THE CIRCADIAN REGULATION OF FEEDING IN ADULT DROSOPHILA MELANOGASTER
|
|
|
- Sylvia Gallagher
- 5 years ago
- Views:
Transcription
1 THE CIRCADIAN REGULATION OF FEEDING IN ADULT DROSOPHILA MELANOGASTER by Shreya Shekhar A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Cell and Systems Biology University of Toronto Copyright by Shreya Shekhar (2010)
2 The Circadian Regulation of Feeding in Adult Drosophila melanogaster Shreya Shekhar Master of Science Department of Cell and Systems Biology University of Toronto Abstract 2010 In nature, all organisms face the daily challenges created by a fluctuating environment. Circadian clocks synchronize behaviour and physiology allowing an organism to adapt to and predict daily changes to environmental conditions. In the fruit fly, Drosophila melanogaster, circadian clocks reside in a set of ~150 neurons in the brain, collectively referred to as the central clock, and in the cells of many peripheral tissues. The central clock regulates daily behavioural rhythms, whereas peripheral clocks are thought to regulate the local metabolic activities of the cells in which they reside. In this thesis, I demonstrate that a peripheral clock resides in the abdominal fat body, a tissue analogous to the mammalian liver and adipocytes. Moreover, I show that flies display a temporal feeding pattern that is partly regulated by a peripheral clock. I propose that the central clock and peripheral clocks coordinate to regulate the timing of fly feeding behaviour. ii
3 Acknowledgments I would like to start by thanking my supervisor, Joel Levine, for his support and guidance through my graduate studies. It was your confidence in me and my capabilities that have allowed me to grow as a researcher and person. I am grateful to Josh Krupp, for being an excellent teacher and friend, whose valuable lessons will guide me throughout my scientific career. I thank both Joel and Josh for editing the thesis and providing helpful comments. I appreciate Jonathan Schneider s assistance with the statistics performed to analyze the data presented here. I could not have understood it without your easy-to-follow explanations. I am grateful to my thesis supervisors, Drs. Marla Sokolowski, Tim Westwood, and Angela Lange whose advice and suggestions have been helpful in my research. Also, thank you to Dr. Sokolowski for her help in preparing for my exit seminar. I would like to thank all the members of the Levine lab for making the past few years in lab an adventure. Research life could not have been half as fun without the birthday celebrations, badminton tournaments and Christmas parties. I would like to thank Jean- Christophe Billeter for being an excellent teacher in Cloning School. And of course I must thank the girls, Farheen Mohammed, Jade Atallah and Sam Jagadeesh, from Cloning School Who knew making competent cells and doing PCRs could be such fun! Also, thanks to all members for their feedback during general lab meetings and in preparation for the defense exam. A big thanks to past lab members including Olga Sizova, Nancy Stepek, Hania Pavlou, Adrienne Chu and Richard Dunbar-Yaffe. iii
4 I would like to thank all the members of the Sokolowski lab, our extended fly family. The afternoon coffee or evening chats in your lab have always the highlight of my day. Also, your advice during thesis writing and in preparation for the exit seminar was very helpful. Finally, the past few years would not have flown by so quickly without my family. I am grateful for having loving parents, who have always provided encouragement and support. And I am thankful to my brother, Mukul, who has, on more than one occasion, been forced to listen to my ramblings about the thrills and disappointments of an experiment. Lastly, I am grateful that Tigger, our new kitten, walked into my life a month ago. She has been a source of amusement in the final leg of thesis writing. iv
5 Table of Contents Abstract...ii Acknowledgments... iii Table of Contents... v List of Tables... vii List of Figures... viii List of Appendices... ix Chapter 1. Introduction to Circadian Clocks... 1 A Network of Neural Clocks Regulate Daily Locomotor Activity Rhythms in Drosophila... 2 The Neuropeptide, Pigment Dispersing Factor, Synchronizes the Cells of the Central Clock... 3 Circadian clocks Residing in Peripheral Tissues Temporally Regulate Local Metabolic Functions... 5 The Molecular Mechanism of the Circadian Clock... 6 cryptochrome-mediated Entrainment of Circadian Clock Cells... 9 Thesis Objectives Chapter 2. A Peripheral Clock in the Fly Fat Body Introduction Methods Strains Fly Stocks Fly Collections for Dissections Fat Body Dissection Procedure Genomic DNA Extraction Protocol Reverse Transcription Polymerase Chain Reactions and Agarose Gel Electrophoresis Quantitative PCR qpcr Analysis Statistical Analysis Results The Core Clock Genes are Expressed in the Abdominal Fat Body The Abdominal Fat Body Contains a Circadian Clock The Fat Body Clock is Dependent on period Expression Discussion v
6 The Abdominal Fat Body is a Peripheral Clock Chapter 3. Circadian Regulation of Fly Feeding Introduction Methods Strains Fly Collections for Behavioural Assays The Capillary Feeder (CAFE) Assay Analysis of Feeding Statistical Analysis Locomotor Activity Assay Results Flies Display a Circadian Feeding Pattern The Feeding Pattern is Regulated by a period-dependent Clock Peripheral Clocks Regulate the Temporal Pattern of Food Intake Light-Entrainment of the Circadian Clock is Essential for Maintaining Feeding Rhythms The Polymorphism in foraging Affects the Circadian Regulation of Feeding Analyzing Fly Meals Discussion Endogenous Clocks Regulate the Temporal Feeding Pattern Continuous Illumination Disrupts Behavioural Rhythms The Relationship between Feeding and Locomotor Activity The Role of the foraging gene in Feeding Rhythms Defining a Fly Meal Chapter 4. Discussion References Appendix A: Detailed Method for Preparing Fly Food Appendix B: Fat Body Timeseries Data Appendix C: Statistical Analysis vi
7 List of Tables Table 1: The relative RNA expression levels of period, timeless and Clock genes in the fat body tissue of Canton-S flies in a light-dark cycle. Table 2: The relative RNA expression levels of period, timeless and Clock genes in the fat body tissue of Canton-S flies under constant darkness. Table 3: The relative RNA expression levels of period, timeless and Clock genes in the fat body tissue of y w flies under constant darkness. Table 4: The relative RNA expression levels of period, timeless and Clock genes in the fat body tissue of per 01 flies under constant darkness. Table 5: The parameters of cosine curves fit to relative RNA expression levels in the fat body tissue of Canton-S flies. Table 6: The parameters of cosine curves fit to relative RNA expression levels in the fat body tissue of y w and per 01 flies under constant darkness. Table 7: The Statistical results of General Linear Model Repeated Measures tests performed to compare feeding amounts between fly strains. vii
8 List of Figures Figure 1.1: The anatomical location and function of central clock neurons in the fly brain. Figure 1.2: A schematic of the molecular clock in the central clock cells of Drosophila melanogaster. Figure 1.3: Daily resetting of the clock involves a CRYPTOCHROME-mediated pathway. Figure 2.1: The location of fat body cells in the adult fruit fly. Figure 2.2: Clock gene expression is detected in the abdominal fat body of male Canton-S flies. Figure 2.3: A period-dependent peripheral clock resides in the abdominal fat body. Figure 3.1: A diagram of the Capillary Feeder (CAFE) assay. Figure 3.2: Wildtype flies show a temporal feeding pattern in a light-dark cycle and constant darkness. Figure 3.3: Feeding patterns appear disrupted in period mutant flies. Figure 3.4: Neuronal clocks show some involvement in regulating the feeding rhythm. Figure 3.5: Constant light disrupts normal feeding patterns. Figure 3.6: Allelic variation in the foraging gene affects the temporal feeding pattern. Figure 3.7: A pattern in the consumption of large meals appears to drive the temporal feeding pattern. Figure 4.1: Clocks in the brain and peripheral tissues may coordinate to regulate the temporal feeding pattern in adult Drosophila. viii
9 List of Appendices Appendix A: Detailed Method for Preparing Fly Food Appendix B: Fat Body Timeseries Data Appendix C: Statistical Analysis ix
10 Chapter 1. Introduction to Circadian Clocks Daily rhythms in behaviour and physiology have been observed in a wide range of organisms from unicellular bacteria to vertebrates (Dunlap et al., 2004). In one of the first accounts of a documented biological rhythm, the Greek naturalist, Androsthenes, observed that legume plants raise their leaves during the day and fold them at night. In 1729, the French astronomer DeMairan found that mimosa plants continue to raise and fold their stems even in constant darkness (DD) (Dunlap et al., 2004). These early observations led to the theory that biological clocks reside within all organisms and regulate the timing of behaviour and physiology. Internal clocks allow an organism to anticipate and adapt to changes in the physical environment. It is common for factors such as light intensity, humidity and temperature to fluctuate on a daily basis. Survival depends on being able to adjust body physiology and behaviour to anticipate and adapt to such changes (Reviewed in Dubruille and Emery, 2008). For instance, many diurnal organisms adjust their activity in response to the reduction in daylight during winter to be able to continue to find food before nightfall. Although an internal clock maintains time autonomously, its rhythm is not exactly 24 hours and it must synchronize to external time-cues or zeitgebers (a German term meaning time giver ) such as light intensity, temperature and food availability (Reviewed in Dubruille and Emery, 2008). In the laboratory, circadian rhythms are measured in a 12hr:12hr light-dark (LD) cycle resembling the solar day-night cycle. Under these conditions, clock-regulated behaviours synchronize to the lighting conditions and exhibit approximately a 24 hour period. In the absence of external time-cues, the clock becomes free-running and the period either 1
11 2 shortens or lengthens, rarely keeping to 24 hrs. Such clocks are thus called circadian ( about a day ) (Dunlap et al., 2004). The fruit fly, Drosophila melanogaster, has served as the preeminent model to study circadian clocks. Before beginning a study of circadian rhythms, it is necessary to provide a brief summary of the extensive circadian research that has been done in fruit flies. This chapter begins with an introduction to neural clocks and the regulation of daily locomotor activity rhythms. It will be followed by a description of clocks in peripheral tissues and their role in the temporal regulation of local cellular functions. To understand the inner-workings of the clock, the current model of the molecular clock mechanism and its entrainment to the light-dark cycle will be provided. The Drosophila clock is for the most part homologous to the mammalian clock, the similarities and differences between the two systems will be discussed throughout the chapter. A Network of Neural Clocks Regulate Daily Locomotor Activity Rhythms in Drosophila Konopka and Benzer (1971) were the first to identify the link between the molecular clock and behaviour in fruit flies. They isolated mutants with altered pupal eclosion and locomotor activity rhythms, two well-known circadian behaviours. Two mutants displayed rhythms with altered periods, while one mutant showed arrhythmic behaviour. Complementation tests determined that all three phenotypes were caused by mutations in a single locus on the X chromosome, which they named period (per) (Konopka and Benzer, 1971). Subsequently, other studies determined a unique characteristic of per, its mrna and protein levels show circadian oscillations (Hardin et al., 1990; Zerr et al., 1990). The cyclic expression of period is important for maintaining normal behavioural rhythms. In a light-dark cycle, wild type flies display an increase in activity at dawn and dusk each day, corresponding to a transition in lighting (Figure 1.1) (Reviewed in Dubruille and Emery, 2008). Flies anticipate the change in lighting by becoming more active several hours before it occurs. In constant conditions, the bimodal activity pattern becomes unimodal, as
12 3 either the morning peak disappears or the two peaks become merged (Reviewed in Dubruille and Emery, 2008). It is believed that different subsets of clock neurons in the brain regulate different components of locomotor activity rhythms (Grima et al., 2004). Clock neurons in the brain, collectively known as the central clock, are divided into lateral, dorsal and lateral-posterior neurons, so named based on their relative locations in the fly brain (Figure 1.1) (Reviewed in Nitabach and Taghert, 2008). Lateral neurons (LNs) are divided into large-ventrolateral neurons (l-ln v s), 5 small-ventrolateral neurons (s-ln v s) and dorsolateral neurons (LN d s) whereas dorsal neurons (DNs) are categorized as DN1s, DN2s and DN3s (Reviewed in Nitabach and Taghert, 2008). Different groups of clock neurons appear to regulate different aspects of locomotor activity rhythms (Grima et al., 2004). 4 s- LN v s are responsible for the morning activity peak (M peak) whereas the LN d s and the 5 th s- LN v regulate the evening peak together (E peak). These clock neurons also regulate the anticipatory increase in activity corresponding to the M and E peaks. In constant conditions s-ln v s alone are able to maintain rhythmic activity, indicating a more important role for these clocks in DD (Grima et al., 2004). However, the LN d s and the 5 th s-ln v are thought to be responsible for maintaining the phase of the activity pattern. It appears that a complex interaction between the morning and evening oscillators in the fly brain drives locomotor activity rhythms. The Neuropeptide, Pigment Dispersing Factor, Synchronizes the Cells of the Central Clock The only molecular output of the central clock that has been identified in Drosophila is the neuropeptide, Pigment Dispersing Factor (PDF). PDF transmits temporal information between clock neurons in different regions of the brain (Reviewed in Stanewsky, 2002). Communication between these clocks is essential for maintaining locomotor activity rhythms, as pdf-null flies display disrupted rhythms in a light-dark cycle and in constant darkness (Renn et al., 1999). pdf is expressed primarily in the s-ln v s, 4 of the l-ln v s in the fly brain and in the posterior region of the ventral ganglia (Park et al., 2000). Although pdf is
13 4 Figure 1.1: The anatomical location and function of central clock neurons in the fly brain. The three types of brain clock cells are lateral neurons (LNs), dorsal neurons (DNs) and lateral-posterior neurons (LPNs), which are named based on their location in the fly brain. These cells are implicated in regulating daily locomotor activity rhythms in flies. Four small-lnv (s-ln v ) neurons, also known as M-cells, are required for maintaining the morning activity peak in a light-dark cycle. The evening activity peak is regulated by the 5th s-ln v and dorsolateral neurons (LN d s), collectively called the E-cells. In constant darkness, the M-cells are able to control locomotor activity rhythms, although the E-cells regulate peak phase. OL indicates the location of the optic lobes. Figure source: Dubruille and Emery (2008). not expressed cyclically, PDF peptide is detected in nerve terminals in a cyclic manner; with highest and lowest levels detected in the early morning and early night hours, respectively (Park et al., 2000). It is thought that the rhythmic release of PDF is important for synchronizing the timing of different clocks and maintaining behavioural rhythms. The PDF receptor has been identified as a G-protein coupled receptor. PDF expression is found in close proximity to cells expressing PDFR (Mertens et al., 2005). However, there are discrepancies regarding where the ligand and receptor are expressed as some LN d s are responsive to PDF but do not express PDFR (Im and Taghert, 2010). Also, mutations in pdf and pdfr show similar but not identical phenotypes (Mertens et al., 2005). This suggests
