512, 607) 0.25 M sucrose, 0.1 M potassium phosphate, ph 7.4 (605) 0.05 M Potassium phosphate, 1 mm EDTA, 20% glycerol (477, 613) 3 mm Tris-HCl buffer, ph 7.5 (212) 0.15 M KCl, 1.5 mm EDTA, 0.1 M KH 2PO 4, ph 7.4 (611) 1.15% KCl (182, 606) 0.1 M potassium phosphate buffer, 20% glycerol, 0.1 mm dithiothreitol, ph 7.4 (608) 0.1 M phosphate buffer, 1.15% KCl, ph 7.4 at 37 C (615) 50 mm Tris-HCl, 0.15 M KCl, 2 mm EDTA, ph 7.4 * Homogeniser Potter-Elvehjam Teflon type (181, 182, 191, 207, 211, 606, 609) * Teflon pestle (180, 212, 518) Teflon-glass (477, 613) Ultra Turrax (191) Dounce (605) Polytron (608) * Waring Blender (477, 613) Not specified (512, 603, 607, 610, 611, 614, 615) 266
267 Microsome Isolation Removal of cell debris/ mitochondria Centrifugal Force Time Reference 9000g 20 min (182, 512, 606) 9000g 15 min (181) 12000g 10 min (180, 211) 600g, 10000g 10 min (each) (191) 10000g 15 min (603, 608) 5000g, 22000g 30 min, 15 min (477, 613) 11000g 30 min (518) 1475g, 25000g 10 min, 10 min (614) 500g, 10000g 5 min, 10 min (609) 9000g 90 min (207) 48000g 30 min (214, 611) 10000g 20 min (212) 15000g 5 min (607) g 20 min * 10800g 20 min (615) Not Specified Not Specified (610) Calcium facilitated precipitation 0.25 M sucrose, 25 mm CaCI 2 added to 12000g supernatant, then centrifuged at 27000g for 30min. Pellet washed with 0.1 M Tris-HCl buffer ph 8.0 (211) Differential Centrifugation Centrifugal Force Time Reference g 30 min (211) g 60 min (180, 181, 191, 207, 212, 512, 603, , 609, 611) * g 90 min (214, 614) 110,000g 70 min (518) 78000g 60 min (477, 613) g Not specified (615) None None (182) Not specified Not Specified (610) Pellet washing buffer 0.15 M KCl (180) 1.15% KCl (606) 0.05 M potassium phosphate, 0.15 M KCl, 20% glycerol, ph 7.7 (477, 613) 10 mm potassium phosphate, 0.15 mm KCl, 1 mm EDTA, ph 7.4 (518) 10 mm Tris-HCl, 150mM KCI, 0.1 mm EDTA, ph 7.0 (614) 0.1 M Tris-HCl buffer, ph 8.0 (211) 0.15 M sucrose (211) 0.25 M sucrose, 10 mm TEA, 1 mm EDTA, ph 7.6 (512, 607) 0.25 M sucrose, 0.1 M potassium phosphate (ph 7.4) (605) 0.1 M KH 2PO 4, 0.15 M KCl, 1.5 mm EDTA, ph 7.4 (611) 0.15 M KCl, 10mM EDTA, ph 7.4 * No pellet washing (181, 182, 191, 603, ) Storage Buffer 0.25 M sucrose (180, 207, 212, 610) * 0.1 M Na 2PO 4, 20% (w/v) glycerol, ph 7.4 (191) 0.25 M sucrose, 5 mm HEPES, ph 7.4 (603) 0.05 M potassium phosphate, 0.1 mm EDTA, 0.02% NaN 3, 20% glycerol, ph 7.7 (477, 613) 0.1 M Tris-HCI, 30% glycerol, ph 7.4 (181) 0.25 M sucrose and 1 mm EDTA, ph 7.4 (518) 0.15 M sucrose (211) 0.25 M sucrose, 10% (v/v) glycerol (607) 0.1 M KH 2PO 4, 0.15 M KCl, 1.5 mm EDTA, 20% (w/v) glycerol, ph 7.4 (611) 0.25 M sucrose, 5 mm HEPES, ph 7.4 (603) 0.1 M potassium phosphate ph 7.4 (608) 20 mm Tris buffer, 0.25 M saccharose, 5.4 mm EDTA, ph 7.4 at 37 C (615) Not Specified (182) Figure 8.1 A summary of different methods to isolate kidney microsomes from tissue collated from the literature. Key: * - Method from a commercial source of human kidney microsomes 267
268 Table 8.4 HPLC elution gradient for mycophenolic acid and warfarin (IS) Time (min) Solvent A (%) Solvent B (%) Solvent C (%) Solvent A: 0.05% formic acid in 90% water, 10% methanol; Solvent B: 0.05% formic acid in 10% water, 90% methanol; Solvent C: 1 mm ammonium acetate in 90%water, 10% methanol 268
269 Recovery Mic (%) G6Pase activity (mmol/ min) A y = 45.27x R² = y = 10.76x R² = % Protein concentration (mg/ ml) B 40% 30% 20% 10% 0% Protein concentration (mg/ ml) Figure 8.2 Assessment of linearity of G6Pase activity in dog kidney with respect to assay protein concentration (A) and impact on relationship between assay protein concentration and the estimated microsomal protein recovery (B). Data are the mean of three incubations from a single experiment. In panel A, symbols indicate homogenate ( ) and microsomes ( ) respectively, with linear lines of best fit and relevant equations shown. 269
270 Recovery Cyt (%) ADH Activity (nmole/ min) % 120% 100% A Protein concentration (mg/ ml) B 80% 60% 40% 20% 0% Protein concentration (mg/ ml) Figure 8.3 Assessment of linearity of ADH activity in rat kidney with respect to assay protein concentration (A) and impact on relationship between assay protein concentration and the estimated cytosolic protein recovery (B). Representative data shown are typically the mean of three incubations from a single experiment, although some data were excluded, in part due to presence of bubbles in assay plate during analysis. In panel A, symbols indicate homogenate ( ) and cytosol ( ) respectively, with linear lines of best fit and relevant equations shown. 270
271 Enrichment factor GST activity (nmole/ min) 6 5 A Protein concentration (µg/ ml) B Protein concentration (µg/ ml) Figure 8.4 Assessment of GST activity linearity in rat kidney respect to assay protein concentration (A) and impact on relationship between assay protein concentration and the estimated cytosolic protein enrichment factor (B). Enrichment factor was calculated as the ratio of the cytosolic GST activity: homogenate GST activity. Each data point represents the mean of three incubations from a single experiment; data shown are from two separate experiments performed on the same day. In panel A, symbols indicate homogenate ( ) and cytosol ( ) respectively. 271
272 Table 8.5 Microsomal protein marker data and MPPG estimates in kidney, liver and intestine for individual dogs. Data for intestine were provided by Dr Oliver Hatley General information Kidney Fresh Tissue Intestine Dog ID # Gender Body Weight (kg) Combined Kidney Weight (g) Liver Weight (g) Homogenate CYP content (nmol/ mg protein) Microsomal CYP content (nmol/ mg protein) MPPGK CYP (mg/ g kidney) Intestine Region Microsome CYP Content (nmol/mg protein) MPPGI CYP (mg/ g intestine) 1 M N/A N/A N/A Prox M N/A N/A N/A Prox F N/A N/A N/A Prox F Prox F Prox F Prox F Prox F Prox F Prox F Prox F Prox F Distal F Distal F Distal M N/A N/A N/A 16 M N/A N/A N/A 17 F N/A N/A N/A Average 4M Standard Deviation 13F CV 19% 19% 37% 24% 23% 22% 27% 61% Range
273 Frozen tissue Kidney Dog ID # Homogenate CYP content (nmol/ mg protein) Microsomal CYP content (nmol/ mg protein) MPPGK CYP (mg/ g kidney) Homogenate G6Psae activity (nmol/ min/ mg protein) Microsome G6Psae activity (nmol/ min/ mg protein) MPPGK G6Pase (mg/ g kidney) Average Standard Deviation CV 16% 15% 18% 16% 16% 16% Range
274 Frozen tissue Liver Dog ID # Homogenate CYP content (nmol/ mg protein) Microsomal CYP content (nmol/ mg protein) MPPGL CYP (mg/ g kidney) Homogenate G6Psae activity (nmol/ min/ mg protein) Microsome G6Psae activity (nmol/ min/ mg protein) MPPGL G6Pase (mg/ g kidney) Average Standard Deviation CV 19% 20% 12% 15% 18% 18% Range
