SPERMATOGENIC CELLS OF THE PREPUBERAL MOUSE
|
|
|
- Angel Katrina Gibson
- 5 years ago
- Views:
Transcription
1 SPERMATOGENIC CELLS OF THE PREPUBERAL MOUSE Isolation and Morphological Characterization ANTHONY R. BELLVI~, J. C. CAVICCHIA, CLARKE F. MILLETFE, DEBORAH A. O'BRIEN, Y. M. BHATNAGAR, and MARTIN DYM From the Departments of Physiology and Anatomy and the Laboratory of Human Reproduction and Reproductive Biology, Harvard Medical School, Boston, Massachusetts ABSTRACT A procedure is described which permits the isolation from the prepuberal mouse testis of highly purified populations of primitive type A spermatogonia, type A spermatogonia, type B spermatogonia, preleptotene primary spermatocytes, leptotene and zygotene primary spermatocytes, pachytene primary spermatocytes, and Sertoli cells. The successful isolation of these prepuberal cell types was accomplished by: (a) defining distinctive morphological characteristics of the cells, (b) determining the temporal appearance of spermatogenic cells during prepuberal development, (c) isolating purified seminiferous cords, after dissociation of the testis with collagenase, (d) separating the trypsin-dispersed seminiferous cells by sedimentation velocity at unit gravity, and (e) assessing the identity and purity of the isolated cell types by microscopy. The seminiferous epithelium from day 6 animals contains only primitive type A spermatogonia and Sertoli cells. Type A and type B spermatogonia are present by day 8. At day 10, meiotic prophase is initiated, with the germ cells reaching the early and late pachytene stages by days 14 and 18, respectively. Secondary spermatocytes and haploid spermatids appear in increasing numbers between days 18 and 20. Cell separations were attempted throughout this developmental period. The purity and optimum day for the recovery of specific cell types are as follows: day 6, Sertoli cells (purity >99%) and primitive type A spermatogonia (90%); day 8, type A spermatogonia (91%) and type B spermatogonia (76%); day 18, preleptotene spermatocytes (93%), leptotene/zygotene spermatocytes (52%), and pachytene spermatocytes (89%). Mammalian spermatogenesis is a continuum of cellular differentiation in which three principal phases can be discerned: spermatogonial renewal and proliferation, meiosis, and spermiogenesis. The initial phase of spermatogonial proliferation occurs in the basal compartment of the seminiferous epithelium (7, 9) and consists of a mitotic proliferation of stem cells which form, in sequence, type A spermatogonia, intermediate sper- matogonia, and type B spermatogonia (19, 30, 31,36). The type B spermatogonia divide to form preleptotene primary spermatocytes which undergo a final replication of nuclear DNA before entering meiotic prophase. The second phase, meiosis, occurs while the spermatocytes remain intercalated between cytoplasmic processes of adjacent Sertoli cells on the adluminai side of the intercellular Sertoli junctions. Meiotic prophase, 68 THE JOURNAL OF CELL Bxot~v- VOLUME 74, 1977" pages 68-85
2 which includes the leptotene, zygotene, pachytene, and diplotene stages, terminates in the first reduction division with the formation of secondary spermatocytes. The latter cells quickly enter the second reduction division to form the haploid spermatids. Spermiogenesis, the final phase of spermatogenesis, consists of a complex morphological transformation of the haploid germ cell that culminates with the release of late spermatids into the lumen of the seminiferous tubule. The isolation of homogeneous populations of the respective spermatogenic cells is an essential prerequisite for definitive biochemical studies of germ cell differentiation. The technique which has been applied to the adult testis most successfully for this purpose utilizes differential sedimentation velocity at unit gravity of cells which differ in volume (14, 18, 20, 23, 24, 35). With the recent refinements introduced by Romrell et al. (35), this technique provides purified populations of pachytene primary spermatocytes, round spermatids, and residual bodies, but does not yield germ cells at any step of development preceding the pachytene stage of meiotic prophase. The low frequency of spermatogonia and early primary spermatocytes in the adult seminiferous epithelium does not permit isolation of these particular cell types. Spermatogenesis is initiated shortly after birth. In consequence, during the prepuberal period the seminiferous epithelium contains only Sertoli cells, spermatogonia, and, with increasing age, primary spermatocytes at progressively more advanced stages of meiotic prophase (3, 10, 27). The feasibility of isolating discrete populations of spermatogonia and primary spermatocytes from prepuberal rats was recently demonstrated by Davis and Schuetz (4). However, their respective populations of germ cells were not sectioned and characterized by light and electron microscopy, and consequently it is difficult to discern the identity and to assess the purity of their cell populations, particularly those at early stages of meiotic prophase. In this report, a procedure is described which permits the recovery from the prepuberal mouse testis of highly purified populations of primitive type A spermatogonia, type A spermatogonia, type B spermatogonia, preleptotene spermatocytes, leptotene/zygotene spermatocytes, pachytene spermatocytes, and prepuberal Sertoli cells. The identity and purity of the isolated germ cell and Sertoli cell populations have been verified by light and electron microscopy, using as criteria distinctive morphological features which are characteristic of the respective cell types. Identification was aided considerably by a concomitant study of the cells in situ within the intact testis and the isolated seminiferous cords. The integrity of the isolated cells has been assessed by Nomarski differential interference microscopy, light and electron microscopy and by the exclusion of trypan blue. MATERIALS AND METHODS Materials Male and female mice CD-1 strain, days of age, were purchased from Charles River Breeding Laboratories, Inc. (Wilmington, Mass.). Bovine serum albumin (BSA, fraction V), trypsin (bovine pancreas, type III), and deoxyribonuclease (DNase-I, DN-EP) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Collagenase (CLS) is a product of Worthington Biochemical Corp. (Freehold, N. J.); the preparation of essential amino acids (x 100) is supplied by Microbiological Associates (Bethesda, Md.), and Eagle's nonessential amino acids (x 100) are obtained from Difco Laboratories (Detroit, Mich.). The glass STA-PUT sedimentation chambers (SP-120 and SP-180) were purchased from Johns Scientific, Toronto, Canada. Nitex filter cloth is a product of Tet/Kressilk, Inc., New York. The enriched Krebs-Ringer bicarbonate medium (EKRB) contained mm NaCl, 4.8 mm KCI, 25.2 mm NaHCOa, 1.2 mm KH2PO4, 1.2 mm MgSO4.7H20, 1.3 mm CaCI2, 11.1 mm glucose, 1 mm glutamine, 10 mi/liter of essential amino acids, 10 ml/liter of nonessential amino acids, 100/zg/ml streptomycin sulfate, and 60 p.g/ml penicillin G (K + salt). Immediately before use, the medium was filtered (0.30-/zm Millipore filter [MiUipore Corp., Boston, Mass.]) and the ph adjusted to 7.3 by a rain aeration with 5% CO2 in air. The siliconized glassware and other equipment were sterilized before Use. Preparation of Seminiferous Epithelial Cells Female mice were naturally mated and observed at 12-h intervals near the end of pregnancy to record the time at which parturition occurred. The day of birth was designated as day 0. Litter size was adjusted to a maximum of ten by removing the appropriate number of female pups. The required number of male pups, depending upon the age, were sacrificed on each day from day 6 to 20, inclusive. The testes were excised and decapsulated by making a small incision in the tunica albuginea and gently expressing the contents onto an aseptic surface. The decapsuiated testes were kept in EKRB at 22~ until all samples were collected, a period which usually did not exceed 30 rain. The method of Romrell et al. (35), with modifications, was used to BELLVI~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 69
3 isolate the seminiferous cords and to prepare the suspension of spermatogenic cells. The decapsulated testes were placed in 20 ml of EKRB containing 0.5 mg/ml collagenase and incubated under 5% COg in air for 15 rain at 330C in a shaking water bath (Precision Seientitle Co., Chicago, IU.) operated at 120 cycles per min. The dispersed seminiferous cords were isolated by allowing them to sediment in EKRB (3-4 min) and then decanting the supernate. This process was repeated three times to ensure removal of the dispersed interstitial tissue (35). The seminiferous cords were incubated in 20 ml of EKRB containing 0.5 mg/ml trypsin and 1 /~g/ml DNase for 15 min at 33~ using the conditions deso'ibed above. Most of the cell aggregates which remained after this treatment were sheared gently by repeated pipetting with a Pasteur pipet for 3 min. The dispersed seminiferous cells were then washed twice, resuspended in EKRB containing 0.5% BSA, filtered through Nitex filter cloth (40 mesh), and the cell concentration was determined with a hemocytometer. Separation of Seminiferous Epithelial Cells Cells of the dissociated seminiferous epithelium were separated by sedimentation velocity at unit gravity at 4~ using a 2-4% BSA gradient in EKRB (35). The cells were bottom-loaded into either the SP-120 or SP- 180 chamber in a volume of 10 or 25 ml, respectively, and at a maximum concentration of 107/ml. A linear gradient was then generated from either 275 ml of 2% BSA and 275 ml of 4% BSA, or 550 ml of 2% BSA and 550 ml of 4% BSA, depending upon the size of the chamber. The time allowed for sedimentation, including generation of the gradient and unloading of the chamber, was standardized at 4 h. The SP-120 and SP-180 chambers were unloaded from the bottom in 5- and 10-ml fractions, respectively, to ensure that the different cell types were recovered in comparable fraction numbers. The first fraction collected was designated as 1 and the remainder were numbered through to 100. The cells in each fraction were pelleted by centrifugation at 200 g for 5 min at 4~ in a Sorvall GLC-2 centrifuge (DuPont Instruments, Sorvall Operations, Newtown, Conn.). The supemate was then aspirated, and the cells were resuspended in 0.5 ml of EKRB containing 0.5% BSA. An aliquot of each fraction was examined by Nomarski differential interference microscopy to assess cellular integrity and, where feasible, to identify the cell types. The mean cell diameter was determined with a calibrated eyepiece micrometer. Fractions containing cells of similar size and morphology were pooled, and an aliquot was taken to determine the percentage of cells which excluded trypan blue. The remaining portion of each sample was prepared for detailed light and electron microscope examination. The criteria required for the identification of isolated germ cells were obtained from a concurrent morphologi- cal study on the postnatal development of the mouse testis. Three mice were sacrificed on each alternative day from day 0 to 20 and the testes excised and prepared for light and electron microscopy. Samples of isolated seminiferous cords, prepared by incubating the testes in collagenase, were also examined. Preparation of Samples for Microscopy Whole prepuberal testes were immersed for 60 min in modified Kamovsky's fixative (17) containing 2.5% ghitaraldehyde, 2% formaldehyde and 0.05% calcium chloride in 0.1 M cacodylate buffer (ph 7.3). After 15 min, the samples were cut into 1 mm cubes, postfixed in 1% osmium tetroxide in 0.1 M cacodylate buffer (ph 7.3) for 2 h, dehydrated in ethanol, and embedded in Epon (21). Isolated seminiferous cords and the respective germ cell populations were initially fixed for 30 min in 2% glutaraldehyde in EKRB (ph 7.3) (35). Secondary fixation was achieved in 1% osmium tetroxide in 0.1 M cacodylate buffer (ph 7.3) for 30 min. The samples were then embedded in low-viscosity Epon (22). 