14 5 there may be additional receptors which receive PDF input or that PDFR may have a second ligand (Im and Taghert, 2010). Circadian clocks Residing in Peripheral Tissues Temporally Regulate Local Metabolic Functions Oscillators also exist in tissues outside of the central clock cells. These peripheral clocks are found in tissues such as the antennae, proboscis, prothoracic gland, gut and oenocytes (Chatterjee et al., 2010; Emery et al., 1997; Reviewed in Hardin, 2005; Krishnan et al., 1999; Krupp et al., 2008). Similar to central clock cells, period mrna levels also cycle in peripheral clock cells (Hardin, 1994). In mammals, clocks in peripheral tissues are unable to maintain rhythmicity and are thought to be entrained by the central clock, a group of neurons in the Suprachiasmatic Nuclei (SCN) of the hypothalamus (Reviewed in Balsalobre, 2002). Using a luminescence assay, it was shown that cultured rat SCN maintains rhythmicity in constant darkness whereas peripheral clocks are less robust and damp after several days (Yamazaki et al., 2000). Since the central clock synchronizes peripheral oscillators, it appears that the peripheral clocks are linked to the SCN in a master-slave relationship (Reviewed in Balsalobre, 2002). A similar relationship between central and peripheral clocks is not observed in fruit flies. Isolated peripheral tissue clocks can entrain to light without cues from central clock cells (Plautz et al., 1997). per-driven bioluminescence was measured in isolated tissue cultures of the proboscis, antenna, legs, and wings. When shifted lighting conditions were imposed, these tissues re-synchronized indicating that peripheral clocks are able to maintain time autonomously independently of the central clock (Plautz et al., 1997). In peripheral tissues, an internal clock is thought to regulate the rhythms of local processes. For example, the clock in oenocytes appears to regulate the synthesis of cuticular hydrocarbons (Krupp et al., 2008). The peripheral oenocyte clock generates these rhythms by controlling the expression of a key enzyme, Desaturase1 (Desat1), involved in cuticular hydrocarbon synthesis. Other peripheral clocks are found in the chemosensory bristles of the antennae and proboscis, the smell and taste organs of the fruit fly (Chatterjee et al., 2010;
15 6 Krishnan et al., 1999). These clocks regulate circadian rhythms in olfactory and gustatory sensitivity. Due to the importance of taste and smell to feeding behaviour, the oscillators in the chemosensory organs are hypothesized to regulate the timing of foraging behaviour and food intake. The Molecular Mechanism of the Circadian Clock Since the identification of period, several other genes which also show circadian regulation have been identified, and a molecular model of the circadian clock has been assembled. In Drosophila, this involves two transcriptional-translational feedback loops which control the expression of several genes (Reviewed in Hardin, 2005). In the first loop (Figure 1.2), the major clock genes involved are Clock (Clk), cycle (cyc), period (per), timeless (tim) and doubletime (dbt). CLOCK and CYCLE are transcription factors containing a PER-ARNT- SIM (PAS) protein-protein binding domain and a basic helix-loop-helix (bhlh) DNA binding domain. Clk is under circadian control and is expressed cyclically, whereas cyc is constitutively expressed and remains at a constant level. Clk mrna and protein levels peak in the early morning and are lowest in the early evening (Reviewed in Hardin, 2005). When expressed, CLK heterodimerizes with CYCLE and upregulates the transcription of two other genes which also encode transcription factors, period and timeless. In the middle of the day, the CLK-CYC heterodimer bind both the E-box sequences, conserved sequences of six nucleotides, in the regulatory regions of per and tim genes (Reviewed in Stanewsky, 2002). This leads to an increase in per and tim transcription in the early evening (Reviewed in Hardin, 2005). The PER and TIM proteins are located in the cytoplasm, where they reach peak levels by late night. When PER is expressed, it is phosphorylated by two kinases, DBT and CASEIN KINASE 2 (CK2), this leads to its destabilization and degradation (Reviewed in Stanewsky, 2002). It is thought that the kinase, PROTEIN PHOSPHATASE 2a (PP2a), counteracts by removing phosphates added to PER. Together, this process regulates the timing at which PER reaches maximum expression levels. As TIM levels increase, it heterodimerizes with
16 7 phosphorylated PER, and increases its stability (Reviewed in Hardin, 2005). The interaction between the two proteins is achieved via PER s PAS domain and TIM s ARMADILLO-like domains (Reviewed in Stanewsky, 2002). It is thought that DBT binds to PER and the entire TIM-PER-DBT complex proceeds to the nucleus (Reviewed in Hardin, 2005). However, the kinases, SHAGGY (SGG) and CK2 first phosphorylate TIM and PER, respectively. Once it enters the nucleus, the TIM-PER-DBT complex binds to the CLK-CYC heterodimer and prevents further expression of per and tim. In the nucleus, DBT phosphorylates PER and CLK, which causes their degradation. TIM is degraded via a light-mediated pathway, which resets the clock mechanism (Reviewed in Hardin, 2005). In the second transcriptional-translational feedback loop, the transcription factors encoded by vrille (vri) and par domain protein 1ε (pdp1ε) genes regulate Clk expression (Reviewed in Hardin, 2005). The CLK-CYC heterodimers bind to the E-box sequences in the promoter regions of these genes and activate transcription. VRI reaches peak levels late in the day, and begins binding to a VRI/PDP1ε box (V/P box) in the regulatory region of Clk and represses its transcription (Reviewed in Hardin, 2005). This repression is offset by PDP1ε. After PDP1ε protein reaches peak levels in the mid to late evening hours, it competes with VRI for the V/P box and when bound it activates Clk transcription (Cyran et al., 2003). As a result Clk mrna and protein levels fluctuate rhythmically each day. The two interacting feedback loops keep time by regulating Clk, per and tim expression. The mammalian clock is similar to the one described in Drosophila however, it is more complex and involves multiple isoforms of some clock proteins. It is composed of two transcriptional-translational feedback loops which regulate gene expression (Reviewed in Reppert and Weaver, 2002). In the positive loop, BMAL1, a homolog of the Drosophila CYCLE, and CLOCK transcriptional factors heterodimerize and upregulate the transcription of three mperiod (mper) and two mcryptochrome (mcry) genes (Reviewed in Reppert and Weaver, 2002). Following protein expression, the mcry proteins act as negative transcription factors that repress transcription by binding to the BMAL1-CLOCK
17 8 Figure 1.2: A schematic of the molecular clock in the central clock cells of Drosophila melanogaster. The cycle begins when a CLOCK-CYCLE heterodimer binds to regulatory elements in the period (per) and timeless (tim) genes, and activates their transcription. Once expressed, per and tim mrnas translocate to the cytoplasm where these are translated into proteins. A number of kinases regulate the stability of these proteins. After TIM and PER proteins accumulate, they form a protein complex with the kinase, DOUBLETIME (DBT). Subsequently, the TIM-PER-DBT complex represses per and tim transcription by binding to CLK-CYC. TIM, PER and CLK are degraded via the proteasomal pathway and the clock restarts. CK2, CASEIN KINASE 2; Sgg, SHAGGY; PP2a, PROTEIN PHOSPHATASE 2a. Figure source: Hardin (2005). heterodimer. The negative transcriptional loop regulates Bmal1 transcription. The BMAL1- CLOCK heterodimer activates transcription of Rev-Erbα, which encodes a transcription factor that binds to a Rev-Erb/ROR regulatory site and represses Bmal1 transcription. The interaction between these loops results in a 12 hour phase difference between the expression of Bmal1 and mper/mcry genes (Reviewed in Reppert and Weaver, 2002). Thus, the
18 9 mammalian clock regulates gene expression via two transcriptional/translational feedback loops homologous to those found in flies. cryptochrome-mediated Entrainment of Circadian Clock Cells Light is thought to act as the strongest zeitgeber in entraining the circadian clock (Reviewed in Hardin, 2005). In flies, light entrainment occurs through three possible routes: external photoreceptors in the compound eyes and possibly the ocelli, extraocular photoreceptors, and a blue-light photoreceptor encoded by cryptochrome (cry) (Helfrich-Forster et al., 2001). As a result, mutant flies which do not have external and extraocular eye structures and are deficient in cry expression are unable to entrain molecular and behaviour cycles to light cues, and instead display free-running rhythms (Helfrich-Forster et al., 2001). The CRY photoreceptor mediates light input into neural and peripheral clock cells, however its mode of action is not well known. Similar to other clock genes, cry transcription is under circadian regulation. CRY protein levels are regulated by the light-dark cycle (Reviewed in Hardin, 2005). When light enters a cell, it either causes an inhibitor to separate from CRY or causes the protein to undergo a conformational change (Figure 1.3). This allows CRY to interact with the TIMELESS protein and trigger its phosphorylation by a tyrosine kinase, and subsequent proteasomal degradation (Reviewed in Hardin, 2005). It has been proposed that the kinase SHAGGY interferes in this process by binding to CRY and preventing TIM degradation (Reviewed in Dubruille and Emery, 2008). An F-box protein JETLAG (JET) is also involved in upregulating TIM degradation, although it is unclear where it fits in the scheme of events (Reviewed in Dubruille and Emery, 2008). Thus, TIM degradation via the CRY photoreceptor leads to the daily resynchronization of the molecular clock.
19 10 Figure 1.3: Daily resetting of the clock involves a CRYPTOCHROMEmediated pathway. When light enters clock cells in the brain, it activates a blue-light photoreceptor called CRYPTOCHROME (CRY) by possibly triggering a conformational change. CRY participates in the phosphorylation of TIM s tyrosine residue. The phosphorylated TIM enters a pathway that involves the JETLAG (JET) protein and is degraded in the proteasome. Figure source: Dubruille and Emery (2008). It has been suggested that CRY also plays a direct role in entraining some peripheral oscillators (Reviewed in Hardin, 2005). A missense mutation in cry was found to disrupt rhythmicity in antennal clock cells whereas the central clock was undisturbed (Krishnan et al., 2001). In constant darkness, cry mutant flies display disrupted olfactory response rhythms. Upon measuring per-driven bioluminescence from the antennae of control and mutant flies, it was discovered that the molecular clock in mutant antennae is less rhythmic than control antennae (Krishnan et al., 2001). This mutation, however, does not affect oscillator function in the brain, indicating that cry may have an additional function in peripheral tissues (Krishnan et al., 2001; Stanewsky et al., 1998). It has also been shown that the cry mutation affects the rhythmic expression of period and timeless in various clocks in the fly body, confirming that cry may be a direct participant in these clocks (Levine et al., 2002).
20 11 Although daily light cues are required for synchronizing the molecular clock, constant light has a detrimental effect on circadian clocks and behaviours. Constant low intensity light affects the period of locomotor activity rhythms in wild type flies (Konopka et al., 1989). Alternatively, high intensity light has an even more drastic effect as some flies become completely arrhythmic (Konopka et al., 1989). cry appears to be involved in this response as cry-knockout flies display normal locomotor activity and eclosion rhythms in constant light (Dolezelova et al., 2007). The disruption of the molecular clock is thought to be a consequence of continuous TIM degradation (Reviewed in Dubruille and Emery, 2008). Thus, the CRY photoreceptor plays a key role in entraining the molecular clock to the solar day. Thesis Objectives The aim of the thesis is to study the circadian regulation of feeding in adult Drosophila melanogaster. Circadian clocks synchronize behaviour to the fluctuating environment (Dunlap et al., 2004). In mammals, feeding behaviour is clock regulated and shows rhythmic changes each day (Rosenwasser et al., 1981). Due to the parallels between the circadian mechanisms of Drosophila and mammals, I hypothesize that clocks regulate feeding in fruit flies. Feeding is directly related to the metabolic needs of the body. Since activity depletes nutrients in the body, it is possible for feeding events to be organized around rest/activity cycles. This suggests that metabolic tissues may contain circadian clocks which regulate the timing of metabolic activities. In Chapter 2, I examine whether the fly fat body, which is homologous to the mammalian hepatocyte and adipose tissues (Reviewed in Canavoso et al., 2001), contains a circadian clock. I find that the core clock genes are circadianly expressed in a period-dependent fashion. Additionally, I assess the temporal pattern of feeding in fruit flies in Chapter three. I report that flies display circadian feeding rhythms, which are regulated in part by the central clock. I propose that the fat body clock and other metabolically related clocks may coordinate with the central clock to regulate feeding. In the final Chapter, I present a model of the circadian regulation of feeding. This study sheds new light on the regulation of a fundamental metabolic behaviour.
21 Chapter 2. A Peripheral Clock in the Fly Fat Body In mammals, peripheral clocks are known to reside in several of the primary metabolic tissues involved in carbohydrate and lipid metabolism, including the liver and adipocytes (Reviewed in Challet, 2010). Given that the fat body of the fly is generally considered to be functionally homologous to these tissues (Reviewed in Canavoso et al., 2001), I hypothesize that it too may contain a circadian clock. In this Chapter, I demonstrate that the core clock genes are expressed in the fat body of male wildtype flies, and that the temporal expression patterns of these genes under various light conditions and in a clock mutant fulfill the basic criteria used to determine the existence of a circadian clock. Introduction In the past sixty years, extensive research examining the feeding behaviour of rodents has allowed humans to gain a better understanding of this innate behaviour. In 1946, Brooks et al. were the first to document the nocturnal feeding rhythm of rats. It was observed that in a 12:12 light-dark cycle, rats consume approximately 70% of their total daily food intake during the night (Brooks et al., 1946). It was later reported that the feeding pattern of rats consists of two peak feeding times occurring at the beginning and at the end of the dark period (Rosenwasser et al., 1981). Although this originally appeared to be a direct response to light cues, experiments conducted in constant darkness demonstrated that rats continue to exhibit a feeding rhythm even in the absence of environmental cues, suggesting that the behaviour is clock-regulated (Rosenwasser et al., 1983). In mammals, it has yet to be determined which tissue specific clocks, or combination thereof, are involved in regulating feeding. Previously, it was shown that nocturnal feeding patterns are abolished in rats with hypothalamic lesions suggesting that the central clock, which resides in the Suprachiasmatic Nuclei (SCN) of the hypothalamus, regulates feeding 12 *Xu et al. (2008) published a study about the fat body clock during this thesis.
