Morphometric Studies on Rat Seminiferous Tubules
|
|
|
- Gary Lucas
- 6 years ago
- Views:
Transcription
1 THE AMERCAN JOURNAL OF ANATOMY 165:1-25 (1982) Morphometric Studies on Rat Seminiferous Tubules TUNG-YANG NG AND A. KENT CHRSTENSEN Department of Anatomy and Cell Biology, The University of Michigan Medical School, Ann Arbor, Michigan ABSTRACT The goal of this morphometric study was to obtain quantitative information on the seminiferous tubules of Sprague-Dawley rats, including changes seen at various stages of the cycle of the seminiferous epithelium. Tissue from perfusion-fixed testes was embedded in Epon-Araldite; and sections were subjected to morphometric measurements at the light microscopic level, using point counting for volume densities and the Floderus equation for numerical densities. Changes occur in the diameter of the seminiferous tubule, as well as in the volume of the seminiferous epithelium and tubule lumen, from stage to stage during the cycle. A significant constriction of the seminiferous tubule accompanies spermiation. The volume of the seminiferous epithelium per unit length of the tubule begins to increase after stage XV, and peaks at stage V of the next cycle. The tubule lumen increases dramatically from stages V to V, at the expense of the epithelium. The number of Sertoli cells is constant per unit length of the seminiferous tubule at all stages of the cycle. This is also true for primary spermatocytes of various developmental phases and for round spermatids from step 1 through step 10 of spermiogenesis. The average number of younger (preleptotene, leptotene, zytgotene) primary spermatocytes per Sertoli cell is (SEM), the number of older (pachytene, diplotene) primary spermatocytes per Sertoli cell is , and the ratio of step 1-10 spermatids to Sertoli cells is By studying tangential views of serially sectioned seminiferous tubules at stage V, it is shown that the number of step-17 spermatids associated with each Sertoli cell averages , although the counts ranged from 6 to 11. The only appreci- able occurrence of cell death after the last spermatogonial mitosis appears to be a 15% loss during the first meiotic division. From our morphometric results, corrected for volume changes during preparation for microscopy, there are 15.7 million ( million) Sertoli cells per gram of fresh rat testis. The length of seminiferous tubule per gram of testis is estimated to be meters, and the tubule surface area per gram testis is * 2.57 cm2. The daily production of mature spermatids is 9.61 million ( million) per gram of testis. Accurate morphometric information can provide answers to important questions about the spermatogenic process, and is also valuable for correlations with physiological and biochemical findings in order to develop a more complete understanding of spermatogenesis. The literature on the rat testis contains a considerable number of quantitative studies on cell numbers in the seminiferous tubule, and on changes in the tubule at various stages of the cycle of the seminiferous epithelium (Roosen- Runge and Giesel, 1950; Clermont and Leblond, 195; Roosen-Runge, 1955a,b; Clermont and Morgentaler, 1955; Clermont and Perey, 1957; Clermont, 1962; Bustos-Obregon, 1970; Clermont and Hermo, 1975; Russell and Clermont, 1977). mprovements in stereological principles and methods over the last decade (eibel and Bolender, 197; Bolender, 1978; Elias and Hyde, 1980) have made it seem Resented in partial fultillment of requirements for the Ph.D. degree in the Department of Anatomy and Cell Biology at the Universky of Michigan Medical School. Dr. ing s current address is- Division of Reproductive Biology. Department of Population Dynamics. School of Hygiene and Public Health. The Johns llopkins University. Baltimore. MD Reprint requests should he sent to Dr. Christensen. Received December Accepted May $04.00 (c: 1982 ALAN R. LSS. NC
2 14 T:Y. NG AND A.K. CHRSTENSEN worthwhile to reassess some of the previous quantitative conclusions, as well as to lookinto some additional morphometric aspects that may have functional correlations. n this study we have sought detailed quantitative information on the rat seminiferous tubule. e have counted the numbers of Sertoli cells and germ cells (from preleptotene primary spermatocytes to step-10 spermatids) that are present in unit volume and in unit length of the seminiferous tubule, as well as in unit volume of the testis. e have related the number of germ cells of various types to the number of Sertoli cells to give some insight into the supportive load carried by Sertoli cells. By comparing the number of germ cells of particular developmental phases (beyond spermatogonia), we have shown where cell death occurs during spermatogenesis. e have also followed variations in size of the seminiferous tubule and its component epithelium and lumen throughout the cycle. e have derived the total length of the seminiferous tubule in a rat testis and have estimated the average number of mature spermatids produced per day by the rat testis. n an effort to obtain quantitative information that is as accurate and useful as possible, we have included various procedures in our study that have not been common in previous quantitative studies on seminiferous tubules. For example, we have used perfusion fixation of the testis with glutaraldehyde fixative and have embedded it in Epon-Araldite to provide favorable structural preservation. e have sought absolute rather than relative values for cell numbers, utilizing the Floderus equation. The resulting estimates of cell number per gram of tissue may be useful in correlating these results with biochemical studies. Our final data have been corrected for the considerable volume changes that take place during preparation for microscopy; thus the data provide values for cell numbers per gram of fresh tissue. The large body of information generated by our counts has been analyzed and formulated by morphometric methods using the central computer system of the University of Michigan to improve the accuracy and flexibility of data handling. Extensive statistical programs available with this computer system have been utilized to test the validity and significance of the results. By these means we have attempted to obtain quantitative data that may serve as a baseline for correlation with studies of various kinds in the future. MATERALS AND METHODS Four male Sprague-Dawley rats of 64,54,77, and 77 days of age, and weighing respectively 256 gm, 284 gm, 29 gm and 18 gm, were used in this study. The animals were acclimated on a 14-hour light and 10-hour dark schedule and fed with normal rat chow and water ad libitum. Animals were sacrificed between 1O:OO a.m. and noon. Under ether anesthesia, both testes of an animal were perfusion-fixed (Christensen, 1965) with 5% glutaraldehyde in 0.1 M s-collidine buffer of ph 7.4, containing 0.005% CaCl,, at room temperature for 45 minutes. The volume of the perfused testis was measured by water displacement. Each testis was sliced into 10 slices perpendicular to the long axis of the organ. The better fixed tesis of the two from an animal was used for the study. Slices were diced into tissue blocks about 2 mm on a side. Thirty tissue blocks, selected randomly, were left in 0.1 M s-collidine buffer (ph 7.4) overnight, dehydrated with graded ethanols (70%, 80%, 95%, and loo%), and embedded in Epon-Araldite. The tissue blocks were oriented to have most of the seminiferous tubules cut in cross section. Sections were cut with glass knives on a Porter-Blum MT-2 ultramicrotome, with the thickness reading set at 4 pm and the speed of the block movement kept constant throughout the sectioning in the study. Three to four sections were placed on a drop of water on a glass slide, dried on a hot plate (about 70 C), and stained with 1% borate-toluidine blue for minutes. The basic morphometric methods used in this study are described in reviews by eibel and Bolender (197) and Elias and Hyde (1980), which can be consulted for background and formula derivations. One section from each of the 0 tissue blocks sampled from each animal was used for analysis. The sections were analyzed under a binocular Zeiss microscope fitted with a 400-point Bausch and Lomb reticle in one of its 8 x eyepieces. n the morphometric measurements, the orientation of the eyepiece, and therefore the reticle, was random. The volume densities (volume per unit volume) of the interstitial tissue and the seminiferous tubule in the testis were measured with a Zeiss 16 x10.40 N.A. neofluar objective. On each section, the volume density was derived from the number of points over the respective structure divided by the total points over the testicular tissue. The mean of the 0 measure-
3 MORPHOMETRY OF SEMNFEROUS TUBULES 15 ments for each animal was taken as thevolume density of the structure in the animal. Every complete seminiferous tubule profile in a section (usually 5-10) was included in the following analyses. The stage of the cycle of the seminiferous epithelium seen in the profile was identified according to Leblond and Clermont (1952a,b). Owing to the lack of reliable criteria to differentiate stages -V in the present material, they were pooled as a single group in analyses. Diameters of the tubule were measured across the minor axis of their profiles. The volume and surface area of the seminiferous tubule were calculated from the diameter, as is described below. The relative volume of the lumen in the tubule (luminal ratio) was measured by point-counting five times for each tubule profile, the orientation of the reticle and the position of the seminiferous tubule profile being changed for each measurement; the multiple measurements permitted a relative standard error of less than 5% to be obtained for the least numerous stages of the cycle. The counts of seminiferous-tubule profile areas were rather consistent, as indicated by a coefficient of variation of 7% or less for each seminiferous tubule used in this study, which is adequate for most morphometric studies. The volume of the seminiferous epithelium or tubular lumen per unit length of the tubule for each stage of the cycle was derived from luminal ratios and diameters. These values, together with the diameter changes during the cycle, yield insight into cyclic events of the seminiferous epithelium. From 150 to 200 seminiferous-tubule profiles were sampled and analyzed for each of the four animals used in this study. nformation about the animal, stage, and original measurements of individual seminiferous-tubule profiles was stored in computer files in the central computer facility of the University of Michigan and processed with a statistical package, MDAS (Michigan nteractive Data Analysis System, Fox and Guire, 1976). Calculations or transformations were carried out in MDAS, and stage differences in seminiferous tubule diameter, luminal ratio and epithelial or luminal volume per unit length of the seminiferous tubule were compared by using one-way ANOVA and Scheffe s test in MDAS (Snedecor and Cochran, 1980). Relationships among volume, length, and surface area of the seminiferous tubule per testis or per cm of testis were derived from a tube model in which the seminiferous tubule was assumed to be a round tube with a radius (r), a length (L) through the tubular center, and a basement-membrane surface area (S). According to the standard equations for such a cylinder, the volume (V) equals m2l, while S = 2mL. Since we can measure the volume and can derive the radius from the measured average diameter of the seminiferous tubule, it is possible to calculate the length and the surface area. The seminiferous tubule of a rat actually shows numerous hairpin turns (Clermont