Towards the Emulation of the Cardiac Conduction System for Pacemaker Testing
|
|
|
- Josephine Hill
- 6 years ago
- Views:
Transcription
1 1 Towards the Emulation of the Cardiac Conduction System for Pacemaker Testing Eugene Yip, Sidharta Andalam, Partha S. Roop, Avinash Malik, Mark Trew, Weiwei Ai, and Nitish Patel arxiv: v2 [cs.sy] 18 Mar 2016 Abstract The heart is a vital organ that relies on the orchestrated propagation of electrical stimuli to coordinate each heart beat. Abnormalities in the heart s electrical behaviour can be managed with a cardiac pacemaker. Recently, the closed-loop testing of pacemakers with an emulation (real-time simulation) of the heart has been proposed. An emulated heart would provide realistic reactions to the pacemaker as if it were a real heart. This enables developers to interrogate their pacemaker design without having to engage in costly or lengthy clinical trials. Many high-fidelity heart models have been developed, but are too computationally intensive to be simulated in real-time. Heart models, designed specifically for the closed-loop testing of pacemakers, are too abstract to be useful in the testing of physical pacemakers. In the context of pacemaker testing, this paper presents a more computationally efficient heart model that generates realistic continuous-time electrical signals. The heart model is composed of cardiac cells that are connected by paths. Significant improvements were made to an existing cardiac cell model to stabilise its activation behaviour and to an existing path model to capture the behaviour of continuous electrical propagation. We provide simulation results that show our ability to faithfully model complex re-entrant circuits (that cause arrhythmia) that existing heart models can not. Index Terms cardiac, electrophysiology, emulation, hybrid, automata, modelling. I. INTRODUCTION The human heart is a vital organ and is responsible for pumping blood around the body to other vital organs. Patients can develop abnormal cardiac behaviour, such as bradycardia (slow heart rate). Cardiac pacemakers can treat bradycardia by monitoring the patient s heart and delivering electrical stimuli to the heart when needed. Pacemakers are life-critical medical devices that must be certified against stringent safety standards, such as IEC [1]. Certification is a costly and time consuming process, yet 1,210 computer-related recalls for medical devices were reported to the US Food and Drug Administration between 2006 and 2011 [2]. Pacemakers must be validated by clinical trials as part of the certification process. This requires the pacemaker to be tested in closed-loop with a patient s heart. Since clinical trials are the only times when a pacemaker is tested on a real heart, they provide a glimpse of how well the pacemaker performs in the real world. Clinical trials are usually performed late in the product development phase, because they are costly and time E. Yip was and S. Andalam, P. S. Roop, A. Malik, W. Ai, and N. Patel are with the Department of Electrical and Computer Engineering, the University of Auckland, New Zealand. M. Trew is with the Auckland Bioengineering Institute, the University of Auckland, New Zealand. s: {eyip002, wai484}@aucklanduni.ac.nz and {sid.andalam, p.roop, avinash.malik, nd.patel, m.trew}@auckland.ac.nz consuming to manage. Thus, issues found during a clinical trial may be costly and time consuming to fix and a new clinical trial may be required to re-evaluate the pacemaker. Some limitations of clinical trials include: potentially small sample of patients that are not representative of the general population, difficulty in recruiting patients with specific heart conditions, difficulty in interrogating a patient s heart to better understand design issues, and inherent risk to the patients. Recently, the emulation of the heart has been proposed to facilitate the closed-loop testing of pacemakers [3]. Emulation is the real-time simulation of a heart model that can react to a pacemaker s electrical shocks and also output the heart s electrical activities for the pacemaker to sense. High-fidelity heart models provide realistic behaviour but are computationally intensive [4], [5], thus, precluding them from emulation. The following benefits can be gained if high-fidelity heart models can be emulated: cheaper and quicker testing than with clinical trials, earlier testing of pacemakers in closedloop in the development phase and outside of clinics, greater testing coverage by emulating a range of heart conditions, better understanding of design issues by interrogating the emulated heart (e.g., replaying problematic test cases), and having minimal risk to the patients. We envision the use of emulated hearts alongside clinical trials to help accelerate the certification process. In the context of testing cardiac pacemakers, a heart model should possess the following properties: Abstraction: The model focusses on the important aspects by ignoring irrelevant details. For example, the cardiac conduction system is the most important aspect because it is responsible for coordinating the heart s electrical activities. Irrelevant details may include hemodynamics (e.g., blood flow), mechanics (e.g., muscle movement), and chemistry (e.g., cellular reactions). Accuracy: The model faithfully represents the cardiac conduction system and demonstrates realistic behaviours. A high-fidelity model provides an accurate reflection of reality but requires high computational power. A lower fidelity model requires less computational power but at the risk of providing an inaccurate reflection of reality. Prediction: The model can answer questions about a real heart, such as How does the heart respond when setting X of the pacemaker is used? Inexpensiveness: The model should be cheaper and faster to construct and use the emulated heart than to conduct a clinical trial. The heart models of Chen et al. [6], Jiang et al. [7], and
2 2 Méry and Singh [8] consider just the emergent features of the cardiac conduction system, which is composed of millions of cells. They model the conduction system as a static, twodimensional, sparse network of cardiac cells. Jiang et al. [3] also developed a hardware prototype that emulates the cardiac conduction system as discrete events. When the logic of a pacemaker s software is tested in closed-loop with a heart model, it may be sufficient to use a heart model that produces and responds to discrete events [7], [8]. However, when a physical implementation of the pacemaker is tested in closedloop with a heart model, it is necessary to use a heart model that produces and responds to continuous-time signals. This is because the physical pacemaker expects a real heart as its environment. The heart model of Chen et al. [6] simulates the conduction system as continuous-time signals. However, the signals are too abstract and bear little resemblance with reality. Thus, the model lacks the accuracy and, therefore, the predictive power. A. Contributions This paper reviews the state-of-the-art heart models [3], [6] [9] that have been designed specifically for the closed-loop testing of cardiac pacemakers. Without introducing significant computational complexity, we propose significant improvements to the modelling of the cardiac conduction system to create a heart model that produces realistic continuous-time electrical signals that a pacemaker would sense. Our model faithfully models forward and backward conduction, which is essential in the modelling of complex re-entrant circuits [10] [12] that cause arrhythmia (abnormal heart rate). Our primary contributions are: We develop a continuous-time model of the conduction system as a two-dimensional network of cardiac cells. Each cell produces an accurate continuous-time signal that represents its electrical activities. These signals are propagated continuously along the paths between the cells. Complex conduction behaviours, such as arrhythmias caused by re-entrant circuits, can be reproduced faithfully by our model. Our heart model is easily customised by modifying various parameters of the conduction system. Each cardiac cell in our heart model is based on the hybrid automaton developed by Ye et al. [13]. We have greatly improved the design of the hybrid automaton to overcome the following limitations: the cell becomes unstable when it is stimulated in quick succession, and the cell is too sensitive to electrical stimulation from its neighbours. Our improvements are elaborated in Section IV. Each path in our heart model is modelled with timed automata. The path model is inspired by that of Jiang et al. [7] that was designed to propagate discrete events rather than continuous-time signals. Our path model is elaborated in Section V. We demonstrate in Section VII that a MathWorks R Simulink R and Stateflow R implementation of our heart model can simulate a wide range of heart conditions with realistic results. Fig. 1. Schematic of the heart and conduction system. B. Paper Layout Section II provides a background to the cardiac conduction system and the important features of electrical activities that are sensed by a pacemaker. Section III reviews the state-ofthe-art heart models for closed-loop testing of pacemakers. Section IV reviews the computationally efficient hybrid automata model of cardiac cells developed by Ye et al. [13]. We identify the limitations encountered with Ye et al. s model during simulation and how we corrected them. Section V describes our path model that handles the propagation of continuous-time signals. In Section VI, we create our proposed heart model by composing instances of our cardiac cell and path models into a network that replicates the conduction pathway. Section VII evaluates the capabilities of our proposed heart model with the recent heart model of Chen et al. [6]. Section VIII concludes this paper and discusses future work for improving the proposed heart model. II. BACKGROUND The heart pumps blood around the body in a rhythmic manner. Figure 1 is a schematic of the heart and shows its four chambers: the right and left atriums and ventricles. The right atrium and ventricle are responsible for pumping deoxygenated blood through the lungs, while the left atrium and ventricle are responsible for pumping oxygenated blood through the body. The contractions of the chambers are coordinated by electrical stimuli that propagate throughout the heart s conduction system. The conduction pathways are shown in Figure 1 as solid black lines with dots at important locations. The names of these locations are labelled with an acronym and their full forms are given in Table I. A. Cardiac Cycle This section describes the major actions of the heart during one cardiac cycle (one heart beat) with the help of Figure 2. In the first phase of the cardiac cycle, Figure 2a, the sinoatrial (SA) node generates an electrical stimulus that spreads quickly throughout the right and left atriums. This causes