22 13 (Brooks et al., 1946). However, given that the central clock is thought to send timing signals to other clocks in the body it is possible that clocks in peripheral tissues may also be involved in regulating the time of feeding (Yamazaki et al., 2000). Feeding is thought to be regulated through the coordination between SCN clock and peripheral clocks. Vertebrate studies suggest that clocks in the liver and adipose tissues, two of the primary metabolic sites, may regulate food intake in mammals. There appears to be a link between feeding and the hepatocyte clock, as restrictive feeding has a direct effect on the peripheral clock. When rats were restrictively fed in the daytime of a light-dark cycle, the liver clock synchronized to the feeding time, shifting 10 hours within two days (Stokkan et al., 2001) whereas the timing of molecular clock in the SCN was unaffected. This result indicates that there exists a connection between feeding and the liver clock that functions independently of the SCN. Adipocytes regulate feeding in mammals via a circadianly regulated hormone, leptin (Froy, 2007; Zhang et al., 1994). Leptin is produced primarily in fat tissues and the level of expression is proportional to the amount of fat in the body (Reviewed in Friedman and Halaas, 1998). When expressed, leptin is released into the blood plasma in a diurnal pattern, with peak levels observed following lights-off (Kalsbeek et al., 2001). Leptin travels to the brain, and binds to receptors in the hypothalamus, where it suppresses food intake (Reviewed in Friedman and Halaas, 1998). The central clock appears to regulate leptin levels as SCN lesioned rats do not show a diurnal pattern in plasma leptin levels (Kalsbeek et al., 2001). This indicates that leptin may act as an indirect route through which the SCN regulates feeding rhythms. Although the regulators of feeding remain unknown, it is hypothesized that the mammalian liver and adipose clocks could be involved. Since the circadian systems of mammals and Drosophila share a high degree of similarity, it is possible that feeding behaviour in flies is also under circadian regulation. If this is the case, it may be possible to determine the source of the feeding rhythm in flies as the brain clock and peripheral clocks are thought to be independent circadian oscillators (Plautz et al.,
23 ). A possible site for the feeding clock may be the fat body, the fly homologue of the mammalian liver and adipose tissue (Reviewed in Canavoso et al., 2001). The fat body is a metabolic tissue that stores lipids, proteins and glycogen (Reviewed in Canavoso et al., 2001). Although it is present throughout the body, a majority of fat body tissue is found in the abdomen (Figure 2.1) (Miller, 1950). In females, a number of fat body cells also reside close to the ovary and play a role in reproduction (Miller, 1950). Fat body tissue is present in the larval, pupal and adult stages of a fly. The larval fat body provides energy during pupation and early adult stages, when the fly is unable to eat (Aguila et al., 2007). Afterwards, cell death occurs and these cells are replaced de novo by the adult tissues (Butterworth et al., 1988; Miller, 1950). Although the larval and adult fat body cells arise at different developmental stages, they are thought to have similar functions. Figure 2.1: The location of fat body cells in the adult fruit fly. Fat body tissue in the head, thorax and abdomen are outlined in blue. In the abdominal segment, fat body cells lie as sheets of tissue above the oenocytes which are found in the segmental overlaps (black circles). Figure source: Miller (1950). One of the primary functions of the larval fat body is to regulate lipid storage and mobilization. Lipid metabolism is a homologous process in mammals and fruit flies. The main differences arise from the organs that are involved and their functions. In mammals, lipids are processed predominantly by the liver and adipose tissues, whereas in Drosophila
24 15 larvae, the fat body and larval oenocytes, a tissue that has been implicated in hydrocarbon synthesis in insects (Fan et al., 2003), coordinate in metabolizing lipids (Gutierrez et al., 2007). In a satiated larva, lipids accumulate predominantly in the fat body and midgut epithelial cells. Once food is ingested, lipids are transported by lipophorin molecules from the midgut to the fat body where they are converted from diacylglycerols (DAGs) to triacylglycerols (TAGs) and stored (Reviewed in Canavoso et al., 2001). In starvation conditions, lipids are transported from the fat body to the larval oenocytes (Gutierrez et al., 2007). Following the movement, energy is mobilized from the oenocytes throughout the body. In this way, the oenocyte and fat body tissue together act as the mammalian hepatocyte and adipocyte. Although not demonstrated, the fat body of the adult fly is thought to perform the identical function of its larval counterpart. Here, I examine the expression of the core clock genes in the adult abdominal fat body using quantitative PCR. I further investigate whether expression levels are altered in the fat body of the clockgene-mutant, [ per0]. I report that clock gene expression oscillates in the fat body in a period-dependent manner, suggesting that a clock resides in this tissue. A peripheral fat body clock may act to synchronize the timing of metabolic processes, and could be involved in influencing circadian behaviours. Methods Strains Canton-S was used as the wild type strain for quantitative PCR experiments. Mutant strains are described within the results. Fly Stocks Flies were raised in polypropylene food bottles (Fisher Scientific catalog no. AS-355) with agar-based fly food. A detailed method for preparing fly food is provided in Appendix A. Stock bottles were kept in an incubator with a 12:12 light-dark cycle (LD cycle). The
25 16 temperature and humidity in the incubator were maintained at approximately 25 C and 40%, respectively. Stock bottles were changed once a week and were thrown out after 16 days of use. Food vials (9.4 cm narrow mouthed, polystyrene vials - Fisher Scientific catalog no. AS-515), which were used to house flies prior to an experiment, also contained standard fly food. Fly Collections for Dissections Flies were collected into food vials on the first day after eclosion (from day old bottles) and placed into an incubator. The next day male flies were sorted on a carbondioxide anaesthetizing pad (CO 2 pad) and females were discarded. Male flies that were collected for mass dissections were ushered into fresh food vials. For timeseries dissections, pairs of males were placed into individual 10x75mm glass culture tubes (VWR catalog no ) filled with 1mL of fly food. Flies were kept in the incubator for a minimum of 3 days prior to dissections in order to entrain them to the light-dark cycle. Fat Body Dissection Procedure Fat body tissue samples were collected from 5-7 day old male flies. An individual fly was first placed on a CO 2 pad for seconds to anaesthetize it. It was then transferred to a dissecting plate where its legs were removed with forceps and it was pinned down with tungsten pins inserted into its neck and genital areas. A 1mL glass pipette was then used to cover the fly with liquid Shields and Sang M3 insect media (Sigma Aldrich catalog no. S3652). Using forceps, a cut was made on the ventral side of the fly extending from the thorax to the genitals. Forceps were used to remove the guts and to separate the thorax from the abdomen. The cuticle was then pinned down on either side of the fly so that the inside of the abdomen lay flat on the plate. Using a tungsten needle, fat body tissue was detached from other abdominal tissues and suspended in a 1.5mL Eppendorf tube containing 1% ß- mercapto-ethanol in RLT buffer. Tissue samples were stored in a -80 C freezer. Prior to running PCR reactions, RNA was first extracted from fat body tissue samples using
26 17 MinElute Spin columns (Qiagen RNeasy Micro Kit catalog no ). It was reverse transcribed to cdna using the QUANTA cdna synthesis kit (Quanta Biosciences catalog no ). Genomic DNA Extraction Protocol 1-2 day old male flies were anaesthetized on a CO 2 pad and collected in an Eppendorf tube. To acquire genomic DNA, standard lab protocol was followed (Hamilton and Zinn, 1994). To purify the DNA, an additional step involved extracting with a phenol, chloroform, isoamyl alcohol solution (25:24:1). After centrifugation, the supernatant containing genomic DNA was transferred into another tube. Using a phenol solution, a phase separation was created and the genomic DNA was extracted into the aqueous phase. Reverse Transcription Polymerase Chain Reactions and Agarose Gel Electrophoresis The gene products of cycle, Clock, timeless, period, cryptochrome and pigment dispersing factor receptor were PCR amplified with Taq DNA polymerase (NEB catalog no. M0320L). The positive PCR controls were set up with genomic DNA. The sequences for the primer sets are the following: cyc F1: 5 -GGA GCT GGA GGA CGT ATC G-3 and cyc R1: 5 - TCA AGA TGA TTA TCC TGC AAG-3 ; Clk F3: 5 -GGA TAA GTC CAC GGT CCT GA-3 and Clk R3: 5 -CTC CAG CAT GAG GTG AGT GT-3 ; tim F11: 5 -CCT ATG TGG TCA ACC CGA AT-3 and tim R11: 5 -TAC ATC ACG TCC ACG GAG AA-3 ; per F12: 5 -GGT TGC TAC GTC CTT CTG GA-3 and per R12: 5 -TGT GCC TCC TCC GAT ATC TT-3 ; cry F1: 5 -ATG TCG GGA GCT GAA TAT CG-3 and cry R1: 5 -CAG GAA GCC CAT GTT GTC TC-3 ; pdfr F1: 5 -GCC ACG ACT AGC GGT CAT AC-3 and pdfr R1: 5 -TGG GTG GCC AGA CTC TTT AG-3. PCR was conducted in a thermal cycler with the following settings: 1 cycle (94 C for 2 min), 25 cycles (94 C for 15s, 55 C for 15s and 72 C for 45s), 1 cycle (72 C for 5 min) and hold (4 C). Following PCR, products were run on a 1.2% agarose gel in a 1% Tris Acetate-EDTA buffer at a speed of 90V/hour. The
27 18 gel was stained in an Ethidium Bromide solution for approximately 25 minutes. It was destained in the presence of magnesium sulphate (MgSO 4 ) salt for 20 minutes and visualized under UV radiation. Quantitative PCR In quantitative PCR (qpcr), the amount of DNA in a sample is estimated based on the level of fluorescence emitted by a reporter molecule in the reaction mix (Stratagene., 2004). SYBR Green is a commonly used reporter dye which fluoresces more brightly once it binds to double stranded DNA. Thus, the level of fluorescence emitted by the reporter is directly proportional to the amount of DNA. Real time qpcr is a sensitive technique where the amount of DNA at the end of several PCR cycles is used to estimate the DNA quantity in the original sample. The output of a qpcr reaction is a threshold cycle (CT) value, which is the 1 st cycle where the fluorescence signal was greater than the background noise (Stratagene., 2004). A reference dye called ROX is also used in the reaction mix to reduce differences caused by factors such as pipetting and plastic transparency between adjacent samples. qpcr reactions were conducted with fat body samples from time series dissection experiments. In this experiment, 5or 8 flies were dissected in 2 hour intervals at 8 timepoints (CT1, 4, 7, 10, 13, 16, 19 and 22) in a 12:12 light-dark cycle or on the first day of constant darkness. As some timepoints were conducted in the dark period, glass culture tubes with flies were wrapped in aluminum foil to prevent light exposure. The fat body samples from two time series dissection experiments were combined to provide sufficient RNA for quantitative PCR. After RNA extraction and reverse transcription duplicate or triplicate qpcr reactions were prepared with the Quanta SYBR Green qpcr kit (Quanta Biosciences catalog no ). The relative RNA levels of ribosomal protein 49 (rp49), tim, per and Clk genes were determined from fat body samples. The primer set used for amplifying rp49 is the following: F1: 5 -ATC GGT TAC GGA TCG AAC AA-3 and R1: 5 -GAC AAT CTC CTT GCG CTT CT-3. The primer sequences for tim, per and Clk were provided earlier. qpcr was conducted in an Mx 3005P Sequence Detection System with the
28 19 following settings: 1 cycle (95 C for 3 min), 40 cycles (95 C for 30s, 60 C for 1min, 72 C for 1min) and 1 cycle (95 C for 1 min, 55 C for 30s, 95 C for 30s). qpcr Analysis After conducting a SYBR Green qpcr experiment, data was acquired from the Mx3005P v.3.20 (Stratagene) program by converting the experiment to a comparative quantitation experiment. The REST relative expression method was used to quantify the relative RNA levels with rp49 as the normalizing gene (Pfaffl, 2001). Microsoft Excel 2007 was used to conduct all calculations and plots were made in Sigmaplot v Statistical Analysis A non-linear regression analysis was performed using SPSS v.16.0 statistical software to fit a cosine curve to the expression data with the following equation, y = a + (b*cos (2π* (CT hours - h))/d). The expression data from one timeseries dissection experiment was used for curve fitting. Additional timeseries data is provided in Tables 1-4 in Appendix B. a, b, and h, and d variables which were used to estimate cosine curve properties are provided in Tables 5 and 6 in Appendix C. a estimates the y-intercept of the curve while b and h represent the amplitude and phase, respectively. d, which estimates the period, was constrained to 24 hours. 95% confidence intervals for b, which were provided in the statistical output, test the null hypothesis b=0, and determine whether a cosine curve is significantly different from a straight line. The r-squared values, which are also provided in the SPSS output, estimate how well cosine curves fit the expression data. Results The Core Clock Genes are Expressed in the Abdominal Fat Body In order to examine whether the abdominal fat body has a circadian clock, standard molecular techniques were used to confirm that the core clock genes are expressed in this
29 20 tissue. First, abdominal fat body tissue was dissected from male wildtype flies. The transcripts for cycle, Clock, timeless, period, cryptochrome and pigment dispersing factor receptor were amplified from fat body cdna via reverse transcription PCR. Genomic DNA served as a positive control for the PCR reactions. The expression of all six clock genes was Figure 2.2: Clock gene expression is detected in the abdominal fat body of male Canton-S flies. cycle, Clock, timeless, period, cryptochrome and pigment dispersing factor receptor were amplified via real time PCR from genomic DNA (G), and fat body cdna (C) samples. Products were run on a 1.2% agarose gel in standard 1% TAE buffer at a speed of 90V/hour. Arrowheads indicate the location of the predicted PCR products. cdna was acquired from dissected fat body samples of 5-7 day old male flies (n=38). Genomic DNA was extracted from male wildtype flies (n=30) and served as the positive control. observed in the abdominal fat body (Figure 2.2). Genomic bands are larger than fat body cdna bands because genomic DNA contains introns which are spliced out once the mrna is produced. I detected the expression of cyc, Clk, tim and per clock genes in the fat body indicating the presence of a molecular clock. The presence of cry expression suggests that a peripheral clock in the fat body may be directly entrained by light. pdfr expression was also
30 21 observed in the fat body, an indication that the fat body clock may interact with the central clock via the neuropeptide pigment dispersing factor. The Abdominal Fat Body Contains a Circadian Clock The circadian clock is characterized by the cyclic expression of the timeless, period and Clock genes, a feature thought to be an important part of the transcriptional-translational feedback loops of the molecular clockworks. To determine whether a clock resides in the abdominal fat body, this tissue was dissected at set time points occurring at 4 hour intervals over a 24 hour period, and the relative expression level of each of the core clock genes was quantified by quantitative PCR. In a light-dark cycle, the expression of timeless, period and Clock genes is significantly cyclic in male Canton-S fat body cells (b, P-value<0.05; Figure 2.3A). Clk mrna levels peak in the morning (~ZT3), whereas tim and per expression reach their highest levels approximately 12 hours later. In constant darkness (Figure 2.3B), the amplitude of the expression profiles for all three genes are statistically significant (b, P- value<0.05). Together these results strongly support the existence of a peripheral clock in the cells of the abdominal fat body. The Fat Body Clock is Dependent on period Expression The rhythmic oscillation in period expression is an integral part of the molecular timekeeping mechanism of the circadian clock. To further study the fat body clock, I examined whether clock gene expression is altered in the period-null mutant, per 01 (in a yellow white (y w) genetic background), a nonsense mutation in the third exon of the period gene (Yu et al., 1987). In male y w genotype control flies, tim and per show significant cycling in constant darkness (b, P-value<0.05, Figure 2.3C), whereas the amplitude for the Clk cosine curve is not statistically significant (b, P-value>0.05). The levels of tim and per mrna peak around CT 17, similar to the time when the same genes peak in Canton-S flies in DD. The expression profile of Clock, however, is reduced compared to the wild type
31 22 Figure 2.3: A period-dependent peripheral clock resides in the abdominal fat body. timeless, period and Clock expression patterns in the fat body of male Canton-S, y w and per 01 flies. In the CS fat body, cyclic clock gene expression is detected in a 24 hour light-dark cycle (A) and in constant darkness (B). y w flies show similar expression levels in constant darkness for per and tim genes (C). Comparatively, per 01 mutant flies show arrhythmic expression for all three genes in DD (D). Expression levels were quantified via quantitative PCR. Cosine curves are fit to RNA expression levels from one experiment (n=1) ± SEM (see Tables 1-4 in Appendix B for additional timeseries data). The white, grey and black horizontal bars underneath plots represent the day, subjective day and night periods, respectively.
32 23 profile. The per[0] mutation disrupted the profile of clock gene expression (Figure 2.3D); the amplitude of expression for all three genes is reduced and not significantly different from a flat line (b, P-value>0.05). Together these results suggest that abdominal fat body cells in Drosophila contain a period-dependent peripheral clock. Discussion The Abdominal Fat Body is a Peripheral Clock I established that the cells of the abdominal fat body of D. melanogaster contain a circadian clock. These cells express the core clock genes cycle, Clock, timeless and period as well as the clock-related genes cryptochrome and pigment dispersing factor receptor. Furthermore, I have demonstrated the existence of a functional fat body clock by illustrating the cyclic expression of per, tim and Clk in a light-dark cycle and constant darkness. In wild type flies, Clk mrna peaked in the morning whereas tim and per levels peaked in the early evening. The temporal expression profile of these genes in the fat body is consistent with that previously observed in the adult head and abdomen (Hardin, 1994; Hardin et al., 1990). The expression of the four main clock genes suggests that both the CLK-CYC and PER-TIM feedback loops exist in the fat body (Reviewed in Hardin, 2005). Recently, it was reported that the abdominal fat body contains a peripheral clock (Xu et al., 2008). Similar to my results, it was shown that tim mrna levels in the abdominal tissue preparation are highest in the evening. Whereas I quantified clock gene levels in isolated fat body tissue, the authors of the aforementioned study quantified gene expression from a dissected preparation which included a mixture of several tissues associated with the abdominal cuticle (i.e. abdominal fat body, epithelial cells, oenocytes and cardiac tissue). Several of these tissues including the oenocytes and epidermal cells have been previously shown to be peripheral clocks (Ito et al., 2008; Krupp et al., 2008), making the interpretation of their data impossible. In addition, I have shown that the cyclic expression of all three clock genes is reduced or completely absent in period-null mutants, confirming that the fat body clock is perioddependent. In the absence of a functional PER protein, it is likely that both transcriptional
33 24 feedback loops are disrupted as they are interconnected, thus preventing the rhythmic expression of all three clock genes (Reviewed in Hardin, 2005). Similarly, the Clk jrk mutation, which causes dysfunctional CLK protein expression (Allada et al., 1998), was previously shown to disrupt the rhythmic expression of timeless in the abdominal cuticle preparation containing fat body tissue (Xu et al., 2008). The fat body is known to store glycogen and lipids, two principal nutrient sources (Reviewed in Canavoso et al., 2001). Adipokinetic hormone (AKH), a homolog of mammalian glucagon, regulates the mobilization of trehalose and lipids from the fly fat body into the hemolymph to provide usable energy (Lee and Park, 2004). Recently, it was found that the corpora cardiaca, the tissue where AKH is produced and released, is also a circadian clock (Personal communication with Ayesha Malik, Joshua Krupp and Joel Levine). While it remains to be determined if the production or the release of AKH is under circadian regulation, it is possible this hormone may act as a synchronizing signal from the clock in the CC to the fat body clock. Tissues involved in lipid metabolism may time lipid mobilization and breakdown around the fly s behavioural activity. Since lipids are thought to move to the oenocyte from the fat body (Gutierrez et al., 2007), perhaps the oenocyte clock is also involved in coordinating the timing of lipid mobilization. pigment dispersing factor or other neuropeptides emanating from the central clock may synchronize the timing of clocks in these tissues. Future experiments for this project involve disrupting the fat body clock and determining its role in regulating feeding rhythms. I plan to do this by expressing Clk-RNAi or cyc-rnai using a fat body driver. It is hypothesized that if the fat body clock is disrupted, the feeding pattern will be attenuated in flies. Thus far, the only obstacle to this experiment has been finding a proper fat body driver, expressed solely in the abdominal fat body cells. Several larval fat body drivers have been studied, but most are not fat body specific in adults. The two fat body drivers that can be used are the larval serum protein 2 (lsp2)-gal4 driver and the r 4 -gal4 driver, both of which are expressed in a tissue-specific manner in adult
34 25 Drosophila (Dauwalder et al., 2002; Lee and Park, 2004). Alternatively, this tissue can be ablated by expressing the pro-apoptotic gene, reaper, in the fat body.
