Performance of Leadless Pacemaker in Japanese Patients vs. Rest of the World. Results From a Global Clinical Trial
|
|
|
- Cameron Robertson
- 6 years ago
- Views:
Transcription
1 Circ J 2017; 81: doi: /circj.CJ ORIGINAL ARTICLE Arrhythmia/Electrophysiology Performance of Leadless Pacemaker in ese Patients vs. Rest of the World Results From a Global Clinical Trial Kyoko Soejima, MD; Taku Asano, MD; Toshiyuki Ishikawa, MD; Kengo Kusano, MD; Toshiaki Sato, MD; Hideo Okamura, MD; Katsumi Matsumoto, MD; Wataru Taguchi, PhD; Kurt Stromberg; Jeff Lande, PhD; Youichi Kobayashi, MD; Micra Transcatheter Pacing Study Group Background: A global study designed to demonstrate the safety and efficacy of a transcatheter pacing system included 38 ese patients enrolled at 4 sites. Subgroup analysis to evaluate the performance of the leadless intracardiac transcatheter pacing system in ese patients was performed. Methods and Results: Safety and efficacy outcomes, patient and implant procedure characteristics, and patient and physician acceptability from the ese population were compared with those from outside. Differences in patient characteristics, implant procedure characteristics and patient acceptability were observed. There were no major complications in ese patients and pacing thresholds remained low and stable throughout follow-up. There were no observable differences between ese patients and patients from outside in the freedom from major complication rate at 12-months post-implant (100.0% vs. 95.7%, P=0.211) or physician acceptability. Conclusions: Although some differences in specific baseline characteristics, such as body size and pacing indication, and in implant procedure characteristics, including anticoagulation strategy and hospitalization period, were observed in the ese patients, transcatheter pacemaker performance was similar to that in the global trial. (Clinical Trial Registration: ClinicalTrials.gov ID NCT ) Key Words: ese patients; Leadless pacemaker; Transcatheter pacing system Permanent cardiac pacing is the only effective treatment for symptomatic bradycardia, and over 50,000 patients receive pacemakers in annually. 1 Traditional pacemakers consist of an electrical generator and one or more transvenous leads and have a history of over half a century; however, 1 in 8 patients experiences a complication. 2,3 Complications include lead-related complications ( %), pocket-related complications ( %), pneumothorax ( %), or device infection ( %). 2,3 Recently, the Transcatheter Pacing System (TPS, Medtronic plc, MN, USA) was introduced to overcome these lead- and pocket-associated problems. In a prospective global clinical study, including ese centers, the TPS was successfully implanted in 99.2% of 725 patients, and met prespecified safety and efficacy criteria. 4 Editorial p 1576 The freedom from major complications at 6-month postimplant among the 725 patients with a TPS implant attempt was 96.0%. The percentage of patients with a low and stable pacing capture threshold measured at 6 months post-implant was 98.3%. Similarly, through the 12 months post-implant, the freedom from major complications rate was 96.0% and pacing thresholds remained low and stable throughout the follow-up period. 5 No significant interregional differences have been reported in traditional leaded pacing therapy, but the TPS is a novel device and requires a new implant procedure. The purpose of this study was to compare outcomes in the global study results Received March 15, 2017; revised manuscript received April 22, 2017; accepted April 25, 2017; released online May 30, 2017 Time for primary review: 19 days Department of Cardiology, Kyorin University Hospital, Tokyo (K. Soejima, T.S.); Department of Cardiology, Showa University Hospital, Tokyo (T.A., Y.K.); Department of Cardiology, Yokohama City University Hospital, Yokohama (T.I., K.M.); Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Suita (K.K., H.O.); Medtronic Co., Ltd., Tokyo (W.T.); Institute for Medical Regulatory Science, Waseda University, Tokyo (W.T.), ; and Medtronic plc, Mounds View, MN (K. Stromberg, J.L.), USA Mailing address: Kyoko Soejima, MD, Department of Cardiology, Kyorin University Hospital, Shinkawa, Mitaka, Tokyo ,. skyoko@ks.kyorin-u.ac.jp ISSN All rights are reserved to the ese Circulation Society. For permissions, please cj@j-circ.or.jp
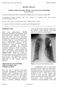
2 1590 SOEJIMA K et al. Figure 1. Transcatheter Pacing System (TPS) in the right ventricle. The TPS is an investigational product and not approved for use in as of <manuscript submission date>. between and outside. Methods Study Design The TPS global study was a prospective, non-randomized, single-arm, multi-site, international clinical study to evaluate the safety and efficacy of the TPS. The study design and primary results have been published previously. 4 6 Patients Enrolled patients met a class I or II guideline-based indication 7 9 for de novo ventricular pacing with no restriction by comorbidity. All patients provided written informed consent. The protocol was approved by the institutional review board at each participating site and was also approved by associated national and local regulatory agencies. Study Device The TPS consists of single-use delivery catheter and pacemaker. The pacemaker is a miniaturized single-chamber ventricular pacemaker with a volume of 0.8 ml, a length of 25.9 mm, an outer diameter of 6.7 mm, and a weight of 2.0 g. It sits in a steerable delivery catheter and is inserted through a femoral vein using a 23-Fr introducer (Figure 1). Follow-up and Endpoints Successfully implanted patients had scheduled study visits at hospital discharge, 1, 3, and 6 months post-implant, and every 6 months thereafter. Adverse events and device function were evaluated at each follow-up visit. The primary safety endpoint was freedom from system-related or procedure-related major complications at 6 months, though for this report we also provide the safety data through 12 months as all the 12-month study visits were completed at the time of this analysis. Major complications were defined as events resulting in death, permanent loss of device function as a result of mechanical or electrical dysfunction, hospitalization, prolongation of hospitalization by at least 48 h, or system revision. The primary efficacy endpoint was the combination of a low ( 2 V at a pulse width of 0.24 ms) and stable (increase 1.5 V from the implant) pacing capture threshold at the 6-month visit; however, for this analysis, we focused on electrical performance through 12 months. Secondary and ancillary endpoints included accuracy of ventricular capture management feature, implant procedure characteristics, estimation of battery longevity and electrical performance. Additional endpoints included reporting on device-handling questionnaire responses by implanting physicians and satisfaction questionnaire responses by patients. For the device-handling questionnaire, the implanting physician selected one of the response choices (extremely difficult, difficult, neutral, easy, and extremely easy) for each question: (1) navigation of the delivery system to the right ventricle, (2) device deployment, (3) pull and hold