3 3 TABLE I FULL NAMES OF NODES ALONG THE CONDUCTION PATHWAYS. AV Atrioventricular LVS Left ventricular septum BB Bachmann s bundle OC Os cordis BH Bundle of His RA Right atrium CS Coronary sinus RBB Right bundle branch CT Crista terminalis RV Right ventricle FP Fast path RVA Right ventricular apex LA Left atrium RVS Right ventricular septum LBB Left bundle branch SA Sinoatrial LV Left ventricle SP Slow path LVA Left ventricular apex Fig. 3. Phases of the action potential. Adapted from [14]. arrhythmias that a heart model should aim to capture. (a) Phase 1 (b) Phase 2 (c) Phase 3 (d) Phase 4 Fig. 2. Phases of the cardiac cycle. Adapted from anatomy-book/contents/m46664.html#sinoatrial-sa-node. the atriums to contract, pumping blood from the atriums into the ventricles. In the second phase, Figure 2b, the electrical stimulus reaches the atrioventricular (AV) node and is delayed momentarily before it continues down into the ventricles. This delay is very important because it gives the atriums enough time to contract and fully fill the (relaxed) ventricles. In the third phase, Figure 2c, the electrical stimulus reaches the right and left ventricular apexes and travels out to the fast conducting Purkinje fibers. This causes the right and left ventricles to contract and pump out blood. In the fourth phase, Figure 2d, the ventricles relax after pumping out all their blood. In a normal heart, each cardiac cycle begins from the SA node, which generates periodic electrical stimuli that spread through the conduction system. The following sections describe the genesis of the heart s electrical activity and how it appears to a pacemaker. Finally, we describe some common B. Action Potentials of Cardiac Cells Most of the heart s electrical activities, that a pacemaker senses, are generated by the myocytes (muscle cells) [15]. A cell s electrical activities result from the movement of ions across its membrane, creating potential differences. The cell s electrical response to an electrical stimulus is described by its action potential [16]. Figure 3 shows the four phases of a typical action potential, which plots the cell s membrane potential over time. In the resting phase, the cell is inactive and has a resting potential of approximately 85mV. The cell enters the stimulated phase when excited electrically by its neighbours or by an artificial pacemaker. The cell returns to the resting phase if its membrane potential fails to cross the threshold voltage V T of approximately 40mV when the excitation stops. Otherwise, the cell enters the upstroke phase and depolarises by allowing ions to move rapidly across its membrane, causing its membrane potential to reach an overshoot voltagev O of approximately+45mv. Then the cell enters the plateau and early repolarisation phase. The cell contracts and starts to repolarise, i.e., its membrane potential starts to return to its resting potential. When the membrane potential is less than the voltagev R of approximately 55mV, the cell has relaxed and returned to the resting phase. All cardiac cells can only respond to subsequent excitations in the later portion of its action potential, called the relative refractory period. However, the membrane potential must cross a higher threshold voltage. Figure 4 shows a normal action potential at 0ms and some possible secondary excitations between 160 and 300ms. For a secondary excitation at 180ms, the resulting action potential has a lower overshoot voltage V O and a shorter action potential duration. The secondary excitation at 300ms results in a more normal action potential because the cell has rested for a longer period. Prominent biophysical cardiac cell models, which explain the genesis of action potentials in terms of ionic flow, include Luo-Rudy [16] and Hodgkin-Huxley [18]. Although biophysical models have high-fidelity, they are computationally intensive. Ye et al. [13], [19] create computationally efficient cardiac cell models by considering just the emergent features of the biophysical models, i.e., the action potential
4 4 (a) Three action potentials (APs) from different regions of the heart and their corresponding electrograms (EGMs). Adapted from [21]. Fig. 4. Dynamic behaviour of secondary excitations. Adapted from [17]. and its dynamic response to secondary excitation. It should be noted that the action potential duration of a human ventricular myocyte is approximately twice that of an atrial myocyte. C. Action Potentials and the Electrogram A key function of any pacemaker is to sense the heart s electrical activity, by using one or more electrodes attached to the inside of the heart wall. The electrical activity of the cardiac cells in the electrode s immediate vicinity are sensed most strongly. A recording of the sensed activities is called an electrogram (EGM) [20]. Figure 5a shows three action potentials and their corresponding EGMs. To help understand the EGM, Figure 5b shows that the EGM deflects up and down whenever an electrical wavefront passes under the electrodes [15]. The faster that the wavefront passes, the steeper the deflection. In Figure 5a, two distinct deflections can be seen in each EGM and they correspond with the upstroke and resting phases of their respective action potential. A heart model that produces distorted action potentials will also produce distorted EGMs. Such distorted EGMs cannot be used to reliably test a pacemaker s ability to discern the timing of important cardiac activities. Moreover, the predictive power of a heart model is compromised when the action potentials are distorted. For example, a heart model with accurate action potentials might predict that a cell goes into its upstroke phase because its neighbours voltages are high enough. However, a heart model with distorted action potentials might instead predict that the cell returns to its resting phase because its neighbours voltages are too low. Thus, arrhythmia would be predicted incorrectly. D. Common Arrhythmias Arrhythmias can be caused by abnormalities in the generation and propagation of action potentials through the conduction system. The abnormalities may be due to congenital defects, side-effects of medication, or cell death. The following (b) EGM deflections due to a travelling electrical wavefront. Adapted from [15]. Fig. 5. Electrograms (EGMs). are some common arrhythmias [11], [12], [20] that a heart model for pacemaker testing should aim to capture: Heart block: This occurs when electrical stimuli has difficulty propagating through the AV node. The propagation of the stimuli may be delayed for longer than usual or may be prevented from propagating altogether. AV node re-entrant tachycardia: This occurs when a re-entry circuit forms around the AV node, causing tachycardia. Bundle branch block: This occurs when electrical stimuli travels slower or not at all down one of the bundle branches. Wolff-Parkinson-White syndrome: This occurs when there is an extra conduction pathway between the atriums and ventricles. The extra pathway allows electrical stimuli to bypass the AV node and create a feedback loop between the atriums and ventricles. Long Q-T syndrome: This occurs when the repolarisation of the ventricles is delayed, i.e., their action potential durations are longer than usual. VA conduction: This occurs when electrical stimuli from the ventricles conduct backwards through the conduction pathways and into the atriums. Pacemakers can also cause arrhythmias when they are unable to correctly sense the timing of the heart s electrical activities. For example, pacemaker-mediated tachycardia is caused by the pacemaker inadvertently conducting electrical stimuli from the ventricles back to the atriums. Pacemakers that deliver electrical stimuli that are not synchronised with
5 5 TABLE II QUALITATIVE COMPARISON OF HEART MODELS THAT ARE DESIGNED FOR TESTING CARDIAC MEDICAL DEVICES. AP = ACTION POTENTIAL. HA = HYBRID AUTOMATA. TA = TIMED AUTOMATA. Cell Model Path Model Spatial Model Reality Less Abstract Heart Models More Abstract Real Heart [22] Hi-Fi [23], [24] UoA Oxford [6] UPenn [3], [7] LORIA [8] MES [9] Continuous APs from biophysical processes [25] Continuous propagations from biophysical processes [10] 3D tissue (layers of bundles of fibers) that deforms Continuous APs from biophysical models [25] Continuous propagations from reactiondiffusion equations [25] 3D finite-volume that deforms Continuous APs from improved Stony Brook HA [13] Continuous propagations from TA and contribution function Continuous APs from simplified Stony Brook HA [19] Continuous propagations from contribution function Discrete APs from TA Discrete propagations from TA Discrete APs from logicomathematics Discrete propagations from cellular automata 2D, static, and sparse network of cells along the conduction pathway Continuous AV signal generators mimic whole heart electrical activity Black boxes of major heart components the heart s cardiac rhythm can cause the heart to fibrillate [26], i.e., twitch uncontrollably. III. RELATED WORK The electrophysiology of the heart has been well researched [10], [27], resulting in the proposal of many theories. These theories are validated by creating high-fidelity whole heart models [23], [24] and ascertaining if they can reproduce experimental observations, i.e., the models are accurate, realistic, and predictive. These high-fidelity models are useful in predicting the prognosis of patient-specific heart conditions [28] and in assisting with interventional cardiology [29]. On the other hand, abstract heart models have been developed with the goal of enabling the closed-loop testing of cardiac pacemakers. Table II provides a qualitative comparison of existing heart models. The abstract heart models from Oxford [6], UPenn [3], [7], LORIA [8], and MES [9] are designed for testing the pacemaker logic. To enable the formal verification of the pacemaker logic, Oxford, UPenn, and LORIA use hybrid automata (HA) or timed automata (TA) to develop formal models of the cardiac conduction system. UPenn and LORIA model the transitions between the resting, upstroke, and early refractory phases of the action potential as discrete events on a continuous timeline. These discrete events are propagated between cells and the propagation is either successful or unsuccessful. These abstractions result in heart models that may produce more behaviours than is possible by a real heart, i.e., an over-approximation. Thus, all problems detected during closed-loop testing must be validated against a more concrete heart model [7]. The heart model from Oxford [6] is more concrete than those from UPenn and LORIA because Oxford models the action potentials as continuous signals. Oxford uses a simplified model of the cardiac cell that Ye et al. [19] developed with hybrid automata. Oxford incorporates a g( v) function into the cell model to capture the continuous electrical activity that a cell receives from its neighbours. However, the g( v) function does not consider the directional behaviour of electrical propagation due to the refractory period of cardiac cells. Noting these limitations, Sections IV and V describe our Fig. 6. Comparison of the action potentials (APs) produced by Luo-Rudy [16], Stony Brook [13], Oxford [6], and our improved version (UoA). Note that the AP of UoA overlaps that of Stony Brook s because they are identical. improvements to the cell and path models. Our heart model can faithfully simulate complex re-entrant circuits without a significant increase in computational complexity. IV. CARDIAC CELL MODEL The Oxford heart model [6] uses a simplified version of the isolated cardiac cell model developed by Stony Brook [19]. The Stony Brook model is itself a simplification of the Luo- Rudy model [16] because it only models the action potential and its dynamic response to secondary excitation. The Stony Brook model uses three piecewise-continuous variables, called v x, v y, and v z, to capture different features of the action potential. The sum of these three variables produces the action potential. The Oxford heart model discards the v y and v z variables and retains just the v x variable. Unfortunately, Figure 6 shows that the resulting action potential is no longer realistic and this diminishes the predictive power of Oxford s heart model (Section II-C). Thus, for our heart model, we retain all three variables and we use an updated Stony Brook cardiac cell model [13]. This section describes the Stony Brook model in more detail and our improvements that overcome some of the model s limitations.