35 Chapter 3. Circadian Regulation of Fly Feeding Feeding behaviour is strictly regulated to meet the metabolic demands of a physically active animal. How feeding behaviour is synchronized with an animal s active state to meet its metabolic requirements is not clear. In mammals, feeding rhythms are generally considered to be under circadian regulation (Rosenwasser et al., 1981), however, the circadian system that regulates feeding patterns, be it the clock in the SCN or a metabolic tissue like the liver, has not been clearly identified. As a means to gain insight into the circadian processes regulating feeding behaviour, I utilized the model organism Drosophila melanogaster. In this Chapter, I examine the temporal organization of fly feeding behaviour, and demonstrate (1) that feeding is under circadian regulation, and (2) that a peripheral clock, at least in part, is involved in modulating feeding rhythms. I hypothesize that the circadian regulation of feeding behaviour in fruit flies involves the coordination of the central clock in the brain and one or more peripheral clocks residing in metabolic tissues. Introduction Rodents have been a useful model system with which to study the circadian regulation of feeding behaviour. Rodents display a nocturnal feeding rhythm with peaks in the beginning and end of the night (Rosenwasser et al., 1981). A rhythm in food intake appears to be governed by both meal size and meal frequency; both parameters also show similar circadian fluctuations. Interestingly, the circadian patterns of meal size and frequency exhibit slight differences in peak phase, suggesting these two components of feeding may be regulated by separate circadian systems (Rosenwasser et al., 1981). Together, this indicates that different circadian clocks may regulate meal size and frequency separately, which together contribute to the overall feeding rhythm. 26 *Xu et al. (2008) published a study about the circadian regulation of feeding during this thesis.
36 27 Similarly, in insects total food intake is also determined by meal size and meal frequency. However, it is unclear if these parameters are under circadian regulation. In fruit flies, meal size appears to be determined in part by the nutritional content of the food source. Flies offered a sucrose-only solution consume significantly larger meals than flies offered a mixed sucrose-yeast solution (Ja et al., 2007). Greater consumption of sucrose-only meals may be necessary to provide adequate nutrients to satisfy hunger in the absence of the richer sucrose yeast food, indicating that meal size is in part dependent on the nutrient content in food. Meal frequency has been shown to be influenced by factors such as gender and the size of the group in which individuals are housed. Females generally consume more food than males by feeding more frequently (Wong et al., 2009). The difference in food consumption is likely linked to the reproductive needs of females. Interestingly, group size also produces an effect on feeding frequency. Flies housed in larger groups consume meals more frequently than flies housed alone (Wong et al., 2009). Since meal size and frequency are affected by different factors, they may be independently regulated perhaps through separate mechanisms. In insects, smell and taste are essential for feeding behaviour and food intake. Gustatory and olfactory cues are used to direct a fly to a food source and to determine if the food is edible (Reviewed in Melcher et al., 2007). Insects detect odorants via olfactory sensilla located on the antenna and maxillary palps. Odorant molecules bind to receptors on the surface of the olfactory receptor neurons in the sensillum (Reviewed in Dahanukar et al., 2005). If the odour is perceived to be pleasant, the fly will extend its proboscis to taste the food (Dethier, 1976). Taste is perceived through gustatory receptor neurons in gustatory sensilla located on the mouthparts, legs and wing margins (Reviewed in Dahanukar et al., 2005). In blowflies, the stimulation of taste receptors is a major factor that regulates the amount of food consumed (Gelperin and Dethier, 1967). For instance, when blowflies are offered a choice between sorbitol, which is nutritious but only weakly stimulating, and fucose, which is nonnutritious but very stimulating, they consume a greater quantity of fucose, suggesting that
37 28 the stimulating power of a food source can override the nutrition it provides (Gelperin and Dethier, 1967). As insects feed, digestive organs including the crop and foregut play a part in regulating food intake. In blowflies, as food is consumed, the crop fills and expands in size, which is thought to trigger stretch receptors in the body wall that limit further food consumption (Dethier and Gelperin, 1967). The foregut also inhibits food intake via stretch receptors that detect the amount of food passing through the digestive system. When the recurrent nerve that connects the foregut stretch receptor to the brain is cut, it causes overeating, suggesting that the foregut stretch receptor is important in the regulation of food intake (Dethier and Gelperin, 1967). Due to their anatomical similarities, it is possible that such modes of control also exist in other dipterans including Drosophila. Feeding-related organs appear to communicate with each other through peptides released from the brain and some metabolic tissues. In fruit flies, the peptide TAKEOUT (TO) regulates food intake based on the nutrient levels in the body. takeout (to) shows increased expression in response to starvation and appears to act as a signal for increasing food intake after starvation (Meunier et al., 2007; Sarov-Blat et al., 2000). In to 1 mutant flies, the amount of food consumed after starvation is reduced compared to that of wild type flies. This response is partly related to sugar sensitivity in the taste neurons. Normally, starvation causes an increase in sugar sensitivity, which leads to increased feeding. In to 1 mutants, starvation does not induce a change in sugar sensitivity, thus flies may not be stimulated to increase food intake (Meunier et al., 2007). The short NEUROPEPTIDE F (snpf) protein is involved in regulating food intake, although the mechanism by which this occurs remains unclear. The localization of snpf to the medulla and the mushroom body calyx, a higher brain centre linked to olfaction (Reviewed in Dahanukar et al., 2005), suggests it may act to regulate feeding based on olfactory cues (Lee et al., 2004). Overexpression of snpf in the central and peripheral nervous systems of adults promotes feeding, whereas loss-of-function mutants are less inclined to feed, suggesting that snpf may increase the appetite (Lee et al., 2004).
38 29 HUGIN (HUG) is a neuropeptide that is involved in making a decision about whether or not to consume a meal (Melcher and Pankratz, 2005). Mutant larvae that overexpress hugin (hug) show an abnormal feeding phenotype; they stop feeding early and move away from a food source. However, when hugin expressing neurons are inactivated with tetanus toxin, feeding behaviour is rescued and larvae continue to feed (Melcher and Pankratz, 2005). In larvae, hug expression is detected in the subesophageal ganglion, a gustatory information processing center (Reviewed in Dahanukar et al., 2005), which suggests it regulates feeding through taste (Melcher and Pankratz, 2005). In adults, hugin appears to be involved in the initiation of feeding. Adult flies with blocked hugin neurons begin eating faster than control flies, even if the meal contains an aversive substance (Melcher and Pankratz, 2005). Together, these results suggest that hugin is involved in evaluating the nutritional content of a potential meal and the decision about whether or not to consume it. The foraging (for) gene, which encodes for a cgmp-dependent protein kinase (PKG), regulates food intake and nutrient absorption. There are two natural alleles in foraging, rovers (for R ) and sitters (for S ) (de Belle et al., 1989). These two populations show differences in nutrient storage and feeding, which appears to influence foraging behaviour. In abundant food, rovers consume less food than sitters but show greater glucose absorption (Kaun et al., 2007). Differences in food intake are reduced when larvae are kept on less nutritious food, although carbohydrate absorption remains higher in rovers. These effects seem to be caused by a difference in PKG expression; rovers display higher expression than sitters (Osborne et al., 1997). foraging is also associated with feeding related behaviours in adult flies, and may also regulate food intake at this stage of the life cycle (Kent et al., 2009). Feeding is an organized process that is regulated by the chemosensory organs, metabolic tissues in the body, and higher brain centres. I propose that feeding in fruit flies is also under circadian control. To examine fly feeding, I measure food intake in flies that carry mutations in clock-regulated genes and report that fly feeding shows temporal regulation. Furthermore,
39 30 I demonstrate that the circadian system regulating feeding rhythms interacts with the foraging gene, a gene known to affect food intake and feeding behaviour. Methods Strains Canton-S was used as the wildtype strain for all behaviour experiments. Rovers, sitters and sitter mutant flies were kindly provided by Marla Sokolowski. The strains used for different feeding experiments are described within the results. Fly Collections for Behavioural Assays Fly stock bottles were emptied hours prior to collections and eclosed flies were collected into food vials. Male flies were sorted on a CO 2 pad and placed into fresh food vials 24 hours later. Flies were entrained to the light-dark cycle for 3-4 days. The Capillary Feeder (CAFE) Assay A modified version of the capillary feeder assay (Figure 3.1) developed by Ja et al. (2007) was used to measure fly feeding. The fly chamber was made from a 9.4cm plastic vial. The base of the vial contained 6mL of distilled water with immersed cotton whereas the top was enclosed by a ~2cm thick sponge. The fly was provided liquid food through a 5μL precalibrated microcapillary (VWR Catalog no ). CAFE food was made by mixing 5% sucrose and 5% autolyzed yeast in distilled water. The solution was autoclaved and stored in a glass bottle at room temperature. Prior to use, food was filter sterilized to remove particles that could clog the microcapillary. Each microcapillary was first filled with a small amount of mineral oil, which acts to reduce food evaporation. The microcapillary was
40 31 Figure 3.1: A diagram of the Capillary Feeder (CAFE) assay. In this assay, an individual fly is kept in a humidified compartment and liquid 5% sucrose-5% yeast food is provided through a pre-calibrated microcapillary. As the fly consumes food from the microcapillary, the oil meniscus descends. The oil level is then measured with reference to a white marking on the capillary as indicated (*). subsequently filled with CAFE food via capillary action and cleaned well to remove excess liquid on the outer surface. The food microcapillary was then fixed into pipette tips and inserted into a premade hole in the sponge sliver at the top of the assay. An individual fly
41 32 was aspirated into the chamber, with a mouth aspirator. This was performed a minimum of 24 hours prior to the start of the experiment in order to acclimatize the fly to the environment. CAFE assays were monitored in a room where humidity was maintained between 30-50% and the temperature was kept between C. Light conditions were maintained at 12:12 light-dark, constant darkness or constant light depending on the experiment requirements. To visualize the capillaries in the dark, a lamp emitting dim red light was kept on during the entire experiment. 2-3 control assays without flies were set up to measure the amount of evaporated food. Assays were set up with cardboard barriers between vials to prevent adjacent flies from observing each other s behaviour. Photographs of this assay were taken in hourly intervals with a Canon PowerShot S5 IS camera using its Remote Capture software. This program allows timed photographs to be captured through a computer. Analysis of Feeding The amount of food consumed was determined by calculating the change in the level of the oil meniscus in the microcapillary over time. Image J v. 1.40g was used to measure the oil level to a reference line (a white marking on the capillary). A number of photographs were stacked together and using the line segment tool measurements were made in pixel units. Using Microsoft Excel 2007, the amount of food consumed per hour was calculated for each fly. The amount of food evaporated was subtracted from hourly food consumption. A 3 point moving average was calculated for each fly. Food intake was recorded for 26 or 50 hour periods, so that a moving average could be calculated for the amount of food consumed at the beginning and end of the experiment. The individual data for flies from the same strain was then averaged and normalized. All plots were made in Sigmaplot v.10.0.
42 33 Statistical Analysis All statistical analyses were carried out with SPSS v The moving average of food intake was binned in 4 hour intervals starting from Zeitgeber/Circadian time 0. As multiple feeding measurements were taken from the same sample (fly), a General Linear Model (GLM) Repeated Measures test was used to compare differences in feeding amounts between any two fly strains. The null hypothesis is that there are no differences in the amount of food consumed between two groups. The GLM repeated measures test measures the effect of genotype (between-subjects factor) and time (within-subjects factor) on feeding differences between two groups. A significant genotypic effect indicates differences in the total amount of food consumed between groups. GLM employs univariate tests to determine the effects of the within-subject variables of time and genotype by time interaction. If the assumption of sphericity tested with the Mauchly s test of sphericity was violated (Mauchly, 1940), the degrees of freedom value was adjusted with Greenhousse-Geisser correction (Greenhouse and Geisser, 1959). A significant time effect indicates that the amount of food consumed changes with time whereas a significant genotype-by-time effect suggests that genotype affects feeding levels that change with time. The F-statistic and P-values for GLM repeated measures tests performed on the feeding data are provided in Table 7 in Appendix C. Locomotor Activity Assay The locomotor activity of individual flies was recorded in glass activity tubes containing food. Activity tubes were prepared with ~1 inch of a 2% bactoagar and 4% sucrose food and sealed on one side with parafilm. A single male fly was aspirated into the tube with a mouth aspirator and the opening was closed with a small piece of cotton. The activity tubes were monitored under the Drosophila Activity Monitoring (DAM2) system (TriKinetics, ) in an incubator. Locomotor activity experiments were conducted for approximately 2 weeks: a light-dark cycle was maintained for the first week, followed by a week in either
43 34 constant darkness or constant light. Activity data was acquired using the DAM System Software v.303 and analyzed using MATLAB R2006b. Results Flies Display a Circadian Feeding Pattern To study daily feeding patterns in Drosophila, I modified the capillary feeder (CAFE) assay developed by Ja et al. (2007) (Figure 3.1). This simple set up allows for the real-time quantification of the food consumed by individual flies. In the CAFE assay, a fly has access to liquid food provided in a microcapillary; as the fly consumes food from the microcapillary the level of food drops. From photographs taken in hourly intervals the amount of food consumed can be calculated by measuring the change in food level over time. To determine whether fly feeding shows a temporal pattern, I measured hourly food consumption in male wild type flies during a 24 hour light-dark cycle. Average food consumption was in the range of nL per hour. The hourly feeding profiles of individual flies (Figure 3.2A) clearly show that feeding events occur at most times of the day. However, the total amount of food consumed at certain hours of the day is larger than at others, with increased food consumption occurring at times corresponding to the transitions in lighting conditions. The feeding profile of a typical individual wild type fly, as shown in Figure 3.2A, illustrates that there is greater food consumption following lights-on (ZT0) and prior to lights-off (ZT12). The 3 hour moving average plot for wild type Canton-S flies (Figure 3.2B) shows a similar pattern. Here, three peaks appear during the day, with two peaks that occur around lights on and lights off. The broader feeding peaks visible in the average plot are due to some variability in the times at which flies increased their feeding. Two short feeding troughs appear during the day, while a large trough is observed in the dark period. Overall there is a difference in daytime and nighttime feeding; the total amount of food consumed during the day is ±78.460nL compared to ±56.419nL consumed during the night. Together these results indicate that fly feeding shows a circadian pattern.
