Gastro retentive Drug Delivery System: A Review
|
|
|
- Rachel Goodwin
- 5 years ago
- Views:
Transcription
1 Volume 1, issue1 (2011), Review Article International Journal of Pharmaceutical Research & Allied Sciences Gastro retentive Drug Delivery System: A Review Shivram Shinde*, Imran Tadwee, Sadhana Shahi Department of pharmaceutics Government College of pharmacy, Osmanpura, Aurangabad (M.S.) INDIA id: shindebs2@gmail.com Abstract A Controlled release dosage forms have been extensively used to improve therapy with several important drugs. However, the development processes are faced with several physiological difficulties such as the inability to restrain and localize the system within the desired region of the gastrointestinal tract and the highly variable nature of the gastric emptying process. This variability may lead to unpredictable bioavailability and times to achieve peak plasma levels. The purpose of writing this review on gastroretentive drug delivery systems was to compile the recent literature with special focus on various gastroretentive approaches that have recently become leading methodologies in the field of site-specific orally administered controlled release drug delivery. In order to understand various physiological difficulties to achieve gastric retention, we have summarized important factors controlling gastric retention. Afterwards, we have reviewed various gastroretentive approaches designed and developed until now, i.e. high density (sinking), floating, bio- or mucoadhesive, expandable, unfoldable, super porous hydrogel and magnetic systems. Finally, advantages of gastroretentive drug delivery systems were covered in detail. Keywords: Gastroretentive, GRDDS, Oral route. Various Approaches Objective The present study attempts to give an insight into the gastroretentive drug delivery systems, and gastric floating tablets, in particular. These have attracted the interest of many formulators due to their advantages over the conventional drug delivery systems, recently. The study highlights these advantages with reference to the various types of gastroretentive drug delivery systems, as well as provides an overview of the recent advances that have taken place in this arena. Introduction Oral administration is the most convenient and preferred means of any delivery to the systemic circulation. Oral controlled release drug delivery have recently been of increasing interest in Pharmaceutical field to achieve improved therapeutic advantages such as ease of dosing administration, patient compliance and flexibility in formulation. Drugs that are easily absorbed from gastro intestinal tract (GIT) and have short half life are eliminated quickly from systemic circulation. Frequent dosing of these drugs is required to achieve therapeutic activity. To avoid these limitations, the development of oral sustained controlled release formulation is an attempt to release the drug slowly into the gastro intestinal tract and maintain an effective drug concentration in the systemic circulation for long time. After oral administration, such a drug delivery would be retain in the stomach and release the drug in a controlled manner so that the drug could be supplied continuously to its absorption site in gastro intestinal tract 1. Gastro retentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastro intestinal tract for local and systemic effect. Gastro retentive dosage form can remain in the gastric region for a longer period and hence significantly prolong the gastric retention time (GRT) of drugs. Over the last few decades several gastro retentive drug delivery approaches been designed and developed, including high density (sinking) system that is retained in the 2,3,4.mucoadhesive systems that causes bioadhesion to stomach mucosa, unfoldable, extendable or swellable system which limits emptying of the 1
2 dosage forms through the pyloric sphincter of the stomach, super porous hydrogel system, magnetic system etc. The current review deals with the various gastro retentive approaches that have been recently become leading methodologies in the field of site-specific orally administered controlled release drug delivery systems. Gastric emptying of dosage form is an extremely variable process and ability to prolong and control the emptying time is a valuable asset for dosage forms, which resides in the stomach for prolong period of time than conventional dosage forms. Several difficulties are faced to designing controlled delivery system for better absorption and enhance bioavailability. One of such difficulty is the inability to confine the dosage form in the desired area of the gastrointestinal tract. Drug absorption from GIT is complex procedure and is subject to many variables. It is widely acknowledged that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa. Thus small intestinal transit time is an important parameter for drugs that are incompletely absorbed. Basic human physiology with the details of gastric emptying, motility patterns and physiological and formulation variables affecting the cosmic emptying is summarized. Gastro retentive systems can remain in the gastric region for several hours and hence significantly prolongs the gastric residence time if drugs. Prolong gastric retention improves bioavailability, reduces drug waste and improves solubility for drugs that are less soluble in high ph environment. It has application also for local drug delivery to the stomach and proximal small intestine 5. Gastro retention helps to provide better availability of new products with new therapeutic possibilities and substantial benefits for patients. The controlled gastric retention of solid dosage forms may be achieved by the mechanism of mucoadhesion 6 floatation, sedimentation, expansion, modified shapes systems or by the simultaneous administration of pharmacological agents 7, 8, 9 that delay gastric emptying. Based on these approaches, classification of floating drug delivery system (FDDS) has been described in detail. In-vivo/ in-vitro evaluation of FDDS has been discussed by scientist to assess the efficiency of such system. Several recent examples have been reported showing the efficiency of such systems for drugs with bioavailability problems. The need for Gastroretentive dosage forms (GRDFs) has led to extensive efforts in both academia and industry towards the development of such drug delivery system. Floating drug delivery system has a bulk density lower than gastric fluids and thus remain buoyant in the stomach for prolong period of time, without affecting the gastric emptying rate. While the system is floating on the gastric content, the drug is released slowly at a desired rate from the stomach. This results in an increase in the GRT and a better control of fluctuation in the plasma drug concentrations. Number of FDDS involving various technologies carrying their own advantages and the limitation were developed such as, single and multiple unit hydrodynamically balanced systems (HBS), single and multiple unit gas generating system, hollow microspheres and raft forming systems. The current review deals with the development of GRDDS, by using natural polymers that has recently become the leading methodology in this field. Natural polymers are good candidate for oral cavity drug delivery. Also because biological property such as non toxicity, biocompatibility and bio degradability. Natural polymer is promising candidate for the enhancement of absorption of drug using floating drug delivery system 10. Basic physiology of Gastrointestinal Tract: Anatomically the stomach is divided into three regions: Fundus, body and antrum (pylorus). The proximal part made of fundus and body act as reservoir for undigested materials where as the antrum is the main site for mixing motions and act as pump from gastric emptying by propelling the actions. Gastric emptying occurs during fasting as well as feed state. The patent of motility is however distinct in 2 states. During fasting state an inter digestive series of electrical events takes place, which cycle both through stomach and intestine every 2 to 3 hours 11. This is called the interdigestive myoelectric cycle or migrating myloelectric cycle (MMC), which is further divided into following 4 phases as described by Wilson and Washington Phase I (basal phase) lasts from 40 to 60 minutes with contractions. 2. Phase II (pre burst phase) lasts for 40 to 60 minutes with intermittent action potential and contractions. As the phase progress the intensity and frequency also increases gradually. 3. Phase III (burst phase) last for 4 to 6 minutes. It includes intense and regular contractions for short period. It is due to this walve that all the undigested 2
3 material is swept out of the stomach down to the small intestine.it is also known as the house keeper wave. 4. Phase IV last for 0 to 5 minutes and occurs between phases III and I of 2 consecutive cycles. After the ingestion of a mixed meal, the pattern of contractions changes from fasted to that of fed state. This is also known as digestive motility pattern and comprises continuous contractions as in phase II of fasted state. These contractions results in reducing the size of food particles (to less than 1mm) which are propelled toward the pylorus in a suspension form. During the fed state onset of MMC is delayed resulting in slowdown of gastric emptying rate. Scintigraphic studies determining gastric emptying rates revealed that orally administered controlled release dosage form are subjected to basically two complications that of short gastric residence time and unpredictable gastric emptying rate. Factors affecting gastric retention: Gastric residence time of an oral dosage form is affected by several factors. To pass through the pyloric valve into the small intestine the particle size should be in the range of 1 to 2 mm 13 The ph of the stomach in fasting state is 1.5 to 2.0 and in fed state is 2.0 to 6.0. A large amount of water administered with an oral dosage form raises the ph of stomach contents to 6.0 to 9.0. stomach doesn.t get time to produce sufficient acid when the liquid empties the stomach, hence generally basic drugs have better chances of dissolving in fed state than in fasting state. The rate of gastric emptying depends mainly on viscosity, volume and caloric content of meals. Nutritive density of meals helps to determine gastric emptying time. It does not make any difference whether the meal has high protein, fat or carbohydrate contents as long as the caloric content is the same. However increase in acidity and caloric value slows down gastric emptying time. Biological factors such as age, body mass index (BMI), gender, posture and diseased states (diabetes, Chron.s disease) influences gastric emptying. In the case of elderly persons, gastric emptying is slowed down. Generally females have slower gastric emptying rate than males. Stress increases rate while depression slows it down 11. The resting volume of the stomach is 25 to 50 ml. volume of liquids administered affects the gastric emptying time. When volume is large, the emptying is faster. Fluids taken at body temperature leave the stomach faster than colder or warmer fluids. Studies have revealed that gastric emptying of a dosage form in fed state can also be influenced by its size. Small size tablets leave the stomach during the digestive phase while the large size tablets are emptied during the housekeeping waves. It has been demonstrated using