1 Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis. N Engl J Med 352;13, March 31, 2005
|
|
|
- Samson Armstrong
- 6 years ago
- Views:
Transcription
1 The risk of ischemic stroke in patients with Intracranial Atherosclerotic Disease (ICAD) ranges from 7 to 24%. 1,2 Developed specifically for the treatment of ICAD, the Wingspan Stent System and Gateway PTA Balloon Catheter deliver new options for ischemic stroke. Designed for Neurovascular Access The Wingspan Stent System features a highly flexible, trackable delivery system designed to facilitate access through challenging neurovascular anatomy. Vessel Wall Apposition The Wingspan Stent employs an extra-flexible, self-expanding stent designed to promote vessel wall apposition in curved and tapered vessels. The stent features a flexible cell design for enhanced conformability and to facilitate access, especially with longer stent lengths. Vessel Lumen Support Wingspan Stents are designed to support the vessel lumen by minimizing vessel recoil following angioplasty and exerting active, controlled, outward radial force. Wingspan Stent System Product Number Diameter x Length WS x 20 mm WS x 20 mm WS x 20 mm WS x 20 mm WS x 20 mm WS x 15 mm WS x 15 mm WS x 15 mm WS x 15 mm WS x 15 mm WS x 9 mm WS x 9 mm WS x 9 mm WS x 9 mm WS x 9 mm Hydrophilic Labeled Stent Self-Expanded Recommended Diameter Stent Diameter Vessel Diameter 2.5 mm mm...>2.0 and 2.5 mm 3.0 mm mm...>2.5 and 3.0 mm 3.5 mm mm...>3.0 and 3.5 mm 4.0 mm mm...>3.5 and 4.0 mm 4.5 mm mm...>4.0 and 4.5 mm 3.5F Unified Over-the-Wire (OTW) Stent Delivery System in Guidewire Compatible 6F (minimum in ID) Guide Catheter Compatible Strut Thickness: in Strut Width: in Stent Foreshortening: % 1 Comparison of Warfarin and Aspirin for Symptomatic Intracranial Arterial Stenosis. N Engl J Med 352;13, March 31, Failure of Extracranial-Intracranial Arterial Bypass to Reduce the Risk of Ischemic Stroke: Results of an International Randomized Trial. N Engl J Med 1985; 313: ETG100 1
2 Gateway PTA Balloon Catheter Hydrophilic Product Number Diameter x Length Product Number Diameter x Length x 20 mm x 20 mm x 20 mm x 20 mm x 20 mm x 20 mm x 20 mm x 20 mm x 20 mm x 20 mm x 15 mm x 15 mm x 15 mm x 15 mm x 15 mm x 15 mm x 15 mm x 15 mm x 15 mm x 15 mm x 9 mm x 9 mm x 9 mm x 9 mm x 9 mm x 9 mm x 9 mm x 9 mm x 9 mm x 9 mm Procedure Overview 1. Gentle Pre-Dilation The Gateway PTA Balloon Catheter features a semi-compliant balloon designed for controlled, low-pressure inflation (6 atm).* Recommended to be undersized to 80% of the target vessel diameter, it provides for gentle pre-dilation prior to stent deployment. 2. Atraumatic Stent Deployment Engineered for atraumatic deployment, the Wingspan Stent is designed to apply minimal pressure less than 0.1 atm* to the vessel wall immediately proximal and distal to the treated lesion. 3. Highly Conformable Stent Design The Wingspan Stent is a self-expanding design with flexible cells to enhance conformability and vessel wall apposition in curved and tapered vessels. 4. Outward Radial Force The Wingspan Stent is designed to support the vessel lumen by minimizing vessel recoil following angioplasty and exerting active, controlled, outward radial force.* * Data on file at Boston Scientific. Bench test results may not be indicative of clinical performance. ETG100 2
3 HDE Safety Study The Wingspan Stent System and Gateway PTA Balloon Catheter have been evaluated in a monitored, adjudicated, multicenter study. In this study, half the lesions treated were in the anterior circulation demonstrating the access capabilities of this groundbreaking system. The clinical performance of the Wingspan Stent System and Gateway PTA Balloon Catheter, as shown in this clinical study, included a procedural success rate of 97.7%. HDE Study Results Multi-Center Study Monitored, adjudicated study at 12 sites; 45 patients enrolled Proven Safety Procedure success = 97.7% (43/44) 0 vessel dissections, 0 vessel ruptures Access 50% of cases in anterior circulation 9 M1 lesions treated Clinical Outcomes 30-day ipsilateral stroke or death rate = 4.4% (2/45) > 50% Stenosis 7.5% (3/40) at 6 months 6-month ipsilateral stroke or death rate = 7.0% (3/43) 24 out of 40 patients experienced increase in lumen diameter (post-procedure vs. 6-month follow-up) Symptomatic Restenosis 0% (0/40) at 6 months ETG100 3
4 See package insert for complete indications, contraindications, warnings, and instructions for use. Humanitarian Device. The Wingspan Stent System with Gateway PTA Balloon Catheter is authorized by United States Federal law for use in improving cerebral artery lumen diameter in patients with intracranial atherosclerotic disease, refractory to medical therapy, in intracranial vessels with 50% stenosis that are accessible to the system. The effectiveness of this device for this use has not been demonstrated. Wingspan Stent System Indications For Use The Wingspan Stent System with Gateway PTA Balloon Catheter is indicated for use in improving cerebral artery lumen diameter in patients with intracranial atherosclerotic disease, refractory to medical therapy, in intracranial vessels with 50% stenosis that are accessible to the system. Contraindications Patients in whom antiplatelet and/or anticoagulation therapy is contraindicated. Lesions that are highly calcified or otherwise could prevent access or appropriate expansion of the Stent. Potential Adverse Effects The potential adverse events listed below, as well as others, may be associated with the use of the Wingspan Stent System with Gateway PTA Balloon Catheter or with the procedure: Observed Adverse Events: Infection; TIA; Stroke; Hematoma; Vasospasm; Hemorrhagic Event; Hypertension; Peripheral vascular diseases; Neurological