Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker
|
|
|
- Joshua Carter
- 6 years ago
- Views:
Transcription
1 Original Article Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker Vivek Y. Reddy, M.D., Derek V. Exner, M.D., M.P.H., Daniel J. Cantillon, M.D., Rahul Doshi, M.D., T. Jared Bunch, M.D., Gery F. Tomassoni, M.D., Paul A. Friedman, M.D., N.A. Mark Estes, III, M.D., John Ip, M.D., Imran Niazi, M.D., Kenneth Plunkitt, M.D., Rajesh Banker, M.D., James Porterfield, M.D., James E. Ip, M.D., and Srinivas R. Dukkipati, M.D., for the LEADLESS II Study Investigators* ABSTRACT BACKGROUND Cardiac pacemakers are limited by device-related complications, notably infection and problems related to pacemaker leads. We studied a miniaturized, fully selfcontained leadless pacemaker that is nonsurgically implanted in the right ventricle with the use of a catheter. METHODS In this multicenter study, we implanted an active-fixation leadless cardiac pacemaker in patients who required permanent single-chamber ventricular pacing. The primary efficacy end point was both an acceptable pacing threshold ( 2.0 V at 0.4 msec) and an acceptable sensing amplitude (R wave 5.0 mv, or a value equal to or greater than the value at implantation) through 6 months. The primary safety end point was freedom from device-related serious adverse events through 6 months. In this ongoing study, the prespecified analysis of the primary end points was performed on data from the first 300 patients who completed 6 months of followup (primary cohort). The rates of the efficacy end point and safety end point were compared with performance goals (based on historical data) of 85% and 86%, respectively. Additional outcomes were assessed in all 526 patients who were enrolled as of June 2015 (the total cohort). RESULTS The leadless pacemaker was successfully implanted in 504 of the 526 patients in the total cohort (95.8%). The intention-to-treat primary efficacy end point was met in 270 of the 300 patients in the primary cohort (90.0%; 95% confidence interval [CI], 86.0 to 93.2, P = 0.007), and the primary safety end point was met in 280 of the 300 patients (93.3%; 95% CI, 89.9 to 95.9; P<0.001). At 6 months, device-related serious adverse events were observed in 6.7% of the patients; events included device dislodgement with percutaneous retrieval (in 1.7%), cardiac perforation (in 1.3%), and pacing-threshold elevation requiring percutaneous retrieval and device replacement (in 1.3%). From the Icahn School of Medicine at Mount Sinai (V.Y.R., S.R.D.) and Weill Cornell Medical Center (J.E.I.) both in New York; Libin Cardiovascular Institute of Alberta, Calgary, Canada (D.V.E.); Cleveland Clinic, Cleveland (D.J.C.); Keck Hospital of University of Southern California, Los Angeles (R.D.), and Premier Cardiology, Newport Beach (R.B.) both in California; Intermountain Medical Center Heart Institute, Salt Lake City, (T.J.B.); Central Baptist Hospital, Lexington, KY (G.F.T.); Mayo Clinic, Rochester, MN (P.A.F.); Tufts University School of Medicine, Boston (N.A.M.E.); Sparrow Clinical Research Institute, Lansing, MI (J.I.); Aurora Medical Group, Milwaukee (I.N.); Naples Community Hospital, Naples, FL (K.P.); and Methodist University Hospital, Memphis, TN (J.P.). Address reprint requests to Dr. Reddy at the Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl., Box 1030, New York, New York 10029, or at vivek.reddy@mountsinai.org. * A complete list of investigators in the LEADLESS II study is provided in the Supplementary Appendix, available at NEJM.org. This article was published on August 30, 2015, at NEJM.org. N Engl J Med 2015;373: DOI: /NEJMoa Copyright 2015 Massachusetts Medical Society. CONCLUSIONS The leadless cardiac pacemaker met prespecified pacing and sensing requirements in the large majority of patients. Device-related serious adverse events occurred in approximately 1 in 15 patients. (Funded by St. Jude Medical; LEADLESS II ClinicalTrials.gov number, NCT ) n engl j med 373;12 nejm.org September 17,
2 Each year, nearly 1 million persons worldwide receive conventional transvenous cardiac pacemakers with active-fixation leads to treat bradycardia and heart block. 1,2 Despite considerable technological advancements since the clinical introduction of these pacemakers six decades ago, pacemaker-related adverse events occur in 1 in 10 patients These events are typically related to the transvenous lead, surgical pocket, or pulse generator. 3,4 The leads are susceptible to dislodgement, fracture, or insulation failure and can also cause infection, cardiac perforation, venous occlusion, and tricuspid regurgitation. Pulse generators have been associated with infection, pocket hematoma, and skin erosion. 3-9,12 A recently developed device is a fully selfcontained, leadless cardiac pacemaker with combined battery, electronics, and electrodes. 13,14 Encapsulated into a small unit (1.0 cc) and deliverable with the use of a catheter through the femoral vein, the leadless cardiac pacemaker is nonsurgically implanted directly within the right ventricle, thereby obviating repetitive lead flexion and potential lead damage with each cardiac cycle. Eliminating the device pocket and transvenous lead also potentially minimizes some longterm complications observed with conventional pacemakers, such as tricuspid valvular regurgitation and thromboembolism across a patent foramen ovale. 15 Feasibility of the leadless cardiac pacemaker in humans was shown in the LEADLESS trial. 13 We now report the outcomes of the LEADLESS II study, a nonrandomized trial examining the clinical safety and efficacy of nonsurgical implantation of the Nanostim leadless cardiac pacemaker in patients who require permanent ventricular pacing. Methods Study Design and Oversight The LEADLESS II trial is a prospective, nonrandomized, multicenter clinical study. The trial is currently ongoing and enrolling patients. The planned interim analysis, reported here, includes the primary analysis of efficacy and safety in the initial 300 patients who were followed for 6 months (the primary cohort) and outcomes for all 526 patients who were enrolled as of June 2015 (the total cohort). This premarket study was sponsored by the manufacturer of the Nanostim leadless cardiac pacemaker (St. Jude Medical) and was approved by the Food and Drug Administration and the institutional review board at each participating center. An international steering committee (see the Supplementary Appendix, available with the full text of this article at NEJM.org), with the participation of the sponsor, was responsible for the design and conduct of the study and the reporting of the findings. Monitoring and collection of the data and initial data analyses were performed by the sponsor in partnership with the steering committee. All the authors vouch for the completeness and accuracy of the data and analyses and for the fidelity of this report to the study protocol, available at NEJM.org. The first and last authors wrote the first draft of the manuscript, which was reviewed and edited by the other authors. The sponsor reviewed the manuscript before submission but was not involved in the writing of the manuscript or in the decision to submit it for publication. Study Participants After obtaining written informed consent, we enrolled patients with indications for permanent single-chamber ventricular pacing, including chronic atrial fibrillation with atrioventricular or bifascicular bundle-branch block, sinus rhythm with second-degree or third-degree atrioventricular block and a low level of physical activity or a shortened expected life span, or sinus bradycardia with infrequent pauses or unexplained syncope with an abnormal electrophysiological study. Patients were excluded if they had a mechanical tricuspid-valve prosthesis, pulmonary arterial hypertension, preexisting endocardial pacing or defibrillation leads, or an inferior vena cava filter or if they had undergone cardiovascular or peripheral vascular surgery within 30 days before enrollment (see the Supplementary Appendix for a full list of inclusion and exclusion criteria). Device Implantation and Follow-up The leadless cardiac pacemaker that we evaluated (Nanostim LP, St. Jude Medical) is an entirely selfcontained, active-fixation, rate-adaptive pacemaker that is 42 mm in length and has a maximum diameter of 5.99 mm (Fig. 1). The pacemaker is delivered to the right ventricle at the end of a 1126 n engl j med 373;12 nejm.org September 17, 2015
