r - D. Brown Q. AI-Awqati
|
|
|
- Luke Griffin
- 6 years ago
- Views:
Transcription
1 r - D. Brown Q. AI-Awqati
2 J. exp. Biol. 172, (1992) 205 Primed in Great Britain The Company of Biologists Limited 1992 THE ROLE OF THE V-ATPase IN RENAL EPITHELIAL H + TRANSPORT BY STEPHEN GLUCK AND RAOUL NELSON Departments of Medicine, Pediatrics, and Cell Biology and Physiology, Washington University School of Medicine; the Division ofnephrology, St Louis Children's Hospital, and the Renal Division, Jewish Hospital, Washington University Medical Center, 216 South Kingshighway Boulevard, St Louis, MO 63110, USA Summary This article provides a brief review of the history of the identification and characterization of the renal V-ATPase, and a review of the physiology of renal bicarbonate reabsorption and H + transport. Introduction To maintain acid base homeostasis, the kidney must reabsorb the filtered bicarbonate and produce new bicarbonate to match daily non-volatile acid production. Over the last 60 years, the understanding of this process has progressed from descriptions of the response of the intact kidney through physiological manipulations down to the beginnings of an understanding of the control of renal acid excretion at the molecular level. The progressive investigation of the nature of renal H + transport ultimately resulted in a collision of classical physiology with the biochemistry and molecular biology of the V-ATPases. The concepts that emerged from the physiological studies of H + transport by urinary epithelia have provided a framework for modeling the regulation of vacuolar acidification. A brief summary of renal anatomy and function The principal function of the kidney is to control the volume and composition of the extracellular fluid. One of the most important aspects of kidney function is the maintenance of the extracellular fluid bicarbonate concentration. Each day, 4000 mmol of bicarbonate are filtered by the glomerulus and reclaimed by the kidney. In addition, mmol of hydrogen ion are produced daily by the catabolism of protein. This acid is titrated by the extracellular fluid bicarbonate and subsequently regenerated in the kidney. The kidney accomplishes both the reabsorption of filtered bicarbonate and the regeneration of bicarbonate by hydrogen ion secretion, a process in which V-ATPases have a pivotal role. Key words: kidney; acid-base; hydrogen ion transport.
3 206 S. GLUCK AND R. NELSON The human kidney is composed of 1 million individual functional units called nephrons (Fig. 1). Each nephron consists of a glomerulus, which forms a nearly protein-free ultrafiltrate of plasma from a capillary tuft, and the renal tubular epithelium, which reabsorbs and secretes solutes along its length, ultimately producing urine out of the filtrate. Tubular processing begins in the proximal tubule, a 'leaky' epithelium that is unable to sustain large electrical concentration or osmotic gradients. The proximal tubule has an apical brush border with dense microvilli providing a large surface area and reabsorbs 60-70% of the glomerular filtrate. The proximal tubule has three morphologically and functionally distinct segments, designated as the SI, S2 and S3 segments. Approximately 80% of the filtered bicarbonate is reabsorbed in the proximal tubule (Fig. 2). After leaving the proximal tubule, the filtrate enters the loop of Henle. The loop consists of the thin descending limb, the thin ascending limb and the thick ascending limb. The thin limbs do not transport solutes actively, but regulate solute and water flux out of the lumen by differential permeabilities to water and solutes. The thick ascending limb of the loop dilutes the urine by transporting solute out in excess of water and creates a hypertonic interstitium that is required for generating a concentrated urine. About 10% of the filtered bicarbonate is reabsorbed by the thick ascending limb. Filtrate leaving the loop enters the distal convoluted tubule, where additional reabsorption of sodium and 1-3% of the filtered bicarbonate takes place. The filtrate then enters the collecting tubule, which is a tight epithelium capable of generating and sustaining an electrical potential difference across the luminal membrane and large concentration and osmotic gradients. Unlike all of the other segments described above, which each consist of a relatively uniform cell type, the collecting duct is composed of two major types of cells, the principal cells (about 60% of the cells) and the intercalated cells. The principal cells reabsorb sodium, secrete potassium and absorb water and all of these transport functions are under the control of regulatory hormones. The intercalated cells secrete acid Afferent arteriole Distal convoluted tubule Connecting tubule Cortical collecting tubule Medullary collecting tubule Efferent arteriole Loop of Henle \ Fig. 1. Diagram of the nephron showing the different segments of the tubule discussed in the text.
