Failure to follow instructions may lead to patient injury.
|
|
|
- Maurice Perry
- 6 years ago
- Views:
Transcription
1 STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient injury. This package insert is designed to provide Instructions for Use of the STABLYX CMC Arthroplasty System; it is not a reference to surgical techniques. Prior to use of the STABLYX CMC Arthroplasty System the surgeon should become familiar with all information contained in this pamphlet. Description: The STABLYX CMC Arthroplasty System is a hemi joint replacement for the base of the first metacarpal of the first Carpometacarpal (CMC) joint. The monoblock prosthesis is a single piece prosthesis having a highly polished, saddle shaped (toroidal) articular surface designed to mirror the normal anatomy of the base of the first metacarpal. The STABLYX prosthesis bears against the mating saddle articular surface of the trapezium. The saddle design of the STABLYX CMC prosthesis allows for flexion-extension, abduction-adduction and opposition motions. It is comprised of Cobalt Chrome (CoCr) with a Titanium Plasma Sprayed (TPS) stem, which is press fit into the medullary canal. Each prosthesis is packaged and provided sterile. The STABLYX CMC Arthroplasty System is comprised of: Multiple sized hemi joint prosthesis System specific instrumentation Indications for Use: The STABLYX CMC Arthroplasty System is intended to replace the base (proximal end) of the first metacarpal in cases of rheumatoid arthritis, traumatic arthritis, osteoarthritis or post fracture deformation of bone loss that present as either a painful, unstable thumb, or a thumb with limited range of motion. This implant is intended for non-cemented, press fit use. Page 1 of 5
2 Contraindications: The STABLYX CMC Arthroplasty System should not be used if any of the following are present: active or latent infection, sepsis, insufficient quantity or quality of bone and/or soft tissue, material sensitivity, or patients who are unwilling or incapable of following post operative care instructions.! Warnings and Precautions: Do not use the STABLYX CMC where the trapezium is severely compromised and the implant cannot be supported. The saddle shape of the implant requires a normal or near normal share of the distal surface of the trapezium. Avoid injury to the Flexor Carpi Radialis and Flexor Policis Longus tendons while exposing the palmer aspect of the CMC joint, resecting bone and removing osteophytes. Avoid a longitudinal capsulotomy, as it is more likely to devascularize the trapezium. Excise suffient bone and osteophytes from the trapazium to allow proper seating of the prosthesis. When reducing the CMC joint, be sure to avoid injury to the articular surface of the trapezium. Assure the CMC joint is congruent throughout its full arc of motion, and that adequate soft tissue tensioning has been achieved. Improper selection, placement, positioning, alignment or fixation of the implants may result in unusual stress conditions which may lead to subsequent reduction in the service life of the prosthetic components, postoperative complications, or ineffective treatment. Malalignment of the components or inaccurate implantation can lead to excessive wear and/or failure of the prosthesis or anatomical structures. When using the optional suture attachment point, keep the suture line taut during impaction of the prosthesis so that the suture is not kinked or damaged. For safe and effective use of the implant, the surgeon must be thoroughly familiar with the surgical technique for the device, implant, and associated instruments. Improper insertion of the device during implantation may also increase the possibility of loosening, migration and failure of the device or the treatment. The device is not designed to withstand the stress of weight bearing, load bearing, or excessive physical activity. Device loosening, bone or soft tissue injury may occur when the implant is subjected to excessive loading. The information in this document should be shared with the patient. Potential STABLYX CMC Arthroplasty System construct failures such as stress fractures of the bones, loosening of the construct and/or fixation, subsidence, soft tissue irritation, or incomplete healing may occur as a result of non compliance to post operative