MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
|
|
|
- Molly Daniels
- 5 years ago
- Views:
Transcription
1 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System
2 MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart, no leads required New ultra-low power circuit design delivers a 12-year longevity 3 Together, we can provide new opportunities to redefine the patient experience and reduce complications associated with traditional pacing technology. 2 Redefined Patient Experience No chest scar No bump No visible or physical reminder of a pacemaker under the skin Fewer post-implant activity restrictions Eliminated Pocket-related Complications 5 Infection Hematoma Erosion SOPHISTICATED. Engineered for a minimally invasive approach Atraumatic FlexFix TM nitinol tines provide secure capsule placement 7,8 Integrated delivery system facilitates a streamlined implant procedure via a percutaneous, femoral approach COMPLETE. The only transcatheter pacing system to offer a complete feature set 3,9,10 12-year battery longevity 3 MRI SureScan TM Technology, which allows the patient to be safely scanned using either a 1.5T or 3T full body MRI 3 Accelerometer-based rate response CareLink TM 2090 and Encore programmer compatible, no accessories required Capture Management TM Eliminated Lead-related Complications 5 Fractures Insulation breaches Venous thrombosis and obstruction > 99 % Implant Success 3,4 63 % Fewer Major Complications 4 Tricuspid regurgitation Long-term Lead- and Pocket-related Complications with Traditional Systems Pocket-related complications 8% at five years 5 Lead-related complications 11% at five years 5 FlexFix tines Circuit board Battery
3 MINIATURIZED. SOPHISTICATED. COMPLETE. PACING CAPSULE 10 Parameter Pacing Mode Mass Volume Electrode Spacing Programmable 3-axis Accelerometer Micra VVIR 1.75 g 0.8 cc 18 mm Device life cycle management options Micra is designed to offer options Micra can be programmed off at the end of service and can be differentiated from additional Micra devices, if subsequent devices are implanted The Micra design incorporates a proximal retrieval feature to enable acute retrieval Successful retrieval demonstrated after 28 months in chronic animal models 11 ANODE Bipolar pacing Primary prespecified safety, effectiveness, and long-term safety objectives were met (n = 726) 3,12 96% of patients experienced no major complications by 12 months follow-up 12 0 dislodgements or systemic infections Low (0.4%) revision rate Pacing thresholds remained low and stable through twelve months 3 Yielding an estimated battery longevity on average of 12.1 years Real-world experience reinforces safety and long-term performance of Micra (n = 1,817) 4 High implant success rate (99.1%) Low major complication rate through 12 months (2.7%) Low dislodgement rate (0.06%) Low procedure-related infection rate (0.17%) 63% fewer major complications than traditional pacemakers *4 Major Complication Rate (%) 10% 8% 6% 4% 2% Micra IDE Micra Global Registry Reference Dataset HR (Registry vs. Ref) 0.37 (95% CI: ) P value: < HR (Registry vs. IDE) 0.71 (95% CI: ) P value: PROXIMAL RETRIEVAL FEATURE 0% Months from Implant Number at Risk Micra IDE Global 1,817 1, Reference 2,667 2,260 1,965 1,698 1,526 1,319 1,212 1,137 1,002 * Historical cohort comprised of 2,667 patients from six trials of commercially available technology (HR: 0.46, 95% CI: ; P-value < 0.001). To adjust for difference in patient populations, propensity matching to a subset of the historical control confirmed a reduction in major complications with Micra. CATHODE Steroid-eluting electrode Separated from FlexFix tines to ensure optimal contact with myocardium FlexFix Nitinol Tines Multidimensional redundancy: two tines have 15 times the holding force necessary to hold the device in place 8 Designed to minimize tissue trauma during deployment, repositioning, and retrieval 7 Optimal electrode tissue interface allows for low and stable chronic thresholds 13
4 > 99% IMPLANT SUCCESS 3,4 STREAMLINED IMPLANT PROCEDURE WITH INTEGRATED DELIVERY SYSTEM Micra Delivery Catheter 105 cm long catheter system with a handle that controls deflection and deployment of the Micra pacing capsule 3 Device Deployment Curve Deflection Tether Lock Tether Pin Flush Port Micra Delivery Catheter Micra Pacing Capsule SMOOTH VESSEL NAVIGATION WITH THE MICRA INTRODUCER Radiopaque Marker Band Micra Introducer Radiopaque Marker Band Lubricious hydrophilic coating 23 Fr inner diameter (27 Fr outer diameter) Silicone oil-coated dilator tip Delivery catheter provides visual feedback when adequate tip pressure has been achieved and retracts during deployment. 10 Linear one-step deployment facilitates consistent capsule placement, no torque required cm (22 in) working length Extended Distal Taper Side Port with 3-way Stopcock
5 References 1 Nippoldt, Doug; Whiting, Jon. Micra Transcatheter Pacing System: Device Volume Characterization Comparison. November Medtronic data on file. 2 Ritter P, Duray GZ, Zhang S, et al. The rationale and design of the Micra Transcatheter Pacing Study: safety and efficacy of a novel miniaturized pacemaker. Europace. May 2015;17(5): Reynolds D, Duray GZ, Omar R, et al. A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med. February 11, 2016;374(6): El-Chami M, et al. Leadless Pacemaker Implant in Patients with Pre-Existing Infections: Results from the Micra Post-Approval Registry. Presented at HRS May 2018, Boston, MA. 5 Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. May 2012;9(5): Williams, Eric; Whiting, Jon. Micra Transcatheter Pacing System Size Comparison. November Medtronic data on file. 7 Eggen, Mike. FlexFix Tine Design. April Medtronic data on file. 8 Eggen MD, Grubac V, Bonner MD. Design and Evaluation of a Novel Fixation Mechanism for a Transcatheter Pacemaker. IEEE Trans Biomed Eng. September 2015;62(9): St. Jude Medical Nanostim Leadless Pacemaker, Nanostim Delivery System Catheter Instructions for Use, January Medtronic Micra