symptoms; Pain; AMI; Angina; Arrhythmia; Creatinine increase; Hematuria; Hypoglycemia/hyperglycemia; Asymptomatic Thromboembolic Event; Bradycardia (35 min); Broken middle-foot left/v-fracture; Chronical antrum gastritis; Death; Elevated bilirubin, GOT, GPT; Fever; Hiatus hernia; Hypervolemia; New distal in stent stenosis; Pulmonary edema; respiratory failure; Seizure; Syncope Potential Adverse Events: Cerebral aneurysm; Coagulopathy; Emboli (air, tissue, or thrombotic tissue); Intimal dissection; Pseudoaneurysm; Stent migration; Stent misplacement; Stent occlusion; Stent embolization; Stent thrombosis; Vessel perforation; Vessel rupture; Vessel thrombosis; Vessel trauma requiring repair or surgical intervention Please be aware that potential adverse effects may arise even with the proper use of medical devices. Accordingly, this device should only be used by persons qualified in the procedures for which it is indicated. Cautions/Precautions The Wingspan Stent System and the Gateway PTA Balloon Catheter are provided STERILE for single use only. Do not resterilize. Store in a cool, dry place. Use the Wingspan Stent System and Gateway PTA Balloon Catheter prior to the Use By date printed on the package. Select a Stent size (length and diameter) that extends a minimum of 3mm on both sides of the lesion. Carefully inspect the sterile package and Wingspan Stent System prior to use to verify that neither has been damaged during shipment. Do not use kinked or damaged components. Typical antiplatelet and anticoagulation regimen used for interventional intracranial procedures is an important adjunct to Stent treatment. Patients must be advised to take their prescribed medications after the Stent is implanted and should be counseled on the risk of not complying with medical therapy. In-stent thrombosis may occur during the procedure if proper antiplatelet and anticoagulation therapy is not administered. Do not steam shape the tip of the Wingspan Stent System because it could damage the Stent or Delivery System. Implanting a Stent may lead to dissection of the vessel distal or proximal to the Stent and may cause other complications (vasospasm/acute closure) of the vessel requiring additional intervention (i.e., further dilation, placement of stents). Do not deploy the Stent if it is not properly positioned in the vessel. Placement of the Stent may compromise side branch patency. Follow the Wingspan Stent System preparation and use instructions carefully. Previous studies have shown that some metal stents may be incompatible with MRI scanning. The Wingspan Stent System has been shown to be MRI compatible in MRI systems operating at field strengths of 3.0 Tesla or lower. MRI laboratory evaluation demonstrated that no significant image distortion or heating was created by the presence of the Stents at scanning sequences commonly used during MRI procedures. Do not use the Wingspan Stent System or the Gateway PTA Balloon Catheter for repositioning or recapturing the Stent. Exercise caution when crossing the deployed Stent with guidewires or other devices. In tortuous vessels, a stiff guidewire may cause binding within the Wingspan Stent System or the Gateway Balloon Catheter during deployment. In such cases, use only soft guidewires, and position the floppy section of the guidewire within the Stent. After deployment, the Stent may foreshorten up to 2.4% in 2.5 mm Stents and up to 7.1% in 4.5 mm Stents. Stent retrieval methods (use of additional wires, snares and/or forceps) may result in additional trauma to the vasculature and/or the vascular access site. Complications may include bleeding, hematoma, or pseudoaneurysm. ETG100 4
5 Warnings The Wingspan Stent System with Gateway PTA Balloon Catheter should only be used by physicians who have received appropriate training in interventional neuroradiology and treatment of intracranial atherosclerotic disease. The Wingspan Stent System is not designed or intended for contrast injections or injections other than heparinized saline. If excessive resistance is encountered during the use of the Wingspan Stent System or with the Gateway PTA Balloon Catheter at any time during the procedure, discontinue use of the System. Movement of the System against resistance may result in damage to the vessel, or a System component. In animal evaluations, the severity of vessel stenosis/neointimal thickness appears to be correlated with the degree of trauma inflicted on the arterial walls by Stent placement or Stent radial expansion. Experience with stent implants indicates that there is a risk of restenosis. Subsequent restenosis may require repeat dilation of the vessel segment containing the stent. The risks and long-term outcome following repeat dilation of endothelialized stents is unknown at present. If the stent is implanted adjacent to or contacting other implanted metal, such as another stent or embolic coil, the metals should be of similar composition to avoid galvanic corrosion potential. Gateway PTA Balloon Catheter Indications for Use The Gateway PTA Balloon Catheter is indicated for balloon dilation of the stenotic portion of intracranial arteries prior to stenting for the purpose of improving intracranial perfusion. Contraindications The Gateway PTA balloon catheter is contraindicated for use in: Patients in whom antiplatelet and/or anticoagulation therapy is contraindicated. Patients who are judged to have a lesion which prevents effective angioplasty. Potential Adverse Effects Adverse events (in alphabetical order) may be associated with the use of an intracranial angioplasty in stenotic lesions of the intracranial arteries: Death; Dissection; Drug reactions to antiplatelet agents/contrast medium; Distal emboli (air, tissue, or thrombotic