test (used to verify device fixation, as previously described 10 and (4) overall impression, following each implant procedure. For the satisfaction questionnaire, evaluated at the 3-month visit, the patient selected one of the response choices (very satisfied, satisfied, neutral, dissatisfied, and very dissatisfied) for each question: (1) recovery, (2) cosmetic appearance and (3) level of activity. Statistical Analysis The study protocol and sample size allowed for up to 3 planned interim analyses of the 2 primary endpoints when 300, 450, and 600 patients completed the 6-month visit. The study also had a prespecified long-term safety objective that occurred when all patients had the opportunity to complete the 12-month visit. Because the long-term safety assessment has been completed, 5 the 12-month major complication free rates are reported. Baseline demographics, medical history, implant procedure characteristics, devicehandling questionnaire, and the patient satisfaction questionnaire were compared between patients at the ese centers ( patients) and those outside ( Outside of patients) using t-tests or the Wilcoxon rank-sum test (continuous variables) or Fisher s exact test (discrete variables). The major complication rates through 12 months were compared between the and Outside of patients using a log-rank test. The accuracy of Micra s ventricular capture management feature was compared between the and patients using Fisher s exact test. The analysis cohort for the longterm safety endpoint included all patients with a Micra implant attempt. The analysis cohort for the secondary
3 Leadless Pacemaker in ese Patients 1591 endpoint of ventricular capture management feature accuracy included all patients with pacing thresholds measured both manually and using Micra s ventricular capture management feature at the 6-month visit. Electrical parameters were summarized at each study visit using means and standard deviations. Battery longevity was projected using Monte Carlo methods by combining bench-measured static current drain distributions combined with actual-use conditions obtained via 12-month device interrogation files, plus 6 half-hour telemetry sessions per year. Results Patients A total of 745 patients were enrolled at 56 centers in 19 countries worldwide, and 726 patients underwent an implantation attempt. In, 38 patients were enrolled at 4 centers and 36 patients underwent an implant attempt by 6 operators. The 2 patients left the study before pacemaker implantation was attempted because they did not meet eligibility criteria; 1 was likely to relocate during the follow-up and the other had a narrow femoral vein. The analysis included 726 patients (including 36 ese patients) who underwent pacemaker implantation, 695 patients (including 35 ese patients) who completed the 3-month visit, 684 patients (including 36 ese patients) who completed the 6-month visit, and 656 (including 36 ese patients) who completed the 12-month visit (Figure 2). Baseline characteristics of the and patients are shown in Table 1 and Table 2. patients were significantly shorter and smaller than the Outside of patients (P<0.001). Pacing indication associated with atrial fibrillation (AF) was lower in patients compared with patients (42% vs. 65%, P=0.007). Of the patients, 21 (58.3%) had indications unrelated to AF and included: sinus node dysfunction (n=15, 41.7%), atrioventricular (AV) block (n=5, 13.9%), and trifascicular block with presyncope (n=1, 2.8%). The rationales for single-chamber pacemaker selection in the patients, which were not mutually exclusive, were: Figure 2. Patient flow diagram. Geo, geographic regions. infrequent pacing (66.7%), old age (61.1%), persistent AF (41.7%), low activity (16.7%), post- or planned AV node ablation (5.6%), anatomical difficulty with atrial lead insertion (2.8%), serious comorbidity that affected prognosis (2.8%), and possible high risk of complications with a dualchamber system (2.8%). None of the patients had a history of cardiomyopathy and more patients presented with congestive heart failure caused by bradycardia (39% vs. 17%, P=0.003). Fewer patients had coronary artery disease compared with the patients (11% vs. 29%, P=0.021). Implant Procedure Characteristics Procedural characteristics of the and Outside of patients are shown in Table 3. All attempts at Micra implant were successful in the 36 (100%) patients. Procedure time from introducer placement to introducer removal was longer in the patients compared with the patients (39.3±16.9 vs. 34.5±24.4 min, Table 1. Characteristics of the Patients at Baseline (n=690*) P value Age (years) Mean ± SD 78.2± ± Min. max Sex Male, n (%) 24 (66.7) 403 (58.4) 0.39 Height (cm) Mean ± SD 159.1± ±10.4 <0.001 Min. max Weight (kg) Mean ± SD 58.8± ±18.1 <0.001 Min. max BMI Mean ± SD 23.1± ±5.3 <0.001 Min. max *Height and weight for 2 patients not available. Calculated using Fisher s exact test for discrete variables and t-test for continuous variables. BMI, body mass index; max., maximum; min., minimum; SD, standard deviation.
4 1592 SOEJIMA K et al. Table 2. Characteristics of the Patients Medical Histories (n=690) Pacing indication associated with AF 41.7% 65.1% Cardiomyopathy 0.0% 11.7% Congestive heart failure 38.9% 17.0% Coronary artery disease 11.1% 29.1% Hypertension 69.4% 79.1% 0.21 Myocardial infarction 8.3% 10.7% 1.00 Pulmonary hypertension 2.8% 11.7% 0.11 Valve dysfunction, tricuspid 19.4% 26.5% 0.44 COPD 8.3% 12.9% 0.61 Diabetes 25.0% 28.7% 0.71 Renal dysfunction 30.6% 20.0% 0.14 Chronic lung disease 33.3% 29.4% 0.58 *Calculated using Fisher s exact test for discrete variables and t-tests for continuous variables. AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease. Table 3. Characteristics of the Implant Procedure (n=684) Implant success rate, % Procedure time, min Mean ± SD 39.3± ± ** Median Device location, n (%) Apex 23 (63.9%) 452 (66.1%) 0.68 Septum/mid-septum 13 (36.1%) 214 (31.3%) Other 0 (0.0%) 18 (2.6%) No. of deployments 5, n (%) 32 (88.9%) 655 (95.8%) 0.08 Preprocedure OAC/antiplatelet use, n (%) 28 (77.8%) 446 (65.2%) 0.15 Intraprocedure anticoagulation, n (%) 36 (100.0%) 341 (49.9%) <0.001 Anticoagulation antagonist, n (%) 23 (63.9%) 46 (6.7%) <0.001 Closure method: manual pressure+suture, n (%) 36 (100.0%) 354 (51.8%) <0.001 Time to ambulation (h), mean ± SD 18.6± ±6.9 <0.001 Days of hospitalization for implant, mean ± SD 5.1± ±2.7 <0.001 *Calculated using Fisher s exact test for discrete variables and t-test for continuous variables; **calculated using Wilcoxon rank-sum test. Denominator: 690 patients outside of with implant attempt. Procedure time: from the beginning of introducer placement to the end of introducer removal. OAC, oral anticoagulant. P=0.007). The location of the device was similar, and was positioned with 5 deployments in the majority of patients (88.9% vs. 95.8%; P=0.08) in both groups. Most patients were anticoagulated prior to the implant, and all patients received intraprocedural anticoagulation, whereas only 49.9% of the patients received anticoagulation during the procedure (P<0.001). Prior to sheath removal and hemostasis, an anticoagulation antagonist was used more frequently in the patients (63.9% vs. 