and Huckins, 1961), but geometric considerations indicate that these turns would not affect our analyses. The curved portion of a hairpin turn can be represented geometrically as a segment of a torus with a radius of curvature (R), and with r, S, and L defined as above. The volume of a torus is V = 27rY2R, the surface areais S = 47r2rR, while the length is L = 27rR. From the last equation it is clear that R = L/(27r), and so V = m2l and S = 2mL, which is the same as for a cylinder. t can be shown that the same equations are valid for any segment of a torus. The relationship between volume, surface area, and length used in the present study for seminiferous tubules are therefore appropriated whether the tubule is straight or bent. The number of germ cells and Sertoli cells per unit volume of the seminiferous tubule (numerical density) was derived with the Floderus (1944) equation N, = N,/(T + D - 2h) where N, is the numerical density, N, is the number of nuclei or nucleoli counted per unit area of the seminiferous tubule profile, T is the section thickness, D is the diameter of the object, and h is the height of the smallest visible cap sections (see below). Methods used to secure a reliable estimation of each parameter need further explanation. The counts that yield N, were carried out under oil immersion with a Zeiss 100 x 11.0 N.A. planapochromatic objective. The nuclear profile of each germ cell in the seminiferous tubule, from preleptotene primary spermatocytes to step-10 spermatids, was counted in the respective seminiferous tubules. The number of Sertoli-cell nucleoli was also recorded. The seminiferous-tubule profile area was determined by point counting. Throughout this study, no more than one distinguishable nucleolus was ever observed in a Sertoli-cell nuclear profile. Extensive analysis of serial sections showed that each Sertoli cell
4 16 T.-Y. NG AND A.K. CHRSTENSEN contains one and only one prominent nucleolus, which was therefore used to represent the Sertoli cell number. These findings are in agreement with those of Bustos-Obregon (1970). The corrected thickness of the sections (T) used in the equation was derived from the measurement of cross sections of 28 reembedded sections. The thickness was found to be pm (SEM). Measurements of the diameter (D) of Sertoli-cell nucleoli and germ-cell nuclei were carried out by the method of Christensen and Peacock (1980). Measurements of long and short axes of these structures showed that they were essentially spherical. The height of the smallest visible cap sections (h) of a nucleus or nucleolus was assumed to be x0 of the diameter of the structure (Mori and Christensen, 1980). An exception was made for the very large primary spermatocytes at pachytene and diplotene phases, for which the value of h was taken to be 0.5 pm. n morphometric studies it is customary to express volumes in terms of cm. However, for biochemical applications of morphometric data, it is generally more useful to have the volume information in terms of grams. The two values cm and grams are essentially interchangeable, since the specific gravity of fresh testis tissue from rats is very near unity, * SEM (Mori and Christensen, 1980). e have therefore described the numerical densities presented in Table in terms of number per gram of testis tissue, although in a strict sense they are actually number per cm of testis tissue. The number of cells of a given type per unit length of the seminiferous tubule (N,) was derived by multiplying the numerical density of the cell in the seminiferous tubule (N,) by the average cross-sectional area of the seminiferous tubule of this stage, of N, = N,m2. This relationship is derived as follows: f r is the average radius of the tubule, n is the cell number in the seminiferous-tubule segment, and V is the volume and L is the length of the segment, then by definition N, = n/v and N, = n/l, yielding VN, = LN, or N, = N,(V/L). However, for a cylinder, V = m2l or VL = m2, which gives N, = Nv7rr2. Calculations or transformations of numerical densities and cell numbers per unit length of the seminiferous tubule were performed in MDAS. Stage differences in these values were compared with one-way ANOVA and Scheffe s test in MDAS at the 95% level, on the basis of individual seminiferous tubule profiles (Snedecor and Cochran, 1980). The number of germ cells per Sertoli cell was obtained by comparing average cell numbers in a unit length of the seminiferous tubule. The rate of cell death during spermatogenesis was derived by similar numerical comparisons. The number of step-17 spermatids in a cluster within individual Sertoli-cell trunks was determined by studying serial sections of longitudinally oriented tubules in each of the four animals used in this study. These sections allowed the spermatid clusters to be viewed in cross section in successive serial sections, and the number of spermatids constituting the cluster to be counted. Tubules at stage V of the cycle were analyzed in 2-pm serial sections photographed at 400x magnification, and 100 clusters were analyzed. The clusters were used only if the spermatids could be traced throughout their length in the seminiferous epithelium. t was important to determine the change in volume that the testis tissue undergoes during preparation for microscopy in order to relate the data obtained on sections to values for the fresh testis. The alterations during tissue preparation might be expected to occur during perfusion-fixation, dehydration and embedding, and during sectioning. Changes during perfusion-fixation were measured in four testes from two Sprague- Dawley rats. Testes were taken from the anesthetized animals, the surrounding connective tissue was trimmed away, and their volumes were measured by fluid displacement before and after the perfusion-fixation. The perfusionfixed testis was 1.4% % (SEM) larger than the fresh testis, corresponding to an increase in linear dimension of 2.8% (1.14 = 1.028). The change during dehydration and embedding was measured in 10 tissue blocks from each of the four animals used in the morphometric measurements. The length of an axis in a tissue block was measured with a Zeiss 2.5 plan objective. The tissue blocks were then processed through the usual procedure of dehydration and embedding. After the polymerization of the resin, the axial length was measured again. The index of shrinkage in one dimension was derived as the ratio of the length of the axis in resin to the length of the same axis before dehydration and embedding. The value obtained was * (SEM), or a reduction of 22.87%. All axes showed similar indices. This change in linear dimension during dehydration and embedding would cause the volume of the embedded tissue to be reduced to 45.88% ( = ) of the value the tissue had after perfusion-fixation.
5 MORPHOMETRY OF SEMNFEROUS TUBULES 17 The effect of compression during sectioning was verified by comparing the vertical height of every trimmed tissue block with the corresponding length of sections cut from the block. The change amounted to a factor of only f (SEM, n = 120). Since this factor is very small, and would be present only in one dimension of the section, it has been ignored in the data corrections. The combined effect of the changes described above can be evaluated by multiplying the change factors for the successive stages of specimen preparation. Thus the overall change in linear dimension from the fresh testis to the tissue in the final sections is a factor of 0.79 (= x 0.771), which reflects a reduction of 21% in linear dimension during processing for microscopy. Since volume is the cube of linear dimension, the change in volume is more striking. During the complete procedure in the present study, the testis tissue was reduced to a volume 52.21% (= 1.18 x ) that of the fresh tissue. These correction factors have been applied to all the data in this paper in order to provide values expressed in terms of the intact testis. For example, for numerical densities, the number of cells counted per unit volume of tissue was multiplied by 0.52 to provide the number of cells per cm of fresh tissue. The length of seminiferous tubule as calculated from measurements was multiplied by ( to the power) in order to obtain the length of seminiferous tubule per cm of fresh testis. The surface area of basement membrane of the seminiferous tubule, derived from the sections, was multiplied by (the cube root of ) to give the total surface area per cm of intact testis. RESULTS The quality of preservation in the perfusionfixed, plastic-embedded material used in this study (Fig. 1,2) makes it favorable for morphometric analysis. Tissue relationships are well maintained, and stages of the cycle of the seminiferous epithelium (Leblond and Clermont, 1952a,b) can be identified clearly, except for stages to V which were analyzed as a single group for the purposes of this study. Changes in the diameter of the seminiferous tubule at various stages of the cycle are shown in Figure. The diameter increases significantly (p < 0.05) from stages -V to stages V and V, and then undergoes a significant decrease (p < 0.05) from stages V to X. Thereafter, the diameter remains significantly smaller than that of stages V and V until stage V of the next cycle. No differences in diameters are found associated with the meiotic divisions. There is a significant increase in volume of the lumen per centimeter of seminiferous tubule (Fig. ) between stages V and V, leading up to spermiation; this is followed by a significant decrease in the luminal volume which occurs simultaneously with the decrease in tubular diameter. The prominent decrease in the diameter of the seminiferous tubule is caused by the depletion of the luminal contents instead of by the loss of the germinal epithelium, since the latter remains relatively constant over the portion of the cycle in which the tubule diameter is decreasing (Fig. ). The volume of the germinal epithelium per unit length of seminiferous tubule increases significantly (p < 0.05) from stage XV to reach a maximum at stage V of the next cycle; thereafter there are significant decreases from stages V to V and from stages V to V. Figure 4 shows cell numbers per unit length of the seminiferous tubule at various stages of the cycle. No significant difference in cell number for a given cell category can be found between any two stages of the cycle. The distribution of Sertoli cells or a particular category of germ cells along the seminiferous-tubule length is thus concluded to be constant throughout the cycle in spite of the size fluctuations in the tubule. Figure 4 does not include secondary spermatocytes. The number of secondary spermatocytes per cm length of the seminiferous tubule in which they occur is 6.5 x lo4 f 0.52 x lo4 (SEM, n = 4). Table 1 shows the numerical ratios of various germ-cell categories to one another, on the basis of their numbers per unit length of seminiferous tubule. The numerical ratio of the younger (preleptotene, leptotene, and zygotene phases) and the older (pachytene and diplotene) generations of primary spermatocytes indicates that there is no cell loss as the younger generation develops into the more mature primary spermatocytes. The numerical ratio of secondary spermatocytes to the older generation of primary spermatocytes is 1.70, which is less than the value of 2.0 that would be expected if no cell loss occurred during the first meiotic division. The cell death rate during the first meiotic division is therefore about 15% (14.8% +.070, SEM). Table 1 also shows a numerical ratio of 1.98 between spermatids (steps 1-10) and secondary spermatocytes, indicating an absence of cell death during the second meiotic division.