radiolabelled technique that there is a difference between gastric emptying times of a liquid, digestible solid and indigestible solids. It was suggested that emptying of large (91mm) indigestible objects from stomach was dependent upon inter digestive migrating complex. When liquid and indigestible solids are present in the stomach, it contracts 3 to 4 times per minute leading to the movement of the contents through partially opened pylorus. Indigestible solids larger than the pyloric opening are propelled back and several phases of myoelectric activity take place, when the pyloric opening increases in size during the housekeeping wave and allows the sweeping of the indigestible solids. Studies have shown that the gastric residence time (GRT) can be significantly increases under the fed state since the MMC is delayed 12. Several formulation parameters can affect the gastric residence time. More reliable gastric emptying patterns are observed for multiparticulate formulations as compared with single unit formulations, which suffer from.all or none concept.. As the units of multiparticulate to a lesser extent by the transit time of food compared with single unit formulation 13. Size and shape of dosage unit also affects the gastric emptying, it is reported that tetrahedron trans ring shaped devices have better gastric residence time as compared with other shapes. The diameter of dosage unit is also equally important as a formulation parameter. Dosage form having a diameter of more than 7.5 mm shows a better gastric residence time compared with one having 9.9 mm. The density of a dosage form also affects the gastric emptying rate. A buoyant dosage form having density of less than that of the gastric fluids floats. Since it is away from the pyloric sphincter, the dosage unit is retain in the stomach for prolong period. It is been studied the effect of buoyancy, posture and the nature of meals on the gastric emptying processes invivo using gamma scintigraphy To perform these studies, floating and non floating capsules of three different sizes having a diameter of 4.8 mm (small units), 7.5mm 3
4 (medium units) and 9.9 mm (large units) were formulated. On comparison of floating and non floating dosage units, it was concluded that regardless of their sizes the floating dosage units remain buoyant on the gastric contents throughout their residence in the gastrointestinal tract, while the non floating dosage unit sinks and remain in the lower part of stomach. Floating units away from the gastro duodenal junction were protected from the peristaltic waves during digestive phase while the non floating forms stayed close to the pylorus and were subjected to propelling and retropelling waves of the digestive phase. It was also observed that of the floating and non floating units, the floating units were had a longer gastric residence time for small and medium units while no significant difference was seen between two types of large unit dosage forms. When subjects were kept in the supine position, it was observed that the floating form could only prolong just stay because of their size, otherwise buoyancy remains no longer and advantage for gastric retention. A comparison was made to study the affect of fed and non fed stages on gastric emptying. For these study all subjects remaining in an upright position were given a light breakfast and another similar group was fed with a succession of meals given at a normal time intervals. It was concluded that as meals were given at the time when previous digestive phase had not completed, the floating form buoyant in the stomach could retain its position for another digestive phase as it was carried by the peristaltic wave in the upper part of stomach. Suitable drug candidates for gastro retention: Various drugs have their greatest therapeutic effect when released in the stomach, particularly when the release is prolong in a continuous, controlled manner. Drugs delivered in this manner have a lower level of side effect and provide their therapeutic effects without the need for repeated dosage with a low dosage frequency. Sustain release in the stomach is also useful for therapeutic agents that the stomach does not readily absorbed, since sustain release prolongs contact time of the agent in the stomach or in the upper part of small intestine, which is where absorption occur and contact time is limited under normal or average condition, Example. material passes through the small intestine in as little as 1-3 hrs 14. In general, appropriated candidate CRGRDF are molecules that have poor colonic absorption but are characterizes by better absorption, properties at the upper part of GIT: Narrow absorption window in GIT, E.g. Riboflavin and Levodopa. Primarily absorbed from stomach and upper part of GIT, Example: Calcium supplements, Chlordizepoxide and Cinnarazine. Drugs that are locally in the stomach, Example. Antacids and Misoprostol. Drugs that degrade in the colon, Example. Ranitidine HCl and Metronidazole. Drugs that disturbs normal colonic bacteria, Example. Amoxicilline trihydrate. Table 1: Good candidates for gastroretentive drug delivery system 15 S.N Drug & Category o 1 Verapamil Calcium channel blocker 2 Nifedipine Calcium channel blocker 3 Omeprazole Proton pump inhibitor 4 Atenolol Antihypertesive 5 Propranolol Antihypertensive Bioavalibility 20-35% 45-65% 35-60% 40-50% 4-26% 6 Verapamil 18-35% Antihypertensive 7 Diltiazem Calcium 40% channel blocker 8.Lidocaine Local 35% anaesthetic 9 Clarithromycin 50% Antibiotic 10 Ramipril ACE inhibitor 28% The need for gastro retentive dosage form (GRDFs) has led to extensive efforts in both academic and industry towards the development of such delivery systems. These efforts resulted in GRDFs that were designated, in large part, based on following approaches. 4
5 Approaches to gastric retention: Various approaches have been pursued to increase the retention of an oral dosage form in the stomach, for example, bioadhesive approach in which the adhesive capacity of some polymer with glycoprotein is closely applied to the epithelial surface of stomach. Other approaches include: high density and low density approach.fig.1 1) High density approach: For preparing such type of formulations, the density of the pellets should be higher than the stomach fluid. It would be at least 1.50 g/ml. In this type, the drug can be coated or mixed with heavy, nontoxic materials such as barium sulfate, titanium dioxide, etc. 2) Low density approach: Floating systems come under low density approach. In this approach, the density of pellets should be less than 1 g/ml, so as to float the pellets or tablets in the gastric fluid and, release the drug slowly for a longer period of time. This type is also called as Hydrodynamically Balanced System (HBS). retention principle, a minimal level of floating force (F) is also required to keep the dosage form reliably buoyant on the surface of the meal. To measure the floating force kinetics, a novel apparatus for determination of resultant weight has been reported in the literature. The apparatus operates by measuring continuously the force equivalent to F (as a function of time) that is required to maintain the submerged object. The object floats better if F is on the higher positive side as shown in fig. 2. This apparatus helps in optimizing FDDS with respect to stability and durability of floating forces produced in order to prevent the drawbacks of unforeseeable intragastric buoyancy capability variations 16. F = F buoyancy F gravity = (Df Ds) gv Where, F= total vertical force, Df = fluid density, Ds= object density, v = volume and g = acceleration due to gravity Fig. 2: Mechanism of floating systems, GF= Gastric fluid Fig. 1: Diagram of Gastro retentive drug delivery system (low density and high density systems) 3) Floating Drug Delivery systems and its mechanism: Floating drug delivery systems (FDDS) have bulk density lesser than gastric fluids, so they remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents, the drug is released slowly at the desired rate from the system as shown in fig. 2(a). However, besides a minimal gastric content needed to allow the proper achievement of the buoyancy Classification of floating system: 1). Single Unit Floating Dosage Systems a) Effervescent system b) Non-effervescent Systems 2). Multiple Unit Floating Dosage Systems a) Effervescent Systems b) Non-effervescent Systems c) Hollow microspheres 3). Raft forming system 1). Single Unit Floating Dosage Systems: a) Effervescent systems Effervescent floating drug delivery systems generate gas (CO2), thus reduce the density of the system, and remain buoyant in the stomach for a prolonged period of time and release the drug slowly at a desired rate. The main ingredients of effervescent system include swellable polymers like chitosan, methyl cellulose and effervescent compounds such as citric acid, sodium bicarbonate, citric acid and tartaric acid 17. Penners et al prepared 5
6 an expandable tablet containing mixture of polyvinyl lactams and polyacrylates that swells rapidly in an aqueous environment and thus, stays in stomach over an extended period of time. In addition to this, gas-forming agents were also incorporated so as soon as the gas formed, the density of the system was reduced and thus, the system tended to float in the gastric environment 18. prepared the effervescent floating tablet offamotidine. They found that the addition of gelforming polymermethocel (K100 and K15M) and gas-generating agent sodium bicarbonate along with citric acid was essential to achieve in vitro buoyancy. The drug release from the tablets was sufficiently sustained and non-fickian transport of the drug from tablets was confirmed 19. b) Non effervescent system Non-effervescent systems commonly use gelforming or highly swellable cellulose type hydrocolloids, polysaccharides and matrix forming polymers such as polycarbonate, polyacrylate, polymethacrylate, and polystyrene. The formulation method includes a simple approach of thoroughly mixing the drug and the gel-forming hydrocolloid. After oral administration, this dosage form swells in contact with gastric fluids and attains a bulk density of less than 1 g/ml. The air entrapped within the swollen matrix imparts buoyancy to the dosage form. Iannuccelli et al prepared air compartment multiple unit system for prolonged gastric residence. These units were composed of a calcium alginate core separated by an air compartment from membrane of calcium alginate. The porous structure generated by leaching of polyvinyl alcohol (PVA), which was employed as water soluble additive in coating composition, was found to increase the membrane permeability preventing the air compartment shrinkage. The ability of floatation increases with increase in PVA, molecular Weight 20. Wu et al prepared floating sustained release tablets of nimodipine by using HPMC and PEG Prior to formulation of floating tablets, nimodipine was incorporated into poloxamer-188 solid dispersion after which it was directly compressed into floating tablets. It was observed that by increasing the HPMC and decreasing the PEG 6000 content, a decline in in vitro release of nimodipine occurred. 21 Single unit formulations are associated with problems such as sticking together or being obstructed in the gastrointestinal tract, which may have a potential danger of producing irritation. The main drawback of such system is all or none phenomenon. In such cases, there is a danger of passing of the dosage form to intestinal part at the time of house-keeper waves. To overcome this difficulty multiple, unit dosage forms are designed. Fig. 3: a) Different layers b) Mechanism of floatation via CO2 liberation Chen et al studied the effect of formulation variables on in vitro performance of floating sustained release of verapamil. The formulations were comprised of variables like polymers excipients, polymer content, density of capsule and amount of effervescent Agents 22. b) Non effervescent systems: Not many reports were found in the literature on non-effervescent multiple unit systems, as compared to the effervescent systems. However, few workers have reported the possibility of developing such system containing indomethacin, using chitosan as the polymeric excipient. A multiple unit HBS containing indomethacin as a model drug prepared by extrusion process is reported. A mixture of drug, chitosan and acetic acid is extruded through a needle, and the extrudate is cut and dried. Chitosan hydrates and floats in the acidic media, and the required drug release could be obtained by modifying the drug-polymer ratio 23. 2). Multiple Unit Floating Systems: Multiple unit dosage forms may be an attractive alternate since they have been shown to reduce inter and intra-subject variabilities in drug absorption as well as to lower the possibility of dose dumping. Various multiple unit floating systems have been developed in 6