6.7%; P<0.001). Hemostasis with suture and manual pressure was the only closure method used in patients, but was used less frequently in the Outside of patients (100.0% vs. 51.8%; P<0.001). Time to ambulation and days of hospitalization for the implant were longer in patients (18.6± 6.2 vs. 10.1±6.9 h; P<0.001 and 5.1±1.8 vs. 2.0±2.7 days; P<0.001, respectively). Long-Term Safety Outcome: System- or Procedure-Related Major Complications There were 5 complications related to the system or procedure experienced by 5 of the patients (presyncope, incision site hematoma, incision site hemorrhage, pericardial effusion, and hypotension). None of these complications met the criteria for a major complication which was defined as an event resulting in death, permanent loss of device function, hospitalization, prolongation of hospitalization, or system revision. Presyncope occurred during the implant procedure, possibly caused by vagal reflex. Incision site hematoma and hemorrhage were related to the implant procedure and introducer. Pericardial effusion was classified as a complication because it required an invasive intervention (pericardiocentesis) for resolution. Hypotension was observed during the implant procedure and was an adverse effect of the sedative agent. The freedom from
5 Leadless Pacemaker in ese Patients 1593 Figure 3. (A C) Electrical performance characteristics by study visit. Data in the graphs are mean values, and I bars represent mean ± standard deviations. N values are the numbers of patients for whom data were available at each time point. PHD, pre-hospital discharge. system- or procedure-related major complications through 12 months was 100.0% (95% confidence interval (CI) %) in the patients and was not significantly different from (95.7%, 95% CI %, P=0.211). Electrical Performance The average pacing capture threshold among patients at both implant and the 12-month visit was 0.54 V compared with 0.63 V and 0.61 V for patients outside (Figure 3A). The average R-wave amplitude among patients at implant was 11.3 mv and 14.2 mv at 12-month visit compared with 11.2 mv and 15.1 mv, respectively, in patients outside (Figure 3B). The average pacing impedance among patients was 753 ohms at implant and 566 ohms at the 12-month visit compared with 722 ohms and 597 ohms, respectively, among patients outside of (Figure 3C). Based on the use conditions of the 36 patients through 12 months, mean estimated battery longevity was 12.5 years with a range of years. Responses to Device-Handling Questionnaire by Implant Physicians Physicians responses to the questionnaire were similar in both groups (Table 4). The majority of physicians felt the navigation delivery was extremely easy/easy (91.6% vs. 89.0% for patients vs. patients, P=0.173), device deployment was extremely easy/easy (94.4% vs. 94.6% for patients vs. patients, P=0.557), pull and hold test was extremely easy/ easy (91.7% vs. 77.8% for patients vs. Outside of patients, P=0.153), and overall impression was extremely easy/easy (91.7% vs. 88.4% for vs. Outside of patients, P=0.638).
6 1594 SOEJIMA K et al. Table 4. Physicians Responses to Device-Handling Questionnaire Device-handling questions (n=683) Navigation of delivery system to RV Extremely difficult/difficult 3 (8.3%) 32 (4.7%) Neutral 0 (0.0%) 43 (6.3%) Extremely easy/easy 33 (91.6%) 608 (89.0%) Device deployment Extremely difficult/difficult 0 (0.0%) 13 (1.9%) Neutral 2 (5.6%) 24 (3.5%) Extremely easy/easy 34 (94.4%) 646 (94.6%) Tug test Extremely difficult/difficult 0 (0.0%) 36 (5.3%) Neutral 3 (8.3%) 116 (17.0%) Extremely easy/easy 33 (91.7%) 531 (77.8%) Overall impression Extremely difficult/difficult 2 (5.5%) 30 (4.4%) Neutral 1 (2.8%) 49 (7.2%) Extremely easy/easy 33 (91.7%) 604 (88.4%) *Calculated using Fisher s exact test for discrete variables and t-tests for continuous variables. RV, right ventricle. Table 5. Patient Satisfaction at 3 Months Satisfaction questions (n=35) (n=658) Recovery Very dissatisfied/dissatisfied 2 (5.7%) 20 (3.0%) Neutral 7 (20.0%) 34 (5.2%) Very satisfied/satisfied 26 (74.3%) 604 (91.8%) Cosmetic appearance Very dissatisfied/dissatisfied 0 (0.0%) 3 (0.5%) Neutral 3 (8.6%) 22 (3.3%) Very satisfied/satisfied 32 (91.4%) 633 (96.2%) Level of Activity Very dissatisfied/dissatisfied 2 (5.7%) 35 (5.3%) Neutral 12 (34.3%) 131 (19.9%) Very satisfied/satisfied 21 (60.0%) 492 (74.9%) *Calculated using Fisher s exact test for discrete variables and t-tests for continuous variables. Responses to Satisfaction Questionnaire by Patients All 35 patients and 658 of 660 patients with a 3-month visit at the time of the primary analysis completed satisfaction questionnaires (Table 5). Patients responses were similar for cosmetic appearance and level of activity. However, more patients outside of were very satisfied or satisfied with their recovery than patients (74.3% vs. 91.8%, P=0.002). Discussion In this study, there were no significant differences in longterm safety, electrical performance, or patient and physician acceptability observed between patients inside and outside, except in patient satisfaction with recovery. This is despite there being differences in specific baseline characteristics and implant procedure characteristics between the 2 groups. These findings suggested that the favorable safety and efficacy results from the global study may be extended to ese patients, despite -specific medical practices and environments. Implantation There were 5 events in 5 patients (13.9%) that were considered complications requiring invasive intervention such as intravenous medications to resolve the event. None were considered major complications, and no dislodgements or systemic infections were observed. There was 1 case of pericardial effusion that occurred in a ese patient during an implant procedure. The patient was an 81-yearold male with a history of chronic obstructive pulmonary disease, emphysema, and pleural effusion. The pericardial effusion was classified as a minor complication because it required pericardiocentesis, but it did not meet the definition of a major complication because the event did not result in death, hospitalization, prolongation of hospitalization, loss of device functionality, or system revision. Because the TPS only has one size and shape and the
