Effects and interactions of LH and LHRH agonist on testicular morphology and function in hypophysectomized rats
|
|
|
- Mariah Chase
- 5 years ago
- Views:
Transcription
1 Effects and interactions of LH and LHRH agonist on testicular morphology and function in hypophysectomized rats J. B. Kerr and R. M. Sharpe M.R.C. Reproductive Biology Unit, Centrefor Reproductive Biology, 37 Chalmers Street, Edinburgh EH3 9EW, U.K. Summary. The effects of single or combined daily treatment with an LHRH agonist and low or high doses of LH upon the testes of adult hypophysectomized rats were studied for up to 2 weeks in which changes in testicular histology, particularly the interstitial tissue, were examined by morphometry and related to functional assessment of the Leydig cells in vivo and in vitro. Compared to saline-treated controls, LHRH agonist treatment did not alter testis volume or the composition of the seminiferous epithelium or any of the interstitial tissue components although serum testosterone and in-vitro testosterone production by isolated Leydig cells were significantly reduced. With 2 \g=m\glh for treatment, testis volume was increased, spermatogenesis was qualitatively normal, total Leydig cell volume was increased, serum testosterone values were initially elevated but subsequently declined and in-vitro testosterone production was enhanced. Testis volume with 20 \g=m\g LH treatment was unchanged compared to saline treatment, the seminiferous epithelium exhibited severe disruption but total Leydig cell volume was greatly increased due to interstitial cell hyperplasia. This group showed elevated serum testosterone concentrations and major increases in testosterone production in vitro. Treatment with LHRH agonist with either dose of LH resulted in reduced testis volume, moderate to very severe focal spermatogenic disruption and increased total Leydig cell volume although serum testosterone values and in-vitro testosterone production were markedly reduced compared to control rats. It is concluded that, in the absence of the pituitary, LHRH agonist fails to disrupt spermatogenesis and the previously described antitesticular action of LHRH agonists in intact rats is therefore dependent upon the presence of LH, which alone or in combination with LHRH agonist, may focally disrupt spermatogenesis in hypophysectomized rats whereas the Leydig cells undergo hyperplasia. The findings show that impairment of spermatogenesis is accompanied by alterations of the interstitial tissue and suggest that communication between these two compartments is involved in the regulation of testicular function. Introduction Although it is now well established that treatment with LHRH agonists can result in major inhibi tion of testicular function in a number of species, including man (see reviews by Hsueh & Jones, 1981; Sandow, 1982; Jones & Hsueh, 1984), at least two important aspects of this inhibition remain unclear and controversial. The first of these centres on the mechanism of action of the LHRH agonists in bringing about impaired spermatogenesis and infertility. Do their inhibitory effects result from modulation of pituitary gonadotrophin release or do they arise, wholly or in part, from * Present address: Department of Anatomy, Monash University, Clayton, Victoria 3168, Australia.
2 direct effects on the testis? There is evidence to support the latter possibility since in the rat at least, LHRH agonists may exert both stimulatory and inhibitory effects directly on the Leydig cells (Sharpe, Doogan & Cooper, 1983a; Sharpe, 1984), the nature of the effect depending on the duration of exposure to the LHRH agonist and the degree of concomitant stimulation with LH (Sharpe, Doogan & Cooper, 1983b). The second unresolved question is what effect do LHRH agonists have upon spermatogenesis and testicular morphology in general, as reports in the literature are somewhat equivocal? Thus, in certain species, notably man (Linde et al., 1981; Rabin, Linde, Doelle & Alexander, 1981), monkeys (Akhtar, Marshall, Wickings & Nieschlag, 1983; Akhtar, Wickings & Nieschlag, 1984) and the dog (Tremblay & Belanger, 1984; Vickery et ai, 1984), suppression of sperm output can be achieved by peripheral administration of LHRH agonists, whilst in the rat only a partial reduction in sperm output occurs (Heber & Swerdloff, 1981) and fertility is often maintained (Vickery et ai, 1983). Suppression of spermatogenesis by LHRH agonists is commonly considered to be secondary to impairment of Leydig cell steroidogenesis and testosterone secretion which occurs both in intact (Rivier, Rivier & Vale, 1979; Labrie et al., 1980a, b; Wang et al., 1983) and in hypophysectomized rats (Hsueh & Erickson, 1979; Bambino, Schreiber & Hsueh, 1980; Wang et al., 1983). Despite impairment of steroidogenesis, the morphology of the Leydig cells has been reported to be either unchanged from normal (Labrie et al., 1978, 1980b; Pelletier et al, 1978, 1980; Lefebvre, Belanger, Pelletier & Labrie, 1984), atrophie (Rivier et al., 1979; Rajfer, Swerdloff & Heber, 1984) or hyperplastic (Vickery et al., 1983). However, the degree of histological preservation of the testes in these studies was not of sufficient quality to evaluate these conclusions. Little information is available on the changes in testicular morphology which precede the reduction in sperm output and, as yet, no study has provided a clear indication of the morphological consequences of the direct actions of LHRH agonists on the testis. The aim of the present study was therefore to clarify some of the above points by examining changes in testicular structure, with particular attention to the Leydig cells, in hypophysectomized adult rats treated for days with a low or high dose of LH, an LHRH agonist or the two hormones in combination. The morphological and morphometric data obtained were related to functional assessment of the Leydig cells in vitro and in vivo. Materials and Methods Animals and treatments. Sprague-Dawley rats hypophysectomized at 52 days of age were obtained from Charles River (U.K.) Ltd, Margate, Kent, U.K. and treatment begun 5-6 days postoperatively. Rats were maintained under standard conditions except that glucose (2-5 mg/ml) was added to the drinking water. Groups of 8 rats were then treated once daily at 09:00-10:00 h (subcutaneous injection) for 12 or 14 days with one of the following: (1) 0-5 ml vehicle, consisting of 0-9% (w/v) NaCl, 2-5 mg gelatin/ml (Sigma, Poole, U.K.) and 2-5 mg bovine serum albumin/ml (BSA, fraction V; Sigma); (2) 100 ng LHRH agonist [(D-Ser-r-bu6,des-Gly-NH210) LHRH ethylamide; Hoechst, A.G., F.R.G] in 0-5 ml vehicle; (3) 2 µg NIAMDD ovine LH-S24 in 0-5 ml vehicle; (4) 20 µg ovine LH-S24 in 0-5 ml vehicle; (5) 2 µg ovine LH-S ng LHRH agonist in 0-5 ml vehicle; (6) 20 µg ovine LH-S ng LHRH agonist in 0-5 ml vehicle. Hormone solutions were made up fresh every 48 h and kept at 4 C. All animals selected for treat ment had body weights within the range g at the start of the experiment and the change in bodyweight was monitored throughout the treatment period. Animals showing a bodyweight increment in excess of 10 g from Days 1 to 8 (5 rats) were discarded from the experiment and all
3 of these were subsequently confirmed to have remnants of pituitary tissue present upon visual inspection of the pituitary fossa. A further rat was discarded on the latter basis at Day 14. Tissuefixation. The testes of 3 animals from each treatment group together with 3 age-matched intact animals were fixed by perfusion on Day 12. Under deep ether anaesthesia the thoracic aorta was cannulated, the right atrium severed and testes were perfused for 30 sec with 0-9% (w/v) NaCl. A mixture of 3% glutaraldehyde, 2% formaldehyde and 001% trinitrophenol buffered with 0-2 M-cacodylate, ph 7-2, was perfused for 10 min and then 5% glutaraldehyde, 3% formaldehyde and 001% trinitrophenol in the same buffer was perfused for 30 min. Testes were removed, trimmed of fat and connective tissue and the volume of each pair of decapsulated testes was measured by water displacement (Elias & Hyde, 1980). Slices of testes from apical, equatorial and caudal regions were cut with razor blades into blocks 1-2 mm on edge and fixed for 2 h in the latter fixative. Blocks were post-fixed in osmium tetroxide, stained en bloc with uranyl acetate, dehydrated in graded ethanols, cleared in propylene oxide and embedded in Epon-araldite. All specimens were processed identically and on the same day. Sections were cut at 1 µ with glass knives on a Reichert OmU3 ultratome, were stained with toluidine blue and examined and photographed with a Zeiss photomicroscope. Morphometric analysis. Blocks from all testes were examined by light microscopy to exclude from study any tissue which showed evidence of fixation or preparation artefacts. Any blocks exhibiting artefactual spaces between the tunica propria of the seminiferous tubules and the inter stitial tissue, shrinkage artefacts within the interstitial spaces or unsatisfactory preservation of the interstitial cells were not assigned for quantitative analysis. Of the blocks exhibiting superior morphological preservation, 10 were selected from each testis in which seminiferous tubules were predominantly cut in transverse section. Stained sections were examined by light microscopy using 10 eyepieces and a 25 objective lens. The volumetric densities of Leydig cells, interstitial spaces (lymphatic sinusoids), blood vessel lumina (arteries, veins, capillaries) and remaining inter stitial cells (endothelium, blood vessel pericytes, macrophages, leucocytes and connective tissue cells) were measured by a point-counting method (Weibel, 1979) using an eyepiece graticule of 121 points (11 11 squares) inserted into one eyepiece. The volumetric density of each component was determined on 6 different areas of each section and expressed as a percentage of the total number of points superimposed upon the tissue section. For each animal points were counted (121 points 6 areas 10 blocks 2 testes). Average volumetric densities for the measured com ponents were calculated for each testis and for each animal. The volume of the testis occupied by each component was obtained by multiplying the volumetric density by the previously measured volume of the same perfusion-fixed testis. Changes in the volume of testes which may occur during post-fixation and processing of tissue blocks were not measured since the principal interest of the morphometric study was to compare the morphology of the testis from saline-treated rats with testes from hormone-treated rats. Conversion of the quantitative data from fixed tissues to values relating to the fresh testis was therefore unnecessary. Average volumetric densities and absolute volumes of the components within a group were determined by calculation of the mean ± standard deviation for that group. Collection of blood samples. Blood samples (1-1-5 ml) were obtained at 2 h after the daily injection on Days 1, 3, 7 and 13 of the experiment by incision of the tail vein under light ether anaesthesia, and the serum was stored at 20 C. Animals (3/group) in which the testes were fixed by perfusion fixation (Day 12) had blood samples collected only on Days 1, 3 and 7. Collection of seminal vesicles, testes andpreparation of isolated Leydig cells. Other than animals used for perfusion fixation, the remainder were killed using solid C02-generated C02 on Day 14 of the experiment, i.e. 24 h after the last injection. After inspection of the pituitary fossa for remnants of tissue, the seminal vesicles were dissected and weighed and the paired testes were removed, weighed and decapsulated. Using 8 testes/treatment group, a suspension of isolated testicular cells