44 35 Figure 3.2: Wildtype flies show a temporal feeding pattern in a light-dark cycle and constant darkness. The feeding pattern of Canton-S flies in 24 hours of LD (plotted twice) and 48 hours of DD as measured by CAFE assays. In LD, the feeding profiles of an individual fly (A) and the 3-point moving average (B, n=23) show increased feeding around lights-on and lights-off. Food intake at night appears reduced compared to daytime feeding. In constant darkness, the greatest amount of feeding occurs during each subjective day in the individual (C) and moving average (D, n=14) plots. Actograms (double-plotted) display average locomotor activity rhythms of CS flies (n=27) in 4 days of LD (E) and 4 days of constant darkness (F). The moving average of feeding values ± SEM is plotted (B. D). White, grey and black horizontal bars indicate day, subjective day and night, respectively. The feeding of wild type Canton-S flies was examined in constant darkness in order to determine if this feeding pattern persists in the absence of light cues. In the feeding plot of a typical individual fly (Figure 3.2C), there is an increase in feeding in the early hours of the first subjective day and also around CT12 and CT36, times when lights would turn off in a
45 36 LD cycle. Between these peaks, there is an overall reduction in the amount of food consumed. Although the pattern is similar to the one seen in LD, the phase of peak feeding times appears shifted in DD. When the moving average is examined (Figure 3.2D), two feeding peaks are visible on the first subjective day in DD, around CT5 and CT11 followed by a large feeding trough that lasts until the middle of the second subjective day. Another broad peak appears around CT36 followed by a second feeding trough. The continuation of the temporal pattern suggests that feeding is under clock regulation and independent of light cues. The temporal pattern in feeding appears to parallel the bimodal pattern in locomotor activity (Figure 3.2E-F). In a light-dark cycle, peaks in locomotor activity are visible at dawn and dusk each day corresponding to the increase in feeding around these times. Similar to locomotor activity, feeding appears to anticipate lights-on and lights-off as the level of feeding increases prior to fluctuations in lighting. In constant darkness, locomotor activity and feeding show similar patterns with one broad peak around the end of the subjective day. The similarity in the two patterns suggests an interaction in mechanisms regulating feeding and locomotor activity. The Feeding Pattern is Regulated by a period-dependent Clock The period gene is vital for maintaining circadian rhythms in behaviour. To investigate whether feeding is under circadian regulation, I quantified feeding rhythms in period-null mutant flies. per 01 flies display arrhythmic locomotor activity in constant conditions (Konopka and Benzer, 1971). In LD, the moving average plot for y w control flies (Figure 3.3A) shows a pattern with notable peaks after the onset of light and darkness; the latter peak appearing larger than the former one. Comparatively, wild type Canton-S flies display a third
46 37 Figure 3.3: Feeding patterns appear disrupted in period mutant flies. The moving average of feeding for y w control and per 01 null-mutants in a 24-hour light-dark cycle (plotted twice) and 2 days of constant darkness. In LD, y w flies (A, n=19) display a feeding pattern, with increased feeding around light-to-dark and dark-to-light transitions. In the per 01 moving average (B, n=17), a solitary feeding peak appears several hours after lights-off. In constant darkness, a feeding pattern is visible in y w flies (C, n=12) but the feeding pattern of mutant flies (D, n=24) appears disrupted. Actograms (double-plotted) display average locomotor activity patterns in 3 days of LD and 2 days of DD. y w flies (E, n=30) display rhythmic locomotor activity patterns in both lighting conditions whereas per 01 flies (F, n=25) display arrhythmic activity in DD. The moving average of feeding values ± SEM is plotted. White, grey and black horizontal bars indicate day, subjective day and night, respectively. feeding peak at midday, when a trough appears in y w flies. The results show that only time has a significant effect on feeding differences between y w flies and wild type flies (time, F 3.24/41 =6.90, P<0.005; genotype, F 1/41 =0.27, P=0.61; genotype by time, F 3.24/41 =0.58, P=0.64). period-null mutants (Figure 3.3B) display a feeding pattern that is significantly
47 38 different from y w controls (genotype, F 1/35 =5.65, P<0.05; time, F 3.58/35 =4.62, P<0.005). per 01 flies consume a constant amount of food during the lights-on period; however after lights-off, feeding does increase and is followed by a trough. In y w flies the evening peak begins prior to lights-off whereas in per 01 flies, it appears following lights-off, which is an indication that per 01 flies do not anticipate lights-off. The feeding profiles of per 01 and y w flies suggests that period-dependent clocks regulate feeding rhythms. In constant darkness, y w flies continue to show a temporal feeding pattern whereas per 01 flies display disrupted feeding. The moving average profile of y w flies (Figure 3.3C) shows an increase in feeding at subjective dawn and dusk each day. The exception to this pattern is a feeding peak that occurs in the middle of the first night instead of appearing on the early subjective morning of the second day in DD. An extended trough in feeding appears to follow this peak. Peaks are also seen on the second day but appear weaker than the previous day. The feeding plot of per 01 flies (Figure 3.3D) displays a strong feeding peak at the beginning of the first day in DD. Subsequently, a greater number of minor peaks appear until the end of the second day. This pattern neither resembles y w flies in DD nor the feeding pattern of per 01 flies in a light-dark cycle, which suggests period is required for normal feeding rhythms. Time has a significant effect (F 7.01/35 =2.12, P<0.05) on feeding differences between y w and per 01 flies in DD. per-expressing clock cells are found in the brain and peripheral tissues, this raises the question as to which clock cells regulate feeding. Peripheral Clocks Regulate the Temporal Pattern of Food Intake In order to examine whether peripheral or central clock cells are responsible for regulating food consumption, I measured food intake in the per :2 fly strain (in a ry 506 genotypic background). In these flies, a functional period gene is only expressed in the central nervous system, thus central clocks are active while peripheral oscillators are disrupted (Zehring et al., 1984). In a light-dark cycle, the feeding pattern of per :2 flies (Figure 3.4B) is similar to the one observed in the ry 506 control flies (Figure 3.4A), although time (F 3.31/30 =8.93, P<0.001) and genotype (F 1/30 =8.64, P<0.01) have significant effects on
48 39 differences in the amount of food consumed. Both control and experimental flies display a feeding peak at dawn and dusk with a single trough before and after the evening peak. The two feeding profiles also show a small third peak between the morning and evening peaks, which is more distinct in per :2 flies. In constant darkness, a pattern similar to the LD feeding profile is still visible in the ry 506 flies, whereas the feeding pattern of per :2 flies changes after the first day in DD. ry 506 flies (Figure 3.4C) continue to increase feeding around dawn and dusk each day, corresponding to times when lights would turn on or off. per :2 flies (Figure 3.4D) show a pattern on the first day of DD, which is similar to the one in LD; however, the frequency of feeding peaks increases in the beginning of the second day. The results show that time significantly affects the amount of food consumed by ry 506 and per :2 flies (F 6.47/27 =4.21, P<0.001). This suggests that input from a peripheral clock is required to maintain feeding rhythms in DD. The synchronization of oscillators in the fly brain occurs via PDF and is essential for retaining locomotor activity rhythms (Reviewed in Stanewsky, 2002). Since feeding and locomotor activity show several similarities, perhaps clocks that regulate feeding also communicate via the PDF neuropeptide. To investigate whether pdf is required for maintaining the temporal pattern in feeding, I measured feeding in pdf-null mutants (in a Canton-S genotypic background); pdf 01 is a null allele created by a nonsense mutation (Renn et al., 1999). Under light-dark conditions, pdf 01 mutant flies (Figure 3.4E) show a single peak in the morning followed by a combination of three smaller peaks which occur around the light-to-dark transition. These flies appear to feed in a similar manner to Canton-S control flies (Figure 3.2B) during the day; however the nighttime Canton-S feeding trough is less obvious in the pdf 01 feeding plot. There is a significant effect of time on feeding (F 3.19/40 =3.14, P<0.05).
49 40 Figure 3.4: Neuronal clocks show some involvement in regulating the feeding rhythm. The moving average feeding profiles of ry 506, per :2 and pdf 01 flies in 1 LD day (plotted twice) and 2 days of DD. In a light-dark cycle, the feeding pattern of ry 506 control (A, n=17) and per :2 (B, n=14) flies appears similar. Constant conditions do not alter the feeding pattern of ry 506 (C, n=17) flies but seem to affect
50 41 the feeding pattern of per :2 flies (D, n=11) by the second day. A feeding pattern is visible in the pdf 01 profile in LD (E, n=18), but peak times are not very consistent in DD (F, n=19). However, both LD and DD patterns are similar to the Canton-S control flies (Figure 3.2). Average locomotor activity rhythms in 3 days of LD and 2 days of DD are shown in double-plotted actograms. ry 506 (G, n=25) and per :2 flies (H, n=28) display rhythmic locomotor activity patterns in LD and DD. The actogram for pdf 01 flies (I, n=26) shows rhythmic locomotor activity in LD; however in DD this pattern appears disrupted by the 2 nd day in DD. The moving average of feeding values ± SEM is plotted. White, grey and black horizontal bars indicate day, subjective day and night, respectively. In constant conditions, a feeding pattern is visible in pdf 01 flies (Figure 3.4F) but is different from its own LD profile. On the first day of DD, the pdf 01 flies show a morning peak several hours after the start of the subjective day. This is followed by a broad evening peak and another peak a few hours later, occurring prior to the morning of the subsequent day. Finally, two peaks occur around CT36 and CT48, times when there would be a transition in lighting in a LD cycle. Although mutant flies continue to display fluctuations in constant conditions, it seems that peak feeding times are affected by the pdf mutation. However, the DD pattern is similar to the one observed for wildtype control flies in DD (Figure 3.2D). The results show that although time (F 5.14/32 =1.67, P>0.05) does not have a significant effect on feeding differences, genotype affects the total amount of food consumed (F 1/32 =8.39, P<0.01). This suggests that pdf plays a role in the regulation of the temporal pattern of feeding. Light-Entrainment of the Circadian Clock is Essential for Maintaining Feeding Rhythms In flies, the molecular clock synchronizes to light each day to maintain behavioural rhythms. The blue-light photoreceptor Cryptochrome (CRY) is integral to the synchronization of clocks to light cues (Reviewed in Hardin, 2005). To determine whether a cry mutation affects the temporal feeding pattern, I examined the feeding pattern of cry-null mutant flies (a w 1118 genotypic background) in a light-dark cycle and constant darkness. In the LD feeding profile of w 1118 control flies (Figure 3.5A), a morning peak and two afternoon peaks appear, with two peaks corresponding to the lights-on and lights-off times. The feeding profile of cry 0 mutants (Figure 3.5B) shows a small peak at dawn and a large peak at dusk. The results show that genotype does not have a significant difference (F 1/43 =0.01, P=0.94)
51 42 on feeding levels in LD although the effect of time is significant (F 2.99/43 =4.30, P<0.01). In constant darkness, the feeding pattern of w 1118 flies (Figure 3.5C) appears to be disrupted as flies only show increased feeding on the first day. It remains to be understood why w 1118 flies do not show a feeding pattern in constant darkness. cry 0 flies (Figure 3.5D) show a clear feeding pattern with a combination of a narrow and broad peak, on both days in DD. Feeding troughs are observed between peaks on both days, similar to the trough observed in LD. Time has a significant effect (F 6.19/30 =5.14, P<0.01) on the feeding differences between w 1118 and cry 0 flies. Thus, a mutation in cry does not seem to disrupt the fly feeding pattern. Constant light disrupts the locomotor activity rhythms of fruit flies (Konopka et al., 1989). This effect is thought to be caused by constantly activating CRY, which leads to TIM degradation (Reviewed in Dubruille and Emery, 2008). I determined whether feeding patterns are also affected by constant light. To control for the effects of constant light of feeding, Canton-S and y w flies were examined. The Canton-S feeding profile in LL (Figure 3.5E) shows a solitary peak on the first morning of the experiment followed by a constant level of feeding until CT32 when feeding once again increases. For an unknown reason, the afternoon peaks which were observed in LD, are not visible on the first day of LL. Compared to the patterns observed in LD and DD (Figure 3.2B, D), it appears that feeding is disrupted in constant light. Moreover, y w flies display a feeding pattern (Figure 3.5F) on the first day in LL, but feeding becomes abnormal soon after. On the first day of LL, three peaks appear which correspond with times when there would be a transition in lighting if it were LD. Subsequently, small feeding peaks occur randomly throughout the second day. Thus, it appears that constant light disrupts normal fly feeding patterns. Furthermore, the feeding profile of w 1118 control flies (Figure 3.5G) shows a disrupted pattern in constant light; only one large feeding peak occurs at CT12 on the first day after which the level of feeding remains constant. Comparatively, the cry 0 feeding pattern in LL
52 43 Figure 3.5: Constant light disrupts normal feeding patterns. The temporal feeding pattern of control and mutant flies in a 24-hour light-dark cycle (plotted twice), 2 days of constant darkness and constant light. w 1118 control (A, n=24) and cry 0 mutant (B, n=20) flies display a feeding pattern in LD; with peaks at dawn and dusk. However, in constant darkness, w 1118 fly feeding (C, n=12) appears disrupted while mutants (D, n=19) appear to feed rhythmically. The feeding patterns of CS (E, n=18), y w (F,
53 44 n=15), and w 1118 (G, n=11) flies are diminished in constant light; although cry 0 flies (H, n=17) continue to display a feeding pattern. Double-plotted actograms show average locomotor activity rhythms of w 1118 and cry 0 flies in 3 days of LD and 2 days of LL. w 1118 flies (I, n=24) become arrhythmic in constant light whereas cry 0 mutants (J, n=19) retain rhythmic activity. The moving average of feeding values ± SEM is plotted. White and black horizontal bars indicate day and night, respectively. Grey bars and diagonally patterned bars indicate subjective day and subjective night, respectively. (Figure 3.5H) is similar to its LD feeding profile. On the first day of constant light, a small peak appears at dawn followed by two feeding peaks in the evening (before and after CT12). This is in contrast to the single large peak that occurs in the evening in LD. On the following day, a single large feeding peak is visible around CT36; indicating the persistence of a feeding pattern. Feeding differences between cry-null flies and w 1118 flies in LL appear to be caused by time (F 6.10/27 =6.04, P<0.001). Constant illumination disrupts the molecular clock through a CRY-mediated pathway, thereby interfering with the circadian regulation of feeding. The Polymorphism in foraging Affects the Circadian Regulation of Feeding The foraging gene is thought to link feeding-related behaviours to metabolic processes in Drosophila (Reviewed in Kaun and Sokolowski, 2009). In order to determine whether metabolic activities within the fly body direct the time of feeding, I examined the effects of allelic differences in foraging expression on feeding patterns by quantifying food intake in rovers (for R ) and sitters (for s ). In addition, I measured food intake in sitter mutants (for s2 ), a rover strain that expresses the sitter allele (de Belle et al., 1989; Pereira and Sokolowski, 1993). Under light-dark conditions, rovers display feeding patterns that differ from those of natural and mutant sitter flies. for R flies (Figure 3.6A) display a temporal pattern with three feeding peaks during the day followed by a feeding trough during the night. This pattern is distinct from the feeding patterns of sitter flies. The feeding profile of for s flies (Figure 3.6B) shows two large feeding peaks after dawn and around ZT12, when lights turn off. The results indicate that a genotype-by-time interaction has a significant effect on feeding differences
54 45 Figure 3.6: Allelic variation in the foraging gene affects the temporal feeding pattern. The moving average of feeding for rovers, natural sitters and sitter mutants in one day of a light-dark cycle (plotted twice). The feeding patterns of for R (A, n=8) appear different from the feeding profiles of for s (B, n=8) and for s2 (C, n=6) flies. The 3-point moving average of feeding values ± SEM is plotted. White and black horizontal bars indicate day and night, respectively. between rovers and natural sitters (F 3.04/15 =4.16, P<0.05). These feeding differences are likely to be linked to the foraging gene, as sitter mutants (Figure 3.6C) display similar patterns to natural sitters. for s2 flies show greater feeding around dawn and dusk, although they display a larger evening peak whereas natural sitters show a larger morning peak. However, time does have a significant effect on the amount of food consumed by natural and mutant sitters (F 2.31/13 =10.18, P<0.001). The feeding pattern of for s2 flies is significantly different from for R flies, an effect of a genotype-by-time interaction (F 5/13 =4.09, P<0.005). The foraging-allele specific differences in feeding patterns suggest that PKG may interact with the circadian system regulating food intake.
55 46 Analyzing Fly Meals Mammalian studies have shown that rhythms in feeding frequency and meal size contribute to the temporal pattern in total feeding (Rosenwasser et al., 1981). To further analyze the circadian regulation of feeding, I categorized hourly meals of each fly and evaluated the number of flies that consumed each meal type. Meals were grouped into small, medium or large meal categories based on two threshold levels. The higher threshold level was the average amount of food consumed per hour in a 24-hour period while the lower threshold level was half of the average amount of food consumed. Meals that were below the half average threshold were considered small. Medium meals were any meals that were in between the half average and average threshold levels. And large meals were meals that were larger than the average threshold. For each strain the number of small, medium and large hourly meals were summed and plotted as frequency against time. In LD and DD, a temporal pattern is visible in the frequency of large meals in Canton-S (Figure 3.7A-B) and y w flies (Figure 3.7C-D). A greater number of flies consume large meals around the light-to-dark and dark-to-light transition times in LD. Similarly, in DD, the frequency of flies consuming large meals increases around the times when lights would turn on or off in a light-dark cycle. These patterns resemble the temporal feeding patterns shown earlier (CS, Figures 3.2 B, D and y w, Figures 3.3 A, C); with peaks occurring at the same time in both plots. In contrast, the frequency pattern for small and medium meals seems to occur out of phase with the large meal pattern. A pattern is visible in the frequency of per 01 flies that consume large meals in LD (Figure 3.7E); however, in DD (Figure 3.7F) the pattern appears disrupted. This is consistent with the temporal feeding patterns observed for per 01 flies earlier (Figures 3.3 B, D). The statistics for this data is yet to be resolved. Greater consumption of food at particular times of the day may result from flies either increasing the meal size or meal frequency.