emboli); Hematoma; Hemorrhage, requiring transfusion; Hypotension/Hypertension; Infection and pain at insertion site; Ischemia/Infarct; Perforation; Pseudoaneurysm, (femoral and intracranial); Restenosis of the dilated vessel; Spasm; Stroke/TIA; Total occlusion of the intracranial artery. Please be aware that potential adverse effects may arise even with the proper use of medical devices. Accordingly, this device should only be used by persons qualified in the procedures for which it is indicated. Cautions/Precautions Store in a dry, dark, cool place. Do not resterilize. Note product Use By date. Follow the Gateway PTA Balloon Catheter preparation and use instructions carefully. Do not prepare or pre-inflate the balloon other than as directed. Use the balloon purging technique described in this Instructions for Use. Typical antiplatelet and anticoagulation regimen used for interventional intracranial procedure is an important adjunct to balloon angioplasty treatment. Do not use the Gateway PTA Balloon Catheter in patients in whom antiplatelet and/or anticoagulation therapy is contraindicated. Vessel thrombosis may occur during the procedure if proper antiplatelet and anticoagulation therapy is not administered. Angioplasty may lead to dissection of the vessel and may cause other complications (vasospasm/acute closure) of the vessel requiring additional intervention (i.e., further dilation, placement of stents). Use only the appropriate balloon inflation media. Do not use air or any gaseous medium to inflate the balloon because it may cause uneven inflation and complications. If unexpected difficulty is experienced during inflation, do not continue; remove the device and do not attempt to use it. To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the lesser of the vessel diameters just proximal and distal to the stenosis. Do not use a guidewire having a diameter greater than in/0.36 mm. When the delivery catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Infusion of any medium other than a flush of heparinized normal saline through the guidewire lumen may compromise device performance. Do not attempt to reposition a partially deployed balloon. Attempted repositioning of a partially deployed balloon may result in severe vessel damage. Balloon pressures should be monitored during inflation. Do not exceed rated burst pressure indicated on the product label. Use of pressures higher than those specified on the product label may result in a ruptured balloon and potential intimal damage and dissection. The rated burst pressure is based on the results of in vitro testing. At least 99.9% of the balloons (with a 95% confidence interval) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization. ETG100 5
6 Cautions/Precautions (continued) Before withdrawing the device, visually confirm complete balloon deflation by fluoroscopy. If the balloon has already been inflated and difficulty is experienced deflating (a non-deflate), connect a large-barrel syringe and manually attempt to deflate the device. In order to achieve optimal performance of Gateway catheters and Boston Scientific steerable guidewires and to maintain the lubricity of the Bioslide surface, it is critical that a continuous flow of appropriate flush solution be maintained between a) the Gateway catheter and guide catheter, and b) the Gateway catheter and any intraluminal device. In addition, flushing aids in preventing contrast crystal formation and/or clotting on both the guidewire and inside the catheter lumen. The recommended continuous flush set up requires two stopcocks and two rotating hemostatic valves (RHV); the RHV s provide a fluid tight seal and are attached to the guide catheter and Gateway catheter. The stopcocks attach to the RHV sidearms, which become infusion ports for appropriate flush or contrast medium injection. Warnings Since the use of this device carries the associated risk of subacute thrombosis, vascular complication and/or bleeding events, judicious selection of patients is necessary. Only physicians who have received training should perform intracranial angioplasty. Angioplasty and stenting procedures should only be performed at hospitals where emergency intracranial surgery can be readily performed in the event of a potentially injurious or life-threatening complication. Trademarks Wingspan, Gateway and Bioslide are trademarks of Boston Scientific Corporation or its affiliates. ETG100 6
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM
 Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
Assurant Cobalt Iliac BALLOON EXPANDABLE STENT SYSTEM Innovating for life. CONFORMABILIT Y 6 F S H E AT H C O M PAT I B I L I T Y THE ASSURANT COBALT ILIAC STENT, WITH ITS UNIQUE COBALT CHROMIUM MODULAR
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System
 100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Understanding aneurysms and flow diversion treatment
 Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
Surpass Streamline Flow Diverter See package insert for complete indications, contraindications, warnings and instructions for use. INTENDED USE / INDICATIONS FOR USE The Surpass Streamline Flow Diverter
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
Advanced Innovation for Exceptional Performance
 Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Single Pass MCA Revascularization with Trevo
 Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU)
 Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Talent Abdominal Stent Graft
 Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
Talent Abdominal with THE Xcelerant Hydro Delivery System Expanding the Indications for EVAR Treat More Patients Short Necks The Talent Abdominal is the only FDA-approved device for proximal aortic neck
System.
