Leadless Cardiac Pacemaker
|
|
|
- Stanley Colin Holt
- 5 years ago
- Views:
Transcription
1 Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine Place(s) of Service: Inpatient I. Description Pacemakers are intended to be used as a substitute for the heart s intrinsic pacing system to correct cardiac rhythm disorders. Conventional pacemakers consist of 2 components: a pulse generator and electrodes (also referred to as leads). Pacemakers are considered life-sustaining, lifesupporting class III devices for patients with variety of bradyarrhythmias. Even though the efficacy and safety profile of conventional pacemakers is excellent, in a small proportion of patients, they may result in complications particularly related to leads and requirement for surgical pocket. Further, some patients are medically ineligible for conventional pacemakers due to lack of venous access and recurrent infection. Leadless pacemakers are single unit devices that are implanted in the heart via femoral access, thereby eliminating potential for complications as a result of leads and surgical pocket. Micra Transcatheter Pacing System is the only commercially available leadless pacemaker in the United States approved by the Food and Drug Administration. For individuals with guidelines-based indication for a ventricular pacing system who are medically eligible to receive a conventional pacing system who are treated with Micra transcatheter pacing system, the evidence includes a pivotal prospective cohort study a 1 postapproval prospective cohort study. Relevant outcomes are other test performance, treatment-related mortality, and treatment-related morbidity. Results at 6 months and 1 year for the pivotal study reported high procedural success (above 99%) and device effectiveness (pacing capture threshold met in 98% patients). Majority of the system or procedural-related complications occur within 30 days. At 1 year, the incidence of major complication did not increase substantially from 6 months (3.5% at 6 months vs 4% at 1 year). Results of the postapproval study were consistent with pivotal study and showed a lower incidence of major complications at -30 days as well as 1 year (1.5% and 2.7%, respectively). In both studies, the point estimates of major complication were lower than the pooled estimates from 6 studies of conventional pacemakers used as a historical comparator. While Micra Transcatheter Pacing System eliminates lead- and surgical pocket-related complications, its use can result in potentially more serious complication related to implantation/release of the device (traumatic cardiac injury) and less serious complications related to the femoral access site (groin hematomas and access site bleeding). Considerable uncertainties and unknowns remain in terms of durability of device and end of life device issues. Early and limited experience suggests that retrieval of these devices is unlikely because, in due course, the
2 Leadless Cardiac Pacemaker 2 devices will be encapsulated. There is limited data on device-device interactions (both electrical and mechanical), which may occur when there is a deactivated Micra device alongside another leadless pacemaker or when a leadless pacemaker and transvenous device are both present. While the current evidence is encouraging, overall benefit with broad use of Micra transcatheter pacing system compared to conventional pacemakers has not been shown. The evidence is insufficient to determine the effects of technology on health outcomes. For individuals with guidelines-based indication for a ventricular pacing system who are medically ineligible for a conventional pacing system who are treated with Micra transcatheter pacing system, the evidence includes subgroup analysis of a pivotal prospective cohort study and a postapproval prospective cohort study. Relevant outcomes are other test performance, treatmentrelated mortality, and treatment-related morbidity. Information on the outcomes in the subgroup of patients from the postapproval study showed that Micra was successfully implanted in 98% of cases and safety outcomes were similar to the original cohort. Even though, the evidence is limited and long-term effectiveness and safety is unknown, the short-term benefits outweigh the risks as the complex tradeoff of adverse events for these devices need to be assessed in the context of lifesaving potential of pacing systems in patients who are ineligible for conventional pacing systems. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. Background CONVENTIONAL PACEMAKERS Pacemakers are intended to be used as a substitute for the heart s intrinsic pacing system to correct cardiac rhythm disorders. By providing an appropriate heart rate and heart rate response, cardiac pacemakers can reestablish effective circulation and more normal hemodynamics that are compromised by a slow heart rate. Pacemakers vary in system complexity and can have multiple functions as a result of the ability to sense and/or stimulate both the atria and the ventricles. Transvenous pacemakers or pacemakers with leads (here after referred as conventional pacemakers) consist of 2 components: a pulse generator (also referred to as battery component) and electrodes (also referred to as leads). The pulse generator consists of a power supply and electronics that can provide periodic electrical pulses to stimulate the heart. It is commonly implanted in the infraclavicular region of the anterior chest wall and generally placed in a prepectoral position, but in some cases a sub-pectoral position is advantageous. It generates the electrical impulse which is transmitted to the myocardium via the electrodes affixed to the myocardium to sense and pace the heart as needed. Conventional pacemakers are also referred to as single-chamber or dual-chamber systems. In single-chamber systems, only 1 lead is placed, typically in the right ventricle. In dual-chamber pacemakers, 2 leads are placed one in the right atrium and the other in right ventricle. Singlechamber ventricular pacemakers are much more commonly used in practice. Annually, approximately 200,000 pacemakers are implanted in the United States and 1 million worldwide. Implantable pacemakers are considered life-sustaining, life-supporting class III devices for patients with variety of bradyarrhythmias. Pacemaker systems have matured over the years with well-established, acceptable performance. As per the Food and Drug Administration (FDA), the
3 Leadless Cardiac Pacemaker 3 early performance of conventional pacemaker systems from implant through 60 to 90 days has usually demonstrated acceptable pacing capture thresholds and sensing. Intermediate performance from 90 days through more than 5 years has usually demonstrated reliability of the pulse generator and lead technology. Chronic performance from 5 to 10 years includes a predictable decline in battery life and mechanical reliability but a vast majority of patients receive excellent pacing and sensing free of operative or mechanical reliability failures. Even though the safety profile of conventional pacemakers is excellent, nevertheless they are associated with complications particularly related to the requirement of leads. Most of safety data on the use of conventional pacemakers comes from registries from Europe, particularly from Denmark where all pacemaker implants are recorded in a national registry. This data is compiled in Table 1. It is important to recognize that valid comparison of complication rates is limited by differences in definitions of complications resulting in a wide variance of outcomes, as well as by the strongly varying time windows of follow-up, use of single chamber or dual chamber systems, and reporting over more than 2 decades. As such, the following data is contemporary and is limited to single-chamber systems when reported separately. Table 1. Reported Complication Rates with Conventional Pacemakers Complications Rates, % a Traumatic complications RV perforation 0.2 to 0.8 RV perforation with tamponade 0.07 to 0.4 Pneumo(hemo)thorax 0.7 to 2.2 Pocket complications Including all hematomas, difficult to control bleeding, infection, discomfort, skin erosion 4.75 Including only those requiring invasive correction or reoperation 0.66 to 1.0 Lead-related complications Including lead fracture, dislodgement, insulation problem, infection, stimulation threshold problem, 1.6 to 3.8 diaphragm or pocket stimulation, other All system related infections requiring reoperation or extraction 0.5 to 0.73 Adapted from Food and Drug Administration executive summary memorandum. 6 a Rates are for new implants only and ventricular single chamber devices when data were available. Some rates listed in this column are for single and dual chamber devices when data were not separated in the publication. Note that Micra Transcatheter Pacing System is a single chamber device. Potential Advantages of Leadless Cardiac Pacemakers Over Conventional Pacemakers The potential advantages of leadless pacemakers fall into 3 categories: avoidance of risks associated with intravascular leads in conventional pacemakers, avoidance of risks associated with pocket creation for placement of conventional pacemakers, and an additional option for patients who require a single chamber pacer. Lead complications include lead failure, lead fracture, insulation defect, pneumothorax, infections requiring lead extractions/replacements that can results in a torn subclavian vein or tricuspid valve. In addition, there are potential risk of venous thrombosis and occlusion of the subclavian system from the leads. Use of a leadless system eliminates such potential risks with the added advantage that patient has vascular access preserved for other medical conditions (eg, dialysis or chemotherapy). Pocket complications include infections, erosions and pain that can be eliminated with leadless pacemakers. Further, a leadless cardiac pacemaker may be more comfortable and