4 was then prepared using collagenase as described elsewhere (Sharpe & Fraser, 1983). The isolated cells were suspended in Medium 199, to which was added Earle's salts, NaHC03 (0-22%), l- glutamine (2 mm; Flow Labs, Irvine, U.K.), transferrin (5 µg/ml; Sigma), insulin (10 µg/ml; Sigma), ceruloplasmin (1 U/ml; Sigma), penicillin (100 i.u./ml; Flow Labs), streptomycin (100µg/ml; Flow Labs), fungizone (2-5 µg/ml; Flow Labs) and BSA (0-5 mg/ml; Sigma). For each treatment group, samples of IO6 nucleated cells were dispensed into plastic multiwell dishes (Nunc; Kamstrup, Denmark) and incubated in a final volume of 0-65 ml in the absence or presence of a supra-maximally stimulating concentration of hcg (10~7m; Chorulon, Intervet, Cambridge, U.K.) or an LHRH agonist (10~7 m; details given above). Incubation was for 4 h at 32 C under a humidified atmosphere of 95% air; 5% C02, after which the medium was aspirated, centrifuged for 5 min at 1000 g and then stored at 20 C for testosterone measurement. Air-dried smears of cells from each of the treatment groups were also prepared, fixed in acetone and stained for 3ß-hydroxysteroid dehydrogenase (3ß-HSD; EC ) in the presence of sodium cyanide (to suppress the diaphorase enzyme), to identify the proportion of Leydig (3ß-HSD-positive) cells; full details of this procedure have been given elsewhere (Sharpe & Fraser, 1983). Testosterone production by the isolated cells has been expressed in ng/106 3ß-HSD-positive cells. Measurement of testosterone. Testosterone concentrations in serum were measured by radio immunoassay after extraction with 10 volumes of hexane:diethyl ether (4:1, v/v) as described and validated by Corker & Davidson (1978). Assay of Leydig cell incubation media before and after extraction, as described above, gave comparable testosterone values (P > 0-2 by Student's t test; = 48), and unextracted diluted incubation media gave displacement curves parallel to the testosterone standard (P > 0-2 in a test of non-parallelism using 2-factor analysis of variance with replication). Incubation media were therefore assayed directly after appropriate dilution (between 1/20 and 1/500), and the limit of detection of the assay was 5 pg testosterone/sample. All samples of incubation medium or serum were assayed together in a single assay and the intra-assay coefficient of variation was <5%. Statistical analysis. For the functional studies, results were analysed using 2-factor analysis of variance (with replication) and Student's t test. For morphometric studies, the effects of hormone treatment compared to saline treatment of hypophysectomized rats were analysed using Duncan's new multiple range test. Comparison between values for the normal testis and saline-treated hypophysectomized rats was made using two-tailed Student's t tests. Gross morphology Results After perfusion fixation the mean + s.d. volume of testes from saline-treated hypophy sectomized rats ( cm3) was significantly smaller ( < 005) than that of testes from normal intact rats ( cm3). Amongst the groups of hormone-treated hypophysectomized rats, testes from rats treated with 2 µg LH (0-741 ± cm3) were significantly (P < 001) larger than those of saline-treated rats, whilst testes from rats treated with 20 µg LH + LHRH agonist (0-281 ± 0-28cm3) were significantly (P < 005) smaller than those from saline-treated rats. No significant differences were noted for other hormone treatments (see Fig. 11). Comparable differ ences as above were obtained for the weights of fresh testes allocated for functional studies. Visual examination of fixed testes from rats treated with 20 µg LH, 2 µg LH + LHRH agonist or 20 µg LH + LHRH agonist revealed the presence of opaque intratesticular densities beneath the tunica albugínea, particularly in the caudal pole of the testes. Although the weight of seminal vesicles from rats treated with 20 µg LH alone was larger (69 ± 34 mg, mean ± s.d.) compared to salinetreated control rats (33 ± 14 mg), this difference and other comparisons did not reach statistical significance.
5 Fig. 1. Normal adult rat testis illustrating full spermatogenesis and interstitial tissue of normal morphology. Leydig cells (L), macrophages (M) and Sertoli cell nuclei (S) are shown, 400. Fig. 2. Vehicle treatment of hypophysectomized rats, illustrating degenerating germ cells (arrows), lipid inclusions (arrowheads) and atrophie Leydig cells (L) in the interstitial tissue, 400.
6 Fig. 3. Treatment of hypophysectomized rats with LHRH agonist. Seminiferous epithelium at stage II (upper) and stage VI (right) lack their usual numbers of elongated spermatids. Macrophages shown by arrows. Leydig cells (L) similar in size to vehicle-treated rats, Treatment of hypophysectomized rats with 20 µg LH, illustrating degeneration of seminiferous epithelium and expansion of the interstitial tissue. Sertoli cell nuclei (S) dense intratubular debris (arrow) and spaces (asterisks) are shown. There are abundant Leydig cells (L) and large lymphatic spaces (LS) Fig.
7 Histology Normal rats. All stages of the spermatogenic cycle were observed in each testis and none of the seminiferous tubules showed evidence of degenerative changes or morphological alterations (Fig. 1). Sertoli cell nuclei were readily visible lying adjacent to the basement membrane of the semin iferous tubules. The interstitial tissue appeared normal, containing clusters of Leydig cells identi fied by their oval or irregular shape. Leydig cell nuclei exhibited a prominent nucleolus while the cytoplasm was basophilic and contained dense granules representing mitochondria. Macrophages were fewer in number than Leydig cells and were characterized by a pale-staining nucleus and cytoplasm containing numerous vacuoles and inclusion bodies. Thin elongated endothelial cells formed the walls of the interstitial lymphatic sinusoids which appeared as uniformly stained pale spaces partly or at times completely surrounding the interstitial cells, a feature characteristic of the morphology of the interstitial space when optimally fixed by perfusion. Saline-treated hypophysectomized rats. The seminiferous tubules showed patchy disruption of spermatogenesis with some tubules exhibiting more extensive degeneration of the seminiferous epithelium than others (Fig. 2). All seminiferous tubules contained abnormal accumulations of spherical lipid inclusions. Many tubules lacked one or more generations of germ cells, the elongat ing and fully developed spermatids being moderately to severely reduced in numbers. Numerous degenerating and pycnotic germ cells were observed, together with vacuoles present within the basal or adluminal regions of the seminiferous epithelium. In the interstitial tissue the principal changes compared to the normal testis were an apparent reduction in the cross-sectional area of the Leydig cells and narrowing of the interstitial lymphatic spaces. Macrophages and other interstitial cells were also present. LHRH-agonist treatment. The seminiferous tubules showed variable spermatogenic activity with some exhibiting a morphologically normal seminiferous epithelium while the majority showed degenerating and pycnotic germ cells and at times loss of the mature generation of elongated spermatids (Fig. 3). The morphology of the remaining germ cells and the Sertoli cells were, at the light microscope level, indistinguishable from that of the normal testis. However, the interstitial tissue contained Leydig cells with a smaller cross-sectional area than those of the normal testis. Other features of the interstitial tissue did not appear significantly altered when compared to interstitial tissue of saline-treated rats. Treatment with 2pg LH. Seminiferous tubules were smaller in cross-sectional area compared to the normal testis but larger than those seen in saline-treated rats. Spermatogenesis was qualitatively normal except for the final phase of spermiogenesis when abnormal retention of elongated spermatids was noted together with bizarre profiles of residual bodies at the luminal aspect of the seminiferous tubules. All other germ cells appeared normal in morphology. Most tubules contained increased numbers of lipid inclusions and degenerating germ cells were commonly observed. Plenti ful numbers of fully developed spermatids seen at stages VII and VIII in the normal testis were not often observed in this treatment group. The interstitial tissue appeared qualitatively unchanged compared to the normal testis except that the Leydig cells often contained cytoplasmic lipid inclusions, a feature not seen in normal Leydig cells. Macrophages, other interstitial cells and the interstitial space were always observed and appeared unchanged compared to the normal testis. Treatment with 20 ßg LH. The cross-sectional area of all seminiferous tubules was markedly reduced compared to the treatment groups described above and different degrees of spermatogenic disruption had occurred ranging from severe to very severe damage to the seminiferous epithelium (Fig. 4). In severely damaged tubules, the seminiferous epithelium consisted of reduced numbers of germ cells with round spermatids being the most mature type present. These tubules also contained variable quantities of lipid inclusions and/or many spherical or irregularly-shaped vacuoles. The most severely disrupted tubules contained relatively few germ cells or at times the complete absence
8 5. Treatment of hypophysectomized rats with 20 µg LH, showing Leydig cell hyperplasia within a very large interstitial area. Note Leydig cell mitotic figure (arrowhead). Macrophages (M) together with other interstitial cells (arrows) are illustrated, 400. Fig. Fig. 6. Treatment of hypophysectomized rats with 20 µg LH + LHRH agonist, illustrating collapse of the seminiferous tubules which contain Sertoli cells (S), spermatogonia (SG) and numerous lipid inclusions (arrowheads). Note interstitial cell hyperplasia consisting of Leydig cells (L) and other interstitial cells. Lymphatic spaces (LS) are prominent, 400.