6 6 q 0 : Resting & FR v x = α 0 xv x v y = α 0 yv y v z = α 0 zv z v = v x v y +v z {v V R } [v V R ] q 3 : Plateau & ER v x = α 3 xv x v y = α 3 yf(θ)v y v z = α 3 zv z v = v x v y +v z {v V R } [V in 0] θ = v/v R [V in 0 v < V T ] [v V O 80.1 θ] q 1 : Stimulated v x = α 1 xv x +β x V in v y = α 1 yv y +β y V in v z = α 1 zv z +β z V in v = v x v y +v z {v V T } [v V T ] q 2 : Upstroke v x = α 2 xv x v y = α 2 yv y v z = α 2 zv z v = v x v y +v z {v V O 80.1 θ} Fig. 7. Stony Brook cardiac cell model [13]. TABLE III COEFFICIENTS AND CONSTANTS IN THE STONY BROOK CARDIAC CELL MODEL [13]. α 0 x = α 1 x = α 2 x = α 3 x = α 0 y = α 1 y = α 2 y = α 3 y = α 0 z = α 1 z = α 2 z = α 3 z = V R = 30 β x = V T = 44.5 β y = V O = β z = Fig. 8. Action potentials shorten unnaturally for the Stony Brook model. Stimulated at 200ms intervals 150 v (mv ) * 0 * 200 * 400 * 600 * 800 * 1,000 1,200 Time (ms) Fig. 9. Action potentials for the UoA model when stimulated at 200ms intervals. A. Stony Brook Cardiac Cell Model The Stony Brook model [13] models the time course of the action potential with the hybrid automaton (HA) shown in Figure 7. The four phases of the action potential, described in Section II-B, are represented as four locations in the HA. In each location, the membrane potential is defined by the variable v as the sum of the variables v x, v y, and v z. The rates at which the variables v x, v y, and v z change are defined by their derivatives v x, v y, and v z, respectively. The values of the coefficients and constants are given in Table III. Note that the Stony Brook model offsets all the voltages such that the resting potential is at 0mV. By default, the HA always starts in location q 0, which is the resting phase of the action potential. It stays in q 0 as long as the invariant v V R is true. The HA transitions from locationq 0 toq 1 when the voltagev in around the cell is greater than 0mV. During this transition, the last values of v x, v y, and v z when leaving q 0 are used to set their initial values when entering q 1. Recall from Figure 4 that the amplitude and duration of the action potential depends on how long the cell had been in the resting phase when it is stimulated. This is approximated by assuming that the closer the cell s membrane potential is to0mv, the longer the cell has been in locationq 0. Thus, the time that the cell had been in the resting phase is approximated by normalising the membrane potential v against the voltage V R, i.e., θ = v/v R. In location q 1, the rate at which the cell s membrane potential increases depends on the strength of V in. The HA transitions back to location q 0 if the voltage V in around the cell fails to stimulate the cell above the threshold voltage V T. However, if the cell s membrane voltage v is stimulated above V T, then the HA transitions to location q 2. The amplitude of the cell s membrane potential is calculated as V O 80.1 θ, i.e., it depends on how long the cell had been in the resting phase. The HA transitions to location q 3 and the cell s membrane potential starts to drop. The rate of repolarisation is determined by the function f(θ) and depends on how long the cell had been in the resting phase: f(θ) = 0.29e 62.89θ +0.70e 10.99θ (1) A higher value for f(θ) means a faster rate of repolarisation. Once the cell s membrane voltage v drops below the resting voltage V R, the HA transitions back to q 0. B. Improvement: Stabilising the Action Potential The bottom plot in Figure 8 shows the action potentials produced by the Stony Brook model when stimulated at 200ms intervals. We can see that the duration of the action potentials shorten towards zero over time. This is unnatural because the action potentials should settle to a constant duration when stimulated at a constant interval [30]. The top three plots in Figure 8 show the values of v x, v y, and v z, respectively, over time. Note that v x describes the initial voltage drop of the
7 7 q 0 : Resting & FR v x = α 0 xv x v y = α 0 yv y v z = α 0 zv z v = v x v y +v z {v V T } [v V R ] q 3 : Plateau & ER v x = α 3 xf(θ)v x v y = α 3 yf(θ)v y [h( v) > V T ] θ = v/v R [h( v) 0 v < V T ] [v V O 80.1 θ] q 1 : Stimulated v x = α 1 xv x +β x h( v) v y = α 1 yv y +β y h( v) v z = α 1 zv z +β z h( v) v = v x v y +v z {v V T } [v V T ] q 2 : Upstroke v x = α 2 xv x v y = α 2 yv y Fig. 11. Relationship between the action potential duration (APD) and the value of f(θ). v z = α 3 zv z v = v x v y +v z {v V R } Fig. 10. Improved cardiac cell model (UoA). v z = α 2 zv z v = v x v y +v z {v V O 80.1 θ} plateau phase. We can see that thev x variable takes too long to decrease in location q 0. Thus, each time the cell is stimulated, it enters location q 1 with a slightly higher value for v x. This causes the values of θ and f(θ) to increase over time. An increasing value of f(θ) causes a faster rate of repolarisation and, hence, shorter action potential durations. To prevent the unnatural shortening of the action potential, the value of v x needs to be closer to zero when the cell transitions from location q 3 to q 0. Thus, we increase the rate at which v x decreases towards zero by including the function f(θ) in location q 3 for v x. The improved HA is shown in Figure 10. Figure 9 shows that the action potentials produced by the improved HA have constant durations when stimulated at 200ms intervals. C. Improvement: Bounding the Rate of Repolarisation In the Stony Brook model, the action potential duration approaches zero if the cell was stimulated shortly after entering location q 0. This behaviour is unnatural because the minimum action potential duration that a cardiac cell can achieve is approximately 40ms. This problem is due to the function f(θ). When the HA returns to location q 0 from q 3, the value of v is V R. An immediate stimulation to location q 1 would set θ = V R /V R = 1. Consequently, f(1) = , which causes an extremely fast rate of repolarisation. To prevent such a fast rate of repolarisation from occurring, we limit the maximum value that function f(θ) can return. Figure 11 shows that action potential durations of 35 ms are produced when f(θ = 0.04) = Thus, we impose a maximum value of for the function f(θ): { 0.29e f(θ) = 62.89θ +0.70e 10.99θ if θ < if θ 0.04 D. Discussion We improved the original Stony Brook cardiac cell model [13] to stabilise the action potentials and to impose a reasonable minimum action potential duration. Figure 9 shows that the shape of the action potentials generated by the improved model remains realistic. However, it is important to demonstrate that the action potential durations vary realistically in response to the timing of secondary excitations (see Figure 4 for illustration). This dynamic behaviour can be evaluated with a restitution curve [30]. To plot the restitution curve, the cardiac cell is electrically stimulated at a constant time period, called the base cycle length (BCL). The BCL consists of two time intervals: the action potential duration followed by the diastolic interval. The diastolic interval begins when the action potential falls to 10% of its peak amplitude. For a range of BCLs, the action potential duration from the tenth BCL is plotted against the diastolic interval from the ninth BCL. Figure 12 shows the restitution curves for the Stony Brook [13], Oxford [6], and our improved (UoA) models. These models were implemented in MathWorks R Simulink R /Stateflow R. The Oxford implementation was provided by the original authors [6]. The restitution curve of the Stony Brook model has been demonstrated [13] to behave realistically compared to the Luo-Rudy model. However, because the Stony Brook model is unstable, we could only reproduce its restitution curve by taking the action potential duration and diastolic interval of the first BCL. The restitution curve for our improved model shows dynamic behaviour that is very similar to Stony Brook. The difference is due to the changes described in Section IV-B. The parameters in the cell model can be tuned to produce a variety of restitution curves and is particularly useful when modelling diseased cardiac cells. The Oxford model, on the other hand, shows unrealistic behaviour. The action potential duration is either 9ms or 98ms, depending on whether the diastolic interval is greater or less than 51ms. V. CARDIAC PATH MODEL The Oxford heart model [6] models the conduction system as a sparse network of cells (Figure 1). The cells are connected electrically by a voltage contribution function g k ( v), which calculates the voltage induced at cell k by its neighbours: g k ( v) = n v i (t δ ki )a ki v k d k (2) i=1
8 8 (a) Propagation from cell 1. (b) Propagation has reached cell 5. (c) Another propagation from cell 10. (d) Propagations collide. Fig. 12. Electrical restitution curves comparing the dynamic behaviour of Luo-Rudy [16], Stony Brook [13], Oxford [6], and our improved version (UoA). Cell 1 gk( v) Cell 2 Cell 1 (mv ) Cell 2 (mv ) * Time (ms) Fig. 13. Two cardiac cells connected electrically by the Oxford voltage contribution function g k ( v). For a given cell k with n connected neighbours, v = (v 1 v n ) is a vector of all the neighbours membrane potentials, such that v i is the membrane potential of neighbour i. The membrane potential of cell k is v k. The time for cell i s action potential to propagate to cell k, called the conduction time, is represented by δ ki. Thus, v i (t δ ki )a ki represents the membrane potential of cell i that reaches cell k after a delay of δ ki and with a gain of a ki. The strength of cell k s membrane potential relative to its neighbours is taken into account by v k d k, where d k is a distance coefficient. Figure 13 plots the action potentials of two cardiac cells connected by Oxford s function g k ( v). Only cell 1 receives an external stimulus at 10ms and the time delay between the cells is 90ms. The expected behaviour is for cell 1 to produce an action potential that stimulates cell 2 to produce an action potential. Cell 2 s action potential would not propagate back to cell 1 because of the refractory feature of cardiac cells [11]. However, Figure 13 shows that both cells produce a sequence of action potentials. This is because the term v i (t δ ki ) in equation (2) requires cell 2 s action potential to be propagated back to cell 1 after a time delay. This incorrect positive feedback behaviour is shown in Figure 13 as arrows between the action potentials. To properly model the propagation of action potentials along a path, its behaviour in real cardiac tissue needs to be reviewed. (e) Propagations annihilated. (f) The path model mimics the propagation along a chain of cells. Fig. 14. Propagation and collision of electrical stimuli along cardiac tissue. Figure 14a shows a chain of cardiac cells where only cell 1 has entered its upstroke phase. The cells membrane potentials are plotted above the cells along with their corresponding HA location. In Figure 14a, cell 1 s membrane potential starts to stimulate cell 2 to enter the upstroke phase. Cell 2 will then stimulate its neighbour and so on. In Figure 14b, cell 5 enters the upstroke phase, while cells 1 to 4 have entered the plateau and early repolarisation phase. Cells 1 to 4 are unresponsive to any electrical stimuli applied to them. This refractory feature forces an action potential to propagate in one direction along a path. When two action potentials propagate towards each other (Figures 14c), they will collide (Figure 14d) and annihilate each other (Figure 14e), i.e., the action potentials will not pass through each other. A computationally efficient heart model can be created by replacing chains of cardiac cells with paths that mimic the propagation of action potentials (Figure 14f). The path model of UPenn [7] does consider the refractory feature of cardiac cells and can model the collision of electrical stimuli. However, only the propagation of discrete action potential events, rather than continuous-time signals, are modelled. Moreover, a cell can only be stimulated by the electrical activity of one neighbour at a time. We propose a new path model that handles the collision of continuous-time action potentials and calculates the overall voltage induced by a cell s neighbours with a reaction-diffusion [10] function. A. Improved Path Model Our timed automaton (TA) for determining whether an action potential can propagate along a path is shown in Figure 15a. To keep the path model simple, we assume that the path is only long enough for one complete action potential to propagate through. That is, we assume that the duration of the propagating action potential is longer than its conduction