56 47 Figure 3.7: A pattern in the consumption of large meals appears to drive the temporal feeding pattern. The frequency of Canton-S, y w and per 01 flies that consume small, medium and large hourly meals in a lightdark cycle (plotted twice) and constant darkness. In CS (A-B) and y w (C-D) plots, the number of flies consuming large meals increases around the light-to-dark and dark-to-light transitions. This pattern resembles the temporal pattern in feeding. Patterns in the small and medium meal profiles appear to be shifted by a few hours from the large meal pattern. In LD, per 01 mutant flies (E) show a pattern in the number of small, medium and large meals consumed but this is not visible in constant darkness (F). A small meal was any meal that was smaller than half the average hourly meal. A large meal was larger than the average hourly meal. And medium meals were any meals that fell between the small and large meal categories. Grey bars, small meals; blue bars, medium meals; black bars, large meals. White, grey and black horizontal bars indicate day, subjective day and night, respectively.
Clicker Question. The Need to Decompose. Mechanism and Reduction: Decomposing Circadian Clocks
 Mechanism and Reduction: Decomposing Circadian Clocks Clicker Question On the Deductive-Nomological (DN) model of reduction, which of the following does not figure in providing the explanation (i.e., is
Mechanism and Reduction: Decomposing Circadian Clocks Clicker Question On the Deductive-Nomological (DN) model of reduction, which of the following does not figure in providing the explanation (i.e., is
Supplemental Data. Shin et al. Plant Cell. (2012) /tpc YFP N
 MYC YFP N PIF5 YFP C N-TIC TIC Supplemental Data. Shin et al. Plant Cell. ()..5/tpc..95 Supplemental Figure. TIC interacts with MYC in the nucleus. Bimolecular fluorescence complementation assay using
MYC YFP N PIF5 YFP C N-TIC TIC Supplemental Data. Shin et al. Plant Cell. ()..5/tpc..95 Supplemental Figure. TIC interacts with MYC in the nucleus. Bimolecular fluorescence complementation assay using
Supplementary Table 3. 3 UTR primer sequences. Primer sequences used to amplify and clone the 3 UTR of each indicated gene are listed.
 Supplemental Figure 1. DLKI-DIO3 mirna/mrna complementarity. Complementarity between the indicated DLK1-DIO3 cluster mirnas and the UTR of SOX2, SOX9, HIF1A, ZEB1, ZEB2, STAT3 and CDH1with mirsvr and PhastCons
Supplemental Figure 1. DLKI-DIO3 mirna/mrna complementarity. Complementarity between the indicated DLK1-DIO3 cluster mirnas and the UTR of SOX2, SOX9, HIF1A, ZEB1, ZEB2, STAT3 and CDH1with mirsvr and PhastCons
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 U1 inhibition causes a shift of RNA-seq reads from exons to introns. (a) Evidence for the high purity of 4-shU-labeled RNAs used for RNA-seq. HeLa cells transfected with control
Supplementary Figure 1 U1 inhibition causes a shift of RNA-seq reads from exons to introns. (a) Evidence for the high purity of 4-shU-labeled RNAs used for RNA-seq. HeLa cells transfected with control
c Tuj1(-) apoptotic live 1 DIV 2 DIV 1 DIV 2 DIV Tuj1(+) Tuj1/GFP/DAPI Tuj1 DAPI GFP
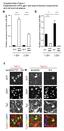 Supplementary Figure 1 Establishment of the gain- and loss-of-function experiments and cell survival assays. a Relative expression of mature mir-484 30 20 10 0 **** **** NCP mir- 484P NCP mir- 484P b Relative
Supplementary Figure 1 Establishment of the gain- and loss-of-function experiments and cell survival assays. a Relative expression of mature mir-484 30 20 10 0 **** **** NCP mir- 484P NCP mir- 484P b Relative
Biological Clocks. Lu Chen, Ph.D. MCB, UC Berkeley. What is biological clock?
 Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 What is biological clock? All eukaryotes and some prokaryotes display changes in gene activity, biochemistry, physiology, and behavior that wax and wane
Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 What is biological clock? All eukaryotes and some prokaryotes display changes in gene activity, biochemistry, physiology, and behavior that wax and wane
Biological Clocks. Lu Chen, Ph.D. MCB, UC Berkeley. Why Does Melatonin Now Outsell Vitamin C??
 Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 Why Does Melatonin Now Outsell Vitamin C?? Wake / sleep complaints are extremely prevalent. Much melatonin is consumed in an attempt to overcome the
Biological Clocks Lu Chen, Ph.D. MCB, UC Berkeley 1 Why Does Melatonin Now Outsell Vitamin C?? Wake / sleep complaints are extremely prevalent. Much melatonin is consumed in an attempt to overcome the
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sherman SI, Wirth LJ, Droz J-P, et al. Motesanib diphosphate
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Sherman SI, Wirth LJ, Droz J-P, et al. Motesanib diphosphate
a) Primary cultures derived from the pancreas of an 11-week-old Pdx1-Cre; K-MADM-p53
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1. Induction of p53 LOH by MADM. a) Primary cultures derived from the pancreas of an 11-week-old Pdx1-Cre; K-MADM-p53 mouse revealed increased p53 KO/KO (green,
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1. Induction of p53 LOH by MADM. a) Primary cultures derived from the pancreas of an 11-week-old Pdx1-Cre; K-MADM-p53 mouse revealed increased p53 KO/KO (green,
The Drosophila melanogaster life cycle
 The Drosophila melanogaster life cycle Eclosion The first phenotype described in Drosophila as an endogenous rhythm (1954): Number of eclosed flies Hamblen et al., Gene3cs 1998 rhythm with a periodicity
The Drosophila melanogaster life cycle Eclosion The first phenotype described in Drosophila as an endogenous rhythm (1954): Number of eclosed flies Hamblen et al., Gene3cs 1998 rhythm with a periodicity
Abbreviations: P- paraffin-embedded section; C, cryosection; Bio-SA, biotin-streptavidin-conjugated fluorescein amplification.
 Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Supplementary Document
 Supplementary Document 1. Supplementary Table legends 2. Supplementary Figure legends 3. Supplementary Tables 4. Supplementary Figures 5. Supplementary References 1. Supplementary Table legends Suppl.
Supplementary Document 1. Supplementary Table legends 2. Supplementary Figure legends 3. Supplementary Tables 4. Supplementary Figures 5. Supplementary References 1. Supplementary Table legends Suppl.
Supplementary Table 2. Conserved regulatory elements in the promoters of CD36.
 Supplementary Table 1. RT-qPCR primers for CD3, PPARg and CEBP. Assay Forward Primer Reverse Primer 1A CAT TTG TGG CCT TGT GCT CTT TGA TGA GTC ACA GAA AGA ATC AAT TC 1B AGG AAA TGA ACT GAT GAG TCA CAG
Supplementary Table 1. RT-qPCR primers for CD3, PPARg and CEBP. Assay Forward Primer Reverse Primer 1A CAT TTG TGG CCT TGT GCT CTT TGA TGA GTC ACA GAA AGA ATC AAT TC 1B AGG AAA TGA ACT GAT GAG TCA CAG
Supplementary Figure 1. ROS induces rapid Sod1 nuclear localization in a dosagedependent manner. WT yeast cells (SZy1051) were treated with 4NQO at
 Supplementary Figure 1. ROS induces rapid Sod1 nuclear localization in a dosagedependent manner. WT yeast cells (SZy1051) were treated with 4NQO at different concentrations for 30 min and analyzed for
Supplementary Figure 1. ROS induces rapid Sod1 nuclear localization in a dosagedependent manner. WT yeast cells (SZy1051) were treated with 4NQO at different concentrations for 30 min and analyzed for
BIOLOGY 621 Identification of the Snorks
 Name: Date: Block: BIOLOGY 621 Identification of the Snorks INTRODUCTION: In this simulation activity, you will examine the DNA sequence of a fictitious organism - the Snork. Snorks were discovered on
Name: Date: Block: BIOLOGY 621 Identification of the Snorks INTRODUCTION: In this simulation activity, you will examine the DNA sequence of a fictitious organism - the Snork. Snorks were discovered on
The Success of Decomposition
 11/21/11 Mechanism and Levels of Organization: Recomposing and Situating Circadian Clocks The Success of Decomposition Moving beyond per, researchers in the 1990s and early 2000s identified many clock
11/21/11 Mechanism and Levels of Organization: Recomposing and Situating Circadian Clocks The Success of Decomposition Moving beyond per, researchers in the 1990s and early 2000s identified many clock
Toluidin-Staining of mast cells Ear tissue was fixed with Carnoy (60% ethanol, 30% chloroform, 10% acetic acid) overnight at 4 C, afterwards
 Toluidin-Staining of mast cells Ear tissue was fixed with Carnoy (60% ethanol, 30% chloroform, 10% acetic acid) overnight at 4 C, afterwards incubated in 100 % ethanol overnight at 4 C and embedded in
Toluidin-Staining of mast cells Ear tissue was fixed with Carnoy (60% ethanol, 30% chloroform, 10% acetic acid) overnight at 4 C, afterwards incubated in 100 % ethanol overnight at 4 C and embedded in
Supplementary Materials
 Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
Supplementary Materials 1 Supplementary Table 1. List of primers used for quantitative PCR analysis. Gene name Gene symbol Accession IDs Sequence range Product Primer sequences size (bp) β-actin Actb gi
CIRCADIAN SIGNALING NETWORKS
 Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P. Matthias and RG Clerc Roger G. Clerc 07.05.2014 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «Slave clocks»
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P. Matthias and RG Clerc Roger G. Clerc 07.05.2014 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «Slave clocks»
The Nobel Assembly at Karolinska Institutet has today decided to award. the 2017 Nobel Prize in Physiology or Medicine. jointly to
 The Nobel Assembly at Karolinska Institutet has today decided to award the 2017 Nobel Prize in Physiology or Medicine jointly to Jeffrey C. Hall, Michael Rosbash and Michael W. Young for their discoveries
The Nobel Assembly at Karolinska Institutet has today decided to award the 2017 Nobel Prize in Physiology or Medicine jointly to Jeffrey C. Hall, Michael Rosbash and Michael W. Young for their discoveries
Beta Thalassemia Case Study Introduction to Bioinformatics
 Beta Thalassemia Case Study Sami Khuri Department of Computer Science San José State University San José, California, USA sami.khuri@sjsu.edu www.cs.sjsu.edu/faculty/khuri Outline v Hemoglobin v Alpha
Beta Thalassemia Case Study Sami Khuri Department of Computer Science San José State University San José, California, USA sami.khuri@sjsu.edu www.cs.sjsu.edu/faculty/khuri Outline v Hemoglobin v Alpha
Supplementary Figure 1 a
 Supplementary Figure a Normalized expression/tbp (A.U.).6... Trip-br transcripts Trans Trans Trans b..5. Trip-br Ctrl LPS Normalized expression/tbp (A.U.) c Trip-br transcripts. adipocytes.... Trans Trans
Supplementary Figure a Normalized expression/tbp (A.U.).6... Trip-br transcripts Trans Trans Trans b..5. Trip-br Ctrl LPS Normalized expression/tbp (A.U.) c Trip-br transcripts. adipocytes.... Trans Trans
Neurons and Hormones 1. How do animals perform the right behaviors at the right time? In the right context?
 Neurons and Hormones 1 How do animals perform the right behaviors at the right time? In the right context? Active at night only What if conflicting signals? Magnetic cues are always present But migrate
Neurons and Hormones 1 How do animals perform the right behaviors at the right time? In the right context? Active at night only What if conflicting signals? Magnetic cues are always present But migrate
CD31 5'-AGA GAC GGT CTT GTC GCA GT-3' 5 ' -TAC TGG GCT TCG AGA GCA GT-3'
 Table S1. The primer sets used for real-time RT-PCR analysis. Gene Forward Reverse VEGF PDGFB TGF-β MCP-1 5'-GTT GCA GCA TGA ATC TGA GG-3' 5'-GGA GAC TCT TCG AGG AGC ACT T-3' 5'-GAA TCA GGC ATC GAG AGA
Table S1. The primer sets used for real-time RT-PCR analysis. Gene Forward Reverse VEGF PDGFB TGF-β MCP-1 5'-GTT GCA GCA TGA ATC TGA GG-3' 5'-GGA GAC TCT TCG AGG AGC ACT T-3' 5'-GAA TCA GGC ATC GAG AGA
Welcome to Bi !
 Welcome to Bi156 2012! Professors: Paul Patterson (php@caltech.edu) Kai Zinn (zinnk@caltech.edu) TAs: Janna Nawroth Yanan Sui ysui@caltech.edu Student presentations Select a topic
Welcome to Bi156 2012! Professors: Paul Patterson (php@caltech.edu) Kai Zinn (zinnk@caltech.edu) TAs: Janna Nawroth Yanan Sui ysui@caltech.edu Student presentations Select a topic
Supplementary Figure 1 MicroRNA expression in human synovial fibroblasts from different locations. MicroRNA, which were identified by RNAseq as most
 Supplementary Figure 1 MicroRNA expression in human synovial fibroblasts from different locations. MicroRNA, which were identified by RNAseq as most differentially expressed between human synovial fibroblasts
Supplementary Figure 1 MicroRNA expression in human synovial fibroblasts from different locations. MicroRNA, which were identified by RNAseq as most differentially expressed between human synovial fibroblasts
Sleep-Wake Cycle I Brain Rhythms. Reading: BCP Chapter 19
 Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Food Intake Regulation & the Clock. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
Citation for published version (APA): Oosterveer, M. H. (2009). Control of metabolic flux by nutrient sensors Groningen: s.n.