9 Fig. 7. Treatment of hypophysectomized rats with 2 µg LH + LHRH agonist, showing (a) disrupted seminiferous epithelia containing multinucleated germ cells (G), degenerating germ cells (arrowheads) extracellular spaces, vacuoles and vesicles (arrows), and (b) extreme degeneration of the seminiferous epithelium, now filled with a variety of dense tissue debris (arrow). Nuclei of Sertoli cells and germ cells are absent, 300. of germ cells. Large extracellular spaces and vacuoles were frequently noted, together with a variable content of lipid inclusions. Sertoli cell nuclei present within such tubules were reduced in numbers and they exhibited pleomorphic morphologies not seen in the above-mentioned treatment groups. Frequently the lumina of the collapsed seminiferous tubules became obliterated, being filled with dense accumulations of cellular debris and in such tubules, germ cells and Sertoli cells could not be identified. Interstitial tissue was markedly expanded compared to the above treatment groups and exhibited interstitial cell hyperplasia (Fig. 5). Leydig cells were especially abundant although they appeared smaller and far more irregular in shape compared to the normal testis. Macrophages and other interstitial cells were commonly observed and many capillaries were present within the interstitial tissue. Treatment with 2 or 20 pg LH + LHRH-agonist. These two groups are described together since the morphological response of the testis was similar. The seminiferous tubules were reduced in cross-sectional area due to collapse of the seminiferous epithelium which exhibited a variety of cellular damage ranging from moderate loss of germ cells to elimination of all cells from the seminiferous tubules (Figs 6, 7a & b, and 8a & b). In those tubules containing germ cells the most advanced cell type seen was the round spermatid although occasionally small numbers of elongat ing spermatids were present. Seminiferous epithelia of this type contained increased numbers of lipid inclusions and many vacuoles and large empty extracellular spaces. Sertoli cells exhibited
10 Fig. 8. Treatment of hypophysectomized rats with 20 µg LH + LHRH agonist showing (a) degenerating germ cells (arrows) and extracellular vacuoles (asterisks), and (b) marked reduction in germ cells and interdigitated processes of Sertoli cell cytoplasm. Nuclei of Sertoli cells (s) and spermatogonia (arrowheads), 300. LH + LHRH agonist showing inter Fig. 9. Treatment of hypophysectomized rats with 20 µg Downloaded from Bioscientifica.com at 11/13/ :57:24AM stitial cell hyperplasia. Leydig cells (L), macrophages (arrowheads) and other interstitial cells indicated. abundant and Note lymphatic spaces (LS) empty capillaries, (arrows) are
11 10" Leydig cells *** r-i 6- LJl Interstitial space Vascular lumina ÏL JL_^ n_ít""fr""it I # J, I ÎhJ=Û Normal Hypox. LHRH-A 2 p.g LH 20 µ LH 2 µ, LH+ 20 µ9 LH + LHRH-A LHRH-A Fig. 10. Volumetric densities of the interstitial compartment and its components in the testis of the normal intact adult rat and in the testis of saline- or hormone-treated adult hypophy sectomized rats. Values expressed as means ± s.d., 3 in each = group., < 0-05 compared with value for normal testis; * < 005, ** < 001, *** < 0001 compared with value for saline-treated hypophysectomized rats. nuclei of irregular shapes, often displaced towards the lumen of the tubules and their cytoplasm formed branching networks. In the most severely damaged tubules, germ cells were markedly depleted or absent, the lumen was either filled with interdigitating processes of Sertoli cell cytoplasm or contained remarkable accumulations of dense basophilic material suggestive of undigested cellular debris. Sertoli cells and germ cells were often not present within such tubules. The morphology of the interstitial tissue was similar to that observed in the 20 µg LH treatment group, with expansion in size and containing many Leydig cells usually surrounded by extensive lymphatic spaces. Blood vessels were especially numerous (Fig. 9). Morphometric analysis The percentage of testis volume (volumetric density) occupied by Leydig cells, the interstitial space, other interstitial cells and the total interstitial compartment was significantly increased
12 14-, Testis E > Leydig cells Ü 20- _ÛI írzdl -* íbdl 50-1 Interstitial space I 10- jtjï Vascular lumina, I I JL E > 50-, Other interstitial cells 30- JL , Total interstitial area Normal Hypox. LHRH-A 2 µ LH 20 µ9 LH 2 µ9 LH+ 20 µ9 LH+ LHRH-A LHRH-A Fig. 11. Absolute volumes of testes, the interstitial compartment and its components in the normal intact adult rat and in saline- or hormone-treated adult hypophysectomized rats. Values expressed as means ± s.d., = 3 in each group., < 005 compared with value for normal testis; * < 005, ** < 001, *** < 0001 compared with value for saline-treated hypophysectomized rats. ( < ) when the effects of 20 µg LH and 2 or 20 µg LH + LHRH agonist treatment were compared to those in saline-treated hypophysectomized rats (Fig. 10). No significant differences were noted for the volumetric density of vascular lumina for any hormone treatment. Treatment with LHRH agonist or 2 µg LH did not significantly change the above parameters compared to
13 saline treatment, except for the volumetric density of other interstitial cells which was significantly less (P < 0-01) after 2 µg treatment. When volumetric density data were converted to absolute volumes per whole testis, total Leydig cell volume was significantly increased (P < ) when treatment with 2 or 20 µg LH and 2 or 20 µg LH + LHRH agonist was compared to salinetreated rats (Fig. 11). Compared to saline treatment, the total interstitial tissue volume was significantly increased (P < 005) by 20 µg LH treatment. Total volume of other interstitial cells was significantly increased (P < 005) with 2 µg LH + LHRH agonist treatment, and the total volume of the interstitial compartment was significantly increased (P < ) after 20 µg LH and 2 µg LH + LHRH agonist treatment. The total volume of blood vessel lumina was not significantly changed when hormone-treated groups were compared to saline-treated rats. Serum concentrations of testosterone Vehicle-injected (control) rats had constant low serum values ( ng/ml) of testosterone at each of the times studied, and these values are within the range for castrated males measured in our 15-1 lo ss, VEHICLE»_ Day of treatment Fig. 12. Serum concentrations of testosterone 2 h after injection of vehicle, LH, LHRH agonist (LHRH-A), or the two hormones in combination, during prolonged daily treatment of hypophysectomized adult rats. Each point is the mean ± s.d. for 7 (Days 1, 3 and 7) or 4 (Day 13) rats.
14 Fig. 13. Testosterone production in vitro by isolated Leydig cells from hypophysectomized adult rats treated for 13 days with vehicle, LH, LHRH agonist (LHRH-A) or the two hormones in combination. Cells were prepared 24 h after the last injection. Bars are the mean + s.d. for triplicate incubations. Asterisks have been used to indicate where in-vivo treat ment with LHRH-A, on its own or with LH, has significantly (P < 0001) reduced testosterone production by isolated Leydig cells in vitro, when compared with cells from the corresponding group treated in vivo with vehicle or LH alone. laboratory (unpublished data). Injection of loong LHRH agonist on Day 1 resulted in an 8-fold increase (P < 0001) in the values of testosterone 2 h later, compared with controls, but at subse quent sampling times this stimulatory effect was not evident (Fig. 12). At 2 h after injection of 2 µg LH there was a significant (P < 0001) increase in testosterone concentrations on Days 1,3 and 7 of treatment but on Day 13 no significant increase occurred. In contrast, injection of 20 µg LH resulted in a significant (P < 0001) and relatively constant increase in serum testosterone levels at all of the sampling times (Fig. 12). Injection of 100 ng LHRH agonist together with either of the doses of LH, had no significant effect on testosterone values on Day 1, but thereafter grossly attenuated the LH-stimulated increase in testosterone concentrations (P < 005 to < 0001), an effect that became progressively more marked with time (Fig. 12). Testosterone production by isolated Leydig cells Leydig cells isolated from the testes of vehicle-injected rats produced low amounts of testo sterone basally, but responded to addition of LHRH agonist or hcg by increasing testosterone production 7- and 47-fold, respectively (Fig. 13). Leydig cells isolated from the testes of rats treated with LHRH agonist alone showed an 85% reduction in basal testosterone production, when com pared with cells from control rats, and whereas there was still a significant ( < 0001) response to LHRH agonist (3-fold) or hcg (43-fold), the levels of testosterone achieved were only 6% and 14%, respectively, of the comparable control values. Compared with controls, treatment of rats with 2 or 20µg LH caused major (P < 0001) increases in basal testosterone production and in responsiveness of the Leydig cells to LHRH agonist or hcg; the last two values were also significantly higher (P < 0001) for rats treated with 20 as opposed to 2 µg LH (Fig. 13).