4 V-ATPase in kidney H + transport 207 PCT TALH DCT Nephron segment CCT OMCD 1MCD Fig. 2. Sites for bicarbonate reabsorption along the nephron. PCT, proximal convoluted tubule; TALH, thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; CCT, cortical collecting tubule; OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct. and actively reabsorb potassium; a subpopulation of intercalated cells also secretes bicarbonate and reabsorbs chloride. A historical perspective on urinary acidification Studies in the 1930s demonstrated that the kidney could reabsorb all of the filtered bicarbonate ion and could excrete quantities of acid exceeding the amount filtered by the glomerulus and therefore must be secreting acid into the urine (Steinmetz, 1974). The next level in understanding the process of renal bicarbonate reabsorption came with the advent of methods to analyze the contributions of different parts of the nephron. The micropuncture method developed in the 1950s allows one only to sample tubular fluid by micropipette from the portions of the nephron that appear on the kidney surface. This method limited the analysis of the segmental regulation of bicarbonate reabsorption to the proximal tubule, the distal convoluted tubule and the cortical collecting tubule. Studies with the micropuncture method revealed that, although most of the bicarbonate is reabsorbed in the proximal tubule, the final regulation of bicarbonate reabsorption occurred in the distal parts of the nephron beyond the loop of Henle. For example, when animals were given a high-acid diet, they showed very little change in proximal tubular bicarbonate reabsorption, but greatly increased their excretion of acid in the distal nephron. After an initial understanding of the sites of bicarbonate reabsorption had developed, investigations focused on the cellular mechanism of bicarbonate reabsorption. Was bicarbonate ion itself transported, or was bicarbonate reabsorbed as a result of hydrogen ion secretion titrating the bicarbonate to CO2, which then diffused across the epithelium? It had been known for some time tfiat the kidney was rich in the enzyme carbonic anhydrase, which catalyzed the dehydration of carbonic acid to CO2 and water and several inhibitors of the enzyme had been identified. It was demonstrated that
5 208 S. GLUCK AND R. NELSON inhibition of carbonic anhydrase produced an 'acid disequilibrium ph' in the lumen of the kidney tubule, defined as a ph in the fluid of the tubular lumen that was lower than the ph of the same fluid allowed to come to equilibrium. This result suggested that the kidney secreted hydrogen ion as a mechanism for bicarbonate reabsorption. Since estimates of intracellular ph from the distribution of weak acids showed that the cytoplasmic ph of the renal tubular cell was very close to the ph of extracellular fluid, it was clear from thermodynamic considerations that the secretion of hydrogen ion must be due to an active transporter, probably present in the luminal membrane (Steinmetz, 1974). The next advances in elucidating the cellular mechanisms responsible for active hydrogen ion secretion in epithelia were made in the amphibian and reptilian bladder. The bladder of these lower vertebrates performs many of the functions carried out by the mammalian collecting duct. The bladder could be mounted as a flat sheet separating two chambers and the fluxes of ions and current could be easily measured. The turtle bladder reabsorbed sodium and acidified its lumen; treatment with ouabain on the serosal side eliminated sodium transport and allowed the characteristics of hydrogen ion secretion to be examined. The turtle bladder could be shown to acidify its lumen in the virtual absence of CO2, again supporting the model of hydrogen ion secretion as a means for bicarbonate reabsorption. After its transport of sodium had been eliminated with ouabain, the bladder generated a lumen-positive potential difference. When acidification of the lumen was studied in bladders under short-circuited conditions, which allows the net charge transferred across the bladder to be measured simultaneously with ion fluxes, it was found that the rate of hydrogen ion secretion (measured with an automatic titration device) exactly matched the addition of positive charge to the lumen. This result suggested that the pump was electrogenic. The imposition of an adverse ph gradient or a lumen-positive potential difference across the lumen slowed the rate of proton transport equally if the magnitude of the ph gradient were converted to an equivalent Nernst potential. These findings also supported the model that the pump was electrogenic. The luminal acidification was dependent on metabolism and was nullified under conditions where bladder ATP production was inhibited. These findings and the electrogenic nature of the transporter suggested that an active proton pump was present. Studies with inhibitors showed that acidification was eliminated by the addition of dicyclohexylcarbodiimide (DCCD) to the lumen, suggesting that the pump might resemble the F-ATPases, but further characterization of the enzyme became difficult in the intact cell (Al-Awqati, 1978; Steinmetz, 1986). During the time that these advances in understanding the cellular basis of proton transport in epithelia were emerging from studies in the turtle urinary bladder, physiological methods for studying the transport function of individual segments of the nephron had improved greatly. With the isolated perfused tubule technique, individual segments of almost any part of the nephron could be isolated by microdissection and perfused, allowing their transport properties to be determined. This approach revealed that the collecting duct of the kidney had an electrogenic proton pump and an assortment of other ion transporters nearly identical to those identified earlier in the turtle urinary bladder (Steinmetz, 1986). The next stride in identification of the nature of the renal epithelial proton pump came
6 V-A TPase in kidney H + transport 209 with the development of methods to examine proton transport in isolated membrane vesicles. Studies in mitochondrial inner membrane vesicles, in membrane vesicles from the stomach, chromaffin granules and other tissues had shown that addition of ATP to the vesicles caused the membranes to take up a weak base upon acidification of the vesicle interior. Membranes isolated from the turtle urinary bladder were found to have an electrogenic ATP-initiated acidification that was not inhibited by the mitochondrial F- ATPase inhibitors azide and oligomycin, distinguishing the proton pump in these vesicles from contaminating submitochondrial vesicles. The kidney membrane proton pump was also resistant to vanadate, distinguishing it from the P-ATPases, but was sensitive to DCCD and sulfhydryl reagents. The proton pump in turtle bladder membranes therefore had the properties that subsequently would be found in all V-ATPases (Gluck et al. 1982). A proton pump with properties identical to those in the turtle bladder was found in the mammalian kidney (Gluck and Al-Awqati, 1984). Simultaneously, reports were emerging from numerous laboratories that proton pumps acidifying a variety of different vacuolar compartments also had the same properties (Forgac, 1989). Was the proton pump studied in the kidney the same enzyme responsible for urinary acidification, or were the proton-transporting membranes in the bladder and kidney merely contaminants from vacuolar compartments? If this enzyme was responsible for urinary acidification, what were the properties of this new type of proton pump? These