Implant Manual. April Bonner MD, Neafus N, Byrd CL, et al. Extraction of the Micra transcatheter pacemaker system. Heart Rhythm. May 2014;11(5):S Duray GZ, Ritter P, El-Chami M, et al. Long-term performance of a transcatheter pacing system: 12-Month results from the Micra Transcatheter Pacing Study. Heart Rhythm. May 2017;14(5) Bonner M, Eggen M, Haddad T, et al. Early Performance and Safety of the Micra Transcatheter Pacemaker in Pigs. Pacing Clin Electrophysiol. November 2015;38(11): Brief Statement Micra Transcatheter Pacing System VVIR Single Chamber with SureScan MRI Indications Micra Model MC1VR01 is indicated for patients with: Symptomatic paroxysmal or permanent high grade AV block in the presence of AF Symptomatic paroxysmal or permanent high grade AV block in the absence of AF, as an alternative to dual chamber pacing when atrial lead placement is considered difficult, high risk, or not deemed necessary for effective therapy Symptomatic bradycardia-tachycardia syndrome or sinus node dysfunction (sinus bradycardia/sinus pauses), as an alternative to atrial or dual chamber pacing when atrial lead placement is considered difficult, high risk, or not deemed necessary for effective therapy Rate-responsive pacing is indicated to provide increased heart rate appropriate to increasing levels of activity. Contraindications Micra Model MC1VR01 is contraindicated for patients who have the following types of medical devices implanted: an implanted device that would interfere with the implant of the Micra device in the judgment of the implanting physician, an implanted inferior vena cava filter, a mechanical tricuspid valve, or an implanted cardiac device providing active cardiac therapy that may interfere with the sensing performance of the Micra device. The device is contraindicated for patients who have the following conditions: femoral venous anatomy unable to accommodate a 7.8 mm (23 French) introducer sheath or implant on the right side of the heart (for example, due to obstructions or severe tortuosity), morbid obesity that prevents the implanted device from obtaining telemetry communication within 12.5 cm (4.9 in), or known intolerance to the materials listed in the Instructions for Use, or to heparin, or sensitivity to contrast media that cannot be adequately premedicated. Steroid use Do not use in patients for whom a single dose of 1.0 mg of dexamethasone acetate cannot be tolerated. Warnings and Precautions End of Service (EOS) When the EOS condition is met, the clinician has the option of permanently programming the device to Off and leaving it in the heart, or retrieving the device, provided the device has not yet become encapsulated. Removal of the Micra device after it has become encapsulated may be difficult because of the development of fibrotic tissue. If removal of the device is required, it is recommended that the removal be performed by a clinician who has expertise in the removal of implanted leads. MRI conditions for use Before an MRI scan is performed on a patient implanted with the Micra device, the cardiology and radiology professionals involved in this procedure must understand the requirements specific to their tasks as defined in the device manuals. Rate-responsive mode may not be appropriate for patients who cannot tolerate pacing rates above the programmed Lower Rate. Asynchronous VVIR pacing with sinus rhythm may not be appropriate when competitive pacing is considered undesirable or causes symptoms of pacemaker syndrome. The patient s age and medical condition should be considered by physicians and patients as they select the pacing system, mode of operation, and implant technique best suited to the individual. Precautions should be taken before administering anticoagulant agents, antiplatelet agents, or contrast media in patients with known hypersensitivity to these agents. The use of deactivated Micra devices in situ and an active Micra device, or an active transvenous pacemaker or defibrillator, has not been clinically tested to determine whether EMI or physical interaction is clinically significant. Bench testing supports that implantation of an active Micra device, or an active transvenous pacemaker or defibrillator, next to an inactivated Micra device is unlikely to cause EMI or physical interaction. Post-approval studies are planned to characterize risks of co-implanted, deactivated Micra devices. Currently recommended end-of-device-life care for a Micra device may include the addition of a replacement device with or without explantation of the Micra device, which should be turned off. Potential Complications Potential complications include, but are not limited to, toxic/allergic reaction, oversensing, acceleration of tachycardia, myocardial infarction, and surgical complications such as cardiac perforation, pericardial effusion, cardiac tamponade, death, device embolization, access site hematoma and AV fistulae, vessel spasm, infection, inflammation, and thrombosis. See the device manuals for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, MRI conditions for use, and potential complications/adverse events. For further information, please call Medtronic at and/or consult the Medtronic website at medtronic.com. Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician. Medtronic 710 Medtronic Parkway Minneapolis, MN USA Toll-free in USA: Worldwide: medtronic.com UC e EN 2018 Medtronic. Minneapolis, MN. All Rights Reserved. Printed in USA. 05/2018
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW
 MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
Call Medtronic at 1 (800) to verify the patient s current implanted system
 MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
New generations pacemakers and ICDs: an update
 Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker
 Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker Patient Scheduling and Screening Q: Is there any special (other than regular MRI consent) consent form that should be signed by the patient?
Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker Patient Scheduling and Screening Q: Is there any special (other than regular MRI consent) consent form that should be signed by the patient?
Left Atrial Appendage Closure: The Good, The Bad and The Ugly
 Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Leadless Cardiac Pacemaker
 Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
MRI SURESCAN SYSTEMS. Patient Scanning Process PATIENT PRESCREENING. SureScan Pacing, Defibrillation, and CRT-D Systems Verification
 MRI SURESCAN SYSTEMS Patient Scanning Process PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT-D Systems Verification Verify that patient has a complete SureScan pacing, defibrillation, or
MRI SURESCAN SYSTEMS Patient Scanning Process PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT-D Systems Verification Verify that patient has a complete SureScan pacing, defibrillation, or
Leadless Pacing. Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt
 Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
REVIEW ARTICLE. Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1
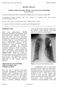 Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience
 Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS
 EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS MRI SureScan Pacing, ICD, and CRT (CRT-D and CRT-P) Transvenous Implantable Cardiac Systems Timothy S. E. Albert, M.D. President CVMR Solutions, Inc. Carmel
EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS MRI SureScan Pacing, ICD, and CRT (CRT-D and CRT-P) Transvenous Implantable Cardiac Systems Timothy S. E. Albert, M.D. President CVMR Solutions, Inc. Carmel
NOVEL DEVICE TECHNOLOGIES
 NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
Model 5392 EPG Temporary Pacer
 Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Active Fixation Models: 7740, 7741 and 7742 Passive Fixation Models: 7731, 7732, 7735 and 7736
 The INGEVITY MRI pacing leads are 6F (2.0 mm) steroid-eluting, endocardial pace / sense leads designed for permanent implantation for either atrial or ventricular applications. INGEVITY MRI is the only
The INGEVITY MRI pacing leads are 6F (2.0 mm) steroid-eluting, endocardial pace / sense leads designed for permanent implantation for either atrial or ventricular applications. INGEVITY MRI is the only
CARDIAC DEVICE MR-CONDITIONAL PRODUCT SUMMARY CHART
 CARDIAC DEVICE MR-CONDITIONAL PRODUCT SUMMARY CHART December 2015 This chart encompasses all Medtronic cardiac devices FDA-Approved as MR Conditional and included in the MRI SureScan portfolio. If a device
CARDIAC DEVICE MR-CONDITIONAL PRODUCT SUMMARY CHART December 2015 This chart encompasses all Medtronic cardiac devices FDA-Approved as MR Conditional and included in the MRI SureScan portfolio. If a device
PACING AT THE BUNDLE OF HIS
 PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
Talent Abdominal Stent Graft
 Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
Watchman. Left Atrial Appendage Closure Device. Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8
 TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
Yes, out of sight out of mind.
 Micra TRANSCATHETER PACING SYSTEM 25.9 mm Yes, out of sight out of mind. cardiocapsule Meet Micra The Invisible Pacemaker Micra is the world s smallest pacemaker 1. It leaves no bump under the skin, no
Micra TRANSCATHETER PACING SYSTEM 25.9 mm Yes, out of sight out of mind. cardiocapsule Meet Micra The Invisible Pacemaker Micra is the world s smallest pacemaker 1. It leaves no bump under the skin, no
Leadless pacemakers a panacea for bradyarrhythmias?
 Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Essentials of Pacemakers and ICD s. Rajesh Banker, MD, MPH
 Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
INNOVATIONS IN DEVICE THERAPY:
 INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
Update on Device Innovation (S-ICD, Wearable, Leadless)
 Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Pacing Without Wires: Leadless Cardiac Pacing
 REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
Lead Selection and Subcutaneous ICD Considerations. The Future of CIEDs
 Lead Selection and Subcutaneous ICD Considerations The Future of CIEDs February, 2013 Steven P. Kutalek, MD Director, Cardiac Electrophysiology Drexel University College of Medicine New Technologies 2012
Lead Selection and Subcutaneous ICD Considerations The Future of CIEDs February, 2013 Steven P. Kutalek, MD Director, Cardiac Electrophysiology Drexel University College of Medicine New Technologies 2012
ENGINEERED TO ENABLE THE FUTURE OF CONNECTED HEALTH
 ENGINEERED TO ENABLE THE FUTURE OF CONNECTED HEALTH Introducing Azure with BlueSync Technology Completely Redesigned Improved Longevity 1 AF Detection and Reduction 2 Secure Wireless Communication with
ENGINEERED TO ENABLE THE FUTURE OF CONNECTED HEALTH Introducing Azure with BlueSync Technology Completely Redesigned Improved Longevity 1 AF Detection and Reduction 2 Secure Wireless Communication with
Pediatric Pacemaker Implantation Endocardial or Epicardial
 Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Industry-Tested. Stacks Up To the Competition 2
 With READY TM Industry-Tested Stacks Up To the Competition 2 INGEVITY TM MRI Pacing Lead, the industry s leading-edge pacing technology, 1 is also the most extensively studied. 2 % of leads 10% 9% 8% 7%
With READY TM Industry-Tested Stacks Up To the Competition 2 INGEVITY TM MRI Pacing Lead, the industry s leading-edge pacing technology, 1 is also the most extensively studied. 2 % of leads 10% 9% 8% 7%
Performance of Leadless Pacemaker in Japanese Patients vs. Rest of the World. Results From a Global Clinical Trial
 Circ J 2017; 81: 1589 1595 doi: 10.1253/circj.CJ-17-0259 ORIGINAL ARTICLE Arrhythmia/Electrophysiology Performance of Leadless Pacemaker in ese Patients vs. Rest of the World Results From a Global Clinical
Circ J 2017; 81: 1589 1595 doi: 10.1253/circj.CJ-17-0259 ORIGINAL ARTICLE Arrhythmia/Electrophysiology Performance of Leadless Pacemaker in ese Patients vs. Rest of the World Results From a Global Clinical
Leadless pacing: Technical challenges and concerns. Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital
 Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
Access More Patients. Customize Each Seal.
 Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Micra MC1VR01. MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR)
 Micra MC1VR01 MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR) MRI Technical Manual The following list includes trademarks or registered
Micra MC1VR01 MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR) MRI Technical Manual The following list includes trademarks or registered
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Comparator cohort In order to compare the Micra pacing thresholds with transvenous thresholds, we analyzed transvenous pacing thresholds
 Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: Results from the Micra Transcatheter Pacing System Global Clinical Trial Jonathan P. Piccini, MD,
Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: Results from the Micra Transcatheter Pacing System Global Clinical Trial Jonathan P. Piccini, MD,
Pocket Adaptor Kit FOR SPINAL CORD STIMULATION. Tip Cards
 Pocket Adaptor Kit FOR SPINAL CORD STIMULATION Tip Cards model 74001 (1 4) model 74002 (2 4) The Pocket Adaptor Tip Cards are to be used for educational purposes only. Please refer to the Pocket Adaptor
Pocket Adaptor Kit FOR SPINAL CORD STIMULATION Tip Cards model 74001 (1 4) model 74002 (2 4) The Pocket Adaptor Tip Cards are to be used for educational purposes only. Please refer to the Pocket Adaptor
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Clinical Data Summary: Avoid FFS Study
 Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Outcomes of Defibrillator Lead Implants Performed by High Volume Operators vs. Low Volume Operators:
 Outcomes of Defibrillator Lead Implants Performed by High Volume Operators vs. Low Volume Operators: Results from the Pacemaker and Implantable Defibrillator Leads Survival Study ( PAIDLESS ) Partially
Outcomes of Defibrillator Lead Implants Performed by High Volume Operators vs. Low Volume Operators: Results from the Pacemaker and Implantable Defibrillator Leads Survival Study ( PAIDLESS ) Partially
SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000. SJM-EDTR Item approved for U.S. use only.
 SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000 1 APPROVED MRI SCAN CONDITIONS 1.5 TESLA FULL BODY 2 SETUP SJM MRI ACTIVATOR HANDHELD DEVICE The SJM MRI Activator handheld device must be enabled
SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000 1 APPROVED MRI SCAN CONDITIONS 1.5 TESLA FULL BODY 2 SETUP SJM MRI ACTIVATOR HANDHELD DEVICE The SJM MRI Activator handheld device must be enabled
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Solara CRT-P MRI SureScan W1TR06
 Solara CRT-P MRI SureScan W1TR06 MR Conditional pacemaker with cardiac resynchronization therapy, SureScan technology, and Bluetooth wireless telemetry (OAE-DDDR) Device Manual The following list includes
Solara CRT-P MRI SureScan W1TR06 MR Conditional pacemaker with cardiac resynchronization therapy, SureScan technology, and Bluetooth wireless telemetry (OAE-DDDR) Device Manual The following list includes
Product Performance Report. Implantable devices. Pacing leads. Pacemakers. Defibrillators SNIA GROUP
 Product Performance Report Implantable devices Pacing leads Pacemakers Defibrillators SNIA GROUP A message from the chairman For ELA, quality is an essential criterion in the everyday decision making process.
Product Performance Report Implantable devices Pacing leads Pacemakers Defibrillators SNIA GROUP A message from the chairman For ELA, quality is an essential criterion in the everyday decision making process.