15 Treatment of rats with LH -f- LHRH agonist completely blocked (P < 0001) the stimulatory effects of the LH treatment and lowered the responsiveness of Leydig cells to LHRH agonist or hcg, to levels below that of cells from control rats (P < 0001; Fig. 13). Compared with cells from rats treated with LH alone, testosterone production by cells from rats treated with LH + LHRH agonist was reduced by the following amounts: basal values: 92% (2 µg LH) and 82% (20 µg LH) reduction; LHRH agonist-stimulated values: 94% and 96% reduction; hcg-stimulated values: 80% and 88% reduction. Discussion Administration of LHRH and its agonists to immature or adult rats is known to reduce LH (hcg) receptors in the testis and to impair the steroidogenic capacity of the Leydig cells and this has been proposed to explain the resulting disruption of the seminiferous epithelium which is normally dependent upon stimulation by testosterone. Three possible mechanisms have been suggested to explain these antigonadal actions of LHRH and its agonists (Hsueh & Jones, 1981): (i) chronic stimulation by LHRH agonists may desensitize the anterior pituitary, resulting in diminished circulating gonadotrophins and atrophy of testicular functions; (ii) high doses of LHRH agonists may cause hypersécrétion of LH from the pituitary, causing desensitization of Leydig cells and sub sequent spermatogenic impairment; and (iii) LHRH agonists may exert direct inhibitory actions on the testis. To test the involvement of pituitary factors in bringing about the anti-testicular effects of LHRH agonists, previous studies have shown that LHRH agonist treatment of immature or adult hypophysectomized rats does not result in any histological changes in the testis compared to saline-treated hypophysectomized rats (Rivier et al., 1979; Labrie et al., 1980b; Pelletier et ai, 1980), suggesting the importance of the pituitary in mediating the anti-spermatogenic actions of LHRH agonists. The present quantitative analysis supports these qualitative findings since treat ment of hypophysectomized rats with LHRH agonist alone did not significantly alter testis weight or the morphology of the seminiferous epithelium and interstitial tissue, compared to saline-treated control rats. Despite the lack of morphological changes in the testis of hypophysectomized rats after LHRH agonist treatment, direct inhibitory effects of LHRH agonist upon Leydig cell func tion were indicated by significant impairment of the ability of isolated Leydig cells to secrete testosterone in vitro when stimulated with LHRH agonist or hcg, in agreement with other studies (Hsueh & Erickson, 1979; Bambino et al., 1980; Wang et ai, 1983). Since LHRH agonists act directly upon Leydig cells (Sharpe et ai, 1983b) but are unable to disrupt the spermatogenic process in hypophysectomized animals, then the moderate to severe spermatogenic damage caused by treatment of intact animals with LHRH agonists may be related to additional effects of agonists upon the pituitary resulting in disturbances of gonadotrophin secretion and subsequent major impairment of Leydig cell function. However, concomitant administration of testosterone or dihydrotestosterone to LHRH agonist treated intact rats is unable to prevent seminiferous tubular disruption (Rivier et al., 1979; Pelletier et al., 1980) whereas in the absence of the pituitary, andro gens plus LHRH agonists support qualitatively normal spermatogenesis (Rivier et al., 1979). These findings exclude the possibility that the anti-spermatogenic effects of LHRH agonists given to intact rats are due to a decrease in androgen concentrations within the testis, and again point to pituitary factors in mediating the actions of LHRH agonists on the testis. Alternatively, the anti gonadal effects of LHRH agonists in intact rats suggest that pituitary factors may themselves exert deleterious effects upon the testis, particularly in combination with LHRH agonist treatment. Our results suggest that LH, either alone or in combination with LHRH agonist, brings about marked disruption of spermatogenesis and alteration of Leydig cell function in hypophysectomized rats. Treatment of hypophysectomized rats with 20 µg LH or 2 or 20 µg LH + LHRH agonist resulted in a decline in testis weight, focal disruption of the seminiferous epithelium and a significant increase in the total volume of Leydig cells within the testis, However, administration of a lower dose of 2 µg LH to hypophysectomized rats stimulated spermatogenesis, significantly increased the
16 total Leydig cell volume and increased testis volume compared to saline-treated control rats. The accompanying morphometric results allow two conclusions: (1) the anti-spermatogenic effects of LHRH agonist were expressed in the presence of low or high concentrations of LH and (2) LH alone may exert deleterious effects upon spermatogenesis but this action is dependent upon high concentrations of the hormone. The response of the testis of hypophysectomized rats treated with 20 µg LH and 2 or 20 µg LH + LHRH agonist ranged from moderate to severe disruption of spermatogenesis and in the latter two treatment groups, tubules devoid of nuclei (and therefore of cells) were commonly observed. Remarkably, tubules often devoid of epithelial cells were com monly observed adjacent to those showing only moderate cellular disruption, normally lacking elongating and mature spermatids. A similar patchy but less severe disruption of spermatogenesis has been observed after chronic LHRH agonist treatment of intact rats (Rivier et al., 1979; Labrie et al., 1980a, b; Pelletier et ai, 1980; Vickery et ai, 1983; Lefebvre et al., 1984). The central role played by LH in initiating and sustaining variable spermatogenic disruption is supported by other studies in which hcg + PMSG given to intact adult rats for 1-2 weeks caused degeneration of the seminiferous epithelium (Rivier et ai, 1979). These findings and those reported here for 20 µg LH treatment suggest that a simple deficiency in testosterone concentration within the testis cannot explain the inhibition of spermatogenesis. Comparable degenerative changes in spermatogenesis occurred when serum testosterone and in-vitro Leydig cell testosterone production was high (with the 20 µg LH treatment) or lowered (as seen with the 2 or 20 µg LH + LHRH agonist treatments). In our studies using 20 µg LH for treatment, although spermatogenesis was impaired, Leydig cells were abundant, their in-vitro steroidogenic responses to hcg or LHRH agonist stimulation were markedly enhanced compared with those of saline-treated controls (1100 and 300 ng testosterone/106 Leydig cells respectively) and serum testosterone values were always within or above the normal range for intact rats. In contrast, similar disruptive effects of 2 or 20 µg LH + LHRH agonist treatment upon spermatogenesis occurred when the capacity of the increased total volume of Leydig cells to secrete testosterone was always impaired compared to correspond ing treatment with 2 or 20 µg LH alone. It is possible that the variations in secretory function of Leydig cells from groups treated with LH alone or in combination with LHRH agonist result in abnormal patterns of testosterone production depending on the dose of hormone treatment and whether the treatments exert an overall stimulation or inhibition of Leydig cell function as previously suggested by Sharpe et al. (1983a). Although the mechanism which brings about significant increases in the total volume of Leydig cells was not studied, it probably occurs via a combination of mitosis of Leydig cells (often seen with 20 µg LH treatment) and differentiation of new Leydig cells from a more primitive interstitial cell type. A similar increase in the Leydig cell population occurs in rats chronically stimulated with daily injections of hcg (Christensen & Peacock, 1980). While the present data do not provide a full explanation for the anti-spermatogenic effects of the treatment regimens, the results suggest that LH alone or in combination with LHRH agonist can focally inhibit spermatogenesis and, in certain seminiferous tubule areas, destroy all the cells of the seminiferous epithelium whereas the nearby Leydig cells undergo striking hyperplasia. The deleterious effects upon the seminiferous epithelium cannot be attributed solely to a lack of testosterone, and as they only occur in the presence of LH it is possible that some other LHregulated Leydig cell factor(s) is involved but their identity and mode of action remain unknown. The histological data suggest that the patchy disruption of the seminiferous tubules represents some stage-dependent sensitivity of the spermatogenic cycle to the factors which mediate these changes. Disappearance of Sertoli cells and germ cells and their replacement with dense aggre gations of extracellular material has also been reported in LHRH agonist treated intact rats (Vickery et al., 1983) but tubules with normal seminiferous epithelia were also seen in the same testis. It is therefore likely that the focal degeneration of seminiferous tubules represents a very localized toxicological effect from which a proportion of seminiferous tubules fail to recover their spermatogenic function (Labrie et ai, 1980b; Pelletier et ai, 1980; Lefebvre et al, 1984).