issues were not resolved until the proton pump had been isolated and its structure and location in the kidney established. The studies of the proton pump in kidney membrane vesicles had provided an assay for the enzyme: vanadate- and azide-resistant A L ethylmaleimide (NEM)-sensitive ATPase activity (Gluck and Al-Awqati, 1984). With the use of this assay, the V-ATPase from the kidney was isolated by a multiple step chromatographic procedure (Gluck and Caldwell, 1987, 1988). Its structure was strikingly similar to that of the V-ATPase from yeast (Anraku; Stevens; Kane; this volume), Neurospora (B. Bowman and E. Bowman, this volume), plant (Sze; Taiz; this volume) and coated vesicle (Forgac; Stone; this volume). The multi-subunit structure of these enzymes and their biochemical properties are discussed in detail in this volume. Monoclonal and polyclonal antibodies were prepared against the isolated kidney V- ATPase, allowing its location in the kidney to be established by immunocytochemistry (Brown et al. 1988a,i>). These experiments confirmed that the kidney V-ATPase resides on the plasma membrane of the renal tubular epithelial cells in the same segments of die nephron in which proton pumps had been identified by physiological studies. Cellular mechanisms of proton and bicarbonate transport in the nephron Proximal tubule Fig. 3 shows a schematic drawing of transporters engaged in proton and bicarbonate transport in the proximal tubule. (Note that this drawing excludes many of the other transport proteins of the proximal tubule, such as the sodium cotransporters for glucose, phosphate, amino acid and organic anion transport.) The proximal tubule is a leaky epithelium responsible for reabsorption of over 60% of the glomerular filtrate and has a dense apical brush border, composed of microvilli, enhancing its capacity for bulk
7 210 S. GLUCK AND R. NELSON CA1V H2O + CO2 Fig. 3. Diagram of proximal tubule bicarbonate transport. CA, carbonic anhydrase. transport. Each of the three segments of the proximal tubule, SI, S2 and S3, is composed of a uniform population of cells whose morphology and transport properties vary from those of the other proximal segments. The proximal tubule reabsorbs approximately 80% of the bicarbonate entering the lumen in the glomerular filtrate. The secretion of H + results in the formation of luminal carbonic acid, which is then dehydrated efficiently to CO2 and water by type IV carbonic anhydrase, a glycophosphatidylinositol-linked ectoenzyme residing in the brush border. Two-thirds of apical H + secretion originates from a brush-border Na + /H + antiporter; the remainder arises from a V-ATPase present in the microvilli only in the initial part of the proximal tubule and in the apical invaginations at the base of the microvilli in the entire proximal tubule (Brown et al. 1988a,b). The brush-border V-ATPase has enzymatic and structural differences from the V-ATPase in microsomes (Wang and Gluck, 1990; Simon and Burckhardt, 1990), much of which may originate from intercalated cells and lysosomes (Gluck, this volume). Hydroxyl ions generated intracellularly by the Na + /H + antiporter and V-ATPase in the brush border are converted into bicarbonate ion by cytosolic type II carbonic anhydrase. Bicarbonate exits the basolateral membrane mostly through an electrogenic Na + /HCO3~ symporter that probably transports lna +, lhccb" and 1CO3 2 ~ together, resulting in net exit of two negative charges, driven by the inside-negative potential. An electroneutral C1~/HCO3~ antiporter accounts for less than 10% of basolateral bicarbonate exit (Alpern, 1990). Regulation of bicarbonate reabsorption in the proximal tubule In the proximal tubule, proton secretion varies with the plasma bicarbonate concentration, the plasma Pco 2» the plasma potassium concentration, plasma angiotensin II levels and several other factors. All of these factors affect the rate of luminal Na + /H + antiporter activity; it has not been established whether they also affect the luminal V- ATPase activity. The brush-border Na + /H + antiporter has a regulatory site in the cytoplasmic domain that increases the rate of antiport greatly at ph values below 7.2. An or increase in the Pco 2 a decrease in the plasma bicarbonate concentration acidifies the cytosol and stimulates the antiporter; the opposite changes in Pco* and plasma bicarbonate concentration suppress antiporter activity. Elevations in the plasma
8 V-ATPase in kidney H + transport 211 potassium concentration depolarize the proximal tubule cell, reducing the voltage-driven basolateral exit of bicarbonate; the resulting elevation of intracellular ph suppresses the luminal Na + /H + antiporter. Lowering the plasma potassium concentration hyperpolarizes the cell, increases bicarbonate exit and consequently stimulates the luminal Na + /H + antiporter. Angiotensin stimulates the luminal antiporter directly through an undetermined mechanism, by signalling mechanisms involving a change in intracellular calcium concentration. Since the proximal tubule absorbs 80% of the filtered bicarbonate, suppression of proximal H + secretion by only 10% can increase bicarbonate delivery to the loop of Henle by 30%, resulting in up to a two fold increase in bicarbonate delivery to the collecting tubule; the extra bicarbonate is excreted unless there are stimuli for increased collecting tubule H + secretion (discussed below). This capacity of the proximal tubule to excrete bicarbonate efficiently allows the kidney to avoid elevations in the plasma bicarbonate concentration. In several clinical conditions, for example extracellular fluid volume depletion and vomiting, factors that stimulate H + secretion, such as angiotensin II and potassium depletion, may impair the capacity to excrete bicarbonate and produce an elevated serum bicarbonate concentration (alkalosis). Thick ascending limb The apical membrane of the thick ascending limb has a Na + /H + antiporter that is responsible for 80% of the hydrogen ion secretion in this segment; a V-ATPase in the apical membrane secretes the remaining 20% (Good, 1990; Borensztein et al. 1991). Bicarbonate generated intracellularly exits the basolateral membrane, probably through a Na + /HCO3~ cotransporter similar to the one in the proximal tubule. Regulation of bicarbonate transport in the thick ascending limb In the thick ascending limb, proton secretion is affected by vasopressin, glucagon and the non-volatile acid intake or production of the animal. Vasopressin, glucagon and any agent that raises cell cyclic AMP levels suppress H + secretion; much of this effect is probably on the apical Na + /H + antiporter, although an effect on the plasma membrane V- ATPase has not been excluded. Acidosis (acidification of the extracellular fluid of the animal) stimulates thick ascending limb H + secretion, possibly by lowering cell ph and stimulating the apical Na + /H + antiporter through an internal ph-sensitive modifier site. Distal convoluted tubule The distal convoluted tubule consists of a uniform cell type whose primary functions are sodium reabsorption and potassium secretion. In normal animals, bicarbonate reabsorption rates are low in the distal convoluted tubule, but increase fivefold in animals subjected to a sustained increase in acid intake, or to potassium depletion (Lucci et al. 1982; Capasso et al. 1986). The mechanism responsible for this increase is unknown. An apical plasma membrane V-ATPase appears to be the sole transporter engendering luminal hydrogen ion secretion in this segment and the mechanism for basolateral bicarbonate exit has not been established.
