Long-Term Mathematical Model Involving Renal Sympathetic Nerve Activity, Arterial Pressure, and Sodium Excretion
|
|
|
- Scott Francis
- 5 years ago
- Views:
Transcription
1 Annals of Biomedical Engineering, Vol. 33, No. 11, November 2005 ( 2005) pp DOI: /s Long-Term Mathematical Model Involving Renal Sympathetic Nerve Activity, Arterial Pressure, and Sodium Excretion FATIH KARAASLAN, 1 YAGMUR DENIZHAN, 2 ABIDIN KAYSERILIOGLU, 3 and H. OZCAN GULCUR 1 1 Institute of Biomedical Engineering, Bogazici University; 2 Electrical and Electronics Engineering Department, Bogazici University; and 3 Sports Medicine Department, Istanbul Medical Faculty, Istanbul University (Received 13 September 2004; accepted 16 May 2005) Abstract This paper presents a physiological long-term model of the cardiovascular system. It integrates the previous models developed by Guyton, Uttamsingh and Coleman. Additionally it introduces mechanisms of direct effects of the renal sympathetic nerve activity (rsna) on tubular sodium reabsorption and renin secretion in accordance with experimental data from literature. The resulting mathematical model constitutes the first long-term model of the cardiovascular system accounting for the effects of rsna on kidney functions in such detail. The objective of developing such a model is to observe the consequences of long-term rsna increase and impairment of rsna inhibition under volume loading. This model provides an understanding of the rsna-related mechanisms, which cause mean arterial pressure increase in hypertension and total sodium amount increase (sodium retention) in congestive heart failure, nephrotic syndrome and cirrhosis. Keywords Long-term cardiovascular system model, Hypertension, Congestive heart failure, Nephrotic syndrome, Cirrhosis. INTRODUCTION It is known that kidneys play an important role in the maintenance of the long-term arterial pressure and body fluid volume regulation. During the first half of the 20th century it was accepted that renal nerves have little functional importance. However, later, it has been demonstrated that renal nerves innervate on functionally important parts of the kidney. Next it was shown that renal nerves directly affect some important kidney functions. 15 Furthermore, clinical and experimental observations indicate an increase in renal sympathetic nerve activity (rsna) in cases of congestive heart failure, nephrotic syndrome and hepatic cirrhosis, which have common clinical presentation of sodium retaining edema formation. The contribution of increased rsna to renal sodium retention in diseases mentioned above was demonstrated by several clinical and experimental studies. 12 Address correspondence to Yagmur Denizhan, Electrical and Electronics Engineering Department, Bogazici University, 34242, Bebek, Istanbul, Turkey. Electronic mail: denizhan@boun.edu.tr 1607 In view of the clinical importance of the long-term interaction between rsna, arterial pressure and sodium excretion, a model has been developed, which integrates and improves the mathematical models of the cardiovascular system existing in the literature. The most extensive mathematical model of the cardiovascular system known in the literature is the one developed by Guyton et al. 22 Other models related to the cardiovascular system include Uttamsingh s and Coleman s models 7,40 which basically focus on the renal system. Although these models include most of the mechanisms responsible for blood volume control, the effects of the renal nerve are accounted for in only Guyton s model 22 and this in a very limited manner. Uttamsingh s and Coleman s models provide a more detailed representation of the kidney, whereas Guyton represents it in a more compact manner. The model proposed in this paper integrates the advantageous parts of these models and also introduces the renal sympathetic nerve effect in a more extensive manner. The organization of the paper is as follows: Section II gives a comparison of the mathematical models of the cardiovascular/renal system known in the literature. In section III the proposed model is presented. Section IV includes the simulation results of the proposed model and their comparison with clinical and experimental data. Section V the conclusions are summarized including the limitations of the model. SOME MATHEMATICAL MODELS OF THE CARDIOVASCULAR/RENAL SYSTEM In 1967 Guyton published his first cardiovascular model 20 where he provided a basic understanding of the mutual effects of basic cardiovascular variables like mean arterial pressure, total peripheral resistance, cardiac output etc. His later model 22 can be considered as the first extensive mathematical model of the long-term (a few weeks) behavior of the cardiovascular system simulated on the computer. This model consists of a large set of coupled non-linear differential equations. The representation as a block diagram /05/ /1 C 2005 Biomedical Engineering Society
2 1608 KARAASLAN et al. includes, beside some integrators and gains, blocks with outputs representing physiological variables. Such blocks express the physiological variable under consideration as a function of other variables. When obtaining these functions, Guyton has integrated experimental and clinical data from a variety of sources. Since the model is supposed to account for the long-term behavior of the system, Guyton et al. have left the fast systolic and diastolic dynamics out of the scope and have represented the arterial pressure in terms of the average of the systolic and diastolic pressures. Similarly, dynamics much slower than observable within the considered time scale (i.e., months) are disregarded, i.e., the corresponding variables are taken as constants. In 1970 s several attempts have been made to model various aspects of the cardiovascular/kidney system. 3,6,27 However, these will not be considered in this paper, since they do not introduce any novelty to Guyton s model as far as the renal sympathetic nerve activity is concerned. In the subsequent years Uttamsingh has developed a detailed mathematical model of the long-term renal system dynamics 40 also including some basic cardiovascular dynamics. The main novelty in Uttamsingh s model 40 is the dependence of renin secretion on the macula densa sodium flow. A later and more extensive contribution to renal system models is the one by Coleman 7 which focuses on the sodium flow in the macula densa, and provides a detailed representation of the renin secretion and tubulo-glomerular feedback mechanisms (Fig. 1). It also includes the effect of angiotensin on the tubulo-glomerular feedback signal. Table 1 shows a qualitative comparison of the causal relations between some variables and renal processes as used by various models. PROPOSED MODEL The objective of this study is to develop a computerbased model of the long-term cardiovascular system response to increased renal sympathetic nerve activity and to inhibition of renal sympathetic nerve activity. The model combines major parts of Guyton s extensive cardiovascular model with two renal models in the literature (Coleman s and Uttamsingh s models) and improves them with some further experimental findings from the literature, such that the simulation results sufficiently resemble the behavior of the arterial pressure and sodium excretion under long-term rsna increase and under volume loading/rsna inhibition. Short-term dynamics are not considered in this model. Guyton s models 20,22 provide a very good and extensive basis for any long-term model of the human cardiovascular system. However, keeping the aim of accounting for the renal sympathetic effects in mind, the model presented in Guyton et al. 22 needs to be improved, because it represents the renal tubular system as a single block and is therefore not suitable for analyzing the influence of renal sympathetic nerve activity at different parts of this system. In Guyton s model, 22 the effect of the renal nerve on kidney functions is confined to the influence on the afferent arteriolar resistance (Table 1). However, in DiBona et al., 12 it has been reported that renal nerves directly affect some activity in different parts of the renal tubular system: Renin secretion from the juxtaglomerular complex and tubular sodium reabsorption. The proposed model adopts the representation of the renal tubular system in terms of its anatomical subregions as given by Coleman 7 (Fig. 1), which allows not only a detailed account for different effects of the renal sympathetic nerve activity (rsna) but also a detailed study of sodium reabsorption from different parts of the renal tubular system. Furthermore, some hormonal effects represented in closed form in Guyton s model have been replaced by more detailed representations in Uttamsingh et al. 40 Thus, based on medical knowledge, the proposed model integrates to Guyton s models for the human cardiovascular system 20,22 the above mentioned physiological functions as represented in Coleman s and Uttamsingh s kidney models and some quantitative findings from medical literature. These quantitative findings include: Dependence of right atrial pressure on atrial natriuretic peptide 31 FIGURE 1. Renal tubular system as modeled by (a) Guyton and (b) Coleman.