7 different forms, and using principles such as air compartment multiple unit system, hollow microspheres prepared by emulsion solvent diffusion method, beads prepared by emulsion gelation method. Use of effervescent and swellable polymer is another approach for preparing multiple unit FDDS. a) Effervescent system: Ichikawa et al developed a new multiple type of floating dosage system composed of effervescent layers and swellable membrane layers coated on sustained release pills. The inner layer of effervescent agents containing sodium bicarbonate and tartaric acid was divided into two sublayers to avoid direct contact between the two agents. These sublayers were surrounded by a swellable polymer membrane containing polyvinyl acetate and purified shellac. When this system was immersed in the buffer at 37ºC, it settled down and the solution permeated into the effervescent layer through the outer swellable membrane. CO2 was generated by the neutralization reaction between the two effervescent agents, producing swollen pills (like balloons) with a density less than 1.0 g/ml. It was found that the system had good floating ability independent of ph and viscosity and the drug (Para-amino benzoic acid) released in a sustained manner as shown in fig.3 (a), (b) Thanoo et al. developed polycarbonate microspheres by solvent evaporation technique. Polycarbonate in dichloromethane was found to give hollow microspheres that floated on water and simulated biofluids, as evidenced by scanning electron microscopy (SEM). High drug loading was achieved and drug-loaded microspheres were able to float on gastric and intestinal fluids. It was found that increasing the drugn to polymer ratio increased both their mean particle size and release rate of drug 24. Sheth et al. developed hydrodynamically balanced capsules containing mixture of drug and hydrocolloids containing a homogeneous mixture of drug and the hydrocolloid in a capsule, which upon contact with gastric fluid acquired and maintained a bulk density of less than 1, thereby being buoyant on the gastric contents of stomach, until all the drug was released as shown in fig Fig. 4: Working principle of hydro dynamically balanced system c) Hollow microspheres: Both natural and synthetic polymers have been used to prepare floating microspheres. Joseph et al. developed a floating dosage form of piroxicam based on hollow polycarbonate microspheres. The microspheres were prepared by the solvent evaporation technique. Encapsulation efficiency of ~95% was achieved. In vivo studies were performed in healthy male albino rabbits. Pharmacokinetic analysis was derived from plasma concentration versus time plot and revealed that the bioavailability from the piroxicam microspheres alone was 1.4 times that of the free drug and 4.8 times that of a dosage form consisting of microspheres plus the loading dose and was capable of sustained delivery of the drug over a prolongedperiod 26. 3) Raft forming system: Raft forming systems have received much attention for the drug delivery for gastrointestinal infections and disorders. The mechanism involved in the raft formation includes the formation of viscous cohesive gel in contact with gastric fluids, wherein each portion of the liquid swells forming a continuous layer called a raft. This raft floats on gastric fluids because of low bulk density created by the formation of CO2. Usually, the system ingredients includes a gel forming agent and alkaline bicarbonates or carbonates responsible for the formation of CO2 to make the system less dense and float on the gastric fluids. Jorgen et al described an antacid raft forming floating system. The system contains a gel forming agent (e.g. 7
8 sodium alginate), sodium bicarbonate and acid neutralizer, which forms a foaming sodium alginate gel (raft), which when comes in contact with gastric fluids, the raft floats on the gastric fluids and prevents the reflux of the gastric contents (i.e. gastric acid) into the esophagus by acting as a barrier between the stomach and esophagus 27. Evaluation of Floating Drug delivery system 1) Evaluation of powder blend a) Angle of Repose b) Bulk Density c) Percentage porosity 2) Evaluation of tablets a) Buoyancy capabilities b) In vitro floating and dissolution behaviour c) Weight variation d) Hardness & friability e) Particle size analysis, surface characterization (for floating microspheres and beads): f) X-Ray/Gamma Scintigraphy g) Pharmacokinetic studies 1) Evaluation of powder blend 28 a) Angle of repose Angle of repose is defined as the maximum angle possible between the surface of the pile of powder and the horizontal plane. Lower the angle of repose, better the flow properties. The angle of repose may be calculated by measuring the height (h) of the pile and the radius of the base(r) with ruler. Tan θ = h/r... 1 b) Bulk density Bulk density denotes the total density of the material. It includes the true volume of interparticle spaces and intraparticle pores. The packing of particles is mainly responsible for bulk.bulk density is defined as: Bulk density = Weight of the powder / Bulk volume of powder...2 When particles are packed, it is possible that a large amount of gaps may be present between the particles. Therefore, trapping of powder allows the particles to shift and remove the voids to minimum volume. The volume occupied by the powder in this condition represents the bulk volume. Substituting this volume for a given weight of powder in equation (2) gives the bulk density. c) Percentage porosity Whether the powder is porous or nonporous, the total porosity expression for the calculation remains the same. Porosity provides information about hardness, disintegration, total porosity etc. % porosity, = void volume x100 Bulk volume % porosity, = (bulk volumetrue volume) x100 True density 2) Evaluation of floating tablets a) Measurement of buoyancy capabilities of the FDDS: The floating behaviour is evaluated with resultant weight measurements. The experiment is carried out in two different media, deionised water and simulated meal. The results showed that higher molecular weight polymers with slower rate of hydration had enhanced floating behaviour and it was observed more in simulated meal medium compared to deionised water 29. b) In Vitro floating and dissolution behaviour: The dissolution tests are generally performed on various drugs using USP dissolution apparatus. USP 28 states the dosage unit is allowed to sink to the bottom of the vessel before rotation of the blade is started. A small, loose piece of nonreactive material with not more than a few turns of a wire helix may be attached to the dosage units that would otherwise float. However, standard USP or BP methods have not been shown to be reliable predictors of in vitro performance of floating dosage forms 29. Pillay et al applied a helical wire sinker to the swellable floating system of theophylline, which is sparingly soluble in water and concluded that the swelling of the system was inhibited by the wire helix and the drug release also slowed down. To overcome this limitation, a method was developed in which the floating drug delivery system was fully submerged under a ring or mesh assembly, and an increase in drug release was observed. Also, it was shown that the method was more reproducible and consistent. However, no significant change in the drug release was observed when the proposed method was applied to a swellable floating system of diltiazem, which is a highly water soluble drug. It was thus concluded that the drug release from swellable floating systems was dependent upon uninhibited swelling, surface exposure, and the solubility of the drug in water 30 c) Weight variation: 8
9 In practice, composite samples of tablets (usually 10) are taken and weighed throughout the compression process. The composite weight divided by 10, however provides an average weight but contains a problem of averaged value. To help alleviate this problem, the United States pharmacopeia (USP) provides limits for the permissible variations in the weights of individual tablets expressed as a percentage of the average weight of the sample. The USP provides the weight variation test by weighing 20 tablets individually, calculating the average weight, and comparing the individual tablet weights to the average. The tablets meet the USP test if no more than 2 tablets are outside the percentage limit, and if no tablet differs by more than 2 times the percentage limit 31. d) Hardness & friability: Hardness is defined as the force required to break a tablet in diametric compression test. Hardness is hence, also termed as the tablet crushing strength. Some devices which are used to test hardness are Monsanto tester, strong Cobb tester, Pfizer tester, etc. The laboratory friability tester is known as the Roche Friabilator. This consists of a device which subjects a number of tablets to the combined effects of abrasion and shock by utilizing a plastic chamber that revolves at 25 rpm & drop the tablet to a distance of six inches with each revolution. Normally, a pre weighed tablet sample is placed in the friabilator which is then operated for 100 revolutions. Conventional compressed tablets that lose less than 0.5 to 1.0 % of their weight are generally considered acceptable. Most of the effervescent tablets undergo high friability weight losses, which accounts for the special stack packaging,that may be required for these types of tablets 32. e) Particle size analysis, surface characterization (for floating microspheres and beads): The particle size and the size distribution of beads or microspheres are determined in the dry state using the optical microscopy method. The external and crosssectional morphology (surface characterization) is done by scanning electron microscope (SEM) 29. gastrointestinal tract, by which one can predict and correlate the gastric emptying time and the passage of dosage form in the GIT. Here the inclusion of a radio opaque material into a solid dosage form enables it to be visualized