9 212 S. GLUCK AND R. NELSON Collecting tubule Fig. 4 shows a schematic drawing of transporters engaged in proton and bicarbonate transport in the cortical collecting tubule. The collecting tubule has two major cell types, the principal cells and the intercalated cells and is made up of several morphologically and functionally distinct segments: the connecting tubule, the cortical collecting tubule and the outer medullary collecting tubule, consisting of the outer and inner stripe. The inner medullary collecting tubule (or papillary collecting tubule) has intercalated cells only in the first third; the last two-thirds of the segment consists of a uniform cell type called principal cells. The connecting tubule principal cells resemble the cells of the distal convoluted tubule in having low levels of a V-ATPase on the apical plasma membrane and resemble principal cells of the cortical collecting tubule in having an apical amiloride-sensitive sodium channel and an apical potassium channel. The mechanism for basolateral bicarbonate exit has not been established. The Na + /K + -ATPase in the basolateral membrane maintains a low cell sodium concentration and an inside-negative potential that provides a driving force for luminal sodium entry down its electrochemical gradient. The connecting tubule normally has a lumen-negative voltage of 5 to 30 mv as a result of the diffusion potential created by sodium entering through conductive apical channels. This lumen-negative potential is an important driving force for potassium secretion through apical potassium channels in the principal cell and for electrogenic H + secretion by the A-type intercalated cell. In both the connecting tubule and cortical collecting tubule, there are two major types of intercalated cells, the acid-secreting 'A-type' (or a) intercalated cells and the bicarbonate-secreting 'B-type' (or /3) intercalated cells (Brown et al. \98Sa,b; Steinmetz, 1986; Schuster, l990a,b). The A-type intercalated cell is designed for electrogenic transepithelial H + secretion. A-type intercalated cells have a V-ATPase in the luminal (apical) pole of the cell that is present both in the plasma membrane and in a specialized \ Na + m r K + Principal cell 1K + B-type intercalated cell ci- Na + II n v K + Principal cell A. A. 1. HCO3-» rci- A-type intercalated cell K + K + Na + HCO 3 - Cyclic AMP LUMEN -5 to -30 mv Aldosterone Fig. 4. Diagram of cortical collecting tubule bicarbonate and proton transport.
10 V-ATPase in kidney H + transport 213 intracellular tubulovesicular system (Brown et al. \9%&a,b, 1989) from which additional V-ATPase-containing membrane may be recruited to the plasmalemma in response to an appropriate physiological stimulus (Schwartz and Al-Awqati, 1985; Bastani et al. 1991). Cytosolic hydroxyl ions generated by the plasma membrane V-ATPase are converted to HCCb" by type II carbonic anhydrase (Brown, 1989; Kim et al. 1990; Brechue et al. 1991) and the bicarbonate exits the basolateral membrane through a Cl~/HC03~antiporter that is an exon splice variant of the red cell anion transporter (Brosius et al. 1989), known commonly as band 3 protein; antibodies to red cell band 3 stain the basolateral membrane of the A-type intercalated cell (Drenckhahn et al. 1985; Schuster et al. 1986; Verlander et al. 1988; Alper et al. 1989). The chloride taken up by Cl~/HCO3~antiport exits the cell through a basolateral chloride channel (Died et al. 1991). The location of these transporters in the A-type intercalated cell preserves the electroneutrality of cellular ion fluxes, but maintains electrogenic transepithelial hydrogen ion secretion. The A-type intercalated cells also have an electroneutral H + /K + - ATPase, similar to the enzyme in the stomach, in the apical membrane (Wingo et al. 1990); the major physiological role of this transporter is probably not proton secretion, but active potassium reabsorption during potassium depletion. The B-type intercalated cell is designed for electroneutral chloride reabsorption and bicarbonate secretion. B-type intercalated cells have a V-ATPase on the basolateral membrane (Brown et al. 1988a,i»). Bicarbonate generated intracellularly exits on the luminal side through an electroneutral C1~/HCO3~ antiporter (Schuster, 1991) that does not cross-react with antibodies to band 3 (Schuster et al. 1986; Verlander et al. 1988; Alper et al. 1989) and whose structure is unknown. This system of transporters provides a mechanism for active transepithelial chloride reabsorption. Chloride enters from the lumen through the electroneutral transporter down a concentration gradient; cell chloride concentration is low because of the inside-negative potential of the cell. Chloride normally exits, driven by the insidenegative potential, through a chloride channel in the basolateral membrane. As discussed below, /3-adrenergic agents open a chloride channel in the luminal membrane (Schuster and Stokes, 1987). The transport properties of the cortical collecting tubule have very few differences from those of the connecting tubule. The principal cells of the cortical collecting tubule have no detectable plasma membrane V-ATPase; they have an apical amiloride-sensitive sodium channel and an apical potassium channel. The cortical collecting tubule also maintains a lumen-negative voltage of 5 to 30 mv as a result of the sodium diffusion potential. The cortical collecting tubule has both A-type and B-type intercalated cells that have nearly the same transport properties as those in the connecting tubule. The A-type intercalated cell in the cortical collecting tubule has a Na + /H + antiporter in the basolateral membrane that is the primary regulator of intracellular ph (Weiner and Hamm, 1990); it has not yet been determined whether the A-type intercalated cells of the connecting tubule also have this transporter. Fig. 5 shows a schematic drawing of transporters engaged in proton and bicarbonate transport in the outer medullary collecting tubule. The outer medulla is the portion of the renal medulla that contains thick ascending limbs. It contains two anatomical regions: the outer stripe, which contains S3 segments of the proximal tubule (also called the pars