 System www.penumbrainc.com POD System Case Examples 45 cm of POD Packing Coil in 1.5 to 3 mm diameter dilating vessel Bronchial Artery Embolization Dr. Amit Kakkar and Dr. Aksim Rivera, Bronx, NY Inflow:
System www.penumbrainc.com POD System Case Examples 45 cm of POD Packing Coil in 1.5 to 3 mm diameter dilating vessel Bronchial Artery Embolization Dr. Amit Kakkar and Dr. Aksim Rivera, Bronx, NY Inflow:
DURABLE. CONSISTENT. SAFE. IN.PACT Admiral Drug-Coated Balloon
 DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
INSTRUCTIONS FOR USE
 PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
Ruby Coil. Large Volume Detachable Coils
 Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Ruby Coil Ruby Case Examples 38 mm Hepatic Artery Aneurysm Pulmonary AVM 2 Coils Dr. James Benenati Miami Cardiac and Vascular Institute, FL Y90 Embolization Type 2 Endoleak Dr. J Moskovitz Florida Hospital,
Instructions for Use 1
 Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
PTCA Dilatation Catheter. Instructions for Use
 PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
PTCA Dilatation Catheter Instructions for Use Rx ONLY Caution: Federal law restricts this device to sale by or on the order of a physician STERILE. Sterilized with ethylene oxide gas Distributed by Medinol
Finally, the Control You Need to Deliver Accurate Treatment
 Finally, the Control You Need to Deliver Accurate Treatment LIFESTREAM Covered Stent. has joined BD When you reach for a balloon expandable stent, you require accuracy. The LIFESTREAM Balloon Expandable
Finally, the Control You Need to Deliver Accurate Treatment LIFESTREAM Covered Stent. has joined BD When you reach for a balloon expandable stent, you require accuracy. The LIFESTREAM Balloon Expandable
Innovation by design. Technology that sets a new standard
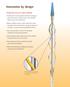 Innovation by design Technology that sets a new standard Flexible nitinol scoring element with three rectangular spiral struts works in tandem with a semi-compliant balloon to score the target lesion Balloon
Innovation by design Technology that sets a new standard Flexible nitinol scoring element with three rectangular spiral struts works in tandem with a semi-compliant balloon to score the target lesion Balloon
BTK Case Studies Joseph Cardenas, MD AZ Heart & Vascular, Yuma, AZ
 BTK Case Studies Joseph Cardenas, MD AZ Heart & Vascular, Yuma, AZ 1 Case 1 78 yr. old female Rutherford Class II/III lesion 1 block claudicant 2 Pre Treatment Post Treatment Anterior Tibial Artery Occlusion
BTK Case Studies Joseph Cardenas, MD AZ Heart & Vascular, Yuma, AZ 1 Case 1 78 yr. old female Rutherford Class II/III lesion 1 block claudicant 2 Pre Treatment Post Treatment Anterior Tibial Artery Occlusion
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
Access More Patients. Customize Each Seal.
 Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
Access More. Customize Each Seal. The Least Invasive Path Towards Proven Patency ULTRA LOW PROFILE TO EASE ADVANCEMENT The flexible, ultra-low 12F ID Ovation ix delivery system enables you to navigate
CP STENT. Large Diameter, Balloon Expandable Stent
 CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
CP STENT Large, Expandable CP STENT OPTIONS 12mm to Expansion 26mm to Expansion CP Matrix (number of zigs) 1.6 2.2 2.8 3.4 3.9 4.5 5 5.5 6 12 14 15 16 18 20 22 24 26 28 30 CP Details: CP is composed of
Neuroform Microdelivery Stent System
 Neuroform Microdelivery Stent System Patient Information Booklet TABLE OF CONTENTS What is the Purpose of This Booklet?...2 What is a Wide Neck, Intracranial Aneurysm?...2 What is the Treatment for Wide
Neuroform Microdelivery Stent System Patient Information Booklet TABLE OF CONTENTS What is the Purpose of This Booklet?...2 What is a Wide Neck, Intracranial Aneurysm?...2 What is the Treatment for Wide
Neuroform EZ Stent System
 Neuroform EZ Stent System Directions for Use 2 Neuroform EZ Stent System 1 cm 1.9 cm 135 cm 185 cm ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning
Neuroform EZ Stent System Directions for Use 2 Neuroform EZ Stent System 1 cm 1.9 cm 135 cm 185 cm ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning
ENDOVASCULAR THERAPIES FOR ACUTE STROKE
 ENDOVASCULAR THERAPIES FOR ACUTE STROKE Cerebral Arteriogram Cerebral Anatomy Cerebral Anatomy Brain Imaging Acute Ischemic Stroke (AIS) Therapy Main goal is to restore blood flow and improve perfusion
ENDOVASCULAR THERAPIES FOR ACUTE STROKE Cerebral Arteriogram Cerebral Anatomy Cerebral Anatomy Brain Imaging Acute Ischemic Stroke (AIS) Therapy Main goal is to restore blood flow and improve perfusion
Emerge. PTCA Dilatation Catheter ONLY. monorail. over-the-wire
 90990229-01 Emerge monorail over-the-wire PTCA Dilatation Catheter Table of Contents 2014-09 < EN > warning...1 device description...1 Table 1. Emerge Balloon Coatings...1 Contents...1 Intended use/indications
90990229-01 Emerge monorail over-the-wire PTCA Dilatation Catheter Table of Contents 2014-09 < EN > warning...1 device description...1 Table 1. Emerge Balloon Coatings...1 Contents...1 Intended use/indications
ROTABLATORTM. Peripheral. Rotational Atherectomy System. Quick Reference Cards
 Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING
 Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING 1 2013 Spectranetics. All Rights Reserved. Approved for External
Excimer Laser for Coronary Intervention: Case Study RADIAL APPROACH: CORONARY LASER ATHERECTOMY FOR CTO OF THE LAD FOLLOWED BY PTCA NO STENTING 1 2013 Spectranetics. All Rights Reserved. Approved for External
Corporate Medical Policy
 Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER. Instructions for Use
 Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English cs Čeština da Dansk nl Nederlands fi Suomi fr Français de Deutsch el Ελληνικά hu Magyar it Italiano lt Lietuvių no Norsk pl Polski pt Português es Español sv Svenska
INSTRUCTIONS FOR USE FOR: en English cs Čeština da Dansk nl Nederlands fi Suomi fr Français de Deutsch el Ελληνικά hu Magyar it Italiano lt Lietuvių no Norsk pl Polski pt Português es Español sv Svenska
Chronic Total Occlusion (CTO) Technologies
 to receive our latest news and key activities. Chronic Total Occlusion (CTO) Technologies Re-open vital channels LinkedIn page Follow us on CORDIS EMEA OUTBACK LTD Re-Entry Catheter True Lumen Re-Entry
to receive our latest news and key activities. Chronic Total Occlusion (CTO) Technologies Re-open vital channels LinkedIn page Follow us on CORDIS EMEA OUTBACK LTD Re-Entry Catheter True Lumen Re-Entry
Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully
 Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
ASAHI Corsair INSTRUCTIONS FOR USE
 ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW
 JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW DISCOVER THE VALUE OF VERSATILITY Versatility means not having to guess the morphology! Peripheral arterial lesions can present with
JETSTREAM Atherectomy System DELIVERING VERSATILITY TO RESTORE FLOW DISCOVER THE VALUE OF VERSATILITY Versatility means not having to guess the morphology! Peripheral arterial lesions can present with
Express LD Iliac. Premounted Stent System ONLY. over-the-wire
 50455844-01 STERILE - DO NOT RESTERILIZE - SINGLE USE ONLY ONLY 2017-03 < EN > Express LD Iliac over-the-wire Premounted Stent System Caution: Federal Law (USA) restricts this device to sale by or on the
50455844-01 STERILE - DO NOT RESTERILIZE - SINGLE USE ONLY ONLY 2017-03 < EN > Express LD Iliac over-the-wire Premounted Stent System Caution: Federal Law (USA) restricts this device to sale by or on the
SAMMPRIS. Stenting and Aggressive Medical Management for Preventing Recurrent Stroke and Intracranial Stenosis. Khalil Zahra, M.D
 SAMMPRIS Stenting and Aggressive Medical Management for Preventing Recurrent Stroke and Intracranial Stenosis Khalil Zahra, M.D Major points Patients with recent TIA or stroke and intra-cranial artery
SAMMPRIS Stenting and Aggressive Medical Management for Preventing Recurrent Stroke and Intracranial Stenosis Khalil Zahra, M.D Major points Patients with recent TIA or stroke and intra-cranial artery
VERNACULAR Trial & Clinical Experience with the VENOVO Venous Stent
 Stephen Black, MD VERNACULAR Trial & Clinical Experience with the VENOVO Venous Stent 1 Speaker Disclaimers The speakers presentation today is on behalf of Bard Peripheral Vascular, Inc. Any discussion
Stephen Black, MD VERNACULAR Trial & Clinical Experience with the VENOVO Venous Stent 1 Speaker Disclaimers The speakers presentation today is on behalf of Bard Peripheral Vascular, Inc. Any discussion
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Figure 1: Revolution TM Peripheral Atherectomy System Diagram. Table 1: Revolution TM Peripheral Atherectomy System Specifications Minimum Burr
 Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Complex ilio-caval revascularization in chronic venous obstruction with the Venovo Stent. Michael K. W. Lichtenberg MD, FESC
 Complex ilio-caval revascularization in chronic venous obstruction with the Venovo Stent Michael K. W. Lichtenberg MD, FESC German Venous Center Arnsberg, Germany Not available for sale or distribution
Complex ilio-caval revascularization in chronic venous obstruction with the Venovo Stent Michael K. W. Lichtenberg MD, FESC German Venous Center Arnsberg, Germany Not available for sale or distribution
FROM THE EVERYDAY TO THE EXTRAORDINARY
 FROM THE EVERYDAY TO THE EXTRAORDINARY Created with the collaboration of more than 250 physicians around the world, ENDURANT empowers you to create stronger outcomes for more patients, including those
FROM THE EVERYDAY TO THE EXTRAORDINARY Created with the collaboration of more than 250 physicians around the world, ENDURANT empowers you to create stronger outcomes for more patients, including those
LeMaitre Embolectomy Catheter
 Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Introduction 3. What is Peripheral Vascular Disease? 5. What Are Some of the Symptoms of Peripheral Vascular Disease? 6
 Patient Information Table of Contents Introduction 3 What is Peripheral Vascular Disease? 5 What Are Some of the Symptoms of Peripheral Vascular Disease? 6 What Causes Peripheral Vascular Disease? 7 How
Patient Information Table of Contents Introduction 3 What is Peripheral Vascular Disease? 5 What Are Some of the Symptoms of Peripheral Vascular Disease? 6 What Causes Peripheral Vascular Disease? 7 How
MICHAEL R. JAFF, DO MASSACHUSETTS, UNITED STATES. Medtronic Further. Together
 DRUG-COATED BALL0ON TREATMENT FOR PATIENTS WITH INTERMITTENT CLAUDICATION: INSIGHTS FROM THE IN.PACT GLOBAL FULL CLINICAL COHORT MICHAEL R. JAFF, DO MASSACHUSETTS, UNITED STATES Medtronic Further. Together
DRUG-COATED BALL0ON TREATMENT FOR PATIENTS WITH INTERMITTENT CLAUDICATION: INSIGHTS FROM THE IN.PACT GLOBAL FULL CLINICAL COHORT MICHAEL R. JAFF, DO MASSACHUSETTS, UNITED STATES Medtronic Further. Together
Proven Performance Through Innovative Design *
 Proven Performance Through Innovative Design * Deliver Our Next Generation AV Covered Stent Results The COVERA Vascular Covered Stent builds upon proven technologies from the category leader in AV Access.