by X-rays. Similarly, the inclusion of a γ-emitting radionuclide in a formulation allows indirect external observation using a γ-camera or scintiscanner. In case of γ- scintigraphy, the γ-rays emitted by the radionuclide are focused on a camera, which helps to monitor the location of the dosage form in the GIT 29. g) Pharmacokinetic studies: Pharmacokinetic studies are an integral part of the in vivo studies and several works have been reported on these. Sawicki studied the pharmacokinetics of verapamil, from the floating pellets containing drug, filled into a capsule, and compared with the conventional verapamil tablets of similar dose (40 mg). The tmax and AUC (0- infinity) values (3.75 h and ng/ml /1h respectively) for floating pellets were comparatively higher than those obtained for the conventional verapamil tablets. (tmax value 1.21 h, and AUC value mg/ml/1h) 29. Recent advances in stomach specific floating dosage forms: Sungthongjeen et al have prepared a floating multilayer coated tablets based on gas formation. The system consists of a drugcontaining core tablet coated with a protective layer (hydroxylpropyl methyl cellulose), a gas forming layer (sodium bicarbonate) and a gas-entrapped membrane, respectively. Eudragit RL 30D was chosen as a gas-entrapped membrane due to its high flexibility and high water permeability. The obtained tablets enabled to float due to the CO2 gas formation and the gas entrapment by polymeric membrane. The effect of formulation variables on floating properties and drug release was investigated. The floating tablets using directcompressed cores had shorter the time to float and faster drug release than those using wet granulated cores. The increased amount of a gas forming agent did not affect time to float but increased the drug release from the floating tablets, f) XRay/ gamma scintigraphy: X-Ray/Gamma Scintigraphy is a very popular evaluation parameter for floating dosage form nowadays. It helps to locate dosage form in the 9
10 Sr. No Brand name 1 Topalkan 2 Conviron 3 Cifran OD 4 Valreleas e 5 Madopar Table 2: Some of the marketed formulations available as GRDDS 33 Delivery system Drug Category Company name Floating liquid alginate preparation colloidal gel forming FDDS Gas generating floating form Floating capsule Diazepam Floating, CR capsule while increasing the coating level of gas entrapped membrane increased the time to float (more then 8 hours) and slightly retarded, but sustained drug release 34. Rajnikanth et al have developed a floating in situ gelling system of clarithromycin (FIGC) using gellan as gelling polymer and calcium carbonate as floating agent for potentially treating gastric ulcers, associated with Helicobacter pylori (H.pylori). Gellan based FIGC was prepared by dissolving varying concentrations of gellan in deionized water, to which varying concentrations of the drug and sucralfate were dispersed well. The addition of sucralfate to the formulation significantly suppressed the degradation of clarithromycin at low ph. FIGC showed a Gastroretentive products available in market 36 Al Mg Antacid Pierre Fabre Drug, France Ferrous sulphate Ranbaxy, India Antianemic Ciprofloxacin Antibiotic Ranbaxy, India CNS depressant LaRoche, USA Hoffmann Benserazide and L-Dopa Roche Products, USA Antiparkinsons significant antih. pylori effect than that of clarithromycin suspension. The in situ gel formulation with sucralfate cleared H.pylori more effectively than that of formulation without sucralfate. In addition, the required amount of clarithromycin for eradication of H.pylori was found to be less from FIGC than from the corresponding clarithromycin suspension. It was concluded that prolonged gastrointestinal residence time and enhanced clarithromycin stability resulting from the floating in situ gel of clarithromycin might contribute better for complete clearance of H. pylori than the corresponding clarithromycin suspension 35. Table 3 Gastroretentive Products Available in Market Brand Name Drug Cifran OD Ciprofloxacin Madopar L-DOPA and Benserazide Valrelease Diazepam Topalkan Aluminum -magnesium antaci Almagate FlatCoat Aluminum -magnesium antacid Liquid Gavison Aluminium hydroxide, Conviron Ferrous sulfate Liquid Gavison Alginic acid, Sodium bicarbonate 10
11 Companies involved in developing gastric retention technologies 37 Company Intec Pharma, Israel Kos Pharmaceuticals, New Jersey DepoMed, Inc., California Ranbaxy, India Akina Pharmaceuticals,Indiana Spherics Inc., Rhode Island Flamel Technologies, France Table 4 Names of Companies Devloping GR products Technology Expandable (unfolding) Expandable (swelling absorbent, porous hydrogel) Expandable (absorbing, swelling tablet) Expandable, floating (gas generation) Expandable (swellingabsorbent, porous hydrogel) Mucoadhesion Mucoadhesion Patents on FDDS 38 Table 5 Patents on Floating Drug Delivery System S.No Type of formulation Patent no. Ref 1 Gastro retentive dosage form U.S-7,413,752. Devane et al., Multiple unit floating dosage form European patent (EP) Vanderbist et al., Bilayer tablet EP Lohray et al., Floating Tablet U.S-66, Kolter et al., Microspheres U.S Illum et al., layer tablet U.S Conte et al., Foams (or) hollow bodies U.S Muller et al., Floating tablet U.S Baichwal et al., Granule U.S Ichikawa et al., Floating capsules U.S ,-79 Sheth et al., Tiny pills U.S Urguhart et al., Floating capsule U.S Sheth et al., Floating device U.S Harrigan et al., Empty globular shells U.S Watanabe et al., 1976 Dosage forms Floating Tablets Floating Capsules Floating Microspheres Floating Granules Powders Films Commonly used drugs in Formulation of FDDS Table 6: Commonly used FDDS Formulations Drugs Acetaminophen, Acetylsalicylic acid, Ampicillin, Amoxicillin trihydrate, Atenolol, Captopril, Cinnerzine, Chlorpheniramine maleate, Ciprofloxacin, Diltiazem, Fluorouracil, Isosorbide dinitrate, Isosorbid mononitrate, p- Aminobenzoic acid(paba), Prednisolone, Nimodipine, Sotalol, Theophylline, Verapamil,Nicardipine,Nimodipine,piretanide Chlordiazepoxide HCl, Diazepam, Furosemide, L-DOPA and Benserazide, Nicardipine, Misoprostol, Propranolol, Pepstatin Aspirin, Griseofulvin, P-nitroaniline, Ibuprofen, Terfenadine, Tranilast,ketoprofen Diclofenac sodium, Indomethacin, Prednisolone,Diltiazem, Riboflavin, sotalol,theophylline Cinnarizine,Piretanide,Prednisolone,Quinidine guconate 11
12 Conclusion: Recently many drugs have been formulated as floating drug delivery systems with an objective of sustained release and restricting the region of drug release to stomach. The principle of buoyant preparation offers a simple and practical approach to achieve increased gastric residence time for the dosage form and sustained drug release. The currently available polymermediated noneffervescent and effervescent FDDS, designed on the basis of delayed gastric emptying and buoyancy principles, appear to be a very much effective approach to the modulation of controlled oral drug delivery. The most important criteria which has to be looked into for the productions of a floating drug delivery system is that the density of the dosage form should be less than that of gastric fluid. And hence, it can be concluded that these dosage forms serve the best in the treatment of diseases related to the GIT and for extracting a prolonged action from a drug with a short half life Cite this article Shivram Shinde, Imran Tadwee, Sadhana Shahi Gastro retentive Drug Delivery System: A Review Int. J. of Pharm. Res. & All. Sci., Volume 1, issue 1 (2011), References: 1. Nayak AK, Maji R, Das B. Gastroretentive drug delivery system: A review ISSN Streubel A, Siepmann J. Bodmeier R. Multiple unit Gastroretentive drug delivery: A new preparation method for low density microparticles. J Microcapsule 2003;20: Goole J, Vanderbist F, Aruighi K. Development and Evaluation of new multipleunit Levodopa sustainedrelease floating dosage forms. Int J Pharm 2007:334: Sharma S, Pawar A. Low density multiparticulate system for pulasatile release of Meloxicam. Int J.Pharm 2006:313: Santus G, Lazzarini G, Bottoni G, Sandefer EF, Doll WJ, Ryo UY, Digenis GA. An in vitro / in vivo investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm1997:44: Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J control Release 2003:90: Deshpande AA, Shah N, Rhodes CT, Malik W. Development of novel controlled release system for gastro retention. Pharm res 1997:14: Park K. Enzyme digestible swelling as platforms for long term oral drug delivery: synthesis and characterization. Biomaterials 1988:9: Radi Hejazi, Mansoor Amiji. Chitosan based gastrointestinal delivery systems. Journal of Controlled Release 203:89: Mojaverian P, Ferguson RK, Vlasses PH. Estimation of gastric residence time of the Heidelberg capsules in humans: effect of varying food composition, gastroetrenology.1885:89:392y Benchgaard H, Ladefoged K. Distribution of pellets in gastrointestinal tract: The influence on transit time exerted by the density or diameter of pellets. J Pharm Pharmacol 1978:30:690Y Vantrappen GR, Petters TL, Janssens J. The secretory component of inter digestive migratory motor complex in man. Scand. J Gastroenterol. 1979:14663:Y Wilson CG. Washington N. The stomach: its role in oral drug delivery. In: Rubinstein MH, Physiloogical Pharmaceutical:Biological Barriers to drug Absorption. Chichester,UK: Ellis Horwood;1989:47Y Nayak AK, Maji R., Das B. Gastroretentive drug delivery system: A Review. IISN Seth SD.Text book of pharmacology, Reed Elsevier Ltd Garg S, Sharma S. Gastroretentive Drug Delivery System. Business Briefing: Pharmatech.2003; Rubinstein A, Friend DR. Specific delivery to the gastrointestinal tract, in: Domb A.J (Ed.), Polymeric SiteSpecific Pharmacotherapy, Wiley, Chichester 1994; Penners G, Lustig K, Jorg PVG. Expandable pharmaceutical forms.us patent 1997; 5:651, Jaimini M, Rana AC, Tanwar YS. Formulation and evaluation of famotidine floating tablets. Current Drug Delivery 2007; 4: Innuccelli V, Coppi G, Bernabei M T, Cameroni R. Air compartment multiple-unit system for prolonged gastric residence. Int. J. Pharm.1998; 174:
13 21. Wu W, Zhou Q, Zhang HB, Ma GD, Fu CD. Studies on nimodipine sustained release tablet capable of floating on gastric fluids with prolonged gastric resident time. Yao Xue Bao.1997; 32: Ichikawa M, Watanabe S, Miyake Y. A new multiple unit oral floating dosage system. I: Preparation and in vitro evaluation of floating and sustained release kinetics. Pharm Sci.1991; 80: Chen GL, Hao WH. In vitro performance of floating sustained release capsule of verapamil. Drug Dev. Ind. Pharm.1998; 24(11): Arora S, Ali J, Ahuja A, Khar KR, Baboota S. Floating Drug Delivery Systems: A Review. AAPS Pharm. Sci. Tech. 2005; 6 (3) Article Thanoo BC, Sunny MC, Jayakrishnan A. Oral sustained release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluids. J. Pharm. Pharmacol.1993; 45: Sheth PR, Tossounian JL. Sustained release pharmaceutical capsules.us patent1978; 4:126, Joseph NH, Laxmi S, Jayakrishnan A. A floating type oral dosage from for piroxicam based on hollow microspheres: in vitro and in vivo evaluation in rabbits. J.Cont. Rel. 2002; 79: Jorgen F, Toftkjor H. Antacid composition.us Patent : Subrahmanyam CVS, Setty JT.Laboratory manual of physical pharmaceutics, Jain MK for vallabh prakashan Shah SH, Patel JK, Patel NV. Stomach specific floating drug delivery system: A Review, Int. J. Pharm. Res. CODEN (USA): IJPRIF ISSN: ; 3: Pillay, Shinde AKJ. Gastroretentive Drug Delivery System: An Overview.2008; 13: Koner P, Saudagar RB, Daharwal SJ. Gastro Retentive Drugs: A Novel Approach Towards Floating Therapy, 2007; 5 (1): Wu W, Zhou Q, Zhang HB, Ma GD, Fu CD. Studies on nimodipine sustained release tablet capable of floating on gastric fluids with prolonged gastric resident time. Yao Xue Xue Bao.1997; 32: Sungthongjeena S, Sriamornsak P, Puttipipatkhachorn S. Design and evaluation of floating multi-layer coated tablets based on gas formation. Eur. J. Pharm. And Biopharm.2008; 69: Rajnikanth P, Mishra B. Floating in situ gelling system for stomach site specific delivery of clarithromycin to eradicate H.pylori. J. Cont. Rel.2008; 25: Gopalakrishnan,S.; Chenthilnathan, A. Floating Drug Delivery Systems: A Review Journal of Pharmaceutical Science and Technology. 2011, 3 (2), Jagadeesh, N.; Shayeda. Floating Drug Delivery Systems. International Journal of Pharmaceutical Sciences and Nanotechnology. 2009, 2 ( 3), Vyas, S.P.; Khar, R.Ks. Gastroretentive systems. In: Controlled drug Delivery. Vallabh Prakashan, Delhi, India pp