rehabilitation, excessive activities or construct overloading. The patient must be cautioned, preferably in writing, about the use, limitations and possible adverse effects of this device including the possibility of device or treatment failure as a result of loose fixation, loosening, stress, excess activity, or weight bearing or load bearing, and the possibility of nerve or soft tissue damage related to either surgical trauma or the presence of the device. The patient should be informed about the importance of following the postoperative rehabilitation prescribed in order to fully understand the possible limitations in activities of daily living. The patient must be warned that failure to follow postoperative care instructions may cause the prosthesis or treatment to fail. DO NOT reuse any of the STABLYX CMC Arthroplasty System implants. Reuse may compromise the structural integrity of the construct and/or lead to failure, which may result in patient injury. The STABLYX CMC implant is supplied sterile using gamma radiation sterilization. DO NOT use if sterile barrier is damaged or if the USE BY date has expired. Any implantable components used with an expired USE BY date will void the product warranty. The implantable components are for single use only; DO NOT reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization of the implantable components may: o Compromise the structural integrity of the construct o Lead to failure resulting in patient injury o Create a risk of contamination of the device causing patient infection or cross-contamination o Lead to the transmission of infectious disease(s) from one patient to another Protect the implants against scratching or nicking as such stress concentration may lead to construct failure. Before using the STABLYX CMC Arthroplasty System, inspect all prosthesis and instruments for wear, disfiguration and physical damage. If evidence of wear, disfiguration or physical damage is found, DO NOT use and contact your local Skeletal Dynamics representative or the Skeletal Dynamics Customer Care Department. The STABLYX CMC Arthroplasty System has not been evaluated for safety and compatibility in the MR environment. It has not been tested for heating, migration or image artifact in the MR environment. The safety of the device in the MR environment in unknown. Scanning a patient who has this device may result in patient injury. Page 2 of 5
3 To maintain product traceability, record the Lot number for each of the implanted system components. DO NOT mix implant components from different manufacturers for metallurgical, biomechanical, and functional reasons. The benefits from implant surgery may not meet the patient s expectations or may deteriorate over time, requiring revision surgery to replace the implant or to carry out alternative procedures. The STABLYX CMC Arthroplasty System is to be used only with Skeletal Dynamics instruments, implants and accessories. Dispose of contaminated implants and instruments per established facilities guidelines and protocols. Caution should be taken for interference to pacemakers during electrocautery or by uncertified drills. Seek medical help immediately if the implant malfunctions. Potential Adverse Events: The following potential risks or discomforts have been associated with carpometacarpal arthroplasty: Disassociation, loosening or migration of the prosthesis, infection, erosion and desvascularization of the trapezium, material sensitivity reaction, tendon and nerve injuries, undesirable shortening or lengthening of thumb, stiffness of the CMC joint, dislocation or subluxation due to improper positioning, wear and deformation of the articular surfaces, intraoperative and postoperative bone fracture and/or postoperative pain and infection. Directions for Use: CMC arthroplasty requires skill and training by the medical professional. The STABLYX CMC Arthroplasty System should only be used by surgeons who have experience with the system. Each surgeon must evaluate the appropriateness for the use of the STABLYX CMC Arthroplasty System during CMC arthroplasty procedures based on their experience with the STABLYX CMC Arthroplasty System. Please refer to the STABLYX CMC Arthroplasty Surgical Technique Guide (MKT ) to review the surgical approach to CMC arthroplasty as