11 214 S. GLUCK AND R. NELSON Cl- HC03- Na+ K+ HCO3- LUMEN +5mV Fig. 5. Diagram of medullary collecting tubule bicarbonate and proton transport. recta) and the inner stripe, which lacks S3 segments. The outer medullary collecting tubule in the outer and inner stripes contains principal cells, which do not have any demonstrable plasma membrane V-ATPase. No sodium reabsorption has been found in this segment. The transepithelial potential is normally at least +5 mv as a result of electrogenic proton secretion by the intercalated cells. All of the intercalated cells in the outer medulla are A-type cells, with a V-ATPase in the plasmalemma and a tubulovesicular system at the apical pole, an H + /K + -ATPase in the apical membrane, and a band-3-immunoreactive Cl~/HCO3~antiporter, a chloride channel and a Na + /H + antiporter (Breyer and Jacobson, 1989) in the basolateral membrane. Some evidence suggests that the electroneutrality of transepithelial H + transport in the outer medullary collecting tubule may be maintained by a paracellular chloride permeability (Stone et al. 1983). Regulation of ion transport in the collecting tubule The rate of hydrogen ion transport in the collecting duct is influenced by a number of factors. In the cortical and outer medullary collecting tubule, proton secretion by the A- type intercalated cells varies with the proton electrochemical gradient across the apical plasma membrane, plasma aldosterone levels, the plasma bicarbonate concentration, the plasma Pco 2 and the non-volatile acid intake or production of the animal. ph and electrical gradients vary the rate of electrogenic H + secretion, presumably by direct kinetic effects of imposing an adverse electrochemical gradient across the V-ATPase. An increase in the lumen-negative potential stimulates proton secretion. A decrease in urinary ph suppresses H + secretion; net acid secretion stops at an apical ph of between 4.5 and 5.0. The quantity of hydrogen ion secreted depends on the concentration of urinary buffers. The principal buffers are phosphate and ammonia. As urine ph declines, the partition of ammonia into the collecting duct lumen increases, permitting more
12 V-A TPase in kidney H + transport 215 protons to be excreted. Increased acid intake and several other factors that stimulate hydrogen ion secretion also increase the generation of ammonia by the proximal tubule. Aldosterone increases H + secretion by elevating the apical sodium conductance of principal cells (in the cortical collecting tubule), thus increasing the lumen-negative potential. It also stimulates H + secretion directly, by an undetermined mechanism, independently of changes in the transepithelial potential. An increase or decrease in the plasma bicarbonate concentration suppresses or stimulates, respectively, H + secretion. The mechanism of these effects is poorly understood. Decreasing the plasma bicarbonate level lowers the intracellular ph (phi) of the intercalated cell; cytosolic acidification produces a transient and sustained elevation in intracellular calcium activity (Hays and Alpern, 1991). The effects of the elevated cell calcium activity are complex. The sustained elevation of calcium activity causes an immediate inhibition of V-ATPase activity; the transient elevation produces a delayed increase in V-ATPase that is inhibited by cytochalasin D, possibly indicating membrane insertion (Hays and Alpern, 1991). Elevations in the plasma Pco 2 stimulate H + secretion; at least part of this effect entails inducing recruitment of V-ATPase to the apical membrane by exocytosis (Gluck et al. 1982; Schwartz and Al-Awqati, 1985; Steinmetz, 1986; Verlander et al. 1987; Brown, V9%9a,b), but changes in the kinetics of the V-ATPase also appear to participate (McKinney and Davidson, 1987, 1988). Administration of HC1 to rats produces a profound redistribution of V-ATPase from the intracellular tubulovesicular compartment to the apical plasma membrane in A-type intercalated cells (Madsen and Tisher, 1984; Bastani et al. 1991); the magnitude of the response does not appear to be controlled directly by the plasma bicarbonate concentration (Bastani etal. 1991). Chloride reabsorption and bicarbonate secretion by the B-type intercalated cells, which are present only in the connecting tubule and cortical collecting tubule, vary with the luminal chloride concentration, /3-adrenergic stimulation and the non-volatile acid intake or production of the animal. Apical chloride delivery increases Cl~/HC03~ antiport by concentration-dependent kinetic effects (Schuster and Stokes, 1987; Levine et al. 1990). /3-Adrenergic stimulation and other agents that raise cyclic AMP levels, stimulates apical C1~/HCO3~ antiport by an undetermined mechanism. The same agents may also increase the apical chloride conductance of the B-type intercalated cell, allowing chloride to exit on the apical side, endowing a net effect of electrogenic bicarbonate secretion (Schuster, 1989, 1991). The physiological role of