7 Leadless Pacemaker in ese Patients 1595 catheter requires a large-bore introducer, the adaptability of the system to small or short patients was a major concern prior to starting the study. Although the ese patients enrolled in the study were considerably smaller than patients from the rest of the world, TPS implantation was successful in all of them and there were no procedurerelated major complications, including groin puncture site major complications. In addition, all ese patients had low and stable pacing threshold, sensitivity, and impedance. The most common device location was the apex, and the distribution of placement location was similar in patients inside and outside of. One ese patient left the study prior to implant attempt because a narrow femoral vein confirmed by preoperative CT scan. Preoperative imaging, such as MRI, CT scan or venogram, is thought to be useful to confirm if the femoral vein s size and anatomy can accommodate a 23-Fr introducer, especially in patients with small stature. The TPS is ideal for elderly and thin patients, as it does not require a subcutaneous pocket. It is expected that ese patients, who tend to be thin, will receive more benefit from the TPS. For ese patients, implant procedure time was longer, most likely because of anticoagulation, monitoring of the activated clotting time during the procedure, and reversing anticoagulation prior to sheath removal. Also, hemostasis with figure-of-eight suture and manual pressure was used for all ese patients. These findings suggested that there was more concern surrounding potential bleeding complications in the ese patients. Time before ambulation and length of hospital stay were longer for ese patients, which might be explained by the use of the same clinical pathway that is used for conventional pacemaker implantation. However, given the physician satisfaction survey results and patient condition following the implant, hospital stays could be shortened, which will have additional benefit, especially for elderly and frail patients. Satisfaction Survey A total of 9 of 35 ese patients were not satisfied with their recovery and there was a significant difference in patient response regarding recovery between and outside. It is plausible that the medical history of the patients and adverse events associated with the implant procedure had a negative effect on their satisfaction with recovery. In fact, 6 of the 9 ese patients, including 2 patients who were very dissatisfied or dissatisfied with recovery, had congestive heart failure. The remaining 3 patients were experiencing an adverse event: worsening of AF or epilepsy at their 3-month visit. One ese patient who was very dissatisfied with recovery had congestive heart failure and reported cardiac failure deterioration at the 3-month visit. None of the ese patients developed pacemaker syndrome. Study Limitations The TPS global study was not designed to evaluate interregional differences between and outside. The number of ese patients who underwent an implant attempt with the TPS was only 36, accounting for approximately 5% of the entire study cohort. However, the study was conducted simultaneously around the globe following a single protocol. This makes it easier to generalize the study results to individual geographic regions. Conclusions TPS was successfully implanted in 100% of ese patients. Despite some differences in baseline characteristics and implant procedure characteristics in ese patients, safety and efficacy performance were favorable and similar to the global trial performance. Further, the TPS was favorably accepted by implanting physicians and patients. None. Name of Grants Disclosures K. Stromberg and J.L. work for Medtronic. W.T. was an employee of Medtronic. References 1. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009: A World Society of Arrhythmia s project. Pacing Clin Electrophysiol 2011; 34: Udo EO, Zuithoff NPA, Van Hemel NM, De Cock CC, Hendriks T, Doevendans PA, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm 2012; 9: Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35: Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374: Duray GZ, Ritter P, El-Chami M, Narasimhan C, Omar R, Tolosana JM, et al. Long-term performance of a transcatheter pacing system: 12-month results from the Micra Transcatheter Pacing Study. Heart Rhythm 2017; 14: Ritter P, Duray GZ, Zhang S, Narasimhan C, Soejima K, Omar R, et al. The rationale and design of the Micra Transcatheter Pacing Study: Safety and efficacy of a novel miniaturized pacemaker. Europace 2015; 17: Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 51: e1 e Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006; 8: JCS Joint Working Group. Guidelines for non-pharmacotherapy of cardiac arrhythmias (JCS 2011): Digest version. Circ J 2013; 77: El-Chami MF, Roberts PR, Kypta A, Omdahl P, Bonner MD, Kowal RC, et al. How to implant a leadless pacemaker with a tine-based fixation. J Cardiovasc Electrophysiol 2016; 27:
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience
 Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
Performance of a Miniaturized Transcatheter Pacing System: First-in-human experience Philippe Ritter, MD Gabor Z Duray, MD, PhD, FESC; Clemens Steinwender, MD, FESC; Kyoko Soejima, MD; Razali Omar, MD;
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
New generations pacemakers and ICDs: an update
 Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
REVIEW ARTICLE. Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1
 Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Leadless Pacing. Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt
 Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study
 Accepted Manuscript Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study Gabor Z. Duray, MD, PhD, Philippe Ritter, MD, Mikhael El-Chami, MD,
Accepted Manuscript Long-term Performance of a Transcatheter Pacing System: 12 month results from the Micra Transcatheter Pacing Study Gabor Z. Duray, MD, PhD, Philippe Ritter, MD, Mikhael El-Chami, MD,
Comparator cohort In order to compare the Micra pacing thresholds with transvenous thresholds, we analyzed transvenous pacing thresholds
 Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: Results from the Micra Transcatheter Pacing System Global Clinical Trial Jonathan P. Piccini, MD,
Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: Results from the Micra Transcatheter Pacing System Global Clinical Trial Jonathan P. Piccini, MD,
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
Pacing Without Wires: Leadless Cardiac Pacing
 REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
A Leadless Intracardiac Transcatheter Pacing System
 Original Article A Leadless Intracardiac Transcatheter Pacing System Dwight Reynolds, M.D., Gabor Z. Duray, M.D., Ph.D., Razali Omar, M.D., Kyoko Soejima, M.D., Petr Neuzil, M.D., Shu Zhang, M.D., Calambur
Original Article A Leadless Intracardiac Transcatheter Pacing System Dwight Reynolds, M.D., Gabor Z. Duray, M.D., Ph.D., Razali Omar, M.D., Kyoko Soejima, M.D., Petr Neuzil, M.D., Shu Zhang, M.D., Calambur
Cardiac implantable electronic devices (CIEDs) in children include pacemakers and implantable cardioverter defibrillators (ICDs).
 Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
NOVEL DEVICE TECHNOLOGIES
 NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
Leadless pacemakers a panacea for bradyarrhythmias?
 Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Leadless Cardiac Pacemaker
 Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc.
 Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
Top 5 Things to Know about Pacemakers and ICD s. Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017.
 Top 5 Things to Know about Pacemakers and ICD s Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017. Top 5 Things 1. Remote Monitoring leads to better care and outcomes. 2. MRI s CAN be done on device patients.
Top 5 Things to Know about Pacemakers and ICD s Jeffrey S. Osborn, M.D., C.C.D.S. March 4, 2017. Top 5 Things 1. Remote Monitoring leads to better care and outcomes. 2. MRI s CAN be done on device patients.