9 9 [t δ ij ] start i! last := i t := 0 relay i t δ j [t δ j ] [t δ i ] relay j t δ i [t δ ji ] start j! last := j t := 0 wait i t δ ij [last = 0 last = i] [last = j ap j (t δ ji ) = q 0 ] direction i t = 0 [ap i = q 2 ap j q 2 ] t := 0 [ap i = q 2 ap j = q 2 ] [last = j ap j (t δ ji ) q 0 ] idle [ap j = q 2 ap i q 2 ] t := 0 [ap i q 2 ap j q 2 ] last := 0 direction j t = 0 [last = i ap i (t δ ij ) q 0 ] [last = 0 last = j] [last = i ap i (t δ ij ) = q 0 ] wait j t δ ji [ap j = q 2 ] annihilate [ap i = q 2 ] (a) TA to determine which action potentials can propagate. (b) TA to relay the action potential of cell i at cell j. (c) TA to relay the action potential of cell j at cell i. Fig. 15. Improved path model (UoA) consisting of a TA to detect collisions and two TA to relay the action potentials. time through the path. An action potential from cell i will fail to propagate to cell j under the following circumstances: Cell j enters the upstroke phase during the conduction time. A recent propagation from cell j to cell i failed to stimulate cell i, i.e., a partial propagation, and the cells in between have not returned back to their resting phase. To recognise these circumstances, the TA requires the following information of the cardiac cells (Figure 10): ap i and ap j : The phase of cell i and j s action potential. ap i (t δ ij ): The phase of cell i s action potential when it reaches cell j after a conduction time of δ ij. ap j (t δ ji ): The phase of cell j s action potential when it reaches cell i after a conduction time of δ ji. In addition, the local variable last is needed to remember the direction of the last propagation: last = 0 if the last propagation was annihilated i if the last propagation started from cell i j if the last propagation started from cell j In Figure 15a, the left half of the TA determines if an action potential from cell i can propagate to cell j. The TA begins in the idle location and transitions to the annihilate location if both cells i and j go into the upstroke phase q 2. A transition back to the idle location is made when both cells exit their upstroke phase q 2. If only cell i goes into the upstroke phase q 2, then a transition from the idle to direction i location is made. Next, if a partial propagation from cell j occurred recently, then a transition to the annihilate location is made. Otherwise, a transition to the wait i location is made. The TA transitions to the annihilate location if cell j enters the upstroke phase q 2 during cell i s conduction time δ ij. Otherwise, the TA remains in the wait i location until the conduction time has elapsed, at which point the signalstart i is emitted (denoted by! ) and a transition to the relay i location is made. Next, a transition to the idle location is made after a time of δ j has elapsed to ignore cell j s upstroke phase. The right half of the TA uses similar logic to determine if an action potential from cell j can propagate to cell i. The TA in Figure 15b relays cell i s action potential at cell j when the signal start i is received (denoted by? ). The relay stops when cell j has successfully depolarised or when cell i s action potential reaches the resting phase q 0. Similar logic is used by the TA in Figure 15c to relay cell j s action potential at cell i when the signal start i is emitted. A cell can be connected to multiple neighbours and, therefore, receive multiple action potentials simultaneously. We determine the voltage induced at cellk by itsnneighbours with the following reaction-diffusion [10] function: h k ( v) = n i=1 Γ ik σ ik A m C m (v out i v k ) (3) where Γ ik is the cross-sectional area (units of mm 2 ) from cell i tok, σ ik is the electrical conductivity (units ofms/mm) from cell i to k, A m is cell k s surface area to volume (units of mm 2 ), C m is cell k s specific membrane capacitance (units of µf/mm 2 ), and vi out is cell i s membrane potential after propagating along the path. B. Discussion We have developed an improved path model specifically for continuous-time action potentials. We can successfully model propagations that are annihilated by a full or partial propagation from the opposite direction. This allows us to model conduction block that only occur in one direction, e.g., due to the source-sink relationship [11], which the UPenn path model [7] can not. Bartocci et al. [31] modelled cardiac tissue with a high-resolution grid of Stony Brook cells [13]. In
10 10 Cell i Path Cell j Cell i Cell j Path ik Path jk Cell k (a) Full and partial propagations. (b) Multiple neighbours needed to stimulate a cell. Fig. 16. Examples of propagation behaviour that can be modelled by the improved path model, but not by the Oxford [6] or UPenn [7] path models. that model, the cells are connected electrically by a reactiondiffusion equation because the conduction times between the cells are not significant. Figure 16a demonstrates two cells i and j with a conduction block from cell j to i. The conduction time between the cells is 30ms. At 10ms, cell i generates an action potential that successfully stimulates cell j. At 260ms, cell j generates an action potential that does not stimulate cell i because of conduction block. Shortly after at 360ms, cell i generates an action potential that does not reach cell j because the cells along the path have not returned back to the resting phase q 0. At 710ms, cell i generates an action potential that successfully stimulates cell j. Figure 16b demonstrates a cell k that will only stimulate if the action potentials of its neighbours, cells i and j, arrive together. At times 10ms and 260ms, cells i and j, respectively, generate action potentials that take 30ms to propagate to cell k. However, the voltage induced on cell k is neither strong enough nor long enough to stimulate cell k. At time 610ms, both neighbours generate action potentials that arrive simultaneously at cell k. This time, the voltage induced on cell k is enough to stimulate it. Figure 17 demonstrates atrioventricular node re-entrant tachycardia (AVNRT [12]), caused by dual pathways around the atrioventricular (AV) node shown in Figure 17a. An electrical stimulus propagates faster down the left pathway than the right pathway, called the fast and slow pathways, respectively. In normal cardiac behaviour, shown in Figure 17a, an electrical stimulus from the atriums splits and propagates down the fast and slow pathways. The stimulus in the fast pathway will reach the bottom of the AV node before the stimulus in the slow pathway. The stimulus in the fast pathway continues to propagate down into the ventricles and up the slow pathway where it will annihilate the existing stimulus. Thus, only the stimulus that propagates down the fast pathway will enter the ventricles. Figure 17c demonstrates our ability to replicate this normal behaviour by modelling the dual pathways around the AV node with only four cells and paths. An early electrical stimulus from the atriums can end up propagating continuously around the AV node, shown in Figure 17b. This is because the early stimulus cannot propagate down the fast pathway, which has cells that are still repolarising. The cells in the fast pathway take longer to repolarise than those in the slow pathway and, hence, have longer action potential durations. Due to this, the stimulus can propagate down the slow pathway because its cells have repolarised. By the time the stimulus reaches the bottom of the AV node, the cells in the fast pathway have reached their resting phase. Thus, the stimulus continues by propagating up the fast pathway, during which time the cells in the slow pathway reach their resting phase. The stimulus continues by propagating back down the slow pathway and the cycle continues. Every time the stimulus passes the bottom and top of the AV node, a stimulus is propagated into the ventricles and atriums, respectively. Figure 17d demonstrates our ability to replicate this abnormal behaviour by modelling the dual pathways around the AV node with only four cells and paths. VI. UOA HEART MODEL Following the approach of Oxford s heart model [6], we model the cardiac conduction system with a two-dimensional network of 33 cardiac cells, shown in Figure 1. Our heart model uses the improved cardiac cell and path models (Sections IV-B, IV-C, and V-A). The following cell and path model parameters were modified to capture the range of action potential durations, conduction velocities, and electrical conductivities present in the conduction system: The value of α 3 y in Figure 10 was increased to increase the cell s rate of repolarisation and, therefore, decrease its action potential duration. The values of δ ij and δ ji in Figure 15 were increased to increase the conduction time and, therefore, decrease the conduction velocity. The value ofσ ik in equation (3) was increased to increase the conductivity. Cardiac electrophysiology data in the literature [22], [32] was used to estimate the parameters. The data of interest include the morphology of myocyte action potentials and the conduction velocities of electrical stimuli along the pathways. Table IV provides a qualitative comparison of Oxford s heart model [6] with our improved heart model (UoA). Thanks to improvements in our cell and path models, we are able to model more heart conditions and with realistic results. This is
11 11 (a) Schematic of the fast and slow pathways around the AV node. (b) Re-entry due to an early stimulus from the atriums. (c) Normal behaviour simulated. Cell 1 stimulated at 10 and 260ms. (d) Abormal behaviour simulated. Cell 1 stimulated at 10 and 160ms. Fig. 17. Atrioventricular node re-entrant tachycardia. demonstrated in the next section where we provide simulation results of the common arrhythmias described in Section II-D. VII. SIMULATION RESULTS We implemented our heart model in MathWorks R Simulink R and Stateflow R R2015b Pro. A fixed simulation time step of0.0005ms and the Dormand-Prince ODE45 solver was used. Figure 19 shows the propagation of electrical stimuli through the cardiac conduction system (Figure 1) during a normal cardiac cycle. The membrane potential of each node is denoted by a shade of grey, according to Figure 18. In the following, we provide simulation results of the arrhythmias described in Section II-D. A. Heart Block This is modelled by reducing the voltage that the neighbouring atrial cells induce on the AV node and by increasing the conduction time through the AV node. Figure 20 shows a simulation of heart block where an electrical stimulus does not propagate through the AV node. B. Bundle Branch Block This is modelled similarly to heart block. For one of the bundle branches, the voltage induced by the AV node is reduced and the conduction time along the branch is increased. Figure 21 shows a simulation of bundle branch block where propagation down the right branch is slower than the left branch. C. Long Q-T Syndrome This is modelled by decreasing the value of α 3 y in the ventricular cells to decrease their rate of repolarisation, thereby creating a longer action potential duration. Figure 22 shows a simulation of the syndrome. D. VA Conduction This is modelled by stimulating a ventricular node before the SA node generates a stimulus. Figure 23 shows a simulation of VA conduction by stimulating the RV1 node in the right ventricle.
12 12 Fig. 18. Grayscale colourmap used to represent the voltage of each node. (a) 35ms. Electrical stimulus propagates from the SA node. (b) 88ms. Electrical stimulus arrives at the AV node. Fig. 19. Simulation of a normal cardiac cycle. The SA node depolarises at 10ms. (c) 193ms. Electrical stimulus propagates into the ventricles. (d) 245ms. Electrical stimulus propagates up the ventricles. (a) 35ms. Electrical stimulus propagates from the SA node. (b) 88ms. Electrical stimulus is blocked at the AV node. Fig. 20. Simulation of heart block. The SA node depolarises at 10ms. (c) 193ms. Electrical stimulus fails to enter the ventricles. (d) 245ms. Ventricles fail to depolarise. (a) 35ms. Electrical stimulus propagates from the SA node. (b) 88ms. Electrical stimulus arrives at the AV node. Fig. 21. Simulation of right bundle branch block. The SA node depolarises at 10ms. (c) 193ms. Electrical stimulus propagates slower into the right ventricle. (d) 245ms. Right ventricle depolarises after the left ventricle has depolarised.
13 13 (a) 35ms. Electrical stimulus propagates from the SA node. (b) 193ms. Electrical stimulus propagates into the ventricles. Fig. 22. Simulation of long Q-T syndrome. The SA node depolarises at 10ms. (c) 640ms. Repolarisation of normal ventricles would already have completed. (d) 740ms. Repolarisation of the ventricles finally completes. (a) 35ms. Electrical stimulus propagates from the RV1 node. (b) 150ms. Electrical stimulus arrives at the AV node. Fig. 23. Simulation of VA conduction. The RV1 node depolarises at 10ms. (c) 220ms. Electrical stimulus propagates into the atriums. (d) 311ms. Electrical stimulus propagates down the atriums. (a) 35ms. Electrical stimulus propagates from the SA node. (b) 125ms. Electrical stimulus propagates into the left ventricle via the LA1 LV1 accessory pathway. Fig. 24. Simulation of Wolff-Parkinson-White syndrome. The SA node depolarises at 10ms. (c) 176ms. Left ventricle depolarises before the right ventricle. (d) 245ms. Ventricles have depolarised.