the response to /3-adrenergic agents may be to facilitate potassium excretion by increasing the lumen-negative potential in the cortical collecting tubule. Administration of HC1 to rats or rabbits suppresses cortical collecting tubule bicarbonate secretion by an unknown mechanism (McKinney and Burg, 1977; Levine etal. 1988). In chloride depletion, apical C1~/HCO3~ antiport is stimulated (Galla et al. 1991), accompanied by an increase in the amount of immunocytochemically detectable V-ATPase in the basolateral membrane of the B-type intercalated cells (Verlander et al. 1992). The arrangement of transporters in the cortical and outer medullary collecting tubule provides a great deal of flexibility in regulating ion reabsorption and secretion. In response to extracellular fluid volume depletion, aldosterone stimulates conductive sodium reabsorption, electrogenic hydrogen ion secretion and electroneutral C1~/HCO3~
13 216 S. GLUCK AND R. NELSON antiport, resulting in the net reabsorption of NaCl and NaHC03. Increased acid intake suppresses electroneutral Cl~/HC03~" antiport, giving an increased reabsorption and generation of HC03~. We thank Debbie Windle for assistance in preparing the manuscript. This manuscript was supported by NIH grants DK38848, DK09976 and AR32087, by a grant from the Monsanto-Washington University Fund and by the Washington University George M. O'Brien Kidney and Urological Diseases Center P50-DK S.L.G. is a Sandoz Pharmaceutical Corporation Established Investigator of the American Heart Association. References AL-AWQATI, Q. (1978). H + transport in urinary epithelia. Am. J. Physiol. 235, F77-F88. ALPER, S., NATALE, J., GLUCK, S., LODISH, H. AND BROWN, D. (1989). Intercalated cell subtypes in rat kidney collecting duct defined using antibodies against erythrocyte band 3 and renal vacuolar H + ATPase. Proc. natn. Acad. Sci. U.S.A. 86, ALPERN, R. J. (1990). Cell mechanisms of proximal tubule acidification. Physiol. Rev. 70, BASTANI, B., PURCELL, H., HEMKEN, P., TRIGC, D. AND GLUCK, S. (1991). Expression and distribution of renal vacuolar H + -ATPase in response to chronic acid and alkali loads in the rat. J. din. Invest. 88, BORENSZTEIN, P., LEVIEL, F., FROISSART, M., HOUILLIER, P., POGGIOLI, J., MARTY, E., BICHARA, M. AND PAILLARD, M. (1991). Mechanisms of H + /HC03~ transport in the medullary thick ascending limb of rat kidney. Kidney Int. (Suppl.) 33, S43-S46. BRECHUE, W. F., KINNE-SAFFRAN, E., KINNE, R. K. AND MAREN, T. H. (1991). Localization and activity of renal carbonic anhydrase (CA) in CA-IT deficient mice. Biochim. biophys. Ada 1066, BREYER, M. D. AND JACOBSON, H. R. (1989). Regulation of rabbit medullary collecting duct cell ph by basolateral Na + /H + and Cr/base exchange. J. din. Invest. 84, BROSIUS, F. C, ALPER, S. L., GARCIA, A. M. AND LODISH, H. F. (1989). The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J. biol. Chem, 264, BROWN, D. (1989a). Vesicle recycling and cell-specific function in kidney epithelial cells. A. Rev. Physiol. 51, BROWN, D. (1989b). Membrane recycling and epithelial cell function. Am, J. Physiol. 256, F1-F12. BROWN, D., HIRSCH, S. AND GLUCK, S. (1988fl). An H + -ATPase is inserted into opposite plasma membrane domains in subpopulations of kidney epithelial cells. Nature 331, BROWN, D., HIRSCH, S. AND GLUCK, S. (1988 ). Localization of a proton pumping ATPase in rat kidney. J. din. Invest. 82, BROWN, D., ZHU, X. L. AND SLY, W. S. (1990). Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. Proc. natn. Acad. Sci. U.S.A. 87, CAPASSO, G., KJNNE, R., MALNIC, G. AND GIEBISCH, G. (1986). Renal bicarbonate reabsorption in the rat. I. Effects of hypokalemia and carbonic anhydrase. J. din. Invest. 78, DIETL, P., SCHWIEBERT, E. AND STANTON, B. A. (1991 ) Cellular mechanisms of chloride transport in the cortical collecting duct. Kidney. Int. (Suppl.) 33, S125-S130. DRENCKHAHN, D., SCHLUTER, K., ALLEN, D. P. AND BENNETT, V. (1985). Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science 230, FORGAC, M. (1989). Structure and function of vacuolar class of ATP-driven proton pumps. Physiol. Rev. 69, GALLA, J. H., GIFFORD, J. D., LUKE, R. G. AND ROME, L. (1991). Adaptations to chloride-depletion alkalosis. Am. J. Physiol. 261, R771-R781. GLUCK, S. AND AL-AWQATI, Q. (1984). An electrogenic proton-translocating adenosine triphosphate from bovine kidney medulla. J. din. Invest. 73, GLUCK, S. AND CALDWELL, J. (1987). Immunoaffinity purification and characterization of vacuolar H + - ATPase from bovine kidney. J. biol. Chem. 262,
14 V-A TPase in kidney H + transport 217 GLUCK, S. AND CALDWELL, J. (1988). Proton translocating ATPase from bovine kidney medulla. Am. J. Physiol. 254.F71-F79. GLUCK, S., CANNON, C. AND AL-AWQATI, Q. (1982). Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H + pumps into the apical membrane. Proc. natn. Acad. Sci. U.S.A. 79, 4327^1331. GLUCK, S., KELLY, S. AND AL-AWQATI, Q. (1982). The proton translocating ATPase responsible for urinary acidification. J. biol. Chem. 257, GOOD, D. (1990). Bicarbonate absorption by the thick ascending limb of Henle's loop. Semin, Nephrol. 10, HAYS, S. R. AND ALPERN, R. J. (1991). Inhibition of Na( + )-independent H + pump by Na( + )-induced changes in cell Ca 2+. J. gen. Physiol. 98, KIM, J., TISHER, C. C, LINSER, P. J. AND MADSEN, K. M. (1990). Ultrastructural localization of carbonic anhydrase II in subpopulations of intercalated cells of the rat kidney. J. Am. Soc. Nephrol. 1, LEVINE, D. Z., IACOVITTI, M., NASH, L. AND VANDORPE, D. (1988). Secretion of bicarbonate by rat distal tubules in vivo. Modulation by overnight fasting. J. din. Invest. 