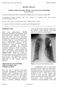
3 Percutaneous Implantation of a Leadless Pacemaker A ANTERIOR VIEW OF HEART Anode RIGHT VENTRICLE RIGHT ATRIUM Coiled spring Delivery catheter ENDOCARDIUM Cathode RIGHT VENTRICULAR APEX RIGHT VENTRICLE Nanostim leadless cardiac pacemaker LEFT VENTRICLE INFERIOR VENA CAVA Docking interface B C Figure 1. The Leadless Cardiac Pacemaker. The Nanostim leadless cardiac pacemaker is shown attached to the right ventricular apex (Panel A). The cathode is in the center of the coiled spring, which affixes the device to the endocardium. The anode of the pacemaker is the uncoated part of the titanium case. The proximal portion of the device has a docking interface that enables attachment to the delivery and retrieval catheters. Chest radiographs of a patient who underwent implantation of this leadless cardiac pacemaker are shown in the posteroanterior (Panel B) and lateral (Panel C) projections. The arrows indicate the location of the device, which is situated in the region of the distal right ventricular septum and right ventricular apex. n engl j med 373;12 nejm.org September 17,
4 300 Were in the group of initial consecutive patients enrolled 300 Underwent implantation attempt 289 Had successful attempt 11 Had unsuccessful attempt 289 Underwent follow-up assessment before hospital discharge and at 2 wk, 6 wk, and 3 mo 30 Underwent Holter monitoring at 3 mo 300 Were included in the primary cohort at 6 mo of follow-up and were assessed for primary efficacy and safety end points A video showing the leadless cardiac pacemaker is available at NEJM.org 526 Patients were enrolled in the study Continued follow-up 226 Were in the ongoingenrollment group 226 Underwent implantation attempt 215 Had successful attempt 11 Had unsuccessful attempt 215 Underwent follow-up assessments for <6 mo 526 Were included in the total cohort and were assessed for device-related and non devicerelated serious adverse events Figure 2. Enrollment, Study Intervention, and Follow-up. The first 300 enrolled patients made up the primary cohort; data from these patients were analyzed for the primary efficacy and safety end points at 6 months, as prespecified in the protocol. An additional 226 patients were enrolled as part of the ongoing trial; data from these patients were analyzed together with data from the primary cohort that had extended follow-up beyond 6 months. This total cohort of 526 patients was assessed for device-related and non device-related serious adverse events. percutaneous delivery catheter and is anchored in the right ventricular apex with the use of a helical screw-in fixation electrode at the distal end of the device. Further details regarding this pacemaker and its implantation technique are provided in Video 1, and in Figure S1 in the Supplementary Appendix. After the device was implanted and before the patient was discharged from the hospital, the pacemaker was interrogated and the patient underwent chest radiography and standard 12-lead electrocardiography. Subsequent follow-up assessments were performed at 2 weeks, 6 weeks, 3 months, 6 months, and every 6 months thereafter. The programming of the pacemaker was left to the physician s discretion. Primary Efficacy and Safety End Points The primary outcome analysis was a prespecified assessment of the primary efficacy and safety end points in the first 300 patients who were followed for 6 months (primary cohort) (Fig. 2). The composite primary efficacy end point was both a therapeutically acceptable pacing capture threshold ( 2.0 V at 0.4 msec) and a therapeutically acceptable sensing amplitude (R wave 5.0 mv, or a value equal to or greater than the value at implantation) through 6 months. The primary safety end point was freedom from device-related serious adverse events during the initial 6 months after implantation. All adverse events were adjudicated by an independent clinical-events committee (see the Supplementary Appendix). A serious adverse event was defined as any untoward medical occurrence that led to death or to a serious deterioration in the health of a patient that resulted in life-threatening illness or injury, permanent impairment of a body structure or a body function, inpatient or prolonged hospitalization, or a medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or a body function. Serious adverse events were classified as device-related if they were considered by the clinical-events committee to be attributable to the investigational device or procedure. Secondary Outcomes The primary cohort was also evaluated for all non device-related serious adverse events during 6 months of follow-up. Such events were considered to be unrelated to the investigational device or procedure. Because the LEADLESS II trial is ongoing, secondary analyses were performed on data from additional patients who were enrolled as of June 2015, combined with data from the first 300 patients, who had extended follow-up beyond 6 months (total cohort) (Fig. 2). Additional analyses in the total cohort included determination of all device-related and non devicerelated serious adverse events during follow-up and the influence of operator experience. Statistical Analysis We estimated that if 300 patients were followed for 6 months, the study would have 90% power, at a two-sided 5.0% significance level, to show 1128 n engl j med 373;12 nejm.org September 17, 2015
5 Percutaneous Implantation of a Leadless Pacemaker rates of safety and efficacy that would be superior to predetermined performance goals for safety and efficacy. The performance goal for the primary efficacy end point of both a therapeutically acceptable pacing capture threshold and a therapeutically acceptable sensing amplitude through 6 months was 85%, and the study was powered under the assumption that the rate of this end point would be 91.5% or higher. The performance goal for efficacy was based on an ongoing pacemaker study (ClinicalTrials.gov number, NCT ) that is sponsored by St. Jude Medical. The performance goal for the primary safety end point of freedom from devicerelated serious adverse events through 6 months was 86%, and the study was powered under the assumption that the event-free rate would be 92%. (See the Supplementary Appendix for explanation of the performance goals.) All the analyses were conducted with the use of exact confidence intervals for binomial proportions. The null hypotheses would be rejected if the lower boundaries of the two-sided 95% confidence intervals for the rate of the primary safety and efficacy end points would be greater than the respective performance goals. The primary safety and efficacy end points were assessed in the intention-to-treat population, which included the first 300 patients (primary cohort) who met the enrollment criteria and provided written informed consent and in whom the implantation of a leadless cardiac