Gastro Retentive Drug Delivery System
 Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
SHRI JAGDISHPRASAD JHABARMAL TIBREWALA UNIVERSITY 1
 INTRODUCTION Oral delivery of drugs is by far the most preferred route of drug delivery due to ease of administration, patient compliance and flexibility in formulation 1. Conventional oral dosage forms
INTRODUCTION Oral delivery of drugs is by far the most preferred route of drug delivery due to ease of administration, patient compliance and flexibility in formulation 1. Conventional oral dosage forms
MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW
 MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW * Patel N. 1, Raiyani D. 1, Patel A. 1, Patel N. 1, Jain H. 1 and Upadhyay U. 1 Department of Pharmaceutics, Sigma Institute of
MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW * Patel N. 1, Raiyani D. 1, Patel A. 1, Patel N. 1, Jain H. 1 and Upadhyay U. 1 Department of Pharmaceutics, Sigma Institute of
Volume 8, Issue 2, May June 2011; Article-029
 Review Article A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEM: AN EMERGING APPROACH TO IMPROVE THE GASTRIC RESIDENCE TIME OF SOLID DOSAGE FORMS Manmohan *, Shukla T P, Mathur A, Upadhyay N, Sharma S
Review Article A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEM: AN EMERGING APPROACH TO IMPROVE THE GASTRIC RESIDENCE TIME OF SOLID DOSAGE FORMS Manmohan *, Shukla T P, Mathur A, Upadhyay N, Sharma S
FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE
 FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE Chandira. Margret R. *, Sahu Chandra Mohan and Jayakar B. Vinayaka Mission s College of Pharmacy Vinayaka Missions University,
FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE Chandira. Margret R. *, Sahu Chandra Mohan and Jayakar B. Vinayaka Mission s College of Pharmacy Vinayaka Missions University,
INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE
 INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE FLOATING DRUG DELIVERY SYSTEM AS A NOVEL VITAL CONCEPT BABITA SHARMA, NITAN BHARTI GUPTA Sri Sai College of Pharmacy, Pathankot, India.
INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE FLOATING DRUG DELIVERY SYSTEM AS A NOVEL VITAL CONCEPT BABITA SHARMA, NITAN BHARTI GUPTA Sri Sai College of Pharmacy, Pathankot, India.
EVALUATION OF EFFERVESCENT FLOATING TABLETS. 6.7 Mathematical model fitting of obtained drug release data
 EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
Int. J. Pharm. Sci. Rev. Res., 27(2), July August 2014; Article No. 15, Pages: Review on Gastro Retentive Drug Delivery System (GRDDS)
 Review Article Review on Gastro Retentive Drug Delivery System (GRDDS) Sachin A. Nitave*, Vishin A. Patil, Amrita A. Kagalkar Anil Alias Pintu Magdum Memorial Pharmacy College, Dharangutti, Shirol, Kolhapur,
Review Article Review on Gastro Retentive Drug Delivery System (GRDDS) Sachin A. Nitave*, Vishin A. Patil, Amrita A. Kagalkar Anil Alias Pintu Magdum Memorial Pharmacy College, Dharangutti, Shirol, Kolhapur,
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION 1.1 ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEMS The oral route is the most promising and convenient route of drug administration. Conventional immediate release system achieves
1 CHAPTER 1 INTRODUCTION 1.1 ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEMS The oral route is the most promising and convenient route of drug administration. Conventional immediate release system achieves
Available Online through Research Article
 ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
LIST OF TABLES. Page No. No List of US Patents for FDDS Gastroretentive products available in the market
 LIST OF TABLES Sl. Table Title of the table 1 3.01 List of US Patents for FDDS 27 2 3.02 List of drugs explored for various floating dosage forms 35 3 3.03 Gastroretentive products available in the market
LIST OF TABLES Sl. Table Title of the table 1 3.01 List of US Patents for FDDS 27 2 3.02 List of drugs explored for various floating dosage forms 35 3 3.03 Gastroretentive products available in the market
A Review on Gastroretentive Drug Delivery System
 A Review on Gastroretentive Drug Delivery System Pranav Joshi*, Priyank Patel,Hiren Modi, Dr.M.R. Patel, Dr.K.R.Patel, Dr.N.M.Patel Department of pharmaceutics, Shri B.M.Shah College of Pharmaceutical
A Review on Gastroretentive Drug Delivery System Pranav Joshi*, Priyank Patel,Hiren Modi, Dr.M.R. Patel, Dr.K.R.Patel, Dr.N.M.Patel Department of pharmaceutics, Shri B.M.Shah College of Pharmaceutical
FLOATING DRUG DELIVERY OF RANITIDINE HYDROCHLORIDE
 Int. J. LifeSc. Bt & Pharm. Res. 2012 Varsha Deva and Manoj Kr Gautam, 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 2012 IJLBPR. All Rights Reserved FLOATING DRUG DELIVERY
Int. J. LifeSc. Bt & Pharm. Res. 2012 Varsha Deva and Manoj Kr Gautam, 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 2012 IJLBPR. All Rights Reserved FLOATING DRUG DELIVERY
FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN
 FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN Ms. Jyoti Rathore 1*, Mr. Hitesh Kumar Parmar 1 Ujjain Institute of Pharmaceutical Sciences, Ujjain. Email- hkparmar7@rediffmail.com ABSTRACT
FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN Ms. Jyoti Rathore 1*, Mr. Hitesh Kumar Parmar 1 Ujjain Institute of Pharmaceutical Sciences, Ujjain. Email- hkparmar7@rediffmail.com ABSTRACT
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED RELEASE FLOATING MATRIX TABLETS OF METFORMIN HYDROCHLORIDE
 ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 8 (Research article) Received 11 April, 2010; received in revised form 17 June, 2010; accepted 13 July, 2010 DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED
ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 8 (Research article) Received 11 April, 2010; received in revised form 17 June, 2010; accepted 13 July, 2010 DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED
This PDF is available for free download
 Research Paper Formulation Strategy for Low Absorption Window Antihypertensive Agent M. J. QURESHI, J. ALI*, ALKA AHUJA AND SANJULA BABOOTA Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard,
Research Paper Formulation Strategy for Low Absorption Window Antihypertensive Agent M. J. QURESHI, J. ALI*, ALKA AHUJA AND SANJULA BABOOTA Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard,
FLOATING DRUG DELIVERY SYSTEM (FDDS): A REVIEW
 25 P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 5(1): January-February 2016 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND
25 P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 5(1): January-February 2016 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND
OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
 Digest Journal of Nanomaterials and Biostructures Vol. 9, No. 3, July September 2014, p. 1077-1084 OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
Digest Journal of Nanomaterials and Biostructures Vol. 9, No. 3, July September 2014, p. 1077-1084 OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN
 Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems
 Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems 1 1 1 2 U.V. Bhosale*, V. Kusum Devi, Nimisha Jain and P.V. Swamy 1 Al-Ameen College
Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems 1 1 1 2 U.V. Bhosale*, V. Kusum Devi, Nimisha Jain and P.V. Swamy 1 Al-Ameen College
Gastroretentive Drug Delivery Systems: A Review
 J Chronother Drug Deliv Vol 5 Issue 1 2014: 1-7 Authors Journal of Chronotherapy and Drug Delivery REVIEW ARTICLE Gastroretentive Drug Delivery Systems: A Review Rupa Gupta 1 *, PK Sharma 1, Ashish Arora
J Chronother Drug Deliv Vol 5 Issue 1 2014: 1-7 Authors Journal of Chronotherapy and Drug Delivery REVIEW ARTICLE Gastroretentive Drug Delivery Systems: A Review Rupa Gupta 1 *, PK Sharma 1, Ashish Arora
Biswajit Biswal IRJP 2 (7)
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN 2230 8407 Available online http://www.irjponline.com Research Article DESIGN DEVELOPMENT AND EVALUATION OF TRIMETAZIDINE DIHYDROCHLORIDE FLOATING BILAYER
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN 2230 8407 Available online http://www.irjponline.com Research Article DESIGN DEVELOPMENT AND EVALUATION OF TRIMETAZIDINE DIHYDROCHLORIDE FLOATING BILAYER
Journal of Pharmaceutical and Scientific Innovation Review Article
 Journal of Pharmaceutical and Scientific Innovation www.jpsionline.com Review Article FLOATING DRUG DELIVERY SYSTEM: A NOVEL APPROACH Mahale G. S.* and Derle Nikita D. Department of Pharmaceutics, M.V.P.s
Journal of Pharmaceutical and Scientific Innovation www.jpsionline.com Review Article FLOATING DRUG DELIVERY SYSTEM: A NOVEL APPROACH Mahale G. S.* and Derle Nikita D. Department of Pharmaceutics, M.V.P.s
Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium
 Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium Florida Sharmin 1, Md. Abdullah Al Masum 1, S. M. Ashraful Islam 1 and Md. Selim Reza 2 1 Department of Pharmacy,
Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium Florida Sharmin 1, Md. Abdullah Al Masum 1, S. M. Ashraful Islam 1 and Md. Selim Reza 2 1 Department of Pharmacy,
Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist
 Human Journals Review Article January 2017 Vol.:8, Issue:2 All rights are reserved by Dharmendra Solanki et al. Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist Keywords: Floating
Human Journals Review Article January 2017 Vol.:8, Issue:2 All rights are reserved by Dharmendra Solanki et al. Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist Keywords: Floating
DISSOLUTION RATE LIMITED ABSORPTION:
 INTRODUCTION Oral drug delivery is the most widely utilized route of administration among all the routes that have been explored for systemic delivery of drugs via pharmaceutical products of different
INTRODUCTION Oral drug delivery is the most widely utilized route of administration among all the routes that have been explored for systemic delivery of drugs via pharmaceutical products of different
7. SUMMARY, CONCLUSION AND RECOMMENDATIONS
 211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
211 7. SUMMARY, CONCLUSION AND RECOMMENDATIONS Drug absorption from the gastro intestinal tract can be limited by various factors with the most common one being poor aqueous solubility and poor permeability
Formulation and Evaluation of Gastroretentive Dosage form of Ciprofloxacin Hydrochloride.
 Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 3(4), 105-109 Research Article ISSN: 0976-822X Formulation and Evaluation of Gastroretentive Dosage
Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 3(4), 105-109 Research Article ISSN: 0976-822X Formulation and Evaluation of Gastroretentive Dosage
Factors responsible for the impaired bioavailability and instability rifampicin FDC
 Table of contents 1.0 Introduction 1.1 Oral multiparticulate drug delivery system...2 1.2 Methods of preparing pellets...3 1.3 Extrusion-spheronization...3 1.4 Theory of pellet formation and growth...4
Table of contents 1.0 Introduction 1.1 Oral multiparticulate drug delivery system...2 1.2 Methods of preparing pellets...3 1.3 Extrusion-spheronization...3 1.4 Theory of pellet formation and growth...4
A review on Gastroretentive Drug Delivery Systems
 29 A review on Gastroretentive Drug Delivery Systems Hemendrasinh J Rathod*, Dhruti P. Mehta, Jitendra Singh Yadav Department of Pharmaceutics, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat, India.
29 A review on Gastroretentive Drug Delivery Systems Hemendrasinh J Rathod*, Dhruti P. Mehta, Jitendra Singh Yadav Department of Pharmaceutics, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat, India.
International Journal of Medicine and Pharmaceutical Research
 International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
Formulation and Evaluation of Atenolol Floating Tablets Using Different Polymers: Guargum, Sodium Alginate, Hpmc100cps and Carbopol940
 ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(4):1146-1151 ORIGINAL RESEARCH ARTICLE Formulation and Evaluation of Atenolol Floating
ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(4):1146-1151 ORIGINAL RESEARCH ARTICLE Formulation and Evaluation of Atenolol Floating
DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG M Seth *, DS Goswami,
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG M Seth *, DS Goswami,
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
Asian Journal of Pharmacy and Life Science ISSN Vol.4 (2), April-June, 2014 REVIEW ON- GASTRORETENTIVE DRUG DELIVERY SYSTEM
 REVIEW ON- GASTRORETENTIVE DRUG DELIVERY SYSTEM PRAFULL GHORPADE* 1, SHRENIK KAMBLE 2 1 Marathwada Mitra Mandal s College of Pharmacy, Kalewadi (Thergaon), Pune, Maharashtra, India *Corresponding author
REVIEW ON- GASTRORETENTIVE DRUG DELIVERY SYSTEM PRAFULL GHORPADE* 1, SHRENIK KAMBLE 2 1 Marathwada Mitra Mandal s College of Pharmacy, Kalewadi (Thergaon), Pune, Maharashtra, India *Corresponding author
Review on Gastro Retentive Drug Delivery System
 ISSN: 2277-7695 CODEN Code: PIHNBQ ZDB-Number: 2663038-2 IC Journal No: 7725 Vol. 1 No. 8 2012 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION Review on Gastro Retentive Drug Delivery
ISSN: 2277-7695 CODEN Code: PIHNBQ ZDB-Number: 2663038-2 IC Journal No: 7725 Vol. 1 No. 8 2012 Online Available at www.thepharmajournal.com THE PHARMA INNOVATION Review on Gastro Retentive Drug Delivery
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
A NOVEL APPROACH FOR FLOATING DRUG DELIVERY SYSTEM
 ISSN: 2230-7346 Suresh Rewar et al. / JGTPS / 6(1)-(2015) 2417 2422 (Review Article) Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com A NOVEL APPROACH FOR FLOATING DRUG
ISSN: 2230-7346 Suresh Rewar et al. / JGTPS / 6(1)-(2015) 2417 2422 (Review Article) Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com A NOVEL APPROACH FOR FLOATING DRUG
MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY FLOATING APPROCHES
 Pooja et al Journal of Drug Delivery & Therapeutics; 213, 3(3), 37-41 37 Available online at http://jddtonline.info RESEARCH ARTICLE MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY
Pooja et al Journal of Drug Delivery & Therapeutics; 213, 3(3), 37-41 37 Available online at http://jddtonline.info RESEARCH ARTICLE MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY
Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System
 Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System Santosh Shep *, Sham Dodiya, Sandeep Lahoti, Rahul Mayee Dr. Vedprakash Patil Pharmacy College, Aurangabad, India INDO GLOBAL
Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System Santosh Shep *, Sham Dodiya, Sandeep Lahoti, Rahul Mayee Dr. Vedprakash Patil Pharmacy College, Aurangabad, India INDO GLOBAL
Formulation and Evaluation of Floating Mefenamic Acid Drug Delivery Tablet
 International Journal of Pharmacy and Medical Sciences (1): 01-06, 01 ISSN -5854 IDOSI Publications, 01 DOI: 10.589/idosi.ijpms.01..1.641 Formulation and Evaluation of Floating Mefenamic Acid Drug Delivery
International Journal of Pharmacy and Medical Sciences (1): 01-06, 01 ISSN -5854 IDOSI Publications, 01 DOI: 10.589/idosi.ijpms.01..1.641 Formulation and Evaluation of Floating Mefenamic Acid Drug Delivery
Venkateswara Rao et.al Indian Journal of Research in Pharmacy and Biotechnology ISSN: (Print) ISSN: (Online)
 Design and development of Metformin hydrochloride Tried sustained release tablets Venkateswara Rao T 1 *, Bhadramma N 1, Raghukiran CVS 2 and Madubabu K 3 Bapatla College of Pharmacy, Bapatla, Guntur-522101
Design and development of Metformin hydrochloride Tried sustained release tablets Venkateswara Rao T 1 *, Bhadramma N 1, Raghukiran CVS 2 and Madubabu K 3 Bapatla College of Pharmacy, Bapatla, Guntur-522101
Figure 1.1: (a) Drug release mechanism and (b) Floating drug delivery systems
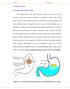 1 1. INTRODUCTION 1.1. Floating drug delivery system Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug
1 1. INTRODUCTION 1.1. Floating drug delivery system Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug
Formulation and In-Vitro Evaluation of Floating Microspheres of Anti-Diabetic Drug Prepared by Solvent Evaporation Method
 Research Article Formulation and In-Vitro Evaluation of Floating Microspheres of Anti-Diabetic Drug Prepared by Solvent Evaporation Method Vadaliya SK 1*, Vadaliya KR 1, Desai HT 1 and Patel JK 2 1 School
Research Article Formulation and In-Vitro Evaluation of Floating Microspheres of Anti-Diabetic Drug Prepared by Solvent Evaporation Method Vadaliya SK 1*, Vadaliya KR 1, Desai HT 1 and Patel JK 2 1 School
Design and Evaluation of Atenolol Floating Drug Delivery System
 Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 4(1), 1-26 Research Article ISSN: 976-822X Design and Evaluation of Atenolol Floating Drug Delivery
Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 4(1), 1-26 Research Article ISSN: 976-822X Design and Evaluation of Atenolol Floating Drug Delivery
A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES
 Review Article A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES Kamini Vasava, Rajesh Ks, Lalit Lata Jha * Department
Review Article A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES Kamini Vasava, Rajesh Ks, Lalit Lata Jha * Department
Design and In-vitro Evaluation of Silymarin Bilayer Tablets
 CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Design and In-vitro Evaluation of Silymarin
CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Design and In-vitro Evaluation of Silymarin
Asian Journal of Biochemical and Pharmaceutical Research
 ISSN: 2231-2560 Research Article Asian Journal of Biochemical and Pharmaceutical Research Design and Evaluation of Gastroretentive Floating Tablets of Dipyridamole: Influence of Formulation Variables A.