6 18 T:Y. NG AND A.K. CHRSTENSEN Fig. 1. Light micrograph of rat testis fixed by perfusion and embedded in Epon-Araldite. The seminiferous tubules and interstitial tissue are well-maintained, providing favor- able material for morphometric analysis. Stagesof thecycleof the germinal epithelium are labeled. x 215. Bar = 100 pm.
7 MORPHOMETRY OF SEMNFEROUS TUBULES 19 Fig. 2. Representative stages of the cycle of the seminiferous epithelium viewed at higher magnification, showing the level of preservation. Shown are A) stages LV, which are pooled in the present study, B) stage V, C) stage V, D) stage X, E) stage X, and F) stage XV. Labeled in the figures are Sertoli cells (SER); type B (B) and intermediate (NT spermatogonia; primary spermatocytes in preleptotene (PRE), leptotene (LEP), zygotene (ZYG), pachytene (PAC), and diplotene (DP) phases; secondary spermatocytes (SEC); and spermatids at various steps (arabic numerals) of spermiogenesis. x 575. Bar = 50 pm.
8 20 T.-Y. NG AND A.K. CHRSTENSEN E, [r n $ 175- m / Ld-4\ T T SPERM1 AT ON \ 1 ' volume '\ of lumen 1 MEOTC DVSONS 1 i -V v V V Vlll X x X X1 Xlll XV -V STAGES OF THE CYCLE p 5 - E x J LL ' LL > E \ b - _ m - LL 0 \ 4.00 k a.50 g r.00 d > Fig.. Diameter of the seminiferous tubule, and volume of the epithelium and the lumen per unit length of seminifer- ous tubule at various stages of the cycle. Each value represents the mean of four animals (i SEM). TABLE 1. Numerical ratios of various germ cell generations* Ratios Ratio of To (.t SEM) Pachytene and diplotene primary spermatocytes Secondary spermatocytes Young generation spermatids (steps 1-10) Young generation spermatids (steus 1-10) Preleutotene. Pachytene and diplotene primary 1.70 i spermatocytes Secondary 1.98 f spermatocytes Pachytene and diplotene primary.4 f suermatocvtes *Ratios derived from cell numbers per unit length lcml of the seminiferous tubule. n order to verify further the overall cell death rate over the two meiotic divisions, the numerical ratios of spermatids to the older-generation primary spermatocytes is seen in Table 1 to be.4, which is about 15% (16.4% *.970, Cell type TABLE 2. Number of zerm cells wer Sertoli cell* Average ( + SEM) Preleptotene, leptotene, or zygotene primary 2.4.t 0.82 spermatocytes Pachytene or diplotene primary spermatocytes 2.7 f Secondary spermatocytes 4.04 f 0.2 Spermatids (steps 1-10] 7.89 f *Ratios derived from cell numbers per unit length (cm) of the seminiferous tubule. SEM) lower than the value, 4.0, that would be expected in the absence of any cell death. Germ-cell-to-Sertoli-cell numerical ratios have been derived by dividing the average number of a category of germ cells per unit length of the seminiferous tubule by the number of Sertoli cells contained in that length. As is shown in Table 2, the average number of younger generation (preleptotene, leptotene, zygotene) primary spermatocytes per Sertoli cell is ; the number of older gener-
9 MORPHOMETRY OF SEMNFEROUS TUBULES 21 YOUNG GENERATON SPEFlMATlDS PACHYTENE AND DPLOTENE PRMARY SPERMATOCYTES 11 f 9 PRELEPTOTENE, LEPTOTENE AND ZYGOTENE PRMARY SPEFMAlOCYTES E P P SERTOL CELLS P f P P -V v V V vlll X x X X1 xlll XV -V STAGES OF THE CYCLE Fig. 4. Number of various categories of cells per unit length of the seminiferous tubule at successive stages of the cycle. There is no significant difference in cell number for a given category between any two stages. ation (pachytene, diplotene) primary spermatocytes per Sertoli cell is 2.7 * (SEM); and there are 4.04 * 0.2 secondary spermatocytes and spermatids for each Sertoli cell. At stage V of the cycle, the step-17 spermatids are located in clusters in the Sertoli-cell cytoplasmic trunks. The results of our counts on serial sections (Fig. 5) show an average of 8.5 f (SEM, n = 100) spermatids per cluster, but the number ranged from 6 to 11. n no case did two clusters converge on the same Sertoli nucleus. Sertoli-cell and germ-cell numbers per testis and per gram of testis can be found in Table. A gram of testis contains 15.7 million Sertoli cells, 20.5 million younger generation primary spermatocytes, 5.8 million older generation (pachytene, diplotene) primary spermatocytes, 2.5 million secondary spermatocytes, and 9.4 million step 1-10 spermatids. The average length of seminiferous tubule in a gram of rat testis is 12.9 * 0.56 m, and the surface area of these tubules per gram testis is cm2. The corresponding values per average testis in the present study are 17.4 m of seminiferous tubule and cm2 of surface area. n assessing the daily rate of sperm production, based on counts of step 1-10 spermatids, it is important to know whether there is significant loss of spermatids after step 10. From the number of step-17 spermatids we found clustered in Sertoli cell cytoplasm, as well as the fact that we did not observe evidence for a reported loss at about stages 9-10 (see Discussion), we felt justified in concluding that no significant loss of spermatids occurs during the
10 22 T.-Y. NG AND A.K. CHRSTENSEN 19 steps of spermiogenesis. The time necessary to process the total number of step 1-10 spermatids in a testis (or gram of testis) would thus be the time period from stages through X of the cycle. The complete cycle in Sprague-Dawley rats has a duration of 12.9 days (Clermont and Harvey, 1965). The time necessary for the cycle to proceed from stage to stage X is 75.58% % of the total cycle (Clermont and Harvey, 1965; Perey et al., 1961; Leblond and Clermont, 1952a; and measurements car- 0 lfi,,,,,, NUMBER OF SPERMATDS Fig. 5. Number of step-7 spermatids per cluster at stage V of the cycle. Each cluster is embedded in a trunk of Sertoli cell cytoplasm. The range of values, from 6 to 11, shows that the mechanism by which the spermatids are segregated to the Sertoli cell trunks allows a certain flexibility in the number of spermatids included in the clusters. ried out in the present study), which would correspond to a duration of 9.75 * days. The average daily production of sperm per testis is therefore 1.42 x lo x lo6 (SEM), and the rate per gram (or cm) of testis is 9.61 x x lo6. DSCUSSON n this study we have assessed various quantitative aspects of rat seminiferous tubules, using current morphometric methods, with care to provide adequate sampling and statistical analysis. The effect of volume change during tissue preparation has been corrected for in the present study to give values corresponding to the fresh organ. n this study, the net shrinkage of testicular tissue from the fresh condition to sections was found to be 21% in one dimension, thus making the volume of the tissue in sections only 52.2% of its volume in the fresh condition. Such corrections have not been utilized commonly in the literature on testicular quantitation; an exception is de Jong and Sharpe (1977a,b). Many of the previous quantitative studies on the rat spermatogenic process have been concerned with where cell death occurs during spermatogenesis. Those studies have utilized various sampling, histological, and analytical techniques. Roosen-Runge and his co-workers (1951; 1955a,b; Roosen-Runge and Giesel, 1950) did early work on the quantitative changes in the seminiferous tubule at different stages of the cycle of the seminiferous epithelium. Roosen- Runge (1955a; 1962) counted germ-cell-to-sertoli-cell ratios on whole-mount preparations and claimed a 22% cell loss from preleptotene primary spermatocytes to maturation-phase spermatids. From the percentage of degener- TABLE. Sertoli and germ cell numbers per testis and per gram* of testis (+ SEM)? Per testis Per gram of testis.~ ~- Sertoli cells 21.9 x lo6 i 1.70 x lo x lo6 f 0.99 x lo6 Preleptotene, leptotene, and zygotene 28.8 x lo6 f.59 x lo x lo6 f 2.11 x lo6 primary spermatocytes Pachytene and diplotene primary spermatocytes 50.2 x lo6 f 5.25 x lo6 5.8 x lo x lo6 Secondary spermatocytes.51 x lo6 f x lo x lo6 f x lo6 Suermatids (steus 1-10) 10.6 x lo x lo6 9.4 x lo6 f 4.7 x lo6 *Since the specific gravity of fresh testis in the rat is nearly unity (1.040 * f SEM. Mori and Christensen. 1980). values per gram are interchangeable with values per cm.?derived from the estimates of four animals and corrected for the volume change in tissue preparation.