1-p,m sections were stained with tohiidine blue and examined by light microscopy. Thin sections were stained with uranyl acetate and lead citrate (34) and examined by electron microscopy. RESULTS Prepuberal Development of the Seminiferous Epithelium It was essential to define the morphological characteristics and the temporal appearance of spermatogenic cells in the prepuberal seminiferous epithelium before attempting to separate the cells. These two objectives were accomplished by a light and electron microscope examination of the intact testis at successive intervals during prepuberal development. The seminiferous epithelium of the newborn mouse testis contains two distinct cell types, gonocytes and Sertoli cells (3). The former are evident in the center of the cords as large round cells, ~m in diameter, having a spherical nucleus with dispersed homogeneous chromatin and a central filamentous nucleolus. The cytoplasm contains spherical mitochondria in relatively low numbers. By 6 days after birth, the germ cells are attached to the basement membrane and have differentiated to form primitive type A spermatogonia (Fig. 1). These cells are similar in appearance to the gonocytes except that they are smaller, /~m in diameter, and the nucleus contains scattered flakes of heterochromatin. Furthermore, the nu- 70 THE JOURNAL OF CELL BIOLOGY' VOLUME 74, 1977
4 deoli now have a prominent reticulated nucleolonema, an irregular shape, and occupy an eccentric position in the nucleus. Primitive type A spermatogonia comprise 16% of all cells in the seminfferous epithelium at day 6 (Table I). In contrast to primitive type A spermatogonia, the more numerous Sertoli cells exhibit frequent irregularities in both nuclear and cytoplasmic contour (Figs. 1 and 2). Scattered areas of heterochromatin occur around the periphery of the nucleus, and the cytoplasm contains many spherical and elongated mitochondria. At this stage in development, the Sertoli cell lacks both the paired nucleolar karyosomes and cytoplasmic lipid inclusions and is considerably smaller in volume than the adult cell. Type A and type B spermatogonia are present at day 8 after birth (Table I). In comparison to primitive type A spermatogonia, type A spermatogonia have a reduced nuclear and cytoplasmic volume. Nuclear chromatin is again homogeneous, and multiple reticulated nucleoli are seen in close proximity to the nuclear membrane (Figs. 2 and 4). Type B spermatogonia have a diameter of -8-9 /zm and are therefore considerably smaller than type A (Fig. 2). These cells have an increased amount of heterochromatin. A thin rim of condensed chromatin around the nuclear membrane is interspersed with larger areas of more highly condensed chromatin. A single reticulated nucleolus is usually situated near the center of the nucleus. Primary spermatocytes at the preleptotene and leptotene stages of meiotic prophase are present at day 10 of development (Table I). The preleptotene spermatocytes are the smallest of germ cells with a diameter of only /~m (Fig. 5) and are usually separated from the basement membrane by Sertoli cell processes (28, 29, 40). Localized areas of condensed chromatin occur scattered throughout the nucleus and occasionally in close proximity to the nuclear membrane. The remaining chromatin is dispersed in a homogeneous manner, but is more densely granular than in type B spermatogonia. Characteristically, the preleptotene spermatocyte contains a limited amount of cytoplasm. Leptotene primary spermatocytes are characterized by the appearance of thin axial elements in the nucleus which signify the initiation of chromosome condensation. In other areas, the chromatin of leptotene spermatocytes is homogeneous and granular, with the condensed chromatin seen at the preleptotene stage being compressed against the nuclear membrane (Fig. 3). Fragments of nucleolonema are frequently observed. These cells have a diameter of ~8-10/xm and are therefore similar in size to type B spermatogonia. Zygotene primary spermatocytes, first observed on day 12 postnatal (Table I), are characterized by the appearance of short segments of synaptonemal complexes (Fig. 4). Condensation of the X and Y chromosomes to form the "sex vesicle" is also initiated at this stage of meiotic prophase. A nucleolus with a reticulated nucleolonema is either tangentially attached to or located in the vicinity of the sex vesicle. These cells have a diameter of ~10-12 /~m and are therefore larger than leptotene spermatocytes. The cytoplasm contains increasing amounts of endoplasmic reticulum and piles of narrow cisternae (29). Mitochondria become more elongated with a few dilated cristae. The transition from zygotene to the pachytene stage of meiotic prophase is gradual (Fig. 5). The autosomes, now paired by the synaptonemal complexes, become increasingly condensed during the prolonged pachytene period. A prominent nucleolar cap later forms over the sex vesicle which becomes closely applied to the nuclear membrane. Increasing numbers of mitochondria with dilated cristae aggregate in clusters as the pachytene stage advances. Both endoplasmic reticulum and areas of thin cisternae also increase substantially during this stage. Primary spermatocytes reach an early pachytene stage by day 14 (Table I), but late pachytene spermatocytes are not observed until days During the pachytene stage, cell diameter increases from -12/zm to a maximum of 18/~m. The seminiferous cord has acquired a lumen in many areas by day 16. Secondary spermatocytes and round spermatids occur in increasing numbers by day 20, thereby signifying the onset of spermiogenesis. Separation of Seminiferous Epithelial Cells by Sedimentation Velocity It is evident that (a) primitive germ cells differentiate in a defined temporal sequence during prepuberal development, (b) cell volume decreases progressively with successive divisions of spermatogonia and then increases substantially between the preleptotene and late pachytene stage of meiotic prophase, and (c) these cells can be identified by virtue of their distinctive morphological characteristics. This information was applied in BELLVE ET AL. Spermatogenic Cells of the Prepuberal Mouse 71
5 FIGURE 1 Electron micrograph of day 6 mouse seminiferous epithelium showing several Sertoli cells (S) and a large primitive type A spermatogonium (PA). The latter contains a centrally located nucleolus with reticulated nucleolonema (arrow). 4,800. FIGURE 2 Electron micrograph of day 8 mouse seminiferous epithelium showing type A spermatogonium (A), type B spermatogonium (B), and several Sertoli cells (S). Note differences between spermatogonia in the size of mitochondria and position of nucleoli (arrows). x 4,800.
6 TABLE I The Temporal Appearance of Spermatogenic Cells in the Prepuberal Mouse Testis Days after birth Celltype Primitive type A spermatogonia Type A spermatogonia Type B spermatogonia Preleptene spermatocytes Leptotene spermatocytes Zygotene spermatocytes Pachytene spermatocytes Secondary spermatocytes Round spermatids Sertoli cells Data are expressed as percentage of total cells in the seminiferous epithelium. The cell counts were determined by classifying nuclei present in 50 cross sections of seminiferous cords chosen at random from the testes of three mice sacrificed at each designated age. Degenerating and unidentified cells (2-4%) have not been included in these data. an effort to isolate and identify discrete populations of spermatogenic cells from the prepuberal seminiferous epithelium. The sequential enzymatic procedure was found to be effective for both the isolation of seminiferous cords and the preparation of cell suspensions from the prepuberal testes. The yield of seminiferous epithelial cells per testis ranges from 7 x 105 at day 6 to 18 x 105 at day 18. Examination of the cells by light and electron microscopy demonstrates that they retain their general morphological characteristics. Furthermore, between 96 and 98% of the cells in the initial suspension and after separation continue to exclude trypan blue. Erythrocytes, lymphocytes, macrophages, myoid cells, and Leydig cells were observed rarely in the isolated cell populations. When seminiferous cells of day 6 testes are subjected to sedimentation velocity at unit gravity, three distinct "cell populations" are recovered. Those "cells" having the lowest sedimentation velocity are recovered in fractions By Nomarski differential interference microscopy, these cells are 4-6 /zm in diameter, smooth in outline, and appear to lack nuclei or cytoplasmic inclusions (Fig. 6). Examination of thin sections reveals that this fraction contains cytoplasts of varying electron density (Fig. 7). Although many of these cytoplasts exhibit concentric whorls of membrane, only a few contain any mitochondria. Other cytoplasts contain numerous small vesicles. Since 98% of the cytoplasts exclude trypan blue, it is assumed that the limiting cytoplasmic membrane is intact. Whether the cytoplasts originate from the cytoplasm of spermatogonia or Sertoli cells is at present unresolved, although the latter origin is more likely. An extremely homogeneous population of Sertoli cells is obtained on day 6 by pooling fractions These cells have a diameter of /zm and an irregular outline, and appear almost granular in nature under Nomarski optics (Fig. 8). The latter characteristics make it difficult to discern any nuclear morphology by light microscopy. Electron microscope examination, however, reveals the characteristic irregularities in nuclear configuration and the typical pattern of heterochromatin and dense nucleoli in close association with the nuclear membrane (Fig. 9). The purity of the Sertoli cell population, as determined from both thin and thick sections, is >99% (Table II). The primitive type A spermatogonia are recovered from the day 6 testis by pooling fractions (Fig. 10). These cells have a diameter of /~m, which is comparable to their size in vivo. They are spherical in outline with large round nuclei which contain flakes of heterochromatin and two to three eccentrically placed nucleoli (Fig. 11). The minimal purity of the primitive type A spermatogonia is 90%, with Sertoli cells constituting the only source of contamination (Table II). Cell suspensions were prepared from day 8 testes and the separated cells again characterized by light and electron microscopy. Large spherical cells, /~m in diameter, are recovered in BELLVI~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 73