Technical option of surgical approach for trouble-shooting
 JHRS Corner Device and lead trouble-shooting - standard strategy and technical option - Technical option of surgical approach for trouble-shooting Katsuhiko IMAI Department of Cardiovascular surgery, Hiroshima
JHRS Corner Device and lead trouble-shooting - standard strategy and technical option - Technical option of surgical approach for trouble-shooting Katsuhiko IMAI Department of Cardiovascular surgery, Hiroshima
Left Atrial Appendage Closure: The Good, The Bad and The Ugly
 Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Left Atrial Appendage Closure: The Good, The Bad and The Ugly John D. Hummel, MD Director of Electrophysiology Research Ross Heart Hospital, The Ohio State University Columbus, Ohio USA Disclosures Modest
Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life
 Chapter 3 Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life Guido H. van Welsenes, MS, Johannes B. van Rees, MD, Joep Thijssen, MD, Serge
Chapter 3 Primary prevention ICD recipients: the need for defibrillator back-up after an event-free first battery service-life Guido H. van Welsenes, MS, Johannes B. van Rees, MD, Joep Thijssen, MD, Serge
Transvenous Pacemaker Procedures
 Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW
 MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France
 How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
How to treat Cardiac Resynchronization Therapy complications? C. Leclercq Departement of Cardiology Centre Cardio-Pneumologique Rennes, France Presenter Disclosure Information Christophe Leclercq, MD,
Long-term Preservation of Left Ventricular Function and Heart Failure Incidence with Ablate and Pace Therapy Utilizing Biventricular Pacing
 The Journal of Innovations in Cardiac Rhythm Management, 3 (2012), 976 981 HEART FAILURE RESEARCH ARTICLE Long-term Preservation of Left Ventricular Function and Heart Failure Incidence with Ablate and
The Journal of Innovations in Cardiac Rhythm Management, 3 (2012), 976 981 HEART FAILURE RESEARCH ARTICLE Long-term Preservation of Left Ventricular Function and Heart Failure Incidence with Ablate and
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
EBR Systems, Inc. 686 W. Maude Ave., Suite 102 Sunnyvale, CA USA
 Over 200,000 patients worldwide are estimated to receive a CRT device each year. However, limitations prevent some patients from benefiting. CHALLENGING PROCEDURE 5% implanted patients fail to have coronary
Over 200,000 patients worldwide are estimated to receive a CRT device each year. However, limitations prevent some patients from benefiting. CHALLENGING PROCEDURE 5% implanted patients fail to have coronary
Transvenous Pacemaker Implantation 22 years after the Mustard Procedure
 Case Report Transvenous Pacemaker Implantation 22 years after the Mustard Procedure Masato Sakamoto MD, Yoshie Ochiai MD, Yutaka Imoto MD, Akira Sese MD, Mamie Watanabe MD, Kunitaka Joo MD Department of
Case Report Transvenous Pacemaker Implantation 22 years after the Mustard Procedure Masato Sakamoto MD, Yoshie Ochiai MD, Yutaka Imoto MD, Akira Sese MD, Mamie Watanabe MD, Kunitaka Joo MD Department of
Original Article Fragmented QRS as a Predictor of Appropriate Implantable Cardioverter-defibrillator Therapy
 4 Original Article Fragmented QRS as a Predictor of Appropriate Implantable Cardioverter-defibrillator Therapy Sirin Apiyasawat, Dujdao Sahasthas, Tachapong Ngarmukos, Pakorn Chandanamattha, Khanchit Likittanasombat
4 Original Article Fragmented QRS as a Predictor of Appropriate Implantable Cardioverter-defibrillator Therapy Sirin Apiyasawat, Dujdao Sahasthas, Tachapong Ngarmukos, Pakorn Chandanamattha, Khanchit Likittanasombat
Right Ventricular Outflow Tract Septal Pacing in Hue Central Hospital
 Right Ventricular Outflow Tract Septal Pacing in Hue Central Hospital Huỳnh Văn Minh Nguyễn Cửu Lợi Tô Hưng Thụy 1 Introduction Apical pacing has been introduced over the past 5 decades to save and improve
Right Ventricular Outflow Tract Septal Pacing in Hue Central Hospital Huỳnh Văn Minh Nguyễn Cửu Lợi Tô Hưng Thụy 1 Introduction Apical pacing has been introduced over the past 5 decades to save and improve
Girish M Nair, Seeger Shen, Pablo B Nery, Calum J Redpath, David H Birnie
 268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
268 Case Report Cardiac Resynchronization Therapy in a Patient with Persistent Left Superior Vena Cava Draining into the Coronary Sinus and Absent Innominate Vein: A Case Report and Review of Literature
Clinical Data Summary: Avoid FFS Study
 Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Percutaneous Mitral Valve Repair
 Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Percutaneous Mitral Valve Repair MitraClip: Procedure, Data, Patient Selection Chad Rammohan, MD FACC Director, Cardiac Cath Lab El Camino Hospital Mountain View, California Mitral Regurgitation MitraClip
Summary, conclusions and future perspectives
 Summary, conclusions and future perspectives Summary The general introduction (Chapter 1) of this thesis describes aspects of sudden cardiac death (SCD), ventricular arrhythmias, substrates for ventricular
Summary, conclusions and future perspectives Summary The general introduction (Chapter 1) of this thesis describes aspects of sudden cardiac death (SCD), ventricular arrhythmias, substrates for ventricular
Cigna - Prior Authorization Procedure List Cardiology
 Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
CIPG Transcatheter Aortic Valve Replacement- When Is Less, More?
 CIPG 2013 Transcatheter Aortic Valve Replacement- When Is Less, More? James D. Rossen, M.D. Professor of Medicine and Neurosurgery Director, Cardiac Catheterization Laboratory and Interventional Cardiology
CIPG 2013 Transcatheter Aortic Valve Replacement- When Is Less, More? James D. Rossen, M.D. Professor of Medicine and Neurosurgery Director, Cardiac Catheterization Laboratory and Interventional Cardiology
Call Medtronic at 1 (800) to verify the patient s current implanted system
 MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
Case Report Hemostasis of Left Atrial Appendage Bleed With Lariat Device
 273 Case Report Hemostasis of Left Atrial Appendage Bleed With Lariat Device Amena Hussain MD, Muhamed Saric MD, Scott Bernstein MD, Douglas Holmes MD, Larry Chinitz MD NYU Langone Medical Center, United
273 Case Report Hemostasis of Left Atrial Appendage Bleed With Lariat Device Amena Hussain MD, Muhamed Saric MD, Scott Bernstein MD, Douglas Holmes MD, Larry Chinitz MD NYU Langone Medical Center, United
Pediatric Pacemaker Implantation Endocardial or Epicardial
 Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany
 Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
ENABLE MRI CLINICAL STUDY SUMMARY
 CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
CLINICAL STUDY SUMMARY ENABLE MRI CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in device implant and follow-up procedures. TABLE OF CONTENTS
Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker
 Original Article Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker Vivek Y. Reddy, M.D., Derek V. Exner, M.D., M.P.H., Daniel J. Cantillon, M.D., Rahul Doshi, M.D., T. Jared Bunch,
Original Article Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker Vivek Y. Reddy, M.D., Derek V. Exner, M.D., M.P.H., Daniel J. Cantillon, M.D., Rahul Doshi, M.D., T. Jared Bunch,
CRT-D or CRT-P: HOW TO CHOOSE THE RIGHT PATIENT?