11 MORPHOMETRY OF SEMNFEROUS TUBULES 2 ating figures, there appeared to be an approximate 10% decline in leptotene and zygotene primary spermatocytes, a 2% loss at the first meiotic division, no loss in the second meiotic division, and a 10% reduction in the number of cells during spermiogenesis in step 9-10 spermatids. n the present study, we did not observe a significant change in the number of leptotene or zygotene primary spermatocytes over pertinent stages of the cycle (Fig. 4). Neither did we discern any degenerating step 9-10 spermatids. Clermont (1962) compared the cell numbers of various generations of germ cells per crosssection of rat seminiferous tubules (with correlated autoradiography) and concluded that there was a 12% cell loss during the first meiotic division and a 15% loss in the second. Our counts show a 15% loss in the first division but do not disclose any further loss in the second meiotic division. The autoradiographic observations by Clermont (1962) suggested that most of the degeneration figures described by Roosen-Runge (1955a) as young (leptotene, zygotene) primary spermatocytes or step 9-10 spermatids were probably degenerating type A spermatogonia. Russell and Clermont (1977) reported cell death at other phases of spermatogenesis, but those losses were infrequent and thus may fail to cause a discernible change in the cell population during sperm development. The 15% incidence of cell death among cells undergoing the first meiotic division may be caused by chromosomal problems resulting from the complex chromosomal interactions (Moens, 197) that take place during the long prophase preceding that division. ncluded in the events of that prophase are synapsis, possible crossing over, and disjunction. The first meiotic division could thus constitute a decisive moment when any difficulties encountered during those chromosomal interactions may manifest themselves as an inability to complete the division. There is an intriguing similarity in the number of Sertoli cells reported here, about 16 million per gram of testis, and the 21 million Leydig cells per gram of testis reported previously from our laboratory (Mori and Christensen, 1980). The significance, if any, of this similarity is not clear. De Jong and Sharpe (1977a,b) carried out cell counts per seminiferous-tubule cross section in testes of Hooded Liverpool rats as part of a study on the effect of neonatal irradiation on rat testes, leading to the conclusion that Sertoli cells are the most likely source of inhibin. They counted nuclei of type A spermatogonia and pachytene primary spermatocytes, as well as nucleoli of Sertoli cells, and converted the average values to cells per animal by means of Abercrombie s (1946) formula (with nuclear diameters calculated from the literature), a factor for conversion to cells per testis from the literature, and a factor compensating for shrinkage of the testis during preparation for light microscopy. n their 81-day-old adult controls, they reported an average of 60.4 million Sertoli cells and million pachytene primary spermatocytes per animal. The corresponding values in our study would be 4.8 million Sertoli cells and million pachytene primary spermatocytes (the average age of our four Sprague-Dawley rats was 68 days). Sertoli cell counts by Bustos-Obregon (1970) in whole-mounts of fixed tubules at stages 11-V, V, and V of the cycle yielded average values of about 18.2 Sertoli cells per 10,000 pm2 of tubule surface. The corresponding value in the present study is 1.1 Sertoli cells per hm2 of tubule surface, obtained by dividing the number of Sertoli cells per gram testis by the tubule surface area per gram testis (see Results), and then adjusting the dimensions. The lower value obtained here may reflect the fact that our data are corrected to correspond to values in the fresh testis; conventional histological fixation by immersion would tend to shrink the tubules somewhat, which could give rise to a larger number of nuclei per unit area of tubule surface. Each Sertoli cell is in contact with a considerable number of germ cells, most of which are shared between adjacent Sertoli cells. However, the net workload for the individual Sertoli cell is indicated by the number of a particular type of germ cell per Sertoli cell. This value is 2.4 for young (preleptotene, leptotene, zygotene) primary spermatocytes, 2.7 for older (pachytene, diplotene) primary spermatocytes, 4.04 for secondary spermatocytes, and 7.89 for round spermatids (steps 1-10). Our values for young and old primary spermatocytes, averaged for all pertinent stages, are similar to the 2.47 count obtained for primary spermatocytes at one stage by Roosen-Runge (1955a) in whole-mount preparations. Other workers have studied the number of older spermatids constituting individual clusters in the Sertoli-cell trunks. Rolshoven (1941), in counts on serial sections, obtained a bimodal distribution, with a tall, narrow peak at eight spermatids per Sertoli cell and a second, smaller peak at 12 spermatids per Sertoli cell. Roosen-Runge (1955a), on the other hand, using whole mounts, found a peak at nine sper-
12 24 T:Y. NG AND A.K. CHRSTENSEN matids (average 8.85) per Sertoli cell, but encountered no second peak. e also failed to find any second peak (Fig. 5). Our average of 8.5 step-17 spermatids per Sertoli cell is somewhat lower than that of Roosen-Runge (1955a), although our range (6-1 1 spermatids) is very similar to his (6-12 spermatids). A comparison of our 8.5 average of older spermatids per Sertoli cell with our mean value of 7.89 for step 1-10 spermatids per Sertoli cell (see Results), suggests that a small fraction of Sertoli cells may not be associated with maturing spermatid clusters during stage V of the cycle. The rather wide range in the number of spermatids in the clusters, from 6 to 11, betrays a certain latitude in the process by which younger spermatids become clustered into the Sertoli cytoplasmic trunks after stage V of the cycle. Results of the present study show that the numbers of Sertoli cells and major germ-cell types per unit length of seminiferous tubule remain essentially constant throughout all stages of the cycle of the seminiferous epithelium. This is consistent with the finding of Bustos-Obregon (1970), from Sertoli-cell counts in whole mounts of seminiferous tubules at stages 11-V, V, and V, and extends his results to all stages of the cycle. n the present study, this is demonstrated by studying the number of cells per unit length of seminiferous tubule. t could not be established accurately by measuring only numerical densities of the cells in sections since, as is shown in this study, the diameter of the seminiferous tubule, which is the reference volume for the numerical density, changes significantly at some stages of the cycle. Since the numerical density, by definition, is a ratio (e.g., cell number per cm of tissue), a change in the reference volume which constitutes the denominator will change the value of the ratio, independently of changes in cell number. The approach used in this paper was devised to avoid this difficulty. Our estimate of daily sperm production can be compared with two previous estimates. Amann (1970) predicted that daily sperm production in rats would be only one-half of the value in rabbits, which he had estimated at 26 million sperm per dayiper gram of testis. That estimate would be close to the findings in the present study, in which the daily sperm production per gram of rat testis has been found to be close to 10 million. The daily sperm production per gram of rat testis reported by Johnson et al. (1980), 21.1 million per gram testis or twice our value, is rather close to the estimate that would have been obtained in the present study if the necessary correction for volume change had not been carried out. Those authors measured the diameter of spermatid nuclei before and after dehydration and embedding, and concluded that the shrinkage of Epon-Araldite embedded tissue was negligible, which differs from our findings. Many of the quantitative changes in the seminiferous tubule shown in this study presumably relate to functional alterations taking place during the cycle of the seminiferous epithelium in the tubule. The evolutions of our knowledge on the hormonal control of spermatogenesis has been reviewed by several authors (Courot et al., 1970; Steinberger, 1971; Steinberger and Steinberger, 197, 1975; Hansson et al., 1976). There appear to be striking regional differences in hormone binding and other functions along the seminiferous tubule. Parvinen et al. (1980) have shown that FSH binding is highest in seminiferous tubules in stages X-, the most elevated CAMP response is at stages 11-V, while the maxiumum storage of androgen-binding protein (ABP) is at stages V-V. Parvinen and Vanha-Perttula (1972) showed a regional difference in lytic enzyme contents along the length of the seminiferous tubule, with stages V-V containing the highest activities. n the process of spermiation, significant decreases in the lumen and diameter of the seminiferous tubule (present findings), decreased amounts of demonstrable lipid droplets (Niemi and Kormano, 1965), decreased acid-phosphatase activities (Parvinen and Vanha-Perttula, 1972), and decreased ABP production (Parvinen et al., 1980) have been shown. Studies on various aspects of spermatogenesis require a knowledge of the interactions between germ cells and Sertoli cells, and also of how these change during successive stages of the cycle. t is hoped that the present investigation may help elucidate quantitative aspects of these relationships. LTERATURE CTED Abercrombie, M Estimation of nuclear population from microtome sections. Anat. Rec., 94: Amann, R.P Sperm production rates. n: The Testis. Vol.1, Development, Anatomy and Physiology. A.D. Johnson,.R. Gomes, and N.L. Van Demark, eds. Academic Press, New York, pp Bolender, R.P Correlation of morphometry and stereology with biochemical analysis of cell fractions. nt. Rev. Cytol., 55: Bustos-Obregon, E On Sertoli cell number and distribution in rat testis. Arch. Biol. (Liege), Christensen, A.K The fine structure of testicular interstitial cells in guinea pigs. J. Cell Biol., 26:
13 MORPHOMETRY OF SEMNFEROUS TUBULES 25 Christensen, A.K., and K.C. Peacock 1980 ncrease in Leydig cell number in testes of adult rats treated chronically with an excess of human chorionic gonadotropin. Biol. Reprod., Clermont, Y Quantitative analysis of spermatogenesis of the rat: A revised model for the renewal of spermatogonia. Am.. Anat., 111:lll-10. Clermont, Y., and S.C. Harvey 1965 Duration of the cycle of the seminiferous epithelium of normal, hypophysectomized and hypophysectomized-hormone treated albino rats. Endocrinology, Clermont, Y., and L. Hermo 1975 Spermatogonial stem cells in the albino rat. Am. J. Anat.. 142: Clermont, Y., and C. Huckins 1961 Microscopic anatomy of the sex cords and seminiferous tubules in growing and adult male albino rats. Am. J. Anat.. 108: Clermont, Y., and C.P. Leblond 195 Renewal of spermatogonia in the rat. Am. J. Anat.. 9: Clermont, Y., and H. Morgentaler 1955 Quantitative study of spermatogenesis in the hypophysectomized rat. Endocrinology, 57: Clermont, Y.. and B. Perey 1957 Quantitative study of the cell population of the seminiferous tubules in immature rats. Am. J. Anat., 100: Courot, M., M.-T. Hochereau-de Reviers. and R. Ortavant 1970 Spermatogenesis. n: The Testis. Vol. 1, Development, Anatomy and Physiology. A.D. Johnson,.R. Gomes, and M.L. Van Demark, eds. Academic Press, New York, pp de Jong, F.H., and R.M. Sharpe 1977a The onset and establishment of spermatogenesis in rats in relation to gonadotrophin and testosterone levels. J. Endocrinology, 75: de Jong, F.H., and R.M. Sharpe Gonadotrophins, testosterone and spermatogenesis in neonatnlly irradiated male rats: Evidence for a role of the Sertoli cell in follicle-stimulating hormone feedback. J. Endocrinology, 75: Elias, H., and D.M. Hyde 1980 An elementary introduction to stereology (quantitative microscopy). Am. J. Anat., 159: Floderus, S Untersuchungen uber den Bau der menschlichen Hypophyse mit besonderer Berucksichtigung der quantitativen mikromorphologischen Verhaltnisse. Acta path. microhiol. Scand. Suppl., 5:l-276. Fox, D.J., andk.e.guire 1976 Documentation for MDAS, rd ed. Statistical Research Laboratory, Univ. Michigan, Ann Arbor. Hansson, V., R. Calandra, K. Purvis, M. Ritzen. and F.S. French 1976 Hormonal regulation of spermatogenesis. Vit. Horm., Johnson, L., C.S. Petty, and.b. Neaves 1980 A comparative study of daily sperm production and testicular composition in humans and rats. Biol. Reprod., 22: Lehlond, C.P., and Y. Clermont 1952a Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N.Y. Acad. Sci., 55: Leblond, C.P., and Y. Clermont 1952b Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am. J. Anat., 90; Moens. P.B. 197 Mechanisms of chromosome synapsis at meiotic prophase. nt. Rev. Cytol., Mori, H., and A.K. Christensen 1980 Morphometric analysis of Leydig cells in the normal rat testis. J. Cell Biol Niemi, M., and M. Kormono 1965 Cyclical changes in and significance of lipids and acid phosphatase activity in the seminiferous tubules of the rat testis. Anat. Rec., 151: Parviuen, M., and T. Vanha-Perttula 1972 dentification and enzyme quantitation of the stages of the seminiferous epithelial wave in the rat. Anat. Rec., 174: Parvinen, M., R. Marana, D.M. Robertson, V. Hansson, and and E.M. Rizen 1980 Functional cycle of rat Sertoli cells: Differential binding and action of follicle stimulating hormone at various stages of the spermatogenic cycle. n: Testicular Development, Structure, and Function. A. Steinberger, and E. Steinherger, eds. Raven Press, New York, pp Perey, B., Y. Clermont, and C.P. Lehlond 1961 The wave of the seminiferous epithelium in the rat. Am. J. Anat., 108: Rolshoven, E Zur Frage des Alterns der generativen Elemente in den Hodenkanalchen. Anat. Anz., 91: 1-8. Roosen-Runge, E.C Quantitative studies on spermatogenesis in the albino rat. 11. The duration of spermatogenesis and some effects of colchicine. Am. J. Anat., 8t1: Roosen-Runge, E.C. 1955a Untersuchungen uber die Degeneration Samenbildender Zellen in der normalen Spermatogenese der Ratte. Zeit. f. Zellforsh. Mikros. Anat Roosen-Runge, E.C Quantitative studies on spermatogenesis in the albino rat Volume changes in the cells of the seminiferous tubules. Anat. Rec Roosen-Runge, E.C The process of spermatogenesis in mammals. Biol. Rev., 7:4-77. Roosen-Runge, E.C., and L.O. Giesel. Jr., 1950 Quantitative studies on spermatogenesis in the albino rat. Am. J. Anat., 87:l-0. Russell, L.D., and Y. Clermont 1977 Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat. Rec., 187: Snedecor, G.., and.g. Cochran 1980 Statistical Methods, 7th ed. owa State Univ. Press, Ames, owa. Steinberger, E Hormonal control of mammalian spermatogenesis. Physiol. Rev., 51:l-22. Steinberger, A., and E. Steinberger 197 Hormonal control of mammalian spermatogenesis. n: The Regulation of Mammalian Reproduction. C.J. Segal, R. Crozier, P.A. Corfman, and P.G. Condliffe, eds. Charles C. Thomas Publisher, Springfield, L, pp Steinberger, E.. and A. Steinberger 1975 Spermatogenic function of the testis. n: Handbook of Physiology, Sec. 7, Vol. 5. D.. Hamilton, and R.O. Greep, eds. Am. Physiol. Soc., ashington, D.C., pp eibel, E.R., and R.P. Bolender 197 Stereological techniques for electron microscopic morphometry. n: Principles and Techniques for Electron Microscopy, Vol. M.A. Hayat. ed. Van Nostrand-Reinhold, New York, pp
Identification of the spermatogenic stages in living seminiferous tubules of man
 Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Cycle of the Seminiferous Epithelium of the Guinea Pig
 Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Morphogenesis of the residual body of the mouse testis
 93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
Spermatogenesis in Man
 Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men
 BIOLOGY OF REPRODUCTION 31, 779-784 (1984) Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men LARRY JOHNSON,2 CHARLES S.
BIOLOGY OF REPRODUCTION 31, 779-784 (1984) Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men LARRY JOHNSON,2 CHARLES S.
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Production of Fertile Sperm. Animal Science 434. Hormonal Regulation of the Testis. hormonal regulation of the testis
 roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
Action of Testosterone, Dihydrotestosterone and 5a Androstane 3tr, 17(3 Diol on the Spermatogenesis of Immature Rats
 BIOLOGY OF REPRODUCTION 14, 332-338 (1976) Action of Testosterone, Dihydrotestosterone and 5a Androstane 3tr, 17(3 Diol on the Spermatogenesis of Immature Rats H. E. CHEMES1, E. PODESTA and M. A. RIVAROLA
BIOLOGY OF REPRODUCTION 14, 332-338 (1976) Action of Testosterone, Dihydrotestosterone and 5a Androstane 3tr, 17(3 Diol on the Spermatogenesis of Immature Rats H. E. CHEMES1, E. PODESTA and M. A. RIVAROLA
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Understanding spermatogenesis is central to probing
 Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From
Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From
IN normal male fowls, four developmental stages of spermatogenetic activity
 Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
The Effects of Selective Withdrawal of FSH or LH. on Spermatogenesis in the Immature Rat
 BIOLOGY OF REPRODUCTION 14,489-494(1976) The Effects of Selective Withdrawal of FSH or LH on Spermatogenesis in the Immature Rat H. G. MADHWA RAJ and MARTIN DYM Departments of Obstetrics and Gynaecology
BIOLOGY OF REPRODUCTION 14,489-494(1976) The Effects of Selective Withdrawal of FSH or LH on Spermatogenesis in the Immature Rat H. G. MADHWA RAJ and MARTIN DYM Departments of Obstetrics and Gynaecology
GROWTH AND OBSERVATIONS OF CHINESE HAMSTER SEMINIFEROUS EPITHELIUM IN VITRO
 J. Cell Sci. 6, 19S-205 (1970) Printed in Great Britain GROWTH AND OBSERVATIONS OF CHINESE HAMSTER SEMINIFEROUS EPITHELIUM IN VITRO D. J. ELLINGSON AND K. T. S. YAO U.S. Department of Health, Education
J. Cell Sci. 6, 19S-205 (1970) Printed in Great Britain GROWTH AND OBSERVATIONS OF CHINESE HAMSTER SEMINIFEROUS EPITHELIUM IN VITRO D. J. ELLINGSON AND K. T. S. YAO U.S. Department of Health, Education
androgens (including testosterone) has been demonstrated in Sertoli cells (Grootegoed Effects of FSH and testosterone on Sertoli cells
 Effects of FSH and testosterone on Sertoli cells and spermatocytes from rat testis F. F. G. ROMMERTS J. A. GROOTEGOED, H. J. VAN DER MOLEN Department of Biochemistry (Division of Chemical Endocrinology),
Effects of FSH and testosterone on Sertoli cells and spermatocytes from rat testis F. F. G. ROMMERTS J. A. GROOTEGOED, H. J. VAN DER MOLEN Department of Biochemistry (Division of Chemical Endocrinology),
Pathology of Male Reproductive System 1
 Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
Review: Spermatogenesis in the bull
 Animal (2018), 12:S1, pp s27 s35 The Animal Consortium 2018. This is an Open Access article, distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/),
Animal (2018), 12:S1, pp s27 s35 The Animal Consortium 2018. This is an Open Access article, distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/),
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Influence of three different histological methods on the morphology and morphometrical data in human testis