Proven Performance Through Innovative Design * Deliver Our Next Generation AV Covered Stent Results The COVERA Vascular Covered Stent builds upon proven technologies from the category leader in AV Access.
COIL SYSTEM ORDERING INFORMATION
 EMBOLIZATION SYSTEM RUBY COIL SYSTEM FRAME with Ruby Standard FILL with Ruby Soft Now with 3 & 40 mm sizes! SYSTEM ANCHOR with Now designed to embolize 3 4 mm vessels! PACK with Coil Now with WAVE Shape
EMBOLIZATION SYSTEM RUBY COIL SYSTEM FRAME with Ruby Standard FILL with Ruby Soft Now with 3 & 40 mm sizes! SYSTEM ANCHOR with Now designed to embolize 3 4 mm vessels! PACK with Coil Now with WAVE Shape
NC EMERGE TM PTCA Dilatation Catheter
 LEARN MORE Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for use only in countries with applicable health
LEARN MORE Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for use only in countries with applicable health
Introduction What Causes Peripheral Vascular Disease? How Do Doctors Treat Peripheral Vascular Disease?... 9
 Patient Information Table of Contents Introduction... 3 What is Peripheral Vascular Disease?... 5 What Are Some of the Symptoms of Peripheral Vascular Disease?... 7 What Causes Peripheral Vascular Disease?...
Patient Information Table of Contents Introduction... 3 What is Peripheral Vascular Disease?... 5 What Are Some of the Symptoms of Peripheral Vascular Disease?... 7 What Causes Peripheral Vascular Disease?...
Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use
 Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use DEVICE DESCRIPTION The Detachable Azur CX 35 Peripheral Coil System (Azur system) consists of a coil implant attached to a delivery system.
Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use DEVICE DESCRIPTION The Detachable Azur CX 35 Peripheral Coil System (Azur system) consists of a coil implant attached to a delivery system.
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Patient Brochure. Clearstream Technologies, Ltd. Moyne Upper Enniscorthy Co. Wexford, Ireland. PK Rev. 0 05/17
 Patient Brochure Clearstream Technologies, Ltd. Moyne Upper Enniscorthy Co. Wexford, Ireland PK1411100 Rev. 0 05/17 LIFESTREAM Patient Brochure If you or a member of your family has been diagnosed with
Patient Brochure Clearstream Technologies, Ltd. Moyne Upper Enniscorthy Co. Wexford, Ireland PK1411100 Rev. 0 05/17 LIFESTREAM Patient Brochure If you or a member of your family has been diagnosed with
Chronic Total Occlusion (CTO) Technologies. Re-open vital channels
 Chronic Total Occlusion (CTO) Technologies Re-open vital channels OUTBACK LTD Re-Entry Catheter True Lumen Re-Entry Technology Get back into the true lumen with ease and precision There are a number of
Chronic Total Occlusion (CTO) Technologies Re-open vital channels OUTBACK LTD Re-Entry Catheter True Lumen Re-Entry Technology Get back into the true lumen with ease and precision There are a number of
Sirolimus drug chemical structure
 STERILE. Sterilized with ethylene oxide gas. Non pyrogenic. For one procedure only. Do not re-sterilize. Do not use opened or damaged packages. Keep in a cool, dark and dry place. Use the product before
STERILE. Sterilized with ethylene oxide gas. Non pyrogenic. For one procedure only. Do not re-sterilize. Do not use opened or damaged packages. Keep in a cool, dark and dry place. Use the product before
Combine the best of both worlds
 S c i e n c e B a s e d E v o l u t i o n s l e a d t o R e v o l u t i o n a r y S o l u t i o n s PIONEER CoCr STENT ON DEB Combine the best of both worlds A next step in cardiovascular treatment should
S c i e n c e B a s e d E v o l u t i o n s l e a d t o R e v o l u t i o n a r y S o l u t i o n s PIONEER CoCr STENT ON DEB Combine the best of both worlds A next step in cardiovascular treatment should
Instructions for Use ANGIOGUARD XP Emboli Capture Guidewire System
 10257072-3 Instructions for Use ANGIOGUARD XP Emboli Capture Guidewire System 1 2 This page has been This page has been 3 CAUTION: Federal (USA) law restricts this device to sale by or on the order of
10257072-3 Instructions for Use ANGIOGUARD XP Emboli Capture Guidewire System 1 2 This page has been This page has been 3 CAUTION: Federal (USA) law restricts this device to sale by or on the order of
Pocket Reference Guide For (CTO) Technologies
 to receive our latest news and key activities. Pocket Reference Guide For (CTO) Technologies LinkedIn page Follow us on CORDIS EMEA Table of Contents Essential prescribing information for both FRONTRUNNER
to receive our latest news and key activities. Pocket Reference Guide For (CTO) Technologies LinkedIn page Follow us on CORDIS EMEA Table of Contents Essential prescribing information for both FRONTRUNNER
Indications. The AngioVac cannula is intended for use as a venous drainage cannula and for the removal of fresh, soft thrombi or emboli
 Indications Straight Cannula or Cannula with 20 O Angle 17F Working Channel The AngioVac cannula is intended for use as a venous drainage cannula and for the removal of fresh, soft thrombi or emboli Radiopaque
Indications Straight Cannula or Cannula with 20 O Angle 17F Working Channel The AngioVac cannula is intended for use as a venous drainage cannula and for the removal of fresh, soft thrombi or emboli Radiopaque
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
DISRUPT PAD. (( Data Summary )) DISRUPT PAD Data Summary SPL Rev. B 2016 Shockwave Medical Inc. All rights reserved.