275 Newcastle University CMFT Biobank Table 8.6 Demographics, protein recovery marker activities, subcellular protein content estimates, and mycophenolic acid in vitro glucuronidation data and IVIVE in individual human kidney samples. Demographics Source Donor ID Age (year) Gender Nationality/ Ethnicity Weight (kg) Height (m) Smoking Alcohol consumption (level/ units per week) B Male White British No Yes (20) B Male White British NA NA No No B Female White British No No B Male White British Ex a Yes (Socially) B Female British Yes Heavy in past B Female White British No NA B Male White British Ex a NA B Male White British No Yes (6) B Male NA Yes (rarely) Yes (4-6) B Male NA No Yes (35) B Male NA NA NA B Male NA Ex a Yes (Occasionally) B Male NA Ex a Yes (Occasionally) NC1 NA NA NA NA NA NA NA NC2 NA NA NA NA NA NA NA NC3 NA NA NA NA NA NA NA NC4 NA NA NA NA NA NA NA NC5 NA NA NA NA NA NA NA NC6 NA NA NA NA NA NA NA NC7 NA NA NA NA NA NA NA NC8 NA NA NA NA NA NA NA NC9 NA NA NA NA NA NA NA NC10 NA NA NA NA NA NA NA NC11 NA NA NA NA NA NA NA NC12 NA NA NA NA NA NA NA NC13 NA NA NA NA NA NA NA NC14 NA NA NA NA NA NA NA NC15 NA NA NA NA NA NA NA NC16 NA NA NA NA NA NA NA NC17 NA NA NA NA NA NA NA NC18 NA NA NA NA NA NA NA n Average Standard Deviation CV 19% 25% 9% Range
276 Newcastle University CMFT Biobank Marker assays Source Donor ID Glucose-6-phosphatase activity (nmol/ min/ mg protein) Glutathione-S-transferase activity (nmol/ min/ mg protein) Homogenate Microsomes Homogenate Microsomes Cytosol B B B B B B B B B B B B B NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC n Average Standard Deviation CV 64% 57% 44% 48% 43% Range
277 Newcastle University CMFT Biobank Subcellular fractions (mg / g kidney) Mycophenolic acid Source Donor ID MPPGK CPPGK S9PPGK CL int,u,ugt,hkm (µl/ min/ mg protein) Scaled CL int,u,ugt,hkm (ml/ min/ g kidney) Old MPPGK New MPPGK B B B B B B B B B B B B B NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC n Average Standard Deviation CV 26% 34% 26% 52% 52% 50% Range
278 Newcastle University CMFT Biobank Mycophenolic acid Source Donor ID CL R,met,UGT (ml/ min/ kg) Predicted CL UGT (ml/ min/ kg) Old MPPGK + Whole Kidney b New MPPGK + Whole kidney b New MPPGK + Cortex only b Old MPPGK, Whole kidney b New MPPGK + Whole kidney b New MPPGK + Cortex only b B B B B B B B B B B B B B NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC NC n Average Standard Deviation CV 49% 46% 47% 11% 17% 13% Range a Stopped smoking > 5 years before surgery; b See main text and Table 2.3 for full details on IVIVE scaling and prediction of mycophenolic acid glucuronidation clearance; NA Demographics data not available; - No data generated. 278
279 CPPGK (mg/ g kidney) MPPGK (mg/ g kidney) Figure 8.5 Comparison of MPPGK and CPPGK for human kidney microsomes from 31 donors 279
280 Figure 8.6 Fraction of mycophenolic acid (MPA) remaining over time in individual donor or pooled human kidney microsomes (0.25 mg/ ml) during glucuronidation substrate depletion assay. and represent incubations in the presence and absence of cofactor (UDPGA) respectively. * Incubation conditions were modified for donor B1140 (protein concentration of 0.5 mg/ ml and 90 min incubation), as k could not be reliably quantified under standard conditions. Each point represents the mean of three measurements in a single experiment. 280
281 8.3 Appendix to Chapter Calculation of collecting duct surface area The surface area of the collecting duct was calculated following a procedure similar to that published for a rodent collecting duct model (616). The cortical collecting ducts (CCD) are each formed following the merging of approximately 10 tubules, as shown in Figure 1.2 (161, 165, 617). Assuming there are 900,000 nephrons per kidney, the surface area of the CCD is calculated following the assumption of 90,000 cylindrical tubules per kidney. Whereas the diameter of CCD (50 µm) is taken from the literature (see Table 1.6), values for length were not found. Therefore it was assumed that the CCD is formed immediately adjacent to the renal capsule, and therefore the length is equivalent to the thickness of the cortex (8 mm; Table 1.7). Although this assumption may slightly overestimate the surface area of the CCD, it is expected that this will be offset by the absence of the connecting tubule from this model, and is unlikely to have any noteworthy impact on overall F reab predictions anyway. The collecting ducts traverse the outer medulla (OMCD) without fusing, and therefore surface area was calculated as for CCD (length = outer medulla width = 2mm; diameter = 50 µm). Upon reaching the inner medulla, the collecting ducts (IMCD) undergo successive dichotomous fusions Figure 1.2. In each human kidney, there are a reported 250 terminal/ papillary collecting ducts (tcd), known as ducts of Bellini, which are distributed across 8 18 renal papillae at the ends of the renal pyramids. tcds empty from the papillae apices into the renal pelvis of each kidney (161, 618). Assuming an initial 90,000 IMCD, this suggests an average of 360 IMCD per tcd, requiring an average of 8.49 dichotomous fusion events per tcd. This is in agreement with a published value of 8 fusion events (165). Although the diameter, and therefore circumference, of individual IMCD increase as they extend towards the papilla apex, the total circumference decreases due to reducing number of CD. The total circumference of IMCD throughout the inner medulla at x mm from the papilla apex (C x ) can be mathematically expressed as an exponential function (Eq. 8.1) (619). The IMCD surface area is the area under the curve of this function between 0 and n, where n is the length of the IMCD (length = inner medulla width = 11 mm). C x = (d 0 NCD 0 π)e( ( x F n ) ln 2 1 d 0 F ( dn ) ) 8.1 Where d 0 and d n are the diameter of IMCD at the papilla apex and at the outer medulla-inner medulla boundary, NCD 0 is the number of IMCDs at the papilla apex, and F is the number of fusion events. The resulting exponential function is shown in Figure 8.7, with the area under the curve (i.e. IMCD surface area) calculated by integration. 281