 Histol Histopathol (2017) 32: 27-34 http://www.hh.um.es Histology and Histopathology From Cell Biology to Tissue Engineering Influence of three different histological methods on the morphology and morphometrical
Histol Histopathol (2017) 32: 27-34 http://www.hh.um.es Histology and Histopathology From Cell Biology to Tissue Engineering Influence of three different histological methods on the morphology and morphometrical
Spermatogonial Cell Proliferation in Organ Culture of Immature Rat Testis'
 BIOLOGY OF REPRODUCTION 48, 761-767 (1993) Spermatogonial Cell Proliferation in Organ Culture of Immature Rat Testis' CARLA BOITANI, 2 MARIA GIUDITTA POLITI, and TIZIANA MENNA Institute of Histology and
BIOLOGY OF REPRODUCTION 48, 761-767 (1993) Spermatogonial Cell Proliferation in Organ Culture of Immature Rat Testis' CARLA BOITANI, 2 MARIA GIUDITTA POLITI, and TIZIANA MENNA Institute of Histology and
TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES*
 FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES* ARNOLD B. BARR, M.D. Department
FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES* ARNOLD B. BARR, M.D. Department
Sertoli cell efficiency and daily sperm production in goats (Capra hircus)
 Anim. Reprod. v.1, n.1, p.122-128, Oct/Dec. 2004 Sertoli cell efficiency and daily sperm production in goats (Capra hircus) M. C. Leal, S. C. Becker-Silva, H. Chiarini-Garcia, L. R. França 1,2 1 Laboratory
Anim. Reprod. v.1, n.1, p.122-128, Oct/Dec. 2004 Sertoli cell efficiency and daily sperm production in goats (Capra hircus) M. C. Leal, S. C. Becker-Silva, H. Chiarini-Garcia, L. R. França 1,2 1 Laboratory
ABNORMAL SPERMATOGENESIS IN XYY MALES: A REPORT ON 4 CASES ASCERTAINED THROUGH A POPULATION STUDY*
 FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. ABNORMAL SPERMATOGENESIS IN XYY MALES: A REPORT ON 4 CASES ASCERTAINED THROUGH A POPULATION
FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. ABNORMAL SPERMATOGENESIS IN XYY MALES: A REPORT ON 4 CASES ASCERTAINED THROUGH A POPULATION
The Male Reproductive System
 The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
(Received 6th October 1972)
 EFFECT OF TESTOSTERONE POLYDIMETHYL- SILOXANE IMPLANTS UPON SPERM PRODUCTION, LIBIDO AND ACCESSORY SEX ORGAN FUNCTION IN RABBITS L. L. EWING, L. G. STRATTON and C. DESJARDINS Department of Physiological
EFFECT OF TESTOSTERONE POLYDIMETHYL- SILOXANE IMPLANTS UPON SPERM PRODUCTION, LIBIDO AND ACCESSORY SEX ORGAN FUNCTION IN RABBITS L. L. EWING, L. G. STRATTON and C. DESJARDINS Department of Physiological
A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL CRYPTORCHIDISM: A PRELIMINARY REPORT*
 FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
Basic histology 5/4/2015
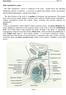 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Reproductive Toxicology
 Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
川北医学院讲稿. Under low power note the testis is enclosed by a strong fibrous. layer of serous epithelium. These fibrous tissue
 川北医学院讲稿 Experiment 5: Male and Female Reproductive System Hello, everybody, class is begin,keep quiet, please. And this is the last experimental class. Today we will learn 5 slices and review all structures
川北医学院讲稿 Experiment 5: Male and Female Reproductive System Hello, everybody, class is begin,keep quiet, please. And this is the last experimental class. Today we will learn 5 slices and review all structures
HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY
 FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
FERTILItY AND STI!RILITY Copyright 1974 The American Fertility Society Vol. 25, No.8, August 1974 PTillted in U.S.AI HISTOLOGIC CHANGES IN THE SEMINIFEROUS TUBULES AFTER VASECTOMY FLETCHER C. DERRICK,
Hormones of brain-testicular axis
 (Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
(Hormone Function) Hormones of brain-testicular axis anterior pituitary drives changes during puberty controlled by GnRH from hypothalamus begins to secrete FSH, LH LH targets interstitial endocrinocytes
Dynamics of mono, di and tri-methylated histone H3 lysine 4 during male meiotic prophase I. Nuclei were co-stained for H3.1/H3.2. Progressing stages
 Dynamics of mono, di and tri-methylated histone H3 lysine 4 during male meiotic prophase I. Nuclei were co-stained for H3.1/H3.2. Progressing stages of spermatogenesis are shown from left to right. Arrows/dotted
Dynamics of mono, di and tri-methylated histone H3 lysine 4 during male meiotic prophase I. Nuclei were co-stained for H3.1/H3.2. Progressing stages of spermatogenesis are shown from left to right. Arrows/dotted
describe the parts and function of semen and the glands that contribute to it
 You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
You need to be able to: describe spermatogenesis (How is sperm made?) describe the anatomy of a sperm describe the parts and function of semen and the glands that contribute to it How is sperm made? Spermatogenesis
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Knockout TM SR : ; ; ; : R ; R : A : X(2013) , ,, B. , (Knockout TM
 33 1 Vol.33 No.1 013 1 Dec. 013 Reproduction & Contraception doi: 10.7669/j.issn.03-37X.013.1.0804 E-mail: randc_journal@163.com Knockout TM SR ; ; ; 400014 : FBS Knockout TM SRKSR : FBS KSR HE TUNEL RT-PCR
33 1 Vol.33 No.1 013 1 Dec. 013 Reproduction & Contraception doi: 10.7669/j.issn.03-37X.013.1.0804 E-mail: randc_journal@163.com Knockout TM SR ; ; ; 400014 : FBS Knockout TM SRKSR : FBS KSR HE TUNEL RT-PCR
REPRODUCTIVE TRACT OF ANIMALS FED A DIET DEFICIENT IN VITAMIN A ALCOHOL BUT CON TAINING VITAMIN A ACID. I. THE MALE RAT
 HISTOLOGY OF THE LESIONS PRODUCED IN THE REPRODUCTIVE TRACT OF ANIMALS FED A DIET DEFICIENT IN VITAMIN A ALCOHOL BUT CON TAINING VITAMIN A ACID. I. THE MALE RAT J. McC. HOWELL, J. N. THOMPSON and G. A.
HISTOLOGY OF THE LESIONS PRODUCED IN THE REPRODUCTIVE TRACT OF ANIMALS FED A DIET DEFICIENT IN VITAMIN A ALCOHOL BUT CON TAINING VITAMIN A ACID. I. THE MALE RAT J. McC. HOWELL, J. N. THOMPSON and G. A.
Developmental stages of fetal-type Leydig cells in prepubertal rats
 Development 107, 213-220 (1989) Printed in Great Britain The Company of Biologists Limited 1989 213 Developmental stages of fetal-type Leydig cells in prepubertal rats T. KUOPIO 1 *, J. TAPANAINEN 2, L.
Development 107, 213-220 (1989) Printed in Great Britain The Company of Biologists Limited 1989 213 Developmental stages of fetal-type Leydig cells in prepubertal rats T. KUOPIO 1 *, J. TAPANAINEN 2, L.
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Gametogenesis. Dr Corinne de Vantéry Arrighi Dr Hervé Lucas
 WHO Collaborating Center for Research in Human Reproduction Clinic for Infertility and Gynecological Endocrinology University Hospital, Geneva, Switzerland Gametogenesis Dr Corinne de Vantéry Arrighi Dr
WHO Collaborating Center for Research in Human Reproduction Clinic for Infertility and Gynecological Endocrinology University Hospital, Geneva, Switzerland Gametogenesis Dr Corinne de Vantéry Arrighi Dr
Seminiferous Tubules
 Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Plasma and testicular testosterone levels, volume density and number of Leydig cells and spermatogenic efficiency of rabbits
 Brazilian Journal of Medical and Biological Research (2002) 35: 493-498 Testosterone levels and number of Leydig cells ISSN 0100-879X 493 Plasma and testicular testosterone levels, volume density and number
Brazilian Journal of Medical and Biological Research (2002) 35: 493-498 Testosterone levels and number of Leydig cells ISSN 0100-879X 493 Plasma and testicular testosterone levels, volume density and number
Sertoli cell and spermatogenic efficiencies in Pêga Donkey (Equus asinus)
 Anim. Reprod., v.11, n.4, p.517-525, Oct./Dec. 2014 Sertoli cell and spermatogenic efficiencies in Pêga Donkey (Equus asinus) E.M. Neves 1, G.M.J. Costa 2, L.R. França 2,3 1 Department of Anatomy, Federal
Anim. Reprod., v.11, n.4, p.517-525, Oct./Dec. 2014 Sertoli cell and spermatogenic efficiencies in Pêga Donkey (Equus asinus) E.M. Neves 1, G.M.J. Costa 2, L.R. França 2,3 1 Department of Anatomy, Federal
SEASONAL CHANGES IN HISTOLOGYOF THE THYROID GLAND CALOTIS VERSICOLOR
 SEASONAL CHANGES IN HISTOLOGYOF THE THYROID GLAND CALOTIS VERSICOLOR M. D. Kulkarni And A. H. Shinde Department of Zoology Yashwantrao Chavan Arts & Science College, Mangrulpir Dist. Washim. (Received
SEASONAL CHANGES IN HISTOLOGYOF THE THYROID GLAND CALOTIS VERSICOLOR M. D. Kulkarni And A. H. Shinde Department of Zoology Yashwantrao Chavan Arts & Science College, Mangrulpir Dist. Washim. (Received
Seminiferous Tubules and Daily Sperm Production in Older. Adult Men with Varied Numbers of Leydig Cells
 eminiferous Tubules and Daily perm Production in Older BOLOGY OF REPRODUCTON 36, 30 1-308 (1987) Adult Men with Varied Numbers of Leydig Cells WLLAM B NEAVE,2 3 LARRY JOHNON,3 and CHARLE PETTY4 Departments
eminiferous Tubules and Daily perm Production in Older BOLOGY OF REPRODUCTON 36, 30 1-308 (1987) Adult Men with Varied Numbers of Leydig Cells WLLAM B NEAVE,2 3 LARRY JOHNON,3 and CHARLE PETTY4 Departments
Sami Ventelä, 1,2 Hiroshi Ohta, 3 Martti Parvinen, 2 and Yoshitake Nishimune 3
 BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
of Rats Injected with Radioactive Cesium 137 KITABATAKE Takashi
 Spermatogenesis of Rats Injected with Radioactive Cesium 137 KITABATAKE Takashi (Received in June 1961) ABSTRACT Solution of radioactive cesium, 137Cs, was injected to rats intra peritoneally, and the
Spermatogenesis of Rats Injected with Radioactive Cesium 137 KITABATAKE Takashi (Received in June 1961) ABSTRACT Solution of radioactive cesium, 137Cs, was injected to rats intra peritoneally, and the
Introduction. Materials and methods ORIGINAL ARTICLE. Rodi O. Ojoo Æ George E. Otiang a-owiti Dominic Oduor-Okelo Æ Daniel W.