Pediatric pacemakers & ICDs:
 Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
PERFORMANCE YOU CAN TRUST. EverFlex Self-expanding Peripheral Stent with Entrust Delivery System
 PERFORMANCE YOU CAN TRUST EverFlex Self-expanding Peripheral Stent with Entrust Delivery System The Entrust Delivery System is designed to provide improved patient outcomes and procedural efficiency. The
PERFORMANCE YOU CAN TRUST EverFlex Self-expanding Peripheral Stent with Entrust Delivery System The Entrust Delivery System is designed to provide improved patient outcomes and procedural efficiency. The
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
FROM THE EVERYDAY TO THE EXTRAORDINARY
 FROM THE EVERYDAY TO THE EXTRAORDINARY Created with the collaboration of more than 250 physicians around the world, ENDURANT empowers you to create stronger outcomes for more patients, including those
FROM THE EVERYDAY TO THE EXTRAORDINARY Created with the collaboration of more than 250 physicians around the world, ENDURANT empowers you to create stronger outcomes for more patients, including those
We Catch. What Others Miss
 We Catch What Others Miss GORE Embolic Filter Diamond Frame Circumferential Filter Attachment eptfe Filter Media with 100-Micron Pores and Hydrophilic Heparin Coating Diamond Frame designed to enhance
We Catch What Others Miss GORE Embolic Filter Diamond Frame Circumferential Filter Attachment eptfe Filter Media with 100-Micron Pores and Hydrophilic Heparin Coating Diamond Frame designed to enhance
FINELINE II STEROX EZ Models 4469/4470/4471/4472/4473/4474 Guidant Implantable Pacing Lead
 FINELINE II STEROX EZ Models 4469/4470/4471/4472/4473/4474 Guidant Implantable Pacing Lead CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in
FINELINE II STEROX EZ Models 4469/4470/4471/4472/4473/4474 Guidant Implantable Pacing Lead CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in
Sinus rhythm with premature atrial beats 2 and 6 (see Lead II).
 Cardiac Pacemaker Premature Beats When one of ectopic foci becomes irritable, it may spontaneously fire, leading to one or more premature beats. Atrial and junctional foci may become irritable from excess
Cardiac Pacemaker Premature Beats When one of ectopic foci becomes irritable, it may spontaneously fire, leading to one or more premature beats. Atrial and junctional foci may become irritable from excess
11 YEARS PROVEN PERFORMANCE. Sprint Quattro Defibrillation Leads
 11 YEARS PROVEN PERFORMANCE Sprint Quattro Defibrillation Leads PROVEN PERFORMANCE. PROVEN RELIABLE. We made intentional choices and took proactive steps from lead design to active product monitoring to
11 YEARS PROVEN PERFORMANCE Sprint Quattro Defibrillation Leads PROVEN PERFORMANCE. PROVEN RELIABLE. We made intentional choices and took proactive steps from lead design to active product monitoring to
Advanced ICD Concepts
 1 4 2 5 7 3 6 8 Advanced ICD Concepts This presentation is provided with the understanding that the slide content must not be altered in any manner as the content is subject to FDA regulations. This presentation
1 4 2 5 7 3 6 8 Advanced ICD Concepts This presentation is provided with the understanding that the slide content must not be altered in any manner as the content is subject to FDA regulations. This presentation
What s New in CIEDs. Keimyung University Dongsan Medical Center Hyoung-Seob Park
 What s New in CIEDs Keimyung University Dongsan Medical Center Hyoung-Seob Park Transvenous CIEDs Technology: - Highly mature & reliable - Still includes generator, connectors and leads Procedure : -