282 Total circumference (mm) Distance from papilla apex (mm) Exit as Ducts of Bellini (tcd) Direction of filtrate flow Entry into inner medulla Figure 8.7 Exponential function used to calculate TSA IMCD. Upon descending into the IMCD from the OMCD, the collecting ducts begin fusing, reducing the overall surface area. This reduction is described by an exponential function used to calculate the total circumference of IMCD as it descends towards the papilla apex, where the final urine pass through the ducts of Bellini (aka terminal ducts ). The total circumference (mm) can also be considered as the surface area density (mm 2 / mm IMCD length). The grey shaded area represents the area under the curve, which is the surface area of the IMCD Microvilli expansion factors As indicated above, microvilli are present, to differing extents, on the apical membranes of cells of both renal tubules and Caco-2 cell monolayers. Whereas Caco-2 cells and PT cells have extensive microvilli making up a brush border, the microvilli on the cells of other tubular sections of the nephron tend to be shorter and of fewer number (165, 167, 359, 360, 620). For example the PT microvilli are between approximately 1.5 and 2.5 µm in length, whereas the microvilli in the descending limb of the LoH, the microvilli are approximately 0.4 µm in length (621). The density and dimensions of microvilli may vary between species (165). In rabbit PT the apical and basolateral membranes have similar surface areas, after accounting for microvilli (apical) and basolateral membrane folding (359). In the absence of data on the apical membrane surface area associated with microvilli, human PT surface area was calculated using data published for the basolateral membrane of PT (167). Comparison with the surface area calculated using the assumption of a cylinder, as recently published for a model of PT reabsorption (71), revealed a 7.5-fold difference. Preliminary sensitivity analysis was performed during the development of the minimal model to assess the importance of changes in surface area of this order of magnitude on prediction of F reab. Using only the PT section as an exemplar, a 7.5 fold change of TSA PT had a substantial impact on predicted F reab,pt. Therefore, in order to account for the scarcity/ lack of microvilli in the LoH, DT and CD, verses presence of microvilli in Caco-2 282
283 cells, the TSA i for these segments in the final model included a surface area correction factor as a 7.5 fold decrease on that calculated using the assumption of open cylinder. Larger values of microvilli expansion factors have been reported for rabbit proximal tubule (15 to 40-fold), rat jejunum (24-fold) and human duodenum, jejunum and ileum (9.2, 14.1 and 15.7 fold), although a smaller value was calculated for the colon (6.4 fold), from which Caco-2 cells are derived (359, ) Derivation of P app calibration Starting point: Hill function 1 (Eq. 8.2): Best fit to the predicted F reab by minimal reabsorption model vs. original P app data (P 1 ), with slope factor a 1 and F 0.5 (P app at which F reab = 0.5) b 1. Hill 1 = P 1 a 1 b 1 a 1 + P 1 a Hill function 2 (Eq. 8.3): Best fit of Hill function to F reab vs. original P app data of reference drugs (P 1 ), with slope factor a 2 and F 0.5 b 2. Hill 2 = P 1 a 2 b 2 a 2 + P 1 a Aim: Hill function 3 (Eq. 8.4): Minimal reabsorption model vs. calibrated P app data (P 2 ), with slope factor a 1 and F 0.5 b 1. Hill 3 = P 2 a 1 b 1 a 1 + P 2 a a 1 and b 1 are obtained from the minimal model (i.e. Hill 1 ). P 2 must be obtained. Rearrangement to obtain P 2: The calibration procedure will make Hill function 2 and Hill function 3 effectively equivalent (Eq. 8.5 and 8.6) Hill 2 = Hill P 1 a 2 b 2 a 2 + P 1 a 2 = P 2 a 1 b 1 a 1 + P 2 a
284 Rearrange to find P 2 (Eq ) P 2 a 1 = (b 1 a 1 + P 2 a 1 ) ( P 1 a 2 b 2 a 2 + P 1 a 2 ) 8.7 P 2 a 1 = ( b 1 a 1 P 1 a 2 b 2 a 2 + P 1 a 2 ) P 1 a 2 1- ( a b 2 a 2 + P 2 ) P 2 = ( (b 1 a 1 a 1 P 2 1 ) a1 a b 2 ) P 2 = b ( a 2) 1 P a 1 1 b 2 ( a 2 a 1 )
285 8.3.4 Results Table 8.7 Database of clinical CL R values collated from the scientific literature. CL R values were calculated using plasma and urine drug concentration data measured in the same healthy subjects, and were normalised for subject weight and body surface area where necessary. Drug Overall weighted mean CL R (ml/ min) Overall weighted standard deviation CL R (ml/ min) Number of trials Number of observations/ measurements f u,p References Acebutolol ( ) Acecainide (631, 632) Acetaminophen (633, 634) Acyclovir ( ) Adefovir (639, 640) Allopurinol (641, 642) Almotriptan (643, 644) Amantadine (645) Amifloxacin (646, 647) Amoxicillin ( ) Ampicillin (649, 651, 652) Antipyrine ( ) Apalcillin (656) Aprindine ( ) Atenolol ( ) Azlocillin ( ) Aztreonam (671, 672) Benzylpenicillin (652, 669, 673) Betamethasone (674) Betaxolol ( ) Bisoprolol ( ) Caffeine (377, 390, 391, 398, 682) Captopril (683, 684) (683) Carbenicillin (652, 669, 685) Cefamandole (686, 687) Cefazolin ( ) 285
286 Drug Overall weighted mean CL R (ml/ min) Overall weighted standard deviation CL R (ml/ min) Number of trials Number of observations/ measurements f u,p References Cefepime ( ) Cefixime (699, 700) Cefmetazole (701, 702) Cefodizime ( ) Cefonicid ( ) Cefoperazone (698, ) Ceforanide (688, 690, 717) Cefotaxime (268, 698, ) Cefotetan (689, ) Cefotiam (727, 728) Cefpirome ( ) Ceftazidime (686, 687, 698, 716, 720, 733) Ceftizoxime (718, 734, 735) Chloroquine ( ) Chlorpheniramine (740, 741) Chlorpropamide (742) Chlorthalidone ( ) Cimetidine (655, 747, 748) Ciprofloxacin ( ) Citalopram ( ) Clinafloxacin ( ) Dapsone (764) Desipramine ( ) Dexrazoxane (769, 770) Difloxacin (753, 771, 772) Digoxin (417, 439, 449, 451, 460, ) Diltiazem (655, ) Dofetilide ( ) Doxepin ( ) 286
287 Drug Overall weighted mean CL R (ml/ min) Overall weighted standard deviation CL R (ml/ min) Number of trials Number of observations/ measurements f u,p References Enoxacin (753, ) Enoximone ( ) Enprofylline ( ) Fexofenadine (647, 803, 804) (803, 804) Fleroxacin (753, 772, 805, 806) Flucloxacillin (669, 807, 808) Fluconazole (655, 754, ) Frovatriptan (812) Furosemide ( ) Gabapentin ( ) Galantamine ( ) Garenoxacin (827, 828) Gatifloxacin (750, ) Gefitinib ( ) Gemifloxacin ( ) Grepafloxacin (750, 753, 840) Imipramine (739, 766, ) Irbesartan ( ) Isoxicam ( ) Lamivudine (647, ) Lamotrigine ( ) Lenalidomide ( ) Levetiracetam (864, 865) Levofloxacin (750, 754, 772, 830, 866, 867) Linezolid (389, 394, 868, 869) Lomefloxacin (814, ) (814, 870, 872) Lorazepam ( ) Maraviroc (878, 879) MDMA ( ) 287
288 Drug Overall weighted mean CL R (ml/ min) Overall weighted standard deviation CL R (ml/ min) Number of trials Number of observations/ measurements f u,p References Melagatran ( ) Memantine ( ) Mesna ( ) Metformin (267, ) Methadone ( ) Metoprolol ( ) Metronidazole ( ) Mexiletine ( ) Moclobemide (915, 916) Morphine (817, ) Moxalactam ( ) Moxifloxacin (750, ) Nafcillin (652, 669, 933) Ofloxacin (753, 772, 934, 935) Olmesartan ( ) Oseltamivir carboxylate (939, 940) Oxprenolol (663, 941) Oxytetracycline (942, 943) Pefloxacin (753, 772, ) Penciclovir (947, 948) Pilsicainide ( ) Pindolol (663, ) Piperacillin (669, 956) Pravastatin ( ) Prednisolone ( ) Prednisone (962, 964) Probenecid (55, 965, 966) Procainamide ( ) 288
289 Drug Overall weighted mean CL R (ml/ min) Overall weighted standard deviation CL R (ml/ min) Number of trials Number of observations/ measurements f u,p References Promethazine (754, 973, 974) Propafenone ( ) Propylthiouracil (980, 981) Pyrazinamide ( ) Quinidine (655, ) Raltegravir (993, 994) Remoxipride ( ) Resveratrol ( ) Ribavirin ( ) Rifabutin (1005, 1006) Rifampin (1007, 1008) Risperidone ( ) Rivaroxaban ( ) Ropivacaine ( ) Rosuvastatin (1020, 1021) Rufloxacin (753, 772, ) Salbutamol (1025, 1026) Sematilide (1027, 1028) Sitagliptin ( ) Sparfloxacin (753, 772, ) Sulfamethoxazole (754, ) Telbivudine ( ) Temafloxacin (772, 1044, 1045) Temocillin (669, 1046, 1047) Tenofovir (639, 1048, 1049) Terodiline ( ) Tetracycline (1050) Theophylline (634, 970, ) Timolol (663, 1058, 1059) 289