17 The observations presented in this study further emphasize that agents that disrupt the spermatogenic process also interfere with the Sertoli cells and Leydig cells and provide evidence for the concept that communication between these cell types is involved in the regulation of spermatogenesis. We thank Jürgen Sandow and Hoechst A.G. for the gift of LHRH agonist; the NIAMDD for ovine LH; Irene Cooper, Ken Donachie and Denis Doogan for technical assistance; and Professor Sir Alistair Currie, Department of Pathology, University of Edinburgh, for access to ultramicrotome equipment. This study was performed while J.B.K. was supported by a C. J. Martin Fellowship from the National Health and Medical Research Council of Australia. References Akhtar, F.B., Marshall, G.R., Wickings, E.J. & Nieschlag, E. (1983) Reversible induction of azoospermia in rhesus monkeys by constant infusion of a gonadotropin-releasing hormone agonist using osmotic minipumps. J. clin. Endocr. Metab. 56, Akhtar, F.B., Wickings, E.J. & Nieschlag, E. (1984) Male fertility control with an LHRH agonist: primate studies. In LHRH and its Analogs. Contraceptive and Therapeutic Applications, pp Eds B. H. Vickery, J. J. Nestor and E. S. E. Hafez. MTP Press, Lancaster. Bambino, T.H., Schreiber, J.R. & Hsueh, A.J.W. (1980) Gonadotropin-releasing hormone and its agonist inhibit testicular luteinizing hormone receptor and steroidogenesis in immature and adult hypophy sectomized rats. Endocrinology 107, Christensen, A.K. & Peacock, K.C. (1980) Increase in Leydig cell number in testes of adult rats treated chronically with an excess of human chorionic gonadotropin. Biol. Reprod. 22, Corker, C.S. & Davidson, D.W. (1978) Radioimmuno assay of testosterone in various biological fluids without chromatography. J. Steroid Biochem. 9, Elias, H. & Hyde, D.M. (1980) An elementary intro duction to stereology (quantitative microscopy). Am. J.Anat. 159,412^146. Heber, D. & Swerdloff, R.S. (1981) Gonadotropinreleasing hormone analog and testosterone synergistically inhibit spermatogenesis. Endocrinology 108, Hsueh, A.J.W. & Erickson, G.F. (1979) Extra-pituitary inhibition of testicular function by luteinising hor mone releasing hormone. Nature, Lond. 281, Hsueh, A.J.W. & Jones, P.B.C. (1981) Extrapituitary actions of gonadotropin-releasing hormone. Endo crine Rev. 2,437^161. Jones, P.B.C. & Hsueh, A.J.W. (1984) Direct antigonadal actions of LHRH. In LHRH and its Analogs. Contraceptive and Therapeutic Applications, pp Eds B. H. Vickery, J. J. Nestor and E. S. E. Hafez. MTP Press, Lancaster. Labrie, F., Auclair, C, Cusan, L., Kelly, P.A., Pelletier, G. & Ferland, L. (1978) Inhibitory effect of LHRH and its agonists on testicular gonadotrophin recep tors and spermatogenesis in the rat. Int. J. Androl., Suppl. 2, Labrie, F., Belanger,., Cusan, L., Sequin, C, Pelletier, G., Kelly, P.A., Reeves, J.J., Lefebvre, F.A., Lemay,., Gomdeau, Y. & Raynaud, J.P. (1980a) Antifertility effects of LHRH agonists in the male. J. Andro!. 1, Labrie, F., Cusan, L., Sequin, C, Belanger,., Pelletier, G., Reeves, J., Kelly, P.A., Lemay, A. & Raynaud, J.P. (1980b) Antifertility effects of LHRH agonists in the male rat and inhibition of testicular steroido genesis in man. Int. J. Fértil. 25, Lefebvre, F.A., Belanger,., Pelletier, G. & Labrie, F. (1984) Recovery of gonadal functions in the adult male rat following cessation of five-month daily treatment with an LHRH agonist. J. Andro!. 5, Linde, R., Docili-. G.C., Alexander,., Kirchner, F., Vale, W., Rivier, J. & Rabin, D. (1981) Reversible inhibition of testicular steroidogenesis and spermato genesis by a potent gonadotropin-releasing hormone agonist in normal men. N. Eng!. J. Med. 305, Pelletier, G., Cusan, L., Auclair, C, Kelly, P.A., Desy, L. & Labrie, F. (1978) Inhibition of spermatogenesis in the rat by treatment with [D-Ala6, Des-Gly-NH210] LHRH ethylamide. Endocrinology 103, Pelletier, G., Cusan, G.L., Belanger,., Sequin, C, Kelly, P.A. & Labrie, F. (1980) Further studies on the inhibitory effect of [D-Ala6, des-gly-nh210] LHRH ethylamide on spermatogenesis and steroidogenesis in the rat: reversibility and effect of androgen administration. J. Andro!. 1, Rabin. D., Linde, R., Doelle, G. & Alexander, N. (1981) Experience with a potent gonadotropin-releasing hormone agonist in normal men: an approach to the development of a male contraceptive. In LHRH Peptides as Female and Male Contraceptives, pp Eds G. I. Zatuchni, J. D. Shelton and J. J. Sciarra. Harper and Row, Philadelphia. Rajfer, J., Swerdlofi, R.S. & Heber, D.M. (1984) Testicular histology following chronic gonadotropinreleasing hormone agonist treatment. Fert. Steril. 42, Rivier, C, Rivier, J. & Vale, W. (1979) Chronic effects of [D-Trp6,Pro9-NEt] luteinizing hormone-releasing factor on reproductive processes in the male rat. Endocrinology 105, Sandow, J. (1982) Gonadotropic and antigonadotropic actions of LHRH analogues. In Neuroendocrine
18 Perspectives, pp Eds E. E. Muller & R. M. MacLeod. Elsevier, Amsterdam. Sharpe, R.M. (1984) Intratesticular factors controlling testicular function. Biol. Reprod. 30, Sharpe, R.M. & Fraser, H.M. (1983) The role of LH in regulation of Leydig cell responsiveness to an LHRH agonist. Molec. cell. Endocr. 33, Sharpe, R.M., Doogan, D.G. & Cooper, I. (1983a) Factors determining whether the direct effects of an LHRH agonist on Leydig cell function in vivo are stimulatory or inhibitory. Molec. cell. Endocr. 32, Sharpe, R.M., Doogan, D.G. & Cooper, I. (1983b) Direct effects of a luteinizing hormone-releasing hormone agonist on intratesticular levels of testosterone and interstitial fluid formation in intact male rats. Endocrinology 113, Tremblay, Y. & Belanger,. (1984) Reversible inhibition of gonadal functions by a potent gonadotropinreleasing hormone agonist in adult dogs. Contracep tion 30, Vickery, B., McRae, G.I., Bergstrom, K., Briones, W., Worden,. & Seidenberg, R. (1983) Inability of longterm administration of D-Nal(2)6-LHRH to abolish fertility in male rats. J. Androl. 4, Vickery, B.H., McRae, G.I., Briones, W., Worden,., Seidenberg, R., Schanbacher, B.D. & Falvo, R. (1984) Effects of an LHRH analog upon sexual function in male dogs. Suppression, reversibility, and effect of testosterone replacement. J. Androl. 5, 28^12. Wang, N.G., Sundaram, K., Pavlou, S., Rivier, J., Vale, W. & Bardin, C.W. (1983) Mice are insensitive to the antitesticular effects of luteinizing hormone-releasing hormone agonists. Endocrinology 112, Weibel, E.R. (1979) Stereological Methods. Vol. 1, Prac tical Methodsfor Biological Morphomelry. Academic Press, London. Received 4 April 1985
Testicular interstitial fluid as a monitor for changes in the intratesticular environment in the rat
 Testicular interstitial fluid as a monitor for changes in the intratesticular environment in the rat R. M. Sharpe and Irene Cooper M.R.C. Reproductive Biology Unit, Centre for Reproductive Biology, 37
Testicular interstitial fluid as a monitor for changes in the intratesticular environment in the rat R. M. Sharpe and Irene Cooper M.R.C. Reproductive Biology Unit, Centre for Reproductive Biology, 37
Effect of a gonadotropin-releasing hormone agonist on luteinizing hormone and testosterone secretion and testicular histology in male rhesus monkeys*
 FERTILITY AND STERILITY Copyright c 1985 The American Fertility Society Printed in U.SA. Effect of a gonadotropin-releasing hormone agonist on luteinizing hormone and testosterone secretion and testicular
FERTILITY AND STERILITY Copyright c 1985 The American Fertility Society Printed in U.SA. Effect of a gonadotropin-releasing hormone agonist on luteinizing hormone and testosterone secretion and testicular
Testicular histology following chronic gonadotropin-releasing hormone agonist treatment
 FERTILITY AND STERILITY Copyright 1984 The American Fertility Society Printed in U.8A. Testicular histology following chronic gonadotropin-releasing hormone agonist treatment Jacob Rajfer, M.D.*t Ronald
FERTILITY AND STERILITY Copyright 1984 The American Fertility Society Printed in U.8A. Testicular histology following chronic gonadotropin-releasing hormone agonist treatment Jacob Rajfer, M.D.*t Ronald
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
$ To whom all correspondence should be addressed. Anita H. Payne,+ Kar-Lit Wong, and Margarita M. Vega
 THE JOURNAL OF B~OLOCICAL CHEMISTRY Vul. 255. No. 15. Issue of August 10. pp. 7118-7122, 1980 Prmted cn (I S. A. Differential Effects of Single and Repeated Administrations of Gonadotropins on Luteinizing
THE JOURNAL OF B~OLOCICAL CHEMISTRY Vul. 255. No. 15. Issue of August 10. pp. 7118-7122, 1980 Prmted cn (I S. A. Differential Effects of Single and Repeated Administrations of Gonadotropins on Luteinizing
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
The Male Reproductive System
 The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
Spermatogenesis Following Experimental Testicular Ischemia
 Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Neelakanta Ravindranath, Ph_D_t:\: Venkatarayappa Ramesh, M.Sc. t Heganahalli Narasimha Sastry Krishnamurthy, M.Sc.