 DISRUPT PAD (( Data Summary )) DISRUPT PAD Data Summary SPL 60971 Rev. B 1 Summary of the key findings from the DISRUPT PAD Study 99% of femoropopliteal lesions treated were moderately or severely calcified.
DISRUPT PAD (( Data Summary )) DISRUPT PAD Data Summary SPL 60971 Rev. B 1 Summary of the key findings from the DISRUPT PAD Study 99% of femoropopliteal lesions treated were moderately or severely calcified.
Guide Wires design and selection Abbott Vascular. All rights reserved.
 Guide Wires design and selection the cardiac catheter was...the key in the lock Cournand AF, Nobel lecture December 11, 1956; nowadays, the coronary guidewire is the master key to all locks Colombo et
Guide Wires design and selection the cardiac catheter was...the key in the lock Cournand AF, Nobel lecture December 11, 1956; nowadays, the coronary guidewire is the master key to all locks Colombo et
MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES
 MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES PUSHING BOUNDARIES Terumo Interventional Systems is committed to your success with innovative procedural solutions and ongoing support for your most challenging
MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES PUSHING BOUNDARIES Terumo Interventional Systems is committed to your success with innovative procedural solutions and ongoing support for your most challenging
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Watchman. Left Atrial Appendage Closure Device. Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8
 TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
TM Watchman Left Atrial Appendage Closure Device PROOF OFLEADERSHIP Uniquely engineered for the LAA 1-3 with proven safety and longterm efficacy. 4-8 Patients with AF have a 5x increased risk of stroke.
Solving the Dilemma of Ostial Stenting: A Case Series Illustrating the Flash Ostial System
 Volume 1, Issue 1 Case Report Solving the Dilemma of Ostial Stenting: A Case Series Illustrating the Flash Ostial System Robert F. Riley * and Bill Lombardi University of Washington Medical Center, Division
Volume 1, Issue 1 Case Report Solving the Dilemma of Ostial Stenting: A Case Series Illustrating the Flash Ostial System Robert F. Riley * and Bill Lombardi University of Washington Medical Center, Division
Subclavian artery Stenting
 Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
PERFORMANCE YOU CAN TRUST. EverFlex Self-expanding Peripheral Stent with Entrust Delivery System
 PERFORMANCE YOU CAN TRUST EverFlex Self-expanding Peripheral Stent with Entrust Delivery System The Entrust Delivery System is designed to provide improved patient outcomes and procedural efficiency. The
PERFORMANCE YOU CAN TRUST EverFlex Self-expanding Peripheral Stent with Entrust Delivery System The Entrust Delivery System is designed to provide improved patient outcomes and procedural efficiency. The
Wolverine Coronary Cutting Balloon
 50482216-01 2017-02 < en > Wolverine Coronary MONORAIL OVER-THE-WIRE Microsurgical Dilatation Device Table of Contents Warning...1 device description...1 Contents...1 Quantity Material...1 Intended use/indications
50482216-01 2017-02 < en > Wolverine Coronary MONORAIL OVER-THE-WIRE Microsurgical Dilatation Device Table of Contents Warning...1 device description...1 Contents...1 Quantity Material...1 Intended use/indications
SCAI Fall Fellows Course Subclavian/Innominate Case Presentation
 SCAI Fall Fellows Course 2012 Subclavian/Innominate Case Presentation Daniel J. McCormick DO, FACC, FSCAI Director, Cardiovascular Interventional Therapy Pennsylvania Hospital University of Pennsylvania
SCAI Fall Fellows Course 2012 Subclavian/Innominate Case Presentation Daniel J. McCormick DO, FACC, FSCAI Director, Cardiovascular Interventional Therapy Pennsylvania Hospital University of Pennsylvania
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine
 Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
Stellarex Drug-coated 0.035" Angioplasty Balloon. Featuring EnduraCoat Technology. The Clear DCB Choice
 Stellarex Drug-coated 0.035" Angioplasty Balloon Featuring EnduraCoat Technology The Clear DCB Choice Top-tier outcomes with the lowest therapeutic drug dose in all patients common to complex 89.0 % patency
Stellarex Drug-coated 0.035" Angioplasty Balloon Featuring EnduraCoat Technology The Clear DCB Choice Top-tier outcomes with the lowest therapeutic drug dose in all patients common to complex 89.0 % patency
CTO Product Portfolio
 CTO Product Portfolio 1 2 CTO Recanalization Via ATHERECTOMY Occluded anterior tibial artery Tip of Crosser Catheter 3 4 Central lumen vessel recanalization by the Crosser Catheter The Crosser Catheter
CTO Product Portfolio 1 2 CTO Recanalization Via ATHERECTOMY Occluded anterior tibial artery Tip of Crosser Catheter 3 4 Central lumen vessel recanalization by the Crosser Catheter The Crosser Catheter
TYSHAK DILATATION CATHETER. Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician.