290 Drug Overall weighted mean CL R (ml/ min) Overall weighted standard deviation CL R (ml/ min) Number of trials Number of observations/ measurements f u,p References Tinidazole ( ) Tizanidine ( ) Tocainide ( ) Tomopenem ( ) Topiramate ( ) Trimethoprim (1039, ) Trovafloxacin (753, 772, 932, ) Valproic Acid (1084, 1085) Valsartan (1086, 1087) Varenicline ( ) Venlafaxine (830, ) Verapamil (655, 1098) Voriconazole ( ) Zanamivir ( ) Zidovudine (852, ) Zopiclone ( ) 290
291 Table 8.8 Human kidney drug transporters reported to interact with drugs in database of 157 drugs (at substrate level). Drug Kidney Transporters References Acebutolol Acecainide Acetaminophen Acyclovir OAT1, MATE1, MATE2K (241, 358) Adefovir OAT1, OAT3, MRP4 (241, 358, 524) Allopurinol Almotriptan Amantadine OCT2 (241, 358, 1118) Amifloxacin Amoxicillin Conflicting data on OAT1 (241, 1119, 1120) Ampicillin MRP2, MRP4 ( ) Antipyrine Apalcillin Aprindine Atenolol OCT2 (1124) Azlocillin In vivo DDI evidence only (1125) Aztreonam Benzylpenicillin OAT1, OAT3, MRP2, MRP4, OATP4C1 (358, 1123, ) Betamethasone P-gp (358) Betaxolol Bisoprolol P-gp, In vivo DDI (1130, 1131) Caffeine Captopril OAT1, OAT3 (353, 1132, 1133) Carbenicillin In vivo DDI evidence only (685) Cefamandole In vivo DDI evidence; Conflicting data on MRP2 (1134, 1135) Cefazolin OAT3, MRP4 (353, 410, 1133, 1136) Cefepime Cefixime Weak MRP4 interaction (358, 1137) Cefmetazole MRP4, In vivo DDI (241, 1123, 1136) Cefodizime Cefonicid In vivo DDI evidence only (241) Cefoperazone MRP2, MRP4 (1136, 1138, 1139) Ceforanide Cefotaxime OAT3, MRP4 (268, 1136) Cefotetan MRP2 (1139) Cefotiam OAT3, MRP2 (268, 1139) Cefpirome Ceftazidime MRP4 (1123) Ceftizoxime OAT1, OAT3, MRP4 (241, 1133, 1136) Chloroquine MATE1 (1140) Chlorpheniramine Chlorpropamide Chlorthalidone Cimetidine OAT1, OAT3, OCT2, MATE1, MATE2K, P-gp, BCRP (241, 358, 1141) Ciprofloxacin MATE-1, P-gp, BCRP, In vivo DDI (241, ) Citalopram Clinafloxacin Dapsone Desipramine Dexrazoxane Difloxacin Digoxin P-gp, OATP4C1 (49, 241, 358) Diltiazem P-gp (358) 291
292 Drug Kidney Transporters References Dofetilide In vivo DDI evidence only (241) Doxepin Enoxacin Weak P-gp interaction, In vivo DDI (1143, ) Enoximone Enprofylline In vivo DDI evidence only (800) Fexofenadine OAT3, MATE1, P-gp, In vivo DDI (241, 358, 804, ) Fleroxacin Weak P-gp interaction; In vivo DDI (805, 1153) Flucloxacillin In vivo DDI evidence only (807) Fluconazole P-gp (1154) Frovatriptan OCT2 (334) Furosemide OAT1, OAT3, MRP2, MRP4, In vivo DDI (241, 358, 1126, 1155, 1156) Gabapentin OCTN1 (820) Galantamine Garenoxacin Gatifloxacin In vivo DDI evidence only (829) Gefitinib P-gp, BCRP ( ) Gemifloxacin P-gp, MRP2, BCRP, In vivo DDI (838, 1160, 1161) Grepafloxacin P-gp (1162, 1163) Imipramine Irbesartan Isoxicam Lamivudine OCT2, MATE1, MATE2K, BCRP, Weak P-gp interaction (82, 241, ) Lamotrigine P-gp ( ) Lenalidomide Conflicting data on P-gp (1173, 1174) Levetiracetam Levofloxacin P-gp (353) Linezolid Lomefloxacin In vivo DDI evidence only (241, 1147) Lorazepam Maraviroc P-gp (879) MDMA Melagatran P-gp (1175) Memantine OCT2 (241, 358, 1118) Mesna MATE1, P-gp, MRP2, OAT4, In vivo DDI (891) Metformin OCT2, MATE1 MATE2K (241, 353, 553, 1145, ) Methadone Conflicting data on P-gp (358, ) Metoprolol OCT2 (1184) Metronidazole Mexiletine Moclobemide Morphine P-gp (358) Moxalactam Moxifloxacin P-gp, MRP2 (1185, 1186) Nafcillin P-gp, Weak BCRP interaction, In vivo DDI (933, 1144, 1187) Ofloxacin OCT2 (1124) Olmesartan OAT1, OAT3, MRP2, MRP4 (241, 358, 1188, 1189) Oseltamivir carboxylate OAT1, OAT3, MRP4 (1119, 1190) Oxprenolol Oxytetracycline P-gp (1191) Pefloxacin P-gp (1192) Penciclovir MRP4; Conflicting data on OAT1 and OAT3 (547, 1193, 1194) Pilsicainide In vivo DDI evidence only (241) Pindolol OCT2, In vivo DDI (241, 1124) Piperacillin MRP4 (1123) Pravastatin OAT3, OAT4, MRP2, MRP4 (241, 1123, 1195, 1196) 292
293 Drug Kidney Transporters References Prednisolone P-gp (358, 1197) Prednisone Weak P-gp interaction (358, 1197) Probenecid Procainamide OCT2, MATE1, MATE2K, In vivo DDI (241, 972, 1198, 1199) Promethazine Propafenone Propylthiouracil Pyrazinamide Quinidine OCTN1, OCTN2 (241, 1200, 1201) Raltegravir OAT1 (1202) Remoxipride Resveratrol BCRP (1203) Ribavirin Rifabutin Rifampin Risperidone P-gp (1204) Rivaroxaban P-gp (1205) Ropivacaine Rosuvastatin OAT3, P-gp, MRP2, MRP4, BCRP (241, ) Rufloxacin Salbutamol OCT2 (1209) Sematilide Sitagliptin OAT3, P-gp, OATP4C1 (428) Sparfloxacin P-gp (1153) Sulfamethoxazole Telbivudine Temafloxacin In vivo evidence only (1210) Temocillin Tenofovir OAT1, OAT3, P-gp, MRP4, BCRP (524, 1211, 1212) Terodiline Tetracycline Weak OAT3 interaction (241, 1213) Theophylline Timolol Tinidazole Tizanidine Tocainide Tomopenem Topiramate MATE2K (1214) Trimethoprim Trovafloxacin Valproic Acid Valsartan Weak P-gp interaction, MRP2 (241, 1215) Varenicline OCT2, MATE1, MATE2K (241, 1089, 1216) Venlafaxine Verapamil P-gp, OCTN1, OCTN2 (433, 1201, 1217) Voriconazole Zanamivir Zidovudine OAT1, OAT3, OAT4, Weak P-gp interaction (241, 1165, 1166, 1218) Zopiclone 293
294 Table 8.9 Specific studies or trials excluded from the database of CL R values, and reasons for exclusion Study/ trial Reason Atenolol 10 mg IV infusion (664) Pilsicainide 50 mg Oral dose (1219) Verapamil (1220) Anomalous result. CL R values reported in this trial were much higher than other studies for this drug, including other trials in the same publication. Anomalous result. CL R values reported in this study were much higher than other studies for this drug. Much higher renal clearance reported compared with other available data (1098). Other studies report a low fraction of dose excreted in urine (1221). 294
295 Figure 8.8 Comparison of CL R predicted using the minimal model of tubular reabsorption in combination with Caco-2 P app from the ph7.4:7.4 configured assay, with observed CL R (n=32 drugs). Symbols indicate neutral ( ), basic ( ), acidic ( ), zwitterion ( ) and amphoteric ( ) drugs respectively. Solid and dashed lines represent line of unity and 3-fold error respectively. P app data was obtained from historical average values reported by AstraZeneca internal databases. Data were acquired using a variety of assay formats. AAFE was 3.09 for all drugs, with predicted CL R within 3-fold of observed values for 18 drugs. Less than half of basic drugs were predicted within 3-fold of observed CL R. 295
PBPK modeling of renal impairment what is missing?