4 Leadless Cardiac Pacemaker 4 appealing as unlike conventional pacemakers, patients are unable to see or feel or have implant scar on the chest wall with leadless pacemakers. Lastly, leadless pacemakers may also be a better option than a surgical endocardial pacemaker for patients with no vascular access due to renal failure or congenital heart disease. Leadless Cardiac Pacemakers in Clinical Development Leadless pacemakers are self-contained in a hermetically sealed capsule. The capsule houses a battery and electronics to operate the system. Similar to most pacing leads, the tip of the capsule includes a fixation mechanism and a monolithic controlled release device. The controlled release device elutes glucocorticosteroid to reduce acute inflammation at the implantation site. Leadless pacemakers have rate responsive functionality, and current device longevity estimates are based on bench data. Estimates have shown that these devices may last over 10 years depending on the programmed parameters. There are 3 systems currently being evaluated in clinical trials: (1) Micra Transcatheter Pacing System (TPS, Medtronic, 2013), (2) Nanostim Leadless Pacemaker System (LPS, St. Jude Medical, 2012); and (3) WiCS system (Wireless Cardiac Stimulation, EBR systems). The first 2 devices are free-standing capsule sized devices that are delivered via femoral venous access using a steerable delivery sheath. However, the fixing mechanism differs between the 2 devices. In the Micra Transcatheter Pacing System, the fixation system consists of 4 self-expanding nitinol tines, which anchor into the myocardium while in the Nanostim LCP, there is a screw-in helix that penetrates about 1 mm into the myocardium, with nylon tines that provide a secondary fixation mechanism. In both devices, the cathode is steroid eluting and delivers pacing current while the anode is located in a titanium case. The third device, WiCS System, is different from other devices that involves implanting a pulse generator subcutaneously near the heart and then wirelessly transmits ultrasound energy to a receiver electrode implanted inside the left ventricle. The receiver electrode converts the ultrasound energy, then delivers electrical stimulation to the heart sufficient to pace the left ventricle synchronously with the right. Of these 3, only Micra Transcatheter Pacing System is approved by FDA and commercially available in the United States. Multiple clinical studies of Nanostim have been published but trials have been halted due to issues with the migration of the docking button in the device. Evidence on Nanostim is not reviewed further as it is not yet FDA approved. Transcatheter Pacing System is about 26 mm in length and introduced through a 23 French catheter via the femoral vein to the right ventricle. It weighs about 2 grams and has an accelerometer-based rate response. Nanostim is about 40 mm in length and introduced through an 18 French catheter to the right ventricle. It is also about 2 grams in weight and uses a temperature-based rate response sensor.13 Regulatory Status In April 2016, Micra Transcatheter Pacing System (Medtronic) was approved by FDA through the premarket approval process for use in patients who have experienced 1 or more of the following conditions:
5 Leadless Cardiac Pacemaker 5 symptomatic paroxysmal or permanent high-grade AV block in the presence of atrial fibrillation (AF) paroxysmal or permanent high-grade AV block in the absence of AF, as an alternative to dual chamber pacing, when atrial lead placement is considered difficult, high risk, or not deemed necessary for effective therapy symptomatic bradycardia-tachycardia syndrome or sinus node dysfunction (sinus bradycardia or sinus pauses), as an alternative to atrial or dual chamber pacing, when atrial lead placement is considered difficult, high risk, or not deemed necessary for effective therapy. II. Criteria/Guidelines Micra Transcatheter Pacing System is covered (subject to Limitations and Administrative Guidelines) when conditions A through D are met: A. Patient has symptomatic paroxysmal or permanent high-grade AV block or symptomatic bradycardia-tachycardia syndrome or sinus node dysfunction (sinus bradycardia or sinus pauses) B. One of the following criteria (1. or 2.) must be met: 1. Patient has any of the following that precludes implantation of a single chamber ventricular pacemaker: a. Need for persistent anticoagulation therapy b. Persistent severe bleeding tendencies c. Severe lung disease and positive end-expiratory pressure ventilation that precludes internal jugular and subclavian access d. Congenital acquired venous anomalies that preclude transvenous access to the heart OR 2. Presence of any of the following and delay in implanting a single chamber ventricular pacemaker could be life threatening: a. Persistent or recurrent local infection at implantation site b. Persistent or recurrent active systemic infection with bacteremia C. Patient does not have any of the following device(s) implanted: 1. An implanted device that would interfere with the implant of the Micra device in the judgment of the implanting physician 2. An implanted inferior vena cava filter 3. A mechanical tricuspid valve 4. An implanted cardiac device providing active cardiac therapy that may interfere with the sensing performance of the Micra device. D. Patient does not have any of the following conditions: 1. Femoral venous anatomy unable to accommodate a 7.8 mm (23 French) introducer sheath 2. Unable to accommodate implant on the right side of the heart (e.g., due to obstructions or severe tortuosity) 3. Morbid obesity that prevents the implanted device from obtaining telemetry communication within 12.5 cm (4.9 in)
6 Leadless Cardiac Pacemaker 6 III. Limitations 4. Known intolerance to titanium, titanium nitride, parylene C, primer for parylene C, PEEK, siloxane, nitinol, platinum, iridium, liquid silicone rubber, and silicone medical adhesive and heparin 5. Known sensitivity to contrast media that cannot be adequately premedicated. 6. Cannot tolerate a single dose of 1.0 mg of dexamethasone acetate. Micra Transcatheter Pacing System is not covered in all other situations as it has not been shown to improve health outcomes. IV. Administrative Guidelines A. Precertification is required for the insertion of a leadless cardiac pacemaker. To pre-certify, complete HMSA s Precertification Request form and fax or mail the form with the following documentation: 1. Clinical notes to include specific documentation demonstrating that Section II. A-D criteria have all been met. B. Applicable codes: CPT Codes Description Insertion, replacement, or removal and replacement of permanent leadless pacemaker Removal of permanent leadless pacemaker. ICD-10 Codes I44.1-I44.2 Description Atrioventricular block; second degree and complete I45.2 Bifascicular block I45.3 Trifascicular block I45.5 Other specified heart block; includes sinoatrial block I47.2 Ventricular tachycardia R00.1 Bradycardia unspecified; includes sinus bradycardia I48.0-I48.92 Atrial fibrillation and flutter code range I49.3 Ventricular premature depolarization (PVC s) I49.5 Sick sinus syndrome (tachycardia-bradycardia syndrome) V. Background This evidence review was created in August 2018 with a search of the MEDLINE database through July 18, 2018.
7 Leadless Cardiac Pacemaker 7 Evidence reviews assess the clinical evidence to determine whether the use of a technology improves the net health outcome. Broadly defined, health outcomes are length of life, quality of life, and ability to function-including benefits and harms. Every clinical condition has specific outcomes that are important to patients and to managing the course of that condition. Validated outcome measures are necessary to ascertain whether a condition improves or worsens; and whether the magnitude of that change is clinically significant. The net health outcome is a balance of benefits and harms. To assess whether the evidence is sufficient to draw conclusions about the net health outcome of a technology, 2 domains are examined: the relevance and the quality and credibility. To be relevant, studies must represent 1 or more intended clinical use of the technology in the intended population and compare an effective and appropriate alternative at a comparable intensity. For some conditions, the alternative will be supportive care or surveillance. The quality and credibility of the evidence depend on study design and conduct, minimizing bias and confounding that can generate incorrect findings. The randomized controlled trial (RCT) is preferred to assess efficacy; however, in some circumstances, nonrandomized studies may be adequate. RCTs are rarely large enough or long enough to capture less common adverse events and long-term effects. Other types of studies can be used for these purposes and to assess generalizability to broader clinical populations and settings of clinical practice. Conventional pace makers systems have been in use for over 50 years and the current available technology has matured with significant similarities of device design across models. Extensive bench testing experience with conventional pacemakers and a good understanding of operative and early postimplant safety and effectiveness is available which limits the need for collection of clinical data to understand their safety and effectiveness with regard to implant, tip fixation, electrical measures, and rate response. As such, a randomized trial comparing the leadless pacemakers with conventional pacemakers was not required by the Food and Drug Administration (FDA). INDIVIDUALS WITH GUIDELINES-BASED INDICATION FOR A VENTRICULAR PACING SYSTEM WHO ARE MEDICALLY ELIGIBLE TO RECEIVE A CONVENTIONAL PACING SYSTEM Clinical Context and Therapy Purpose The purpose of Micra Transcatheter Pacing System in patients with a class I or II guidelines-based indication for implantation of a single chamber ventricular pacemaker is to provide a treatment option that is an alternative to or an improvement on conventional pacing systems. The question addressed in this evidence review is: Does use of the Micra Transcatheter Pacing System improve the net health outcome in patients with patients with a class I or II guidelinesbased indication for implantation of a single-chamber ventricular pacemaker who are medically eligible to receive a conventional pacing systems? The following PICOTS were used to select literature to inform this review. Patients The relevant population of interest is patients with a class I or II guidelines-based indication for implantation of a single chamber ventricular pacemaker who are medically eligible to receive conventional pacing system.