81, LEVINE, D. Z., VANDORPE, D., IACOVITTI, M. AND HARRISON, V, (1990). Luminal chloride modulates rat distal tubule bidirectional bicarbonate flux in vivo. J. din. Invest. 85, Lucci, M. S., PUCACCO, L. R., CARTER, N. W. AND DUBOSE, T. D. (1982). Evaluation of bicarbonate transport in rat distal tubule: effects of acid-base status. Am. J. Physiol. 243, F335-F341. MADSEN, K. M. AND TISHER, C. C. (1984). Response of intercalated cells of rat outer medullary collecting duct to chronic metabolic acidosis. 1Mb. Invest. 51, MCKINNEY, T. D. AND BURG, M. B. (1977). Bicarbonate transport by rabbit cortical collecting tubules: effect of acid and alkali loads in vivo on transport in vitro. J. din. Invest. 60, MCKINNEY, T. D. AND DAVIDSON, K. K. (1987). Bicarbonate transport in collecting tubules from outer stripe of outer medulla of rabbit kidneys. Am. J. Physiol. 253, F816-F822. MCKJNNEY, T. D. AND DAVIDSON, K. K. (1988). Effects of respiratory acidosis on HCC>3~ transport by rabbit collecting tubules. Am. J. Physiol. 255, F656-F665. SCHUSTER, V. (1990a). Bicarbonate reabsorption and secretion in die cortical and outer medullary collecting tubule. Semin. Nephrol. 10, SCHUSTER, V. J., BONSIB, S. M. AND JENNINGS, M. L. (1986). Two types of collecting duct mitochondriarich (intercalated) cells: lectin and band 3 cytochemistry. Am. J. Physiol. 251, C347-C355. SCHUSTER, V. L. (1989). Physiology and cell biology update: control mechanisms for bicarbonate secretion. Am. J. Kidney Dis. 13, SCHUSTER, V. L. (19906). Organization of collecting duct intercalated cells. Kidney Int. 38, SCHUSTER, V. L. (1991). Cortical collecting duct bicarbonate secretion. Kidney Int. (Suppl.) 33, S47-S50. SCHUSTER, V. L. AND STOKES, J. B. (1987). Chloride transport by the cortical and outer medullary collecting duct. Am. J. Physiol. 253, F203-F2I2. SCHWARTZ, G. J. AND AL-AWQATI, Q. (1985). Carbon dioxide causes exocytosis of vesicles containing H + pumps in isolated perfused proximal and collecting tubules. J. din. Invest. 75, SIMON, B. J. AND BURCKHARDT, G. (1990). Characterization of inside-out oriented H( + )-ATPases in cholate-pretreated renal brush-border membrane vesicles. J. Membr. Biol. 117, STEINMETZ, P. R. (1974). Cellular mechanisms of urinary acidification. Physiol. Rev. 54, STEINMETZ, P. R. (1986). Cellular organization of urinary acidification. Am. J. Physiol. 251, F173-F187. STONE, D. K., KOKKO, J. P. AND JACOBSON, H. R. (1983). Anion dependence of rabbit medullary collecting duct acidification. J. din. Invest. 71, VERLANDER, J., MADSEN, K. M. AND TISHER, C. C. (1987). Effect of acute respiratory acidosis on two populations of intercalated cells in rat cortical collecting duct. Am. J. Physiol. 253, Fl 142 F1156. VERLANDER, J. W., MADSEN, K. M., GALLA, J. H., LUKE, R. G. AND TISHER, C. C. (1992). Response of intercalated cells to chloride depletion metabolic alkalosis. Am. J. Physiol. 262, F309-F319. VERLANDER, J. W., MADSEN, K. M., LOW, P. S., ALLEN, D. P. AND TISHER, C. C. (1988). Immunocytochemical localization of band 3 protein in the rat collecting duct. Am. J. Physiol. 255, F115-F125. WANG, Z.-Q. AND GLUCK, S. (1990). Isolation and properties of bovine kidney brush-border vacuolar H + -ATPase: A proton pump with enzymatic and structural differences from kidney microsomal H + - ATPase. J. biol. Chem. 265,
15 218 S. GLUCK AND R. NELSON H + -ATPase: A proton pump with enzymatic and structural differences from kidney microsomal H + - ATPase. / biol. Chem. 265, WEINER, D. AND HAMM, L. L. (1990). Regulation of intracellular ph in the rabbit cortical collecting tubule. J. din. Invest. 85, WINGO, C. S., MADSEN, K. M., SMOLKA, A. AND TISHER, C. C. (1990). H-K-ATPase immunoreactivity in cortical and outer medullary collecting duct. Kidney Int. 38,
The regulation of renal acid secretion: New observations from studies of distal nephron segments
 Kidney International, Vol. 29 (1986), pp. 1099 1109 EDITORIAL REVIEW The regulation of renal acid secretion: New observations from studies of distal nephron segments Forty years ago, in a landmark paper,
Kidney International, Vol. 29 (1986), pp. 1099 1109 EDITORIAL REVIEW The regulation of renal acid secretion: New observations from studies of distal nephron segments Forty years ago, in a landmark paper,
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Normal Renal Function
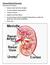 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Sodium and chlorine transport
 Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Identify and describe. mechanism involved in Glucose reabsorption
 Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Diuretics having the quality of exciting excessive excretion of urine. OED. Inhibitors of Sodium Reabsorption Saluretics not Aquaretics
 Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
MS1 Physiology Review of Na+, K+, H + /HCO 3. /Acid-base, Ca+² and PO 4 physiology
 MS1 Physiology Review of,, / /Acidbase, Ca+² and PO 4 physiology I. David Weiner, M.D. Professor of Medicine and Physiology University of Florida College of Medicine Basic principles Proximal tubule Majority
MS1 Physiology Review of,, / /Acidbase, Ca+² and PO 4 physiology I. David Weiner, M.D. Professor of Medicine and Physiology University of Florida College of Medicine Basic principles Proximal tubule Majority
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
ACID-BASE BALANCE URINE BLOOD AIR
 ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
organs of the urinary system
 organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis.
 Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
The Urinary S. (Chp. 10) & Excretion. What are the functions of the urinary system? Maintenance of water-salt and acidbase
 10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
28/04/2013 LEARNING OUTCOME C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS STUDENT ACHIEVEMENT INDICATORS URINARY SYSTEM & EXCRETION
 LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
PARTS OF THE URINARY SYSTEM
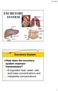 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
April 08, biology 2201 ch 11.3 excretion.notebook. Biology The Excretory System. Apr 13 9:14 PM EXCRETORY SYSTEM.
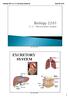 Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Chapter 13 The Urinary System
 Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Diagram of the inner portions of the kidney
 Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
12/7/10. Excretory System. The basic function of the excretory system is to regulate the volume and composition of body fluids by:
 Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
BLOCK REVIEW Renal Physiology. May 9, 2011 Koeppen & Stanton. EXAM May 12, Tubular Epithelium
 BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
Hill et al. 2004, Fig. 27.6
 Lecture 25, 15 November 2005 Osmoregulation (Chapters 25-28) Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 1. Osmoregulation 2. Kidney Function Text: Chapters
Lecture 25, 15 November 2005 Osmoregulation (Chapters 25-28) Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 1. Osmoregulation 2. Kidney Function Text: Chapters
Homeostatic Regulation
 Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-
 Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
Urinary System. Dr. ZHANG Xiong. Dept. of Physiology. ZJU School of Medicine. QUESTION 6
 Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine http://10.71.121.158 Copyright@ Xiong Zhang QUESTION 6 How is the filtrate reabsorbed in tubular system? Copyright@ Xiong Zhang
Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine http://10.71.121.158 Copyright@ Xiong Zhang QUESTION 6 How is the filtrate reabsorbed in tubular system? Copyright@ Xiong Zhang
PRINCIPLES OF DIURETIC ACTIONS:
 DIURETIC: A drug that increases excretion of solutes Increased urine volume is secondary All clinically useful diuretics act by blocking Na + reabsorption Has the highest EC to IC ratio = always more sodium
DIURETIC: A drug that increases excretion of solutes Increased urine volume is secondary All clinically useful diuretics act by blocking Na + reabsorption Has the highest EC to IC ratio = always more sodium
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
1. remove: waste products: urea, creatinine, and uric acid foreign chemicals: drugs, water soluble vitamins, and food additives, etc.
 Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Making Water! OR is it really Just Water Just Ask the Nephron!! Author: Patricia L. Ostlund ostlundp@faytechcc.edu (910) 678-9892 Fayetteville Technical Community College Fayetteville, NC 28303 Its just
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
DIURETICS-2. Dr. Shariq Syed. Shariq AIKC/TYB/2014
 DIURETICS-2 Dr. Syed Structure of Kidney Blood filtered by functional unit: Nephron Except for cells, proteins, other large molecules, rest gets filtered Structure of Kidney 3 major regions of nephron
DIURETICS-2 Dr. Syed Structure of Kidney Blood filtered by functional unit: Nephron Except for cells, proteins, other large molecules, rest gets filtered Structure of Kidney 3 major regions of nephron
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Renal System and Excretion
 Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
RENAL. 6. Renal acid secretion is affected by all of the following except a. pco 2 b. K c. Carbonic anhydrase d. Aldosterone e. Ca
 RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Therapeutics of Diuretics
 (Last Updated: 08/22/2018) Created by: Socco, Samantha Therapeutics of Diuretics Thambi, M. (2017). The Clinical Use of Diuretics. Lecture presented at PHAR 503 Lecture in UIC College of Pharmacy, Chicago.
(Last Updated: 08/22/2018) Created by: Socco, Samantha Therapeutics of Diuretics Thambi, M. (2017). The Clinical Use of Diuretics. Lecture presented at PHAR 503 Lecture in UIC College of Pharmacy, Chicago.
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
Fifth Year Biology. Excretion. Miss Rochford
 Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
I. Metabolic Wastes Metabolic Waste:
 I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
Histology / First stage The Urinary System: Introduction. Kidneys
 The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19](/thumbs/80/80449526.jpg) 1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Dept. of Physiology. ZJU School of Medicine.
 Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine Http://10.10.10.151/Able.Acc2.Web/Template/View.aspx?action =view&coursetype=0&courseid=26519 QUESTION 6 How is the filtrate reabsorbed
Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine Http://10.10.10.151/Able.Acc2.Web/Template/View.aspx?action =view&coursetype=0&courseid=26519 QUESTION 6 How is the filtrate reabsorbed