 PBPK modeling of renal impairment what is missing? Aleksandra Galetin Centre for Applied Pharmacokinetic Research, University of Manchester, UK Outline of the presentation Physiological changes in renal
PBPK modeling of renal impairment what is missing? Aleksandra Galetin Centre for Applied Pharmacokinetic Research, University of Manchester, UK Outline of the presentation Physiological changes in renal
Muhammad Fawad Rasool Feras Khalil Stephanie Läer
 Clin Pharmacokinet (2015) 54:943 962 DOI 10.1007/s40262-015-0253-7 ORIGINAL RESEARCH ARTICLE A Physiologically Based Pharmacokinetic Drug Disease Model to Predict Carvedilol Exposure in Adult and Paediatric
Clin Pharmacokinet (2015) 54:943 962 DOI 10.1007/s40262-015-0253-7 ORIGINAL RESEARCH ARTICLE A Physiologically Based Pharmacokinetic Drug Disease Model to Predict Carvedilol Exposure in Adult and Paediatric
Prediction of the Effects of Renal Impairment on the Clearance for Organic Cation Drugs that. undergo Renal Secretion: A Simulation-Based Study
 DMD Fast Forward. Published on February 28, 2018 as DOI: 10.1124/dmd.117.079558 This article has not been copyedited and formatted. The final version may differ from this version. Prediction of the Effects
DMD Fast Forward. Published on February 28, 2018 as DOI: 10.1124/dmd.117.079558 This article has not been copyedited and formatted. The final version may differ from this version. Prediction of the Effects
Microsomal and cytosolic scaling factors in dog and human kidney cortex and application for
 Title Microsomal and cytosolic scaling factors in dog and human kidney cortex and application for in vitro-in vivo extrapolation of renal metabolic clearance Authors Daniel Scotcher, Sarah Billington,
Title Microsomal and cytosolic scaling factors in dog and human kidney cortex and application for in vitro-in vivo extrapolation of renal metabolic clearance Authors Daniel Scotcher, Sarah Billington,
Proteomic Quantification of Kidney Transporters: Methodological Challenges, Interindividual Variability and Application in IVIVE
 Proteomic Quantification of Kidney Transporters: Methodological Challenges, Interindividual Variability and Application in IVIVE Bhagwat Prasad, Ph.D. University of Washington, Seattle, WA (bhagwat@uw.edu)
Proteomic Quantification of Kidney Transporters: Methodological Challenges, Interindividual Variability and Application in IVIVE Bhagwat Prasad, Ph.D. University of Washington, Seattle, WA (bhagwat@uw.edu)
Under prediction of hepatic clearance from in vitro studies: prospects for resolution. J Brian Houston
 DDI, Seattle, June 2018 Under prediction of hepatic clearance from in vitro studies: prospects for resolution J Brian Houston Centre for Applied Pharmacokinetic Research (CAPkR) Scaling up in vitro parameters
DDI, Seattle, June 2018 Under prediction of hepatic clearance from in vitro studies: prospects for resolution J Brian Houston Centre for Applied Pharmacokinetic Research (CAPkR) Scaling up in vitro parameters
Comparison Between the US FDA, Japan PMDA and EMA In Vitro DDI Guidance: Are we Close to Harmonization?
 Comparison Between the US FDA, Japan PMDA and EMA In Vitro DDI Guidance: Are we Close to Harmonization? Brian Ogilvie, Ph.D. VP Scientific Consulting XenoTech, LLC bogilvie@xenotechllc.com 14 Jun, 2018
Comparison Between the US FDA, Japan PMDA and EMA In Vitro DDI Guidance: Are we Close to Harmonization? Brian Ogilvie, Ph.D. VP Scientific Consulting XenoTech, LLC bogilvie@xenotechllc.com 14 Jun, 2018
Basic Concepts in Pharmacokinetics. Leon Aarons Manchester Pharmacy School University of Manchester
 Basic Concepts in Pharmacokinetics Leon Aarons Manchester Pharmacy School University of Manchester Objectives 1. Define pharmacokinetics 2. Describe absorption 3. Describe distribution 4. Describe elimination
Basic Concepts in Pharmacokinetics Leon Aarons Manchester Pharmacy School University of Manchester Objectives 1. Define pharmacokinetics 2. Describe absorption 3. Describe distribution 4. Describe elimination
XTreme 200 Human Liver Microsomes Lot No Human Liver Microsomes Pool of 200 (100 Male and 100 Female) Suspension medium: 250 mm sucrose
 XTreme 200 Human Liver Microsomes Lot No. 1710084 Human Liver Microsomes Pool of 200 (100 Male and 100 Female) Suspension medium: 250 mm sucrose H2610 0.5 ml at 20 mg/ml H2620 1.0 ml at 20 mg/ml H2630
XTreme 200 Human Liver Microsomes Lot No. 1710084 Human Liver Microsomes Pool of 200 (100 Male and 100 Female) Suspension medium: 250 mm sucrose H2610 0.5 ml at 20 mg/ml H2620 1.0 ml at 20 mg/ml H2630
BASIC PHARMACOKINETICS
 BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
BASIC PHARMACOKINETICS MOHSEN A. HEDAYA CRC Press Taylor & Francis Croup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Table of Contents Chapter
Drug Dosing in Renal Insufficiency. Coralie Therese D. Dimacali, MD College of Medicine University of the Philippines Manila
 Drug Dosing in Renal Insufficiency Coralie Therese D. Dimacali, MD College of Medicine University of the Philippines Manila Declaration of Conflict of Interest For today s lecture on Drug Dosing in Renal
Drug Dosing in Renal Insufficiency Coralie Therese D. Dimacali, MD College of Medicine University of the Philippines Manila Declaration of Conflict of Interest For today s lecture on Drug Dosing in Renal
Characterization of in vitro glucuronidation clearance of a range of drugs in human kidney
 DMD Fast This Forward. article has not Published been copyedited on and January formatted. 24, The 2012 final version as doi:10.1124/dmd.111.043984 may differ from this version. Characterization of in
DMD Fast This Forward. article has not Published been copyedited on and January formatted. 24, The 2012 final version as doi:10.1124/dmd.111.043984 may differ from this version. Characterization of in
Invited Review. Introduction
 BIOPHARMACEUTICS & DRUG DISPOSITION Biopharm. Drug Dispos. 34: 2 28 (2013) Published online 8 October 2012 in Wiley Online Library (wileyonlinelibrary.com).1810 Invited Review Absolute abundance and function
BIOPHARMACEUTICS & DRUG DISPOSITION Biopharm. Drug Dispos. 34: 2 28 (2013) Published online 8 October 2012 in Wiley Online Library (wileyonlinelibrary.com).1810 Invited Review Absolute abundance and function
Drug Interactions, from bench to bedside
 Drug Interactions, from bench to bedside Candidate to Market, The Paterson Institute for Cancer Research, Manchester, UK Michael Griffin PhD Overview of presentation To understand the importance of drug-drug
Drug Interactions, from bench to bedside Candidate to Market, The Paterson Institute for Cancer Research, Manchester, UK Michael Griffin PhD Overview of presentation To understand the importance of drug-drug
Renal Disease and PK/PD. Anjay Rastogi MD PhD Division of Nephrology
 Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Evaluation and Quantitative Prediction of Renal Transporter-Mediated Drug-Drug Interactions. Bo Feng, Ph.D. DDI 2017 June 19-21, 2017
 Evaluation and Quantitative Prediction of Renal Transporter-Mediated Drug-Drug Interactions Bo Feng, Ph.D. DDI 2017 June 19-21, 2017 Outline Background of renal transporters. Clinically observed transporter-mediated
Evaluation and Quantitative Prediction of Renal Transporter-Mediated Drug-Drug Interactions Bo Feng, Ph.D. DDI 2017 June 19-21, 2017 Outline Background of renal transporters. Clinically observed transporter-mediated
Evaluation of Drug-Drug Interactions FDA Perspective
 Evaluation of Drug-Drug Interactions FDA Perspective Kellie Schoolar Reynolds, Pharm.D. Deputy Director Division of Clinical Pharmacology IV Office of Clinical Pharmacology Office of Translational Sciences
Evaluation of Drug-Drug Interactions FDA Perspective Kellie Schoolar Reynolds, Pharm.D. Deputy Director Division of Clinical Pharmacology IV Office of Clinical Pharmacology Office of Translational Sciences
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Caveat: Validation and Limitations of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters
 Caveat: Validation and Limitations of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 How to Safeguard that Metrics Reflect E/T Activity? in healthy
Caveat: Validation and Limitations of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 How to Safeguard that Metrics Reflect E/T Activity? in healthy
Strategy on Drug Transporter Investigation Why, How, Which & When. Jasminder Sahi
 Strategy on Drug Transporter Investigation Why, How, Which & When Jasminder Sahi Intestine Drug Absorption PEPT1 OATPs MCTs AE2 Epithelial Cell MCTs MRP3 Liver Excretion via Liver Kidney MRPs OATPs N PT1
Strategy on Drug Transporter Investigation Why, How, Which & When Jasminder Sahi Intestine Drug Absorption PEPT1 OATPs MCTs AE2 Epithelial Cell MCTs MRP3 Liver Excretion via Liver Kidney MRPs OATPs N PT1
The Future of In Vitro Systems for the Assessment of Induction and Suppression of Enzymes and Transporters
 The Future of In Vitro Systems for the Assessment of Induction and Suppression of Enzymes and Transporters AAPS, San Diego November 6 th, 2014 Andrew Parkinson, XPD Consulting Lisa Almond, Simcyp-Certara
The Future of In Vitro Systems for the Assessment of Induction and Suppression of Enzymes and Transporters AAPS, San Diego November 6 th, 2014 Andrew Parkinson, XPD Consulting Lisa Almond, Simcyp-Certara
MODULE PHARMACOKINETICS WRITTEN SUMMARY
 MODULE 2.6.4. PHARMACOKINETICS WRITTEN SUMMARY m2.6.4. Pharmacokinetics Written Summary 2013N179518_00 TABLE OF CONTENTS PAGE 1. BRIEF SUMMARY...4 2. METHODS OF ANALYSIS...5 3. ABSORPTION...6 4. DISTRIBUTION...7
MODULE 2.6.4. PHARMACOKINETICS WRITTEN SUMMARY m2.6.4. Pharmacokinetics Written Summary 2013N179518_00 TABLE OF CONTENTS PAGE 1. BRIEF SUMMARY...4 2. METHODS OF ANALYSIS...5 3. ABSORPTION...6 4. DISTRIBUTION...7
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Excretion of Drugs. Prof. Hanan Hagar Pharmacology Unit Medical College
 Excretion of Drugs Prof. Hanan Hagar Pharmacology Unit Medical College Excretion of Drugs By the end of this lecture, students should be able to! Identify main and minor routes of excretion including renal
Excretion of Drugs Prof. Hanan Hagar Pharmacology Unit Medical College Excretion of Drugs By the end of this lecture, students should be able to! Identify main and minor routes of excretion including renal
It the process by which a drug reversibly leaves blood and enter interstitium (extracellular fluid) and/ or cells of tissues.