described by Jorge L. Orbay, M.D. of the Miami Hand Institute located in Miami, Florida. Cleaning: The recommended manual cleaning instructions are set forth below. Other cleaning methods must be validated by the user. Instrument Cleaning The STABLYX CMC Arthroplasty System instrumentation must be cleaned thoroughly before re-use to achieve sterilization.! Warnings & Precautions System instruments and accessories should be decontaminated immediately after completion of the surgical procedure. Contaminated instruments should not be allowed to dry prior to cleaning/reprocessing. Excess blood or debris should be wiped off to prevent it from drying. Only qualified personnel with documented evidence of training and competency should clean the instruments. Training should be inclusive of current applicable guidelines and standards and healthcare facility policies. Avoid the use of metal brushes or scouring pads during the cleaning process. Instruments should be rinsed of cleaning agents to prevent residue. Do not use mineral oil or silicone lubricants on instruments. Neutral ph enzymatic and cleaning agents are recommended for cleaning instruments. It is important that alkaline cleaning agents are thoroughly neutralized and rinsed from instruments. Prior to sterilization, instruments should be inspected for cleanliness of surfaces, joints, lumens, proper function, and wear and tear. Cleaning Instructions Cleaning should begin at the point of use prior to processing. Keep instruments moist after use to prevent soil from drying on them. An enzymatic detergent (Enzol) was used to validate the cleaning process. 1. Disassemble instrumentation, if applicable. 2. Rinse components thoroughly under running cool tap water. While rinsing, use a soft bristle brush to loosen and remove as much visible soil as possible from components. 3. Soak components in a neutral enzymatic cleaner for a minimum of ten (10) minutes. Components must be fully immersed in the cleaner. Follow the cleaner manufacturer s instructions for cleaner preparation and exposure time. Page 3 of 5
4 4. Thoroughly rinse the components with cool water. While rinsing, use soft bristle brushes, pipettes or a water jet to clean out lumens, holes, and other challenging features. 5. Manually scrub the components thoroughly in newly made, clean, neutral ph enzymatic cleaner using soft bristle brushes or pipettes. All lumens, holes, hinged components, mating surfaces, and crevices, and challenging components should be thoroughly scrubbed. Actuate all moveable features and expose all areas to cleaner and to the brush or pipette. Note: When scrubbing rasps, a stiff bristle brush will be required. 6. Rinse components thoroughly with deionized or purified water; using pipettes or a water jet to clean out lumens, holes, and other hard to reach or challenging features. Actuate all movable features to fully irrigate all areas. 7. Visually inspect components for soil. Repeat the cleaning procedure until no visible soil remains on the components. 8. Perform a final rinse on the components using deionized water or purified water. 9. Dry the clean components using compressed air or a soft, lint free, clean cloth. Sterilization: The STABLYX CMC Arthroplasty System implantable components have been sealed then sterilized by gamma radiation. The implants are provided in an undamaged package. If any of the implants or the package appears damaged, expiration date has been exceeded, or if sterility is questionable, the implant should not be used. DO NOT re-sterilize the implantable components. Trial components are available in the system to avoid opening the sterile package prior to prosthesis implantation. The implants should be removed from their sterile package only after the implant site has been prepared and properly sized. The accessories and instruments of the STABLYX CMC Arthroplasty Implant Set are provided non sterile. They are intended for steam sterilization at the healthcare facility. Steam sterilization may be accomplished using one of the cycles shown below. Cycle Type Temperature Duration Drying Time Pre-Vacuum Autoclave 270 F (132 C) 4 minutes (wrapped) 20 minutes Gravity Autoclave 270 F (132 C) 15 minutes (wrapped) 20 minutes Follow ANSI/AAMI ST79: Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Flash