8 Leadless Cardiac Pacemaker 8 Interventions The therapy being considered is the Micra Transcatheter Pacing System. Comparators The following therapy is currently being used to make decisions about managing patients requiring a pacemaker: a conventional pacemaker. Outcomes The general outcomes of interest are treatment-related mortality and morbidity. Specifically, the short-term outcomes include acute complication-free survival rate, electrical performance of the device including pacing capture threshold and adverse events including procedural and postprocedural complications. Long-term outcomes include chronic complication-free survival rate, electrical performance of the device including pacing impedance and pacing thresholds and chronic complications including any system explant, replacement (with and without system explant) and repositions. Further, analysis summary statistics regarding battery length are deemed crucial as well. Timing To assess short-term safety, the first 30 days postimplant is generally considered appropriate as majority of device and procedural complications occur within this time frame. To assess long-term efficacy and safety as well issues related to end of life of the device, follow up to 9 to 12 years postimplant with adequate sample size are required to characterize device durability and characterize infrequent complications with sufficient certainty. Setting Cardiac pacemaker implant is performed by interventional cardiologists in the electrophysiology laboratory. Nonrandomized Controlled Trials Pivotal Trial The pivotal investigational device exemption (IDE) trial was a prospective single cohort study in which 744 patients with class I or II indication for implantation of a single chamber ventricular pacemaker according to ACC/AHA/HRS 2008 guidelines and any national guidelines were enrolled. The details on the design14 and results of the IDE trial have been published. Trial characteristics and results at 6 months are summarized in Table 2 and 3, respectively. System performance from the pivotal trial has been published but results are not discussed further. Of the 744 patients, the implantation of the Micra Transcatheter Pacing System was attempted in 725 patients of whom 719 (99.2%) were successfully implanted. The demographics of the trial population were typical for a single chamber pacemaker study performed in the United States with 42% being female and average age was 76 years. Sixty-four percent had a pacing indication associated with persistent or permanent atrial arrhythmias, 72.6% had any atrial fibrillation at baseline, and 27.4% did not have a history of atrial fibrillation. Among those 27.4% (n=199) without atrial fibrillation, 16.1% (n=32) had a primary indication of sinus bradycardia and 3.5% (n=7) had a primary indication of tachycardia-bradycardia.
9 Leadless Cardiac Pacemaker 9 The IDE trial had 2 primary end points related to safety and efficacy. The trial would have met the safety end point if the lower bound of the 95% confidence interval (CI) for the rate of freedom from major complications related to the Micra Transcatheter Pacing System or implantation procedure exceeded 83% at 6 months. Major complications were defined as those resulting in any of the following; death, permanent loss of device function due to mechanical or electrical dysfunction of the device (eg, pacing function disabled, leaving device abandoned electrically), hospitalization, prolonged Hospitalization by at least 48 hours or system revision (reposition, replacement, explant). The trial would have met the efficacy end point if the lower bound of the 95% CI for the proportion of patients with adequate pacing capture thresholds (PCT) exceeded 80% at 6 months. PCT as an effectiveness objective is a common electrical measure of pacing efficacy and is consistent with recent studies. Pacing capture threshold measured in volts is defined as the minimum amount of energy needed to capture the myocardial tissue electrically. Unnecessary high pacing output adversely shortens the battery life of the pacemaker and is influenced by physiologic and pharmacologic factors. As per FDA, demonstrating that PCT is less than 2 Volts for the vast majority of subjects will imply that the Micra Transcatheter Pacing System will have a longevity similar to current pacing systems since Micra s capture management feature will nominally set the safety margin to 0.5 volts above the PCT with hourly confirmation of the PCT. Results of the IDE trial are summarized in Table 3. At 6 months, the trial met both the efficacy and safety primary end points including freedom from major complications related to the system or procedure in 96.0% of the patients (95% CI, 93.9% to 97.3%), compared with a performance goal of 83%, and an adequate pacing capture threshold in 98.3% of the patients (95% CI, 96.1% to 99.5%), compared with a performance goal of 80%. The results of the IDE trial were compared post hoc with a historical cohort of 2667 patients generated from the six previous pacemaker studies conducted between 2005 and 2012 by Medtronic that evaluated performance requirement at 6 months postimplant of right ventricle pacing leads (single-chamber rates obtained by excluding any adverse events that were only related to the right atrial lead from the analysis). Micra Transcatheter Pacing System was associated with fewer complication than the historical control (4.0% vs 7.4%; hazard ratio [HR], 0.49; 95% CI, 0.33 to 0.75; p=0.001). Because there were differences in the baseline patient characteristics between the 2 cohorts (patients in the historical cohort were younger and with lower prevalence of coexisting conditions vs the IDE trial), an additional propensity-matched analysis was also conducted that showed similar result (HR=0.46; 95% CI, 0.28 to 0.74). As per FDA, lower rate of major complication with Micra Transcatheter Pacing System were driven by reductions in access site events (primarily implant site hematoma and implant site infections), pacing issues (primarily device capture and device pacing issues), and fixation events (there were no device/lead dislodgements in the Micra IDE trial). While the overall rate of complication was low, the rate of major complications related to cardiac injury (ie., pericardial effusion or perforation) was higher in the Micra IDE trial than in the 6 reference Medtronic pacemaker studies (1.6% vs 1.1%, p=0.288). Thus, there appears to be a tradeoff between types of adverse events with Micra Transcatheter Pacing System and conventional pacemakers. While adverse events related to leads and pocket are eliminated or minimized with Micra Transcatheter Pacing System, certain adverse events such as groin vascular complications
10 Leadless Cardiac Pacemaker 10 and vascular/cardiac bleeding occur at a higher frequency or are additive (new events) than conventional pacemakers. Of these, procedural complications such as acute cardiac perforations that were severe enough to resulting result in tamponade and emergency surgery were most concerning. In addition to lack of adequate data on long-term safety, effectiveness, reliability, and incidence of late device failures and battery longevity, there is also inadequate clinical experience with issues related to devices that have reached end of life including whether to extract or leave the device in situ and possibility of device-device interactions. There is no data on device-device interactions (both electrical and mechanical), which may occur when there is a deactivated Micra device alongside another leadless pacemaker or when a leadless pacemaker and transvenous device are both present. Even though, there have only been few device retrievals and very limited experience with time course of encapsulation of these devices in humans, it is highly likely that these devices will be fully encapsulated by the end of its typical battery life, and therefore device retrieval is unlikely. Current recommendations for end-of-device-life care for a Micra device may include the addition of a replacement device with or without explanation of the Micra device, which should be turned off. Post Approval Study The FDA approval of the Micra Transcatheter Pacing System is contingent on multiple postapproval studies to ensure reasonable assurance of continued safety and effectiveness of the device. Among these, the Micra Transcatheter Pacing System Post-Approval Study, a global, prospective, observational, multi-center study, enrolled 1830 patients to ensure that data is available for 1741 patients to estimate acute complication rate within 30 day of the implant, 500 patients to estimate 9-year complication free survival rate, and a minimum of 200 patients with a Micra Transcatheter Pacing System revision for characterizing end of device service. As per the protocol, if a subsequent device is placed and the Micra is deactivated or explanted, Medtronic would contact the implanting center and request the patient's clinical data surrounding the revision. All such data would be summarized including the type of system revision, how the extraction was attempted, success rate, and any associated complications. Study characteristics and results at 1 year (reported in FDA documents and as conference presentation) are summarized in Table 2 and 3, respectively. The postapproval study completed enrollment in early March The definition of major complication in the postapproval study was same as the Micra IDE trial. It is unclear if any patients who participated in the IDE trial were also enrolled in the post approval study. Results summarized in Table 3 report the data at 30 days published by Roberts et al (2017) and Chami et al (2018) with a mean follow-up of 6.8 months of 1817 patients of whom 465 patients had a follow-up for more than 1 year. At 30 days, the major complication rate was 1.51% (95% CI, 0.78 to 2.62%). The major complication rate was lower in the postapproval study compared with IDE trial (odds ratio [OR], 0.58; 95% CI, 0.27 to 1.25) although this did not reach statistical difference. The lower major complications was associated with a decrease in events that led to hospitalization, prolonged hospitalization, or loss of device function in the postapproval study compared to the IDE trial.