14 14 (a) 88ms. Electrical stimulus arrives at the AV node via fast path. (b) 222ms. Early stimulation of the SA node. (c) 243ms. Early stimulus propagates through the atriums. (d) 320ms. Early stimulus arrives at the AV node via the slow path. (e) 400ms. Early stimulus re-enters the atriums via the fast path. (f) 430ms. Early stimulus re-enters the slow path a second time. (g) 515ms. Refractory of FP1 node prevents a second re-entry into the atriums. Fig. 25. Simulation of AV node re-entrant tachycardia. The SA node depolarises at 10 and 220ms. (h) 570ms. Atriums and ventricles repolarise. TABLE IV DETAILED QUALITATIVE COMPARISON OF HEART MODELS FROM UOA AND OXFORD. Heart Anatomy (Figure 1) Morphology of action potentials (Figure 6) Electrical Restitution (Figure 12) Path Model (Figure 16) Disease Conditions (Figures 20 to 25) UoA Oxford [6] Realistic because a more recent Stony Brook HA model [13] is used (with the improvements from Sections IV-B and IV-C) Shows good dynamic behaviour 2D conduction system Unrealistic because a simplified Stony Brook HA model [19] is used Shows limited dynamic behaviour Electrical propagation is time delayed, bi-directional, and anisotropic. Correctly disallows colliding electrical stimuli from passing through each other Bradycardia and tachycardia by modifying the SA rate, AV node re-entrant tachycardia, VA conduction, heart blocks, and long Q-T syndrome Incorrectly allows colliding electrical stimuli to pass through each other Bradycardia and tachycardia by modifying the SA rate, and heart blocks E. Wolff-Parkinson-White Syndrome This is modelled by creating an additional pathway between the left atrium and left ventricle. Figure 24 shows a simulation of the syndrome, which causes the left ventricle to depolarise earlier than usual. F. AV Node Re-entrant Tachycardia This is modelled by the fast and slow pathways around the SA node, demonstrated previously in Figure 17. Figure 25 simulates re-entrant tachycardia by stimulating the SA node a second time with an early stimulus. G. Time to Simulate the Cell and Heart Models The execution time needed to simulate the cell and heart models of Oxford and UoA are compared in this section. For a fair comparison, we wanted to avoid the execution overhead of the Simulink R environment. Thus, for each model, Simulink Coder TM was used to generate a single-threaded executable C program that is optimised for execution speed. Each C program was then executed on a single core of a 3.40GHz Intel Core i processor with 16GB of RAM and running Microsoft Windows 7 Enterprise. Each reported execution time is the average of ten runs of the same program. The Oxford cell model takes s to simulate 60s of cell activity with a 1s base cycle length, compared with 9.586s for the UoA improved cell model. The Oxford cell
15 15 model takes longer to simulate because it uses a Stateflow R block to manually check the transiton guards and produce a corresponding Boolean event whenever a guard is true. The Boolean event triggers another Stateflow R block to take transitions between the hybrid automaton states. For our UoA improved cell model, the hybrid automaton is modelled by a single Stateflow R block. The hybrid automaton transition guards are modelled directly as Stateflow R transition conditions. Stateflow R (automatically) handles the checking of transitions, which leads to a more efficient simulation, even though the Oxford cell model only uses the v x variable of the Stony Brook 2005 cell model. Moreover, the UoA improved cell model uses Simulink R components that are more efficient to simulate wherever possible. The Oxford and UoA heart models use the same arrangement of 33 nodes, as shown in Figure 1. The Oxford heart model has a total of 34 paths between the nodes and takes 8.516s to simulate 1s of a normal cardiac cycle. The UoA improved heart model has a total of 68 forward and backward paths between the nodes and takes s to simulate 1s of a normal cardiac cycle and s to simulate 1s of AV node re-entrant tachycardia. The UoA improved heart model takes longer to simulate because it implements a much more complex path model that considers the backward propagation and annihilation of electrical impulses throughout the cardiac conduction system. Thus, it is imperative to further improve the efficiency of the path model without sacrificing its predictive power. The execution time of the UoA improved heart model could be reduced by parallelising the simulation of the nodes over multi-cores [5]. VIII. CONCLUSION We carefully reviewed the heart models designed for the closed-loop testing of pacemakers and identified key areas of improvement. To this end, we advanced the state-of-theart in three ways: (1) stabilising and enforcing a minimum action potential duration on the Stony Brook cardiac cell model, (2) developing a path model that handles the partial and full propagation of continuous-time action potentials, and (3) demonstrating the predictive power of our heart model through the extensive simulation of arrhythmias. We are able to model many more arrhythmias than are possible with the existing heart models. For future work, we look to implement our heart model with more computationally efficient methods to achieve the goal of real-time heart simulation, i.e., heart emulation. We also look to investigate the automatic generation and parameterisation of a network of cardiac cells and paths for patient-specific heart modelling. ACKNOWLEDGMENT This research was funded by the University of Auckland FRDF Postdoctoral grant No The authors would like to express their gratitude to Prof. Marta Kwiatkowsk and her research group from the University of Oxford for providing an implementation of their heart model. REFERENCES [1] IEC :2015, International Electrotechnical Commission Std. 2.0, Jan [2] H. Alemzadeh, R. K. Iyer, Z. Kalbarczyk, and J. Raman, Analysis of Safety-Critical Computer Failures in Medical Devices, IEEE Security Privacy, vol. 11, no. 4, pp , [3] Z. Jiang, M. Pajic, and R. Mangharam, Cyber-Physical Modeling of Implantable Cardiac Medical Devices, Proceedings of the IEEE, vol. 100, no. 1, pp , Jan [4] R. Bordas, B. Carpentieri, G. Fotia, F. Maggio, R. Nobes, J. Pitt-Francis, and J. Southern, Simulation of Cardiac Electrophysiology on Next- Generation High-Performance Computers, Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, vol. 367, no. 1895, pp , [5] E. Bartocci, E. M. Cherry, J. Glimm, R. Grosu, S. A. Smolka, and F. H. Fenton, Toward Real-time Simulation of Cardiac Dynamics, in Proceedings of the 9th International Conference on Computational Methods in Systems Biology, ser. CMSB 11. ACM, 2011, pp [6] T. Chen, M. Diciolla, M. Kwiatkowska, and A. Mereacre, Quantitative Verification of Implantable Cardiac Pacemakers over Hybrid Heart Models, Information and Computation, vol. 236, pp , 2014, special Issue on Hybrid Systems and Biology. [7] Z. Jiang, M. Pajic, R. Alur, and R. Mangharam, Closed-Loop Verification of Medical Devices with Model Abstraction and Refinement, International Journal on Software Tools for Technology Transfer, vol. 16, no. 2, pp , [8] D. Méry and N. K. Singh, Formalization of Heart Models Based on the Conduction of Electrical Impulses and Cellular Automata, in Foundations of Health Informatics Engineering and Systems, ser. Lecture Notes in Computer Science, Z. Liu and A. Wassyng, Eds. Springer Berlin Heidelberg, 2012, vol. 7151, pp [9] J. Lian, H. Krätschmer, and D. Müssig, Open Source Modeling of Heart Rhythm and Cardiac Pacing, The Open Pacing, Electrophysiology & Therapy Journal, vol. 3, pp , [10] A. G. Kléber and Y. Rudy, Basic Mechanisms of Cardiac Impulse Propagation and Associated Arrhythmias, Physiological Reviews, vol. 84, no. 2, pp , [11] P. Spector, Principles of Cardiac Electric Propagation and Their Implications for Re-entrant Arrhythmias, Circulation: Arrhythmia and Electrophysiology, vol. 6, no. 3, pp , Jun [12] B. C. Mani and B. B. Pavri, Dual Atrioventricular Nodal Pathways Physiology: A Review of Relevant Anatomy, Electrophysiology, and Electrocardiographic Manifestations, Indian Pacing and Electrophysiology Journal, pp , Jan [13] P. Ye, E. Entcheva, S. A. Smolka, and R. Grosu, Modelling Excitable Cells Using Cycle-Linear Hybrid Automata, IET Systems Biology, vol. 2, no. 1, pp , Jan [14] C.-H. Luo and Y. Rudy, A Dynamic Model of the Cardiac Ventricular Action Potential: I. Simulations of Ionic Currents and Concentration Changes, Circulation Research, vol. 74, no. 6, pp , Feb [15] T. J. Bunch, D. L. Hayes, C. D. Swerdlow, S. J. Asirvatham, and P. A. Friedman, Pacing and Defibrillation: Clinically Relevant Basics for Practice. Wiley-Blackwell, 2013, pp [16] C.-H. Luo and Y. Rudy, A Model of the Ventricular Cardiac Action Potential: Depolarization, Repolarization, and Their Interaction, Circulation Research, vol. 68, no. 6, pp , Jun [17] F. Ou, The Effects of Heart Structure on Action Potentials, Cardiac Electrophysiology Group, Auckland Bioengineering Institute, Tech. Rep., 15. [18] A. L. Hodgkin and A. F. Huxley, A Quantitative Description of Membrane Current and its Application to Conduction and Excitation in Nerve, The Journal of Physiology, vol. 117, no. 4, pp , [19] P. Ye, E. Entcheva, R. Grosu, and S. A. Smolka, Efficient Modeling of Excitable Cells Using Hybrid Automata, in Proceedings of Computational Methods in System Biology, 2005, pp [20] F. Kusumoto, Understanding Intracardiac EGMs and ECGs, 1st ed. Wiley-Blackwell, [21] C. W. Haws and R. L. Lux, Correlation Between In Vivo Transmembrane Action Potential Durations and Activation-Recovery Intervals From Electrograms. Effects of Interventions That Alter Repolarization Time, Circulation, vol. 81, no. 1, pp , 1990.