What s New in CIEDs Keimyung University Dongsan Medical Center Hyoung-Seob Park Transvenous CIEDs Technology: - Highly mature & reliable - Still includes generator, connectors and leads Procedure : -
ΤΟ ΗΚΓ ΣΤΟΝ ΒΗΜΑΤΟΔΟΤΟΥΜΕΝΟ ΑΣΘΕΝΗ
 ΤΟ ΗΚΓ ΣΤΟΝ ΒΗΜΑΤΟΔΟΤΟΥΜΕΝΟ ΑΣΘΕΝΗ ΤΖΩΡΤΖ ΔΑΔΟΥΣ ΕΠΙΚΟΥΡΟΣ ΚΑΘΗΓΗΤΗΣ Α.Π.Θ. ΜΑΡΙΑ ΚΑΡΑΛΙΟΛΙΟΥ ΕΙΔΙΚΕΥΟΜΕΝΗ ΙΑΤΡΟΣ ΚΑΡΔΙΟΛΟΓΟΣ Β ΚΑΡΔΙΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ Α.Π.Θ. ΙΠΠΟΚΡΑΤΕΙΟ Γ.Ν.Θ. 5 Why a Pacemaker is Implanted
ΤΟ ΗΚΓ ΣΤΟΝ ΒΗΜΑΤΟΔΟΤΟΥΜΕΝΟ ΑΣΘΕΝΗ ΤΖΩΡΤΖ ΔΑΔΟΥΣ ΕΠΙΚΟΥΡΟΣ ΚΑΘΗΓΗΤΗΣ Α.Π.Θ. ΜΑΡΙΑ ΚΑΡΑΛΙΟΛΙΟΥ ΕΙΔΙΚΕΥΟΜΕΝΗ ΙΑΤΡΟΣ ΚΑΡΔΙΟΛΟΓΟΣ Β ΚΑΡΔΙΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ Α.Π.Θ. ΙΠΠΟΚΡΑΤΕΙΟ Γ.Ν.Θ. 5 Why a Pacemaker is Implanted
CONSULTA CRT-P Model C4TR01
 CONSULTA CRT-P Model C4TR01 Physical characteristics Volume a 15 cm 3 Mass H x W x D Surface area of titanium device can Surface area of each titanium nitride LECG electrode Radiopaque ID Materials in
CONSULTA CRT-P Model C4TR01 Physical characteristics Volume a 15 cm 3 Mass H x W x D Surface area of titanium device can Surface area of each titanium nitride LECG electrode Radiopaque ID Materials in
INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS
 INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS BEFORE INTERPRETING THE ECG: Nora Goldschlager, M.D. MACP, FACC, FAHA, FHRS. Cardiology San Francisco General Hospital UCSF Disclosures: None 1 2 QUESTIONS
INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS BEFORE INTERPRETING THE ECG: Nora Goldschlager, M.D. MACP, FACC, FAHA, FHRS. Cardiology San Francisco General Hospital UCSF Disclosures: None 1 2 QUESTIONS
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD
 Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
CRF procedure PROCEDURE FLOW. PATIENT ASSESSMENT Symptoms and indication. Pacemaker & ICD registration. Procedures. Procedure ICD.
 Pacemaker & ICD registration Procedures CRF procedure PROCEDURE FLOW Procedure ICD ICD PM ICM lead only PATIENT ASSESSMENT Symptoms and indication Symptoms and events (multiple possibilities) Asymptomatic
Pacemaker & ICD registration Procedures CRF procedure PROCEDURE FLOW Procedure ICD ICD PM ICM lead only PATIENT ASSESSMENT Symptoms and indication Symptoms and events (multiple possibilities) Asymptomatic
Transvenous Pacemaker Procedures
 Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
UNLOCK THE ANSWER. The Reveal LINQ TM System lets your physician learn about your heart while you live your life.
 UNLOCK THE ANSWER The Reveal LINQ TM System lets your physician learn about your heart while you live your life. WHY LONG-TERM MONITORING MAY BE RIGHT FOR YOU Accurate information is the key to better
UNLOCK THE ANSWER The Reveal LINQ TM System lets your physician learn about your heart while you live your life. WHY LONG-TERM MONITORING MAY BE RIGHT FOR YOU Accurate information is the key to better
Managing Metallic Artifacts in MRI
 Managing Metallic Artifacts in MRI William Faulkner, BS, RT (R) (MR) (CT), FSMRT Background Magnetism is a fundamental property of matter. As such, all substances have some type of magnetic property. Magnetic
Managing Metallic Artifacts in MRI William Faulkner, BS, RT (R) (MR) (CT), FSMRT Background Magnetism is a fundamental property of matter. As such, all substances have some type of magnetic property. Magnetic
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany
 Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
King s Research Portal
 King s Research Portal DOI: 10.1016/j.tcm.2016.03.003 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Rinaldi, C. A. (2016).
King s Research Portal DOI: 10.1016/j.tcm.2016.03.003 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Rinaldi, C. A. (2016).