11 Leadless Cardiac Pacemaker 11 After a mean follow-up of 6.8 months, the major complication rate was 2.7% (95% CI, 2.0% to 3.6%). Authors compared these results with the same historical cohort of 2667 patients used in the IDE trial and reported a 63% reduction in the risk for major complications through 12 months with Micra Transcatheter Pacing System relative to conventional pacemakers (HR=0.37; 95% CI, 0.27 to 0.52). Additionally, the risk for major complication was lower in the Micra postapproval study than in the IDE trial but it was statistically significant different (HR= 0.71, 95% CI, 0.44 to 1.1). However, details of events classified as major complications were not reported for the historical control or for the IDE trial cohort at 1 year and therefore it is unclear as to which events were decreased in the post approval study or if any events increased with Micra Transcatheter Pacing System. Table 2. Summary of Key Nonrandomized Trial Characteristics Reynolds et al (2016); NCT Study Study Type Country Date Prospecti s 2013 ve single - cohort 2015 Roberts et al (2017); Chami et al (2018); NCT Micra Post Approval Study Prospecti ve single cohort 19 countries in North America, Europe, Asia, Australia, and Africa 23 countries in North America, Europe, Asia, Participants Patients who met class I or II guidelinesbased indications for pacing and suitable candidates for single-chamber ventricular Any patient to be implanted with a Micra a 30-day results reported by Roberts et al (2017). a Results after a mean follow-up of 6.8 months reported by Chami et al (2018) 22 as an abstract. Treatment Micra transcath eter pacemak er (n=744) Micra transcath eter pacemak er (n=795 a Follow-Up, mo a 6.8 b Table 3. Summary of Key Nonrandomized Trial Results Study Freedom From System- or Procedure- Related Major Percentage of Patients With Adequate Pacing Capture Major Complications Complications Thresholds at Reynolds at 6 Months 6 Months et al (2016) 16 ; 6 Major Complications Criteria months N 719 a ; 300 b Micra, n (%) 96.0% 98.3% ( 2.0 V) Total major complications: 28 in 25 patients (3.5%) DVT: 1 (0.1%) Pulmonary TE: 1 (0.1%) Events at groin puncture site: 5 (0.7%) Cardiac perforation: 11 (1.6%) Pacing issues: 2 (0.3%) Death: 1 (0.1%) Loss of device function: 1 (0.1%) Hospitalization: 13 (2.3%) Prolonged hospitalization ( 48 h): 16 (2.6%) System revision c : 3 (0.4%) 95% CI 93.9% to 97.3% 95.4% to 99.6% Others: 8 (1.7%) NA NA Durray et al (2017) 23 ; N NA
12 Leadless Cardiac Pacemaker 12 Micra, n (%) 96.0% NR (93%) Total major complications: 32 in 29 patients (4.0%) DVT: 1 (0.1%) Pulmonary TE: 1 (0.1%) Events at groin puncture site: 5 (0.7%) Cardiac perforation: 11 (1.6%) 95% CI 94.2% to 97.2% NA Post- Approval Study Roberts et al Pacing issues: 2 (0.3%) Others: 11 (1.7%) Death: NR (0.1%) Loss of device function: NR (0.1%) Hospitalization: NR (2.3%) Prolonged hospitalization ( 48 h): NR (2.2%) System revision c : (0.7%) Loss of device function: NR (0.3%) (2017) N 21 ; 795 NA Micra, n (%) 97.3% d 87.2% ( 1.0 V) 97.0% ( 2.0 V) Total major complications: 13 in 12 patients (1.51% [95% CI, 0.78% to 2.62%] DVT: 1 (0.13%) Events at groin puncture site: 6 (0.75%) Cardiac effusion/perforation: 1 (0.13%) Device dislodgement: 1 (0.13%) Death: 1 (0.13%) Hospitalization: 4 (0.50%) Prolonged hospitalization ( 48 h): 9 (1.01%) System revision c : 2 (0.25%) OR (95% CI) 0.58 (0.27 to NA Pacing issues: NA 1 (0.13%) NA Post- 1.25) e Approval Study Chami et al (2018) 22 ; NA 1817 NA Micra, n (%) 97.3% d NA Total major complications: NA 46 in 41 patients (2.7% [95% CI, 2.0% to 3.6%] Pericardial effusions: 8 (0.44%) Dislodgement: 1 (0.06%) Procedure-related infections: 3 (0.17%) Procedure-related deaths: 5 (0.28%) As per FDA: Complications f : 61 in 53 Deaths: Procedure-related: 4 Unknown relatedness: 3 HR (95% CI) 0.71 (0.44 to 1.1) e 0.37 (0.27 to 0.52) g NA Pending adjudication: NA 3 NA CI: confidence interval; DVT: deep vein thrombosis; FDA: Food and Drug Administration; HR: hazard ratio; NA; not available; NR: not reported; TE: thromboembolism. a Total number of patients who received the implant successfully. b Number of patients for whom data was available for 6-month evaluation. c Device explant, reposition or replacement d Calculations performed by BCBSA based on the major complication rate (2.7%; 95% CI 2.0 to 3.6%) reported by Chami et al (2018) e Major complication compared against IDE trial. f Unclear if the complications meet the definition of a major complication as events leading to death, hospitalization, prolonged hospitalization by 48 hours, system revision, or loss of device therapy. gmajor complication compared against historical control. The purpose of the gaps tables (see Tables 4 and 5) is to display notable gaps identified in each study. This information is synthesized as a summary of the body of evidence following each table and provides the conclusions on the sufficiency of the evidence supporting the position statement.
13 Leadless Cardiac Pacemaker 13 Table 4. Relevance Gaps Study Population a Intervention b Comparator c Outcomes d Follow-Up e Reynolds et al (2016) 16 6 months; 2. This was a single cohort Durray et al study; there was (2017) no comparator months Roberts et al (2017) 21 Post- Approval Study 30-day; Chami et al (2018) 22 Post- Approval Registry 2. This was a single cohort study; there was no comparator 1. Not sufficient duration for benefit 2. Not sufficient duration for harms 1. Not sufficient duration for benefit 2. Not sufficient duration for harms The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. a Population key: 1. Intended use population unclear; 2. Clinical context is unclear; 3. Study population is unclear; 4. Study population not representative of intended use. b Intervention key: 1. Not clearly defined; 2. Version used unclear; 3. Delivery not similar intensity as comparator; 4.Not the intervention of interest. c Comparator key: 1. Not clearly defined; 2. Not standard or optimal; 3. Delivery not similar intensity as intervention; 4. Not delivered effectively. d Outcomes key: 1. Key health outcomes not addressed; 2. Physiologic measures, not validated surrogates; 3. No CONSORT reporting of harms; 4. Not establish and validated measurements; 5. Clinical significant difference not prespecified; 6. Clinical significant difference not supported. e Follow-Up key: 1. Not sufficient duration for benefit; 2. Not sufficient duration for harms. Table 5. Study Design and Conduct Gaps Study Allocation a Blinding b Reynolds et al (2016) 16 6 months; Durray et al (2017) months Roberts et al (2017) 21 Post- Approval Study 30-day; Chami et al (2018) Participants not randomly allocated; design was prospective single cohort study 1. Participants not randomly allocated; design was prospective registry 3. Not blinded to treatment assignment 4. Not blinded outcome assessment. However, adverse events analyzed by an independent clinical event committee. Trial oversight provided by an independent data and safety monitoring committee. 3. Not blinded to treatment assignment 4. Not blinded outcome assessment 5. Outcome assessed by treating physician Selecti ve Reporti Data Completene ss e Pow er d Statistic al f 1-year The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. a Allocation key: 1. Participants not randomly allocated; 2. Allocation not concealed; 3. Allocation concealment unclear; 4. Inadequate control for selection bias. b Blinding key: 1. Not blinded to treatment assignment; 2. Not blinded outcome assessment; 3. Outcome assessed by treating physician. c Selective Reporting key: 1. Not registered; 2. Evidence of selective reporting; 3. Evidence of selective publication. d Data Completeness key: 1. High loss to follow-up or missing data; 2. Inadequate handling of missing data; 3. High number of crossovers; 4. Inadequate handling of crossovers; 5. Inappropriate exclusions; 6. Not intent to treat analysis (per protocol for noninferiority trials). e Power key: 1. Power calculations not reported; 2. Power not calculated for primary outcome; 3. Power not based on clinically important difference. f Statistical key: 1. Analysis is not appropriate for outcome type: (a) continuous; (b) binary; (c) time to event; 2. Analysis is not appropriate for multiple observations per patient; 3. Confidence intervals and/or p values not reported; 4. Comparative treatment effects not calculated.