 FERTILITY AND STERILITY Copyright e 1992 The American Fertility Society Vol. 57, No.3, March 1992 Printed on acid-free paper in U.S.A. Chronic suppression of testicular function by constant infusion of
FERTILITY AND STERILITY Copyright e 1992 The American Fertility Society Vol. 57, No.3, March 1992 Printed on acid-free paper in U.S.A. Chronic suppression of testicular function by constant infusion of
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE
 ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
Identification of the spermatogenic stages in living seminiferous tubules of man
 Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Efferent Ducts and Epididymis
 increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
Hormonal Control of Male Sexual Function
 Hormonal Control of Male Sexual Function A majority of the control of sexual functions in the male (and the female) begins with secretions of gonadotropin-releasing hormone (GnRH) by the hypothalamus.
Hormonal Control of Male Sexual Function A majority of the control of sexual functions in the male (and the female) begins with secretions of gonadotropin-releasing hormone (GnRH) by the hypothalamus.
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
Seminiferous Tubules
 Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Basic histology 5/4/2015
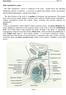 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
androgen on the seminal vesicles it had neither a blocking effect on the penile
 MORPHOLOGICAL AND BEHAVIOURAL EFFECTS OF AN 'ANTIANDROGEN' IN MALE RATS F. A. BEACH and W. H. WESTBROOK Department of Psychology, University of California, Berkeley, California 94720, U.S.A. (Received
MORPHOLOGICAL AND BEHAVIOURAL EFFECTS OF AN 'ANTIANDROGEN' IN MALE RATS F. A. BEACH and W. H. WESTBROOK Department of Psychology, University of California, Berkeley, California 94720, U.S.A. (Received
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
IN normal male fowls, four developmental stages of spermatogenetic activity
 Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Spermatogenesis in Man
 Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
川北医学院讲稿. Under low power note the testis is enclosed by a strong fibrous. layer of serous epithelium. These fibrous tissue
 川北医学院讲稿 Experiment 5: Male and Female Reproductive System Hello, everybody, class is begin,keep quiet, please. And this is the last experimental class. Today we will learn 5 slices and review all structures
川北医学院讲稿 Experiment 5: Male and Female Reproductive System Hello, everybody, class is begin,keep quiet, please. And this is the last experimental class. Today we will learn 5 slices and review all structures
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
Treatment of Oligospermia with Large Doses of Human Chorionic Gonadotropin
 Treatment of Oligospermia with Large Doses of Human Chorionic Gonadotropin A Preliminary Report S. J. GLASS, M.D., and H. M. HOLLAND, M.D. BEFORE discussing gonadotropic therapy of oligospermia, it is
Treatment of Oligospermia with Large Doses of Human Chorionic Gonadotropin A Preliminary Report S. J. GLASS, M.D., and H. M. HOLLAND, M.D. BEFORE discussing gonadotropic therapy of oligospermia, it is
Effect of Gonadotropic Hormones on Hypophysectomized (Anterior Lobe) Male Rana Pipiens
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 54, Issue 3 (May, 1954) 19545 Effect of Gonadotropic Hormones on Hypophysectomized
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 54, Issue 3 (May, 1954) 19545 Effect of Gonadotropic Hormones on Hypophysectomized
(Received 6th October 1972)
 EFFECT OF TESTOSTERONE POLYDIMETHYL- SILOXANE IMPLANTS UPON SPERM PRODUCTION, LIBIDO AND ACCESSORY SEX ORGAN FUNCTION IN RABBITS L. L. EWING, L. G. STRATTON and C. DESJARDINS Department of Physiological
EFFECT OF TESTOSTERONE POLYDIMETHYL- SILOXANE IMPLANTS UPON SPERM PRODUCTION, LIBIDO AND ACCESSORY SEX ORGAN FUNCTION IN RABBITS L. L. EWING, L. G. STRATTON and C. DESJARDINS Department of Physiological
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
GARY S. KLEDZIK LIONEL CUSAN CLAUDE AUCLAIR, PH.D. PAUL A. KELLY, PH.D. FERNAND LABRIE, M.D., PH.D.*
 FERTILITY AND STERILITY Copyright 1978 The American Fertility Society Vol. 3, No.3, September 1978 Printed in U S.A. INHIBITION OF OVARIAN LUTEINIZING HORMONE (LH) AND FOLLICLE-STIMULATING HORMONE RECEPTOR
FERTILITY AND STERILITY Copyright 1978 The American Fertility Society Vol. 3, No.3, September 1978 Printed in U S.A. INHIBITION OF OVARIAN LUTEINIZING HORMONE (LH) AND FOLLICLE-STIMULATING HORMONE RECEPTOR
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Production of Fertile Sperm. Animal Science 434. Hormonal Regulation of the Testis. hormonal regulation of the testis
 roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
Morphogenesis of the residual body of the mouse testis
 93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
MALE REPRODUCTIVE SYSTEM
 1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
The Fine Structure of the Epithelial Cells of the Mouse Prostate* II. Ventral Lobe Epithelium
 Published Online: 1 June, 1960 Supp Info: http://doi.org/10.1083/jcb.7.3.511 Downloaded from jcb.rupress.org on September 28, 2018 The Fine Structure of the Epithelial Cells of the Mouse Prostate* II.
Published Online: 1 June, 1960 Supp Info: http://doi.org/10.1083/jcb.7.3.511 Downloaded from jcb.rupress.org on September 28, 2018 The Fine Structure of the Epithelial Cells of the Mouse Prostate* II.
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
NEW EVIDENCE DEMONSTRATING THE MULTIVALENT NATURE OF HUMAN CHORIONIC GONADOTROPIN*
 FERTILITY AND STERR.ITY Copyright 1971 by The Williams & Wilkins Co. Vol. 22, No.1, January 1971 Printed in U.S.A. NEW EVIDENCE DEMONSTRATING THE MULTIVALENT NATURE OF HUMAN CHORIONIC GONADOTROPIN* S.
FERTILITY AND STERR.ITY Copyright 1971 by The Williams & Wilkins Co. Vol. 22, No.1, January 1971 Printed in U.S.A. NEW EVIDENCE DEMONSTRATING THE MULTIVALENT NATURE OF HUMAN CHORIONIC GONADOTROPIN* S.
Hormones of brain-testicular axis
 (Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
(Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
Fine Structure of the Normal Trigeminal Ganglion in the Cat and Monkey*
 Fine Structure of the Normal Trigeminal Ganglion in the Cat and Monkey* DAVID S. MAXWELL, PH.D. Principal Contributor and Leader of Discussion HE inclusion of animal material m a y be justified as a means
Fine Structure of the Normal Trigeminal Ganglion in the Cat and Monkey* DAVID S. MAXWELL, PH.D. Principal Contributor and Leader of Discussion HE inclusion of animal material m a y be justified as a means
Transport of Sperm. Endocrinology of the Epididymis and Sperm Maturation. Vas Efferentia. John Parrish Department of Animal Sciences
 Endocrinology of the Epididymis and Sperm Maturation John Parrish Department of Animal Sciences References: he Physiology of Reproduction, Knobil and Neill, 2006; Chapter on the Epididymis by Robaire ransport
Endocrinology of the Epididymis and Sperm Maturation John Parrish Department of Animal Sciences References: he Physiology of Reproduction, Knobil and Neill, 2006; Chapter on the Epididymis by Robaire ransport
(Haltmeyer & Eik-Neis, 1969; Katongole, Naftolin & Short, 1971). rutting behaviour and a rise in the male hierarchy (Lincoln, Youngson &
 THE RELATIONSHIP BETWEEN SOCIAL STATUS AND REPRODUCTIVE ACTIVITY IN MALE IMPALA, AEPYCEROS MELAMPUS P. S. BRAMLEY and W. B. NEAVES Departments of Animal Physiology and Anatomy, University of Nairobi, P.O.
THE RELATIONSHIP BETWEEN SOCIAL STATUS AND REPRODUCTIVE ACTIVITY IN MALE IMPALA, AEPYCEROS MELAMPUS P. S. BRAMLEY and W. B. NEAVES Departments of Animal Physiology and Anatomy, University of Nairobi, P.O.