16 16 [22] D. Durrer, R. T. van Dam, G. E. Freud, M. J. Janse, F. L. Meijler, and R. C. Arzbaecher, Total Excitation of the Isolated Human Heart, Circulation, vol. 41, pp , Jun [23] M. Sermesant, H. Delingette, and N. Ayache, An Electromechanical Model of the Heart for Image Analysis and Simulation, IEEE Transactions on Medical Imaging, vol. 25, no. 5, pp , May [24] B. H. Smaill and P. J. Hunter, Computer Modeling of Electrical Activation: From Cellular Dynamics to the Whole Heart, in Cardiac Electrophysiology Methods and Models, D. C. Sigg, P. A. Iaizzo, Y.-F. Xiao, and B. He, Eds. Springer US, 2010, pp [25] R. Clayton, O. Bernus, E. Cherry, H. Dierckx, F. Fenton, L. Mirabella, A. Panfilov, F. Sachse, G. Seemann, and H. Zhang, Models of Cardiac Tissue Electrophysiology: Progress, Challenges and Open Questions, Progress in Biophysics and Molecular Biology, vol. 104, no. 1 3, pp , [26] A. A. McLeod and P. P. Jokhi, Pacemaker Induced Ventricular Fibrillation in Coronary Care Units, British Medical Journal, May [27] P. C. Franzone, L. F. Pavarino, and S. Scacchi, Eds., Mathematical Cardiac Electrophysiology, ser. Modeling, Simulation & Applications. Springer International Publishing, 2014, vol. 13. [28] N. A. Trayanova, Your Personal Virtual Heart, IEEE Spectrum, vol. 51, no. 11, pp , Nov [29] S. M. Green, A. J. Klein, S. Pancholy, S. V. Rao, D. Steinberg, R. Lipner, J. Marshall, and J. C. Messenger, The Current State of Medical Simulation in Interventional Cardiology: A Clinical Document from the Society for Cardiovascular Angiography and Intervention s (SCAI) Simulation Committee, Catheterization and Cardiovascular Interventions, vol. 83, no. 1, pp , [30] J. B. Nolasco and R. W. Dahlen, A Graphic Method for the Study of Alternation in Cardiac Action Potentials, Journal of Applied Physiology, vol. 25, no. 2, [31] E. Bartocci, F. Corradini, M. R. D. Berardini, E. Entcheva, S. A. Smolka, and R. Grosu, Modeling and Simulation of Cardiac Tissue using Hybrid I/O Automata, Theoretical Computer Science, vol. 410, no , pp , [32] D. C. Sigg, P. A. Iaizzo, Y.-F. Xiao, and B. He, Eds., Cardiac Electrophysiology Methods and Models. Springer US, Eugene Yip received his B.E. (Hons) and Ph.D degrees in electrical and computer systems engineering from the University of Auckland, New Zealand. At the start of 2015, he joined the Heart-on-FPGA research group in the Department of Electrical and Computer Engineering, University of Auckland, as a research assistant working on the real-time modelling of cardiac electrical activity. In the middle of 2015, he joined the SWT research group at the University of Bamberg as a research assistant working on synchronous languages. His current research interests include synchronous mixed-criticality systems, parallel programming, static timing analysis, formal methods for organ modelling, and biomedical devices. Partha S. Roop received his Ph.D degree in computer science (software engineering) from the University of New South Wales, Sydney, Australia, in He is currently an Associate Professor and is the Director of the Computer Systems Engineering Program with the Department of Electrical and Computer Engineering, the University of Auckland, New Zealand. Partha is an associated team member of the SPADES team INRIA, Rhone-Alpes, France, and held a visiting position in CAU Kiel, Germany, and Iowa State University, USA. His research interests include the design and verification of embedded systems. In particular, he is developing techniques for the design of embedded applications in automotive, robotics, and intelligent transportation systems that meet functional-safety standards. Avinash Malik is a lecturer at the University of Auckland, New Zealand. His main research interest lies in programming languages for multicore and distributed systems and their formal semantics and compilation. He has worked at organisations such as INRIA in France, Trinity College Dublin, IBM research Ireland, and IBM Watson on the design and the compilation of programming languages. He holds B.E. and Ph.D degrees from the University of Auckland. Mark Trew attained a Bachelor of Engineering in Engineering Science in 1992 and a Ph.D Engineering Science in 1999, both from the University of Auckland, New Zealand. He is currently a Senior Research Fellow at the Auckland Bioengineering Institute. Mark constructs computer models and analysis tools for interpreting and understanding detailed images of cardiac tissue and cardiac electrical activity. Weiwei Ai received the B.S. degree in 2003 from Qingdao University, China, and the M.E. degree in 2006 from Beijing University of Technology, China. From 2006 to 2013, she worked as a reliability and failure analysis engineer in CEC Huada Electronic Design Co.,Ltd. She is currently working towards the Ph.D. degree at the the University of Auckland, New Zealand. Her research interests are in verification and validation with a focus on medical devices. Sidharta Andalam received his Ph.D degree from the University of Auckland, New Zealand, in 2013, where his thesis focused on developing a predictable platform for safety-critical systems. He is currently a research fellow in embedded systems at the University of Auckland, New Zealand. His principle research interest is in the design, implementation, and analysis of safety-critical applications. He has worked at TUM CREATE, Singapore, exploring safety-critical applications in the automotive domain. Nitish Patel received the B.E. degree from Mangalore University, Karnataka, India, and the Ph.D. degree from the University of Auckland, New Zealand. He is currently a Senior Lecturer at the University of Auckland. His research interests include embedded systems, robotics, artificial neural networks, control systems, and hardware implementation of real-time systems for control.
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Medical Cyber-Physical Systems
 Medical Cyber-Physical Systems Electrophysiology basics Lecture 10 Principles of Modeling for Cyber-Physical Systems Instructor: Madhur Behl Many thanks to: Zhihao Jiang, Houssam Abbas, and Rahul Mangharam,
Medical Cyber-Physical Systems Electrophysiology basics Lecture 10 Principles of Modeling for Cyber-Physical Systems Instructor: Madhur Behl Many thanks to: Zhihao Jiang, Houssam Abbas, and Rahul Mangharam,
Introduction. Circulation
 Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Abstract: Cardiac arrhythmias occur when blood flow to the
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Abstract: Cardiac arrhythmias occur when blood flow to the
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
PART I. Disorders of the Heart Rhythm: Basic Principles
 PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1
 BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
A Dynamic model of Pulmonary Vein Electrophysiology. Harry Green 2 nd year Ph.D. student University of Exeter
 A Dynamic model of Pulmonary Vein Electrophysiology Harry Green 2 nd year Ph.D. student University of Exeter Background to the Project Cardiac disease is the leading cause of death in most developed countries
A Dynamic model of Pulmonary Vein Electrophysiology Harry Green 2 nd year Ph.D. student University of Exeter Background to the Project Cardiac disease is the leading cause of death in most developed countries
Where are the normal pacemaker and the backup pacemakers of the heart located?
 CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
ECG. Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13
 ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
Outline. Electrical Activity of the Human Heart. What is the Heart? The Heart as a Pump. Anatomy of the Heart. The Hard Work
 Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Full file at
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
Electrocardiogram and Heart Sounds
 Electrocardiogram and Heart Sounds Five physiologic properties of cardiac muscle Automaticity: SA node is the primary pacemaker of the heart, but any cells in the conduction system can initiate their
Electrocardiogram and Heart Sounds Five physiologic properties of cardiac muscle Automaticity: SA node is the primary pacemaker of the heart, but any cells in the conduction system can initiate their
Epicardio Simulation. Introductory Guide
 Epicardio Simulation Introductory Guide Introduction Epicardio Simulation is a fully validated advanced cloud-based 3D interactive real-time simulation of the heart that shortens the Cardiology learning
Epicardio Simulation Introductory Guide Introduction Epicardio Simulation is a fully validated advanced cloud-based 3D interactive real-time simulation of the heart that shortens the Cardiology learning
Electrocardiography Biomedical Engineering Kaj-Åge Henneberg
 Electrocardiography 31650 Biomedical Engineering Kaj-Åge Henneberg Electrocardiography Plan Function of cardiovascular system Electrical activation of the heart Recording the ECG Arrhythmia Heart Rate
Electrocardiography 31650 Biomedical Engineering Kaj-Åge Henneberg Electrocardiography Plan Function of cardiovascular system Electrical activation of the heart Recording the ECG Arrhythmia Heart Rate
Cardiac Conduction System
 Cardiac Conduction System What causes the Heart to Beat? Heart contracts by electrical signals! Cardiac muscle tissue contracts on its own an electrical signal is sent out by the heart so that all cells
Cardiac Conduction System What causes the Heart to Beat? Heart contracts by electrical signals! Cardiac muscle tissue contracts on its own an electrical signal is sent out by the heart so that all cells
The Heart. Happy Friday! #takeoutyournotes #testnotgradedyet
 The Heart Happy Friday! #takeoutyournotes #testnotgradedyet Introduction Cardiovascular system distributes blood Pump (heart) Distribution areas (capillaries) Heart has 4 compartments 2 receive blood (atria)
The Heart Happy Friday! #takeoutyournotes #testnotgradedyet Introduction Cardiovascular system distributes blood Pump (heart) Distribution areas (capillaries) Heart has 4 compartments 2 receive blood (atria)
The Electrocardiogram
 The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
CARDIOVASCULAR SYSTEM
 CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
Paramedic Rounds. Tachyarrhythmia's. Sean Sutton Dallas Wood
 Paramedic Rounds Tachyarrhythmia's Sean Sutton Dallas Wood Objectives At the end of this session, the paramedic will be able to: State the key components of the cardiac conduction pathway, along with the
Paramedic Rounds Tachyarrhythmia's Sean Sutton Dallas Wood Objectives At the end of this session, the paramedic will be able to: State the key components of the cardiac conduction pathway, along with the
Cardiac Cycle. Each heartbeat is called a cardiac cycle. First the two atria contract at the same time.
 The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
The Rate-Adaptive Pacemaker: Developing Simulations and Applying Patient ECG Data
 The Rate-Adaptive Pacemaker: Developing Simulations and Applying Patient ECG Data Harriet Lea-Banks - Summer Internship 2013 In collaboration with Professor Marta Kwiatkowska and Alexandru Mereacre September
The Rate-Adaptive Pacemaker: Developing Simulations and Applying Patient ECG Data Harriet Lea-Banks - Summer Internship 2013 In collaboration with Professor Marta Kwiatkowska and Alexandru Mereacre September
A Cellular Automata Model For Dynamics and Control of Cardiac Arrhythmias DANNY GALLENBERGER (CARROLL UNIVERSITY) XIAOPENG ZHAO (UTK) KWAI WONG (UTK)
 A Cellular Automata Model For Dynamics and Control of Cardiac Arrhythmias DANNY GALLENBERGER (CARROLL UNIVERSITY) XIAOPENG ZHAO (UTK) KWAI WONG (UTK) Introduction Sudden cardiac arrest is responsible for
A Cellular Automata Model For Dynamics and Control of Cardiac Arrhythmias DANNY GALLENBERGER (CARROLL UNIVERSITY) XIAOPENG ZHAO (UTK) KWAI WONG (UTK) Introduction Sudden cardiac arrest is responsible for
The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits:
 1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
1 The cardiovascular system is composed of the heart and blood vessels that carry blood to and from the body s organs. There are 2 major circuits: pulmonary and systemic. The pulmonary goes out to the
UNDERSTANDING YOUR ECG: A REVIEW
 UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Cardiac physiology. b. myocardium -- cardiac muscle and fibrous skeleton of heart
 I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
EPicardio Simulation. User Reference Guide
 EPicardio Simulation User Reference Guide If you require technical assistance please contact support@epicardio.com For product details see here: www.epicardio.com Introduction Epicardio Simulation is a
EPicardio Simulation User Reference Guide If you require technical assistance please contact support@epicardio.com For product details see here: www.epicardio.com Introduction Epicardio Simulation is a
This presentation will deal with the basics of ECG description as well as the physiological basics of
 Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Biology 212: Anatomy and Physiology II. Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures
 Biology 212: Anatomy and Physiology II Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures References: Saladin, KS: Anatomy and Physiology, The Unity of Form
Biology 212: Anatomy and Physiology II Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures References: Saladin, KS: Anatomy and Physiology, The Unity of Form
Paroxysmal Supraventricular Tachycardia PSVT.