pacemaker was attempted. The primary efficacy end point was also analyzed in the subgroup of patients in whom implantation was successful. Statistical calculations were performed with the use of SAS software (SAS Institute) and were validated according to the operating procedures of the sponsor. Results Patient and Procedural Characteristics Between February 2014 and June 2015, a total of 526 patients were enrolled at 56 clinical sites (employing 100 operators) in three countries. The 300-patient primary cohort completed the 6-month follow-up in June 2015, thereby triggering the prespecified formal primary analyses (Fig. 2). The 526-patient total cohort was followed for a mean (±SD) of 6.9±4.2 months. The characteristics of these two cohorts are shown in Table 1. The mean age in the total cohort was 75.8±12.1 years, and 61.8% of the participants were male. Pacemaker indications were atrial fibrillation with atrioventricular block in 294 patients (55.9%), sinus rhythm with high-grade atrioventricular block in 46 patients (8.7%), and sinus bradycardia with infrequent pauses or syncope in 186 patients (35.4%). Pacemaker implantation was successful in 504 of the 526 patients (95.8%). Procedural and fluoroscopy times were 28.6±17.8 minutes and 13.9±9.1 minutes, respectively. Most patients (70.2%) did not require device repositioning after initial deployment. The pacemaker required repositioning more than two times in 22 patients (4.4%). The duration of hospital stay from implantation to discharge was 1.1±1.7 days (range, 0 to 33). Device Efficacy In the intention-to-treat analysis, 270 of the 300 participants in the primary cohort (90.0%; twosided 95% confidence interval [CI], 86.0 to 93.2) reached the primary efficacy end point; the lower boundary of the 95% CI exceeded the prespecified performance goal of 85% (P = 0.007). Device implantation was unsuccessful in 11 patients; among the remaining 289 patients in whom implantation was successful, 270 reached the primary efficacy end point (93.4%; two-sided 95% CI, 89.9 to 96.0; P<0.001). The reasons for failure to reach the primary efficacy end point in the 19 patients with successful implantation included inadequate pacing capture threshold in 4 patients and inadequate sensed R-wave amplitudes in 16 patients. One patient had both inadequate pacing and inadequate sensing. Among the 289 patients with successful implantation, complete 6-month follow-up data were available for 266 patients and the last observation was carried forward for 23 patients (owing to death in 13 patients, missing data on the 6-month visit in 4 patients, and withdrawal from the study in 6 patients). In the total cohort, the mean sensing and pacing threshold values improved significantly over time from the values observed at the time of pacemaker implantation; at 12 months, the mean R-wave amplitude was 9.2±2.9 mv, and the mean pacing capture threshold (at 0.4 msec) was 0.58±0.31 V (Fig. S2 in the Supplementary Appendix). The percentage of ventricular pacing n engl j med 373;12 nejm.org September 17,
6 Table 1. Patient Characteristics at Baseline and Procedural Characteristics.* Characteristic Primary Cohort (N = 300) Total Cohort (N = 526) Patient characteristics Age yr Mean 75.7± ±12.1 Range Body-mass index Mean 29.2± ±6.8 Range Sex no. (%) Male 193 (64.3) 325 (61.8) Female 107 (35.7) 201 (38.2) Race or ethnic group no. (%) White 269 (89.7) 478 (90.9) Black 21 (7.0) 35 (6.7) American Indian or Alaska Native 1 (0.3) 1 (0.2) Asian 7 (2.3) 10 (1.9) Other 2 (0.7) 2 (0.4) Hispanic or Latino ethnic group no. (%) Hispanic or Latino 13 (4.3) 17 (3.2) Non-Hispanic or non-latino 287 (95.7) 508 (96.6) Unknown 0 1 (0.2) Coronary artery disease no. (%) 121 (40.3) 201 (38.2) History of coronary-artery bypass grafting no. (%) 48 (16.0) 84 (16.0) History of myocardial infarction no. (%) 42 (14.0) 73 (13.9) History of percutaneous coronary intervention no. (%) 47 (15.7) 86 (16.3) Hypertension no. (%) 252 (84.0) 420 (79.8) Diabetes mellitus no. (%) 82 (27.3) 143 (27.2) Hyperlipidemia no. (%) 208 (69.3) 355 (67.5) Peripheral vascular disease no. (%) 45 (15.0) 69 (13.1) Congestive heart failure no. (%) 43 (14.3) 82 (15.6) NHYA class I 11 (3.7) 18 (3.4) NYHA class II 20 (6.7) 36 (6.8) NYHA class III 3 (1.0) 9 (1.7) NYHA class IV 0 0 NYHA class unknown 9 (3.0) 19 (3.6) History of arrhythmia no. (%) Supraventricular 231 (77.0) 399 (75.9) Ventricular 15 (5.0) 28 (5.3) Tricuspid-valve disease no. (%) Regurgitation or prolapse 59 (19.7) 106 (20.2) Stenosis 0 0 Repair or replacement 3 (1.0) 3 (0.6) Left ventricular ejection fraction % 57.1± ±8.1 Medications no. (%) Beta-blockers 120 (40.0) 199 (37.8) Angiotensin-converting enzyme inhibitors 80 (26.7) 149 (28.3) Angiotensin-receptor blockers 62 (20.7) 91 (17.3) Anticoagulants 180 (60.0) 310 (58.9) Antiplatelets 143 (47.7) 247 (47.0) Antiarrhythmic drugs: class I or III 28 (9.3) 48 (9.1) 1130 n engl j med 373;12 nejm.org September 17, 2015
7 Percutaneous Implantation of a Leadless Pacemaker Table 1. (Continued.) Characteristic Primary Cohort (N = 300) Total Cohort (N = 526) Procedural characteristics Duration of implantation min Total: sheath insertion to removal 50.0± ±25.3 Procedure: insertion of delivery catheter to removal 30.4± ±17.8 Duration of fluoroscopy min 14.9± ±9.1 Device repositioning no. of patients/total no. (%) None 199/289 (68.9) 354/504 (70.2) 1 53/289 (18.3) 89/504 (17.7) 2 24/289 (8.3) 39/504 (7.7) >2 13/289 (4.5) 22/504 (4.4) Final device position in right ventricle no. of patients/ total no. (%) Apex 140/289 (48.4) 192/504 (38.1) Apical septum 5/289 (1.7) 96/504 (19.0) Outflow, septum, or other 144/289 (49.8) 215/504 (42.7) Missing data 0/289 1/504 (0.2) * Plus minus values are means ±SD. NYHA denotes New York Heart Association. The body-mass index is the weight in kilograms divided by the square of the height in meters. Race and ethnic group were self-reported. Data are for patients in whom implantation of the leadless cardiac pacemaker was successful (289 in the primary cohort and 504 in the total cohort). was 38.7±36.9 before hospital discharge and 51.6±39.1 at 12 months. Device Safety The primary safety end point was met in 280 of the 300 patients in the primary cohort (93.3%; two-sided 95% CI, 89.9 to 95.9); the lower boundary of the 95% CI exceeded the prespecified performance goal of 86% (P<0.001). A total of 22 devicerelated serious adverse events were observed in 20 patients (6.7%) over a period of 6 months (Table 2, and Fig. S3 in the Supplementary Appendix). The rates of cardiac perforation, device dislodgement, and elevated pacing thresholds necessitating device retrieval and replacement were 1.3%, 1.7%, and 1.3%, respectively. Vascular complications were reported in 1.3% of the patients. In the total cohort of 526 patients, the rate of device-related serious adverse events was 6.5%, including cardiac perforation in 1.5% of the patients, device dislodgement in 1.1%, and device retrieval due to elevated pacing thresholds in 0.8% (Table 2, and Fig. S4 in the Supplementary Appendix). The six device dislodgements were identified at 8.0±6.4 days after implantation (range, 1 to 14). Device migration to the pulmonary artery or right femoral vein occurred in 4 and 2 patients, respectively. All six devices were retrieved percutaneously. There was no significant difference in the dislodgement rate between devices positioned in the right ventricular apex and those in non-apical positions (P = 0.42). In the total cohort, there were 28 deaths (5.3%) during follow-up; the mean age of patients who died was 79.1±10.9 years (range, 40 to 97). A total of 19 deaths (68%) occurred within 6 months, 8 (29%) between 6 and 12 months, and 1 (4%) beyond 12 months. The deaths were classified as having a cardiac cause in 4 patients, a noncardiac cause in 14 patients, and an unknown cause in 10 patients (Table S1 in the Supplementary Appendix). There were no deaths that were considered to be device-related. However there were 2 deaths (0.4%) that were classified by the clinical-events committee as procedure-related (see the Supplementary Appendix for details). The rate of non device-related serious adverse events was 6.3% in the primary cohort and 5.5% in the total cohort (Table 3). The influence of operator experience on the rate of device-related serious adverse events was assessed. Cases were stratified according to n engl j med 373;12 nejm.org September 17,