ISSN: 2231-2560 Research Article Asian Journal of Biochemical and Pharmaceutical Research Design and Evaluation of Gastroretentive Floating Tablets of Dipyridamole: Influence of Formulation Variables A.
Development And Evaluation Of Gastroretentive Floating Tablet Of Rosuvastatin
 Development And Evaluation Of Gastroretentive Floating Tablet Of Rosuvastatin Amiya kumar Prusty, Archana P. Chand Institute of Pharmacy and Technology, salipur, Biju Patnaik University of technology,
Development And Evaluation Of Gastroretentive Floating Tablet Of Rosuvastatin Amiya kumar Prusty, Archana P. Chand Institute of Pharmacy and Technology, salipur, Biju Patnaik University of technology,
Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate
 Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate Lokesh Kumar*, Virendra Singh, Rakesh Kumar Meel Department of Pharmaceutics, Goenka College of Pharmacy, NH-11,
Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate Lokesh Kumar*, Virendra Singh, Rakesh Kumar Meel Department of Pharmaceutics, Goenka College of Pharmacy, NH-11,
Formulation and Development of Sustained Release Tablets of Valsartan Sodium
 INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Formulation and Development of Sustained Release Tablets of Valsartan Sodium G. Sandeep * and A. Navya Department of
INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Formulation and Development of Sustained Release Tablets of Valsartan Sodium G. Sandeep * and A. Navya Department of
Chapter 2 Rationale and Objective
 Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the
Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the
The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation
 METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
Scholars Research Library
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(1): 121-137 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(1): 121-137 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
A Comparative Evaluation of Cross Linked Starch Urea-A New Polymer and Other Known Polymers for Controlled Release of Diclofenac
 Asian Journal of Chemistry Vol. 22, No. 6 (2010), 4239-4244 A Comparative Evaluation of Cross Linked Starch Urea-A New Polymer and Other Known Polymers for Controlled Release of Diclofenac K.P.R. CHOWDARY*
Asian Journal of Chemistry Vol. 22, No. 6 (2010), 4239-4244 A Comparative Evaluation of Cross Linked Starch Urea-A New Polymer and Other Known Polymers for Controlled Release of Diclofenac K.P.R. CHOWDARY*
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
A. Incorrect! Histamine is a secretagogue for stomach acid, but this is not the only correct answer.
 Pharmacology - Problem Drill 21: Drugs Used To Treat GI Disorders No. 1 of 10 1. Endogenous secretagogues for stomach acid include: #01 (A) Histamine (B) Gastrin (C) PGE1 (D) A and B (E) A, B and C Histamine
Pharmacology - Problem Drill 21: Drugs Used To Treat GI Disorders No. 1 of 10 1. Endogenous secretagogues for stomach acid include: #01 (A) Histamine (B) Gastrin (C) PGE1 (D) A and B (E) A, B and C Histamine
Formulation Development and In-Vitro Evaluation of Gastroretentive Floating tablets of Atenolol
 Formulation Development and In-Vitro Evaluation of Gastroretentive Floating tablets of Atenolol K.Viveksarathi, K.Kannan*, S.Selvamuthu Kumar, R.Manavalan Department of Pharmacy, Faculty of Engineering
Formulation Development and In-Vitro Evaluation of Gastroretentive Floating tablets of Atenolol K.Viveksarathi, K.Kannan*, S.Selvamuthu Kumar, R.Manavalan Department of Pharmacy, Faculty of Engineering
REVISION OF MONOGRAPH ON TABLETS. Tablets
 March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
Journal of Chemical and Pharmaceutical Research, 2015, 7(5): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(5):1225-1231 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Formulation and evaluation of raft forming sustained
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(5):1225-1231 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Formulation and evaluation of raft forming sustained
Development of Metclopramide Floating Tablets Based on HPMC Matrices: A Comparison Study with Marketed Formulation
 Development of Metclopramide Floating Tablets Based on HPMC Matrices: A Comparison Study with Marketed Formulation Shammy Jindal*, Amit Sharma & Kamya Jindal Laureate Institute of Pharmacy, Kathog - Himachal
Development of Metclopramide Floating Tablets Based on HPMC Matrices: A Comparison Study with Marketed Formulation Shammy Jindal*, Amit Sharma & Kamya Jindal Laureate Institute of Pharmacy, Kathog - Himachal
Formulation and evaluation of sublingual tablets of lisinopril
 Journal of GROVER Scientific & Industrial AGARWAL: Research FORMULATION AND EVALUATION OF SUBLINGUAL TABLETS OF LISINOPRIL Vol. 71, June 2012, pp. 413-417 413 Formulation and evaluation of sublingual tablets
Journal of GROVER Scientific & Industrial AGARWAL: Research FORMULATION AND EVALUATION OF SUBLINGUAL TABLETS OF LISINOPRIL Vol. 71, June 2012, pp. 413-417 413 Formulation and evaluation of sublingual tablets
Volume: 2: Issue-3: July-Sept ISSN FORMULATION AND EVALUATION OF SUSTAINED RELEASE MATRIX TABLETS OF NICORANDIL
 Volume: 2: Issue-3: July-Sept -2011 ISSN 0976-4550 FORMULATION AND EVALUATION OF SUSTAINED RELEASE MATRIX TABLETS OF NICORANDIL Ajaykumar Patil*, Ashish Pohane, Ramya Darbar, Sharanya Koutika, Alekhya
Volume: 2: Issue-3: July-Sept -2011 ISSN 0976-4550 FORMULATION AND EVALUATION OF SUSTAINED RELEASE MATRIX TABLETS OF NICORANDIL Ajaykumar Patil*, Ashish Pohane, Ramya Darbar, Sharanya Koutika, Alekhya
Formulation and Evaluation
 Chapter-5 Formulation and Evaluation 5.1 OBJECTIVE After successful taste masking and solubility enhancement of drugs in preliminary studies, by using Mannitol Solid Dispersion, next step includes the
Chapter-5 Formulation and Evaluation 5.1 OBJECTIVE After successful taste masking and solubility enhancement of drugs in preliminary studies, by using Mannitol Solid Dispersion, next step includes the
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS
 International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
DESIGN AND OPTIMIZATION OF FLOATING MATRIX TABLETS OF FAMOTIDINE BY CENTRAL COMPOSITE DESIGN
 Academic Sciences Asian Journal of Pharmaceutical and Clinical Research Vol, Suppl 1, 2012 ISSN - 0974-2441 Research Article DESIGN AND OPTIMIZATION OF FLOATING MATRIX TABLETS OF FAMOTIDINE BY CENTRAL
Academic Sciences Asian Journal of Pharmaceutical and Clinical Research Vol, Suppl 1, 2012 ISSN - 0974-2441 Research Article DESIGN AND OPTIMIZATION OF FLOATING MATRIX TABLETS OF FAMOTIDINE BY CENTRAL
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(3):159-164 Studies on formulation and in vitro evaluation
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(3):159-164 Studies on formulation and in vitro evaluation
FORMULATION AND IN VITRO EVALUATION OF FAMOTIDINE FLOATING TABLETS BY LIPID SOLID DISPERSION SPRAY DRYING TECHNIQUE
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article FORMULATION AND IN VITRO EVALUATION OF FAMOTIDINE FLOATING TABLETS BY LIPID SOLID DISPERSION
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article FORMULATION AND IN VITRO EVALUATION OF FAMOTIDINE FLOATING TABLETS BY LIPID SOLID DISPERSION
Optimization of Valsartan SR Floating Tablet Formulation by 2 2 Factorial Design and Multiple Regression Technique
 Optimization of Valsartan SR Floating Tablet by 2 2 Factorial Design and Multiple Regression Technique K. Srinivasa Reddy 1, Surya Kanta Swain 2, K. P. R. Chowdary *3, S. V. U. M. Prasad 4 1 School of
Optimization of Valsartan SR Floating Tablet by 2 2 Factorial Design and Multiple Regression Technique K. Srinivasa Reddy 1, Surya Kanta Swain 2, K. P. R. Chowdary *3, S. V. U. M. Prasad 4 1 School of
A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEMS
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 5.210 Volume 4, Issue 11, 1313-1332 Review Article ISSN 2278 4357 A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEMS Sayyed Sarfaraz
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES SJIF Impact Factor 5.210 Volume 4, Issue 11, 1313-1332 Review Article ISSN 2278 4357 A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEMS Sayyed Sarfaraz
Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride
 Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride R. Margret Chandira*, C.M. Sahu and B. Jayakar Department of Pharmaceutics Vinayaka Mission s College of
Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride R. Margret Chandira*, C.M. Sahu and B. Jayakar Department of Pharmaceutics Vinayaka Mission s College of
Principals and Dosage Forms in the Therapy Modified Drug Release. Institute of Pharmaceutical Technology and Biopharmacy
 Principals and Dosage Forms in the Therapy Modified Drug Release Institute of Pharmaceutical Technology and Biopharmacy Dosage forms Definition: Dosage forms are the means by which drug molecules are delivered
Principals and Dosage Forms in the Therapy Modified Drug Release Institute of Pharmaceutical Technology and Biopharmacy Dosage forms Definition: Dosage forms are the means by which drug molecules are delivered
FORMULATION DEVELOPMENT AND IN-VITRO CHARACTERIZATION OF BILAYER TABLETS OF AMOXICILLIN AND FAMOTIDINE
 ISSN: 2395 1338 FORMULATION DEVELOPMENT AND IN-VITRO CHARACTERIZATION OF BILAYER TABLETS OF AMOXICILLIN AND FAMOTIDINE B. Venkateswara Reddy *, K. Navaneetha Department of Pharmaceutics, St.Paul s College
ISSN: 2395 1338 FORMULATION DEVELOPMENT AND IN-VITRO CHARACTERIZATION OF BILAYER TABLETS OF AMOXICILLIN AND FAMOTIDINE B. Venkateswara Reddy *, K. Navaneetha Department of Pharmaceutics, St.Paul s College
Formulation and evaluation of immediate release salbutamol sulphate
 5 Formulation, optimization and evaluation of immediate release layer of salbutamol sulphate Salbutamol is moderately selective beta (2)-receptor agonist similar in structure to terbutaline and widely
5 Formulation, optimization and evaluation of immediate release layer of salbutamol sulphate Salbutamol is moderately selective beta (2)-receptor agonist similar in structure to terbutaline and widely
FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels
 Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
FORMULATION AND EVALUATIONOF AMOXYCILLIN: THREE-LAYER GUAR GUM MATRIX TABLET
 Gupta and Singh, IJPSR, 2013; Vol. 4(7): 2683-2690. ISSN: 0975-8232 IJPSR (2013), Vol. 4, Issue 7 (Research Article) Received on 05 March, 2013; received in revised form, 29 April, 2013; accepted, 29 June,
Gupta and Singh, IJPSR, 2013; Vol. 4(7): 2683-2690. ISSN: 0975-8232 IJPSR (2013), Vol. 4, Issue 7 (Research Article) Received on 05 March, 2013; received in revised form, 29 April, 2013; accepted, 29 June,
Important Characterization of Nicorandil Buccal Tablets
 Human Journals Research Article February 2015 Vol.:2, Issue:3 All rights are reserved by Anil Kumar R et al. Important Characterization of Nicorandil Buccal Tablets Keywords: Surface ph, Mucoadhesive strength,
Human Journals Research Article February 2015 Vol.:2, Issue:3 All rights are reserved by Anil Kumar R et al. Important Characterization of Nicorandil Buccal Tablets Keywords: Surface ph, Mucoadhesive strength,
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p.
 Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING
 SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING Dr. Dieter Lubda MilliporeSigma is a business of Merck KGaA, Darmstadt, Germany Agenda: Sustained release
SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING Dr. Dieter Lubda MilliporeSigma is a business of Merck KGaA, Darmstadt, Germany Agenda: Sustained release
What is Nanotechnology?
 Use of Nanotechnology to Optimize Delivery of Probiotics and Prebiotics to Target Sites Ian G Tucker Professor of Pharmaceutical Sciences University of What is Nanotechnology? Some people say they have
Use of Nanotechnology to Optimize Delivery of Probiotics and Prebiotics to Target Sites Ian G Tucker Professor of Pharmaceutical Sciences University of What is Nanotechnology? Some people say they have
Gastroretentive drug delivery system: a concise review
 International Journal of Research in Pharmacy and Science Yadav et al., Int J Res Pharm Sci 2016, 6(2) ; 19 24 Review Article Gastroretentive drug delivery system: a concise review Yadav S 1,2, Nyola NK
International Journal of Research in Pharmacy and Science Yadav et al., Int J Res Pharm Sci 2016, 6(2) ; 19 24 Review Article Gastroretentive drug delivery system: a concise review Yadav S 1,2, Nyola NK
ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE
 PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
Floating Drug Delivery Systems: A Review
 610 : A Review Vinay Kumar Katakam, Jagan Mohan Somagoni, Sunil Reddy, Chandra Mohan Eaga, Bala Ramesha Chary Rallabandi, Madhusudan Rao Yamsani* Centre for Biopharmaceutics and Pharmacokinetics, University
610 : A Review Vinay Kumar Katakam, Jagan Mohan Somagoni, Sunil Reddy, Chandra Mohan Eaga, Bala Ramesha Chary Rallabandi, Madhusudan Rao Yamsani* Centre for Biopharmaceutics and Pharmacokinetics, University
Volume 1, issue 4 (2012), *
 Review Article ISSN 2277-3657 Available online at www.ijpras.com Volume 1, issue 4 (2012),20-28 International Journal of Pharmaceutical Research & Allied Sciences Floating Drug Delivery System Bhalla.Neetika
Review Article ISSN 2277-3657 Available online at www.ijpras.com Volume 1, issue 4 (2012),20-28 International Journal of Pharmaceutical Research & Allied Sciences Floating Drug Delivery System Bhalla.Neetika
Formulation and Evaluation of Gastroretentive Floating Drug Delivery System of Atenolol
 DOI:10.2126/ijprhs.2016.0.06 H Kaur et al. CODEN (USA)-IJPRUR, e-issn: 2348-646 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article
DOI:10.2126/ijprhs.2016.0.06 H Kaur et al. CODEN (USA)-IJPRUR, e-issn: 2348-646 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article
Available online through ISSN
 Kumar D et al / IJRAP 2011, 2 (3) 767-774 Review Article Available online through www.ijrap.net ISSN 2229-3566 APPROACHES, TECHNIQUES AND EVALUATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEMS: AN OVERVIEW
Kumar D et al / IJRAP 2011, 2 (3) 767-774 Review Article Available online through www.ijrap.net ISSN 2229-3566 APPROACHES, TECHNIQUES AND EVALUATION OF GASTRORETENTIVE DRUG DELIVERY SYSTEMS: AN OVERVIEW
INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES
 Research Article [Dhakar et al., 3(2): Feb., 12] ISSN: 9767126 INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES Formulation and evaluation of floating tablet of rosiglitazone malate Koushlendra Singh,
Research Article [Dhakar et al., 3(2): Feb., 12] ISSN: 9767126 INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES Formulation and evaluation of floating tablet of rosiglitazone malate Koushlendra Singh,
Formulation and Evaluation of Floating Microsphere Containing Anti Diabetic Drug
 Research Article Formulation and Evaluation of Floating Microsphere Containing Anti Diabetic Drug Manish Dubey 1, Prashant Kesharwani 2 *, Amit Tiwari 2, Roshni Chandel 2, K. Raja 1 and T. Sivakumar 1
Research Article Formulation and Evaluation of Floating Microsphere Containing Anti Diabetic Drug Manish Dubey 1, Prashant Kesharwani 2 *, Amit Tiwari 2, Roshni Chandel 2, K. Raja 1 and T. Sivakumar 1
Antacid therapy: Antacids side effects:
 Antacids Lec:5 When the general public ask why it takes antacids, the answer will include: 1.that uncomfortable feeling from overeating. 2. heart burn. 3. a growing hungry feeling between meals. Antacids
Antacids Lec:5 When the general public ask why it takes antacids, the answer will include: 1.that uncomfortable feeling from overeating. 2. heart burn. 3. a growing hungry feeling between meals. Antacids
FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 974-434 Vol.2, No.1, pp 341-347, Jan-Mar 1 FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS Kothawade S. N. 1 *, Kadam
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 974-434 Vol.2, No.1, pp 341-347, Jan-Mar 1 FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS Kothawade S. N. 1 *, Kadam
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN Research Article
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION DEVELOPMENT AND EVALUATION OF CLARITHROMYCIN ORAL DOSAGE FORM AGAINST HELICOBACTER PYLORI INFECTION
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION DEVELOPMENT AND EVALUATION OF CLARITHROMYCIN ORAL DOSAGE FORM AGAINST HELICOBACTER PYLORI INFECTION
Innovations in Design: NIA-West. Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017
 Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Formulation and evaluation of gastro retentive floating drug delivery system of Atenolol
 2014; 3(5): 11-18 ISSN: 2277-7695 TPI 2014; 3(5): 11-18 2013 TPI www.thepharmajournal.com Received: 05-06-2014 Accepted: 12-06-2014 Kameswara Rao Sankula Assistant Professor, Department of Pharmaceutics,
2014; 3(5): 11-18 ISSN: 2277-7695 TPI 2014; 3(5): 11-18 2013 TPI www.thepharmajournal.com Received: 05-06-2014 Accepted: 12-06-2014 Kameswara Rao Sankula Assistant Professor, Department of Pharmaceutics,
Design and Characterization of Gastroretentive Bilayer Tablet of Amoxicillin Trihydrate and Ranitidine Hydrochloride for H.
 Pharmaceutical Research Design and Characterization of Gastroretentive Bilayer Tablet of Amoxicillin Trihydrate and Ranitidine Hydrochloride for H. pylori Infection Shikha Jain, Vikas Jain, S.C. Mahajan
Pharmaceutical Research Design and Characterization of Gastroretentive Bilayer Tablet of Amoxicillin Trihydrate and Ranitidine Hydrochloride for H. pylori Infection Shikha Jain, Vikas Jain, S.C. Mahajan
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Effect of Excipients on Dissolution: Case Studies with Bio-relevant/ Hydro-alcoholic Media
 Effect of Excipients on Dissolution: Case Studies with Bio-relevant/ Hydro-alcoholic Media Sandip B. Tiwari, Ph.D. Technical Director: South Asia Colorcon Asia Pvt. Ltd., Goa, India DISSO-INDIA 2013, May
Effect of Excipients on Dissolution: Case Studies with Bio-relevant/ Hydro-alcoholic Media Sandip B. Tiwari, Ph.D. Technical Director: South Asia Colorcon Asia Pvt. Ltd., Goa, India DISSO-INDIA 2013, May