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
COMMONLY BILLED CODES AND ASSOCIATED 2018 MEDICARE RATES
 CRHF REIMBURSEMENT & HEALTH POLICY Pacemaker Therapy COMMONLY BILLED CODES AND ASSOCIATED 2018 MEDICARE RATES This document reflects commonly billed codes for Pacemaker Therapy and their associated National
CRHF REIMBURSEMENT & HEALTH POLICY Pacemaker Therapy COMMONLY BILLED CODES AND ASSOCIATED 2018 MEDICARE RATES This document reflects commonly billed codes for Pacemaker Therapy and their associated National
Azure XT DR MRI SureScan W2DR01
 Azure XT DR MRI SureScan W2DR01 MR Conditional dual chamber pacemaker with SureScan technology and Bluetooth wireless telemetry (OAE-DDDR) Device Manual The following list includes trademarks or registered
Azure XT DR MRI SureScan W2DR01 MR Conditional dual chamber pacemaker with SureScan technology and Bluetooth wireless telemetry (OAE-DDDR) Device Manual The following list includes trademarks or registered
Lead extraction. Dr. Mervat Abo El Maaty Professor of Cardiology Ain shams University 2013
 Lead extraction Dr. Mervat Abo El Maaty Professor of Cardiology Ain shams University 2013 Agenda Introduction History of consensus Definitions Complications Indications Lead management environment Extraction
Lead extraction Dr. Mervat Abo El Maaty Professor of Cardiology Ain shams University 2013 Agenda Introduction History of consensus Definitions Complications Indications Lead management environment Extraction
12 YEARS PROVEN PERFORMANCE. Sprint Quattro Defibrillation Leads
 12 YEARS PROVEN PERFORMANCE Sprint Quattro Defibrillation Leads PROVEN PERFORMANCE. PROVEN RELIABLE. We made intentional choices and took proactive steps from lead design to active product monitoring to
12 YEARS PROVEN PERFORMANCE Sprint Quattro Defibrillation Leads PROVEN PERFORMANCE. PROVEN RELIABLE. We made intentional choices and took proactive steps from lead design to active product monitoring to
HOW TO PERFORM HIS-BUNDLE PACING. Training Presentation
 HOW TO PERFORM HIS-BUNDLE PACING Training Presentation AGENDA Overview of His-Bundle Pacing SelectSecure Lead Overview C315 Catheter Family Overview His-Bundle Pacing Implant Technique Including locating
HOW TO PERFORM HIS-BUNDLE PACING Training Presentation AGENDA Overview of His-Bundle Pacing SelectSecure Lead Overview C315 Catheter Family Overview His-Bundle Pacing Implant Technique Including locating
Advisa MRI and Ensura MRI SureScan pacing systems
 Advisa MRI and Ensura MRI SureScan pacing systems MR Conditional dual chamber and single chamber SureScan pacing systems with SureScan leads MRI Technical Manual The following list includes trademarks
Advisa MRI and Ensura MRI SureScan pacing systems MR Conditional dual chamber and single chamber SureScan pacing systems with SureScan leads MRI Technical Manual The following list includes trademarks
DO YOU HAVE PAROXYSMAL ATRIAL FIBRILLATION?
 DO YOU HAVE PAROXYSMAL ATRIAL FIBRILLATION? Do you have atrial fibrillation ()? Do you think you might have it? If so, the time to take control is now. There are three important things to do. 1. If you
DO YOU HAVE PAROXYSMAL ATRIAL FIBRILLATION? Do you have atrial fibrillation ()? Do you think you might have it? If so, the time to take control is now. There are three important things to do. 1. If you
How to Implant a Leadless Pacemaker With a Tine-Based Fixation
 Techniques, Technology, and Innovations Section Editor: Samuel J. Asirvatham, M.D. How to Implant a Leadless Pacemaker With a Tine-Based Fixation MIKHAEL F. EL-CHAMI, M.D., PAUL R. ROBERTS, M.D., ALEX
Techniques, Technology, and Innovations Section Editor: Samuel J. Asirvatham, M.D. How to Implant a Leadless Pacemaker With a Tine-Based Fixation MIKHAEL F. EL-CHAMI, M.D., PAUL R. ROBERTS, M.D., ALEX
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE. Updated September 2018
 CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
CATHETER ABLATION CODING & REIMBURSEMENT GUIDE Updated September 2018 TABLE OF CONTENTS Diagnosis Codes...3 ICD-10-CM Diagnosis Codes Coverage for Catheter Ablation Procedures....4 Medicare Other Payers
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
ENABLE MRI CLINICAL STUDY SUMMARY
 CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
MODEL EPG SINGLE CHAMBER TEMPORARY EXTERNAL PACEMAKER
 MODEL 53401 EPG SINGLE CHAMBER TEMPORARY EXTERNAL PACEMAKER In-service Guide 53401 TRAINING LEARNING OBJECTIVES Identify the control knobs and be able to explain how to use them to program device. Distinguish
MODEL 53401 EPG SINGLE CHAMBER TEMPORARY EXTERNAL PACEMAKER In-service Guide 53401 TRAINING LEARNING OBJECTIVES Identify the control knobs and be able to explain how to use them to program device. Distinguish
Top 5 Things to Know about Pacemakers and ICD s. Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017.
 Top 5 Things to Know about Pacemakers and ICD s Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017. Top 5 Things 1. Remote Monitoring leads to better care and outcomes. 2. MRI s CAN be done on device patients.
Top 5 Things to Know about Pacemakers and ICD s Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017. Top 5 Things 1. Remote Monitoring leads to better care and outcomes. 2. MRI s CAN be done on device patients.
Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study
 Accepted Manuscript Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study Gabor Z. Duray, MD, PhD, Philippe Ritter, MD, Mikhael El-Chami, MD,
Accepted Manuscript Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study Gabor Z. Duray, MD, PhD, Philippe Ritter, MD, Mikhael El-Chami, MD,
NIRxcell CoCr Coronary Stent System
 cordis.com/nirxcell NIRxcell CoCr Coronary Stent System Exceptional Outcomes by Design Exceptionally low TLR rate of 5.1 % at 9 months 1 Superior Deliverability and Crossability 2 Best-in-class Conformability
cordis.com/nirxcell NIRxcell CoCr Coronary Stent System Exceptional Outcomes by Design Exceptionally low TLR rate of 5.1 % at 9 months 1 Superior Deliverability and Crossability 2 Best-in-class Conformability
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Extracorporeal Circuit Cannulae { VENOUS}
 Extracorporeal Circuit Cannulae { VENOUS} Medtronic are designed to provide optimal venous drainage while providing flexibility and ease of use. We offer a variety of single stage venous cannulae with
Extracorporeal Circuit Cannulae { VENOUS} Medtronic are designed to provide optimal venous drainage while providing flexibility and ease of use. We offer a variety of single stage venous cannulae with
November 2016 POWERFULLY SIMPLE CARDIAC MONITORING. SEEQ External Cardiac Monitor (ECM) System. From the leaders who brought you Reveal LINQ ICM
 November 2016 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ External Cardiac Monitor (ECM) System From the leaders who brought you Reveal LINQ ICM TRADITIONAL HOLTER MONITORING TYPICALLY 1-2 DAYS NOT LONG
November 2016 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ External Cardiac Monitor (ECM) System From the leaders who brought you Reveal LINQ ICM TRADITIONAL HOLTER MONITORING TYPICALLY 1-2 DAYS NOT LONG
Percepta CRT-P MRI SureScan W1TR01
 Percepta CRT-P MRI SureScan W1TR01 MR Conditional pacemaker with cardiac resynchronization therapy, SureScan technology, and Bluetooth wireless telemetry (OAE-DDDR) Device Manual Caution: Federal law (USA)
Percepta CRT-P MRI SureScan W1TR01 MR Conditional pacemaker with cardiac resynchronization therapy, SureScan technology, and Bluetooth wireless telemetry (OAE-DDDR) Device Manual Caution: Federal law (USA)
Zenith Alpha T HORACIC ENDOVASCULAR GRAFT
 Device description Zenith Alpha T HORACIC ENDOVASCULAR GRAFT www.cookmedical.com AI-D21181-EN-F Modular design The two-piece modular system allows the physician to customize a graft system to fit each
Device description Zenith Alpha T HORACIC ENDOVASCULAR GRAFT www.cookmedical.com AI-D21181-EN-F Modular design The two-piece modular system allows the physician to customize a graft system to fit each
Girish M Nair, Seeger Shen, Pablo B Nery, Calum J Redpath, David H Birnie
 268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
DURABLE. CONSISTENT. SAFE. IN.PACT Admiral Drug-Coated Balloon
 DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
Azure S DR MRI SureScan W3DR01
 Azure S DR MRI SureScan W3DR01 MR Conditional dual chamber pacemaker with SureScan technology and Bluetooth wireless telemetry (OOE-DDDR) Device Manual The following list includes trademarks or registered
Azure S DR MRI SureScan W3DR01 MR Conditional dual chamber pacemaker with SureScan technology and Bluetooth wireless telemetry (OOE-DDDR) Device Manual The following list includes trademarks or registered
Pacing troubleshooting. NASPE training Lancashire & South Cumbria Cardiac Network
 Pacing troubleshooting NASPE training Lancashire & South Cumbria Cardiac Network Pacing stimulus present failure to capture Lead dislodgement Early unstable position Late twiddlers syndrome Abnormal ECG
Pacing troubleshooting NASPE training Lancashire & South Cumbria Cardiac Network Pacing stimulus present failure to capture Lead dislodgement Early unstable position Late twiddlers syndrome Abnormal ECG
Versatility and Proven Safety
 Versatility and Proven Safety Indicated for: Temporary or Permanent placement Standard or Over-The-Wire Delivery Jugular, Femoral, Antecubital, and Popliteal Access > Proven Safety Initial Clinical Trial
Versatility and Proven Safety Indicated for: Temporary or Permanent placement Standard or Over-The-Wire Delivery Jugular, Femoral, Antecubital, and Popliteal Access > Proven Safety Initial Clinical Trial
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM
 Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
The EFFORTLESS SICD Registry. The EFFORTLESS SICD Registry. SICD Implant 11/14/2016
 11/14/2016 VuMedi Webinar: Safety and Efficacy of the Subcutaneous ICD Primary Results of The EFFORTLESS SICD Registry November 21, 2016 Philip Chang, MD, FACC, FHRS Assistant Professor of Medicine and
11/14/2016 VuMedi Webinar: Safety and Efficacy of the Subcutaneous ICD Primary Results of The EFFORTLESS SICD Registry November 21, 2016 Philip Chang, MD, FACC, FHRS Assistant Professor of Medicine and
A Leadless Intracardiac Transcatheter Pacing System
 Original Article A Leadless Intracardiac Transcatheter Pacing System Dwight Reynolds, M.D., Gabor Z. Duray, M.D., Ph.D., Razali Omar, M.D., Kyoko Soejima, M.D., Petr Neuzil, M.D., Shu Zhang, M.D., Calambur
Original Article A Leadless Intracardiac Transcatheter Pacing System Dwight Reynolds, M.D., Gabor Z. Duray, M.D., Ph.D., Razali Omar, M.D., Kyoko Soejima, M.D., Petr Neuzil, M.D., Shu Zhang, M.D., Calambur