8 Table 2. Device-Related Serious Adverse s.* Primary Cohort (N = 300) Total Cohort (N = 526) s Patients Rate s Patients Rate % % Total Cardiac perforation Cardiac tamponade with intervention Cardiac perforation requiring intervention Pericardial effusion with no intervention Vascular complication Bleeding Arteriovenous fistula Pseudoaneurysm Failure of vascular closure device requiring intervention Arrhythmia during device implantation Asystole Ventricular tachycardia or ventricular fibrillation Cardiopulmonary arrest during implantation procedure Device dislodgement Device migration during implantation owing to inadequate fixation Pacing threshold elevation with retrieval and implantation of new device Other Hemothorax Angina pectoris Pericarditis Acute confusion and expressive aphasia Dysarthria and lethargy after implantation Contrast-induced nephropathy Orthostatic hypotension with weakness Left-leg weakness during implantation Probable pulmonary embolism Ischemic stroke * s were classified as device-related if they were considered by the clinical-events committee to be attributable to the investigational device or procedure. Some patients had more than one event, and therefore the number of patients is less than the number of events. the first 10 devices implanted by an operator (470 implants) versus subsequent implants by the same operator (56 implants). The rate of device-related serious adverse events was 6.8% for the initial 10 cases versus 3.6% for the subsequent implants (P = 0.56) n engl j med 373;12 nejm.org September 17, 2015
9 Percutaneous Implantation of a Leadless Pacemaker Retrievability of the Implanted Devices In seven patients in the total cohort (excluding the six patients with dislodgements), the leadless cardiac pacemakers were successfully retrieved at 160±180 days (median, 100; range, 1 to 413) without complications. The reasons for retrieval were elevated pacing thresholds in four patients, worsening heart failure in two patients, and elective explantation in one patient. Three patients received new leadless cardiac pacemakers, two received conventional pacemakers, and the two patients with heart failure received cardiacresynchronization therapy with either direct Hisbundle pacing or biventricular pacing. Holter-Monitor Findings A prespecified subgroup of 30 patients underwent 24-hour ambulatory electrocardiography to assess pacing function. The percentage of ventricular pacing was 50.3±39.9 (range, 0 to 98). The mean minimum and maximum heart rates were 58.2±9.2 beats per minute and 111.1±21.1 beats per minute, respectively. The mean heart rate in all 30 patients was 71.2±9.8 beats per minute. The rate-adaptive feature was active in 16 patients. There were no pauses exceeding 2.0 seconds, no episodes of undersensing, and no instances of failure to capture. Four patients had T-wave oversensing, no instances of which resulted in a prolonged pause, symptoms in the patient, or reported adverse events. Discussion This analysis from an ongoing multicenter study showed that the Nanostim leadless cardiac pacemaker was capable of providing effective pacemaker function in a varied group of patients who had indications for long-term pacing therapy. The coexisting conditions in this patient cohort were similar to those in patients who receive conventional single-chamber pacemakers, but the rates of hypertension, hyperlipidemia, and diabetes were higher in our cohort. 16 The rate of successful implantation was 95.8%, and the efficacy goals for pacing and sensing were met in 90% of the participants. The mean pacing threshold and sensing values at 6 months were similar to those observed with conventional transvenous leads, 17 and these values were stable over time. Furthermore, effective pacemaker function was verified in a subgroup of patients by means of ambulatory electrocardiography. Finally, this pacemaker was safely retrievable; however, most of the devices that were retrieved were explanted within 1 year after implantation, and there are few data on the feasibility of the removal of leadless cardiac pacemakers beyond this point. Device-related serious adverse events were observed in 6.7% of the 300 patients in the primary cohort, including device dislodgement in 1.7%, cardiac perforation in 1.3%, elevated pacing thresholds requiring device retrieval and reimplantation in 1.3%, and vascular complications in 1.3%. For comparison, implantation of conventional ventricular pacemakers is associated with complications (excluding lead fracture) in 3.2% of patients, including pneumothorax in 1.1%, lead dislodgement in 0.8%, and infection in 0.5%. 18 The rate of cardiac perforation in our study (1.5% among the 526 patients in the total cohort) is similar to the rate observed with transvenous leads, which ranges from 0.6 to 5.0%. 3,19,20 Perforations related to leadless cardiac pacemakers may be due in part to the relatively large diameter of the device. A recent report of the early performance of a different leadless cardiac pacemaker (Micra, Medtronic) that was implanted in 140 patients who were followed for a mean of 2 months showed an 18.6% rate of procedure-related adverse events. 21 Although the rate of cardiac perforation (0.7%) was somewhat lower than that observed in our study, the rate of vascular complications was higher, including bleeding in 2.1% of patients, hematoma in 1.4%, and pseudoaneurysm in 1.4%. It is difficult, however, to compare these two systems directly. Premature battery depletion was not observed, but the limited duration of follow-up precludes robust confidence in the battery longevity of the leadless cardiac pacemaker. However, on the basis of the observed device-use conditions (e.g., heart rate, percentage of ventricular pacing, and pacing impedance) of the 300-patient cohort followed for 6 months, the battery longevity is estimated to be 15.0±6.7 years (95% CI, 14.2 to 15.8). This study was limited by the observational design that did not directly compare the leadless cardiac pacemaker with conventional pacemakers, thereby limiting our ability to draw conclusions about the relative safety and efficacy of these devices. In addition, the performance goal n engl j med 373;12 nejm.org September 17,