 It the process by which a drug reversibly leaves blood and enter interstitium (extracellular fluid) and/ or cells of tissues. Primarily depends on: 1.Regional blood flow. 2.Capillary permeability. 3.Protein
It the process by which a drug reversibly leaves blood and enter interstitium (extracellular fluid) and/ or cells of tissues. Primarily depends on: 1.Regional blood flow. 2.Capillary permeability. 3.Protein
PREDICTING PHARMACOKINETICS FOLLOWING TOPICAL APPLICATION USING NON-ANIMAL METHODS IAN SORRELL, MI-YOUNG LEE, RICHARD CUBBERLEY
 PREDICTING PHARMACOKINETICS FOLLOWING TOPICAL APPLICATION USING NON-ANIMAL METHODS IAN SORRELL, MI-YOUNG LEE, RICHARD CUBBERLEY UNILEVER APPLICATIONS KINETICS Need for estimates of local and systemic concentrations
PREDICTING PHARMACOKINETICS FOLLOWING TOPICAL APPLICATION USING NON-ANIMAL METHODS IAN SORRELL, MI-YOUNG LEE, RICHARD CUBBERLEY UNILEVER APPLICATIONS KINETICS Need for estimates of local and systemic concentrations
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
Click to edit Master title style
 A Short Course in Pharmacokinetics Chris Town Research Pharmacokinetics Outline Pharmacokinetics - Definition Ideal Pharmacokinetic Parameters of a New Drug How do we optimize PK for new compounds Why
A Short Course in Pharmacokinetics Chris Town Research Pharmacokinetics Outline Pharmacokinetics - Definition Ideal Pharmacokinetic Parameters of a New Drug How do we optimize PK for new compounds Why
BLOCK REVIEW Renal Physiology. May 9, 2011 Koeppen & Stanton. EXAM May 12, Tubular Epithelium
 BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
RSC, February Interplay between enzymes and. clearance and intracellular concentration of drugs. Centre for Applied Pharmacokinetic Research
 RSC, February 2014 Interplay between enzymes and transporters in defining hepatic drug clearance and intracellular concentration of drugs J Brian Houston Centre for Applied Pharmacokinetic Research (CAPkR)
RSC, February 2014 Interplay between enzymes and transporters in defining hepatic drug clearance and intracellular concentration of drugs J Brian Houston Centre for Applied Pharmacokinetic Research (CAPkR)
Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook with Computer Simulations
 Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook with Computer Simulations Rosenbaum, Sara E. ISBN-13: 9780470569061 Table of Contents 1 Introduction to Pharmacokinetics and Pharmacodynamics.
Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook with Computer Simulations Rosenbaum, Sara E. ISBN-13: 9780470569061 Table of Contents 1 Introduction to Pharmacokinetics and Pharmacodynamics.
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Transporters DDI-2018
 Transporters DDI-2018 Mark S. Warren, Ph.D. June 16, 2018 Senior Director of Assay Services DDI-2018: 21 st Conference on DDIs FDA guidance documents: A 21 year history 1997 2006 2012 2017 Each year, large
Transporters DDI-2018 Mark S. Warren, Ph.D. June 16, 2018 Senior Director of Assay Services DDI-2018: 21 st Conference on DDIs FDA guidance documents: A 21 year history 1997 2006 2012 2017 Each year, large
Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization
 Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization Nico Holmstock Scientist, Janssen R&D M CERSI 2017, BALTIMORE (USA) Canagliflozin An
Development of Canagliflozin: Mechanistic Absorption Modeling During Late-Stage Formulation and Process Optimization Nico Holmstock Scientist, Janssen R&D M CERSI 2017, BALTIMORE (USA) Canagliflozin An
Section 5.2: Pharmacokinetic properties
 Section 5.2: Pharmacokinetic properties SmPC training presentation Note: for full information refer to the European Commission s Guideline on summary of product characteristics (SmPC) SmPC Advisory Group
Section 5.2: Pharmacokinetic properties SmPC training presentation Note: for full information refer to the European Commission s Guideline on summary of product characteristics (SmPC) SmPC Advisory Group
Renal Excretion of Drugs
 Renal Excretion of Drugs 3 1 Objectives : 1 Identify main and minor routes of Excretion including renal elimination and biliary excretion 2 Describe its consequences on duration of drugs. For better understanding:
Renal Excretion of Drugs 3 1 Objectives : 1 Identify main and minor routes of Excretion including renal elimination and biliary excretion 2 Describe its consequences on duration of drugs. For better understanding:
INTRODUCTION TO PHARMACOKINETICS
 INTRODUCTION TO PHARMACOKINETICS 1 http://www.biology.iupui.edu/biocourses/biol540/4pipeline2css.html 2 PHARMACOKINETICS 1. ABSORPTION 2. DISTRIBUTION 3. METABOLISM 4. EXCRETION ALL THESE PROCESSES ARE
INTRODUCTION TO PHARMACOKINETICS 1 http://www.biology.iupui.edu/biocourses/biol540/4pipeline2css.html 2 PHARMACOKINETICS 1. ABSORPTION 2. DISTRIBUTION 3. METABOLISM 4. EXCRETION ALL THESE PROCESSES ARE
PHA5128 Dose Optimization II Case Study I Spring 2013
 Silsamicin is an investigational compound being evaluated for its antimicrobial effect. The route of administration for this drug is via intravenous bolus. Approximately 99.9% of this drug is eliminated
Silsamicin is an investigational compound being evaluated for its antimicrobial effect. The route of administration for this drug is via intravenous bolus. Approximately 99.9% of this drug is eliminated
Building innovative drug discovery alliances. Hepatic uptake and drug disposition o in vitro and in silico approaches
 Building innovative drug discovery alliances Hepatic uptake and drug disposition o in vitro and in silico approaches Dr Beth Williamson Evotec AG, 2017 Outline Importance of predicting clearance In vitro
Building innovative drug discovery alliances Hepatic uptake and drug disposition o in vitro and in silico approaches Dr Beth Williamson Evotec AG, 2017 Outline Importance of predicting clearance In vitro
Evaluation of Proposed In Vivo Probe Substrates and Inhibitors for Phenotyping Transporter Activity in Humans
 Supplement Article Evaluation of Proposed In Vivo Probe Substrates and Inhibitors for Phenotyping Transporter Activity in Humans The Journal of Clinical Pharmacology (2016), 56(S7) S82 S98 C 2016, The
Supplement Article Evaluation of Proposed In Vivo Probe Substrates and Inhibitors for Phenotyping Transporter Activity in Humans The Journal of Clinical Pharmacology (2016), 56(S7) S82 S98 C 2016, The
Current Approaches and Applications of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters
 Current Approaches and Applications of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 Definition Phenotyping is quantifying the in vivo activity
Current Approaches and Applications of Phenotyping Methods for Drug Metabolizing Enzymes and Transporters Uwe Fuhr, University Hospital Cologne 1 Definition Phenotyping is quantifying the in vivo activity
FDA s Clinical Drug Interaction Studies Guidance (2017 Draft Guidance)
 FDA s Clinical Drug Interaction Studies Guidance (2017 Draft Guidance) Kellie S. Reynolds, Pharm.D. Deputy Director, Division of Clinical Pharmacology IV Office of Clinical Pharmacology (OCP) Office of
FDA s Clinical Drug Interaction Studies Guidance (2017 Draft Guidance) Kellie S. Reynolds, Pharm.D. Deputy Director, Division of Clinical Pharmacology IV Office of Clinical Pharmacology (OCP) Office of
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Monica Edholm Medica Medic l a Pr oducts Agency
 EMA EFPIA workshop Break-out session no. 2 Case Study Title: M&S for dose adjustment in impaired patients M&S for dose adjustment in renally Monica Edholm MedicalProducts Agency Disclaimer: The views expressed
EMA EFPIA workshop Break-out session no. 2 Case Study Title: M&S for dose adjustment in impaired patients M&S for dose adjustment in renally Monica Edholm MedicalProducts Agency Disclaimer: The views expressed
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Quantitative Evaluation of the Effect of P-Glycoprotein on Oral Drug Absorption
 Quantitative Evaluation of the Effect of P-Glycoprotein on Oral Drug Absorption -Assessment of Drug Permeability to Rat Small Intestine- College of Pharmacy, Setsunan University, Osaka, Japan Yoshiyuki
Quantitative Evaluation of the Effect of P-Glycoprotein on Oral Drug Absorption -Assessment of Drug Permeability to Rat Small Intestine- College of Pharmacy, Setsunan University, Osaka, Japan Yoshiyuki
Chapter-V Drug use in renal and hepatic disorders. BY Prof. C.Ramasamy, Head, Dept of Pharmacy Practice SRM College of Pharmacy, SRM University
 Chapter-V Drug use in renal and hepatic disorders. BY Prof. C.Ramasamy, Head, Dept of Pharmacy Practice SRM College of Pharmacy, SRM University Estimating renal function An accurate estimation of renal
Chapter-V Drug use in renal and hepatic disorders. BY Prof. C.Ramasamy, Head, Dept of Pharmacy Practice SRM College of Pharmacy, SRM University Estimating renal function An accurate estimation of renal
Pharmacokinetics of Drugs. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Pharmacokinetics of Drugs Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Absorption Is the transfer of a drug from its site of administration to the bloodstream.