sterilization is not recommended, but if used, should only be performed according to the requirements of ANSI/AAMI ST79: Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Usage of an FDA cleared wrap is recommended to ensure the accessories and instruments are sterile prior to use. Subsequent instrument sterilization needs to be performed in the caddy provided. For reuse and sterilization, instruments should be arranged within the caddy in the manner supplied by the company. Handling and Storage: When not in use, store the clean and disinfected STABLYX CMC Arthroplasty Set within the Sterilization Tray. Prior to use, inspect the instrumentation for serviceability and the sterile packaged implants for signs of tampering or water contamination. Disclaimer of Warranty and Limited Remedies: Skeletal Dynamics, LLC makes no express or implied warranty, including any implied warranty of merchantability or fitness for a particular purpose, on the product(s) described in this publication. Skeletal Dynamics, LLC shall not be liable under any circumstances for any direct, incidental or consequential damages other than as expressly provided by specific law. No person has authority to bind Skeletal Dynamics, LLC to any representation or warranty except as specifically set forth in this publication. Descriptions or specifications provided by Skeletal Dynamics, LLC in any publication are only included to generally describe the product when manufactured and do not constitute any express warranties. Page 4 of 5
5 STABLYX CMC Arthroplasty Set - Cat.# STX-CMA-SYS Catalog # Nomenclature Implants STX-CMA-IS1 STABLYX, CMC Arthroplasty Implant, Sz. 1 STX-CMA-IS2 STABLYX, CMC Arthroplasty Implant, Sz. 2 STX-CMA-IS3 STABLYX, CMC Arthroplasty Implant, Sz. 3 STX-CMA-IS4 STABLYX, CMC Arthroplasty Implant, Sz. 4 STX-CMA-IS5 STABLYX, CMC Arthroplasty Implant, Sz. 5 Trials STX-CMA-TS1 STABLYX, CMC Arthroplasty Trial, Sz. 1 STX-CMA-TS2 STABLYX, CMC Arthroplasty Trial, Sz. 2 STX-CMA-TS3 STABLYX, CMC Arthroplasty Trial, Sz. 3 STX-CMA-TS4 STABLYX, CMC Arthroplasty Trial, Sz. 4 STX-CMA-TS5 STABLYX, CMC Arthroplasty Trial, Sz. 5 System Instrumentation HNDL-UQC-FXD Handle, Universal Quick Connect, Fixed FRCP-BHS-RTC Forceps, Bone Holding Small, Ratcheting STX-VCE STABLYX, Volar Capsule Elevator / Shoehorn STX-VOR STABLYX, Volar Osteophyte Rasp STX-MR1-SP STABLYX, Metacarpal Rasp, Sz. 1 STX-MR2-SP STABLYX, Metacarpal Rasp, Sz. 2 STX-MR3-SP STABLYX, Metacarpal Rasp, Sz. 3 STX-MR4-SP STABLYX, Metacarpal Rasp, Sz. 4 STX-MR5-SP STABLYX, Metacarpal Rasp, Sz. 5 STX-MP1 STABLYX, Metacarpal Planar, Sz. 1 STX-CMA-SZ1 STABLYX, Trapezial Sizer, Sz. 1 STX-CMA-SZ2 STABLYX, Trapezial Sizer, Sz. 2 STX-CMA-SZ3 STABLYX, Trapezial Sizer, Sz. 3 STX-CMA-SZ4 STABLYX, Trapezial Sizer, Sz. 4 STX-CMA-SZ5 STABLYX, Trapezial Sizer, Sz. 5 STX-CMA-TC1 STABLYX, Trapezial Contouring Tool STX-CMA-IMP STABLYX, CMC Impactor OST-CRV Osteotome, Curved STX-CMA-STP AWL-STR-020 Sterilization Trays STX-CMC-TRAY STABLYX, Norman Suture Passer Awl, Straight, 2.0mm STABLYX CMC Arthroplasty System Sterilization Tray Customer Care Center: Skeletal Dynamics, LLC / 8905 SW 87 th Ave. / Suite 201 / Miami, FL / United States / Emergo Europe, Molenstraat 15, 2513 BH The Hague, The Netherlands Page 5 of 5
ALIGN Radial Head System INSTRUCTIONS FOR USE
 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand Institute Miami, Florida. System Overview The ALIGN Radial Head System is designed to restore the kinematics of the native radial
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand Institute Miami, Florida. System Overview The ALIGN Radial Head System is designed to restore the kinematics of the native radial
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 EXPOSURE Make an 8cm to 10cm longitudinal incision centered over Lister s tubercle
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 EXPOSURE Make an 8cm to 10cm longitudinal incision centered over Lister s tubercle
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Positioning Sleeve Surgical Technique
 Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Integra. Silicone Toe System SURGICAL TECHNIQUE
 Silicone Toe System SURGICAL TECHNIQUE Silicone Toe System Table of Contents Indications...2 Contraindications...2 Warnings...2 Precautions...2 Adverse Events...2 Flexible Great Toe (FGT)...3 Surgical