 Atrial Tachycardia; is the name for an arrhythmia caused by a disorder of the impulse generation in the atrium or the AV node. An area in the atrium sends out rapid signals, which are faster than those
Atrial Tachycardia; is the name for an arrhythmia caused by a disorder of the impulse generation in the atrium or the AV node. An area in the atrium sends out rapid signals, which are faster than those
Basic Electrophysiology Protocols
 Indian Journal of Cardiology ISSN-0972-1622 2012 by the Indian Society of Cardiology Vol. 15, (3-4), 27-37 [ 27 Review Article Shomu Bohora Assistant Professor, Deptt. of Cardiology, U.N. Mehta Institute
Indian Journal of Cardiology ISSN-0972-1622 2012 by the Indian Society of Cardiology Vol. 15, (3-4), 27-37 [ 27 Review Article Shomu Bohora Assistant Professor, Deptt. of Cardiology, U.N. Mehta Institute
Electrocardiography Abnormalities (Arrhythmias) 7. Faisal I. Mohammed, MD, PhD
 Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY]](/thumbs/96/126998162.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] Learning Objectives: Describe the ionic basis of action potentials in cardiac contractile and autorhythmic cells Explain the relationship
QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] Learning Objectives: Describe the ionic basis of action potentials in cardiac contractile and autorhythmic cells Explain the relationship
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES
 UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
Electrocardiography I Laboratory
 Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
ELECTROCARDIOGRAPHY (ECG)
 ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology
 EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important
EHRA Accreditation Exam - Sample MCQs Invasive cardiac electrophysiology Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important
Nerve. (2) Duration of the stimulus A certain period can give response. The Strength - Duration Curve
 Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
CARDIAC PHYSIOLOGY. Amelyn U. Ramos-Rafael,M.D. Functional Anatomy of the Heart
 CARDIAC PHYSIOLOGY Amelyn U. Ramos-Rafael,M.D. Functional Anatomy of the Heart 1 Functional Anatomy of The Heart The Atria relatively thin walled The Ventricles ventricular walls thicker than atrial walls
CARDIAC PHYSIOLOGY Amelyn U. Ramos-Rafael,M.D. Functional Anatomy of the Heart 1 Functional Anatomy of The Heart The Atria relatively thin walled The Ventricles ventricular walls thicker than atrial walls
current, and acting like
 Heart 10 IV. HEART PHYSIOLOGY - How the heart beats. How the heart depolarizes the myocardium, which leads to a contraction. A) INTRINSIC CONTROL - Heart controls its own rhythm. HOW? The presence of gap
Heart 10 IV. HEART PHYSIOLOGY - How the heart beats. How the heart depolarizes the myocardium, which leads to a contraction. A) INTRINSIC CONTROL - Heart controls its own rhythm. HOW? The presence of gap
Introduction to ECG Gary Martin, M.D.
 Brief review of basic concepts Introduction to ECG Gary Martin, M.D. The electrical activity of the heart is caused by a sequence of rapid ionic movements across cell membranes resulting first in depolarization
Brief review of basic concepts Introduction to ECG Gary Martin, M.D. The electrical activity of the heart is caused by a sequence of rapid ionic movements across cell membranes resulting first in depolarization
Enhanced Cellular Automation Model For Detecting Heart Fibrillation
 Enhanced Cellular Automation Model For Detecting Heart Fibrillation R. Touqan, A. Nasser & N. Hamdy Arab Academy for Science and Technology (AAST), email< rtouqan@hotmail.com Abstract: A new technique
Enhanced Cellular Automation Model For Detecting Heart Fibrillation R. Touqan, A. Nasser & N. Hamdy Arab Academy for Science and Technology (AAST), email< rtouqan@hotmail.com Abstract: A new technique
Cardiovascular System
 Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
Cardiovascular System The Heart Cardiovascular System The Heart Overview What does the heart do? By timed muscular contractions creates pressure gradients blood moves then from high pressure to low pressure
THE CARDIOVASCULAR SYSTEM. Heart 2
 THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
Chapter 12: Cardiovascular Physiology System Overview
 Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS
 NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
d) Cardiovascular System Higher Human Biology
 d) Cardiovascular System Higher Human Biology What can your remember about the heart and blood vessels? What is the Cardiovascular System? The cardiovascular system, also known as the circulatory system,
d) Cardiovascular System Higher Human Biology What can your remember about the heart and blood vessels? What is the Cardiovascular System? The cardiovascular system, also known as the circulatory system,
Death, dynamics and disorder: Terminating reentry in excitable media by dynamically-induced inhomogeneities
 PRAMANA c Indian Academy of Sciences Vol. 64, No. 4 journal of April 2005 physics pp. 553 562 Death, dynamics and disorder: Terminating reentry in excitable media by dynamically-induced inhomogeneities
PRAMANA c Indian Academy of Sciences Vol. 64, No. 4 journal of April 2005 physics pp. 553 562 Death, dynamics and disorder: Terminating reentry in excitable media by dynamically-induced inhomogeneities
1 Cardiology Acute Care Day 22 April 2013 Arrhythmia Tutorial Course Material
 1 Cardiology Acute Care Day 22 April 2013 Arrhythmia Tutorial Course Material Arrhythmia recognition This tutorial builds on the ECG lecture and provides a framework for approaching any ECG to allow the
1 Cardiology Acute Care Day 22 April 2013 Arrhythmia Tutorial Course Material Arrhythmia recognition This tutorial builds on the ECG lecture and provides a framework for approaching any ECG to allow the
EKG Abnormalities. Adapted from:
 EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
Cardiac muscle is different from other types of muscle in that cardiac muscle
 6 E X E R C I S E Cardiovascular Physiology O B J E C T I V E S 1. To define autorhythmicity, sinoatrial node, pacemaker cells, and vagus nerves 2. To understand the effects of the sympathetic and parasympathetic
6 E X E R C I S E Cardiovascular Physiology O B J E C T I V E S 1. To define autorhythmicity, sinoatrial node, pacemaker cells, and vagus nerves 2. To understand the effects of the sympathetic and parasympathetic
Chapter 16: Arrhythmias and Conduction Disturbances
 Complete the following. Chapter 16: Arrhythmias and Conduction Disturbances 1. Cardiac arrhythmias result from abnormal impulse, abnormal impulse, or both mechanisms together. 2. is the ability of certain
Complete the following. Chapter 16: Arrhythmias and Conduction Disturbances 1. Cardiac arrhythmias result from abnormal impulse, abnormal impulse, or both mechanisms together. 2. is the ability of certain
Comparison of Different ECG Signals on MATLAB
 International Journal of Electronics and Computer Science Engineering 733 Available Online at www.ijecse.org ISSN- 2277-1956 Comparison of Different Signals on MATLAB Rajan Chaudhary 1, Anand Prakash 2,
International Journal of Electronics and Computer Science Engineering 733 Available Online at www.ijecse.org ISSN- 2277-1956 Comparison of Different Signals on MATLAB Rajan Chaudhary 1, Anand Prakash 2,
Systems Biology Across Scales: A Personal View XXVII. Waves in Biology: Cardiac Arrhythmia. Sitabhra Sinha IMSc Chennai
 Systems Biology Across Scales: A Personal View XXVII. Waves in Biology: Cardiac Arrhythmia Sitabhra Sinha IMSc Chennai The functional importance of biological waves Spiral Waves Cardiac Arrhythmias Arrhythmias:
Systems Biology Across Scales: A Personal View XXVII. Waves in Biology: Cardiac Arrhythmia Sitabhra Sinha IMSc Chennai The functional importance of biological waves Spiral Waves Cardiac Arrhythmias Arrhythmias:
The Heart. Size, Form, and Location of the Heart. 1. Blunt, rounded point; most inferior part of the heart.
 12 The Heart FOCUS: The heart is composed of cardiac muscle cells, which are elongated, branching cells that appear striated. Cardiac muscle cells behave as a single electrical unit, and the highly coordinated
12 The Heart FOCUS: The heart is composed of cardiac muscle cells, which are elongated, branching cells that appear striated. Cardiac muscle cells behave as a single electrical unit, and the highly coordinated
Lab #3: Electrocardiogram (ECG / EKG)
 Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Lab #3: Electrocardiogram (ECG / EKG) An introduction to the recording and analysis of cardiac activity Introduction The beating of the heart is triggered by an electrical signal from the pacemaker. The
Section 5.1 The heart and heart disease
 Section 5.1 The heart and heart disease Mammals are too large to rely on diffusion. They need a circulatory system to move substances around the body. Blood moves down pressure gradients, from high to
Section 5.1 The heart and heart disease Mammals are too large to rely on diffusion. They need a circulatory system to move substances around the body. Blood moves down pressure gradients, from high to
Contents. Example: Pacemaker in DDD Mode. I. Introduction II. Website and Relevant Documents III. Background Information IV.
 Contents I. Introduction II. Website and Relevant Documents III. Background Information IV. Pacemaker System V. i. Components ii. Operating Modes iii. Parameters Example: Pacemaker in DDD Mode Pacemaker
Contents I. Introduction II. Website and Relevant Documents III. Background Information IV. Pacemaker System V. i. Components ii. Operating Modes iii. Parameters Example: Pacemaker in DDD Mode Pacemaker
Unit 6: Circulatory System. 6.2 Heart
 Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 (www.robots.ox.ac.uk/~neil/teaching/lectures/med_elec) The story so far
 Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 (www.robots.ox.ac.uk/~neil/teaching/lectures/med_elec) The story so far The heart pumps blood around the body. It has four chambers which contact
Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 (www.robots.ox.ac.uk/~neil/teaching/lectures/med_elec) The story so far The heart pumps blood around the body. It has four chambers which contact
Catheter Ablation. Patient Education
 Catheter Ablation Patient Education Allina Health System Your heart has four chambers. Two upper chambers (atria) pump blood to the two lower chambers (ventricles). In order for the heart to pump, it requires
Catheter Ablation Patient Education Allina Health System Your heart has four chambers. Two upper chambers (atria) pump blood to the two lower chambers (ventricles). In order for the heart to pump, it requires
V. TACHYCARDIAS Rapid rhythm abnormalities
 V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
Neurogenic and Myogenic hearts
 Neurogenic and Myogenic hearts In animals with open circulatory system the heart is usually sac-like or tubular. It has ostia or lateral openings which get closed when heart contracts and opens when heart
Neurogenic and Myogenic hearts In animals with open circulatory system the heart is usually sac-like or tubular. It has ostia or lateral openings which get closed when heart contracts and opens when heart
Arrival of excitation at right ventricular apical endocardium in Wolff-Parkinson-White syndrome type A, with and without right bundle-branch block'
 British Heart Journal, 973, 35, 594-600. Arrival of excitation at right ventricular apical endocardium in Wolff-Parkinson-White syndrome type A, with and without right bundle-branch block' Cesar A. Castillo,
British Heart Journal, 973, 35, 594-600. Arrival of excitation at right ventricular apical endocardium in Wolff-Parkinson-White syndrome type A, with and without right bundle-branch block' Cesar A. Castillo,
Lake EMS Basic EKG Review: Atrial Rhythms. The Lake EMS Quality Development Team
 Lake EMS Basic EKG Review: Atrial Rhythms The Lake EMS Quality Development Team This program is the Intellectual Property of Lake Emergency Medical Services Use of this program is limited to training and
Lake EMS Basic EKG Review: Atrial Rhythms The Lake EMS Quality Development Team This program is the Intellectual Property of Lake Emergency Medical Services Use of this program is limited to training and
Class XI Chapter 18 Body Fluids and Circulation Biology
 Question 1: Name the components of the formed elements in the blood and mention one major function of each of them. The component elements in the blood are: (1) Erythrocytes: They are the most abundant