 CRT-D or CRT-P: HOW TO CHOOSE THE RIGHT PATIENT? Alessandro Lipari, MD Chair and Department of Cardiology University of Study and Spedali Civili Brescia -Italy The birth of CRT in Europe, 20 years ago
CRT-D or CRT-P: HOW TO CHOOSE THE RIGHT PATIENT? Alessandro Lipari, MD Chair and Department of Cardiology University of Study and Spedali Civili Brescia -Italy The birth of CRT in Europe, 20 years ago
Device Interrogation- Pacemakers, ICD and Loop Recorders. Dulce Obias-Manno, RN, MHSA, CCDS,CEPS, FHRS Device Clinic Coordinator, MHVI
 Device Interrogation- Pacemakers, ICD and Loop Recorders Dulce Obias-Manno, RN, MHSA, CCDS,CEPS, FHRS Device Clinic Coordinator, MHVI Disclosures Consultant: Medtronic Speaker s Bureau: St. Jude Medical
Device Interrogation- Pacemakers, ICD and Loop Recorders Dulce Obias-Manno, RN, MHSA, CCDS,CEPS, FHRS Device Clinic Coordinator, MHVI Disclosures Consultant: Medtronic Speaker s Bureau: St. Jude Medical
INNOVATIONS IN DEVICE THERAPY:
 INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
How to Implant a Leadless Pacemaker With a Tine-Based Fixation
 Techniques, Technology, and Innovations Section Editor: Samuel J. Asirvatham, M.D. How to Implant a Leadless Pacemaker With a Tine-Based Fixation MIKHAEL F. EL-CHAMI, M.D., PAUL R. ROBERTS, M.D., ALEX
Techniques, Technology, and Innovations Section Editor: Samuel J. Asirvatham, M.D. How to Implant a Leadless Pacemaker With a Tine-Based Fixation MIKHAEL F. EL-CHAMI, M.D., PAUL R. ROBERTS, M.D., ALEX
Results from RE-LY and RELY-ABLE
 Results from RE-LY and RELY-ABLE Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in longterm stroke prevention EXECUTIVE SUMMARY Dabigatran etexilate (Pradaxa ) has shown a consistent
Results from RE-LY and RELY-ABLE Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in longterm stroke prevention EXECUTIVE SUMMARY Dabigatran etexilate (Pradaxa ) has shown a consistent
Implantable cardioverter defibrillator, Inappropriate shock, Lead failure
 Inappropriate Discharges of Intravenous Implantable Cardioverter Defibrillators Owing to Lead Failure Takashi WASHIZUKA, 1 MD, Masaomi CHINUSHI, 1 MD, Ryu KAZAMA, 1 MD, Takashi HIRONO, 1 MD, Hiroshi WATANABE,
Inappropriate Discharges of Intravenous Implantable Cardioverter Defibrillators Owing to Lead Failure Takashi WASHIZUKA, 1 MD, Masaomi CHINUSHI, 1 MD, Ryu KAZAMA, 1 MD, Takashi HIRONO, 1 MD, Hiroshi WATANABE,
Update on Device Innovation (S-ICD, Wearable, Leadless)
 Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
This is What I do to Improve CRT Response for CRT Non-Responders
 This is What I do to Improve CRT Response for CRT Non-Responders Michael R Gold, MD, PhD Medical University of South Carolina Charleston, SC Disclosures: Steering Committees (unpaid) and Clinical Trials,
This is What I do to Improve CRT Response for CRT Non-Responders Michael R Gold, MD, PhD Medical University of South Carolina Charleston, SC Disclosures: Steering Committees (unpaid) and Clinical Trials,
Supplemental Material
 Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
The learning curve associated with the implantation of the Nanostim leadless pacemaker
 Journal of Interventional Cardiac Electrophysiology (2018) 53:239 247 https://doi.org/10.1007/s10840-018-0438-8 MULTIMEDIA REPORT The learning curve associated with the implantation of the Nanostim leadless
Journal of Interventional Cardiac Electrophysiology (2018) 53:239 247 https://doi.org/10.1007/s10840-018-0438-8 MULTIMEDIA REPORT The learning curve associated with the implantation of the Nanostim leadless
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE. Reducing the risk of stroke in atrial fibrillation
 A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
UnitedHealthcare Medicare Advantage Cardiology Prior Authorization Program
 Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS
 INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS BEFORE INTERPRETING THE ECG: Nora Goldschlager, M.D. MACP, FACC, FAHA, FHRS. Cardiology San Francisco General Hospital UCSF Disclosures: None 1 2 QUESTIONS
INTERPRETING THE ECG IN PATIENTS WITH PACEMAKERS BEFORE INTERPRETING THE ECG: Nora Goldschlager, M.D. MACP, FACC, FAHA, FHRS. Cardiology San Francisco General Hospital UCSF Disclosures: None 1 2 QUESTIONS
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018; 162:XX.
 Extraction of a dislocated leadless pacemaker in a patient with infective endocarditis and repeated endocardial and epicardial pacing system infections Milos Taborsky a, Tomas Skala a, Martin Kocher b,
Extraction of a dislocated leadless pacemaker in a patient with infective endocarditis and repeated endocardial and epicardial pacing system infections Milos Taborsky a, Tomas Skala a, Martin Kocher b,
Finally, a pacemaker may be either permanent or temporary, which will also factor into your code selections.
 2015 Cardiology Survival Guide Chapter 15: Pacemakers With more than 30 codes and many variables to choose from, you must weigh your options carefully when reporting insertion, revision, or removal of
2015 Cardiology Survival Guide Chapter 15: Pacemakers With more than 30 codes and many variables to choose from, you must weigh your options carefully when reporting insertion, revision, or removal of
COMMONLY BILLED CODES AND ASSOCIATED 2018 MEDICARE RATES
 CRHF REIMBURSEMENT & HEALTH POLICY Pacemaker Therapy COMMONLY BILLED CODES AND ASSOCIATED 2018 MEDICARE RATES This document reflects commonly billed codes for Pacemaker Therapy and their associated National
CRHF REIMBURSEMENT & HEALTH POLICY Pacemaker Therapy COMMONLY BILLED CODES AND ASSOCIATED 2018 MEDICARE RATES This document reflects commonly billed codes for Pacemaker Therapy and their associated National
Essentials of Pacemakers and ICD s. Rajesh Banker, MD, MPH
 Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
SURGICAL ABLATION OF ATRIAL FIBRILLATION DURING MITRAL VALVE SURGERY THE CARDIOTHORACIC SURGICAL TRIALS NETWORK
 SURGICAL ABLATION OF ATRIAL FIBRILLATION DURING MITRAL VALVE SURGERY THE CARDIOTHORACIC SURGICAL TRIALS NETWORK Marc Gillinov, M.D. For the CTSN Investigators ACC Late Breaking Clinical Trials March 16,
SURGICAL ABLATION OF ATRIAL FIBRILLATION DURING MITRAL VALVE SURGERY THE CARDIOTHORACIC SURGICAL TRIALS NETWORK Marc Gillinov, M.D. For the CTSN Investigators ACC Late Breaking Clinical Trials March 16,
Indication, Timing, Assessment and Update on TAVI
 Indication, Timing, Assessment and Update on TAVI Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Starr- Edwards Mechanical
Indication, Timing, Assessment and Update on TAVI Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Starr- Edwards Mechanical
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
14ο Βορειοελλαδικό Καρδιολογικό Συνέδριο ΚΕΒΕ Βηματοδότηση: Νεότερες εξελίξεις Προοπτικές
 14ο Βορειοελλαδικό Καρδιολογικό Συνέδριο ΚΕΒΕ Βηματοδότηση: Νεότερες εξελίξεις Προοπτικές Α.Γ. Κατσίβας Συντονιστής Διευθυντής, Α Καρδιολογική Κλινική, ΓΝΑ «Κοργιαλένειο-Μπενάκειο Ε.Ε.Σ» BRADY - ARRHYTHMIA
14ο Βορειοελλαδικό Καρδιολογικό Συνέδριο ΚΕΒΕ Βηματοδότηση: Νεότερες εξελίξεις Προοπτικές Α.Γ. Κατσίβας Συντονιστής Διευθυντής, Α Καρδιολογική Κλινική, ΓΝΑ «Κοργιαλένειο-Μπενάκειο Ε.Ε.Σ» BRADY - ARRHYTHMIA
Supplementary Online Content
 Supplementary Online Content Tseng ZH, Hayward RM, Clark NM, et al. Sudden death in patients with cardiac implantable electronic devices. JAMA Intern Med. Published online June 22, 2015. doi:10.1001/jamainternmed.2015.2641.