Control of testicular vasomotion by testosterone and tubular factors in rats
 Control of testicular vasomotion by testosterone and tubular factors in rats O. Colin, A. Bergh, J-E. Damber and A. Widmark Departments of ^Anatomy, 2Pathology, and 3Urology and Andrology, 4Physiology,
Control of testicular vasomotion by testosterone and tubular factors in rats O. Colin, A. Bergh, J-E. Damber and A. Widmark Departments of ^Anatomy, 2Pathology, and 3Urology and Andrology, 4Physiology,
LYMPH GLAND. By : Group 1
 LYMPH GLAND By : Group 1 ANATOMY LYMPH NODE Lymphatic Organs Red bone marrow Thymus gland Lymph nodes Lymph nodules Spleen Primary organs Secondary organs Lymph Nodes Firm, smooth-surfaced, bean-shaped
LYMPH GLAND By : Group 1 ANATOMY LYMPH NODE Lymphatic Organs Red bone marrow Thymus gland Lymph nodes Lymph nodules Spleen Primary organs Secondary organs Lymph Nodes Firm, smooth-surfaced, bean-shaped
HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY
 FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses
 Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses using an anti-cre antibody; testes at 1 week (left panel),
Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses using an anti-cre antibody; testes at 1 week (left panel),
The Effects of Selective Withdrawal of FSH or LH. on Spermatogenesis in the Immature Rat
 BIOLOGY OF REPRODUCTION 14,489-494(1976) The Effects of Selective Withdrawal of FSH or LH on Spermatogenesis in the Immature Rat H. G. MADHWA RAJ and MARTIN DYM Departments of Obstetrics and Gynaecology
BIOLOGY OF REPRODUCTION 14,489-494(1976) The Effects of Selective Withdrawal of FSH or LH on Spermatogenesis in the Immature Rat H. G. MADHWA RAJ and MARTIN DYM Departments of Obstetrics and Gynaecology
Reproductive System Purpose General Structures Male Structures Functions Female Anatomy Structures Functions Clinical Applications
 The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
The Reproductive System: Male, Ch 23 Outline of class lecture After studying the male reproductive system you should be able to: 1. Define the purpose of reproduction and identify the general organs of
Leydig cell number and function in the adult cynomolgus
 Leydig cell number and function in the adult cynomolgus monkey (Macaca fascicularis) is increased by daily hcg treatment but not by daily FSH treatment K. J. Teerds, F. F. G. Rommerts, H. J. G. van de
Leydig cell number and function in the adult cynomolgus monkey (Macaca fascicularis) is increased by daily hcg treatment but not by daily FSH treatment K. J. Teerds, F. F. G. Rommerts, H. J. G. van de
IF SEXUALLY MATURE male rats are subjected to experimental cryptorchidism,
 Cryptorchidism Its Pre- and Postpubertal Effects on the Hypophysis of the Rat JAMES R. MOREHEAD, PH.D.,* and CHARLES F. MORGAN, Pn.D:r IF SEXUALLY MATURE male rats are subjected to experimental cryptorchidism,
Cryptorchidism Its Pre- and Postpubertal Effects on the Hypophysis of the Rat JAMES R. MOREHEAD, PH.D.,* and CHARLES F. MORGAN, Pn.D:r IF SEXUALLY MATURE male rats are subjected to experimental cryptorchidism,
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Caleb A. Awoniyi, Ph.D.t Woo Kyoon Kim, M.D. Bradley S. Hurst, M.D. William D. Schlaff, M.D.
 FERTILITY A STERILITY Copyright 1992 The American Fertility Society Printed on acid-free paper in U.S.A. Immunoneutralization of gonadotropin-releasing hormone and subsequent treatment with testosterone
FERTILITY A STERILITY Copyright 1992 The American Fertility Society Printed on acid-free paper in U.S.A. Immunoneutralization of gonadotropin-releasing hormone and subsequent treatment with testosterone
(b) Stomach s function 1. Dilution of food materials 2. Acidification of food (absorption of dietary Fe in small intestine) 3. Partial chemical digest
 (1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
(1) General features a) Stomach is widened portion of gut-tube: between tubular and spherical; Note arranged of smooth muscle tissue in muscularis externa. 1 (b) Stomach s function 1. Dilution of food
Pathology of Male Reproductive System 1
 Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells
 Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells By Setsuko Ogata Department of Anatomy, Tokyo Women's Medical College, Shinjuku, Tokyo, Japan (Director : Prof. Dr. Kura Kubota)
Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells By Setsuko Ogata Department of Anatomy, Tokyo Women's Medical College, Shinjuku, Tokyo, Japan (Director : Prof. Dr. Kura Kubota)
The Male Reproductive System
 The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
Chapter 14 Reproduction Review Assignment
 Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Date: Mark: _/45 Chapter 14 Reproduction Review Assignment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Use the diagram above to answer the next question.
Hypothalamus & Pituitary Gland
 Hypothalamus & Pituitary Gland Hypothalamus and Pituitary Gland The hypothalamus and pituitary gland form a unit that exerts control over the function of several endocrine glands (thyroid, adrenals, and
Hypothalamus & Pituitary Gland Hypothalamus and Pituitary Gland The hypothalamus and pituitary gland form a unit that exerts control over the function of several endocrine glands (thyroid, adrenals, and
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL CRYPTORCHIDISM: A PRELIMINARY REPORT*
 FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
Motility and eosin uptake of formaldehyde-treated ram
 Motility and eosin uptake of formaldehyde-treated ram spermatozoa O. A. Osinowo, J. O. Bale, E. O. Oyedipe and L. O. Eduvie Department ofanimal Reproduction, National Animal Production Research Institute,
Motility and eosin uptake of formaldehyde-treated ram spermatozoa O. A. Osinowo, J. O. Bale, E. O. Oyedipe and L. O. Eduvie Department ofanimal Reproduction, National Animal Production Research Institute,
The effect of thyroid activity on adult rat spermatogenesis
 The effect of thyroid activity on adult rat spermatogenesis Ai, J. 1* ; Zarifkar, A. 2 ; Takhshid, M. A. 3 ; Alavi, J. 1 and Moradzadeh, M. 2 1 Department of Anatomical Sciences, School of Medicine, University
The effect of thyroid activity on adult rat spermatogenesis Ai, J. 1* ; Zarifkar, A. 2 ; Takhshid, M. A. 3 ; Alavi, J. 1 and Moradzadeh, M. 2 1 Department of Anatomical Sciences, School of Medicine, University
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Epstein-Barr Virus: Stimulation By 5 '-Iododeoxy uridine or 5 '-Brom odeoxy uridine in Human Lymphoblastoid Cells F ro m a Rhabdom yosarcom a*
 A n n a ls o f C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, No. 6 Copyright 1973, Institute for Clinical Science Epstein-Barr Virus: Stimulation By 5 '-Iododeoxy uridine or 5 '-Brom odeoxy
A n n a ls o f C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, No. 6 Copyright 1973, Institute for Clinical Science Epstein-Barr Virus: Stimulation By 5 '-Iododeoxy uridine or 5 '-Brom odeoxy
Chapter 28: REPRODUCTIVE SYSTEM: MALE
 Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
Chapter 28: REPRODUCTIVE SYSTEM: MALE I. FUNCTIONAL ANATOMY (Fig. 28.1) A. Testes: glands which produce male gametes, as well as glands producing testosterone 2. Seminiferous tubules (Fig.28.3; 28.5) a.