 Anat Embryol (2005) 209: 381 389 DOI 10.1007/s00429-004-0452-8 ORIGINAL ARTICLE Rodi O. Ojoo Æ George E. Otiang a-owiti Dominic Oduor-Okelo Æ Daniel W. Onyango Frequency of stages of the seminiferous cycle
Anat Embryol (2005) 209: 381 389 DOI 10.1007/s00429-004-0452-8 ORIGINAL ARTICLE Rodi O. Ojoo Æ George E. Otiang a-owiti Dominic Oduor-Okelo Æ Daniel W. Onyango Frequency of stages of the seminiferous cycle
Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells
 Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells By Setsuko Ogata Department of Anatomy, Tokyo Women's Medical College, Shinjuku, Tokyo, Japan (Director : Prof. Dr. Kura Kubota)
Cytological Studies on Human Spermatogenic and Sustentacular (Sertoli) Cells By Setsuko Ogata Department of Anatomy, Tokyo Women's Medical College, Shinjuku, Tokyo, Japan (Director : Prof. Dr. Kura Kubota)
Quantitative differences between variants of
 Quantitative differences between variants of A spermatogonia in man R. Paniagua, M. Nistal, P. Amat, M. C. Rodr\l=i'\guez,and J. R. Alonso "Department of Cytology and Histology, Faculty ofbiology, University
Quantitative differences between variants of A spermatogonia in man R. Paniagua, M. Nistal, P. Amat, M. C. Rodr\l=i'\guez,and J. R. Alonso "Department of Cytology and Histology, Faculty ofbiology, University
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
ULAR LESIONS CAUSED BY CROTALUS VENOM.*
 AN EXPERIMENTAL STUDY OF THE LATE GLOMER- ULAR LESIONS CAUSED BY CROTALUS VENOM.* BY RICHARD M. PEARCE, M.D. (From the John Herr Musser Department of Research Medicine of the University of Pennsylvania,
AN EXPERIMENTAL STUDY OF THE LATE GLOMER- ULAR LESIONS CAUSED BY CROTALUS VENOM.* BY RICHARD M. PEARCE, M.D. (From the John Herr Musser Department of Research Medicine of the University of Pennsylvania,
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
The effect of thyroid activity on adult rat spermatogenesis
 The effect of thyroid activity on adult rat spermatogenesis Ai, J. 1* ; Zarifkar, A. 2 ; Takhshid, M. A. 3 ; Alavi, J. 1 and Moradzadeh, M. 2 1 Department of Anatomical Sciences, School of Medicine, University
The effect of thyroid activity on adult rat spermatogenesis Ai, J. 1* ; Zarifkar, A. 2 ; Takhshid, M. A. 3 ; Alavi, J. 1 and Moradzadeh, M. 2 1 Department of Anatomical Sciences, School of Medicine, University
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
ELECTRON-MICROSCOPIC AUTO- RADIOGRAPHY OF TRITIATED TESTOSTERONE IN RAT TESTIS
 J. Cell Set. 26, 339-346 (i977) 339 Printed in Great Britain ELECTRON-MICROSCOPIC AUTO- RADIOGRAPHY OF TRITIATED TESTOSTERONE IN RAT TESTIS P. M. FREDERIK* AND H. J. VAN DER MOLEN Department of Biochemistry
J. Cell Set. 26, 339-346 (i977) 339 Printed in Great Britain ELECTRON-MICROSCOPIC AUTO- RADIOGRAPHY OF TRITIATED TESTOSTERONE IN RAT TESTIS P. M. FREDERIK* AND H. J. VAN DER MOLEN Department of Biochemistry
New aspect of hepatic nuclear glycogenosis
 J. clin. Path. (1968), 21, 19 New aspect of hepatic nuclear glycogenosis in diabetes1 F. CARAMIA, F. G. GHERGO, C. BRANCIARI, AND G. MENGHINI From the Institute of General Pathology, University of Rome,
J. clin. Path. (1968), 21, 19 New aspect of hepatic nuclear glycogenosis in diabetes1 F. CARAMIA, F. G. GHERGO, C. BRANCIARI, AND G. MENGHINI From the Institute of General Pathology, University of Rome,
All You Wanted to Know About Spermatogonia but Were Afraid to Ask
 All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
Gametogenesis. Omne vivum ex ovo All living things come from eggs.
 Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SPERMATOGENIC CELLS OF THE PREPUBERAL MOUSE
 SPERMATOGENIC CELLS OF THE PREPUBERAL MOUSE Isolation and Morphological Characterization ANTHONY R. BELLVI~, J. C. CAVICCHIA, CLARKE F. MILLETFE, DEBORAH A. O'BRIEN, Y. M. BHATNAGAR, and MARTIN DYM From
SPERMATOGENIC CELLS OF THE PREPUBERAL MOUSE Isolation and Morphological Characterization ANTHONY R. BELLVI~, J. C. CAVICCHIA, CLARKE F. MILLETFE, DEBORAH A. O'BRIEN, Y. M. BHATNAGAR, and MARTIN DYM From
Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey
 J. Biosci., Vol. 2, Number 3, September 1980, pp. 261-266. Printed in India. Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey ASHA PRAKASH, M. R. N. PRASAD and T.C. ANAND KUMAR
J. Biosci., Vol. 2, Number 3, September 1980, pp. 261-266. Printed in India. Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey ASHA PRAKASH, M. R. N. PRASAD and T.C. ANAND KUMAR
Spermatogenesis Following Experimental Testicular Ischemia
 Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Exercise 6. Procedure
 Exercise 6 Procedure Growing of root tips Select a few medium-sized onion bulbs. Carefully remove the dry roots present. Grow root tips by placing the bulbs on glass tubes (of about 3 4 cm. diameter) filled
Exercise 6 Procedure Growing of root tips Select a few medium-sized onion bulbs. Carefully remove the dry roots present. Grow root tips by placing the bulbs on glass tubes (of about 3 4 cm. diameter) filled
Effects of Ablation of the Submaxillary Gland in Guinea Pigs IV. Cause of deterioration of the tubules in the testes
 1961 475 Effects of Ablation of the Submaxillary Gland in Guinea Pigs IV. Cause of deterioration of the tubules in the testes Kazuo Suzuki Received August 1, 1960 Shakujii Institute, Tokyo Medical College,
1961 475 Effects of Ablation of the Submaxillary Gland in Guinea Pigs IV. Cause of deterioration of the tubules in the testes Kazuo Suzuki Received August 1, 1960 Shakujii Institute, Tokyo Medical College,
MALE REPRODUCTIVE SYSTEM
 1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
1 MALE REPRODUCTIVE SYSTEM SCPA 602 Anatomical Basis for Pathological Study Updated: 20.09.2018 Lect. Nisamanee Charoenchon, PhD nisamanee.cha@mahidol.ac.th Department of Pathobiology, Mahidol University
relatively unpredictable environmental factors. PATTERNS OF CHANGE IN THE REPRODUCTIVE ORGANS OF THE MALE POCKET GOPHER, GEOMYS PINETIS
 PATTERNS OF CHANGE IN THE REPRODUCTIVE ORGANS OF THE MALE POCKET GOPHER, GEOMYS PINETIS KATHERINE CARTER EWEL Department of Zoology, University offlorida, Gainesville, Florida {Received 6th April 1971,
PATTERNS OF CHANGE IN THE REPRODUCTIVE ORGANS OF THE MALE POCKET GOPHER, GEOMYS PINETIS KATHERINE CARTER EWEL Department of Zoology, University offlorida, Gainesville, Florida {Received 6th April 1971,
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE
 ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Effect of methotrexate (mtx) administration on spermatogenesis: an experimental on animal model
 Effect of methotrexate (mtx) administration on spermatogenesis: an experimental on animal model S Shrestha, 1 S Dhungel, 1 AK Saxena, 2 S Bhattacharya 1 and D Maskey 1 1 Department of Anatomy, B. P. Koirala
Effect of methotrexate (mtx) administration on spermatogenesis: an experimental on animal model S Shrestha, 1 S Dhungel, 1 AK Saxena, 2 S Bhattacharya 1 and D Maskey 1 1 Department of Anatomy, B. P. Koirala
Downloaded From: on 07 Mar 2019 Terms of Use:
 The First and The Second Mitotic Phases of Spermatogonial Stage in Xenopus laevis: Secondary Spermatogonia Which Have Differentiated after Completion of The First Mitotic Phase Acquire an Ability of Mitosis
The First and The Second Mitotic Phases of Spermatogonial Stage in Xenopus laevis: Secondary Spermatogonia Which Have Differentiated after Completion of The First Mitotic Phase Acquire an Ability of Mitosis
Treatment of Defective Spermatogenesis tvith Human Gonadotropins
 Treatment of Defective Spermatogenesis tvith Human Gonadotropins W. Z. POLISHUK, M.D., Z. PALTI, M.D., and A. LAUFER, M.D. TREATMENT of male sterility due to defective spermatogenesis is not satisfactory.