Supplementary Online Content Tseng ZH, Hayward RM, Clark NM, et al. Sudden death in patients with cardiac implantable electronic devices. JAMA Intern Med. Published online June 22, 2015. doi:10.1001/jamainternmed.2015.2641.
A Prospective Study Comparing the Sensed R Wave in Bipolar and Extended Bipolar Configurations: The PropR Study
 A Prospective Study Comparing the Sensed R Wave in Bipolar and Extended Bipolar Configurations: The PropR Study ANEESH V. TOLAT, M.D.,* MELISSA WOICIECHOWSKI, M.S.N.,* ROSEMARIE KAHR, R.C.I.S.,* JOSEPH
A Prospective Study Comparing the Sensed R Wave in Bipolar and Extended Bipolar Configurations: The PropR Study ANEESH V. TOLAT, M.D.,* MELISSA WOICIECHOWSKI, M.S.N.,* ROSEMARIE KAHR, R.C.I.S.,* JOSEPH
Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases
 Case Report Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases Koji Tsutsumi MD, Tatsuru Niibori MD, Keiichiro Katsumoto MD Department
Case Report Implantation of a Permanent Tined Endocardial Electrode into Right Atrium during Open-heart Surgery: Report of 3 Cases Koji Tsutsumi MD, Tatsuru Niibori MD, Keiichiro Katsumoto MD Department
AF Today: W. For the majority of patients with atrial. are the Options? Chris Case
 AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
Cardiac Imaging in abnormal rhythm Role of MDCT
 Cardiac Imaging in abnormal rhythm Role of MDCT Cardiac Imaging in abnormal rhythm Role of MDCT Scope of the problem CT in Atrial Fibrillation CT and pacing Ventricular arrhythmia Other applications 1
Cardiac Imaging in abnormal rhythm Role of MDCT Cardiac Imaging in abnormal rhythm Role of MDCT Scope of the problem CT in Atrial Fibrillation CT and pacing Ventricular arrhythmia Other applications 1
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
King s Research Portal
 King s Research Portal DOI: 10.1016/j.tcm.2016.03.003 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Rinaldi, C. A. (2016).
King s Research Portal DOI: 10.1016/j.tcm.2016.03.003 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Rinaldi, C. A. (2016).
2010 Canadian Cardiovascular Society/ Canadian Heart Rhythm Society Training and Maintenance of Competency in Adult Clinical Cardiac
 2010 Canadian Cardiovascular Society/ Canadian Heart Rhythm Society Training and Maintenance of Competency in Adult Clinical Cardiac Electrophysiology Martin S. Green, Chair, CHRS Education Committee Peter
2010 Canadian Cardiovascular Society/ Canadian Heart Rhythm Society Training and Maintenance of Competency in Adult Clinical Cardiac Electrophysiology Martin S. Green, Chair, CHRS Education Committee Peter
Management of RT patients with implanted cardiac devices: From recommendation to implementation
 Management of RT patients with implanted cardiac devices: From recommendation to implementation Coen Hurkmans Clinical Physicist Catharina Hospital Eindhoven The Netherlands 1/22 Outline CIED relocation.
Management of RT patients with implanted cardiac devices: From recommendation to implementation Coen Hurkmans Clinical Physicist Catharina Hospital Eindhoven The Netherlands 1/22 Outline CIED relocation.
Transcatheter Aortic Valve Implantation Procedure (TAVI)
 Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Leadless pacing: Technical challenges and concerns. Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital
 Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
EKG Competency for Agency
 EKG Competency for Agency Name: Date: Agency: 1. The upper chambers of the heart are known as the: a. Atria b. Ventricles c. Mitral Valve d. Aortic Valve 2. The lower chambers of the heart are known as
EKG Competency for Agency Name: Date: Agency: 1. The upper chambers of the heart are known as the: a. Atria b. Ventricles c. Mitral Valve d. Aortic Valve 2. The lower chambers of the heart are known as
ESC Stockholm Arrhythmias & pacing
 ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
ESC Stockholm 2010 Take Home Messages for Practitioners Arrhythmias & pacing Prof. Panos E. Vardas Professor of Cardiology Heraklion University Hospital Crete, Greece Disclosures Small teaching fees from
PACING AT THE BUNDLE OF HIS
 PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
Cardiac Resynchronisation Therapy Patient Information
 Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
CAPTIVATE SUMMARY CLINICAL SUMMARY. CAPTure Information Via Automatic Threshold Evaluation
 CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
CLINICAL SUMMARY CAPTIVATE SUMMARY CAPTure Information Via Automatic Threshold Evaluation CAUTION: Federal law restricts this device to sale by or on the order of a physician trained or experienced in
Repair or Replacement
 Surgical intervention post MitraClip Device: Repair or Replacement Saudi Heart Association, February 21-24 Rüdiger Lange, MD, PhD Nicolo Piazza, MD, FRCPC, FESC German Heart Center, Munich, Germany Division
Surgical intervention post MitraClip Device: Repair or Replacement Saudi Heart Association, February 21-24 Rüdiger Lange, MD, PhD Nicolo Piazza, MD, FRCPC, FESC German Heart Center, Munich, Germany Division
Subclinical AF: Implications of device based episodes
 Subclinical AF: Implications of device based episodes Michael R Gold, MD, PhD Medical University of South Carolina Charleston, SC Disclosures: Clinical Trials and Consulting: Medtronic, Boston Scientific
Subclinical AF: Implications of device based episodes Michael R Gold, MD, PhD Medical University of South Carolina Charleston, SC Disclosures: Clinical Trials and Consulting: Medtronic, Boston Scientific
INTERNAL CARDIOVERSION. Lancashire & South Cumbria Cardiac Network
 INTERNAL CARDIOVERSION Lancashire & South Cumbria Cardiac Network DC Cardioversion AF & AFL safe 1,2 efficacious 3,4 (60-94%) 5 SR - Increases exercise tolerance 6 Maintainence SR unlikely in patients
INTERNAL CARDIOVERSION Lancashire & South Cumbria Cardiac Network DC Cardioversion AF & AFL safe 1,2 efficacious 3,4 (60-94%) 5 SR - Increases exercise tolerance 6 Maintainence SR unlikely in patients
Cardiac Rhythm Device Management. PBL STOP Your acronym for a standardized follow-up
 Cardiac Rhythm Device Management PBL STOP Your acronym for a standardized follow-up What s in it for you? What do you need to feel comfortable with a pacemaker/icd follow-up? 2 CORE OBJECTIVE Provide a