 University of Groningen Control of metabolic flux by nutrient sensors Oosterveer, Maaike IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
University of Groningen Control of metabolic flux by nutrient sensors Oosterveer, Maaike IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
A smart acid nanosystem for ultrasensitive. live cell mrna imaging by the target-triggered intracellular self-assembly
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 A smart ZnO@polydopamine-nucleic acid nanosystem for ultrasensitive live cell mrna imaging
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 A smart ZnO@polydopamine-nucleic acid nanosystem for ultrasensitive live cell mrna imaging
Beta Thalassemia Sami Khuri Department of Computer Science San José State University Spring 2015
 Bioinformatics in Medical Product Development SMPD 287 Three Beta Thalassemia Sami Khuri Department of Computer Science San José State University Hemoglobin Outline Anatomy of a gene Hemoglobinopathies
Bioinformatics in Medical Product Development SMPD 287 Three Beta Thalassemia Sami Khuri Department of Computer Science San José State University Hemoglobin Outline Anatomy of a gene Hemoglobinopathies
Figure S1. Analysis of genomic and cdna sequences of the targeted regions in WT-KI and
 Figure S1. Analysis of genomic and sequences of the targeted regions in and indicated mutant KI cells, with WT and corresponding mutant sequences underlined. (A) cells; (B) K21E-KI cells; (C) D33A-KI cells;
Figure S1. Analysis of genomic and sequences of the targeted regions in and indicated mutant KI cells, with WT and corresponding mutant sequences underlined. (A) cells; (B) K21E-KI cells; (C) D33A-KI cells;
Transcription Regulation And Gene Expression in Eukaryotes (Cycle G2 # )
 Transcription Regulation And Gene Expression in Eukaryotes (Cycle G2 #13709-01) CIRCADIAN SIGNALING NETWORKS RG. Clerc May 19. 2010 www.fmi.ch/training/teaching Circadian rythms : most physiological processes
Transcription Regulation And Gene Expression in Eukaryotes (Cycle G2 #13709-01) CIRCADIAN SIGNALING NETWORKS RG. Clerc May 19. 2010 www.fmi.ch/training/teaching Circadian rythms : most physiological processes
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. H3F3B expression in lung cancer. a. Comparison of H3F3B expression in relapsed and non-relapsed lung cancer patients. b. Prognosis of two groups of lung cancer
Supplementary Figures Supplementary Figure 1. H3F3B expression in lung cancer. a. Comparison of H3F3B expression in relapsed and non-relapsed lung cancer patients. b. Prognosis of two groups of lung cancer
 www.lessonplansinc.com Topic: Protein Synthesis - Sentence Activity Summary: Students will simulate transcription and translation by building a sentence/polypeptide from words/amino acids. Goals & Objectives:
www.lessonplansinc.com Topic: Protein Synthesis - Sentence Activity Summary: Students will simulate transcription and translation by building a sentence/polypeptide from words/amino acids. Goals & Objectives:
Table S1. Oligonucleotides used for the in-house RT-PCR assays targeting the M, H7 or N9. Assay (s) Target Name Sequence (5 3 ) Comments
 SUPPLEMENTAL INFORMATION 2 3 Table S. Oligonucleotides used for the in-house RT-PCR assays targeting the M, H7 or N9 genes. Assay (s) Target Name Sequence (5 3 ) Comments CDC M InfA Forward (NS), CDC M
SUPPLEMENTAL INFORMATION 2 3 Table S. Oligonucleotides used for the in-house RT-PCR assays targeting the M, H7 or N9 genes. Assay (s) Target Name Sequence (5 3 ) Comments CDC M InfA Forward (NS), CDC M
Stochastic simulations
 Stochastic simulations Application to circadian clocks Didier Gonze Circadian rhythms Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Circadian rhythms
Stochastic simulations Application to circadian clocks Didier Gonze Circadian rhythms Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Circadian rhythms
Supporting Information
 Supporting Information Malapeira et al. 10.1073/pnas.1217022110 SI Materials and Methods Plant Material and Growth Conditions. A. thaliana seedlings were stratified at 4 C in the dark for 3 d on Murashige
Supporting Information Malapeira et al. 10.1073/pnas.1217022110 SI Materials and Methods Plant Material and Growth Conditions. A. thaliana seedlings were stratified at 4 C in the dark for 3 d on Murashige
Advanced Subsidiary Unit 1: Lifestyle, Transport, Genes and Health
 Write your name here Surname Other names Edexcel GCE Centre Number Candidate Number Biology Advanced Subsidiary Unit 1: Lifestyle, Transport, Genes and Health Thursday 8 January 2009 Morning Time: 1 hour
Write your name here Surname Other names Edexcel GCE Centre Number Candidate Number Biology Advanced Subsidiary Unit 1: Lifestyle, Transport, Genes and Health Thursday 8 January 2009 Morning Time: 1 hour
Circadian Rhythm Disturbances: What Happens When Your Biological Clock Is In The Wrong Time Zone
 Circadian Rhythm Disturbances: What Happens When Your Biological Clock Is In The Wrong Time Zone Steven A. Thau MD Chief, Pulmonary, Sleep Department. Phelps Hospital, Northwell Health Internal Clock Examples
Circadian Rhythm Disturbances: What Happens When Your Biological Clock Is In The Wrong Time Zone Steven A. Thau MD Chief, Pulmonary, Sleep Department. Phelps Hospital, Northwell Health Internal Clock Examples
Culture Density (OD600) 0.1. Culture Density (OD600) Culture Density (OD600) Culture Density (OD600) Culture Density (OD600)
 A. B. C. D. E. PA JSRI JSRI 2 PA DSAM DSAM 2 DSAM 3 PA LNAP LNAP 2 LNAP 3 PAO Fcor Fcor 2 Fcor 3 PAO Wtho Wtho 2 Wtho 3 Wtho 4 DTSB Low Iron 2 4 6 8 2 4 6 8 2 22 DTSB Low Iron 2 4 6 8 2 4 6 8 2 22 DTSB
A. B. C. D. E. PA JSRI JSRI 2 PA DSAM DSAM 2 DSAM 3 PA LNAP LNAP 2 LNAP 3 PAO Fcor Fcor 2 Fcor 3 PAO Wtho Wtho 2 Wtho 3 Wtho 4 DTSB Low Iron 2 4 6 8 2 4 6 8 2 22 DTSB Low Iron 2 4 6 8 2 4 6 8 2 22 DTSB
SUPPLEMENTARY DATA. Supplementary Table 1. Primer sequences for qrt-pcr
 Supplementary Table 1. Primer sequences for qrt-pcr Gene PRDM16 UCP1 PGC1α Dio2 Elovl3 Cidea Cox8b PPARγ AP2 mttfam CyCs Nampt NRF1 16s-rRNA Hexokinase 2, intron 9 β-actin Primer Sequences 5'-CCA CCA GCG
Supplementary Table 1. Primer sequences for qrt-pcr Gene PRDM16 UCP1 PGC1α Dio2 Elovl3 Cidea Cox8b PPARγ AP2 mttfam CyCs Nampt NRF1 16s-rRNA Hexokinase 2, intron 9 β-actin Primer Sequences 5'-CCA CCA GCG
What Is There Left to Learn about the Drosophila Clock?
 What Is There Left to Learn about the Drosophila Clock? J. BLAU, F. BLANCHARD, B. COLLINS, D. DAHDAL, A. KNOWLES, D. MIZRAK, AND M. RUBEN Department of Biology, New York University, New York, New York
What Is There Left to Learn about the Drosophila Clock? J. BLAU, F. BLANCHARD, B. COLLINS, D. DAHDAL, A. KNOWLES, D. MIZRAK, AND M. RUBEN Department of Biology, New York University, New York, New York
LESSON 4.5 WORKBOOK How do circuits regulate their output?
 DEFINITIONS OF TERMS Homeostasis tendency to relatively stable equilibrium. Feed-forward inhibition control mechanism whereby the output of one pathway inhibits the activity of another pathway. Negative
DEFINITIONS OF TERMS Homeostasis tendency to relatively stable equilibrium. Feed-forward inhibition control mechanism whereby the output of one pathway inhibits the activity of another pathway. Negative
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1: Cryopreservation alters CD62L expression by CD4 T cells. Freshly isolated (left) or cryopreserved PBMCs (right) were stained with the mix of antibodies described
Supplementary Figure 1 Supplementary Figure 1: Cryopreservation alters CD62L expression by CD4 T cells. Freshly isolated (left) or cryopreserved PBMCs (right) were stained with the mix of antibodies described
Neural circuits underlying circadian behavior in Drosophila melanogaster
 Behavioural Processes 71 (2006) 211 225 Review Neural circuits underlying circadian behavior in Drosophila melanogaster Dennis C. Chang Department of Biology, Brandeis University, 415 South Street, MS-008,
Behavioural Processes 71 (2006) 211 225 Review Neural circuits underlying circadian behavior in Drosophila melanogaster Dennis C. Chang Department of Biology, Brandeis University, 415 South Street, MS-008,
Pigment-Dispersing Factor Modulates Pheromone Production in Clock Cells that Influence Mating in Drosophila
 rticle Pigment-Dispersing Factor Modulates Pheromone Production in Clock Cells that Influence Mating in Drosophila Joshua J. Krupp, Jean-Christophe illeter,,2 my Wong, Charles Choi, 3 Michael N. Nitabach,
rticle Pigment-Dispersing Factor Modulates Pheromone Production in Clock Cells that Influence Mating in Drosophila Joshua J. Krupp, Jean-Christophe illeter,,2 my Wong, Charles Choi, 3 Michael N. Nitabach,
The period (per) gene in Drosophila melanogaster plays a central
 Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C B. H. Collins*, E. Rosato, and C. P. Kyriacou* Departments of *Genetics and Biology, University
Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C B. H. Collins*, E. Rosato, and C. P. Kyriacou* Departments of *Genetics and Biology, University
Stochastic simulations
 Circadian rhythms Stochastic simulations Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Application to circadian clocks Didier Gonze Circadian rhythms
Circadian rhythms Stochastic simulations Circadian rhythms allow living organisms to live in phase with the alternance of day and night... Application to circadian clocks Didier Gonze Circadian rhythms
Interactions between circadian neurons control temperature synchronization of Drosophila behavior
 University of Massachusetts Medical School escholarship@umms GSBS Student Publications Graduate School of Biomedical Sciences 2007-10-05 Interactions between circadian neurons control temperature synchronization
University of Massachusetts Medical School escholarship@umms GSBS Student Publications Graduate School of Biomedical Sciences 2007-10-05 Interactions between circadian neurons control temperature synchronization
Phylogenetic analysis of human and chicken importins. Only five of six importins were studied because
 Supplementary Figure S1 Phylogenetic analysis of human and chicken importins. Only five of six importins were studied because importin-α6 was shown to be testis-specific. Human and chicken importin protein
Supplementary Figure S1 Phylogenetic analysis of human and chicken importins. Only five of six importins were studied because importin-α6 was shown to be testis-specific. Human and chicken importin protein
Molecular and Neuronal Analysis of Circadian Photoresponses in Drosophila: A Dissertation
 University of Massachusetts Medical School escholarship@umms GSBS Dissertations and Theses Graduate School of Biomedical Sciences 2007-10-25 Molecular and Neuronal Analysis of Circadian Photoresponses
University of Massachusetts Medical School escholarship@umms GSBS Dissertations and Theses Graduate School of Biomedical Sciences 2007-10-25 Molecular and Neuronal Analysis of Circadian Photoresponses
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05883 SUPPLEMENTARY INFORMATION Supplemental Figure 1 Prostaglandin agonists and antagonists alter runx1/cmyb expression. a-e, Embryos were exposed to (b) PGE2 and (c) PGI2 (20μM) and
doi: 10.1038/nature05883 SUPPLEMENTARY INFORMATION Supplemental Figure 1 Prostaglandin agonists and antagonists alter runx1/cmyb expression. a-e, Embryos were exposed to (b) PGE2 and (c) PGI2 (20μM) and
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Significance. 1 To whom correspondence should be addressed.
 Pacemaker-neuron dependent disturbance of the molecular clockwork by a Drosophila CLOCK mutant homologous to the mouse Clock mutation Euna Lee a,b, Eunjoo Cho c, Doo Hyun Kang a,b, Eun Hee Jeong b, Zheng
Pacemaker-neuron dependent disturbance of the molecular clockwork by a Drosophila CLOCK mutant homologous to the mouse Clock mutation Euna Lee a,b, Eunjoo Cho c, Doo Hyun Kang a,b, Eun Hee Jeong b, Zheng
Advance in circadian rhythm genetics in mammals
 16 2 2004 4 Chinese Bulletin of Life Sciences Vol. 16, No. 2 Apr., 2004 1004-0374 (2004) 02-0104-05 1 100101 2 434025 9 24, Q41 A Advance in circadian rhythm genetics in mammals XU Zu-Yuan 1,2 (1 Beijing
16 2 2004 4 Chinese Bulletin of Life Sciences Vol. 16, No. 2 Apr., 2004 1004-0374 (2004) 02-0104-05 1 100101 2 434025 9 24, Q41 A Advance in circadian rhythm genetics in mammals XU Zu-Yuan 1,2 (1 Beijing
Bio-Rhythms. Biorhythms. Presented by: Dr. Magdy Akladios 1. What is a Biorhythm. Biorhythms Theory. SENG/ INDH 5334: Human Factors Engineering
 SENG/ INDH 5334: Human Factors Engineering Bio-Rhythms By: Magdy Akladios, PhD, PE, CSP, CPE, CSHM 1 What is a Biorhythm A biorhythm is a hypothetical cyclic pattern of alterations in physiology, emotions,
SENG/ INDH 5334: Human Factors Engineering Bio-Rhythms By: Magdy Akladios, PhD, PE, CSP, CPE, CSHM 1 What is a Biorhythm A biorhythm is a hypothetical cyclic pattern of alterations in physiology, emotions,
RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: The anatomical basis of a neuropeptide s circadian functions
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: The anatomical basis
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: The anatomical basis
BHP 2-7 and Nthy-ori 3-1 cells were grown in RPMI1640 medium (Hyclone) supplemented with 10% fetal bovine serum (Gibco), 2mM L-glutamine, and 100 U/mL
 1 2 3 4 Materials and Methods Cell culture BHP 2-7 and Nthy-ori 3-1 cells were grown in RPMI1640 medium (Hyclone) 5 supplemented with 10% fetal bovine serum (Gibco), 2mM L-glutamine, and 100 U/mL 6 penicillin-streptomycin.
1 2 3 4 Materials and Methods Cell culture BHP 2-7 and Nthy-ori 3-1 cells were grown in RPMI1640 medium (Hyclone) 5 supplemented with 10% fetal bovine serum (Gibco), 2mM L-glutamine, and 100 U/mL 6 penicillin-streptomycin.