3 Long-Term Mathematical Model Involving RSNA 1609 TABLE 1. Comparison of different models in the literature with respect to some renal mechanisms. Guyton s model Uttamsingh s model Coleman s model (Guyton, 1972) (Uttamsingh, 1985) (Coleman, 1992) Proposed model Factor(s) influencing Macula densa sodium Macula densa sodium Macula densa sodium flow renin secretion flow flow nerve Renal sympathetic activity Factor(s) influencing Sodium concentration Renin concentration Renin concentration Renin concentration angiotensin secretion Renal blood flow Factor(s) influencing Potassium concentration Angiotensin concentration Angiotensin Potassium aldosterone secretion Sodium concentration concentration Sodium concentration Angiotensin concentration Angiotensin concentration Arterial pressure Arterial pressure Factor(s) influencing Right atrial pressure Sodium concentration Right atrial pressure antidiuretic hormone Sodium concentration Excess fluid in extracellular Sodium concentration secretion Autonomic nervous compartment Autonomic nervous system activity system activity Factor(s) influencing Natriuretic factor a Right atrial pressure atrial natriuretic peptide secretion Variable(s) affected Afferent arteriolar Afferent arteriolar resistance by renal sympathetic resistance Renin secretion rate nerve activity Tubular sodium reabsorption rate a In 1970s although the existence of natriuretic peptide regulation was recognized, 27 the detailed regulation mechanism was not known yet. Therefore, Guyton has used a hypothetical regulation loop, which he called natriuretic factor. Effect of atrial natriuretic peptide on sodium excretion 25 Dependence of rsna on right atrial pressure 11 and arterial pressure 10 Effects of rnsa on the renin secretion rate 8 and the tubular sodium reabsorption rate 8,35 Data on sodium excretion and renin concentration 36,39 Since sodium flow in macula densa and renin secretion rate do not lend themselves to practical measurement, some assumptions have been made in order to estimate them from measurable variables reported in the data given in. 36,39 These assumptions are as follows: (i) It has been assumed that the changes in the sodium flow in macula densa is similar to the changes in sodium excretion in urine, with some modulation by the distal tubule and collecting duct sodium reabsorption rate. Anatomically, after macula densa, sodium goes through the distal tubule and the collecting duct [Fig. 1(b)], where some part of it is reabsorbed, and then leaves the body in the urine. This assumption is actually a consequence of a more basic assumption that both the distal tubule sodium reabsorption rate and the collecting duct sodium reabsorption rate are constant percentages of the respective inflows. The assumption on the fractional distal tubule sodium reabsorption rate is justified by the experimental findings of Barba et al., 1 who report an increase of only 3% (small enough to be assumed as 0%) in the fractional distal tubule sodium reabsorption as a response to dropping the sodium intake down to 62.5% of its normal value. On the other hand, the assumption on the fractional collecting duct sodium reabsorption rate is based on the following argumentation of Mejia et al. 34 because of relatively small amount of NaCl reabsorbed in the collecting duct system, control mechanisms at this site can only mediate relatively small changes the NaCl excretion rate. (ii) It has been assumed that at steady state the percentage change in the renin secretion is proportional to renin concentration change in the blood. The justification of this assumption is based on the fact that at steady-state renin secretion and destruction rates are equal. Consequently, a percentage change in steady-state renin secretion can be said to cause the same percentage change in the steady-state renin concentration. (iii) It has been assumed that renin is secreted only from the kidneys according to. 24 All basic assumptions in the proposed model, including those mentioned above, can be listed as follows: The kidneys are modeled as if they constitute a single huge nephron. It is assumed that the change in sodium flow in macula densa is similar to the change in sodium excretion in urine, with some modulation by the distal tubule and collecting duct sodium reabsorption rate.
4 1610 KARAASLAN et al. It is assumed that at steady state the percentage change in the renin secretion is proportional to the percentage change in renin concentration. It is assumed that renin is secreted only from the kidneys. It is assumed that the direct influence of renal nerves on tubular sodium reabsorption is confined to the proximal tubule. It is assumed that there is no baroreceptor mediated rsna adaptation. It is assumed that baroreceptor mechanism has no influence on renin secretion. The effect of potassium concentration on aldosterone secretion is assumed to be constant. The sodium reabsorption effect of aldosterone is assumed to be confined to distal tubule. Throughout the model the hormone regulation mechanisms have been represented in accordance with Guyton s approach. This approach is based on a simplified model, which relates the hormone concentration to the hormone secretion level. Since at the steady-state the same amount of hormone is destroyed as is secreted, a percentage change in the hormone secretion will result in the same percentage change in the steady-state value of the hormone concentration. This steady-state concentration will obviously be reached with a certain time delay. This behavior can be approximated by an exponential convergence with a specific time constant T, which corresponds to the time delay for the concentration to reach approximately 63% of the steady-state level. From control theory point of view, this exponential convergence of the percentage change in hormone concentration (x) to the percentage change in hormone secretion level (x s ) can easily be represented by a first order differential equation dx = 1 dt T (x s x). Instead of the differential equation, the above relation is used in the integral form in the simulations. x(t) = 1 T t 0 (x s x)dτ (1) Figure 2 shows the block diagram of the proposed model. Each block represents the dependence of a physical or FIGURE 2. The block diagram of the basic physiological mechanisms involved in blood volume regulation. Each block expresses the dependence of a physical or physiological variable on other variables. Continuous and dotted arrows are used to represent the stimulating and inhibiting effects, respectively.