Cardiac Rhythm Device Management PBL STOP Your acronym for a standardized follow-up What s in it for you? What do you need to feel comfortable with a pacemaker/icd follow-up? 2 CORE OBJECTIVE Provide a
Management of Anticoagulation during Device Implants; Coumadin to Novel Agents
 Management of Anticoagulation during Device Implants; Coumadin to Novel Agents DR D Birnie Invited Faculty Core Curriculum Heart Rhythm Society May 8 th 2014 Disclosures Boehringer Ingleheim Research Support
Management of Anticoagulation during Device Implants; Coumadin to Novel Agents DR D Birnie Invited Faculty Core Curriculum Heart Rhythm Society May 8 th 2014 Disclosures Boehringer Ingleheim Research Support
Left Atrial Appendage Occlusion
 Left Atrial Appendage Occlusion A new strategy to prevent stroke in atrial fibrillation Ashok Talreja MD and Arijit Chanda MD VHVI symposium 24th February 2018 Outline of presentation 1. Risk of stroke
Left Atrial Appendage Occlusion A new strategy to prevent stroke in atrial fibrillation Ashok Talreja MD and Arijit Chanda MD VHVI symposium 24th February 2018 Outline of presentation 1. Risk of stroke
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD
 Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
evicore cardiology procedures and services requiring prior authorization
 evicore cardiology procedures and services requiring prior authorization Moda Health Commercial Group and Individual Members* *Check EBT to verify member enrollment in evicore program Radiology Advanced
evicore cardiology procedures and services requiring prior authorization Moda Health Commercial Group and Individual Members* *Check EBT to verify member enrollment in evicore program Radiology Advanced
Pacemaker System Malfunction Resulting from External Electrical Cardioversion: A Case Report
 Case Report Pacemaker System Malfunction Resulting from External Electrical Cardioversion: A Case Report Taku Nishida MD, Tamio Nakajima MD, PhD, Yutaka Goryo MD, Ken-ichi Ishigami MD, PhD, Hiroyuki Kawata
Case Report Pacemaker System Malfunction Resulting from External Electrical Cardioversion: A Case Report Taku Nishida MD, Tamio Nakajima MD, PhD, Yutaka Goryo MD, Ken-ichi Ishigami MD, PhD, Hiroyuki Kawata
Electrophysiology. Jim Collins, CPC, CCC President, CardiologyCoder.Com, Inc. Two Types of Diagnostic EP Studies
 Electrophysiology Jim Collins, CPC, CCC President, CardiologyCoder.Com, Inc. 1 Two Types of Diagnostic EP Studies 93619 Basic, Comprehensive Study RA Record His Record RV Record RA Pace RV Pace 93620 Comprehensive
Electrophysiology Jim Collins, CPC, CCC President, CardiologyCoder.Com, Inc. 1 Two Types of Diagnostic EP Studies 93619 Basic, Comprehensive Study RA Record His Record RV Record RA Pace RV Pace 93620 Comprehensive
2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction
 Summary of Expert Consensus Statement for CLINICIANS 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction This is a summary of the Heart Rhythm
Summary of Expert Consensus Statement for CLINICIANS 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction This is a summary of the Heart Rhythm
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
Atrial Fibrillaiton and Heart Failure: Anticoagulation therapy in all cases?
 Atrial Fibrillaiton and Heart Failure: Anticoagulation therapy in all cases? Nicolas Lellouche Fédération de Cardiologie Hôpital Henri Mondor Créteil Disclosure Statement of Financial Interest I currently
Atrial Fibrillaiton and Heart Failure: Anticoagulation therapy in all cases? Nicolas Lellouche Fédération de Cardiologie Hôpital Henri Mondor Créteil Disclosure Statement of Financial Interest I currently
Surgical Ablation for Lone AF: What have we learned after 30 years?
 Surgical Ablation for Lone AF: What have we learned after 30 years? Ralph J. Damiano, Jr., MD Evarts A. Graham Professor of Surgery Chief of Cardiothoracic Surgery Vice Chairman, Department of Surgery
Surgical Ablation for Lone AF: What have we learned after 30 years? Ralph J. Damiano, Jr., MD Evarts A. Graham Professor of Surgery Chief of Cardiothoracic Surgery Vice Chairman, Department of Surgery
Concurrent Failure of Active and Redundant Ventricular Epicardial Electrodes in Children
 Concurrent Failure of Active and Redundant Ventricular Epicardial Electrodes in Children ERALD A. SERWER, M.D., MACDONALD DICK 11, M.D., KAREN UZARK, R.N., Ph.D., WILLIAM A. SCOTT, M.D., and EDWARD L.
Concurrent Failure of Active and Redundant Ventricular Epicardial Electrodes in Children ERALD A. SERWER, M.D., MACDONALD DICK 11, M.D., KAREN UZARK, R.N., Ph.D., WILLIAM A. SCOTT, M.D., and EDWARD L.
Mitral Valve Disease, When to Intervene
 Mitral Valve Disease, When to Intervene Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Current ACC/AHA guideline Stages
Mitral Valve Disease, When to Intervene Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Current ACC/AHA guideline Stages
Biventricular Pacing - Hemodynamic Benefit for Patients with Congestive Heart Failure
 428 December 2000 Biventricular Pacing - Hemodynamic Benefit for Patients with Congestive Heart Failure K. MALINOWSKI Helios Clinics, Aue, Germany Summary Congestive heart failure afflicts a large and
428 December 2000 Biventricular Pacing - Hemodynamic Benefit for Patients with Congestive Heart Failure K. MALINOWSKI Helios Clinics, Aue, Germany Summary Congestive heart failure afflicts a large and
Remote monotoring of cardiac rhythm devices: present and future Pacemaker and ICD
 Remote monotoring of cardiac rhythm devices: present and future Pacemaker and ICD Philippe Mabo University Hospital, Rennes, France ESC congress, Paris 30 August 2011 U642 Disclosures Biotronik: research
Remote monotoring of cardiac rhythm devices: present and future Pacemaker and ICD Philippe Mabo University Hospital, Rennes, France ESC congress, Paris 30 August 2011 U642 Disclosures Biotronik: research
Patients selection criteria for LAA occlusion. Young Keun On, MD, PhD, FHRS Samsung Medical Center Sungkyunkwan University School of Medicine
 Patients selection criteria for LAA occlusion Young Keun On, MD, PhD, FHRS Samsung Medical Center Sungkyunkwan University School of Medicine Atrial Fibrillation The most common cardiac arrhythmia. Confers
Patients selection criteria for LAA occlusion Young Keun On, MD, PhD, FHRS Samsung Medical Center Sungkyunkwan University School of Medicine Atrial Fibrillation The most common cardiac arrhythmia. Confers