Pharmacokinetics of Drugs Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Absorption Is the transfer of a drug from its site of administration to the bloodstream.
Strategies for Developing and Validating PBPK Models for Extrapolation to Unstudied Population
 Strategies for Developing and Validating PBPK Models for Extrapolation to Unstudied Population Nina Isoherranen Department of Pharmaceutics University of Washington Processes driving drug disposition are
Strategies for Developing and Validating PBPK Models for Extrapolation to Unstudied Population Nina Isoherranen Department of Pharmaceutics University of Washington Processes driving drug disposition are
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Anatomy/Physiology Study Guide: Unit 9 Excretory System
 Anatomy/Physiology Study Guide: Unit 9 Excretory System 1) In the space below, list the primary structures (organs) and their corresponding functions. Structures: Functions: KIDNEY 1) URETER BLADDER URETHRA
Anatomy/Physiology Study Guide: Unit 9 Excretory System 1) In the space below, list the primary structures (organs) and their corresponding functions. Structures: Functions: KIDNEY 1) URETER BLADDER URETHRA
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
In Vitro In Vivo Extrapolation (IVIVE): Why It Is Not As Easy As You May Think
 EMA Workshop on MPS - 2017 In Vitro In Vivo Extrapolation (IVIVE): Why It Is Not As Easy As You May Think Amin Rostami Professor of Systems Pharmacology University of Manchester, UK & Chief Scientific
EMA Workshop on MPS - 2017 In Vitro In Vivo Extrapolation (IVIVE): Why It Is Not As Easy As You May Think Amin Rostami Professor of Systems Pharmacology University of Manchester, UK & Chief Scientific
TDM. Measurement techniques used to determine cyclosporine level include:
 TDM Lecture 15: Cyclosporine. Cyclosporine is a cyclic polypeptide medication with immunosuppressant effect. It has the ability to block the production of interleukin-2 and other cytokines by T-lymphocytes.
TDM Lecture 15: Cyclosporine. Cyclosporine is a cyclic polypeptide medication with immunosuppressant effect. It has the ability to block the production of interleukin-2 and other cytokines by T-lymphocytes.
BIOPHARMACEUTICS and CLINICAL PHARMACY
 11 years papers covered BIOPHARMACEUTICS and CLINICAL PHARMACY IV B.Pharm II Semester, Andhra University Topics: Absorption Distribution Protein binding Metabolism Excretion Bioavailability Drug Interactions
11 years papers covered BIOPHARMACEUTICS and CLINICAL PHARMACY IV B.Pharm II Semester, Andhra University Topics: Absorption Distribution Protein binding Metabolism Excretion Bioavailability Drug Interactions
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Osmoregulation and the Excretory System
 Honors Biology Study Guide Chapter 25.4 25.10 Name Osmoregulation and the Excretory System FUNCTIONS OF THE EXCRETORY SYSTEM OSMOREGULATION Freshwater: Marine: Land Animals: Sources of Nitrogenous Wastes?
Honors Biology Study Guide Chapter 25.4 25.10 Name Osmoregulation and the Excretory System FUNCTIONS OF THE EXCRETORY SYSTEM OSMOREGULATION Freshwater: Marine: Land Animals: Sources of Nitrogenous Wastes?
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Pharmacokinetic Modeling & Simulation in Discovery and non-clinical Development
 Pharmacokinetic Modeling & Simulation in Discovery and non-clinical Development Where do we stand? Disclaimer I am not a bioinformatician, mathematician or biomedical engineer. I am a simple minded pharmacist,
Pharmacokinetic Modeling & Simulation in Discovery and non-clinical Development Where do we stand? Disclaimer I am not a bioinformatician, mathematician or biomedical engineer. I am a simple minded pharmacist,
12/7/10. Excretory System. The basic function of the excretory system is to regulate the volume and composition of body fluids by:
 Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
USING PBPK MODELING TO SIMULATE THE DISPOSITION OF CANAGLIFLOZIN
 USING PBPK MODELING TO SIMULATE THE DISPOSITION OF CANAGLIFLOZIN Christophe Tistaert PDMS Pharmaceutical Sciences Preformulation & Biopharmaceutics AAPS 2015, FLORIDA (USA) Canagliflozin An orally active
USING PBPK MODELING TO SIMULATE THE DISPOSITION OF CANAGLIFLOZIN Christophe Tistaert PDMS Pharmaceutical Sciences Preformulation & Biopharmaceutics AAPS 2015, FLORIDA (USA) Canagliflozin An orally active
General Principles of Pharmacology and Toxicology
 General Principles of Pharmacology and Toxicology Parisa Gazerani, Pharm D, PhD Assistant Professor Center for Sensory-Motor Interaction (SMI) Department of Health Science and Technology Aalborg University
General Principles of Pharmacology and Toxicology Parisa Gazerani, Pharm D, PhD Assistant Professor Center for Sensory-Motor Interaction (SMI) Department of Health Science and Technology Aalborg University
Supporting information
 Supporting information Intracellular drug bioavailability: a new predictor of system dependent drug disposition Mateus, André 1* ; Treyer, Andrea 1* ; Wegler, Christine 1,2 ; Karlgren, Maria 1 ; Matsson,
Supporting information Intracellular drug bioavailability: a new predictor of system dependent drug disposition Mateus, André 1* ; Treyer, Andrea 1* ; Wegler, Christine 1,2 ; Karlgren, Maria 1 ; Matsson,
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Introduction to Pharmacokinetics (PK) Anson K. Abraham, Ph.D. Associate Principal Scientist, PPDM- QP2 Merck & Co. Inc., West Point, PA 5- June- 2017
 Introduction to Pharmacokinetics (PK) Anson K. Abraham, Ph.D. Associate Principal Scientist, PPDM- QP2 Merck & Co. Inc., West Point, PA 5- June- 2017 1 Outline Definition & Relevance of Pharmacokinetics
Introduction to Pharmacokinetics (PK) Anson K. Abraham, Ph.D. Associate Principal Scientist, PPDM- QP2 Merck & Co. Inc., West Point, PA 5- June- 2017 1 Outline Definition & Relevance of Pharmacokinetics
Development of a Dynamic Physiologically Based Mechanistic Kidney Model to Predict Renal Clearance
 Citation: CPT Pharmacometrics Syst. Pharmacol. (2018) 7, 593 602; doi:10.1002/psp4.12321 ARTICLE Development of a Dynamic Physiologically Based Mechanistic Kidney Model to Predict Renal Clearance Weize
Citation: CPT Pharmacometrics Syst. Pharmacol. (2018) 7, 593 602; doi:10.1002/psp4.12321 ARTICLE Development of a Dynamic Physiologically Based Mechanistic Kidney Model to Predict Renal Clearance Weize
1. remove: waste products: urea, creatinine, and uric acid foreign chemicals: drugs, water soluble vitamins, and food additives, etc.
 Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Diuretic Agents Part-2. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Diuretic Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Potassium-sparing diuretics The Ion transport pathways across the luminal and basolateral
Diuretic Agents Part-2 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Potassium-sparing diuretics The Ion transport pathways across the luminal and basolateral
Exploiting BDDCS and the Role of Transporters
 Exploiting BDDCS and the Role of Transporters (Therapeutic benefit of scientific knowledge of biological transporters, understanding the clinical relevant effects of active transport on oral drug absorption)
Exploiting BDDCS and the Role of Transporters (Therapeutic benefit of scientific knowledge of biological transporters, understanding the clinical relevant effects of active transport on oral drug absorption)
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Biopharmaceutics Drug Disposition Classification System (BDDCS) --- Its Impact and Application
 Biopharmaceutics Drug Disposition Classification System (BDDCS) --- Its Impact and Application Leslie Z. Benet, Ph.D. Professor of Bioengineering and Therapeutic Sciences Schools of Pharmacy and Medicine
Biopharmaceutics Drug Disposition Classification System (BDDCS) --- Its Impact and Application Leslie Z. Benet, Ph.D. Professor of Bioengineering and Therapeutic Sciences Schools of Pharmacy and Medicine
Application of a Systems Approach to the Bottom-Up Assessment of Pharmacokinetics in Obese Patients
 Page 1 of 14 Clinical Pharmacokinetics Application of a Systems Approach to the ottom-up Assessment of Pharmacokinetics in Patients Expected Variations in Clearance C. Ghobadi, et al. Supplemental Digital
Page 1 of 14 Clinical Pharmacokinetics Application of a Systems Approach to the ottom-up Assessment of Pharmacokinetics in Patients Expected Variations in Clearance C. Ghobadi, et al. Supplemental Digital
PHAR 7632 Chapter 16
 PHAR 7632 Chapter 16 Routes of Excretion Routes of Excretion Student Objectives for this Chapter After completing the material in this chapter each student should:- be able to describe the various routes
PHAR 7632 Chapter 16 Routes of Excretion Routes of Excretion Student Objectives for this Chapter After completing the material in this chapter each student should:- be able to describe the various routes
Saliva Versus Plasma Bioequivalence of Rusovastatin in Humans: Validation of Class III Drugs of the Salivary Excretion Classification System
 Drugs R D (2015) 15:79 83 DOI 10.1007/s40268-015-0080-1 ORIGINAL RESEARCH ARTICLE Saliva Versus Plasma Bioequivalence of Rusovastatin in Humans: Validation of Class III Drugs of the Salivary Excretion
Drugs R D (2015) 15:79 83 DOI 10.1007/s40268-015-0080-1 ORIGINAL RESEARCH ARTICLE Saliva Versus Plasma Bioequivalence of Rusovastatin in Humans: Validation of Class III Drugs of the Salivary Excretion
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
Principles of Renal Physiology. 4th Edition
 Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Can PK and Modelling Help?
 Safeguarding public health Can PK and Modelling Help? Terry Shepard Pharmacokinetics Assessor, Statistics Unit MHRA, London EMA Workshop: Ensuring safe and effective medicines for an ageing population
Safeguarding public health Can PK and Modelling Help? Terry Shepard Pharmacokinetics Assessor, Statistics Unit MHRA, London EMA Workshop: Ensuring safe and effective medicines for an ageing population
Biomath M263 Clinical Pharmacology
 Training Program in Translational Science Biomath M263 Clinical Pharmacology Spring 2013 www.ctsi.ucla.edu/education/training/webcasts Wednesdays 3 PM room 17-187 CHS 4/3/2013 Pharmacokinetics and Pharmacodynamics
Training Program in Translational Science Biomath M263 Clinical Pharmacology Spring 2013 www.ctsi.ucla.edu/education/training/webcasts Wednesdays 3 PM room 17-187 CHS 4/3/2013 Pharmacokinetics and Pharmacodynamics
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
EVALUATION OF DRUG-DRUG INTERACTION POTENTIAL BETWEEN SACUBITRIL/VALSARTAN (LCZ696) AND STATINS USING A PHYSIOLOGICALLY- BASED PHARMACOKINETIC MODEL
 Drug metabolism and Pharmacokinetics/PK Sciences EVALUATIN F DRUG-DRUG INTERACTIN PTENTIAL BETWEEN SACUBITRIL/VALSARTAN (LCZ696) AND STATINS USING A PHYSILGICALLY- BASED PHARMACKINETIC MDEL Imad Hanna,
Drug metabolism and Pharmacokinetics/PK Sciences EVALUATIN F DRUG-DRUG INTERACTIN PTENTIAL BETWEEN SACUBITRIL/VALSARTAN (LCZ696) AND STATINS USING A PHYSILGICALLY- BASED PHARMACKINETIC MDEL Imad Hanna,
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
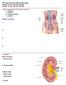 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
Culture Hepatocytes in Human Plasma to Count the free Concentration of Drug in Evaluation of Drug-drug Interaction. Chuang Lu
 Culture Hepatocytes in Human Plasma to Count the free Concentration of Drug in Evaluation of Drug-drug nteraction Chuang Lu Millennium, The Takeda Oncology Company Cambridge, MA, USA DD 205, Seattle, 6/29/205
Culture Hepatocytes in Human Plasma to Count the free Concentration of Drug in Evaluation of Drug-drug nteraction Chuang Lu Millennium, The Takeda Oncology Company Cambridge, MA, USA DD 205, Seattle, 6/29/205
Presentation of the SEURAT-1 COSMOS Project: Prediction of Systemic Toxicity Following Dermal Exposure
 Presentation of the SEURAT-1 COSMOS Project: Prediction of Systemic Toxicity Following Dermal Exposure Mark Cronin 1, Elena Fioravanzo 2, Judith Madden 1, Andrea Richarz 1, Lothar Terfloth 3, Fabian Steinmetz
Presentation of the SEURAT-1 COSMOS Project: Prediction of Systemic Toxicity Following Dermal Exposure Mark Cronin 1, Elena Fioravanzo 2, Judith Madden 1, Andrea Richarz 1, Lothar Terfloth 3, Fabian Steinmetz
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Chris Bohl, Ph.D. Global Technical Support Manager- Products
 Chris Bohl, Ph.D. Global Technical Support Manager- Products cbohl1@xenotechllc.com Sekisui XenoTech Overview GLP-compliant in vitro ADME-DMPK CRO founded in 1994 at the University of Kansas Medical Center
Chris Bohl, Ph.D. Global Technical Support Manager- Products cbohl1@xenotechllc.com Sekisui XenoTech Overview GLP-compliant in vitro ADME-DMPK CRO founded in 1994 at the University of Kansas Medical Center
Chapter 13 The Urinary System
 Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE
 UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE FACULTY OF MEDICAL SCIENCES SCHOOL OF PHARMACY BACHELOR OF SCIENCE IN PHARMACY DEGREE COURSE SYLLABUS COURSE TITLE: COURSE CODE: BIOPHARMACEUTICS, NEW DRUG DELIVERY
UNIVERSITY OF THE WEST INDIES, ST AUGUSTINE FACULTY OF MEDICAL SCIENCES SCHOOL OF PHARMACY BACHELOR OF SCIENCE IN PHARMACY DEGREE COURSE SYLLABUS COURSE TITLE: COURSE CODE: BIOPHARMACEUTICS, NEW DRUG DELIVERY
PHA First Exam Fall 2003
 PHA 5127 First Exam Fall 2003 On my honor, I have neither given nor received unauthorized aid in doing this assignment. Name Question/Points 1. /14 pts 2. /6 pts 3. /15 pts 4. /12 pts 5. /20 pts 6. /10pts
PHA 5127 First Exam Fall 2003 On my honor, I have neither given nor received unauthorized aid in doing this assignment. Name Question/Points 1. /14 pts 2. /6 pts 3. /15 pts 4. /12 pts 5. /20 pts 6. /10pts