Silicone Toe System SURGICAL TECHNIQUE Silicone Toe System Table of Contents Indications...2 Contraindications...2 Warnings...2 Precautions...2 Adverse Events...2 Flexible Great Toe (FGT)...3 Surgical
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Integra. PyroSphere System SURGICAL TECHNIQUE
 Integra PyroSphere System SURGICAL TECHNIQUE Table of Contents Indications For Use...02 Contraindications...02 Warnings...03 Precautions...03 Patient Positioning...03 Surgical Technique... 04 Step 1 Initial
Integra PyroSphere System SURGICAL TECHNIQUE Table of Contents Indications For Use...02 Contraindications...02 Warnings...03 Precautions...03 Patient Positioning...03 Surgical Technique... 04 Step 1 Initial
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
Silicone PIP, MCP & MCP-X (PreFlex)
 Silicone PIP, MCP & MCP-X (PreFlex) Finger Joint Arthroplasty Operative Technique Silicone PIP Silicone MCP Silicone PreFlex (MCP-X) Stryker Disclaimer This publication sets forth detailed recommended
Silicone PIP, MCP & MCP-X (PreFlex) Finger Joint Arthroplasty Operative Technique Silicone PIP Silicone MCP Silicone PreFlex (MCP-X) Stryker Disclaimer This publication sets forth detailed recommended
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
TMJ Fossa-Eminence and Condylar Prosthesis System. Instructions for Use. For total reconstruction (arthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence and Condylar Prosthesis System Instructions for Use For total reconstruction (arthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF:
TMJ Fossa-Eminence and Condylar Prosthesis System Instructions for Use For total reconstruction (arthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF:
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Integra. Silicone PIP Implant SURGICAL TECHNIQUE
 Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
LMH. Minimal Bone Resection. Lesser Metatarsal Head Implant. Thin, low-profile design for minimal bone resection
 Minimal Bone Resection LMH Lesser Metatarsal Head Implant Thin, low-profile design for minimal bone resection Stem offset dorsally for anatomically correct alignment in medullary canal Rectangular shape
Minimal Bone Resection LMH Lesser Metatarsal Head Implant Thin, low-profile design for minimal bone resection Stem offset dorsally for anatomically correct alignment in medullary canal Rectangular shape
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
Description Materials Indications Patient selection factors to be considered include: Contraindications Absolute contraindications include:
 Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 ELBOW LANDMARKS With the elbow flexed 90 0, palpate and mark the lateral epicondyle.
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 ELBOW LANDMARKS With the elbow flexed 90 0, palpate and mark the lateral epicondyle.
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
TriboFit Hip System. Issue 3 - August 2013 English
 Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
MICROPORT KNEE SYSTEMS The following languages are included in this packet:
 EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
Instructions for use for Adler Cemented Total Hip Replacement Prosthesis
 0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM
 STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
STERILE IMPLANT PRODUCT INSERT PATIENT-FITTED TEMPOROMANDIBULAR JOINT RECONSTRUCTION PROSTHESIS SYSTEM CAUTION United States Federal Law restricts this device to sale by or on the order of a physician.
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Important notice: the device(s) can be prescribed and implanted only by a doctor legally authorized to perform this type of surgery.
 rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
OSKAR Radial Head Prosthesis
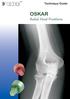 Technique Guide OSKAR Radial Head Prosthesis DESCRIPTION The OSKAR Radial Head Implant is a pliable, onepiece intramedullary-stemmed cuffed implant designed to help preserve the joint space and relationships
Technique Guide OSKAR Radial Head Prosthesis DESCRIPTION The OSKAR Radial Head Implant is a pliable, onepiece intramedullary-stemmed cuffed implant designed to help preserve the joint space and relationships