 TYSHAK DILATATION CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional Systems Inc. 824 Twelfth
TYSHAK DILATATION CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional Systems Inc. 824 Twelfth
INSTRUCTIONS FOR USE FOR: English
 INSTRUCTIONS FOR USE FOR: English INSTRUCTIONS FOR USE FOR: Carefully read all instructions prior to use. Observe all warnings and precautions noted throughout these instructions. Failure to do so may
INSTRUCTIONS FOR USE FOR: English INSTRUCTIONS FOR USE FOR: Carefully read all instructions prior to use. Observe all warnings and precautions noted throughout these instructions. Failure to do so may
Zenith Renu AAA Converter Graft. Device Description Planning and Sizing Deployment Sequence Patient Follow-Up
 Zenith Renu AAA Converter Graft Device Description Planning and Sizing Deployment Sequence Patient Follow-Up Device description: Device indications The Zenith Renu AAA Converter Graft with Z-Trak Introduction
Zenith Renu AAA Converter Graft Device Description Planning and Sizing Deployment Sequence Patient Follow-Up Device description: Device indications The Zenith Renu AAA Converter Graft with Z-Trak Introduction
Lutonix AV Clinical Trial
 Lutonix AV Clinical Trial Long Term Effects of LUTONIX 035 DCB Catheter 18 month Interim Results Scott O. Trerotola, MD Stanley Baum Professor of Radiology Professor of Surgery Associate Chair and Chief,
Lutonix AV Clinical Trial Long Term Effects of LUTONIX 035 DCB Catheter 18 month Interim Results Scott O. Trerotola, MD Stanley Baum Professor of Radiology Professor of Surgery Associate Chair and Chief,
MULTILAYER FLOW MODULATOR (MFM ) FOR PERIPHERAL ANEURYSMS TREATMENT (LOWER LIMBS, VISCERALS AND SUBCLAVIAN)
 0459 MULTILAYER FLOW MODULATOR (MFM ) FOR PERIPHERAL ANEURYSMS TREATMENT (LOWER LIMBS, VISCERALS AND SUBCLAVIAN) WITH ITS ANGIOGRAPHIC CONTROL DELIVERY SYSTEM INSTRUCTIONS FOR USE (I.F.U.) English Français
0459 MULTILAYER FLOW MODULATOR (MFM ) FOR PERIPHERAL ANEURYSMS TREATMENT (LOWER LIMBS, VISCERALS AND SUBCLAVIAN) WITH ITS ANGIOGRAPHIC CONTROL DELIVERY SYSTEM INSTRUCTIONS FOR USE (I.F.U.) English Français
Promus ELITE. Everolimus-Eluting Platinum Chromium Coronary Stent System. Patient Information Guide CV01
 Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System Patient Information Guide CV01 Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System PATIENT INFORMATION GUIDE
Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System Patient Information Guide CV01 Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System PATIENT INFORMATION GUIDE
Sustained Release. Superior Results.
 ELUVIA Drug-Eluting Vascular Stent System Sustained Release. Superior Results. Superiority determined in Post Hoc Superiority Analysis. 12-Month Primary Patency rate of 86.8% in the Eluvia arm (n=309)
ELUVIA Drug-Eluting Vascular Stent System Sustained Release. Superior Results. Superiority determined in Post Hoc Superiority Analysis. 12-Month Primary Patency rate of 86.8% in the Eluvia arm (n=309)
NuDEL STENT DELIVERY SYSTEM COARCTATION OF THE AORTA INSTRUCTIONS FOR USE
 NuDEL STENT DELIVERY SYSTEM COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 5 Right Ventricular Outflow Tract
NuDEL STENT DELIVERY SYSTEM COARCTATION OF THE AORTA AND RIGHT VENTRICULAR OUTFLOW TRACT INSTRUCTIONS FOR USE General Instructions.. Page 2 Coarctation Indication..Page 5 Right Ventricular Outflow Tract
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
FLEXIBLE, BALOON EXPANDABLE
 EARLY RESULTS OF A CLINICAL TRIAL OF FLEXIBLE, BALOON EXPANDABLE COVERED STENT GRAFT IN ILIAC OCCLUSIVE DISEASE Chris LeCroy Coastal Vascular and Interventional Pensacola, Florida Clinical Trial WL GORE
EARLY RESULTS OF A CLINICAL TRIAL OF FLEXIBLE, BALOON EXPANDABLE COVERED STENT GRAFT IN ILIAC OCCLUSIVE DISEASE Chris LeCroy Coastal Vascular and Interventional Pensacola, Florida Clinical Trial WL GORE
Understanding Peripheral
 Patient Information Guide Understanding Peripheral Artery Disease Innova Vascular Self-Expanding Stent System Table of Contents Glossary... 2 What is Peripheral Artery Disease?... 4 Treating Peripheral
Patient Information Guide Understanding Peripheral Artery Disease Innova Vascular Self-Expanding Stent System Table of Contents Glossary... 2 What is Peripheral Artery Disease?... 4 Treating Peripheral
Every heartbeat is precious. Thanks to you, he ll have 100 million more.
 Every heartbeat is precious. Thanks to you, he ll have 100 million more. Master the Complex Thrombectomy Portfolio FETCH 2 Aspiration Catheter ANGIOJET Ultra Thrombectomy System EVERY HEARTBEAT IS PRECIOUS
Every heartbeat is precious. Thanks to you, he ll have 100 million more. Master the Complex Thrombectomy Portfolio FETCH 2 Aspiration Catheter ANGIOJET Ultra Thrombectomy System EVERY HEARTBEAT IS PRECIOUS
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Cook Medical. Zenith Flex AAA Endovascular Graft with Z-Trak Introduction System Physician Training
 Cook Medical Zenith Flex AAA Endovascular Graft with Z-Trak Introduction System Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full-thickness,
Cook Medical Zenith Flex AAA Endovascular Graft with Z-Trak Introduction System Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full-thickness,
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