10 Table 3. Non Device-Related Serious Adverse s.* Primary Cohort (N = 300) Total Cohort (N = 526) s Patients Rate s Patients Rate % % Total Acute renal failure Angina pectoris Atrial fibrillation with rapid ventricular rates Bacteremia Bell s palsy Bilateral pulmonary emboli with pulmonary infarction Change in mental status Dizziness Heart failure Heart failure and gout Hypertensive emergency Lung cancer Mechanical fall Methicillin-resistant Staphylococcus aureus infection Myocardial infarction Palpitations Pericardial effusion after placement of epicardial lead Reduction in ejection fraction: new onset Seizure: new onset Sepsis Shortness of breath Stroke Syncope: unknown cause Syncope: vasovagal Urinary retention Ventricular tachycardia or ventricular fibrillation Vertigo * These events were considered by the clinical-events committee to be unrelated to the investigational device or procedure. Some patients had more than one event, and therefore the number of patients is less than the number of events. for efficacy was based on an ongoing pacemaker study, the data from which are not publicly available. Furthermore, the mean follow-up was only 6 months, again limiting our understanding of long-term efficacy and pacemaker-related complications, particularly in comparison with conventional pacemaker systems. Currently, the leadless cardiac pacemaker can serve as only a single-chamber ventricular pacemaker, which accounts for a minority of implanted pacemakers 1134 n engl j med 373;12 nejm.org September 17, 2015
11 Percutaneous Implantation of a Leadless Pacemaker in the United States. 2 The leadless cardiac pacemaker also cannot provide electrographic data. Refinements in device-to-device communication, atrial affixation, and device diagnostics would be necessary for this device to fully replace conventional dual-chamber pacemakers. In summary, the Nanostim leadless cardiac pacemaker met prespecified pacing and sensing requirements in 90% of the patients in whom an implantation was attempted and in 93.4% of the patients in whom the implantation was successful. At 6 months, serious adverse events were observed in 6.7% of the patients. Supported by St. Jude Medical. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. References 1. Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 51(21): e1-e Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009 a World Society of Arrhythmia s project. Pacing Clin Electrophysiol 2011; 34: Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35: Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of shortand long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012; 9: Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm 2007; 4: Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116: Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of consecutive patients. Eur Heart J 2011; 32: Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace 2007; 9: Korkeila P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol 2007; 30: Palmisano P, Accogli M, Zaccaria M, et al. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace 2013; 15: Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000; 85: Al-Mohaissen MA, Chan KL. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J Am Soc Echocardiogr 2012; 25: Reddy VY, Knops RE, Sperzel JS, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014; 129: Knops RE, Tjong FVY, Neuzil P, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEAD- LESS trial. J Am Coll Cardiol 2015; 65: DeSimone CV, Friedman PA, Noheria A, et al. Stroke or transient ischemic attack in patients with transvenous pacemaker or defibrillator and echocardiographically detected patent foramen ovale. Circulation 2013; 128: Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002; 346: Wilkoff BL, Bello D, Taborsky M, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm 2011; 8: Healey JS, Toff WD, Lamas GA, et al. Cardiovascular outcomes with atrialbased pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation 2006; 114: Mahapatra S, Bybee KA, Bunch TJ, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005; 2: Acha MR, Keaney JJ, Lubitz SA, et al. Increased perforation risk with an MRIconditional pacing lead: a single-center study. Pacing Clin Electrophysiol 2015; 38: Ritter P, Duray GZ, Steinwender C, et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J 2015 June 4 (Epub ahead of print). Copyright 2015 Massachusetts Medical Society. specialties and topics at nejm.org Specialty pages at the Journal s website (NEJM.org) feature articles in cardiology, endocrinology, genetics, infectious disease, nephrology, pediatrics, and many other medical specialties. These pages, along with collections of articles on clinical and nonclinical topics, offer links to interactive and multimedia content and feature recently published articles as well as material from the NEJM archive ( ). n engl j med 373;12 nejm.org September 17,
Essentials of Pacemakers and ICD s. Rajesh Banker, MD, MPH
 Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
REVIEW ARTICLE. Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1
 Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
New generations pacemakers and ICDs: an update
 Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
Leadless Pacing. Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt
 Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Pacing Without Wires: Leadless Cardiac Pacing
 REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience
 Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
Leadless pacemakers a panacea for bradyarrhythmias?
 Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
INNOVATIONS IN DEVICE THERAPY:
 INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
Cardiac implantable electronic devices (CIEDs) in children include pacemakers and implantable cardioverter defibrillators (ICDs).
 Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Performance of Leadless Pacemaker in Japanese Patients vs. Rest of the World. Results From a Global Clinical Trial
 Circ J 2017; 81: 1589 1595 doi: 10.1253/circj.CJ-17-0259 ORIGINAL ARTICLE Arrhythmia/Electrophysiology Performance of Leadless Pacemaker in ese Patients vs. Rest of the World Results From a Global Clinical
Circ J 2017; 81: 1589 1595 doi: 10.1253/circj.CJ-17-0259 ORIGINAL ARTICLE Arrhythmia/Electrophysiology Performance of Leadless Pacemaker in ese Patients vs. Rest of the World Results From a Global Clinical
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
The learning curve associated with the implantation of the Nanostim leadless pacemaker
 Journal of Interventional Cardiac Electrophysiology (2018) 53:239 247 https://doi.org/10.1007/s10840-018-0438-8 MULTIMEDIA REPORT The learning curve associated with the implantation of the Nanostim leadless
Journal of Interventional Cardiac Electrophysiology (2018) 53:239 247 https://doi.org/10.1007/s10840-018-0438-8 MULTIMEDIA REPORT The learning curve associated with the implantation of the Nanostim leadless
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD
 Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Technical option of surgical approach for trouble-shooting
 JHRS Corner Device and lead trouble-shooting - standard strategy and technical option - Technical option of surgical approach for trouble-shooting Katsuhiko IMAI Department of Cardiovascular surgery, Hiroshima
JHRS Corner Device and lead trouble-shooting - standard strategy and technical option - Technical option of surgical approach for trouble-shooting Katsuhiko IMAI Department of Cardiovascular surgery, Hiroshima
CAPTIVATE SUMMARY CLINICAL SUMMARY. CAPTure Information Via Automatic Threshold Evaluation
 CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France
 How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Update on Device Innovation (S-ICD, Wearable, Leadless)
 Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
A Leadless Intracardiac Transcatheter Pacing System
 Original Article A Leadless Intracardiac Transcatheter Pacing System Dwight Reynolds, M.D., Gabor Z. Duray, M.D., Ph.D., Razali Omar, M.D., Kyoko Soejima, M.D., Petr Neuzil, M.D., Shu Zhang, M.D., Calambur
Original Article A Leadless Intracardiac Transcatheter Pacing System Dwight Reynolds, M.D., Gabor Z. Duray, M.D., Ph.D., Razali Omar, M.D., Kyoko Soejima, M.D., Petr Neuzil, M.D., Shu Zhang, M.D., Calambur
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc.
 Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
Occlusion de l'auricule gauche: Niche ou réel avenir? D Gras, MD, Nantes, France
 Occlusion de l'auricule gauche: Niche ou réel avenir? D Gras, MD, Nantes, France LAA Occlusion Is there a real future? Background Protect AF Trial Other Studies CAP, ASAP, Prevail Left Atrial Appendage
Occlusion de l'auricule gauche: Niche ou réel avenir? D Gras, MD, Nantes, France LAA Occlusion Is there a real future? Background Protect AF Trial Other Studies CAP, ASAP, Prevail Left Atrial Appendage
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
PERFORMANCE MEASURE TECHNICAL SPECIFICATIONS. HRS-3: Implantable Cardioverter-Defibrillator (ICD) Complications Rate
 PERFORMANCE MEASURE TECHNICAL SPECIFICATIONS HRS-3: Implantable Cardioverter-Defibrillator (ICD) Complications Rate Measure Title Description Measure Type Data Source Level of Analysis Numerator HRS-3:
PERFORMANCE MEASURE TECHNICAL SPECIFICATIONS HRS-3: Implantable Cardioverter-Defibrillator (ICD) Complications Rate Measure Title Description Measure Type Data Source Level of Analysis Numerator HRS-3:
Leadless Cardiac Pacemaker
 Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
NOVEL DEVICE TECHNOLOGIES
 NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
Implantation of Cardioverter Defibrillator After Percutaneous Closure of Atrial Septal Defect
 The Ochsner Journal 10:27 31, 2010 f Academic Division of Ochsner Clinic Foundation Implantation of Cardioverter Defibrillator After Percutaneous Closure of Atrial Septal Defect Anas Bitar, MD, Maria Malaya
The Ochsner Journal 10:27 31, 2010 f Academic Division of Ochsner Clinic Foundation Implantation of Cardioverter Defibrillator After Percutaneous Closure of Atrial Septal Defect Anas Bitar, MD, Maria Malaya
DECREASE-HF CLINICAL SUMMARY
 CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. CLINICAL SUMMARY DECREASE-HF Boston Scientific Corporation
CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. CLINICAL SUMMARY DECREASE-HF Boston Scientific Corporation
Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS
 Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS NBG Codes 1st Letter 2nd Letter 3rd Letter A V D Chamber(s) Paced = atrium = ventricle = dual (both atrium and ventricle) Chamber(s) Sensed A = atrium
Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS NBG Codes 1st Letter 2nd Letter 3rd Letter A V D Chamber(s) Paced = atrium = ventricle = dual (both atrium and ventricle) Chamber(s) Sensed A = atrium
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders
 C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
Leadless Pacemakers: practice and promise in congenital heart disease
 Clarke et al. Journal of Congenital Cardiology (2017) 1:4 DOI 10.1186/s40949-017-0007-5 Journal of Congenital Cardiology REVIEW Leadless Pacemakers: practice and promise in congenital heart disease T.S.O.
Clarke et al. Journal of Congenital Cardiology (2017) 1:4 DOI 10.1186/s40949-017-0007-5 Journal of Congenital Cardiology REVIEW Leadless Pacemakers: practice and promise in congenital heart disease T.S.O.
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Index. cardiology.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute ischemic stroke TOAST classification of, 270 Acute myocardial infarction (AMI) cardioembolic stroke following, 207 208 noncardioembolic
Index Note: Page numbers of article titles are in boldface type. A Acute ischemic stroke TOAST classification of, 270 Acute myocardial infarction (AMI) cardioembolic stroke following, 207 208 noncardioembolic
Supplemental Material
 Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
LONGITUDINAL SURVEILLANCE REGISTRY OF ACUITY SPIRAL LEAD
 CLINICAL SUMMARY LONGITUDINAL SURVEILLANCE REGISTRY OF ACUITY SPIRAL LEAD CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and
CLINICAL SUMMARY LONGITUDINAL SURVEILLANCE REGISTRY OF ACUITY SPIRAL LEAD CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in device implant and
EBR Systems, Inc. 686 W. Maude Ave., Suite 102 Sunnyvale, CA USA
 Over 200,000 patients worldwide are estimated to receive a CRT device each year. However, limitations prevent some patients from benefiting. CHALLENGING PROCEDURE 5% implanted patients fail to have coronary
Over 200,000 patients worldwide are estimated to receive a CRT device each year. However, limitations prevent some patients from benefiting. CHALLENGING PROCEDURE 5% implanted patients fail to have coronary
MEDICAL POLICY Cardioverter Defibrillators
 POLICY........ PG-0224 EFFECTIVE......06/01/09 LAST REVIEW... 01/27/17 MEDICAL POLICY Cardioverter Defibrillators GUIDELINES This policy does not certify benefits or authorization of benefits, which is
POLICY........ PG-0224 EFFECTIVE......06/01/09 LAST REVIEW... 01/27/17 MEDICAL POLICY Cardioverter Defibrillators GUIDELINES This policy does not certify benefits or authorization of benefits, which is
A bs tr ac t. n engl j med 363;25 nejm.org december 16,
 The new england journal of medicine established in 1812 december 16, 2010 vol. 363 no. 25 Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure Anthony S.L. Tang, M.D., George A. Wells,
The new england journal of medicine established in 1812 december 16, 2010 vol. 363 no. 25 Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure Anthony S.L. Tang, M.D., George A. Wells,
Clinical Policy: Holter Monitors Reference Number: CP.MP.113
 Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
Clinical Policy: Reference Number: CP.MP.113 Effective Date: 05/18 Last Review Date: 04/18 Coding Implications Revision Log Description Ambulatory electrocardiogram (ECG) monitoring provides a view of
file://c:\documents and Settings\admin\My Documents\CV\92.htm
 Page 1 of 5 Amir Farjam Fazelifar, M.D. Assistant Professor of Cardiac Electrophysiology Academic Address: Shaheed Rajaei Cardiovascular, Medical & Research Center, Vali- Asr Avenue Tehran- Iran Tel /
Page 1 of 5 Amir Farjam Fazelifar, M.D. Assistant Professor of Cardiac Electrophysiology Academic Address: Shaheed Rajaei Cardiovascular, Medical & Research Center, Vali- Asr Avenue Tehran- Iran Tel /
Watchman. Left Atrial Appendage Closure Device. Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8
 TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
ESC Stockholm Arrhythmias & pacing
 ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey
 Europace (2018) 20, 555 559 doi:10.1093/europace/eux381 EP WIRE Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey Serge Boveda 1 *, Radoslaw Lenarczyk 2, Kristina
Europace (2018) 20, 555 559 doi:10.1093/europace/eux381 EP WIRE Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey Serge Boveda 1 *, Radoslaw Lenarczyk 2, Kristina
Call Medtronic at 1 (800) to verify the patient s current implanted system
 MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany
 Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction
 Summary of Expert Consensus Statement for CLINICIANS 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction This is a summary of the Heart Rhythm