14 Leadless Cardiac Pacemaker 14 Section Summary: Individuals With Guidelines-Based Indication for a Ventricular Pacing System who are Medically Eligible to Receive a Conventional Pacing System The evidence for use of Micra Transcatheter Pacing System consists of a pivotal prospective cohort study and a postapproval prospective cohort study. Results at 6 months and 1 year for the pivotal study reported high procedural success (above 99%) and device effectiveness (pacing capture threshold met in 98% patients). Majority of the system or procedural-related complications occur within 30 days. At 1 year, the incidence of major complication did not increase substantially from 6 months (3.5% at 6 months versus 4% at 1 year). Results of the postapproval study were consistent with pivotal study and showed a lower incidence of major complications at -30 days as well as 1 year (1.5% and 2.7%, respectively). In both studies, the point estimates of major complication were lower than the pooled estimates from 6 studies of conventional pacemakers used as a historical comparator. While Micra Transcatheter Pacing System eliminates adverse events associated with lead and pocket issue, its use results in additional complication related to the femoral access site (groin hematomas and access site bleeding) and implantation/release of the device (traumatic cardiac injury). Considerable uncertainties and unknowns remain in terms of durability of device and end of life device issues. Early and limited experience suggests that retrieval of these devices is unlikely because in due course of time, the devices will be encapsulated. There is limited data on device-device interactions (both electrical and mechanical), which may occur when there is a deactivated Micra device alongside another leadless pacemaker or when a leadless pacemaker and transvenous device are both present. INDIVIDUALS WITH GUIDELINES-BASED INDICATION FOR A VENTRICULAR PACING SYSTEM WHO ARE MEDICALLY INELIGIBLE FOR A CONVENTIONAL PACING SYSTEM Clinical Context and Therapy Purpose The purpose of Micra Transcatheter Pacing System in patients with a class I or II guidelines-based indication for implantation of a single chamber ventricular pacemaker is to provide a treatment option that is an alternative to or an improvement on conventional pacing systems. The question addressed in this evidence review is: Does use of the Micra Transcatheter Pacing System improve the net health outcome in patients with patients with a class I or II guidelinesbased indication for implantation of a single chamber ventricular pacemaker who are medically ineligible for a conventional pacing system? The following PICOTS were used to select literature to inform this review. Patients The relevant population of interest is patients with a class I or II guidelines-based indication for implantation of a single chamber ventricular pacemaker who are medically ineligible for a conventional pacing system. Interventions The therapy being considered is Micra Transcatheter Pacing System. Comparators
15 Leadless Cardiac Pacemaker 15 The following therapy and practice are currently being used to make decisions about managing patients ineligible for a conventional pacemaker: medical management and/or conventional pacemakers. Outcomes The general outcomes of interest are treatment-related mortality and morbidity. Specifically, the short-term outcomes include acute complication-free survival rate, electrical performance of the device including pacing capture threshold and adverse events including procedural and postprocedural complications. Long-term outcomes include chronic complication-free survival rate, electrical performance of the device including pacing impedance and pacing thresholds and chronic complications including any system explant, replacement (with and without system explant) and repositions. Further, analysis summary statistics regarding battery length are deemed crucial as well. Timing To assess short-term safety, the first 30 days postimplant is generally considered appropriate as majority of device and procedural complications occur within this time frame. To assess long-term efficacy and safety as well issues related to end of life of the device, follow up to 9 to 12 years postimplant with adequate sample size are required to characterize device durability and characterize infrequent complications with sufficient certainty. Setting Cardiac pacemaker implant is performed by interventional cardiologists in the electrophysiology laboratory. Nonrandomized Controlled Trials No studies that exclusively enrolled patients who were medically ineligible to receive a conventional pacing system were identified. In the IDE trial, 6.2% or 45 patients received the Micra Transcatheter Pacing System because they were medically ineligible to receive a conventional pacing system such as compromised venous access, the need to preserve veins for hemodialysis, thrombosis, a history of infection, or the need for an indwelling venous catheter. A stratified analysis of these 45 patients was not presented in the published paper or the FDA documents. In the postapproval registry whose early results have been published only as an abstract, authors reported stratified results of 99 of 1744 patients who had previous cardiac implantable electronic device (CIED) infection.22 Of these 99, 78 (79%) were classified as medically ineligible to receive a conventional pacemaker in the opinion of physician. A stratified analysis of these 78 patients was not presented in the abstract. Trial characteristics and results are summarized in Tables 6 and 7, respectively. In this cohort of patients with CIED infection, Micra was implanted successfully in 98 patients and the previous CIED was explanted the same day as Micra was implanted in 36% of patients. Major complications were reported in 2% of patients with an average follow-up of 5.5 months. S x deaths were reported but none was related to the Micra Transcatheter Pacing System or the procedure.
16 Leadless Cardiac Pacemaker 16 Table 6. Summary of Key Nonrandomized Trial Characteristics in Patients Ineligible to Receive Conventional Pacing Systems and/or Previous Cardiac Implantable Electronic Device Infection Study Study Type Country Dates Participants Treatment Follow-Up, mo Chami et Prospective 23 countries in Any patient to Micra 5.5 (range, 0- al (2018) 22 single North 2018 be implanted transcath 24.7) NCT02536 cohort America, with a Micra eter 118 Europe, Asia, with a CIED pacemak Micra Australia, infection er Post- and Africa (N=99) CIED: Approv cardiac implantable electronic device. Table 7. Summary of Key Nonrandomized Trial Results in Patients Ineligible to Receive Conventional Pacing Systems and/or Previous Cardiac Implantable Electronic Device Infection No. of Patients With System- Study or Procedure-Related Major Complications Major Complications Average Pacing Threshol d Chami et a (2018) 22 Post- Approval s try N Micra, n (%) 2% (2/99) 0.64 ± 0.42 V Total major complications: 4 in 2 patients Patient 1. Effusion requiring pericardiocentesis Patient 2. Elevated thresholds, complication of device removal (IVC filter entanglement), and subsequent abdominal IVC: in cava filter The purpose of the gaps tables (see Tables 8 and 9) is to display notable gaps identified in each study. This information is synthesized as a summary of the body of evidence following each table and provides the conclusions on the sufficiency of the evidence supporting the position statement. Table 8. Relevance Gaps Study Population a Intervention b Comparator c Outcomes d Follow-Up e Chami et al 2. This was a single cohort 1. Not sufficient duration for (2018) 2 study; there was benefit 2 no comparator 2. Not sufficient Post- duration for Approval harms The Registry evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. a Population key: 1. Intended use population unclear; 2. Clinical context is unclear; 3. Study population is unclear; 4. Study population not representative of intended use. b Intervention key: 1. Not clearly defined; 2. Version used unclear; 3. Delivery not similar intensity as comparator; 4.Not the intervention of interest. c Comparator key: 1. Not clearly defined; 2. Not standard or optimal; 3. Delivery not similar intensity as intervention; 4. Not delivered effectively. d Outcomes key: 1. Key health outcomes not addressed; 2. Physiologic measures, not validated surrogates; 3. No CONSORT reporting of harms; 4. Not establish and validated measurements; 5. Clinical significant difference not prespecified; 6. Clinical significant difference not supported. e Follow-Up key: 1. Not sufficient duration for benefit; 2. Not sufficient duration for harms. Table 9. Study Design and Conduct Gaps Study Allocation a Blinding b Selective Reporting d Data Completene ss e Pow er d Statistic al f
17 Leadless Cardiac Pacemaker 17 Chami et al (2018) 2 2 Post- Approval Registry 1 year Not blinded to Participant treatment assignment s not 6. Not blinded randomly outcome allocated; assessment. design was 7. Outcome assessed prospectiv by treating physician The evidence gaps stated in this table are those notable in the current review; this is not a comprehensive gaps assessment. a Allocation key: 1. Participants not randomly allocated; 2. Allocation not concealed; 3. Allocation concealment unclear; 4. Inadequate control for selection bias. b Blinding key: 1. Not blinded to treatment assignment; 2. Not blinded outcome assessment; 3. Outcome assessed by treating physician. c Selective Reporting key: 1. Not registered; 2. Evidence of selective reporting; 3. Evidence of selective publication. d Data Completeness key: 1. High loss to follow-up or missing data; 2. Inadequate handling of missing data; 3. High number of crossovers; 4. Inadequate handling of crossovers; 5. Inappropriate exclusions; 6. Not intent to treat analysis (per protocol for noninferiority trials). e Power key: 1. Power calculations not reported; 2. Power not calculated for primary outcome; 3. Power not based on clinically important difference. f Statistical key: 1. Analysis is not appropriate for outcome type: (a) continuous; (b) binary; (c) time to event; 2. Analysis is not appropriate for multiple observations per patient; 3. Confidence intervals and/or p values not reported; 4. Comparative treatment effects not calculated. Section Summary: Individuals with Guidelines-Based Indication for a Ventricular Pacing System who are Medically Ineligible for a Conventional Pacing System No studies that exclusively enrolled patients who were medically ineligible to receive a conventional pacing system were identified. However, a subgroup of patients in whom use of conventional pacemakers was precluded was enrolled in the pivotal as well as the postapproval trial. Information on the outcomes in these subgroups of patients from the postapproval study showed that Micra was successfully implanted in 98% of cases and safety outcomes were similar to the original cohort. Even though, the evidence is limited and long-term effectiveness and safety is unknown, the shortterm benefits outweigh the risks as the complex tradeoff of adverse events for these devices need to be assessed in the context of lifesaving potential of pacing systems in patients who are ineligible for conventional pacing systems on the market. SUMMARY OF EVIDENCE For individuals with guidelines-based indication for a ventricular pacing system who are medically eligible to receive a conventional pacing system who are treated with Micra transcatheter pacing system, the evidence includes a pivotal prospective cohort study a 1 postapproval prospective cohort study. Relevant outcomes are other test performance, treatment-related mortality, and treatment-related morbidity. Results at 6 months and 1 year for the pivotal study reported high procedural success (above 99%) and device effectiveness (pacing capture threshold met in 98% patients). Majority of the system or procedural- related complications occur within 30 days. At 1 year, the incidence of major complication did not increase substantially from 6 months (3.5% at 6 months vs 4% at 1 year). Results of the postapproval study were consistent with pivotal study and showed a lower incidence of major complications at -30 days as well as 1 year (1.5% and 2.7%, respectively). In both studies, the point estimates of major complication were lower than the pooled estimates from 6 studies of conventional pacemakers used as a historical comparator. While Micra Transcatheter Pacing System eliminates lead- and surgical pocket-related complications, its use can result in potentially more serious complication related to implantation/release of the device
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
NOVEL DEVICE TECHNOLOGIES
 NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
Leadless Pacing. Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt
 Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
New generations pacemakers and ICDs: an update
 Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
INNOVATIONS IN DEVICE THERAPY:
 INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW
 MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
Essentials of Pacemakers and ICD s. Rajesh Banker, MD, MPH
 Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
REVIEW ARTICLE. Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1
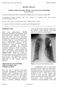 Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Transvenous Pacemaker Procedures
 Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
Update on Device Innovation (S-ICD, Wearable, Leadless)
 Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Pediatric Pacemaker Implantation Endocardial or Epicardial
 Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
National Coverage Determination (NCD) for Cardiac Pacemakers (20.8)
 Page 1 of 12 Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for Cardiac Pacemakers (20.8) Tracking Information Publication Number 100-3 Manual Section Number 20.8 Manual
Page 1 of 12 Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for Cardiac Pacemakers (20.8) Tracking Information Publication Number 100-3 Manual Section Number 20.8 Manual
Cardiac implantable electronic devices (CIEDs) in children include pacemakers and implantable cardioverter defibrillators (ICDs).
 Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Leadless pacemakers a panacea for bradyarrhythmias?
 Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Sinus rhythm with premature atrial beats 2 and 6 (see Lead II).
 Cardiac Pacemaker Premature Beats When one of ectopic foci becomes irritable, it may spontaneously fire, leading to one or more premature beats. Atrial and junctional foci may become irritable from excess
Cardiac Pacemaker Premature Beats When one of ectopic foci becomes irritable, it may spontaneously fire, leading to one or more premature beats. Atrial and junctional foci may become irritable from excess
Leadless pacing: Technical challenges and concerns. Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital
 Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
AF Today: W. For the majority of patients with atrial. are the Options? Chris Case
 AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
Pacing Without Wires: Leadless Cardiac Pacing
 REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
Figure 2. Normal ECG tracing. Table 1.
 Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
PERFORMANCE MEASURE TECHNICAL SPECIFICATIONS. HRS-3: Implantable Cardioverter-Defibrillator (ICD) Complications Rate
 PERFORMANCE MEASURE TECHNICAL SPECIFICATIONS HRS-3: Implantable Cardioverter-Defibrillator (ICD) Complications Rate Measure Title Description Measure Type Data Source Level of Analysis Numerator HRS-3:
PERFORMANCE MEASURE TECHNICAL SPECIFICATIONS HRS-3: Implantable Cardioverter-Defibrillator (ICD) Complications Rate Measure Title Description Measure Type Data Source Level of Analysis Numerator HRS-3:
Cardiac Pacemakers» 2013 HOSPITAL REIMBURSEMENT GUIDE
 Cardiac Pacemakers» 2013 HOSPITAL REIMBURSEMENT GUIDE 2 Contents Page Introduction Medicare Coding and Payment Overview Hospital Inpatient Hospital Outpatient HCPCS Device Category C-Codes Coverage for
Cardiac Pacemakers» 2013 HOSPITAL REIMBURSEMENT GUIDE 2 Contents Page Introduction Medicare Coding and Payment Overview Hospital Inpatient Hospital Outpatient HCPCS Device Category C-Codes Coverage for
RN-BC, MS, CCRN, FAHA
 Presented By: Barbara Furry, RN-BC, MS, CCRN, FAHA Director The Center of Excellence in Education Director of HERO Follow me on Twitter! CEE Med Updates@BarbaraFurryRN Like me on Facebook! 1 A. Atropine
Presented By: Barbara Furry, RN-BC, MS, CCRN, FAHA Director The Center of Excellence in Education Director of HERO Follow me on Twitter! CEE Med Updates@BarbaraFurryRN Like me on Facebook! 1 A. Atropine
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany
 Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Call Medtronic at 1 (800) to verify the patient s current implanted system
 MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience
 Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD
 Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
ΤΟ ΗΚΓ ΣΤΟΝ ΒΗΜΑΤΟΔΟΤΟΥΜΕΝΟ ΑΣΘΕΝΗ
 ΤΟ ΗΚΓ ΣΤΟΝ ΒΗΜΑΤΟΔΟΤΟΥΜΕΝΟ ΑΣΘΕΝΗ ΤΖΩΡΤΖ ΔΑΔΟΥΣ ΕΠΙΚΟΥΡΟΣ ΚΑΘΗΓΗΤΗΣ Α.Π.Θ. ΜΑΡΙΑ ΚΑΡΑΛΙΟΛΙΟΥ ΕΙΔΙΚΕΥΟΜΕΝΗ ΙΑΤΡΟΣ ΚΑΡΔΙΟΛΟΓΟΣ Β ΚΑΡΔΙΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ Α.Π.Θ. ΙΠΠΟΚΡΑΤΕΙΟ Γ.Ν.Θ. 5 Why a Pacemaker is Implanted
ΤΟ ΗΚΓ ΣΤΟΝ ΒΗΜΑΤΟΔΟΤΟΥΜΕΝΟ ΑΣΘΕΝΗ ΤΖΩΡΤΖ ΔΑΔΟΥΣ ΕΠΙΚΟΥΡΟΣ ΚΑΘΗΓΗΤΗΣ Α.Π.Θ. ΜΑΡΙΑ ΚΑΡΑΛΙΟΛΙΟΥ ΕΙΔΙΚΕΥΟΜΕΝΗ ΙΑΤΡΟΣ ΚΑΡΔΙΟΛΟΓΟΣ Β ΚΑΡΔΙΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ Α.Π.Θ. ΙΠΠΟΚΡΑΤΕΙΟ Γ.Ν.Θ. 5 Why a Pacemaker is Implanted
Presented By: Barbara Furry, RN-BC, MS, CCRN, FAHA Director The Center of Excellence in Education Director of HERO
 Presented By: Barbara Furry, RN-BC, MS, CCRN, FAHA Director The Center of Excellence in Education Director of HERO Follow me on Twitter! CEE Med Updates@BarbaraFurryRN Like me on Facebook! What is a
Presented By: Barbara Furry, RN-BC, MS, CCRN, FAHA Director The Center of Excellence in Education Director of HERO Follow me on Twitter! CEE Med Updates@BarbaraFurryRN Like me on Facebook! What is a
PACING AT THE BUNDLE OF HIS
 PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
Technical option of surgical approach for trouble-shooting
 JHRS Corner Device and lead trouble-shooting - standard strategy and technical option - Technical option of surgical approach for trouble-shooting Katsuhiko IMAI Department of Cardiovascular surgery, Hiroshima
JHRS Corner Device and lead trouble-shooting - standard strategy and technical option - Technical option of surgical approach for trouble-shooting Katsuhiko IMAI Department of Cardiovascular surgery, Hiroshima
Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker
 Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker Patient Scheduling and Screening Q: Is there any special (other than regular MRI consent) consent form that should be signed by the patient?
Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker Patient Scheduling and Screening Q: Is there any special (other than regular MRI consent) consent form that should be signed by the patient?
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Pacing Lead Implant Testing. Document Identifier
 Pacing Lead Implant Testing 1 Objectives Upon completion of this presentation, the participant should be able to: Name the two primary surgical options for implanting pacing leads Describe three significant
Pacing Lead Implant Testing 1 Objectives Upon completion of this presentation, the participant should be able to: Name the two primary surgical options for implanting pacing leads Describe three significant
Active Fixation Models: 7740, 7741 and 7742 Passive Fixation Models: 7731, 7732, 7735 and 7736
 The INGEVITY MRI pacing leads are 6F (2.0 mm) steroid-eluting, endocardial pace / sense leads designed for permanent implantation for either atrial or ventricular applications. INGEVITY MRI is the only
The INGEVITY MRI pacing leads are 6F (2.0 mm) steroid-eluting, endocardial pace / sense leads designed for permanent implantation for either atrial or ventricular applications. INGEVITY MRI is the only
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
About atrial fibrillation (AFib) Atrial Fibrillation (AFib) What is AFib? What s the danger? Who gets AFib?
 Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS
 Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS NBG Codes 1st Letter 2nd Letter 3rd Letter A V D Chamber(s) Paced = atrium = ventricle = dual (both atrium and ventricle) Chamber(s) Sensed A = atrium
Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS NBG Codes 1st Letter 2nd Letter 3rd Letter A V D Chamber(s) Paced = atrium = ventricle = dual (both atrium and ventricle) Chamber(s) Sensed A = atrium
Pediatric pacemakers & ICDs:
 Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE. Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology
 PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology Medical University of Łódź The heart is made up of four chambers Left Atrium Right Atrium Left Ventricle
PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology Medical University of Łódź The heart is made up of four chambers Left Atrium Right Atrium Left Ventricle
2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction
 Summary of Expert Consensus Statement for CLINICIANS 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction This is a summary of the Heart Rhythm
Summary of Expert Consensus Statement for CLINICIANS 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction This is a summary of the Heart Rhythm
Supplemental Material
 Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
CAPTIVATE SUMMARY CLINICAL SUMMARY. CAPTure Information Via Automatic Threshold Evaluation
 CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
SARASOTA MEMORIAL HOSPITAL
 SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE PACEMAKERS AND DETERMINING PACEMAKER DATE: REVIEWED: PAGES: 10/99 11/18 1 of 7 PS1094 ISSUED FOR: Nursing RESPONSIBILITY: *Qualified RN PURPOSE: To establish
SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE PACEMAKERS AND DETERMINING PACEMAKER DATE: REVIEWED: PAGES: 10/99 11/18 1 of 7 PS1094 ISSUED FOR: Nursing RESPONSIBILITY: *Qualified RN PURPOSE: To establish
Finally, a pacemaker may be either permanent or temporary, which will also factor into your code selections.
 2015 Cardiology Survival Guide Chapter 15: Pacemakers With more than 30 codes and many variables to choose from, you must weigh your options carefully when reporting insertion, revision, or removal of
2015 Cardiology Survival Guide Chapter 15: Pacemakers With more than 30 codes and many variables to choose from, you must weigh your options carefully when reporting insertion, revision, or removal of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA88; Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA88; Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block
SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000. SJM-EDTR Item approved for U.S. use only.
 SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000 1 APPROVED MRI SCAN CONDITIONS 1.5 TESLA FULL BODY 2 SETUP SJM MRI ACTIVATOR HANDHELD DEVICE The SJM MRI Activator handheld device must be enabled
SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000 1 APPROVED MRI SCAN CONDITIONS 1.5 TESLA FULL BODY 2 SETUP SJM MRI ACTIVATOR HANDHELD DEVICE The SJM MRI Activator handheld device must be enabled
Occlusion de l'auricule gauche: Niche ou réel avenir? D Gras, MD, Nantes, France
 Occlusion de l'auricule gauche: Niche ou réel avenir? D Gras, MD, Nantes, France LAA Occlusion Is there a real future? Background Protect AF Trial Other Studies CAP, ASAP, Prevail Left Atrial Appendage
Occlusion de l'auricule gauche: Niche ou réel avenir? D Gras, MD, Nantes, France LAA Occlusion Is there a real future? Background Protect AF Trial Other Studies CAP, ASAP, Prevail Left Atrial Appendage
Lead extraction. Dr. Mervat Abo El Maaty Professor of Cardiology Ain shams University 2013
 Lead extraction Dr. Mervat Abo El Maaty Professor of Cardiology Ain shams University 2013 Agenda Introduction History of consensus Definitions Complications Indications Lead management environment Extraction
Lead extraction Dr. Mervat Abo El Maaty Professor of Cardiology Ain shams University 2013 Agenda Introduction History of consensus Definitions Complications Indications Lead management environment Extraction
Girish M Nair, Seeger Shen, Pablo B Nery, Calum J Redpath, David H Birnie
 268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
Cigna - Prior Authorization Procedure List Cardiology
 Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Catheter Ablation as Treatment for Atrial Fibrillation
 Catheter Ablation as Treatment for Atrial Fibrillation Policy Number: Original Effective Date: MM.06.011 09/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/29/2018 Section:
Catheter Ablation as Treatment for Atrial Fibrillation Policy Number: Original Effective Date: MM.06.011 09/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/29/2018 Section:
Innovations. Outcomes 2013
 Innovations The Global Cardiovascular Innovation Center (GCIC) is an incubator facility that is part of the Cleveland Clinic Innovation Center. The GCIC has awarded 56 commercialization grants totaling
Innovations The Global Cardiovascular Innovation Center (GCIC) is an incubator facility that is part of the Cleveland Clinic Innovation Center. The GCIC has awarded 56 commercialization grants totaling
The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node.
 PACEMAKER Natural pacemaker: The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node. Artificial pacemaker: It is a small, battery-operated device that helps the heart beat in
PACEMAKER Natural pacemaker: The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node. Artificial pacemaker: It is a small, battery-operated device that helps the heart beat in
Complications of Lead Extraction: Prevention and treatment. Maria Grazia Bongiorni, MD, FESC
 Complications of Lead Extraction: Prevention and treatment Maria Grazia Bongiorni, MD, FESC Director of Cardiovascular Division University Hospital of Pisa (Italy) ourtesy of Dr Eivind Platou Potential
Complications of Lead Extraction: Prevention and treatment Maria Grazia Bongiorni, MD, FESC Director of Cardiovascular Division University Hospital of Pisa (Italy) ourtesy of Dr Eivind Platou Potential
INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS
 INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS BEFORE INTERPRETING THE ECG: Nora Goldschlager, M.D. MACP, FACC, FAHA, FHRS. Cardiology San Francisco General Hospital UCSF Disclosures: None 1 2 QUESTIONS
INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS BEFORE INTERPRETING THE ECG: Nora Goldschlager, M.D. MACP, FACC, FAHA, FHRS. Cardiology San Francisco General Hospital UCSF Disclosures: None 1 2 QUESTIONS
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
PACEMAKER INTERPRETATION AND DEVICE MANAGEMENT PART I
 1 PACEMAKER INTERPRETATION AND DEVICE MANAGEMENT PART I Cynthia Webner DNP, RN, CCNS, CCRN-CMC Karen Marzlin DNP, RN, CCNS, CCRN-CMC 2 PROFESSIONAL NURSING PRACTICE CAN ONLY ADVANCE AS MUCH AS INDIVIDUAL
1 PACEMAKER INTERPRETATION AND DEVICE MANAGEMENT PART I Cynthia Webner DNP, RN, CCNS, CCRN-CMC Karen Marzlin DNP, RN, CCNS, CCRN-CMC 2 PROFESSIONAL NURSING PRACTICE CAN ONLY ADVANCE AS MUCH AS INDIVIDUAL
EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS
 EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS MRI SureScan Pacing, ICD, and CRT (CRT-D and CRT-P) Transvenous Implantable Cardiac Systems Timothy S. E. Albert, M.D. President CVMR Solutions, Inc. Carmel
EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS MRI SureScan Pacing, ICD, and CRT (CRT-D and CRT-P) Transvenous Implantable Cardiac Systems Timothy S. E. Albert, M.D. President CVMR Solutions, Inc. Carmel
Cardiology. Objectives. Chapter
 1:44 M age 1121 Chapter Cardiology Objectives art 1: Cardiovascular natomy and hysiology, ECG Monitoring, and Dysrhythmia nalysis (begins on p. 1127) fter reading art 1 of this chapter, you should be able
1:44 M age 1121 Chapter Cardiology Objectives art 1: Cardiovascular natomy and hysiology, ECG Monitoring, and Dysrhythmia nalysis (begins on p. 1127) fter reading art 1 of this chapter, you should be able
Advanced ICD Concepts
 1 4 2 5 7 3 6 8 Advanced ICD Concepts This presentation is provided with the understanding that the slide content must not be altered in any manner as the content is subject to FDA regulations. This presentation
1 4 2 5 7 3 6 8 Advanced ICD Concepts This presentation is provided with the understanding that the slide content must not be altered in any manner as the content is subject to FDA regulations. This presentation
Cardiac Pacing. Learning outcomes. Introduction. The cardiac impulse - its formation and its failure CHAPTER. To understand:
 Cardiac Pacing CHAPTER 10 Learning outcomes To understand: The indications for cardiac pacing in the peri-arrest setting How to perform percussion pacing How to apply non-invasive, transcutaneous electrical
Cardiac Pacing CHAPTER 10 Learning outcomes To understand: The indications for cardiac pacing in the peri-arrest setting How to perform percussion pacing How to apply non-invasive, transcutaneous electrical
RESPECT Safety Findings