5 Long-Term Mathematical Model Involving RSNA 1611 physiological variable on other physical or physiological variables. These blocks are briefly explained below. The mathematical relations are given in the respective equations. The normal steady-state values of the variables are given in the glossary of terms (Table 2). Blocks 1, 7, 11, 32, and 35 include novel representations or mechanisms, which are not present in the previous models Block 1: Renal nerves from brain to kidneys (efferent renal nerves) are primarily affected by arterial pressure, cardiac pressure, and angiotensin hormone. It was shown that an increase in arterial pressure and right atrial pressure causes the renal sympathetic nerve activity to decrease in DiBona et al. 10,11 It was also shown that a short-term increase in the angiotensin hormone TABLE 2. Glossary of terms. Normal steady-state Symbol Name/Definition values/unit α map Effect of mean arterial pressure on renal sympathetic nerve activity 1 α rap Effect of right atrial pressure on renal sympathetic nerve activity 1 β rsna Effect of renal sympathetic nerve activity on afferent arteriolar resistance 1 δ ra Effect of right atrial pressure on normalized antidiuretic hormone secretion rate 0 γ at Effect of angiotensin hormone concentration on fractional proximal sodium reabsorption 1 γ filsod Effect of the filtered sodium load on fractional proximal sodium reabsorption 1 γ rsna Effect of renal sympathetic nerve activity on fractional proximal sodium reabsorption 1 ε aum Autonomic multiplier effect 1 η cd sodreab Fractional collecting duct sodium reabsorption 0.93 η dt sodreab Fractional distal tubule sodium reabsorption 0.5 η pt sodreab Fractional proximal sodium reabsorption 0.8 λ anp Effect of atrial natriuretic peptide on collecting duct sodium reabsorption rate 1 λ dt Effect of distal tubule sodium outflow on collecting duct sodium reabsorption rate 1 µ adh Effect of antidiuretic concentration on tubular water reabsorption rate 1 µ al Effect of aldosterone concentration on tubular water reabsorption rate 1 ν md sod Effect of macula densa sodium flow on normalized renin secretion rate 1 ν rsna Effect of renal sympathetic nerve activity on normalized renin secretion rate 1 ξ at Effect of angiotensin hormone on normalized aldosterone secretion rate 1 ξ k/sod Effect of potassium to sodium concentration ratio on normalized aldosterone secretion rate 1 ξ map Effect of mean arterial pressure on normalized aldosterone secretion rate 1 tgf Tubuloglomerular feedback signal 1 cd sodreab Absolute collecting duct sodium reabsorption rate meq/min co Cardiac output 5l/min dt sod Distal tubule sodium outflow 1.8 meq/min dt sodreab Absolute distal tubule sodium reabsorption rate 1.8 meq/min filsod Filtered sodium load 18 meq/min gfilt Glomerular filtration rate l/min md sod Macula densa sodium flow 3.6 meq/min pt sodreab Absolute proximal tubular sodium reabsorption rate 14.4 meq/min rb The renal blood flow 1.2 l/min sodin Sodium intake meq/min t wreab Tubular water reabsorption rate l/min u Urine flow rate l/min u sod Urine sodium flow meq/min vr Venous return 5l/min win Water intake l/min ψ al Effect of aldosterone concentration on fractional distal sodium reabsorption 1 a auto Autonomus system activity 1 a baro Baroreceptor activity 0.75 a chemo Chemoreceptor activity 0.25 C adh Antidiuretic hormone concentration 4 munits/l C al Aldosterone concentration 85 ng/l Ĉ anp Normalized atrial natriuretic peptide concentration 1 C at Angiotensin concentration 20 ng/l C gcf Glomerular capillary filtration coefficient C k Potassium concentration 5 meq/l Ĉ r Normalized renin concentration 1 C sod Sodium concentration 144 meq/l K bar Coefficient relating basic arterial resistance to vascularity 16.6 mmhg min l 1 K vd Vascularity destruction coefficient
6 1612 KARAASLAN et al. TABLE 2. Continued. Normal steady-state Symbol Name/Definition values/unit M sod Total amount of sodium 2160 meq N adh Normalized antidiuretic hormone concentration 1 N als Normalized aldosterone secretion rate 1 n ε dt Normal value of fractional distal sodium reabsorption 0.5 n η cd Normal value of fractional collecting duct sodium reabsorption 0.93 n η pt Normal value of fractional proximal sodium reabsorption 0.8 N al Normalized aldosterone concentration 1 N rs Normalized renin secretion rate 1 N rsna Normalized renal sympathetic nerve activity 1 P B Bowman hydrostatic pressure 18 mmhg P f Net filtration pressure 16 mmhg P gh Glomerular hydrostatic pressure 62 mmhg P go Glomerular osmotic pressure 28 mmhg P ma Mean arterial pressure 100 mmhg P mf Mean filling pressure 7 mmhg P ra Right atrial pressure 0 mmhg R a Arterial resistance 16.6 mmhg min l 1 R aa Afferent arteriolar resistance mmhg min l 1 R ba Basic arterial resistance 16.6 mmhg min l 1 R bv Basic venous resistance 3.4 mmhg min l 1 R ea Efferent arteriolar resistance mmhg min l 1 rsna Renal sympathetic nerve activity 1 R tp Total peripheral resistance 20 mmhg min l 1 R r Renal vascular resistance mmhg min l 1 R vr Resistance to venous return 1.4 mmhg min l 1 N adhs Normalized antidiuretic hormone secretion rate 1 T adh Time constant for antidiuretic hormone secretion 6 min T al Time constant for aldosterone hormone secretion 30 min T r Time constant for renin secretion 15 min vas Vascularity 1 vas d Vascularity destruction rate vas f Vascularity formation rate V b Blood volume 5 l V ecf Extracellular fluid volume 15 l concentration increases renal sympathetic nerve activity (rsna) in DiBona et al. 9 However, Lohmeier 31 has mentioned an indirect measurement showing that hypertension induced by long-term angiotensin infusion causes a decrease in rsna. Finally, in 2003 Barrett et al. 2 have been able to measure directly that long-term (7 days) angiotensin infusion causes a sustained increase in mean arterial pressure and a sustained decrease in rsna. Consequently, in the proposed long-term model no direct effect of angiotensin hormone on rsna has been included. Rsna is modeled as the product of normalized rsna (N rsna ), the effect of mean arterial pressure on rsna (α map ) and the effect of right atrial pressure on rsna (α rap ). The relationship between mean arterial pressure and heart rate exhibits the typical adaptation behavior, such that the effect of a change in mean arterial pressure level vanishes within 1 2 days. However, in Barrett et al., 2 it is reported that no similar resetting has been observed in the relationship between mean arterial pressure and rsna (i.e., baroreflex control of rsna) within 7 days after a change in the mean arterial pressure has been induced. Consequently, no adaptation mechanism has been included in the equation of α map. The equations for α map and α rap have been obtained by curve fitting from the figures given in DiBona et al. 10,11 This block consists of a novel representation of rsna not given in the considered previous cardiovascular/renal models [Eqs. (2), (3), and (4)]. rsna = N rsna α map α rap (2) 1.1 α map = e (P ma 100 mmhg)/15 mmhg (3) α rap = (mmHg) 1 P ra (4) Block 2: The renal vascular resistance (R r ) is composed of the afferent and efferent arteriolar resistances (R aa and R ea ). The efferent arteriolar resistance (R ea ) has been modeled as a function of the angiotensin hormone concentration (C at ), the equation for which has
7 Long-Term Mathematical Model Involving RSNA 1613 been extracted from experimental data given by Guyton et al. 21 On the other hand, the afferent arteriolar resistance (R aa ) is modeled as the product of a constant normal value (R aa ss = mmhg min l 1 ), the effect of renal sympathetic nerve activity on afferent arteriolar resistance (β rsna ) and the effect of tubuloglomerular feedback signal on afferent arteriolar resistance ( tgf ). β rsna is expressed as a function of rsna. The relating equations have been obtained from Guyton et al. 22 R r = R aa + Rea (5) R aa = R aa ss β rsna tgf (6) β rsna = 1.5(rsna 1) + 1 (7) R ea = (8) e log 10 C at Block 3: The renal blood flow ( rb ) is obtained by dividing the mean arterial pressure (P ma ) by the renal vascular resistance (R r ) in accordance with Guyton s model. 22 Here it is assumed that the mean arterial pressure (P ma ) sufficiently well approximates the renal arterial pressure. rb = P ma /R r (9) Block 4: The relations in this block are borrowed from Guyton et al. 22 The glomerular filtration rate ( gfilt )is expressed as the product of the net filtration pressure (P f ) and a constant glomerular capillary filtration coefficient (C gcf ). The net filtration pressure (P f ) is obtained by subtracting the sum of Bowman hydrostatic pressure (P B ) and the glomerular osmotic pressure (P go ) from the glomerular hydrostatic pressure (P gh ). The glomerular hydrostatic pressure (P gh ) is calculated as the difference of mean arterial pressure (P ma ) and the mean afferent arteriolar pressure, which is represented as the product of the renal blood flow ( rb ) and the afferent arteriolar resistance (R aa ). P B and P go were assumed constant at their normal steady-state values. gfilt = P f C gcf (10) P f = P gh (P B + P go ) (11) P gh = P ma rb R aa (12) Block 5: The sodium flow sensed at macula densa gives rise to a signal, which is mainly fed back to the afferent arteriole in order to regulate the glomerular filtration rate. The tubuloglomerular feedback signal ( tgf )was modeled as a function of macula densa sodium flow ( md sod ). 26 tgf = e ( md sod 3.859)/( ) (13) Block 6: The filtered sodium load ( filsod ) represents the amount of sodium filtered from the glomerulus to the proximal tubule per unit time, and can be expressed as the product of the glomerular filtration rate ( gfilt ) and the sodium concentration (C sod ) as proposed by Coleman. 7 filsod = gfilt C sod (14) Block 7: Some fraction (the so-called fractional proximal sodium reabsorption, η pt sodreab ) of the filtered sodium load ( filsod ) is reabsorbed from the proximal tubule to the blood. Hence the absolute proximal tubular sodium reabsorption rate ( pt sodreab ) can be represented as the product of filtered sodium load ( filsod ) and η pt sodreab [Eq. (15)]. The fractional proximal sodium reabsorption (η pt sodreab ) is affected by filtered sodium load, 7 angiotensin hormone concentration (C at ) 7 and rsna. 8,35 Following Coleman s approach, 7 the fractional proximal sodium reabsorption (η pt sodreab ) has been modeled as the product of its normal value (n η pt = 0.8) and the effects of the filtered sodium load, the angiotensin hormone concentration and rsna on fractional proximal sodium reabsorption, γ filsod, γ at, and γ rsna, respectively, where γ rsna is a novel contribution not present in Coleman s model [Eq. (16)]. An increase in filtered sodium load ( filsod ) has a decreasing influence on fractional proximal sodium reabsorption. This effect (γ filsod ) has been modeled by curve fitting to the piece-wise linear representation given in 7 [Eq. (17)]. An increase in angiotensin concentration (C at ) has an increasing influence on fractional proximal sodium reabsorption. This effect (γ at ) has also been modeled by curve fitting to the piece-wise linear representation given in 7 [Eq. (18)]. The results presented by Denton et al. 8 and Miki et al. 35 show that an increase in rsna has a direct increasing effect on tubular sodium reabsorption rate. On the other hand, the evidence provided by Nomora et al. 37 shows that rsna primarily affects the proximal tubule sodium reabsorption, hence it seems plausible to exclude the effects of rsna on sodium reabsorption at other parts of the renal tubular system. The effect of rsna on fractional proximal sodium reabsorption (γ rsna ) has been modeled by curve fitting to the data provided in 8 and 35 [Eq. (19)]. pt sodreab = filsod η pt sodreab (15) η pt sodreab = n η pt γ filsod γ at γ rsna (16) γ filsod = γ at = e 1+[( filsod 14 meq/ min)/(138 meq/ min)] e log 10 [(C at)/(1 ng/l)] (17) (18)
8 1614 KARAASLAN et al. 0.7 γ rsna = (19) 1 + e (1 rsna)/2.18 Block 8: Since the part of the filtered sodium load ( filsod ), which is not reabsorbed into the blood, goes to macula densa, the macula densa sodium flow ( md sod ) is calculated as the difference of the filtered sodium load ( filsod ) and the absolute proximal tubule sodium reabsorption rate ( pt sodreab ). md sod = filsod pt sodreab (20) Block 9: Some fraction (η dt sodreab ) of the macula densa sodium flow ( md sod ) is reabsorbed into the blood at the distal tubule. Thus, the absolute distal tubule sodium reabsorption rate ( dt sodreab ) can be expressed as the product of the macula densa sodium flow ( md sod ) and the so-called fractional distal tubule sodium reabsorption (η dt sodreab ). The fractional distal tubule sodium reabsorption (η dt sodreab ) is affected by aldosterone hormone concentration. 7 The fractional distal sodium reabsorption (η dt sodreab ) is represented as the product of its normal value (n ε dt = 0.5) and the effect of aldosterone hormone concentration on fractional distal sodium reabsorption (ψ al ). The piece-wise linear relationship given by Uttamsingh et al. 40 has been modified to represent ψ al as a function of the aldosterone hormone concentration (C al ). dt sodreab = md sod η dt sodreab (21) η dt sodreab = n ε dt ψ al (22) ψ al = e [ log 10 (C al/(1 ng/l))]/0.88 (23) Block 10: The distal tubule sodium outflow ( dt sod )is calculated as the difference of macula densa sodium flow ( md sod ) and absolute distal tubule sodium reabsorption rate ( dt sodreab ). dt sod = md sod dt sodreab (24) Block 11: At the collecting duct some fraction (η cd sodreab ) of the sodium contained in the flow is reabsorbed into the blood. Hence, in accordance with, 7 the absolute collecting duct sodium reabsorption rate ( cd sodreab ) is modeled as the product of the distal tubule sodium outflow ( dt sod ) and so-called fractional collecting duct sodium reabsorption (η cd sodreab ). The fractional collecting duct sodium reabsorption (η cd sodreab ) can be represented as the product of its normal value (n η cd = 0.93) and the effects of distal tubule sodium outflow ( dt sod ) and atrial natriuretic peptide hormone concentration (Ĉ anp ) on fractional collecting duct sodium reabsorption, λ dt (adapted from Coleman et al. 7 ) and λ anp (derived from Huang et al. 25 ), respectively. The effect of atrial natriuretic peptide hormone concentration is particularly dominant at collecting duct of renal tubular system; 19 hence, this effect has not been considered at other parts of the renal tubular system. This block includes a novel representation of the effect of atrial natriuretic peptide on sodium excretion not present in previous models [Eqs. (26) and (28)]. cd sodreab = dt sod η cd sodreab (25) η cd sodreab = n η cd λ dt λ anp (26) λ dt = e ( dt sod 1.6mEq/ min)/2meq/ min (27) λ anp = 0.1 Ĉ anp (28) Block 12: Sodium remaining in the collecting duct constitutes the urine sodium flow ( u sod ), which can be expressed as the difference of the distal tubule sodium outflow ( dt sod ) and the absolute collecting duct sodium reabsorption rate ( cd sodreab ). u sod = dt sod cd sodreab (29) Block 13: Although the water intake of a person can be considered as a voluntary act, hence an independent variable, there are many physiological factors that affect the level of thirst. Guyton points out the parallelism between antidiuretic hormone secretion and various stimuli known to affect thirst (e.g., high sodium concentration and hypovolemia, both of which have thirst-inducing effects, are accompanied by high antidiuretic hormone secretion). 22 In the proposed model Guyton s approach is used and water intake ( win ) is represented as a function of antidiuretic hormone concentration (C adh ) l/ min win = ( ) l/ min C adh 1 milliunits/l (30) Block 14: The extracellular fluid volume (V ecf ), being the accumulation of net fluid intake rate, is calculated as the time integral of the difference between the water intake ( win ) and the urine output rate ( u ), as proposed by Guyton. 20 In the simulations the initial condition (IC) has been taken equal to the normal value of 15 l. t V ecf (t) = IC + ( win u )dτ, IC = 15 l (31) 0
9 Long-Term Mathematical Model Involving RSNA 1615 Block 15: Based on Guyton s model, 20 the blood volume (V b ) is represented as a function of extracellular fluid volume (V ecf ) l V b = l + (32) 1 + e (V ecf l) ( l 1 ) Block 16: Since the blood pressure is non-uniform within the circulatory system, an average variable needs to be defined. Therefore, Guyton defines mean filling pressure (P mf ) as the average of all pressures in all individual segments of the systemic circulation when each of these pressures is weighted in proportion to the compliance to the compliance of the respective segment. 20 He indicates that it can be measured by stopping the circulation and rapidly pumping blood from arteries to the veins, thus bringing the pressures in the two major compliance areas of the systemic circulation into equilibrium within a few seconds. 20 The average compliance of all systemic circulation segments is affected by the autonomous nervous system. Thus, mean filling pressure (P mf ) is expressed as a function of blood volume (V b ) and the autonomic multiplier effect (ε aum ). P mf = (7.436 mmhg/l V b mmhg) ε aum (33) Block 17: The venous return ( vr ), which is blood flow into the heart, can be calculated dividing the difference between mean filling pressure (P mf ) and right atrial pressure (P ra ) by the resistance to venous return (R vr ). 20 vr = P mf P ra (34) R vr Block 18: At steady state, the input to and the output from the heart must be equal. 23 Hence, cardiac output ( co ) is taken equal to venous return ( vr ). Block 19: The right atrial pressure (P ra ) is calculated as a function of the cardiac output ( co ) in accordance with the Frank-Starling Law. 20 P ra = mmhg e co ( min /l) (35) Block 20: Vascularity gives an average measure of the number and diameter of blood vessels in the body. When there is excess blood flow in the circulatory system lasting for long periods of time, the number of tissue vessels and their diameters slowly increase to accommodate the extra flow. Similarly, when there is too little flow, vascularity of the tissues decreases slowly. This long-term auto-regulation of the blood vessels is accounted for by expressing the vascularity (vas) as the time integral of the net vascularity increase rate, which is the difference between vascularity formation rate (vas f ) and vascularity destruction rate (vas d ). The vascularity destruction rate (vas d ) can be taken as a constant fraction K vd = (the numerical value of the related parameter k 2 in Figs. 11-5, p. 184 in Ref. 20 has been obtained via personal communication with Guyton, A.C) of the existing vascularity, while the vascularity formation rate (vas f ) can be expressed as a function of the cardiac output ( co ). 20 t vas(t) = IC + 0 (vas f vas d )dτ, IC = 1 (36) vas f = e co ( min /L) (37) vas d = vas K vd (38) Block 21: The arterial resistance (R a ) is modeled as the basic arterial resistance (R ba ) modified by the effect of autonomous nervous system (autonomic multiplier effect, ε aum ), where the basic arterial resistance (R ba ) is inversely proportional to vascularity (vas) 20 with a constant of proportionality K bar = 16.6 mmhg min l (the numerical value of the related parameter k 1 in Figs. 11-5, p. 184 in Ref. 20 has been obtained via personal communication with Guyton, A.C). R a = R ba ε aum (39) R ba = K bar /vas (40) Block 22: The resistance to venous return is defined as the average resistance from each segment of the systemic circulation to the right atrium when each of these resistances is weighted in proportion to the compliance of the respective segment. 16 The empirical formula borrowed from Guyton s model 20 expresses the resistance to venous return (R vr ) as a function of a constant basic venous resistance (R bv ) and the arterial resistance (R a ). R vr = (8 R bv + R a )/31 (41) Block 23: The total peripheral resistance (R tp ) is calculated as the sum of arterial resistance (R a ) and constant basic venous resistance (R bv ). 20 R tp = R a + R bv (42) Block 24: Since the fluctuation between systolic and diastolic pressures is ignorable from the perspective of long-term cardiovascular dynamics, it is reasonable to use their mean, i.e., mean arterial pressure. The mean arterial pressure (P ma ) is calculated as the product of cardiac output ( co ) and the total peripheral resistance (R tp ). Angiotensin has a strong direct vasoconstrictor effect, which persists only for 1 or 2 min, while its indirect influence of increasing sodium and water reabsorption
10 1616 KARAASLAN et al. from the kidneys becomes much more dominant in the long term. 19 Consequently, in this long-term model only the indirect influence of angiotensin has been included. P ma = co R tp (43) Block 25: Guyton et al. 20 have represented the effect of the autonomous nervous system on various variables in terms of a normalized variable, the autonomic multiplier effect (ε aum ). The autonomic multiplier effect (ε aum )is composed of chemoreceptor ( a chemo ) and baroreceptor ( a baro ) activities, which are functions of the mean arterial pressure (P ma ) (expressed via an intermediate variable a auto ). As opposed to the chemoreceptor activity, the baroreceptor activity (a baro ) exhibits an adaptation behavior accounted for by the integral equation, where the factor min 1 is the inverse of the adaptation time constant. ε aum = a chemo + a baro (44) a auto = e P ma mmhg 1 (45) a chemo = 1/4a auto { (46) a baro (t) = 3/4 a auto ( min) 1 t 0 } (a baro 1) dτ (47) Block 26: The antidiuretic hormone concentration (C adh ) can be expressed as the product of the normalized antidiuretic hormone concentration (N adh ) and normal value of antidiuretic hormone concentration (4 milli-units/l) [Eq. (48)]. Equation (49) accounts for the regulation mechanism, by which the normalized antidiuretic hormone concentration (N adh ) is regulated to a normalized antidiuretic hormone secretion rate (N adhs ). Here the time constant T adh is taken as 6 min 16 and the initial condition (IC) as equal to the normal value = 1. As indicated in 18 and used in, 22 the antidiuretic hormone secretion rate is mainly determined by the sodium concentration (C sod ), the autonomic multiplier effect (ε aum ) and the effect of right atrial pressure (δ ra ) in an additive manner Eq. (50)]. Since C sod contributes to the antidiuretic hormone secretion rate from a certain concentration level (taken as 141 meq/l) onwards (when sodium concentration is slightly below and above than its normal value, antidiuretic hormone is secreted), in this block C sod has been limited to values above this level. This lower bound corresponds to a value 3 meq/l below the normal sodium concentration of 144 meq/l, where 3 meq/l is a value borrowed from. 22 Similarly, since blood volume decrease below its normal value, hence arterial pressure decrease and consequently increase of ε aum above its normal value 1 contributes to the antidiuretic hormone secretion rate, in this block ε aum has been limited to values above 1. The effect of right atrial pressure (δ ra ) is not a simple function of the right atrial pressure (P ra ) but also involves a self adaptation mechanism accounted for in Eq. (51) and borrowed from. 22 C adh = 4 milli units/l N adh (48) N adh (t) = 1 t (N adhs N adh )dτ + IC, IC = 1 T adh 0 (49) [ ] (Csod 141 meq/l) N adhs = + (ε aum 1) δ ra /3 1mEq/l (50) t δ ra (t) = (0.2 mmhg 1 )P ra ( min) 1 δ ra dτ 0 (51) Block 27: In this block the tubular water reabsorption rate ( t wreab ) is calculated in terms of the effects of aldosterone concentration and antidiuretic hormone concentration (µ al and µ adh ) and the glomerular filtration rate ( gfilt ) as proposed by Guyton. 22 However, the relations for µ al and µ adh have been derived from Uttamsingh s model l/ min t wreab = l/ min gfilt µ al µ adh (52) µ al = e [ log 10 (C al/(1 ng/l))]/0.88 (53) 0.8 µ adh = (54) 1 + e log 10 (C adh/(1 meq/l)) Block 28: The urine flow rate ( u ) is calculated as the difference between the glomerular filtration rate ( gfilt ) and the tubular water reabsorption rate ( t wreab ). In the simulations, u has been confined to values above l/min as used in Guyton s model. 22 Such a lower bound for the urine flow rate has been included in order to guarantee the excretion of the daily minimum obligatory urine volume ( 0.5 l/day) needed for getting rid of the waste products of the metabolism as indicated in. 19 u = gfilt t wreab (55)
11 Long-Term Mathematical Model Involving RSNA 1617 Block 29: The sodium intake ( sodin ) has been taken as an independent variable with a normal steady-state value of meq/min. Block 30: The total amount of sodium (M sod ) is represented as the time integral of the net sodium intake rate, i.e., the difference between sodium intake ( sodin ) and urine sodium flow ( u sod ). The initial condition (IC) is taken equal to the normal value = 2160 meq. t M sod (t) = IC + 0 ( sodin u sod )dτ, IC = 2160 meq (56) Block 31: Sodium concentration (C sod ) is expressed as the ratio of the total amount of sodium (M sod ) to the extracellular fluid volume (V ecf ). C sod = M sod /V ecf (57) Block 32: This block represents the mechanism, by which the normalized renin concentration (Ĉ r ) is regulated to a normalized renin secretion rate (N rs ), in a similar way Guyton has represented the regulation mechanisms of other hormones. The factors that determine this normalized renin secretion rate (N rs ) include macula densa sodium flow ( md sod ), renal sympathetic nerve activity (rsna) and the blood pressure sensed by the baroreceptors at the juxtaglomerular complex. 12 However, Guyton has observed that the baroreceptor mechanism has a negligible direct effect on renin secretion. 21 Hence, in the proposed model the normalized renin secretion rate (N rs )is calculated in terms of the effect of macula densa sodium flow on normalized renin secretion rate (ν md sod ) and effect of renal sympathetic nerve activity on normalized renin secretion rate (ν rsna ). T r represents the time constant for renin secretion and is taken as 15 min. 21 The initial condition (IC) is taken equal to the normal value, i.e., 1. This block includes a novel representation of the rsna effect on renin secretion not present in previous models [Eq. (61)]. Ĉ r (t) = 1 T r t 0 (N rs Ĉ r )dτ + IC, IC = 1 (58) N rs = ν md sod ν rsna (59) ν md sod = ν rsna = e ( md sod meq/ min)/( meq/ min) (60) e (rsna ) (61) Block 33: The angiotensin concentration (C at ) is assumed to be proportional to the normalized renin concentration (Ĉ r ). 7 C at = 20 ng/l Ĉ r (62) Block 34: This block represents the mechanism, by which the normalized aldosterone concentration (N al )isregulated to the normalized aldosterone secretion rate (N als ). The time constant T al governing the regulation of aldosterone concentration is taken as 60 min, as used by Guyton et al. 22 The normalized aldosterone secretion rate (N als ) is calculated in terms of the effects of potassium to sodium concentration ratio, mean arterial pressure and angiotensin concentration (ξ k/sod, ξ map, and ξ at, respectively) as suggested by Guyton et al. 22 Here, in spite of its important contribution to aldosterone secretion, the potassium concentration C K has been assumed to remain constant at its normal level of 5 meq/l, since the objective of this model is to investigate the relationship between renal sympathetic activity, arterial pressure and sodium excretion. Although no direct effect of mean arterial pressure on aldosterone secretion rate has been reported in general, Guyton indicates that a direct effect may exist in cases of hypotension. 21 Based on this, the effect of the mean arterial pressure on aldosterone secretion (ξ map ) has been assumed as 1 (no effect) for mean arterial pressures above normal, while for hypotension cases (P ma < 100 mmhg) the relation provided by Guyton et al. 22 has been adopted. The effect of angiotensin hormone concentration on aldosterone secretion rate (ξ at ) has been obtained by smoothing the piece-wise linear representation provided by Uttamsingh et al. 40 In the simulations aldosterone concentration (C al ) has been limited to a range between ng/l in order to guarantee the stability of the overall algorithm. Similarly, ξ k/sod has been confined to non-negative values only. Aldosterone concentration (C al ) is equal to the product of the normalized aldosterone concentration (N al ) and the normal value of aldosterone concentration (85 ng/l). The initial condition (IC) is taken equal to the normal value, i.e., 1. C al = N al 85 ng/l (63) N al (t) = 1 T al t 0 (N als N al )dτ + IC, IC = 1 (64) N als = ξ k/sod ξ map ξ at (65) ξ k/sod = C K/C sod (66) { e ( mmhg 1 ) P ma if P ξ map = ma 100 mmhg 1 if P ma > 100 mmhg (67)
12 1618 KARAASLAN et al. 2.4 ξ at = (68) 1 + e [ log 10 (C at/(1 ng/l))]/0.8 Block 35: The normalized atrial natriuretic peptide concentration (Ĉ anp ) is calculated as a function of the right atrial pressure (P ra ) in accordance with Lohmeier et al. 33 For human beings the actual value at normal conditions is about 36 ng/l Ĉ anp = (69) 1 + e (P ra mmhg)/(1 mmhg) Block 36: This block represents the potassium concentration, which in this model has been assumed to remain constant at its normal value (C k = 5 meq/l). The curve-fitting tool of Matlab has been used when obtaining non-linear equations from the findings in the literature represented in terms of piecewise linear equations or in graphical form. The proposed cardiovascular system model has been implemented using Matlab/Simulink. Runge Kutta 4 numerical method with fixed step size of 10 min has been used to solve the nonlinear differential equations in the model. Results of the sensitivity analysis performed by changing nine parameters one at a time by ±10% of their nominal values are summarized in Table 3. The resulting changes in the steady-state values of the 15 physiological variables are in general relatively small compared to the ±10% parameter change. The largest parameter sensitivity (10.9%) is observed in the renal blood flood as a response to a 10% change in the steady-state efferent arteriolar resistance, while all other changes remain below 10% in magnitude. Thus it can be said that the proposed model is quite robust with respect to parameter changes. RESULTS AND DISCUSSION Due to various reasons the evaluation of the validity of the proposed overall model is a non-trivial issue. The model consists of blocks, which are based on open-loop experimental data and are put together in order to function as a closed-loop system. As far as comparisons to real observations are concerned, we are confined to the limited amount of closed-loop clinical and experimental data available in the literature. Due to the difficulty of long-term experiments, observations and measurements, also the amount of long-term data is rather small. Some clinical and experimental data in the literature are not given in terms of the same units. In order to provide a common basis for fair comparison such data have been first expressed as percentages of the normal steady-state values as given in the related publication, and then converted into the units used in the simulations taking the normal steady-state values of the model as a basis. Since in practice physiological variables are considered as normal within certain ranges, the mean values of such ranges have been taken as normal steady-state values. Experimental results (represented by dots in Figs. 3 and 4) have been converted into the units used in the simulations Behavior Under Varying Sodium Intake in Healthy Subjects Varying sodium intake is a good method for testing whether of the closed-loop model of the blood volume and pressure regulation mechanisms work properly, because a change in the sodium intake can be considered as an indirect way of causing long-term blood volume changes. Furthermore among all variables considered in the model, sodium intake is one of the few, which can be approximated as an independent variable. The publication of Feng et al. 16 has been chosen as a basis of comparison, because this publication reports the results of a long-term experiment involving both increase and decrease in sodium intake. The experiment consists of high sodium intake (approximately by 200%) during the first 5 days and low sodium intake (approximately 94%) for the next 5 days in healthy human subjects [Fig. 3(A)]. As expected, extracellular fluid volume [Fig. 3(B)] and blood volume [Fig. 3(C)] increase during increasing of salt intake. An increase in the blood volume stimulates blood pressure and cardiac receptors, therefore reflex rsna inhibition is expected 2 [Fig. 3(D)]. As expected, an increase in the blood volume increases the right atrial pressure, and hence also the expansion of the right atrium causes secretion of atrial natriuretic peptide [Fig. 3(E)]. An increase in sodium intake causes the filtered sodium load and the macula densa sodium flow to increase. The increase in macula densa sodium flow and rsna decrease cause to decrease the renin secretion rate, therefore renin concentration decrease [Fig. 3(F)]. The decrease of renin concentration causes a decrease in angiotensin and aldosterone concentrations [Fig. 3(G)]. As a result of the decrease of angiotensin and aldosterone concentrations, the renal tubular sodium reabsorption decreases. In addition to this, increasing of atrial natriuretic peptide decreases the tubular sodium reabsorption. Consequently, the sodium excretion increases [Fig. 3(H)] during sodium loading [Fig. 3(A)]. Hence, in the long run the difference between sodium intake and sodium urine goes to zero [Fig. 3(I)] and the sodium balance is established. The model also predicts that the difference between water intake and urine volume will go to zero [Fig. 3(J)] establishing the water balance. The increase in sodium intake increases the antidiuretic hormone concentration, as expected. During the subsequent 5 days of sodium intake reduction compensatory mechanisms work in the opposite direction. All together, the cooperation of several control mechanisms results in a relatively small change in the sodium concentration [Fig. 3(L)], in spite of the large variation in sodium intake.
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Regulation of Sodium and Volume. Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM
 Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
Renal Regulation of Sodium and Volume Dr. Dave Johnson Associate Professor Dept. Physiology UNECOM Maintaining Volume Plasma water and sodium (Na + ) are regulated independently - you are already familiar
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D.
 Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Regulation of fluid and electrolytes balance
 Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Blood Pressure Regulation. Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure
 Sheet physiology(18) Sunday 24-November Blood Pressure Regulation Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure MAP= Diastolic Pressure+1/3 Pulse Pressure CO=MAP/TPR
Sheet physiology(18) Sunday 24-November Blood Pressure Regulation Slides 9-12 Mean Arterial Pressure (MAP) = 1/3 systolic pressure + 2/3 diastolic pressure MAP= Diastolic Pressure+1/3 Pulse Pressure CO=MAP/TPR
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal System and Excretion
 Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Blood Pressure Fox Chapter 14 part 2
 Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Mechanism: 1- waterretention from the last part of the nephron which increases blood volume, venous return EDV, stroke volume and cardiac output.
 Blood pressure regulators: 1- Short term regulation:nervous system Occurs Within secondsof the change in BP (they are short term because after a while (2-3 days) they adapt/reset the new blood pressure
Blood pressure regulators: 1- Short term regulation:nervous system Occurs Within secondsof the change in BP (they are short term because after a while (2-3 days) they adapt/reset the new blood pressure
Fluids and electrolytes
 Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Blood Pressure Regulation. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
Blood Pressure Regulation Faisal I. Mohammed, MD,PhD 1 Objectives Outline the short term and long term regulators of BP Know how baroreceptors and chemoreceptors work Know function of the atrial reflex.
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
Fluid and electrolyte balance, imbalance
 Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Physiology (4) 2/4/2018. Wael abu-anzeh
 Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Fal Fal P h y s i o l o g y 6 1 1, S a n F r a n c i s c o S t a t e U n i v e r s i t y
 Fall 12 OSMOTIC REGULATION OF THE RENAL SYSTEM: Effects of fasting and ingestion of water, coke, or Gatorade on urine flow rate and specific gravity Dorette Franks The purpose of the physiology experiment
Fall 12 OSMOTIC REGULATION OF THE RENAL SYSTEM: Effects of fasting and ingestion of water, coke, or Gatorade on urine flow rate and specific gravity Dorette Franks The purpose of the physiology experiment
CASE 13. What neural and humoral pathways regulate arterial pressure? What are two effects of angiotensin II?
 CASE 13 A 57-year-old man with long-standing diabetes mellitus and newly diagnosed hypertension presents to his primary care physician for follow-up. The patient has been trying to alter his dietary habits
CASE 13 A 57-year-old man with long-standing diabetes mellitus and newly diagnosed hypertension presents to his primary care physician for follow-up. The patient has been trying to alter his dietary habits
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
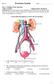 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Chapter 17: Urinary System
 Introduction Chapter 17: Urinary System Organs of the Urinary System REFERENCE FIGURE 17.1 2 kidneys filters the blood 2 ureters transport urine from the kidneys to the urinary bladder Urinary bladder
Introduction Chapter 17: Urinary System Organs of the Urinary System REFERENCE FIGURE 17.1 2 kidneys filters the blood 2 ureters transport urine from the kidneys to the urinary bladder Urinary bladder
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
Anatomy/Physiology Study Guide: Unit 9 Excretory System
 Anatomy/Physiology Study Guide: Unit 9 Excretory System 1) In the space below, list the primary structures (organs) and their corresponding functions. Structures: Functions: KIDNEY 1) URETER BLADDER URETHRA
Anatomy/Physiology Study Guide: Unit 9 Excretory System 1) In the space below, list the primary structures (organs) and their corresponding functions. Structures: Functions: KIDNEY 1) URETER BLADDER URETHRA
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Outline Urinary System
 Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
Urinary System and Excretion Bio105 Lecture Packet 20 Chapter 16 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure B. Urine formation 1. Hormonal regulation
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
The ability of the kidneys to regulate extracellular fluid volume by altering sodium
 REGULATION OF EXTRACELLULAR FLUID VOLUME BY INTEGRATED CONTROL OF SODIUM EXCRETION Joey P. Granger Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi
REGULATION OF EXTRACELLULAR FLUID VOLUME BY INTEGRATED CONTROL OF SODIUM EXCRETION Joey P. Granger Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi
Hyperaldosteronism: Conn's Syndrome
 RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
RENAL AND ACID-BASE PHYSIOLOGY 177 Case 31 Hyperaldosteronism: Conn's Syndrome Seymour Simon is a 54-year-old college physics professor who maintains a healthy lifestyle. He exercises regularly, doesn't
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Blood Pressure Regulation -1
 CVS Physiology Lecture 18 Blood Pressure Regulation -1 Please study the previous sheet before studying this one, even if the first part in this sheet is revision. In the previous lecture we were talking
CVS Physiology Lecture 18 Blood Pressure Regulation -1 Please study the previous sheet before studying this one, even if the first part in this sheet is revision. In the previous lecture we were talking
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: 100 20% of grade in class 1) During exercise, plasma levels of Renin increase moderately. Why should Renin levels be elevated during
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: 100 20% of grade in class 1) During exercise, plasma levels of Renin increase moderately. Why should Renin levels be elevated during
BODY FLUID. Outline. Functions of body fluid Water distribution in the body Maintenance of body fluid. Regulation of fluid homeostasis
 BODY FLUID Nutritional Biochemistry Yue-Hwa Chen Dec 13, 2007 Chen 1 Outline Functions of body fluid Water distribution in the body Maintenance of body fluid Intake vs output Regulation of body fluid Fluid
BODY FLUID Nutritional Biochemistry Yue-Hwa Chen Dec 13, 2007 Chen 1 Outline Functions of body fluid Water distribution in the body Maintenance of body fluid Intake vs output Regulation of body fluid Fluid
Kidneys in regulation of homeostasis
 Kidneys in regulation of homeostasis Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important terms
Kidneys in regulation of homeostasis Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important terms
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Counter-Current System Regulation of Renal Functions
 Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System)
 NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
NOTES: CH 44 Regulating the Internal Environment (Homeostasis & The Urinary System) HOMEOSTASIS **Recall HOMEOSTASIS is the steady-state physiological condition of the body. It includes: 1) Thermoregulation:
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Renal physiology II. Basic renal processes. Dr Alida Koorts BMS
 Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Principles of Renal Physiology. 4th Edition
 Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Principles of Renal Physiology 4th Edition Principles of Renal Physiology 4th Edition Chris Lote Professor of Experimental Nephrology, University of Birmingham, UK SPRINGER SCIENCE+BUSINESS MEDIA, B.V.
Chapter 26 The Urinary System
 Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Hormonal Control of Osmoregulatory Functions *
 OpenStax-CNX module: m44828 1 Hormonal Control of Osmoregulatory Functions * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of
OpenStax-CNX module: m44828 1 Hormonal Control of Osmoregulatory Functions * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Chapter 10: Urinary System & Excretion
 Chapter 10: Urinary System & Excretion Organs of Urinary System Kidneys (2) form urine Ureters (2) Carry urine from kidneys to bladder Bladder Stores urine Urethra Carries urine from bladder to outside
Chapter 10: Urinary System & Excretion Organs of Urinary System Kidneys (2) form urine Ureters (2) Carry urine from kidneys to bladder Bladder Stores urine Urethra Carries urine from bladder to outside
(D) (E) (F) 6. The extrasystolic beat would produce (A) increased pulse pressure because contractility. is increased. increased
 Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Therefore MAP=CO x TPR = HR x SV x TPR
 Regulation of MAP Flow = pressure gradient resistance CO = MAP TPR Therefore MAP=CO x TPR = HR x SV x TPR TPR is the total peripheral resistance: this is the combined resistance of all blood vessels (remember
Regulation of MAP Flow = pressure gradient resistance CO = MAP TPR Therefore MAP=CO x TPR = HR x SV x TPR TPR is the total peripheral resistance: this is the combined resistance of all blood vessels (remember
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