Summary of Expert Consensus Statement for CLINICIANS 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction This is a summary of the Heart Rhythm
Implantable cardioverter defibrillator, Inappropriate shock, Lead failure
 Inappropriate Discharges of Intravenous Implantable Cardioverter Defibrillators Owing to Lead Failure Takashi WASHIZUKA, 1 MD, Masaomi CHINUSHI, 1 MD, Ryu KAZAMA, 1 MD, Takashi HIRONO, 1 MD, Hiroshi WATANABE,
Inappropriate Discharges of Intravenous Implantable Cardioverter Defibrillators Owing to Lead Failure Takashi WASHIZUKA, 1 MD, Masaomi CHINUSHI, 1 MD, Ryu KAZAMA, 1 MD, Takashi HIRONO, 1 MD, Hiroshi WATANABE,
Pediatric Pacemaker Implantation Endocardial or Epicardial
 Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Biventricular Pacing for Atrioventricular Block and Systolic Dysfunction
 original article for Atrioventricular Block and Systolic Dysfunction Anne B. Curtis, M.D., Seth J. Worley, M.D., Philip B. Adamson, M.D., Eugene S. Chung, M.D., Imran Niazi, M.D., Lou Sherfesee, Ph.D.,
original article for Atrioventricular Block and Systolic Dysfunction Anne B. Curtis, M.D., Seth J. Worley, M.D., Philip B. Adamson, M.D., Eugene S. Chung, M.D., Imran Niazi, M.D., Lou Sherfesee, Ph.D.,
Revisions to the BC Guide for Physicians in Determining Fitness to Drive a Motor Vehicle
 Revisions to the BC Guide for Physicians in Determining Fitness to Drive a Motor Vehicle Thank you for taking the time to review the draft Cardiovascular Diseases and Disorders chapter. Please provide
Revisions to the BC Guide for Physicians in Determining Fitness to Drive a Motor Vehicle Thank you for taking the time to review the draft Cardiovascular Diseases and Disorders chapter. Please provide
Transvenous Pacemaker Procedures
 Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
A Prospective Study Comparing the Sensed R Wave in Bipolar and Extended Bipolar Configurations: The PropR Study
 A Prospective Study Comparing the Sensed R Wave in Bipolar and Extended Bipolar Configurations: The PropR Study ANEESH V. TOLAT, M.D.,* MELISSA WOICIECHOWSKI, M.S.N.,* ROSEMARIE KAHR, R.C.I.S.,* JOSEPH
A Prospective Study Comparing the Sensed R Wave in Bipolar and Extended Bipolar Configurations: The PropR Study ANEESH V. TOLAT, M.D.,* MELISSA WOICIECHOWSKI, M.S.N.,* ROSEMARIE KAHR, R.C.I.S.,* JOSEPH
Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life
 Chapter 3 Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life Guido H. van Welsenes, MS, Johannes B. van Rees, MD, Joep Thijssen, MD, Serge
Chapter 3 Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life Guido H. van Welsenes, MS, Johannes B. van Rees, MD, Joep Thijssen, MD, Serge
G Lin, R F Rea, S C Hammill, D L Hayes, P A Brady
 Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA Correspondence to: Dr Peter A Brady, MD, FRCP, Mayo Clinic, 200 First Street SW, Rochester, MN, 55905, USA; brady.peter@mayo.edu Accepted
Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA Correspondence to: Dr Peter A Brady, MD, FRCP, Mayo Clinic, 200 First Street SW, Rochester, MN, 55905, USA; brady.peter@mayo.edu Accepted
Percutaneous Mitral Valve Repair
 Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs
 EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important step for
EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important step for
SURGICAL ABLATION OF ATRIAL FIBRILLATION DURING MITRAL VALVE SURGERY THE CARDIOTHORACIC SURGICAL TRIALS NETWORK
 SURGICAL ABLATION OF ATRIAL FIBRILLATION DURING MITRAL VALVE SURGERY THE CARDIOTHORACIC SURGICAL TRIALS NETWORK Marc Gillinov, M.D. For the CTSN Investigators ACC Late Breaking Clinical Trials March 16,
SURGICAL ABLATION OF ATRIAL FIBRILLATION DURING MITRAL VALVE SURGERY THE CARDIOTHORACIC SURGICAL TRIALS NETWORK Marc Gillinov, M.D. For the CTSN Investigators ACC Late Breaking Clinical Trials March 16,
Transvenous Pacemaker Implantation 22 years after the Mustard Procedure
 Case Report Transvenous Pacemaker Implantation 22 years after the Mustard Procedure Masato Sakamoto MD, Yoshie Ochiai MD, Yutaka Imoto MD, Akira Sese MD, Mamie Watanabe MD, Kunitaka Joo MD Department of
Case Report Transvenous Pacemaker Implantation 22 years after the Mustard Procedure Masato Sakamoto MD, Yoshie Ochiai MD, Yutaka Imoto MD, Akira Sese MD, Mamie Watanabe MD, Kunitaka Joo MD Department of
Girish M Nair, Seeger Shen, Pablo B Nery, Calum J Redpath, David H Birnie
 268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
Complications of Lead Extraction: Prevention and treatment. Maria Grazia Bongiorni, MD, FESC
 Complications of Lead Extraction: Prevention and treatment Maria Grazia Bongiorni, MD, FESC Director of Cardiovascular Division University Hospital of Pisa (Italy) ourtesy of Dr Eivind Platou Potential
Complications of Lead Extraction: Prevention and treatment Maria Grazia Bongiorni, MD, FESC Director of Cardiovascular Division University Hospital of Pisa (Italy) ourtesy of Dr Eivind Platou Potential
AF Today: W. For the majority of patients with atrial. are the Options? Chris Case
 AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
What s New in CIEDs. Keimyung University Dongsan Medical Center Hyoung-Seob Park
 What s New in CIEDs Keimyung University Dongsan Medical Center Hyoung-Seob Park Transvenous CIEDs Technology: - Highly mature & reliable - Still includes generator, connectors and leads Procedure : -
What s New in CIEDs Keimyung University Dongsan Medical Center Hyoung-Seob Park Transvenous CIEDs Technology: - Highly mature & reliable - Still includes generator, connectors and leads Procedure : -
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW
 MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
Left Atrial Appendage Closure: The Good, The Bad and The Ugly
 Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Cigna - Prior Authorization Procedure List Cardiology
 Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Seek and Ye Shall Find: Surprising Findings When Using the ILR-LINQ
 Seek and Ye Shall Find: Surprising Findings When Using the ILR-LINQ Suneet Mittal, MD, FACC, FHRS Director, Electrophysiology Laboratory Valley Health System www.arrhythmia.org; @drsuneet October 31, 2015
Seek and Ye Shall Find: Surprising Findings When Using the ILR-LINQ Suneet Mittal, MD, FACC, FHRS Director, Electrophysiology Laboratory Valley Health System www.arrhythmia.org; @drsuneet October 31, 2015
Pediatric pacemakers & ICDs:
 Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Cardiology/Cardiothoracic
 Cardiology/Cardiothoracic ICD-9-CM to ICD-10-CM Code Mapper 800-334-5724 www.contexomedia.com 2013 ICD-9-CM 272.0 Pure hypercholesterolemia 272.2 Mixed hyperlipidemia 272.4 Other and hyperlipidemia 278.00
Cardiology/Cardiothoracic ICD-9-CM to ICD-10-CM Code Mapper 800-334-5724 www.contexomedia.com 2013 ICD-9-CM 272.0 Pure hypercholesterolemia 272.2 Mixed hyperlipidemia 272.4 Other and hyperlipidemia 278.00
CRF procedure PROCEDURE FLOW. PATIENT ASSESSMENT Symptoms and indication. Pacemaker & ICD registration. Procedures. Procedure ICD.