 CO-1 SCAI Town Hall Meeting Monday, October 31, 2016 Washington, DC RESPECT Safety Findings John D. Carroll, M.D., MSCAI Professor of Medicine Cardiology University of Colorado School of Medicine University
CO-1 SCAI Town Hall Meeting Monday, October 31, 2016 Washington, DC RESPECT Safety Findings John D. Carroll, M.D., MSCAI Professor of Medicine Cardiology University of Colorado School of Medicine University
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES
 UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE. Updated September 2018
 CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
Puzzling Pacemakers Cheryl Herrmann, APN, CCRN, CCNS-CSC-CMC
 Puzzling Pacemakers Cheryl Herrmann, APN, CCRN, CCNS-CSC-CMC Pacemaker: An electric device implanted in the body to regulate the heart beat. Delivers electrical stimuli over leads with electrodes in contact
Puzzling Pacemakers Cheryl Herrmann, APN, CCRN, CCNS-CSC-CMC Pacemaker: An electric device implanted in the body to regulate the heart beat. Delivers electrical stimuli over leads with electrodes in contact
1. Technology Name: WATCHMAN Left Atrial Appendage Closure Technology. 3. Trade Brand of Technology: WATCHMAN Left Atrial Appendage Closure Technology
 1. Technology Name: REASANZ (serelaxin) Injection 1 mg/ml Solution for I.V. Infusion 2. Manufacturer Name: Novartis Pharmaceuticals Corporation 3. Trade Brand of Technology: The brand name of the new technology
1. Technology Name: REASANZ (serelaxin) Injection 1 mg/ml Solution for I.V. Infusion 2. Manufacturer Name: Novartis Pharmaceuticals Corporation 3. Trade Brand of Technology: The brand name of the new technology
VIRTUS: Trial Design and Primary Endpoint Results
 VIRTUS: Trial Design and Primary Endpoint Results Mahmood K. Razavi, MD St. Joseph Cardiac and Vascular Center Orange, CA, USA IMPORTANT INFORMATION: These materials are intended to describe common clinical
VIRTUS: Trial Design and Primary Endpoint Results Mahmood K. Razavi, MD St. Joseph Cardiac and Vascular Center Orange, CA, USA IMPORTANT INFORMATION: These materials are intended to describe common clinical
Left Atrial Appendage Closure: The Good, The Bad and The Ugly
 Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Title and contents page 1 Who should read this document 2 Scope of the Guideline 2 Background 2 What is new in this version 2
 Temporary Transvenous Pacing Guideline Classification: Clinical Guideline Lead Author: Dr Peter Woolfson Additional author(s): Dr Alan Fitchet Sister Joanne Hughes, Matron Julie Winstanley Authors Division:
Temporary Transvenous Pacing Guideline Classification: Clinical Guideline Lead Author: Dr Peter Woolfson Additional author(s): Dr Alan Fitchet Sister Joanne Hughes, Matron Julie Winstanley Authors Division:
Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases
 Case Report Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases Koji Tsutsumi MD, Tatsuru Niibori MD, Keiichiro Katsumoto MD Department
Case Report Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases Koji Tsutsumi MD, Tatsuru Niibori MD, Keiichiro Katsumoto MD Department
Leadless pacing. P.E. Vardas Professor of Cardiology University of Crete, Greece
 Leadless pacing P.E. Vardas Professor of Cardiology University of Crete, Greece DISCLOSURES Small teaching fees from: Bayer/J&J, Boeringer, Menarini Small consulting fees from: Medtronic 1957: First External
Leadless pacing P.E. Vardas Professor of Cardiology University of Crete, Greece DISCLOSURES Small teaching fees from: Bayer/J&J, Boeringer, Menarini Small consulting fees from: Medtronic 1957: First External
Devices to Protect Against Stroke in Atrial Fibrillation
 Devices to Protect Against Stroke in Atrial Fibrillation Jonathan C. Hsu, MD, MAS Associate Clinical Professor Division of Cardiology, Section of Cardiac Electrophysiology June 2, 2018 Disclosures Honoraria
Devices to Protect Against Stroke in Atrial Fibrillation Jonathan C. Hsu, MD, MAS Associate Clinical Professor Division of Cardiology, Section of Cardiac Electrophysiology June 2, 2018 Disclosures Honoraria
Danish Pacemaker and ICD Register Annual report 2015
 Danish Pacemaker and ICD Register Annual report 2015 Preface The Danish Pacemaker Register was founded in 1982 by physicians from all Danish hospitals where pacemakers were implanted. When the first implantable
Danish Pacemaker and ICD Register Annual report 2015 Preface The Danish Pacemaker Register was founded in 1982 by physicians from all Danish hospitals where pacemakers were implanted. When the first implantable
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc.
 Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France
 How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
ESC Stockholm Arrhythmias & pacing
 ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
PERIOPERATIVE MANAGEMENT: CARDIAC PACEMAKERS AND DEFIBRILLATORS
 PERIOPERATIVE MANAGEMENT: CARDIAC PACEMAKERS AND DEFIBRILLATORS DR SUSAN CORCORAN CARDIOLOGIST ONCE UPON A TIME.. Single chamber pacemakers Programmed at 70/min VVI 70 UNIPOLAR SYSTEMS A Unipolar Pacing
PERIOPERATIVE MANAGEMENT: CARDIAC PACEMAKERS AND DEFIBRILLATORS DR SUSAN CORCORAN CARDIOLOGIST ONCE UPON A TIME.. Single chamber pacemakers Programmed at 70/min VVI 70 UNIPOLAR SYSTEMS A Unipolar Pacing
Transcatheter Aortic-Valve Implantation for Aortic Stenosis
 Transcatheter Aortic-Valve Implantation for Aortic Stenosis Policy Number: Original Effective Date: MM.06.019 10/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/27/2018
Transcatheter Aortic-Valve Implantation for Aortic Stenosis Policy Number: Original Effective Date: MM.06.019 10/01/2012 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/27/2018
Patient Resources: Arrhythmias and Congenital Heart Disease
 Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Kadlec Regional Medical Center Cardiac Electrophysiology WATCHMAN Left Atrial Appendage Closure Device
 Patient Information Guide Kadlec Regional Medical Center Cardiac Electrophysiology WATCHMAN Left Atrial Appendage Closure Device Your doctor has recommended that you consider undergoing a procedure to
Patient Information Guide Kadlec Regional Medical Center Cardiac Electrophysiology WATCHMAN Left Atrial Appendage Closure Device Your doctor has recommended that you consider undergoing a procedure to
WATCHMAN PROTECT AF Study Rev. 6
 WATCHMAN PROTECT AF Study Rev. 6 Protocol Synopsis Title WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation (PROTECT AF) Sponsor Atritech/Boston Scientific
WATCHMAN PROTECT AF Study Rev. 6 Protocol Synopsis Title WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation (PROTECT AF) Sponsor Atritech/Boston Scientific
OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices
 OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices Ontario Health Technology Advisory Committee January 2012 Issue Background The Ontario
OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices Ontario Health Technology Advisory Committee January 2012 Issue Background The Ontario
Clinical Data Summary: Avoid FFS Study
 Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
What s New in CIEDs. Keimyung University Dongsan Medical Center Hyoung-Seob Park
 What s New in CIEDs Keimyung University Dongsan Medical Center Hyoung-Seob Park Transvenous CIEDs Technology: - Highly mature & reliable - Still includes generator, connectors and leads Procedure : -
What s New in CIEDs Keimyung University Dongsan Medical Center Hyoung-Seob Park Transvenous CIEDs Technology: - Highly mature & reliable - Still includes generator, connectors and leads Procedure : -
Watchman. Left Atrial Appendage Closure Device. Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8
 TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
Patient guide: pfm Nit-Occlud PDA coil occlusion system. Catheter occlusion of. Patent Ductus Arteriosus. with the
 Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
ATRIAL FIBRILLATION ANSWERS. A Patient Education Handbook on Electrophysiology
 ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
Cardiac Rhythm Device Management. PBL STOP Your acronym for a standardized follow-up
 Cardiac Rhythm Device Management PBL STOP Your acronym for a standardized follow-up What s in it for you? What do you need to feel comfortable with a pacemaker/icd follow-up? 2 CORE OBJECTIVE Provide a
Cardiac Rhythm Device Management PBL STOP Your acronym for a standardized follow-up What s in it for you? What do you need to feel comfortable with a pacemaker/icd follow-up? 2 CORE OBJECTIVE Provide a
UnitedHealthcare Medicare Advantage Cardiology Prior Authorization Program
 Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
11/21/18. EKG Pop Quiz. Michael Giocondo, MD Cardiac Electrophysiology Saint Luke s Cardiovascular Consultants
 EKG Pop Quiz Michael Giocondo, MD Cardiac Electrophysiology Saint Luke s Cardiovascular Consultants 1 Disclosures No financial relationships to disclose. EKG #1 75 y/o woman with a dual-chamber pacemaker
EKG Pop Quiz Michael Giocondo, MD Cardiac Electrophysiology Saint Luke s Cardiovascular Consultants 1 Disclosures No financial relationships to disclose. EKG #1 75 y/o woman with a dual-chamber pacemaker