Question 1: Name the components of the formed elements in the blood and mention one major function of each of them. The component elements in the blood are: (1) Erythrocytes: They are the most abundant
37 1 The Circulatory System
 H T H E E A R T 37 1 The Circulatory System The circulatory system and respiratory system work together to supply cells with the nutrients and oxygen they need to stay alive. a) The respiratory system:
H T H E E A R T 37 1 The Circulatory System The circulatory system and respiratory system work together to supply cells with the nutrients and oxygen they need to stay alive. a) The respiratory system:
WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES
 WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES Michelle Henry Barton DVM, PhD, DACVIM University of Georgia, Athens, GA INTRODUCTION The purpose of this talk is to review
WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES Michelle Henry Barton DVM, PhD, DACVIM University of Georgia, Athens, GA INTRODUCTION The purpose of this talk is to review
Omar Sami. Muhammad Abid. Muhammad khatatbeh
 10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT
 Link download full: http://testbankair.com/download/test-bank-for-ecgs-made-easy-5thedition-by-aehlert/ TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT Chapter 5 TRUE/FALSE 1. The AV junction consists
Link download full: http://testbankair.com/download/test-bank-for-ecgs-made-easy-5thedition-by-aehlert/ TEST BANK FOR ECGS MADE EASY 5TH EDITION BY AEHLERT Chapter 5 TRUE/FALSE 1. The AV junction consists
PHASE RESPONSE OF MODEL SINOATRIAL NODE CELLS - AN INVESTIGATION OF THE INFLUENCE OF STIMULUS PARAMETERS
 PHASE RESPONSE OF MODEL SINOATRIAL NODE CELLS - AN INVESTIGATION OF THE INFLUENCE OF STIMULUS PARAMETERS A. C. F. Coster, B. G. Celler Biomedical Systems Laboratory, School of Electrical Engineering, University
PHASE RESPONSE OF MODEL SINOATRIAL NODE CELLS - AN INVESTIGATION OF THE INFLUENCE OF STIMULUS PARAMETERS A. C. F. Coster, B. G. Celler Biomedical Systems Laboratory, School of Electrical Engineering, University
Question Expected Answers Marks Additional Guidance two marks for correct answer 1 (a) a single value between 67 and 80 ; ; max 2
 Question Expected Answers Marks Additional Guidance two marks for correct answer 1 (a) a single value between 67 and 80 ; ; max 2 If answer incorrect, allow one mark for appropriate working i.e. 60 divided
Question Expected Answers Marks Additional Guidance two marks for correct answer 1 (a) a single value between 67 and 80 ; ; max 2 If answer incorrect, allow one mark for appropriate working i.e. 60 divided
Cardiovascular Physiology
 Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Cardiovascular Physiology The mammalian heart is a pump that pushes blood around the body and is made of four chambers: right and left atria and right and left ventricles. The two atria act as collecting
Cardiac Resynchronisation Therapy Patient Information
 Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
The Nuts and Bolts of ICD Therapy
 Electrical Management of Cardiac Rhythm Disorders For Cardiology Fellows December 5-8 Austin, Texas The Nuts and Bolts of ICD Therapy 1 2 Action Potential Localized Differences in Conduction Conduction
Electrical Management of Cardiac Rhythm Disorders For Cardiology Fellows December 5-8 Austin, Texas The Nuts and Bolts of ICD Therapy 1 2 Action Potential Localized Differences in Conduction Conduction
PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE. Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology
 PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology Medical University of Łódź The heart is made up of four chambers Left Atrium Right Atrium Left Ventricle
PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology Medical University of Łódź The heart is made up of four chambers Left Atrium Right Atrium Left Ventricle
Lab 7. Physiology of Electrocardiography
 7.1 Lab 7. Physiology of Electrocardiography The heart is a muscular pump that circulates blood throughout the body. To efficiently pump the blood, cardiac contractions must be coordinated and are regulated
7.1 Lab 7. Physiology of Electrocardiography The heart is a muscular pump that circulates blood throughout the body. To efficiently pump the blood, cardiac contractions must be coordinated and are regulated
Chapter 20 (2) The Heart
 Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 13 The Cardiovascular System: Cardiac Function
 Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
4. The two inferior chambers of the heart are known as the atria. the superior and inferior vena cava, which empty into the left atrium.
 Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Answer each statement true or false. If the statement is false, change the underlined word to make it true. 1. The heart is located approximately between the second and fifth ribs and posterior to the
Lab 2. The Intrinsic Cardiac Conduction System. 1/23/2016 MDufilho 1
 Lab 2 he Intrinsic Cardiac Conduction System 1/23/2016 MDufilho 1 Figure 18.13 Intrinsic cardiac conduction system and action potential succession during one heartbeat. Superior vena cava ight atrium 1
Lab 2 he Intrinsic Cardiac Conduction System 1/23/2016 MDufilho 1 Figure 18.13 Intrinsic cardiac conduction system and action potential succession during one heartbeat. Superior vena cava ight atrium 1
Fast Facts: Cardiac Arrhythmias
 Fast Facts Fast Facts: Cardiac Arrhythmias Gerry Kaye, Steve Furniss and Robert Lemery Fast Facts Fast Facts: Cardiac Arrhythmias Gerry Kaye MBChB MD FRCP FRACP Consultant Cardiologist and Associate Professor
Fast Facts Fast Facts: Cardiac Arrhythmias Gerry Kaye, Steve Furniss and Robert Lemery Fast Facts Fast Facts: Cardiac Arrhythmias Gerry Kaye MBChB MD FRCP FRACP Consultant Cardiologist and Associate Professor
CASE 10. What would the ST segment of this ECG look like? On which leads would you see this ST segment change? What does the T wave represent?
 CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
The conduction system
 The conduction system In today s lecture we will discuss the conducting system of the heart. If we placed the heart in a special solution that contains Ca+ it will keep on contracting, keep in mind that
The conduction system In today s lecture we will discuss the conducting system of the heart. If we placed the heart in a special solution that contains Ca+ it will keep on contracting, keep in mind that
BME 5742 Bio-Systems Modeling and Control. Lecture 41 Heart & Blood Circulation Heart Function Basics
 BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
BME 5742 Bio-Systems Modeling and Control Lecture 41 Heart & Blood Circulation Heart Function Basics Dr. Zvi Roth (FAU) 1 Pumps A pump is a device that accepts fluid at a low pressure P 1 and outputs the
Figure 2. Normal ECG tracing. Table 1.
 Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
The Electrocardiogram part II. Dr. Adelina Vlad, MD PhD
 The Electrocardiogram part II Dr. Adelina Vlad, MD PhD Basic Interpretation of the ECG 1) Evaluate calibration 2) Calculate rate 3) Determine rhythm 4) Determine QRS axis 5) Measure intervals 6) Analyze
The Electrocardiogram part II Dr. Adelina Vlad, MD PhD Basic Interpretation of the ECG 1) Evaluate calibration 2) Calculate rate 3) Determine rhythm 4) Determine QRS axis 5) Measure intervals 6) Analyze
CORONARY ARTERIES. LAD Anterior wall of the left vent Lateral wall of left vent Anterior 2/3 of interventricluar septum R & L bundle branches
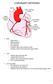 CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
CORONARY ARTERIES RCA Right atrium Right ventricle SA node 55% AV node 90% Posterior wall of left ventricle in 90% Posterior third of interventricular septum 90% LAD Anterior wall of the left vent Lateral
Dr David Begley Papworth Hospital, Cambridge HRUK Certificate of Accreditation Course: Core Heart Rhythm Congress 2011
 Dr David Begley Papworth Hospital, Cambridge HRUK Certificate of Accreditation Course: Core Heart Rhythm Congress 2011 The AV node is the soul of the heart, and whoever understands its anatomy and electrophysiology
Dr David Begley Papworth Hospital, Cambridge HRUK Certificate of Accreditation Course: Core Heart Rhythm Congress 2011 The AV node is the soul of the heart, and whoever understands its anatomy and electrophysiology
CIRCULATORY SYSTEM BLOOD VESSELS
 Name: Block: CIRCULATORY SYSTEM Multicellular organisms (above the level of roundworms) rely on a circulatory system to bring nutrients to, and take wastes away from, cells. In higher organisms such as
Name: Block: CIRCULATORY SYSTEM Multicellular organisms (above the level of roundworms) rely on a circulatory system to bring nutrients to, and take wastes away from, cells. In higher organisms such as
The Function of an ECG in Diagnosing Heart Conditions. A useful guide to the function of the heart s electrical system for patients receiving an ECG
 The Function of an ECG in Diagnosing Heart Conditions A useful guide to the function of the heart s electrical system for patients receiving an ECG Written by Erhan Selvi July 28, 2014 Audience and Scope
The Function of an ECG in Diagnosing Heart Conditions A useful guide to the function of the heart s electrical system for patients receiving an ECG Written by Erhan Selvi July 28, 2014 Audience and Scope
Modeling of Anatomy, Electrophysiology and Tension Development in the Human Heart
 European Functional Cardiac Modeling Meeting Modeling of Anatomy, Electrophysiology and Tension Development in the Human Heart Dr.-Ing. Gunnar Seemann Overview Electrophysiology Tension development Anatomy
European Functional Cardiac Modeling Meeting Modeling of Anatomy, Electrophysiology and Tension Development in the Human Heart Dr.-Ing. Gunnar Seemann Overview Electrophysiology Tension development Anatomy
The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node.
 PACEMAKER Natural pacemaker: The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node. Artificial pacemaker: It is a small, battery-operated device that helps the heart beat in
PACEMAKER Natural pacemaker: The heart's "natural" pacemaker is called the sinoatrial (SA) node or sinus node. Artificial pacemaker: It is a small, battery-operated device that helps the heart beat in
Circulatory system of mammals
 Circulatory system of mammals Explain the cardiac cycle and its initiation Discuss the internal factors that control heart action Blood flows through the heart as a result of pressure differences Blood
Circulatory system of mammals Explain the cardiac cycle and its initiation Discuss the internal factors that control heart action Blood flows through the heart as a result of pressure differences Blood
Heart. Heart 2-Tunica media: middle layer (media ='middle') muscle fibers (smooth or cardiac).
 t. innermost lumenal General Circulatory system heart and blood vessels walls have 3 layers (inside to outside) 1-Tunica interna: aka tunica intima layer--lumenal layer epithelium--endothelium simple squamous
t. innermost lumenal General Circulatory system heart and blood vessels walls have 3 layers (inside to outside) 1-Tunica interna: aka tunica intima layer--lumenal layer epithelium--endothelium simple squamous
Principles and Applications of Electrical Circuits and Signal Theories in EP
 Principles and Applications of Electrical Circuits and Signal Theories in EP Graydon Beatty, VP Therapy Development Atrial Fibrillation Division, St. Jude Medical, Inc. CardioRhythm 2009 Background Biophysics
Principles and Applications of Electrical Circuits and Signal Theories in EP Graydon Beatty, VP Therapy Development Atrial Fibrillation Division, St. Jude Medical, Inc. CardioRhythm 2009 Background Biophysics
Antiarrhythmic Drugs
 Antiarrhythmic Drugs DR ATIF ALQUBBANY A S S I S T A N T P R O F E S S O R O F M E D I C I N E / C A R D I O L O G Y C O N S U L T A N T C A R D I O L O G Y & I N T E R V E N T I O N A L E P A C H D /
Antiarrhythmic Drugs DR ATIF ALQUBBANY A S S I S T A N T P R O F E S S O R O F M E D I C I N E / C A R D I O L O G Y C O N S U L T A N T C A R D I O L O G Y & I N T E R V E N T I O N A L E P A C H D /
Chapter 20: Cardiovascular System: The Heart
 Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs
 EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important step for
EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important step for
