Duct Acidification Effects of Potassium, HCO3. Harry R. Jacobson. Internal Medicine, 5323 Harry Hines Blvd., Dallas, Texas 75235
|
|
|
- Aileen Park
- 5 years ago
- Views:
Transcription
1 Medullary Collecting Duct Acidification Effects of Potassium, HCO3 Concentration, and pco2 Harry R. Jacobson University of Texas Health Science Center, Department of Internal Medicine, 5323 Harry Hines Blvd., Dallas, Texas Abstract. The medullary collecting duct (MCD) from renal outer medulla possesses significant HCO3 absorptive capacity. In vitro microperfusion studies have shown that HCO3 absorption in this segment is carbonic anhydrase dependent, affected by peritubular and luminal chloride concentrations, is independent of the presence of Na or the presence of Na transport, and is stimulated by mineralocorticoid hormone. The present in vitro microperfusion studies defined regulatory influences on MCD acidification as assessed by acute changes in the extracellular K and HCO3 concentrations and pco2. These studies showed that acute changes in the peritubular K concentration to either 0 mm K or 50 mm K have no significant effect on HCO3 absorption in MCD. Intracellular voltage recordings showed that elevation of peritubular K concentration from 5 to 50 mm produced only a 2.8 mv depolarization of the basolateral cell membrane of MCD cells. In addition, acute reduction of peritubular K from 5 to 0 mm had no significant effect on intracellular voltage. Studies that were designed to assess the effects of HCO3 concentration and pco2 on acidification showed that acute reduction of peritubular HCO3 concentration from 25 to 5 mm (ph change from 7.4 to 6.8) increased lumen-positive voltage from 30.2±3.8 to 40.0±4.4 mv, and simultaneously increased net HCO3 absorption from 15.6±1.9 to 22.9±2.9 pmol* mm- *min-'. Elevation of peritubular HCO3 concentration from 25 to 50 mm (ph change from 7.4 to 7.8) significantly decreased lumenpositive voltage from 33.8±2.4 to 26.7± 1.5 mv and Dr. Jacobson is the recipient of National Institutes of Health Research Career Development Award 1-KO1-AM Received for publication 23 January 1984 and in revised form 22 May J. Clin. Invest. The American Society for Clinical Investigation, Inc /84/12/2107/08 $ 1.00 Volume 74, December 1984, simultaneously decreased net HCO3 absorption from 17.9±1.2 to 12.8±1.3 pmol *mm-' *min-'. In addition, acute reduction of peritubular pco2 from 40 to <14 mmhg (final ph ) significantly decreased lumenpositive voltage from 31±4.4 to 15.7±1.0 mv. Coincidentally, HCO3 absorption decreased significantly from 11.0±3.7 to 5.3±0.7 pmol * mm' * min-'. We conclude that: (a) alteration of peritubular K concentration from 0 to 50 mm in vitro does not affect HCO3 absorption in the MCD, and that this lack of effect appears to be related to a low basolateral cell membrane K conductance; (b) net HCO3 absorption and the associated lumen-positive voltage can be modulated by in vitro changes in peritubular HCO3 and pco2 (or ph); and (c) the MCD perfused in vitro appears to be a good model for studying the mechanisms and regulation of distal nephron acidification. Introduction We, and more recently others, have demonstrated via in vitro microperfusion studies that the medullary collecting duct (MCD)' from renal outer medulla possesses significant HCO3 absorptive capacity (1-4). Indeed, when the rabbit MCD from inner stripe of outer medulla is perfused and bathed in vitro with symmetrical Ringer's HCO3 solutions, transepithelial voltage is always oriented lumen positive and net HCO3 absorption occurs at a rate of pmol mm' min-' (1-4). Recent studies using in vitro microperfusion of rat MCD demonstrate virtually identical features, except that the rate of acidification is even greater with net HCO3 absorptive rates of pmol* mm-' * min-' (5). Certain characteristics of the MCD acidification process have been elucidated. First, proton secretion (HCO3 absorption) occurs against an electrical and chemical gradient and thus appears to be active (1). Second, the net acidification process as well as the lumen-positive transepithelial voltage is obliterated by exposure of the in vitro 1. Abbreviations used in this paper. CCT, cortical collecting tubule(s); MCD, medullary collecting duct(s); Jv, net volume flux; VT, transepithelial voltage Regulation ofacidification in Medullary Collecting Duct
2 perfused MCD to carbonic anhydrase inhibitors (1). Third, there appears to be an important role for the anion chloride in net acidification by the MCD, since total removal of peritubular chloride results in ablation of net HCO3 absorption and removal of luminal chloride results in augmentation of net HCO3 absorption (2). Fourth, proton secretion in this nephron segment is molecularly independent of sodium, in that it is not affected by peritubular ouabain, luminal amiloride, or total replacement of luminal and peritubular sodium (3, 4). Finally, proton secretion appears to be under control, in part, by adrenal corticosteroid hormones, since adrenalectomy results in significant inhibition and DOCA administration results in a significant increase in net HCO3 absorption, and since in vitro addition of aldosterone to rabbit MCD harvested from adrenalectomized animals results in significant stimulation of net HCO3 absorption. In summary, then, we have a distal nephron segment with significant proton secretory capacity that is carbonic anhydrase-dependent, affected by peritubular and luminal chloride concentrations, is independent of the presence of sodium or the presence of sodium transport, and is stimulated by mineralocorticoid hormone. Other than the mineralocorticoid dependence of proton secretion in the MCD (3) we know very little about regulation of proton secretion in this nephron segment. Specifically, there is no information available on the effects of extracellular K concentration, as well as extracellular ph, pco2, and HCO3 concentration on the acidification process in the MCD. Because in both the rabbit and the rat this appears to be the distal nephron segment with the highest capacity for proton secretion, it is important to define those factors that regulate the acidification process. The studies presented here represent initial attempts to define regulatory influences on MCD acidification by acute changes in the extracellular environment in vitro. They demonstrate that acidification in this segment is unaffected by alteration of peritubular K concentration from 0 to 50 mm. In addition, these studies show that net acidification in this nephron segment is sensitive to the peritubular HCO3 concentration and pco2 (or ph). Methods Outer MCD segments were dissected and perfused in vitro by techniques previously described (6). Briefly, female New Zealand white rabbits that weighed kg were killed by decapitation. One kidney was removed and a 1-mm coronal slice was placed in chilled bath medium at ph 7.4. Segments were dissected free-hand with the aid of a dissecting microscope and sharpened forceps. The freed segments were transferred to a thermostatically controlled lucite perfusion chamber and perfused between concentric glass pipettes at 380±1 C. Several solutions were used as perfusate and bath in these studies. The major ionic composition of the various solutions are outlined in Table I. The control perfusate was an artificial plasma ultrafiltrate with the following composition in millimolar NaCI 105; KGI 5; NaHCO3 25; Na acetate 10; NaHPO4 2.5; CaCl 1.8; MgSO4 1; glucose 8.3; alanine 5. The control bath was identical to the perfusate except for the addition of 5% by volume fetal calf serum. The bath was exchanged at a rate of 0.6 ml/min. Unless stated otherwise, all solutions were bubbled with 95% 02/5% CO2 gas mixture to the point of equilibration at 370C and drawn anaerobically into plastic syringes and refrigerated until use. Previous studies using identical methods have documented that solutions handled in such a manner, irrespective of the [HCO3J or pco2, result in bath ph that remains constant and at the desired level (7). Strict attention was given to the origin of all segments studied. Outer MCD segments were dissected in such a manner that the proximal portion originated at the junction of the outer and inner stripe of the outer medulla, and the distal portion ended at the junction of the inner stripe of outer medulla with the inner medulla. After initiation of perfusion, tubules were allowed to equilibrate for 60 min at 380C in the control bath. Transepithelial voltage (VT) was monitored throughout all experiments. Net volume absorption was determined by the use of exhaustively dialyzed 3H-inulin as volume marker. Net volume flux (Jv) was determined from the changes in the 3H-inulin activity between perfused and collected fluid via equations previously described (1). Net total CO2 flux (JHCO3) was calculated from the total CO2 content of perfused and collected fluid measured by microcalorimetry (8) and calculated by the following equation: JHCO3 = [VA(CI - Co)]/[L], where VI is the perfusion rate, Cl is the perfusate total C02, CO is the collected fluid total C02, and L is the tubule length. The equation assumes a negligible change in HCO3 concentration due to water movement, which was substantiated by measuring Jv and discarding experiments in which the Jv - ±0.05 nl. mm- I min-'. Total CO2 measurements were made by collecting into 3060-nl pipettes that were then used to inject samples into the phosphoric acid chamber of the picapnotherm. Measurements were made immediately after collection, and reproducibility was ±2.5% when a single sample was measured multiple times. Note that in all studies reported, tubule length ranged from 1.0 to 1.8 mm and perfusion rates ranged between 1.8 and 3.0 nl.min-'. Thus, in all reports of significant difference between control and experimental periods, the change in collected fluid total CO2 was over 10%, which was a difference that was easily detectable by the methods used. In some experiments, net Cl transport was determined by measurement of Cl concentration in perfused and collected fluid utilizing the electrometric titration technique (9). Chloride flux was calculated in a manner identical to that of net HCO3 flux, which was described above. In some tubules, both transepithelial voltage and intracellular voltage were monitored simultaneously. Intracellular voltage was measured by puncturing individual cells with 1 M KCG-filled Ling electrodes using methods identical to those published previously (10). Ling electrodes were pulled with an ultrafine micropipette puller (Frederick Haer & Co., Brunswick, ME). Electrode resistances were measured to be between 100 and 200 megaohms. All intracellular voltages were referenced to the bath and were measured using a high input impedance electrometer (FD223, W-P Instruments, Inc., New Haven, CT). Tubular cells were impaled by use of a hydraulic drive micromanipulator (David Kopf Instruments, model 1207B, Tujunga, CA). Stable intracellular voltage recordings were obtained and maintained for as long as 90 min. Criteria for adequate impalements were the same as those described as well as graphically presented previously ( 1). Briefly, four types of recordings were obtained. The first type, an initial rapid negative deflection followed by a steady decay in voltage over 1-3 min, was seen intermittently but no more than 20% of the time. Such a tracing was uniformly associated with visible evidence of swelling of the impaled cell, and all such punctures were ignored. The second type of puncture consisted of an initial negative deflection with 2108 H. R. Jacobson
3 a steady increase in the negativity to a stable value after a few minutes. Such punctures were considered acceptable and constituted about onethird of the successful impalements. The third type of tracing consisted of an initial negative deflection, with an almost immediate fall in the magnitude of this negative voltage followed by a gradual 1-3-min increase in intracellular negativity, to a plateau level more negative than the initial spike. Such punctures constituted about two-thirds of the successful impalements. Finally, the fourth type of tracing was identical to the second and third form described above, but the plateau voltage was maintained for too short a time to complete the appropriate protocols, i.e., stable voltage for <10 min. This short-lived but technically successful impalement occurred with almost half of all impalement attempts. Data obtained with successful impalements was only acceptable if the recorded voltage, upon removal of the microelectrode from the cell, was < ±5 mv different from the prepuncture voltage. (This occurred in all but one impalement.) The frequency of successful impalements using such methods and criteria are a function mostly of experience. In MCD cells, the success rate is roughly 20-25%, while in our hands the success rate in cortical collecting tubules is closer to 50%, and that in proximal tubules is even higher (these success rates refer to percentage of tubules studied). Several groups of studies were performed. Group I studies examined the effects of alterations in peritubular K concentration on net HCO3 absorption, transepithelial voltage, and intracellular voltage. After control measurements were made, the bath was changed to an identical one except for alteration in K concentration. Bath K concentration changes were of two types. In some tubules the bath K concentration was reduced to 0 by replacing 5 mm of KCI with 5 mm NaCl. In these experiments, fetal calf serum was omitted from the bath to ensure that [K] was unmeasurable by standard flame photometry. In other tubules, bath K concentration was increased to 50 mm by replacing 45 mm of bath NaCl with KCl (see Table I). An additional group of tubules was studied with simultaneous changes in both luminal and peritubular K concentration. In these experiments, control perfusate and bath were replaced by a high K low Cl solution. The K concentration in perfusate and bath was elevated to 75 mm by replacement of 70 mm NaCl with 65 mm K gluconate and 5 mm KCl (see Table I). In these experiments, both net HCO3 absorption and net Cl transport were measured. The second major group of studies investigated the effects of alteration in peritubular HCO3 concentration and pco2 on VT and net HCO3 absorption. After control measurements were obtained, bath solution was changed to one that contained either 5 mm HCO3 (acute metabolic acidosis) or one that contained 50 mm HCO3 (acute metabolic alkalosis). When HCO3 concentration was reduced to 5 mm Table I. Solutions* Used in Studies on Rabbit MCD Zero High High K/ Low High Normal K K Low Cl HCO3 HCO3 Na K Cl HCO Gluconate pht * In mm. t ph determined at 37 C after equilibration with 95% 02/5% CO2- it was done by replacing 20 mm NaHCO3 with 20 mm NaCG, and when bath HCO3 was elevated it was done by replacing 25 mm NaCG with 25 mm NaHCO3 (Table I). Both solutions were equilibrated with 95% 02/5% CO2, so that the final phs were 6.8 and 7.7, respectively. In a final group of tubules, after control collections were obtained, the response to low ambient pco2 was determined. Bath was changed to one that was identical to control, except that the experimental bath was equilibrated with 98% 02/2% CO2. The pco2 and the ph of this hypocapneic bath were mmhg and , respectively. In all experiments where perfusate and bath were asymmetrical with respect to ionic composition, the observed VT and, when applicable, intracellular voltages were corrected for calculated liquid junction potentials as previously described (12). Statistical analysis was performed using the paired t test, and P 0.05 was considered to indicate a significant difference. Results Effects of acute changes in peritubular K concentration (Table II). In six tubules, transepithelial voltage and net HCO3 absorption were measured during control circumstances (bath K concentration 5 mm) and after an increase in bath K concentration to 50 mm. Control VT was 20.6±3.7 mv lumen positive. After an increase in bath K concentration to 50 mm, there was a slight but significant (P < 0.05) increase in lumenpositive voltage to 24.3±3.5 mv. Presumably, this slight increase in lumen-positive voltage is secondary to paracellular diffusion of K, since it has been previously demonstrated that the MCD of the rabbit has a higher permeability to K than Na. In contrast to the effect on VT, elevation of peritubular K concentration to 50 mm had no significance on net HCO3 absorption in these tubules. Net HCO3 absorption during control circumstances was 12.2±1.86 pmol * mm-' min', and was unchanged at 12.2±2.0 pmol* mm-' * min' when bath K concentration was 50 mm. Just as with elevation of peritubular K, acute removal of peritubular K had no effect on MCD transport. Net HCO3 absorption during control conditions was 16.0±1.46 pmol. mm-'.min-', and was unchanged when bath K was completely removed at 14.9±1.70 pmol mm-'.min-'. Similarly, there was no significant effect on transepithelial voltage, with control VT being 27.4±2.6 mv and VT with 0 K bath being 27.9±2.7 mv. In our previous studies, we observed that when MCD were perfused in vitro with symmetrical Ringer's HCO3 solutions, net HCO3 absorption was matched virtually 1:1 by net Cl secretion. The route of this net C1 secretion is unknown, but the high lumen-positive voltage could facilitate paracellular movement of Cl from bath to lumen, assuming that the paracellular shunt pathway C1 permeability is sufficiently high. Similarly, the high lumen-positive voltage would be expected to provide a significant driving force for net movement of K as well as Na (in the absorptive direction). However, previous studies (13) have demonstrated that the rabbit MCD, when perfused in vitro with symmetrical Ringer's HCO3 solutions, does not transport significant amounts of Na or K. Thus, the 2109 Regulation ofacidification in Medullary Collecting Duct
4 Table II. Effects of Bath [K], [HCO3], and pco2 on Net HCO3 Absorption and Voltage in MCD VT in mv JHCO3 in pmol * mm-'* min-' Protocol N Control Experimental Control Experimental Bath [K] (50 mm) ± ±3.5* 12.2± ±2.0 Bath [K] (0 mm) ± ± ± ±1.7 Bath [HCO3J (5 mm) ± ±4.4$ 15.6± ±2.9* Bath [HCO3J (50 mm) ± ±1.5* 17.9± ±1.3* Bath pco2 (10-14 mmhg) ± ±1.Ot 11.0± ±0.6t * P < 0.05, control vs. experimental. t P < 0.01, control vs. experimental. JHCO3, net total CO2 flux. finding of equal amounts of HCO3 absorption and Cl secretion in our previous studies raises the possibility that there is some more direct linkage between proton secretion and Cl secretion than just electrogenic proton secretion shunted by paracellular Cl movement. To address this issue, we perfused and bathed a group of five MCD with solutions designed to favor shunting of the electrogenic proton secretion by K rather than C1. Tubules were perfused and bathed with the high K, low Ca solution (see Table I). Net HCO3 absorption and net Ca secretion were measured in these tubules. The results of these studies are presented graphically in Fig. 1, where net Cl secretion in pmol *mm' * min-' is plotted against net HCO3 absorption in the same units. For comparison, included in Fig. 1 are our previous data in MCD perfused and bathed with symmetrical Ringer's HCO3 solutions. All paired collections for Ca and HCO3 flux in tubules perfused and bathed with high K, low Cl solution demonstrated significantly greater HCO3 absorption than Ca secretion. In no circumstance were the Ca and HCO3 fluxes equal. These experiments demonstrated JHCO3 pmol * mmr1- min1 Figure 1. Net Cl secretion plotted against net HCO3 absorption (both in pmol -mm' * min-') in tubules perfused and bathed with high K, low Cl solutions is illustrated by the open circles. In all experiments, net HCO3 absorption exceeded net Cl secretion. For comparison is illustrated the relationship between Cl secretion and HCO3 absorption when tubules are perfused and bathed with Ringer's HCO3 solutions containing 5 mm K and 115 mm Cl (closed circles). See text for discussion. that net HCO3 absorption in MCD is not obligatorily coupled to Ca secretion. Although K fluxes were not measured in these experiments, presumably the difference between HCO3 absorption and Cl secretion was represented mostly by net K absorption. In comparing the net HCO3 absorptive rates in the present studies with those of our previous studies (2), there was a tendency for net HCO3 flux to be slightly lower when MCD are perfused with the high K, low Cl solution. Since these experiments are unpaired, it is premature to make any conclusions from this data. However, should these differences prove to be real, they may be secondary to partial reduction in proton secretion by the lower peritubular Cl concentration used in these experiments. Irrespective of the magnitude of the net HCO3 absorption in these studies, and whether or not there is a significant difference between the rates of HCO3 absorption in these experiments as compared with our previous experiments using Ringer's HCO3 solutions as bath and perfusate, the purpose of this group of experiments was to determine whether the relative rates of HCO3 absorption and Cl secretion can differ in MCD. Intracellular voltage recordings. Since elevation of bath [K] to 50 mm and reduction of bath [K] to 0 mm produced no major effects on acidification in MCD, we were interested in determining the response of intracellular voltage to these two maneuvers. We thus performed individual cell punctures of isolated perfused MCD. In these experiments, we measured the control intracellular voltage with reference to the bath and also measured the response of intracellular voltage to elevation of peritubular K concentration from 5 to 50 mm and to reduction of peritubular K concentration from 5 to 0 mm. In 14 punctures of 9 tubules, control intracellular voltage averaged -36.1±2.84 mv with respect to the bath. In these same tubules, transepithelial voltage was +29.0±1.3. Increasing the bath K concentration from 5 to 50 mm resulted in a small but significant (P < 0.01) depolarization of the basolateral cell membrane to -33.3±2.91 mv. This small depolarization is in contrast to the magnitude of basolateral cell membrane voltage depolarization that was observed by others and ourselves in proximal tubules and cortical collecting tubules (CCT) (10, 11, 14). Indeed, this very small depolarization in response to a log increase in peritubular K concentrations suggests that 2110 H. R. Jacobson
5 the basolateral cell membrane of the MCD has a relatively low K conductance. Removal of K from the bath (11 punctures, 6 tubules) produced no significant change in basolateral cell membrane or transepithelial voltage, with the former being -40.1±1.79 mv under control conditions and -40.5±1.83 mv at zero K bath, and the latter being +30.3±1.37 and 31.0±1.34 at 5 and 0 mm bath K, respectively. If we can assume that removal of bath K inhibits Na-K-ATPase, this observation is compatible with the recent demonstration that ouabain in the peritubular fluid does not affect HCO3 transport in the MCD (4). Effects of peritubular HCO3 concentration and pco2 on MCD (table II). In six MCD the effects of acute reduction of peritubular HCO3 concentration from 25 to 5 mm (ph ) was determined. Reduction of bath HCO3 concentration resulted in a significant increase in lumen-positive voltage from 30.2±3.8 to 40.0±4.4 mv, P < Simultaneously, net HCO3 absorption increased significantly from 15.6±1.9 to 22.9±2.9 pmol * mm-' * min-', P < In two of these tubules, the peritubular HCO3 replacement was not with C1 but with gluconate. Thus, peritubular C1 concentration remained the same as during control conditions. Results of these two tubules were the same as the remaining four and the data for all six tubules was pooled. In another group of six tubules, the effects of an acute increase in peritubular HCO3 concentration from 25 mm to 50 mm (ph ) were determined. Elevation of peritubular HCO3 concentration produced a significant decrease in lumenpositive voltage from 33.8±2.4 to 26.7±1.5 (P < 0.05). Simultaneously, net HCO3 absorption decreased from 17.9±1.1 to 12.8±1.3 pmol * mm-' * min-' (P < 0.01). The final group of experiments determined the effect of acute reduction in peritubular pco2 on acidification in six MCD. In these experiments, after control collections were made at a pco2 of 40 mmhg, the bath was changed to one that had been equilibrated to 2% CO2. The measured pco2 in all experiments was always between 10 and 14 mmhg, and bath ph ranged between 7.80 and This acute reduction in peritubular pco2 and increase in ph produced a major decrease in a lumen-positive voltage from 31±4.4 to 15.7±1.0. Coincident with this reduction in lumen-positive voltage was a major decrease in net HCO3 absorption from 11.0±3.7 to 5.3±0.6 pmol mm-' * min-'. - Discussion The medullary collecting duct from the inner stripe of outer medulla has been identified as a major capacity segment for distal nephron hydrogen ion secretion. This was initially demonstrated in in vitro studies of rabbit tubules (1-4) and has subsequently been documented by similar studies on rat tubules that were perfused in vitro (5). Note that in all of these in vitro microperfusion studies, H+ secretion was assessed by net HCO3 absorption, since the studies used 25 mm HCO3 in perfusion solutions. While 25 mm HCO3 may not be the usual concentration for this buffer in luminal fluid at the level of the MCD in rabbit or rat, use of such a HCO3 concentration allows for easy measurement of the proton secretory capacity of this segment. Because of the potential importance of the MCD to net distal nephron acidification, it is important to determine which factors modulate proton secretion in this segment. As mentioned previously, we have provided evidence that hydrogen ion secretion in this segment is responsive to mineralocorticoid hormones (3). In addition, we have demonstrated that acidification can occur independent of the Na ion and is dependent upon the presence of peritubular Cl (2, 3). However, there are several possible modulators of acidification in this segment that have not been examined. The present studies were designed to elucidate some of the regulatory mechanisms. By virtue of its anatomic location in the kidney, the MCD is expected to be exposed to major changes in the peritubular K concentration that may reflect not only changes in K balance but also changes in the hydration status. For this reason, we tested the effects of acute alterations in peritubular K concentration on acidification in this segment. Our studies clearly demonstrate that changes in the K concentration from 0 to 50 mm have no significant effect on net HCO3 absorption by the MCD. It should be noted that these studies do not address the question of whether chronic changes in K balance affect MCD acidification. Indeed, such studies will require studying MCD harvested from animals who have been maintained on low and high K-containing diets. Because we failed to find a significant effect of acute alterations in peritubular K concentration on acidification in this segment, we further investigated the effects of these K concentration changes on intracellular voltage. There were several reasons for performing these electrophysiologic measurements. Based on our earlier studies (2), we believe that the MCD cell is characterized, in part, by an apical cell membrane electrogenic proton secretory mechanism coupled with a basolateral cell membrane neutral Cl-HCO3 exchanger that allows for base exit from cell to peritubular fluid. The luminal cell membrane electrogenic proton pump should be affected by the electrical potential across the apical cell membrane, while the basolateral cell membrane Cl-HCO3 exchanger should be insensitive to the voltage across the basolateral cell membrane but sensitive to the relative concentrations of Cl and HCO3 across this membrane. Since elevation of peritubular K concentration to 50 mm in proximal convoluted and proximal straight tubule cells as well as CCT cells results in major depolarization of intracellular voltage (10, 11, 14), we would expect to observe an effect on net HCO3 absorption if the basolateral cell membrane of the MCD cell also possessed high K conductance. Thus, if elevation of the peritubular K concentration to 50 mm significantly depolarizes the cell, one would expect an increase in proton secretion due to improvement of the electrical gradient against which the proton pump 2111 Regulation ofacidification in Medullary Collecting Duct
6 must work. We did not see such an increase in proton secretion (HCO3 absorption). Thus, we tested the effect of a log increase in the peritubular K concentration on intracellular voltage. Indeed, our studies clearly show that this maneuver results in minimal depolarization of the basolateral cell membrane voltage. Recently presented studies by Koeppen (15) are in agreement. Our studies also show that reduction of peritubular K to 0 mm does not affect transepithelial voltage, net HCO3 absorption, or intracellular voltage. We have previously demonstrated that acidification can persist unchanged in the MCD when all luminal and peritubular fluid Na is removed (3). In addition, Laski and Kurtzman (4) have recently demonstrated that ouabain added to the peritubular side of the rabbit MCD perfused in vitro has no significant effect on transepithelial voltage nor on net acidification. Our results with zero K bath are compatible with the observation that acidification in MCD is not affected by inhibition of basolateral membrane Na-K- ATPase. A second purpose of our studies was to evaluate the stoichiometry of net HCO3 absorption and net Cl secretion. As mentioned previously, when MCD of rabbit are perfused in vitro with symmetrical Ringer's HCO3 solutions, net HCO3 absorption is matched by net Cl secretion. From the magnitude of the transepithelial voltage and the previous observations by Stokes (13) demonstrating significant Na and K permeabilities in this segment, one would expect that HCO3 absorption might, in part, be matched by voltage-driven passive movements of Na or K. Because of the demonstration by Stokes (13) that the MCD is more permeable to K, and because our previous studies (2) examined the MCD with solutions in which the Cl concentration was >20-fold that of the K concentration, we wanted to perform experiments in which the tubules were perfused and bathed in solutions that would allow significant voltage-driven net K transport. It is clear from our experiments using the high K, low C1 perfusate and bath that net HCO3 absorption and net Cl secretion are not obligatorily coupled 1:1. Since it is not the purpose of these studies to define physiologic relevance of voltage-driven K transport in this segment, but rather to determine whether proton secretion (HCO3 absorption) and Cl secretion are tightly linked, we do not want to speculate on the physiologic role of the MCD with respect to K transport. Since HCO3 absorption in our studies always exceeded net Cl secretion, it is clear that bulk electroneutrality was maintained by the movement of a cation from lumen to bath. Which cation moved and the route of its movement are not important for the present studies. In comparing the data from the present studies and those reported by us earlier, it is important to point out that no contradictions exist. When MCD are perfused and bathed with Ringer's HCO3, the equality of net HCO3 absorption and net Cl secretion is compatible not only with transcellular Cl movement, which is somehow coupled to proton secretion, but also with paracellular movement of Cl which is driven by the lumen-positive voltage. Such paracellular C1 movement, since it appears to account for the entire bulk electroneutrality of proton secretion, would presumably occur through an anion selective paracellular pathway. Using electrophysiologic techniques we have demonstrated that, in the MCD, passive C1 permeability exceeds passive Na permeability (2). However, the difference between these two ionic permeabilities is not sufficient to account for the totally preferential C1 movement. Recent demonstration that the basolateral cell membrane of MCD cells is highly C1 conductive (15) makes our earlier electrophysiologic measurements of passive C1 permeability unreliable. Isotopic permeability coefficients, utilizing 3Cl and excluding significant exchange diffusion of Cl, will be necessary. The present studies cannot be used to quantitatively or qualitatively address the route(s) of transepithelial Cl movement. Rather, they help to clarify our earlier observations, in that they exclude 1:1 coupled, i.e., HCl secretion. Some or all of the Cl may be secreted via transcellular or paracellular routes. Determination of apical cell membrane C1 conductance and the electrochemical gradient for Cl across the apical cell membrane will be helpful in addressing this issue further. In our first studies on the rabbit MCD perfused in vitro, we tested whether manipulation of the acid base status of the rabbit from which the MCD was harvested had any significant effect on expression of in vitro acidification (1). The rationale for such studies stemmed from the observations by McKinney and Burg (16, 17) in which the in vitro CCT of the rabbit either reabsorbed or secreted HCO3, depending upon the acid base status of the animal from which the CCT was harvested. In our earlier studies, we were unable to find an effect of either ammonium chloride or NaHCO3 pretreatment of the rabbits on MCD acidification as studied in vitro (1). At that time, we were concerned that this observation might in part be explained by blunting of in vitro expression of in vivo acidification rates, due to the fact that all in vitro studies were performed at normal ph. In other words, we believed it was possible that the MCD in vivo responded appropriately to acid base stimuli, but that this in vivo response was rapidly lost in vitro when tubules were exposed to a peritubular HCO3 concentration of 25 mm and a pco2 of 40 mmhg with a ph of 7.4. For this reason we tested the effects of in vitro changes in acid base status on acidification by the MCD. When peritubular fluid was acutely changed to one simulating acute metabolic acidosis, i.e., 5 mm as opposed to 25 mm peritubular HCO3 concentration, net acidification was significantly increased, as was lumen-positive transepithelial voltage. In contrast, when peritubular fluid was acutely changed to one which simulated acute metabolic alkalosis with a peritubular HCO3 concentration of 50 mm and a peritubular PCO2 of 40, net HCO3 absorption was significantly suppressed, as was lumen-positive transepithelial voltage. In both these groups of experiments it is impossible to determine whether the effect observed was due to a change in the concentration of HCO3 or a change in ph. Since our earlier studies are 2112 H. R. Jacobson
7 compatible with a basolateral cell membrane Cl-HCO3 exchanger (2), it is possible that the major mechanism behind our observations is the effect of the chemical concentration of peritubular HCO3 on the Cl-HCO3 exchanger. While isohydric changes in the peritubular HCO3 concentration (i.e., with appropriate changes in pco2 to maintain ph constant) would help to address this question, it is clear that a complete understanding of the mechanism behind our observations will require definition of the electrical and chemical driving forces for proton and base movement across both basolateral and apical cell membranes. This can only be accomplished by additional studies examining the effects ofchanges in peritubular HCO3 concentration, both isohydric and nonisohydric, on intracellular voltage and ph. The importance of the present studies is just the documentation that rates of acidification in the MCD can be altered by changes in peritubular HCO3 concentration or ph. Further support for regulation of acidification by the MCD when studied in vitro comes from recent studies by Laski and Kurtzman (4). These investigators demonstrated a significant correlation between net acidification rates and in vivo urine ph of the rabbit from which the MCD was harvested. As opposed to our studies in the rabbit and studies by Atkins and Burg (5) in the rat, the recent studies by Laski and Kurtzman (4) used a different animal model (starvation as opposed to ammonium chloride loading). As an additional attempt to demonstrate regulation of MCD acidification in vitro, we examined the effects of a reduction in peritubular pco2. When pco2 is reduced to a level between 10 and 14 mmhg and ph as a result rises to - 7.8, there is a drastic reduction in net acidification as well as the lumen-positive voltage in MCD. We did not test lesser decreases in pco2, nor did we examine the effects of an elevated PCO2 on acidification in this segment. Such studies will be required to determine the physiologic significance of acute changes in pco2 on distal nephron acidification. However, the importance of our studies using this major decrease in PCO2 relates to the additional demonstration that acidification in this segment is responsive to acid base changes. The mechanism behind the reduction in acidification observed in the present studies is unclear. It is possible that the reduction of PCO2 to the range significantly reduces substrate for buffering of the intracellular hydroxyl generated behind the proton pump, or that the effect is secondary to an intracellular ph-induced change on proton secretion at the apical cell membrane (i.e., production of a less favorable chemical gradient for proton secretion due to alkalinization of the cell). Note that the effects of pco2 and possibly- also the effects of peritubular HCO3 concentration changes mentioned above may also be expressed via alterations in the number of proton pumps at the apical cell membrane. Recent studies have suggested that acidification by urinary bladder epithelial cells may in part be regulated by recycling of proton pumps from the apical cell membrane to storage vesicles in the cell cytoplasm and vice versa (18). Thus, appropriate stimuli to increased acidification result in migration of these preformed pumps to the apical cell membrane with resultant fusion. In converse, circumstances which would inhibit acidification might result in endocytosis of apical cell membrane proton pumps. Such possibilities remain to be tested in the rabbit MCD. It should be emphasized that the purpose of the present studies was to investigate if net acidification by MCD can be regulated by changes in peritubular [K] as well as peritubular acid base conditions. While significant changes in net acidification were observed with alteration of peritubular [HCO31 and pco2, the present studies cannot define the mechanism(s) responsible for these changes. Nor can the present studies determine ifthe predominant factor regulating net acidification by this nephron segment is peritubular ph or peritubular [HCO3] or even intracellular ph. These questions can best be addressed in future studies using isohydric changes in peritubular [HCO3] and pco2, as well as appropriate measurements of intracellular voltage and, if possible, intracellular ph during manipulation of net acidification. In summary, rabbit MCD were studied in vitro to examine the influence of acute changes in peritubular K concentration as well as acute changes in peritubular acid base status on acidification and transepithelial voltage in this nephron segment. In addition, studies were conducted to examine intracellular voltage and the effects of peritubular [K] on intracellular voltage. These studies demonstrate that (a) alteration of peritubular K from 0 to 50 mm in vitro does not affect net HCO3 absorption in the MCD; (b) MCD cells, in contrast to proximal tubule and CCT cells, possess a low basolateral cell membrane K conductance; (c) net HCO3 absorption and Cl secretion are not obligatorily coupled; and (d) net HCO3 absorption and the associated lumen-positive voltage, which is thought to be due to electrogenic proton secretion, can be modulated acutely by in vitro changes in peritubular HCO3 concentration and pco2. Thus, the MCD perfused in vitro appears to be a good model for studying the regulation of distal nephron acidification. Acknowledgments The author acknowledges the skillful technical assistance of Ms. Susan Corona and Ms. Amy Womack. This work was supported in part by National Institutes of Health research grants 2-ROI-AM and 3-RO-AM S1. References 1. Lombard, W. E., J. P. Kokko, and H. R. Jacobson Bicarbonate transport in cortical and outer medullary collecting tubules. Am. J. Physiol. 244:F289-F Stone, D. K., D. W. Seldin, J. P. Kokko, and H. R. Jacobson Anion dependence of rabbit medullary collecting duct acidification. J. Clin. Invest. 71: Stone, D. K., D. W. Seldin, J. P. Kokko, and H. R. Jacobson Mineralocorticoid modulation of rabbit medullary collecting 2113 Regulation ofacidification in Medullary Collecting Duct
8 duct acidification. A sodium-independent effect. J. Clin. Invest. 72: Laski, M. E., and N. A. Kurtzman Characterization of acidification in the cortical and medullary collecting tubule of the rabbit. J. Clin. Invest. 72: Atkins, J. L., and M. B. Burg Secretion and absorption of bicarbonate by rat collecting ducts. Clin. Res. 31 :423A. 6. Burg, M., J. Grantham, M. Abramow, and J. Orloff Preparation and study of fragments of single rabbit nephrons. Am. J. Physiol. 210: Jacobson, H. R Effects of CO2 and acetazolamide on bicarbonate and fluid transport in rabbit proximal tubule. Am. J. Physiol. 240:F54-F Vurek, G. G., D. G. Warnock, and R. Corsey Measurement of picomole amounts of carbon dioxide by calorimetry. Anal. Chem. 47: Ramsay, J. A., R. H. Brown, and P. C. Croghan Electrometric titration of chloride in small volumes. J. Exp. Biol. 32: Biagi, B., K. Takahiro, M. Sohtell, and G. Giebisch Intracellular potentials in rabbit proximal tubule perfused in vitro. Am. J. Physiol. 240:F200-F210. I. Koeppen, B. M., B. A. Biagi, and G. H. Giebisch Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am. J. Physiol. 244:F35-F Jacobson, H. R., and J. P. Kokko Intrinsic differences in various segments of the proximal convoluted tubule. J. Clin. Invest. 57: Stokes, J. B Sodium and potassium transport across the cortical and outer medullary collecting tubule of the rabbit: evidence for diffusion across the outer medullary portion. Am. J. Physiol. 242:F514-F Molony, D. A., J. P. Kokko, D. Seldin, and H. R. Jacobson Acid peritubular ph suppresses sodium reabsorption in cortical collecting tubules. Kidney Int. 25: Koeppen, B. M Conductive properties of rabbit outer medullary collecting duct. Kidney Int. 25: McKinney, T. D., and M. B. Burg Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am. J. Physiol. 234:F141- F McKinney, T. D., and M. B. Burg Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J. Clin. Invest. 61: Gluck, S., C. Cannon, and Q. Al-Awqati Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc. Nati. Acad. Sci. USA. 79: H. R. Jacobson
The regulation of renal acid secretion: New observations from studies of distal nephron segments
 Kidney International, Vol. 29 (1986), pp. 1099 1109 EDITORIAL REVIEW The regulation of renal acid secretion: New observations from studies of distal nephron segments Forty years ago, in a landmark paper,
Kidney International, Vol. 29 (1986), pp. 1099 1109 EDITORIAL REVIEW The regulation of renal acid secretion: New observations from studies of distal nephron segments Forty years ago, in a landmark paper,
Characterization of Acidification in the Cortical and Medullary Collecting Tubule of the Rabbit
 Characterization of Acidification in the Cortical and Medullary Collecting Tubule of the Rabbit MELVIN E. LASKI and NEIL A. KURTZMAN, with the technical assistance of VICKY MORGAN, Section of Nephrology,
Characterization of Acidification in the Cortical and Medullary Collecting Tubule of the Rabbit MELVIN E. LASKI and NEIL A. KURTZMAN, with the technical assistance of VICKY MORGAN, Section of Nephrology,
Intrinsic Differences in Various Segments
 Intrinsic Differences in Various Segments of the Proximal Convoluted Tubule HARRY R. JACOBSON and JuHA P. KoKKo From the Department of Internal Medicine, The University of Texas Southwestern Medical School,
Intrinsic Differences in Various Segments of the Proximal Convoluted Tubule HARRY R. JACOBSON and JuHA P. KoKKo From the Department of Internal Medicine, The University of Texas Southwestern Medical School,
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
Sodium and chlorine transport
 Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Defect in Urinary Acidification Induced In Vitro by Amphotericin B
 Defect in Urinary Acidification Induced In Vitro by Amphotericin B PILIP R. STEINMETZ and Lois R. LAWSON From the Departments of Medicine, Harvard Medical School and the Beth Israel Hospital, Boston,-
Defect in Urinary Acidification Induced In Vitro by Amphotericin B PILIP R. STEINMETZ and Lois R. LAWSON From the Departments of Medicine, Harvard Medical School and the Beth Israel Hospital, Boston,-
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
MATURATION OF RAT PROXIMAL TUBULE CHLORIDE PERMEABILITY. Michel Baum and Raymond Quigley. Departments of Pediatrics and Internal Medicine
 Articles in PresS. Am J Physiol Regul Integr Comp Physiol (July 28, 2005). doi:10.1152/ajpregu.00257.2005 R-00257-2005.R2 MATURATION OF RAT PROXIMAL TUBULE CHLORIDE PERMEABILITY Michel Baum and Raymond
Articles in PresS. Am J Physiol Regul Integr Comp Physiol (July 28, 2005). doi:10.1152/ajpregu.00257.2005 R-00257-2005.R2 MATURATION OF RAT PROXIMAL TUBULE CHLORIDE PERMEABILITY Michel Baum and Raymond
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Introduction. Acids, Bases and ph; a review
 0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
0 P a g e Introduction In this sheet, we discuss acidbase balance in our body and the role of kidneys in its establishment. Arrangement of topics is different from that of the lecture, to assure consistency
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
MS1 Physiology Review of Na+, K+, H + /HCO 3. /Acid-base, Ca+² and PO 4 physiology
 MS1 Physiology Review of,, / /Acidbase, Ca+² and PO 4 physiology I. David Weiner, M.D. Professor of Medicine and Physiology University of Florida College of Medicine Basic principles Proximal tubule Majority
MS1 Physiology Review of,, / /Acidbase, Ca+² and PO 4 physiology I. David Weiner, M.D. Professor of Medicine and Physiology University of Florida College of Medicine Basic principles Proximal tubule Majority
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion
 Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
ACID-BASE BALANCE URINE BLOOD AIR
 ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
ACIDBASE BALANCE URINE BLOOD AIR H 2 PO 4 NH 4 HCO 3 KIDNEY H H HCO 3 CELLS Hb H LUNG H 2 CO 3 HHb CO 2 H 2 O ph = 7.4 [HCO 3 ] = 24 meq/l PCO 2 = 40 mm Hg CO 2 PRIMARY RENAL MECHANISMS INVOLVED IN ACIDBASE
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Cortical distal nephron Cl transport in volume homeostasis and blood pressure regulation
 Am J Physiol Renal Physiol 305: F427 F438, 2013. First published May 1, 2013; doi:10.1152/ajprenal.00022.2013. Review Cortical distal nephron Cl transport in volume homeostasis and blood pressure regulation
Am J Physiol Renal Physiol 305: F427 F438, 2013. First published May 1, 2013; doi:10.1152/ajprenal.00022.2013. Review Cortical distal nephron Cl transport in volume homeostasis and blood pressure regulation
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Dr. Suzana Voiculescu Discipline of Physiology and Fundamental Neurosciences Carol Davila Univ. of Medicine and Pharmacy
 Dr. Suzana Voiculescu Discipline of Physiology and Fundamental Neurosciences Carol Davila Univ. of Medicine and Pharmacy Definition All the processes inside the body which keep the H+ concentration within
Dr. Suzana Voiculescu Discipline of Physiology and Fundamental Neurosciences Carol Davila Univ. of Medicine and Pharmacy Definition All the processes inside the body which keep the H+ concentration within
Effects of Amiloride on the Transport of Sodium and Other Ions in the Alga Hydrodictyon reticulatum
 Gen. Physiol. Biophys. (1987). 6, 255-263 255 Effects of Amiloride on the Transport of Sodium and Other Ions in the Alga Hydrodictyon reticulatum R. RYBOVÁ, R. METLIČKA and K. JANÁČEK Academy of Sciences.
Gen. Physiol. Biophys. (1987). 6, 255-263 255 Effects of Amiloride on the Transport of Sodium and Other Ions in the Alga Hydrodictyon reticulatum R. RYBOVÁ, R. METLIČKA and K. JANÁČEK Academy of Sciences.
DIURETICS-2. Dr. Shariq Syed. Shariq AIKC/TYB/2014
 DIURETICS-2 Dr. Syed Structure of Kidney Blood filtered by functional unit: Nephron Except for cells, proteins, other large molecules, rest gets filtered Structure of Kidney 3 major regions of nephron
DIURETICS-2 Dr. Syed Structure of Kidney Blood filtered by functional unit: Nephron Except for cells, proteins, other large molecules, rest gets filtered Structure of Kidney 3 major regions of nephron
Renal Bicarbonate Reabsorption in the Rat
 Renal Bicarbonate Reabsorption in the Rat Ill. Distal Tubule Perfusion Study of Load Dependence and Bicarbonate Permeability Y. L. Chan, G. Malnic, and G. Giebisch Department of Cellular and Molecular
Renal Bicarbonate Reabsorption in the Rat Ill. Distal Tubule Perfusion Study of Load Dependence and Bicarbonate Permeability Y. L. Chan, G. Malnic, and G. Giebisch Department of Cellular and Molecular
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
Normal Renal Function
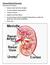 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Identify and describe. mechanism involved in Glucose reabsorption
 Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
COMMUNICATION BRIEF EFFECTS OF AMMONIUM AND BICARBONATE-CO2 ON INTRACELLULAR CHLORIDE LEVELS IN APLYSIA NEURONS
 BRIEF COMMUNICATION EFFECTS OF AMMONIUM AND BICARBONATE-CO2 ON INTRACELLULAR CHLORIDE LEVELS IN APLYSIA NEURONS JOHN M. RUSSELL, Department of Physiology and Biophysics, University of Texas Medical Branch,
BRIEF COMMUNICATION EFFECTS OF AMMONIUM AND BICARBONATE-CO2 ON INTRACELLULAR CHLORIDE LEVELS IN APLYSIA NEURONS JOHN M. RUSSELL, Department of Physiology and Biophysics, University of Texas Medical Branch,
Diuretics having the quality of exciting excessive excretion of urine. OED. Inhibitors of Sodium Reabsorption Saluretics not Aquaretics
 Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
RENAL TUBULAR ACIDOSIS An Overview
 RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
RENAL TUBULAR ACIDOSIS An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY CLINICAL BIOCHEMISTRY PBL MBBS IV VJ. Temple 1 What is Renal Tubular
Transport processes in urinary acidification
 Kidney International, Vol. 9 (1976) p. 172 1 88 Transport processes in urinary acidification GERHARD MALNIC and PHILIP R. STEINMETZ Department of Physiology, Instituto de Ciências Biomédicas, University
Kidney International, Vol. 9 (1976) p. 172 1 88 Transport processes in urinary acidification GERHARD MALNIC and PHILIP R. STEINMETZ Department of Physiology, Instituto de Ciências Biomédicas, University
What location in the gastrointestinal (GI) tract has tight, or impermeable, junctions between the epithelial cells?
 CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
CASE 32 A 17-year-old boy presents to his primary care physician with complaints of diarrhea for the last 2 days. The patient states that he just returned to the United States after visiting relatives
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Electrical Potential Differences and Electromotive Forces in Epithelial Tissues
 LETTER TO THE EDITOR [Brief letters to the Editor that make specific scientific reference to papers published previously in THE JOURNAL OF GENERAL PHYSIOLOGY are invited. Receipt of such letters will be
LETTER TO THE EDITOR [Brief letters to the Editor that make specific scientific reference to papers published previously in THE JOURNAL OF GENERAL PHYSIOLOGY are invited. Receipt of such letters will be
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Transport of Solutes and Water
 Transport of Solutes and Water Across cell membranes 1. Simple and Facilitated diffusion. 2. Active transport. 3. Osmosis. Simple diffusion Simple diffusion - the red particles are moving from an area
Transport of Solutes and Water Across cell membranes 1. Simple and Facilitated diffusion. 2. Active transport. 3. Osmosis. Simple diffusion Simple diffusion - the red particles are moving from an area
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL FUNCTION An Overview
 RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
RENAL FUNCTION An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY & MOLECULAR BIOLOGY PBL MBBS II SEMINAR VJ. Temple 1 Kidneys
CHAPTER 27 LECTURE OUTLINE
 CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
Transport of Ammonia in the Rabbit Cortical Collecting Tubule
 Transport of Ammonia in the Rabbit Cortical Collecting Tubule L. Lee Hamm, D. Trigg, D. Martin, C. Gillespie, and J. Buerkert Renal Division, Department ofmedicine, Washington University School ofmedicine,
Transport of Ammonia in the Rabbit Cortical Collecting Tubule L. Lee Hamm, D. Trigg, D. Martin, C. Gillespie, and J. Buerkert Renal Division, Department ofmedicine, Washington University School ofmedicine,
הנפרון. Introduction to Physiology (Course # 72336) THE KINDEY regulation of water and inorganic ions. Adi Mizrahi
 Introduction to Physiology (Course # 72336) 1 THE KINDEY regulation of water and inorganic ions Adi Mizrahi mizrahia@cc.huji.ac.il הכליה עקרונות של תפקוד ומבנה בסיסיים 2 הנפרון הכליה רגולציית מים ויונים
Introduction to Physiology (Course # 72336) 1 THE KINDEY regulation of water and inorganic ions Adi Mizrahi mizrahia@cc.huji.ac.il הכליה עקרונות של תפקוד ומבנה בסיסיים 2 הנפרון הכליה רגולציית מים ויונים
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis.
 Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
Potassium regulation. -Kidney is a major regulator for potassium Homeostasis. Normal potassium intake, distribution, and output from the body. Effects of severe hyperkalemia Partial depolarization of cell
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Acid-Base Tutorial 2/10/2014. Overview. Physiology (2) Physiology (1)
 Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
Overview Acid-Base Tutorial Nicola Barlow Physiology Buffering systems Control mechanisms Laboratory assessment of acid-base Disorders of H + ion homeostasis Respiratory acidosis Metabolic acidosis Respiratory
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
H secretion in renal cortical tubules: Kinetic aspects1
 Kidney International, Vol. 32 (1987), pp. 136 15 ROBRT F. PITTS MMORIAL LCTUR H secretion in renal cortical tubules: Kinetic aspects1 GRHARD MALNIC Department of Physiology and Biophysics, Institute Ciencias
Kidney International, Vol. 32 (1987), pp. 136 15 ROBRT F. PITTS MMORIAL LCTUR H secretion in renal cortical tubules: Kinetic aspects1 GRHARD MALNIC Department of Physiology and Biophysics, Institute Ciencias
BLOCK REVIEW Renal Physiology. May 9, 2011 Koeppen & Stanton. EXAM May 12, Tubular Epithelium
 BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
Acid and Base Balance
 Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-
 Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
Graphics are used with permission of : adam.com (http://www.adam.com/) Benjamin/Cummings Publishing Co (http://www.awl.com/bc) -42-74. (1) Carbon dioxide arrives at the kidney tubule cell in the proximal
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
CASE 27. What is the response of the kidney to metabolic acidosis? What is the response of the kidney to a respiratory alkalosis?
 CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
CASE 27 A 21-year-old man with insulin-dependent diabetes presents to the emergency center with mental status changes, nausea, vomiting, abdominal pain, and rapid respirations. On examination, the patient
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Acid - base equilibrium
 Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Membrane Transport. Anatomy 36 Unit 1
 Membrane Transport Anatomy 36 Unit 1 Membrane Transport Cell membranes are selectively permeable Some solutes can freely diffuse across the membrane Some solutes have to be selectively moved across the
Membrane Transport Anatomy 36 Unit 1 Membrane Transport Cell membranes are selectively permeable Some solutes can freely diffuse across the membrane Some solutes have to be selectively moved across the
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Homeostatic Regulation
 Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Urinary System. Dr. ZHANG Xiong. Dept. of Physiology. ZJU School of Medicine. QUESTION 6
 Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine http://10.71.121.158 Copyright@ Xiong Zhang QUESTION 6 How is the filtrate reabsorbed in tubular system? Copyright@ Xiong Zhang
Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine http://10.71.121.158 Copyright@ Xiong Zhang QUESTION 6 How is the filtrate reabsorbed in tubular system? Copyright@ Xiong Zhang
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Cortical Collecting Tubule of Adrenalectomized Rabbits
 Dietary Modulation of Active Potassium Secretion in the Cortical Collecting Tubule of Adrenalectomized Rabbits CHARLS S. WINGO, DONALD W. SLDIN, JUHA P. KOKKO, and HARRY R. JACOBSON, University of Texas
Dietary Modulation of Active Potassium Secretion in the Cortical Collecting Tubule of Adrenalectomized Rabbits CHARLS S. WINGO, DONALD W. SLDIN, JUHA P. KOKKO, and HARRY R. JACOBSON, University of Texas
Excretion of Drugs. Prof. Hanan Hagar Pharmacology Unit Medical College
 Excretion of Drugs Prof. Hanan Hagar Pharmacology Unit Medical College Excretion of Drugs By the end of this lecture, students should be able to! Identify main and minor routes of excretion including renal
Excretion of Drugs Prof. Hanan Hagar Pharmacology Unit Medical College Excretion of Drugs By the end of this lecture, students should be able to! Identify main and minor routes of excretion including renal
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
3/19/2009. The task of the kidney in acid-base balance Excretion of the daily acid load. Buffering of an acid load. A o B - + H + B - A o +OH - C +
 The task of the kidney in acid-base balance Excretion of the daily acid load Buffering of an acid load Oxidation of amino acids, fats and carbohydrates often lead to acid production. On an average American
The task of the kidney in acid-base balance Excretion of the daily acid load Buffering of an acid load Oxidation of amino acids, fats and carbohydrates often lead to acid production. On an average American
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19](/thumbs/80/80449526.jpg) 1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
r - D. Brown Q. AI-Awqati
 r - D. Brown Q. AI-Awqati J. exp. Biol. 172, 205-218 (1992) 205 Primed in Great Britain The Company of Biologists Limited 1992 THE ROLE OF THE V-ATPase IN RENAL EPITHELIAL H + TRANSPORT BY STEPHEN GLUCK
r - D. Brown Q. AI-Awqati J. exp. Biol. 172, 205-218 (1992) 205 Primed in Great Britain The Company of Biologists Limited 1992 THE ROLE OF THE V-ATPase IN RENAL EPITHELIAL H + TRANSPORT BY STEPHEN GLUCK
Dr. Suzana Voiculescu
 Dr. Suzana Voiculescu Definition All the processes inside the body which keep the H+ concentration within normal values. Depends on water and ion balance blood gas homeostasis Blood acidity may be expressed
Dr. Suzana Voiculescu Definition All the processes inside the body which keep the H+ concentration within normal values. Depends on water and ion balance blood gas homeostasis Blood acidity may be expressed
*Department of Pharmacology, Jichi Medical School,. Tochigi , Japan
 Journal of Physiology (1993), 462, pp. 275-289 275 With 8 ftgures Printed in Great Britain EFFECTS OF PROSTAGLANDIN E2 ON MEMBRANE VOLTAGE OF THE CONNECTING TUBULE AND CORTICAL COLLECTING DUCT FROM RABBITS
Journal of Physiology (1993), 462, pp. 275-289 275 With 8 ftgures Printed in Great Britain EFFECTS OF PROSTAGLANDIN E2 ON MEMBRANE VOLTAGE OF THE CONNECTING TUBULE AND CORTICAL COLLECTING DUCT FROM RABBITS
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Lab 19 The Urinary System
 Lab 19 The Urinary System Laboratory Objectives Identify and describe the micro- and macroscopic anatomy of the kidney. Track the blood flow in and out of the kidney. Compare blood, glomerular filtrate,
Lab 19 The Urinary System Laboratory Objectives Identify and describe the micro- and macroscopic anatomy of the kidney. Track the blood flow in and out of the kidney. Compare blood, glomerular filtrate,
DIURETICS-4 Dr. Shariq Syed
 DIURETICS-4 Dr. Shariq Syed AIKTC - Knowledge Resources & Relay Center 1 Pop Quiz!! Loop diuretics act on which transporter PKCC NKCC2 AIKTCC I Don t know AIKTC - Knowledge Resources & Relay Center 2 Pop
DIURETICS-4 Dr. Shariq Syed AIKTC - Knowledge Resources & Relay Center 1 Pop Quiz!! Loop diuretics act on which transporter PKCC NKCC2 AIKTCC I Don t know AIKTC - Knowledge Resources & Relay Center 2 Pop
Diarrhea is almost always associated with changes in. HCO 3 Secretion in the Rat Colonic Crypt Is Closely Linked to Cl Secretion
 GASTROENTEROLOGY 2000;118:101 107 HCO 3 Secretion in the Rat Colonic Crypt Is Closely Linked to Cl Secretion JOHN P. GEIBEL,*, SATISH SINGH,, VAZHAIKKURICHI M. RAJENDRAN, and HENRY J. BINDER, Departments
GASTROENTEROLOGY 2000;118:101 107 HCO 3 Secretion in the Rat Colonic Crypt Is Closely Linked to Cl Secretion JOHN P. GEIBEL,*, SATISH SINGH,, VAZHAIKKURICHI M. RAJENDRAN, and HENRY J. BINDER, Departments
STEIN IN-TERM EXAM -- BIOLOGY APRIL 19, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- APRIL 19, 2018 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There is
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- APRIL 19, 2018 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There is
Ch 3 Membrane Transports
 Ch 3 Membrane Transports what's so dynamic about cell membranes? living things get nutrients and energy from the envrionment this is true of the entire organism and each cell this requires transport in/out
Ch 3 Membrane Transports what's so dynamic about cell membranes? living things get nutrients and energy from the envrionment this is true of the entire organism and each cell this requires transport in/out
STEIN IN-TERM EXAM -- BIOLOGY APRIL 18, PAGE
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- APRIL 18, 2019 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There is
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- APRIL 18, 2019 -- PAGE 1 of 9 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There is
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Role of NH 3 and NH 4 transporters in renal acid-base transport
 Am J Physiol Renal Physiol 300: F11 F23, 2011. First published November 3, 2010; doi:10.1152/ajprenal.00554.2010. Role of NH 3 and NH 4 transporters in renal acid-base transport I. David Weiner 1,2 and
Am J Physiol Renal Physiol 300: F11 F23, 2011. First published November 3, 2010; doi:10.1152/ajprenal.00554.2010. Role of NH 3 and NH 4 transporters in renal acid-base transport I. David Weiner 1,2 and