GARY S. KLEDZIK, PH.D. LIONEL CUSAN CLAUDE AUCLAIR, PH.D. PAUL A. KELLY, PH.D. FERNAND LABRIE, M.D., PH.D.*
 , FERTILITY AND STERILITY Copyright" 19 The American Fertility Society Vol. 29, No.5, May 19 Printed in U.S.A. INHIBITORY EFFECT OF A LUTEINIZING HORMONE (LH)-RELEASING HORMONE AGONIST ON RAT OVARIAN LH
, FERTILITY AND STERILITY Copyright" 19 The American Fertility Society Vol. 29, No.5, May 19 Printed in U.S.A. INHIBITORY EFFECT OF A LUTEINIZING HORMONE (LH)-RELEASING HORMONE AGONIST ON RAT OVARIAN LH
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
African Trypanosomes
 African Trypanosomes Giemsa-stained blood smear of African trypanosomes viewed under the 100X objective lens. The block arrows denote trypomastigote forms of the African trypanosomes found within the blood
African Trypanosomes Giemsa-stained blood smear of African trypanosomes viewed under the 100X objective lens. The block arrows denote trypomastigote forms of the African trypanosomes found within the blood
DR. DARWISH H. BADRAN. Parathyroid glands
 Parathyroid glands History 1849 - Sir Richard owen provided 1st accurate description of normal parathyroid glands after examining Indian Rhinoceros 1879 - Anton Wölfer described tetany in a patient
Parathyroid glands History 1849 - Sir Richard owen provided 1st accurate description of normal parathyroid glands after examining Indian Rhinoceros 1879 - Anton Wölfer described tetany in a patient
The Effect of Cortisone on Cell Proliferation and Migration in Peripheral Nerves undergoing Wallerian degeneration
 The Effect of Cortisone on Cell Proliferation and Migration in Peripheral Nerves undergoing Wallerian by G. A. THOMAS 1 From the Department of Anatomy, Guy's Hospital Medical School, London INTRODUCTION
The Effect of Cortisone on Cell Proliferation and Migration in Peripheral Nerves undergoing Wallerian by G. A. THOMAS 1 From the Department of Anatomy, Guy's Hospital Medical School, London INTRODUCTION
Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization
 Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization may occur! A. Scrotum 1. Muscular pouch that holds the
Male Reproductive Structures I. Overview A. Main functions: 1. Produce a haploid male gamete (sperm) 2. Deposit sperm in the female so fertilization may occur! A. Scrotum 1. Muscular pouch that holds the
Reproductive FSH. Analyte Information
 Reproductive FSH Analyte Information 1 Follicle-stimulating hormone Introduction Follicle-stimulating hormone (FSH, also known as follitropin) is a glycoprotein hormone secreted by the anterior pituitary
Reproductive FSH Analyte Information 1 Follicle-stimulating hormone Introduction Follicle-stimulating hormone (FSH, also known as follitropin) is a glycoprotein hormone secreted by the anterior pituitary
REAPPRAISAL OF THE VALUE OF TESTICULAR BIOPSY IN THE INVESTIGATION OF INFERTILITY
 FERTWTY AND STEIuLlTY Copyright 1980 The American Fertility Society Vol., No.1 January 1980 Prinwl in U.S.A. REAPPRAISAL OF THE VALUE OF TESTICULAR BIOPSY IN THE INVESTIGATION OF INFERTILITY TERENCE
FERTWTY AND STEIuLlTY Copyright 1980 The American Fertility Society Vol., No.1 January 1980 Prinwl in U.S.A. REAPPRAISAL OF THE VALUE OF TESTICULAR BIOPSY IN THE INVESTIGATION OF INFERTILITY TERENCE
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
Physiologic Anatomy of the Male Sexual Organs
 Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
Reproductive and Hormonal Functions of the Male The reproductive functions of the male can be divided into three major subdivisions: (1) spermatogenesis, which means simply the formation of sperm; (2)
PATHOLOGY Intracellular Degeneration LAB 1
 PATHOLOGY Intracellular Degeneration LAB 1 Cellular swelling Liver Organ :- Liver Lesion :- 1. Narrowing of hepatic sinusoids due to the swelling of hepatocyte. 2. The cytoplasm of affected hepatocyte
PATHOLOGY Intracellular Degeneration LAB 1 Cellular swelling Liver Organ :- Liver Lesion :- 1. Narrowing of hepatic sinusoids due to the swelling of hepatocyte. 2. The cytoplasm of affected hepatocyte
Note: The cause of testicular neoplasms remains unknown
 - In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
- In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
different ratios of PMSG and HCG on the occurrence of follicular haemorrhage THE induction of ovulation with PMSG and HCG in the rat has been studied
 Q. Jl exp. Physiol. (1968) 53, 129-135 THE INDUCTION OF OVULATION IN IMMATURE RATS TREATED WITH PREGNANT MARE'S SERUM GONADOTROPHIN AND HUMAN CHORIONIC GONADOTROPHIN. By S. F. LUNN and E. T. BELL. From
Q. Jl exp. Physiol. (1968) 53, 129-135 THE INDUCTION OF OVULATION IN IMMATURE RATS TREATED WITH PREGNANT MARE'S SERUM GONADOTROPHIN AND HUMAN CHORIONIC GONADOTROPHIN. By S. F. LUNN and E. T. BELL. From
Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey
 J. Biosci., Vol. 2, Number 3, September 1980, pp. 261-266. Printed in India. Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey ASHA PRAKASH, M. R. N. PRASAD and T.C. ANAND KUMAR
J. Biosci., Vol. 2, Number 3, September 1980, pp. 261-266. Printed in India. Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey ASHA PRAKASH, M. R. N. PRASAD and T.C. ANAND KUMAR
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
12/3/12. Managing Bull Development to Optimize Fertility Rearing bulls for fertility
 Managing Bull Development to Optimize Fertility Rearing bulls for fertility Effect of post weaning nutrition (after normal calf hood nutrition) - testis size - age at puberty - semen quality Effect of
Managing Bull Development to Optimize Fertility Rearing bulls for fertility Effect of post weaning nutrition (after normal calf hood nutrition) - testis size - age at puberty - semen quality Effect of
INTRODUCTION MATERIALS AND METHODS
 SPERM DENSITY AND ULTRASTRUCTURE OF SERTOLI CELLS IN MALE RATS TREATED WITH KAEMPFERIA PARVIFLORA WALL. EX BAKER EXTRACT Paiwan Sudwan 1,2, Kanokporn Saenphet 1, Supap Saenphet 1 and Chalobol Wongsawad
SPERM DENSITY AND ULTRASTRUCTURE OF SERTOLI CELLS IN MALE RATS TREATED WITH KAEMPFERIA PARVIFLORA WALL. EX BAKER EXTRACT Paiwan Sudwan 1,2, Kanokporn Saenphet 1, Supap Saenphet 1 and Chalobol Wongsawad
ROLE OF SOME ANTIOXIDANTS ON MERCURY CHLORIDE INDUCED SPERMATOGENESIS IN SWISS ALBINO MICE DURING PRE PUBERTAL PHASE OF LIFE
 Indian J.Sci.Res.1(2) : 19-25, 2010 ROLE OF SOME ANTIOXIDANTS ON MERCURY CHLORIDE INDUCED SPERMATOGENESIS IN SWISS ALBINO MICE DURING PRE PUBERTAL PHASE OF LIFE a1 DUGESH NANDINI SHARMA AND LATA BHATTACHARYA
Indian J.Sci.Res.1(2) : 19-25, 2010 ROLE OF SOME ANTIOXIDANTS ON MERCURY CHLORIDE INDUCED SPERMATOGENESIS IN SWISS ALBINO MICE DURING PRE PUBERTAL PHASE OF LIFE a1 DUGESH NANDINI SHARMA AND LATA BHATTACHARYA
Developmental stages of fetal-type Leydig cells in prepubertal rats
 Development 107, 213-220 (1989) Printed in Great Britain The Company of Biologists Limited 1989 213 Developmental stages of fetal-type Leydig cells in prepubertal rats T. KUOPIO 1 *, J. TAPANAINEN 2, L.
Development 107, 213-220 (1989) Printed in Great Britain The Company of Biologists Limited 1989 213 Developmental stages of fetal-type Leydig cells in prepubertal rats T. KUOPIO 1 *, J. TAPANAINEN 2, L.
KENJI OHYAMA, MASANORI OHTA, YosHIKo NAKAGOMI, YOSHIE SHIMURA, T0M0AKI SANG, KAZUMASA SATO, SHINPEI NAKAZAWA, AND HIROMICHI ISHIKAWA*
 Endocrine Journal 1997, 44 (4), 459-465 Restoration of Seminiferous Tubular Function after Discontinuation of Long-Term Gonadotropin-Releasing Hormone Agonist Administration in Premature Male Rats KENJI
Endocrine Journal 1997, 44 (4), 459-465 Restoration of Seminiferous Tubular Function after Discontinuation of Long-Term Gonadotropin-Releasing Hormone Agonist Administration in Premature Male Rats KENJI
DAX1, testes development role 7, 8 DFFRY, spermatogenesis role 49 DMRT genes, male sex differentiation role 15
 Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
gonadotrophin-induced disruption of spermatogenesis
 Macrophage activation enhances the human chorionic gonadotrophin-induced disruption of spermatogenesis in the rat J. B. Kerr and R. M. Sharpe Department of Anatomy, Monash University, Clayton, Melbourne,
Macrophage activation enhances the human chorionic gonadotrophin-induced disruption of spermatogenesis in the rat J. B. Kerr and R. M. Sharpe Department of Anatomy, Monash University, Clayton, Melbourne,
Male reproductive system The physiology of sexual act
 Male reproductive system The physiology of sexual act Gabriella Kékesi 65. The development and physiology of the male reproductive system. The physiology of the sexual act Define chromosomal, gonadal and
Male reproductive system The physiology of sexual act Gabriella Kékesi 65. The development and physiology of the male reproductive system. The physiology of the sexual act Define chromosomal, gonadal and
Department of Medicine Harbor-UCLA Medical Center Torrance, California ABSTRACT
 BOLOGY OF REPRODUCTON 37, 5 5-59 (1987) Testosterone Selectively ncreases Serum Follicle-Stimulating Hormonal (FSH) But Not Luteinizing Hormone (LH) in Gonadotropin-Releasing Hormone Antagonist-Treated
BOLOGY OF REPRODUCTON 37, 5 5-59 (1987) Testosterone Selectively ncreases Serum Follicle-Stimulating Hormonal (FSH) But Not Luteinizing Hormone (LH) in Gonadotropin-Releasing Hormone Antagonist-Treated
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1
 Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
Prelab #4 BLOOD; BONE MARROW; RESPIRATORY; INTEGUEMENT Page 1 Blood Slide 101 This a classic slide of blood cells using a Wright stain. Inspect red blood cells and their appearance. Note the approximate