7 74 THE JOURNAL OF CELL BXOLOGY" VOLUME 74, 1977
8 fractions (Fig. 12). They are identified as type A spermatogonia since multiple nucleoli are seen in close proximity to the nuclear membrane, the chromatin is relatively homogenous, and the nuclear/cytoplasmic ratio has increased substantially (Fig. 13). Populations of type A spermatogonia can be prepared so as to exceed 91% purity (Table II). Contaminating cell types include Sertoli cells and type B spermatogonia. Fractions contain type B spermatogonia (Fig. 14). These cells are smaller (8-10 /zm in diameter) than type A spermatogonia and contain greater quantities of heterochromatin and small spherical mitochondria (Fig. 15). Many cells are seen in metaphase and late prophase configurations of mitosis. This population of cells may also include intermediate spermatogonia which are difficult to distinguish from type B spermatogonia. On this basis, the population of type B is assessed at 76% purity. The principal source of contamination is Sertoli cells (Table II). Most of the Sertoli cells, however, are recovered in fractions The greater sedimentation velocity of the Sertoli cell population on day 8 reflects a 15% increase in cell volume that has occurred since day 6 of postnatal development. Cytoplasts, comparable to those of day 6, are also recovered on day 8 but generally in lower numbers. Cell separations were also performed over the developmental period of days 9-16, inclusive. Although relatively homogeneous populations of primary spermatocytes can be obtained, the level of purity is inconsistent. The variability is due to contamination of the primary spermatocyte populations by spermatogonia and Sertoli cells. Type A and type B spermatogonia contaminate preparations of zygotene and leptotene spermatocytes, respectively. Furthermore, a proportion of the Sertoli cells loses some cytoplasm during the preparative procedures, and some form aggregates of one or more cells after loading into the sedimentation chamber. The sedimentation velocity of these cells is either less than or greater than that of single intact Sertoli cells. Consequently, they contaminate cell populations in other regions of the gradient. Frequently, two to five preleptotene spermatocytes are seen attached to a single Sertoli cell, thereby forming an aggregate which may also cosediment with pachytene spermatocytes. The recovery of Sertoli cells and spermatogonia, relative to primary spermatocytes, decreases between days 10 and 18. This reduction is due, in part, to the decreasing proportion of Sertoli cells and spermatogonia in the seminiferous epithelium as development progresses (Table I). In addition, however, both of these cell types are apparently lost differentially during the filtration steps. Attempts were made to isolate primary spermatocytes at successive stages of meiotic prophase from the day 18 seminiferous epithelium. Examination by Nomarski differential interference microscopy reveals cells which are similar in morphology but which differ substantially in size over successive fractions of the gradient. In order to identify which fractions contain cells at the desired stages of meiotic prophase, the following experimental protocol was adopted. Every two successive fractions throughout the gradient were pooled and the mean diameter of cells in each pool was determined by light microscopy using a calibrated eyepiece micrometer. The cells present in each pool were then identified by light and electron microscopy. In subsequent separation attempts, cell diameter was used to select with precision those fractions which could be pooled to yield populations of spermatocytes at specific stages of meiotic prophase. On this basis, populations of preleptotene, leptotene/zygotene, and pachytene primary spermatocytes were recovered. Preleptotene primary spermatocytes, which FIGURE 3 Electron micrograph of day 18 mouse seminiferous epithelium showing leptotene primary spermatocytes (L). Note homogeneous density of nucleoplasm and relatively pale cytoplasm. Cytoplasmic bridge connects two adjacent cells (arrow). 4,800. FIGURE 4 Electron micrograph of day 18 mouse seminiferous epithelium showing zygotene primary spermatocytes (Z). Nuclei contain patchy condensed chromatin and short segments of synaptonemal complex (arrow). x 4,800. FIGURE 5 Section of day 18 mouse seminiferous epithelium depicting preleptotene primary spermatocytes (PL) near basal lamina, and pachytene primary spermatocytes (P). The latter contains a condensed sex vesicle (arrow) in close proximity to the nudear membrane. 4,800. BELLVI ~. ET AL. Spermatogenic Cells of the Prepuberal Mouse 75
9 76 THE JOURNAL OF CELL BIOLOGY" VOLUME 74, 1977
10 TABLE li Purity and Composition of Cell Populations Isolated from the Prepuberal Mouse Seminiferous Epithelium Cell populations Cell type PA A B PL L/Z p S % Primitive type A spermatogonia <1 Type A spermatogonia < 1 - Type B spermatogonia Preleptotene spermatocytes Leptotene spermatocytes Zygotene spermatocytes <1 - Pachytene spermatocytes Sertoli cells >99 Data are expressed as percent of total cells recovered in each isolated cell population. Those cells (3-5%) which could not be identified because of necrosis or unfavorable section are not included. The population of type B spermatogonia may contain a small proportion of intermediate spermatogonia which have a greater cell diameter (-10-11/zrn) but cannot always be identified with certainty. Cell populations include primitive type A spermatogonia (PA), type A spermatogonia (A), type B spermatogonia (B), preleptotene spermatocytes (PL), pooled leptotene and zygotene spermatocytes (L/Z), pachytene spermatocytes (P), and Sertoli cells (S). range in diameter from 7.6 to 8.2/zm (mean 7.8 /zm), are recovered by pooling fractions Except for an occasional Sertoli cell, this cell population appears homogeneous when examined by Nomarski microscopy (Fig. 16). In thin sections, these cells are seen to have regions of condensed heterochromatin throughout the nucleus as well as attached to the nuclear membrane (Fig. 17). Both nuclear and cytoplasmic volumes are smaller than in type B spermatogonia. The purity of the preleptotene spermatocytes is assessed at 93% (Table II). Leptotene and zygotene primary spermatocytes have cell diameters ranging from -8.2 to 10/xm and -10 to 12 /zm, respectively. These cells are recovered as a single enriched population by pooling fractions (Fig. 18). Leptotene spermatocytes are identified by their larger nuclear volume, homogeneous chromatin, and the occurrence of a few elongated mitochondria in the cyto- plasm (Fig. 19). The diffuse threads of axial elements are not always evident in the isolated cells. Zygotene spermatocytes can be discerned by the increase in localized areas of heterochromatin, initiation of the sex vesicle, and short but infrequent segments of synaptonemal complex. This cell population, however, is invariably contaminated with Sertoli cells and less frequently with binucleate preleptotene spermatocytes and early pachytene spermatocytes (Table II and Fig. 19). Due to the contamination by Sertoli cells, separation of leptotene and zygotene spermatocytes, although feasible, was not attempted. Furthermore, it is not possible to isolate homogeneous populations of these cells after day 18 in development, since round spermatids, which occur in increasing numbers (Table I), cosediment with the population of leptotene and zygotene spermatocytes. Pachytene primary spermatocytes range from 12 to 18/zm in diameter and can be recovered on FIGURE 6 Cytoplasts isolated from the seminiferous epithelium of the day 6 mouse testis by differential sedimentation velocity. Nomarski photomicrograph, x 850. FIGURE 7 Electron micrograph of cytoplasts isolated from the day 6 mouse seminiferous epithelium. Note concentric whorls of membrane and absence of nuclei, x 2,800. FIGURE 8 Sertoli cells isolated from the day 6 mouse seminiferous epithelium by differential sedimentation velocity. Nomarski photomicrograph, x 850. FIGURE 9 Electron micrograph of isolated prepuberal Sertoli cells. Note irregular configuration of nucleus and cytoplasm, and heterochromatin in association with the nuclear membrane, x 2,800. BELLV~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 77
11 '/8 THE JOURNAL OF CELL BIOLOGY" VOLUME 74, 1977
12 this basis in fractions These cells are identified by their patchy condensed chromatin and prominent sex vesicle with, at later stages, its associated nucleolar cap. Mitochondria with dilated cristae and the extensive endoplasmic reticulum can also be discerned. The purity of this cell population usually exceeds 89% (Table II). Unfortunately, it is not possible to obtain valid estimates of cell yield after enzymatic dissociation of the testis. Calculations based on cell number are impractical since serial sections would be required to accurately assess the total population of testicular cells. The problem is complicated further by the removal of interstitial tissue and by a nonselective loss of germ cells from the ends of tubules during the collagenase treatment. Furthermore, estimates of yield based on DNA content are confounded by the differential recovery of cells having a 4C, 2C, and 1C complement of DNA. However, of the total cells present in the final cell suspension, % are recovered within the pooled populations of specific cell types. The remaining cells are recovered as heterogeneous populations and are therefore discarded. The approximate number of cells recovered per testis at the appropriate ages is as follows: day 6, 1.6 x 105 cytoplasts, 4.2 x 105 Sertoli cells, and 1.4 x 105 primitive type A spermatogonia; day 8, 1.6 x 105 type A spermatogonia and 1.5 x 105 type B spermatogonia; day 18, 2.1 x 105 preleptotene spermatocytes, 2.6 x 105 leptotene and zygotene spermatocytes, and 7.3 x 105 pachytene spermatocytes. DISCUSSION In the adult testis, spermatogenic cells exist in complex cellular associations and are classified into stages of the cycle of the seminiferous epithelium on the basis of steps in spermatid differentiation (19, 30, 31). In this system, identification of the different classes of spermatogonia and early primary spermatocytes is facilitated by their association with spermatids at specific steps of spermiogenesis. Since spermatids are not present in the prepuberal seminiferous epithelium, a precise identification of early germ cells is considerably more difficult (3, 32). This particular problem has been surmounted in the present report by defining distinctive morphological characteristics of the respective seminiferous epithelial cells in the adult and using these criteria to identify the prepuberal cell types. The criteria used are in general concordance with the findings of previous light (3, 19, 30, 31) and electron microscope studies (8, 10-12, 29, 38-40). The isolation of essentially pure populations of Sertoli cells was fortuitous. Although the prepuberal Sertoli cell lacks the distinctive paired karyosomes of the nucleolus in the adult, the irregular nuclear and cytoplasmic configuration, the peripherally located heterochromatin, and the occurrence of elongated mitochondria make identification of this cell type unambiguous. Previous efforts to isolate Sertoli cells from either the prepuberal (5, 42) or adult rat (41) have involved isolation of seminiferous tubules followed by a period of in vitro culture. During the initial period in culture, many of the contaminating germ cells are phagocytosed by the Sertoli cells. Also, this type of procedure may not entirely remove the peritubular cells which can proliferate and eventually form a fibroblastic contaminant of the Sertoli cell cultures. The present technique for the isolation of Sertoli cells has the distinct advantages of simplicity, brevity, and extreme purity. Furthermore, since the Sertoli cells are isolated during a period of mitotic activity, they may well continue to proliferate in vitro for several days before entering mitotic arrest. Previous studies have reported that the recovery FmtlRE 10 Primitive type A spermatogonia isolated from the day 6 mouse seminiferous epithelium by differential sedimentation velocity. Nomarski photomicrograph, x 850. FtGURE 11 Electron micrograph of isolated primitive type A spermatogonia. Note flakes of heterochromatin and eccentrically placed nucleoli, x 2,800. FIGURE 12 Type A spermatogonia isolated from the day 8 mouse seminiferous epithelium by differential sedimentation velocity. Nomarski photomicrograph, x 850. FmURE 13 Electron micrograph of isolated type A spermatogonia. Note smaller nuclear and cytoplasmic volume with nudeoli in close proximity to nuclear membrane, x 2,800. BELLVI~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 79
13 80 THE JOURNAL OF CELL BIOLOGY" VOLUME 74, 1977
14 of Sertoli cells and spermatogonia from the adult testis is substantially lower than expected (24, 35). One suggestion (4, 24) is that these particular cells may be more vulnerable to lysis during the enzymatic dissociation and therefore do not appear in the final cell suspension. This proposal is unlikely, however, since spermatogonia and Sertoli cells are recovered from the seminiferous epithelium on day 6 in normal yields and with viabilities of 96-98%. The current observations support alternative and more plausible explanations. The occluding Sertoli junctions form during the postnatal period of development (13, 26). In the mouse 6 days after birth, the Sertoli junctions are seen merely as localized networks of intramembranous particles (26). Subsequently, however, the junctional complexes increase in area and length until at day 16 they appear as parallel linear arrays of intramembranous particles which course circumferentially around the basal surface of the cell. The appearance at this time of a lumen in many areas of the seminiferous cords attests to the impermeability of the Sertoli junctions. With maturation of these tight junctions, the seminiferous epithelium is effectively divided into two compartments, a basal compartment in which the spermatogonia and perhaps early primary spermatocytes reside and an adluminal compartment in which the more advanced spermatocytes and spermatids are secluded (6). Clearly, the excessive decline in recovery of Sertoli cells and spermatogonia between days 10 and 18 coincides with the development of the Sertoli junctions. In this regard, it is pertinent that the tight junctions which exist between adjacent exocrine cells of the pancreas are not disrupted by digestion with chymotrypsin although dissociation does occur in a medium which either lacks Ca ++ or contains EDTA (1). On the basis of this evidence, it is proposed that the Sertoli junctions are relatively resistant to tryptic dissociation. Consequently, the Sertoli cells remain as large aggregates in association with fragments of the basal lamina, and, together with spermatogonia entrapped in the basal compartment, are lost during the filtration steps. Thus, the liberation of spermatogonia from the day 20 rat testis in the presence of EDTA, but not after tryptic dissociation (4), may be due to dissociation of the Sertoli junctions. Alternatively, EDTA may cause the release of spermatogonia from the basal lamina although there is no direct evidence to support this contention. The precise stage in differentiation at which spermatocytes traverse the Sertoli junctions is not as yet clearly delineated (cf. reference 6, 7, 9, 28, and 29). It is feasible, for instance, that preleptotene primary spermatocytes pass into the adluminal compartment and therefore may exist in both compartments of the seminiferous epithelium at different phases of their development. This would account for the high recovery of preleptotene spermatocytes which is observed after enzymatic dissociation of the day 18 mouse seminiferous epithelium. An alternative proposal which could account for the recovery of preleptotene spermatocytes, but not spermatogonia, is not immediately apparent. The isolation of seminiferous cells from the prepuberal testis is facilitated primarily by (a) the use of animals at specific stages of development, (b) the isolation of seminiferous cords before preparing the cell suspension, (c) the differential volume of these cells which enables their separation by sedimentation velocity, and (d) the identification of isolated cells by virtue of their distinctive morphological features. The sequential enzymatic dissociation permits isolation of intact seminiferous tubules and also ensures removal of the dispersed cellular components of the vascular system, interstitial cells, and virtually all of the peritubular Fl~tr~E 14 Type B spermatogonia isolated from the day 8 mouse semintferous epithelium by differential sedimentation velocity. Nomarski photomicrograph, x 850. FIGURE 15 Electron micrograph of isolated type B spermatogonia. Note decrease in cell size and characteristic pattern of heterochromatin. 2,800. FIbUlaE 16 Preleptotene primary spermatocytes isolated from the day 18 mouse seminiferous epithelium by differential sedimentation velocity. Nomarski photomicrograph, x 850. Fl6ol~ 17 Electron micrograph of isolated preleptotene primary spermatocytes. Note diminished cytoplasmic volume and localized areas of condensed chromatin. 2,800. BELLVI~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 81
15 82 THE JOURNAL OF CELL BIOLOGY-VOLUME 74, 1977
16 FIGURE 22 Schematic diagram of spermatogenesis in the prepuberal and adult mouse testis showing the relative volumes and characteristic morphology of the respective cell types. This complex process occurs in three phases: the mitotic proliferation of spermatogonia (ascending axis); meiosis with its prolonged meiotic prophase (horizontal axis) and two reduction divisions which yield 2 x secondary spermatocytes and then 4x haploid round spermatids; and spermiogenesis (descending axis) which culminates in the formation of spermatozoa. Enriched populations of each cell type, except intermediate spermatogonia and secondary spermatocytes, can now be isolated (see text). FI6URE 18 Pooled population of ieptotene and zygotene primary spermatocytes isolated from the day 18 mouse seminiferous epithelium by differential sedimentation velocity. Contaminating Sertoli cells are slightly refractile and irregular in outline. Nomarski photomicrograph, x 850. FmuRE 19 Electron micrograph of pooled leptotene and zygotene primary spermatocytes after isolation. Contaminating Sertoli cell (lower center) and a binucleate preleptotene primary spermatocyte (lower left) are also present, x 2,800. FIGURE 20 Pachytene primary sperrnatocytes isolated from the day 18 mouse seminiferous epithelium by differential sedimentation velocity. Nomarski photomicrograph FmtIRE 21 Electron micrograph of isolated pachytene primary spermatocytes. Note patchy areas of condensed chromatin, sex vesicle, nucleolar cap, and mitochondria with dilated cristae, x 2,800. BELLVl~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 83
17 cells. The interstitial tissue is thereby effectively eliminated as a source of contamination. Furthermore, the existence of the Sertoli junctions appears to facilitate the isolation of preleptotene spermatocytes and leptotene/zygotene spermatocytes with minimal contamination by type B and type A spermatogonia, respectively. Caution must be taken, however, to prevent contamination of the leptotene/zygotene spermatocytes by round spermatids which first appear in appreciable numbers during days 19 and 20 of development. By utilizing these physical parameters of the seminiferous epithelium, it proved possible to isolate highly enriched populations of primitive type A spermatogonia, type A spermatogonia, type B spermatogonia, preleptotene spermatocytes, and pachytene spermatocytes. An enriched population of leptotene and zygotene spermatocytes can also be obtained. Furthermore, since the volume of the pachytene spermatocytes ranges from 900/~m 3 to 3,050/xm 3, it may also be feasible to isolate populations of early, mid, and late pachytene spermatocytes. The isolation of spermatogenic cells at these earlier stages of differentiation complements those obtained previously from the adult seminiferous epithelium, namely, late pachytene spermatocytes, round spermatids, and residual bodies (Fig. 22) (35). The extremely short duration of both the diplotene primary spermatocyte and secondary spermatocyte stages does not permit the isolation of these particular cell types. It should be noted that the population of type A spermatogonia may contain, in addition to types Al to A4, a small number of the nonproliferating (Ao) and renewal stem cells (As, Apt, Aal) (2, 16, 32, 33). The isolated seminiferous cells continue to exclude trypan blue (96-98%) and retain their morphological integrity as determined by light and electron microscopy. Moreover, spermatogenic cells isolated from the adult testis under identical conditions show appreciable levels of oxygen consumption (35). The incidence of degenerating prepuberal spermatogenic cells (2-4%) is not greater than expected under the conditions used for their isolation. This suggests that the large number of spermatogenic cells which undergo normal degeneration in situ (30, 37) are either quickly phagocytosed by Sertoli cells (15) or else are removed by the enzymatic treatment. Although further cytological and biochemical characterization is required, the successful isolation of spermatogenic cells representing virtually all major stages of spermatogenesis (Fig. 22) is a major advance towards elucidating the regulatory mechanisms involved in this complex differentiative process. The isolated spermatogenic cells, for instance, have proved an invaluable asset in recent studies on the expression of membrane antigens during mouse spermatogenesis (25). The authors extend their appreciation to Steven Borack of the Photographic Unit, to Ms. Bonita Rup for expert technical assistance, and to Mrs. Mary Forte for preparation of the manuscript. This research project was supported by the National Institute of Child Health and Human Development (NICHHD) grants HD and HD 06969, Additional support was provided by NICHHD Center Grant HD and Rockefeller Foundation grant Dr. J. C. Cavicchia was supported by the Consejo Nacional de Investigaciones Cientificas y Trcnicas, Republica Argentina; Dr. C. F. Millette is a Special Rockefeller Foundation Postdoctoral Fellow; Ms. D. A. O'Brien, a Danforth Foundation Fellow; and Dr. Y. M. Bhatnagar, a Population Council Postdoctoral Fellow. Received for publication 2 December 1976, and in revised form 28 February REFERENCES 1. AMSTERDAM, A., and J. D. JAMIESON Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J. Cell Biol. 63: CLERMONT, Y., and L. HERMO Spermatogonial stem cells in the albino rat. Am. J. Anat. 142: CLERMONT, Y., and B. PEREY Quantitative study of the cell population of the seminiferous tubules in immature rats. Am. J. Anat. 100: DAvis, J. C., and A. W. SCnUETZ Separation of germinal cells from immature rat testes by sedimentation at unit gravity. Exp. Cell Res. 91: DORRINGTON, J. H., N. F. ROLLER, and I. B. Fgrrz Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol. Cell. Endocrinol. 3: DYM, M The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat. Rec. 175: DVM, M., and D. W. FAWCEI"r The bloodtestis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol. Reprod. 3: FAWCETr, D. W The fine structure of chromosomes in the meiotic prophase of vertebrate spermatocytes. J. Biophys. Biochem. Cytol. 2: FAWCETT, D. W., L. V. LEAK, and P. M. HEIDGER Electron microscopic observations on the structural components of the blood-testis barrier. J. 84 THE JOURNAL OF CELL BIOLOGY 'VOLUME 74, 1977
18 Reprod. FeaR. 10(Suppl.): FLICKrSGER, C. J The postnatal development of the Sertoli cells of the mouse. Z. Zellforsch. Mikrosk. Anat. 78: FLICKINGER, C. J., and D. W. FAWCErr The junctional specializations of Sertoli cells in the seminiferous epithelium. Anat. Rec. 158: GAgDNER, P. J., and E. A. HOLYOKE Fine structure of the seminiferous tubule of the Swiss mouse. I. The limiting membrane, Sertoli cell, spermatogonia, and spermatocytes. Anat. Rec. 150: GILULA, N. B., D. W. FAWCETr, and A. Aom The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev. Biol. 50: Go, V. L. W., R. G. VERNON, and I. B. Farrz Studies on spermatogenesis in rats. I. Application of the sedimentation velocity technique to an investigation of spermatogenesis. Can. J. Biochem. 49: GONDOS, B., and C. J. HOBEL Ultrastructure of germ cell development in the human fetal testis. Z. Zellforsch. Mikrosk. Anat. 119: HocKlUS, C The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat. Rec. 169: KAaNOVSKY, M. J A formaldehyde-glutaraidehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 27(2, Pt.2)-'137 a. (Abstr.). 18. LAst, D. M. K., R. FtmmsR, and W. R. B~UCE The separation, physical characterization, and differentiation kinetics of spermatogonial cells of the mouse. Proc. Natl. Acad. Sci. U. S. A. 65: LEBLOND, C. P., and Y. CLERMONT Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the "periodic acid-fusehsin sulfurous acid" technique. Am. J. Anat. 90: LOlR, M., and LANNEAU, M Separation of ram spermatids by sedimentation at unit gravity. Exp. Cell Res. 83: Lurr, J. H Improvements in epoxy embedding methods. J. Biophys. Biochem. Cytol. 9: Lurr, J. H Embedding media-old and new. In Advanced Techniques in Biological Electron Microscopy. J. K. Koehler, editor. Springer-Verlag, New York MEISTmCH, M. L Separation of mouse spermatogenic cells by velocity sedimentation. J. Cell. Physiol. 80: MEISTmCH, M. L., W. R. BRUCE, and Y. CLER- MONT Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp. Cell Res. 79: MILLEXTE, C. F., and A. R. BELLViL Temporal expression of membrane antigens during mouse spermatogenesis. J. Cell Biol. 74: NAGANO, T,, and F. SUZUKi The postnatal development of the junctional complexes of the mouse Sertoli cells as revealed by freeze-fracture. Anat. Rec. 185: NEBEL, B. R., A. P. AMAROSE, and E. M. HACK- Err Calendar of gametogenic development in the prepuberal male mouse. Science (Wash. D.C.). 134: NICANDER, L An electron microscopical study of cell contacts in the seminiferous tubules of some animals. Z. Zellforsch. Mikrosk. Anat. 83: NICANDER, L., and L. PLOEN Fine structure of spermatogonia and primary spermatocytes in rabbits. Z. Zellforsch. Mikrosk. Anat. 99: OAKaERG, E. F A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am. J. Anat. 99: OAKaEaG, E. F Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 99: OAIOERG, E. F A new concept of spermatogonial stem-cell renewal in the mouse and its relationship to genetic defects. Murat. Res. 11: OAKBERG, E. F Spermatogonial stem-cell renewal in the mouse. Anat. Rec. 169: REYNOLDS, E. S The use of lead citrate at high ph as an electron-opaque stain in electron microscopy. J. Cell Biol. 17: ROmRELL, L. J., A. R. BELLV~, and D. W. FAW- CETr Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev. Biol. 49: ROOSEN-RUNGE, E. C Kinetics of spermatogenesis in mammals.ann. N. Y. Acad. Sci. $$: ROOSEN-RuNGE, E. C Germinal-cell loss in normal metazoan spermatogenesis. J. Reprod. Fertil. 35: SAr'SFORD, C. S The development of the Sertoli cell of the rat and mous.e: Its existence as a mononucleate unit. J. Anat. 97: SCnLEn=RMACnER, E., and W. SCrIMIOT The local control of mammalian spermatogenesis. Humangenetik. 19: SOLAPa, A. J The evolution of the ultrastructure of the sex chromosomes (sex vesicle) during meiotic prophase in mouse spermatocytes. J. Ultrastruc. Res. 27: STEINBERGER, A., J. J. HEINDEL, J. N. LINDSEY, J. S. H. ELKIUGTON, B. M. SANBORN, and E. STEINaERt3ER Isolation and culture of FSH responsive Sertoli cells. Endocrine Res. Commun. 2: WELSH, M. J., and J. P. WmBE Rat Sertoll cells: A rapid method for obtaining viable cells. Endocrinology. 96: BELLV~ ET AL. Spermatogenic Cells of the Prepuberal Mouse 85
Adapted from Preg. & Part., Senger
 MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
MALE ENDOCRINOLOGY AND SPERMATOGENESIS (Chapter 10) AVS 222 (Instructor: Dr. Amin Ahmadzadeh) I. MALE ENDOCRINOLOGY (Figure10-1 to 10-3) A. Glands and their respective hormones 1) Hypothalamic hormone:
Male Reproductive System
 Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Male Reproductive System Constitution of male reproductive system Genital gland ----testis Genital ducts epididymis / ductus deferens / urinary duct Accessory sex glands Penis prostate gland Seminal vesicle
Spermatogenesis in Man
 Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Spermatogenesis in Man I. Nuclear Morphology During Spermatogenesis in Man BRUNETTO CHIARELLI, PH.D., ARTHUR FALEK, PH.D., KAREN J. BACK, B.S., and C. THOMAS COWART, M.D. THE SEQUENCE of transformations
Histology of Male Reproductive system (1)
 Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Histology of Male Reproductive system (1) Prof. Dr. Malak A. Al-yawer Learning Objectives At the end of this lecture, the medical student will be able to: State the organization of the testis Define seminiferous
Identification of the spermatogenic stages in living seminiferous tubules of man
 Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Identification of the spermatogenic stages in living seminiferous tubules of man V. Nikkanen, K.-O. S\l=o"\derstr\l=o"\m and M. Parvinen Department of Obstetrics and Gynecology, Turku University Central
Morphogenesis of the residual body of the mouse testis
 93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
93 Morphogenesis of the residual body of the mouse testis By CASIMIR F. FIRLIT and JOSEPH R. DAVIS (From the Department of Pharmacology and Therapeutics, Stritch School of Medicine, and Graduate School,
Cycle of the Seminiferous Epithelium of the Guinea Pig
 Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
Cycle of the Seminiferous Epithelium of the Guinea Pig A Method for Identification of the Stages Yves Clermont, Ph.D. IN THE GUINEA PIG, the cells of the seminiferous epithelium are arranged in definite
The Use of Rabbits in Male Reproductive Toxicology
 Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
Environmental Health Perspectives Vol. 77, pp. 5-9, 1988 The Use of Rabbits in Male Reproductive Toxicology by Daniel Morton* The rabbit is the smallest and least expensive laboratory animal in which serial
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
The spermatogenesis CHARACTERISTICS OF THE SPERMATOZOON 26/04/2017. Reproductive Biotechnologies Andrology I. Prof. Alberto Contri
 Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
Reproductive Biotechnologies Andrology I The spermatogenesis Prof. Alberto Contri CHARACTERISTICS OF THE SPERMATOZOON 1) Aploid cell with high condensed DNA 2) Forward motility - flagellum 3) Enzymes for
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER)
 THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
THE EFFECTS OF REPEATED INJECTIONS OF CHORIONIC GONADOTROPIN ON THE TESTES OF THE LEOPARD FROG (RANA PIPIENS SCHREBER) ROBERT P. McCOURT Department of Zoology and Entomology, The Ohio State University,
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
ABSTRACT. The objective of this study was to assess the effectiveness of a Nycodenz gradient
 ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of
ABSTRACT MILLER, STEPHANIE RENEE. Assessment of nycodenz gradient on enrichment and culture of perinatal porcine spermatogonial stem cells. (Under the direction of Robert M. Petters). The objective of
Seminiferous Tubules
 Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Testes The testes are compound tubular glands that lie within a scrotal sac, suspended from the body by a spermatic cord. The testes are dual organs that act as exocrine glands producing a holocrine secretion,
Production of Fertile Sperm. Animal Science 434. Hormonal Regulation of the Testis. hormonal regulation of the testis
 roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
roduction of Fertile Sperm hormonal regulation of the testis nimal Science 434 Lecture 12: Spermatogenesis mitotic division of spermatogonia meiotic divisions of spermatocytes morphologic transformation
Quantitative differences between variants of
 Quantitative differences between variants of A spermatogonia in man R. Paniagua, M. Nistal, P. Amat, M. C. Rodr\l=i'\guez,and J. R. Alonso "Department of Cytology and Histology, Faculty ofbiology, University
Quantitative differences between variants of A spermatogonia in man R. Paniagua, M. Nistal, P. Amat, M. C. Rodr\l=i'\guez,and J. R. Alonso "Department of Cytology and Histology, Faculty ofbiology, University
5 15/3/2012. Malik Al-Momani
 5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
5 15/3/2012 Malik Al-Momani بسم هللا الرحمن الرحيم Spermatogenesis Note : Please refer to slides so see photos. Quick Revision : - Testis is divided by septum into testicular lobules, inside the lobules
Medical School Histology Basics Male Reproductive System. VIBS 289 lab
 Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
Medical School Histology Basics Male Reproductive System VIBS 289 lab Larry Johnson Texas A&M University OBJECTIVE To conduct a histologic examination of the testis (which produce spermatozoa), excretory
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
GROWTH AND OBSERVATIONS OF CHINESE HAMSTER SEMINIFEROUS EPITHELIUM IN VITRO
 J. Cell Sci. 6, 19S-205 (1970) Printed in Great Britain GROWTH AND OBSERVATIONS OF CHINESE HAMSTER SEMINIFEROUS EPITHELIUM IN VITRO D. J. ELLINGSON AND K. T. S. YAO U.S. Department of Health, Education
J. Cell Sci. 6, 19S-205 (1970) Printed in Great Britain GROWTH AND OBSERVATIONS OF CHINESE HAMSTER SEMINIFEROUS EPITHELIUM IN VITRO D. J. ELLINGSON AND K. T. S. YAO U.S. Department of Health, Education
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM
 LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
LABORATORY EXERCISES FOR MALE REPRODUCTIVE SYSTEM Slide #101 (1096). Testis, rat. sustentacular ( Sertoli ) cells Nuclei of Sustentacular cells Leydig cells Spermatogonia Spermatocytes Spermatids pale
The Male Reproductive System
 The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
The Male Reproductive System YONG-MEI CHEN ( 陈咏梅 ) Dept. of Anatomy, Histology & Embryology Peking Union Medical College Tel:69156461 E-mail address: pumc_he@126.com Content Spermatogenesis Spermiogenesis
Basic histology 5/4/2015
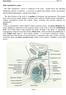 Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Male reproductive system The male reproductive system is composed of the testes, genital ducts (the adjoining epididymis, and the vas deferens, a accessory sex glands (the seminal vesicles, the prostrate
Morphogenesis by dissociated immature rat testicular cells in primary culture
 /. Einhryol. exp. Morph. Vol. 44, pp. 297-302, 1978 297 Printed in Great Britain (p Company of Biologists Limited 1978 SHORT PAPER Morphogenesis by dissociated immature rat testicular cells in primary
/. Einhryol. exp. Morph. Vol. 44, pp. 297-302, 1978 297 Printed in Great Britain (p Company of Biologists Limited 1978 SHORT PAPER Morphogenesis by dissociated immature rat testicular cells in primary
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Pathology of Male Reproductive System 1
 Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
Pathology of Male Reproductive System 1 Professor dr Ali Hassan Altimimi Professor of Pathology& Histology MSc, PHD, MD(UK) MALE REPRODUCTIVE SYSTEM The internal male genitalia consist of the testes with
TEMPORAL EXPRESSION OF MEMBRANE ANTIGENS
 Published Online: 1 July, 1977 Supp Info: http://doi.org/10.1083/jcb.74.1.86 Downloaded from jcb.rupress.org on August 20, 2018 TEMPORAL EXPRESSION OF MEMBRANE ANTIGENS DURING MOUSE SPERMATOGENESIS CLARKE
Published Online: 1 July, 1977 Supp Info: http://doi.org/10.1083/jcb.74.1.86 Downloaded from jcb.rupress.org on August 20, 2018 TEMPORAL EXPRESSION OF MEMBRANE ANTIGENS DURING MOUSE SPERMATOGENESIS CLARKE
Dynamics of mono, di and tri-methylated histone H3 lysine 4 during male meiotic prophase I. Nuclei were co-stained for H3.1/H3.2. Progressing stages
 Dynamics of mono, di and tri-methylated histone H3 lysine 4 during male meiotic prophase I. Nuclei were co-stained for H3.1/H3.2. Progressing stages of spermatogenesis are shown from left to right. Arrows/dotted
Dynamics of mono, di and tri-methylated histone H3 lysine 4 during male meiotic prophase I. Nuclei were co-stained for H3.1/H3.2. Progressing stages of spermatogenesis are shown from left to right. Arrows/dotted
SPERMATOGENESIS IN VITRO
 SPERMATOGENESIS IN VITRO INDUCTION OF PROLIFERATION, MEIOSIS AND DIFFERENTIATION Mário Sousa Lab Cell Biology Institute of Biomedical Sciences (ICBAS) University of Porto msousa@icbas.up.pt Spermatogonia
SPERMATOGENESIS IN VITRO INDUCTION OF PROLIFERATION, MEIOSIS AND DIFFERENTIATION Mário Sousa Lab Cell Biology Institute of Biomedical Sciences (ICBAS) University of Porto msousa@icbas.up.pt Spermatogonia
Male Reproductive System
 Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Male Reproductive System organs that function in: gamete and hormone production not all in abdominal cavity paired testicles = controlled by LH & FSH duct systems accessory glands Testis: Gross Histology
Isolation of rat gonocytes by velocity sedimentation at unit gravity
 Isolation of rat gonocytes by velocity sedimentation at unit gravity F. M. F. van Dissel-Emiliani, D. G. de Rooji and M. L. Meistrich *Department ofexperimental Radiotherapy, University of Texas, M.D.
Isolation of rat gonocytes by velocity sedimentation at unit gravity F. M. F. van Dissel-Emiliani, D. G. de Rooji and M. L. Meistrich *Department ofexperimental Radiotherapy, University of Texas, M.D.
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
MALE REPRODUCTIVE SYSTEM The male reproductive system consists of primary sex organs (testes) and secondary or accessory sex organs. The secondary organs consist of a series of genital ducts (ductules
Some Observations on the Fine Structure of the Goblet Cells. Special Reference to the Well-Developed Agranular Endoplasmic Reticulum
 Okajimas Folia Anat. Jpn., 58(4-6) : 583-594, March 1982 Some Observations on the Fine Structure of the Goblet Cells in the Nasal Respiratory Epithelium of the Rat, with Special Reference to the Well-Developed
Okajimas Folia Anat. Jpn., 58(4-6) : 583-594, March 1982 Some Observations on the Fine Structure of the Goblet Cells in the Nasal Respiratory Epithelium of the Rat, with Special Reference to the Well-Developed
ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF BLUETONGUE VIRUS*
 Onderstepoort J. vet. Res. (1968), 35 (1), 139-150 Printed in the Repub. of S. Afr. by The Government Printer, Pretoria ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF BLUETONGUE VIRUS* G. LECATSAS, Veterinary
Onderstepoort J. vet. Res. (1968), 35 (1), 139-150 Printed in the Repub. of S. Afr. by The Government Printer, Pretoria ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF BLUETONGUE VIRUS* G. LECATSAS, Veterinary
THE FORM OF HAEMOGLOBIN IN THE ERYTHROCYTES OF THE COD, GADUS CALLARIAS
 J. Cell Set. 8, 407-412 (1971) 407 Printed in Great Britain THE FORM OF HAEMOGLOBIN IN THE ERYTHROCYTES OF THE COD, GADUS CALLARIAS N.W.THOMAS Department of Anatomy, Marischal College, Aberdeen, Scotland
J. Cell Set. 8, 407-412 (1971) 407 Printed in Great Britain THE FORM OF HAEMOGLOBIN IN THE ERYTHROCYTES OF THE COD, GADUS CALLARIAS N.W.THOMAS Department of Anatomy, Marischal College, Aberdeen, Scotland
FOLLICLE CELL BRIDGES IN THE MOSQUITO OVARY: SYNCYTIA FORMATION AND BRIDGE MORPHOLOGY
 jf. Cell Set. 31, 137-143 (1978) 137 Printed in Great Britain Company of Biologists Limited I
jf. Cell Set. 31, 137-143 (1978) 137 Printed in Great Britain Company of Biologists Limited I
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE
 TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
TRANSFER OF PREMELANOSOMES INTO THE KERATINIZING CELLS OF ALBINO HAIR FOLLICLE PAUL F. PARAKKAL. From the Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION
STUDIES OF THE HUMAN UNFERTILIZED TUBAL OVUM*t
 FERTILITY AND STERILITY Copyright @ 1973 by The Williams & Wilkins Co. Vol. 24, No.8, August 1973 Printed in U.S.A. STUDIES OF THE HUMAN UNFERTILIZED TUBAL OVUM*t C. NORIEGA, M.D., AND C. OBERTI, M.D.
FERTILITY AND STERILITY Copyright @ 1973 by The Williams & Wilkins Co. Vol. 24, No.8, August 1973 Printed in U.S.A. STUDIES OF THE HUMAN UNFERTILIZED TUBAL OVUM*t C. NORIEGA, M.D., AND C. OBERTI, M.D.
Embryology 3. Spermatogenesis:
 Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
Embryology 3 Spermatogenesis: The 2 testis in males are each divided into lobes and lobules by connective tissue septa forming 250 lobule and in each lobule there are 1 to 4 seminefrous tubule ( so almost
ELECTRON MICROSCOPIC STUDIES ON EQUINE ENCEPHALOSIS VIRUS
 Onderstepoort]. vet. Res. 40 (2), 53-58 (1973) ELECTRON MICROSCOPIC STUDIES ON EQUINE ENCEPHALOSIS VIRUS G. LECATSAS, B. J. ERASMUS and H. J. ELS, Veterinary Research Institute, Onderstepoort ABSTRACT
Onderstepoort]. vet. Res. 40 (2), 53-58 (1973) ELECTRON MICROSCOPIC STUDIES ON EQUINE ENCEPHALOSIS VIRUS G. LECATSAS, B. J. ERASMUS and H. J. ELS, Veterinary Research Institute, Onderstepoort ABSTRACT
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
New aspect of hepatic nuclear glycogenosis
 J. clin. Path. (1968), 21, 19 New aspect of hepatic nuclear glycogenosis in diabetes1 F. CARAMIA, F. G. GHERGO, C. BRANCIARI, AND G. MENGHINI From the Institute of General Pathology, University of Rome,
J. clin. Path. (1968), 21, 19 New aspect of hepatic nuclear glycogenosis in diabetes1 F. CARAMIA, F. G. GHERGO, C. BRANCIARI, AND G. MENGHINI From the Institute of General Pathology, University of Rome,
Gametogenesis. Omne vivum ex ovo All living things come from eggs.
 Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Omne vivum ex ovo All living things come from eggs. William Harvery, 1651 Gametogenesis This lecture is the preface, so to speak, to embryology; that is, it introduces the development of the specialized
Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey
 J. Biosci., Vol. 2, Number 3, September 1980, pp. 261-266. Printed in India. Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey ASHA PRAKASH, M. R. N. PRASAD and T.C. ANAND KUMAR
J. Biosci., Vol. 2, Number 3, September 1980, pp. 261-266. Printed in India. Ultrastructural studies on the epididymal spermatozoa in the rhesus monkey ASHA PRAKASH, M. R. N. PRASAD and T.C. ANAND KUMAR
Reproductive Toxicology
 Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Reproductive Toxicology 32 (2011) 395 406 Contents lists available at SciVerse ScienceDirect Reproductive Toxicology jo u r n al hom epa ge: ww w.elsevier.com/locate/reprotox Effects of multiple doses
Continuous Cell Culture From a Patient With Chronic Myelogenous Leukemia. I. Propagation and Presence of Philadelphia Chromosome 1
 Continuous Cell Culture From a Patient With Chronic Myelogenous Leukemia. I. Propagation and Presence of Philadelphia Chromosome 1 LINDA S. LUCAS,2 JACQUELINE J. K..WHANG,3 J. H. TJIO,4 ROBERT A. MANAKER,2
Continuous Cell Culture From a Patient With Chronic Myelogenous Leukemia. I. Propagation and Presence of Philadelphia Chromosome 1 LINDA S. LUCAS,2 JACQUELINE J. K..WHANG,3 J. H. TJIO,4 ROBERT A. MANAKER,2
IN normal male fowls, four developmental stages of spermatogenetic activity
 Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
Development of the Testis Tubule in the Fowl By GAMAL A. R. KAMAR (From the Animal Production Department, Faculty of Agriculture, Cairo University, Giza, Egypt) With three plates (figs. 1-3) SUMMARY Three
A Compact and a Dispersed Form of the Golgi Apparatus
 A Compact and a Dispersed Form of the Golgi Apparatus of Fish Liver 1 D. James Morre and Carole A. Lembi Department of Botany and Plant Pathology Purdue University, Lafayette, Indiana 47907, and H. H.
A Compact and a Dispersed Form of the Golgi Apparatus of Fish Liver 1 D. James Morre and Carole A. Lembi Department of Botany and Plant Pathology Purdue University, Lafayette, Indiana 47907, and H. H.
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Ch 2: The Cell. Goals: Anatomy of a typical cell Cell Membrane Discussion of internal structure of a cell with emphasis on the various organelles
 Ch 2: The Cell Goals: Anatomy of a typical cell Cell Membrane Discussion of internal structure of a cell with emphasis on the various organelles Developed by John Gallagher, MS, DVM Some Terminology: 1.
Ch 2: The Cell Goals: Anatomy of a typical cell Cell Membrane Discussion of internal structure of a cell with emphasis on the various organelles Developed by John Gallagher, MS, DVM Some Terminology: 1.
Cell Overview. Hanan Jafar BDS.MSc.PhD
 Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Electron Microscopy of Small Cells: Mycoplasma hominis
 JOURNAL of BAcTRiowOY, Dc. 1969, p. 1402-1408 Copyright 0 1969 American Society for Microbiology Vol. 100, No. 3 Printed In U.S.A. NOTES Electron Microscopy of Small Cells: Mycoplasma hominis JACK MANILOFF
JOURNAL of BAcTRiowOY, Dc. 1969, p. 1402-1408 Copyright 0 1969 American Society for Microbiology Vol. 100, No. 3 Printed In U.S.A. NOTES Electron Microscopy of Small Cells: Mycoplasma hominis JACK MANILOFF
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
Lesson 1. Quiz (short) Cell cycle Chromosomes Mitosis phases
 Lesson 1 Quiz (short) Cell cycle Chromosomes Mitosis phases 2 Cell division is needed for Growth (Mitosis) Repair (Mitosis) Reproduction (Meiosis) 3 Mitosis consists of 4 phases (division of the nuclear
Lesson 1 Quiz (short) Cell cycle Chromosomes Mitosis phases 2 Cell division is needed for Growth (Mitosis) Repair (Mitosis) Reproduction (Meiosis) 3 Mitosis consists of 4 phases (division of the nuclear
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Sami Ventelä, 1,2 Hiroshi Ohta, 3 Martti Parvinen, 2 and Yoshitake Nishimune 3
 BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
BIOLOGY OF REPRODUCTION 66, 1422 1429 (2002) Development of the Stages of the Cycle in Mouse Seminiferous Epithelium after Transplantation of Green Fluorescent Protein-Labeled Spermatogonial Stem Cells
BIOL 2402 Reproductive Systems
 Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
Collin College Dr. Chris Doumen BIOL 2402 Reproductive Systems 1 Reproductive System Most systems between males and females in the human body are similar in structure. The exception of course are the organs
EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES
 EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
10 The Golgi Apparatus: The First 100 Years
 2 Structure With no cell compartment or organelle has morphology served such a pivotal role in its discovery and investigation as with the apparatus of Golgi. The original description of the apparato reticulo
2 Structure With no cell compartment or organelle has morphology served such a pivotal role in its discovery and investigation as with the apparatus of Golgi. The original description of the apparato reticulo
Published Online: 25 November, 1956 Supp Info: on November 16, 2018 jcb.rupress.org Downloaded from
 Published Online: 25 November, 1956 Supp Info: http://doi.org/10.1083/jcb.2.6.799 Downloaded from jcb.rupress.org on November 16, 2018 B~IEF NOrmS 799 Permanganate--A New Fixative for Electron Microscopy.*
Published Online: 25 November, 1956 Supp Info: http://doi.org/10.1083/jcb.2.6.799 Downloaded from jcb.rupress.org on November 16, 2018 B~IEF NOrmS 799 Permanganate--A New Fixative for Electron Microscopy.*
psittaci by Silver-Methenamine Staining and
 JOURNAL OF BACTERIOLOGY, July 1972, p. 267-271 Copyright 1972 American Society for Microbiology Vol. 111, No. 1 Printed in U.S.A. Location of Polysaccharide on Chlamydia psittaci by Silver-Methenamine
JOURNAL OF BACTERIOLOGY, July 1972, p. 267-271 Copyright 1972 American Society for Microbiology Vol. 111, No. 1 Printed in U.S.A. Location of Polysaccharide on Chlamydia psittaci by Silver-Methenamine
Animal Science 434! Tonic and Preovulatory Surge of GnRH! Tonic and Preovulatory Surge of GnRH! Lecture 11: The Follicular Phase of the Estrous Cycle!
 Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
Tonic and Preovulatory Surge of GnRH! Animal Science 434! Lecture 11: The Follicular Phase of the Estrous Cycle!! (-)! Hypothalamus! GnRH! Estradiol! (-)! Tonic and Preovulatory Surge of GnRH! Anterior!
The Male Reproductive System
 The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
The Male Reproductive System The male reproductive system Testes Genital ducts Accessory sex glands: seminal vesicles prostate bulbourethral glands External genitalia: penis Structure of the Testis Tunica
川北医学院讲稿. Under low power note the testis is enclosed by a strong fibrous. layer of serous epithelium. These fibrous tissue
 川北医学院讲稿 Experiment 5: Male and Female Reproductive System Hello, everybody, class is begin,keep quiet, please. And this is the last experimental class. Today we will learn 5 slices and review all structures
川北医学院讲稿 Experiment 5: Male and Female Reproductive System Hello, everybody, class is begin,keep quiet, please. And this is the last experimental class. Today we will learn 5 slices and review all structures
The Fine Structure of the Epithelial Cells of the Mouse Prostate* II. Ventral Lobe Epithelium
 Published Online: 1 June, 1960 Supp Info: http://doi.org/10.1083/jcb.7.3.511 Downloaded from jcb.rupress.org on September 28, 2018 The Fine Structure of the Epithelial Cells of the Mouse Prostate* II.
Published Online: 1 June, 1960 Supp Info: http://doi.org/10.1083/jcb.7.3.511 Downloaded from jcb.rupress.org on September 28, 2018 The Fine Structure of the Epithelial Cells of the Mouse Prostate* II.
THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS
 Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Copyright 1974 The American Fertility Society FERTILITY AND STERILITY Vol. 25, No.3, March, 1974 Printed in U.S.A. THE EFFECTS OF LIGATION OF CAUDA EPIDIDYMIDIS ON THE DOG TESTIS A. M. VARE, M.B.B.S.,
Spermatogenesis: An Overview
 Spermatogenesis: An Overview 2 Rakesh Sharma and Ashok Agarwal Abstract The purpose of this chapter is to provide a comprehensive overview of spermatogenesis and the various steps involved in the development
Spermatogenesis: An Overview 2 Rakesh Sharma and Ashok Agarwal Abstract The purpose of this chapter is to provide a comprehensive overview of spermatogenesis and the various steps involved in the development
All You Wanted to Know About Spermatogonia but Were Afraid to Ask
 All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
All You Wanted to Know About Spermatogonia but Were Afraid to Ask Review DIRK G. DE ROOIJ* AND LONNIE D. RUSSELL From the *Department of Cell Biology, University Medical Center Utrecht, Utrecht, The Netherlands;
Cell Cycle and Cell Division
 122 Cell Cycle and Cell Division 1. Meiosis I is reductional division. Meiosis II is equational division due to [1988] (a) pairing of homologous chromosomes (b) crossing over (c) separation of chromatids
122 Cell Cycle and Cell Division 1. Meiosis I is reductional division. Meiosis II is equational division due to [1988] (a) pairing of homologous chromosomes (b) crossing over (c) separation of chromatids
A Photographic Representation of Mitosis and Meiosis in the Male of Rattus norvegicus
 422 Cytologia 23 A Photographic Representation of Mitosis and Meiosis in the Male of Rattus norvegicus S. Ohno, W. D. Kaplan and R. Kinosita Department of Cytology and Genetics, Medical Research Institute,
422 Cytologia 23 A Photographic Representation of Mitosis and Meiosis in the Male of Rattus norvegicus S. Ohno, W. D. Kaplan and R. Kinosita Department of Cytology and Genetics, Medical Research Institute,
ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION*
 ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION* The description of the lesion in the title of this rcport is intentionally non-committal. Diagnosed clinically as a lentigo, it was removed as
ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION* The description of the lesion in the title of this rcport is intentionally non-committal. Diagnosed clinically as a lentigo, it was removed as
ON THE PRESENCE OF A CILIATED COLUMNAR EPITHELIAL CELL TYPE WITHIN THE BOVINE CERVICAL MUCOSA 1
 ON THE PRESENCE OF A CILIATED COLUMNAR EPITHELIAL CELL TYPE WITHIN THE BOVINE CERVICAL MUCOSA 1 R. I. Wordinger, 2 J. B. Ramsey, I. F. Dickey and I. R. Hill, Jr. Clemson University, Clemson, South Carolina
ON THE PRESENCE OF A CILIATED COLUMNAR EPITHELIAL CELL TYPE WITHIN THE BOVINE CERVICAL MUCOSA 1 R. I. Wordinger, 2 J. B. Ramsey, I. F. Dickey and I. R. Hill, Jr. Clemson University, Clemson, South Carolina
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
Exam 2 spring 2016 Page 1
 xam 2 spring 2016 Page 1 Name: ate: 1 ll of the following are composed of eukaryotic cells XPT animals plants fungi bacteria 2 The function of the cytoplasm is to keep the cell contents wet provide a medium
xam 2 spring 2016 Page 1 Name: ate: 1 ll of the following are composed of eukaryotic cells XPT animals plants fungi bacteria 2 The function of the cytoplasm is to keep the cell contents wet provide a medium
Supporting Information
 Supporting Information Tanaka et al. 10.1073/pnas.1517466112 SI Materials and Methods Collection of Spermatogenic Cells from Azoospermic Men. We performed semen analysis at least twice for each man. When
Supporting Information Tanaka et al. 10.1073/pnas.1517466112 SI Materials and Methods Collection of Spermatogenic Cells from Azoospermic Men. We performed semen analysis at least twice for each man. When
Gametogenesis. To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis. Introduction
 Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
Gametogenesis To complete this worksheet, select: Module: Continuity Activity: Animations Title: Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell
FINE STRUCTURE OF SERTOLI CELLS AND MYOID CELLS IN MICE WITH TESTICULAR FEMINIZATION
 FERTILITY AND STERILITY Copyright 1974 The American Fertility Society Vol. 25, No.4, April 1974 Printed in U.S.A. FINE STRUCTURE OF SERTOLI CELLS AND MYOID CELLS IN MICE WITH TESTICULAR FEMINIZATION KYUNG
FERTILITY AND STERILITY Copyright 1974 The American Fertility Society Vol. 25, No.4, April 1974 Printed in U.S.A. FINE STRUCTURE OF SERTOLI CELLS AND MYOID CELLS IN MICE WITH TESTICULAR FEMINIZATION KYUNG
Understanding spermatogenesis is central to probing
 Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From
Journal of Andrology, Vol. 29, No. 5, September/October 2008 Copyright E American Society of Andrology The Cycle of the Seminiferous Epithelium in Humans: A Need to Revisit? Review RUPERT P. AMANN From
TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES*
 FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES* ARNOLD B. BARR, M.D. Department
FERTILITY AND STERILITY Copyright 1973 by The Williams & Wilkins Co. Vol. 24, No.5, May 1973 Printed in U.S.A. TIMING OF SPERMATOGENESIS IN FOUR NONHUMAN PRIMATE SPECIES* ARNOLD B. BARR, M.D. Department
Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men
 BIOLOGY OF REPRODUCTION 31, 779-784 (1984) Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men LARRY JOHNSON,2 CHARLES S.