 Pacemaker & ICD registration Procedures CRF procedure PROCEDURE FLOW Procedure ICD ICD PM ICM lead only PATIENT ASSESSMENT Symptoms and indication Symptoms and events (multiple possibilities) Asymptomatic
Pacemaker & ICD registration Procedures CRF procedure PROCEDURE FLOW Procedure ICD ICD PM ICM lead only PATIENT ASSESSMENT Symptoms and indication Symptoms and events (multiple possibilities) Asymptomatic
Long-term Preservation of Left Ventricular Function and Heart Failure Incidence with Ablate and Pace Therapy Utilizing Biventricular Pacing
 The Journal of Innovations in Cardiac Rhythm Management, 3 (2012), 976 981 HEART FAILURE RESEARCH ARTICLE Long-term Preservation of Left Ventricular Function and Heart Failure Incidence with Ablate and
The Journal of Innovations in Cardiac Rhythm Management, 3 (2012), 976 981 HEART FAILURE RESEARCH ARTICLE Long-term Preservation of Left Ventricular Function and Heart Failure Incidence with Ablate and
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Recurrent Implantable Defibrillator Discharges (ICD) Discharges ICD Storm
 Recurrent Implantable Defibrillator Discharges (ICD) Discharges ICD Storm Guy Amit, MD, MPH Soroka University Medical Center Ben-Gurion University of the Negev Beer-Sheva, Israel Disclosures Consultant:
Recurrent Implantable Defibrillator Discharges (ICD) Discharges ICD Storm Guy Amit, MD, MPH Soroka University Medical Center Ben-Gurion University of the Negev Beer-Sheva, Israel Disclosures Consultant:
AF :RHYTHM CONTROL BY DR-MOHAMMED SALAH ASSISSTANT LECTURER CARDIOLOGY DEPARTMENT
 AF :RHYTHM CONTROL BY DR-MOHAMMED SALAH ASSISSTANT LECTURER CARDIOLOGY DEPARTMENT 5-2014 Atrial Fibrillation therapeutic Approach Rhythm Control Thromboembolism Prevention: Recommendations Direct-Current
AF :RHYTHM CONTROL BY DR-MOHAMMED SALAH ASSISSTANT LECTURER CARDIOLOGY DEPARTMENT 5-2014 Atrial Fibrillation therapeutic Approach Rhythm Control Thromboembolism Prevention: Recommendations Direct-Current
Clinical Results with the Dual-Chamber Cardioverter Defibrillator Phylax AV - Efficacy of the SMART I Discrimination Algorithm
 April 2000 107 Clinical Results with the Dual-Chamber Cardioverter Defibrillator Phylax AV - Efficacy of the SMART I Discrimination Algorithm B. MERKELY Semmelweis University, Dept. of Cardiovascular Surgery,
April 2000 107 Clinical Results with the Dual-Chamber Cardioverter Defibrillator Phylax AV - Efficacy of the SMART I Discrimination Algorithm B. MERKELY Semmelweis University, Dept. of Cardiovascular Surgery,
Supplementary Online Content
 Supplementary Online Content Tseng ZH, Hayward RM, Clark NM, et al. Sudden death in patients with cardiac implantable electronic devices. JAMA Intern Med. Published online June 22, 2015. doi:10.1001/jamainternmed.2015.2641.
Supplementary Online Content Tseng ZH, Hayward RM, Clark NM, et al. Sudden death in patients with cardiac implantable electronic devices. JAMA Intern Med. Published online June 22, 2015. doi:10.1001/jamainternmed.2015.2641.
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of
National Coverage Determination (NCD) for Cardiac Pacemakers (20.8)
 Page 1 of 12 Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for Cardiac Pacemakers (20.8) Tracking Information Publication Number 100-3 Manual Section Number 20.8 Manual
Page 1 of 12 Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for Cardiac Pacemakers (20.8) Tracking Information Publication Number 100-3 Manual Section Number 20.8 Manual
Adult Echocardiography Examination Content Outline
 Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Subclinical AF: Implications of device based episodes
 Subclinical AF: Implications of device based episodes Michael R Gold, MD, PhD Medical University of South Carolina Charleston, SC Disclosures: Clinical Trials and Consulting: Medtronic, Boston Scientific
Subclinical AF: Implications of device based episodes Michael R Gold, MD, PhD Medical University of South Carolina Charleston, SC Disclosures: Clinical Trials and Consulting: Medtronic, Boston Scientific
Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases
 Case Report Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases Koji Tsutsumi MD, Tatsuru Niibori MD, Keiichiro Katsumoto MD Department
Case Report Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases Koji Tsutsumi MD, Tatsuru Niibori MD, Keiichiro Katsumoto MD Department
Catheter Ablation: Atrial fibrillation (AF) is the most common. Another Option for AF FAQ. Who performs ablation for treatment of AF?
 : Another Option for AF Atrial fibrillation (AF) is a highly common cardiac arrhythmia and a major risk factor for stroke. In this article, Dr. Khan and Dr. Skanes detail how catheter ablation significantly
: Another Option for AF Atrial fibrillation (AF) is a highly common cardiac arrhythmia and a major risk factor for stroke. In this article, Dr. Khan and Dr. Skanes detail how catheter ablation significantly
Left Atrial Appendage Occlusion
 Left Atrial Appendage Occlusion A new strategy to prevent stroke in atrial fibrillation Ashok Talreja MD and Arijit Chanda MD VHVI symposium 24th February 2018 Outline of presentation 1. Risk of stroke
Left Atrial Appendage Occlusion A new strategy to prevent stroke in atrial fibrillation Ashok Talreja MD and Arijit Chanda MD VHVI symposium 24th February 2018 Outline of presentation 1. Risk of stroke
Finally, a pacemaker may be either permanent or temporary, which will also factor into your code selections.
 2015 Cardiology Survival Guide Chapter 15: Pacemakers With more than 30 codes and many variables to choose from, you must weigh your options carefully when reporting insertion, revision, or removal of
2015 Cardiology Survival Guide Chapter 15: Pacemakers With more than 30 codes and many variables to choose from, you must weigh your options carefully when reporting insertion, revision, or removal of
Atrial fibrillation (AF) is a disorder seen
 This Just In... An Update on Arrhythmia What do recent studies reveal about arrhythmia? In this article, the authors provide an update on atrial fibrillation and ventricular arrhythmia. Beth L. Abramson,
This Just In... An Update on Arrhythmia What do recent studies reveal about arrhythmia? In this article, the authors provide an update on atrial fibrillation and ventricular arrhythmia. Beth L. Abramson,
Patient guide: pfm Nit-Occlud PDA coil occlusion system. Catheter occlusion of. Patent Ductus Arteriosus. with the
 Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study
 Accepted Manuscript Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study Gabor Z. Duray, MD, PhD, Philippe Ritter, MD, Mikhael El-Chami, MD,
Accepted Manuscript Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study Gabor Z. Duray, MD, PhD, Philippe Ritter, MD, Mikhael El-Chami, MD,
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Transcoronary Chemical Ablation of Atrioventricular Conduction
 757 Transcoronary Chemical Ablation of Atrioventricular Conduction Pedro Brugada, MD, Hans de Swart, MD, Joep Smeets, MD, and Hein J.J. Wellens, MD In seven patients with symptomatic atrial fibrillation
757 Transcoronary Chemical Ablation of Atrioventricular Conduction Pedro Brugada, MD, Hans de Swart, MD, Joep Smeets, MD, and Hein J.J. Wellens, MD In seven patients with symptomatic atrial fibrillation
Unit 6: Circulatory System. 6.2 Heart
 Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
PACING AT THE BUNDLE OF HIS
 PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP140 Section: Medical Benefit Policy Subject: Automatic Implantable Cardioverter-Defibrillator I. Policy: Automatic Implantable Cardioverter-Defibrillator II. Purpose/Objective:
POLICIES AND PROCEDURE MANUAL Policy: MP140 Section: Medical Benefit Policy Subject: Automatic Implantable Cardioverter-Defibrillator I. Policy: Automatic Implantable Cardioverter-Defibrillator II. Purpose/Objective:
