GCE BIOLOGY - BY1. Mark Scheme - January 2013
|
|
|
- Edith Evans
- 6 years ago
- Views:
Transcription
1 GCE BIOLOGY - BY Mark Scheme - January 03. (a) (i) Stage A telophase; Stage C metaphase; (ii) Centromeres split/ divide; Chromatids/ chromosomes are being pulled to (opposite) poles; (due to) contraction/ shortening of the spindle (fibres); (i) Interphase; (ii) The (quantity of) DNA has doubled / (quantity of) DNA changes from 6 to ; NOT increase (iii) Meiosis; (correct spelling) (At the end of the cell cycle) the (quantity) of DNA has been halved (and halved again) / can describe with numbers /involves (consecutive) divisions; Ignore reference to chromosomes total [8]
2 . (a) DNA RNA Double stranded Single stranded helical Not helical Deoxyribose/ C 5 H 0 O 4 / one Ribose/ C 5 H 0 O 5 / one more less oxygen atom in pentose oxygen atom in pentose NOT deoxyribonucleic acid NOT ribonucleic acid sugar Contains thymine Contains uracil Not letters Not letters Can list all bases present Can list all bases present Max 3 Only one type (Relatively) long/ larger molecule 3 types (mrna, trna & rrna) (relatively) short/ smaller molecule 3% guanine therefore 3% cytosine; (54% made up of adenine and thymine) Adenine = 7(%); Correct answer = marks total [5]
3 3. (a) (i) Phagocytosis/ endocytosis; the (cell) membrane {invaginates/infolds/ surrounds/ wraps around/ engulfs} (to form a vesicle (allow vacuole) )around the {food particle/ algae}; (ii) Golgi {Body/apparatus}; (iii) Exocytosis; (i) (Site of aerobic) respiration / production of ATP; NOT production of energy alone (ii) Carry out {endo/exo/ phago}cytosis / synthesis of digestive enzymes/ movement/ form lysosomes; Reject active transport unqualified NOT digestion/ feeding (c).no nucleus/nuclear membrane/ DNA free in cytoplasm;. No membrane bound organelles / named example/ possess mesosome; 3. A loop of DNA / circular DNA/ ORA DNA {linear/ on chromosome/ associated with histone}; 4. Smaller/70S ribosomes; 5. Cell wall; Reject reference to cellulose 6. Capsule/ flagellum/ plasmid; NOT reference to size (can be neutral) Max 3 3 Total [9] 3
4 4. (a) Quaternary/ 4 ; (i) (Labelled) arrow in correct position; (ii) COOH/ carboxyl/ carboxylic acid; (iii) Disulphide {bond/ bridges} / ionic bonds / hydrogen / hydrophobic interactions / Van der Waals; (Any ) NOT peptide / S-S (covalent neutral) (c) Mark points must be comparative phospholipid triglyceride fatty acids 3 fatty acids; phosphate (head) do not contain a phosphate (head) polar/hydrophilic head and non-polar/hydrophobic; non-polar/hydrophobic tails Max (d) (i) {Heads/ phosphates} are {hydrophilic/ polar} and are {attracted to/ in} the water; {Tails/ fatty acids} are {hydrophobic/ non polar} and are {repelled by/ above/ avoid} water; NOT react/ dissolve with water (ii) 6.(m ); The phospholipids are {arranged in/ formed} a {bilayer/ double layer} in the membrane; Ref to phospholipid bilayer alone- insufficient 4 Total [0] 4
5 5. (a) (i) Oxygen by (simple) diffusion; through the phospholipid (bilayer); (ii) Phosphate ions by {facilitated diffusion/active transport}; through {carrier /channel}proteins/ protein pumps (active transport); (not channel proteins with active transport) NOT intrinsic Pass through hydrophilic pore; (not with active transport) Max (i) Active transport; (Between 0-30au) the concentration of phosphate ions is lower outside (the root)/higher inside (the root)/ Ions are being taken up against a concentration gradient; With oxygen present (aerobic) respiration can occur; Providing {ATP/ energy} (for active transport)/ active transport needs {energy/ ATP}; Max (ii) There are a {limited/fixed} number of {carriers/ proteins/ channels} (for phosphate ions) in the membrane; (The curve levels off/the rate of uptake becomes constant) when all of the {carriers/ channels/ proteins} are in use; (iii) (Ions are being taken up by) facilitated diffusion; Uptake {only begins/ occurs} when the external concentration is higher than the concentration inside the root hair cells/ down a concentration gradient; (c) They are a {component of/required to synthesise} {DNA/ RNA/ ATP/ NAD/ FAD/ NADP/ nucleotides/ nucleic acids}; 5 Total [] 5
6 6. (a) (i) Molecule of water (drawn with arrow towards the O atom of the glycosidic bond); NOT water going out Monosaccharides drawn with OH groups in correct position on C and C4 ( involved in bond); (ii) Hydrolysis; NOT hydrolysation (ignore reference to acid) (iii) Glycosidic; (iv) Glucose and galactose; ignore alpha/ beta (i) An enzyme that has been fixed to an inert {matrix/support/ substance}; (ii) The enzyme can easily be recovered/ reused; The product is free from contamination; Enzyme is {stable at / tolerates/ withstand} higher temperatures/denatures at a higher temperature/ functions over a wide range of ph; NOT wider range of temperature alone Several enzymes with differing optima can be used at the same time; More control over the reaction/enzymes easily added or removed/ can be used in a continuous process; Max 6
7 (c) (i) Heat with Benedict s solution/reagent; NOT warm/ water bath/ ref to acid Blue to{red/ orange/ green/ yellow/ brown}; (ii) Instrument/equipment that can detect a specific molecule/metabolite (in a mixture of molecules/bodily fluid). (iii) Any one from: The biosensor would give quantitative data/ it would detect {a particular product/glucose/galactose}/ Can detect even at {very low concentrations/ small volumes}; (d). (The concentration of reducing sugars) would decrease;. {Lactose/ substrate} concentration is lower (in the sour milk); 3. Lactic acid lowers the ph; 4. Enzyme would be inactivated/denatured; 5. Hydrogen/ ionic bonds (maintaining the 3D shape) would break; 6. This will change the shape/charge of the active site (of lactase); 7. Fewer enzyme-substrate complexes would be formed/fewer successful collisions; 8. Benedicts would remain {blue/ change to {orange/ yellow/ green/ brown}/ negative} Max 4 6 Total [6] 7
8 7. (a) Describe and explain the effect of inhibitors on enzyme action. [0] A Enzymes are globular proteins/biological catalysts; enzymes B C Active site (of the enzyme) has a specific 3D/ tertiary shape; lower activation energy of a reaction; D Inhibitors reduce the rate of (an enzyme catalysed) reaction; E Competitive inhibitors; F Have a shape similar to the substrate/complementary to the active site; NOT same shape competitive G H Fit/ bind into the active site; Prevent the substrate molecule entering the active site/block the active site; I Max. rate of reaction can be achieved at higher substrate concentrations/ Increasing the concentration of the substrate reduces the effect of the inhibitor; allow correctly labelled graph non-competitive J K L M N O Non-competitive inhibitors; Bind to the allosteric site/site other than the active site; Causes a change in the shape of the active site; Substrate can no longer fit into the active site/ active site is no longer complementary; Fewer/ no enzyme-substrate complexes form/ fewer successful collisions; Max. rate of reaction cannot be achieved/increasing the concentration of the substrate has no effect on inhibition; allow correctly labelled graph 8
9 A Describe the effects of placing animal and plant cells in solutions of differing solute concentration. Osmosis is the (net) movement of water molecules down a water potential gradient/from a higher water potential to a lower water potential; B C D E F G H I J K through a partially/selectively permeable membrane; Hypotonic solutions have a higher water potential than the (cytoplasm of the) cells; Water moves into the cells (by osmosis); Animal cells swell /burst/ref osmotic lysis; reject turgid Plant cells the cytoplasm swells up/cell contents/plasma membrane pushes against the cell wall; (plant cells) becomes turgid/ψ p >0/cell wall prevents osmotic lysis; Hypertonic solutions have a lower water potential than the (cytoplasm of the ) cells; Water moves out of the cells (by osmosis); Animal cells shrink/crenated; reject flaccid In plant cells the cytoplasm shrinks / the (plasma) membrane is pulled away from the cell wall; L Plant cell becomes plasmolysed/ ψ p =0; M N Isotonic solutions have the same water potential as the cytoplasm of the cell; (In isotonic solutions) there is no net movement of water molecules; O At incipient plasmolysis 50% of the cells in a plant tissue will be turgid and 50% will be plasmolysed; 9
Name: Date: Block: Biology 12
 Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
/ The following functional group is a. Aldehyde c. Carboxyl b. Ketone d. Amino
 Section A: Multiple Choice Select the answer that best answers the following questions. Please write your selected choice on the line provided, in addition to circling the answer. /25 1. The following
Section A: Multiple Choice Select the answer that best answers the following questions. Please write your selected choice on the line provided, in addition to circling the answer. /25 1. The following
PMT GCE MARKING SCHEME SUMMER 2016 BIOLOGY - BY1 (LEGACY) 1071/01. WJEC CBAC Ltd.
 GCE MARKING SCHEME SUMMER 016 BIOLOGY - BY1 (LEGACY) 1071/01 INTRODUCTION This marking scheme was used by WJEC for the 016 examination. It was finalised after detailed discussion at examiners' conferences
GCE MARKING SCHEME SUMMER 016 BIOLOGY - BY1 (LEGACY) 1071/01 INTRODUCTION This marking scheme was used by WJEC for the 016 examination. It was finalised after detailed discussion at examiners' conferences
BIOCHEMISTRY. How Are Macromolecules Formed? Dehydration Synthesis or condensation reaction Polymers formed by combining monomers and removing water.
 BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
Review Quizzes Chapters 1-5
 Review Quizzes Chapters 1-5 1.Which of the following constitutes the quarternary level of protein structure? a. bonding between side chains of amino acids b. sequence of amino acids joined by peptide bonds
Review Quizzes Chapters 1-5 1.Which of the following constitutes the quarternary level of protein structure? a. bonding between side chains of amino acids b. sequence of amino acids joined by peptide bonds
Biological Molecules. Carbohydrates, Proteins, Lipids, and Nucleic Acids
 Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Sample Questions BSC1010C Chapters 5-7
 Sample Questions BSC1010C Chapters 5-7 1. Which type of lipid is most important in biological membranes? a. oils b. fats c. wax d. phospholipids e. triglycerides 2. Which type of interaction stabilizes
Sample Questions BSC1010C Chapters 5-7 1. Which type of lipid is most important in biological membranes? a. oils b. fats c. wax d. phospholipids e. triglycerides 2. Which type of interaction stabilizes
Biochemistry Macromolecules and Enzymes. Unit 02
 Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
PMT BY1. Contains a pentose sugar. Found in the nucleus. Thymine is never present. Consists of a double helix. Molecules short lived
 BY1 1. DNA RNA Contains a pentose sugar Found in the nucleus Thymine is never present Consists of a double helix Molecules short lived Associated with ribosomes [Total 6 marks] 2. Organelle Mitochondrion;
BY1 1. DNA RNA Contains a pentose sugar Found in the nucleus Thymine is never present Consists of a double helix Molecules short lived Associated with ribosomes [Total 6 marks] 2. Organelle Mitochondrion;
1. Describe the difference between covalent and ionic bonds. What are the electrons doing?
 Exam 1 Review Bio 212: 1. Describe the difference between covalent and ionic bonds. What are the electrons doing? 2. Label each picture either a Carbohydrate, Protein, Nucleic Acid, or Fats(Lipid). a.
Exam 1 Review Bio 212: 1. Describe the difference between covalent and ionic bonds. What are the electrons doing? 2. Label each picture either a Carbohydrate, Protein, Nucleic Acid, or Fats(Lipid). a.
CARBOHYDRATES. Produce energy for living things Atoms? Monomer Examples? Carbon, hydrogen, and oxygen in 1:2:1 ratio.
 CARBOHYDRATES Produce energy for living things Atoms? Carbon, hydrogen, and oxygen in 1:2:1 ratio Monomer Examples? Sugars, starches MONOSACCHARIDES--- main source of energy for cells Glucose Know formula?
CARBOHYDRATES Produce energy for living things Atoms? Carbon, hydrogen, and oxygen in 1:2:1 ratio Monomer Examples? Sugars, starches MONOSACCHARIDES--- main source of energy for cells Glucose Know formula?
The Chemical Building Blocks of Life. Chapter 3
 The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Biological Molecules
 The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
2.1. thebiotutor. Unit F212: Molecules, Biodiversity, Food and Health. 1.1 Biological molecules. Answers
 thebiotutor Unit F212: Molecules, Biodiversity, Food and Health 1.1 Biological molecules Answers 1 1. δ + H hydrogen bond δ + H O δ - O δ - H H δ + δ+ 1 hydrogen bond represented as, horizontal / vertical,
thebiotutor Unit F212: Molecules, Biodiversity, Food and Health 1.1 Biological molecules Answers 1 1. δ + H hydrogen bond δ + H O δ - O δ - H H δ + δ+ 1 hydrogen bond represented as, horizontal / vertical,
Cells. Variation and Function of Cells
 Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Questions in Cell Biology
 Name: Questions in Cell Biology Directions: The following questions are taken from previous IB Final Papers on the subject of cell biology. Answer all questions. This will serve as a study guide for the
Name: Questions in Cell Biology Directions: The following questions are taken from previous IB Final Papers on the subject of cell biology. Answer all questions. This will serve as a study guide for the
Carbon. Isomers. The Chemical Building Blocks of Life
 The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES
 Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES You Must Know The role of dehydration synthesis in the formation of organic compounds and hydrolysis in the digestion of organic compounds.
Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES You Must Know The role of dehydration synthesis in the formation of organic compounds and hydrolysis in the digestion of organic compounds.
CH 7.2 & 7.4 Biology
 CH 7.2 & 7.4 Biology LABEL THE MEMBRANE Phospholipids Cholesterol Peripheral proteins Integral proteins Cytoskeleton Cytoplasm Extracellular fluid Most of the membrane A phospholipid bi-layer makes up
CH 7.2 & 7.4 Biology LABEL THE MEMBRANE Phospholipids Cholesterol Peripheral proteins Integral proteins Cytoskeleton Cytoplasm Extracellular fluid Most of the membrane A phospholipid bi-layer makes up
1. (a) (i) Ability to distinguish points (close together); 1 (ii) Electrons have a shorter wavelength; 1
 1. (a) (i) Ability to distinguish points (close together); 1 Electrons have a shorter wavelength; 1 (b) (i) Golgi / nucleus / mitochondrion / endoplasmic reticulum / chromosome / larger ribosomes; R Membrane
1. (a) (i) Ability to distinguish points (close together); 1 Electrons have a shorter wavelength; 1 (b) (i) Golgi / nucleus / mitochondrion / endoplasmic reticulum / chromosome / larger ribosomes; R Membrane
Biomolecules. Biomolecules. Carbohydrates. Biol 219 Lec 3 Fall Polysaccharides. Function: Glucose storage Fig. 2.2
 Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
1. I can explain the structure of ATP and how it is used to store energy.
 1. I can explain the structure of ATP and how it is used to store energy. ATP is the primary energy molecule for the cell. It is produced in the mitochondria during cellular respiration, which breaks down
1. I can explain the structure of ATP and how it is used to store energy. ATP is the primary energy molecule for the cell. It is produced in the mitochondria during cellular respiration, which breaks down
AMERICAN NATIONAL SCHOOL General Certificate of Education Advanced Level
 MERIN NTIONL SHOOL General ertificate of Education dvanced Level IOLOGY 9700/01 Paper 1 Multiple hoice lass 1 dditional Materials: Multiple hoice nswer Sheet Soft clean eraser Soft pencil (type or H is
MERIN NTIONL SHOOL General ertificate of Education dvanced Level IOLOGY 9700/01 Paper 1 Multiple hoice lass 1 dditional Materials: Multiple hoice nswer Sheet Soft clean eraser Soft pencil (type or H is
Transport. Slide 1 of 47. Copyright Pearson Prentice Hall
 & Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
& Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
Biological Molecules
 Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Biology 5A Fall 2010 Macromolecules Chapter 5
 Learning Outcomes: Macromolecules List and describe the four major classes of molecules Describe the formation of a glycosidic linkage and distinguish between monosaccharides, disaccharides, and polysaccharides
Learning Outcomes: Macromolecules List and describe the four major classes of molecules Describe the formation of a glycosidic linkage and distinguish between monosaccharides, disaccharides, and polysaccharides
2 3 Carbon Compounds. Proteins. Proteins
 2 3 Carbon Compounds Proteins Proteins Proteins are macromolecules that contain nitrogen, carbon, hydrogen, and oxygen. Proteins are polymers of molecules called amino acids. There are 20 amino acids,
2 3 Carbon Compounds Proteins Proteins Proteins are macromolecules that contain nitrogen, carbon, hydrogen, and oxygen. Proteins are polymers of molecules called amino acids. There are 20 amino acids,
The Plasma Membrane. 5.1 The Nature of the Plasma Membrane. Phospholipid Bilayer. The Plasma Membrane
 5.1 The Nature of the Plasma Membrane The Plasma Membrane Four principal components in animals Phospholipid bilayer Molecules of cholesterol interspersed within the bilayer. Membrane proteins embedded
5.1 The Nature of the Plasma Membrane The Plasma Membrane Four principal components in animals Phospholipid bilayer Molecules of cholesterol interspersed within the bilayer. Membrane proteins embedded
Cells. Prokaryotic vs. Eukaryotic Euakryotic cells are generally one to one hundred times bigger than prokaryotic cells
 Cell Theory Cells 1. All living things are composed of one or more cell 2. Cell is the basic unit of life 3. All cells come from the division of pre-existing cells Cells are divided into 2 categories:
Cell Theory Cells 1. All living things are composed of one or more cell 2. Cell is the basic unit of life 3. All cells come from the division of pre-existing cells Cells are divided into 2 categories:
Macromolecules. copyright cmassengale
 Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Organic Compounds. Compounds that contain CARBON are called organic. Macromolecules are large organic molecules.
 Macromolecules Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
3. Hydrogen bonds form between which atoms? Between an electropositive hydrogen and an electronegative N, O or F.
 Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
Concept 7.1: Cellular membranes are fluid mosaics of lipids and proteins
 Concept 7.1: Cellular membranes are fluid mosaics of lipids and proteins Lipids: Non-polar substances such as fat that contain C, H, O. Phospholipids: Lipid with phosphate group, very abundant in plasma
Concept 7.1: Cellular membranes are fluid mosaics of lipids and proteins Lipids: Non-polar substances such as fat that contain C, H, O. Phospholipids: Lipid with phosphate group, very abundant in plasma
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4
 Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Topic 1 (Old Curriculum) Past Exam Questions Extended Response SOLUTIONS
 1. skeletal muscle fibres are larger / have many nuclei / are not typical cells; fungal hyphae are (sometimes) not divided up into individual cells; unicellular organisms can be considered acellular; because
1. skeletal muscle fibres are larger / have many nuclei / are not typical cells; fungal hyphae are (sometimes) not divided up into individual cells; unicellular organisms can be considered acellular; because
What are the molecules of life?
 Molecules of Life What are the molecules of life? Organic Compounds Complex Carbohydrates Lipids Proteins Nucleic Acids Organic Compounds Carbon- hydrogen based molecules From Structure to Function Ø Carbon
Molecules of Life What are the molecules of life? Organic Compounds Complex Carbohydrates Lipids Proteins Nucleic Acids Organic Compounds Carbon- hydrogen based molecules From Structure to Function Ø Carbon
Human Anatomy & Physiology C H A P T E R
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 2 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 2 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids
 9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids o o o Food is a good source of one or more of the following: protein,
9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids o o o Food is a good source of one or more of the following: protein,
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 3 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 3 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Macro molecule = is all the reactions that take place in cells, the sum of all chemical reactions that occur within a living organism Anabolism:
 Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Biology Chapter 5. Biological macromolecules
 Biology Chapter 5 Biological macromolecules Small molecules (like water and NaCl) have certain properties that arise from the bonds which hold atoms together in a particular arrangement. Many of the molecules
Biology Chapter 5 Biological macromolecules Small molecules (like water and NaCl) have certain properties that arise from the bonds which hold atoms together in a particular arrangement. Many of the molecules
The Amazing Molecule: Water
 The Amazing Molecule: Water All living things are made of chemicals. Understanding life requires an understanding of chemistry. Biochemistry- the chemistry of life helps us understand todays biological
The Amazing Molecule: Water All living things are made of chemicals. Understanding life requires an understanding of chemistry. Biochemistry- the chemistry of life helps us understand todays biological
AQA Biology A-level Topic 1: Biological Molecules
 AQA Biology A-level Topic 1: Biological Molecules Notes Monomers and polymers Monomers are small units which are the components of larger molecules, examples include monosaccharides such as glucose, amino
AQA Biology A-level Topic 1: Biological Molecules Notes Monomers and polymers Monomers are small units which are the components of larger molecules, examples include monosaccharides such as glucose, amino
Movement across the Membrane
 Chapter 8. Movement across the Membrane 2003-2004 1 Cell membrane Cells have an inside & an outside Cell membrane is the boundary Can it be an impenetrable boundary? NO! Why not? The cell needs materials
Chapter 8. Movement across the Membrane 2003-2004 1 Cell membrane Cells have an inside & an outside Cell membrane is the boundary Can it be an impenetrable boundary? NO! Why not? The cell needs materials
(impermeable; freely permeable; selectively permeable)
 BIOL 2457 CHAPTER 3 Part 1 SI 1 1. A is the basic structure of life. 2. The gelatinous inside of the cell is called the. 3. Name the structure that increases the cell s surface area? 4. Name the structure
BIOL 2457 CHAPTER 3 Part 1 SI 1 1. A is the basic structure of life. 2. The gelatinous inside of the cell is called the. 3. Name the structure that increases the cell s surface area? 4. Name the structure
Biology Kevin Dees. Biology Chapter 5. Biological macromolecules
 Biology Chapter 5 Biological macromolecules Small molecules (like water and NaCl) have certain properties that arise from the bonds which hold atoms together in a particular arrangement. Many of the molecules
Biology Chapter 5 Biological macromolecules Small molecules (like water and NaCl) have certain properties that arise from the bonds which hold atoms together in a particular arrangement. Many of the molecules
In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 CHAPTER 3 TEST Cell Structure Circle T if the statement is true or F if it is false. T F 1. Small cells can transport materials and information more quickly than larger cells can. T F 2. Newly made proteins
CHAPTER 3 TEST Cell Structure Circle T if the statement is true or F if it is false. T F 1. Small cells can transport materials and information more quickly than larger cells can. T F 2. Newly made proteins
MEMBRANE STRUCTURE & FUNCTION
 MEMBRANE STRUCTURE & FUNCTION Chapter 8 KEY CONCEPTS Cellular s are fluid mosaics of lipids and proteins Membrane structure results in selective permeability Passive transport is diffusion of a substance
MEMBRANE STRUCTURE & FUNCTION Chapter 8 KEY CONCEPTS Cellular s are fluid mosaics of lipids and proteins Membrane structure results in selective permeability Passive transport is diffusion of a substance
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
Carbohydrates. Mark Scheme. Save My Exams! The Home of Revision. Exam Board 3.1 Biological Molecules Carbohydrates. Page 1.
 Carbohydrates Mark Scheme Level Subject Exam Board Module Topic Booklet A Level Biology AQA 3.1 Biological Molecules 3.1. Carbohydrates Mark Scheme Time Allowed: 59 minutes Score: /4 Percentage: /100 Grade
Carbohydrates Mark Scheme Level Subject Exam Board Module Topic Booklet A Level Biology AQA 3.1 Biological Molecules 3.1. Carbohydrates Mark Scheme Time Allowed: 59 minutes Score: /4 Percentage: /100 Grade
Inorganic compounds: Usually do not contain carbon H 2 O Ca 3 (PO 4 ) 2 NaCl Carbon containing molecules not considered organic: CO 2
 Organic Chemistry The study of carbon-containing compounds and their properties. Biochemistry: Made by living things All contain the elements carbon and hydrogen Inorganic: Inorganic compounds: All other
Organic Chemistry The study of carbon-containing compounds and their properties. Biochemistry: Made by living things All contain the elements carbon and hydrogen Inorganic: Inorganic compounds: All other
Bio 12 Important Organic Compounds: Biological Molecules NOTES Name:
 Bio 12 Important Organic Compounds: Biological Molecules NOTES Name: Many molecules of life are.(means many molecules joined together) Monomers: that exist individually Polymers: Large organic molecules
Bio 12 Important Organic Compounds: Biological Molecules NOTES Name: Many molecules of life are.(means many molecules joined together) Monomers: that exist individually Polymers: Large organic molecules
History of the Cell. History of the Cell 10/24/2013. Unit 3: Cellular Structure and Function. Robert Hooke (1665) Robert Hooke (1665)
 Unit 3: Cellular Structure and Function Mr. Hulse BVHS 2013-2014 Unit 3: Learning Targets 1-9 History of the Cell Robert Hooke (1665) 1 st person to see a cell Observed a piece of cork using a microscope
Unit 3: Cellular Structure and Function Mr. Hulse BVHS 2013-2014 Unit 3: Learning Targets 1-9 History of the Cell Robert Hooke (1665) 1 st person to see a cell Observed a piece of cork using a microscope
Membranes. Chapter 5
 Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Membrane Structure. Membrane Structure. Membrane Structure. Membranes
 Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Organic Compounds. Compounds that contain CARBON are called organic. Macromolecules are large organic molecules.
 Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Gateway to the Cell 11/1/2012. The cell membrane is flexible and allows a unicellular organism to move FLUID MOSAIC MODEL
 Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Gateway to the Cell The cell membrane is flexible and allows a unicellular organism to move Isolates the cell, yet allows communication with its surroundings fluid mosaics = proteins (and everything else)
Most life processes are a series of chemical reactions influenced by environmental and genetic factors.
 Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Plasma Membranes. Plasma Membranes WJEC GCE BIOLOGY 4.6
 4.6 Repeat Fig 3.20A here Fluid Mosaic Model of the Plasma Membrane Carbohydrate chain Glycoprotein Intrinsic Protein Non-polar hydrophobic fatty acid Phospholipids Appearance of the Cell Membrane Seen
4.6 Repeat Fig 3.20A here Fluid Mosaic Model of the Plasma Membrane Carbohydrate chain Glycoprotein Intrinsic Protein Non-polar hydrophobic fatty acid Phospholipids Appearance of the Cell Membrane Seen
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
chloroplasts cell membrane nucleus nucleus cell wall vacuole cytoplasm Animal cell Plant cell Investigating Cells Summary Booklet page 1
 1. General: state that cells are the basic units of living things. Sections of living tissue, when examined under a microscope are seen to be made up of similar units. These units consist of cytoplasm,
1. General: state that cells are the basic units of living things. Sections of living tissue, when examined under a microscope are seen to be made up of similar units. These units consist of cytoplasm,
The Building blocks of life. Macromolecules
 The Building blocks of life Macromolecules 1 copyright cmassengale 2 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 3 LIFE ON EARTH IS CARBON-BASED
The Building blocks of life Macromolecules 1 copyright cmassengale 2 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 3 LIFE ON EARTH IS CARBON-BASED
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
The Cell Membrane AP Biology
 The Cell Membrane 2007-2008 Warm Up What would happen if you gave a patient an IV of pure water? a. Their blood cells would shrink. b. Their blood cells would burst. c. The patient would slowly become
The Cell Membrane 2007-2008 Warm Up What would happen if you gave a patient an IV of pure water? a. Their blood cells would shrink. b. Their blood cells would burst. c. The patient would slowly become
Biology 12 Cell Structure and Function. Typical Animal Cell
 Biology 12 Cell Structure and Function Typical Animal Cell Vacuoles: storage of materials and water Golgi body: a series of stacked disk shaped sacs. Repackaging centre stores, modifies, and packages proteins
Biology 12 Cell Structure and Function Typical Animal Cell Vacuoles: storage of materials and water Golgi body: a series of stacked disk shaped sacs. Repackaging centre stores, modifies, and packages proteins
Aim: What are the molecules of life?
 Aim: What are the molecules of life? Do Now: List the elements & compounds cycled through ecosystems. Homework: Read pp. 59 63 P. 63 # 1,2,3,4,5 Vocabulary: Carbohydrate, lipid, protein, amino acid, nucleic
Aim: What are the molecules of life? Do Now: List the elements & compounds cycled through ecosystems. Homework: Read pp. 59 63 P. 63 # 1,2,3,4,5 Vocabulary: Carbohydrate, lipid, protein, amino acid, nucleic
Cell Compounds and Biological Molecules. Biology 12 Unit 2 Cell Compounds and Biological Molecules Inquiry into Life pages 20-44
 Cell Compounds and Biological Molecules Biology 12 Unit 2 Cell Compounds and Biological Molecules Inquiry into Life pages 20-44 Basic Chemistry Matter anything that has mass and volume Element comprises
Cell Compounds and Biological Molecules Biology 12 Unit 2 Cell Compounds and Biological Molecules Inquiry into Life pages 20-44 Basic Chemistry Matter anything that has mass and volume Element comprises
CELL TRANSPORT and THE PLASMA MEMBRANE. SB1d. Explain the impact of water on life processes (i.e., osmosis, diffusion).
 CELL TRANSPORT and THE PLASMA MEMBRANE SB1d. Explain the impact of water on life processes (i.e., osmosis, diffusion). What if What would happen if an organism could not get energy or get rid of wastes?
CELL TRANSPORT and THE PLASMA MEMBRANE SB1d. Explain the impact of water on life processes (i.e., osmosis, diffusion). What if What would happen if an organism could not get energy or get rid of wastes?
BSC Exam I Lectures and Text Pages
 BSC 2010 - Exam I Lectures and Text Pages I. Intro to Biology (2-29) II. Chemistry of Life Chemistry review (30-46) Water (47-57) Carbon (58-67) Macromolecules (68-91) III. Cells and Membranes Cell structure
BSC 2010 - Exam I Lectures and Text Pages I. Intro to Biology (2-29) II. Chemistry of Life Chemistry review (30-46) Water (47-57) Carbon (58-67) Macromolecules (68-91) III. Cells and Membranes Cell structure
Outline. Membrane Structure and Function. Membrane Models Fluid-Mosaic. Chapter 5
 Membrane Structure and Function Chapter 5 Membrane Models Fluid-Mosaic Outline Plasma Membrane Structure and Function Protein Functions Plasma Membrane Permeability! Diffusion! Osmosis! Transport Via Carrier
Membrane Structure and Function Chapter 5 Membrane Models Fluid-Mosaic Outline Plasma Membrane Structure and Function Protein Functions Plasma Membrane Permeability! Diffusion! Osmosis! Transport Via Carrier
The Transport of Materials Across Cell Membranes
 The Transport of Materials Across Cell Membranes EK 2.B.1.b. LO 2.10 The Plasma Membrane 2 EK 2.B.1.b. LO 2.10 The Plasma Membrane The cell membrane is said to be semi permeable or selectively permeable
The Transport of Materials Across Cell Membranes EK 2.B.1.b. LO 2.10 The Plasma Membrane 2 EK 2.B.1.b. LO 2.10 The Plasma Membrane The cell membrane is said to be semi permeable or selectively permeable
Chapter 2. Chemical Composition of the Body
 Chapter 2 Chemical Composition of the Body Carbohydrates Organic molecules that contain carbon, hydrogen and oxygen General formula C n H 2n O n -ose denotes a sugar molecule Supply energy Glucose Complex
Chapter 2 Chemical Composition of the Body Carbohydrates Organic molecules that contain carbon, hydrogen and oxygen General formula C n H 2n O n -ose denotes a sugar molecule Supply energy Glucose Complex
Biology EOC Review. Saturday Session
 Biology EOC Review Saturday Session Cells DNA Ribosome Cytoplasm Cell Membrane Prokaryote Eukaryote Prokaryotic Bacteria Flagellum Cell Membrane (Plasma) Cell Wall Eukaryotic Animal Mitochondria Ribosome
Biology EOC Review Saturday Session Cells DNA Ribosome Cytoplasm Cell Membrane Prokaryote Eukaryote Prokaryotic Bacteria Flagellum Cell Membrane (Plasma) Cell Wall Eukaryotic Animal Mitochondria Ribosome
9700 BIOLOGY 9700/22 Paper 2 (Structured Questions AS), maximum raw mark 60
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the October/November 2009 question paper for the guidance of teachers 9700 BIOLOGY
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the October/November 2009 question paper for the guidance of teachers 9700 BIOLOGY
Plasma Membrane Function
 Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Ch3: Cellular Transport Review KEY
 Ch3: Cellular Transport Review KEY OSMOSIS Label the pictures below ( isotonic, hypertonic, or hypotonic environments) hypotonic hypertonic isotonic hypertonic means there is a GREATER concentration of
Ch3: Cellular Transport Review KEY OSMOSIS Label the pictures below ( isotonic, hypertonic, or hypotonic environments) hypotonic hypertonic isotonic hypertonic means there is a GREATER concentration of
Macromolecules. 3. There are several levels of protein structure, the most complex of which is A) primary B) secondary C) tertiary D) quaternary
 Macromolecules 1. If you remove all of the functional groups from an organic molecule so that it has only carbon and hydrogen atoms, the molecule become a molecule. A) carbohydrate B) carbonyl C) carboxyl
Macromolecules 1. If you remove all of the functional groups from an organic molecule so that it has only carbon and hydrogen atoms, the molecule become a molecule. A) carbohydrate B) carbonyl C) carboxyl
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Cells and Their Environment Chapter 8. Cell Membrane Section 1
 Cells and Their Environment Chapter 8 Cell Membrane Section 1 Homeostasis Key Idea: One way that a cell maintains homeostasis is by controlling the movement of substances across the cell membrane. Homeostasis
Cells and Their Environment Chapter 8 Cell Membrane Section 1 Homeostasis Key Idea: One way that a cell maintains homeostasis is by controlling the movement of substances across the cell membrane. Homeostasis
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 3 The Cellular Level of Organization Introduction The purpose of the chapter is to: 1. Introduce the parts of a cell 2. Discuss the importance
Principles of Anatomy and Physiology 14 th Edition CHAPTER 3 The Cellular Level of Organization Introduction The purpose of the chapter is to: 1. Introduce the parts of a cell 2. Discuss the importance
Review: Cellular Transport
 Review: Cellular Transport OSMOSIS 1. Label the pictures below ( isotonic, hypertonic, or hypotonic). The dots represent solutes. A. B. C. 2. means there is a GREATER concentration of solute molecules
Review: Cellular Transport OSMOSIS 1. Label the pictures below ( isotonic, hypertonic, or hypotonic). The dots represent solutes. A. B. C. 2. means there is a GREATER concentration of solute molecules
Unit 2 Warm Ups. Equilibrium
 Unit 2 Warm Ups Equilibrium 1. Cell wall 2. Mitochondria 3. Chloroplast 4. Vesicle 5. Vacuole 6. Rough Endoplasmic Reticulum 7. Smooth Endoplasmic Reticulum 8. Cytoskeleton 9. Lysosomes 10.Cell Membrane
Unit 2 Warm Ups Equilibrium 1. Cell wall 2. Mitochondria 3. Chloroplast 4. Vesicle 5. Vacuole 6. Rough Endoplasmic Reticulum 7. Smooth Endoplasmic Reticulum 8. Cytoskeleton 9. Lysosomes 10.Cell Membrane
First discovered in 1665 since then every organism observed with microscopes shows cells
 The Cell Cell theory (1838): 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
The Cell Cell theory (1838): 1. All organisms are composed of one or more cells, and the life processes of metabolism and heredity occur within these cells. 2. Cells are the smallest living things, the
Cytoskeleton. Provide shape and support for the cell. Other functions of the cytoskeleton. Nucleolus. Nucleus
 Chapter 4: Cell Structure and Function Cytoskeleton The cytoskeleton is a network of fibers that organizes structures and activities in the cell. Microtubules (the largest) Intermediate fibers Microfilaments
Chapter 4: Cell Structure and Function Cytoskeleton The cytoskeleton is a network of fibers that organizes structures and activities in the cell. Microtubules (the largest) Intermediate fibers Microfilaments
Bio. 221 Exam 1 W 09/26/07 8 AM, 9 AM
 Bio. 221 Exam 1 W NAME 09/26/07 8 AM, 9 AM Multiple Choice/Matching: Use Scantron for Questions 1-25 1 pt each. 1) When a membrane-bound vesicle in the cytoplasm fuses with the cell membrane and releases
Bio. 221 Exam 1 W NAME 09/26/07 8 AM, 9 AM Multiple Choice/Matching: Use Scantron for Questions 1-25 1 pt each. 1) When a membrane-bound vesicle in the cytoplasm fuses with the cell membrane and releases
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Week 1 Multiple Choice Questions: 1. A substrate molecule may be bound to the active site of an enzyme by all of the following EXCEPT
 WEEK 1: Chemistry of Life (7%) Week 1 Concepts: How do the unique chemical and physical properties of water make life on earth possible? What is the role of carbon in the molecular diversity of life? How
WEEK 1: Chemistry of Life (7%) Week 1 Concepts: How do the unique chemical and physical properties of water make life on earth possible? What is the role of carbon in the molecular diversity of life? How
Chapter 8 Cells and Their Environment
 Chapter Outline Chapter 8 Cells and Their Environment Section 1: Cell Membrane KEY IDEAS > How does the cell membrane help a cell maintain homeostasis? > How does the cell membrane restrict the exchange
Chapter Outline Chapter 8 Cells and Their Environment Section 1: Cell Membrane KEY IDEAS > How does the cell membrane help a cell maintain homeostasis? > How does the cell membrane restrict the exchange
Membranes. Chapter 5. Membrane Structure
 Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Chapter 2: The Chemical Level of. Organization. Copyright 2009, John Wiley & Sons, Inc.
 Chapter 2: Organization The Chemical Level of Question Of the following functions, the major propose of RNA is to A. Function in the synthesis of protein. B. Transmit genetic information to offspring.
Chapter 2: Organization The Chemical Level of Question Of the following functions, the major propose of RNA is to A. Function in the synthesis of protein. B. Transmit genetic information to offspring.
Slide 2 of 47. Copyright Pearson Prentice Hall. End Show
 2 of 47 7-3 Cell Boundaries All cells are surrounded by a thin, flexible barrier known as the cell membrane. Many cells also produce a strong supporting layer around the membrane known as a cell wall.
2 of 47 7-3 Cell Boundaries All cells are surrounded by a thin, flexible barrier known as the cell membrane. Many cells also produce a strong supporting layer around the membrane known as a cell wall.
Unit 3 : Homeostasis and Cell Transport
 Unit 3 : Homeostasis and Cell Transport A. Maintaining Homeostasis 1. Homeostasis process of regulating and maintaining a constant internal env. 2. Need to maintain homeostasis of water levels, glucose
Unit 3 : Homeostasis and Cell Transport A. Maintaining Homeostasis 1. Homeostasis process of regulating and maintaining a constant internal env. 2. Need to maintain homeostasis of water levels, glucose
Movement of substances across the cell membrane
 Ch 4 Movement of substances across the cell membrane Think about (Ch 4, p.2) 1. The structure of the cell membrane can be explained by the fluid mosaic model. It describes that the cell membrane is mainly
Ch 4 Movement of substances across the cell membrane Think about (Ch 4, p.2) 1. The structure of the cell membrane can be explained by the fluid mosaic model. It describes that the cell membrane is mainly
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
The further from the nucleus, the higher the electron s energy Valence shell electrons participate in biological reactions
 Chemistry of Life Revision: The further from the nucleus, the higher the electron s energy Valence shell electrons participate in biological reactions Atoms exchange electrons with other elements to form
Chemistry of Life Revision: The further from the nucleus, the higher the electron s energy Valence shell electrons participate in biological reactions Atoms exchange electrons with other elements to form
Cell Membrane (Transport) Notes
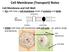 Cell Membrane (Transport) Notes Cell Membrane and Cell Wall: ALL cells have a cell membrane made of proteins and lipids protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump SOME cells
Cell Membrane (Transport) Notes Cell Membrane and Cell Wall: ALL cells have a cell membrane made of proteins and lipids protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump SOME cells
Describe the Fluid Mosaic Model of membrane structure.
 Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membranebound organelles. In this topic, we will examine the structure and
Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membranebound organelles. In this topic, we will examine the structure and