BIOLOGY OF REPRODUCTION 31, 779-784 (1984) Germ Cell Degeneration During Postprophase of Meiosis and Serum Concentrations of Gonadotropins in Young Adult and Older Adult Men LARRY JOHNSON,2 CHARLES S.
BIOH122 Session 26 Gametogenesis. Introduction. 1. a. Define gametogenesis. b. What cells are gametes?
 BIOH122 Session 26 Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell division processes that occur during the cell cycle? d. Define the cell cycle.
BIOH122 Session 26 Gametogenesis Introduction 1. a. Define gametogenesis. b. What cells are gametes? c. What are the two cell division processes that occur during the cell cycle? d. Define the cell cycle.
Male Reproductive Physiology
 Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
Male Reproductive Physiology Overview Anatomy Function Endocrine and spermatogenesis Testis epididymus,vas deferens,seminal vesicles and prostate Hypothalamic pituitary testicular axis Hormones of the
HISTOLOGY OF THE MALE REPRODUCTIVE SYSTEM
 HISTOLOGY OF THE MALE REPRODUCTIVE SYSTEM Learning Objectives: 1. Describe the histology of and identify, in order, the passageways through which sperm pass as they exit from the body. 2. Describe the
HISTOLOGY OF THE MALE REPRODUCTIVE SYSTEM Learning Objectives: 1. Describe the histology of and identify, in order, the passageways through which sperm pass as they exit from the body. 2. Describe the
Exercise 6. Procedure
 Exercise 6 Procedure Growing of root tips Select a few medium-sized onion bulbs. Carefully remove the dry roots present. Grow root tips by placing the bulbs on glass tubes (of about 3 4 cm. diameter) filled
Exercise 6 Procedure Growing of root tips Select a few medium-sized onion bulbs. Carefully remove the dry roots present. Grow root tips by placing the bulbs on glass tubes (of about 3 4 cm. diameter) filled
Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses
 Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses using an anti-cre antibody; testes at 1 week (left panel),
Supplemental Figure 1. (A) The localization of Cre DNA recombinase in the testis of Cyp19a1-Cre mice was detected by immunohistchemical analyses using an anti-cre antibody; testes at 1 week (left panel),
MITOSIS IN DEVELOPING CARDIAC MUSCLE. FRANCIS J. MANASEK. From the Department of Anatomy, Harvard Medical School, Boston, Massachusetts 02115
 Published Online: 1 April, 1968 Supp Info: http://doi.org/10.1083/jcb.37.1.191 Downloaded from jcb.rupress.org on June 30, 2018 MITOSIS IN DEVELOPING CARDIAC MUSCLE FRANCIS J. MANASEK. From the Department
Published Online: 1 April, 1968 Supp Info: http://doi.org/10.1083/jcb.37.1.191 Downloaded from jcb.rupress.org on June 30, 2018 MITOSIS IN DEVELOPING CARDIAC MUSCLE FRANCIS J. MANASEK. From the Department
The Ultrastructure of Immature Sertoli Cells. Maturation-Like Changes During Culture and the Maintenance of Mitotic Potentiality
 BIOLOGY OF REPRODUCTION 18, 329-345 (1978) The Ultrastructure of Immature Sertoli Cells. Maturation-Like Changes During Culture and the Maintenance of Mitotic Potentiality ALBERTO J. SOLARI and IRVING
BIOLOGY OF REPRODUCTION 18, 329-345 (1978) The Ultrastructure of Immature Sertoli Cells. Maturation-Like Changes During Culture and the Maintenance of Mitotic Potentiality ALBERTO J. SOLARI and IRVING
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted
 Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Histological and Ultrastructural studies of Caecal tonsil in Chicken (Gallus domesticus)
 ISSN: 2319-7706 Volume 4 Number 6 (2015) pp. 63-68 http://www.ijcmas.com Original Research Article Histological and Ultrastructural studies of Caecal tonsil in Chicken (Gallus domesticus) T.A.Kannan 1*,
ISSN: 2319-7706 Volume 4 Number 6 (2015) pp. 63-68 http://www.ijcmas.com Original Research Article Histological and Ultrastructural studies of Caecal tonsil in Chicken (Gallus domesticus) T.A.Kannan 1*,
ENHANCEMENT OF THE GRANULATION OF ADRFNERGIC STORAGE VESICLES IN DRUG-FREE SOLUTION
 ENHANCEMENT OF THE GRANULATION OF ADRFNERGIC STORAGE VESICLES IN DRUG-FREE SOLUTION TAKASHI IWAYAMA and J. B. FURNESS. From the Department of Zoology, University of Melbourne, Victoria, Australia. Dr.
ENHANCEMENT OF THE GRANULATION OF ADRFNERGIC STORAGE VESICLES IN DRUG-FREE SOLUTION TAKASHI IWAYAMA and J. B. FURNESS. From the Department of Zoology, University of Melbourne, Victoria, Australia. Dr.
Male reproduction. Cross section of Human Testis ผศ.ดร.พญ.ส ว ฒณ ค ปต ว ฒ ภาคว ชาสร รว ทยา คณะแพทยศาสตร ศ ร ราชพยาบาล 1. Aims
 Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
Aims Male reproduction Male reproductive structure Spermatogenesis ส ว ฒณ ค ปต ว ฒ ห อง 216 โทร: 7578 Hypothalamo-pituitary-testicular axis Male sex hormone action Male reproductive structure Male reproductive
AN ELECTRON-MICROSCOPIC STUDY OF THE STARCH-CONTAINING PLASTIDS IN THE FERN TODEA BARBARA
 J. Cell Sci. 4, 211-221 (1969) 211 Printed in Great Britain AN ELECTRON-MICROSCOPIC STUDY OF THE STARCH-CONTAINING PLASTIDS IN THE FERN TODEA BARBARA H. M. SMITH* AND D. S. SMITHf Department of Biology,
J. Cell Sci. 4, 211-221 (1969) 211 Printed in Great Britain AN ELECTRON-MICROSCOPIC STUDY OF THE STARCH-CONTAINING PLASTIDS IN THE FERN TODEA BARBARA H. M. SMITH* AND D. S. SMITHf Department of Biology,
Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice
 CELL STRUCTURE AND FUNCTION 8, 339-346 (1984) C by Japan Society for Cell Biology Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice Ikuo Yamaoka, Sumie Katsuta and Yoshimi
CELL STRUCTURE AND FUNCTION 8, 339-346 (1984) C by Japan Society for Cell Biology Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice Ikuo Yamaoka, Sumie Katsuta and Yoshimi
Spermatogenesis Following Experimental Testicular Ischemia
 Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
Spermatogenesis Following Experimental Testicular Ischemia Frank Hinman, Jr, MD, and Gilbert I Smith, MD REGENERATION of the spermatogenic elements of the testis after depression by testosterone and by
A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL CRYPTORCHIDISM: A PRELIMINARY REPORT*
 FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
FERTILITY AND STERILITY Copyright 1970 by the Williams & Wilkins Co. Vol. 21, No. 11, November 1970 Printed in U.S.A. A COMPARATIVE STUDY OF GERM CELL KINETICS IN THE TESTES OF CHILDREN WITH UNILATERAL
MORPHOLOGIC CHARACTERISTICS OF THE CHEMICALLY INDUCED ACROSOME REACTION IN HUMAN SPERMATOZOA*
 FERTILITY AND STERILITY Copyright 1979 The American Fertility Society Vol. 32, No.1, July 1979 Printed in U.SA. MORPHOLOGIC CHARACTERISTICS OF THE CHEMICALLY INDUCED ACROSOME REACTION IN HUMAN SPERMATOZOA*
FERTILITY AND STERILITY Copyright 1979 The American Fertility Society Vol. 32, No.1, July 1979 Printed in U.SA. MORPHOLOGIC CHARACTERISTICS OF THE CHEMICALLY INDUCED ACROSOME REACTION IN HUMAN SPERMATOZOA*