Plasmids Western blot analysis and immunostaining Flow Cytometry Cell surface biotinylation RNA isolation and cdna synthesis
 Plasmids psuper-retro-s100a10 shrna1 was constructed by cloning the dsdna oligo 5 -GAT CCC CGT GGG CTT CCA GAG CTT CTT TCA AGA GAA GAA GCT CTG GAA GCC CAC TTT TTA-3 and 5 -AGC TTA AAA AGT GGG CTT CCA GAG
Plasmids psuper-retro-s100a10 shrna1 was constructed by cloning the dsdna oligo 5 -GAT CCC CGT GGG CTT CCA GAG CTT CTT TCA AGA GAA GAA GCT CTG GAA GCC CAC TTT TTA-3 and 5 -AGC TTA AAA AGT GGG CTT CCA GAG
(Phodopus sungorus) Honors Research Thesis. Presented in Partial Fulfillment of the Requirements for graduation "with Honors Research
 The Effects of Light at Night on Immune Organ Clock Gene Expression in Siberian Hamsters (Phodopus sungorus) Honors Research Thesis Presented in Partial Fulfillment of the Requirements for graduation "with
The Effects of Light at Night on Immune Organ Clock Gene Expression in Siberian Hamsters (Phodopus sungorus) Honors Research Thesis Presented in Partial Fulfillment of the Requirements for graduation "with
Single-Molecule Analysis of Gene Expression Using Two-Color RNA- Labeling in Live Yeast
 Supplemental Figures, Tables and Results Single-Molecule Analysis of Gene Expression Using Two-Color RNA- Labeling in Live Yeast Sami Hocine 1, Pascal Raymond 2, Daniel Zenklusen 2, Jeffrey A. Chao 1 &
Supplemental Figures, Tables and Results Single-Molecule Analysis of Gene Expression Using Two-Color RNA- Labeling in Live Yeast Sami Hocine 1, Pascal Raymond 2, Daniel Zenklusen 2, Jeffrey A. Chao 1 &
Description of Supplementary Files. File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables
 Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables Supplementary Figure 1: (A), HCT116 IDH1-WT and IDH1-R132H cells were
Description of Supplementary Files File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables Supplementary Figure 1: (A), HCT116 IDH1-WT and IDH1-R132H cells were
Parathyroid Hormone, But Not Melatonin, Resets The Bone Circadian Clock
 Parathyroid Hormone, But Not Melatonin, Resets The Bone Circadian Clock Naoki Okubo 1,2,3, Yoichi Minami 1,3, Hiroyoshi Fujiwara 2, Tatsuya Kunimoto 1,2,3, Toshihiro Hosokawa 1,2,3, Ryo Oda 2, Toshikazu
Parathyroid Hormone, But Not Melatonin, Resets The Bone Circadian Clock Naoki Okubo 1,2,3, Yoichi Minami 1,3, Hiroyoshi Fujiwara 2, Tatsuya Kunimoto 1,2,3, Toshihiro Hosokawa 1,2,3, Ryo Oda 2, Toshikazu
*To whom correspondence should be addressed. This PDF file includes:
 www.sciencemag.org/cgi/content/full/science.1212182/dc1 Supporting Online Material for Partial Retraction to Detection of an Infectious Retrovirus, XMRV, in Blood Cells of Patients with Chronic Fatigue
www.sciencemag.org/cgi/content/full/science.1212182/dc1 Supporting Online Material for Partial Retraction to Detection of an Infectious Retrovirus, XMRV, in Blood Cells of Patients with Chronic Fatigue
Nature Immunology: doi: /ni.3836
 Supplementary Figure 1 Recombinant LIGHT-VTP induces pericyte contractility and endothelial cell activation. (a) Western blot showing purification steps for full length murine LIGHT-VTP (CGKRK) protein:
Supplementary Figure 1 Recombinant LIGHT-VTP induces pericyte contractility and endothelial cell activation. (a) Western blot showing purification steps for full length murine LIGHT-VTP (CGKRK) protein:
Circadian Rhythms in Physiology and Behavior. The Persistence of Memory, Salvador Dali, 1931
 Circadian Rhythms in Physiology and Behavior The Persistence of Memory, Salvador Dali, 1931 Homeostasis and Rhythms? Homeostasis (Bernard, 1878): All the vital mechanisms, however varied they may be, have
Circadian Rhythms in Physiology and Behavior The Persistence of Memory, Salvador Dali, 1931 Homeostasis and Rhythms? Homeostasis (Bernard, 1878): All the vital mechanisms, however varied they may be, have
Integration Solutions
 Integration Solutions (1) a) With no active glycosyltransferase of either type, an ii individual would not be able to add any sugars to the O form of the lipopolysaccharide. Thus, the only lipopolysaccharide
Integration Solutions (1) a) With no active glycosyltransferase of either type, an ii individual would not be able to add any sugars to the O form of the lipopolysaccharide. Thus, the only lipopolysaccharide
Simulation of Drosophila Circadian Oscillations, Mutations, and Light Responses by a Model with VRI, PDP-1, and CLK
 Title: Simulation of Drosophila Circadian Oscillations, Mutations, and Light Responses by a Model with VRI, PDP-1, and CLK Authors: Paul Smolen, Paul E. Hardin *, Brian S. Lo, Douglas A. Baxter, and John
Title: Simulation of Drosophila Circadian Oscillations, Mutations, and Light Responses by a Model with VRI, PDP-1, and CLK Authors: Paul Smolen, Paul E. Hardin *, Brian S. Lo, Douglas A. Baxter, and John
Supplementary Information. Bamboo shoot fiber prevents obesity in mice by. modulating the gut microbiota
 Supplementary Information Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota Xiufen Li 1,2, Juan Guo 1, Kailong Ji 1,2, and Ping Zhang 1,* 1 Key Laboratory of Tropical Plant Resources
Supplementary Information Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota Xiufen Li 1,2, Juan Guo 1, Kailong Ji 1,2, and Ping Zhang 1,* 1 Key Laboratory of Tropical Plant Resources
Mutation Screening and Association Studies of the Human UCP 3 Gene in Normoglycemic and NIDDM Morbidly Obese Patients
 Mutation Screening and Association Studies of the Human UCP 3 Gene in Normoglycemic and NIDDM Morbidly Obese Patients Shuichi OTABE, Karine CLEMENT, Séverine DUBOIS, Frederic LEPRETRE, Veronique PELLOUX,
Mutation Screening and Association Studies of the Human UCP 3 Gene in Normoglycemic and NIDDM Morbidly Obese Patients Shuichi OTABE, Karine CLEMENT, Séverine DUBOIS, Frederic LEPRETRE, Veronique PELLOUX,
A basic helix loop helix transcription factor controls cell growth
 A basic helix loop helix transcription factor controls cell growth and size in root hairs Keke Yi 1,2, Benoît Menand 1,3, Elizabeth Bell 1, Liam Dolan 1,4 Supplementary note Low soil phosphate availability
A basic helix loop helix transcription factor controls cell growth and size in root hairs Keke Yi 1,2, Benoît Menand 1,3, Elizabeth Bell 1, Liam Dolan 1,4 Supplementary note Low soil phosphate availability
L I F E S C I E N C E S
 1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 OCTOBER 31, 2006
1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 OCTOBER 31, 2006
Transcription Regulation And Gene Expression in Eukaryotes FS 2016 Graduate Course G2
 Transcription Regulation And Gene Expression in Eukaryotes FS 2016 Graduate Course G2 P. Matthias and RG Clerc Pharmazentrum Hörsaal 2 16h15-18h00 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «slave
Transcription Regulation And Gene Expression in Eukaryotes FS 2016 Graduate Course G2 P. Matthias and RG Clerc Pharmazentrum Hörsaal 2 16h15-18h00 CIRCADIAN SIGNALING NETWORKS Master pacemaker SCN «slave
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Lats1/2 deleted ihbs and ihps showed decreased transcripts of hepatocyte related genes (a and b) Western blots (a) and recombination PCR (b) of control and
Supplementary Figure 1 Supplementary Figure 1. Lats1/2 deleted ihbs and ihps showed decreased transcripts of hepatocyte related genes (a and b) Western blots (a) and recombination PCR (b) of control and
Drosophila Free-Running Rhythms Require Intercellular Communication
 Drosophila Free-Running Rhythms Require Intercellular Communication Ying Peng 1,2, Dan Stoleru 1,2, Joel D. Levine 1, Jeffrey C. Hall 1, Michael Rosbash 1,2* PLoS BIOLOGY 1 Department of Biology, Brandeis
Drosophila Free-Running Rhythms Require Intercellular Communication Ying Peng 1,2, Dan Stoleru 1,2, Joel D. Levine 1, Jeffrey C. Hall 1, Michael Rosbash 1,2* PLoS BIOLOGY 1 Department of Biology, Brandeis
The effect of light exposure on Drosophila melanogaster survival
 The effect of light exposure on Drosophila melanogaster survival Grace M. Chen, Caroline M. Kim, Steve Lee, Elsie Ng ABSTRACT The purpose of our study was to observe how exposure to light affects the survival
The effect of light exposure on Drosophila melanogaster survival Grace M. Chen, Caroline M. Kim, Steve Lee, Elsie Ng ABSTRACT The purpose of our study was to observe how exposure to light affects the survival
Supplementary Materials and Methods
 DD2 suppresses tumorigenicity of ovarian cancer cells by limiting cancer stem cell population Chunhua Han et al. Supplementary Materials and Methods Analysis of publicly available datasets: To analyze
DD2 suppresses tumorigenicity of ovarian cancer cells by limiting cancer stem cell population Chunhua Han et al. Supplementary Materials and Methods Analysis of publicly available datasets: To analyze
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION
 Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
PHYSIOLOGY AND MAINTENANCE Vol. V - Biological Rhythms - Tarja Porkka-Heiskanen, Jarmo T. Laitinen
 BIOLOGICAL RHYTHMS Tarja Porkka-Heiskanen, Institute of Biomedicine, University of Helsinki, Finland Jarmo T. Laitinen Department of Physiology, University of Kuopio, Finland Keywords: Light, melatonin,
BIOLOGICAL RHYTHMS Tarja Porkka-Heiskanen, Institute of Biomedicine, University of Helsinki, Finland Jarmo T. Laitinen Department of Physiology, University of Kuopio, Finland Keywords: Light, melatonin,
Astaxanthin prevents and reverses diet-induced insulin resistance and. steatohepatitis in mice: A comparison with vitamin E
 Supplementary Information Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E Yinhua Ni, 1,2 Mayumi Nagashimada, 1 Fen Zhuge, 1 Lili
Supplementary Information Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E Yinhua Ni, 1,2 Mayumi Nagashimada, 1 Fen Zhuge, 1 Lili
Supplementary Figure 1
 Supplementary Figure 1 3 3 3 1 1 Bregma -1.6mm 3 : Bregma Ref) Http://www.mbl.org/atlas165/atlas165_start.html Bregma -.18mm Supplementary Figure 1 Schematic representation of the utilized brain slice
Supplementary Figure 1 3 3 3 1 1 Bregma -1.6mm 3 : Bregma Ref) Http://www.mbl.org/atlas165/atlas165_start.html Bregma -.18mm Supplementary Figure 1 Schematic representation of the utilized brain slice
Supplemental Information. Th17 Lymphocytes Induce Neuronal. Cell Death in a Human ipsc-based. Model of Parkinson's Disease
 Cell Stem Cell, Volume 23 Supplemental Information Th17 Lymphocytes Induce Neuronal Cell Death in a Human ipsc-based Model of Parkinson's Disease Annika Sommer, Franz Maxreiter, Florian Krach, Tanja Fadler,
Cell Stem Cell, Volume 23 Supplemental Information Th17 Lymphocytes Induce Neuronal Cell Death in a Human ipsc-based Model of Parkinson's Disease Annika Sommer, Franz Maxreiter, Florian Krach, Tanja Fadler,
Neurobiology of Circadian Rhythms
 ARC-IBRO ISN Joined Neuroscience School Behavioural Bioassays in Neuroscience: Brain and Behavior From Invertabrates To Small Mammals 4-14 December 2014 ICIPE, Nairobi KENYA Neurobiology of Circadian Rhythms
ARC-IBRO ISN Joined Neuroscience School Behavioural Bioassays in Neuroscience: Brain and Behavior From Invertabrates To Small Mammals 4-14 December 2014 ICIPE, Nairobi KENYA Neurobiology of Circadian Rhythms
Supplementary Figure 1a
 Supplementary Figure 1a Hours: E-cadherin TGF-β On TGF-β Off 0 12 24 36 48 24 48 72 Vimentin βactin Fig. S1a. Treatment of AML12 cells with TGF-β induces EMT. Treatment of AML12 cells with TGF-β results
Supplementary Figure 1a Hours: E-cadherin TGF-β On TGF-β Off 0 12 24 36 48 24 48 72 Vimentin βactin Fig. S1a. Treatment of AML12 cells with TGF-β induces EMT. Treatment of AML12 cells with TGF-β results
Neural clocks and Neuropeptide F/Y regulate circadian gene expression in a peripheral metabolic tissue
 RESEARCH ARTICLE Neural clocks and Neuropeptide F/Y regulate circadian gene expression in a peripheral metabolic tissue Renske Erion 1, Anna N King 1, Gang Wu 2, John B Hogenesch 3, Amita Sehgal 1 * 1
RESEARCH ARTICLE Neural clocks and Neuropeptide F/Y regulate circadian gene expression in a peripheral metabolic tissue Renske Erion 1, Anna N King 1, Gang Wu 2, John B Hogenesch 3, Amita Sehgal 1 * 1
Oligo Sequence* bp %GC Tm Hair Hm Ht Position Size Ref. HIVrt-F 5 -CTA-gAA-CTT-TRA-ATg-CAT-ggg-TAA-AAg-TA
 Human immunodeficiency virus (HIV) detection & quantitation by qrt-pcr (Taqman). Created on: Oct 26, 2010; Last modified by: Jul 17, 2017; Version: 3.0 This protocol describes the qrt-pcr taqman based
Human immunodeficiency virus (HIV) detection & quantitation by qrt-pcr (Taqman). Created on: Oct 26, 2010; Last modified by: Jul 17, 2017; Version: 3.0 This protocol describes the qrt-pcr taqman based
Behavioral and molecular analyses suggest that circadian output is disrupted by disconnected mutants
 The EMBO Journal vol. 1 1 no. 1 pp. 1-6, 1992 Behavioral and molecular analyses suggest that circadian output is disrupted by disconnected mutants in D.melanogaster Paul E.Hardin1,2,3, Jeffrey C.Hall2
The EMBO Journal vol. 1 1 no. 1 pp. 1-6, 1992 Behavioral and molecular analyses suggest that circadian output is disrupted by disconnected mutants in D.melanogaster Paul E.Hardin1,2,3, Jeffrey C.Hall2
L I F E S C I E N C E S
 1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 NOVEMBER 2, 2006
1a L I F E S C I E N C E S 5 -UUA AUA UUC GAA AGC UGC AUC GAA AAC UGU GAA UCA-3 5 -TTA ATA TTC GAA AGC TGC ATC GAA AAC TGT GAA TCA-3 3 -AAT TAT AAG CTT TCG ACG TAG CTT TTG ACA CTT AGT-5 NOVEMBER 2, 2006
Make sure you remember the Key Concepts
 A2 Psychology Term 1 Module 4 Physiological Psychology Biological Rhythms, Sleep and Dreaming Area of Study: Biological Rhythms. Lesson 7 Getting you Thinking pg 403 Make sure you remember the Key Concepts
A2 Psychology Term 1 Module 4 Physiological Psychology Biological Rhythms, Sleep and Dreaming Area of Study: Biological Rhythms. Lesson 7 Getting you Thinking pg 403 Make sure you remember the Key Concepts
The CREB-binding protein affects the circadian regulation of behaviour
 The CREB-binding protein affects the circadian regulation of behaviour Christian Maurer, Tobias Winter, Siwei Chen, Hsiu-Cheng Hung and Frank Weber Biochemistry Center Heidelberg, University of Heidelberg,
The CREB-binding protein affects the circadian regulation of behaviour Christian Maurer, Tobias Winter, Siwei Chen, Hsiu-Cheng Hung and Frank Weber Biochemistry Center Heidelberg, University of Heidelberg,
SUPPLEMENTAL FIGURE 1
 SUPPLEMENTL FIGURE 1 C Supplemental Figure 1. pproach for removal of snorns from Rpl13a gene. () Wild type Rpl13a exonintron structure is shown, with exo in black and intronic snorns in red rectangles.
SUPPLEMENTL FIGURE 1 C Supplemental Figure 1. pproach for removal of snorns from Rpl13a gene. () Wild type Rpl13a exonintron structure is shown, with exo in black and intronic snorns in red rectangles.
Modeling Rhythms on Differents Levels: Cells, Tissues, and Organisms
 Modeling Rhythms on Differents Levels: Cells, Tissues, and Organisms Hanspeter Herzel Institute for Theoretical Biology (ITB) Charité and Humboldt University Berlin Molecular Chronobiology SCN-neuron nucleus
Modeling Rhythms on Differents Levels: Cells, Tissues, and Organisms Hanspeter Herzel Institute for Theoretical Biology (ITB) Charité and Humboldt University Berlin Molecular Chronobiology SCN-neuron nucleus
Supplementary Information
 Supplementary Information Remodeling of heterochromatin structure slows neuropathological progression and prolongs survival in an animal model of Huntington s disease Junghee Lee, Yu Jin Hwang, Yunha Kim,
Supplementary Information Remodeling of heterochromatin structure slows neuropathological progression and prolongs survival in an animal model of Huntington s disease Junghee Lee, Yu Jin Hwang, Yunha Kim,
Regulation of Feeding and Metabolism by Neuronal and Peripheral Clocks in Drosophila
 Article Regulation of Feeding and Metabolism by Neuronal and Peripheral Clocks in Drosophila Kanyan Xu, 1 Xiangzhong Zheng, 2 and Amita Sehgal 1, * 1 Howard Hughes Medical Institute, Department of Neuroscience,
Article Regulation of Feeding and Metabolism by Neuronal and Peripheral Clocks in Drosophila Kanyan Xu, 1 Xiangzhong Zheng, 2 and Amita Sehgal 1, * 1 Howard Hughes Medical Institute, Department of Neuroscience,
An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action
 An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action Supplementary Figure : Expression levels of toll-like receptor 4 (Tlr4) in muse lung does not change throughout the
An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action Supplementary Figure : Expression levels of toll-like receptor 4 (Tlr4) in muse lung does not change throughout the
Organization of the Drosophila Circadian Control Circuit
 Current Biology 18, R84 R93, January 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2007.11.061 Organization of the Drosophila Circadian Control Circuit Review Michael N. Nitabach 1
Current Biology 18, R84 R93, January 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2007.11.061 Organization of the Drosophila Circadian Control Circuit Review Michael N. Nitabach 1
Inheritance of Aldehyde Oxidase in Drosophila melanogaster
 Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Supplemental Figures: Supplemental Figure 1
 Supplemental Figures: Supplemental Figure 1 Suppl. Figure 1. BM-DC infection with H. pylori does not induce cytotoxicity and treatment of BM-DCs with H. pylori sonicate, but not heat-inactivated bacteria,
Supplemental Figures: Supplemental Figure 1 Suppl. Figure 1. BM-DC infection with H. pylori does not induce cytotoxicity and treatment of BM-DCs with H. pylori sonicate, but not heat-inactivated bacteria,
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels Amranul Haque, Pantea Gheibi, Yandong Gao, Elena
SUPPORTING INFORMATION Biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels Amranul Haque, Pantea Gheibi, Yandong Gao, Elena