Treatment of Defective Spermatogenesis tvith Human Gonadotropins W. Z. POLISHUK, M.D., Z. PALTI, M.D., and A. LAUFER, M.D. TREATMENT of male sterility due to defective spermatogenesis is not satisfactory.
KENJI OHYAMA, MASANORI OHTA, YosHIKo NAKAGOMI, YOSHIE SHIMURA, T0M0AKI SANG, KAZUMASA SATO, SHINPEI NAKAZAWA, AND HIROMICHI ISHIKAWA*
 Endocrine Journal 1997, 44 (4), 459-465 Restoration of Seminiferous Tubular Function after Discontinuation of Long-Term Gonadotropin-Releasing Hormone Agonist Administration in Premature Male Rats KENJI
Endocrine Journal 1997, 44 (4), 459-465 Restoration of Seminiferous Tubular Function after Discontinuation of Long-Term Gonadotropin-Releasing Hormone Agonist Administration in Premature Male Rats KENJI
Infertility is not an uncommon problem in Western
 Review Article A Practical Approach to Testicular Biopsy Interpretation for Male Infertility Lisa A. Cerilli, MD; Wayne Kuang, MD; David Rogers, MD Infertility is not an uncommon problem in Western societies
Review Article A Practical Approach to Testicular Biopsy Interpretation for Male Infertility Lisa A. Cerilli, MD; Wayne Kuang, MD; David Rogers, MD Infertility is not an uncommon problem in Western societies
Quantitative studies on spermatogenesis in buffalo (Bubalus bubalis)
 Quantitative studies on spermatogenesis in buffalo (Bubalus bubalis) G. S. Bilaspuri, S. S. Guraya To cite this version: G. S. Bilaspuri, S. S. Guraya. Quantitative studies on spermatogenesis in buffalo
Quantitative studies on spermatogenesis in buffalo (Bubalus bubalis) G. S. Bilaspuri, S. S. Guraya To cite this version: G. S. Bilaspuri, S. S. Guraya. Quantitative studies on spermatogenesis in buffalo
For more information about how to cite these materials visit
 Author: A. Kent Christensen, Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author: A. Kent Christensen, Ph.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
(LOXODONTA AFRICANA)
 THE TESTIS OF THE AFRICAN ELEPHANT (LOXODONTA AFRICANA) IL DEVELOPMENT, PUBERTY AND WEIGHT OSCAR W. JOHNSON and IRVEN O. BUSS Department of Biology, Moorhead State College, Moorhead, Minnesota, and Department
THE TESTIS OF THE AFRICAN ELEPHANT (LOXODONTA AFRICANA) IL DEVELOPMENT, PUBERTY AND WEIGHT OSCAR W. JOHNSON and IRVEN O. BUSS Department of Biology, Moorhead State College, Moorhead, Minnesota, and Department
LOCAL CONTROL OF TESTICULAR FUNCTION
 Quarterly Journal of Experiniental Physiology (1983), 68, 265-287 Printed in Great Britain REVIEW ARTICLE LOCAL CONTROL OF TESTICULAR FUNCTION RICHARD M. SHARPE MRC Reproductive Biology UAit, Centre for
Quarterly Journal of Experiniental Physiology (1983), 68, 265-287 Printed in Great Britain REVIEW ARTICLE LOCAL CONTROL OF TESTICULAR FUNCTION RICHARD M. SHARPE MRC Reproductive Biology UAit, Centre for
G.R. Marshall 2 ' 3 '4,5 and T.M. Plant 4
 BIOLOGY OF REPRODUCTION 54, 1192-1199 (1996) Puberty Occurring Either Spontaneously or Induced Precociously in Rhesus Monkey (Macaca mulatta) Is Associated with a Marked Proliferation of Sertoli Cells
BIOLOGY OF REPRODUCTION 54, 1192-1199 (1996) Puberty Occurring Either Spontaneously or Induced Precociously in Rhesus Monkey (Macaca mulatta) Is Associated with a Marked Proliferation of Sertoli Cells
THE EFFECT OF OESTRIN ON THE TESTIS OF THE ADULT MOUSE
 389 THE EFFECT OF OESTRIN ON THE TESTIS OF THE ADULT MOUSE BY MARJORIE ALLANSON. (Harold Row Research Scholar, King's College, London.) (Received 5th March, 1931.) (With One Plate.) I. INTRODUCTION. THE
389 THE EFFECT OF OESTRIN ON THE TESTIS OF THE ADULT MOUSE BY MARJORIE ALLANSON. (Harold Row Research Scholar, King's College, London.) (Received 5th March, 1931.) (With One Plate.) I. INTRODUCTION. THE
The Structure of the Germinal Epithelium of the Fowl Testis with Special Reference to the Presence of Multinuclear Cells. By P. E.
 48 7 The Structure of the Germinal Epithelium of the Fowl Testis with Special Reference to the Presence of Multinuclear Cells By P. E. LAKE (From the Poultry Research Centre, King's Buildings, West Mains
48 7 The Structure of the Germinal Epithelium of the Fowl Testis with Special Reference to the Presence of Multinuclear Cells By P. E. LAKE (From the Poultry Research Centre, King's Buildings, West Mains
Morphogenesis by dissociated immature rat testicular cells in primary culture
 /. Einhryol. exp. Morph. Vol. 44, pp. 297-302, 1978 297 Printed in Great Britain (p Company of Biologists Limited 1978 SHORT PAPER Morphogenesis by dissociated immature rat testicular cells in primary
/. Einhryol. exp. Morph. Vol. 44, pp. 297-302, 1978 297 Printed in Great Britain (p Company of Biologists Limited 1978 SHORT PAPER Morphogenesis by dissociated immature rat testicular cells in primary
Some Observations on the Fine Structure of the Goblet Cells. Special Reference to the Well-Developed Agranular Endoplasmic Reticulum
 Okajimas Folia Anat. Jpn., 58(4-6) : 583-594, March 1982 Some Observations on the Fine Structure of the Goblet Cells in the Nasal Respiratory Epithelium of the Rat, with Special Reference to the Well-Developed
Okajimas Folia Anat. Jpn., 58(4-6) : 583-594, March 1982 Some Observations on the Fine Structure of the Goblet Cells in the Nasal Respiratory Epithelium of the Rat, with Special Reference to the Well-Developed
Testicular Toxicity: Evaluation During Drug Development Guidance for Industry
 Testicular Toxicity: Evaluation During Drug Development Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Testicular Toxicity: Evaluation During Drug Development Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
ANATOMY. lecture # : 2 1 Date : Lecturer : Maher Hadidi
 ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
ANATOMY lecture # : 2 1 Date : Lecturer : Maher Hadidi Spermatogenesis Transformation of Spermatogonia into mature sperm that begins at puberty into old age. Provide motility of the sperm...-c:tail to
Effect of cadmium chloride on the serum levels of follicle stimulating hormone (FSH), luteinizing hormone (LH) and androgens in the adult male rat
 Proc. Indian Acad. Sci., Vol. 87 B, (Experimental Biology-3), No.7, July 1978, pp. 161-167, @ printed in India Effect of cadmium chloride on the serum levels of follicle stimulating hormone (FSH), luteinizing
Proc. Indian Acad. Sci., Vol. 87 B, (Experimental Biology-3), No.7, July 1978, pp. 161-167, @ printed in India Effect of cadmium chloride on the serum levels of follicle stimulating hormone (FSH), luteinizing
Effect of Gonadotropic Hormones on Hypophysectomized (Anterior Lobe) Male Rana Pipiens
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 54, Issue 3 (May, 1954) 19545 Effect of Gonadotropic Hormones on Hypophysectomized
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 54, Issue 3 (May, 1954) 19545 Effect of Gonadotropic Hormones on Hypophysectomized
BIOL 2402 Reproductive Systems
 Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
ON THE PRESENCE OF A CILIATED COLUMNAR EPITHELIAL CELL TYPE WITHIN THE BOVINE CERVICAL MUCOSA 1
 ON THE PRESENCE OF A CILIATED COLUMNAR EPITHELIAL CELL TYPE WITHIN THE BOVINE CERVICAL MUCOSA 1 R. I. Wordinger, 2 J. B. Ramsey, I. F. Dickey and I. R. Hill, Jr. Clemson University, Clemson, South Carolina
ON THE PRESENCE OF A CILIATED COLUMNAR EPITHELIAL CELL TYPE WITHIN THE BOVINE CERVICAL MUCOSA 1 R. I. Wordinger, 2 J. B. Ramsey, I. F. Dickey and I. R. Hill, Jr. Clemson University, Clemson, South Carolina
Role of FSH in the Control of Testicular Function
 Archives of Andrology Journal of Reproductive Systems ISSN: 0148-5016 (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/iaan19 Role of FSH in the Control of Testicular Function A. G. Davies
Archives of Andrology Journal of Reproductive Systems ISSN: 0148-5016 (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/iaan19 Role of FSH in the Control of Testicular Function A. G. Davies
